Abstract
Background:
Progressive supranuclear palsy (PSP) is a neurodegenerative condition, typically presenting with, but not limited to, impairments of postural instability, gait, and gaze stability.
Purpose:
This case report describes the multifactorial assessment and rehabilitation of a patient with atypical PSP who has significant gaze deficits, asymmetrical stepping responses, trunk rigidity, and reduced posterior excursion on limits of stability.
Case Description:
Evaluation utilized computerized gait and balance assessments, foot clearance analysis, a squat test, and a timed stepping test. The intervention included boxing, stepping tasks, and treadmill training each with eye movement challenges. A total of 15 hours of physical therapy was provided; 1 hour, 2 times a week.
Outcomes:
Post-intervention improvements were noted subjectively, on eye-body coordination, and objectively, on limits of stability, foot clearance, and task performance (squats, timed stepping). Follow-up demonstrated some decline from post-test results; however, patient-reported adherence to the program was less than recommended.
Conclusion:
A multifactorial rehabilitation program can improve balance, eye-body coordination and strength in a high functioning patient with atypical PSP. Longitudinal randomized controlled studies are suggested to further investigate this interventional approach in high functioning individuals diagnosed with atypical PSP.
Keywords: Progressive Supranuclear Palsy, rehabilitation, balance, stepping, exercise
INTRODUCTION
Progressive supranuclear palsy (PSP) is a neurodegenerative disease and the most common atypical Parkinsonian syndrome (Litvan et al, 1996). It has been referred to as a ‘Parkinson-plus disorder’ (Agarwal and Gilbert, 2020). Initial motor symptoms are symmetrical and axial with associated gait difficulties and falls (Agarwal and Gilbert, 2020). As the condition progresses a differentiating sign of slowed vertical gaze worsens along with dysarthria, dysphagia, and frontal cognitive difficulties (Agarwal and Gilbert, 2020). It is rare with the highest prevalence rate, found during a brief literature review, reported at 6.4 per 100,000 (Schrag, Ben-Shlomo, and Quinn, 1999). Multiple PSP phenotypes have been noted, making diagnosis difficult (Agarwal and Gilbert, 2020; Lopez, Bayulkem, and Hallett, 2016). Thus, diagnosis is usually made by the presence and progression of multiple signs including, but not limited to: postural instability, supranuclear opthalmoplegia primarily of vertical gaze, pseudobulbar dysfunction, dystonic rigidity of the neck and upper trunk and mild cognitive dysfunction (Richardson, Steele, and Olszewski 1963). Additionally, ruling out other diagnoses by noting treatment response is a common approach in diagnosing movement disorders (National Institute of Neurological Disorders and Stroke web-site https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Progressive-Supranuclear-Palsy-Fact-Sheet#3281_5).
Motor abnormalities can be disabling within a few years of the disease onset. According to Goetz et al, under the typical disease course, 48% of patients will either lose their ability to walk independently, to stand unassisted or require a wheelchair within 4 years of onset of the disease (Goetz, Leurgans, Lang, and Litvan, 2003). No pharmacological intervention has provided effective treatment (Lubarsky and Juncos, 2008). To date, rehabilitation seems to be a means of countering some of the presenting impairments (Clerici et al, 2017; Nicolai et al, 2010; Sosner, Wall, and Sznajder, 1993; Suteerawattananon, MacNeill, and Protas, 2002; Zampieri and Di Fabio, 2008; Zampieri and Di Fabio, 2009) however, authors are unaware of reports of exercise intervention in high functioning patients diagnosed with atypical PSP. Due to the progressive nature of the disease, the typical patient may appear to have met a ceiling in rehabilitation potential- leading to questions of treatment effectiveness. In a recent review on therapeutic exercise for people with PSP, Slade, Finkelstein, McGinley, and Morris (2020) noted while a variety of interventions have been studied, including gait, gaze, and balance training, there is limited evidence of their benefits especially in advanced PSP. For those with PSP there is no consensus on which interventions are most effective, nor is there standardization of performance-based assessments or available evidence of psychometric analysis of performance-based assessments.
This case report describes a patient with balance and gaze palsy concerns which interfered with high level leisure activities. To understand the impairments and complex effect of the disease on balance function, objective tests such as gait analysis, foot clearance analysis, and computerized posturography are used, along with a few selected clinical tests (Nath et al, 2003). Improved understanding of the disease amongst healthcare workers and the public can enhance recognition and diagnosis of the disease and atypical presentations (Höglinger et al, 2016; Lopez, Bayulkem, and Hallett, 2016). Therefore, therapists may seek published case reports for guidance. This case report provides guidance by offering a detailed description of patient assessment and an exercise intervention for a high functioning patient with atypical PSP.
CASE DESCRIPTION
This patient is a 63 year old Caucasian, who initially reported mild symptoms of dysarthria and balance impairments 11 years prior to this report. He was then diagnosed with Parkinson Disease and his symptoms remained stable for approximately 7 years. Because he was a litigator, speech concerns impaired his ability to perform as he would like at work. He retired, and consequently, began a self-prescribed fitness routine. The routine included 5-6 times weekly aerobic and strengthening exercises. He had an active lifestyle which included daily hiking with his two dogs. He reported taking no medications.
At the age of 59, upon ophthalmology examination, vertical supranuclear palsy was noted secondary to slow vertical saccades. He was then described by the opthalomologist as having ‘parkinsonian syndrome with supranuclear palsy’. The diagnosis was also confirmed as atypical PSP by a movement disorder specialist. This patient was diagnosed with atypical PSP by the absence of frequent falls, early onset, and long disease course. Specifically, he was enjoying an unusual longevity with sustained high level of functioning after initial symptom presentation. However, gaze stability, blepharospasm, and postural stability were more frequently affecting his daily living i.e. preventing carefree hiking. Minimal comorbidities were revealed by MRI, including mild cervical spine degeneration and small and stable white matter lesions. He demonstrated strong cognitive skills as noted with his ability to follow directions and performance on neuropsychological testing.
His chief complaint was blepharospasm (e.g. forcible closure and delayed opening of eye lids) and lack of confidence in his ability to control gaze during daily hikes. The inability to quickly change visual targets caused foot placement difficulty when using rocks to cross a stream. Additionally, he was not confident taking backwards steps. Noting gaze concerns, he began occupational therapy (OT). Results of OT examination showed that smooth eye pursuit was accurate horizontally but slowed vertically. Saccades were hypometric and slowed horizontally and vertically. He received practical walking training as well as head and eye movement training with a Dynavision 2™ viso-motor computerized system (TSS Technologies, West Chester Ohio). After six OT sessions, improvements in reaction time while finding targets in lower quadrants and carryover of eye-head movement patterns during practical walking tasks were noted. He reported improvement in eye-aiming tasks in the vertical direction and more comfort and confidence with activities. Challenges persisted in the ability to train eye-head-body movement patterns, specifically training eye-head-limb coordination while stepping on uneven terrain. Physical therapy was requested to augment OT. The patient specifically expressed he wanted to improve his ability to hike over rocks.
Clinical Findings
Prior to our examination of the patient, results of the OT examination were obtained from the patient’s chart. The patient scored 24/100 on the PSP Rating Scale. The PSP rating scale is a measure of disability rather than a diagnostic tool (Golbe and Ohman-Strickland, 2007). In this study, it was used to characterize disease severity. The scale rates activities of daily living by interview, behaviour, and limb motor control with the worst score being 100 points; each item is rated on a Likert scale (Golbe and Ohman-Strickland, 2007). Intra-class correlation coefficients have revealed good inter-rater reliability (Golbe and Ohman-Strickland, 2007). Additionally, survival distribution calculations done by Golbe and Ohman-Strickland (2007) noted that only 25% of their subjects who were diagnosed younger than 65 survived 9.78 years. According to this scale his disease severity was mild. The PSP rating was obtained from the patient’s chart and did not contain itemized scores. However, based on our observation, impairments which lowered his PSP rating scale score were concentrated in bulbar, oculomotor, and gait/midline items. His oculomotor exam, which was also obtained via chart review, revealed slow saccades primarily in the vertical direction and to some extent in the horizontal direction, with normal smooth pursuit. His score on the Berg Balance Scale was 52/56. Those with Parkinson Disease, who score similarly on the Berg Balance Scale can be characterized as having mild balance problems and low risk of falls (Qutubuddin et al, 2005). Although Qutubuddin et al. (2005) results cannot be strictly applied to this case, PSP, known as a ‘Parkinson-Plus Disorder’, is inherently similar in clinical presentation. The results of the Berg Balance Scale helped to confirm mild symptom status.
Physical Therapy Assessment and Pre-Test Findings
The following assessments and findings were considered important to his overall function. He was able to balance on unilateral stance for 10 seconds; however, he was unable to perform a unilateral squat without using hands for support. His strength was excellent judging by his scores of 5/5 on manual muscle testing for all major muscle groups. Moderate axial rigidity was noted secondary to poor trunk rotation and decreased right arm swing during gait. “En block” trunk motions were also noticed during turns at the end of a walking pass.
Computerized Posturography
A NeuroCom SMART Equitest System (previously Natus, Inc., Clackamas, OR) was used to administer the following assessments: Sensory Organization Test (SOT), Motor Control Test (MCT), and the Limits of Stability Test (LOS). These tests have been previously utilized in the assessment of individuals with Parkinson’s disease (Harro, Marquis, Piper, and Burdis, 2016).
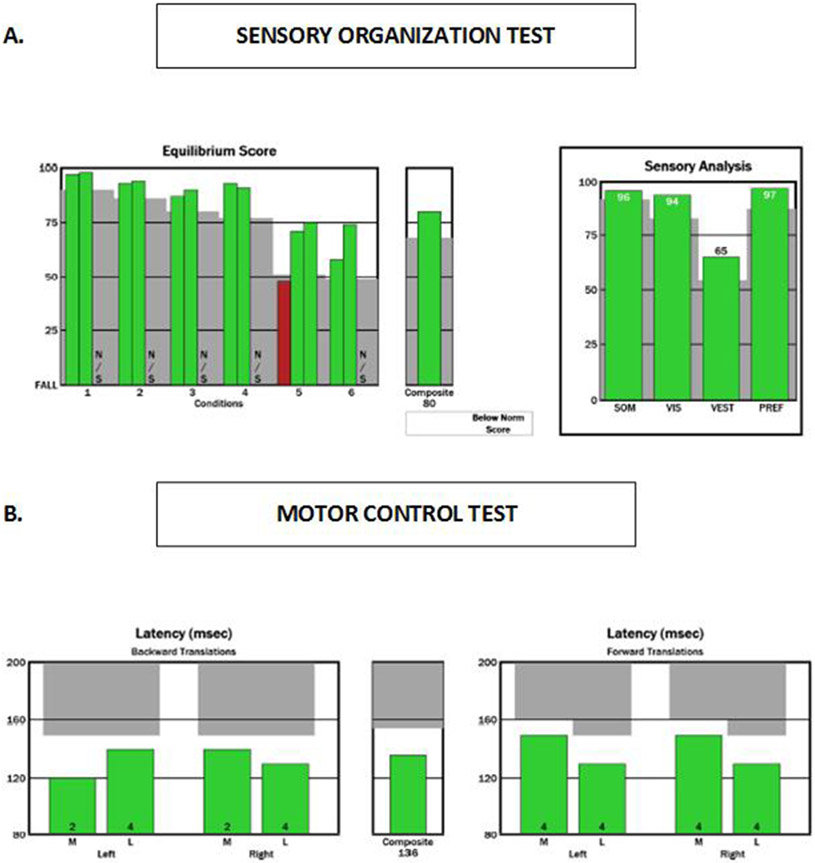
SOT results were normal (Figure 1A), indicating preserved ability to integrate three sensory systems: 1) somatosensory; 2) visual; and 3) vestibular. MCT results were also normal (Figure 1B), indicating preserved ability to automatically generate a rapid postural response to external perturbations (Jacobson and Kartush, 1997).
Figure 1. NeuroCom report showing pre-test (baseline) results on the SOT (Figure 1A) and MCT (Figure 1B).
Green bars on all plots indicate results within normal limits for this patient’s age group. NS means that particular test condition was not completed. In the SOT the patient stands quietly for 20 seconds each trial under the following six conditions: (1) eyes open, no sway reference; (2) eyes closed, no sway reference; (3) eyes open, visual/surround sway reference; (4) eyes open, support surface sway reference; (5) eyes closed, support surface sway reference; and (6) eyes open, support surface and visual/surround sway reference. Sway reference is the displacement of the platform and/or the visual surround. Higher scores reflect less body sway (i.e., better balance).The top left plot reflects the patient’s performance on each trial per condition. The top right plot reflects the patient’s performance on the somatosensory (SOM), vestibular (VES), visual (VIS) and preference (PREF) ratios. Which are calculated using the average equilibrium score for each condition. In the MCT (lower plot) the patient is required to stand still and keep a straight posture in response to anterior and posterior perturbations of the forceplate surface. The average amplitude of latency response (in milliseconds) between the shift of the forceplate and the torque generated by the individual on the forceplate. Responses are measured for each foot for medium and large perturbations in the forward and backward directions.
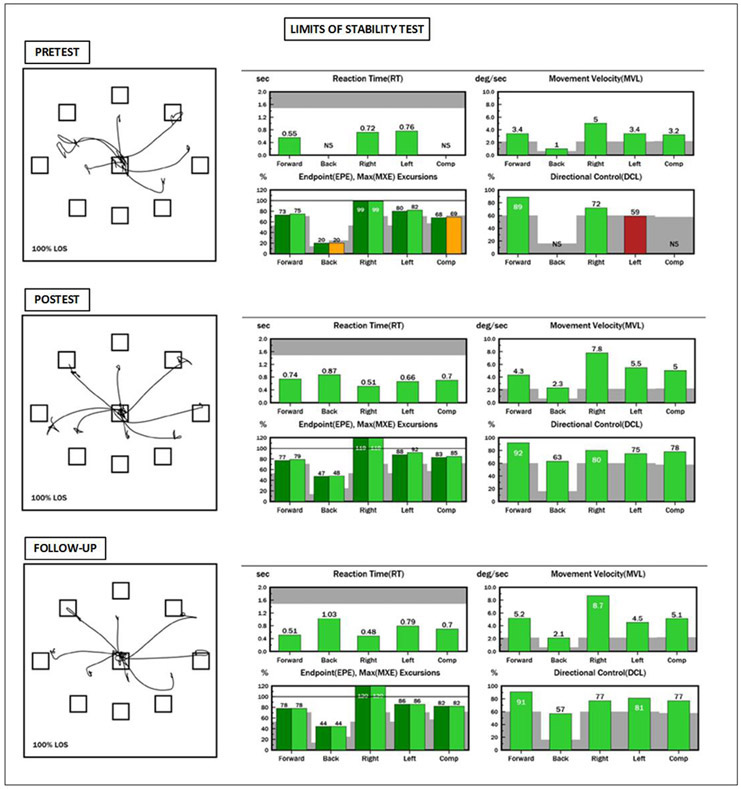
Results on the LOS revealed several deficits (Figure 2). Reaction time was slower than normal in the posterior and left directions, affecting the composite score. An NS (not scored) was generated by NeuroCom for reaction time and directional control in the posterior direction revealing his inability to lean backward (Figure 2 - Pre-test, upper left bar graph). Reduced excursions were found in the forward, posterior, and left directions; all of which contributed to abnormal excursion composite scores (Figure 2 - Pre-test, bottom left bar graph). Directional control was just below normal in the left direction and a score was not generated for directional control in the backward direction; therefore, a composite score could not be determined (Figure 2 - Pre-test, bottom right bar graph).
Figure 2. NeuroCom report showing pre-test (baseline), post-test (post intervention) and follow-up results on the LOS test.
In this test the patient is required to purposefully shift their weight toward targets in eight directions while receiving continuous visual feedback about the movement of their COG. These targets represent 100% of their theoretical limits of stability based upon their height. During the leaning task, the patient must maintain a straight posture, only moving at their ankles, like an inverted pendulum. The top left star-shaped tracing of each testing time point represents the trajectory of the patient’s center of gravity. To the right of that are the scores for each variable and each direction. Green bars indicate normal results and yellow or red bars indicate abnormal results. Gray represents the area where results are more than 2 standard deviations away from the normative mean. NS means not scored and it was generated because the patient was not able to perform the leaning task on that particular direction. Note the important increase on backwards excursion at post intervention compared to baseline, and maintenance of scores within normal limits at follow-up
Gait and Foot Clearance Analysis
Spatiotemporal gait analysis and foot clearance analysis were performed with a 10 camera MX system (Vicon Motion Systems, Denver, CO). The patient walked independently without any orthoses or assistive devices. The walking task was performed at regular pace over a 500 centimeters (cm) walking path. Three trials were collected and the average was calculated for the following parameters: velocity (cm/second), step length (cm), step time (seconds), and double support time (percent of gait cycle).
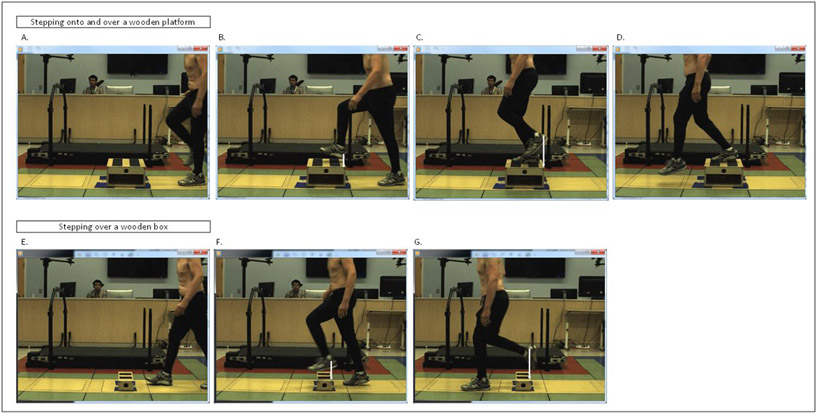
Our method of foot clearance analysis is illustrated in Figure 3. Motion analysis was used to record foot kinematics during a step up task (wooden platform: 10.16 cm high, 33.02 cm deep, 45.72 cm wide), and a step over task (wooden box: 15.24 cm high, 20 cm deep, 44.45 cm wide). Three reflective markers were placed on each foot, and four markers were placed on the top corners of the wooden platform and wooden box. Two testing conditions were used: the patient took a short walk about 300 cm before he either had to: 1) a step onto and over the wooden platform; or 2) step over the wooden box. Instructions were to look straight ahead at the beginning of the walk, and look where necessary during walking. Foot clearance of the leading foot on the sagittal plane was measured as the vertical height of the heel marker when the foot was above the front of the wooden platform or the wooden box. Three trials were obtained for each foot. To keep gait as natural as possible, the patient was not told which foot to choose to lead the stepping task. Several trials were collected until a minimum of 3 trials were obtained for each foot. All trials were performed at his comfortable pace.
Figure 3. Sample trial for foot clearance analysis.
Illustration of the methodology used for the stepping onto and over a platform (A-D), and over obstacle task (E-G). Each task happens from left to right. The top images show the patient walking toward the box, stepping on it with the right, and then stepping over it with the left foot. The bottom images show the patient walking toward the obstacle and stepping over, leading with the left foot. Foot clearance is represented by the white vertical arrow on the leading foot (B) and lagging foot (C) for the platform task and leading foot (F) and lagging foot (G) for the box task.
Results for gait and foot clearance analysis are shown on Table 1, under the pre-test time point column. The patient scored within normal limits for spatiotemporal gait parameters. Since this is an experimental test, there are no normative data for comparison; therefore, we collected pre and post-intervention data to assess intervention effects.
Table 1.
Results from Gait and Foot Clearance Analysis
| Task | Variable | Pre-test | Post-test | Follow-up |
|---|---|---|---|---|
| Gait | Velocity (cm/s) | 136 | 128 | 133 |
| step length (cm) | 54 | 58 | 55 | |
| step time (s) | 0.73 | 0.73 | 0.72 | |
| double support (% cycle) | 25.37 | 24.14 | 25.57 | |
| Platform Step up | L heel clearance (cm) | 29 | 31 | 21 |
| R heel clearance (cm) | 31 | 32 | 24 | |
| Box Step up | L heel clearance (cm) | 29 | 29 | 21 |
| R heel clearance (cm) | 39 | 41 | 34 |
Timed Stepping and Unilateral Squat Tests
Two clinically feasible tests were used to extrapolate performance during hiking: timed stepping and unilateral squats. This patient was asked to repeatedly initiate stepping with the same foot onto a 10.16 cm high, by 91.44 cm wide by 35.56 cm deep stool (10 times), come to double limb stance on the stool and then step down with the initiating foot. For this patient ten steps were required to note error. No use of hand support was allowed. The pattern of stepping was demonstrated to the patient and followed by a prompt to discern understanding. If there was a change in the pattern the test would be stopped then restarted. It was repeated with the opposite side initiating the stepping. Pre-test results are shown in Table 2. The patient took longer to complete 10 steps when leading with his left foot, and had less accurate placement of the lagging (right) foot; his right toes hit the step three times out of ten steps. He was also unable to perform the unilateral squat task without hand hold support at pre-test.
Table 2.
Results from Timed Stepping and Unilateral Squat Test
| Pre-Intervention | Post-Intervention | |
|---|---|---|
| Repeated Stepping 10 x | ||
| Right foot leading | 29.4 seconds | 27.19 seconds |
| Accuracy: no errors | Accuracy: no errors | |
| Left foot leading | 31.5 seconds | 27.49 seconds |
| Accuracy: trailing foot hit step prematurely 3x | Accuracy: no errors | |
| Unilateral squat | Unable to perform without hand hold assist. | Performed 7 prior to hand hold assist. |
Physical Therapy Diagnosis and Prognosis
As it is characteristic of parkinsonian syndromes, this patient presented with axial rigidity combined with postural instability although mild, which coincide with two of the four cardinal signs of Parkinson Disease (e.g. tremor, bradykinesia, rigidity, and postural instability). These findings were primarily complicated by the co-existing blepharospasm and slowed vertical saccadic movement which led to challenges during higher level mobility and motor function tasks. His current longevity with this condition and level of robust physical activity helped foster expectations for a reasonably good prognosis with physical therapy intervention. His limits of stability and eye-body coordination seemed the most significant impairment and most feasibly addressed by exercises that simultaneously challenged both constraints. A potential lack of ability to improve further could not be ruled out. As stated previously, authors are unaware of reports of exercise intervention in high functioning patients diagnosed with atypical PSP, and thus an intervention was designed and reported below.
Therapeutic Intervention
Based on assessments, intervention was developed to address LOS, eye-body coordination, and strength. The following intervention was to improve LOS and eye-body coordination and provide the patient with better motor control to more safely and confidently perform higher level activities such as hiking. Boxing, stepping tasks and treadmill training were the three modalities selected. Please see Table 3 for details. The following is a description of each exercise as related to patient constraints: 1) Boxing was used to improve rigidity and bradykinesia, to promote axial rotation and normal sequencing of head first-trunk second-foot third movements, to evoke gaze-shifts in the horizontal direction, to practice visually guided locomotion, as well as to increase directional control and excursion of movements in the sideways and backwards directions. Given LOS findings, backwards walking while boxing was used as part of the intervention; 2) Stepping tasks were used to improve gaze-shift strategies in the vertical direction along with eye-body coordination. Additionally, this exercise was used to help improve confidence while moving backwards; and 3) Treadmill training was used to improve gaze-shift strategies in the vertical direction while moving. He was asked to visually seek targets in random spots lower than his hips and then shift his gaze upwards to eye level, while walking on the treadmill. Secondly, increasing confidence levels and directional control sideways and backwards through practice of stepping reactions was sought. Challenges of anterior- posterior and medial-lateral stability were added by randomly, suddenly stopping the treadmill during forward walking and side stepping, respectively. This activity also challenged reactive postural control and protective stepping.
Table 3.
Supervised Physical Therapy Intervention for Stepping, Limits of Stability, and Eye/Head/Body Movement Patterns
| Activity | Initial Exercises | Progressions* |
|---|---|---|
| Boxing | ||
|
Gaze with eye/head/trunk movement A punching bolster was placed on the patient’s left side to maximize trunk rotation. Patient was instructed to take a step and look straight ahead with arms in “boxer’s stance”. Patient turned his head then his trunk to face the target, punch the target and then return to “boxer’s stance”. This was repeated 10 times. Task was then repeated on the patient’s right side. |
No visual targets Visual targets Visual targets scattered to increase visual searching Symbols added to visual targets for dual task challenge |
|
|
Backwards stepping A punching bolster was placed anterolateral to patient’s midline and moved during backwards progression. Patient visually focused on point in front with arms in “boxer’s stance”. The patient followed instructions below: -Take two steps backwards while maintaining focus on a point in front. -Direct gaze towards target. -Punch the target. -Return gaze and trunk to original position. This was done with the target on his left side as well as his right. |
Each session encouraged maximal step length and speed until close to falling (decline in confidence to avoid fall) | |
| Step Training | ||
|
Gaze shift with stepping Patient stood on a 6 inch step focusing on fixed spot. Laser was pointed on ground in random locations. Patient located laser with vision, immediately return gaze to starting point, and then stepped down from step. |
No visual targets Visual targets Visual targets scattered to increase visual searching Symbols added to visual targets for dual task challenge |
|
|
Accuracy and Coordination of stepping Leading with right lower extremity, the patient stepped onto 6 inch step, tapped step with left foot then stepped down- leading with left. Then repeated the stepping pattern leading with left lower extremity. |
Each session focus was on increasing speed and accuracy until close to loss of balance (decline in confidence to avoid fall) | |
|
Stepping responses Patient was instructed to lean to his limit of stability anteriorly, posteriorly, left and right. Instructed to lean far enough that taking a step would be necessary to prevent fall, then stop a fall with step. |
Added symbols to encourage larger stepping response. | |
| Treadmill | ||
|
Stepping Responses and Postural Stability Patient was strapped into a harness for safety. Treadmill pace was set at 0.5 miles per hour. Patient was instructed to walk while maintaining proper body alignment and gait technique. This was done twice for 2 minutes each stepping to the left, right and backwards. The treadmill was stopped if patient’s body alignment or gait technique suffered. |
Attempted to increase the length of time spent doing the exercise before adjusting body alignment or gait technique. | |
|
Gaze shift during stepping Patient was strapped into a harness for safety. Treadmill pace was set at 0.5 miles per hour. Patient was instructed to walk while looking at a fixed point ahead. Laser was pointed at the ground and patient had to locate laser with vision, and immediately return gaze to starting point. |
Focused on moving the head and eyes together progressed by speed of head movement if possible. Verbally cued to increase pace of gaze shift until close to loss of balance. Challenged patient until unable to maintain eye and head movement in succinct pattern. | |
|
Stepping Responses Patient was strapped into a harness for safety. Treadmill was randomly started and stopped throughout treatment. |
Stopping was performed randomly to avoid practice effect. Attempts to reduce hand support during activity. | |
Each session patient was challenged to increase speed and/or difficulty of task until either accuracy, ability to coordinate task or confidence to avoid fall declined.
Two treatment sessions per week were broken into three 20 minute segments (i.e. boxing, stepping tasks, and treadmill training). A total of 15 hours of physical therapy intervention was provided over the duration of 2 ½ months. Please see Table 3 for progressions. In OT, eye-body movement strategies were employed while ambulating in the community and performing activities of daily living to improve visual scanning and targeting.
A secondary goal was to improve strength, balance, and neuromuscular control evidenced by his inability to perform a unilateral squat. Therefore, he was advised to perform closed chain exercises. Exercises included stepping up steps two at a time progressing in 12 weeks to a 4 pound ankle weight, practicing the unilateral squat gradually reducing handhold assist and maintaining a lateral plank progressing to more narrow leg placement and thus smaller base of support. He completed these five times weekly during his normal exercise regimen.
Post-Test and Follow-Up Outcomes
Not all tests were repeated at the end of the intervention and follow-up. The PSP rating scale and the Berg Balance Scale were not repeated because they were obtained via chart review and describe the presentation of the patient. The SOT and MCT NeuroCom balance tests were not repeated, because the patient scored within normal limits at pre-test, and therefore, were not targeted by the intervention. The following tests were repeated during post-testing and at a 6 month follow-up: LOS, gait and foot clearance analysis, performance-based tests of timed stepping and unilateral squats.
Post-Test Findings
Improvement was noted in many of the repeated tests. The patient’s reaction time and directional control could not be scored in the posterior direction prior to intervention and improved to be within normal limits for his age group. Endpoint and maximum excursions increased in the posterior direction, and slight improvements were observed for the forward, left and composite scores. Improvements were notable because of the change from abnormal to within normal limit scores. Directional control improved toward posterior and left directions reaching normal limits. Tracings of his COG path (star shaped graph) at post-test compared to pre-test also illustrated improvements. There was little change in gait spatiotemporal parameters, which remained within normal limits at post-test (Table 1). Foot clearance scores increased around 0.2 to 2 cm bilaterally at post-test compared to pre-test during both, stepping up and stepping over tasks (Table 1). Results on the repeated stepping test and the squats during unilateral stance also improved. Increased speed, symmetry and accuracy were recorded on the repeated stepping test; the patient no longer contacted the step prematurely with the trailing foot (Table 2). He was able to perform seven unilateral squats prior to hand hold assist irrespective of side. Gains in strength were noted by improvements in his home exercise regimen. He added ankle weights while stepping up two steps, reduced hand hold assist during unilateral squats and was able to maintain a lateral plank while holding a narrower lower limb base of support. Improvements were also noted by his subjective reports of increased speed upon stair climbing and increased speed and comfort with using his vision to walk with his dog on trails. Such improvements were noted along with the need for conscious effort to incorporate eye-body movement patterns during high level activity (i.e. biking and hiking).
Follow-Up Findings
Follow-up results six months after intervention revealed the patient was able to maintain the balance improvements. LOS results were similar to post-test and remained within normal limits (Figure 1). However, foot clearance analysis revealed stepping up and stepping over scores decreased on both tasks to levels that were not only below post-intervention but below initial testing (Table 1). Performance-based tests revealed that asymmetries remained. He was able to perform 15 unilateral squats with his right lower extremity while only 2 on his left side before losing balance. Repeated step test demonstrated no errors in accuracy and no remarkable time differences: 28.1 seconds right foot leading and 28.7 seconds left foot leading (Table 2).
DISCUSSION
To our knowledge, this is the first description of rehabilitation of a high functioning patient with atypical PSP. Objective assessment revealed specific deficits and helped tailor intervention that improved balance, eye-body coordination and strength. His performance on NeuroCom balance tests, including the SOT and MCT showed intact sensory and reactive postural control of balance, but revealed mild LOS abnormalities. This finding supports an atypical presentation. Patients with PSP are expected to show significant SOT deficits in less than 4 years of diagnosis diagnosis (Ondo et al, 2000). Although LOS deficits were mild compared to typical cases (Ondo et al, 2012), LOS deficits can be detrimental to one’s ability to perform tasks that require reaching, bending and/or postural transitions (Jacobson and Kartush, 1997), all of which are required during hiking. Therefore, this finding likely related to our patient’s main complaint of difficulty hiking.
The methods we utilized for foot clearance analysis were a modification of those pioneered by Di Fabio, Zampieri, and Tuite (2008) in patients with PSP. Although our patient was atypical given his preserved function, foot clearance deficits seemed to fall under the typical presentation of PSP and revealed concerns of gaze deficits influencing his ability to coordinate stepping responses. There is evidence in patients with PSP that gaze deficits negatively affect foot clearance (Di Fabio, Zampieri, and Tuite, 2008). In a comparison of two groups of patients with PSP, those with severe gaze deficits demonstrated significantly reduced trailing foot clearance on a stepping task versus those with mild oculomotor deficits (Di Fabio, Zampieri, and Tuite, 2008). During foot clearance analysis, as he approached the wooden platform and/or box, his head would pitch down in an attempt to look at the wooden box, but his eyes appeared to counter rotate up, demonstrating a fixated gaze at the horizon. Suppression of the vestibular-ocular reflex is a phenomenon that has been previously documented (Di Fabio, Zampieri, and Tuite, 2007). This leads to restrictions in patients’ downward gaze and their ability to localize objects on the ground during stepping activity. Not surprisingly, one of our patient’s subjective complaints was that his eyes would get ‘stuck’ while he was walking his dog.
Literature supports a direct link between foot clearance abilities and the risk of falls (Begg and Sparrow, 2000; Chou and Draganich, 1998; Di Fabio, Kurszewski, Jorgenson, and Kunz, 2004). These results, which demonstrated an increase of heel clearance of around 0.02 (m) with an overall step height ranging between 0.2 to 0.3 (m) may not reach clinical significance. Alternatively, authors were unable to locate published evidence of what may be considered deleterious hypermetria. Hypermetric leg movement has been observed in individuals with cerebellar damage and with leg kinematics which are considered ‘undistinguishable from control subject(s)’ (Morton, Dordevic, and Bastian, 2004). Importantly, performance on a repeated stepping test, provided at the same time intervals, appeared to suggest improvement. Direct comparisons of foot clearance values are limited, as obstacle dimensions and tasks are different between studies. Our patient’s eye-body coordination problems seem to have been a determining factor on his possibly excessive foot clearance, which may reflect a more cautious obstacle clearance strategy. We base our conclusions on the framework by Patla (1997). According to that framework, vision plays a crucial role on gait stability and adaptability as it allows for the implementation of avoidance strategies (e.g. selection of alternate foot placement, increased ground clearance to avoid hitting an obstacle on ground, and changing the direction of locomotion) which are the most effective mechanism to ensure balance during locomotion.
Our patient’s performance on a unilateral squat test helped us understand his ability to hike over boulders commonly found on the trail he took with his dogs. The squat task mimicked the motor control and strength required to lift one’s body weight while stepping onto a boulder or walking up a hill, a skill frequently required during hiking. Similarly, one must lower their body weight while progressing from an elevated surface (e.g. a boulder) to the ground or manage change in trail elevation. At pre-test he was unable to perform the task without hand hold support. This finding suggests, not only possible balance impairment, but potentially strength deficits not detected with manual muscle testing. Improvements demonstrated on the unilateral squat likely contributed to his desire and report of being able to hike over rocks.
In a recent review, Brown, Rowe, Passamonti, and Rittman (2020) noted that PSP cases with clinical presentations similar to Parkinson’s disease may benefit from treatments proven to be effective in Parkinson’s disease. Therefore, our intervention modalities were selected to mirror previous studies not only in PSP but also in Parkinson’s disease (Abbruzzese, Marchese, Avanzino, and Pelosin, 2016; Combs et al, 2011; King and Horak, 2009; King et al, 2015; van der Kolk and King, 2013; Zampieri and Di Fabio, 2008; Zampieri and Di Fabio, 2009). These studies were successful adopting a multidimensional rather than single modality approach. The following comparisons of this intervention to the literature helps to inform practice. Of the three exercise modalities used, boxing was the only one not yet investigated in individuals with PSP. Our boxing modality combined elements from two studies that involved people with Parkinson’s Disease. One study was specifically designed to study boxing exercises (Combs et al, 2011) and a second study proposed an agility program incorporating boxing drills (King and Horak, 2009). This intervention was planned to occur twice weekly over 2 months, however with patient scheduling constraints, intervention was provided over 2 ½ months and included15 hours of physical therapy. In Combs et al. (2011), patients completed more sessions (24-36) over a longer duration of 3 months. In an agility program designed by King and Horak (2009) intervention lasted 1 month and incorporated 12 sessions. Regarding clinical presentation, this patient had better balance than the more impaired group of individuals with PD: Berg Balance Score: 52 vs 47, respectively. Although Combs et al. (2011) noted improvements in most of their subjects in many outcome measures; further comparisons between studies are limited secondary to methodological differences.
Step training included step up and down exercises similar to previous reports (Zampieri and DiFabio, 2008; Zampieri, and DiFabio, 2009). Eye-body movement patterns were reinforced during step training. Since previous OT service had included visual targeting while playing basketball and walking on level surfaces, we decided to incorporate stepping patterns using platforms. This offered a varied challenge and an approximation of skills related to goals of hiking on uneven terrain. Our intervention was similar to previous rehabilitation studies (Zampieri and DiFabio, 2008; Zampieri and DiFabio, 2009), in terms of intensity and duration. However, in addition to the oculomotor work, our step training also focused on improving our patient’s limits of stability, similarly to a study on Tai Chi exercises for PD (Fuzhong et al, 2012). As in Tai Chi, which uses weight shifting exercises and trunk rotation movements to improve balance and trunk range-of-motion, our intervention added the challenge of alternating between narrow and wide base of support. We also used visual targets to direct trunk rotation movements -to counter rigidity. Although our intervention was much shorter (2½ months versus six months) but with the same frequency and intensity, very similar results were achieved as compared to the Tai Chi study (Fuzhong et al, 2012) as measured with the NeuroCom LOS test.
Finally, our third modality, treadmill training, was comparable to a case report that used body weight support treadmill training with an individual with PSP (Suteerawattananon, MacNeill, and Protas, 2002). As in that case report, perturbations (suddenly stopping and starting the treadmill) were used to challenge anterior posterior stability, and side stepping to challenge medial lateral stability. Aside from the intervention and duration being relatively similar, the clinical presentation of the patient and evaluation tools utilized were different. While our patient was high functioning with mild balance problems, their patient with PSP was more typical, with poor balance and frequent falls. Consequently, none of the balance tools that served as outcomes for their study were applicable to ours. Our treadmill training also incorporated gaze shift exercises using a similar technique to a recently published randomized controlled trial that studied 24 patients with PSP (Clerici et al, 2017). Results of that trial showed visual target oculomotor exercises in association with traditional treadmill training were more effective than robot-assisted treadmill training in improving PSP Rating Scale scores (Clerici et al, 2017). Their intervention was done at a much higher intensity than ours, as they included an inpatient stay and 100 hours of rehabilitation.
The outcomes from our case report contribute to the evidence suggesting that rehabilitation improved postural control and eye-body coordination in this individual with PSP. We refer to the work by Petzinger et al. 2013 to speculate the mechanisms behind the positive effects of our intervention. As shown in their review, in the context of a progressive movement disorder, the central nervous system can respond to exercise depending on the stimulus it is offered. Aerobic exercise seems to stimulate blood flow and angiogenesis in the brain, whereas task-specific exercise seems to promote the creation of new connections and enhance the activity of existing ones. These modalities may induce neuroplasticity. Therefore, the ideal rehabilitation scenario may be a combination of aerobic exercise and task-specific exercise. Our patient seems to be a good example of that ideal scenario. Since he had been an avid exerciser for years, he may have been able to benefit from neuroprotective effects of aerobic exercise (Petzinger et al, 2013) and possibly benefit more than a sedentary patient would from the task-specific exercises we offered. Nevertheless, at the six month follow-up, he reported that he had reverted to his former exercise regimen and ceased to perform the task-specific routine. Our follow-up results confirmed that some of the improvements he had gained were lost. This seems to indicate there is a continued need for targeted therapy to maintain specific benefits, which may be influenced by patient education to elicit behavioural change, or by monitoring compliance for improved patient adherence to key exercises between bouts of skilled physical therapy as the disease progresses.
There are limitations to this case report. Firstly, we used chart review to obtain information on this patient’s PSP rating scale and were only able to report his total score. It would have been helpful to report itemized scores to have a better idea of the specific areas of impairment. However, his level of involvement scored as mild on the scale and given the clinical picture the items most likely to show impairments involved axial rigidity and oculomotor items. Another limitation was that we chose not to repeat the SOT and MCT tests at post-test and follow-up as the patient had tested within normal range, and consequently, we were unable to determine if there were any longitudinal changes on those tests. Given that PSP is a progressive disease it is possible that during the course of treatment, test scores could have declined. Additionally, his gait function assessment would have been strengthened had we included a clinical test of gait stability and adaptability such as the Functional Gait Assessment, which has been validated for Parkinson’s Disease (Yang et al, 2014). We also acknowledge the lack of step monitoring and trips/slips monitoring could be a limitation. Adding GPS tracking data to capture walking excursions outside the house per week during and after treatment could strengthening this case report. Also, a metronome could be applied during intervention to monitor his pace of eye-body coordination activities.
In conclusion, this case report provides evidence in support of the benefits of a multifactorial rehabilitation program with boxing, step training and treadmill training in improving balance, eye-body coordination and strength in a high functioning patient with PSP. Longitudinal randomized controlled studies are suggested as the next step to investigate this interventional approach in a group of individuals with PSP who are clinically alike.
ACKNOWLEDGEMENTS
This work was supported by the intramural research program of the NIH Clinical Center (protocol 90-cc-0168).
Footnotes
Declaration of Interest
The authors report no conflict of interest.
REFERENCES
- Abbruzzese G, Marchese R, Avanzino L, Pelosin E 2016. Rehabilitation for Parkinson's disease: Current outlook and future challenges. Parkinsonism and Related Disorders 22: S60–64. [DOI] [PubMed] [Google Scholar]
- Agarwal S, Gilbert R 2020. Progressive Supranuclear Palsy. Treasure Island (FL): StatPearls Publishing. [PubMed] [Google Scholar]
- Begg RK, Sparrow WA 2000. Gait characteristics of young and old individuals negotiating a raised surface: Implications for the prevention of falls: Journal of Gerontology: Medical Sciences 55: M147–M154. [DOI] [PubMed] [Google Scholar]
- Brown FS, Rowe JB, Passamonti L, Rittman T 2020. Falls in Progressive Supranuclear Palsy. Movement Disorders Clinical Practice 7: 16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou LS, Draganich LF 1998. Placing the trailing foot closer to an obstacle reduces flexion of the hip, knee, and ankle to increase the risk of tripping. Journal of Biomechanics 31: 685–691. [DOI] [PubMed] [Google Scholar]
- Clerici I, Ferrazzoli D, Maestri R, Bossio F, Zivi I, Canesi M, Pezzoli G, Frazzitta G 2017. Rehabilitation in progressive supranuclear palsy: Effectiveness of two multidisciplinary treatments. PloS One 12: e0170927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs SA, Diehl MD, Staples WH, Conn L, Davis K, Lewis N, Schaneman K 2011. Boxing training for patients with Parkinson disease: A case series. Physical Therapy 91: 132–142. [DOI] [PubMed] [Google Scholar]
- Di Fabio RP, Kurszewski WM, Jorgenson EE, Kunz RC 2004. Footlift asymmetry during obstacle avoidance in high-risk elderly. Journal of the American Geriatric Society 52: 2088–2093. [DOI] [PubMed] [Google Scholar]
- Di Fabio RP, Zampieri C, Tuite P 2007. Gaze-shift strategies during functional activity in progressive supranuclear palsy. Experimental Brain Research 178: 351–362. [DOI] [PubMed] [Google Scholar]
- Di Fabio RP, Zampieri C, Tuite P 2008. Gaze control and foot kinematics during stair climbing: Characteristics leading to fall risk in progressive supranuclear palsy. Physical Therapy 88: 240–250. [DOI] [PubMed] [Google Scholar]
- Fuzhong L, Harmer P, Fitzgerald K, Eckstrom E, Stock R, Galver J, Maddalozzo G, Batya S 2012. Tai chi and postural stability in patients with Parkinson Disease. New England Journal of Medicine 366: 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz CG, Leurgans S, Lang AE, Litvan I 2003. Progression of gait, speech and swallowing deficits in progressive supranuclear palsy. Neurology 60: 917–922. [DOI] [PubMed] [Google Scholar]
- Golbe LI, Ohman-Strickland PA 2007. A clinical rating scale for progressive supranuclear palsy. Brain 130: 1552–1565. [DOI] [PubMed] [Google Scholar]
- Harro CC, Marquis A, Piper N, Burdis C 2016. Reliability and validity of force platform measures of balance impairment in individuals with Parkinson disease. Physical Therapy 96: 1955–1964. [DOI] [PubMed] [Google Scholar]
- Höglinger GU, Respondek G, Stameou M, Kurz C, Josephs KA, Lang AE, Mollenhauer B, Muller U, Nilsson C, Whitwell JL et al. 2017. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Movement Disorders 32: 853–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson G, Kartush J 1997. Handbook of Balance Function Testing. San Diego, CA: Singular Publishing Group, Inc. [Google Scholar]
- King LA, Horak FB 2009. Delaying mobility disability in people with Parkinson disease using a sensorimotor agility exercise program. Physical Therapy 89: 384–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LA, Wilhelm J, Chen Y, Blehm R, Nutt J, Chen Z, Serdar A, Horak FB 2015. Effects of group, individual, and home exercise in persons with parkinson disease: A randomized clinical trial. Journal of Neurologic Physical Therapy 39: 204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvan I, Agid Y, Jankovic J, Goetz C, Brandel JP, Lai EC, Wenning G, D'Olhaberriague L, Verny M, Chaudhuri KR et al. 1996. Accuracy of clinical criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome). Neurology 46: 922–930. [DOI] [PubMed] [Google Scholar]
- Lopez G, Bayulkem K, Hallett M 2016. Progressive supranuclear palsy (PSP): Richardson syndrome and other PSP variants. Acta Neurologica Scandinavica 134: 242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubarsky M, Juncos J 2008. Progressive supranuclear palsy: A current review. Neurologist 14: 79–88. [DOI] [PubMed] [Google Scholar]
- Morton SM, Dordevic GS, Bastian AJ 2004. Cerebellar damage produces context-dependent deficits in control of leg dynamics during obstacle avoidance. Experimental Brain Research 156: 149–163. [DOI] [PubMed] [Google Scholar]
- Nath U, Ben-Shlomo Y, Thomson RG, Lees AJ, Burn DJ 2003. Clinical features and natural history of progressive supranuclear palsy: A clinical cohort study. Neurology 60: 910–916. [DOI] [PubMed] [Google Scholar]
- Nicolai S, Mirelman A, Herman T, Zijlstra A, Mancini M, Becker C, Lindemann U, Berg D, Maetzler W 2010. Improvement of balance after audio-biofeedback. A 6 week intervention study in patients with progressive supranuclear palsy. Zeitschrift fur Gerontologie und Geriatrie 43: 224–228. [DOI] [PubMed] [Google Scholar]
- Ondo W, Warrior D, Overby A, Calmes J, Hendersen N, Olson S, Jankovic J 2000. Computerized posturography analysis of progressive supranuclear palsy: A case-control comparison with Parkinson's disease and healthy controls. Archives of Neurology 57: 1464–1469. [DOI] [PubMed] [Google Scholar]
- Patla AE 1997. Understanding the roles of vision in the control of human locomotion. Gait and Posture 5: 54–69 [Google Scholar]
- Petzinger GM, Fisher BE, McEwen S, Beeler JA, Walsh JP, Jakowec MW 2013. Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson's disease. Lancet. Neurology 12: 716–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qutubuddin AA, Pegg PO, Cifu DX, Brown R, McNamee S, Carne W 2005. Validating the Berg Balance Scale for patients with Parkinson's disease: A key to rehabilitation evaluation. Archives of Physical Medicine and Rehabilitation 86: 789–792. [DOI] [PubMed] [Google Scholar]
- Richardson JC, Steele J, Olszewski J 1963. Supranuclear ophthalmoplegia, pseudobulbar palsy, nuchal dystonia and dementia. A clinical report on eight cases of heterogenous system degeneration. Transactions of the American Neurological Association 88: 25–29. [PubMed] [Google Scholar]
- Schrag A, Ben-Shlomo Y, Quinn NP 1999. Prevalence of progressive supranuclear palsy and multiple system atrophy: A cross-sectional study. Lancet 354 (9192): 1771–1775. [DOI] [PubMed] [Google Scholar]
- Slade SC, Finkelstein DI, McGinley JL, Morris ME 2020. Exercise and physical activity for people with Progressive Supranuclear Palsy: A systematic review. Clinical Rehabilitation 34: 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosner J, Wall GC, Sznajder J 1993. Progressive supranuclear palsy: Clinical presentation and rehabilitation of two patients. Archives of Physical Medicine Rehabilitation 74: 537–539. [DOI] [PubMed] [Google Scholar]
- Suteerawattananon M, MacNeill B, Protas EJ 2002. Supported treadmill training for gait and balance in a patient with progressive supranuclear palsy. Physical Therapy 82: 485–495. [PubMed] [Google Scholar]
- van der Kolk NM, King LA 2013. Effects of exercise on mobility in people with Parkinson's disease. Movement Disorders 28: 1587–1596. [DOI] [PubMed] [Google Scholar]
- Yang Y, Wang Y, Zhou Y, Chen C, Xing D, Wang C 2014. Validity of the functional gait assessment in patients with Parkinson disease: Construct, concurrent, and predictive validity. Physical Therapy 94: 392–400. [DOI] [PubMed] [Google Scholar]
- Zampieri C, Di Fabio RP 2008. Balance and eye movement training to improve gait in people with progressive supranuclear palsy: Quasi-randomized clinical trial. Physical Therapy 88: 1460–1473. [DOI] [PubMed] [Google Scholar]
- Zampieri C, Di Fabio RP 2009. Improvement of gaze control after balance and eye movement training in patients with progressive supranuclear palsy: A quasi-randomized controlled trial. Archives of Physical Medicine and Rehabilitation 90: 263–270. [DOI] [PubMed] [Google Scholar]