Abstract
Stilbenes are a class of polyphenolic compounds, naturally found in a wide variety of dietary sources such as grapes, berries, peanuts, red wine, and some medicinal plants. There are several well-known stilbenes including trans-resveratrol, pterostilbene, and 3′-hydroxypterostilbene. The core chemical structure of stilbene compounds is 1,2-diphenylethylene. Recently, stilbenes have attracted extensive attention and interest due to their wide range of health-beneficial effects such as anti-inflammation, -carcinogenic, -diabetes, and -dyslipidemia activities. Moreover, accumulating in vitro and in vivo studies have reported that stilbene compounds act as inducers of multiple cell-death pathways such as apoptosis, cell cycle arrest, and autophagy for chemopreventive and chemotherapeutic agents in several types of cancer cells. The aim of this review is to highlight recent molecular findings and biological actions of trans-resveratrol, pterostilbene, and 3′-hydroxypterostilbene.
Keywords: 3′-hydroxypterostilbene, bioavailability, biological actions, pterostilbene, resveratrol, stilbenes
1. Introduction
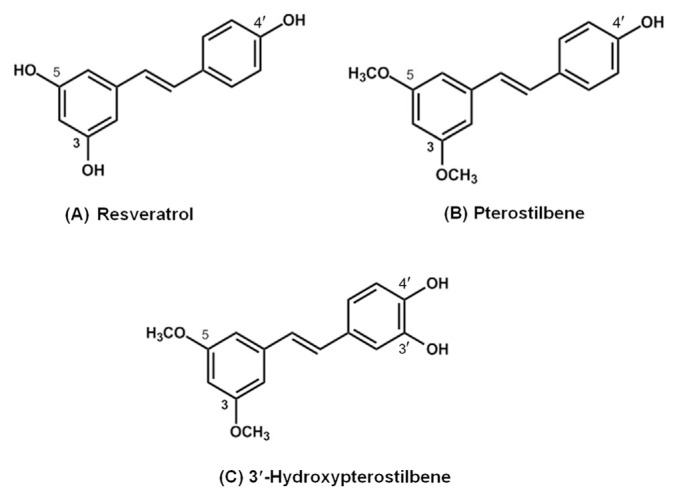
The natural active compounds from plant or herbal origins are being investigated for their bioactivities [1]. Stilbenes are phytochemicals with ~200–300 g/mol molecular weight, a subclass of polyphenolic compounds [2]. They are naturally found in a wide variety of dietary sources such as grapes, blueberries, red wine, and some other plants [3–5]. Family members of the stilbene have a C6-C2-C6 basic skeleton and consist of two phenyl groups linked by an ethene double bond. There are several well-known stilbenes such as trans-resveratrol, pterostilbene, and 3′-hydroxypterostilbene (Figure 1).
Figure 1.
Chemical structures of (A) resveratrol, (B) pterostilbene, and (C) 3′-hydroxypterostilbene.
Resveratrol (3,4′,5-trihydroxy-trans-stilbene) is the most extensively studied stilbene found in grape skin, berries, peanuts, and some medicinal plants [3,4,6]. Hundreds of studies have shown that resveratrol plays a critical role in human health and diseases due to its diverse biological and pharmacological actions including antioxidation, -inflammation, -carcinogenic, and -diabetic potencies [7–13]. However, one potential issue surrounding resveratrol is that resveratrol has a very low bioavailability that may lower its biological effectiveness [14–16].
Pterostilbene, the 3,5-dimethoxy motif at the A-phenyl ring of resveratrol, has recently received tremendous attention due to its promising chemopreventive and chemotherapeutic properties [17–26]. Due to its structural characteristic, pterostilbene is more lipophilic, exhibits better bioavailability, and is more biologically active than resveratrol [16]. Pterostilbene is primarily found in a tree wood, Pterocarpus marsupium, which is a traditional herbal medicine used for the treatment of diabetes [27]. Moreover, in recent studies, pterostilbene have been reported to have powerful growth-inhibitory effects in several different types of cancer cells, notably breast, colon, and prostate cancer cells. [18,25,28]. In multiple research findings, pterostilbene was shown to be an effective apoptotic and autophagic agent that is able to inhibit cancer cell viability, induce cell cycle arrest, alter apoptosis expression gene, promote autophagy-related proteins, and inhibit cancer cells from metastasizing [18,25,28,29].
3′-Hydroxypterostilbene (trans-3,5-dimethoxy-3′,4′-dihydroxystilbene), one of metabolites of pterostilbene [30], can also be isolated from whole plant of the herb Sphaerophysa salsula, a shrub widely distributed in central Asia and northwest China [20,31]. The latest researches showed that 3′-hydroxypterostilbene appeared to contribute stronger biological activities than pterostilbene on human colon cancer cells [28].
This article reviews the recent advances in understanding the biological activities, molecular effects, and bioavailability of resveratrol, pterostilbene, and 3′-hydroxypterostilbene, and summarizes their clinical potential for the prevention and treatment of chronic diseases.
2. Resveratrol
Resveratrol (trans-3,5,4′-trihydroxystilbene), is a natural polyphenolic phytoalexin compound produced by plants to protect them from injury, uv irradiation, and fungal attack [32,33]. It is one of well-studied stilbenes mainly found in grapes and red wine; however, the presence of resveratrol has also been detected in other plants such as peanuts, pistachios, and berries [3,4,34]. In the past few years, the interest in resveratrol has extensively increased and a lot of scientific evidence has demonstrated that resveratrol exerts a plethora of biological functions, especially in the protective effects of chronic diseases, such as anti-inflammation, -cancer, -diabetes, and -obesity activities [7–10,12] (Table 1).
Table 1.
The biological activities and molecular effects of trans-resveratrol.
| Modes/treatments | Model used | Mechanism | References |
|---|---|---|---|
| Inflammation | |||
| Resveratrol mixed in AIN-93 diet (300 ppm) | DSS-induced colitis C57BL/6 mice | ↓iNOS, ↓COX-2, ↓TNF-α, ↓% of neutrophils in mesenteric lymph nodes & lamina propria, ↓CD3+ T-cell counts | Cui et al, 2010 [36] |
| Resveratrol (3 mg/kg BW) | DSS-induced colitis C57BL/6 mice | ↓Disease activity index, ↑weight/length of the colon, ↓Histological signs of cell damage | Sánchez-Fidalgo et al, 2010 [37] |
| Resveratrol (10–40μM) | RAW 264.7 | ↓TNF-α, ↓IL-1β, ↓IL-10, ↓iNOS, ↓PGES-1, ↓COX-2, ↓p-p38, ↓NO, ↓PGE2, ↓iNOS, ↓COX-2, ↓IL-1β, ↓IL-6, ↓TNF-α, | Lee et al, 2015 [9] |
| Co-administration of resveratrol (25mg/kg) & apigenin (50 mg/kg) | Carrageenan-induced paw edema in mice | ↓Edema, ↑Apigenin bioavailability, ↓apigenin glucuronides | Lee et al, 2015 [9] |
| Breast cancer | |||
| Resveratrol (16–64μM) | MCF-7casp-3 (caspase sensitive) | ↓Cell viability, ↑Fragmented & condensed nuclei, ↑caspase-3/7, ↑PARP cleavage, ↑ autophagosome, ↑LC3II, ↓Akt/PKB phosphorylation,↓p70S6K phosphorylation | Scarlatti et al, 2008 [38] |
| Resveratrol (16–64μM) | MCF-7vc (caspase insensitive) | ↓Cell viability, ↑Fragmented & condensed nuclei, ↑autophagosome, ↑LC3II, ↓Akt/PKB phosphorylation, ↓p70S6K phosphorylation | Scarlatti et al, 2008 [38] |
| Resveratrol (10–50μM) | MDA-MB-231, MCF-7, & BT-549 | ↓Cell viability, ↑rapamycin sensitivity, ↑apoptosis, ↑cell cycle arrest, ↓Akt phosphorylation | He et al, 2011 [39] |
| Prostate cancer | |||
| Resveratrol (5–100μM) | PC-3-M-MM2 | ↓Cell viability, ↑apoptosis, ↑TUNEL, ↓cell migration (wound healing assay), ↓cell invasion (Boyden chamber invasion assay), ↓miR-21, ↑maspin, ↑PDCD4, ↓Akt phosphorylation | Sheth et al, 2012 [44] |
| Resveratrol (20 mg/kg BW) | PC-3M-MM2 xenograft SCID mice | ↓Tumor volume, ↓tumor weight, ↓lung metastasis, ↓Akt phosphorylation, ↓miR-21, ↑PDCD4 | Sheth et al, 2012 [44] |
| Resveratrol (25–100μM) | PC-3 & DU145 | ↓Cell viability, ↓STIM1, ↓STIM1-TRPC1, ↓store-mediated Ca2+ entry, ↑LC3B-II, ↑GFP-LC3B, ↑GRP78, ↑CHOP, ↓p-mTOR, ↑p-AMPK, ↓p- p70S6K | Selvaraj et al, 2015 [43] |
| Colon cancer | |||
| Resveratrol mixed in AIN-93 diet (300 ppm) | AOM/DSS-induced colonic carcinogenesis C57BL/6 mice | ↓Colon tumor incidence, ↓tumor multiplicity, ↓tumor volume | Cui et al, 2010 [36] |
| Resveratrol (5–100μM) | HCT-116, HT-29, & Caco-2 | ↓Cell viability, ↓colony formation capacity, ↑apoptosis, ↑caspase-3, ↑PARP | Nutakul et al, 2011 [15] |
| Resveratrol (25–150μM) | HT-29 & COLO 201 | ↓Cell viability, ↓colony formation capacity, ↑apoptosis, ↑caspase-3, ↑caspase-8, ↑PARP, ↑autophagy, ↑LC3B-II, ↑autophagosome, ↑ROS production | Miki et al, 2012 [45] |
| Diabetes | |||
| Resveratrol (0.02% w/w) | C57BL/KsJ-db/db mice | ↓Blood glucose, ↓free fatty acid, ↓triglyceride, ↓ApoB/Apo AI, ↑adiponectin, ↓hemoglobin A1c, ↓PEPCK, ↓G6P, ↑GK, ↑SREBP, ↓hepatic glycogen, ↓ hepatic triglyceride, ↑pancreatic insulin protein, ↑insulin, ↑GLUT4, ↑p-AMPK, ↑uncoupling protein | Do et al, 2012 [47] |
| Resveratrol (250 mg/d) | Patients with type II diabetes | ↓Hemoglobin A1c, ↓systolic blood pressure | Bhatt et al, 2012 [46] |
| Resveratrol (5 mg/kg/d) | Streptozotocin-nicotinamide induced type II diabetes rats | ↑Glucose tolerance, ↑pancreatic insulin, ↑Cardiac antioxidant enzymes activities, ↑catalase, ↑superoxide peroxidase, ↓nitrate/nitrate, ↓8-isoprostane, ↓NF-kB | Mohammadshahi et al, 2014 [12] |
| Resveratrol (10 mg/kg BW) | C57BL/KsJ-Lepdb/+ | ↑Glucose metabolism, ↑insulin tolerance, ↑pAMPK, ↓pHDAC4, ↓G6Pase, ↑reproductive outcome | Yao et al, 2015 [7] |
| Obesity | |||
| Resveratrol (0.4%) | High-fat diet-induced obese mice | ↓Body weight gain, ↓epididymal adipose tissue, ↓perirenal adipose tissue, ↓mesenteric adipose tissue, ↓retroperitoneal adipose tissue, ↓triglyceride, ↓free fatty acid, ↓glucose, ↓HOMA-IR, ↓TNF-α, ↓MCP-1, ↓GalR1, ↓GalR2, ↓PKCδ, ↓Cyc-D, ↓E2F1, ↓PPARγ, ↓C/EBPα, ↓SREBP1c, ↓LXR, ↓FAS, ↓LPL, ↓aP2, ↓Leptin, ↓p-ERK | Kim et al, 2011 [48] |
| Resveratrol (150 mg/d) | Healthy obese men (BMI 28–36 kg/m2) | ↓Adipocyte diameter, ↓the proportions of very-large adipocytes (>90μM), ↓the proportions of large adipocytes (70–89μM), ↑the proportions of small adipocytes (< 50μM), ↓Wnt signaling pathway, ↓Notch signaling pathway, ↑lysosomal pathways, ↑phagosomal pathway | Konings et al, 2014 [13] |
| Resveratrol (0.03–10μM) | 3T3-L1 adipocyte | ↓Adipogenesis, ↓PPARγ, ↓perilipin, ↓lipolysis | Chang et al, 2016 [10] |
| Resveratrol (1-, 10-, 30-mg/kg BW) | High-fat diet-induced obese mice | ↓Body weight gain, ↓subcutaneous adipose tissue, ↓epididymal adipose tissue, ↓fat droplet accumulations | Chang et al, 2016 [10] |
| Aging | |||
| Resveratrol (2.5–10μM) | Saccharomyces cerevisiae | ↑Sir2, ↑lifespan | Howitz et al, 2003 [51] |
| Resveratrol (6.25–100μM) | Drosophila melanogaster & Caenorhabditis elegans | ↑Sir2, ↑lifespan | Wood et al, 2004 [52] |
| Resveratrol (22.4 mg/kg BW) | Mice on a high-calorie diet | ↑Lifespan, ↑insulin sensitivity, ↓IGF-1, ↑AMPK, ↑PGC-1α, ↑mitochondrial number, ↑motor function | Baur et al, 2006, [55] |
| Resveratrol (100-, 400- & 2400-mg/kg food) | Elderly mice (12-mo-old) | ↑Insulin sensitivity, ↑cardiovascular function, ↑bone density, ↑motor coordination, ↓inflammation, ↓vascular endothelium apoptosis, ↓cataract formation | Pearson et al, 2008 [56] |
| Resveratrol (30μM & 130μM) | Honey bees | ↑Lifespan, ↓the food consumption | Rascón et al, 2012 [54] |
| Resveratrol (0.05μM, 0.5μM, & 1μM) | D. melanogaster | ↑Lifespan, ↑stress resistance, ↓age-dependent decline of locomotor activity | Danilov et al, 2015 [57] |
| Resveratrol (200 μg/g diet) | Annual fish Nothobranchius guentheri | ↓ROS, ↑CAT, ↑SOD, ↑GPx, ↓carbonyl contents, ↓MDA, ↓lipofuscin | Liu et al, 2015 [59] |
| Resveratrol (20 mg/kg BW) | Aged rats (18-mo-old) | ↑The dendritic length & spine density in pyramidal neurons of prefrontal cortex, dorsal hippocampus, & dentate gyrus | Hernández-Hernández et al, 2016 [58] |
ApoAI = Apolipoprotein AI; ApoB = Apolipoprotein B; CAT = catalase; CD3+ T-cell = cluster of differentiation 3+ T-cell; C/EBPα = CCAAT-enhancer-binding protein alpha; COX-2 = cyclooxygenase-2; Cyc-D = cyclin-D; FAS = fatty acid synthase; G6P = glucose-6 phosphate; G6Pase = glucose-6 phosphatase; GalR1 = galanin receptor R1; GalR2 = galanin receptor R2; GFP-LC3B = green fluorescent protein-microtubule-associated protein 1 light chain 3 beta; GK = glucokinase; GLUT4 = glucose transporter 4; GPx = glutathione peroxidase; GRP78 = 78 kDa glucose-regulated protein; HOMA-IR = homeostatic model assessment-insulin resistance; IGF-1 = insulin-like growth factor-1; IL-1β = interleukin 1 beta; IL-6 = interleukin 6; IL-10 = interleukin 10; iNOS = inducible nitric oxide synthase; LC3 II = autophagy-related protein light chain 3 II; LPL = lipoprotein lipase; LXR = liver X receptor; MCP-1 = monocyte chemoattractant protein-1; MDA = malondialdehyde; miR-21 = microRNA-21; NF-κB = nuclear factor kappa B; NO = nitric oxide; p70S6K = 70 kDa ribosomal protein S6 kinase; p-AMPK = phosphorylated adenosine monophosphate activated protein kinase; PARP = poly ADP-ribose polymerase; PDCD4 = programmed cell death 4; PEPCK = phosphoenolpyruvate carboxykinase; p-ERK = phosphorylated extracellular signal-related kinase; PGC-1α = peroxisome proliferator-activated receptor gamma coactivator 1-alpha; PGE2 = prostaglandin E2; PGES-1 = prostaglandin E synthases-1; pHDAC4 = phospho-histone deacetylase 4; PKB = protein kinase B; PKCδ = protein kinase C delta; p-mTOR = phosphor-mammalian target of rapamycin; p-p38 = phopho-p38; PPARα = peroxisome proliferator activated receptor alpha; PPARγ = peroxisome proliferator activated receptor gamma; Sir2 = sirtuin 2; SOD = superoxide dismutase; ROS = reactive oxygen species; SREBP = sterol regulatory element-binding protein; TNF-α = tumor necrosis factor-α; TRPC1 = transient receptor potential cation channel subfamily C member 1; TUNEL = terminal deoxynucleotidyl transferase dUTP nick end labeling.
2.1. Inflammation
Chronic inflammation has been recognized as the root cause of various human diseases. The inflammatory processes may induce DNA mutations in cells via oxidative or nitrosative stress, which may influence normal cell functions and consequently lead to inflammatory diseases and cancer [35]. In an experiment conducted by Cui et al [36], it was demonstrated that resveratrol significantly suppressed inflammation markers such as inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), and tumor necrosis factor-α (TNF-α) in dextran sulfate sodium (DSS) mouse model of colitis. In addition, resveratrol inhibited neutrophils infiltration in the mesenteric lymph nodes and lamina propria, and decreased the numbers of CD3+ T cells that express TNF-α and interferon gamma (IFN-γ) in DSS-treated mice [36]. Sánchez-Fidalgo and co-workers [37] also found similar results. In their study using DSS-induced colitis model, it was determined that resveratrol supplementation attenuated chronic colonic inflammation with reduced proinflammatory cytokines, including interleukin-1 beta (IL-1β), IL-10, prostaglandin E synthases-1 (PGES-1), TNF-α, iNOS, and COX-2, via downregulation of the p38 MAPK (mitogen-activated protein kinases) signaling pathway [37].
Recently, the study performed by Lee et al [9] suggested that resveratrol could increase the bioavailability of apigenin, which is a bioactive flavonoid with strong anti-inflammation activities. Cotreatment of apigenin and resveratrol increased the levels of plasma apigenin up to 2.39 times compared to the apigenin-alone group. Moreover, cotreatment of apigenin and resveratrol significantly reduced the paw edema caused by carrageenan-induced inflammation in mice. These findings indicated that resveratrol could act as a biological enhancer to strengthen the anti-inflammatory activity of apigenin [9].
2.2. Breast cancer
A number of researchers have considered the anticarcinogenic effects of resveratrol in breast cancer [38–40]. A study conducted by Scarlatti et al [38] reported that resveratrol could inhibit human breast cancer cell proliferation and promote death via multiple pathways including apoptosis, cell cycle arrest in the S phase, and autophagy. Resveratrol was shown to induce cell death in both caspase-3 sensitive (MCF-7casp-3) and caspase-3 insensitive (MCF-7vc) human breast cancer cells. Thus, the effects of resveratrol on breast cancer cell death might not be fully dependent on caspase-3 [38]. Besides, resveratrol stimulated autophagy by activating noncanonical (Beclin-1 independent) routes in both cell lines. The molecular mechanism involved resveratrol-induced autophagy was associated with the suppression of Akt/protein kinase B (PKB) phosphorylation and mammalian target of rapamycin (mTOR)/S6K signaling pathway [38]. In another study, He et al [39] found that resveratrol and rapamycin combination might be a novel combinatorial strategy for breast cancer therapy. The findings of this study indicated that resveratrol significantly potentiate the anticancer activity of mTOR inhibitor rapamycin by suppressing rapamycin-induced AKt signaling pathway in MDA-MB-231, MCF-7, and BT-549 human breast cancer cells [39].
2.3. Prostate cancer
Prostate cancer epithelial cells possess large amounts of calcium channels that mediate multiple cellular processes such as cell proliferation, tumorigenesis, and migration [41,42]. Thus, modulation of calcium homeostasis seems to play an important role in the treatment of prostate cancer. In a recent study, Selvaraj et al [43] found that resveratrol could inhibit cell proliferation and survival of prostate cancer PC-3 and DU145 cells via downregulation of stromal interaction molecular 1 (STIM1) which acts as calcium sensor in endoplasmic reticulum (ER). Resveratrol treatment reduced the expression of STIM1, thereby, inhibiting the interaction of STIM1 and transient receptor potential channel 1 (TRPC1), which could attenuate Ca2+ entry and induce prostate cancer cell death [43]. In addition, resveratrol treatment also induced ER stress by decreasing ER calcium storage and storing operated calcium entry (SOCE). Moreover, resveratrol was shown to induce autophagic cell death via activation of AMP-activated protein kinase (AMPK) and inhibition of Akt/mTOR pathway [43].
Another study conducted by Sheth et al [44] in 2012 determined the chemopreventive properties of resveratrol in androgen insensitive and highly aggressive human prostate cancer cells, PC-3M-MM2. The results showed that resveratrol negatively regulated prostate cancer cell proliferation by inducing apoptosis. Resveratrol treatment substantially inhibited PC-3M-MM2 cell invasion and migration in Boyden chamber invasion and wound healing assays. Also, resveratrol enhanced tumor suppressor proteins, maspin, and programmed cell death 4 (PDCD4) expressions, via downregulation Akt /MicroRNA-21 (MiR-21) signaling pathway [44].
To further understand the antitumor efficacy of resveratrol in vivo, Sheth et al [44] performed a study in a PC-3M-MM2 xenograft-severe combined immunodeficient (SCID) mouse model. The findings showed that resveratrol treatment (20 mg/kg BW) for 5 weeks caused significant reduction of prostate tumor growth and the incidence and number of lung metastasis in SCID mice. The mechanisms involved in these antitumor and -metastatic effects of resveratrol were inhibition of Akt/MiR-21 pathway and upregulation of PDCD4 levels [44].
2.4. Colon cancer
Resveratrol also possesses significant anti-colon cancer activity [15,45]. In 2011, Nutakul et al [15] demonstrated that resveratrol exhibited anti-carcinogenic effects in three human colorectal carcinoma cells, Caco-2, HCT-116, and HT-29. Resveratrol was shown to induce apoptosis by activation of caspase-3 and poly (ADP-ribose) polymerase (PARP). Another study by Miki et al [45] indicated that resveratrol effectively induced cytotoxic effects by reactive oxygen species (ROS)-mediated apoptosis and autophagy in HT-29 and COLO 201 human colon cancer cells. Treated HT-29 and COLO 201 cells with resveratrol could trigger apoptosis via increasing the levels of caspase-8, -3, and PARP but have no effect on Bax and Bcl-xL levels. Thus, the proposed mechanisms involved resveratrol-induced apoptosis might undergo caspase-8/-3 activation. Besides, resveratrol was found to cause autophagic cell death by upregulating LC3 II protein level and increase the autophagosome formation in HT-29 and COLO 201 cells [45]. Moreover, resveratrol-induced apoptosis and autophagy are mediated by intracellular ROS production. Elevated level of ROS is associated with increased caspase-8 and -3 cleavage and LC3 II expression [45]. Therefore, the combination of resveratrol with ROS inducer might be useful for colon cancer treatment.
In vivo studies, Cui et al [36] demonstrated that resveratrol significantly suppressed the development of colon cancer. In azoxymethane (AOM)/DSS-induced colon cancer model, resveratrol effectively lowers the incidence of colon tumors. The tumor incidence in AOM/DSS-treated mice was 80% whereas only 20% of mice treated with AOM + DSS + resveratrol had tumor incidence [36]. Likewise, resveratrol treatment greatly decreased the tumor multiplicity (number of tumors per animal). AOM/DSS-treated mice had an average tumor multiplicity of 2.4 ± 0.7 while AOM + DSS + resveratrol group only had 0.2 ± 0.13 tumors per mouse. Thus, resveratrol showed great potential as a useful agent for the treatment of colon cancer.
2.5. Diabetes mellitus
Resveratrol has been reported to have beneficial effects in diabetes and diabetic vascular complication [7,8,46,47]. Studies on rodent models of diabetes have suggested that chronic resveratrol administration potentiates improving hyperglycemia, glucose tolerance, and dyslipidemia and protecting against pancreatic β-cell failure as well as diabetic cardiomyopathy [11,12,47]. In 2012, Do et al [47] conducted a study using C57BL/KsJ-db/db mice fed with resveratrol (0.02% w/w) for 6 weeks. Treatment with resveratrol significantly reduced the fasting blood glucose and hemoglobin A1c, which has a similar hypoglycemic effect as an antidiabetic drug, rosiglitazone [47]. Moreover, resveratrol effectively suppressed the expression of hepatic gluconeogenic enzymes, phosphoenolpyruvate carboxykinase (PEPCK), and glucose 6-phosphate (G6P), while increased the hepatic glycolytic gene expression, pyruvate kinase (PK), and sterol regulatory element binding protein 1c (SREBP1c) leading to decrease glycogen synthesis in liver. The dyslipidemia, free fatty acid, triacylglycerol, and ApoB/ApoAI levels were also regulated through enhancing AMPK activity [47]. Similarly, in 2015 Yao et al [7] found that resveratrol ameliorated gestational diabetes mellitus symptoms via activation of AMPK. Supplementation of resveratrol effectively improved glycemic control, insulin resistances, and reproductive outcome in pregnant C57BL/KsJ-Lepdb/+ mice.
In a recent clinical study, 62 patients with type II diabetes were oral supplementation with resveratrol (250 mg/d) for 3 months. The results showed that treatment with resveratrol significantly reduced the hemoglobin A1c and systolic blood pressure in type II diabetic patients [46]. These findings demonstrated that dietary resveratrol might have promising protective effects of diabetes.
2.6. Obesity
Resveratrol has potential as an antiobesity agent via down-regulation of adipogenic processes [10,48]. A study conducted by Chang et al [10] showed that resveratrol suppressed adipogenic differentiation in 3T3-L1 cells by downregulating both peroxisome proliferator-activated receptor gamma (PPARγ) and perilipin proteins expression, which are two key regulators of adipogenesis and lipogenesis. Additionally, in order to better understand the antiobese effects in vivo, the authors performed an experiment using a high-fat diet (HFD)-induce obese mice model. Administration of resveratrol (1–30 mg/kg BW) for 10 weeks significantly reduced body weight gain in a dose-dependent manner [10]. Also, resveratrol significantly decreased the weight of subcutaneous and epididymal adipose tissues in HFD-treated mice, but had no effect on brown adipose tissue. Furthermore, it was shown that resveratrol greatly reduced fat droplet accumulations in adipose tissue. Similarly, Kim et al [48] reported that resveratrol supplementation effectively reduced the body weight gain, the weight of visceral adipose tissues, plasma triacylglycerol, free fatty acid, glucose, TNF-α, and monocyte chemoattractant protein-1 (MCP-1) via downregulation of galanin-mediated signaling modulators (GalR1, GalR2, PKCδ, Cyc-D, E2F1, and p-ERK) and adipogenic genes expression (PPARγ, C/EBPα, SREBP1c, LXR, FAS, LPL, aP2, and Leptin) [48].
Recently, a human study by Konings et al [13] investigated the effects of resveratrol treatment on adipose tissue morphology and gene expression in healthy obese men. Eleven healthy obese men with body mass index (BMI) between 28 kg/m2 and 36 kg/m2 were administrated with placebo and resveratrol (150 mg/d) for 30 days. The results showed that resveratrol significantly reduced abdominal subcutaneous adipocyte size (placebo 74.7 ± 3.5μM; resveratrol 65 ± 4.4μM). Resveratrol treatment greatly decreased the proportion of large (70–89μM) and very-large (> 90μM) adipocytes, while increasing the small adipocytes (< 50μM) proportion [13]. Moreover an interesting finding was observed, resveratrol activated lysosomal and phagosomal signaling pathways in obese men which might be an alternative pathway of lipolysis through lysosome-dependent autophagy [13].
2.7. Aging
It has been demonstrated that resveratrol possesses the potential of lifespan extension in various organism and animal models [49,50]. Most reports suggested that the mechanism in regard to longevity promotion by resveratrol is related to calorie restriction. In a study conducted by Howitz et al [51] indicated that resveratrol enhanced DNA stability and prolonged 70% lifespan of budding yeast Saccharomyces cerevisiae by activating Sir2, a member of the sirtuin family induced by energy restriction, mimicking the effects of calorie restriction. Furthermore, resveratrol lengthened lifespan through activation of Sir2 in Drosophila melanogaster and Caenorhabditis elegans [52] as well as induction of Sirt-1-dependent autophagy in C. elegans [53]. Thirty micrometres and 130μM of resveratrol treatments were shown to extend average lifespan of honey bees by 38% and 33%, respectively, as well as maximum and median lifespan [54]. They found that resveratrol significantly decreased food consumption in bees, compared to unsupplemented controls, which means that the lifespan extension effect of resveratrol may be mediated via caloric restriction. Regarding vertebrates, Baur et al [55] demonstrated that dietary resveratrol significantly increased the lifespan of high-calorie diet-fed mice through improved their physiological conditions associated with health, including enhanced insulin sensitivity, reduced IGF-I levels, increased AMPK and PGC-1α activities, elevated mitochondrial number, and improved motor function. In another study, Pearson et al [56] found that long-term resveratrol treatment of elderly mice could mimic transcriptional changes induced by dietary restriction and showed a marked reduction of diverse signs of aging, including improved insulin sensitivity, cardiovascular function, bone density, and motor coordination, and reduced inflammation, apoptosis in the vascular endothelium, and cataract formation.
In addition to calorie restriction, other mechanisms for the anti-aging benefit of resveratrol have also been studied. Danilov and colleagues [57] investigated the effects of nonsteroidal anti-inflammatory drugs (NSAIDs), such as aspirin, valeroyl salicylate, trans-resveratrol, valdecoxib, licofelone, on longevity of D. melanogaster. They found that treatment of D. melanogaster with NSAIDs, including resveratrol, caused a prolongation in lifespan. Moreover, resveratrol delayed age-dependent decline of locomotor activity and increased stress resistance in D. melanogaster. It was suggested that the anti-aging effects of NSAIDs in Drosophila model is mediated by Pkh2-ypk1-lem3-tat2 signaling pathway [57]. A study conducted by Hernández-Hernández et al [58] showed that daily oral administration of 20 mg/kg bw of resveratrol to aged rats (18-month-old) for 60 days significantly induced modification of dendritic morphology in prefrontal cortex, dorsal hippocampus, and dentate gyrus, and these changes could explain the therapeutic effect of resveratrol in aging and in Alzheimer’s disease. Furthermore, resveratrol was shown to lengthen lifespan of annual fish, Nothobranchius guentheri, and to improve several oxidative stress-related factors, including reducing the levels of ROS, enhancing the activities of antioxidant enzymes, and decreasing the degree of oxidative damage, which were induced by aging [59].
3. Pterostilbene
Pterostilbene (trans-3,5-dimethoxy-4′-hydroxystilbene), natural dimethylated analogue of resveratrol, is a stilbenoid phytochemical compound primarily found in blueberries, grapes, and a tree wood, Pterocarpus marsupium [2,25,31,60]. Pterostilbene content varies from different type of berries. Vaccinium ashei (rabbiteye blueberry) contains 99 ng/g dry sample, while Vaccinium stamineum (deerberry) contains 560 ng/g dry sample [3,61]. Substantial studies demonstrate that pterostilbene have diverse pharmacological activities for the prevention and treatment of diseases including inflammation, cancer, diabetes, and dyslipidemia [29,60,62–66] (Table 2).
Table 2.
The biological activities and molecular effects of pterostilbene.
| Modes/treatments | Model used | Mechanism | References |
|---|---|---|---|
| Inflammation | |||
| pterostilbene (1–10μM) | 3T3-L1 & RAW 264.7 coculture | ↓IL-6 & TNF-α secretion ↓COX-2, ↓iNOS, ↓IL-6, ↓TNF-α, ↓PAI-1, ↓CRP, ↓MCP-1, ↓resistin, ↓leptin, ↓Migration of macrophages toward adipocytes |
Hsu et al, 2013 [67] |
| Pterostilbene (0.1–1μM) | HUVECs | ↓Monocyte binding, ↓sICAM1, ↓IL-8, ↓MCP-1, ↓sE-selectin, ↓p-eIF2α, ↓ICAM1, ↓MMP9, ↓CRP78 | Liu et al, 2016 [68] |
| Breast cancer | |||
| Pterostilbene (40–80μM) | MCF-7 | ↓Cell viability, ↑Apoptosis, ↑Caspase-3, ↑Bax, ↓Bcl-2, ↑ROS generation, ↓MMP, ↑AMACR | Chakraborty et al, 2010 [69] |
| Pterostilbene (50–100μM) | MCF-7 & Bcap-37 | ↓Cell viability, ↑Apoptosis, ↑PARP, ↑G1 phase arrest, ↓cyclin D1, ↓β-catenin, ↑autophagy, ↑LC3 II | Wang et al, 2012 [18] |
| Pterostilbene (15–50μM) | MCF-7 | ↑Autophagy, ↑Beclin 1, ↑LC3 II, ↑ROS generation | Chakraborty et al, 2012 [70] |
| Prostate cancer | |||
| Pterostilbene (1–25μM) | LNCaP | ↓Cell viability, ↑G1 phase arrest, ↑CDNK1A, ↑CDNK1B, ↓prostate-specific antigen | Wang et al, 2010 [71] |
| Pterostilbene (40–80μM) | PC-3 | ↑Apoptosis, ↑caspase-3, ↑Bax, ↓Bcl-2, ↑ROS generation, ↓MMP, ↑AMACR | Chakraborty et al, 2010 [69] |
| Pterostilbene (40–100μM) | LNCaP | ↓Cell viability, ↑G1 phase arrest, ↑p53, ↑p21, ↑p-AMPK, ↓fatty acid synthase, ↓acetyl CoA carboxylase | Lin et al, 2012 [25] |
| Pterostilbene (40–100μM) | PC-3 | ↓Cell viability, ↑apoptosis, ↑caspase-3, ↑Caspase-9, ↑p-AMPK, ↓fatty acid synthase, ↓acetyl CoA carboxylase | Lin et al, 2012 [25] |
| Colon cancer | |||
| Pterostilbene (1–30μM) | HT-29 | ↓Cell viability, ↓cyclin D1, ↓c-Myc, ↑PARP, ↓TNF-α, ↓IL-1β, ↓IFN-γ, ↓iNOS, ↓COX-2 | Paul et al, 2009 [19] |
| Pterostilbene (0.004%) | AOM-induced colonic carcinogenesis rat | ↓Tumor multiplicity, ↓PCNA, ↓TNF-α, ↓IL-1β, ↓IL-4 | Paul et al, 2010 [63] |
| Pterostilbene (50μM) | HT-29 | ↓β-catenin, ↓cyclin D1, ↓c-Myc, ↓IκBα,↓phosphorylation of p65 | Paul et al, 2010 [63] |
| Pterostilbene (5–100μM) | HCT-116, HT-29, & Caco-2 | ↓Cell viability, ↓colony formation capacity, ↑apoptosis, ↑caspase-3, ↑PARP | Nutakul et al, 2011 [15] |
| Pterostilbene (50 ppm & 250 ppm) | AOM-induced colonic carcinogenesis mice | ↓Aberrant crypt foci, lymphoid nodules & tumors, ↓NF-κB, ↓iNOS,↓COX-2, ↑heme oxygenase-1, ↑Glutathione reductase, ↑Nrf2 | Chiou et al, 2011 [79] |
| Pterostilbene (5–50μM) | COLO 205, HCT-116 & HT-29 | ↑Apoptosis, ↑caspase-3, -8, -9, ↓mTOR/p70S6K, ↓PI3K/Akt, ↓MAPKs, ↓p-ERK1/2, ↓p-JNK1/2, ↑autophagy, ↑LC3 II | Cheng et al, 2014 [28] |
| Pterostilbene (10 mg/kg BW) | COLO 205 xenograft nude mice | ↓Tumor volume, ↓tumor weight, ↓COX-2, ↓MMP-9, ↓VEGF, ↓cyclin D1, ↑caspase-3 | Cheng et al, 2014 [28] |
| Diabetes | |||
| Pterostilbene (40 mg/kg BW) | Diabetic rats | ↓Blood glucose, ↓Glycosylated hemoglobin, ↑Hexokinase, ↓Glucose-6-phosphatase, ↓Fructose-1,6-bisphosphatase | Pari et al, 2006 [66] |
| Pterostilbene (4μM & 8μM) | INS-1E (insulin-secreting rat insulinoma) β-cell line | ↑Nuclear Nrf2, ↑HO-1, ↑CAT, ↑SOD, ↑GPx, ↑Bcl-2, ↓Bax, ↓caspase-3 | Bhakkiyalakshmi et al, 2014 [72] |
| Pterostilbene (15 mg/kg & 50 mg/kg BW) | Wistar rats fed an obesogenic diet | ↓HOMA-IR, ↑GLUT4, ↑p-Akt/total Akt ratio, ↑cardiotrophin-1, ↑glucokinase | Gómez-Zorita et al, 2015 [73] |
| Dyslipidemia | |||
| Pterostilbene (40 mg/kg BW) | Streptozotocin-nicotinamide induced type II diabetes rats | ↓VLDL-C, ↓LDL-C, ↑HDL-C, ↓triglycerides, ↓free fatty acids, ↓phospholipids | Satheesh & Pari, 2008 [74] |
| Pterostilbene (15 mg/kg & 30 mg/kg BW) | Wistar rats fed an obesogenic diet | ↓ Adipose tissue weight, ↓ ME, ↓FAS, ↓G6PDH, ↓CPT-1a, ↓ACO | Gómez-Zorita et al, 2014 [75] |
| Pterostilbene (10–50μM) | H4IIEC3 cells | PPARα ligand, ↑PPARα gene expression | Rimando et al, 2015 [76] |
| Aging | |||
| Pterostilbene (0.004% or 0.016%) | Aged male Fischer rats (19-mo-old) | ↓Cognitive behavioral deficits, ↓dopamine release, ↑pterostilbene levels in hippocampus, ↑working memory | Joseph et al, 2008 [77] |
| Pterostilbene (120 mg/kg diet) | SAMP8 mice | ↓The number of errors over 2-day radial arm water maze test, ↑MnSOD, ↑PPAR-α, ↓phosphorylated JNK, ↓PHF | Chang et al, 2012 [78] |
ACO = acetyl-coA carboxylase; AMACR = α-methylacyl-CoA recemase; Bcl-2 = B-cell leukemia/lymphoma 2; CAT = catalase; CDNK1A = cyclin-dependent kinase inhibitor 1A; CDNK1B = cyclin-dependent kinase inhibitor 1B; COX-2 = cyclooxygenase-2; CPT-1a = carnitine palmitoyl-transferase 1a; CRP = C-reactive protein; FAS = fatty acid synthase; G6PDH = glucose-6-phosphate dehydrogenase; GLUT4 = glucose transporter 4; GPx = glutathione peroxidase; HDL-C = high density lipoprotein cholesterol; HO-1 = heme oxygenase-1; HOMA-IR = homeostatic model assessment-insulin resistance; IFN-γ = interferon gamma; IκBα = inhibitor of kappa B; IL-1β = interleukin 1 beta; IL-4 = interleukin 4; IL-6 = interleukin 6; IL-8 = interleukin 8; iNOS = inducible nitric oxide synthase; LC3 II = autophagy-related protein light chain 3 II; LDL-C = low density lipoprotein cholesterol; MAPKs = mitogen-activated protein kinases; MCP-1 = monocyte chemoattractant protein-1; ME = malic enzyme; MMP = matrix metallopeptidase; mTOR = mammalian target of rapamycin; Nrf2 = NF-E2-related factor 2; p70S6K = 70 kDa ribosomal protein S6 kinase; PAI-1 = plasminogen activator inhibitor-1; p-AMPK = phosphorylated adenosine monophosphate activated protein kinase; PARP = poly ADP-ribose polymerase; PCNA = proliferating cell nuclear antigen; p-eIF2α = phospho-eIF2α; p-ERK1/2 = phosphorylated-extracellular signal-regulated kinase 1/2; PHF = paired helical filaments; PI3K = phosphatidylinositol 3-kinase; p-JNK1/2 = phospho-JNK1/2; sICAM1 = soluble intercellular adhesion molecule-1; PPARα = peroxisome proliferator activated receptor alpha; ROS = reactive oxygen species; SOD = superoxide dismutase; TNF-α = tumor necrosis factor-α; VEGF = vascular endothelial growth factor; VLDL-C = very low density lipoprotein cholesterol.
3.1. Inflammation
A study conducted by Hsu et al [67] showed that pterostilbene exhibited strong inflammatory inhibitory effects in 3T3-L1 adipocytes and RAW264.7 macrophages coculture. It showed that pterostilbene significantly inhibited cytokines IL-6 and TNF-α secretion and proinflammatory gene expression including COX-2, iNOS, IL-6, TNF-α, MCP-1, and plasminogen activator inhibitor-1 (PAI-1) and also decreased the migratory ability of macrophages toward adipocytes [67]. Further study was performed by Liu and colleagues [68] evaluating the anti-inflammation effects of pterostilbene in endothelial HUVECs cells. Pterostilbene treatment markedly attenuated inflammatory responses by reducing the production of cytokines including soluble intercellular adhesion molecule-1 (sICAM1), IL-8, MCP-1, and sE-selectin and inhibiting the adhesion of U937 monocytes to HUVECs cells. Also, pterostilbene decreased the expression of endoplasmic reticulum stress (ERS)-related proteins such as, eIF2α, ICAM1, matrix metallopeptidase 9 (MMP9), and GRP78 [68].
3.2. Breast cancer
Pterostilbene has been shown to exhibit anticarcinogenic activities in breast cancer [18,69,70]. Chakraborty and colleagues [69] found that pterostilbene induced cytotoxic effects by generating reactive oxygen species (ROS) in MCF-7 breast cancer cells. Treated MCF-7 cells with pterostilbene could trigger apoptosis via increasing the levels of caspase-3 and apoptotic protein Bax, and decreasing the antiapoptotic protein Bcl-2 levels and mitochondrial membrane potential [69]. In 2012, the same research group further demonstrated that pterostilbene caused autophagy by upregulating the expression of autophagy-related proteins including Beclin-1 and LC3 II in MCF-7 cells [70]. Furthermore, Wang et al [18] also indicated that pterostilbene could simultaneously stimulate apoptosis, cell cycle arrest, and autophagy in human breast cancer MCF-7 and Bcap-37 cells.
3.3. Prostate cancer
In prostate cancer, Wang et al [71] reported that treatment of p53 wild type human prostate cancer LNCaP cell with 25μM pterostilbene could inhibit cell viability and induce cell cycle arrest at G1/S phase by increasing the cyclin-dependent kinase inhibitor CDNK1A and CDNK1B. Furthermore, pterostilbene treatment had strong inhibitory effect on prostate-specific antigen (PSA) expression. In addition, Chakraborty et al [69] treated p53 null type human prostate cancer PC-3 cells with pterostilbene and found that pterostilbene induced apoptosis by increasing the cellular ROS, caspase-3, and apoptotic protein Bax and decreasing antiapoptotic protein Bcl-2. Pterostilbene also inhibited MMP-9 and α-methylacyl-CoA recemase (AMACR), which are two notable metastasis activators. Similarly, other studies conducted by Lin et al [25] in 2012 indicated the same results as previous studies. Pterostilbene treatment induced differential effects in p53 positive and negative human prostate cancer cell that could upregulate cell cycle arrest at G1 phase in LNCaP cells, while caused the PC-3 cells undergo apoptosis [25]. Moreover, Lin et al [25] discovered that pterostilbene inhibited prostate cancer cell growth and proliferation through activation of AMPK activity and suppression of lipogenesis by decreasing the expression of fatty acid synthase and acetyl-CoA carboxylase.
3.4. Colon cancer
The anticarcinogenic effects of pterostilbene have been demonstrated in various human colorectal carcinoma cells including Caco-2, COLO 205, HCT-116, and HT-29 cells, and COLO 205 xenograft tumors [15,28]. Pterostilbene induced apoptosis by downregulating mammalian target of rapamycin (mTOR), phosphatidylinositol 3-kinase (PI3K)/Akt, and mitogen-activated protein kinases (MAPKs) signaling pathways. Pterostilbene also caused autophagy via increasing the production of Beclin-1 and LC3 II in COLO 205 cells [28]. The studies conducted by Paul et al [19,63] indicated that pterostilbene inhibited cell proliferation of HT-29 cells by regulating the Wnt/β-catenin-signaling pathway and significantly reduced the inflammatory regulators. Moreover, in vivo, Chiou et al [64] suggested that treatment with pterostilbene was able to inhibit colorectal aberrant crypt foci and colon carcinogenesis via suppressing the inflammatory signaling regulators including NF-κB, iNOS, and COX-2 in azoxymethane (AOM)-induced colonic carcinogenesis in mice. Another research group demonstrated that pterostilbene could reduce colon tumor multiplicity and proliferating cell nuclear antigen (PCNA) and also decreased mucosal levels of the proinflammatory cytokines, TNF-α, IL-1β, and IL-4 in AOM-induced colon carcinogenesis in rats [63].
3.5. Diabetes mellitus
Regarding to antidiabetic activities, pterostilbene was demonstrated to have cytoprotective effects on pancreatic β-cell, as it was found to eliminate oxidative stress by mediating the NF-E2-related factor 2 (Nrf2) downstream target genes expression including heme oxygenas-1 (HO-1), superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) [72]. Also, pterostilbene increased the expression of anti-apoptotic protein, Bcl-2 and downregulated the proapoptotic Bax and caspase-3 expression, which protects pancreatic β-cell from apoptosis in diabetes [72]. Additionally, pterostilbene significantly lower blood glucose and glycosylated hemoglobin concentration in streptozotocin- and nicotinamide-induced diabetic rats [66]. Treatment with pterostilbene significantly increased the expression of glycolysis enzyme, hexokinase, and decreased the expression of gluconeogenesis enzymes, glucose-6-phosphatase and fructose-1,6-bisphosphatase, which regulate glucose homeostasis [66]. Moreover, in a recent study, Gómez-Zorita et al [73] demonstrated that pterostilbene improved glycemic control. Pterostilbene was shown to decrease insulin resistance index (HOMA-IR) and increase GLUT4 protein expression, phosphorylated-Akt/total Akt ratio, cardiotrophin-1, and glucokinase activity in insulin-resistant rats induced by obesogenic diet [73].
3.6. Dyslipidemia
Dyslipidemia results in various health problems such as obesity, hypertension, type 2 diabetes, atherosclerosis, and coronary heart disease. Satheesh and Pari [74] indicated that pterostilbene could effectively modify the dyslipidemia, as it can decrease low density lipoprotein cholesterol (LDL-C) and very low density lipoprotein cholesterol (VLDL-C) and increase the high density lipoprotein cholesterol (HDL-C) in streptozotocin-nicotinamide induced diabetic rats. In addition, pterostilbene significantly reduced the levels of serum and tissue triacylglycerols, free fatty acids, and phospholipids [74]. Moreover, Gómez-Zorita et al [75] evaluated the hypolipidemic effects of pterostilbene in Wistar rats which were fed obesogenic diet with or without pterostilbene for 6 weeks. The findings of this study showed that pterostilbene greatly reduced total adipose tissue weights including perirenal, mesenteric, and subcutaneous fat mass. The activities of lipogenic enzymes, malic enzyme (ME), fatty acid synthase (FAS), and acetyl-CoA carboxylase (ACC) were significantly reduced in white adipose tissue. The glucose-6-phosphate dehydrogenase (G6PDH) and malic enzyme (ME) involved in liver lipogenesis were also reduced. Moreover, the activities of fatty acid oxidation enzymes, carnitine palmitoyl-transferase 1a (CPT-1a), and acyl-CoA oxidase, were markedly increased in liver. Thus, the proposed mechanisms for reducing body fat by pterostilbene are reduction of lipogenesis in adipose tissue and liver and induction of fatty acid oxidation in liver.
Furthermore, a recent study by Rimando et al [76] provided insight that pterostilbene acts as a peroxisome proliferator activated receptor α (PPARα) ligand, which regulates fatty acid β-oxidation. Pterostilbene markedly increased PPARα gene expression in a dose dependent manner in H4IIEC3 rat hepatoma cells. Moreover, the activation of PPARα gene expression of pterostilbene at 10 μmol/L was greater than that of fenofibrate at 100 μmol/L and 200 μmol/L, which is a clinical drug used to treat high cholesterol levels [76]. Thus, dietary pterostilbene may be effective in correcting the dyslipidemia.
3.7. Aging
Recent reports indicate that pterostilbene possesses high potential in bioactivities than its analogue resveratrol. However, there were only few studies investigating the anti-aging effects of pterostilbene. A study conducted by Joseph et al [77] examined the preventive effect of pterostilbene on age-related behavioral changes in aged rats. Treatment of aged rats with 0.004% and 0.016% concentration of pterostilbene in diet improved the cognitive behavioral deficits, as well as dopamine release, and work memory, which was correlated with pterostilbene levels in the hippocampus [77]. Moreover, pterostilbene, but not resveratrol, was demonstrated to significantly decrease number of errors over a 2-day radial arm water maze test in SAMP8 mice, a model of accelerated aging that is increasingly being validated as a model of sporadic and age-related Alzheimer’s disease. Pterostilbene also improved the cellular stress, inflammation, and Alzheimer’s disease pathology through upregulation of PPAR-α expression, showing higher modulator potential than resveratrol [78].
4. 3′-Hydroxypterostilbene
3′-Hydroxypterostilbene (trans-3,5-dimethoxy-3′,4′-dihydroxystilbene), one of metabolites of pterostilbene [30], can also be isolated from whole plant of the herb Sphaerophysa salsula, a shrub widely distributed in central Asia and northwest China [31]. A recent study indicated that 3′-hydroxypterostilbene possesses anti-adipogenic, -inflammatory, -oxidant, and Sirt-1 inhibitory activities [79]. However, there is a paucity of published studies involving biological activities of 3′-hydroxypterostilbene (Table 3).
Table 3.
The biological activities and molecular effects of 3′-hydroxypterostilbene.
| Modes/Treatments | Model used | Mechanism | Reference |
|---|---|---|---|
| Leukemia | |||
| 3′-hydroxypterostilbene (0.7–5μM) | HL60, K562, HUT78 HL60-R, K562-ADR | ↓Cell proliferation, ↑apoptosis, ↓mitochondrial membrane potential | Tolomeo et al, 2005 [20] |
| Colon cancer | |||
| 3′-hydroxypterostilbene (5–50μM) | COLO 205, HCT-116, HT-29 | ↑Apoptosis, ↑caspase-3, -8, -9, ↓mTOR/p70S6K, ↓PI3K/Akt, ↓MAPKs, ↓p-ERK1/2, ↓p-JNK1/2, ↑autophagy, ↑LC3 II | Cheng et al, 2014 [28] |
| 3′-hydroxypterostilbene (10 mg/kg BW) | COLO 205 Xenograft nude mice | ↓Tumor volume, ↓tumor weight, ↓COX-2, ↓MMP-9, ↓VEGF, ↓cyclin D1,↑caspase-3 | Cheng et al, 2014 [28] |
| 3′-hydroxypterostilbene (12–50 μg/mL) | HCT-116, MDA-MB-231, PC-3, HepG2, 3T3-L1, enzyme incubation | ↓Cell viability, ↓adipogenesis,↓Sirt-1 | Takemoto et al, 2015 [79] |
COX-2 = cyclooxygenase-2; LC3 II = autophagy-related protein light chain 3 II; MAPKs = mitogen-activated protein kinases; MMP-9 = matrix metallopeptidase 9; mTOR = mammalian target of rapamycin; p70S6K = 70 kDa ribosomal protein S6 kinase; p-ERK1/2 = phosphorylated-extracellular signal-regulated kinase 1/2; PI3K = phosphatidylinositol 3-kinase; p-JNK1/2 = phospho-JNK1/2; VEGF = vascular endothelial growth factor.
4.1. Leukemia
In a study conducted by Tolomeo et al [20], it was reported that 3′-hydroxypterostilbene exerted cytotoxicity in five different human leukemia cell lines (HL60, K562, HUT78, HL60-R, and K562-ADR) but showed low toxicity in normal hemopoietic stem cells. The proposed mechanism of antiproliferation potential of 3′-hydroxypterostilbene in leukemia cells was due to intrinsic apoptotic cell death by disrupting mitochondrial membrane potential [20].
4.2. Colon cancer
Recently, 3′-hydroxypterostilbene was demonstrated to have antiproliferation effects in human colon cancer COLO 205, HCT-116, and HT-29 cells [28]. The results of the study showed that 3′-hydroxypterostilbene induced apoptosis and autophagy by increasing the activities of caspase-3, -8, and -9 and inhibiting mTOR/p70S6K, PI3K/Akt, and MAPKs signaling pathways. In the same study, further experiments were conducted to evaluate the antitumor effect of 3′-hydroxypter-ostilbene in vivo using COLO 205 xenograft nude mice model. The findings suggested that 3′-hydroxypterostilbene significantly inhibited the tumor volume and tumor weight via downregulating the protein expressions of COX-2, MMP-9, vascular endothelial growth factor (VEGF), and cyclin D1. Moreover, the anticancer effects of 3′-hydroxypterostilbene were more potent than that of pterostilbene in human colon cancer cells and xenograft nude mice [28].
5. Bioavailability of resveratrol and pterostilbene
Although resveratrol has been reported to possess many pharmacological effects, one potential issue surrounding resveratrol is that resveratrol has low systemic bioavailability which may lower its effectiveness [14]. Pterostilbene shares many pharmacological similarities with resveratrol such as antioxidant, -inflammation, -cancer, -diabetes, and hypolipidemic activity [18,19,25,73]; however, pterostilbene exhibits much better bioavailability and is more biologically active than resveratrol [15,19,80]. Based on the chemical structure, pterostilbene could possess better bioavailability as it contains two extra methoxy groups at the A-phenyl ring of resveratrol. The two methoxy groups cause pterostilbene to have relatively higher lipophilicity, which may enhance the cell membrane permeability and increase its oral absorption [60].
In a study conducted by Nutakul et al [15], it was indicated that the amount of intracellular pterostilbene was two to four times higher than those of resveratrol following treatments of pterostilbene or resveratrol at the same concentration in Caco-2, HT-29, and HCT-116 human colon cancer cells [15]. The same study also showed that pterostilbene exhibited much stronger antiproliferation and apoptotic effects than those of resveratrol. The IC50 of pterostilbene was two to five times lower than those of resveratrol in cell viability among three human colon cancer cells. Intravenous administrations of resveratrol, pterostilbene and 3,5,4′-trimethoxy-trans-stilbene (TMS) to rats showed that pterostilbene and TMS had greater plasma exposure and lower clearances, as well as longer half-life [81–83]. It was suggested that methoxylation on hydroxyl groups of pterostilbene or TMS could hinder their metabolism and appeared to have better pharmacokinetic characteristics than their natural occurring analog, resveratrol [83]. Moreover, Chiou et al [79] demonstrated that pterostilbene was more potent than resveratrol in preventing colon tumorigenesis via activation of the Nrf2-mediated antioxidant signaling pathway. Likewise, other research group indicated that pterostilbene was more effective than resveratrol as an anti-inflammatory agent for inhibition of colon carcinogenesis in HT-29 human adenocarcinoma cell line [19]. Therefore, the biological activities of pterostilbene were usually found to be more potent than resveratrol might due to its higher bioavailability.
Biological functions of resveratrol might be somewhat limited by first-pass metabolism [16]. Accumulating studies demonstrated that Phase II conjugation, sulfate, and glucuronide conjugates, mainly occur on the hydroxyl groups of stilbenoid compounds in the body [16,21,84,85], since resveratrol containing three hydroxyl groups is rapidly converted to its glucuronide and sulfate conjugates that lower its bioavailability. However, in order to further understand whether the metabolites of resveratrol possess any biological functions, Mikstis et al [40] evaluated the anticancer activity of resveratrol and its sulfated conjugates against breast cancer cell lines. The results of this study showed that the cytotoxicity of human sulfated metabolites (trans-resveratrol 3-O-sulfate, trans-resveratrol 4′-O-sulfate, and trans-resveratrol 3-O-4′-O-disulfate) were less potent than that of resveratrol. The cytotoxicity effects of all sulfated metabolites were reduced about 10 times in comparison with resveratrol against three different breast cancer cell lines (MCF-7, ZR-75-1 and MDA-MB-231) [40].
Recent studies showed that the methoxylation on the free hydroxyl groups of resveratrol could reduce its metabolization and increased its plasma exposure [81,83]. Furthermore, in a pharmacokinetic study, pterostilbene was shown to have 80% oral bioavailability in comparison to only 20% for resveratrol [16]. Thus, the findings of these studies indicated that pterostilbene, dimethyl ether of resveratrol, showed much greater bioavailability than resveratrol.
6. Summary
This review provides important evidence that stilbenoid compounds such as resveratrol, pterostilbene, and 3′-hydroxypterostilbene have promising applications for the management and treatment of chronic disorders, such as heart disease, stroke, cancer, diabetes, and obesity. Methylated modification of resveratrol found in pterostilbene and 3′-hydroxypterostilbene appeared to have stronger biological properties. Low bioavailability might be one of the factors that affect the bioactivities of resveratrol, while more studies are required to elucidate the molecular mechanism of their bioactive actions. Moreover, pterostilbene, dimethyl ether of resveratrol, exhibits much greater bioavailability and bioactivity than resveratrol, making it an ideal chemopreventive and chemotherapeutic agent. However, the human studies of stilbenoid compounds are still lacking, future clinical research for these compounds in chronic diseases is necessary to investigate their physiological and pharmacological effects and safety.
Footnotes
Conflicts of interest
All authors declare no conflicts of interest.
REFERENCES
- 1. Murtaza G, Sajjad A, Mehmood Z, Shah SH, Siddiqi AR. Possible molecular targets for therapeutic applications of caffeic acid phenethyl ester in inflammation and cancer. J Food Drug Anal. 2015;23:11–8. doi: 10.1016/j.jfda.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roupe KA, Remsberg CM, Yáñez JA, Davies NM. Pharmacometrics of stilbenes: seguing towards the clinic. Curr Clin Pharmacol. 2006;1:81–101. doi: 10.2174/157488406775268246. [DOI] [PubMed] [Google Scholar]
- 3. Rimando AM, Kalt W, Magee JB, Dewey J, Ballington JR. Resveratrol, pterostilbene, and piceatannol in vaccinium berries. J Agric Food Chem. 2004;52:4713–9. doi: 10.1021/jf040095e. [DOI] [PubMed] [Google Scholar]
- 4. Medina-Bolivar F, Condori J, Rimando AM, Hubstenberger J, Shelton K, O’Keefe SF, Bennett S, Dolan MC. Production and secretion of resveratrol in hairy root cultures of peanut. Phytochem. 2007;68:1992–2003. doi: 10.1016/j.phytochem.2007.04.039. [DOI] [PubMed] [Google Scholar]
- 5. Wang Z, Huang Y, Zou J, Cao K, Xu Y, Wu JM. Effects of red wine and wine polyphenol resveratrol on platelet aggregation in vivo and in vitro. Int J Mol Med. 2002;9:77–9. [PubMed] [Google Scholar]
- 6. Sanders TH, McMichael RW, Hendrix KW. Occurrence of resveratrol in edible peanuts. J Agric Food Chem. 2000;48:1243–6. doi: 10.1021/jf990737b. [DOI] [PubMed] [Google Scholar]
- 7. Yao L, Wan J, Li H, Ding J, Wang Y, Wang X, Li M. Resveratrol relieves gestational diabetes mellitus in mice through activating AMPK. Reprod Biol Endocrinol. 2015;13:1–7. doi: 10.1186/s12958-015-0114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Taguchi K, Hida M, Matsumoto T, Kobayashi T. Resveratrol ameliorates clonidine-induced endothelium-dependent relaxation involving Akt and endothelial nitric oxide synthase regulation in type 2 diabetic mice. Biol Pharm Bull. 2015;38:1864–72. doi: 10.1248/bpb.b15-00403. [DOI] [PubMed] [Google Scholar]
- 9. Lee JA, Ha SK, Cho E, Choi I. Resveratrol as a bioenhancer to improve anti-inflammatory activities of apigenin. Nutrients. 2015;7:9650–61. doi: 10.3390/nu7115485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chang CC, Lin KY, Peng KY, Day YJ, Hung LM. Resveratrol exerts anti-obesity effects in high-fat diet obese mice and displays differential dosage effects on cytotoxicity, differentiation, and lipolysis in 3T3-L1 cells. Endocr J. 2016;63:169–78. doi: 10.1507/endocrj.EJ15-0545. [DOI] [PubMed] [Google Scholar]
- 11. Bagul PK, Dinda AK, Banerjee SK. Effect of resveratrol on sirtuins expression and cardiac complications in diabetes. Biochem Biophys Res Commun. 2015;468:221–7. doi: 10.1016/j.bbrc.2015.10.126. [DOI] [PubMed] [Google Scholar]
- 12. Mohammadshahi M, Haidari F, Soufi FG. Chronic resveratrol administration improves diabetic cardiomyopathy in part by reducing oxidative stress. Cardiol J. 2014;21:39–46. doi: 10.5603/CJ.a2013.0051. [DOI] [PubMed] [Google Scholar]
- 13. Konings E, Timmers S, Boekschoten M, Goossens G, Jocken J, Afman L, Müller M, Schrauwen P, Mariman E, Blaak E. The effects of 30 days resveratrol supplementation on adipose tissue morphology and gene expression patterns in obese men. Int J Obes. 2014;38:470–3. doi: 10.1038/ijo.2013.155. [DOI] [PubMed] [Google Scholar]
- 14. Walle T, Hsieh F, DeLegge MH, Oatis JE, Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32:1377–82. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 15. Nutakul W, Sobers HS, Qiu P, Dong P, Decker EA, McClements DJ, Xiao H. Inhibitory effects of resveratrol and pterostilbene on human colon cancer cells: a side-by-side comparison. J Agric Food Chem. 2011;59:10964–70. doi: 10.1021/jf202846b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kapetanovic IM, Muzzio M, Huang Z, Thompson TN, McCormick DL. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother Pharmacol. 2011;68:593–601. doi: 10.1007/s00280-010-1525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen RJ, Ho CT, Wang YJ. Pterostilbene induces autophagy and apoptosis in sensitive and chemoresistant human bladder cancer cells. Mol Nutr Food Res. 2010;54:1819–32. doi: 10.1002/mnfr.201000067. [DOI] [PubMed] [Google Scholar]
- 18. Wang Y, Ding L, Wang X, Zhang J, Han W, Feng L, Sun J, Jin H, Wang XJ. Pterostilbene simultaneously induces apoptosis, cell cycle arrest and cyto-protective autophagy in breast cancer cells. Am J Transl Res. 2012;4:44–51. [PMC free article] [PubMed] [Google Scholar]
- 19. Paul S, Rimando AM, Lee HJ, Ji Y, Reddy BS, Suh N. Anti-inflammatory action of pterostilbene is mediated through the p38 mitogen-activated protein kinase pathway in colon cancer cells. Cancer Prev Res. 2009;2:650–7. doi: 10.1158/1940-6207.CAPR-08-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tolomeo M, Grimaudo S, Di Cristina A, Roberti M, Pizzirani D, Meli M, Dusonchet L, Gebbia N, Abbadessa V, Crosta L, Barucchello R, Grisolia G, Invidiata F, Simoni D. Pterostilbene and 3′-hydroxypterostilbene are effective apoptosis-inducing agents in MDR and BCR-ABL-expressing leukemia cells. Int J Biochem Cell Biol. 2005;37:1709–26. doi: 10.1016/j.biocel.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 21. Remsberg CM, Yáñez JA, Ohgami Y, Vega-Villa KR, Rimando AM, Davies NM. Pharmacometrics of pterostilbene: preclinical pharmacokinetics and metabolism, anticancer, antiinflammatory, antioxidant and analgesic activity. Phytother Res. 2008;22:169–79. doi: 10.1002/ptr.2277. [DOI] [PubMed] [Google Scholar]
- 22. Schneider JG, Alosi JA, McDonald DE, McFadden DW. Pterostilbene inhibits lung cancer through induction of apoptosis. J Surg Res. 2010;161:18–22. doi: 10.1016/j.jss.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 23. Ferrer P, Asensi M, Segarra R, Ortega A, Benlloch M, Obrador E, Varea MT, Asensio G, Jorda L, Estrela JM. Association between pterostilbene and quercetin inhibits metastatic activity of B16 melanoma. Neoplasia. 2005;7:37–47. doi: 10.1593/neo.04337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McCormack DE, Mannal P, McDonald D, Tighe S, Hanson J, McFadden D. Genomic analysis of pterostilbene predicts its antiproliferative effects against pancreatic cancer in vitro and in vivo. J Gastrointest Surg. 2012;16:1136–43. doi: 10.1007/s11605-012-1869-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lin VC, Tsai YC, Lin JN, Fan LL, Pan MH, Ho CT, Wu JY, Way TD. Activation of AMPK by pterostilbene suppresses lipogenesis and cell-cycle progression in p53 positive and negative human prostate cancer cells. J Agric Food Chem. 2012;60:6399–407. doi: 10.1021/jf301499e. [DOI] [PubMed] [Google Scholar]
- 26. Pan MH, Chang YH, Badmaev V, Nagabhushanam K, Ho CT. Pterostilbene induces apoptosis and cell cycle arrest in human gastric carcinoma cells. J Agric Food Chem. 2007;55:7777–85. doi: 10.1021/jf071520h. [DOI] [PubMed] [Google Scholar]
- 27. Modak M, Dixit P, Londhe J, Ghaskadbi S, Devasagayam TPA. Indian herbs and herbal drugs used for the treatment of diabetes. J Clin Biochem Nutr. 2007;40:163. doi: 10.3164/jcbn.40.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cheng TC, Lai CS, Chung MC, Kalyanam N, Majeed M, Ho CT, Ho YS, Pan MH. Potent anti-cancer effect of 3′-hydroxypterostilbene in human colon xenograft tumors. PLoS One. 2014;9:e111814. doi: 10.1371/journal.pone.0111814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mak KK, Wu AT, Lee WH, Chang TC, Chiou JF, Wang LS, Wu CH, Huang CY, Shieh YS, Chao TY, Ho CT, Yen GC, Yeh CT. Pterostilbene, a bioactive component of blueberries, suppresses the generation of breast cancer stem cells within tumor microenvironment and metastasis via modulating NF-kappaB/microRNA 448 circuit. Mol Nutr Food Res. 2013;57:1123–34. doi: 10.1002/mnfr.201200549. [DOI] [PubMed] [Google Scholar]
- 30. Shao X, Chen X, Badmaev V, Ho CT, Sang S. Structural identification of mouse urinary metabolites of pterostilbene using liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2010;24:1770–8. doi: 10.1002/rcm.4579. [DOI] [PubMed] [Google Scholar]
- 31. Ma ZJ, Li X, Li N, Wang JH. Stilbenes from Sphaerophysa salsula. Fitoterapia. 2002;73:313–5. doi: 10.1016/s0367-326x(02)00074-6. [DOI] [PubMed] [Google Scholar]
- 32. Schouten A, Wagemakers L, Stefanato FL, Kaaij RM, van Kan JA. Resveratrol acts as a natural profungicide and induces self-intoxication by a specific laccase. Mol Microbiol. 2002;43:883–94. doi: 10.1046/j.1365-2958.2002.02801.x. [DOI] [PubMed] [Google Scholar]
- 33. Jeandet P, Douillet-Breuil AC, Bessis R, Debord S, Sbaghi M, Adrian M. Phytoalexins from the Vitaceae: biosynthesis, phytoalexin gene expression in transgenic plants, antifungal activity, and metabolism. J Agric Food Chem. 2002;50:2731–41. doi: 10.1021/jf011429s. [DOI] [PubMed] [Google Scholar]
- 34. Gentile C, Tesoriere L, Butera D, Fazzari M, Monastero M, Allegra M, Livrea MA. Antioxidant activity of Sicilian pistachio (Pistacia vera L. var. Bronte) nut extract and its bioactive components. J Agric Food Chem. 2007;55:643–8. doi: 10.1021/jf062533i. [DOI] [PubMed] [Google Scholar]
- 35. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cui X, Jin Y, Hofseth AB, Pena E, Habiger J, Chumanevich A, Poudyal D, Nagarkatti M, Nagarkatti PS, Singh UP. Resveratrol suppresses colitis and colon cancer associated with colitis. Cancer Prev Res. 2010;3:549–59. doi: 10.1158/1940-6207.CAPR-09-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sánchez-Fidalgo S, Cárdeno A, Villegas I, Talero E, de la Lastra CA. Dietary supplementation of resveratrol attenuates chronic colonic inflammation in mice. Eur J Pharmacol. 2010;633:78–84. doi: 10.1016/j.ejphar.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 38. Scarlatti F, Maffei R, Beau I, Codogno P, Ghidoni R. Role of non-canonical Beclin 1-independent autophagy in cell death induced by resveratrol in human breast cancer cells. Cell Death Differ. 2008;15:1318–29. doi: 10.1038/cdd.2008.51. [DOI] [PubMed] [Google Scholar]
- 39. He X, Wang Y, Zhu J, Orloff M, Eng C. Resveratrol enhances the anti-tumor activity of the mTOR inhibitor rapamycin in multiple breast cancer cell lines mainly by suppressing rapamycin-induced AKT signaling. Cancer Lett. 2011;301:168–76. doi: 10.1016/j.canlet.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 40. Miksits M, Wlcek K, Svoboda M, Kunert O, Haslinger E, Thalhammer T, Szekeres T, Jäger W. Antitumor activity of resveratrol and its sulfated metabolites against human breast cancer cells. Planta Med. 2009;75:1227–30. doi: 10.1055/s-0029-1185533. [DOI] [PubMed] [Google Scholar]
- 41. Prevarskaya N, Skryma R, Shuba Y. Calcium in tumour metastasis: new roles for known actors. Nat Rev Cancer. 2011;11:609–18. doi: 10.1038/nrc3105. [DOI] [PubMed] [Google Scholar]
- 42. Monteith GR, Davis FM, Roberts-Thomson SJ. Calcium channels and pumps in cancer: changes and consequences. J Biol Chem. 2012;287:31666–73. doi: 10.1074/jbc.R112.343061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Selvaraj S, Sun Y, Sukumaran P, Singh BB. Resveratrol activates autophagic cell death in prostate cancer cells via downregulation of STIM1 and the mTOR pathway. Mol Carcinog. 2016;55:818–31. doi: 10.1002/mc.22324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sheth S, Jajoo S, Kaur T, Mukherjea D, Sheehan K, Rybak LP, Ramkumar V. Resveratrol reduces prostate cancer growth and metastasis by inhibiting the Akt/MicroRNA-21 pathway. PLoS One. 2012;7:e51655. doi: 10.1371/journal.pone.0051655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Miki H, Uehara N, Kimura A, Sasaki T, Yuri T, Yoshizawa K, Tsubura A. Resveratrol induces apoptosis via ROS-triggered autophagy in human colon cancer cells. Int J Oncol. 2012;40:1020–8. doi: 10.3892/ijo.2012.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bhatt JK, Thomas S, Nanjan MJ. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr Res. 2012;32:537–41. doi: 10.1016/j.nutres.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 47. Do GM, Jung UJ, Park HJ, Kwon EY, Jeon SM, McGregor RA, Choi MS. Resveratrol ameliorates diabetes-related metabolic changes via activation of AMP-activated protein kinase and its downstream targets in db/db mice. Mol Nutr Food Res. 2012;56:1282–91. doi: 10.1002/mnfr.201200067. [DOI] [PubMed] [Google Scholar]
- 48. Kim S, Jin Y, Choi Y, Park T. Resveratrol exerts anti-obesity effects via mechanisms involving down-regulation of adipogenic and inflammatory processes in mice. Biochem Pharmacol. 2011;81:1343–51. doi: 10.1016/j.bcp.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 49. Pan MH, Lai CS, Tsai ML, Wu JC, Ho CT. Molecular mechanisms for anti-aging by natural dietary compounds. Mol Nutr Food Res. 2012;56:88–115. doi: 10.1002/mnfr.201100509. [DOI] [PubMed] [Google Scholar]
- 50. Kasiotis KM, Pratsinis H, Kletsas D, Haroutounian SA. Resveratrol and related stilbenes: their anti-aging and anti-angiogenic properties. Food Chem Toxicol. 2013;61:112–20. doi: 10.1016/j.fct.2013.03.038. [DOI] [PubMed] [Google Scholar]
- 51. Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–6. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 52. Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–9. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 53. Morselli E, Maiuri MC, Markaki M, Megalou E, Pasparaki A, Palikaras K, Criollo A, Galluzzi L, Malik SA, Vitale I, Michaud M, Madeo F, Tavernarakis N, Kroemer G. Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death Dis. 2010;1:e10. doi: 10.1038/cddis.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rascón B, Hubbard BP, Sinclair DA, Amdam GV. The lifespan extension effects of resveratrol are conserved in the honey bee and may be driven by a mechanism related to caloric restriction. Aging (Albany NY) 2012;4:499–508. doi: 10.18632/aging.100474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–42. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–68. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Danilov A, Shaposhnikov M, Shevchenko O, Zemskaya N, Zhavoronkov A, Moskalev A. Influence of non-steroidal anti-inflammatory drugs on Drosophila melanogaster longevity. Oncotarget. 2015;6:19428–44. doi: 10.18632/oncotarget.5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hernández-Hernández EM, Serrano-Garcia C, Vazquez-Roque RA, Diaz A, Monroy E, Rodriguez-Moreno A, Floran B, Flores G. Chronic administration of resveratrol prevents morphological changes in prefrontal cortex and hippocampus of aged rats. Synapse. 2016;70:206–17. doi: 10.1002/syn.21888. [DOI] [PubMed] [Google Scholar]
- 59. Liu T, Qi H, Ma L, Liu Z, Fu H, Zhu W, Song T, Yang B, Li G. Resveratrol attenuates oxidative stress and extends life span in the annual fish Nothobranchius guentheri. Rejuvenation Res. 2015;18:225–33. doi: 10.1089/rej.2014.1618. [DOI] [PubMed] [Google Scholar]
- 60. McCormack D, McFadden D. Pterostilbene and cancer: current review. J Surg Res. 2012;173:e53–61. doi: 10.1016/j.jss.2011.09.054. [DOI] [PubMed] [Google Scholar]
- 61. Rodríguez-Bonilla P, López-Nicolás JM, Méndez-Cazorla L, García-Carmona F. Development of a reversed phase high performance liquid chromatography method based on the use of cyclodextrins as mobile phase additives to determine pterostilbene in blueberries. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:1091–7. doi: 10.1016/j.jchromb.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 62. McFadden D. A review of pterostilbene antioxidant activity and disease modification. Oxidative Med Cell Longevity. 2013;2013:575482. doi: 10.1155/2013/575482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Paul S, DeCastro AJ, Lee HJ, Smolarek AK, So JY, Simi B, Wang CX, Zhou R, Rimando AM, Suh N. Dietary intake of pterostilbene, a constituent of blueberries, inhibits the β-catenin/p65 downstream signaling pathway and colon carcinogenesis in rats. Carcinog. 2010;31:1272–8. doi: 10.1093/carcin/bgq004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chiou YS, Tsai ML, Wang YJ, Cheng AC, Lai WM, Badmaev V, Ho CT, Pan MH. Pterostilbene inhibits colorectal aberrant crypt foci (ACF) and colon carcinogenesis via suppression of multiple signal transduction pathways in azoxymethane-treated mice. J Agric Food Chem. 2010;58:8833–41. doi: 10.1021/jf101571z. [DOI] [PubMed] [Google Scholar]
- 65. Rimando AM, Nagmani R, Feller DR, Yokoyama W. Pterostilbene, a new agonist for the peroxisome proliferator-activated receptor α-isoform, lowers plasma lipoproteins and cholesterol in hypercholesterolemic hamsters. J Agric Food Chem. 2005;53:3403–7. doi: 10.1021/jf0580364. [DOI] [PubMed] [Google Scholar]
- 66. Pari L, Satheesh MA. Effect of pterostilbene on hepatic key enzymes of glucose metabolism in streptozotocin-and nicotinamide-induced diabetic rats. Life Sci. 2006;79:641–5. doi: 10.1016/j.lfs.2006.02.036. [DOI] [PubMed] [Google Scholar]
- 67. Hsu CL, Lin YJ, Ho CT, Yen GC. The inhibitory effect of pterostilbene on inflammatory responses during the interaction of 3T3-L1 adipocytes and RAW 264.7 macrophages. J Agric Food Chem. 2013;61:602–10. doi: 10.1021/jf304487v. [DOI] [PubMed] [Google Scholar]
- 68. Liu J, Fan C, Yu L, Yang Y, Jiang S, Ma Z, Hu W, Li T, Yang Z, Tian T. Pterostilbene exerts an anti-inflammatory effect via regulating endoplasmic reticulum stress in endothelial cells. Cytokine. 2016;77:88–97. doi: 10.1016/j.cyto.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 69. Chakraborty A, Gupta N, Ghosh K, Roy P. In vitro evaluation of the cytotoxic, anti-proliferative and anti-oxidant properties of pterostilbene isolated from Pterocarpus marsupium. Toxicol In Vitro. 2010;24:1215–28. doi: 10.1016/j.tiv.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 70. Chakraborty A, Bodipati N, Demonacos MK, Peddinti R, Ghosh K, Roy P. Long term induction by pterostilbene results in autophagy and cellular differentiation in MCF-7 cells via ROS dependent pathway. Mol Cell Endocrinol. 2012;355:25–40. doi: 10.1016/j.mce.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 71. Wang TT, Schoene NW, Kim YS, Mizuno CS, Rimando AM. Differential effects of resveratrol and its naturally occurring methylether analogs on cell cycle and apoptosis in human androgen-responsive LNCaP cancer cells. Mol Nutr Food Res. 2010;54:335–44. doi: 10.1002/mnfr.200900143. [DOI] [PubMed] [Google Scholar]
- 72. Bhakkiyalakshmi E, Shalini D, Sekar TV, Rajaguru P, Paulmurugan R, Ramkumar KM. Therapeutic potential of pterostilbene against pancreatic beta-cell apoptosis mediated through Nrf2. Br J Pharmacol. 2014;171:1747–57. doi: 10.1111/bph.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gómez-Zorita S, Fernandez-Quintela A, Aguirre L, Macarulla M, Rimando A, Portillo M. Pterostilbene improves glycaemic control in rats fed an obesogenic diet: involvement of skeletal muscle and liver. Food Funct. 2015;6:1968–76. doi: 10.1039/c5fo00151j. [DOI] [PubMed] [Google Scholar]
- 74. Satheesh MA, Pari L. Effect of pterostilbene on lipids and lipid profiles in streptozotocin–nicotinamide induced type 2 diabetes mellitus. J Appl Biomed. 2008;6:31–7. [Google Scholar]
- 75. Gómez-Zorita S, Fernandez-Quintela A, Lasa A, Aguirre L, Rimando AM, Portillo MP. Pterostilbene, a dimethyl ether derivative of resveratrol, reduces fat accumulation in rats fed an obesogenic diet. J Agric Food Chem. 2014;62:8371–8. doi: 10.1021/jf501318b. [DOI] [PubMed] [Google Scholar]
- 76. Rimando AM, Khan SI, Mizuno CS, Ren G, Mathews ST, Kim H, Yokoyama W. Evaluation of PPARα activation by known blueberry constituents. J Sci Food Agric. 2016;96:1666–71. doi: 10.1002/jsfa.7269. [DOI] [PubMed] [Google Scholar]
- 77. Joseph JA, Fisher DR, Cheng V, Rimando AM, Shukitt-Hale B. Cellular and behavioral effects of stilbene resveratrol analogues: implications for reducing the deleterious effects of aging. J Agric Food Chem. 2008;56:10544–51. doi: 10.1021/jf802279h. [DOI] [PubMed] [Google Scholar]
- 78. Chang J, Rimando A, Pallas M, Camins A, Porquet D, Reeves J, Shukitt-Hale B, Smith MA, Joseph JA, Casadesus G. Low-dose pterostilbene, but not resveratrol, is a potent neuromodulator in aging and Alzheimer’s disease. Neurobiol Aging. 2012;33:2062–71. doi: 10.1016/j.neurobiolaging.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 79. Takemoto JK, Remsberg CM, Davies NM. Pharmacologic activities of 3′-hydroxypterostilbene: cytotoxic, anti-Oxidant, anti-adipogenic, anti-inflammatory, histone deacetylase and sirtuin 1 inhibitory activity. J Pharm Pharm Sci. 2015;18:713–27. doi: 10.18433/j33w4c. [DOI] [PubMed] [Google Scholar]
- 80. Chiou YS, Tsai ML, Nagabhushanam K, Wang YJ, Wu CH, Ho CT, Pan MH. Pterostilbene is more potent than resveratrol in preventing azoxymethane (AOM)-induced colon tumorigenesis via activation of the NF-E2-related factor 2 (Nrf2)-mediated antioxidant signaling pathway. J Agric Food Chem. 2011;59:2725–33. doi: 10.1021/jf2000103. [DOI] [PubMed] [Google Scholar]
- 81. Lin HS, Ho PC. A rapid HPLC method for the quantification of 3, 5, 4′-trimethoxy-trans-stilbene (TMS) in rat plasma and its application in pharmacokinetic study. J Pharm Biomed Anal. 2009;49:387–92. doi: 10.1016/j.jpba.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 82. Das S, Lin HS, Ho PC, Ng KY. The impact of aqueous solubility and dose on the pharmacokinetic profiles of resveratrol. Pharm Res. 2008;25:2593–600. doi: 10.1007/s11095-008-9677-1. [DOI] [PubMed] [Google Scholar]
- 83. Lin HS, Yue BD, Ho PC. Determination of pterostilbene in rat plasma by a simple HPLC-UV method and its application in pre-clinical pharmacokinetic study. Biomed Chromatogr. 2009;23:1308–15. doi: 10.1002/bmc.1254. [DOI] [PubMed] [Google Scholar]
- 84. Azzolini M, La Spina M, Mattarei A, Paradisi C, Zoratti M, Biasutto L. Pharmacokinetics and tissue distribution of pterostilbene in the rat. Mol Nutr Food Res. 2014;58:2122–32. doi: 10.1002/mnfr.201400244. [DOI] [PubMed] [Google Scholar]
- 85. Muzzio M, Huang Z, Hu SC, Johnson WD, McCormick DL, Kapetanovic IM. Determination of resveratrol and its sulfate and glucuronide metabolites in plasma by LC–MS/MS and their pharmacokinetics in dogs. J Pharm Biomed Anal. 2012;59:201–8. doi: 10.1016/j.jpba.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]