Abstract
Objective:
Examine factors associated with recovery from posttraumatic stress disorder (PTSD) and evaluate the role of deployment mild traumatic brain injury (mTBI) in the relationship between PTSD recovery and functional outcomes.
Methods:
Post 9/11 combat Veterans with lifetime history of PTSD (N = 124, 84.7% male) completed the Mid-Atlantic MIRECC Assessment of Traumatic Brain Injury (MMA-TBI), Salisbury Blast Interview (SBI), Clinician Administered PTSD scale (CAPS-5), cognitive assessment battery, and measures of depression, PTSD symptoms, neurobehavioral symptoms, sleep quality, pain interference, and quality of life.
Results:
Analyses of variance (ANOVA) results revealed significant differences in most behavioral health outcomes based on PTSD recovery, with participants who have recovered from PTSD showing less severe neurobehavioral and depressive symptoms, better sleep quality, less functional pain interference, and higher quality of life. No differences were found in cognitive functioning between those who have recovered from PTSD and those who have not. History of deployment mTBI did not significantly moderate the relationship between PTSD recovery and most functional and cognitive outcomes with the exception of two measures of processing speed. Specifically, among participants with history of deployment mTBI, those who have recovered from PTSD displayed better cognitive functioning than those who have not. Additionally, participants who have not recovered from PTSD had higher levels of blast exposure during military service.
Conclusions:
PTSD recovery was associated with better psychological functioning and higher quality of life, but not with objective cognitive functioning. Deployment mTBI history moderated only the relationship between PTSD recovery status and tests of processing speed.
Keywords: PTSD recovery, functional outcomes, quality of life, cognition, blast exposure
Posttraumatic stress disorder (PTSD) is the most commonly diagnosed psychiatric condition among post-deployment Iraq and Afghanistan Veterans (Ramsey et al., 2017). In this cohort of Veterans, the prevalence of PTSD has been estimated at 23% (Fulton et al., 2015), and approximately 52% of Veterans with at least one mental health diagnosis screen positive for PTSD (Seal et al., 2007). The severity and course of PTSD symptoms is variable, and many individuals experience full recovery (Santiago et al., 2013; Steinert et al., 2015). In the general population, approximately 35% of individuals recover from PTSD within four months post-diagnosis (Santiago et al., 2013), and up to 50% experience recovery within three to seven years (Steinert et al., 2015). Similarly, approximately half of post-9/11 Veterans (who served in Operations Enduring Freedom [OEF] and Iraqi Freedom [OIF]) no longer meet diagnostic criteria for PTSD three years after screening positive (Armenta et al., 2018). However, less is known about symptom presentation and functional outcomes associated with PTSD recovery in this population.
Research indicates that a number of behavioral health comorbidities may be negatively associated with the trajectory of PTSD symptoms. For example, depression has been linked with persistent non-remitting PTSD (Armenta et al., 2018), and it has been implicated in significantly lower rates of recovery from PTSD (Tural et al., 2012). Additionally, a relationship between physical health factors and the incidence and severity of PTSD symptoms has been observed. For example, there is evidence of a negative association between PTSD and health-related quality of life (HRQoL) among OEF/OIF Veterans (Pittman et al., 2012), with some studies suggesting that resolution of PTSD symptoms may be correlated with improved HRQoL (Gill et al., 2013). Higher PTSD symptoms have also been associated with greater pain severity and pain interference (Bourn et al., 2016), and research has demonstrated that people who do not recover from PTSD report more pain-related disability (Ravn et al., 2019); nevertheless, it is still unclear whether the opposite is true and whether individuals who experience resolution of PTSD symptoms would report less functional pain interference. Research has been more consistent regarding sleep disturbances and PTSD in that poor sleep quality has been associated with PTSD symptomatology (Armenta et al., 2018; Gilbert et al., 2015; Swinkels et al., 2013), and PTSD recovery has been associated with improvements in sleep quality (Gilbert et al., 2015). Finally, a relationship between PTSD symptoms and poorer cognition has been well documented, especially in the domains of verbal memory, processing speed, attention, and working memory (Scott et al., 2015). Yet, the association between PSTD recovery and cognition remains under-researched. In summary, literature examining psychological and cognitive outcomes of PTSD recovery is limited, and it remains largely unknown whether PTSD recovery extends beyond PTSD symptomatology and translates into a clinically meaningful reduction in non-PTSD symptoms or improvement in functional outcomes.
Multiple trauma variables have also been linked with PTSD recovery. One study found that higher cumulative exposure to traumatic events was related to lower rates of PTSD remission (Kolassa et al., 2010). These findings are particularly relevant for Iraq and Afghanistan Veterans as they are more likely to have been exposed to numerous traumatic events across multiple deployments. Subsequently, they may present with a different course and recovery from PTSD than Veterans from other eras. For example, one of the unique features of this cohort of Veterans is exposure to various blasts and explosions during military service. Blast exposure is the leading cause of injury among Iraq and Afghanistan Veterans, with some estimates suggesting that blasts may account for up to 78% of combat injuries (Owens et al., 2008). Blast exposure has been correlated with more severe PTSD symptoms (Reid et al., 2014), and combat injuries secondary to blast exposure have been associated with the incidence and severity of PTSD-like symptoms (Kennedy et al., 2010; Tschiffely et al., 2015).
Additionally, exposure to primary blast waves can lead to traumatic brain injury (TBI) (Song et al., 2018; Taber et al., 2015; Wolf et al., 2009), and approximately 27–44% of Veterans with history of mild TBI (mTBI) have clinically significant symptoms of PTSD (Hoge et al., 2008; Kontos et al., 2013). Deployment TBI may have significant effects on the development, severity, and course of PTSD. Specifically, TBI experienced during deployment has been identified as a risk factor in the development of PTSD (Yurgil et al., 2014), and primary blast TBI has been associated with more severe PTSD symptoms (Kennedy et al., 2010; Petrie et al., 2014; Tschiffely et al., 2015). Deployment-related TBI (mild and moderate) has also been shown to increase severity of symptoms and worsen functional outcomes in Veterans with PTSD (Vasterling et al., 2018). Outcomes related to PTSD and TBI are further complicated by a significant overlap and reciprocal influence of symptoms (Brenner et al., 2010; Walter et al., 2012). For example, individuals with both PTSD and TBI report more severe neurocognitive symptoms (Tanev et al., 2014). Moreover, both PTSD and deployment TBI have been linked to worse behavioral health outcomes (Martindale et al., 2018) and worse cognitive functioning (Martindale et al., 2020). However, less work has focused on the role of deployment TBI in PTSD recovery and in the relationship between PTSD and functional outcomes. Notably, many studies examining post-9/11 deployment TBI include samples that report history of either exclusively mTBI (e.g., Brenner et al., 2010; Kennedy et al., 2010; Martindale et al., 2020) or primarily mTBI, with 86–90% of individuals with TBI history endorsing only mTBI (e.g., Martindale et al., 2018; Vasteling et al., 2018; Walter et al., 2012). Thus, it is important to examine factors associated with PTSD recovery in the context of deployment mTBI.
In summary, many combat Veterans recover from PTSD over time, but factors associated with PTSD recovery are still not well understood. Identifying these factors may provide important clinical insight to improve treatment planning and functioning in this population. The first aim of this study was to examine neuropsychological and functional outcomes associated with recovery from PTSD. It was hypothesized that individuals who have recovered from PTSD would display lower levels of psychiatric symptoms, higher quality of life, and better cognitive functioning. The second aim of the study was to elucidate the role of deployment mTBI in the relationship between PTSD recovery and behavioral health outcomes. It was hypothesized that history of deployment mTBI would moderate this relationship, such that individuals with mTBI who had not recovered from PTSD would display more severe psychiatric symptoms and poorer cognitive functioning compared to other groups. Finally, we aimed to conduct an exploratory analysis of participant characteristics associated with PTSD recovery and expected that PTSD recovery would be related to fewer deployment mTBIs, fewer exposures to blast, and less combat exposure.
Method
Participants and Procedure
This cross-sectional analysis used data from an independent parent study funded by the Chronic Effects of Neurotrauma Consortium (CENC) investigating the effects of combat deployment and mTBI on OEF/OIF/OND Veterans. Participants were recruited through targeted mailings, flyers, and brochures located throughout the medical center, as well as through advertisements at community events and community centers serving veterans. This study was approved by the local Institutional Review Board. All participants provided verbal and written informed consent prior to study activities.
Inclusion criteria for the parent study were: at least one OEF/OIF/OND combat deployment [combat defined as any score of > 17 (minimum score) on the Deployment Risk and Resiliency Inventory-2, Section D (Vogt, Smith, King, & King, 2012)], English speaking, at least 18 years of age, able to comply with instructions to complete study tasks, and able to provide informed consent. Exclusion criteria were: any penetrating head injury; non-deployment related TBI with loss of consciousness (to reduce likelihood of greater than mild TBI severity at recruitment); and presence of a neurologic disorder, severe mental illness, dementia, current substance use disorder, or psychotic symptoms.
The initial sample collected for the parent study was N = 338. Participants were excluded from the present analytic sample if they: experienced a traumatic (Criterion A) event either prior to or after military service; had history of TBI that was greater than mild in severity; failed symptom validity assessment, defined by the published cut-off score (Wisdom, Callahan, & Shaw, 2010) on the Structured Inventory of Malingered Symptoms (SIMS; Smith & Burger, 1997); failed performance validity tests, defined by failing the Medical Symptom Validity Test (MSVT; Green, 2004) or the b Test (Boone et al., 2002) based on the cutoffs and procedures published in test manuals. In the original sample (N = 338), 46 participants (13.6%) failed the SIMS at >23 cutoff, and 69 participants (20.41%) failed at least one performance validity test. These groups were not mutually exclusive, as some participants (n = 22; 6.5%) failed both symptom validity and performance validity tests. Additionally, participants were excluded if they did not have lifetime history of PTSD diagnosis related to military service (n = 104). The final sample size used for analyses in the present study was N = 124.
Measures
Clinical Interviews
Demographic variables (age, sex, education, employment, race, and disability status) were obtained via a semi-structured interview. Disability status was rated as present if the participant was rated for any level of service connection for a mental health or physical health disability. Service connection is a disability rating from 0–100% determined by outpatient compensation and pension evaluations in the VA system. Disability ratings from other sources were not evaluated.
The Clinician-Administered PTSD Scale for DSM5 (CAPS-5; Weathers et al., 2017) was used to determine the presence of lifetime and current PTSD diagnosis. The CAPS-5 evaluates PTSD during the current month (the prior 30 days) and the worst month (the month of the most severe symptoms since the trauma) according to DSM-5 criteria. If an individual meets full criteria during the current month, they are considered to have current PTSD. If an individual met criteria for PTSD in their worst month and did not meet criteria for PTSD in their current month, then they are considered to have a lifetime diagnosis of PTSD (i.e., they met criteria for PTSD at one point in their lifetime, but do not currently). The CAPS-5 total score indicates the severity of symptoms for the current and worst month. All participants included in this analytic sample met criteria for lifetime PTSD. Recovery from PTSD (“PTSD recovery” group) was operationalized as a positive lifetime diagnosis of PTSD, but no current diagnosis of PTSD. Participants in the “PTSD Recovery” group may not have been free of PTSD symptoms, but any symptoms that were currently present did not meet threshold criteria for PTSD diagnosis. Participants who continued to meet criteria for current PTSD were included in the “current PTSD” group.
The Mid-Atlantic MIRECC Assessment of Traumatic Brain Injury (MMA-TBI; Rowland, Martindale, Shura, et al., 2020) was used to evaluate lifetime TBI history. This semi-structured interview evaluates potential concussive events across the lifespan according to the VA/DOD definition of TBI. History of deployment-related mild TBI was coded as binary (present/absent) for analyses.
The Salisbury Blast Interview (SBI; Rowland, Martindale, Spengler, et al., 2020) evaluated blast exposure history. The SBI gathers details about events involving blasts/explosions across the lifespan, regardless of distance or severity. Participants reporting the experience of any pressure change (score of 1 [slightly, noticeable but not uncomfortable] or above on the SBI) were categorized as having been exposed to a blast (yes/no). Maximum and average pressure experienced (i.e., severity), was measured using a behaviorally-anchored Likert scale ranging from 0 to 5 (0 = no pressure; 5 = strong pressure, resulted in greater than minor injury). Notably, though higher reported blast pressure is more likely to result in a TBI (Rowland, Martindale, Spengler et al., 2020), this is not a guaranteed outcome. Therefore, blast pressure severity and TBI represent independent, but potentially related constructs. The number of exposures (i.e., frequency), and the minimum distance from a blast event were also included as outcome variables.
Self-Report Measures
Study participants also completed several self-report questionnaires. The Patient Health Questionnaire (PHQ-9; Kroenke et al., 2001) is a 9-item self-report measure evaluating depressive symptoms over the past two weeks. The PHQ-9 is scored on a scale of 0–27 with higher scores indicating greater severity of depressive symptoms. The PTSD Checklist for DSM-5 (PCL-5; Blevins et al., 2015) is a 20-item questionnaire scored on a total scale of 0–80 that measures how bothered an individual has been by PTSD symptoms over the past month with higher scores indicating greater distress related to PTSD symptoms. The Neurobehavioral Symptom Inventory (NSI; Cicerone & Kalmar, 1995) is a 22-item self-report questionnaire assessing the severity of somatic/sensory, cognitive, and affective symptoms (King et al., 2012) over the past two weeks. The total score ranges from 0–88, with higher scores indicating more severe symptom burden. The Patient Reported Outcomes Measurement Information System Pain Interference (PROMIS-PI; Amtmann et al., 2010) is an 8-item questionnaire scored on a scale of 8–40 that measures the interference in daily activities caused by pain over the past seven days, with higher scores indicating higher pain interference. The Pittsburgh Sleep Quality Index (PSQI; Buysse et al., 1989) is a 9-item questionnaire that provides a global sleep quality score (over the past month) ranging from 0 to 21, with higher score indicating poorer sleep quality. Quality of Life After Brain Injury (QOLIBRI; von Steinbüchel et al., 2010) evaluates quality of life during the past week across several domains: cognition, self, daily life and autonomy, social relationships, emotions, and physical problems. Subscales were calculated according to recommended procedures (von Steinbüchel et al., 2010). Higher scores reflect better quality of life. Administration of the QOLIBRI was altered slightly: two questions requiring the presence of a brain injury to answer were removed as not all participants had history of TBI. Total scores were used in analyses for all measures.
Cognitive Tests
Additionally, study participants completed a neurocognitive assessment battery including the Wechsler Adult Intelligence Scale, fourth edition (WAIS-IV; Wechsler, 2008), Trail Making Test (TMT; Reitan & Wolfson, 1985) forms A and B, Controlled Oral Word Association Test (COWAT; Benton & Hamsher, 1989), and Semantic Fluency (Animal Naming; Benton & Hamsher, 1989). Scores on all cognitive measures were converted to demographically-corrected (sex, age, race, and education) T-scores (M = 50, SD = 10). WAIS-IV T-scores were derived from the WAIS-IV Advanced Clinical Solutions (ACS) demographically adjusted norms. T-scores for Animal Naming, COWAT, and TMT were derived from norms developed by Heaton and colleagues (2004).
Data Analysis
Data were analyzed using SAS 9.2 (SAS, Inc., Cary, NC). Factorial analyses of variance (ANOVA) were conducted to evaluate differences in continuous outcome variables based on PTSD recovery status, deployment mTBI status, and interaction of PTSD recovery and deployment mTBI, with follow up t-tests as needed. Chi-square analysis was utilized for dichotomous variables. Independent variables were dichotomously coded to denote: (1) current PTSD or PTSD recovery and (2) presence/absence of deployment mTBI history. Significance was set to α = .05, and effect sizes are reported in tables (Cohen’s d; phi, φ). False discovery rate (FDR) was used to adjust for multiple comparisons (Benjamini & Hochberg, 1995). FDR was applied to all outcome variables (demographics, functional outcomes, and cognitive tests) and significance is indicated after FDR correction throughout the text and tables.
Results
Demographics and Military History
Table 1 displays sample demographic information. The total sample was comprised of mostly male (n = 105; 84.68%) Veterans with an average age of 41.61 years (SD = 9.97). Most participants were White (n = 67; 54.03%), with 15.10 (SD = 2.04) years of education. The size of the two comparison groups was relatively equal, with n = 66 recovered from PTSD (i.e., PTSD Recovery Group), and n = 58 who met current criteria for a PTSD diagnosis (i.e., Current PTSD Group). The majority of participants in this sample reported history of at least one blast exposure (n = 100), with the modal number of exposures being 1 (n = 15). Median number of experienced blast events across the entire sample was 9.
Table 1.
Participant Demographics and Characteristics (N = 124)
| PTSD Recovery (n = 66) | Current PTSD (n = 58) | p | Effect Size d or φ | |||
|---|---|---|---|---|---|---|
| M or % | SD | M or % | SD | |||
| Age | 42.26 | 9.98 | 40.88 | 9.99 | .445 | d = 0.14 |
| Years of Education | 15.35 | 2.22 | 14.83 | 1.79 | .156 | d = 0.26 |
| Men | 86.4% | 82.8% | .578 | φ = 0.05 | ||
| Racial/ Ethnic Minority | 47.0% | 44.8% | .811 | φ = 0.02 | ||
| Employed | 68.2% | 56.9% | .194 | φ = 0.12 | ||
| Disability | 84.9% | 89.7% | .426 | φ = −0.07 | ||
| Percent of Service Connection | 70.36 | 26.42 | 74.09 | 25.68 | .452 | φ = −0.25 |
| Tours Served | 3.64 | 6.25 | 2.47 | 2.69 | .170 | φ = 0.24 |
| Deployment mTBI History | 56.5% | 43.6% | .472 | φ = 0.07 | ||
| Time Since Trauma (Days) | 4532 | 2391 | 4479 | 2564 | .907 | d = 0.02 |
| Combat Exposure (DRRI-2) | 35.05 | 13.69 | 37.05 | 14.08 | .423 | d = −0.14 |
| Blast Exposeda | 78.79% | 82.76% | .577 | φ = − 0.05 | ||
| Number of Blast Events | 240 | 921 | 235 | 557 | .970 | d = 0.01 |
| Minimum Blast Distance (Feet) | 238 | 730 | 364 | 1146 | .462 | d = −0.13 |
| Average Pressure Rating b | 1.06 | 0.82 | 1.60 | 1.20 | .004 | d = −0.52 |
| Maximum Pressure Rating c | 1.85 | 1.33 | 2.59 | 1.61 | .006 | d = −0.50 |
Note. PTSD = posttraumatic stress disorder; M = mean; SD = standard deviation; PTSD = posttraumatic stress disorder; d = Cohen’s d. φ = phi coefficient; disability status was rated as present if the participant was rated for any level of service connection for a mental health or physical health disability; service connection is a disability rating from 0–100% determined by outpatient compensation and pension evaluations in the VA system; mTBI = mild traumatic brain injury; DRRI-2 = Deployment Risk and Resiliency Inventory-2, Section D (Combat Experiences), total score;
blast exposed = percentage of participants who were exposed to a blast event with at least a low pressure wave, equal to a rating of 1 on the SBI pressure wave Likert scale ranging from 0 (no blast exposure) to 5 (most severe blast exposure);
average pressure rating = the average of all pressure wave ratings across experienced blast events;
maximum pressure rating = the highest pressure wave rated across all of a participant’s experienced blast events. Bold font indicates statistical significance after False Discovery Rate correction for multiple comparisons.
As shown in Table 1, demographic variables were not significantly associated with PTSD recovery (p > .05 for all demographic variables). Specifically, age, years of education, sex, racial minority status, disability status, and employment status were not associated with PTSD recovery. Also, there were no differences between the two groups (those who have recovered from PTSD and those who had a current PTSD diagnosis) in terms of most military variables, including the number of tours served, history of deployment mTBI, time since traumatic event, and combat exposure, but significant differences were found between the two groups in terms of some blast exposure variables. Although the percentage of participants who reported blast exposure and the number of experienced blast events were similar between the two groups, differences were observed in terms of average and maximum pressure ratings. Participants who have not recovered from PTSD reported significantly higher average pressure and maximum pressure ratings associated with blast events.
Behavioral Health, Functional, and Cognitive Outcomes
There were significant differences in most behavioral health outcomes based on PTSD recovery status (see Table 2). Participants who had recovered from PTSD reported significantly lower symptoms of depression and PTSD, lower levels of neurobehavioral symptoms and pain interference, better sleep quality, and higher quality of life (in terms of cognition, self-esteem, emotional health, and physical health) as compared to those who had current PTSD diagnosis (Table 3). However, no differences in cognitive performance were noted between those who recovered from PTSD and those who did not (Table 3). Participants with deployment mTBI history reported lower quality of life in the physical health domain, but no other behavioral health differences were present. Additionally, participants with deployment mTBI history had lower scores on TMT-A and TMT-B. Finally, interactions between PTSD recovery status and deployment mTBI history were observed for the Processing Speed Index of the WAIS-IV and TMT-A. Graphic representations of these interactions are presented in Figures 1 and 2. The pattern of interaction was similar for both variables: participants with history of deployment mTBI who have recovered from PTSD displayed better cognitive functioning than those who have not recovered.
Table 2.
ANOVA Results for Behavioral Health and Cognitive Outcomes by PTSD Recovery Status and Deployment Mild TBI History (N = 124)
| Omnibus Model | PTSD Recovery | Deployment mTBI | Interaction | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measures | F | p | R2 | η2 | F | p | ηp2 | F | p | ηp2 | F | p | ηp2 |
| Behavioral Health | |||||||||||||
| NSI Total | 4.87 | .003 | .099 | .10 | 11.01 | .001 | .075 | 2.92 | .090 | .020 | 0.57 | .454 | .004 |
| NSI Affective | 6.80 | <.001 | .133 | .13 | 18.42 | <.001 | .120 | 1.51 | .222 | .010 | 0.27 | .605 | .002 |
| NSI Cognitive | 2.84 | .040 | .060 | .06 | 7.59 | .007 | .054 | 1.10 | .297 | .008 | 0.07 | .786 | .001 |
| NSI Somatic | 4.17 | .007 | .086 | .09 | 7.86 | .006 | .054 | 2.92 | .090 | .020 | 1.45 | .231 | .010 |
| PCL-5 | 10.97 | <.001 | .198 | .20 | 28.98 | <.001 | .175 | 2.03 | .157 | .012 | 1.13 | .289 | .007 |
| PHQ-9 | 7.47 | <.001 | .144 | .14 | 21.70 | <.001 | .140 | 0.00 | .968 | <.001 | 0.14 | .709 | .001 |
| PROMIS-PI | 3.07 | .030 | .065 | .06 | 5.87 | .017 | .041 | 3.14 | .079 | .022 | 0.26 | .609 | .002 |
| PSQI | 3.41 | .020 | .071 | .07 | 8.47 | .004 | .060 | 0.00 | .948 | <.001 | 1.09 | .298 | .008 |
| QOLIBRI Total | 3.09 | .030 | .065 | .07 | 8.06 | .005 | .057 | 0.05 | .823 | <.001 | 0.59 | .443 | .004 |
| Cognition | 2.07 | .120 | .045 | .04 | 5.79 | .018 | .042 | 0.09 | .764 | <.001 | 0.14 | .708 | .001 |
| Self-Esteem | 4.93 | .003 | .100 | .10 | 14.34 | <.001 | .010 | 0.06 | .806 | <.001 | 0.04 | .845 | <.001 |
| Independence | 0.70 | .554 | .016 | .02 | 1.63 | .204 | .012 | 0.25 | .616 | .002 | 0.08 | .779 | <.001 |
| Social Relationships | 2.43 | .068 | .052 | .05 | 1.16 | .283 | .008 | 2.84 | .094 | .020 | 2.61 | .109 | .019 |
| Emotional Health | 2.83 | .041 | .060 | .06 | 7.54 | .007 | .053 | 0.01 | .918 | <.001 | 0.47 | .494 | .003 |
| Physical Health | 3.81 | .012 | .079 | .08 | 4.46 | .037 | .031 | 6.94 | .010 | .048 | 0.23 | .632 | .002 |
| Cognitive Functioning | |||||||||||||
| WAIS-IV: | |||||||||||||
| PRI | 1.42 | .241 | .031 | .03 | 0.27 | .604 | .002 | 0.96 | .329 | .007 | 3.07 | .082 | .022 |
| VCI | 1.47 | .225 | .032 | .03 | 0.34 | .562 | .003 | 0.12 | .732 | <.001 | 3.77 | .054 | .023 |
| WMI | 1.00 | .397 | .022 | .02 | 0.54 | .464 | .004 | 0.44 | .507 | .003 | 1.88 | .172 | .014 |
| PSI | 4.58 | .004 | .094 | .09 | 1.42 | .235 | .010 | 2.03 | .157 | .014 | 9.87 | .002 | .067 |
| Semantic Fluency | 1.66 | .178 | .037 | .04 | 3.73 | .056 | .027 | 0.97 | .327 | .007 | 0.36 | .545 | .003 |
| Phonemic Fluency | 1.23 | .300 | .027 | .03 | 0.01 | .934 | <.001 | 3.47 | .065 | .026 | 0.26 | .610 | .002 |
| TMT-A | 7.68 | <.001 | .149 | .15 | 1.48 | .226 | .010 | 16.27 | <.001 | .105 | 5.49 | .021 | .035 |
| TMT-B | 3.56 | .016 | .075 | .07 | 2.90 | .091 | .020 | 6.00 | .016 | .042 | 1.75 | .188 | .012 |
Note. Type III SS reported for omnibus models including two main effects and an interaction effect. mTBI = mild traumatic brain injury; PTSD = posttraumatic stress disorder; Interaction = interaction of deployment mTBI and PTSD recovery variables; η2 = eta squared; ηp2 = partial eta squared; NSI = Neurobehavioral Symptoms Inventory; PCL-5 = PTSD Checklist for DSM-5; PHQ-9 = Patient Health Questionnaire; PROMIS-PI = Patient Reported Outcomes Measurement Information System, Pain Interference; PSQI = Pittsburgh Sleep Quality Index; QOLIBRI = Quality of Life After Brain Injury; WAIS-IV = Wechsler Adult Intelligence Scale, fourth edition; PRI = Perceptual Reasoning Index; VCI = Verbal Comprehension Index; WMI = Working Memory Index; PSI = Processing Speed Index; Semantic Fluency = Animal Naming Test; Phonemic Fluency = Controlled Oral Word Association Test; TMT-A = Trail Making Test A; TMT-B = Trail Making Test B. Bold font indicates statistical significance after False Discovery Rate correction for multiple comparisons.
Table 3.
PTSD Recovery and Behavioral Health Outcomes (N = 124)
| Outcome Measures | PTSD Recovery (n = 66) | Current PTSD (n = 58) | p | Cohen’s d | ||
|---|---|---|---|---|---|---|
| M | SD | M | SD | |||
| Behavioral Health | ||||||
| PHQ-9 Total | 9.76 | 6.15 | 14.53 | 5.50 | <.001 | 0.82 |
| PCL-5 Total | 27.58 | 16.31 | 41.48 | 14.21 | <.001 | 0.91 |
| Worst Month CAPS-5 | 39.50 | 12.54 | 47.83 | 13.62 | <.001 | 0.64 |
| Current Month CAPS-5 | 13.53 | 8.73 | 33.02 | 8.88 | <.001 | 2.21 |
| NSI Total | 21.77 | 16.00 | 30.00 | 11.76 | .002 | 0.59 |
| NSI Affective | 9.47 | 6.75 | 14.02 | 5.20 | <.001 | 0.75 |
| NSI Cognitive | 4.67 | 3.71 | 6.22 | 3.17 | .014 | 0.45 |
| NSI Somatic | 7.48 | 6.47 | 10.79 | 6.54 | .006 | 0.51 |
| PROMIS Pain Interference | 17.61 | 9.19 | 20.91 | 8.45 | .040 | 0.37 |
| PSQI Global | 10.53 | 4.33 | 12.33 | 3.45 | .013 | 0.46 |
| QOLIBRI | 56.34 | 18.92 | 47.69 | 13.38 | .004 | −0.53 |
| Cognition | 16.71 | 6.32 | 14.19 | 5.31 | .019 | −0.43 |
| Self-Esteem/Motivation | 14.94 | 6.55 | 10.62 | 5.09 | <.001 | −0.74 |
| Independence | 17.26 | 6.07 | 15.62 | 4.96 | .106 | −0.30 |
| Social Relationships | 12.89 | 5.53 | 11.67 | 5.96 | .239 | −0.21 |
| Emotional Health | 12.91 | 5.24 | 10.71 | 4.67 | .016 | −0.44 |
| Physical Health | 8.67 | 2.78 | 7.78 | 2.45 | .062 | −0.34 |
| Cognitive Functioning | ||||||
| Semantic Fluency | 48.41 | 9.24 | 51.16 | 8.28 | .087 | 0.31 |
| Phonemic Fluency | 47.02 | 8.96 | 47.26 | 10.24 | .889 | 0.03 |
| TMT-A | 48.91 | 9.66 | 47.60 | 12.41 | .514 | −0.12 |
| TMT-B | 49.78 | 8.48 | 46.83 | 11.80 | .110 | −0.29 |
| WAIS-IV: | ||||||
| Block Design | 49.83 | 8.08 | 50.79 | 10.28 | .562 | 0.10 |
| Similarities | 48.68 | 9.16 | 48.40 | 9.51 | .865 | −0.03 |
| Digit Span | 47.76 | 9.54 | 46.22 | 10.42 | .394 | −0.15 |
| Matrix Reasoning | 50.45 | 9.43 | 50.48 | 11.31 | .988 | 0.00 |
| Vocabulary | 50.47 | 8.78 | 49.21 | 9.15 | .435 | −0.14 |
| Arithmetic | 45.62 | 10.05 | 44.79 | 11.42 | .668 | −0.08 |
| Symbol Search | 51.11 | 9.19 | 49.93 | 10.90 | .516 | −0.12 |
| Visual Puzzles | 50.89 | 10.64 | 53.00 | 10.45 | .270 | 0.20 |
| Information | 48.65 | 10.61 | 50.03 | 9.64 | .451 | 0.14 |
| Coding | 48.86 | 10.43 | 46.34 | 11.13 | .196 | −0.23 |
Note. PTSD = Posttraumatic stress disorder; M = mean; SD = standard deviation;
all differences with p values below .05 remained statistically significant after False Discovery Rate correction at p < .05; PHQ-9 = Patient Health Questionnaire; PCL-5 = PTSD Checklist for DSM-5; CAPS-5 = Clinician-Administered PTSD Scale-5; NSI = Neurobehavioral Symptom Inventory; PROMIS = Patient-Reported Outcomes Measurement Information System; PSQI = Pittsburgh Sleep Quality Index; QOLIBRI = Quality of Life After Brain Injury; Semantic Fluency = Animal Naming Test; Phonemic Fluency = Controlled Oral Word Association Test; TMT-A = Trail Making Test A; TMT-B = Trail Making Test B; WAIS-IV = Wechsler Adult Intelligence Scale, 4th edition. Bold font indicates statistical significance after False Discovery Rate correction for multiple comparisons.
Figure 1. PSI Standard Scores Based on PTSD Recovery Status and Deployment Mild TBI History.
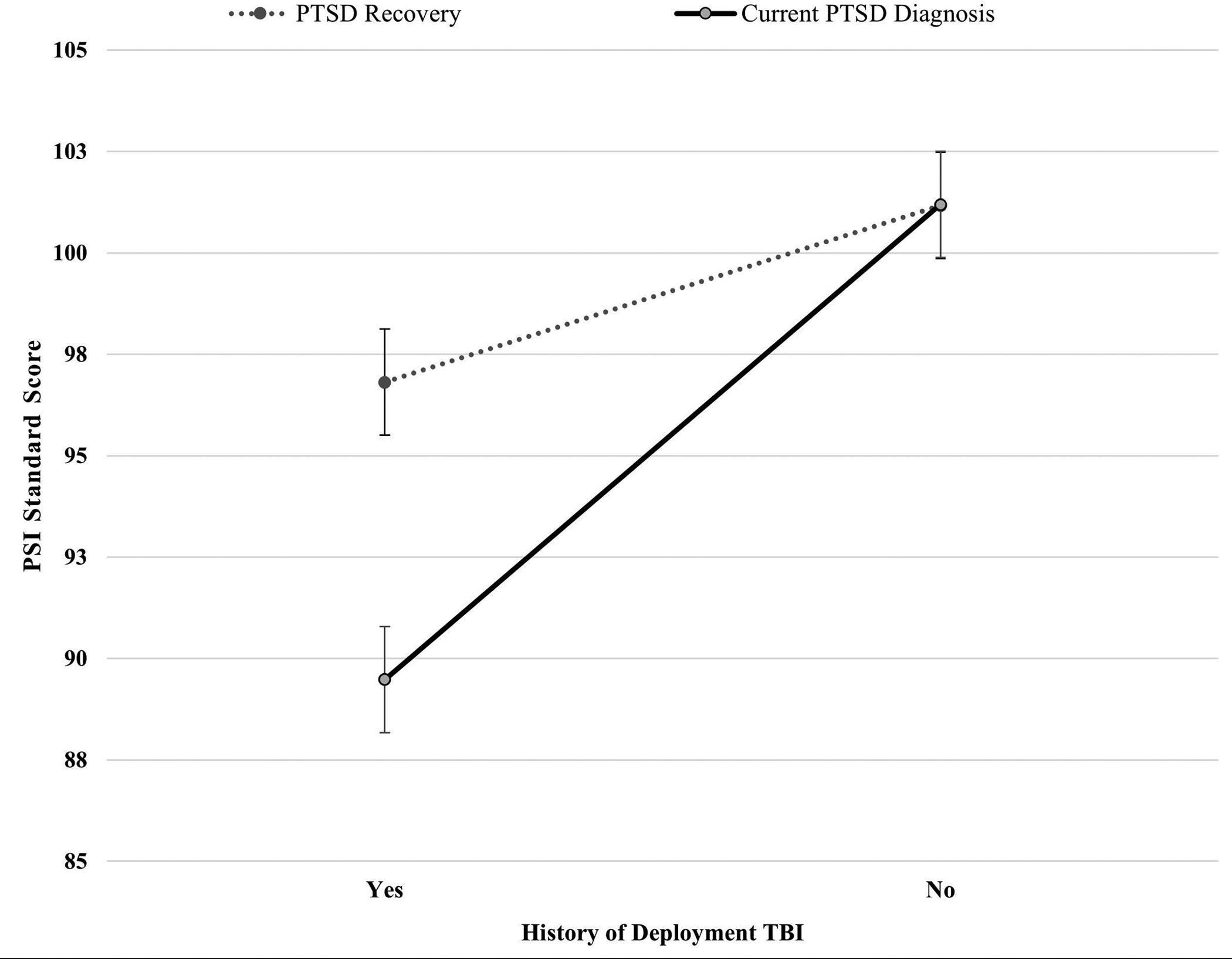
Note. Standard scores have a mean of 100 and standard deviation of 15, with higher scores indicating better cognitive functioning. PSI = Processing Speed Index of the Wechsler Adult Intelligence Scale, 4th edition; PTSD = posttraumatic stress disorder; Deployment TBI = deployment-related mild traumatic brain injury. Among participants without history of deployment mild TBI, the difference in PSI scores based on PTSD recovery status was not statistically significant. However, among participants with history of deployment mild TBI, the difference between those who have recovered from PTSD (M = 96.81; SD = 12.41) and those who have not recovered (M = 89.48; SD = 11.05) was statistically significant (p = .003) with a medium effect size (Cohen’s d = 0.50). Error bars represent standard error.
Figure 2. TMT-A T-Scores Based on PTSD Recovery Status and Deployment Mild TBI History.
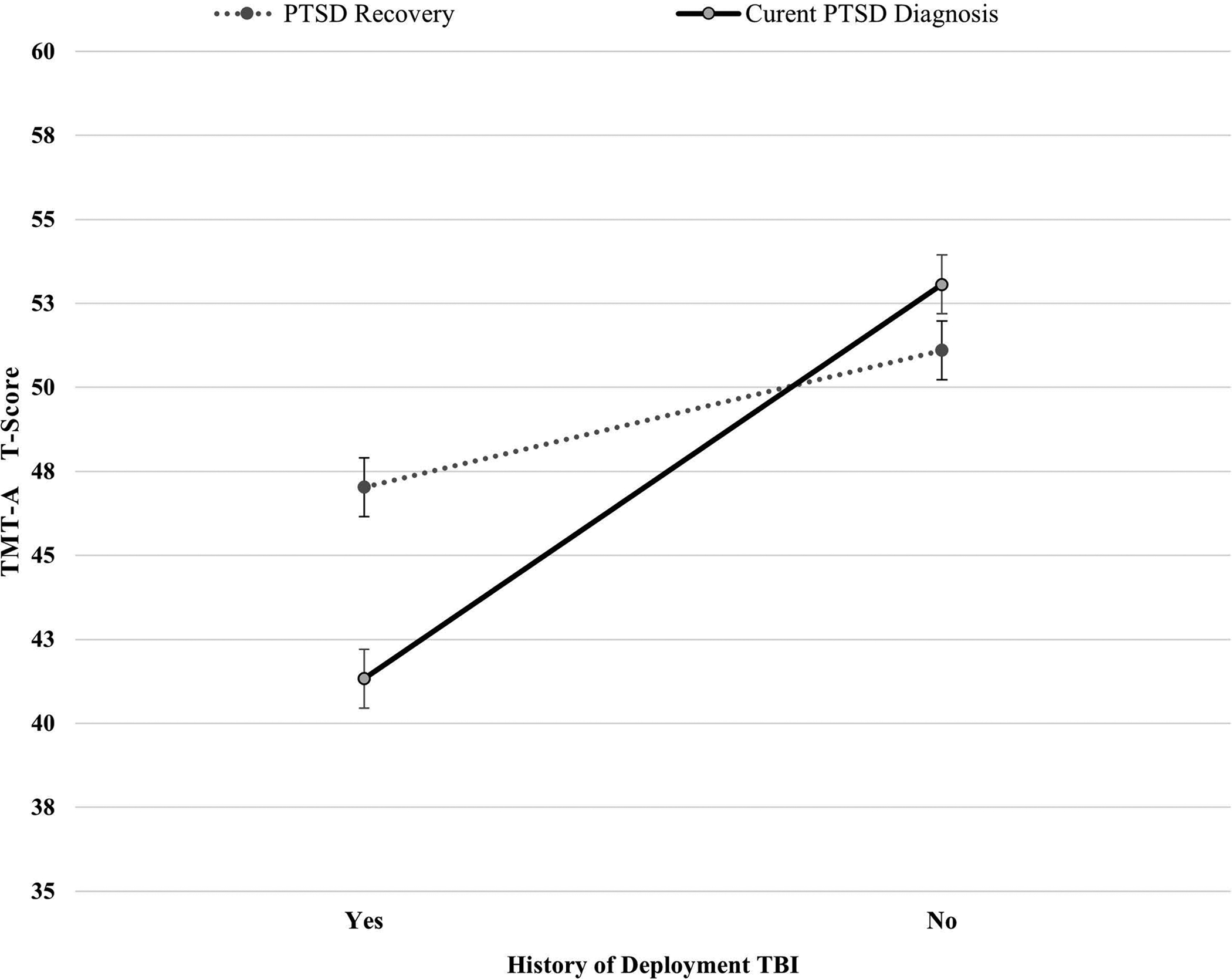
Note. T-scores have a mean of 50 and standard deviation of 10, with higher scores indicating better cognitive functioning. TMT-A = Trail Making Test A; PTSD = posttraumatic stress disorder; Deployment TBI = deployment-related mild traumatic brain injury. Among participants without history of deployment mild TBI, the difference in TMT-A scores based on PTSD recovery status was not statistically significant. However, among participants with history of deployment mild TBI, the difference between those who have recovered from PTSD (M = 47.03; SD = 9.62) and those who have not recovered (M = 41.33; SD = 12.30) was statistically significant (p = .018) with a medium effect size (Cohen’s d = 0.52). Error bars represent standard error.
Discussion
This study evaluated the relationship between recovery from PTSD and a number of factors across a variety of biopsychosocial domains, including neurobehavioral and psychiatric symptoms, cognitive functioning, quality of life, and participant characteristics. Results indicated that PTSD recovery was associated with better functioning across most behavioral health symptoms and quality of life, highlighting the broad spectrum of positive functional outcomes associated with recovery from PTSD. No significant differences were observed in cognitive functioning based on PTSD recovery status alone. However, interactions were noted between PTSD recovery and deployment mTBI history for two cognitive measures of processing speed (the PSI on the WAIS-IV and TMT-A), indicating that participants with deployment mTBI history who had recovered from PTSD performed better on those measures than those who had not. Results also revealed that participants who had not recovered from PTSD were more likely to have experienced blasts of higher severity. No other military variables or demographic characteristics were different between those who had or had not recovered from PTSD.
The finding that participants who had recovered from PTSD reported lower severity of blast exposure than those who had not raises the possibility that higher severity of blast exposure may be related to increased chronicity of PTSD. The exact mechanism through which this relationship could occur is still not clear. It is possible that blast events may be inherently more traumatic and therefore likely to result in chronic symptomology. It is also possible that blast exposures of higher severity may affect brain structure or function in a manner that alters the PTSD recovery process. In fact, there is accumulating evidence that blasts can affect brain structure and function (Davenport et al., 2012; Hayes, Morey, & Tupler, 2012; Song et al., 2018; Taber et al., 2015), although the effect appears to be diffuse and non-specific. In addition, there is evidence that the experience of a pressure wave may contribute to allostatic load (maladaptive function of neural circuitry that calibrates behavioral and physical responses to stress within the brain), thus putting the brain in a vulnerable state when attempting to adapt to stress-related physical and mental conditions (McEwen et al., 2012; McEwen & Gianaros, 2011). Overall, findings of the present study raise the possibility that it is the severity of blast exposure (but not necessarily the number of exposures) that may play an important role in recovery from PTSD.
Moreover, recovery from PTSD was associated with lower report of symptoms across several domains (neurobehavioral symptoms, depression, pain interference, and sleep quality) as well as greater satisfaction with cognitive ability, self-esteem/motivation, emotional health, and physical health. The findings of the present study are generally consistent with published research demonstrating that PTSD recovery has been associated with improvements in sleep quality (Gilbert et al., 2015), lower levels of depression and somatic symptoms (Armenta et al., 2018; Forbes et al., 2003), and better health-related quality of life (Gill et al., 2013). While many of these findings were not surprising, they illustrate the depth and breadth of the effect that recovery from PTSD may have on behavioral health functioning and emphasize the importance of considering recovery across many areas of psychological, social, and physical functioning. Yet, it is important to note that the data utilized in the present study are cross-sectional; hence, it is unclear if these differences existed pre-trauma and consequently led to a better prognosis. At the same time, functional outcomes in the present study were assessed based on the period of 1–2 weeks prior to the study, suggesting that the outcome measures assess current (not premorbid) levels of behavioral health symptoms. Therefore, it is possible that better functioning and less severe psychological symptoms may be a result of resolution of PTSD symptoms, or perhaps PTSD recovery may be more likely to occur in the absence of comorbid psychopathology. Given the cross-sectional design, it is outside of scope for this study to comment on the temporal or causal relationship between these constructs, but future research can examine longitudinal associations between PTSD recovery and functional outcomes in more depth and detail.
Further, much research has highlighted the importance of social support in recovery across psychological and medical disorders/conditions (Birkeland et al., 2017; Hendryx et al., 2009). However, the current study found no difference in satisfaction with social relationships between those with current PTSD and those who have recovered. Since this was not a longitudinal study, we cannot assess the change in satisfaction with social relationships over the course of PTSD recovery. It remains possible that participants who have recovered from PTSD were less satisfied with their social relationships prior to recovery. Another possibility is that participants who have recovered from PTSD may continue to experience subthreshold levels of PTSD symptoms, including those affecting social engagements such as detachment from others or lack of positive emotions. Lastly, actual social support and social satisfaction may not be commensurate. Future studies may examine this distinction more closely.
The present study also sought to evaluate the moderating effects of deployment mTBI on the relationship between PTSD and functional outcomes. No significant interactions between mTBI history and PTSD recovery status were found for any behavioral health outcomes and for the majority of cognitive outcomes. This is consistent with some research demonstrating that PTSD and mTBI may not have synergistic negative effects on cognition, and that PTSD does not necessarily have an exacerbating effect on cognitive functioning in Veterans with mTBI (Gordon et al., 2011). At the same time, other studies have found differential results for cognitive and functional outcomes. For example, Merritt and colleagues (2019) reported no significant group differences in neuropsychological performance among Veterans with comorbid mTBI and PTSD as compared to those with PTSD only, mTBI only, or the combat-exposed control group, but they found that the comorbid mTBI and PTSD group had worse functional outcomes (Merritt et al., 2019).
Nonetheless, two notable interaction effects between mTBI history and PTSD recovery status were observed in the present study on measures of processing speed. Specifically, among participants with history of deployment mTBI, those who had recovered from PTSD displayed better cognitive functioning than those who had not. And, in fact, some research has revealed similar findings, albeit in different cognitive domains. For example, Gilmore et al. (2018) found that Veterans with comorbid PTSD and history of remote mild TBI demonstrated diminished brain response during a sustained visual attention task (Gilmore et al., 2018). Further, Pagulayan et al. (2018) reported that Veterans with mTBI and PTSD displayed significantly lower performance on measures of prospective memory compared to controls (Pagulayan et al., 2018). The mechanisms underlying these findings are not quite clear. It is possible that history of mild neurotrauma related to deployment mTBI may exacerbate non-remitting PTSD symptoms, which may in turn result in poorer cognitive functioning in some domains, thus demonstrating a synergistic negative effect between mTBI and PTSD on cognition. Yet, it should be noted that effect sizes for cognitive variables in the present study were relatively small, and that mean scores on cognitive tests in all groups were in the low average to average (not impaired) range. This is a salient caveat to consider when interpreting results of the present study. More studies are needed to elucidate the interplay between deployment mTBI and PTSD recovery in relation to functional outcomes.
It is also important to further highlight the clinical interpretation of scores in this study. On average, the participants who experienced PTSD recovery reported PCL-5 total scores below the clinical cutoff score of 33 (M = 27.58, SD = 16.31), whereas participants who were still meeting criteria for PTSD diagnosis at the time of the study had PCL-5 scores well above the clinical cutoff (M = 41.48, SD = 14.21). However, many participants in the PTSD recovery group still endorsed some distressing symptoms judging by the standard deviation of scores in that group. Moreover, participants in the PTSD recovery group, on average, reported a clinically mild to moderate range of depressive symptoms based on their PHQ-9 scores (M = 9.76, SD = 6.15), as compared to those in the current PTSD group who endorsed moderate to severe symptoms of depression (M = 14.53, SD = 5.5). Additionally, all participants in the present study reported poor sleep quality above the clinical cutoff of 5 on the PSQI, regardless of their PTSD recovery status. Although there were statistically significant differences between participants with PTSD recovery and current PTSD, these data reveal that Veterans who have experienced PTSD recovery may still have clinically relevant symptoms. Overall, findings indicate that while individuals who have recovered from PTSD likely experience less severe symptom burden, clinically significant levels of symptomatology may still be present. Such individuals may benefit from additional treatment targeting areas in which they are exhibiting clinically elevated symptoms (e.g., depression, sleep, etc.). Of note, this pattern was not found in cognitive outcomes. Furthermore, effect sizes for behavioral health variables were much larger as compared to cognitive variables. This suggests that resolution of PSTD symptoms is likely to have a stronger association with psychological and neurobehavioral symptoms, but a weaker correlation with cognitive functioning.
Findings of the present study have important clinical and research implications. Our results showed generally no significant differences in cognitive functioning based solely on PTSD recovery history, and mean scores on cognitive tests for all groups were in the low average to average (not impaired) range. These findings are promising, and clinicians may utilize them when treating individuals with PTSD. Specifically, patients may be encouraged that even if they have current PSTD symptoms, they would not be necessarily expected to display significant deficits in cognitive functioning based on PTSD symptomatology alone. However, if individuals have history of deployment mTBI combined with unremitting symptoms of PTSD, clinicians may expect mild reductions in processing speed in some (but not all) patients. Consequently, implementing cognitive rehabilitation interventions that focus on processing speed may be clinically indicated for those patients. Similar to results of the present study, Nelson et al. (2009) reported significant differences in processing speed based on the presence of PTSD comorbid with mTBI history in post-9/11 Veterans, and asserted that rehabilitation interventions may need to be adjusted for slowed processing speed in this population. Clinicians may wish to adapt existing “gold standard” approaches to PTSD treatment (e.g., Cognitive Processing Therapy) by allotting more time for patients to organize their thoughts and reflections (Nelson et al., 2009). It would also be reasonable to suggest that clinicians may design compensatory strategies with these findings in mind (e.g., patients may be encouraged to allow additional time when completing complex or timed tasks, and they may require more time to complete certain assignments in occupational or educational settings).
Further, our study underscores the importance of targeting PTSD symptoms when addressing behavioral health concerns in Veterans with history of deployment mTBI, as our findings suggested that resolution of PTSD symptoms may carry over into other areas of functioning, including lower symptoms of depression, better sleep quality, less functional pain interference, and better quality of life. Because of these associations between PTSD symptoms and other behavioral health outcomes, clinicians working towards rehabilitation of patients with PTSD may consider integrative and multidisciplinary approaches that would focus on combinations of symptoms. Due to a high rate of comorbidity between PTSD and depression in post-9/11 Veterans, various innovative approaches have been developed for the treatment of both conditions. For example, Strachan et al. (2012) described an integrated approach to delivering exposure-based treatment for symptoms of PTSD and depression in this cohort of Veterans. They reported promising results of various applications of Behavioral Activation and Therapeutic Exposure (BA-TE) treatment (both in person and via home-based telehealth). Specifically, they found that BA-TE resulted in symptom reduction for both PTSD and depression. Therefore, clinicians involved in rehabilitation of Veterans with comorbid psychiatric conditions may explore treatments that simultaneously address several clusters of symptoms.
Additionally, clinicians working in rehabilitation settings may consider integrating sleep-focused interventions in the treatment of Veterans with PTSD, as our findings revealed a significant association between PTSD symptoms and sleep quality; Veterans in the “PTSD recovery” group reported significantly better sleep quality compared to those in the “Current PTSD” group. A number of behavioral health treatments targeting sleep quality – including CBT for insomnia (CBT-i) – may be beneficial. For example, Rusch et al. (2015) examined the role of CBT-i (along with other interventions) in the improvement of sleep quality and comorbid symptoms among military personnel. They reported that sleep improvement corresponded with significant declines in symptoms of depression and PTSD, whereas deterioration in sleep quality was related to a decrease in health-related quality of life. The investigators concluded that sleep-focused treatments may be an effective way to facilitate psychiatric recovery (Rusch et al., 2015). Similarly, Ord et al. (2020) demonstrated that sleep was significantly associated with quality of life beyond symptoms of PTSD and deployment TBI history in post-9/11 Veterans, and suggested that behavioral sleep treatments may be beneficial as an adjunct or first-line treatment for Veterans with PTSD and/or TBI history. Taken as a whole, results of the present study and extant research underscore the importance of comprehensive rehabilitation approaches when treating individuals with PTSD.
Finally, regarding implications for future research, the current study provides an impetus for further examination of the complex interaction between PTSD symptoms and history of deployment mTBI in the context of behavioral health and cognitive functioning. Some published studies have identified deployment TBI as a risk factor in the development of PTSD (Yurgil et al., 2014), and others have linked blast TBI with more severe PTSD symptoms (Kennedy et al., 2010; Petrie et al., 2014). Yet, published literature examining associations between deployment TBI, PTSD, and neuropsychological functioning continues to produce mixed results. Some studies have shown that cognitive outcomes do not differ based solely on history of mTBI (Brenner et al., 2010; Verfaellie et al., 2014), but are rather associated with PTSD (Shandera-Ochsner et al., 2013; Storzbach et al., 2015; Verfaellie et al., 2014). Conversely, other research has reported that history of deployment TBI – but not current PTSD – may be associated with poorer cognitive functioning (Martindale et al., 2020). Given inconsistent findings regarding neuropsychological outcomes of comorbid mTBI and PTSD, additional research is warranted to further investigate these multifaceted relationships.
The findings of the present study ought to be considered in the context of several strengths and limitations. A major strength of our study is the use of the gold-standard PTSD diagnostic interview (CAPS-5) in order to conceptualize PTSD recovery. Much of the current literature utilizes self-report screeners, such as the PCL-5, to track PTSD symptomatology; yet, the PCL-5 is not traditionally a diagnostic measure. The use of a well-validated diagnostic interview conducted by trained mental health professionals allows for a more accurate diagnosis of lifetime and current PTSD, which leads to more reliable conclusions regarding PTSD recovery and functional outcomes associated with it. Another strength of the study is the utilization of published and validated comprehensive interviews assessing blast exposure and lifetime TBI history. However, even though these measures were administered by staff trained in mental health assessment, information obtained through these measures is ultimately self-reported, and self-reported data may be affected by participants inaccurately recalling information about events that occurred a long time ago or by lack of veracity or accuracy in responding (whether intentional or not). To address the latter concern, all participants who failed symptom validity testing were excluded from the present study.
A notable limitation is the cross-sectional nature of the study which did not allow for a longitudinal examination of PTSD symptom improvement. Future studies may utilize longitudinal designs and track PTSD symptoms over time, concurrently with other behavioral health and cognitive outcomes, to ascertain whether resolution of PTSD symptoms temporally corresponds to improvements in other domains of functioning. An additional limitation with Veteran research is small sample sizes for female Veterans. In this study, the sample size for female Veterans was too small to draw statistical conclusions. Because female Veterans are the population with the greatest projected growth in the military, future studies examining PTSD recovery should aim to include a higher percentage of female participants. Additionally, treatment data were not collected as part of the parent study; consequently, we were unable to evaluate whether specific types of treatment may have affected PTSD recovery status. Finally, based on exclusion criteria of the parent study, Veterans with previous history of civilian mTBI with loss of consciousness had been excluded from recruitment, although Veterans with prior mTBI with alteration of consciousness may have been included in the sample. Removal of these participants from the analytic sample did not significantly affect the pattern of findings. Nevertheless, further studies are needed to evaluate differential effects of mTBI acquired in deployment versus non-deployment settings.
Conclusions
In conclusion, results of this study revealed that Veterans who had recovered from PTSD reported lower levels of symptoms across many behavioral health domains, suggesting that PTSD recovery extends beyond PTSD symptoms and is associated with a number of positive functional outcomes, including lower levels of neurobehavioral and depressive symptoms, better sleep quality, lower pain interference, and higher quality of life. No significant differences were found between participants who had recovered from PTSD and those who still met criteria for PTSD on measures of cognitive functioning. Blast events of higher severity were associated with lower recovery rates from PTSD. Lastly, history of deployment mTBI was not associated with the majority of behavioral health or cognitive outcomes, and it did not appear to moderate the relationship between PTSD recovery and most functional outcomes. However, two significant interactions between deployment mTBI history and PTSD recovery status were noted on measures of processing speed. Specifically, among participants with history of deployment mTBI, those who had recovered from PTSD displayed better cognitive functioning than those who had not. Results should be interpreted in the context of relatively small effect sizes for cognitive variables and mean scores in the low average to average (not impaired) range for all groups on cognitive tests.
Impact.
Veterans who have recovered from posttraumatic stress disorder (PTSD) show less severe neurobehavioral and depressive symptoms, better sleep quality, less functional pain interference, and higher quality of life. No significant differences were generally found in cognitive functioning between those who have recovered from PTSD and those who have not.
Findings highlight the importance of PTSD treatment in combat Veterans, as reduction in PTSD symptoms may correspond with better sleep quality, less severe psychiatric symptoms, and improved functional outcomes.
Among Veterans with history of deployment mild traumatic brain injury (mTBI), those who have recovered from PTSD displayed better cognitive functioning on tests of processing speed than those who have not (although effect sizes were small to medium). This finding underscores the importance of targeting PTSD symptoms when addressing behavioral health concerns in Veterans with history of deployment mTBI, as resolution of PTSD symptoms may correlate with improved cognitive functioning in some domains.
Acknowledgements
We would like to thank the Veterans and Service Members who participated in this research. We would also like to thank Mary Peoples, David J. Curry, MSW, Alana M. Higgins, MA, Christine Sortino, MS, and G. Melissa Evans, MA, for their contributions to this project.
Footnotes
Disclosure
There are no conflicts of interest to disclose. This work was supported by grant funding from the Department of Defense, Chronic Effects of Neurotrauma Consortium (CENC) Award W81XWH-13-2-0095 and Department of Veterans Affairs CENC Award I01 CX001135. This work was also supported by resources of the Research & Academic Affairs Service Line, Salisbury Veterans Affairs Healthcare System, Mid-Atlantic Mental Illness Research Education and Clinical Center (MIRECC), and Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness, Research, and Treatment (MIRT).
Publisher's Disclaimer: Disclaimer
Publisher's Disclaimer: The views, opinions, and/or findings contained in this article are those of the authors and should not be construed as an official US Department of Veterans Affairs or US Department of Defense position, policy or decision, unless so designated by other official documentation.
References
- Amtmann D, Cook KF, Jensen MP, Chen WH, Choi S, Revicki D, Cella D, Rothrock N, Keefe F, Callahan L, & Lai JS (2010). Development of a PROMIS item bank to measure pain interference. PAIN, 150(1), 173–182. 10.1016/j.pain.2010.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armenta RF, Rush T, LeardMann CA, Millegan J, Cooper A, Hoge CW, & Millennium Cohort Study team. (2018). Factors associated with persistent posttraumatic stress disorder among US military service members and veterans. BMC Psychiatry, 18(1), 48. 10.1186/s12888-018-1590-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B, 57(1), 289–300. [Google Scholar]
- Benton AI, & Hamsher K (1989). Multilingual aphasia examination. AJA Associates. [Google Scholar]
- Birkeland MS, Nielsen MB, Hansen MB, Knardahl S, & Heir T (2017). Like a bridge over troubled water? A longitudinal study of general social support, colleague support, and leader support as recovery factors after a traumatic event. European Journal of Psychotraumatology, 8(1), 1–10. 10.1080/20008198.2017.1302692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blevins CA, Weathers FW, Davis MT, Witte TK, & Domino JL (2015). The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): Development and initial psychometric evaluation. Journal of Traumatic Stress, 28(6), 489–498. 10.1002/jts.22059 [DOI] [PubMed] [Google Scholar]
- Boone KB, Lu P, & Herzberg D (2002). The b Test. Western Psychological Services. [Google Scholar]
- Bourn LE, Sexton MB, Porter KE, & Rauch SA (2016). Physical activity moderates the association between pain and PTSD in treatment-seeking veterans. Pain Medicine, 17(11), 2134–2141. 10.1093/pm/pnw089 [DOI] [PubMed] [Google Scholar]
- Brenner LA, Terrio H, Homaifar BY, Gutierrez PM, Staves PJ, Harwood JEF, Reeves D, Adler LE, Ivins BJ, Helmick K, & Warden D (2010). Neuropsychological test performance in soldiers with blast-related mild TBI. Neuropsychology, 24(2), 160–167. 10.1037/a0017966 [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, & Kupfer DJ (1989). The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research, 28, 193–213. [DOI] [PubMed] [Google Scholar]
- Cicerone KD, & Kalmar K (1995). Persistent postconcussion syndrome: The structure of subjective complaints after mild traumatic brain injury. The Journal of Head Trauma Rehabilitation, 10(3), 1–17. 10.1097/00001199-199510030-00002 [DOI] [Google Scholar]
- Davenport ND, Lim KO, Armstrong MT, & Sponheim SR (2012). Diffuse and spatially variable white matter disruptions are associated with blast-related mild traumatic brain injury. Neuroimage, 59(3), 2017–2024. 10.1016/j.neuroimage.2011.10.050 [DOI] [PubMed] [Google Scholar]
- Forbes D, Creamer M, Hawthorne G, Allen N, & McHugh T (2003). Comorbidity as a predictor of symptom change after treatment in combat-related posttraumatic stress disorder. The Journal of Nervous and Mental Disease, 191(2), 93–99. 10.1097/00005053-200302000-00005 [DOI] [PubMed] [Google Scholar]
- Fulton JJ, Calhoun PS, Wagner HR, Schry AR, Hair LP, Feeling N, Elbogen E, & Beckham JC (2015). The prevalence of posttraumatic stress disorder in Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) veterans: A meta-analysis. Journal of Anxiety Disorders, 31, 98–107. 10.1016/j.janxdis.2015.02.003 [DOI] [PubMed] [Google Scholar]
- Gilbert KS, Kark SM, Gehrman P, & Bogdanova Y (2015). Sleep disturbances, TBI and PTSD: implications for treatment and recovery. Clinical Psychology Review, 40, 195–212. 10.1016/j.cpr.2015.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill JM, Saligan L, Lee H, Rotolo S, & Szanton S (2013). Women in recovery from PTSD have similar inflammation and quality of life as non-traumatized controls. Journal of Psychosomatic Research, 74(4), 301–306. 10.1016/j.jpsychores.2012.10.013 [DOI] [PubMed] [Google Scholar]
- Gilmore CS, Marquardt CA, Kang SS, & Sponheim SR (2018). Reduced P3b brain response during sustained visual attention is associated with remote blast mTBI and current PTSD in US military veterans. Behavioural Brain Research, 340, 174–182. 10.1016/j.bbr.2016.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P (2004). Test manual for the Medical Symptom Validity Test. Green’s Publishing. [Google Scholar]
- Hayes JP, Morey RA, & Tupler LA (2012). A case of frontal neuropsychological and neuroimaging signs following multiple primary-blast exposure. Neurocase, 18(3), 258–269. 10.1080/13554794.2011.588181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, & Grant I (2004). Revised comprehensive norms for an expanded Halstead-Reitan battery: Demographically adjusted neuropsychological norms for African American and Caucasian adults. Psychological Assessment Resources. [Google Scholar]
- Hendryx M, Green CA, & Perrin NA (2009). Social support, activities, and recovery from serious mental illness: STARS study findings. The Journal of Behavioral Health Services & Research, 36(3), 320–329. 10.1007/s11414-008-9151-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, & Castro CA (2008). Mild traumatic brain injury in U.S. Soldiers returning from Iraq. The New England Journal of Medicine, 358(5), 453–463. 10.1056/NEJMoa072972 [DOI] [PubMed] [Google Scholar]
- Kennedy JE, Leal FO, Lewis JD, Cullen MA, & Amador RR (2010). Posttraumatic stress symptoms in OIF/OEF service members with blast-related and non-blast-related mild TBI. NeuroRehabilitation, 26(3), 223–231. [DOI] [PubMed] [Google Scholar]
- King PR, Donnelly KT, Donnelly JP, Dunnam M, Warner G, Kittleson CJ, Bradshaw CB, Alt M, & Meier ST (2012). Psychometric study of the neurobehavioral symptom inventory. Journal of Rehabilitation Research & Development, 49(6), 879–888. 10.1682/JRRD.2011.03.0051 [DOI] [PubMed] [Google Scholar]
- Kolassa I-T, Ertl V, Eckart C, Kolassa S, Onyut LP, & Elbert T (2010). Spontaneous remission from PTSD depends on the number of traumatic event types experienced. Psychological Trauma: Theory, Research, Practice, and Policy, 2(3), 169–174. 10.1037/a0019362 [DOI] [Google Scholar]
- Kontos AP, Kotwal RS, Elbin RJ, Lutz RH, Forsten RD, Benson PJ, & Guskiewicz KM (2013). Residual effects of combat-related mild traumatic brain injury. Journal of Neurotrauma, 30(8), 680–686. 10.1089/neu.2012.2506 [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, & Williams JB (2001). The PHQ-9: validity of a brief depression severity measure. Journal of General Internal Medicine, 16(9), 606–613. 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martindale SL, Epstein EL, Taber KH, VA Mid-Atlantic MIRECC Workgroup, & Rowland JA (2018). Behavioral and health outcomes associated with deployment and nondeployment acquisition of traumatic brain injury in Iraq and Afghanistan Veterans. Archives of Physical Medicine and Rehabilitation, 99(12), 2485–2495. 10.1016/j.apmr.2018.04.029 [DOI] [PubMed] [Google Scholar]
- Martindale SL, Ord AS, Lad SS, Miskey HM, Taber KH, & Rowland JA (2020). Differential effects of deployment and nondeployment mild TBI on neuropsychological outcomes. Rehabilitation Psychology. Advance online publication. 10.1037/rep0000374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Eiland L, Hunter R, & Miller M (2012). Stress and anxiety: structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology, 62(1), 3–12. 10.1016/j.neuropharm.2011.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, & Gianaros PJ (2011). Stress- and allostasis-induced brain plasticity. Annual Review of Medicine, 62, 431–445. 10.1146/annurev-med-052209-100430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt VC, Jurick SM, Crocker LD, Hoffman SN, Keller AV, DeFord N, & Jak AJ (2019). Evaluation of objective and subjective clinical outcomes in combat veterans with and without mild TBI and PTSD: A four-group design. Journal of Clinical and Experimental Neuropsychology, 41(7), 665–679. 10.1080/13803395.2019.1610161 [DOI] [PubMed] [Google Scholar]
- Nelson LA, Yoash-Gantz RE, Pickett TC, & Campbell TA (2009). Relationship between processing speed and executive functioning performance among OEF/OIF veterans: implications for postdeployment rehabilitation. The Journal of Head Trauma Rehabilitation, 24(1), 32–40. 10.1097/HTR.0b013e3181957016 [DOI] [PubMed] [Google Scholar]
- Ord AS, Lad SS, Shura RD, Rowland JA, Taber KH, & Martindale SL (2020). Pain interference and quality of life in combat Veterans: Examining the roles of posttraumatic stress disorder, traumatic brain injury, and sleep quality. Rehabilitation Psychology. Advance online publication. 10.1037/rep0000333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens BD, Kragh JF Jr, Wenke JC, Macaitis J, Wade CE, & Holcomb JB (2008). Combat wounds in operation Iraqi Freedom and operation Enduring Freedom. Journal of Trauma and Acute Care Surgery, 64(2), 295–299. [DOI] [PubMed] [Google Scholar]
- Pagulayan KF, Rau H, Madathil R, Werhane M, Millard SP, Petrie EC, Parmenter B, Peterson S, Sorg S, Hendrickson R, Mayer C, Meabon JS, Huber BR, Raskind M, Cook DG, & Peskind ER (2018). Retrospective and prospective memory among OEF/OIF/OND veterans with a self-reported history of blast-related mTBI. Journal of the International Neuropsychological Society, 24(4), 324–334. 10.1017/S1355617717001217 [DOI] [PubMed] [Google Scholar]
- Petrie EC, Cross DJ, Yarnykh VL, Richards T, Martin NM, Pagulayan K, … Peskind ER (2014). Neuroimaging, behavioral, and psychological sequelae of repetitive combined blast/impact mild traumatic brain injury in Iraq and Afghanistan war veterans. Journal of Neurotrauma, 31, 425–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman JO, Goldsmith AA, Lemmer JA, Kilmer MT, & Baker DG (2012). Posttraumatic stress disorder, depression, and health-related quality of life in OEF/OIF veterans. Quality of Life Research, 21(1), 99–103. 10.1007/s11136-011-9918-3 [DOI] [PubMed] [Google Scholar]
- Ramsey C, Dziura J, Justice AC, Altalib HH, Bathulapalli H, Burg M, Decker S, Driscoll M, Goulet J, Haskell S, Kulas J, Wang KH, Mattocks K, & Brandt C (2017). Incidence of mental health diagnoses in veterans of Operations Iraqi Freedom, Enduring Freedom, and New Dawn, 2001–2014. American Journal of Public Health, 107(2), 329–335. 10.2105/AJPH.2016.303574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravn SL, Karstoft K, Sterling M, & Andersen TE (2019). Trajectories of posttraumatic stress symptoms after whiplash: A prospective cohort study. European Journal of Pain, 23(3), 515–525. 10.1002/ejp.1325 [DOI] [PubMed] [Google Scholar]
- Reid MW, Miller KJ, Lange RT, Cooper DB, Tate DF, Bailie J, … Kennedy JE (2014). A Multisite study of the relationships between blast exposures and symptom reporting in a post-deployment active duty military population with mild traumatic brain injury. Journal of Neurotrauma, 31(23), 1899–1906. 10.1089/neu.2014.3455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM, & Wolfson D (1985). The Halstead-Reitan neuropsychological test battery: Theory and clinical interpretation. Neuropsychology Press. [Google Scholar]
- Rosen RC, Marx BP, Maserejian NN, Holowka DW, Gates MA, Sleeper LA, Vasterling JJ, Kang HK, & Keane TM (2012). Project VALOR: Design and methods of a longitudinal registry of post‐traumatic stress disorder (PTSD) in combat‐exposed veterans in the Afghanistan and Iraqi military theaters of operations. International Journal of Methods in Psychiatric Research, 21(1), 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland JA, Martindale SL, Shura RD, Miskey H, Bateman J, Epstein E, Stern M, Hurley R, & Taber KH (2020). Initial validation of the Mid-Atlantic MIRECC Assessment of Traumatic Brain Injury. Journal of Neurotrauma. Advance online publication. 10.1089/neu.2019.6972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland JA, Martindale SL, Spengler KM, Shura RD, & Taber KH (2020). Sequelae of blast events in Iraq and Afghanistan war veterans using the Salisbury Blast Interview: A CENC study. Brain Injury, 34(5), 642–652. 10.1080/02699052.2020.1729418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusch HL, Guardado P, Baxter T, Mysliwiec V, & Gill JM (2015). Improved sleep quality is associated with reductions in depression and PTSD arousal symptoms and increases in IGF-1 concentrations. Journal of Clinical Sleep Medicine, 11(6), 615–623. 10.5664/jcsm.4770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago PN, Ursano RJ, Gray CL, Pynoos RS, Spiegel D, Lewis-Fernandez R, Friedman MJ, & Fullerton CS (2013). A systematic review of PTSD prevalence and trajectories in DSM-5 defined trauma exposed populations: intentional and non-intentional traumatic events. PloS One, 8. 10.1371/journal.pone.0059236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal KH, Bertenthal D, Miner CR, Sen S, & Marmar C (2007). Bringing the war back home: mental health disorders among 103,788 US veterans returning from Iraq and Afghanistan seen at Department of Veterans Affairs facilities. Archives of Internal Medicine, 167(5), 476–482. [DOI] [PubMed] [Google Scholar]
- Scott JC, Matt GE, Wrocklage KM, Crnich C, Jordan J, Southwick SM, Krystal JH, & Schweinsburg BC (2015). A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychological Bulletin, 141(1), 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shandera-Ochsner AL, Berry DT, Harp JP, Edmundson M, Graue LO, Roach A, & High WM Jr (2013). Neuropsychological effects of self-reported deployment-related mild TBI and current PTSD in OIF/OEF veterans. The Clinical Neuropsychologist, 27(6), 881–907. [DOI] [PubMed] [Google Scholar]
- Smith GP, & Burger GK (1997). Detection of malingering: validation of the Structured Inventory of Malingered Symptomatology (SIMS). Journal of the American Academy of Psychiatry and the Law, 25(2), 183–189. [PubMed] [Google Scholar]
- Song H, Cui J, Simonyi A, Johnson CE, Hubler GK, DePalma RG, & Gu Z (2018). Linking blast physics to biological outcomes in mild traumatic brain injury: narrative review and preliminary report of an open-field blast model. Behavioural Brain Research, 340, 147–158. 10.1016/j.bbr.2016.08.037 [DOI] [PubMed] [Google Scholar]
- Steinert C, Hofmann M, Leichsenring F, & Kruse J (2015). The course of PTSD in naturalistic long-term studies: high variability of outcomes. A systematic review. Nordic Journal of Psychiatry, 69(7), 483–496. 10.3109/08039488.2015.1005023 [DOI] [PubMed] [Google Scholar]
- Storzbach D, O’Neil ME, Roost SM, Kowalski H, Iverson GL, Binder LM, Fann JR, & Huckans M (2015). Comparing the neuropsychological test performance of Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) Veterans with and without blast exposure, mild traumatic brain injury, and posttraumatic stress symptoms. Journal of the International Neuropsychological Society: JINS, 21(5), 353–363. 10.1017/S1355617715000326 [DOI] [PubMed] [Google Scholar]
- Strachan M, Gros DF, Ruggiero KJ, Lejuez CW, & Acierno R (2012). An integrated approach to delivering exposure-based treatment for symptoms of PTSD and depression in OIF/OEF veterans: preliminary findings. Behavior Therapy, 43(3), 560–569. 10.1016/j.beth.2011.03.003 [DOI] [PubMed] [Google Scholar]
- Swinkels CM, Ulmer CS, Beckham JC, Buse N, VA Mid-Atlantic MIRECC Registry Workgroup, & Calhoun PS (2013). The association of sleep duration, mental health, and health risk behaviors among US Afghanistan/Iraq era veterans. Sleep, 36(7), 1019–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber KH, Hurley RA, Haswell CC, Rowland JA, Hurt SD, Lamar CD, & Morey RA (2015). White matter compromise in veterans exposed to primary blast forces. The Journal of Head Trauma Rehabilitation, 30(1), E15–E25. 10.1097/HTR.0000000000000030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanev KS, Pentel KZ, Kredlow MA, & Charney ME (2014). PTSD and TBI comorbidity: scope, clinical presentation and treatment options. Brain Injury, 28(3), 261–270. 10.3109/02699052.2013.873821 [DOI] [PubMed] [Google Scholar]
- Tschiffely AE, Ahlers ST, & Norris JN (2015). Examining the relationship between blast-induced mild traumatic brain injury and posttraumatic stress-related traits. Journal of Neuroscience Research, 93(12), 1769–1777. 10.1002/jnr.23641 [DOI] [PubMed] [Google Scholar]
- Tural Ü, Önder E, & Aker T (2012). Effect of depression on recovery from PTSD. Community Mental Health Journal, 48(2), 161–166. [DOI] [PubMed] [Google Scholar]
- Vasterling JJ, Aslan M, Lee LO, Proctor SP, Ko J, Jacob S, & Concato J (2018). Longitudinal associations among posttraumatic stress disorder symptoms, traumatic brain injury, and neurocognitive functioning in army soldiers deployed to the Iraq War. Journal of the International Neuropsychological Society: JINS, 24(4), 311–323. 10.1017/S1355617717001059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verfaellie M, Lafleche G, Spiro A III, & Bousquet K (2014). Neuropsychological outcomes in OEF/OIF veterans with self-report of blast exposure: Associations with mental health, but not MTBI. Neuropsychology, 28(3), 337. 10.1037/neu0000027 [DOI] [PubMed] [Google Scholar]
- Vogt D, Smith B, King D, & King L (2012). Manual for the Deployment Risk and Resilience Inventory-2 (DRRI-2): A collection of measures for studying deployment-related experiences of military veterans. National Center for PTSD. [Google Scholar]
- von Steinbüchel N, Wilson L, Gibbons H, Hawthorne G, Höfer S, Schmidt S, … Truelle J-L (2010). Quality of Life after Brain Injury (QOLIBRI): Scale development and metric properties. Journal of Neurotrauma, 27(7), 1167–1185. 10.1089/neu.2009.1076 [DOI] [PubMed] [Google Scholar]
- Walter KH, Kiefer SL, & Chard KM (2012). Relationship between posttraumatic stress disorder and postconcussive symptom improvement after completion of a posttraumatic stress disorder/traumatic brain injury residential treatment program. Rehabilitation Psychology, 57(1), 13–17. 10.1037/a0026254 [DOI] [PubMed] [Google Scholar]
- Weathers FW, Bovin MJ, Lee DJ, Sloan DM, Schnurr PP, Kaloupek DG, … Marx BP (2017). The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5): Development and initial psychometric evaluation in military Veterans. Psychological Assessment. 10.1037/pas0000486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (2008). Wechseler Adult Intelligence Scale - Fourth Edition. Pearson. [Google Scholar]
- Wisdom NM, Callahan JL, & Shaw TG (2010). Diagnostic utility of the Structured Inventory of Malingered Symptomatology to detect malingering in a forensic sample. Archives of Clinical Neuropsychology, 25(2), 118–125. 10.1093/arclin/acp110 [DOI] [PubMed] [Google Scholar]
- Wolf SJ, Bebarta VS, Bonnett CJ, Pons PT, & Cantrill SV (2009). Blast injuries. Lancet, 374, 405–415. 10.1016/S0140-6736(09)60257-9 [DOI] [PubMed] [Google Scholar]
- Yurgil KA, Barkauskas DA, Vasterling JJ, Nievergelt CM, Larson GE, Schork NJ, … & Baker DG (2014). Association between traumatic brain injury and risk of posttraumatic stress disorder in active-duty Marines. JAMA Psychiatry, 71(2), 149–157. 10.1001/jamapsychiatry.2013.3080 [DOI] [PubMed] [Google Scholar]


