Significance
Metabolic suppression helps dormant insects survive prolonged periods without food. Although metabolic suppression is widespread, we do not understand the mechanisms underlying shifts to a hypometabolic state. We show that metabolic suppression in dormant Colorado potato beetles is a consequence of shifts in flight muscle mitochondrial homeostasis. Dormant beetles activate mitophagy in their muscle, degrading most mitochondria, thereby reducing their metabolism. In anticipation of the end of dormancy, beetles regrow their flight muscle mitochondria. We show a unique pattern by which beetles regulate mitochondrial homeostasis to contribute to whole-animal shifts in energy metabolism. Other adult insects also degenerate their flight muscle during dormancy; thus, it is possible that remodeling flight muscle mitochondrial homeostasis to save energy is a widespread strategy.
Keywords: mitophagy, dormancy, insect flight muscle, mitochondria
Abstract
Many insects enter a state of dormancy (diapause) during winter in which they lower their metabolism to save energy. Metabolic suppression is a hallmark of diapause, yet we know little about the mechanisms underpinning metabolic suppression in winter or how it is reversed in the spring. Here, we show that metabolic suppression in dormant Colorado potato beetles results from the breakdown of flight muscle mitochondria via mitophagy. Diapausing Colorado potato beetles suppress their metabolism by 90%, and this lowered metabolic rate coincides with a similar reduction in flight muscle mitochondrial function and density. During early diapause, beetles increase the expression of mitophagy-related transcripts (Parkin and ATG5) in their flight muscle coincident with an increase in mitophagy-related structures in the flight muscle. Knocking down Parkin expression with RNA interference in diapausing beetles prevented some mitochondrial breakdown and partially restored the whole animal metabolic rate, suggesting that metabolic suppression in diapausing beetles is driven by mitophagy. In other animals and in models of disease, such large-scale mitochondrial degradation is irreversible. However, we show that as diapause ends, beetles reverse mitophagy and increase the expression of PGC1α and NRF1 to replenish flight muscle mitochondrial pools. This mitochondrial biogenesis is activated in anticipation of diapause termination and in the absence of external stimuli. Our study provides a mechanistic link between mitochondrial degradation in insect tissues over the winter and whole-animal metabolic suppression.
When resources are limited during the winter, animals face energetic stress (1). To save energy during these periods, many animals become dormant and suppress their metabolic rate (2). Metabolic suppression is widespread in dormant animals and is facilitated by reduced rates of adenosine triphosphate (ATP) production in the mitochondria, thereby allowing animals to consume less stored food energy (3). Many dormant vertebrates, such as hibernating ground squirrels and diapausing killifish embryos, reduce ATP production by lowering the activity of mitochondrial electron transport system enzymes (4, 5). Insects also suppress their metabolic rate during diapause, a hypometabolic dormant state that insects enter before the onset of harsh environmental conditions (6). However, we know less about the mechanisms that underpin metabolic suppression in insects than in vertebrates. Further, we do not know how insects reverse this suppression and increase their metabolic rate in the spring to power energy-costly activities such as flight and reproduction.
The Colorado potato beetle (Leptinotarsa decemlineata) is a pest of potato plants and lives in temperate North America, Europe, and Asia (7). In late summer and early autumn, short day lengths cue decreased levels of circulating developmental hormones (especially juvenile hormone), which initiate diapause in adult beetles (8, 9). During this stage of diapause initiation, adults feed voraciously, arrest their reproductive development, accumulate lipid energy stores, and eventually burrow into the soil where they overwinter in diapause (10). Diapausing Colorado potato beetles suppress their metabolic rates (11) and increase their tolerance to stresses such as low temperatures and desiccation (12). In the spring, they terminate their diapause, emerge from the soil, and immediately disperse to fly to search for food and a mate (13).
During diapause, Colorado potato beetles degrade their flight muscle and rapidly regrow it postwinter (14). It is not clear which components of muscle contribute to this degradation. In a previous study we found that beetle flight muscle increases the expression of transcripts associated with protein turnover and mitochondrial homeostasis during diapause, including mitochondrial import inner membrane translocase protein (TIM16), autophagy-related protein 10 (ATG10), and biogenesis of lysosome-related organelles complex 1 subunit 2 (15). From these changes in transcript abundance, we hypothesized that flight muscle is not just degraded but could also play a role in modulating mitochondria-dependent energy metabolism during diapause. Mitochondria make up approximately 40% of asynchronous insect flight muscle (16). Thus, flight muscle degradation is a good candidate for exploring the mechanisms underlying decreased whole-animal metabolic rate. Further, Colorado potato beetles regrow their flight muscle and recover high metabolic rates in the spring, but a mechanistic link between flight muscle regeneration and the re-establishment of high metabolic rates in diapausing insects has not been made.
Here we show that whole-animal metabolic suppression in a diapausing insect is driven by flight muscle mitochondrial breakdown (mitophagy). During diapause, Colorado potato beetles activate Parkin-mediated mitophagy in their flight muscle, which remodels mitochondrial homeostasis and results in a lower metabolic rate. Furthermore, they reverse this mitophagy just before diapause ends without any changes to external stimuli. In anticipation of their emergence from diapause, Colorado potato beetles use transcriptionally mediated mitochondrial biogenesis to regrow their flight muscle mitochondrial pool and concomitantly recover a high metabolic rate. Our results provide insight into how remodeling mitochondrial homeostasis at the tissue level can drive changes in metabolism at the whole-animal level and suggest ways to activate mitochondrial proliferation in diseased states.
Results and Discussion
Diapausing Beetles Reduce Metabolism and Break Down Flight Muscle Mitochondria.
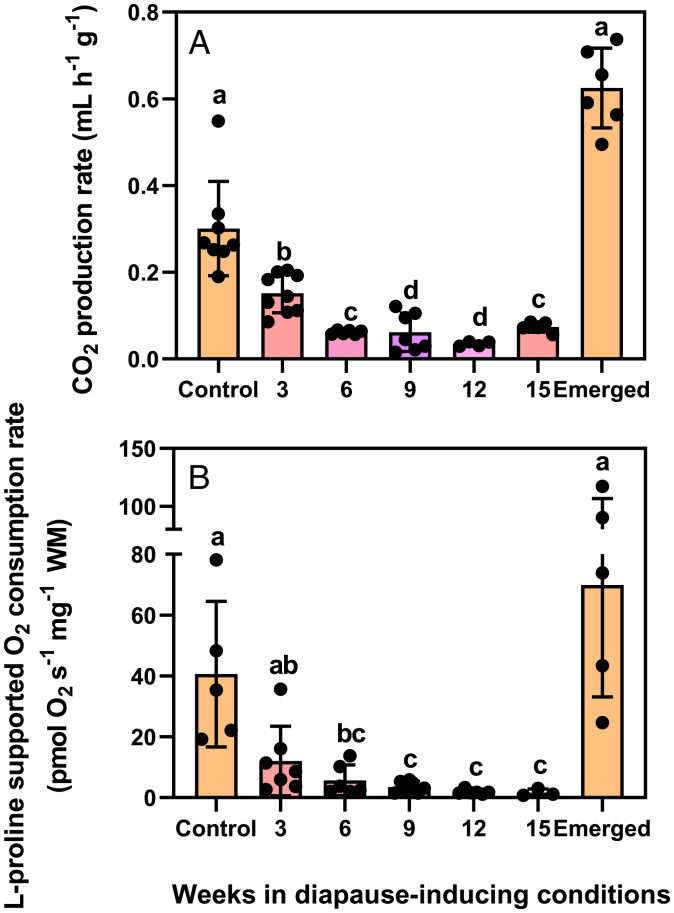
Laboratory-cultured Colorado potato beetles enter diapause when reared from egg to adult under a short daylength (24 °C 8 h: 16 h, L:D) and transferred to diapause-inducing conditions (15 °C 8 h: 16 h, L:D). After approximately 9 wk in these diapause-inducing conditions, they suppress their metabolic rates and burrow into the soil. At this point, the whole-animal metabolic rate reaches a nadir ∼90% lower than that of nondiapausing beetles (Fig. 1A). In diapause, beetles maintain this low metabolic rate for approximately 9 more wk and by 17 to 20 wk in these constant, diapause-inducing conditions, they spontaneously break diapause and rapidly increase their metabolic rates to levels higher than prediapause values (Fig. 1A).
Fig. 1.
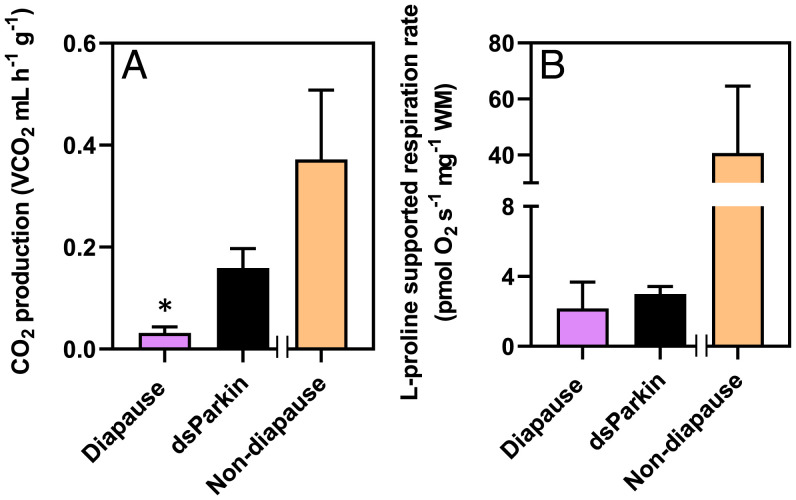
Colorado potato beetles suppress their whole-animal metabolic rates and mitochondrial respiration rates in the same pattern during diapause. (A) Mean ± SD. CO2 production rate in beetles entering diapause (3–9 wk), during diapause (9–15 wk), and upon emergence from diapause (emerged, after 17–20 wk). (B) Mean ± SD proline-supported mitochondrial O2 consumption in saponin-permeabilized flight muscle fibers (standardized to wet tissue mass [WM]) in the same treatment groups as (A). Each point represents an individual beetle (A) or beetle’s flight muscle (B). Groups were compared using a 1-way ANCOVA with beetle mass (A) and flight muscle (B) used as covariates. Different letters denote significant differences among treatment groups (P < 0.05; SI Appendix, Table S1).
We hypothesized that the reduced metabolic rate we observed in diapausing Colorado potato beetles was driven by reduced mitochondrial respiratory capacity. We focused on flight muscle because these beetles appear to histolyze this metabolically costly tissue during diapause (14), and the extent to which histolysis contributes to whole-organism metabolic suppression is not clear. Using high-resolution respirometry, we found that the mitochondrial respiration rates of saponin-permeabilized flight muscle during diapause (state 3, saturating proline and adenosine diphosphate) decline in the same temporal pattern as the whole-animal metabolic rate and to the same extent compared with nondiapausing beetles (Fig. 1B). Respiration rates did not increase after the addition of the uncoupler carbonyl cyanide m-chlorophenyl hydrozone (CCCP; maximal uncoupled rates; SI Appendix, Figs. S2 and S3), suggesting that any decreases during diapause were due to reduced electron transport system (ETS) oxidative capacities rather than active suppression of ATP synthase, a strategy used by other diapausing ectotherms (5). Such a decrease could result from either a change in mitochondrial abundance or changes in flux through the electron transport system.
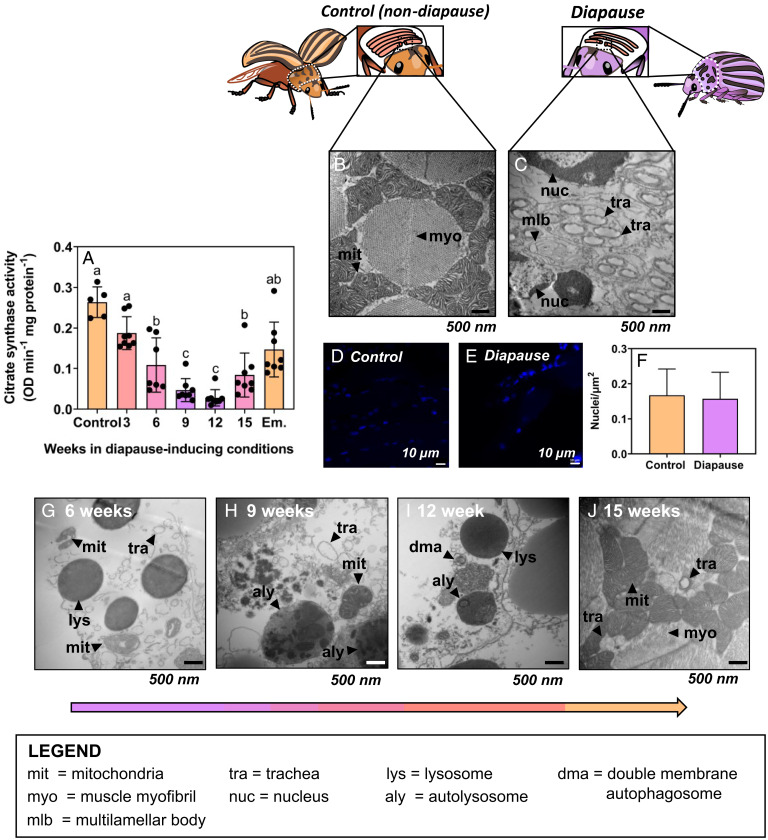
Two lines of evidence support decreased mitochondrial abundance as the cause of suppressed flight muscle metabolism in Colorado potato beetles. First, citrate synthase activity (a proxy for mitochondrial abundance [17]) decreased concurrently with, and to the same extent as, mitochondrial respiration rates (Fig. 2A). Second, transmission electron microscopy revealed that mitochondria are nearly absent from the flight muscle of diapausing beetles (Fig. 2B and C). This corroborates early work suggesting that diapause coincides with the degeneration of flight muscle sarcosomes in this species (18). However, we found that diapausing beetles degrade flight muscle mitochondria without a concomitant decrease in the number of nuclei (and presumably cells; Fig. 2D–F). Diapausing beetles also appear to retain their flight muscle trachea (Fig. 2C), and these gas exchange structures were present in both diapausing and nondiapausing flight muscle (Dataset S2, Control 1). Thus, beetles appear to selectively degrade metabolically costly mitochondria via mitophagy (mitochondrion-specific autophagy) without entirely degrading the flight muscle cells themselves.
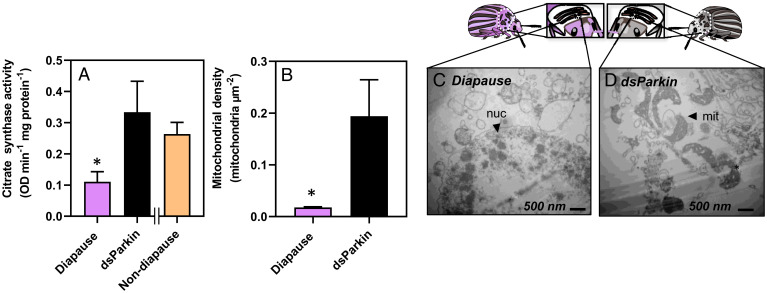
Fig. 2.
Functional mitochondria are absent from Colorado potato beetle flight muscle during diapause, but beetles reverse this mitochondrial breakdown upon diapause emergence. (A) Mean ± SD citrate synthase activity as a proxy for mitochondrial abundance in flight muscle of beetles as they enter diapause (3–9 wk), during diapause (9–15 wk), and upon emergence (Em). Groups were compared using a 1-way ANCOVA with protein content as a covariate, and different letters denote significant differences among treatments (P < 0.05; SI Appendix, Table S1). (B, C) Representative transmission electron micrographs of flight muscle cross sections from (B) nondiapausing and (C) diapausing beetles (19,000× magnification). Myofibrils (myo) and mitochondria (mit) are mostly absent from diapausing flight muscle; mlb, multilamellar body. (D–F) DAPI staining of flight muscle nuclei in nondiapausing (D) and diapausing (E) beetles indicates no differences in nuclear integrity of flight muscle cells between treatments. (G–J) Representative transmission electron micrographs of flight muscle cross sections from beetles entering diapause (G–I) and emerged beetles (J). Control beetle flight muscle has densely packed mit surrounding large muscle myo, while diapausing beetle flight muscle lack mit but still have nuclei (nuc) and trachea (tra). As beetles enter diapause and during diapause maintenance (6–15 wk; G–I), there are lysosomes (lys), double membrane autophagosomes (dma), and autolysosomes (aly) containing broken-down mit inside, indicating active autophagy in diapausing flight muscle. When beetles emerge from diapause, their flight muscle mitochondrial abundance is recovered (J) and the autophagy machinery (lys, aly, dma) observed during diapause is gone.
Many insects irreversibly histolyze their flight muscle to redirect those resources to other processes such as reproduction (19–21). However, those insects lose flight capability permanently, whereas Colorado potato beetles, like other insects that diapause as adults, fly in early spring. Therefore, we propose that they selectively degrade mitochondria during diapause rather than incur the cost of regrowing all components of their muscle. Such a strategy should be advantageous because it reduces muscle metabolic demands during winter but avoids even costlier hyperplasia at the end of diapause. We did not explore this phenomenon in other species, but it is possible that other adult diapausing insects that appear to degrade their flight muscle during dormancy could also be selectively degrading mitochondria to save energy.
Parkin-Mediated Mitophagy Is Active in Diapausing Flight Muscle.
To maintain mitochondrial and metabolic homeostasis in their cells, active animals balance the selective removal of dysfunctional mitochondria (mitophagy) with the biogenesis of new mitochondria (22). We hypothesized that a shift toward mitophagy during diapause drives the decline in mitochondrial abundance (and consequently mitochondrial metabolism) that we observed in flight muscle.
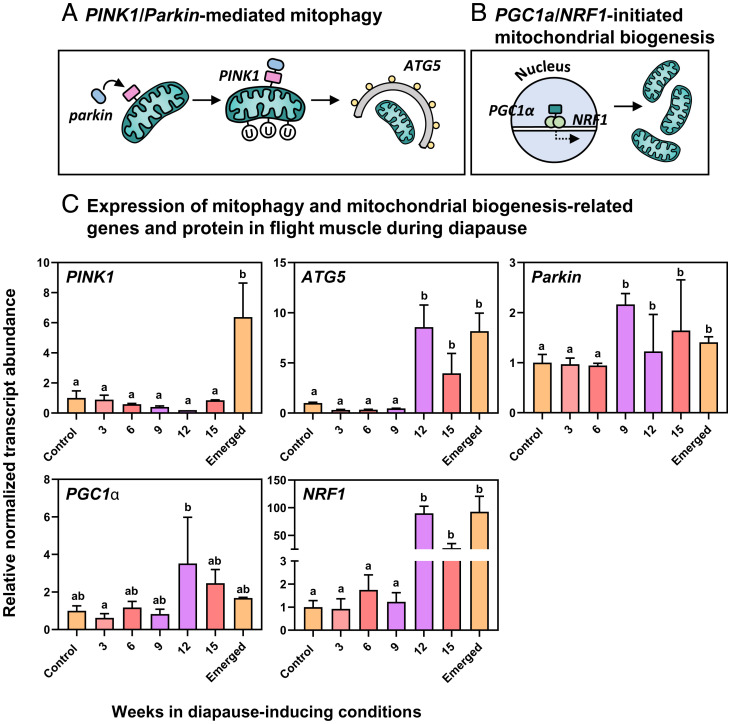
Mitophagy can be driven by the PTEN-induced putative kinase protein 1 (PINK1)/Parkin pathway (23). The decreased mitochondrial membrane potential of a dysfunctional or damaged mitochondrion induces PINK1 to stabilize to the outer mitochondrial membrane. PINK1 then recruits the E3-ubiquitin ligase Parkin, which ubiquitinates proteins on the outer membrane of the damaged mitochondrion and tags it for autophagic removal (24). When cells require more mitochondria, such as during prolonged exercise or exercise training when energy demand is high, mitochondrial biogenesis is initiated by the transcription factor peroxisome proliferator activated receptor gamma coactivator 1 alpha (PGC1α [25]). PGC1α forms a heterodimer with nuclear respiratory factors (NRF1 and 2), which initiate the transcription and translation of mitochondrial proteins and coordinate the synthesis of new mitochondria (26).
We assessed the potential for Parkin-mediated mitophagy to drive the decreased abundance of flight muscle mitochondria during diapause by measuring mitophagy markers using microscopy and qPCR. We observed a range of autophagic structures inside diapausing flight muscle including multilamellar bodies, lysosomes, autolysosomes containing degraded mitochondrial contents, and double-membrane autophagosomes (Fig. 2F–I). The development of these structures was consistent with activated Parkin-mediated mitophagy in Drosophila flight muscle (27, 28) and mammalian skeletal muscle (29, 30). Further, these autophagy-related structures were absent from the flight muscle of nondiapausing beetles, suggesting that mitophagy was uniquely activated during diapause.
We measured the mRNA abundance of PINK1, Parkin, and ATG5, all of which are crucial to the initiation and execution of mitophagy (Fig. 3A). mRNA abundance of PINK1, Parkin, and ATG5 remained stable and low in early diapause (Fig. 3C). However, after 9 wk of the 20-wk diapause period, Parkin abundance doubled and remained high throughout the diapause period (Fig. 3C). ATG5 abundance peaked after 12 wk in diapause-inducing conditions, and PINK1 abundance was highest upon emergence from diapause (Fig. 3C). We previously reported increased transcript abundance of several additional mitophagy markers in diapausing flight muscle, including TIM16 (involved in stabilizing PINK1 to the inner mitochondrial membrane [31]), the biogenesis of lysosome-related organelles complex 1 subunit 2 (involved in lysosome assembly [32]), and ATG10 (which forms a complex with ATG5 and initiates autophagosome formation [33]). Taken together, these results suggest that Parkin-mediated mitophagy is activated in flight muscle early in diapause and that mitophagy continues throughout diapause maintenance, leading to mitochondrial degradation. Because we observed consistent expression of Parkin and ATG5 throughout diapause maintenance (9–15 wk in diapause-inducing conditions) in concert with the persistence of mitophagy-related structures (lysosomes and autophagosomes), we propose that mitophagy persists through the entire diapause maintenance period.
Fig. 3.
Colorado potato beetles increase the abundance of mitophagy-related transcripts during diapause and increase the abundance of mitochondrial biogenesis-related transcripts in anticipation of emergence from diapause. (A) A schematic of PINK1/Parkin-mediated mitophagy. (B) A schematic of PGC1α/NRF1-initiated mitochondrial biogenesis. (C) Mean ± SD normalized abundance of selected mitophagy and mitochondrial biogenesis-related transcripts in beetles as they enter diapause (3–9 wk), during diapause (9–15 wk), and upon emergence from diapause. Expression values are normalized to control values and two reference genes according to the ΔΔCt method. Groups were compared with a 1-way ANOVA, and different letters denote significant differences among treatment groups (P < 0.05; SI Appendix, Table S1).
In the first 9 wk of diapause, Colorado potato beetles appear to shift mitochondrial homeostasis toward mitophagy. Transcript abundance of PGC1α and NRF1 (which drive mitochondrial biogenesis) are low and stable in the first 9 wk of diapause (Fig. 3C), suggesting that mitophagy has increased without altering mitochondrial biogenesis. This imbalance in mitochondrial homeostasis is analogous to some mammalian disease phenotypes. For example, amyotrophic lateral sclerosis is associated with enhanced mitophagy (34), and sarcopenia is associated with deficient mitochondrial biogenesis (35, 36). The excessive muscle atrophy in disease states associated with this increased mitophagy is irreversible. By contrast, the enhanced mitophagy we observed in diapausing beetles was spontaneously reversed.
Mitophagy Drives Whole-Animal Metabolic Suppression during Diapause.
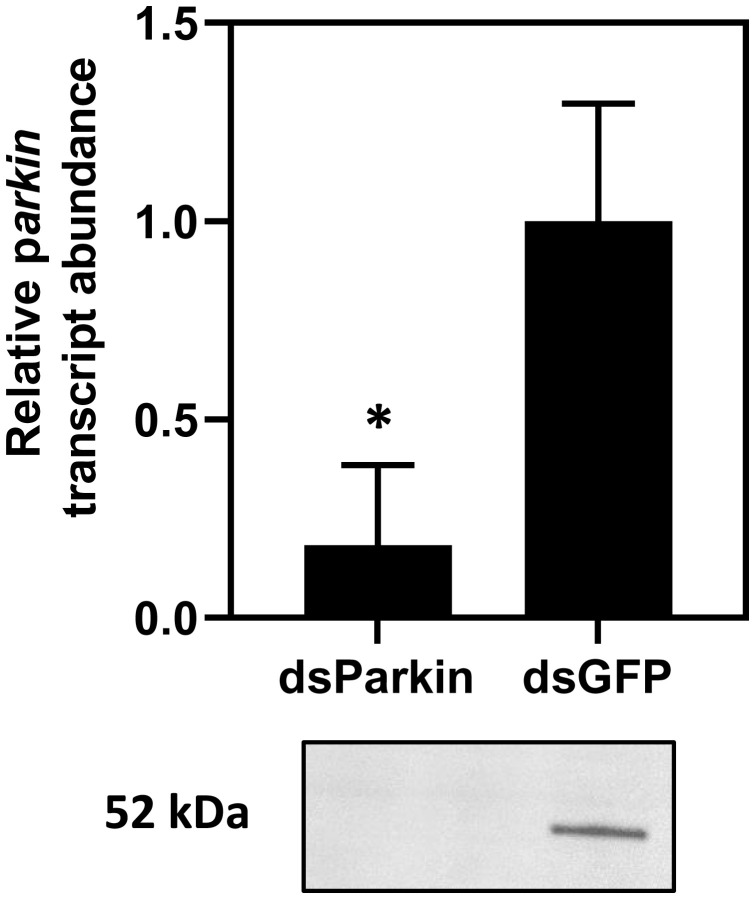
Mitochondrial ATP production accounts for the vast majority of an animal’s oxygen consumption (37), so we hypothesized that Parkin-mediated mitophagy drives the metabolic suppression we observed in diapausing beetles. We predicted that knocking down Parkin transcript abundance in beetles entering diapause would inhibit mitophagy and prevent metabolic rate suppression. We used RNA interference (RNAi) to knock down Parkin mRNA and reduce Parkin protein abundance after 7 wk in diapause-inducing conditions, 2 wk before peak Parkin expression. Five days after injecting double stranded Parkin (dsParkin) into diapausing beetles, Parkin mRNA and protein abundance were reduced 80% relative to dsGFP (green fluorescent protein) controls (Fig. 4 and SI Appendix, Figs. S5 and S6). In all subsequent experiments, we sampled beetles at this 5-d time point, where Parkin abundance was 80% reduced. We refer to knockdown beetles as dsParkin and beetles injected with dsGFP as control diapausing.
Fig. 4.
RNA interference knockdown of Parkin transcript and protein abundance in flight muscle of diapausing Colorado potato beetles. Verification of transcript and protein knockdown via qPCR and Western blot, respectively, in beetles injected with dsParkin compared to beetles injected with a negative control dsRNA construct, dsGFP. All values are represented as ± SD. Asterisk denotes significant differences between treatments according to a Welch’s t-test (P < 0.05; SI Appendix, Table S2).
The dsParkin treatment increased flight muscle citrate synthase activity threefold compared to the control diapausing beetles (Fig. 5A), which suggests an increase in mitochondrial abundance in dsParkin beetles relative to diapausing beetles. Transmission electron microscopy showed that mitochondrial density in the flight muscle of dsParkin beetles was 9.4-fold higher than in control diapausing beetles but that knocking down Parkin did not fully restore the nondiapausing phenotype as we might have expected based on citrate synthase activity (Fig. 5B–D). Thus, although an 80% reduction in Parkin abundance using RNAi prevented some mitophagy from occurring during the early stages of diapause, it did not halt mitophagy and reverse the diapause phenotype altogether. We speculate that during diapause Parkin could participate in suppressing glycolytic or tricarboxylic acid (TCA) cycle flux, independent of its role in mitophagy (38). If this were the case, then knocking down Parkin would maximize TCA cycle flux in the remaining mitochondria, leading to our observed increases in citrate synthase activity without fully restoring mitochondrial abundance.
Fig. 5.
Knocking down Parkin transcript abundance increases flight muscle citrate synthase activity and mitochondrial density. Mean ± SD citrate synthase activity (optical density, OD, per minute per mg of protein) as a proxy for mitochondrial abundance (A) and mean ± SD mitochondrial density measured with transmission electron microscopy in diapausing beetles (C) and diapausing beetles injected with dsParkin (D). Asterisks denote significant differences between diapause and dsParkin knockdown beetles according to a Welch’s t test (P < 0.05; SI Appendix, Table S2). X-axis separates the nondiapause data, which were collected in previous experiments (data from Figs. 1–3) to show the extent of the knockdown phenotype; mit, mitochondria; nuc, nuclei.
Parkin knockdown also prevented a decline in the whole-animal metabolic rate. dsParkin beetles had CO2 production rates 40% higher than diapausing controls (Fig. 6A). Nonetheless, metabolic rates of dsParkin beetles were still lower than those of nondiapausing beetles, which suggests that that knocking down Parkin partially prevented metabolic suppression but did not completely break diapause. The reversal of whole-animal metabolic suppression in dsParkin beetles did match the magnitude of mitochondrial abundance increase that we observed. However, this increased metabolic rate did not appear to be driven by changes in mitochondrial respiration, because dsParkin beetles did not restore mitochondrial oxidative capacity (Fig. 6B). We posit that either 1) the increased whole-animal CO2 production we observed resulted from increased TCA cycle activity, indicating that Parkin may regulate metabolism upstream of mitophagy, or 2) beetles have additional mechanisms that suppress respiration in the mitochondria that remain intact during diapause. We speculate that there may also be Parkin-independent pathways associated with regulating mitophagy in this species that could still activate mitophagy in dsParkin beetles. For example, pathways initiated by the Bcl2 E1B 19 kDa protein-interacting protein 3 or the fission protein drp1 (22) can compensate and drive mitochondrial degradation in Parkin-deficient Drosophila models (39). Thus, the precise role of Parkin in mitophagy-mediated metabolic suppression (and the involvement of other pathways) during diapause merits further investigation.
Fig. 6.
Knocking down Parkin transcript abundance partially recovers whole-animal metabolic rate but not mitochondrial respiration rates. Mean ± SD. CO2 production rate (A) and mean ± SD mitochondrial respiration rates (B) in diapausing beetles and diapausing beetles injected with dsParkin. Asterisk denotes significant differences between diapause and dsParkin knockdown beetles according to a Welch’s t test (P < 0.05; SI Appendix, Table S2). X-axis separates the nondiapause data, which are presented in orange bars in Figs. 1–3 to show the extent of the knockdown phenotype; WM, wet tissue mass.
Because knocking down Parkin did not break diapause or fully reverse the diapausing phenotype in beetles, we suggest that there are complex regulatory pathways upstream of Parkin that commit flight muscle cells to mitophagy upon exposure to diapause-inducing conditions. For example, fork head box-O (FOXO) transcription factors are well-established central regulators of diapause in insects (40) and energy metabolism in most animal systems (41). FOXO can activate the expression of mitophagy-related genes (42, 43) and could thus play an important role upstream of Parkin in regulating mitochondrial degradation and metabolic rate in diapausing Colorado potato beetles, even in the absence of Parkin expression in dsParkin beetles. While we did not observe any changes in FOXO expression during diapause initiation in the flight muscle in this study (SI Appendix, Fig. S4), it is well established that FOXO expression increases in the fat body of diapausing Colorado potato beetles (44), so we cannot rule out that changes in FOXO expression in one tissue can exert effects on cellular function in other tissues, such as the flight muscle.
Mitochondrial Biogenesis Drives Metabolic Recovery after Diapause.
After 17 to 20 wk in diapause-inducing conditions, laboratory-reared Colorado potato beetles spontaneously emerged from diapause and increased their metabolic rates (Fig. 1A). Under natural conditions, metabolic rates return to prediapause levels as beetles search for food, resume reproductive development, and seek a mate. We hypothesized that the reversal of metabolic suppression after diapause was driven by the recovery of flight muscle mitochondrial abundance via mitochondrial biogenesis. Beetles emerging from diapause recovered their flight muscle mitochondrial respiration rates in the same pattern as their whole-animal metabolic rates; by 17 to 20 wk in diapause-inducing conditions, proline-supported mitochondrial respiration (state 3 respiration) in permeabilized flight muscle increased to rates that surpassed prediapause values (Fig. 1B), indicating that mitochondrial respiration was no longer suppressed. Indeed, citrate synthase activity in flight muscle increased to nondiapause levels (Fig. 2A), and the flight muscle of the emerged beetles contained newly synthesized mitochondria (Fig. 2I). This concordance of increased mitochondrial respiration rates, TCA cycle enzymes, and mitochondrial abundance in emerged postdiapause beetles mirrored whole-animal metabolic rates (Figs. 1A and 2). Thus, the reproliferation of mitochondria appears to underlie an increased metabolic rate upon diapause emergence.
Because mitochondrial homeostasis shifted toward mitophagy during diapause, we hypothesized that the proliferation of mitochondria results from a return to mitochondrial biogenesis as spontaneous diapause emergence approaches. At 12 wk in diapause-inducing conditions (during the diapause maintenance period), beetles increased the abundance of PGC1α and NRF1 by approximately two- and 100-fold, respectively, driving mitochondrial proliferation (Fig. 3C). During this time beetles also increased the abundance of mTOR, which encodes a protein that regulates mitochondrial biogenesis upstream of PGC1α (SI Appendix, Fig. S4). Mitochondrial homeostasis appears to be maintained toward the end of diapause and upon diapause emergence: Beetles not only activated mitochondrial biogenesis but also maintained a level of mitophagy (both Parkin and PINK1 expression remained high; Fig. 3C). Maintenance of mitochondrial homeostasis by balancing mitophagy and mitochondrial biogenesis is crucial for healthy cell function (22). Imbalances in mitochondrial homeostasis are normally irreversible and are associated with aging-related diseases such as sarcopenia (35). Diapausing beetles can readily reverse this damaging phenotype and re-establish mitochondrial homeostasis while concomitantly recovering their metabolic rates toward the end of diapause. Thus, these beetles could provide insight into mitigating the adverse effects of aging on mitochondrial homeostasis.
Colorado potato beetles activated mitochondrial biogenesis even though conditions remained constant throughout their diapause, suggesting that mitochondrial homeostasis (and ultimately metabolic rate) are regulated by an endogenous rhythm in this species. PGC1α expression also increases during hibernation in ground squirrels (45) and bats (46) when these animals are otherwise immobile and overwintering in microhabitats with constant conditions. However, ground squirrels and bats typically maintain muscle mass and keep their mitochondria throughout hibernation (47), whereas we show that Colorado potato beetles degrade mitochondria during diapause. Thus, in contrast to hibernating mammals, these beetles endogenously activate mitochondrial biogenesis in atrophied and mitochondria-deficient muscle, without changes in external stimuli, and in anticipation of an increased energy demand in the spring. Early work on Colorado potato beetles showed that flight muscle degeneration during diapause is hormonally regulated (14), but the mechanisms linking seasonal rhythms of hormone production and recovery of mitochondria to emergence from diapause merit further investigation.
Conclusions
Insects from at least two orders degenerate and regenerate flight muscle during the winter (21, 48), and here we reveal a role for Parkin-mediated mitophagy in this process. While other dormant animals suppress their metabolic rates by reducing mitochondrial respiration, diapausing Colorado potato beetles remove mitochondria altogether. It remains unclear whether other diapausing insects employ similar mechanisms to lower their metabolic rate. We predict that the degradation of flight muscle mitochondria during winter could be widespread in adult-diapausing species when the cost to insects of degrading mitochondria is lower than the cost of maintaining mitochondrial function over a prolonged winter. Furthermore, this mitophagy is reversible; beetles activate transcriptionally mediated mitochondrial biogenesis to recover metabolic rate and re-establish mitochondrial homeostasis in their flight muscle in anticipation of emergence from diapause. Our study establishes a mechanistic link between metabolic suppression and mitochondrial degradation in insect tissues over the winter and provides insight into the diversity of energy-saving adaptations that animals use in resource-limited environments.
Materials and Methods
Experimental Animals.
We reared beetles and induced diapause according to ref. (15) (SI Appendix, Materials and Methods). Briefly, to produce control (nondiapausing) beetles, we reared eggs, larvae, and adults at 24 °C under a long daylength (16 h:8 h L:D). We induced diapause by rearing eggs to adults at 24 °C under a short daylength (8 h:16 h, L:D), and then transferred those adults to diapause-inducing conditions (8 h:16 h, L:D, 15 °C).
Whole-Animal Metabolic Rate, Mitochondrial Respiration Rate, and Citrate Synthase Enzyme Activity.
We measured CO2 production as a proxy for metabolic rate using flow-through respirometry, used high-resolution respirometry to measure state 3 mitochondrial respiration rates in saponin-permeabilized beetle flight muscle, and used a kinetic enzyme assay (described in ref. [4]) to measure citrate synthase activity in beetle flight (SI Appendix, Materials and Methods).
Fluorescence and Electron Microscopy.
We used fluorescence microscopy to image nuclei in the flight muscle of nondiapause (control) and diapausing beetles and used transmission electron microscopy to visualize the structure and presence of mitochondria in fthe light muscle cells of beetles during diapause as described in SI Appendix, Materials and Methods.
mRNA Abundance Quantification.
We used qPCR to measure changes in the abundance of mitophagy (Parkin, ATG5, PINK1) and mitochondrial biogenesis–related (PGC1a, NRF1) transcripts in beetle flight muscle. qPCR primers are listed in SI Appendix, Table S2, and protocols for RNA extraction, cDNA synthesis, and cycling conditions are described in SI Appendix, Materials and Methods.
dsRNA Production and RNA Interference Knockdown of Parkin.
We designed and synthesized dsRNA complementary to Parkin and GFP (as a negative control that would not elicit a knockdown) as described in SI Appendix, Materials and Methods. To knock down Parkin in diapausing beetles, we injected them with 1 µg dsRNA complementary to Parkin (dsParkin) and injected a second group with dsGFP as a negative control. We used qPCR to verify transcript knockdown and Western blot analysis to verify protein knockdown in individuals injected with dsParkin (as described in SI Appendix, Materials and Methods). We measured whole-animal metabolic rate, flight muscle mitochondrial respiration rate, citrate synthase enzyme activity, and mitochondrial abundance in knockdown beetles 5 d postinjection, when transcript knockdown was at its peak (SI Appendix, Fig. S5 and SI Appendix, Materials and Methods).
Supplementary Material
Acknowledgments
We thank the staff at the Biotron Integrated Microscopy Facility at Western University for their technical assistance with microscopy; Chris Guglielmo for use of laboratory equipment; staff at the Western Biology Greenhouses; Adam Smith, Aaron Hoang, Joseph Atem, Ahmad Butt, and Babafemi Adewusi for assistance with plant and beetle care; Cam Donly and Robert Cumming for important discussion early in the project; Amanda Lynn Stubley and Meghan Duell for comments on early versions of the manuscript; and two anonymous reviewers whose comments greatly improved the manuscript. This work was supported by the Natural Sciences and Engineering Research Council of Canada via Discovery grants to B.J.S. and J.F.S., postgraduate doctoral scholarships to J.E.L. and K.F.T., and an Ontario Graduate Scholarship to K.E.M., and also by a Genome Canada, Genome British Columbia, and Genome Quebec (LSARP 10106; Genome Canada) grant for the Biosurveillance of Alien Forest Enemies (BioSAFE) project.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2201089119/-/DCSupplemental.
Data Availability
All study data are available in the main text and SI Appendix.
References
- 1.Sinclair B. J., Linking energetics and overwintering in temperate insects. J. Therm. Biol. 54, 5–11 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Wilsterman K., Ballinger M. A., Williams C. M., A unifying, eco‐physiological framework for animal dormancy. Funct. Ecol. 35, 11–31 (2021). [Google Scholar]
- 3.Staples J. F., Buck L. T., Matching cellular metabolic supply and demand in energy-stressed animals. Comp. Biochem. Physiol. A 153, 95–105 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Mathers K. E., McFarlane S. V., Zhao L., Staples J. F., Regulation of mitochondrial metabolism during hibernation by reversible suppression of electron transport system enzymes. J. Comp. Physiol. B 187, 227–234 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Duerr J. M., Podrabsky J. E., Mitochondrial physiology of diapausing and developing embryos of the annual killifish Austrofundulus limnaeus: Implications for extreme anoxia tolerance. J. Comp. Physiol. B 180, 991–1003 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Kostál V., Eco-physiological phases of insect diapause. J. Insect Physiol. 52, 113–127 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Grapputo A., Boman S., Lindström L., Lyytinen A., Mappes J., The voyage of an invasive species across continents: Genetic diversity of North American and European Colorado potato beetle populations. Mol. Ecol. 14, 4207–4219 (2005). [DOI] [PubMed] [Google Scholar]
- 8.De Kort C., Bergot B., Schooley D., The nature and titre of juvenile hormone in the Colorado potato beetle, Leptinotarsa decemlineata. J. Insect Physiol. 28, 471–474 (1982). [Google Scholar]
- 9.De Wilde J., Duintjer C., Mook L., Physiology of diapause in the adult Colorado beetle (Leptinotarsa decemlineata Say)—I The photoperiod as a controlling factor. J. Insect Physiol. 3, 75–85 (1959). [Google Scholar]
- 10.Yocum G. D., Buckner J. S., Fatland C. L., A comparison of internal and external lipids of nondiapausing and diapause initiation phase adult Colorado potato beetles, Leptinotarsa decemlineata. Comp. Biochem. Physiol. B 159, 163–170 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Lehmann P., Piiroinen S., Lyytinen A., Lindström L., Responses in metabolic rate to changes in temperature in diapausing Colorado potato beetle Leptinotarsa decemlineata from three European populations. Physiol. Entomol. 40, 123–130 (2015). [Google Scholar]
- 12.Boiteau G., Coleman W., Cold tolerance in the Colorado potato beetle, Leptinotarsa decemlineata (Say) (Coleoptera: Chrysomelidae). Can. Entomol. 128, 1087–1099 (1996). [Google Scholar]
- 13.Ferro D. N., Alyokhin A. V., Tobin D. B., Reproductive status and flight activity of the overwintered Colorado potato beetle. Entomol. Exp. Appl. 91, 443–448 (1999). [Google Scholar]
- 14.Stegwee D., Kimmel E. C., de Boer J. A., Henstra S., Hormonal control of reversible degeneration of flight muscle in the Colorado potato beetle, Leptinotarsa decemlineata Say (Coleoptera). J. Cell Biol. 19, 519–527 (1963). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lebenzon J. E., Torson A. S., Sinclair B. J., Diapause differentially modulates the transcriptomes of fat body and flight muscle in the Colorado potato beetle. Comp. Biochem. Physiol. D 40, 100906 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Iwamoto H., Structure, function and evolution of insect flight muscle. Biophysics (Nagoya-Shi) 7, 21–28 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larsen S., et al. , Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J. Physiol. 590, 3349–3360 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stegwee D., Respiratory chain metabolism in the Colorado potato beetle—II. Respiration and oxidative phosphorylation in “sarcosomes” from diapausing beetles. J. Insect Physiol. 10, 97–102 (1964). [Google Scholar]
- 19.Sun B. J., et al. , Nocturnal dispersal flight of crickets: Behavioural and physiological responses to cool environmental temperatures. Funct. Ecol. 34, 1907–1920 (2020). [Google Scholar]
- 20.Gunn A., Gatehouse A., The migration syndrome in the African armyworm moth, Spodoptera exempts: Allocation of resources to flight and reproduction. Physiol. Entomol. 18, 149–159 (1993). [Google Scholar]
- 21.Edwards F., Environmental control of flight muscle histolysis in the bug Dysdercus intermedius. J. Insect Physiol. 15, 2013–2020 (1969). [DOI] [PubMed] [Google Scholar]
- 22.Palikaras K., Tavernarakis N., Mitochondrial homeostasis: The interplay between mitophagy and mitochondrial biogenesis. Exp. Gerontol. 56, 182–188 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Jin S. M., Youle R. J., PINK1- and Parkin-mediated mitophagy at a glance. J. Cell Sci. 125, 795–799 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Narendra D. P., et al. , PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 8, e1000298 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gureev A. P., Shaforostova E. A., Popov V. N., Regulation of mitochondrial biogenesis as a way for active longevity: Interaction between the Nrf2 and PGC-1α signaling pathways. Front. Genet. 10, 435 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scarpulla R. C., Vega R. B., Kelly D. P., Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol. Metab. 23, 459–466 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cornelissen T., et al. , Deficiency of parkin and PINK1 impairs age-dependent mitophagy in Drosophila. eLife 7, e35878 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Si H., et al. , Overexpression of pink1 or parkin in indirect flight muscles promotes mitochondrial proteostasis and extends lifespan in Drosophila melanogaster. PLoS One 14, e0225214 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vainshtein A., Desjardins E. M., Armani A., Sandri M., Hood D. A., PGC-1α modulates denervation-induced mitophagy in skeletal muscle. Skelet. Muscle 5, 9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rogers R. S., et al. , Impaired mitophagy plays a role in denervation of neuromuscular junctions in ALS mice. Front. Neurosci. 11, 473 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin S. M., et al. , Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J. Cell Biol. 191, 933–942 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang A., et al. , Biogenesis of lysosome-related organelles complex-1 subunit 1 (BLOS1) interacts with sorting nexin 2 and the endosomal sorting complex required for transport-I (ESCRT-I) component TSG101 to mediate the sorting of epidermal growth factor receptor into endosomal compartments. J. Biol. Chem. 289, 29180–29194 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han Z., et al. , ATG10 (autophagy-related 10) regulates the formation of autophagosome in the anti-virus immune response of Pacific oyster (Crassostrea gigas). Fish Shellfish Immunol. 91, 325–332 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Evans C. S., Holzbaur E. L. F., Autophagy and mitophagy in ALS. Neurobiol. Dis. 122, 35–40 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joseph A.-M., et al. , Dysregulation of mitochondrial quality control processes contribute to sarcopenia in a mouse model of premature aging. PLoS One 8, e69327 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiao J., Demontis F., Skeletal muscle autophagy and its role in sarcopenia and organismal aging. Curr. Opin. Pharmacol. 34, 1–6 (2017). [DOI] [PubMed] [Google Scholar]
- 37.White C. R., Kearney M. R., Determinants of inter-specific variation in basal metabolic rate. J. Comp. Physiol. B 183, 1–26 (2013). [DOI] [PubMed] [Google Scholar]
- 38.Liu K., et al. , Parkin regulates the activity of pyruvate kinase M2. J. Biol. Chem. 291, 10307–10317 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Villa E., Marchetti S., Ricci J.-E., No Parkin Zone: Mitophagy without Parkin. Trends Cell Biol. 28, 882–895 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Sim C., Denlinger D. L., Insulin signaling and FOXO regulate the overwintering diapause of the mosquito Culex pipiens. Proc. Natl. Acad. Sci. U.S.A. 105, 6777–6781 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Webb A. E., Brunet A., FOXO transcription factors: Key regulators of cellular quality control. Trends Biochem. Sci. 39, 159–169 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mei Y., et al. , FOXO3a-dependent regulation of Pink1 (Park6) mediates survival signaling in response to cytokine deprivation. Proc. Natl. Acad. Sci. U.S.A. 106, 5153–5158 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim S., Koh H., Role of FOXO transcription factors in crosstalk between mitochondria and the nucleus. J. Bioenerg. Biomembr. 49, 335–341 (2017). [DOI] [PubMed] [Google Scholar]
- 44.Lehmann P., et al. , Photoperiodic effects on diapause-associated gene expression trajectories in European Leptinotarsa decemlineata populations. Insect Mol. Biol. 23, 566–578 (2014). [DOI] [PubMed] [Google Scholar]
- 45.Xu R., et al. , Hibernating squirrel muscle activates the endurance exercise pathway despite prolonged immobilization. Exp. Neurol. 247, 392–401 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eddy S. F., Storey K. B., Differential expression of Akt, PPARgamma, and PGC-1 during hibernation in bats. Biochem. Cell Biol. 81, 269–274 (2003). [DOI] [PubMed] [Google Scholar]
- 47.Cotton C. J., Skeletal muscle mass and composition during mammalian hibernation. J. Exp. Biol. 219, 226–234 (2016). [DOI] [PubMed] [Google Scholar]
- 48.Unnithan G. C., Nair K. K., Ultrastructure of juvenile hormone-induced degenerating flight muscles in a bark beetle, Ips paraconfusus. Cell Tissue Res. 185, 481–490 (1977). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are available in the main text and SI Appendix.