Abstract
Hematopoietic stem cell (HSC) regeneration is the remarkable process by which extremely rare, normally inactive cells of the bone marrow can replace an entire organ if called to do so by injury or harnessed by transplantation. HSC research is arguably the first quantitative single-cell science and the foundation of adult stem cell biology. Bone marrow transplant is the oldest and most refined technique of regenerative medicine. Here we review the intertwined history of the discovery of HSCs and bone marrow transplant, the molecular and cellular mechanisms of HSC self-renewal, and the use of HSCs and their derivatives for cell therapy.
DISCOVERY OF HSCs AND THE BIRTH OF BONE MARROW TRANSPLANT
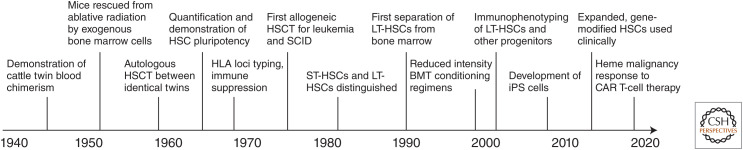
As a free-flowing liquid, blood is unlike any other organ in the body. So while the invention of microscopy provided histological insights into the identification of adult stem cells of many tissues based on their defined spatial relationships and morphological characteristics (such as the basal layer of epidermis or crypts of intestinal villi), the complex array of blood-forming cells in bone marrow prohibited identification of hematopoietic stem cells (HSCs) by direct visualization. Instead, discovery of HSCs in the absence of morphological or other direct descriptors required the dawn of atomic science, cell transplantation, and clonogenic assays to functionally identify multipotent cells based on their progeny (Fig. 1).
Figure 1.
Timeline of hematopoietic stem cell (HSC) and blood marrow transplant (BMT) discoveries. (HSCT) Hematopoietic stem cell transplant, (SCID) severe combined immunodeficiency, (ST-HSC) short-term HSC, (LT-HSC) long-term HSC, (iPS) induced pluripotent stem cells, (HLA) human leukocyte antigen, (CAR) chimeric antigen receptor.
The concept of a multipotent HSC in the bone marrow giving rise to all blood lineages had been hypothesized as early as the 1800s by others including Franz Neumann and Alexander Maximow. However, Owen (1945) provided the first experimental evidence for such a claim by showing that fraternal twin cattle sharing a placenta maintained lifelong presence of both siblings’ blood types. Soon afterward, the advent of nuclear radiation and lethal bone marrow failure experienced by victims of radiation toxicity inspired the first experiments showing that intravenous transplants of normal adult bone marrow cells could protect mice from otherwise lethal radiation doses by providing an alternative and sustainable source of blood cells (Jacobson et al. 1951; Lorenz et al. 1951; Ford et al. 1956). The chimeric blood composition of the mice engrafted in these experiments suggested that HSCs could be quantified by measuring their progeny. McCulloch and Till (1960) and Till and McCulloch (1961) performed the first HSC-limiting dilution assays in which defined numbers of adult mouse bone marrow donor cells were injected and linearly correlated to the number of cells required to protect recipients from radiation and the number of large spleen nodules that formed in recipients 2 weeks later. These spleen nodules represented the first colony-forming units (CFU-S) and were demonstrated to be true genetic clonal derivatives of individual HSCs (Becker et al. 1963); to contain mixtures of all blood cell types including erythroid, megakaryocytic, granulocyte/macrophage (Wu et al. 1967), and lymphoid lineages (Wu et al. 1968), as well as daughter cells capable of recapitulation of the colony in secondary transplant recipients (Siminovitch et al. 1963); and to have highly stochastically variable lineage and maturity content between HSC clones (Till et al. 1964). Together, these experiments proved that HSCs were capable of self-renewal and differentiation into all blood cell lineages and established HSC research as the first quantitative single-cell science.
Basic HSC research using bone marrow transplants in mice inspired clinical progress in human bone marrow transplants (or hematopoietic stem cell transplant [HSCT]) shortly after. The first unsuccessful bone marrow transplant attempted was likely a patient with aplastic anemia who received a small amount of intravenous marrow from a sibling (Osgood et al. 1939). But the technique was not formally developed until the advances in mice of the 1950s led to the first successful engraftment of bone marrow transplants from identical twin donors for patients with refractory leukemia (Thomas et al. 1957, 1959). These first bone marrow transplants followed the typical operating characteristics that would be expected from genetically identical donor grafts; they gave prompt cell line recovery after radiation (no graft failure), and did not cause the secondary wasting syndrome that had been first described previously in mice receiving bone marrow grafts from unrelated donors—graft-versus-host disease (GVHD) (Trentin 1956)—but also gave little graft-versus-leukemia (GVL) effect in that recipients relapsed a few months posttransplant. In contrast, the first transplants between genetically nonidentical but immunologically compatible siblings (allogeneic HSCT) could give durable leukemia treatment responses for years (Thomas et al. 1975, 1977, 1979) but occurred much later as they awaited the discovery and methods to type the human leukocyte antigen (HLA) system of genes on chromosome 6 governing immunologic rejection between donor and graft (van Rood et al. 1968), extensive experiments with canine sibling transplants (Storb and Thomas 1972), and the development of immunosuppression (Santos and Owens 1969; Storb et al. 1970; Kolb et al. 1973). The first successful human bone marrow transplants were major breakthroughs in hematology and immunology, and gave hope to patients with leukemias and other blood disorders.
The first bone marrow transplants used heterogenous mixtures of cells from bone marrow that included and proved the existence of HSCs in animals and humans. However, research to isolate and identify the specific HSC cell population continued. Whereas initially the cells capable of producing spleen colonies were taken to be identical to HSCs, early experiments suggested that there was greater heterogeneity in CFU-S than previously thought. It was first observed that spleen colonies appeared and disappeared rapidly after transplant and that later day 14 colonies were not formed from the first day 7 colonies (Hodgson and Bradley 1979; Magli et al. 1982). The different temporal origins of spleen colonies suggested heterogeneity in the underlying transplanted cells, and opened the question of whether cells capable of long-term hematopoiesis in the bone marrow were distinct from CFU-S and cells giving immediate radioprotection. It was later confirmed by cell centrifugation and separation experiments that HSCs with long-term repopulating ability (long-term repopulating cells [LTRCs]/LT-HSCs) could be physically separated from less primitive progenitor cells, such as CFU-S and lineage-restricted progenitors giving rise to granulocytes and macrophages (CFU-GM) (Jones et al. 1990). Physically larger CFU-S and CFU-GM were responsible for the early engraftment and hematopoiesis required to save animals from the initial aplasia of radiation injury, while long-term engraftment was achieved by LTRCs that were smaller and morphologically lymphocyte-like. These LTRCs among total adult bone marrow cells were very rare (1/10,000), as quantified in competitive repopulating unit (CRU) assays developed contemporaneously (Szilvassy et al. 1990). The field was further revolutionized by the first experiments to sort HSCs using flow cytometry and fluorescence-activated cell sorting (FACS) (Spangrude et al. 1988). The use of centrifugal cell separation and immunophenotyping marked a new era of hematopoietic research in which HSCs and other blood cell lineages were prospectively sorted using defined physical markers. LTRCs could be further defined and purified through cell surface immunophenotypes such as Lin−CKit+Sca1+CD135−CD34Lo/− CD150+ in mouse or Lin−CD34+CD38−CD90+ CD45RA− in human, or by Rhodamine-123 and Hoechst33342 dye efflux side population experiments as Rho−SP+ (Baum et al. 1992; Osawa et al. 1996; Kiel et al. 2005; Dykstra et al. 2007; and see Weissman and Shizuru 2008 for a history of the discovery of CD antigens to distinguish HSCs from other progenitors and differentiated blood cells).
The discovery of cell surface markers for HSCs finally gave a molecular description to the rare, morphologically indistinct cells of the bone marrow vitally responsible for long-term regeneration of blood and powerfully used in bone marrow transplants. It also gave hematologists the ability to purify HSCs and thereby study their unique molecular properties.
HSC SELF-RENEWAL: DORMANCY AND PROLIFERATION
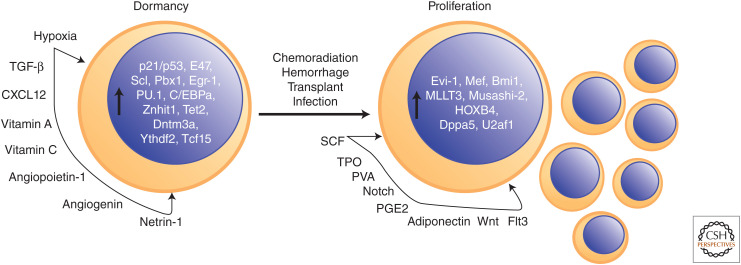
Following the development of techniques to prospectively isolate and purify LT-HSCs, extensive molecular and cellular biology has occurred to characterize the mechanisms of HSC self-renewal. The long-term repopulating capacity of HSCs is a complex phenotype that includes both cell proliferation in response to extrinsic signaling and stress and dominant long-term quiescence to prevent exhaustion from continued cell cycling (Fig. 2). Both HSC dormancy and proliferation are exquisitely regulated by mechanisms both internal in HSCs themselves and external in cells of the bone marrow niche, and are increasingly elegantly refined by techniques with single-cell resolution. Here we review the factors affecting HSC self-renewal in both steady-state conditions as well as following injury and transplantation.
Figure 2.
Factors promoting hematopoietic stem cell (HSC) dormancy and proliferation. (SCF) Stem cell factor, (TPO) Thrombopoietin, (PVA) polyvinyl alcohol.
HSC Dormancy
Both initial characterization and refined modern methods have confirmed that adult HSCs of the bone marrow are largely dormant in vivo. The short-term (ST)-HSCs in initial transplants were found to be largely in a G0, nondividing cell state and therefore resistant to chemotherapy compared to malignant cells (Becker et al. 1965; Bruce et al. 1966). Multiple studies using dye incorporation, limiting dilution assays, and transgenic histone H2B-GFP pulse label experiments have shown that LTRCs as well rarely divide in vivo, as infrequently as every 145 days in mice (Bradford et al. 1997; Cheshier et al. 1999; Sudo et al. 2000; Wilson et al. 2008; Busch et al. 2015). Indeed, recent evidence suggests that murine HSCs divide only four times in adulthood with retention of LTRC activity, which is lost on the fifth cell division (Bernitz et al. 2016). Dormancy in vivo is in contrast to HSC self-renewal in the context of transplant, where they are essential for long-term repopulating activity and can expand up to 1000-fold (Ema et al. 2005). The dormancy of adult HSCs is also in contrast to fetal life, where HSCs in the fetal liver expand nearly 100-fold (Morrison et al. 1995; Ema and Nakauchi 2000), and after which the number of LTRCs remains fairly constant throughout life (Yamamoto et al. 2018; Ganuza et al. 2019), although with declining function in aging (de Haan and Lazare 2018). The dormancy of HSCs suggests that they are not the predominant cell type responsible for the massive hematopoiesis (billions of blood cells per day) required at steady state in vivo. Instead, LTRCs at the top of the hematopoietic hierarchy give rise to the ST-HSC (CFU-S) discovered earlier, which in turn differentiate into multipotent progenitors (MPPs) with no self-renewal abilities (Yang et al. 2005) that are predominantly responsible for hematopoiesis under steady-state conditions in vivo (Sun et al. 2014; McRae et al. 2019).
The limited number of HSC cell divisions is accomplished through an exquisitely regulated ground state of HSC dormancy that preserves prolonged self-renewal capacity for stress conditions (including transplantation into ablated hosts). Numerous HSC-intrinsic factors as well as interactions with cells of the bone marrow niche regulate HSC quiescence to uncontrolled division and exhaustion of LTRC capacity. The hypoxia of the bone marrow niche is a key environmental regulator of HSC quiescence to alter cellular metabolism to limit proliferation and exhaustion. Despite the dense vascularity of bone marrow, intermediate filament Nestin+ periarteriolar mesenchymal cells of the quiescent HSC niche are characterized by low regional perfusion (Parmar et al. 2007; Kunisaki et al. 2013) and have been shown in elegant microscale optical imaging experiments to maintain absolute local pO2 levels as low as ∼23 mm Hg for HSCs (Spencer et al. 2014) compared to 80–100 mm Hg in arterial blood. These hypoxic conditions make HSCs dependent on glycolysis for energy and give HSCs lower basal and maximal respiration rates than proliferative MPPs irrespective of mitochondrial content (Simsek et al. 2010; Romero-Moya et al. 2013; Takubo et al. 2013; Ito and Suda 2014; de Almeida et al. 2017). Use of glycolysis due to hypoxia limits energy production for proliferation as well as intracellular reactive oxygen species (ROS) production (Jang and Sharkis 2007; Mantel et al. 2012) and DNA damage (Walter et al. 2015)—but others have also suggested that dormancy is characterized by error-prone DNA repair mechanisms such as nonhomologous end joining owing to lack of HSC cell-cycle entry (Mohrin et al. 2010; Beerman et al. 2014). The need for DNA damage repair and anaerobic respiration due to the hypoxic environment of HSCs is controlled through quantitative levels of transcription factors such as HIF-1, Meis1, and Foxo3a (Miyamoto et al. 2007; Tothova et al. 2007; Simsek et al. 2010; Takubo et al. 2010). HSC dormancy also requires the antioxidant vitamins A and C (Agathocleous et al. 2017; Cabezas-Wallscheid et al. 2017). In addition to low oxygen utilization, other conservative metabolic requirements for HSC dormancy include low rates of protein synthesis and dependence on recycling organelles through autophagy and mitophagy (Signer et al. 2014; Cao et al. 2015; Ito et al. 2016; Ho et al. 2017).
Apart from metabolic requirements, extrinsic signaling factors and internal epigenetic modulators and transcriptional programs act to maintain HSC dormancy. Extracellular cytokines have prominent roles promoting HSC dormancy by limiting excessive cell division. Signaling ligand TGF-β is produced by numerous cell types of the bone marrow niche including vascular cells, megakaryocytes, and nervous system Schwann cells (Yamazaki et al. 2011; Zhao et al. 2014); limits in vitro and in vivo HSC proliferation through its receptor TGFBR2 and transcriptional effector SMAD4 (Keller et al. 1990; Yamazaki et al. 2009, 2011); and prevents exhaustion after stress-induced HSC proliferation (Brenet et al. 2013). The ligand CXCL12 (SDF-1) through its receptor CXCR4 promotes HSC quiescence and is produced by numerous niche cells including Leptin receptor, Lepr+ perivascular stromal cells, CXCL12-abundant reticular cells, endothelial cells, megakaryocytes, and perivascular mesenchymal stem cells (Sugiyama et al. 2006; Nie et al. 2008; Greenbaum et al. 2013; Bruns et al. 2014). CXCL12 also inhibits HSC egressing from the bone marrow and is under the control of circadian nervous system cycles and bone marrow macrophages (Méndez-Ferrer et al. 2008; Chow et al. 2011). The ligand Netrin-1 is also produced by periarteriolar endothelial and stromal cells and promotes HSC quiescence (Renders et al. 2021). The ligand Angiopoietin-1 promotes HSC quiescence and is produced by osteoblasts in the niche (Arai et al. 2004). In addition to cytokines and growth factors above, single-cell RNA sequencing has provided new insights into novel niche factors regulating HSCs such as Angiogenin, a secreted RNase that also promotes HSC quiescence (Goncalves et al. 2016; Silberstein et al. 2016). Downstream of extracellular signaling, numerous transcription factors and epigenetic modulators also promote HSC dormancy including p21 and p53 (Cheng et al. 2000; van Os et al. 2007; Liu et al. 2009) under the regulation of E47 (Yang et al. 2011), Scl (Lacombe et al. 2010), Pbx1 (Ficara et al. 2008), Egr-1 (Min et al. 2008), Gfi-1 (Hock et al. 2004; Lee et al. 2018) under regulation of Mysm1 (Wang et al. 2013), PU.1 (Staber et al. 2013), C/EBPa (Ye et al. 2013; Hasemann et al. 2014), Znhit1 (Sun et al. 2020), Tet2 (Moran-Crusio et al. 2011), Dnmt3a (Jeong et al. 2018), Ythdf2 (Li et al. 2018b), and Tcf15 (Rodriguez-Fraticelli et al. 2020).
HSC Proliferation
Whereas LT-HSCs spend most of adult life in a dormant state, signaling from the niche and stressors from the body can awaken HSCs and stimulate cell proliferation. In addition to contributing to our understanding of hematopoietic homeostasis, the study of HSC proliferation is vital to HSC regenerative medicine in improving bone marrow transplant and ex vivo culture of HSCs for translational applications. HSCs are awakened from their dormant ground state by both physiologic stressors including hemorrhage and infection and iatrogenic stressors such as DNA damage through radiation injury or chemotherapy, and bone marrow transplant mobilizing and conditioning regimens. Infection and inflammation in response to various pathogens or pathogen-associated molecular patterns such as lipopolysaccharide that activate proinflammatory Toll-like receptor, interferon, IL-6, TNF-α, and NF-κB protein signaling networks have been shown to increase HSC cell-cycle entry and proliferation (Essers et al. 2009; Baldridge et al. 2010; Chen et al. 2010; Esplin et al. 2011; King and Goodell 2011; Megías et al. 2012; Zhao et al. 2013). However, such studies have also shown that proliferative signaling through chronic immune stimulation also injures HSC LTRC capabilities and skews differentiation potential toward myeloid lineages. The repopulating capacity of HSCs injured by interferon signaling is opposed by Irf2, a negative transcriptional regulator of interferon signaling (Sato et al. 2009). Proliferation is also stimulated by the common pre-stem-cell transplantation mobilization and conditioning methods including the cytokine G-CSF and chemotherapy (Wilson et al. 2008; Busch et al. 2015; Schoedel et al. 2016). In addition to chemotherapy, DNA damage occurring from accumulated ROS damage and aging also stimulates HSC proliferation (Morrison et al. 1996; Akunuru and Geiger 2016; Li et al. 2016). Blood loss also increases HSC proliferation (Cheshier et al. 2007).
As in dormancy, HSC proliferation is stimulated by extracellular cytokines and growth factors. Two classic HSC proliferative cytokines include c-kit ligand (stem cell factor [SCF], steel factor) and Thrombopoietin (TPO). c-kit ligand is secreted by perivascular and endothelial cells of the niche and increases survival and proliferation of HSCs and downstream progenitors both in vivo and in vitro in combination with other cytokines such as IL-3, -6, -11, -12, -27, and/or TPO (Geissler et al. 1981; Carow et al. 1991; Metcalf and Nicola 1991; Ogawa et al. 1991; Li and Johnson 1994; Sharma et al. 2007; Ding et al. 2012; Dahlin et al. 2018). The fact that HSCs rely on c-kit and possess the c-kit receptor can be used by coating HSCs with anti-c-kit antibodies for destruction by antibody-dependent cell-mediated cytotoxicity and may one day allow for bone marrow transplants with novel biological-based preconditioning regimens (rather than radiation or chemotherapy that has been traditionally used, as discussed below) (Chhabra et al. 2016). TPO produced by megakaryocytes and osteoblasts is another critical cytokine responsible for expansion of HSCs in vivo and in vitro in combination with SCF and IL-3 (Sitnicka et al. 1996; Kimura et al. 1998; Ema et al. 2000; Fox et al. 2002), but also promotes quiescence and ensures lack of exhaustion of HSCs (Qian et al. 2007; Yoshihara et al. 2007). TPO is under the negative regulation of the Lnk adaptor protein in HSCs, with deletion of Lnk leading to large increases in HSC self-renewal (Ema et al. 2005; Seita et al. 2007). A recent study highlighted the importance of TPO and c-kit ligand in showing that synergistic dosing of TPO and c-kit with no other cytokines provided long-term ex vivo expansion of murine HSCs in vitro to a scale suitable for engraftment without preconditioning (Wilkinson et al. 2019). However, in this study, a fibronectin-coated substrate and replacement of fetal bovine serum with polyvinyl alcohol (PVA) to serve as a bioactive cytokine and lipid carrier were also used, which highlights the importance of insoluble and noncytokine factors in optimizing ex vivo HSC culture.
Apart from c-kit and TPO, Wnt and Notch extracellular signaling factors have also been shown to stimulate HSC expansion. Following injury, hematopoietic and stromal cells of the bone marrow up-regulate Wnt ligands, which trigger β-catenin-mediated signaling to increase proliferation and repopulation, and decrease intracellular ROS, thereby promoting HSC regeneration (Reya et al. 2003; Duncan et al. 2005; Nemeth et al. 2007; Congdon et al. 2008; Lento et al. 2014). Notch ligands such as DLL4 and DLL1 primarily expressed through vascular endothelial cells of the niche and signaling through the Notch1 intracellular domain and transcription factor Hes1 have been shown to promote HSC proliferation in vivo and in vitro and prevent myeloid skewing or predifferentiation of HSCs (Varnum-Finney et al. 2000; Stier et al. 2002; Kunisato et al. 2003; Tikhonova et al. 2019). The ligand Adiponectin from adipocytes in bone marrow has also been shown to increase HSC proliferation in vitro and in vivo through the p38 MAPK pathway without sacrificing long-term repopulating activity (DiMascio et al. 2007). Flt3 ligand is another cytokine that stimulates HSC proliferation, largely in potentiation of other growth factors (Tsapogas et al. 2017). Prostaglandin E2 is a nonpeptide eicosanoid factor that has also been shown to increase HSC proliferation and self-renewal (Hoggatt et al. 2009).
Following stimulation by stress or extracellular factors as described above, HSCs shift metabolism toward increased oxidative phosphorylation and nucleic acid metabolism necessary for cell proliferation (Ito et al. 2012; Karigane et al. 2016; Karigane and Takubo 2017). Calcium and vitamin D are also extrinsic metabolic requirements for HSC self-renewal and proliferation (Cortes et al. 2016; Umemoto et al. 2018). In addition to niche cell signaling and metabolic environmental cues, a strong role for intrinsic factors in HSC proliferation is also evident from transplantation experiments showing greater than 500-fold clonal variations in proliferation between individual LT-HSCs (Sieburg et al. 2011; Benz et al. 2012). Furthermore, the preexisting epigenetic state of HSCs dictates the diverse individual clonal responses to stress signaling (Yu et al. 2017). Transcription factors and epigenetic modulators increasing HSC proliferation include Evi-1 (Goyama et al. 2008), Mef (Elf4) (Lacorazza et al. 2006), Bmi1 (Park et al. 2003; Iwama et al. 2004), MLLT3 (Calvanese et al. 2019), and HOXB4 (Antonchuk et al. 2002). RNA-binding factors also contribute to HSC maintenance and proliferation. For example, Musashi-2 is an RNA-binding protein that alters translation of target gene RNA and promotes HSC maintenance and proliferation by repression at the protein level of the Notch-inhibiting membrane protein Numb (Ito et al. 2010; Rentas et al. 2016). Dppa5 is an RNA-binding protein required for endoplasmic reticulum (ER) stress regulation whose overexpression leads to HSC expansion (Miharada et al. 2014). U2af1 is a splicing factor required for promoting HSC proliferative gene expression (Dutta et al. 2021).
The discovery of factors promoting the various components of HSC self-renewal—HSC dormancy, maintenance of LTRC activity, and HSC proliferation—has enriched our understanding of HSC contributions to hematopoiesis and allowed for the development of ex vivo HSC culture conditions. These methods to grow HSCs are also a requirement for novel translational research areas seeking to create synthetic HSCs from various sources and expand HSCs and their derivatives for therapeutic applications.
CELL THERAPY BY HSCs AND DERIVATIVES
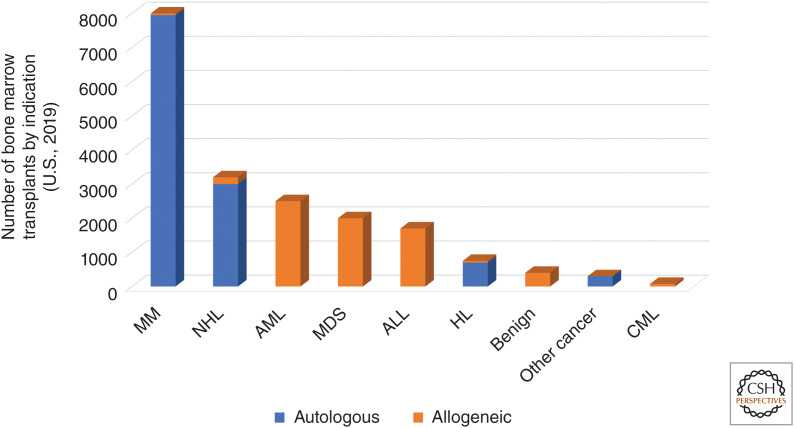
Discoveries on HSC self-renewal and their utility in regenerative medicine for HSCT have developed in parallel since the beginning of HSC research. For over 60 years, HSCT has been deployed for both solid and hematologic malignancies and a wide range of nonmalignant diseases such as bone marrow failure and congenital immunodeficiency syndromes. Whereas in principle HSCT can cure any hematologic disorder, in practice HSCT is limited by donor availability, patient eligibility, treatment-related morbidity and mortality (including infection, organ failure, and GVHD), cost (Khera et al. 2014), and relapse of the primary disease. The therapeutic GVL effect of allogeneic HSCT has also been further refined in chimeric antigen receptor (CAR) cell therapy using HSC derivatives (e.g., CAR T cells). Here we review the sources of donor HSCs for HSCT, including the translational potential of iPS cells as new sources for HSCs, the common therapeutic indications for HSCT (Fig. 3), and the recent expansion of anticancer cell therapies by HSC derivatives.
Figure 3.
Current blood marrow transplant (BMT) indications separated by source of donor hematopoietic stem cells (HSCs) (autologous vs. allogeneic). (MM) Multiple myeloma, (NHL) non-Hodgkin lymphoma, (AML) acute myeloid leukemia, (MDS) myelodysplastic syndromes, (ALL) acute lymphocytic leukemia, (HL) Hodgkin's lymphoma, (CML) chronic myeloid lymphoma. Benign disorders include bone marrow failure syndromes such as aplastic anemia, congenital diseases, and other nonmalignant conditions. (Figure adapted from Phelan et al. 2020. Full complement of slides related to this figure are available at www.cibmtr.org.)
Sources for transplanted HSCs either come from the patient themselves or a healthy donor. Autologous HSCT involves withdrawal and preservation of a patient's own HSCs for reintroduction after administration of an otherwise lethal dose of radiation or chemotherapy (conditioning regimen). Autologous HSCT is the most common type of bone marrow transplant at over 14,000 performed yearly in the United States with multiple myeloma and non-Hodgkin lymphoma being the dominant indications (Fig. 3; D'Souza et al. 2020). Allogeneic HSCT involves conditioning followed by the delivery of donor HSCs from another individual—in order of frequency, an HLA-matched unrelated donor, HLA-matched sibling, partially HLA-matched (haploidentical) family member, followed by other sources at lower frequency—and is most commonly used in acute leukemias (acute myeloid leukemia [AML] and acute lymphocytic leukemia [ALL]) and myelodysplastic syndromes (MDS) (Fig. 3; D'Souza et al. 2020). Typically, four or five HLA genes (8–10 alleles) on chromosome 6-A, -B, -C, -DRB1, and -DQB1 are assessed to determine matching for allogeneic HSCT. Thus, a fully HLA-matched sibling or an ideal matched unrelated donor is referred to as an 8/8 or 10/10 match and a haploidentical family member is a 4/8 or 5/10 match (Howard et al. 2015). The greater degree of HLA mismatch increases the risk of both host-mediated graft rejection (Ciurea et al. 2009) and GVHD, but not necessarily greater GVL (Arora et al. 2009; Ringdén et al. 2009; Pidala et al. 2014; Sweeney and Vyas 2019). GVHD can also be minimized by high-dose cyclophosphamide in the immediate posttransplant induction phase of HSCT (Luznik et al. 2012) and use of a bone marrow rather than peripheral blood source for HSCs (Anasetti et al. 2012), making this the preferred source for patients with nonmalignant conditions (for whom there is no theoretical advantage to greater GVL from a peripheral blood source) withstanding donor availability for the more invasive procedure. Peripheral blood, with harvesting of HSCs by apheresis after mobilization by G-CSF, chemotherapy, and, more recently, CXCR4 inhibition (DiPersio et al. 2009), is nonetheless the most common source accounting for 80% of transplants in adults (D'Souza et al. 2020). Ethnic representation in the National Marrow Donor Program (NMDP) limits donor sources and therefore certain disease indications for allogeneic transplants: matched unrelated donors can be found for 79% of Caucasian patients but just 33% of African-Americans (Switzer et al. 2013). This limits allogeneic HSCT for these patients in malignant and nonmalignant diseases and is especially relevant to sickle cell anemia, for which allogeneic HSCT can be an effective treatment option (Hsieh et al. 2009; Leonard et al. 2020).
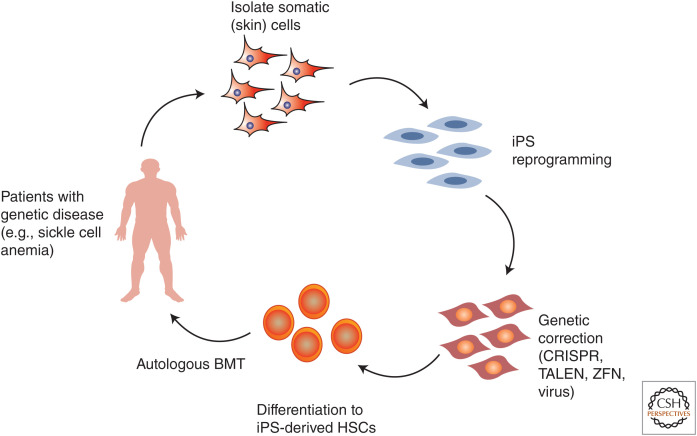
Given issues of donor availability and GVHD from traditional sources of HSCs, there has been intense preclinical scientific interest in creating autologous HSC sources from patient-derived human-induced pluripotent stem cells, iPS (Takahashi and Yamanaka 2006; Takahashi et al. 2007; Yu et al. 2007). In this process, a patient's somatic cells are reprogrammed into iPS cells (classically by virally inserted transcription factors, but more recently through a variety of potentially safer vector-free or small molecule techniques [Yu et al. 2009; Hu et al. 2011; Kim et al. 2020]) and then would be differentiated into HSCs for delivery back to a patient (Fig. 4). The patient's iPS cells could also be genetically manipulated to correct a congenital condition. Hanna et al. (2007) showed an impressive proof-of-principle for this approach using mouse iPS cells in which mouse skin fibroblasts were taken from a sickle cell anemia mouse model, reprogrammed to iPS cells, genetically corrected to replace hemoglobin genes with non–sickle cell alleles, then differentiated to hematopoietic progenitors in vitro and finally transplanted back into the mice. The mice then recovered from their sickle cell phenotype. Despite this impressive demonstration, the derivation of bona fide LT-HSCs from human iPS cells with long-term production of all blood lineages has since been a major technological hurdle. Reports of deriving HSCs from embryonic cell populations or induced pluripotent stem cells have used specialized feeder cells, cell sorting, transcription factor expression, and cytokines to show short-term myeloid-biased engraftment but low lymphocyte production and lack of long-term engraftment (Kyba et al. 2002; Doulatov et al. 2013; Guo et al. 2020; Shan et al. 2020). These reports of pluripotent cell-derived HSC immaturity are in line with the relative immaturity of pluripotent stem cell derivatives from other tissues, such as pancreatic β cells, neurons, and cardiomyocytes (Pagliuca et al. 2014; Weick 2016; Biermann et al. 2019). A major question therefore is whether HSC immaturity is a reflection of the synthetic limitations of in vitro culture, or intrinsic to iPS cells as sources. But arguing against the latter, it has been shown by different groups that iPS cells engrafted into immunocompromised animals as teratomas in vivo (xenografts classically created to assess the differentiation potential of pluripotent iPS cells in that they contain tissues from ectoderm, mesoderm, and endodermal germ layers) contain serially transplantable HSCs with multilineage differentiation and long-term engraftment (Amabile et al. 2013; Suzuki et al. 2013; Tsukada et al. 2017). Whereas use of teratomas would not be suitable for translational development, these studies suggest that more faithful recapitulation of the in vivo development of HSCs may aid maturation. Most recently, Sugimura et al. (2017) differentiated iPS cells first to hemogenic endothelial cells, the origin site of HSCs during embryonic development, and then expressed a larger number of transcription factors (ERG, HOXA5, HOXA9, HOXA10, LCOR, RUNX1, SPI1) to specify HSCs. These iPS-HSCs showed impressive, up to 16-week, engraftment in primary and secondary recipients often with multilineage differentiation potential. Thus, while technical limitations remain to generating LT-HSCs from human iPS cells, iPS-HSCs may provide novel sources of genetically correctable HSCs to widen the range of indications for autologous HSCT.
Figure 4.
Process of the creation of patient-specific iPS stem cells, genetic correction, and differentiation to hematopoietic stem cells (HSCs) for the treatment of hematological genetic diseases (such as sickle cell anemia). (BMT) Bone marrow transplant, (iPS) induced pluripotent stem cells.
Therapeutic indications for HSCT have changed over time. For example, autologous HSCT was once widely used for metastatic breast cancer, but eventually randomized controlled trials argued against a benefit to the procedure (Vogl and Stadtmauer 2006). Similarly, chronic myelogenous leukemia (CML) was often treated with allogeneic HSCT before the development of the tyrosine kinase inhibitor imatinib, and allogeneic HSCT for CML is now mainly used for disease refractory to targeted therapy or in leukemic transformation (Benyamini and Rowe 2013). Today, therefore, autologous HSCT is most commonly performed for multiple myeloma and non-Hodgkin lymphoma, and allogeneic HSCT for acute leukemias and MDS.
Multiple myeloma is the most lethal plasma cell dyscrasia and involves clonal plasma cells causing renal failure, bone destruction, and anemia. Autologous HSCT for consolidation after primary induction therapy has been standard of care for patients healthy enough to receive it after the first randomized trials showed deepened remissions (Attal et al. 1996). Whereas primary therapy for myeloma has advanced substantially since then—specifically, the addition of proteasome inhibitors (such as bortezomib and carfilzomib) and immunomodulatory imide drugs (lenalidomide, pomalidomide) to create triple therapy regimens in combination with steroids—progression-free survival benefits on the order of years continue to favor the addition of autologous HSCT for consolidation therapy (Palumbo et al. 2014; Cavo et al. 2020). Non-Hodgkin lymphoma, the most common histologic subtype being diffuse large B-cell lymphoma (DLBCL), is the second leading indication for autologous HSCT. DLBCL is an aggressive malignancy of clonal B cells in the lymph nodes and extranodal sites of the body that is usually responsive to combination chemotherapy regimens with rituximab, anti-CD20 (Coiffier et al. 2010). However, in the setting of disease that is relapsed or refractory to initial treatment, salvage chemotherapy followed by autologous HSCT in eligible patients provides a large benefit on response rates and overall survival compared to salvage therapy alone (Philip et al. 1995; Oliansky et al. 2011).
In both myeloma and DLBCL, the goal of autologous HSCT is to use patients’ own HSCs as rescue to deliver a myeloablative dose of chemotherapy. However, in the postremission management of acute leukemias (AML and ALL), allogeneic HSCT is added for its GVL effect. AML and ALL are aggressive proliferations of malignant clones of myeloid or lymphoid progenitors and blasts in the blood and bone marrow. AML mostly affects adults, while ALL is the most common cancer of childhood. Despite these different patient populations, allogeneic HSCT confers relapse prevention and survival benefits compared to consolidative chemotherapy alone in eligible patients with high-risk disease based on molecular and cellular features (Yanada et al. 2005; Schrauder et al. 2006; Cornelissen et al. 2007; Seibel et al. 2008; Koreth et al. 2009; Schrappe et al. 2012). The clinical existence of GVL as the mechanism of benefit from allogeneic HSCT was suggested by minimal efficacy of allogeneic HSCT grafts that were either T-cell depleted or from identical twin donors, as observed in the first identical twin bone marrow transplants for leukemias in the 1950s described above and systematically studied by Horowitz et al. (1990). Furthermore, it was observed that donor lymphocyte infusion alone could promote durable remissions in CML and ALL (Kolb et al. 1990; Poon et al. 2013). These studies supported the existence of GVL in allogeneic HSCT and thereby allowed for the development of reduced intensity conditioning chemotherapy regimens that could extend allogeneic HSCT eligibility for older or less medically fit patients (Slavin et al. 1998; Dreger et al. 2000; Nagler et al. 2000). They also suggested that donor lymphocytes are the cellular effectors of GVL.
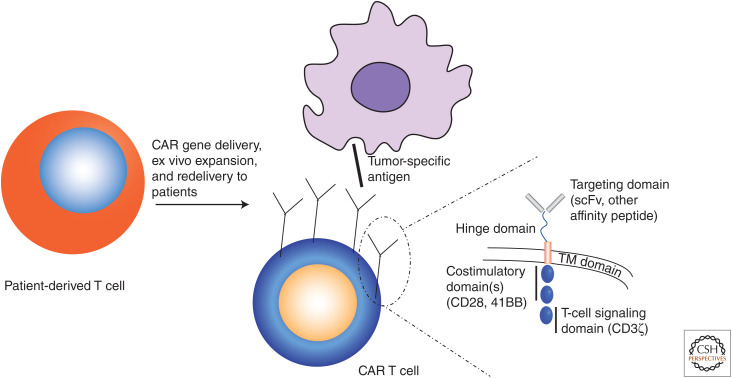
Investigating the cellular mechanisms of GVL has continued to yield clinical and preclinical cancer cell therapy advances. Cytotoxic CD8+ T lymphocytes were the first cells identified to be capable of causing GVL, but at the expense of GVHD, which occurs through graft recognition of minor and unmatched major histocompatibility antigens on host leukemic cells and normal tissues, respectively (Tsoi et al. 1980; Falkenburg et al. 1991; Niederwieser et al. 1993; Warren et al. 1998; Bonnet et al. 1999). These results spurred investigations to synthetically manipulate graft T cells to maximize GVL without triggering greater GVHD. Principal among these is genetic manipulation to express CARs. CARs are single-chain cell-surface receptors that combine fragments of multiple different immune cell surface receptors to create a single construct that can be transfected into patient cells that can then be transplanted back to mediate GVL in the patient (Fig. 5). CARs typically contain an extracellular cancer cell–recognition domain (e.g., a single-chain variable fragment antibody) and an intracellular signaling domain usually containing both costimulatory (CD28, 4-1BB) and stimulatory (CD3ζ) fragments (Fig. 5, inset). Patient-derived CAR T cells have so far been used for refractory hematologic malignancies including ALL (Maude et al. 2018; Park et al. 2018), DLBCL (Neelapu et al. 2017; Schuster et al. 2019), and multiple myeloma (Raje et al. 2019). Whereas CAR T cells have induced impressive response rates in trials, their use can cause potentially fatal cytokine-release syndrome, and they are further limited by the expense of the need to collect and transfect a recipient's cells each time to create an autologous product to avoid GVHD. Another attempt to improve GVL without increasing GVHD relies on donor natural killer cells (NKs), which are large granular lymphocytes that express MHC class I–specific killer-cell immunoglobulin-like receptors (KIRs) such that NK cells will kill targets lacking their allotypic MHC-I. Ruggeri et al. (1999, 2007) showed that NK alloreactive grafts (grafts with KIR mismatch between donor and recipient) were impressively correlated with lower relapse rates and survival in allogeneic HSCT for AML—without increased GVHD reactions. Because they do not mediate GVHD in an MHC class II–dependent fashion, the possibility exists of using standardized, expanded NK cells as an “off-the-shelf” cell therapy for cancer. Therefore, clinical trials (Hodgins et al. 2019) and improvements to NK cell cytotoxicity using iPS technology and modification with NK-specific CARs (Li et al. 2018a) are currently underway.
Figure 5.
Creation of patient-specific chimeric antigen receptor (CAR) T-cell therapy, in which patient T cells after delivery of the CAR cassette—a synthetic transmembrane (TM) receptor recombining multiple functional domains (inset, right)—can target cells bearing tumor-specific antigens. (scFv) Single-chain variable fragment.
CONCLUDING REMARKS
HSC regeneration is the complex process by which rare cells of the bone marrow that normally maintain a long-term dormant state through conservative metabolism and external signaling from cells of the bone marrow niche are awakened by proliferative niche factors released in the context of bone marrow injury by radiation, drugs, infection, or trauma. These same factors can be harnessed to mobilize and collect HSCs for transplant. The story of HSC research and bone marrow transplant has progressed from its origin in the exigencies of radiation injury and preliminary transplants of unpurified bone marrow cell populations in animals and humans to advanced single-cell science on mechanisms of HSC regeneration and the use of bone marrow transplant for tens of thousands of patients today. HSC research and its intertwined history and practice in bone marrow transplant shows the synergy of basic and applied science. In the future, single-cell methods will continue to deepen our understanding of the mechanisms of HSC regeneration. Techniques such as single-cell RNA sequencing are providing enhanced understanding of transcriptional heterogeneity within the HSC cellular compartment as well as cells of the niche. These tools can provide useful molecular information to further subclassify HSCs through their occupancy of different transcriptional states, to understand the heterogeneity and different functions of cells of the bone marrow niche, and to uncover dynamic signaling factors and mechanisms expressed by subsets of cells invisible to methods conducted on bulk populations. Finally, this improved understanding of the heterogeneity and signaling of HSCs and their niche will enable further developments in engineering HSCs and their derivatives for treatment of a wide and growing range of therapeutic indications.
Footnotes
Editors: Kenneth D. Poss and Donald T. Fox
Additional Perspectives on Regeneration available at www.cshperspectives.org
REFERENCES
- Agathocleous M, Meacham CE, Burgess RJ, Piskounova E, Zhao Z, Crane GM, Cowin BL, Bruner E, Murphy MM, Chen W, et al. 2017. Ascorbate regulates haematopoietic stem cell function and leukaemogenesis. Nature 549: 476–481. 10.1038/nature23876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akunuru S, Geiger H. 2016. Aging, clonality, and rejuvenation of hematopoietic stem cells. Trends Mol Med 22: 701–712. 10.1016/j.molmed.2016.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amabile G, Welner RS, Nombela-Arrieta C, D'Alise AM, Di Ruscio A, Ebralidze AK, Kraytsberg Y, Ye M, Kocher O, Neuberg DS, et al. 2013. In vivo generation of transplantable human hematopoietic cells from induced pluripotent stem cells. Blood 121: 1255–1264. 10.1182/blood-2012-06-434407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anasetti C, Logan BR, Lee SJ, Waller EK, Weisdorf DJ, Wingard JR, Cutler CS, Westervelt P, Woolfrey A, Couban S, et al. 2012. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med 367: 1487–1496. 10.1056/NEJMoa1203517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonchuk J, Sauvageau G, Humphries RK. 2002. HOXB4-induced expansion of adult hematopoietic stem cells ex vivo. Cell 109: 39–45. 10.1016/S0092-8674(02)00697-9 [DOI] [PubMed] [Google Scholar]
- Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. 2004. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 118: 149–161. 10.1016/j.cell.2004.07.004 [DOI] [PubMed] [Google Scholar]
- Arora M, Weisdorf DJ, Spellman SR, Haagenson MD, Klein JP, Hurley CK, Selby GB, Antin JH, Kernan NA, Kollman C, et al. 2009. HLA-identical sibling compared with 8/8 matched and mismatched unrelated donor bone marrow transplant for chronic phase chronic myeloid leukemia. J Clin Oncol 27: 1644–1652. 10.1200/JCO.2008.18.7740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, Casassus P, Maisonneuve H, Facon T, Ifrah N, et al. 1996. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Français du Myélome. N Engl J Med 335: 91–97. 10.1056/NEJM199607113350204 [DOI] [PubMed] [Google Scholar]
- Baldridge MT, King KY, Boles NC, Weksberg DC, Goodell MA. 2010. Quiescent haematopoietic stem cells are activated by IFN-γ in response to chronic infection. Nature 465: 793–797. 10.1038/nature09135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum CM, Weissman IL, Tsukamoto AS, Buckle AM, Peault B. 1992. Isolation of a candidate human hematopoietic stem-cell population. Proc Natl Acad Sci 89: 2804–2808. 10.1073/pnas.89.7.2804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker AJ, McCulloch EA, Till JE. 1963. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature 197: 452–454. 10.1038/197452a0 [DOI] [PubMed] [Google Scholar]
- Becker AJ, McCulloch EA, Siminovitch L, Till JE. 1965. The effect of differing demands for blood cell production on DNA synthesis by hemopoietic colony-forming cells of mice. Blood 26: 296–308. 10.1182/blood.V26.3.296.296 [DOI] [PubMed] [Google Scholar]
- Beerman I, Seita J, Inlay MA, Weissman IL, Rossi DJ. 2014. Quiescent hematopoietic stem cells accumulate DNA damage during aging that is repaired upon entry into cell cycle. Cell Stem Cell 15: 37–50. 10.1016/j.stem.2014.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyamini N, Rowe JM. 2013. Is there a role for allogeneic transplantation in chronic myeloid leukemia? Expert Rev Hematol 6: 759–765. 10.1586/17474086.2013.849571 [DOI] [PubMed] [Google Scholar]
- Benz C, Copley MR, Kent DG, Wohrer S, Cortes A, Aghaeepour N, Ma E, Mader H, Rowe K, Day C, et al. 2012. Hematopoietic stem cell subtypes expand differentially during development and display distinct lymphopoietic programs. Cell Stem Cell 10: 273–283. 10.1016/j.stem.2012.02.007 [DOI] [PubMed] [Google Scholar]
- Bernitz JM, Kim HS, MacArthur B, Sieburg H, Moore K. 2016. Hematopoietic stem cells count and remember self-renewal divisions. Cell 167: 1296–1309.e10. 10.1016/j.cell.2016.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biermann M, Cai W, Lang D, Hermsen J, Profio L, Zhou Y, Czirok A, Isai DG, Napiwocki BN, Rodriguez AM, et al. 2019. Epigenetic priming of human pluripotent stem cell-derived cardiac progenitor cells accelerates cardiomyocyte maturation. Stem Cells 37: 910–923. 10.1002/stem.3021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet D, Warren EH, Greenberg PD, Dick JE, Riddell SR. 1999. CD8+ minor histocompatibility antigen-specific cytotoxic T lymphocyte clones eliminate human acute myeloid leukemia stem cells. Proc Natl Acad Sci 96: 8639–8644. 10.1073/pnas.96.15.8639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford GB, Williams B, Rossi R, Bertoncello I. 1997. Quiescence, cycling, and turnover in the primitive hematopoietic stem cell compartment. Exp Hematol 25: 445–453. [PubMed] [Google Scholar]
- Brenet F, Kermani P, Spektor R, Rafii S, Scandura JM. 2013. TGFβ restores hematopoietic homeostasis after myelosuppressive chemotherapy. J Exp Med 210: 623–639. 10.1084/jem.20121610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce WR, Meeker BE, Valeriote FA. 1966. Comparison of the sensitivity of normal hematopoietic and transplanted lymphoma colony-forming cells to chemotherapeutic agents administered in vivo. J Natl Cancer Inst 37: 233–245. [PubMed] [Google Scholar]
- Bruns I, Lucas D, Pinho S, Ahmed J, Lambert MP, Kunisaki Y, Scheiermann C, Schiff L, Poncz M, Bergman A, et al. 2014. Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nat Med 20: 1315–1320. 10.1038/nm.3707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch K, Klapproth K, Barile M, Flossdorf M, Holland-Letz T, Schlenner SM, Reth M, Höfer T, Rodewald HR. 2015. Fundamental properties of unperturbed haematopoiesis from stem cells in vivo. Nature 518: 542–546. 10.1038/nature14242 [DOI] [PubMed] [Google Scholar]
- Cabezas-Wallscheid N, Buettner F, Sommerkamp P, Klimmeck D, Ladel L, Thalheimer FB, Pastor-Flores D, Roma LP, Renders S, Zeisberger P, et al. 2017. Vitamin A-retinoic acid signaling regulates hematopoietic stem cell dormancy. Cell 169: 807–823.e19. 10.1016/j.cell.2017.04.018 [DOI] [PubMed] [Google Scholar]
- Calvanese V, Nguyen AT, Bolan TJ, Vavilina A, Su T, Lee LK, Wang Y, Lay FD, Magnusson M, Crooks GM, et al. 2019. MLLT3 governs human haematopoietic stem-cell self-renewal and engraftment. Nature 576: 281–286. 10.1038/s41586-019-1790-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Zhang A, Cai J, Yuan N, Lin W, Liu S, Xu F, Song L, Li X, Fang Y, et al. 2015. Autophagy regulates the cell cycle of murine HSPCs in a nutrient-dependent manner. Exp Hematol 43: 229–242. 10.1016/j.exphem.2014.11.002 [DOI] [PubMed] [Google Scholar]
- Carow CE, Hangoc G, Cooper SH, Williams DE, Broxmeyer HE. 1991. Mast cell growth factor (c-kit ligand) supports the growth of human multipotential progenitor cells with a high replating potential. Blood 78: 2216–2221. 10.1182/blood.V78.9.2216.2216 [DOI] [PubMed] [Google Scholar]
- Cavo M, Gay F, Beksac M, Pantani L, Petrucci MT, Dimopoulos MA, Dozza L, van der Holt B, Zweegman S, Oliva S, et al. 2020. Autologous haematopoietic stem-cell transplantation versus bortezomib-melphalan-prednisone, with or without bortezomib-lenalidomide-dexamethasone consolidation therapy, and lenalidomide maintenance for newly diagnosed multiple myeloma (EMN02/HO95): a multicentre, randomised, open-label, phase 3 study. Lancet Haematol 7: e456–e468. 10.1016/S2352-3026(20)30099-5 [DOI] [PubMed] [Google Scholar]
- Chen C, Liu Y, Liu Y, Zheng P. 2010. Mammalian target of rapamycin activation underlies HSC defects in autoimmune disease and inflammation in mice. J Clin Invest 120: 4091–4101. 10.1172/JCI43873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T, Rodrigues N, Shen H, Yang Y, Dombkowski D, Sykes M, Scadden DT. 2000. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science 287: 1804–1808. 10.1126/science.287.5459.1804 [DOI] [PubMed] [Google Scholar]
- Cheshier SH, Morrison SJ, Liao X, Weissman IL. 1999. In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proc Natl Acad Sci 96: 3120–3125. 10.1073/pnas.96.6.3120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheshier SH, Prohaska SS, Weissman IL. 2007. The effect of bleeding on hematopoietic stem cell cycling and self-renewal. Stem Cells Dev 16: 707–718. 10.1089/scd.2007.0017 [DOI] [PubMed] [Google Scholar]
- Chhabra A, Ring AM, Weiskopf K, Schnorr PJ, Gordon S, Le AC, Kwon HS, Ring NG, Volkmer J, Ho PY, et al. 2016. Hematopoietic stem cell transplantation in immunocompetent hosts without radiation or chemotherapy. Sci Transl Med 8: 351ra105. 10.1126/scitranslmed.aae0501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow A, Lucas D, Hidalgo A, Méndez-Ferrer S, Hashimoto D, Scheiermann C, Battista M, Leboeuf M, Prophete C, van Rooijen N, et al. 2011. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med 208: 261–271. 10.1084/jem.20101688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciurea SO, de Lima M, Cano P, Korbling M, Giralt S, Shpall EJ, Wang X, Thall PF, Champlin RE, Fernandez-Vina M. 2009. High risk of graft failure in patients with anti-HLA antibodies undergoing haploidentical stem-cell transplantation. Transplantation 88: 1019–1024. 10.1097/TP.0b013e3181b9d710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, Lefort S, Marit G, Macro M, Sebban C, et al. 2010. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d'Etudes des Lymphomes de l'Adulte. Blood 116: 2040–2045. 10.1182/blood-2010-03-276246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congdon KL, Voermans C, Ferguson EC, DiMascio LN, Uqoezwa M, Zhao C, Reya T. 2008. Activation of Wnt signaling in hematopoietic regeneration. Stem Cells 26: 1202–1210. 10.1634/stemcells.2007-0768 [DOI] [PubMed] [Google Scholar]
- Cornelissen JJ, van Putten WLJ, Verdonck LF, Theobald M, Jacky E, Daenen SMG, van Marwijk Kooy M, Wijermans P, Schouten H, Huijgens PC, et al. 2007. Results of a HOVON/SAKK donor versus no-donor analysis of myeloablative HLA-identical sibling stem cell transplantation in first remission acute myeloid leukemia in young and middle-aged adults: benefits for whom? Blood 109: 3658–3666. 10.1182/blood-2006-06-025627 [DOI] [PubMed] [Google Scholar]
- Cortes M, Chen MJ, Stachura DL, Liu SY, Kwan W, Wright F, Vo LT, Theodore LN, Esain V, Frost IM, et al. 2016. Developmental vitamin D availability impacts hematopoietic stem cell production. Cell Rep 17: 458–468. 10.1016/j.celrep.2016.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlin JS, Hamey FK, Pijuan-Sala B, Shepherd M, Lau WWY, Nestorowa S, Weinreb C, Wolock S, Hannah R, Diamanti E, et al. 2018. A single-cell hematopoietic landscape resolves 8 lineage trajectories and defects in Kit mutant mice. Blood 131: e1–e11. 10.1182/blood-2017-12-821413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida MJ, Luchsinger LL, Corrigan DJ, Williams LJ, Snoeck H-W. 2017. Dye-independent methods reveal elevated mitochondrial mass in hematopoietic stem cells. Cell Stem Cell 21: 725–729.e4. 10.1016/j.stem.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan G, Lazare SS. 2018. Aging of hematopoietic stem cells. Blood 131: 479–487. 10.1182/blood-2017-06-746412 [DOI] [PubMed] [Google Scholar]
- DiMascio L, Voermans C, Uqoezwa M, Duncan A, Lu D, Wu J, Sankar U, Reya T. 2007. Identification of adiponectin as a novel hemopoietic stem cell growth factor. J Immunol 178: 3511–3520. 10.4049/jimmunol.178.6.3511 [DOI] [PubMed] [Google Scholar]
- Ding L, Saunders TL, Enikolopov G, Morrison SJ. 2012. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481: 457–462. 10.1038/nature10783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPersio JF, Stadtmauer EA, Nademanee A, Micallef INM, Stiff PJ, Kaufman JL, Maziarz RT, Hosing C, Früehauf S, Horwitz M, et al. 2009. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood 113: 5720–5726. 10.1182/blood-2008-08-174946 [DOI] [PubMed] [Google Scholar]
- Doulatov S, Vo LT, Chou SS, Kim PG, Arora N, Li H, Hadland BK, Bernstein ID, Collins JJ, Zon LI, et al. 2013. Induction of multipotential hematopoietic progenitors from human pluripotent stem cells via respecification of lineage-restricted precursors. Cell Stem Cell 13: 459–470. 10.1016/j.stem.2013.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreger P, Glass B, Seyfarth B, Humpe A, Claviez A, von Neuhoff N, Suttorp M, Schoch R, Schmitz N. 2000. Reduced-intensity allogeneic stem cell transplantation as salvage treatment for patients with indolent lymphoma or CLL after failure of autologous SCT. Bone Marrow Transplant 26: 1361–1362. 10.1038/sj.bmt.1702722 [DOI] [PubMed] [Google Scholar]
- D'Souza A, Fretham C, Lee SJ, Arora M, Brunner J, Chhabra S, Devine S, Eapen M, Hamadani M, Hari P, et al. 2020. Current use of and trends in hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant 26: e177–e182. 10.1016/j.bbmt.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan AW, Rattis FM, DiMascio LN, Congdon KL, Pazianos G, Zhao C, Yoon K, Cook JM, Willert K, Gaiano N, et al. 2005. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat Immunol 6: 314–322. 10.1038/ni1164 [DOI] [PubMed] [Google Scholar]
- Dutta A, Yang Y, Le BT, Zhang Y, Abdel-Wahab O, Zang C, Mohi G. 2021. U2af1 is required for survival and function of hematopoietic stem/progenitor cells. Leukemia 35: 2382–2398. 10.1038/s41375-020-01116-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykstra B, Kent D, Bowie M, McCaffrey L, Hamilton M, Lyons K, Lee SJ, Brinkman R, Eaves C. 2007. Long-term propagation of distinct hematopoietic differentiation programs in vivo. Cell Stem Cell 1: 218–229. 10.1016/j.stem.2007.05.015 [DOI] [PubMed] [Google Scholar]
- Ema H, Nakauchi H. 2000. Expansion of hematopoietic stem cells in the developing liver of a mouse embryo. Blood 95: 2284–2288. 10.1182/blood.V95.7.2284 [DOI] [PubMed] [Google Scholar]
- Ema H, Takano H, Sudo K, Nakauchi H. 2000. In vitro self-renewal division of hematopoietic stem cells. J Exp Med 192: 1281–1288. 10.1084/jem.192.9.1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ema H, Sudo K, Seita J, Matsubara A, Morita Y, Osawa M, Takatsu K, Takaki S, Nakauchi H. 2005. Quantification of self-renewal capacity in single hematopoietic stem cells from normal and Lnk-deficient mice. Dev Cell 8: 907–914. 10.1016/j.devcel.2005.03.019 [DOI] [PubMed] [Google Scholar]
- Esplin BL, Shimazu T, Welner RS, Garrett KP, Nie L, Zhang Q, Humphrey MB, Yang Q, Borghesi LA, Kincade PW. 2011. Chronic exposure to a TLR ligand injures hematopoietic stem cells. J Immunol 186: 5367–5375. 10.4049/jimmunol.1003438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essers MAG, Offner S, Blanco-Bose WE, Waibler Z, Kalinke U, Duchosal MA, Trumpp A. 2009. IFNα activates dormant haematopoietic stem cells in vivo. Nature 458: 904–908. 10.1038/nature07815 [DOI] [PubMed] [Google Scholar]
- Falkenburg JH, Goselink HM, van der Harst D, van Luxemburg-Heijs SA, Kooy-Winkelaar YM, Faber LM, de Kroon J, Brand A, Fibbe WE, Willemze R. 1991. Growth inhibition of clonogenic leukemic precursor cells by minor histocompatibility antigen-specific cytotoxic T lymphocytes. J Exp Med 174: 27–33. 10.1084/jem.174.1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficara F, Murphy MJ, Lin M, Cleary ML. 2008. Pbx1 regulates self-renewal of long-term hematopoietic stem cells by maintaining their quiescence. Cell Stem Cell 2: 484–496. 10.1016/j.stem.2008.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford CE, Hamerton JL, Barnes DW, Loutit JF. 1956. Cytological identification of radiation-chimæras. Nature 177: 452–454. 10.1038/177452a0 [DOI] [PubMed] [Google Scholar]
- Fox N, Priestley G, Papayannopoulou T, Kaushansky K. 2002. Thrombopoietin expands hematopoietic stem cells after transplantation. J Clin Invest 110: 389–394. 10.1172/JCI0215430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganuza M, Hall T, Finkelstein D, Wang YD, Chabot A, Kang G, Bi W, Wu G, McKinney-Freeman S. 2019. The global clonal complexity of the murine blood system declines throughout life and after serial transplantation. Blood 133: 1927–1942. 10.1182/blood-2018-09-873059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler EN, McFarland EC, Russell ES. 1981. Analysis of pleiotropism at the dominant white-spotting (W) locus of the house mouse: a description of ten new W alleles. Genetics 97: 337–361. 10.1093/genetics/97.2.337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves KA, Silberstein L, Li S, Severe N, Hu MG, Yang H, Scadden DT, Hu GF. 2016. Angiogenin promotes hematopoietic regeneration by dichotomously regulating quiescence of stem and progenitor cells. Cell 166: 894–906. 10.1016/j.cell.2016.06.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyama S, Yamamoto G, Shimabe M, Sato T, Ichikawa M, Ogawa S, Chiba S, Kurokawa M. 2008. Evi-1 is a critical regulator for hematopoietic stem cells and transformed leukemic cells. Cell Stem Cell 3: 207–220. 10.1016/j.stem.2008.06.002 [DOI] [PubMed] [Google Scholar]
- Greenbaum A, Hsu YMS, Day RB, Schuettpelz LG, Christopher MJ, Borgerding JN, Nagasawa T, Link DC. 2013. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature 495: 227–230. 10.1038/nature11926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo R, Hu F, Weng Q, Lv C, Wu H, Liu L, Li Z, Zeng Y, Bai Z, Zhang M, et al. 2020. Guiding T lymphopoiesis from pluripotent stem cells by defined transcription factors. Cell Res 30: 21–33. 10.1038/s41422-019-0251-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J, Wernig M, Markoulaki S, Sun C-W, Meissner A, Cassady JP, Beard C, Brambrink T, Wu LC, Townes TM, et al. 2007. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science 318: 1920–1923. 10.1126/science.1152092 [DOI] [PubMed] [Google Scholar]
- Hasemann MS, Lauridsen FKB, Waage J, Jakobsen JS, Frank A-K, Schuster MB, Rapin N, Bagger FO, Hoppe PS, Schroeder T, et al. 2014. C/EBPα is required for long-term self-renewal and lineage priming of hematopoietic stem cells and for the maintenance of epigenetic configurations in multipotent progenitors. PLoS Genet 10: e1004079. 10.1371/journal.pgen.1004079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho TT, Warr MR, Adelman ER, Lansinger OM, Flach J, Verovskaya EV, Figueroa ME, Passegué E. 2017. Autophagy maintains the metabolism and function of young and old stem cells. Nature 543: 205–210. 10.1038/nature21388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock H, Hamblen MJ, Rooke HM, Schindler JW, Saleque S, Fujiwara Y, Orkin SH. 2004. Gfi-1 restricts proliferation and preserves functional integrity of haematopoietic stem cells. Nature 431: 1002–1007. 10.1038/nature02994 [DOI] [PubMed] [Google Scholar]
- Hodgins JJ, Khan ST, Park MM, Auer RC, Ardolino M. 2019. Killers 2.0: NK cell therapies at the forefront of cancer control. J Clin Invest 129: 3499–3510. 10.1172/JCI129338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson GS, Bradley TR. 1979. Properties of haematopoietic stem cells surviving 5-fluorouracil treatment: evidence for a pre-CFU-S cell? Nature 281: 381–382. 10.1038/281381a0 [DOI] [PubMed] [Google Scholar]
- Hoggatt J, Singh P, Sampath J, Pelus LM. 2009. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood 113: 5444–5455. 10.1182/blood-2009-01-201335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, Rimm AA, Ringdén O, Rozman C, Speck B. 1990. Graft-versus-leukemia reactions after bone marrow transplantation. Blood 75: 555–562. 10.1182/blood.V75.3.555.555 [DOI] [PubMed] [Google Scholar]
- Howard A, Fernandez-Vina MA, Appelbaum FR, Confer DL, Devine SM, Horowitz MM, Mendizabal A, Laport GG, Pasquini MC, Spellman SR. 2015. Recommendations for donor human leukocyte antigen assessment and matching for allogeneic stem cell transplantation: consensus opinion of the blood and marrow transplant clinical trials network (BMT CTN). Biol Blood Marrow Transplant 21: 4–7. 10.1016/j.bbmt.2014.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh MM, Kang EM, Fitzhugh CD, Link MB, Bolan CD, Kurlander R, Childs RW, Rodgers GP, Powell JD, Tisdale JF. 2009. Allogeneic hematopoietic stem-cell transplantation for sickle cell disease. N Engl J Med 361: 2309–2317. 10.1056/NEJMoa0904971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Yu J, Suknuntha K, Tian S, Montgomery K, Choi K-D, Stewart R, Thomson JA, Slukvin II. 2011. Efficient generation of transgene-free induced pluripotent stem cells from normal and neoplastic bone marrow and cord blood mononuclear cells. Blood 117: e109–e119. 10.1182/blood-2010-07-298331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Suda T. 2014. Metabolic requirements for the maintenance of self-renewing stem cells. Nat Rev Mol Cell Biol 15: 243–256. 10.1038/nrm3772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Kwon HY, Zimdahl B, Congdon KL, Blum J, Lento WE, Zhao C, Lagoo A, Gerrard G, Foroni L, et al. 2010. Regulation of myeloid leukaemia by the cell fate determinant Musashi. Nature 466: 765–768. 10.1038/nature09171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Carracedo A, Weiss D, Arai F, Ala U, Avigan DE, Schafer ZT, Evans RM, Suda T, Lee CH, et al. 2012. A PML-PPARδ pathway for fatty acid oxidation regulates haematopoietic stem cell maintenance. Nat Med 18: 1350–1358. 10.1038/nm.2882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Turcotte R, Cui J, Zimmerman SE, Pinho S, Mizoguchi T, Arai F, Runnels JM, Alt C, Teruya-Feldstein J, et al. 2016. Self-renewal of a purified Tie2+ hematopoietic stem cell population relies on mitochondrial clearance. Science 354: 1156–1160. 10.1126/science.aaf5530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwama A, Oguro H, Negishi M, Kato Y, Morita Y, Tsukui H, Ema H, Kamijo T, Katoh-Fukui Y, Koseki H, et al. 2004. Enhanced self-renewal of hematopoietic stem cells mediated by the polycomb gene product Bmi-1. Immunity 21: 843–851. 10.1016/j.immuni.2004.11.004 [DOI] [PubMed] [Google Scholar]
- Jacobson LO, Simmons EL, Marks EK, Eldredge JH. 1951. Recovery from radiation injury. Science 113: 510–511. 10.1126/science.113.2940.510 [DOI] [PubMed] [Google Scholar]
- Jang YY, Sharkis SJ. 2007. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood 110: 3056–3063. 10.1182/blood-2007-05-087759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong M, Park HJ, Celik H, Ostrander EL, Reyes JM, Guzman A, Rodriguez B, Lei Y, Lee Y, Ding L, et al. 2018. Loss of Dnmt3a immortalizes hematopoietic stem cells in vivo. Cell Rep 23: 1–10. 10.1016/j.celrep.2018.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RJ, Wagner JE, Celano P, Zicha MS, Sharkis SJ. 1990. Separation of pluripotent haematopoietic stem cells from spleen colony-forming cells. Nature 347: 188–189. 10.1038/347188a0 [DOI] [PubMed] [Google Scholar]
- Karigane D, Takubo K. 2017. Metabolic regulation of hematopoietic and leukemic stem/progenitor cells under homeostatic and stress conditions. Int J Hematol 106: 18–26. 10.1007/s12185-017-2261-x [DOI] [PubMed] [Google Scholar]
- Karigane D, Kobayashi H, Morikawa T, Ootomo Y, Sakai M, Nagamatsu G, Kubota Y, Goda N, Matsumoto M, Nishimura EK, et al. 2016. P38α activates purine metabolism to initiate hematopoietic stem/progenitor cell cycling in response to stress. Cell Stem Cell 19: 192–204. 10.1016/j.stem.2016.05.013 [DOI] [PubMed] [Google Scholar]
- Keller JR, Mcniece IK, Sill KT, Ellingsworth LR, Quesenberry PJ, Sing GK, Ruscetti FW. 1990. Transforming growth factor beta directly regulates primitive murine hematopoietic cell proliferation. Blood 75: 596–602. 10.1182/blood.V75.3.596.596 [DOI] [PubMed] [Google Scholar]
- Khera N, Emmert A, Storer BE, Sandmaier BM, Alyea EP, Lee SJ. 2014. Costs of allogeneic hematopoietic cell transplantation using reduced intensity conditioning regimens. Oncologist 19: 639–644. 10.1634/theoncologist.2013-0406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. 2005. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121: 1109–1121. 10.1016/j.cell.2005.05.026 [DOI] [PubMed] [Google Scholar]
- Kim Y, Jeong J, Choi D. 2020. Small-molecule-mediated reprogramming: a silver lining for regenerative medicine. Exp Mol Med 52: 213–226. 10.1038/s12276-020-0383-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S, Roberts AW, Metcalf D, Alexander WS. 1998. Hematopoietic stem cell deficiencies in mice lacking c-Mpl, the receptor for thrombopoietin. Proc Natl Acad Sci 95: 1195–1200. 10.1073/pnas.95.3.1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King KY, Goodell MA. 2011. Inflammatory modulation of HSCs: viewing the HSC as a foundation for the immune response. Nat Rev Immunol 11: 685–692. 10.1038/nri3062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb HJ, Storb R, Graham TC, Kolb H, Thomas ED. 1973. Antithymocyte serum and methotrexate for control of graft-versus-host disease in dogs. Transplantation 16: 17–23. 10.1097/00007890-197307000-00004 [DOI] [PubMed] [Google Scholar]
- Kolb HJ, Mittermüller J, Clemm C, Holler E, Ledderose G, Brehm G, Heim M, Wilmanns W. 1990. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood 76: 2462–2465. 10.1182/blood.V76.12.2462.2462 [DOI] [PubMed] [Google Scholar]
- Koreth J, Schlenk R, Kopecky KJ, Honda S, Sierra J, Djulbegovic BJ, Wadleigh M, DeAngelo DJ, Stone RM, Sakamaki H, et al. 2009. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA 301: 2349–2361. 10.1001/jama.2009.813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, Mizoguchi T, Wei Q, Lucas D, Ito K, et al. 2013. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 502: 637–643. 10.1038/nature12612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunisato A, Chiba S, Nakagami-Yamaguchi E, Kumano K, Saito T, Masuda S, Yamaguchi T, Osawa M, Kageyama R, Nakauchi H, et al. 2003. HES-1 preserves purified hematopoietic stem cells ex vivo and accumulates side population cells in vivo. Blood 101: 1777–1783. 10.1182/blood-2002-07-2051 [DOI] [PubMed] [Google Scholar]
- Kyba M, Perlingeiro RCR, Daley GQ. 2002. Hoxb4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell 109: 29–37. 10.1016/S0092-8674(02)00680-3 [DOI] [PubMed] [Google Scholar]
- Lacombe J, Herblot S, Rojas-Sutterlin S, Haman A, Barakat S, Iscove NN, Sauvageau G, Hoang T. 2010. Scl regulates the quiescence and the long-term competence of hematopoietic stem cells. Blood 115: 792–803. 10.1182/blood-2009-01-201384 [DOI] [PubMed] [Google Scholar]
- Lacorazza HD, Yamada T, Liu Y, Miyata Y, Sivina M, Nunes J, Nimer SD. 2006. The transcription factor MEF/ELF4 regulates the quiescence of primitive hematopoietic cells. Cancer Cell 9: 175–187. 10.1016/j.ccr.2006.02.017 [DOI] [PubMed] [Google Scholar]
- Lee J-M, Govindarajah V, Goddard B, Hinge A, Muench DE, Filippi M-D, Aronow B, Cancelas JA, Salomonis N, Grimes HL, et al. 2018. Obesity alters the long-term fitness of the hematopoietic stem cell compartment through modulation of Gfi1 expression. J Exp Med 215: 627–644. 10.1084/jem.20170690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lento W, Ito T, Zhao C, Harris JR, Huang W, Jiang C, Owzar K, Piryani S, Racioppi L, Chao N, et al. 2014. Loss of β-catenin triggers oxidative stress and impairs hematopoietic regeneration. Genes Dev 28: 995–1004. 10.1101/gad.231944.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard A, Tisdale J, Abraham A. 2020. Curative options for sickle cell disease: haploidentical stem cell transplantation or gene therapy? Br J Haematol 189: 408–423. 10.1111/bjh.16437 [DOI] [PubMed] [Google Scholar]
- Li CL, Johnson GR. 1994. Stem cell factor enhances the survival but not the self-renewal of murine hematopoietic long-term repopulating cells. Blood 84: 408–414. 10.1182/blood.V84.2.408.408 [DOI] [PubMed] [Google Scholar]
- Li T, Zhou ZW, Ju Z, Wang ZQ. 2016. DNA damage response in hematopoietic stem cell ageing. Genomics Proteomics Bioinformatics 14: 147–154. 10.1016/j.gpb.2016.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Hermanson DL, Moriarity BS, Kaufman DS. 2018a. Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell Stem Cell 23: 181–192.e5. 10.1016/j.stem.2018.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Qian P, Shao W, Shi H, He XC, Gogol M, Yu Z, Wang Y, Qi M, Zhu Y, et al. 2018b. Suppression of m6A reader Ythdf2 promotes hematopoietic stem cell expansion. Cell Res 28: 904–917. 10.1038/s41422-018-0072-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Elf SE, Miyata Y, Sashida G, Liu Y, Huang G, Di Giandomenico S, Lee JM, Deblasio A, Menendez S, et al. 2009. P53 regulates hematopoietic stem cell quiescence. Cell Stem Cell 4: 37–48. 10.1016/j.stem.2008.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz E, Uphoff D, Reid TR, Shelton E. 1951. Modification of irradiation injury in mice and Guinea pigs by bone marrow injections. J Natl Cancer Inst 12: 197–201. [PubMed] [Google Scholar]
- Luznik L, O'Donnell PV, Fuchs EJ. 2012. Post-transplantation cyclophosphamide for tolerance induction in HLA-haploidentical bone marrow transplantation. Semin Oncol 39: 683–693. 10.1053/j.seminoncol.2012.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magli MC, Iscove NN, Odartchenko N. 1982. Transient nature of early haematopoietic spleen colonies. Nature 295: 527–529. 10.1038/295527a0 [DOI] [PubMed] [Google Scholar]
- Mantel C, Messina-Graham S, Moh A, Cooper S, Hangoc G, Fu X-Y, Broxmeyer HE. 2012. Mouse hematopoietic cell-targeted STAT3 deletion: stem/progenitor cell defects, mitochondrial dysfunction, ROS overproduction, and a rapid aging-like phenotype. Blood 120: 2589–2599. 10.1182/blood-2012-01-404004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers GD, et al. 2018. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 378: 439–448. 10.1056/NEJMoa1709866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch EA, Till JE. 1960. The radiation sensitivity of normal mouse bone marrow cells, determined by quantitative marrow transplantation into irradiated mice. Radiat Res 13: 115–125. 10.2307/3570877 [DOI] [PubMed] [Google Scholar]
- McRae HM, Voss AK, Thomas T. 2019. Are transplantable stem cells required for adult hematopoiesis? Exp Hematol 75: 1–10. 10.1016/j.exphem.2019.05.007 [DOI] [PubMed] [Google Scholar]
- Megías J, Yáñez A, Moriano S, O'Connor JE, Gozalbo D, Gil M-L. 2012. Direct toll-like receptor-mediated stimulation of hematopoietic stem and progenitor cells occurs in vivo and promotes differentiation toward macrophages. Stem Cells 30: 1486–1495. 10.1002/stem.1110 [DOI] [PubMed] [Google Scholar]
- Méndez-Ferrer S, Lucas D, Battista M, Frenette PS. 2008. Haematopoietic stem cell release is regulated by circadian oscillations. Nature 452: 442–447. 10.1038/nature06685 [DOI] [PubMed] [Google Scholar]
- Metcalf D, Nicola NA. 1991. Direct proliferative actions of stem cell factor on murine bone marrow cells in vitro: effects of combination with colony-stimulating factors. Proc Natl Acad Sci 88: 6239–6243. 10.1073/pnas.88.14.6239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miharada K, Sigurdsson V, Karlsson S. 2014. Dppa5 improves hematopoietic stem cell activity by reducing endoplasmic reticulum stress. Cell Rep 7: 1381–1392. 10.1016/j.celrep.2014.04.056 [DOI] [PubMed] [Google Scholar]
- Min IM, Pietramaggiori G, Kim FS, Passegué E, Stevenson KE, Wagers AJ. 2008. The transcription factor EGR1 controls both the proliferation and localization of hematopoietic stem cells. Cell Stem Cell 2: 380–391. 10.1016/j.stem.2008.01.015 [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Araki KY, Naka K, Arai F, Takubo K, Yamazaki S, Matsuoka S, Miyamoto T, Ito K, Ohmura M, et al. 2007. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell 1: 101–112. 10.1016/j.stem.2007.02.001 [DOI] [PubMed] [Google Scholar]
- Mohrin M, Bourke E, Alexander D, Warr MR, Barry-Holson K, Le Beau MM, Morrison CG, Passegué E. 2010. Hematopoietic stem cell quiescence promotes error-prone DNA repair and mutagenesis. Cell Stem Cell 7: 174–185. 10.1016/j.stem.2010.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran-Crusio K, Reavie L, Shih A, Abdel-Wahab O, Ndiaye-Lobry D, Lobry C, Figueroa ME, Vasanthakumar A, Patel J, Zhao X, et al. 2011. Tet2 loss leads to increased hematopoietic stem cell self-renewal and myeloid transformation. Cancer Cell 20: 11–24. 10.1016/j.ccr.2011.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Hemmati HD, Wandycz AM, Weissman IL. 1995. The purification and characterization of fetal liver hematopoietic stem cells. Proc Natl Acad Sci 92: 10302–10306. 10.1073/pnas.92.22.10302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Wandycz AM, Akashi K, Globerson A, Weissman IL. 1996. The aging of hematopoietic stem cells. Nat Med 2: 1011–1016. 10.1038/nm0996-1011 [DOI] [PubMed] [Google Scholar]
- Nagler A, Slavin S, Varadi G, Naparstek E, Samuel S, Or R. 2000. Allogeneic peripheral blood stem cell transplantation using a fludarabine-based low intensity conditioning regimen for malignant lymphoma. Bone Marrow Transplant 25: 1021–1028. 10.1038/sj.bmt.1702392 [DOI] [PubMed] [Google Scholar]
- Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T, Lin Y, et al. 2017. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 377: 2531–2544. 10.1056/NEJMoa1707447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth MJ, Topol L, Anderson SM, Yang Y, Bodine DM. 2007. Wnt5a inhibits canonical Wnt signaling in hematopoietic stem cells and enhances repopulation. Proc Natl Acad Sci 104: 15436–15441. 10.1073/pnas.0704747104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Y, Han YC, Zou YR. 2008. CXCR4 is required for the quiescence of primitive hematopoietic cells. J Exp Med 205: 777–783. 10.1084/jem.20072513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederwieser D, Grassegger A, Aubock J, Herold M, Nachbaur D, Rosenmayr A, Gachter A, Nussbaumer W, Gaggl S, Ritter M. 1993. Correlation of minor histocompatibility antigen-specific cytotoxic T lymphocytes with graft-versus-host disease status and analyses of tissue distribution of their target antigens. Blood 81: 2200–2208. 10.1182/blood.V81.8.2200.2200 [DOI] [PubMed] [Google Scholar]
- Ogawa M, Matsuzaki Y, Nishikawa S, Hayashi S, Kunisada T, Sudo T, Kina T, Nakauchi H, Nishikawa S. 1991. Expression and function of c-kit in hemopoietic progenitor cells. J Exp Med 174: 63–71. 10.1084/jem.174.1.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliansky DM, Czuczman M, Fisher RI, Irwin FD, Lazarus HM, Omel J, Vose J, Wolff SN, Jones RB, McCarthy PL, et al. 2011. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the treatment of diffuse large B cell lymphoma: update of the 2001 evidence-based review. Biol Blood Marrow Transplant 17: 20–47.e30. 10.1016/j.bbmt.2010.07.008 [DOI] [PubMed] [Google Scholar]
- Osawa M, Hanada K, Hamada H, Nakauchi H. 1996. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science 273: 242–245. 10.1126/science.273.5272.242 [DOI] [PubMed] [Google Scholar]
- Osgood E, Riddle M, Mathews T. 1939. Aplastic anemia treated with daily transfusions and intravenous marrow; case report. Ann Intern Med 13: 357–367. 10.7326/0003-4819-13-2-357 [DOI] [Google Scholar]
- Owen RD. 1945. Immunogenetic consequences of vascular anastomoses between bovine twins. Science 102: 400–401. 10.1126/science.102.2651.400 [DOI] [PubMed] [Google Scholar]
- Pagliuca FW, Millman JR, Gürtler M, Segel M, Van Dervort A, Ryu JH, Peterson QP, Greiner D, Melton DA. 2014. Generation of functional human pancreatic β cells in vitro. Cell 159: 428–439. 10.1016/j.cell.2014.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo A, Cavallo F, Gay F, Di Raimondo F, Ben Yehuda D, Petrucci MT, Pezzatti S, Caravita T, Cerrato C, Ribakovsky E, et al. 2014. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med 371: 895–905. 10.1056/NEJMoa1402888 [DOI] [PubMed] [Google Scholar]
- Park I, Qian D, Kiel M, Becker MW, Pihalja M, Weissman IL, Morrison SJ, Clarke MF. 2003. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature 423: 302–305. 10.1038/nature01587 [DOI] [PubMed] [Google Scholar]
- Park JH, Rivière I, Gonen M, Wang X, Sénéchal B, Curran KJ, Sauter C, Wang Y, Santomasso B, Mead E, et al. 2018. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med 378: 449–459. 10.1056/NEJMoa1709919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD. 2007. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci 104: 5431–5436. 10.1073/pnas.0701152104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan R, Arora M, Chen M. 2020. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR US summary slides, 2020. The Medical College of Wisconsin and the National Marrow Donor Program.
- Philip T, Guglielmi C, Hagenbeek A, Somers R, Van der Lelie H, Bron D, Sonneveld P, Gisselbrecht C, Cahn JY, Harousseau JL, et al. 1995. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphoma. N Engl J Med 333: 1540–1545. 10.1056/NEJM199512073332305 [DOI] [PubMed] [Google Scholar]
- Pidala J, Lee SJ, Ahn KW, Spellman S, Wang H-L, Aljurf M, Askar M, Dehn J, Fernandez Viña M, Gratwohl A, et al. 2014. Nonpermissive HLA-DPB1 mismatch increases mortality after myeloablative unrelated allogeneic hematopoietic cell transplantation. Blood 124: 2596–2606. 10.1182/blood-2014-05-576041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon LM, Hamdi A, Saliba R, Rondon G, Ledesma C, Kendrick M, Qazilbash M, Hosing C, Jones RB, Popat UR, et al. 2013. Outcomes of adults with acute lymphoblastic leukemia relapsing after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 19: 1059–1064. 10.1016/j.bbmt.2013.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H, Buza-Vidas N, Hyland CD, Jensen CT, Antonchuk J, Månsson R, Thoren LA, Ekblom M, Alexander WS, Jacobsen SEW. 2007. Critical role of thrombopoietin in maintaining adult quiescent hematopoietic stem cells. Cell Stem Cell 1: 671–684. 10.1016/j.stem.2007.10.008 [DOI] [PubMed] [Google Scholar]
- Raje N, Berdeja J, Lin Y, Siegel D, Jagannath S, Madduri D, Liedtke M, Rosenblatt J, Maus MV, Turka A, et al. 2019. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med 380: 1726–1737. 10.1056/NEJMoa1817226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renders S, Svendsen AF, Panten J, Rama N, Maryanovich M, Sommerkamp P, Ladel L, Redavid AR, Gibert B, Lazare S, et al. 2021. Niche derived netrin-1 regulates hematopoietic stem cell dormancy via its receptor neogenin-1. Nat Commun 12: 608. 10.1038/s41467-020-20801-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentas S, Holzapfel N, Belew MS, Pratt G, Voisin V, Wilhelm BT, Bader GD, Yeo GW, Hope KJ. 2016. Musashi-2 attenuates AHR signalling to expand human haematopoietic stem cells. Nature 532: 508–511. 10.1038/nature17665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL. 2003. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature 423: 409–414. 10.1038/nature01593 [DOI] [PubMed] [Google Scholar]
- Ringdén O, Pavletic SZ, Anasetti C, Barrett AJ, Wang T, Wang D, Antin JH, Di Bartolomeo P, Bolwell BJ, Bredeson C, et al. 2009. The graft-versus-leukemia effect using matched unrelated donors is not superior to HLA-identical siblings for hematopoietic stem cell transplantation. Blood 113: 3110–3118. 10.1182/blood-2008-07-163212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Fraticelli AE, Weinreb CS, Wang S-W, Migueles RP, Jankovic M, Usart M, Klein AM, Lowell S, Camargo FD. 2020. Single-cell lineage tracing unveils a role for Tcf15 in haematopoiesis. Nature 583: 585–589. 10.1038/s41586-020-2503-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Moya D, Bueno C, Montes R, Navarro-Montero O, Iborra FJ, López LC, Martin M, Menendez P. 2013. Cord blood-derived CD34+ hematopoietic cells with low mitochondrial mass are enriched in hematopoietic repopulating stem cell function. Haematologica 98: 1022–1029. 10.3324/haematol.2012.079244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri L, Capanni M, Casucci M, Volpi I, Tosti A, Perruccio K, Urbani E, Negrin RS, Martelli MF, Velardi A. 1999. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood 94: 333–339. 10.1182/blood.V94.1.333.413a31_333_339 [DOI] [PubMed] [Google Scholar]
- Ruggeri L, Mancusi A, Capanni M, Urbani E, Carotti A, Aloisi T, Stern M, Pende D, Perruccio K, Burchielli E, et al. 2007. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. Blood 110: 433–440. 10.1182/blood-2006-07-038687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos GW, Owens AH. 1969. Allogeneic marrow transplants in cyclophosphamide treated mice. Transplant Proc 1: 44–46. [PubMed] [Google Scholar]
- Sato T, Onai N, Yoshihara H, Arai F, Suda T, Ohteki T. 2009. Interferon regulatory factor-2 protects quiescent hematopoietic stem cells from type I interferon-dependent exhaustion. Nat Med 15: 696–700. 10.1038/nm.1973 [DOI] [PubMed] [Google Scholar]
- Schoedel KB, Morcos MNF, Zerjatke T, Roeder I, Grinenko T, Voehringer D, Göthert JR, Waskow C, Roers A, Gerbaulet A. 2016. The bulk of the hematopoietic stem cell population is dispensable for murine steady-state and stress hematopoiesis. Blood 128: 2285–2296. 10.1182/blood-2016-03-706010 [DOI] [PubMed] [Google Scholar]
- Schrappe M, Hunger SP, Pui CH, Saha V, Gaynon PS, Baruchel A, Conter V, Otten J, Ohara A, Versluys AB, et al. 2012. Outcomes after induction failure in childhood acute lymphoblastic leukemia. N Engl J Med 366: 1371–1381. 10.1056/NEJMoa1110169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrauder A, Reiter A, Gadner H, Niethammer D, Klingebiel T, Kremens B, Peters C, Ebell W, Zimmermann M, Niggli F, et al. 2006. Superiority of allogeneic hematopoietic stem-cell transplantation compared with chemotherapy alone in high-risk childhood T-cell acute lymphoblastic leukemia: results from ALL-BFM 90 and 95. J Clin Oncol 24: 5742–5749. 10.1200/JCO.2006.06.2679 [DOI] [PubMed] [Google Scholar]
- Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, Jäger U, Jaglowski S, Andreadis C, Westin JR, et al. 2019. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med 380: 45–56. 10.1056/NEJMoa1804980 [DOI] [PubMed] [Google Scholar]
- Seibel NL, Steinherz PG, Sather HN, Nachman JB, Delaat C, Ettinger LJ, Freyer DR, Mattano LA, Hastings CA, Rubin CM, et al. 2008. Early postinduction intensification therapy improves survival for children and adolescents with high-risk acute lymphoblastic leukemia: a report from the Children's Oncology Group. Blood 111: 2548–2555. 10.1182/blood-2007-02-070342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seita J, Ema H, Ooehara J, Yamazaki S, Tadokoro Y, Yamasaki A, Eto K, Takaki S, Takatsu K, Nakauchi H. 2007. Lnk negatively regulates self-renewal of hematopoietic stem cells by modifying thrombopoietin-mediated signal transduction. Proc Natl Acad Sci 104: 2349–2354. 10.1073/pnas.0606238104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan W, Wang B, Xu Y, Li X, Li X, Wang H, Lin Y, Tie R, Zhao Q, Wang J, et al. 2020. Generation of hematopoietic cells from mouse pluripotent stem cells in a 3D culture system of self-assembling peptide hydrogel. J Cell Physiol 235: 2080–2090. 10.1002/jcp.29110 [DOI] [PubMed] [Google Scholar]
- Sharma Y, Astle CM, Harrison DE. 2007. Heterozygous kit mutants with little or no apparent anemia exhibit large defects in overall hematopoietic stem cell function. Exp Hematol 35: 214.e1–214.e9. 10.1016/j.exphem.2006.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieburg HB, Rezner BD, Muller-Sieburg CE. 2011. Predicting clonal self-renewal and extinction of hematopoietic stem cells. Proc Natl Acad Sci 108: 4370–4375. 10.1073/pnas.1011414108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signer RAJ, Magee JA, Salic A, Morrison SJ. 2014. Haematopoietic stem cells require a highly regulated protein synthesis rate. Nature 509: 49–54. 10.1038/nature13035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberstein L, Goncales KA, Kharchenko PV, Turcotte R, Kfoury Y, Mercier F, Baryawno N, Severe N, Bachand J, Spencer JA, et al. 2016. Proximity-based differential single-cell analysis of the niche to identify stem/progenitor cell regulators. Cell Stem Cell 19: 530–543. 10.1016/j.stem.2016.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siminovitch L, McCulloch EA, Till JE. 1963. The distribution of colony-forming cells among spleen colonies. J Cell Comp Physiol 62: 327–336. 10.1002/jcp.1030620313 [DOI] [PubMed] [Google Scholar]
- Simsek T, Kocabas F, Zheng J, Deberardinis RJ, Mahmoud AI, Olson EN, Schneider JW, Zhang CC, Sadek HA. 2010. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell 7: 380–390. 10.1016/j.stem.2010.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitnicka E, Lin N, Priestley GV, Fox N, Broudy VC, Wolf NS, Kaushansky K. 1996. The effect of thrombopoietin on the proliferation and differentiation of murine hematopoietic stem cells. Blood 87: 4998–5005. 10.1182/blood.V87.12.4998.bloodjournal87124998 [DOI] [PubMed] [Google Scholar]
- Slavin S, Nagler A, Naparstek E, Kapelushnik Y, Aker M, Cividalli G, Varadi G, Kirschbaum M, Ackerstein A, Samuel S, et al. 1998. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood 91: 756–763. 10.1182/blood.V91.3.756 [DOI] [PubMed] [Google Scholar]
- Spangrude GJ, Heimfeld S, Weissman IL. 1988. Purification and characterization of mouse hematopoietic stem cells. Science 241: 58–62. 10.1126/science.2898810 [DOI] [PubMed] [Google Scholar]
- Spencer JA, Ferraro F, Roussakis E, Klein A, Wu J, Runnels JM, Zaher W, Mortensen LJ, Alt C, Turcotte R, et al. 2014. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 508: 269–273. 10.1038/nature13034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staber PB, Zhang P, Ye M, Welner RS, Nombela-Arrieta C, Bach C, Kerenyi M, Bartholdy BA, Zhang H, Alberich-Jordà M, et al. 2013. Sustained PU.1 levels balance cell-cycle regulators to prevent exhaustion of adult hematopoietic stem cells. Mol Cell 49: 934–946. 10.1016/j.molcel.2013.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stier S, Cheng T, Dombkowski D, Carlesso N, Scadden DT. 2002. Notch1 activation increases hematopoietic stem cell self-renewal in vivo and favors lymphoid over myeloid lineage outcome. Blood 99: 2369–2378. 10.1182/blood.V99.7.2369 [DOI] [PubMed] [Google Scholar]
- Storb R, Thomas ED. 1972. Bone marrow transplantation in randomly bred animal species and in man. In Proceedings of the Sixth Leucocyte Culture Conference, pp. 805–840. Academic Press, New York. [Google Scholar]
- Storb R, Epstein RB, Graham TC, Thomas ED. 1970. Methotrexate regimens for control of graft-versus-host disease in dogs with allogeneic marrow grafts. Transplantation 9: 240–246. 10.1097/00007890-197003000-00007 [DOI] [PubMed] [Google Scholar]
- Sudo K, Ema H, Morita Y, Nakauchi H. 2000. Age-associated characteristics of murine hematopoietic stem cells. J Exp Med 192: 1273–1280. 10.1084/jem.192.9.1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimura R, Jha DK, Han A, Soria-Valles C, da Rocha EL, Lu YF, Goettel JA, Serrao E, Rowe RG, Malleshaiah M, et al. 2017. Haematopoietic stem and progenitor cells from human pluripotent stem cells. Nature 545: 432–438. 10.1038/nature22370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T, Kohara H, Noda M, Nagasawa T. 2006. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 25: 977–988. 10.1016/j.immuni.2006.10.016 [DOI] [PubMed] [Google Scholar]
- Sun J, Ramos A, Chapman B, Johnnidis JB, Le L, Ho YJ, Klein A, Hofmann O, Camargo FD. 2014. Clonal dynamics of native haematopoiesis. Nature 514: 322–327. 10.1038/nature13824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Jiang N, Jiang Y, He Q, He H, Wang X, Yang L, Li R, Liu F, Lin X, et al. 2020. Chromatin remodeler Znhit1 preserves hematopoietic stem cell quiescence by determining the accessibility of distal enhancers. Leukemia 34: 3348–3358. 10.1038/s41375-020-0988-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Yamazaki S, Yamaguchi T, Okabe M, Masaki H, Takaki S, Otsu M, Nakauchi H. 2013. Generation of engraftable hematopoietic stem cells from induced pluripotent stem cells by way of teratoma formation. Mol Ther 21: 1424–1431. 10.1038/mt.2013.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney C, Vyas P. 2019. The graft-versus-leukemia effect in AML. Front Oncol 9: 1217. 10.3389/fonc.2019.01217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switzer GE, Bruce JG, Myaskovsky L, DiMartini A, Shellmer D, Confer DL, Abress LK, King RJ, Harnaha AG, Ohngemach S, et al. 2013. Race and ethnicity in decisions about unrelated hematopoietic stem cell donation. Blood 121: 1469–1476. 10.1182/blood-2012-06-437343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilvassy SJ, Humphries RK, Lansdorp PM, Eaves AC, Eaves CJ. 1990. Quantitative assay for totipotent reconstituting hematopoietic stem cells by a competitive repopulation strategy. Proc Natl Acad Sci 87: 8736–8740. 10.1073/pnas.87.22.8736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. 2006. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676. 10.1016/j.cell.2006.07.024 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. 2007. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131: 861–872. 10.1016/j.cell.2007.11.019 [DOI] [PubMed] [Google Scholar]
- Takubo K, Goda N, Yamada W, Iriuchishima H, Ikeda E, Kubota Y, Shima H, Johnson RS, Hirao A, Suematsu M, et al. 2010. Regulation of the HIF-1α level is essential for hematopoietic stem cells. Cell Stem Cell 7: 391–402. 10.1016/j.stem.2010.06.020 [DOI] [PubMed] [Google Scholar]
- Takubo K, Nagamatsu G, Kobayashi CI, Nakamura-Ishizu A, Kobayashi H, Ikeda E, Goda N, Rahimi Y, Johnson RS, Soga T, et al. 2013. Regulation of glycolysis by Pdk functions as a metabolic checkpoint for cell cycle quiescence in hematopoietic stem cells. Cell Stem Cell 12: 49–61. 10.1016/j.stem.2012.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas ED, Lochte HL, Lu WC, Ferrebee JW. 1957. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med 257: 491–496. 10.1056/NEJM195709122571102 [DOI] [PubMed] [Google Scholar]
- Thomas ED, Lochte HL, Cannon JH, Sahler OD, Ferrebee JW. 1959. Supralethal whole body irradiation and isologous marrow transplantation in man. J Clin Invest 38: 1709–1716. 10.1172/JCI103949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E, Storb R, Clift RA, Fefer A, Johnson FL, Neiman PE, Lerner KG, Glucksberg H, Buckner CD. 1975. Bone-marrow transplantation (first of two parts). N Engl J Med 292: 832–843. 10.1056/NEJM197504172921605 [DOI] [PubMed] [Google Scholar]
- Thomas ED, Buckner CD, Banaji M, Clift RA, Fefer A, Flournoy N, Goodell BW, Hickman RO, Lerner KG, Neiman PE, et al. 1977. One hundred patients with acute leukemia treated by chemotherapy, total body irradiation, and allogeneic marrow transplantation. Blood 49: 511–533. 10.1182/blood.V49.4.511.511 [DOI] [PubMed] [Google Scholar]
- Thomas ED, Buckner CD, Clift RA, Fefer A, Johnson FL, Neiman PE, Sale GE, Sanders JE, Singer JW, Shulman H, et al. 1979. Marrow transplantation for acute nonlymphoblastic leukemia in first remission. N Engl J Med 301: 597–599. 10.1056/NEJM197909133011109 [DOI] [PubMed] [Google Scholar]
- Tikhonova AN, Dolgalev I, Hu H, Sivaraj KK, Hoxha E, Cuesta-Domínguez Á, Pinho S, Akhmetzyanova I, Gao J, Witkowski M, et al. 2019. The bone marrow microenvironment at single-cell resolution. Nature 569: 222–228. 10.1038/s41586-019-1104-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till JE, McCulloch EA. 1961. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res 14: 213–222. 10.2307/3570892 [DOI] [PubMed] [Google Scholar]
- Till JE, McCulloch EA, Siminovitch L. 1964. A stochastic model of stem cell proliferation, based on the growth of spleen colony-forming cells. Proc Natl Acad Sci 51: 29–36. 10.1073/pnas.51.1.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, et al. 2007. Foxos are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell 128: 325–339. 10.1016/j.cell.2007.01.003 [DOI] [PubMed] [Google Scholar]
- Trentin JJ. 1956. Mortality and skin transplantability in x-irradiated mice receiving isologous, homologous or heterologous bone marrow. Proc Soc Exp Biol Med 92: 688–693. 10.3181/00379727-92-22582 [DOI] [PubMed] [Google Scholar]
- Tsapogas P, Mooney CJ, Brown G, Rolink A. 2017. The cytokine Flt3-ligand in normal and malignant hematopoiesis. Int J Mol Sci 18: 1115. 10.3390/ijms18061115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoi MS, Storb R, Dobbs S, Medill L, Thomas ED. 1980. Cell-mediated immunity to non-HLA antigens of the host by donor lymphocytes in patients with chronic graft-vs-host disease. J Immunol 125: 2258–2262. [PubMed] [Google Scholar]
- Tsukada M, Ota Y, Wilkinson AC, Becker HJ, Osato M, Nakauchi H, Yamazaki S. 2017. In vivo generation of engraftable murine hematopoietic stem cells by Gfi1b, c-Fos, and Gata2 overexpression within teratoma. Stem Cell Reports 9: 1024–1033. 10.1016/j.stemcr.2017.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemoto T, Hashimoto M, Matsumura T, Nakamura-Ishizu A, Suda T. 2018. Ca2+-mitochondria axis drives cell division in hematopoietic stem cells. J Exp Med 215: 2097–2113. 10.1084/jem.20180421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Os R, Kamminga LM, Ausema A, Bystrykh LV, Draijer DP, van Pelt K, Dontje B, de Haan G. 2007. A limited role for p21Cip1/Waf1 in maintaining normal hematopoietic stem cell functioning. Stem Cells 25: 836–843. 10.1634/stemcells.2006-0631 [DOI] [PubMed] [Google Scholar]
- van Rood JJ, van Leeuwen A, Schippers A, Balner H. 1968. Human histocompatibility antigens in normal and neoplastic tissues. Cancer Res 28: 1415–1422. [PubMed] [Google Scholar]
- Varnum-Finney B, Xu L, Brashem-Stein C, Nourigat C, Flowers D, Bakkour S, Pear WS, Bernstein ID. 2000. Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nat Med 6: 1278–1281. 10.1038/81390 [DOI] [PubMed] [Google Scholar]
- Vogl DT, Stadtmauer EA. 2006. High-dose chemotherapy and autologous hematopoietic stem cell transplantation for metastatic breast cancer: a therapy whose time has passed. Bone Marrow Transplant 37: 985–987. 10.1038/sj.bmt.1705366 [DOI] [PubMed] [Google Scholar]
- Walter D, Lier A, Geiselhart A, Thalheimer FB, Huntscha S, Sobotta MC, Moehrle B, Brocks D, Bayindir I, Kaschutnig P, et al. 2015. Exit from dormancy provokes DNA-damage-induced attrition in haematopoietic stem cells. Nature 520: 549–552. 10.1038/nature14131 [DOI] [PubMed] [Google Scholar]
- Wang T, Nandakumar V, Jiang XX, Jones L, Yang AG, Huang XF, Chen SY. 2013. The control of hematopoietic stem cell maintenance, self-renewal, and differentiation by Mysm1-mediated epigenetic regulation. Blood 122: 2812–2822. 10.1182/blood-2013-03-489641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren EH, Greenberg PD, Riddell SR. 1998. Cytotoxic T-lymphocyte-defined human minor histocompatibility antigens with a restricted tissue distribution. Blood 91: 2197–2207. 10.1182/blood.V91.6.2197 [DOI] [PubMed] [Google Scholar]
- Weick JP. 2016. Functional properties of human stem cell-derived neurons in health and disease. Stem Cells Int 2016: 4190438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman IL, Shizuru JA. 2008. The origins of the identification and isolation of hematopoietic stem cells, and their capability to induce donor-specific transplantation tolerance and treat autoimmune diseases. Blood 112: 3543–3553. 10.1182/blood-2008-08-078220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson AC, Ishida R, Kikuchi M, Sudo K, Morita M, Crisostomo RV, Yamamoto R, Loh KM, Nakamura Y, Watanabe M, et al. 2019. Long-term ex vivo hematopoietic stem cell expansion allows nonconditioned transplantation. Nature 571: 117–121. 10.1038/s41586-019-1244-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, Laurenti E, Oser G, van der Wath R, Blanco-Bose W, Jaworski M, Offner S, Dunant CF, Eshkind L, Bockamp E, et al. 2008. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell 135: 1118–1129. 10.1016/j.cell.2008.10.048 [DOI] [PubMed] [Google Scholar]
- Wu AM, Till JE, Siminovitch L, McCulloch EA. 1967. A cytological study of the capacity for differentiation of normal hemopoietic colony-forming cells. J Cell Physiol 69: 177–184. 10.1002/jcp.1040690208 [DOI] [PubMed] [Google Scholar]
- Wu AM, Till JE, Siminovitch L, McCulloch EA. 1968. Cytological evidence for a relationship between normal hematopoietic colony-forming cells and cells of the lymphoid system. J Exp Med 127: 455–464. 10.1084/jem.127.3.455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto R, Wilkinson AC, Ooehara J, Lan X, Lai CY, Nakauchi Y, Pritchard JK, Nakauchi H. 2018. Large-scale clonal analysis resolves aging of the mouse hematopoietic stem cell compartment. Cell Stem Cell 22: 600–607.e4. 10.1016/j.stem.2018.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S, Iwama A, Takayanagi S, Eto K, Ema H, Nakauchi H. 2009. TGF-β as a candidate bone marrow niche signal to induce hematopoietic stem cell hibernation. Blood 113: 1250–1256. 10.1182/blood-2008-04-146480 [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Ema H, Karlsson G, Yamaguchi T, Miyoshi H, Shioda S, Taketo MM, Karlsson S, Iwama A, Nakauchi H. 2011. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell 147: 1146–1158. 10.1016/j.cell.2011.09.053 [DOI] [PubMed] [Google Scholar]
- Yanada M, Matsuo K, Emi N, Naoe T. 2005. Efficacy of allogeneic hematopoietic stem cell transplantation depends on cytogenetic risk for acute myeloid leukemia in first disease remission: a metaanalysis. Cancer 103: 1652–1658. 10.1002/cncr.20945 [DOI] [PubMed] [Google Scholar]
- Yang L, Bryder D, Adolfsson J, Nygren J, Månsson R, Sigvardsson M, Jacobsen SEW. 2005. Identification of Lin− Sca1+kit+CD34+Flt3− short-term hematopoietic stem cells capable of rapidly reconstituting and rescuing myeloablated transplant recipients. Blood 105: 2717–2723. 10.1182/blood-2004-06-2159 [DOI] [PubMed] [Google Scholar]
- Yang Q, Esplin B, Borghesi L. 2011. E47 regulates hematopoietic stem cell proliferation and energetics but not myeloid lineage restriction. Blood 117: 3529–3538. 10.1182/blood-2010-07-297689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M, Zhang H, Amabile G, Yang H, Staber PB, Zhang P, Levantini E, Alberich-Jordà M, Zhang J, Kawasaki A, et al. 2013. C/EBPa controls acquisition and maintenance of adult haematopoietic stem cell quiescence. Nat Cell Biol 15: 385–394. 10.1038/ncb2698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara H, Arai F, Hosokawa K, Hagiwara T, Takubo K, Nakamura Y, Gomei Y, Iwasaki H, Matsuoka S, Miyamoto K, et al. 2007. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell 1: 685–697. 10.1016/j.stem.2007.10.020 [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. 2007. Induced pluripotent stem cell lines derived from human somatic cells. Science 318: 1917–1920. 10.1126/science.1151526 [DOI] [PubMed] [Google Scholar]
- Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, Thomson JA. 2009. Human induced pluripotent stem cells free of vector and transgene sequences. Science 324: 797–801. 10.1126/science.1172482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu VWC, Yusuf RZ, Oki T, Wu J, Saez B, Wang X, Cook C, Baryawno N, Ziller MJ, Lee E, et al. 2017. Epigenetic memory underlies cell-autonomous heterogeneous behavior of hematopoietic stem cells. Cell 168: 944–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Ling F, Wang HC, Sun XH. 2013. Chronic TLR signaling impairs the long-term repopulating potential of hematopoietic stem cells of wild type but not Id1 deficient mice. PLoS ONE 8: e55552. 10.1371/journal.pone.0055552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Perry JM, Marshall H, Venkatraman A, Qian P, He XC, Ahamed J, Li L. 2014. Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nat Med 20: 1321–1326. 10.1038/nm.3706 [DOI] [PubMed] [Google Scholar]