Graphical abstract
Keywords: SARS-CoV-2, COVID-19, Aspergillus, Candida, Pneumocystis, Histoplasma, Mucormycosis, Cryptococcus, Fungal infections
Abstract
Since December 2019 SARS-CoV-2 infections have affected millions of people worldwide. Along with the increasing number of COVID-19 patients, the number of cases of opportunistic fungal infections among the COVID-19 patients is also increasing. There have been reports of the cases of aspergillosis and candidiasis in the COVID-19 patients. The COVID-19 patients have also been affected by rare fungal infections such as histoplasmosis, pneumocystosis, mucormycosis and cryptococcosis. These fungal infections are prolonging the stay of COVID-19 patients in hospital. In this study several published case reports, case series, prospective and retrospective studies were investigated to explore and report the updated information regarding candidiasis, crytptococcosis, aspergillosis, mucormycosis, histoplasmosis, and pneumocystosis infections in COVID-19 patients. In this review, the risk factors of these co-infections in COVID-19 patients have been reported. There have been reports that the comorbidities and the treatment with corticoids, monoclonal antibodies, use of mechanical ventilation, and use of antibiotics during COVID-19 management are associated with the emergence of fungal infections in the COVID-19 patients. Hence, this review analyses the role of these therapies and comorbidities in the emergence of these fungal infections among COVID-19 patients. This review will help to comprehend if these fungal infections are the result of the co-morbidities, and treatment protocol followed to manage COVID-19 patients or directly due to the SARS-CoV-2 infection. The analysis of all these factors will help to understand their role in fungal infections among COVID-19 patients which can be valuable to the scientific community.
Introduction
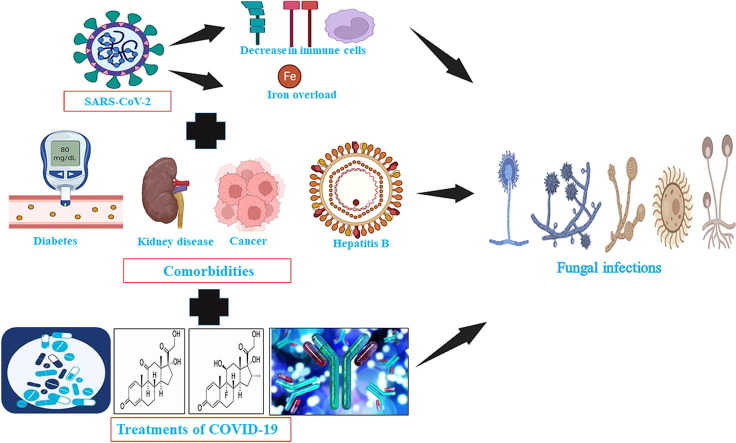
The first case of COVID-19 was reported in Wuhan, China in December 2019 (Spiteri et al., 2019). Since then; it has spread worldwide and taken the form of a pandemic. As of 6th June 2022, approximately 529,410,287 people have been infected and 6,296,771 people have succumbed to COVID-19 (WHO, WHO Coronavirus (COVID-19) Dashboard, 2021). The SARS-CoV-2 infection causes fever; dry cough, dyspnea, diarrhoea, headache, muscle pain and malaise in mild and moderate cases (Hassan et al., 2020). About 5 % of the patients affected by COVID-19 become critically ill suffering from severe pneumonia and acute respiratory distress syndrome thus requiring admission to the intensive care unit and mechanical ventilation (Murthy et al., 2020). Along with the SARS-CoV-2 infections; the COVID-19 patients are being co-infected with various other pathogens. There have been reports of viral bacterial and fungal co-infections among COVID-19 patients (Lai et al., 2020). There have been increasing reports of candidiasis; aspergillosis and mucormycosis in the COVID-19 patients (Hughes et al., 2020, Lahmer et al., 2021, Mehta and Pandey, 2020). These fungal co-infections affect the course of COVID-19 disease. Studies show that these infections are prolonging the stay of COVID-19 patients in hospitals and also increase the mortality rate (Lahmer et al., 2021, Koehler et al., 2020). Also; during the earlier viral pandemic caused by H1N1 and SARS-CoV-1 there was an increase in fungal infections (Patti et al., 2020).
This review of literature aims to explore and report the updated information regarding candidiasis, cryptococcosis, aspergillosis, mucormycosis, histoplasmosis, and pneumocystosis infections in COVID-19 patients. In this review, the risk factors of these co-infections in COVID-19 patients have been reported. Apart from the co-morbidities, the treatment with corticoids, antibiotics, monoclonal antibodies, and the use of mechanical ventilation during COVID-19 management could also cause fungal infections in the COVID-19 patients. The use of inexpensive glucocorticoids and anti-inflammatory therapies can also make the COVID-19 patients predisposed to secondary fungal infections (Garg et al., 2021, Kimmig et al., 2020). The use of antibiotics during the treatment of COVID-19 will overwhelm the normal microflora, which will allow the establishment of pathogenic fungi (Gandra et al., 2021). Chakraborti et al have reported that long-term mechanical ventilation is related to pulmonary and urinary fungal infections (Chakraborti et al., 2018). Hence, we also aimed to review if the treatment with corticoids, monoclonal antibodies, use of mechanical ventilation, and use of antibiotics during COVID-19 treatment along with other co-morbidities were associated with various fungal in COVID-19 patients. The effects of these co-infections on the course of COVID-19 such as prolonged stay in ICU have also been discussed. The review also provides information regarding co-morbidities of the patients who were diagnosed with SARS-CoV-2 and later on were infected with pathogenic fungi. This will help to comprehend if the fungal infections were the result of the co-morbidities, treatment protocol followed to manage COVID-19 patients or directly due to the SARS-CoV-2 infection. The information about the duration after which the fungal infections were diagnosed after the confirmation of SARS-CoV-2 infection has also been provided. The analysis of all these factors will help to understand their role in fungal infections among COVID-19 patients which can be valuable to the scientific community. The antifungal therapy used for the treatment of these infections has also been summarized which can be beneficial to the health practitioners.
Risk factors of fungal infections in COVID-19 patients
Most of the fungal infections are caused by the fungi of exogenous origin. However, the fungi found endogenously inside the host or mycobiome which comprises a very small proportion of the total microbiota have been complice in the onset of different diseases (Huffnagle and Noverr, 2013). The fungal microbiome can act as a reservoir for pathogenic fungi where they exist without causing diseases for a long time as a commensal but whenever the host is immunocompromised or under antibiotic treatment, they bloom to cause harmful diseases. Candida species are the most common colonizers of the mucosal surfaces, 30–70 % of healthy adults carry them, and under favourable conditions such as during antibiotic treatment and immunosuppression, they can cause candidemia and mycoses. Similarly, Pneumocystis and Cryptococcus neoformans can also exist as commensal in the lung but cause life-threatening infections during immunosuppression (Huffnagle and Noverr, 2013).
COVID-19 patients with comorbidities such as diabetes, hypertension, malignancy, multiple uses of antibiotics, chronic obstructive pulmonary diseases (COPD), Hepatitis B infection, chronic kidney diseases, renal failure, cerebrovascular diseases and cardiovascular diseases are at high risk of having poor clinical outcomes (Guan et al., 2020, Villanueva-Lozano et al., 2021). Furthermore, the critically ill COVID-19 patients have low levels of CD4+ and CD8+ T cells and monocytes, thus making them immunocompromised (Monneret et al., 2020, Tavakolpour et al., 2020, Yang et al., 2020). The comorbidities along with the compromised immunity of COVID-19 patients can make them more susceptible to fungal infections. COVID-19 causes hyper-ferritinemic syndrome where there is an increase in ferritin levels which eventually causes excess release of free iron and iron overload (John et al., 2021). The excessive release of free iron along with iron overload is some of the major risk factors of mucormycosis (John et al., 2021). Prolonged stays of COVID-19 patients at the hospital, mechanical ventilation and admission to intensive care units can also increase the risk of fungal infections in the COVID-19 patients (Song et al., 2020). The use of inexpensive glucocorticoids and anti-inflammatory therapies can also make the COVID-19 patients predisposed to secondary fungal infections (Garg et al., 2021, Kimmig et al., 2020). The use of corticosteroids in COVID-19 patients makes them predisposed to fungal infections such as mucormycosis by subduing the immune system and elevating the level of blood glucose (Gandra et al., 2021). The use of immunosuppressive drugs in COVID-19 patients with organ transplants can also make the patients prone to fungal infections (Khatri et al., 2021). Apart from immune dysregulation and the aforementioned risk factors, lack of oral hygiene can also increase the chances of candidiasis in the oral parts such as the tongue, gingiva and palate of the COVID-19 patients (Iranmanesh et al., 2020). Moreover. the viral infections also cause immunosuppression and damage to the pulmonary epithelium which can facilitate the fungal infection (Koehler et al., 2020). Ichai et al have hypothesized that the use of negative pressure in the ICU which is used to protect the staff from SARS-CoV-2 infections can also make the COVID-19 patients prone to opportunistic Aspergillus species infections (Ichai et al., 2020). Improper disinfection and cleaning can also contribute to the cases of nosocomial fungal infections in COVID-19 patients (Bhatt et al., 2021). Furthermore, the use of antibiotics during the course of treatment of COVID-19 will overwhelm the normal microflora, which will allow the establishment of pathogenic fungi (Gandra et al., 2021). Moreover, the hypoxia induced by SARS-CoV2 infections can also further deteriorate the tissues affected by angioinvasion in cases of mucormycosis (Gandra et al., 2021).
Cryptococcosis, histoplasmosis and pneumocystosis following SARS-CoV-2 infections
Cryptococcus species are a major cause of highly fatal cryptococcal meningitis which mostly affects immunocompromised patients and has a high mortality rate of 21 % in general patients (Brizendine et al., 2013, Iyer et al., 2021). Almost 95 % of the infections are caused by the pathogenic yeast Cryptococcus neoformans (serotype A) while the remaining infections are due to C. neoformans (serotype B) or Cryptococcus gatti (Maziarz and Perfect, 2016). Generally, impaired immunity, HIV infection, cancer, solid organ transplant and use of steroid therapy make the patients more susceptible to Cryptococcus species infections (Henao-Martínez et al., 2016). Histoplasmosis is caused by Histoplasma capsulatum and it is endemic in Latin America and the United States of America (Bertolini et al., 2020). Similarly, pneumocystosis is caused by Pneumocystosis jirovecii. Both pneumocystosis and histoplasmosis affect immunocompromised individuals with low CD4 cell counts such as HIV patients, individuals with malignancies and organ transplants (Hochhegger et al., 2021). Studies have reported that severe COVID-19 patients have low CD4 cell counts (Chen et al., 2020, Jiang et al., 2020). Moreover, some reports show that SARS-CoV-2 infections cause lymphopenia which makes the patients more susceptible to pneumocystosis and other secondary fungal infections (Blaize et al., 2020). These studies suggest that the severe SARS-CoV-2 infection predisposes the patients to histoplasmosis due to the lack of CD4 cells which are vital for maintaining a robust immune response. As SARS-CoV2 infections are also reported to affect the immune status of the patients and corticosteroids as well as other immunomodulatory drugs are routinely being used by clinicians to manage the COVID-19 patients, it is more likely that COVID-19 patients will become more prone to these fungal infections. PUBMED, Scopus, Web of Science, and Google Scholar databases were searched using the keywords (“Cryptococcus” OR “Cryptococcosis” OR “Cryptococcal meningitis”), (“Pneumocystis” OR “Pneumocystosis” OR “Pneumocystis jirovecii”), (“Histoplasma”, “Histoplasmosis” OR “Histoplasma capsulatum”) and (“SARS-CoV-2” OR “COVID-19”) AND (“SARS-CoV-2” and “COVID-19”) without date until June 10th 2021, to find relevant studies.
Altogether five case reports were identified where COVID-19 patients were also suffering from Cryptococcosis. One case report was excluded, as in that case, the patient did not acquire Cryptococcus infection after SARS-COV-2 infection (Chiappe Gonzalez et al., 2020). The patient has already been infected with C. neoformans and the patient acquired the SARS-CoV-2 infection after his stay at the hospital (Chiappe Gonzalez et al., 2020). In the other four cases, the patients acquired the C. neoformans infection after they tested positive for SARS-CoV-2. Similar to the results by Henao-Martinez et al, most of the cases were male (3/4) (Henao-Martínez et al., 2016). All the patients were above 60 years old and are more likely to have impaired immunity (Saltzman and Peterson, 1987). Hence, they have more chances of getting affected by SARS-CoV-2 and fungal infections such as cryptococcosis. All of the patients were under corticosteroid therapy and one patient received tocilizumab which may have made the patients more susceptible to Cryptococcus species infection by subduing the immune system. Furthermore, all the patients were under mechanical ventilation and two of the patients also received antibiotics during COVID-19 treatment. Three of the patients were presented with comorbidities that may also have aided in the SARS-CoV-2 infection which could have eventually led to the secondary fungal infection. C. neoformans can also exist as commensal in the lung and cause life-threatening infections during immunosuppression (Huffnagle and Noverr, 2013). There was a report of pulmonary C. neoformans infection in a patient following SARS-CoV-2 infection (Cafardi et al., 2021). This case suggests that there is a likelihood of activation of latent C. neoformans infections in patients due to the overwhelming effect of SARS-CoV-2 and the corticoids on the patient’s immune system. There are also chances that the patients were infected with hospital-borne or environmental C. neoformans which in due course overwhelmed the patients due to co-morbidities, SARS-CoV-2 infection and use of steroids. As, there is limited number of reports and due to the involvement of plethora of factors, it is very difficult to establish the role of SARS-CoV-2 and the therapy used in its management to these secondary infections. Overall, three out of the four patients died. However, it is difficult to affirm the cause of deaths due to the Cryptococcus infections. The details of the patients reported in these case reports are represented in Table 1 .
Table 1.
Reported cases of cryptococcosis, histoplasmosis and pneumocystosis following SARS-CoV-2 infections along with the information regarding the co-morbidities and therapy used to manage the COVID-19 patients.
| Case/Country | Age | Gender | Co-morbidities | Use of antibiotics during COVID-19 management | Use of immune-modulatory drugs during COVID-19 management | Mechanical ventilation during COVID-19 management | Days after which infection was confirmed following a positive RT-PCR result | Antifungal treatment | Outcome | Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| Disseminated/Cryptococcus neoformans/USA | 75 | Male | Cirrhosis of liver, hypertension, kidney transplant recipient | Yes; ceftriaxone and clarithromycin | Yes, Prednisone | Yes | 12 days | Fluconazole | Death after 18 days of admission due to septic shock | (Passarelli et al., 2020) |
| Disseminated/Cryptococcus neoformans/Qatar | 60 | Male | Diabetes, hypertension, ischemic heart disease | NA | Yes, methylprednisolone, hydrocortisone, tocilizumab | Yes | 48 days | Flucytosine with Amphotericin B | Death due to sepsis after 10 days of cryptococcemia | (Khatib et al., 2020) |
| Meningoencephalitis/Cryptococcus neoformans/USA | 73 | Female | None | Yes, azithromycin | Yes, dexamethasone | Yes | 12 days | Flucytosine with Amphotericin B | Survived | (Ghanem et al., 2021) |
| Pulmonary/Cryptococcus neoformans/USA | 78 | Male | COPD, hypertension | No | Yes; methylprednisolone | Yes | 20 days | Amphotericin B changed to isavuconazole due to renal injury | Death after 39 days of hospital admission | (Cafardi et al., 2021) |
| Histoplasma capsulatum/USA | 62 | Female | Diabetes | Yes; levofloxacin and azithromycin | Yes, tocilizumab and dexamethasone | NA | 26 days | Liposomal amphotericin B which was later changed to isavuconazole | Survived. Discharged after 89 days from hospital. | (Cafardi et al., 2021) |
| Pulmonary/Histoplasma capsulatum/Brazil | 20 | Male | None | NA | NA | NA | 4 months | Itraconazole | Survived | (de Macedo et al., 2021) |
| Pulmonary/Histoplasma capsulatum/Brazil | 32 | Male | None | NA | Yes; methylprednisolone | NA | NA | Itraconazole | Survived | (de Macedo et al., 2021) |
| China/Pneumocystis jirovecii | 72 | Female | Rheumatoid arthritis | Yes; cefoperazone | Yes; methylprednisolone, tocilizumab | No | 20 days | Caspofungin | Survived | (Cai et al., 2020) |
| Italy/Pneumocystis jirovecii | 65 | Male | Kidney transplant recipient, diabetes, hypertension | Yes; azithromycin and piperacillin/tazobactam | Yes; methylprednisolone | No | 2 days | Trimethoprim-sulfamethoxazole | Death due to respiratory failure and multiple organ dysfunction | (De Francesco et al., 2020) |
| Italy/Pneumocystis jirovecii | Male | No | Yes; ceftaroline | Yes; dexamethasone | Yes | 18 days | trimethoprim-sulphamethoxazole | Survived | (Viceconte et al., 2021) | |
| USA/Pneumocystis jirovecii | 38 | Male | HIV | No | Yes; prednisone | Yes | 15 days | Trimethoprim-sulfamethoxazole | Death after 22 days of hospitalization due to respiratory failure | (Merchant et al., 2021) |
Altogether seven case reports were identified where COVID-19 patients were also suffering from Histoplasmosis. Three of the case reports were from Brazil, two from Argentina and 1 from the USA. In four of the case reports including 3 patients suffering from AIDS, histoplasmosis was already detected before the diagnosis of COVID-19 patients (Bertolini et al., 2020, Basso et al., 2021, Messina et al., 2020, Stasiak et al., 2021). In the remaining three cases histoplasmosis was confirmed after the patients underwent treatment for COVID-19 (Table 1) (Cafardi et al., 2021, de Macedo et al., 2021). Two of the cases were adults (20 and 32 years) and had no co-morbidity. The third patient in which histoplasmosis was confirmed after the confirmation of COVID-19 was old (62 years) and diabetic. In this old and diabetic patient, the impaired immunity could have also aided in the secondary Histoplasma infection. One of the patients came in contact with the potential environmental source of Histoplasma capsulatum after recovering from SARS-CoV-2 infection (de Macedo et al., 2021). This case report suggests that the pulmonary damage due to SAR-CoV-2 infection could have facilitated the Histoplasma infection after the possible inhalation of Histoplasma conidia from the environment by the patient. The case of the other two patients suggests that the treatment of COVID-19 patients with corticosteroids or monoclonal antibodies could have reactivated the latent Histoplasma or made the patients more prone to infection from environmental or nosocomial sources. As, there are only three cases of Histoplasmosis in patients after SARS-CoV-2 infections, the hypotheses that the SARS-CoV-2 infection and the therapy used to manage COVID-19 are associated with Histoplasma infection cannot be corroborated. As of now, it is impossible to conclude whether there is coincidental, causal or contributory relation between the effect of SARS-CoV-2 on patients’ immunity and treatment protocol with the cases of histoplasmosis. However, the clinicians should consider in mind the possibility of H. capsulatum infections in COVID-19 patients during or even after the treatment of COVID-19, especially in USA and South American countries. All the patients whose cases are reviewed in this study that had histoplasmosis infections following SARS-CoV-2 infection survived (Table 1).
Altogether 12 case reports were identified where COVID-19 patients were also diagnosed with Pneumocystis jirovecii infections. In two case reports identified in this study, there were concurrent SARS-CoV-2 and P. jirovecii infections in HIV patients (Coleman et al., 2020, Bhat et al., 2020). In another case report where a patient was admitted to the hospital due to COVID-19 complications was also found to be HIV positive, later this patient also tested positive for P. jirovecii infection (Mang et al., 2020). As, these patients were HIV positive, a major risk factor of pneumocystosis, it cannot be concluded that the P. jirovecii infections were a result of SARS-CoV-2 infection or the consequence of the therapy used to manage the COVID-19 patients. Similarly, in another case report also an 83 years old female patient with leucocytosis and lymphocytopenia was diagnosed with concurrent SAR-CoV-2 and P. jirovecii infections (Menon et al., 2020). In one case report, a 46 years old woman with Raynaud’s syndrome was admitted to the hospital due to pneumonia-like symptoms and was later diagnosed simultaneously with HIV, SARS-CoV-2 and P. jirovecii (Larzábal et al., 2020). In another case, a 52 years old male with several co-morbidities ischemic heart disease, chronic alcohol liver disease, hypertension, and hepatic steatosis died within 17 h after admission to the hospital and the necropsy revealed the simultaneous co-infection of SARS-CoV-2 and P. jirovecii (Jeican et al., 2021). In two of the case reports the patients (n = 3) were present with respiratory symptoms and suspected as COVID-19 patients (Choy and Wong, 2020, Kelly et al., 2020). However, later they tested positive for HIV and P. jirovecii was also detected which implied that the respiratory symptoms were due to pneumocytosis. Similar cases were also reported in Denmark where patients with suspected COVID-19 disease were later diagnosed with P. jirovecii pneumonia and HIV (Borchmann and Hansen, 2021). These two case reports that along with COVID-19 clinicians should also be wary about pathogens that mimic COVID-19 such as P. jirovecii. Two fatalities were reported, one with HIV infection and another with anti-melanoma differentiation-associated gene 5 juvenile dermatomyositis, who had a simultaneous diagnosis of SARS-CoV-2 and P. jirovecii (Broadhurst et al., 2021, Quintana-Ortega et al., 2021). Both these patients died because of respiratory failure and the contribution of these infections to disease severity was unclear (Broadhurst et al., 2021, Quintana-Ortega et al., 2021). The case reports reviewed in this section suggest in patients with HIV infection who present with pneumonia-like symptoms; the clinicians should test for both P. jirovecii and SARS-CoV-2 infections as these patients are more prone to these infections because of their weakened immunity. Furthermore, there is a chance of misdiagnosis as both COVID-19 and P. jirovecii show similar clinical and radiological features. The simultaneous infections of P. jirovecii and SARS-CoV-2 also make it difficult to understand the role of these pathogens in the disease progression in the patients. The case reports discussed above do not support the hypotheses that the pulmonary inflammation/pulmonary damage, immunosuppression caused by SAR-CoV-2 infections and therapy used to manage the COVID-19 patients facilitated the pneumocystosis in COVID-19 patients as these patients were either already infected with P. jirovecii or the SARS-COV-2 infection and P. jirovecii were diagnosed simultaneously. Furthermore, these patients were also diagnosed with HIV or immunosuppression which are risk factors for P. jirovecii infections.
However, in four case reports, P. jirovecii was detected after the patients were confirmed of SARS-CoV-2 infection and the treatment for COVID-19 had begun. The details of these case reports are listed in Table 1. The cases of P. jirovecii infections were confirmed after 2–20 days of a positive result for SARS-CoV-2 infection. All of the patients were under the steroids for management of COVID-19 and 3 patients were treated with antibiotics during COVID-19 treatment. One of the patients was HIV positive and another was the recipient of a kidney transplant (De Francesco et al., 2020, Merchant et al., 2021). As HIV and solid organ transplant are major risk factors for P. jirovecii infections it is difficult to determine whether the P. jirovecii infections were result of these co-morbidities or due to the SARS-CoV-2 infection and the therapy used to manage the COVID-19 patients. Based on just four case reports it becomes impossible to confirm if the P. jirovecii were the result of SARS-CoV-2 infection or the therapy used to manage the COVID-19 patients.
Treatment of cryptococcosis, histoplasmosis and pneumocytosis in COVID-19 patients
The cases of cryptococcosis in COVID-19 patients reviewed in this study were treated with fluconazole or the combination of Amphotericin B and flucytosine which are also recommended by Centres for Disease Control and Prevention (CDC), USA (CDC, Treatment for C. neoformans Infection, 2021). The COVID-19 patients who were also infected by Histoplasma capsulatum received liposomal amphotericin B and itraconazole for the treatment of histoplasmosis which is also recommended by an expert panel of Infectious Diseases Society of America for the management of histoplasmosis (Wheat et al., 2007). For the treatment of pneumocytosis in COVID-19 patients, trimethoprim-sulphamethoxazole was used in the cases analysed in this study. According to CDC, trimethoprim-sulphamethoxazole is the most common form of treatment for pneumocystosis in general patients (CDC, Pneumocystis pneumonia, 2021).
Mucormycosis following SARS-CoV-2 infection
Mucormycosis is mostly caused by fungi belonging to Mucor and Rhizopus species (CDC, Where Mucormycosis Comes From | Mucormycosis | CDC, 2021). Other fungi genera of the order Mucorales such as Rhizomucor, Apophysomyses, Absidia, Cunninghamella and Syncephalastrum can also cause mucormycosis (Prabhu and Patel, 2004). Generally, these fungi are not harmful, but in patients with compromised immunity, they can infect different body parts and become lethal (CDC, Where Mucormycosis Comes From | Mucormycosis | CDC, 2021). The incidence rate of mucormycosis is between 0.005 and 1.7 cases per million population and the fatality rate is 46 % in the general population (Jeong et al., 2019). It is common in transplant recipients and patients with haematological malignancy (Jeong et al., 2019). These infections are very rare and hard to diagnose, and a delay in their diagnosis can significantly increase the 30-day mortality rate (Werthman-Ehrenreich, 2021). During mucormycosis, the spores are inhaled in the airways or seeded on the susceptible epithelial tissues where these spores germinate and undergo angioinvasion by using the general host conditions like iron overload, hyperglycemia, neutropenia and ketoacidosis (Ahmadikia et al., 2021, Petrikkos and Tsioutis, 2018). Then, the mucormycosis causes necrosis, thrombosis and local hemorrhage which eventually gets disseminated to different organs thus increasing the risk of fatality (Ahmadikia et al., 2021, Petrikkos and Tsioutis, 2018). As SARS-CoV-2 infection also affects the patients’ immunity, the cases of mucormycosis are expected to increase in the COVID-19 patients similar to the cases of Aspergillosis and Candidiasis. Furthermore, the use of immunomodulatory drugs, broad-spectrum antibiotics and corticosteroids during the treatment of COVID 19 patients also makes them more vulnerable to mucormycosis. Generally, mucormycosis can be rhino-orbital-cerebral, cutaneous, pulmonary, gastro-intestinal, and disseminated (Riley et al., 2016). In COVID-19 patients rhino-orbital-cerebral, rhino-orbital, pulmonary and gastrointestinal mucormycosis have been reported (Mehta and Pandey, 2020, Werthman-Ehrenreich, 2021, Monte Junior et al., 2020, Placik et al., 2020). In a review of 41 cases of COVID-19 associated mucormycosis (CAM) by John et al, it was reported that CAM was mostly diagnosed (94 % of patients) with diabetes mellitus (John et al., 2021). A retrospective, interventional study performed by Sen et al reported 6 cases of rhino-orbital mucormycosis in COVID-19 patients at two different ophthalmic centers in India (Sen et al., 2021). These patients were male, aged between 46 and 73 years, diabetic, and received systemic corticosteroids for treating COVID-19 (Sen et al., 2021). All the patients who were under liposomal amphotericin B treatment along with posaconazole eventually survived, however, all of them lost their vision (Sen et al., 2021). Ravani et al reported 31 cases of mucormycosis in COVID-19 patients (Ravani et al., 2021). All these patients were diabetic, liposomal amphotericin B was used to treat all these patients and mortality was reported in one case (Ravani et al., 2021). However, they did not report the days after which mucormycosis was diagnosed after positive RT-PCR test for SARS-CoV-2, the use of corticosteroids during the treatment of COVID-19 and individual age and sex of the COVID-19 patients with mucormycosis (Ravani et al., 2021). Table 3 provides information about the cases in which mucormycosis was diagnosed following SARS-CoV-2 infection. Apart from the cases mentioned in Table 2 different cases mentioned in various case series and retrospective studies were also included in this study (Sen et al., 2021, Ravani et al., 2021, Bayram et al., 2021, Nehara et al., 2021, Sarkar et al., 2021, Sharma et al., 2021, Moorthy et al., 2021).
Table 3.
Reported cases of mucormycosis following SARS-CoV-2 infections along with the information regarding the co-morbidities and therapy used to manage the COVID-19 patients.
| Case/Country | Age | Gender | Co-morbidities | Use of antibiotics during COVID-19 management | Use of immune-modulatory drugs during COVID-19 management | Mechanical ventilation during COVID-19 management | Days after which mucormycosis was confirmed following a positive RT-PCR result | Antifungal treatment | Outcome | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Rhino-orbital mucomycosis/India | 60 | Male | Diabetes | Yes; meropenem and vancomycin | Yes, dexamethasone and methylprednisolone | Yes | 10 | Amphotericin B | Death after 6 days of admission | (Mehta and Pandey, 2020) |
| Rhino-orbital mucormycosis/USA | 33 | Female | Diabetes, asthma, hypertension | Yes; piperacillin-tazobactam and Vancomycin | NA | NA | NA | Amphotericin B | Death after 26 days of admission | (Werthman-Ehrenreich, 2021) |
| Gastrointestinal Mucormycosis/Brazil | 86 | Male | Arterial hypertension | Yes, azithromycin and ceftriaxone | Yes, hydrocortisone | Yes | 5 days | No | Death after 7 days of admission | (Monte Junior et al., 2020) |
| Rhino-orbital mucormycosis/USA, Rhizopus, Proven | 60 | Male | Diabetes, asthma, hypertension, hyperlipidemia | Yes; cefepime and vancomycin | Yes; dexamethasone | Yes | 4 days | Liposomal Amphotericin B, posaconazole and caspofungin | Death after 31 days of admission | (Mekonnen et al., 2021) |
| Rhino-cerebral/USA | 41 | Male | Diabetes | Yes, cefepime | Yes | No | NA | Liposomal Amphotericin B | Discharged | (Alekseyev et al., 2021) |
| Pulmonary/USA/Rhizopus | 49 | Male | None | Yes; ceftriaxone and azithromycin | Yes; dexamethasone | Yes | 14 days | Amphotericin B | Death after 21 days of admission | (Placik et al., 2020) |
| Pulmonary/India/Probable/Rhizopus | 55 | Male | Diabetes, kidney disease | Yes; meropenem | Yes; dexamethasone | Yes | 21 days | Liposomal Amphotericin B | Discharged after 54 days | (Garg et al., 2021) |
| Pulmonary/Italy/Rhizopus | 66 | Male | Hypertension | Yes, piperacillin-tazobactam, levofloxacin, meropenem | No | Yes | 14 days | Liposomal Amphotericin B | Death after 62 days of admission | (Pasero et al., 2021) |
| Pulmonary/USA/Rhizopus | 79 | Male | Diabetes, hypertension | Yes; ceftriaxone and azithromycin | Yes; dexamethasone | Yes | 29 days | Liposomal Amphotericin B | Discharged after 36 days to acute care facility | (Johnson et al., 2021) |
| Disseminated mucormycosis/UK | 22 | Male | Obesity, hypothyroidism | Yes; azithromycin | Yes | NA | After 20 days in autopsy | NA | Death after 20 days of admission | (Krishna et al., 2021) |
| Rhino-orbital-cerebral mucormycosis/Iran | 40 | Female | None | Yes; vancomycin and meropenem | Yes; dexamethasone | Yes | 8 days | IV Amphotericin B | Death after 3 months of admission due to cerebral infection | (Veisi et al., 2022) |
| Rhino-orbital mucormycosis/Iran | 54 | Male | Diabetes | Yes, Levofloxacin | Yes; dexamethasone | Yes | 8 days | IV Amphotericin B | Survived | (Veisi et al., 2022) |
| Rhino-orbital/India/Rhizopus oryzaae | 38 | Male | None | No | Yes; dexamethasone and methylprednisolone | No | 18 days | Amphotericin B | Survived | (Maini et al., 2021) |
| Rhino-orbital/Mexico/Absidia | 24 | Female | Obesity, Diabetes | Yes; imipenem/linezolid | NA | Yes | NA | Amphotericin | Death due to septic shock | (Waizel-Haiat et al., 2021) |
| Cutaneous/USA/Rhizopus microspores | 68 | Male | Heart transplant recipient, diabetes, hypertension, chronic kidney disease | Yes; vancomycin and meropenem | NA | NA | 3 months | Liposomal Amphotericin B, posaconazole | Death after 175 days due to septic shock | (Khatri et al., 2021) |
| Rhino-orbital/India | 47 | Male | Renal transplant recipient, diabetes | NA | Yes, Tacrolimus, steroids. But for treating COVID-19 no steroids were used. | NA | 14 days | Liposomal amphotericin B | Death after 51 days of admission | (Meshram et al., 2021) |
| Pulmonary/India | 25 | Male | Renal transplant recipient, diabetes | NA | Yes, Tacrolimus, steroids, mycophenolic acid. But for treating COVID-19 no steroids were used. | NA | 10 days | Liposomal amphotericin B | Death after 49 days of admission | (Meshram et al., 2021) |
| Rhino-orbital-cerebral/USA | 36 | Male | Diabetes | No | Yes | No | NA | intravenous amphotericin, isavuconazole, and micafungin |
Death due to extension of infection to the cranial cavity after 4 days of hospital admission. | (Dallalzadeh et al., 2021) |
| Rhino-orbital-cerebral/USA | 48 | Male | Diabetes | NA | Yes, dexamethasone | NA | 6 days | Amphotericin B and isavuconazole | Death due to extension of infection to the cranial cavity | (Dallalzadeh et al., 2021) |
| Rhino-orbital/Iran | 61 | Female | No | No | Yes | No | 21 days | Amphotericin B | NA | (Karimi-Galougahi et al., 2019) |
| Rhino-orbital/India | 66 | Male | Diabetes | NA | Yes | NA | 12 days | Amphotericin B | Survived after orbital exenteration | (Rao et al., 2021) |
| Pulmonary/USA/Rhizopus azygosporus | 56 | Male | Renal disease | No | Yes; methylprednisolone and tocilizumab | No | 19 days | Liposomal Amphotericin B | Dead after 17 days in hospital due to cardiac arrest. | (Kanwar et al., 2021) |
| Pulmonary/Austria/Rhizopus microspores | 53 | Male | Acute myeloid leukaemia | Yes, piperacillin/tazobactam | Yes; prednisolone and tocilizumab | Yes | After 24 days in autopsy | No | Dead after 24 days | (Zurl et al., 2021) |
| Rhino-orbital/Spain/Rhizopus oryzae | 62 | Male | Diabetes, recipient of kidney transplant | Yes, ceftriaxone and azithromycin | Yes, dexamethasone | Yes | 17 days | Liposomal Amphotericin B, posaconazole | Survived | (Arana et al., (2021)) |
| Musculoskeletal/Spain/ Lichtheimia ramose | 48 | Male | Kidney disease, recipient of kidney transplant | Yes, azithromycin | Yes, tocilizumab | No | 21 days | Liposomal Amphotericin B and isavuconazole | Survived | (Arana et al., n/a (2021)) |
| Pulmonary/France/ Rhizopus microspores | 55 | Male | Follicular lymphoma | NA | Yes | Yes | 15 days | Liposomal Amphotericin B | Dead after 0 days in hospital | (Bellanger et al., 2021) |
| Rhino-orbital/Iran/Rhizopus oryzae | 50 | Female | Diabetes, hypertension | NA | Yes, dexamethasone | No | 26 days | Liposomal Amphotericin B | Survived | (Tabarsi et al., 2021) |
| Pulmonary/India | 72 | Male | Hypothyroid, hypertension, diabetes | Yes, imipenem | Yes, methylprednisolone | No | NA | Liposomal Amphotericin B and posaconazole | Survived | (Chennamchetty et al., 2021). |
| Chile | 62 | Male | None | Yes | Yes | Yes | 12 days | None | Survived | (Rabagliati et al., 2021) |
| Chile | 55 | Male | Diabetes, hypertension | Yes | Yes | Yes | 5 days | Liposomal Amphotericin B | Death | (Rabagliati et al., 2021) |
| USA/Pulmonary | 44 | Female | Diabetes | Yes, cefepime and vancomycin | Yes, methylprednisolone | No | 13 days | Liposomal Amphotericin B | Death after 17 days of admission | (Khan et al., 2020) |
| Rhino-orbital/Egypt | 65 | Female | Diabetes | NA | NA | NA | 14 days | Amphotericin B | Survived | (Ashour et al., 2021) |
| Rhino-orbital/Egypt | 67 | Male | Chronic Kidney disease | NA | NA | NA | 14 days | Amphotericin B | Death after 28 days of admission | (Ashour et al., 2021) |
| Rhino-orbital/Egypt | 42 | Male | Diabetes | NA | NA | NA | NA | Amphotericin B | Survived | (Ashour et al., 2021) |
| Rhino-orbital/Egypt | 63 | Female | Diabetes | NA | NA | NA | NA | Amphotericin B | Survived | (Ashour et al., 2021) |
| Rhino-orbital/Egypt | 41 | Female | Diabetes | NA | NA | NA | 14 days | Amphotericin B | Survived | (Ashour et al., 2021) |
| Rhino-orbital/Egypt | 42 | Male | Diabetes, Chronic Kidney disease, hypertension | NA | NA | Yes | 16 days | Amphotericin B | Death after 31 days of admission | (Ashour et al., 2021) |
| Rhino-orbital/Egypt | 50 | Male | Diabetes | NA | NA | NA | 14 days | NA | Death after 31 days of admission | (Ashour et al., 2021) |
Table 2.
Classification of the reported cases of mucormycosis following SARS-CoV-2 infections in different age groups.
| Age group | Number of cases (in %) |
|---|---|
| 20–29 | 5 (5.74) |
| 30–39 | 8 (9.19) |
| 40–49 | 18 (20.68) |
| 50–59 | 16 (18.39) |
| 60–69 | 25 (28.73) |
| 70–79 | 12 (13.79) |
| 80–89 | 3 (3.44) |
PUBMED, Web of Science, Scopus and Google Scholar databases were searched using the keywords such as (“Mucormycosis” OR “Mucor” OR “Black fungus” OR “Mucorales”) and (“SARS-CoV-2” OR “COVID-19”) without date until June 10, 2021. Altogether 128 cases of mucormycosis were identified in this study. Most of the cases were reported in males (103 out of 128). In 87 cases, age-related information was available. For these cases, the mean age was 59.55 years. The categorization of these cases in different age groups is shown in Table 2. The age group 60–69 had the highest cases of mucormycosis in patients with confirmed SARS-CoV-2 infections. Patients between the ages of 40–79 accounted for 81.6 % of the total cases. Rhino-orbital mucormycosis was most common in the patients (n = 114). Cutaneous (n = 1), pulmonary (n = 10), musculoskeletal (n = 1), disseminated (n = 1) and gastrointestinal mucormycosis (n = 1) were also detected. Diabetes was the most common comorbidity among these patients. 107 out of the 128 cases were diabetic (83.59 %). four of the patients had no comorbidities. Other comorbidities included hypertension, obesity, asthma, kidney disease, hypothyroidism, kidney disease, organ transplant reception and cancer. Amphotericin B was the most commonly used antifungal drug to treat mucormycosis infection. Out of all the cases, nine patients did not receive any steroids for the treatment of COVID-19 whereas 96 patients (75 %) received steroids such as dexamethasone, and methylprednisolone. For the rest of the cases, no data related to the use of steroids during COVID-19 treatment was available. Three of the cases had tocilizumab treatment for COVID-19. Out of these three cases, two had the use of both antibody and steroid therapy for treating COVID-19. For 47 cases data related to the days after which mucormycosis was confirmed following a positive RT-PCR result for SARS-CoV-2 was available (Table 4 ). Mucormycosis was detected in the patients between 3 days to 3 months of SARS-CoV-2 confirmation. The average days after which mucormycosis was confirmed following a positive RT-PCR result for SARS-CoV-2 is 16.95 days. 26 out of the 47 cases (55.31 %) of mucormycosis were detected after 11–20 days of a positive RT-PCR result for SARS-CoV-2. 25 of the cases required mechanical ventilation during COVID-19 treatment whereas 9 patients did not require mechanical ventilation; and for the rest of the cases, no related information about mechanical ventilation during COVID-19 management was available (Table 3). Recently Patel et al also reported that the inappropriate use of glucocorticoids and COVID-19 associated hypoxemia were independently associated with COVID-19 associated mucormycosis among patients in India (Patel et al., 2021). Furthermore, the immunosuppression and the increase of blood glucose levels in diabetic COVID-19 patients due to the use of corticosteroids creates ketoacidotic environment which fosters the growth of opportunistic pathogenic fungal infections (Petrikkos and Tsioutis, 2018, Dallalzadeh et al., 2021). Dallalzadeh et al also postulated that the SARS-CoV-2 associated immunosuppression and mechanical ventilation can be risk factors for mucormycosis in COVID-19 patients (Dallalzadeh et al., 2021). The cases studied in this review support the hypotheses that the immunosuppression caused by SARS-CoV-2 and the therapy used to control COVID-19 such as the use of glucocorticoids, monoclonal antibodies and mechanical ventilation are likely to be associated with mucormycosis infections in COVID-19 patients.
Table 4.
Days after which mucormycosis was confirmed following a positive RT-PCR result for SARS-CoV-2.
| Days | Number of cases (in %) |
|---|---|
| 0–10 | 11 (23.40) |
| 11–20 | 26 (55.31) |
| 21–30 | 8 (17.02) |
| 31+ | 2 (4.25) |
Treatment of mucormycosis in COVID-19 patients
In the cases reviewed in this study, mostly liposomal amphotericin was used for the treatment of mucormycosis in COVID-19 patients. A COVID-19 patient suffering from mucormycosis was transitioned from liposomal amphotericin to posaconazole due to acute kidney damage (Mekonnen et al., 2021). Similarly, the combination of amphotericin B, micafungin and isavuconazole has also been used to treat mucormycosis in COVID-19 patients (Dallalzadeh et al., 2021). The combination of liposomal amphotericin B with posaconazole has also been used for the treatment of mucormycosis in COVID-19 patients (Khatri et al., 2021). According to the European Confederation of Medical Mycology high dose liposomal amphotericin B is recommended as first-line treatment whereas moderate strength isavuconazole or posaconazole can be used as salvage treatment of mucormycosis in general patients (Cornely et al., 2019).
Infection of Candida species in COVID-19 patients
Candida species are opportunistic pathogens which can cause nosocomial infections in COVID-19 patients and further exacerbate the patient’s health condition. The COVID-19 patients are at increased risk of Candida infections due to their poor immunity and other risk factors discussed in the earlier section. PUBMED, Web of Science, Scopus and Google Scholar databases were searched using the keywords such as (“Candidiasis” OR “Candida infection” OR “Candida albicans”, “non-Candida albicans”) and (“SARS-CoV-2” OR “COVID-19”) without date until June 10, 2021. In a retrospective cohort study at two hospitals in the United Kingdom, 21.4 % of the respiratory samples collected from COVID-19 patients tested positive for secondary Candida infection (Hughes et al., 2020). The study suggested that these isolates were part of a normal microbiome rather than pulmonary candidiasis. However, in three patients who were admitted to the intensive care unit, there were three incidents of hospital-acquired, central line-associated Candida albicans infections (Hughes et al., 2020). Different studies have reported the oral manifestation of Candidiasis in the labial mucosa, buccal mucosa, gingiva, tongue, oropharynx, and palate of the COVID-19 patients (Corchuelo and Ulloa, 2020, Riad et al., 2021). Prevalence of Xerostomia (dry mouth) in the COVID-19 patients along with the inability of the patients to maintain proper oral hygiene could be the possible causes of oral Candidiasis (Riad et al., 2021). Apart from Candida albicans, other non-albicans Candida species have also been reported to be isolated from COVID-19 patients. Villanueva-Lozano et al reported the isolation of C. auris from blood and urine of the COVID-19 patients in the intensive care unit (ICU) of COVID-19 facility in Mexico (Villanueva-Lozano et al., 2021). Furthermore, three C. auris isolates were obtained from the environmental samples such as bed rails and infusion pumps (Villanueva-Lozano et al., 2021). These COVID-19 patients with C. auris co-infections were under mechanical ventilation and also had prolonged stay in ICU, and also had the insertion of urinary catheter and peripherally inserted central line (Villanueva-Lozano et al., 2021). 8 of the 15C. auris isolates were resistant to both Amphotericin B and fluconazole and an 83.3 % mortality rate was reported among the patients with Candidemia (Villanueva-Lozano et al., 2021). Similarly, C. auris isolates were obtained from deep tracheal aspirates of 7 patients who previously had COVID-19 pneumonia at an ICU unit in Lebanon (Allaw et al., 2021). All these patients with co-infection of C. auris were under mechanical ventilation, had prolonged stay at the hospital, were elderly, had central venous and urinary catheters and also had the intake of steroids (Allaw et al., 2021). In Florida, USA also C. auris were isolated from the 52 % of 67 COVID-19 patients (Kuehn, 2021). Chowdhary et al reported the isolation of C. auris from 10 COVID-19 patients in India where 6 out of these 10 patients eventually died; thus suggesting a very high fatality rate of 60 % (Chowdhary et al., 2020). Posteraro et al reported the detection of invasive C. glabrata infection in a diabetic patient who was also diagnosed with COVID-19 (Posteraro et al., 2020). However, after 13 days of treatment with caspofungin, the fungi developed pan-echinocandin resistance and the patient eventually died of septic shock before he could benefit from treatment with another antifungal therapy (Posteraro et al., 2020). From another COVID-19 patient who had prolonged stay at hospital in Oman also C. glabrata was isolated (Al-Hatmi et al., 2021). Also from the same intensive care unit, C. tropicalis along with C. albicans was isolated from the blood culture of a patient with COVID pneumonia (Al-Hatmi et al., 2021). In a study in Iran, apart from C. albicans and C. glabrata, other fungi such as C. krusei, C. parapsilosis, C. tropicalis and C. dublinienesis were isolated were isolated from the COVID-19 patients who also suffered from lymphopaenia (Salehi et al., 2020). These studies show that the C. albicans is the most common cause of Candidemia and there is an upsurge in the cases of non-albicans Candida species among the COVID-19 patients. The studies also show that the co-infection with the Candida species can worsen the disease course of COVID-19 and cause increase in the mortality.
Treatment of the Candida infections in COVID-19 patients
For the treatment of oral Candidiasis, various antifungal protocols have been used. Topical applications of nystatin and miconazole have been used for the successful treatment of oral Candidiasis in COVID-19 patients (Corchuelo and Ulloa, 2020, Riad et al., 2021). Intravenous administration of fluconazole has also been used in combination with oral/topical administration of nystatin or miconazole (Riad et al., 2021, Amorim dos Santos et al., 2020). Along with the antifungal drugs, Chlorhexidine gluconate (0.12 to 2 %) and hydrogen peroxide (1 %) were also prescribed to the COVID-19 patients suffering from oral Canididasis (Corchuelo and Ulloa, 2020, Riad et al., 2021, Amorim dos Santos et al., 2020). For the treatment of COVID-19 patients co-infected with C. auris echinocandins such as anidulafungin and caspofungin were used at an ICU in Lebanon (Allaw et al., 2021). Similarly, for the treatment of Candidemia, the combination of voriconazole and caspofungin; and a combination of amphotericin B and caspofungin has also been used (Al-Hatmi et al., 2021).
Infection of Aspergillus species in COVID-19 patients
Aspergillus species can cause invasive pulmonary aspergillosis, aspergilloma (fungal ball), allergic bronchopulmonary aspergillosis and tracheobronchial aspergillosis in general patients (Patti et al., 2020). Globally the cases of COVID-19 associated pulmonary aspergillosis (CAPD) have increased and it is associated with the exacerbation of the course of COVID-19 disease and also increasing mortality (Koehler et al., 2020). PUBMED, Web of Science, Scopus and Google Scholar databases were searched using the keywords such as (“Aspergillosis” OR “Aspergilloma” OR “Aspergillus”) and (“SARS-CoV-2” OR “COVID-19”) without date until June 10, 2021. Most of the cases of CAPD are caused by Aspergillus fumigatus followed by Aspergillus flavus (Lai and Yu, 2021). Along with these Aspergillus species, other species such as A. lentulus, A. niger, A. nidulans and A. citrinoterreus have also been reported (Bartoletti et al., 20212021, Falces‐Romero et al., 2020, Machado et al., 2021). In the study by Machado et al in Spain, CAPD was diagnosed in 0.3 % of the 2723 COVID-19 patients which accounted for 3.3 % of the 239 COVID-19 patients in the ICU (Machado et al., 2021). Arkel et al reported a high incidence rate of Aspergillosis (19.4 %) among 31 ICU patients (van Arkel et al., 2020). However, in a study in China, CAPA was diagnosed in 7.75 of the COVID-19 patients which accounted for 30.7 % of the ICU patients (Wang et al., 2020). In a recent review by Chong and Neu, the overall incidence rate of CAPD in COVID-19 patients was reported as 13 %, with a range from 2.5 to 35 % (Chong and Neu, 2021). The treatment of the COVID-19 patients with corticosteroids such as dexamethasone and monoclonal antibodies such as tocilizumab which can affect the patients’ immune system has contributed to the increase in the cases of CAPA (Salmanton-García et al., 2021). Furthermore, viral pneumonia elevates the risk of Aspergillus infections because of the damage caused to the airway epithelium and alveolar endothelium which can aid the Aspergillus invasion (Koehler et al., 2020, Herold et al., 2015). SARS-CoV-2 has also shown the ability to damage the integrity of human airway epithelial cell culture which suggests that the COVID-19 patients are at increased risk of pulmonary Aspergillosis (Zhu et al., 2020). The SARS-CoV-2 infection also causes loss of cilia in the airway epithelia (Zhu et al., 2020, Hao et al., 2020). These cilia play an important role in trapping and then transporting microbes such as Aspergillus out of the airway. Hence, the loss of cilia in the airway epithelia due to SARS-CoV-2 makes the COVID-19 more susceptible to co-infection from pulmonary Aspergillosis. In a report by Salmanton-Garcia, most of the cases (81.7 %) Aspergillosis were diagnosed within 10 days after the positive RT-PCR test for COVID-19 (Salmanton-García et al., 2021). Another study reported that Aspergillosis was diagnosed after a median of 15 days of mechanical ventilation (Machado et al., 2021). Chong and Neu reported the diagnosis of CAPA between 8 and 16 days from the onset of illness and 4–15 days from the ICU admission (Chong and Neu, 2021). Apart from invasive pulmonary aspergillosis, there have also been reports of fungal balls, and aspergilloma, in a COVID-19 patient (Patti et al., 2020). Fungal rhinosinusitis (inflammation of sinuses due to fungal infection) has also been reported in a COVID-19 patient in India which was caused by dual infection of Aspergillus fumigatus and Rhizopus (Sebastian et al., 2021). The patient who developed fungal sinusitis was under corticosteroid treatment and developed the fungal infection after 10–15 days of onset of COVID-19 illness (Sebastian et al., 2021).
Lahmer et al reported that the COVID-19 associated pulmonary aspergillosis (CAPA) is associated with the increased mortality rate (Lahmer et al., 2021). Their study showed that in the patients with CAPA the ICU mortality rate was 36 % in comparison to the COVID-19 patients without CAPA (9.5 % mortality rate) (Lahmer et al., 2021). Koehler et al also reported a similar outcome in their study where 44 % 30 days mortality rate was observed in CAPA patients in comparison to those without CAPA (19 % mortality rate) (Koehler et al., 2020). In a review of literature done by Machado et al, a mortality rate of 56.3 % was reported (Machado et al., 2021). Similarly, another study reported a very high CAPA mortality rate of 48.4 %, ranging between 22.2 % and 100 % mortality rate (Chong and Neu, 2021).
Treatment of aspergillosis in COVID-19 patients
Voriconazole is preferred for the treatment of patients with invasive Aspergillosis because of the fewer adverse effects and better results in patients in comparison to Amphotericin B (Herbrecht et al., 2002). The consensus guidance issued by the European Confederation for Medical Mycology (ECMM) and the International Society for Human and Animal Mycology (ISHAM) recommend intravenous administration of either voriconazole or isavuconazole for the treatment of COVID-19 associated pulmonary aspergillosis (Koehler et al., 2020). The ECMM/ISHAM group also recommends the use of liposomal Amphotericin B (3 mg/kg/day) if resistance to azoles is observed (Koehler et al., 2020). Salmanton-Garcia et al reported that the treatment of CAPD with voriconazole was associated with reduced death (Salmanton-García et al., 2021). For the treatment of fungal sinusitis voriconazole and liposomal Amphotericin B have been used and for treating fungal balls intravenous voriconazole has been used (Patti et al., 2020, Sebastian et al., 2021).
Conclusion
The studies discussed here show the increase in the cases of fungal infections among the COVID-19 patients. However, due to the limited number of cases/studies particularly among the COVID-19 patients with pneumocytosis, cryptococcosis or histoplasmosis, it becomes impossible to conclude if the fungal infections are due to SARS-CoV-2 infection, other co-morbidities or the therapy used to manage the COVID-19 patients. In the cases of mucormycosis, the studies reviewed here suggest that comorbidities such as diabetes along with the use of steroid therapy and monoclonal antibodies are likely to be associated with the increase of cases of mucormycosis among the COVID-19 patients. In the cases of aspergillosis and candidiasis in COVID-19 patients, also the co-morbidities and the therapy used to manage the COVID-19 play an important role. However, it is difficult to determine if the cases of these fungal infections and COVID-19 are causal, coincidental, or contributory. These studies suggest that the amalgamation of the effect of the SARS-CoV-2 on host immunity, co-morbidities, immunocompromised conditions along with the treatment regimen followed to manage the COVID-19 patients affect the immune system to create optimum conditions for various pathogenic fungal infections. The studies reviewed here also imply that these fungal infections are associated with the prolonged stay of the COVID-19 patients in the hospital. Further studies are required among both the healthy patients and patients with co-morbidities to establish or dismiss the role of SARS-CoV-2 infections and its treatment protocol in the increasing cases of secondary fungal infections, worldwide.
CRediT authorship contribution statement
Nahid Akhtar: Conceptualization. Atif Khurshid Wani: Conceptualization. Surya Kant Tripathi: Conceptualization. Ajit Prakash: Conceptualization. M. Amin-ul Mannan: Conceptualization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The lab funding from the Scientific and Engineering Research (SERB), Core Research Grant, file no. EMR/2017/002299, India is duly acknowledged.
References
- Ahmadikia K., Hashemi S.J., Khodavaisy S., Getso M.I., Alijani N., Badali H., Mirhendi H., Salehi M., Tabari A., Mohammadi Ardehali M., Kord M., Roilides E., Rezaie S. The double-edged sword of systemic corticosteroid therapy in viral pneumonia: A case report and comparative review of influenza-associated mucormycosis versus COVID-19 associated mucormycosis. Mycoses. 2021;64(8):798–808. doi: 10.1111/myc.13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alekseyev K., Didenko L., Chaudhry B. Rhinocerebral Mucormycosis and COVID-19 Pneumonia. J. Med. Cases. 2021;12:85–89. doi: 10.14740/jmc3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hatmi A.M.S., Mohsin J., Al-Huraizi A., Khamis F. COVID-19 associated invasive candidiasis. J. Infect. 2021;82:e45–e46. doi: 10.1016/j.jinf.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allaw F., Kara Zahreddine N., Ibrahim A., Tannous J., Taleb H., Bizri A.R., Dbaibo G., Kanj S.S. First Candida auris Outbreak during a COVID-19 Pandemic in a Tertiary-Care Center in Lebanon. Pathogens. 2021;10:157. doi: 10.3390/pathogens10020157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amorim dos Santos J., Normando A.G.C., Carvalho da Silva R.L., De Paula R.M., Cembranel A.C., Santos-Silva A.R., Guerra E.N.S. Oral mucosal lesions in a COVID-19 patient: New signs or secondary manifestations? Int. J. Infect. Dis. 2020;97:326–328. doi: 10.1016/j.ijid.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- C. Arana, R.E.C. Ramírez, M. Xipell, J. Casals, A. Moreno, S. Herrera, M. Bodro, F. Cofan, F. Diekmann, N. Esforzado, Mucormycosis associated with covid19 in two kidney transplant patients, Transpl. Infect. Dis. n/a (2021) e13652. https://doi.org/10.1111/tid.13652. [DOI] [PMC free article] [PubMed]
- Ashour M.M., Abdelaziz T.T., Ashour D.M., Askoura A., Saleh M.I., Mahmoud M.S. Imaging spectrum of acute invasive fungal rhino-orbital-cerebral sinusitis in COVID-19 patients: A case series and a review of literature. J. Neuroradiol. 2021;48(5):319–324. doi: 10.1016/j.neurad.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- M. Bartoletti, R. Pascale, M. Cricca, M. Rinaldi, A. Maccaro, L. Bussini, G. Fornaro, T. Tonetti, G. Pizzilli, E. Francalanci, L. Giuntoli, A. Rubin, A. Moroni, S. Ambretti, F. Trapani, O. Vatamanu, V.M. Ranieri, A. Castelli, M. Baiocchi, R. Lewis, M. Giannella, P. Viale, PREDICO Study Group, Epidemiology of Invasive Pulmonary Aspergillosis Among Intubated Patients With COVID-19: A Prospective Study, Clin. Infect. Dis. (2020). https://doi.org/10.1093/cid/ciaa1065. [DOI] [PMC free article] [PubMed]
- Basso R.P., Poester V.R., Benelli J.L., Stevens D.A., Zogbi H.E., Vasconcellos I.C.d.S., Pasqualotto A.C., Xavier M.O. COVID-19-Associated Histoplasmosis in an AIDS Patient. Mycopathologia. 2021;186(1):109–112. doi: 10.1007/s11046-020-00505-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayram N., Ozsaygılı C., Sav H., Tekin Y., Gundogan M., Pangal E., Cicek A., Özcan İ. Susceptibility of severe COVID-19 patients to rhino-orbital mucormycosis fungal infection in different clinical manifestations. Jpn. J. Ophthalmol. 2021;65(4):515–525. doi: 10.1007/s10384-021-00845-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellanger A.-P., Navellou J.-C., Lepiller Q., Brion A., Brunel A.-S., Millon L., Berceanu A. Mixed mold infection with Aspergillus fumigatus and Rhizopus microsporus in a severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) patient. Infect. Dis. Now. 2021 doi: 10.1016/j.idnow.2021.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolini M., Mutti M.F., Barletta J.A., Falak A., Cuatz D., Sisto A., Ragusa M.A., Fernandez Claros N.O., Rolón M.J. COVID-19 associated with AIDS-related disseminated histoplasmosis: a case report. Int. J. STD AIDS. 2020;31:1222–1224. doi: 10.1177/0956462420957518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat P., Noval M., Doub J.B., Heil E. Concurrent COVID-19 and Pneumocystis jirovecii pneumonia in a severely immunocompromised 25-year-old patient. Int. J. Infect. Dis. 2020;99:119–121. doi: 10.1016/j.ijid.2020.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt K., Agolli A., H. Patel M., Garimella R., Devi M., Garcia E., Amin H., Domingue C., Del Castillo R.G., Sanchez-Gonzalez M. High mortality co-infections of COVID-19 patients: mucormycosis and other fungal infections. Discoveries. 2021;9(1):e126. doi: 10.15190/d.2021.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaize M., Mayaux J., Luyt C.-E., Lampros A., Fekkar A. COVID-19–related Respiratory Failure and Lymphopenia Do Not Seem Associated with Pneumocystosis. Am. J. Respir. Crit. Care Med. 2020;202:1734–1736. doi: 10.1164/rccm.202007-2938LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchmann O., Hansen A.-B.-E. Pneumocystis pneumonia and HIV infection in two patients suspected with COVID-19. Ugeskr. Laeger. 2021;183:V09200673. [PubMed] [Google Scholar]
- K.D. Brizendine, J.W. Baddley, P.G. Pappas, Predictors of Mortality and Differences in Clinical Features among Patients with Cryptococcosis According to Immune Status, PLoS ONE. 8 (2013) e60431. https://doi.org/10.1371/journal.pone.0060431. [DOI] [PMC free article] [PubMed]
- Broadhurst A.G.B., Lalla U., Taljaard J.J., Louw E.H., Koegelenberg C.F.N., Allwood B.W. The diagnostic challenge of pneumocystis pneumonia and COVID-19 co-infection in HIV. Respirol. Case Rep. 2021;9 doi: 10.1002/rcr2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafardi J., Haas D., Lamarre T., Feinberg J. Opportunistic Fungal Infection Associated with COVID-19. Open Forum Infect. Dis. 2021 doi: 10.1093/ofid/ofab016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai S., Sun W., Li M., Dong L. A complex COVID-19 case with rheumatoid arthritis treated with tocilizumab. Clin. Rheumatol. 2020;39:2797–2802. doi: 10.1007/s10067-020-05234-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC, Pneumocystis pneumonia, (2021). https://www.cdc.gov/fungal/diseases/pneumocystis-pneumonia/index.html (accessed June 8, 2022).
- CDC, Treatment for C. neoformans Infection, (2021). https://www.cdc.gov/fungal/diseases/cryptococcosis-neoformans/treatment.html (accessed June 11, 2021).
- CDC, Where Mucormycosis Comes From | Mucormycosis | CDC, (2021). https://www.cdc.gov/fungal/diseases/mucormycosis/causes.html (accessed June 8, 2021).
- Chakraborti A., Jaiswal A., Verma P.K., Singhal R. A Prospective Study of Fungal Colonization and Invasive Fungal Disease in Long-Term Mechanically Ventilated Patients in a Respiratory Intensive Care Unit, Indian J. Crit. Care Med. Peer-Rev. Off. Publ. Indian Soc. Crit. Care Med. 2018;22:597–601. doi: 10.4103/ijccm.IJCCM_181_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Qi T., Liu L., Ling Y., Qian Z., Li T., Li F., Xu Q., Zhang Y., Xu S., Song Z., Zeng Y., Shen Y., Shi Y., Zhu T., Lu H. Clinical progression of patients with COVID-19 in Shanghai, China. J. Infect. 2020;80:e1–e6. doi: 10.1016/j.jinf.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- V.K. Chennamchetty, S. Adimulapu, B.P. Kola, M.D. Padua, A. C, M.K. Verma, M.V.R. Rao, Post-COVID pulmonary mucormycosis- A case report, IP Indian J. Immunol. Respir. Med. 6 (2021) 62–66. https://doi.org/10.18231/j.ijirm.2021.014.
- Chiappe Gonzalez A.J., Montenegro-Idrogo J.J., Vargas Vadillo A.R., Slee Torres M., Vargas Matos I., Resurrección Delgado C.P. Hospital-acquired SARS-CoV-2 pneumonia in a person living with HIV. Int. J. STD AIDS. 2020;31:1320–1322. doi: 10.1177/0956462420957528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong W.H., Neu K.P. Incidence, diagnosis and outcomes of COVID-19-associated pulmonary aspergillosis (CAPA): a systematic review. J. Hosp. Infect. 2021;113:115–129. doi: 10.1016/j.jhin.2021.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- A. Chowdhary, B. Tarai, A. Singh, A. Sharma, Multidrug-Resistant Candida auris Infections in Critically Ill Coronavirus Disease Patients, India, April-July 2020, Emerg. Infect. Dis. 26 (2020) 2694–2696. https://doi.org/10.3201/eid2611.203504. [DOI] [PMC free article] [PubMed]
- Choy C.Y., Wong C.S. It’s not all about COVID-19: pneumocystis pneumonia in the era of a respiratory outbreak. J. Int. AIDS Soc. 2020;23 doi: 10.1002/jia2.25533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman H., Snell L.B., Simons R., Douthwaite S.T., Lee M.J. COVID-19 and Pneumocystis jirovecii pneumonia: a diagnostic dilemma in HIV. AIDS Lond. Engl. 2020;34:1258–1260. doi: 10.1097/QAD.0000000000002571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corchuelo J., Ulloa F.C. Oral manifestations in a patient with a history of asymptomatic COVID-19: Case report. Int. J. Infect. Dis. 2020;100:154–157. doi: 10.1016/j.ijid.2020.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornely O.A., Alastruey-Izquierdo A., Arenz D., Chen S.C.A., Dannaoui E., Hochhegger B., Hoenigl M., Jensen H.E., Lagrou K., Lewis R.E., Mellinghoff S.C., Mer M., Pana Z.D., Seidel D., Sheppard D.C., Wahba R., Akova M., Alanio A., Al-Hatmi A.M.S., Arikan-Akdagli S., Badali H., Ben-Ami R., Bonifaz A., Bretagne S., Castagnola E., Chayakulkeeree M., Colombo A.L., Corzo-León D.E., Drgona L., Groll A.H., Guinea J., Heussel C.-P., Ibrahim A.S., Kanj S.S., Klimko N., Lackner M., Lamoth F., Lanternier F., Lass-Floerl C., Lee D.-G., Lehrnbecher T., Lmimouni B.E., Mares M., Maschmeyer G., Meis J.F., Meletiadis J., Morrissey C.O., Nucci M., Oladele R., Pagano L., Pasqualotto A., Patel A., Racil Z., Richardson M., Roilides E., Ruhnke M., Seyedmousavi S., Sidharthan N., Singh N., Sinko J., Skiada A., Slavin M., Soman R., Spellberg B., Steinbach W., Tan B.H., Ullmann A.J., Vehreschild J.J., Vehreschild M.J.G.T., Walsh T.J., White P.L., Wiederhold N.P., Zaoutis T., Chakrabarti A. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect. Dis. 2019;19:e405–e421. doi: 10.1016/S1473-3099(19)30312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallalzadeh L.O., Ozzello D.J., Liu C.Y., Kikkawa D.O., Korn B.S. Secondary infection with rhino-orbital cerebral mucormycosis associated with COVID-19. Orbit Amst. Neth. 2021:1–4. doi: 10.1080/01676830.2021.1903044. [DOI] [PubMed] [Google Scholar]
- De Francesco M.A., Alberici F., Bossini N., Scolari F., Pascucci F., Tomasoni G., Caruso A. Pneumocystis jirevocii and SARS-CoV-2 Co-Infection: A Common Feature in Transplant Recipients? Vaccines. 2020;8:544. doi: 10.3390/vaccines8030544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Macedo P.M., Freitas A.D’Ávila., Bártholo T.P., Bernardes-Engemann A.R., Almeida M.d.A., Almeida-Silva F., Zancopé-Oliveira R.M., Almeida-Paes R. Acute Pulmonary Histoplasmosis Following COVID-19: Novel Laboratorial Methods Aiding Diagnosis. J. Fungi. 2021;7(5):346. doi: 10.3390/jof7050346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falces‐Romero I., Ruiz‐Bastián M., Díaz‐Pollán B., Maseda E., García‐Rodríguez J. Isolation of Aspergillus spp. in respiratory samples of patients with COVID-19 in a Spanish Tertiary Care Hospital. Mycoses. 2020;63(11):1144–1148. doi: 10.1111/myc.13155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandra S., Ram S., Levitz S.M. The “Black Fungus” in India: The Emerging Syndemic of COVID-19–Associated Mucormycosis. Ann. Intern. Med. 2021;174(9):1301–1302. doi: 10.7326/M21-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg D., Muthu V., Sehgal I.S., Ramachandran R., Kaur H., Bhalla A., Puri G.D., Chakrabarti A., Agarwal R. Coronavirus Disease (Covid-19) Associated Mucormycosis (CAM): Case Report and Systematic Review of Literature. Mycopathologia. 2021;186(2):289–298. doi: 10.1007/s11046-021-00528-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- H. Ghanem, G. Sivasubramanian, Cryptococcus neoformans Meningoencephalitis in an Immunocompetent Patient after COVID-19 Infection, Case Rep. Infect. Dis. 2021 (2021) e5597473. https://doi.org/10.1155/2021/5597473. [DOI] [PMC free article] [PubMed]
- Guan W., Liang W., Zhao Y., Liang H., Chen Z., Li Y., Liu X., Chen R., Tang C., Wang T., Ou C., Li L., Chen P., Sang L., Wang W., Li J., Li C., Ou L., Cheng B., Xiong S., Ni Z., Xiang J., Hu Y., Liu L., Shan H., Lei C., Peng Y., Wei L., Liu Y., Hu Y., Peng P., Wang J., Liu J., Chen Z., Li G., Zheng Z., Qiu S., Luo J., Ye C., Zhu S., Cheng L., Ye F., Li S., Zheng J., Zhang N., Zhong N., He J. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur. Respir. J. 2020;55:2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao S., Ning K., Kuz C.A., Vorhies K., Yan Z., Qiu J. Long-Term Modeling of SARS-CoV-2 Infection of In Vitro Cultured Polarized Human Airway Epithelium. MBio. 2020;11:e02852–e02920. doi: 10.1128/mBio.02852-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan S.A., Sheikh F.N., Jamal S., Ezeh J.K., Akhtar A. Coronavirus (COVID-19): A Review of Clinical Features. Diagnosis, and Treatment, Cureus. 2020;12 doi: 10.7759/cureus.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henao-Martínez A.F., Gross L., Mcnair B., McCollister B., DeSanto K., Montoya J.G., Shapiro L., Beckham J.D. RISK FACTORS FOR CRYPTOCOCCAL MENINGITIS — A SINGLE UNITED STATES CENTER EXPERIENCE. Mycopathologia. 2016;181:807–814. doi: 10.1007/s11046-016-0048-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbrecht R., Denning D.W., Patterson T.F., Bennett J.E., Greene R.E., Oestmann J.-W., Kern W.V., Marr K.A., Ribaud P., Lortholary O., Sylvester R., Rubin R.H., Wingard J.R., Stark P., Durand C., Caillot D., Thiel E., Chandrasekar P.H., Hodges M.R., Schlamm H.T., Troke P.F., de Pauw B. Invasive Fungal Infections Group of the European Organisation for Research and Treatment of Cancer and the Global Aspergillus Study Group, Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 2002;347:408–415. doi: 10.1056/NEJMoa020191. [DOI] [PubMed] [Google Scholar]
- Herold S., Becker C., Ridge K.M., Budinger G.R.S. Influenza virus-induced lung injury: pathogenesis and implications for treatment. Eur. Respir. J. 2015;45:1463–1478. doi: 10.1183/09031936.00186214. [DOI] [PubMed] [Google Scholar]
- Hochhegger B., Zanon M., Altmayer S., Mandelli N.S., Stüker G., Mohammed T.-L., Verma N., Meirelles G.S.P., Marchiori E. COVID-19 mimics on chest CT: a pictorial review and radiologic guide. Br. J. Radiol. 2021;94:20200703. doi: 10.1259/bjr.20200703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffnagle G.B., Noverr M.C. The emerging world of the fungal microbiome. Trends Microbiol. 2013;21(7):334–341. doi: 10.1016/j.tim.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S., Troise O., Donaldson H., Mughal N., Moore L.S.P. Bacterial and fungal coinfection among hospitalized patients with COVID-19: a retrospective cohort study in a UK secondary-care setting. Clin. Microbiol. Infect. 2020;26:1395–1399. doi: 10.1016/j.cmi.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichai P., Saliba F., Baune P., Daoud A., Coilly A., Samuel D. Impact of negative air pressure in ICU rooms on the risk of pulmonary aspergillosis in COVID-19 patients. Crit. Care. 2020;24:538. doi: 10.1186/s13054-020-03221-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iranmanesh B., Khalili M., Amiri R., Zartab H., Aflatoonian M. Oral manifestations of COVID-19 disease: A review article. Dermatol. Ther. 2020;34 doi: 10.1111/dth.14578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer K.R., Revie N.M., Fu C., Robbins N., Cowen L.E. Treatment strategies for cryptococcal infection: challenges, advances and future outlook. Nat. Rev. Microbiol. 2021;19:454–466. doi: 10.1038/s41579-021-00511-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeican I.I., Inișca P., Gheban D., Tăbăran F., Aluaș M., Trombitas V., Cristea V., Crivii C., Junie L.M., Albu S. COVID-19 and Pneumocystis jirovecii Pulmonary Coinfection—The First Case Confirmed through Autopsy. Medicina (Mex.) 2021;57:302. doi: 10.3390/medicina57040302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong W., Keighley C., Wolfe R., Lee W.L., Slavin M.A., Kong D.C.M., Chen S.-C.-A. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2019;25:26–34. doi: 10.1016/j.cmi.2018.07.011. [DOI] [PubMed] [Google Scholar]
- Jiang M., Guo Y., Luo Q., Huang Z., Zhao R., Liu S., Le A., Li J., Wan L. T cell subset counts in peripheral blood can be used as discriminatory biomarkers for diagnosis and severity prediction of COVID-19. J. Infect. Dis. 2020;222:198–202. doi: 10.1093/infdis/jiaa252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John T.M., Jacob C.N., Kontoyiannis D.P. When Uncontrolled Diabetes Mellitus and Severe COVID-19 Converge: The Perfect Storm for Mucormycosis. J. Fungi. 2021;7:298. doi: 10.3390/jof7040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A.K., Ghazarian Z., Cendrowski K.D., Persichino J.G. Pulmonary aspergillosis and mucormycosis in a patient with COVID-19. Med. Mycol. Case Rep. 2021;32:64–67. doi: 10.1016/j.mmcr.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar A., Jordan A., Olewiler S., Wehberg K., Cortes M., Jackson B.R. A Fatal Case of Rhizopus azygosporus Pneumonia Following COVID-19. J. Fungi. 2021;7:174. doi: 10.3390/jof7030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- M. Karimi-Galougahi, S. Arastou, S. Haseli, Fulminant mucormycosis complicating coronavirus disease 2019 (COVID-19), Int. Forum Allergy Rhinol. 11 (2021) 1029–1030. https://doi.org/10.1002/alr.22785. [DOI] [PMC free article] [PubMed]
- Kelly S., Waters L., Cevik M., Collins S., Lewis J., Wu M.-S., Blanchard T.J., Geretti A.M. Pneumocystis pneumonia, a COVID-19 mimic, reminds us of the importance of HIV testing in COVID-19. Clin. Med. 2020;20:590–592. doi: 10.7861/clinmed.2020-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N., Gutierrez C.G., Martinez D.V., Proud K.C. A case report of COVID-19 associated pulmonary mucormycosis. Arch Clin Cases. 2020;07(03):46–51. doi: 10.22551/2020.28.0703.10172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatib M.Y., Ahmed A.A., Shaat S.B., Mohamed A.S., Nashwan A.J. Cryptococcemia in a patient with COVID-19: A case report. Clin. Case Rep. 2020;9:853–855. doi: 10.1002/ccr3.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri A., Chang K.-M., Berlinrut I., Wallach F. Mucormycosis after Coronavirus disease 2019 infection in a heart transplant recipient – Case report and review of literature. J. Mycol. Medicale. 2021;31(2):101125. doi: 10.1016/j.mycmed.2021.101125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmig L.M., Wu D., Gold M., Pettit N.N., Pitrak D., Mueller J., Husain A.N., Mutlu E.A., Mutlu G.M. IL-6 Inhibition in Critically Ill COVID-19 Patients Is Associated With Increased Secondary Infections. Front. Med. 2020;7 doi: 10.3389/fmed.2020.583897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- P. Koehler, M. Bassetti, A. Chakrabarti, S.C.A. Chen, A.L. Colombo, M. Hoenigl, N. Klimko, C. Lass-Flörl, R.O. Oladele, D.C. Vinh, L.-P. Zhu, B. Böll, R. Brüggemann, J.-P. Gangneux, J.R. Perfect, T.F. Patterson, T. Persigehl, J.F. Meis, L. Ostrosky-Zeichner, P.L. White, P.E. Verweij, O.A. Cornely, Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance, Lancet Infect. Dis. 21 (2021) e149–e162. https://doi.org/10.1016/S1473-3099(20)30847-1. [DOI] [PMC free article] [PubMed]
- Krishna V., Morjaria J., Jalandari R., Omar F., Kaul S. Autoptic identification of disseminated mucormycosis in a young male presenting with cerebrovascular event, multi-organ dysfunction and COVID-19 infection. IDCases. 2021;25 doi: 10.1016/j.idcr.2021.e01172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn B.M. Drug-Resistant Yeast Infections Spread in COVID-19 Unit. JAMA. 2021;325:714. doi: 10.1001/jama.2021.1031. [DOI] [PubMed] [Google Scholar]
- Lahmer T., Kriescher S., Herner A., Rothe K., Spinner C.D., Schneider J., Mayer U., Neuenhahn M., Hoffmann D., Geisler F., Heim M., Schneider G., Schmid R.M., Huber W., Rasch S., Magira E. Invasive pulmonary aspergillosis in critically ill patients with severe COVID-19 pneumonia: Results from the prospective AspCOVID-19 study. PLoS ONE. 2021;16(3):e0238825. doi: 10.1371/journal.pone.0238825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.-C., Yu W.-L. COVID-19 associated with pulmonary aspergillosis: A literature review. J. Microbiol. Immunol. Infect. Wei Mian Yu Gan Ran Za Zhi. 2021;54:46–53. doi: 10.1016/j.jmii.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.-C., Wang C.-Y., Hsueh P.-R. Co-infections among patients with COVID-19: The need for combination therapy with non-anti-SARS-CoV-2 agents? J. Microbiol. Immunol. Infect. 2020;53:505–512. doi: 10.1016/j.jmii.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larzábal F.J., Vilela A., Brusca S., Saluzzi I., Ghergo G.E., Angiono M.A. Simultaneous diagnosis and favorable evolution of infection with Pneumocystis jirovecii, SARS-CoV-2 and advanced HIV. Medicina (Mex.) 2020;80:554–556. [PubMed] [Google Scholar]
- Machado M., Valerio M., Álvarez‐Uría A., Olmedo M., Veintimilla C., Padilla B., De la Villa S., Guinea J., Escribano P., Ruiz‐Serrano M.J., Reigadas E., Alonso R., Guerrero J.E., Hortal J., Bouza E., Muñoz P., Alcalá L., Aldámiz T., Álvarez B., Arias A., Arroyo L.A., Bermúdez E., Burillo A., Candela A., Carrillo R., Catalán P., Cercenado E., Cobos A., Díez C., Estévez A., Fanciulli C., Galar A., García M.D., Viedma D.G., Gijón P., González A., Guillén H., Haces L.V., Kestler M., López J.C., Losada C.N., Marín M., Martín P., Montilla P., Moure Z., Palomo M., Parras F., Pérez‐Granda M.J., Pérez L., Pérez L., Pescador P., Rincón C., Rodríguez B., Rodríguez S., Rojas A., Sánchez C., Sánchez M., Serrano J., Tejerina F., Vesperinas L., Vicente T. Invasive pulmonary aspergillosis in the COVID-19 era: An expected new entity. Mycoses. 2021;64(2):132–143. doi: 10.1111/myc.13213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maini A., Tomar G., Khanna D., Kini Y., Mehta H., Bhagyasree V. Sino-orbital mucormycosis in a COVID-19 patient: A case report. Int. J. Surg. Case Rep. 2021;82 doi: 10.1016/j.ijscr.2021.105957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mang S., Kaddu-Mulindwa D., Metz C., Becker A., Seiler F., Smola S., Maßmann A., Becker S.L., Papan C., Bals R., Lepper P.M., Danziger G. Pneumocystis jirovecii Pneumonia and Severe Acute Respiratory Syndrome Coronavirus 2 Coinfection in a Patient With Newly Diagnosed HIV-1 Infection. Clin. Infect. Dis. 2020;72:1487–1489. doi: 10.1093/cid/ciaa906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maziarz E.K., Perfect J.R. Cryptococcosis. Infect. Dis. Clin. North Am. 2016;30:179–206. doi: 10.1016/j.idc.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta S., Pandey A. Rhino-Orbital Mucormycosis Associated With COVID-19. Cureus. 2020;12 doi: 10.7759/cureus.10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekonnen Z.K., Ashraf D.C., Jankowski T., Grob S.R., Vagefi M.R., Kersten R.C., Simko J.P., Winn B.J. Acute Invasive Rhino-Orbital Mucormycosis in a Patient With COVID-19-Associated Acute Respiratory Distress Syndrome, Ophthal. Plast. Reconstr. Surg. 2021;37:e40–e80. doi: 10.1097/IOP.0000000000001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon A.A., Berg D.D., Brea E.J., Deutsch A.J., Kidia K.K., Thurber E.G., Polsky S.B., Yeh T., Duskin J.A., Holliday A.M., Gay E.B., Fredenburgh L.E. A Case of COVID-19 and Pneumocystis jirovecii Coinfection. Am. J. Respir. Crit. Care Med. 2020;202:136–138. doi: 10.1164/rccm.202003-0766LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant E.A., Flint K., Barouch D.H., Blair B.M. Co-infection with coronavirus disease 2019, previously undiagnosed human immunodeficiency virus. Pneumocystis jirovecii pneumonia and cytomegalovirus pneumonitis, with possible immune reconstitution inflammatory syndrome, IDCases. 2021;24 doi: 10.1016/j.idcr.2021.e01153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshram H.S., Kute V.B., Chauhan S., Desai S. Mucormycosis in post-COVID-19 renal transplant patients: A lethal complication in follow-up. Transpl. Infect. Dis. Off. J. Transplant. Soc. 2021 doi: 10.1111/tid.13663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina F.A., Marin E., Caceres D.H., Romero M., Depardo R., Priarone M.M., Rey L., Vázquez M., Verweij P.E., Chiller T.M., Santiso G. Coronavirus Disease 2019 (COVID-19) in a Patient with Disseminated Histoplasmosis and HIV-A Case Report from Argentina and Literature Review. J. Fungi Basel Switz. 2020;6:275. doi: 10.3390/jof6040275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monneret G., de Marignan D., Coudereau R., Bernet C., Ader F., Frobert E., Gossez M., Viel S., Venet F., Wallet F. Immune monitoring of interleukin-7 compassionate use in a critically ill COVID-19 patient. Cell. Mol. Immunol. 2020;17:1001–1003. doi: 10.1038/s41423-020-0516-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monte Junior E.S.d., Santos M.E.L.D., Ribeiro I.B., Luz G.d.O., Baba E.R., Hirsch B.S., Funari M.P., de Moura E.G.H. Rare and Fatal Gastrointestinal Mucormycosis (Zygomycosis) in a COVID-19 Patient: A Case Report. Clin. Endosc. 2020;53(6):746–749. doi: 10.5946/ce.2020.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorthy A., Gaikwad R., Krishna S., Hegde R., Tripathi K.K., Kale P.G., Rao P.S., Haldipur D., Bonanthaya K. SARS-CoV-2, Uncontrolled Diabetes and Corticosteroids—An Unholy Trinity in Invasive Fungal Infections of the Maxillofacial Region? A Retrospective, Multi-centric Analysis. J. Maxillofac. Oral Surg. 2021;20(3):418–425. doi: 10.1007/s12663-021-01532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy S., Gomersall C.D., Fowler R.A. Care for Critically Ill Patients With COVID-19. JAMA. 2020;323:1499. doi: 10.1001/jama.2020.3633. [DOI] [PubMed] [Google Scholar]
- Nehara H.R., Puri I., Singhal V., Ih S., Bishnoi B.R., Sirohi P. Rhinocerebral mucormycosis in COVID-19 patient with diabetes a deadly trio: Case series from the north-western part of India, Indian. J. Med. Microbiol. 2021;39(3):380–383. doi: 10.1016/j.ijmmb.2021.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasero D., Sanna S., Liperi C., Piredda D., Branca G.P., Casadio L., Simeo R., Buselli A., Rizzo D., Bussu F., Rubino S., Terragni P. A challenging complication following SARS-CoV-2 infection: a case of pulmonary mucormycosis. Infection. 2021;49(5):1055–1060. doi: 10.1007/s15010-020-01561-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarelli V.C., Perosa A.H., de Souza Luna L.K., Conte D.D., Nascimento O.A., Ota-Arakaki J., Bellei N. Detected SARS-CoV-2 in Ascitic Fluid Followed by Cryptococcemia: a Case Report, Sn Compr. Clin. Med. 2020;2:2414–2418. doi: 10.1007/s42399-020-00574-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A., Agarwal R., Rudramurthy S.M., Shevkani M., Xess I., Sharma R., Savio J., Sethuraman N., Madan S., Shastri P., Thangaraju D., Marak R., Tadepalli K., Savaj P., Sunavala A., Gupta N., Singhal T., Muthu V., Chakrabarti A. MucoCovi Network3, Multicenter Epidemiologic Study of Coronavirus Disease-Associated Mucormycosis, India. Emerg. Infect. Dis. 2021;27(9):2349–2359. doi: 10.3201/eid2709.210934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R.K. Patti, N.R. Dalsania, N. Somal, A. Sinha, S. Mehta, M. Ghitan, C. Seneviratne, Y. Kupfer, Subacute Aspergillosis “Fungal Balls” Complicating COVID-19, J. Investig. Med. High Impact Case Rep. 8 (2020) 2324709620966475. https://doi.org/10.1177/2324709620966475. [DOI] [PMC free article] [PubMed]
- Petrikkos G., Tsioutis C. Recent Advances in the Pathogenesis of Mucormycoses. Clin. Ther. 2018;40:894–902. doi: 10.1016/j.clinthera.2018.03.009. [DOI] [PubMed] [Google Scholar]
- Placik D.A., Taylor W.L., Wnuk N.M. Bronchopleural fistula development in the setting of novel therapies for acute respiratory distress syndrome in SARS-CoV-2 pneumonia. Radiol. Case Rep. 2020;15:2378–2381. doi: 10.1016/j.radcr.2020.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posteraro B., Torelli R., Vella A., Leone P.M., De Angelis G., De Carolis E., Ventura G., Sanguinetti M., Fantoni M. Pan-Echinocandin-Resistant Candida glabrata Bloodstream Infection Complicating COVID-19: A Fatal Case Report. J. Fungi Basel Switz. 2020;6:163. doi: 10.3390/jof6030163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhu R.M., Patel R. Mucormycosis and entomophthoramycosis: a review of the clinical manifestations, diagnosis and treatment. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2004;10:31–47. doi: 10.1111/j.1470-9465.2004.00843.x. [DOI] [PubMed] [Google Scholar]
- Quintana-Ortega C., Remesal A., de Valbuena M.R., de la Serna O., Laplaza-González M., Álvarez-Rojas E., Udaondo C., Alcobendas R., Murias S. Fatal outcome of anti-MDA5 juvenile dermatomyositis in a paediatric COVID-19 patient: a case report. Mod. Rheumatol. Case Rep. 2021;5:101–107. doi: 10.1080/24725625.2020.1832755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabagliati R., Rodríguez N., Núñez C., Huete A., Bravo S., Garcia P. COVID-19–Associated Mold Infection in Critically Ill Patients, Chile. Emerg. Infect. Dis. 2021;27:1454–1456. doi: 10.3201/eid2705.204412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao R., Shetty A.P., Nagesh C.P. Orbital infarction syndrome secondary to rhino-orbital mucormycosis in a case of COVID-19: Clinico-radiological features. Indian J. Ophthalmol. 2021;69:1627–1630. doi: 10.4103/ijo.IJO_1053_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravani S.A., Agrawal G.A., Leuva P.A., Modi P.H., Amin K.D. Rise of the phoenix: Mucormycosis in COVID-19 times. Indian J. Ophthalmol. 2021;69:1563–1568. doi: 10.4103/ijo.IJO_310_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riad A., Gomaa E., Hockova B., Klugar M. Oral candidiasis of COVID-19 patients: Case report and review of evidence. J. Cosmet. Dermatol. 2021;20:1580–1584. doi: 10.1111/jocd.14066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley T.T., Muzny C.A., Swiatlo E., Legendre D.P. Breaking the Mold: A Review of Mucormycosis and Current Pharmacological Treatment Options. Ann. Pharmacother. 2016;50:747–757. doi: 10.1177/1060028016655425. [DOI] [PubMed] [Google Scholar]
- Salehi M., Ahmadikia K., Mahmoudi S., Kalantari S., Jamalimoghadamsiahkali S., Izadi A., Kord M., Manshadi S.A.D., Seifi A., Ghiasvand F., Khajavirad N., Ebrahimi S., Koohfar A., Boekhout T., Khodavaisy S. Oropharyngeal candidiasis in hospitalised COVID-19 patients from Iran: Species identification and antifungal susceptibility pattern. Mycoses. 2020;63:771–778. doi: 10.1111/myc.13137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmanton-García J., Sprute R., Stemler J., Bartoletti M., Dupont D., Valerio M., Garcia-Vidal C., Falces-Romero I., Machado M., de la Villa S., Schroeder M., Hoyo I., Hanses F., Ferreira-Paim K., Giacobbe D.R., Meis J.F., Gangneux J.-P., Rodríguez-Guardado A., Antinori S., Sal E., Malaj X., Seidel D., Cornely O.A., Koehler P. COVID-19–Associated Pulmonary Aspergillosis. Emerg. Infect. Dis. 2021;27:1077–1086. doi: 10.3201/eid2704.204895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltzman R.L., Peterson P.K. Immunodeficiency of the elderly. Rev. Infect. Dis. 1987;9:1127–1139. doi: 10.1093/clinids/9.6.1127. [DOI] [PubMed] [Google Scholar]
- Sarkar S., Gokhale T., Choudhury S.S., Deb A.K. COVID-19 and orbital mucormycosis. Indian J. Ophthalmol. 2021;69:1002–1004. doi: 10.4103/ijo.IJO_3763_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian S.K., Kumar V.B., Gupta M., Sharma Y. Covid Assossiated Invasive Fungal Sinusitis, Indian. J. Otolaryngol. Head Neck Surg. 2021:1–4. doi: 10.1007/s12070-021-02471-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen M., Lahane S., Lahane T.P., Parekh R., Honavar S.G. Mucor in a Viral Land: A Tale of Two Pathogens. Indian J. Ophthalmol. 2021;69:244–252. doi: 10.4103/ijo.IJO_3774_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S., Grover M., Bhargava S., Samdani S., Kataria T. Post coronavirus disease mucormycosis: a deadly addition to the pandemic spectrum. J. Laryngol. Otol. 2021;135(5):442–447. doi: 10.1017/S0022215121000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G.e., Liang G., Liu W. Fungal Co-infections Associated with Global COVID-19 Pandemic: A Clinical and Diagnostic Perspective from China. Mycopathologia. 2020;185(4):599–606. doi: 10.1007/s11046-020-00462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- G. Spiteri, J. Fielding, M. Diercke, C. Campese, V. Enouf, A. Gaymard, A. Bella, P. Sognamiglio, M.J. Sierra Moros, A.N. Riutort, Y.V. Demina, R. Mahieu, M. Broas, M. Bengnér, S. Buda, J. Schilling, L. Filleul, A. Lepoutre, C. Saura, A. Mailles, D. Levy-Bruhl, B. Coignard, S. Bernard-Stoecklin, S. Behillil, S. van der Werf, M. Valette, B. Lina, F. Riccardo, E. Nicastri, I. Casas, A. Larrauri, M. Salom Castell, F. Pozo, R.A. Maksyutov, C. Martin, M. Van Ranst, N. Bossuyt, L. Siira, J. Sane, K. Tegmark-Wisell, M. Palmérus, E.K. Broberg, J. Beauté, P. Jorgensen, N. Bundle, D. Pereyaslov, C. Adlhoch, J. Pukkila, R. Pebody, S. Olsen, B.C. Ciancio, First cases of coronavirus disease 2019 (COVID-19) in the WHO European Region, 24 January to 21 February 2020, Eurosurveillance. 25 (2020) 2000178. https://doi.org/10.2807/1560-7917.ES.2020.25.9.2000178.
- Stasiak C.E.S., Nigri D.H., Cardoso F.R., Mattos R.S.A.R., Gonçalves Martins P.A., Carvalho A.R.S., Altino de Almeida S., Rodrigues R.S., Rosado-de-Castro P.H. Case Report: Incidental Finding of COVID-19 Infection after Positron Emission Tomography/CT Imaging in a Patient with a Diagnosis of Histoplasmosis and Recurring Fever. Am. J. Trop. Med. Hyg. 2021;104(5):1651–1654. doi: 10.4269/ajtmh.20-0952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabarsi P., Khalili N., Pourabdollah M., Sharifynia S., Naeini A.S., Ghorbani J., Mohamadina A., Abtahian Z., Askari E. Res; Sq: 2021. COVID-19 associated rhinosinusitis mucormycosis due to Rhizopus arrhizus: A rare but potentially fatal infection occurring after treatment with corticosteroids. https://doi.org/10.21203/rs.3.rs-398594/v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavakolpour S., Rakhshandehroo T., Wei E.X., Rashidian M. Lymphopenia during the COVID-19 infection: What it shows and what can be learned. Immunol. Lett. 2020;225:31–32. doi: 10.1016/j.imlet.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Arkel A.L.E., Rijpstra T.A., Belderbos H.N.A., van Wijngaarden P., Verweij P.E., Bentvelsen R.G. COVID-19–associated Pulmonary Aspergillosis. Am. J. Respir. Crit. Care Med. 2020;202:132–135. doi: 10.1164/rccm.202004-1038LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veisi A., Bagheri A., Eshaghi M., Rikhtehgar M.H., Rezaei Kanavi M., Farjad R. Rhino-orbital mucormycosis during steroid therapy in COVID-19 patients: A case report. Eur. J. Ophthalmol. 2022;32(4):NP11–NP16. doi: 10.1177/11206721211009450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viceconte G., Buonomo A.R., Lanzardo A., Pinchera B., Zappulo E., Scotto R., Schiano Moriello N., Vargas M., Iacovazzo C., Servillo G., Gentile I. “Federico II” COVID-19 Team, Pneumocystis jirovecii pneumonia in an immunocompetent patient recovered from COVID-19. Infect. Dis. Lond. Engl. 2021;53(5):382–385. doi: 10.1080/23744235.2021.1890331. [DOI] [PubMed] [Google Scholar]
- Villanueva-Lozano H., Treviño-Rangel R.d.J., González G.M., Ramírez-Elizondo M.T., Lara-Medrano R., Aleman-Bocanegra M.C., Guajardo-Lara C.E., Gaona-Chávez N., Castilleja-Leal F., Torre-Amione G., Martínez-Reséndez M.F. Outbreak of Candida auris infection in a COVID-19 hospital in Mexico. Clin. Microbiol. Infect. 2021;27(5):813–816. doi: 10.1016/j.cmi.2020.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waizel-Haiat S., Guerrero-Paz J.A., Sanchez-Hurtado L., Calleja-Alarcon S., Romero-Gutierrez L. A Case of Fatal Rhino-Orbital Mucormycosis Associated With New Onset Diabetic Ketoacidosis and COVID-19. Cureus. 2021;13 doi: 10.7759/cureus.13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Yang Q., Zhang P., Sheng J., Zhou J., Qu T. Clinical characteristics of invasive pulmonary aspergillosis in patients with COVID-19 in Zhejiang, China: a retrospective case series. Crit. Care. 2020;24:299. doi: 10.1186/s13054-020-03046-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werthman-Ehrenreich A. Mucormycosis with orbital compartment syndrome in a patient with COVID-19. Am. J. Emerg. Med. 2021;42(264):e5–264.e8. doi: 10.1016/j.ajem.2020.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L.J. Wheat, A.G. Freifeld, M.B. Kleiman, J.W. Baddley, D.S. McKinsey, J.E. Loyd, C.A. Kauffman, Clinical Practice Guidelines for the Management of Patients with Histoplasmosis: 2007 Update by the Infectious Diseases Society of America, Clin. Infect. Dis. 45 (2007) 807–825. https://doi.org/10.1086/521259. [DOI] [PubMed]
- WHO, WHO Coronavirus (COVID-19) Dashboard, (2021). https://covid19.who.int (accessed June 5, 2021).
- Yang W., Cao Q., Qin L.e., Wang X., Cheng Z., Pan A., Dai J., Sun Q., Zhao F., Qu J., Yan F. novel coronavirus disease (COVID-19): A multi-center study in Wenzhou city, Zhejiang, China. J. Infect. 2020;80(4):388–393. doi: 10.1016/j.jinf.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Wang W., Liu Z., Liang C., Wang W., Ye F., Huang B., Zhao L., Wang H., Zhou W., Deng Y., Mao L., Su C., Qiang G., Jiang T., Zhao J., Wu G., Song J., Tan W. Morphogenesis and cytopathic effect of SARS-CoV-2 infection in human airway epithelial cells. Nat. Commun. 2020;11:3910. doi: 10.1038/s41467-020-17796-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurl C., Hoenigl M., Schulz E., Hatzl S., Gorkiewicz G., Krause R., Eller P., Prattes J. Autopsy Proven Pulmonary Mucormycosis Due to Rhizopus microsporus in a Critically Ill COVID-19 Patient with Underlying Hematological Malignancy. J. Fungi. 2021;7:88. doi: 10.3390/jof7020088. [DOI] [PMC free article] [PubMed] [Google Scholar]