Abstract
HIV-related cardiovascular disease research is predominantly from Europe and North America. Of the estimated 37∙9 million people living with HIV worldwide, 25∙6 million live in sub-Saharan Africa. Although mechanisms for HIV-related cardiovascular disease might be the same in all people with HIV, the distribution of cardiovascular disease risk factors varies by geographical location. Sub-Saharan Africa has a younger population, higher prevalence of elevated blood pressure, lower smoking rates, and lower prevalence of elevated cholesterol than western Europe and North America. These variations mean that the profile of cardiovascular disease differs between low-income and high-income countries. Research in, implementation of, and advocacy for risk reduction of cardiovascular disease in the global context of HIV should account for differences in the distribution of traditional cardiovascular disease risk factors (eg, hypertension, smoking), consider non-traditional cardiovascular disease risk factors (eg, access to antiretroviral therapy with more benign cardiovascular disease side effect profiles, indoor air pollution), and encourage the inclusion of relevant risk reduction approaches for cardiovascular disease in HIV-care guidelines. Future research priorities include implementation science to scale up and expand integrated HIV and cardiovascular disease care models, which have shown promise in sub-Saharan Africa; HIV and cardiovascular disease epidemiology and mechanisms in women; and tobacco cessation for people living with HIV.
Introduction
People living with HIV have an excess risk of cardiovascular disease compared with people without HIV.1,2 The mechanisms driving this risk include HIV-specific, and traditional and non-traditional cardiovascular disease risk factors. Data linking HIV and clinical cardiovascular disease, risk factors, and risk assessment come predominantly from Europe and North America. Of the estimated 37∙9 million people living with HIV worldwide, 25∙6 million live in sub-Saharan Africa, where less is known about incidence of cardiovascular disease and the burden of risk factors driving cardiovascular disease.
Epidemiology of HIV and cardiovascular disease
With access to life-preserving combination antiretroviral therapy (ART), people with HIV are living longer and could have increased risk for diseases of ageing, such as cardiovascular disease. A model based on the AIDS Therapy Evaluation in the Netherlands (ATHENA) cohort showed that the median age of people with HIV on ART will increase from 43∙9 years in 2010, to 56∙5 years in 2030, by which time 78% of people with HIV will have been diagnosed with cardiovascular disease.3
The increase in relative risk of myocardial infarction among people with HIV ranges from 20% to 100%, compared with people without HIV (table 1). This increased relative risk persists among those with viral suppression,14 exists for type 1 and type 2 acute myocardial infarction events;15 and might be more pronounced in women than in men (for coronary heart disease).16 Although the absolute rates of acute myocardial infarction are lower among people with fewer risk factors for this condition, the relative risk of acute myocardial infarction remains higher among people with HIV including those with good cardiac health.17 Ischaemic stroke accounts for approximately 80% of all strokes in people with HIV,18 with the rest accounted for by haemorrhagic stroke.
Table 1:
Studies on the association of HIV status and clinical cardiovascular disease
| Location | Number of participants | Number of HIV cases | Number of CVD events | Mean age (SD) | Outcome | Measure of effect | Effect estimate (95% CI) | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Stroke | ||||||||
| Qureshi et al (1997) | Grady Memorial Hospital, USA | 236 | 113 | 68 | 35 (6) | Cerebral infarction | Odds ratio | 3·2 (1·1–8·9) |
| Cole et al (2004) | Baltimore-Washington Cooperative Young Stroke Study, USA | 386 | 6 | 386 | 36 | Ischaemic stroke | Odds ratio | 13·70 (6·10–30·80) |
| Chow et al (2012) | Massachusetts General Hospital and Brigham and Women’s Hospital, USA | 36 731 | 4308 | 914 | 41 (12) | Ischaemic stroke | Hazard ratio | 1·21 (1·01–1·46) |
| Mateen et al (2013) | Multicenter AIDS Cohort Study, USA | 3945 | 1776 | 114 | 42 | All stroke | Relative risk | 2·16 (1·39–3·31) |
| Walker et al (2013) | Rural Hai district in northern Tanzania and urban Dar-es-Salaam, Tanzania | 201 | 25 | 201 | 61 (13) | All stroke | Odds ratio | 5·61 (2·41–13·09) |
| Marcus et al (2014)4 | Kaiser Permanente Southern California and Northern California, USA | 282 368 | 24 768 | 1279 | 40 (10) | Ischaemic stroke | Incidence rate ratio | 1·4 (1·2–1·7) |
| Rasmussen et al (2015) | Danish HIV Cohort Study | 58970 | 5897 | 1785 | Median 37 (IQR 31–44) | Stroke | Incidence rate ratio | 1·84 (1·60–2·13) |
| Sico et al (2015) | Veterans Aging Cohort Study, USA | 76 835 | 25 434 | 910 | 49 (9) | Ischaemic stroke | Hazard ratio | 1·17 (1·01–1·36) |
| Benjamin et al (2016)5 | Malawi urban hospital; stroke cases and community controls | 725 | 69 | 222 | 59 | All stroke | Odd ratio | 3·28 (2·05–5·25) |
| Alonso et al (2019)6 | Truven Health MarketScan Commercial Claims and Encounter and the Medicare Supplemental and Coordination of Benefits databases, USA | 79 100 | 19 798 | 93 | 43 (13) | Stroke | Hazard ratio | 2·3 (1·5–3·6) |
| Myocardial infarction | ||||||||
| Triant et al (2009) | Massachusetts General Hospital and Brigham and Women’s Hospital, USA | 70 357 | 487 | .. | Mid-50s | Acute myocardial infarction | Odds ratio | 1·93(1·21–2·93) |
| Lang et al (2010)7 | French hospital database on HIV | 74 958 | 74 958 | 360 | Not provided | Myocardial infarction | Standardised morbidity ratio | 1·5 (1·3–1·7) men; 1·4 (1·3–1·6) women |
| Durand et al (2011) | Régie de l’Assurance maladie du Québec, Canada | 27 734 | 7053 | 365 | 40 (11) | Acute myocardial infarction | Hazard ratio | 2·11 (1·69–2·63) |
| Klein et al (2015) | Kaiser Permanente Southern California and Northern California, USA | 282 368 | 24 768 | 2803 | 40 | Myocardial infarction | Incidence rate ratio | 1·40 (1·20–1·60) |
| Althoff et al (2015) | Veterans Aging Cohort Study, USA | 83 527 | 56 274 | 689 | Mid-50s | Acute myocardial infarction | Hazard ratio | 1·76 (1·49–2·07) |
| Rasmussen et al (2015) | Danish HIV cohort | 58 970 | 5897 | 1238 | Median 37 (IQR 31–44) | Myocardial infarction | Incidence rate ratio | 2·02 (1·71–2·38) |
| Alonso et al (2019)6 | Truven Health MarketScan Commercial Claims and Encounter and Medicare Supplemental and Coordination of Benefits databases, USA | 79 100 | 19 798 | 154 | 43 (13) | Myocardial infarction | Hazard ratio | 1·2 (0·8–1·8) |
| Heart failure | ||||||||
| Freiberg et al (2017)8 | Veterans Aging Cohort Study, USA | 98 015 | 31 523 | 2636 | 48 (10) | Congestive heart failure | Hazard ratio | 1·41 (1·29–1·54) |
| Feinstein et al (2018)9 | HIV Electronic Comprehensive Cohort of CVD Complications (Northwestern Medicine), USA | 7371 | 4640 | 152 | 40 (11) | Adjudicated heart failure | Hazard ratio | 2·10 (1·38–3·21) |
| Alonso et al (2019)6 | Truven Health MarketScan Commercial Claims and Encounter and Medicare Supplemental and Coordination of Benefits databases, USA | 79 100 | 19 798 | 223 | 43 (13) | Heart failure | Hazard ratio | 2·8 (2·0–3·8) |
| Peripheral artery disease | ||||||||
| Alonso et al (2019)6 | Truven Health MarketScan Commercial Claims and Encounter and Medicare Supplemental and Coordination of Benefits databases, USA | 79 100 | 19 798 | 98 | 43 (13) | Peripheral artery disease | Hazard ratio | 0·9 (0·5–1·4) |
| Beckman et al (2019)10 | Veterans Aging Cohort Study, USA | 91 953 | 28 714 | 7708 | 48 (10) | Peripheral artery disease | Hazard ratio | 1·19 (1·13–1·25) |
| Lai et al (2018) | Taiwan Centers for Disease Control, HIV Surveillance Database | 2 000 000 | 26 272 | 55 | 32 (10) | Peripheral artery disease | Standardised Incidence rate | 0·87 (0·65–1·13) |
| Sudden cardiac death | ||||||||
| Tseng et al (2012)11 | HIV specialty clinic in San Francisco, California, USA | 2860 | 2860 | 30 | Median 39 (IQR 33–45) | Sudden cardiac death | Standardised mortality ratio | 4·46 |
| Lai et al (2018)12 | Taiwan Centers for Disease Control, HIV Surveillance Database | General population | 26272 | 82 | 32 (10) | Sudden cardiac death | Standardised Incidence rate | 3·01 (2·39–3·73) |
| Alvi et al (2019)13 | Bronx Lebanon Hospital Center, Icahn School of Medicine at Mount Sinai, USA | 2149 | 344 | 191 | 60 (9) | Sudden cardiac death | Odds ratio | 3·0 (1·78–4·24) |
| Tseng et al (CROI 2019, unpublished) | HIV specialty clinic in San Francisco, California, USA | 552 | 47 | 552 | 51–63 | Sudden cardiac death | Incidence rate ratio | 1·86 (1·39–2·50) |
| Freiberg et al (CROI 2019, unpublished) | Veterans Aging Cohort Study, USA | 144 336 | 43 407 | 3035 | 50 (11) | Sudden cardiac death | Hazard ratio | 1·14 (1·04–1·25) |
For cardiovascular disease types where data are sparse, studies using population-based HIV-negative groups and conference abstracts are included. Studies with references were not included in a meta-analysis by Shah and others.2 All other studies (appendix p 1) were included in this meta-analysis. CVD=cardiovascular disease. CROI=conference on retroviruses and opportunistic Infections.
The scope and presentation of heart failure in HIV has evolved with improved uptake and access to ART. Overt AIDS cardiomyopathy has become less common while other causes of heart failure, including preserved ejection fraction, ischaemic post-myocardial infarction, and reduced ejection fraction heart failure, have become more common. The excess in HIV-associated risk could be greater for reduced than for preserved ejection fraction heart failure.8
Peripheral artery disease is relatively understudied among people with HIV. Peripheral artery disease in the USA is one of the most common clinical presentations of atherosclerosis. The few available studies on the association between HIV and peripheral artery disease report inconsistent results.6,10,12,19 Estimates from South Africa on peripheral artery disease suggest a prevalence of 7% in HIV populations with a mean age 46 years,20 compared with the general population with prevalence estimates of 3∙9% in people aged 40–49 years.21 The largest study examining HIV infection as a risk factor for peripheral artery disease involved over 90 000 participants from the Veterans Aging Cohort Study in the USA and reported a 19% increase in risk of incident peripheral artery disease among people with HIV versus without HIV.10
In a recent meta-analysis,2 people living with HIV had more than twice increased risk of cardiovascular disease overall. All the cohorts analysed, except one from Tanzania, were either from western Europe or North America. The distribution of cardiovascular disease risk factors varies by geographical location and by HIV prevalence. Sub-Saharan Africa has the greatest prevalence of HIV, along with a younger population, higher elevated blood pressure prevalence, lower tobacco smoking rates, and lower prevalence of elevated cholesterol than western Europe and North America (figure 1).22,23 In addition, several unique exposures such as indoor air pollution and repeated exposure to infectious agents might compound the effect of HIV on cardiac and pulmonary health24,25 in sub-Saharan Africa, but could have minimal relevance in high-income settings.
Figure 1: Prevalence distribution of HIV and risk factors for cardiovascular disease.
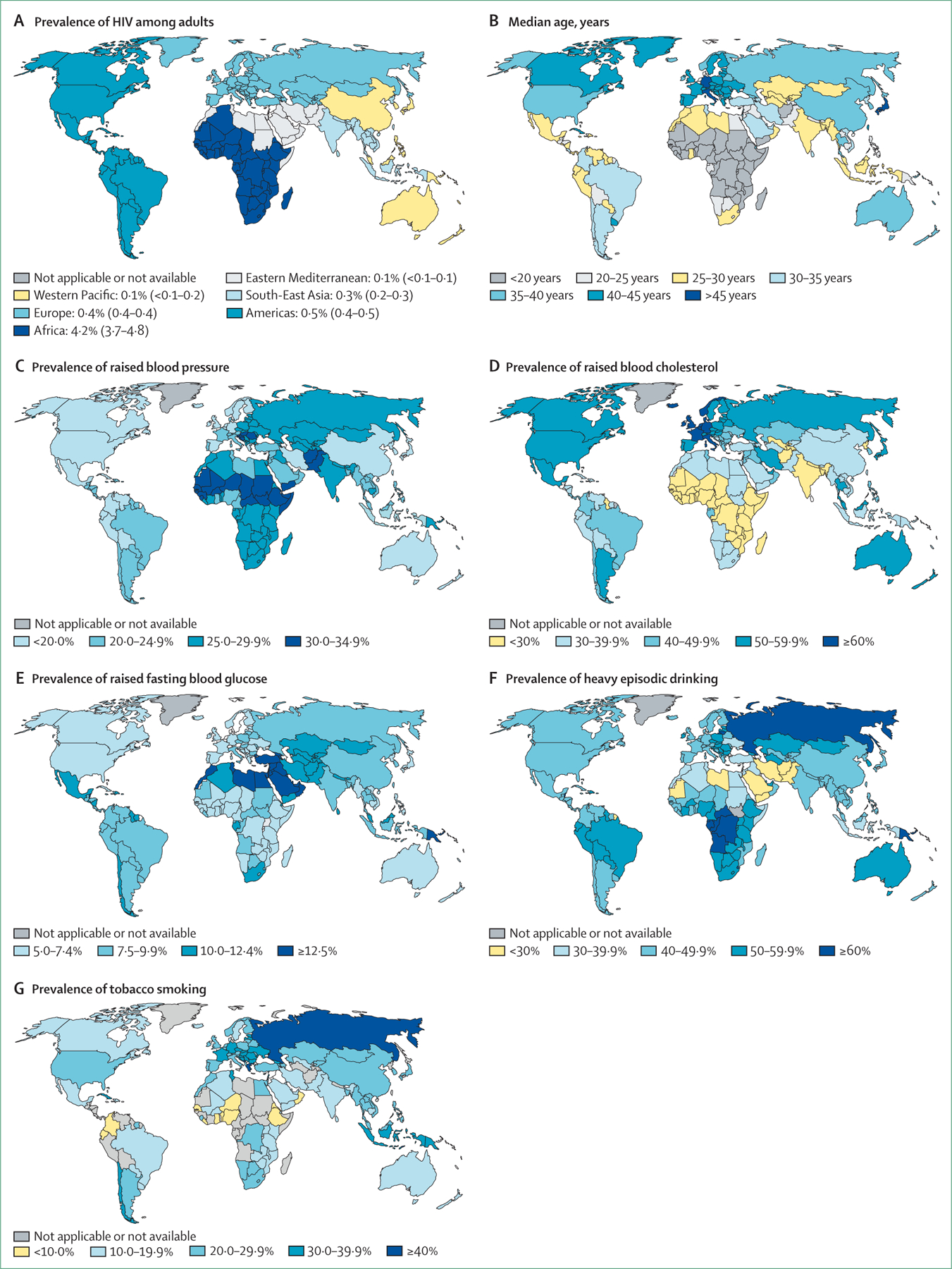
World maps showing increased HIV prevalence, age, raised blood pressure, raised blood cholesterol, raised fasting blood glucose, heavy episodic drinking, and tobacco smoking. Permission from WHO and the Institut National d’Etudes Démographiques. (A) Prevalence of HIV among adults aged 15–49, 2016 classified by WHO regions. Global prevalence of HIV: 0·8 (average 0·7–0·9). Numbers in brackets show global prevalence of HIV on average. (B) Median age (years), 2017. (C) Prevalence of raised blood pressure (systolic blood pressure ≥140 mm Hg; diastolic blood pressure ≥90 mm Hg), ages over 18 years, 2015. Age-standardised estimate for both sexes. (D) Prevalence of raised blood cholesterol (≥5·0 mmol/L), ages over 25 years, 2008. Age-standardised estimate for both sexes. (E) Prevalence of raised fasting blood glucose (≥7·0 mmol/L or on medication for raised blood glucose), ages over 18 years, 2014. Age-standardised estimate for both sexes. (F) Prevalence of heavy episodic drinking among both sexes, ages over 15 years and older, 2016. (G) Prevalence of tobacco smoking among people aged 15 years and older, 2016. Age-standardised estimate.
Cardiovascular disease mechanisms in HIV
Virus-related mechanisms
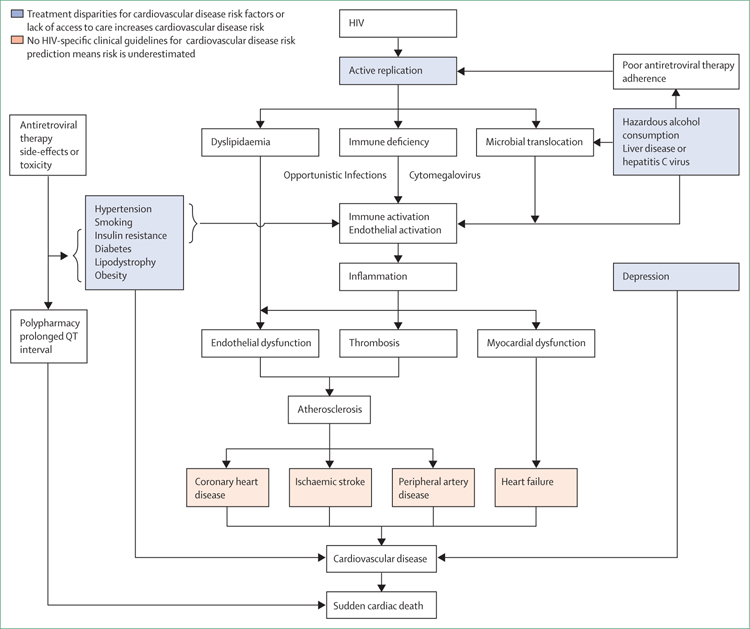
Virus-related mechanisms include pro-inflammatory effects of HIV proteins released from the HIV virus, CD4+ T-cell depletion, increased intestinal permeability, microbial translocation, and altered cholesterol metabolism (figure 2). Biomarkers of chronic inflammation, monocyte activation, and altered coagulation are elevated in people living with HIV compared with people without HIV.26 Many of these biomarkers are associated with atherogenesis, an inflammatory process, leading to atherosclerotic cardiovascular disease (eg, myocardial infarction). Importantly, sex-based differences in response to an acute HIV infection might persist into chronic infection, resulting in increased immune activation in women compared with men.16 These sex-based differences could explain higher rates of myocardial infarction among women,16 which is an important reason for greater inclusion of women and sex-stratified analyses in this field. Greater inclusivity should extend to pre-menopausal and post-menopausal women, and gender minorities for whom sex hormone profiles might (independently and synergistically with HIV) be important drivers of cardiovascular disease risk.
Figure 2:
Mechanisms of HIV-associated cardiovascular disease
CD4+ T-cell depletion among people with HIV is associated with higher rates and risk of incident acute myocardial infarction, heart failure, peripheral artery disease, and ischaemic stroke (table 1). Weakened adaptive immunity could lead to opportunistic infections, which in turn drive an inflammatory response that might lead to cardiovascular disease (eg, infectious myocarditis).27 A study by Grody and colleagues28 also suggests that HIV itself can cause cardiomyopathy via direct infection of cardiac myocytes.29 The depletion of CD4+ T cells in the gut mucosa, caused by HIV infection, damages the lining of the gut and increases its permeability to microbial translocation products. Microbial translocation is a process whereby microbial products from the gut leak across the disrupted gut lining and into the portal circulation on the way to the liver. This process leads to chronic immune activation and inflammation.30 HIV, like other viral infections, is associated with altered cholesterol metabolism resulting in atherogenic lipid and cholesterol profiles.31
In the CNS, HIV could alter the blood–brain barrier through infected monocytes, which routinely surveil the brain from the peripheral circulation. In the closed brain compartment, HIV can infect perivascular macrophages, microglial, and mural support tissue, and establish a reservoir.32,33 This microenvironment of HIV-infected cells can accentuate inflammatory mediators and contribute to endothelial dysfunction, and vessel wall remodelling.33,34 Whether the mechanism in the brain differs from the periphery is yet to be established, but clinical stroke phenotypes suggest differences do exist.35
ART-related mechanisms
Any adverse effect of ART on cardiovascular disease risk should be balanced against the life-preserving effects of ART and the effects of these drugs on reducing HIV viraemia.36 Older ART regimens (eg, abacavir, lopinavir, and ritonavir) used in low-income settings had side-effect profiles detrimental to cardiovascular health such as altered glucose and lipid metabolism, mitochondrial toxicity and subsequent cardiac myopathy, or impaired left ventricular function.37 Other regimens (eg, dolutegravir or atazanavir) might have less detrimental effects on cardiovascular health.38,39 Whether these cardiotoxic-ART effects differ by sex is unclear.40
Weight gain after initiation of ART and HIV viral suppression in part reflects ART side-effects41 and a return to health after resolution of overt HIV replication (a catabolic state).42 It also reflects fluctuating trends in weight gain in low-income and high-income settings, which are not unique to HIV. Combined, these sources of weight gain have been associated with incident diabetes, a cardiovascular disease risk factor, and this association is dependent on body physique before starting ART.42
People ageing with HIV have multiple chronic comorbidities often requiring multidrug regimens and resulting in polypharmacy.43 The potential for drug–drug interactions increases with increasing number of medications which in turn could contribute to QT interval prolongation.44 Prolonged QT intervals are associated with HIV infection and increased risk of sudden cardiac death. People with HIV have a 4-times increased risk of a sudden cardiac death compared with people without HIV.11
Non-HIV specific mechanisms
Non-HIV specific mechanisms contributing to increased risk of cardiovascular disease include traditional and non-traditional cardiovascular disease risk factors. Traditional risk factors include smoking, diabetes, dyslipidaemia, hypertension, and biological sex. Although not unique to people living with HIV, populations in some regions might have greater exposure to these risk factors (figure 1). Women with HIV might have higher odds of developing metabolic syndrome than men.40 When these risk factors are absent, the relative risk of acute myocardial infarction among people with HIV compared with people without HIV is still 2-times higher, but the absolute rates of acute myocardial infarction are low. Increasing exposure to these risk factors leads to an exponential increase in cardiovascular disease risk regardless of HIV status.17
These risk factors in combination with HIV infection have been variably associated with subclinical atherosclerosis including carotid intima-media thickness, coronary artery calcification, and other structural and functional vascular alterations.45–49 In addition to variability in the geographical distribution of traditional cardiovascular disease risk factors, variability in the association of these risk factors with subclinical cardiovascular disease highlight the need for geographically diverse studies for HIV-related cardiovascular disease risk reduction globally.
Non-traditional cardiovascular disease risk factors are also accentuated in HIV and include unhealthy alcohol consumption, depression, hepatitis C, and possibly cytomegalovirus co-infection. Unhealthy alcohol consumption, independent of HIV infection, can cause microbial translocation, activating Kupffer cells, which drive chronic inflammation. Liver fibrosis can itself be associated with increased heart failure risk.50 Unhealthy alcohol consumption is also associated with ART non-adherence,51 which causes increased HIV viral replication and might consequently increase cardiovascular disease risk.
Major depressive disorder can affect between 5% and 10% of people with HIV. Depression is a risk factor for both acute myocardial infarction and heart failure among people with HIV.52,53 Although the exact mechanism is unknown, some reports link depression with autonomic nervous system dysregulation, inflammation, and platelet activation.
Hepatitis C and cytomegalovirus are common co-infections among people with HIV. In many, but not all, studies, hepatitis C has been linked to incident cardiovascular disease events.14,54 Underlying mechanisms are thought to be related to chronic inflammation, endothelial dysfunction, and exacerbating microbial translocation through hepatic damage. The role of cytomegalovirus as a risk factor for cardiovascular disease is less clear. Observational studies in the general population link cytomegalovirus antibody status to incident cardiovascular disease.55 Among people with HIV, no such studies exist, although some evidence links cytomegalovirus status to immunosenescence,56 which might predispose to cardiovascular disease risk.
Disparities in cardiovascular care occur with studies reporting that people with HIV are less likely to receive aspirin for primary cardiovascular disease prevention, HMG-CoA reductase inhibitor therapy for diabetes, cardiovascular disease, or dyslipidaemia;57 and invasive procedures for myocardial infarction compared with people without HIV.58 These disparities by HIV status might be worsened by substance use disorders, female sex,40 and among racial and ethnic minorities.
Clinical guidelines for treating cardiovascular disease in HIV
Scarce data on HIV and cardiovascular disease risk, from sub-Saharan Africa, are the reason for insufficient cardiovascular disease risk stratification tools and guidelines tailored for this setting.59,60 Most existing cardiovascular disease risk calculators are derived from HIV uninfected populations in high-income countries. Although an HIV-specific risk estimation model exists, it is derived from a largely white European population and relies on data from before the modern ART era, therefore limiting its current applicability.61 Cardiovascular disease risk prediction for people living with HIV in sub-Sahara Africa faces another problem in that the epidemiology of traditional cardiovascular disease risk factors is unique from that in high-income countries. The results of studies including REPRIEVE (NCT02344290), SEARCH (NCT01864603), and EVERLAST48 might provide important incidence and risk factor data to inform cardiovascular disease risk prediction for people with HIV in low-income and middle-income countries.
The American Heart Association (AHA) recently provided guidance62 on applying the atherosclerotic cardiovascular disease risk calculator in people living with HIV (table 2). For patients with HIV-related cardiovascular disease risk-enhancing factors (eg, prolonged viraemia, fatty liver disease), clinicians might adjust the calculated risk estimate upward by 1·5–2 times on the basis that most risk calculators tend to underestimate cardiovascular disease risk in this group,76 and that specific HIV-related factors (eg, low nadir and current CD4 count) are associated with cardiovascular disease risk elevation. Traditional and non-traditional atherosclerotic cardiovascular disease risk factors should be considered. Intervention involves a combination of lifestyle optimisation regardless of risk and consideration of pharmacotherapy with rosuvastatin, atorvastatin, pravastatin, or pitavastatin for people at sufficiently high risk.
Table 2:
Guidelines on cardiovascular disease prevention, prediction, and risk reduction
| Cardiovascular disease risk prediction | Tobacco | Hypertension | Diabetes | Dyslipidaemia | Drug interactions | |
|---|---|---|---|---|---|---|
|
| ||||||
| Kenya National Guidelines for cardiovascular disease management (2018)63 | No HIV-specific recommendation | No HIV-specific recommendation | As in general population with exceptions on interactions of antihypertensive drugs with ART | Assess glucose at baseline and then annually | Assess fasting lipids at baseline and annually if abnormal | Yes; antihypertensive drugs interacting with ART |
| South African dyslipidaemia guideline (2018) and National Consolidated HIV Guidelines (2015)64,65 | “There is no validated risk score for HIV-infected black South Africans; Framingham risk tables may be used to aid decision-making” | No HIV-specific recommendation | No HIV specific recommendation | No HIV-specific recommendation | Full lipogram recommended after ART initiation; repeated at 3 months after starting protease inhibitor and periodically thereafter | Yes; lipid lowering drugs interacting with ART |
| Malawi HIV Testing Services Guidelines (2016)66 | Not addressed | Recommends stopping smoking | Assess blood pressure at ART initiation; repeat annually; identified specific blood pressure thresholds; lifestyle changes or pharmacotherapy management | Recommends screening as part of hypertension management | Not addressed | Not addressed |
| Infectious Diseases Society of America HIV Guidelines (2013)67 | Coronary heart disease risk assessment recommended but not specified; Framingham risk scores assumed given reference to NCEP guidelines | Recommends smoking cessation as part of regular patient education | Blood pressure check annually in all patients | Consider NHANES HbA1c cutoff of 5·8%; check fasting plasma glucose and HbA1c every 6–12 months | Recommends fasting lipid profile 1–3 months after and before ART initiation; management per NCEP guidelines | Yes; lipid lowering drugs interacting with ART |
| American Heart Association Statement on HIV and Cardiovascular Disease62 | Proposes upward adjustment of risk assessment by 1·5–2-times using ACC, AHA ASCVD risk estimator in the presence of risk-enhancing factors | Smoking cessation and online resources | No HIV-specific recommendation | No HIV-specific recommendation | Lifestyle optimisation; pharmacotherapy with a start low, go slow strategy because of side-effects | Mentioned with reference to online resource; interaction of lipid lowering drugs, anticoagulant, and ART |
| European AIDS Clinical Society Guidelines Version 9·1 (2018)68 | Framingham score every two years in men (>40 years) and women (>50 years) without cardiovascular disease; HIV-specific calculators as alternatives | Provides screening algorithm, treatment strategies motivational, cognitive behavioural counselling, and pharmacotherapy | Extensive risk assessment algorithm; includes drug sequencing algorithm | Diagnostic criteria, risk assessment algorithm, and management algorithm | Risk assessment algorithm | Yes; antihypertensive drugs and cholesterol lowering drugs interacting with ART |
| British HIV Association guidelines (2019 interim update)69 | QRISK2 for patients aged >40 years annually if no vascular disease | Auditable targets include patients with a smoking history documented in the last 2 years (90%) and blood pressure recorded in the last 15 months (90%) | Annual screens for those with cardiovascular disease and at increased risk (10-year risk >10%) | Annual screens for those with cardiovascular disease and at increased risk (10-year risk >10%) | Annual screens for those with established cardiovascular disease and at increased risk (10-year risk >10%); high-dose (80 mg) atorvastatin for established cardiovascular disease | Recommend all medications to be reviewed and documented at each clinic visit to identify potential drug–drug interactions |
Recommendations in selected HIV-care guidelines from sub-Saharan Africa, North America, and Europe. ART=antiretroviral therapy. NCEP=National Centers for Environmental Prediction. NHANES=National Health and Nutrition Examination Survey. ACC=American College of Cardiology. AHA=American Heart Association. ASCVD=atherosclerotic cardiovascular disease. QRISK2=Quality Cardiovascular Risk Score 2.
This risk adjustment approach in the AHA scientific statement on HIV is also incorporated in the European Society for Cardiology guidelines71 that consider people with HIV at high risk for cardiovascular disease and suggest treating LDL-cholesterol to a goal of less than 70 mg/dL. These guidelines seem reasonable to apply in low-income and middle-income countries in the absence of a clear alternative. However, in South Africa, people living with HIV are considered to be at low risk for atherosclerotic cardiovascular disease and guidelines applicable to the general population are suggested for people living with HIV with no upward adjustment in risk.64
The 2010 South African guidelines72 for the management of ischaemic stroke and transient ischaemic attack have provided comprehensive guidance, but they were not specific about HIV infection and the risk of stroke. Extrapolating AHA guidance to existing cardiovascular risk scores for stroke risk prediction in low-income and middle-income countries is reasonable, considering previously discussed limitations.73 An additional caveat is that intracranial, large to medium sized ischaemic strokes might be driven by non-traditional vascular risk factors and therefore might not be applicable.74
Combining stroke epidemiology with cardiovascular disease, an oversight to be rectified in the International Classification of Diseases and Related Health Problems-11,75 reduces emphasis on stroke in high-income settings.76 The AHA acknowledged that the cause of stroke is dependent on the stage of HIV infection, transitioning from opportunistic infection, coagulopathy, and HIV-associated vasculitis in advanced disease, to atherosclerosis and an undefined (non-vasculitic, non-atherosclerotic) vasculopathy among those with stable disease and on ART.35,62,77 In high-income countries, the stage of HIV is less advanced than in low-income countries but opportunistic infections can still arise when ART fails, or in the late presentation of HIV.78 An inflammatory vasculopathy can occur in the context of a cerebrospinal fluid HIV escape syndrome, among those with stable disease and those on ART.79 Although antiplatelets, statins, antiglycaemic drugs or antihypertensive drugs are the main therapies for primary and secondary prevention of cardiovascular disease in high-risk groups, other targeted therapies might be required after a stroke (eg, antithrombotic drugs, antiviral drugs for co-infection, and ART in those with more potent CNS penetration to manage cerebrospinal fluid HIV escape syndrome).
No guidelines exist for HIV-related heart failure prevention and treatment, given the absence of clinical trial data and few observational data on heart failure in the modern ART era. Accordingly, the 2019 AHA scientific statement suggests that heart failure examination and therapy for people with HIV should be similar to that for those without HIV, given the uncertainty about the mechanisms of heart failure in HIV. Diuretics, renally-excreted β blockers, angiotensin-converting enzyme (ACE) inhibitors, and angiotensin receptor blockers (ARBs), with the exception of losartan and irbesartan, do not appear to have prohibitive drug–drug interactions with most ART. Providers should be aware of heart failure medication metabolised by the cytochrome P450 3A4 enzyme because of potential interactions with ART that can inhibit this enzyme, such as ritonavir and cobicistat. People living with HIV should be offered surgical, device, and mechanical therapies for heart failure on the basis of clinical indications applicable to the general population.62
Experts recommend following the guidelines for peripheral artery disease and sudden cardiac death provided by the American College of Cardiology, AHA,80 European Society for Vascular Surgery, and European Society of Cardiology.81 These guidelines recommend full assessment and screening of patients, and a physical examination. If the assessment is suggestive of peripheral artery disease, additional testing (eg, ankle-brachial index) is needed. Revascularisation, assessment of left ventricular ejection fraction, and consideration of electrophysiology and an implantable cardiac defibrillator can be considered as preventative strategies for reducing the risk of sudden cardiac death.80
Cardiovascular disease risk reduction in HIV
Sub-Saharan Africa and low-income and middle-income countries
Country-level HIV programmes helped transition health systems that were traditionally focused on acute, episodic care to develop some of the first chronic care programmes. As a result, much of the effort to reduce coronary heart disease risk in low-income and middle-income countries has been deployed and evaluated in these national HIV programmes. Most approaches to cardiovascular disease risk reduction in people with HIV begin with HIV management while also addressing coronary heart disease risk screening, referral, and risk factor management to varying degrees. Active HIV replication and immune dysfunction are important drivers of coronary heart disease risk. Strengths and weaknesses of screening programmes at the community-level and clinic-level have been described.82 Using HIV platforms to incorporate comprehensive chronic-disease management and exploring population health approaches is key.83
Lessons learned include the importance of efficiently testing new deployments of existing resources in sub-Saharan Africa.84 Recent illustrative examples come from Malawi85 and Uganda.86 Following population-based integrated HIV and non-communicable disease screening in Uganda,86 people with HIV and hypertension were referred to receive integrated care with 45% successful linkage to care. Among factors linked to care, blood pressure control increased from 15% at baseline to 46% over subsequent follow-up. In a Malawi HIV-care programme,85 29 359 people living with HIV were screened for hypertension over a 1-year period revealing an 11% prevalence. Subsequently, 85% of people with HIV and hypertension received treatment, or lifestyle modification advice, or both, with blood pressure control rates at 6 months of 38% in people with HIV who had mild hypertension and 30% in people with HIV who had moderate hypertension.
These risk reduction strategies also apply to stroke in people with HIV. Antiplatelet therapy is added as a secondary preventive agent in individuals with haemorrhagic stroke and brain imaging can help to risk-stratify these patients. Effective prevention and treatment of heart failure in people with HIV in low-income and middle-income countries are scarce. Use of ACE inhibitors, ARBs, β blockers, or mineralocorticoid antagonists is low, and diuretics are prescribed in only 51–67% of cases.87,88 Data on the application of device and mechanical therapies for heart failure in people with HIV are insufficient, as are concerted efforts of heart failure prevention.89
Risk reduction approaches for coronary heart disease also apply to peripheral artery disease. A 2009 South African study90 of patients with advanced peripheral artery disease and poor HIV control used CD4+ T-cell count to guide vascular intervention decisions. Lower CD4 counts led to more conservative surgical approaches. Standard surgical techniques were used, except for patients with AIDS for whom broad spectrum antibiotics and fluconazole were used prophylactically instead of the standard cefazolin prophylaxis. Smoking rates in this study were high (79%) and are an important intervention point. Little availability of services to diagnose and treat cardiac arrhythmias in several low-income countries in sub-Saharan Africa exists,91 which further limits our ability to prevent sudden cardiac death in these settings.
High-income countries
Coronary heart disease and stroke prevention might not be adequately addressed in the current primary care of people with HIV. A large international study which surveyed people with HIV found that only 19% discussed cardiovascular disease with their providers, and only 31% had ever discussed hypertension, hypercholesterolaemia, family history of cardiovascular disease, or smoking.92 In the USA, rates of statin prescription among people with HIV who are most likely to benefit from these drugs remain low.93 Under-prescription is particularly evident in racial and ethnic minorities in people with HIV.94 However, low rates of appropriate cardiovascular disease risk-factor management could relate more to poor risk factor control, rather than HIV-specific shortfalls. Studies in the USA95 and sub-Saharan Africa96 have shown better control of coronary heart disease risk factors presumably affected by access to care for people with HIV than for people without HIV. Whether or not individuals with HIV will benefit from lower thresholds for lipid, blood pressure, or glucose control remains unknown.
In high-income countries most stroke patients with HIV will be managed in stroke units. With the rapid patient assessment now required to offer life-saving and disability-saving therapy, stroke interventions will frequently be offered without knowledge of a HIV diagnosis. Although no high-quality studies to give guidance on the safety and effectiveness of these therapies exist, retrospective studies indicate no substantial harm is caused.97
Despite the considerable improvement in HIV life expectancy and promising outcomes of advanced heart failure therapies (including transplantation) for people with HIV, many health centres lack experience and expertise caring for people with HIV who have advanced heart failure. Some clinicians still consider HIV as a contraindication to heart transplantation.98 Strategies to prevent or treat heart failure with preserved ejection fraction in HIV remain undefined.
The risk of peripheral artery disease is highest among those with unsuppressed HIV viraemia or low CD4 T-cell counts, suggesting that HIV management is an important factor in reducing risk. Traditional risk factors such as smoking and diabetes are also strongly associated with peripheral artery disease.
All data on approaches to reducing sudden cardiac death are from the general population and not specific to people with HIV. Among individuals who have ischaemic heart disease, electrophysiological evaluation and consideration of an implantable cardiac defibrillator are recommended in some clinical scenarios.80 Individuals with particular infiltrative conditions such as sarcoidosis might be considered for an implantable cardiac defibrillator based on clinical history, structural heart disease, or cardiac MRI findings.80 Whether or not HIV-infected individuals will benefit from different defibrillator thresholds will require further research.
Current approaches to reducing cardiovascular disease risk and their effectiveness
Sub-Saharan Africa and low-income and middle-income countries
Prevalence of coronary heart disease risk factors for people with HIV in low-income and middle-income countries varies—eg, hypertension (21%), elevated LDL cholesterol (23%), hypertriglyceridaemia (27%), low LDL cholesterol (52%), overweight (21%), and obesity (8%).99 Underdiagnosis of these risk factors continues and screening programmes have identified 33–47% undiagnosed hypertension cases100 and 66% elevated cholesterol cases. Studies with coronary heart disease-related clinical endpoints in low-income and middle-income countries are needed.
Models for integrating HIV care and coronary heart disease risk reduction include facility-based and community-based integrated care.85,101 Although preliminary findings on their feasibility and effect on existing HIV-care delivery are promising,100,102 data on clinical outcomes in general, including coronary heart disease risk reduction, are unavailable.
Reducing stroke burden among people with HIV is not the current focus of attention in many low-income and middle-income countries especially as progress is needed to reduce stroke burden in the general population. Although stroke incidence and disability decreased globally over the last 20 years, stroke incidence in southern Africa has increased.103 Given that HIV infection is attributable to 15–25% of incident stroke cases in this region,5,62 developing stroke preventive strategies for people with HIV at a policy-level might improve the disproportionate increase of stroke incidence in HIV endemic regions.
Precursors to heart failure are common among people living with HIV in low-income and middle-income countries. Hypertensive heart disease, rheumatic heart disease, and cardiomyopathy account for approximately two-thirds of cases of heart failure among hospitalised patients; however, ischaemic heart disease is much less common.104 Hypertension is a recognised risk factor for heart failure. The age-standardised prevalence of hypertension increased by 8% in low-income and middle-income countries between 2000 and 2010.105 Heart failure outcomes are worse for people with HIV in low-income and middle-income countries than in the general population. The sub-Saharan Africa Survey of Heart Failure (THESUS-HF)106 showed that HIV infection conferred a 62% greater risk of all-cause mortality at 180 days after a heart failure admission.
Little is known about the burden of peripheral artery disease over time among people with HIV in low-income and middle-income countries. Presentation for peripheral artery disease among people with HIV in places such as South Africa might be delayed. In one study, 90% of largely untreated HIV patients admitted to a vascular unit presented with advanced stage vascular disease (rest pain or gangrene, corresponding to Fontaine stage III or IV) resulting in a high primary limb amputation rate of 32%.90 A study from Nigeria suggested higher peripheral artery disease prevalence among people with HIV, but similar prevalence in virologically-suppressed people with HIV, compared with people without HIV.107
Few studies of sudden cardiac death have been reported. A study in Cameroon108 reported a 9·4% rate of sudden cardiac death with crude incident rate of 31·3% per 100 000 person-years. Thus, it remains challenging to establish how approaches to reduce ischaemic heart disease are affecting sudden cardiac death risk for people with HIV.
High-income countries
Few data exist regarding temporal trends of clinical coronary heart disease incidence among people with HIV (table 3). A study in Europe112 followed up 8762 people with HIV since 2000, and showed that coronary heart disease risk factor burden is high, but that modification of risk factors such as hypertension appears to be improving over time. Identifying the role of coronary heart disease risk-factor management in temporal changes in cardiovascular disease mortality for people with HIV is difficult given concomitant changes in ART timing and regimens which affect absolute and relative risks for coronary heart disease (table 3).
Table 3:
Time trends of myocardial infarction incidence among people with HIV in high-income countries
| Number of people with HIV | Number of AMI events | HIV positive effect estimate | HIV negative effect estimate | Relative effect estimate by HIV (95% CI) | |
|---|---|---|---|---|---|
|
| |||||
| Kaiser Permanente Southern California and Northern California health plans * 109 | |||||
| 1996–2011† | 24 768 | 2803 | 268 | 165 | 1·4 (1·2–1·6) |
| 1996–1999 | .. | .. | 276 | 136 | 1·8 (1·3–2·6) |
| 2000–2003 | .. | .. | 324 | 162 | 1·7 (1·4–2·1) |
| 2004–2007 | .. | .. | 270 | 178 | 1·3 (1·0–1·6) |
| 2008–2009 | .. | .. | 245 | 167 | 1·3 (0·9–1·7) |
| 2010–2011 | .. | .. | 195 | 165 | 1·0 (0·7–1·4) |
| French hospital database on HIV * 110 | |||||
| 2000–2002 early cART era | 43 628 | 132 men | 196 | 148·0 | 1·35 (1·14–1·61) |
| 2003–2005 intermediate cART era | 51 007 | 205 men | 229·5 | 133·4 | 1·74 (1·51–1·99) |
| 2006–2009 late cART era | 58 866 | 259 men | 185·2 | 161·1 | 1·12 (0·99–1·27) |
| 2000–2002 early cART era | 43 628 | 15 women | 68·4 | 25·5 | 2·81 (1·58–4·64) |
| 2003–2005 intermediate cART era | 51 007 | 16 women | 47·6 | 23·6 | 2·18 (1·25–3·55) |
| 2006–2009 late cART era | 58 866 | 36 women | 67·5 | 32·9 | 1·99 (1·39–2·75) |
| Cohort of HIV adults of the AIDS research network (Spain) ‡ 111 | |||||
| 2004–2009 | 10 760 | 18 (HIV) | 279 (265, 293) | 199 (188, 209) | 1·41 (1·26–1·55) |
| 2010–2015 | 10 760 | 34 (HIV) | 222 (211, 233) | 173 (163, 183) | 1·28 (1·15–1·43) |
Measure of effect was the incidence rate per 100 000 person-years.
The total number of people with HIV for the entire period (1996–2011) was 24 768. During this time period, 2803 AMI events occurred (320 among people with HIV and 2483 among people without HIV).
Measure of effect was the standardised incidence rate in men (vs general Spanish male population). AMI=acute myocardial infarction. cART=combination antiretroviral therapy.
Absolute rates of cardiovascular disease mortality for people with HIV have declined since 1999, primarily because of improved HIV treatment uptake and related continued improvement in CD4 cell count.113 However, the relative contribution of cardiovascular disease to mortality is increasing because of the ageing population of people with HIV in high-income countries and decreasing risks of AIDS-related deaths.114 Between 1997 and 2006, a US-based longitudinal analysis showed that despite the increasing uptake of ART, and accounting for health disparities, the rate of ischaemic stroke increased by 60% for people living with HIV.115 This finding was supported by reports which showed that global HIV-associated cardiovascular disease tripled between 1990 and 2015.2
In high-income settings, the epidemiology of HIV-associated heart failure has most likely shifted in the ART era from severe left ventricular systolic dysfunction in the setting of uncontrolled HIV replication and AIDS, to diastolic and systolic dysfunction in the setting of chronic, cumulative comorbidities. How heart failure risk factors interact with HIV-related cardiovascular disease risk factors to drive myocardial dysfunction and heart failure is unknown. The prognosis of HIV-associated heart failure has also shifted from shortened survival among people living with HIV or AIDS to outcomes comparable with people without HIV.
Future studies are needed to ascertain the effect of HIV control and risk factor control on peripheral artery disease risk in HIV. Ischaemic heart disease is the most common underlying condition associated with sudden cardiac death. In the general population, the incidence of sudden cardiac death in the setting of ischaemic heart disease is decreasing.116 By contrast, structural heart disease including cardiomyopathy associated with myocardial fibrosis and left ventricular hypertrophy are increasing.117 Scarce data on sudden cardiac death in HIV make it challenging to assess how the approaches to reduce ischaemic heart disease are affecting sudden cardiac death risk in people with HIV.
Future approaches to reducing cardiovascular risk
Sub-Saharan Africa and low-income and middle-income countries
First, coronary heart disease risk factor screening should be part of routine care for people with HIV.84 Given few resources and the absence of cost-effective data on routine coronary heart disease risk factor screening in facility-based HIV-care programmes,118 it might be advisable to reserve routine screening for people with HIV older than 40 years for whom feasibility has been shown.119 This is a practical approach, although barriers to scale up and expand into other low-income and middle-income countries still exist.119 Contextually appropriate innovations such as targeted screening of people with HIV at high risk for coronary heart disease, task redistribution, and the use of point-of-care diagnostics would help to overcome some of these barriers.119 Improving risk awareness for coronary heart disease among treated people with HIV might also augment surveillance efforts.120 In addition, early diagnosis could attenuate the need for pharmacotherapy in most cases, which is an important consideration for low-income and middle-income health-system resources.121
Second, there should be a pathway to care for people with HIV who screen positive for coronary heart disease risk factors including factors unique to or dominant in low-income and middle-income countries.84,122 This pathway should also improve access to essential medicines for coronary heart disease risk factors.60 An integrated care approach (ie, providing coronary heart disease care in the same clinic visit as HIV care) is feasible102 and would be optimal to improve linkage and retention in care, which is crucial for chronic disease management.123 Uptake of this approach remains slow in low-income and middle-income countries,100,124,125 and data on cost-effectiveness and clinical outcomes are sparse.101,102,118
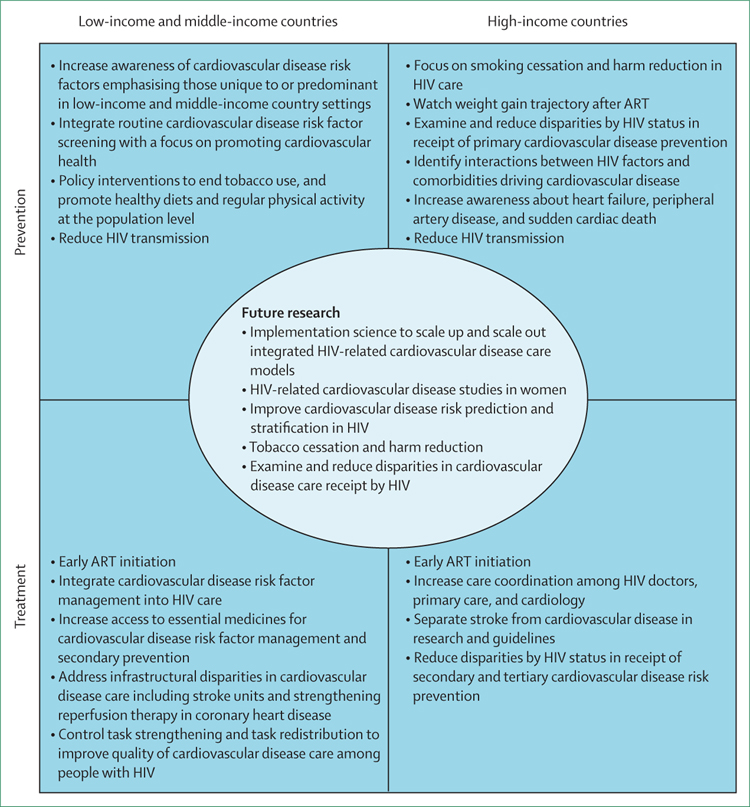
Third, timely policy interventions for people with HIV can decrease exposure to coronary heart disease risk factors such as tobacco, high cholesterol diets, and excessive alcohol usage. The prevalence of cigarette smoking, dyslipidaemia, and obesity are lower in many low-income and middle-income countries than high-income countries, although these factors are on the rise because of urbanisation.99 Public education, tobacco-product taxation, and legislation to reduce availability of trans-fatty acids are effective and cost-effective methods that are urgently required in low-income and middle-income countries as part of primary prevention for coronary heart disease with potential effects beyond HIV populations.126,127 Fourth, rigorous implementation approaches are required to inform how best to scale up and scale out interventions for preventing coronary heart disease among people with HIV in low-income and middle-income countries.102,128 These recommendations apply to other cardiovascular disease causes discussed in this Review (figure 3).
Figure 3: Intervention points to reduce HIV-associated cardiovascular disease risk stratified by income status.
ART=antiretroviral therapy.
We should leverage large HIV-population cohorts in sub-Saharan Africa and low-income and middle-income countries to develop tailored risk scores for stroke prevention. In regions with the highest burden of HIV infection in sub-Saharan Africa, priority should be given to developing stroke diagnostic and treatment pathways, centred around stroke units where brain imaging is available, and where complex management and rehabilitation can be provided. Government-operated stroke units are not commonplace in sub-Saharan Africa and are currently clustered in South Africa, Nigeria, and Ghana.129,130 Early admission to a stroke unit saves lives and reduces disability.131 Important barriers such as financial constraints, delay in presentation, sociocultural factors, and denial of a stroke are more frequent factors encountered in sub-Saharan Africa.132
Research to understand the disparity in heart failure outcomes for people with HIV is needed. Screening of individuals at greatest risk for heart failure62 should occur in the same environment as the screening for HIV and related conditions. Care delivery for heart failure could thus mimic some of the successful innovations from the HIV-care framework such as task redistribution. In Rwanda, nurses have been successfully deployed for decentralised heart failure diagnosis and care delivery since 2006, showing their ability to apply basic echocardiography skills to diagnose common presentations of heart failure.133 By using tailored algorithms to manage heart failure, patients in this nurse-led programme had a 36% 5-year mortality rate.134
Public education about the signs and symptoms of peripheral artery disease should be targeted toward people with HIV in low-income and middle-income countries to reduce the occurrence of late disease presentation and incidence of avoidable amputations. Increased awareness of sudden death and underlying mechanisms is needed. The development of tools for risk stratification and symptom identification as a preventive strategy135 is warranted.
High-income countries
First, more research is needed to identify optimal thrombotic risk reduction strategies for HIV in regions where the comorbid burden of cardiovascular disease and HIV is greatest. Second, lifestyle modification, particularly smoking cessation and reduction, should be emphasised in HIV care to reduce atherosclerotic cardiovascular disease risk. Similarly maintaining a healthy diet is essential to avoiding excess weight gain and related metabolic complications, particularly after ART initiation.42 Implementing these lifestyle optimisation strategies is challenging, but an opportunity exists to embed such strategies with HIV primary care. Third, appropriate management of cardiovascular disease risk factors is essential for diabetes, hypertension, and dyslipidaemia, in particular, with pharmacotherapy as indicated. Improved understanding of HIV-related cardiovascular disease risk factors is also important. Fourth, the impact of anti-inflammatory strategies, which might decrease atherosclerotic cardiovascular disease in the general population,136 merit additional investigation in the setting of HIV. Attention should be paid to sex-based differences in response to HIV and the potential effect on cardiovascular disease risk. Strategies to lower inflammation that could be HIV-specific include therapies aimed at limiting or repairing intestinal barrier dysfunction, co-infection with cytomegalovirus, or HIV cure. Fifth, the cardiovascular benefit of early initiation of ART, and cardiovascular disease risk associated with newer ART regimens remain unknown.
Recognising stroke as a separate outcome to cardiovascular disease, in line with the 2017 WHO International Classification of Diseases reclassification,75 will help to focus risk scores and population effect metrics from clinical trials and cohort studies in people with HIV. Additionally, mechanistic studies with a focus on intracranial HIV-associated vasculopathy are needed to guide drug discoveries.
It is important to supplement existing epidemiological data on increased heart failure in HIV with deeper clinical phenotyping of heart failure presentations and triggers among people with HIV. These studies would enable identification of targets for prevention and early interventions aimed at controlling the onset and progression of heart failure in HIV. Effective therapies for heart failure with preserved ejection fraction are still absent in the general population. Investigating the immunopathogenesis of HIV-associated heart failure might inform broader understanding and targeting of immunological and inflammatory contributors to heart failure with preserved ejection fraction.
A more complete estimation of the prevalence, incidence, and severity of peripheral artery disease among people with HIV is needed. Mechanistic studies should evaluate the mediators of increased peripheral artery disease and sudden cardiac death for people with HIV. Such studies provide insight for treatment modalities beyond ensuring HIV viral suppression, immune restoration, and management of traditional risk factors for atherosclerosis. Smoking is a strong modifiable risk factor for peripheral artery disease. Efforts to reduce smoking in people living with HIV are almost certain to reduce peripheral artery disease incidence and progression. Additionally, increased awareness about peripheral artery disease and sudden cardiac death is needed. One approach to increase awareness that is worth investigating is routine ankle-brachial index screening.
Conclusion
In summary, a geographical imbalance between HIV disease burden and the populations included in published HIV-related cardiovascular disease research exists. There is strong evidence linking HIV infection to myocardial infarction, stroke, and heart failure. There are scarce data that also link HIV infection to peripheral artery disease and sudden cardiac death. Although mechanisms for these associations might be the same for all people with HIV, the distribution of cardiovascular disease risk factors varies by geographical location. These variations result in different profiles of cardiovascular disease risk in low-income and middle-income countries compared with high-income countries. To reduce cardiovascular disease risk globally among people with HIV calls for thoughtful balancing of public and individual health approaches, evidence-informed strategies, expert health-care and patient opinions, and basic translational and implementation research in areas with the highest HIV burden.
Supplementary Material
Search strategy and selection criteria.
References for this Review were identified by PubMed searches from June 10, 2019, to Nov 1, 2019, for HIV or AIDS and types of cardiovascular disease. Search terms included “HIV”, “human immunodeficiency virus”, “AIDS”, “acquired immunodeficiency syndrome”, “myocardial infarction”, “stroke”, “coronary heart disease”, “heart failure”, “peripheral artery disease”, “ankle brachial index”, “sudden cardiac death”, “atherosclerosis”, “Africa”, “Kenya”, “Uganda”, “Malawi”, “Nigeria”, “Thailand”, “India”, “China”, “Russia”, “Asia”, “South America”, “low income country”, “middle income country”, “LMIC”, and “meta-analysis”. Manuscripts that were from the last 5 years and assessed incident clinical events, or included meta-analyses comprehensively summarising existing data were preferred. Only papers published in English were reviewed.
Footnotes
Declaration of interests
PH reports honoraria from Gilead Sciences and Merck outside the submitted work. LAB reports grants from Global Health Catalyst (Africa Non-Communicable Diseases Open Lab), outside the submitted work. All other authors declare no competing interests.
Editorial note: the Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
For the World Population Maps see https://www.ined.fr/en/everything_about_population/graphs-maps/world-maps-interactiv/
For the WHO Map Gallery see http://gamapserver.who.int/mapLibrary/app/searchResults.aspx
Contributor Information
Kaku So-Armah, Boston University School of Medicine, Boston, MA, USA.
Laura A Benjamin, UCL Queen Square Institute of Neurology, University College London, London, UK; Institute of Infection and Global Health, University of Liverpool, Liverpool, UK.
Gerald S Bloomfield, Duke Global Health Institute, Duke University, Durham, North Carolina, NC, USA.
Matthew J Feinstein, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
Prof Priscilla Hsue, University of California, San Francisco, CA, USA.
Benson Njuguna, Moi Teaching and Referral Hospital, Eldoret, Kenya.
Matthew S Freiberg, Prof, Vanderbilt University Medical Center, Nashville VA Medical Center, VA Tennessee Valley Healthcare System, Nashville, TN, USA.
References
- 1.Islam FM, Wu J, Jansson J, Wilson DP. Relative risk of cardiovascular disease among people living with HIV: a systematic review and meta-analysis. HIV Med 2012; 13: 453–68. [DOI] [PubMed] [Google Scholar]
- 2.Shah ASV, Stelzle D, Lee KK, et al. Global burden of atherosclerotic cardiovascular disease in people living with HIV. Circulation 2018; 138: 1100–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smit M, Brinkman K, Geerlings S, et al. Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis 2015; 15: 810–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marcus JL, Leyden WA, Chao CR, et al. HIV infection and incidence of ischemic stroke. AIDS 2014; 28: 1911–19. [DOI] [PubMed] [Google Scholar]
- 5.Benjamin LA, Corbett EL, Connor MD, et al. HIV, antiretroviral treatment, hypertension, and stroke in Malawian adults: a case-control study. Neurology 2016; 86: 324–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alonso A, Barnes AE, Guest JL, Shah A, Shao IY, Marconi V. HIV infection and incidence of cardiovascular diseases: an analysis of a large healthcare database. J Am Heart Assoc 2019; 8: e012241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lang S, Mary-Krause M, Cotte L, et al. Increased risk of myocardial infarction in HIV-infected patients in France, relative to the general population. AIDS 2010; 24: 1228–30. [DOI] [PubMed] [Google Scholar]
- 8.Freiberg MS, Chang CH, Skanderson M, et al. Association between HIV infection and the risk of heart failure with reduced ejection fraction and preserved ejection fraction in the antiretroviral therapy era: results from the veterans aging cohort study. JAMA Cardiol 2017; 2: 536–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feinstein MJ, Steverson AB, Ning H, et al. Adjudicated heart failure in HIV-infected and uninfected men and women. J Am Heart Assoc 2018; 7: e009985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beckman JA, Duncan MS, Alcorn CW, et al. Association of human immunodeficiency virus infection and risk of peripheral artery disease. Circulation 2018; 138: 255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tseng ZH, Secemsky EA, Dowdy D, et al. Sudden cardiac death in patients with human immunodeficiency virus infection. J Am Coll Cardiol 2012; 59: 1891–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai YJ, Chen YY, Huang HH, Ko MC, Chen CC, Yen YF. Incidence of cardiovascular diseases in a nationwide HIV/AIDS patient cohort in Taiwan from 2000 to 2014. Epidemiol Infect 2018; 146: 2066–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alvi RM, Neilan AM, Tariq N, et al. The risk for sudden cardiac death among patients living with heart failure and human immunodeficiency virus. JACC Heart Fail 2019; 7: 759–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freiberg MS, Chang CC, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 2013; 173: 614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crane HM, Paramsothy P, Drozd DR, et al. Types of myocardial infarction among human immunodeficiency virus-infected individuals in the United States. JAMA Cardiol 2017; 2: 260–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abelman RA, Mugo BM, Zanni MV. Conceptualizing the risks of coronary heart disease and heart failure among people aging with HIV: sex-specific considerations. Curr Treat Options Cardiovasc Med 2019; 21: 41. [DOI] [PubMed] [Google Scholar]
- 17.Paisible AL, Chang CC, So-Armah KA, et al. HIV infection, cardiovascular disease risk factor profile, and risk for acute myocardial infarction. J Acquir Immune Defic Syndr 2015; 68: 209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdallah A, Chang JL, O’Carroll CB, et al. Stroke in human immunodeficiency virus-infected individuals in sub-Saharan Africa (SSA): a systematic review. J Stroke Cerebrovasc Dis 2018; 27: 1828–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cedarbaum E, Ma Y, Scherzer R, et al. Contributions of HIV, hepatitis C virus, and traditional vascular risk factors to peripheral artery disease in women. AIDS 2019; 33: 2025–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamdem F, Mapoure Y, Hamadou B, et al. Prevalence and risk factors of peripheral artery disease in black Africans with HIV infection: a cross-sectional hospital-based study. Vasc Health Risk Manag 2018; 14: 401–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fowkes FG, Thorogood M, Connor MD, Lewando-Hundt G, Tzoulaki I, Tollman SM. Distribution of a subclinical marker of cardiovascular risk, the ankle brachial index, in a rural African population: SASPI study. Eur J Cardiovasc Prev Rehabil 2006; 13: 964–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lumsden RH, Bloomfield GS. The causes of HIV-associated cardiomyopathy: a tale of two worlds. BioMed Res Int 2016; 2016: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaziano TA, Abrahams-Gessel S, Gomez-Olive FX, et al. Cardiometabolic risk in a population of older adults with multiple co-morbidities in rural South Africa: the HAALSI (health and aging in Africa: longitudinal studies of INDEPTH communities) study. BMC Public Health 2017; 17: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lagat DK, DeLong AK, Wellenius GA, et al. Factors associated with isolated right heart failure in women: a pilot study from western Kenya. Glob Heart 2014; 9: 249–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bloomfield GS, Khazanie P, Morris A, et al. HIV and noncommunicable cardiovascular and pulmonary diseases in low- and middle-income countries in the ART era: what we know and best directions for future research. J Acquir Immune Defic Syndr 2014; 67 (suppl 1): S40–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neuhaus J, Jacobs DR Jr, Baker JV, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis 2010; 201: 1788–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barbaro G, Di Lorenzo G, Grisorio B, Barbarini G. Cardiac involvement in the acquired immunodeficiency syndrome: a multicenter clinical-pathological study. Gruppo Italiano per lo Studio Cardiologico dei pazienti affetti da AIDS Investigators. AIDS Res Hum Retroviruses 1998; 14: 1071–77. [DOI] [PubMed] [Google Scholar]
- 28.Brenchley JM, Schacker TW, Ruff LE, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med 2004; 200: 749–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grody WW, Cheng L, Lewis W. Infection of the heart by the human immunodeficiency virus. Am J Cardiol 1990; 66: 203–06. [DOI] [PubMed] [Google Scholar]
- 30.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006; 12: 1365–71. [DOI] [PubMed] [Google Scholar]
- 31.Riddler SA, Smit E, Cole SR, et al. Impact of HIV infection and HAART on serum lipids in men. JAMA 2003; 289: 2978–82. [DOI] [PubMed] [Google Scholar]
- 32.Veenstra M, León-Rivera R, Li M, Gama L, Clements JE, Berman JW. Mechanisms of CNS viral seeding by HIV+ CD14+ CD16+ monocytes: establishment and reseeding of viral reservoirs contributing to HIV-associated neurocognitive disorders. MBio 2017; 8: e01280–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bertrand L, Méroth F, Tournebize M, Leda AR, Sun E, Toborek M. Targeting the HIV-infected brain to improve ischemic stroke outcome. Nat Commun 2019; 10: 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watters SA, Mlcochova P, Gupta RK. Macrophages: the neglected barrier to eradication. Curr Opin Infect Dis 2013; 26: 561–66. [DOI] [PubMed] [Google Scholar]
- 35.Benjamin LA, Allain TJ, Mzinganjira H, et al. The role of human immunodeficiency virus-associated vasculopathy in the etiology of stroke. J Infect Dis 2017; 216: 545–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El-Sadr WM, Lundgren J, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med 2006; 355: 2283–96. [DOI] [PubMed] [Google Scholar]
- 37.Worm SW, Sabin C, Weber R, et al. Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D:A:D) study. J Infect Dis 2010; 201: 318–30. [DOI] [PubMed] [Google Scholar]
- 38.Gatell JM, Assoumou L, Moyle G, et al. Switching from a ritonavir-boosted protease inhibitor to a dolutegravir-based regimen for maintenance of HIV viral suppression in patients with high cardiovascular risk. AIDS 2017; 31: 2503–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ryom L, Lundgren JD, El-Sadr W, et al. Cardiovascular disease and use of contemporary protease inhibitors: the D:A:D international prospective multicohort study. Lancet HIV 2018; 5: e291–300. [DOI] [PubMed] [Google Scholar]
- 40.Solomon D, Sabin CA, Mallon PWG, Winston A, Tariq S. Cardiovascular disease in women living with HIV: a narrative review. Maturitas 2018; 108: 58–70. [DOI] [PubMed] [Google Scholar]
- 41.Bourgi K, Rebeiro PF, Turner M, et al. Greater weight gain in treatment naïve persons starting dolutegravir-based antiretroviral therapy. Clin Infect Dis 2019; ciz407. [DOI] [PMC free article] [PubMed]
- 42.Herrin M, Tate JP, Akgün KM, et al. Weight gain and incident diabetes among HIV-infected veterans initiating antiretroviral therapy compared with uninfected individuals. J Acquir Immune Defic Syndr 2016; 73: 228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holtzman C, Armon C, Tedaldi E, et al. Polypharmacy and risk of antiretroviral drug interactions among the aging HIV-infected population. J Gen Intern Med 2013; 28: 1302–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fantoni M, Autore C, Del Borgo C. Drugs and cardiotoxicity in HIV and AIDS. Ann N Y Acad Sci 2001; 946: 179–99. [DOI] [PubMed] [Google Scholar]
- 45.Sun D, Wu Y, Yuan Y, Wang Y, Liu W, Yang J. Is the atherosclerotic process accentuated under conditions of HIV infection, antiretroviral therapy, and protease inhibitor exposure? Meta-analysis of the markers of arterial structure and function. Atherosclerosis 2015; 242: 109–16. [DOI] [PubMed] [Google Scholar]
- 46.D’Ascenzo F, Cerrato E, Calcagno A, et al. High prevalence at computed coronary tomography of non-calcified plaques in asymptomatic HIV patients treated with HAART: a meta-analysis. Atherosclerosis 2015; 240: 197–204. [DOI] [PubMed] [Google Scholar]
- 47.Vos AG, Hoeve K, Barth RE, et al. Cardiovascular disease risk in an urban African population: a cross-sectional analysis on the role of HIV and antiretroviral treatment. Retrovirology 2019; 16: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sarfo FS, Nichols M, Gebregziabher M, et al. Evaluation of vascular event risk while on long-term anti-retroviral suppressive therapy [EVERLAST]: protocol for a prospective observational study. eNeurologicalSci 2019; 15: 100189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nonterah EA, Boua PR, Klipstein-Grobusch K, et al. Classical cardiovascular risk factors and HIV are associated with carotid intima-media thickness in adults from Sub-Saharan Africa: findings from H3Africa AWI-Gen study. J Am Heart Assoc 2019; 8: e011506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.So-Armah KA, Lim JK, Lo Re V, et al. FIB-4 stage of liver fibrosis predicts incident heart failure among HIV-infected and uninfected patients. Hepatology 2017; 66: 1286–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Braithwaite RS, Bryant KJ. Influence of alcohol consumption on adherence to and toxicity of antiretroviral therapy and survival. Alcohol Res Health 2010; 33: 280–87. [PMC free article] [PubMed] [Google Scholar]
- 52.Khambaty T, Stewart JC, Gupta SK, et al. Association between depressive disorders and incident acute myocardial infarction in human immunodeficiency virus–infected adults: veterans aging cohort study. JAMA Cardiol 2016; 1: 929–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.White JR, Chang CC, So-Armah KA, et al. Depression and human immunodeficiency virus infection are risk factors for incident heart failure among veterans: veterans aging cohort study. Circulation 2015; 132: 1630–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Forde KA, Haynes K, Troxel AB, et al. Risk of myocardial infarction associated with chronic hepatitis C virus infection: a population-based cohort study. J Viral Hepat 2012; 19: 271–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang H, Peng G, Bai J, et al. Cytomegalovirus infection and relative risk of cardiovascular disease (ischaemic heart disease, stroke, and cardiovascular death): a meta-analysis of prospective studies up to 2016. J Am Heart Assoc 2017; 6: e005025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee SA, Sinclair E, Hatano H, et al. Impact of HIV on CD8+ T cell CD57 expression is distinct from that of CMV and aging. PLoS One 2014; 9: e89444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burkholder GA, Tamhane AR, Salinas JL, et al.Underutilization of aspirin for primary prevention of cardiovascular disease among HIV-infected patients. Clin Infect Dis 2012; 55: 1550–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pearce D, Ani C, Espinosa-Silva Y, et al. Comparison of in-hospital mortality from acute myocardial infarction in HIV sero-positive versus sero-negative individuals. Am J Cardiol 2012; 110: 1078–84. [DOI] [PubMed] [Google Scholar]
- 59.Mosepele M, Hemphill LC, Palai T, et al. Cardiovascular disease risk prediction by the American College of Cardiology (ACC)/American Heart Association (AHA) atherosclerotic cardiovascular disease (ASCVD) risk score among HIV-infected patients in sub-Saharan Africa. PLoS One 2017; 12: e0172897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hyle EP, Mayosi BM, Middelkoop K, et al. The association between HIV and atherosclerotic cardiovascular disease in sub-Saharan Africa: a systematic review. BMC Public Health 2017; 17: 954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Friis-Møller N, Ryom L, Smith C, et al. An updated prediction model of the global risk of cardiovascular disease in HIV-positive persons: the data-collection on adverse effects of anti-HIV drugs (D:A:D) study. Eur J Prev Cardiol 2016; 23: 214–23. [DOI] [PubMed] [Google Scholar]
- 62.Feinstein MJ, Hsue PY, Benjamin LA, et al. Characteristics, prevention, and management of cardiovascular disease in people living with HIV: a scientific statement from the American Heart Association. Circulation 2019; 140: e98–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ministry of Health. Kenya national guidelines for cardiovascular diseases management. 2018. http://www.health.go.ke/wp-content/uploads/2018/06/Cardiovascular-guidelines-2018_A4_Final.pdf (accessed Jan 24, 2020).
- 64.Klug E, Raal FJ, Marais AD, et al. South African dyslipidaemia guideline consensus statement: 2018 update a joint statement from the South African Heart Association (SA Heart) and the Lipid and Atherosclerosis Society of Southern Africa (LASSA). S Afr Med J 2018; 108: 973–1000. [PubMed] [Google Scholar]
- 65.Department of Health Republic of South Africa. National consolidated guidelines for the prevention of mother-to-child transmission of HIV (PMTCT) and the management of HIV in children, adolescents and adults. 2014. http://www.kznhealth.gov.za/family/HIV-Guidelines-Jan2015.pdf (accessed Jan 24, 2020).
- 66.Ministry of Health. Malawi HIV testing services guidelines. 2016. https://aidsfree.usaid.gov/sites/default/files/htc_malawi_2016.pdf (accessed Jan 24, 2020).
- 67.Aberg JA, Gallant JE, Ghanem KG, Emmanuel P, Zingman BS, Horberg MA. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 58: 1–10. [DOI] [PubMed] [Google Scholar]
- 68.European AIDS Clinical Society. Guidelines version 9·1. 2018. https://www.eacsociety.org/files/2018_guidelines-9.1-english.pdf (accessed Jan 24, 2020).
- 69.British HIV Association. BHIVA guidelines for the routine investigation and monitoring of adult HIV-1-positive individuals (2019 interim update). 2019. https://www.bhiva.org/file/DqZbRxfzlYtLg/Monitoring-Guidelines.pdf (accessed Jan 24, 2020). [DOI] [PubMed]
- 70.Triant VA, Perez J, Regan S, et al. Cardiovascular risk prediction functions underestimate risk in HIV infection. Circulation 2018; 137: 2203–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020; 41: 111–88. [DOI] [PubMed] [Google Scholar]
- 72.Bryer A, Connor MD, Haug P, et al. The South African guideline for the management of ischemic stroke and transient ischemic attack: recommendations for a resource-constrained health care setting. Int J Stroke 2011; 6: 349–54. [DOI] [PubMed] [Google Scholar]
- 73.Mateen FJ, Post WS, Sacktor N, et al. Long-term predictive value of the Framingham risk score for stroke in HIV-positive vs HIV-negative men. Neurology 2013; 81: 2094–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bae HJ, Yoon BW, Kang DW, et al. Correlation of coronary and cerebral atherosclerosis: difference between extracranial and intracranial arteries. Cerebrovasc Dis 2006; 21: 112–19. [DOI] [PubMed] [Google Scholar]
- 75.Shakir R, Norrving B. Stroke in ICD-11: the end of a long exile. Lancet 2017; 389: 2373. [DOI] [PubMed] [Google Scholar]
- 76.Ryom L, Boesecke C, Bracchi M, et al. Highlights of the 2017 European AIDS Clinical Society (EACS) guidelines for the treatment of adult HIV-positive persons version 9.0. HIV Med 2018; 19: 309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Benjamin LA, Bryer A, Lucas S, et al. Arterial ischemic stroke in HIV: defining and classifying etiology for research studies. Neurol Neuroimmunol Neuroinflamm 2016; 3: e254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gutierrez J, Goldman J, Dwork AJ, Elkind MS, Marshall RS, Morgello S. Brain arterial remodeling contribution to nonembolic brain infarcts in patients with HIV. Neurology 2015; 85: 1139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dravid AN, Natrajan K, Kulkarni MM, et al. Discordant CSF/plasma HIV-1 RNA in individuals on virologically suppressive antiretroviral therapy in Western India. Medicine (Baltimore) 2018; 97: e9969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Circulation 2018; 138: e272–391. [DOI] [PubMed] [Google Scholar]
- 81.Aboyans V, Ricco JB, Bartelink MEL, et al. 2017 ESC Guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries endorsed by: the European Stroke Organization (ESO) the task force for the diagnosis and treatment of peripheral arterial diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J 2018; 39: 763–816. [DOI] [PubMed] [Google Scholar]
- 82.Chamie G, Kwarisiima D, Clark TD, et al. Leveraging rapid community-based HIV testing campaigns for non-communicable diseases in rural Uganda. PLoS One 2012; 7: e43400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vedanthan R, Kamano JH, Bloomfield GS, Manji I, Pastakia S, Kimaiyo SN. Engaging the entire care cascade in Western Kenya: a model to achieve the cardiovascular disease secondary prevention roadmap goals. Glob Heart 2015; 10: 313–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Njuguna B, Vorkoper S, Patel P, et al. Models of integration of HIV and noncommunicable disease care in sub-Saharan Africa: lessons learned and evidence gaps. AIDS 2018; 32 (suppl 1): S33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Patel P, Speight C, Maida A, et al. Integrating HIV and hypertension management in low-resource settings: lessons from Malawi. PLoS Med 2018; 15: e1002523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kwarisiima D, Atukunda M, Owaraganise A, et al. Hypertension control in integrated HIV and chronic disease clinics in Uganda in the SEARCH study. BMC Public Health 2019; 19: 511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sliwa K, Carrington MJ, Becker A, Thienemann F, Ntsekhe M, Stewart S. Contribution of the human immunodeficiency virus/acquired immunodeficiency syndrome epidemic to de novo presentations of heart disease in the Heart of Soweto Study cohort. Eur Heart J 2012; 33: 866–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bloomfield GS, DeLong AK, Akwanalo CO, et al. Markers of atherosclerosis, clinical characteristics, and treatment patterns in heart failure: a case-control study of middle-aged adult heart failure patients in rural Kenya. Glob Heart 2016; 11: 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Serhal M, Longenecker CT. Preventing heart failure in inflammatory and immune disorders. Curr Cardiovasc Risk Rep 2014; 8: 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.van Marle J, Mistry PP, Botes K. HIV-occlusive vascular disease. S Afr J Surg 2009; 47: 36–42. [PubMed] [Google Scholar]
- 91.Talle MA, Bonny A, Scholtz W, et al. Status of cardiac arrhythmia services in Africa in 2018: a PASCAR sudden cardiac death task force report. Cardiovasc J Afr 2018; 29: 115–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sherer R, Solomon S, Schechter M, Nachega JB, Rockstroh J, Zuniga JM. HIV provider-patient communication regarding cardiovascular risk: results from the AIDS Treatment for Life International Survey. J Int Assoc Provid AIDS Care 2014; 13: 342–45. [DOI] [PubMed] [Google Scholar]
- 93.Kelly SG, Krueger KM, Grant JL, et al. Statin prescribing practices in the comprehensive care for HIV-infected patients. J Acquir Immune Defic Syndr 2017; 76: e26–29. [DOI] [PubMed] [Google Scholar]
- 94.Riestenberg RA, Furman A, Cowen A, et al. Differences in statin utilization and lipid lowering by race, ethnicity, and HIV status in a real-world cohort of persons with human immunodeficiency virus and uninfected persons. Am Heart J 2019; 209: 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hanna DB, Jung M, Xue X, et al. Trends in nonlipid cardiovascular disease risk factor management in the women’s interagency HIV study and association with adherence to antiretroviral therapy. AIDS Patient Care STDS 2016; 30: 445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Feinstein MJ, Kim JH, Bibangambah P, et al. Ideal cardiovascular health and carotid atherosclerosis in a mixed cohort of HIV-infected and uninfected Ugandans. AIDS Res Hum Retroviruses 2017; 33: 49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.AbdelRazek MA, Gutierrez J, Mampre D, et al. Intravenous thrombolysis for stroke and presumed stroke in human immunodeficiency virus-infected adults: a retrospective, multicenter US study. Stroke 2018; 49: 228–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Uriel N, Nahumi N, Colombo PC, et al. Advanced heart failure in patients infected with human immunodeficiency virus: is there equal access to care? J Heart Lung Transplant 2014; 33: 924–30. [DOI] [PubMed] [Google Scholar]
- 99.Patel P, Rose CE, Collins PY, et al. Noncommunicable diseases among HIV-infected persons in low-income and middle-income countries: a systematic review and meta-analysis. AIDS 2018; 32 (suppl 1): S5–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rabkin M, Nishtar S. Scaling up chronic care systems: leveraging HIV programs to support noncommunicable disease services. J Acquir Immune Defic Syndr 2011; 57 (suppl 2): S87–90. [DOI] [PubMed] [Google Scholar]
- 101.Wroe EB, Kalanga N, Mailosi B, et al. Leveraging HIV platforms to work toward comprehensive primary care in rural Malawi: the integrated chronic care clinic. Healthc (Amst) 2015; 3: 270–76. [DOI] [PubMed] [Google Scholar]
- 102.Ojo T, Lester L, Iwelunmor J, et al. Feasibility of integrated, multilevel care for cardiovascular diseases (CVD) and HIV in low- and middle-income countries (LMICs): a scoping review. PLoS One 2019; 14: e0212296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Collaborators GBDS. Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019; 18: 439–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ntsekhe M, Mayosi BM. Cardiac manifestations of HIV infection: an African perspective. Nat Clin Pract Cardiovasc Med 2009; 6: 120–27. [DOI] [PubMed] [Google Scholar]
- 105.Mills KT, Bundy JD, Kelly TN, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation 2016; 134: 441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sliwa K, Davison BA, Mayosi BM, et al. Readmission and death after an acute heart failure event: predictors and outcomes in sub-Saharan Africa: results from the THESUS-HF registry. Eur Heart J 2013; 34: 3151–59. [DOI] [PubMed] [Google Scholar]
- 107.Agu CE, Uchendu IK, Nsonwu AC, Okwuosa CN, Achukwu PU. Prevalence and associated risk factors of peripheral artery disease in virologically suppressed HIV-infected individuals on antiretroviral therapy in Kwara state, Nigeria: a cross sectional study. BMC Public Health 2019; 19: 1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bonny A, Tibazarwa K, Mbouh S, et al. Epidemiology of sudden cardiac death in Cameroon: the first population-based cohort survey in sub-Saharan Africa. Int J Epidemiol 2017; 46: 1230–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Klein DB, Leyden WA, Xu L, et al. Declining relative risk for myocardial infarction among HIV-positive compared with HIV-negative individuals with access to care. Clin Infect Dis 2015; 60: 1278–80. [DOI] [PubMed] [Google Scholar]
- 110.Baldé A, Lang S, Wagner A, et al. Trends in the risk of myocardial infarction among HIV-1-infected individuals relative to the general population in France: Impact of gender and immune status. PLoS One 2019; 14: e0210253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Masiá M, Padilla S, García JA, et al. Decreasing rates of acute myocardial infarction in people living with HIV: a nationwide cohort study in Spain, 2004–2015. HIV Med 2018; 19: 491–96. [DOI] [PubMed] [Google Scholar]
- 112.Shahmanesh M, Schultze A, Burns F, et al. The cardiovascular risk management for people living with HIV in Europe: how well are we doing? AIDS 2016; 30: 2505–18. [DOI] [PubMed] [Google Scholar]
- 113.Smith CJ, Ryom L, Weber R, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet 2014; 384: 241–48. [DOI] [PubMed] [Google Scholar]
- 114.Feinstein MJ, Bahiru E, Achenbach C, et al. Patterns of cardiovascular mortality for HIV-infected adults in the United States: 1999 to 2013. Am J Cardiol 2016; 117: 214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ovbiagele B, Nath A. Increasing incidence of ischemic stroke in patients with HIV infection. Neurology 2011; 76: 444–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Junttila MJ, Hookana E, Kaikkonen KS, Kortelainen ML, Myerburg RJ, Huikuri HV. Temporal trends in the clinical and pathological characteristics of victims of sudden cardiac death in the absence of previously identified heart disease. Circ Arrhythm Electrophysiol 2016; 9: e003723. [DOI] [PubMed] [Google Scholar]
- 117.Hookana E, Junttila MJ, Puurunen VP, et al. Causes of nonischemic sudden cardiac death in the current era. Heart Rhythm 2011; 8: 1570–75. [DOI] [PubMed] [Google Scholar]
- 118.Nugent R, Barnabas RV, Golovaty I, et al. Costs and cost-effectiveness of HIV/noncommunicable disease integration in Africa: from theory to practice. AIDS 2018; 32 (suppl 1): S83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rabkin M, Palma A, McNairy ML, et al. Integrating cardiovascular disease risk factor screening into HIV services in Swaziland: lessons from an implementation science study. AIDS 2018; 32 (suppl 1): S43–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Patel P, Sabin K, Godfrey-Faussett P. Approaches to improve the surveillance, monitoring, and management of noncommunicable diseases in hiv-infected persons: viewpoint. JMIR Public Health Surveill 2018; 4: e10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Divala OH, Amberbir A, Ismail Z, et al. The burden of hypertension, diabetes mellitus, and cardiovascular risk factors among adult Malawians in HIV care: consequences for integrated services. BMC Public Health 2016; 16: 1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mitambo C, Khan S, Matanje-Mwagomba BL, et al. Improving the screening and treatment of hypertension in people living with HIV: an evidence-based policy brief by Malawi’s knowledge translation platform. Malawi Med J 2017; 29: 224–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Knight L, Schatz E, Mukumbang FC. “I attend at Vanguard and I attend here as well”: barriers to accessing healthcare services among older South Africans with HIV and non-communicable diseases. Int J Equity Health 2018; 17: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Muddu M, Tusubira AK, Sharma SK, Akiteng AR, Ssinabulya I, Schwartz JI. Integrated hypertension and HIV care cascades in an HIV treatment program in eastern Uganda: a retrospective cohort study. J Acquir Immune Defic Syndr 2019; 81: 552–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Iwelunmor J, Ezechi O, Obiezu-Umeh C, et al. Capabilities, opportunities and motivations for integrating evidence-based strategy for hypertension control into HIV clinics in southwest Nigeria. PLoS One 2019; 14: e0217703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shang C, Lee HM, Chaloupka FJ, Fong GT, Thompson M, O’Connor RJ. Association between tax structure and cigarette consumption: findings from the International Tobacco Control Policy Evaluation (ITC) project. Tob Control 2019; 28 (suppl 1): s31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hyseni L, Bromley H, Kypridemos C, et al. Systematic review of dietary trans-fat reduction interventions. Bull World Health Organ 2017; 95: 821–830G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kemp CG, Weiner B, Sherr K, et al. Implementation science for evidence-based integration of HIV and non-communicable disease services in Sub-Saharan Africa: a systematic review. AIDS 2018; 32 (suppl 1): S93–S105. [DOI] [PubMed] [Google Scholar]
- 129.Burton A South Africa: stroke units out of the blue. Lancet Neurol 2016; 15: 359–60. [DOI] [PubMed] [Google Scholar]
- 130.Jenkins C, Ovbiagele B, Arulogun O, et al. Knowledge, attitudes and practices related to stroke in Ghana and Nigeria: A SIREN call to action. PLoS One 2018; 13: e0206548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Howells DW, Donnan GA. Where will the next generation of stroke treatments come from? PLoS Med 2010; 7: e1000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Baatiema L, de-Graft Aikins A, Sav A, Mnatzaganian G, Chan CKY, Somerset S. Barriers to evidence-based acute stroke care in Ghana: a qualitative study on the perspectives of stroke care professionals. BMJ Open 2017; 7: e015385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kwan GF, Bukhman AK, Miller AC, et al. A simplified echocardiographic strategy for heart failure diagnosis and management within an integrated noncommunicable disease clinic at district hospital level for sub-Saharan Africa. JACC Heart Fail 2013; 1: 230–36. [DOI] [PubMed] [Google Scholar]
- 134.Eberly LA, Rusingiza E, Park PH, et al. 10-Year heart failure outcomes from nurse-driven clinics in rural sub-Saharan Africa. J Am Coll Cardiol 2019; 73: 977–80. [DOI] [PubMed] [Google Scholar]
- 135.Marijon E, Uy-Evanado A, Dumas F, et al. Warning symptoms are associated with survival from sudden cardiac arrest. Ann Intern Med 2016; 164: 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017; 377: 1119–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.