Abstract
Calcium and phosphate are critical for numerous physiological processes. Consequently, the plasma concentration of these ions are tightly regulated. Calcitriol, the active form of vitamin D, is a positive modulator of mineralization as well as calcium and phosphate metabolism. The molecular and physiological effects of calcitriol are well documented. Calcitriol increases blood calcium and phosphate levels by increasing absorption from the intestine, and resorption of bone. Calcitriol synthesis is a multistep process. A precursor is first made via skin exposure to UV, it is then 25-hydroxylated in the liver to form 25-hydroxyitamin D. The next hydroxylation step occurs in the renal proximal tubule via the 1-αhydroxylase enzyme (encoded by CYP27B1) thereby generating 1,25-dihydroxyvitamin D, that is, calcitriol. At the same site, the 25-hydroxyvitamin D 24-hydroxlase enzyme encoded by CYP24A1 can hydroxylate 25-hydroxyvitamin D or calcitriol to deactivate the hormone. Plasma calcitriol levels are primarily determined by the regulated expression of CYP27B1 and CYP24A1. This occurs in response to parathyroid hormone (increases CYP27B1), calcitriol itself (decreases CYP27B1 and increases CYP24A1), calcitonin (increases or decreases CYP24A1 and increases CYP27B1), FGF23 (decreases CYP27B1 and increases CYP24A1) and potentially plasma calcium and phosphate levels themselves (mixed effects). Herein, we review the regulation of CYP27B1 and CYP24A1 transcription in response to the action of classic phophocalciotropic hormones and explore the possibility of direct regulation by plasma calcium.
Keywords: Vitamin D, CYP27B1, CYP24A1, calcium, kidney, PTH, CaSR, calcitriol
Impact Statement
Fundamental to the maintenance of calcium and phosphate homeostasis is the regulation of the phosphocalciotropic hormone 1,25-dihydroxyvitamin D or calcitriol. This occurs in the renal proximal tubule via the regulation of the expression of two enzymes. 1-alpha-hyrdoxylase encoded by CYP27B1 results in the 1-hydroxylation of calcitriol and thus more active vitamin D. In contrast, 24-hydroxylase encoded by CYP24A1, results in the 24- hydroxylation of calcitriol and its precursor 25-hydroxyvitamin D, decreasing the levels of the circulating active hormone. This review summarizes what is known about the regulation of these enzymes.
Introduction
Calcium and phosphate homeostasis
Calcium is a vital mineral found predominantly in the structural matrix of bone. However, calcium is also essential for a diversity of physiological functions including muscle contraction, neurotransmitter release, intracellular signal transduction, and blood clotting.1,2 Phosphate is also a vital mineral and a significant structural element in bone. It is also a component of ATP, nucleic acids and phospholipids. 1 Due to their critical importance in physiological and cellular processes, calcium and phosphate concentrations are tightly regulated within a narrow range in the circulation. Moreover, when the level of one ion becomes sufficiently elevated they can precipitate forming extraosseous calcifications as seen in patients with renal insufficiency. 3 As such, the plasma levels of both minerals are regulated via the coordinated action of a group of hormones, referred to as phosphocalciotropic hormones that includes calcitriol (active vitamin D or 1,25-dihydroxyvitamin D), parathyroid hormone (PTH), and fibroblast growth factor 23 (FGF23) (N.B. sex hormones also affect calcium and mineral homeostasis but are not considered phophocalciotropic hormones). Importantly, plasma concentrations of calcium and phosphate are interdependent. Many of these hormones regulate calcium and phosphate balance by increasing the production or inactivation of calcitriol (Figure 1). This is thought to occur primarily by regulating transcription of the 1- or 24-hydroxylating enzymes that activate and deactivate 25-hydoxyvitamin D and calcitriol respectively. Although there is evidence that 1-alpha hydroxylase can be phosphorylated, the effect of this on protein abundance and function is unclear due to a lack of adequate antibodies to study the phosphoenzyme.4,5 1-alpha hydroxylase is a mixed function oxidase encoded by the gene CYP27B1 within the nuclear genome. 6 This enzyme localizes to the inner membrane of mitochondria where it hydroxylates 25-hydroxyvitamin D at the 1-alpha position to produce 1,25-dihydroxyvitamin2D3 (calcitriol), the biologically active form of the hormone. 7 Calcitriol is deactivated by a 25-hydroxyvitamin D-24-hydroxylase enzyme which is encoded by the gene CYP24A1. It is also a mitochondrial enzyme that catalyzes the hydroxylation of both calcitriol and its precursor 25-hydroxyvitamin D3 to 1,24,25-trihydroxyvitamin D3 or 24,25-dihydroxyvitamin D3. 24 hydroxylation inactivates these hormones as these forms of vitamin D are unable to bind to the vitamin D receptor (VDR). 8
Figure 1.
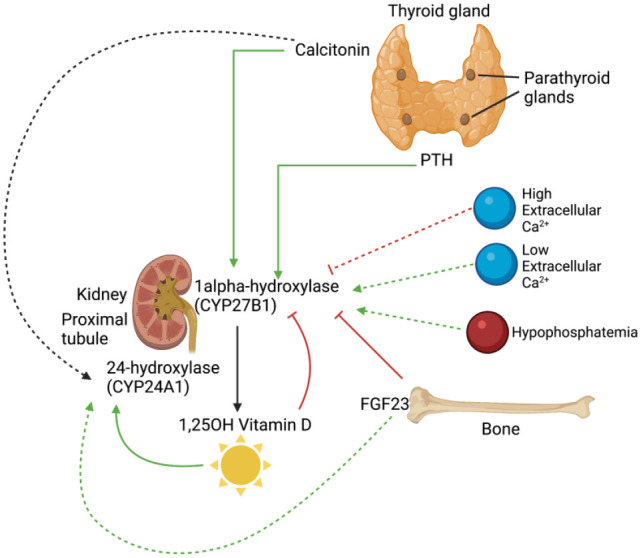
Regulation of CYP27B1 and CYP24A1 transcription by phosphocalciotropic hormones, calcium and phosphate. Calcitonin and PTH are secreted from the thyroid and parathyroid glands, respectively. Both act to upregulate the transcription of CYP27B1. In addition, calcitonin affects CYP24A1 by either upregulating or attenuating its transcription depending on the situation. High extracellular calcium has been associated with a decrease in CYP27B1 transcription, while low extracellular calcium and hypophosphatemia are associated with increased CYP27B1 transcription. FGF23 production in bone suppresses CYP27B1 expression while enhancing CYP24A1 production. Finally, calcitriol itself feedback inhibits its own production by inhibiting CYP27B1 transcription and promoting its own inactivation by increasing CYP24A1 transcription. (Created with BioRender.com, supported by previous studies9–15). (A color version of this figure is available in the online journal.)
Calcitriol synthesis
The synthesis of calcitriol occurs in multiple steps. First, pre-vitamin D3 is formed from 7-dehydrocholesterol in the skin following UV irradiation or absorption from the diet. Following thermal isomerization to produce vitamin D3, the compound then travels, bound to vitamin D binding protein, to the liver where it is hydroxylated to form 25-hydroxyvitamin D by 25-hydroxylase.16–18 This initial hydroxylation step is not tightly regulated, and is only limited by the amount of pre-vitamin D3 in the circulation. 19 The secondary hydroxylation step, which produces 1,25-dihydroxyvitamin D3 or calcitriol, is the most regulated and occurs most substantially in the mitochondria of the renal proximal tubule by 1-alpha hydroxylase encoded by CYP27B1.7,20,21 For this to occur 25-hydroxyvitamin D bound to the vitamin D-binding protein (DBP) must be filtered through the glomerulus and subsequently endocytosed into the proximal tubule after binding to megalin and cubilin. Cubilin sequesters the 25-hydroxyvitamin D-DBP and megalin stimulates endocytosis of the complex. After trafficking to lysosomes 25-hydroxytamin D is liberated and can be shuttled to the mitochondria. 22 Calcitriol is the active form of vitamin D, which can bind to the vitamin D receptor (VDR) and exert its downstream effects on calcium and phosphate metabolism.23–26 CYP27B1 is primarily expressed in the proximal tubule, where the majority of calcitriol production occurs. 21 Interestingly the proximal tubule is also the site of the majority (60–70%) of calcium reabsorption from the glomerular filtrate, via paracellular pathways.27–29 The majority of tubular phosphate reabsorption also occurs in this segment but by a transcellular pathway. 30 There is also extra-renal expression of 1-alpha hydroxylase, encoded by CYP27B1, though the effect on plasma calcitriol levels is negligible and thus these sites of calcitriol synthesis are mostly relevant for local, paracrine vitamin D action.9,31–33 Consistent with this, a kidney-specific Cyp27b1 pseudo-null mouse model displays a phenotype similar to the global null animal. 32 Extra-renal synthesis of 1-alpha hydroxylase appears to be regulated separately to the proximal tubule, as they do not contain the same regulatory module as the kidney gene, and is not considered herein. 32 Importantly, an increase in expression of Cyp27b1 is closely tied to increased calcitriol production, consistent with transcription being the major mode of regulation in calcitriol production. 34
Calcitriol actions
Calcitriol is a steroid hormone that acts by crossing the plasma membrane and binding the VDR, which heterodimerizes with the retinoid X receptor (RXR) (Figure 1). This complex then translocates to the nucleus where it transcriptionally regulates target genes via binding to vitamin D response elements (VDREs).24,25,35 Through this process, calcitriol has a significant impact on calcium and phosphate homeostasis by indirectly influencing the handling of these minerals in the intestine, kidney, and bone.16,19,25,35–39 The effects of calcitriol are primarily observed in the duodenum and colon, where transcellular calcium absorption is increased via increasing TRPV6 expression. 37 Calcitriol likely also increases paracellular calcium absorption from the jejunum and ileum by increasing claudin 2 expression. 40 In the distal renal tubule calcitriol increases TRPV5 expression thereby increasing the predominant transcellular reabsorption pathway for calcium. 41 Calcitriol also increases the reabsorption of phosphate from the proximal tubule and absorption of phosphate from the intestine, although the mechanism behind the former processes requires further elucidation. 42 In the intestine, calcitriol increases the expression and/or posttranscriptional modulation of the sodium-dependent phosphate transport protein 2b (NaPi-IIb), resulting in increased absorption of phosphate and increased serum phosphate concentrations. 43 In bone, high levels of calcitriol can trigger resorption to mobilize calcium and phosphate stores into the circulation when required, but calcitriol can also support bone mineralization by raising calcium and phosphate levels through the stimulation of intestinal absorption.25,26,36,44,45
Clinical importance of calcitriol
Abnormalities in calcitriol metabolism highlight the importance of this hormone in the maintenance of calcium and phosphate homeostasis. Calcitriol deficiency results in hypocalcemia, hypophosphatemia, and vitamin D-dependent rickets. Rickets is associated with abnormalities of bone weakness, fractures, pain, as well as abnormal bone bending and deformity of the tibiae and femora. Consistent with this, mutations in the CYP27B1 gene cause vitamin D hydroxylation-deficient rickets type IA, an autosomal recessive disorder characterized by an inability to synthesize 1,25-dihydroxyvitamin D, and consequently hypocalcemia, hypophosphatemia, secondary hyperparathyroidism, and rickets. 46 1-alpha hydroxylase deficiency is rare, but there is an unusually high frequency in the French-Canadian population due to a founder effect. Fortunately, this condition responds to treatment with exogenous calcitriol.47,48 However, calcitriol supplementation is more commonly prescribed to patients with chronic kidney disease to treat secondary hyperparathyroidism and hypocalcemia which is the result of decreased calcitriol production secondary to lower levels of 1-alpha hydroxylase occurring as a result of decreasing renal mass. 49
In contrast, loss of function mutations in the CYP24A1 gene cause hypercalcemia due to the inability to inactivate calcitriol and can present as idiopathic infantile hypercalcemia or as a rare genetic cause of nephrolithiasis.8,50–53 Granulomatous diseases such as subcutaneous fat necrosis, sarcoidosis, tuberculosis, and lymphoma can contribute excess 1-alpha hydroxylase production due to macrophage activation. This results in increased calcitriol levels and hypercalcemia.54–56 These diseases highlight the importance of CYP27B1 and CYP24A1 in maintaining appropriate concentrations of calcitriol in the circulation and the impact of dysregulated activity on calcium and phosphate homeostasis. The remainder of this review therefore focuses on the signaling mechanisms regulating the expression of the CYP27B1 and CYP24A1 genes in the renal proximal tubule, and thus calcitriol levels in plasma.
Classic signaling pathways
Parathyroid hormone-mediated regulation of CYP27B1 and CYP24A1
PTH is a peptide hormone secreted by the chief and oxyphil cells of the parathyroid gland. PTH acts to increase plasma calcium by increasing renal reabsorption and bone resorption while promoting excretion of phosphate. The secretion of PTH is predominantly regulated by the extracellular calcium concentration, which is detected by the calcium-sensing receptor (CaSR) on the surface of chief and oxyphil cells of the parathyroid.57,58 Calcium ions bind to the extracellular domain of the CaSR, which at high levels activates the receptor, thereby suppressing PTH secretion when the blood calcium concentration is sufficiently high.59,60
Phosphate also affects CaSR signaling as a noncompetitive inhibitor, whereas calcium is an agonist to the receptor. 60 Elevated blood phosphate levels inhibit CaSR signaling, thus stimulating PTH secretion. 61 In the kidney, PTH inhibits phosphate reabsorption from the proximal tubule and increases renal calcium reabsorption from the distal nephron. These actions in the proximal tubule are the result of PTH binding to the G-protein-coupled type 1 PTH receptor (PTHR1) on both the apical and basolateral surfaces of proximal tubule epithelial cells. The major effects of PTH are mediated by the coupling of PTHR1 to Gs- and Gq/11-proteins, stimulating the protein kinase A (PKA) and protein kinase C (PKC) pathways, respectively. These kinases phosphorylate the sodium hydrogen exchanger regulatory factor 1 (NHERF1) a PDZ domain-containing scaffold protein, which triggers degradation of sodium-dependent phosphate transport protein 2A (NaPi-IIa), thereby attenuating phosphate reabsorption from the proximal tubule. 62
PTH also acts to increase transcription of the rate-limiting enzyme in calcitriol production, 1-alpha hydroxylase, in the proximal tubule (Figure 2).10,11,63–71 This enables PTH to further increase the calcium concentration in the blood through the actions of calcitriol on the intestine and bone. This is the classical pathway through which PTH indirectly increases intestinal calcium absorption. Simultaneously, the half-life of CYP24A1 mRNA is reduced approximately fourfold in the presence of PTH, thereby slowing the inactivation of calcitriol. 72
Figure 2.
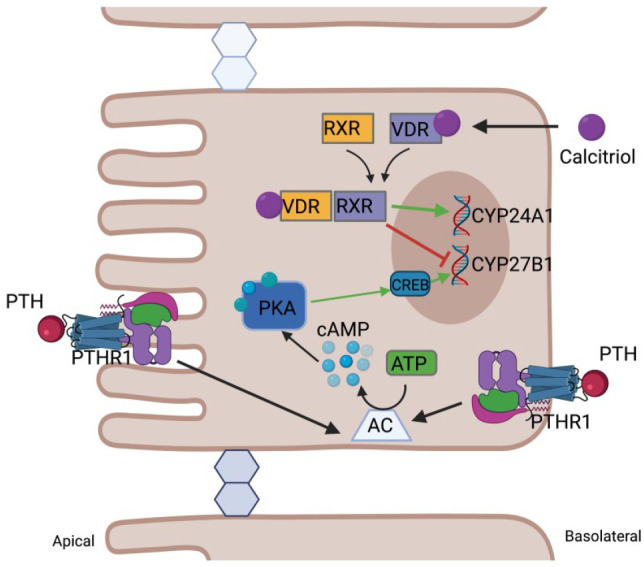
Transcriptional regulation of CYP27B1 and CYP24A1 by PTH and calcitriol in proximal tubular epithelial cells. PTH-induced transcriptional regulation occurs primarily by a PKA-mediated pathway. Upon binding to the G-protein-coupled receptor in the apical or basolateral membrane, adenylate cyclase is activated producing cAMP, which in turn activates protein kinase A (PKA). PKA phosphorylates CREB, activating it and permitting binding to CRE sites in the promoter of CYP27B1. Calcitriol signaling occurs via binding to the intracellular vitamin D receptor (VDR), which heterodimerizes with the retinoid X receptor (RXR) prior to DNA binding. Together, liganded VDR-RXR enter the nucleus where it can bind to vitamin D response elements of target genes. This increases CYP24A1 transcription, resulting in decreased calcitriol levels, and decreases CYP27B1 transcription, reducing calcitriol production. (Created with BioRender.com, supported by previous studies11,24,68,71,73). (A color version of this figure is available in the online journal.)
Evidence of the important role PTH plays in regulating calcitriol production comes from animal models. When parathyroidectomized animals are fed a low calcium diet, they fail to increase circulating levels of calcitriol, yet have increased levels of the inactive hormone produced by 24-hydroxylase. 10 However, calcitriol production was induced by administering either a parathyroid extract or PTH, consistent with PTH stimulating the production of calcitriol via increasing Cyp27b1 and reducing Cyp24a1 expression. 10 These experiments highlight the important role the parathyroid glands contribute through PTH which positively modulates the levels of calcitriol.
Further studies on parathyroidectomized and calcitriol-deficient rats have implicated cAMP as an important intracellular signal involved in the stimulation of Cyp27b1 transcription in response to PTH. 71 Notably, infusion of cAMP into parathyroidectomized rats mimicked the effect of PTH infusion by stimulating calcitriol production in a dose-dependent manner. 71 Moreover, renal adenylate cyclase activity was enhanced by PTH in rats made calcitriol-deficient by feeding them a vitamin D-deficient diet. In this study, the administration of PTH caused an immediate increase in renal cAMP levels. 71 Brenza and DeLuca 11 also provide evidence that cAMP is a second messenger involved in the induction of CYP27B1 transcription in a pig cell line, AOK-B50 cells, as well as the human HCK-8 proximal tubule cell line. In this cell culture work, forskolin, an adenylate cyclase activator that increases intracellular cAMP, increased the activity of a CYP27B1 reporter. 11 However, direct application of PTH to these cell models resulted in a significantly greater response, suggesting that PTH is potentially activating more than one second-messenger pathway to increase transcription of CYP27B1.11,68 Work done by Korkor et al. in mouse cortical kidney cell cultures (a model system composed predominantly of proximal tubule epithelial cells) further implicates cAMP as an important second messenger for PTH signaling. They found that calcitriol synthesis was dependent on de novo production of 1-alpha hydroxylase, and that calcitriol synthesis was proportional to the amount of cellular cAMP. 74 Furthermore, addition of protein or mRNA synthesis inhibitors prevented cAMP-mediated stimulation of calcitriol production, leading to the conclusion that increased calcitriol production is dependent on an increase in novel protein synthesis. 74 Brenza et al. 68 also found that the promoter for CYP27B1 contains cyclic AMP response element (CRE) sites. These are DNA binding sites for the CREB transcription factor, which is activated in response to phosphorylation by the PKA pathway. 75 These findings further implicate cAMP as the predominant second messenger mediating PTH-induced stimulation of CYP27B1 transcription. 68
Work by Zierold et al. 70 in a porcine kidney cell line (AOK-B50) identified other specific transcription factors that upregulate CYP27B1 gene production following exposure to PTH. NR4A2 (Nurr1), a nuclear receptor that binds to DNA sequences in the promoter region of the genes it regulates, has been implicated in the upregulation of CYP27B1 in response to PTH. NR4A2 over-expression increased CYP27B1 mRNA by binding to the promoter and NR4A2 mRNA levels were increased in response to PTH. 70 C/EBP was also implicated in the regulation of CYP27B1 transcription in these studies, acting directly on the promoter to decrease transcription and indirectly by reducing the amount of NR4A2 produced. 70 C/EBP is also known to upregulate CYP24A1 transcription. The overall effect of C/EBP in this work was thus a decrease in calcitriol production. However, these results are in contrast to work interrogating the role of calcitonin in regulating calcitriol expression, which is discussed below.
Feedback inhibition by calcitriol
Calcitriol exerts negative feedback on CYP27B1 transcription thereby inhibiting its own production. This negative effect on CYP27B1 expression occurs downstream of the binding of calcitriol to VDR. Calcitriol-bound VDR dimerizes with the retinoid X receptor (RXR), which in turn can bind to vitamin D response elements (VDREs) within DNA. Consistent with this concept of calcitriol feedback inhibition via nuclear signaling, inhibition of Cyp27b1 expression is absent in VDR knockout mice, who have significantly higher calcitriol levels compared with wild-type animals.24–26,36 Further, animals made calcitriol deficient by being fed a low vitamin D containing diet and limiting exposure to UV radiation were supplemented with calcitriol, they had reduced Cyp27b1 expression. 24
This is supported by work in rats. The administration of calcitriol to calcitriol-deficient rats reduced the transcription of Cyp27b1 in both the absence and presence of PTH. 11 Calcitriol also inhibits PTH gene transcription in the parathyroid to further suppress its own production.11,76 This suggests that calcitriol can override PTH-mediated stimulation of CYP27B1 expression when the concentration of calcitriol is sufficiently high, although the exact level is currently unclear. 11 It is unclear whether the ultimate effect of these two competing inputs depends on their relative concentrations or another input. Clearly, more studies are needed to determine their roles in different conditions. Finally, calcitriol also upregulates C/EBP in rat kidney, which acts directly on the Cyp27b1 promoter to inhibit its activity and reduce CYP27B1 transcription. 70 C/EBP also inhibits the stimulatory action of NR4A2 on the promoter, thereby reducing the impact of NR4A2 in the presence of PTH and reducing CYP27B1 transcription. 70
Calcitriol also acts to limit its own production by increasing the expression of CYP24A1, which encodes the 24-hydroxylase that inactivates calcitriol.6,11,77 When porcine AOK-B50 kidney cells were treated with calcitriol, CYP24A1 gene expression was stimulated in concert with suppressed CYP27B1 gene expression. 11 Transcription of CYP24A1 was upregulated by calcitriol via VDR-dependent control of VDREs in the CYP24A1 promoter.11,73 Calcitriol-induced upregulation of C/EBP in the kidney not only serves to repress CYP27B1 transcription, but also to upregulate CYP24A1 expression via C/EBP sites in the 24-hydroxylase promoter. 78 This is further supported by the observation that there is marked attenuation of CYP24A1 transcription when this site is mutated. 78 Thus, not only is there a feedback loop that inhibits further production of calcitriol, there is also feedback to promote the inactivation of calcitriol and its precursor, 25-hydroxyitamin D.
Nonclassic signaling pathways
Calcitonin
Calcitonin is a hormone released from the C-cells of the thyroid gland in response to high blood calcium levels. It binds to the G-protein-coupled calcitonin receptor, which then signals through both the PKC and PKA pathways. 12 Calcitonin protects against hypercalcemia largely by exerting a negative effect on osteoclasts in bone to prevent bone resorption and thus increases bone mineralization an effect potentially augmented by decreasing urinary calcium excretion.79–81 In this way, calcitonin may redirect calcium from the blood and urine into bone mineralization. There is evidence, however, that calcitonin also plays a role in regulating calcitriol production by increasing CYP27B1 transcription. Under hypercalcemic conditions, calcitonin may be a significant regulator of CYP27B1 expression, rather than PTH which is likely more relevant in the hypocalcemic state. 34 While PTH signals through both the PKA and PKC pathways to induce CYP27B1 transcription, calcitonin preferentially increases CYP27B1 expression via the PKC pathway as demonstrated in renal porcine LLCPK cells. 12 Although administration of the PKA activator 8-bromo-cAMP had a stimulatory effect on CYP27B1 mRNA expression, the PKA inhibitors Rp-cAMPS and H-89 had no effect on calcitonin-induced CYP27B1 expression. In contrast, the PKC activator phorbol 12-myristate 13-acetate (PMA) increased CYP27B1 mRNA levels to a similar extent as seen with calcitonin treatment, while administration of the PKC inhibitor staurosporine attenuated CYP27B1 transcription induced by calcitonin in a dose-dependent manner. 12 This strongly implicates PKC in mediating the stimulatory effect of calcitonin on calcitriol production.
The transcription factor C/EBP is also a downstream effector of calcitonin (Figure 3). Expression of this transcription factor increased following exposure to calcitonin. 13 C/EBP can bind to the promoter of CYP27B1 thereby increasing 1-alpha hydroxylase production. Further, the transfection with a dominant negative modulator of C/EBPs binding sites inhibited calcitonin induced Cyp27b1 transcription in a dose-dependent manner. 13 This is a potential mechanism by which calcium retention is increased despite a normal blood calcium concentration, thereby increasing calcium availability in the circulation in times of increased demand, such as pregnancy and lactation. 13 There is evidence therefore, supporting a role for C/EBP in both positive and negative regulatory pathways, as it is also associated with decreased CYP27B1 expression after calcitriol exposure. These seemingly contradictory effects might be explained by other yet to be identified signaling events affecting C/EBPβ gene modulation. This issue requires further investigation to elucidate the differing effects of C/EBP on Cyp27b1 expression.
Figure 3.

Transcriptional regulation of CYP27B1 and CYP24A1 by calcium, phosphate, FGF23, and calcitonin. Extracellular calcium activates the calcium sensing receptor, which in turn activates mitogen-activated protein kinase (MAPK) and protein kinase C pathways. These pathways are proposed to interact with the CYP27B1 promoter to increase its activity at low extracellular calcium concentrations and to decrease promoter activity at high extracellular calcium concentrations. Hypophosphatemia is linked to increased expression of CYP27B1. However, the mechanism behind this is unknown. Fibroblast growth factor 23 (FGF23) binds to the FGF receptor (FGFR) and its cofactor klotho to activate the MAPK pathway, phosphorylating ERK1/2, which inhibits CYP27B1 transcription. FGF23 signaling in the proximal tubule is also associated with increased CYP24A1 levels, although the mechanism behind this is unknown. Calcitonin binds to its G-protein-coupled receptor, activating both the PKA and PKC pathways. PKC increases the expression of the C/EBP transcription factor thereby promoting CYP27B1 transcription. Calcitonin can also signal through the Sp1 and NF-Y transcription factors to upregulate CYP24A1 transcription, although there may be an additional mechanism whereby CYP24A1 transcription is attenuated. (Created with BioRender.com, supported by references9,12–15,82,83.) (A color version of this figure is available in the online journal.)
There is conflicting evidence surrounding the role of calcitonin on CYP24A1 expression. Research in thyroparathyroidectomized rats, in which both PTH and calcitonin production are lost, fed a low calcium diet found twofold increased Cyp24a1 expression, however, calcitonin administration reduced Cyp24a1 mRNA expression. 82 In contrast to this, in vitro work in human embryonic kidney (HEK-293) cells that were transfected with the calcitonin receptor found that calcitonin stimulates CYP24A1 expression. In addition, H89 and calphostin C, inhibitors of the PKA and PKC pathways respectively, reduced calcitonin-induced CYP24A1 expression by 60%. 83 It was proposed that calcitonin induced the PKA or PKC pathway which phosphorylates and activates the transcription factors Sp1 and NF-Y, which were both shown to increase expression of CYP24A1. 83 Although these studies are seemingly contradictory, there is a possibility that calcitonin can exert a different effect depending on calcium and calcitriol levels, promoting calcitriol degradation when blood calcium levels are high and suppressing degradation of calcitriol when calcium levels are low. Further work is required to determine the role of calcitonin in calcitriol metabolism.
FGF23/klotho
Fibroblast growth factor-23 (FGF23) is a peptide hormone produced in bone by osteoblasts and osteocytes that inhibits renal tubular phosphate reabsorption and calcitriol production to reduce intestinal calcium and phosphate absorption.64,84 FGF23 additionally indirectly lowers serum calcium levels by decreasing PTH levels. FGF23 binds to fibroblast growth factor receptor isoforms 3 and 4, thereby activating the receptors and inducing tyrosine autophosphorylation and the stimulation of its intrinsic tyrosine kinase activity.85,86 This leads to the activation of the MAP kinase pathway and downstream phosphorylation of extracellular signal-regulated kinase-1 and -2 (ERK1/2). 87 The coreceptor, klotho, is required for binding of FGF23 to the receptor enabling its subsequent activation in the kidney.87,88 FGF23 and klotho have a suppressive role in renal CYP27B1 transcription through ERK1/2.84,87,88 HEK-293 cells transfected with the CYP27B1 promoter had suppressed CYP27B1 promoter activity when exposed to FGF23. The suppressive effect of FGF23 was blocked by a ERK1/2 inhibitor. Moreover, FGF23-null mice display threefold increased CYP27B1 promoter activity in the kidney compared with WT mice with normal FGF23 levels, consistent with FGF23 suppressing CYP27B1 expression. 9 This supports the role of FGF23 in suppressing CYP27B1 transcription.
FGF23 also lowers blood calcitriol levels by promoting renal CYP24A1 transcription, resulting in calcitriol inactivation.9,89 Consistent with this, FGF23-null mice display 63% lower Cyp24a1 mRNA expression in the kidney compared with wild-type mice. 9 Further work is needed to elucidate the mechanism driving the FGF23-mediated increase in CYP24A1 expression. Research into this area is of great interest because of potential implications in chronic kidney disease, where elevated FGF23 presents in the early phases and is accompanied by severe calcitriol deficiency. 90 Increased CYP24A1 transcription, in response to FGF23, may exacerbate calcitriol deficiency and contribute to the progression of chronic kidney disease.91,92
Hypophosphatemia
Regulation of CYP27B1 expression also occurs in response to alterations in serum phosphate concentration. Hypophosphatemia, as caused by a low phosphate containing diet, results in increased Cyp27b1 expression independently of PTH and markedly elevated serum calcitriol levels.93,14 This is supported by observations that phosphate-depleted mice exhibit enhanced calcitriol production threefold compared with control-fed mice. 94 However, this effect is abolished in these mice post-hypophysectomy suggesting that the pituitary gland has a role in sensing circulating phosphate levels and altering serum calcitriol production in response. 14 Consistent with this is evidence that pituitary hormones may exert an effect on calcitriol production through transcriptional regulation of Cyp27b1. 14 Due to the previous association of growth hormone deficiency with hypovitaminosis D, growth hormone represents a potential candidate for this role. 95 However, the exact mechanism whereby extracellular phosphate sensing occurs, or how pituitary hormones affect CYP27B1 expression remains to be elucidated.
Calcium and the calcium-sensing receptor
In addition to plasma phosphate, plasma calcium concentration also modulates calcitriol production, independent of PTH or other calciotropic hormones. Thyroparathyroidectomized rats are unable to secrete PTH or calcitonin in response to altered blood calcium levels. However, the direct infusion of CaCl2 into these animals resulted in suppression of renal Cyp27b1 expression. 65 Further, infusion of these rats with calcium and PTH simultaneously resulted in increased blood calcium levels, however, Cyp27b1 activity was suppressed compared with rats given PTH alone. Conversely, when PTH was administered with EGTA, a calcium chelator that prevents a rise in blood calcium levels, Cyp27b1 expression was stimulated. 65 These results demonstrate that higher blood calcium levels counteract the effects of PTH to suppress production of calcitriol when blood calcium levels are high. Further work is needed to fully delineate the effect of increased blood calcium on CYP24A1 expression.
The calcium-sensing receptor (CaSR) is a seven transmembrane G-protein-coupled receptor that senses the extracellular calcium concentration and negatively regulates PTH production and secretion from the parathyroid gland in response. The CaSR also has effects outside of the parathyroid gland, with expression in organs such as the brain, kidney, and intestines where it has effects on neuropathological conditions and modulate renal calcium reabsorption and intestinal calcium absorption.60,96–99 It acts to modulate the expression and activity of calcium and phosphate transporters, channels and pores, including tight junction proteins that regulate both paracellular and transcellular calcium (re)absorption across renal and intestinal epitehlia.59,97,100–102 CaSR activity has also recently been linked to the modulation of CYP27B1 expression in a HEK-293 cell model. CaSR expressing HEK-293 cells were transfected with a CYP27B1 promoter reporter construct and exposed to increasing levels of extracellular calcium. Up to and including 3 mM extracellular calcium resulted in increased reporter activity. 15 This suggests that activation of the CaSR at higher calcium concentrations signals to the CYP27B1 promoter to increase gene transcription. This is in contrast to the in vivo experiments described in the above section whereby increased blood calcium levels supressed CYP27B1 expression. Interestingly, when the extracellular concentration of calcium was raised above 3 mM there was suppression of promoter activity. 15 This group further suggests that the CaSR signals through either a PKC or ERK1/2 pathway to regulate CYP27B1 expression, since administration of inhibitors of these pathways simultaneously blocked the effect of calcium on CYP27B1 transcription, though administration of each inhibitor individually had little effect. 15 Further, whether CaSR alters CYP24A1 expression is not known. Thus, further work is required to determine the exact effects of renal CaSR activation on CYP27B1 and CYP24A1 expression and the signaling pathways involved.
Future directions
Regulation of 1-alpha and 24-hydroxylases and therefore circulating calcitriol levels is relatively unexplored beyond the actions of PTH and calcitriol. Thus, several questions remain to be answered. The signaling pathways that regulate CYP27B1 and CYP24A1 transcription need to be further delineated, particularly with respect to the direct effects of calcium and phosphate and the potential role of the CaSR in this regulation. In addition, given that the majority of the reabsorption of these minerals occurs in the proximal tubule, the same site of the majority of 1-alpha hydroxylase production, there may be a potential connection between the renal reabsorption of calcium and phosphate and the production of the enzymes that modulate calcitriol production. Further studies should investigate a potential link.
The curious results that phosphate depleted mice are unable to increase calcitriol production post-hypophysectomy requires confirmation and follow up. Further studies aimed to determine the roles of pituitary hormones on calcitriol metabolism should be completed, in particular to determine the potential role of growth hormone. Moreover, the mechanisms behind phosphate sensing and pituitary hormone effects on CYP27B1 and CYP24A1 expression remains to be determined.
Given the important role of 24-hydroxylase in regulating circulating calcitriol levels, the effect of phosphocalciotropic hormones on the expression of CYP24A1 and the mechanisms behind these actions also demands further attention.
Conclusions
This review highlights our knowledge of the regulation of renal calcitriol production. Due to its importance in calcium and phosphate regulation, it is critical to tightly control the expression of CYP27B1, which encodes the rate limiting enzyme in calcitriol production. PTH signals increased CYP27B1 expression and thus calcitriol production through cAMP and the transcription factors CREB and NR4A2. Calcitriol inhibits its own production and promotes its inactivation by decreasing CYP27B1 and increasing CYP24A1 expression, respectively. There are a number of less well-studied effectors of CYP27B1 expression and thus calcitriol levels. This includes repression by FGF23, and calcium itself possibly through CaSR signaling and stimulation by calcitonin. These less-studied pathways are key to fully understanding the regulation of calcitriol metabolism. This knowledge will be crucial in understanding vitamin D associated pathologies and help uncover new drug targets for their treatment.
Footnotes
Authors’ Contributions: KY wrote the first draft of the manuscript and MRB, CG and RTA edited for important scientific content
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Work in the Alexander Laboratory is funded by grants from the Women and Children’s Health Research Institute, which is supported by the Stollery Children’s Hospital Foundation, the Canadian Institutes of Health Research and the National Sciences and Engineering Research Council. Dr. Alexander is the Canada Research Chair in Epithelial Transport Physiology and a Stollery Science Laboratory Distinguished Research.
ORCID iDs: Megan R Beggs  https://orcid.org/0000-0002-2690-1785
https://orcid.org/0000-0002-2690-1785
R Todd Alexander  https://orcid.org/0000-0001-7396-7894
https://orcid.org/0000-0001-7396-7894
References
- 1. Renkema KY, Alexander RT, Bindels RJ, Hoenderop JG. Calcium and phosphate homeostasis: concerted interplay of new regulators. Ann Med 2008;40:82–91 [DOI] [PubMed] [Google Scholar]
- 2. Hoenderop JG, Nilius B, Bindels RJ. Calcium absorption across epithelia. Physiol Rev 2005;85:373–422 [DOI] [PubMed] [Google Scholar]
- 3. Ketteler M, Rothe H, Kruger T, Biggar PH, Schlieper G. Mechanisms and treatment of extraosseous calcification in chronic kidney disease. Nat Rev Nephrol 2011;7:509–16 [DOI] [PubMed] [Google Scholar]
- 4. Kagawa T, Kozai M, Masuda M, Harada N, Nakahashi O, Tajiri M, Yoshikawa R, Nakao M, Takei Y, Iwano M, Takeda E, Taketani Y, Yamamoto H. Sterol regulatory element binding protein 1 trans-activates 25-hydroxy vitamin D3 24-hydroxylase gene expression in renal proximal tubular cells. Biochem Biophys Res Commun 2018;500:275–82 [DOI] [PubMed] [Google Scholar]
- 5. Ghazarian JG, Yanda DM. Inhibition of 25-hydroxyvitamin D 1α-hydroxylase by renal mitochondrial protein kinase-catalyzed phosphorylation. Biochem Biophys Res Commun 1985;132:1095–102 [DOI] [PubMed] [Google Scholar]
- 6. Chandler JS, Chandler SK, Pike JW, Haussler MR. 1,25-dihydroxyvitamin D3 induces 25-hydroxyvitamin D3-24-hydroxylase in a cultured monkey kidney cell line (LLC-MK2) apparently deficient in the high affinity receptor for the hormone. J Biol Chem 1984;259:2214–22 [PubMed] [Google Scholar]
- 7. Fraser DR, Kodicek E. Unique biosynthesis by kidney of a biological active vitamin D metabolite. Nature 1970;228:764–6 [DOI] [PubMed] [Google Scholar]
- 8. Jones G, Prosser DE, Kaufmann M. 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): its important role in the degradation of vitamin D. Arch Biochem Biophys 2012;523:9–18 [DOI] [PubMed] [Google Scholar]
- 9. Chanakul A, Zhang MY, Louw A, Armbrecht HJ, Miller WL, Portale AA, Perwad F. FGF-23 regulates CYP27B1 transcription in the kidney and in extra-renal tissues. PLoS ONE 2013;8:e72816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garabedian M, Holick MF, Deluca HF, Boyle IT. Control of 25-hydroxycholecalciferol metabolism by parathyroid glands. Proc Natl Acad Sci U S A 1972;69:1673–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brenza HL, DeLuca HF. Regulation of 25-hydroxyvitamin D3 1α-hydroxylase gene expression by parathyroid hormone and 1,25-dihydroxyvitamin D3. Arch Biochem Biophys 2000;381:143–52 [DOI] [PubMed] [Google Scholar]
- 12. Yoshida N, Yoshida T, Nakamura A, Monkawa T, Hayashi M, Saruta T. Calcitonin induces 25-hydroxyvitamin D3 1-hydroxylase mRNA expression via protein kinase C pathway in LLC-PK1 cells. J Am Soc Nephrol 1999;10:2474–9 [DOI] [PubMed] [Google Scholar]
- 13. Zhong Y, Armbrecht HJ, Christakos S. Calcitonin, a regulator of the 25-hydroxyvitamin D3 1α-hydroxylase gene. J Biol Chem 2009;284: 11059–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yoshida T, Yoshida N, Monkawa T, Hayashi M, Saruta T. Dietary phosphorus deprivation induces 25-hydroxyvitamin D3 1a-hydroxylase gene expression. Endocrinol 2001;142:1720–6 [DOI] [PubMed] [Google Scholar]
- 15. Huang A, Binmahfouz L, Hancock DP, Anderson PH, Ward DT, Conigrave AD. Calcium-sensing receptors control CYP27B1-luciferase expression: transcriptional and posttranscriptional mechanisms. J Endocr Soc 2021;5:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fleet JC. The role of vitamin D in the endocrinology controlling calcium homeostasis. Mol Cell Endocrinol 2017;453:36–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Holick MF, Maclaughlin JA, Clark MB, Holick SA, Potts JT, Anderson RR, Blank IH, Parrish JA, Elias P. Photosynthesis of previtamin D3 in human skin and the physiologic consequences. Science 1980;210:203–5 [DOI] [PubMed] [Google Scholar]
- 18. Holick MF, Frommer JE, Mcneill SC, Richtand NM, Henley JW, Potts JT. Photometabolism of 7-dehydrocholesterol to previtamin D3 in skin. Biochem Biophys Res Commun 1977;76:107–14 [DOI] [PubMed] [Google Scholar]
- 19. Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev 2016;96:365–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brunette MG, Chan M, Ferrierem C, Roberts KD. Site of 1,25(OH)2 vitamin D3 synthesis in the kidney. Nature 1978;276:287–9 [DOI] [PubMed] [Google Scholar]
- 21. Kawashima H, Torikai S, Kurokawa K. Localization of 25-hydroxyvitamin D3 la-hydroxylase and 24-hydroxylase along the rat nephron. Proc Natl Acad Sci USA 1981;78:1199–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chesney RW. Interactions of vitamin D and the proximal tubule. Pediatr Nephrol 2016;31:7–14 [DOI] [PubMed] [Google Scholar]
- 23. Bajwa A, Forster MN, Maiti A, Woolbright BL, Beckman MJ. Specific regulation of CYP27B1 and VDR in proximal versus distal renal cells. Arch Biochem Biophys 2008;477:33–42 [DOI] [PubMed] [Google Scholar]
- 24. Wang Y, Zhu J, Deluca HF, Deluca HF. The vitamin D receptor in the proximal renal tubule is a key regulator of serum 1,25-dihydroxyvitamin D 3. Am J Physiol Endocrinol Metab 2015;308:201–5 [DOI] [PubMed] [Google Scholar]
- 25. Xue Y, Fleet JC. Intestinal vitamin D receptor is required for normal calcium and bone metabolism in mice. Gastroenterology 2009;136:1317–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Panda DK, Miao D, Bolivar I, Li J, Huo R, Hendy GN, Goltzman D. Inactivation of the 25-hydroxyvitamin D 1α-hydroxylase and vitamin D receptor demonstrates independent and interdependent effects of calcium and vitamin D on skeletal and mineral homeostasis. J Biolog Chem 2004;279:16754–66 [DOI] [PubMed] [Google Scholar]
- 27. Downie ML, Alexander RT. Molecular mechanisms altering tubular calcium reabsorption. Pediatr Nephrol. Epub ahead of print 1 April 2021. DOI: 10.1007/s00467-021-05049-0. [DOI] [PubMed] [Google Scholar]
- 28. Beggs MR, Young K, Pan W, O’neill DD, Saurette M, Plain A, Rievaj J, Doschak MR, Cordat E, Dimke H, Todd Alexander R. Claudin-2 and claudin-12 form independent, complementary pores required to maintain calcium homeostasis. PNAS 2021;118:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alexander RT, Dimke H, Cordat E. Proximal tubular NHEs: sodium, protons and calcium? Am J Physiol Renal Physiol 2013;305:F229–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wagner CA, Rubio-Aliaga I, Hernando N. Renal phosphate handling and inherited disorders of phosphate reabsorption: an update. Pediatr Nephrol 2019;34:549–59. [DOI] [PubMed] [Google Scholar]
- 31. Meyer MB, Benkusky NA, Kaufmann M, Lee SM, Onal M, Jones G, Pike JW. A kidney-specific genetic control module in mice governs endocrine regulation of the cytochrome P450 gene Cyp27b1 essential for vitamin D3 activation. J Biol Chem 2017;292:17541–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Meyer MB, Benkusky NA, Kaufmann M, Lee SM, Redfield RR, Jones G, Pike JW. Targeted genomic deletions identify diverse enhancer functions and generate a kidney-specific, endocrine-deficient Cyp27b1 pseudo-null mouse. Journal of Biological Chemistry 2019;294:9518–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bikle DD, Patzek S, Wang Y. Physiologic and pathophysiologic roles of extra renal CYP27b1: case report and review. Bone Rep 2018;8:255–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shinki T, Ueno Y, DeLuca HF, Suda Shinki T. Calcitonin is a major regulator for the expression of renal 25-hydroxyvitamin D3-1a-hydroxylase gene in normocalcemic rats. Proc Natl Acad Sci USA 1999;96:8253–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wongdee K, Charoenphandhu N. Vitamin D-enhanced duodenal calcium transport. Vitam Horm 2015; 98: 407–40 [DOI] [PubMed] [Google Scholar]
- 36. Haussler MR, Whitfield GK, Kaneko I, Haussler CA, Hsieh D, Hsieh JC, Jurutka PW. Molecular mechanisms of vitamin D action. Calc Tis Int 2013;92:77–98 [DOI] [PubMed] [Google Scholar]
- 37. Christakos S, Dhawan P, Ajibade D, Benn BS, Feng J, Joshi SS. Mechanisms involved in vitamin D mediated intestinal calcium absorption and in non-classical actions of vitamin D. J Steroid Biochem Mol Biol 2010;121:183–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Christakos S, Dhawan P, Porta A, Mady LJ, Seth T. Vitamin D and intestinal calcium absorption. Mol Cell Endocrinol 2011;347:25–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fleet JC, Eksir F, Hance KW, Wood RJ. Vitamin D-inducible calcium transport and gene expression in three Caco-2 cell lines. Am J Physiol Gastrointest Liver Physiol 2002;283:G618–125 [DOI] [PubMed] [Google Scholar]
- 40. Zhang YG, Wu S, Lu R, Zhou D, Zhou J, Carmeliet G, Petrof E, Claud EC, Sun J. Tight junction CLDN2 gene is a direct target of the Vitamin D receptor. Sci Rep 2015;5:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hoenderop JGJ, van der Kemp AWCM, Urben CM, Strugnell SA, Bindels RJM. Effects of vitamin D compounds on renal and intestinal Ca2+ transport proteins in 25-hydroxyvitamin D3-1α-hydroxylase knockout mice. Kidney Int 2004;66:1082–9 [DOI] [PubMed] [Google Scholar]
- 42. Levi M, Gratton E, Forster IC, Hernando N, Wagner CA, Biber J, Sorribas V, Murer H. Mechanisms of phosphate transport. Nat Rev Nephrol 2019;15:482–500 [DOI] [PubMed] [Google Scholar]
- 43. Hernando N, Gagnon K, Lederer E. Phosphate transport in epithelial and nonepithelial tissue. Physiol Rev 2021;101:1–35 [DOI] [PubMed] [Google Scholar]
- 44. Kim S, Yamazaki M, Shevde NK, Pike JW. Transcriptional control of receptor activator of nuclear factor-B ligand by the protein kinase A activator forskolin and the transmembrane glycoprotein 130-activating cytokine, oncostatin M, is exerted through multiple distal enhancers. Mol Endocrinol 2007;21:197–214. [DOI] [PubMed] [Google Scholar]
- 45. Li YC, Amling M, Pirro AE, Priemel M, Meuse J, Baron R, Delling G, Demay MB. Normalization of mineral ion homeostasis by dietary means prevents hyperparathyroidism, rickets, and osteomalacia, but not alopecia in vitamin D receptor-ablated mice. Endocrinology 1998;139:4391–6 [DOI] [PubMed] [Google Scholar]
- 46. Liberman UA. Disorders in vitamin D action. In: Feingold KR, Anawalt B, Boyce A, et al. (eds) Endotext. South Dartmouth, MA: MDText.com, 2014, pp.1–13 [Google Scholar]
- 47. De Braekeleer M, Larochelle J. Population genetics of vitamin D-dependent rickets in northeastern Quebec. Ann Hum Genet 1991;55: 283–90 [DOI] [PubMed] [Google Scholar]
- 48. Fraser D, Kooh SW, Kind P, Holick MF, Tanaka Y, DeLuca HF. Pathogenesis of hereditary vitamin-D—dependent rickets—an inborn error of vitamin D metabolism involving defective conversion of 25-hydroxyvitamin D to 1α,25-dihydroxyvitamin D. New Engl J Med 1973;289:817–22. [DOI] [PubMed] [Google Scholar]
- 49. Nigwekar SU, Tamez H, Thadhani RI. Vitamin D and chronic kidney disease-mineral bone disease (CKD-MBD). Bonekey Reports 2014;3:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. de Paolis E, Scaglione GL, de Bonis M, Minucci A, Capoluongo E. CYP24A1 and SLC34A1 genetic defects associated with idiopathic infantile hypercalcemia: from genotype to phenotype. Clin Chem Lab Med 2019;57:1650–67 [DOI] [PubMed] [Google Scholar]
- 51. Jiráčková J, Hyšpler R, Alkanderi S, Pavlíková L, Palicka V, Sayer JA. Novel CYP24A1 mutation in a young male patient with nephrolithiasis: case report. Kidney Blood Press Res 2019;44:870–7 [DOI] [PubMed] [Google Scholar]
- 52. Tebben PJ, Singh RJ, Kumar R. Vitamin D-mediated hypercalcemia: mechanisms, diagnosis, and treatment. Endocr Rev 2016;37:521–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schlingmann KP, Kaufmann M, Weber S, Irwin A, Goos C, John U, Misselwitz J, Klaus G, Kuwertz-Bröking E, Fehrenbach H, Wingen AM, Güran T, Hoenderop JG, Bindels RJ, Prosser DE, Jones G, Konrad M. Mutations in CYP24A1 and idiopathic infantile hypercalcemia. New Engl J Med 2011;365:410–21 [DOI] [PubMed] [Google Scholar]
- 54. Inui N, Murayama A, Sasaki S, Suda T, Chida K, Kato S, Nakamura H. Correlation between 25-hydroxyvitamin D3 1-hydroxylase gene expression in alveolar macrophages and the activity of sarcoidosis. Am J Med 2001;110:687–93 [DOI] [PubMed] [Google Scholar]
- 55. Donovan PJ, Sundac L, Pretorius CJ, d’Emden MC, McLeod DS. Calcitriol-mediated hypercalcemia: causes and course in 101 patients. J Clin Endocrinol Metab 2013;98:4023–9 [DOI] [PubMed] [Google Scholar]
- 56. Ghirri P, Bottone U, Coccoli L, Bernardini M, Vuerich M, Cuttano A, Riparbelli C, Pellegrinetti G, Boldrini A. Symptomatic hypercalcemia in the first months of life- calcium-regulating hormones and treatment. J Endocrinol Invest 1999;22:349–53 [DOI] [PubMed] [Google Scholar]
- 57. Chen RA, Goodman WG. Role of the calcium-sensing receptor in parathyroid gland physiology. Am J Physiol Renal Physiol 2004;286:F1005–111 [DOI] [PubMed] [Google Scholar]
- 58. Ritter CS, Haughey BH, Miller B, Brown AJ. Differential gene expression by oxyphil and chief cells of human parathyroid glands. J Clin Endocrinol Metab 2012;97:E1499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Conigrave AD, Ward DT. Calcium-sensing receptor (CaSR): pharmacological properties and signaling pathways. Best Pract Res Clin Endocrinol Metab 2013;27:315–31 [DOI] [PubMed] [Google Scholar]
- 60. Tan RSG, Lee CHL, Dimke H, Todd Alexander R. The role of calcium-sensing receptor signaling in regulating transepithelial calcium transport. Exp Biol Med (Maywood) 2021;246:2407–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Centeno PP, Herberger A, Mun HC, Tu C, Nemeth EF, Chang W, Conigrave AD, Ward DT. Phosphate acts directly on the calcium-sensing receptor to stimulate parathyroid hormone secretion. Nat Commun 2019;10:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lee JJ, Plain A, Beggs MR, Dimke H, Alexander RT. Effects of phospho- and calciotropic hormones on electrolyte transport in the proximal tubule. F1000Res 2017;6:1797–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fraser DR, Kodicek E. Regulation of 25-hydroxycholecalciferol-1-hydroxylase activity in kidney by parathyroid hormone. Nature 1973;241:163–6 [DOI] [PubMed] [Google Scholar]
- 64. Blau JE, Collins MT. The PTH-vitamin D-FGF23 axis. Rev Endocr Metab Disord 2015;16:165–74 [DOI] [PubMed] [Google Scholar]
- 65. Matsumoto T, Ikeda K, Morita K, Fukumoto S, Takahashi H, Ogata E. Blood Ca2+ modulates responsiveness of renal 25(OH)D3-Ia-hydroxylase to PTH in rats. Am J Physiol 1987;253:E503–157 [DOI] [PubMed] [Google Scholar]
- 66. Duque EJ, Elias RM, Moysés RMA. Parathyroid hormone: a uremic toxin. Toxins 2020;12:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Goltzman D, Mannstadt M, Marcocci C. Physiology of the calcium-parathyroid hormone-vitamin D axis. Front Horm Res 2018;50:1–13 [DOI] [PubMed] [Google Scholar]
- 68. Brenza HL, Kimmel-Jehan C, Jehan F, Shinki T, Wakino S, Anazawa H, Suda T, Deluca HF. Parathyroid hormone activation of the 25-hydroxyvitamin D3-1α-hydroxylase gene promoter. Proc Natl Acad Sci USA 1998;95:1387–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Armbrecht HJ, Boltz MA, Ritter CS, Brown AJ. Parathyroid hormone stimulation of the renal 25-hydroxyvitamin D-1α-hydroxylase-effect of age and free radicals. J Ster Biochem Mol Biol 2007;103:330–3 [DOI] [PubMed] [Google Scholar]
- 70. Zierold C, Nehring JA, Deluca HF. Nuclear receptor 4A2 and C/EBPβ regulate the parathyroid hormone-mediated transcriptional regulation of the 25-hydroxyvitamin D3-1α-hydroxylase. Arch Biochem Biophys 2007;460:233–9 [DOI] [PubMed] [Google Scholar]
- 71. Horiuchi N, Suda T, Takahashi H, Shimazawa E, Ogata E. In vivo evidence for the intermediary role of 3,’5’-cyclic AMP in parathyroid hormone-induced stimulation of 1α,25-dihydroxy vitamin D3 synthesis in rats. Endocrinol 1977;101:969–74 [DOI] [PubMed] [Google Scholar]
- 72. Zierold C, Mings JA, Deluca HF. Parathyroid hormone regulates 25-hydroxyvitamin D3-24-hydroxylase mRNA by altering its stability. PNAS 2001;98:13572–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zierold C, Darwish HM, Deluca HF. Two vitamin D response elements function in the rat 1,25-dihydroxyvitamin D 24-hydroxylase promoter. J Biol Chem 1995;270:1675–8 [DOI] [PubMed] [Google Scholar]
- 74. Korkor AB, Gray RW, Henry HL, Kleinman JG, Blumenthal SS, Garancis JC. Evidence that stimulation of 1,25(OH)2D3 production in primary cultures of mouse kidney cells by cyclic AMP requires new protein synthesis. J Bone Miner Res 1987;2:517–24 [DOI] [PubMed] [Google Scholar]
- 75. Sassone-Corsi P. The cyclic AMP pathway. Cold Spring Harbor Pers Biol 2012;4:1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Russell J, Lettieri D, Sherwood LM. Supression by 1,25(OH)2D3 of transcription of the pre-proparathyroid hormone gene. Endocrinol 1986;119:2864–6 [DOI] [PubMed] [Google Scholar]
- 77. Tanaka Y, DeLuca HF. Rat renal 25-hydroxyvitamin D3 1- and 24-hydroxylases: their in vivo regulation. Am J Physiol 1984;246:E168–73 [DOI] [PubMed] [Google Scholar]
- 78. Dhawan P, Peng X, Sutton ALM, MacDonald PN, Croniger CM, Trautwein C, Centrella M, McCarthy TL, Christakos S. Functional cooperation between CCAAT/enhancer-binding proteins and the vitamin D receptor in regulation of 25-hydroxyvitamin D 3 24-hydroxylase. Mol Cell Biol 2005;25:472–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Turner AG, Tjahyono F, Chiu WS, Skinner J, Sawyer R, Moore AJ, Morris HA, Findlay DM, Zajac JD, Davey RA. The role of the calcitonin receptor in protecting against induced hypercalcemia is mediated via its actions in osteoclasts to inhibit bone resorption. Bone 2011;48:354–61 [DOI] [PubMed] [Google Scholar]
- 80. Davey RA, Turner AG, McManus JF, Chiu WS, Tjahyono F, Moore AJ, Atkins GJ, Anderson PH, Ma C, Glatt V, MacLean HE, Vincent C, Bouxsein M, Morris HA, Findlay DM, Zajac JD. Calcitonin receptor plays a physiological role to protect against hypercalcemia in mice. J Bone Miner Res 2008;23:1182–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hsu YJ, Dimke H, Hoenderop JG, Bindels RJ. Calcitonin-stimulated renal Ca2+ reabsorption occurs independentlyof TRPV5. Nephrol Dial Transplant 2010;25:1428–35 [DOI] [PubMed] [Google Scholar]
- 82. Beckman MJ, Goff JP, Reinhardt TA, Beitz DC, Horst RL. In vivo regulation of rat intestinal 24-hydroxylase: potential new role of calcitonin. Endocrinology 1994;135:1951–5 [DOI] [PubMed] [Google Scholar]
- 83. Gao XH, Dwivedi PP, Omdahl JL, Morris HA, May BK. Calcitonin stimulates expression of the rat 25-hydroxyvitamin D3-24-hydroxylase (CYP24) promoter in HEK-293 cells expressing calcitonin receptor: identification of signaling pathways. J Mol Endocrinol 2004;32:87–98 [DOI] [PubMed] [Google Scholar]
- 84. Lang F, Leibrock C, Pandyra AA, Stournaras C, Wagner CA, Föller M. Phosphate homeostasis, inflammation and the regulation of FGF-23. Kidney Blood Press Res 2018;43:1742–8 [DOI] [PubMed] [Google Scholar]
- 85. Erben RG. Pleiotropic actions of FGF23. Toxicol Pathol 2017;45:904–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Li H, Martin A, David V, Quarles LD. Compound deletion of Fgfr3 and Fgfr4 partially rescues the Hyp mouse phenotype. Am J Physiol Endocrinol Metab 2011;300:E508–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Perwad F, Zhang MYH, Tenenhouse HS, Portale AA. Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1α-hydroxylase expression in vitro. Am J Physiol—Renal Physiol 2007;293:F1577–83 [DOI] [PubMed] [Google Scholar]
- 88. Takenaka T, Inoue T, Miyazaki T, Hayashi M, Suzuki H. Xeno-klotho inhibits parathyroid hormone signaling. J Bone Miner Res 2016;31:455–62 [DOI] [PubMed] [Google Scholar]
- 89. Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 2004;19:429–35 [DOI] [PubMed] [Google Scholar]
- 90. Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, Andress DL. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int 2007;71:31–8 [DOI] [PubMed] [Google Scholar]
- 91. Quarles LD. Skeletal secretion of FGF-23 regulates phosphate and vitamin D metabolism. Nat Rev Endocrinol 2015;8:276–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Williams S, Malatesta K, Norris K. Vitamin D and chronic kidney disease. Ethnic Dis 2009;19:1–7 [PMC free article] [PubMed] [Google Scholar]
- 93. Tanaka Y, Deluca HF. The control of 25-hydroxyvitamin D metabolism by inorganic phosphorus. Arch Biochem Biophys 1973;154:566–74 [DOI] [PubMed] [Google Scholar]
- 94. Fujiwara I, Aravindan R, Horst RL, Drezner MK. Abnormal regulation of renal 25-hydroxyvitamin D-1a-hydroxylase activity in X-linked hypophosphatemia: a translational or post-translational defect. J Bone Min Res 2003;18:434–42 [DOI] [PubMed] [Google Scholar]
- 95. Esposito S, Leonardi A, Lanciotti L, Cofini M, Muzi G, Penta L. Vitamin D and growth hormone in children: a review of the current scientific knowledge. J Transl Med 2019;17:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Yano S, Brown EM, Chattopadhyay N. Calcium-sensing receptor in the brain. Cell Calcium 2004;35:257–64 [DOI] [PubMed] [Google Scholar]
- 97. Topala CN, Schoeber JP, Searchfield LE, Riccardi D, Hoenderop JG, Bindels RJ. Activation of the Ca2+-sensing receptor stimulates the activity of the epithelial Ca2+ channel TRPV5. Cell Calc 2009;45:331–9 [DOI] [PubMed] [Google Scholar]
- 98. Toka HR, Pollak MR, Houillier P. Calcium sensing in the renal tubule. Physiol (Bethesda) 2015;30:317–26 [DOI] [PubMed] [Google Scholar]
- 99. Giudice M, lo Mihalik B, Dinnyés A, Kobolák J. The nervous system relevance of the calcium sensing receptor in health and disease. Molecules 2019;24:1–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Brown EM. Role of the calcium-sensing receptor in extracellular calcium homeostasis. Best Pract Res Clin Endocrinol Metab 2013;27:333–43. [DOI] [PubMed] [Google Scholar]
- 101. Lee JJ, Liu XO, ’ Neill D, Beggs MR, Weissgerber P, Flockerzi V, Chen XZ, Dimke H, Todd Alexander R. Activation of the calcium-sensing receptor attenuates TRPV6-dependent intestinal calcium absorption. JCI Insight 2019;4:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Dimke H, Desai P, Borovac J, Lau A, Pan W, Alexander RT. Activation of the Ca2+-sensing receptor increases renal claudin-14 expression and urinary Ca2+ excretion. Am J Physiol Renal Physiol 2013;304:F761–79 [DOI] [PMC free article] [PubMed] [Google Scholar]


