Abstract
This scientific commentary refers to ‘Sex differences in the genetic architecture of cognitive resilience to Alzheimer’s disease’ by Eissman et al. (https://doi.org/10.1093/brain/awac177).
This scientific commentary refers to ‘Sex differences in the genetic architecture of cognitive resilience to Alzheimer’s disease’ by Eissman et al. (https://doi.org/10.1093/brain/awac177).
The accumulation of the neuropathological hallmarks of Alzheimer’s disease, i.e. amyloid-β plaques and neurofibrillary tau tangles, is associated with neurodegeneration and cognitive decline, eventually resulting in mild cognitive impairment and dementia. However, some individuals show remarkable resilience to Alzheimer’s disease by retaining better cognitive performance than expected based on levels of pathology. This is illustrated by studies indicating that many nonagenarians and centenarians remain cognitively healthy despite high levels of neuropathology.1
Inter-individual differences in resilience to Alzheimer’s disease are thus a robust finding in the literature but, due to the complex pathogenesis of Alzheimer’s disease, the mechanisms that underlie this resilience are incompletely understood. Nevertheless, evidence is mounting that one’s genetic architecture might be instrumental in conferring resilience. For example, those who maintain high levels of cognitive health until extreme ages have lower polygenic risk scores for Alzheimer’s disease, indicating that they harbour fewer genetic variants associated with an increased Alzheimer’s disease risk and relatively more genetic variants that protect against Alzheimer’s disease.2
Furthermore, a recent genome-wide association study (GWAS) reported that a cluster of single nucleotide polymorphisms (SNPs) on chromosome 18 is related to resilience. The cluster is located upstream of ATP8B1, which encodes the enzyme aminophospholipid translocase, highlighting the association between brain health and the transport of specific phospholipids from the extracellular space to the cytoplasmic leaflet.3 Accordingly, rare variants in the ATP8B4 gene, which also encodes a phospholipid transporter, were found to be associated with an increased risk of Alzheimer’s disease.4 Such findings may prove valuable in developing new therapeutic interventions to promote the maintenance of brain health.
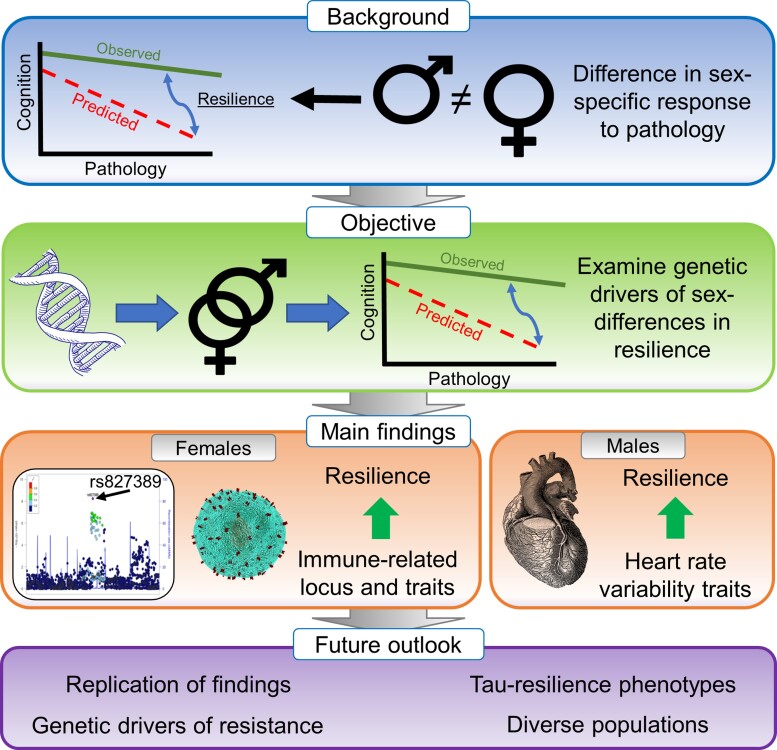
Intriguingly, sex-specific differences in responses to brain pathology have also been reported. While outcomes with regard to these sex-specific associations vary across assessments, several studies have now indicated that females accumulate pathology at faster rates than males,5 while at the same time females have also been shown to better preserve brain structure compared to males with similar levels of tau pathology.6 This suggests that females may have lower resistance to the accumulation of pathology but higher resilience to its downstream detrimental effects. The mechanisms underlying these sex-dependent associations between pathology and cognition are still unclear. In this issue of Brain, Eissman and colleagues7 investigate whether sex-specific genetic factors contribute to cognitive resilience against Alzheimer’s disease pathology (Fig. 1).
Figure 1.
Overview of the study and key findings of Eissman and colleagues. 7
In any assessment of cognitive resilience, which is defined as the (im)balance between cognition and pathology, the selection of cognitive and pathological markers is key. In this work, the authors used amyloid-β as the pathological marker, which is commonly observed first in the Alzheimer’s disease neuropathological cascade (amyloid-β pathology → neocortical tau pathology → neurodegeneration → cognitive decline) and typically becomes abnormal long before symptom onset. To obtain amyloid pathology measures, the authors harmonized two autopsy cohorts [Adult Changes in Thought (ACT); and Religious Order Study/Memory and Aging Project (ROS/MAP)], and two amyloid-PET cohorts [Alzheimer’s Disease Neuroimaging Initiative (ADNI) and Anti-Amyloid Treatment in Asymptomatic Alzheimer’s Disease (A4-study)]. This resulted in a total (i.e. autopsy and amyloid-PET combined) sample size of 5024 subjects (2093 males and 2931 females) including both cognitively unimpaired and impaired (mild cognitive impairment and dementia) subjects.
To measure the other essential component of cognitive resilience, namely cognitive performance, the authors assessed several sensitive markers of early Alzheimer’s disease-related cognitive decline. Using linear regression models, the authors then determined for each participant the deviation of their cognitive performance relative to what is expected based on their degree of amyloid pathology (i.e. the residual approach). An individual with better cognitive performance (Y variable) than expected based on their level of amyloid pathology (X variable) is considered resilient, whereas poorer performance than expected indicates low cognitive resilience. In a second resilience metric, the authors additionally incorporated educational attainment. Of note, this combined resilience score, as well as educational attainment, were higher in males, whereas amyloid positivity and Alzheimer’s disease dementia were both more prevalent in females.
The primary analyses comprised sex-aware genetic analyses of cognitive resilience. On the autopsy and amyloid-PET datasets individually, and for each cognitive resilience measure separately, the authors performed a sex-stratified GWAS and sex-interaction GWAS by comparing resilient individuals with non-resilient individuals, whereafter the results were meta-analysed. They applied this approach to the entire sample; to cognitively unimpaired individuals only; to the autosomal chromosomes; and to the X chromosome only. Further, the authors focused on known Alzheimer’s disease-associated variants identified in previous case–control GWASs. To examine whether relationships between the polygenic architecture of resilience and the genetic architecture that predisposes to a range of complex traits differ between the sexes, the authors performed genetic correlation analyses.
Across all comparisons, the authors identified a novel genome-wide significant female-specific locus on chromosome 10 (rs827389), which was associated with a higher combined resilience score but only among cognitively unimpaired subjects. This locus is expressed in various tissues including foetal and adult cortex, and the locus maps within chromatin loops that interact with the promoter regions of multiple genes involved in RNA processing. These include GATA3, which is, in turn, associated with neuronal development, immune T-cell fate and amyloid autoantibody production. Using genetic correlation analyses, the authors found that higher resilience was associated with reduced genetic risk for autoimmune traits like lupus, multiple sclerosis and coeliac disease among females, whereas increased genetic risk was observed among males. The identification of the immune-related locus on chromosome 10, in conjunction with the genetic correlation analyses pointing towards autoimmune-related pathways, hints at possibilities for interventions targeting immune-related pathways in females specifically. For males, previously established Alzheimer’s disease genetic loci MS4A6A and SORL1 were associated with higher resilience, while PTK2B and KAT8 were associated with lower resilience; these associations were not observed in females. PICALM was associated with lower resilience in males and with higher resilience in females. Notably, the authors did not find an APOE-by-sex effect. Lastly, genetic correlation analyses revealed that possible targets to enhance resilience for males might reside in pathways relating to cardiovascular health, specifically heart rate variability.
The main findings thus suggest that sex differences in resilience to amyloid pathology may be driven by genetic factors. However, as the authors acknowledge, sex-specific genetic drivers of resilience need to be further explored in future studies, and the findings of the present study should be interpreted in light of some potential limitations. First, studying the genetic contribution to a complex phenotype like ‘resilience to amyloid pathology’ requires a large sample size, and amyloid-PET and autopsy datasets were pooled to feed into the resilience phenotype. This hampers the interpretability of genetic associations as there may be inherent differences between post-mortem autopsy cohorts and in vivo neuroimaging cohorts relating to differences in recruitment strategies and demographic characteristics. Moreover, it should be noted that neuropathology does not equal ‘PEThology’.
Second, resilience was defined based on amyloid pathology. There is a long delay between the emergence of amyloid and the manifestation of clinical symptoms, and many downstream processes like neocortical tau accumulation and neurodegeneration occur after widespread amyloidosis. It is therefore unclear whether the sex-specific genetic drivers of resilience observed here are related to amyloid-β specifically or to (combinations of) the downstream processes. Further, amyloid pathology, especially cross-sectionally, is only modestly related to cognition. When determining resilience based on residuals, the association between predictor and outcome needs to be of sufficient magnitude to provide an estimate of resilience that has substantially more predictive power than the original cognitive score on which the residual is based.8 The effects of sex-specific genetic drivers of resilience reported here may therefore be driven by differences in cognitive performance, rather than reflecting resilience to Alzheimer’s disease pathology. The operationalization of resilience using amyloid as the pathological marker could also explain why an APOE × sex effect was not found, as the Alzheimer’s disease risk-modifying effects of APOE mainly operate through amyloidogenic pathways.
Third, as acknowledged by the authors, the generalizability of the results may be limited by the predominance of non-Hispanic white, highly educated study participants. Fourth, genetic analyses need samples of considerable size, and the required sample size increases when assessing a complex phenotype like resilience (heritability among cognitively unimpaired subjects of both sexes estimated at 20–25% by the authors), compared to a more robust phenotype such as ‘having Alzheimer’s disease’ (estimated heritability ∼70% based on twin studies9). This is further exacerbated by sex stratification. While the sample size of ∼5000 well-phenotyped individuals is commendable because a resilience measure requires the availability of both a pathological and a cognitive marker at a similar time point, we stress the importance of replicating these findings in independent, and preferentially larger, samples.
To conclude, previous literature has shown that females are less resistant but more resilient to Alzheimer’s disease pathology than males. The current work suggests that genetic factors may contribute to the emergence of these sex-specific effects. While the findings in this work need replication in an independent dataset, they should encourage increased focus on sex stratification and sex interactions in genetic studies. Future possible avenues of research include the assessment of sex-specific genetic drivers of resilience to tau pathology or neurodegenerative markers, which show a stronger link with cognition and may provide a more robust estimate of resilience. Finally, assessment of genetic factors that underlie resistance to pathology (i.e. preventing its accumulation despite the presence of risk factors) will also be key, and these may be even more genetically determined than resilience.10
Contributor Information
Colin Groot, Alzheimer Center Amsterdam, Neurology, Vrije Universiteit Amsterdam, Amsterdam UMC location VUmc, Amsterdam, The Netherlands; Amsterdam Neuroscience, Neurodegeneration, Amsterdam, The Netherlands; Lund University, Clinical Memory Research Unit, Lund, Sweden.
Henne Holstege, Alzheimer Center Amsterdam, Neurology, Vrije Universiteit Amsterdam, Amsterdam UMC location VUmc, Amsterdam, The Netherlands; Amsterdam Neuroscience, Neurodegeneration, Amsterdam, The Netherlands; Genomics of Neurodegenerative Diseases and Aging, Human Genetics, Vrije Universiteit Amsterdam, Amsterdam UMC location VUmc, Amsterdam, The Netherlands.
Rik Ossenkoppele, Alzheimer Center Amsterdam, Neurology, Vrije Universiteit Amsterdam, Amsterdam UMC location VUmc, Amsterdam, The Netherlands; Amsterdam Neuroscience, Neurodegeneration, Amsterdam, The Netherlands; Lund University, Clinical Memory Research Unit, Lund, Sweden.
Competing interests
The authors report no competing interests.
References
- 1. Beker N, Sikkes SAM, Hulsman M, et al. . Longitudinal maintenance of cognitive health in centenarians in the 100-plus study. JAMA Netw Open. 2020;3:e200094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tesi N, van der Lee SJ, Hulsman M, et al. . Centenarian controls increase variant effect sizes by an average twofold in an extreme case-extreme control analysis of Alzheimer's disease. Eur J Hum Genet. 2019;27:244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dumitrescu L, Mahoney ER, Mukherjee S, et al. . Genetic variants and functional pathways associated with resilience to Alzheimer's disease. Brain. 2020; 143:2561–2575. doi: 10.1093/brain/awaa209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Holstege H, Hulsman M, Charbonnier C, et al. . Exome sequencing identifies rare damaging variants in ATP8B4 and ABCA1 as novel risk factors for Alzheimer’s disease. medRxiv. [Preprint] doi: 10.1101/2020.07.22.20159251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buckley RF, Scott MR, Jacobs HIL, et al. . Sex mediates relationships between regional tau pathology and cognitive decline. Ann Neurol. 2020;88:921–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ossenkoppele R, Lyoo CH, Jester-Broms J, et al. . Assessment of demographic, genetic, and imaging variables associated with brain resilience and cognitive resilience to pathological tau in patients with Alzheimer disease. JAMA Neurol. 2020;77:632–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eissman JM, Dumitrescu L, Mahoney ER, et al. . Sex differences in the genetic architecture of cognitive resilience to Alzheimer's disease. Brain. 2022;145(7):2541–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bocancea DI, van Loenhoud A C, Groot C, Barkhof F, van der Flier WM, Ossenkoppele R. Measuring resilience and resistance in aging and Alzheimer disease using residual methods: A systematic review and meta-analysis. Neurology. 2021;97:474–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gatz M, Reynolds CA, Fratiglioni L, et al. . Role of genes and environments for explaining Alzheimer disease. Arch Gen Psychiatry. 2006;63:168–174. [DOI] [PubMed] [Google Scholar]
- 10. Arenaza-Urquijo EM, Vemuri P. Improving the resistance and resilience framework for aging and dementia studies. Alzheimers Res Ther. 2020;12:41. [DOI] [PMC free article] [PubMed] [Google Scholar]