Abstract
Background:
Disinfection byproducts (DBPs) in public water systems (PWS) are an unintended consequence resulting from reactions between mostly chlorine-based disinfectants and organic and inorganic compounds in source waters. Epidemiology studies have shown that exposure to DBP (specifically trihalomethanes) was associated with an increased risk of bladder cancer.
Objective:
Our goal was to characterize the relative differences in exposures and estimated potential bladder cancer risks for people served by different strata of PWS in the United States and to evaluate uncertainties associated with these estimates.
Methods:
We stratified PWS by source water type (surface vs. groundwater) and population served (large, medium, and small) and calculated population-weighted mean trihalomethane-4 (THM4) concentrations for each stratum. For each stratum, we calculated a population attributable risk (PAR) for bladder cancer using odds ratios derived from published pooled epidemiology estimates as a function of the mean THM4 concentration and the fraction of the total U.S. population served by each stratum of systems. We then applied the stratum-specific PARs to the total annual number of new bladder cancer cases in the U.S. population to estimate bladder cancer incidence in each stratum.
Results:
Our results show that approximately 8,000 of the 79,000 annual bladder cancer cases in the United States were potentially attributable to DBPs in drinking water systems. The estimated attributable cases vary based on source water type and system size. Approximately 74% of the estimated attributable cases were from surface water systems serving populations of people. We also identified several uncertainties that may affect the results from this study, primarily related to the use of THM4 as a surrogate measure for DBPs relevant to bladder cancer.
Discussion:
Despite significant reductions in exposure over the past several decades, our study suggests that of the bladder cancer cases in the United States may still be attributed to exposure to DBPs found in drinking water systems. https://doi.org/10.1289/EHP9985
Introduction
More than 250 million people in the United States are served by public water systems (PWS) that chemically disinfect their water to kill or inactivate pathogenic microbial contaminants.1 The control of infectious diseases resulting from clean water and improved sanitation has been noted as one of the most significant public health advancements of the 20th century.2 Although not nearly as significant a risk as undisinfected, high-risk waters, chemical disinfection may also pose health risks of its own due to the formation of disinfection byproducts (DBPs).3,4 DBPs, including trihalomethane-4 (THM4, comprising chloroform, bromodichloromethane, chlorodibromomethane, and bromoform), are formed from reactions between chlorine-based disinfectants and organic and inorganic compounds in source waters.5 Researchers have identified more than 700 different DBPs that can form during the chlorination process.6 In addition to chlorine, the formation of these DBPs can also incorporate bromine, iodine, and nitrogen depending on precursor presence in the drinking water source.7
Over the past 40 y, the U.S. Environmental Protection Agency (U.S. EPA) has sequentially promulgated three regulations to reduce exposures to DBPs in PWS and their associated health risks: the interim Total Trihalomethane (TTHM) Rule, and the Stage 1 and Stage 2 Disinfectants and Disinfection Byproducts Rules (D/DBPRs).4,8,9 These regulations include maximum contaminant levels (MCLs) as well as requirements for treatment techniques and monitoring. The U.S. EPA also promulgated corresponding Surface Water Treatment Rules to prevent increases in microbial risk while systems made changes to comply with the DBP rules (see Supplemental Material, “Additional Information about U.S. EPA DBP Regulations.”).10–12 Other countries that have developed DBP regulations or guidelines include Australia, Canada, Germany, Japan, the Netherlands, New Zealand, South Africa, Sweden, and the United Kingdom.13 To our knowledge, 90 countries have legislation and/or conducted monitoring for total THM (i.e., THM4).14,15
Epidemiology studies have shown that increased exposure to THM4 in drinking water was associated with a higher risk of bladder cancer,16–23 and other health outcomes.24–27 Despite the considerable epidemiological evidence, specific causative DBPs and their mode of action relevant to bladder cancer have not been clearly established; the lack of a good animal model for THM-associated bladder carcinogenicity is also a limitation.28 Nonetheless, the U.S. EPA used THM4 for development of its dose–response function to estimate baseline and potential reductions in bladder cancer risk resulting from promulgation of the Stage 1 and Stage 2 D/DBPRs.29
In its economic analysis for Stage 2, the U.S. EPA estimated the annual number of potential bladder cancer cases in the United States attributable to chlorination DBPs (i.e., those formed due to any use of chlorine within the treatment plant) in drinking water and the expected reduction of these cases from implementation of the Stage 1 and Stage 2 D/DBPRs.29 The U.S. EPA developed a dose–response function examining the relationship between THM4 concentration in drinking water and increased bladder cancer risk based on a pooled-data analysis of six case–control studies.21 Using national THM4 occurrence data from 1997 to 1998 combined with this dose–response function, the U.S. EPA estimated that the proportion of lifetime bladder cancer [i.e., population attributable risk (PAR)] associated with chlorination DBPs in drinking water was 17.1% as a pre-Stage 1 baseline risk (i.e., the baseline risk prior to implementation of the Stage 1 D/DBPR).29 However, in the Stage 2 economic analysis, the U.S. EPA also noted that a causal relationship between bladder cancer and exposure to any individual DBP or combinations of DBPs had not yet been established and that the lower bound of the potential risk estimates could be as low as zero.29 More recently, Regli et al.30 reexamined the Stage 2 D/DBPR dose–response function to further evaluate the uncertainty around the estimates derived from its application to THM4 concentration levels. One source of uncertainty in the estimation of nationwide THM4 levels was the use of older data from the Information Collection Rule31 collected from PWS serving a population people during the period July 1997 to December 1998.32 More recent data collected from the period 2006–2011 as part of the third Six-Year Review (SYR3) of national primary drinking water regulations represent the most complete summary of DBP occurrence at PWS in the United States since the 1996 rule.33,34
Given the updated and more representative occurrence data, the purpose of this analysis is to characterize the relative potential differences in exposures from THM4 in drinking water and the associated potential bladder cancer cases. Second, we expanded the scope to add more specificity on potential risk related to groups of people served by different strata of PWS based on the type of source water (surface vs. groundwater) and the size of the PWSs serving that stratum (i.e., small, medium, and large PWS). In addition, we evaluate uncertainties associated with the limits of these characterizations, with an emphasis on those associated with the use and potential relevance of THM4 as a surrogate for the suite of chlorination DBPs and the potential changes in DBP mixtures over the past few decades.
Methods
Stratification of PWS
For the analyses performed here, we stratified the PWS into six groups based on different combinations reflecting the following:
Two source water categories as defined by the U.S. EPA: surface water (all types of surface waters including Groundwater Under the Direct Influence of Surface Water) or groundwater (all types of groundwater systems)
Three size groups based on population served by PWS: small ( people served), medium (10,000–100,000 people served), or large ( people served).
The surface water vs. groundwater distinction is important because surface water–supplied systems typically have higher concentrations of organic DBP precursors and DBPs than groundwater systems.35 The PWS-size groups help to inform potential differences in DBP levels, and hence DBP exposures, across these groups. For example, small PWS were first required to meet a THM4 MCL limit about 20 y later than the medium and large PWS (which were required to comply with the 1979 interim MCL of ). Additionally, large PWS were part of the extensive study conducted in the period 1997–1998 about DBP occurrence and treatment,32 which allows for a more detailed evaluation of changes in those systems over time than is possible with the small or medium systems.
The PWS in each source-water/size stratum were also grouped into two THM4 concentration range bins: and based on system annual mean THM4 concentrations. In the Stage 1 and Stage 2 D/DBPRs,4,9 is the threshold level representing the concentration below which systems may qualify for reduced monitoring.
Calculation of Population-Weighted Mean THM4 Concentrations
As shown in Figure 1, we used the compliance monitoring data from the SYR3 data set to calculate a population-weighted mean THM4 concentration for PWS in each of the six strata.36 The SYR3 data set used in this study consists of THM4 concentrations and number of people served by individual PWS as reported by states and other primacy agencies. Prior to calculation of the population-weighted mean THM4 concentrations, the SYR3 data set was examined to ensure that it met minimum data quality assurance and quality control (QA/QC) criteria.34 The examination of implausible values for DBPs included errors in reported units of measurement, sample type, sample location (i.e., only from distribution systems; no entry point data were included), sample date, and outliers (i.e., THM4 concentrations greater than , or 10 times the MCL of , were excluded as likely reporting errors). Fewer than 0.05% of records were excluded due to missing measurement units and erroneous sample types or as outliers, and 2% were excluded for not being distribution system samples. THM4 concentrations reported below the reporting limit were counted as zero to estimate.
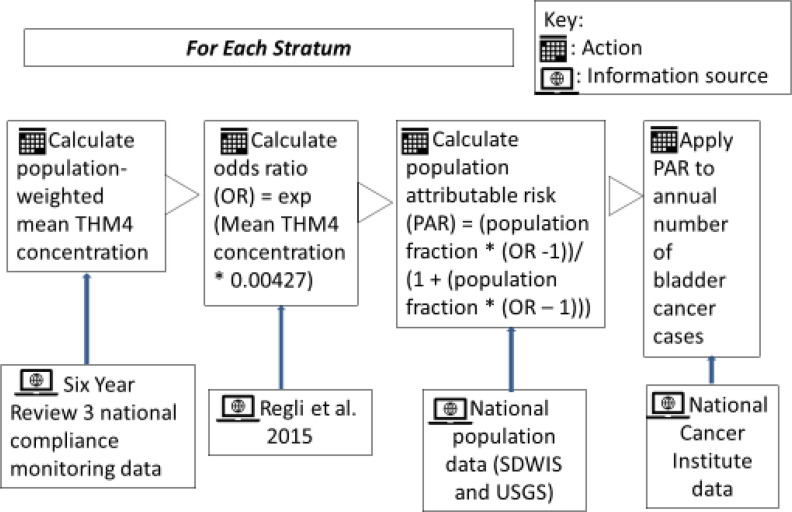
Figure 1.
Overview of methodology used to estimate U.S. national exposures and potential bladder cancer cases for each stratum. This illustrates information sources and actions applied for each stratum.
Our calculation of the population-weighted mean THM4 concentrations focused on concentration data from community water systems (CWS) and nontransient non-CWS (NTNCWS) because they were subject to the Stage 1 and Stage 2 D/DBPRs. CWS are PWS that serve at least 15 service connections used by year-round residents or regularly serve at least 25 year-round residents. NTNCWS are PWS that are not CWS and that regularly serve at least 25 of the same people for more than 6 months per year. CWS account for about 99% of the population served by systems in our analysis. We assumed that individuals receiving water from CWS and NTNCWS experience long-term exposure.
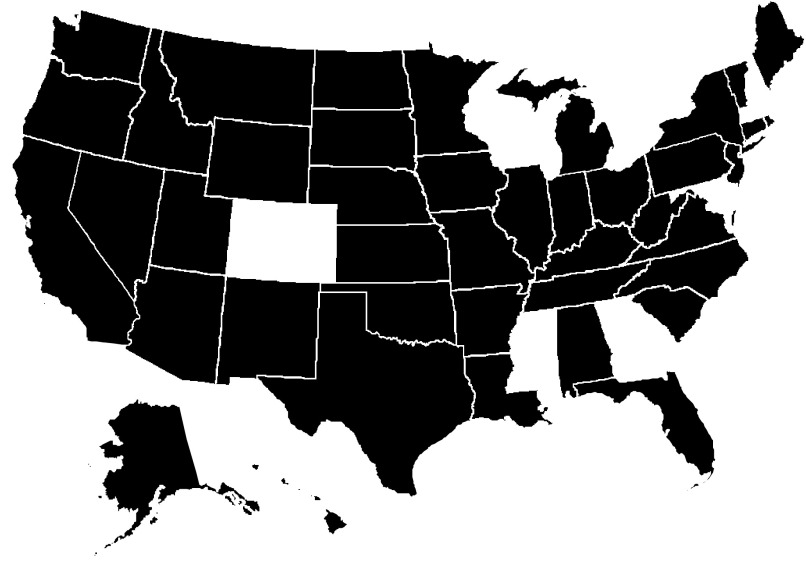
Although the SYR3 data set contains compliance monitoring data for 6 y (2006–2011), our study was limited to data from only calendar year, 2011, because it is the most recent year in the data set. The 2011 data used in this study were from 20,760 disinfecting systems serving a total of nearly 198 million people in 42 states/tribal lands (see Figure 2, which shows the states and tribal lands that submitted THM4 compliance monitoring data for 2011). Approximately 86,000 THM4 monitoring records were available from those systems; the average number of records varied based on system size and source water type (additional information about the number of THM4 records for 2011 and supporting data showing representatives of the 42 states is provided in Tables S1 and S2, respectively). Data from 2011 (referred to in this paper as post-Stage 1) also reflects occurrence following the effective date for the Stage 1 D/DBPR for all PWS (generally considered as spanning from 2001 to 2004), but prior to the effective date for the Stage 2 D/DBPR (generally considered as spanning from 2012 to 2014).
Figure 2.
States and tribal lands that submitted THM4 compliance monitoring data for calendar year 2011 and comprising Six-Year Review 3 data set (shown with shading). Data for 20,760 disinfecting systems serving a total of nearly 198 million people. See Supplemental Material Table S1 for information about number of THM4 records by system size and source water type. Map was created in R (version 4.0.0; R Development Core Team). Note: THM4, trihalomethane-4.
For each PWS in a given stratum, its THM4 annual mean concentration was multiplied by its population served (i.e., as reported by states and other primacy agencies in the SYR3 data set). Those products for each stratum were summed and divided by the total population served by all systems in that stratum to arrive at a population-weighted mean concentration for each stratum. Although the population-weighted mean THM4 concentration for each stratum is based on data for both CWS and NTNCWS, the national analysis shown in the “Results” section represents only CWS to avoid potential double-counting of those residents/users who obtain some portion of their drinking water from a CWS and another portion from an NTNCWS.
For groundwater strata that was derived from the SYR3 data, we accounted for the population served by nondisinfecting groundwater systems to arrive at a national population-weighted mean reflecting both disinfecting and nondisinfecting groundwater systems. We used data from the U.S. EPA Stage 2 economic analysis29 showing the percent of the population served by disinfecting and nondisinfecting groundwater systems to make this adjustment. We assumed that the THM4 concentrations at nondisinfecting groundwater systems were . An example of the approach for applying this assumption is for small groundwater systems, which had a population-weighted mean for disinfecting systems estimated to be from the SYR3 data. Because approximately 17% of the population served by small groundwater systems is served from nondisinfecting groundwater systems,29 the national population-weighted mean concentration for all small groundwater systems was calculated to be [i.e., ].
Post-Stage 2 THM4 Concentration Methodology
To estimate the post-Stage 2 THM4 mean concentrations, we used summary-level data from the U.S. EPA Stage 2 economic analysis29 on the predicted percent reduction in average THM4 concentration from post-Stage 1 to post-Stage 2 and applied those estimated reductions to the post-Stage 1 concentrations obtained from the SYR3 data described above. In its Stage 2 economic analysis, the U.S. EPA estimated a reduction in overall average THM4 concentration of 7.8%, with a range (based on system size) of 7.2%–9.2% for surface water systems and 1.4%–2.0% for groundwater systems (see Exhibit 5.23 of the U.S. EPA Stage 2 economic analysis29 for fifth and 95th percentile estimates). Based on that information for surface water systems, and assuming a similar degree of uncertainty for the percentage reduction in groundwater systems, the estimated mean percentage reduction estimates (with fifth and 95th percentile values) were:
Surface water serving people: 9.2% (Fifth percentile: 5.1%, 95th percentile: 13.5%)
Surface water serving people: 7.2% (Fifth percentile: 4.7%, 95th percentile: 9.7%)
Groundwater serving people: 1.4% (Fifth percentile: 0.8%, 95th percentile: 2.1%)
Groundwater serving people: 2.0% (Fifth percentile: 1.3%, 95th percentile: 2.7%)
All systems: 7.8% (Fifth percentile: 4.5%, 95th percentile: 11.2%).
The U.S. EPA Stage 2 economic analysis29 showed a breakdown of the mean percent reductions by water source and system size but did not provide separate removal estimates for the concentration range bins used in this evaluation (i.e., and ). We anticipate that most of the reduction in THM4 concentration would be reflected by PWS in the range rather than in the range. For this study, we assumed that 100% of the THM4 reductions would be in the range, and no reductions in the range, for both surface water and groundwater systems. Footnote “d” in Table 2 provides an example showing how this approach was applied to estimating post-Stage 2 THM4 mean concentrations for one specific stratum (i.e., surface water systems serving people).
Table 2.
Estimate of THM4 mean concentrations among U.S. community water systems in year 2011 post-Stage 1 D/DBPR and any year post-Stage 2 D/DBPR (see Excel Table S2 for underlying data).
| Source water type | System size (population served) | Post-Stage 1 THM4 mean concentrationsa () | Percentage reduction from Stage 1 to Stage 2b,c | Post-Stage 2 THM4 mean concentrationsd () | ||||
|---|---|---|---|---|---|---|---|---|
| Total | All systems | Total | ||||||
| Surface water systems | 22.5 | 54.7 | 39.0 | 9.2% | 22.5 | 48.0 | 36.2 | |
| 10,000–100,000 | 23.6 | 52.4 | 34.2 | 9.2% | 23.6 | 43.7 | 31.1 | |
| 23.4 | 49.4 | 33.4 | 7.2% | 23.4 | 41.4 | 30.4 | ||
| Subtotal | 23.4 | 51.0 | 34.2 | — | 23.4 | 42.9 | 31.1 | |
| Groundwater systems | 6.3 | 56.4 | 10.9 | 1.4% | 6.3 | 48.7 | 10.7 | |
| 10,000–100,000 | 11.0 | 53.8 | 17.1 | 1.3% | 11.0 | 51.5 | 16.8 | |
| 16.4 | 50.5 | 19.6 | 1.7% | 16.4 | 47.6 | 19.4 | ||
| Subtotal | 10.2 | 54.2 | 15.2 | — | 10.2 | 50.0 | 14.9 | |
| All systems | Total | 18.5 | 51.4 | 28.7 | 7.8% | 18.5 | 43.7 | 26.4 |
Note: —, not applicable; D/DBPR, U.S. Environmental Protection Agency Disinfectants and Disinfection Byproducts Rule; THM4, trihalomethane-4.
Stage 1 Disinfectants and Disinfection Byproducts Rule.
From Stage 2 economic analysis, Exhibit 5.23 with groundwater system reductions adjusted for nondisinfecting systems.
Using the fifth and 95th percentile estimates of percent reduction from Stage 1 to Stage 2, the overall range of percent reduction was estimated to be 4.4%–11.2%, resulting in an overall range for the post-Stage 2 THM4 concentration of . Calculation of the post-Stage 2 concentrations is based on two assumptions: a) All Stage 2 reductions will occur in the systems, and therefore the post-Stage 2 concentrations for the systems are the same as the Stage 1 concentrations; and b) For each source and size strata, the post-Stage 2 concentration for all systems is a population-weighted average of the concentrations in the two ranges of interest.
The to concentrations can be expressed as . Using the data for the surface water systems serving people as an example to calculate the THM4 post-Stage 2 concentration for the range results in: .
Calculation of the Odds Ratio (OR)
As described in Regli et al.30 the stratum-specific ORs derived from pooled data of Villanueva et al.21 were based on the following Equation 1:
| (1) |
where is the mean THM4 concentration for the ith stratum, and 0.00427 [95% confidence interval (CI): 0.00164, 0.00626] is the model parameter (i.e., the weighted slope for ORs as a function of THM4 concentration). Villanueva et al.21 combined data from six case–control studies conducted in the United States and Europe that included 2,806 cases and 5,254 controls for whom estimates of THM4 exposure were available for at least 70% of the exposure window. The authors used logistic regression to obtain ORs that were adjusted for study site, age, sex, education, smoking status (never, current, ex-smoker), work in a high-risk occupation for bladder cancer, heavy coffee consumption, and total fluid intake.21 Although it may be unclear whether they are related to DBP exposures, these potential confounding factors are important to control for because of their contribution to or influence on bladder cancer incidence. Despite this uncertainty, given the magnitude of some of these bladder cancer risk factors, such as smoking, sex, and occupational hazards, control for these covariates in this pooled analysis by Villanueva et al. was warranted.37 Given that we also calculated stratum-specific ORs based on upper and lower CIs noted above, this analysis did not further evaluate the confounding factors associated with the population served by the PWS in each stratum.
Calculation of the Population Attributable Risk
For each stratum, we calculated a PAR for bladder cancer using stratum-specific ORs derived from Villanueva et al. as a function of the mean THM4 concentration and the fraction of the total U.S. population served by each stratum of systems. The calculation of the PAR for each stratum used Equation 2, with ORs used as a measure of relative risk:
| (2) |
where equals the PAR for the ith stratum, equals the fraction of the total population in the ith stratum, and equals the OR for the ith stratum as calculated above.
The population fraction for a given stratum is an estimate of the portion of the total population in the United States that is served by the CWS stratum (269.3 million people, as shown in Table 1). There were approximately 38.7 million people in the United States who get drinking water from private wells,38 and we accounted for them in our estimate of the fraction of the total population in each of the strata. We assumed that all private wells are undisinfected (i.e., none are using chlorinated water) and that their THM4 concentrations are zero.
Table 1.
Number of U.S. community water systems and populations served by strata in year 2011 post-Stage 1 D/DBPR (see Excel Table S1 for underlying data).
| Source water type | System size (population served) | Number of systems with THM4 mean concentrationsa | Population served by systems with THM4 mean concentrations in ranges indicated (millions) | ||||
|---|---|---|---|---|---|---|---|
| Total | Total | ||||||
| Surface water systems | 4,213 | 4,748 | 8,961 | 8.2 | 9.5 | 17.7 | |
| 10,000–100,000 | 1,404 | 938 | 2,342 | 40.3 | 23.9 | 64.2 | |
| 242 | 111 | 353 | 66.9 | 42.3 | 109.2 | ||
| Subtotal | 5,859 | 5,797 | 11,656 | 115.4 | 75.7 | 191.1 | |
| Groundwater systems | 35,009 | 2,821 | 37,830 | 26.8 | 3.1 | 29.9 | |
| 10,000–100,000 | 1,293 | 186 | 1,479 | 28.8 | 4.9 | 33.6 | |
| 61 | 8 | 69 | 13.3 | 1.4 | 14.7 | ||
| Subtotal | 36,364 | 3,014 | 39,378 | 68.9 | 9.3 | 78.2 | |
| All systems | Total | 42,223 | 8,812 | 51,034 | 184.3 | 85.0 | 269.3 |
Note: D/DBPR, U.S. Environmental Protection Agency Disinfectants and Disinfection Byproducts Rule; MCL, maximum contaminant level; PWS, public water system; THM4, trihalomethane-4.
PWS with THM4 mean concentrations were assumed to reduce their concentrations to achieve compliance with the MCL over time and included in the range bin with the average concentration for systems in that bin.
The for the systems with THM4 mean concentrations and systems with THM4 mean concentrations were obtained by applying the SYR3 population fractions for each concentration range to the total population values nationally. Fewer than 3% of the systems had THM4 mean concentrations , and those were assumed to reduce their concentrations to achieve compliance with the MCL over time. Those systems all served relatively small populations (i.e., people) and account for approximately 1% of the total population in this study. Those systems were not excluded from this analysis but rather were included in the range bin and assumed to have the average concentration for systems in that bin.
Application of the PAR
The final step in the calculation was to multiply the PAR value by the number of annual new bladder cancer cases in the United States, estimated to be 79,030, based on National Cancer Institute (NCI) data for 2017.39 We used this number to estimate the number of post-Stage 1 and post-Stage 2 D/DBPR attributable bladder cancer cases. Additional information on perspectives about historical NCI data is provided in the Supplemental Material, “National Cancer Institute Bladder Cancer Data Description.” This estimation was done separately for each stratum and then summed to obtain the national total number of cases attributable to drinking water sources.
Evaluation of Relative Uncertainties Associated with Estimating National Exposures and Potential Bladder Cancer Cases
To evaluate uncertainties associated with the use of THM4 as a surrogate for the suite of chlorination DBPs, we examined the effects of brominated DBPs on the exposure characterizations underlying the ORs. We assessed relative differences in brominated DBPs between surface water and groundwater systems by examining the bromine incorporation factor (BIF), which gives a molar-based estimate of the fraction of brominated species, for large systems using free chlorine (distinguished from those using a chloramine residual in the distribution system), on a PWS-specific basis, relative to THM4. For this evaluation, we used THM4 data from the SYR3 2011 data set and disinfectant type information from the Third Unregulated Contaminant Monitoring Rule (UCMR3).40
To evaluate uncertainties associated with potential changes in the distribution of DBP mixtures over the past few decades and how this may affect the surrogate value of THM4, we examined metrics informing changes over time in organic content and the formation of brominated DBPs. We used the ratio of haloacetic acid-5 (HAA5) to THM4 (HAA5:THM4) as a surrogate metric to inform changes in natural organic matter (consistent with studies by Liang and Singer41), and we used the ratio of THM3 to THM4, which gives a mass-based result (THM3 refers to the three brominated species of THM4), and BIF to inform potential relative changes in the content of brominated DBPs. Changes in natural organic matter and/or brominated DBP could lead to potential changes in the distribution of DBP mixtures. To the extent that there have been increases in bromide levels in the United States, the relative distribution of brominated species—not just THMs but also other DBP classes like HAAs and haloacetonitriles that include brominated compounds—would likely be relatively higher.30
Results
A total of 51,034 CWS serving 269.3 million people were stratified based on source water type and population served, along with THM4 concentration ranges used in this evaluation, as shown in Table 1. These represent all CWS in the United States as of 2011 (supporting information for Table 1 is provided in Excel Table S1 of the Supplemental Material). Table 2 shows a national-level population-weighted mean THM4 concentration of for post-Stage 1 and a corresponding post-Stage 2 THM4 concentration of . That table also shows mean THM4 concentration estimated for each of the strata and concentration range bins.
Based on post-Stage 1 data, 9,080 of the 79,000 annual bladder cancer cases were potentially attributable to THM4 in drinking water (Table 3) [i.e., an overall PAR value of 11.5% (9,080/79,030)]. Approximately 84% (7,670) of the cases are from surface water systems, and more than half of those (4,610, or 60%) are attributable to DBPs from surface water systems with mean THM4 concentrations in the range. More than half of those cases (2,470) in the higher range of THM4 concentrations are for large systems serving people. The total population estimate in the United States from 2011 includes populations served by both CWS (269.3 million) and private wells (38.6 million). Based on post-Stage 2 data, there would be approximately 8,240 attributable annual new cases of bladder cancer potentially attributable to chlorination DBP among U.S. CWS (Table 3). The post-Stage 2 estimate corresponds to an overall PAR value of 10.4% (8,240/79,030) [supporting information for Tables 2 and 3 is provided in Excel Tables S2 (occurrence and cases for post-Stage 1) and S3 (occurrence and cases for post-Stage 2), respectively].
Table 3.
Estimate of bladder cancer cases potentially attributable to chlorination DBPs among U.S. community water systems in year 2011 post-Stage 1 D/DBPR and any year post-Stage 2 D/DBPR (see Excel Table S3 for underlying data).
| Source water type | System size (population served) | OR | PAR | Number of annual cases attributable to DBPs | ||||
|---|---|---|---|---|---|---|---|---|
| Total | ||||||||
| Surface water systems–post-Stage 1a | 1.1009 | 1.2631 | 0.27% | 0.80% | 212 | 635 | 847 | |
| 10,000–100,000 | 1.1061 | 1.2510 | 1.37% | 1.91% | 1,080 | 1,510 | 2,590 | |
| 1.1051 | 1.2347 | 2.23% | 3.12% | 1,770 | 2,470 | 4,230 | ||
| Subtotal | 1.1052 | 1.2434 | 3.79% | 5.64% | 3,060 | 4,610 | 7,670 | |
| Groundwater systems–post-Stage 1a | 1.0272 | 1.2725 | 0.24% | 0.27% | 186 | 215 | 401 | |
| 10,000–100,000 | 1.0479 | 1.2583 | 0.44% | 0.41% | 352 | 321 | 673 | |
| 1.0728 | 1.2407 | 0.30% | 0.11% | 248 | 85 | 333 | ||
| Subtotal | 1.0445 | 1.2603 | 0.99% | 0.78% | 786 | 621 | 1,410 | |
| All systems–post-Stage 1a | Total | 1.0821 | 1.2452 | 4.68% | 6.34% | 3,850 | 5,240 | 9,080 |
| Surface water systems–post-Stage 2b | 1.1009 | 1.2273 | 0.27% | 0.70% | 212 | 549 | 761 | |
| 10,000–100,000 | 1.1061 | 1.2049 | 1.37% | 1.57% | 1,080 | 1,240 | 2,320 | |
| 1.1052 | 1.1933 | 2.23% | 2.59% | 1,770 | 2,040 | 3,810 | ||
| Subtotal | 1.1052 | 1.2011 | 3.79% | 4.71% | 3,060 | 3,830 | 6,890 | |
| Groundwater systems–post-Stage 2b | 1.0272 | 1.2311 | 0.24% | 0.23% | 186 | 183 | 369 | |
| 10,000–100,000 | 1.0479 | 1.2458 | 0.45% | 0.39% | 352 | 306 | 658 | |
| 1.0728 | 1.2252 | 0.31% | 0.10% | 248 | 79 | 327 | ||
| Subtotal | 1.0445 | 1.2379 | 0.99% | 0.72% | 786 | 568 | 1,350 | |
| All systems–post-Stage 2b | Total | 1.0821 | 1.2051 | 4.68% | 5.36% | 3,850 | 4,400 | 8,240 |
Note: Estimates on this table reflect rounding. Example calculation for OR, PAR, and number of cases [shown for post-Stage 1, surface water systems serving people and with a population-weighted THM4 mean of (see Table 2)]: . Fraction of . . (shown in Table 3 as 2,470 due to rounding). DBP, disinfection byproducts; D/DBPR, U.S. Environmental Protection Agency Disinfectants and Disinfection Byproducts Rule; OR, odds ratio; PAR, population attributable risk; THM4, trihalomethane-4; U.S. EPA, U.S. Environmental Protection Agency.
Stage 1 U.S. EPA Disinfectants and Disinfection Byproducts Rule.
Stage 2 U.S. EPA Disinfectants and Disinfection Byproducts Rule.
As noted in the “Methods” section, we were able to quantify two sources of uncertainty that potentially affected the post-Stage 2 attributable bladder cancer estimate: a) uncertainty in the percent reduction in THM4 concentrations from post-Stage 1 to post-Stage 2, and b) uncertainty in the dose–response function derived from Villanueva et al.21 The uncertainty in the percent reductions in THM4 concentrations results in a relatively narrow range of 7,900 to 8,600 cases, whereas the uncertainty in the dose–response function results in a much wider range of 3,060 to 12,400 cases relative to the central estimate of 8,240 annual cases identified above. Note that both are assuming there is a causal relationship between exposure to chlorination DBP in drinking water and bladder cancer and that THM4 can be used as a surrogate for such exposure. The data and equations used to estimate the uncertainty in the percent reductions in THM4 concentrations are provided in Excel Tables S4 and S5, whereas the data and equations used to estimate the uncertainty in the dose–response function are provided in Excel Tables S6 and S7.
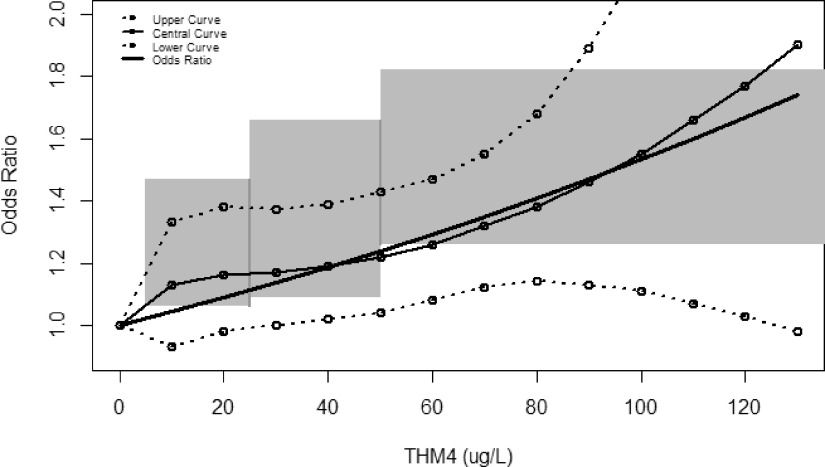
Figure 3 illustrates the relative consistency between the estimated slope and confidence regions for several of the key studies used to compare ORs to THM4 exposure, including the confidence regions that can be inferred from the Costet et al.18 meta-analysis for males, the central curve from the Villanueva et al.21 pooled analysis, and the OR plot from Regli et al.30 This figure shows much of the confidence region inferred from the most updated meta-analysis18 falling within the CIs of the dose–response function in Villanueva et al.21 Each of these studies describes the basis and methodology used to estimate their respective confidence regions. The shaded region of Figure 3 is based on the lower and upper CIs for the four exposure category results from Costet et al.,18 which presents information only for males, and consists of three adjoining rectangular regions, one for each THM4 exposure range in comparison with the reference level of . The R script used to develop Figure 3 and for the two model parameters based on the lower- and upper-95% CI curves in Villanueva et al.21 are provided in the Supplemental Material, “R Script of Data and Equations for Figure #3 (R Core Team).”
Figure 3.
Comparison of THM4 and bladder cancer epidemiological dose–response information. The slope shows the odds ratio plot from Regli et al.30; also shown is the central, upper, and lower curves from the Villanueva et al.21 pooled analysis; the shaded region is based on the lower and upper confidence intervals for the four exposure category results (males) from Costet et al.18 See Supplemental Material, “R Script of Data and Equations,” for Figure 3.
The Costet et al.18 meta-analysis, which included data from Villanueva et al.23 and from five of the six studies used by Villanueva et al.21 provided the largest data set to date for informing the association of THM4 with bladder cancer and included data from both North American and European studies (see the total international meta-analysis from their Table 4 for more details). Their analysis showed statistically significant increasing ORs relative to increasing THM4 concentrations () for males when considering both North American and European populations together. Costet et al. used males for their evaluation as the most reliable data for comparing European vs. North American studies and did so because that was where significance was identified in Villanueva et al.21 Additionally, males have a much greater bladder cancer incidence rate than females. Costet et al.18 saw no evidence of a differential exposure–response relation for THM4 and bladder cancer between Europe and North America. Use of data only for males in the shaded regions of Figure 3 (from Costet et al.) translates to results that are slightly higher than the ORs from Regli et al.30 and the lower curve from Villanueva et al.21 both of which include information for both males and females.
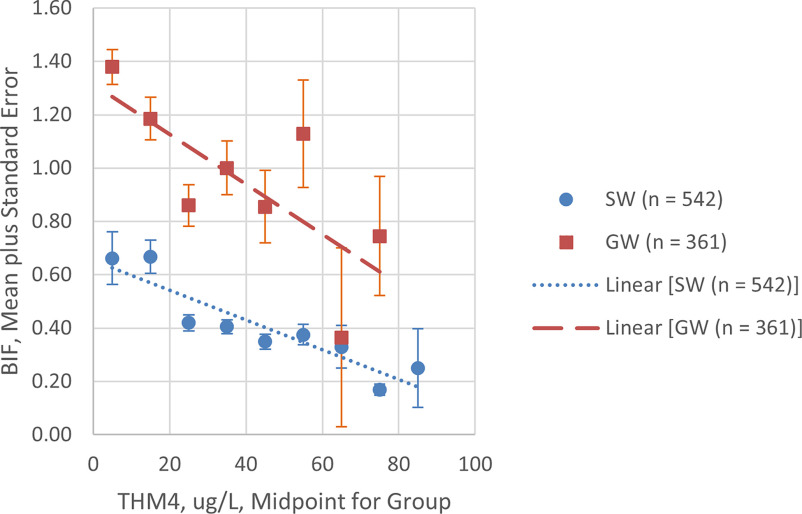
In examining the effects of brominated DBP on the exposure characterizations underlying the ORs, we found an inverse relationship between THM4 concentrations and BIF (Figure 4, where n values are the total number of systems within the groupings; Excel Table S8 includes the underlying data). In contrast, we found a positive relationship between the use of groundwater systems and BIF, indicating a likelihood for a higher proportion of brominated species in the THM4 mixture. We saw a statistically significant () difference between BIFs for surface water and groundwater systems.
Figure 4.
Bromine Incorporation Factor (BIF) vs. THM4 for 903 free chlorine systems serving people stratified by water source. BIF was calculated for each annual system-level average THM4 sample based on the molar concentration and number of bromine atoms for each THM specie. Systems were grouped in increments based on system average THM4 concentrations. For each group, standard error was estimated as the standard deviation divided by the square root. See Excel Table S8 for underlying data. Note: THM4, trihalomethane-4.
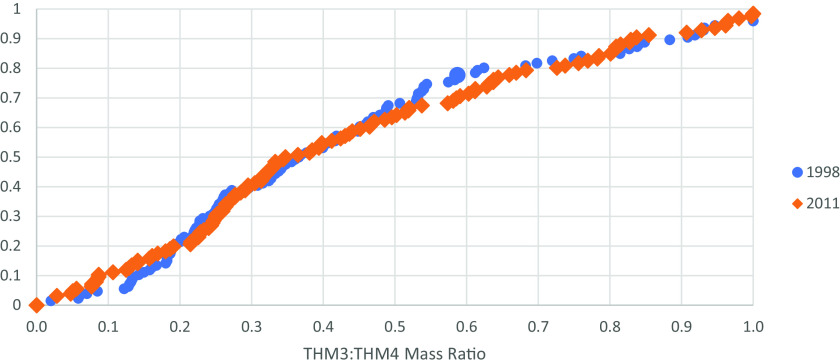
For the brominated DBP parameters (the ratio of THM3 to THM4 and BIF), we found, in general, minimal temporal differences from 1998 to 2011. Based on the cumulative distributions for common systems of the ratio of THM3 to THM4 in 1998 vs. 2011 (Figure 5; Excel Table S9 includes the underlying data), we saw no differences in mean values () or variances () that were compared. A similar evaluation of BIF for common systems in 1998 vs. 2011 showed no differences in means () or variances ().
Figure 5.
Cumulative distribution for the mass-based ratio of THM3 (i.e., brominated THMs) to THM4 for common systems between 1998 and 2011 data sets (). Researchers calculated mass-based ratio for each system from the 1997–1998 Information Collection Rule data set that provided both THM3 and THM4 concentrations and compared to matching ratio from 2011 Six-Year Review 3 data set. Statistical testing performed using R (version 4.1.2; R Development Core Team) for the hypothesis that the means and variances were not different. See Excel Table S9 for underlying data. Note: THM3, three brominated species of THM4; THM4, trihalomethane-4.
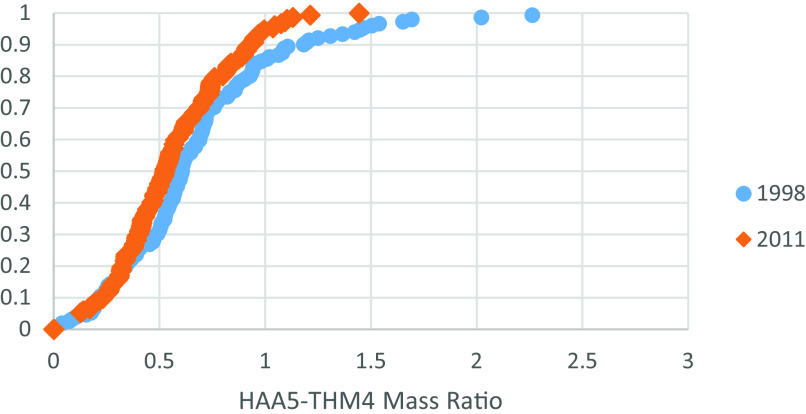
For the parameter used to inform qualitative changes in natural organic matter (HAA5:THM4), we saw a slight decrease in the ratio, potentially related to changes in system operation or source waters following the Stage 1 D/DBPR (Figure 6; Excel Table S10 includes the underlying data) [means are significantly different (); variances are significantly different ()]. This was also evaluated for PWS with THM4 concentrations greater than in 1998 and 2011; testing about the means showed results comparable to those described for Figures 5 and 6.
Figure 6.
Cumulative distribution for the ratio of HAA5 to THM4 for common systems between 1998 and 2011 data sets (). Researchers calculated mass-based ratio for each system from the 1997–1998 Information Collection Rule data set that provided both HAA5 and THM4 concentrations and compared to matching ratio from 2011 Six-Year Review 3 data set. Statistical testing performed using R (version 4.1.2; R Development Core Team) for the hypothesis that the means and variances were not different. See Excel Table S10 for underlying data. Note: HAA5, haloacetic acid-5; THM4, trihalomethane-4.
Discussion
We estimated the bladder cancer PAR of 10.4% based on the most current data and information (post-Stage 2); as expected, this is lower than estimates based on earlier periods such as post-Stage 1 () and pre-Stage 1 (). This current estimate advances earlier efforts because it is based on the most comprehensive and representative THM4 data set from 2011 capturing 42 of 50 states/tribes in the United States. A strength of the SYR3 data relied on here is that it contained THM4 data representing 269.4 million people served by CWS. Another sensitivity analysis addressed the representatives of the most complete year (2011) of monitoring data used for the PAR and showed limited variation across other years from the SYR3. Last, a comparison of our post-Stage 2 occurrence estimates showed that they were generally consistent with the information in Seidel et al.42 for systems serving more than 100,000 people (additional information is provided in the Supplemental Material, “Literature Information about Post-Stage 2 THM4 Concentration Estimates”), and, more broadly, with the post-Stage 2 occurrence estimates in Evans et al.43
Our analyses were based on various assumptions that may lead to some uncertainty in our PAR estimates. Some factors that may change over time can impact overall exposure estimates that are not addressable in our analysis but may not be completely characterized and integrated in the underlying epidemiological studies; for example, residential mobility (people may move during their lifetime to locations with different exposure levels), use of household interventions such as in-home filters, and variable exposure route-related activities (e.g., exposure from swimming) are factors that may change over time. In addition, our estimates for both post-Stage 1 and post-Stage 2 conditions assume full compliance with the Stage 1 and Stage 2 D/DBPRs as well as constant THM4 levels over time. They are based on estimated lifetime exposure to THM4 (and co-occurring DBP) for each of the six strata. These assumptions do not take into consideration the gradual attenuation of risk from lower levels of THM4 resulting from compliance with the Stage 1 and Stage 2 D/DBPRs (i.e., cessation lag). By not accounting for cessation lag and assuming full compliance with the DBP rules, we are underestimating the potential risks due to both current and historical DBP concentrations. We used this approach to enable comparisons with previous occurrence estimates and the associated remaining post-Stage 1 and post-Stage 2 risks that were made in the Stage 2 economic analysis. As discussed there, we also recognize, given the anticipated cessation lag, that the reduction in the number of bladder cancer cases from pre-Stage 1 to post-Stage 1 and to post-Stage 2 would take many years to be fully realized if this relationship is causal.29
One novel aspect of our current analysis was the ability to estimate stratum-specific estimates of PAR across water source type and system size. The relatively large percentage of cases from surface water systems with mean THM4 concentrations in the range is noteworthy, given that the population served by those PWS is 34% lower than the population served by surface water PWS in the range (75.7 million vs. 115.4 million people). The reductions from post-Stage 1 to post-Stage 2 in THM4 concentrations in those systems within the range is consistent with the trends we expect in that period, given the emphasis in the Stage 2 D/DBPR of seeking to reduce concentrations at locations with the higher post-Stage 1 occurrence levels. One limitation of the stratified analysis was the assumption that all PWSs reduced the average THM4 concentrations to because our approach collapsed all PWS into between and . We anticipate this source of uncertainty to result in a slight underestimation of the potential risks.
Use of THM4 as a Surrogate
Although there is some uncertainty as to whether THM4 is the most toxicologically relevant surrogate to gauge risks presented by the broad suite of chlorination DBPs in drinking water,44–48 many existing studies use it as a surrogate measure for chlorination DBPs.18,19,21 However, other than recognizing that in general, as THM4 increases many other classes of chlorination DBPs also increase, relationships between THM4 and DBP classes can vary widely depending on conditions for specific water systems as shown by other studies.41,44,49 Many factors such as source water characteristics that impact DBP formation [e.g., organic matter, typically considered as total organic carbon (TOC), and inorganic matter, most significantly bromide] may result in lack of comparability of DBP mixtures across distribution systems being examined by THM4 levels in epidemiological studies. For example, Richardson and Plewa50 and Li and Mitch45 have raised concerns about potential changes in the composition or distribution of different classes of DBP in DBP mixtures over the past few decades, such as with effluent organic matter,51 and algal matter in some PWS. Changes in the DBP mixtures from pre-Stage 1 to our current understanding may have been affected by a variety of factors—for example, increases in source water concentrations of bromide52–55 or changes to the composition of organic matter caused by harmful algal blooms.56,57 Some PWS have also changed treatment practices by shifting the point of chlorination, removing more precursors before the addition of chlorine, and/or using chloramination before entering the distribution systems, all of which can change the composition of the mixture.5,58 Nonetheless, of all the known DBP, THM4 is considered to be the predominant chlorination DBP on a mass basis in drinking water.
The OR vs. THM4 dose–response relationship shown in Figure 3 may be influenced, in part, by the extent to which the proportion of brominated THMs vary as a function of THM4 concentration and type of source water. Brominated DBP are generally considered to have greater toxicity than their chlorinated analogs.6 Our observation about BIF decreasing with higher concentrations of THM4 (Figure 4) is relevant when considering the data used to calculate ORs from low or unexposed reference groups (for example, those with THM4 levels below in Costet et al.18). If brominated species pose greater risk than nonbrominated species and if BIFs are higher in the low exposure referent concentration category, it would be more difficult to discern and interpret differences in risk due to increasing THM4 concentrations. Unrecognized health effects in the low or unexposed reference groups of THM4 levels would bias the derived ORs and attenuate some of the THM4 risk among groups with higher THM4 levels (for example, exposure ranges represented by the shaded rectangles of Figure 3). These data also suggest that the estimates for the populations served by systems having lower THM4 concentrations (i.e., ) may have higher bladder cancer risks on a per-microgram/liter basis than indicated in Table 3. Although we understand the likely direction of this bias (to underestimate the risk of THM4 and associated DBP exposure), gauging its magnitude is appropriate for future research once additional data are available.
Regarding changes in the composition of DBP mixtures in time, our observations about relatively little difference in the content of brominated DBPs from the 1998 to 2011 time periods (Figure 5) indicates a lack of bias in the OR vs. THM4 dose–response relationship. However, for changes in natural organic matter, our observation about a slight decrease over time in the ratio of HAA5:THM4 (Figure 6) implies that there may be differences in the nature of natural organic matter over this time period, and those differences may be associated with a different distribution of the mixture of chlorination DBPs. Overall, there is insufficient information at this time to determine whether these findings would lead to any bias (either up or down) of the bladder cancer risks for the U.S. population based on potential changes in the distribution of DBP mixtures over the past few decades.
One feature not considered in our study is the relative impact on nitrogenous DBPs (e.g., nitrosamines) as a function of the increasing numbers of systems using chloramination. Several of the nitrosamine compounds [e.g., N-nitrosodimethylamine (NDMA), which is the nitrosamine with the highest observed occurrence in U.S. drinking waters] are recognized human carcinogens, and they have increased occurrence in systems using chloramination, especially those with wastewater impacts on their source waters.59,60 NDMA has generally been associated with liver, lung, and gastrointestinal cancers but not with bladder cancer, and it was thus not considered further in this study about bladder cancer.
Weight of Evidence
Regli et al. provided a rationale for why the weight of evidence supporting causality has increased since the promulgation of the Stage 2 D/DBPR.30 Their reasons include an increased understanding of the role of genetically susceptible populations due to specific polymorphisms for THMs and HAAs, and exposure routes for THMs (oral, inhalation, and dermal) that impact risk. For example, they discussed the genotoxicity and mutagenicity of specific DBPs as well as the proportion of the susceptible U.S. population based on specific genotypes. Cantor et al. suggested that the GST theta 1 [GSTT1 ] genotype (found in approximately 80% of the population) could increase susceptibility to brominated THM-induced cancer, due to a greater generation of mutagenic intermediates.17
More recent information has further strengthened the overall weight of evidence supporting causality.61,62 Kenyon et al.61 developed a human physiologically based model to evaluate the impact of DBP exposure on internal dose which showed the relatively large contributions of dermal and inhalation exposure routes to the internal dose reaching the systemic circulation. Pegram et al.62 provided the first demonstration of brominated THM-induced genotoxicity in human urothelial cells supporting the hypothesis (Pegram et al.63) that increased bladder cancer risk from brominated THMs depends on the GSTT1 genotype. Freeman et al. reported that they found suggestive evidence for a multiplicative interaction between THM4 exposure and genotypes of rs907611 in two case–control studies in Spain and New England.64 We recognize the continued importance of research to develop new information to further inform the question of causality between bladder cancer and exposure to any individual DBP or combinations of DBP.
Conclusion
Our results provide updated estimates of national THM4 exposures and the number of potential attributable annual bladder cancer cases in the United States among specific water system strata and concentration range bins. Our PAR results were developed using data sources relevant to occurrence from public drinking water systems and help to inform an understanding of both post-Stage 1 and post-Stage 2 conditions. Our results have risk management implications as they show the relatively larger number of attributable cases are for people who are served by large surface water systems ( of the cases associated with chlorination DBP, but of the population) than smaller surface water systems or groundwater systems. Despite the increased weight of evidence established in recent years toward inferring a causal relationship between DBP exposure and bladder cancer, more work is needed to understand the possible mechanisms involved in that relationship, clarify different sources of uncertainty, and address the utility of THM4 as a surrogate measure of risk from the most relevant DBP mixtures of toxicological interest.
Supplementary Material
Acknowledgments
The authors wish to acknowledge J. Chen, J. Donohue, M. Elovitz, B. Jacobs, R. Pegram, J.E. Simmons, and M. Wright from the U.S. EPA, and A. Cullity and E. Mateo from The Cadmus Group, who provided support in several of the analyses described in this paper.
The views expressed in this article are those of the authors and do not necessarily represent the views or policies of the U.S. EPA.
References
- 1.U.S. EPA (U.S. Environmental Protection Agency). 2018. Safe Drinking Water Information System (SDWIS) Federal Reporting Services. https://www.epa.gov/ground-water-and-drinking-water/safe-drinking-water-information-system-sdwis-federal-reporting [accessed 22 February 2018].
- 2.U.S. Centers for Disease Control and Prevention. 1999. A Century of Water Chlorination and Treatment: One of the Ten Greatest Public Health Achievements of the 20th Century. MMWR Morb Mortal Wkly Rep 48(29):621–629. https://www.cdc.gov/healthywater/drinking/history.html#:∼:text=A%20Century%20of%20U.S.%20Water,quality%20in%20the%20sUnited%20States [accessed 5 March 2018].10458535 [Google Scholar]
- 3.WHO (World Health Organization). 2018. Developing drinking-water quality regulations and standards; general guidance with a special focus on countries with limited resources. https://www.who.int/publications/i/item/9789241513944 [accessed 10 May 2018].
- 4.U.S. EPA. 1998. National Primary Drinking Water Regulations: Disinfectants and Disinfection Byproducts; Final Rule. Fed Reg 63:69390. https://www.govinfo.gov/content/pkg/FR-1998-12-16/pdf/98-32887.pdf#page=1 [accessed 2 January 2018].
- 5.McGuire MJ, Karanfil T, Krasner SW, Reckhow DA, Roberson JA, Summers RS, et al. 2014. Not your granddad’s disinfection by-product problems and solutions. J Am Water Works Assoc 106(8), 10.5942/jawwa.2014.106.0128. [DOI] [Google Scholar]
- 6.Richardson SD, Plewa MJ, Wagner ED, Schoeny R, DeMarini DM. 2007. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: a review and roadmap for research. Mutat Res 636(1–3):178–242, PMID: , 10.1016/j.mrrev.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Richardson SD, Postigo C. 2011. Drinking water disinfection by-products. In: Emerging Organic Contaminants and Human Health: The Handbook of Environmental Chemistry, vol. 20. Barceló D, ed. Berlin, Germany: Springer. 10.1007/698_2011_125. [DOI] [Google Scholar]
- 8.U.S. EPA. 1979. National Interim Primary Drinking Water Regulations; Control of Trihalomethanes in Drinking Water; Final Rule. Fed Reg 44:68624. https://www.epa.gov/dwreginfo/stage-1-and-stage-2-disinfectants-and-disinfection-byproducts-rules [accessed 2 August 2018].
- 9.U.S. EPA. 2006. National Primary Drinking Water Regulations: Stage 2 Disinfectants and Disinfection Byproducts Rule; Final Rule. Fed Reg 71:388. https://www.federalregister.gov/ [accessed 2 January 2018].
- 10.U.S. EPA. 1998. National Primary Drinking Water Regulations: Interim Enhanced Surface Water Treatment; Final Rule. Fed Reg 63:69478. https://www.federalregister.gov/ [accessed 2 January 2018].
- 11.U.S. EPA. 2006. National Primary Drinking Water Regulations: Long-Term 2 Enhanced Surface Water Treatment Rule; Final Rule. Fed Reg 71:654. https://www.federalregister.gov/ [accessed 2 January 2018]. [PubMed]
- 12.DeMarini DM. 2020. A review on the 40th anniversary of the first regulation of drinking water disinfection by‐products. Environ Mol Mutagen 61(6):588–601, 10.1002/em.22378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson P, Hall T, Young W, Rumsby P. 2008. A Review of Different National Approaches to the Regulation of THMs in Drinking Water. Report No. Defra7831. http://dwi.defra.gov.uk/research/completed-research/reports/DWI70_2_216%20THMs%20in%20drinking%20water.pdf [accessed 2 April 2018].
- 14.Evlampidou I, Font-Ribera L, Rojas-Rueda D, Gracia-Lavedan E, Costet N, Pearce N, et al. 2020. Trihalomethanes in drinking water and bladder cancer burden in the European Union. Environ Health Perspect 128(1):017001, PMID: , 10.1289/EHP4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kogevinas M, Evlampidou I, Krasner S, Richardson SD, Villanueva CM. 2020. Global assessment of trihalomethanes in drinking water. Abstract for international society for environmental epidemiology. Environ Health Perspect, https://ehp.niehs.nih.gov/doi/abs/10.1289/isee.2020.virtual.P-1265.
- 16.Cantor KP, Lynch CF, Hildesheim M, Dosemeci M, Lubin J, Alavanja M, et al. 1998. Drinking water source and chlorination byproducts. I. Risk of bladder cancer. Epidemiology 9(1):21–28, PMID: , 10.1097/00001648-199801000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Cantor KP, Villanueva CM, Silverman DT, Figueroa JD, Real FX, Garcia-Closas M, et al. 2010. Polymorphisms in GSTT1, GSTZ1, and CYP2E1, disinfection by-products, and risk of bladder cancer in pain. Environ Health Perspect 118(11):1545–1550, PMID: , 10.1289/ehp.1002206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costet N, Villanueva CM, Jaakkola JJK, Kogevinas M, Cantor KP, King WD, et al. 2011. Water disinfection by-products and bladder cancer: is there a European specificity? A pooled and meta-analysis of European case-control studies. Occupational and Environmental Medicine 68(5):379–385, 10.1136/oem.2010.062703. [DOI] [PubMed] [Google Scholar]
- 19.Freeman LB, Cantor KP, Baris D, Nuckols JR, Johnson A, Colt JS, et al. 2017. Bladder cancer and water disinfection by-product exposures through multiple routes: a population based case–control study (New England, USA). Environ Health Perspect 125(6):067010, PMID: , 10.1289/EHP89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King WD, Marrett LD. 1996. Case-control study of bladder cancer and chlorination by-products in treated water (Ontario, Canada). Cancer Causes Control 7(6):596–604, PMID: , 10.1007/BF00051702. [DOI] [PubMed] [Google Scholar]
- 21.Villanueva CM, Cantor KP, Cordier S, Jaakkola JJK, King WD, Lynch CF, et al. 2004. Disinfection byproducts and bladder cancer a pooled analysis. Epidemiology 15(3):357–367, PMID: , 10.1097/01.ede.0000121380.02594.fc. [DOI] [PubMed] [Google Scholar]
- 22.Villanueva CM, Cantor KP, King WD, Jaakkola JJK, Cordier S, Lynch CF, et al. 2006. Total and specific fluid consumption as determinants of bladder cancer risk. Int J Cancer 118(8):2040–2047, PMID: , 10.1002/ijc.21587. [DOI] [PubMed] [Google Scholar]
- 23.Villanueva CM, Cantor KP, Grimalt JO, Malats N, Silverman D, Tardon A, et al. 2006. Bladder cancer and exposure to water disinfection by-products through ingestion, bathing, showering, and swimming in pools. Am J Epidemiol 165(2):148–156, PMID: , 10.1093/aje/kwj364. [DOI] [PubMed] [Google Scholar]
- 24.Säve-Söderbergh M, Toljander J, Donat-Vargas C, Åkesson A. 2021. Drinking water disinfection by-products and congenital malformations: a nationwide register-based prospective study. Environ Health Perspect 129(9):097012, PMID: , 10.1289/EHP9122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaufman JA, Wright JM, Evans A, Rivera-Núñez Z, Meyer A, Narotsky MG. 2020. Disinfection by-product exposures and the risk of musculoskeletal birth defects. Environ Epidemiol 4(1):e081, PMID: , 10.1097/EE9.0000000000000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rivera-Núñez Z, Wright JM. 2013. Association of brominated trihalomethane and haloacetic acid exposure with fetal growth and preterm delivery in Massachusetts. J Occup Environ Med 55(10):1125–1134, PMID: , 10.1097/JOM.0b013e3182a4ffe4. [DOI] [PubMed] [Google Scholar]
- 27.Rahman MB, Driscoll T, Cowie C, Armstrong BK. 2010. Disinfection by-products in drinking water and colorectal cancer: a meta-analysis. Int J Epidemiol 39(3):733–745, PMID: , 10.1093/ije/dyp371. [DOI] [PubMed] [Google Scholar]
- 28.U.S. EPA. 2016. Six-Year Review 3 Technical Support Document for Disinfectants/Disinfection Byproducts Rules (refer to Section 4.1.1.3.3). EPA-810-R-16-012. https://www.epa.gov/sites/default/files/2016-12/documents/810r16012.pdf [accessed 2 January 2018].
- 29.U.S. EPA. 2005. Economic Analysis for the Final Stage 2 Disinfectants and Disinfection Byproducts Rule, EPA 815-R-05-010. https://nepis.epa.gov/Exe/ZyPDF.cgi?Dockey=P1005OOX.txt [accessed 2 January 2018].
- 30.Regli S, Chen J, Messner M, Elovitz MS, Letkiewicz FJ, Pegram RA, et al. 2015. Estimating potential increased bladder cancer risk due to increased bromide concentrations in sources of disinfected drinking waters. Environ Sci Technol 49(22):13094–13102, PMID: , 10.1021/acs.est.5b03547. [DOI] [PubMed] [Google Scholar]
- 31.U.S. EPA. 1996. National Primary Drinking Water Regulations: Monitoring Requirements for Public Drinking Water Supplies; Final Rule (Information Collection Rule). Fed Reg 61:24354. https://www.federalregister.gov/ [accessed 2 January 2018].
- 32.U.S. EPA. 2000. ICR Auxiliary 1 Database. EPA-815-C-00-002. Updated. https://www.epa.gov/dwsixyearreview/contaminant-occurrence-and-related-data-six-year-review-drinking-water-standards [accessed 2 January 2018].
- 33.U.S. EPA. 2016. Six-Year Review 3 Technical Support Document for Disinfectants/Disinfection Byproducts Rules. EPA-810-R-16-012. https://www.epa.gov/sites/default/files/2016-12/documents/810r16012.pdf [accessed 2 January 2018].
- 34.U.S. EPA. 2016. The Data Management and Quality Assurance/Quality Control Process for the Third Six-Year Review Information Collection Rule Dataset. EPA 810-R-16-015. https://www.epa.gov/dwsixyearreview/support-documents-epas-third-review-existing-drinking-water-standards [accessed 4 January 2018].
- 35.McGuire MJ, McLain JL, Obolensky A. 2002. Information Collection Rule Data Analysis. AWWA Research Foundation and AWWA. https://www.waterrf.org/research/projects/information-collection-rule-data-analysis [accessed 1 May 2018].
- 36.U.S. EPA. 2018. Six-Year Review 3 Compliance Monitoring Data (2006–2011). https://www.epa.gov/dwsixyearreview/contaminant-occurrence-and-related-data-six-year-review-drinking-water-standards [accessed 22 February 2018].
- 37.Saginala K, Barsouk A, Aluru JS, Rawla P, Padala SA, Barsouk A. 2020. Epidemiology of bladder cancer. Med Sci (Basel) 8(1):15, PMID: , 10.3390/medsci8010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.U.S. Geological Survey. 2009. Contamination in U.S. Private Wells. https://water.usgs.gov/edu/gw-well-contamination.html [accessed 5 June 2018].
- 39.National Cancer Institute. 2018. Surveillance, Epidemiology, and End Results (SEER): Cancer Stat Facts: Bladder Cancer. Bethesda, MD. https://seer.cancer.gov/statfacts/html/urinb.html [accessed 2 January 2018].
- 40.U.S. EPA. 2018. Occurrence Data from the Unregulated Contaminant Monitoring Rule 3. https://www.epa.gov/dwucmr/occurrence-data-unregulated-contaminant-monitoring-rule [accessed 29 May 2018].
- 41.Liang L, Singer PC. 2003. Factors influencing the formation and relative distribution of haloacetic acids and trihalomethanes in drinking water. Environ Sci Technol 37(13):2920–2928, PMID: , 10.1021/es026230q. [DOI] [PubMed] [Google Scholar]
- 42.Seidel CJ, Samson CC, Bartrand T, Ergul A, Summers RS. 2017. Disinfection byproduct occurrence at large water systems after stage 2 DBPR. J Am Water Works Assoc 109(7):17–30, 10.5942/jawwa.2017.109.0082. [DOI] [Google Scholar]
- 43.Evans S, Campbell C, Naidenko OV. 2020. Analysis of cumulative cancer risk associated with disinfection byproducts in United States drinking water. Int J Environ Res Public Health 17(6):2149, PMID: , 10.3390/ijerph17062149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Furst KE, Bolorinos J, Mitch WA. 2021. Use of trihalomethanes as a surrogate for haloacetonitrile exposure introduces misclassification bias. Water Res X 11:100089, PMID: , 10.1016/j.wroa.2021.100089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li XF, Mitch W. 2018. Drinking water disinfection byproducts (DBPs) and human health effects: multidisciplinary challenges and opportunities. Environ Sci Technol 52(4):1681–1689, PMID: , 10.1021/acs.est.7b05440. [DOI] [PubMed] [Google Scholar]
- 46.Plewa MJ, Wagner ED, Richardson SD. 2017. TIC-Tox: a preliminary discussion on identifying the forcing agents of DBP-mediated toxicity of disinfected water. J Environ Sci (China) 58:208–216, PMID: , 10.1016/j.jes.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 47.Wagner ED, Plewa MJ. 2017. CHO cell cytotoxicity and genotoxicity analyses of disinfection by-products: an updated review. J Environ Sci (China) 58:64–76, PMID: , 10.1016/j.jes.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 48.Hrudey SE, Backer LC, Humpage AR, Krasner SW, Michaud DS, Moore LE, et al. 2015. Evaluating evidence for association of human bladder cancer with drinking-water chlorination disinfection by-products. J Toxicol Environ Health B Crit Rev 18(5):213–241, PMID: , 10.1080/10937404.2015.1067661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chhipi-Shrestha G, Rodriguez MR, Sadiq R. 2018. Unregulated disinfection by-products in drinking water in Quebec: a meta analysis. J Environ Manage 223:984–1000, PMID: , 10.1016/j.jenvman.2018.06.082. [DOI] [PubMed] [Google Scholar]
- 50.Richardson S, Plewa MJ. 2020. To regulate or not to regulate? What to do with more toxic disinfection by-products? J Environ Chem Eng 8(4):103939, 10.1016/j.jece.2020.103939. [DOI] [Google Scholar]
- 51.Rice J, Westerhoff P. 2015. Spatial and temporal variation in de facto wastewater reuse in drinking water systems across the U.S.A. Environ Sci Technol 49(2):982–989, 10.1021/es5048057. [DOI] [PubMed] [Google Scholar]
- 52.Good KD, VanBriesen JM. 2019. Coal-fired power plant wet flue gas desulfurization bromide discharges to U.S. watersheds and their contributions to drinking water sources. Environ Sci Technol 53(1): 213–223, PMID: , 10.1021/acs.est.8b03036. [DOI] [PubMed] [Google Scholar]
- 53.Good KD, VanBriesen JM. 2016. Current and potential future bromide loads from coal-fired power plants in the Allegheny River Basin and their effects on downstream concentrations. Environ Sci Technol 50(17):9078–9088, PMID: , 10.1021/acs.est.6b01770. [DOI] [PubMed] [Google Scholar]
- 54.Kolb C, Francis RA, VanBriesen JM. 2017. Disinfection byproduct regulatory compliance surrogates and bromide-associated risk. J Environ Sci (China) 58:191–207, PMID: , 10.1016/j.jes.2017.05.043. [DOI] [PubMed] [Google Scholar]
- 55.McTigue NE, Cornwell DA, Graf K, Brown R. 2014. Occurrence and consequences of increased bromide in drinking water sources. J Am Water Works Assoc 106(11):492–508, 10.5942/jawwa.2014.106.0141. [DOI] [Google Scholar]
- 56.Foreman K, Vacs Renwick D, McCabe M, Cadwallader A, Holsinger H, Kormondy C, et al. 2021. Effects of harmful algal blooms on regulated disinfection byproducts: findings from five utility case studies. AWWA Water Sci 3(3):e1223, 10.1002/aws2.1223. [DOI] [Google Scholar]
- 57.Tomlinson A, Drikas M, Brookes J. 2016. The role of phytoplankton as pre-cursors for disinfection by-product formation upon chlorination. Water Res 102:229–240, PMID: , 10.1016/j.watres.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 58.AWWA Disinfection Committee. 2021. Emerging trends in disinfection: lessons from AWWA’s disinfection survey. J Am Water Works Assoc 113:20–28, 10.1002/awwa.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rice J, Via S, Westerhoff P. 2015. Extent and impacts of unplanned wastewater reuse in US Rivers. J Am Water Works Assoc 107: E571–E581, 10.5942/jawwa.2015.107.0178. [DOI] [Google Scholar]
- 60.U.S. EPA. 2016. Six-Year Review 3 Technical Support Document for Nitrosamines. EPA-810-R-16-009. https://www.epa.gov/sites/default/files/2016-12/documents/810r16009.pdf [accessed 16 April 2018].
- 61.Kenyon EM, Eklund C, Leavens T, Pegram RA. 2015. Development and application of a human PBPK model for bromodichloromethane to investigate the impacts of multi-route exposure. J Appl Toxicol 36(9):1095–1111, PMID: , 10.1002/jat.3269. [DOI] [PubMed] [Google Scholar]
- 62.Pegram R, Campbell JA, Simmons SO, Ross TM, DeMarini DM, Chorley BN. 2016. Brominated trihalomethane toxicity in human urothelial cell lines. Abstract for Society of Toxicology. https://cfpub.epa.gov/si/si_public_record_report.cfm?dirEntryId=311581&Lab=NHEERL [accessed 12 March 2018]. [Google Scholar]
- 63.Pegram RA, Andersen ME, Warren SH, Ross TM, Claxton LD. 1997. Glutathione S-transferase-mediated mutagenicity of trihalomethanes in Salmonella typhimurium: contrasting results with bromodichloromethane off chloroform. Toxicol Appl Pharmacol 144(1):183–188, PMID: , 10.1006/taap.1997.8123. [DOI] [PubMed] [Google Scholar]
- 64.Freeman LEB. 2022. Disinfection by-products in drinking water and bladder cancer: evaluation of risk modification by common genetic polymorphisms in two case-control studies. Environ Health Perspect 130(5):57006, PMID: , 10.1289/EHP9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.