Vaccines have been a game changer in the global response to the coronavirus disease 2019 (COVID-19) pandemic, reducing severe disease and death in all populations. Global inequities in vaccine distribution indicate the need for additional vaccines with properties that enhance their deployability [1], [2]. The continuing spread and evolution of the virus, with new virus variants of concern (VoC) such as omicron exhibiting increased transmissibility and partial evasion of vaccine immune responses, indicates the need for new vaccines with greater breadth and duration of protection, especially against severe disease. As more people have been infected and vaccinated, evaluating new vaccines in placebo-controlled trials is becoming more difficult, leading to interest among developers, regulators, and the scientific community in other robust ways to rapidly evaluate vaccine effectiveness. Here, we present a framework of considerations for use of immunogenicity data to support science-based predictions about the effectiveness of some vaccines. Addressing these considerations will facilitate rigorous decision-making about effectiveness of new vaccines.
Neutralizing antibodies (which reduce the ability of cell-free virus to infect cells), at sufficiently high levels, confer protection against infection and disease [3]. When measured soon after vaccination, they can predict short-term vaccine effectiveness against symptomatic illness [4]. Neutralizing responses correlate less well with duration of protection against severe illness (substantial protection against severe illness is retained as neutralizing titers fall [5]) including that caused by evolving variants, which have become less susceptible to neutralizing antibodies to the original Wuhan strain. This indicates that other non-neutralizing protective responses, including cell-mediated immunity (CMI) and non-neutralizing humoral responses, likely also play an important role in these outcomes and that new vaccines that fail to introduce such responses might not be as effective as those that do. Non-neutralizing protective responses may be less important for vaccines that induce high, sustained, and broad neutralizing responses, but the duration of neutralizing responses and the rate at which the virus will evolve to evade them is difficult to predict. Thus, the potential efficacy of new vaccines should not be predicted solely from their ability to induce neutralizing antibodies; non-neutralizing protective responses, including CMI, also need to be considered [5]. Although measurement of CMI is difficult to standardize, because immune responses depend upon antigen presentation, a specific vaccine is likely to reproducibly induce components of the overall immune response in similar proportions. Under certain circumstances this could allow the overall response, including these non-neutralizing protective responses, to be predicted by neutralizing responses [6].
Similarity of peak neutralizing responses induced by a new vaccine and a comparator, with adequate efficacy against severe disease caused by the most recently circulating VoC, is thus likely to predict effectiveness of the new vaccine, if it is explicitly determined that the neutralizing responses also predict similarity of the combined effects of neutralizing, non-neutralizing humoral, and CMI responses to each vaccine. This evaluation should be supported by appropriate animal studies and human immunogenicity studies.
International Coalition of Medicines Regulatory Authorities (ICMRA) discussions indicated that many regulators would accept data showing that immune responses to a new vaccine are non-inferior vs. a highly efficacious vaccine, or superior vs. a vaccine with modest efficacy [7]. Inferring the effectiveness of a new vaccine by comparing a vaccine-induced immune response biomarker (such as neutralizing antibody) against that elicited by a vaccine for which efficacy was previously demonstrated is known as “immunobridging”. Indeed, regulators from some countries have stated that they would authorize new vaccines based on superior neutralizing responses vs. two doses of Vaxzevria (AstraZeneca, Cambridge, United Kingdom of Great Britain and Northern Ireland), a globally distributed vaccine that is available for use in comparative studies. A demonstration of superior neutralizing immune responses might suggest adequate short-term efficacy, but if CMI or other non-neutralizing responses induced by the new vaccine were weaker, reduced effectiveness against future variants or duration of protection against severe disease would be possible.
A clear understanding of the immune response characteristics and the effectiveness of the comparator vaccine is critically important. Both the nature of observational studies used to determine the comparator’s effectiveness against severe disease caused by circulating variants and potential changes in the variants themselves could introduce substantial uncertainty into assessment of the comparator’s current effectiveness. The clinical utility of the new vaccine will depend on its ability to protect against severe disease caused by current and future circulating VoC [1], so immune responses (assessed in standardized assays) associated with known effectiveness against this endpoint should be used. Because future variants may be derived from a previous variant, neutralizing responses against previously circulating variants (including the ancestral virus) may be useful in predicting breadth of coverage against mild disease, and may influence labeling. Where feasible, comparisons should be made with vaccines with high efficacy against severe disease caused by circulating VoC, and we believe that manufacturers of high-efficacy vaccines should make their vaccines available for such comparisons. Statistical non-inferiority of neutralizing antibody responses vs. a comparator that is highly effective against severe disease caused by circulating VoC may predict similarly high effectiveness. Where the effectiveness of the comparator is less certain or the comparator has more modest effectiveness, it would be prudent to require that the immune response to the new vaccine be superior to that of the comparator, recognizing that non-inferiority comparisons allow for the rare possibility of actual inferiority. If this uncertainty is high, or no comparator with adequate efficacy is available, it may be possible to contemplate authorization only if superiority of neutralizing antibody response is demonstrated by a substantial margin that could be determined only after careful consideration by regulators, who might also require that additional criteria be met. If demonstrated to achieve a more meaningful level of effectiveness, it may be possible to use additional doses of the comparator.
An evaluation of the likelihood that neutralizing responses will predict non-neutralizing protective responses may include information about the vaccine antigen presentation technologies (i.e., platforms) and antigens, and/or data from humans and animals, which should be evaluated as a whole. Neutralizing responses will more likely predict non-neutralizing protective responses relative to a known effective comparator if both vaccines are manufactured using the same platform and the same antigens. If the platforms are different, broad comparison of cellular immune responses to both vaccines could help to support a conclusion that the new vaccine will induce adequate cellular responses. Vaccines that produce antigens in the cells of recipients, such as live virus vaccines, nucleic acid vaccines, and replication-defective viral vaccines typically induce the strongest CMI. Some adjuvants can promote cross-presentation of antigens that can also induce strong CD8-positive T lymphocyte CMI responses, but this is not a consistent finding with all adjuvants. If a new vaccine includes fewer antigenic sequences than the comparator vaccine (for example, if a monovalent RBD-only vaccine is compared with a vaccine that includes the full spike protein), the new vaccine would be expected to induce weaker cellular and non-neutralizing humoral responses because it likely does not include as many epitopes important for long-term protection and resiliency to future variants.
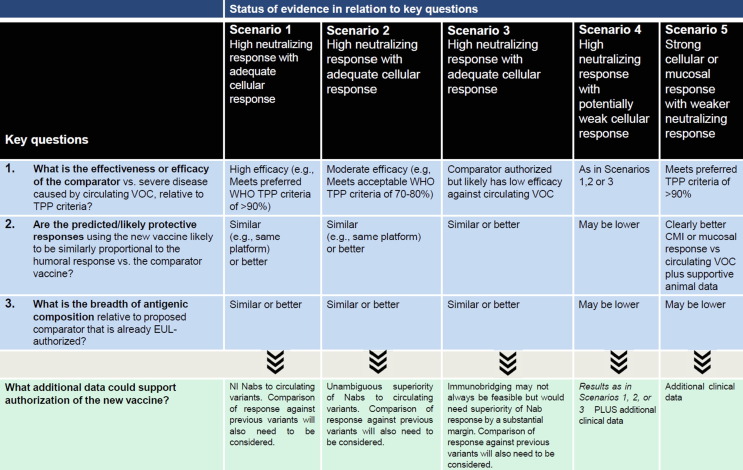
Key components of this framework for evaluating COVID-19 vaccine effectiveness (Fig. 1 ) are now part of the evaluation for WHO Emergency Use Listing [EUL] [8]. Five scenarios are considered, based on: (1) What is the effectiveness or efficacy of the comparator vs. severe disease caused by circulating VOC, relative to TPP criteria? (2) Are the predicted/likely protective responses using the new vaccine likely to be similarly proportional to the humoral response vs. the comparator vaccine, and (3) What is the breadth of antigenic composition of the new vaccine relative to the proposed comparator? Some vaccines that fall outside of these scenarios may be effective, but current consensus may not support evaluating their effectiveness without more rigorous placebo-controlled trials, although this becoming more difficult.
Fig. 1.
A framework to define approaches to evaluating the effectiveness of new COVID-19 vaccines for possible WHO Emergency Use listing. The Figure should be viewed in concert with the accompanying text, and decisions should be based on the totality of data. Deviations from the considerations in the framework can be justified with additional data and rationales not detailed in the table. Different scenarios (columns) are defined by assessments of key questions (1–3) related to a new vaccine and its proposed comparator. Additional data that could support a favorable evaluation are described in the final row. Scenarios 1–3 implicitly assume that neutralizing responses will be predictive of other (especially cellular) protective responses, but each contemplates using a comparator with different efficacy against severe disease caused by circulating variants, which influences the criteria that could support effectiveness of the new vaccine. In Scenario 1, where the comparator retains high efficacy against severe disease caused by circulating VOCs (e.g. meets the preferred WHO TPP criteria of >90% efficacy), non-inferiority criteria could be used. In Scenario 2, where there is uncertainty about the effectiveness of the comparator or where it is not considered to be highly effective (e.g. meets the “acceptable” WHO TPP criteria of 70–80%) against circulating VOCs, concerns about uncertainty in establishing the efficacy of the comparator and the possibility that non-inferiority criteria could allow for listing of an inferior vaccine, suggest the prudence of using superiority criteria. In cases where this uncertainty is great, or no comparator with adequate efficacy is available (Scenario 3), immunobridging may not be feasible, or it may be possible to contemplate authorization only if superiority of immune response is demonstrated by a substantial margin that could be determined only after careful consideration by regulators, who might also require that additional criteria be met. Where new vaccines with strong humoral responses but a significant likelihood that cellular responses are weaker than the comparator or the antigenic composition is less broad (e.g., Scenario 4), or strong cellular (or perhaps mucosal) responses with the knowledge that systemic humoral responses are too weak to support immunobridging based on neutralizing responses, regardless of breadth of antigenic composition (e.g., Scenario 5), there may be a need for additional clinical effectiveness data (as may be obtained in in-deployment studies or from human challenge studies, if feasible) before issuance of EUL.
Where effectiveness of a new vaccine cannot be directly inferred by immunobridging, key knowledge gaps could be rapidly and efficiently addressed before authorization or EUL listing with additional clinical data. Such data could be obtained in simple in-deployment studies [9] or in human challenge studies [10] (which would require challenge strains that keep up with evolving variants). Real-world data could also contribute to an understanding of effectiveness of vaccines that are already authorized in some countries. In-deployment studies collect randomized efficacy data on a provisionally deployed vaccine. Instead of assigning vaccination appointments based on non-random operational considerations, the timing of each appointment is randomized, permitting unbiased comparison of rates of severe COVID-19 between vaccinated and unvaccinated people. This approach would be the most rapid way to collect reliable and relevant data on efficacy against severe disease, allowing rapid decision-making regarding next steps, whether vaccine efficacy proved adequate or not.
Whenever new vaccines are made available, rigorous evaluation of safety to support a favorable assessment of benefits relative to risks will be essential. Appropriate post-marketing follow-up to assess effectiveness against circulating VoC should also occur for all vaccines that are approved, authorized, or EUL-listed, whether evaluated via immunobridging or clinical trials.
Additional COVID-19 vaccines are needed to meet worldwide public health goals. Effectiveness of certain vaccines may be predicted using immunogenicity data, supported by understanding of protective immune responses. Immunobridging may be most appropriate when new vaccines have substantial advantages over existing vaccines in aspects, such as deployability, availability or cost. Immunobridging approaches should be informed by the best available science, which over time will likely provide further insight into approaches for evaluating new vaccines.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We gratefully acknowledge the worldwide scientific community for their contributions to discussions at WHO-sponsored meetings at which this framework was discussed. We also gratefully acknowledge Marco Cavaleri for helpful comments on this manuscript. The opinions in this manuscript do not necessarily reflect those of the authors’ institutions.
References
- 1.WHO Target Product Profiles for COVID-19 Vaccines, Revised version, April 2022. Geneva: World Health Organization; 2022. <https://cdn.who.int/media/docs/default-source/blue-print/tpp-6apr-2022-final.pdf> [retrieved 31 May 2022].
- 2.Nohynek H., Wilder-Smith A. Does the world still need new covid-19 vaccines? N Engl J Med. 2022;386(22):2140–2142. doi: 10.1056/NEJMe2204695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Earle K.A., Ambrosino D.M., Fiore-Gartland A., Goldblatt D., Gilbert P.B., Siber G.R., et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021;39(32):4423–4428. doi: 10.1016/j.vaccine.2021.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 5.Developing a framework for evaluating new COVID-19 vaccines. Geneva: World Health Organization; 2022. <https://www.who.int/news-room/events/detail/2022/02/23/default-calendar/developing-a-framework-for-evaluating-new-covid-19-vaccines> [retrieved 31 May 2022].
- 6.Plotkin S.A., Gilbert P.B., Plotkin S.A., Gilbert P.B. Nomenclature for immune correlates of protection after vaccination. Clin Infect Dis. 2012;54:1615–1617. doi: 10.1093/cid/cis238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujiwara Y and Raine J. ICMRA COVID-19 Vaccine development: Future steps Workshop 24 June 2021. <https://www.icmra.info/drupal/en/covid-19/24june2021> [retrieved 31 May 2022].
- 8.Considerations for evaluation of Covid-19 vaccines (revised: Points to consider for manufacturers of COVID19 vaccines, Version 30 March 2022. Geneva: World Health Organization; 2022. <https://extranet.who.int/pqweb/sites/default/files/documents/Considerations_Assessment_Covid-19_Vaccines_v30March2022.pdf> [retrieved 31 May 2022].
- 9.Krause P.R., Fleming T.R., Longini I.M., Peto R., Briand S., Heymann D.L., et al. SARS-CoV-2 variants and vaccines. N Engl J Med. 2021;385(2):179–186. doi: 10.1056/NEJMsr2105280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rapeport G, Smith E, Gilbert A, Catchpole A, McShane H, Chiu C. SARS-CoV-2 Human Challenge Studies - Establishing the Model during an Evolving Pandemic. N Engl J Med. 2021;385(11):961–4. <https://doi.org/10.1056/NEJMp2106970 PMID:34289273>. [DOI] [PubMed]