Summary
Background
Plasmodium falciparum (Pf) Sporozoite (SPZ) Chemoprophylaxis Vaccine (PfSPZ-CVac) involves concurrently administering infectious PfSPZ and malaria drug, often chloroquine (CQ), to kill liver-emerging parasites. PfSPZ-CVac (CQ) protected 100% of malaria-naïve participants against controlled human malaria infection. We investigated the hypothesis that PfSPZ-CVac (CQ) is safe and efficacious against seasonal, endemic Pf in malaria-exposed adults.
Methods
Healthy 18–45 year olds were enrolled in a double-blind, placebo-controlled trial in Bougoula–Hameau, Mali, randomized 1:1 to 2.048 × 105 PfSPZ (PfSPZ Challenge) or normal saline administered by direct venous inoculation at 0, 4, 8 weeks. Syringes were prepared by pharmacy staff using online computer-based enrolment that randomized allocations. Clinical team and participant masking was assured by identical appearance of vaccine and placebo. Participants received chloroquine 600mg before first vaccination, 10 weekly 300mg doses during vaccination, then seven daily doses of artesunate 200mg before 24-week surveillance during the rainy season. Safety outcomes were solicited adverse events (AEs) and related unsolicited AEs within 12 days of injections, and all serious AEs. Pf infection was detected by thick blood smears performed every four weeks and during febrile illness over 48 weeks. Primary vaccine efficacy (VE) endpoint was time to infection at 24 weeks. NCT02996695.
Findings
62 participants were enrolled in April/May 2017. Proportions of participants experiencing at least one solicited systemic AE were similar between treatment arms: 6/31 (19.4%, 95%CI 9.2-36.3) of PfSPZ-CVac recipients versus 7/31 (22.6%, 95%CI 29.2-62.2) of controls (p value = 1.000). Two/31 (6%) in each group reported related, unsolicited AEs. One unrelated death occurred. Of 59 receiving 3 immunizations per protocol, fewer vaccinees (16/29, 55.2%) became infected than controls (22/30, 73.3%). VE was 33.6% by hazard ratio (p = 0.21, 95%CI -27·9, 65·5) and 24.8% by risk ratio (p = 0.10, 95%CI -4·8, 54·3). Antibody responses to PfCSP were poor; 28% of vaccinees sero-converted.
Interpretation
PfSPZ-CVac (CQ) was well-tolerated. The tested dosing regimen failed to significantly protect against Pf infection in this very high transmission setting.
Funding
U.S. National Institutes of Health, Sanaria.
Registration number
ClinicalTrials.gov identifier (NCT number): NCT02996695.
Keywords: Malaria vaccine, Plasmodium falciparum, Sporozoite, PfSPZ Vaccine, PfSPZ-CVac
Abbreviations: Pf, Plasmodium falciparum; SPZ, sporozoite; CHMI, Controlled Human Malaria Infection; PfSPZ-CVac, Plasmodium falciparum Sporozoite Chemoprophylaxis Vaccine; CQ, chloroquine; DVI, direct venous inoculation; VE, vaccine efficacy; CSP, circumsporozoite protein; ALT, alanine aminotransferase; TBS, thick blood smear; SMC, safety monitoring committee; DOT, directly observed therapy; ELISA, enzyme linked immunosorbent assay; PCR, polymerase chain reaction; HR, hazard ratio
Research in context.
Evidence before this study
We searched PubMed, the Cochrane Library, Google Scholar, Scopus, and Web of Science on December 30, 2019, for English-language articles on randomised controlled trials of malaria vaccines based whole organisms. We searched using the terms (“malaria vaccines” [MeSH Terms] OR “malaria” [All Fields] AND “vaccines” [All Fields]) OR “malaria vaccines” [All Fields] OR (“malaria” [All Fields] AND “vaccine” [All Fields]) OR “malaria vaccine” [All Fields]) AND (PfSPZ [All Fields] AND PfSPZ Vaccine [All Fields]). For the Cochrane Library and other data sources, we used the key search terms “PfSPZ”, “malaria vaccines”, “adults”, AND “clinical trials”. We did not identify studies that assess the safety and protective efficacy of more than 51,200 unattenuated whole malaria sporozoites administered with chemoprophylaxis to a malaria-endemic population.
Added value of this study
This is the first study in malaria-experienced adults that assessed the safety, tolerability, and protective efficacy against field exposure of PfSPZ-CVac and contributed to evidence for feasibility of vaccine administration in a resource-limited setting where malaria is highly prevalent.
Implications of all the available evidence
We have shown that direct venous inoculation of up to 2.048 × 105 non-attenuated, infectious P. falciparum sporozoites is safe and well tolerated. In this small study, PfSPZ-CVac did not confer significant protective efficacy; however, results are consistent with a protective vaccine. Higher doses may be needed to increase the level of sterile protection in semi-immune adults.
Alt-text: Unlabelled box
Introduction
A safe and effective malaria vaccine would be an important tool for malaria prevention, control, and elimination.1 Radiation attenuated Plasmodium falciparum (Pf) sporozoites (SPZ) administered by mosquito bite have been known to protect recipients against controlled human malaria infection (CHMI) for decades.2 More recently, mosquito-bite administration of infectious PfSPZ to subjects taking chloroquine (CQ) chemoprophylaxis has been shown to provide even more potent protection.3,4
Sanaria Inc. developed a product called Sanaria® PfSPZ Challenge (NF54) comprised of infectious West African NF54 strain PfSPZ. PfSPZ Challenge (NF54) has infected 100% (79/79) of malaria-naïve volunteers after direct venous inoculation (DVI) by needle and syringe of 3.2 × 103 PfSPZ.5 PfSPZ Chemoprophylaxis Vaccine (PfSPZ-CVac) involves the administration of PfSPZ Challenge together with prophylactic CQ. When PfSPZ-CVac (CQ) was tested in malaria-naïve individuals in Germany, three doses of 5.12 × 104 PfPZ administered at four week intervals, there was 100% vaccine efficacy (VE) (nine/nine volunteers) against CHMI administered by DVI ten weeks after last immunization (9.5 weeks after CQ prophylaxis was discontinued).6 In this study, the CHMI was homologous; the challenge parasite was the same strain as in the vaccine. On a dose for dose basis, this vaccination approach appeared ten to 20 times more potent than the first generation whole PfSPZ approach, radiation-attenuated PfSPZ (PfSPZ Vaccine).7 Recently, three doses of 2 × 105 PfSPZ of PfSPZ-CVac (CQ) protected 100% of six participants who underwent CHMI with a heterologous South American Pf parasite (Pf7G8) 12 weeks after last vaccine dose.8 Pf7G8 is more genetically distant from the vaccine strain (PfNF54) than any of more than 700 African Pf parasites tested.9
Based on these promising results with PfSPZ-CVac (CQ), we conducted this first field trial of PfSPZ-CVac (CQ) in malaria-experienced Malian adults to determine if immunization with PfSPZ-CVac (CQ) was safe, well tolerated, immunogenic, and protective against naturally transmitted Pf in Mali.
Methods
Study design
We conducted a double-blind, randomized, placebo-controlled phase 1 trial in Mali, recruiting participants from Bougoula–Hameau and surrounding villages in a suburban district of 6900 inhabitants located five kilometres from Sikasso. The study was done at the Bougoula–Hameau research centre. The rainy season lasts from May to December, such that malaria transmission peaks from September through November.
Participants
Healthy, malaria-exposed adult men and non-pregnant women aged 18-45 years old were eligible if they provided informed consent and planned to reside in the study area for the duration of follow-up. Women of child-bearing potential provided documentation of reliable contraception during the vaccination phase. Persons were excluded for known allergies or contraindications to any study intervention (PfSPZ Challenge, chloroquine, artemether or lumefantrine); previous malaria vaccination; abnormal screening laboratory findings; or recent receipt of antimalarial medications, investigational products, immunosuppressive medications, or blood products. We excluded persons with chronic illness; clinically significant abnormalities on an electrocardiogram; and positive tests for HIV, hepatitis B or C, or sickle cell disease or trait. The trial was conducted in accordance with Good Clinical Practices and the Declaration of Helsinki. Community permission to implement the study was obtained from village leaders as described by Diallo et al.10 All participants provided written informed consent, obtained at the research centre in Bougoula–Hameau. The study was approved by the ethics review board in Mali (Faculté de Médecine de Pharmacie et d'Odonto-Stomatologie [FMPOS], Bamako, Mali), the Mali National Regulatory Authority, and the University of Maryland institutional review board, and was conducted under FDA IND 16889. The study protocol is available in the Supplementary Material.
Randomization and masking
Individual participants were randomized within a single cohort of 62 participants in a 1:1 ratio without stratification to receive three doses of either 2.048 × 105 PfSPZ of Sanaria® PfSPZ Challenge or 0.9% NaCl via IV injection at 0, 4 and 8 weeks. Both vaccines and controls were administered CQ. Randomization was done online using a computer-based enrolment module that assigned a treatment code to each participant after demographic and eligibility data were entered. Participants were enrolled by the investigators. A sealed opaque envelope containing the list of randomization codes and treatment assignments generated by the statistician was delivered to study pharmacist before the first immunization. Study product was prepared behind closed doors and provided to the vaccinators labelled only with the participant's study identification number. PfSPZ Challenge and NaCl are colourless products, indistinguishable by odour or consistency, and were administered in 0.5 mL using identical syringes to assure blinding. The unblinded study pharmacist did not conduct post-vaccination assessments or follow-up of study participants. Vaccinators were physicians not involved in any other study activity who administered injections behind curtains in rooms adjacent to the vaccine preparation room. Participants who received the first vaccination were not replaced.
Procedures
PfSPZ Challenge contains aseptic, purified, cryopreserved P. falciparum strain NF54 sporozoites.8 Sterile isotonic NaCl (Hospira, Lake Forest, IL, USA) was procured in the USA. 0.5 mL of PfSPZ Challenge or NaCl were administered by DVI over several seconds into a participant's arm or hand vein using a 1 mL syringe with 25G 16mm needle on study days 3 (V1), 31 (V2), and 59 (V3). PfSPZ Challenge was administered within 30 minutes of thawing. Two tablets of CQ phosphate (600 mg CQ base) were administered as a loading dose via directly observed therapy (DOT) on study day 1 before the first injection, and one tablet was administered weekly thereafter by DOT for 10 additional doses, with the last dose on study day 64. Beginning on study day 71, 12 days after V3, seven daily doses of artesunate 200 mg/dose were administered under DOT to clear any existing parasitemia. CQ and artesunate were given with at least 20 mL of liquid. The efficacy surveillance period began after artesunate treatment, and extended for 24 weeks during the rainy season for the primary VE endpoint; follow-up was then continued for an additional 24 weeks during the dry season, after which the final study analysis was completed. The duration of active follow-up for the primary study analysis was 35 weeks (i.e., the ten-week vaccination period, one-week artesunate administration period, and 24 weeks of post-artesunate surveillance spanning one malaria season).
Participants were observed for at least 30 min after each vaccination. Local and systemic reactogenicity events were documented on days of vaccination and one, three, seven, and 12 days after vaccinations. Blood was drawn for laboratory safety testing on vaccination days and 12 days later to measure serum alanine aminotransferase (ALT), serum creatinine, haemoglobin, platelets, and white blood cells. At each clinic visit, concomitant medications were reviewed, vital signs assessed, and targeted physical exam performed if indicated. Pulse, blood pressure, and respiration measurements taken just before first injections were considered baseline. Safety and other data were entered within 72 hours directly into an internet data system.
Blood specimens for malaria testing by thick blood smear (TBS) microscopy and quantitative polymerase chain reaction (qPCR) were collected every 28 days and when malaria illness was suspected for 48 weeks after artesunate clearance. TBS microscopy was conducted by local malaria microscopy experts using a two-reader technique with a third reader serving as tiebreaker when needed. The theoretical limit of detection for TBS was two parasites/uL. qPCR analyses were conducted retrospectively at the University of Maryland School of Medicine using an optimized method with a lower limit of detection of ∼40 parasites/mL.11 Antibodies against P. falciparum circumsporozoite protein (CSP) by enzyme linked immunosorbent assay (ELISA) were measured at baseline on the day of Dose 1 (V1), 12 days later, and 12 days after V3 by Sanaria as described.6 Antibody data units are the reciprocal serum dilution at which optical density (OD) was equal to 1.0 and are referred to as “OD 1.0”.
Safety oversight included an independent safety monitor and a safety monitoring committee (SMC). Safety assessments through 12 days after V1 (including laboratory results) were reviewed by the SMC to determine whether second doses could be administered.
Outcomes
The primary objective was to assess safety and tolerability of PfSPZ Challenge compared to NaCl among malaria-experienced adults taking CQ prophylaxis. Primary outcomes included solicited local and systemic AEs in the 12 days after injections (day of injection and 11 subsequent days); unsolicited, related AEs in the same time period; and serious AEs (SAEs) during the entire study period. Although the protocol-specified outcome for unsolicited AEs was restricted to the 12-day post-vaccination follow-up period, unsolicited AEs were tracked throughout the study period.
Secondary objectives were (1) to assess the VE of PfSPZ-CVac (CQ) against naturally transmitted Pf malaria infection diagnosed by microscopy and by real-time polymerase chain reaction (PCR) at 24 weeks and 48 weeks after artesunate clearance, and (2) to examine immune responses to immunization using antibody levels to Pf circumsporozoite protein (CSP) and other Pf proteins and markers of cell-mediated immunity.
Exploratory objectives included assessment of potential immune correlates of protection against infection by naturally transmitted P. falciparum malaria within 24 and 48 weeks after artesunate clearance. Exploratory outcomes included correlation of antibody levels against P. falciparum proteins to time to first parasitemia by microscopy and by qPCR using Cox proportional hazards modelling.
Statistical analysis
We aimed to test the hypothesis that time to first P. falciparum infection was longer in the PfSPZ-CVac group versus controls. Sample size calculations were based on a type 1 error of 0·05 (alpha) and a requirement for 90% power. Under these assumptions, if PfSPZ-CVac provided 60% efficacy, and the incidence rate in the control group was 75%, a sample size of 28 was required in each group. Anticipating up to 10% loss to follow-up, 31 volunteers were enrolled in each group.
VE against malaria infection, as measured by TBS, was assessed within 168 days of completing the post-vaccination antimalarial treatment and was calculated with multiple approaches:
-
•
The hazard ratio approach: VE = 1 - HR, where HR is the unadjusted hazard ratio. This approach is based on comparing the time to first infection between treatment arms over 24 weeks.
-
•
The adjusted hazard ratio approach: VE = 1 - HR, where HR is the adjusted hazard ratio based on a Cox proportional hazards model including pre-specified covariates (age and log-transformed antibody level).
-
•
The proportional approach: VE = 1 - RR, where RR is the risk ratio. This approach measures efficacy by comparing the probabilities of infection between treatment arms over 24 weeks.
-
•
The incidence rate ratio approach: VE = 1 - IRR, where IRR is the incidence rate ratio. This approach analyses multiple infections per individual.
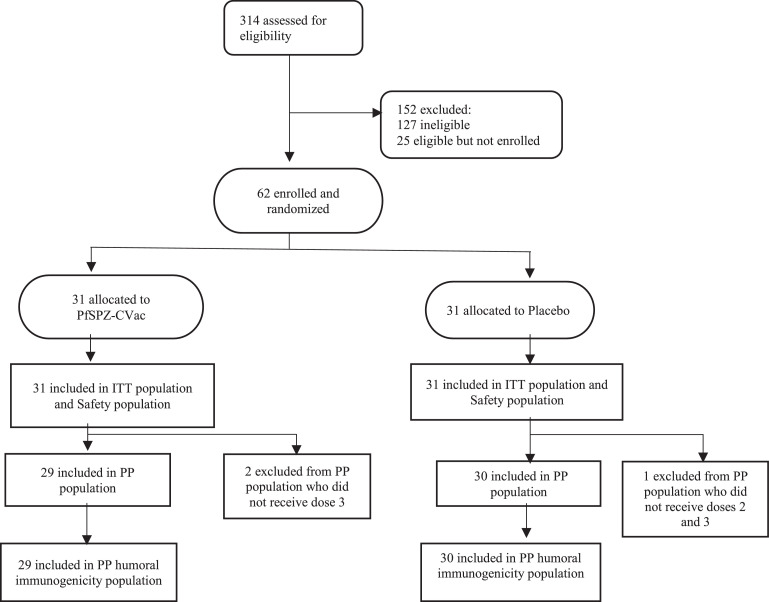
The hazard ratio approach for the 24-week interval was the primary efficacy analysis for this study as it addresses the protocol-specified endpoint. Because this estimate is considered primary and others secondary, efficacy estimates were not adjusted for multiple testing. The hazard ratio was obtained by Cox regression. A score confidence interval was calculated, and the p-value was calculated by inverting the score interval. For the proportional approach to estimate VE, the risk ratio of infection within 24 weeks of follow-up was calculated based on Kaplan-Meier estimates of survival curves for treatment and control groups. Variance estimates for 24-week survival probabilities were obtained by Greenwood's formula, and the confidence interval for the risk ratio was calculated by applying the delta method.12,13 The efficacy analyses were performed on the per-protocol (PP) population, which excluded three participants because they did not complete their treatment regimen (Figure 1) and repeated on the intention-to-treat (ITT) population. Refer to the Supplementary Material for further details regarding the analysis populations and efficacy methodology.
Figure 1.
CONSORT flow diagram.
All statistical analyses were computed with SAS statistical software version 9.4 or R statistical software version 3.4.0 or higher. This trial is registered at ClinicalTrials.gov, number NCT02996695.
Role of the funding source
The funders were involved in the study design, study management, data collection, data analysis, data interpretation, and report writing. The principal investigators (MAT, MBL) had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
214 volunteers were screened, 152 excluded (127 ineligible and 25 eligible but not enrolled) and 62 enrolled between 26 April and 02 May 2017, before the rainy season start (Figure 1). Volunteers did not meet inclusion/exclusion criteria because of spousal refusal, planned travel outside the study area, and refusal of required birth control for females, abnormal screening laboratory values (serum ALT, serum creatinine, haemoglobin, platelets, white blood cells, positive hepatitis B or C testing, positive HIV test, presence of sick cell trait), abnormal screening electrocardiogram, pregnancy, and conditions that could jeopardize participant safety. Twenty five participants were eligible but were not enrolled as the sample size required was achieved.
Thirty-one participants were randomized to the PfSPZ-CVac group and 31 to the control group. One control participant received one injection, two PfSPZ-CVac participants received two injections, and 59 (95%) received all three scheduled injections. All 62 participants were included in the safety and ITT populations, and all 59 participants who received all three injections were included in the PP analysis.
Most participants were male (74%) (Table 1). The sex distribution differed between the two treatment groups with males representing 65% of PfSPZ-CVac participants and 84% of control participants. Mean age at enrolment was 30·4 years (range: 18–44 years) with a median age of 32. Age was comparable across treatment groups. No participants were P. falciparum positive at baseline by thick blood smear microscopy (Table 1).
Table 1.
Summary of categorical demographic and baseline characteristics by treatment group, all enrolled participants.
| PfSPZ-CVac (N = 31) | Control (N = 31) | ||
|---|---|---|---|
| Variable | Characteristic | n (%) | n (%) |
| Sex | Male | 20 (65) | 26 (84) |
| Female | 11 (35) | 5 (16) | |
| Baseline malaria infection by thick smear microscopy | Yes | 0 (0) | 0 (0) |
| No | 31 (100) | 31 (100) | |
| Age (years) | |||
| Mean Age (SD) | 31·5 (8·9) | 29·3 (8·0) | |
| Age Range | 18-44 | 18-42 | |
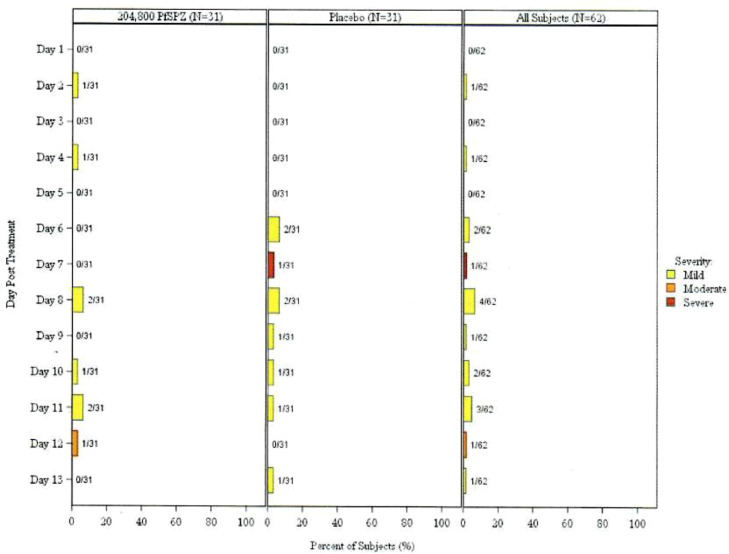
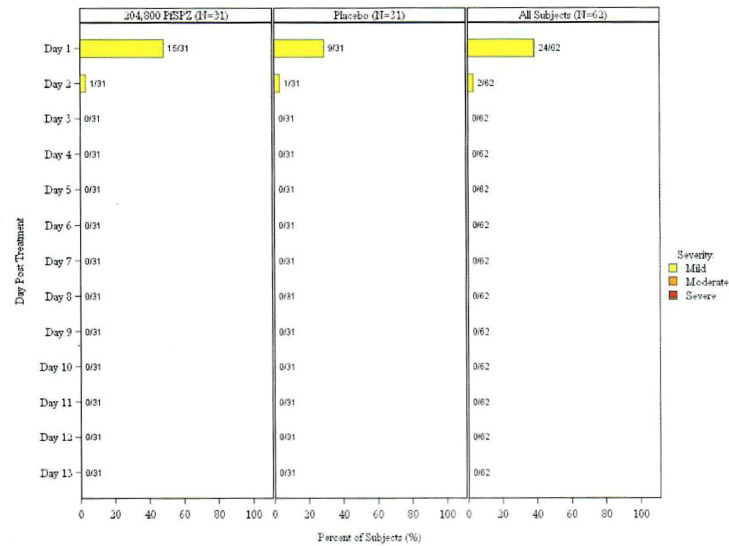
Table 2 and Figure 2, Figure 3 summarize the safety outcomes: local and systemic AEs and unsolicited related AEs within 12 days of vaccination, as well as SAEs throughout the study period. Unsolicited AEs throughout the study period are also summarized, although this was not a protocol-specified outcome. The most common adverse events deemed related to vaccination in vaccines and controls were malaise (6% and 10%, respectively) and headache (10% and 10%, respectively). Injection site pain was the most common local solicited event with 48% in vaccine group and 29% in control group. Most events were mild in severity. Proportions of participants experiencing at least one solicited systemic AE were similar between treatment arms: 6/31 (19.4%, 95% CI 9.2-36.3) of PfSPZ-CVac recipients versus 7/31 (22.6%, 95% CI 29.2-62.2) of controls (p value = 1.000, Fisher's Exact Test) (Table 2). The proportion of participants experiencing at least one local AE was higher in the PfSPZ-CVac arm (15/31 or 48.4%, 95% CI 32.0-65.2) than the control arm (9/31 or 29.0%, 95 CI 16.1-46.6), but the difference was not statistically significant (p value = 0.192, Fisher's Exact Test) (Table 2). Two participants in each arm experienced at least one related unsolicited AE within 12 days of vaccination; these events were all mild. In the PfSPZ-CVac group, one participant reported moderate malaise. In the control group, severe fever was reported in one participant during an upper respiratory infection due to presumed viral aetiology as the TBS was negative. One SAE, cranial trauma due to motorcycle accident that led to death, was reported, and was not considered related to the study product. No additional deaths or SAEs were reported.
Table 2.
Summary of safety outcomes.
| Overall Summary of Adverse Events | ||
|---|---|---|
| PfSPZ-CVac (N = 31) | Control (N = 31) | |
| Participantsa with: | n (%) | n (%) |
| At least one local solicited adverse event within 12 days of vaccination | 15 (48) | 9 (19) |
| At least one systemic solicited adverse event within 12 days of vaccination | 6 (19) | 7 (23) |
| At least one unsolicited adverse event during the study period | 25 (81) | 28 (90) |
| At least one related unsolicited adverse event within 12 days of vaccination | 2 (6) | 2 (6) |
| Mild (Grade 1) | 2 (6) | 2 (6) |
| Moderate (Grade 2) | 0 | 0 |
| Severe (Grade 3) | 0 | 0 |
| At least one severe (Grade 3) unsolicited adverse event during the study period | 1 (3) | 0 |
| Related | 0 | 0 |
| Unrelated | 1 (3) | 0 |
| At least one serious adverse event during the study period | 1b (3) | 0 |
| At least one related, serious adverse event during the study period | 0 | 0 |
| At least one adverse event during the study period leading to early termination | 1b (3) | 0 |
| Solicited events within 12 days of vaccination | ||
| PfSPZ-CVac (N = 31) | Control (N = 31) | |
| Symptoms/signs | n (%) | n (%) |
| Any Symptom/sign | 18 (58·1) | 14 (45·2) |
| Any Systemic Symptom/sign | 6 (19·4) | 7 (22·6) |
| Any Local Symptom/sign | 15 (48·4) | 9 (29·0) |
| Arthralgia/Joint Pain | 1 (3.2) | 0 |
| Chills | 0 | 1 (3·2) |
| Feverishness | 1 (3·2) | 1 (3·2) |
| Headache | 3 (9·7) | 3 (9·7) |
| Malaise | 2 (6·5) | 3 (9·7) |
| Myalgia/Body Aches | 0 | 0 |
| Nausea | 0 | 1 (3·2) |
| Fever | 0 | 1 (3·2) |
| Ecchymosis/Bruising Measurement | 0 | 0 |
| Erythema/Redness Measurement | 0 | 0 |
| Induration/Swelling Measurement | 1 (3·2) | 0 |
| Induration/Swelling Severity | 1 (3·3) | 0 |
| Pain at Injection Site | 15 (48·4) | 9 (29·0) |
| Tenderness at Injection Site | 0 | 0 |
N = Number of participants in the Safety Population; n = Number of participants in the Safety population experiencing the symptom after at least one dose.
Participants are counted once for each category regardless of the number of events.
Refers to a single adverse event occurring in one participant.
Figure 2.
Maximum severity of solicited systemic symptoms per participant by day post treatment- all doses, safety population.
Figure 3.
Maximum severity of solicited local symptoms per participant by day post treatment- all doses, safety population.
During the primary 24 week surveillance period after artesunate clearance, fewer participants in the PfSPZ-CVac (CQ) group were infected with P. falciparum compared to controls by TBS microscopy [55·2% (95% CI 37·5-71·6) vs. 73·3% (95% CI 55·6-85·8)] although the proportions were not significantly different. VE by hazard ratio, the primary method for VE assessment, was estimated at 33·6% (95% CI -27·9, 65·5, p = 0.221), meaning that the estimated hazard of infection for participants receiving PfSPZ-CVac was 33.6% lower than that of controls but the estimate was not statistically significant (Supplementary Material, Table S1). Among 59 participants in the PP population, 58 (98%) completed the 48 week efficacy follow-up period, so potential bias from dropout was minimal.
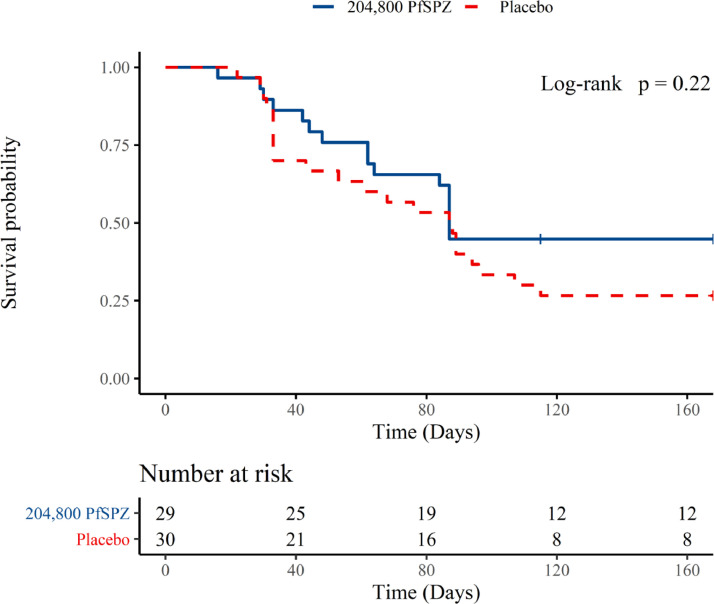
During weeks 25–48 after artesunate clearance, 1 of 13 previously uninfected vaccinees experienced a first infection, and 1 of 8 previously uninfected controls experienced a first infection, with rates of infection reflecting the reduced rainfall and lower vector densities during the second 24 week interval (dry season) compared to the first (wet season). Thus, proportions infected by TBS microscopy over the entire 48-week surveillaince period were similar to those for the 24-week period [58·6% of vaccines vs. 76·7% of controls; 95% CIs 40·7-74·5 and 59·1-88·2, respectively] (Supplementary Material, Table S1), though the proportions were not significantly different. Efficacy by hazard ratio was estimated at 33·4% (95% CI -26·0, 64·8) (Supplementary Material, Table S1), but this was not statistically significant (p = 0·211). Efficacy estimates by the risk ratio and incidence rate ratio approaches also failed to detect a significant difference between the study arms (Supplementary Material, Table S1). Results for the ITT population were similar (data not shown). Kaplan-Meier survival curves for the two treatment groups are displayed in Figure 5. Although the proportional hazards assumption is not met, the p-value from the score test for the Cox regression is equivalent to that from the log rank test, which does not require this assumption and tests for any difference between the two curves.
Figure 5.
Kaplan meier curves of time to parasitemia within 6 months after last vaccination and artesunate treatment– thick blood smear, PP population. Protective efficacy was analysed by time to first positive blood smear, within 6 months after the last vaccination. HR=0·336, 95%CI -0·279-0·655. The p-value from a log-rank test was 0·22.
Although clinical malaria was not a prespecified efficacy endpoint, it was tracked as an AE throughout the follow-up period and was experienced by 35% (11/31) of PfSPZ-CVac group and 55% (17/31) of controls over the entire follow-up period (35.3% VE, p = 0.20, Fisher's exact test).
For parasitemia measured by qPCR, 24-week infection rates were 26/29 (89·7%) in the PfSPZ-CVac arm and 29/30 (96·7%) for controls, showing extremely high rates of infection during the study period and similar rates between arms (Supplementary Material, Table S1). The proportions positive within 48 weeks were 27/29 (93.1%) and 29/30 (96.7%) in PfSPZ Challenge and control arms, respectively, and were not significantly different. Estimates of efficacy by qPCR by all statistical methods were low and nonsignificant (data not shown). On Day 3 of artesunate monotherapy, 24·1% (7/29, 95% CI 12·2-42·1) in the PfSPZ-CVac arm and 10% (3/30, 95% CI 3·5-25·6) of controls were positive for Pf by qPCR.
Results for efficacy analyses for the ITT population were similar to those for the PP population for both TBS and qPCR, for both 24-week and 48-week intervals, and for all methods applied (data not shown).
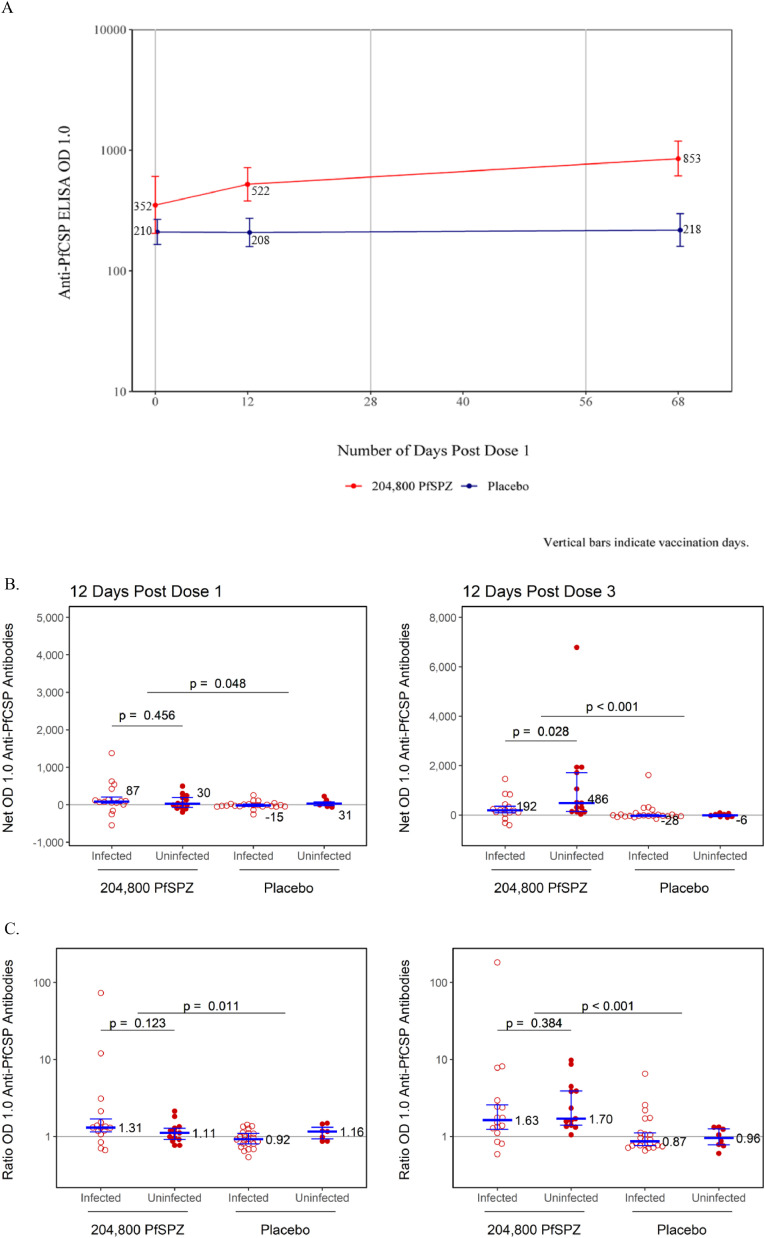
Geometric mean anti-PfCSP antibody levels in the PfSPZ-CVac group were higher than the control group at both post-injection time points (Figure 4A and Supplementary Material, Table S2). The geometric mean anti-PfCSP level in PfSPZ-CVac recipients at baseline was 351·5 (95% CI 204·1-605·5) and 210·2 (95% CI 165·7- 266·6) in controls. Twelve days post Dose 1, it increased to 521·9 (95% CI 379·8-717·2) in PfSPZ-CVac recipients and remained unchanged at 208·1 (95% CI 158·8-272·8) in controls. Twelve days post Dose 3, it increased further to 852·8 (95% CI 611·8-1188·9) in PfSPZ-CVac recipients and remained unchanged at 218·3 (95%CI 159·7-298·2) in controls. Antibody time trends and the corresponding reverse cumulative distribution (RCD) plots over time are shown in Supplementary Material (Figure S1). The proportion of participants achieving seroresponse (i.e., net OD 1.0 ≥50 and ratio OD 1.0 ≥3) from baseline to 12 days after third injections was 28% in PfSPZ-CVac recipients versus 3% of controls (p = 0·0122).
Figure 4.
Antibodies to PfCSP by ELISA (serum dilution at which the optical density was 1.0).
A: Geometric Mean Time Trends of Anti-PfCSP ELISA OD 1.0 with 95% Confidence Intervals by Treatment Group PP Population. Red line represents the Anti-PfCSP ELISA OD 1.0 for vaccine group and the blue line represents the Anti-PfCSP ELISA OD 1.0 for control group. Population analysed: 29 in vaccine group and 30 in control group.
B: Additive Change (net OD 1.0) from Baseline of Anti-PfCSP ELISA OD 1.0 by Treatment Group, PP Population. The filled red circles represent the uninfected and the unfilled red circles represent the infected. The blue lines represent the median ratio OD 1.0 and interquartile ranges.
C: Multiplicative Change from Baseline of Anti-PfCSP ELISA OD 1.0 by Treatment Group PP Population. The solid red circles represent the multiplicative change from baseline at 12 Days Post Dose 1 of the Anti-PfCSP antibodies and the light circle represent the control group. The blue lines represent the median ratio OD 1.0 and interquartile ranges.
For anti-PfCSP antibodies, additive changes from baseline (Net OD 1.0), were calculated by subtracting the pre-vaccination from the post-vaccination antibody value. Distributions of Net OD 1.0 are displayed in Figure 4B by treatment group and infection status. Net OD 1.0 values were significantly higher in the PfSPZ-CVac than in the control group (p = <0.001), similar between infected and uninfected vaccines at 12 days post Dose 1, and significantly (p = 0.028) higher in uninfected than infected vaccines at 12 days post Dose 3. Multiplicative changes in anti-PfCSP antibody from baseline (Ratio OD 1.0) were calculated for each participant by dividing the post-vaccination antibody level by the pre-vaccination level. Distributions of ratios are displayed in Figure 4B by treatment group and infection status. Ratios were higher in the PfSPZ-CVac than in the control group, and similar between uninfected and infected participants within the vaccine group.
Discussion
PfSPZ-CVac (CQ) is the most potent method of whole PfSPZ immunization in malaira-naïve adults, as evidenced by the 100% protection achieved against heterologous CHMI performed 12 weeks after immunization with three doses of 2.0 × 105 PfSPZ.8 The heterologous parasite used for CHMI in the US was Pf7G8, a South America strain, which is more genetically distant than any other sequenced parasite.9 These results strongly support further development of PfSPZ-CVac. The prospects for a high efficacy vaccine have been tempered, however, by concerns regarding the malaria-related signs and symptoms experienced by naïve individuals during the transient parasitemia occurring days 7-9 after PfSPZ-CVac (CQ) administration.3,5,8,14,15 This first field trial in Mali was designed to assess whether the same degree of reactogenity occurred in malaria-exposed adults possessing naturally acquired immunity, and whether the same high-level protection seen following CHMI was achieved against naturally transmitted Pf.
PfSPZ-CVac showed excellent safety and tolerability in the 31 malaria-exposed adults who were vaccinated in the current trial. No grade 3 malaria-related AEs occurred, unlike trials in malaria-naïve individuals,5,6,8 and no differences were noted between vaccine and control groups in solicited or unsolicited AEs recorded through day 12 post vaccination, of any severity grade. Although qPCR was not performed in this study during the periods when transient parasitemia may have occurred, naturally acquired immunity likely contributed to the greatly improved tolerability by reducing the density of parasites in the blood as well as the level of rectogenitiy to any parasites that were present.16
VE was less than expected: 34% by time-to-event analysis (1-hazard ratio) and 25% by the proportional method (1-risk ratio). Neither result achieved statistical significance in this small trial, which had been powered to detect a 60% reduction in the 24-week infection rate from 75% to 30%. While the control group infection rate was as expected (73.3%), VE was less than half what had been predicted.
Why was VE so much lower in this study, despite administering nearly the same dose of PfSPZ (3 × 2.048 × 105) as in the US study where heterologous protection against a divergent parasite was 100% (3 × 2.0 × 105)?8 We think the most likely explanation is that the Malians did not mount protective immune responses comparable to those mounted by vaccines in the US. With the exact same immunization regimen, antibodies to PfCSP two weeks after the last dose of vaccine showed a median net OD 1.0 in this trial of 242 and in the US trial of 8060,8 a 33-fold difference. Evidence suggests that tissue resident T cells in the liver are the primary mediators of protection,17 and that decreased antibody responses serve as an imperfect marker of decreased T cell responses, as seen in other studies in malaria-exposed African adults where both were measured.18, 19, 20, 21 In our study, only 28% of vaccines met criteria for seroconversion, compared to 100% in the studies in Germany6 and the US.8 Interestingly, even though antibody responses were low, as in most of our field studies,21, 22, 23 the net increase in antibodies to PfCSP was significantly higher in uninfected vs. infected vaccines (Figure 4B). We think this net response is an indication of vaccine take, not that these low levels of antibodies mediate protection, which is mediated primarily by T cells.24
We believe that three factors contributed to vaccine hyporesponsiveness. The first is that the partial anti-parasite immunity resulting from lifelong exposure to Pf parasites may have interdicted the PfSPZ used for immunization before they could invade, replicate and induce protective immune responses, reducing the effective dose to much less than 2.048 × 105 PfSPZ. In future studies, it should be possible to overcome immunogen neutralization by increasing the PfSPZ dose. The study of PfSPZ-CVac (CQ) at the Institute for Tropical Medicine at the University of Tübingen showed a strong dose response in terms of VE: at 3.2 × 103 PfSPZ/dose VE was 33%, at 1.28 × 104 PfSPZ/dose VE was 67% and at 5.12 × 104 PfSPZ/dose it was 100%.6 Thus, it is possible that increasing the dose of PfSPZ Challenge will lead to increased VE by providing more replicating late liver stage parasites. Indeed, results from a follow-on CVac study in Mali where a roughly 2-fold increased dose of 4.0 × 105 PfSPZ was used showed statistically significant protection (Sagara, unpublished).
The second factor is suspected to be the immune dysregulation that results from lifelong exposure to Pf malaria. Those living in malaria endemic areas have diminished responses to malaria-specific antigens25 and also to other vaccines and pathogens.26, 27, 28 Administration of a higher dose of PfSPZ or an adjuvant might help to overcome immune dysregulation. Because T cell responses to immunization with the PfSPZ Vaccine (radiation-attenuated PfSPZ) are best in 6–10 year old children,19 focusing on this age group would be another approach to increasing the magnitude of immune responses to PfSPZ-CVac.
The third factor suspected of contributing to vaccine hyporesponsiveness was failure to presumptively clear any existing sub-patent parasitemias before the first and third doses of vaccine, a key procedure in other field trials. At the time this trial was designed, we did not fully appreciate that even submicroscopic densities of parasites, as are present in the Sahel at the end of the dry season when the study subjects were immunized,29,30 can abrogate the induction of protective immunity. It has since been shown for example that immunizing naïve US adults when there is parasitemia detectable by qPCR but not by TBS, eliminates the protective efficacy of PfSPZ-CVac.5 VE data from the other studies in Africa in which presumptive treatment was administered before the first dose of either PfSPZ Vaccine or PfSPZ-CVac (Sirima and Diawara, both unpublished),21,22 and again before the last dose of vaccine, to prevent the immune-inhibitory effects of undetected, low-density parasitemia, and there was significant VE in all 5 studies over the first malaria transmission season, ranging from 39 to 56%, with efficacy maintained at a similar level through a second malaria season in three trials. In addition there have been 2 field studies in Africa in which presumptive treatment was not given before the first dose of PfSPZ Vaccine, but given before the last dose (Diawara unpublished),23 and there was no significant protection. In Mali when the same immunization regimen of PfSPZ Vaccine was administered with and without presumptive treatment before the first dose, there was significant VE only in the study in which presumptive treatment before the first dose was given. Pf parasites in the bloodstream, spleen or bone marrow may be eliminating the protective cellular immune response required for protection by PfSPZ, as has been shown to occur in animal models (Sirima and Diawara, both unpublished).21,22,31,32
Our PfSPZ-CVac trial is now the third field trial where presumptive treatment was not done and statistically significant VE was not achieved. Presumptive treatment was administered to all study subjects, but it was administered after the third dose, not before, so in fact all three immunizations may have been compromised by subpatent parasitemias. Evidence is provided by the finding that on Day 3 of artesunate monotherapy after the 3rd dose of vaccine, 24·1% (7/29, 95% CI 12·2-42·1) of vaccines were indeed parasitemic, and more may have been parasitemic at levels too low for qPCR detection.
The artesunate therapy after the third dose was supposed to clear parasitemia so we could unambiguously measure new infections. Because of the findings on Day 3 mentioned above, we are not sure that artesunate monotherapy was successful, which would have made it difficult to assess VE against new infection. However, the qPCR results may have been in part due to the fact that qPCR detects presence of gametocytes which can persist in the blood for months, and that artesunate clearance worked as planned.
Another explanation for the poor VE in this study is that the protective immune responses induced did not recognize the breakthrough parasites we detected because of sequence variability. We think this is unlikely, but sequencing is ongoing.
In summary, we have demonstrated safety and tolerability of PfSPZ-CVac (CQ) in a malaria-experienced population, and have a preliminary indication that there is some VE against new Pf infection detected by TBS and clinical malaria. Our next steps to improve VE will be to increase the dose of PfSPZ and to presumptively treat all subjects before the first and last immunizations. These studies are currently ongoing in Mali.
Contributors
MAT, MBL were the principal investigators. MAT, MBL, OKD, CVP, YA, JKK, GEP, EN, GAD, TLR, ERJ, BKLS and SLH, wrote the study protocol with contributions from all authors in the review of the approved final version, and additional amendments. DC, AKK, KT collected the data. BK, CA, AZ, KEL and NKC completed the study laboratory endpoints. BKLS, TLR, ERJ and SLH developed the vaccine. AD, AN and YA completed the procedures and syringe preparations for injection. EN provided regulatory and project management support during the study. JKK and the Emmes Corporation supported database design and management. GEP and SMG completed the statistical analysis. MAT, MBL, BK, CA, DC, GEP, GAD, TLR and SLH interpreted data and results.
Data sharing statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declaration of interests
TLR, YA, BKLS, ERJ, NKC and SLH are salaried, full-time employees of Sanaria, the developer and sponsor of Sanaria PfSPZ Vaccine. SLH and BKLS also have financial interests in Sanaria. BKLS, and SLH are inventors on patents and applications for patent that have been assigned to Sanaria. DC, AKK, KT, AN, BK, CA, AZ, AD, MAT are employees of MRTC and were paid for the implementation of the study through the sub contract HHSN2722013000221/HHSN27200015-10018602. MBL was supported by the NIH HHSN272201300022I contract.
EN participated in the study as the NIH/DMID Clinical Project Manager and helped coordinate and organize DSMB meetings and communications. SMG became a Novavax Inc employee after contributing to this manuscript. All other authors declare no competing interests.
Acknowledgments
This project has been funded in whole or in part with Federal funds from the National Institutes of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, HHSN272201300022I. Sanaria manufactured the vaccine and supported the costs of transportation and syringe preparation. The manufacture of Sanaria PfSPZ Challenge was supported in part by Small Business Innovation Research grant from the NIAID (2R44AI058375, “Universal attenuated sporozoite vaccine and challenge system”). This project is part of the EDCTP2 programme supported by the European Union. We thank first, and foremost the study volunteers and the local guides for their participation in the study, and the local communities of Bougoula–Hameau, Karamokobougou, Fincolo and Kafela for their support. We also thank the FMPOS Ethics Committee, NIAID IRB, Malian Ministry of Health and Public Hygiene, and Malian Department of Pharmacy, and Medicine. We are grateful to the USTTB Rector and his office. We are grateful to community health centre of Bougoula–Hameau. We thank our Malaria Research and Training Center (MRTC) colleagues within the clinical laboratory team. We thank Walter Jones of DMID for clinical trial coordination support. We thank David Diemert, Michele Spring and Davidson Hamer as members of the Safety Monitoring Committee (SMC). We thank EMMES data management, statistical analysis, and the production of the clinical statistical report. We also thank the Sanaria, and Protein Potential Teams for the manufacturing of the investigational products, PfSPZ Challenge, and diluents, preparation of investigational material at the clinical site. We thank UMB for regulatory aspects, and antibody assays performance. MRTC for site activities performance. The authors alone are responsible for the views expressed in this manuscript, and they do not necessarily represent the decisions, policy, or views of the US Government. Mention of trade names, commercial products, or organizations does not imply endorsement by the US Government. We dedicate this manuscript to our late director, Ogobara K. Doumbo, whose commitment, clinical acumen, and compassion underpinned the success of this trial.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101579.
Appendix. Supplementary materials
References
- 1.Ouattara A, Laurens MB. Vaccines against malaria. Clin Infect Dis. 2015;60(6):930–936. doi: 10.1093/cid/ciu954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffman SL, Goh LM, Luke TC, et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis. 2002;185(8):1155–1164. doi: 10.1086/339409. [DOI] [PubMed] [Google Scholar]
- 3.Roestenberg M, McCall M, Hopman J, et al. Protection against a malaria challenge by sporozoite inoculation. N Engl J Med. 2009;361(5):468–477. doi: 10.1056/NEJMoa0805832. [DOI] [PubMed] [Google Scholar]
- 4.Roestenberg M, Teirlinck AC, McCall MB, et al. Long-term protection against malaria after experimental sporozoite inoculation: an open-label follow-up study. Lancet. 2011;377(9779):1770–1776. doi: 10.1016/S0140-6736(11)60360-7. [DOI] [PubMed] [Google Scholar]
- 5.Murphy SC, Deye GA, Sim BKL, et al. PfSPZ-CVac efficacy against malaria increases from 0% to 75% when administered in the absence of erythrocyte stage parasitemia: a randomized, placebo-controlled trial with controlled human malaria infection. PLoS Pathog. 2021;17(5) doi: 10.1371/journal.ppat.1009594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mordmuller B, Surat G, Lagler H, et al. Sterile protection against human malaria by chemoattenuated PfSPZ vaccine. Nature. 2017;542(7642):445–449. doi: 10.1038/nature21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seder RA, Chang LJ, Enama ME, et al. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science. 2013;341(6152):1359–1365. doi: 10.1126/science.1241800. [DOI] [PubMed] [Google Scholar]
- 8.Mwakingwe-Omari A, Healy SA, Lane J, et al. Two chemoattenuated PfSPZ malaria vaccines induce sterile hepatic immunity. Nature. 2021;595(7866):289–294. doi: 10.1038/s41586-021-03684-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silva JC, Dwivedi A, Moser KA, et al. Plasmodium falciparum 7G8 challenge provides conservative prediction of efficacy of PfNF54-based PfSPZ Vaccine in Africa. Nat Commun. 13(1), 2022, 3390. [DOI] [PMC free article] [PubMed]
- 10.Diallo DA, Doumbo OK, Plowe CV, Wellems TE, Emanuel EJ, Hurst SA. Community permission for medical research in developing countries. Clin Infect Dis. 2005;41(2):255–259. doi: 10.1086/430707. [DOI] [PubMed] [Google Scholar]
- 11.Lyke KE, Laurens M, Adams M, et al. Plasmodium falciparum malaria challenge by the bite of aseptic Anopheles stephensi mosquitoes: results of a randomized infectivity trial. PLoS One. 2010;5(10):e13490. doi: 10.1371/journal.pone.0013490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenwood M. The natural duration of cancer. Reports on Public Health and Medical Subjects. 1926;33:1–26. [Google Scholar]
- 13.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Statist Assoc. 1958;(53):457–481. [Google Scholar]
- 14.Mordmuller B, Supan C, Sim KL, et al. Direct venous inoculation of Plasmodium falciparum sporozoites for controlled human malaria infection: a dose-finding trial in two centres. Malar J. 2015;14:117. doi: 10.1186/s12936-015-0628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sulyok Z, Fendel R, Eder B, et al. Heterologous protection against malaria by a simple chemoattenuated PfSPZ vaccine regimen in a randomized trial. Nat Commun. 2021;12(1):2518. doi: 10.1038/s41467-021-22740-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doolan DL, Dobano C, Baird JK. Acquired immunity to malaria. Clin Microbiol Rev. 2009;22(1):13–36. doi: 10.1128/CMR.00025-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Epstein JE, Tewari K, Lyke KE, et al. Live attenuated malaria vaccine designed to protect through hepatic CD8(+) T cell immunity. Science. 2011;334(6055):475–480. doi: 10.1126/science.1211548. [DOI] [PubMed] [Google Scholar]
- 18.Jongo SA, Shekalaghe SA, Church LWP, et al. Safety, immunogenicity, and protective efficacy against controlled human malaria infection of Plasmodium falciparum sporozoite vaccine in Tanzanian adults. Am J Trop Med Hyg. 2018;99(2):338–349. doi: 10.4269/ajtmh.17-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jongo SA, Church LWP, Mtoro AT, et al. Safety and differential antibody and T-cell responses to the Plasmodium falciparum sporozoite malaria vaccine, PfSPZ vaccine, by age in Tanzanian adults, adolescents, children, and infants. Am J Trop Med Hyg. 2019;100(6):1433–1444. doi: 10.4269/ajtmh.18-0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jongo SA, Church LWP, Mtoro AT, et al. Increase of dose associated with decrease in protection against controlled human malaria infection by PfSPZ vaccine in Tanzanian adults. Clin Infect Dis. 2020;71(11):2849–2857. doi: 10.1093/cid/ciz1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sissoko MS, Healy SA, Katile A, et al. Safety and efficacy of a three-dose regimen of Plasmodium falciparum sporozoite vaccine in adults during an intense malaria transmission season in Mali: a randomised, controlled phase 1 trial. Lancet Infect Dis. 2021;22(3):377–389. doi: 10.1016/S1473-3099(21)00332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sissoko MS, Healy SA, Katile A, et al. Safety and efficacy of PfSPZ Vaccine against Plasmodium falciparum via direct venous inoculation in healthy malaria-exposed adults in Mali: a randomised, double-blind phase 1 trial. Lancet Infect Dis. 2017;17(5):498–509. doi: 10.1016/S1473-3099(17)30104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oneko M, Steinhardt LC, Yego R, et al. Safety, immunogenicity and efficacy of PfSPZ Vaccine against malaria in infants in western Kenya: a double-blind, randomized, placebo-controlled phase 2 trial. Nat Med. 2021;27(9):1636–1645. doi: 10.1038/s41591-021-01470-y. [DOI] [PubMed] [Google Scholar]
- 24.Bijker EM, Teirlinck AC, Schats R, et al. Cytotoxic markers associate with protection against malaria in human volunteers immunized with Plasmodium falciparum sporozoites. J Infect Dis. 2014;210(10):1605–1615. doi: 10.1093/infdis/jiu293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ho M, Webster HK, Looareesuwan S, et al. Antigen-specific immunosuppression in human malaria due to Plasmodium falciparum. J Infect Dis. 1986;153(4):763–771. doi: 10.1093/infdis/153.4.763. [DOI] [PubMed] [Google Scholar]
- 26.McGregor IA, Barr M. Antibody response to tetanus toxoid inoculation in malarious and non-malarious Gambian children. Trans R Soc Trop Med Hyg. 1962;(56):364–367. [Google Scholar]
- 27.Williamson WA, Greenwood BM. Impairment of the immune response to vaccination after acute malaria. Lancet. 1978;1(8078):1328–1329. doi: 10.1016/s0140-6736(78)92403-0. [DOI] [PubMed] [Google Scholar]
- 28.Nyirenda TS, Nyirenda JT, Tembo DL, et al. Loss of humoral and cellular immunity to invasive nontyphoidal Salmonella during current or convalescent Plasmodium falciparum infection in Malawian children. Clin Vaccine Immunol. 2017;24(7):1–13. doi: 10.1128/CVI.00057-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sonden K, Doumbo S, Hammar U, et al. Asymptomatic multiclonal Plasmodium falciparum infections carried through the dry season predict protection against subsequent clinical malaria. J Infect Dis. 2015;212(4):608–616. doi: 10.1093/infdis/jiv088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adomako-Ankomah Y, Chenoweth MS, Durfee K, et al. High Plasmodium falciparum longitudinal prevalence is associated with high multiclonality and reduced clinical malaria risk in a seasonal transmission area of Mali. PLoS One. 2017;12(2) doi: 10.1371/journal.pone.0170948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orjih AU, Nussenzweig RS. Plasmodium berghei: suppression of antibody response to sporozoite stage by acute blood stage infection. Clin Exp Immunol. 1979;38(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- 32.Ocana-Morgner C, Mota MM, Rodriguez A. Malaria blood stage suppression of liver stage immunity by dendritic cells. J Exp Med. 2003;197(2):143–151. doi: 10.1084/jem.20021072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.