ABSTRACT
A decade-old meta-analytic work indicated that l-arginine supplementation might have a blood pressure (BP)–lowering effect in different populations. However, several relevant investigations have emerged in the last 10 y, and an up-to-date systematic review and meta-analysis on this topic is currently lacking. Therefore, we aimed to examine the impact of l-arginine supplementation on BP by conducting a systematic review and dose–response meta-analysis of randomized placebo-controlled clinical trials (RCTs). We searched online databases using relevant keywords up to April 2021 to identify RCTs using oral l-arginine on systolic BP (SBP) and diastolic BP (DBP) in adults. Inclusion criteria were adult participants and an intervention duration ≥4 d. Exclusion criteria were the use of l-arginine infusion and acute interventions. A random-effects model was used to estimate the weighted mean difference (WMD) and 95% CI. Twenty-two RCTs with 30 effect sizes were included in this meta-analysis. The pooled analysis demonstrated significant decreases in SBP (WMD = −6.40 mmHg; 95% CI: −8.74, −4.05; P < 0.001) and DBP (WMD = −2.64 mmHg; 95% CI: −3.94, −1.40; P < 0.001) after l-arginine supplementation. Subgroup analysis showed significant reductions in SBP and DBP regardless of baseline BP category (normotensive, hypertensive), study duration (≤24 d, >24 d), sex (female, male), health status (healthy, unhealthy), and BMI (normal, overweight, obese). No significant changes were observed with dosages >9 g/d, trial duration >24 d, or in obese individuals. l-Arginine supplementation also appears to decrease DBP more effectively in females than in males. Moreover, meta-regression analysis for DBP demonstrated a significant relation between the dose of l-arginine intake and changes in DBP (P = 0.020). In the nonlinear dose–response analysis, the effective dosage of l-arginine supplementation was detected to be ≥4 g/d for SBP (P = 0.034), independent of trial duration. Overall, l-arginine supplementation may be effective for decreasing BP. This study was registered at PROSPERO as CRD42021242772.
Keywords: arginine, blood pressure, systematic review, metabolic disease, nutritional supplements, nutrition
Statement of Significance: This meta-analysis was performed for the following reasons: 1) The last meta-analysis on this topic was done in 2010, and since then many relevant studies have been published. Therefore, there was a need for an up-to-date systematic review and meta-analysis on this topic. 2) To establish the appropriate clinical dose and duration of l-arginine supplementation. 3) To evaluate the effects of l-arginine supplementation in adults with different baseline blood pressure categories, BMIs, genders, and health statuses. 4) To conduct a new analysis (nonlinear dose–response analysis by 1-stage robust error meta-regression model analysis), which was not previously performed in the decade-old meta-analytic work.
Introduction
According to the WHO, the prevalence of hypertension in adults is expected to increase from 26.4% to 29.2% by 2025, affecting more than 1 billion people (1, 2). Indeed, the single-most-important and modifiable risk factor for heart failure with preserved ejection fraction, hemorrhagic stroke, and cognitive impairment is high blood pressure (BP), which has been linked to millions of deaths per year (3, 4). To combat the hypertension epidemic, effective dietary interventions are desperately needed.
l-Arginine is an amino acid that our body obtains either from dietary sources or endogenous metabolism. It is the substrate for numerous enzymatic pathways involved in regulating vascular tone, immune activation, and cell growth (5). l-Arginine is metabolized by NO synthase (NOS) into NO and l-citrulline in endothelial cells (5). l-Arginine supplementation has been shown to exert several beneficial effects on cardiometabolic markers, including BP, microvascular endothelium–dependent dilation, adiposity, and insulin resistance (6–9). Several studies have examined the impact of l-arginine on resting BP. For instance, a diet rich in l-arginine significantly reduced both systolic BP (SBP) and diastolic BP (DBP) in healthy participants (10). Similarly, 3 to 12 g/d l-arginine supplementation reduced both SBP and DBP in patients with mild hypertension (11) and pre-eclamptic women (12), indicating an antihypertensive effect. A meta-analysis of 11 randomized controlled trials (RCTs) has shown a decreased SBP and DBP in those who received a median dose of 9 g (4–24 g/d) for a median duration of 4 wk (2–24 wk), which was impacted by little to considerable heterogeneity (I2 = 73.3% and 34.4% for SBP and DBP, respectively). However, no significant correlation was found between dosage, duration of supplementation, or baseline BP on net alteration of SBP and DBP (13). In contrast, studies have found that l-arginine supplementation failed to induce significant BP reductions in pregnant women with mild chronic hypertension (14) and in young, healthy individuals (15).
Consequently, research on the association between l-arginine supplementation and hypertension has shown mixed findings, which may be due to several factors, including diverse BP categories, dosages, and durations. Although there is a previous meta-analysis on this topic (13), this investigation was conducted a decade ago, and several relevant articles have emerged since then. Therefore, we conducted a thorough meta-analysis including the latest literature on the impact of l-arginine supplementation on SBP and DBP in adults.
Methods
The current research used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) declaration (16). The present study was registered at PROSPERO (CRD42021242772).
Search strategy
To find relevant prospective studies on the effects of l-arginine supplementation on BP published up to March 2021, we executed a systematic literature search in scientific databases including PubMed/Medline, Scopus, Web of Science, EMBASE, the Cochrane databases, and Google Scholar. Moreover, the bibliography of related studies, including prior meta-analytic work (13), was scrutinized to find potential missing studies. There were no restrictions on the length of time or language of publications. The PICO (Participant, Intervention, Comparison/Control, Outcome) search framework was used to search for items related to l-arginine supplementation and BP. The combination of Medical Subject Heading (MeSH) and non-MESH terms was used for the search, as follows: (arginine OR l-arginine) AND (“blood pressure” OR systolic OR diastolic OR SBP OR DBP OR hypertension OR hypertensive OR HTN) AND (Intervention OR “Intervention Study” OR “Intervention Studies” OR “controlled trial” OR randomized OR randomized OR random OR randomly OR placebo OR “clinical trial” OR Trial OR “randomized controlled trial” OR “randomized clinical trial” OR RCT OR blinded OR “double blind” OR “double blinded” OR trial OR “clinical trial” OR trials OR “Pragmatic Clinical Trial” OR “Cross-Over Studies” OR “Cross-Over” OR “Cross-Over Study” OR parallel OR “parallel study” OR “parallel trial”).
Study selection
We incorporated studies that satisfied the following criteria: 1) RCTs (parallel or crossover), 2) used oral intake of l-arginine, 3) examined the effects of l-arginine supplementation on SBP and DBP, 4) had an intervention duration of at least 4 d (RCTs with ≥2 eligible arms were considered as separate studies), 5) were performed in adults (≥18 y old), and 6) provided means and SDs for SBP and DBP, or any other effect sizes from which the calculation of mean and SD was possible. The searches were limited to human studies with no language restrictions. Two authors (FS and OA) independently screened the title and abstracts of the included publications, extracted results, and assessed the validity of the qualifying studies to determine whether they qualified. Any disputes were settled with discussion. Exclusion criteria included animal studies, reviews, and in vitro studies. Moreover, studies in children and adolescents, gray literature, conference abstracts, editorial papers, books, and RCTs without a placebo or control group were also excluded. Further exclusion criteria consisted of studies in which l-arginine was administered by infusion, consumed for less than 4 d, in combination with vitamins or minerals, and those that reported mean arterial BP as the only outcome. In addition, studies assessing BP responses to exercise (post-exercise) were excluded unless resting BP data at pre– and post–l-arginine supplementation were reported.
Data extraction
All eligible RCTs were re-checked separately, and the following information was extracted by 2 independent investigators (OA and FS). The first author's name, country, publication year, type of clinical trial, participant characteristics (mean age, BMI, sex), randomization, blinding, sample size, the number of participants in the intervention and control groups, the form and dose of supplemented arginine, study duration, and related data were extracted for further measures. If the l-arginine doses were reported in milligrams per day, they were converted to grams per day. The mean and SD for SBP and DBP at the start and end of each intervention (for both parallel and crossover trials) were also collected. If these data were not present, the mean difference was calculated by subtracting the mean value at baseline from the mean value at the end of the study.
Quality assessment
The Cochrane Collaboration tool was used to assess the quality of the studies (17). All studies were judged for any source of bias, which included randomized sequence generation, allocation concealment, participant and staff blinding, outcome assessor blinding, inadequate outcome data, selective reporting, and other biases. As a result, 3 groups were created: high risk of bias, low risk of bias, and uncertain risk of bias (Table 1). Two reviewers (FS and OA) separately assessed the quality of the work, and any conflicting opinions were settled by discussion.
TABLE 1.
Quality assessment (a summary of the risk of bias according to Cochrane criteria) of included studies investigating the effects of l-arginine supplementation on blood pressure in adults1
| Study (ref) | Random-sequence generation | Allocation concealment | Selective reporting | Other sources of bias | Blinding (participants and personnel) | Blinding (outcome assessment) | Incomplete outcome data | Random sequence generation | Quality |
|---|---|---|---|---|---|---|---|---|---|
| Chin-Dusting et al., 1996 (15) | U | U | L | U | L | U | L | U | Good |
| Clarkson et al., 1996 (41) | U | U | L | U | L | U | L | U | Good |
| Piatti et al., 2001 (42) | U | U | L | U | L | U | L | U | Good |
| Sydow et al., 2002 (43) | U | U | L | U | L | U | L | U | Good |
| Rytlewski et al., 2005 (12) | U | U | L | H | L | U | L | U | Good |
| West et al., 2005 (44) | U | U | L | U | L | U | L | U | Good |
| Hladunewich et al., 2006 (45) | L | L | L | U | L | U | L | L | Good |
| Lucotti et al., 2006 (47) | U | U | L | U | L | U | L | U | Good |
| Facchinetti et al., 2007 (48) | U | U | L | U | L | U | L | U | Good |
| Fontanive et al., 2009 (49) | U | U | L | U | L | U | H | U | Fair |
| Lucotti et al., 2009 (46) | U | U | L | U | L | U | L | U | Good |
| Ast et al. 2010 (11) | U | U | L | U | L | U | L | U | Good |
| Neri et al., 2010 (14) | U | U | L | U | L | U | L | U | Good |
| Battaglia et al., 2010 (50) | L | L | L | U | L | U | L | L | Good |
| Willoughby et al., 2011 (51) | U | L | H | L | L | U | L | U | Good |
| Ast et al., 2012(52) | U | U | L | U | L | U | L | U | Good |
| de Lima et al., 2012 (39) | U | U | L | U | U | U | L | U | Fair |
| Malfatti et al., 2014 (53) | U | U | H | U | U | U | H | U | Bad |
| Pahlavani et al., 2014 (54) | L | L | L | L | L | U | L | L | Good |
| Camarena Pulido et al., 2016 (55) | U | L | L | U | L | U | L | U | Good |
| McNeal et al., 2018 (56) | U | L | L | L | L | U | L | U | Good |
| Salmani et al., 2021 (40) | L | L | L | U | L | L | L | L | Good |
Bad, <2 low risk of bias; Good, >2 low risk of bias; Fair, = 2 low risk of bias. H, high risk of bias; L, low risk of bias; ref, reference; U, unclear risk of bias.
Statistical analysis
The statistical analysis was conducted using Stata version 11.0 (StataCorp). All tests were 2-tailed, and P values <0.05 were considered statistically significant. A random-effects model was used for the calculation of the pooled weighted mean difference (WMD) to account for existing heterogeneity (18). We calculated mean differences in SBP and DBP from baseline to the postintervention between l-arginine supplementation and control groups. The SD of the mean difference was calculated using the following formula: SD = square root [(SD at baseline)2 + (SD at the end of study)2 – (2 r × SD at baseline × SD at the end of study)] (19). We used the Hozo et al. (20) approach to transform SEs, 95% CIs, and IQRs to SDs, in each study that reported SE instead of SD. The formula SD = SE × √n (n = the number of individuals in each group) was used to calculate SD. A correlation coefficient of 0.8 was used for r (21). Subgroup analysis was conducted to determine the source of heterogeneity. Subgroups were selected based on the required minimum number of studies according to the criteria set forth by Fu and colleagues (22, 23), where there should be at least 6 to 10 studies for continuous and a minimum of 4 studies for categorical subgroup variables. The analyses of baseline SBP (<130 mmHg, ≥130 mmHg) and DBP (<80 mmHg, ≥80 mmHg) were based on the American Heart Association's BP categories (24), whereas those for intervention duration (≤24 wk, >24 wk) and dosage of l-arginine (>9 g/d) were based on the median values of the included studies. Other subgroup analyses were performed according to sex (male, female), health status (healthy, unhealthy), and baseline BMI [kg/m²; normal (18.5–24.9), overweight (25–29.9), and obese (≥30)]. In the meta-analyses, the I2 or Cochrane's Q test was used to measure statistical heterogeneity (25), with values greater than 40% indicating strong heterogeneity (26).
Publication bias was checked by the funnel plot test (27, 28). To analyze each study's effect on the pooled effect size, the leave-one-out method (i.e., removing 1 trial at a time and recalculating the impact size) sensitivity analysis was conducted to see how many inferences were dependent on a specific sample. We used the trim-and-fill method to detect and adjust the impact of the publication bias (29). Meta-regression was performed for assessing the potential effects of l-arginine (g/d) dosage and duration on SBP and DBP. Furthermore, for the dose–response analysis between l-arginine supplementation and SBP and DBP, we used a 1-stage robust error meta-regression (REMR) model focused on inverse variance–weighted least-squares regression and cluster robust error variances for dealing with the synthesis of the correlated dose–response data from different studies (30, 31).
Certainty assessment
The overall certainty of evidence across the studies was assessed and summarized using the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) approach, as previously described (32).
Results
Flow of study selection
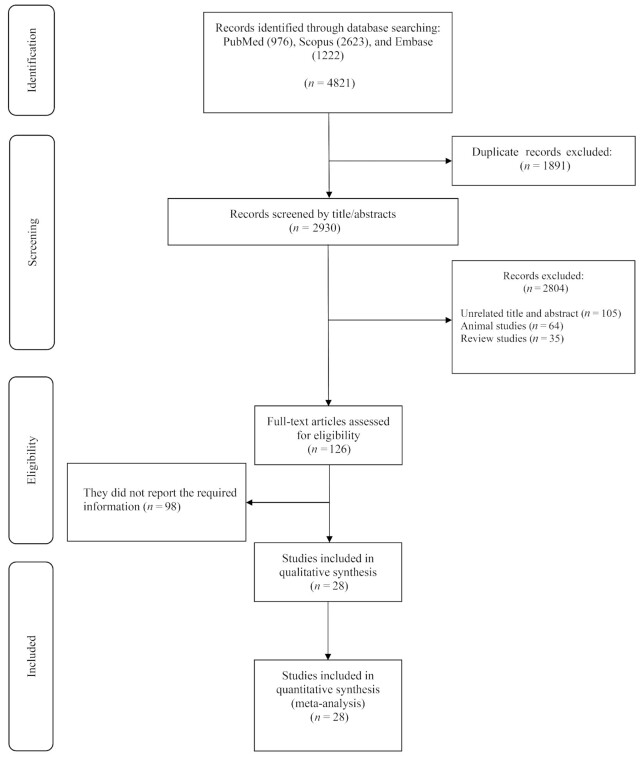
The flow chart presented in Figure 1 describes the selection process and the references retrieved from the database. A total number of 4821 studies were identified in the first step of the literature search of electronic databases. After excluding duplicate (n = 1891) and irrelevant studies based on titles and abstracts (n = 2804), 126 potentially relevant articles were considered for full-text review. After the screening, 98 studies were excluded due to the following reasons: insufficient outcome data reported, intravenous infusion, acute oral ingestion, or short duration of supplementation (<4 d). Finally, 28 studies were included in the qualitative synthesis, but we could not find the full text of a study in which BP was a secondary outcome (33). Of the 28 reports retrieved for detailed assessment, 6 studies, including Siani et al. (10), Orea-Tejeda et al. (34), Miller et al. (35), Kelly et al. (36), and Jahangir et al. (37), were excluded because they were not placebo-controlled. The study by Pezza et al. (38) was not included due to the uncertainty of the washout period and the possibility of carryover effect and the combined effects of BP medication with l-arginine supplementation (38). All of the studies were written in English, while one was in Portuguese (39). Finally, 22 studies were included (11, 12, 14, 15, 39, 41–56) in the present meta-analysis, and their characteristics are illustrated in Table 2.
FIGURE 1.
Flowchart of study selection for trial inclusion in the systematic review of the effects of l-arginine supplementation on blood pressure in adults.
TABLE 2.
Characteristics of included studies investigating the effects of l-arginine supplementation on blood pressure in adults1
| Sample size | Trial duration, | Intervention | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Sample size | Age, y | BMI, kg/m2 | Intervention | Control | Adverse | ||||||||
| Study (ref) | Country | design | Participants | and sex | IG | CG | d | IG | CG | IG | CG | dose, g/d | group | effect |
| Chin-Dusting et al., 1996 (15) | China | Parallel, R, PC, DB | Healthy adults | M (16) | 8 | 8 | 28 | 21.9 ± 0.6 | 20.9 ± 1.0 | NR | NR | 20 | Placebo (deionized water (600 mL) and compound hydroxybenzoate solution (5 mL) made up to 900 mL with either aromatic or orange syrup | No adverse effect |
| Clarkson et al., 1996 (41) | England | Cross over, R, PC, DB | Hypercholesterolemic adults (>162 mg/dL, with cholesterol mean: 238 ± 43; who were not hypertensive or diabetic) | M/F (F: 9, M: 18) | 15 | 15 | 28 | 29 ± 5 | 29 ± 5 | NR | NR | 21 | Placebo (identical powder formulations, which when mixed with water made a palatable drink) | Stool frequency and abdominal bloating |
| Piatti et al., 2001 (42) | Italy | Parallel, R, PC, DB | Adults with T2D | M/F (F: 4, M: 8) | 6 | 6 | 28 | 58 ± 7.34 | 58 ± 7.34 | 25.3 ± 2.20 | 25.3 ± 2.20 | 9 | Placebo (identical powder formulations, which were mixed with water and 200 mg aspartame) | No statement |
| Sydow et al., 2002 (43) | Germany | Parallel, R, PC, DB | Adults with peripheral arterial occlusive disease | M/F (F: 3, M: 15) | 9 | 9 | 56 | 64 ± 12.3 | 68.8 ± 8.4 | 25.44 | 27.02 | 24 | Placebo | No statement |
| Rytlewski et al., 2005 (12) | Poland | Prospective, R, PC | Adults with pre-eclampsia | F (61) | 30 | 31 | 21 | 29.3 ± 6.7 | 29.2 ± 5.9 | NR | NR | 3 | Placebo | No adverse effect |
| West et al., 2005 (44) | France | Cross over, R, PC, DB | Hypercholesterolemic adults | M (16) | 8 | 8 | 21 | 45 ± 5.37 | 45 ± 5.37 | 28 ± 3.11 | 28 ± 3.11 | 12 | Placebos (supplied by Jarrow Formulas) | No statement |
| Hladunewich et al., 2006 (45) | USA | Parallel, R, PC, DB | Pregnant women with pre-eclampsia | F (45) | 19 | 20 | 7 | 29 ± 6 | 28 ± 7 | NR | NR | 14 | Placebo | No adverse effect |
| Lucotti et al., 2006 (47) | Italy | Parallel, R, PC, DB | Adults with T2D | M/F (F: 25, M: 8) | 16 | 17 | 21 | 56.4 ± 8.04 | 56.4 ± 8.04 | 39.1 ± 2.87 | 39.1 ± 2.87 | 8.3 + hypocaloric diet and exercise training program | Placebo + hypocaloric diet and exercise training program | No adverse effect |
| Facchinetti et al., 2007 (48) | Italy | Parallel, R, PC, DB | Adults with pre-eclampsia | F (74) | 39 | 35 | 28 | 32.8 ± 5.4 | 32.6 ± 4.9 | 29.0 ± 7.7 | 27.4 ± 5.3 | 4 + (20 g/500 mL) intravenously daily, for 5 d followed by 4 g/d orally for 14 d | Saline infusion, 500 mL intravenously over 4 h given daily for 5 d, then oral placebo for 14 d | Diarrhea in three patients |
| Fontanive et al., 2009 (49) | Italy | Parallel, R, PC, DB | Adults with mild-to-moderate systolic HF and LV ejection fraction ≤45% | M/F (F: 23, M: 45) | 37 | 31 | 21 | 64 ± 11 | 64 ± 8 | NR | NR | 6 | Placebo | Three patients in the l-arginine group consisting of; asthenia, skin rash, eosinophilia |
| Lucotti et al., 2009 (46) | Italy | Parallel, R, PC, DB | Adults with coronary artery disease | M/F (F: 2, M: 28) | 16 | 14 | 180 | 65 ± 10 | 64 ± 11 | NR | NR | 6.4 | Placebo | No adverse effect |
| Ast et al., 2010(a) (11) | Poland | Parallel, R, PC, DB | Healthy adults | M/F (F: 24, M: 30) | 7 | 3 | 28 | 38.3 ± 11.2 | 35.5 ± 5.7 | 23.9 ± 3.3 | 22.9 ± 3.7 | 6 | Placebo | No adverse effect |
| Ast et al., 2010(b) (11) | Poland | Parallel, R, PC, DB | Healthy adults | M/F (F: 24, M: 30) | 6 | 3 | 28 | 39.2 ± 7.9 | 35.5 ± 5.7 | 27.2 ± 2.2 | 22.9 ± 3.7 | 12 | Placebo | — |
| Ast et al., 2010(c) (11) | Poland | Parallel, R, PC, DB | Healthy adults | M/F (F: 24, M: 30) | 12 | 5 | 28 | 36.4 ± 6.7 | 39.1 ± 10.4 | 28.4 ± 3.7 | 25.4 ± 2.9 | 12 | Placebo | — |
| Ast et al., 2010(d) (11) | Poland | Parallel, R, PC, DB | Adults with mild hypertension | M/F (F: 24, M: 30) | 13 | 5 | 28 | 36.4 ± 6.7 | 41.1 ± 12.0 | 27.2 ± 3.6 | 25.4 ± 2.9 | 6 | Placebo | — |
| Neri et al., 2010 (14) | Italy | Parallel, R, PC, DB | Adults with mild chronic hypertension | F (79) | 39 | 40 | 84 | 34.4 + 4.1 | 33.7 + 3.8 | 31.3 + 6.6 | 31.7 + 7.1 | 4 | Placebo | No adverse effect |
| Battaglia et al., 2010 (50) | Italy | Prospective, R, PC, DB | Female adults with polycystic ovary syndrome | F (28) | 13 | 15 | 180 | 24.3 + 3.5 | 25.2 + 5.1 | 23.5 + 4.2 | 24.4 + 4.4 | 8 + Yasmin: drospirenone + ethinylestradiol (Bayer-Schering Italia) | Placebo ++Yasmin: drospirenone + ethinylestradiol | No adverse effect |
| Willoughby et al., 2011 (51) | USA | Parallel, R, PC, DB | Male healthy adults, physically active men, resistance-trained | M (24) | 12 | 12 | 7 | 22.58 ± 3.31 | 21.75 ± 2.17 | NR | NR | 12 | Placebo (apple pectin, General Nutrition Corp.) | No adverse effect |
| Ast et al., 2012(a) (52) | Poland | Parallel, R, PC, DB | Healthy adults | M/F (F: 9, M: 10) | 6 | 3 | 28 | 37.7 ± 10.3 | 35.3 ± 5.43 | 23.9 ± 2.99 | 23.3 ± 3.48 | 6 | Placebo | No adverse effect |
| Ast et al., 2012(b) (52) | Poland | Parallel, R, PC, DB | Healthy adults | M/F (F: 9, M: 10) | 7 | 3 | 28 | 40.7 ± 7.58 | 35.3 ± 5.43 | 26.7 ± 3.4 | 23.3 ± 3.48 | 12 | Placebo | — |
| de Lima et al., 2012 (39) | Portugal | Parallel, R, PC, DB | Hypertensive adults | F (20) | 10 | 10 | 30 | 50.0 ± 1.8 | 51.5 ± 1.6 | 26.8 ± 1.0 | 27.6 ± 1.0 | 6 | Placebo | No statement |
| Malfatti et al., 2014(a) (53) | Brazil | Parallel, PC | Hypertensive adults | M (8) | 2 | 2 | 4 | 50.8 ± 9.4 | 50.8 ± 9.4 | NR | NR | 2 | Placebo | No statement |
| Malfatti et al., 2014(b) (53) | Brazil | Parallel, PC | Hypertensive adults | M (8) | 2 | 2 | 4 | 50.8 ± 9.4 | 50.8 ± 9.4 | NR | NR | 4 | Placebo | — |
| Pahlavani et al., 2014 (54) | Iran | Parallel, R, PC, DB | Healthy adults | M (54) | 25 | 27 | 60 | 21.32 ± 4.59 | 20.40 ± 4.04 | 23.34 ± 4.02 | 24.01 ± 4.53 | 2 | Placebo (maltodextrin) | No adverse effect |
| Camarena Pulido et al., 2016 (55) | Mexico | Parallel, R, PC, DB | Pregnant women who had high-risk factors for developing pre-eclampsia | F (96) | 49 | 47 | 140 | 20 ± 5.4 | 20 ± 4.7 | 28 ± 4.8 | 26.7 ± 2.9 | 3 | Placebo | Dyspepsia, in placebo group (n = 5) compared with the l-arginine group (n = 14). 3 patients, abdominal pain (placebo group, n = 1; l-arginine group, n = 2); 1 patient in each of the groups had diarrhea |
| McNeal et al., 2018(a) (56) | Austria | Parallel, R, PC, DB | Healthy overweight or obese women [BMI (kg/m2) ≥25] | F (26) | 7 | 7 | 90 | 40 ± 3.2 | 40 ± 2.6 | 34.3 ± 1.9 | 36.6 ± 1.8 | 15 | Placebo | No adverse effect |
| McNeal et al., 2018(b) (56) | Austria | Parallel, R, PC, DB | Healthy overweight or obese women (BMI ≥25) | F (25) | 6 | 6 | 90 | 34 ± 2.6 | 40 ± 2.6 | 35.4 ± 1.6 | 36.6 ± 1.8 | 30 | Placebo | — |
| McNeal et al., 2018(c) (56) | Austria | Parallel, R, PC, DB | Healthy overweight or obese women (BMI ≥25) | M (25) | 7 | 7 | 90 | 33 ± 2.6 | 35 ± 2.5 | 32.9 ± 1.7 | 36.1 ± 2.5 | 15 | Placebo | — |
| McNeal et al., 2018(d) (56) | Austria | Parallel, R, PC, DB | Healthy overweight or obese women (BMI ≥25) | M (24) | 7 | 6 | 90 | 33 ± 1.5 | 35 ± 2.5 | 32.9 ± 0.8 | 36.1 ± 2.5 | 30 | Placebo | — |
| Salmani et al., 2021 (40) | Iran | Parallel, R, PC, DB | Adults with ischemic heart failure | M/F (F: 13, M: 31) | 12 | 11 | 70 | 56.6 ± 3.3 | 55.8 ± 7.9 | 29.1 ± 4.2 | 28 ± 4 | 3 | Placebo (maltodextrin) | No adverse effect |
CG, control group; CO, controlled; DB, double-blinded; HF, heart failure; IG, intervention group; LV, left ventricular; NR, not reported; PC, placebo-controlled; R, randomized; ref, reference; SB, single-blinded; T2D, type 2 diabetes.
Study characteristics
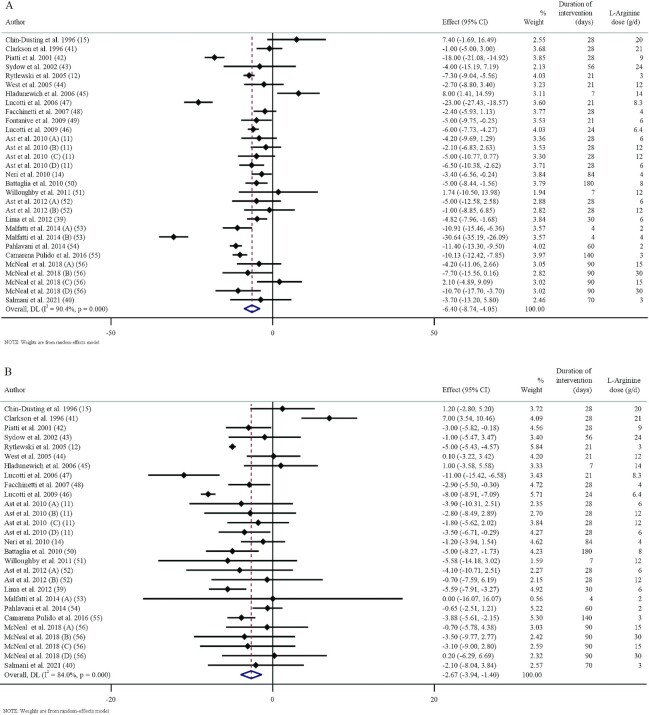
These studies were published between 1996 and 2021 and originated in Iran (40, 54), China (15), Italy (14, 42, 46–50), Poland (11, 12, 52), Portugal (39), Brazil (53), the United Kingdom (41), Austria (56), Germany (43), France (44), the United States (45, 51), and Mexico (55). The study design characteristics are presented in Table 2. The WMD and 95% CI of SBP and DBP and their changes are presented in Figure 2A and B, respectively. There were 18 parallel (11, 14, 39, 40, 43, 45–49, 51–56), 2 crossover (41, 44), and 2 prospective designed (12, 50) studies. The mean age, baseline BMI, and SBP ranged from 20 to 64 y, 23.34 to 39.1, and 108 to 151 mmHg in the intervention group, respectively. The duration of supplementation ranged from 4 to 180 d. Thirteen studies included only males or females, and 9 included both sexes. The daily dosage of l-arginine supplementation ranged from 2 to 30 g. Studies included participants with hypercholesterolemia (41, 44), type 2 diabetes (42, 47), peripheral arterial disease (43), pre-eclampsia (12, 45, 48), pregnancy with high risk of developing pre-eclampsia (55), ischemic heart failure (40), coronary artery disease (46), hypertension (14, 39, 53), mild and moderate systolic heart failure (49), polycystic ovary syndrome (50), overweight and obese but otherwise healthy (56), as well as normotensives (11, 51, 52, 54). Ast et al. (11) and Malfatti et al. (53) included 2 different l-arginine doses in their studies. The studies by de Lima et al. (39), Salmani et al. (40), and Willoughby et al. (51) evaluated the effects of l-arginine consumption on BP responses to exercise; therefore, only resting SBP and DBP data at pre- and postintervention were included. In the investigation by McNeal et al. (56), 2 doses of l-arginine (15 and 30 g/d) in both females and males were assessed so that 4 arms were considered for this study. Out of the 22 RCTs, 10 showed a significant antihypertensive effect of l-arginine supplementation on SBP (11, 12, 15, 44, 47, 48, 50, 53, 55, 56) and 9 on DBP (11, 12, 15, 40, 44, 47, 48, 50, 55).
FIGURE 2.
Forest plots detailing weighted mean WMDs and 95% CIs of the effects of l-arginine supplementation on SBP (A) and DBP (B) in adults. Horizontal lines represent 95% CIs. Diamonds represent pooled estimates from random-effects analysis. DBP, diastolic blood pressure; SBP, systolic blood pressure; WMD, weighted mean difference.
Adverse events
Information on adverse effects was mentioned in the studies by Clarkson et al. (41) (increased stool frequency and abdominal bloating), Facchinetti et al. (48) (diarrhea), Fontanive et al. (49) (asthenia, skin rash, and eosinophilia), and Camarena Pulido et al. (55) (dyspepsia and abdominal pain).
Qualitative data assessment
Based on the Cochrane Risk-of-Bias Assessment tool, 19 studies were considered high-quality (Good) (11, 12, 14, 15, 40–48, 50–52, 54–56) with a total low risk of bias for all domains of this tool. Two studies had moderate quality (Fair) in which 1 or more domains had an unclear risk of bias (39, 49). Only 1 of the studies had low quality since it had a high risk of bias for 1 or more domains (53) (Table 1).
Effect of l-arginine supplementation on SBP
Overall, 30 effect sizes from 22 studies with a total sample size of 431 in the intervention group and 394 in the placebo group for SBP were included in the analysis (11, 12, 14, 15, 39, 41–56). l-Arginine supplementation significantly reduced SBP [WMD = −6.40 mmHg; 95% CI: −8.74 to −4.05; P < 0.001 (Figure 2A)]. Between-study heterogeneity was also observed (I2 = 90.4%). Additionally, subgroup analyses showed that l-arginine supplementation significantly reduced SBP in all subgroups, except in the high-dose interventions (>9 g/d) (11, 15, 41, 43–45, 48, 51, 52, 56), duration longer than 24 d, and studies conducted in obese participants (BMI: >30) (14, 47, 56) (Table 3).
TABLE 3.
Subgroup analyses of populations in the included studies investigating the effects of l-arginine supplementation on blood pressure in adults1
| Heterogeneity | ||||||
|---|---|---|---|---|---|---|
| Number of studies | WMD (95% CI) | P | I 2 | Tau-squared | P between subgroups | |
| Subgroup analyses of l-arginine supplementation on SBP | ||||||
| Overall effect | 30 | −6.40 (−8.74, −4.05) | <0.001 | 90.4% | 34.77 | |
| Baseline SBP | 0.831 | |||||
| <130 mmHg | 14 | −5.39 (−8.26, −2.53) | <0.001 | 88.2% | 22.79 | |
| ≥130 mmHg | 16 | −7.38 (−11.45, −3.30) | <0.001 | 92.2% | 60.11 | |
| Trial duration | 0.150 | |||||
| ≤24 d | 9 | −8.96 (−14.57, −3.34) | 0.002 | 95.4% | 65.91 | |
| >24 d | 21 | −5.26 (−7.66, −2.87) | <0.001 | 84.1% | 23.21 | |
| Intervention dose | <0.001 | |||||
| ≤9 g/d | 17 | −9.36 (−12.24, −6.49) | <0.001 | 93.0% | 32.00 | |
| >9 g/d | 13 | −1.66 (−4.36, 1.03) | 0.226 | 50.8% | 11.71 | |
| Sex | <0.001 | |||||
| Male | 8 | −7.52 (−14.79, −0.26) | 0.042 | 93.7% | 97.47 | |
| Female | 9 | −4.62 (−7.19, −2.05) | <0.001 | 79.9% | 10.93 | |
| Both | 13 | −6.82 (−10.69, −2.95) | 0.001 | 89.8% | 41.81 | |
| Health status | 0.101 | |||||
| Healthy | 9 | −4.64 (−8.15, −1.13) | 0.010 | 80.2% | 19.09 | |
| Unhealthy | 21 | −7.22 (−10.29, −4.16) | <0.001 | 92.3% | 43.84 | |
| Baseline BMI (kg/m2) | 0.142 | |||||
| Normal (18.5–24.9) | 4 | −6.86 (−11.36, −2.36) | 0.003 | 80.3% | 15.54 | |
| Overweight (25–29.9) | 11 | −5.91 (−9.47, −2.35) | <0.001 | 86.2% | 28.17 | |
| Obese (≥30) | 6 | −7.93 (−15.87, 0.001) | 0.050 | 91.9% | 88.35 | |
| Subgroup analyses of l-arginine supplementation on DBP | ||||||
| Overall effect | 28 | −2.66 (−3.93, −1.39) | <0.001 | 84.0% | 7.14 | |
| Baseline DBP | 0.785 | |||||
| <80 mmHg | 18 | −2.17 (−4.35, −0.004) | 0.050 | 87.6% | 16.26 | |
| ≥80 mmHg | 10 | −3.44 (−5.15, −1.73) | <0.001 | 71.4% | 4.24 | |
| Trial duration | <0.001 | |||||
| ≤24 d | 7 | −4.82 (−7.21, −2.43) | <0.001 | 89.9% | 5.97 | |
| >24 d | 21 | −1.99 (−3.27, −0.71) | 0.002 | 60.9% | 4.65 | |
| Intervention dose | <0.001 | |||||
| ≤9 g/d | 16 | −4.26 (−5.61, −2.91) | <0.001 | 83.6% | 4.28 | |
| >9 g/d | 14 | −0.15 (−1.99, 1.68) | 0.869 | 45.5% | 4.94 | |
| Sex | <0.001 | |||||
| Male | 7 | −0.51 (−1.91, 0.88) | 0.471 | 0.0% | 0.00 | |
| Female | 9 | −3.60 (−4.86, −2.35) | <0.001 | 59.6% | 1.68 | |
| Both | 12 | −2.95 (−6.01, 0.10) | 0.058 | 89.0% | 23.53 | |
| Health status | <0.001 | |||||
| Healthy | 9 | −2.09 (−3.53, −0.65) | 0.004 | 24.0% | 1.04 | |
| Unhealthy | 19 | −2.86 (−4.40, −1.31) | <0.001 | 86.6% | 7.64 | |
| Baseline BMI (kg/m2) | <0.001 | |||||
| Normal (18.5–24.9) | 4 | −2.86 (−5.60, −0.12) | 0.041 | 49.7% | 3.64 | |
| Overweight (25–29.9) | 11 | −3.17 (−4.14, −2.20) | <0.001 | 6.3% | 0.17 | |
| Obese (≥30) | 6 | −3.32 (−6.87, 0.22) | 0.067 | 68.7% | 12.88 | |
P < 0.05 was considered significant. DBP, diastolic blood pressure; SBP, systolic blood pressure; WMD, weighted mean difference.
Effect of l-arginine supplementation on DBP
Overall, 30 effect sizes from 22 studies with a total sample size of 392 participants in the intervention and 361 in the placebo group were included in the analysis (11, 12, 14, 15, 39, 41–56). Pooled effect sizes indicated a significant decrease in DBP [WMD = −2.64 mmHg; 95% CI: −3.94, −1.40; P < 0.001 (Figure 2B)]. Between-study heterogeneity was also observed (I2 = 84.00). Moreover, the subgroup analysis revealed that l-arginine supplementation did not affect DBP when a high dose of l-arginine (>9 g/d) was used or in studies conducted in males, both sexes, and obese participants (BMI: >30) (Table 3).
Publication bias
Although the visual inspection of funnel plots showed slight asymmetries, no significant publication bias was detected for SBP, but it was considerable for DBP (Supplemental File 1).
Nonlinear dose–response analysis by REMR model analysis
For the dose–response analysis between l-arginine supplementation and SBP and DBP, we used a 1-stage REMR model. The dose–response analyses revealed nonlinear associations between l-arginine supplementation and SBP. A significant negative association between the dose and SBP was found. Moreover, the effective dosage of l-arginine supplementation was detected to be ≥4 g/d (coefficients = −0.435, P = 0.028; Figure 3A). However, there was no significant association between dose–response and DBP (coefficients = −0.151, P = 0.119; Figure 3B).
FIGURE 3.
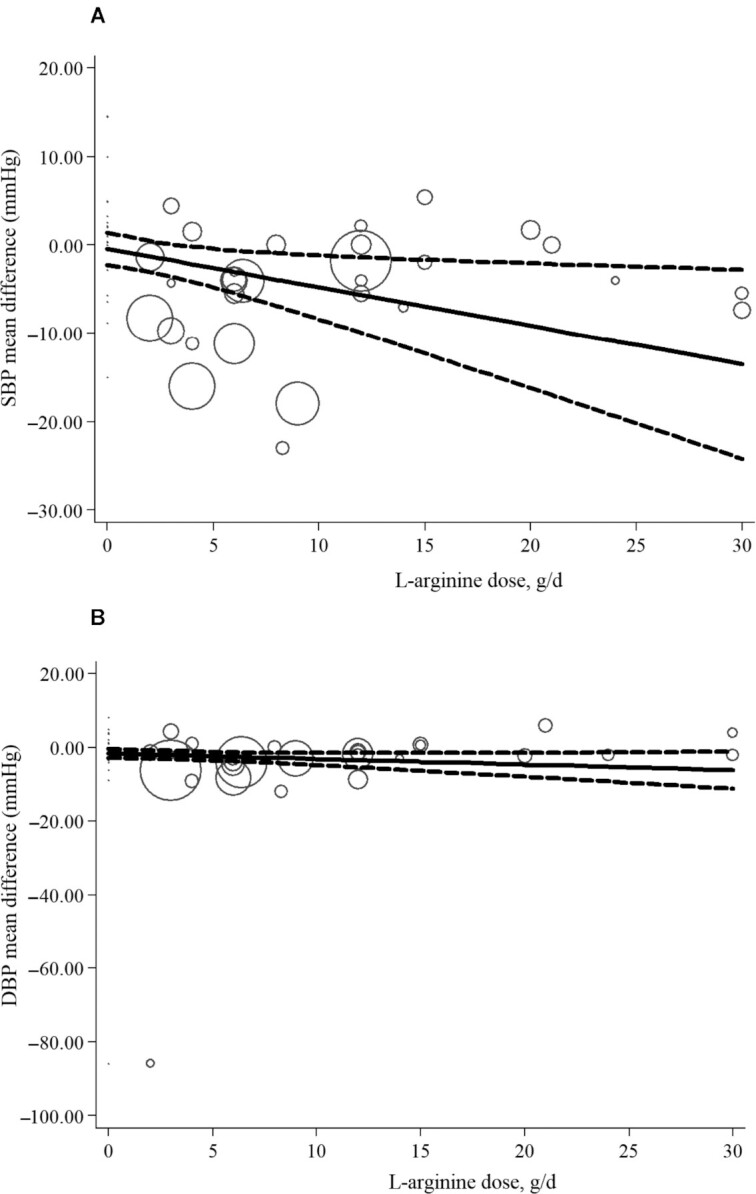
Nonlinear dose–response analysis of the effects of l-arginine dose on SBP (A) and DPB (B) in adults (REMR model analysis). Individual trials are represented by circles, with their weight in the overall analysis represented by circle size. The solid lines represent the estimated dose-response for l-arginine supplementation on (A) SBP, (B) DBP. The dashed lines represent 95% CI. DBP, diastolic blood pressure; REMR, robust error meta-regression; SBP, systolic blood pressure.
Meta-regression analysis for SBP and DBP
Meta-regression analyses were performed to assess whether BP was affected by l-arginine doses and intervention durations. We did not find a significant linear relation between dose (g/d) (coefficient = 0.264; 95% CI: −0.09, 0.62; P = 0.145) and duration (d) (coefficient = 0.853; CI: −1.87, 2.25; P = 0.853) of intervention and changes in SBP (Figure 4). In addition, there was no significant linear relation between duration of intervention and changes in DBP (coefficient = −0.56; CI: −5.67, 4.53; P = 0.821). However, we found a significant relationship between the dose of intervention and changes in DBP (coefficient = 0.977; CI: 0.12, 1.83; P = 0.028) (Figure 5).
FIGURE 4.
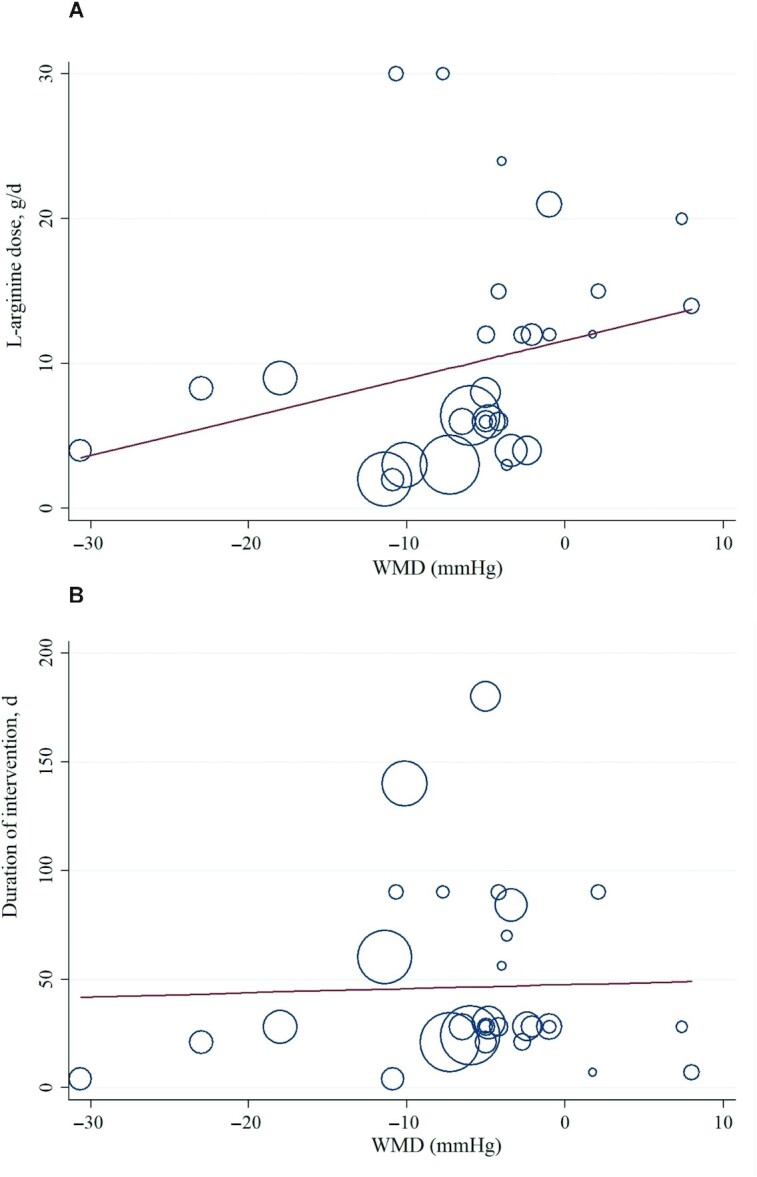
Random-effects meta-regression plots of the associations between dose of l-arginine (A) or duration of intervention (B) and WMD of SBP in adults. Line show linear prediction; each trial is represented by a circle that shows the actual coordinates (observed effect size by latitude) for that study. The size (specifically, the area) of each circle is proportional to that study’s weight in the analysis. SBP, systolic blood pressure; WMD, weighted mean difference.
FIGURE 5.
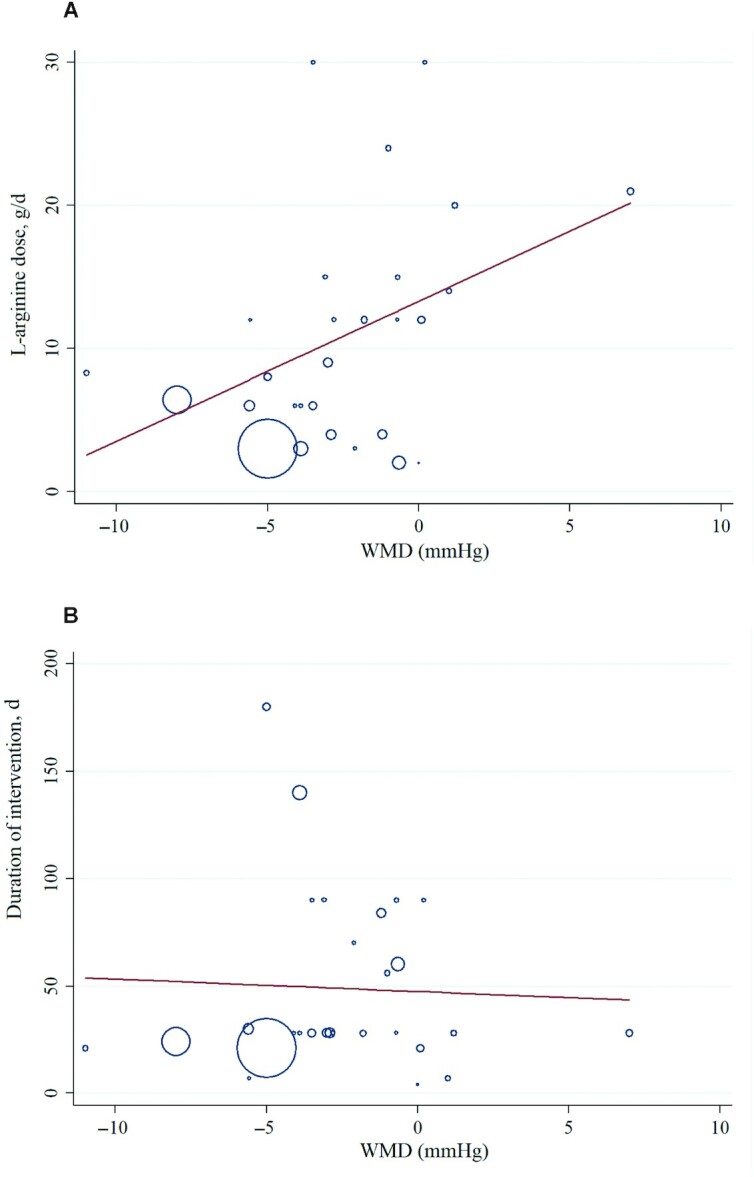
Random-effects meta-regression plots of the association between dose of l-arginine (A) or duration of intervention (B) and WMD of DBP in adults. Line show linear prediction; each trial is represented by a circle that shows the actual coordinates (observed effect size by latitude) for that study. The size (specifically, the area) of each circle is proportional to that study’s weight in the analysis. DBP, diastolic blood pressure; WMD, weighted mean difference.
Sensitivity analysis
Upon removing individual study effects for sensitivity analysis, no study was found to affect the overall results.
GRADE assessment
Using the GRADE evidence profile, the certainty in outcomes of l-arginine supplementation on BP is shown in Table 4. Because of severe inconsistencies and indirectness for both SBP and DBP, the quality of evidence was low.
TABLE 4.
GRADE profile of included studies investigating the effects of l-arginine supplementation on blood pressure in adults1
| Summary of findings | ||||||||
|---|---|---|---|---|---|---|---|---|
| Quality assessment | Number of | |||||||
| Outcomes | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | intervention/control | WMD (95% CI) | Quality of evidence5 |
| SBP | No serious limitations | Very serious limitations2 | Serious limitations3 | No serious limitations | No serious limitations | 431/394 | −6.40 (−8.74, −4.05) | ⊕⊕◯◯ Low |
| DBP | No serious limitations | Very serious limitations4 | Serious limitations3 | No serious limitations | No serious limitations | 392/361 | −2.66 (−3.93, −1.39) | ⊕⊕◯◯ Low |
DBP, diastolic blood pressure; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; SBP, systolic blood pressure; WMD, weighted mean difference.
The test for heterogeneity is significant, and the I2 is moderate, 90.4%.
Studies conducted in participants with various conditions.
The test for heterogeneity is significant, and the I2 is moderate, 84.0%
⊕ symbol shows +1 quality evidence; for every serious limitation, one of this quality evidence is lost and changes to ◯ symbol; ⊕⊕◯◯: low (The true effect may differ significantly from the estimate).
Discussion
We performed an updated systematic review and meta-analysis to investigate the effects of l-arginine supplementation on BP by summarizing the data from published RCTs. The results showed that l-arginine supplementation significantly lowered SBP and DBP in adults. Subgroup analyses portrayed a novel finding that l-arginine supplementation may effectively decrease BP regardless of resting BP category (normal, elevated BP, or hypertension), BMI (normal, overweight, or obese), and health status. l-Arginine supplementation also appears to decline DBP effectively in females but not in males.
In a decade-old meta-analysis, Dong and colleagues (13) performed a study in 387 participants in 11 RCTs with oral l-arginine interventions ranging from 4 to 24 g/d. The findings were in line with the present study demonstrating that oral l-arginine intake could considerably reduce both SBP and DBP. An inverse relation between baseline SBP and net shift in SBP was found in the previous meta-regression analysis, but it was not significant. Moreover, our findings highlighting the BP-lowering effect of l-arginine supplementation are corroborated by several investigations in both human and animal models (11, 12, 15, 40, 44, 47, 48, 50, 53, 55–57). Clinically, declines of 2 mmHg in resting SBP and DBP can reduce the mortality from stroke by 6% and 15% and from heart disease by 4% and 9%, respectively (58, 59). Therefore, the results from our pooled analysis showing significant reductions in resting SBP (−6.40 mmHg) and DBP (−2.64 mmHg) support the clinical significance of l-arginine supplementation as a nonpharmacological strategy for BP control.
The positive effects of l-arginine on BP may be attributed to several signaling pathways. Arginine is a precursor to NO, which induces endothelium-dependent dilation (8), increases blood supply to tissues, and stimulates the secretion of hormones such as insulin and human growth hormone (60, 61). NO is an endogenous messenger molecule that plays a role in several processes that ameliorate the atherosclerotic process by decreasing monocyte adhesion and atheroma formation (62, 63). Although limited information exists on the factors regulating NO synthesis in hypertension, l-arginine has been shown to increase NO bioavailability by increasing its formation and subsequent superoxide scavenging (64, 65). Moreover, elevated extracellular l-arginine increases NO output in both the endothelial and inner medullary collecting duct (IMCD) cells of the kidneys (5). Improved NO in epithelial cells, like the IMCD, causes reduced sodium reabsorption, resulting in heightened sodium and water excretion, decreased extracellular and plasma volume, and consequently lower BP. Similarly, an improvement in l-arginine transport in endothelial and epithelial cells lowers BP by reducing vascular resistance or increasing sodium and water excretion (66).
Increased plasma NO concentrations by l-arginine blunted angiotensin II–induced hypertension and prevented renal damage (66). This finding adds support to the concept that, in addition to reducing arterial pressure, antihypertensive therapies that improve l-arginine and NO bioavailability can attenuate hypertension-related renal damage (67). In rat aortic smooth muscle cells, angiotensin II improves l-arginine transport and the expression of the cationic amino acid transporter 1 (CAT1) mRNA (68). Increased extracellular l-arginine to promote endogenous NO synthesis should therefore be implemented in angiotensin II–induced hypertension. It seems that CAT1 is upregulated in angiotensin II–induced hypertension, allowing the antihypertensive effects of l-arginine to be more efficient (66, 68). Moreover, l-arginine has also been shown to suppress the angiotensin-converting enzyme in humans (69).
Endothelin-1 is a potent endothelium-derived vasoconstrictor synthesized in conditions of high cardiovascular risk such as hypertension. l-Arginine lowers endothelin-1 concentrations in humans via endothelial NO-induced inhibition of endothelin-1 synthesis (70, 71). NO reduces endothelin-1 secretion and endothelin-1 gene expression since it promotes superoxide synthesis during chronic angiotensin II infusion. Therefore, reducing endothelin-1 concentrations lowers superoxide bioavailability and raises NO concentrations (72, 73).
Insulin induces vasodilation by enhancing the production of NO over that of endothelin-1. Thus, insulin resistance has been associated with endothelial dysfunction and hypertension (74, 75). Evidence from a few RCTs has shown that l-arginine supplementation with 6.4 to 30 g/d reduced blood glucose and insulin, indicating improved glucose control and insulin sensitivity in adults with normoglycemia (56), prediabetes, and type 2 diabetes (42, 46, 76). Therefore, l-arginine supplementation may reduce BP via improved insulin-mediated control of endothelial function (42, 46, 56).
Another role attributed to l-arginine is that of an ROS scavenger, preventing endothelial cells from releasing oxygen-free radicals (77). Notably, l-arginine can generate NO by reacting with hydrogen peroxide in a nonenzymatic pathway (78). In hypertension, uncoupled NOS releases more superoxide than NO, resulting in a vicious loop that dramatically reduces NO. Under such circumstances, the nonenzymatic pathway that produces NO from l-arginine could play a key role in increasing NO output (79). l-Arginine can “recouple” uncoupled NOS in the kidneys and disrupt the vicious loop that decreases NO bioavailability. These findings suggest that l-arginine supplementation can increase NO output not only through the traditional pathway but also through a variety of other accessory pathways (67). This is in line with the “arginine paradox” concept, which refers to the fact that NO synthesis and vasodilatory function are dependent on the extracellular concentration of l-arginine (5). Multiple mechanisms have been proposed to clarify this concept. For instance, the cytoplasmic compartmentalization of l-arginine presents the possibility that CAT1 and endothelial NOS1 (eNOS) appear in the plasma membrane of endothelial cells and may be unable to reach intracellular l-arginine pools (80). Since eNOS and CAT1 are similar, eNOS may prefer to use l-arginine transferred from the extracellular fluid by CAT1. Endogenous NOS antagonists, such as asymmetric dimethylarginine, have also decreased NOS sensitivity to l-arginine (81). Moreover, increased extracellular l-arginine bioavailability can be used to counteract the inhibitory effects of this endogenous compound (81). An additional possible mechanism that may play a part in the BP-lowering effect of l-arginine supplementation is the mediating effect of NO on cardiac autonomic function. Prior findings indicate that NO may enhance vagal and diminish sympathetic activity (82–84), which may result from an improved baroreflex sensitivity (85). Indeed, poor baroreflex sensitivity has been inversely associated with BP in different populations (86, 87).
This meta-analysis has various strengths and limitations. The accuracy of the individual studies determined the validity of this meta-analysis. While all studies were randomized, allocation concealment, randomization efficiency, and withdrawal information were not continuously published. Specific study sample sizes were minimal, limiting the ability of randomization to reduce the possible effects of confounding variables. Individuals with varying degrees of health statuses were included in the studies, contributing to a heterogeneous sample. Moreover, a few trials had concerns about the harmful effects of l-arginine supplementation and the inefficiency of long-term supplementation due to a rise in arginase activity, leading to a reduction in NO bioavailability and an elevation in ROS production (88). Indeed, arginase overactivation by high-dose, long-term l-arginine supplementation, as indicated by catabolism of l-arginine to ornithine and urea, may explain the lack of antihypertensive effects in males with overweight and obesity (56). In the present meta-analysis, the majority of included studies were short-term, lasting less than 3–4 wk. Our present findings demonstrate that daily oral l-arginine becomes ineffective in decreasing BP after 24 d of supplementation. Therefore, the effects of chronic l-arginine supplementation on BP and its vascular protection need further research. Some RCTs were not primarily designed to assess the effects of l-arginine supplementation on BP. The absence of controls for baseline BP in some studies and different study designs should also be considered. Last, the assessment of BP differed across studies as some trials used the supine, while others utilized the seated or standing position. A strength of this study is the inclusion of all RCTs with no limitation on language and time of interventions. Moreover, studies were included based on inclusion criteria, which made it possible to analyze several subgroup variables. Furthermore, most RCTs were double-blinded and placebo-controlled. In addition, sensitivity tests in this study were used to identify potential sources of heterogeneity among studies.
This meta-analysis indicated that l-arginine supplementation had a positive effect on decreasing SBP and DBP. Future homogeneous and well-designed RCTs and mechanistic studies are needed to understand the effects of l-arginine on SBP and DBP in humans. Based on our findings, it seems that consuming low dosages of l-arginine (≤9 g/d) for a short term (≤24 d) is more effective than consuming higher dosages for longer durations. l-Arginine is beneficial for healthy and unhealthy individuals, hypertensive and normotensive, in all BMI categories, and regardless of sex, but especially favorable for women (39, 48, 56). Moreover, RCTs included in the current meta-analysis revealed no major side effects following the high dosages of l-arginine supplementation.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—FS: designed the study; FS and OA: developed the search strategy and extracted the data; OA, FS, and RB: conducted the analyses and drafted the manuscript; FS, OA, and KM: assessed the risk of bias of the meta-analyses and interpreted the results; AW, RB, and AF revised the manuscript; and all authors: read and approved the final manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: The authors report no conflicts of interest.
Supplemental File 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: BP, blood pressure; CAT1, cationic amino acid transporter 1; DBP, diastolic blood pressure; eNOS, endothelial nitric oxide synthase; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; IMCD, inner medullary collecting duct; MeSH, Medical Subject Heading; NOS, nitric oxide synthase; RCT, randomized clinical trial; REMR, robust error meta-regression; ROS, reactive oxygen species; SBP, systolic blood pressure; WMD, weighted mean difference.
Contributor Information
Farideh Shiraseb, Department of Community Nutrition, School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences (TUMS), Tehran, Iran.
Omid Asbaghi, Cancer Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Reza Bagheri, Department of Exercise Physiology, University of Isfahan, Isfahan, Iran.
Alexei Wong, Department of Health and Human Performance, Marymount University, Arlington, VA, USA.
Arturo Figueroa, Department of Kinesiology and Sport Management, Texas Tech University, Lubbock, TX, USA.
Khadijeh Mirzaei, Department of Community Nutrition, School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences (TUMS), Tehran, Iran.
References
- 1. James WPT. The epidemiology of obesity: the size of the problem. J Intern Med. 2008;263(4):336–52. [DOI] [PubMed] [Google Scholar]
- 2. Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, AlMazroa MA, Amann M, Anderson HR, Andrews KG. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet North Am Ed. 2012;380(9859):2224–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999-2000. JAMA. 2002;288(14):1723–7. [DOI] [PubMed] [Google Scholar]
- 4. Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, Alexander L, Estep K, Abate KH, Akinyemiju TF. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990-2015. JAMA. 2017;317(2):165–82. [DOI] [PubMed] [Google Scholar]
- 5. Khalaf D, Krüger M, Wehland M, Infanger M, Grimm D. The effects of oral L-arginine and L-citrulline supplementation on blood pressure. Nutrients. 2019;11(7):1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McNeal CJ, Meininger CJ, Reddy D, Wilborn CD, Wu G. Safety and effectiveness of arginine in adults. J Nutr. 2016;146(12):2587S–93S. [DOI] [PubMed] [Google Scholar]
- 7. Ástvaldsdóttir Á, Naimi-Akbar A, Davidson T, Brolund A, Lintamo L, Granath AA, Tranæus S, Östlund P. Arginine and caries prevention: a systematic review. Caries Res. 2016;50(4):383–93. [DOI] [PubMed] [Google Scholar]
- 8. Melik Z, Zaletel P, Virtic T, Cankar K. L-arginine as dietary supplement for improving microvascular function. Clin Hemorheol Microcirc. 2017;65(3):205–17. [DOI] [PubMed] [Google Scholar]
- 9. Zarezadeh M, Emami MR, Kord-Varkane H, Mousavi SM, Alizadeh H, Asbaghi O, Olang B, Khorshidi M. The effect of oral L-arginine supplementation on asymmetric dimethylarginine levels: a systematic review and meta-analysis of randomized clinical trials. Adv Integr Med. 2020;7(2):61–6. doi: https://doi.org/10.1016/j.aimed.2019.06.003. [Google Scholar]
- 10. Siani A, Pagano E, Iacone R, Iacoviello L, Scopacasa F, Strazzullo P. Blood pressure and metabolic changes during dietary L-arginine supplementation in humans. Am J Hypertens. 2000;13(5):547–51. [DOI] [PubMed] [Google Scholar]
- 11. Ast J, Jablecka A, Bogdanski P, Smolarek I, Krauss H, Chmara E. Evaluation of the antihypertensive effect of L-arginine supplementation in patients with mild hypertension assessed with ambulatory blood pressure monitoring. Med Sci Monit. 2010;16(5):CR266–71. [PubMed] [Google Scholar]
- 12. Rytlewski K, Olszanecki R, Korbut R, Zdebski Z. Effects of prolonged oral supplementation with L-arginine on blood pressure and nitric oxide synthesis in preeclampsia. Eur J Clin Invest. 2005;35(1):32–37. [DOI] [PubMed] [Google Scholar]
- 13. Dong J-Y, Qin L-Q, Zhang Z, Zhao Y, Wang J, Arigoni F, Zhang W. Effect of oral L-arginine supplementation on blood pressure: a meta-analysis of randomized, double-blind, placebo-controlled trials. Am Heart J. 2011;162(6):959–65. [DOI] [PubMed] [Google Scholar]
- 14. Neri I, Monari F, Sgarbi L, Berardi A, Masellis G, Facchinetti F. L-arginine supplementation in women with chronic hypertension: impact on blood pressure and maternal and neonatal complications. J Matern Fetal Neonatal Med. 2010;23(12):1456–60. [DOI] [PubMed] [Google Scholar]
- 15. Chin-Dusting JP, Alexander CT, Arnold PJ, Hodgson WC, Lux AS, Jennings GL. Effects of in vivo and in vitro L-arginine supplementation on healthy human vessels. J Cardiovasc Pharmacol. 1996;28(1):158–66. [DOI] [PubMed] [Google Scholar]
- 16. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Higgins J. Cochrane handbook for systematic reviews of interventions [Internet]. Version 5.1. 0 [updated March 2011]. The Cochrane Collaboration. Available from: www.cochrane-handbook.org.
- 18. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 19. Asbaghi O, Sadeghian M, Mozaffari-Khosravi H, Maleki V, Shokri A, Hajizadeh-Sharafabad F, Alizadeh M, Sadeghi O. The effect of vitamin D-calcium co-supplementation on inflammatory biomarkers: a systematic review and meta-analysis of randomized controlled trials. Cytokine. 2020;129:155050. [DOI] [PubMed] [Google Scholar]
- 20. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Method. 2005;5(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Higgins JP. Cochrane handbook for systematic reviews of interventions version 5.0.1 [Internet]. The Cochrane Collaboration; 2008. Available from: http://www cochrane-handbook.org.
- 22. Fu R, Gartlehner G, Grant M, Shamliyan T, Sedrakyan A, Wilt TJ, Griffith L, Oremus M, Raina P, Ismaila A. Conducting quantitative synthesis when comparing medical interventions: AHRQ and the Effective Health Care Program. J Clin Epidemiol. 2011;64(11):1187–97. [DOI] [PubMed] [Google Scholar]
- 23. Agency for Healthcare Research and Quality . Methods guide for effectiveness and comparative effectiveness reviews [Internet]. 2018. Available from: https://effectivehealthcare.ahrq.gov/products/collections/cer-methods-guide. [PubMed] [Google Scholar]
- 24. Flack JM, Adekola B. Blood pressure and the new ACC/AHA hypertension guidelines. Trends Cardiovasc Med. 2020;30(3):160–4. doi: https://doi.org/10.1016/j.tcm.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 25. Namazi N, Larijani B, Azadbakht L. Low-carbohydrate-diet score and its association with the risk of diabetes: a systematic review and meta-analysis of cohort studies. Horm Metab Res. 2017;49(8):565–71. [DOI] [PubMed] [Google Scholar]
- 26. Brondani LA, Assmann TS, de Souza BM, Bouças AP, Canani LH, Crispim D. Meta-analysis reveals the association of common variants in the uncoupling protein (UCP) 1–3 genes with body mass index variability. PLoS One. 2014;9(5):e96411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101. [PubMed] [Google Scholar]
- 28. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Duval S. Chapter 8: The trim and fill method. In: Publication bias in meta-analysis: prevention, assessment and adjustments. Rothstein HR, Sutton AJ, Borenstein M (editors). Chichester (UK): John Wiley & Sons; 2005. p. 127–44. [Google Scholar]
- 30. Xu C, Doi SA. The robust error meta-regression method for dose–response meta-analysis. Int J Evid Based Healthc. 2018;16(3):138–44. [DOI] [PubMed] [Google Scholar]
- 31. Xie Y, Gou L, Peng M, Zheng J, Chen L. Effects of soluble fiber supplementation on glycemic control in adults with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr. 2021;40(4):1800–10. [DOI] [PubMed] [Google Scholar]
- 32. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang N, Xiong A-H, Xiao X, Li L-P. Effect and mechanism of L-arginine therapy for fetal growth retardation due to pregnancy-induced hypertension. J South Med Univ. 2007;27(2):198–200. [PubMed] [Google Scholar]
- 34. Orea-Tejeda A, Orozco-Gutiérrez JJ, Castillo-Martínez L, Keirns-Davies C, Montano-Hernández P, Vázquez-Díaz O, Valdespino-Trejo A, Infante O, Martínez-Memije R. The effect of L-arginine and citrulline on endothelial function in patients in heart failure with preserved ejection fraction. Cardiol J. 2010;17(5):464–70. [PubMed] [Google Scholar]
- 35. Miller AL. The effects of a sustained-release L-arginine formulation on blood pressure and vascular compliance in 29 healthy individuals. Altern Med Rev. 2006;11(1):23–9. [PubMed] [Google Scholar]
- 36. Kelly BS, Alexander JW, Dreyer D, Greenberg NA, Erickson A, Whiting JF, Ogle CK, Babcock GF, First MR. Oral arginine improves blood pressure in renal transplant and hemodialysis patients. J Parenter Enter Nutr. 2001;25(4):194–202. [DOI] [PubMed] [Google Scholar]
- 37. Jahangir E, Vita JA, Handy D, Holbrook M, Palmisano J, Beal R, Loscalzo J, Eberhardt RT. The effect of L-arginine and creatine on vascular function and homocysteine metabolism. Vasc Med. 2009;14(3):239–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pezza V, Bernardini F, Pezza E, Pezza B, Curione M. Study of supplemental oral L-arginine in hypertensives treated with enalapril+ hydrochlorothiazide. Am J Hypertens. 1998;11(10):1267. [DOI] [PubMed] [Google Scholar]
- 39. de Lima JM, Silva AS, Alves NFB, Porpino SKP, de Almeida AEM, Lima RT. L-arginina aumenta a produįão endotelial de óxido nítrico e reduz a pressão arterial de repouso sem alterar as respostas pressóricas do exercício. Motricidade. 2012;8(3):19–29. [Google Scholar]
- 40. Salmani M, Alipoor E, Navid H, Farahbakhsh P, Yaseri M, Imani H. Effect of L-arginine on cardiac reverse remodeling and quality of life in patients with heart failure. Clin Nutr. 2021;40(5):3037–44. [DOI] [PubMed] [Google Scholar]
- 41. Clarkson P, Adams MR, Powe AJ, Donald AE, McCredie R, Robinson J, McCarthy SN, Keech A, Celermajer DS, Deanfield JE. Oral L-arginine improves endothelium-dependent dilation in hypercholesterolemic young adults. J Clin Invest. 1996;97(8):1989–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Piatti P, Monti LD, Valsecchi G, Magni F, Setola E, Marchesi F, Galli-Kienle M, Pozza G, Alberti KGM. Long-term oral L-arginine administration improves peripheral and hepatic insulin sensitivity in type 2 diabetic patients. Diabetes Care. 2001;24(5):875–80. [DOI] [PubMed] [Google Scholar]
- 43. Sydow K, Schwedhelm E, Arakawa N, Bode-Böger SM, Tsikas D, Hornig B, Frölich JC, Böger RH. ADMA and oxidative stress are responsible for endothelial dysfunction in hyperhomocyst(e)inemia: effects of L-arginine and B vitamins. Cardiovasc Res. 2003;57(1):244–52. [DOI] [PubMed] [Google Scholar]
- 44. West SG, Likos-Krick A, Brown P, Mariotti F. Oral L-arginine improves hemodynamic responses to stress and reduces plasma homocysteine in hypercholesterolemic men. J Nutr. 2005;135(2):212–17. [DOI] [PubMed] [Google Scholar]
- 45. Hladunewich MA, Derby GC, Lafayette RA, Blouch KL, Druzin ML, Myers BD. Effect of L-arginine therapy on the glomerular injury of preeclampsia: a randomized controlled trial. Obstet Gynecol. 2006;107(4):886–95. [DOI] [PubMed] [Google Scholar]
- 46. Lucotti P, Monti L, Setola E, La Canna G, Castiglioni A, Rossodivita A, Pala MG, Formica F, Paolini G, Catapano AL. Oral L-arginine supplementation improves endothelial function and ameliorates insulin sensitivity and inflammation in cardiopathic nondiabetic patients after an aortocoronary bypass. Metabolism. 2009;58(9):1270–76. [DOI] [PubMed] [Google Scholar]
- 47. Lucotti P, Setola E, Monti LD, Galluccio E, Costa S, Sandoli EP, Fermo I, Rabaiotti G, Gatti R, Piatti P. Beneficial effects of a long-term oral L-arginine treatment added to a hypocaloric diet and exercise training program in obese, insulin-resistant type 2 diabetic patients. Am J Physiol Endocrinol Metab. 2006;291(5):E906–12. [DOI] [PubMed] [Google Scholar]
- 48. Facchinetti F, Saade GR, Neri I, Pizzi C, Longo M, Volpe A. L-arginine supplementation in patients with gestational hypertension: a pilot study. Hypertens Pregnancy. 2007;26(1):121–30. [DOI] [PubMed] [Google Scholar]
- 49. Fontanive P, Saponati G, Iurato A, Volterrani C, Boni A, Piccioni L, Dini FL. Effects of L-arginine on the Minnesota Living with Heart Failure Questionnaire quality-of-life score in patients with chronic systolic heart failure. Med Sci Monit. 2009;15(12):CR606–11. [PubMed] [Google Scholar]
- 50. Battaglia C, Mancini F, Battaglia B, Facchinetti F, Artini PG, Venturoli S. L-arginine plus drospirenone-ethinyl estradiol in the treatment of patients with PCOS: a prospective, placebo controlled, randomised, pilot study. Gynecol Endocrinol. 2010;26(12):861–68. [DOI] [PubMed] [Google Scholar]
- 51. Willoughby DS, Boucher T, Reid J, Skelton G, Clark M. Effects of 7 days of arginine-alpha-ketoglutarate supplementation on blood flow, plasma L-arginine, nitric oxide metabolites, and asymmetric dimethyl arginine after resistance exercise. Int J Sport Nutr Exerc Metab. 2011;21(4):291–99. [DOI] [PubMed] [Google Scholar]
- 52. Ast J, Cieslewicz A, Korzeniowska K, Bogdanski P, Kazmierczak E, Olszewski J, Skołuda A, Jabłecka A. Supplementation with L-arginine does not influence arterial blood pressure in healthy people: a randomized, double blind, trial. Eur Rev Med Pharmacol Sci. 2011;15(12):1375–84. [PubMed] [Google Scholar]
- 53. Malfatti CRM, Pavla JLL, Da Silva LA, Medeiros TE, Alonso KC, Artoni RF, Nogaroto V, Túrmina JA, Osiecki A, Osiecki R. Effects of L-arginine supplementation on blood pressure reduction pre-, peri-, and post-cardiovascular stimulus in hypertensive subjects with different angiotensin–converting-enzyme genotypes. Hum Mov. 2014;15(2):116–19. [Google Scholar]
- 54. Pahlavani N, Jafari M, Sadeghi O, Rezaei M, Rasad H, Rahdar HA, Entezari MH. L-arginine supplementation and risk factors of cardiovascular diseases in healthy men: a double-blind randomized clinical trial. F1000Research. 2014;3:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Camarena Pulido E, García Benavides L, Panduro Barón J, Pascoe Gonzalez S, Madrigal Saray A, García Padilla F, Totsuka Sutto S. Efficacy of L-arginine for preventing preeclampsia in high-risk pregnancies: a double-blind, randomized, clinical trial. Hypertens Pregnancy. 2016;35(2):217–25. [DOI] [PubMed] [Google Scholar]
- 56. McNeal CJ, Meininger CJ, Wilborn CD, Tekwe CD, Wu G. Safety of dietary supplementation with arginine in adult humans. Amino Acids. 2018;50(9):1215–29. [DOI] [PubMed] [Google Scholar]
- 57. Dumont Y, D'Amours M, Lebel M, Larivière R. Supplementation with a low dose of L-arginine reduces blood pressure and endothelin-1 production in hypertensive uraemic rats. Nephrol Dialysis Transplant. 2001;16(4):746–54. [DOI] [PubMed] [Google Scholar]
- 58. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–71. [DOI] [PubMed] [Google Scholar]
- 59. Cook NR, Cohen J, Hebert PR, Taylor JO, Hennekens CH. Implications of small reductions in diastolic blood pressure for primary prevention. Arch Intern Med. 1995;155(7):701–9. [PubMed] [Google Scholar]
- 60. Adams MR, Forsyth CJ, Jessup W, Robinson J, Celermajer DS. Oral L-arginine inhibits platelet aggregation but does not enhance endothelium-dependent dilation in healthy young men. J Am Coll Cardiol. 1995;26(4):1054–61. [DOI] [PubMed] [Google Scholar]
- 61. Moazami N, Dembitsky WP, Adamson R, Steffen RJ, Soltesz EG, Starling RC, Fukamachi K. Does pulsatility matter in the era of continuous-flow blood pumps?. J Heart Lung Transplant. 2015;34(8):999–1004. [DOI] [PubMed] [Google Scholar]
- 62. Toda N. Nitric oxide and dietary factors: part V summary/conclusion and references. Vasc Dis Prev. 2007;4(1):85–110. [Google Scholar]
- 63. Lekakis JP, Papathanassiou S, Papaioannou TG, Papamichael CM, Zakopoulos N, Kotsis V, Dagre AG, Stamatelopoulos K, Protogerou A, Stamatelopoulos SF. Oral L-arginine improves endothelial dysfunction in patients with essential hypertension. Int J Cardiol. 2002;86(2-3):317–23. [DOI] [PubMed] [Google Scholar]
- 64. Kakoki M, Kim H-S, Arendshorst WJ, Mattson DL. L-Arginine uptake affects nitric oxide production and blood flow in the renal medulla. Am J Physiol Regul Integr Comp Physiol. 2004;287(6):R1478–R85. [DOI] [PubMed] [Google Scholar]
- 65. Kakoki M, Kim H-S, Edgell C-JS, Maeda N, Smithies O, Mattson DL. Amino acids as modulators of endothelium-derived nitric oxide. Am J Physiol Renal Physiol. 2006;291(2):F297–304. [DOI] [PubMed] [Google Scholar]
- 66. Rajapakse NW, Mattson DL. Role of L-arginine in nitric oxide production in health and hypertension. Clin Exp Pharmacol Physiol. 2009;36(3):249–55. [DOI] [PubMed] [Google Scholar]
- 67. Rajapakse NW, De Miguel C, Das S, Mattson DL. Exogenous L-arginine ameliorates angiotensin II-induced hypertension and renal damage in rats. Hypertension. 2008;52(6):1084–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Low BC, Grigor MR. Angiotensin II stimulates system y+ and cationic amino acid transporter gene expression in cultured vascular smooth muscle cells. J Biol Chem. 1995;270(46):27577–83. [DOI] [PubMed] [Google Scholar]
- 69. Higashi Y, Oshima T, Ono N, Hiraga H, Yoshimura M, Watanabe M, Matsuura H, Kambe M, Kajiyama G. Intravenous administration of L-arginine inhibits angiotensin-converting enzyme in humans. J Clin Endocrinol Metab. 1995;80(7):2198–202. [DOI] [PubMed] [Google Scholar]
- 70. Lerman A, Burnett JC Jr, Higano ST, McKinley LJ, Holmes DR Jr. Long-term L-arginine supplementation improves small-vessel coronary endothelial function in humans. Circulation. 1998;97(21):2123–8. [DOI] [PubMed] [Google Scholar]
- 71. Piatti P, Fragasso G, Monti LD, Setola E, Lucotti P, Fermo I, Paroni R, Galluccio E, Pozza G, Chierchia S. Acute intravenous L-arginine infusion decreases endothelin-1 levels and improves endothelial function in patients with angina pectoris and normal coronary arteriograms: correlation with asymmetric dimethylarginine levels. Circulation. 2003;107(3):429–36. [DOI] [PubMed] [Google Scholar]
- 72. Kelly LK, Wedgwood S, Steinhorn RH, Black SM. Nitric oxide decreases endothelin-1 secretion through the activation of soluble guanylate cyclase. Am J Physiol Lung Cell Mol Physiol. 2004;286(5):L984–91. [DOI] [PubMed] [Google Scholar]
- 73. Laplante M-A, Wu R, Moreau P, de Champlain J. Endothelin mediates superoxide production in angiotensin II-induced hypertension in rats. Free Radic Biol Med. 2005;38(5):589–96. [DOI] [PubMed] [Google Scholar]
- 74. Sowers JR. Diabetes mellitus and vascular disease. Hypertension. 2013;61(5):943–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Muniyappa R, Yavuz S. Metabolic actions of angiotensin II and insulin: a microvascular endothelial balancing act. Mol Cell Endocrinol. 2013;378(1-2):59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Monti L, Setola E, Lucotti P, Marrocco-Trischitta M, Comola M, Galluccio E, Poggi A, Mammì S, Catapano A, Comi G. Effect of a long-term oral L-arginine supplementation on glucose metabolism: a randomized, double-blind, placebo-controlled trial. Diabetes Obesity Metab. 2012;14(10):893–900. [DOI] [PubMed] [Google Scholar]
- 77. Wascher TC, Posch K, Wallner S, Hermetter A, Kostner GM, Graier WF. Vascular effects of L-arginine: anything beyond a substrate for the NO-synthase?. Biochem Biophys Res Commun. 1997;234(1):35–8. [DOI] [PubMed] [Google Scholar]
- 78. Nagase S, Takemura K, Ueda A, Hirayama A, Aoyagi K, Kondoh M, Koyama A. A novel nonenzymatic pathway for the generation of nitric oxide by the reaction of hydrogen peroxide and D-or L-arginine. Biochem Biophys Res Commun. 1997;233(1):150–3. [DOI] [PubMed] [Google Scholar]
- 79. Pritchard KA Jr, Groszek L, Smalley DM, Sessa WC, Wu M, Villalon P, Wolin MS, Stemerman MB. Native low-density lipoprotein increases endothelial cell nitric oxide synthase generation of superoxide anion. Circ Res. 1995;77(3):510–18. [DOI] [PubMed] [Google Scholar]
- 80. McDonald KK, Zharikov S, Block ER, Kilberg MS. A caveolar complex between the cationic amino acid transporter 1 and endothelial nitric-oxide synthase may explain the “arginine paradox”. J Biol Chem. 1997;272(50):31213–16. [DOI] [PubMed] [Google Scholar]
- 81. Leone A, Moncada S, Vallance P, Calver A, Collier J. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet North Am Ed. 1992;339(8793):572–5. [DOI] [PubMed] [Google Scholar]
- 82. Conlon K, Collins T, Kidd C. Modulation of vagal actions on heart rate produced by inhibition of nitric oxide synthase in the anaesthetized ferret. Exp Physiol. 1996;81(3):547–50. [DOI] [PubMed] [Google Scholar]
- 83. Hare JM, Keaney JF, Balligand J-L, Loscalzo J, Smith TW, Colucci WS. Role of nitric oxide in parasympathetic modulation of beta-adrenergic myocardial contractility in normal dogs. J Clin Invest. 1995;95(1):360–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zanzinger J, Czachurski J, Seller H. Inhibition of basal and reflex-mediated sympathetic activity in the RVLM by nitric oxide. Am J Physiol Regul Integr Comp Physiol. 1995;268(4):R958–62. [DOI] [PubMed] [Google Scholar]
- 85. Chowdhary S, Nuttall SL, Coote JH, Townend JN. L-arginine augments cardiac vagal control in healthy human subjects. Hypertension. 2002;39(1):51–6. [DOI] [PubMed] [Google Scholar]
- 86. Hesse C, Charkoudian N, Liu Z, Joyner MJ, Eisenach JH. Baroreflex sensitivity inversely correlates with ambulatory blood pressure in healthy normotensive humans. Hypertension. 2007;50(1):41–6. [DOI] [PubMed] [Google Scholar]
- 87. Fitzgibbon LK, Coverdale NS, Phillips AA, Shoemaker JK, Klentrou P, Wade TJ, Cairney J, O'Leary DD. The association between baroreflex sensitivity and blood pressure in children. Appl Physiol Nutr Metab. 2012;37(2):301–7. [DOI] [PubMed] [Google Scholar]
- 88. Huang J, Ladeiras D, Yu Y, Ming X-F, Yang Z. Detrimental effects of chronic L-arginine rich food on aging kidney. Front Pharmacol. 2021;11:582155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.