ABSTRACT
The recent Food and Agricultural Organization/World Health Organization/United Nations University expert consultations on protein requirements and quality have emphasized the need for the new Digestible Indispensable Amino Acid Score (DIAAS), as a measure of protein quality. This requires human measurements of the true ileal digestibility of individual indispensable amino acids (IAAs) until the end of the small intestine. Digestibility is measured using standard oro-ileal balance methods, which can only be achieved by an invasive naso-ileal intubation in healthy participants or fistulation at the terminal ileum. Significant efforts have been made over the last 2 decades to develop noninvasive or minimally invasive methods to measure IAA digestibility in humans. The application of intrinsically labeled (with stable isotopes like 13C, 15N, and 2H) dietary proteins has helped in circumventing the invasive oro-ileal balance techniques and allowed the differentiation between endogenous and exogenous protein. The noninvasive indicator amino acid oxidation (IAAO) technique, which is routinely employed to measure IAA requirements, has been modified to estimate metabolic availability (a sum of digestibility and utilization) of IAA in foods, but provides an estimate for a single IAA at a time and is burdensome for participants. The recently developed minimally invasive dual isotope tracer method measures small intestinal digestibility of multiple amino acids at once and is suitable for use in vulnerable groups and disease conditions. However, it remains to be validated against standard oro-ileal balance techniques. This review critically evaluates and compares the currently available stable isotope-based protein quality evaluation methods with a focus on the digestibility and metabolic availability measurements in humans. In view of building a reliable DIAAS database of various protein sources and subsequently supporting protein content claims in food labeling, a re-evaluation and harmonization of the available methods are necessary.
Keywords: protein quality, stable isotopes, protein digestibility, metabolic availability, intrinsic labeling, oro-ileal balance, dual isotope tracer technique, indicator amino acid oxidation
Statement of Significance: This review is the first of its kind that exhaustively reports on, and critically compares, stable isotope-based and direct measurements of amino acid digestibility/bioavailability in humans, using the ileal balance, dual isotope, and IAAO methods. The review provides details on the principles, advantages, and drawbacks of different methods, as well as details on efficient approaches of intrinsic labeling of food proteins; in addition, this review details all available human measurements of IAA digestibility and metabolic availability of food proteins.
Introduction
Dietary protein provides nitrogen and amino acids (AAs), particularly indispensable amino acids (IAAs), which are required in adequate quantity and proportion for the synthesis of protein and other nitrogen- and AA-related compounds with various structural and biological functions in the body (1, 2). The quality of dietary protein source has been directly assessed by measuring the utilization and retention of dietary nitrogen and AAs in the body, but this approach is difficult due to the complexity of the physiological and metabolic processes of protein digestion, absorption, and metabolic utilization of AAs (2–5). Alternatively, the quality of a dietary protein is defined by its ability to meet age-specific nitrogen and IAA requirements for growth and maintenance. Therefore, it can be assessed by the widely accepted chemical scoring approach that compares the IAA pattern of a protein with the age-specific IAA requirements corrected for protein or IAA digestibility, through 2 simple indexes, the Protein Digestibility Corrected Amino Acid Score (PD-CAAS) and the Digestible Indispensable Amino Acid Score (DIAAS) (2, 5–7). A critical aspect of these indexes is measurement of protein and IAA digestibility to correct the chemical score. Several methods for measuring digestibility are currently available, where stable isotope labeling, especially intrinsically labeled dietary proteins with 15N, 2H, and 13C tracers, has been used. This review critically evaluates and compares the methodological concerns of the stable isotope-based protein quality evaluation methods with a focus on the digestibility and metabolic availability (MA) measurements in humans and their applicability in various pathophysiological conditions.
Concepts of chemical scoring and correction for digestibility
The quality of a dietary protein can be assessed by its chemical score, which is a ratio of its IAA content to the age-specific IAA requirement pattern (4, 6, 7). The lowest chemical score of a food is corrected for the crude protein digestibility to obtain a protein quality metric called the PD-CAAS (Supplementary Material 1). Although practical and widely used, the PD-CAAS has been subject to criticism, mainly for using a single fecal nitrogen digestibility value for correction. Indeed, fecal digestibility is not always a good proxy of digestibility, especially for proteins of low digestibility (8), possibly due to the contribution of colonic microbes to nitrogen transactions via the fermentation of undigested protein entering the colon. As the PD-CAAS is truncated to 100% this does not allow indication of the potential of a high-quality protein to optimize the AA composition of food mixtures with low protein quality. The advantages and limitations of the PD-CAAS have been extensively reviewed previously (9–11). To overcome the concerns related to PD-CAAS, the World Health Organization/Food and Agriculture Organization/United Nations University (WHO/FAO/UNU) expert consultations recommended that the chemical score of each IAA should be corrected for their true ileal digestibility values measured in humans, and the lowest score thus obtained be termed the DIAAS (Supplementary Material 1) (4, 6). Unlike the PD-CAAS, a score of >100% for a single food or mixed diets in the DIAAS is not truncated to indicate the potential of a high-quality protein to complement low-quality protein in mixed diets. Both the DIAAS and PD-CAAS can be used to inform protein content claims in food labeling. Since measuring true ileal AA digestibility in humans is invasive and expensive, pigs and alternatively rats are used to determine the ileal digestibility coefficients for regulatory purposes (4, 6, 12). The reliability of the PD-CAAS and DIAAS is also dependent on factors such as the determination of AA composition, the reference AA profile, the nitrogen to protein conversion factor, and the complexities and uncertainties around the measurements of dietary protein/IAA digestibility.
Digestibility issues: fecal versus ileal digestibility, apparent versus true digestibility, protein versus AA digestibility
The simplest and long-standing method of measuring protein and AA digestibility has been the oro-fecal balance method (5, 7). Although noninvasive, a major concern of the fecal digestibility measurements is the hindgut microbial modification of the undigested dietary nitrogen exiting the terminal ileum. As dietary nitrogen and AA absorption essentially occurs in the small intestine, the ileal digestibility, measured at the terminal ileum is considered to be a more accurate assay. The ileo-fecal differences in nitrogen (2–9%) and AA digestibility (0.4–15%) have been reported in monogastric animals (including humans) for highly digestible proteins (13, 14), and these differences were reported to be as high as 20% in rats for less digestible plant proteins possibly due to microbial fermentation of dietary fiber (15) and undigested AA during colonic transit (16).
The traditional assessment of digestibility is based on the measurements of total nitrogen and AA losses (endogenous and exogenous) in digesta and is termed “apparent” digestibility (9, 17, 18). Different methods have been used to measure endogenous losses; the advantages and limitations of these methods have been previously discussed (17, 19). When apparent digestibility is corrected for endogenous protein and AA losses measured by feeding with protein-free diets (20, 21) or by differentiating between endogenous and exogenous losses using intrinsically labeled proteins (22), it is termed “true” and “real” digestibility, respectively (Supplementary Material 1) (19). For simplicity, the term “true” is often used in place of “real” digestibility.
An additional concern in the assessment of dietary protein digestibility is the uncertainty associated with assuming overall protein (nitrogen) digestibility as a proxy for individual AA digestibility. A modest variation in ileal digestibility of IAAs has been reported in humans (14, 20, 22, 23) ranging from 89% (threonine) to 95% (lysine) with a nitrogen digestibility of 94% in soy protein isolate (23). Considerable differences have been observed in less digestible whole-plant protein sources, such as pea cultivars, where ileal digestibility of IAAs varied from 75% (tryptophan) to 89% (methionine) with a nitrogen digestibility of 76% in pigs (24), suggesting the need to measure the digestibility of each IAA to evaluate the quality of dietary protein.
Intrinsic labeling of dietary protein for measuring protein and AA digestibility in humans
Intrinsically labeled dietary proteins with stable isotopes have been used to measure true or real digestibility for the last 25 y. Intravenous, oral, or ruminal administration of single/multiple labeled free AAs or 15N ammonium sulphate have been used for labeling milk (25, 26), eggs (27), and meat (28), whereas deuterium oxide (2H2O) and 15N fertilizers (29–31), and more rarely 13CO2 (32), have been used to intrinsically label plant proteins (Table 1). For milk proteins, the intravenous administration of labeled AAs is more efficient compared with oral administration as it avoids losses due to fermentation in the rumen, feed refusals, and impaired absorption due to the feed matrix. However, due to the high cost, the protein is generally labeled with 1 (33) or 2 AAs (34). Another consideration relates to the type of tracer being used, which depends on the method (direct or indirect) of determining digestibility. For instance, 15N-labeled milk, bovine meat, and plant proteins (lupin, soybean, wheat, rapeseed, and pea) have been widely used to measure ileal nitrogen and AA digestibility using direct ileal balance methods in humans (23, 28, 30, 31, 35, 36). However, due to the exchange and loss of 15N during transamination of AAs (Supplementary Material 1), the use of this label has major limits especially for the indirect methods which measure plasma appearances of labeled AAs (25, 37).
TABLE 1.
Approaches used for intrinsic labeling of proteins to measure protein and AA digestibility in humans
| Proteins | Label | Form of label | Method of administration |
|---|---|---|---|
| Milk and meat from ruminants | 15N | 15N ammonium sulphate | • Perfusion in rumen (22, 23, 26, 28)• Oral administration of dose (23, 78) |
| 2H | 2H-labeled crystalline AAs | • Intravenous infusion in jugular vein (98) | |
| Deuterium oxide (2H2O) | • Oral administration of dose dissolved in water (38)• Feeding with 2H-labeled fodder (43) | ||
| 13C | 13C labeled crystalline AAs | • Intravenous infusion in jugular vein (87, 91, 99) | |
| Egg and chicken meat | 15N, 2H, and 13C | 15N-, 2H-, and 13C- labeled crystalline AAs | • Oral administration of dose dissolved in water (59)• Diets supplemented with labeled AAs (100–103) |
| Plant proteins | 15N | 15N ammonium nitrate, 15N potassium nitrate, 15N ammonium chloride | • Fertilization of soil with labeled salt (29, 35, 104)• Foliar spraying before flowering (23, 30, 80, 105) |
| 2H | Deuterium oxide (2H2O) | • Pulse dosing at flowering (37, 59) | |
| 13C | 13CO2 | • Atmospheric labeling (32, 45) |
AA, amino acid.
Goat milk protein has been previously labeled with 2H by administering 2H2O to a lactating goat (38). In this method, labeling occurs by the exchange between the hydrogen atoms at the α-H position of AAs with 2H from enriched body water during the transamination reaction, with a small quantity of 2H labeled AAs being synthesized de novo (39–42). Similar to 15N, AAs can lose the α-2H label during the same reaction, underestimating the absorbed 2H AAs (39). 2H-labeled milk and meat could also be produced by feeding with 2H-labeled fodders, obtained by watering fodder plants with 2H2O (43). Since plants are autotrophs and synthesize AAs de novo, this would lead to 2H labeling in multiple random positions of the AAs, including α-H (44). Although use of 2H2O enables efficient labeling of plant proteins, labeling milk using 2H-labeled fodder is cumbersome, expensive, and leads to lower enrichments in milk particularly for the limiting AAs in the plant.
The transamination losses of the 2H label can be addressed by using correction factors determined by controlled experiments (37) (Table 2, Supplementary Material 1). Transamination correction factors (FTCF) have also been proposed for 15N but experimental validation is necessary due to their high intraindividual variability and the estimates differed with the amount of protein in test meals (25). This could be attributed to the higher potential for exchange of 15N, owing to a smaller pool of amino nitrogen compared with hydrogen in the body water (25, 37). Therefore, the best isotopic label to use is 13C, as this is not modified by transamination or other metabolic processes, and intrinsic labeling could be achieved by oral administration of 13C-labeled AAs to animals, or by growing plants/algae in a 13CO2-enriched environment (32, 45). Although 13C labeling is feasible for animal proteins, this might not be the case for plant proteins, as it is difficult and expensive to obtain the high grain yields required for digestibility protocols (46). Overall, it is important to choose an appropriate tracer for intrinsic labeling of dietary proteins based on the method used for measuring digestibility with careful consideration of the labeled atom position to avoid possible label losses due to metabolic rearrangements.
TABLE 2.
Transamination correction factors for IAAs using 2H1
| IAA | 2H label |
|---|---|
| Methionine | 1.058 ± 0.005 |
| Phenylalanine | 1.053 ± 0.006 |
| Threonine | 1.016 ± 0.002 |
| Lysine | 1.002 ± 0.002 |
| Leucine | 1.081 ± 0.002 |
| Isoleucine | 1.070 ± 0.004 |
| Valine | 1.048 ± 0.003 |
IAA, indispensable amino acid.
Values are mean ± SD, n = 6; Reproduced with permission from Devi et al. (37).
Direct method for true ileal protein and AA digestibility: oro-ileal balance method
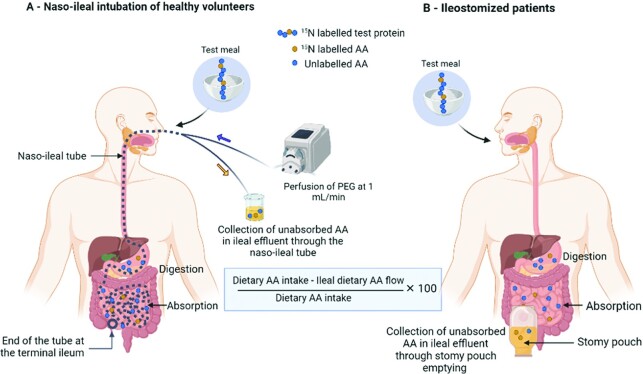
The classical and standard method for measuring true ileal protein and AA digestibility entails collection of the digesta at the terminal ileum during the postprandial period to determine the amount of undigested nitrogen or/and AA. In humans, this requires invasive procedures unless studied specifically in ileostomates (20). In healthy volunteers with an intact intestine, a radio-opaque, triple lumen tube is introduced through the nose and allowed to migrate through the intestine by peristaltic motility until the leading tip reaches the ileum (Figure 1). The location of the tube can be assessed through radiography. After test meal administration, ileal effluents are continuously collected for 8 h through one of the lumens. The infusion of a nonabsorbable marker (polyethylene glycol 4000, PEG-4000) into the intestine through another lumen of the tube allows determination of the total ileal effluent flow during this period. Combined with the administration of 15N intrinsically labeled dietary protein, this method provides accurate estimates of nitrogen or AA digestibility at the ileal level and has been in use for over 25 y. A PubMed search for related studies was conducted with key terms of “ileal digestibility” OR “ileal protein digestibility” OR “ileal amino acid digestibility” AND “human” and the digestibility coefficients of animal and plant proteins processed by different methods are provided in Supplementary Table 1. The true ileal digestibility of AAs ranged from 91% (glycine) to 99% (tyrosine) in milk protein, and from 89% (threonine) to 97% (tyrosine) in soy protein (23). The true ileal AA digestibility of unlabeled whey protein and zein was recently reported with low values for zein, ranging from 24% for cysteine to 64% for glutamine (21). Ileal digestibility of casein, whey protein, soy isolate, and concentrate, were also determined in ileostomates and were found to be high, varying from 97% to 100% (20).
FIGURE 1.
Schematic representation of the principle of the oro-ileal balance method to measure ileal AA digestibility with (A) intubated healthy volunteers or with (B) ileostomized patients using labeled protein. With the intubation method, the volunteers are equipped with a triple lumen naso-ileal tube. One lumen is dedicated to inflate a balloon at the end of the tube to facilitate the migration of the tube through the intestinal tract. When the tube is at the terminal ileum, a perfusion of PEG (unabsorbable marker) is started through the second lumen, in order to evaluate intestinal flow by the slow marker method. The test meal containing intrinsically 15N-labeled test protein undergoes digestion and absorption and ileal effluents containing nonabsorbed dietary AAs are continuously collected from the third lumen during the 8-h postprandial period. In ileostomates, where the colon and rectum have been partially or totally removed and the terminal ileum exteriorized, the ileal effluents are directly collected into the pouch. The digestibility of each AA is determined by the ratio of the absorbed AA to the intake. AA, amino acid; PEG, polyethylene glycol.
As summarized in Table 3, the procedure in ileostomates is relatively noninvasive, and is convenient for a crossover design; with the collection of total digesta, the use of nonabsorbable markers could be avoided. However, these patients might suffer from different gut disorders and their ileum will have morphological and microbiological modifications (47, 48); hence the measured digestibility values might differ from healthy volunteers. Additionally, the recruitment capacity of patients with a permanent ileostomy is limited. The naso-ileal intubation model is limited by the invasiveness of the procedure and a variable tolerance of the tube among subjects as well as interindividual variability for the tube migration. The placement of the tube could cause a concertinaing effect on the small intestine (49) which might confound the di- and tri-peptidase digestion and subsequent AA absorption, however, this needs to be confirmed. Use of nonabsorbable markers could introduce errors in measuring the ileal effluent flow rate (50). Lastly, the recycling of the isotope from test proteins in the endogenous protein leads to a minor underestimation (∼1%) of ileal nitrogen digestibility (50). Moreover, neither model is suitable for routine digestibility measurements in humans, particularly in vulnerable age groups and with pathophysiological conditions. However, long-term experience in the implementation of this technique allows for the minimization of bias and the provision of accurate and repeatable values.
TABLE 3.
Principle, strengths, and limitations of the AA digestibility and metabolic availability assays used in humans
| Methods | Principle of measurement and equation | Strength | Limitation | Reference |
|---|---|---|---|---|
| Direct balance | • Measures disappearance of ingested protein-derived AAs from intestinal lumen• Calculated as the difference between the amount of ingested dietary AAs and that recovered in the terminal ileum For intrinsically labeled test proteins: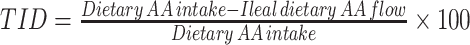 For unlabeled test proteins: For unlabeled test proteins: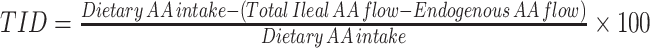 • Two models are adopted in humans: naso-ileal intubation and ileostomy model • Two models are adopted in humans: naso-ileal intubation and ileostomy model |
• Standard and direct method for measuring true ileal digestibility with repeatable results• Provides digestibility estimates of all AAs in a single trial• Ileostomy model is noninvasive and does not require a nonabsorbable marker to estimate flow rates of ileal effluents | • Naso-ileal intubation model is invasive, requires sampling of ileal digesta and not suitable for routine application in humans• Uncertainties around the recovery of the nonabsorbable markers in intubation model• Expensive assay• Ileostomy model cannot be employed for healthy humans• Errors associated with the measurement of endogenous AA losses if test proteins are not intrinsically labeled• Overestimates AA availability of heat-treated and chemically processed foods• Microbial colonization in terminal ileum of ileostomates could confound the digestibility estimates• Discounts colonic absorption of AAs, if any• Not applicable for routine measurements, vulnerable age groups, and pathological conditions with altered digestion and absorption | (6, 19, 23, 48, 50, 106–108) |
| Dual isotope tracer | • Measures appearance of ingested protein-derived AAs in systemic circulation• Compares appearance of labeled AAs in plasma from intrinsically labeled food proteins to that of a simultaneously ingested but differently labeled reference protein of known digestibility in relation to the meal administered• An example equation for 2H-labeled test protein and 13C-labeled reference protein: 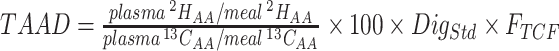
|
• Minimally invasive method requires blood collection• Provides digestibility estimates of almost all AAs in a single trial• Measures digestibility of proteins in habitually consumed meal preparations• Suitable for application in humans and vulnerable groups | • Expensive, as requires test and reference proteins to be intrinsically labeled• Indirect method. Digestibility of reference protein needs to be established in target population• Uncertainty introduced by the transamination/deamination reactions• Analytical complexity | (25, 37, 59, 107) |
| IAAO slope ratio | • Oxidation of a 13C-labeled IAA (indicator) is used as a surrogate for protein synthesis• Compares oxidation response slope of an indicator IAA to graded intakes of test IAA from test protein to that of reference crystalline AAs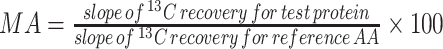 • Bioavailability of crystalline IAA assumed to be 100%• Test IAA must be the first limiting IAA in the diet• Oxidation rates of indicator must be linear to the graded intakes of test IAA• IAAO slopes for test protein and reference AAs must have a common origin at a fixed base intake of test IAA in crystalline form • Bioavailability of crystalline IAA assumed to be 100%• Test IAA must be the first limiting IAA in the diet• Oxidation rates of indicator must be linear to the graded intakes of test IAA• IAAO slopes for test protein and reference AAs must have a common origin at a fixed base intake of test IAA in crystalline form |
• Noninvasive, requires breath collection and rate of CO2 production• Analytically simple and relatively less expensive• Does not require test proteins to be intrinsically labeled, less expensive compared to other methods | • Repeated measures design with higher subject burden, difficult for routine application in vulnerable groups• Provides MA estimate of 1 IAA at a time• The interindividual variability of the MA estimates is not reported | (67, 70, 107) |
AA, amino acid; DigStd, digestibility of the reference protein; FTCF, transamination correction factor; IAA, indispensable amino acid; IAAO, indicator amino acid oxidation; MA, metabolic availability; TAAD, true amino acid digestibility; TID, true ileal digestibility.
Indirect methods for measuring AA digestibility
Dual isotope tracer method
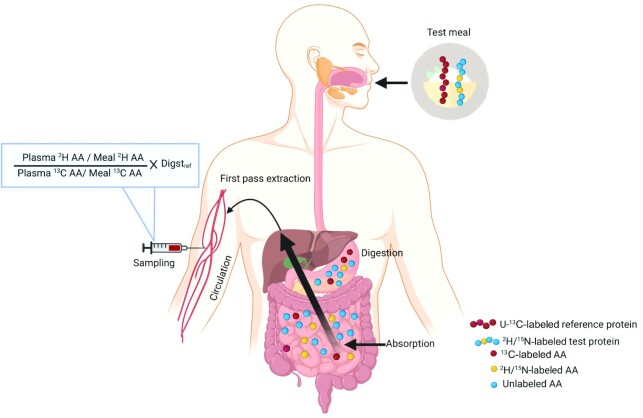
A minimally invasive dual isotope tracer technique to measure true ileal AA digestibility has been recently developed to overcome the invasiveness and complexities around collecting ileal digesta in direct methods (37). In this method, 2 differently intrinsically labeled proteins, a test protein (2H or 15N) and a reference protein (13C) of predetermined digestibility are simultaneously fed in a plateau feeding protocol. The ratio of plasma appearance of AAs from test to reference proteins at plateau with respect to the meal administered and corrected for AA digestibility of the reference protein allows determination of the true ileal AA digestibility of the test protein (Figure 2) (6, 37). Since the method measures plasma appearance of AAs to represent protein digestion and AA absorption throughout the small intestine, the term true AA digestibility (TAAD) will be used henceforth to differentiate from direct methods that measure digestibility at the terminal ileum. The method assumes that AAs from both test and reference proteins undergo similar first pass and splanchnic metabolism and enter a common pool after digestion and absorption; the ratio of test to standard AAs from this pool cancels out this uptake and metabolism. Another assumption of the method is the equivalent absorption of differently labeled AAs from the test and reference proteins, which is reasonable as stable isotopic differentiations are not known (51, 52). An advantage of using the plateau feeding protocol is the attainment of a steady isotopic plasma enrichment which negates the effect of slow or fast response proteins in the test meal that could influence the rate of AA metabolism (53). This pattern of feeding is not habitual and AA digestibility could differ when administered as a bolus meal as is habitually consumed. However, the reserve capacity of pancreatic proteases is much higher (∼10–15 times) than the volume required for protein digestion under normal physiological conditions, suggesting that the digestibility estimates might not drastically vary with different meal patterns (54, 55), although an effect on protein of low digestibility cannot be excluded and must be evaluated.
FIGURE 2.
Schematic representation of the principle of the dual isotope tracer method to measure small intestinal AA digestibility. The test meal containing intrinsically 2H/15N-labeled test protein (yellow and blue circles) and uniformly 13C-labeled reference protein (red circles) undergoes digestion. After absorption and first-pass splanchnic extraction, AAs from both the proteins enter the systemic circulation. The plasma appearance of individual AAs from 2H/15N-labeled test protein is compared with that of 13C-labeled reference protein of known digestibility with respect to the test meal to determine the small intestinal AA digestibility in the test proteins. AA, amino acid; Digstref, digestibility of reference protein.
The viable options of reference proteins could be U-13C-labeled proteins that are commercially available and commonly consumed, such as spirulina protein (Supplementary Table 1), which is well digested and the constituent AAs have a stable isotopic carbon backbone (37). Although animal source proteins could also serve as good reference proteins, the production of intrinsically labeled casein or egg or meat protein in high quantities will be expensive. The digestibility of reference protein can be determined against a free AA mix (2H or 15N labeled) which is considered to be representative of a completely digested protein (37). The use of U-2H is preferred over an 15N-labeled AA mixture, as the exchange of 15N during transamination does not allow tracing back to the original carbon skeleton of the AA. Although a U-13C-free AA mixture can also be used as a reference, a protein versus protein comparison for the test and reference has been preferred since the rates of absorption, metabolism, and utilization of AAs derived from peptides are different from those of free AAs (56, 57). Here too, the label must be carefully chosen as the phenylalanine digestibility of 15N-labeled spirulina was found to be 15% lower than [U]-13C-labeled spirulina (37, 58). Additionally, the TAAD of legume protein (mung bean) has been found to be similar with the use of either U-13C spirulina protein or U-13C free AAs as the reference, in healthy individuals (59) (Table 4) in a plateau feeding protocol, but this needs to be tested in a bolus feeding or a shorter repeated meal protocol. A mixture of free labeled AAs would also be a preferred choice for patients with impaired digestion and absorption functions such as those with cystic fibrosis, environmental enteropathy, and others (58, 60, 61). Another advantage is that the high digestibility of free AAs ensures a lower interindividual variability, as digestibility has an inverse association with variability, and thus provides more reliable reference values (62). The TAAD of U-13C spirulina had a mean IAA digestibility of ∼85% (37) and this has been used as a correction factor while measuring the digestibility of different test proteins in the same population. This might introduce an error when measuring protein digestibility in a different group of individuals, as the mean variability of IAA digestibility in spirulina was found to be ∼ 6% ranging from 3% for threonine and 12% for lysine (37). This could be addressed by measuring the reference protein digestibility in the same participant using a crossover design.
TABLE 4.
True AA digestibility of mung bean with spirulina and crystalline AAs as reference proteins using dual isotope tracer method in healthy adults1,2
| True AA digestibility (%) | ||
|---|---|---|
| IAA | MB3,4 | MB-13C AA3,4 |
| Methionine | 52.2 ± 7.2 | 48.7 ± 6.3 |
| Phenylalanine | 73.4 ± 6.3 | 74.6 ± 1.4 |
| Threonine | 42.5 ± 1.2 | 42.7 ± 3.2 |
| Lysine | 63.0 ± 5.4 | 69.3 ± 3.4 |
| Leucine | 67.5 ± 3.2 | 69.3 ± 5.0 |
| Iso-leucine | 75.8 ± 2.6 | 76.6 ± 5.0 |
| Valine | 67.8 ± 6.0 | 66.7 ± 5.1 |
| Mean IAA | 63.2 ± 1.5 | 64.0 ± 2.4 |
Values are mean ± SD; AA, amino acid; IAA, indispensable amino acid; MB, mung bean true AA digestibility referenced to spirulina protein (n = 6); MB-13C AA, mung bean small intestinal IAA digestibility referenced to standard 13C IAA mixture (n = 5).
Reproduced with permission from Kashyap et al. (59)
Paired t-test between MB compared with MB-13CAA (n = 5), no significant differences were observed in IAA digestibility between the groups.
No significant difference in true AA digestibility between MB compared with MB-13C AA.
A PubMed search was carried out to retrieve TAAD values of different proteins using keywords of “ileal AA digestibility” OR “ileal digestibility” AND “dual isotope tracer” AND “humans,” and is presented in Supplementary Table 1. High-quality proteins such as egg white, whole boiled egg, and chicken meat using the dual isotope method have reported digestibility estimates of 87%, 90%, and 92%, respectively (63), which were similar to digestibility estimates obtained by the direct ileal balance method (27, 28); however, plant protein digestibility values were lower than other studies (64, 65). This could be attributed to the differences in the test meal matrix between these experiments and type of processing of the test proteins (Supplementary Table 1). The interindividual variability of the digestibility estimates around the mean is reasonably small (overall CV <6%) when measured in apparently healthy humans. A major advantage of the dual isotope tracer method (Table 3) is enabling AA digestibility determination in habitually consumed diets in vulnerable populations of infants, children, pregnant women, and older adults, and in pathophysiological conditions.
Indicator AA oxidation slope ratio method
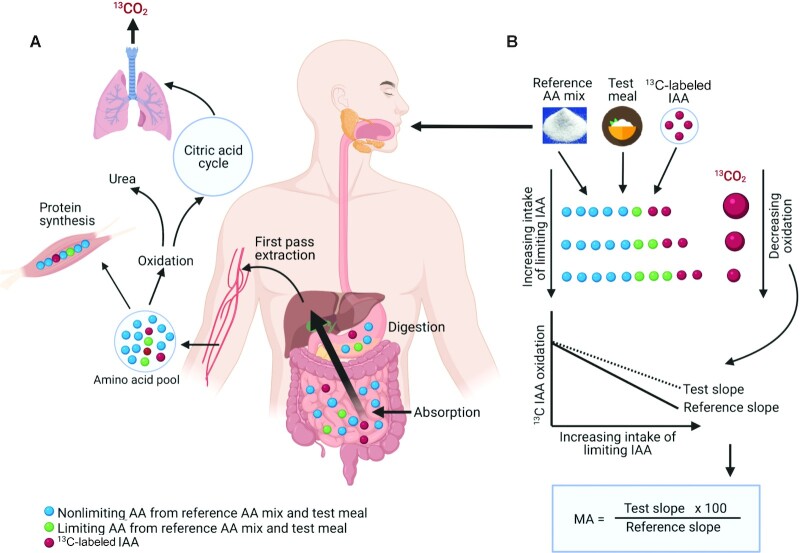
This method was developed using the principles of the traditional slope ratio growth assay in which growth and feed efficiency (by carcass weight) in animals have been measured to estimate bioavailability of an IAA in a dietary protein (66). Although the slope ratio growth assay is an absolute standard of estimating IAA bioavailability, the requirement for several groups of animals for bioavailability assessment of a single IAA, prolonged adaptation to a test IAA intake, and invasiveness precludes its routine application in humans (66). The indicator amino acid oxidation (IAAO) slope ratio method is a noninvasive adaptation of the growth assay, in which oxidation of an orally administered 13C-labeled “indicator” IAA is used as a proxy to assess the contribution of an unlabeled test dietary IAA to protein synthesis; the higher the oxidation of indicator IAA, the lower the protein synthesis (67). This method estimates the MA (includes digestibility, absorption, and utilization) of a single selected IAA in a dietary protein. It does so by comparing the oxidation response slopes of the labeled indicator IAA (usually 1-13C phenylalanine) to graded intakes of the selected test IAA from a test protein at subrequirement concentrations, to that of a reference crystalline IAA mixture (Figure 3). The IAAO slope ratio method has been validated against the growth assay with a mean difference of 4–7% for the MA estimates of lysine in differently processed pea proteins (68).
FIGURE 3.
Schematic representation of the principle and application of the IAAO method to estimate MA of limiting AAs in test proteins. (A) Represents the digestion, absorption, and metabolic handling of free or protein-derived unlabeled nonlimiting (blue circles), limiting/test (green circles), and 13C-labeled indicator IAA (red circles). (B) Subjects are provided with increasing intakes of limiting IAA (green circles) at the subrequirement concentration from a reference AA mixture or a combination of reference AA mixture and test protein with constant intake of 13C-labeled indicator IAA (red circles) across the study days in a repeated measures design. With increasing intake of limiting/test IAA (green circles), the incorporation of 13C-labeled indicator IAA (red circles) into tissue protein synthesis increases with the subsequent reduction in its oxidation, which is measured as 13CO2 in breath. The IAAO response slopes are obtained by measuring both reference AA mixture and test protein at the same concentrations of limiting/test IAA intakes. The MA of limiting/test IAA in a test protein is computed by comparing the estimated IAAO response slope of test protein to the estimated slope of reference AA mixture, which is assumed to have 100% MA. AA, amino acid; IAA, indispensable amino acid; IAAO, indicator amino acid oxidation; MA, metabolic availability.
A key condition of the method is that the IAAO response must be linearly and inversely related to the test IAA intake concentrations, which requires the intakes to be at subrequirement concentrations, preferably <60% of the requirements (6). Due to the known high variability of IAA requirements, of ∼25–40% (69), this critical condition of linearity could be challenging to satisfy, as has been observed previously, where the oxidation of indicator was reported to plateau, when it should not have, at subrequirement concentrations of test IAA intakes (70). Further, it has been argued that a highly digestible protein (casein and hydrolyzed casein) could be a better control, as food-derived peptides have been shown to influence gut protein metabolism, stimulate gut endogenous AA secretions, and are better absorbed than free AAs (56, 57, 71).
An advantage of the IAAO slope ratio method is that it is noninvasive and analytically simple, involving only the measurement of 13CO2 enrichment in noninvasive breath samples (Table 3). It does not require intrinsically labeled test proteins and is relatively cost-effective, particularly for mixed meals where all food protein sources in a mixed meal would need to be intrinsically labeled. The method provides reproducible results and is sensitive in the detection of postprocessing (heat treatment) reduction in MA as has been shown for lysine in peas, rice, and milk (68, 72–74). A PubMed search using key terms of “amino acid metabolic availability” OR “amino acid bioavailability” AND “indicator amino acid oxidation” AND “humans,” was performed to obtain MA of IAAs in different proteins, as measured in humans, and the results are presented in Supplementary Table 1. The method does not report interindividual variability of the MA estimates, however, the CV appears to range from 15% to 52% across the studies when the SE of the slope was considered (70, 72, 73, 75). Nevertheless, the method provides reasonable MA estimates for foods and as expected, has been found to be lower than true ileal IAA digestibility or TAAD values (23, 43, 59, 72–74), except for rice, which could be due to the differences in milling processes (73, 75, 76).
The IAAO slope ratio method provides MA of a single IAA through multiple experiments with 3–4 graded intakes of test IAA in a repeated measures study design, which requires significant time, subject compliance, and meticulous control of their body composition, physiological status, and dietary intakes for the study duration. Moreover, the method provides a measure of metabolic utilization of a dietary protein. With rigorous application, the noninvasive IAAO slope ratio method has the potential to determine MA of limiting IAAs of dietary proteins in habitually consumed meal preparations in humans.
Other methods of protein quality evaluation using stable isotopes
In addition to the direct and indirect methods detailed above, isotopic methods allow the determination of postprandial utilization of dietary proteins and their contribution to protein synthesis. The net postprandial protein utilization (NPPU) can be determined using intrinsically 15N-labeled dietary proteins, based on the difference between total ingested dietary protein corrected by real/true ileal protein digestibility, and dietary nitrogen transfer to ammonia/urea by deamination (Supplementary Material 1). Using double labeled 15N-13C eggs, metabolic loss of AAs was ∼18% as assessed by 13CO2 and 15N recovery for an 8-h period (77). Nevertheless, there are almost no studies using 13C-labeled protein, due to the labeling cost as mentioned above. The NPPU estimates have been determined for different protein sources in adults with adequate protein adaptation and ranged from 72 to 78% for high-quality proteins, such as milk (78, 79) or soy protein (80), to 63–66% for lower quality proteins such as wheat (31).
The intravenous infusion of an isotopically labeled AA also allows the determination of dietary postprandial protein utilization (PPU) (81). In this method, leucine oxidation is measured in the postabsorptive and postprandial phases to determine leucine balance in relation to ingested leucine and a continuous infusion of 1-13C leucine (81, 82) (Supplementary Material 1). Dietary protein utilization was calculated by converting leucine balance to nitrogen balance in relation to nitrogen intake (82, 83). Depending on the feeding protocol, the PPU of wheat protein was 61–68%, which was consistent with NPPU estimates. However, the PPU of milk (93–100%) was found to be considerably higher than the NPPU estimates (79, 82, 84). The approach has not been used to determine digestibility of dietary proteins due to the theoretical assumptions and uncertainties associated with splanchnic sequestration of labeled AA, tracer recycling, issues with measurement of precursor enrichment, and the assumed conversion factor of leucine to nitrogen content in tissue proteins (6).
Another method uses the combined ingestion of intrinsically labeled protein with a single AA (1-13C,15N or 2H5 phenylalanine, or 1-13C leucine) and an intravenous infusion of the same AA but differently labeled (2H5 phenylalanine, or 2H3 or 1-13C leucine) to measure endogenous and exogenous AA fluxes, and the contribution of exogenous AAs to whole-body and skeletal muscle protein synthesis (85). Postprandial protein handling and metabolism have been previously measured in high-quality animal and plant proteins (53, 86–91). The method can be used to assess the effect of protein source, protein load, exercise and meal timing on whole-body protein synthesis as well as muscle protein anabolism when associated with muscle biopsies (89, 92, 93). Although the technique could be used as a proxy for measuring protein quality, the use of a single intrinsically labeled test protein could underestimate the rate of plasma appearance of exogenous AAs due to the dilution and recycling of the tracer in the splanchnic bed and gastrointestinal tract (94). Additionally, in comparison to some of the other methods described in this review, it is invasive and not suitable for routine use.
Conclusion
The 2014 FAO expert consultation on protein quality evaluation recommended the use of DIAAS as the preferred metric for assessing the protein quality of individual foods and mixed diets (6). This requires the determination of individual AA digestibility at the ileal level which, until recently, was impossible to achieve without an invasive intubation or exteriorization of the intestine. However, together with the use of stable isotopes to intrinsically label dietary proteins, this is the only direct method that exists and has allowed accurate and precise digestibility estimates over the past 25 y and can be considered as a reference method for assessing protein and AA digestibility. Stable isotopes have been used as key tools in the field of protein and AA digestibility investigation and more largely protein quality, including in the development of minimal or noninvasive methods. The recently developed minimally invasive dual isotope tracer method can measure TAAD of almost all AAs in a single trial from dietary proteins in habitually consumed meal preparations in different age groups and shows promise to be applicable to vulnerable age groups and in those with pathophysiological conditions. However, the method is relatively new and needs to be validated against conventional assays which measure digestibility at the terminal ileum. The IAAO slope ratio method provides MA of one IAA at a time and demands significant time and compliance input from subjects in a crossover study design, which limits its use in vulnerable groups. Nevertheless, it is of interest due to its noninvasiveness and analytical simplicity.
Although efforts have been made to determine protein and AA digestibility of different foods, the agreement between the methods has not been rigorously evaluated by measuring the digestibility of the same protein source across methods. This is critical for harmonizing the digestibility values obtained from different methods considering the variable effect of the food matrix, of processing, and species-specific differences (particularly for plant proteins) on protein digestibility (95–97). As a start, the mean IAA and lysine digestibility of similarly processed whole-milk powders were found to be comparable across the methods (Supplementary Table 1), however, this needs to be expanded for other protein sources with low (<75%) and medium (<85%) digestibility. Until then, the values of true ileal protein and IAA digestibility/MA of different foods that are presented in Supplementary Table 1, could serve as a guide for field-level use. Further studies are also required to establish the acceptable limits of interlaboratory variability in true ileal protein and IAA digestibility/MA measurements of foods.
With the development of DIAAS, the re-evaluation and harmonization of these methods is important for supporting protein content claims of food labeling systems across countries. Moreover, efforts to determine the protein and AA digestibility in relevant food groups and combinations using minimally invasive methods need to be continued to build into the expanding database of DIAAS and to inform the agricultural supplementary nutrition programs and industrial regulatory frameworks and policies.
Supplementary Material
ACKNOWLEDGEMENTS
Figures were created with BioRender.com. The authors’ responsibilities were as follows—SB, SK, DT, CG, AVK, and JC: formulated the original draft of the manuscript; SB, SK, CG, JC, AVK, and DT: refined the manuscript with comments provided by the coauthors until submission; CG, AVK, DT, JC, SD, and DAM: gave comments and suggestions to improve the subsequent drafts before finalization; CG and AVK: have responsibility for the final content; and all authors: read and approved the final manuscript.
Notes
This work is sponsored by the Dutch Dairy Association (NZO) to UMR PNCA and funded by the Margdarshi Fellowship of the India Alliance to AVK. The funding agencies had no role in the analysis and drafting the manuscript.
Author disclosures: The authors report no conflicts of interest.
Supplemental Material 1 and Supplemental Table 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: AA, amino acid; DIAAS, Digestible Indispensable Amino Acid Score; IAA, indispensable amino acid; IAAO, indicator amino acid oxidation; MA, metabolic availability; NPPU, net postprandial protein utilization; PD-CAAS, Protein Digestibility Corrected Amino Acid Score; PPU, postprandial protein utilization; TAAD, true amino acid digestibility.
Contributor Information
Sulagna Bandyopadhyay, Division of Nutrition, St. John's Research Institute, St. John's National Academy of Health Sciences, Bangalore, India.
Sindhu Kashyap, Division of Nutrition, St. John's Research Institute, St. John's National Academy of Health Sciences, Bangalore, India.
Juliane Calvez, Université Paris-Saclay, AgroParisTech, INRAE (National Research Institute for Agriculture, Food, and Environment), UMR PNCA (Research Unit for Nutrition Physiology and Dietary Behavior), Paris, France.
Sarita Devi, Division of Nutrition, St. John's Research Institute, St. John's National Academy of Health Sciences, Bangalore, India.
Dalila Azzout-Marniche, Université Paris-Saclay, AgroParisTech, INRAE (National Research Institute for Agriculture, Food, and Environment), UMR PNCA (Research Unit for Nutrition Physiology and Dietary Behavior), Paris, France.
Daniel Tomé, Université Paris-Saclay, AgroParisTech, INRAE (National Research Institute for Agriculture, Food, and Environment), UMR PNCA (Research Unit for Nutrition Physiology and Dietary Behavior), Paris, France.
Anura V Kurpad, Division of Nutrition, St. John's Research Institute, St. John's National Academy of Health Sciences, Bangalore, India; Department of Physiology, St. John's Medical College, St. John's National Academy of Health Sciences, Bangalore, India.
Claire Gaudichon, Université Paris-Saclay, AgroParisTech, INRAE (National Research Institute for Agriculture, Food, and Environment), UMR PNCA (Research Unit for Nutrition Physiology and Dietary Behavior), Paris, France.
References
- 1. Wu G. Functional amino acids in growth, reproduction, and health. Adv Nutr. 2010;1(1):31–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tomé D. Criteria and markers for protein quality assessment – a review. Br J Nutr. 2012;108(S2):S222–9. [DOI] [PubMed] [Google Scholar]
- 3. Kurpad AV. Protein: quality and sources. Encyclopedia of Human Nutrition. Amsterdam, Netherlands: Elsevier Inc; 2012. p. 123–30. [Google Scholar]
- 4. FAO . Dietary protein quality evaluation in human nutrition: report of an FAO expert consultation. FAO Food and Nutrition Paper No. 92. Rome: FAO; 2013. [PubMed] [Google Scholar]
- 5. FAO/WHO . Protein quality evaluation in human diets. Report of a Joint FAO/WHO Expert Consultation (FAO Food Nutrition Paper No. 51). Rome (Italy): Food and Agriculture Organization of the United Nations; 1991. [Google Scholar]
- 6. FAO . Research Approaches and Methods for Evaluating the Protein Quality of Human Foods: Report of a FAO Expert Working Group. Rome (Italy): FAO; 2014. [DOI] [PubMed] [Google Scholar]
- 7. WHO/FAO/UNU Expert Consultation . Protein and amino acid requirements in human nutrition. Report of a Joint WHO/FAO/UNU Expert Consultation. WHO Technical Report Series, No 935. Geneva; 2007. [Google Scholar]
- 8. Rutherfurd SM, Fanning AC, Miller BJ, Moughan PJ. Protein digestibility-corrected amino acid scores and digestible indispensable amino acid scores differentially describe protein quality in growing male rats. J Nutr. 2015;145(2):372–9. [DOI] [PubMed] [Google Scholar]
- 9. Darragh AJ, Hodgkinson SM. Quantifying the digestibility of dietary protein. J Nutr. 2000;130(7):1850S–6S. [DOI] [PubMed] [Google Scholar]
- 10. Sarwar G. The protein digestibility-corrected amino acid score method overestimates quality of proteins containing antinutritional factors and of poorly digestible proteins supplemented with limiting amino acids in rats. J Nutr. 1997;127(5):758–64. [DOI] [PubMed] [Google Scholar]
- 11. Schaafsma G. The protein digestibility-corrected amino acid score. J Nutr. 2000;130(7):1865S–7S. [DOI] [PubMed] [Google Scholar]
- 12. Hendriks WH, van Baal J, Bosch G. Ileal and faecal protein digestibility measurement in humans and other non-ruminants – a comparative species view. Br J Nutr. 2012;108(S2):S247–57. [DOI] [PubMed] [Google Scholar]
- 13. Rowan AM, Moughan PJ, Wilson MN, Maher K, Tasman-Jones C. Comparison of the ileal and faecal digestibility of dietary amino acids in adult humans and evaluation of the pig as a model animal for digestion studies in man. Br J Nutr. 1994;71(1):29–42. [DOI] [PubMed] [Google Scholar]
- 14. Sauer WC, Mosenthin R, Ahrens F, Den Hartog LA. The effect of source of fiber on ileal and fecal amino acid digestibility and bacterial nitrogen excretion in growing pigs. J Anim Sci. 1991;69(10):4070–7. [DOI] [PubMed] [Google Scholar]
- 15. Davila AM, Blachier F, Gotteland M, Andriamihaja M, Benetti PH, Sanz Y, Tomé D. Re-print of “Intestinal luminal nitrogen metabolism: role of the gut microbiota and consequences for the host.”. Pharmacol Res. 2013;69(1):114–26. [DOI] [PubMed] [Google Scholar]
- 16. Moughan PJ, Souffrant WB, Hodgkinson SM. Physiological approaches to determining gut endogenous amino acid flows in the mammal. Arch Tierernahr. 1998;51(2–3):237–52. [DOI] [PubMed] [Google Scholar]
- 17. Miner-Williams W, Deglaire A, Benamouzig R, Fuller MF, Tomé D, Moughan PJ. Endogenous proteins in terminal ileal digesta of adult subjects fed a casein-based diet. Am J Clin Nutr. 2012;96(3):508–15. [DOI] [PubMed] [Google Scholar]
- 18. Stein HH, Sève B, Fuller MF, Moughan PJ, De Lange CF. Invited review: amino acid bioavailability and digestibility in pig feed ingredients: terminology and application. J Anim Sci. 2007;85(1):172–80. [DOI] [PubMed] [Google Scholar]
- 19. Fuller MF, Tomé D. In vivo determination of amino acid bioavailability in humans and model animals. J AOAC Int. 2005;88(3):923–34. [PubMed] [Google Scholar]
- 20. Moughan PJ, Butts CA, van Wijk H, Rowan AM, Reynolds GW. An acute ileal amino acid digestibility assay is a valid procedure for use in human ileostomates. J Nutr. 2005;135(3):404–9. [DOI] [PubMed] [Google Scholar]
- 21. Calvez J, Benoit S, Piedcoq J, Khodorova N, Azzout-Marniche D, Tomé D, Benamouzig R, Airinei G, Gaudichon C. Very low ileal nitrogen and amino acid digestibility of zein compared to whey protein isolate in healthy volunteers. Am J Clin Nutr. 2021;113(1):70–82. [DOI] [PubMed] [Google Scholar]
- 22. Deglaire A, Bos C, Tomé D, Moughan PJ. Ileal digestibility of dietary protein in the growing pig and adult human. Br J Nutr. 2009;102(12):1752–9. [DOI] [PubMed] [Google Scholar]
- 23. Gaudichon C, Bos C, Morens C, Petzke KJ, Mariotti F, Everwand J, Benamouzig R, Daré S, Tomé D, Metges CC. Ileal losses of nitrogen and amino acids in humans and their importance to the assessment of amino acid requirements. Gastroenterology. 2002;123(1):50–9. [DOI] [PubMed] [Google Scholar]
- 24. Hess V, Ganier P, Thibault JN, Seve B. Comparison of the isotope dilution method for determination of the ileal endogenous amino acid losses with labelled diet and labelled pigs. Br J Nutr. 2000;83(2):123–30. [PubMed] [Google Scholar]
- 25. van der Wielen N, Khodorova NV, Gerrits WJJ, Gaudichon C, Calvez J, Tomé D, Mensink M. Blood 15N:13C enrichment ratios are proportional to the ingested quantity of protein with the dual-tracer approach for determining amino acid bioavailability in humans. J Nutr. 2020;150(9):2346–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mahé S, Fauquant J, Gaudichon C, Roos N, Maubois JL, Tome D. 15 N-Labelling and preparation of milk, casein and whey proteins. Le Lait. 1994;74(4):307–12. [Google Scholar]
- 27. Evenepoel P, Geypens B, Luypaerts A, Hiele M, Ghoos Y, Rutgeerts P. Digestibility of cooked and raw egg protein in humans as assessed by stable isotope techniques. J Nutr. 1998;128(10):1716–22. [DOI] [PubMed] [Google Scholar]
- 28. Oberli M, Marsset-Baglieri A, Airinei G, Santé-Lhoutellier V, Khodorova N, Rémond D, Foucault-Simonin A, Piedcoq J, Tomé D, Fromentin G, Benamouzig R. High true ileal digestibility but not postprandial utilization of nitrogen from bovine meat protein in humans is moderately decreased by high-temperature, long-duration cooking. J Nutr. 2015;145(10):2221–8. [DOI] [PubMed] [Google Scholar]
- 29. Gausseres N, Mahe S, Benamouzig R, Luengo C, Ferriere F, Rautureau J, Tomé D. [15N]-labeled pea flour protein nitrogen exhibits good ileal digestibility and postprandial retention in humans. J Nutr. 1997;127(6):1160–5. [DOI] [PubMed] [Google Scholar]
- 30. Mariotti F, Pueyo ME, Tomé D, Mahé S. The bioavailability and postprandial utilisation of sweet lupin (Lupinus albus)-flour protein is similar to that of purified soyabean protein in human subjects: a study using intrinsically 15 N-labelled proteins. Br J Nutr. 2002;87(4):315–23. [DOI] [PubMed] [Google Scholar]
- 31. Bos C, Juillet B, Fouillet H, Turlan L, Daré S, Luengo C, N'tounda R, Benamouzig R, Gausserès N, Tomé D, Gaudichon C. Postprandial metabolic utilization of wheat protein in humans. Am J Clin Nutr. 2005;81(1):87–94. [DOI] [PubMed] [Google Scholar]
- 32. Edwards CA, Zavoshy R, Khanna S, Slater C, Morrison DJ, Preston T, Weaver LT. Production of 13C labelled pea flour for use in human digestion and fermentation studies. Isotopes Environ Health Stud. 2002;38(3):139–47. [DOI] [PubMed] [Google Scholar]
- 33. Gorissen SH, Trommelen J, Kouw IW, Holwerda AM, Pennings B, Groen BB, Wall BT, Churchward-Venne TA, Horstman AM, Koopman R, Burd NA. Protein type, protein dose, and age modulate dietary protein digestion and phenylalanine absorption kinetics and plasma phenylalanine availability in humans. J Nutr. 2020;150(8):2041–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Holwerda AM, Trommelen J, Kouw IWK, Senden JM, Goessens JPB, van Kranenburg J, Gijsen AP, Verdijk LB, van Loon LJC. Exercise plus presleep protein ingestion increases overnight muscle connective tissue protein synthesis rates in healthy older men. Int J Sport Nutr Exerc Metab. 2021;31(3):217–26. [DOI] [PubMed] [Google Scholar]
- 35. Bos C, Airinei G, Mariotti F, Benamouzig R, Bérot S, Evrard J, Fénart E, Tomé D, Gaudichon C. The poor digestibility of rapeseed protein is balanced by its very high metabolic utilization in humans. J Nutr. 2007;137(3):594–600. [DOI] [PubMed] [Google Scholar]
- 36. Fouillet H, Mariotti F, Gaudichon C, Bos C, Tomé D. Peripheral and splanchnic metabolism of dietary nitrogen are differently affected by the protein source in humans as assessed by compartmental modeling. J Nutr. 2002;132(1):125–33. [DOI] [PubMed] [Google Scholar]
- 37. Devi S, Varkey A, Sheshshayee MS, Preston T, Kurpad AV. Measurement of protein digestibility in humans by a dual-tracer method. Am J Clin Nutr. 2018;107(6):984–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tessier R, Khodorova N, Calvez J, Kapel R, Quinsac A, Piedcoq J, Tomé D, Gaudichon C. 15N and ²H intrinsic labeling demonstrate that real digestibility in rats of proteins and amino acids from sunflower protein isolate is almost as high as that of goat whey. J Nutr. 2020;150(3):450–7. [DOI] [PubMed] [Google Scholar]
- 39. Commerford SL, Carsten AL, Cronkite EP. The distribution of tritium among the amino acids of proteins obtained from mice exposed to tritiated water. Radiat Res. 1983;94(1):151–5. [PubMed] [Google Scholar]
- 40. Atasoglu C, Valdés C, Walker ND, Newbold CJ, Wallace RJ. De novo synthesis of amino acids by the ruminal bacteria Prevotella bryantii B14, Selenomonas ruminantium HD4, and Streptococcus bovis ES1. Appl Environ Microbiol. 1998;64(8):2836–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Atasoglu C, Valdés C, Newbold CJ, Wallace RJ. Influence of peptides and amino acids on fermentation rate and de novo synthesis of amino acids by mixed micro-organisms from the sheep rumen. Br J Nutr. 1999;81(4):307–14. [PubMed] [Google Scholar]
- 42. Hungate RE. The Rumen and its Microbes. Amsterdam, Netherlands: Elsevier; 2013. [Google Scholar]
- 43. Kashyap S, Shivakumar N, Sejian V, Deutz NE, Preston T, Sreeman S, Devi S, Kurpad AV. Goat milk protein digestibility in relation to intestinal function. Am J Clin Nutr. 2021;113(4):845–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Evans BR, Shah R. Development of approaches for deuterium incorporation in plants. In: Kelman Z(editor). Methods in Enzymology. Amsterdam, Netherlands: Elsevier; 2015. Vol. 565, pp. 213–43. [DOI] [PubMed] [Google Scholar]
- 45. Berthold HK, Hachey DL, Reeds PJ, Thomas OP, Hoeksema S, Klein PD. Uniformly 13C-labeled algal protein used to determine amino acid essentiality in vivo. Proc Natl Acad Sci. 1991;88(18):8091–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Grusak MA. Intrinsic stable isotope labeling of plants for nutritional investigations in humans. J Nutr Biochem. 1997;8(4):164–71. [Google Scholar]
- 47. Fuller M. Determination of protein and amino acid digestibility in foods including implications of gut microbial amino acid synthesis. Br J Nutr. 2012;108(S2):S238–46. [DOI] [PubMed] [Google Scholar]
- 48. Fuller MF, Milne A, Harris CI, Reid TM, Keenan R. Amino acid losses in ileostomy fluid on a protein-free diet. Am J Clin Nutr. 1994;59(1):70–3. [DOI] [PubMed] [Google Scholar]
- 49. Cook GC, Carruthers RH. Reaction of human small intestine to an intraluminal tube and its importance in jejunal perfusion studies. Gut. 1974;15(7):545–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Deglaire A. Gut endogenous protein flows and postprandial metabolic utilization of dietary amino acids in simple-stomached animals and humans (Doctoral dissertation, agroparistech). 2008. [Google Scholar]
- 51. Krempf M, Hoerr RA, Marks L, Young VR. Phenylalanine flux in adult men: estimates with different tracers and route of administration. Metabolism. 1990;39(6):560–2. [DOI] [PubMed] [Google Scholar]
- 52. Marchini JS, Castillo L, Chapman TE, Vogt JA, Ajami A, Young VR. Phenylalanine conversion to tyrosine: comparative determination with l-[ring-2H5]phenylalanine and l-[1-13C]phenylalanine as tracers in man. Metabolism. 1993;42(10):1316–22. [DOI] [PubMed] [Google Scholar]
- 53. Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL, Beaufrère B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci. 1997;94(26):14930–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Keller J. Gastrointestinal digestion and absorption. In: Lennarz WJ, Lane MD (editors). Encyclopedia of Biological Chemistry. Amsterdam, Netherlands: Elsevier; 2013. pp. 354–9. [Google Scholar]
- 55. el-Khoury AE, Sánchez M, Fukagawa NK, Gleason RE, Tsay RH, Young VR. The 24-h kinetics of leucine oxidation in healthy adults receiving a generous leucine intake via three discrete meals. Am J Clin Nutr. 1995;62(3):579–90. [DOI] [PubMed] [Google Scholar]
- 56. Matthews DM, Adibi SA. Peptide absorption. Gastroenterology. 1976;71(1):151–61. [PubMed] [Google Scholar]
- 57. Moughan PJ, Fuller MF, Han KS, Kies AK, Miner-Williams W. Food-derived bioactive peptides influence gut function. Int J Sport Nutr Exerc Metab. 2007;17(s1):S5–S22. [DOI] [PubMed] [Google Scholar]
- 58. Engelen MP, Com G, Anderson PJ, Deutz NE. New stable isotope method to measure protein digestibility and response to pancreatic enzyme intake in cystic fibrosis. Clin Nutr. 2014;33(6):1024–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kashyap S, Varkey A, Shivakumar N, Devi S, Reddy BHR, Thomas T, Preston T, Sreeman S, Kurpad AV. True ileal digestibility of legumes determined by dual-isotope tracer method in Indian adults. Am J Clin Nutr. 2019;110(4):873–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Baker SJ. Subclinical intestinal malabsorption in developing countries. Bull World Health Organ. 1976;54(5):485–94. [PMC free article] [PubMed] [Google Scholar]
- 61. Swaminathan N, Mathan VI, Baker SJ, Radhakrishnan AN. Disaccharidase levels in jejunal biopsy specimens from American and South Indian control subjects and patients with tropical sprue. Clin Chim Acta. 1970;30(3):707–12. [DOI] [PubMed] [Google Scholar]
- 62. Gaudichon C, Calvez J. Determinants of amino acid bioavailability from ingested protein in relation to gut health. Current Opinion in Clinical Nutrition & Metabolic Care. 2021;24(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kashyap S, Shivakumar N, Varkey A, Duraisamy R, Thomas T, Preston T, Devi S, Kurpad AV. Ileal digestibility of intrinsically labeled hen's egg and meat protein determined with the dual stable isotope tracer method in Indian adults. Am J Clin Nutr. 2018;108(5):980–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rutherfurd SM, Bains K, Moughan PJ. Available lysine and digestible amino acid contents of proteinaceous foods of India. Br J Nutr. 2012;108(S2):S59–68. [DOI] [PubMed] [Google Scholar]
- 65. Yin YL, Li TJ, Huang RL, Liu ZQ, Kong XF, Chu WY, Tan BE, Deng D, Kang P, Yin FG. Evaluating standardized ileal digestibility of amino acids in growing pigs. Anim Feed Sci Technol. 2008;140(3–4):385–401. [Google Scholar]
- 66. Batterham ES. Availability and utilization of amino acids for growing pigs. Nutr Res Rev. 1992;5(1):1–18. [DOI] [PubMed] [Google Scholar]
- 67. Elango R, Levesque C, Ball RO, Pencharz PB. Available versus digestible amino acids – new stable isotope methods. Br J Nutr. 2012;108(S2):S306–14. [DOI] [PubMed] [Google Scholar]
- 68. Moehn S, Bertolo RF, Pencharz PB, Ball RO. Development of the indicator amino acid oxidation technique to determine the availability of amino acids from dietary protein in pigs. J Nutr. 2005;135(12):2866–70. [DOI] [PubMed] [Google Scholar]
- 69. Elango R, Humayun MA, Ball RO, Pencharz PB. Indicator amino acid oxidation is not affected by period of adaptation to a wide range of lysine intake in healthy young men. J Nutr. 2009;139(6):1082–7. [DOI] [PubMed] [Google Scholar]
- 70. Rafii M, Elango R, Ball RO, Pencharz PB, Courtney-Martin G. Metabolic availability of the limiting amino acids lysine and tryptophan in cooked white African cornmeal assessed in healthy young men using the indicator amino acid oxidation technique. J Nutr. 2018;148(6):917–24. [DOI] [PubMed] [Google Scholar]
- 71. Butts CA, Moughan PJ, Smith WC, Reynolds GW, Garrick DJ. The effect of food dry matter intake on endogenous ileal amino acid excretion determined under peptide alimentation in the 50 kg liveweight pig. J Sci Food Agric. 1993;62(3):235–43. [Google Scholar]
- 72. Humayun MA, Elango R, Moehn S, Ball RO, Pencharz PB. Application of the indicator amino acid oxidation technique for the determination of metabolic availability of sulfur amino acids from casein versus soy protein isolate in adult men. J Nutr. 2007;137(8):1874–9. [DOI] [PubMed] [Google Scholar]
- 73. Bandyopadhyay S, Kuriyan R, Shivakumar N, Ghosh S, Ananthan R, Devi S, Kurpad AV. Metabolic availability of lysine in milk and a vegetarian cereal-legume meal determined by the indicator amino acid oxidation method in Indian men. J Nutr. 2020;150(10):2748–54. [DOI] [PubMed] [Google Scholar]
- 74. Rafii M, Pencharz PB, Ball RO, Tomlinson C, Elango R, Courtney-Martin G. Bioavailable methionine assessed using the indicator amino acid oxidation method is greater when cooked chickpeas and steamed rice are combined in healthy young men. J Nutr. 2020;150(7):1834–44. [DOI] [PubMed] [Google Scholar]
- 75. Prolla IR, Rafii M, Courtney-Martin G, Elango R, da Silva LP, Ball RO, Pencharz PB. Lysine from cooked white rice consumed by healthy young men is highly metabolically available when assessed using the indicator amino acid oxidation technique. J Nutr. 2013;143(3):302–6. [DOI] [PubMed] [Google Scholar]
- 76. Shivakumar N, Kashyap S, Kishore S, Thomas T, Varkey A, Devi S, Preston T, Jahoor F, Sheshshayee MS, Kurpad AV. Protein-quality evaluation of complementary foods in Indian children. Am J Clin Nutr. 2019;109(5):1319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Fromentin C, Tomé D, Nau F, Flet L, Luengo C, Azzout-Marniche D, Sanders P, Fromentin G, Gaudichon C. Dietary proteins contribute little to glucose production, even under optimal gluconeogenic conditions in healthy humans. Diabetes. 2013;62(5):1435–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gaudichon C, Mahé S, Benamouzig R, Luengo C, Fouillet H, Daré S, Van Oycke M, Ferrière F, Rautureau J, Tomé D. Net postprandial utilization of [15N]-labeled milk protein nitrogen is influenced by diet composition in humans. J Nutr. 1999;129(4):890–5. [DOI] [PubMed] [Google Scholar]
- 79. Bos C, Mahé S, Gaudichon C, Benamouzig R, Gausserès N, Luengo C, Ferrière F, Rautureau J, Tomé D. Assessment of net postprandial protein utilization of 15 N-labelled milk nitrogen in human subjects. Br J Nutr. 1999;81(3):221–6. [PubMed] [Google Scholar]
- 80. Mariotti F, Mahe S, Benamouzig R, Luengo C, Daré S, Gaudichon C, Tomé D. Nutritional value of [15N]-soy protein isolate assessed from ileal digestibility and postprandial protein utilization in humans. J Nutr. 1999;129(11):1992–7. [DOI] [PubMed] [Google Scholar]
- 81. Millward DJ, Pacy PJ. Postprandial protein utilization and protein quality assessment in man. Clin Sci. 1995;88(6):597–606. [DOI] [PubMed] [Google Scholar]
- 82. Millward DJ, Fereday A, Gibson NR, Cox MC, Pacy PJ. Efficiency of utilization of wheat and milk protein in healthy adults and apparent lysine requirements determined by a single-meal [1-13C] leucine balance protocol. Am J Clin Nutr. 2002;76(6):1326–34. [DOI] [PubMed] [Google Scholar]
- 83. Millward DJ. An adaptive metabolic demand model for protein and amino acid requirements. Br J Nutr. 2003;90(2):249–60. [DOI] [PubMed] [Google Scholar]
- 84. Millward DJ, Fereday A, Gibson NR, Pacy PJ. Human adult amino acid requirements: [1-13C] leucine balance evaluation of the efficiency of utilization and apparent requirements for wheat protein and lysine compared with those for milk protein in healthy adults. Am J Clin Nutr. 2000;72(1):112–21. [DOI] [PubMed] [Google Scholar]
- 85. Trommelen J, van Loon LJ. Assessing the whole-body protein synthetic response to feeding in vivo in human subjects. Proc Nutr Soc. 2021;80(2):139–47. [DOI] [PubMed] [Google Scholar]
- 86. Gorissen SH, Horstman AM, Franssen R, Kouw IW, Wall BT, Burd NA, De Groot LC, van Loon LJ. Habituation to low or high protein intake does not modulate basal or postprandial muscle protein synthesis rates: a randomized trial. Am J Clin Nutr. 2017;105(2):332–42. [DOI] [PubMed] [Google Scholar]
- 87. Pennings B, Pellikaan WF, Senden JM, van Vuuren AM, Sikkema J, van Loon LJ. The production of intrinsically labeled milk and meat protein is feasible and provides functional tools for human nutrition research. J Dairy Sci. 2011;94(9):4366–73. [DOI] [PubMed] [Google Scholar]
- 88. Reitelseder S, Tranberg B, Agergaard J, Dideriksen K, Højfeldt G, Merry ME, Storm AC, Poulsen KR, Hansen ET, van Hall G, Lund P. Phenylalanine stable isotope tracer labeling of cow milk and meat and human experimental applications to study dietary protein-derived amino acid availability. Clin Nutr. 2020;39(12):3652–62. [DOI] [PubMed] [Google Scholar]
- 89. Tang JE, Moore DR, Kujbida GW, Tarnopolsky MA, Phillips SM. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J Appl Physiol. 2009;107(3):987–92. [DOI] [PubMed] [Google Scholar]
- 90. Van Vliet S, Shy EL, Abou Sawan S, Beals JW, West DW, Skinner SK, Ulanov AV, Li Z, Paluska SA, Parsons CM, Moore DR. Consumption of whole eggs promotes greater stimulation of postexercise muscle protein synthesis than consumption of isonitrogenous amounts of egg whites in young men. Am J Clin Nutr. 2017;106(6):1401–12. [DOI] [PubMed] [Google Scholar]
- 91. van Loon LJ, Boirie Y, Gijsen AP, Fauquant J, De Roos AL, Kies AK, Lemosquet S, Saris WH, Koopman R. The production of intrinsically labeled milk protein provides a functional tool for human nutrition research. J Dairy Sci. 2009;92(10):4812–22. [DOI] [PubMed] [Google Scholar]
- 92. Churchward-Venne TA, Snijders T, Linkens AM, Hamer HM, van Kranenburg J, van Loon LJ. Ingestion of casein in a milk matrix modulates dietary protein digestion and absorption kinetics but does not modulate postprandial muscle protein synthesis in older men. J Nutr. 2015;145(7):1438–45. [DOI] [PubMed] [Google Scholar]
- 93. Koopman R, Crombach N, Gijsen AP, Walrand S, Fauquant J, Kies AK, Lemosquet S, Saris WH, Boirie Y, van Loon LJ. Ingestion of a protein hydrolysate is accompanied by an accelerated in vivo digestion and absorption rate when compared with its intact protein. Am J Clin Nutr. 2009;90(1):106–15. [DOI] [PubMed] [Google Scholar]
- 94. Wolfe RR, Park S, Kim IY, Starck C, Marquis BJ, Ferrando AA, Moughan PJ. Quantifying the contribution of dietary protein to whole body protein kinetics: examination of the intrinsically labeled proteins method. American Journal of Physiology-Endocrinology and Metabolism. 2019;317(1):E74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Melito C, Tovar J. Cell walls limit in vitro protein digestibility in processed legume seeds. Food Chem. 1995;53(3):305–7. [Google Scholar]
- 96. Zahir M, Fogliano V, Capuano E. Food matrix and processing modulate in vitro protein digestibility in soybeans. Food Func. 2018;9(12):6326–36. [DOI] [PubMed] [Google Scholar]
- 97. Drulyte D, Orlien V. The effect of processing on digestion of legume proteins. Foods. 2019;8(6):224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Dangin M, Guillet C, Garcia-Rodenas C, Gachon P, Bouteloup-Demange C, Reiffers-Magnani K, Fauquant J, Ballèvre O, Beaufrère B. The rate of protein digestion affects protein gain differently during aging in humans. J Physiol. 2003;549(2):635–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Boirie Y, Fauquant J, Rulquin H, Maubois JL, Beaufrère B. Production of large amounts of [13C] leucine-enriched milk proteins by lactating cows. J Nutr. 1995;125(1):92–8. [DOI] [PubMed] [Google Scholar]
- 100. McCue MD, Arquisola B, Albach E, Pollock ED. Hens produce artificially enriched 13C egg proteins for metabolic tracer studies. International Journal of Biology. 2013;5(2):69–84. [Google Scholar]
- 101. Fromentin C, Sanders P, Nau F, Anton M, Fromentin G, Tomé D, Thibault JN, Gaudichon C. A pilot study for the intrinsic labeling of egg proteins with 15N and 13C. Rapid Commun Mass Spectrom. 2012;26(1):43–8. [DOI] [PubMed] [Google Scholar]
- 102. Evenepoel P, Hiele M, Luypaerts A, Geypens B, Buyse J, Decuypere E, Rutgeerts P, Ghoos Y. Production of egg proteins, enriched with L-leucine-13C1, for the study of protein assimilation in humans using the breath test technique. J Nutr. 1997;127(2):327–31. [DOI] [PubMed] [Google Scholar]
- 103. van Vliet S, Beals JW, Parel JT, Hanna CD, Utterback PL, Dilger AC, Ulanov AV, Li Z, Paluska SA, Moore DRet al. Development of intrinsically labeled eggs and poultry meat for use in human metabolic research. J Nutr. 2016;146(7):1428–33. [DOI] [PubMed] [Google Scholar]
- 104. Gausserès N, Mahé S, Benamouzig R, Luengo C, Drouet H, Rautureau J, Tomé D. The gastro-ileal digestion of 15N-labelled pea nitrogen in adult humans. Br J Nutr. 1996;76(1):75–85. [DOI] [PubMed] [Google Scholar]
- 105. Mariotti F, Pueyo ME, Tome D, Berot S, Benamouzig R, Mahe S. The influence of the albumin fraction on the bioavailability and postprandial utilization of pea protein given selectively to humans. J Nutr. 2001;131(6):1706–13. [DOI] [PubMed] [Google Scholar]
- 106. Rutherfurd SM, Moughan PJ. Available versus digestible dietary amino acids. Br J Nutr. 2012;108(S2):S298–305. [DOI] [PubMed] [Google Scholar]
- 107. Moughan PJ, Wolfe RR. Determination of dietary amino acid digestibility in humans. J Nutr. 2019;149(12):2101–9. [DOI] [PubMed] [Google Scholar]
- 108. Columbus D, de Lange CF. Evidence for validity of ileal digestibility coefficients in monogastrics. Br J Nutr. 2012;108(S2):S264–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.