Abstract
Platelets are key mediators of thrombus formation and inflammation during the acute phase of ischaemic stroke. Particularly, the platelet glycoprotein (GP) receptors GPIbα and GPVI have been shown to mediate platelet adhesion and activation in the ischaemic brain. GPIbα and GPVI blockade could reduce infarct volumes and improve functional outcome in mouse models of acute ischaemic stroke, without concomitantly increasing intracerebral haemorrhage. However, the functional role of platelets during long-term stroke recovery has not been elucidated so far.
Thus, we here examined the impact of platelet depletion on post-stroke recovery after transient middle cerebral artery occlusion (tMCAO) in adult male mice. Platelet depleting antibodies or isotype control were applied from day 3–28 after tMCAO in mice matched for infarct size. Long-term functional recovery was assessed over the course of 28 days by behavioural testing encompassing motor and sensorimotorical functions, as well as anxiety-like or spontaneous behaviour. Whole brain flow cytometry and light sheet fluorescent microscopy were used to identify resident and infiltrated immune cell types, and to determine the effects of platelet depletion on the cerebral vascular architecture, respectively.
We found that delayed platelet depletion does not improve long-term functional outcome in the tMCAO stroke model. Immune cell abundance, the extent of thrombosis and the organisation of the cerebral vasculature were also comparable between platelet-depleted and control mice. Our study demonstrates that, despite their critical role in the acute stroke setting, platelets appear to contribute only marginally to tissue reorganisation and functional recovery at later stroke stages.
Keywords: Behaviour, Cerebral ischaemia, Long-term, Platelet depletion, tMCAO, Stroke, Platelets, Transient middle cerebral artery occlusion, Recovery
Highlights
-
•
Stable and safe global platelet depletion can be achieved for a prolonged period.
-
•
Platelets only play a minor role in neurological recovery during the chronic phase.
-
•
Platelet depletion after infarct maturation does not alter inflammatory response.
-
•
Cerebral architecture after stroke is not influenced by delayed platelet depletion.
1. Introduction
Ischaemic stroke is the second leading cause of death and third leading cause of long-lasting disability worldwide (Collaborators, 2019). Mechanical thrombectomy and lysis therapy with recombinant tissue plasminogen activator (rt-PA) remain the only treatment options. The application of both approaches is restricted to the acute phase after stroke. To date, there are no pharmacological treatments available to enhance neurological recovery. Unlike acute stroke treatment, focusing on the recanalisation of the occluded vessel, recovery treatments target processes such as brain remodelling and plasticity. Preclinical research over the past decades has identified several promising candidates for the treatment of stroke sequelae, but none have been translated to the clinic. Latest clinical trials, e.g. treatment with fluoxetine, failed, regardless of promising data from experimental animal studies (Lundstrom et al., 2021).
As central cellular players in haemostasis and thrombosis, platelets have been thoroughly investigated as mediators of thrombus formation leading to ischaemic stroke. An emerging body of evidence increasingly indicates a wider role of platelets in the complex pathophysiology of stroke, as well as other diseases such as atherosclerosis, Alzheimer's disease and in the context of immune-related functions (Birnie et al., 2019; Ghoshal and Bhattacharyya, 2014; Golebiewska and Poole, 2015; Jenne et al., 2013; Nording and Langer, 2018; Wuescher et al., 2015). Since thrombosis and inflammation are closely intertwined during ischaemic stroke, a phenomenon referred to as thromboinflammation, platelets become increasingly important in stroke research.
We previously demonstrated that blocking the platelet glycoprotein receptors GPIbα or GPVI in cerebral ischaemia results in decreased infarct sizes and improved neurological performance, without increasing the risk of intracerebral haemorrhage (Kleinschnitz et al., 2007). Inhibition of GPIbα has been associated with reduced inflammatory responses (Schuhmann et al., 2017), and targeting GPVI attenuated infarct progression (Bieber et al., 2021). These protective effects were retained in aged and comorbid mice (Kraft et al., 2015) and even in combination with thrombolysis via rt-PA (Schuhmann et al., 2019). In particular, platelets appear to modify post-stroke neuroinflammation and angiogenesis through interaction with various immune cells (Nording et al., 2021; Packham et al., 2014; Schuhmann et al., 2020).
To date, the multifactorial contribution of platelets to stroke and ischaemia/reperfusion injury (Burkard et al., 2020; Stegner et al., 2019) was exclusively demonstrated in the acute phase up to 24 h after cerebral ischaemia. The potential for platelets to also contribute to long-term restorative processes in the central nervous system remains unknown.
Differences in study designs between experimental and clinical research are likely accountable for failed clinical translation. In almost all experimental studies, infarct volume was reported as the main end point, whereas in most clinical trials, the focus was put on neurological outcomes. In addition, the onset of treatment is significantly delayed in the clinical trials compared to experimental trials (Schmidt-Pogoda et al., 2020). In the study presented herein, we took these difficulties into account.
We subjected male C57BL/6 mice to 30 min of transient middle cerebral artery occlusion (tMCAO). Treatment with either platelet depletion or isotype control antibodies was initiated after infarct maturation 3 days post ischaemia (dpi) and continued until the end of the observation period at 28 dpi. To assess long-term functional recovery with clinically relevant parameters, we performed extensive behavioural testing, as well as flow cytometry and light sheet microscopy. With this comprehensive approach we aimed to unravel the functional role of platelets in a long-term observational period after cerebral ischaemia.
2. Methods
2.1. Study design
In this study, a total of 68 male C57BL/6N mice aged 10–12 weeks Charles River Laboratories, Sulzfeld, Germany) were included. Mice were housed in groups of 5 in individually ventilated cages (IVC) at constant room temperature (22 °C) and humidity (55 ± 5%). Circadian rhythm (12:12 h) was inverted to allow for behavioural testing during the day corresponding to the animals’ active phase in the dark. Water and food were accessible ad libitum.
All animal experiments were performed in agreement with the ARRIVE guidelines (Kilkenny et al., 2010) as well as the IMPROVE guidelines (Percie du Sert et al., 2017), approved by local state authorities (Landesamt für Natur, Umwelt und Verbraucherschutz NRW, LANUV) and conducted in agreement with the German Animal Welfare Act (German Ministry of Agriculture, Health, and Economic Cooperation). Mice showing comparable infarct sizes after infarct visualisation via magnetic resonance imaging (MRI) and volumetric analysis on day 3 after tMCAO were randomly assigned to treatment groups by a blinded person not involved in data acquisition and analysis. Antibody for in vivo platelet depletion in mice (#R300) or isotype control (#C301) were obtained from Emfret Analytics (Germany) and diluted 1:1 with phosphate buffered saline (PBS). Antibodies were administered via intraperitoneal (i.p.) injection with an initial dose at 3 dpi [3 μg/g] and injections every other day [1,5 μg/g] from day 10 onwards. Investigators performing surgery, behavioural testing and analysis of all collected data were blinded to group allocation. Unblinding was performed after completion of statistical analyses. Mice were not included in the study if they suffered from intracranial bleedings, haemorrhagic infarcts or had no infarct at all after MRI on 3 dpi. Mice were excluded from final analysis if they died before the experimental end point 28 days after onset of ischaemia. Of 68 tMCAO-operated animals, 53 were included in the final analysis. 15 mice were excluded for one or more of the reasons stated above, equally distributed between antibody and isotype control treatments.
2.2. Ischaemia model
Focal cerebral ischaemia was induced by 30 min of intraluminal transient middle cerebral artery occlusion (tMCAO) as previously described (Leinweber et al., 2021). Mice were anaesthetised with 4% isoflurane (Piramal) in 100% O2 for 2–4 min (World Precision Instruments, Small Animal Anesthesia System, EZ-7000). Anaesthesia was maintained with ∼2% isoflurane and body temperature kept at 37 °C during surgery, using a feedback-controlled warming device (World Precision Instruments, Animal Temperature Controller, ATC-2000). Following a midline skin incision in the neck, the right common carotid artery (CCA) and the external carotid artery (ECA) were dissected and ligated. The internal carotid artery (ICA) was temporarily closed with a vascular clip (Fine Science Tools Inc., Foster City, CA, USA). For induction of cerebral ischaemia, a silicon rubber-coated monofilament (#6023912, Doccol, Corporation, USA) was introduced through the common carotid artery and advanced into the right internal carotid artery to occlude the origin of the right middle cerebral artery (MCA). The intraluminal suture was left in place for 30 min. After that, mice were re-anaesthetised, and the monofilament withdrawn to allow reperfusion. The common carotid artery was ligated before suturing the skin wound.
For the assessment of general neurological deficits and motor function, the following Bederson Score (Bederson et al., 1986) was applied on days 1–7, 14, 21 and 28 post ischaemia: 0 no impairment, 1 flexion of one front limb, 2 flexion of one front limb and decreased lateral startle reflexes, 3 unidirectional circling, 4 circling and occasional turning around the longitudinal axis, 5 no spontaneous movement. Testing took place on a straight, rough plane to allow enough grip for the mice to move.
2.3. Magnetic resonance imaging
To analyse infarct size prior to treatment initiation and to scan for possible intracerebral bleeding, MRI was performed on a 3 T MRI unit (Biograph mMR, Siemens Healthineers, Germany) at 3 dpi. A commercially available dual channel surface coil designed for examining mice (Rapid Biomedical GmbH, Germany) was used. The imaging protocol included a coronal T2-weighted turbo spin-echo (TSE) sequence (resolution 0.2 x 0.2 × 0.8 mm³, 15 slices, TE = 105 ms, TR = 1660 ms, TA = 3:47 min) that was used for identifying infarct volume and a susceptibility weighted coronal T2-weighted gradient echo sequence (SWI, 0.1 x 0.1 × 0.2 mm³, 40 slices, TE = 20.6 ms, TR = 38 ms, TA = 6:01 min) for detecting haemorrhage. Refined localization was done beforehand with the help of short T1-weighted TSE sequences along each axis (0.2 x 0.2 × 1 mm³, 7 slices, TA = 17s).
The measurement was conducted under constant ketamine/xylazine anaesthesia. The T2-weighted TSE sequences were used for planimetric evaluation of oedema corrected infarct volume using the indirect measurement technique as well as evaluation of oedema formation. Calculation of infarct size was performed using ImageJ (National Institutes of Health, USA) according to the following equation: Vindirect (mm2) = [Vinfarct × (1-(Vih – Vch)/Vch)] × 1,6, whereas Vinfarct represents the hyper-intense area, Vih represents the ipsilateral hemisphere, Vch represents the contralateral hemisphere and 1,6 represents the spacing between the acquired individual images. Brain oedema was calculated according to the following equation: Oedema [%] = Vtotal × 100/(Vch × 2) - 100, whereas Vtotal represents the total brain volume and Vch represents the contralateral hemisphere. Calculation was performed for every second image and results summed up to determine infarct size or oedema formation, respectively.
2.4. Atrophy calculation
At 28 dpi, mice were sacrificed by ketamin/xylazin overdose, followed by transcardial perfusion with 20 ml PBS and extracted brains were cut in 3 * 2 mm thick brain slices (Mouse Brain Slicer Matrix, # BSMAS001-1, Zivic Instruments, USA). Slices were immediately collected into cold PBS and scanned. Atrophy was calculated as: Atrophy [%] = 100 – ((Vtotal28dpi/Vtotal3dpi) × 100, where Vtotal28dpi represents the total brain volume on day 28 (overlay scanner) and Vtotal3dpi represents the total brain volume on day 3 (MRI images). Loss of tissue mass is expressed as percentage compared to initial values at treatment start for each individual animal.
2.5. Haemogram profiling
30 μl–50 μl of blood were collected in tubes containing EDTA and carefully mixed to avoid clotting. The ProCyte Dx haematology analyser (IDEXX, USA) was used for the generation of a complete haemogram.
2.6. Behavioural testing
Mice were familiarised with handling and the experimental procedures and surroundings on a daily basis for 7 days prior to study. To ensure comparability of results all behaviour experiments were conducted in the same order throughout different sets of experiments. Testing always took place during the day (10 a.m.–4 p.m.) corresponding to the animal's active phase and were carried out in dim red light and low noise environment (behavioural unit) (Peirson et al., 2018). Mice from experimental sets not assigned to behavioural testing were handled and housed the same way to avoid inconsistencies (Leiter et al., 2019; Peirson et al., 2018).
2.7. Rotarod
To assess motoric deficits, Rotarod Test was performed using the RotaRod Advanced for Mice (TSE Systems, Germany) (Balkaya et al., 2013; Bouet et al., 2007; Jones and Roberts, 1968). Mice were trained for the task before induction of ischaemia, including 1 trial per day on 3 consecutive days. During training sessions, the rod was set to constant speed of 4 rpm for 180 s. Animals were placed on the moving rod, facing away from the experimenter. If animals fell from the rod they were immediately placed back on top. Before induction of ischaemia a baseline measurement was recorded under standard testing conditions. This included accelerated speed from 4 rpm to 40 rpm within 300 s. Animals were placed on a constantly moving rod, facing away from the experimenter, before starting testing profile. Mice falling from the rod were placed back on top 3 times during the whole trial before trial was stopped for the respective animal. All mice were evaluated once before starting the second trial, with 2 trials being conducted on each indicated testing day. Total time on the rod was recorded for both trials and the mean value used for analysis. Mice not showing sufficient motivation to perform the task were excluded from analysis for the specific time point.
2.8. Adhesive Removal Test
To assess sensorimotorical deficits, the Adhesive Removal Test was performed (Balkaya et al., 2013; Bouet et al., 2007; Schallert et al., 1982) Mice were trained for the task before induction of ischaemia, including 1 trial per day on 3 consecutive days. Mice were placed inside a round arena (15 cm diameter, opaque bottom, transparent walls) for a habituation period of 60 s. Afterwards the animal was taken out of the arena and adhesive tapes placed on the palms of both front paws (3 × 4 mm) before placing the animal back inside the arena until removal of the tapes. Baseline measurement was performed before induction of ischaemia under standard testing conditions. Mice were again placed inside the arena for 60 s without intervention before the adhesive tapes were placed on both front paws. Time to removal for each of the tapes was recorded. If the tapes were not removed after 120 s, they were removed by the experimenter and the mouse placed back to the home cage. On each indicated testing day, 3 consecutive trials were conducted with each animal. The Δ time to removal between both paws was determined for each trial before using the mean of the best 2 trials for analysis. Mice not showing sufficient motivation to perform the task in the given time were excluded from analysis for the specific time point.
2.9. Grip Strength
To assess hind limb muscle strength a hand-held force gauge (Physimeter 906 MC-B, Erichsen, Germany) was used (Alamri et al., 2018; Cabe et al., 1978). The mouse was handled by the experimenter with a tight grip behind its head, still allowing the hind limbs to move freely. Afterwards the hind limbs were gently pulled over a metal bar and the maximum force applied before the mouse lost its grip was recorded. Per side, 3 measurements were conducted while trials were interrupted by short resting phases. The mean of all three trials per side was calculated for analysis. Trials with mice either not showing sufficient motivation to perform the task or defending actively against the experimenters’ grip were excluded from analysis for the specific time point or redone, respectively.
2.10. Elevated Plus Maze
To investigate the effects of platelet depletion on anxiety and anxiety-like behaviour the Elevated Plus Maze Test was performed. The maze is characterised by its cross-shape with equally sized arms (W 5 cm x L 25 cm). It consists of 2 open arms and 2 arms being flanked by opaque walls (H 17 cm), connected by a common intersection (5 cm × 5 cm) with the maze being elevated 70 cm above the ground. The test is able to detect anxiety due to the natural tendency of mice to avoid open areas and preferably reside near walls or objects, called thigmotaxis (Lister, 1987; Walf and Frye, 2007). Animals were placed in the central intersection, facing away from the experimenter and towards an open arm. Mice were then allowed to explore the maze for 5 min without interruption. Camera recording was performed using an automatic tracking system (EthoVision XT15, Noldus, Netherlands) and data exported for statistical analysis. Parameters like total time spent in open or closed arms, total time the mice spent moving or not moving and the time spent in different body elongation states were analysed.
2.11. Open Field
To investigate the effects of platelet depletion on spontaneous motor and exploration behaviours, the Open Field Test was performed (Kraeuter et al., 2019; Reinboth et al., 2016). The arena is square-shaped (W 50 cm x L 50 cm), flanked by opaque walls (H 30 cm) and contains no visual clues for the mouse to allow for spontaneous exploration behaviour. The arena was based on infrared (IR) translucent material, placed upon an infrared light-box (850 nm, TSE Systems, Germany) to increase contrast for video recording. Animals were placed in the centre, facing away from the experimenter. Mice were then allowed to explore the arena for 5 min without interruption. Camera recording was performed using an automatic tracking system (EthoVision XT15, Noldus, Netherlands) and data exported for statistical analysis. Parameters like total time spent in the centre or borders of the arena, total time the mice spent moving or not moving and the time spent in different body elongation states were analysed.
2.12. Haematoxylin & eosin staining
Histological stainings with haematoxylin and eosin (HE) were performed with samples obtained at 28 dpi. Brain slices were prepared as aforementioned. The desired plane containing the striatum as a region of interest was placed in a cryomold, embedded in Tissue Tek O.C.T. Compound (# 4583, Sakura Finetek, Netherlands) and fresh frozen on dry ice. Samples were stored at −80 °C until further processing. 7 μm thick cryosections were cut and stored at −20 °C until staining.
Sections were post-fixed with 4% paraformaldehyde (PFA) for 10 min and briefly washed with distilled water before incubation in haemalaun solution (#T865.2, Carl Roth, Germany) for 10 min. After washing with warm, flowing tap water for 15 min, sections were again washed with distilled water for 2 min before incubation in eosin solution (#X883.2, Carl Roth, Germany) containing acetic acid for 45 s. Sections were again thoroughly washed with tap water before undergoing treatment with ascending ethanol concentrations (70%–96% - 100%). Subsequently, sections were washed in xylene (2 × 3 min) and mounted with Cytoseal (# 8312-4, ThermoFisher Scientific, USA) to prevent bleaching.
2.13. Immunohistochemistry
Immunohistochemistry was performed with samples obtained at 28 dpi. Tissue sampling, preparation and storage were conducted as stated before.
Sections were post-fixed with 4% PFA for 10 min and washed (3 × 4 min) with PBS containing 0.1% Triton-X for tissue permeabilisation. After blocking for 1 h with 2.5% bovine serum albumin, 2.5% normal donkey serum and 0.2% Triton X-100 in PBS at room temperature to prevent unspecific binding, sections were incubated with primary antibodies in a 1:1 mixture of blocking solution and PBS overnight at 4 °C. Incubation was continued for 30 min at room temperature the next day followed by washing (4 × 4 min) with PBS. Secondary antibodies were prepared in a mixture of primary antibody solution (Blocking solution and PBS) and a stock solution containing 600 nM 4’,6-diamidino-2-phenylindole,dihydrochloride (DAPI) (1:1) and incubated for 1 h at room temperature. Subsequently, sections were washed (4 × 4 min) in PBS and mounted with a medium containing Mowiol and 1,4-Diazabicyclo-(2,2,2)octan (DABCO) to prevent bleaching.
The following antibodies were used for immunohistochemistry at the given dilution: CD31 (# MCA2388, Bio-Rad, USA; diluted 1:100); GPIX (#M051-0, Emfret Analytics, Germany; diluted 1:100); NeuN (# ABN91, Merck, Germany; diluted 1:1000). Highly Cross-Absorbed Alexa Flour Secondary Antibodies (ThermoFisher Scientific, USA) were used in a 1:500 dilution.
Images were taken with a Leica DMi8 microscope using a Hamamatsu C11440-22 CU camera for fluorescence stainings and a DMC2900 camera for non-fluorescence stainings as well as the associated Leica Application Suite X Software (LasX 3.0.2.16120). Image processing was performed using ImageJ (National Institutes of Health, USA).
2.14. Flow cytometry
Flow cytometry analysis was performed at 28 dpi. Animals were deeply anaesthetised and transcardially perfused with 20 ml of PBS. Sample processing was performed according to the protocol for the dissociation of inflamed neural tissue using the Multi Tissue Dissociation Kit 1 for the gentleMACS Octo Dissociator (# 130-110-201, Miltenyi Biotec, Germany). Briefly, brains were removed, washed in cold PBS, and manually shredded. Dissociation was performed using a dedicated enzyme mix. After debris and red blood cell removal samples were stained for flow cytometric analysis with the MACSQuant Analyzer 10 (Miltenyi Biotec, Germany) using the following antibodies: CD45 (# 103115, BioLegend, USA), CD3 (# 100203, BioLegend, USA), CD4 (# 100534, BioLegend, USA), CD8 (# 100751, BioLegend, USA), CD11b (# 550993, BD Biosciences, USA), Ly6G (# 553128, BD Biosciences, USA). Analysis of cell populations was performed using FlowJo 10.8.0 Software (BD Biosciences, USA).
2.15. Light sheet fluorescent microscopy
Light sheet fluorescent microscopy (LSFM) was performed with samples obtained at 28 dpi. Brains were prepared for LSFM as described elsewhere (Mohamud Yusuf et al., 2020). Briefly, animals were deeply anaesthetised and transcardially perfused with 40 ml of PBS, followed by 40 ml of 4% PFA, followed by 8 ml hydrogel containing FITC conjugated albumin. Mice were placed head down in ice water to allow hardening of the hydrogel. Brains were removed and post-fixed overnight in 4% PFA before dehydration using tetrahydrofuran (THF, Sigma-Aldrich, USA) and final clearing using ethyl cinnamate (ECi, # 112372, Sigma-Aldrich, USA). Samples were stored in ECi until image acquisition.
Image acquisition and analysis was performed as formerly described (Mohamud Yusuf et al., 2020). Summarily, brains were imaged using an Ultramicroscope (LaVision BioTec, Germany) and vascular quantification in striatal areas was performed using the Imaris Software (Oxford Instruments, UK) and ImageJ (National Institutes of Health, USA).
2.16. Statistical analyses
Statistical evaluation was performed with the GraphPad Prism 8.0 software package (GraphPad Software, USA). To test for normal distribution of the data sets D'Agostino and Pearson omnibus normality test was used. If required, Shapiro-Wilk test was used. In case of normal distribution unpaired, two-tailed Student's t-test corrected by Holm-Sidak method was used to compare 2 groups, otherwise Mann-Whitney-U test was used. Survival rates displayed as Kaplan-Meier curves were evaluated using Log-rank (Mantel-Cox) test. Statistical significance is indicated by asterisks with P ≤ 0.05*, P ≤ 0.01**, P ≤ 0.001*** and P ≤ 0,0001****. Results are either displayed as scatter plots or bar graphs with means ± standard deviation (SD).
3. Results
3.1. Stable platelet depletion does not alter the haemogram profile
To investigate the effects of platelet depletion solely on the recovery phase after stroke, treatment was initiated after infarct maturation on day 3. Moreover, to ensure comparable baseline conditions, MRI scans based on T2* sequences were performed. Only mice with comparable infarct volumes (Supplemental Fig. 1) and showing no intracerebral haemorrhage were randomly assigned to treatment groups before starting therapy with either platelet depletion antibody or the respective isotype control (Fig. 1A).
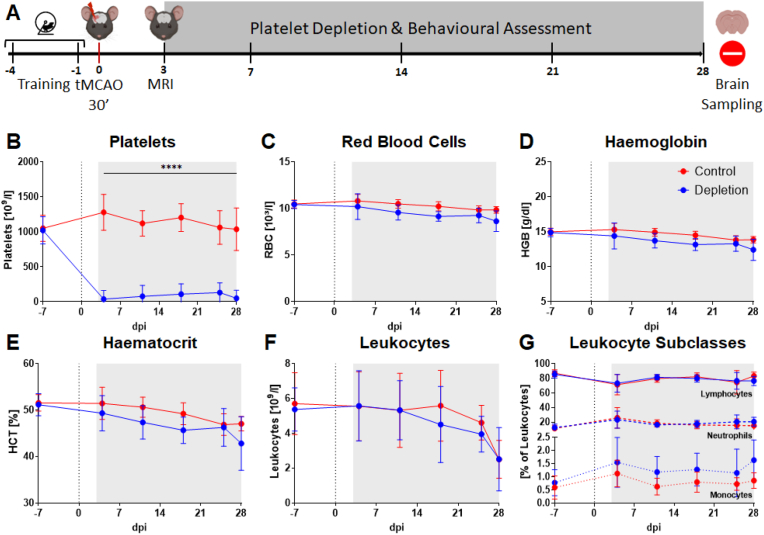
Fig. 1.
Study design and longitudinal assessment of peripheral blood parameters until 28dpi. Training and baseline recording for various behavioural tests was performed before induction of tMCAO (d0). Animals showing comparable infarct sizes were randomly assigned to treatment groups. Antibody for in vivo platelet depletion in mice or isotype control were administered from day 3 to day 28 after stroke. Behavioural testing was performed constantly until the end of the experiment 28 days after tMCAO (A). Sufficient depletion of platelets could be observed after the first antibody injection until the end of the experiment (B). All other blood parameters showed no or only minor differences between the treatment groups (C, D, E, F, G). Grey area: duration of treatment. In case of normal distribution unpaired, two-tailed Student's t-test corrected by Holm-Sidak method was used for statistical analysis, otherwise Mann-Whitney-U test was applied. N = 17/17.
First, to confirm sufficient and stable platelet depletion and assess changes in haematological parameters, blood sampling was performed once a week, including a baseline measurement one week before surgery, throughout the 28-day experimental period. Indeed, stable depletion of platelets was achieved over the entire period (Fig. 1B) while other haematological parameters including erythrocytes, haemoglobin, haematocrit, and leukocytes remained largely unaffected in both treatment groups (Fig. 1C–G). Importantly, HE staining from animals after stroke revealed no increased susceptibility to microbleedings in the ischaemic brain (Supplemental Fig. 2). Moreover, no additional severe bleeding events in organs or tissue were evident during sacrificing and tissue preparation, indicative of a high specificity and tolerability of the platelet depletion strategy.
3.2. Effect of platelet depletion on long-term functional outcome after stroke
Comparison of survival rates, displayed as Kaplan-Meier curves, revealed no reduced mortality in animals treated with platelet depletion antibodies. Moreover, platelet depletion treatment did not ameliorate body weight loss compared to isotype treated animals until 28 dpi (Fig. 2A and B).
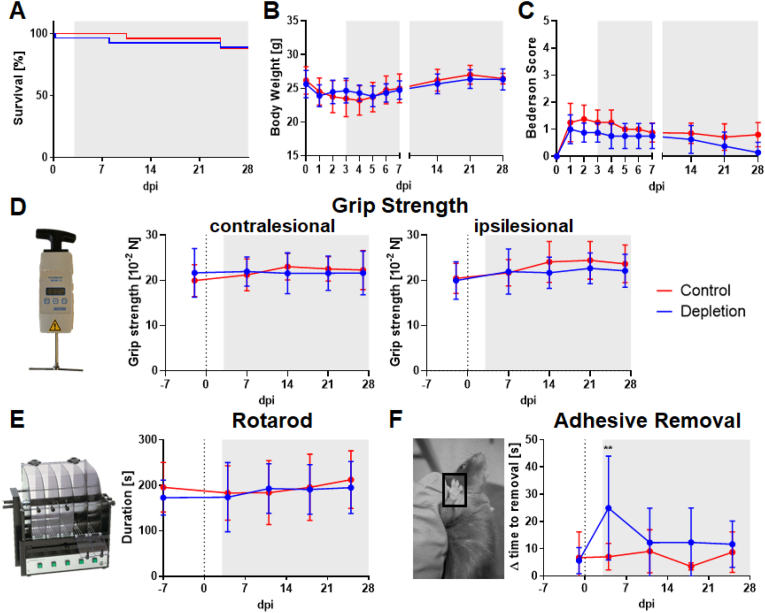
Fig. 2.
Basic parameters as well as extensive behavioural testing did not indicate changes between the treatment groups. Survival curves showed no significant increase in mortality in any of the treatment groups (A). Animals typically lost weight during the first week after tMCAO and fully recovered until 28 dpi (B). 24 h after tMCAO motor performance, assessed by the Bederson score, was slightly impaired, however after 28 days, both treatment groups recovered equally (C). Several behavioural tests were performed over the 28 days observation period, including Grip Strength (D), Rotarod (E) and Adhesive Removal (F). Until the end of the experiment, none of these tests detected any significant differences between platelet depleted and control animals. Grey area: duration of treatment. In case of normal distribution unpaired, two-tailed Student's t-test corrected by Holm-Sidak method was used for statistical analysis, otherwise Mann-Whitney-U test was applied. N = 26/27 (Survival), N = 15/16 (Body Weight), N = 8/8 (Bederson score), N = 17/18 (Grip Strength, Rotarod, Adhesive Removal).
Moreover, four independent neuro- and sensorimotor outcome parameters were assessed to identify potential functional recovery within the experimental period. Surprisingly, no significant improvement was detected in platelet-depleted animals compared to isotype-treated mice as shown by the Bederson Score (Fig. 2C), the Grip Strength (Fig. 2D) and the Rotarod test (Fig. 2E), where muscle strength and coordination is evaluated. Additionally, the Adhesive Removal Test was used to evaluate sensorimotoric deficits upon stroke. Only the left forepaw is impaired, indicated by a higher delta time, whereas a decrease of delta time indicates recovery (Fig. 2F). Only a temporary decrease in delta time was noted in platelet depleted animals on day 4 after a 30 min transient occlusion of the middle cerebral artery but this effect disappeared afterwards.
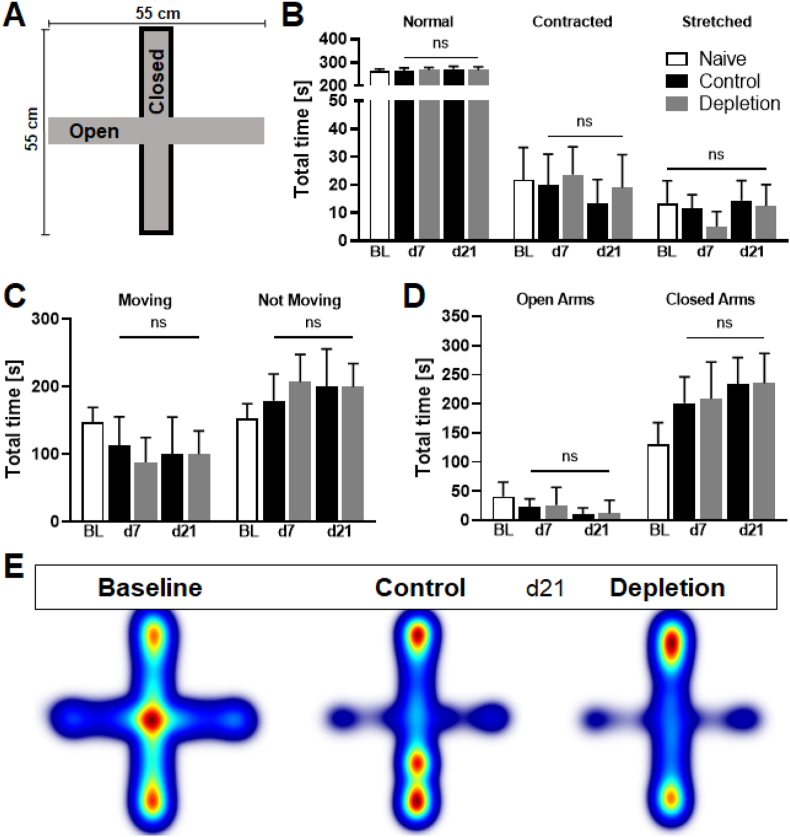
Platelet depletion is capable of acutely reducing anxiety-like behaviour, presumably due to a decreased abundance of Iba1+ and CD3+ cells in the hippocampus (Kocovski et al., 2019). Thus, the Elevated Plus Maze was performed to investigate the long-term effect of delayed and sustained platelet depletion on anxiety-like behaviour (Fig. 3A). Parameters such as preferred areas of residency, body elongation states, and the total time spent moving were also used for analysis. Interestingly, none of these parameters changed significantly between the treatment groups at any time point (Fig. 3B–D). Heatmaps generated from data obtained at 21 dpi visualise the measured preferences to stay in closed arms in both experimental groups (Fig. 3E).
Fig. 3.
Anxiety-like behaviour was not altered between treatment groups. During the Elevated Pus Maze Test (A) numerous parameters like the time spent in different body elongation states (B), the total time the mouse was moving or not moving (C) and the total time spent either in open or closed arms (D) were measured and analysed. None of the investigated parameters changed significantly between platelet depletion and control animals at any analysed time point. Heatmaps generated from data collected at day 21 post ischaemia are used to visualise the areas mice reside the most, with red coloured areas meaning more time spent and blue coloured areas less time spent (E). In case of normal distribution unpaired, two-tailed Student's t-test corrected by Holm-Sidak method was used for statistical analysis, otherwise Mann-Whitney-U test was applied. N = 17/17. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
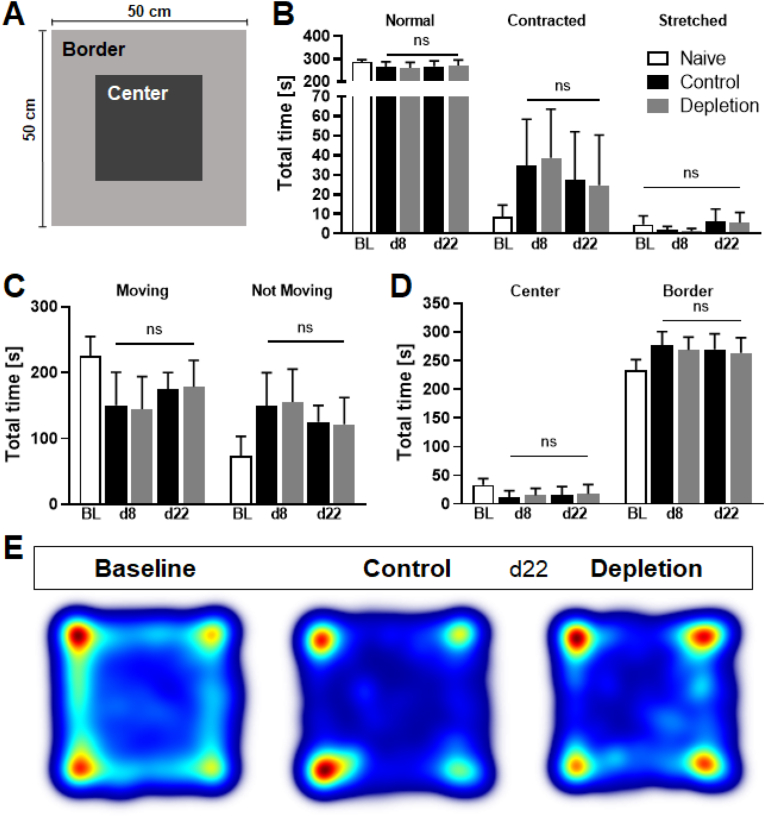
Additionally, to assess spontaneous exploration behaviour in depleted animals, the Open Field test was used (Fig. 4A). Similarly, parameters such as preferred areas of residency, time spent in different body elongation states, or time spent moving remained comparable in control and platelet-depleted groups (Fig. 4B–D). As previously shown, heatmaps were generated from data collected at 22 dpi to visualise the preferred areas mice resided (Fig. 4E). Considering that mice after stroke tend to spend more time in border zones of the arena than in central regions, our data indicate that platelets do not interfere with anxiety-like or spontaneous behaviour after cerebral ischaemia.
Fig. 4.
Spontaneous or exploration behaviour was not altered between treatment groups. During the Open Field Test (A) numerous parameters like the time spent in different body elongation states (B), the total time the mouse was moving or not moving (C) and the total time spent either in the centre or the border of the arena (D) were measured and analysed. None of the investigated parameters changed significantly between platelet depletion and control animals at any analysed time point. Heatmaps generated from data collected at day 22 post ischaemia are used to visualise the areas mice reside the most, with red coloured areas meaning more time spent and blue coloured areas less time spent (E). In case of normal distribution unpaired, two-tailed Student's t-test corrected by Holm-Sidak method was used for statistical analysis, otherwise Mann-Whitney-U test was applied. N = 17/17. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.3. Role of long-term platelet depletion in microthrombosis, neuronal density and vascular architecture after stroke
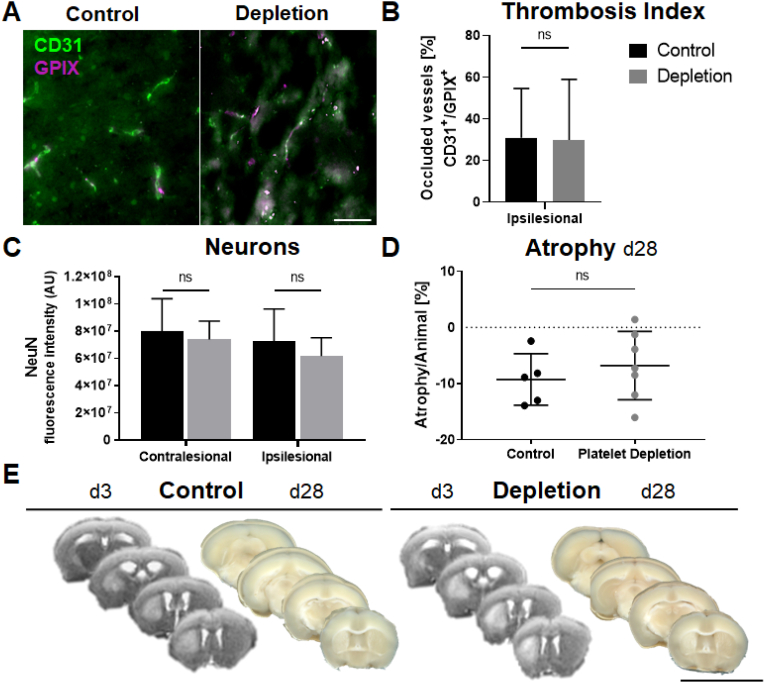
Research on the prolonged occurrence of thrombus formation after ischaemic events remains controversial (Gob et al., 2021; Tang et al., 2014). Therefore, to evaluate the impact of platelet depletion on thrombus formation, brain tissue from day 28 post stroke was co-stained to identify platelet clots (GPIX+) in blood vessels (CD31+). Then, the thrombosis index was calculated as a ratio of occluded vs. open vessels. Interestingly, platelet depletion did not affect thrombi formation from day 3 after stroke onwards compared to isotype control treatment (Fig. 5A and B), suggesting that thrombus formation is already completed upon this time point.
Fig. 5.
Platelet depletion does not affect neuronal density, microthrombi formation or atrophy in the chronic phase after ischaemic stroke. Disregarding differences between contra- and ipsilesional hemispheres, both treatment groups showed the same levels of CD31+/GPIX+ vessel staining (A, B) as well as NeuN+ (C) neuronal staining. Atrophy of brain tissue was calculated based on scans from brain slices at the end of the experiment on day 28 (D). No difference occurred between platelet depleted and control antibody treated animals. Representative images were taken from each group to show infarct size and atrophy (E). Infarcts are visible as brighter areas in MRI scans, located in the striatum. Unstained brain scans from day 28 post ischaemia show bright areas in rostral slices as a residue from the infarcted area. In case of normal distribution unpaired, two-tailed Student's t-test corrected by Holm-Sidak method was used for statistical analysis, otherwise Mann-Whitney-U test was applied. Scale bar indicates 50 μm [B]; 1 cm [E]. N = 5/7 (Atrophy), N = 5/6 (Immunohistochemistry).
Furthermore, platelets are known to contribute to neurogenesis via secretion of various trophic factors (Hayon et al., 2012, 2013). Thus, we also analysed whether platelet depletion interferes with neuronal density on day 28 after stroke. However, immunohistochemistry analysis using the neuronal marker NeuN revealed no differences in neuronal density while comparing platelet depleted animals to their matched control groups (Fig. 5C). To calculate the proportion of atrophic brain tissue as a surrogate of tissue remodelling and repair at the end of the observational period, whole brains were sliced, scanned, and volumes compared to MRI scans acquired at 3 dpi. Surprisingly, data analysis revealed no difference in atrophy patterns in both experimental groups (Fig. 5D and E), implying that delayed platelet depletion does not interfere with dismantlement of brain tissue after stroke.
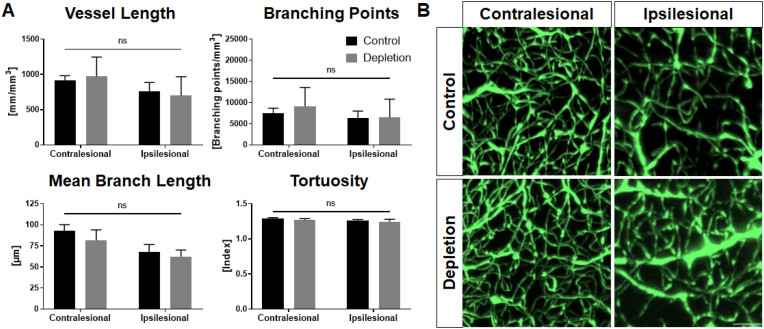
Various studies investigating vascularisation provide conflictive results on the contribution of platelets. Indeed, platelet-derived microparticles (PMP) similar to other platelet-derived factors appeared to be positive regulators of neovascularisation (Hayon et al., 2012, 2013; Packham et al., 2014), although these findings still remain controversial (Nording et al., 2021). Hence, we performed light sheet fluorescent microscopy (LSFM) analysis using brains 28 days after stroke to further identify a potential effect of platelet depletion in vascularisation. Specifically, various parameters including vessel length, the number of branching points, mean branch length and tortuosity of the entire vasculature were assessed in the striatum as a region of interest due to infarct localisation. However, LSFM and subsequent data analysis did not unveil changes in the cerebral vasculature of both treatment groups (Fig. 6). Disregarding alterations between the ipsi- and contralesional hemisphere no differences concerning the platelet depletion and the control group occurred. Representative images were taken to visualise the aforementioned differences between the hemispheres (Fig. 6B).
Fig. 6.
Light sheet analysis of whole brains did not expose any changes in the cerebral vascular system. Mice were sacrificed at 28 dpi and whole brains prepared for light sheet analysis of the vascular system. Vessel length, number of branching points, mean branch length and tortuosity were evaluated. None of the parameters were altered in any of the treatment groups (A). Representative images were taken to visualise differences between the ipsi- and contralesional hemispheres (B). In case of normal distribution unpaired, two-tailed Student's t-test corrected by Holm-Sidak method was used for statistical analysis, otherwise Mann-Whitney-U test was applied. Scale bar indicates 50 μm. N = 5/6.
3.4. Impact of platelet depletion on post-stroke immune cell infiltration
Platelets are deeply involved in immune cell recruitment resulting in the formation of platelet-leukocyte aggregates (PLAs) and the promotion of inflammatory processes leading to worsening stroke outcomes (Nording et al., 2022; Schuhmann et al., 2017). Furthermore, soluble CD84, shed from platelets, stimulates T cells and shapes their migratory behaviour after cerebral ischaemia (Schuhmann et al., 2020).
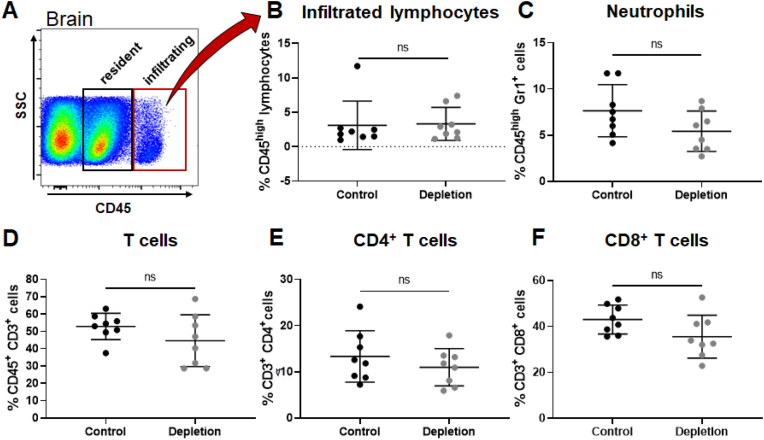
Therefore, we assessed the ability of platelets to modify the local cellular inflammatory response upon stroke by examination of infiltrated immune cells. Relative expression of the global lymphocyte marker CD45 was used for a clear discrimination between resident and infiltrating lymphocytes (Fig. 7A). Interestingly, brain tissue collected at 28 dpi revealed no change in infiltrated immune cell populations including infiltrated lymphocytes, neutrophils and T-cell numbers, including subclasses (Fig. 7B–F).
Fig. 7.
Flow cytometry analysis of whole brains revealed no differences in the abundance of various immune cell subsets 28 dpi. Whole brains from 28 dpi were used to investigate infiltrated immune cells in both groups (A). Both treatment groups did not show differences the number of infiltrated lymphocytes (B), neutrophils (C). Besides, proportion of T cells (D), including T-cell subclasses (E, F) were not altered in the brain parenchyma of platelet depleted or isotype treated animals. In case of normal distribution unpaired, two-tailed Student's t-test corrected by Holm-Sidak method was used for statistical analysis, otherwise Mann-Whitney-U test was applied. N = 8/8.
4. Discussion
Acute platelet depletion has been shown to blunt the immediate neuroinflammation after ischaemic stroke in experimental models. We here show that a delayed and sustained depletion of platelets beginning after completion of the acute stroke phase (from 3 dpi onwards), does not improve long-term stroke recovery in mice subjected to tMCAO. In keeping with this, platelet depletion had no mitigating effect on late-stage microthrombosis, local inflammatory responses, angiogenesis or neuronal density in the post-infarcted mouse brain.
Previous studies in which platelet inhibition was commenced in the acute phase of ischaemic stroke were able to demonstrate reduced infarct sizes as well as improved neurological outcome at 24 h after tMCAO. Here, both inhibition of GPIbα and GPVI using blocking antibodies showed protective effects (Kleinschnitz et al., 2007; Schuhmann et al., 2017). The observation of these protective effects were confirmed with a novel GPIbα inhibitor (Anfibatide) (Chen et al., 2018; Li et al., 2015) which has therefore been subject of clinical trials (Li et al., 2021; Zheng et al., 2021). In contrast, inhibition of platelet aggregation by blocking GPIIb/IIIa failed to be protective and increased the risk of intracerebral haemorrhage (Kleinschnitz et al., 2007; Kraft et al., 2015; Stegner et al., 2019). To date, studies addressing platelet blockade in ischaemic stroke have been limited to the acute phase, and end point analysis has occurred no later than 24 h after ischaemia.
Only few studies have examined the long-term significance of platelet signalling, and these have yielded largely contradictory findings. In an animal model of multiple sclerosis, experimental autoimmune encephalomyelitis (EAE), platelet depletion was able to improve anxiety-like behaviour in the course of EAE until day 14 (Kocovski et al., 2019). On the other hand, supplementation with platelet lysate in a permanent model of stroke improved long-term (90 dpi) behavioural deficits, indicating that one or more factors contained within platelets exert sustained protective actions (Hayon et al., 2013). The identity of these factors, whether they are actually secretion products or normally retained within platelets, and their effect if applied after infarct maturation, remain to be defined. Results obtained in dissimilar models such as EAE and ischaemic stroke are not overall comparable and need to be carefully reviewed, given that multiple sclerosis and stroke are fundamentally different disease entities that, however, share relevant molecular pathomechanisms involved in CNS damage (Li et al., 2018).
In our long-term stroke outcome model, platelet depletion was initiated after complete infarct maturation at day 3 after stroke and continued until day 28. In contrast to preceding studies that focussed on short-term outcome, we detected no effect of platelet depletion on neurological post-stroke recovery, compared to control animals. Our results suggest that platelet involvement in stroke sequelae is restricted to the acute phase, with only a minor role in behavioural recovery during the chronic phase after ischaemic stroke. The improved neurological outcomes seen after interfering with platelet signalling in the acute phase can likely be attributed to smaller infarct sizes, rather than to a direct influence of platelets on behavioural recovery once the infarct has matured. This implies that antiplatelet strategies applied thereafter are likely to be ineffective.
A recently published study supports thrombotic activity persisting to 24 h after reperfusion (Gob et al., 2021). A prolonged thrombogenesis lasting up to 28 dpi has been reported (Tang et al., 2014), but the employed photoactivated dye model is rather artificial and unlikely to reflect the actual in vivo stroke setting. In our model of tMCAO with platelet depletion initiated after infarct maturation on day 3 after ischaemia, we did not observe differences in the number of residual thrombi compared to controls, indicating that thrombus formation predominantly occurs within the acute phase of ischaemia and reperfusion, and is not modified by delayed removal of platelets. Surprisingly, also brain atrophy as a surrogate of tissue remodelling and repair was unaltered in platelet depleted animals on day 28 post stroke compared to isotype control treated animals.
Of emerging interest is the role of platelets and platelet-secreted factors in post-stroke neurogenesis and angiogenesis. Platelets store and release considerable amounts of trophic factors that are critical for neuro-as well as angiogenic processes, such as platelet factor 4 (PF4), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF) and brain-derived neurotrophic factor (BDNF) (Leiter et al., 2019; Mazzucco et al., 2010). The picture however remains controversial. In a model of permanent MCAO, rat brains were covered with polymer gel foam containing platelet derived microparticles (PMP). Infarcted brains after permanent occlusion exposed to PMP-containing gels displayed increased numbers of blood vessels and newly generated and proliferating neural cells in the affected areas, compared to controls (Hayon et al., 2012, 2013). In a muscle overload in vivo study, inhibition or depletion of platelets resulted in decreased sprouting and longitudinal splitting of vessels (Packham et al., 2014), underscoring the involvement of platelet-derived factors in angiogenesis. Conversely, platelet-secreted PF4 negatively regulated neovascularisation in part by inhibiting endothelial cell functions such as tube formation and migration in vitro and in vivo after hind limb ischaemia (Nording et al., 2021).
Thus, the precise role of platelets in angiogenesis, and particularly neurogenesis after cerebral infarction, remains obscure. The influence of platelets on angiogenic effects in a long-term model of transient cerebral ischaemia has not been investigated before. In this study we could not detect any differences in post-stroke vascular architecture or neuronal density after delayed platelet depletion compared to control animals.
In keeping with the lack of improvement in behavioural recovery and prolonged thrombogenesis, we also found no effect of platelet depletion on immune cell infiltration to the cerebral parenchyma, nor on cell counts in peripheral blood. This was unexpected, given that several lines of evidence implicate platelets in various inflammatory pathophysiologies (Golebiewska and Poole, 2015; Irving et al., 2004; Jenne et al., 2013; Nording and Langer, 2018; Rossaint et al., 2018), by interacting directly with different immune cell types such as T cells and leukocytes, to orchestrate the inflammatory response. Platelet-leukocyte aggregates (PLA) are avidly formed as a result of platelet P-selectin binding to leukocyte P-selectin glycoprotein ligand (PSGL) −1, while PLA adhesion is stabilised through the specific interaction between GPIbα externalised on the platelet surface and macrophage-1 integrin on leukocytes (Nording and Langer, 2018; Nording et al., 2022; Rawish et al., 2020). Accordingly, blocking GPIbα or depleting platelets diminishes PLA formation in vitro or in vivo. PLA levels are known to increase after stroke and promote transmigration of leukocytes to the brain parenchyma, resulting in ongoing neuroinflammation and worsening of stroke outcome (Beuker et al., 2021; Jayaraj et al., 2019; Marquardt et al., 2009). In addition, a study using the tMCAO model revealed that blockade of GPIbα in the acute phase attenuated the local inflammatory response, as evidenced by diminished infiltration of monocytes and CD4+ T cells into the infarcted area as well as decreased expression of pro-inflammatory cytokines. (Schuhmann et al., 2017).
The type of experimental model that is used, and the time-point of study, can critically impact on the extent and quality of the detected immune cell infiltration (Zhou et al., 2013). For instance, significantly fewer immune cells can be found in brain parenchyma 5 days after tMCAO compared to permanent occlusion (pMCAO). Neutrophils are amongst the first immune cells to infiltrate the brain after a transient ischaemic insult, peaking at approximately 1–3 days after the event, and represent critical determinants for neurological outcome. T cells infiltrate the ischaemic hemisphere around 24 h after stroke, with most lymphocytes being found near blood vessels between day 3 and 7 (Gelderblom et al., 2009; Jayaraj et al., 2019). Accumulation of cytotoxic (CD8+) T cells and T helper cells (CD4+), peaks within the brain tissue at around 14 days after permanent vessel occlusion (Heindl et al., 2021). The delayed onset of platelet-depleting antibody treatment on 3 dpi presumably does not interfere with these very early infiltration processes. Although we cannot exclude dynamic alterations in immune cell composition between 3 and 28 dpi, platelet depletion is unlikely to impact substantially on this, given that all parameters assessed at 28 dpi, including behavioural outcome and inflammatory markers, did not differ between the groups.
Despite the novel approach, this study has its own limitations in terms of treatment regimen and observational end points, since former studies typically aimed to rescue the penumbra or attenuate IRI in the acute or sub-acute phase after ischaemic stroke. However, adjusting the start of platelet depletion treatment to earlier time points was not a feasible option due to the fact that an earlier platelet depletion treatment would most likely provoke haemorrhage. Furthermore, we aimed to examine the effects of platelets on processes solely included in recovery after complete infarct maturation at 3 dpi. Therefore, the end point was chosen based on the translational approach and the overarching need in general stroke research to discover targets that execute regenerative effects until the long-term stage after cerebral ischaemia. Collectively, our findings indicate that the delayed depletion of platelets, i.e. after completion of the acute stroke phase, exerts no beneficial impact on long-term stroke recovery. Given that infarct size and brain inflammation are significantly reduced on day 1 after tMCAO following platelet inhibition in the acute phase (Kleinschnitz et al., 2007; Kraft et al., 2015; Schuhmann et al., 2017, 2020), the pathophysiological role of platelets obviously differs depending on the different stroke stages, specifically the acute vs. chronic context. Identification of the specific pathways leading to these divergent platelet-mediated effects will be the demanding objective of upcoming investigations.
Author contributions
RS, FL, SM and CK conceived the study. RS, FS, CD and AMY conducted the experiments. RS analysed the data and wrote the manuscript. All authors carefully revised the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We thank Steffi Hezel and Kristina Wagner for excellent technical assistance, as well as Dr. Marcel Gratz for guidance and help with all MRI related questions. We also thank the Light Microscopy Unit of the Imaging Center Essen (IMCES). This study was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Projektnummer 374031971 – TRR 240. AIC was supported by the DFG Walter Benjamin Program (ref. DFG CA 2642/1-1) and the Förderprogramm der Corona-Stiftung im Stifterverband.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2022.100493.
Contributor Information
Rebecca D. Steubing, Email: rebecca.steubing@uk-essen.de.
Fabian Szepanowski, Email: fabian.szepanowski@uk-essen.de.
Christina David, Email: christina.david@uk-essen.de.
Ayan Mohamud Yusuf, Email: ayan.mohamudyusuf@uk-essen.de.
Stine Mencl, Email: stine.mencl@uk-essen.de.
Anne-Kathrin Mausberg, Email: anne-kathrin.mausberg@uk-essen.de.
Harald F. Langer, Email: harald.langer@uksh.de.
Manuela Sauter, Email: manuela.sauter@uksh.de.
Cornelius Deuschl, Email: cornelius.deuschl@uk-essen.de.
Michael Forsting, Email: michael.forsting@uk-essen.de.
Anke C. Fender, Email: anke.fender@uk-essen.de.
Dirk M. Hermann, Email: dirk.hermann@uk-essen.de.
Ana I. Casas, Email: anaisabel.casasguijarro@uk-essen.de.
Friederike Langhauser, Email: friederike.langhauser@uk-essen.de.
Christoph Kleinschnitz, Email: christoph.kleinschnitz@uk-essen.de.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Alamri F.F., Shoyaib A.A., Biggers A., Jayaraman S., Guindon J., Karamyan V.T. Applicability of the grip strength and automated von Frey tactile sensitivity tests in the mouse photothrombotic model of stroke. Behav. Brain Res. 2018;336:250–255. doi: 10.1016/j.bbr.2017.09.008. [DOI] [PubMed] [Google Scholar]
- Balkaya M., Krober J., Gertz K., Peruzzaro S., Endres M. Characterization of long-term functional outcome in a murine model of mild brain ischemia. J. Neurosci. Methods. 2013;213:179–187. doi: 10.1016/j.jneumeth.2012.12.021. [DOI] [PubMed] [Google Scholar]
- Bederson J.B., Pitts L.H., Tsuji M., Nishimura M.C., Davis R.L., Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- Beuker C., Strecker J.K., Rawal R., Schmidt-Pogoda A., Ruck T., Wiendl H., Klotz L., Schabitz W.R., Sommer C.J., Minnerup H., Meuth S.G., Minnerup J. Immune cell infiltration into the brain after ischemic stroke in humans compared to mice and rats: a systematic review and meta-analysis. Transl. Stroke Res. 2021;12:976–990. doi: 10.1007/s12975-021-00887-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieber M., Schuhmann M.K., Kollikowski A.M., Stegner D., Nieswandt B., Pham M., Stoll G. Targeting platelet glycoprotein VI attenuates progressive ischemic brain damage before recanalization during middle cerebral artery occlusion in mice. Exp. Neurol. 2021;344 doi: 10.1016/j.expneurol.2021.113804. [DOI] [PubMed] [Google Scholar]
- Birnie E., Claushuis T.A.M., Koh G., Limmathurotsakul D., Day N.P.J., Roelofs J., Ware J., Hou B., de Vos A.F., van der Poll T., van 't Veer C., Wiersinga W.J. Thrombocytopenia impairs host defense against burkholderia pseudomallei (melioidosis) J. Infect. Dis. 2019;219:648–659. doi: 10.1093/infdis/jiy541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouet V., Freret T., Toutain J., Divoux D., Boulouard M., Schumann-Bard P. Sensorimotor and cognitive deficits after transient middle cerebral artery occlusion in the mouse. Exp. Neurol. 2007;203:555–567. doi: 10.1016/j.expneurol.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Burkard P., Vogtle T., Nieswandt B. Platelets in thrombo-inflammation: concepts, mechanisms, and therapeutic strategies for ischemic stroke. Hämostaseologie. 2020;40:153–164. doi: 10.1055/a-1151-9519. [DOI] [PubMed] [Google Scholar]
- Cabe P.A., Tilson H.A., Mitchell C.L., Dennis R. A simple recording grip strength device. Pharmacol. Biochem. Behav. 1978;8:101–102. doi: 10.1016/0091-3057(78)90131-4. [DOI] [PubMed] [Google Scholar]
- Chen C., Li T., Zhao Y., Qian Y., Li X., Dai X., Huang D., Pan T., Zhou L. Platelet glycoprotein receptor Ib blockade ameliorates experimental cerebral ischemia-reperfusion injury by strengthening the blood-brain barrier function and anti-thrombo-inflammatory property. Brain Behav. Immun. 2018;69:255–263. doi: 10.1016/j.bbi.2017.11.019. [DOI] [PubMed] [Google Scholar]
- Collaborators G.B.D.S. Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:439–458. doi: 10.1016/S1474-4422(19)30034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelderblom M., Leypoldt F., Steinbach K., Behrens D., Choe C.U., Siler D.A., Arumugam T.V., Orthey E., Gerloff C., Tolosa E., Magnus T. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40:1849–1857. doi: 10.1161/STROKEAHA.108.534503. [DOI] [PubMed] [Google Scholar]
- Ghoshal K., Bhattacharyya M. Overview of platelet physiology: its hemostatic and nonhemostatic role in disease pathogenesis. Sci. World J. 2014;2014 doi: 10.1155/2014/781857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gob V., Voll M.G., Zimmermann L., Hemmen K., Stoll G., Nieswandt B., Schuhmann M.K., Heinze K.G., Stegner D. Infarct growth precedes cerebral thrombosis following experimental stroke in mice. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-02360-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golebiewska E.M., Poole A.W. Platelet secretion: from haemostasis to wound healing and beyond. Blood Rev. 2015;29:153–162. doi: 10.1016/j.blre.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayon Y., Dashevsky O., Shai E., Brill A., Varon D., Leker R.R. Platelet microparticles induce angiogenesis and neurogenesis after cerebral ischemia. Curr. Neurovascular Res. 2012;9:185–192. doi: 10.2174/156720212801619018. [DOI] [PubMed] [Google Scholar]
- Hayon Y., Dashevsky O., Shai E., Varon D., Leker R.R. Platelet lysates stimulate angiogenesis, neurogenesis and neuroprotection after stroke. Thromb. Haemostasis. 2013;110:323–330. doi: 10.1160/TH12-11-0875. [DOI] [PubMed] [Google Scholar]
- Heindl S., Ricci A., Carofiglio O., Zhou Q., Arzberger T., Lenart N., Franzmeier N., Hortobagyi T., Nelson P.T., Stowe A.M., Denes A., Edbauer D., Liesz A. Chronic T cell proliferation in brains after stroke could interfere with the efficacy of immunotherapies. J. Exp. Med. 2021;218 doi: 10.1084/jem.20202411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving P.M., Macey M.G., Shah U., Webb L., Langmead L., Rampton D.S. Formation of platelet-leukocyte aggregates in inflammatory bowel disease. Inflamm. Bowel Dis. 2004;10:361–372. doi: 10.1097/00054725-200407000-00007. [DOI] [PubMed] [Google Scholar]
- Jayaraj R.L., Azimullah S., Beiram R., Jalal F.Y., Rosenberg G.A. Neuroinflammation: friend and foe for ischemic stroke. J. Neuroinflammation. 2019;16:142. doi: 10.1186/s12974-019-1516-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenne C.N., Urrutia R., Kubes P. Platelets: bridging hemostasis, inflammation, and immunity. Int. J. Lab. Hematol. 2013;35:254–261. doi: 10.1111/ijlh.12084. [DOI] [PubMed] [Google Scholar]
- Jones B.J., Roberts D.J. The quantiative measurement of motor inco-ordination in naive mice using an acelerating rotarod. J. Pharm. Pharmacol. 1968;20:302–304. doi: 10.1111/j.2042-7158.1968.tb09743.x. [DOI] [PubMed] [Google Scholar]
- Kilkenny C., Browne W.J., Cuthill I.C., Emerson M., Altman D.G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschnitz C., Pozgajova M., Pham M., Bendszus M., Nieswandt B., Stoll G. Targeting platelets in acute experimental stroke: impact of glycoprotein Ib, VI, and IIb/IIIa blockade on infarct size, functional outcome, and intracranial bleeding. Circulation. 2007;115:2323–2330. doi: 10.1161/CIRCULATIONAHA.107.691279. [DOI] [PubMed] [Google Scholar]
- Kocovski P., Jiang X., D'Souza C.S., Li Z., Dang P.T., Wang X., Chen W., Peter K., Hale M.W., Orian J.M. Platelet depletion is effective in ameliorating anxiety-like behavior and reducing the pro-inflammatory environment in the Hippocampus in murine experimental autoimmune encephalomyelitis. J. Clin. Med. 2019;8 doi: 10.3390/jcm8020162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraeuter A.K., Guest P.C., Sarnyai Z. The open Field test for measuring locomotor activity and anxiety-like behavior. Methods Mol. Biol. 2019;1916:99–103. doi: 10.1007/978-1-4939-8994-2_9. [DOI] [PubMed] [Google Scholar]
- Kraft P., Schuhmann M.K., Fluri F., Lorenz K., Zernecke A., Stoll G., Nieswandt B., Kleinschnitz C. Efficacy and safety of platelet glycoprotein receptor blockade in aged and comorbid mice with acute experimental stroke. Stroke. 2015;46:3502–3506. doi: 10.1161/STROKEAHA.115.011114. [DOI] [PubMed] [Google Scholar]
- Leinweber J., Mizurini D.M., Francischetti I.M.B., Fleischer M., Hermann D.M., Kleinschnitz C., Langhauser F. Elastase inhibitor agaphelin protects from acute ischemic stroke in mice by reducing thrombosis, blood-brain barrier damage, and inflammation. Brain Behav. Immun. 2021;93:288–298. doi: 10.1016/j.bbi.2020.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiter O., Seidemann S., Overall R.W., Ramasz B., Rund N., Schallenberg S., Grinenko T., Wielockx B., Kempermann G., Walker T.L. Exercise-induced activated platelets increase adult hippocampal precursor proliferation and promote neuronal differentiation. Stem Cell Rep. 2019;12:667–679. doi: 10.1016/j.stemcr.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B.X., Dai X., Xu X.R., Adili R., Neves M.A.D., Lei X., Shen C., Zhu G., Wang Y., Zhou H., Hou Y., Ni T., Pasman Y., Yang Z., Qian F., Zhao Y., Gao Y., Liu J., Teng M., Marshall A.H., Cerenzia E.G., Li M.L., Ni H. In vitro assessment and phase I randomized clinical trial of anfibatide a snake venom derived anti-thrombotic agent targeting human platelet GPIbalpha. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-91165-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Chen L., Ma X., Cui P., Lang W., Hao J. Shared gene expression between multiple sclerosis and ischemic stroke. Front. Genet. 2018;9:598. doi: 10.3389/fgene.2018.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T.T., Fan M.L., Hou S.X., Li X.Y., Barry D.M., Jin H., Luo S.Y., Kong F., Lau L.F., Dai X.R., Zhang G.H., Zhou L.L. A novel snake venom-derived GPIb antagonist, anfibatide, protects mice from acute experimental ischaemic stroke and reperfusion injury. Br. J. Pharmacol. 2015;172:3904–3916. doi: 10.1111/bph.13178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister R.G. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- Lundstrom E., Isaksson E., Greilert Norin N., Nasman P., Wester P., Martensson B., Norrving B., Wallen H., Borg J., Hankey G.J., Hackett M.L., Mead G.E., Dennis M.S., Sunnerhagen K.S. Effects of fluoxetine on outcomes at 12 Months after acute stroke: results from EFFECTS, a randomized controlled trial. Stroke. 2021;52:3082–3087. doi: 10.1161/STROKEAHA.121.034705. [DOI] [PubMed] [Google Scholar]
- Marquardt L., Anders C., Buggle F., Palm F., Hellstern P., Grau A.J. Leukocyte-platelet aggregates in acute and subacute ischemic stroke. Cerebrovasc. Dis. 2009;28:276–282. doi: 10.1159/000228710. [DOI] [PubMed] [Google Scholar]
- Mazzucco L., Borzini P., Gope R. Platelet-derived factors involved in tissue repair-from signal to function. Transfus. Med. Rev. 2010;24:218–234. doi: 10.1016/j.tmrv.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Mohamud Yusuf A., Hagemann N., Schulten S., Rausch O., Wagner K., Hussner T., Qi Y., Totzeck M., Kleinschnitz C., Squire A., Gunzer M., Hermann D.M. Light sheet microscopy using FITC-albumin followed by immunohistochemistry of the same rehydrated brains reveals ischemic brain injury and early microvascular remodeling. Front. Cell. Neurosci. 2020;14 doi: 10.3389/fncel.2020.625513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nording H., Baron L., Haberthur D., Emschermann F., Mezger M., Sauter M., Sauter R., Patzelt J., Knoepp K., Nording A., Meusel M., Meyer-Saraei R., Hlushchuk R., Sedding D., Borst O., Eitel I., Karsten C.M., Feil R., Pichler B., Erdmann J., Verschoor A., Chavakis E., Chavakis T., von Hundelshausen P., Kohl J., Gawaz M., Langer H.F. The C5a/C5a receptor 1 axis controls tissue neovascularization through CXCL4 release from platelets. Nat. Commun. 2021;12:3352. doi: 10.1038/s41467-021-23499-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nording H., Langer H.F. Complement links platelets to innate immunity. Semin. Immunol. 2018;37:43–52. doi: 10.1016/j.smim.2018.01.003. [DOI] [PubMed] [Google Scholar]
- Nording H., Sauter M., Lin C., Steubing R., Geisler S., Sun Y., Niethammer J., Emschermann F., Wang Y., Zieger B., Nieswandt B., Kleinschnitz C., Simon D.I., Langer H.F. Activated platelets upregulate beta2 integrin mac-1 (CD11b/CD18) on dendritic cells, which mediates heterotypic cell-cell interaction. J. Immunol. 2022 doi: 10.4049/jimmunol.2100557. [DOI] [PubMed] [Google Scholar]
- Packham I.M., Watson S.P., Bicknell R., Egginton S. In vivo evidence for platelet-induced physiological angiogenesis by a COX driven mechanism. PLoS One. 2014;9 doi: 10.1371/journal.pone.0107503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirson S.N., Brown L.A., Pothecary C.A., Benson L.A., Fisk A.S. Light and the laboratory mouse. J. Neurosci. Methods. 2018;300:26–36. doi: 10.1016/j.jneumeth.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percie du Sert N., Alfieri A., Allan S.M., Carswell H.V., Deuchar G.A., Farr T.D., Flecknell P., Gallagher L., Gibson C.L., Haley M.J., Macleod M.R., McColl B.W., McCabe C., Morancho A., Moon L.D., O'Neill M.J., Perez de Puig I., Planas A., Ragan C.I., Rosell A., Roy L.A., Ryder K.O., Simats A., Sena E.S., Sutherland B.A., Tricklebank M.D., Trueman R.C., Whitfield L., Wong R., Macrae I.M. The IMPROVE guidelines (ischaemia models: procedural refinements of in vivo experiments) J. Cerebr. Blood Flow Metabol. 2017;37:3488–3517. doi: 10.1177/0271678X17709185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawish E., Nording H., Munte T., Langer H.F. Platelets as mediators of neuroinflammation and thrombosis. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.548631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinboth B.S., Koster C., Abberger H., Prager S., Bendix I., Felderhoff-Muser U., Herz J. Endogenous hypothermic response to hypoxia reduces brain injury: implications for modeling hypoxic-ischemic encephalopathy and therapeutic hypothermia in neonatal mice. Exp. Neurol. 2016;283:264–275. doi: 10.1016/j.expneurol.2016.06.024. [DOI] [PubMed] [Google Scholar]
- Rossaint J., Margraf A., Zarbock A. Role of platelets in leukocyte recruitment and resolution of inflammation. Front. Immunol. 2018;9:2712. doi: 10.3389/fimmu.2018.02712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallert T., Upchurch M., Lobaugh N., Farrar S.B., Spirduso W.W., Gilliam P., Vaughn D., Wilcox R.E. Tactile extinction: distinguishing between sensorimotor and motor asymmetries in rats with unilateral nigrostriatal damage. Pharmacol. Biochem. Behav. 1982;16:455–462. doi: 10.1016/0091-3057(82)90452-x. [DOI] [PubMed] [Google Scholar]
- Schmidt-Pogoda A., Bonberg N., Koecke M.H.M., Strecker J.K., Wellmann J., Bruckmann N.M., Beuker C., Schabitz W.R., Meuth S.G., Wiendl H., Minnerup H., Minnerup J. Why most acute stroke studies are positive in animals but not in patients: a systematic comparison of preclinical, early phase, and phase 3 clinical trials of neuroprotective agents. Ann. Neurol. 2020;87:40–51. doi: 10.1002/ana.25643. [DOI] [PubMed] [Google Scholar]
- Schuhmann M.K., Guthmann J., Stoll G., Nieswandt B., Kraft P., Kleinschnitz C. Blocking of platelet glycoprotein receptor Ib reduces "thrombo-inflammation" in mice with acute ischemic stroke. J. Neuroinflammation. 2017;14:18. doi: 10.1186/s12974-017-0792-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhmann M.K., Kraft P., Bieber M., Kollikowski A.M., Schulze H., Nieswandt B., Pham M., Stegner D., Stoll G. Targeting platelet GPVI plus rt-PA administration but not alpha2beta1-mediated collagen binding protects against ischemic brain damage in mice. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20082019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhmann M.K., Stoll G., Bieber M., Vogtle T., Hofmann S., Klaus V., Kraft P., Seyhan M., Kollikowski A.M., Papp L., Heuschmann P.U., Pham M., Nieswandt B., Stegner D. CD84 links T cell and platelet activity in cerebral thrombo-inflammation in acute stroke. Circ. Res. 2020;127:1023–1035. doi: 10.1161/CIRCRESAHA.120.316655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegner D., Klaus V., Nieswandt B. Platelets as modulators of cerebral ischemia/reperfusion injury. Front. Immunol. 2019;10:2505. doi: 10.3389/fimmu.2019.02505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y.H., Vital S., Russell J., Seifert H., Senchenkova E., Granger D.N. Transient ischemia elicits a sustained enhancement of thrombus development in the cerebral microvasculature: effects of anti-thrombotic therapy. Exp. Neurol. 2014;261:417–423. doi: 10.1016/j.expneurol.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf A.A., Frye C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007;2:322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuescher L.M., Takashima A., Worth R.G. A novel conditional platelet depletion mouse model reveals the importance of platelets in protection against Staphylococcus aureus bacteremia. J. Thromb. Haemostasis. 2015;13:303–313. doi: 10.1111/jth.12795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B., Li J., Jiang J., Xiang D., Chen Y., Yu Z., Zeng H., Ge J., Dai X., Liu J., Li B., Huo Y. Safety and efficacy of a platelet glycoprotein Ib inhibitor for patients with non-ST segment elevation myocardial infarction: a phase Ib/IIa study. Pharmacotherapy. 2021;41:828–836. doi: 10.1002/phar.2620. [DOI] [PubMed] [Google Scholar]
- Zhou W., Liesz A., Bauer H., Sommer C., Lahrmann B., Valous N., Grabe N., Veltkamp R. Postischemic brain infiltration of leukocyte subpopulations differs among murine permanent and transient focal cerebral ischemia models. Brain Pathol. 2013;23:34–44. doi: 10.1111/j.1750-3639.2012.00614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.