Summary
Different immune-mediated diseases have been described after SARS-CoV-2 vaccination, with antineutrophil cytoplasmic antibodies (ANCA)-associated vasculitis (AAV) being one of the possible side effects. In this study, a total of 35 patients presented ANCA for the first time during 2021, with the number during 2019 being 15. Twenty-seven out of thirty-five patients developed ANCA after vaccination. Two of them developed these antibodies after receiving the first dose (7.4%), and 25 patients developed ANCA after the second dose of the vaccine (92.6%), with BNT162b2 being the main vaccine received by these patients. In 97.1% of the patients who developed ANCA during 2021, the positivity of ANCA was accompanied by systemic involvement, with renal and respiratory tracts being the main organs affected. Therefore, an increase in the development of AAV has been observed during 2021 in comparison with 2019, which could be due to the administration of SARS-CoV-2 vaccine.
Subject areas: Health sciences, Clinical finding, Disease
Graphical abstract
Highlights
-
•
The development of ANCA and AAV could be one of the side effects caused by vaccination
-
•
Incidence of new ANCA positive patients in 2021 has increased in comparison with 2019
-
•
An increase in the frequency of anti-PR3 antibodies during 2021 has been observed
-
•
A predominance of respiratory manifestations has been observed in AAV during 2021
Health sciences; Clinical finding; Disease
Introduction
Mass vaccination against SARS-CoV-2 has been the most effective strategy to combat against SARS-CoV-2 infection, conferring a 95% protection against COVID-19 and reducing hospitalization and mortality (Mathieu et al., 2021). As of 10 January 2022, 90.4% of Spanish population over 12 years had received complete vaccination schedule against SARS-CoV-2, being BNT162b2 (Pfizer-BioNTech) (69.4%), mRNA-1273 (Moderna) (17.9%), the main vaccines administrated. Likewise, although to a lesser extent, AZD1222 (Oxford-AstraZeneca) (10.2%) and Ad26.CoV2.S (Janssen) (2.5%) vaccines have also been used (“https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/vacunaCovid19.htm,” n.d.). The mentioned vaccines have been shown to be safe and effective, and the most prevalent short-term side effects have mostly involved injection site reaction. Severe related adverse events have been rare. However, different immune-mediated diseases, including cases of myocarditis or glomerulonephritis (GN) have been reported, antineutrophil cytoplasmic antibodies (ANCA)-associated vasculitis being one of the possible side effects caused by the mass-scale vaccination (Gargano et al., 2021; Sekar et al., 2021). Vasculitis is a disorder characterized by damage of mural structures of blood vessels caused by the infiltration of mononuclear cells in vessel walls. ANCA-associated vasculitis (AAV) is characterized by the development of autoantibodies against antigens in cytoplasmic granules of neutrophils affecting predominantly to small vessels, with myeloperoxidase (MPO) or proteinase 3 (PR3) being the main antigens toward which these autoantibodies are directed. Although the development of ANCA after influenza vaccination has been previously reported, most of the studies that analyze the possible relationship between SARS-CoV-2 vaccination and the development of ANCA and AAV correspond to case reports (Chen et al., 2021; Davidovic et al., 2021; Dube et al., 2021; Feghali et al., 2021; Felzer et al., 2021; Hakroush and Tampe, 2021; Okuda et al., 2021). Likewise, the few published works that include series of patients and review the temporal association between glomerular disease and SARS-CoV-2 vaccination only include very few cases of AAV, as these studies consider the effect of the vaccine on the development of different types of glomerulonephritis, such as immunoglobulin A (IgA) nephropathy, membranous nephropathy, minimal change disease, collapsing glomerulopathy, or lupus nephritis (Caza et al., 2021; Fenoglio et al., 2022; Klomjit et al., 2021). Therefore, to our knowledge, this is the largest study that include patients who debuted with ANCA and AAV during 2021 after receiving vaccination against SARS-CoV-2 without suffering from COVID-19, in comparison with 2019, before the COVID-19 pandemic.
Results
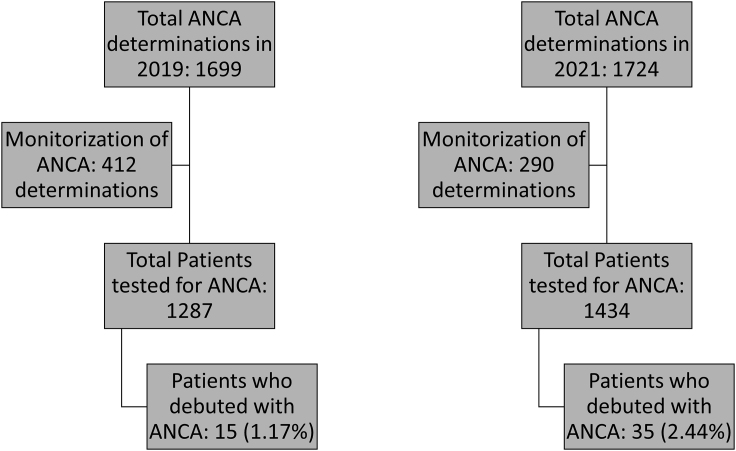
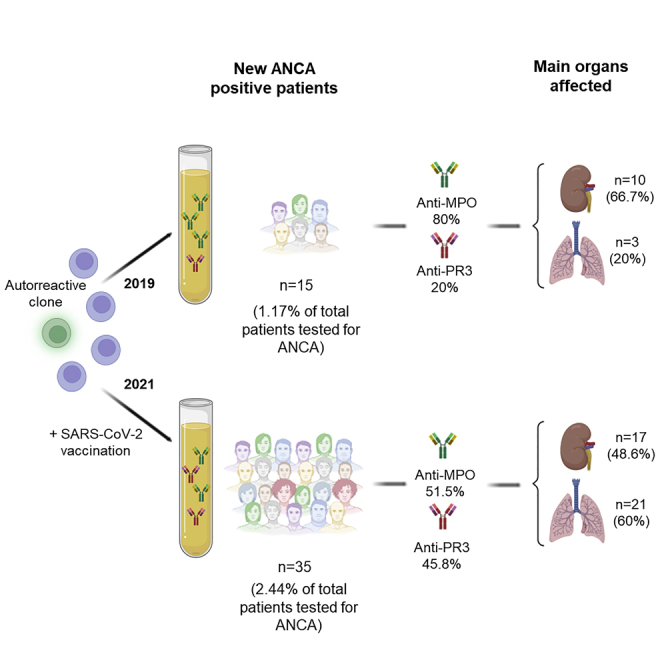
A total of 35 patients presented ANCA and/or anti-GBM antibodies for the first time during 2021, with the number during 2019 being 15. Throughout 2019 and 2021, a similar number of ANCA and/or anti-GBM antibodies determinations were carried out. Specifically, this study was performed in 1,287 patients in 2019 and 1,434 patients during 2021. Monitorization of the mentioned autoantibodies was not taken into account. Consequently, the percentage of patients who debuted with these autoantibodies in 2019 was 1.17%, whereas in 2021 this percentage increased to 2.44%, the differences observed being statistically significant (p = 0.020) (Figure 1). Mean age of the patients included was 65.8 and 63.1 years in 2019 and 2021, respectively. No statistically significant difference on gender was observed. In 2019, the most frequent ANCA was anti-MPO, being present in 80.0% of the patients. However, in 2021, an increase in the positivity for anti-PR3 antibody was observed, which was presented in 45.8% of the patients. Despite nonsignificant differences in the frequency of anti-PR3 antibodies between 2019 and 2021, an important increase of the detection of this autoantibody was observed in 2021 (Table 1). The median titer of anti-MPO antibodies in 2019 was 191.0 CU (interquartile range [IQR] 92.0–718.4 CU) and 115.2 CU (IQR 34.5–689.0 CU) in 2021, with no significant differences between both years (p = 0.481). In addition, the median titer of anti-PR3 antibodies in 2019 and 2021 were 100.0 CU (IQR 62.5–139.5 CU) and 53.2 CU (IQR 30.0–334.3 CU), respectively. No significant differences between both years (p = 0.824) were found.
Figure 1.
STROBE flow chart for patient identification and selection
Table 1.
Demographic characteristics and laboratory findings of ANCA-positive patients in 2019 and 2021
| 2019 (n = 15) | 2021 (n = 35) | p | |
|---|---|---|---|
| Age, years, mean (SD) | 65.8 (14.6) | 63.1 (16.4) | 0.969 |
| Sex, females, n (%) | 6 (40.0%) | 19 (54.3%) | 0.537 |
| ANCA or anti-GBM Ab, n (%) | |||
| Anti-MPO Ab | 11 (73.3%) | 16 (45.7%) | 0.137 |
| Anti-PR3 Ab | 2 (13.3%) | 15 (42.9%) | 0.090 |
| Anti-GBM Ab | 1 (6.7%) | 2 (5.7%) | 0.603 |
| Anti-MPO + anti-PR3 Ab | 1 (6.7%) | 1 (2.9%) | 0.875 |
| Anti-MPO + anti-GBM Ab | — | 1 (2.9%) | — |
SD, standard deviation; ANCA, antineutrophil cytoplasmic antibodies; MPO, myeloperoxidase; PR3, proteinase 3; GBM, glomerular basal membrane; Ab, antibody.
Only 8 out of 35 patients detected in 2021 developed ANCA before receiving SARS-CoV-2 vaccine. Of the remaining 27 patients, 2 patients developed ANCA after receiving the first dose (7.4%), being BNT162b2 the vaccine administrated in all cases, and 25 patients developed ANCA after the second dose of the vaccine (92.6%), being the vaccination schedule received at that moment: two doses of BNT162b2, two doses of mRNA-1273, two doses of AZD1222, and one dose of Ad26.CoV2.S plus one dose of mRNA-1273, in 87.5%, 4.2%, 4.2%, and 4.2%, respectively. The median time for ANCA detection was 24 days after the first dose (IQR 6–42 days) and 102 days (IQR 46.5–130.5 days) after the second dose of the vaccine.
Four patients included in the present study presented SARS-CoV-2 infection during the follow-up. However, in all four cases the developed of ANCA occurred after vaccination and before the viral infection.
In 97.1% of the patients who developed ANCA during 2021, the positivity of ANCA was accompanied by systemic involvement (Table 2). The activity index following BVAS score of the patients who debuted with AAV during 2021 was 18.8 (SD 6.5) in comparison with those patients who debuted in 2019 in whom a mean of 11.4 (SD 5.2) was observed, with no statistically significant differences (p = 0.741). Renal and respiratory tracts were the main organs affected. Kidney biopsy was carried out in 8 of the patients who presented renal manifestations during 2019 (80%) and 2021 (47%). In the rest of the cases, the kidney biopsy could not be carried out due to the clinical status of the patients. In all of the biopsied patients who debuted with ANCA during 2019 and 2021, the biopsy showed data of pauci-immune extracapillary glomerulonephritis. The data regarding renal function and the need for renal replacement therapy in patients with renal involvement can be seen in Table 3.
Table 2.
Clinical characteristics of ANCA-positive patients in 2019 and 2021
| 2019 (n = 15) | 2021 (n = 35) | p | |
|---|---|---|---|
| SARS-CoV-2 infection, n (%) | — | 4 (11.4%) | — |
| Vaccination schedule, n (%) | |||
| 2 doses BNT162b2 (Pfizer) | — | 12 (34.3%) | — |
| 3 doses BNT162b2 (Pfizer) | — | 19 (54.3%) | — |
| 2 doses mRNA-1273 (Moderna) + 1 dose BNT162b2 (Pfizer) | — | 1 (2.9%) | — |
| 3 doses mRNA-1273 (Moderna) | — | 1 (2.9%) | — |
| 2 doses AZD1222 (AstraZeneca) + 1 dose mRNA-1273 (Moderna) | — | 1 (2.9%) | — |
| 1 dose Ad26.CoV2.S (Janssen) + 1 dose mRNA-1273 (Moderna) | — | 1 (2.9%) | — |
| ANCA positivity occurs, n (%) | |||
| Before vaccination | — | 8 (22.9%) | — |
| After 1st dose | — | 2 (5.7%) | — |
| After 2nd dose | — | 25 (71.4%) | — |
| After 3rd dose | — | — | — |
| Organ involvement, n (%) | |||
| Renal | 10 (66.7%) | 17 (48.6%) | 0.386 |
| Respiratory | 3 (20.0%) | 21 (60.0%) | 0.022 |
| Sinuses | 2 (13.3%) | 7 (20.0%) | 0.872 |
| Ophthalmic | — | 4 (11.4%) | — |
| Neural | 1 (6.7%) | — | — |
| Gastrointestinal | — | 2 (5.7%) | — |
| Cutaneous | — | — | — |
| Musculoskeletal | 1 (6.7%) | 4 (11.4%) | 1.000 |
| Constitutional syndrome | 6 (60.0%) | 12 (34.3%) | 0.949 |
| None | 1 (6.7%) | 2 (5.7%) | 0.603 |
ANCA, antineutrophil cytoplasmic antibodies.
Table 3.
Renal function in ANCA-positive patients in 2019 and 2021 with renal involvement
| 2019 (n = 10) | 2021 (n = 17) | p | |
|---|---|---|---|
| Serum creatinine (mg/dL), mean (SD) | 2.9 (1.6) | 4.1 (3.5) | 0.331 |
| GFR (mL/min), median (IQR) | 19.5 (10.8–42.5) | 17.5 (12.8–30.8) | 0.763 |
| Proteinuria (mg/24 h), median (IQR) | 1,265 (644–2050) | 1,365 (320–3588) | 0.977 |
| Acute hemodialysis, n (%) | 3 (30%) | 4 (23.5%) | 0.933 |
| Requirement chronic hemodialysis, n (%) | 2 (20%) | 3 (17.6%) | 0.718 |
GFR, glomerular filtration rate; SD, standard deviation; IQR, interquartile range.
Besides, a significant increase of respiratory manifestations was observed in patients who developed ANCA in 2021 in comparison with those patients who presented ANCA for the first time during 2019 (p = 0.022). Taking this into account, the most frequent respiratory manifestations are depicted in Table 4. Likewise, although in 2019 none of the patients presented ophthalmic or gastrointestinal manifestations, in 2021 11.4% and 5.7% of new ANCA positive patients suffered from ophthalmic and gastrointestinal involvement, respectively.
Table 4.
Respiratory manifestations of ANCA-positive patients in 2019 and 2021 with lung involvement
| 2019 (n = 3) | 2021 (n = 21) | p | |
|---|---|---|---|
| ILD, n (%) | 2 (66.6%) | 12 (57.1%) | 0.754 |
| Haemoptysis/alveolar hemorrhage, n (%) | 1 (33.3%) | 5 (23.8%) | 0.722 |
| Nodules or cavities, n (%) | — | 2 (9.5%) | — |
| Others, n (%) | — | 2 (9.5%) | — |
ILD, interstitial lung disease.
In relation to the treatments used, no differences were observed between both years (Table 5).
Table 5.
Treatments administrated to ANCA positive patients in 2019 and 2021
| Treatment, n (%) | 2019 (n = 15) | 2021 (n = 35) | p |
|---|---|---|---|
| Steroid | 14 (93.3%) | 24 (68.6%) | 0.129 |
| Plasmapheresis | 3 (20.0%) | 3 (8.6%) | 0.506 |
| IVIG | 3 (20.0%) | 4 (11.4%) | 0.722 |
| Cyclophosphamide | 6 (40.0%) | 6 (17.1%) | 0.170 |
| Rituximab | 2 (13.3%) | 5 (14.3%) | 0.722 |
| MMF | — | 2 (5.7%) | — |
IVIG, intravenous immunoglobulin; MMF, mycophenolate mofetil.
Discussion
To date, only a case series including patients who developed new or relapsing glomerulonephritis (Caza et al., 2021; Fenoglio et al., 2022; Klomjit et al., 2021), or case reports of unique patients who presented AAV following different types of SARS-CoV-2 vaccines (Anderegg et al., 2021; Baier et al., 2022; Garcia et al., 2022; Gupta and Ellis, 2022; Ibrahim et al., 2022; Obata et al., 2021; Prabhahar et al., 2022; Prema et al., 2021; Shakoor et al., 2021; Villa et al., 2021), have been published.
Our case series is the largest series reported on new cases of patients with ANCA after SARS-CoV-2 vaccination. Importantly, the finding of ANCA positivity remained specific as in 2019 and was associated with AAV and systemic involvement with a significant predominance of respiratory manifestations. The two most common vaccines currently in use in our country include mRNA-based (BNT162b2 and mRNA-1273) and adenovirus vector (AZD1222 and Ad26.CoV2.S) vaccines.
Nevertheless, the BNT162b2 (Pfizer-BioNTech) vaccine was the one received by most of the patients included in the study. This vaccine was administrated in 88.6% of the patients included in the study and in 88.9% of the patients who developed ANCA after receiving SARS-CoV-2 vaccine. The results obtained could be attributable to the administration of such vaccine. However, because majority of subjects are vaccinated with BNT162b2, it cannot be excluded a secondary effect from the polyclonal activation induced by the vaccine that can include autoreactive clones specific for MPO or PR3. In any case, the ANCA production was observed at the time of clinical debut and clearly associated with AAV development. Thus, it cannot be considered as an epiphenomenon.
Most patients in our series developed ANCA after the second dose of the vaccine (68.6%), but the detection of these autoantibodies varied from 6 days after the first dose to more than 5 months after the second dose.
Unlike expected, in Southern European countries including Spain, the incidence of anti-MPO antibodies usually is higher than anti-PR3 antibodies. However, in the present study a relevant increase in the frequency of anti-PR3 antibodies in 2021 (45.8%) compared with 2019 (20.0%) was observed. There is no clear explanation for such a finding. A possible limitation of the present study could be that ANCA were only tested using chemiluminescent methods for anti-MPO and anti-PR3 antibodies. ANCA were not tested using IIF, so other ANCA specificities different from those mentioned could not be detected.
New-onset autoimmune manifestations following SARS-CoV-2 vaccination are being reported extensively, molecular mimicry, the production of particular autoantibodies, and the role of certain vaccine adjuvants being the main mechanisms through which these vaccines trigger autoimmune phenomena. Previous studies indicate that the use of human papillomavirus, hepatitis B, and influenza vaccines may trigger the onset or exacerbations of autoimmune diseases by molecular mimicry inducing autoimmunity (Segal and Shoenfeld, 2018). However, whether the association between SARS-CoV-2 vaccine and autoimmune manifestations is coincidental or causal remains to be elucidated. Further studies and longer-term follow-up studies are necessary to elucidate the underlying mechanisms and identify the exact cause of these autoimmune manifestations.
Limitations of the study
A possible limitation of the present study could be that ANCA were only tested using chemiluminescent methods for anti-MPO and anti-PR3 antibodies that are the same routine tests in 2019 and 2021. ANCA were not tested using indirect immunofluorescence on human neutrophils, so other ANCA specificities different from those mentioned could not be detected.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Critical commercial assays | ||
| QUANTA Flash® MPO | Werfen | 701133 |
| QUANTA Flash® PR3 | Werfen | 701138 |
| QUANTA Flash® GBM | Werfen | 701143 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed and will be fulfilled by the Lead Contact, Marcos López-Hoyos (marcos.lopez@scsalud.es).
Materials availability
This paper does not generate any new material.
Experimental model and subject details
Human study
All patients from University Hospital Marqués de Valdecilla, a tertiary referral hospital for the Autonomous Community of Cantabria, who debuted with the presence of ANCA (anti-MPO, anti-PR3), and/or anti-glomerular basement membrane (GBM) antibodies during 2021 with suspected vasculitis, discarding the presence of inflammatory bowel disease, and who received vaccination against SARS-CoV-2 were included. This hospital is the unique center that performances the determinations of autoantibodies in the Autonomous Community of Cantabria,with a reference population of 580,000 inhabitants. Likewise, patients who debuted with these autoantibodies in 2019 were also included in order to stablish the corresponding comparisons.
Study approval
The study was conducted according to the guidelines of the Declaration of Helsinki and it was approved by the Regional Ethics Committee (CEIm, internal code 2020.167).
Method details
ANCA and anti-GBM determination
Serum measurement of ANCA and anti-GBM antibodies was performed by chemiluminescence assay using BIO-FLASH (Inova, San Diego, CA) according to the instructions of the manufacturer A value of 20 chemiluminescent units (CU) was stablished as cut-off point to consider positive results for ANCA or anti-GBM antibodies. The measuring range for anti-MPO antibodies was 3.2–739.8 CU, for anti-PR3 antibodies was 2.3–3285.3 CU, and for anti-GBM antibodies was 2.9–1437.8 CU. ANCA were not tested by indirect immunofluorescence (IIF).
BVAS score
Disease activity was determined using the scoring system Birmingham Vasculitis Activity Score (BVAS) (Mukhtyar et al., 2009).
Quantification and statistical analysis
Statistical analysis
Retrospective observational single-center study. Parametric and non-parametric tests were chosen as appropriate for descriptive comparisons of continuous variables, and chi-squared test for categorical variables. Fisher exact test was used for the comparisons in smaller groups. Comparisons were based on the unpaired T-Student test or U-Mann–Whitney U test for parametric and non-parametric continuous data, respectively. A p-value < 0.05 was considered to be significant. All p-values are reported two-sided. Analyses were performed using IBM SPSS Statistics 19.0 (IBM Corp. Armonk, NY, USA).
Acknowledgments
This research received no external funding.
Author contributions
Conceptualization: M.L.H. and G.F.F.; Methodology: J.I.V., L.B.V., E.G.L., C.C.H.; Software: J.I.V. and L.V.B.; Validation: J.I.V., L.B.V., G.F.F., and M.L.H.; Formal Analysis: J.I.V. and L.B.V.; Investigation: J.I.V., L.B.V., E.R.C., M.H.V., J.C.R.S.M., G.F.F., and M.L.H.; Data Curation: J.I.V. and L.B.V.; Writing—Original Draft: J.I.V. and L.B.V.; Writing—Review & Editing: all authors.
Declaration of interests
All the authors declared no competing interests.
Published: August 19, 2022
Data and code availability
All data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- Anderegg M.A., Liu M., Saganas C., Montani M., Vogt B., Huynh-Do U., Fuster D.G. De novo vasculitis after mRNA-1273 (Moderna) vaccination. Kidney Int. 2021;100:474–476. doi: 10.1016/J.KINT.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier E., Olgemöller U., Biggemann L., Buck C., Tampe B. Dual-positive MPO- and PR3-ANCA-associated vasculitis following SARS-CoV-2 mRNA booster vaccination: a case report and systematic review. Vaccines. 2022;10:653. doi: 10.3390/VACCINES10050653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caza T.N., Cassol C.A., Messias N., Hannoudi A., Haun R.S., Walker P.D., May R.M., Seipp R.M., Betchick E.J., Amin H., et al. Glomerular disease in temporal association with SARS-CoV-2 vaccination: a series of 29 cases. Kidney360. 2021;2:1770–1780. doi: 10.34067/KID.0005372021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.C., Chen H.Y., Lu C.C., Lin S.H. Case report: anti-neutrophil cytoplasmic antibody-associated vasculitis with acute renal failure and pulmonary hemorrhage may occur after COVID-19 vaccination. Front. Med. 2021;8:2195. doi: 10.3389/FMED.2021.765447/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidovic T., Schimpf J., Sprenger-Mähr H., Abbassi-Nik A., Soleiman A., Zitt E., Lhotta K. Case report de novo and relapsing glomerulonephritis following SARS-CoV-2 mRNA vaccination in microscopic polyangiitis. Case Report. 2021;2021:8400842. doi: 10.1155/2021/8400842. [DOI] [Google Scholar]
- Dube G.K., Benvenuto L.J., Batal I. Antineutrophil cytoplasmic autoantibody-associated glomerulonephritis following the pfizer-BioNTech COVID-19 vaccine. Kidney Int. Rep. 2021;6:3087–3089. doi: 10.1016/J.EKIR.2021.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feghali E.J., Zafar M., Abid S., Santoriello D., Mehta S. De-Novo antineutrophil cytoplasmic antibody-associated vasculitis following the mRNA-1273 (moderna) vaccine for COVID-19. Cureus. 2021;13:e19616. doi: 10.7759/CUREUS.19616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felzer J.R., Fogwe D.T., Samrah S., Michet C.J., Specks U., Baqir M., Kubbara A.F. Association of COVID-19 antigenicity with the development of antineutrophilic cytoplasmic antibody vasculitis. Respirol. Case Rep. 2021;10:e0894. doi: 10.1002/RCR2.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoglio R., Lalloni S., Marchisio M., Oddone V., de Simone E., del Vecchio G., Sciascia S., Roccatello D. New onset biopsy-proven nephropathies after COVID vaccination. Am. J. Nephrol. 2022;53:325–330. doi: 10.1159/000523962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia D.S., Martins C., da Fonseca E.O., de Carvalho V.C.P., de Rezende R.P.V. Clinical Images: severe proteinase 3 antineutrophil cytoplasmic antibody glomerulonephritis temporally associated with Sinovac Biotech’s inactivated SARS-CoV-2 vaccine. ACR Open Rheumatol. 2022;4:277–278. doi: 10.1002/ACR2.11397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargano J.W., Wallace M., Hadler S.C., Langley G., Su J.R., Oster M.E., Broder K.R., Gee J., Weintraub E., Shimabukuro T., et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the advisory committee on immunization practices — United States, June 2021. MMWR Morb. Mortal. Wkly. Rep. 2021;70:977–982. doi: 10.15585/MMWR.MM7027E2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R.K., Ellis B.K. Concurrent antiglomerular basement membrane nephritis and antineutrophil cytoplasmic autoantibody-mediated glomerulonephritis after second dose of SARS-CoV-2 mRNA vaccination. Kidney Int. Rep. 2022;7:127–128. doi: 10.1016/J.EKIR.2021.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakroush S., Tampe B. Case report: ANCA-associated vasculitis presenting with rhabdomyolysis and pauci-immune crescentic glomerulonephritis after pfizer-BioNTech COVID-19 mRNA vaccination. Front. Immunol. 2021;12:3957. doi: 10.3389/FIMMU.2021.762006/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim H., Alkhatib A., Meysami A. Eosinophilic granulomatosis with polyangiitis diagnosed in an elderly female after the second dose of mRNA vaccine against COVID-19. Cureus. 2022;14:e21176. doi: 10.7759/CUREUS.21176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klomjit N., Alexander M.P., Fervenza F.C., Zoghby Z., Garg A., Hogan M.C., Nasr S.H., Minshar M.A., Zand L. COVID-19 vaccination and glomerulonephritis. Kidney Int. Rep. 2021;6:2969–2978. doi: 10.1016/J.EKIR.2021.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu E., Ritchie H., Ortiz-Ospina E., Roser M., Hasell J., Appel C., Giattino C., Rodés-Guirao L. A global database of COVID-19 vaccinations. Nat. Hum. Behav. 2021;5:947–953. doi: 10.1038/S41562-021-01122-8. [DOI] [PubMed] [Google Scholar]
- Mukhtyar C., Lee R., Brown D., Carruthers D., Dasgupta B., Dubey S., Flossmann O., Hall C., Hollywood J., Jayne D., et al. Modification and validation of the Birmingham vasculitis activity score (version 3) Ann. Rheum. Dis. 2009;68:1827–1832. doi: 10.1136/ARD.2008.101279. [DOI] [PubMed] [Google Scholar]
- Obata S., Hidaka S., Yamano M., Yanai M., Ishioka K., Kobayashi S. MPO-ANCA-associated vasculitis after the Pfizer/BioNTech SARS-CoV-2 vaccination. Clin. Kidney J. 2021;15:357–359. doi: 10.1093/CKJ/SFAB181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda S., Hirooka Y., Sugiyama M. Propylthiouracil-induced antineutrophil cytoplasmic antibody-associated vasculitis after COVID-19 vaccination. Vaccines. 2021;9:842. doi: 10.3390/VACCINES9080842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhahar A., Naidu G., Chauhan P., Sekar A., Sharma A., Sharma A., Kumar A., Nada R., Rathi M., Kohli H.S., Ramachandran R. ANCA-associated vasculitis following ChAdOx1 nCoV19 vaccination: case-based review. Rheumatol. Int. 2022;42:749–758. doi: 10.1007/S00296-021-05069-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prema J., Muthukumaran A., Haridas N., Fernando E., Seshadri J., Kurien A.A. Two cases of double-positive antineutrophil cytoplasmic autoantibody and antiglomerular basement membrane disease after BBV152/covaxin vaccination. Kidney Int. Rep. 2021;6:3090–3091. doi: 10.1016/J.EKIR.2021.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal Y., Shoenfeld Y. Vaccine-induced autoimmunity: the role of molecular mimicry and immune crossreaction. Cell. Mol. Immunol. 2018;15:586–594. doi: 10.1038/CMI.2017.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekar A., Campbell R., Tabbara J., Rastogi P. ANCA glomerulonephritis after the Moderna COVID-19 vaccination. Kidney Int. 2021;100:473–474. doi: 10.1016/J.KINT.2021.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakoor M.T., Birkenbach M.P., Lynch M. ANCA-associated vasculitis following pfizer-BioNTech COVID-19 vaccine. Am. J. Kidney Dis. 2021;78:611–613. doi: 10.1053/J.AJKD.2021.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa M., Díaz-Crespo F., Pérez de José A., Verdalles Ú., Verde E., Almeida Ruiz F., Acosta A., Mijaylova A., Goicoechea M. A case of ANCA-associated vasculitis after AZD1222 (Oxford-AstraZeneca) SARS-CoV-2 vaccination: casualty or causality? Kidney Int. 2021;100:937–938. doi: 10.1016/J.KINT.2021.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.