Abstract
In this report we describe the functional expression of EmrE, a 110-amino-acid multidrug transporter from Escherichia coli, in the yeast Saccharomyces cerevisiae. To allow for phenotypic complementation, a mutant strain sensitive to a series of cationic lipophilic drugs was first identified. A hemagglutinin epitope-tagged version of EmrE (HA-EmrE) conferring resistance to a wide variety of drugs, including acriflavine, ethidium, methyl viologen, and the neurotoxin 1-methyl-4-phenylpyridinium (MPP+), was functionally expressed in this strain. HA-EmrE is expressed in yeast at relatively high levels (0.5 mg/liter), is soluble in a mixture of organic solvents, and can be functionally reconstituted in proteoliposomes. In bacterial cells, EmrE removes toxic compounds by active transport through the plasma membrane, lowering their cytosolic concentration. However, yeast cells expressing HA-EmrE take up 14C-methyl viologen as well as control cells do. Thus, we investigated the basis of the enhanced resistance to the above compounds. Using Cu2+ ions or methylamine, we could selectively permeabilize the plasma membrane or deplete the proton electrochemical gradients across the vacuolar membrane, respectively. Incubation of yeast cells with copper ions caused an increase in 14C-methyl viologen uptake. In contrast, treatment with methylamine markedly diminished the extent of uptake. Conversely, the effect of Cu2+ and methylamine on a plasma membrane uptake system, proline, was essentially the opposite: while inhibited by the addition of Cu2+, it remained unaffected when cells were treated with methylamine. To examine the intracellular distribution of HA-EmrE, a functional chimera between HA-EmrE and the green fluorescent protein (HA-EmrE-GFP) was prepared. The pattern of HA-EmrE-GFP fluorescence distribution was virtually identical to that of the vacuolar marker FM 4-64, indicating that the transporter is found mainly in this organelle. Therefore, HA-EmrE protects yeast cells by lowering the cytoplasmic concentrations through removal of the toxin to the vacuole. This novel way of detoxification has been previously suggested to function in organisms in which a large vacuolar compartment exists. This report represents the first molecular description of such a mechanism.
Multidrug transporters (MDTs) are proteins that recognize a wide substrate range with relatively high affinity and remove the substrates from the cytoplasm in an energy-dependent process. Since many of the substrates happen to be toxic compounds, the MDTs frequently have been associated with multidrug resistance, a phenomenon that poses a serious threat to the treatment of resistant cancers and infectious diseases. Based on primary amino acid sequence similarities, five different families of MDTs have been identified. The MiniTEXANs or SMR family includes more than 40 proteins in eubacteria, a few of which have been studied in detail. One of them, EmrE, is an Escherichia coli MDT which confers resistance to a wide variety of toxicants by actively exchanging them with hydrogen ions (15, 26, 29, 34). EmrE is a highly hydrophobic 12-kDa protein that has been purified by taking advantage of its unique solubility in organic solvents. After solubilization and purification, the protein retains its ability to transport, as judged from the fact that it can be reconstituted in a functional form (45). Hydrophobicity analysis of the sequence yielded four putative transmembrane domains of similar sizes. Results from transmission Fourier transform infrared measurements agree remarkably well with this hypothesis and yielded α-helical estimates of 78 and 80% for EmrE in chloroform-methanol and 1,2-dimyristoyl phosphocholine, respectively (2). That EmrE is functional as a homo-oligomer is suggested by coreconstitution experiments of the wild-type protein with three different inactive mutants in which negative dominance has been observed (46).
MDTs are used in various gene therapy projects for selection of transfected cells and to promote gene amplification (24). EmrE is a relatively simple H+-driven MDT; it is a small polypeptide, and no known posttranslational modifications are required for its function. We therefore explored the possibility that it may serve as a versatile gene for biotechnology projects. Saccharomyces cerevisiae provides a good model system to study transport processes in more complex organisms because of the vast genetic tools available and its wide use in heterologous expression of membrane proteins. In this work, HA-EmrE, a hemagglutinin (HA) epitope-tagged version of EmrE, was functionally expressed in S. cerevisiae. Yeast cells expressing HA-EmrE exhibit enhanced resistance to a wide variety of cytotoxic compounds. Since overall drug uptake levels were similar in HA-EmrE-expressing and control cells, we investigated the mode of protection exerted in yeast by the transporter. The subcellular protein distribution was determined by using a fluorescent dye specific for the vacuolar membrane and a functional chimera between the transporter and green fluorescent protein (GFP). Our results strongly suggest that HA-EmrE protects yeast cells by sequestration of the cytotoxic compounds in the yeast vacuole. This novel way of detoxification might be found in other organisms in which large vacuolar compartments exist.
MATERIALS AND METHODS
Materials.
14C-methyl viologen (paraquat) was from Sigma. [3H]proline (26 Ci/mmol) was from Amersham, and FM 4-64 was from Molecular Probes. Other reagents were from commercially available sources.
Strains.
E. coli DH5α (17) was used for propagation of recombinant plasmids. The S. cerevisiae strains used in this study were YAE65 (MATa ade2-119 ilv1-92 trp5-b sge1 ura3Δ5) and YHE4 (MATa ade2-119 ilv1-92 trp5-b ura3Δ5) (9, 10) (kindly supplied by C. Senstag, University of Zurich) as well as BWT-1 (matα GAL his3 lys2 ura3 leu2 trp1 met10; from the A. Levitzki lab stock).
Media.
Yeast strains were grown in standard media. Complete medium (YPD) contained 1% yeast extract, 2% Bacto Peptone (both from Difco), and 2% glucose or 2% glycerol. Minimal medium (SD; 0.67% yeast nitrogen base without amino acids [Difco] and 2% glucose) was supplemented according to auxotrophic requirements as described in reference 16. Yeast cells were transformed by the method of Elble (11).
Plasmids.
Plasmid pNKY85 (1) was digested with BglII and used to transform YAE65 and YHE4 from LEU2 ura3 to leu2::URA3. Transformed colonies were picked and tested for auxotrophicity to leucine in uracil-lacking media. BFG-1 (2μm, 3-phosphoglycerate kinase promoter and terminator, LEU2) contains an internal ATG followed by three copies of the HA epitope (41) separated by a BamHI site (38). The codons in this epitope favor efficient translation since they are among those preferred by yeast (4).
Cloning procedures.
The open reading frame of EmrE was amplified by PCR from pKK56 (45), using as the sense primer EP485-3 (5′-TTCGAAGCTTGGATCCATGAACCCTTATATTTATCTTG) and as the antisense primer RP (5′-CCGAATTCTCGAGTTAATGTGGTGTGCTGCTTCGTGAC), and digested with BamHI and XhoI. The DNA segment was ligated with BFG-1, creating BFG-1/HA-EmrE. In this way, EmrE is inserted in frame at the BamHI site and as a result contains 24 additional amino acids at the N terminus bearing two copies of the HA epitope (YPYDVPDYA) (Fig. 1A).
FIG. 1.
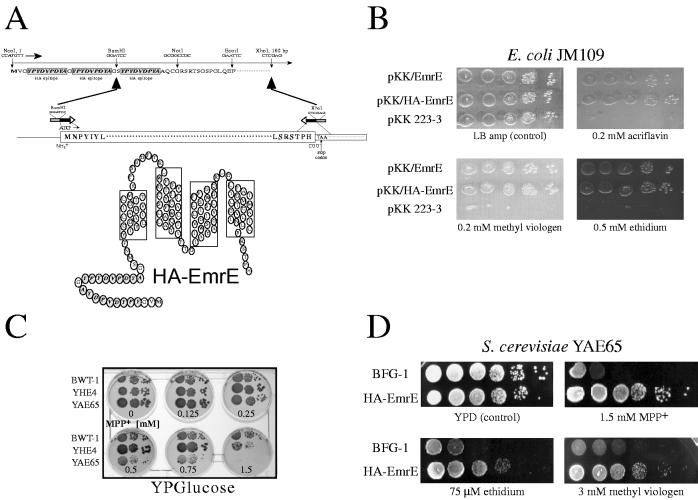
HA-EmrE protects bacteria and yeast cells from a wide range of cytotoxic compounds. (A) Diagrammatic presentation of the generation of HA-EmrE. EmrE was inserted in frame at the BamHI site of the BFG-1 vector, creating the epitope-tagged HA-EmrE. HA-EmrE contains 24 additional amino acids at the N-terminus comprising two repeats of the HA epitope (YPYDVPDYA). (B) HA-EmrE, like the wild-type EmrE, confers resistance to bacterial cells. Five-microliter aliquots of logarithmic dilutions (1:102 to 1:107) from an overnight culture were spotted on LB plates in the absence (control) or presence of either 0.2 mM acriflavine, 0.2 mM methyl viologen, or 0.5 mM ethidium. (C) YAE65 is an S. cerevisiae strain particularly sensitive to MPP+. Overnight cultures were tested for susceptibility to the toxin MPP+. In comparison to the yeast wild-type strain BWT-1, YHE4 shows an increased sensitivity surpassed only by that of the extremely sensitive isogenic strain YAE65. (D) Yeast cells expressing HA-EmrE show an increased resistance to pleiotropic drugs. YAE65 cells were grown overnight in minimal medium. Serial dilutions (1:1 to 1:105) were performed in sterile water, and 5-μl suspensions were spotted on YPD plates containing the indicated drug concentrations. After 3 days of incubation at 30°C, the plates were photographed.
HA-EmrE was inserted in plasmid pKK223-3 (Pharmacia Biotech Inc.), using as the template BFG-1/HA-EmrE, the sense primer 5′-ATCGCCCGGGGAATTCACGCGTCCCATGGTTGGTTACCCCATAC, and the antisense primer 5′-CCGAATTCAAGCTTAATGTGGTGTGCTTCGTGAC. The PCR product was digested with EcoRI and HindIII and inserted in pKK223-3 at the same sites.
To create a chimera between HA-EmrE and the GFP (28) mutant S65T (18), a reverse primer (RP-EmrE-GFP), which inserts an EcoRI site immediately before the stop codon, was synthesized. The EcoRI site adds two amino acids, Glu and Phe, to the C terminus of HA-EmrE; RP-EmrE-GFP has the sequence 5′-CCGAGCTCGAGAAGCTTAGAATTCATGTGGTGTGCTTCGTGA. A PCR product of the primer EP485-3 and RP-EmrE-GFP was digested with BamHI and XhoI and inserted in BFG-1, creating BFG-1/HA-EmrE II. The GFP mutant Ser65Thr from pRSETb/GFP (18) was excised with EcoRI and XhoI and inserted in BFG-1/HA-EmrE II at the same sites, creating BFG-1/HA-EmrE-GFP. Therefore, BFG-1/HA-EmrE-GFP expresses a gene fusion coding for a 372-amino-acid polypeptide which includes two copies of the HA epitope, EmrE and GFP.
Drug sensitivity assays.
E. coli JM109 (43) was transformed with pKK/HA-EmrE, pKK/EmrE (pKK56) (45), or pKK223-3 (mock vector). After overnight growth at 37°C, the culture was diluted serially 10-fold and 5-μl aliquots of the suspensions were spotted on LB plates in the presence or absence of the various compounds at the indicated concentrations. After 24 h of growth, the plates were photographed. Similarly, yeast strain YAE65 transformed with the corresponding plasmids was grown in liquid minimal medium to late log phase. Five-microliters aliquots of logarithmic dilutions from these cultures were spotted on YPD or YPG plates with various concentrations of the tested drug. YPD plates were incubated at 30°C for 2 to 3 days.
Membrane purification.
Membranes of yeast cells were prepared by the glass beads protocol (12) modified as follows. Cells were grown on minimal medium to early logarithmic phase, harvested, and washed once with sterile distilled water and once with ice-cold lysis buffer containing 140 mM NaCl 10 mM K-HEPES (pH 7.4), 10 mM MgCl2, 10% glycerol, and 10 mM β-mercaptoethanol. The pellet was resuspended with 2 ml per g of cell pellet in lysis buffer supplemented with a cocktail of protease inhibitors (0.2 mM phenylmethylsulfonyl fluoride, 1 μM pepstatin, 1 μM aprotinin, 5 μM leupeptin, and 10 μM chymostatin). An equal volume of acid-washed glass beads was added, and the suspension was vortexed in a cold room six times for 30 s each. The lysate was centrifuged at 4°C for 5 min at 5,000 × g to discard whole cells. The supernatant was then ultracentrifuged for 45 min at 200,000 × g, and the pellet was resuspended in a minimal volume of lysis buffer supplemented with antiproteases. The membrane suspension was immediately frozen in liquid nitrogen and stored at −70°C until thawed for further manipulations.
Western blotting.
Membrane lysates were mixed with an equal volume of protein sample buffer, and proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 16% Tricine gels as described elsewhere (33). The protein was transferred to a polyvinylidene difluoride membrane (Millipore) by using a semidry blotter for 45 min at 1 mA/cm2. Transfer buffer contained 48 mM glycine, 25 mM Tris-HCl (pH 8.3), and 10% methanol. The membrane was then blocked for 1 h in 1% blocking solution (Boehringer Mannheim) in TBS-T (137 mM NaCl, 50 mM Tris-HCl [pH 7.5], 0.05% Tween 20). The blot was then incubated at least for 2 h with 1:5,000 dilution of monoclonal antibody against the HA epitope (12CA5; BAbCo, Berkeley, Calif.). After five washes with TBS-T, the blot was incubated with anti-mouse secondary antibody (Boehringer Mannheim), and developed as recommended in the protocol for the Boehringer Mannheim chemiluminescence Western blotting kit.
Extraction and reconstitution of HA-EmrE from yeast membranes.
The method used is modified from one described elsewhere (45). In brief, 27-μl aliquots of E. coli phospholipids (50 mg/ml) were added to 400 μl of yeast membranes (10 mg/ml) and mixed with 6 ml of chloroform- to methanol (1:1). After 30 min on ice, 1 ml of water was added, and the mixture was vortexed and centrifuged at 4°C for 5 min at 5,000 × g. The upper phase was removed, and the lower phase was dried with argon. The pellet was resuspended in 80 μl of 0.18 M NH4Cl–15 mM Tris-HCl (pH 7.0). The mixture was divided in aliquots and frozen at −70°C.
Transport of 14C-methyl viologen in proteoliposomes.
Transport was as described in reference 45. In brief, before the transport assay, the proteoliposome suspension was thawed and sonicated in a bath-type sonicator for a few seconds until clear. Then 3 μl of this suspension was diluted in 200 μl of an ammonium-free medium containing 140 mM KCl, 10 mM Tricine, and 5 mM MgCl2 (pH 8.5) in the presence of 70 μM 14C-methyl viologen (150 nCi/assay) and the correspondent tracers and inhibitors as indicated for each experiment. At given times, the reaction was stopped by dilution with 2 ml of ice-cold solution without the radioactive substrate, filtering through Schleicher & Schuell filters (0.2-μm pore size), and washing with an additional 2 ml of solution. The radioactivity on the filters was assessed by liquid scintillation. In each experiment, a control reaction with 5 μM nigericin was used to subtract the nonspecific radioactivity.
Transport in whole cells.
The method used is essentially as described by Kitamoto et al. (21, 27). Briefly, cells were grown to the early logarithmic phase in minimal glucose medium, counted, harvested, and washed twice with sterile distilled water. Cells were then resuspended to a density of 1.5 × 109/ml in buffer B (0.6 M sorbitol, 10 mM glucose, 20 mM morpholineethanesulfonic acid-Tris [pH 6.0]) and incubated for 30 min at 30°C, in the absence or presence of 0.5 mM CuSO4 and/or 10 mM methylamine. The suspension was diluted 10-fold in transport buffer with the correspondent tracers in the presence of 10 μCi of 14C-methyl viologen or [3H]proline per ml. Aliquots of 100 μl were withdrawn at the indicated times, diluted in 3 ml of ice-cold buffer B and rapidly filtered through HAWP filters (pore size, 0.45 μm; Millipore). After the filters were washed three times with 3 ml of ice-cold transport buffer and briefly dried, radioactivity was assessed by liquid scintillation. Each experiment was repeated independently at least three times.
Vacuolar staining with FM 4-64.
Cells were grown in minimal medium to early logarithmic phase, harvested, and resuspended to an optical density at 600 nm (OD600) of 5 in minimal medium supplemented at 10 μg/ml with the lipophilic styryl dye N-(3-triethylammoniumpropyl)-4-(p-diethylaminophenyl-hexatrienyl) pyridinium dibromide (FM 4-64) (40). After 60 min of incubation at 30°C, cells were spun, washed twice, and resuspended at an OD600 of 0.2 for at least 1 h in minimal medium (chase). For confocal microscopy, an aliquot of the suspension was spun resuspended in a minimal volume of growth medium, mixed with an equal volume of 2.6% low-melting-point agarose, poured on a slide, sealed, and immediately visualized in a confocal microscope.
Scanning laser confocal microscopy.
A Bio-Rad MRC-1024 confocal scanhead coupled to a Zeiss Axiovert 135M inverted microscope was used to acquire images of the stained cells, with a 63× oil objective (numerical aperture, 1.4). Excitation light was provided by a 100-mW air-cooled argon ion laser run in the multiline mode. The excitation wavelength of 488 nm was selected with a suitable interference filter. The relative excitation power level was set with either a 3 or 1% neutral density filter. The fluorescence emission was split between three channels with two dichroic mirrors (555DRLP [50% point at 550 nm] and 605DRHP [50% point at 605 nm]). GFP emission was detected on the short-wavelength side of the first dichroic mirror, through an HQ525/40 bandpass filter (525 ± 20 nm). The confocal aperture was 2.5 to 3.0mm. There was no detectable bleedthrough of FM 4-64 to this channel. FM 4-64 emission was detected on the long-wavelength side of the second dichroic mirror, through an HQ655/90 bandpass filter (655 ± 45 nm). The confocal aperture was 4.0 to 4.5 mm. There was some detection of GFP emission in the FM 4-64 channel, but it was weaker than the FM 4-64 emission under the conditions of these experiments. The maximum bleedthrough was estimated to be no more than 15%.
RESULTS
Epitope tagging of EmrE.
EmrE is a miniature MDT from E. coli. It is a 110-amino-acid-long polypeptide with four putative transmembrane domains. Since it lacks significant hydrophilic domains, its immunogenicity is low and it is difficult to raise antibodies against it, even after repeated injections of pure EmrE protein to rabbits (unpublished observations). To identify the transporter by immunological techniques, EmrE was tagged with the HA epitope (YPYDVPDYA). For this purpose, an oligonucleotide was engineered to allow for in-frame insertion of the emrE gene in the BamHI site at the multicloning site of the yeast expression plasmid BFG1-1, creating HA-EmrE (Fig. 1A). As a result, HA-EmrE has 24 additional amino acids at the N terminus of the protein bearing two copies of the HA epitope.
HA-EmrE confers resistance to E. coli cells as well as the wild-type EmrE does.
To determine whether addition of the double epitope has an effect on EmrE function, we assessed the resistance conferred to E. coli cells. E. coli JM109 cells transformed with either pKK/EmrE, pKK/HA-EmrE, or pKK223-3 (mock vector) were grown on LB plates in the absence or presence of various drugs. As shown in Fig. 1B, cells expressing HA-EmrE could grow in the presence of 0.2 mM acriflavine, 0.2 mM methyl viologen, or 0.5 mM ethidium as well as cells expressing the wild-type EmrE. In contrast, cells transformed with the vector alone (pKK223-3) grew only on the control plate (LB supplemented with 50 μg of ampicillin per ml). Identical conclusions were reached when resistance was assessed in liquid media or by measurements of the halo of growth inhibition around filters soaked with concentrated solutions of the above compounds (not shown). The results demonstrate that the epitope-tagged version of EmrE retains the ability to protect the cells and may serve for future studies in which immunological methods are required.
Characterization of a strain sensitive to multiple drugs.
To test phenotypic complementation of an MDT, it was necessary to use an S. cerevisiae strain sensitive to a wide variety of toxicants. We screened a wide variety of mutants and found one which displayed the desired phenotype. The strain chosen was YAE65, an sge1 null mutant that was selected for its sensitivity to crystal violet and harbors an additional unidentified mutation that increases its sensitivity (9, 10). These findings are demonstrated in experiments in which serial dilutions of overnight cultures were plated on YPD plates containing increasing concentrations of the neurotoxin 1-methyl-4-phenylpyridinium (MPP+). The results in Fig. 1C reveal the high sensitivity of strain YAE65 to MPP+ compared to the resistance shown by wild-type strain BWT-1. Whereas at 0.75 mM MPP+ the growth of strain YAE65 is impaired, wild-type BWT-1 grows at 1.5 mM MPP+ practically as well as it grows in its absence. Use of glycerol as the sole carbon source enhances the effectiveness of MPP+. At MPP+ concentrations as low as 0.25 mM in YPGly plates, the growth of BWT-1 is almost nil (not shown). The difference may stem from the fact that the suggested MPP+ site of action is the mitochondrial respiratory chain (31). In YPGly medium, cells depend entirely on the integrity of the mitochondria to support their growth, whereas in YPD growth might be sustained by the glycolytic pathway when mitochondria fail. The overall growth was significantly slower with glycerol than with glucose. The experiments shown below were carried out on glucose-containing media.
Functional expression of HA-EmrE in yeast cells.
As explained earlier, EmrE was inserted in BFG-1, a vector used for expression in S. cerevisiae. BFG-1 is a 2μm plasmid which uses the strong and constitutive 3-phosphoglycerate kinase promoter and terminator (37). The first 24 amino acids of HA-EmrE, corresponding to the appended sequence, are encoded mainly by codons used in highly expressed yeast proteins (Codon Bias Index) (4). This allows for high levels of expression of otherwise unexpressed heterologous proteins (unpublished observations). In the specific case of HA-EmrE, relatively high levels of expression are achieved; it is possible to produce 0.5 mg of HA-EmrE per liter of minimal medium. We examined whether the expression of HA-EmrE causes sensitive yeast cells to display increased resistance to pleiotropic drugs. In the example of such an experiment shown in Fig. 1D, YAE65 cells transformed with BFG-1/HA-EmrE grew better in the presence of toxicants than did control (BFG-1) cells. As shown in Fig. 1D, expression of HA-EmrE in YAE65 cells confers resistance to a wide variety of toxicants: increased resistance to MPP+ (1.5 mM), to methyl viologen (3 mM), and to ethidium (75 μM). In experiments in which the OD600 of overnight cultures grown in the presence of cytotoxic compounds was measured, the results were essentially similar: only yeast cells expressing HA-EmrE were resistant to the toxic effects of the compounds tested. We conclude that HA-EmrE is functionally expressed in S. cerevisiae.
HA-EmrE is soluble in a mixture of chloroform and methanol.
To identify HA-EmrE in total cell membranes prepared from yeast cells, we used a monoclonal antibody (12CA5) against the HA epitope. For Western blots, we used as a negative control membranes from cells transformed with the vector (BFG-1) alone. As shown in Fig. 2A, HA-EmrE appears mainly as a single band of ∼14 kDa (lane 2); in contrast, no immunoreactivity was detected in the control sample (lane 1). Wild-type EmrE can be extracted with a 1:1 mixture of chloroform and methanol (yielding what we refer to as C:M extracts) (45). Therefore, we checked whether HA-EmrE retains this unusual property. Indeed, HA-EmrE is also quantitatively extracted with organic solvents (Fig. 2, lane 5). Noteworthy, the additional 24 amino acids include four negative charges (corresponding to four aspartic acids), changing the overall protein charge from +2 to −2, yet, the transporter is still soluble in the above mixture.
FIG. 2.
HA-EmrE is extractable in a chloroform-methanol mixture and can be functionally reconstituted in proteoliposomes. (A) HA-EmrE is soluble in a chloroform-methanol mixture. Membranes and C:M extracts were produced from yeast cells, separated on a 16% Tricine gel (33), and assayed for protein expression with a monoclonal antibody against the HA epitope. Membrane samples (lanes 1 to 4) and C:M (Chl:Meth) extracts (lanes 5 to 7) consisted of cells transformed with BFG-1 mock vector (control; lane 1), HA-EmrE (lanes 2 and 5), HA-EmrE II (lanes 3 and 6), HA-EmrE-GFP (lane 4 and 7). (B) HA-EmrE can be reconstituted in proteoliposomes. Transport of 14C-methyl viologen was assayed in proteoliposomes prepared with C:M extracts from either BFG-1- or HA-EmrE-transformed cells as described in Materials and Methods. (C) Substrates of EmrE inhibit the uptake of 14C-methyl viologen. Uptake of 14C-methyl viologen was measured in the presence of increasing concentrations of ethidium bromide. The inhibition curve is in agreement with previous studies (45). Similar results were achieved with acriflavine (not shown).
HA-EmrE transport activity can be reconstituted in vitro.
HA-EmrE expressed in yeast is also functional in vitro, as demonstrated by the fact that the extracted and highly purified protein can be reconstituted in proteoliposomes. In this system, HA-EmrE catalyzes ΔpH driven accumulation of 14C-methyl viologen against its concentration gradient. Proteoliposomes prepared from C:M extracts of control membranes (BFG-1) showed no uptake activity. In contrast, those prepared from extracts of membranes containing HA-EmrE showed 100-fold-higher uptake activity (Fig. 2B). We also determined whether the recombinant protein has the same specificity range as the wild type. We measured the ability of competitors to inhibit the uptake of 14C-methyl viologen in proteoliposomes and found that the 50% inhibitory concentrations of each tested substrate (acriflavine [10 μM] and ethidium [1.27 μM] were similar to those measured for the wild-type transporter (45). An example of such an assay is shown in Fig. 2C, where ethidium totally inhibited 14C-methyl viologen uptake in a concentration-dependent manner.
HA-EmrE protects yeast cells by intracellular compartmentalization of the toxins.
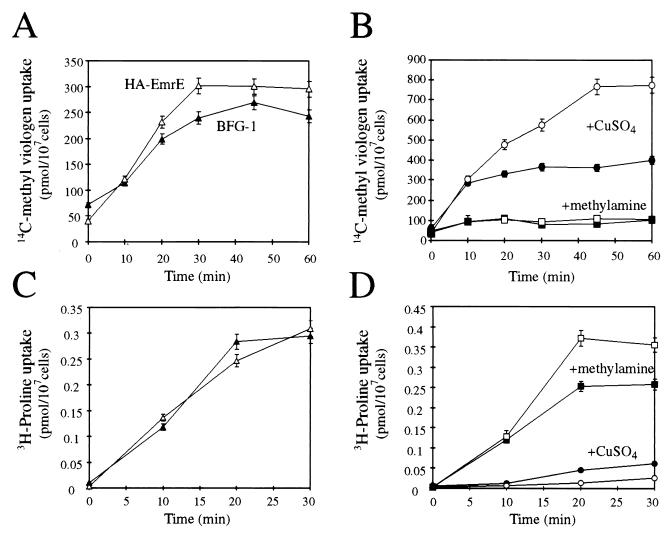
EmrE protects bacterial cells against cytotoxic compounds by active extrusion of the offending compounds across the plasma membrane, thereby lowering their concentration near their target sites. An electrochemical gradient of protons, of the proper direction and magnitude, that may sustain HA-EmrE activity is found also across the plasma membrane of yeast cells. Hence, we investigated whether a similar HA-EmrE-driven removal mechanism protects the resistant cells. Using a rapid filtration method, we measured the uptake of 14C-methyl viologen in whole cells. As shown in Fig. 3A, the content of 14C-methyl viologen in HA-EmrE is actually slightly higher than the uptake level of control cells. Based on these results, we set up experiments to investigate the mechanism by which cells expressing HA-EmrE display an increased resistance even though they absorb the same extent of toxicants. Anraku and collaborators (21, 27) have shown that it is possible to selectively permeabilize the plasma membrane by the addition of Cu2+ ions to cells resuspended in a medium of low ionic strength. The Cu2+ permeabilization technique allows the specific extraction of cytosolic pools of ions, amino acids, and other metabolites. We measured the uptake of 14C-methyl viologen in the presence of 0.5 mM CuSO4 and found that whereas in control cells the uptake increased only slightly, HA-EmrE-expressing cells showed a twofold increase in the magnitude of uptake (Fig. 3B). A possible explanation for this phenomenon is that HA-EmrE is located in a subcellular compartment in which ΔμH+ is generated across its membrane and serves as the driving force for active sequestration of the toxicants. The plasma membrane constitutes a diffusion barrier for methyl viologen; hence, the addition of Cu2+ abolishes this restriction and allows an enhanced intracellular HA-EmrE-mediated uptake. In line with this contention, Cu2+ has no effect on the activity of the purified protein reconstituted in proteoliposomes. It is possible to diminish the magnitude of H+ gradients by using weak bases such as ammonia or other amines such as methylamine (14). We determined the effect of 10 mM methylamine on the uptake of 14C-methyl viologen and found that it causes a drastic reduction in the extent of methyl viologen incorporation. The dramatic results were quantitatively similar for control and HA-EmrE-expressing cells (Fig. 3B). Similar results were achieved with 100 μM carbonyl cyanide m-chlorophenylhydrazone, a proton ionophore (not shown). For a transport system located in the plasma membrane, the effects of Cu2+ and methylamine are expected to be essentially the opposite. Indeed, when we tested the influence of the above compounds on the uptake of [3H]proline, the inverse effect was found. Proline is transported by plasma membrane transporters and stored in the cytosol (21, 32). As shown in Fig. 3C, we found no significant differences between the cells in the uptake of proline. As expected for a plasma membrane transport system, treatment with methylamine did not change the transport values significantly. In contrast, Cu2+ addition abolished the uptake of [3H]proline (Fig. 3D). Taken as a whole, the results of both transport experiments strongly favor the hypothesis that HA-EmrE catalyzes the sequestration of toxicants on subcellular acidic compartments.
FIG. 3.
HA-EmrE catalyzes the compartmentalization of toxins in an acidic intracellular compartment. (A) HA-EmrE-expressing and control (BFG-1) cells take up similar amounts of 14C-methyl viologen. HA-EmrE-expressing and BFG-1 cells were assayed for total uptake of 14C-methyl viologen by a rapid filtration method. (B) Permeabilized HA-EmrE-expressing cells take up increased amounts of 14C-methyl viologen. Uptake of 14C-methyl viologen was measured on BFG-1 (closed symbols) and HA-EmrE-expressing (open symbols) cells treated with either 0.5 mM CuSO4 (•, ○) (27) or 10 mM methylamine (■, □) (14) as described in Materials and Methods. (C and D) The effects of Cu2+ and methylamine on a plasma membrane transport system are essentially the opposite. HA-EmrE (open symbols)- and BFG-1 (closed symbols)-transformed cells were assayed for the ability to transport [3H]proline in the presence of 0.5 mM CuSO4 (•, ○) or 10 mM methylamine (■, □) or in the absence of further additions (▵, ▴).
HA-EmrE-GFP is located at the vacuolar membrane.
Various organelles may fulfill the acidic requirements necessary for HA-EmrE activity, among them secretory vesicles, endosomes, and the vacuole. To examine the intracellular localization of the transporter, we constructed a fusion of HA-EmrE with GFP. This fusion protein is fully functional, as judged by its ability to confer resistance to MPP+ (Fig. 4A), acriflavine, ethidium, and methyl viologen (not shown). This protein is expressed at approximately the same levels as HA-EmrE (Fig. 2A, lane 4). We also detected a minor band of approximately ∼16 kDa, probably a result of some proteolysis occurring in the membrane isolation process. As expected from the large hydrophilic tail attached to HA-EmrE, it is not extractable into organic solvents (Fig. 2A, lane 7). In contrast, HA-EmrE II, a protein created for the subsequent insertion of GFP, is soluble in the above mixture (Fig. 2A, lane 6). HA-EmrE II has a total protein charge of −3, as it contains two additional amino acids, glutamate and phenylalanine, at the C terminus.
FIG. 4.
HA-EmrE-GFP confers resistance to yeast cells and shows a distinct pattern of vacuolar staining. (A) The HA-EmrE-GFP chimera confers resistance to yeast cells against toxic compounds. YAE65 cells transformed with either BFG-1 (control), HA-EmrE, HA-EmrE II, or HA-EmrE-GFP were grown overnight in minimal medium. Serial dilutions (1:1 to 1:104) were performed, and 5-μl aliquots of the suspensions were spotted on YPD plates either lacking or containing 1.5 mM MPP+; 3 days later, plates were photographed. (B and C) HA-EmrE-GFP shows a typical vacuolar distribution. Cells expressing HA-EmrE-GFP (B) and HA-GFP (C) were stained for 60 min with 10 μM FM 4-64 (40), washed, and visualized in a confocal microscope as described in Materials and Methods. HA-GFP shows a consistent cytosolic distribution. HA-EmrE-GFP specifically locates in the tonoplast; FM 4-64 and HA-EmrE-GFP images are clearly overlapping.
Preliminary observations showed that the cellular distribution of HA-EmrE-GFP fluorescence closely resembled that in yeast cells immunostained with antibodies raised against the vacuolar H+/ATPase (20). We therefore decided to take advantage of the lipophilic styryl dye FM 4-64, used as a specific vacuolar membrane marker (40). FM 4-64 has a reddish fluorescence that is not detected by using filters directed to record the fluorescence of the GFP. It is therefore possible to measure both signals independently. Cells expressing HA-EmrE-GFP were grown in minimal medium, stained with FM 4-64, and visualized in a confocal microscope. As shown in Fig. 4B, similar patterns of distribution were found for the green fluorescence and the FM 4-64 signal. The results indicate that the fluorescence emitted by HA-EmrE-GFP was entirely coincident with that of the vacuolar membranes stained with FM 4-64. As a control, we expressed GFP alone. GFP is a soluble protein from the jellyfish Aequorea victoria that shows a spread cytoplasmic fluorescence. Figure 4C shows the results from the confocal studies performed with the GFP-expressing cells. The green fluorescence is distributed throughout the cytoplasm but not in the large subcellular compartments (black circles within the cell). Since the FM 4-64 signal stains the boundary of the vacuole, it was possible to confirm that the above compartments are vacuoles, given that the FM 4-64 rings match the limit of the circles described above. The results unequivocally demonstrate that HA-EmrE-GFP is located mainly in the vacuole membrane. Therefore, we can infer that the intracellular compartment to which HA-EmrE removes the toxicants is predominantly the cell vacuole.
DISCUSSION
The evidence presented in this paper demonstrates that EmrE protects yeast cells by sequestration of the toxicants in the yeast vacuole. HA-EmrE expression in YAE65, an S. cerevisiae strain particularly sensitive to lipophilic cations (9, 10), caused an increased resistance to a variety of cytotoxic compounds. However, control and HA-EmrE-expressing cells took up comparable levels of 14C-methyl viologen. After selective permeabilization of the plasma membrane by using Cu2+ ions (27), HA-EmrE-expressing cells display an increased ability to take up methyl viologen. The increase might be explained by the elimination of a permeability barrier and the HA-EmrE-associated ability to remove toxicants from the cytoplasm to acidic intracellular compartments. Transport from the cytoplasm reduces the concentration of noxious chemicals near their targets, and cells become resistant. To test the role of the proton gradient across the vacuolar membrane, we used methylamine, a weak base known to impair the magnitude of those gradients (14). Methylamine completely abolished the uptake of 14C-methyl viologen and, notably, also reduced the extent of 14C-methyl viologen uptake in control (BFG-1) cells. The results may reflect the activity of an existing endogenous H+-driven transport system, which extrudes its substrates into subcellular acidic compartments. As a control for the method used, we tested the effects of Cu2+ and methylamine on a plasma membrane transport system. Proline is taken up by a specific permease (PUT4) (39). As expected, whereas methylamine did not inhibit [3H]proline uptake, addition of Cu2+ drastically diminished the extent of this uptake. These results imply that the data presented here reflect genuine HA-EmrE-mediated toxin sequestration into subcellular compartments. Distribution of the HA-EmrE-GFP fluorescence closely resembled that of the fluorescent signal of FM 4-64. The results support the contention that although several intracellular membranes exist, HA-EmrE-GFP is found mainly in the tonoplast. Taking the results as a whole, we conclude that the mechanism of protection mediated by HA-EmrE is the active removal of cytotoxic compounds into the yeast vacuole.
Protection by compartmentation of toxicants seems to be widespread in organisms in which large vacuoles exist. The vacuole plays an important role in the detoxification and as a homeostatic mechanism to balance the cytosolic metal contents within the nontoxic range. It has been suggested that in yeast, compartmentalization is the mechanism underlying resistance to metals such as cadmium (23), nickel (19, 30), tellurium, iron and copper (36), chromium and selenium (13), and presumably cobalt and zinc (8). In plants, the lack of specific detoxification organs forces this organism to supply the cells with specific autonomous mechanisms of protection. Indeed, the vacuole has been suggested to provide a storage compartment for defense proteins and secondary metabolites (allelochemicals) (42) and to act as a transient storage compartment for further detoxification of heavy metals (47). Although the P-glycoprotein or MDR is the major protein conferring resistance to numerous cell types, some findings could not be explained solely by this mechanism. In several cases, the P-glycoprotein-mediated resistance was not correlated with decreased transport, suggesting that compartmentation may play a role in the resistance exhibited by these cells (5, 35). In fact, the multidrug resistance-related protein was shown to catalyze the sequestration of toxins into secretory vesicles (3, 6, 7).
Mammalian cells heterologously expressing a vesicular monoamine transporter (VMAT) become resistant to the neurotoxin MPP+ by intracellular compartmentalization of the toxin (25). Moreover, the VMAT family exhibits a distinct homology to bacterial MDTs. In addition, VMAT and EmrE have similar substrate specificities (44), suggesting that neurotransmitter transport may have evolved as a intracellular mechanism to protect the cells against possible toxicity of those compounds. Our results for methyl viologen uptake in the presence of methylamine suggest that a cryptic H+-driven transport system, similar to those found for metals, may exist in yeast for xenobiotics.
No consensus sequences have been defined for yeast vacuole proteins (22). It is interesting that the vast majority of a heterologous expressed membrane protein is sorted to the vacuolar membrane. Because of its small size and high expression levels, EmrE can provide a good model system for studying vacuolar targeting. We hereby show that a protein which acts as a toxin removal transporter in prokaryotes confers resistance by toxin sequestration into the vacuole of a eukaryotic cell.
ACKNOWLEDGMENTS
We thank Sharon Levy for the cloning of EmrE in BFG-1, Jeffrey Gerst (Weizmann Institute) for help with the FM 4-64 studies, and Aryeh Weiss for technical assistance with confocal microscopy.
This work was supported by grants from the Deutsche-Israeli Program, National Institutes of Health (NS16708), and Israel Science Foundation.
REFERENCES
- 1.Alani E, Cao L, Kleckner N. A method for gene disruption that allows repeated use of URA3 selection in the construction of multiply disrupted yeast strains. Genetics. 1987;116:541–545. doi: 10.1534/genetics.112.541.test. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arkin I, Russ W, Lebendiker M, Schuldiner S. Determining the secondary structure and orientation of EmrE, a multi-drug transporter, indicates a transmembrane four helix bundle. Biochemistry. 1996;35:7233–7238. doi: 10.1021/bi960094i. [DOI] [PubMed] [Google Scholar]
- 3.Benderra Z, Morjani H, Trussardi A, Manfait M. Role of the vacuolar H+-ATPase in daunorubicin distribution in etoposide-resistant MCF7 cells overexpressing the multidrug-resistance associated protein. Int J Oncol. 1998;12:711–715. doi: 10.3892/ijo.12.3.711. [DOI] [PubMed] [Google Scholar]
- 4.Bennetzen J L, Hall B D. Codon selection in yeast. J Biol Chem. 1982;257:3026–3031. [PubMed] [Google Scholar]
- 5.Bobichon H, Colin M, Depierreux C, Liautaud-Roger F, Jardillier J C. Ultrastructural changes related to multidrug resistance in CEM cells: role of cytoplasmic vesicles in drug exclusion. J Exp Ther Oncol. 1996;1:49–61. [PubMed] [Google Scholar]
- 6.Breuninger L M, Paul S, Gaughan K, Miki T, Chan A, Aaronson S A, Kruh G D. Expression of multidrug resistance-associated protein in NIH/3T3 cells confers multidrug resistance associated with increased drug efflux and altered intracellular drug distribution. Cancer Res. 1995;55:5342–5347. [PubMed] [Google Scholar]
- 7.Cleary I, Doherty G, Moran E, Clynes M. The multidrug-resistant human lung tumour cell line, DLKP-A10, expresses novel drug accumulation and sequestration systems. Biochem Pharmacol. 1997;53:1493–1502. doi: 10.1016/s0006-2952(97)00003-8. [DOI] [PubMed] [Google Scholar]
- 8.Conklin D S, Culbertson M R, Kung C. Interactions between gene products involved in divalent cation transport in Saccharomyces cerevisiae. Mol Gen Genet. 1994;244:303–311. doi: 10.1007/BF00285458. [DOI] [PubMed] [Google Scholar]
- 9.Ehrenhofer-Murray A E, Seitz M U, Sengstag C. The Sge1 protein of Saccharomyces cerevisiae is a membrane-associated multidrug transporter. Yeast. 1998;14:49–65. doi: 10.1002/(SICI)1097-0061(19980115)14:1<49::AID-YEA199>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 10.Ehrenhofer-Murray A E, Wurgler F E, Sengstag C. The Saccharomyces cerevisiae SGE1 gene product: a novel drug-resistance protein within the major facilitator superfamily. Mol Gen Genet. 1994;244:287–294. doi: 10.1007/BF00285456. [DOI] [PubMed] [Google Scholar]
- 11.Elble R. A simple and efficient procedure for transformation of yeasts. BioTechniques. 1992;13:18–20. [PubMed] [Google Scholar]
- 12.Franzusoff A J, Cirillo V P. Glucose transport activity in isolated plasma membrane vesicles from Saccharomyces cerevisiae. J Biol Chem. 1983;258:3608–3614. [PubMed] [Google Scholar]
- 13.Gharieb M M, Gadd G M. Evidence for the involvement of vacuolar activity in metal(loid) tolerance: vacuolar-lacking and -defective mutants of Saccharomyces cerevisiae display higher sensitivity to chromate, tellurite and selenite. Biometals. 1998;11:101–106. doi: 10.1023/a:1009221810760. [DOI] [PubMed] [Google Scholar]
- 14.Greenfield N J, Hussain M, Lenard J. Effects of growth state and amines on cytoplasmic and vacuolar pH, phosphate and polyphosphate levels in Saccharomyces cerevisiae: a 31P-nuclear magnetic resonance study. Biochim Biophys Acta. 1987;926:205–214. doi: 10.1016/0304-4165(87)90205-4. [DOI] [PubMed] [Google Scholar]
- 15.Grinius L, Dreguniene G, Goldberg E B, Liao C H, Projan S J. A staphylococcal multidrug resistance gene product is a member of a new protein family. Plasmid. 1992;27:119–129. doi: 10.1016/0147-619x(92)90012-y. [DOI] [PubMed] [Google Scholar]
- 16.Guthrie C, Fink G R, editors. Methods in Enzymology. Vol. 194. San Diego, Calif: Academic Press; 1991. [Google Scholar]
- 17.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 18.Heim R, Cubitt A B, Tsien R Y. Improved green fluorescence. Nature. 1995;373:663–664. doi: 10.1038/373663b0. [DOI] [PubMed] [Google Scholar]
- 19.Joho M, Ishikawa Y, Kunikane M, Inouhe M, Tohoyama H, Murayama T. The subcellular distribution of nickel in Ni-sensitive and Ni-resistant strains of Saccharomyces cerevisiae. Microbios. 1992;71:149–159. [PubMed] [Google Scholar]
- 20.Kane P M, Kuehn M C, Howald-Stevenson I, Stevens T H. Assembly and targeting of peripheral and integral membrane subunits of the yeast vacuolar H(+)-ATPase. J Biol Chem. 1992;267:447–454. [PubMed] [Google Scholar]
- 21.Kitamoto K, Yoshizawa K, Ohsumi Y, Anraku Y. Dynamic aspects of vacuolar and cytosolic amino acid pools of Saccharomyces cerevisiae. J Bacteriol. 1988;170:2683–2686. doi: 10.1128/jb.170.6.2683-2686.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klionsky D J. Nonclassical protein sorting to the yeast vacuole. J Biol Chem. 1998;273:10807–10810. doi: 10.1074/jbc.273.18.10807. [DOI] [PubMed] [Google Scholar]
- 23.Li Z S, Lu Y P, Zhen R G, Szczypka M, Thiele D J, Rea P A. A new pathway for vacuolar cadmium sequestration in Saccharomyces cerevisiae: YCF1-catalyzed transport of bis(glutathionato)cadmium. Proc Natl Acad Sci USA. 1997;94:42–47. doi: 10.1073/pnas.94.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Licht T, Herrmann F, Gottesman M M, Pastan I. In vivo drug-selectable genes: a new concept in gene therapy. Stem Cells. 1997;15:104–111. doi: 10.1002/stem.150104. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Peter D, Roghani A, Schuldiner S, Prive G, Eisenberg D, Brecha N, Edwards R. A cDNA that suppresses MPP+ toxicity encodes a vesicular amine transporter. Cell. 1992;70:539–551. doi: 10.1016/0092-8674(92)90425-c. [DOI] [PubMed] [Google Scholar]
- 26.Morimyo M, Hongo E, Hama-Inaba H, Machida I. Cloning and characterization of the mvrC gene of Escherichia coli K12 which confers resistance against methyl viologen toxicity. Nucleic Acids Res. 1992;20:3159–3165. doi: 10.1093/nar/20.12.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohsumi Y, Kitamoto K, Anraku Y. Changes induced in the permeability barrier of the yeast plasma membrane by cupric ion. J Bacteriol. 1988;170:2676–2682. doi: 10.1128/jb.170.6.2676-2682.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prasher D C, Eckenrode V K, Ward W W, Prendergast F G, Cormier M J. Primary structure of the Aequorea victoria green-fluorescent protein. Gene. 1992;111:229–233. doi: 10.1016/0378-1119(92)90691-h. [DOI] [PubMed] [Google Scholar]
- 29.Purewal A S, Jones I G, Midgley M. Cloning of the ethidium efflux gene from Escherichia coli. FEMS Microbiol Lett. 1990;68:73–76. doi: 10.1016/0378-1097(90)90127-c. [DOI] [PubMed] [Google Scholar]
- 30.Ramsay L M, Gadd G M. Mutants of Saccharomyces cerevisiae defective in vacuolar function confirm a role for the vacuole in toxic metal ion detoxification. FEMS Microbiol Lett. 1997;152:293–298. doi: 10.1111/j.1574-6968.1997.tb10442.x. [DOI] [PubMed] [Google Scholar]
- 31.Ramsay R R, Krueger M J, Youngster S K, Singer T P. Evidence that the inhibition sites of the neurotoxic amine 1-methyl-4-phenylpyridinium (MPP+) and of the respiratory chain inhibitor piericidin A are the same. Biochem J. 1991;273:481–484. doi: 10.1042/bj2730481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sato T, Ohsumi Y, Anraku Y. Substrate specificities of active transport systems for amino acids in vacuolar-membrane vesicles of Saccharomyces cerevisiae. Evidence of seven independent proton/amino acid antiport systems. J Biol Chem. 1984;259:11505–11508. [PubMed] [Google Scholar]
- 33.Schagger H, von Jagow G. Tricine-SDS PAGE for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 34.Schuldiner S, Lebendiker M, Yerushalmi H. EmrE, the smallest ion coupled transporter, provides a unique paradigm for structure function studies. J Exp Biol. 1997;200:335–341. doi: 10.1242/jeb.200.2.335. [DOI] [PubMed] [Google Scholar]
- 35.Shapiro A B, Fox K, Lee P, Yang Y D, Ling V. Functional intracellular P-glycoprotein. Int J Cancer. 1998;76:857–864. doi: 10.1002/(sici)1097-0215(19980610)76:6<857::aid-ijc15>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 36.Szczypka M S, Zhu Z, Silar P, Thiele D J. Saccharomyces cerevisiae mutants altered in vacuole function are defective in copper detoxification and iron-responsive gene transcription. Yeast. 1997;13:1423–1435. doi: 10.1002/(SICI)1097-0061(199712)13:15<1423::AID-YEA190>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 37.Tuite M F, Dobson M J, Roberts N A, King R M, Burke D C, Kingsman S M, Kingsman A J. Regulated high efficiency expression of human interferon-alpha in Saccharomyces cerevisiae. EMBO J. 1982;1:603–608. doi: 10.1002/j.1460-2075.1982.tb01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tyers M, Tokiwa G, Nash R, Futcher B. The Cln3-Cdc28 kinase complex of S. cerevisiae is regulated by proteolysis and phosphorylation. EMBO J. 1992;11:1773–1784. doi: 10.1002/j.1460-2075.1992.tb05229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vandenbol M, Jauniaux J C, Grenson M. Nucleotide sequence of the Saccharomyces cerevisiae PUT4 proline- permease-encoding gene: similarities between CAN1, HIP1 and PUT4 permeases. Gene. 1989;83:153–159. doi: 10.1016/0378-1119(89)90413-7. [DOI] [PubMed] [Google Scholar]
- 40.Vida T A, Emr S D. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J Cell Biol. 1995;128:779–792. doi: 10.1083/jcb.128.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson I A, Niman H L, Houghten R A, Cherenson A R, Connolly M L, Lerner R A. The structure of an antigenic determinant in a protein. Cell. 1984;37:767–778. doi: 10.1016/0092-8674(84)90412-4. [DOI] [PubMed] [Google Scholar]
- 42.Wink M. The plant vacuole: a multifunctional compartment. J Exp Bot. 1993;44:231–246. [Google Scholar]
- 43.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–199. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 44.Yelin R, Schuldiner S. The pharmacological profile of the vesicular monoamine transporter resembles that of multidrug transporters. FEBS Lett. 1995;377:201–207. doi: 10.1016/0014-5793(95)01346-6. [DOI] [PubMed] [Google Scholar]
- 45.Yerushalmi H, Lebendiker M, Schuldiner S. EmrE, an Escherichia coli 12-kDa multidrug transporter, exchanges toxic cations and H+ and is soluble in organic solvents. J Biol Chem. 1995;270:6856–6863. doi: 10.1074/jbc.270.12.6856. [DOI] [PubMed] [Google Scholar]
- 46.Yerushalmi H, Lebendiker M, Schuldiner S. Negative dominance studies demonstrate the oligomeric structure of EmrE, a multidrug antiporter from Escherichia coli. J Biol Chem. 1996;271:31044–31048. doi: 10.1074/jbc.271.49.31044. [DOI] [PubMed] [Google Scholar]
- 47.Zenk M H. Heavy metal detoxification in higher plants—a review. Gene. 1996;179:21–30. doi: 10.1016/s0378-1119(96)00422-2. [DOI] [PubMed] [Google Scholar]