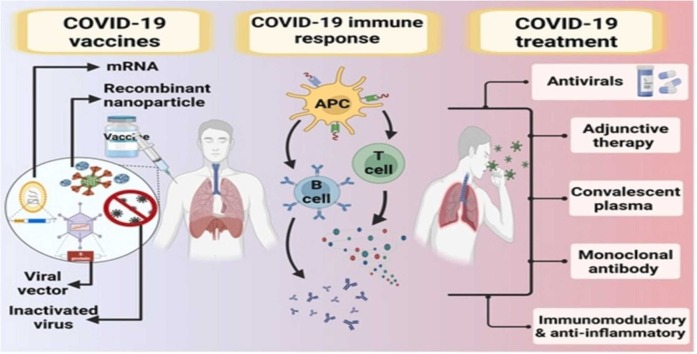
Abstract
The healthy immune system eliminates pathogens and maintains tissue homeostasis through extraordinarily complex networks with feedback systems while avoiding potentially massive tissue destruction. Many parameters influence humoral and cellular vaccine responses, including intrinsic and extrinsic, environmental, and behavioral, nutritional, perinatal and administrative parameters. The relative contributions of persisting antibodies and immune memory as well as the determinants of immune memory induction, to protect against specific diseases are the main parameters of long-term vaccine efficacy. Natural and vaccine-induced immunity and monoclonal antibody immunotherapeutic, may be evaded by SARS-CoV-2 variants. Besides the complications of the production of COVID-19 vaccinations, there is no effective single treatment against COVID-19. However, administration of a combined treatment at different stages of COVID-19 infection may offer some cure assistance. Combination treatment of antiviral drugs and immunomodulatory drugs may reduce inflammation in critical COVID-19 patients with cytokine release syndrome. Molnupiravir, remdesivir and paxlovid are the approved antiviral agents that may reduce the recovery time. In addition, immunomodulatory drugs such as lactoferrin and monoclonal antibodies are used to control inflammatory responses in their respective auto-immune conditions. Therefore, the widespread occurrence of highly transmissible variants like Delta and Omicron indicates that there is still a lot of work to be done in designing efficient vaccines and medicines for COVID-19. In this review, we briefly discussed the immunological response against SARS-CoV-2 and the vaccines approved by the World Health Organization (WHO) for COVID-19, their mechanisms, and side effects. Moreover, we mentioned various treatment trials and strategies for COVID-19.
Keywords: COVID-19, Antiviral, Immunomodulators, Vaccination, Treatment
Abbreviations: +ssRNA, Positive-sense single-stranded RNA; 3CL, 3C-like; ACE2, Angiotensin-converting enzyme 2; CCCs, Common cold’ coronaviruses; ChAd, Chimpanzee-derived adenovirus; CRS, Cytokine release syndrome; DPP, Dipeptidyl peptidase; GRAS, Generally recognized as safe; HSPGs, Heparan sulfate proteoglycans; IL-6, Interleukin-6; mAb, Monoclonal antibody; miRNA, MicroRNA; PAMPs, Pathogen-associated molecular patterns; PRRs, Pattern recognition receptors; RdRp, RNA dependent RNA polymerase; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; SSRI, Selective serotonin reuptake inhibitor; Th, T-helper; TLRs, Toll-like receptors; Tregs, Regulatory T-cells; VLPs, Virus-like particles; VOCs, Variants of concern
Graphical Abstract
1. Introduction
The recent COVID-19 pandemic outbreak, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is regarded as the biggest global challenge to healthcare organizations. SARS-CoV-2 is a member of the family Coronaviridae that is classified under the order Nidovirales [1]. SARS-CoV-2 is included in the betacoronavirus genotype together with two highly pathogenic viruses, SARS-CoV and MERS-CoV that are estimated as novel human-infecting viruses [1]. Coronaviruses are a type of enveloped and positive-sense single-stranded RNA (+ssRNA) viruses that are more likely to infect people with chronic comorbidities such as cardiovascular and cerebrovascular diseases and diabetes [2]. Severe manifestations may also be associated with coinfections with bacteria and/or fungi [3]. SARS-CoV-2 is considered a highly transmitted virus with a fast-increasing incidence of infections and its transmission can be done by asymptomatic carriers [4]. The envelope spike (S) protein receptor-binding domain of SARS-CoV-2 was demonstrated to be structurally analogous to that of SARS-CoV, despite amino acid variation at some key residues [5]. Further structural analysis strongly suggests that SARS-CoV-2 may enter cells via the host receptor angiotensin-converting enzyme 2 (ACE2) [6]. However, it is largely unknown how SARS-CoV-2 manages to evade the immune response and drive pathogenesis. SARS-CoV-2 and SARS-CoV have similar clinical features [7] and may share pathogenesis mechanisms [8]. The infection is usually combined with severe pneumonia, with fever and dry cough as common symptoms at the onset of illness [9], [10]. Some patients progressed fast with Acute Respiratory Stress Syndrome (ARDS) and septic shock, which was eventually followed by multiple organ failure, and about 10% of patients died [10]. SARS-CoV-2 patients have higher plasma levels of pro-inflammatory cytokines such as IL1, IL-2, IL7, TNF-, GSCF, and MCP1 than healthy adults [9].
SARS-CoV-2, like other RNA viruses, adapted to their new hosts with highly genetic mutations, leading to the emergence of new mutant variants that might have changed characteristics from their ancestral strains. To date, several mutant variants of SARS-CoV-2 have been modified genetically through the outbreak of this pandemic disease. World health organization (WHO) identified a few variants of concern (VOCs), which assume their influence on global public health. Both innate and acquired immunity are included in recognizing and eradicating invading viral pathogens. COVID-19 happens through the transmission of viral particles from person to person, and the infection is developed according to the interaction of the immune system with the invaded virus [11]. Based on previous preclinical studies on exploring vaccines against SARS-CoV or MERS-CoV, the spike (S) protein of SARS-CoV-2 is considered the most important target antigen for the development of vaccines against COVID-19. Owing to quickly responding to the COVID-19 outbreaks, a wide range of vaccines have been studied on COVID-19 using several platforms and approaches, including inactivated vaccines, live attenuated vaccines, nucleic acid (RNA, DNA) vaccines, viral vectored vaccines, and protein subunit vaccines [12]. Many efforts have been made to develop vaccines, that primarily target the entry of viral particles into host cells, and then various strategies have been developed to improve the quality and quantity of the used vaccine. Accordingly, many types of vaccines will be developed to meet specific requests across various target populations. This might involve the opportunity of using heterologous vaccines depending on boosting capacities, health status, and the demand for balanced Th1-directed cellular and humoral immune responses [13].
On the other hand, in the absence of a decisive treatment protocol, the treatment of COVID-19 patients mainly depends on the symptomatic circumstances and availability of therapeutic drugs. Antiviral therapies, antibiotics, systemic corticosteroids, and anti-inflammatory drugs are the most used regimens. In addition to routine therapies, RNA synthesis inhibitors, neuraminidase inhibitors, convalescent plasma, and alternative medicines have also been considered in the COVID-19 treatment [5]. Due to the emergence of drug-resistant mutants to antiviral drugs, treatment with indirect drugs that affect host cell proteins is more likely. These indirect agents might act as potential drugs against SARS-CoV-2 entry, such as baricitinib, included in the endocytosis of angiotensin-converting enzyme 2 (ACE2) and targeting the Janus kinase [14]. Although COVID-19 infection presents a critical challenge to the development of both vaccination and drugs, it also provides a unique perspective for scientists and the clinical pharmacology community to explore and assist in the clinical development of new vaccine and therapy strategies. In the present review, we summarize the immunological aspects of the COVID-19 pandemic, focusing on vaccination, antiviral agents, and immunomodulatory therapeutic proteins.
2. Constitutive immunity beyond infection
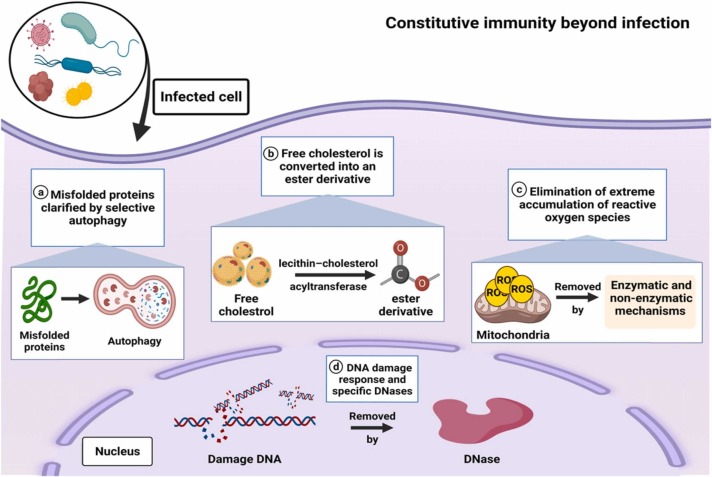
The immune responses to microbial infections are often disruptive and can cause marked tissue damage [15], [16]. The mammalian immune system, as we understand it today, is induced mainly by two types of receptor systems; the germline-encoded pattern recognition receptors (PRRs), which initiate innate immune responses, and the antigen-specific receptors generated through gene rearrangement after antigen encounter, which initiate adaptive immune responses [17], [18], [19]. The immune responses induced by PRRs, such as Toll-like receptors (TLRs), interact with those induced by antigen-specific receptors; this interaction is notably represented by dendritic cells, which rely on PRR-driven cues to initiate dendritic cell maturation for the stimulation of lymphocytes through antigen-specific receptors [20]. Infections are protected by constitutive immune mechanisms. However, they are also involved in the elimination of sterile danger. For example, DNA damage in the nucleus and the aggregation of DNA in extranuclear compartments are eliminated by the DNA damage response and specific DNases, respectively ( Fig. 1) [21]. In addition, the accumulation of misfolded proteins produces the formation of aggresomes, which are clarified by selective autophagy [22], [23]. The extreme accumulation of reactive oxygen species induces the death of the oxygen-stressed cells [24], and free cholesterol is converted into an ester derivative by lecithin–cholesterol acyltransferase, thus enabling transport to the liver by high-density lipoprotein and eventual degradation [25]. Disorders in these constitutive and potential danger-eliminating mechanisms produce the aggregation of danger-associated molecular patterns and the activation of PRR-based immunity. The cGAS–STING system, for example, increases type I interferon production in cells with abnormalities in either the DNA damage response or additional nuclear DNases when DNA accumulates. Likewise, defective elimination of protein aggregates or cholesterol leads to the encouragement of IL-1β production through activation of the NLRP3 inflammasome [26], [27].
Fig. 1.
Molecular mechanisms of constitutive immunity beyond microbial infection.
3. Immune response in COVID-19
The immune system boosts our body’s natural strength to defend against pathogens including viruses, bacteria, fungi, protozoan, and worm infections [28]. As long as the immune system runs smoothly, we do not recognize infections like COVID-19. Our immune system can be classified into three groups, including innate immunity (rapid response), adaptive immunity (slow response), and passive immunity. Passive immunity has two types, and they are natural immunity that we receive from our mother and artificial immunity that we receive from medicine. However, when our body meets any germs or viruses for the first time, the immune system cannot work properly, and we become sick, and the same thing has happened in the case of COVID-19 [29]. Attributed to the high genetic homology of SARS-CoV-2 to other closely related coronaviruses, the collective data from SARS-CoV and MERS-CoV previous infections paved the way to realizing how SARS-CoV-2 escapes the host’s immune response [5]. Compared to SARS-CoV and other closely related coronaviruses, its spike S protein is 20–30 amino acids longer. Thus, SARS-CoV-2 has similar immune evasive strategies, but an additional mechanism is still undiscovered [30], [31]. While SARS-CoV and SARS-CoV-2 seem to participate in the entry receptor of ACE2, MERS-CoV utilizes dipeptidyl peptidase (DPP)− 4 as a specific receptor [32]. Whether SARS-CoV-2 infects any immune cells is unknown yet, but only minimum percentages of monocytes and macrophages in the lung express ACE2 [33]. In an antiviral response, innate immune cells recognize the invasion of the virus, often by pathogen-associated molecular patterns (PAMPs). For RNA viruses such as coronavirus, it is known that PAMPs in the form of viral genomic RNA or the intermediates during viral replication, including dsRNA, are recognized by each of the endosomal RNA receptors, TLR3, and TLR7, and the cytosolic RNA sensor, RIG-I/MDA5 [34]. The Th1 type of immune reaction plays a dominant function in adaptive immunity to viral infections. In SARS-CoV, both T-and B-cell epitopes were comprehensively mapped for the structural proteins S, N, M, and E protein [35].
4. Pre-existing immunity to SARS-CoV-2
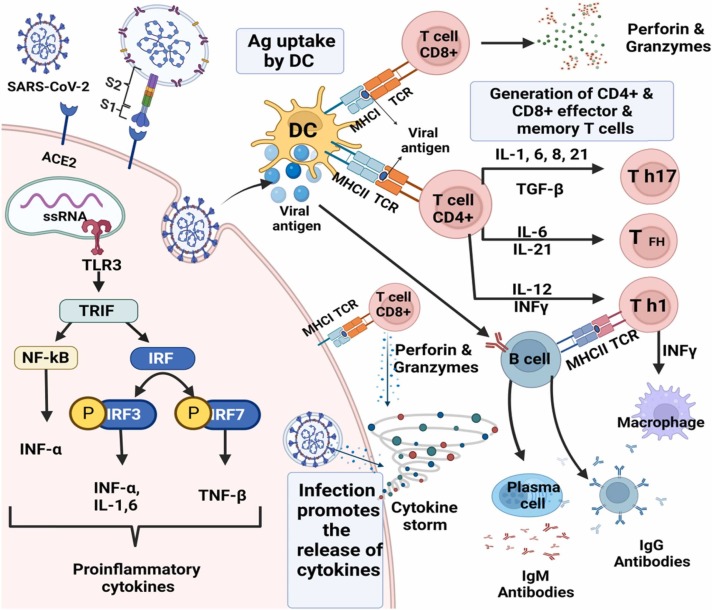
Lymphocytes from uninfected donors are reactive to SARS-CoV-2 antigen peptide pools in 20–50% of cases [36], [37]. The SARS-CoV-2 reaction with T-cells was predominantly associated with CD4 + T cells, with a smaller contribution by CD8 + T-cells ( Fig. 2 ). Braun et al., declared positive T-cell responses versus spike peptides in 34% of SARS-CoV-2 seronegative healthy donors (CD4 + and CD8 + T-cells were not distinguished) [38]. Similarly, Le Bert et al., announced T-cell reactions to nucleocapsid protein nsp7 or nsp13 in 50% of subjects with no history of COVID-19, or contact with patients with COVID-19 [39]. A study by Meckiff also disclosed reactivity in unexposed subjects. It has been speculated that the SARS-CoV-2 specific T-cells in unexposed individuals might originate from memory T-cells derived from exposure to ‘common cold’ coronaviruses (CCCs), such as HCoV-OC43, HCoV-HKU1, HCoV-NL63, and HCoV-229E, which vastly circulate in human individuals and are in charge of mild self-limiting respiratory symptoms [40]. Over 90% of the human population is seropositive for at least three of the CCCs [41]. Braun and colleagues reported that the T-cell reactivity was highest against a collection of SARS-CoV-2 spike peptides that had higher homology to CCCs, but the variation was not significant [42]. Memory CD4 + and CD8 + T-cells might also facilitate direct antiviral immunity in the lungs and nasopharynx early after exposure, in keeping with our understanding of antiviral CD4 + T-cells in the lungs versus the related SARS-CoV [43], and our common understanding of the value of memory CD8 + T-cells in protection from viral infections. As a result, a cytokine storm or cytokine release syndrome (CRS) begins with a widespread activation of cytokine-secreting cells, which is accompanied by both innate and adaptive immune responses (Fig. 2), which both contribute to an undesired outcome [44]. SARS-CoV-2 invades type II pneumocytes through ACE2 in the respiratory system, resulting in fast viral replication and a pro-inflammatory state with high levels of cytokines such as IL-1, IL-6, CXCL8, and TNF [45]. The term "cytokine storm" was immediately used to characterize the immunopathology associated with severe COVID-19 infection. Moreover, the concept of cytokine storm has been enlarged to cover any inflammatory disorders with high circulating cytokines that cause systemic inflammation and secondary organ dysfunction regardless of absolute cytokine concentrations [46].
Fig. 2.
Immune response during COVID-19 disease. SARS-CoV-2 leads to infection of ACE2 expressing target cells such as alveolar type 2 cells or other unknown target cells. the virus may inhibit anti-viral IFN responses resulting in uncontrolled viral replication. The flow of neutrophils and monocytes/macrophages results in the hyper-production of pro-inflammatory cytokines. The immunopathology of the lung may be the result of the “cytokine storms”. Specific Th1/Th17 may be activated and contributes to exacerbating inflammatory responses. B cells/plasma cells produce SARS-CoV-2 specific antibodies that may help neutralize viruses.
5. Vaccine immunology
Vaccines provide protection by stimulating effector mechanisms (cells or molecules) that are capable of rapidly controlling replicating pathogens or inactivating their toxic components. Vaccine-induced immune effectors are antibodies produced by B lymphocytes that can specifically connect to a toxin or pathogen [47]. Other potential effectors are cytotoxic CD8 + T lymphocytes that may limit the spread of infectious agents by recognizing and killing infected cells or secreting specific antiviral cytokines and CD4 + T-helper (Th) lymphocytes. These Th cells may participate in defense through cytokine production and supply the generation and maintenance of B and CD8 + T-cell responses. Effector CD4 + T cells were originally subdivided into T-helper 1 (Th1), or T-helper 2 (Th2) based on their primary cytokine production (interferon-or interleukin [IL]−4), respectively. This dichotomy became outdated as Th cells were shown to comprise a large number of subsets with distinct cytokine-producing and homing capacities [48]. A recently identified critical subset of vaccine-induced CD4 + Th cells are follicular T-helper (Tfh) cells. They are specially equipped and positioned in the lymph nodes to support potent B-cell activation and differentiation into antibody-secreting cells [49], and have been identified as directly controlling antibody responses and mediating an adjuvant [50], [51], [52]. T-helper 17 (Th17) cells are another important subset, that essentially defends against extracellular bacteria that colonize the skin and mucosa, recruiting neutrophils and promoting local inflammation. [53], [54]. These effectors are dominated by regulatory T-cells (Tregs) implicated in preserving immune tolerance [55]. Most antigens and vaccines trigger B-and T-cell reactions, such that there is no rationale for opposing vaccines favoring antibody production (humoral immunity) and T-cell responses (cellular immunity). Furthermore, most antibody responses require CD4 + T-cells, whereas antibodies have a significant influence on T-cell responses to intracellular pathogens [56].
6. COVID-19 vaccine strategies
It is imperative to develop various vaccine platforms and strategies in parallel. Indeed, since the outbreak began, researchers around the world have been racing to develop COVID-19 vaccines, with at least 166 vaccine candidates currently in preclinical and clinical development [57] . To meet the urgent need for a vaccine, a new pandemic vaccine development paradigm has been proposed that compresses the development timeline from 10 to 15 years to 1–2 years [12]. It is estimated that 40–75% of infections are mild or asymptomatic [58], [59], and asymptomatic people may have significantly longer viral shedding than symptomatic people [60]. Vaccine design concerns the selection of antigens, vaccine platforms, vaccination routes, and regimens. In the case of SARS-CoV, it has been shown that only antibodies directed to S protein can neutralize the virus and prevent infection [61]. Inclusion of viral enzymes such as the RNA-dependent RNA polymerase in a vaccine design may ensure that it targets all emerging variant strains, as these proteins are highly conserved [62], [63], [64], even across other bat-derived coronaviruses that could emerge as a threat to humans in the future. In general, vaccine platforms are divided into six categories: live attenuated viruses, recombinant viral-vectored vaccines that are bioengineered to express target pathogen antigens in vivo, inactivated or killed viruses, protein subunit vaccines, virus-like particles (VLPs), and nucleic acid-based (DNA or mRNA) vaccines [65]. The route of vaccination is an essential consideration of vaccine strategies [66], [67]. This is especially important for mucosal pathogens such as SARS-CoV-2 that require not only neutralizing antibodies but also innate and adaptive cellular immunity [68], [69]. The parenteral route of vaccination is unable to effectively induce mucosal IgA antibodies or TRM cells in the lungs [70], [71]. By comparison, the respiratory mucosal route of vaccination is adept at inducing antibodies and TRM cells in the respiratory mucosa as well as macrophage-mediated trained immunity [72], [73]. Inactivated virus, protein subunit, and nucleic acid vaccines cannot be administered by the respiratory mucosal route owing to their requirement for potentially unsafe immune adjuvants and repeated delivery. By contrast, recombinant viral-vectored vaccines, particularly those using human serotype 5 adenoviruses (Ad5) or chimpanzee-derived adenovirus (ChAd), are safe and highly effective for respiratory mucosal vaccination [74].
7. The development of effective COVID-19 vaccines
Global immune deficiency is a risk factor for anti-COVID-19 vaccine efficacy, particularly in the elderly who have been exposed to a myriad of factors that participate in the weakening of the immune system. These factors include weakness of antigen recognition, decreased immune cell quantity and functionality, increased level/length, and timing of humoral immune modifications of components, decreased initiation of cellular reactions, and memory cell disorders. Other factors associated with immunodeficiency include age-dependent humoral and immune cell changes, immunosenescence, malnutrition, [75]; protein energy micronutrient deficiency, and telomere shortening [76]. In addition, past or current treatments impact the scalable ineffectiveness of vaccines in both older adults [77] and children [78], especially in the immunocompromised [79]. Immunosuppressive drug use is a risk factor for anti-SARS-CoV-2 vaccine ineffectiveness [80] due to higher levels of IL-6 and decreased IgG concentrations [81]. Parasitic infections and respiratory tract infections, such as sophisticated pneumonia [82], can also affect the subsequent immune response to anti-SARS-CoV-2 vaccination [83]. Adjuvant purity and safety, knowledge gaps about the relative contribution of the innate and adaptive responses to preservation versus individual pathogens, and the accurate mode of action of individual adjuvants are existing negative factors in vaccine efficacy [84]. The safety of the vaccine was initially estimated in laboratory studies with mice or rabbits. If the animals do not display signs of disease after being given the vaccine, then the tests begin in humans, and the number of subjects gradually increases [85]. The period of the clinical trial, on average, for a classical vaccine (after preclinical stage in vitro and in vivo tests) is as follows [86], [87].
In phase I, also called the first human test, the vaccine is administered to a small group of healthy volunteers (10−100) to test if the vaccine is safe or whether it causes severe side effects. In phase II, the candidate vaccine is given to a larger group of subjects (100−1000) and in phase III, to an even larger group (1000−100,000). Separate studies may be in demand for adults, children, and the elderly. Continuous surveillance is important in the case of complications that occur with late effect [88]. The ongoing COVID-19 pandemic is a global priority, and several vaccines have already been authorized or agreed for use in humans to combat the pandemic [89], [90]. Vaccines versus the SARS-CoV-2 virus are in the first stage and this by a derogation from the rule, skipped animal studies. At the time of writing, 9 vaccines have been authorized for emergency or full use by at least one WHO-recognized strict regulatory body (BNT162b2, mRNA-1273, ChAdOx1, Ad26. COV2. S, BBIBP-CorV, CoronaVac, COVAXIN, COVOVAX, and NUVAXOVID) as listed in ( Table 1) and presented in ( Fig. 3). The lipid nanoparticle-encapsulated mRNA-based vaccines BNT162b2 and mRNA1273 encode the full-length SARS-CoV-2 spike [91], [92]. Pfizer-BioNTech COVID-19 vaccine is the first approved vaccine by the U.S. Food and Drug Administration [93]. The other vaccine is the ChAdOx1 nCoV-19 vaccine (AZD1222) that comprises the SARS-CoV-2 structural surface glycoprotein antigen (spike protein; nCoV-19) gene in a replication-deficient chimpanzee adenoviral vector ChAdOx1 [94]. The Ad26. COV2. S vaccine is a replication-incompetent human adenovirus type 26 (Ad26)-based vector that encodes full-length SARS-CoV-2 spike protein in a prefusion-stabilized shape [95]. Solforosi et al., [96] found that although both two-dose regimens of Ad26. COV2. S vaccine was more immunogenic than the one-dose regimen, neutralizing antibody titers were greater in nonhuman primates (NHPs) that received the two vaccination doses 8 weeks apart compared to the 4-week interval. This demonstrates that an extra period between vaccination doses can increase the amplitude and/or quality of the antibody response considerably [97]. The vaccine is produced by genetic engineering and attempts to teach the body to recognize the coronavirus protein S, which is delivered via a type-5 adenovirus [98]. Vaccines induce immunity to the virus but can also encourage inflammation in the body.
Table 1.
List of the WHO approved vaccine for emergency use.
| Vaccine type | No. | Scientific name | Manufacturer | Specification | Side effects | Approved | Doses | efficacy | Country | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| mRNA | 1 | BNT162b2 | Pfizer-BionTech | A lipid nanoparticle-formulated, nucleoside-modified mRNA vaccine encoding the prefusion spike glycoprotein of SARS-CoV-2 | Fatigue, headache, muscle pain, chills, myocarditis and pericarditis | 31 December 2020 | 2 doses 21 days apart | 95–87.5% | USA | [99], [100] |
| 2 | mRNA-1273SPIKEVAX | Moderna | Based on mRNA and is a cutting- edge approach that uses genetically engineered RNA to generate a protein that itself safely prompts an immune response. | Pain at the injection site, nausea, vomiting, fever, fatigue, myocarditis and pericarditis | 30 April 2021 | 2 doses 28 days apart | 94.1% | USA | [101] | |
| Viral vector | 3 | ChAdOx1-S or AZD1222 or Vaxzevria | Astra-Zeneca/Oxford | Based on Adenovirus Viral vector vaccines use a virus that has been genetically engineered so that it cannot cause disease but produces coronavirus proteins to safely generate an immune response. | Rare cases of blood clots and Guillain-Barré syndrome [GBS] | 15 February 2021 | 2 doses 4–12 weeks apart | 70% | UK | [99], [102] |
| 4 | Ad.26. COV2. S or JNJ- 78436725 | Janssen (Johnson & Johnson) | Is a recombinant, replication-incompetent adenovirus serotype 26 (Ad26) vector vaccine, encoding the stabilized prefusion spike glycoprotein of virus | Rare case of blood clots, thrombocytopenia and Guillain-Barré syndrome [GBS] | 12 March 2021 | 1 dose | 72–57% | USA | [99], [103] | |
| Inactivated | 5 | BBIBP-CorV Sinopharm | Beijing Institute of Biological Products Co., Ltd. (BIBP) | Inactivated virus (HB02 strain) vaccine that introduces a dead copy of SARS-CoV-2 into the body | Pain at the vaccination site, fatigue, lethargy, headache and tenderness | 7 May 2021 | 2 doses 14–12 days apart | 79% | China | [104] |
| 6 | CoronaVac | Sinovac | Inactivated or weakened virus (CZ02 strain) vaccines are based on a form of the virus that has been inactivated or weakened so that it does not cause disease but is still able to generate an immune response. | Injection site reactions, included fatigue, diarrhea, and muscle pain | 1 June 2021 | 2 doses 14 days apart | 65.9% | China | [105] | |
| 7 | COVAXIN | Bharat Biotech | Whole virion inactivated SARS-CoV-2 vaccine | Pain at the injection site, followed by headache, fever, and fatigue | 3 November 2021 | 2 doses 4 weeks apart | 78% | India | [106], [107], [108] | |
| Recombinant nanoparticle and virus-like particle | 8 | COVOVAX | Serum Institute of India Pvt. Ltd | (SARS-CoV-2 rS Protein Nanoparticle [Recombinant]) | 17 December 2021 | India | [109] | |||
| 9 | NVX-CoV2373 NUVAXOVID | Novavax | SARS-CoV-2 rS [Recombinant, adjuvanted]. Synthetic nanoparticle coated with trimer spike protein with matrix M adjuvant | Injection-site tenderness, fever, headache, muscle pain, and fatigue | 20 December 2021 | 2 doses 21 days apart | 89.7% | Czech Republic | [110] |
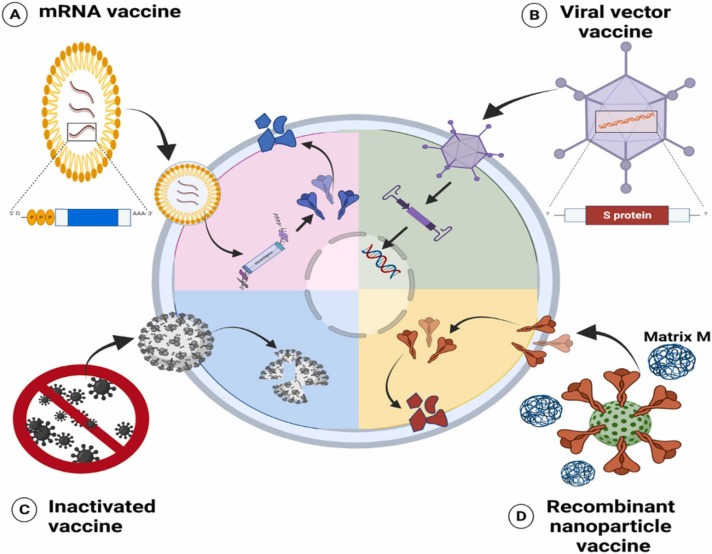
Fig. 3.
Different types of WHO-approved vaccines for emergency use including; (A) mRNA enter cells and act as a translational template for spike protein synthesis, (B) Viral vector vaccine that delivers antigen-encoding DNA to cells and initiates the inflammatory response and immunity, (C) Inactivated virus vaccine which is up taken and broken down by cells and (D) Recombinant nanoparticle and virus-like particle which release viral synthetic spikes is when up taken then processed into multiple epitopes by cells.
8. COVID-19 vaccine development challenges
In the vaccine emergence process, adequate animal models are crucial to testing protective responses. SARS-CoV-2 does not grow in wild-type animals and stimulates mild disease in transgenic animals expressing ACE2 [111]. Several vaccine candidates have passed through intensive clinical testing protocols in a bid to prove their efficaciousness and safety [112]. However, there is an unrealistically high expectation for the COVID-19 vaccine. Many people believe that COVID-19 vaccines are completely safe and effective, even though they have yet to pass several rigorous approval protocols and conditions. If there is no “repentance” from the extremely high expectations for the COVID-19 vaccine, they might end up catalyzing a narrative that challenges the quality and reliability of vaccines in a bigger way [113], [114]. Another striking problem is on the side of vaccine and drug makers. This time, it is not about which vaccine or therapeutic platform they should operate with, but rather about the paths of conventional clinical development, which have proven to be cumbersome and demanding for a long time in addressing the present public health threat. In response to this challenge, several groups and researchers are now retooling the development process and protocols in a bid to find.
9. Current drugs for COVID-19 treatment
The COVID-19 pandemic is forcing scientific organizations to develop novel therapies as soon as possible. Furthermore, the prolonged time required for the synthesis, formulation, and evaluation of new drugs from preclinical to phase III trials is a milestone in pandemic control. Several drugs are being considered for COVID-19 therapy, including those that specifically target the viral replication cycle, viral entry, viral translocation to the nucleus, enhance the innate antiviral immune response, or other targets [115], [116]. The pathogenesis of COVID-19 is assumed to be driven by two primary mechanisms. In the early clinical phase, SARS-CoV-2 replication is the main cause of the illness. In the late clinical phase, tissue damage caused by a dysregulated immune or inflammatory response to SARS-CoV-2 appears to be its main cause. According to this knowledge, it is predicted that treatments that specifically target SARS-CoV-2 would have the most impact early in the course of the illness, whereas immunosuppressive or anti-inflammatory treatments are expected to be more helpful in COVID-19's latter stages [117]. Currently, the antiviral drugs being considered for COVID-19 infection therapies are directed at various stages of viral infection, along with viral entry, translation, proteolysis, viral RNA replication, viral protein assembly, and viral release. As a result, COVID-19 treatment strategies are constantly providing clinicians with relative guidance on how to provide healthcare to COVID-19 patients [118]. For COVID-19 hospitalized patients, there are three principal pharmacological goals as presented in ( Table 2 ); (1) Antiviral drugs that modulate cellular mechanisms and aid in viral replication control, such as molnupiravir, paxlovid, remdesivir. (2) Anti-inflammatory drugs, such as colchicine and methylprednisolone, for controlling inflammation and immune response caused by viral infection; and (3) Adjunct drugs, such as antibiotics, anticoagulants, and vitamins, for reducing the risk of viral complications [119], [120], [121]. However, the National Institutes of Health (NIH) panel recommends against the use of antibacterial therapy in the absence of another indication of bacterial infection in COVID-19 patients [117].
Table 2.
Common antiviral and anti-inflammatory drugs used in COVID-19 treatment and their anti-Omicron variant activity.
| Drug | Structure | Approval | Main action | Adverse effects | specifications | Administration | Activity against Omicron | Ref. |
|---|---|---|---|---|---|---|---|---|
| Paxlovid (Ritonavir-boosted nirmatrelvir) | C23H32F3N5O4 | The highest recommended drug by NIH in non-hospitalized COVID-19. FDA EUA for mild, moderate, and high-risk COVID-19 outpatients | 3 C-like (3CL) protease inhibitor that inhibit the enzymes for protein synthesis | Dysgeusia, diarrhea, increased blood pressure, myalgia, and it is a cytochrome P450 3A inhibitor, raising the concern for significant drug-drug interactions. | Antiviral | Oral | Active against all Omicron subvariants and conformed in preclinical trials | [122], [123], [124] |
| Remdesivir | C27H35N6O8P | FDA approval or the treatment of hospitalized COVID-19 patients | RdRp inhibitor. Prevents viral transcription and replication | Nausea, fever, chills, or shivering, swelling in face, pounding in neck or ears, and headache | Antiviral | Intravenous injection | Active against Omicron VOCs | [124], [125], [126] |
| Molnupiravir | C13H19N3O7 | FDA EUA for mild, moderate, and high-risk COVID-19 outpatients | RdRp inhibitor with mutagenic effect on viral genome | Diarrhea, dizziness, headache, hives, itching, skin rash, nausea, redness of the skin, vomiting, possible risk of genotoxicity, and fetal harm | Antiviral | Oral | Active against all Omicron subvariants and conformed in preclinical trials | [124], [127], [128] |
| Bamlanivimab plus etesevimab | FDA EUA approval for treatment of COVID-19 patients | Neutralizing mAbs bind to spike protein of SARS-CoV-2 | Drug-drug interaction, Hypersensitivity, anaphylaxis, diarrhea, nausea, vomiting, and dizziness | Monoclonal antibody | Intravenous injection | Inactive | [117], [129] | |
| Casirivimab plus imdevimab | FDA EUA approval for treatment of COVID-19 patients | Recombinant human mAbs bind to SARS-CoV-2 spike protein | Drug-drug interaction, Hypersensitivity, anaphylaxis, diarrhea, nausea, vomiting, and dizziness | Monoclonal antibody | Intravenous injection | Inactive | [117], [130] | |
| Sotrovimab | FDA EUA approval for treatment of COVID-19 patients | mAbs bind to conserved spike protein between SARS-CoV and SARS-CoV-2 | Drug-drug interaction, Hypersensitivity, anaphylaxis, diarrhea, nausea, vomiting, and dizziness | Monoclonal antibody | Intravenous injection | Active against Omicron BA.1 and BA.1.1 subvariants but not BA.2 | [117], [131] | |
| Bebtelovimab | FDA EUA approval for treatment of COVID-19 patients | recombinant neutralizing human mAb binding to SARS-CoV-2 spike protein | Drug-drug interaction, Hypersensitivity, anaphylaxis, diarrhea, nausea, vomiting, and dizziness | Monoclonal antibody | Intravenous injection | active in vitro against all circulating Omicron subvariants | [117], [132], [133], [134] | |
| Tixagevimab plus cilgavimab | FDA EUA approval for treatment of COVID-19 patients | Recombinant human anti-SARS-CoV-2 mAbs bind to virus spike protein | Drug-drug interaction, Hypersensitivity, anaphylaxis, diarrhea, nausea, vomiting, and dizziness | Monoclonal antibody | Intravenous injection | active in vitro against Omicron subvariants with high dose | [117], [132] | |
| Systemic glucocorticoids(Dexamethasone) | C22H29FO5 | Used in hospitalized patients with COVID-19 who required supplemental oxygen | hyperglycemia, secondary infections, avascular necrosis and psychiatric effects | Anti-inflammatory | Intravenous injection | NA | [117], [135] | |
| Anti-interleukin-6 receptor monoclonal antibodies (Sarilumab and tocilizumab) | FDA approved for treatment of rheumatic disorder | anti-IL-6 receptor mAb | Neutropenia, thrombocytopenia, increasing liver enzymes, HBV reactivation, and secondary infections | Anti-inflammatory | NA | [117], [136], [137] |
FDA; Food and drug administration, EUA; emergency use authorization, RdRp; RNA dependent RNA polymerase, mAb; monoclonal antibody, IL-6; interleukin 6, and NA; not applicable.
9.1. Antiviral drugs
The absence of relevant therapy for SARS-CoV-2 infection, in addition to the genetic homology with previous coronaviruses, encourages the application of already existing antiviral agents that have proved active in previous infections. Remdesivir is the only FDA approved drug for the treatment of COVID-19 patients and among the most promising candidate. Remdesivir is a phosphoramidite prodrug of adenosine C-nucleoside with broad antiviral activity that was initially used to treat Ebola virus infection. Remdesivir was administered intravenously at a dose of 200 mg on day one and 100 mg on days two through 10 subsequently [126]. Remdesivir inhibits viral replication by prematurely terminating RNA transcription through binding to the viral RNA-dependent RNA polymerase [125]. The difference in response to remdesivir is most likely owing to insufficient tissue levels, rather than resistance [138]. Remdesivir in combination with the immunomodulatory drug tocilizumab is suggested for COVID-19 treatment in solid-organ transplant patients as part of combination therapy. [139]. Remdesivir has a role in early mild to moderate COVID-19 disease and a limited role in hospitalized high-flow patients [140].
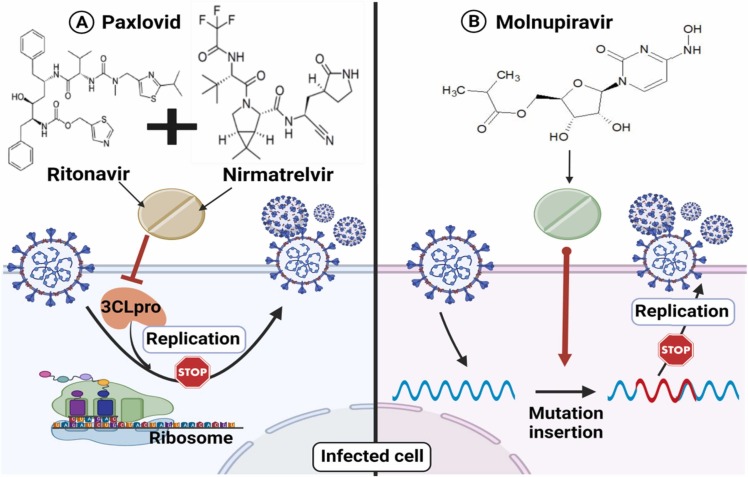
Currently, two novel antiviral drugs have been approved by the FDA for oral administration in COVID-19 patients and are marketed under the names of paxlovid and molnupiravir. Paxlovid is the first FDA-approved oral drug for the treatment of COVID-19 cases under the emergency use authorization (EUA) [141]. On November 5, 2021, Pfizer, based in New York City, officially confirmed that its antiviral drug paxlovid lowered hospitalizations by 89% [122], [123]. Paxlovid is a therapeutic hybrid of different compounds such as nirmatrelvir (PF-07321332) and ritonavir that helps to protect liver enzymes from breaking down the antiviral before it has an opportunity to disable the coronavirus [142]. Paxlovid is a 3CL protease inhibitor that works by blocking an enzyme required for viral protein synthesis [122]. The antiviral mechanisms of action of molnupiravir and paxlovid were illustrated in ( Fig. 4 ). Molnupiravir is the second FDA-approved oral drug under the EUA for the treatment of COVID-19 patients [143]. On November 4, 2021, the UK's medicines regulator issued a temporary authorization for the antiviral drug molnupiravir, developed by Merck, for the treatment of mild to moderate COVID-19 in adults with at least one risk factor for acute symptoms [144]. It was announced that early treatment with molnupiravir minimizes the chances of hospitalization or death by 50% [145]. Molnupiravir functions by inserting mutations into the viral genome during replication, potentially causing so many errors that the virus cannot survive [146].
Fig. 4.
Antiviral mechanism of action of the two FDA-approved oral drugs under the emergency use authorization (EUA) for the treatment of COVID-19 patients (A) Paxlovid which inhibits the 3CLpro and (B) Molnupiravir which inserts mutations into the viral genome during replication.
Several medications with antiviral and/or immunomodulatory activities in vitro have also been tested for SARS-CoV-2 infection, including approved antimalarial (chloroquine) and anti-protozoan drugs (emetine and ivermectin) [147]. However, the Infectious Diseases Society of America (IDSA) and NIH panels made a strong recommendation against the use of chloroquine alone or in combination with azithromycin because of its high toxicity with uncertain benefit [117], [140]. Emetine is a protein synthesis inhibitor that has been approved as an anti-protozoan treatment for amoebiasis; it also inhibits malaria by binding to the ribosomal E site of Plasmodium falciparum [148]. Emetine inhibits a wide range of DNA and RNA viruses, including Zika virus, Ebolavirus, Cytomegalovirus, Rabies virus, HIV-1, echovirus 1, buffalo poxvirus, bovine herpesvirus 1, and influenza [149]. Emetine an isoquinoline alkaloid, has shown potent in vitro activity against the coronavirus (SARS-CoV-2). It can prevent viruses from entering cells by inhibiting viral replication enzymes and intracellular transport, as well as preventing viral protein translation [150]. Ivermectin is a low-cost, FDA-approved anti-parasitic drug that has broad-spectrum in vitro antiviral activity against several viruses [151]. It inhibits the proliferation of COVID-19 in cell culture and acts by inhibiting nuclear import (IMPα/β1) of virus and host proteins. Ivermectin may also act on the SARS-CoV-2 virus via three proposed mechanisms, including inhibiting host nuclear import, interfering with spike attachment to the human cell membrane, and inhibiting helicase activity [152], [153]. However, the clinical outcomes for most already existing antimalarial and anti-protozoal drugs come with insignificant results or/and severe undesired side effects, which encourages many health organizations to recommend against their implementation in the for the treatment of COVID-19 in hospitalized and non-hospitalized patients COVID-19 regimen [117], [154], [155].
9.2. Immunomodulatory and anti-inflammatory agents in COVID-19 treatment
The hyperactivation of the immune system response is a hallmark of COVID-19 infection. In an effort to fight against viral infection, the immune system unleashes a huge amount of inflammatory factors and cytokine interleukins, which are collectively known as a “cytokine storm”. This cytokine storm leads to many adverse complications in the COVID-19 patients, including dysfunction of air exchange, organ damage, pneumonia, cardiac injury, thromboembolism, and edema, which in severe conditions could lead to patient death [156]. Based on the complications of viral infection, alleviating the immune system’s hyper response through immunomodulatory agents could have potential treatment that reduces the risk of hospitalization and mortality [157]. Several studies were dedicated to evaluating the clinical outcomes of immunomodulatory and anti-inflammatory agents such as interleukin inhibitors, interferons, corticosteroids, and lactoferrin in COVID-19 patients.
9.2.1. Interferon
In addition to the antiviral activities of interferon against several viral infections, its immunomodulatory effects are widely reported and accepted [158], [159]. Interferon is a natural protein produced as a part of the immune system response to tolerate viral infections. Interferon production imbalance and suppression during COVID-19 infection were highlighted in many studies [160], and hence its administration was hypothesized to alleviate or reduce the COVID-19 manifestations of infection. Interferon proteins were classified into three classes: alpha, beta, and gamma interferon. Three main classes were recognized in interferon proteins alpha, beta and gamma interferon. Collectively, the three interferon classes were enrolled in the COVID-19 treatment regimen alone or as combined therapy along with standard care. The clinical outcomes are controversial as some of the preclinical studies reported significant results [161], [162], whereas larger and well-controlled clinical studies reported non-significance and even adverse complications attributed to interferon administration [163], [164]. Recently, the NIH strongly recommended against the application of any interferon types in hospitalized and non-hospitalized COVID-19 patients, considering the insignificant clinical results with diverse side effects expected [117].
9.2.2. Corticosteroids
Regarding the anti-inflammatory potent activity of some corticosteroids, they are used to reduce the inflammatory response in many autoimmune diseases. As a result of the severe effects of COVID-19 infection on the immune system, particularly in cytokine dysregulation, corticosteroids, which were previously applied in severe SARS-infected patients, have become a part of the COVID-19 treatment protocols [165], [166]. Two of the potent and widely applied corticosteroid drugs in lung treatment were implemented in the COVID-19 standard treatment protocols, namely methylprednisolone and dexamethasone. Methylprednisolone is a synthetic glucocorticoid used mainly as an anti-inflammatory or immunosuppressive agent [167]. The mechanism of action is via binding to the intracellular glucocorticoid receptor. The methylprednisolone-glucocorticoid receptor complex blocks the promoter sites of pro-inflammatory genes, resulting in the inhibition of inflammatory cytokine synthesis by inhibiting the function of transcription factors such as nuclear factor-kappa-B (NF-kB) [168], [169], [170].
The efficacy of methylprednisolone and dexamethasone was compared by Ranjbar and his colleagues to 86 hospitalized COVID-19 hypoxic patients allocated into three groups; one treated with methylprednisolone (2 mg/kg/day) and the second with dexamethasone (6 mg/day) with the control group. The results showed no significant difference between the two groups subjected to intravenous corticosteroid treatment, with some potency of methylprednisolone over dexamethasone. However, significant differences were reported compared to the control group, where the corticosteroid treated groups revealed better clinical status after ten days of corticosteroid administration (P = 0.001) with a significant reduction in ventilation dependence (about 20%, P = 0.040) and hospitalization time (about 3 days, P = 0.015). The outcome results of corticosteroid application in the COVID-19 treatment regimen are inconclusive. In a study conducted in 2014, hospitalized COVID-19 patients receiving dexamethasone (6 mg/daily) intravenously for ten days in addition to the standard treatment protocol, the 28-day mortality rate was significantly decreased compared to 4321 patients receiving usual care (control groups). The administration of dexamethasone in patients with mild COVID-19 manifestations resulted in harmful effects [171]. Lie et al., reported not only the infectiveness of corticosteroids in declining the fever duration, hospitalization period, or the viral clearance time in the mild COVID-19 pneumonia corticosteroid-treated group (55 out of 475 patients) but their adverse effects in expanding the infection complications [172]. In their study, Muflihah et al., reported an increase in the mortality rate (six times) in patients subjected to immunomodulatory drugs (methylprednisolone and dexamethasone with tocilizumab) in their treatment regime compared to the untreated group [173]. On the other side, other studies related the delay in viral clearance upon corticosteroid administration in moderate-severe COVID-19 pneumonia to the age and health conditions of patients with nothing to do with early corticosteroid administration [174]. In conclusion, dexamethasone is recommended by the IDSA and NIH panels for patients with severe COVID-19 who required supplemental oxygen. The guidelines recommend against using glucocorticoids in non-hospitalized COVID-19 individuals who do not have hypoxemia and do not require extra oxygen [117], [140].
9.2.3. Interleukin 6 inhibitors
The FDA-approved interleukin-6 (IL-6) inhibitors including anti-IL-6 receptor monoclonal antibodies (mAbs) such as sarilumab and tocilizumab, and anti-IL-6 mAbs such as siltuximab. Tocilizumab is an immunomodulatory monoclonal antibody that revealed promising clinical outcomes in severe COVID-19 patients. The underlying mechanism for tocilizumab is based upon its ability to block IL-6 receptors, as severe COVID-19 infection is usually associated with a high titer of IL-6 [175]. The role of IL-6 in stimulating endothelial dysfunction and increasing vascular permeability in complicating the severity of COVID-19 infection, and thus tocilizumab, could play a role in alleviating manifestations in severe COVID-19 patients [176]. In a single-center study that included 100 hospitalized patients, tocilizumab intravenous administration proved significant clinical outcomes in the treated group (43 patients) compared to the control group (55 patients) [177]. However, in a phase III study, 438 hospitalized COVID-19 patients (with severe manifestation) were allocated into two groups; the first (294 patients) received a single dose of tocilizumab (8 mg/kg), while the control group (144 patients) received a placebo. The results indicated no significant differences between the tocilizumab group and the placebo in terms of health condition or 28-day mortality rate reduction [178]. The NIH panel suggested that tocilizumab or sarilumab should only be administered in combination with dexamethasone or another corticosteroid in sever hospitalized COVID-19 patients who require supplemental oxygen [117]. The NIH panel recommends against the use of siltuximab for the treatment of COVID-19 patients [117].
9.2.4. Lactoferrin
Lactoferrin is a natural glycoprotein with multi-biological activities including antiviral, antioxidant, and immunomodulatory effects, as presented in ( Fig. 5 ) [179], [180], [181]. Lactoferrin plays a major role in iron transportation and homeostasis through reversible chelating of two Fe (III) atoms per molecule [182]. Lactoferrin is constitutively produced by neutrophils and exocrine glands in most mammals as a part of innate immunity and is generally recognized as safe (GRAS). Several studies elucidated the potency of lactoferrin against many viral infections by retarding the viral entry into the host cells either by blocking the cell receptor or quenching the virus particles [183], [184], [185]. This mechanism has been reported in many coronaviruses by blocking the HSPGs surface protein in vitro, which is a key co-receptor for COVID-19 entry [186]. In addition, lactoferrin also revealed significant immunomodulatory and anti-inflammatory characteristics [187], which may be the reason behind its inclusion in some COVID-19 treatment regimens. The anti-inflammatory mechanism of lactoferrin is based on its ability to regulate pro-inflammatory gene expression within the nucleus of the host cells [188]. Salaris et al., investigated the in vitro effects of lactoferrin in COVID-19 infected Caco-2 cells. The study reported significant induction in many antiviral cytokine genes, including IFNA1, IFNB1, TLR3, TLR7, IRF3, IRF7, and MAVS genes, with partial inhibition of COVID-19 infection and replication [189]. In another study, Campione and his colleagues proved through in silico study the direct interaction between lactoferrin and the spike surface protein of the COVID-19 virus, which hinders the viral invasion into target cells. The in vitro study stressed the significance of lactoferrin concentration, applied cell lines, and infection multiplicity in the interaction efficacy [190]. The lactoferrin potency against COVID-19 infection was confirmed in a preclinical study that enrolled 92 patients with mild-moderate symptoms, divided into three groups: 32 patients received bovine lactoferrin (oral and intranasal administration), 32 hospitalized patients receiving standard care, and 28 patients served as control (home-based isolated). The results indicated a significant recovery in the lactoferrin-treated group compared to the standard care group, with a remarkable reduction in inflammatory and coagulant markers (D-dimers, IL-6, and serum ferritin) attributed to lactoferrin administration [191].
Fig. 5.
Lactoferrin mechanism of action as antiviral and immunomodulatory by (1) Induction of antiviral cytokines, (2) Binding to viral spike proteins, (3) Binding to Heparan sulfate proteoglycans (HSPGs) receptor and preventing viral entry into the host cell, and (4) Inhibiting viral polymerase and prevent viral replication.
9.3. Adjunctive therapy in COVID-19 infection
9.3.1. Anticoagulants
In addition to severe pneumonia resulting from severe COVID-19 infection, thromboembolism is recognized as the second mortality risk in hospitalized patients [192]. Many studies have reported an increase in the incidence of the coagulation parameters (D-dimer, fibrinogen, and prolonged thrombin time) upon viral infection [193], [194]. The mechanism behind thromboembolism induction in the COVID-19 infection is still unknown. However, it is hypothesized that the severe inflammatory response generated through viral infection could lead to endothelial dysfunction [195]. The COVID-19 infection is usually combined with a high titer of cytokines, especially IL-6, which is characterized by hyper-coagulation induction [196]. A prophylactic anticoagulant included in the treatment protocol was suggested, elucidating a decrease in the mortality rate and good clinical outcomes. Heparin, a potent and most applied anticoagulant, represents the first choice in COVID-19 patients. In addition to its anticoagulant activity, heparin reveals anti-inflammatory and potential antiviral activities [197]. The heparin application in COVID-19 patients revealed significant clinical outcomes with no adverse consequences, as highlighted in many studies [198], [199]. In a cohort study of 4297 hospitalized COVID-19 patients in the USA, the effects of prophylactic inhalation of anticoagulants after 24 h of hospitalization were revealed. The results revealed a decrease in the mortality rate (30-day mortality) by 27% in the anticoagulant inhalator group (84%), with no risk of bleeding [200]. The results are in accordance with other larger studies conducted on 7824 hospitalized COVID-19 patients who received antiplatelet therapy. The clinical outcome revealed a decline in the mortality risk and mechanical ventilation period in the antiplatelet receiving groups compared to the control group [201]. Despite widespread agreement on the use of anticoagulants in treatment regimens, the dosage, timing, and duration remain variable and empirically determined. However, the NIH panel in the treatment guideline advises against using antiplatelet medication to stop COVID-19 progression or mortality in individuals who are not severely ill. There is insufficient data for the NIH panel to recommend for or against anticoagulants in critically ill COVID-19 patients who are hospitalized or discharged from the hospital [117].
9.3.2. Mineral and vitamin supplements (zinc, vitamin C, and vitamin D)
With no effective-approved treatment protocol, enhancing the immune system represents an ideal target for both COVID-19 prophylaxes and curing [202]. Zinc and vitamin C are common supplements in viral-influenza treatment that enhance the efficiency of the immune system against the infection. Ascorbic acid (vitamin C) is a well-known antioxidant, whereas zinc has potential immunomodulatory and antibody-induction effects [203]. In addition, the reported in vitro antiviral activity of zinc in many viral infections by inhibiting the RNA-dependent RNA polymerase pointed to the importance of zinc supplementation in a dose of 10–20 mg/daily for better clinical outcomes [204], [205]. The enhancement of lactoferrin antiviral activity in a high concentration of zinc also supported their combined applications for better clinical outcomes in COVID-19 patients [206]. In the same regard, the physiological role of vitamin D in providing balanced health conditions and improving the immune system is well known, as its deficiency is usually associated with the development of respiratory infections [207], [208]. In addition, vitamin D has indirect antimicrobial activity through activating the monocytes’ differentiation into macrophages, which enhances the microbial chemotaxis process [209]. The association between vitamin D deficiencies and the severity of COVID-19 manifestation and higher mortality risk as declared in a retrospective study emphasizes the need for vitamin D supplementation during infection [210]. The proposed prophylactic dose of vitamin D is between 400 and 1200 IU/day for 3–6 months, which is hypothesized to improve the outcomes of the COVID-19 treatment regimen [211]. However, the results of mineral and vitamin supplementation in the COVID-19 regimen are inconclusive, with rising questions about the association between vitamin deficiency (especially vitamin D) and the severity of the COVID-19 infection [212], [213]. Thomas and his colleagues reported the insignificant effects of zinc or/and ascorbic acid oral supplementation on the health conditions of COVID-19 hospitalized patients, even in high concentrations up to 8000 and 50 mg/day of ascorbic acid and zinc gluconate, respectively [214]. In the same regard, a recent review asserted the inconclusive results of vitamins and mineral supplementation (vitamin A, E, C, and D with zinc) during COVID-19 infection. However, the authors encourage their administration for a short time, especially with the risk of their deficiency [215]. To date, there is insufficient data for the NIH panel to recommend either with or against the use of vitamins and zinc for the treatment of COVID-19 [117].
9.4. Convalescent plasma
The high titer of antibodies in convalescent patient plasma reported in many viral infections represents a promising tool for preventing viral replication with a high potential for complete viral clearance [216]. As a result, convalescent plasma from COVID-19 patients was proposed to be effective in alleviating severe manifestations in individuals with severe complications [217]. Several investigations in a large cohort of COVID-19 hospitalized patients indicated a reduction in mortality and the need for mechanical breathing after receiving convalescent plasma. According to recent findings from a follow-up study on 35,322 transfused COVID-19 patients, patients who received high IgG plasma (> 18.45 signal-to-cutoff ratio (S/Co)) had lower mortality (8.9%) than those who received medium (4.6218.45 S/Co) or low IgG plasma. On the other hand, the availability, safety, and efficacy of the convalescent plasma application in COVID-19 patients are still challenging. Potent convalescent plasma should have a high titer of COVID-19 specific antibody (or neutralizing antibody titer NAT) which peaked 12 weeks after infections [218]. In addition, the health conditions, age, weight, agreement, and percentages of the recovered donors limited the availability of the convalescent plasms for wide application. Plasma-transfusion considered as safe treatment option and no sever adverse effects were reported [219]. One percent of patients having plasma transfusions may experience febrile and minor allergic reactions in the form of urticaria, which are treated with supportive treatment. Jaundice, phlebitis, and briefly elevated body temperature are the other frequent side effects. Administration of non-matched ABO blood can result in significant consequences, such as anaphylaxis. With the use of the current screening tools, the risk of blood pathogen transmission due to transfusion is extremely low [220]. However, in one patient receiving convalescent plasma, a study described a very tiny side effect of an evanescent face red spot, but there were no other negative side effects [221]. Unfortunately, some reported results of convalescent plasma application were underwhelming. The clinical outcomes of a randomized controlled trial on 921 hospitalized COVID-19 patients allocated 2:1 and receiving 500 ml of convalescent plasma revealed no reduction in the hospitalization period or 30-day mortality risk, and even this group revealed a high risk for serious complications compared to the control group receiving standard care only [222]. The analysis of clinical outcomes of 33 trials for COVID-19 patients (a total of 16477 patients) showed that 51.6% of patients receiving convalescent plasma and 48.4% were in the control group. The results indicate a non-significant association between the application of convalescent plasma and a reduction in mortality rate [223]. The World Health Organization (WHO) guidelines issued in December 2021 advised against it, particularly in non-severe patients due to insignificant clinical outcomes [224] and restricted its application to emergencies [225]. IDSA panel suggested that COVID-19 convalescent plasma may be more beneficial if administered at high titers at the early onset of hospitalization, in patients with no or low titers of antibodies against SARS-CoV-2, or in patients with humoral immune impairment [140], [226], [227] Concerning the use of convalescent plasma with SARS-CoV-2 VOCs, the NIH panel recommends against the use of convalescent plasma that was collected before the emergence of the Omicron variant [117].
9.5. Monoclonal antibody (mAb)
To overcome the availability issues and potential transfusion risks reported from convalescent plasma application, intensive research was directed towards monoclonal antibody application in COVID-19 treatment [228]. Based on the clinical outcomes of monoclonal antibody applications in previous viral infections like SARS, MERS, and Ebola, the same strategy could represent a step toward reducing the severity and spread of the pandemic [229], [230]. In addition to their usage as medicines, monoclonal antibodies are now being used in SARS-CoV-2 detection assays, such as antigen and immunoglobulin testing [231]. Monoclonal antibodies with specific anti-COVID-19 can be developed from convalescent plasma of cured COVID-19 patients. FDA has granted EUA for five anti-SARS-CoV-2 mAb products including bamlanivimab plus etesevimab, casirivimab plus imdevimab, sotrovimab, bebtelovimab, and tixagevimab plus cilgavimab. The monoclonal antibody neutralizes the virus through interacting with the viral S-protein responsible for recognition of human-ACE2 (cell receptor), hence the viral entry into the host cell is inhibited. Bamlanivimab and etesevimab are two monoclonal antibodies developed by Lilly (USA) with potent antiviral activity [129]. In a study for bamlanivimab efficacy based upon interim analysis for a phase II trial (which included 452 outpatients with moderate symptoms), the bamlanivimab intravenous administration within 3 days of a first positive test (single dose of 2800-mg) revealed a reduction in viral load, severity progress, and needs for hospitalization compared to the placebo group [178].
The concerns about viral resistance spreading among COVID-19 variants forced the application of a monoclonal antibody cocktail for effective results [232]. The REGN-COV2 is a mix of two monoclonal antibodies (casirivimab and imdevimab) developed by the Regeneron company (USA). The antibody cocktail included one antibody from humanized VI mice and the other derived from the conversant plasma of COVID-19 patients in a ratio of 1:1. The REGN-COV2 cocktail inhibits the viral attaching to the host cells by neutralizing the viral particles in two non-overlapping sites, hence revealing enhanced activity with less resistance induction potency [233]. Weinreich et al., studied the efficacy of the REGN-COV2 cocktail in 275 outpatients allocated to three groups (1:1:1), one for placebo and the other two receiving 2.4 or 8.0 mg of REGN-COV2 cocktail. The interim analysis result declared a significant reduction in the viral load in the REGN-COV2 cocktail groups (compared to the placebo group), especially in the early stages of the infection [130]. The potency of the REGN-COV2 cocktail to reduce the hospitalization rate was also confirmed in a retrospective study enrolling 696 patients (revealing mild-moderate symptoms) who received the REGN-COV2 cocktail with 696 patients as control. The study revealed a significant reduction in hospitalization rate in the treated group compared to the control [234]. To date, the efficacy of the monoclonal antibody has been asserted only in the early stages of infection in patients with mild to moderate symptoms, whereas in severe COVID-19 infection, treatment outcomes are usually insignificant or may have adverse consequences [235]. From another perspective, the prolonged time for monoclonal antibody production at a commercial level and the high cost involved, in addition to its administration nature, represent challenges for widespread applications [236]. Unfortunately, the first two mAbs are inactive against the Omicron variants and thus the NIH panel strongly recommended against their use [117]. Sotrovimab is a mAb binds to conserved spike protein between SARS-CoV and SARS-CoV-2. Sotrovimab is active against Omicron BA.1 and BA.1.1 subvariants but not BA.2 for that the NIH panel recommend against its use [117]. Tixagevimab plus cilgavimab are recombinant human anti-SARS-CoV-2 mAbs in the original dose they are inactive against Omicron variants. The FDA update the dose of Tixagevimab plus cilgavimab and it has retained in vitro activity against the Omicron BA.2 subvariant [132]. Bebtelovimab is a recombinant neutralizing human mAb binding to SARS-CoV-2 spike protein [134]. Bebtelovimab is the only recommended mAbs by the NIH panel when paxlovid and remdesivir are not available, feasible to use, or clinically appropriate. Bebtelovimab is active in vitro against all circulating Omicron subvariants [117].
10. SARS-CoV-2 variants and their response to treatment and vaccination
SARS-CoV-2 is continually mutated, rendering vaccines less powerful, probably because certain variations evade the immune response. SARS-CoV-2's pathogenesis is altered by several processes, including recombination, single point mutation, insertion, and deletion, leading to a variety of variations [237]. The spike protein and receptor binding domains are both prone to significant mutations, resulting in many SARS-CoV-2 variants [238]. Present vaccines are designed for the first SARS-CoV-2 variant that appeared; it is unclear if the old version of spike glycoprotein can protect against subsequent SARS-CoV-2 variants. The spike glycoprotein and other proteins in the Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.617.2), and Omicron (B.1.1.529) variants of concern (VOCs) differ significantly from the initial SARS-CoV-2 variant from Wuhan [237], [239]. A study found that while some SARS-CoV-2 variants may partially evade the humoral immune response generated by the Pfizer vaccine or SARS-CoV-2 infection, they cannot evade specific CD4 +T cell responses, such as Alpha and Beta. To achieve a high level of neutralizing antibody production and a cell-mediated immune response, two doses of the BTN162b2 vaccination were required [240]. Another study revealed that successive vaccination with two inactivated vaccine doses followed by a third booster dose of receptor-binding domain (RBD) vaccine significantly produced neutralizing antibodies against Alpha, Iota, Kappa, Beta, and Delta SARS-CoV-2 variants of interest (VOIs) [241].
An in vitro study found that the Omicron spike appears to promote higher effective cell entry, leading to improved infectivity. Whereas the Beta variation is resistant to humoral immunity induced by vaccination, the infectivity of Beta strain was weaker than the wild variant, which could explain why Beta's epidemic spread was slower [242]. The Delta strain has a 2-fold increase in pseudo-virus entrance efficiency compared to the wild type, indicating more effective spike binding, which may have been related to its rapid spread [243]. Omicron is capable of escaping the immunity induced by vaccination series with mRNA-1273, BNT162b2, or Ad26. COV2. S, increasing transmission possibilities. A third dosage of the COVID-19 mRNA vaccine-induced humoral immunity is sufficient to neutralize this variant [244]. The genome of Omicron has over 50 mutations, with about 36 on the spike protein that binds to target cell receptors. This is significant since antibodies neutralizing the virus caused by vaccination or normal infection mainly target the S protein. These might be serious mutations that affect vaccination reactions, treatment outcomes, and infectivity [239]. A significant modification in the Omicron's pathogenesis was suggested, which has shifted entrance pathways, preferring endosomal fusion over TMPRSS2. This change in cell entry, causing a reduction in cell fusion in infected cells, is expected to diminish cell-to-cell transmission in other variations [245]. Additional modifications appear to be the same as those identified in other strains linked to increased transmission. Early investigations suggest that the SARS-CoV-2 Omicron variant has a significant transmissibility and milder severity of symptoms than the Delta variant and has shown partial vaccine evasion. [246]. In an in vitro antiviral study, they found that remdesivir, molnupiravir, and nirmatrelvir are active against SARS-CoV-2 VOCs, including Omicron [247]. Regarding the clinical effectiveness of remdesivir against the novel SARS-CoV-2 mutations, there is no available information [248]. Molnupiravir and nirmatrelvir alone and in combination have synergistic antiviral effects, where the SARS-CoV-2 Delta and Omicron variant infections were potently suppressed by them [249], [250]. Bamlanivimab/etesevimab and casirivimab/imdevimab, the first two recognized monoclonal antibody combinations, were mostly ineffective against the Omicron variants. The significance of generating MAbs that target conserved sections of spike that are not targeted by the antibodies generated in infected individuals is further highlighted by the notable loss of activity of several MAbs against the Omicron variants [251]. The NIH panel recommends against the use of these MAbs in Omicron and its subvariants [117]. The activities of different SARS-CoV-2 medications against Omicron variants are presented in ( Table 2 ).
11. Current promising trials for COVID-19 treatment and prophylaxis
Currently, with the absence of authorized effective treatment for the SARA-CoV-2 virus, many trials are ongoing for already existing pharmaceutical drugs as a potential treatment for COVID-19 infection and complications. The rationale behind the ongoing trials is derived from the cumulative information from previous coronavirus infections (SARS and MERS) and the pharmacokinetics of the tested drugs. Fluvoxamine is a selective serotonin reuptake inhibitor (SSRI) that is approved by the FDA as an antidepressant drug with anti-inflammatory and anticoagulant activities, representing a potential candidate for COVID-19 infection [252]. In a randomized, adaptive platform study including 1497 outpatients, they were allocated to the treatment group (741 patients) receiving 10 mg/twice daily of fluvoxamine for 10 days, and a placebo group (756 patients). The results indicated a reduction in the hospitalization requirement for the fluvoxamine group compared to placebo. In addition to the availability of fluvoxamine, the 10 days treatment cost was found to be around US $4, which elucidates its applicability for worldwide applications [253]. With an uncertain mechanism for the potency in COVID-19 patients, some hypotheses may be considered, including the anti-inflammatory activity through indirect regulation of cytokine production [254]. In addition, the antiplatelet activity of fluvoxamine (due to serotonin quenching) decreased the risk of thromboembolism in highly recognized severe COVID-19 infections [255]. Another potential mechanism was also reported, which stated the role of a low dose of fluvoxamine (80 nM) in enhancing and up-regulating non-specific endocytosis for COVID-19 spike proteins by the HEK293T cell line [256]. However, IDSA panel recommends the use of fluvoxamine only in the context of a clinical trial [140] Additionally, NIH panel neither make a recommendation for or against the use of fluvoxamine in the management of COVID-19 due to a lack of sufficient data [117].
In the same regard, stem cell therapy, especially umbilical cord mesenchymal stem cells (UC-MSCs), has also been proposed as a potential treatment for COVID-19 infection. Based upon its significant anti-inflammatory and immunomodulatory activities, it is proposed to play a role in alleviating the side effects of the cytokine storm that is generated upon severe viral infection. The application of umbilical cord mesenchymal stem cells in COVID-19 infection represents a cost-effective alternative with promising preclinical outcomes [257]. The efficacy of (UC-MSCs) on critically ill COVID-19 patients (40 patients) as adjuvant treatment was studied in a randomized controlled trial. Patients were allocated to two groups, one intravenously infused with 100 ml of UC-MSCs (1 ×106 /kg in) and the second group receiving 100 ml of 0.9% saline solution (control group). The study results indicated a significant enhancement in the survival rate in the test group over control, with a significant reduction in the IL-6 levels in recovered patients. However, there was no change in the hospitalization time and ventilation requirements in the two groups [258].
There are other medications in the phase three clinical trial with a good safety profile and promising activity against COVID-19. AT-527 and S-217622 (ensitrelvir) are drugs used orally as RdRp and 3CL protease inhibitors, respectively [259], [260], [261]. Plitidepsin is an anti-tumor agent showing promising results in preclinical studies against SARS-CoV-2. The antiviral activity of plitidepsin against SARS-CoV-2 is mediated through inhibition of the known host protein eEF1A (eukaryotic translation elongation factor 1 alpha), which could reduce the future virus resistance to antiviral agents [262], [263], [264].
Natural compounds originating from algal, fungal, and plant origins were shown to be capable of regulating the secretion of pro-inflammatory cytokines, interfering with viral development in infected cells, and altering key molecular mechanisms. Such natural chemicals might be used in COVID-19 treatment and/or as dietary supplements for prophylaxis [265]. Chitsike and Duerksen-Hughes demonstrated that treatment techniques incorporating multivalent antibodies, recombinant ACE2, and mini-proteins are effective not only for pre-exposure prophylaxis but also for guarding against SARS-CoV-2 random mutation [266]. Furthermore, using different administration routes such as aerosolization may also be beneficial in reducing viral propagation to the lungs when using nasal sprays, considering that the nasal channel is the most common and first site of infection [267]. MicroRNA (miRNA) plays a key role in a variety of biological processes, including viral infection, as post-transcriptional regulators of gene expression [268]. MiRNAs have recently been discovered to play an important role in virus-host interactions, such as inhibiting the production of proteins involved in the SARS-CoV-2 life cycle, like ACE2 and TMPRSS2 [269]. Another study suggested the use of dietary and non-dietary miRNA to control COVID-19 complications [270].
12. Conclusion and future prospective
Scientists and medical professionals are looking for safe, effective vaccinations and therapies to control the COVID-19 outbreak in both healthy and infected people. To date, no definite vaccines and/or single drugs have been developed to eliminate this disease. Also, due to the high prevalence of COVID-19, several therapies and drugs developed during previous viral infections have been tested for this infection. As is indicated from the present review, many different vaccines and drugs are used in combination as an efficient candidate against COVID-19 infection. Importantly, the application and development of new vaccines to control COVID-19 has changed the understanding of exploring vaccines, besides leading to great revolutionary modifications in the development of vaccines. In addition, understanding the actions of viral enzymes such as RdRp, replicase, transcriptase, and proteases provide insight into recognizing the RNA replication process of coronaviruses, leading to the development and exploration of new competitors and inhibitors for these enzymes, which assist in controlling COVID-19 infection. Many new vaccines and drugs have been developed owing to the role of spike S protein and RBD during infection with SARS-CoV-2 in the induction of protective immunity and humoral immunity via T-cell responses and neutralizing antibodies. Owing to the high morbidity and mortality rates of COVID-19, future studies on SARS-CoV-2 are essential to provide essential information for developing and designing novel vaccines and strategies for treatment and prophylaxis against the newly emerging variants of this virus. Thus, it is crucial to validate the newly developed vaccines using animal models to confirm that they are safe and compatible for human use. Future COVID-19 vaccines should efficiently protect children, young people and the elderly from infection by newly discovered SARS-CoV-2 variants that may cause future pandemics. To explore and determine more future secrets beyond the COVID-19 pandemics, scientists and investigators should study carefully various factors such as their mortality and morbidity rates, in vivo efficacy, studies on lethal-challenge and elderly models, and zoonotic virus infection determination. Finally, discovering the mechanism of COVID-19 infection and improving the immunity of populations will potentially lead to a decrease in their mortality and morbidity rates.
Funding
This research received no external funding.
CRediT authorship contribution statement
Yasser Mohamed: Conceptualization, Formal analysis, Data curation, Collected literature data, wrote, and edited the manuscript. Yousra A. El-Maradny: Conceptualization, Formal analysis, Data curation, Collected literature data, wrote, and edited the manuscript. Ahmed K. Saleh: Conceptualization, Formal analysis, Data curation, Collected literature data, wrote, and edited the manuscript. AbdElAziz A. Nayl: Conceptualization, Formal analysis, Data curation, Collected literature data, wrote, and edited the manuscript. Hamada El-Gendi: Conceptualization, Formal analysis, Data curation, Collected literature data, wrote, and edited the manuscript. Esmail M. El-Fakharany: Conceptualization, Formal analysis, Data curation, Collected literature data, wrote, and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kramer A., Schwebke I., Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis. 2006;6:130. doi: 10.1186/1471-2334-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao S., Lin Q., Ran J., Musa S.S., Yang G., Wang W., Lou Y., Gao D., Yang L., He D., Wang M.H. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: A data-driven analysis in the early phase of the outbreak. Int. J. Infect. Dis. 2020;92:214–217. doi: 10.1016/j.ijid.2020.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020;94 doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Totura A.L., Baric R.S. SARS coronavirus pathogenesis: host innate immune responses and viral antagonism of interferon. Curr. Opin. Virol. 2012;2:264–275. doi: 10.1016/j.coviro.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borges R.C., Hohmann M.S., Borghi S.M. Dendritic cells in COVID-19 immunopathogenesis: insights for a possible role in determining disease outcome. Int Rev. Immunol. 2021;40:108–125. doi: 10.1080/08830185.2020.1844195. [DOI] [PubMed] [Google Scholar]
- 12.Lurie N., Saville M., Hatchett R., Halton J. Developing Covid-19 vaccines at pandemic speed. N. Engl. J. Med. 2020;382:1969–1973. doi: 10.1056/NEJMp2005630. [DOI] [PubMed] [Google Scholar]
- 13.Tscherne A., Schwarz J.H., Rohde C., Kupke A., Kalodimou G., Limpinsel L., Okba N.M.A., Bošnjak B., Sandrock I., Odak I., Halwe S., Sauerhering L., Brosinski K., Liangliang N., Duell E., Jany S., Freudenstein A., Schmidt J., Werner A., Gellhorn Serra M., Klüver M., Guggemos W., Seilmaier M., Wendtner C.-M., Förster R., Haagmans B.L., Becker S., Sutter G., Volz A. Immunogenicity and efficacy of the COVID-19 candidate vector vaccine MVA-SARS-2-S in preclinical vaccination. Proc. Natl. Acad. Sci. 2021;118 doi: 10.1073/pnas.2026207118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richardson P., Griffin I., Tucker C., Smith D., Oechsle O., Phelan A., Stebbing J. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395:e30–e31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coban C., Lee M.S.J., Ishii K.J. Tissue-specific immunopathology during malaria infection. Nat. Rev. Immunol. 2018;18:266. doi: 10.1038/nri.2017.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinman R.M., Mellman I. Immunotherapy: bewitched, bothered, and bewildered no more. Science. 1979;305(2004):197–200. doi: 10.1126/science.1099688. [DOI] [PubMed] [Google Scholar]
- 17.Takeuchi O., Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 18.Iwasaki A., Medzhitov R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015;16:343–353. doi: 10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flajnik M.F., Kasahara M. Origin and evolution of the adaptive immune system: genetic events and selective pressures. Nat. Rev. Genet. 2010;11:47–59. doi: 10.1038/nrg2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwasaki A., Medzhitov R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015;16:343–353. doi: 10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haber J.E. Deciphering the DNA damage response. Cell. 2015;162:1183–1185. doi: 10.1016/j.cell.2015.08.034. [DOI] [PubMed] [Google Scholar]
- 22.Johnston J.A., Ward C.L., Kopito R.R. Aggresomes: a cellular response to misfolded proteins. J. Cell Biol. 1998;143:1883–1898. doi: 10.1083/jcb.143.7.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fortun J., Dunn W.A., Joy S., Li J., Notterpek L. Emerging role for autophagy in the removal of aggresomes in Schwann cells. J. Neurosci. 2003;23:10672–10680. doi: 10.1523/JNEUROSCI.23-33-10672.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holze C., Michaudel C., Mackowiak C., Haas D.A., Benda C., Hubel P., Pennemann F.L., Schnepf D., Wettmarshausen J., Braun M. Oxeiptosis, a ROS-induced caspase-independent apoptosis-like cell-death pathway. Nat. Immunol. 2018;19:130–140. doi: 10.1038/s41590-017-0013-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu X.-H., Zhang D.-W., Zheng X.-L., Tang C.-K. Cholesterol transport system: An integrated cholesterol transport model involved in atherosclerosis. Prog. Lipid Res. 2019;73:65–91. doi: 10.1016/j.plipres.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Halle A., Hornung V., Petzold G.C., Stewart C.R., Monks B.G., Reinheckel T., Fitzgerald K.A., Latz E., Moore K.J., Golenbock D.T. The NALP3 inflammasome is involved in the innate immune response to amyloid-β. Nat. Immunol. 2008;9:857. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duewell P., Kono H., Rayner K.J., Sirois C.M., Vladimer G., Bauernfeind F.G., Abela G.S., Franchi L., Nuñez G., Schnurr M. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chowdhury M.A., Hossain N., Kashem M.A., Shahid M.A., Alam A. Immune response in COVID-19: A review. J. Infect. Public Health. 2020 doi: 10.1016/j.jiph.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaussabel D., Pascual V., Banchereau J. Assessing the human immune system through blood transcriptomics. BMC Biol. 2010;8:84. doi: 10.1186/1741-7007-8-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prompetchara E., Ketloy C., Palaga T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 2020;38:1–9. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- 31.Felsenstein S., Herbert J.A., McNamara P.S., Hedrich C.M. COVID-19: immunology and treatment options. Clin. Immunol. 2020 doi: 10.1016/j.clim.2020.108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Wit E., Van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: Recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016;14:523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu W.J., Zhao M., Liu K., Xu K., Wong G., Tan W., Gao G.F. T-cell immunity of SARS-CoV: Implications for vaccine development against MERS-CoV. Antivir. Res. 2017;137:82–92. doi: 10.1016/j.antiviral.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R., Rawlings S.A., Sutherland A., Premkumar L., Jadi R.S. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020 doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.A. Mostaghimi, M.-J. Antonini, D. Plana, P.D. Anderson, B. Beller, E.W. Boyer, A. Fannin, J. Freake, R. Oakley, M.S. Sinha, Rapid prototyping and clinical testing of a reusable face shield for health care workers responding to the COVID-19 pandemic, MedRxiv, 2020. [DOI] [PMC free article] [PubMed]
- 38.Braun J., Loyal L., Frentsch M., Wendisch D., Georg P., Kurth F., Hippenstiel S., Dingeldey M., Kruse B., Fauchere F. Presence of SARS-CoV-2 reactive T cells in COVID-19 patients and healthy donors. medRxiv. Prepr. Post. Online April. 2020;22 doi: 10.1038/s41586-020-2598-9. [DOI] [PubMed] [Google Scholar]
- 39.N. Le Bert, A.T. Tan, K. Kunasegaran, C.Y.L. Tham, M. Hafezi, A. Chia, M. Chng, M. Lin, N. Tan, M. Linster, Different pattern of pre-existing SARS-COV-2 specific T cell immunity in SARS-recovered and uninfected individuals, BioRxiv, 2020.
- 40.B.J. Meckiff, C. Ramírez-Suástegui, V. Fajardo, S.J. Chee, A. Kusnadi, H. Simon, A. Grifoni, E. Pelosi, D. Weiskopf, A. Sette, Single-cell transcriptomic analysis of SARS-CoV-2 reactive CD4+ T cells, Available at SSRN 3641939, 2020. [DOI] [PMC free article] [PubMed]
- 41.Gorse G.J., Patel G.B., Vitale J.N., O’Connor T.Z. Prevalence of antibodies to four human coronaviruses is lower in nasal secretions than in serum. Clin. Vaccin. Immunol. 2010;17:1875–1880. doi: 10.1128/CVI.00278-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Braun J., Loyal L., Frentsch M., Wendisch D., Georg P., Kurth F., Hippenstiel S., Dingeldey M., Kruse B., Fauchere F. Presence of SARS-CoV-2 reactive T cells in COVID-19 patients and healthy donors. medRxiv. Prepr. Post. Online April. 2020;22 doi: 10.1038/s41586-020-2598-9. [DOI] [PubMed] [Google Scholar]
- 43.Zhao J., Zhao J., Mangalam A.K., Channappanavar R., Fett C., Meyerholz D.K., Agnihothram S., Baric R.S., David C.S., Perlman S. Airway memory CD4+ T cells mediate protective immunity against emerging respiratory coronaviruses. Immunity. 2016;44:1379–1391. doi: 10.1016/j.immuni.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Azkur A.K., Akdis M., Azkur D., Sokolowska M., van de Veen W., Brüggen M.C., O’Mahony L., Gao Y., Nadeau K., Akdis C.A. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy: Eur. J. Allergy Clin. Immunol. 2020;75:1564–1581. doi: 10.1111/all.14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mulchandani R., Lyngdoh T., Kakkar A.K. Deciphering the COVID-19 cytokine storm: Systematic review and meta-analysis. Eur. J. Clin. Investig. 2021;51 doi: 10.1111/eci.13429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schultze J.L., Aschenbrenner A.C. COVID-19 and the human innate immune system. Cell. 2021;184:1671–1692. doi: 10.1016/j.cell.2021.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cooper N.R., Nemerow G.R. The role of antibody and complement in the control of viral infections. J. Invest. Dermatol. 1984;83:121–127. doi: 10.1111/1523-1747.ep12281847. [DOI] [PubMed] [Google Scholar]
- 48.Geginat J., Paroni M., Maglie S., Alfen J.S., Kastirr I., Gruarin P., De Simone M., Pagani M., Abrignani S. Plasticity of human CD4 T cell subsets. Front Immunol. 2014;5:630. doi: 10.3389/fimmu.2014.00630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crotty S. A brief history of T cell help to B cells. Nat. Rev. Immunol. 2015;15:185–189. doi: 10.1038/nri3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bentebibel S.-E., Lopez S., Obermoser G., Schmitt N., Mueller C., Harrod C., Flano E., Mejias A., Albrecht R.A., Blankenship D. Induction of ICOS+ CXCR3+ CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Sci. Transl. Med. 2013;5 doi: 10.1126/scitranslmed.3005191. 176ra32-176ra32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spensieri F., Borgogni E., Zedda L., Bardelli M., Buricchi F., Volpini G., Fragapane E., Tavarini S., Finco O., Rappuoli R. Human circulating influenza-CD4+ ICOS1+ IL-21+ T cells expand after vaccination, exert helper function, and predict antibody responses. Proc. Natl. Acad. Sci. 2013;110:14330–14335. doi: 10.1073/pnas.1311998110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gavillet B.M., Eberhardt C.S., Auderset F., Castellino F., Seubert A., Tregoning J.S., Lambert P.-H., de Gregorio E., Del Giudice G., Siegrist C.-A. MF59 mediates its B cell adjuvanticity by promoting T follicular helper cells and thus germinal center responses in adult and early life. J. Immunol. 2015;194:4836–4845. doi: 10.4049/jimmunol.1402071. [DOI] [PubMed] [Google Scholar]
- 53.Lin Y., Slight S.R., Khader S.A. Semin Immunopathol. Springer; 2010. Th17 cytokines and vaccine-induced immunity; pp. 79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kumar P., Chen K., Kolls J.K. Th17 cell based vaccines in mucosal immunity. Curr. Opin. Immunol. 2013;25:373–380. doi: 10.1016/j.coi.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sakaguchi S., Miyara M., Costantino C.M., Hafler D.A. FOXP3+ regulatory T cells in the human immune system. Nat. Rev. Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 56.Igietseme J.U., Eko F.O., He Q., Black C.M. Antibody regulation of T-cell immunity: implications for vaccine strategies against intracellular pathogens. Expert Rev. Vaccin. 2004;3:23–34. doi: 10.1586/14760584.3.1.23. [DOI] [PubMed] [Google Scholar]
- 57.W.H. Organization, Draft landscape of COVID-19 candidate vaccines—23 April 2020, 2020.
- 58.Chau N.V.V., Thanh Dung N., Yen L.M., Minh N.N.Q., Hung L.M., et al. The natural history and transmission potential of asymptomatic SARS-CoV-2 infection. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.P. Poletti, M. Tirani, D. Cereda, F. Trentini, G. Guzzetta, G. Sabatino, V. Marziano, A. Castrofino, F. Grosso, G. Del Castillo, Probability of symptoms and critical disease after SARS-CoV-2 infection, ArXiv Preprint ArXiv:2006.08471. (2020).
- 60.Long Q.-X., Tang X.-J., Shi Q.-L., Li Q., Deng H.-J., Yuan J., Hu J.-L., Xu W., Zhang Y., Lv F.-J. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 61.Buchholz U.J., Bukreyev A., Yang L., Lamirande E.W., Murphy B.R., Subbarao K., Collins P.L. Contributions of the structural proteins of severe acute respiratory syndrome coronavirus to protective immunity. Proc. Natl. Acad. Sci. 2004;101:9804–9809. doi: 10.1073/pnas.0403492101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ahmed S.F., Quadeer A.A., McKay M.R. Preliminary identification of potential vaccine targets for the COVID-19 Coronavirus (SARS-CoV-2) Based on SARS-CoV Immunological Studies. Viruses. 2020;12 doi: 10.3390/v12030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stephensen C.B., Casebolt D.B., Gangopadhyay N.N. Phylogenetic analysis of a highly conserved region of the polymerase gene from 11 coronaviruses and development of a consensus polymerase chain reaction assay. Virus Res. 1999;60:181–189. doi: 10.1016/S0168-1702(99)00017-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gao Y., Yan L., Huang Y., Liu F., Zhao Y., Cao L., Wang T., Sun Q., Ming Z., Zhang L. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science. 1979;368(2020):779–782. doi: 10.1126/science.abb7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rauch S., Jasny E., Schmidt K.E., Petsch B. New vaccine technologies to combat outbreak situations. Front Immunol. 2018;9:1963. doi: 10.3389/fimmu.2018.01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jeyanathan M., Yao Y., Afkhami S., Smaill F., Xing Z. New tuberculosis vaccine strategies: taking aim at un-natural immunity. Trends Immunol. 2018;39:419–433. doi: 10.1016/j.it.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 67.Afkhami S., Yao Y., Xing Z. Methods and clinical development of adenovirus-vectored vaccines against mucosal pathogens. Mol. Ther. -Methods Clin. Dev. 2016;3:16030. doi: 10.1038/mtm.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sariol A., Perlman S. Lessons for COVID-19 immunity from other coronavirus infections. Immunity. 2020 doi: 10.1016/j.immuni.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.L. Moreno-Fierros, I. García-Silva, S. Rosales-Mendoza, Development of SARS-CoV-2 vaccines: should we focus on mucosal immunity?, 2020. [DOI] [PubMed]
- 70.Belyakov I.M., Ahlers J.D. What role does the route of immunization play in the generation of protective immunity against mucosal pathogens? J. Immunol. 2009;183:6883–6892. doi: 10.4049/jimmunol.0901466. [DOI] [PubMed] [Google Scholar]
- 71.Jeyanathan M., Yao Y., Afkhami S., Smaill F., Xing Z. New tuberculosis vaccine strategies: taking aim at un-natural immunity. Trends Immunol. 2018;39:419–433. doi: 10.1016/j.it.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 72.Yao Y., Jeyanathan M., Haddadi S., Barra N.G., Vaseghi-Shanjani M., Damjanovic D., Lai R., Afkhami S., Chen Y., Dvorkin-Gheva A. Induction of autonomous memory alveolar macrophages requires T cell help and is critical to trained immunity. Cell. 2018;175:1634–1650. doi: 10.1016/j.cell.2018.09.042. [DOI] [PubMed] [Google Scholar]
- 73.Zhao J., Zhao J., Mangalam A.K., Channappanavar R., Fett C., Meyerholz D.K., Agnihothram S., Baric R.S., David C.S., Perlman S. Airway memory CD4+ T cells mediate protective immunity against emerging respiratory coronaviruses. Immunity. 2016;44:1379–1391. doi: 10.1016/j.immuni.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Afkhami S., Yao Y., Xing Z. Methods and clinical development of adenovirus-vectored vaccines against mucosal pathogens. Mol. Ther. -Methods Clin. Dev. 2016;3:16030. doi: 10.1038/mtm.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wouters-Wesseling W., Rozendaal M., Snijder M., Graus Y., Rimmelzwaan G., de Groot L., Bindels J. Effect of a complete nutritional supplement on antibody response to influenza vaccine in elderly people. J. Gerontol. Ser. A: Biol. Sci. Med. Sci. 2002;57:M563–M566. doi: 10.1093/gerona/57.9.m563. [DOI] [PubMed] [Google Scholar]
- 76.Tsoukalas D., Fragkiadaki P., Docea A.O., Alegakis A.K., Sarandi E., Vakonaki E., Salataj E., Kouvidi E., Nikitovic D., Kovatsi L. Association of nutraceutical supplements with longer telomere length. Int J. Mol. Med. 2019;44:218–226. doi: 10.3892/ijmm.2019.4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ventura M.T., Casciaro M., Gangemi S., Buquicchio R. Immunosenescence in aging: between immune cells depletion and cytokines up-regulation, Clinical and Molecular. Allergy. 2017;15:21. doi: 10.1186/s12948-017-0077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Savy M., Edmond K., Fine P.E.M., Hall A., Hennig B.J., Moore S.E., Mulholland K., Schaible U., Prentice A.M. Landscape analysis of interactions between nutrition and vaccine responses in children. J. Nutr. 2009;139:2154S–2218S. doi: 10.3945/jn.109.105312. [DOI] [PubMed] [Google Scholar]
- 79.Arvas A. Vaccination in patients with immunosuppression. Turk. Arch. Pediatr. /Türk Pediatr. Arşivi. 2014;49:181. doi: 10.5152/tpa.2014.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pickering L.K., Baker C.J., Kimberlin D.W., Red Book (2012): Report of the Committee on Infectious Diseases. Am. Acad. Pediatr. 2012 [Google Scholar]
- 81.Eliakim A., Swindt C., Zaldivar F., Casali P., Cooper D.M. Reduced tetanus antibody titers in overweight children. Autoimmunity. 2006;39:137–141. doi: 10.1080/08916930600597326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Călina D., Roșu L., Roșu A.F., Ianoşi G., Ianoşi S., Zlatian O., Mitruț R., Docea A.O., Rogoveanu O., Mitruț P. Etiological diagnosis and pharmacotherapeutic management of parapneumonic pleurisy. Farmacia. 2016;64:946–952. [Google Scholar]
- 83.Skalny A.V., Rink L., Ajsuvakova O.P., Aschner M., Gritsenko V.A., Alekseenko S.I., Svistunov A.A., Petrakis D., Spandidos D.A., Aaseth J. Zinc and respiratory tract infections: Perspectives for COVID-19. Int. J. Mol. Med. 2020;46:17–26. doi: 10.3892/ijmm.2020.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Garçon N., Stern P.L., Cunningham A.L. Elsevier,; 2011. Understanding Modern Vaccines: Perspectives in Vaccinology. [Google Scholar]
- 85.DeStefano F., Bodenstab H.M., Offit P.A. Principal controversies in vaccine safety in the United States. Clin. Infect. Dis. 2019;69:726–731. doi: 10.1093/cid/ciz135. [DOI] [PubMed] [Google Scholar]
- 86.Pronker E.S., Weenen T.C., Commandeur H., Claassen E.H., Osterhaus A.D.M.E. Risk in vaccine research and development quantified. PLoS One. 2013;8 doi: 10.1371/journal.pone.0057755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Goetz K.B., Pfleiderer M., Schneider C.K. First-in-human clinical trials with vaccines—what regulators want. Nat. Biotechnol. 2010;28:910–916. doi: 10.1038/nbt0910-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Guerra Mendoza Y., Garric E., Leach A., Lievens M., Ofori-Anyinam O., Pirçon J.-Y., Stegmann J.-U., Vandoolaeghe P., Otieno L., Otieno W. Safety profile of the RTS, S/AS01 malaria vaccine in infants and children: additional data from a phase III randomized controlled trial in sub-Saharan Africa. Hum. Vaccin Immunother. 2019;15:2386–2398. doi: 10.1080/21645515.2019.1586040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., Bibi S., Briner C., Cicconi P., Collins A.M., Colin-Jones R., Cutland C.L., Darton T.C., Dheda K., Duncan C.J.A., Emary K.R.W., Ewer K.J., Fairlie L., Faust S.N., Feng S., Ferreira D.M., Finn A., Goodman A.L., Green C.M., Green C.A., Heath P.T., Hill C., Hill H., Hirsch I., Hodgson S.H.C., Izu A., Jackson S., Jenkin D., Joe C.C.D., Kerridge S., Koen A., Kwatra G., Lazarus R., Lawrie A.M., Lelliott A., Libri V., Lillie P.J., Mallory R., Mendes A.V.A., Milan E.P., Minassian A.M., McGregor A., Morrison H., Mujadidi Y.F., Nana A., O’Reilly P.J., Padayachee S.D., Pittella A., Plested E., Pollock K.M., Ramasamy M.N., Rhead S., Schwarzbold A.V., Singh N., Smith A., Song R., Snape M.D., Sprinz E., Sutherland R.K., Tarrant R., Thomson E.C., Török M.E., Toshner M., Turner D.P.J., Vekemans J., Villafana T.L., Watson M.E.E., Williams C.J., Douglas A.D., Hill A.V.S., Lambe T., Gilbert S.C., Pollard A.J., Aban M., Abayomi F., Abeyskera K., Aboagye J., Adam M., Adams K., Adamson J., Adelaja Y.A., Adewetan G., Adlou S., Ahmed K., Akhalwaya Y., Akhalwaya S., Alcock A., Ali A., Allen E.R., Allen L., Almeida T.C.D.S.C., Alves M.P.S., Amorim F., Andritsou F., Anslow R., Appleby M., Arbe-Barnes E.H., Ariaans M.P., Arns B., Arruda L., Azi P., Azi L., Babbage G., Bailey C., Baker K.F., Baker M., Baker N., Baker P., Baldwin L., Baleanu I., Bandeira D., Bara A., Barbosa M.A.S., Barker D., Barlow G.D., Barnes E., Barr A.S., Barrett J.R., Barrett J., Bates L., Batten A., Beadon K., Beales E., Beckley R., Belij-Rammerstorfer S., Bell J., Bellamy D., Bellei N., Belton S., Berg A., Bermejo L., Berrie E., Berry L., Berzenyi D., Beveridge A., Bewley K.R., Bexhell H., Bhikha S., Bhorat A.E., Bhorat Z.E., Bijker E., Birch G., Birch S., Bird A., Bird O., Bisnauthsing K., Bittaye M., Blackstone K., Blackwell L., Bletchly H., Blundell C.L., Blundell S.R., Bodalia P., Boettger B.C., Bolam E., Boland E., Bormans D., Borthwick N., Bowring F., Boyd A., Bradley P., Brenner T., Brown P., Brown C., Brown-O’Sullivan C., Bruce S., Brunt E., Buchan R., Budd W., Bulbulia Y.A., Bull M., Burbage J., Burhan H., Burn A., Buttigieg K.R., Byard N., Cabera Puig I., Calderon G., Calvert A., Camara S., Cao M., Cappuccini F., Cardoso J.R., Carr M., Carroll M.W., Carson-Stevens A., de Y., Carvalho M., Carvalho J.A.M., Casey H.R., Cashen P., Castro T., Castro L.C., Cathie K., Cavey A., Cerbino-Neto J., Chadwick J., Chapman D., Charlton S., Chelysheva I., Chester O., Chita S., Cho J.S., Cifuentes L., Clark E., Clark M., Clarke A., Clutterbuck E.A., Collins S.L.K., Conlon C.P., Connarty S., Coombes N., Cooper C., Cooper R., Cornelissen L., Corrah T., Cosgrove C., Cox T., Crocker W.E.M., Crosbie S., Cullen L., Cullen D., Cunha D.R.M.F., Cunningham C., Cuthbertson F.C., Da Guarda S.N.F., da Silva L.P., Damratoski B.E., Danos Z., Dantas M.T.D.C., Darroch P., Datoo M.S., Datta C., Davids M., Davies S.L., Davies H., Davis E., Davis J., Davis J., De Nobrega M.M.D., De Oliveira Kalid L.M., Dearlove D., Demissie T., Desai A., Di Marco S., Di Maso C., Dinelli M.I.S., Dinesh T., Docksey C., Dold C., Dong T., Donnellan F.R., Dos Santos T., dos Santos T.G., Dos Santos E.P., Douglas N., Downing C., Drake J., Drake-Brockman R., Driver K., Drury R., Dunachie S.J., Durham B.S., Dutra L., Easom N.J.W., van Eck S., Edwards M., Edwards N.J., El Muhanna O.M., Elias S.C., Elmore M., English M., Esmail A., Essack Y.M., Farmer E., Farooq M., Farrar M., Farrugia L., Faulkner B., Fedosyuk S., Felle S., Ferreira Da Silva C., Field S., Fisher R., Flaxman A., Fletcher J., Fofie H., Fok H., Ford K.J., Fowler J., Fraiman P.H.A., Francis E., Franco M.M., Frater J., Freire M.S.M., Fry S.H., Fudge S., Furze J., Fuskova M., Galian-Rubio P., Galiza E., Garlant H., Gavrila M., Geddes A., Gibbons K.A., Gilbride C., Gill H., Glynn S., Godwin K., Gokani K., Goldoni U.C., Goncalves M., Gonzalez I.G.S., Goodwin J., Goondiwala A., Gordon-Quayle K., Gorini G., Grab J., Gracie L., Greenland M., Greenwood N., Greffrath J., Groenewald M.M., Grossi L., Gupta G., Hackett M., Hallis B., Hamaluba M., Hamilton E., Hamlyn J., Hammersley D., Hanrath A.T., Hanumunthadu B., Harris S.A., Harris C., Harris T., Harrison T.D., Harrison D., Hart T.C., Hartnell B., Hassan S., Haughney J., Hawkins S., Hay J., Head I., Henry J., Hermosin Herrera M., Hettle D.B., Hill J., Hodges G., Horne E., Hou M.M., Houlihan C., Howe E., Howell N., Humphreys J., Humphries H.E., Hurley K., Huson C., Hyder-Wright A., Hyams C., Ikram S., Ishwarbhai A., Ivan M., Iveson P., Iyer V., Jackson F., De Jager J., Jaumdally S., Jeffers H., Jesudason N., Jones B., Jones K., Jones E., Jones C., Jorge M.R., Jose A., Joshi A., Júnior E.A.M.S., Kadziola J., Kailath R., Kana F., Karampatsas K., Kasanyinga M., Keen J., Kelly E.J., Kelly D.M., Kelly D., Kelly S., Kerr D., de R., Kfouri Á., Khan L., Khozoee B., Kidd S., Killen A., Kinch J., Kinch P., King L.D.W., King T.B., Kingham L., Klenerman P., Knapper F., Knight J.C., Knott D., Koleva S., Lang M., Lang G., Larkworthy C.W., Larwood J.P.J., Law R., Lazarus E.M., Leach A., Lees E.A., Lemm N.M., Lessa A., Leung S., Li Y., Lias A.M., Liatsikos K., Linder A., Lipworth S., Liu S., Liu X., Lloyd A., Lloyd S., Loew L., Lopez Ramon R., Lora L., Lowthorpe V., Luz K., MacDonald J.C., MacGregor G., Madhavan M., Mainwaring D.O., Makambwa E., Makinson R., Malahleha M., Malamatsho R., Mallett G., Mansatta K., Maoko T., Mapetla K., Marchevsky N.G., Marinou S., Marlow E., Marques G.N., Marriott P., Marshall R.P., Marshall J.L., Martins F.J., Masenya M., Masilela M., Masters S.K., Mathew M., Matlebjane H., Matshidiso K., Mazur O., Mazzella A., McCaughan H., McEwan J., McGlashan J., McInroy L., McIntyre Z., McLenaghan D., McRobert N., McSwiggan S., Megson C., Mehdipour S., Meijs W., Mendonça R.N.Á., Mentzer A.J., Mirtorabi N., Mitton C., Mnyakeni S., Moghaddas F., Molapo K., Moloi M., Moore M., Moraes-Pinto M.I., Moran M., Morey E., Morgans R., Morris S., Morris S., Morris H.C., Morselli F., Morshead G., Morter R., Mottal L., Moultrie A., Moya N., Mpelembue M., Msomi S., Mugodi Y., Mukhopadhyay E., Muller J., Munro A., Munro C., Murphy S., Mweu P., Myasaki C.H., Naik G., Naker K., Nastouli E., Nazir A., Ndlovu B., Neffa F., Njenga C., Noal H., Noé A., Novaes G., Nugent F.L., Nunes G., O’Brien K., O’Connor D., Odam M., Oelofse S., Oguti B., Olchawski V., Oldfield N.J., Oliveira M.G., Oliveira C., Oosthuizen A., O’Reilly P., Osborne P., Owen D.R.J., Owen L., Owens D., Owino N., Pacurar M., Paiva B.V.B., Palhares E.M.F., Palmer S., Parkinson S., Parracho H.M.R.T., Parsons K., Patel D., Patel B., Patel F., Patel K., Patrick-Smith M., Payne R.O., Peng Y., Penn E.J., Pennington A., Peralta Alvarez M.P., Perring J., Perry N., Perumal R., Petkar S., Philip T., Phillips D.J., Phillips J., Phohu M.K., Pickup L., Pieterse S., Piper J., Pipini D., Plank M., Du Plessis J., Pollard S., Pooley J., Pooran A., Poulton I., Powers C., Presa F.B., Price D.A., Price V., Primeira M., Proud P.C., Provstgaard-Morys S., Pueschel S., Pulido D., Quaid S., Rabara R., Radford A., Radia K., Rajapaska D., Rajeswaran T., Ramos A.S.F., Ramos Lopez F., Rampling T., Rand J., Ratcliffe H., Rawlinson T., Rea D., Rees B., Reiné J., Resuello-Dauti M., Reyes Pabon E., Ribiero C.M., Ricamara M., Richter A., Ritchie N., Ritchie A.J., Robbins A.J., Roberts H., Robinson R.E., Robinson H., Rocchetti T.T., Rocha B.P., Roche S., Rollier C., Rose L., Ross Russell A.L., Rossouw L., Royal S., Rudiansyah I., Ruiz S., Saich S., Sala C., Sale J., Salman A.M., Salvador N., Salvador S., Sampaio M., Samson A.D., Sanchez-Gonzalez A., Sanders H., Sanders K., Santos E., Santos Guerra M.F.S., Satti I., Saunders J.E., Saunders C., Sayed A., Schim van der Loeff I., Schmid A.B., Schofield E., Screaton G., Seddiqi S., Segireddy R.R., Senger R., Serrano S., Shah R., Shaik I., Sharpe H.E., Sharrocks K., Shaw R., Shea A., Shepherd A., Shepherd J.G., Shiham F., Sidhom E., Silk S.E., da Silva Moraes A.C., Silva-Junior G., Silva-Reyes L., Silveira A.D., Silveira M.B.V., Sinha J., Skelly D.T., Smith D.C., Smith N., Smith H.E., Smith D.J., Smith C.C., Soares A., Soares T., Solórzano C., Sorio G.L., Sorley K., Sosa-Rodriguez T., Souza C.M.C.D.L., Souza B.S.D.F., Souza A.R., Spencer A.J., Spina F., Spoors L., Stafford L., Stamford I., Starinskij I., Stein R., Steven J., Stockdale L., Stockwell L.V., Strickland L.H., Stuart A.C., Sturdy A., Sutton N., Szigeti A., Tahiri-Alaoui A., Tanner R., Taoushanis C., Tarr A.W., Taylor K., Taylor U., Taylor I.J., Taylor J., te Water Naude R., Themistocleous Y., Themistocleous A., Thomas M., Thomas K., Thomas T.M., Thombrayil A., Thompson F., Thompson A., Thompson K., Thompson A., Thomson J., Thornton-Jones V., Tighe P.J., Tinoco L.A., Tiongson G., Tladinyane B., Tomasicchio M., Tomic A., Tonks S., Towner J., Tran N., Tree J., Trillana G., Trinham C., Trivett R., Truby A., Tsheko B.L., Turabi A., Turner R., Turner C., Ulaszewska M., Underwood B.R., Varughese R., Verbart D., Verheul M., Vichos I., Vieira T., Waddington C.S., Walker L., Wallis E., Wand M., Warbick D., Wardell T., Warimwe G., Warren S.C., Watkins B., Watson E., Webb S., Webb-Bridges A., Webster A., Welch J., Wells J., West A., White C., White R., Williams P., Williams R.L., Winslow R., Woodyer M., Worth A.T., Wright D., Wroblewska M., Yao A., Zimmer R., Zizi D., Zuidewind P. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., McGettigan J., Khetan S., Segall N., Solis J., Brosz A., Fierro C., Schwartz H., Neuzil K., Corey L., Gilbert P., Janes H., Follmann D., Marovich M., Mascola J., Polakowski L., Ledgerwood J., Graham B.S., Bennett H., Pajon R., Knightly C., Leav B., Deng W., Zhou H., Han S., Ivarsson M., Miller J., Zaks T. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/nejmoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V., Cooper D., Frenck R.W., Hammitt L.L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D.B., Mather S., Dormitzer P.R., Şahin U., Jansen K.U., Gruber W.C. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/nejmoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., McGettigan J., Khetan S., Segall N., Solis J., Brosz A., Fierro C., Schwartz H., Neuzil K., Corey L., Gilbert P., Janes H., Follmann D., Marovich M., Mascola J., Polakowski L., Ledgerwood J., Graham B.S., Bennett H., Pajon R., Knightly C., Leav B., Deng W., Zhou H., Han S., Ivarsson M., Miller J., Zaks T. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/nejmoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.FDA . U.S. Food and Drug Administration,; 2021. FDA Approves First COVID-19 Vaccine. [Google Scholar]
- 94.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., Bibi S., Briner C., Cicconi P., Collins A.M., Colin-Jones R., Cutland C.L., Darton T.C., Dheda K., Duncan C.J.A., Emary K.R.W., Ewer K.J., Fairlie L., Faust S.N., Feng S., Ferreira D.M., Finn A., Goodman A.L., Green C.M., Green C.A., Heath P.T., Hill C., Hill H., Hirsch I., Hodgson S.H.C., Izu A., Jackson S., Jenkin D., Joe C.C.D., Kerridge S., Koen A., Kwatra G., Lazarus R., Lawrie A.M., Lelliott A., Libri V., Lillie P.J., Mallory R., Mendes A.V.A., Milan E.P., Minassian A.M., McGregor A., Morrison H., Mujadidi Y.F., Nana A., O’Reilly P.J., Padayachee S.D., Pittella A., Plested E., Pollock K.M., Ramasamy M.N., Rhead S., Schwarzbold A.V., Singh N., Smith A., Song R., Snape M.D., Sprinz E., Sutherland R.K., Tarrant R., Thomson E.C., Török M.E., Toshner M., Turner D.P.J., Vekemans J., Villafana T.L., Watson M.E.E., Williams C.J., Douglas A.D., Hill A.V.S., Lambe T., Gilbert S.C., Pollard A.J., Aban M., Abayomi F., Abeyskera K., Aboagye J., Adam M., Adams K., Adamson J., Adelaja Y.A., Adewetan G., Adlou S., Ahmed K., Akhalwaya Y., Akhalwaya S., Alcock A., Ali A., Allen E.R., Allen L., Almeida T.C.D.S.C., Alves M.P.S., Amorim F., Andritsou F., Anslow R., Appleby M., Arbe-Barnes E.H., Ariaans M.P., Arns B., Arruda L., Azi P., Azi L., Babbage G., Bailey C., Baker K.F., Baker M., Baker N., Baker P., Baldwin L., Baleanu I., Bandeira D., Bara A., Barbosa M.A.S., Barker D., Barlow G.D., Barnes E., Barr A.S., Barrett J.R., Barrett J., Bates L., Batten A., Beadon K., Beales E., Beckley R., Belij-Rammerstorfer S., Bell J., Bellamy D., Bellei N., Belton S., Berg A., Bermejo L., Berrie E., Berry L., Berzenyi D., Beveridge A., Bewley K.R., Bexhell H., Bhikha S., Bhorat A.E., Bhorat Z.E., Bijker E., Birch G., Birch S., Bird A., Bird O., Bisnauthsing K., Bittaye M., Blackstone K., Blackwell L., Bletchly H., Blundell C.L., Blundell S.R., Bodalia P., Boettger B.C., Bolam E., Boland E., Bormans D., Borthwick N., Bowring F., Boyd A., Bradley P., Brenner T., Brown P., Brown C., Brown-O’Sullivan C., Bruce S., Brunt E., Buchan R., Budd W., Bulbulia Y.A., Bull M., Burbage J., Burhan H., Burn A., Buttigieg K.R., Byard N., Cabera Puig I., Calderon G., Calvert A., Camara S., Cao M., Cappuccini F., Cardoso J.R., Carr M., Carroll M.W., Carson-Stevens A., de Y., Carvalho M., Carvalho J.A.M., Casey H.R., Cashen P., Castro T., Castro L.C., Cathie K., Cavey A., Cerbino-Neto J., Chadwick J., Chapman D., Charlton S., Chelysheva I., Chester O., Chita S., Cho J.S., Cifuentes L., Clark E., Clark M., Clarke A., Clutterbuck E.A., Collins S.L.K., Conlon C.P., Connarty S., Coombes N., Cooper C., Cooper R., Cornelissen L., Corrah T., Cosgrove C., Cox T., Crocker W.E.M., Crosbie S., Cullen L., Cullen D., Cunha D.R.M.F., Cunningham C., Cuthbertson F.C., Da Guarda S.N.F., da Silva L.P., Damratoski B.E., Danos Z., Dantas M.T.D.C., Darroch P., Datoo M.S., Datta C., Davids M., Davies S.L., Davies H., Davis E., Davis J., Davis J., De Nobrega M.M.D., De Oliveira Kalid L.M., Dearlove D., Demissie T., Desai A., Di Marco S., Di Maso C., Dinelli M.I.S., Dinesh T., Docksey C., Dold C., Dong T., Donnellan F.R., Dos Santos T., dos Santos T.G., Dos Santos E.P., Douglas N., Downing C., Drake J., Drake-Brockman R., Driver K., Drury R., Dunachie S.J., Durham B.S., Dutra L., Easom N.J.W., van Eck S., Edwards M., Edwards N.J., El Muhanna O.M., Elias S.C., Elmore M., English M., Esmail A., Essack Y.M., Farmer E., Farooq M., Farrar M., Farrugia L., Faulkner B., Fedosyuk S., Felle S., Ferreira Da Silva C., Field S., Fisher R., Flaxman A., Fletcher J., Fofie H., Fok H., Ford K.J., Fowler J., Fraiman P.H.A., Francis E., Franco M.M., Frater J., Freire M.S.M., Fry S.H., Fudge S., Furze J., Fuskova M., Galian-Rubio P., Galiza E., Garlant H., Gavrila M., Geddes A., Gibbons K.A., Gilbride C., Gill H., Glynn S., Godwin K., Gokani K., Goldoni U.C., Goncalves M., Gonzalez I.G.S., Goodwin J., Goondiwala A., Gordon-Quayle K., Gorini G., Grab J., Gracie L., Greenland M., Greenwood N., Greffrath J., Groenewald M.M., Grossi L., Gupta G., Hackett M., Hallis B., Hamaluba M., Hamilton E., Hamlyn J., Hammersley D., Hanrath A.T., Hanumunthadu B., Harris S.A., Harris C., Harris T., Harrison T.D., Harrison D., Hart T.C., Hartnell B., Hassan S., Haughney J., Hawkins S., Hay J., Head I., Henry J., Hermosin Herrera M., Hettle D.B., Hill J., Hodges G., Horne E., Hou M.M., Houlihan C., Howe E., Howell N., Humphreys J., Humphries H.E., Hurley K., Huson C., Hyder-Wright A., Hyams C., Ikram S., Ishwarbhai A., Ivan M., Iveson P., Iyer V., Jackson F., De Jager J., Jaumdally S., Jeffers H., Jesudason N., Jones B., Jones K., Jones E., Jones C., Jorge M.R., Jose A., Joshi A., Júnior E.A.M.S., Kadziola J., Kailath R., Kana F., Karampatsas K., Kasanyinga M., Keen J., Kelly E.J., Kelly D.M., Kelly D., Kelly S., Kerr D., de R., Kfouri Á., Khan L., Khozoee B., Kidd S., Killen A., Kinch J., Kinch P., King L.D.W., King T.B., Kingham L., Klenerman P., Knapper F., Knight J.C., Knott D., Koleva S., Lang M., Lang G., Larkworthy C.W., Larwood J.P.J., Law R., Lazarus E.M., Leach A., Lees E.A., Lemm N.M., Lessa A., Leung S., Li Y., Lias A.M., Liatsikos K., Linder A., Lipworth S., Liu S., Liu X., Lloyd A., Lloyd S., Loew L., Lopez Ramon R., Lora L., Lowthorpe V., Luz K., MacDonald J.C., MacGregor G., Madhavan M., Mainwaring D.O., Makambwa E., Makinson R., Malahleha M., Malamatsho R., Mallett G., Mansatta K., Maoko T., Mapetla K., Marchevsky N.G., Marinou S., Marlow E., Marques G.N., Marriott P., Marshall R.P., Marshall J.L., Martins F.J., Masenya M., Masilela M., Masters S.K., Mathew M., Matlebjane H., Matshidiso K., Mazur O., Mazzella A., McCaughan H., McEwan J., McGlashan J., McInroy L., McIntyre Z., McLenaghan D., McRobert N., McSwiggan S., Megson C., Mehdipour S., Meijs W., Mendonça R.N.Á., Mentzer A.J., Mirtorabi N., Mitton C., Mnyakeni S., Moghaddas F., Molapo K., Moloi M., Moore M., Moraes-Pinto M.I., Moran M., Morey E., Morgans R., Morris S., Morris S., Morris H.C., Morselli F., Morshead G., Morter R., Mottal L., Moultrie A., Moya N., Mpelembue M., Msomi S., Mugodi Y., Mukhopadhyay E., Muller J., Munro A., Munro C., Murphy S., Mweu P., Myasaki C.H., Naik G., Naker K., Nastouli E., Nazir A., Ndlovu B., Neffa F., Njenga C., Noal H., Noé A., Novaes G., Nugent F.L., Nunes G., O’Brien K., O’Connor D., Odam M., Oelofse S., Oguti B., Olchawski V., Oldfield N.J., Oliveira M.G., Oliveira C., Oosthuizen A., O’Reilly P., Osborne P., Owen D.R.J., Owen L., Owens D., Owino N., Pacurar M., Paiva B.V.B., Palhares E.M.F., Palmer S., Parkinson S., Parracho H.M.R.T., Parsons K., Patel D., Patel B., Patel F., Patel K., Patrick-Smith M., Payne R.O., Peng Y., Penn E.J., Pennington A., Peralta Alvarez M.P., Perring J., Perry N., Perumal R., Petkar S., Philip T., Phillips D.J., Phillips J., Phohu M.K., Pickup L., Pieterse S., Piper J., Pipini D., Plank M., Du Plessis J., Pollard S., Pooley J., Pooran A., Poulton I., Powers C., Presa F.B., Price D.A., Price V., Primeira M., Proud P.C., Provstgaard-Morys S., Pueschel S., Pulido D., Quaid S., Rabara R., Radford A., Radia K., Rajapaska D., Rajeswaran T., Ramos A.S.F., Ramos Lopez F., Rampling T., Rand J., Ratcliffe H., Rawlinson T., Rea D., Rees B., Reiné J., Resuello-Dauti M., Reyes Pabon E., Ribiero C.M., Ricamara M., Richter A., Ritchie N., Ritchie A.J., Robbins A.J., Roberts H., Robinson R.E., Robinson H., Rocchetti T.T., Rocha B.P., Roche S., Rollier C., Rose L., Ross Russell A.L., Rossouw L., Royal S., Rudiansyah I., Ruiz S., Saich S., Sala C., Sale J., Salman A.M., Salvador N., Salvador S., Sampaio M., Samson A.D., Sanchez-Gonzalez A., Sanders H., Sanders K., Santos E., Santos Guerra M.F.S., Satti I., Saunders J.E., Saunders C., Sayed A., Schim van der Loeff I., Schmid A.B., Schofield E., Screaton G., Seddiqi S., Segireddy R.R., Senger R., Serrano S., Shah R., Shaik I., Sharpe H.E., Sharrocks K., Shaw R., Shea A., Shepherd A., Shepherd J.G., Shiham F., Sidhom E., Silk S.E., da Silva Moraes A.C., Silva-Junior G., Silva-Reyes L., Silveira A.D., Silveira M.B.V., Sinha J., Skelly D.T., Smith D.C., Smith N., Smith H.E., Smith D.J., Smith C.C., Soares A., Soares T., Solórzano C., Sorio G.L., Sorley K., Sosa-Rodriguez T., Souza C.M.C.D.L., Souza B.S.D.F., Souza A.R., Spencer A.J., Spina F., Spoors L., Stafford L., Stamford I., Starinskij I., Stein R., Steven J., Stockdale L., Stockwell L.V., Strickland L.H., Stuart A.C., Sturdy A., Sutton N., Szigeti A., Tahiri-Alaoui A., Tanner R., Taoushanis C., Tarr A.W., Taylor K., Taylor U., Taylor I.J., Taylor J., te Water Naude R., Themistocleous Y., Themistocleous A., Thomas M., Thomas K., Thomas T.M., Thombrayil A., Thompson F., Thompson A., Thompson K., Thompson A., Thomson J., Thornton-Jones V., Tighe P.J., Tinoco L.A., Tiongson G., Tladinyane B., Tomasicchio M., Tomic A., Tonks S., Towner J., Tran N., Tree J., Trillana G., Trinham C., Trivett R., Truby A., Tsheko B.L., Turabi A., Turner R., Turner C., Ulaszewska M., Underwood B.R., Varughese R., Verbart D., Verheul M., Vichos I., Vieira T., Waddington C.S., Walker L., Wallis E., Wand M., Warbick D., Wardell T., Warimwe G., Warren S.C., Watkins B., Watson E., Webb S., Webb-Bridges A., Webster A., Welch J., Wells J., West A., White C., White R., Williams P., Williams R.L., Winslow R., Woodyer M., Worth A.T., Wright D., Wroblewska M., Yao A., Zimmer R., Zizi D., Zuidewind P. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sadoff J., Gray G., Vandebosch A., Cárdenas V., Shukarev G., Grinsztejn B., Goepfert P.A., Truyers C., Fennema H., Spiessens B., Offergeld K., Scheper G., Taylor K.L., Robb M.L., Treanor J., Barouch D.H., Stoddard J., Ryser M.F., Marovich M.A., Neuzil K.M., Corey L., Cauwenberghs N., Tanner T., Hardt K., Ruiz-Guiñazú J., Le Gars M., Schuitemaker H., Van Hoof J., Struyf F., Douoguih M. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N. Engl. J. Med. 2021;384:2187–2201. doi: 10.1056/nejmoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Solforosi L., Kuipers H., Jongeneelen M., Huber S.K.R., Van Der Lubbe J.E.M., Dekking L. Immunogenicity and efficacy of one and two doses of Ad26. COV2. S COVID vaccine in adult and aged NHP. J. Exp. Med. 2021;218 doi: 10.1084/jem.20202756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Solforosi L., Kuipers H., Jongeneelen M., Huber S.K.R., Van Der Lubbe J.E.M., Dekking L. Immunogenicity and efficacy of one and two doses of Ad26. COV2. S COVID vaccine in adult and aged NHP. J. Exp. Med. 2021;218 doi: 10.1084/jem.20202756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sadoff J., Gray G., Vandebosch A., Cárdenas V., Shukarev G., Grinsztejn B., Goepfert P.A., Truyers C., Fennema H., Spiessens B., Offergeld K., Scheper G., Taylor K.L., Robb M.L., Treanor J., Barouch D.H., Stoddard J., Ryser M.F., Marovich M.A., Neuzil K.M., Corey L., Cauwenberghs N., Tanner T., Hardt K., Ruiz-Guiñazú J., Le Gars M., Schuitemaker H., Van Hoof J., Struyf F., Douoguih M. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N. Engl. J. Med. 2021;384:2187–2201. doi: 10.1056/nejmoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mascellino M.T., Di Timoteo F., De Angelis M., Oliva A. Overview of the Main Anti-SARS-CoV-2 Vaccines: Mechanism of Action, Efficacy and Safety. Infect. Drug Resist. 2021;14:3459–3476. doi: 10.2147/IDR.S315727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Oliver S.E., Gargano J.W., Marin M., Wallace M., Curran K.G., Chamberland M., McClung N., Campos-Outcalt D., Morgan R.L., Mbaeyi S., Romero J.R., Talbot H.K., Lee G.M., Bell B.P., Dooling K. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Moderna COVID-19 Vaccine - United States, December 2020. MMWR Morb. Mortal. Wkly. Rep. 2021;69:1653–1656. doi: 10.15585/mmwr.mm695152e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Boivin Z., Martin J. Untimely Myocardial Infarction or COVID-19 Vaccine Side Effect. Cureus. 2021;13 doi: 10.7759/cureus.13651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Knoll M.D., Wonodi C. Oxford-AstraZeneca COVID-19 vaccine efficacy. Lancet. 2021;397:72–74. doi: 10.1016/S0140-6736(20)32623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Oliver S.E., Gargano J.W., Scobie H., Wallace M., Hadler S.C., Leung J., Blain A.E., McClung N., Campos-Outcalt D., Morgan R.L., Mbaeyi S., MacNeil J., Romero J.R., Talbot H.K., Lee G.M., Bell B.P., Dooling K. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Janssen COVID-19 Vaccine - United States, February 2021. MMWR Morb. Mortal. Wkly Rep. 2021;70:329–332. doi: 10.15585/mmwr.mm7009e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Babamahmoodi F., Saeedi M., Alizadeh-Navaei R., Hedayatizadeh-Omran A., Mousavi S.A., Ovaise G., Kordi S., Akbari Z., Azordeh M., Ahangarkani F., Alikhani A. Side effects and Immunogenicity following administration of the Sputnik V COVID-19 vaccine in health care workers in Iran. Sci. Rep. 2021;11:21464. doi: 10.1038/s41598-021-00963-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chuaychoosakoon C., Parinyakhup W., Tanutit P., Maliwankul K., Klabklay P. Shoulder injury related to Sinovac COVID-19 vaccine: A case report. Ann. Med. Surg. 2021;68 doi: 10.1016/j.amsu.2021.102622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ella R., Reddy S., Blackwelder W., Potdar V., Yadav P., Sarangi V., Aileni V.K., Kanungo S., Rai S., Reddy P., Verma S., Singh C., Redkar S., Mohapatra S., Pandey A., Ranganadin P., Gumashta R., Multani M., Mohammad S., Bhatt P., Kumari L., Sapkal G., Gupta N., Abraham P., Panda S., Prasad S., Bhargava B., Ella K., Vadrevu K.M., Aggarwal P., Aglawe V., Ali A., Anand N., Awad N., Bafna V., Balasubramaniyam G., Bandkar A., Basha P., Bharge V., Bhate A., Bhate S., Bhavani V., Bhosale R., Chalapathy D., Chaubal C., Chaudhary D., Chavan A., Desai P., Dhodi D., Dutta S., Garg R., Garg K., George M., Goyal P., Guleria R., Gupta S., Jain M., Jain M.K., Jindal S., Kalra M., Kant S., Khosla P., Kulkarni P., Kumar P., Kumar Y., Majumdar A., Meshram P., Mishra V., Mohanty S., Nair J., Pandey S., Panigrahi S.K., Patil B., Patil V., Rahate P., Raj V., Ramanand S., Rami K., Ramraj B., Rane S., Rao E.V., Rao N., Raphael R., Reddy G., Redkar V., Redkar S., Sachdeva A., Saha J., Sahoo J., Sampath P., Savith A., Shah M., Shanmugam L., Sharma R., Sharma P., Sharma D., Singh A., Singh J., Singh P., Sivaprakasam S., Subramaniam S., Sudheer D., Tandon S., Tariq M., Tripathi V., Vable M., Verma R., Waghmare S. Efficacy, safety, and lot-to-lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): interim results of a randomised, double-blind, controlled, phase 3 trial. Lancet. 2021;398:2173–2184. doi: 10.1016/s0140-6736(21)02000-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ella R., Reddy S., Jogdand H., Sarangi V., Ganneru B., Prasad S., Das D., Raju D., Praturi U., Sapkal G., Yadav P., Reddy P., Verma S., Singh C., Redkar S.V., Gillurkar C.S., Kushwaha J.S., Mohapatra S., Bhate A., Rai S., Panda S., Abraham P., Gupta N., Ella K., Bhargava B., Vadrevu K.M. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial. Lancet Infect. Dis. 2021;21:950–961. doi: 10.1016/S1473-3099(21)00070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ganneru B., Jogdand H., Daram V.K., Das D., Molugu N.R., Prasad S.D., Kannappa S.V., Ella K.M., Ravikrishnan R., Awasthi A., Jose J., Rao P., Kumar D., Ella R., Abraham P., Yadav P.D., Sapkal G.N., Shete-Aich A., Desphande G., Mohandas S., Basu A., Gupta N., Vadrevu K.M. Th1 skewed immune response of whole virion inactivated SARS CoV 2 vaccine and its safety evaluation. IScience. 2021;24 doi: 10.1016/j.isci.2021.102298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pawar H.A., Pawar A.H., Pawar S.A. (An International Multidisciplinary Peer Review Open Access monthly Journal) COVID-19 Vaccines approved for use and under W development in India: An overview. J. Pharm. Adv. Res. 2021;4:1277–1284. [Google Scholar]
- 110.Dunkle L.M., Kotloff K.L., Gay C.L., Áñez G., Adelglass J.M., Barrat Hernández A.Q., Harper W.L., Duncanson D.M., McArthur M.A., Florescu D.F., McClelland R.S., Garcia-Fragoso V., Riesenberg R.A., Musante D.B., Fried D.L., Safirstein B.E., McKenzie M., Jeanfreau R.J., Kingsley J.K., Henderson J.A., Lane D.C., Ruíz-Palacios G.M., Corey L., Neuzil K.M., Coombs R.W., Greninger A.L., Hutter J., Ake J.A., Smith K., Woo W., Cho I., Glenn G.M., Dubovsky F. Efficacy and Safety of NVX-CoV2373 in Adults in the United States and Mexico. N. Engl. J. Med. 2021:1–13. doi: 10.1056/nejmoa2116185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Verdecchia P., Cavallini C., Spanevello A., Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur. J. Intern. Med. 2020;76:14–20. doi: 10.1016/j.ejim.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.P.J. Hotez, M.E. Bottazzi, Developing a low-cost and accessible COVID-19 vaccine for global health, 2020. [DOI] [PMC free article] [PubMed]
- 113.T.T. Le, Z. Andreadakis, A. Kumar, R.G. Román, S. Tollefsen, & M.S., S. Mayhew, The COVID-19 vaccine development landscape, 2020. [DOI] [PubMed]
- 114.P.J. Hotez, M.E. Bottazzi, Developing a low-cost and accessible COVID-19 vaccine for global health, 2020. [DOI] [PMC free article] [PubMed]
- 115.Bergman S., Cennimo D.J., Miller M.M., Olsen K.M. COVID-19 Treatment: Investigational Drugs and Other Therapies. Medscape. 2021:1–49. [Google Scholar]
- 116.El-Fakharany E., El-Maradny Y., Othman A., Gerges M., Belal F., Behery E. COVID-19 coronavirus: pathogenesis, clinical features, diagnostics, epidemiology, prevention and control. Microb. Biosyst. 2020;5:7–20. doi: 10.21608/mb.2020.33405.1018. [DOI] [Google Scholar]
- 117.COVID-19 Treatment Guidelines Panel . National Institutes of Health,; 2022. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines.〈https://www.covid19treatmentguidelines.nih.gov/〉 [PubMed] [Google Scholar]
- 118.Pau A.K., Aberg J., Baker J., Belperio P.S., Coopersmith C., Crew P., Grund B., Gulick R.M., Harrison C., Kim A., Lane H.C., Masur H., Sheikh V., Singh K., Yazdany J., Tebas P. Convalescent Plasma for the Treatment of COVID-19: Perspectives of the National Institutes of Health COVID-19 Treatment Guidelines Panel. Ann. Intern Med. 2021;174:93–95. doi: 10.7326/M20-6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.CDC . Vol. 2020. Public Health Media Library,; 2019. p. 2020. (Information for Clinicians on Investigational Therapeutics for Patients with COVID-19). [Google Scholar]
- 120.Khani E., Khiali S., Entezari-Maleki T. Potential COVID-19 Therapeutic Agents and Vaccines: An Evidence-Based Review. J. Clin. Pharm. 2021;61:429–460. doi: 10.1002/jcph.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Scavone C., Mascolo A., Rafaniello C., Sportiello L., Trama U., Zoccoli A., Bernardi F.F., Racagni G., Berrino L., Castaldo G., Coscioni E., Rossi F., Capuano A. Therapeutic strategies to fight COVID-19: Which is the status artis? Br. J. Pharm. 2021 doi: 10.1111/bph.15452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ledford H. COVID antiviral pills: what scientists still want to know. Nature. 2021;599:358–359. doi: 10.1038/d41586-021-03074-5. [DOI] [PubMed] [Google Scholar]
- 123.Mahase E. Covid-19: Pfizer’s paxlovid is 89% effective in patients at risk of serious illness, company reports. BMJ. 2021;375:n2713. doi: 10.1136/bmj.n2713. [DOI] [PubMed] [Google Scholar]
- 124.National Institutes of Health COVID-19 Treatment Guidelines. Food and Drug Administration, Nonhospitalized Adults: Therapeutic Management. COVID-19 Treat. Guidel. 2022:113–119. 〈https://www.covid19treatmentguidelines.nih.gov/management/clinical-management/nonhospitalized-adults--therapeutic-management/〉 accessed July 3, 2022. [Google Scholar]
- 125.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., Hohmann E., Chu H.Y., Luetkemeyer A., Kline S., Lopez de Castilla D., Finberg R.W., Dierberg K., Tapson V., Hsieh L., Patterson T.F., Paredes R., Sweeney D.A., Short W.R., Touloumi G., Lye D.C., Ohmagari N., Oh M., Ruiz-Palacios G.M., Benfield T., Fätkenheuer G., Kortepeter M.G., Atmar R.L., Creech C.B., Lundgren J., Babiker A.G., Pett S., Neaton J.D., Burgess T.H., Bonnett T., Green M., Makowski M., Osinusi A., Nayak S., Lane H.C. Remdesivir for the Treatment of Covid-19 — Final Report. N. Engl. J. Med. 2020;383:1813–1826. doi: 10.1056/nejmoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Imran M., Kumar Arora M., Asdaq S.M.B., Khan S.A., Alaqel S.I., Alshammari M.K., Alshehri M.M., Alshrari A.S., Mateq Ali A., Al-Shammeri A.M., Alhazmi B.D., Harshan A.A., Alam M.T., Abida Discovery, development, and patent trends on molnupiravir: A prospective oral treatment for covid-19. Molecules. 2021;26 doi: 10.3390/molecules26195795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mahase E. Covid-19: UK becomes first country to authorise antiviral molnupiravir. BMJ. 2021;375 doi: 10.1136/bmj.n2697. [DOI] [PubMed] [Google Scholar]
- 129.Duan J., Yan X., Guo X., Cao W., Han W., Qi C., Feng J., Yang D., Gao G., Jin G. A human SARS-CoV neutralizing antibody against epitope on S2 protein. Biochem. Biophys. Res. Commun. 2005;333:186–193. doi: 10.1016/j.bbrc.2005.05.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Weinreich D.M., Sivapalasingam S., Norton T., Ali S., Gao H., Bhore R., Musser B.J., Soo Y., Rofail D., Im J., Perry C., Pan C., Hosain R., Mahmood A., Davis J.D., Turner K.C., Hooper A.T., Hamilton J.D., Baum A., Kyratsous C.A., Kim Y., Cook A., Kampman W., Kohli A., Sachdeva Y., Graber X., Kowal B., DiCioccio T., Stahl N., Lipsich L., Braunstein N., Herman G., Yancopoulos G.D. REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19. N. Engl. J. Med. 2021;384:238–251. doi: 10.1056/nejmoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gupta A., Gonzalez-Rojas Y., Juarez E., Crespo Casal M., Moya J., Rodrigues Falci D., Sarkis E., Solis J., Zheng H., Scott N., Cathcart A.L., Parra S., Sager J.E., Austin D., Peppercorn A., Alexander E., Yeh W.W., Brinson C., Aldinger M., Shapiro A.E. Effect of Sotrovimab on Hospitalization or Death among High-risk Patients with Mild to Moderate COVID-19: A Randomized Clinical Trial. JAMA - J. Am. Med. Assoc. 2022;327:1236–1246. doi: 10.1001/jama.2022.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cameroni E., Bowen J.E., Rosen L.E., Saliba C., Zepeda S.K., Culap K., Pinto D., VanBlargan L.A., de Marco A., di Iulio J., Zatta F., Kaiser H., Noack J., Farhat N., Czudnochowski N., Havenar-Daughton C., Sprouse K.R., Dillen J.R., Powell A.E., Chen A., Maher C., Yin L., Sun D., Soriaga L., Bassi J., Silacci-Fregni C., Gustafsson C., Franko N.M., Logue J., Iqbal N.T., Mazzitelli I., Geffner J., Grifantini R., Chu H., Gori A., Riva A., Giannini O., Ceschi A., Ferrari P., Cippà P.E., Franzetti-Pellanda A., Garzoni C., Halfmann P.J., Kawaoka Y., Hebner C., Purcell L.A., Piccoli L., Pizzuto M.S., Walls A.C., Diamond M.S., Telenti A., Virgin H.W., Lanzavecchia A., Snell G., Veesler D., Corti D. Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift. Nature. 2022;602:664–670. doi: 10.1038/s41586-021-04386-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mahase E. Covid-19: Has the spread of omicron BA.2 made antibody treatments redundant? BMJ. 2022;377:o1009. doi: 10.1136/bmj.o1009. [DOI] [PubMed] [Google Scholar]
- 134.Plichta J., Kuna P., Panek M. Monoclonal Antibodies as Potential COVID-19 Therapeutic Agents. COVID. 2022;2:599–620. doi: 10.3390/covid2050045. [DOI] [Google Scholar]
- 135.Stauffer W.M., Alpern J.D., Walker P.F. COVID-19 and Dexamethasone: A Potential Strategy to Avoid Steroid-Related Strongyloides Hyperinfection. JAMA - J. Am. Med. Assoc. 2020;324:623–624. doi: 10.1001/jama.2020.13170. [DOI] [PubMed] [Google Scholar]
- 136.Rosas I.O., Bräu N., Waters M., Go R.C., Hunter B.D., Bhagani S., Skiest D., Aziz M.S., Cooper N., Douglas I.S., Savic S., Youngstein T., del Sorbo L., Cubillo Gracian A., de La Zerda D.J., Ustianowski A., Bao M., Dimonaco S., Graham E., Matharu B., Spotswood H., Tsai L., Malhotra A. Tocilizumab in Hospitalized Patients with Severe Covid-19 Pneumonia. N. Engl. J. Med. 2021;384:1503–1516. doi: 10.1056/nejmoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gupta S., Wang W., Hayek S.S., Chan L., Mathews K.S., Melamed M.L., Brenner S.K., Leonberg-Yoo A., Schenck E.J., Radbel J., Reiser J., Bansal A., Srivastava A., Zhou Y., Finkel D., Green A., Mallappallil M., Faugno A.J., Zhang J., Velez J.C.Q., Shaefi S., Parikh C.R., Charytan D.M., Athavale A.M., Friedman A.N., Redfern R.E., Short S.A.P., Correa S., Pokharel K.K., Admon A.J., Donnelly J.P., Gershengorn H.B., Douin D.J., Semler M.W., Hernán M.A., Leaf D.E. Association between Early Treatment with Tocilizumab and Mortality among Critically Ill Patients with COVID-19. JAMA Intern. Med. 2021;181:41–51. doi: 10.1001/jamainternmed.2020.6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Boshier F.A.T., Pang J., Penner J., Parker M., Alders N., Bamford A., Grandjean L., Grunewald S., Hatcher J., Best T., Dalton C., Bynoe P.D., Frauenfelder C., Köeglmeier J., Myerson P., Roy S., Williams R., de Silva T.I., Goldstein R.A., Breuer J. Evolution of viral variants in remdesivir-treated and untreated SARS-CoV-2-infected pediatrics patients. J. Med. Virol. 2022;94:161–172. doi: 10.1002/jmv.27285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shafiekhani M., Shahabinezhad F., Niknam T., Tara S.A., Haem E., Mardani P., Zare Z., Jafarian S., Mirzad Jahromi K., Arabsheybani S., Moeini Y.S., Alavi J., Jalali S.S., Salimi M., Shahriarirad R., Malekhosseini S.A. Evaluation of the therapeutic regimen in COVID-19 in transplant patients: where do immunomodulatory and antivirals stand? Virol. J. 2021;18:1–10. doi: 10.1186/s12985-021-01700-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.A. Adarsh Bhimraj, R.L. Morgan, A. Hirsch Shumaker, V. Lavergne, L. Baden, V. Chi-Chung Cheng, K.M. Edwards, R. Gandhi, J. Gallagher, W.J. Muller, J.C. O, S. Shoham, M. Hassan Murad, R.A. Mustafa, S. Sultan, Y. Falck-Ytter, Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19, n.d. www.idsociety.org/COVID19guidelines. [DOI] [PMC free article] [PubMed]
- 141.FDA, Coronavirus (COVID-19) Update: FDA Authorizes First Oral Antiviral for Treatment of COVID-19, 2021.
- 142.Mahase E. Covid-19: UK stockpiles two unapproved antiviral drugs for treatment at home. BMJ. 2021;375:n2602. doi: 10.1136/bmj.n2602. [DOI] [PubMed] [Google Scholar]
- 143.FDA, Coronavirus (COVID-19) Update: FDA Authorizes Additional Oral Antiviral for Treatment of COVID-19 in Certain Adults, (2021).
- 144.Mahase E. Covid-19: UK stockpiles two unapproved antiviral drugs for treatment at home. BMJ. 2021;375:n2602. doi: 10.1136/bmj.n2602. [DOI] [PubMed] [Google Scholar]
- 145.Jayk Bernal A., Gomes da Silva M.M., Musungaie D.B., Kovalchuk E., Gonzalez A., Delos Reyes V., Martín-Quirós A., Caraco Y., Williams-Diaz A., Brown M.L., Du J., Pedley A., Assaid C., Strizki J., Grobler J.A., Shamsuddin H.H., Tipping R., Wan H., Paschke A., Butterton J.R., Johnson M.G., De Anda C., MOVe-OUT Study Group Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N. Engl. J. Med. 2021:1–12. doi: 10.1056/NEJMoa2116044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kabinger F., Stiller C., Schmitzová J., Dienemann C., Kokic G., Hillen H.S., Höbartner C., Cramer P. Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis. Nat. Struct. Mol. Biol. 2021;28:740–746. doi: 10.1038/s41594-021-00651-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Devaux C.A., Rolain J.-M., Colson P., Raoult D. file:///C:/Users/USER/Downloads/32251767.nbib. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Choy K.-T., Wong A.Y.-L., Kaewpreedee P., Sia S.F., Chen D., Hui K.P.Y., Chu D.K.W., Chan M.C.W., Cheung P.P.-H., Huang X., Peiris M., Yen H.-L. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antivir. Res. 2020;178 doi: 10.1016/j.antiviral.2020.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yang S., Xu M., Lee E.M., Gorshkov K., Shiryaev S.A., He S., Sun W., Cheng Y.S., Hu X., Tharappel A.M., Lu B., Pinto A., Farhy C., Huang C.T., Zhang Z., Zhu W., Wu Y., Zhou Y., Song G., Zhu H., Shamim K., Martínez-Romero C., García-Sastre A., Preston R.A., Jayaweera D.T., Huang R., Huang W., Xia M., Simeonov A., Ming G., Qiu X., Terskikh A.V., Tang H., Song H., Zheng W. Emetine inhibits Zika and Ebola virus infections through two molecular mechanisms: Inhibiting viral replication and decreasing viral entry. Cell Discov. 2018;4:1–14. doi: 10.1038/s41421-018-0034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bleasel M.D., Peterson G.M. Emetine, Ipecac, Ipecac Alkaloids and Analogues as Potential Antiviral Agents for Coronaviruses. Pharm. (Basel) 2020;13 doi: 10.3390/ph13030051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020;178 doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.de Oliveira O.V., Rocha G.B., Paluch A.S., Costa L.T. Repurposing approved drugs as inhibitors of SARS-CoV-2 S-protein from molecular modeling and virtual screening. J. Biomol. Struct. Dyn. 2021;39:3924–3933. doi: 10.1080/07391102.2020.1772885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lehrer S., Rheinstein P.H. Ivermectin Docks to the SARS-CoV-2 Spike Receptor-binding Domain Attached to ACE2. Vivo. 2020;34:3023–3026. doi: 10.21873/invivo.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Popp M., Stegemann M., Metzendorf M.-I., Gould S., Kranke P., Meybohm P., Skoetz N., Weibel S. Ivermectin for preventing and treating COVID-19. Cochrane Database Syst. Rev. 2021;7 doi: 10.1002/14651858.CD015017.pub2. CD015017–CD015017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Singh B., Ryan H., Kredo T., Chaplin M., Fletcher T. Chloroquine or hydroxychloroquine for prevention and treatment of COVID‐19. Cochrane Database Syst. Rev. 2021 doi: 10.1002/14651858.CD013587.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tang Y., Liu J., Zhang D., Xu Z., Ji J., Wen C. Cytokine Storm in COVID-19: The Current Evidence and Treatment Strategies. Front. Immunol. 2020;11:1–13. doi: 10.3389/fimmu.2020.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hertanto D.M., Wiratama B.S., Sutanto H., Wungu C.D.K. Immunomodulation as a potent COVID-19 pharmacotherapy: Past, present and future. J. Inflamm. Res. 2021;14:3419–3428. doi: 10.2147/JIR.S322831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kasper L.H., Reder A.T. Immunomodulatory activity of interferon-beta. Ann. Clin. Transl. Neurol. 2014;1:622–631. doi: 10.1002/acn3.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rizza P., Moretti F., Belardelli F. Recent advances on the immunomodulatory effects of IFN-alpha: implications for cancer immunotherapy and autoimmunity., Autoimmunity. 2010;43:204–209. doi: 10.3109/08916930903510880. [DOI] [PubMed] [Google Scholar]
- 160.Akamatsu M.A., de Castro J.T., Takano C.Y., Ho P.L. Off balance: Interferons in COVID-19 lung infections. EBioMedicine. 2021;73 doi: 10.1016/j.ebiom.2021.103642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Arabi Y.M., Shalhoub S., Mandourah Y., Al-Hameed F., Al-Omari A., Al Qasim E., Jose J., Alraddadi B., Almotairi A., Al Khatib K., Abdulmomen A., Qushmaq I., Sindi A.A., Mady A., Solaiman O., Al-Raddadi R., Maghrabi K., Ragab A., Al Mekhlafi G.A., Balkhy H.H., Al Harthy A., Kharaba A., Gramish J.A., Al-Aithan A.M., Al-Dawood A., Merson L., Hayden F.G., Fowler R. Ribavirin and Interferon Therapy for Critically Ill Patients With Middle East Respiratory Syndrome: A Multicenter Observational Study. Clin. Infect. Dis.: Off. Publ. Infect. Dis. Soc. Am. 2020;70:1837–1844. doi: 10.1093/cid/ciz544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Alavi Darazam I., Shokouhi S., Pourhoseingholi M.A., Naghibi Irvani S.S., Mokhtari M., Shabani M., Amirdosara M., Torabinavid P., Golmohammadi M., Hashemi S.P., Azimi A., Jafarazadeh Maivan M.H., Rezaei O., Zali A., Hajiesmaeili M., Shabanpour Dehbsneh H., Hoseyni Kusha A., Taleb Shoushtari M., Khalili N., Soleymaninia A., Gachkar L., Khoshkar A. Role of interferon therapy in severe COVID-19: the COVIFERON randomized controlled trial. Sci. Rep. 2021;11:1–11. doi: 10.1038/s41598-021-86859-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Arasteh O., Khalili H. Do interferons play a role in COVID-19? Int. J. Clin. Pract. 2021;75:3–5. doi: 10.1111/ijcp.14721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kalil A.C., Mehta A.K., Patterson T.F., Erdmann N., Gomez C.A., Jain M.K., Wolfe C.R., Ruiz-Palacios G.M., Kline S., Regalado Pineda J., Luetkemeyer A.F., Harkins M.S., Jackson P.E.H., Iovine N.M., Tapson V.F., Oh M.-D., Whitaker J.A., Mularski R.A., Paules C.I., Ince D., Takasaki J., Sweeney D.A., Sandkovsky U., Wyles D.L., Hohmann E., Grimes K.A., Grossberg R., Laguio-Vila M., Lambert A.A., Lopez de Castilla D., Kim E., Larson L., Wan C.R., Traenkner J.J., Ponce P.O., Patterson J.E., Goepfert P.A., Sofarelli T.A., Mocherla S., Ko E.R., Ponce de Leon A., Doernberg S.B., Atmar R.L., Maves R.C., Dangond F., Ferreira J., Green M., Makowski M., Bonnett T., Beresnev T., Ghazaryan V., Dempsey W., Nayak S.U., Dodd L., Tomashek K.M., Beigel J.H. Efficacy of interferon beta-1a plus remdesivir compared with remdesivir alone in hospitalised adults with COVID-19: a double-bind, randomised, placebo-controlled, phase 3 trial., Lancet. Respir. Med. 2021;9:1365–1376. doi: 10.1016/S2213-2600(21)00384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Y.M. Arabi, F. Ya, Corticosteroid Therapy for Critically ill Patients with the Middle East Respiratory Syndrome, 2017: 1–46.
- 167.Langhoff E., Ladefoged J. Relative immunosuppressive potency of various corticosteroids measured in vitro. Eur. J. Clin. Pharm. 1983;25:459–462. doi: 10.1007/BF00542111. [DOI] [PubMed] [Google Scholar]
- 168.Jeronimo C.M.P., Farias M.E.L., Val F.F.A., Sampaio V.S., Alexandre M.A.A., Melo G.C., Safe I.P., Borba M.G.S., Netto R.L.A., Maciel A.B.S., Neto J.R.S., Oliveira L.B., Figueiredo E.F.G., Oliveira Dinelly K.M., de Almeida Rodrigues M.G., Brito M., Mourão M.P.G., Pivoto João G.A., Hajjar L.A., Bassat Q., Romero G.A.S., Naveca F.G., Vasconcelos H.L., de Araújo Tavares M., Brito-Sousa J.D., Costa F.T.M., Nogueira M.L., Baía-da-Silva D.C., Xavier M.S., Monteiro W.M., Lacerda M.V.G. file:///C:/Users/USER/Downloads/19028526.nbib. Clin. Infect. Dis. 2021;72:e373–e381. doi: 10.1093/cid/ciaa1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Leneva I.A., Russell R.J., Boriskin Y.S., Hay A.J. Characteristics of arbidol-resistant mutants of influenza virus: Implications for the mechanism of anti-influenza action of arbidol. Antivir. Res. 2009;81:132–140. doi: 10.1016/j.antiviral.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 170.Liu J., Zheng X., Huang Y., Shan H., Huang J. Successful use of methylprednisolone for treating severe COVID-19. J. Allergy Clin. Immunol. 2020;146:325–327. doi: 10.1016/j.jaci.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Group T.R.C. Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021;384:693–704. doi: 10.1056/nejmoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Li Q., Li W., Jin Y., Xu W., Huang C., Li L., Huang Y., Fu Q., Chen L. Efficacy Evaluation of Early, Low-Dose, Short-Term Corticosteroids in Adults Hospitalized with Non-Severe COVID-19 Pneumonia: A Retrospective Cohort Study. Infect. Dis. Ther. 2020;9:823–836. doi: 10.1007/s40121-020-00332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Muflihah H., Bhekti Rahimah S., Widiyanto T., Mahwati Y., Parumasivam T., Sastramihardja H.S. Clinical use of antiviral, antibiotic and immunomodulatory drugs in hospitalized COVID-19 patients: a retrospective study in Bandung, Indonesia. F1000Res. 2021;10:1091. doi: 10.12688/f1000research.73606.1. [DOI] [Google Scholar]
- 174.Spagnuolo V., Guffanti M., Galli L., Poli A., Querini P.R., Ripa M., Clementi M., Scarpellini P., Lazzarin A., Tresoldi M., Dagna L., Zangrillo A., Ciceri F., Castagna A., Andolina A., Redaelli M.B., Baldissera E., Bigai G., Bigoloni A., Boffini N., Borio G., Bossolasco S., Bruzzesi E., Calabrò M.G., Calvisi S., Campochiaro C., Canetti D., Canti V., Castellani J., Castiglioni B., Cavalli G., Cavallo L., Cernuschi M., Chiurlo M., Cilla M., Cinel E., Cinque P., Conte C., Da Prat V., Danise A., De Lorenzo R., De Luca G., Dell’Acqua A., Dell’Acqua R., Della Torre E., Della Torre L., Di Terlizzi G., Dumea I., Farolfi F., Ferrante M., Frangi C., Fumagalli L., Gallina G., Germinario B., Gianotti N., Hasson H., Lalla F., Landoni G., Lanzillotta M., Voti R.L., Mastrangelo A., Messina E., Moizo E., Montagna M., Monti G., Morsica G., Muccini C., Nozza S., Oltolini C., Pascali M., Patrizi A., Pieri M., Prestifilippo D., Ramirez G., Ranzenigo M., Sapienza J., Sartorelli S., Seghi F., Tambussi G., Din C.T., Tomelleri A., Turi S., Uberti-Foppa C., Vinci C. Viral clearance after early corticosteroid treatment in patients with moderate or severe covid-19. Sci. Rep. 2020;10:1–7. doi: 10.1038/s41598-020-78039-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Aziz M., Fatima R., Assaly R. Elevated interleukin-6 and severe COVID-19: A meta-analysis. J. Med. Virol. 2020;92:2283–2285. doi: 10.1002/jmv.25948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pons S., Fodil S., Azoulay E., Zafrani L. The vascular endothelium: The cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit. Care. 2020;24:4–11. doi: 10.1186/s13054-020-03062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Toniati P., Piva S., Cattalini M., Garrafa E., Regola F., Castelli F., Franceschini F., Airò P., Bazzani C., Beindorf E.A., Berlendis M., Bezzi M., Bossini N., Castellano M., Cattaneo S., Cavazzana I., Contessi G.B., Crippa M., Delbarba A., De Peri E., Faletti A., Filippini M., Frassi M., Gaggiotti M., Gorla R., Lanspa M., Lorenzotti S., Marino R., Maroldi R., Metra M., Matteelli A., Modina D., Moioli G., Montani G., Muiesan M.L., Odolini S., Peli E., Pesenti S., Pezzoli M.C., Pirola I., Pozzi A., Proto A., Rasulo F.A., Renisi G., Ricci C., Rizzoni D., Romanelli G., Rossi M., Salvetti M., Scolari F., Signorini L., Taglietti M., Tomasoni G., Tomasoni L.R., Turla F., Valsecchi A., Zani D., Zuccalà F., Zunica F., Focà E., Andreoli L., Latronico N. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy. Autoimmun. Rev. 2020;19 doi: 10.1016/j.autrev.2020.102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Rosas I.O., Bräu N., Waters M., Go R.C., Hunter B.D., Bhagani S., Skiest D., Aziz M.S., Cooper N., Douglas I.S., Savic S., Youngstein T., Del Sorbo L., Cubillo Gracian A., De La Zerda D.J., Ustianowski A., Bao M., Dimonaco S., Graham E., Matharu B., Spotswood H., Tsai L., Malhotra A. Tocilizumab in Hospitalized Patients with Severe Covid-19 Pneumonia. N. Engl. J. Med. 2021;384:1503–1516. doi: 10.1056/nejmoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ikeda M., Nozaki A., Sugiyama K., Tanaka T., Naganuma A., Tanaka K., Sekihara H., Shimotohno K., Saito M., Kato N. Characterization of antiviral activity of lactoferrin against hepatitis C virus infection in human cultured cells. Virus Res. 2000;66:51–63. doi: 10.1016/S0168-1702(99)00121-5. [DOI] [PubMed] [Google Scholar]
- 180.El-Fakharany E.M. Nanoformulation of lactoferrin potentiates its activity and enhances novel biotechnological applications. Int. J. Biol. Macromol. 2020;165:970–984. doi: 10.1016/j.ijbiomac.2020.09.235. [DOI] [PubMed] [Google Scholar]
- 181.Redwan E.M., Uversky V.N., El-Fakharany E.M., Al-Mehdar H. Potential lactoferrin activity against pathogenic viruses. Comptes Rendus - Biol. 2014;337:581–595. doi: 10.1016/j.crvi.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 182.Campione E., Cosio T., Rosa L., Lanna C., Di Girolamo S., Gaziano R., Valenti P., Bianchi L. Lactoferrin as protective natural barrier of respiratory and intestinal mucosa against coronavirus infection and inflammation. Int. J. Mol. Sci. 2020;21:1–14. doi: 10.3390/ijms21144903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wakabayashi H., Oda H., Yamauchi K., Abe F. Lactoferrin for prevention of common viral infections. J. Infect. Chemother. 2014;20:666–671. doi: 10.1016/j.jiac.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 184.El-Fakharany E.M., El-Baky N.A., Linjawi M.H., Aljaddawi A.A., Saleem T.H., Nassar A.Y., Osman A., Redwan E.M. Influence of camel milk on the hepatitis C virus burden of infected patients. Exp. Ther. Med. 2017;13 doi: 10.3892/etm.2017.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Albar A.H., El-Fakharany E.M., Almehdar H.A., Uversky V.N., Redwan E.M. In vitro exploration of the anti-hcv potential of the synthetic spacer peptides derived from human, bovine, and camel lactoferrins. Protein Pept. Lett. 2017;24 doi: 10.2174/0929866524666161111111320. [DOI] [PubMed] [Google Scholar]
- 186.Clausen T.M., Sandoval D.R., Spliid C.B., Pihl J., Perrett H.R., Painter C.D., Narayanan A., Majowicz S.A., Kwong E.M., McVicar R.N., Thacker B.E., Glass C.A., Yang Z., Torres J.L., Golden G.J., Bartels P.L., Porell R.N., Garretson A.F., Laubach L., Feldman J., Yin X., Pu Y., Hauser B.M., Caradonna T.M., Kellman B.P., Martino C., Gordts P.L.S.M., Chanda S.K., Schmidt A.G., Godula K., Leibel S.L., Jose J., Corbett K.D., Ward A.B., Carlin A.F., Esko J.D. SARS-CoV-2 Infection Depends on Cellular Heparan Sulfate and ACE2. Cell. 2020;183:1043–1057.e15. doi: 10.1016/j.cell.2020.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Siqueiros-Cendón T., Arévalo-Gallegos S., Iglesias-Figueroa B.F., García-Montoya I.A., Salazar-Martínez J., Rascón-Cruz Q. Immunomodulatory effects of lactoferrin. Acta Pharm. Sin. 2014;35:557–566. doi: 10.1038/aps.2013.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Frioni A., Conte M.P., Cutone A., Longhi C., Musci G., Di Patti M.C.B., Natalizi T., Marazzato M., Lepanto M.S., Puddu P., Paesano R., Valenti P., Berlutti F. Lactoferrin differently modulates the inflammatory response in epithelial models mimicking human inflammatory and infectious diseases. BioMetals. 2014;27:843–856. doi: 10.1007/s10534-014-9740-9. [DOI] [PubMed] [Google Scholar]
- 189.Salaris C., Scarpa M., Elli M., Bertolini A., Guglielmetti S., Pregliasco F., Blandizzi C., Brun P., Castagliuolo I. Protective effects of lactoferrin against sars-cov-2 infection in vitro. Nutrients. 2021;13:1–12. doi: 10.3390/nu13020328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Campione E., Lanna C., Cosio T., Rosa L., Conte M.P., Iacovelli F., Romeo A., Falconi M., Del Vecchio C., Franchin E., Lia M.S., Minieri M., Chiaramonte C., Ciotti M., Nuccetelli M., Terrinoni A., Iannuzzi I., Coppeda L., Magrini A., Bernardini S., Sabatini S., Rosapepe F., Bartoletti P.L., Moricca N., Di Lorenzo A., Andreoni M., Sarmati L., Miani A., Piscitelli P., Valenti P., Bianchi L. Lactoferrin Against SARS-CoV-2: In Vitro and In Silico Evidences. Front. Pharmacol. 2021;12:1–10. doi: 10.3389/fphar.2021.666600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Campione E., Lanna C., Cosio T., Rosa L., Conte M.P., Iacovelli F., Romeo A., Falconi M., Del Vecchio C., Franchin E., Lia M.S., Minieri M., Chiaramonte C., Ciotti M., Nuccetelli M., Terrinoni A., Iannuzzi I., Coppeta L., Magrini A., Bernardini S., Sabatini S., Rosapepe F., Bartoletti P.L., Moricca N., Di Lorenzo A., Andreoni M., Sarmati L., Miani A., Piscitelli P., Squillaci E., Valenti P., Bianchi L. Lactoferrin as antiviral treatment in COVID-19 management: Preliminary evidence. Int. J. Environ. Res. Public Health. 2021;18:1–15. doi: 10.3390/ijerph182010985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Miesbach W., Makris M. COVID-19: Coagulopathy, Risk of Thrombosis, and the Rationale for Anticoagulation. Clin. Appl. Thromb. /Hemost. 2020;26 doi: 10.1177/1076029620938149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Arachchillage D.R.J., Laffan M. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18:1233–1234. doi: 10.1111/jth.14820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sadd C., Rowe T., Nazeef M., Kory P., Sultan S., Faust H. Thromboelastography to Detect Hypercoagulability and Reduced Fibrinolysis in Coronavirus Disease 2019 Acute Respiratory Distress Syndrome Patients. Crit. Care Explor. 2020;2 doi: 10.1097/CCE.0000000000000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chandra A., Chakraborty U., Ghosh S., Dasgupta S. Anticoagulation in COVID-19: Current concepts and controversies. Postgrad. Med. J. 2021:1–8. doi: 10.1136/postgradmedj-2021-139923. [DOI] [PubMed] [Google Scholar]
- 196.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Godino C., Scotti A., Maugeri N., Mancini N., Fominskiy E., Margonato A., Landoni G. Antithrombotic therapy in patients with COVID-19? -Rationale and Evidence. Int J. Cardiol. 2021;324:261–266. doi: 10.1016/j.ijcard.2020.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Negri E.M., Piloto B.M., Morinaga L.K., Jardim C.V.P., Lamy S.A.E.-D., Ferreira M.A., D’Amico E.A., Deheinzelin D. Heparin Therapy Improving Hypoxia in COVID-19 Patients - A Case Series. Front Physiol. 2020;11 doi: 10.3389/fphys.2020.573044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Shi C., Wang C., Wang H., Yang C., Cai F., Zeng F., Cheng F., Liu Y., Zhou T., Deng B., Vlodavsky I., Li J.P., Zhang Y. The Potential of Low Molecular Weight Heparin to Mitigate Cytokine Storm in Severe COVID-19 Patients: A Retrospective Cohort Study. Clin. Transl. Sci. 2020;13:1087–1095. doi: 10.1111/cts.12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Rentsch C.T., Beckman J.A., Tomlinson L., Gellad W.F., Alcorn C., Kidwai-Khan F., Skanderson M., Brittain E., King J.T., Ho Y.L., Eden S., Kundu S., Lann M.F., Greevy R.A., Ho P.M., Heidenreich P.A., Jacobson D.A., Douglas I.J., Tate J.P., Evans S.J.W., Atkins D., Justice A.C., Freiberg M.S. Early initiation of prophylactic anticoagulation for prevention of coronavirus disease 2019 mortality in patients admitted to hospital in the United States: Cohort study. BMJ. 2021;372 doi: 10.1136/bmj.n311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Santoro F., Nuñez-Gil I.J., Vitale E., Viana-Llamas M.C., Reche-Martinez B., Romero-Pareja R., Feltez Guzman G., Fernandez Rozas I., Uribarri A., Becerra-Muñoz V.M., Alfonso-Rodriguez E., Garcia-Aguado M., Huang J., Ortega-Armas M.E., Garcia Prieto J.F., Corral Rubio E.M., Ugo F., Bianco M., Mulet A., Raposeiras-Roubin S., Jativa Mendez J.L., Espejo Paeres C., Albarrán A.R., Marín F., Guerra F., Akin I., Cortese B., Ramakrishna H., Macaya C., Fernandez-Ortiz A., Brunetti N.D. Antiplatelet therapy and outcome in COVID-19: the Health Outcome Predictive Evaluation Registry. Heart. 2022;108:130–136. doi: 10.1136/heartjnl-2021-319552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Murni I.K., Prawirohartono E.P., Triasih R. Potential Role of Vitamins and Zinc on Acute Respiratory Infections Including Covid-19. Glob. Pediatr. Health. 2021;8 doi: 10.1177/2333794X211021739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Gammoh N.Z., Rink L. Zinc Infect. Inflamm. 2017 doi: 10.3390/nu9060624. [DOI] [Google Scholar]
- 204.Read S.A., Obeid S., Ahlenstiel C., Ahlenstiel G. Role Zinc Antivir. Immun. 2019:696–710. doi: 10.1093/advances/nmz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Pecora F., Persico F., Argentiero A., Neglia C., Esposito S. The role of micronutrients in support of the immune response against viral infections. Nutrients. 2020;12:1–45. doi: 10.3390/nu12103198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wakabayashi H., Oda H., Yamauchi K., Abe F. Lactoferrin for prevention of common viral infections. J. Infect. Chemother. 2014;20:666–671. doi: 10.1016/j.jiac.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 207.Lubberts E. Vitam. D. Autoimmun.: Mol. Mech. Ther. Potential. 2017;7 doi: 10.3389/fimmu.2016.00697. [DOI] [Google Scholar]
- 208.Pecora F., Persico F., Argentiero A., Neglia C., Esposito S. The role of micronutrients in support of the immune response against viral infections. Nutrients. 2020;12:1–45. doi: 10.3390/nu12103198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.G. Costagliola, E. Spada, P. Comberiati, D.G. Peroni, Could nutritional supplements act as therapeutic adjuvants in COVID-19 ?, 0 (2021) 1–5. [DOI] [PMC free article] [PubMed]
- 210.Di G.E.C.V., Quaranta L.V.N., Buonamico A.Z.E., Palumbo E.C.A. Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID - 19. J. Endocrinol. Investig. 2021;44:765–771. doi: 10.1007/s40618-020-01370-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.G. Costagliola, E. Spada, P. Comberiati, D.G. Peroni, Could nutritional supplements act as therapeutic adjuvants in COVID-19 ?, 0 (2021) 1–5. [DOI] [PMC free article] [PubMed]
- 212.Butler-Laporte G., Nakanishi T., Mooser V., Morrison D.R., Abdullah T., Adeleye O., Mamlouk N., Kimchi N., Afrasiabi Z., Rezk N., Giliberti A., Renieri A., Chen Y., Zhou S., Forgetta V., Brent Richards J. Vitamin D and COVID-19 susceptibility and severity in the COVID-19 host genetics initiative: A Mendelian randomization study. PLoS Med. 2021;18:1–14. doi: 10.1371/journal.pmed.1003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Desai A.P., Dirajlal-Fargo S., Durieux J.C., Tribout H., Labbato D., McComsey G.A. Vitamin K & D Deficiencies are independently associated with COVID-19 disease severity. Open Forum Infect. Dis. 2021;8:1–8. doi: 10.1093/ofid/ofab408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Thomas S., Patel D., Bittel B., Wolski K., Wang Q., Kumar A., Il’Giovine Z.J., Mehra R., McWilliams C., Nissen S.E., Desai M.Y. Effect of High-Dose Zinc and Ascorbic Acid Supplementation vs Usual Care on Symptom Length and Reduction among Ambulatory Patients with SARS-CoV-2 Infection: The COVID A to Z Randomized Clinical Trial. JAMA Netw. Open. 2021;4:1–10. doi: 10.1001/jamanetworkopen.2021.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Murni I.K., Prawirohartono E.P., Triasih R. Potential Role of Vitamins and Zinc on Acute Respiratory Infections Including Covid-19. Glob. Pediatr. Health. 2021;8 doi: 10.1177/2333794X211021739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Pau A.K., Aberg J., Baker J., Belperio P.S., Coopersmith C., Crew P., Grund B., Gulick R.M., Harrison C., Kim A., Lane H.C., Masur H., Sheikh V., Singh K., Yazdany J., Tebas P. Convalescent Plasma for the Treatment of COVID-19: Perspectives of the National Institutes of Health COVID-19 Treatment Guidelines Panel. Ann. Intern Med. 2021;174:93–95. doi: 10.7326/M20-6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Devasenapathy N., Ye Z., Loeb M., Fang F., Najafabadi B.T., Xiao Y., Couban R., Bégin P., Guyatt G. Efficacy and safety of convalescent plasma for severe COVID-19 based on evidence in other severe respiratory viral infections: a systematic review and meta-analysis. Cmaj. 2020;192:E745–E755. doi: 10.1503/cmaj.200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Li G., Chen X., Xu A. Profile of specific antibodies to the SARS-associated coronavirus. N. Engl. J. Med. 2003;349:508–509. doi: 10.1056/NEJM200307313490520. [DOI] [PubMed] [Google Scholar]
- 219.Khan T.N.S., Mukry S.N., Masood S., Meraj L., Devrajani B.R., Akram J., Fatima N., Maqsood S., Mahesar A., Siddiqui R., Ishaque S., Afzal M.B., Mukhtar S., Ahmed S., Naz A., Shamsi T.S. Usefulness of convalescent plasma transfusion for the treatment of severely ill COVID-19 patients in Pakistan. BMC Infect. Dis. 2021;21 doi: 10.1186/s12879-021-06451-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Seshan J., Dube S.K., Rajagopalan V., Panda P.S., Rath G.P. Convalescent Plasma Therapy for COVID-19: Current Status and Future Directions. J. Neuroanaesth. Crit. Care. 2020;7:140–147. doi: 10.1055/s-0040-1716594. [DOI] [Google Scholar]
- 221.Duan K., Liu B., Li C., Zhang H., Yu T., Qu J., Zhou M., Chen L., Meng S., Hu Y., Peng C., Yuan M., Huang J., Wang Z., Yu J., Gao X., Wang D., Yu X., Li L., Zhang J., Wu X., Li B., Xu Y., Chen W., Peng Y., Hu Y., Lin L., Liu X., Huang S., Zhou Z., Zhang L., Wang Y., Zhang Z., Deng K., Xia Z., Gong Q., Zhang W., Zheng X., Liu Y., Yang H., Zhou D., Yu D., Hou J., Shi Z., Chen S., Chen Z., Zhang X., Yang X. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117/-/DCSupplemental. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Bégin P., Callum J., Jamula E., Cook R., Heddle N.M., Tinmouth A., Zeller M.P., Beaudoin-Bussières G., Amorim L., Bazin R., Loftsgard K.C., Carl R., Chassé M., Cushing M.M., Daneman N., Devine D.V., Dumaresq J., Fergusson D.A., Gabe C., Glesby M.J., Li N., Liu Y., McGeer A., Robitaille N., Sachais B.S., Scales D.C., Schwartz L., Shehata N., Turgeon A.F., Wood H., Zarychanski R., Finzi A., Marceau D., Huang A., Carr H., Lin Y., Lall R., Graham C., Arsenault C., Sales V., Sidhu D., Semret M., Hamm C., Arhanchiague E., Solh Z., Srour N., Soliman K., Yee C., Laroche V., Nahirniak S., Greenaway C., Pai M., Côté A., Tsang J.L.Y., Cserti-Gazdewich C., Talbot D., Poulin S., Guimaraes R., Rushton-Marovac M., Langlois A., Ning S., Shih A., Boileau M., Singh H., Ledingham D., Ponnampalam A., Yan M., Prokopchuk-Gauk O., Poirier A., Girouard G., Pavenski K., Drouin O., Harris D., Durand M., Rimmer E., Ovakim D., Ménard F., Cuccarolo G., Carruthers J., Lucier K., Auclair M.C., Avram M., Brassard M., Cerro S., Martinez V., Morin J., Saint-Jacques M., Veillette M., Armali C., Kron A., Modi D., Duncan J., Justumus P., John M.S., St-Onge G., Hadzi-Tosev M., Dion P.M., McGillivary L., de Moulliac A.V., Nyman S.A., Perilli S., Van Vliet P.J., Lane S., Pavenski K., Pereira R., Sirotich E., Abelson J., Greene S., Khandelwal A., Thakar S., Longo S., Anand S.P., Benlarbi M., Bourassa C., Boutin M., Descôteaux-Dinelle J., Gendron-Lepage G., Goyette G., Laumaea A., Medjahed H., Prévost J., Richard J., Kaufmann D., Brunet-Ratnasingham E., Chaumont N., Drebot M., Robinson A., Mendoza E., Dimitrova K., Manguiat K., Phillipson C., Chan M., Evans D., Lin J., Boyer L., Cloutier M., Drouin M., Ducas É., Dussault N., Fournier M.J., Landy P., Nolin M.È., Perreault J., Tremblay T., Nazy I., Xie F., Liu D., Wong M., Silverio G., Walkus K., Barton M., Haveman K., Mueller D., Scott A., Moher M., Wood G., Roarty T., Auld F., Carney G., Thomson V., Onell R., Walley K., Donohoe K., Brunk C., Hernandez G., Jacobucci T., Lazosky L., Mann P., Raval G., Zampieri L.A., Sekhon M., Wright A., James N., Chang G., Chen R., Deol K., Gantioqui J., Larsen E., Ramdin N., Roche M., Rosinski K., Sham L., Storms M., Gillrie M., Mahe E., Suryanarayan D., Ugarte-Torres A., Robinson T., Gibbs M., Hewsgirard J., Holmes M., McCarthy J., Ody M., Doucette K., Sligl W., Sonpar A., Robertson K., Narayan J., Ravindran L., Stewart B., Zapernick L., Lee S., Sy E., Wong A., Gryzb K., Craddock S., Fuchs D., Myrah D., Sunny S., Harding S.R., Kogilwaimath S., Hodgson N., Johnson D., Meier S., Thomson K., Heendeniya A., Houston B., Kenyan Y., Lother S., Olafson K., Rush B., Wuerz T., Solvason D., Albensi L., Alias S., Choi N., Curtis L., Hutmacher M., Kashani H., Lane D., Marten N., Pronyk-Ward T., Rigaux L., Silva R., Tays Q., Naidu R., Mathews J., Mai M., Miceli V., Molson L., Radhakrishnan G., Schaefer L., Haddad M., Landry S., Chernish R., Kruisselbrink R., Liu T., Jeromin J., Siddiqui A., Girolametto C., Krokoszynski K., Main C., Fox-Robichaud A., Rochwerg B., Kruja E., Ellingham D., Sampat D., Tang N., Leto D., Karunakaran M., Ricciuto D., Fusco K., Ghate T., Robinson H., Ball I., Shalhoub S., Slessarev M., Silverman M., Nano E., Bentall T., Campbell E., Kinney J., Parvathy S., Fera E., La Delfa A., Nadarajah J., Solow H., Mendoza E., Engel K., Monaco D., Kononow L., Suntharalingam S., Fralick M., Munshi L., Saeed S., Hajjaj O., Hsu E., Ali K., Duan E., Farjou G., Jenson L., Salib M., Patterson L., Anant S., Ding J., Jomy J., Das P., Geagea A., Ingber S., Owen E., Lostun A., Albano T., Chatterjee A., Giraldo M., Hickey J., Lee I., Okada N., Pasquale N., Ponzielli R., Rahmat M., Sabur S., Schlag M., Aguiar L., Damani A., Hong S., Kokabi M., Perkins C., Cowan J., Giulivi T., MacFadden D., Cyr J., Pecarskie A., Porteous R., Vedder P.O., Watpool I., Berardi P., Bustani L., Graver A., Iyengar A., Kisilewicz M., Majewski J., Marovac M., Murthy R., Sharma K., Walcer M., Chagla Z., Cheung J., Duan E., Clarke F., Matic K., Giraldo M., Hickey J., Lee I., Okada N., Pasquale N., Ponzielli R., Rahmat M., Sabur S., Schlag M., Carpenter T., Schwartz K., Suthar P., Jiwajee A., Lindsay D., Malik A., Tse B., Matukas L., Ray J., Bell S., Krok E., Guo R., John S., Joshi V., Keen J., Lazongas C., Ostro J., Shore K., Wang J., Choi J., Nallapati P., Irwin T., Wang V., Sheldrake P., Adhikari N., Wunsch H., Bailey J., Meirovich H., Colavecchia C., Kahwash E., Sud S., Romano M., Coburn B., Del Sorbo L., Granton J., Husain S., Pendergrast J., Sharkawy A., Wilcox L., Saeed S., Hajjaj O., Kulikova M., Massin S., Kennette W., Mazzetti I., Naccarato K., Park G., Pennetti A., Primeau C., Vilag C., Lapointe Y., Lemay A.S., Duceppe E., Rioux-Massé B., Tremblay C., Arlotto P., Bouchard C., Matte S., Messier-Peet M., Francoeur C.L., Lauzier F., Leblanc G., Bellemare D., Cloutier È., Costerousse O., Chénard É.C., Daher R., Daigle M., Grenier S., Guilbeault G., Rioux M.P., St-Onge M., Tremblay A., Beaudoin B., Lanthier L., Larrivée P., Morin P.A., Carbonneau É., Lacasse R., Autmizguine J., Boucoiran I., Du Pont-Thibodeau G., La Haye A., Lague V., Léveillé K., Quach-Thanh C., Émériaud G., Jouvet P., Haddad É., Turgeon-Provost C., Fox S., Baldé D., Ménard L., Morissette S., Schnorr-Meloche M., Turcotte A.A., Vallée C., Castonguay S., Nguyen T., Rivest N., Roussos M., Simoneau E., Belecciu A., Bouchard M.H., Daviau E., Martin C., Sabourin N., Tremblay S., Gagné É., Gagné N.L., Larouche J., Larouche V., Tremblay V., Tremblay V., Blanchette P., Claveau D., Lamarre M., Tapps D., Albert M., Duca A., Leduc J.M., Boudreault-Pedneault J.S., Barsalou A., Deschênes-Dion S., Ibrahim S., Ridyard S., Rousseau J., Ahern S., Arsenault M.P., Dufresne S.F., Mollica L., Wang H.T., Beau S., Beaupré D., Dégarie M., Delorme I., Farkas M., Gratton M.O., Guertin A., Jalbert G., Meilleur M., Labrecque C.R., Santos É., Lu J.T., Auger J., Lessard M.C., Mardini L., Pesant Y., Delves L., Delves L., Denault S., Grigorova S., Lambert M., Langille N., Langlois C., Rock C., Sardin-Laframboise Y., Archambault P., Bélanger-Pelletier J., Duquet-Deblois E., Dupuis-Picard V., Hamelin Y., Leduc S., Richard M., Fortin M., Gervais P., Boulay M.È., Ferland C., Guertin J., Lepage J., Roy A., Assouline S., Caplan S., Kong L., Canticas C., Mayhew C., Ouedraogo J., Tep T.S., Batist G., Cheng M., Klein M., Kronfli N., Pelletier P., Qureshi S., Vinh D., Dziarmaga R., Peiris H., Proulx-Boucher K., Roger J., Rothschild M.A., Yuen C.Y., Barkati S., Routy J.P., Sinanan-Pelletier S., LeBlanc R., St-Hilaire E., Thibeault P., Morin K., Caissie G., Collette J.C., Daigle L., Daigle M., Gendron B., Godin N., Lapointe A., Moreau G., Ouellette-Bernier L., Rockburn J., Sonier-Ferguson B., Wilson C., DeSimone R., Ellsworth G., Fry R., Goss N., Gulick R., Vaamonde C., Wilkin T., Arar C., Berardi J., Chen D., Garcia-Miller C., Goldbach A., Gripp L., Hayden D., Kane K., Li J., Marcelin K.A., Megill C., Nelson M., Paguntalan A., Raab G., Resso G., Rosario R., Rossen N., Tamura S., Zhao E., Goss C., Kim Y., Patel E., Paul S., Romero T., ElBadri N., Flores L., Sandoval T., Kapadia S., Vasovic L., Griffiths S.K., Alvarado D., Goudy F., Lewis M., Loizou M., Louie R., Pambrun C., Torrance S., Drews S., McManus J., Cuevas O., Lafresne W., Ruoso P., Shin C., Steed T., Ward R., Allard I., Germain M., Girard S., Parent É., Pigeon C.M., Lopes M.E., Pêcego M., Rosario N., da Costa Silva C.A., Oliveira T., Lopes M.C., Mateos S., Hall L., Paradiso S., Strauss D., Arnold D.M. Convalescent plasma for hospitalized patients with COVID-19: an open-label, randomized controlled trial. Nat. Med. 2021;27:2012–2024. doi: 10.1038/s41591-021-01488-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Axfors C., Janiaud P., Schmitt A.M., van’t Hooft J., Smith E.R., Haber N.A., Abayomi A., Abduljalil M., Abdulrahman A., Acosta-Ampudia Y., Aguilar-Guisado M., Al-Beidh F., Alejandria M.M., Alfonso R.N., Ali M., AlQahtani M., AlZamrooni A., Anaya J.M., Ang M.A.C., Aomar I.F., Argumanis L.E., Averyanov A., Baklaushev V.P., Balionis O., Benfield T., Berry S., Birocco N., Bonifacio L.B., Bowen A.C., Bown A., Cabello-Gutierrez C., Camacho B., Camacho-Ortiz A., Campbell-Lee S., Cao D.H., Cardesa A., Carnate J.M., Castillo G.J.J., Cavallo R., Chowdhury F.R., Chowdhury F.U.H., Ciccone G., Cingolani A., Climacosa F.M.M., Compernolle V., Cortez C.F.N., Costa Neto A., D’Antico S., Daly J., Danielle F., Davis J.S., De Rosa F.G., Denholm J.T., Denkinger C.M., Desmecht D., Díaz-Coronado J.C., Díaz Ponce-Medrano J.A., Donneau A.F., Dumagay T.E., Dunachie S., Dungog C.C., Erinoso O., Escasa I.M.S., Estcourt L.J., Evans A., Evasan A.L.M., Fareli C.J., Fernandez-Sanchez V., Galassi C., Gallo J.E., Garcia P.J., Garcia P.L., Garcia J.A., Garigliany M., Garza-Gonzalez E., Gauiran D.T.V., Gaviria García P.A., Giron-Gonzalez J.A., Gómez-Almaguer D., Gordon A.C., Gothot A., Grass Guaqueta J.S., Green C., Grimaldi D., Hammond N.E., Harvala H., Heralde F.M., Herrick J., Higgins A.M., Hills T.E., Hines J., Holm K., Hoque A., Hoste E., Ignacio J.M., Ivanov A.V., Janssen M., Jennings J.H., Jha V., King R.A.N., Kjeldsen-Kragh J., Klenerman P., Kotecha A., Krapp F., Labanca L., Laing E., Landin-Olsson M., Laterre P.F., Lim L.L., Lim J., Ljungquist O., Llaca-Díaz J.M., López-Robles C., López-Cárdenas S., Lopez-Plaza I., Lucero J.A.C., Lundgren M., Macías J., Maganito S.C., Malundo A.F.G., Manrique R.D., Manzini P.M., Marcos M., Marquez I., Martínez-Marcos F.J., Mata A.M., McArthur C.J., McQuilten Z.K., McVerry B.J., Menon D.K., Meyfroidt G., Mirasol M.A.L., Misset B., Molton J.S., Mondragon A.V., Monsalve D.M., Moradi Choghakabodi P., Morpeth S.C., Mouncey P.R., Moutschen M., Müller-Tidow C., Murphy E., Najdovski T., Nichol A.D., Nielsen H., Novak R.M., O’Sullivan M.V.N., Olalla J., Osibogun A., Osikomaiya B., Oyonarte S., Pardo-Oviedo J.M., Patel M.C., Paterson D.L., Peña-Perez C.A., Perez-Calatayud A.A., Pérez-Alba E., Perkina A., Perry N., Pouladzadeh M., Poyato I., Price D.J., Quero A.K.H., Rahman M.M., Rahman M.S., Ramesh M., Ramírez-Santana C., Rasmussen M., Rees M.A., Rego E., Roberts J.A., Roberts D.J., Rodríguez Y., Rodríguez-Baño J., Rogers B.A., Rojas M., Romero A., Rowan K.M., Saccona F., Safdarian M., Santos M.C.M., Sasadeusz J., Scozzari G., Shankar-Hari M., Sharma G., Snelling T., Soto A., Tagayuna P.Y., Tang A., Tatem G., Teofili L., Tong S.Y.C., Turgeon A.F., Veloso J.D., Venkatesh B., Ventura-Enriquez Y., Webb S.A., Wiese L., Wikén C., Wood E.M., Yusubalieva G.M., Zacharowski K., Zarychanski R., Khanna N., Moher D., Goodman S.N., Ioannidis J.P.A., Hemkens L.G. Vol. 21. 2021. Association between convalescent plasma treatment and mortality in COVID-19: a collaborative systematic review and meta-analysis of randomized clinical trials; pp. 1–23. (BMC Infectious Diseases). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.W.H. Organization, Therapeutics and COVID-19, 2021.
- 225.Zhao Q., He Y. Challenges of Convalescent Plasma Therapy on COVID-19. J. Clin. Virol. 2020;127 doi: 10.1016/j.jcv.2020.104358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.de Candia P., Prattichizzo F., Garavelli S., la Grotta R., de Rosa A., Pontarelli A., Parrella R., Ceriello A., Matarese G. Effect of time and titer in convalescent plasma therapy for COVID-19. IScience. 2021;24 doi: 10.1016/j.isci.2021.102898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Thompson M.A., Henderson J.P., Shah P.K., Rubinstein S.M., Joyner M.J., Choueiri T.K., Flora D.B., Griffiths E.A., Gulati A.P., Hwang C., Koshkin V.S., Papadopoulos E.B., Robilotti E.V., Su C.T., Wulff-Burchfield E.M., Xie Z., Yu P.P., Mishra S., Senefeld J.W., Shah D.P., Warner J.L. Association of Convalescent Plasma Therapy with Survival in Patients with Hematologic Cancers and COVID-19. JAMA Oncol. 2021;7:1167–1175. doi: 10.1001/jamaoncol.2021.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Marovich M., Mascola J.R., Cohen M.S. Monoclonal antibodies for prevention and treatment of COVID-19. JAMA. 2020;324:131–132. doi: 10.1001/jama.2020.10245. [DOI] [PubMed] [Google Scholar]
- 229.Petrosillo N., Viceconte G., Ergonul O., Ippolito G., Petersen E. COVID-19, SARS and MERS: are they closely related? Clin. Microbiol. Infect. 2020;26:729–734. doi: 10.1016/j.cmi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Saghazadeh A., Rezaei N. Towards treatment planning of COVID-19: Rationale and hypothesis for the use of multiple immunosuppressive agents: Anti-antibodies, immunoglobulins, and corticosteroids. Int. Immunopharmacol. 2020;84 doi: 10.1016/j.intimp.2020.106560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Hwang Y.-C., Lu R.-M., Su S.-C., Chiang P.-Y., Ko S.-H., Ke F.-Y., Liang K.-H., Hsieh T.-Y., Wu H.-C. Monoclonal antibodies for COVID-19 therapy and SARS-CoV-2 detection. J. Biomed. Sci. 2022;29:1–50. doi: 10.1186/s12929-021-00784-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Baum A., Fulton B.O., Wloga E., Copin R., Pascal K.E., Russo V., Giordano S., Lanza K., Negron N., Ni M., Wei Y., Atwal G.S., Murphy A.J., Stahl N., Yancopoulos G.D., Kyratsous C.A. Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science. 1979;369(2020):1014–1018. doi: 10.1126/science.abd0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Weinreich D.M., Sivapalasingam S., Norton T., Ali S., Gao H., Bhore R., Musser B.J., Soo Y., Rofail D., Im J., Perry C., Pan C., Hosain R., Mahmood A., Davis J.D., Turner K.C., Hooper A.T., Hamilton J.D., Baum A., Kyratsous C.A., Kim Y., Cook A., Kampman W., Kohli A., Sachdeva Y., Graber X., Kowal B., DiCioccio T., Stahl N., Lipsich L., Braunstein N., Herman G., Yancopoulos G.D. REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19. N. Engl. J. Med. 2021;384:238–251. doi: 10.1056/nejmoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Razonable R.R., Pawlowski C., O’Horo J.C., Arndt L.L., Arndt R., Bierle D.M., Borgen M.D., Hanson S.N., Hedin M.C., Lenehan P., Puranik A., Seville M.T., Speicher L.L., Tulledge-Scheitel S.M., Venkatakrishnan A., Wilker C.G., Badley A.D., Ganesh R. Casirivimab–Imdevimab treatment is associated with reduced rates of hospitalization among high-risk patients with mild to moderate coronavirus disease-19. EClinicalMedicine. 2021;40 doi: 10.1016/j.eclinm.2021.101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Rosas I.O., Bräu N., Waters M., Go R.C., Hunter B.D., Bhagani S., Skiest D., Aziz M.S., Cooper N., Douglas I.S., Savic S., Youngstein T., Del Sorbo L., Cubillo Gracian A., De La Zerda D.J., Ustianowski A., Bao M., Dimonaco S., Graham E., Matharu B., Spotswood H., Tsai L., Malhotra A. Tocilizumab in Hospitalized Patients with Severe Covid-19 Pneumonia. N. Engl. J. Med. 2021;384:1503–1516. doi: 10.1056/nejmoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Sparrow E., Friede M., Torvaldsen S. Ther. antibodies Infect. Dis. 2017:235–237. doi: 10.2471/BLT.16.178061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Challen R., Brooks-Pollock E., Read J.M., Dyson L., Tsaneva-Atanasova K., Danon L. Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: Matched cohort study. BMJ. 2021;372:1–10. doi: 10.1136/bmj.n579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Kumar A., Dowling W.E., Román R.G., Chaudhari A., Gurry C., Le T.T., Tollefson S., Clark C.E., Bernasconi V., Kristiansen P.A. Status Report on COVID-19 Vaccines Development. Curr. Infect. Dis. Rep. 2021;23:1–2. doi: 10.1007/s11908-021-00752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Petersen E., Ntoumi F., Hui D.S., Abubakar A., Kramer L.D., Obiero C., Tambyah P.A., Blumberg L., Yapi R., Al-Abri S., de T., Pinto C.A., Yeboah-Manu D., Haider N., Asogun D., Velavan T.P., Kapata N., Bates M., Ansumana R., Montaldo C., Mucheleng’anga L., Tembo J., Mwaba P., Himwaze C.M., Hamid M.M.A., Mfinanga S., Mboera L., Raj T., Aklillu E., Veas F., Edwards S., Kaleebu P., McHugh T.D., Chakaya J., Nyirenda T., Bockarie M., Nyasulu P.S., Wejse C., Muyembe-Tamfum J.-J., Azhar E.I., Maeurer M., Nachega J.B., Kock R., Ippolito G., Zumla A. Emergence of new SARS-CoV-2 Variant of Concern Omicron (B.1.1.529) - highlights Africa’s research capabilities, but exposes major knowledge gaps, inequities of vaccine distribution, inadequacies in global COVID-19 response and control efforts. Int. J. Infect. Dis. 2022;114:268–272. doi: 10.1016/j.ijid.2021.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Geers D., Shamier M.C., Bogers S., den Hartog G., Gommers L., Nieuwkoop N.N., Schmitz K.S., Rijsbergen L.C., van Osch J.A.T., Dijkhuizen E., Smits G., Comvalius A., van Mourik D., Caniels T.G., van Gils M.J., Sanders R.W., Oude Munnink B.B., Molenkamp R., de Jager H.J., Haagmans B.L., de Swart R.L., Koopmans M.P.G., van Binnendijk R.S., de Vries R.D., GeurtsvanKessel C.H. SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees. Sci. Immunol. 2021;6:1–15. doi: 10.1126/sciimmunol.abj1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Song S., Zhou B., Cheng L., Liu W., Fan Q., Ge X., Peng H., Fu Y.-X., Ju B., Zhang Z. Sequential immunization with SARS-CoV-2 RBD vaccine induces potent and broad neutralization against variants in mice. Virol. J. 2022;19:1–5. doi: 10.1186/s12985-021-01737-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Simnani F.Z., Singh D., Kaur R. COVID-19 phase 4 vaccine candidates, effectiveness on SARS-CoV-2 variants, neutralizing antibody, rare side effects, traditional and nano-based vaccine platforms: a review. 3 Biotech. 2022;12:1–30. doi: 10.1007/s13205-021-03076-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zhang J., Xiao T., Cai Y., Lavine C.L., Peng H., Zhu H., Anand K., Tong P., Gautam A., Mayer M.L., Walsh R.M., Rits-Volloch S., Wesemann D.R., Yang W., Seaman M.S., Lu J., Chen B. Membrane fusion and immune evasion by the spike protein of SARS-CoV-2 Delta variant. Science. 1979;374(2021):1353–1360. doi: 10.1126/science.abl9463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Garcia-Beltran W.F., St Denis K.J., Hoelzemer A., Lam E.C., Nitido A.D., Sheehan M.L., Berrios C., Ofoman O., Chang C.C., Hauser B.M., Feldman J., Gregory D.J., Poznansky M.C., Schmidt A.G., Iafrate A.J., Naranbhai V., Balazs A.B. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell. 2021:1–10. doi: 10.1101/2021.12.14.21267755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.B.J. Willett, J. Grove, O.A. Maclean, C. Wilkie, N. Logan, G. De Lorenzo, W. Furnon, S. Scott, M. Manali, A. Szemiel, S. Ashraf, The hyper-transmissible SARS-CoV-2 Omicron variant exhibits significant antigenic change, vaccine escape and a switch in cell entry mechanism, MedRxiv. (2022).
- 246.Nyberg T., Ferguson N.M., Nash S.G., Webster H.H., Flaxman S., Andrews N., Hinsley W., Bernal J.L., Kall M., Bhatt S., Blomquist P., Zaidi A., Volz E., Aziz N.A., Harman K., Funk S., Abbott S., Lopez Bernal J., Abdul Aziz N., Hope R., Charlett A., Chand M., Ghani A.C., Seaman S.R., Dabrera G., de Angelis D., Presanis A.M., Thelwall S. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399:1303–1312. doi: 10.1016/S0140-6736(22)00462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Vangeel L., Chiu W., de Jonghe S., Maes P., Slechten B., Raymenants J., André E., Leyssen P., Neyts J., Jochmans D. Remdesivir, Molnupiravir and Nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern. Antivir. Res. 2022;198 doi: 10.1016/j.antiviral.2022.105252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Marco Cascella A., Rajnik M., Cuomo A., Dulebohn S.C., di Napoli R. StatPearls Publishing,; 2022. Features, Evaluation, and Treatment of Coronavirus (COVID-19). Statpearls [internet]〈https://www.ncbi.nlm.nih.gov/books/NBK554776/?report=printable〉 [PubMed] [Google Scholar]
- 249.Li P., Wang Y., Lavrijsen M., Lamers M.M., de Vries A.C., Rottier R.J., Bruno M.J., Peppelenbosch M.P., Haagmans B.L., Pan Q. SARS-CoV-2 Omicron variant is highly sensitive to molnupiravir, nirmatrelvir, and the combination. Cell Res. 2022;32:322–324. doi: 10.1038/s41422-022-00618-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Cavazzoni P. US Food and Drug Administration,; 2022. Coronavirus (COVID-19) update: FDA limits use of certain monoclonal antibodies to treat COVID-19 due to the Omicron variant.〈https://www.fda.gov/news-〉 [Google Scholar]
- 251.Tao K., Tzou P.L., Kosakovsky Pond S.L., Ioannidis J.P.A., Shafer R.W. Susceptibility of SARS-CoV-2 Omicron Variants to Therapeutic Monoclonal Antibodies: Systematic Review and Meta-analysis. Microbiol. Spectr. 2022 doi: 10.1128/spectrum.00926-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Sukhatme V.P., Reiersen A.M., Vayttaden S.J., Sukhatme V.V. Fluvoxamine: A Review of Its Mechanism of Action and Its Role in COVID-19. Front. Pharmacol. 2021;12:1–9. doi: 10.3389/fphar.2021.652688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Reis G., dos Santos Moreira-Silva E.A., Silva D.C.M., Thabane L., Milagres A.C., Ferreira T.S., dos Santos C.V.Q., de Souza Campos V.H., Nogueira A.M.R., de Almeida A.P.F.G., Callegari E.D., de Figueiredo Neto A.D., Savassi L.C.M., Simplicio M.I.C., Ribeiro L.B., Oliveira R., Harari O., Forrest J.I., Ruton H., Sprague S., McKay P., Glushchenko A.V., Rayner C.R., Lenze E.J., Reiersen A.M., Guyatt G.H., Mills E.J. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Glob. Health. 2022;10:e42–e51. doi: 10.1016/s2214-109x(21)00448-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Taler M., Gil-Ad I., Lomnitski L., Korov I., Baharav E., Bar M., Zolokov A., Weizman A. Immunomodulatory effect of selective serotonin reuptake inhibitors (SSRIs) on human T lymphocyte function and gene expression. Eur. Neuropsychopharmacol. 2007;17:774–780. doi: 10.1016/j.euroneuro.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 255.Sukhatme V.P., Reiersen A.M., Vayttaden S.J., Sukhatme V.V. Fluvoxamine: A Review of Its Mechanism of Action and Its Role in COVID-19. Front. Pharmacol. 2021;12:1–9. doi: 10.3389/fphar.2021.652688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Glebov O.O. Low-Dose Fluvoxamine Modulates Endocytic Trafficking of SARS-CoV-2 Spike Protein: A Potential Mechanism for Anti-COVID-19 Protection by Antidepressants. Front. Pharmacol. 2021;12:1–6. doi: 10.3389/fphar.2021.787261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Atluri S., Manchikanti L., Hirsch J.A. Expanded umbilical cord mesenchymal stem cells (UC-MSCs) as a therapeutic strategy in managing critically ILL COVID-19 patients: The case for compassionate use. Pain. Physician. 2020;23:E71–E84. doi: 10.36076/ppj.2020/23/e71. [DOI] [PubMed] [Google Scholar]
- 258.Dilogo I.H., Aditianingsih D., Sugiarto A., Burhan E., Damayanti T., Sitompul P.A., Mariana N., Antarianto R.D., Liem I.K., Kispa T., Mujadid F., Novialdi N., Luviah E., Kurniawati T., Lubis A.M.T., Rahmatika D. Umbilical cord mesenchymal stromal cells as critical COVID-19 adjuvant therapy: A randomized controlled trial. Stem Cells Transl. Med. 2021;10:1279–1287. doi: 10.1002/sctm.21-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Khiali S., Entezari-Maleki T. New Variants of SARS-CoV-2 and Next Generation of COVID-19 Treatments. J. Clin. Pharmacol. 2022 doi: 10.1002/jcph.2067. [DOI] [PubMed] [Google Scholar]
- 260.Shannon A., Fattorini V., Sama B., Selisko B., Feracci M., Falcou C., Gauffre P., el Kazzi P., Delpal A., Decroly E., Alvarez K., Eydoux C., Guillemot J.C., Moussa A., Good S.S., la Colla P., Lin K., Sommadossi J.P., Zhu Y., Yan X., Shi H., Ferron F., Canard B. A dual mechanism of action of AT-527 against SARS-CoV-2 polymerase. Nat. Commun. 2022;13 doi: 10.1038/s41467-022-28113-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Good S.S., Westover J., Jung K.H., Zhou X.J., Moussa A., la Colla P., Collu G., Canard B., Sommadossi J.P. AT-527, a double prodrug of a guanosine nucleotide analog, is a potent inhibitor of SARS-CoV-2 in vitro and a promising oral antiviral for treatment of covid-19. Antimicrob. Agents Chemother. 2021;65 doi: 10.1128/AAC.02479-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Sachse M., Tenorio R., Fernández de Castro I., Muñoz-Basagoiti J., Perez-Zsolt D., Raïch-Regué D., Rodon J., Losada A., Avilés P., Cuevas C., Paredes R., Segalés J., Clotet B., Vergara-Alert J., Izquierdo-Useros N., Risco C. Unraveling the antiviral activity of plitidepsin against SARS-CoV-2 by subcellular and morphological analysis. Antivir. Res. 2022;200 doi: 10.1016/j.antiviral.2022.105270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Varona J.F., Landete P., Lopez-Martin J.A., Estrada V., Paredes R., Guisado-Vasco P., de Orueta L.F., Torralba M., Fortun J., Vates R., Barberan J., Clotet B., Ancochea J., Carnevali D., Cabello N., Porras L., Gijon P., Monereo A., Abad D., Zuñiga S., Sola I., Rodon J., Vergara-Alert J., Izquierdo-Useros N., Fudio S., Pontes M.J., de Rivas B., de Velasco P.G., Nieto A., Gomez J., Aviles P., Lubomirov R., Belgrano A., Sopesen B., White K.M., Rosales R., Yildiz S., Reuschl A.K., Thorne L.G., Jolly C., Towers G.J., Zuliani-Alvarez L., Bouhaddou M., Obernier K., McGovern B.L., Rodriguez M.L., Enjuanes L., Fernandez-Sousa J.M., Krogan N.J., Jimeno J.M., Garcia-Sastre A. Preclinical and randomized phase I studies of plitidepsin in adults hospitalized with COVID-19. Life Sci. Alliance. 2022;5 doi: 10.26508/lsa.202101200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.K.M. White, R. Rosales, S. Yildiz, T. Kehrer, L. Miorin, E. Moreno, S. Jangra, M.B. Uccellini, R. Rathnasinghe, L. Coughlan, C. Martinez-Romero, J. Batra, A. Rojc, M. Bouhaddou, J.M. Fabius, K. Obernier, M. Dejosez, M.J. Guillén, A. Losada, P. Avilés, M. Schotsaert, T. Zwaka, M. Vignuzzi, K.M. Shokat, N.J. Krogan, A. García-Sastre, Plitidepsin has potent preclinical efficacy against SARS-CoV-2 by targeting the host protein eEF1A, n.d. https://www.science.org. [DOI] [PMC free article] [PubMed]
- 265.Saied E.M., El-Maradny Y.A., Osman A.A., Darwish A.M.G., Nahas H.H.A., Niedbała G., Piekutowska M., Abdel-Rahman M.A., Balbool B.A., Abdel-Azeem A.M. A comprehensive review about the molecular structure of severe acute respiratory syndrome coronavirus 2 (Sars-cov-2): Insights into natural products against covid-19. Pharmaceutics. 2021;13:1759. doi: 10.3390/pharmaceutics13111759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Chitsike L., Duerksen-Hughes P. Keep out! SARS-CoV-2 entry inhibitors: their role and utility as COVID-19 therapeutics. Virol. J. 2021;18:1–17. doi: 10.1186/s12985-021-01624-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Hou Y.J., Okuda K., Edwards C.E., Martinez D.R., Asakura T., Dinnon K.H., Kato T., Lee R.E., Yount B.L., Mascenik T.M., Chen G., Olivier K.N., Ghio A., Tse L.V., Leist S.R., Gralinski L.E., Schäfer A., Dang H., Gilmore R., Nakano S., Sun L., Fulcher M.L., Livraghi-Butrico A., Nicely N.I., Cameron M., Cameron C., Kelvin D.J., de Silva A., Margolis D.M., Markmann A., Bartelt L., Zumwalt R., Martinez F.J., Salvatore S.P., Borczuk A., Tata P.R., Sontake V., Kimple A., Jaspers I., O’Neal W.K., Randell S.H., Boucher R.C., Baric R.S. SARS-CoV-2 Reverse Genetics Reveals a Variable Infection Gradient in the Respiratory Tract. Cell. 2020;182 doi: 10.1016/j.cell.2020.05.042. 429-446.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Vickers K.C., Palmisano B.T., Shoucri B.M., Shamburek R.D., Remaley A.T. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat. Cell Biol. 2011;13:423–435. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Paul S., Bravo Vázquez L.A., Reyes-Pérez P.R., Estrada-Meza C., Aponte Alburquerque R.A., Pathak S., Banerjee A., Bandyopadhyay A., Chakraborty S., Srivastava A. The role of microRNAs in solving COVID-19 puzzle from infection to therapeutics: A mini-review. Virus Res. 2022;308 doi: 10.1016/j.virusres.2021.198631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Hassab Elnabi S.E. New strategies for treatment of COVID-19 and evolution of SARS-CoV-2 according to biodiversity and evolution theory. Egypt. J. Basic Appl. Sci. 2020;7:226–232. doi: 10.1080/2314808x.2020.1789815. [DOI] [Google Scholar]