Abstract
Site- and enantioselective incorporation of deuterium into organic compounds is of broad interest in organic synthesis, especially within the pharmaceutical industry. While catalytic approaches relying on two-electron reaction manifolds have allowed for stereoselective delivery of a formal deuteride (D–) or deuteron (D+) at benzylic positions, complementary strategies that make use of one-electron deuterium atom transfer and target non-benzylic positions remain elusive. Here we report a photochemical approach for asymmetric radical deuteration by utilizing readily available peptide- or sugar-derived thiols as the catalyst and inexpensive deuterium oxide as the deuterium source. This metal-free platform enables four types of deuterofunctionalization reactions of exocyclic olefins and allows deuteration at non-benzylic positions with high levels of enantioselectivity and deuterium incorporation. Computational studies reveal that attractive non-covalent interactions are responsible for stereocontrol. We anticipate that our findings will open up new avenues for asymmetric deuteration.
Subject terms: Asymmetric catalysis, Synthetic chemistry methodology, Photocatalysis
Catalytic asymmetric deuterations rely largely on two-electron reaction manifolds and are mostly limited to benzylic positions. Here, a metal-free platform using peptide- or sugar-derived chiral thiols and deuterium oxide allows for asymmetric open-shell deuteration at non-benzylic positions under visible-light irradiation.
Introduction
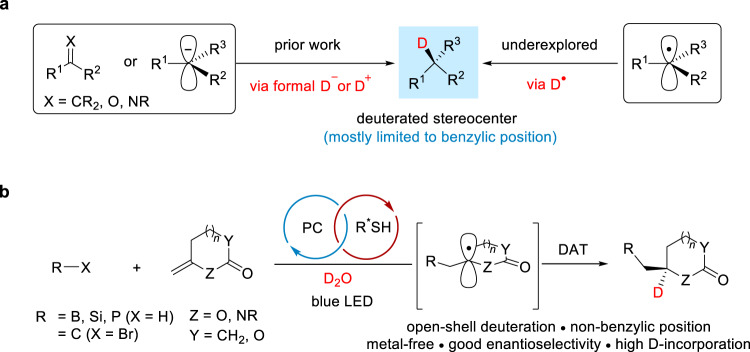
Deuterium is a stable and non-radioactive isotope of hydrogen and deuterium-labeled compounds are widely used in a broad range of disciplines1–6. While some applications need deuterated compounds with high overall deuterium content without considering site- and stereoselectivity, others require deuteration at a distinct position and/or in a stereoselective manner. In the pharmaceutical industry, for example, site- and enantioselective incorporation of deuterium into drug molecules can slow down drug metabolism and potential epimerization of stereocenters, among other benefits, thereby improving drug efficacy6. Approval of the first deuterated drug, deutetrabenazine, by the US Food and Drug Administration in 2017 has further spurred the development of novel deuteration methods. While considerable progress has been made on regioselective non-asymmetric deuteration2–4, asymmetric deuteration remains underexplored. In this regard, protocols employing enantioenriched starting materials have emerged, allowing for stereoretentive hydrogen isotope exchange7–11 and highly diastereoselective deuteration12,13. In contrast, catalytic asymmetric deuteration approaches using prochiral or racemic substrates are still limited, mainly due to the challenges associated with identifying a chiral catalyst capable of binding with the commonly used deuterating reagents such as deuterium gas, deuterium oxide, and deuterated solvents. While the use of chiral transition metal complexes14–17, enzymes18–20, and small-molecule catalysts such as chiral phosphoric acids21–23 have met with some success, deuterations are mostly restricted to benzylic positions in these studies. Moreover, from a mechanistic perspective, the deuteration event in the existing approaches typically proceeds through a two-electron manifold where the deuterium atom is introduced to stereocenters as a formal deuteride (D–) or deuteron (D+) (Fig. 1a), with notable exceptions being disclosed recently in the deuterium labeling experiments of Hyster’s18,19 and Jiang’s23 work, where a radical deuteration pathway24 is operative when a deuterated enzyme cofactor or deuterated Hantzsch ester is utilized as the deuterium source.
Fig. 1. Strategies for the construction of deuterated stereocenters.
a Catalytic asymmetric deuteration via a closed-shell mechanism involving formal deuteride (D–) or deuteron (D+) or via an open-shell deuterium atom transfer (DAT) pathway. b This work, enantioselective deuterofunctionalization of olefins using a chiral thiol catalyst and deuterium oxide (D2O). PC photocatalyst, LED light-emitting diode.
Motivated by the growing interest in merging asymmetric organocatalysis with photocatalysis25–32 and in view of the paucity of practical methods for asymmetric radical deuterations, we recently questioned whether a photocatalytic radical deuteration could be achieved in a highly enantioselective and cost-effective fashion. In particular, we hypothesized that a combination of chiral thiols with deuterium oxide (D2O) might be a potential solution, given the widespread use of achiral thiols as a catalyst for non-asymmetric radical deuteration4,33–36 and the encouraging stereocontrol that chiral thiol catalysts exerted in a handful of prior work37–40. Additionally, the following features make this strategy promising: 1) uncatalyzed background deuteration—a common issue when carbon anions are involved—would be inhibited as prochiral carbon radicals are virtually unreactive towards D2O due to the high bond dissociation energy (BDE) of the O–D bond (119 kcal/mol for HO–H bond)24,41, 2) deuterium atom would be covalently bonded to the chiral thiol catalyst through facile in-situ hydrogen/deuterium exchange4, thereby enhancing enantiofacial discrimination for the deuteration event. If successful, this strategy would not only introduce a complementary and mechanistically distinct approach to construct deuterated stereocenters, but also enable asymmetric deuteration in a metal-free manner, a feature that would be appealing to the pharmaceutical industry.
Herein, we report a photochemical approach for catalytic, asymmetric radical deuteration at non-benzylic positions in the context of deuteroboration, deuterosilylation, deuterophosphinoylation, and deuterodifluoroalkylation of exocyclic olefins using inexpensive D2O and readily available thiol catalysts derived from peptides or sugars (Fig. 1b).
Results and discussion
Reaction development
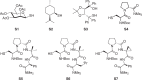
During our investigations on photoinduced hydroalkylation of olefins under the joint catalysis of Lewis base-borane and thiol42, we serendipitously observed the addition of N-heterocyclic carbene (NHC)–BH3 complex43,44 1a onto olefins. While photoinduced hydroboration of olefins using NHC–BH3 complexes have been disclosed recently by several groups45–49, asymmetric version of the reaction remains unexplored. We thus chose the reaction of 1a and exocyclic olefin 2a as a model reaction to evaluate our hypothesis using chiral thiols that are easily prepared from comercially available chiral sources such as sugars, amino acids, and peptides (Table 1). While Roberts have shown that sugar-derived thiols such as S1 are competent catalysts for radical hydrosilylation of olefins under thermal conditions, only a single product with high enantiomeric ratio (97.5:2.5 er) was obatained using a sterically very demanding substrate, with low to moderate enantioselectivity for all the other substrates37. We started our investigation by using S1 as the deuterium atom transfer (DAT) catalyst and readily available 4DPAIPN as the organophotocatalyst in a binary solvent mixture of toluene and D2O (3:1) at 10 °C. While the desired product 3a was obatined in 56% yield with 96% D upon isolation, a very low er was observed (entry 1). Other chiral pool-derived thiols such as S2–S4 also provided the product in almost racemic form (entries 2–4). To our delight, when cysteine-derived β-turn-containing peptidic thiol S5, which was recently developed by Miller and Knowles for the deracemization of ureas39, was tested under our conditions, 3a was obatined in 67% yield with 93:7 er and high levels of deuterium incorporation (94% D) at the α–N position (entry 5). Thiol S6 with a leucine unit gave same er but lower yield of 3a while thiol S7 with a cyclopropane moiety provided slightly lower er (entries 6 and 7). We then examined the influence of D2O on the reactivity and enantioselectivity of the reaction. Increasing the amount of D2O (toluene:D2O = 1:1) significantly lowered the yield of 3a but increased the deuterium incorporation to 97% (entry 8). In contrast, decreasing the amount of D2O (toluene:D2O = 4:1) had negnigible influence on the reaction yield but diminished the deuterium incorporation to 90% (entry 9). Interestingly, the enantioselectivity reamined the same under these conditions. However, when the reaction was carried out in the absence of D2O, the er dropped to 88:12, although the reaction efficiency was maintained (entry 10). Extending the reaction time to 72 h further improved the yield of 3a to 73% (entry 11). Importantly, control experiments confirmed that the photocatalyst, visible light, and the thiol are essential for the reaction (entries 12–14).
Table 1.
Reaction optimizationa
| Entry | R*SH | Solvent | Yield /%b | D /%c | erd |
|---|---|---|---|---|---|
| 1 | S1 | toluene:D2O (3:1) | 56 | 96 | 48:52 |
| 2 | S2 | toluene:D2O (3:1) | 79 | 90 | 51:49 |
| 3 | S3 | toluene:D2O (3:1) | 42 | 90 | 53:47 |
| 4 | S4 | toluene:D2O (3:1) | 50 | 95 | 58:42 |
| 5 | S5 | toluene:D2O (3:1) | 67 | 94 | 93:7 |
| 6 | S6 | toluene:D2O (3:1) | 49 | 96 | 93:7 |
| 7 | S7 | toluene:D2O (3:1) | 68 | 94 | 92:8 |
| 8 | S5 | toluene:D2O (1:1) | 43 | 97 | 93:7 |
| 9 | S5 | toluene:D2O (4:1) | 71 | 90 | 93:7 |
| 10 | S5 | toluene | 73 | – | 88:12 |
| 11e | S5 | toluene:D2O (3:1) | 73 | 94 | 93:7 |
| 12f | S5 | toluene:D2O (3:1) | N.D. | – | – |
| 13g | S5 | toluene:D2O (3:1) | N.D. | – | – |
| 14h | – | toluene:D2O (3:1) | N.D. | – | – |
 | |||||
er enantiomeric ratio, N.D. Not detected.
aUnless otherwise noted, all reactions were carried with 1a (0.2 mmol), 2a (0.1 mmol), 4DPAIPN (1 mol%), R*SH (15 mol%), toluene (0.75 mL), D2O (0.25 mL) under 10 °C for 48 h with irradiation from a 30 W blue LED.
bIsolated yield of 3a.
cDetermined by 1H NMR analysis of the isolated product.
dDetermined by chiral HPLC analysis.
eReaction time: 72 h.
fNo photocatalyst.
gWithout light irradiation.
hNo thiol catalyst.
Reaction scope
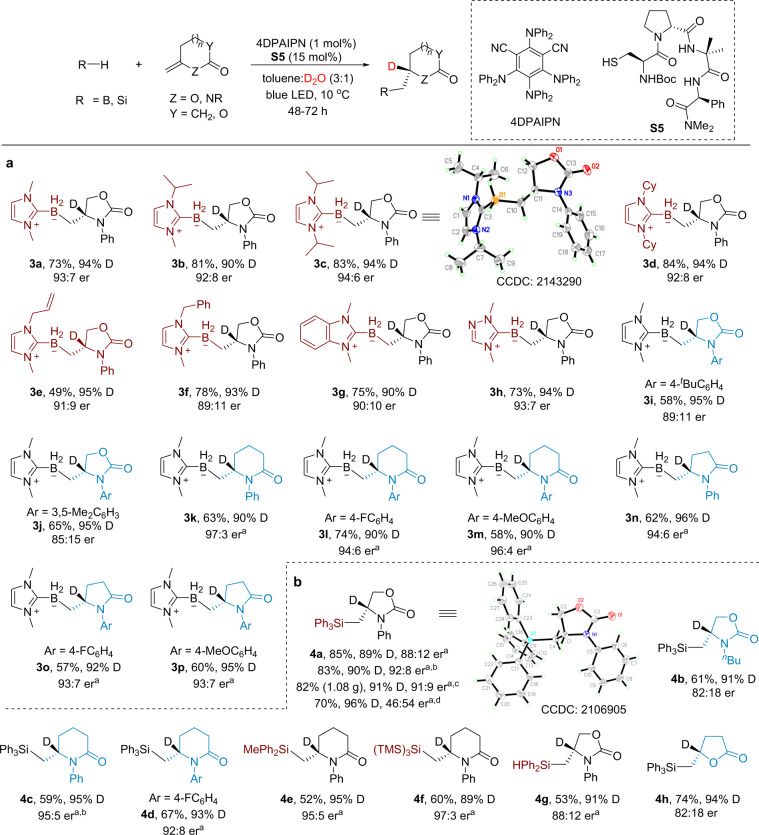
With the optimized conditions in hand, the scope and limitations of the deuteroboration reaction was explored (Fig. 2a). NHC boranes with various substituents on nitrogen and 1,2,4-triazol-5-ylidene borane all underwent the reaction smoothly, providing the desired products 3a–3h in good yields with high levels of deuterium incorporation and with er ranging from 89:11 to 94:6. Other Lewis base-borane complexes such as Ph3P–BH3 and DMAP–BH3 were evaluated but no reactivity was observed under the current conditions. In addition to 2-oxazolidinone-based olefins, exocyclic olefins on 2-piperidinones and 2-pyrrolidinones are also viable substrates, furnishing the corresponding products 3i–3p in 57-74% yield with er up to 97:3. Interestingly, high resolution mass spectra (HRMS) analysis indicated that the BH2 moieties in all these products were also partially deuterated. We also briefly examined the deuterosilylation reactions given that Roberts’ early studies on the hydrosilylation reaction under thermal conditions mostly gave low to moderate enantioselectivities37. Under room temperature, the reaction of triphenylsilane with 2a in the presence of S5 afforded the desired product 4a in 85% yield with 89% D and 88:12 er (Fig. 2b). The er was improved to 92:8 upon using S7 as the thiol catalyst. To demonstrate the practicality of the reaction, we scaled up the reaction with lower catalyst loadings and 1.08 gram of 4a was obtained with comparable results as that of the small scale reaction. By comparison, the reaction using Roberts’ optimal thiol S1 under otherwise identical conditions provided 4a in 70% yield with 96% D but with very low enantioselectivity (46:54 er). Replacing the phenyl group on nitrogen with n-butyl group produced 4b with a decreased er (82:18) while the use of 2-piperidinone-based olefins afforded 4c and 4d in moderate yields with good enantioselectivities. Other silanes such as diphenylmethylsilane, tris(trimethylsilyl)silane, and diphenylmethylsilane were also tolerated (4e–4g). γ-Lactone-based olefin afforded the product 4h in good yield with modest levels of enantioselectivity. 1,1-Disubstituted olefins in acyclic systems were tested but typically gave the corresponding products in racemic form (Supplementary Fig. 1), in line with the long-standing challenge of controlling the stereoselectivity of acyclic radicals50.The absolute configurations of the deuterated stereocenters were determined by X-ray crystallographic analysis to be R and S for products 3c and 4a, respectively. At the end of the reactions, the D2O can be recovered using a separatory funnel if desired. When the recycled D2O was subjected to the standard conditions for the synthesis of 4a, the deuterium content of 4a dropped from 90% to ~80%, although the yield and enantioselectivity were similar to those of the standard conditions (Supplementary Fig. 2). These observations suggest that partial H/D exchange occurred for the D2O during the reaction, leading to a decreased deuterium content for the recycled D2O.
Fig. 2. Peptidic thiol-catalyzed enantioselective deuterofunctionalization of exocyclic olefins.
a Enantioselective deuteroboration. b Enantioselective deuterosilylation. Yield, deuterium incorporation, and er are for isolated products; see Supplementary Note 2.3 for experimental details. aReaction was conducted at rt. bS7 was used instead of S5. cGram-scale reaction with 4DPAIPN (0.5 mol%) and S7 (10 mol%). dS1 was used instead of S5.
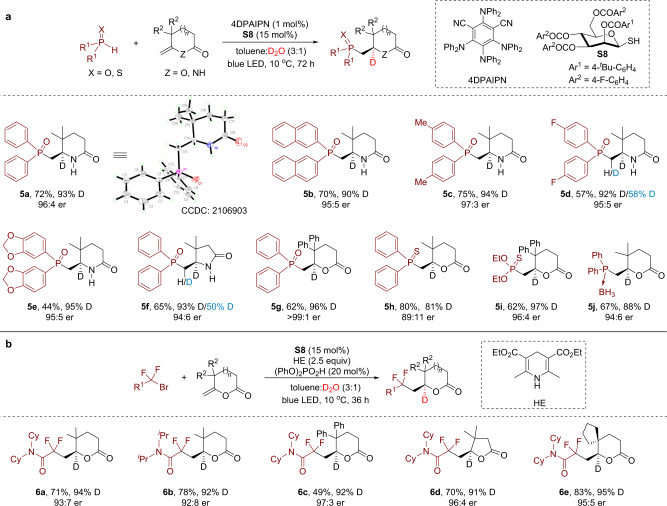
Next, we turned our attention to explore the applicability of this deuteration strategy in other photocatalytic systems. Given the versatile utilities of organophosphorous compounds and the similar bond strength of P–H bond (BDE = 79 kcal/mol for diphenylphosphine oxide)51 and B–H bond of NHC–boranes (BDE = 74–80 kcal/mol)43, we hypothesized that phosphorous-centered radicals might be formed in a similar fashion and engage in alkene deuterophosphinoylation. While inital trials with peptidic thiol catalysts proved fruitless, we were pleased to find that highly enantioselective deuterophosphinoylation could be achieved using a new β-mannose-derived thiol catalyst S8 (Fig. 3a). Using diarylphosphine oxides as the phosphinoyl radical precursor, various methylenelactams and methylenelactones were well tolerated to deliver the desired products 5a–5g in 44–75% yields with good levels of enantioselectivity (94:6–>99:1 er). The structure and absolute configuration of 5a was determined to be R using single-crystal X-ray diffraction. Interestingly, for products 5d and 5f, partial deuteration at the α-P position was also observed. Other phosphorous-centered radical precursors such as diphenylphosphine sulfide and diethyl thiophosphite are also compatible (5h and 5i). Of particular note is that diphenylphosphine borane complex is also a suitable substrate for this transformation, as exemplified by the synthesis of 5j. For deuterophosphinoylation and deuterodifluoroalkylation reactions, the substitutes at the allylic positions were found to be very important as much lower conversions and enantioselectivities were observed using an olefin devoid of such substituents (Supplementary Fig. 3). The reason behind this observation remains unknown and is currently under investigation.
Fig. 3. Sugar-derived thiol-catalyzed enantioselective deuterofunctionalization of exocyclic olefins.
a Enantioselective deuterophosphinoylation. b Enantioselective deuterodifluoroalkylation. See Supplementary Note 2.3 for experimental details.
Having demonstrated the generality of this enantioselective deuterofunctionalization strategy for introducing heteroatoms to olefins, we sought to extend it to alkene deuteroalkylation. We chose α-bromodifluoroacetamides as alkyl radical precursors given their widespread applications in radical chemistry and the importance of difluoroalkylated compounds52. To our delight, using thiol S8 as the DAT catalyst and Hantzsch ester (HE) as an electron donor, photoreductive deuterodifluoroalkylation reactions ocurred smoothly to furnish gem-difluoro- and deuterium-containing products 6a−6e in modest to good yields with high levels of enantioselectivity and deuterium incorporation (Fig. 3b).
Product derivatization
The deuterated products obtained in this study can be easily elaborated to provide versatile chiral building blocks without erosion of enantiopurity and deuterium content (Fig. 4). For example, NHC-borane 3a was treated with 2M HCl and pinacol to provide synthetically useful alkyl pinacol boronic ester 746,49. In addition, 3a could be transformed into difluoroborane 8 using Curran’s approach53. Hydrolysis under basic conditions afforded valuable α-deuterated 1,2-amino alcohol derivative 9. For 2-oxazolidinone 4a, manipulation of the C–Si bond under oxidative conditions54 provides synthetically useful 4-hydroxymethyl-substituted oxazolidinone 10 or 4-methyl-substituted oxazolidinone 11 while hydrolysis afforded silicon-containing 1,2-amino alcohol derivative 12.
Fig. 4. Product derivatization.
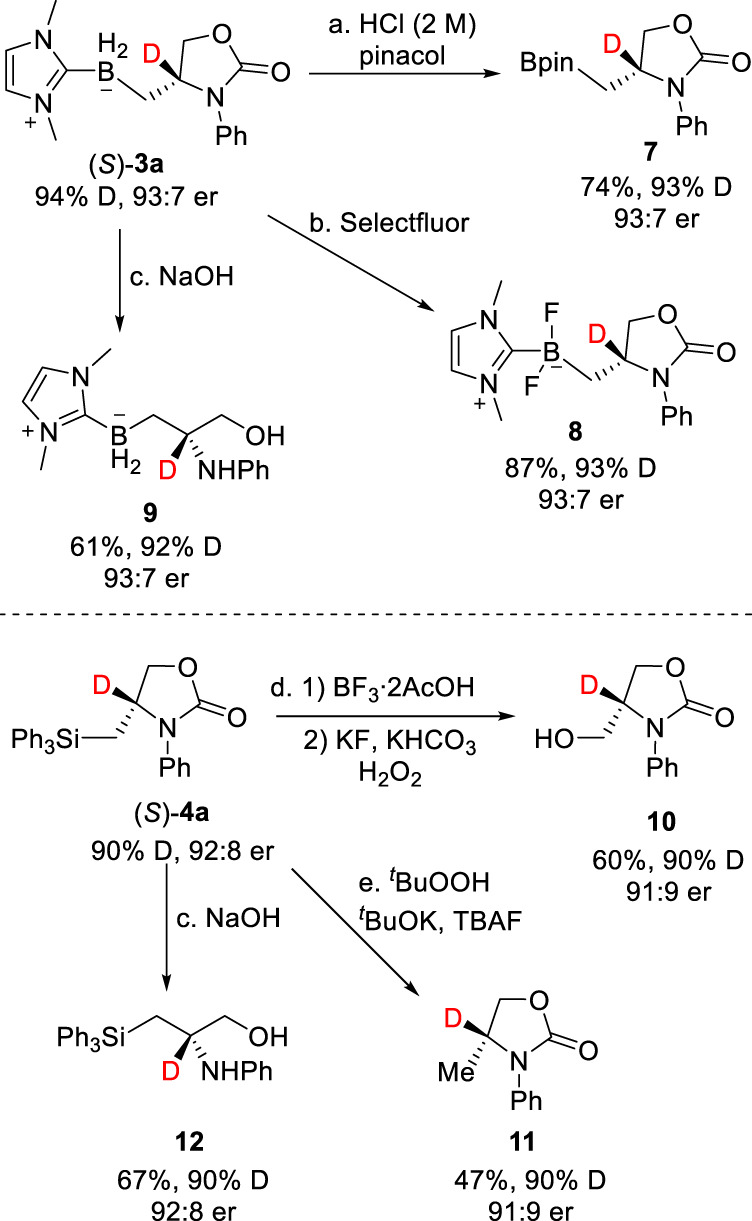
See Supplementary Note 2.4 for experimental details.
Mechanistic studies
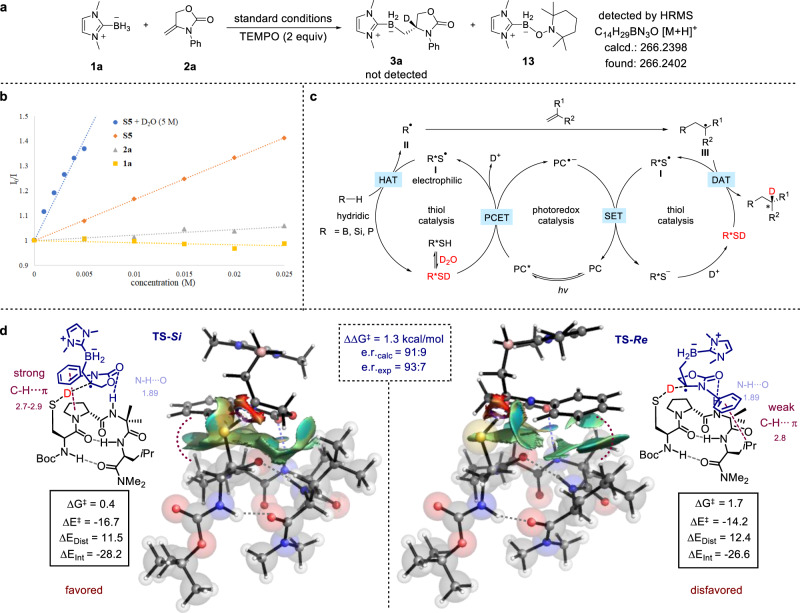
Subsequently, preliminary mechanistic studies were carried out to shed some light on the mechanism of the reactions. We focused on the deuteroboration reaction of 1a and 2a catalyzed by thiol S5. First, addition of 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) to the reaction mixture completely inhibited the reaction with radical adduct 13 being detected by HRMS (Fig. 5a), suggesting a radical-based pathway. Stern−Volmer quenching experiments revealed that the excited state of the photoredox catalyst 4DPAIPN [E1/2(PC*/PC•−) = +0.90 V vs SCE]55 could be quenched by the peptidic thiol catalyst S5 (Ep/2 = +1.41 V versus SCE) but not by the NHC–borane 1a (Ep/2 = +0.76 V versus SCE)49, although reductive quenching by 1a is thermodynamically more favorable (Fig. 5b). Intrigued by these observations, we further investigated the influence of water on redox potentials and the quenching rate. While the oxidation potential of S5 and the reduction potential of 4DPAIPN were largely unchanged in the presence of water (Supplementary Figs. 13 and 14), the quenching rate of thiol increased significantly when D2O was present (blue line in Fig. 5b), suggesting that D2O is not merely a deuterium source in the reaction. As concerted proton−coupled electron transfer (PCET) with water being the the proton acceptor is a very common process in biological systems56–58, we attributed the increased quenching rate of thiol in the presence of D2O to a concerted oxidative PCET process where water is the proton acceptor. In line with this proposal, we observed that the reaction of 1a and 2a was much faster in the presence of D2O than in its absence (Supplementary Table 7). Finally, the quantum yield of the reaction of 1a and 2a was determined to be 0.76%, indicating that a radical chain-based mechanism is unlikely. While further mechanistic investigations for the other three types of reactions are currently underway in our laboratory, a plausible mechanism was proposed based on prior work44,46,59 and our experimental observations (Fig. 5c). Photoexcitation of the photocatalyst (PC) with visible light would produce the excited stated PC*, which oxidizes a thiol catalyst to generate an electrophilic thiyl radical I via a PCET process. A polarity-matched hydrogen atom transfer (HAT) event then occurs between the thiyl radical and a hydridic R–H (R=B, Si or P) bond of the substrate60,61. Subsequent radical addition to the olefin furnishes a prochiral and nucleophilic carbon-centered radical III, which undergoes polarity-matched and stereoselective DAT with the in-situ generated deuterated chiral thiol (R*SD) to deliver the desired deuterated product and regenerate the thiyl radical. Finally, single-electron reduction of the thiyl radical by the reduced state of the photocatalyst (PC•–) would regenerate the ground-state photocatalyst and the deuterated thiol after protonation.
Fig. 5. Experimental and computational studies of the mechanistic aspects of the reaction.
a Radical trap experiment with TEMPO. b Stern−Volmer Plot. c Proposed catalytic cycle. d DFT calculated Re and Si transition states, including non-covalent interactions (NCI), and distortion/interaction analyses. Energies are in kcal/mol and distances are in Å. See Supplementary Note 2.11 for full details. TEMPO = 2,2,6,6-tetramethyl-1-piperidinyloxy.
Computational studies
To elucidate the origin of enantioselectivity for the DAT step, we performed density functional theory (DFT) calculations at the CPCM(Toluene) PBE0/6-311++G(3d,2p)//ωB97xD/6-31+G(d,p) level of theory using thiol S6 as the catalyst for the reaction of 1a and 2a (Fig. 5d, see Supplementary Information for computational details). After conformation analysis of the peptide catalyst based on Miller’s pioneering studies on β-turn-containing tetrapeptides62,63, it was found that the approach of the radical adduct in the transition state (TS) is dictated by the C=O···H–N interaction in the backbone (highlighted with blue dash lines). Moreover, the Si and Re faces of the carbon radical interact differently with the peptidic thiol in the transition states due to non-covalent dispersion interactions64,65, with TS-Si displaying strong C–H···π interactions between the proline and the phenyl ring of the radical adduct. In contrast, only weak C−H···π interactions are identified in TS-Re. As depicted in the NCI plot and quantified in the distortion/interaction analysis66, the interaction between the radical adduct and the thiol catalyst is stronger by 1.6 kcal/mol in TS-Si. In addition, the overall activation energy difference considering entropic contributions is calculated to be 1.3 kcal/mol (ΔΔG≠), corresponding to a theoretical er of 91:9 at 10 °C in favor of R enantiomer, which is in close agreement with the experimentally observed sense and magnitude of enantioinduction (93:7 er).
In summary, by merging organocatalysis with photoredox catalysis, highly enantioselective radical deuteration at non-benzylic positions has been achieved using peptide- or sugar-derived thiol catalysts and D2O. This metal-free approach is uniformly effective for deuteroboration, deuterosilylation, deuterophosphinoylation, and deuterodifluoroalkylation of exocyclic olefins. We anticipate that this catalytic asymmetric deuteration strategy will be applicable to other radical reactions terminated with a hydrogen atom transfer event and will be of guiding significance and practical utility.
Methods
General procedure for the deuteroboration of olefins
To an oven-dried 16 × 60 mm vial containing a dry Teflon stir bar were charged with 4DPAIPN (0.8 mg, 0.001 mmol), thiol catalyst S5 (9.0 mg, 0.015 mmol), and NHC–BH3 1a (22.2 mg, 0.2 mmol). After sequential addition of dry toluene (0.75 mL), D2O (0.25 mL), and olefin 2a (17.5 mg, 0.1 mmol), the reaction mixture was flushed with nitrogen gas for two minutes and then the vial was sealed with a cap and parafilm. The vial was placed in a cooling station and a 30 W blue LED (λmax = 441 nm) was then placed at the top of the cooling station, which is connected to a chiller to maintain the temperature of the cooling water at 10 °C. The reaction mixture was stirred at 10 °C under irradiation with a stirring rate of 400 r/min for 72 h. When the reaction is complete as monitored by thin layer chromatography and gas chromatography–mass spectrometry, CH2Cl2 (10 mL) and H2O (5 mL) were added, the organic layer was separated and the aqueous layer was extracted with CH2Cl2 (10 mL ×3). The combined organic layer was washed with brine and dried over anhydrous Na2SO4. After filtration and evaporation, the residue was purified by chromatography on silica gel to afford the desired product.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
Financial support from the National Natural Science Foundation of China (grant no. 22001163, J.Y.), “Thousand Youth Talents Plan”, and Shanghai Jiao Tong University (SJTU) are acknowledged. We thank Prof. Franziska Schoenebeck (RWTH Aachen University) for helpful discussions during the preparation of the manuscript. J.Y. thanks Profs. Xianjie Fang, Gang Chen, and Changkun Li for access to their analytical instrumentation and Prof. Zhaoguo Zhang for sharing laboratory space during the initial stage of this project (all from SJTU). I.F.A. and B.P. thank the University of La Rioja for financial support and the computer cluster “Beronia” for providing computational resources. Mr. Xiaoyu Yan and Mr. Xiao Yang are acknowledged for synthesizing some substrates and reproducing the results of compounds 4a, 5f, 6b, 6d and 7.
Author contributions
J.Y. conceived and directed the project. Q.S., M.X., R.C., and D.R. performed the experiments and analyzed the data. B.P. and I.F.A. carried out the computational studies. J.Y. and I.F.A. wrote the manuscript with input from all authors.
Peer review
Peer review information
Nature Communications thanks Xiao-Song XUE, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Data availability
All data generated or analyzed during this study are included in this Article and the Supplementary Information and Supplementary Data files. Details about materials and methods, experimental procedures, mechanistic studies, characterization data, computational details, NMR and HPLC spectra are available in the Supplementary Information. Calculated coordinates are available in the Supplementary Data file. Crystallographic data for compounds 3c, 4a, and 5a are available free of charge from the Cambridge Crystallographic Data Centre (CCDC) under reference number 2143290, 2106905, and 2106903, respectively (https://www.ccdc.cam.ac.uk/structures).
Competing interests
A patent application by J.Y., Q.S., and M.X. detailing part of this research was filed through the Patent Office of the People’s Republic of China (November 2021). J.Y., Q.S., and M.X. declare no other competing interests. The other authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Qinglong Shi, Meichen Xu
Contributor Information
Ignacio Funes-Ardoiz, Email: ignacio.funesa@unirioja.es.
Juntao Ye, Email: juntaoye@sjtu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-32238-8.
References
- 1.Simmons EM, Hartwig JF. On the interpretation of deuterium kinetic isotope effects in C−H bond functionalizations by transition-metal complexes. Angew. Chem. Int. Ed. 2012;51:3066–3072. doi: 10.1002/anie.201107334. [DOI] [PubMed] [Google Scholar]
- 2.Kopf S, et al. Recent developments for the deuterium and tritium labeling of organic molecules. Chem. Rev. 2022;122:6634–6718. doi: 10.1021/acs.chemrev.1c00795. [DOI] [PubMed] [Google Scholar]
- 3.Shi H, Kang Q-K. Catalytic hydrogen isotope exchange reactions in late-stage functionalization. Synlett. 2022;33:329–338. doi: 10.1055/a-1354-0367. [DOI] [Google Scholar]
- 4.Zhou R, Ma L, Yang X, Cao J. Recent advances in visible-light photocatalytic deuteration reactions. Org. Chem. Front. 2021;8:426–444. doi: 10.1039/D0QO01299H. [DOI] [Google Scholar]
- 5.Atzrodt J, Derdau V, Kerr WJ, Reid M. Deuterium- and tritium-labelled compounds: applications in the life sciences. Angew. Chem. Int. Ed. 2018;57:1758–1784. doi: 10.1002/anie.201704146. [DOI] [PubMed] [Google Scholar]
- 6.Pirali T, Serafini M, Cargnin S, Genazzani AA. Applications of deuterium in medicinal chemistry. J. Med. Chem. 2019;62:5276–5297. doi: 10.1021/acs.jmedchem.8b01808. [DOI] [PubMed] [Google Scholar]
- 7.Taglang C, et al. Enantiospecific C−H activation using ruthenium nanocatalysts. Angew. Chem. Int. Ed. 2015;54:10474–10477. doi: 10.1002/anie.201504554. [DOI] [PubMed] [Google Scholar]
- 8.Hale LVA, Szymczak NK. Stereoretentive deuteration of α-chiral amines with D2O. J. Am. Chem. Soc. 2016;138:13489–13492. doi: 10.1021/jacs.6b07879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palmer WN, Chirik PJ. Cobalt-catalyzed stereoretentive hydrogen isotope exchange of C(sp3)–H bonds. ACS Catal. 2017;7:5674–5678. doi: 10.1021/acscatal.7b02051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou R, et al. Visible-light-mediated deuteration of silanes with deuterium oxide. Chem. Sci. 2019;10:7340–7344. doi: 10.1039/C9SC02818H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang, Q. K. et al. Rhodium-catalyzed stereoselective deuteration of benzylic C–H bonds via reversible η6 -coordination. Angew. Chem. Int. Ed. 6110.1002/anie.202117381 (2022). [DOI] [PubMed]
- 12.Xiao M, et al. Transition-metal-free hydrogen autotransfer: diastereoselective N-alkylation of amines with racemic alcohols. Angew. Chem. Int. Ed. 2019;58:10528–10536. doi: 10.1002/anie.201905870. [DOI] [PubMed] [Google Scholar]
- 13.Ji P, et al. Synthesis of enantioenriched α-deuterated α-amino acids enabled by an organophotocatalytic radical approach. Org. Lett. 2020;22:1557–1562. doi: 10.1021/acs.orglett.0c00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyazaki D, et al. Enantioselective borodeuteride reduction of aldimines catalyzed by cobalt complexes: preparation of optically active deuterated primary amines. Org. Lett. 2003;5:3555–3558. doi: 10.1021/ol0349920. [DOI] [PubMed] [Google Scholar]
- 15.Li B, Chen J, Zhang Z, Gridnev ID, Zhang W. Nickel-catalyzed asymmetric hydrogenation of N-sulfonyl imines. Angew. Chem. Int. Ed. 2019;58:7329–7334. doi: 10.1002/anie.201902576. [DOI] [PubMed] [Google Scholar]
- 16.Yang P, et al. Nickel-catalyzed asymmetric transfer hydrogenation and α-selective deuteration of N-sulfonyl imines with alcohols: access to α-deuterated chiral amines. Org. Lett. 2020;22:8278–8284. doi: 10.1021/acs.orglett.0c02921. [DOI] [PubMed] [Google Scholar]
- 17.Guo S, Wang X, Zhou JS. Asymmetric umpolung hydrogenation and deuteration of alkenes catalyzed by nickel. Org. Lett. 2020;22:1204–1207. doi: 10.1021/acs.orglett.0c00112. [DOI] [PubMed] [Google Scholar]
- 18.Emmanuel MA, Greenberg NR, Oblinsky DG, Hyster TK. Accessing non-natural reactivity by irradiating nicotinamide-dependent enzymes with light. Nature. 2016;540:414–417. doi: 10.1038/nature20569. [DOI] [PubMed] [Google Scholar]
- 19.Biegasiewicz KF, et al. Photoexcitation of flavoenzymes enables a stereoselective radical cyclization. Science. 2019;364:1166–1169. doi: 10.1126/science.aaw1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rowbotham JS, Ramirez MA, Lenz O, Reeve HA, Vincent KA. Bringing biocatalytic deuteration into the toolbox of asymmetric isotopic labelling techniques. Nat. Commun. 2020;11:1454. doi: 10.1038/s41467-020-15310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakamoto T, Mori K, Akiyama T. Chiral phosphoric acid catalyzed enantioselective transfer deuteration of ketimines by use of benzothiazoline as a deuterium donor: synthesis of optically active deuterated amines. Org. Lett. 2012;14:3312–3315. doi: 10.1021/ol3012869. [DOI] [PubMed] [Google Scholar]
- 22.Shao T, et al. Photoredox-catalyzed enantioselective α-deuteration of azaarenes with D2O. iScience. 2019;16:410–419. doi: 10.1016/j.isci.2019.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kong M, et al. Catalytic reductive cross coupling and enantioselective protonation of olefins to construct remote stereocenters for azaarenes. J. Am. Chem. Soc. 2021;143:4024–4031. doi: 10.1021/jacs.1c01073. [DOI] [PubMed] [Google Scholar]
- 24.Soulard V, Villa G, Vollmar DP, Renaud P. Radical deuteration with D2O: catalysis and mechanistic insights. J. Am. Chem. Soc. 2018;140:155–158. doi: 10.1021/jacs.7b12105. [DOI] [PubMed] [Google Scholar]
- 25.Gentry EC, Knowles RR. Synthetic applications of proton-coupled electron transfer. Acc. Chem. Res. 2016;49:1546–1556. doi: 10.1021/acs.accounts.6b00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silvi M, Melchiorre P. Enhancing the potential of enantioselective organocatalysis with light. Nature. 2018;554:41–49. doi: 10.1038/nature25175. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, Lu L-Q, Yu D-G, Zhu C-J, Xiao W-J. Visible light-driven organic photochemical synthesis in China. Sci. China Chem. 2019;62:24–57. doi: 10.1007/s11426-018-9399-2. [DOI] [Google Scholar]
- 28.Proctor RSJ, Colgan AC, Phipps RJ. Exploiting attractive non-covalent interactions for the enantioselective catalysis of reactions involving radical intermediates. Nat. Chem. 2020;12:990–1004. doi: 10.1038/s41557-020-00561-6. [DOI] [PubMed] [Google Scholar]
- 29.Lv X, Xu H, Yin Y, Zhao X, Jiang Z. Visible light-driven cooperative DPZ and chiral hydrogen-bonding catalysis. Chin. J. Chem. 2020;38:1480–1488. doi: 10.1002/cjoc.202000306. [DOI] [Google Scholar]
- 30.Grosskopf J, Kratz T, Rigotti T, Bach T. Enantioselective photochemical reactions enabled by triplet energy transfer. Chem. Rev. 2022;122:1626–1653. doi: 10.1021/acs.chemrev.1c00272. [DOI] [PubMed] [Google Scholar]
- 31.Genzink MJ, Kidd JB, Swords WB, Yoon TP. Chiral photocatalyst structures in asymmetric photochemical synthesis. Chem. Rev. 2022;122:1654–1716. doi: 10.1021/acs.chemrev.1c00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mondal, S. et al. Enantioselective radical reactions using chiral catalysts. Chem. Rev. 122, 5842–5976 (2022). [DOI] [PubMed]
- 33.Loh YY, et al. Photoredox-catalyzed deuteration and tritiation of pharmaceutical compounds. Science. 2017;358:1182–1187. doi: 10.1126/science.aap9674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Constantin T, et al. Aminoalkyl radicals as halogen-atom transfer agents for activation of alkyl and aryl halides. Science. 2020;367:1021–1026. doi: 10.1126/science.aba2419. [DOI] [PubMed] [Google Scholar]
- 35.Wang L, Xia Y, Derdau V, Studer A. Remote site-selective radical C(sp3)−H monodeuteration of amides using D2O. Angew. Chem. Int. Ed. 2021;60:18645–18650. doi: 10.1002/anie.202104254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y, et al. Organophotocatalytic selective deuterodehalogenation of aryl or alkyl chlorides. Nat. Commun. 2021;12:2894. doi: 10.1038/s41467-021-23255-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haque MB, Roberts BP, Tocher DA. Enantioselective radical-chain hydrosilylation of alkenes using homochiral thiols as polarity-reversal catalysts. J. Chem. Soc. Perkin Trans. 1998;1:2881–2890. doi: 10.1039/a803280g. [DOI] [Google Scholar]
- 38.Hashimoto T, Kawamata Y, Maruoka K. An organic thiyl radical catalyst for enantioselective cyclization. Nat. Chem. 2014;6:702–705. doi: 10.1038/nchem.1998. [DOI] [PubMed] [Google Scholar]
- 39.Shin NY, Ryss JM, Zhang X, Miller SJ, Knowles RR. Light-driven deracemization enabled by excited-state electron transfer. Science. 2019;366:364–369. doi: 10.1126/science.aay2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turek AK, Sak MH, Miller SJ. Kinetic analysis of a cysteine-derived thiyl-catalyzed asymmetric vinylcyclopropane cycloaddition reflects numerous attractive noncovalent interactions. J. Am. Chem. Soc. 2021;143:16173–16183. doi: 10.1021/jacs.1c07323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luo, Y.-R. Handbook of bond dissociation energies in organic compounds (CRC Press, 2003).
- 42.Lei G, Xu M, Chang R, Funes-Ardoiz I, Ye J. Hydroalkylation of unactivated olefins via visible-light-driven dual hydrogen atom transfer catalysis. J. Am. Chem. Soc. 2021;143:11251–11261. doi: 10.1021/jacs.1c05852. [DOI] [PubMed] [Google Scholar]
- 43.Ueng SH, et al. Complexes of borane and N-heterocyclic carbenes: a new class of radical hydrogen atom donor. J. Am. Chem. Soc. 2008;130:10082–10083. doi: 10.1021/ja804150k. [DOI] [PubMed] [Google Scholar]
- 44.Taniguchi T. Advances in chemistry of N-heterocyclic carbene boryl radicals. Chem. Soc. Rev. 2021;50:8995–9021. doi: 10.1039/D1CS00385B. [DOI] [PubMed] [Google Scholar]
- 45.Xia PJ, et al. Photoinduced single-electron transfer as an enabling principle in the radical borylation of alkenes with NHC-borane. Angew. Chem. Int. Ed. 2020;59:6706–6710. doi: 10.1002/anie.201913398. [DOI] [PubMed] [Google Scholar]
- 46.Xu W, Jiang H, Leng J, Ong HW, Wu J. Visible-light-induced selective defluoroborylation of polyfluoroarenes, gem-difluoroalkenes, and trifluoromethylalkenes. Angew. Chem. Int. Ed. 2020;59:4009–4016. doi: 10.1002/anie.201911819. [DOI] [PubMed] [Google Scholar]
- 47.Dai W, Geib SJ, Curran DP. 1,4-hydroboration reactions of electron-poor aromatic rings by N-heterocyclic carbene boranes. J. Am. Chem. Soc. 2020;142:6261–6267. doi: 10.1021/jacs.0c00490. [DOI] [PubMed] [Google Scholar]
- 48.Zhu C, et al. Photoredox-controlled β-regioselective radical hydroboration of activated alkenes with NHC-boranes. Angew. Chem. Int. Ed. 2020;59:12817–12821. doi: 10.1002/anie.202005749. [DOI] [PubMed] [Google Scholar]
- 49.Qi J, et al. New radical borylation pathways for organoboron synthesis enabled by photoredox catalysis. Angew. Chem. Int. Ed. 2020;59:12876–12884. doi: 10.1002/anie.201915619. [DOI] [PubMed] [Google Scholar]
- 50.Giese B. The stereoselectivity of intermolecular free radical reactions. Angew. Chem. Int. Ed. Engl. 1989;28:969–980. doi: 10.1002/anie.198909693. [DOI] [Google Scholar]
- 51.Cai B-G, Xuan J, Xiao W-J. Visible light-mediated c p bond formation reactions. Sci. Bull. 2019;64:337–350. doi: 10.1016/j.scib.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 52.Huang W, et al. Thiyl-radical-catalyzed photoreductive hydrodifluoroacetamidation of alkenes with Hantzsch ester as a multifunctional reagent. ACS Catal. 2016;6:7471–7474. doi: 10.1021/acscatal.6b02420. [DOI] [Google Scholar]
- 53.Nerkar S, Curran DP. Synthesis and suzuki reactions of N-heterocyclic carbene difluoro(aryl)-boranes. Org. Lett. 2015;17:3394–3397. doi: 10.1021/acs.orglett.5b01101. [DOI] [PubMed] [Google Scholar]
- 54.Jones GR, Landais Y. The oxidation of the carbon-silicon bond. Tetrahedron. 1996;52:7599–7662. doi: 10.1016/S0040-4020(96)00038-5. [DOI] [Google Scholar]
- 55.Garreau M, Le Vaillant F, Waser J. C-terminal bioconjugation of peptides through photoredox catalyzed decarboxylative alkynylation. Angew. Chem. Int. Ed. 2019;58:8182–8186. doi: 10.1002/anie.201901922. [DOI] [PubMed] [Google Scholar]
- 56.Migliore A, Polizzi NF, Therien MJ, Beratan DN. Biochemistry and theory of proton-coupled electron transfer. Chem. Rev. 2014;114:3381–3465. doi: 10.1021/cr4006654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gagliardi CJ, Murphy CF, Binstead RA, Thorp HH, Meyer TJ. Concerted electron–proton transfer (EPT) in the oxidation of cysteine. J. Phys. Chem. C. 2015;119:7028–7038. doi: 10.1021/acs.jpcc.5b00368. [DOI] [Google Scholar]
- 58.Dongare P, Maji S, Hammarstrom L. Direct evidence of a tryptophan analogue radical formed in a concerted electron-proton transfer reaction in water. J. Am. Chem. Soc. 2016;138:2194–2199. doi: 10.1021/jacs.5b08294. [DOI] [PubMed] [Google Scholar]
- 59.Pan X, Lacôte E, Lalevée J, Curran DP. Polarity reversal catalysis in radical reductions of halides by N-heterocyclic carbene boranes. J. Am. Chem. Soc. 2012;134:5669–5674. doi: 10.1021/ja300416f. [DOI] [PubMed] [Google Scholar]
- 60.Roberts BP. Polarity-reversal catalysis of hydrogen-atom abstraction reactions: concepts and applications in organic chemistry. Chem. Soc. Rev. 1999;28:25–35. doi: 10.1039/a804291h. [DOI] [Google Scholar]
- 61.Cao H, Tang X, Tang H, Yuan Y, Wu J. Photoinduced intermolecular hydrogen atom transfer reactions in organic synthesis. Chem. Catal. 2021;1:523–598. doi: 10.1016/j.checat.2021.04.008. [DOI] [Google Scholar]
- 62.Metrano AJ, et al. Asymmetric catalysis mediated by synthetic peptides, version 2.0: expansion of scope and mechanisms. Chem. Rev. 2020;120:11479–11615. doi: 10.1021/acs.chemrev.0c00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Metrano AJ, et al. Diversity of secondary structure in catalytic peptides with β-turn-biased sequences. J. Am. Chem. Soc. 2017;139:492–516. doi: 10.1021/jacs.6b11348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wheeler SE, Seguin TJ, Guan Y, Doney AC. Noncovalent interactions in organocatalysis and the prospect of computational catalyst design. Acc. Chem. Res. 2016;49:1061–1069. doi: 10.1021/acs.accounts.6b00096. [DOI] [PubMed] [Google Scholar]
- 65.Sterling AJ, Zavitsanou S, Ford J, Duarte F. Selectivity in organocatalysis—from qualitative to quantitative predictive models. WIREs Comput Mol. Sci. 2021;11:e1518. doi: 10.1002/wcms.1518. [DOI] [Google Scholar]
- 66.Bickelhaupt FM, Houk KN. Analyzing reaction rates with the distortion/interaction-activation strain model. Angew. Chem. Int. Ed. 2017;56:10070–10086. doi: 10.1002/anie.201701486. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
All data generated or analyzed during this study are included in this Article and the Supplementary Information and Supplementary Data files. Details about materials and methods, experimental procedures, mechanistic studies, characterization data, computational details, NMR and HPLC spectra are available in the Supplementary Information. Calculated coordinates are available in the Supplementary Data file. Crystallographic data for compounds 3c, 4a, and 5a are available free of charge from the Cambridge Crystallographic Data Centre (CCDC) under reference number 2143290, 2106905, and 2106903, respectively (https://www.ccdc.cam.ac.uk/structures).