Abstract
The clinical relevance of blood levels of adipokines in individuals with postmenopausal osteoporosis (PMOP) has not been previously clarified. We performed this meta‐analysis to clarify the association between three common adipokines levels and the occurrence of PMOP. PubMed, Embase, Cochrane library, and China National Knowledgement Infrastructure (CNKI) were searched for collecting articles published before 31 October 2021, without language and status restrictions. Fourteen studies met the selection criteria. Meta‐analysis revealed that blood leptin level was remarkably lower (mean difference [MD], −1.94; 95% confidence interval [CI], −3.83 to −0.06; I2 = 96%) and adiponectin level was remarkably higher (MD, 3.48; 95% CI, 2.36 to 4.60; I2 = 90%) in individuals with PMOP than healthy individuals with normal bone mineral density (BMD). However, the statistical difference in leptin level was changed after eliminating the confounding influence of leptin sources and assay approaches. Furthermore, a positive association (r = 0.28) between leptin level and body mass index (BMI) as well as a negative association (r = −0.33) between adiponectin level and BMD was found. Moreover, adiponectin had the highest probability of predicting PMOP (84%). Current evidence suggests that leptin positively affects BMI and adiponectin negatively affects BMD, and adiponectin is the most relevant adipokine negatively associated with PMOP.
Keywords: adipokines, adiponectin, leptin, meta‐analysis, osteoporosis, postmenopausal, resistin
1. INTRODUCTION
Osteoporosis (OP) refers to an imbalance condition between bone resorption and bone formation, which results in a decrease in bone mineral density (BMD) after increasing bone resorption. Issued data revealed that OP has become a prevalent public health problem worldwide, and its prevalence is estimated to rise from approximately 10 million to more than 14 million by 2020 in the United States. 1 The diagnosis of OP remains challenging because individuals only experience an asymptomatic condition before it manifests as a low‐trauma fracture of the hip, spine, proximal humerus, pelvis, and/or wrist. 2 Therefore, more attention should be paid to clarifying the potential mechanisms of OP, which can benefit preventing occurrence or delay progression of OP.
Actually, studies have revealed several factors contributing to the occurrence and progression of OP. Of which, menopause has been especially emphasized because it causes a predominance of bone resorption and therefore, increases the incidence of OP and the risk of bone fractures. 3 So, postmenopausal individuals will suffer from OP principally because of the detrimental bone turnover caused by oestrogen decline and ageing. 3 Considering the complexity of regulating physiological and pathological bone biology, other factors were also speculated to regulate the metabolism of bone tissue. Interestingly, epidemiological studies reported a significant positive association between body mass index (BMI) and BMD, and also revealed that reduction in body weight may contribute to bone loss. 4 , 5
So far, studies revealed multiple mechanisms that construct the specific association between bone metabolism and changes in fat. Traditionally, fat mass was considered to play an important role in mechanical load or oestrogen secretion as an endocrine organ. However, except oestrogen synthesis in postmenopausal women, adipocytes are also responsible for producing adipokines, such as leptin, adiponectin, and resistin 6 which act to stimulate inflammatory and anti‐inflammatory responses. 7 , 8 It is noted that the synthesis and secretion of adipokines also regulate and modulate energy homeostasis and metabolism. 9 Moreover, adipokines have also been found to involve in the mediation of bone biology and remodelling. 10 , 11
A previous meta‐analysis investigated the influence of blood levels of adipokines on BMD, osteoporotic status, and fracture risk in healthy men and women, 11 and another Chinese meta‐analysis investigated the difference in adipokines between postmenopausal individuals with OP and healthy individuals with normal BMD 12 ; however, the clinical relevance of blood levels of adipokines in postmenopausal individuals with OP has not been definitively clarified in previous meta‐analyses. Therefore, we conducted this meta‐analysis to further investigate the influence of blood levels of three common adipokines, including leptin, adiponectin, and resistin, on the occurrence of OP, BMI, and BMD in postmenopausal individuals.
2. MATERIALS AND METHODS
We conducted this meta‐analysis following the Preferred Reporting Items for Systematic Reviews and Meta‐analysis (PRISMA) for Network Meta‐analysis (PRISMA‐NMA) 13 and the Cochrane methods. 6 The completed PRISMA checklist has been listed in Table S1. Ethical approval from Institutional Review Board and Informed consent was not applicable to our meta‐analysis.
2.1. Search strategy
In 2020, a meta‐analysis investigated the association between adipocytokines and postmenopausal osteoporosis (PMOP), which was published in the Chinese language and identified eligible studies published before February 2020. 12 We therefore identified potential studies through performed an updated search in PubMed, Embase, the Cochrane library, and China National Knowledgement Infrastructure (CNKI) databases from January 2020 through October 2021, with no language limitations. We used the following key terms to develop the search strategy with Boolean logic operators: leptin, adiponectin, resistin, and PMOP. Additional studies were also manually searched by checking review articles and other relevant material. The type of study was restricted to human studies only. Detailed search strategies are documented in Table S2.
2.2. Eligibility and study selection
Two independent authors evaluated and selected relevant studies according to the following criteria: (1) postmenopausal individuals were confirmed as PMOP with recognized criteria in the case group and healthy postmenopausal individuals with normal BMD in the control group; (2) observational studies were considered to be eligible if data of three common adipokines between both groups were available; and (3) studies were published in full‐texts. We also developed the following exclusion criteria: abstracts, studies without data of interest and ineligible design, animal experiments, or the methodological quality were recognized as weak (with a score of <5 in the Newcastle–Ottawa Scale [NOS]). Any disagreements were resolved by consultation with a third senior author.
2.3. Data extraction
From the included articles, two independently authors extracted the following information: (1) name of the first author and publication year; (2) country; (3) basic characteristics of individuals including sample size, mean age, mean BMI, and mean time since menopause; (4) details of biochemical examination including sources of indicators and assay approaches; and (5) details of methodology. Moreover, we also extracted correlation coefficients 14 to further evaluate the relationship between the levels of different adipokines and BMI and BMD. When Spearman's correlation coefficients were reported, we converted it into Pearson's correlation coefficients according to the methods described in previous studies. 15 , 16
2.4. Quality assessment
We used the NOS to assess the methodological quality of the included studies, which was developed based on a star‐based system. 17 The methodological quality of an individual study was determined from three aspects as follows: the selection of the study groups, the comparability of the groups, and the ascertainment of the outcome of interest. The total score of >7, 5–6, and <5 was considered high‐, medium‐, and low methodological quality, respectively.
2.5. Outcomes of interest
In this meta‐analysis, we defined the difference in leptin, adiponectin, and resistin concentrations between individuals with PMOP and normal individuals as the primary outcomes, and the relationship between the concentrations of adipokines and BMI and BMD as the secondary outcomes.
2.6. Statistical analysis
All statistical analyses were conducted using RevMan 5.4 software (Nordic Cochrane Centre, Copenhagen, Denmark). The levels of adipokines were continuous variables, and thus we used the mean difference (MD) with a 95% confidence interval (CI) to express the pooled results because all indicators were calculated using comparable data units. Statistical heterogeneity across studies in individual outcomes was evaluated using Cochrane Q examination 18 and I2 statistic. 19 Nevertheless, we only used the random‐effects model to perform a meta‐analysis because a relatively definitive conclusion has been achieved about variations between studies in the real world. 20 Moreover, we first calculated the transformed values of correlation coefficient values using Fisher's transformation method. Then, we performed a meta‐analysis to calculate the summary of Fisher's Z values based on a generic inverse‐variance model. Finally, summary r values were converted from the summary of Fisher's Z values using recognized formulas. We also tested the robustness of all pooled results using the leave‐one‐out method. It is noted that we calculated standardized levels of adipokines using Z‐scores methods for the purpose of further evaluating the strength of relationships in the different comparisons by introducing the surface under the cumulative ranking curve (SUCRA), 21 which was estimated using the Bayesian network meta‐analysis with the Aggregate Data Drug Information System version 1.16 (Drug Information Systems, Groningen, The Netherlands). Meanwhile, we also checked publication bias for individual comparison with an accumulated number of more than 10 through generating a funnel plot. 22 A p‐value of <0.05 was considered statistically significant.
2.7. GRADE assessment
We used the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) framework to rate the certainty of evidence. In the GRADE system, the certainty of evidence will be classified as “very low,” “low,” “moderate,” and “high.” 23 The certainty of evidence begins with a “high” rating for observational studies. 24 The certainty could be downgraded according to the risk of bias, imprecision, inconsistency, and indirectness; however, the reviewer can upgrade the certainty according to publication bias, large effect, plausible confounding, or dose–response gradient. 25
3. RESULTS
3.1. Literature search
We identified 43 records after performing the updated search in the initial retrieval, including Pubmed (n = 13), Embase (n = 24), the Cochrane library (n = 2), and CNKI (n = 4). We removed 9 duplicate records through running software, then 33 ineligible studies were excluded after carefully screening the titles and abstracts. A total of 14 studies were identified from a previous meta‐analysis which was published in the Chinese language, and thus, a total of 15 studies were retained for further eligibility assessment. After screening full texts, 14 studies 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 were included in the meta‐analysis. The process of the literature retrieval and screening is shown in Figure 1.
FIGURE 1.
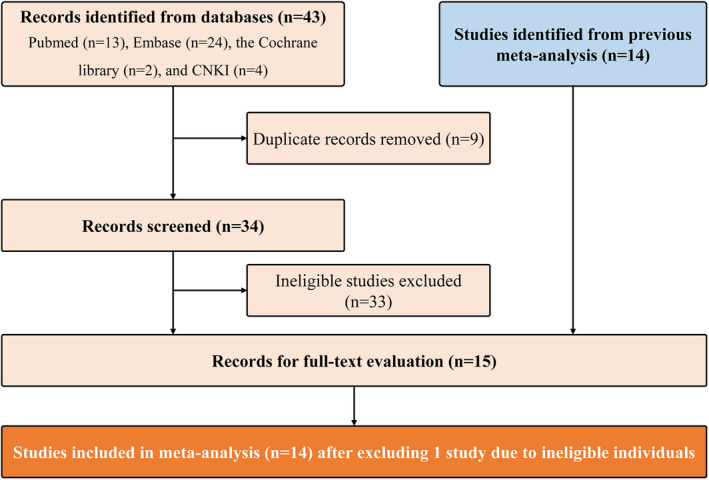
Flow diagram of literature screening. CNKI, China National Knowledgement Infrastructure
3.2. Study characteristics
A total of 14 studies were included in this meta‐analysis finally, which enrolled 615 individuals with PMOP and 499 healthy patients with normal BMD. Of these studies, 8 studies 30 , 31 , 33 , 34 , 35 , 37 , 38 , 39 were published in China, and the remaining studies were in Turkey, 29 , 32 , 36 Italy, 28 France, 27 and Iraq. 26 Eleven studies 26 , 27 , 29 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 only reported one of three common adipokines and 3 studies 28 , 30 , 39 reported multiple indicators of adipokines. Three studies measured plasma adipokines levels using enzyme‐linked immunosorbent assay (ELISA) 29 , 36 and radioimmunoassay (RIA), 32 and eleven studies measured serum adipokines levels using ELISA 26 , 28 , 30 , 31 , 33 , 35 , 37 , 39 and RIA. 27 , 34 , 38 The basic characteristics of the included studies are shown in Table 1.
TABLE 1.
Characteristics of the included studies (n = 14)
| Study | Country | No. of patients | Mean age, years | Mean BMI, kg/m2 | Mean time since menopause, years | Source | Indicators | Assay approach | r a | Score b | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PMOP | Control | PMOP | Control | PMOP | Control | PMOP | Control | |||||||
| Xia, 2011 | China | 41 | 39 | 64.82 | 65.47 | 23.69 | 23.87 | 15.81 | 16.59 | Serum | ADP | ELlSA | BMD | 7 |
| Zhang, 2012 | China | 35 | 30 | 62.48 | 61.75 | n.a. | n.a. | 10.47 | 10.07 | Serum | LP | RIA | n.a. | 6 |
| Wang, 2011 | China | 26 | 30 | 62.08 | 60.91 | 25.27 | 24.5 | 10.66 | 9.95 | Serum | LP | RIA | n.a. | 7 |
| Lv, 2006 | China | 32 | 30 | 59.61 | 56.2 | 26.66 | 24.46 | 10.09 | n.a. | Serum | LP | ELlSA | n.a. | 6 |
| Zhang, 2008 | China | 30 | 30 | 61.3 | 56.8 | 26.16 | 25.82 | n.a. | n.a. | Serum | LP | ELlSA | BMD | 5 |
| Wang, 2015 | China | 112 | 60 | 66.3 | 65.7 | 23.5 | 10.7 | Serum | ADP | ELlSA | BMD | 8 | ||
| Zuo, 016 | China | 58 | 30 | 68.9 | 58.9 | 22.2 | 24.8 | 20 | 10 | Serum | ADP, RT | ELlSA | n.a. | 6 |
| Liu, 2010 | China | 60 | 56 | 57.2 | 5.5 | 23.2 | n.a. | Serum | LP, RT | ELlSA | n.a. | 8 | ||
| Cervellati, 2016 | Italy | 43 | 31 | 58.5 | 55.3 | 23.6 | 26 | 7.5 | 3.3 | Serum | LP, ADP, RT | ELlSA | BMD | 6 |
| Breuil, 2011 | France | 20 | 16 | 73.25 | 67.3 | n.a. | n.a. | 12.1 | 12.9 | Serum | LP | RIA | BMI, BMD | 5 |
| Odabasi, 2000 | Turkey | 50 | 50 | 61.18 | 58.3 | 28.91 | 29.46 | 14 | 12 | Plasma | LP | RIA | BMI, BMD | 7 |
| Yilmazi, 2005 | Turkey | 36 | 30 | 54.52 | 54.25 | 29.33 | 29.25 | 9.1 | 9.02 | Plasma | LP | ELlSA | BMI, BMD | 7 |
| Kocyigit, 2013 | Turkey | 42 | 37 | 58.2 | 59.2 | 28.6 | 30 | 12.1 | 11.6 | Plasma | LP | ELlSA | BMI, BMD | 7 |
| Al‐Osami, 2018 | Iraq | 30 | 30 | 62.33 | 60.18 | 26.45 | 26.86 | 15.3 | 16.3 | Serum | ADP | ELlSA | BMI, BMD | 5 |
Abbreviations: ADP, adiponectin; BMI, body mass index; ELISA, enzyme ‐linked immunosorbent assay; LP, leptin; n.a., not applicable; PMOP, postmenopausal osteoporosis; RIA, radioimmunoassay; RT, resistin.
Pearson's or Spearman's correlation coefficient.
The methodological quality was determined using the Newcastle–Ottawa Scale (NOS).
3.3. Methodological quality
The NOS was used to assess the methodological quality of the included articles. Three studies scored 5, four studies scored 6, five studies scored 7, and the remaining two studies scored 8. In general, the overall methodological quality of included studies was at a moderate level.
3.4. Meta‐analysis of adipokines levels in all patients with PMOP
Among 14 studies included, 10 reported leptin levels in 374 individuals with PMOP and 340 controls. The pooled result suggested that individuals with PMOP had significantly lower leptin levels compared to controls (MD = −1.94 ng/mL, 95% CI, −3.83 to −0.06, p = 0.04; Figure 2). Substantial statistical heterogeneity was detected (I2 = 96%, p < 0.001), we therefore conducted a series of subgroup analyses based on leptin sources and assay approaches to explore potential influencing factors. Pooled results were reversely changed to have no statistical significance although statistical heterogeneity was not obviously decreased, as shown in Figure S1. Moreover, the results of sensitivity analysis also suggested a significant change after omitting one study at one time (Table S3).
FIGURE 2.
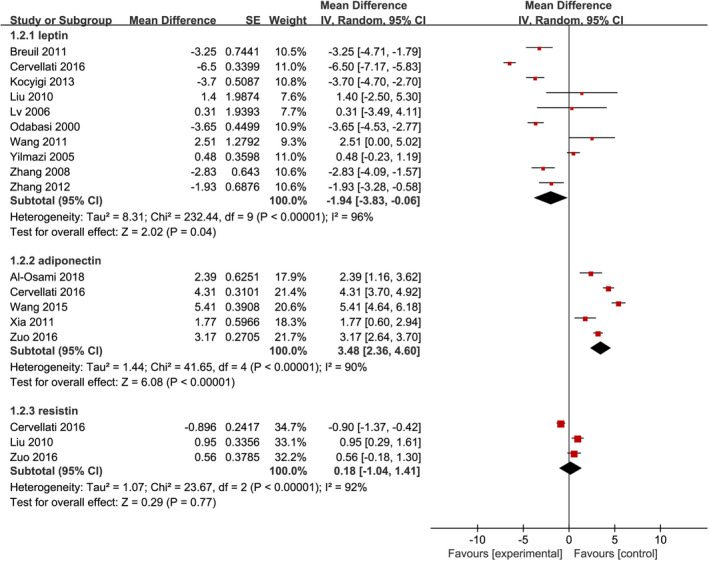
Forest plot of meta‐analysis of differences in blood adipocytokines levels between postmenopausal individuals with osteoporosis and healthy individuals with normal bone mineral density
A total of 5 studies reported adiponectin levels in 284 individuals with PMOP and 190 controls. As shown in Figure 2, individuals with PMOP had significantly higher adiponectin levels compared to controls (MD = 3.48 μg/mL, 95% CI, 2.36 to 4.60, p < 0.001). Although substantial statistical heterogeneity was detected (I2 = 90%, p < 0.001), no subgroup analysis was conducted because serum adiponectin levels were measured using the ELISA method in these studies. It is noted that the results of sensitivity analysis confirmed the robustness of the pooled result because it was not significantly changed after omitting one study at one time (Table S3).
Among 14 studies included, 3 reported resistin levels in 161 individuals with PMOP and 117 controls. As shown in Figure 2, adiponectin level between individuals with PMOP and healthy controls with normal BMD was comparable (MD = 0.18 pg/mL, 95% CI, −1.04 to 1.41, p = 0.77). No subgroup analysis was conducted because serum adiponectin levels were measured using the ELISA method in these studies. We detected substantial statistical heterogeneity for this indicator (I2 = 92%, p < 0.001), however, we did not conduct a subgroup analysis because serum resistin levels were measured using the ELISA method in these studies. Unfortunately, the pooled result should be cautiously interpreted because there was statistical changes after omitting one study at one time (Table S3).
3.5. Meta‐analysis of the relationship of adipokines levels with the BMD
Among the included studies, 9 studies examined the relationship between adipokines levels and BMD in patients with PMOP and reported the Pearson's or Spearman's correlation coefficients. We performed a pooled analysis of the relationship between adipokines levels and BMD among individuals with PMOP using Fisher's Z transformation. As shown in Figure 3, the meta‐analysis generated a summary Fisher's Z value of 0.27 (95% CI, −0.07 to 0.61, p = 0.12), −0.34 (95% CI, −0.59 to −0.09, p = 0.009), and 0.14 (95% CI, −0.09 to 0.37, p = 0.24) for leptin, adiponectin, and resistin, respectively. Therefore, a summary r value was 0.26, −0.33, and 0.14 for leptin, adiponectin, and resistin, respectively. This meta‐analysis of correlation coefficients revealed a significantly negative relationship between adiponectin levels and BMD. However, sensitivity analysis did not confirm the robustness of the pooled results (Table S3).
FIGURE 3.
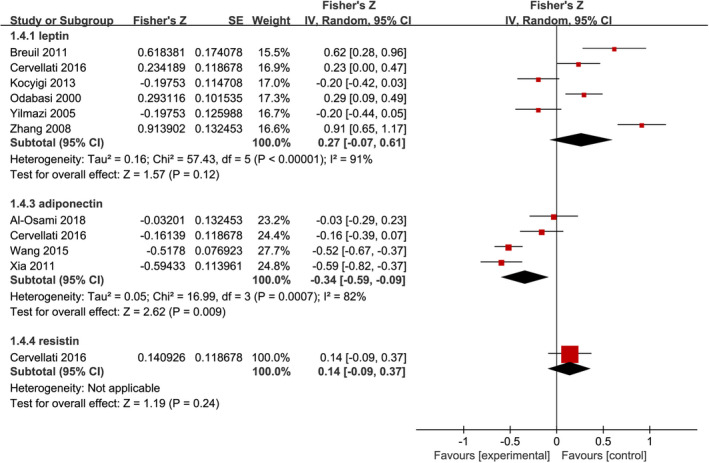
Forest plot of meta‐analysis of the relationship between adipokines and bone mineral density
3.6. Meta‐analysis of the relationship of adipokines levels with the BMI
Five studies examined the relationship between leptin or adiponectin levels and BMI in patients with PMOP and reported the Pearson's or Spearman's correlation coefficients. We performed a pooled analysis of the relationship between leptin or adiponectin levels and BMI among individuals with PMOP. As shown in Figure 4, the meta‐analysis generated a summary Fisher's Z value of 0.57 (95% CI, 0.38 to 0.76, p < 0.001) and 0.22 (95% CI, −0.04 to 0.48, p = 0.09) for leptin and adiponectin, respectively. Therefore, a summary r value was 0.52 and 0.22 for leptin and adiponectin, respectively. This meta‐analysis of correlation coefficients revealed a significantly positive relationship between leptin levels and BMI, which was further confirmed by the results of sensitivity analysis (Table S3).
FIGURE 4.
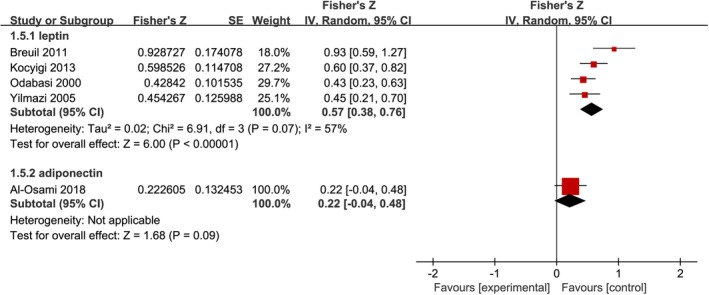
Forest plot of meta‐analysis of the relationship between adipokines and body mass index
3.7. Certainty of evidence
The concentration of leptin, adiponectin, and resistin was rated with very low, high, and very low certainty, respectively. Association of leptin, adiponectin, and resistin with BMD was rated with very low, moderate, and very low certainty, respectively. The association of leptin and adiponectin with BMI was rated with moderate and very low certainty, respectively. Details of the certainty of evidence assessment of each outcome were presented in Table S4 .
3.8. Comparative probabilities of the strength of relationships
We estimated SUCRA which was calculated following the parameters including 4 chains, 20,000 tuning iterations, 100,000 simulation iterations, the thinning interval of 10, 10,000 inference samples, and a variance scaling factor of 2.5 40 to rank three available relationships of different adipokines with the occurrence of PMOP. As shown in Figure 5, ranking probability revealed that the relationship between adiponectin levels and the occurrence of PMOP ranked first (84%), followed by the relationship of leptin with PMOP (39%) and the relationship of resistin with PMOP (61%).
FIGURE 5.
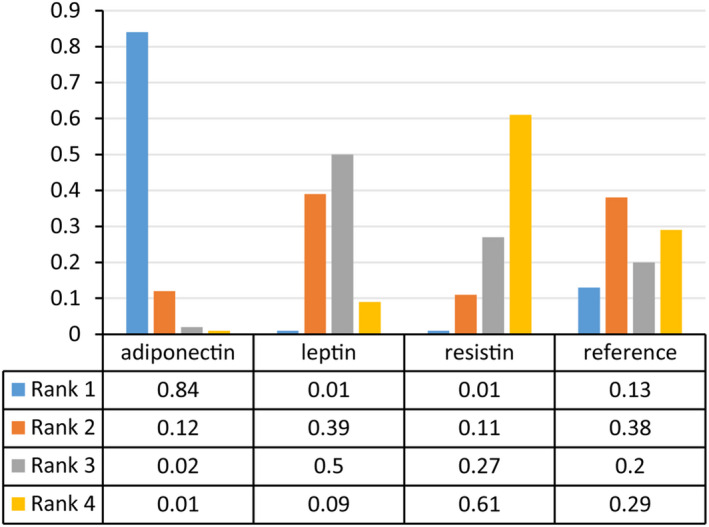
Ranking probability of strength of relationships between adipokines and the occurrence of PMOP. PMOP, postmenopausal osteoporosis
3.9. Publication bias
A funnel plot was generated to assess the possibility of publication bias among the enrolled studies involving the relationship between leptin levels and the occurrence of PMOP. The symmetrical funnel plot did not identify any publication bias across the studies of patients with PMOP, as shown in Figure S2.
4. DISCUSSION
The blood adipokines levels have been found to be associated with BMI and BMD, however, the clinical prevalence of specific adipokines in PMOP remains unclear. We performed this meta‐analysis to further clarify the association between blood levels of three common adipokines including leptin, adiponectin, and resistin, and PMOP through including 14 studies assessing the difference between adipokines and the occurrence of PMOP in postmenopausal individuals. Our meta‐analysis reveals that adiponectin levels are remarkably higher in individuals with PMOP than in healthy individuals with normal BMD. Meanwhile, we also demonstrate a positive relationship between leptin levels and BMI as well as a negative relationship between adiponectin levels and BMD. More, our meta‐analysis also demonstrates that adiponectin is the most relevant adipokine negatively associated with the occurrence of PMOP.
As one of the adipokines, leptin can regulate appetite and weight and is also involved in the proliferation and differentiation of osteoblast 41 , 42 and osteoclasts. 41 Certainly, leptin also affects bone through its actions on the central nervous system, and the role of leptin in regulating bone remodelling has been reported in some clinical epidemiological studies. 11 In the present meta‐analysis, a positive association between leptin and BMI is demonstrated, however, a definitive association between leptin and BMD is not determined. More importantly, leptin levels in individuals with PMOP are comparable to that in healthy individuals with normal BMD. Many factors may confound the association between leptin levels and BMD, we therefore conducted a subgroup analysis to explore possible confounding factors. Unfortunately, leptin levels are still revealed to be unrelated to the BMD and the occurrence of PMOP. Therefore, more studies are needed to clarify this definitive association.
As a new one of adipokines, adiponectin is exclusively expressed by adipocytes, 7 and studies reported an inverse relationship between adiponectin levels and visceral fat mass and BMI. Unfortunately, in this meta‐analysis, only one study reported a correlation coefficient between adiponectin levels and BMI and did not reveal a significant association. However, our meta‐analysis demonstrated a significantly negative association between adiponectin levels and BMD, which is consistent with previous findings. 43 , 44 More importantly, our meta‐analysis also demonstrated that higher adiponectin concentration is associated with a higher occurrence of PMOP. It is noted that results of SUCRA further revealed that adiponectin may be prevalent adipokine involved in the occurrence of PMOP.
Resistin is mainly expressed by bone marrow and peripheral mononuclear cells (29), and has been speculated to have an association with BMD due to its polymorphisms. 45 Meanwhile Oh et al. found that, in middle‐aged men, serum resistin levels were inversely associated with lumber BMD. 46 However, a definitive association between resistin and BMI, BMD, or the occurrence of PMOP has not yet been generated. 11 In this meta‐analysis, we do not also detect a statistical difference in resistin levels between individuals with PMOP and healthy individuals with normal BMD. Meanwhile, a definitive association between resistin level and BMD is not identified.
It is noted that our meta‐analysis encountered substantial overall statistical heterogeneity for the individual outcome (I2 > 90%), and most importantly is that subgroup analysis changed the estimates in terms of the difference in blood leptin levels between postmenopausal individuals with OP and healthy individuals with normal BMD, suggesting that the estimates for leptin should be cautiously interpreted. Meanwhile, heterogeneity of each subgroup remained high after subgroup analyses for available factors, suggesting that several unknown factors contributed to the observed study heterogeneity, such as mean BMI and mean time since menopause.
The present meta‐analysis has several strengths. First, our meta‐analysis firstly investigated the relationships between three common adipokines with the occurrence of PMOP. Second, we introduced the method of calculating SUCRA to determine the relative strength of association of different adipokines with PMOP. Third, we simultaneously estimated the difference of adipokines in levels and the relationships of different adipokines with BMD and BMI. Fourth, although most of the results showed significant heterogeneity, the quality of included studies was at a moderate level.
Despite the novelty of our findings, the following limitations should be further interpreted. First, although we tried our best to control the confounding factors such as adipokines sources and assay approaches, some potential confounding factors such as mean time since menopause may affect the conclusion more or less. Second, since this study lacked effective longitudinal cohort studies, we could not infer the causality of the association between adipokines levels and the occurrence of PMOP. Third, a paucity of studies with extreme sample sizes investigated the association between blood resistin levels and the occurrence of PMOP; thus, the evidence to support it is low. Fourth, we did not register the formal protocol of this meta‐analysis on any public platform, which may introduce bias to the pooled results. However, we strictly followed the methodological framework recommended by the Cochrane handbook to reduce the risk of bias. Fifth, we checked references of topic‐related reviews and included studies to identify the studies missed from the electronic search; however, we did not search other sources for grey literature, which may introduce the risk of missing potentially eligible studies. Sixth, the vast majority of studies originated from Eastern countries; thus, these results should be cautiously extrapolated to Western populations. Seventh, significant heterogeneity was detected for outcomes, which might significantly undermine the validity of the results. However, we conducted a sensitivity analysis to confirm the robustness of some results.
In conclusion, current evidence suggests that blood leptin positively affects BMI and adiponectin negatively affects BMD, as well as adiponectin is the most relevant adipokine negatively associated with the occurrence of PMOP. However, the associations between blood leptin and resistin levels and the occurrence of PMOP, BMI, and BMD should be further verified in future high‐quality studies because most findings were generated from evidence with low or very low certainty in this meta‐analysis.
AUTHOR CONTRIBUTIONS
Yimu Fu: Data curation (equal); formal analysis (equal); writing – original draft (equal). Linyuan Shu: Conceptualization (equal); data curation (equal); formal analysis (equal); writing – original draft (equal). Hui Sun: Conceptualization (equal); data curation (equal); formal analysis (equal); writing – review and editing (equal).
CONFLICT OF INTEREST
The authors confirm that there are no conflicts of interest.
Supporting information
Figure S1
Figure S2
Table S1
Table S2
Table S3
Table S4
ACKNOWLEDGEMENTS
We would like to deeply appreciate all authors who performed all eligible studies which have been included in the present network meta‐analysis. This work was supported by the National Natural Science Foundation of China (Grant No. 81501862).
Shu L, Fu Y, Sun H. The association between common serum adipokines levels and postmenopausal osteoporosis: A meta‐analysis. J Cell Mol Med. 2022;26:4333‐4342. doi: 10.1111/jcmm.17457
Linyuan Shu and Yimu Fu should be considered joint first author.
DATA AVAILABILITY STATEMENT
Full datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. Additional data are available in the supplementary materials.
REFERENCES
- 1. Burge R, Dawson‐Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis‐related fractures in the United States, 2005‐2025. J Bone Miner Res. 2007;22(3):465‐475. doi: 10.1359/jbmr.061113 [DOI] [PubMed] [Google Scholar]
- 2. Cosman F, de Beur SJ, LeBoff MS, et al. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359‐2381. doi: 10.1007/s00198-014-2794-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weitzmann MN, Pacifici R. Estrogen deficiency and bone loss: an inflammatory tale. J Clin Invest. 2006;116(5):1186‐1194. doi: 10.1172/jci28550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wardlaw GM. Putting body weight and osteoporosis into perspective. Am J Clin Nutr. 1996;63(3 Suppl):433s‐436s. doi: 10.1093/ajcn/63.3.433 [DOI] [PubMed] [Google Scholar]
- 5. Reid IR. Relationships among body mass, its components, and bone. Bone. 2002;31(5):547‐555. doi: 10.1016/s8756-3282(02)00864-5 [DOI] [PubMed] [Google Scholar]
- 6. Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated march 2011]. The Cochrane Collaboration; 2011. www.handbookcochrane.org [Google Scholar]
- 7. Sepidarkish M, Rezamand G, Qorbani M, et al. Effect of omega‐3 fatty acids supplementation on adipokines: a systematic review and meta‐analysis of randomized controlled trials. Crit Rev Food Sci Nutr. 2021;1‐15. doi: 10.1080/10408398.2021.1915743 [DOI] [PubMed] [Google Scholar]
- 8. von Frankenberg AD, Silva FM, de Almeida JC, et al. Effect of dietary lipids on circulating adiponectin: a systematic review with meta‐analysis of randomised controlled trials. Br J Nutr. 2014;112(8):1235‐1250. doi: 10.1017/s0007114514002013 [DOI] [PubMed] [Google Scholar]
- 9. Gray B, Steyn F, Davies PS, Vitetta L. Omega‐3 fatty acids: a review of the effects on adiponectin and leptin and potential implications for obesity management. Eur J Clin Nutr. 2013;67(12):1234‐1242. doi: 10.1038/ejcn.2013.197 [DOI] [PubMed] [Google Scholar]
- 10. Thomas T, Burguera B, Melton LJ 3rd, et al. Role of serum leptin, insulin, and estrogen levels as potential mediators of the relationship between fat mass and bone mineral density in men versus women. Bone. 2001;29(2):114‐120. doi: 10.1016/s8756-3282(01)00487-2 [DOI] [PubMed] [Google Scholar]
- 11. Biver E, Salliot C, Combescure C, et al. Influence of adipokines and ghrelin on bone mineral density and fracture risk: a systematic review and meta‐analysis. J Clin Endocrinol Metab. 2011;96(9):2703‐2713. doi: 10.1210/jc.2011-0047 [DOI] [PubMed] [Google Scholar]
- 12. Chen JY, Lv ZH, Zhang B, Li WJ, Chen H, Wu ZX. Association between adipocytokines and postmenopausal osteoporosis: a meta‐analysis. Chin J Osteoporos. 2020;26(11):1640‐1645. doi: 10.3969/j.issn.1006-7108.2020.11.015 [DOI] [Google Scholar]
- 13. Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. doi: 10.1136/bmj.n160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peterson RA, Brown SP. On the use of beta coefficients in meta‐analysis. J Appl Psychol. 2005;90(1):175‐181. doi: 10.1037/0021-9010.90.1.175 [DOI] [PubMed] [Google Scholar]
- 15. Li X, He J. The association between serum/plasma leptin levels and obstructive sleep apnea syndrome: a meta‐analysis and meta‐regression. Front Endocrinol. 2021;12:696418. doi: 10.3389/fendo.2021.696418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Surov A, Meyer HJ, Wienke A. Associations between apparent diffusion coefficient (ADC) and KI 67 in different tumors: a meta‐analysis. Part 1: ADC(mean). Oncotarget. 2017;8(43):75434‐75444. doi: 10.18632/oncotarget.20406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol. 2010;25(9):603‐605. doi: 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 18. Bowden J, Tierney JF, Copas AJ, Burdett S. Quantifying, displaying and accounting for heterogeneity in the meta‐analysis of RCTs using standard and generalised Q statistics. BMC Med Res Methodol. 2011;11:41. doi: 10.1186/1471-2288-11-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21(11):1539‐1558. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 20. Zeng D, Lin DY. On random‐effects meta‐analysis. Biometrika. 2015;102(2):281‐294. doi: 10.1093/biomet/asv011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Singh S, Murad MH, Chandar AK, et al. Comparative effectiveness of pharmacological interventions for severe alcoholic hepatitis: a systematic review and network meta‐analysis. Gastroenterology. 2015;149(4):958‐70.e12. doi: 10.1053/j.gastro.2015.06.006 [DOI] [PubMed] [Google Scholar]
- 22. Palma Perez S, Delgado Rodriguez M. Practical considerations on detection of publication bias. Gac Sanit. 2006;20(Suppl 3):10‐16. doi: 10.1157/13101085 [DOI] [PubMed] [Google Scholar]
- 23. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924‐926. doi: 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schünemann HJ, Cuello C, Akl EA, et al. GRADE guidelines: 18. How ROBINS‐I and other tools to assess risk of bias in nonrandomized studies should be used to rate the certainty of a body of evidence. J Clin Epidemiol. 2019;111:105‐114. doi: 10.1016/j.jclinepi.2018.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401‐406. doi: 10.1016/j.jclinepi.2010.07.015 [DOI] [PubMed] [Google Scholar]
- 26. Al‐Osami MH, Hameed EK. Serum adiponectin level in osteoporotic postmenopausal women with type 2 diabetes mellitus. Diabetes Metab Syndr. 2018;12(6):939‐942. doi: 10.1016/j.dsx.2018.05.019 [DOI] [PubMed] [Google Scholar]
- 27. Breuil V, Amri EZ, Panaia‐Ferrari P, et al. Oxytocin and bone remodelling: relationships with neuropituitary hormones, bone status and body composition. Joint Bone Spine. 2011;78(6):611‐615. doi: 10.1016/j.jbspin.2011.02.002 [DOI] [PubMed] [Google Scholar]
- 28. Cervellati C, Bonaccorsi G, Bergamini CM, et al. Association between circulatory levels of adipokines and bone mineral density in postmenopausal women. Menopause. 2016;23(9):984‐992. doi: 10.1097/gme.0000000000000655 [DOI] [PubMed] [Google Scholar]
- 29. Kocyigit H, Bal S, Atay A, Koseoglu M, Gurgan A. Plasma leptin values in postmenopausal women with osteoporosis. Bosn J Basic Med Sci. 2013;13(3):192‐196. doi: 10.17305/bjbms.2013.2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu W, Li H, Xie H, et al. Relationship between serum leptin, Resistin, Visfatin, apelin and bone turnover biological marker in postmenopausal women [in Chinese]. Pract Prev Med. 2010;17(3):417‐420. doi: 10.3969/j.issn.1006-3110.2010.03.001 [DOI] [Google Scholar]
- 31. Lv K. Clinical Study on the Levels of Serum E2, IL‐6, IGF‐I and Leptin in Patients with Postmenopausal Osteoporosis [in Chinese]. Shandong University; 2006. [Google Scholar]
- 32. Odabaşi E, Ozata M, Turan M, et al. Plasma leptin concentrations in postmenopausal women with osteoporosis. Eur J Endocrinol. 2000;142(2):170‐173. doi: 10.1530/eje.0.1420170 [DOI] [PubMed] [Google Scholar]
- 33. Wang J, Jiang XF, Li XM, Hong Y. Correlation between serum adiponectin and biochemical indexes of bone metabolism in elderly patients with postmenopausal osteoporosis [in Chinese]. Chin J Gerontol. 2015;35(2):360‐361. doi: 10.3969/j.issn.1005-9202.2015.02.034 [DOI] [Google Scholar]
- 34. Wang LL, Shi Z, Wei J, Jiang HJ. Expression of leptin in peripheral serum of postmenopausal osteoporosis patients with fracture [in Chinese]. Chin J Gerontol. 2011;31(7):1146‐1147. doi: 10.3969/j.issn.1005-9202.2011.07.023 [DOI] [Google Scholar]
- 35. Xia JN. Relationship between serum adiponectin and bone mineral density in postmenopausal women [in Chinese]. Zhejiang Pract Med. 2011;16(3):168‐169. doi: 10.3969/j.issn.1007-3299.2011.03.005 [DOI] [Google Scholar]
- 36. Yilmazi M, Keleş I, Aydin G, et al. Plasma leptin concentrations in postmenopausal women with osteoporosis. Endocr Res. 2005;31(2):133‐138. doi: 10.1080/07435800500229276 [DOI] [PubMed] [Google Scholar]
- 37. Zhang H, Chen GF, Wu JZ, Chen YX. Significance of testing serum leptin, NO, IL‐6, BGP and E2 in patients with postmenopausal osteoporosis [in Chinese]. Chin J Trad Med Traumatol Orthoped. 2008;16(2):1‐3. doi: 10.3969/j.issn.1005-0205.2008.02.001 [DOI] [Google Scholar]
- 38. Zhang SG, Zhang YS, Cao GF, Yuan FS, Wang XY. Expression of leptin, estrogen and the biochemical markers of bone turnover in peripheral blood of women with postmenopausal osteoporosis [in Chinese]. Chin J Misdiagnostics. 2012;12(1):34. [Google Scholar]
- 39. Zuo XY, Yang Y, Li ST, et al. Association between bone mineral density and lipid metabolism in postmenopausal women [in Chinese]. Chin Gen Pract. 2016;19(16):1903‐1906. doi: 10.3969/j.issn.1007-9572.2016.16.012 [DOI] [Google Scholar]
- 40. Cipriani A, Higgins JPT, Geddes JR, Salanti G. Conceptual and technical challenges in network meta‐analysis. Ann Intern Med. 2013;159(2):130‐137. doi: 10.7326/0003-4819-159-2-201307160-00008 [DOI] [PubMed] [Google Scholar]
- 41. Cornish J, Callon KE, Bava U, et al. Leptin directly regulates bone cell function in vitro and reduces bone fragility in vivo. J Endocrinol. 2002;175(2):405‐415. doi: 10.1677/joe.0.1750405 [DOI] [PubMed] [Google Scholar]
- 42. Thomas T, Gori F, Khosla S, Jensen MD, Burguera B, Riggs BL. Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology. 1999;140(4):1630‐1638. doi: 10.1210/endo.140.4.6637 [DOI] [PubMed] [Google Scholar]
- 43. Peng XD, Xie H, Zhao Q, Wu XP, Sun ZQ, Liao EY. Relationships between serum adiponectin, leptin, resistin, visfatin levels and bone mineral density, and bone biochemical markers in Chinese men. Clin Chim Acta. 2008;387(1–2):31‐35. doi: 10.1016/j.cca.2007.08.012 [DOI] [PubMed] [Google Scholar]
- 44. Zhang H, Xie H, Zhao Q, et al. Relationships between serum adiponectin, apelin, leptin, resistin, visfatin levels and bone mineral density, and bone biochemical markers in post‐menopausal Chinese women. J Endocrinol Investig. 2010;33(10):707‐711. doi: 10.1007/bf03346674 [DOI] [PubMed] [Google Scholar]
- 45. Beckers S, Zegers D, Van Camp JK, et al. Resistin polymorphisms show associations with obesity, but not with bone parameters in men: results from the Odense androgen study. Mol Biol Rep. 2013;40(3):2467‐2472. doi: 10.1007/s11033-012-2327-z [DOI] [PubMed] [Google Scholar]
- 46. Oh KW, Lee WY, Rhee EJ, et al. The relationship between serum resistin, leptin, adiponectin, ghrelin levels and bone mineral density in middle‐aged men. Clin Endocrinol. 2005;63(2):131‐138. doi: 10.1111/j.1365-2265.2005.02312.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Figure S2
Table S1
Table S2
Table S3
Table S4
Data Availability Statement
Full datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. Additional data are available in the supplementary materials.


