Abstract
Opioid use disorder is a serious public health issue in the United States. Animal models of opioid dependence are fundamental for studying the etiology of addictive behaviors. We tested the hypothesis that extended access to heroin self-administration leads to increases in heroin intake and produces somatic signs of opioid dependence in both male and female mice. Adult C57BL/6J mice were trained to nosepoke (fixed-ratio 1) to obtain intravenous heroin in six daily 1-h sessions (30–60 μg/kg/infusion). The mice were divided into short access (ShA; 1 h) and long access (LgA; 6 h) groups. Immediately after the 10th escalation session, the mice received a challenge dose of naloxone (1 mg/kg), and somatic signs of withdrawal were recorded. The mice readily acquired intravenous heroin self-administration. LgA mice escalated their drug intake in the first hour across sessions and had significantly higher scores of somatic signs of naloxone-precipitated opioid withdrawal compared with ShA mice. Female mice exhibited increases in heroin intake compared with male mice. Male and female mice exhibited similar levels of somatic signs of withdrawal. Because of the wide availability of genetically modified mouse lines, the present mouse model may be particularly useful for better understanding genetic and sex differences that underlie the transition to compulsive-like opioid taking and seeking.
1. Introduction
Opioid use disorder is defined as a chronically relapsing disorder that is characterized by compulsive opioid intake, loss of control in limiting drug intake, and the presence of a negative emotional state when access to the drug is prevented (Koob et al., 2014). According to the Substance Abuse and Mental Health Services Administration (2017), 11.8 million people over the age of 12 misused opioids in 2016. The model-adjusted prevalence of having prescription opioid use disorder among nonmedical users increased from 12.7% in 2003 to 16.9% in 2013 (Han et al., 2015). In 2015, drug overdoses were the leading cause of accidental death in the United States (American Society of Addiction Medicine, 2016). From 2002 to 2016, the number of heroin users increased by 135%, and the number of deaths that were attributable to heroin increased by 533% in the United States (Substance Abuse and Mental Health Services Administration, 2017). Among individuals aged 18 to 64, rates of overdose deaths that involved prescription opioids increased from 4.5 per 100,000 in 2003 to 7.8 per 100,000 in 2013 (Han et al., 2015). Further research of the neurobiology of opioid use disorder is needed to identify novel strategies for prevention, diagnosis, and treatment.
Animal models of opioid addiction are essential for understanding the etiology of the disease because they permit investigations of specific elements of the drug addiction process, such as the acquisition and escalation of drug intake, abstinence, and relapse in operant models. Rat self-administration models have demonstrated that various opioid drugs serve as reinforcers that maintain stable patterns of self-administration (e.g., Wade et al., 2015). Limited access to opioids (e.g., 1–3 h per day) in both animal models and humans produces drug consumption that remains relatively stable over time. This pattern of moderate intake has been hypothesized to model controlled drug intake in the initial stages of drug addiction (Harding and Zinberg 1983). In contrast, with extended access to intravenous opioids (e.g., 6–23 h per day), rodents develop several somatic and motivational signs of opioid dependence that have similarities to drug consumption (i.e., drug dependence) that is observed in later stages of drug addiction in humans. For example, rats exhibit the escalation of drug intake over time, higher levels of drug seeking (Ahmed et al., 2000; Barbier et al., 2013; Lenoir and Ahmed, 2006, 2007; Schmeichel et al., 2015; Vendruscolo et al., 2011; Wade et al., 2015), and greater sensitivity to heroin- and stress-induced reinstatement (Ahmed et al., 2000; Lenoir and Ahmed, 2006, 2007). Additionally, upon opioid withdrawal, rats exhibit elevations of intracranial self-stimulation thresholds (i.e., “hypohedonia”; Kenny et al., 2006), allodynia (Barbier et al., 2013; Edwards et al., 2012; Park et al., 2015), anxiety-like behavior (Park et al., 2013), and somatic signs of opioid withdrawal (Vendruscolo et al., 2011). Thus, rat models of extended vs. limited drug access have been used to model the transition from controlled drug use to compulsive drug seeking and taking and to investigate the underlying neurobiological mechanisms (Koob and Volkow, 2016; Tunstall et al., 2017). Despite the wide availability of genetically modified mouse lines to study the neurobiology of opioid addiction, few mouse studies have investigated extended access to intravenous drug self-administration.
Although significant sex differences in the vulnerability to drug addiction have been reported in both human and rodent studies, the vast majority of preclinical studies have been conducted in male subjects only. In humans, men are more likely than women to abuse drugs, but this current sex difference may be the result of differences in opportunity and not necessarily the vulnerability to drug abuse (Becker and Chartoff, 2018; Becker and Hu, 2008). The number of women who use and abuse drugs is on the rise (Becker and Hu, 2008). Clinical reports suggest that women progress through the key stages of addiction from initial use to dependence at a faster rate than men (Becker and Koob, 2016; Brady and Randall, 1999; Kosten et al., 1993). Similar results have been found in preclinical studies. Female rodents were shown to develop specific characteristics of addiction faster or following less drug exposure than male rodents (Anker and Carroll, 2010; Becker and Koob, 2016; Lynch, 2006, 2018; Lynch and Taylor, 2004; Priddy et al., 2017).
The present study compared male and female mice that were allowed to intravenously self-administer heroin in short- and long-access sessions. We also evaluated the somatic signs of naloxone-precipitated withdrawal.
2. Material and Methods
2.1. Subjects
Adult male (n = 19) and female (n = 19) C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME, USA) were used. All of the mice were individually housed in a temperature-controlled (22°C) vivarium on a 12 h/12 h light/dark cycle (lights on at 6:30 PM) with ad libitum access to food (Envigo – formula 7017; calories from protein: 24%; calories from fat: 14%, calories from carbohydrate: 62%) and water. All of the procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the National Institute on Drug Abuse Animal Care and Use Committee.
2.2. Surgery
After acclimating to the animal facility for at least 7 days, the mice were surgically implanted with intravenous catheters in the right jugular vein as previously reported (Thomsen and Caine, 2007). The mice were anesthetized with isoflurane (2–3%). The midscapular region was shaved, and Nair™ was applied anteromedially to the right forearm to remove the fur. Both areas were cleaned with 70% alcohol and povidone-iodine. An incision was made on the mouse’s back and neck, and a chronic silastic catheter (SAI Infusion Technologies, Lake Villa, IL, USA) was passed subcutaneously to exit dorsally via the midscapular region. After isolating the right jugular vein under 2× magnification, the vein was punctured with a 23-gauge needle, and the catheter was inserted to the point that a silicone bead that was positioned 1.2 cm from the catheter tip was situated at the point of vein puncture. Blood was drawn to confirm correct placement in the vein, and the catheter was secured in place with silk-thread sutures immediately above and below the silicone bead. An additional suture was used to anchor these two sutures to each other. The catheter port was situated in the midscapular incision, and the incisions on the back and neck were sutured and glued with veterinarian adhesive (VetBond, Fisher Scientific, Asheville, NC, USA). The catheters were immediately flushed after surgery and daily during post-operative recovery with 0.02 ml sterile heparinized (20 USP units/ml) saline that contained antibiotic (67 mg/kg Cefazolin). All of the mice were allowed to recover for 5–7 days before behavioral testing. Thereafter, the catheters were flushed with 0.02–0.03 ml of heparinized saline (200 U/ml) before and after each self-administration session. The patency of the catheters was confirmed for individual subjects during training and at the completion of the study for all mice using a sedative cocktail (15 mg/ml ketamine and 0.75 mg/ml midazolam).
2.3. Self-administration chambers
The self-administration sessions were conducted in mouse operant chambers (Med Associates, St. Albans, VT, USA). The chambers (21.4 cm length × 20.3 cm width × 12.7 cm height) were located in a dark room and individually enclosed in wooden sound-attenuating cubicles that were fitted with a ventilation fan that additionally masked external noise. The operant chamber consisted of clear polycarbonate side walls and ceiling, with a modular aluminum front and back walls that allowed operant manipulanda to be mounted. The floor consisted of twenty-four 3.2 mm diameter steel rods that were spaced 8.9 mm apart. Two illuminated nosepoke holes (1.3 cm diameter × 1 cm depth) that were equipped with an infrared beam were mounted 1.5 cm above the floor on the front wall. A spring-covered Tygon tube was connected to the mouse’s catheter through a fluid swivel, and the swivel was connected to a syringe that contained the heroin solution that was placed outside the chambers. The syringe was placed inside a syringe pump (Med Associates, St. Albans, VT, USA; 3.3 revolutions per minute) that was placed on top of the wooden cubicle. MedPC software controlled the delivery of fluids and presentation of visual stimuli and recorded the behavioral data.
2.4. Self-administration procedure
The experimental procedure is shown in Fig. 1. All of the behavioral tests were conducted during the dark phase of the light/dark cycle, 3–5 days per week. The mice were trained to nosepoke in one of two nosepoke holes (i.e., the active nosepoke hole) on a fixed-ratio 1 (FR1) schedule of reinforcement (i.e., each operant response resulted in drug delivery) to obtain 20 μl of heroin (National Institute on Drug Abuse, Intramural Research Program Pharmacy, Baltimore, MD, USA; 30 μg/kg/infusion) that was delivered over 1.4 s in daily 1 h sessions. Each session was initiated with the programmed delivery of a single priming infusion. All reinforced responses were followed by a 30-s timeout period, in which a cue light that was above the active nosepoke was turned on. Additional nosepokes during the timeout period did not result in additional infusions. Nosepokes in the other nosepoke hole (i.e., the inactive nosepoke hole) had no programmed consequences. No food restriction was used to establish operant responding. Peanut butter (Jif, Orrville, OH, USA) was placed in the active nosepoke hole before the first acquisition session to encourage the mice to locate the active nosepoke hole. Once the peanut butter was eaten by the mouse, behavior was maintained solely by heroin reinforcement. During the acquisition of heroin self-administration, food and water were unavailable in the test chambers. After the acquisition of heroin self-administration (5 days of 1-h sessions), the dose was increased to 60 μg/kg/infusion in one 1-h session. Following the last acquisition session, the mice were divided into two groups that were matched for active nosepokes in the last session and given either 1-h or 6-h access to heroin self-administration in 10 sessions. During the escalation phase, the sessions were conducted three times per week with 1–2 days between sessions (i.e., typically a Monday-Wednesday-Friday schedule), based on our unpublished data with rats that indicated a robust and reliable escalation using this procedure.
Figure 1.
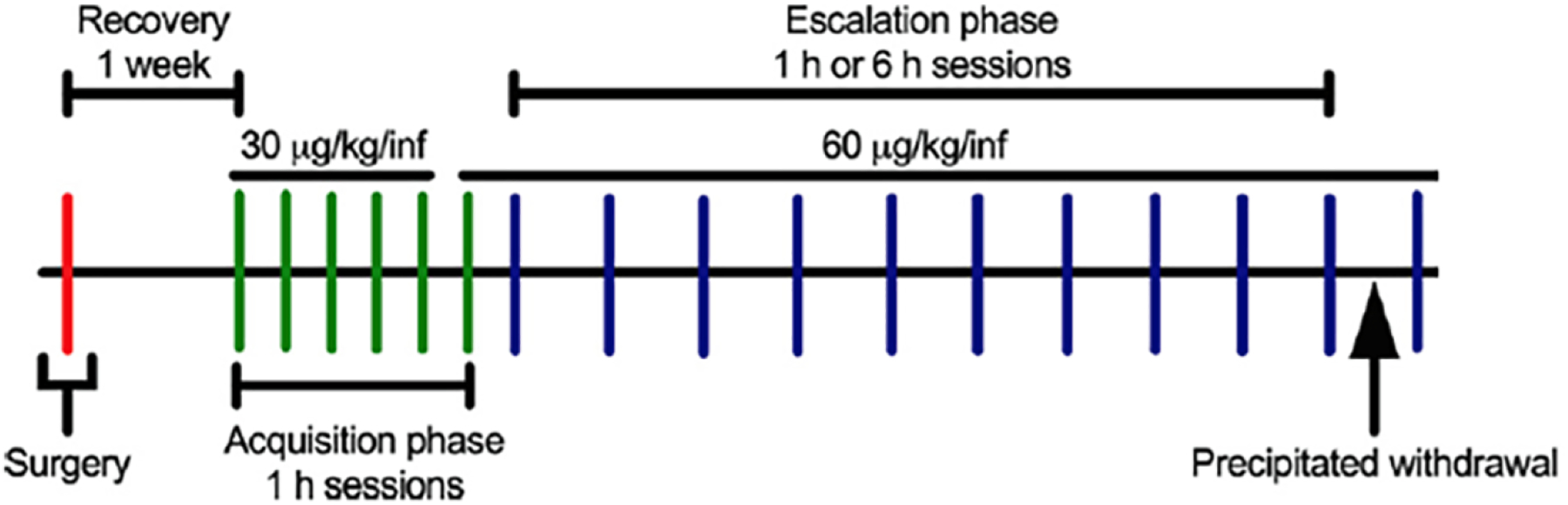
Timeline of the study. Adult male and female C57BL/6J mice underwent surgery and were implanted with a catheter in the right jugular vein and a back-mounted port. The mice were allowed 1 week to recover before the first acquisition session. During the acquisition phase, the mice were trained to nosepoke on an FR1 schedule to obtain intravenous heroin (30 μg/kg/infusion in sessions 1–5; 60 μg/kg/infusion in session 6 onward). The mice then underwent the escalation phase and were divided into short access (ShA; 1 h) or long access (LgA; 6 h) groups. During the escalation phase, the mice were allowed to self-administer heroin in 10 sessions on a Monday-Wednesday-Friday schedule. Immediately after the 10th escalation session, somatic signs of opioid withdrawal (e.g., paw tremors, jumps, etc.) were recorded for 15 min following administration of the preferential μ-opioid receptor antagonist naloxone (1 mg/kg) to precipitate withdrawal.
2.5. Measurement of naloxone-precipitated withdrawal
Immediately after the 10th session, the mice in the 1- and 6-h groups were administered the preferential μ-opioid receptor antagonist naloxone (1 mg/kg, intraperitoneally; Mylan Institutional, Galway, Ireland) and placed in a Plexiglas box (8 in length × 8 in width × 11.5 in height) to observe somatic signs of naloxone-precipitated opioid withdrawal. Five minutes after the injection, the number of paw tremors (i.e., “clapping paws” in front of the chest) and jumps were counted for 15-min. The mice were also observed for less frequent signs of withdrawal, including abnormal posture (counted once per session), scratches, wet dog shakes, abdominal constriction, salivation, ptosis, and genital grooming. All of the observations of withdrawal signs were weighted equally (i.e., one score per observation) and totaled to yield the withdrawal score. The two observers were blind to the experimental conditions during the somatic withdrawal testing.
2.6. Statistical analysis
The data are expressed as the mean and standard error of the mean (SEM). The data were analyzed using between-subjects, within-subjects, or mixed two-way analysis of variance (ANOVA) as appropriate. First-hour intake during the escalation phase was analyzed using a three-way mixed ANOVA, with Group (1 h or 6 h), Sex (male or female), and Session as factors. A two-way repeated-measures ANOVA (Sex × Session) was used to compare 6-h intake across escalation in the male and female LgA groups. Post hoc comparisons were performed using the Duncan test when appropriate. Somatic signs of opioid withdrawal were analyzed using a two-way mixed ANOVA (Sex × Group). Additionally, planned pairwise-comparisons were conducted using Fisher’s LSD to explore the effect of heroin access on escalation in the 1st h self-administration in males and females analyzed separately (i.e., despite no significant Sex × Group × Session interaction). Eta-squared (η2) was used as a measure of effect size. Values of p < 0.05 were considered statistically significant.
3. Results
All mice were given access to 30 μg/kg and 60 μg/kg doses of heroin. Because all the sessions for the ShA mice were the same length during access to these doses (i.e., 1 h), we analyzed their 5 acquisition sessions at 30 μg/kg and their next 5 sessions at the 60 μg/kg dose to determine a self-administration dose-response in male and female mice. During the acquisition phase of intravenous heroin self-administration at the 30 μg/kg/infusion dose, the female mice exhibited increased heroin self-administration compared with male mice (Fig. 2, left panel; Sex Effect: F1,14 = 4.8, p < 0.05; η2 = 0.14; female > male; Session effect: F4,56 = 16.7, p < 0.0001; η2 = 0.18; increased responding across sessions; Sex × Session interaction: F4,56 = 9.3, p < 0.0001; η2 = 0.10). The posthoc comparisons indicated that females self-administered more heroin compared with males from session 3 onward (p < 0.05). At the 60 μg/kg/infusion dose of heroin (Fig. 2, center panel), the ANOVA did not yield any significant effects (Sex effect: F1,14 = 2.4, p = 0.14; Session effect: F4,56 = 1.5, p = 0.23; Sex × Session interaction: F4,56 = 1.0, p = 0.41). To analyze the self-administration dose-response function (Fig. 2, right panel), we calculated the average of the last three sessions at the 30 μg/kg/infusion dose shown in Fig. 2, left panel, compared with the average of the last three sessions at the 60 μg/kg/infusion dose shown in Fig. 2, center panel. The ANOVA yielded a significant Sex × Dose interaction: F1,14 = 9.5, p < 0.01; η2 = 0.10 (Sex effect: F1,14 = 8.1, p <0.05; η2 = 0.23; female > male; Dose effect: F1,14 = 10.3, p < 0.01; η2 = 0.11). The posthoc comparisons indicated that females self-administration significantly more compared with males at the dose of 30 μg/kg/infusion, but not at 60 μg/kg/infusion (p < 0.001), suggesting a rightward-shift in intravenous heroin self-administration dose-response function in female mice compared with male mice.
Figure 2.
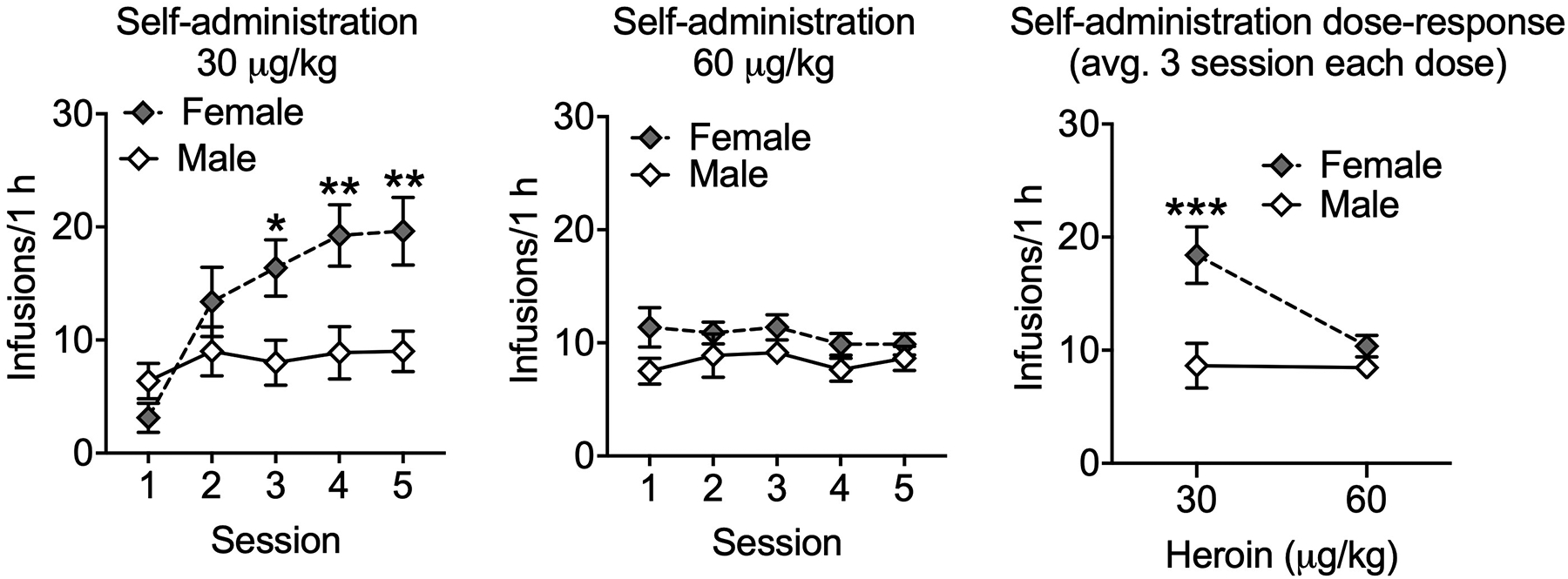
Dose-dependent heroin self-administration in mice of both sexes. Left panel: Increased heroin self-administration at 30 μg/kg/infusion in female mice compared with male mice. Center panel: Male and female mice did not differn in heroin self-administration at 60 μg/kg/infusion. Right panel: Female mice change their heroin self-administration in function of the dose, whereas the male mice did not. The graph shows the average of the last three sessions at the 30 μg/kg/infusion dose shown in the left panel compared with the average of the last three sessions at the 60 μg/kg/infusion dose shown in the center panel. Note: only the mice assigned to the ShA group were used (1 h sessions for both doses). *p<0.05, **p<0.01, ***p<0.001.
Fig. 3 shows the number of intravenous heroin infusions during the escalation phase that were received in ShA and LgA mice in the first hour of the session during the escalation phase. In the ShA sessions and first hour of the LgA sessions, the three-way ANOVA yielded significant effects of Sex (F1,34 = 5.4, p < 0.05; η2 = 0.08; female > male), Group (F1,34 = 6.6, p < 0.05; η2 = 0.10; LgA > ShA), and Session (F9,306 = 6.2, p < 0.0001; η2 = 0.04) and a significant Group × Session interaction (F9,306 = 3.7, p < 0.001; η2 = 0.02) but not a significant Sex × Group interaction (F1,34 = 2.2, p = 0.15), a Sex × Session interaction (F9,306 = 0.5, p = 0.88) or a Sex × Group × Session interaction (F9,306 = 1.2, p = 0.28). The posthoc comparisons on the Group × Session interaction indicated that LgA mice received significantly more heroin infusions in the first hour compared with ShA mice in sessions 6–10 (p < 0.05). LgA mice exhibited an increase in heroin self-administration in the first hour of sessions 5–10 compared with session 1 (p < 0.05). Additional planned comparisons explored male and female behavior separately. For male LgA mice, the comparisons indicated that sessions 7–10 were elevated compared to session 1 (p < 0.05). For female LgA mice, sessions 4–10 were elevated compared to session 1 (p < 0.05). Neither ShA males nor ShA females demonstrated responding on any session that was different from responding on session 1 (p > 0.05). These data suggest escalation in both male and female mice given long-access to heroin. However, when comparing LgA and ShA groups on a session-by-session basis, the comparisons indicated that male LgA mice did not respond more than male ShA mice on any session (p > 0.05). Females LgA mice responded more than Female ShA mice on sessions 4 and 6–10 (p < 0.05). These comparisons suggest that the escalation in female mice was more dramatic than in male mice.
Figure 3.
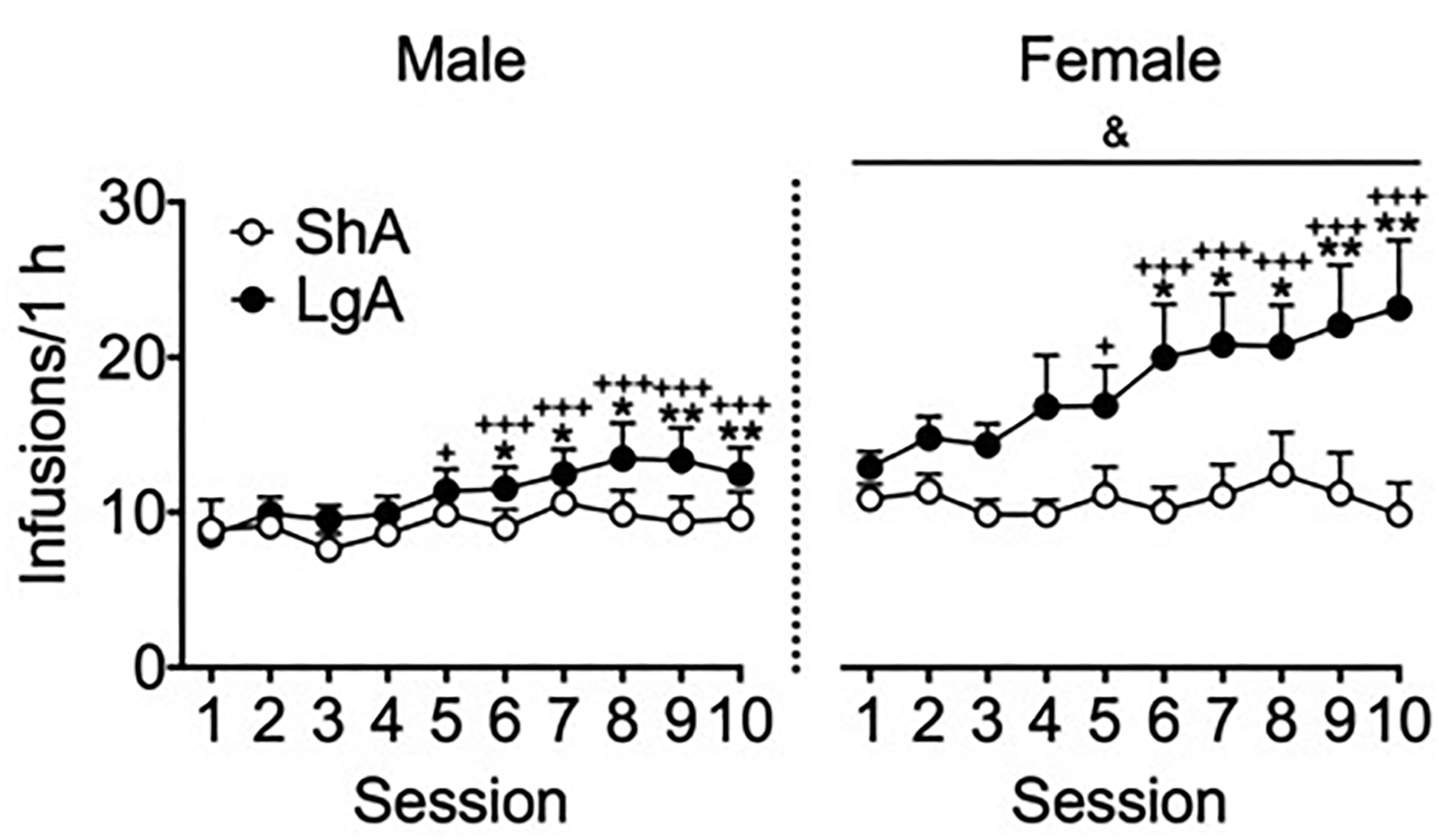
Extended access to heroin resulted in the escalation of heroin intake during the first hour in LgA mice. All of the mice received identical operant training during the acquisition phase. The mice learned to nosepoke in the active nosepoke hole to receive heroin infusions in 1-h sessions (30 μg/kg/infusion in sessions 1–5; 60 μg/kg/infusion in session 6 onward). In the escalation phase, the mice were allowed continuous access to heroin infusions for 1 h (ShA) or 6 h (LgA) per day for 10 sessions. The ShA groups maintained steady intake over repeated sessions. The LgA groups escalated their heroin intake during the first hour of the LgA sessions across time. Female mice self-administered significantly more heroin than male mice during the ShA and LgA sessions (see Results for details). The data are expressed as mean ± SEM. n = 8–11. *p < 0.05, **p < 0.01, different from ShA (male and female data combined); +p < 0.05, +++p < 0.001, different from session 1 (male and female data combined); &p < 0.05, different from male (overall effect of sex).
Table 1 shows the number of intravenous heroin infusions in 6 h during the FR1 schedule of reinforcement in LgA mice. In LgA mice, the two-way repeated-measures ANOVA yielded a significant effect of Sex (F 1,20 = 11.09, p < 0.01, η2 = 0.30; female > male) but not a significant Session effect (F9,180 = 0.43, p=0.92) or a significant Sex × Session interaction (F9,180 = 1.39, p=0.20).
Table 1.
Female mice self-administered more heroin than male mice in the LgA (6 h) sessions.
| Sex | Session 1 | Session 2 | Session 3 | Session 4 | Session 5 | Session 6 | Session 7 | Session 8 | Session 9 | Session 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Male | 52 ± 6 | 53 ± 6 | 44 ± 4 | 52 ± 5 | 57 ± 6 | 56 ± 8 | 62 ± 8 | 54 ± 10 | 60 ± 10 | 55 ± 8 |
| Female | 90 ± 7&& | 91 ± 9&& | 102 ± 11&& | 95 ± 12&& | 92 ± 10&& | 89 ± 11&& | 95 ± 14&& | 95 ± 13&& | 90 ± 11&& | 93 ± 10&& |
The data are expressed as mean ± SEM. n = 8–11.
p < 0.01, different from males (overall effect of sex).
We did not find sex or group differences for inactive nosepokes (data not shown). For nosepokes in the active operandum during the timeout, the ANOVA indicated that LgA females (average of 10 sessions: 627.4 ± 267.1) exhibited increased responding compared with males (average of 10 sessions: 54.3 ± 17.0) during the 6 h session (sex effect: F(1,20)=6.58; p<0.05; session effect: F(9,180)=1.10; p=0.36; sex × session interaction: F(9,180)=1.12; p=0.35). The high responding and variability in LgA females was likely due to exploratory behavior (e.g., sniffing) inside the nosepoke port during the timeout because the program was set to continuously record responses (i.e., breaks of the infrared beam inside the nosepoke port) during the timeout without a debounce time (i.e., after a response was recorded, there was no delay time until the next response could be recorded). For ShA mice, we did not find difference between female (average of 10 sessions: 15.9 ± 7.8) and male (average of 10 sessions: 6.1 ± 1.5) mice (sex effect: F(1,14)=1.51; p=0.24; session effect: F(9,126)=1.58; p=0.13; sex × session interaction: F(9,126)=0.97; p=0.47)
Fig. 4 shows total scores for somatic signs of naloxone-precipitated withdrawal in ShA and LgA mice. The two-way ANOVA yielded a significant effect of Group (F1,34 = 24.2, p < 0.0001, η2 = 0.40), in which LgA mice exhibited more signs of opioid withdrawal compared with ShA mice. The ANOVA did not yield a significant effect of Sex (F1,34 = 0.46, p = 0.50) or a significant Sex × Group interaction (F1,34 = 1.9, p = 0.18).
Figure 4.
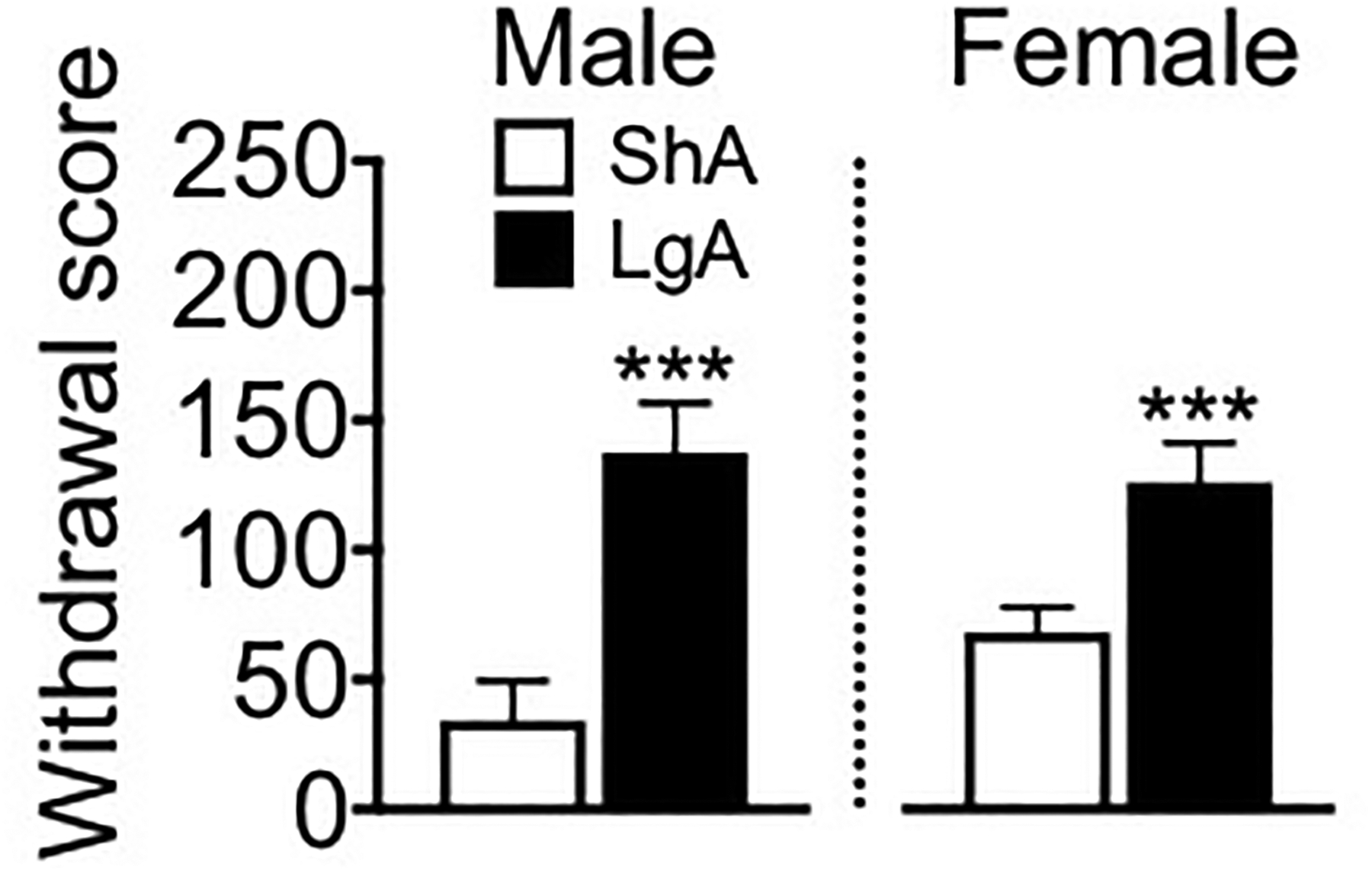
LgA mice exhibited more somatic signs of opioid withdrawal than ShA mice. The mice were administered the preferential μ-opioid receptor antagonist naloxone (1 mg/kg, intraperitoneally), and somatic signs of naloxone-precipitated withdrawal were recorded for 15 min. The withdrawal score comprised the total number of observations of various signs of somatic withdrawal (see Methods). The data are expressed as mean ± SEM. n = 8–11. ***p < 0.001, different from ShA (male and female data combined).
Table 2 shows the number of paw tremors, jumps, and miscellaneous somatic signs of naloxone-precipitated withdrawal in ShA and LgA mice. The two-way ANOVA yielded a significant effect of Group on paw tremors (F1,34 = 11.3, p < 0.01, η2 = 0.24; LgA > ShA; Sex effect: F1,34 = 0.44, p = 0.51; Sex × Group interaction: F1,34 = 0.96, p = 0.33) and jumps (F1,34 = 8.3, p < 0.01, η2 = 0.19; LgA > ShA; Sex effect: F1,34 = 0.01, p = 0.91; Sex × Group interaction: F1,34 = 0.37, p = 0.55) but not on other miscellaneous signs (Group effect: F1,34 = 0.06, p = 0.80; Sex effect: F1,34 = 0.45, p = 0.51; Sex × Group interaction: F1,34 = 0.81, p = 0.37).
Table 2.
LgA mice exhibited more paw tremors and jumps than ShA mice.
| Group | Sex | Paw Tremors | Jumps | Miscellaneous signs1 |
|---|---|---|---|---|
| ShA | Male | 32.25 ± 14.87 | 0.38 ± 0.38 | 1.63 ± 0.38 |
| LgA | Male | 106.09 ± 16.64** | 28.27 ± 7.79** | 3.00 ± 0.51 |
| ShA | Female | 60.13 ± 7.92 | 4.38 ± 2.85 | 3.50 ± 2.66 |
| LgA | Female | 100.64 ± 20.09** | 22.55 ± 10.89** | 2.73 ± 0.38 |
Wet dog shakes, abnormal posture, scratching, profuse salivation, and genital grooming.
The data are expressed as mean ± SEM. n = 8–11.
p < 0.01, different from ShA (male and female data combined; overall effect of group).
Discussion
In the present study, we adapted and validated in mice a model that was originally developed in rats (Ahmed and Koob, 1998) of limited-access, stable heroin self-administration vs. extended-access, compulsive-like heroin self-administration. LgA mice of both sexes exhibited the escalation of intravenous heroin self-administration, and an increase in somatic signs of naloxone-precipitated withdrawal compared with ShA mice. Additionally, female mice self-administered more heroin compared with male mice.
Although mice of both sexes readily acquired intravenous heroin self-administration at the 30 μg/kg/infusion dose, the females exhibited about twice as much responding for heroin from session 3 to 5 compared with males. No sex differences were found when the dose was increased to 60 μg/kg/infusion. That females self-administered more heroin at the lower dose suggest that females may be less sensitive to the effects of heroin (e.g., reinforcing or rate-limiting effects) compared to males (i.e., a right-shift of the dose-response curve). Future parametric studies with a wider range of doses will address this point. Alternatively, the lack of titration exhibited by males across the 30 and 60 μg/kg/infusion heroin doses may be sex specific, as a similar result was found in male rats self-administering heroin across a wider range of doses (30 −120 μg/kg/infusion; Wade et al., 2014). Critically, the finding that ShA male and female mice did not differ in responding for heroin at the 60 μg/kg/infusion dose suggests that the enhanced escalation of heroin intake in female LgA mice was not due to differences in baseline self-administration responding resulting from potential differences between sexes in propensitiy to perform an operant response or in behavioral activity in general.
By giving the mice short or long access to heroin, we generated groups of male and female mice with a limited drug self-administration history (ShA) and a greater drug self-administration history that caused somatic and motivational signs of drug dependence (LgA). As demonstrated previously for intravenous opioid self-administration in rats and in the present study in mice, ShA mice exhibited a regular and stable pattern of heroin consumption when given limited access to heroin. In contrast, LgA mice that were given extended access to heroin exhibited a different pattern of heroin intake, which included an initial “loading” in drug intake in the first hour of heroin access. The increase in “drug-loading” behavior that was observed during the first hour of the LgA session may reflect the emergence of a negative emotional-like state during withdrawal, which has been hypothesized to drive compulsive-like self-administration via negative reinforcement (Koob et al., 2014). These results suggest that extended access to heroin imparts an additional source of motivation to seek and take the drug in mice. This mouse model may be particularly useful for studying both the appetitive properties of opioids that drive the acquisition phase of drug taking (modeled in ShA mice) and the motivational and neurobiological adaptations that comprise the transition to drug dependence (modeled by LgA mice).
Previous studies reported that rats that were maintained on a 6-h access model (Lenoir and Ahmed, 2006, 2007) escalated their heroin intake in the first hour of the session and across the entire 6-h LgA session. The previous findings that extended access leads to a greater intensity of drug taking in rats were similarly demonstrated in the present study with mice. Specifically, LgA but not ShA mice escalated their heroin intake in the first hour of the sessions. In contrast to the majority of rat studies (Ahmed et al., 2000; Barbier et al., 2013; Lenoir and Ahmed, 2006, 2007; Schmeichel et al., 2015; Vendruscolo et al., 2011; Wade et al., 2015), LgA mice did not escalate their total (i.e., 6 h) heroin intake across sessions. In addition to species, procedural differences, such as dose, session length, the number of sessions, may explain these discrepancies. Such procedural differences in rat studies can alter the development and expression of escalated drug-taking behavior (e.g., Wade et al., 2015). Future studies should refine these parameters in mice to further describe drug escalation and dependence.
Several studies have reported that mice will intravenously self-administer drugs, including cocaine (Caine et al., 2007; Zhang, 2006) and heroin (Zhang et al., 2015), similarly to rats (for review, see Kmiotek et al., 2012; Thomsen and Caine, 2007). However, we could only find one previous study that explicitly compared short-access vs. extended-access drug self-administration in mice. Zhang et al. (2014) gave C57BL/6J mice short (1-h) or long (4-h) access to oxycodone (0.25 mg/kg/infusion) across 14 consecutive daily sessions and observed an escalation of oxycodone intake in the LgA group but not in the ShA group, providing convergent evidence that supports the present findings (i.e., the escalation of drug intake in mice following LgA vs. ShA opioid access). Importantly, however, this experiment did not include an acquisition phase, thus making it difficult to discern the specific contributions of operant learning from the escalation of drug intake that is caused by the development of drug dependence over LgA sessions.
Notably, in the present study, female mice self-administered more heroin during the 1-h ShA session and the first hour and 6 h of the LgA session. These results are consistent with Cicero et al. (2003), who reported that female rats self-administered significantly greater amounts of heroin than male rats. Evidence also indicates that single housing animals might have differential effects in male and female rodents (Becker and Koob, 2016). Males are more territorial, and females are more social. In the present study, all of the mice were single-housed rather than group-housed. Thus, the housing conditions may have been more stressful to female mice and contributed to their increase in drug taking. Zhang et al. (2015) did not report a significant difference in heroin self-administration between male and female mice. This could be attributable to the fact that the mice were group-housed after surgery and the high dose of heroin that was used in their study. Previous studies of conditioned place preference in rats reported that low doses of morphine were more rewarding in females than in males (Karami and Zarrindast, 2008). This opens interesting new avenues for studying the effects of environment, sex, and drug availability on opioid self-administration and motivation. However, a limitation of the present study is the use of a single dose of heroin in the escalation phase. As suggested by the dose-effect function in ShA mice, females were putatively more resistant to the effects of heroin than males (i.e., a right shift of the dose-response function in females) and this might have contributed to the increased escalation of drug intake observed in females.
In the present study, we also observed significantly more somatic signs of naloxone-precipitated withdrawal in LgA mice compared with ShA mice, reflecting the development of physical dependence on opioids in LgA mice. These results are consistent with previous studies in rats that reported a significant increase in somatic signs of naloxone-precipitated withdrawal in LgA rodents compared with ShA rodents (Vendruscolo et al., 2011). Although male mice self-administered less heroin than female mice, they exhibited slightly more somatic signs of withdrawal compared with females. Although females self-administered more heroin than males, we did not find sex differences in the expression of somatic signs of withdrawal. A possible explanation for this finding is that females appear to be more resistant to opioid withdrawal signs than male mice (Diaz et al., 2005; see, for review, Becker and Koob, 2016). Alternatively, the lack of differences between males and females in the present study might simply be attributable to a ceiling effect. Our 15-min period of observing signs of heroin withdrawal may have been too short to see a significant difference between males and females. Evidence indicates that the time course of the manifestation of withdrawal symptoms is different between males and females. Cicero et al. (2002) reported that withdrawal symptoms in male rats were more prolonged compared with female rats.
In summary, the present study suggests that mice with extended access to heroin escalate their heroin intake and exhibit a “drug-loading” pattern of intake, and the development of physical dependence. Significant sex differences were observed. Female mice that had extended access to heroin exhibited more robust escalation. We propose that mice that are given extended vs. limited access to opioids constitutes a useful model for studying the neurobiological adaptations that underlie opioid addiction in both sexes.
Highlights.
Mice with extended access to heroin escalated their drug intake
Mice with extended access to heroin exhibited somatic signs of opioid withdrawal
Females showed increased heroin intake compared with males
Acknowledgements
The authors thank Michael Arends for proofreading the manuscript and Barak Caine for technical guidance.
Funding
This work was supported by the National Institute on Drug Abuse Intramural Research Program.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Ahmed SH, Walker JR, Koob GF, 2000. Persistent Increase in the Motivation to Take Heroin in Rats with a History of Drug Escalation. Neuropsychopharmacology 22, 413–421. 10.1016/S0893-133X(99)00133-5 [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF, 1998. Transition from Moderate to Excessive Drug Intake: Change in Hedonic Set Point. Science 282, 298–300. 10.1126/science.282.5387.298 [DOI] [PubMed] [Google Scholar]
- American Society of Addiction Medicine, 2016. Opioid Addiction 2016 Facts & Figures. Available at: https://www.asam.org/docs/default-source/advocacy/opioid-addiction-disease-facts-figures.pdf. Accessed July 11 2018.
- Anker JJ, Carroll ME, 2010. Females Are More Vulnerable to Drug Abuse than Males: Evidence from Preclinical Studies and the Role of Ovarian Hormones, in: Neill JC, Kulkarni J (Eds.), Biological Basis of Sex Differences in Psychopharmacology. Springer Berlin Heidelberg, Berlin, Heidelberg, pp. 73–96. 10.1007/7854_2010_93 [DOI] [PubMed] [Google Scholar]
- Barbier E, Vendruscolo LF, Schlosburg JE, Edwards S, Juergens N, Park PE, Misra KK, Cheng K, Rice KC, Schank J, Schulteis G, Koob GF, Heilig M, 2013. The NK1 Receptor Antagonist L822429 Reduces Heroin Reinforcement. Neuropsychopharmacology 38, 976–984. 10.1038/npp.2012.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Chartoff E, 2018. Sex differences in neural mechanisms mediating reward and addiction. Neuropsychopharmacology. 10.1038/s41386-018-0125-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Hu M, 2008. Sex differences in drug abuse. Front. Neuroendocrinol 29, 36–47. 10.1016/j.yfrne.2007.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Koob GF, 2016. Sex Differences in Animal Models: Focus on Addiction. Pharmacol. Rev 68, 242–263. 10.1124/pr.115.011163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady KT and Randall CL, 1999. Gender Differences in Substance Use Disorders. Psychiatric Clinics of North America. 22, 241–252. 10.1016/S0193-953X(05)70074-5 [DOI] [PubMed] [Google Scholar]
- Caine SB, Thomsen M, Gabriel KI, Berkowitz JS, Gold LH, Koob GF, Tonegawa S, Zhang J, Xu M, 2007. Lack of Self-Administration of Cocaine in Dopamine D1 Receptor Knock-Out Mice. J. Neurosci 27, 13140–13150. 10.1523/JNEUROSCI.2284-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Aylward SC, Meyer ER, 2003. Gender differences in the intravenous self-administration of mu opiate agonists. Pharmacol. Biochem. Behav 74, 541–549. 10.1016/S0091-3057(02)01039-0 [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Nock B, Meyer ER, 2002. Gender-linked differences in the expression of physical dependence in the rat. Pharmacol. Biochem. Behav 72, 691–697. 10.1016/S0091-3057(02)00740-2 [DOI] [PubMed] [Google Scholar]
- Diaz SL, Kemmling AK, Rubio MC, Balerio GN, 2005. Morphine withdrawal syndrome: Involvement of the dopaminergic system in prepubertal male and female mice. Pharmacol. Biochem. Behav 82, 601–607. 10.1016/j.pbb.2005.10.012 [DOI] [PubMed] [Google Scholar]
- Edwards S, Vendruscolo LF, Schlosburg JE, Misra KK, Wee S, Park PE, Schulteis G, Koob GF, 2012. Development of mechanical hypersensitivity in rats during heroin and ethanol dependence: Alleviation by CRF1 receptor antagonism. Neuropharmacology 62, 1142–1151. 10.1016/j.neuropharm.2011.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Compton WM, Jones CM, Cai R Nonmedical Prescription Opioid Use and Use Disorders Among Adults Aged 18 Through 64 Years in the United States, 2003–2013 JAMA. 2015;314(14):1468–1478. [DOI] [PubMed] [Google Scholar]
- Harding WM, Zinberg NE Occasional opiate use. Advances in Substance Abuse 3, 27 (1983). [PubMed] [Google Scholar]
- Karami M, Zarrindast MR, 2008. Morphine sex-dependently induced place conditioning in adult Wistar rats. Eur. J. Pharmacol 582, 78–87. 10.1016/j.ejphar.2007.12.010 [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Chen SA, Kitamura O, Markou A, Koob GF, 2006. Conditioned Withdrawal Drives Heroin Consumption and Decreases Reward Sensitivity. J. Neurosci 26, 5894–5900. 10.1523/JNEUROSCI.0740-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmiotek EK, Baimel C, Gill KJ, 2012. Methods for Intravenous Self Administration in a Mouse Model. J. Vis. Exp 10.3791/3739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Buck CL, Cohen A, Edwards S, Park PE, Schlosburg JE, Schmeichel B, Vendruscolo LF, Wade CL, Whitfield TW, George O, 2014. Addiction as a stress surfeit disorder. Neuropharmacology 76, 370–382. 10.1016/j.neuropharm.2013.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND, 2016. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 3, 760–773. 10.1016/S2215-0366(16)00104-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, Gawin FH, Kosten TR, Rounsaville BJ, 1993. Gender differences in cocaine use and treatment response. J. Subst. Abuse Treat 10, 63–66. 10.1016/0740-5472(93)90100-G [DOI] [PubMed] [Google Scholar]
- Lenoir M, Ahmed SH, 2007. Supply of a Nondrug Substitute Reduces Escalated Heroin Consumption. Neuropsychopharmacology 33, 2272–82. 10.1038/sj.npp.1301602 [DOI] [PubMed] [Google Scholar]
- Lenoir M, Ahmed SH, 2006. Heroin-Induced Reinstatement is Specific to Compulsive Heroin Use and Dissociable from Heroin Reward and Sensitization. Neuropsychopharmacology 32, 616–24. 10.1038/sj.npp.1301083 [DOI] [PubMed] [Google Scholar]
- Lynch WJ, 2018. Modeling the development of drug addiction in male and female animals. Pharmacol. Biochem. Behav 164, 50–61. 10.1016/j.pbb.2017.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, 2006. Sex differences in vulnerability to drug self-administration. Exp. Clin. Psychopharmacol 14, 34–41. 10.1037/1064-1297.14.1.34 [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Taylor JR, 2004. Sex Differences in the Behavioral Effects of 24-h/day Access to Cocaine under a Discrete Trial Procedure. Neuropsychopharmacology 29, 943–951. 10.1038/sj.npp.1300389 [DOI] [PubMed] [Google Scholar]
- National Opioid Overdose Crisis, 2018. Opioid Overdose Crisis. Available at: https://www.drugabuse.gov/drugs-abuse/opioids/opioid-overdose-crisis#six. Accessed July 11 2018.
- Park PE, Schlosburg JE, Vendruscolo LF, Schulteis G, Edwards S, Koob GF, 2015. Chronic CRF 1 receptor blockade reduces heroin intake escalation and dependence-induced hyperalgesia: CRF 1 R in heroin dependence. Addict. Biol 20, 275–284. 10.1111/adb.12120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park PE, Vendruscolo LF, Schlosburg JE, Edwards S, Schulteis G, Koob GF, 2013. Corticotropin-releasing factor (CRF) and α 2 adrenergic receptors mediate heroin withdrawal-potentiated startle in rats. Int. J. Neuropsychopharmacol 16, 1867–1875. 10.1017/S1461145713000308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priddy BM, Carmack SA, Thomas LC, Vendruscolo JCM, Koob GF, Vendruscolo LF, 2017. Sex, strain, and estrous cycle influences on alcohol drinking in rats. Pharmacol., Biochem. and Behav 152, 61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeichel BE, Barbier E, Misra KK, Contet C, Schlosburg JE, Grigoriadis D, Williams JP, Karlsson C, Pitcairn C, Heilig M, Koob GF, Vendruscolo LF, 2015. Hypocretin Receptor 2 Antagonism Dose-Dependently Reduces Escalated Heroin Self-Administration in Rats. Neuropsychopharmacology 40, 1123–1129. 10.1038/npp.2014.293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, 2017. SAMHSA Shares Latest Behavioral Health Data, Including Opioid Misuse. Available at: https://newsletter.samhsa.gov/2017/10/12/samhsa-new-data-mental-health-substance-use-including-opioids/. Accessed July 11 2018.
- Thomsen M, Caine SB, 2007. Intravenous Drug Self-administration in Mice: Practical Considerations. Behav. Genet 37, 101–118. 10.1007/s10519-006-9097-0 [DOI] [PubMed] [Google Scholar]
- Tunstall BJ, Carmack SA, Koob GF, Vendruscolo LF, 2017. Dysregulation of brain stress systems mediates compulsive alcohol drinking. Curr. Opin. Behav. Sci 13, 85–90. 10.1016/j.cobeha.2016.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Schlosburg JE, Misra KK, Chen SA, Greenwell TN, Koob GF, 2011. Escalation patterns of varying periods of heroin access. Pharmacol. Biochem. Behav 98, 570–574. 10.1016/j.pbb.2011.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade CL, Vendruscolo LF, Schlosburg JE, Hernandez DO, Koob GF, 2015. Compulsive-Like Responding for Opioid Analgesics in Rats with Extended Access. Neuropsychopharmacology 40, 421–428. 10.1038/npp.2014.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Svenningsson P, Picetti R, Schlussmand SD, Nairn AC, Greengard P, and Kreek MJ, 2006. Cocaine Self-Administration in Mice Is Inversely Related to Phosphorylation at Thr34 (Protein Kinase A Site) and Ser130 (Kinase CK1 Site) of DARPP-32. J. Neurosci 26, 2645–2651. 10.1523/JNEUROSCI.3923-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Mayer-Blackwell B, Schlussman SD, Randesi M, Butelman ER, Ho A, Ott J, Kreek MJ, 2014. Extended access oxycodone self-administration and neurotransmitter receptor gene expression in the dorsal striatum of adult C57BL/6 J mice. Psychopharmacology (Berl.) 231, 1277–1287. 10.1007/s00213-013-3306-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Picetti R, Butelman ER, Ho A, Blendy JA, Kreek MJ, 2015. Mouse Model of the OPRM1 (A118G) Polymorphism: Differential Heroin Self-Administration Behavior Compared with Wild-Type Mice. Neuropsychopharmacology 40, 1091–1100. 10.1038/npp.2014.286 [DOI] [PMC free article] [PubMed] [Google Scholar]


