Abstract
Background
No dietary factors have yet been shown to conclusively impact the incidence of microscopic colitis (MC). Here, we sought to examine the relationship between alcohol intake and the risk of MC.
Methods
We conducted a prospective cohort study of 209,902 participants (age range, 28.5–66.7 years) enrolled in the Nurses’ Health Study (NHS) and Nurses’ Health Study II (NHSII). Validated data on alcohol consumption were collected at baseline in 1986 in the NHS and 1991 in the NHSII and updated every 4 years. Diagnoses of MC were confirmed via review of histopathology data. We used Cox proportional hazards modeling to estimate adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs).
Results
Through 2016 in the NHS and 2017 in the NHSII, we confirmed 352 incident cases of MC over 4,994,324 person-years. Higher alcohol consumption was associated with an increased risk of MC (Ptrend < .001). Compared to non-users, the aHRs of MC were 1.20 (95% CI, 0.86–1.67) for consumers of 0.1–4.9 g/day of alcohol, 1.90 (95% CI, 1.34–2.71) for consumers of 5–14.9 g/day, and 2.31 (95% CI, 1.54–3.46) for consumers of ≥15 g/day. The associations were consistent across the histologic subtypes of collagenous and lymphocytic colitis (Pheterogeneity = .523). When stratified by alcohol type, the risk according to every 2 servings/week appeared to be strongest with consumption of wine (aHR, 1.08; 95% CI, 1.04–1.12) as compared to beer (aHR, 1.01; 95% CI, 0.91–1.12) or liquor (aHR, 1.00; 95% CI, 0.92–1.09).
Conclusions
Alcohol consumption was associated with an increased risk of MC. Further studies are needed to determine the mechanism underlying these associations, as well as the impact of reducing alcohol intake in patients with MC.
Keywords: microscopic colitis, alcohol, wine, beer, liquor
Introduction
Microscopic colitis (MC) is a chronic inflammatory disease of the large intestine characterized by watery, non-bloody diarrhea and histologic findings of lymphocytic infiltration, with or without expansion of the lamina propria.1 The disease most often occurs among older women, and has a comparable incidence to the more traditional inflammatory bowel diseases: Crohn’s disease (CD) and ulcerative colitis (UC).1–3 While the precise pathogenesis of MC remains unknown, it is believed to result from a dysregulated mucosal immune response to luminal antigens in genetically predisposed individuals.4 Prior epidemiological studies have largely been limited to identifying pharmacologic triggers for MC,5,6 with a particular focus on medications that are known to modulate the epithelial barrier or the gut microbiome function, including non-steroidal anti-inflammatory drugs (NSAIDs), selective serotonin reuptake inhibitors (SSRIs), proton pump inhibitors (PPIs), statins, and menopausal hormone therapy. In contrast, the roles of dietary factors, which also have a significant impact on shaping the gut microenvironment and mucosal barrier function,7 have largely been unexplored. A few dietary factors have been studied, but none have yet been conclusively associated with risk of MC.8,9 Specifically, several studies have investigated whether alcohol intake increases the risk of MC,8,10–12 but these studies have yielded conflicting results and been limited by a combination of small sample sizes, failure to control for known risk factors for MC, a lack of detailed data on amounts and types of alcohol use, and an inability to prospectively ascertain other lifestyle factors and diagnoses of MC.
Alcohol consumption has known deleterious effects on the gastrointestinal system. Regular alcohol use may impair epithelial barrier function and alter the gut microbial composition, ultimately resulting in mucosal injury.13 These biologic effects of alcohol intake are in large part thought to be responsible for the development of gastritis, peptic ulcer disease, and non-infectious diarrhea.14 Interestingly, many of these complications are more pronounced in older adults where there is evidence of declines in mucosal integrity and microbial diversity.15 We therefore sought to examine the relationship between alcohol intake and the risk of MC using 2 large, prospective cohort studies of US women: the Nurses’ Health Study (NHS) and Nurses’ Health Study II (NHSII). As both the NHS and NHSII include more than 30 years of follow-up data with repeated measurements of validated dietary and lifestyle factors, these cohorts provide us with a unique opportunity to examine this relationship while also accounting for other important confounders.
Methods
Study Population
Our study population was comprised of participants enrolled in the NHS and NHSII. The NHS consists of 121,701 female US nurses who have completed health-related questionnaires by mail every 2 years since 1976. Similarly, the NHSII includes 116,667 female US nurses who have completed health-related questionnaires by mail every other year since 1989. Overall, participants enrolled in the NHS and NHSII have returned follow-up questionnaires at response rates greater than 90%.
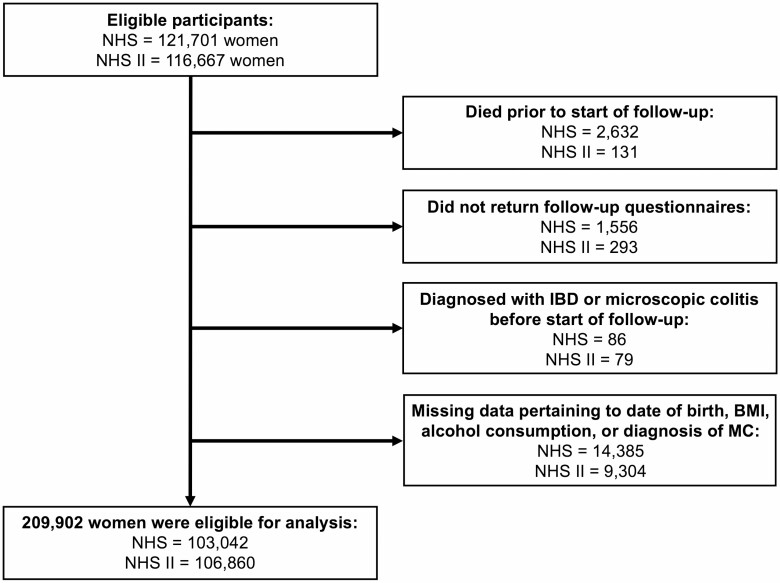
As physicians were not routinely collecting random biopsies as part of the diagnostic workup for suspected MC until at least the mid-1980s16 and the dietary data were first collected in 1991 in NHSII, we limited our follow-up to 1986–2016 in the NHS and 1991–2017 in the NHSII. We excluded participants who died before the start of follow-up; who were diagnosed with CD, UC, indeterminate colitis, or MC prior to the start of follow-up; or who were lost to follow-up prior to 1986 in the NHS and 1991 in the NHSII. Similarly, participants were excluded for missing data pertaining to the date of birth, body mass index (BMI), alcohol consumption, or diagnosis of MC (Fig. 1). Our study was approved by the Institutional Review Board of Mass General Brigham (Boston, MA).
Figure 1.
Flowchart of eligible participants in the study. Abbreviations: BMI, body mass index; IBD, inflammatory bowel disease; MC, microscopic colitis; NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II.
Assessment of Alcohol Consumption
The NHS began assessing alcohol consumption in 1980 via semiquantitative food frequency questionnaires (SFFQ). These dietary questionnaires were repeated in 1984, 1986, and then every 4 years thereafter. Likewise, the SFFQ was first administered to NHSII participants in 1991 and was updated every 4 years.
Participants were asked to report the average number of servings of alcohol consumed using non-linear categories ranging from 1–3 servings/month to ≥6 servings/day, both in total and with respect to each alcohol type (light beer, regular beer, white wine, red wine, liquor), as well as the average number of days per week that they consumed alcohol. The total quantity of alcohol consumed was calculated by multiplying the average number of servings consumed daily by the average alcohol content of each type of alcoholic beverage (11.3 g of alcohol per 12 ounces of light beer, 12.8 g per 12 ounces of beer, 11.0 g per 5 ounces of wine, and 14.0 g per 1.5 ounces of liquor). For the purposes of our analysis, alcohol consumption was collected at baseline in 1986 in the NHS and 1991 in the NHSII and updated every 4 years thereafter. The average quantity of daily alcohol consumption was sub-classified as 0 g, 0.1–4.9 g, 5–14.9 g, and ≥15 g. The average number of servings of alcohol consumed per unit of time was sub-classified as 0 servings/month, 1 serving/month to 6 servings/week, and ≥7 servings/week. When alcohol use was analyzed as a continuous variable, every unit increase was defined as 2 servings per week.
The assessment of alcohol consumption from the SFFQ has previously been validated. In a 1991 study of 173 women in the NHS and 136 men in the Health Professional Follow-Up Study, alcohol intake reported through a self-administered SFFQ had excellent correlations with alcohol consumption estimated from multiple 7-day diet records (Spearman correlation coefficients: 0.90 for women, 0.86 for men).17
Assessment of Covariates
At baseline and every 2 years thereafter, age, height, weight, menopause status, use of menopausal hormone therapy (never, prior, current), and smoking status (never, prior, current) were assessed and updated. The BMI was calculated from the reported height and weight values. Self-reported weights have been previously validated in a 1990 subset of NHS participants, and had a high correlation overall with in-person weight measurements (r = 0.97).18 Data on physical activity were also collected in both cohorts, starting in 1984 in the NHS and 1989 in the NHSII and updated every 4 years. Briefly, we estimated the total metabolic equivalent (MET)-hours/week of physical activity using the reported average time per week spent doing any of the following activities: walking or hiking outdoors; jogging; running; bicycling; swimming laps; playing tennis; calisthenics, aerobics, aerobic dance, or using a rowing machine; playing squash or racquet ball; and performing other vigorous activities. We also assessed diet quality using the Alternative Healthy Eating Index (AHEI) score, which was constructed based on dietary factors that have been consistently associated with a lower risk of chronic diseases.19 This information was updated every 4 years with each SFFQ.
We also collected data pertaining to the use of NSAIDs, PPIs, SSRIs, and statins. Consistent with our prior studies, we defined regular use of NSAIDs as intake of ≥2 tablets per week.6 Of note, the NHS questionnaire did not collect data pertaining to NSAID use until 1990, while the NHSII has collected data on NSAID use since the cohort inception in 1989. Likewise, the use of PPIs, SSRIs, and statins was first consistently recorded starting in 2000 and 2001 for the NHS and NHSII, respectively. Lastly, we used updated median income data derived from census tracts as a measure of socioeconomic status.
Outcome Ascertainment
At the start of both the NHS and NHSII, participants reported diagnoses of colitis on the questionnaires through open-ended responses. Starting in 1982 for the NHS and 1991 for the NHSII, questionnaires were expanded to specifically ask participants whether they had been diagnosed with any form of inflammatory bowel disease. A follow-up supplementary questionnaire was sent to distinguish whether they had been diagnosed with CD, UC, MC, or other types of colitis (eg, ischemic, etc.), as well as to obtain permission to request their medical records for review. Starting in 2014 for the NHS and 2015 for the NHSII, the general questionnaires were expanded to separately ask participants about diagnoses of CD, UC, and MC.
For all patients reporting a diagnosis of MC, medical records were reviewed independently by 2 gastroenterologists blinded to patient exposures. Pertinent clinical, endoscopic, and histopathologic findings were extracted, and cases of MC were confirmed and sub-typed as either lymphocytic colitis (LC) or collagenous colitis (CC) based on a review of histopathology reports. Participants who declined a medical record review were excluded from the analyses.
Statistical Analysis
Person-time of follow-up was calculated from the baseline questionnaire (1986 for the NHS and 1991 for the NHSII) until a diagnosis of MC, CD, or UC; the last returned questionnaire; the end of follow-up (2016 for the NHS and 2017 for the NHSII); or death, whichever occurred first. We used Cox proportional hazards modeling with time-varying exposures to estimate adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs). Models were stratified by age and time period (2-year intervals), and multivariable models were additionally adjusted for BMI (≤25, 25 <30, ≥30 kg/m2), menopausal hormone therapy (pre-menopausal, never, former, current), physical activity (MET hours/week, by quartile), NSAID use (<2 tablets/week, ≥2 tablets/week), smoking status (never, former, current), and diet quality (AHEI score, by quartiles). Tests for linear trend were performed using alcohol intake as a continuous variable and did not include non-users.
We conducted several exploratory analyses. First, we assessed the presence of a non-linear relationship between alcohol intake and risk of MC using restricted cubic spline curves.20 Second, we examined the relationship between alcohol intake and risk of MC according to subgroups defined by age (<50 years vs. ≥50 years), BMI (≤25 kg/m2 vs. >25 kg/m2), NSAID use (<2 tablets/week vs. ≥2 tablets/week), smoking status (never vs. ever), and use of menopausal hormone therapy (never vs. ever). We tested for potential effect modification using the log likelihood ratio test. Third, we explored whether the associations differed by alcohol subtype. Lastly, we explored whether the associations between alcohol intake and risk of MC differed by the subtypes of CC and LC. The P values for heterogeneity were calculated using the log likelihood ratio test, comparing model fit between models allowing separate associations across disease subtypes and models assuming a common effect.21
In a sensitivity analysis, we investigated whether use of PPIs, SSRIs, or statins significantly confounded the relationship between alcohol consumption and risk of MC by limiting our follow-up analyses to data collected after 2000 in the NHS and 2001 in the NHSII, when these medications were widely available in the market and consistently ascertained in our cohorts. Additionally, we conducted latency analyses evaluating the relationship between alcohol consumption and risk of MC according to various exposure time windows (ie, baseline, 0 to <4 years, 4 to <8 years, 8 to <12 years). Furthermore, since the NHS and NHSII participants first reported diagnoses of MC via primary (rather than supplementary) questionnaires in 2014 and 2015, respectively, we also conducted an analysis limiting our study population to participants who returned these particular questionnaires. Last, we conducted an analysis of the relationship between alcohol intake and risk of MC while adjusting for the median annual income, to control for socioeconomic status.
For all analyses, P values ≤ .05 were considered statistically significant. All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
Ethical Approval
The study protocol was approved by the Institutional Review Board of Mass General Brigham, which allowed participants’ completion of questionnaires to be considered as implied consent.
Results
Study Population Characteristics
After exclusions, our study population at baseline was comprised of 103,042 participants from the NHS and 106,860 participants from the NHSII, for a total study size of 209,902 (Fig. 1). The mean age of participants at baseline was 45.5 years old (SD, 9.4 years; range, 28.5–66.7 years; Table 1), and the majority of participants were post-menopausal (74.7%) and White (93.6%). As shown in Table 1, participants consuming higher amounts of alcohol were more likely to be smokers, were more likely to use menopausal hormone therapy and NSAIDs, had a higher level of physical activity, and had a lower BMI. Diet quality as measured by AHEI was similar across different categories of alcohol consumption.
Table 1.
Baseline characteristics of participants according to alcohol consumption
| Baseline consumption of alcohol (g/day) | ||||
|---|---|---|---|---|
| 0 (n = 50,744) | 0.1–4.9 (n = 99,204) | 5–14.9 (n = 43,600) | ≥15 (n = 16,354) | |
| Cohort | ||||
| NHS, n (%) | 27,472 (54.1) | 44,844 (45.2) | 20,314 (46.6) | 10,412 (63.7) |
| NHSII, n (%) | 23,272 (45.9) | 54,360 (54.8) | 23,286 (53.4) | 5942 (36.3) |
| Age, years, mean (SD) | 47.0 (9.8) | 44.6 (9.2) | 44.8 (9.1) | 48.0 (8.9) |
| White, % | 90.1 | 94.0 | 96.1 | 95.9 |
| BMI, kg/m2, mean (SD) | 27.8 (6.9) | 27.4 (6.3) | 25.6 (5.2) | 24.8 (5.0) |
| BMI, kg/m2, categories | ||||
| 18.5 ≤ 25 | 39.8 | 40.7 | 52.5 | 58.0 |
| 25 < 30 | 29.2 | 31.3 | 30.4 | 28.2 |
| ≥30 | 31.1 | 28.0 | 17.1 | 13.7 |
| Postmenopausal, % | 66.9 | 76.5 | 79.1 | 76.4 |
| Menopausal hormone therapy use: | ||||
| Never use, % | 50.0 | 44.9 | 41.5 | 39.2 |
| Prior use, % | 41.2 | 43.9 | 44.7 | 49.1 |
| Current use, % | 8.8 | 11.3 | 13.8 | 11.7 |
| Physical activity, MET-hr/wk (SD) | 14.7 (21.2) | 17.8 (21.9) | 21.0 (23.1) | 19.2 (21.9) |
| Physical activity, by quartile: | ||||
| Q1, % | 32.8 | 24.0 | 19.1 | 22.0 |
| Q2, % | 26.3 | 25.5 | 22.7 | 24.6 |
| Q3, % | 22.8 | 25.8 | 25.9 | 24.9 |
| Q4, % | 18.2 | 24.7 | 32.4 | 28.5 |
| NSAID use ≥ 2x/wk, % | 34.0 | 46.2 | 49.3 | 45.2 |
| Smoking status: | ||||
| Never smoker, % | 66.2 | 55.2 | 44.2 | 28.3 |
| Prior smoker, % | 28.0 | 38.8 | 49.0 | 59.7 |
| Current smoker, % | 5.8 | 6.0 | 6.8 | 12.0 |
| Total pack years, years (SD) | 26.1 (23.3) | 20.5 (19.6) | 19.6 (19.4) | 27.1 (24.4) |
| Diet quality, AHEI average | 49.2 (10.1) | 50.6 (9.4) | 52.4 (9.1) | 50.8 (9.1) |
| Diet quality, in AHEI quartiles: | ||||
| Q1, % | 31.8 | 24.8 | 18.2 | 23.3 |
| Q2, % | 24.9 | 25.6 | 23.6 | 25.5 |
| Q3, % | 21.8 | 25.1 | 27.9 | 26.6 |
| Q4, % | 21.4 | 24.5 | 30.4 | 24.6 |
Abbreviations: AHEI, Alternate Healthy Eating Index; BMI, body mass index; METs, metabolic equivalents; NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II; NSAID, nonsteroidal anti-inflammatory drugs; Q, quartile; SD, standard deviation.
We documented 352 incident causes of MC over 31 years and 4,994,324 person-years of follow-up. The overall incidence of MC in our study population was 7.0 cases per 100,000 person-years.
Alcohol Consumption and Risk of MC
In our pooled analysis, higher alcohol consumption was associated with increased incidences of MC in both age-adjusted (Ptrend < .001) and multivariable-adjusted (Ptrend < .001) models (Table 2). Compared to non-users, the aHRs of MC were 1.20 (95% CI, 0.86–1.67) for consumers of 0.1–4.9 g/day of alcohol, 1.90 (95% CI, 1.34–2.71) for consumers of 5–14.9 g/day, and 2.31 (95% CI, 1.54–3.46) for consumers of ≥15 g/day (Table 2). We also examined the associations between alcohol consumption and risk of MC according to histologic subtypes of CC and LC, and observed no evidence for heterogeneity (Pheterogeneity = .523). Specifically, compared to non-users, the aHRs with consumption of ≥15g/day of alcohol were 2.78 (95% CI, 1.49–5.18; Ptrend = .005) for CC and 2.52 (95% CI, 1.43–4.45; Ptrend = .001) for LC.
Table 2.
Alcohol consumption and risk of microscopic colitis
| Cumulative average consumption of alcohol (g/day) | Ptrend | Pheterogeneity | ||||
|---|---|---|---|---|---|---|
| 0 | 0.1–4.9 | 5–14.9 | ≥ 15 | |||
| Person-years of follow-up | 1,151,326 | 2,386,316 | 1,060,502 | 396,180 | ||
| Microscopic colitis: | ||||||
| Number of cases, n | 47 | 139 | 111 | 55 | .523 | |
| Age-adjusted, HR (95% CI) | 1 (ref) | 1.35 (0.97–1.89) | 2.37 (1.68–3.34) | 2.99 (2.02–4.42) | <.001 | |
| MV-adjusted, HR (95% CI)a | 1 (ref) | 1.20 (0.86–1.67) | 1.90 (1.34–2.71) | 2.31 (1.54–3.46) | <.001 | |
| Lymphocytic colitis: | ||||||
| Number of cases, n | 25 | 68 | 49 | 27 | ||
| Age-adjusted, HR (95% CI) | 1 (ref) | 1.25 (0.79–1.98) | 1.95 (1.20–3.16) | 2.80 (1.62–4.83) | <.001 | |
| MV-adjusted, HR (95% CI)a | 1 (ref) | 1.17 (0.74–1.86) | 1.74 (1.06–2.87) | 2.52 (1.43–4.45) | .001 | |
| Collagenous colitis: | ||||||
| Number of cases, n | 17 | 66 | 56 | 28 | ||
| Age-adjusted, HR (95% CI) | 1 (ref) | 1.76 (1.03–3.00) | 3.30 (1.92–5.69) | 4.15 (2.27–7.60) | <.001 | |
| MV-adjusted, HR (95% CI)a | 1 (ref) | 1.46 (0.85–2.50) | 2.39 (1.36–4.18) | 2.78 (1.49–5.18) | .005 |
Abbreviations: CI, confidence interval; HR, hazard ratio; MV, multivariable.
aAdjusted for age, body mass index, menopausal hormone therapy, physical activity, nonsteroidal anti-inflammatory drug use, smoking status, Alternative Healthy Eating Index, and cohort.
Exploratory Analyses
We conducted several exploratory analyses. First, we evaluated the presence of a non-linear association between alcohol consumption and risk of MC by constructing cubic spline curves (Supplementary Fig. 1). There was a linear dose-responsive association between alcohol consumption and risk of MC (Plinear trend < .001). Although the association between alcohol consumption and risk of MC appeared to peak at moderate consumption of 10–15 g/day (HR ~ 2.3), the risk continued to be significant up to 30 g/day of consumption (HR ~ 1.9). Second, we explored whether the association between alcohol consumption and risk of MC was consistent according to selected strata defined by cohort, age, BMI, smoking, and use of NSAIDs or menopausal hormone therapy, and observed no evidence of an effect modification (all Pinteraction values ≥ .171; Table 3). Third, we explored whether the association differed by alcohol subtype. The associations appeared to be particularly significant with wine consumption (aHR per every 2 servings/week, 1.08; 95% CI, 1.04–1.12), while intake of beer (aHR, 1.01; 95% CI, 0.91–1.12) and liquor (aHR, 1.00; 95% CI, 0.92–1.09) were not associated with increased risk (Table 4). Furthermore, the associations between wine consumption and risk of MC were similar regardless of whether white or red wine was consumed. Compared to non-users, the aHRs of MC in those consuming ≥7 servings/week were 2.17 (95% CI, 1.45–3.25) for red wine and 2.00 (95% CI, 1.35–2.96) for white wine (Supplementary Table 1).
Table 3.
Association between alcohol consumption and risk of microscopic colitis according to selected strata
| Alcohol intake (g) | Cases | Person-years | MVa (95% CI) | Pinteraction |
|---|---|---|---|---|
| NHS: | .992 | |||
| 0 | 26 | 653,381 | 1.00 (1.00–1.00) | |
| 0.1–4.9 | 84 | 1,160,302 | 1.46 (0.93–2.27) | |
| 5–14.9 | 55 | 528,005 | 1.83 (1.13–2.97) | |
| ≥15 | 40 | 260,514 | 2.71 (1.62–4.52) | |
| NHSII: | ||||
| 0 | 21 | 497,945 | 1.00 (1.00–1.00) | |
| 0.1–4.9 | 55 | 1,226,014 | 0.95 (0.57–1.58) | |
| 5–14.9 | 56 | 532,497 | 2.00 (1.18–3.37) | |
| ≥15 | 15 | 135,665 | 1.80 (0.91–3.58) | |
| Age ≤ 50 years: | .620 | |||
| 0 | 34 | 729,772 | 1.00 (1.00–1.00) | |
| 0.1–4.9 | 101 | 1,740,450 | 1.09 (0.74–1.62) | |
| 5–14.9 | 79 | 773,763 | 1.68 (1.11–2.55) | |
| ≥15 | 42 | 242,217 | 2.47 (1.54–3.96) | |
| Age > 50 years: | ||||
| 0 | 13 | 421,553 | 1.00 (1.00–1.00) | |
| 0.1–4.9 | 38 | 645,866 | 1.56 (0.82–2.94) | |
| 5–14.9 | 32 | 286,739 | 2.62 (1.34–5.11) | |
| ≥15 | 13 | 153,962 | 2.00 (0.90–4.43) | |
| BMI ≤ 25 kg/m2: | .263 | |||
| 0 | 20 | 458,945 | 1.00 (1.00–1.00) | |
| 0.1–4.9 | 67 | 970,100 | 1.39 (0.84–2.31) | |
| 5–14.9 | 69 | 555,509 | 2.26 (1.35–3.77) | |
| ≥15 | 27 | 228,502 | 1.98 (1.09–3.60) | |
| BMI > 25 kg/m2: | ||||
| 0 | 27 | 692,381 | 1.00 (1.00–1.00) | |
| 0.1–4.9 | 72 | 1,416,216 | 1.11 (0.70–1.74) | |
| 5–14.9 | 42 | 504,993 | 1.71 (1.03–2.83) | |
| ≥15 | 28 | 167,678 | 3.28 (1.89–5.70) | |
| <2 NSAID tables per week: | .312 | |||
| 0 | 16 | 698,150 | 1.00 (1.00–1.00) | |
| 0.1–4.9 | 55 | 1,193,189 | 1.74 (0.99–3.07) | |
| 5–14.9 | 31 | 498,979 | 2.07 (1.11–3.86) | |
| ≥15 | 23 | 196,641 | 3.62 (1.86–7.05) | |
| ≥2 NSAID tablets per week: | ||||
| 0 | 31 | 453,175 | 1.00 (1.00–1.00) | |
| 0.1–4.9 | 84 | 1,193,127 | 0.97 (0.64–1.48) | |
| 5–14.9 | 80 | 561,523 | 1.77 (1.15–2.72) | |
| ≥15 | 32 | 199,538 | 1.80 (1.08–3.01) | |
| Never smoker: | .171 | |||
| 0 | 28 | 776,512 | 1.00 (1.00–1.00) | |
| 0.1–4.9 | 64 | 1,320,582 | 1.22 (0.78–1.91) | |
| 5–14.9 | 39 | 468,628 | 1.89 (1.15–3.12) | |
| ≥15 | 14 | 114,702 | 2.69 (1.40–5.18) | |
| Prior or current smoker: | ||||
| 0 | 19 | 374,814 | 1.00 (1.00–1.00) | |
| 0.1–4.9 | 75 | 1,065,734 | 1.19 (0.71–1.97) | |
| 5–14.9 | 72 | 591,874 | 1.87 (1.12–3.13) | |
| ≥15 | 41 | 281,478 | 2.22 (1.28–3.87) | |
| MHT non-user: | .267 | |||
| 0 | 15 | 693,031 | 1.00 (1.00–1.00) | |
| 0.1–4.9 | 37 | 1,267,432 | 1.11 (0.60–2.05) | |
| 5–14.9 | 35 | 519,451 | 2.25 (1.19–4.24) | |
| ≥15 | 14 | 186,555 | 2.47 (1.16–5.29) | |
| MHT user: | ||||
| 0 | 32 | 458,295 | 1.00 (1.00–1.00) | |
| 0.1–4.9 | 102 | 1,118,884 | 1.24 (0.83–1.85) | |
| 5–14.9 | 76 | 541,051 | 1.83 (1.19–2.81) | |
| ≥15 | 41 | 209,624 | 2.30 (1.42–3.72) |
Abbreviations: BMI, body mass index; CI, confidence interval; MHT, menopausal hormone therapy; MV, multivariable; NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II; NSAIDs, nonsteroidal anti-inflammatory drugs.
aAdjusted for age, body mass index, menopausal hormone therapy, physical activity, nonsteroidal anti-inflammatory drug use, smoking status, Alternative Healthy Eating Index, and cohort, minus the selected strata.
Table 4.
Association between alcohol intake and risk of microscopic colitis according to every 2 servings per week of each alcohol type
| Beer | Wine | Liquor | |
|---|---|---|---|
| Number of cases | 85 | 217 | 83 |
| Age-adjusted HR (95% CI) | 1.03 (0.93–1.14) | 1.10 (1.07–1.14) | 1.03 (0.95–1.11) |
| P trend | .512 | <.001 | .523 |
| MV HRa (95% CI) | 1.01 (0.91–1.12) | 1.08 (1.04–1.12) | 1.00 (0.92–1.09) |
| P trend | .878 | <.001 | .971 |
| MV HRb (95% CI) | 0.96 (0.86–1.08) | 1.05 (1.01–1.10) | 0.94 (0.85–1.03) |
| P trend | .520 | .029 | .166 |
Alcohol intake was analyzed as a continuous variable, with every unit increase set as equal to 2 additional servings per week. Abbreviations: CI, confidence interval; HR, hazard ratio; MV, multivariable
aAdjusted for age, body mass index, menopausal hormone therapy, physical activity, nonsteroidal anti-inflammatory drug use, smoking status, Alternative Healthy Eating Index, and cohort.
bAdditionally adjusted for total alcohol intake.
Sensitivity Analysis
We considered the possibility that our observed associations may have been confounded by other established pharmacologic risk factors for MC, such as use of PPIs, SSRIs, and statins. We therefore restricted our follow-up analysis to data collected after 2000 for the NHS and 2001 for the NHSII, when this information was consistently collected in our cohorts, and observed similar associations (Ptrend < .001). Compared to non-users, participants consuming 5–14.9 g/day (aHR, 1.93; 95% CI, 1.32–2.81) and ≥15 g/day (aHR, 2.52; 95% CI, 1.65–3.85) of alcohol were at increased risk of MC, while participants consuming 0.1–4.9 g/day (aHR, 1.18; 95% CI, 0.82–1.69) were not at significantly increased risk. Additionally, when stratifying according to the current use of these medications, we observed no evidence of an effect modification (PPIs, Pinteraction = .677; SSRIs, Pinteraction = .788; statins, Pinteraction = .748; Supplementary Table 2). We also examined the associations according to various exposure time windows. Alcohol intake was associated with increased risk of MC at 0 to <4 years (primary analyses), 4 to <8 years, and 8 to <12 years (all Ptrend values < .001), but the strongest association was at 8 to <12 years prior to diagnosis (Supplementary Table 3). Furthermore, limiting our study population to participants that returned the 2014 questionnaire in the NHS and the 2015 questionnaire in the NHSII did not materially alter our estimates. Compared to non-users, the aHRs of MC were 1.11 (95% CI, 0.79–1.56) for consumers of 0.1–4.9 g/day of alcohol, 1.77 (95% CI, 1.23–2.55) for consumers of 5–14.9 g/day, and 2.15 (95% CI, 1.42–3.26) for consumers of ≥15 g/day. Last, when controlling for socioeconomic status, higher consumption of alcohol intake remained significantly associated with increased risk of MC (Ptrend < .001). Compared to non-users, the aHR of MC with ≥15 g/day of alcohol consumption was 2.23 (95% CI, 1.49–3.35).
Discussion
In this study of 2 large, prospective cohorts of US female nurses, we found that alcohol consumption was associated with increased risk of developing MC. The relationship between alcohol intake and risk of MC appeared to be dose-responsive and independent of other known risk factors for MC, including known pharmacologic risk factors,5,6 smoking,10,22 and BMI.23
Our findings are consistent with 2 of the 4 prior studies that examined the relationship between alcohol intake and risk of MC. In 2011, Yen and colleagues10 showed in a case-control study (n = 340 cases) that patients with MC were more likely to consume alcohol on a daily or weekly basis compared to controls (P = .036), although notably this study did not control for potential confounders. More recently, Larsson et al.8 performed a prospective cohort study (n = 135 cases) in Sweden examining the impact of a variety of dietary and lifestyle factors on risk of MC, including the average quantity of alcohol consumed per day. In this study, alcohol intake was associated with increased risk of MC (highest quartile of alcohol use: aHR, 1.89; 95% CI, 0.82–4.33; Ptrend = .032) when adjusted for sex, age, and smoking. Our findings are in contrast with 2 prior studies. In a case-control study of 131 MC cases, Roth et al.11 showed that any alcohol consumption within the prior month was not associated with risk of MC (OR, 1.25; 95% CI, 0.52–2.97). Likewise, Verhaegh et al.12 conducted a case-control study (n = 171 cases) in the Netherlands to broadly identify potential risk factors for MC. No association was seen between alcohol use and risk of MC (OR, 1.65; 95% CI, 0.84–3.25; P = .147), with alcohol intake defined as a period of excessive alcohol consumption (>21 units/week for ≥3 months) occurring any time before the participant was diagnosed with MC. Our prospective cohort study of over 200,000 participants and 350 incident cases of MC with repeated measurements of amounts and types of alcohol consumption and other important lifestyle factors and medications over more than 30 years significantly expands upon these prior studies and represents the most comprehensive study to date on the relationship between alcohol consumption and risk of MC.
Our findings are biologically plausible. Studies have shown that alcohol intake degrades the integrity of the intestinal epithelial barrier, increasing the trans-epithelial and paracellular passage of luminal antigens into the lamina propria and bloodstream.13,14,24 Additionally, alcohol consumption is associated with dysbiosis and intestinal bacterial overgrowth, leading to an increased abundance of endotoxin-producing bacteria.13,14 While the pathogenesis of MC is largely unknown, growing evidence suggests that development of MC is related to an impaired barrier function and changes in the composition and function of the gut microbiome.25,26 Therefore, it is possible that alcohol consumption influences the risk of MC through its effect on the gut microbiota and barrier function. Along the same lines, alcoholic beverages (particularly wine and beer) contain high concentrations of sulphites, which have been shown to cause colonocyte damage that leads to increased intestinal permeability and inflammation, and thus may also play a role in precipitating MC.8,27 Nevertheless, our suggestive finding that the association may be stronger with or limited to wine consumption could also point to a role for a number of polyphenol compounds that are uniquely found in wine, such as non-flavonoids that originate from the pulp, skin, and seeds of grapes.28 These compounds have been shown to have a dramatic effect on the gut microbiota composition and to exert a prebiotic effect.29,30
Our study has a number of strengths. First, it represents the largest study to date to examine the association between alcohol intake and risk of MC. Second, the prospective design of our study minimized the risk of selection and recall biases, which are a limitation of prior case-control studies. Third, we had detailed, updated, and validated data on alcohol consumption over a long period of time, which allowed us to comprehensively examine the relationship between alcohol intake and risk of MC. The repeated assessments and high correlation between our method of assessment and diet records minimized the potential for measurement errors. Lastly, to reduce confounding, we controlled for other lifestyle factors, dietary patterns, and pharmacologic risk factors associated with MC.
We acknowledge several limitations. The majority of participants in our study were White, female nurses living in the United States, potentially limiting the generalizability of our findings. While alcohol consumption habits certainly vary by country,31 we note that postmenopausal women represent the highest risk category5 and, to our knowledge, there are no data that suggest differences in MC according to race and ethnicity. Although cases of MC were confirmed through a medical record review, we relied on self-reports of diagnoses for the initial query; during early periods of follow-up in our study, MC was not widely appreciated and biopsies of normal-appearing tissue were not always performed. Therefore, underreporting of diagnoses or undiagnosed instances of disease may have led to outcome misclassifications. However, we highlight that our participants are health professionals who generally have good access to health care and are health-care literate. Additionally, the incidence of MC in our study population is similar to that of other population-based studies.1,2 Lastly, we note that MC continues to be an understudied disease; therefore, it is possible that our observed association may be related to residual confounding from unrecognized risk factors.
Conclusion
In summary, we found that alcohol consumption was associated with increased risk of MC in a large, prospective cohort study of female health professionals. Furthermore, the association of alcohol intake and increased risk of MC appears to be predominantly driven by the consumption of wine. Our findings add to the limited existing evidence that alcohol consumption is a risk factor for MC. Further studies are needed to elucidate the pathophysiology underlying the associations observed in this study, including investigations of the impact of the different types of alcohol on the gut microbiota and epithelial barrier function in older adults. Additionally, prospective studies are warranted to determine whether reduction in alcohol intake among patients with established MC leads to improvements in symptoms.
Supplementary data
Supplementary data are available at Inflammatory Bowel Diseases online.
Supplemental Figure 1: Alcohol Intake and Risk of Microscopic Colitis.
Acknowledgments
We presented our study as a virtual oral presentation on May 22, 2021, at Digestive Disease Week 2021.
Contributor Information
Blake Niccum, Department of Internal Medicine, Massachusetts General Hospital, Boston, MA, USA.
Kevin Casey, Division of Gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
Kristin Burke, Division of Gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA; Clinical and Translational Epidemiology Unit, Massachusetts General Hospital, Boston, MA, USA.
Emily W Lopes, Division of Gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA; Clinical and Translational Epidemiology Unit, Massachusetts General Hospital, Boston, MA, USA.
Paul Lochhead, Division of Gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA; Clinical and Translational Epidemiology Unit, Massachusetts General Hospital, Boston, MA, USA.
Ashwin Ananthakrishnan, Division of Gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA; Clinical and Translational Epidemiology Unit, Massachusetts General Hospital, Boston, MA, USA.
James M Richter, Division of Gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA; Clinical and Translational Epidemiology Unit, Massachusetts General Hospital, Boston, MA, USA.
Jonas F Ludvigsson, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden; Department of Pediatrics, Orebro University Hospital, Orebro, Sweden; Division of Epidemiology and Public Health, School of Medicine, University of Nottingham, UK; Department of Medicine, Columbia University College of Physicians and Surgeons, New York, NY, USA.
Andrew T Chan, Division of Gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA; Clinical and Translational Epidemiology Unit, Massachusetts General Hospital, Boston, MA, USA.
Hamed Khalili, Division of Gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA; Clinical and Translational Epidemiology Unit, Massachusetts General Hospital, Boston, MA, USA; Institute of Environmental Medicine, Nutrition Epidemiology, Karolinska Institutet, Stockholm, Sweden; Broad Institute of MIT and Harvard, Cambridge, MA, USA.
Author Contributions
• B.N.: Study design, critical feedback on results, drafting of the manuscript;
• K.C.: Study design, acquisition of data, statistical analysis, critical review of the manuscript;
• E.W.L.: Critical feedback on results, acquisition of data, critical review of the manuscript;
• J.M.R.: Critical feedback on results, acquisition of data, critical review of the manuscript;
• P.L.: Verification of underlying data, critical feedback on results, critical review of the manuscript;
• A.A.: Critical feedback on results, critical review of the manuscript;
• K.B.: Critical feedback on results, critical review of the manuscript;
• J.F.L.: Critical feedback on results, critical review of the manuscript;
• A.T.C.: Study design, critical feedback on results, critical review of the manuscript; and
• H.K.: Study design, statistical analysis, critical feedback on results, drafting of the manuscript.
All of the above authors had full access to all of the data in the study, approve of the final version of the manuscript, agree to be accountable for all aspects of the work, and accept responsibility to submit for publication.
Funding
This work was supported by the National Institute on Aging (R01 AG068390 to H.K. and J.F.L.), a Nurses’ Health Study cohort infrastructure grant (UM1 CA186107), and a Nurses’ Health Study II cohort infrastructure grant (U01 CA176726). This study has received funding from the Janssen corporation. H.K. is also supported by a senior research grant from the Crohn’s and Colitis Foundation, a grant from Beker Foundation, and a clinical research award from the American College of Gastroenterology. A.T.C. is also supported by a senior research award from the Crohn’s and Colitis Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of Interest
H.K. has received consulting fees from Takeda and grant funding from Takeda and Pfizer, and is supported by a senior research grant from the Crohn’s and Colitis Foundation, a grant from Beker Foundation, and a clinical research award from the American College of Gastroenterology. A.T.C. has received consulting fees from Bayer Pharma AG, Pfizer Inc, and Boeheringer Ingelheim and is supported by a senior research award from the Crohn’s and Colitis Foundation. J.F.L. coordinates a study on behalf of the Swedish inflammatory bowel diseases quality register (SWIBREG). J.M.R. has received consulting fees from Takeda and Iterative Scopes. No other authors have financial disclosures or potential conflicts of interest.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Pardi DS. Diagnosis and management of microscopic colitis. Am j Gastroenterol. 2017;112:78–85. [DOI] [PubMed] [Google Scholar]
- 2. Bergman D, Clements MS, Khalili H, et al. A nationwide cohort study of the incidence of microscopic colitis in Sweden. Aliment Pharmacol Ther. 2019;49:1395–1400. [DOI] [PubMed] [Google Scholar]
- 3. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390: 2769–2778. [DOI] [PubMed] [Google Scholar]
- 4. Pisani LF, Tontini GE, Vecchi M, Pastorelli L. Microscopic colitis: what do we know about pathogenesis? Inflamm Bowel Dis. 2016;22:450–458. [DOI] [PubMed] [Google Scholar]
- 5. Tong J, Zheng Q, Zheng Q, et al. Incidence, prevalence, and temporal trends of microscopic colitis: a systematic review and meta-analysis. Am j Gastroenterol. 2015;110:265–2 76; quiz 277. [DOI] [PubMed] [Google Scholar]
- 6. Burke KE, Ananthakrishnan AN, Lochhead P, et al. Identification of menopausal and reproductive risk factors for microscopic colitis-results from the nurses’ health study. Gastroenterology. 2018;155:1764–1775.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zmora N, Suez J, Elinav E. You are what you eat: diet, health and the gut microbiota. Nat Rev Gastroenterol Hepatol. 2019;16:35–56. [DOI] [PubMed] [Google Scholar]
- 8. Larsson JK, Sonestedt E, Ohlsson B, et al. The association between the intake of specific dietary components and lifestyle factors and microscopic colitis. Eur j Clin Nutr. 2016;70:1309–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu PH, Lebwohl B, Burke KE, et al. Dietary gluten intake and risk of microscopic colitis among US women without celiac disease: a prospective cohort study. Am j Gastroenterol. 2019;114: 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yen EF, Pokhrel B, Du H, et al. Current and past cigarette smoking significantly increase risk for microscopic colitis. Inflamm Bowel Dis. 2012;18:1835–1841. [DOI] [PubMed] [Google Scholar]
- 11. Roth B, Gustafsson RJ, Jeppsson B, et al. Smoking and alcohol habits in relation to the clinical picture of women with microscopic colitis compared to controls. bmc Womens Health. 2014;14:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Verhaegh BPM, Pierik MJ, Goudkade D, et al. Early life exposure, lifestyle, and comorbidity as risk factors for microscopic colitis: a case-control study. Inflamm Bowel Dis. 2017;23:1040–1046. [DOI] [PubMed] [Google Scholar]
- 13. Bishehsari F, Magno E, Swanson G, et al. Alcohol and gut-derived inflammation. Alcohol Res. 2017;38:163. [PMC free article] [PubMed] [Google Scholar]
- 14. Bode C, Bode JC. Alcohol’s role in gastrointestinal tract disorders. Alcohol Health Res World. 1997;21:76. [PMC free article] [PubMed] [Google Scholar]
- 15. DeJong EN, Surette MG, Bowdish DME. The gut microbiota and unhealthy aging: disentangling cause from consequence. Cell Host Microbe. 2020;28:180–189. [DOI] [PubMed] [Google Scholar]
- 16. Pardi DS, Kelly CP. Microscopic colitis. Gastroenterology. 2011;140:1155–1165. [DOI] [PubMed] [Google Scholar]
- 17. Giovannucci E, Colditz G, Stampfer MJ, et al. The assessment of alcohol consumption by a simple self-administered questionnaire. Am j Epidemiol. 1991;133:810–817. [DOI] [PubMed] [Google Scholar]
- 18. Rimm EB, Stampfer MJ, Colditz GA, et al. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1:466–473. [DOI] [PubMed] [Google Scholar]
- 19. Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. j Nutr. 2012;142:1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–561. [DOI] [PubMed] [Google Scholar]
- 21. Wang M, Spiegelman D, Kuchiba A, et al. Statistical methods for studying disease subtype heterogeneity. Stat Med. 2016;35:782–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vigren L, Sjöberg K, Benoni C, et al. Is smoking a risk factor for collagenous colitis? Scand j Gastroenterol. 2011;46: 1334–1339. [DOI] [PubMed] [Google Scholar]
- 23. Liu PH, Burke KE, Ananthakrishnan AN, et al. Obesity and weight gain since early adulthood are associated with a lower risk of microscopic colitis. Clin Gastroenterol Hepatol. 2019;17:2523–2532.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Swanson GR, Tieu V, Shaikh M, et al. Is moderate red wine consumption safe in inactive inflammatory bowel disease? Digestion. 2011;84:238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Barmeyer C, Erko I, Fromm A, et al. Ion transport and barrier function are disturbed in microscopic colitis. Ann n y Acad Sci. 2012;1258:143–148. [DOI] [PubMed] [Google Scholar]
- 26. Morgan DM, Cao Y, Miller K, et al. Microscopic colitis is characterized by intestinal dysbiosis. Clin Gastroenterol Hepatol. 2020;18:984–986. [DOI] [PubMed] [Google Scholar]
- 27. Teigen LM, Geng Z, Sadowsky MJ, et al. Dietary factors in sulfur metabolism and pathogenesis of ulcerative colitis. Nutrients 2019;11:931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Radonjić S, Maraš V, Raičević J, et al. Wine or beer? Comparison, changes and improvement of polyphenolic compounds during technological phases. Molecules 2020;25:4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Biasi F, Deiana M, Guina T, et al. Wine consumption and intestinal redox homeostasis. Redox Biol. 2014;2:795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dueñas M, Cueva C, Muñoz-González I, et al. Studies on modulation of gut microbiota by wine polyphenols: from isolated cultures to omic approaches. Antioxidants (Basel). 2015; 4:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bloomfield K, Stockwell T, Gmel G, Rehn N. International comparisons of alcohol consumption. Alcohol Res Health. 2003;27:95. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.