Abstract
Despite its omnipresence in everyday interactions and its importance for mental health, mood and its neuronal underpinnings are poorly understood. Computational models can help identify parameters affecting self-reported mood during mood induction tasks. Here, we test if computationally modeled dynamics of self-reported mood during monetary gambling can be used to identify trial-by-trial variations in neuronal activity. To this end, we shifted mood in healthy (N = 24) and depressed (N = 30) adolescents by delivering individually tailored reward prediction errors while recording magnetoencephalography (MEG) data. Following a pre-registered analysis, we hypothesize that the expectation component of mood would be predictive of beta-gamma oscillatory power (25–40 Hz). We also hypothesize that trial variations in the source localized responses to reward feedback would be predicted by mood and by its reward prediction error component. Through our multilevel statistical analysis, we found confirmatory evidence that beta-gamma power is positively related to reward expectation during mood shifts, with localized sources in the posterior cingulate cortex. We also confirmed reward prediction error to be predictive of trial-level variations in the response of the paracentral lobule. To our knowledge, this is the first study to harness computational models of mood to relate mood fluctuations to variations in neural oscillations with MEG.
Keywords: MEG, mood, reward prediction error, reward processing
Introduction
Humans report on their moods in their everyday conversations and subjective mood reports form the basis of clinical assessment and much of research in affective neuroscience. Advances in computational modeling lend support to the idea that mood is intricately linked with reward processing and that it serves to integrate over a person’s history of rewards and punishers (Nettle and Bateson 2012; Rutledge et al. 2014; Keren et al. 2021). Yet, despite its ubiquity and importance, the brain mechanisms underlying mood and its relationship with changes in reward contingencies in the environment are surprisingly understudied.
Mood is understood to integrate over events in the environment and is a potentially emergent property of the coordinated activity of many neural populations. Such activity is thought to manifest as the synchrony of oscillations, which supports functional connections and communication in the brain. In this context, it is noteworthy that oscillatory power is correlated with treatment-induced changes that have been described to occur in mood disorders (Fingelkurts and Fingelkurts 2015; Kaiser et al. 2015; Nugent et al. 2019a, 2019b). Mood is also highly dynamic during development, especially in adolescence (Klimstra et al. 2016) when incidence of depression is up to 13% in the United States according to the 2017 National Survey on Drug Use and Health (NIMH » Major Depression [WWW Document]. 2021). Understanding the temporal structure—including very early responses—to changes in environmental incentives that influence mood can offer important insights into the genesis and remission of mood disorders and can inform the timing of potential interventions (such as through transcranial magnetic stimulation) that aim to modify it for clinical purposes (Tremblay et al. 2019; Zrenner et al. 2020).
Therefore, understanding the role of oscillations and fast neuronal responses in the interplay between mood and environmental incentives is key and magnetoencephalography (MEG) offers a great opportunity to do so non-invasively.
Unlike mood induction paradigms involving emotional music, videos and reminiscence, changes in mood during decision-making, reward and gambling tasks can be modeled by integrating reward outcomes and reward prediction errors (RPEs) experienced over time (Rutledge et al. 2014; Eldar et al. 2016; Vinckier et al. 2018). The selection of a monetary gambling task allows for a more straightforward quantification of task parameters potentially affecting mood.
Recent work by Keren et al. (2021) proposed a new descriptive model of mood dynamics, the primacy mood model, where participants’ responses to rewards and mood changes are more strongly influenced by early experiences. The primacy mood model was shown to describe self-reported mood better than other tested mood models that weighted rewards experienced last more strongly. Together with this model, Keren et al. (2021) developed a monetary gambling task designed to induce mood changes by delivering reward prediction errors based on the difference between the participants current mood and the mood target for that block. This adaptive controller was shown to be effective in parametrically shifting self-reported mood in both healthy volunteers (n = 29) and patients diagnosed with major depression (n = 43). Moreover, this approach to mood induction (Keren et al. 2021) avoids some of the pitfalls related to demand characteristics where participants are explicitly asked to transfer themselves mentally in a negative or positive event.
The primacy mood model combines the reward expectation and the reward prediction errors, with subject specific weighting parameters derived by fitting expectation and RPEs to the reported mood. In the model framework two subject specific weight parameters correspond to mood reactivity to experienced reward outcomes (referred to as expectation) and to the difference between each reward outcome and previous experiences (reward prediction error). Keren et al. (2021) tested neural activity correlates of parameters from the mood model, showing that subject expectation weights positively correlated with BOLD signal activity in ACC and vmPFC, cortical areas involved in mood regulation and reward-driven decision-making (Bush et al. 2000; Zald et al. 2002; Stevens et al. 2011; Etkin et al. 2015; Hiser and Koenigs 2018), measured in the “rest” time period before mood rating.
Here we aim to expand on this work by identifying correlates of the primacy mood model parameters in MEG neural activity data, with our main goal being to identify how the progressive change of mood over time is related to neural responses at a single trial level.
In a pilot analysis (n = 14, age 16.22 years, see Supplementary Materials), we tested with linear mixed effect models whether brain oscillations and trial-level changes in evoked responses would show a relationship with mood or its model components.
Following our pilot analysis we pre-registered three hypotheses, which we test here on a confirmatory sample (pre-registered analysis available on OSF (https://osf.io/djw8h), pre-registration was made before pre-processing of the confirmatory dataset).
Motivated by major concerns about false positive results in neuroscience in general and neuroimaging in particular (Turner et al. 2018; Pavlov et al. 2021), we split our data into pilot and confirmatory samples to finalize our analysis methods before processing the larger confirmatory sample. While a small pilot sample in itself has low statistical power, including a confirmatory sample where we narrow the hypothesis space and research degrees of freedom gives us low type-1 “false positive” error rates (see section “Statistical Power Calculation” in Supplementary Material). We were motivated to make this trade-off because of the major concerns in the field cited above but also specifically for results derived from methods with a potentially vast range of features and therefore statistical testing space, as is common in electrophysiology (Larson and Carbine 2017; Pernet et al. 2020).
Our first question was to see if brain oscillations were related to mood or to its two model predictors, i.e., expectation and RPE. We then state the following hypothesis:
“We hypothesize that beta-gamma oscillatory power (25–40 Hz) measured by MEG in the time interval preceding mood rating will be positively correlated with the reward expectation term derived from the primacy mood model, with a source space cluster covering the frontal superior and medial cortex and the ACC.”
Given the strong predictive effect of reward expectation on mood, we propose that identifying brain activity related to reward expectation could be an important step in further identifying and testing the brain circuits responsible for the experience of mood.
In our pilot analysis, no other standard frequency bands (delta, theta, alpha, beta, high-gamma) showed significant correlations with mood or its predictors (expectation or RPE) in the same time window. The beta-gamma band was defined to include frequencies between 25 and 40 Hz, based on previous work by Marco-Pallarés et al. (2015) who showed modulation of this frequency in response to positive feedback rewards.
Brain response to reward feedback have been related to RPE with both EEG and MEG (Holroyd et al. 2003; Doñamayor et al. 2012; Talmi et al. 2012; Marco-Pallarés et al. 2015), but little is known on the effect of mood on these same processes (Paul and Pourtois 2017). In our pilot sample, we tested if MEG evoked responses to reward feedback had any relation to mood, or its expectation and RPE model components. We found two source space ROIs, paracentral lobule, and precuneus, showing significant negative relationship to primacy model RPE; i.e., negative RPE were correlated with stronger response to reward feedback, and activity in the right insula showing an inverse relationship to mood. We then hypothesize that in our confirmatory sample:
2) “RPE and self-reported mood will be correlated with the variability in the evoked response to the gamble outcome (feedback), in right precuneus and paracentral lobule (at ~500 ms) for RPE and in the right insular cortex (at ~400 ms after feedback presentation) for mood.”
As mood also affects decision-making (Vinckier et al. 2018) in our pilot data, we tested whether mood or expectation might predict MEG evoked responses following the presentation of gambling options and following choice selection (decision to gamble or not). From our pilot data results showing significant effects surviving multiple comparisons we hypothesize that:
3) “The reward expectation term from the primacy mood model will be predictive of the signal in posterior MEG sensors during the 250–400 ms period after presentation of gambling options”.
We initially selected the 250–400 ms time window based on observations on modulations of the P300 component in EEG (De Pascalis et al. 2004; Sur and Sinha 2009). In the pilot data, we did not see any significant effects of mood or of its model predictor following gamble choice selection (related to error monitoring).
While previous studies have looked at influence of mood on trial-averaged responses (Paul and Pourtois 2017), to our knowledge this is the first study to relate the temporal dynamics of mood to trial level variations in evoked responses and oscillatory power with non-invasive electrophysiology in humans. Characterizing the neural substrates that link mood and reward is essential to understanding how disrupted reward processing contributes to mood disorders and may be instrumental in predicting symptom trajectory and response to treatment.
Materials and Methods
Sample
Study participants are adolescent volunteers (age 12–19 years) recruited through mail, online advertisement and direct referrals from clinical sources. Participants provided informed consent to a protocol approved by the NIH Institutional Review Board (clinical trial no. NCT03388606) before completing questionnaires and an in-person evaluation with a medical practitioner at the NIH clinical center to guarantee their suitability to enroll in the study. Both healthy volunteers (not satisfying criteria for any diagnosis according to DSM-5) and patients with a primary diagnosis of major depression (MDD) or sub-threshold depression were included. All participants received the same scripted instructions for their participation in this study. The full list of inclusion and exclusion criteria is outlined in the Supplementary Material.
Following in-person screening, MEG data were collected with a 275-channel CTF scanner (272 working channels, sampling at 1200 Hz, third-order synthetic gradiometer configuration) and a structural MRI (MPRAGE, 1 mm isotropic resolution) of the subject’s head was acquired with a 3 T GE MRI scanner (collected within 6 months of the MEG scan), both housed in the NMR suite of the NIH clinical center.
We collected MEG data from 56 volunteers that passed our inclusion criteria. Of this sample, 2 participants were excluded from all reported analysis, one due to artifacts during data collection, and one from reporting to have misunderstood task instructions at the end of the experiment. Of the included 54 participants (age 16.3 ± 1.8 years, 30 MDDs, 35 females), all were included in sensor based analyses, and 51 (age 16.3 ± 1.8 years, 29 MDDs, 33 females) were included in our source space analysis (two participants did not have a structural MRI due to laboratory shutdown in March 2020 and one participant had large errors >> 5 mm in the initial localization of the MEG fiducial coils). Data from fourteen participants were initially analyzed as an exploratory sample to inform the study hypotheses (as reported in our preregistration available on OSF (https://osf.io/djw8h)), leaving a separate confirmatory sample of 40 participants for analyses at the sensor level and a subsample of 37 participants at the source level.
Task Description
While in the MEG scanner, participants played a monetary gambling task. The task consists of three blocks, where in each block an adaptive closed-loop mood controller delivers reward prediction errors to try to move the participant’s mood to a target value (Keren et al. 2021). The targets of the controller are to reach the highest mood (1) in the first block, lowest (0) in the second block, and then highest mood again in third block. Every 2–3 gambling trials (for a total of 34 times per task), participants are asked to report their mood at that moment on a sliding scale with the words “Unhappy” on the left end and “Happy” on the right end of the scale. The mood ratings are used as inputs of a proportional-integral (PI) mood controller, which accordingly modifies the gambling amounts and the RPE values to push participants mood rating towards the desired end of the mood scale. Within each block, 70% of trials are congruent (delivering positive RPE in the high mood target blocks and negative RPE in the low mood block) and 30% are incongruent (delivering negative RPEs in the high mood target blocks and positive RPEs in the low mood block). While the mood target for each block and the proportion of congruent versus incongruent trials is equal for every participant, the PI mood controller changes the range of gambling values and RPEs received for each participants. From Keren et al. (2021), we expect the mood induction to have the strongest effect in the second negative block and the smallest effect in the first positive block (they report a mean (± SD) effect size per block of 0.92 ± 1.60 in the block 1, −1.75 ± 1.10 in block 2 and 1.45 ± 1.70 in block 3).
Before entering the scanner, participants are instructed on how to perform the task knowing that the final amount they win in the task will be converted to a proportional amount of money but are not informed about the mood manipulation.
Each block consists of 27 trials for a total of 81 trials. An example of a task trial selecting the gambling option is shown in Figure 1. In each trial, participants are presented with a certain amount (displayed on the left side of the screen) and two possible gambling outcomes (on the right side of the screen). The gambling amounts are selected by the closed loop controller based on the mood target and the participant’s self-reported mood. Participants are given 3 s to decide to either gamble or select the fixed outcome, by pressing the right or left button on a fiber optic response pad (FORP). When participants do not make a selection in time, the task controller automatically selects the gambling option.
Figure 1.
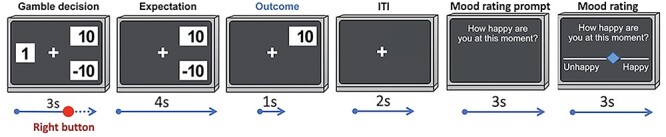
Structure of the closed-loop gambling task. The gambling amounts are presented on the right side of the screen (in this example a possible win of 10 points or loss or − 10 points), while the certain choice (here a win of 1 point) is on the left. If choosing to gamble the certain amount disappears from the screen. The two possible gambling amounts are displayed on the screen for 4 s before the outcome is revealed (here a win of 10 points). There is a 2 s inter trial interval (ITI) before the start of the next trial. Every 2–3 trials participants are prompted to rate their mood. The mood question “How happy are you at this moment?” remains on the screen for 3 s before a sliding scale appears with the words “Unhappy” on the left end and “Happy” on the right, allowing participants to rate their mood with the FORP buttons.
After choice selection, there is a 4 s waiting period, then (for the gamble selection) the gamble outcome is revealed and remains on the screen for 1 s. After each trial, there is a 2 s inter-trial-interval time when only a fixation cross is displayed. Every 2 or 3 trials participants are prompted to rate their current mood on a horizontal slider. The cursor on the slider can be moved continuously by pressing and holding the right and left buttons on the FORP.
At the end of each block, participants are given a break from the task while remaining in the scanner and can proceed to the next block by pressing a button on the FORP.
At the end of the MEG scanning session, participants are debriefed to ask about their experience in the scanner.
Mood Model
Participants rate their mood with a slider between a value of 0 and 1 (with 0 being the lowest and 1 being the highest) every 2 to 3 trials of the gambling task. We can model how reported mood changes over trials with the primacy mood model proposed by Keren et al. (2021), taking into account previous gambling outcomes and reward prediction errors. We selected the primacy mood model as it was shown to better fit self-reported mood, for both healthy and depressed participants, compared to recency models (weighting more heavily recent reward experiences) and other temporal representations. Moreover subject specific parameters (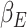 ) calculated from the primacy model were found to correlate with BOLD activity in the anterior cingulate cortex and prefrontal cortex (brain regions implicated in mood regulation) motivating our choice to further explore correlates of the primacy mood model parameters with neural data. According to the primacy model, reward expectation Et at each trial is calculated as the average of all previously received outcomes At:
) calculated from the primacy model were found to correlate with BOLD activity in the anterior cingulate cortex and prefrontal cortex (brain regions implicated in mood regulation) motivating our choice to further explore correlates of the primacy mood model parameters with neural data. According to the primacy model, reward expectation Et at each trial is calculated as the average of all previously received outcomes At:
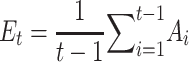 |
The reward prediction error at each trial is then defined as the difference between the received outcome and the expectation:
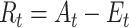 |
(1) |
We model the self-reported mood at time 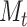 as:
as:
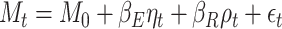 |
(2) |
where we call the contributions of expectation, 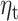 , and RPE,
, and RPE, 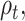 to mood at time
to mood at time 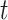 as:
as:
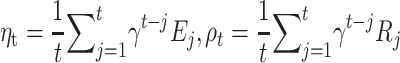 |
(3) |
We calculate the accumulated expectation 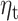 and the accumulated RPE
and the accumulated RPE 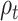 at trial
at trial 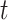 by summing over all past trials (
by summing over all past trials (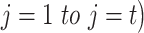 , changing the exponent of parameter
, changing the exponent of parameter 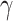 to give a stronger weight to early trials (e.g., for
to give a stronger weight to early trials (e.g., for 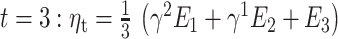 , with the model fit constraint
, with the model fit constraint 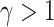 ). The model parameters
). The model parameters 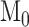 ,
, 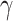 ,
, 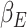 , and
, and 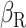 are subject specific and are derived by fitting self-reported mood data to the model:
are subject specific and are derived by fitting self-reported mood data to the model: 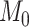 is the participant’s baseline mood,
is the participant’s baseline mood, 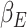 and
and  are subject level parameters representing subject’s sensitivity to expectation and surprise (prediction error), respectively,
are subject level parameters representing subject’s sensitivity to expectation and surprise (prediction error), respectively, 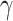 is a parameter representing the subject’s rate of temporal discounting, and
is a parameter representing the subject’s rate of temporal discounting, and  is the error term. The full description of the model and implementation with python’s TensorFlow package is described in Keren et al. (2021).
is the error term. The full description of the model and implementation with python’s TensorFlow package is described in Keren et al. (2021).
All trial dependent parameters obtained from the model fit are sampled at every trial, but participants report mood every 2–3 trials during the gambling task. In order to use self-reported mood in our linear mixed model to test variability over all trials, mood ratings are interpolated with a Piecewise Cubic Hermite Interpolating Polynomial implemented in Matlab.
MEG Data
MEG data analysis is performed on the NIH HPC Biowulf cluster (http://hpc.nih.gov) with Matlab (The MathWorks, Inc., Natick, Massachusetts, United States of America) and functions from the FieldTrip toolbox ((Oostenveld et al. 2011); http://fieldtriptoolbox.org). Following initial visual inspection, MEG data are pre-processed in Matlab: third-order synthetic gradiometer configuration is applied; segments with motion exceeding a threshold of 5 mm or including noticeable artifacts are eliminated; data are bandpass filtered between 0.5 and 300 Hz, baseline corrected, and a 60 Hz notch filter is applied to reduce power line noise. Data are then corrected for eye movements and heartbeat artifacts with ICA fastica algorithm (30 independent components are calculated and a maximum of 4 components, 2 heartbeat, and 2 eye movement ICs, are eliminated for each dataset).
Data Processing
Source data reconstruction are achieved by a beamformer approach and a forward model based on Nolte’s spherical approximation (Veen et al. 1997; Nolte 2003) implemented in FieldTrip. Subjects’ individual brain MRIs are co-registered to the MEG data by identification of three fiducial coils (nasion, right/left preauricular points). Source level activity is reconstructed on a 5 mm grid based on the MNI brain and warped to individual anatomy. Beamformer weights are calculated based on the data covariance over the whole task with a covariance regularization equal to 5% of its maximum singular value (a high regularization is selected to improve SNR and increase spatial smoothness (Brookes et al. 2008); the effects of matrix regularization and forward model selection is explored in supplementary analysis).
Confirmatory Analyses
We apply linear mixed effects models to estimate the contribution of trial level mood, expectation and reward prediction error to response variability in the MEG data. The use of linear mixed effect models allows us to analyze data from all participants in a single model while accounting for inter-subject differences.
Hypothesis 1—Beta-Gamma Oscillatory Power
Following our pre-registered hypotheses, we estimate beta-gamma oscillatory power in the 3 s waiting period preceding mood-rating. MEG signal is first band pass filtered in the frequency band of interest (25–40 Hz) then, for sensor space analysis oscillatory power is estimated by measuring the signal variance in each 3 s window. For source space analysis, oscillatory power in each voxel 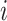 is estimated as
is estimated as
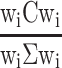 |
(4) |
where 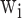 are the beamformer weights,
are the beamformer weights,  is the data covariance matrix in the 3 s window of interest, and
is the data covariance matrix in the 3 s window of interest, and 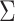 the estimated noise covariance matrix.
the estimated noise covariance matrix.
The effect of expectation on beta-gamma power is tested in our confirmatory sample for the accumulated expectation term 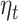 . Our formulation is as follows:
. Our formulation is as follows:
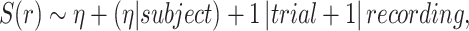 |
(5) |
where 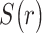 indicates the MEG signal at location
indicates the MEG signal at location 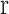 (either sensor or source voxel).
(either sensor or source voxel).
The 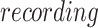 random effect is included to account for participants who had to be repositioned in the scanner during the task due to excessive movement (>5 mm).
random effect is included to account for participants who had to be repositioned in the scanner during the task due to excessive movement (>5 mm).
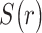 is a vector with dimension
is a vector with dimension  , where
, where 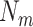 is the number of mood rating trials from all participants (There are 34 mood ratings per task when all trials are included.)
is the number of mood rating trials from all participants (There are 34 mood ratings per task when all trials are included.)
Hypothesis 2—Evoked Response to Feedback
For the analysis of evoked responses, a low-pass filter of 30 Hz is applied following the pre-processing steps before calculation of the beamformer weights.
Regions of interest (ROI) are defined by the Automated Anatomical Labeling (AAL) atlas available in fieldtrip. The signal from an ROI is estimated from its geometrical centroid. We select a time window −200 to 1000 ms with respect to the presentation of the gamble feedback.
There are 81 trials per task where participants can choose to gamble and then wait to receive feedback. For this hypothesis, we consider only trials when participants choose to gamble over selecting a certain reward.
The source data time course is estimated and then downsampled to 300 Hz to reduce the number of time points to test.
It is important to note that with beamforming, the sign of evoked responses in source space is uncertain (i.e., sign may be flipped between participants). For each participant, the sign of the source signal for an ROI or voxel is estimated by maximizing the correlation over subjects of their trial averaged evoked response to the stimulus (gambling option or feedback presentation).
In our confirmatory analysis, we test if mood predicts MEG signal in the right insula, and if the 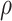 parameter predicts response variation in the right paracentral lobule and precuneus.
parameter predicts response variation in the right paracentral lobule and precuneus.
Our formulation is as follows (here presented for the  fixed effect):
fixed effect):
 |
(6) |
where 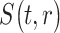 indicates the source reconstructed MEG signal in ROI
indicates the source reconstructed MEG signal in ROI 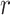 and at time
and at time  with respect to the task event.
with respect to the task event. 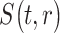 is a vector with dimension
is a vector with dimension 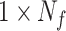 , where
, where 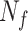 is the number of feedback trials from all participants.
is the number of feedback trials from all participants.
Hypothesis 3—Evoked Response to Gambling Options
Again a low-pass filter of 30 Hz is applied following the pre-processing steps before calculation of the beamformer weights. We select the average response in the time window of 250–400 ms with respect to the presentation of the gambling options for the analysis of the evoked responses at both sensor and source level. For source space analysis, the data time course is first reconstructed at each source voxel and then the evoked response for each trial is estimated as the average signal in the 250–400 ms time window.
We test the effect of trial expectation, as defined by the primacy model (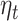 ) on the average evoked response in the 250–400 ms window following gamble options presentation. There are total of 81 gambling trials per subject.
) on the average evoked response in the 250–400 ms window following gamble options presentation. There are total of 81 gambling trials per subject.
Our formulation is as follows:
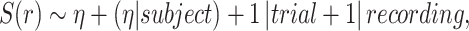 |
(7) |
where 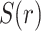 indicates the MEG signal at location
indicates the MEG signal at location 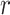 (either sensor or source voxel).
(either sensor or source voxel).
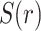 is a vector with dimension
is a vector with dimension 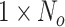 , where
, where 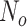 is the number of task trials from all participants.
is the number of task trials from all participants.
We run our statistical model at each sensor and source space voxel.
Random Permutations
For all linear mixed effect models, we test for statistical significance over multiple voxels or sensors by applying threshold free cluster enhancement (TFCE) spatial clustering with parameters E = 0.5, H = 2, dh = 0.1 as indicated in Smith and Nichols (2009).
Null distributions are obtained by running the same linear mixed model after random permutation of S over trials for each participant (10 000 random permutations for the sensor space analysis, 2000 random permutation for the voxel space). Each random permutation is used for all sensors/voxel, TFCE is applied on the t-values for the fixed effect and the maximum (and minimum) spatial cluster value is included in the null distribution.
We then use a two-tailed t-test against the null distribution to infer statistical significance of the spatial clusters (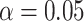 ) corrected with Bonferroni for the multiple hypotheses tested.
) corrected with Bonferroni for the multiple hypotheses tested.
Exploratory Analyses
The following exploratory analyses are run with the whole sample of available participants (N = 51 at the source level).
Comparison with Previous fMRI Results
Previously published results using the same gambling task reported a significant cluster of fMRI BOLD activation in the ACC correlating with the expectation weight, 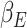 (Keren et al. 2021). The expectation weight
(Keren et al. 2021). The expectation weight 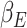 is a subject specific term in the primacy mood model (see equation (2)), derived by best fitting subject’s mood ratings to the model equation. Following our hypothesis that trial variations in beta-gamma power are related to reward expectation, we then test if the subject average beta-gamma power is correlated to
is a subject specific term in the primacy mood model (see equation (2)), derived by best fitting subject’s mood ratings to the model equation. Following our hypothesis that trial variations in beta-gamma power are related to reward expectation, we then test if the subject average beta-gamma power is correlated to  in an analogous analysis. For this analysis, the average beta-gamma power is calculated at the voxel level by averaging the previously estimated MEG power in the 3 s pre mood rating period over all available trials for each participant.
in an analogous analysis. For this analysis, the average beta-gamma power is calculated at the voxel level by averaging the previously estimated MEG power in the 3 s pre mood rating period over all available trials for each participant.
A Pearson correlation between subject average beta-gamma power and 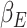 is then run and significant clusters are calculated. Null distributions are obtained by calculating maximum TFCE value in random permutations (N = 5000).
is then run and significant clusters are calculated. Null distributions are obtained by calculating maximum TFCE value in random permutations (N = 5000).
The significant cluster from Keren et al. (2021) is then compared to the MEG cluster in MNI space to check for congruent cross-modality results.
Sensitivity Analysis: Primacy Model Fit
In our initial hypotheses, we set to test how trial variations in MEG data could be predicted by parameters of the primacy mood model, based on previous results from Keren et al. (2021), showing its best performance over alternative models. In order to derive meaningful conclusions on the validity of prediction, we have to check how well the model is fitting our behavioral data. Based on the previous data by Keren et al. (2021), we set a threshold of 0.5 r-squared and a minimum mood range of 0.1 (mood scale limits 0–1) to determine a sensitivity sample that responded to the mood induction and whose behavior was well represented by the model. We then repeat the same mixed effect model analyses outlined in the confirmatory analysis including all participants that pass the sensitivity criteria, to determine whether the correlation between model parameters and MEG neural data will improve.
Effects of Group: MDD/HV and MFQ Scores
In all our analyses as described above, we selected to include in the same sample both adolescents with past or current diagnosis of major depression and adolescents with no history of MDD. We motivated this choice in order to include a better representation of the full spectrum of mood variations. Nevertheless, there could be meaningful variations in the neural activity of MDDs compared to HVs. As an exploratory analysis, we therefore test two alternative prediction models on the sensitivity subsample that confirmed our initial hypotheses:
- (1) a categorical fixed effect term for group (MDD or HV) and its interaction with the other fixed effect (here presented for
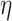 )
)
- (2) a fixed effected term for the short MFQ score collected at the time of the experiment and its interaction with the other fixed effect (here presented for
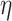 )
)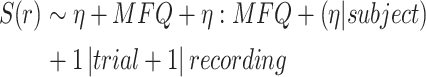
We then repeat the random permutation process with the new models (2000 random permutations each, with TFCE) and report whether MDD group, MFQ scores or their interaction with the main predictor showed a significant effect.
Results
Mood Manipulation
Our gambling task was effective in inducing mood changes with an average effect size of the mood induction of (Cohen’s d, mean ± SD): 0.8 ± 2.3 for block 1, −1.9 ± 2.1 for block 2, and 1.7 ± 2.5 for block 3 (see Fig. 2). The average effect of the negative mood induction is comparable for MDDs and HVs (Cohen’s d, mean ± SD, MDD: −1.8 ± 2.0; HV: −2.1 ± 2.1). The positive mood induction has a stronger effect for MDDs in the first block (Cohen’s d, mean ± SD, MDD: 1.1 ± 2.3; HV: 0.4 ± 2.2) since on average they start with a lower mood. The positive mood induction in the last block shows instead a stronger effect for healthy volunteers (Cohen’s d, mean ± SD, MDD: 1.2 ± 2.7; HV: 2.3 ± 2.3).
Figure 2.
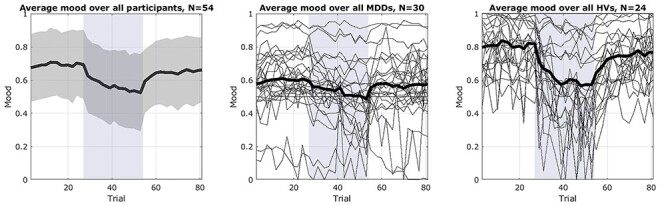
Participants’ self-reported mood during the 3-blocks of the gambling task. Positive blocks have a white background while the negative block is shaded in gray. The left panel shows the average (n = 54) reported mood of all participants as a black line with standard deviation over subjects shaded in gray. The middle and right panels show, respectively, the average mood of all participants diagnosed with MDD (n = 30) and of all healthy volunteers (n = 24), with all individual reported moods shown as thinner black lines.
Confirmatory Analysis
Hypothesis 1: Trial Variations in Beta-Gamma Oscillatory Power Measured in the 3 s Time Interval Preceding Mood Rating Will be Positively Correlated with Expectation 
For our first hypothesis, we set to test if trial variations in beta-gamma (25–40 Hz) oscillatory power are affected by reward expectation.
We report results for the linear mixed model analysis with accumulated expectation (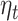 ) as fixed effect of the model. At the sensor level we found a significant cluster of sensors (Fig. 3A) where beta-gamma power is positively related to
) as fixed effect of the model. At the sensor level we found a significant cluster of sensors (Fig. 3A) where beta-gamma power is positively related to  (peak on channel MRC25, fixed effect t-stat = 3.64, uncorrected P-value = 2.8e-04).
(peak on channel MRC25, fixed effect t-stat = 3.64, uncorrected P-value = 2.8e-04).
Figure 3.
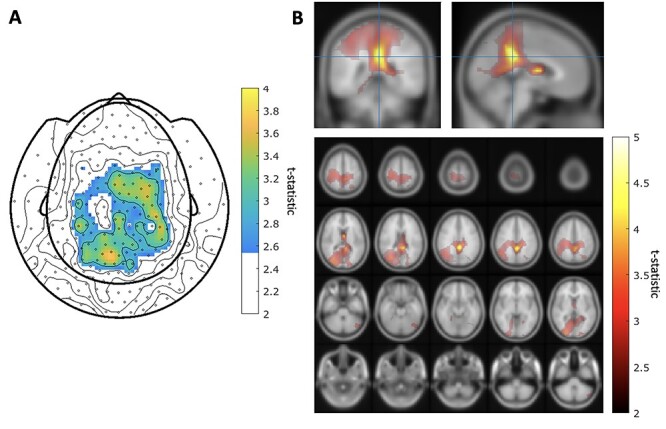
A. Maps of MEG sensors where the expectation parameter 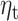 significantly predict beta-gamma power preceding mood rating in confirmatory sample. Sensors surviving clustering correction (
significantly predict beta-gamma power preceding mood rating in confirmatory sample. Sensors surviving clustering correction (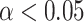 , two-tailed, 10 000 random permutations) are shown in color. Color bar indicates t-statistic of the fixed effect. B. Source space maps showing brain regions where expectation
, two-tailed, 10 000 random permutations) are shown in color. Color bar indicates t-statistic of the fixed effect. B. Source space maps showing brain regions where expectation 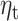 predicts beta-gamma power preceding mood rating in confirmatory sample. Color bar indicates t-statistic of the fixed effect. Top row shows cluster peak in coronal and sagittal views. The plot on the bottom shows the significant cluster over multiple axial slices.
predicts beta-gamma power preceding mood rating in confirmatory sample. Color bar indicates t-statistic of the fixed effect. Top row shows cluster peak in coronal and sagittal views. The plot on the bottom shows the significant cluster over multiple axial slices.
Both predictors show similar significant clusters with a peak in the left posterior cingulate cortex (MNI coordinate [−2, −40, 30 mm) extending to mid cingulate, parietal cortex and the caudate.
At the source level, we found a significant cluster where 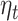 predicted beta-gamma power in the mid to posterior cingulate cortex and extending to the paracentral lobule (Fig. 3B cluster peak at MNI coordinate [−2, −40, 30]mm, T-stat = 4.78, uncorrected P-value = 2.0e-06). Other significant clusters were present in the occipital cortex, caudate and the ACC. By exploration of the covariance regularization parameter (5%, 1%, and 0.2% of the maximum singular value of the covariance matrix), we found significant clusters to be highly dependent on regularization, with clusters in supplementary motor cortex and frontal superior cortex becoming prominent at lower regularization values (Figure S13 in Supplementary Material). Over our explored range of regularization values, the mid-posterior cingulate cortex cluster remained present.
predicted beta-gamma power in the mid to posterior cingulate cortex and extending to the paracentral lobule (Fig. 3B cluster peak at MNI coordinate [−2, −40, 30]mm, T-stat = 4.78, uncorrected P-value = 2.0e-06). Other significant clusters were present in the occipital cortex, caudate and the ACC. By exploration of the covariance regularization parameter (5%, 1%, and 0.2% of the maximum singular value of the covariance matrix), we found significant clusters to be highly dependent on regularization, with clusters in supplementary motor cortex and frontal superior cortex becoming prominent at lower regularization values (Figure S13 in Supplementary Material). Over our explored range of regularization values, the mid-posterior cingulate cortex cluster remained present.
Hypothesis 2: Self-Reported Mood and RPE from the Primacy Model Can Predict Changes in the Response to Reward. 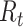 and
and 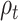 Will Be Correlated with the Variability in the Evoked Response in Right Precuneus and Paracentral Lobule (at ~500 ms) and for
Will Be Correlated with the Variability in the Evoked Response in Right Precuneus and Paracentral Lobule (at ~500 ms) and for 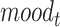 in the Right Insular Cortex (at ~400 ms after Feedback Presentation)
in the Right Insular Cortex (at ~400 ms after Feedback Presentation)
In order to test the effect of mood and RPE on the evoked response following gambling feedback, we ran models independently for the three separate fixed effects. From our exploratory analysis, we hypothesized to find significant clusters in the right insula cortex for the mood predictor and right precuneus and paracentral lobule for 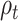 . In our confirmatory analysis, we found no significant effect of self-reported mood on the evoked response source localized to the right insula.
. In our confirmatory analysis, we found no significant effect of self-reported mood on the evoked response source localized to the right insula.
The 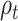 parameter was confirmed to predict the response on the right paracentral lobule at 500 ms, and at an earlier peak at ~250 ms (Fig. 4). We could not predict the response in the right precuneus at 500 ms.
parameter was confirmed to predict the response on the right paracentral lobule at 500 ms, and at an earlier peak at ~250 ms (Fig. 4). We could not predict the response in the right precuneus at 500 ms.
Figure 4.
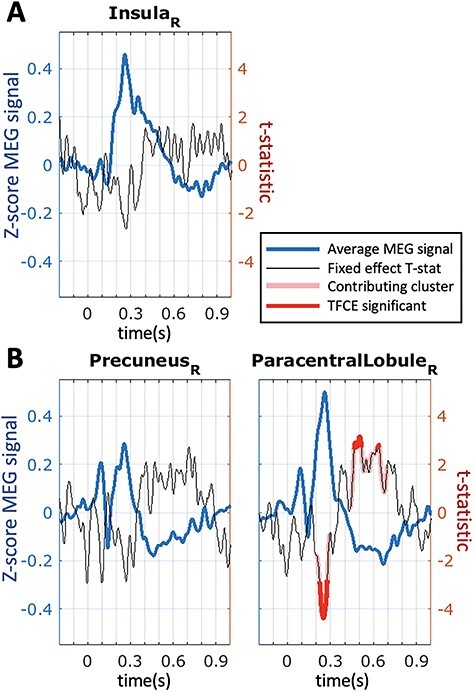
Response to reward feedback in ROIs from the AAL atlas hypothesized to vary with mood or with the reward prediction error parameters. A. Response to feedback in the right insula cortex and prediction from self-reported mood. No significant temporal clusters were found in the confirmatory sample. B. ROIs with significant temporal clusters for the 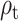 fixed effect.
fixed effect. 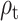 was confirmed to predict responses in right paracentral lobule (cluster peaks at 248 and 502 ms). No significant effect of
was confirmed to predict responses in right paracentral lobule (cluster peaks at 248 and 502 ms). No significant effect of 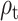 was found in the right precuneus.
was found in the right precuneus.
Hypothesis 3: The Reward Expectation Parameters from the Primacy Mood Model (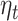 ) Will Be Predictive of the Signal in Posterior MEG Sensors during the 250–400 ms Time Window after the Presentation of Gambling Options
) Will Be Predictive of the Signal in Posterior MEG Sensors during the 250–400 ms Time Window after the Presentation of Gambling Options
For our last hypothesis, we tested whether the trial level variation in evoked response 250–400 ms after presentation of gambling options could be predicted by the model expectation parameters.
Agreeing with our initial hypothesis, we found that 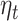 significantly predicted signal response in clusters of MEG axial gradiometers (Fig. 5B). Though at the source level, we could not find a corresponding significant cluster surviving multiple comparison correction (P-value > 0.05).
significantly predicted signal response in clusters of MEG axial gradiometers (Fig. 5B). Though at the source level, we could not find a corresponding significant cluster surviving multiple comparison correction (P-value > 0.05).
Figure 5.
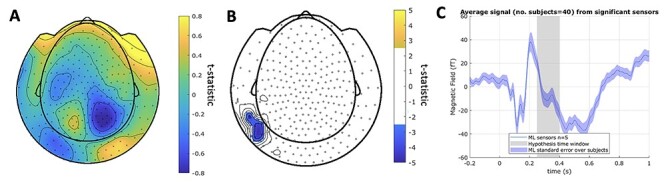
Expectation predicts the evoked response 250–400 ms after presentation of gambling options. A. Topographic map of the z-scored average MEG signal over all trials and subjects in the 250–400 ms time window after presentation of gambling options. B. Maps of MEG sensors where expectation parameter 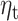 significantly predicts MEG response 250–400 ms following presentation of gambling options. Sensors surviving clustering correction (
significantly predicts MEG response 250–400 ms following presentation of gambling options. Sensors surviving clustering correction (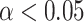 , two-tailed, 10 000 random permutations) are shown in color. Color bar indicates t-statistic of the fixed effect. Predictor
, two-tailed, 10 000 random permutations) are shown in color. Color bar indicates t-statistic of the fixed effect. Predictor 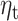 did not show any surviving significant clusters. In source space. C. Time course of the evoked response (average over all trials and subjects in confirmatory sample) over all significant sensors (clusters in Fig. 5B). The time window of interest is highlighted in gray.
did not show any surviving significant clusters. In source space. C. Time course of the evoked response (average over all trials and subjects in confirmatory sample) over all significant sensors (clusters in Fig. 5B). The time window of interest is highlighted in gray.
Exploratory Analysis
Comparison with fMRI Results
We found that subject average beta-gamma power in the pre mood rating period was significantly (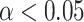 , 5000 random permutations, TFCE cluster correction) correlated with the subject expectation weight
, 5000 random permutations, TFCE cluster correction) correlated with the subject expectation weight 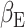 from the primacy mood model (Fig. 6). We found significant clusters with peaks in the ACC (r = 0.6, P-value = 3 × 10–6), caudate and occipital cortex. By comparing (Keren et al. 2021) previous fMRI work, we found overlap between MEG and BOLD fMRI activation correlating to
from the primacy mood model (Fig. 6). We found significant clusters with peaks in the ACC (r = 0.6, P-value = 3 × 10–6), caudate and occipital cortex. By comparing (Keren et al. 2021) previous fMRI work, we found overlap between MEG and BOLD fMRI activation correlating to 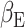 in the ACC.
in the ACC.
Figure 6.
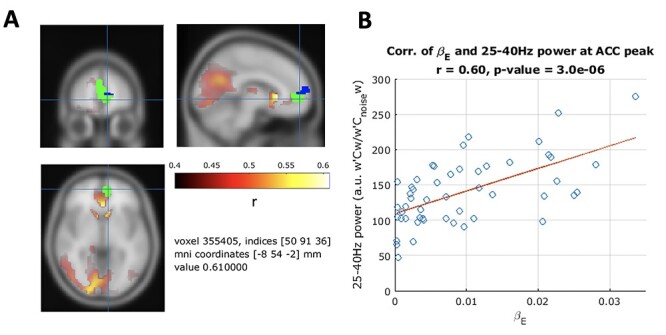
Subject level analysis: subject level expectation weight 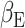 significantly (
significantly (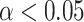 , 5000 random permutations) correlates with subject average beta-gamma power preceding mood rating. Color bar indicates Pearson’s correlation (r) for MEG power. Source map showing significantly correlated clusters has been masked with cortical and subcortical regions included in the AAL atlas. Subject beta-gamma power shows significant correlation clusters in subgenual ACC, caudate and occipital cortex. Voxels of overlap between MEG and fMRI results (Keren et al. 2021) are displayed in green and voxels where only fMRI showed significant correlation are in blue. Both modalities show a subject level brain activity correlating with
, 5000 random permutations) correlates with subject average beta-gamma power preceding mood rating. Color bar indicates Pearson’s correlation (r) for MEG power. Source map showing significantly correlated clusters has been masked with cortical and subcortical regions included in the AAL atlas. Subject beta-gamma power shows significant correlation clusters in subgenual ACC, caudate and occipital cortex. Voxels of overlap between MEG and fMRI results (Keren et al. 2021) are displayed in green and voxels where only fMRI showed significant correlation are in blue. Both modalities show a subject level brain activity correlating with 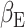 in ACC regions.
in ACC regions.
Exploratory Sensitivity Analysis
As part of our exploratory analysis, we aimed to see if the relationships between expectation and beta-gamma power may be an effect of group differences or depended on poor mood model fit.
We selected a subsample with self-reported mood well described by the primacy model (model fit r-squared > 0.5 and mood range > 0.1). This threshold included 30 participants from the full sample (30/54 = 56% of sample). Two participants in this group did not have a structural anatomical. For our analyses at source level, the sample therefore included 28 participants (age 16.0 ± 1.9 years, 12 MDDs, 18 females).
Beta-Gamma Power Is Positively Related to Expectation
in our sensitivity sample (N = 28) we found that expectation still significantly (P < 0.05, two-tailed null distribution, TFCE) predicted beta-gamma power in a cluster including posterior cingulate. Compared to the results of the confirmatory analysis the significant cluster extended more into left temporal cortex and frontal cortex, with a peak in the ACC and frontal superior medial cortex (Fig. 7A).
Figure 7.
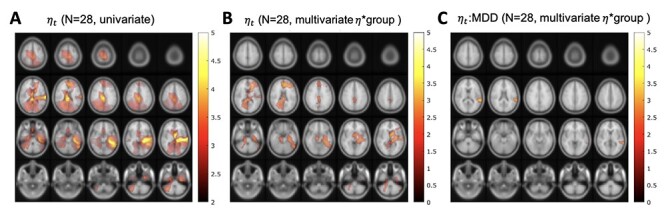
Sensitivity analysis for beta-gamma power and the accumulated expectation 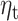 . (A) Source clusters where expectation
. (A) Source clusters where expectation 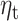 significantly predicts beta-gamma power in the sensitivity sample. (B) Multivariate analysis including fixed effect of group (HV or MDD) shows a reduced effect of
significantly predicts beta-gamma power in the sensitivity sample. (B) Multivariate analysis including fixed effect of group (HV or MDD) shows a reduced effect of 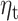 with surviving clusters in the frontal cortex. (C) Significant effect of
with surviving clusters in the frontal cortex. (C) Significant effect of 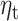 and MDD group interaction in the left temporal cortex.
and MDD group interaction in the left temporal cortex.
Effects of Group: MDD/HV and MFQ Scores
We then tested the effects of MFQ score and MDD diagnosis in the sensitivity sub-sample (n = 30), which responded well to the mood induction.
Relationship between Beta-Gamma Power and Expectation
We found that MFQ score and its interaction with expectation do not have any significant effect on beta-gamma power. MDD diagnosis is also not predictive of beta-gamma power, but its inclusion in the prediction model reduces the trial variance explained by 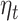 (Fig. 7B) and the interaction of MDD diagnosis with the accumulated expectation (
(Fig. 7B) and the interaction of MDD diagnosis with the accumulated expectation (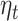 ) shows a significant cluster in the left mid and superior temporal cortex (Fig. 7C).
) shows a significant cluster in the left mid and superior temporal cortex (Fig. 7C).
Discussion
With a confirmatory sample of 40 participants, we found support in our pre-registered hypothesis that reward expectation, defined by the computational primacy mood model, is positively related to beta-gamma power over central MEG sensors. The same linear mixed effect analysis at the source space localized the main significant cluster over the posterior cingulate cortex (PCC), extending to mid cingulate, paracentral lobule, and parietal cortex. No significant clusters were found in superior or medial frontal cortex when including the full confirmatory sample. Our sensitivity analysis, selecting a sub-sample of participants well described by the primacy mood model, showed even better prediction of beta-gamma power from expectation, with a significant cluster also in frontal superior cortex and ACC, as well as a cluster in the left temporal cortex predicted by MDD diagnosis. The cingulate cortex is thought to have an important role in integrative brain functions, being involved in emotional processing, memory, and learning. The PCC in particular is involved in memory processes and strongly connected to the hippocampus as well as being a key node in the resting state network.
It is interesting to note that from our model definition, reward expectation is the equivalent to the average of all experienced rewards during the gambling task, a mental representation that we expect to involve memory processes. Though our results do not link directly beta-gamma power with self-reported mood, our computational mood model support the idea that reward expectation is a key component in mood processes, suggesting that it may be important to identify the separate mental processes that contribute to form mood if we hope to understand its integrative function in the human brain.
With this result, we also identify that brain oscillations may have an important, and measurable, role in mood dynamics, as previously found in studies involving pharmacological manipulation (e.g., ketamine) and group comparisons between MDDs and HVs showing in particular how reduced frontal gamma oscillations may be a marker of depression (Fitzgerald and Watson 2018; Nugent et al. 2019a).
Brain oscillations are believed to be key to brain communication (supported by an expanding literature in MEG functional connectivity) and their function, depending on oscillatory frequency, in conjunction with other techniques (PET, MRS) can be used to explore the specific function of neuronal assemblies (e.g., glutamatergic excitatory vs. GABAergic inhibitory processes). This is key to determine possible dysfunctions in mood disorders such as depression, and possibly developing more effective pharmacological therapies than current antidepressants. Previous work in brain electrophysiology has identified high-frequency gamma oscillations as possible markers of depression, with depressed patient showing reduced gamma power in the frontal cortex (Fitzgerald and Watson 2018).
Beta-gamma oscillations have been observed if multiple EEG studies, synchronizing in response to positive feedback (HajiHosseini and Holroyd 2015; Marco-Pallarés et al. 2015). The naming of the band in the literature is unclear, with different authors also referring to it as high-beta or low-gamma bands. We initially selected the 25–40 Hz band based on these studies and analysis of our exploratory sample with standard frequency bands. We believe these results may be a start for further studies exploring how changes in beta-gamma power and beta-gamma functional connectivity in the cingulate cortex relate to reward presentation (as observed in EEG following reward feedback) and mood.
Looking at evoked responses, we had hypothesized that the response of the insular cortex to reward feedback would be correlated with self-reported mood. The insular cortex is thought to be a key area for reward processes, interoception, and mood (Preuschoff et al. 2008; Singer et al. 2009) with multiple studies identifying changes in insula function in depressed patients. While we observed activation of the right insular cortex following reward feedback (both positive and negative), in this study we could not find significant evidence that insula response is directly influenced by participants’ self-reported mood.
We found instead confirmatory evidence that RPE (a predictor of mood as defined from the primacy model) modulates the evoked potential in the paracentral lobule both at an early 200–300 ms and later time ~ 500 ms from feedback presentation. Further sensitivity analysis showed that in participants whose behavior was well described by the primacy mood model, RPE was predictive of response to feedback between 200–300 ms in a number of ROIs including paracentral lobule, mid and posterior cingulate, and right basal ganglia. The direction of the prediction in all of these results indicated a stronger response to negative reward prediction errors. This is in agreement with previous literature on the feedback related negativity (FRN), extensively observed in EEG. The localization of the FRN, observed on fronto-central sensors in EEG, is still unclear. Doñamayor et al. (2012) localized the FRN from MEG data to the PCC, while other studies combing EEG and fMRI localized the FRN to the dorsal ACC (Hauser et al. 2014).
In our sensitivity analysis, we also found that both self-reported mood and the accumulated RPE were predictive of the peak at ~265 ms in response to feedback in the paracentral lobule. This result, combined with previous EEG work by Paul and Pourtois (2017), showing a likely effect of mood on the FRN, lends support to the idea that we may be able to measure the effect of mood on fast reward processes. We propose that this may be a starting point in exploring of mood is represented in the neural activity and modified by the experience of different reward environments in both healthy volunteers and in major depression.
Though we found some evidence that cerebellum activity is correlated with reward expectation following the presentation of gambling options (see Fig. S3 and S12 in Supplementary Material), this was not confirmed in our source level analysis of the confirmatory sample. Several papers have highlighted the involvement of cerebellum in reward processing and its connection to basal ganglia (Bostan et al. 2010; Wagner et al. 2017; Pierce and Péron 2020), but how well we can measure cerebellar activity with non-invasive electrophysiology remains largely unexplored.
Although we tried to mitigate statistical bias by pre-registering our approach, our analysis still has some technical limitations. We are analyzing the change in MEG signal over trials: this gives lower signal to noise compared to a standard beamformer localization where multiple trials are averaged together. While we are using a linear mixed effects model to include all our available data into one statistical test, source localization in M/EEG always maintains a degree of uncertainty due to the ill posed nature of the inverse problem and its estimate is dependent on signal SNR (as well as choice of head model and co-registration accuracy (Jaiswal et al. 2020)).
We explored the choice of forward model and beamformer parameters in Supplementary Material, finding a strong effect of beamformer parameter choice on our localization results for beta-gamma power. We suggest that for similar analyses it might be beneficial to apply an adaptive regularization as proposed in Woolrich et al. (2011)) or test localization accuracy at similar SNR level with computational models to determine the best source localization parameters.
While most of our hypotheses do not test for the effect of mood directly, we start from the proven relationship between mood and reward environment (the progressive update of reward expectation and prediction errors) to define task parameters that are both correlated to self-reported mood and to measurable brain activity. We can see how key features of rewards that influence mood dynamics (i.e., expectation and reward prediction error) are encoded in multiple brain regions at different times and with different mechanisms (both stimulus evoked responses and oscillations).
To our knowledge, this paper offers new evidence that it is possible to track the effect of changes in mood predictors in neuronal activity (non-invasively) at the minute time scales. Based on our pre-registered exploratory analysis, within our task we only expected to see direct correlates of mood on neural activity on the insula, but this was not confirmed by our results. While univariate analysis did show significant effects of mood, multivariate analysis may be more suited to reveal how our perceived mood affects brain function. We believe mood to be an integrative function, which cannot be accurately reflected by the activity of a single brain area but is the result of activity and communication between multiple cortical and sub-cortical regions. Mood may not be measured as activation of a brain region, but rather a shift in baseline activity and functional connectivity of brain networks, priming the brain to respond more strongly to certain stimuli (and/or “inhibiting” the brain to respond less strongly to others), similar to the effect of attention and arousal (Bowrey et al. 2017). We hope this start may help laying some of the groundwork to determine possible causality in reward and mood processes in humans in vivo by identifying both ROIs and accurate timing.
Supplementary Material
Contributor Information
Lucrezia Liuzzi, Section of Clinical and Computational Psychiatry (CompΨ), Emotion and Development Branch, National Institute of Mental Health, National Institutes of Health, Bethesda, MD 20892, USA.
Katharine K Chang, Department of Psychology, University of Rochester, Rochester, NY 14627, USA.
Charles Zheng, Machine Learning Team, Functional Magnetic Resonance Imaging Facility, National Institutes of Health, Bethesda, MD 20892, USA.
Hanna Keren, Section of Clinical and Computational Psychiatry (CompΨ), Emotion and Development Branch, National Institute of Mental Health, National Institutes of Health, Bethesda, MD 20892, USA.
Dipta Saha, Section of Clinical and Computational Psychiatry (CompΨ), Emotion and Development Branch, National Institute of Mental Health, National Institutes of Health, Bethesda, MD 20892, USA.
Dylan M Nielson, Section of Clinical and Computational Psychiatry (CompΨ), Emotion and Development Branch, National Institute of Mental Health, National Institutes of Health, Bethesda, MD 20892, USA.
Argyris Stringaris, Section of Clinical and Computational Psychiatry (CompΨ), Emotion and Development Branch, National Institute of Mental Health, National Institutes of Health, Bethesda, MD 20892, USA.
Funding
The Intramural Research Program of the National Institute of Mental Health, National Institutes of Health (NIH) (award no. ZIA-MH002957–01 to AS). The views expressed in this article do not necessarily represent the views of the National Institutes of Health, the Department of Health and Human Services, or the United States Government.
Ethics
Human subjects: All participants signed informed consent to a protocol approved by the NIH Institutional Review Board. The protocol is registered under the clinical trial no. NCT03388606.
Notes
This work used the computational resources of the NIH HPC (high-performance computing) Biowulf cluster (http://hpc.nih.gov). Data analysis uses functions from the FieldTrip software toolbox (http://fieldtriptoolbox.org). Conflict of Interest: The authors declare no competing financial interests.
Code Accessibility
All analysis code will be made available on github (https://github.com/nimh-comppsych/matlab_meg-mmi).
Mood modeling code by Keren et al. (doi: 10.7554/eLife.62051) used in our analysis is available at https://osf.io/vw7sz/?view_only=e8cb4ef6782e4735815867203971994a.
Data Accessibility
Study data available on OpenNeuro (https://openneuro.org/datasets/ds003568).
References
- Bostan AC, Dum RP, Strick PL. 2010. The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci. 107:8452–8456. 10.1073/pnas.1000496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowrey HE, James MH, Aston-Jones G. 2017. New directions for the treatment of depression: targeting the photic regulation of arousal and mood (PRAM) pathway. Depress Anxiety. 34:588–595. 10.1002/da.22635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes MJ, Vrba J, Robinson SE, Stevenson CM, Peters AM, Barnes GR, Hillebrand A, Morris PG. 2008. Optimising experimental design for MEG beamformer imaging. Neuroimage. 39:1788–1802. 10.1016/j.neuroimage.2007.09.050. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. 2000. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 4:215–222. 10.1016/S1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- De Pascalis V, Strippoli E, Riccardi P, Vergari F. 2004. Personality, event-related potential (ERP) and heart rate (HR) in emotional word processing. Personal Individ Differ. 36:873–891. 10.1016/S0191-8869(03)00159-4. [DOI] [Google Scholar]
- Doñamayor N, Schoenfeld MA, Münte TF. 2012. Magneto- and electroencephalographic manifestations of reward anticipation and delivery. Neuroimage. 62:17–29. 10.1016/j.neuroimage.2012.04.038. [DOI] [PubMed] [Google Scholar]
- Eldar E, Rutledge RB, Dolan RJ, Niv Y. 2016. Mood as representation of momentum. Trends Cogn Sci. 20:15–24. 10.1016/j.tics.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Büchel C, Gross JJ. 2015. The neural bases of emotion regulation. Nat Rev Neurosci. 16:693–700. 10.1038/nrn4044. [DOI] [PubMed] [Google Scholar]
- Fingelkurts AA, Fingelkurts AA. 2015. Altered structure of dynamic electroencephalogram oscillatory pattern in major depression. Biol Psychiatry, Cortical Oscillations for Cognitive/Circuit Dysfunction in Psychiatric Disorders. 77:1050–1060. 10.1016/j.biopsych.2014.12.011. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PJ, Watson BO. 2018. Gamma oscillations as a biomarker for major depression: an emerging topic. Transl Psychiatry. 8:1–7. 10.1038/s41398-018-0239-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser TU, Iannaccone R, Stämpfli P, Drechsler R, Brandeis D, Walitza S, Brem S. 2014. The feedback-related negativity (FRN) revisited: new insights into the localization, meaning and network organization. Neuroimage. 84:159–168. 10.1016/j.neuroimage.2013.08.028. [DOI] [PubMed] [Google Scholar]
- Hiser J, Koenigs M. 2018. The multifaceted role of ventromedial prefrontal cortex in emotion, decision-making, social cognition, and psychopathology. Biol Psychiatry. 83:638–647. 10.1016/j.biopsych.2017.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, Nieuwenhuis S, Yeung N, Cohen JD. 2003. Errors in reward prediction are reflected in the event-related brain potential. Neuroreport. 14:2481–2484. [DOI] [PubMed] [Google Scholar]
- HajiHosseini A, Holroyd CB. 2015. Reward feedback stimuli elicit high-beta EEG oscillations in human dorsolateral prefrontal cortex. Sci Rep. 5:13021. 10.1038/srep13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal A, Nenonen J, Stenroos M, Gramfort A, Dalal SS, Westner BU, Litvak V, Mosher JC, Schoffelen J-M, Witton C et al. 2020. Comparison of beamformer implementations for MEG source localization. Neuroimage. 216:116797. 10.1016/j.neuroimage.2020.116797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. 2015. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiat. 72:603–611. 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren H, Zheng C, Jangraw DC, Chang K, Vitale A, Rutledge RB, Pereira F, Nielson DM, Stringaris A. 2021. The temporal representation of experience in subjective mood. Elife. 10:e62051. 10.7554/eLife.62051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimstra TA, Kuppens P, Luyckx K, Branje S, Hale WW, Oosterwegel A, Koot HM, Meeus WHJ. 2016. Daily dynamics of adolescent mood and identity. J Res Adolesc. 26:459–473. 10.1111/jora.12205. [DOI] [PubMed] [Google Scholar]
- Larson MJ, Carbine KA. 2017. Sample size calculations in human electrophysiology (EEG and ERP) studies: a systematic review and recommendations for increased rigor. Int J Psychophysiol Rigor and Replication: Towards Improved Best Practices in Psychophysiological Research. 111:33–41. 10.1016/j.ijpsycho.2016.06.015. [DOI] [PubMed] [Google Scholar]
- Marco-Pallarés J, Münte TF, Rodríguez-Fornells A. 2015. The role of high-frequency oscillatory activity in reward processing and learning. Neurosci Biobehav Rev. 49:1–7. 10.1016/j.neubiorev.2014.11.014. [DOI] [PubMed] [Google Scholar]
- Nettle D, Bateson M. 2012. The evolutionary origins of mood and its disorders. Curr Biol. 22:R712–R721. 10.1016/j.cub.2012.06.020. [DOI] [PubMed] [Google Scholar]
- NIMH » Major Depression [WWW Document] . 2021. https://www.nimh.nih.gov/health/statistics/major-depression (accessed 5.4.21).
- Nolte G. 2003. The magnetic lead field theorem in the quasi-static approximation and its use for magnetoencephalography forward calculation in realistic volume conductors. Phys Med Biol. 48:3637–3652. 10.1088/0031-9155/48/22/002. [DOI] [PubMed] [Google Scholar]
- Nugent AC, Ballard E, Gould TD, Park LT, Moaddel R, Brutsche NE, Zarate CA. 2019. Ketamine has distinct electrophysiological and Behavioral effects in depressed and healthy subjects. Mol Psychiatry. 24:1040–1052. 10.1038/s41380-018-0028-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent AC, Wills KE, Gilbert JR, Zarate CA. 2019. Synaptic potentiation and rapid antidepressant response to ketamine in treatment-resistant major depression: a replication study. Psychiatry Res Neuroimaging. 283:64–66. 10.1016/j.pscychresns.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen J-M. 2011. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011:156869. 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul K, Pourtois G. 2017. Mood congruent tuning of reward expectation in positive mood: evidence from FRN and theta modulations. Soc Cogn Affect Neurosci. 12:765–774. 10.1093/scan/nsx010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov YG, Adamian N, Appelhoff S, Arvaneh M, Benwell CSY, Beste C, Bland AR, Bradford DE, Bublatzky F, Busch NA et al. 2021. #EEGManyLabs: investigating the replicability of influential EEG experiments. Cortex. 144:213–229. 10.1016/j.cortex.2021.03.013. [DOI] [PubMed] [Google Scholar]
- Pernet C, Garrido MI, Gramfort A, Maurits N, Michel CM, Pang E, Salmelin R, Schoffelen JM, Valdes-Sosa PA, Puce A. 2020. Issues and recommendations from the OHBM COBIDAS MEEG committee for reproducible EEG and MEG research. Nat Neurosci. 23:1473–1483. 10.1038/s41593-020-00709-0. [DOI] [PubMed] [Google Scholar]
- Pierce JE, Péron J. 2020. The basal ganglia and the cerebellum in human emotion. Soc Cogn Affect Neurosci. 15:599–613. 10.1093/scan/nsaa076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuschoff K, Quartz SR, Bossaerts P. 2008. Human insula activation reflects risk prediction errors as well as risk. J Neurosci. 28:2745–2752. 10.1523/JNEUROSCI.4286-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge RB, Skandali N, Dayan P, Dolan RJ. 2014. A computational and neural model of momentary subjective well-being. Proc Natl Acad Sci. 111:12252–12257. 10.1073/pnas.1407535111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K. 2009. A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci. 13:334–340. 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. 2009. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 44:83–98. 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Stevens FL, Hurley RA, Taber KH, Hurley RA, Hayman LA, Taber KH. 2011. Anterior cingulate cortex: unique role in cognition and emotion. J Neuropsychiatry Clin Neurosci. 23:121–125. 10.1176/jnp.23.2.jnp121. [DOI] [PubMed] [Google Scholar]
- Sur S, Sinha VK. 2009. Event-related potential: an overview. Ind Psychiatry J. 18:70–73. 10.4103/0972-6748.57865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmi D, Fuentemilla L, Litvak V, Duzel E, Dolan RJ. 2012. An MEG signature corresponding to an axiomatic model of reward prediction error. NeuroImage, Neuroergonomics: The human Brain in Action and at Work. 59:635–645. 10.1016/j.neuroimage.2011.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay S, Rogasch NC, Premoli I, Blumberger DM, Casarotto S, Chen R, Di Lazzaro V, Farzan F, Ferrarelli F, Fitzgerald PB et al. 2019. Clinical utility and prospective of TMS–EEG. Clin Neurophysiol. 130:802–844. 10.1016/j.clinph.2019.01.001. [DOI] [PubMed] [Google Scholar]
- Turner BO, Paul EJ, Miller MB, Barbey AK. 2018. Small sample sizes reduce the replicability of task-based fMRI studies. Commun Biol. 1:1–10. 10.1038/s42003-018-0073-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veen BDV, Drongelen WV, Yuchtman M, Suzuki A. 1997. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans Biomed Eng. 44:867–880. 10.1109/10.623056. [DOI] [PubMed] [Google Scholar]
- Vinckier F, Rigoux L, Oudiette D, Pessiglione M. 2018. Neuro-computational account of how mood fluctuations arise and affect decision making. Nat Commun. 9:1708. 10.1038/s41467-018-03774-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner MJ, Kim TH, Savall J, Schnitzer MJ, Luo L. 2017. Cerebellar granule cells encode the expectation of reward. Nature. 544:96–100. 10.1038/nature21726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich M, Hunt L, Groves A, Barnes G. 2011. MEG beamforming using Bayesian PCA for adaptive data covariance matrix regularization. Neuroimage. 57:1466–1479. 10.1016/j.neuroimage.2011.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH, Mattson DL, Pardo JV. 2002. Brain activity in ventromedial prefrontal cortex correlates with individual differences in negative affect. Proc Natl Acad Sci. 99:2450–2454. 10.1073/pnas.042457199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zrenner B, Zrenner C, Gordon PC, Belardinelli P, McDermott EJ, Soekadar SR, Fallgatter AJ, Ziemann U, Müller-Dahlhaus F. 2020. Brain oscillation-synchronized stimulation of the left dorsolateral prefrontal cortex in depression using real-time EEG-triggered TMS. Brain Stimul. 13:197–205. 10.1016/j.brs.2019.10.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Study data available on OpenNeuro (https://openneuro.org/datasets/ds003568).


