Abstract
Background and aim:
Research in the field of Brain-Computer Interfaces (BCIs) has increased exponentially over the past few years, demonstrating their effectiveness and application in several areas. The main purpose of the present paper was to explore the relevance of user engagement during interaction with a BCI prototype (Neuro-Upper, NU), which aimed at brainwave synchronization through audio-visual entrainment, in the improvement of cognitive performance.
Methods:
This paper presents findings on data collected from a sample of 18 subjects with clinical disorders who completed about 55 consecutive sessions of 30 min of audio-visual stimulation. The relationship between engagement and improvement of cognitive function (measured through the Intelligence Quotient - IQ) during NU neuromodulation was evaluated through the Index of Cognitive Engagement (ICE) measured by the Pope ratio (Beta / (Alpha + Theta), and Arousal [(High Beta + Low Beta) / (High Alpha + Low Alpha)].
Results:
A significant correlation between engagement and IQ improvement, but no correlation between arousal and IQ improvement emerged, as expected.
Conclusions:
Future research aiming at clarifying the role of arousal in psychological disorders and related symptoms will be essential. (www.actabiomedica.it)
Keywords: Neuroenhancement, Cognitive functioning, Engagement
Introduction
In the last few years Brain Computer Interaction (BCI) researchers have expanded their applications focusing on neurofeedback training (NFT) using EEG. Through EEG, brain activity can be used to understand brain states. Task engagement can be described with respect to cognitive activity (mental effort), motivational orientation (approach vs. avoidance), and affective changes (positive vs. negative valence) (1). Shifts between states are prompted by user skills and task demands. The following states of intense engagement (that occur for example in gaming) have been described in terms of EEG: bored, lazy, in-flow and anxious. Engagement resembles to the flow concept described as an experience characterized by concentration, motivation, and immersion in ongoing activities, accompanied by increased performance (2-4). In the field of Human-Computer Interaction (HCI), several studies have shown that, when brainwaves are between the Alpha and Theta, the individual can reach a dimension of flow. In this study, we were interested in observing the correlation between an EEG-related measure of engagement and the results of the brainwave audiovisual stimulation. Immersion in a setting is an important dimension of flow (5, 6). Fluctuations in arousal and alertness occur constantly across the day and play an important role in modulating cognitive processes. Arousal is a temporary adaptive condition of the brain, in response to a significant stimulus, characterized by a greater cognitive-attentive state, excitement and sudden reaction to external stimulus. The interaction between emotion and cognition is described in terms of intersections along orthogonal dimensions of task engagement and arousal (7) where few suboptimal states are predictive of degraded performance (ex.: mind wandering, perseveration, inattentional blindness).
Therefore, it is reasonable to target neurophysiological states and their associated mechanisms that account for enhanced human performance. EEG offers accessible and cost-effective metrics for the determination of user engagement and arousal. Previous studies report on combining NFT with audiovisual stimulation and resulting in a flow state induced by brainwave modulation. More specifically, the association of visual stimulation and binaural tones improves cognitive function through the increase of Alpha and the decrease of Beta or Theta (8-10). Classical music and white noise are widely used to module Alpha and Theta and induce the flow state (1, 11-13). Incorporating music into BCI systems offers great potential for emotion-regulation systems, because of eliciting affective responses (14, 15). Music information is processed primary by auditory cortex, which permits the music stimulus to reach consciousness and from the simultaneously activated Brodmann and Wernicke areas. The motor cortex, the cerebellum and the hypothalamus are also activated (16-17). Other researchers (18) hypothesize a model of flow in which the activation of the limbic system occurs by interaction among the three attentional networks: the Default Mode Network (DMN), the Central Executive Network (CEN) and the Salience Network (SN). This configuration has been supported by several investigations (19-21).
Often NFT have been associated with transient changes in brain activity, but it is not known whether they also change neural structure and function outside the task performance. Such training-induced plasticity could be particularly meaningful for potential remedial applications. Further research is needed to characterize the effect of mental states in BCI, especially with longitudinal design that was not found in the literature by the authors. The absence of longitudinal data precludes the differentiation of specific brain states and the interactions between them, many of which may work on various time scales (within-trial, within-session, weeks, months, etc.). Sufficiently large data sets are required to account for both individual fluctuations in neural signals and intra-individual differences that arise from changes in interdependent, time-varying “brain states”.
In the mid-1990s, NASA and the USA Federal Aviation Administration Controls explored ways to objectively identify and measure engagement using EEG recordings of pilots to quantify the level of involvement. The initial study gave rise to the Index of Cognitive Engagement (ICE) (22) where the power of the Beta waves (13-22 Hz), indicating attention, is divided by the combined power of Alpha (8-13 Hz) and Theta waves (4-8 Hz), characteristic of relaxation states, as shown in Formula (1). Further studies have confirmed its reliability (23-25).
Formula (1):

Berka and colleagues (26) demonstrate that the ICE indicates information-gathering, visual scanning, and sustained attention. Combining audiovisual entrainment and NFT, with the aim of enhancing human cognitive performance, Rajamani et al. (27) study revealed a significant improvement in memory performance, processing speed, understanding, and language, ranging between 5% and 16.67% for participants who had maintained an appropriate engagement level during training. Similarly, Coelli et al. (28) measured the engagement and changes in Alpha and Beta waves during sustained attention. Their results suggest a correlation between engagement level and sustained attention test scores. Further research (29) aimed to build a model of the flow state by relating the engagement index with arousal and valence. Under arousal conditions, neurotransmitters such as acetylcholine, norepinephrine, dopamine, and serotonin increased (7). The limbic system (mainly the hypothalamus and amygdala), and the frontal and temporal lobes are activated, and EEG reveals a strong presence of beta rhythms. Exaggerated effort or excessive activation may induce a degree of stress due to an upregulation of the salience network (31), whereas the flow state is regarded as a suitable level of both engagement and arousal. Some evidence confirms that engagement and arousal indices are useful in measuring flow, while the valence index does not appear to be significant (30). Other findings suggested that Theta (4-7 Hz) indexes attentional states and mediates feed-forward influences from the thalamus to the cortex (31-32) constituting an additional physiological marker of flow state (8, 33-34).
Alpha waves (8-12 Hz) are also relevant for entrainment theories, as they accompany conscious relaxation and visual perception, and are related to attentive visual gating in the presence of auditory or visual stimuli (9-10, 35-36). Although the exact causal relation between behavior and the Alpha rhythm remains unknown, NFT and/or non-invasive brain stimulation successfully modulated ongoing Alpha oscillations (38). Clinical applications suggest that Alpha oscillations contribute significantly to cortical plasticity in the motor cortex, causing changes that outlast their entrainment phase. The Alpha frequency is usually separated into different sub-bands including Lower Alpha (6-8 Hz), Medium Alpha (8-10 Hz), and Upper Alpha (10-13 Hz). This distinction was made because in many anxious/depressive disorders High Beta activity is typically present exhibiting hyper-arousal states often referred to as “Excess Beta”. We argued that the arousal index should be considered with caution, given that optimal performance can be expected at a moderate level (39). It is plausible that both Alpha and Beta are involved in top-down processing because the high end of the Alpha frequency range (13-14 Hz) overlaps with the low end of the Beta frequency range. Thus, it is also conceivable that the same physiological activity pattern has been called Beta or Beta 1, 2, or High Alpha in different studies. When arousal progresses towards the extremes of the defined range (High Alpha and Low Delta), performance deteriorates because of maladaptive behavior patterns as in psychiatric disorders (31, 39). An active role of Alpha oscillations in stimulus encoding and memory acquisition has been recognized (40-42). Indeed, plastic changes in sensory areas elicited by perceptual learning may affect large-scale information processing (43), as in the case of entrainment with Neuro-Upper (NU), a BCI prototype used in this study. This indicates that a variety of neural processes may be linked to Alpha rhythms and exploited in clinical and real-life settings. NU is a passive BCI because the user exerts no effort to deliberately elicit their own brain activity while the BCI monitors it (44). It was showed in a previous randomized controlled trial with a clinical sample a significant decrease in symptoms together with gains in cognitive functions only for participants who undergone to the experimental treatment (45).
It is crucial to scrutinize whether engagement in audiovisual entrainment plays a functional role in increasing the flow state, with a consequent increase in cognitive abilities. The present study aims at verifying the hypothesized role of engagement in improving the audiovisual entrainment effects on the cognitive functions. We computed the engagement and arousal indices from EEG recordings gathered in 2017-2019 studies where NU was demonstrated to be effective in cognitive enhancement (45-46). In the present research we focus on the relationship between EEG-derived engagement level during brainwave entrainment sessions and the difference between cognitive functions measured as Intelligence Quotient (IQ) at baseline and post treatment (47-48). The higher IQ scores following the entrainment period will support our hypotheses; we anticipated that individuals who showed a high level of engagement across sessions would exhibit improved cognitive function scores after entrainment. More specifically, our strongest hypothesis is that states of high engagement foster beneficial effects of neuromodulation. We also predicted that optimal performance can be expected at a moderate arousal state (39) and that the valence would not be crucial at this regard.
Materials and methods
Participants
In Table 1. participants socio-demographic characteristics and IQT measures al baseline and posttreatment are reported. We analyzed EEG data from 18 participants who voluntarily participated at previous research already published (45-46) carried out is in accordance with the most recent version of the World Medical Association Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects.
Table 1.
Participants socio-demographic characteristics, diagnoses, and IQT measures at baseline and posttreatment
| SUB. | GEN. | AGE (in years) | PSYCHIATRIC DISORDER Baseline | PSYCHIATRIC DISORDER Posttreatment | IQT Baseline | IQT Posttreat. |
|---|---|---|---|---|---|---|
| S.1 | M | 53 | Anxiety Disorder | None | 102 | 129 |
| S.2 | F | 25 | Anxiety Disorder | None | 139 | 145 |
| S.3 | M | 36 | Depressive Disorder | Depressive Disorder | 110 | 138 |
| S.4 | F | 62 | Obsessive | None | 111 | 140 |
| S.5 | M | 58 | Depressive Disorder and Obsessive-compulsive Disorder | Obsessive-compulsive Disorder | 128 | 140 |
| S.6 | F | 23 | Depressive Disorder and Obsessive-compulsive Disorder | None | 126 | 144 |
| S.7 | M | 31 | Depressive Disorder | None | 114 | 117 |
| S.8 | F | 32 | Depressive Disorder | None | 105 | 106 |
| S.9 | F | 26 | Anxiety Disorder and Insomnia | Anxiety Disorder | 109 | 116 |
| S.10 | F | 33 | Anxiety Disorder | Anxiety Disorder | 106 | 106 |
| S.11 | M | 28 | Major Depressive Disorder | Depressive Disorder | 98 | 122 |
| S.12 | F | 56 | Anxiety Disorder | None | 114 | 124 |
| S.13 | F | 43 | Depressive and Anxiety Disorders; Hypersomnolence | Anxiety Disorder | 108 | 99 |
| S.14 | F | 31 | Depressive and Substance use Disorders | Depressive Disorder in remission | 97 | 100 |
| S.15 | M | 65 | Major Depressive Disorder | Major Depressive Disorder in remission | 95 | 113 |
| S.16 | M | 56 | Depressive and Obsessive-compulsive Disorders | None | 100 | 147 |
| S.17 | F | 44 | Asperger’s syndrome and Anxiety Disorder | Asperger’s Syndrome | 146 | 149 |
| S.18 | M | 56 | Anxiety Disorder | None | 119 | 149 |
Enrollment was based on the inclusion criteria: i) Age between 18-60 years; ii) Depression score above or equal eight at the HAM-D at the Hamilton Rating Scale for Depression (HAM-D, 49); iii) Trait anxiety score above the 95 percentile at the State Trait Anxiety Inventory Form Y (STAI-Y, 50). Exclusion criteria were: i) Symptoms of dementia (scoring < 23) at the Mini Mental State Examination (MMSE, 30), or other severe neurological conditions; ii) Photosensitive epilepsy; iii) History of alcohol abuse or dependency, or suicide risk.
The sample included 10 males and 8 females, their mean age was of 42.11 years (SD = 14.26, range = 23 - 65). All participants completed the baseline assessment and showed single or multiple comorbidities according to the DSM-5 (Diagnostic Statistical Manual of Mental Disorders, 51) criteria for personality disorders. All subjects self-reported both not suffering from neurological illness and not having experienced a severe head injury during their lives. They received no payment for taking part in the procedures and all provided their written informed consent to participate. They showed no sensory impairments and were native Italian speakers. All procedures were non-invasive, and the subjects were free to withdraw at any time without any penalty. Participants who did not complete at least 55 sessions were excluded from the sample.
Assessment Instruments
The Spielberger State Trait Anxiety Inventory (STAI) (50), the Hamilton Rating Scale for Depression (HAM-D) (49), and the Mini Mental State Examination (MMSE) (52) were used to score anxiety, depression, and cognitive function, respectively. Diagnosis on psychiatric disorders was obtained at baseline and after completion of the entrainment phase (T1 and T2) using the Structured Clinical Interview for DSM-IV-R Axis II Disorders (SCID-4-RV) (53), and the Structured Clinical Interview for DSM-5 (SCID-5) (51), which are both comprehensive and standardized tools for evaluating major psychiatric disorders based on DSM definitions and criteria. The procedure of diagnostic included: (a) early interview with the individual by a psychologist, (b) gathering the history data of participant including lifetime course of the disorder, and any previous treatment and recorded psychiatric diagnosis, and (c) the final diagnosis by a supervisor psychologist based on an independent interview with the subject and all the gathered data, according to the DSM‐5. Cognitive function was evaluated through the Wechsler Adult Intelligence Scale Revised (WAIS-R) (54) or Wechsler Adult Intelligence Scale (WAIS-IV) (55).
EEG data acquisition
We used the NU system that included a Mindwave® headset that provides a single channel of EEG recording from a dry electrode placed at the frontal location (FP1) referenced to an electrode placed at the ear lobe, which is used as ground to filter out the electrical noise. The device uses Think-Gear application specific integrated circuit module dry electrode technology that operates at a minimum of 2.7 V and covers a bandwidth of 3 - 100 Hz. The sampling rate for recording EEG data is configured to 512 Hz. Although Mindwave® may not be as accurate as the best research or medical systems, studies of low-cost EEG devices report high accuracy even using only a single electrode (56) and a good option for applications such as tracking attention and meditation (57). Raw data is measured in the time domain. The Mindwave® device via the Think gear technology allows the frequency domain data to be decomposes the raw input signal on several frequency bands at the hardware level using a fast Fourier transform (FFT): Delta (0.5 - 2.75 Hz), Theta (3.5 - 6.75 Hz), Alpha 1 (7.5 - 9.25 Hz), Alpha 2 (10 - 11.75 Hz), Beta 1 (13 - 16.75 Hz), Beta 2 (18 - 29.75 Hz), Gamma 1 (31 - 39.75 Hz) and Gamma 2 (41 - 49.75 Hz). After FFT decomposition, the MindWave device broadcasts the decomposed EEG signal once per second (i.e., the refresh rate is 1Hz) and the NU device transferred for each participants these outputs to an array of lamps producing flickering colored light (58-59) as feedback. The visual effector was positioned at about 210 cm from the user at a height of 180 cm from the floor. Depending on calculations, the delay between the EEG acquisition and the subsequent administration of the flash can be estimated to be between 0.2 - 0.4 msec. Brainwave patterns were continually recorded for each NU session (about 40 minutes). The music consisted of classical, folk and jazz pieces (4 vocal, 4 instrumental) chosen for appropriateness with the target emotional state. Previous research has secured NU procedure as safe, and the developer and researchers are not aware of any severe adverse effect in patients without a history of seizures or photosensitivity. The stimulation was well tolerated although few participants reported brief periods of enhanced awareness of positive and negative emotions (e.g., sleep disturbance, headache, and unrest). In the final interview, users stated they found NU as acceptable.
Entrainment procedure
EEG recordings were collected from daily individual sessions. Arriving at the Laboratory, participants were taken to a darkened room, and asked to relax, wear the headset, and avoid any superfluous head or body movement. They were instructed to observe the flickering lights with eyes open while music was played at a comfortable volume through headphones and relax (Sennheiser HD 419). Visual stimulation consisted of EEG frequencies of participants themselves converted in real-time into flashing lights emitted from the visual effector. Each session started with a 10-sec calibration phase, followed by the automated entrainment period of 30 min.
Engagement and Arousal indices
Five minutes were removed from both the beginning and the end of each EEG recording. Segments of 1 sec were used to obtain the mean and median statistics of each signal (Alpha1, Alpha2, Beta1, Beta2, Delta, Gamma 1, Gamma 2, Theta). A total of 1100 observations were obtained for each band. EEG-based Engagement and Arousal average indices were then computed on the 20-min recording corresponding to each session based on the formula already described and the next one:
Arousal = (High Beta + Low Beta) / (High Alpha + Low Alpha)
Statistical analysis
To the present research, the primary outcome was the differences in WAIS-R or WAIS-IV scores between the two assessment measures together with EEG-based engagement and arousal average indices. A dependent t-Test for paired samples was employed to compare IQT across time. To determine the impact of engagement ad arousal on IQT data, analysis of covariance (ANCOVA) was used. The function of the pretest is to play the role of the baseline measure and was treated as covariates while the posttreatment data was the dependent variable (covariates: QIT baseline; dependent variables: QIT posttreatment). Statistical analyses were conducted in R Version 3.0.2 (60). Significant probability values were established at p < 0.05.
Results
In Figure 1 the Intelligence Quotient Total (IQT) obtained from the cognitive function assessments through the WAIS-R or WAIS IV at baseline (T1) and posttreatment (T2) is showed. Interaction between time and IQT is represented by standard deviation (SD) and interval between the lowest and highest values found in the sample. Table 1 reports demographic characteristics, clinical evaluations, IQT scores, clinical diagnosis, and co-morbidities at T1 and T2 for all participants. Figures 2 and 3 indicated the IQT increase of “Engagement” and “Arousal” at the conclusion of the entrainment phase.
Figure 1.

IQT scores measured with the WAIS-R or WAIS IV before (T1) and after (T2) entrainment phase. Interaction between time and IQT is represented by mean, standard deviation (SD) and interval between the lowest and highest values found in the sample.
Figure 2.
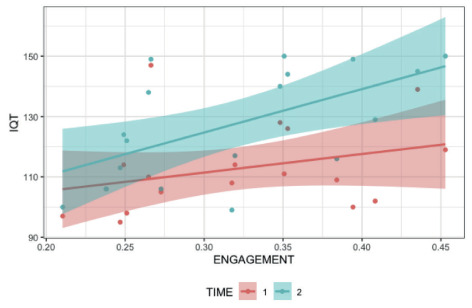
Scatter plot of regression model explaining the 3-way-interaction between Engagement, IQT, and assessment periods. Each symbol represents a unit, green and purple at T1 and T2, respectively.
Figure 3.

Scatter plot of regression model for the 3-way-interaction between Arousal, IQT, and assessment periods. Each symbol represents a unit, green and purple at T1 and T2, respectively. No statistically significant difference was found concerning the Arousal index.
At baseline, participants displayed a prevalence of depressive and anxiety disorders. Other groups of disorders (obsessive-compulsive, sleep-wake disorders, hypersomnolence, and substance use) were less common. Furthermore, nine participants (50%) had at least two or three comorbid psychiatric disorders. Posttreatment revealed a significant remission for depression and anxiety symptoms for nine individuals (50% of the sample) who at posttreatment no longer exhibited any disorder; other five individuals showed a reduction in their comorbidities.
The average of IQT at the baseline T1 fell in the normal range. At the posttreatment, sixteen participants out of the 18 showed a significant increase in IQT, as measured by the WAIS-R or WAIS-IV scores. One participant maintained the same IQT level, and a second subject demonstrated a decrease. A significant increase [t (17) = - 4.116, p = 0.007] of the IQT score for the overall sample was found from T1 (M=109.52, ± SD=11.98) to T2 (M=124.47, ± SD=17.25). To explore the effect of engagement and arousal on IQT considering baseline and posttreatment, two general linear models were created. A main effect was found for the ICE [F (1, 16) = 5.910, p = 0.027)] but not for time; the interaction was also not significant suggesting that engagement regardless of time exert an impact on IQT (see Figure 2).
Time regardless of engagement did not exert impact on IQT and did not emerge no interaction between engagement and time. Indeed, participants who displayed greater engagement also had higher IQT. No statistically significant finding was observed for the arousal index and for the interaction between arousal and time. The IQT did not change as a function of arousal (Fig. 3). Results from ANCOVA failed to produce any significant interaction, as a result no post-hoc comparison was computed.
Discussion
In recent years, research on BCIs has undergone exponential growth, and they have become potentially effective for a wide range of applications, including the enhancement of cognitive functions. This study was interested in the impact of engagement of users interacting with BCI and its potential effect on the outcomes of an audiovisual entrainment treatment. We anticipated that ICE would facilitate the achievement of flow state characterized by the presence of Alpha waves, whereas typical high Beta activity of excitement states of arousal would interfere with performance. We were interested in observing if the variation in intelligence scores, measured at baseline and after the treatment period, could be partially explained by (or at least correlated to) subjective engagement and/or arousal levels during each session. In other words, we investigated whether measures of engagement and arousal predicted subsequent performance in terms of IQT. Our findings partially confirmed these expectations: IQT score variations measured at T2 were, in fact, associated with ICE. It therefore appears that the activation experienced during the entrainment sessions, as measured by the arousal index, has no influence on the IQT.
Our findings further confirm the validity of the Pope index. Emotional responses during audio-visual entrainment may be significantly related to engagement, and a conjunction between low arousal and higher engagement may predict progress in cognitive function. The effect of ICE on IQT in conceivably due to the synchronized the activity of the Default Mode Network (DMN) involved in a broad cognitive context related to memory (61), which can be modulated by listening to music (62). The only exception was one subject who scored lower in T2 than in T1, despite showing a good average engagement index.
The data also confirmed the hypothesis concerning the effect of arousal. Arousal is characterized by a presence of Beta brainwaves, and most subjects showed a strong presence of Beta during the entrainment sessions with an average arousal index of 0.64. The occurrence of a high arousal index may reflect that most participants exhibited symptoms of anxiety disorder. Previous studies have proposed that a specific combination of engagement and arousal reflects flow, or an optimal state, and consequently, greater cognitive processing (29). The present research expands on these findings by investigating the association between engagement and cognitive function as well as arousal level in people suffering from mental disorders.
Limitations and future directions
Our findings are potentially relevant but also preliminary. The research has limitations which may reduce both interpretability and generalizability of its conclusions. First, the overall test results need to be interpreted with caution because procedural learning can have influenced the subtests on the perceptual domain during the re-administration: procedural learning is the acquisition of knowledge or experience that can improve performance on a particular task. Thus, it may affect results when a test like the first is administered after a short interval. In the present study, however, the second assessment occurred after an interval of 3-4 months, which was considered too long to impact on performance. A second limitation can be the absence of a control group. The stimulation encompassed a consistent number of sessions in a quiet environment with the instruction to relax while listening to music: this might produce a placebo effect or raise other unexpected consequences. A third limitation is that the Mindwave Mobile® has only one electrode which is FP1 and FP1 is vulnerable to a lot of noise coming from eye and muscle movements. Future studies could use sham conditions to measure the impact of motivation. Furthermore, the current NU is not equipped to generate accurate records of stimulus onsets that could be synchronized to the EEG recordings, consequently we could not determine the phase relationship between the stimuli and the evoked EEG signal. Future work will take into consideration methods that allow higher precision, such as high-resolution EEG. Because very high levels of arousal may indicate that the user has entered a stressful state, future studies could also identify a threshold of engagement/arousal level which can indicate the onset of a state of stress, boredom, or flow. Real-time assessment opens possibilities for adaptive e-learning or e-assessment environments. For example, it may be possible for a system to warn the users wearing an EEG helmet when their engagement level is declining and prompt them to react. Finally, existing/alternative methods for detecting engagement and arousal can be derived from automatic facial emotion recognition software or from the electrodermal activity encoders/sensors. These techniques may be combined in the future to investigate their accuracy against the valence signal.
Conclusion
Alpha, Beta, and Theta bands were continuously extracted from the EEG recordings and used to calculate users’ engagement and arousal while participants got the audiovisual entrainment. Our results indicated that significantly higher engagement levels along the entrainment sessions predict higher IQT and that, overall, favoring the flow state seems a promising approach for enhancing user cognitive function. This research takes a preliminary step in relating EEG records with entrainment data on the enhancement of cognitive status and reported findings aiming at exploring the usefulness of engagement and arousal indexes to identify an optimal state in individuals with psychiatric disorders. These results are consistent with the scientific literature: the neurocognitive mechanisms underlying the flow state are still uncertain, but a recent study (18) indicated that only the state of engagement is important for achieving the flow state while no relation was detected with either arousal or valence. Our findings add further evidence indicating that the engagement may enhance the effects of entrainment with better cognitive performance. The reason for this finding is not yet obvious but may imply that entrainment is effective in changing alpha and theta activity. This is an important issue that should be addressed in future research.
Authors’ contribution:
O.P. conceptualized and planned the study assisting in the statistical analysis, revising, and editing of the final manuscript. G.R. gathered data, conducted the statistical analysis, and wrote the original draft of the manuscript. All authors approved the final manuscript.
Conflict of Interest:
Each author declares that he or she has no commercial associations (e.g.: consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
References
- Fairclough S, Gilleade K, Ewing K, Roberts J. Capturing user engagement via psychophysiology: Measures and mechanisms for biocybernetic adaptation. Int J Auton Adapt Commun Syst. 2013;6(1):63–79. doi: 10.1504/IJAACS.2013.050694. [Google Scholar]
- Gruzelier JH. A theory of alpha/theta neurofeedback, creative performance enhancement, long distance functional connectivity and psychological integration. Cogn Process. 2008;10:101–109. doi: 10.1007/s10339-008-0248-5. https://doi.org/10.1007/s10339-008-0248-5. [DOI] [PubMed] [Google Scholar]
- Gruzelier JH, Egner T, Vernon D. Neuper C, Klimesch W, editors. Validating the efficacy of neurofeedback for optimising performance. Event-related dynamics of brain oscillations. Prog Brain Res. 2006;159:421–431. doi: 10.1016/S0079-6123(06)59027-2. doi:10.1016/S0079-6123(06)59027-2. [DOI] [PubMed] [Google Scholar]
- Nakamura J, Csikszentmihalyi M. Flow theory and research. Oxford library of psychology. In: Lopez S. J, Snyder C. R, editors. Oxford handbook of positive psychology. Oxford University Press; 2009. pp. 195–206. [Google Scholar]
- Wefald AJ, Downey RG. Construct dimensionality of Engagement and its relation with satisfaction. J Psychol. 2009;143:91–112. doi: 10.3200/JRLP.143.1.91-112. doi: 10.3200/JRLP.143.1.91-112. [DOI] [PubMed] [Google Scholar]
- Whitton N. Game engagement theory and adult learning. Simul Gaming. 2011;42(5):596–609. https://doi.org/10.1177/1046878110378587. [Google Scholar]
- Dehais F, Lafont A, Roy R, Fairclough S. A neuroergonomics approach to mental workload, engagement, and human performance. Front Neurosci. 2020;14:268. doi: 10.3389/fnins.2020.00268. doi: 10.3389/fnins.2020.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanslmayr S, Sauseng P, Doppelmayr M, Schabus M, Klimesch W. Increasing individual upper alpha power by neurofeedback improves cognitive performance in human subjects. Appl Psychophiol Biofeedback. 2005;30(1):1–10. doi: 10.1007/s10484-005-2169-8. doi:10.1007/s10484-005-2169-8. [DOI] [PubMed] [Google Scholar]
- Makada TI, Ozair D, Mohammed M, Abellanoza C. Enhancing memory retention by increasing alpha and decreasing beta brainwaves using music. Proceedings of the 9th ACM International Conference on Pervasive Technologies Related to Assistive Environments (PETRA ‘16). Association for Computing Machinery, New York, NY, USA. 2016:1–4. https://doi.org/10.1145/2910674.2935851. [Google Scholar]
- Mladenović J, Frey J, Bonnet-Save M, Mattout J, Lotte F. The impact of Flow in an EEG-based Brain Computer Interface. Proceedings of the 7th International BCI Conference, Graz, Austria. 2017 https://hal.inria.fr/hal-01527748. [Google Scholar]
- Edge J, Lancaster L. Phenomenological analysis of superior musical performance facilitated by neurofeedback: Enhancing musical performance through neurofeedback: Playing the tune of life. Transpers Psychol Rev. 2004;8:23–35. [Google Scholar]
- Peretz I, Zatorre R. Brain organization for music processing. Annu Rev of Psychol. 2005;56(1):89–114. doi: 10.1146/annurev.psych.56.091103.070225. doi:10.1146/annurev.psych.56.091103.070225. [DOI] [PubMed] [Google Scholar]
- Peretz I, Vuvane D, Lagrois ME, Armony JL. Neural overlap in processing music and speech. Philos Trans R Soc. 2015;B 370:20140090. doi: 10.1098/rstb.2014.0090. http://doi.org/10.1098/rstb.2014.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez R, Palencia-Lefler M, Giraldo S, Vamvakousis Z. Musical neurofeedback for treating depression in elderly people. Front Neurosci. 2015;9:354. doi: 10.3389/fnins.2015.00354. https://doi.org/10.3389/fnins.2015.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sourina O, Liu Y, Nguyen MK. Real-time EEG-based emotion recognition for music therapy. J Multimodal User Interfaces. 2011;5(1-2):27–35. https://doi.org/10.1007/s12193-011-0080-6. [Google Scholar]
- Pereira CS, Teixeira J, Figueiredo P, Xavier J, Castro SL, Brattico E. Music and emotions in the brain: familiarity matters. PLoS ONE. 2011;6:e27241. doi: 10.1371/journal.pone.0027241. doi:10.1371/journal.pone.0027241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimpoor VN, van den Bosch I, Kovacevic N, McIntosh AR, Dagher A, Zatorre RJ. Interactions between the nucleus accumbens and auditory cortices predict music reward value. Science. 2013;340:216–219. doi: 10.1126/science.1231059. doi: 10.1126/science.1231059. [DOI] [PubMed] [Google Scholar]
- Van der Linden D, Tops M, Bakker AB. Go with the flow: A neuroscientific view on being fully engaged. Eur J Neurosci. 2020;00:1–17. doi: 10.1111/ejn.15014. https://doi.org/10.1111/ejn.15014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmat L, de Manzano Ö, Theorell T, Högman L, Fischer H. Ullén F Physiological correlates of the flow experience during computer game playing. Int J Psychophysiol. 2015;97:1–7. doi: 10.1016/j.ijpsycho.2015.05.001. https://doi.org/10.1016/j.ijpsy cho.2015.05.001. [DOI] [PubMed] [Google Scholar]
- Hancock PA, Kaplan AD, Cruit JK, Hancock GM, MacArthur KR, Szalma JL. A meta-analysis of flow effects and the perception of time. Acta Psychologica. 2019;198:102836. doi: 10.1016/j.actpsy.2019.04.007. https://doi.org/10.1016/j.actpsy.2019.04.007. [DOI] [PubMed] [Google Scholar]
- Ulrich M, Keller J, Hoenig K, Waller C, Grön G. Neural correlates of experimentally induced flow experiences. NeuroImage. 2014;86:194–202. doi: 10.1016/j.neuroimage.2013.08.019. https://doi.org/10.1016/j.neuro image.2013.08.019. [DOI] [PubMed] [Google Scholar]
- Pope AT, Bogart EH, Bartolome DS. Biocybernetic system evaluates indices of operator engagement in automated task. Biol Psychol. 1995;40:1–2. 187–195. doi: 10.1016/0301-0511(95)05116-3. https://doi.org/10.1016/0301-0511(95)05116-3. [DOI] [PubMed] [Google Scholar]
- Andujar M, Gilbert JE. Let’s learn! Enhancing user’s engagement levels through passive brain-computer interfaces. CHI ‘13 Extended Abstracts on Human Factors in Computing Systems. 2013:703–708. doi: 10.1145/2468356.2468480. [Google Scholar]
- Rajamani K, Ramalingam A, Bavisetti S, Abujelala M. CBREN: Computer brain entertainment system using neural feedback cognitive enhancement. In Proceedings of the 10th International Conference on Pervasive Technologies Related to Assistive Environments (PETRA ‘17). Association for Computing Machinery, New York, NY, USA. 2017:236–237. https://doi.org/10.1145/3056540.3064971. [Google Scholar]
- Reinecke L, Klatt J, Krämer N. Entertaining media use and the satisfaction of recovery needs: recovery outcomes associated with the use of interactive and non interactive entertaining media. Media Psychol. 2011;14:192–215. doi: 10.1080/15213269.2011.573466. [Google Scholar]
- Berka C, Levendowski DJ, Lumicao MN, Yau A, Davis G, Zivkovic VT, Olmstead RE, Tremoulet PD, Craven PL. EEG correlates of task engagement and mental workload in vigilance, learning, and memory tasks. Aviat, Space, Environ Med. 2007;78(5 Suppl):B231–B244. https://pubmed.ncbi.nlm.nih.gov/17547324/ [PubMed] [Google Scholar]
- Daneshzand M, Faezipour M, Barkana BD. Robust desynchronization of Parkinson’s disease pathological oscillations by frequency modulation of delayed feedback deep brain stimulation. PLoS ONE. 2018;13(11):e0207761. doi: 10.1371/journal.pone.0207761. https://doi.org/10.1371/journal.pone.0207761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelli S, Sclocco R, Barbieri R, Reni G, Zucca C, Bianchi AM. EEG-based index for engagement level monitoring during sustained attention. Conference proceedings - IEEE Engineering in Medicine and Biology Society. 2015:1512–1515. doi: 10.1109/EMBC.2015.7318658. doi: 10.1109/EMBC.2015.7318658. [DOI] [PubMed] [Google Scholar]
- McMahan T, Parberry I, Parsons TD. Evaluating player task engagement and arousal using electroencephalography. Procedia Manuf. 2015;3:2303–2310. https://doi.org/10.1016/j.promfg.2015.07.376. [Google Scholar]
- Maran T, Sachse P, Martini M, Weber B, Pinggera J, Zuggal S, Furtner M. Lost in time and space: States of high arousal disrupt implicit acquisition of spatial and sequential context information. Front Behav Neurosci. 2017;11:206. doi: 10.3389/fnbeh.2017.00206. doi: 10.3389/fnbeh.2017.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Li Y, Stitt IM, Zhou ZC, Sellers KK, Frohlich F. Theta oscillations organize spiking activity in higher-order visual thalamus during sustained attention. eNeuro. 2018:5. doi: 10.1523/ENEURO.0384-17.2018. http://dx. doi.org/10.1523/ENEURO.0384-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich RF, Breska A, Knight RT. Neural entrainment and network resonance in support of top-down guided attention. Curr Opin. 2019;29:82–89. doi: 10.1016/j.copsyc.2018.12.016. https://doi.org/10.1016/j.copsyc.2018.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienitz R, Joscha T, Schmiedt KA, Shapcott K, Saunders RC, Schmid MC. Theta rhythmic neuronal activity and reaction times arising from cortical receptive field interactions during distributed attention. Curr Biol. 2018;28(15):2377–2387.e5. doi: 10.1016/j.cub.2018.05.086. https://doi.org/10.1016/j.cub.2018.05.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Raichle ME. Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron. 2007;56:171–184. doi: 10.1016/j.neuron.2007.08.023. doi: 10.1016/j.neuron.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Händel BF, Haarmeier T, Jensen O. Alpha oscillations correlate with the successful inhibition of unattended stimuli. J Cogni Neurosci. 2011;23(9):2494–502. doi: 10.1162/jocn.2010.21557. doi:10.1162/jocn.2010.21557. [DOI] [PubMed] [Google Scholar]
- Lundqvist M, Herman P, Lansner A. Effect of prestimulus alpha power, phase, and synchronization on stimulus detection rates in a biophysical attractor network model. J Neurosci. 2013;33(29):11817–11824. doi: 10.1523/JNEUROSCI.5155-12.2013. https://doi.org/10.1523/JNEUROSCI.5155-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G, Miniussi C, Gross J. The functional importance of rhythmic activity in the brain. Curr Biol. 2012;22(16):R658–R663. doi: 10.1016/j.cub.2012.06.061. https://doi.org/10.1016/j.cub.2012.06.061. [DOI] [PubMed] [Google Scholar]
- Arns M, Swatzyna RJ, Gunkelman J, et al. Sleep maintenance, spindling excessive beta and impulse control: an RDoC arousal and regulatory systems approach? Neuropsychiatr Electrophysiol. 2015;1:5. https://doi.org/10.1186/s40810-015-0005-9. [Google Scholar]
- Freyer F, Becker R, Dinse HR, Ritter P. State-dependent perceptual learning. J Neurosci. 2013;33(7):2900–2907. doi: 10.1523/JNEUROSCI.4039-12.2013. https://doi.org/10.1523/JNEUROSCI.4039-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamame CM, Cosmelli D, Henriquez R, Aboitiz F. Neural mechanisms of human perceptual learning: electrophysiological evidence for a two-stage process. PLoS ONE. 2011;6:e19221. doi: 10.1371/journal.pone.0019221. doi: 10.1371/journal.pone.0019221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masquelier T, Hugues E, Deco G, Thorpe SJ. Oscillations, phase-of-firing coding, and spike timing-dependent plasticity: An efficient learning scheme. J Neurosci. 2009;29:13484–13493. doi: 10.1523/JNEUROSCI.2207-09.2009. doi:10.1523/JNEUROSCI.2207-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigala R, Haufe S, Roy D, Dinse H, Ritter P. The role of alpha-rhythm states in perceptual learning: insights from experiments and computational models. Front Comput Neurosci. 2014;8:36. doi: 10.3389/fncom.2014.00036. https://doi.org/10.3389/fncom.2014.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol LR, Andreessen LM, Zander TO. Passive Brain-Computer Interfaces: A perspective on increased interactivity. In: Nam CS, Nijholt A, Lotte F, editors. Brain-Computer Interfaces handbook: Technological and theoretical advances. Boca Raton, FL, USA: CRC Press; 2018. pp. 69–86. [Google Scholar]
- Pino O. A randomized controlled trial (RCT) to explore the effect of audio-visual entrainment among psychological disorders.: Neuro-Upper. Acta Biomed. 2021;92(6):e2021408. doi: 10.23750/abm.v92i6.12089. 2022. doi: 10.23750/abm.v92i6.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pino O. Neuro-Upper, a novel technology for Audio-Visual Entrainment. A randomized controlled trial of 11 individuals with anxiety and depressive disorders. BAOJ Med Nursing. 2017;3(2):041. http://dx.doi.org/10.24947/baojmn/3/2/141. [Google Scholar]
- Ng BS, Logothetis NK, Kayser C. EEG phase patterns reflect the selectivity of neural firing. Cereb Cortex. 2013;23:389–398. doi: 10.1093/cercor/bhs031. doi:10.1093/cercor/bhs031. [DOI] [PubMed] [Google Scholar]
- Siegel M, Donner T, Engel A. Spectral fingerprints of large-scale neuronal interactions. Nat Rev Neurosci. 2012;13:121–134. doi: 10.1038/nrn3137. https://doi.org/10.1038/nrn3137. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiat. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC495331/pdf/jnnpsyc00273-0060.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. http://www.apa.org/pi/about/publications/caregivers/practice-settings/assessment/tools/trait-state.aspx. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. https://www.ncbi.nlm.nih.gov/pubmed/1202204. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2000) DSM-IV-TR. Diagnostic and statistical manual of mental disorders. fourth edition. Text Revision. Washington D.C: http://www.psych.org/MainMenu/Research/DSMIV.aspx. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: Author; 2013. doi: 10.1176/appi.books.9780890423349. [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale-Revised (WAIS-R) New York: The Psychological Corporation; 1981. http://journals.sagepub.com/doi/abs/10.1177/073428298300100310. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale - Fourth Edition Administration and Scoring Manual. San Antonio, TX: Pearson; 2008. [Google Scholar]
- LaRocco J, Le MD, Paeng DG. A systemic review of available low-cost EEG headsets used for drowsiness detection. Front Neuroinform. 2020;14:553352. doi: 10.3389/fninf.2020.553352. doi: 10.3389/fninf.2020.553352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasiljevic GAM, de Miranda LC. The influence of graphical elements on user’s attention and control on a neurofeedback-based game. Entertain Comput. 2019 https://doi.org/10.1016/j.entcom.2018.10.003. [Google Scholar]
- Elliot AJ. Color and psychological functioning: A review of theoretical and empirical work. Front Psychol. 2015;6:368. doi: 10.3389/fpsyg.2015.00368. http://dx.doi.org/10.3389/fpsyg.2015.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godinez Tello RM Jr, Torres Muller SM, Ferreira A, Freire Bastos T. Comparison of the influence of stimuli color on Steady-State Visual Evoked Potentials. Res Biomed Eng. 2015;31:218–231. http://dx.doi.org/10.1590/2446-4740.0739. [Google Scholar]
- R Core Team R. A language and environment for statistical computing. Vienna, Austria. http://www.R-project.org, 2014. [Google Scholar]
- Vatansever D, Manktelow A, Sahakian BJ, Menon DK, Stamatakis EA. Default Mode Network engagement beyond self-referential internal mentation. Brain Connect. 2018;8:4. doi: 10.1089/brain.2017.0489. https://doi.org/10.1089/brain.2017.0489. [DOI] [PubMed] [Google Scholar]
- Taruffi L, Pehrs C, Skouras S, Koelsch S. Effects of sad and happy music on mind-wandering and the Default Mode Network. Sci Rep. 2017;7:14396. doi: 10.1038/s41598-017-14849-0. https://doi.org/10.1038/s41598-017-14849-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


