Abstract
In allogeneic stem cell transplant (Allo-SCT) recipients, the cell-mediated and humoral immunogenicity of the 3-dose SARS-CoV-2 vaccination schedule has not been investigated in prospective studies. In a prospective cohort, we recruited 122 Allo-SCT recipients since August 2021, when Ontario began offering a 3-dose vaccine schedule for Allo-SCT recipients. We determined humoral and cell-mediated immunity and adverse effects of the 3-dose SARS-COV-2 vaccination schedule in Allo-SCT recipients. In immunogenicity analysis (n = 95), the median (interquartile range [IQR]) antibody titer against the receptor-binding domain (RBD) of the spike (S) protein after the third dose (10,358.0 U/mL [IQR = 673.9-31,753.0]) was significantly higher than that after the first (10.2 U/mL [IQR = 0.6-37.0]) and the second doses (125.6 U/mL [IQR = 2.8-1251.0]) (P < .0001). The haploidentical donor status was an independent risk factor (adjusted odds ratio = 7.67, 95% confidence interval [CI], 1.86-31.60) for suboptimal antibody response (anti-RBD < 100 U/mL). S-specific CD4+ and CD8+ T-cell responses were measured in a subset of Allo-SCT recipients (n = 20) by flow cytometry. Most developed antigen-specific CD4+ (55%-80%) and CD8+ T-cells (80%) after 2 doses of vaccine. Frequencies of CD4+ polyfunctional (P = .020) and IL-2 monofunctional (P = .013) T-cells significantly increased after the third dose. Twenty-three episodes (23/301 doses [7.6%]) of new-onset or worsening pre-existing graft-versus-host disease (GVHD) occurred, including 4 episodes after the third dose. We observed 4 relapses (3.27%). Seven patients developed SARS-CoV-2 infection despite vaccination, although none required hospitalization. In conclusion, the 3-dose SARS-CoV-2 vaccine schedule provided immunity associated with a low risk of GVHD and other adverse effects. This prospective cohort showed that the third dose of SARS-CoV-2 vaccine in allogeneic stem cell transplant recipients promoted better humoral and cellar immune responses than after the initial series without increasing the risk of GVHD or severe adverse effects.
Key Words: SARS-CoV-2, Hematopoietic stem cell transplantation, Vaccines
Coronavirus disease 2019 (COVID-19) is associated with up to 30% mortality in allogeneic stem cell transplant (Allo-SCT) recipients,1 , 2 and prevention of COVID-19 is urgently required in this population. Vaccination against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has been shown to be a successful preventative strategy in immunocompetent individuals.3, 4, 5 More than 80% of Allo-SCT recipients who received two doses of mRNA vaccines mounted anti- receptor-binding domain (RBD) antibody response; still, titers tend to be lower than in healthy individuals.6 , 7 Because of waning immunity, a 3-dose schedule has been recommended.8 , 9 Some retrospective studies in Allo-SCT recipients suggested a favorable antibody response after the 3-dose mRNA vaccine.10 , 11 However, cell-mediated and humoral immunogenicity has not been investigated in prospective studies. T-cell response is an essential determinant of the clinical outcome of COVID-19 and underpins vaccine efficacy.12 , 13 Retrospective studies did not investigate the role of essential variables such as donor type (i.e., matched related, matched unrelated, haploidentical, mismatched unrelated donors), graft-versus-host disease (GVHD), and immunosuppressive regimens after a 3-dose schedule.
The safety of the 3-dose schedule and the potential for off-target responses, including exacerbation of pre-existing GVHD and development of new-onset GVHD, require further investigation. Some studies suggested increasing organ stage severity and worsening of GVHD after SARS-CoV-2 vaccination.14 We aimed to determine the immunogenicity and outcomes of the 3-dose SARS-CoV-2 vaccination schedule in Allo-SCT recipients. Simultaneously, we explored the risk of new-onset GVHD or exacerbation of pre-existing GVHD.
Methods
Study design and outcomes
This prospective cohort enrolled adult patients (age ≥18 years) who received a SARS-CoV-2 vaccine series 3 months after undergoing allogeneic stem cell transplantation at the Hans Messner Allogeneic Transplant Program, Toronto, Canada. The vaccine was provided through the provincial vaccination campaign. Those who received at least 1 dose of the vaccine until December 15, 2021, were included and followed up until February 14, 2022. We excluded patients with previous COVID-19 diagnoses and individuals with febrile illnesses within a week before vaccination. For the antibody analysis, we excluded patients who received the SARS-CoV-2 vaccine before transplantation, patients who did not return for any anti-RBD antibody titer by February 14, 2022, patients who received at least 1 dose of rituximab within 6 months before the first dose of vaccine, and patients who received intravenous immunoglobulin within 21 days before antibody testing. For cellular immune response measurements, only patients who received vaccine doses before transplantation were excluded.
We obtained blood samples at the time of cohort entry and 4 to 6 weeks after each vaccine dose for testing. A subset of patients also provided consent for additional blood for T-cell immunity studies. The study was approved by the institutional research ethics board, and all patients provided written informed consent. The primary outcome was the proportion of patients with a detectable level of the anti-SARS-CoV-2 spike (S) RBD antibodies. Secondary outcomes included COVID-19 diagnosis, adverse effects after SARS-CoV-2 vaccination, and death. For all outcomes except for death, the closing date of observation was February 14, 2022. The closing date for the outcome of death was March 14, 2022.
Laboratory Methods
Anti-RBD binding antibodies were measured using the Roche Elecsys anti–SARS-CoV-2 S enzyme immunoassay as per the manufacturer's instructions in a certified biochemistry laboratory.15 The assay has a lower limit of quantitation of 0.4 U/mL and a positive response defined as >0.8 U/mL.
We also ascertained the cellular-mediated immune response to the vaccine in a subset of patients after the second and third dose (n = 20). Cryopreserved peripheral blood mononuclear cells were thawed, rested, and incubated overnight with overlapping peptide pools corresponding to the ancestral SARS-CoV-2 S protein. Antigen-specific CD4+ and CD8+ T cells were measured using intracellular cytokine staining flow cytometry, as previously described.16, 17, 18 S-specific CD4+ T cells were defined as monofunctional cells producing only interferon-gamma (IFN-γ) or interleukin-2, or polyfunctional T-cells producing a combination of both simultaneously. In contrast, CD8+ T cells were investigated in terms of total IFN-γ expression. A representative gating strategy for identifying S-specific CD4+ and CD8+ T-cells is provided in Supplementary Figure S1.
Adverse effects and safety
We categorized the adverse events based on the Food and Drug Administration toxicity grading scale for volunteers in vaccine trials as follows: grade 1 (no interference in daily activities), grade 2 (some interference in daily activities), grade 3 (participants unable to perform daily activities), and grade 4 (potentially life-threatening).19 All patients remained under follow-up after vaccination during the observation period. Additionally, study team members contacted all participants every 1 to 2 weeks and reviewed patients' charts for episodes of new-onset or worsening of pre-existing GVHD, COVID-19 diagnosis, hospitalization, or other adverse events until February 14, 2022. Acute GVHD was graded according to the Keystone criteria, and chronic GVHD (cGVHD) was defined as the National Institutes of Health Chronic Graft-versus-Host Disease Consensus criteria.20 , 21 GVHD outcome (i.e., new-onset or worsening of GVHD) was required to have occurred within 40 days of receiving the latest vaccine dose to be included in study results.22 The laboratory diagnosis of SARS-CoV-2 infection was made using reverse-transcription polymerase chain reaction or rapid antigen test from the upper respiratory tract specimens.
Statistical analysis
We used the Chi-square test, Fisher's exact test, and the Mann-Whitney U test in univariate analysis. We also used backward stepwise multivariate logistic regression models to identify the independent risk factors for study outcomes. A 2-sided Wilcoxon matched-pairs signed-rank test was used for the T-cell analysis, with adjusted P values calculated using the Holm-Šídák correction for multiple comparisons. Furthermore, we determined the variables associated with the suboptimal vaccine immunogenicity status after the third dose of vaccine, which was defined as an anti-RBD antibody titer less than 100 U/mL.23 We considered P < .05 as the level of statistical significance.
Results
Overview of the cohort
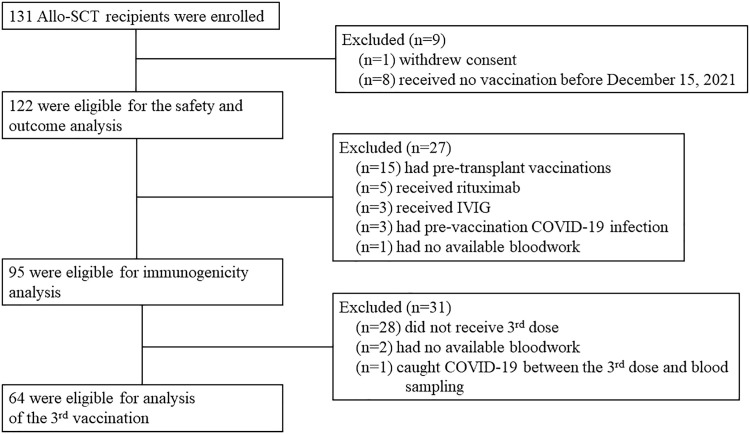
A total of 131 Allo-SCT recipients were enrolled, and eventually, 122 were included in the analysis (Figure 1 ). Of these, 74 (60.7%) received 3 doses of SARS-CoV-2 vaccines. Baseline characteristics of the cohort are provided in Table 1 . The median age was 57 (interquartile range [IQR] = 45-64) years. Sixty-six patients (54.1%) were in the first year after transplantation, and 58 (47.5%) were on immunosuppressive therapy at enrollment. Matched unrelated donor (MUD) was the most common stem cell donor type (44.3%) followed by the matched related donor (MRD [27.9%]) and haploidentical donor (HD [24.6%]). At the time of enrolment, 3 patients had a relapse of underlying hematological diseases. The median intervals between the date of transplantation and the date of the first dose, second dose, and third dose were 185 days (IQR = 115-524), 249 days (IQR = 160-681), and 459 days (IQR = 294-883), respectively.
Figure 1.
Study flow diagram.
Table 1.
Baseline Characteristics of the Patients Who Met the Eligibility Criteria After Exclusion
| Variable | N = 122 (%) |
|---|---|
| Age, median (IQR) | 57 (45-64) |
| Male gender | 60 (49.2) |
| Diagnosis | |
| Acute myeloid leukemia | 56 (45.9) |
| Myelodysplastic syndrome | 14 (11.5) |
| Acute lymphoblastic leukemia | 11 (9.0) |
| Myelofibrosis | 8 (6.6) |
| Chronic myelomonocytic leukemia | 7 (5.7) |
| Non-Hodgkin lymphoma | 5 (4.1) |
| Mixed phenotype acute leukemia | 4 (3.3) |
| Chronic myeloid leukemia | 4 (3.3) |
| Hodgkin lymphoma | 3 (2.5) |
| Aplastic anemia | 3 (2.5) |
| Chronic lymphocytic leukemia | 2 (1.6) |
| Others | 5 (4.1) |
| Comorbidities | |
| Diabetes mellitus | 18 (14.8) |
| Hypertension | 51 (41.8) |
| Liver diseases | 10 (8.2) |
| Chronic kidney disease | 13 (10.7) |
| Donor type | |
| Matched related | 34 (27.9) |
| Matched unrelated | 54 (44.3) |
| Haploidentical | 30 (24.6) |
| Mismatched unrelated donor | 4 (3.3) |
| Conditioning | |
| Myeloablative | 57 (46.7) |
| Reduced-intensity | 65 (53.3) |
| Previous history of allo-SCT | 5 (4.1) |
| Interval between transplantation and enrollment | |
| <6 months | 37 (30.3) |
| 6 months to 12 months | 29 (23.8) |
| >12 to 24 months | 25 (20.5) |
| >24 months | 31 (25.4) |
| Numbers of pre-transplantation vaccination | 15 (12.3) |
| One dose | 2 (1.6) |
| Two doses | 13 (10.7) |
| Numbers of post-transplantation vaccination | |
| One dose | 17 (13.9) |
| BNT162b2 (Pfizer-BioNTech) | 15 (12.3) |
| mRNA-1273 (Moderna) | 2 (1.6) |
| Two doses | 31 (25.4) |
| BNT162b2 (Pfizer-BioNTech) | 30 (24.6) |
| mRNA-1273 (Moderna) | 0 |
| Mixed | 1 (0.8) |
| Three doses | 74 (60.7) |
| BNT162b2 (Pfizer-BioNTech) | 60 (49.2) |
| mRNA-1273 (Moderna) | 8 (6.6) |
| Mixed | 6 (4.9) |
| Transplant complications before post-transplantation vaccine | |
| Relapse | 3 (2.5) |
| Acute GVHD | 8 (6.6) |
| Chronic GVHD | 34 (27.9) |
| Immunosuppressive agents at the time of enrollment | 58 (47.5) |
| Corticosteroid | 28 (23.0) |
| Non-steroid immunosuppressive agents | 51 (41.8) |
| Cyclosporine | 30 (24.6) |
| Mycophenolate | 9 (7.4) |
| Azathioprine | 4 (3.3) |
| Ruxolitinib/Itacitinib | 6 (4.9) |
| Methotrexate | 1 (0.8) |
| History of Rituximab | 13 (10.7) |
| Within 6 months of 1st vaccine post-transplant | 6 (4.9) |
“Other” hematological disorders in were myeloid sarcoma, thalassemia, blastic plasmacytoid dendritic cell neoplasm, paroxysmal nocturnal hemoglobinuria, and autoimmune inflammatory disorder with undetermined cause. Eight patients had active acute GVHD and were still on treatment (7 took oral steroid and 1 took topical steroid) at the date of the first vaccine dose.
Antibody response to the SARS-CoV-2 vaccines
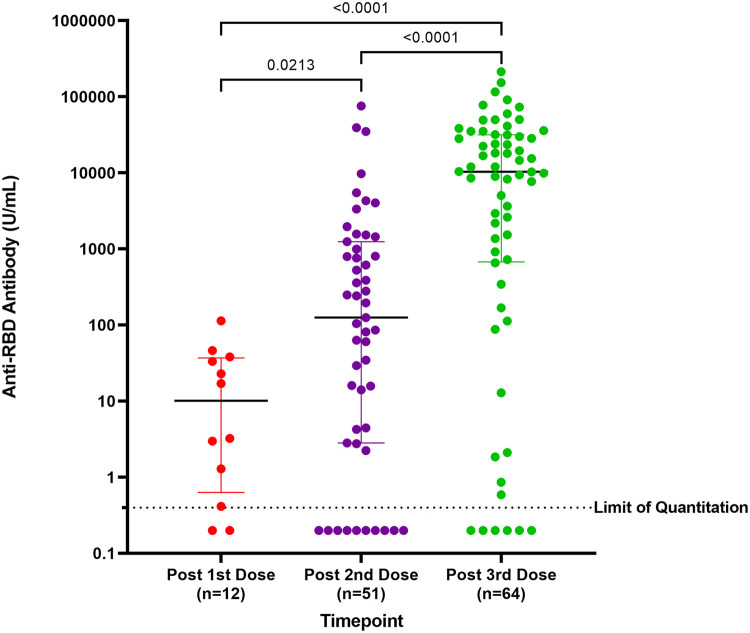
Of the 122 participants, 95 were included in the immunogenicity analysis (Figure 1). The median anti-RBD titer after the first dose, second dose, and third dose was 10.2 U/mL (IQR = 0.6-37.0), 125.6 U/mL (IQR = 2.8-1251.0), and 10,358.0 U/mL (IQR = 673.9-31,753.0), respectively. The median antibody titer after the third dose was significantly higher than that after the first dose (P < .0001) and the second dose (P < .0001; Figure 2 ).
Figure 2.
Anti-RBD titers after each vaccine dose. Each dot represents an individual patient. Horizontal line represents median values and IQR. The Mann-Whitney U test was used for the analysis.
In the immunogenicity analysis, 64 patients received a third dose SARS-CoV-2 mRNA vaccine (Table 2 ). A positive response was observed in 57 (89.1%) patients. Vaccine immunogenicity was suboptimal (anti-RBD antibody titer <100 U/mL) in 12 (18.8%) patients. Chronic kidney disease (41.7% versus 13.5%; P = .04), haploidentical donor status (50% versus 11.5%; P = .01), and median lymphocyte count at the third dose (900.0 cells/μL [IQR = 675.0-1250.0] versus 1450.0 cells/μL [IQR = 1075.0-2250.0], P = .03) were significantly associated with a suboptimal antibody response after the third dose of vaccine. Suboptimal response was not observed in MRD Allo-SCT recipients. In addition, patients who were on immunosuppressive agents had significantly lower antibody titers (median 2400.0 U/mL, IQR = 127.0-9672.0) compared to patients who were not on immunosuppressive therapy (median 22,974.0 U/mL, IQR = 3573.0-39,903.0 [P < .01]). In a multivariate analysis including donor types, chronic kidney disease, and lymphocyte count, only haploidentical donor source was identified as an independent risk factor for a suboptimal antibody response (adjusted odds ratio [aOR] = 7.67; 95% confidence interval [CI], 1.86-31.60; P < .01). Haploidentical donor status was identified as a significant risk factor of suboptimal antibody response (aOR = 9.18, 95% CI, 1.74-48.4; P = .01) even after adjusting for post-transplant cyclophosphamide (PTCY) treatment (aOR = 0.63, 95% CI, 0.11-3.62; P = .60) and immunosuppressive therapy at enrollment (aOR =1.30, 95% CI, 0.32-5.27: P = .72).
Table 2.
Comparison of Allo-SCT Recipients Anti-RBD Antibody Titers After Three Doses of SARS-CoV-2 Vaccine*
| Responder (n = 52) | Suboptimal Responder (n = 12) | P Value† | |
|---|---|---|---|
| Age median (IQR) | 58 (49-64) | 59 (47-66) | .97 |
| Sex male | 29 (55.8) | 5 (41.7) | .52 |
| Underlying hematological diseases | |||
| AML | 26 (50.0) | 9 (75) | .26 |
| MDS | 6 (11.5) | 0 | |
| CML | 1 (1.9) | 1 (8.3) | |
| ALL | 3 (5.8) | 0 | |
| HL | 0 | 1 (8.3) | |
| NHL | 1 (1.9) | 0 | |
| MPAL | 2 (3.8) | 1 (8.3) | |
| AA | 1 (1.9) | 0 | |
| CMML | 6 (11.5) | 0 | |
| MF | 4 (7.7) | 0 | |
| Others | 2 (3.8) | 0 | |
| Comorbidities | |||
| Chronic kidney disease | 7 (13.5) | 5 (41.7) | .04 |
| Liver disease | 13 (25.0) | 4 (33.3) | .72 |
| Diabetes mellitus | 6 (11.5) | 3 (25.0) | .35 |
| Type of transplant | |||
| Matched related donor | 20 (38.5) | 0 | .01 |
| Matched unrelated donor | 22 (42.3) | 6 (50.0) | .75 |
| Haploidentical donor | 6 (11.5) | 6 (50.0) | .01 |
| Mismatched unrelated donor | 4 (7.7) | 0 | 1.00 |
| Patients in the 1st year post-transplant | 22 (42.3) | 5 (41.7) | 1.00 |
| MAC | 11 (21.2) | 2 (16.7) | 1.00 |
| RIC | 11 (21.2) | 3 (25.0) | .75 |
| Total body irradiation | 10 (19.2) | 4 (33.3) | .44 |
| rATG | 34 (65.4) | 8 (66.7) | 1.00 |
| PTCY | 35 (67.3) | 9 (75.0) | .74 |
| Immunosuppressive agents at enrollment | 20 (38.5) | 6 (50.0) | .53 |
| Corticosteroid‡ | 12 (23.1) | 4 (33.3) | .48 |
| Any one or more immunosuppressive agents | 17 (32.7) | 5 (41.7) | .74 |
| Cyclosporine | 7 (13.5) | 1 (8.3) | 1.00 |
| Mycophenolate | 7 (13.5) | 1 (8.3) | 1.00 |
| Azathioprine | 2 (3.8) | 0 | 1.00 |
| Ruxolitinib / Itacitinib | 2 (3.8) | 2 (16.7) | .16 |
| Methotrexate | 0 | 1 (8.3) | .19 |
| Acute GVHD at the first dose | 2 (3.8) | 1 (8.3) | .47 |
| Chronic GVHD at the first dose | 18 (34.6) | 5 (41.7) | .74 |
| Lymphocyte count, median (IQR) | 1450 (1075-2250) | 900 (675-1250) | .03 |
| Neutrophil count, median (IQR) | 2800 (2200-4125) | 2950 (2575-3425) | .71 |
AA indicates aplastic anemia; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CML, chronic myeloid leukemia; CMML, chronic myelomonocytic leukemia; HL, Hodgkin lymphoma; MAC, myeloablative conditioning; MDS, myelodysplastic syndrome; MF, myelofibrosis; MPAL, mixed phenotype acute leukemia; NHL, non-Hodgkin lymphoma; rATG, rabbit anti-thymocyte globulin; RIC, reduced-intensity conditioning.
All patients but 1 received mRNA vaccines. 1 patient received ChAdOx1 nCoV-19/AZD1222-ChAdOx1 nCoV-19/AZD1222-Pfizer-BioNTech BNT162b2.
Univariable analysis.
Median (IQR) prednisone dose in patients with suboptimal immune response [8.75 mg (6.875-11.25)] was not significantly different from other participants [7.50 mg (5.00-14.3750), P = .806].
Cell-Mediated Immunity
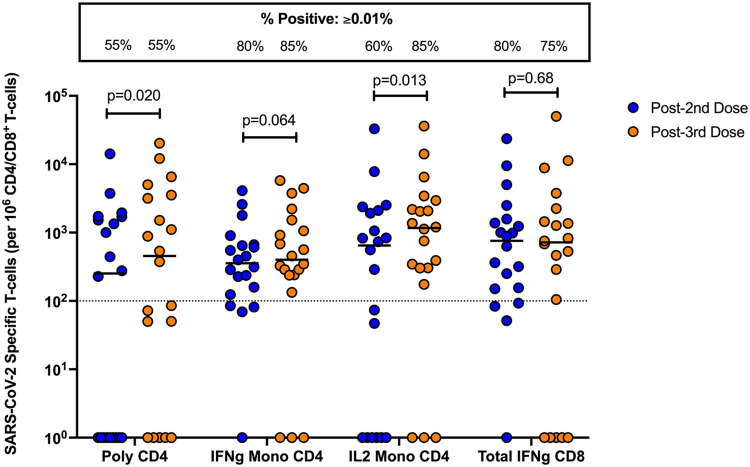
S-specific CD4+ and CD8+ T-cell responses were measured in a subset of Allo-SCT recipients (n = 20; Figure 3 ). After 2 doses of vaccine, the frequency of Allo-SCT recipients with a positive T-cell response (defined as a T-cell frequency ≥0.01%) was 55% for polyfunctional CD4+ T-cells, 80% for IFN-γ monofunctional CD4+ T-cells, 60% for IL-2 monofunctional CD4+ T-cells, and 80% for total IFN-γ expressing CD8+ T-cells. After the third dose, Allo-SCT recipients had a detectable S-specific polyfunctional CD4+ T-cell response (55%), IFN-γ monofunctional CD4+ T-cell response (85%), IL-2 monofunctional CD4+ T-cell response (85%), and CD8+ T-cell response, which was defined as total IFN-γ expression (75%). These proportions were largely unchanged, except for IL-2 monofunctional CD4+ T-cells, which expanded by 25 percentage points after the third dose. A statistically significant increase in the frequency of polyfunctional CD4+ T-cells (median frequency 251.9 versus 455 CD4+ T-cells per 106 CD4+ T-cells, P = .020) and IL-2 monofunctional CD4+ T-cells (646.4 versus 1165.6 CD4+ T-cells per 106 CD4+ T-cells, P = .013) was measured after the third dose. We also observed a trend toward an increase in the frequency of IFN-γ monofunctional CD4+ T-cells after the third dose, but this did not reach statistical significance (356.3 versus 401.1 CD4+ T-cells per 106 CD4+ T-cells, P = .064). No differences in the frequency of S-specific total IFN-γ expressing CD8+ T-cells were measured between second and third doses (758.0 versus 717.9 CD8+ T-cells per 106 CD8+ T-cells, P = .68).
Figure 3.
Cell-mediated immune response in Allo-SCT recipients after 2 or 3 doses of mRNA vaccine. Proportions of polyfunctional, IFN-γ monofunctional and IL-2 monofunctional CD4+, and total IFN-γ expressing CD8+ T-cells in Allo-SCT recipients (n = 20 paired). Each dot represents a patient. Horizontal lines denote the median for each group. A 2-sided Wilcoxon matched-pairs signed-rank test with Holm-Šídák correction for multiple comparisons was used. Adjusted P values are shown above each respective comparison. The box at the top shows the proportion of patients in each group with a positive T-cell response, defined as a frequency equal to or exceeding 0.01%, as indicated by the dashed horizontal line. Poly indicates polyfunctional; IFNg, interferon gamma; IL2, interleukin 2.
Safety analysis
In total, 122 patients received 301 vaccine doses through the public health program (296 doses of mRNA vaccines [265 BNT162b2, Pfizer-BioNTech, and 31 mRNA-1273, Moderna], and 5 ChAdOx1 nCoV-19/AZD1222 vaccines) (Figure 1, Table 1). Of the 5 doses of ChAdOx1 nCoV-19/AZD1222 vaccines, 2 were excluded from the immunogenicity analysis due to receiving intravenous immunoglobulin after vaccination. The other 3 doses of ChAdOx1 nCoV-19/AZD1222 were part of a 3-dose vaccination schedule in 2 patients who developed optimal antibody response.
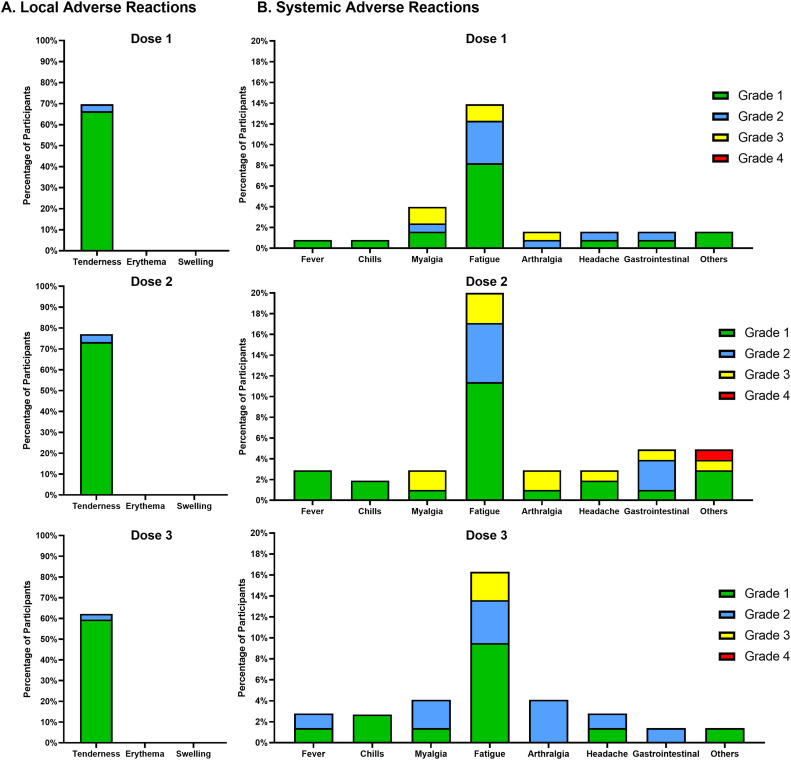
The most common adverse effects were local tenderness (>60% after each dose) and fatigue (≥14% after each dose). There were no grade 4 adverse effects except for a patient who developed Guillain-Barré syndrome four months after the second dose (Figure 4 and Table 3 ). This patient also received other vaccines one month before the diagnosis of Guillain-Barré syndrome and did not receive the 3rd dose of the SARS-CoV-2 vaccine.
Figure 4.
Local and systemic adverse effects of vaccines. *Data are unavailable for 2 patients, because of death. Gastrointestinal symptoms include nausea, vomiting and diarrhea. Others include shortness of breath, rash, dizziness, night sweats, and Guillain-Barré syndrome.
Table 3.
Adverse Effects of Vaccines
| First Dose (N = 122) | Second Dose (N = 105) | Third dose (N = 74) | |
|---|---|---|---|
| Tenderness | 85 (70.0%) | 81 (77.0%) | 46 (62.0%) |
| ≥Grade 2 | 4 | 4 | 2 |
| ≥Grade 3 | 0 | 0 | 0 |
| Erythema | 0 | 0 | 0 |
| ≥Grade 2 | 0 | 0 | 0 |
| ≥Grade 3 | 0 | 0 | 0 |
| Swelling | 0 | 0 | 0 |
| ≥Grade 2 | 0 | 0 | 0 |
| ≥Grade 3 | 0 | 0 | 0 |
| Fever | 1 (0.8%) | 3 (2.9%) | 2 (2.7%) |
| ≥Grade2 | 0 | 0 | 1 |
| ≥Grade3 | 0 | 0 | 0 |
| Chills | 1 (0.8%) | 2 (1.9%) | 2 (2.7%) |
| ≥Grade 2 | 0 | 0 | 0 |
| ≥Grade 3 | 0 | 0 | 0 |
| Myalgia | 5 (4.1%) | 3 (2.9%) | 3 (4.1%) |
| ≥Grade 2 | 3 | 2 | 2 |
| ≥Grade 3 | 2 | 2 | 0 |
| Fatigue | 17 (14.0%) | 21 (20.0%) | 12 (14.0%) |
| ≥Grade 2 | 7 | 9 | 5 |
| ≥Grade 3 | 5 | 3 | 2 |
| Arthralgia | 2 (1.6%) | 3 (2.9%) | 3 (4.1%) |
| ≥Grade 2 | 2 | 2 | 3 |
| ≥Grade 3 | 1 | 2 | 0 |
| Headache | 2 (1.6%) | 3 (2.9%) | 2 (2.7%) |
| ≥Grade 2 | 1 | 1 | 1 |
| ≥Grade 3 | 0 | 1 | 0 |
| Gastrointestinal symptoms | 2 (1.6%) | 5 (4.8%) | 1 (1.4%) |
| ≥Grade 2 | 1 | 4 | 1 |
| ≥Grade 3 | 0 | 1 | 0 |
| Others | 2 (1.6%) | 5 (4.8%) | 1 (1.4%) |
| ≥Grade 2 | 0 | 3 | 0 |
| ≥Grade 3 | 0 | 2 | 0 |
Gastrointestinal symptoms include nausea, vomiting and diarrhea. Others include shortness of breath, rash, dizziness, night sweats, and Guillain-Barré syndrome. Data are unavailable for 2 patients, due to death.
During the study period, 23 episodes (23/301 doses, 7.6%) of new-onset or worsening GVHD occurred in 22 patients (i.e., 11 MRD, 8 MUD, 2 HD, and 1 mismatched unrelated donor). Only 4 of these episodes occurred after the third dose (i.e., 1 new-onset GVHD and 3 worsening of pre-existing GVHD). We observed 4 patients who developed relapse of underlying hematological disorders (acute myeloid leukemia [n = 2], non-Hodgkin's lymphoma [n = 1], and myeloid sarcoma [n = 1]), (Tables 4 and 5 ).
Table 4.
Study Outcomes
| First Dose(N = 122) | Second Dose(N = 105) | Third Dose(N = 74) | Total(N = 301) | |
|---|---|---|---|---|
| GVHD | 11 (9.0%) | 8 (7.6)% | 4 (5.4%) | 23 (7.6%) |
| New onset of acute GVHD | 1 (0.8%) | 1 (1.0%) | 0 | 2 (0.7%) |
| New onset of chronic GVHD | 5 (4.1%) | 4 (3.8%) | 1 (1.4%) | 10 (3.3%) |
| Worsening of pre-existing acute GVHD | 1 (0.8%) | 1 (1.0%) | 0 | 2 (0.7%) |
| Worsening of pre-existing chronic GVHD | 4 (3.3%) | 2 (1.9%) | 3 (4.1%) | 9 (3.0%) |
| Relapse | 2 (1.6%) | 1 (1.05) | 1 (1.4%) | 4 (1.3%) |
| COVID-19 | 1 (0.8%) | 0 | 6 (8.15) | 7 (2.3%) |
| Breakthrough | 1 (0.8%) | 0 | 6 (8.1%) | 7 (2.3%) |
| 14-day all-cause hospitalization | 0 | 0 | 0 | 0 |
| 14-day all-cause ICU admission/mechanical ventilation | 0 | 0 | 0 | 0 |
| 30-day all-cause death | 0 | 0 | 0 | 0 |
| Death, all-cause in the cohort | 1 (0.8%) | 2 (1.9%) | 0 | 3 (1.0%) |
ICU indicates intensive care unit.
Table 5.
Patients With New-Onset GVHD or Worsening of Pre-Existing GVHD After Vaccination
| No. | Age | Gender | Underlying HD | Donor Type | Days from Transplantation | Active GVHD Before Vaccination | Time From Vaccination | Worsening or New-onset of GVHD | GVHD type | Intervention |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 44 | F | MDS | MUD | 172 | No | 9 days after the 2rd dose | New | aGVHD | Prednisone 1 mg/kg/d was started. |
| 2 | 64 | F | AML | MUD | 122 | cGVHD (eye, mouth, liver, and skin) | 17 days after the 1st dose | Worse | cGVHD | Prednisone was increased from 0.25 to 0.5 mg/kg/d. |
| 3 | 71 | M | AML | MUD | 133 | No | 35 days after the 1st dose | New | cGVHD | Prednisone 0.5 mg/kg/d was started. |
| 4 | 23 | M | AML | MRD | 153 | No | 2 days after the 2nd dose | New | cGVHD | Prednisone 0.5 mg/kg/d was started. |
| 5 | 23 | M | AML | MRD | 272 | cGVHD (liver) | 36 days after the 3rd dose | Worse | cGVHD | Prednisone was increased from 0.025 to 0.10 mg/kg/d. |
| 6 | 60 | M | MF | MRD | 111 | cGHVD (liver) | 10 days after the 1st dose | Worse | cGVHD | Prednisone 0.5 mg/kg/day and azathioprine 50 mg/d were started. |
| 7 | 58 | F | CMML | MUD | 112 | No | 7 days after the 1st dose | New | aGVHD | Prednisone 1 mg/kg/d was started. |
| 8 | 52 | F | AML | MRD | 164 | No | 33 days after the 2nd dose | New | cGVHD | Prednisone 0.15 mg/kg/d was started. |
| 9 | 50 | F | MF | HD | 203 | No | 24 days after the 2nd dose | New | cGVHD | Cyclosporine was increased from 100 to 150 mg/d. |
| 10 | 37 | F | MDS | MRD | 125 | No | 27 days after the 1st dose | New | cGVHD | Prednisone 0.5 mg/kg/d was started. |
| 11 | 28 | F | AA | MUD | 198 | aGVHD (skin) | 5 days after the 2nd dose | Worse | aGVHD | Prednisone 1 mg/kg/d was started. |
| 12 | 56 | F | AML | MUD | 277 | cGVHD (liver) | 13 days after the 2nd dose | Worse | cGVHD | Prednisone was increased from 0.08 to 0.16 mg/kg/d. |
| 13 | 56 | F | AML | MRD | 205 | cGVHD (lung, liver, and skin) | 22 days after the 1st dose | Worse | cGVHD | Prednisone was increased to 1 mg/kg/d. |
| 14 | 65 | F | MF | MMUD | 237 | cGVHD (eye, lung, and liver) | 5 days after the 3rd dose | Worse | cGVHD | Prednisone 0.5 mg/kg/d was started. |
| 15 | 45 | F | PNH | HD | 241 | No | 19 days after the 1st dose | New | cGVHD | Azathioprine 50 mg/d was started. |
| 16 | 62 | M | AML | MRD | 2484 | cGVHD (eye, mouth, and lung) | 15 days after the 3rd dose | Worse | cGVHD | Prednisone was increased from 0.25 to 0.5 mg/kg/d. |
| 17 | 68 | F | AML | MRD | 2017 | cGVHD (eye, mouth, skin, and lung) | 36 days after the 1st dose | Worse | cGVHD | Prednisone 1 mg/kg/d was started. |
| 18 | 64 | M | AML | MUD | 114 | aGVHD (liver and skin) | 13 days after the 1st dose | Worse | aGVHD | Prednisone 1 mg/kg/d and cyclosporine 100 mg/day were started. |
| 19 | 58 | F | MPAL | MRD | 134 | aGVHD (gut) | 17 days after the 1st dose | New | cGVHD | Prednisone was increased from 0.125 to 0.5 mg/kg/day. |
| 20 | 63 | M | CMML | MRD | 162 | No | 15 days after the 1st dose | New | cGVHD | Prednisone 1 mg/kg/d was started. |
| 21 | 53 | F | AML | MUD | 183 | No | 12 days after the 2nd dose | New | cGVHD | Prednisone 0.5 mg/kg/d was started. |
| 22 | 56 | F | MDS | MRD | 751 | cGVHD (eye, mouth, liver, and skin) | 33 days after the 3rd dose | New | cGVHD | Prednisone 0.125 mg/kg/d was started. |
| 23 | 46 | F | AML | MRD | 280 | cGVHD (eye, mouth, and lung) | 38 days after the 2nd dose | Worse | cGVHD | Prednisone 1 mg/kg/d was started. |
aGVHD indicates acute graft-versus-host disease; HD, haploidentical donor; MMUD, mismatched unrelated donor; PNH, paroxysmal nocturnal hemoglobinuria
Patients no. 4 and no. 5 are the same patient. Six events (No. 2, No. 5, No. 9, No. 12, No. 16, and No. 19) occurred during tapering of immunosuppressive agents.
In total, 7 patients developed SARS-CoV-2 infection, which was confirmed by reverse-transcription polymerase chain reaction test (n = 6) or rapid antigen test (n = 1). Of these, 6 patients were diagnosed after the third dose, 4 patients were 60 years or older, and only 2 required treatment with sotrovimab. No one developed severe disease requiring hospitalization (Table 4).
Three patients died during the observation period because of relapse of underlying disease (myelofibrosis [n = 1]), polymicrobial pneumonia on the background of lung cGVHD (n = 1), and progressive pulmonary cGVHD after the first dose (n = 1). The last patient did not develop respiratory failure until 2 months after the second dose. This patient did not receive the third dose.
Discussion
Our study is the first prospective cohort evaluating the immunogenicity of 3-dose SARS-CoV-2 vaccine schedule in Allo-SCT recipients. We obtained 4 novel findings. First, we showed humoral anti-RBD responses generated by a majority (89.1%) of Allo-SCT recipients after 3 doses of SARS-CoV-2 vaccine. Second, we identified haploidentical donor status as an independent risk factor for a suboptimal antibody response. Third, we demonstrated the safety of SARS-CoV-2 vaccination in Allo-SCT recipients after a 3-dose schedule. Last, 3 doses of the SARS-CoV-2 vaccine in Allo-SCT recipients resulted in robust T-cell responses that may reduce the severity of COVID-19 in those with breakthrough infections.
Regarding immunogenicity, the antibody titer after the third dose was significantly higher than after the first and second doses (Figure 2). This finding could suggest that the third dose provides a booster effect that establishes robust humoral immunity against SARS-CoV-2 in Allo-SCT recipients. Previous studies have evaluated the immunogenicity of mRNA vaccines in Allo-SCT recipients who received up to 2 doses.6 , 7 , 24, 25, 26, 27, 28 Some of these studies showed that the antibody titer in Allo-SCT recipients was significantly lower than in healthy controls.6 , 7 , 28 The time-dependent waning of vaccine efficacy after an initial vaccination series has been a concern even in the general population.29 In these contexts, the third dose could be an essential solution for these concerns in Allo-SCT settings.
In the present study, haploidentical recipients were more likely to develop a suboptimal antibody response after the third dose of a SARS-CoV-2 vaccine (Table 2). This association remained statistically significant after adjusting for other variables such as PTCY. This finding might be secondary to delayed immune reconstitution in haploidentical recipients compared to other sources of transplantation.30 , 31 Unlike MUD and mismatched unrelated donor, haploidentical recipients routinely received PTCY and a higher dose of anti-thymocyte globulin (4.5 g/kg versus 2 g/kg). They receive a more prolonged immunosuppressive therapy than other sources of transplantation, which might be associated with the suboptimal antibody response. It should be noted that the cutoff of 100 U/mL is arbitrary, and individuals whose immune responses were not included in the “suboptimal” category did not necessarily have protective levels of anti-SARS-CoV-2 antibodies. Further studies with a longer duration of follow-up and assessment of neutralizing antibody levels are required to determine vaccine effectiveness in haploidentical Allo-SCT recipients.
This study provides evidence regarding the safety of a 3-dose SARS-CoV-2 vaccination schedule in an Allo-SCT setting. Nineteen patients (15.6%) experienced new-onset or worsening pre-existing GVHD after the first and second dose (Table 4). This frequency (15.6%) was slightly higher than those in the previous studies (range 10%-13.2%).22 , 26 In addition, the incidence of GVHD after the third dose (5.4%) remained lower than in the first and second doses (9.0% and 7.6%, respectively) (Table 4). This finding was reassuring because some previous studies have suggested that a higher incidence of GVHD may occur with increasing the number of SARS-CoV-2 vaccine doses.14 Notably, 10 of 34 (29.4%) patients with active cGVHD experienced worsening of cGVHD after the vaccination. This finding suggests careful consideration of vaccination in patients with existing cGVHD and supports the results of a similar study.14
SARS-CoV-2 infection was not associated with hospitalization or mortality in our cohort. This finding supports previous studies that demonstrated the safety of a 2-dose SARS-CoV-2 vaccination schedule in Allo-SCT patients.32 Four of 7 patients with COVID-19 breakthrough were 60 years or older, and only 3 of 7 required outpatient therapy. The variant of concern was Omicron (B.1.1.529) in four patients with COVID-19 breakthrough, as the dominant SARS-CoV-2 strain in Ontario during this study.33 The interval between transplantation and the third dose of SARS-CoV-2 vaccine in patients with breakthrough infection was relatively longer than in patients without a breakthrough (541 days [IQR = 418-561] versus 411 days [IQR = 280-851]; P = .395).
Overall, the incidence of adverse effects after each dose of the SARS-CoV-2 vaccine in this study (Figure 4, Table 3) was comparable to those previously reported in the general population and Allo-SCT settings.3 , 4 , 6 , 22 , 27 , 34 Thus the safety of a three-dose schedule of SARS-CoV-2 vaccination in the Allo-SCT setting appears to be similar to the general population.3 , 4 , 34
T-cell immunity is less commonly analyzed in vaccine immunogenicity studies. A strength of our study is paired T-cell data in Allo-SCT recipients after the second and third dose of SARS-CoV-2 vaccines. A study evaluating T-cells responses in 17 Allo-SCT patients found a CD4+ T-cell response in 29.4% of patients after 1 dose and 70.6% of patients after 2 doses, whereas a CD8+ T-cell response was seen in 17.6% after 1 dose and 52.3% after 2 doses.35 These results are similar to our findings. We showed a positive CD4+ T-cell response in 55% to 80% of Allo-SCT recipients (varying based on functionality) and a positive CD8+ T-cell response in 80% of patients. A larger study looking at T-cell responses in 45 Allo-SCT recipients immunized with 2 doses of BNT162b2 mRNA vaccine13 found similar response rates and preferential induction of polyfunctional CD4+ T-cell responses. Other studies looking at T-cell responses after 2 doses have found comparatively lower T-cell response rates.26 , 36 , 37 This may be explained, at least in part, by using different T-cell assays and differences in cohort demographics. To our knowledge, ours is the first prospective cohort to measure SARS-CoV-2 S-specific CD4+ and CD8+ T-cells responses in Allo-SCT after both the second and third dose of COVID-19 vaccine for a paired analysis. Our study showed that although the proportion of patients with positive T-cells does not vary much between second and third doses—except IL-2 monofunctional CD4+ T-cells—we saw an increase in antigen-specific cell-mediated immunity after the third dose, notably IL-2 monofunctional and polyfunctional CD4+ T-cells. This finding suggests that 3 doses of vaccine in Allo-SCT result in robust T-cell responses that may mitigate severe COVID-19 in those with breakthrough infections.
Our study has several limitations. First, our study objectives did not include comparing the immune response to vaccines between Allo-SCT patients and a nontransplant control group. Similarly, we were not able to compare vaccinated versus unvaccinated Allo-SCT recipients because SARS-CoV-2 vaccination was universally recommended. Hence, statistical calculation of the association between vaccination and GVHD events was impossible because of the lack of an unvaccinated control group. Some of the new-onset or worsening GVHD events in the present study might be associated with factors unrelated to vaccination (e.g., flare of GVHD while tapering immunosuppressive agents). These limitations were also present in similar studies demonstrating the safety of a 2-dose schedule.22 , 26 , 32 Second, we started study enrollment in the middle of August 2021, when guidelines recommended the 3-dose schedule. Most of the participants had already received 2 doses of SARS-CoV-2 vaccines before enrollment. Thus the number of blood samples that were obtained after the first and the second dose was smaller than that after the third dose. Third, although the median (IQR) prednisone dose in patients with suboptimal immune response was not significantly different from others, this finding should be cautiously interpreted because most patients were on minimal doses of steroids. Fourth, we did not determine donor vaccination status, which may have augmented some of the responses. Nevertheless, increasing the antibody titers after the third dose supports the hypothesis that a 3-dose schedule may overcome the waning of immunity over time.
In conclusion, the third dose of the COVID-19 vaccination appears to establish a stronger immunity against SARS-CoV-2 than after the initial series in the Allo-SCT setting. In addition, adverse events after each dose were primarily mild and tolerable, although new-onset or worsening of GVHD should be carefully monitored. Our findings will encourage Allo-SCT recipients to receive the three doses of SARS-CoV-2 vaccines.
Financial disclosure
Supported by the Public Health Agency of Canada through the COVID-19 Immunity Task Force and Vaccine Surveillance Reference Group and the University Health Network's PRESERVE-Pandemic Response Biobank for coronavirus samples, UHN Biospecimen Services, REB # 20-5364. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the University Health Network.
Authorship statement
S.M.H-M, D.K., A.M., and J.D. designed the study. M.K., D.K., S.M.H-M, and S.K. analyzed the data. The immunologic analyses and laboratory tests, including antibody-mediated and cell-mediated immunity designed and undergone by B.M-K., M.I., V.H.F., D.K., A.H., and V.K. The interpretation of hematologic data and the GVHD were supervised and reviewed in detail by J.I.M. and I.P. M.K. and S.M.H-M prepared the primary draft. All authors provided feedback and contributed to the revision of the draft. D.K., S.M.H-M., A.H. J.I.M., I.P., A.H., J.I.M., V.H.F., and A.M. interpreted the data. M.K. and V.H.F. contributed equally to this work and should be considered co-first authors.
Conflict of interest
D.K. has received research grants from Roche, GSK and advisory fees from Roche, GSK, Merck, Astellas, Sanofi, Exevir. A.H. has received clinical trials grant from Merck and advisory fees from Merck. SMHM has received Quality Improvement grant from Merck and advisory fees from AstraZeneca and GSK.
Acknowledgments
The authors thank the Canadian Donation and Transplantation Research Program (CDTRP), Catherine Burton, Hébert Marie-Josée, Andres Finzi, Sarah Shalhoub, Lori West, Karina Top, Megan Levings, Sacha DeSerres, Dima Kabbani, Daniel Kaufmann, Héloïse Cardinal, and Manish Sadarangani for their contributions to the study design.
Footnotes
Financial disclosure: See Acknowledgments on page 706.e9.
M.K. and V.H.F. contributed equally.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jtct.2022.07.024.
Appendix. Supplementary materials
References
- 1.Mushtaq MU, Shahzad M, Chaudhary SG, et al. Impact of SARS-CoV-2 in hematopoietic stem cell transplantation and chimeric antigen receptor T cell therapy recipients. Transplant Cell Ther. 2021;27 doi: 10.1016/j.jtct.2021.07.005. 796 e791-796 e797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma A, Bhatt NS, St Martin A, et al. Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: an observational cohort study. Lancet Haematol. 2021;8:e185–e193. doi: 10.1016/S2352-3026(20)30429-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canti L, Humblet-Baron S, Desombere I, et al. Predictors of neutralizing antibody response to BNT162b2 vaccination in allogeneic hematopoietic stem cell transplant recipients. J Hematol Oncol. 2021;14:174. doi: 10.1186/s13045-021-01190-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shem-Tov N, Yerushalmi R, Danylesko I, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in haematopoietic stem cell transplantation recipients. Br J Haematol. 2022;196:884–891. doi: 10.1111/bjh.17918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Center for Disease Control and Prevention . 2022. COVID-19 Vaccine Interim COVID-19 Immunization Schedule for Ages 5 Years and Older. [Google Scholar]
- 9.Public Health Agency of Canada . Canada booster dose recommendation; April 12, 2022. An Advisory Committee Statement (ACS) National Advisory Committee on Immunization (NACI) Published. [Google Scholar]
- 10.Maillard A, Redjoul R, Klemencie M, et al. Antibody response after 2 and 3 doses of SARS-CoV-2 mRNA vaccine in allogeneic hematopoietic cell transplant recipients. Blood. 2022;139:134–137. doi: 10.1182/blood.2021014232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Redjoul R, Le Bouter A, Parinet V, Fourati S, Maury S. Antibody response after third BNT162b2 dose in recipients of allogeneic HSCT. Lancet Haematol. 2021;8:e681–e683. doi: 10.1016/S2352-3026(21)00274-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moss P. The T cell immune response against SARS-CoV-2. Nat Immunol. 2022;23:186–193. doi: 10.1038/s41590-021-01122-w. [DOI] [PubMed] [Google Scholar]
- 13.Clemenceau B, Guillaume T, Coste-Burel M, et al. SARS-CoV-2 T-cell responses in allogeneic hematopoietic stem cell recipients following two doses of BNT162b2 mRNA vaccine. Vaccines (Basel) 2022:10. doi: 10.3390/vaccines10030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trunk AD, Shewan SK, Lee CJ, Parker CJ, Couriel DR. Chronic graft-versus-host disease exacerbation after SARS-CoV-2 vaccination. Bone Marrow Transplant. 2022;57:502–503. doi: 10.1038/s41409-021-01543-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel EU, Bloch EM, Clarke W, et al. Comparative performance of five commercially available serologic assays to detect antibodies to SARS-CoV-2 and identify individuals with high neutralizing titers. J Clin Microbiol. 2021:59. doi: 10.1128/JCM.02257-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall VG, Ferreira VH, Ierullo M, et al. Humoral and cellular immune response and safety of two-dose SARS-CoV-2 mRNA-1273 vaccine in solid organ transplant recipients. Am J Transplant. 2021;21:3980–3989. doi: 10.1111/ajt.16766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferreira VH, Marinelli T, Ierullo M, et al. Severe acute respiratory syndrome coronavirus 2 infection induces greater T-cell responses compared to vaccination in solid organ transplant recipients. J Infect Dis. 2021;224:1849–1860. doi: 10.1093/infdis/jiab542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall VG, Ferreira VH, Wood H, et al. Delayed-interval BNT162b2 mRNA COVID-19 vaccination enhances humoral immunity and induces robust T cell responses. Nat Immunol. 2022;23:380–385. doi: 10.1038/s41590-021-01126-6. [DOI] [PubMed] [Google Scholar]
- 19.Guidance of Industry Toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. U.S. Food and Drug Administration. 2007 [Google Scholar]
- 20.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 21.Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21:389–401. doi: 10.1016/j.bbmt.2014.12.001. e381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ali H, Ngo D, Aribi A, et al. Safety and tolerability of SARS-CoV2 emergency-use authorized vaccines for allogeneic hematopoietic stem cell transplant recipients. Transplant Cell Ther. 2021;27 doi: 10.1016/j.jtct.2021.07.008. 938 e931-938 e936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall VG, Ferreira VH, Ku T, et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med. 2021;385:1244–1246. doi: 10.1056/NEJMc2111462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bergman P, Blennow O, Hansson L, et al. Safety and efficacy of the mRNA BNT162b2 vaccine against SARS-CoV-2 in five groups of immunocompromised patients and healthy controls in a prospective open-label clinical trial. EBioMedicine. 2021;74 doi: 10.1016/j.ebiom.2021.103705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeshurun M, Pasvolsky O, Shargian L, et al. Humoral serological response to the BNT162b2 vaccine after allogeneic haematopoietic cell transplantation. Clin Microbiol Infect. 2022;28 doi: 10.1016/j.cmi.2021.10.007. 303 e301-303 e304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ram R, Hagin D, Kikozashvilli N, et al. Safety and Immunogenicity of the BNT162b2 mRNA COVID-19 Vaccine in Patients after Allogeneic HCT or CD19-based CART therapy-A Single-Center Prospective Cohort Study. Transplant Cell Ther. 2021;27:788–794. doi: 10.1016/j.jtct.2021.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matkowska-Kocjan A, Owoc-Lempach J, Chruszcz J, et al. The COVID-19 mRNA BNT163b2 vaccine was well tolerated and highly immunogenic in young adults in long follow-up after haematopoietic stem cell transplantation. Vaccines (Basel) 2021:9. doi: 10.3390/vaccines9101209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Easdale S, Shea R, Ellis L, et al. Serologic responses following a single dose of SARS-Cov-2 vaccination in allogeneic stem cell transplantation recipients. Transplant Cell Ther. 2021;27 doi: 10.1016/j.jtct.2021.07.011. 880 e881-880 e884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chemaitelly H, Tang P, Hasan MR, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med. 2021;385:e83. doi: 10.1056/NEJMoa2114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elfeky R, Lazareva A, Qasim W, Veys P. Immune reconstitution following hematopoietic stem cell transplantation using different stem cell sources. Expert Rev Clin Immunol. 2019;15:735–751. doi: 10.1080/1744666X.2019.1612746. [DOI] [PubMed] [Google Scholar]
- 31.Seggewiss R, Einsele H. Immune reconstitution after allogeneic transplantation and expanding options for immunomodulation: an update. Blood. 2010;115:3861–3868. doi: 10.1182/blood-2009-12-234096. [DOI] [PubMed] [Google Scholar]
- 32.Le Bourgeois A, Coste-Burel M, Guillaume T, et al. Safety and antibody response after 1 and 2 doses of BNT162b2 mRNA vaccine in recipients of allogeneic hematopoietic stem cell transplant. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.26344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ontario Public Health . 2021. COVID-19 Variant of Concern Omicron (B.1.1.529): Risk Assessment, December 29, 2021. [Google Scholar]
- 34.Munro APS, Janani L, Cornelius V, et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021;398:2258–2276. doi: 10.1016/S0140-6736(21)02717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harrington P, Doores KJ, Saha C, et al. Repeated vaccination against SARS-CoV-2 elicits robust polyfunctional T cell response in allogeneic stem cell transplantation recipients. Cancer Cell. 2021;39:1448–1449. doi: 10.1016/j.ccell.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindemann M, Klisanin V, Thummler L, et al. Humoral and Cellular Vaccination Responses against SARS-CoV-2 in Hematopoietic Stem Cell Transplant Recipients. Vaccines (Basel) 2021:9. doi: 10.3390/vaccines9101075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Enßle JC, Campe J, Schwenger A, et al. Severe impairment of T-cell responses to BNT162b2 immunization in patients with multiple myeloma. Blood. 2022;139:137–142. doi: 10.1182/blood.2021013429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.