Abstract
Given that a large proportion of the bacteria colonizing the roots of plants is capable of producing N-acyl-l-homoserine lactone (AHL) molecules, it appears likely that these bacterial pheromones may serve as signals for communication between cells of different species. In this study, we have developed and characterized novel Gfp-based monitor strains that allow in situ visualization of AHL-mediated communication between individual cells in the plant rhizosphere. For this purpose, three Gfp-based AHL sensor plasmids that respond to different spectra of AHL molecules were transferred into AHL-negative derivatives of Pseudomonas putida IsoF and Serratia liquefaciens MG1, two strains that are capable of colonizing tomato roots. These AHL monitor strains were used to visualize communication between defined bacterial populations in the rhizosphere of axenically grown tomato plants. Furthermore, we integrated into the chromosome of AHL-negative P. putida strain F117 an AHL sensor cassette that responds to the presence of long-chain AHLs with the expression of Gfp. This monitor strain was used to demonstrate that the indigenous bacterial community colonizing the roots of tomato plants growing in nonsterile soil produces AHL molecules. The results strongly support the view that AHL signal molecules serve as a universal language for communication between the different bacterial populations of the rhizosphere consortium.
In recent years, it has become evident that bacteria not only exist as individual cells but also often coordinate their activities and act in a concerted manner similar to that of multicellular organisms. Such interactions require sophisticated cell-cell communication systems to adjust the various functions within a bacterial community. In fact, many gram-negative bacteria have been shown to produce N-acyl-l-homoserine lactone (AHL) signal molecules, which are utilized by the bacteria to monitor their own population densities in a process known as quorum sensing (for reviews, see references 11 and 15). These regulatory systems typically rely on two proteins, an AHL synthase, usually a member of the LuxI family of proteins, and an AHL receptor protein, belonging to the LuxR family of transcriptional regulators. At low population densities, cells produce a basal level of AHL via the activity of the AHL synthase. As the cell density increases, AHLs accumulate in the growth medium. On reaching a critical threshold concentration, the AHL molecule binds to its cognate receptor, which in turn activates or represses the expression of target genes.
AHL-based quorum-sensing systems have been identified in a number of plant-associated bacteria and have been shown to control the expression of various functions in response to the density of the population (for a recent review, see reference 35). In Agrobacterium tumefaciens, the causative agent of crown gall tumors, a quorum-sensing system relying on N-(3-oxo-octanoyl)-l-homoserine lactone (3-oxo-C8-HSL) is involved in the regulation of Ti plasmid conjugative transfer (36, 53). In plant-pathogenic bacteria which belong to the genus Erwinia and which cause soft rot disease, the production of cell wall-degrading enzymes as well as the synthesis of the antibiotic carbapenem is subject to N-(3-oxo-hexanoyl)-l-homoserine lactone (3-oxo-C6-HSL)-mediated gene regulation (28). Similarly, 3-oxo-C6-HSL is produced by Pantoea stuartii, the causative agent of Stewart's wilt and leaf blight of corn, and was shown to control the production of an exopolysaccharide capsule which represents the major virulence determinant of the bacterium (47). It is generally believed that the expression of virulence determinants at high cell densities ensures a rapid and coordinated attack to overwhelm the plant before it is able to mount an efficient defense response.
Furthermore, quorum-sensing systems have also been identified in bacteria which are beneficial for the plant. A well-known example is Pseudomonas aureofaciens strain 30-84, which is used as a biocontrol agent to protect wheat from take-all disease, caused by Gaeumannomyces graminis var. tritici. When this strain is present in the rhizosphere of wheat, the severity of the disease is strongly reduced due to the production of phenazine antibiotics which are active against the ascomycete fungus. The synthesis of these antibiotic compounds has been demonstrated to be regulated by a quorum-sensing system that utilizes the AHL molecule N-hexanoyl-l-homoserine lactone (C6-HSL) (51). Recent work has shown that in Rhizobium leguminosarum, at least four different AHL production loci direct the synthesis of multiple AHLs (27). Among these molecules is N-(3-hydroxy-7-cis-tetradecenoyl)-l-homoserine lactone (18, 40), a compound which was first referred to as small bacteriocin because it inhibits the growth of certain sensitive strains of R. leguminosarum (49). The highly sophisticated quorum-sensing network which operates in R. leguminosarum is involved in the control of various functions, including stationary-phase adaptation (44), the ability to nodulate peas (39), and the conjugal transfer of plasmid pRL1JI (27).
Despite rapidly increasing knowledge of the molecular mechanisms of the quorum-sensing cascades in various plant-associated bacteria, little information exists about whether AHLs are in fact produced under in situ conditions. In previous reports, circumstantial evidence was presented that P. aureofaciens produces C6-HSL when colonizing the rhizosphere of wheat (34, 51). Moreover, based on cross-stimulation of transcriptional fusions of reporter genes lacZ and inaZ to the phenazine biosynthetic operon, it was inferred that AHL molecules can serve as interpopulation signals in the wheat rhizosphere (34). However, no information is currently available on AHL-mediated communication in the rhizosphere of a dicot plant species.
The aim of this study was to develop novel tools that are suitable for in situ visualization of AHL-mediated communication between individual cells in the rhizosphere of tomato plants. This was accomplished by transferring three different Gfp-based AHL sensor plasmids into AHL-negative derivatives of two plant-associated bacteria, Pseudomonas putida IsoF and Serratia liquefaciens MG1. The resulting strains were rigorously characterized with respect to their sensitivities for various AHL molecules. These AHL monitor strains were then used to monitor AHL-based communication between cells of selected bacterial strains in the rhizosphere of axenically grown tomato plants in a nondestructive, online manner. Furthermore, using a P. putida monitor strain carrying a chromosomally integrated long-chain AHL sensor cassette, we demonstrated that the indigenous bacterial community of the tomato rhizosphere in fact produces AHLs.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. All strains were grown aerobically in modified Luria-Bertani (LB) medium (4) containing 4 g of NaCl/liter instead of 10 g of NaCl/liter at 30 or 37°C (Escherichia coli). Solid media contained 15 g of agar/liter. Antibiotics were added as required at final concentrations of 10 mg of tetracycline/liter, 25 mg of gentamicin/liter, 50 mg of kanamycin/liter, and 100 mg of ampicillin/liter. Root-associated bacteria were isolated as follows. Root samples were collected and rinsed in sterile phosphate-buffered saline (PBS) to remove adhering soil particles. One gram of root material was then macerated in 9 ml of sucrose solution (4% [wt/vol]) with a mortar and pestle. Serial 10-fold dilutions of the suspensions were prepared, and 100-μl aliquots were plated on LB agar. Single colonies were further purified and screened for AHL production by cross-streaking against E. coli MT102(pSB403) (50) as described below.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| Bacterial strains | ||
| Pseudomonas putida IsoF | Wild-type isolate from tomato roots | This work |
| Pseudomonas putida F117 | AHL-negative derivative of IsoF; PpuI− | This work |
| Pseudomonas putida KS35 | F117 with integrated PlasB-gfp(ASV) Plac-lasR cassette | This work |
| Pseudomonas putida Z2D | Wild-type isolate from tomato roots | This work |
| Serratia liquefaciens MG44 | AHL-negative mutant of S. liquefaciens MG1; SwrI− | 12 |
| Rahnella aquatilis T13 | Wild-type isolate from tomato roots | This work |
| Rahnella aquatilis TAA | Wild-type isolate from tomato roots | This work |
| Escherichia coli MT102 | araD139 (ara-leu)7697 Δlac thi hsdR | Laboratory collection |
| Escherichia coli HB101 | recA thi pro leu hsdR−M+ Smr | 24 |
| Escherichia coli CC118 (λpir) | Δ(ara-leu) araD ΔlacX74 galE galK phoA20 thi-I rpsE rpoB argE(Am) recA1 λpir lysogen | 20 |
| Plasmids | ||
| pAS-C8 | pBBR1MCS-5 carrying Pcepl-gfp(ASV)-Plac-cepR; Gmr | 38 |
| pKR-C12 | pBBR1MCS-5 carrying PlasB-gfp(ASV)-Plac-lasR; Gmr | 38 |
| pTn5-LAS | pTn5-based delivery plasmid carrying PlasB-gfp(ASV) Plac-lasR; Gmr Apr | Hentzer et al., submitted |
| pJBA132 | pME6031 carrying luxR-PluxR-Plux1-gfp(ASV)-T0-T1; Tetr | 1 |
| pSB403 | Bioluminescent broad-host-range AHL sensor plasmid; Tetr | 50 |
| pUT-Kan-dsred | pUT-Kan carrying Plac-rfp-T0-T1; Kanr Apr | 45 |
| pTn5-Red | Tn5-based delivery plasmid carrying Plac-dsred-T0-T1; Tetr Apr | Hentzer et al., submitted |
| pGEM-3Zf(+) | lacZα cloning vector; Apr | Promega, Madison, Wis. |
| pBBR1MCS-5 | Broad-host-range plasmid; lacZα; Gmr | 25 |
| pRK600 | ColE1 RK2-Mob+ RK2-Tra+; helper plasmid; Cmr | 8 |
To identify strains of interest, their 16S ribosomal DNAs (rDNAs) were sequenced. Amplification of 16S rDNAs (E. coli positions 8 to 1526) was performed using primers 616V (5′-AGAGTTTGATYMTGGCTCAG-3′) and 630R (5′-CAKAAAGGAGGTGATCC-3′). The amplicons were then sequenced using primers 607V (5′-GGGCTACACACGTGC-3′), 609V (5′-GGATTAGATACCCBDGTA-3′), 610RII (5′-ACCGCKRCTGCTGGCAC-3′), and 612II (5′-GTAAGGTTYTNCGCGT-3′). For phylogenetic affiliation of the strains, the sequences were analyzed using the ARB software package (http://www.arb-home.de).
Tagging of bacterial strains with Gfp and DsRed.
To monitor single bacterial cells in the tomato rhizosphere, strains were tagged with green fluorescent protein (Gfp) or red fluorescent protein (DsRed). To avoid loss of the marker in the absence of antibiotic pressure, a PA1/04/03-gfp-T0-T1 (2) or a PA1/04/03-dsred-T0-T1 (45) transposon cassette was randomly inserted into the chromosomes of respective strains using the delivery vector pUTkan or pUTtc. Plasmids were mobilized from E. coli CC118(λpir) to the respective strains by triparental mating using E. coli HB101(pRK600) as a helper as described previously (7). Fluorescent exconjugants that grew indistinguishably from the parental strains on plates and on tomato roots (data not shown) were used in the experiments described here.
Characterization of AHL monitor strains.
To determine the specificity and sensitivity of the different Gfp-based AHL monitor strains, respective overnight cultures were diluted four times into fresh LB medium, incubated for 1 h at 30°C, and then distributed in 200-μl aliquots into wells of a microtiter plate. 3-oxo-C12-HSL [N-(3-oxo-dodecanoyl)-l-homoserine lactone], 3-oxo-C10-HSL [N-(3-oxo-decanoyl)-l-homoserine lactone], 3-oxo-C6-HSL, C12-HSL [N-dodecanoyl-l-homoserine lactone], C10-HSL [N-decanoyl-l-homoserine lactone], C8-HSL [N-octanoyl-l-homoserine lactone], C6-HSL, and C4-HSL [N-butanoyl-l-homoserine lactone] were added to the wells at final concentrations of 10,000, 5,000, 2,500, 1,250, 625, 310, 160, 80, 40, and 20 nM. Following 6 h of incubation at 30°C, green fluorescence was measured using the microtiter plate reader Lambda Fluoro 320 Plus (MWG Biotech, Ebersberg, Germany) with an excitation wavelength of 474 nm and emission detection at 515 nm. Data were processed with KC4 software (Bio-Tek Instruments). The fluorescence measurements were corrected for autofluorescence and plotted as a function of AHL concentrations. Synthetic AHL molecules either were purchased from Fluka Chemie AG, Buchs, Switzerland, or were kindly provided by P. Williams (School of Pharmaceutical Sciences, University of Nottingham, Nottingham, United Kingdom).
Characterization of AHLs produced by rhizosphere isolates.
The production of AHLs by bacterial isolates was investigated with the aid of the bioluminescent sensor plasmid pSB403 (50). This sensor plasmid contains the Photobacterium fischeri luxR gene together with the luxI promoter region as a transcriptional fusion to bioluminescence genes luxCDABE of Photorhabdus luminescens. The quorum-sensing system of P. fischeri relies on 3-oxo-C6-HSL, and the sensor plasmid consequently exhibits the highest sensitivity for this AHL molecule. However, several other AHL molecules are detected by the sensor, albeit with somewhat reduced sensitivity (50). Bioluminescence was detected either with a highly sensitive photon-counting camera (C2400-40; Hamamatsu Photonics, Herrsching, Germany) or by exposure of an X-ray film (X-Ray-90; AGFA-Gevaert, Munich, Germany). We also used different Gfp-based biosensors for the detection of AHLs. For this, the production of AHLs was monitored as the expression of green fluorescence. This was accomplished by illuminating plates with blue light using an HQ 480/40 filter (F44-001; AHF-Analysentechnik, Tübingen, Germany) in combination with a halogen lamp (Intralux 5000-1; Volpi, Schlieren, Switzerland) as a light source. Illumination took place in a dark box equipped with the C2400-40 camera connected to a Pentax CCTV camera lens and an HQ 535/20 filter (F42-001; AHF-Analysentechnik).
For a more detailed analysis, the AHL molecules were extracted from spent culture supernatants of the strains and separated by thin-layer chromatography (TLC), and AHL spots were visualized by overlaying the TLC plates with soft agar seeded with the sensor strain E. coli MT102(pSB403) as described previously (17, 41). Routinely, AHLs were extracted twice with dichloromethane (250:100 supernatant/dichloromethane) from 250 ml of sterile filtered supernatants of cultures grown in LB medium at 30°C to an optical density at 600 nm of 1.0. The combined extracts were dried over anhydrous magnesium sulfate, filtered, and evaporated to dryness. Residues were dissolved in 250 μl of ethyl acetate. Ten-microliter samples were applied to C18 reversed-phase TLC plates (Merck no. 1.15389) and dried with a stream of cold air. Samples were separated by using methanol (60% [vol/vol]) in water as the mobile phase. For the detection of AHLs, each TLC plate was overlaid with a thin film of 0.8% (wt/vol) LB agar (143 ml) seeded with 7 ml of an exponentially grown AHL biosensor and then was incubated at 30°C for 24 h. The tentative identification of AHLs present in spent culture supernatant extracts was achieved by comparison of mobilities (Rf values) to those for the synthetic AHL standards.
Growth of tomato seedlings in a gnotobiotic test system.
Tomato seeds (Lycopersicon esculentum Micro-Tom) (31) provided by B. Nebelung, Everswinkel, Germany, were surface sterilized by soaking first in 70% ethanol for 3 min and subsequently in a 5% sodium hypochlorite solution (with 0.6% Tween 20) for 20 min. Seeds were thoroughly rinsed five times in sterile water and sown in Phytatrays (Sigma, Taufkirchen, Germany) filled with 250 g of sterile quartz sand and 25 ml of sterile nutrient solution (42). The Phytatrays were covered with a lid, sealed with Parafilm, and incubated for 14 to 21 days in a growth chamber at 25°C (day) and 20°C (night) with 70% humidity and a 14 h-10 h day-night cycle. Plants were inoculated with bacteria 10 days after seeding (4 to 5 days after germination). Bacterial strains were grown overnight in 250 ml of LB medium with antibiotics when necessary and harvested by centrifugation. Cells were washed in 10 mM MgSO4 and diluted in 10 mM MgSO4 to a final concentration of 109 CFU/ml. Three milliliters of the bacterial suspension was used to inoculate a Phytatray with five tomato plants. For inoculation of the seedlings with two strains, AHL producer and monitor strains were mixed in a 1:1 ratio, and 3 ml of the mixture was used to inoculate one Phytatray.
Growth of tomato seedlings in potting soil.
To examine molecular communication in the naturally occurring rhizosphere of tomato plants, tomato seedlings were grown in nonsterile substrate (46). Seeds were surface sterilized as described above, sown in potting substrate (Fruehstorfer Einheitserde, type T; Patzer, Simmtal-Jossa, Germany), and incubated as described above. Plants were inoculated five weeks after seeding with 10 ml of bacterial suspension per plant as described above.
Confocal laser scanning microscopy.
All microscopic observations and image acquisitions for root-associated bacteria were performed using an LSM 510 scanning confocal microscope (Zeiss, Jena, Germany) equipped with an Ar ion laser (Gfp: excitation, 488 nm; emission filter BP 505-550) and an HeNe laser (DsRed: excitation, 543 nm; emission filter LP 560) together with the standard software package delivered with the instrument (version 2.1). Tomato roots were sampled for microscopy 1 to 10 days postinoculation. The roots were washed in PBS to remove sand particles, mounted in PBS on a microscope slide, and examined immediately.
RESULTS
Construction and characterization of Gfp-based AHL monitor strains suitable for in situ studies of cell-cell communication in the rhizosphere.
In order to monitor a maximum of different AHL molecules, we used three Gfp-based AHL sensor plasmids in this study. Each of these sensor plasmids is capable of detecting a certain range of AHL molecules, depending on the components used for its construction.
The sensor plasmid pJBA132 (1) is based on components of the lux quorum-sensing system of Vibrio fischeri and contains a PluxI-gfp(ASV) transcriptional fusion together with the luxR gene on the broad-host-range plasmid pME6031. The lux quorum-sensing system utilizes 3-oxo-C6-HSL (10), and the sensor plasmid consequently exhibits the highest sensitivity for this signal molecule. However, this sensor plasmid is also responsive to various related AHL molecules, albeit with decreased sensitivity (1). Noteworthy is the fact that for the construction of pJBA132 (as well as for that of the AHL sensors described below), an unstable variant of Gfpmut3*, namely, Gfp(ASV), was used (2). This Gfp variant carries a C-terminal peptide tag which makes the protein prone to degradation by housekeeping-intracellular tail-specific proteases (Clp). Due to the high rate of turnover of the reporter protein, online monitoring of transient communication is possible.
Both plasmid pKR-C12 (38) and the hybrid transposon mini Tn5-LAS (19a) contain an AHL sensor cassette which is based on components of the las quorum-sensing system of Pseudomonas aeruginosa. Specifically, this cassette consists of a PlasB-gfp(ASV) translational fusion together with the lasR gene placed under the control of Plac. As expected, because the cognate AHL of the las system is 3-oxo-C12-HSL (33), this system is most sensitive for 3-oxo-C12-HSL and other long-chain AHL molecules.
Finally, for the detection of AHL molecules with medium-length acyl side chains, we used plasmid pAS-C8 (38). This sensor plasmid was constructed from components of the cep system of Burkholderia cepacia, which utilizes the signal molecule C8-HSL (17, 26), and contains a PcepI-gfp(ASV) translational fusion together with the cepR gene transcribed from the Plac promoter of the broad-host-range plasmid pBBR1MCS-5. This sensor plasmid responds most efficiently to C8-HSL and, with a lower efficiency, to related AHL molecules.
As we intended to use these sensor plasmids for the analysis of AHL-mediated communication between bacterial cells in the rhizosphere, we transferred them to two strains capable of colonizing tomato roots. P. putida IsoF was originally isolated from the tomato rhizosphere and was subsequently shown to be an excellent root colonizer (data not shown). Since this strain produces various AHL molecules due to the presence of the ppuI AHL synthase gene, a defined ppuI knockout mutant, F117, which no longer produces AHLs (data not shown), was used as the host. Likewise, S. liquefaciens MG44 (12), a defined AHL-negative derivative of wild-type MG1, was used as a host for the AHL sensor plasmids. S. liquefaciens MG1, which was isolated from the surface of a cucumber (16), can persist for extended periods of time in the rhizosphere of tomatoes (data not shown). Both P. putida and S. liquefaciens are typical inhabitants of the rhizosphere, and the presence of members of these genera often promotes the growth of the host plant (22, 29, 48).
The presence of the AHL sensor cassettes on mobilizable broad-host-range vectors enabled us to transfer them to the AHL-negative strains P. putida F117 and S. liquefaciens MG44, giving rise to six different monitor strains. Recent work has shown that both the specificity and the sensitivity of the AHL sensor plasmids described above are dependent to some degree on the strain background (1, 38; Hentzer et al., submitted). We therefore characterized the various monitor strains with respect to their sensitivity for different AHL molecules. This was accomplished by measuring the Gfp fluorescence of cultures exposed to various AHL concentrations in a microtiter dish assay as described in Materials and Methods.
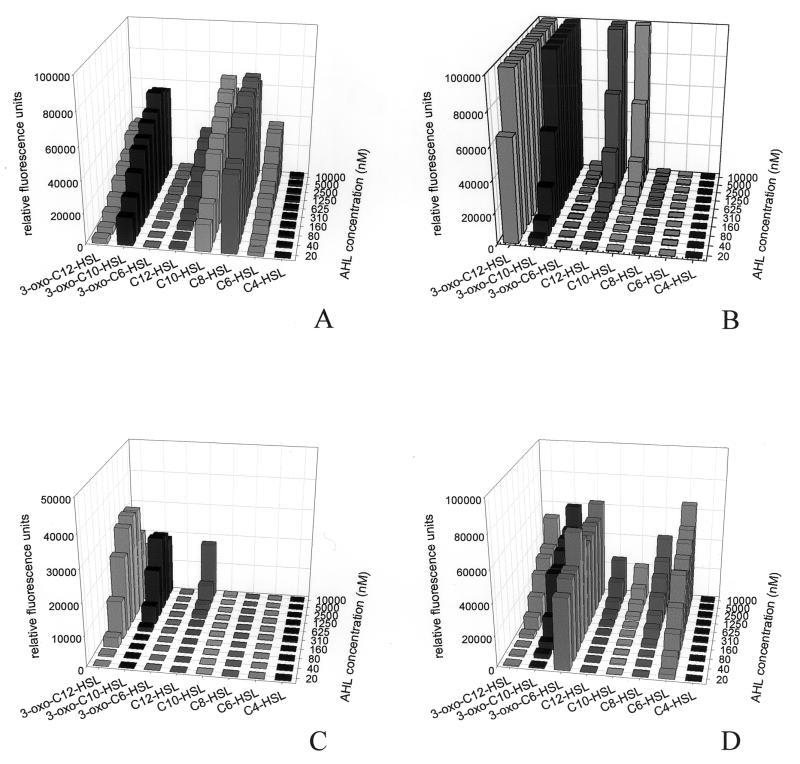
To our great surprise, some of the AHL sensor plasmids worked well in one of the strains but not in the other. For example, in the presence of 10 nM 3-oxo-C6-HSL, pJBA132 gave rise to strong fluorescence in the S. liquefaciens MG44 background; however, it was only weakly stimulated at concentrations of up to 1 μM 3-oxo-C6-HSL in the P. putida F117 background (Fig. 1 and data not shown). In contrast, MG44 harboring plasmid pKR-C12 was very insensitive for 3-oxo-C12-HSL, while the presence of this plasmid in the F117 background allowed the detection of less than a 20 nM concentration of this AHL molecule. Likewise, the sensor plasmid pAS-C8 did not work in the MG44 background, while in the F117 background, it allowed the detection of less than 10 nM C8-HSL. At present, the reasons for the failure of proper functioning of the AHL sensor plasmids in certain strain backgrounds are unclear and will require further investigation. For this study, we used the three sensor plasmids in the strain background that allowed for the most sensitive detection of the respective AHL molecules, i.e., plasmids pKR-C12 and pAS-C8 in P. putida F117 and plasmid pJBA132 in S. liquefaciens MG44.
FIG. 1.
Characterization of the AHL monitor strains used in this study. The monitor strains P. putida F117(pAS-C8) (A), P. putida F117(pKR-C12) (B), P. putida KS35 (F117::Tn5-LAS) (C), and S. liquefaciens MG44(pJBA132) (D) were grown in the presence of different AHL compounds in a concentration range from 0 to 10 μM. The green fluorescence of the cultures was recorded with a microtiter plate reader at 515 nm. The measured values were corrected for autofluorescence and plotted as a function of AHL concentration. The AHL compounds assayed are shown at the bottom of each panel.
The three monitor strains responded to different spectra of AHL molecules. In good agreement with other studies (38; Hentzer et al., submitted), F117 harboring plasmid pKR-C12 responded not only to 3-oxo-C12-HSL (detection limit of less than 10 nM) but also to 3-oxo-C10-HSL, C10-HSL, and C12-HSL, albeit with reduced sensitivity (Fig. 1). As expected, monitor strain F117(pAS-C8) was most sensitive for C8-HSL (detection limit of 10 nM) but was also, with lower efficiency, stimulated by C10-HSL, 3-oxo-C10-HSL, C6-HSL, C12-HSL, and 3-oxo-C12-HSL. MG44 harboring the 3-oxo-C6-HSL sensor plasmid pJBA132 was activated by a broad spectrum of AHL molecules. This sensor exhibited the highest sensitivity for 3-oxo-C6-HSL (detection limit of 5 nM), followed by C6-HSL, 3-oxo-C10-HSL, 3-oxo-C12-HSL, and C8-HSL.
We also determined the detection limits of the three monitor strains for single-cell analysis by inspection of samples by epifluorescence microscopy. For the most efficient AHL molecules, concentrations of less than 5 nM were sufficient to stimulate the sensors to a level allowing the unequivocal detection of green fluorescent cells.
Assessment of interspecies communication in cross-streaking experiments.
To test the monitor strains for their suitability for detecting AHL production in situ, they were cross-streaked against various bacterial strains isolated from tomato roots. Of 300 strains tested, approximately 12% provoked a positive signal with one or more of the monitor strains (data not shown). Three strains gave rise to particularly strong signals with at least one of the monitor strains, and these were chosen for further investigations (Table 2). Sequence analysis of the 16S rDNAs identified strains T13 and TAA as a Rahnella sp. (more than 99% similarity to Rahnella aquatilis) and strain Z2D as a Pseudomonas sp. (more than 98% similarity to P. putida).
TABLE 2.
Activation of the AHL monitor strains in cross-streak experimentsa
| Rhizosphere isolate | Result obtained with:
|
|||
|---|---|---|---|---|
| MG44 (pJBA132) | KS35 | F117 (pKR-C12) | F117 (pAS-C8) | |
| P. putida IsoF | +++ | +++ | +++ | +++ |
| P. putida Z2D | +++ | +++ | +++ | + |
| R. aquatilis T13 | +++ | − | − | ++ |
| R. aquatilis TAA | +++ | + | +++ | +++ |
The four monitor strains, MG44(pJBA132), F117(pKR-C12), F117(pAS-C8), and KS35 (F117::Tn5-LAS), were cross-streaked against various rhizosphere isolates on LB agar plates. Following 24 h of incubation at 30°C, the production of Gfp(ASV) by the monitor strains was visualized by epifluorescence microscopy. Levels of activation are indicated as follows: +++, strong activation, diffusion of AHL of >1 cm; ++, activation, diffusion of AHL of 0.5 to 1 cm; +, weak activation, diffusion of AHL of <0.5 cm; −, no detectable activation.
AHL profiles of the rhizosphere isolates used in this study.
TLC in combination with AHL biosensors provides a simple and rapid technique for characterizing and quantifying the AHL species produced by a given organism (17, 41). Dichloromethane extracts of spent culture supernatants of P. putida IsoF, P. putida Z2D, R. aquatilis TAA, and R. aquatilis T13 were analyzed as described in Materials and Methods. The two P. putida strains, Z2D and IsoF, exhibited very similar AHL patterns (Fig. 2). Using the bioluminescent monitor strain E. coli MT102(pSB403), four different AHL species were detected. Based on their mobilities (Rf values) and by including appropriate reference compounds, these molecules were tentatively identified as 3-oxo-C12-HSL, 3-oxo-C10-HSL, 3-oxo-C8-HSL, and 3-oxo-C6-HSL. The fact that these strains produced several long-chain AHL molecules provides a rationale for the strong stimulation of the F117(pKR-C12) monitor strain (Table 2).
FIG. 2.
TLC analysis of AHLs produced by bacterial strains isolated from the tomato rhizosphere. AHLs extracted from cell-free culture supernatants of P. putida IsoF, P. putida Z2D, R. aquatilis TAA, and R. aquatilis T13 were separated by TLC, and spots were detected with the aid of the AHL biosensor E. coli MT102(pSB403). Synthetic AHLs were included as reference compounds, as indicated.
The two R. aquatilis strains differed in their AHL patterns. While only a single AHL species, which was found to comigrate with 3-oxo-C6-HSL, was detected in the supernatant of strain T13, two AHL species were produced by strain TAA, one comigrating with 3-oxo-C6-HSL and the other comigrating with 3-oxo-C8-HSL. The production of different AHLs by the two strains also explains the observed differences in their abilities to activate the three AHL monitor strains in cross-streaking experiments. While strain T13 strongly stimulated the MG44(pJBA132) sensor, it did not activate F117(pKR-C12). In contrast, both sensors responded equally well to the presence of strain TAA, indicating the production of both long- and short-chain AHL molecules.
Cell-cell communication in the rhizosphere of axenic tomato plants.
To investigate whether bacteria colonizing the rhizosphere do produce AHL molecules in situ, we inoculated axenically cultured tomato plants with an AHL monitor strain together with an AHL-producing rhizosphere isolate. The use of a gnotobiotic system has three major advantages: (i) the sensor and AHL-producing strains can be freely chosen, and thus pairs that show good results in the cross-streaking experiments can be analyzed under in situ conditions; (ii) there is a low autofluorescence background due to the use of sterile quartz sand instead of soil for the growth of tomatoes; and (iii) there is a high level of reproducibility. Four to 5 days after germination of the tomato seeds, the plants were inoculated with a mixture of a sensor strain and an AHL-producing strain. Ten days after inoculation, the plants were harvested and the roots were rinsed with PBS to remove adherent quartz particles. For detection of fluorescent cells, root samples were inspected with a confocal laser scanning microscope.
In all instances in which the sensor strains were coinoculated with an AHL-producing strain capable of activating the sensor strains in cross-streaking experiments, green fluorescent cells were detected, as shown in Fig. 3A and B for the pairs MG44(pJBA132)-T13 and F117(pAS-C8)-Z2D, respectively. As expected, no signals were detected when the sensor strains were inoculated alone (data not shown). In order to visualize the presence of the sensor strains in the rhizosphere, they were chromosomally tagged with DsRed. When these tagged monitor strains were used for inoculation of axenic tomato plants, red fluorescent cells were clearly visible on the surface of the roots, but none of the cells exhibited green fluorescence (Fig. 3C). However, in the presence of an AHL-producing strain, most cells were found to exhibit red as well as green fluorescence. As a consequence of the coexpression of Gfp and DsRed, the cells appeared yellow in the red-green double-exposure photomicrograph shown in Fig. 3D. In these experiments, we observed that activated cells of the monitor strain were not necessarily associated with microcolonies, suggesting that the AHLs may spread within the rhizosphere. To address this issue in better detail, we inoculated a tomato plant with the monitor strain MG44(pJBA132) together with R. aquatilis T13 tagged with DsRed. In support of our hypothesis, we observed that red fluorescent cells, which indicate the location of T13 cells, were often separated from the green fluorescent cells indicating the activated monitor cells (Fig. 3E).
FIG. 3.
AHL-mediated cell-cell communication in the tomato rhizosphere. Axenically grown tomato plants were inoculated with a mixture of a Gfp-based AHL monitor strain and an AHL-producing tomato isolate. (A) S. liquefaciens MG44(pJBA132) together with R. aquatilis T13. (B) P. putida F117(pAS-C8) together with P. putida Z2D. Root samples taken 10 days after inoculation were inspected by confocal laser scanning microscopy. Perception of AHL signal molecules is indicated by the appearance of green fluorescent cells. To visualize the distribution of the monitor strain on the tomato root, the monitor strain F117(pKR-C12) was tagged with DsRed. (D) In the presence of P. putida Z2D, most cells of the red fluorescent monitor strain were found also to be green fluorescent, as indicated by the appearance of yellow cells in the red-green double-exposure photograph. (C) When the monitor strain was inoculated alone, only red fluorescent cells were observed, excluding the possibility that the monitor strain is stimulated by the plant. Green fluorescent cells of the monitor strain were often found to be separated from the AHL-producing strains. This finding was particularly apparent when the monitor strain was coinoculated with a DsRed-tagged AHL-producing strain. (E) Results of a colonization experiment using a mixture of the monitor strain MG44(pJBA132) and a DsRed-tagged derivative of R. aquatilis T13 (red arrow). The green arrow indicates the position of activated sensor cells. (F) The DsRed-tagged monitor strain KS35 (F117::Tn5-LAS) was also stimulated when it was used for the inoculation of tomato plants grown in nonsterile soil, indicating the production of AHL molecules by the indigenous rhizosphere community.
In conclusion, these experiments provide strong evidence that AHLs are produced by bacteria colonizing the rhizosphere of axenically grown tomato plants and that these signals can be perceived by other bacteria. Moreover, our data suggest that AHLs are capable of spreading over relatively long distances in the rhizosphere.
Cell-cell communication in the rhizosphere of tomato plants grown in soil.
The results obtained with the gnotobiotic tomato system encouraged us to investigate the possibility of AHL-mediated communication in the rhizosphere of soil-grown tomato plants. Despite the fact that the sensor plasmids were relatively stable in test tube experiments, e.g., less that 20% plasmid loss after 100 generations (data not shown), we were concerned that, due to increased competition pressure, plasmid loss might be a problem in the nonsterile system. Hence, we integrated the las-based monitor cassette into the chromosome of the DsRed-tagged strain P. putida F117. As shown in Fig. 1C, this monitor strain exhibited virtually the same specificity as the plasmid-based monitor strain, albeit with a slightly reduced sensitivity due to the reduced copy number of the sensor cassette. Half-maximal activation was observed in the presence of approximately 160 nM 3-oxo-C12-HSL in the microtiter assay, and a detection limit of about 40 nM was obtained for single-cell inspection. The specificity remained highest for 3-oxo-C12-HSL, followed by 3-oxo-C10-HSL and C12-HSL.
In a first step, this monitor strain was used to inoculate tomato plants grown in nonsterile soil together with the untagged wild-type strain IsoF. As for the axenically grown plants, we observed that most red fluorescent cells also exhibited green fluorescence, indicating that IsoF also produces AHL in the natural root habitat. However, in contrast to the gnotobiotic system, we also detected green fluorescent cells when the monitor strain was inoculated alone (Fig. 3F). These results clearly demonstrate that the indigenous rhizosphere community produces AHLs at concentrations high enough to activate the monitor strain and thus provide strong evidence that these signal molecules are utilized by the consortium for communication between different populations.
DISCUSSION
Evidence that has accumulated over the past few years has firmly established that a large number of Proteobacteria synthesize AHL signal molecules (for reviews, see references 11 and 15). This appears to be particularly true for bacteria which are associated with plants. In an extensive survey, Cha et al. (5) demonstrated that the majority of plant-associated bacteria produce AHL signal molecules. Specifically, they showed that almost all tested isolates of the genera Agrobacterium, Pantoea, and Rhizobium and about half of the erwinias and pseudomonads tested synthesized detectable levels of AHLs. In contrast, only a few AHL producers could be identified among Xanthomonas isolates. More recently, Elasri et al. (13) screened 137 soilborne and plant-associated strains belonging to different Pseudomonas species for their ability to synthesize AHLs. Of all the strains tested, 54 were positive for AHL production. Most interestingly, however, was the observation that plant-associated and plant-pathogenic bacteria produced AHLs more frequently than did soilborne strains. On the basis of these results, the authors suggested that the closer the relationship of the bacteria with the host plant, the higher the probability that it produces AHLs. We screened over 300 bacterial strains isolated from the rhizosphere of tomato plants on standard laboratory media for their ability to synthesize AHLs. Approximately 12% of the strains provoked a clear response in one or more of the AHL monitor strains described in this report. This result is in very good agreement with the results of Pierson et al. (34), who screened 700 strains of wheat root-associated bacteria with the aid of different AHL biosensors and found that about 8% of the strains were able to activate at least one of the sensors.
Given that many gram-negative bacteria that colonize the roots of tomato plants utilize the same chemical language, it appears likely that AHL signal molecules are used not only as population density sensors in one species but also for communication between cells of different species. The concept of interspecies communication was already introduced in 1979, when it was observed that Vibrio harveyi was stimulated to produce light following the addition of cell-free culture fluid from several species of nonluminous bacteria (19). This stimulation was attributed to the presence of a so-called alloinducer, the structure of which remained unidentified at that time. It was not until recently that it was shown that V. harveyi in fact utilizes two interlinked quorum-sensing systems to control the bioluminescence phenotype (14). One of these systems relies on the AHL molecule 3-hydroxy-l-butanoyl-homoserine lactone (3-OH-C4-HSL), while the cognate signal molecule (HAI-2) of the second system has not yet been identified. However, while HAI-2 has been demonstrated to be produced by a large number of bacteria (43), including E. coli and Salmonella enterica serovar Typhimurium, 3-OH-C4-HSL has so far been detected only in culture supernatants of Vibrio parahaemolyticus and Xenorhabdus nematophilus as well as V. harveyi (3, 9).
Evidence for interpopulation signaling via AHL molecules has been presented in previous studies. McKenney et al. (30) demonstrated that spent culture supernatants of P. aeruginosa enhance virulence factor production in B. cepacia and that this effect was attributable to the presence of AHLs in the P. aeruginosa supernatants. More important in the context of this study is the recent demonstration of AHL-mediated cross-communication in the rhizosphere of wheat (34). Using an AHL-specific reporter strain, P. aureofaciens 30-84Ice/I, which expresses ice nucleation activity from the AHL-controlled phenazine promoter of the organism, the authors demonstrated that AHLs produced by coinoculated bacterial populations are perceived by the reporter strain (34). These results provided strong evidence that AHLs may serve as interpopulation signals in the wheat rhizosphere. However, despite the fact that nucleation activity can be measured with a very high sensitivity even in complex samples, this reporter strain does not allow the detection of gene expression at the single-cell level. Moreover, since the measurement of nucleation activity requires processing of the sample, information about the spatial localization of the cells is lost.
To obtain direct visual evidence for AHL-mediated cross-communication between bacteria, we used three Gfp-based AHL sensor cassettes which respond to different spectra of AHL molecules, depending on the components used for their construction. In previous studies, Gfp-based AHL sensor plasmids were used for the detection of AHL molecules in the lung tissues of mice infected with P. aeruginosa (52), for visualization of interspecies communication between P. aeruginosa and B. cepacia in mixed biofilms (38), and for the analysis of quorum-sensing inhibition by halogenated furanone compounds in swarming colonies of S. liquefaciens MG1 (37) and in P. aeruginosa biofilms (Hentzer et al., submitted). In order to visualize cell-cell communication in the tomato rhizosphere, different AHL sensor plasmids were transferred into P. putida F117 and S. liquefaciens MG44, both of which are good root colonizers. To our surprise, we observed that the functionality of the sensor plasmids was highly dependent on the background strain. While plasmids pKR-C12 and pAS-C8 worked well in P. putida F117, they did not work in S. liquefaciens MG44. Conversely, pJBA132 functioned only in the latter strain. The reasons for these effects are at present unclear. However, given that cells are freely permeable for short-chain AHLs but that long-chain AHLs are actively transported (32), it is tempting to speculate that differences in the presence and/or specificity of long-chain AHL transporters in the two strains may account for the observed differences.
To monitor bacterial cells in the rhizosphere of tomato plants, we integrated a cassette expressing DsRed from Discosoma sp. into the chromosomes of various strains. The tagging of strains served two purposes: (i) to ensure the presence of the AHL monitor strains in the rhizosphere when no activation was observed and (ii) to visualize the spatial arrangement of AHL monitor strains and coinoculated AHL producer strains. These experiments also revealed important information about differences in the colonization patterns of the strains used in this study. In agreement with previous studies of the colonization behavior of pseudomonads (6), we observed that P. putida IsoF cells tended to form microcolonies on the root surface. Similar colonization patterns were also observed with the two Rahnella strains used in this study. In contrast, S. liquefaciens cells were preliminarily found as single cells and did not form aggregates on the surface of the root.
Using axenically grown tomato plants that were inoculated with a mixture of an AHL monitor and an AHL producer, we were able to visualize in situ cell-cell communication between defined bacterial populations. Our experiments revealed that activated cells of the monitor strains were not necessarily associated with microcolonies, indicating that AHLs are capable of spreading over relatively long distances along the root surface. In fact, we observed that microcolonies of the two P. putida F117 monitor strains were usually well separated from those of the cocolonizing AHL producer strains. Previous work has shown that the root epidermis is often covered by a distinct matrix, the so-called mucigel (21). Recent electron microscopy studies provided evidence that microcolonies of a Pseudomonas fluorescens biocontrol strain are embedded in this mucoid matrix (6). Hence, one possible explanation of our observations could be that AHL signal molecules spread within the mucigel layer. This hypothesis is further corroborated by the finding that inoculation of 50 μl of 3-oxo-C12-HSL (100 μM) at a single location on the top part of a tomato root colonized by P. putida KS35 leads to the activation of sensor cells on the entire root surface after 12 h of incubation (data not shown). However, further work will be needed to assess the full extent of the mobility of the different AHL molecules within the rhizosphere.
Despite that fact that only a minority of bacterial isolates from the tomato rhizosphere were capable of synthesizing AHL molecules, we were able to detect the production of AHLs by the indigenous rhizosphere community of plants grown in nonsterile soil. Given that the AHL monitor F117::Tn5-LAS used in these experiments is activated only in the presence of at least 40 nM 3-oxo-C12-HSL, for which the sensor is most sensitive, it is reasonable to assume that the concentrations of AHLs in the rhizosphere are biologically meaningful. For V. fischeri it has been demonstrated that a 3-oxo-C6-HSL concentration as low as 10 nM is sufficient to trigger the induction of bioluminescence (23), representing the archetypic AHL-controlled phenotype.
In conclusion, our data provide strong evidence that AHL molecules are produced by the bacterial consortium naturally colonizing the roots of tomato plants and strongly support the view that these molecules may act as signals for coordinating the functions of the different populations within the community. Work is currently under way to investigate the role of AHL-mediated cell-cell communication in the composition and architecture of the rhizosphere community.
ACKNOWLEDGMENTS
We thank H. P. Schweitzer and M. E. Kovach for providing bacterial strains and plasmids and P. Williams for the generous gift of synthetic AHLs.
This work was supported by the BMBF.
REFERENCES
- 1.Andersen J B, Heydorn A, Hentzer M, Eberl L, Geisenberger O, Christensen B B, Molin S, Givskov M. gfp-based N-acyl-homoserine lactone sensor systems for detection of bacterial communication. Appl Environ Microbiol. 2001;67:575–585. doi: 10.1128/AEM.67.2.575-585.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen J B, Sternberg C, Poulsen L K, Bjørn S P, Givskov M, Molin S. New unstable variants of green fluorescent protein for studies of transient gene expression in bacteria. Appl Environ Microbiol. 1998;64:2240–2246. doi: 10.1128/aem.64.6.2240-2246.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bassler B L, Greenberg E P, Stevens A M. Cross-species induction of luminescence in the quorum-sensing bacterium Vibrio harveyi. J Bacteriol. 1997;179:4043–4045. doi: 10.1128/jb.179.12.4043-4045.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertani G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951;62:293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cha C, Gao P, Chen Y, Shaw P D, Farrand S K. Production of acyl-homoserine lactone quorum-sensing signals by gram-negative plant-associated bacteria. Mol Plant-Microbe Interact. 1998;11:1119–1129. doi: 10.1094/MPMI.1998.11.11.1119. [DOI] [PubMed] [Google Scholar]
- 6.Chin-A-Woeng T F C, de Priester W, van der Bij A J, Lugtenberg B J J. Description of the colonization of a gnotobiotic tomato rhizosphere by Pseudomonas fluorescens biocontrol strain WCS365, using scanning electron microscopy. Mol Plant-Microbe Interact. 1997;10:79–86. [Google Scholar]
- 7.Christensen B B, Sternberg C, Andersen J B, Palmer R J, Jr, Nielsen A T, Givskov M, Molin S. Molecular tools for study of biofilm physiology. Methods Enzymol. 1999;310:20–42. doi: 10.1016/s0076-6879(99)10004-1. [DOI] [PubMed] [Google Scholar]
- 8.de Lorenzo V, Timmis K N. Analysis and construction of stable phenotypes in gram-negative bacteria with Tn5- and Tn10-derived mini-transposons. Methods Enzymol. 1994;235:386–405. doi: 10.1016/0076-6879(94)35157-0. [DOI] [PubMed] [Google Scholar]
- 9.Dunphy G, Miyamoto C, Meighen E. A homoserine lactone autoinducer regulates virulence of an insect-pathogenic bacterium, Xenorhabdus nematophilus (Enterobacteriaceae) J Bacteriol. 1997;179:5288–5291. doi: 10.1128/jb.179.17.5288-5291.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eberhard A, Burlingame A L, Eberhard C, Kenyon G L, Nealson K H, Oppenheimer N J. Structural identification of autoinducer of Photobacterium fischeri luciferase. Biochemistry. 1981;20:2444–2449. doi: 10.1021/bi00512a013. [DOI] [PubMed] [Google Scholar]
- 11.Eberl L. N-Acyl homoserinelactone-mediated gene regulation in gram-negative bacteria. Syst Appl Microbiol. 1999;22:493–506. doi: 10.1016/S0723-2020(99)80001-0. [DOI] [PubMed] [Google Scholar]
- 12.Eberl L, Winson M K, Sternberg C, Stewart G S A B, Christiansen G, Chhabra S R, Bycroft B, Williams P, Molin S, Givskov M. Involvement of N-acyl-l-homoserine lactone autoinducers in controlling the multicellular behaviour of Serratia liquefaciens. Mol Microbiol. 1996;20:127–136. doi: 10.1111/j.1365-2958.1996.tb02495.x. [DOI] [PubMed] [Google Scholar]
- 13.Elasri M, Delorme S, Lemanceau P, Stewart G, Laue B, Glickmann E, Oger P M, Dessaux Y. Acyl-homoserine lactone production is more common among plant-associated Pseudomonas spp. than among soilborne Pseudomonas spp. Appl Environ Microbiol. 2001;67:1198–1209. doi: 10.1128/AEM.67.3.1198-1209.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freeman J A, Bassler B L. Sequence and function of LuxU: a two-component phosphorelay protein that regulates quorum sensing in Vibrio harveyi. J Bacteriol. 1999;181:899–906. doi: 10.1128/jb.181.3.899-906.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuqua C, Winans S C, Greenberg E P. Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum-sensing transcriptional regulators. Annu Rev Microbiol. 1996;50:727–751. doi: 10.1146/annurev.micro.50.1.727. [DOI] [PubMed] [Google Scholar]
- 16.Givskov M, Molin S. Expression of extracellular phospholipase from Serratia liquefaciens is growth-phase-dependent, catabolite-repressed and regulated by anaerobiosis. Mol Microbiol. 1992;6:1363–1374. doi: 10.1111/j.1365-2958.1992.tb00857.x. [DOI] [PubMed] [Google Scholar]
- 17.Gotschlich A, Huber B, Geisenberger O, Tögl A, Steidle A, Riedel K, Hill P, Tümmler B, Vandamme P, Middleton B, Camara M, Williams P, Hardman A, Eberl L. Synthesis of multiple N-acyl-homoserine lactones is wide-spread among the members of the Burkholderia cepacia complex. Syst Appl Microbiol. 2001;24:1–14. doi: 10.1078/0723-2020-00013. [DOI] [PubMed] [Google Scholar]
- 18.Gray K M, Pearson J P, Downie J A, Boboye B E, Greenberg E P. Cell-to-cell signaling in the symbiotic nitrogen-fixing bacterium Rhizobium leguminosarum: autoinduction of stationary phase and rhizosphere-expressed genes. J Bacteriol. 1996;178:372–376. doi: 10.1128/jb.178.2.372-376.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenberg E P, Hastings J W, Ulitzur S. Induction of luciferase synthesis in Beneckea harveyi by other marine bacteria. Arch Microbiol. 1979;120:87–91. [Google Scholar]
- 19a.Hentzer, M., K. Riedel, T. B. Rasmussen, A. Heydron, J. B. Anderson, M. R. Parsek, S. Rice, L. Eberl, S. Molin, S. Kjelleberg, and Giuskov. Interference with Oseydinibas aerygubisa quorum-sensing by a halogenated furanone compound. Microbiology, in press. [DOI] [PubMed]
- 20.Herrero M, de Lorenzo V, Timmis K N. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J Bacteriol. 1990;172:6557–6567. doi: 10.1128/jb.172.11.6557-6567.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenny H, Grossenbacher K. Root-soil boundary zones as seen in the electron microscope. Soil Sci Soc Am Proc. 1963;27:273–277. [Google Scholar]
- 22.Kalbe C, Marten P, Berg G. Strains of the genus Serratia as beneficial rhizobacteria of oilseed rape with antifungal properties. Microbiol Res. 1996;151:433–439. doi: 10.1016/S0944-5013(96)80014-0. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan H B, Greenberg E P. Diffusion of autoinducer is involved in regulation of the Vibrio fischeri luminescence system. J Bacteriol. 1985;163:1210–1214. doi: 10.1128/jb.163.3.1210-1214.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kessler B, de Lorenzo V, Timmis K N. A general system to integrate lacZ fusions into the chromosomes of gram-negative eubacteria: regulation of the Pm promoter of the TOL plasmid studied with all controlling elements in monocopy. Mol Gen Genet. 1992;233:293–301. doi: 10.1007/BF00587591. [DOI] [PubMed] [Google Scholar]
- 25.Kovach M E, Elzer P H, Hill D S, Robertson G T, Farris M A, Roop R M, I. I, Peterson K M. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene. 1995;166:175–176. doi: 10.1016/0378-1119(95)00584-1. [DOI] [PubMed] [Google Scholar]
- 26.Lewenza S, Conway B, Greenberg E P, Sokol P A. Quorum sensing in Burkholderia cepacia: identification of the LuxRI homologs CepRI. J Bacteriol. 1999;181:748–756. doi: 10.1128/jb.181.3.748-756.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lithgow J K, Wilkinson A, Hardman A, Rodelas B, Wisniewski-Dye F, Williams P, Downie J A. The regulatory locus cinRI in Rhizobium leguminosarum controls a network of quorum-sensing loci. Mol Microbiol. 2000;37:81–97. doi: 10.1046/j.1365-2958.2000.01960.x. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Cui Y, Mukherjee A, Chatterjee A K. Characterization of a novel RNA regulator of Erwinia carotovora ssp. carotovora that controls production of extracellular enzymes and secondary metabolites. Mol Microbiol. 1998;29:219–234. doi: 10.1046/j.1365-2958.1998.00924.x. [DOI] [PubMed] [Google Scholar]
- 29.McCullagh M, Utkhede R, Menzies J G, Punja Z K, Paulitz T C. Evaluation of plant growth-promoting rhizobacteria for biological control of Pythium root rot of cucumbers grown in rockwool and effects on yield. Eur J Plant Pathol. 1996;102:747–755. [Google Scholar]
- 30.McKenney D, Brown K E, Allison D G. Influence of Pseudomonas aeruginosa exoproducts on virulence factor production in Burkholderia cepacia: evidence of interspecies communication. J Bacteriol. 1995;177:6989–6992. doi: 10.1128/jb.177.23.6989-6992.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meissner R, Jacobson Y, Melamed S, Levyatuv S, Shalev G, Ashri A, Elkind Y, Levy A. A new model system for tomato genetics. Plant J. 1997;12:1465–1472. [Google Scholar]
- 32.Pearson J P, Van Delden C, Iglewski B H. Active efflux and diffusion are involved in transport of Pseudomonas aeruginosa cell-to-cell signals. J Bacteriol. 1999;181:1203–1210. doi: 10.1128/jb.181.4.1203-1210.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pearson J P, Pesci E C, Iglewski B H. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J Bacteriol. 1997;179:5756–5767. doi: 10.1128/jb.179.18.5756-5767.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pierson E A, Wood D W, Cannon J A, Blachere F M, Pierson L S., III Interpopulation signaling via N-acyl-homoserine lactones among bacteria in the wheat rhizosphere. Mol Plant-Microbe Interact. 1998;11:1078–1084. [Google Scholar]
- 35.Pierson L S, III, Wood D W, Pierson E A. Homoserine lactone-mediated regulation in plant-associated bacteria. Annu Rev Phytopathol. 1998;36:207–225. doi: 10.1146/annurev.phyto.36.1.207. [DOI] [PubMed] [Google Scholar]
- 36.Piper K R, von Bodman S B, Farrand S K. Conjugation factor of Agrobacterium tumefaciens regulates Ti plasmid transfer by autoinduction. Nature. 1993;362:448–450. doi: 10.1038/362448a0. [DOI] [PubMed] [Google Scholar]
- 37.Rasmussen T B, Manefield M, Andersen J B, Eberl L, Anthoni U, Christophersen C, Steinberg P, Kjelleberg S, Givskov M. How Delisea pulchra furanones affect quorum-sensing and swarming motility in Serratia liquefaciens MG1. Microbiology. 2000;146:3237–3244. doi: 10.1099/00221287-146-12-3237. [DOI] [PubMed] [Google Scholar]
- 38.Riedel, K., M. Hentzer, O. Geisenberger, B. Huber, A. Steidle, A. Gotschlich, H. Wu, M. Givskov, S. Molin, and L. Eberl. AHL-mediated intergeneric communication between Pseudomonas aeruginosa and Burkholderia cepacia in mixed biofilms. Microbiology, in press. [DOI] [PubMed]
- 39.Rodelas B, Lithgow J K, Wisniewski-Dye F, Hardman A, Wilkinson A, Economou A, Williams P, Downie J A. Analysis of quorum-sensing-dependent control of rhizosphere-expressed (rhi) genes in Rhizobium leguminosarum bv. viciae. J Bacteriol. 1999;181:3816–3823. doi: 10.1128/jb.181.12.3816-3823.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schripsema J, de Rudder K E, van Vliet T B, Lankhorst P P, de Vroom E, Kijne J W, van Brussel A A. Bacteriocin small of Rhizobium leguminosarum belongs to the class of N-acyl-l-homoserine lactone molecules known as autoinducers and as quorum sensing co-transcription factors. J Bacteriol. 1996;178:366–371. doi: 10.1128/jb.178.2.366-371.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shaw P D, Ping G, Daly S, Cha C, Cronan J E, Jr, Rinehart K L, Farrand S K. Detecting and characterizing N-acyl-homoserine lactone signal molecules by thin-layer chromatography. Proc Natl Acad Sci USA. 1997;94:6036–6041. doi: 10.1073/pnas.94.12.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simons M, van der Bij A J, Brand I, de Weger L A, Wijffelman C A, Lugtenberg B J J. Gnotobiotic system for studying rhizosphere colonization by plant growth promoting Pseudomonas bacteria. Mol Plant-Microbe Interact. 1996;9:600–607. doi: 10.1094/mpmi-9-0600. [DOI] [PubMed] [Google Scholar]
- 43.Surette M G, Miller M B, Bassler B L. Quorum sensing in Escherichia coli, Salmonella typhimurium, and Vibrio harveyi: a new family of genes responsible for autoinducer production. Proc Natl Acad Sci USA. 1999;96:1639–1644. doi: 10.1073/pnas.96.4.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thorne S H, Williams H D. Cell density-dependent starvation survival of Rhizobium leguminosarum bv. phaseoli: identification or the role of an N-acyl-homoserine lactone in adaptation to stationary-phase survival. J Bacteriol. 1999;181:981–990. doi: 10.1128/jb.181.3.981-990.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tolker-Nielsen T, Brinch U C, Ragas P C, Andersen J B, Jacobsen C S, Molin S. Development and dynamics of Pseudomonas sp. biofilms. J Bacteriol. 2000;182:6482–6489. doi: 10.1128/jb.182.22.6482-6489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tuomainen J, Betz C, Kangasjärvi J, Ernst D, Yin Z H, Langebartels C, Sandermann H. Ozone induction of ethylene emission in tomato plants: regulation by differential accumulation of transcripts for the biosynthetic enzymes. Plant J. 1997;12:1151–1162. [Google Scholar]
- 47.Von Bodman S B, Majerczak D R, Coplin D L. A negative regulator mediates quorum-sensing control of exopolysaccharide production in Pantoea stewartii subsp. stewartii. Proc Natl Acad Sci USA. 1998;95:7687–7692. doi: 10.1073/pnas.95.13.7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weller D M. Biological control of soilborne plant pathogens in the rhizosphere with bacteria. Annu Rev Phytopathol. 1988;26:379–407. [Google Scholar]
- 49.Wijffelman C A, Pees E, van Brussel A A N, Hooykaas P J J. Repression of small bacteriocin excretion in Rhizobium leguminosarum and Rhizobium trifolii by transmissible plasmids. Mol Gen Genet. 1983;192:171–176. [Google Scholar]
- 50.Winson M K, Swift S, Fish L, Throup J P, Jørgensen F, Chhabra S R, Bycroft B W, Williams P, Stewart G S A B. Construction and analysis of luxCDABE-based plasmid sensors for investigating N-acyl-homoserine lactone-mediated quorum sensing. FEMS Microbiol Lett. 1998;163:185–192. doi: 10.1111/j.1574-6968.1998.tb13044.x. [DOI] [PubMed] [Google Scholar]
- 51.Wood D W, Gong F, Daykin M M, Williams P, Pierson L S., III N-Acyl-homoserine lactone-mediated regulation of phenazine gene expression by Pseudomonas aureofaciens 30–84 in the wheat rhizosphere. J Bacteriol. 1997;179:7663–7670. doi: 10.1128/jb.179.24.7663-7670.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu H, Song Z, Hentzer M, Andersen J B, Heydorn A, Mathee K, Moser C, Eberl L, Molin S, Høiby N, Givskov M. Detection of N-acyl-homoserine lactones in lung tissues infected with Pseudomonas aeruginosa. Microbiology. 2000;146:2481–2493. doi: 10.1099/00221287-146-10-2481. [DOI] [PubMed] [Google Scholar]
- 53.Zhang L, Murphy P J, Kerr A, Tate E M. Agrobacterium conjugation and gene regulation by N-acyl-l-homoserine lactones. Nature. 1993;362:446–448. doi: 10.1038/362446a0. [DOI] [PubMed] [Google Scholar]