Abstract
Introduction: Children and adolescents with cancer report increased fatigue and decreased physical activity, introducing risk factors for chronic disease and suppressed quality of life. Research suggests an inverse relationship between fatigue and physical activity, but the biological explanation is not well understood. The purpose of this study was to 1) explore metabolites associated with fatigue or physical activity and 2) to identify any shared metabolomic elements. Methods: Children, ages 8–17 years, attending a pediatric oncology summer camp provided Patient-Reported Outcome Measurement System® (PROMIS) Pediatric Fatigue assessments, physical activity data (steps/day), and urine samples pre- and post-camp. Differences in PROMIS Pediatric Fatigue scores and average daily steps were calculated using paired t-tests. Liquid chromatography-tandem mass spectrometry was conducted using a targeted metabolomic approach. Results: Thirty-two enrolled children had complete data. Fatigue scores decreased (pre-camp 45.1; post-camp 42.1; p = 0.04) while steps-per-day increased (pre-camp 6699; post-camp 16,021; p < 0.001). Twenty-seven metabolites significantly differentiated (false discovery rate <0.20) between low, medium, or high physical activity, while 8 metabolites discriminated between high and low fatigue. Indole-3-lactic acid, a tryptophan metabolite, was significantly associated with both physical activity and fatigue. Conclusion: This study provides evidence of metabolome associations with fatigue and physical activity in children with cancer. Overlapping metabolomic elements provide evidence of biological inter-connectivity and suggest areas for future research. Given the known evidence regarding the benefits of physical activity, and the potential interaction with fatigue, nurses should routinely assess patient reports of these elements and provide patient/family education related to fatigue management and physical activity goals.
Keywords: pediatric oncology, physical activity, fatigue, metabolomics, symptoms
Fatigue is one of the most predominant and persistent symptoms during and after childhood cancer therapy occurring in around 68% of children and adolescents with implications for psychological stress and reduced quality of life (Hinds et al., 2021). The exact etiology of cancer-related fatigue remains elusive but is thought to be attributable to multiple factors, including dysregulation of the hypothalamic-pituitary-adrenal axis, inflammation (proinflammatory cytokines), and disruption in energy metabolism, as well as other biological processes (i.e., oxidative stress) altered by the cancer itself and treatment modalities (Berger et al., 2010; Xiao, Beitler, et al., 2016).
Physical activity, a broad term that includes movement activities such as structured exercise, walking, and yoga, has been associated with reduced cancer-related fatigue in both adults and, to a lesser degree, children (Hilfiker et al., 2018; Huang et al., 2019; Huang & Ness, 2011; Tomlinson et al., 2014; Withycombe et al., 2018). The biological explanation for the relationship between fatigue and physical activity may be explained, in part, by cytokine gene expression changes that reduce inflammation, mind–body interactions that decrease cortisol levels, and maintenance of muscle mass which assists with production of ATP (Kinney et al., 2019); However, the precise biological connections between fatigue and physical activity during and after childhood cancer therapy are still relatively unknown.
Metabolomics, a method that uses liquid or gas chromatography with mass spectrometry, offers a way to examine many small molecules to provide insight into complex physiological pathways associated with disease and/or health (Li et al., 2016). Combined with computational algorithms to identify individual metabolites and metabolomic pathways associated with specified conditions, metabolomic analyses may be conducted in multiple sample types (e.g., blood, urine, and tissue) using an untargeted (wide detection of known and unknown metabolites), targeted (focused detection on select, identifiable metabolites), or hybrid approach (Wei et al., 2021).
The use of metabolomics to explore disease changes related to cancer and cancer treatment is rapidly growing, but few researchers have used this method to explore the underlying biological processes of cancer-related symptoms, particularly in children. One study of childhood acute lymphoblastic leukemia patients evaluated metabolomic profiles of cerebral spinal fluid and found associations between fatigue and three metabolites: gamma-glutamylglutamine, asparagine, and dimethylglycine (Brown et al., 2021). Another study in children with cancer found metabolites produced in the gut microbiome to be associated with gastrointestinal and/or psychoneurological symptoms (including fatigue) (Bai et al., 2018). No studies have examined metabolomics in relationship to physical activity in children during or after cancer treatment.
The purpose of this study was to 1) explore urine metabolites associated with fatigue or physical activity in children on and off treatment for cancer and 2) identify any metabolites or pathways that were similar between fatigue and physical activity.
Methods
Study Design and Setting
Research participants were children ages 8–17 years, attending a 6-day/5night, summer camp developed for childhood cancer survivors or children still receiving active cancer therapy. Participants were enrolled over two summers (June 2017 and June 2018). Inclusion criteria included: English speaking, cancer diagnosis or history of childhood cancer, no history of neurological disorders or syndrome that would prohibit self-report of symptoms, and no physical limitations that would interfere with normal physical activity. Parental consent for study participation was obtained verbally (by phone) or in writing during in-person events leading up to camp. Children provided verbal assent. Using a pre-/post-study design, physical activity and fatigue measurements, and urine samples were collected at baseline and end of camp. Height and weight were collected at baseline and used to calculate body mass index (BMI) percentile scores based on age and gender per the Center for Disease Control BMI calculator (Center for Disease Control, 2021).
Physical Activity
Physical activity was measured as steps-per-day and collected using commercially available, wrist-worn Garmin Vivofit® accelerometers. This monitor has a long battery life, waterproof rating, and is validated for measuring step counts in children across multiple ages (Muller et al., 2018). The average number of steps-per-day was calculated for 7 days preceding camp and 5 full days during camp. Intensity levels of physical activity were not measured due to limitations with the output algorithm from the commercially available monitor (i.e., proprietary formula that prohibited benchmarking with other publications).
Fatigue Assessment
Self-reported fatigue was collected 1–2 days prior to camp (electronically) and again at the end of camp, using the Patient-Reported Outcomes Measurement Information System (PROMIS) Peds 37 Profile measuring fatigue, anxiety, depressive symptoms, pain interference and intensity, physical function-mobility, and peer relationships. The PROMIS Pediatric instruments were developed by the National Institutes of Health to provide a domain-based approach for assessing common symptoms/conditions. These instruments are reliable (Varni et al., 2014) and validated for use in children with cancer (Hinds et al., 2013). The recall period for the instruments is the “past 7 days.” For this metabolomics study, only the symptom of fatigue was included in the analysis. The PROMIS measures were scored using the free, online HealthMeasures Scoring Service provided by the PROMIS® Assessment Center SM (https://www.assessmentcenter.net/ac_scoringservice) and reported using the T-score metric with a mean of 50 and a standard deviation of 10 (Varni et al., 2014).
Sample Collection and Processing
Upon checking into camp, and again prior to leaving camp, children provided single void urine samples. Urine samples were collected around noon at both time points. As camp is considered a safe environment free from invasive medical procedures, children were asked to provide a urine sample rather than a blood sample. Urine is commonly utilized in metabolomic analyses and has been well described in the literature, including a published reference library of the human urine metabolome (Bouatra et al., 2013). Collected urine samples were immediately placed in 2 mL aliquots and frozen on dry ice prior to transport to the research lab for storage at -80°C. At the end of data collection, deidentified aliquots were shipped on dry ice to the Arizona Metabolomics Laboratory (at Arizona State University) for processing.
Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS)
The targeted LC-MS/MS method in this study was modeled after that developed and used in a growing number of studies extracting a detailed list of confirmed metabolites (Jasbi et al., 2021; Shi et al., 2019; Zhu et al., 2015). Briefly, we included ∼300 metabolites (additional information available from the authors by request) selected from >35 metabolic pathways of biological significance to energy expenditure (such as glycolysis, TCA cycle, purine metabolism, and amino acid metabolism). All LC-MS/MS parameters, including precursor/produce ion transitions, collision energy (CE), and retention time, were confirmed and validated using metabolite standards. An Agilent 1290 UPLC-6490 QQQ-MS system (Santa Clara, CA) was used for the LC-MS/MS experiments with system control through Agilent MassHunter Workstation software. Dual sample injection was utilized as follows: 10 μL for negative ionization analysis and 4 μL for positive ionization analysis. Chromatographic separations were completed using hydrophilic interaction chromatography via Waters XBridge BEH amide column (150 × 2.1 mm, 2.5 μm particle size, Waters, Milford, MA) with a flow rate of 0.3 mL/min. The column compartment temperature was maintained at 40°C, while the autosampler temperature was set at 4°C. Multiple-reaction-monitoring (MRM) mode was utilized for the targeted data analysis with enhanced sensitivity and specificity. Agilent MassHunter Quantitative Data Analysis software was utilized for the MRM extracted peaks.
Data Analysis
Pre-camp versus post-camp differences in PROMIS Pediatric Fatigue scores and average daily steps were calculated using paired t-tests. For the metabolomics analyses, physical activity and fatigue outcomes were grouped into categories based on published cut points. Fatigue was dichotomized into high versus low categories, using published reference benchmarks (high fatigue defined as PROMIS scores >47.5, while low fatigue were scores of 47.5 and below) (Carle et al., 2021). Physical activity was grouped into one of three category levels: High (>15,000 steps/day), moderate (10,000–14,999 steps/day), or low (<10,000 steps/day) (Tudor-Locke et al., 2011).
Intensities for all 184 reliably measured metabolites (i.e., those that had a coefficient of variation <30% among quality control samples) were log2 transformed and quantile normalized; creatinine levels were examined but not normalized secondary to no correlation with average daily steps or fatigue at either data collection time point. We identified differentially expressed metabolites across the physical activity and fatigue categories using repeated measures Limma with robust variance estimation (Phipson et al., 2016). Limma is a popular R package that uses linear models to assess differential expression of many quantitative biological features simultaneously across multifactor designed experiments, like an ANOVA. As such, also like ANOVA, adjusting for confounders is not possible. We therefore examined the possibility of confounding variables through chi-squared tests exploring age, sex, race, treatment, and BMI associations with the outcomes at time 1 or 2. A secondary check was completed stratifying the metabolic analyses by sex and treatment type. Based on results from these additional tests, the risk of confounding variables was felt to be minimal and the whole sample was retained in the final analyses. Additionally, we used the Benjamini-Hochberg false discovery rate (FDR) to correct for multiple comparisons (Benjamini et al., 2001). A metabolite with an FDR<0.20 was a priori deemed statistically significant given the discovery-based nature of this study and prior publications demonstrating acceptability of this practice (Lyon et al., 2018; Xiao, Beitler, et al., 2016).
Significant metabolites were used to determine pathway enrichment via MetaboAnalyst v5.0 (Over Representation Analysis) mapping to the Kyoto Encyclopedia Genes and Genomes (KEGG) reference library (Pang et al., 2021). Enrichment analysis calculates pathway-specific p-values by comparing the number of metabolite hits to the number expected by chance for each referent pathway. We report enriched metabolic pathways with at least two metabolite hits and p < 0.05. Pathway direction and relative magnitude was obtained by z-score standardizing each significant metabolite (mean = 0; standard deviation = 1) and summing across the metabolites that comprise each pathway.
Results
A total of 43 children enrolled in the study and 32 had complete data (urine samples, self-reports of fatigue, and step data) for inclusion in the metabolomic analysis. The study sample demographics were: White (66%), African American (25%), Asian (6%), and other (3%); Hispanic ethnicity (6%) (Table 1). The majority were off treatment (84%) and male (53%). A plurality had a cancer diagnosis of leukemia/lymphoma (37%).
Table 1.
Characteristics of Study Participants.
| Characteristic | N (32) | % |
|---|---|---|
| Gender | ||
| Male | 17 | 53 |
| Female | 15 | 47 |
| Race | ||
| White | 21 | 66 |
| African American | 8 | 25 |
| Asian | 2 | 6 |
| Other | 1 | 3 |
| Ethnic group | ||
| Hispanic | 2 | 6 |
| Non-Hispanic | 30 | 94 |
| Diagnosis | ||
| Leukemia/lymphoma | 11 | 37 |
| Solid tumors | 9 | 28 |
| CNS tumors | 7 | 22 |
| Other/unknown | 4 | 13 |
| Treatment | ||
| On | 5 | 16 |
| Off | 27 | 84 |
| Age in years, M (SD) | 13.2 (2.6) | Median (IQR) 13 (11–15) |
| Months since Dx, M (SD) | 71 (49.6) | Median (IQR) 67.5 (33–107) |
| Body mass index+ | ||
| Underweight | 2 | 6 |
| Healthy | 14 | 44 |
| Overweight | 8 | 25 |
| Obese | 8 | 25 |
Note. +Body mass index calculated per Center for Disease Control age and gender percentiles for height and weight. Abbreviations: M, mean; SD, standard deviation, Dx, diagnosis; IQR, interquartile range.
Fatigue and Physical Activity Changes during Camp
Mean PROMIS fatigue T-scores decreased from pre-camp to post-camp (45.1 v 42.1, p = 0.04). Lower PROMIS fatigue scores represent a reduction in symptom experience. A 3-point change in the PROMIS mean score is considered a “minimally important difference” (MID), based on prior research including patient and clinician viewpoints, and signifies a clinically important change (Thissen et al., 2016). Thus, the observed 3-point reduction in fatigue score was significant from both a statistical and clinical standpoint. Physical activity increased more than two-fold during camp with an average of 6,699 steps-per-day the week before camp and 16,021 steps-per-day the week of camp (p < 0.001). Although not statistically significant, there tended to be an inverse relationship between fatigue and steps-per-day pre-camp (p = 0.13) and at the end of camp (p = 0.38).
Metabolites and Physical Activity
Using a targeted analysis, 27 metabolites significantly differentiated (FDR < 0.20) between categories of low, medium, or high physical activity (Table 2). Thirteen of these metabolites had an FDR <0.05. Betaine (i.e., N,N,N-trimethylglycine), and N-acetylneuraminic acid (the predominant sialic acid found in human cells), yielded the smallest FDR-correct p-values for the relationship with physical activity. Some of the metabolite associations with physical activity showed a linear relationship. For instance, betaine (a modified amino acid involved in glycine, serine, and threonine metabolism) had a linear, inverse association with physical activity. As steps-per-day increased, betaine levels decreased (low steps = 5.0E+07, medium steps = 2.7E+07, and high steps = 2.5E+07; FDR Q = 0.001). However, a different trend is noted with indole-3-lactic acid, a monocarboxylic acid tryptophan metabolite, as it displayed a non-linear U-shaped dose response with steps (low steps = 12.9E+04, medium steps = 9.0E+04, and high steps = 12.1E+04).
Table 2.
Metabolites Significantly Associated with Low (<10,000), Medium (10,000–15,000), or High (>15,000) Steps-Per-Day.
| Metabolite | KEGG ID | Low a | Medium a | High a | Raw P | FDR Q | KEGG-Annotated Metabolic Pathway |
|---|---|---|---|---|---|---|---|
| Amino acids | |||||||
| Betaine | C00719 | 4.99E+07 | 2.68E+07 | 2.52E+07 | <0.001 | 0.001 | Glycine, serine, and threonine metabolism |
| Asparagine | C00152 | 1.33E+05 | 1.82E+05 | 2.23E+05 | <0.001 | 0.01 | Aminoacyl-tRNA biosynthesis |
| 3-hydroxykynurenine | C02794 | 1.54E+05 | 2.11E+05 | 2.28E+05 | 0.001 | 0.03 | Tryptophan catabolism |
| Tyrosine | C00082 | 13.4E+05 | 9.46E+05 | 1.36E+06 | 0.002 | 0.03 | Tyrosine metabolism |
| 1-methylhistidine | C01152 | 1.01E+07 | 1.95E+07 | 2.08E+07 | 0.01 | 0.07 | Histidine metabolism |
| N-acetylornithine | C00437 | 13.2E+04 | 9.33E+04 | 10.1E+04 | 0.01 | 0.07 | Arginine biosynthesis |
| Phenylalanine | C02057 | 9.85E+06 | 7.03E+06 | 9.29E+06 | 0.01 | 0.08 | Phenylalanine metabolism |
| Tryptophan | C00078 | 3.24E+06 | 2.17E+06 | 3.07E+06 | 0.01 | 0.09 | Tryptophan metabolism |
| Methionine | C00073 | 3.31E+05 | 1.98E+05 | 2.70E+05 | 0.02 | 0.14 | Cysteine and methionine metabolism |
| Valine | C00183 | 2.48E+06 | 1.72E+06 | 1.94E+06 | 0.02 | 0.15 | Valine, leucine, and isoleucine metabolism |
| Sugars | |||||||
| N-acetylneuraminic acid | C19910 | 1.25E+06 | 1.03E+06 | 8.86E+05 | <0.001 | <0.001 | Amino and nucleotide sugar metabolism |
| Glyceric acid | C00258 | 8.98E+04 | 11.8E+04 | 1.31E+05 | 0.001 | 0.03 | Pentose phosphate pathway |
| Erythrose | C01796 | 3.27E+05 | 2.95E+05 | 4.43E+05 | 0.002 | 0.03 | Sugar metabolism |
| Galactose | C00124 | 6.99E+05 | 9.01E+05 | 8.60E+05 | 0.002 | 0.03 | Galactose metabolism |
| Myoinositol | C00137 | 8.89E+05 | 6.79E+05 | 6.01E+05 | 0.01 | 0.07 | Galactose metabolism |
| 3-phosphoglyceric acid | C00197 | 3.22E+03 | 6.32E+03 | 5.66E+03 | 0.01 | 0.09 | Glycolysis |
| Ribose 5-phosphate | C00117 | 2.37E+04 | 1.92E+04 | 1.28E+04 | 0.02 | 0.15 | Pentose phosphate pathway |
| Xylitol | C00379 | 5.98E+05 | 5.06E+05 | 4.66E+05 | 0.02 | 0.16 | Pentose and glucuronate interconversions |
| Nucleotides | |||||||
| Inosine | C00294 | 5.13E+04 | 2.04E+04 | 1.47E+04 | 0.001 | 0.03 | Purine metabolism |
| Xanthine | C00385 | 5.05E+04 | 4.68E+04 | 3.42E+04 | 0.01 | 0.09 | Purine metabolism |
| Cytosine | C00380 | 1.29E+05 | 1.28E+05 | 2.77E+05 | 0.02 | 0.14 | Pyrimidine metabolism |
| Carboxylic acids | |||||||
| 4-hydroxybenzoic acid | C00156 | 1.54E+06 | 2.12E+06 | 3.51E+06 | <0.001 | 0.01 | Aminobenzoic acid degradation |
| m-coumaric acid | C12621 | 1.35E+04 | 1.58E+04 | 1.93E+04 | 0.001 | 0.03 | Phenylalanine metabolism |
| Indole-3-lactic acid | C02043 | 12.9E+04 | 9.03E+04 | 12.1E+04 | 0.002 | 0.03 | Tryptophan metabolism |
| Glyoxylic acid | C00048 | 6.73E+03 | 8.46E+03 | 7.20E+03 | 0.02 | 0.16 | Purine metabolism |
| Lipids | |||||||
| Lauric acid | C02679 | 1.80E+06 | 1.70E+06 | 1.80E+06 | 0.001 | 0.02 | Fatty acid biosynthesis |
| Drugs | |||||||
| 2-pyrrolidinone | C11118 | 4.91E+04 | 6.31E+04 | 6.20E+04 | 0.01 | 0.08 | Xenobiotics metabolism |
aRaw intensity value across the physical activity groups.
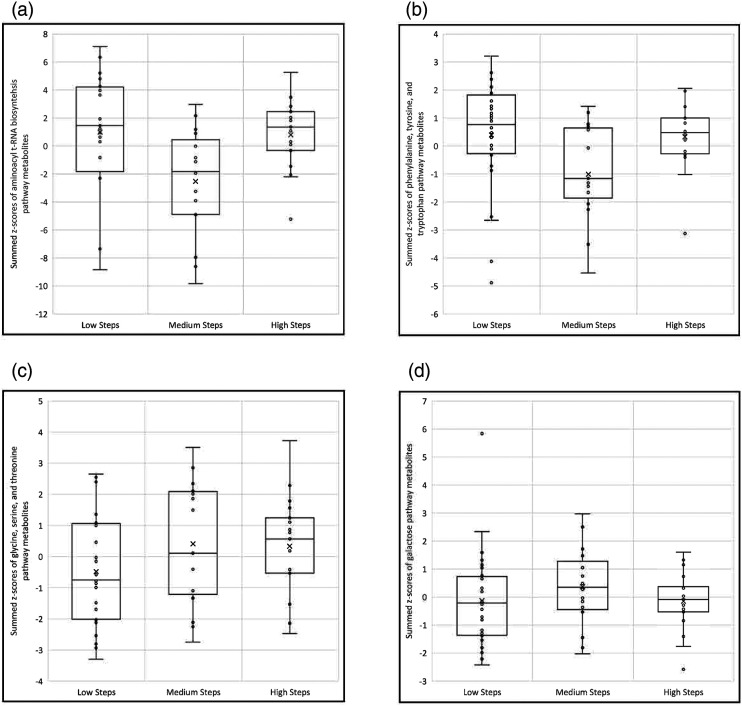
From the 27 metabolites associated with steps-per-day, pathway enrichment analysis identified 10 pathways represented by two or more metabolites (Table 3). Of these enriched pathways, 5 had p-values < 0.05: aminoacyl-tRNA biosynthesis; phenylalanine, tyrosine, and tryptophan biosynthesis; glycine, serine, and threonine metabolism; galactose metabolism; and phenylalanine metabolism; the phenylalanine pathway consisted of the same metabolites as the phenylalanine, tyrosine, and tryptophan biosynthesis pathway. Boxplots of summed metabolite z-scores were created to show the direction of change for the top 4 enhanced pathways across the different categories of physical activity (Figure 1). A U-shaped dose response change is noted in the aminoacyl t-RNA biosynthesis pathway (Figure 1A) as well as the phenylalanine, tyrosine, and tryptophan pathway (Figure 1B) where pathway activity is relatively high among children with low steps-per-day (<10,000), decreases among children with medium physical activity (10,000–15,000 steps-per-day), but returns to higher levels among children with high (>15,000) steps-per-day. Of the metabolic pathways related to physical activity, the glycine, serine, and threonine pathway (Figure 1C and D) is the only one to display a linear relationship with pathway activity increasing as steps increase. The galactose pathway Figure 1D displays an inverted U-shaped dose response with greater pathway activation associated with medium step numbers, compared to low or high steps.
Table 3.
Metabolic Pathway Enrichment Analysis using the 27 Differentially Expressed Metabolites Associated with Low, Medium, or High Physical Activity Categories.
| KEGG Metabolic Pathway | Total Metabolites a | Hits b | p-value | FDR |
|---|---|---|---|---|
| Aminoacyl-tRNA biosynthesis | 48 | 6 | <0.001 | 0.01 |
| Phenylalanine, tyrosine, and tryptophan biosynthesis | 4 | 2 | 0.002 | 0.06 |
| Glycine, serine, and threonine metabolism | 33 | 4 | 0.002 | 0.06 |
| Galactose metabolism | 27 | 3 | 0.01 | 0.21 |
| Phenylalanine metabolism | 10 | 2 | 0.01 | 0.21 |
| Pentose phosphate pathway | 22 | 2 | 0.06 | 0.79 |
| Purine metabolism | 65 | 3 | 0.11 | 0.96 |
| Glyoxylate and dicarboxylate metabolism | 32 | 2 | 0.11 | 0.96 |
| Cysteine and methionine metabolism | 33 | 2 | 0.11 | 0.96 |
| Amino sugar and nucleotide sugar metabolism | 37 | 2 | 0.14 | 0.96 |
aTotal number of metabolites included in each KEGG (Kyoto Encyclopedia of Genes and Genomes) referent metabolic pathways.
bNumber of identified metabolites that map to each KEGG pathway. Enrichment determined by 2 or more hits per pathway. Abbreviations: FDR, false discovery rate.
Figure 1.
Boxplots of metabolic pathway scores (i.e., summed z-scores of pathway metabolites) by average steps-per-day categories (low <10,000; medium = 10,000–15,000; high >15,000). A) Aminoacyl t-RNA biosynthesis metabolic pathway (asparagine, phenylalanine, methionine, valine, tryptophan, and tyrosine metabolites). B) Phenylalanine, tyrosine, and tryptophan metabolic pathway (phenylalanine and tyrosine metabolites). C) Glycine, serine, and threonine metabolic pathway (betaine, 3-phosphoglyceric acid, glyceric acid, and glyoxylic acid metabolites). D) Galactose metabolic pathway (galactose and myoinositol metabolites). The Phenylalanine metabolic pathway shared the same metabolites with the phenylalanine, tyrosine, and tryptophan metabolic pathway.
Metabolites and Fatigue
Eight metabolites were identified to discriminate between high vs low fatigue (FDR<0.20). Among those, two metabolites had an FDR <0.05: acetohydroxamic acid (a urease inhibitor), and trimethylamine-N-oxide (a metabolite produced by gut microbiota from choline and carnitine) (Table 4). Due to the small number of significant metabolites, no enrichment pathways were identified for metabolites related to fatigue.
Table 4.
Urine Metabolites Significantly Associated with Low (≤47.5) v High (>47.5) Mean PROMIS Pediatric Fatigue Scores.
| Metabolite | KEGG ID | Low a fatigue | High a fatigue | Raw P | FDR Q | KEGG-Annotated Metabolic Pathway |
|---|---|---|---|---|---|---|
| Amino acids | ||||||
| Isoleucine | C00407 | 9.62E+06 | 12.8E+06 | 0.004 | 0.14 | Valine, leucine, and isoleucine metabolism. |
| Anthranilic acid | C00108 | 2.69E+04 | 6.21E+04 | 0.01 | 0.18 | Phenylalanine, tyrosine, and tryptophan biosynthesis |
| Sugars | ||||||
| Trehalose | C01083 | 6.67E+05 | 16.9E+05 | 0.002 | 0.11 | Starch and sucrose metabolism |
| Nucleotides | ||||||
| Uridine | C00299 | 9.78E+03 | 12.4E+03 | 0.002 | 0.11 | Pyrimidine metabolism |
| Carboxylic acids | ||||||
| Trimethylamine-N-oxide | C01104 | 13.8E+07 | 9.35E+07 | <0.001 | 0.02 | Microbial gut flora metabolism |
| Muconic acid | C02220 | 6.47E+03 | 17.2E+03 | 0.004 | 0.14 | Tryptophan metabolism |
| Indole-3-lactic acid | C02043 | 1.08E+05 | 1.37E+05 | 0.01 | 0.18 | Tryptophan metabolism |
| Drugs | ||||||
| Acetohydroxamic acid | C06808 | 5.83E+05 | 3.67E+05 | <0.001 | 0.02 | Xenobiotics metabolism |
aRaw intensity value across the fatigue groups.
Assessment of potential confounders
As adjustment for confounders is not possible utilizing LIMMA models, additional analyses were performed. Bivariate associations between age, gender, race, BMI, and treatment type were explored using chi-squared tests, in relationship to physical activity (Supplemental Table 1) and fatigue (Supplemental Table 2) at both time points (pre-camp and during camp). Age, sex, race, treatment, and BMI were not associated with our outcomes at time 1 or 2 (physical activity, Supplemental Table 1; or fatigue, Supplemental Table 2). The exception was sex as girls were less active (p = 0.02) than boys during camp (T2). A secondary check was completed stratifying the metabolic analyses by sex (Supplemental Table 3 for boys and Supplemental Table 4 for girls) and treatment type (Supplemental Table 5). In all instances, metabolite and metabolic pathway results were similar, therefore suggesting that potential confounding variables were a minimal risk.
Intersection of Metabolites and Pathways
Indole-3-lactic acid, a tryptophan metabolite originating from the gut-microbiome, was the only metabolite significantly associated with both physical activity and fatigue. Lower levels of indole-3-latic acid were associated with both decreased fatigue and moderate steps-per-day. Higher indole-3-latic acid levels were associated with higher fatigue and two categories of physical activity (low and high steps-per-day).
In evaluating potential overlap between the enriched KEGG-annotated metabolomic pathways related to fatigue or physical activity (Tables 2 and 4), five overlapping metabolomic pathways were observed: 1) phenylalanine, tyrosine, and tryptophan biosynthesis; 2) pyrimidine metabolism; 3) tryptophan metabolism; 4) valine, leucine, and isoleucine metabolism; and 5) xenobiotics metabolism, involving metabolites significantly associated with fatigue and physical activity in our analyses (Table 5). These intersecting elements may offer additional insight into understanding a potential biological interaction between fatigue and physical activity.
Table 5.
Shared metabolites and Pathways Between Fatigue and Physical Activity.
| Individual Metabolites | KEGG-Annotated Metabolic Pathways |
|---|---|
| Indole-3-lactic acid | Phenylalanine, tyrosine, and tryptophan biosynthesis |
| Pyrimidine metabolism | |
| Tryptophan metabolism | |
| Valine, leucine, and isoleucine metabolism | |
| Xenobiotics metabolism |
Discussion
This is one of the first studies to measure metabolomic changes related to fatigue and physical activity in children during and after cancer treatment. This study demonstrates that metabolomic profile distinctions can be observed between categories of differing physical activity levels and between low versus high fatigue. This study also offers preliminary evidence related to pathways of interest which may intersect in relationship to fatigue and physical activity.
Research studies support “moderate to high intensity” physical activity as a cancer-related fatigue intervention for adult oncology patients (American College of Sports Medicine, 2019; Cramp & Byron-Daniel, 2012; Tomlinson et al., 2014). In our study, children increased their physical activity level during camp by an average of 10,000 steps-per-day, more than doubling their baseline activity levels. Differences were observed in 27 metabolites and three enriched metabolic pathways (FDR ≤0.20) between categories of low, medium, and high steps-per-day. Our findings related to indole-3-lactic acid are in line with emerging research which suggests that the human gut microbiota may be influenced by physical activity (Dorelli et al., 2021).
Our dose-related findings for physical activity are in agreement with other studies showing biological gradient differences in metabolites in response to physical activity intensity and duration (Kelly et al., 2020; Xiao et al., 2016). For three, out of four, enhanced pathways (Figure 1), we observed similar directions for pathway activity associated with both low and high levels of physical activity, while the moderate level of physical activity (defined for 10,000–15,000 steps-per-day for this study) was associated with pathway changes in the opposite direction, displaying a U-shaped curve. Of interest, the top three pathways in our enrichment analysis of metabolites associated with physical activity categories (aminoacyl-tRNA biosynthesis; phenylalanine, tyrosine, and tryptophan metabolism; and glycine, serine, and threonine metabolism) were recently reported in another study of metabolite changes occurring after an acute exercise bout in healthy athletes (Tabone et al., 2021).
It is important to note that inconsistent results have been reported, in the pediatric oncology literature, regarding effectiveness of physical activity interventions on cancer-related fatigue (Wurz, McLaughlin, Lategan, Ellis, et al., 2021). To date, no consensus has been reached to specify the best intervention (walking, resistance exercises, etc.) or the intensity/duration needed to improve fatigue during childhood cancer, although the general consensus is that physical activity is generally of benefit (Malysse et al., 2021; Wurz et al., 2021b). Commonly, discussions related to appropriate levels of physical activity intensity to induce change in patient-reported outcomes (i.e., fatigue) have cited dose response U-shaped curves to depict “low” intensity physical activity as ineffective, “moderate” levels of physical activity as optimal, and “high” levels of physical activity as aversive (Ekkekakis et al., 2005). The pitfall with dose response theory is defining low, moderate, and high levels of physical activity as one-size fits all (or one physical activity intensity recommendation for all) as this may not account for inter-personal variations. This may be particularly true of individuals with cancer as the treatment for cancer and/or the cancer itself can impact patient-reported outcomes such as fatigue.
Additionally, when examining fatigue as an outcome of physical activity, it should be noted that other elements such as sleep and/or depression may influence fatigue presence and severity. While ongoing research is needed to develop evidence-based guidelines for beneficial physical activity levels (frequency, intensity, and duration) during childhood cancer therapy (Wurz, McLaughlin, Chamorro Vina, et al., 2021), studies such as this one may help to provide insight into the relationship between metabolomic pathway variations and possible dose response curves with differing levels of activity.
Our study additionally identified 8 metabolites that distinguished between low versus high fatigue (Table 4). A recent exploratory, untargeted metabolomics study found that fatigue was associated with asparagine, dimethylglycine, and gamma-glutamylglutamine levels in children undergoing treatment for leukemia (Brown et al., 2021) with the authors attributing these metabolites to central nervous system oxidative stress and cancer treatment with asparaginase. The results of our study differed, but the differences in study design should be noted. First, the majority of our participants were off therapy; second, we conducted a targeted metabolomic analysis; and third, we analyzed urine samples rather than cerebral spinal fluid. Our findings indicate that fatigue may be associated with compounds derived by or related to gut microbial metabolism including trimethylamine-N-oxide, a carboxylic acid associated with poor cardiometabolic outcomes, and several metabolites involved in tryptophan metabolism (indole-3-latic acid, muconic acid, and anthranilic acid). Acetohydroxamic acid, a compound derived from xenobiotic metabolism, was also linked to changes in fatigue in this study.
An exciting, yet preliminary finding of this study relates to the identification of indole-3-latic acid and additional expanded metabolomic pathways (phenylalanine, tyrosine, and tryptophan biosynthesis; pyrimidine metabolism; tryptophan metabolism; valine, leucine, and isoleucine metabolism; and xenobiotics metabolism) associated with both different levels of fatigue and physical activity. Indole-3-latic acid is a tryptophan catabolite produced primarily in the gut microbiota. This metabolite, and other tryptophan catabolites, is suggested to have multiple functions, including anti-inflammatory and anti-oxidative effects in humans (Roager & Licht, 2018).
Tryptophan was also identified in two of the five overlapping KEGG-annotated metabolic pathways (Table 5) with fatigue and physical activity (phenylalanine, tyrosine, and tryptophan biosynthesis; tryptophan metabolism). Tryptophan pathways are involved in the production of neurotransmitters such as serotonin and melatonin which are important to sleep and have also been linked to fatigue (Zhao et al., 2020). A previous study demonstrated increased tryptophan levels after exercise in healthy controls and suggested that elevated tryptophan may be linked with fatigue by serving as a precursor for 5-hydroxytryptamine (Castell et al., 1999). These shared pathways (phenylalanine, tyrosine, and tryptophan biosynthesis; tryptophan metabolism) offer evidence supporting the potential biological mechanisms through which cancer-related fatigue and physical activity may be interrelated.
Of note, three other metabolic pathways intersected with fatigue and physical activity: pyrimidine metabolism (related to ATP and energy production); valine, leucine, and isoleucine metabolism (branched chain amino acids necessary for energy production and muscle health); and xenobiotic metabolism (work to excrete foreign items such as drugs or environmental chemicals). These shared metabolic pathways are worthy of consideration in future research studies evaluating fatigue and physical activity connections.
We found only one other study that explored metabolomic associations between “moderate-to-high” intensity exercise and fatigue (Kucharski et al., 2019). This study reports decreased physical fatigue and depression after an intervention of aerobic and resistance exercise in older adults with rheumatoid arthritis. The study also reported a dose response interaction with moderate-to-high exercise being associated with greater decreases in fatigue and depression, and without the effect being observed with lower-level intensity exercise (Kucharski et al., 2019). Changes in multiple serum metabolites were also reported after the exercise intervention, including changes in indole-3-acetic acid and N-acetylglutamic acid, which were also metabolites associated with physical activity in our study. In the Kucharski (2019) study, agmatine was identified as the only metabolite significantly associated with changes in both aerobic exercise and fatigue. Our study included agmatine but did not find significant association with either physical activity or fatigue in children.
Study Limitations
The findings of this study are limited by the small sample size and the heterogeneity of the sample characteristics (large standard deviation in age, BMI, cancer treatment, and cancer diagnosis). Although the children were offered the same foods during camp, no effort was made to control for differences in nutritional intake either prior to or during camp which could have influenced metabolomic findings. Our study data was collected around the time of summer camp attendance which allowed changes in physical activity to be observed in this patient population, yet limited data collection as camp is considered a “safe” space and purposeful efforts are taken to protect children from medical interventions during this time. As such we did not collect data related to current medications or diet and did not perform venipunctures to collect serum samples for metabolomic analysis. Although the majority of patients (27 out of 32; 84%) were off treatment and not receiving chemotherapy during data collection, five children were on-therapy at the time of camp. Although it is possible that received medications could have influenced the observed urine metabolites, this was of lesser concern secondary to the small number of patients on active therapy and the fact that only oral medications were administered during camp (no intensive treatment). Additionally, camp check-in occurred on a Sunday allowing for a minimum of 72 hours between any intravenous chemotherapy and the first urine sample being provided. We did attempt to consolidate the time of urine sample collection (occurring around noon at both time points). An additional limitation is that other elements of camp attendance may have influenced perceptions of fatigue (such as socialization with peers) which were unrelated to physical activity and not captured in this analysis. This study does not seek to imply a causal relationship but instead identifies metabolomic pathways found to be related to both fatigue and physical activity in this sample and setting. This study was also limited in defining physical activity as the commercially available monitor did not provide useful intensity measures which could be easily benchmarked. As a surrogate measure for physical activity intensity, steps-per-day were collected and categorized based on published literature.
Conclusion
This study provides beginning evidence of metabolome differences associated with varying levels of fatigue and physical activity in children during and after cancer treatment. Metabolomic pathway associations with physical activity categories varied in their dose response, with observed U-shaped and linear associations between metabolites and differing categories of physical activity. Overlapping metabolomic pathways were noted between fatigue and physical activity providing further evidence for potential inter-connectivity in children treated for cancer; however, these findings should be interpreted with caution as a causal relationship cannot be inferred. Additional research is needed, and this study suggests that indol-3-lactic acid and/or the tryptophan pathways may be potential targets for future studies with a goal of further evaluating interactions between fatigue and physical activity during childhood cancer therapy. As these elements are related to the gut microbiome, future metabolomics research in this area may benefit from the inclusion of a multi-omics approach which integrates gut microbiome data, to further explore biological connections between physical activity and fatigue. Given the known evidence regarding the benefits of physical activity during and after cancer therapy, and the potential interaction with fatigue, nurses should routinely assess patient reports of these elements throughout the cancer continuum and provide patient and family education related to fatigue management and physical activity goals.
Supplemental Material
Supplemental Material for sj-pdf-1-brn-10.1177_10998004221085029 Metabolites Associated With Fatigue and Physical Activity in Childhood Cancer by Janice S. Withycombe, PhD, RN, FAAN, Ronald Eldridge, PhD, Yan Jin, PhD, Haiwai Gu, PhD, Sharon M. Castellino, MD, and Dorothy D. Sears, PhD in Biological Research For Nursing
Acknowledgments
Dr Withycombe would like to acknowledge participation as a Fellow in the 2018, Transdisciplinary Research in Energetics and Cancer (TREC) National Cancer Institute Training Workshop which helped to inform this work (Grant #R25CA203650; PI: Irwin).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Oncology Nursing Society Foundation; and the American Cancer Society Institutional Research Grant through Winship Cancer Center (IRG-17-181-04).
Author Note: Two facilities are equally affiliated with Dr. Withycombe’s work on this study. Data collection occurred while employed at Nell Hodgson Woodruff School of Nursing (Emory University), with statistical analysis and manuscript efforts occurring while employed at Clemson University.
Supplemental Material: Supplemental material for this article is available online.
ORCID iD
Janice S. Withycombe, PhD, RN, FAAN https://orcid.org/0000-0002-3042-9049
References
- American College of Sports Medicine (2019). ACSM guidelines for exercise and cancer. American College of Sports Medicine. Retrieved August 16, 2021 from. https://www.acsm.org/blog-detail/acsm-certified-blog/2019/11/25/acsm-guidelines-exercise-cancer-download. [Google Scholar]
- Bai J., Behera M., Bruner D. W. (2018). The gut microbiome, symptoms, and targeted interventions in children with cancer: a systematic review. Support Care Cancer, 26(2), 427–439. 10.1007/s00520-017-3982-3. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Drai D., Elmer G., Kafkafi N., Golani I. (2001). Controlling the false discovery rate in behavior genetics research. Behavioural Brain Research, 125(1-2), 279–284. 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- Berger A. M., Abernethy A. P., Atkinson A., Barsevick A. M., Breitbart W. S., Cella D., Cimprich B., Cleeland C., Eisenberger M. A., Escalante C. P., Jacobsen P. B., Kaldor P., Ligibel J. A., Murphy B. A., O'Connor T., Pirl W. F., Rodler E., Rugo H. S., Thomas J., Wagner L. I. (2010). NCCN Clinical Practice Guidelines Cancer-related fatigue. Journal of the National Comprehensive Cancer Network: JNCCN, 8(8), 904–931. 10.6004/jnccn.2010.0067. [DOI] [PubMed] [Google Scholar]
- Bouatra S., Aziat F., Mandal R., Guo A. C., Wilson M. R., Knox C., Bjorndahl T. C., Krishnamurthy R., Saleem F., Liu P., Dame Z. T., Poelzer J., Huynh J., Yallou F. S., Psychogios N., Dong E., Bogumil R., Roehring C., Wishart D. S. (2013). The human urine metabolome. Plos One, 8(9), Article e73076. 10.1371/journal.pone.0073076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. L., Sok P., Taylor O., Woodhouse J. P., Bernhardt M. B., Raghubar K. P., Kahalley L. S., Lupo P. J., Hockenberry M. J., Scheurer M. E. (2021). Cerebrospinal fluid metabolomic profiles associated with fatigue during treatment for pediatric acute lymphoblastic leukemia. J Pain Symptom Manage, 61(3), 464–473. 10.1016/j.jpainsymman.2020.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carle A. C., Bevans K. B., Tucker C. A., Forrest C. B. (2021). Using nationally representative percentiles to interpret PROMIS pediatric measures. Quality of Life Research: an International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation, 30(4), 997–1004. 10.1007/s11136-020-02700-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castell L. M., Yamamoto T., Phoenix J., Newsholme E. A. (1999). The Role of Tryptophan in Fatigue in Different Conditions of Stress. In Huether G., Kochen W., Simat T. J., Steinhart H. (Eds.), Tryptophan, Serotonin, and Melatonin. Advances in Experimental Medicine and Biology (467, pp. 697-704). Boston, MA: Springer. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control (2021). BMI percentile calculator for child and teen. Center for Disease Control. https://www.cdc.gov/healthyweight/bmi/calculator.html. [Google Scholar]
- Cramp F., Byron-Daniel J. (2012). Exercise for the management of cancer-related fatigue in adults. The Cochrane Database of Systematic Reviews Electronic Resource, 11, CD006145. 10.1002/14651858.CD006145.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorelli B., Gallè F., De Vito C., Duranti G., Iachini M., Zaccarin M., Preziosi Standoli J., Ceci R., Romano F., Liguori G., Romano Spica V., Sabatini S., Valeriani F., Cattaruzza M. S. (2021). Can Physical Activity Influence Human Gut Microbiota Composition Independently of Diet? A Systematic Review, 13(6). 10.3390/nu13061890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekkekakis P., Hall E. E., Petruzzello S. J. (2005). Variation and homogeneity in affective responses to physical activity of varying intensities: an alternative perspective on dose-response based on evolutionary considerations. Journal of Sports Sciences, 23(5), 477–500. 10.1080/02640410400021492. [DOI] [PubMed] [Google Scholar]
- Hilfiker R., Meichtry A., Eicher M., Nilsson Balfe L., Knols R. H., Verra M. L., Taeymans J. (2018). Exercise and other non-pharmaceutical interventions for cancer-related fatigue in patients during or after cancer treatment: a systematic review incorporating an indirect-comparisons meta-analysis. British Journal of Sports Medicine, 52(10), 651–658. 10.1136/bjsports-2016-096422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds P. S., Nuss S. L., Ruccione K. S., Withycombe J. S., Jacobs S., DeLuca H., Faulkner C., Liu Y., Cheng Y. I., Gross H. E., Wang J., DeWalt D. A. (2013). PROMIS pediatric measures in pediatric oncology: valid and clinically feasible indicators of patient-reported outcomes. Pediatric Blood & Cancer, 60(3), 402–408. 10.1002/pbc.24233. [DOI] [PubMed] [Google Scholar]
- Hinds P. S., Weaver M. S., Withycombe J. S., Baker J. N., Jacobs S. S., Mack J. W., Maurer S. H., McFatrich M., Pinheiro L. C., Reeve B. B., Wang J. (2021). Subjective toxicity profiles of children in treatment for cancer: A new guide to supportive care? Journal Pain Symptom Manage, 61(6), 1188-1195. 10.1016/j.jpainsymman.2020.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T. T., Ness K. K. (2011). Exercise interventions in children with cancer: a review. International Journal Pediatr, 2011, 461512. 10.1155/2011/461512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H. P., Wen F. H., Yang T. Y., Lin Y. C., Tsai J. C., Shun S. C., Jane S. W., Chen M. L. (2019). The effect of a 12-week home-based walking program on reducing fatigue in women with breast cancer undergoing chemotherapy: A randomized controlled study. Int J Nurs Stud, 99, 103376. 10.1016/j.ijnurstu.2019.06.007. [DOI] [PubMed] [Google Scholar]
- Jasbi P., Shi X., Chu P., Elliott N., Hudson H., Jones D., Serrano G., Chow B., Beach T. G., Liu L., Jentarra G., Gu H. (2021). Metabolic profiling of neocortical tissue discriminates Alzheimer's disease from mild cognitive impairment, high pathology controls, and normal controls. Journal of Proteome Research, 20(9), 4303–4317. 10.1021/acs.jproteome.1c00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R. S., Kelly M. P., Kelly P. (2020). Metabolomics, physical activity, exercise and health: A review of the current evidence. Biochim Biophys Acta Mol Basis Dis, 1866(12), 165936. 10.1016/j.bbadis.2020.165936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney A. Y., Blair C. K., Guest D. D., Ani J. K., Harding E. M., Amorim F., Boyce T., Rodman J., Ford C. G., Schwartz M., Rosenberg L., Foran O., Gardner J., Lin Y., Arap W., Irwin M. R. (2019). Biobehavioral effects of Tai Chi Qigong in men with prostate cancer: Study design of a three-arm randomized clinical trial. Contemp Clin Trials Commun, 16, 100431. 10.1016/j.conctc.2019.100431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharski D., Lange E., Ross A. B., Svedlund S., Feldthusen C., Önnheim K., Mannerkorpi K., Gjertsson I. (2019). Moderate-to-high intensity exercise with person-centered guidance influences fatigue in older adults with rheumatoid arthritis. Rheumatology International, 39(9), 1585–1594. 10.1007/s00296-019-04384-8. [DOI] [PubMed] [Google Scholar]
- Li S., Dunlop A. L., Jones D. P., Corwin E. J. (2016). High-Resolution metabolomics: Review of the field and implications for nursing science and the study of preterm birth. Biological Research for Nursing, 18(1), 12–22. 10.1177/1099800415595463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon D. E., Starkweather A., Yao Y., Garrett T., Kelly D. L., Menzies V., Dereziński P., Datta S., Kumar S., Jackson-Cook C. (2018). Pilot study of metabolomics and psychoneurological symptoms in women with early stage breast cancer. Biological Research for Nursing, 20(2), 227–236. 10.1177/1099800417747411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malysse C., Romero-Galisteo R. P., Merchán-Baeza J. A., Durán-Millán J. I., González-Sánchez M., Galan-Mercant A. (2021). Physical activity promotion programmes in childhood cancer patients and their impact on fatigue and pain: A systematic review. Children (Basel), 8(12). 10.3390/children8121119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller J., Hoch A. M., Zoller V., Oberhoffer R. (2018). Feasibility of physical activity assessment with wearable devices in children aged 4-10 years-a pilot study. Front Pediatr, 6, 5. 10.3389/fped.2018.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Z., Chong J., Zhou G., de Lima Morais D. A., Chang L., Barrette M., Gauthier C., Jacques P. É., Li S., Xia J. (2021). MetaboAnalyst 5.0: narrowing the gap between raw spectra and functional insights. Nucleic Acids Research, 49(W1), W388–W396. 10.1093/nar/gkab382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phipson B., Lee S., Majewski I. J., Alexander W. S., Smyth G. K. (2016). Robust hyperparameter estimation protects against hypervariable genes and improves power to detect differential expression. Ann Appl Stat, 10(2), 946–963. 10.1214/16-AOAS920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roager H. M., Licht T. R. (2018). Microbial tryptophan catabolites in health and disease. Nature Communications, 9(1), 3294. 10.1038/s41467-018-05470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X., Wang S., Jasbi P., Turner C., Hrovat J., Wei Y., Liu J., Gu H. (2019). Database-assisted globally optimized targeted mass spectrometry (dGOT-MS): Broad and reliable metabolomics analysis with enhanced identification. Analytical Chemistry, 91(21), 13737–13745. 10.1021/acs.analchem.9b03107. [DOI] [PubMed] [Google Scholar]
- Tabone M., Bressa C., García-Merino J. A., Moreno-Pérez D., Van E. C., Castelli F. A., Fenaille F., Larrosa M. (2021). The effect of acute moderate-intensity exercise on the serum and fecal metabolomes and the gut microbiota of cross-country endurance athletes. Scientific Reports, 11(1), 3558. 10.1038/s41598-021-82947-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thissen D., Liu Y., Magnus B., Quinn H., Gipson D. S., Dampier C., Huang I. C., Hinds P. S., Selewski D. T, Reeve B. B., Gross H. E., DeWalt D. A. (2016). Estimating minimally important difference (MID) in PROMIS pediatric measures using the sca.le-judgment method. Quality of Life Research: an International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation, 25(1), 13–23. 10.1007/s11136-015-1058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson D., Diorio C., Beyene J., Sung L. (2014). Effect of exercise on cancer-related fatigue: a meta-analysis. Archives of Physical Medicine and Rehabilitation, 93(8), 675–686. 10.1097/PHM.0000000000000083. [DOI] [PubMed] [Google Scholar]
- Tudor-Locke C., Craig C. L., Beets MW, Belton S, Cardon G. M., Duncan S., Hatano Y., Lubans D. R., Olds T. S., Raustorp A., Rowe D. A., Spence J. C., Tanaka S, Blair S. N. (2011). How many steps/day are enough? for children and adolescents. Int J Behav Nutr Phys Act, 8, 78. 10.1186/1479-5868-8-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varni J. W., Magnus B., Stucky B. D., Liu Y., Quinn H., Thissen D., Gross H. E., Huang I. C., DeWalt D. A. (2014). Psychometric properties of the PROMIS (R) pediatric scales: precision, stability, and comparison of different scoring and administration options. Quality of Life Research: an International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation, 23(4), 1233–1243. 10.1007/s11136-013-0544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y., Jasbi P., Shi X., Turner C., Hrovat J., Liu L., Rabena Y., Porter P., Gu H. (2021). Early breast cancer detection using untargeted and targeted metabolomics. Journal of Proteome Research, 20(6), 3124–3133. 10.1021/acs.jproteome.1c00019. [DOI] [PubMed] [Google Scholar]
- Withycombe J. S., Baek M. J., Jordan D. H., Thomas N. J., Hale S. (2018). Pilot study evaluating physical activity and fatigue in adolescent oncology patients and survivors during summer camp. J Adolesc Young Adult Oncol, 7(2), 254–257. 10.1089/jayao.2017.0074. [DOI] [PubMed] [Google Scholar]
- Wurz A, McLaughlin E., Chamorro Viña C., Grimshaw S. L., Hamari L., Götte M., Kesting S., Rossi F., van der Torre P., Guilcher G. M. T., McIntyre K., Culos-Reed S. N. (2021. a). Advancing the Field of Pediatric Exercise Oncology: Research and Innovation Needs. Curr Oncol, 28(1), 619–629. 10.3390/curroncol28010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurz A., McLaughlin E., Lategan C., Chamorro Vina C., Grimshaw S. L., Hamari L., Gotte M., Kesting S., Rossi F., van der Torre P., Guilcher G. M. T., McIntyre K., Culos-Reed S. N. (2021. b). The international pediatric oncology exercise guidelines (iPOEG). Transl Behav Med, 11(10), 1915-1922. 10.1093/tbm/ibab028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurz A., McLaughlin E., Lategan C., Ellis K., Culos-Reed S. N. (2021. c). Synthesizing the literature on physical activity among children and adolescents affected by cancer: evidence for the international Pediatric Oncology Exercise Guidelines (iPOEG). Transl Behav Med, 11(3), 699–708. 10.1093/tbm/ibaa136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C., Beitler J. J., Higgins K. A., Conneely K., Dwivedi B., Felger J., Wommack E. C., Shin D. M., Saba N. F., Ong L. Y., Kowalski J., Bruner D. W., Miller A. H. (2016. a). Fatigue is associated with inflammation in patients with head and neck cancer before and after intensity-modulated radiation therapy. Brain, Behavior, and Immunity, 52, 145–152. 10.1016/j.bbi.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Q., Moore S. C., Keadle S. K., Xiang Y. B., Zheng W., Peters T. M., Leitzmann M. F., Ji B. T., Sampson J. N., Shu X. O., Matthews C. E. (2016. b). Objectively measured physical activity and plasma metabolomics in the Shanghai Physical Activity Study. International Journal Epidemiol, 45(5), 1433–1444. 10.1093/ije/dyw033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M., Tuo H., Wang S., Zhao L. (2020). The effects of dietary nutrition on sleep and sleep disorders. Mediators of Inflammation, 2020, 3142874. 10.1155/2020/3142874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Djukovic D., Deng L., Gu H., Himmati F., Abu Zaid M., Chiorean E. G., Raftery D. (2015). Targeted serum metabolite profiling and sequential metabolite ratio analysis for colorectal cancer progression monitoring. Analytical and Bioanalytical Chemistry, 407(26), 7857–7863. 10.1007/s00216-015-8984-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for sj-pdf-1-brn-10.1177_10998004221085029 Metabolites Associated With Fatigue and Physical Activity in Childhood Cancer by Janice S. Withycombe, PhD, RN, FAAN, Ronald Eldridge, PhD, Yan Jin, PhD, Haiwai Gu, PhD, Sharon M. Castellino, MD, and Dorothy D. Sears, PhD in Biological Research For Nursing