Abstract
Objective: We evaluated the safety and efficacy of a novel combination of Punica granatum fruit rind and Theobroma cocoa seed extracts (LN18178 or Tesnor®) in enhancing serum testosterone level and reducing aging males' symptoms (AMS) in a randomized, double-blind, placebo-controlled investigation (CTRI Reg. No. CTRI/2019/02/017506).
Methods: One hundred twenty healthy male participants (age 36-55 years) were randomized (n=40) to placebo, 200 or 400 mg of LN18178 for a period of fifty-six consecutive days of supplementation. The primary efficacy parameter was the AMS score. The secondary parameters were serum testosterone (free and total) levels, hand-grip strength, and perceived stress scale (PSS-10) score. Measurement of other hormones included in the study was serum dihydrotestosterone (DHT), cortisol, and 17β-estradiol (E2). Total blood chemistry parameters, vital signs, and urinalysis were parts of the safety assessment.
Results: Both doses of LN18178 significantly reduced the mean AMS scores after 56 days of supplementation. Furthermore, it significantly improved general, psychological, and sexual well-being. Serum levels of free testosterone and total testosterone levels were significantly increased in LN18178 supplemented (200 and 400 mg) participants compared to the baseline and placebo. Significant improvements in hand-grip strength and reduced PSS-10 scores were also observed.
Conclusion: LN18178 supplementation reduced AMS scores and improved sexual performance. Also, LN18178 groups exhibited superior muscular strength and reduction in perceived stress. Total blood chemistry and urine analysis demonstrated the broad-spectrum safety.
Keywords: Aging males' symptoms, LN18178, Muscular strength, Tesnor®, Testosterone, Stress alleviation
Introduction
Advancing age is a physiological progression associated with structural and functional decline in many human body organs and complemented by adaptations in behavior, physical activity, body composition, and reductions in sexual energy and endocrine functions 1. Aging males' symptoms (AMS) include declining physical (somatic), psychological and sexual functions with advancing age in men. During aging, men suffer from a gradual decline in androgen level, which is known as androgen deficiency in the aging male (ADAM) or late-onset hypogonadism (LOH) 2. Testosterone, the principal androgen of the male reproductive system, is essential for the regulation of several physiological functions, including sexual arousal, development of secondary sexual characteristics, bone and muscle mass, muscular strength, production of red blood cells (RBC), metabolic homeostasis, healthy sperm profile, psychological wellness 3, 4. In men, testosterone levels decline with age 5. In the '30s, men start declining serum testosterone levels of around 1 - 1.4% per year as testosterone synthesis and bioavailability of free testosterone (FT) decline with advancing age. Total testosterone (TT) level drops at an average of 1.6% per year, while FT level declines by 2-3% in men after 50 years of age 6. A decline in circulating testosterone level also reduces lean body mass (LBM), muscular strength, and physical performance 4. Low testosterone level decreases aging adults' physical performance, mood status, energy level, and quality of life (QOL). A low level of FT causes erectile dysfunction, loss of muscle mass, reduced sexual desire, abnormal abdominal fat gain, low bone mineral density, joint pain, sleep disturbances, fatigue, depression, and a decline in cognitive functions 7-9. Since ancient times, selected phytonutrients have demonstrated enhanced muscle mass, muscle strength, and sexual function 10. Indian folklore medicine comprehends many plants, including Tribulus terrestris, Mucuna prurines, Withania somnifera, and their extracts for general well-being and to increase male hormone level, improve sexual function, penile erection, and spermatogenesis 10, 11.
LN18178 or Tesnor® is a standardized, proprietary blend of Punica granatum (PG) fruit rind and Theobroma cocoa seed (TC) extracts. Recently, in a fifty-six-day human trial, LN18178 significantly increased serum TT and FT in concurrence with increased muscle strength and size in healthy young males 12. This herbal blend increases steroidogenesis via activating CYP17A1 in mouse Leydig cells in vitro (unpublished observation). Punica granatum L., commonly known as the pomegranate, is a long-living deciduous shrub native of Iran, widely cultivated in the Mediterranean region, Southeast Asia, and tropical Africa 13. The fruit rind has various pharmacological activities such as antioxidant, immune-modulatory, anti-diabetic, anti-plasmodial, anti-microbial, wound healing, anti-hyperglycemic, hepatoprotective, and anti-diarrheal 14, 15. Peels of pomegranate fruit were also used to treat infection of male and female sexual organs, acne, mastitis, piles, allergic dermatitis, and timpanists 15. Theobroma cocoa, also known as Cocoa beans, is a widely used raw material in chocolate confectioneries. Cocoa is a tropical tree that originated from the headwaters of the Amazon River, a tropical American region 16. Polyphenolic antioxidants in Cocoa provide several health benefits, including protection against hypertension, endothelial dysfunction, inflammation, platelet activation 17, 18.
The objective of the present fifty-six-day trial was to assess the efficacy of LN18178 (Tesnor®) supplementation on AMS, muscular strength, serum testosterone levels in aging males. Furthermore, we have evaluated the changes in other hormones in the participants. This study also demonstrates that this botanical blend is tolerable to the study participants.
Materials and methods
Study material
LN18178 (Tesnor®) is a standardized herbal blend combining aqueous ethanol extracts of Punica granatum fruit rind (PG) and Theobroma cocoa seed (CT) at a 4:1 ratio. The mixture was formulated into a free-flowing powder with 25% excipients. The final product was standardized to contain at least 3.5% punicalagins and 0.5% theobromine. LN18178 is a patent-pending proprietary composition developed at Laila Nutraceuticals R&D Centre, Vijayawada. Detailed descriptions of the plant raw materials, extraction procedures, and phytochemical analysis using High Performance Liquid Chromatography (HPLC) are mentioned earlier 12.
Study Design
The present randomized, double-blind, placebo-controlled clinical trial was conducted to assess the influence of LN18178 on aging males' symptoms and circulating male hormones, including testosterone in healthy aging males (Age: 36 to 55 yrs). Three independent sites in Bengaluru, Karnataka, India (Shetty's Hospital, Sri Venkateswara Hospital, and Narayana Hrudayalaya Hospital) conducted this study following the ICH-GCP guidelines. Individual ethics committees of these three study centers reviewed and approved the study protocol. The trial was registered (CTRI/2019/02/017506) in the Clinical Trial Registry of India (CTRI).
Study Participants
One hundred and twenty men [age: 36-55 years; body mass index (BMI): 20-29 kg/m2] participated in this study. The participants had aging males' symptoms (AMS) scores between 27 and 43. They expressed their willingness to walk for 30 minutes per day (five days a week) and not to start any therapies for sexual health, consumption of energy supplements, protein supplements, or health drinks during the study. The participants were recruited through a preliminary screening via telephone. The inclusion and exclusion criteria for recruiting the study participants are summarized in Table 1. All subjects were aware of this study's potential risks/benefits. The participants read and signed the informed consent form (ICF).
Table 1.
Inclusion and Exclusion Criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Male subjects (age 36 - 55 yrs, BMI: 22-29 kg/m2 | History of taking medications for erectile dysfunction, oligospermia urinary and clinical history of endocrine disorders eg: pituitary tumors, hypo- and hyper-thyroidism. |
| Subjects with total AMS total between 27 and 43. | Subjects diagnosed with sleep apnea and having history of psychiatric disorder. |
| Willingness to walk for 30 min/day (5 days/week) | Subjects consuming alcohol or smoking and taken any medications that can interfere with muscle mass such as corticosteroids, testosterone replacement. |
| Healthy subjects with willing partners and good health | Subjects under medications including anti-hypertensives, inhaled beta agonists, anti-hyperlipidemics, psychotropic and consume recreational drugs( cocaine) etc. |
| Ability to understand the risks and benefits of the protocol | Subjects having history of Benign Prostate Hyperplasia (BPH), hypertension, diabetes, stroke, angina, life-threatening arrhythmia within the past 6 months. |
| Subjects should written ICF &available thought out the study | Subjects using anorectic agents and endocannabinoid neuromodulators in last 2 weeks are prohibited. |
| Subjects agree to maintain the activity diary. | Subjects with HIV positive or any other STDs and underwent major surgical procedures in last 6 months. |
| Subjects who agree not to start any therapies for sexual health or consuming energy boosting supplements or protein supplements or health drinks during the course of the study. | Subject has illness as per the opinion of investigator. |
| Subjects willing to refrain from drinking coffee or caffeinated drinks or beverages during the study | Subjects participated in any other clinical studies from last 90 days |
Randomization, blinding, and supplementation
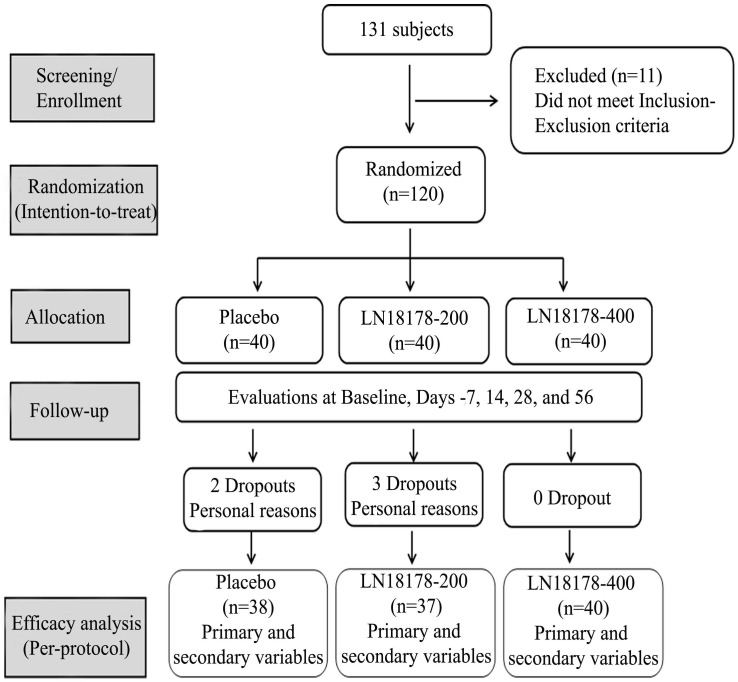
One hundred and twenty eligible subjects were recruited and randomly allocated into three groups (n=40) viz. LN18178-200 mg, LN18178-400 mg or placebo through computer-generated block randomization using PROC-PLAN procedure in SAS (SAS Institute Inc.). Investigational products (IP) were packed in high-density polyethylene (HDPE) bottles identical in size and appearance. IP bottles were marked in a sequence. Once enrolled in the trial, participants were supplied with the following available number in a randomly allocated IP bottle. Investigators and the study team were blinded to the randomization. As advised, each participant consumed one hard gelatin capsule of study product (placebo or LN18178-200 mg or LN18178-400 mg) after breakfast over a period of fifty-six consecutive days. Placebo capsules were identical to LN18178 capsules in color, size, weight, and appearance. The study consisted of screening, randomization, baseline visits, and four follow-up visits on days 7, 14, 28, and 56 (Figure 1). Study site coordinators contacted the participants over the telephone about their adherence to study protocols throughout the study duration. Adverse event monitoring was strictly enforced.
Figure 1.
A CONSORT diagram summarizes the flow of the study, participants' enrollment, follow-up, and analysis.
Primary efficacy measure
Aging males' symptoms score
Change of aging males' symptoms (AMS) score from baseline was the primary efficacy measure of the present study. AMS is a simple and effective measure to characterize a decline in general well-being with low testosterone levels in men. AMS consists of a 17-item self-reported questionnaire distributed into three sub-scales, psychological (7 questions), somatic (5 questions), and sexual (5 questions), with a maximum total score of 85 and a minimum of 17 19. Each item is scored from 1 to 5 depending on the response, with 5 being the most severe. Participants completed the AMS questionnaire at baseline, on days 7, 14, 28, and 56 of supplementation. Twenty milliliters of venous blood samples were collected from the overnight fasted participants between 6.30 and 7.30 AM at each visit. The clotted blood samples were centrifuged (350 × g for 15 min) at 4 °C. The serum samples were stored in aliquots at -20 °C until analyses.
Secondary efficacy measure
Serum hormone analysis
We determined the serum hormones viz. total testosterone (TT), free testosterone (FT), 17β-estradiol (E2), dihydrotestosterone (DHT), and cortisol to evaluate the efficacy of LN18178 at baseline, day 7, 14, 25, and 56 of supplementation. The serum levels of creatinine (Cr), blood urea nitrogen (BUN), aspartate transaminase (AST), alanine transaminase (ALT), and alkaline phosphatase (ALP) were measured at baseline and end of the study. Hormone analysis was performed using commercially available specific and sensitive EIA kits. Ortho Clinical Diagnostics (Raritan, NJ, USA) supplied TT, cortisol, and E2 assay kits. The FT and DHT kits were procured from Diagnostics Biochem Canada Inc. (London, ON, Canada) and BioVendor - Laboratorní medicína a.s. (Karásek, Czechia), respectively. All assays were performed following the manufacturer's protocols. The hormone concentrations were calculated using the standard curves from each assay. The analytical sensitivities of FT, TT, DHT, Cortisol, and E2 assay kits were 0.062 pmol/L, 0.17 nmol/L, 0.080 nmol/L, 2.83 nmol/L, and 23.347 pmol/L, respectively.
Hand-grip Strength
Hand-grip strength of the participants was determined using a digital dynamometer (INCO Instruments & Medical Devices Pvt. Limited, Ambala, India) as an indicator of muscle strength at baseline and follow-up visits. Participants were asked to extend their forearm of the dominant hand on a table and allowed to bend at 90° with the elbow. The dynamometer was gently placed on the hand of the participant and asked to squeeze as hard as possible without any jerking motion. Three measurements were taken at 2 min intervals, and the best performance was recorded.
Perceived Stress Scale
The perceived stress scale (PSS) is a validated measure of the perception of stress, comprising of a 10-item questionnaire. Each item is scored from 0 to 4, and the total PSS score ranges from 0 to 50 20. The participants completed the questionnaire at baseline and all follow-up visits.
Safety parameters
As a part of the safety assessment, total blood chemistry, including a series of hematological, serum biochemical measurements, and urinalysis, were performed at screening and the end of the study. VITROS® 5600 integrated system (Dry Chemistry analyzer, Ortho Clinical Diagnostics, New Jersey, USA) was used for blood chemistry and urine analysis. The clinical chemistry parameters were fasting glucose, serum creatinine, uric acid, blood urea nitrogen (BUN), serum bilirubin and alanine transaminase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), sodium, potassium, serum albumin. The hematology parameters were hemoglobin, platelet count, total leukocyte count, RBC, ESR, differential count. Urinalysis included color, specific gravity, pH, glucose, protein, and RBC. Microscopic examinations were performed under a clinical light microscope (Olympus Opto Systems India Pvt. Ltd. New Delhi, India). Participants' vital signs, including blood pressure (systolic/diastolic), pulse rate, respiratory rate, and oral temperature, were recorded at all study visits.
Sample size calculation and power analysis
Approximately 36 subjects per treatment group were assumed to provide 90% power to detect a treatment effect in the primary efficacy variable at a two-sided significance level of 0.05%. A mean effect size of 1.20 and a deviation of 1.10 were considered in the sample size calculation based on an earlier study on an herbal supplement 21. With an assumption of 10% dropout, 120 subjects (40 subjects per arm) were recruited in this study.
Statistical analysis
The data are expressed as mean ± SD. The efficacy parameters were tested for significance using Student's t-test for 'within-the-group' and ANCOVA for 'between-the-group' comparisons. A one-way ANOVA was used for evaluating baseline characteristics, vital signs, and laboratory parameters. A p-value of <0.05 was considered statistically significant. Statistical analyses were performed using the SAS 9.4 software (SAS Institute, Inc, Cary, NC).
Results
The present study enrolled one hundred and twenty healthy men (age: 36-55 y) with aging males' symptoms (AMS) scores between 27 and 43. Participants consumed one capsule of either placebo or LN18178 200 mg or LN18178 400 mg after breakfast for fifty-six consecutive days. The mean age of participants was 45.90 ± 6.283, 43.66 ± 5.809, and 45.20 ± 5.459 years in the placebo, LN18178 200 mg, and LN18178 400 mg groups, respectively. Baseline demographics were comparable among the study groups and are presented in Table 2. Five subjects discontinued the study because of their personal reasons. A total of 115 subjects completed the study, and the data was analyzed in the per-protocol (PP) population.
Table 2.
Baseline characteristics of the participants of the study
| Variables | Placebo (n=40) | LN18178-200 (n=40); P-value | LN18178-400 (n=40); P-value |
|---|---|---|---|
| Age (y) | 45.90 ± 6.28 | 43.66 ± 5.81; 0.2893 | 45.20 ± 5.46; 0.8615 |
| Body weight (kg) | 73.99 ± 8.25 | 75.26 ± 8.73; 0.7809 | 73.37 ± 8.00; 0.9412 |
| BMI (kg/m2) | 26.11 ± 1.98 | 25.96 ± 1.85; 0.9508 | 25.56 ± 2.28; 0.4664 |
| AMS score | 36.45 ± 4.02 | 35.58 ± 4.54; 0.6406 | 35.13 ± 4.24; 0.3501 |
| Grip strength (Kg) | 35.85 ± 11.25 | 35.56 ± 12.95; 0.9942 | 35.11 ± 13.16; 0.9625 |
| PSS-10 score | 19.90 ± 5.15 | 20.39 ± 5.25; 0.9092 | 19.60 ± 5.36; 0.9647 |
| Free Testosterone (pmol/L) | 42.68 ± 10.62 | 41.64 ± 13.39; 0.9949 | 40.95 ± 11.56; 0.9433 |
| Total Testosterone (nmol/L) | 12.62 ± 4.84 | 12.48 ± 5.47; 0.9997 | 13.69 ± 5.13; 0.4930 |
| Dihydrotestosterone (nmol/L) | 2.22 ± 0.77 | 2.25 ± 0.90; 0.7981 | 2.20 ± 0.93; 0.9940 |
| Cortisol (nmol/L) | 207.55 ± 79.10 | 198.44 ± 52.69; 0.8082 | 221.90 ± 98.26; 0.7033 |
| Estradiol (pmol/L) | 90.86 ± 35.29 | 95.05 ± 30.45; 0.8557 | 91.46 ± 35.90; 0.9966 |
Data present as mean ± SD; Intent-to-treat (ITT) population. No significant differences between the placebo and the LN18178 groups;
Data were analyzed using ANOVA.
Table 3 presents the gradual improvements in the total AMS scores and the scores of AMS sub-scales from baseline to follow-up visits. An early and significant reduction in total AMS scores was observed in LN18178 400 mg group starting from day 7 (P < 0.05) from baseline and placebo, through the end of the trial (p<0.05 vs. baseline and placebo). In the LN18178-200 mg group, improvements in total AMS scores were significant from day 14 through the end of the trial. After 56 days of supplementation, the total AMS scores were significantly (P < 0.05 vs. baseline and placebo) decreased by 15.51% and 19.30% in LN18178 200 and 400 mg groups, respectively. In placebo, the total AMS score was reduced by only 4.55% at the end of supplementation (Table 3).
Table 3.
Effect of LN18178 supplementation on AMS scores of the participants through the course of the trial
| Variable | Evaluation days | Placebo | LN18178-200 | LN18178-400 |
|---|---|---|---|---|
| AMS score | Baseline | 36.26 ± 4.038 | 35.73 ± 4.507 | 35.13 ± 4.238 |
| Day 7 | 36.11 ± 4.279 | 35.41 ± 4.456 (0.0698*; 0.8835#) | 33.75 ± 4.612 (0.0006*; 0.0037#) | |
| Day 14 | 35.75 ± 4.666 | 34.30 ± 4.795 (0.0005*; 0.2427#) | 31.85± 4.666 (<0.0001*; <0.0001#) | |
| Day 28 | 35.18 ± 5.082 | 32.38 ± 4.399 (<0.0001*; 0.0041#) | 30.33 ± 4.911 (<0.0001*; <0.0001#) | |
| Day 56 | 34.61 ± 5.335 | 30.19 ± 4.162 (<0.0001*; <0.0001#) | 28.35 ± 4.828 (<0.0001*; <0.0001#) | |
| Psychological behavior | Baseline | 10.45 ± 1.969 | 10.05 ± 2.134 | 9.3 ± 1.977 |
| Day 7 | 10.45 ± 1.927 | 10.08 ± 2.152 (0.5708*; 0.9981#) | 9.0 ± 2.013 (0.0087*; 0.0468#) | |
| Day 14 | 10.53 ± 2.076 | 9.92 ± 2.126 (0.4636*; 0.515#) | 8.7± 1.937 (0.0034*; 0.0025#) | |
| Day 28 | 10.29 ± 2.229 | 9.59 ± 2.088 (0.0419*; 0.4065#) | 8.48 ± 1.811 (0.0009*; 0.0085#) | |
| Day 56 | 10.26 ± 2.036 | 8.95 ±1.84 (<0.0001*; 0.0009#) | 8.23 ± 1.544 (0.0001*; <0.0001#) | |
| Somatic behavior | Baseline | 17.03 ± 3.209 | 17.3 ± 3.915 | 17.3 ± 3.466 |
| Day 7 | 16.95 ± 3.337 | 16.97 ± 3.685 (0.0319*; 0.6651#) | 16.38 ± 3.372 (0.0003*; 0.0047#) | |
| Day 14 | 16.97 ± 3.69 | 16.11 ± 3.68 (<0.0001*; 0.1106#) | 14.95 ± 3.45 (<0.0001*;0.0001#) | |
| Day 28 | 16.34 ± 3.707 | 14.95 ± 3.448 (<0.0001*; 0.0016#) | 14.23 ± 3.206 (<0.0001*; <0.0001#) | |
| Day 56 | 15.79 ± 3.974 | 13.92 ±2.947 (<0.0001*; 0.0013#) | 12.95 ± 3.17 (<0.0001*; <0.0001#) | |
| Sexual Behavior | Baseline | 8.79 ± 2.591 | 8.38 ± 2.419 | 8.53 ± 2.407 |
| Day 7 | 8.71 ± 2.567 | 8.35 ± 2.418 (0.324*; 0.6651#) | 8.38 ± 2.549 (0.2047*;0.7958#) | |
| Day 14 | 8.58 ± 2.489 | 8.27 ± 2.502 (0.4573*; 0.9517#) | 7.9 ± 2.34 (0.0027*; 0.1082#) | |
| Day 28 | 8.55 ± 2.501 | 7.84± 2.18 (0.0033*; 0.3303#) | 7.63 ± 2.295 (0.0003*; 0.0190#) | |
| Day 56 | 8.55 ± 2.49 | 7.32 ± 2.015 (0.0001*; 0.0088#) | 7.18 ± 2.308 (<0.0001*; 0.0004#) |
Data present as mean ± SD. Placebo (n=38), LN18178-200 (n=37), and LN18178-400 (n=40). In parentheses, * and # indicate P-values in intragroup comparison (vs. baseline) using student t-test and in intergroup comparison (vs. placebo) analyzed using ANCOVA, respectively; NS, not significant.
The AMS subscale score analyses reveal that 56 days of supplementation of low and high dose LN18178 resulted in significant reductions in psychological behavior (10.95% and 11.51%), somatic behavior (19.54% and 25.14%), and sexual behavior (12.65% and 15.83%) from baseline. In contrast, the changes in these subscale scores in the placebo group were minimal and not significant compared to baseline (Table 3).
Table 4 shows gradual improvements in the hand-grip strength of the LN18178-supplemented participants during the study. The low and high-dose herbal supplemented groups showed 18.95% and 24.64% increases in hand-grip strength from baseline. An early and significant increase (P < 0.05) in the muscle strength in both LN18178 groups started from day 7 of supplementation.
Table 4.
Effect of LN18178 supplementation on hand-grip strength and PSS-10 score of the participants through the course of the trial
| Variable | Evaluation days | Placebo | LN18178-200 | LN18178-400 |
|---|---|---|---|---|
| Hand-Grip Strength (Kg) | Baseline | 35.31 ± 11.28 | 35.52 ± 13.13 | 35.11 ± 13.16 |
| Day 7 | 36.59 ± 13.01 | 37.45 ± 12.24 (0.0349*; 0.8292#) | 38.12 ±14.82 (0.0001*; 0.2627#) | |
| Day 14 | 37.12 ± 11.73 | 39.26 ±12.58 (0.0003*; 0.4544#) | 40.96 ±16.14 (0.0003*; 0.0341#) | |
| Day 28 | 37.00 ± 13.62 | 39.94 ± 11.43 (0.0001*; 0.2829#) | 42.75 ± 16.18 (<0.0001*; 0.0031#) | |
| Day 56 | 36.65 ± 11.19 | 42.25 ± 12.37 (<0.0001*; 0.0056#) | 43.76 ± 15.34 (<0.0001*; <0.0001#) | |
| Perceived Stress Scale (PSS-10) score | Baseline | 19.63 ± 5.096 | 20.14 ± 5.067 | 19.60 ± 5.363 |
| Day 7 | 19.45 ± 5.060 | 19.92 ± 5.325 (0.0882*; 0.9854#) | 19.38 ± 5.348 (0.1068*; 0.9752#) | |
| Day 14 | 18.97 ± 4.594 | 18.68± 4.410 (0.0002*; 0.1851#) | 17.85 ± 4.742 (0.0001*; 0.0172#) | |
| Day 28 | 18.89 ± 4.637 | 17.38 ± 4.412 (0.0001*; 0.0031#) | 16.25 ±4.749 (0.0001*; <0.0001#) | |
| Day 56 | 18.92 ± 5.18 | 16.14 ± 4.263 (<0.0001*; 0.0003#) | 14.50 ± 5.149 (<0.0001*; <0.0001#) |
Data present as mean ± SD. Placebo (n=38), LN18178-200 (n=37), and LN18178-400 (n=40). In parentheses, * and # indicate P-values in intragroup comparison (vs. baseline) using student t-test and in intergroup comparison (vs. placebo) analyzed using ANCOVA, respectively; NS, not significant.
From baseline, the mean PSS scores in LN18178 200- and 400 mg groups started significant reductions from day 14 through the end of the study. Following 56 days of supplementation, the mean decreases (P < 0.05) in PSS scores were 15% and 23% in 200- and 400 mg groups, respectively, compared to placebo (Table 4).
Efficacy of LN18178 on serum hormones
Increases in free testosterone (FT) and total testosterone (TT) during 56 consecutive days of LN18178 supplementation are presented in table 5. LN18178 supplementation significantly increased the FT level by 39.16% and 48.28%; the TT level by 21.39% and 24.56% in 200- and 400 mg groups, respectively, from baseline. The LN18178 400 mg group showed early and significant increases in FT and TT levels from baseline following seven days of supplementation. Compared to placebo, increases in FT and TT levels were substantial in 200- and 400 mg LN18178 groups.
Table 5.
Effect of LN18178 supplementation on serum hormones of the participants
| Variable | Evaluation days | Placebo | LN18178-200 | LN18178-400 |
|---|---|---|---|---|
| Free Testosterone (pmol/L) | Baseline | 42.68±10.62 | 41.64±13.39 | 40.95±11.56 |
| Day 7 | 41.99 ± 9.44 | 44.76 ± 12.39 (0.121*; 0.2594#) | 45.46 ± 13.29 (0.0193*; 0.1151#) | |
| Day 14 | 41.64 ±12.21 | 48.23 ±16.34 (0.0115*; 0.0452#) | 51.01 ± 14.75 (0.0001*; 0.0015#) | |
| Day 28 | 42.23 ± 9.79 | 53.09 ± 16.41 (<0.0001*; 0.0006#) | 54.48 ± 15.34 (<0.0001*; <0.0001#) | |
| Day 56 | 43.03 ± 11.31 | 57.95 ± 18.84 (<0.0001*; <.0001#) | 60.72 ±17.73 (<0.0001*; <.0001#) | |
| Total Testosterone (nmol/L) | Baseline | 12.62 ±4.84 | 12.48 ±5.47 | 13.69 ±5.13 |
| Day 7 | 11.99 ± 4.50 | 12.86 ±5.28 (0.4464*; 0.2859#) | 14.95 ±6.15 (0.0512*; 0.0287#) | |
| Day 14 | 11.93 ± 4.91 | 13.10 ± 5.01 (0.1564*; 0.066#) | 14.56 ± 4.91 (0.0071*; 0.0077#) | |
| Day 28 | 12.41 ± 5.94 | 13.97 ± 4.75 (0.0093*; 0.0642#) | 15.49 ± 4.84 (0.0002*; 0.0071#) | |
| Day 56 | 12.96 ± 5.32 | 15.15 ± 6.01 (<0.0001*; 0.0164#) | 17.05 ± 4.98 (<0.0001*; 0.0003#) | |
| Dihydrotestosterone (nmol/L) | Baseline | 2.22 ± 0.77 | 2.25 ± 0.90 | 2.20 ± 0.93 |
| Day 7 | 2.17 ± 0.83 | 2.24 ± 0.97 (0.921*; 0.9257#) | 2.12 ± 0.85 (0.2537*; 0.9199#) | |
| Day 14 | 2.18 ± 0.85 | 2.27 ± 0.94 (0.8117*; 0.8478#) | 2.10 ± 0.85 (0.2487*; 0.819#) | |
| Day 28 | 2.23 ± 0.81 | 2.32 ± 0.94 (0.4704*; 0.8448#) | 2.17 ± 0.88 (0.7256*; 0.9219#) | |
| Day 56 | 2.22 ± 0.68 | 2.32 ± 0.73 (0.3151*; 0.6744#) | 2.37 ± 0.75 (0.1021*; 0.2113#) | |
| Cortisol (nmol/L) | Baseline | 207.55 ± 79.10 | 198.44 ± 52.69 | 221.90 ± 98.25 |
| Day 7 | 222.73 ± 69.52 | 200.65± 60.31 (0.8413*; 0.4793#) | 222.45 ± 91.19 (0.9763*; 0.9512#) | |
| Day 14 | 203.96 ± 66.54 | 200.10 ± 71.32 (0.893*; 0.9998#) | 216.10 ± 99.86 (0.6984*; 0.9443#) | |
| Day 28 | 214.45 ± 76.81 | 212.79 ± 67.73 (0.2384*; 0.9921#) | 211.96 ± 93.37 (0.5651*; 0.8728#) | |
| Day 56 | 232.94 ± 81.72 | 228.25 ± 70.13 (0.0359*; 0.9944#) | 236.80 ± 95.41 (0.4001*; 0.9976#) | |
| Estradiol (pmol/L) | Baseline | 90.86 ± 35.29 | 95.05 ± 30.45 | 91.46 ± 35.90 |
| Day 56 | 92.30 ± 27.83 | 96.99 ± 25.35 (0.6541*; 0.8802#) | 93.25 ± 34.79 (0.7694*; 0.9923#) | |
| Testosterone/Estradiol | Baseline | 154.90±66.70 | 132.27±55.53 | 157.65±71.64 |
| Day 56 | 148.52±62.66 | 161.16±78.53 (0.0718*; 0.4442#) | 194.76±78.61 (0.0302*; 0.0012#) |
Data present as mean ± SD. Placebo (n=38), LN18178-200 (n=37), and LN18178-400 (n=40). In parentheses, * and # indicate P-values in intragroup comparison (vs. baseline) using student t-test and in intergroup comparison (vs. placebo) analyzed using ANCOVA, respectively.
LN18178 supplementation did not significantly change the E2 levels in the participants (Table 5). However, post-trial, the high-dose LN18178 group showed 23.54% (P < 0.05; vs. baseline) and 31.13% (P < 0.05; vs. placebo) increases of the TT and E2 (TT/E2) ratio (Table 5).
At the end of the study, the LN18178 supplemented groups did not significantly alter their serum cortisol and Dihydrotestosterone (DHT) levels compared to baseline and placebo (Table 5).
Safety parameters
The routine hematology, clinical chemistry parameters, and the participants' vital signs were unaltered during the trial and remained within the normal laboratory ranges (data not shown). Also, the metabolic markers, Cr, AST, ALT, ALP, and BUN, were within the normal ranges during the trial (Table 6). Overall, the participants reported only five minor adverse events: fever, cough & cold, constipation, and diarrhea during the study (Table 7).
Table 6.
Effect of LN18178 supplementation on serum metabolic markers of the study participants
| Variable | Evaluation days | Placebo | LN18178-200 | LN18178-400 |
|---|---|---|---|---|
| Creatinine (μmol/L) | Baseline | 77.79 ± 10.17 | 82.21 ± 12.02 | 77.79 ± 13.26 |
| Day 56 | 76.02 ± 12.55 | 83.98 ± 14.14 (0.110*; 0.099#) | 78.67 ± 14.76 (0.5808*; 0.4686#) | |
| BUN (mg/dL) | Baseline | 9.91 ± 2.96 | 9.69 ± 2.68 | 9.38 ± 2.44 |
| Day 56 | 9.86 ± 2.93 | 9.09 ± 1.98 (0.181*; 0.3804#) | 10.54 ± 2.48 (0.006*; 0.136#) | |
| AST (U/L) | Baseline | 24.13 ± 8.30 | 21.97 ± 5.75 | 22.48 ± 6.40 |
| Day 56 | 25.16 ± 10.26 | 23.20 ± 5.85 (0.3661*; 0.3156#) | 23.64 ± 6.23 (0.4977*; 0.4287#) | |
| ALT (U/L) | Baseline | 27.53 ± 13.20 | 23.38 ± 12.30 | 23.65± 8.89 |
| Day 56 | 29.70 ± 15.77 | 24.05 ± 12.35 (0.8164*; 0.0888#) | 25.54 ± 13.30 (0.4507*; 0.2108#) | |
| ALP (U/L) | Baseline | 86.89±17.90 | 84.70 ± 20.87 | 86.23 ± 19.88 |
| Day 56 | 85.21 ± 20.23 | 83.84 ± 20.81 (0.8588*; 0.7729#) | 84.65 ± 19.79 (0.6508; 0.9019#) |
Data present as mean ± SD. Placebo (n=38), LN18178-200 (n=37), and LN18178-400 (n=40). ALP, alkaline phosphatase, ALT, alanine Transaminase; AST, aspartate aminotransferase, BUN, blood urea nitrogen. In parentheses, * and # indicate P-values in intragroup comparison (vs. baseline) using student t-test and in intergroup comparison (vs. placebo) analyzed using ANCOVA, respectively. The changes are not significant.
Table 7.
Summary of adverse events reported by the participants during the trial
| Adverse events | Placebo (n=38) | LN18178-200 (n=37) | LN18178-400 (n=40) |
|---|---|---|---|
| Fever | 1 | 1 | 0 |
| Common cold and cough | 0 | 1 | 0 |
| Constipation | 0 | 0 | 1 |
| Diarrhoea | 1 | 0 | 0 |
| Total number of adverse events | 2 | 2 | 1 |
Discussion
The present human study demonstrates the efficacy and safety of LN18178, a proprietary blend of Punica granatum (PG) rind and Theobroma cocoa (TC) seed extracts in healthy men (age: 36-55 yrs.) with mild to moderate aging 'males' symptoms (AMS) scores. Recent human clinical investigations revealed the potential benefits of botanical and nutraceutical supplementation in reducing the severity of AMS scores and boosting testosterone levels in aging males 21-24. This is the first report demonstrating the benefits of a standardized, herbal blend of PG rind and TC seed extracts (LN18178) in improving AMS, TT, FT, and muscular strength in aging men. PG is traditionally known as a symbol of fertility 25. Also, the juice of the fruit and its pericarp are employed to relieve colic, colitis, menorrhagia, headache, and other conditions in traditional medicine, demonstrating a broad spectrum safety of PG 14, 26. PG rind powder and extracts have extensive application in the dairy industry 27. Cocoa beans are widely used in confectionaries and are rich in potent antioxidants 28. Earlier, a ninety-day repeated dose oral toxicity study in rats and genetic toxicological studies have established a broad-spectrum safety of LN18178. This sub-chronic toxicity study estimated the no-observed-adverse-effect level (NOAEL) of LN18178 in male and female rats was 2500 mg/kg body weight (BW), which is greater than 26 g/day in human of an average BW of sixty-five kg 29. This estimated human equivalent dose is at least 65-fold greater than the higher dose in the present trial. Increases in TT and FT levels and muscle strength in the aging subjects of the present study corroborate with the earlier observation that LN18178 supplementation increased serum testosterone levels associated with increased muscle strength and size in young males 12.
Testosterone replacement therapy is the primary treatment option for correcting the androgen deficiency in males 30, 31. However, because of possible increased risk of heart attacks and strokes, US FDA recommends a restriction of testosterone therapy for certain medical conditions or the subjects with testicular dysfunction or disorder in gonadal-hypophyseal axis, rather than age related androgen deficiency 32. Interestingly, in the recent past, a series of botanical products have been shown to increase testosterone levels in young or aging/older males 33. Rao et al demonstrated that Trigonella foenum-graecum seed extract increased testosterone levels and age related symptoms including sexual function in healthy aging males 22. Further, supplementation of different preparations of Withania somnifera extracts increased testosterone level and demonstrated beneficial effects in healthy and infertile men in independent clinical trials 33.
In the present study, fifty-six days of supplementation of LN18178 significantly improved the total AMS scores compared to baseline and placebo. AMS scale is a validated tool in assessing health-related quality of life (HRQoL) and consequences of advancing age in men 34. Early and significant improvements in AMS scores were observed in LN18178 (400 mg group) from day seven to the end of the study. Significant improvements in psychological well-being, somatic behavior, and sexual behavior in the LN18178-supplemented participants are important observations of the present study.
Another interesting observation of this trial was significant improvements in serum testosterone levels in both intervention groups following fifty-six consecutive days of supplementation. Both serum TT and FT levels gradually started increasing significantly from day 14 till the end of the study, which highlights the potential benefits of LN18178 in improving somatic and sexual behavior. An increase in serum testosterone level supports the mechanistic basis for increased testicular steroidogenesis 35, 36. Testosterone plays an essential role in regulating metabolic homeostasis, maintaining muscle and bone integrity, and fat metabolism 37, 38. Increased testosterone level prevents age-related metabolic dysregulation, muscle and bone mass loss, and fat accumulation 39. Also, increased testosterone level improves libido in men 38. An interesting observation from the present study is a significantly increased TT/E2 ratio in the LN18178 supplemented subjects. It is worth noting that a positive correlation exists between the TT/E2 ratio and increased sexual desire, semen quality, and libido in men 40. Therefore, the improved score in the sexual behavior domain of the AMS questionnaire in association with increased testosterone level in the herbal supplemented subjects is noteworthy.
Hand-grip strength is a widely recognized tool to assess muscular isometric strength 41. Significant improvement in hand-grip strength of the participants on either LN18178 200 or 400 mg supplementation highlights the positive correlation between muscular strength and serum testosterone levels 42. Moreover, LN18178 supplemented participants showed a significant stress reduction. This study's observations align with the earlier findings that suggest an improvement in testosterone level attenuates stress and enhances sexual desire in men 43, 44.
The botanical formulation LN18178 is well tolerated by the study participants. The complete blood chemistry analysis, including serum creatinine, BUN, AST, ALT, and ALP levels in LN18178 supplemented subjects are within the physiological range, which further substantiates the broad-spectrum safety of LN18178.
Conclusion
LN18178 (Tesnor®), a novel botanical proprietary blend of Punica granatum fruit rind, and Theobroma cocoa seed extracts, is well-tolerated and safe for human consumption. This study suggests that LN18178 attenuates the severity of AMS, boosts serum testosterone levels, and improves the psychological, sexual, and general well-being of healthy aging men. LN18178 also increases muscular strength and reduces stress. LN18178 is a well-tolerated, safe, and effective nutraceutical blend that boosts sexual function, testosterone level, and psychological and general well-being in aging males.
Acknowledgments
The authors thank Laila Group of Industries, Vijayawada, India, for financial support to conduct the study. The authors also thank the clinical study participants and the site monitors for their generous contribution.
Funding
The present research was funded (grant no. LN0143121) by Laila Nutraceuticals, Vijayawada, Andhra Pradesh, India.
Data availability statement
The data supporting the present study results are available from the corresponding author upon request.
Abbreviations
- ALP
Alkaline phosphatase
- ALT
Alanine aminotransferase/Glutamic
- AMS
Aging males' symptoms
- AST
Aspartate aminotransferase
- BMI
Body mass index
- BUN
Blood urea nitrogen
- Cr
Creatinine
- DHT
Dihydrotestosterone
- E2
17β- Estradiol
- FBS
Fasting blood sugar
- HRQoL
Health-related quality of life
- ICF
informed consent form
- IP
investigational product
- MUAC
Mid-upper arm circumferences
- PG
Punica granatum
- TT
total testosterone
- FT
Free testosterone
- TC
Theobroma cocoa
References
- 1.Nishimatsu H, Kitamura T, Yamada D. et al. Improvement of symptoms of aging in males by a preparation LEOPIN ROYAL containing aged garlic extract and other five of natural medicines - comparison with traditional herbal medicines (Kampo) Aging Male. 2014;17(2):112–116. doi: 10.3109/13685538.2013.771328. doi: 10.3109/13685538.2013.771328. [DOI] [PubMed] [Google Scholar]
- 2.Traustadóttir T, Harman SM, Tsitouras P, Pencina KM. et al. Long-term testosterone supplementation in older men attenuates age-related decline in aerobic capacity. J Clin Endocrinol Metab. 2018;103(8):2861–2869. doi: 10.1210/jc.2017-01902. doi: 10.1210/jc.2017-01902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen W, Liu ZY, Wang LH. et al. Are the aging male's symptoms (AMS) scale and the androgen deficiency in the aging male (ADAM) questionnaire suitable for the screening of late-onset hypogonadism in aging Chinese men? Aging Male. 2013;16(3):92–96. doi: 10.3109/13685538.2013.805319. doi: 10.3109/13685538.2013.805319. [DOI] [PubMed] [Google Scholar]
- 4.Saad F, Röhrig G, von Haehling S, Traish A. Testosterone deficiency and testosterone treatment in older men. Gerontology. 2017;63(2):144–156. doi: 10.1159/000452499. doi: 10.1159/000452499. [DOI] [PubMed] [Google Scholar]
- 5.Payton S. Unique insight into the physiological functions of testosterone. Nat Rev Urol. 2013;10:616.. doi: 10.1038/nrurol.2013.223. doi: 10.1038/nrurol.2013.223. [DOI] [PubMed] [Google Scholar]
- 6.Stanworth RD, Jones TH. Testosterone for the aging male; current evidence and recommended practice. Clin Interv Aging. 2008;3(1):25–44. doi: 10.2147/cia.s190. doi: 10.2147/cia.s190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeap BB, Almeida OP, Hyde Z, Chubb SA, Hankey GJ, Jamrozik K, Flicker L. Higher serum free testosterone is associated with better cognitive function in older men, while total testosterone is not. The Health In Men Study. Clin Endocrinol. 2008;68(3):404–412. doi: 10.1111/j.1365-2265.2007.03055.x. doi: 10.1111/j.1365-2265.2007.03055.x. [DOI] [PubMed] [Google Scholar]
- 8.Ahn HS, Park CM, Lee SW. The clinical relevance of sex hormone levels and sexual activity in the ageing male. BJU International. 2002;89(6):526–530. doi: 10.1046/j.1464-410x.2002.02650.x. doi: 10.1046/j.1464-410x.2002.02650.x. [DOI] [PubMed] [Google Scholar]
- 9.McBride JA, Carson CC 3rd, Coward RM. Testosterone deficiency in the aging male. Therapeutic Adv Urol. 2016;8(1):47–60. doi: 10.1177/1756287215612961. doi: 10.1177/1756287215612961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chauhan NS, Sharma V, Dixit VK, Thakur M. A review on plants used for improvement of sexual performance and virility. BioMed Res. Int. 2014;2014:868062.. doi: 10.1155/2014/868062. doi: 10.1155/2014/868062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kazim Sahin, Cemal Orhan, Fatih Akdemir. et al. Comparative evaluation of the sexual functions and NF-κB and Nrf2 pathways of some aphrodisiac herbal extracts in male rats. BMC Complement Altern Med. 2016;16(1):318.. doi: 10.1186/s12906-016-1303-x. doi:10.1186/s12906-016-1303-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sreeramaneni PGA, Yalamanchi A, Konda MR, A proprietary herbal blend containing extracts of Punica granatum fruit rind and Theobroma cocoa seeds increases serum testosterone level in healthy young males: A randomized, double-blind placebo-controlled study. J Diet Suppl. 2022; 1-17. doi: 10.1080/19390211. 2022. 2035037. [DOI] [PubMed]
- 13.Shaygannia E, Bahmani M, Zamanzad B, Rafieian-Kopaei M. A review study on Punica granatum L. J Evid-Based Integr Med. 2016;21(3):221–227. doi: 10.1177/2156587215598039. doi: 10.1177/2156587215598039. [DOI] [PubMed] [Google Scholar]
- 14.Ricci D, Giamperi L, Bucchini A, Fraternale D. Antioxidant activity of Punica granatum fruits. Fitoterapia. 2006;77(4):310–312. doi: 10.1016/j.fitote.2006.01.008. doi: 10.1016/j.fitote.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Rahimi HR, Arastoo M, Ostad SN. A comprehensive review of Punica granatum (Pomegranate) properties in toxicological, pharmacological, cellular and molecular biology researches. Iran J Pharm Sci. 2012;11(2):385–400. doi: 10.22037/IJPR.2012.1148. [PMC free article] [PubMed] [Google Scholar]
- 16.Pahlevi AD, Lunian FM, Diógenes HA, Cacao-Theobroma cacao, In: Editor(s): Sueli Rodrigues, Ebenezer Silva, Edy de Brito. Exotic fruits: Reference guide, USA: Academic Press. 2018. p. 69-76. https://doi.org/10.1016/B978-0-12-803138-4.00010-1.
- 17.Desch S, Schmidt J, Kobler D, Sonnabend M. et al. Effect of cocoa products on blood pressure: systematic review and meta-analysis. Am J Hypertens. 2010;23(1):97–103. doi: 10.1038/ajh.2009.213. doi: 10.1038/ajh.2009.213. [DOI] [PubMed] [Google Scholar]
- 18.West SG, McIntyre MD, Piotrowski MJ, Poupin N. et al. Effects of dark chocolate and cocoa consumption on endothelial function and arterial stiffness in overweight adults. Br J Nutr. 2014;111(4):653–661. doi: 10.1017/S0007114513002912. doi: 10.1017/S0007114513002912. [DOI] [PubMed] [Google Scholar]
- 19.Heinemann L. A. J, Zimmermann T, Vermeulen A, et al. A new 'aging males' symptoms' rating scale. Aging Male. 1999;2(2):105–114. doi:10.3109/13685539909003173. [Google Scholar]
- 20.Baik SH, Fox RS, Mills SD, Roesch SC, Sadler GR, Klonoff EA, Malcarne VL. Reliability and validity of the Perceived Stress Scale-10 in Hispanic Americans with English or Spanish language preference. J Health Psychol. 2019;24(5):628–639. doi: 10.1177/1359105316684938. doi: 10.1177/1359105316684938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park HJ, Lee KS, Lee EK, Park NC. Efficacy and safety of a mixed extract of Trigonella foenum-graecum seed and lespedeza cuneata in the treatment of testosterone deficiency syndrome: A randomized, double-blind, placebo-controlled clinical trial. World J Mens Health. 2018;36(3):230–238. doi: 10.5534/wjmh.170004. doi: 10.5534/wjmh.170004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rao A, Steels E, Inder WJ, Abraham S, Vitetta L. Testofen, a specialised Trigonella foenum-graecum seed extract reduces age-related symptoms of androgen decrease, increases testosterone levels and improves sexual function in healthy aging males in a double-blind randomised clinical study. Aging Male. 2016;19(2):134–42. doi: 10.3109/13685538.2015.1135323. doi: 10.3109/13685538.2015.1135323. [DOI] [PubMed] [Google Scholar]
- 23.Choi SW, Jeon SH, Kwon EB. et al. Effect of Korean Herbal Formula (Modified Ojayeonjonghwan) on Androgen Receptor Expression in an Aging Rat Model of Late Onset Hypogonadism. World J. Men's Health. 2019;37(1):105–112. doi: 10.5534/wjmh.180051. doi: 10.5534/wjmh.180051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopresti AL, Drummond PD, Smith SJ. A randomized, double-blind, placebo-controlled, crossover study examining the hormonal and vitality effects of ashwagandha (Withania somnifera) in aging, overweight males. Am J Mens Health. 2019;13(2):155798831983598.. doi: 10.1177/1557988319835985. doi: 10.1177/1557988319835985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langley P. Why a pomegranate? Br Med J. 2000;321(7269):1153–1154. doi: 10.1136/bmj.321.7269.1153. doi: 10.1136/bmj.321.7269.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sreekumar S, Sithul H, Muraleedharan P, Azeez JM, Sreeharshan S. Pomegranate fruit as a rich source of biologically active compounds. BioMed Res Int. 2014;2014:686921.. doi: 10.1155/2014/686921. doi: 10.1155/2014/686921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahajan D, Bhat ZF, Kumar S. Pomegranate (Punica granatum) rind extract as a novel preservative in cheese. Food Biosci. 2015;12:47–53. doi:10.1016/j.fbio.2015.07.005. [Google Scholar]
- 28.Rusconi M, Conti A. Theobroma cacao L. the Food of the Gods: a scientific approach beyond myths and claims. Pharmacol Res. 2010;61(1):5–13. doi: 10.1016/j.phrs.2009.08.008. doi: 10.1016/j.phrs.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Madireddy RK, Alluri KV, Somepalli V, Golakoti T, Sengupta K. Toxicological Assessments of a Proprietary Blend of Punica granatum Fruit Rind and Theobroma cacao Seed Extracts: Acute, Subchronic, and Genetic Toxicity Studies. J Toxicol. 2022. Article ID 3903943, doi:/10.1155/2022/3903943. [DOI] [PMC free article] [PubMed]
- 30.Nieschlag E. Testosterone treatment comes of age: new options for hypogonadal men. Clin Endocrinol. 2006;65(3):275–281. doi: 10.1111/j.1365-2265.2006.02618.x. doi:10.1111/j.1365-2265.2006.02618.x. [DOI] [PubMed] [Google Scholar]
- 31.Bhasin S, Brito JP, Cunningham GR, Hayes FJ. et al. Testosterone Therapy in Men With Hypogonadism: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2018;103(5):1715–1744. doi: 10.1210/jc.2018-00229. doi:10.1210/jc.2018-00229. [DOI] [PubMed] [Google Scholar]
- 32.Santella C, Renoux C, Yin H, Yu OHY, Azoulay L. Testosterone Replacement Therapy and the Risk of Prostate Cancer in Men With Late-Onset Hypogonadism, Am J Epidemiol. 2019; 188(9): 1666-1673. doi-org.eres.qnl.qa/10.1093/aje/kwz138. [DOI] [PubMed]
- 33.Smith SJ, Lopresti AL, Teo SYM, Fairchild TJ. Examining the Effects of Herbs on Testosterone Concentrations in Men: A Systematic Review. Adv Nutr. 2021;12(3):744–765. doi: 10.1093/advances/nmaa134. doi: 10.1093/advances/nmaa134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heinemann LA, Saad F, Zimmermann T, Novak A. et al. The Aging Males' Symptoms (AMS) scale: update and compilation of international versions. Health Qual Life Outcomes. 2003;1:15.. doi: 10.1186/1477-7525-1-15. doi: 10.1186/1477-7525-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith LB, Walker WH. The regulation of spermatogenesis by androgens. Semin Cell Dev Biol. 2014;30:2–13. doi: 10.1016/j.semcdb.2014.02.012. doi: 10.1016/j.semcdb.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luo L, Chen H, Zirkin BR. Leydig cell aging: steroidogenic acute regulatory protein (StAR) and cholesterol side-chain cleavage enzyme. J Androl. 2001;22(1):149–156. doi: 10.1002/j.1939-4640.2001.tb02165.x. [PubMed] [Google Scholar]
- 37.Booth A, Johnson DR, Granger DA. Testosterone and men's health. J Behav Med. 1999;22(1):1–19. doi: 10.1023/a:1018705001117. doi: 10.1023/a:1018705001117. [DOI] [PubMed] [Google Scholar]
- 38.Tyagi V, Scordo M, Yoon RS, Liporace FA, Greene LW. Revisiting the role of testosterone: Are we missing something? Rev Urol. 2017;19(1):16–24. doi: 10.3909/riu0716. doi: 10.3909/riu0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelly DM, Jones TH. Testosterone: a metabolic hormone in health and disease. J Endocrinol. 2013;217(3):R25–45. doi: 10.1530/JOE-12-0455. doi: 10.1530/JOE-12-0455. [DOI] [PubMed] [Google Scholar]
- 40.Liao Y, Chang YK, Wang SM, Chang HC. Ceiling effect of clomiphene citrate on the testosterone to estradiol ratio in eugonadal infertile men. PLoS One. 2022;17(1):e0262924.. doi: 10.1371/journal.pone.0262924. doi: 10.1371/journal.pone.0262924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiu HT, Shih MT, Chen WL. Examining the association between grip strength and testosterone. Aging Male. 2020;23(5):915–922. doi: 10.1080/13685538.2019.1632282. doi: 10.1080/13685538.2019.1632282. [DOI] [PubMed] [Google Scholar]
- 42.Srinivas-Shankar U, Roberts SA, Connolly MJ. et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95(2):639–650. doi: 10.1210/jc.2009-1251. doi: 10.1210/jc.2009-1251. [DOI] [PubMed] [Google Scholar]
- 43.Zitzmann M. Testosterone, mood, behaviour and quality of life. Andrology. 2020;8(6):1598–1605. doi: 10.1111/andr.12867. doi: 10.1111/andr.12867. [DOI] [PubMed] [Google Scholar]
- 44.Van Anders SM. Testosterone and sexual desire in healthy women and men. Archives of Sexual Behavior. 2012;41(6):1471–84. doi: 10.1007/s10508-012-9946-2. doi: 10.1007/s10508-012-9946-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the present study results are available from the corresponding author upon request.