Abstract
Animal models of prostate cancer are essential to identify chemopreventive treatments against this major male malignancy. The N-methyl-N-nitrosourea (MNU) plus testosterone rat model of prostate carcinogenesis is a reliable animal model that recapitulates human prostate cancer in many respects and has been used extensively in chemoprevention studies with good predictive value for the results of human clinical trials. The objective of this article is to describe the induction protocol of this model, demonstrate its robustness and reproducibility over time and across rat strains, provide diagnostic criteria for the identification of prostate lesions, and present the current tumor induction protocol so that others can use this model in a reliable manner. The majority of accessory sex gland tumors in this model are adenocarcinomas originating in the anterior and dorsolateral prostate that metastasize to lungs and abdominal structures. The rat strain used is of critical importance, with the commercially available Wistar WU and Fischer F344 strains yielding the highest tumor incidences. Low dose, long-term testosterone treatment is essential for a high tumor incidence, but in advanced stage, large adenocarcinomas do not appear to be androgen dependent. This rat model is a robust and reproducible prostate cancer animal model of human prostate cancer.
Keywords: prostate cancer, animal model, rat, testosterone, MNU
Introduction
The importance of relevant animal models of cancer for research into prostate cancer etiology and the identification of agents that may prevent malignancy cannot be overstated. For several major human cancers there are reasonably adequate models that are generally accepted as relevant.1 For prostate cancer, there are a number of mouse models that support investigations of the role of specific oncogenes and tumor suppressor genes.2–4 However, the likely pivotal role of hormones, particularly androgens and estrogens, in prostate carcinogenesis has not been elucidated in those models and most murine models have not been used successfully to identify cancer preventive agents.5,6 This limits the use of mouse models for the study of some of the critical factors involved in prostate carcinogenesis and for chemoprevention experiments.
We have developed and refined a rat model of prostate carcinogenesis using a sequential regimen of antiandrogen inhibition of prostate epithelial cell proliferation, followed by short-term stimulation of cell proliferation, and a single injection of the chemical carcinogen, N-methyl-N-nitrosourea (MNU), followed by chronic administration of low dose testosterone. Development of this model was based on our observation that active cell proliferation at the time of carcinogen administration is essential for the induction of adenocarcinomas in the rat prostate.7–9 Addition of subsequent treatment with testosterone at very low doses via slow release Silastic tubing implants proved to have a strong tumor promoting effect.10
This model has been used in more than 25 studies to identify compounds that could prevent prostate cancer or modify its induction. The first such study was published in 199811 and many other studies have followed.12–19 The model accurately predicted the negative outcome of the Selenium and Vitamin E Cancer Prevention Trial (SELECT) randomized study,17,20 a randomized clinical trial with an antiestrogen (toremifene)13,21 and the preventive activity of blocking androgen receptor action in humans.13,18 This predictive nature of this prostate cancer model suggests that it is a useful test system for potential chemopreventive agents and research on hormonal factors that may modulate prostate cancer development.
Since the first full publication of the application of this model to test agents for chemopreventive activity,11 several modifications have been made to the tumor induction protocol; these changes were implemented to lower the incidence of tumors at other sites that might interfere with animal survival during prostate cancer prevention studies, and to address changes in the availability of some reagents used in the induction protocol. In addition, the rat strain that was initially used, the random bred Wistar Unilever (WU) rat, was not always available forcing the use of other rat strains.
The objective of this article is to describe the changes that have been made to the protocol for prostate cancer studies using this model over time in response to changes in availability of reagents and to simplify the approach and reduce the incidence of irrelevant neoplasms that impact survival; demonstrate its robustness and reproducibility over time and across rat strains; provide diagnostic criteria for the identification of prostate lesions; and present the current protocol for induction of prostate cancer in rats by MNU plus testosterone so that others can use the model in a reliable manner.
Materials and Methods
Animals
All animal experiments were approved by the institutional animal care and use committees at CIVO-TNO (“Centraal Instituut voor Voedingsonderzoek” of the Dutch Organization for “Toegepast-Natuurwetenschappelijk Onderzoek), Zeist, The Netherlands, New York University School of Medicine, New York, NY (NYU), Illinois Institute of Technology (IIT) Research Institute, Chicago, IL (IITRI), or the University of Illinois at Chicago, Chicago, IL (UIC).
Rats were obtained from the following sources:
Outbred Wistar Unilever rats (Cpb:WU) from TNO-Central Institute for the Breeding of Laboratory Animals, Zeist, The Netherlands;
Outbred Wistar Unilever rats (HsdCpb:WU) from Harlan Sprague Dawley, Indianapolis, IN;
Outbred Wistar Unilever rats (HsdCpb:WU) from Envigo, Horst, Netherlands;
Outbred Wistar Unilever rats (Crl:WU) from Charles River WIGA, Sulzfeld, Germany;
Inbred Lewis rats (LEW/Crl) from Charles River, Raleigh, NC;
Inbred Copenhagen rats (COP/Cr), Fischer rats (F344/Cr), and Wister Furth rats (WF/Ncr) from Charles River, Kingston, NY;
Outbred Lobund Wistar rats (Lo-CR:WI) that are partially inbred from NCI (National Cancer Institute) Frederick, Frederick, MD;
Outbred Wistar rats (Hsd:WI) from Envigo, Indianapolis, IN.
Reagents
Cyproterone acetate (CAS number 427–51-0) was obtained from Berlex Laboratories, Montville, NJ, or Sigma, St. Louis, MO. Flutamide (CAS 13311–84-7) was obtained from Sigma. Testosterone propionate (CAS 57–85-2) and testosterone (CAS 58–22-0) were obtained from Steraloids, Newport, RI, or Sigma. Testosterone propionate solution for injection (50 mg/mL, Neohombreol) was obtained from Organon, Oss, Netherlands. Carboxymethylcellulose (CAS 9004–32-4) was obtained from Sigma. N-methyl-N-nitrosourea (MNU) (CAS 684–93-5) was obtained in re-crystallized form from Ash-Stevens, Detroit, MI, until its manufacture there was discontinued and it was obtained since in a non-recrystallized form from the NCI Chemical Carcinogen Repository, Midwest Research Institute, Kansas City, MO, and more recently from the MRIGlobal Chemical Carcinogen Repository, MRIGlobal, North Kansas City, MO.
Prostate Cancer Induction Protocol
Prostate cancer was induced by a sequential treatment with an anti-androgen (cyproterone acetate or flutamide when cyproterone acetate became unavailable for a time) for approximately 3 weeks to stop prostatic cell proliferation.8,9 Administration of the antiandrogen was followed by either one or three daily injections with testosterone propionate to create a wave of cell proliferation in the prostate epithelium;9 at the peak of this wave, rats received a single injection with MNU. Beginning one or two weeks after MNU injection, rats received low dose treatment with testosterone via slow-release implants for the duration of the experiments (Figure 1).
Figure 1.

Flow diagram of tumor induction protocol. Oral treatment with antiandrogen, Flutamide (20 mg/kg) or cyproterone acetate (50 mg/kg) is effective when given daily for 18 to 21 days in a 2% aqueous carboxymethylcellulose suspension. Testosterone propionate (100 mg/kg) is given by subcutaneous injection suspended in oil. MNU (30–40 mg/kg) is given by intravenous or subcutaneous injection dissolved in acidified saline. Chronic testosterone is given by Silastic tubing implants for at least one year. MNU indicates N-methyl-N-nitrosourea.
Pre-treatment with antiandrogen and testosterone propionate.
Cyproterone acetate or flutamide was suspended in 2% aqueous carboxymethylcellulose for gavage administration at concentrations that would allow a gavage volume of 1 mL/rat or 10 mL/kg body weight. Testosterone propionate was suspended at 50 mg/mL in corn oil (Sigma) or as Neohombreol (Organon) for subcutaneous injection. The cyproterone acetate dose was 50 mg/kg/day, the dose of flutamide was 20 mg/kg/day, and the testosterone propionate dose was 100 mg/kg.
MNU treatment.
MNU was shipped on dry ice and stored at −20°C or below until it was freshly dissolved immediately prior to use. MNU was dissolved at a concentration of 10 mg/mL in phosphate-citrate-buffered saline (pH 4.7) or in some experiments in acidified saline (pH 5.0), immediately prior to intravenous, intraperitoneal, or subcutaneous injection. MNU is stable at pH 4.8 to 5.0 for at least several hours. Intravenous injections were administered to rats under mild sedation. The MNU dose ranged from 40 to 20 mg/kg.
Testosterone treatment.
Testosterone was administered by slow-release Silastic tubing implants. These implants were made from Silastic tubing (Dow Corning, VWR Scientific; ID 0.078 in.; OD 0.125 in.) filled using vacuum suction (see Supplemental Material) and tightly packed with crystalline testosterone over a length of 3 cm, and sealed with General Electric RTV-108 adhesive sealant. Prior to implantation, the implants were soaked in 70% alcohol for 30 minutes and then washed with sterile phosphate-buffered saline (pH 7.0) using sterile technique, for two days prior to implantation, changing the saline twice daily and once more approximately one hour prior to implantation. Unless indicated otherwise, two implants were placed subcutaneously on the back of each rat while under anesthesia. In some experiments, these implants were replaced at 6-month intervals, but since the implants still contained a visible quantity of testosterone after one year, replacement was omitted in several studies. The resulting increase in circulating testosterone is stably 2.5-fold over control values.10
General Conduct of Experiments
At 8 to 10 weeks of age, rats were assigned to groups of 20 to 40 for the experiments described in this report. The antiandrogens cyproterone acetate or flutamide were administered daily, by gavage, for 18 to 21 consecutive days, followed one day later by one or three daily injections of testosterone propionate. All doses of testosterone propionate were administered at approximately the same time of day. Unless indicated otherwise, precisely 54 hours (±1.5 hours) after the first or only testosterone propionate injection, MNU was injected intravenously via the tail vein or, in some experiments, intraperitoneally or subcutaneously. Animals were weighed at least monthly and were observed at least weekly for signs of illness and to detect humane endpoints. In several long-term experiments, rats were euthanized upon observation of potential moribundity, while in most prostate cancer prevention studies, rats were euthanized when identified as moribund or at 13 to 14 months after MNU injection, whichever was earlier. Rats were euthanized by exsanguination under anesthesia.
The animals were housed in solid bottom plastic cages with corn-cob or, in some studies, hardwood bedding in standard animal rooms with a 12–12 hour light dark cycle in AALAC-accredited facilities. Rats had ad libitum access to tap water and food throughout all studies. The diets used were a cereal-based stock diet produced at CIVO-TNO,22 natural ingredient diets NIH-07 (Zeigler Brothers, Gardners, PA), Teklad 2018 (18% protein) or Teklad 7001 (4% fat) mouse/rat diet (Envigo, Madison, WI), and the semi-purified AIN-93M diet (Envigo, Madison, WI).
In one experiment, testosterone implants were removed under general anesthesia 28 weeks after they had been implanted, and one group of rats were surgically castrated at the same time while another group of rats were left intact.
Because androgen sensitivity is a hallmark of most human prostate cancers, we conducted tumor transplant studies designed to determine the role of androgens in prostate cancer growth in this rat model. To this end, prostatic cancer cells that were isolated from a large dorsolateral prostate region tumor in a WF rat and cultured23 were injected subcutaneously into syngeneic rats (400,000 cells in 0.2 ml of L-15 medium on either side of the hind quarter flank). When tumors were palpable 11 days after tumor cell injection, one half of the rats was surgically castrated while the other half was left intact. Tumor growth was measured by calipers every 3 to 7 days and tumor volumes were calculated using a standard formula (L x W x H x 0.5236).24
Postmortem Procedures and Histopathology
A complete necropsy was performed and in many experiments all tumor-like lesions or masses were collected, including nodules on liver and lung. Any grossly visible lesions on the prostate-seminal vesicle complex were recorded. The prostate-seminal vesicle complex was removed en bloc with urethra and urinary bladder attached. All collected tissues were fixed in neutral buffered formalin, in some experiments followed by placement in 70% ethanol one to two weeks later. After at least one week fixation, the prostate complex was dissected as follows (see also visuals in Supplemental Material): ventral lobes were removed and placed in a cassette; the seminal vesicles with anterior prostate (coagulating gland) were sectioned off and placed in a cassette with the anterior prostate side down; the bladder (used as landmark) was then cut off but the urethra was left in place; the remaining dorsolateral prostate complex was cut midway at a coronal plane at a right angle to the urethra and each half was placed in a cassette with the cut face down. All tissues were then processed to and embedded in a histological grade paraffin wax and 4–5 micron sections were prepared and stained with hematoxylin and eosin. We have shown previously that to detect small lesions in the rat prostate (<5 mm diameter) six-step sections at 250 to 300 micron intervals yield a maximal lesion incidence11; this approach has been used in all experiments included in this report.
We stained some large prostate tumors for androgen receptor using anti-androgen receptor (N-20), purified rabbit IgG from Santa Cruz Biotechnology, Santa Cruz, CA (#sc-816) at a dilution of 1:200 after antigen retrieval with EDTA and microwaving (7X7 minutes); ventral prostate fixed using the same fixation protocol at the same time as the tumor tissue was used as positive control and negative control antibody used was normal rabbit IgG (Santa Cruz, #sc-2027).
Histologic criteria used to identify accessory sex gland lesions have been described previously,11,25–29 and were consistent with the INHAND paper about proliferative and nonproliferative lesions of the rat (and mouse) male reproductive system.30 Lesions classified as adenocarcinomas were invasively growing epithelial processes consisting of atypical cells that ranged from well to poorly differentiated, arranged in growth patterns ranging from clearly glandular to sheets or strands of poorly differentiated cells and often disrupted normal architecture, particularly in the case of larger tumors. Lesions classified as hyperplastic lesions consisted of atypical cells that in most cases abruptly changed from normal epithelium but were not invasively growing were classified as atypical hyperplasia or carcinoma in situ11,25; the incidence of these non- or preneoplastic lesions are not reported here. Non-invasive lesions qualifying as adenomas26 were not encountered. All histopathological evaluation was carried out by a single board-certified pathologist (MCB) who took great care to assure constant diagnostic criteria.
Statistical Methods Used
Lesion incidences were compared using the Fisher exact test (one sided or two-sided as appropriate and indicated in text and tables) and a chi-square test when comparing more than two groups. Continuous data such as age at death were compared using an unpaired t test (with Welch’s correction if differences in standard deviations were identified) or a Mann-Whitney test for non-Gaussian distributed data (one sided or two-sided as appropriate and indicated in text and tables). Differences in subcutaneous tumor cell growth were analyzed using non-linear regression analysis (curve fit for exponential growth). Prizm software (GraphPad, San Diego, CA) was sued for these analyses.
Results
Prostate Cancer Induction in WU Rats
We compared prostate adenocarcinoma incidence at 12 to 15 months (13 months in most studies) after MNU in control groups of 35 experiments with WU rats (n = 1301), most of which were chemoprevention studies (Table 1). The incidence of adenocarcinomas in all accessory sex glands combined ranged from 53% to-96% (mean 73.8 ±SD 9.6%), and the incidence of tumors that were clearly or likely of prostatic origin (not seminal vesicles) ranged from 41% to-85% (mean 64.2 ±SD 11.3%). Most chemoprevention studies were conducted at IITRI under the direction of D.L. McCormick and the remainder at NYU or UIC under the direction of M.C. Bosland.
Table 1.
Summary of Neoplastic Lesions in Accessory Sex Glands of Wistar WU Rats Treated with MNU and Testosterone in the Control Groups of 35 Separate Experimentsa
| Incidence of Lesions | |||||
|---|---|---|---|---|---|
|
| |||||
| Total Number of Animals: 1301 | Number (%) | % Range | % Median | % Mean | 95% CI |
| All Accessory Sex Glands Combined (dorsolateral and anterior prostate plus seminal vesicle): | |||||
| Adenocarcinoma (or carcinosarcomab) | 959 (73.7) | 52.6–96.2 | 74.4 | 73.8 | 70.5, 77.1 |
| Sarcoma [(neuro)fibro or histiocytic sarcoma] | 17 (1.3) | ||||
| Any Prostate Gland Lobe (but not seminal vesicle only): | |||||
| Adenocarcinoma (or carcinosarcomab) | 831 (63.9) | 41.0–84.6 | 66.7 | 64.2 | 60.3, 68.1 |
| Dorsolateral and/or Anterior Prostate (clearly confined to these glands and microscopic size; with or without seminal vesicle lesions): | |||||
| Adenocarcinoma | 557 (42.8) | 7.0–64.7 | 45.2 | 42.6 | 38.1, 47.0 |
| Sarcoma [(neuro)fibro or histiocytic sarcoma] | 4 (0.3) | ||||
| Dorsolateral Prostate Region (originating from dorsolateral, anterior prostate, and/or seminal vesicle): | |||||
| Adenocarcinoma (or carcinosarcomab) | 310 (23.8) | 2.6–57.1 | 20.9 | 24.4 | 19.4, 29.4 |
| Anterior Prostate/Seminal Vesicle Region (originating from anterior prostate and/or seminal vesicle): | |||||
| Adenocarcinoma (or carcinosarcomab) | 125 (9.6) | 0–28.6 | 8.6 | 9.3 | 7.0, 11.6 |
| Sarcoma [(neuro)fibro or histiocytic sarcoma] | 0 | ||||
| Seminal Vesicle Only (clearly confined to this gland): | |||||
| Adenocarcinoma (or carcinosarcomab) | 128 (9.78) | 0–23.1 | 9.3 | 9.6 | 7.5, 11.6 |
| Sarcoma [(neuro)fibro or histiocytic sarcoma]c | 6 (0.5) | ||||
Control groups of 30–43 rats in 35 chemoprevention studies and three other prostate cancer induction studies.
Carcinosarcomas were rare.
One seminal vesicle sarcoma was found in an animal that also had a prostate tumor.
The first prostate adenocarcinomas were observed 8 to 9 months after MNU injection in animals that died or were euthanized because they were moribund or had a large external tumor (e.g., a Zymbal gland tumor) that necessitated euthanasia. Large prostate tumors often caused urinary obstruction (Supplemental Figure 1), resulting in morbidity due to hydronephrosis and ultimately moribundity. We previously reported on the effect of study duration on prostate cancer incidence observed in a limited number of studies.31 The duration did not affect overall tumor incidence, but with increasing duration (12, 13, 14, and 15 months after MNU injection) there was a shift from predominantly smaller tumor to larger tumors.31 The ratio of smaller to larger tumors, considering only the largest tumor if multiple cancers were detected, was 40% to 50% at 13 to 14 months after MNU; this time point was subsequently used in all studies that are presented in Table 1.
Malignant lesions in the accessory sex glands were commonly identified in the dorsolateral and anterior prostate lobes, but were never seen in the ventral prostate. In addition, lower incidences of tumors were found in the seminal vesicles, but these tumors did not drive overall cancer incidence in the accessory sex glands which was determined by adenocarcinomas in the dorsolateral and/or anterior prostate. The precise site of origin of large tumors was often difficult to ascertain, but small tumors (≤5 mm in largest diameter) occurred predominantly in the dorsolateral and particularly the anterior prostate in any single animal, regardless of whether there were lesions in the seminal vesicles. Animals with only seminal vesicle tumors were few (<10%) in each control group (Table 1) although many animals with prostate adenocarcinomas also had tumors in the seminal vesicle (Table 2).
Table 2.
Comparison of Neoplastic Lesions in Accessory Sex Glands and Two Other Major Tumor Sites in Eight Rat Strains Treated with Various Variations of the Tumor Induction Protocol at Various Times between 1986 and 2016 with Four Repeat Experiments with Wistar WU rats
| Strain | LEW | WU-1 | WU-2a | W/Fa | F344–1a | COPa | LoWia | F344–2 | Hsd:WIb | WU-3 | WU-4 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Year(s) | 1986–87 | 1988–89 | 1994–95 | 1994–95 | 1994–95 | 1994–95 | 1994–95 | 1998–99 | 2017–18 | 2010–11 | 2015–16 |
| Source of rats | Hsd US | Crl WIGA | Hsd US | Hsd US | Hsd US | Hsd US | NCI | Taconic | Hsd US | Hsd NL | Hsd NL |
| Type of antiandrogen used | CA | CA | CA | CA | CA | CA | CA | CA | FL | FL | FL |
| No. testosterone propionate injections | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 1 | 1 | 1 | 1 |
| Source of MNU | A-S | A-S | A-S | A-S | A-S | A-S | A-S | A-S | NCI-Repc | NCI-Repc | NCI-Repc |
| Dose of MNU (mg/kg) | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 30 | 30 | 30 | 30 |
| Diet | NIH-07 | NIH-07 | NIH-07 | NIH-07 | NIH-07 | NIH-07 | NIH-07 | NIH-07 | Tekl 18% | AIN-93M | AIN-93M |
|
| |||||||||||
| Number of Animals | 16 | 28 | 30 | 29 | 30 | 30 | 29 | 30 | 38 | 31 | 31 |
| Mean age at death (days) | 297 | 359 | 339 | 334 | 341 | 428 | 251 | 374 | 420 | 341 | 344 |
| Range | 158–469 | 126–568 | 109–497 | 160–460 | 183–503 | 221–559 | 152–320 | 275–405 | 259–431d | 241–400d | 216–406d |
|
| |||||||||||
| Gross tumors, all accessory sex glands | 3 (19) | 15 (54) | 12 (40) | 22 (76) | 12 (43) | 17(57) | 29 (93) | 16 (53) | 1 (3) | 17 (55) | 12 (42) |
| – DLP & SV/AP region | 2 (13) | 9 (32) | 4 (13) | 8 (28) | 6 (21) | 10 (33) | 17 (59) | 7 (23) | 0 | 7 (23) | 10 (32) |
| – DLP region (only) | 0 | 5 (18) | 5 (20) | 10 (34) | 3 (11) | 3 (10) | 1 (3) | 1 (3) | 1 (3) | 3 (10) | 1 (3) |
| – SV/AP region (only) | 1 (6) | 1 (4) | 3 (10) | 4 (14) | 3 (11) | 4 (13) | 9 (31) | 8 (27) | 0 | 7 (23) | 1 (3) |
| All Accessory Sex Glands, adenocarcino(sarco)mas (ACAR): | |||||||||||
| – All adenocarcino(sarco)mas | 14 (88) | 23 (82)e | 25 (83) | 28 (97) | 25 (89) | 23 (77) | 29 (100)f | 26 (87) | 22 (58) | 23 (74) | 23 (74) |
| – Macroscopic size | 3 (19) | 16 (57)e | 13 (43) | 21 (72) | 12 (43) | 16 (53) | 29 (100)f | 17 (57) | 1 (3) | 17 (55) | 14 (45) |
| – Microscopic size | 11 (69) | 7 (25) | 12 (40) | 7 (24) | 13 (46) | 7 (23) | 0 | 9 (30) | 21 (55) | 6 (19) | 9 (29) |
| – All ACARs without SV only | 13 (81) | 21 (75) | 22 (73) | 26 (90) | 23 (77) | 20 (67) | 27 (93) | 20 (67) | 20 (53) | 21 (68) | 21 (68) |
| – Seminal vesicle only | 1 (6) | 2 (7) | 3 (10) | 2 (7) | 2 (7) | 3 (10) | 2(7) | 6 (20) | 1 (3) | 2 (6) | 2 (6) |
| Macroscopic adenocarcinomas: | |||||||||||
| – DLP & SV/AP region | 0 | 8 (29)f | 4 (13) | 12 (41) | 5 (18) | 13 (43) | 17 (59) | 9 (30) | 0 | 4 (13) | 0 |
| – DLP region | 0 | 7 (25)f | 5 (17) | 4 (14) | 4 (14) | 2 (7) | 1 (3) | 1 (3) | 1 (3) | 5 (16) | 13 (42) |
| – SV/AP region (only) | 0 | 0 | 3 (10) | 4 (14) | 3 (11) | 2 (7) | 9 (31)f | 3 (10) | 0 | 8 (26) | 0 |
| – Dorsolateral Prostate (DLP) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| – Anterior Prostate (AP) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| – Seminal Vesicle (SV) | 1 (6) | 1 (4) | 1 (3) | 1 (3) | 0 | 1 (3) | 2 (7) | 1 (3) | 0 | 0 | 2 (6) |
| Microscopic adenocarcino(sarco)mas: | |||||||||||
| – Dorsolateral Prostate | 3 (19) | 3 (11) | 5 (13) | 4 (14) | 1 (4) | 1 (3) | 0 | 0 | 7 (18) | 0 | 2 (6) |
| – Anterior Prostate | 11 (69) | 8 (29) | 12 (40) | 10 (34) | 18 (64) | 5 (17) | 2 (7) | 6 | 18 (47) | 6 (19) | 5 (16) |
| – Seminal Vesicle | 8 (50) | 4 (14) | 9 (30) | 13 (45) | 13 (46) | 9 (30) | 7 (24) | 15 (50) | 1 (3) | 4 (13) | 5 (16) |
| Seminal Vesicle only: all ACARs | 1 (6) | 1 (4) | 3 (10) | 2 (7) | 2 (7) | 3 (10) | 2 (7) | 6 (20) | 1 (3) | 2 (6) | 2 (6) |
| DLP – SV/AP region sarcomas: | 0 | 0 | 0 | 2 (DLP) | 0 | 0 | 0 | 1 (SV/AP) | 1 (DLP) | 1 (DLP) | 0 |
| Adenocarcinoma metastasesg | 0 | 6 (21) | 1 (3) | 3 (10) | 4 (14) | 13 (43) | 15 (52) | 3 (10) | NR | 0 | 5 (16) |
| - Lung | 0 | 0 | 1 (3) | 2 (7) | 3 (10) | 9 (30) | 7 (24) | 1 (3) | NR | 0 | 5 (16) |
| - Abdominal structures | 0 | 6 (21) | 1 (3) | 1 (3) | 3 (10) | 10 (33) | 12 (41) | 3 (10) | NR | 0 | 0 |
| Generalized lymphoma | 6 (38) | 6 (21) | 3 (10) | 0 | 2 (7) | 2 (7) | 0 | 3 (10) | NR | 3 (10) | 2 (6) |
| Zymbal gland tumor | 2 (13) | 1 (4) | 3 (10) | 0 | 3 (10) | 0 | 1 (3) | 10 (33) | NR | 7 (23) | 7 (23) |
These rats strains were compared in a single concurrent experiment; all other rats strains are from separate experiments.
This is a control group of a study carried out at IIT Research Institute.
MNU was administered by intraperitoneal injection in this experiment, but intravenously in all other studies.
These studies were terminated at approximately 14 months after MNU injection; all other experiments were terminated when the last animals died or were euthanized because of moribundicity.
Two tumors were carcinosarcomas
One tumor was a carcinosarcoma
Grossly visible metastatic lesions; no systematic histological searches for metastases in, e.g., the lungs, were conducted.
Abbreviations: Hsd = Harlan Sprague Dawley; US = United States; Crl WIGA = Charles River Germany; NCI = National Cancer Institute; NL = Netherlands; Tekl = Teklad; CA = Cyproterone acetate; FL = Flutamide; A-S = Ash Stevens; NCI-Rep = NCI Carcinogen Repository; MNU = N-Methyl-N-Nitrosourea; DLP = Dorsolateral prostate; AP = Anterior prostate; SV = seminal vesicle; ACAR = Adenocarcinoma; NR = Not Recorded.
Most tumors were classified as adenocarcinomas that were clearly invasive and morphologically epithelial. The morphology of prostate cancers ranged from well-differentiated with gland-like growth patterns to poorly differentiated consisting of strands or fields of tumor cells. However, even poorly differentiated prostate cancers contained areas with cancer cells lining up in pseudo-acinar arrangements or multicellular sheets (Figure 2). Adenocarcinomas did not display comedo or cribriform growth patterns. Carcinosarcomas consisted of areas of adenocarcinoma differentiation that merged into areas of sarcomatous growth suggestive of a mixed morphology rather than confluence of epithelial and mesenchymal tumors; these tumors were mostly found in the seminal vesicle (Figure 3).
Figure 2.
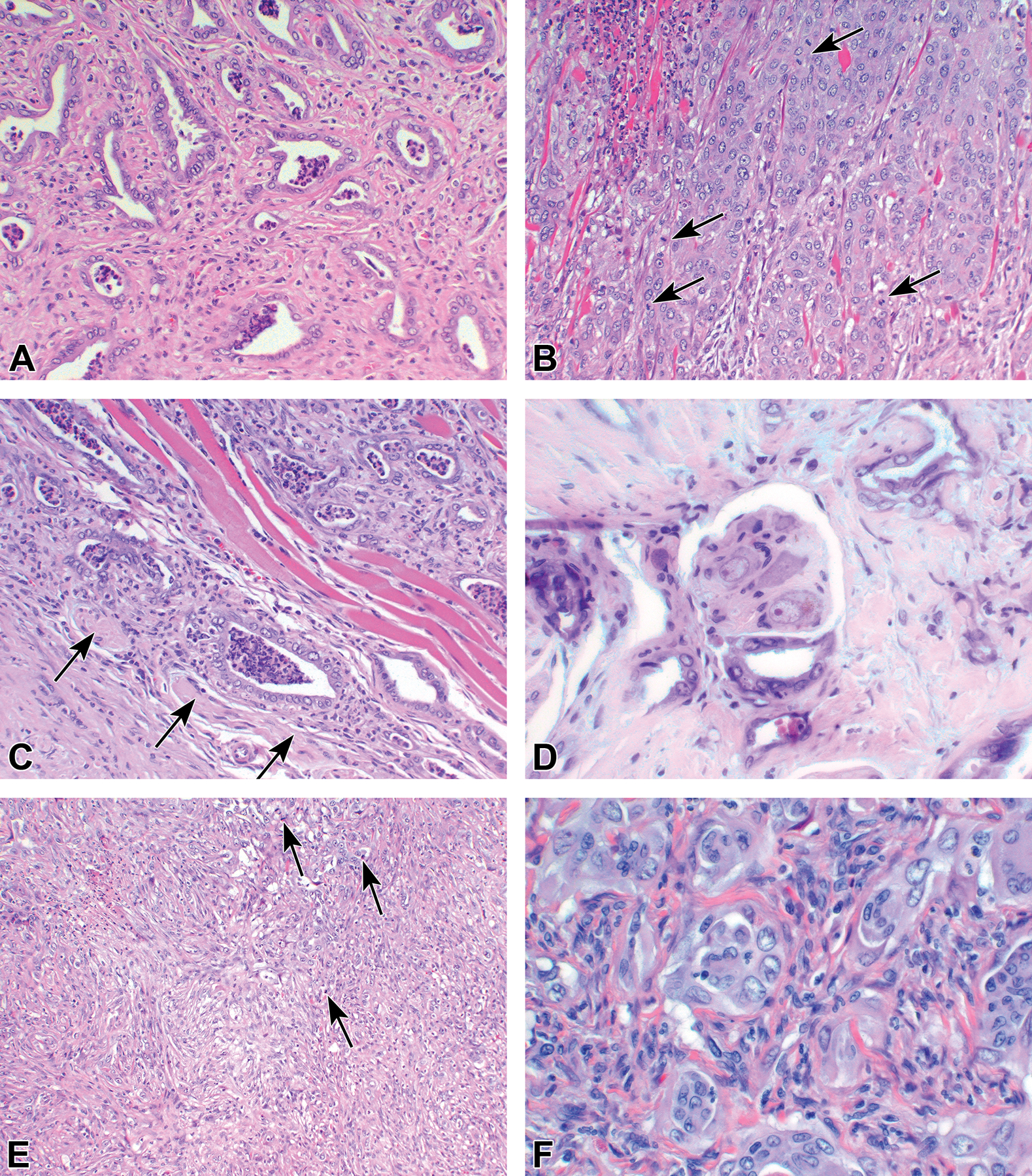
Examples of adenocarcinomas induced in the dorsolateral prostate. (A) Well differentiated tumor. (B) Moderately differentiated tumor showing mitotic figures (arrows). (C) Adenocarcinoma invading muscle and nerves (arrows). (D) High power of perineural invasion. (E and F) poorly differentiated carcinoma showing some mitotic figures in E (arrow).
Figure 3.
Examples of tumors induced in the seminal vesicle. (A) Small adenocarcinoma that penetrated the seminal vesicle capsule. (B) Small adenocarcinoma that invaded into the seminal vesicle capsule originating from an area of atypical hyperplasia (ah). (C) Tumor diagnosed as carcinosarcoma showing epithelial structures (arrows) and areas that resemble sarcoma (S). (D) Tumor diagnosed as carcinosarcoma showing epithelial cells (black arrows), areas that resemble sarcoma (S), and some mitotic figures (white arrow).
The earliest stages of these tumors were locally invasive and mostly well differentiated (Figures 3–5). Larger adenocarcinomas involved multiple accessory sex gland structures (Figure 5), and invaded surrounding tissues, and spread throughout abdominal structures or to the lungs (Figure 6) (Table 2). Mesenchymal tumors in the prostate and seminal vesicle were rare and were diagnosed as either histiocytic sarcoma, fibrosarcoma, or neurofibrosarcoma (Figure 6).
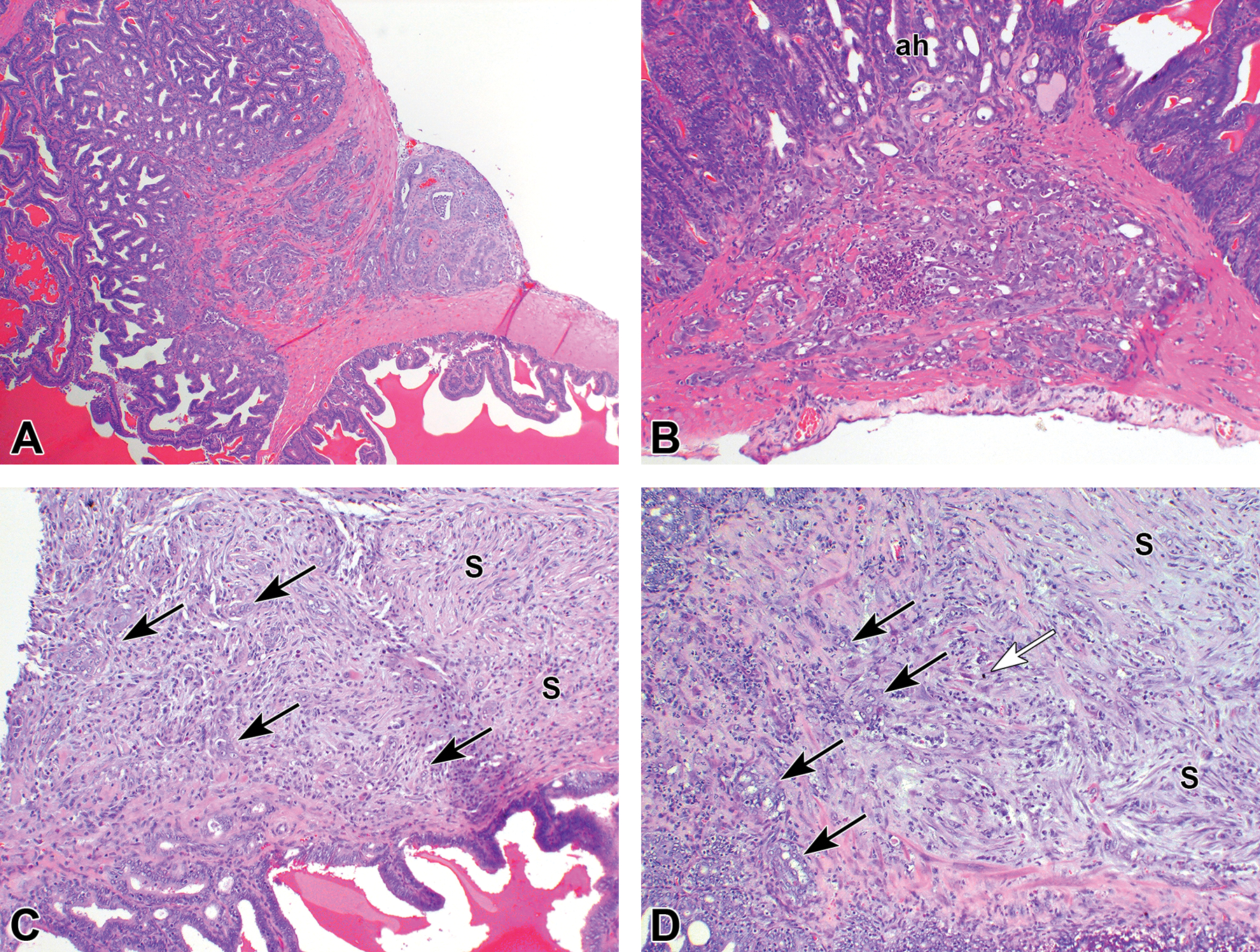
Figure 5.
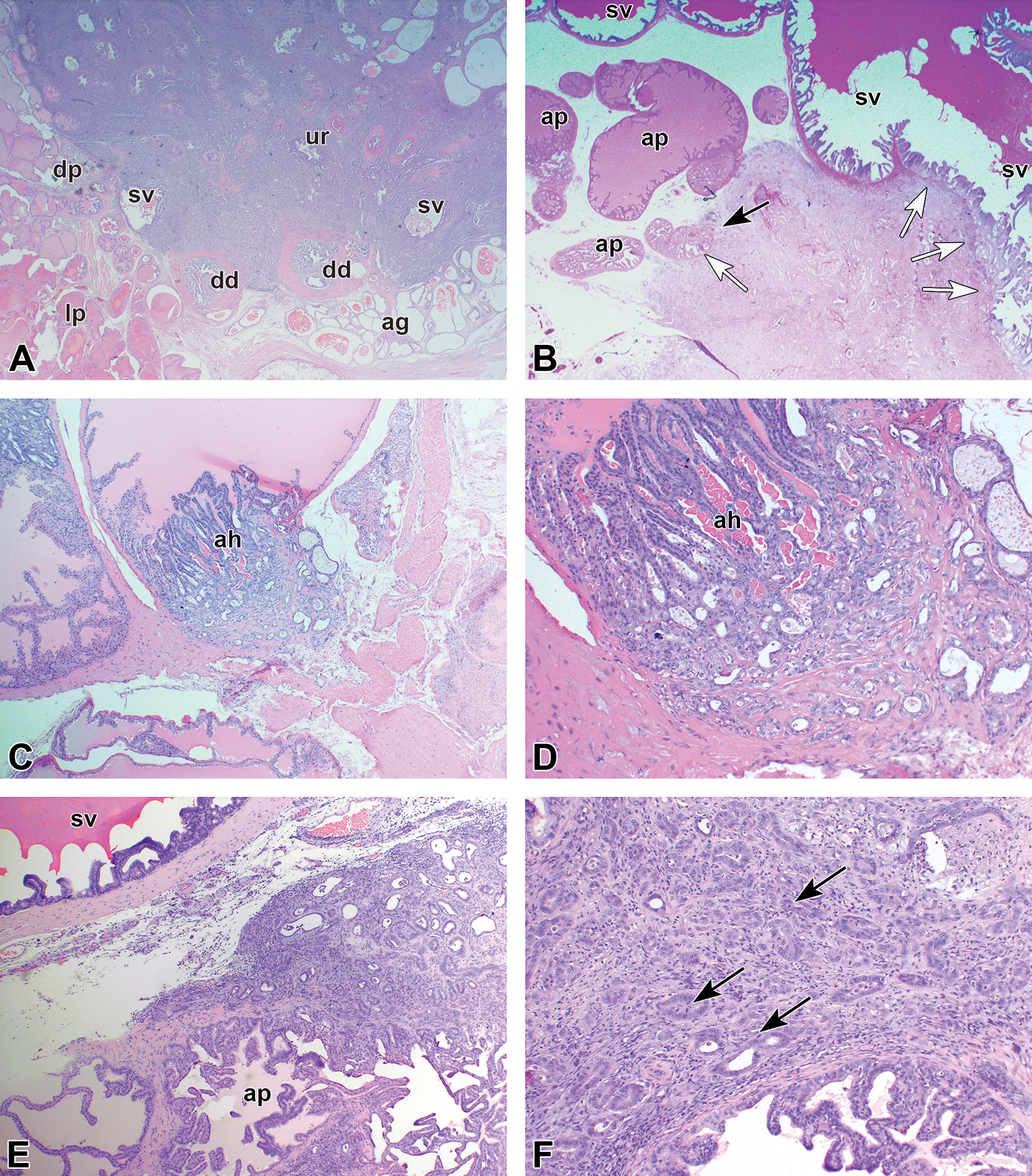
Examples of adenocarcinomas. (A) Large adenocarcinoma in the dorsolateral prostate region involving several structures and ducts of seminal vesicle (sv) and anterior prostate (ap). ag indicates ampullary gland; dd, deferent duct; dp, dorsal prostate; lp, lateral prostate; ur, urethra. (B) Large adenocarcinoma involving both anterior prostate (black arrows) and seminal vesicle (white arrows). (C) Small adenocarcinoma of the anterior prostate originating from an area of atypical hyperplasia (ah). (D) Higher power of panel C. (E) Larger adenocarcinoma confined to the anerior prostate (ap). (F) Higher power of panel E showing some mitotic figures (arrows).
Figure 6.
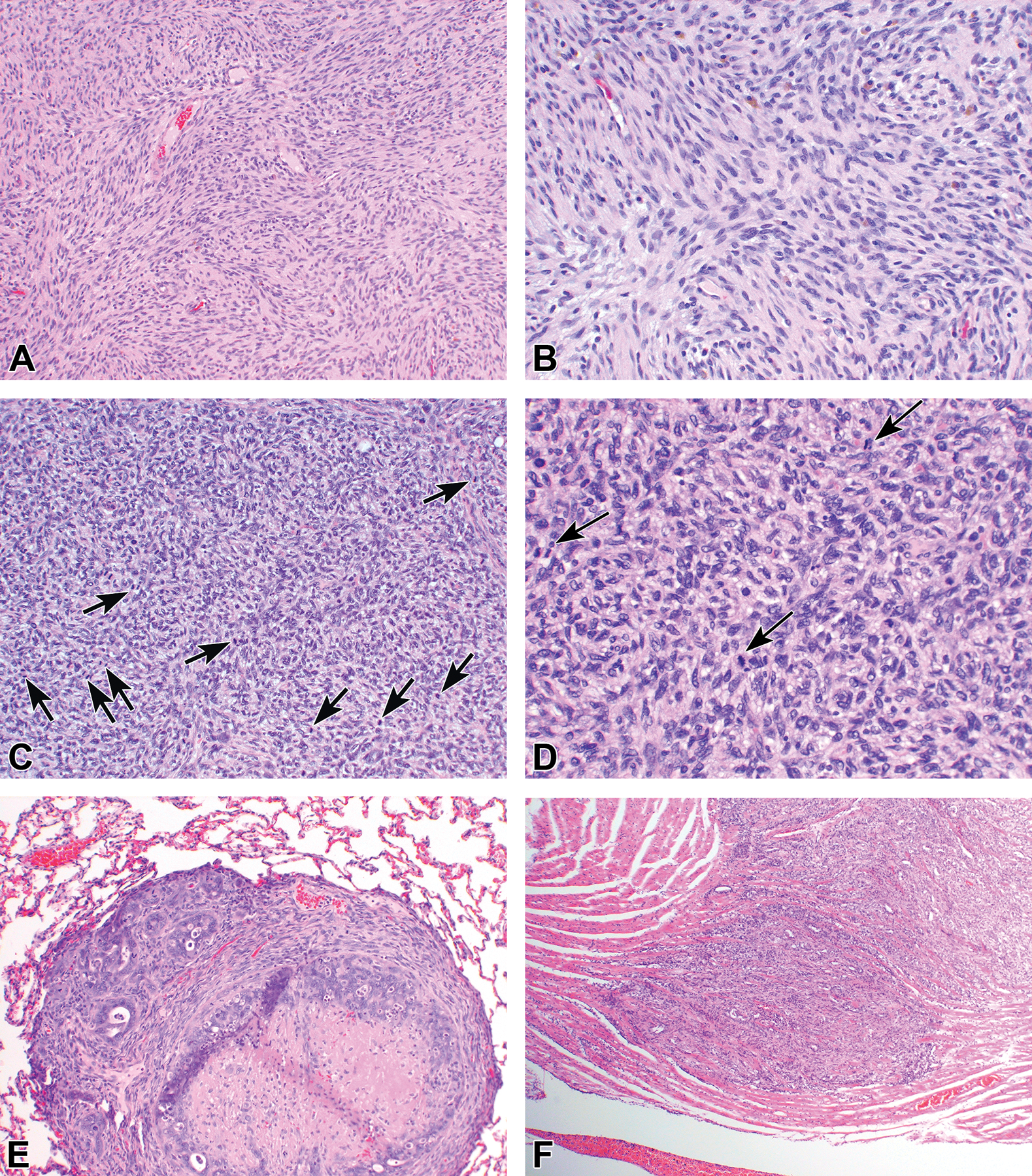
(A-D) Examples of sarcomas induced in the dorsolateral prostate. (A and B) Neurogenic sarcoma. (C and D) Histiocytic sarcoma showing many mitotic figures (arrows). Both types of tumors were rare and also observed occasionally at other organ sites, such as neurogenic sarcomas in the heart. (E) Pulmonary metastasis of prostate carcinoma showing central necrosis. (F) Rare cardiac metastasis of prostate carcinoma.
Prostate Cancer Induction in Different Rat Strains
In Table 2, details are presented of prostate cancer induction studies in seven different rat strains, including four repeat experiments with outbred WU rats and two repeat studies with inbred F344 rats. Details are presented about the lobe-specific incidences by size, separating small (≤5 mm in largest diameter) and larger tumors (>5 mm). Also included are data on the two most common neoplasms observed at other sites (Zymbal gland tumors and lymphomas) and metastases. Information is included about when the experiments were conducted, the source of rats, type of antiandrogen and number of testosterone propionate injections given prior to MNU, source and dose of MNU, and diet used, which are all variables that could affect outcome.
Wistar Furth and Lobund Wistar rats had the highest incidences of accessory sex gland tumors (98%−100%) and particularly dorsolateral and anterior prostate adenocarcinomas (90%−93%). The Harlan Sprague Dawley Wistar rat (Hsd:WI) had the lowest adenocarcinoma incidence in the accessory sex glands (55%) and dorsolateral and anterior prostate adenocarcinomas (53%). The Wistar WU and F344 strains also yielded high incidences of adenocarcinomas in the accessory sex glands (74%−89%) and dorsolateral and anterior prostate (68%−77%). Metastases occurred in a variable frequency, mostly to the lungs and various abdominal structures. Adenocarcinomas in Lobund Wistar rats were more often metastatic than in the other rat strains and resulted in the shortest survival, indicating the highly aggressive nature of accessory sex gland adenocarcinomas in this strain. Metastases were also more frequent in Copenhagen (COP) rats than in other strains, except for Lobund Wistar rats, which may be related to the longer average survival of these animals than rats of other strains. We also conducted a study with Sprague Dawley rats which also developed prostate and seminal vesicle adenocarcinomas (data not shown). However, because we applied a reduced dose of chronic testosterone in that experiment, we cannot compare the results with those shown in Table 2. Tumors occurred at various other sites as well, which is detailed in Table 3. Of note, Wistar Furth rats developed fewer tumors at other sites and Lewis (LEW) rats and, to a lesser extent, Wistar WU rats developed more tumors at other sites than observed in the other strains.
Table 3.
Comparison of Accessory Sex Gland Carcinoma Metastases and Tumors at Other Sites in Eight Rat Strains Treated with Various Variations of the Tumor Induction Protocol at Various Times between 1986 and 2016 Including Four Repeat Experiments with Wistar WU rats.
| Strain | LEW | WU-1 | WU-21 | W/F1 | F3441 | COP1 | LoWi1 | F344–2 | Hsd:WI2 | WU-3 | WU-4 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Year(s) | 1986–87 | 1988–89 | 1994–95 | 1994–95 | 1994–95 | 1994–95 | 1994–95 | 1998–99 | 2017–18 | 2010–11 | 2015–16 |
| Source of rats | Hsd US | Crl WIGA | Hsd US | Hsd US | Hsd US | Hsd US | NCI | Taconic | Hsd US | Hsd NL | Hsd NL |
| Type of antiandrogen used | CA | CA | CA | CA | CA | CA | CA | CA | FL | FL | FL |
| No. testosterone propionate injections | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 1 | 1 | 1 | 1 |
| Source of MNU | A-S | A-S | A-S | A-S | A-S | A-S | A-S | NCI | NCI-Rep | NCI-Rep | NCI-Rep |
| Dose of MNU (mg/kg) | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 30 | 30 | 30 | 30 |
| Diet | NIH-07 | NIH-07 | NIH-07 | NIH-07 | NIH-07 | NIH-07 | NIH-07 | NIH-07 | Tekl 18% | AIN-93M | AIN-93M |
|
| |||||||||||
| Number of Animals | 16 | 28 | 30 | 29 | 30 | 30 | 29 | 30 | 38 | 31 | 31 |
| Mean age at death (days) | 297 | 359 | 339 | 334 | 341 | 428 | 251 | 374 | 420 | 341 | 344 |
| Range | 158–469 | 126–568 | 109–497 | 160–460 | 183–503 | 221–559 | 152–320 | 275–405 | 259–4313 | 241–4003 | 216–4063 |
|
| |||||||||||
| Metastases | 0 | 6 (21) | 1 (3) | 3 (10) | 4 (13) | 13 (43) | 15 (52) | 3 (10) | 1 (3)4 | 04 | 5 (16) |
| – Lung | 0 | 0 | 1 (3) | 2 (7) | 3 (10) | 9 (30) | 7 (24) | 1 (3) | 04 | 04 | 5 (16) |
| – Abdominal | 0 | 6 (21) | 1 (3) | 1 (3) | 3 (10) | 10 (33) | 12 (41) | 3 (10) | 1 (3)4 | 04 | 0 |
| Other tumors: | |||||||||||
| – Abdominal | 0 | 6 (21) | 1 (3) | 1 (3) | 3 (10) | 10 (33) | 12 (41) | 3 (10) | 1 (3)4 | 04 | 0 |
| – Zymbal gland tumors | 2 (13) | 1 (4) | 3 (10) | 0 | 3 (10) | 0 | 1 (3) | 1 (3) | 04 | 7 (23)4 | 7 (23) |
| – Generalized lymphomas | 6 (38) | 7 (25) | 3 (10) | 0 | 1 (3) | 1 (3) | 0 | 1 (3) | 1 (3)4 | 3 (10)4 | 2 (6) |
| – Localized lymphomas | 1 (6) | 0 | 0 | 0 | 1 (3) | 1 (3) | 0 | 0 | 04 | 04 | 0 |
| – Benign skin tumors | 3 (19) | 4 (14) | 2 (7) | 2 (7) | 4 (13) | 1 (3) | 0 | NR | 1 (3)4 | 1 (3)4 | 2 (6) |
| – Malignant skin tumors | 0 | 3 (11) | 1 (3) | 0 | 0 | 1 (3) | 0 | NR | 1 (3)4 | 04 | 0 |
| – Intestinal adenocarcinomas | 0 | 2 (7) | 0 | 0 | 0 | 2 (7) | 0 | 0 | 04 | 1 (3)4 | 0 |
| – Lung-alveologenic tumors | 0 | 0 | 9 (30) | 0 | 2 (7) | 0 | 1 (3) | NR | NR | 04 | 0 |
| – Jaw odontoblastomas | 0 | 0 | 1 (3) | 0 | 1 (3) | 0 | 0 | 0 | 04 | 04 | 0 |
| – Sarcomas: | |||||||||||
| Thoracic neurogenic tumors | 0 | 2 (7) | 2 (7) | 0 | 0 | 0 | 0 | 1 (3) | 04 | 04 | 1 (3) |
| Other sarcomas | 2 (13) | 2 (7) | 4 (13) | 1 (3) | 3 (10) | 3 (10) | 1 (3) | 0 | 1 (3)4 | 04 | 0 |
| – Urothelial tumors | 1 (6) | 1 (4) | 0 | 0 | 0 | 2 (7) | 0 | 2 (7) | 1 (3)4 | 04 | 0 |
| – Mammary tumors | 0 | 0 | 0 | 0 | 3 (10) | 0 | 0 | 1 (3) | 1 (3)4 | 04 | 0 |
| – Forestomach tumors | 0 | 0 | 0 | 0 | 0 | 3 (10) | 0 | 0 | 1 (3)4 | 04 | 0 |
| – Thyroid adenomas | 0 | 0 | 1 (3) | 0 | 0 | 3 (10) | 0 | 1 (3) | 04 | 04 | 0 |
| – Pituitary adenomas | 0 | 0 | 2 (7) | 1 (3) | 1 (3) | 3 (10) | 0 | 4 (13) | 04 | 1 (3)4 | 0 |
| – Adrenal pheochromocytoma | 0 | 1 (4) | 0 | 0 | 0 | 0 | 0 | 0 | 04 | 0 | 0 |
| – Testicle/vas deferens tumors | 0 | 0 | 0 | 1 (3) | 0 | 1 (3) | 0 | 0 | 04 | 0 | 0 |
These rats strains were compared in a single concurrent experiment; all other rats strains are from separate experiments
This is a control group of a study carried out at IIT Research Institute
These studies were terminated at approximately 14 months after MNU injection; all other experiments were terminated when the last animals died or were euthanized because of moribundicity.
Based on gross observations without histological confirmation.
Abberviations: Hsd = Harlan Sprague Dawley; US = United States; Crl WIGA = Charles River Germany; NCI = National Cancer Institute; NL = Netherlands; Tekl = Teklad; CA = Cyproterone acetate; FL = Flutamide; A-S = Ash Stevens; NCI-Rep = NCI Carcinogen Repository; MNU = N-Methyl-N-Nitrosourea.
Effect of Tumor Induction Protocol Modifications
Administration of testosterone propionate prior to MNU was essential to induce a high incidence of prostate and seminal vesicle adenocarcinomas (Table 4). Pretreatment with antiandrogen followed by testosterone propionate was the most effective in inducing adenocarcinomas in any prostate gland lobe in a study with F344 rats (Table 4). The specific antiandrogen (cyproterone acetate or flutamide) used to pretreat animals did not impact the tumor incidence (Table 5). (Note that Table 5 summarizes the results of a study with a lower Silastic implant-delivered testosterone dose (2 cm × 2 cm implants.) than the 2 cm × 3 cm implant dose that was used in the studies shown in Tables 1 and 2). The number of injections of testosterone propionate (1 versus 3) given before MNU treatment also did not impact tumor incidence in F344 rats (Table 2) and in WU rats (data not shown). MNU obtained from Ash Stevens tended to be slightly more effective in inducing tumors than was MNU from the NCI repository, but this difference was not statistically significant (Tables 2 and 5). The dose of MNU (40, 30, or 20 mg/kg) had only a modest impact on prostate cancer incidence (Table 6); the apparently higher incidences of prostate cancer in rats receiving the two lower doses of MNU may be the result of the induction of fewer non-prostate lesions by these doses. The MNU dose was strongly inversely associated with survival and the number of tumors at sites other than the accessory sex glands (Table 6).
Table 4.
Comparison of Neoplastic Lesions in Accessory Sex Glands of F344 Rats Treated with MNU with or without Pretreatment with Cyproterone Acetate and Testosterone Propionate and Followed by Testosteronea
| Pretreatment Prior to MNU injection | Number (%) of rats with: |
||
|---|---|---|---|
| CA plus TP | TP only | None | |
| Number of Rats | 30 | 29 | 24 |
| Mean survival [days] (range) | 374 (275–405) | 385 (275–406) | 376 (227–406) |
| All Accessory Sex Glands Combined (dorsolateral and anterior prostate plus seminal vesicle): | |||
| Adenocarcinoma (or carcinosarcomab) | 26 (87)c,d | 24 (83)c,e | 13 (54)c,d,e |
| Any Prostate Gland Lobe (but not seminal vesicle only): | |||
| Adenocarcinoma (or carcinosarcomab) | 20 (67)f,g | 16 (55)f,h | 4 (17)f,g,h |
| Dorsolateral plus Anterior Prostate (clearly confined to these glands; with or without seminal vesicle lesions): | |||
| Adenocarcinoma | 6 (20) | 11 (38) | 3 (13) |
| Dorsolateral Prostate Region (originating from dorsolateral, anterior prostate, and/or seminal vesicle): | |||
| Adenocarcinoma (or carcinosarcomab) | 10 (33)i,j | 6 (22)i | 1 (4)j.j |
| Anterior Prostate/Seminal Vesicle Region (originating from anterior prostate and/or seminal vesicle): | |||
| Adenocarcinoma (or carcinosarcomab) | 8 (27) | 4 (14) | 4 (17) |
| Seminal Vesicle Only (clearly confined to this gland): | |||
| Adenocarcinoma (or carcinosarcomab) | 6 (20) | 6 (21) | 5 (21) |
| Accessory sex gland metastases: | |||
| All (abdominal, heart, lung, other) | 3 (10) | 4 (14) | 1 (4) |
| Abdominal structures | 3 (10) | 1 (3) | 1 (4) |
| Lung | 1 (3) | 3 (10) | 0 |
| Tumors at other sites than the accessory sex glands: | |||
| Tumors potentially causing moribundicity | 5 (17) | 7 (24) | 6 (25) |
| Lymphoma | 1 (3) | 3 (10) | 1 (4) |
| Zymbal gland tumor | 1 (3) | 2 (7) | 1 (4) |
| Other malignant tumors | 3 (10) | 2 (7) | 5 (21) |
F344 rats were obtained from Taconic, fed NIH-07 diet and sequentially treated with the anti-androgen cyproterone acetate (CA), one injection of testosterone propionate (TP), and 30 mg/kg MNU (from Ash-Stevens) followed by chronic treatment with testosterone by Silastic implants (2 implants filled over 2 cm length).
Carcinosarcomas were very rare; no sarcomas were found.
P = 0.12 (X-square test); P = 0.007 (X-square test for linear trend)
P = 0.014 for difference between these two groups (2-sided Fisher Exact test)
P = 0.036 for difference between these two groups (2-sided Fisher Exact test)
P < 0.001 (X-square test); P < 0.001 (X-square test for linear trend)
P < 0.001 for difference between these two groups (2-sided Fisher Exact test)
P = 0.005 for difference between these two groups (2-sided Fisher Exact test)
P = 0.031 P = 0.12 (X-square test); P = 0.009 (X-square test for linear trend)
P = 0.015 for difference between these two groups (2-sided Fisher Exact test)
Table 5.
Comparison of Neoplastic Lesions in Accessory Sex Glands of Wistar WU Rats Treated with Flutamide or Cyproterone Acetate followed by MNU From Ash-Stevens or the NCI Carcinogen Repository Followed by Testosteronea
| Number (%) of rats with: |
|||
|---|---|---|---|
| Pretreatment Source of MNU |
Flutamide NCI Repository | Cyproterone NCI Repository | Flutamide Ash Stevens |
| Number of Rats | 32 | 18 | 22 |
| Mean survival [days] (range) | 397 (311–409) | 403 (351–409) | 387 (328–408) |
| All Accessory Sex Glands Combined (dorsolateral and anterior prostate plus seminal vesicle): | |||
| Adenocarcinoma (or carcinosarcomab) | 21 (67.6) | 12 (66.7) | 18 (82.6) |
| Sarcoma | 2 (6.3) | 1 (5.6) | 1 (4.3) |
| Any Prostate Gland Lobe (but not seminal vesicle only): | |||
| Adenocarcinoma (or carcinosarcomab) | 19 (59.4) | 9 (1.3) | 15 (65.2)c |
| Sarcoma | 0 | 0 | 0 |
| Dorsolateral plus Anterior Prostate (clearly confined to these glands and microscopic size; with or without seminal vesicle lesions): | |||
| Adenocarcinoma | 19 (59.4) | 8 (1.3) | 15 (65.2) |
| Sarcoma | 0 | 0 | 0 |
| Dorsolateral Prostate Region (originating from dorsolateral, anterior prostate, and/or seminal vesicle): | |||
| Adenocarcinoma (or carcinosarcomab) | 0 | 1 (5.6) | 0 |
| Sarcoma | 1 (3.1)d | 0 | 0 |
| Anterior Prostate/Seminal Vesicle Region (originating from anterior prostate and/or seminal vesicle): | |||
| Adenocarcinoma (or carcinosarcomab) | 1 (3.1) | 2 (11.1) | 2 (8.7) |
| Sarcoma | 0 | 0 | 1 (4.3) |
| Seminal Vesicle Only (clearly confined to this gland): | |||
| Adenocarcinoma (or carcinosarcomab) | 2 (6.3) | 1 (5.6) | 1 (4.3) |
| Sarcoma | 0 | 0z | 1 (4.3)e |
WU rats were obtained from Harlan Sprague Dawley-Netherlands, fed NIH-07 diet and sequentially treated with anti-androgen, one injection of testosterone propionate, and 30 mg/kg MNU followed by chronic treatment with testosterone by Silastic implants (2 implants filled over 2 cm length). No statistically significant differences between groups were identified.
Carcinosarcomas were rare.
Not including one anterior prostate/seminal vesicle region tumor.
One more sarcoma was found in an animal with prostate carcinoma.
Two more sarcomas were found in animals with prostate carcinomas.
Table 6.
Comparison of Neoplastic Lesions in Accessory Sex Glands of Wistar WU Rats Treated with Three Different Doses of MNU from Ash-Stevens after pretreatment with Cyproterone Acetate and Testosterone Propionate and Followed by Testosteronea
| Number (%) of rats with: |
|||
|---|---|---|---|
| Dose of MNU | 40 mg/kg | 30 mg/kg | 20 mg/kg |
| Number of Rats | 29 | 27 | 25 |
| Mean survival [days] (range) | 360 (126–568) | 423 (176–627) | 537 (262–686) |
| Median survival [days] (95% CI) | 351 (322, 397)b,c,d | 415 (381, 465)b,c,e | 547 (497, 577)b,d,e |
| All Accessory Sex Glands Combined (dorsolateral and anterior prostate plus seminal vesicle): | |||
| Adenocarcinoma (or carcinosarcomaf) | 23 (79.3) | 25 (92.6) | 23 (92.0) |
| Any Prostate Gland Lobe (but not seminal vesicle only): | |||
| Adenocarcinoma (or carcinosarcomaf) | 21 (72.4)g | 23 (85.2)g | 23 (92.0)g |
| Dorsolateral plus Anterior Prostate (clearly confined to these glands; with or without seminal vesicle lesions): | |||
| Adenocarcinoma | 19 (65.5)h,i,j | 9 (33.3)h,i | 7 (28.0)h,j,k |
| Dorsolateral Prostate Region (originating from dorsolateral, anterior prostate, and/or seminal vesicle): | |||
| Adenocarcinoma (or carcinosarcomaf) | 16 (55.2) | 17 (63.0) | 17 (68.0) |
| Anterior Prostate/Seminal Vesicle Region (originating from anterior prostate and/or seminal vesicle): | |||
| Adenocarcinoma (or carcinosarcomaf) | 1 (3.4) | 1 (3.7) | 3 (12.0) |
| Seminal Vesicle Only (clearly confined to this gland): | |||
| Adenocarcinoma (or carcinosarcomaf) | 2 (6.9) | 6 (22.2) | 0 0.0232 |
| Accessory sex gland metastases: | |||
| All (abdominal, heart, lung, other) | 5 (17.2)l,m | 8 (29.6)l | 13 (52.0)l,m |
| Abdominal structures | 5 (17.2)l,n | 8 (29.6) l | 12 (48.0)l,n |
| Lung | 0h,n | 3 (11.1)h | 6 (24.0) h,n |
| Tumors at other sites than the accessory sex glands: | |||
| Tumors potentially causing moribundicity | 18 (62.1)o,p,q | 9 (33.3)o,p | 6 (24.0)o,q |
| Lymphoma | 7 (24.1)h,r,s | 1 (3.7)h,r | 0h,s |
| Zymbal gland tumor | 1 (3.4) | 4 (14.8) | 2 (8.0) |
| Other malignant tumors | 11 (37.9) | 4 (14.8) | 4 (16.0) |
WU rats were obtained from Harlan Sprague Dawley-Netherlands, fed NIH-07 diet and sequentially treated with anti-androgen, one injection of testosterone propionate, and 30 mg/kg MNU followed by chronic treatment with testosterone by Silastic implants (2 implants filled over 2 cm length).
P < 0.0001 (Kruskal-Wallis nonparatmetric ANOVA) and P < 0.0001 (X-square test for linear trend)
P = 0.030 for difference between these two groups (2-sided Mann-Whitney test)
P < 0.0001 for difference between these two groups (2-sided Mann-Whitney test)
P < 0.001 for difference between these two groups (2-sided Mann-Whitney test)
Carcinosarcomas were very rare; no sarcomas were found.
P = 0.056 (X-square test for linear trend)
P = 0.005 (X-square test for linear trend)
P = 0.032 for difference between these two groups (2-sided Fisher Exact test)
P = 0.008 for difference between these two groups (2-sided Fisher Exact test)
One of the anterior prostate tumors was a rare squamous cell carcinoma.
P = 0.007 (X-square test for linear trend)
P = 0.001 for difference between these two groups (2-sided Fisher Exact test)
P = 0.001 for difference between these two groups (2-sided Fisher Exact test)
P = 0.011 (X-square test for linear trend)
P = 0.037 for difference between these two groups (2-sided Fisher Exact test)
P = 0.007 for difference between these two groups (2-sided Fisher Exact test)
P = 0.052 for difference between these two groups (2-sided Fisher Exact test)
P = 0.012 for difference between these two groups (2-sided Fisher Exact test)
As time-to-death was markedly increased with decreasing MNU dose, the dose was inversely associated with the occurrence of detectable metastases (Table 6). The dose of Silastic implant-delivered testosterone does make a considerable difference as we have shown previously; comparing the effects of one 3 cm implant with those of two or four 3 cm implants, we showed that two 3 cm implants results in a maximal tumor response.10 The timing of MNU injection appears not very critical because we found comparable accessory sex glands tumor incidences administering MNU at 36, 48, or 56 hours after the testosterone propionate injection given ~24 hours after the last antiandrogen treatment (Table 7). Since MNU injection at 56 hours resulted in most adenocarcinomas directly traceable to an origin in anterior or dorsolateral prostate we used this time point (±2 hours) for most studies in Table 1.
Table 7.
Comparison of Neoplastic Lesions in Accessory Sex Glands of Wistar WU Rats Treated with MNU at Three Different Times after Testosterone Propionate Injectiona
| Number (%) of rats with: |
|||
|---|---|---|---|
| Time of MNU injection after testosterone propionate injection | 36 hours | 48 hours | 56 hours |
| Number of Rats | 23 | 20 | 27 |
| Mean survival [days] (range) | 411 (273–571) | 363 (207–497) | 376 (239–568) |
| Median survival [days] (95% CI) | 398 (381, 440) | 368 (324, 403) | 379 (343, 408) |
| All Accessory Sex Glands Combined (dorsolateral and anterior prostate plus seminal vesicle): | |||
| Adenocarcinoma (or carcinosarcomab) | 22 (96) | 16 (80) | 23 (85) |
| Any Prostate Gland Lobe (but not seminal vesicle only): | |||
| Adenocarcinoma (or carcinosarcomab) | 22 (96) | 16 (80) | 21 (78) |
| Dorsolateral plus Anterior Prostate (clearly confined to these glands; with or without seminal vesicle lesions | |||
| Adenocarcinoma | 2 (9)c | 4 (20) | 9 (33)c |
| Dorsolateral Prostate Region (originating from dorsolateral, anterior prostate, and/or seminal vesicle): | |||
| Adenocarcinoma (or carcinosarcomab) | 17 (74) | 12 (60) | 16 (59) |
| Anterior Prostate/Seminal Vesicle Region (originating from anterior prostate and/or seminal vesicle): | |||
| Adenocarcinoma (or carcinosarcomab) | 3 (13) | 1 (5) | 1 (4) |
| Seminal Vesicle Only (clearly confined to this gland): | |||
| Adenocarcinoma (or carcinosarcomab) | 0 | 0 | 2 (7) |
| Accessory sex gland metastases: | |||
| All (abdominal, heart, lung, other) | 13 (57)d | 8 (40) | 6 (22)d |
| Abdominal structures | 12 (52) | 8 (40) | 6 (22) |
| Lung | 3 (13) | 3 (15) | 0 |
| Tumors at other sites than the accessory sex glands: | |||
| Tumors potentially causing moribundicity | 7 (30) | 9 (45) | 15 (56) |
| Lymphoma | 3 (13) | 5 (25) | 7 (26) |
| Zymbal gland tumor | 3 (13) | 3 (15) | 1 (4) |
| Other malignant tumors | 2 (9) | 2 (10) | 7 (26) |
WU rats were obtained from Harlan Sprague Dawley-Netherlands, fed NIH-07 diet and sequentially treated with anti-androgen, one injection of testosterone propionate, and 30 mg/kg MNU followed by chronic treatment with testosterone by Silastic implants (2 implants filled over 2 cm length). of from Ash-Stevens after pretreatment with Cyproterone Acetate and Followed by Testosterone
Carcinosarcomas were very rare; no sarcomas were found.
P = 0.049 for difference between these two groups (2-sided Mann-Whitney test)
P = 0.020 for difference between these two groups (2-sided Mann-Whitney test)
Prostate Tumor Characteristics
As indicated above, the induced prostate adenocarcinomas had the capacity to metastasize; metastases were most commonly identified in rats bearing large prostate cancers (>1 cm diameter). Tumor spread occurred to abdominal structures, most likely though local invasion and seeding, and to the lungs and occasionally other sites (Figure 6). Grossly visible skeletal metastases were not observed, but no attempts were made to search for small skeletal lesions. It is not clear whether pelvic lymph nodes were metastatic sites because larger tumors often obscured pelvic structures.
Induction of a high incidence of prostate adenocarcinomas in this model requires chronic low-dose testosterone treatment after MNU administration,10 which strongly suggests that these tumors are androgen-dependent. Indeed, when we removed testosterone implants 28 weeks after they were implanted, prostate tumor incidence dropped significantly in animals that were castrated at that time and in rats that were left intact (Table 8). However, we could not detect androgen receptor expression by immunohistochemistry when we stained sections of large tumors. We previously reported the culture of cells isolated from large prostate adenocarcinomas in Wistar Furth rats and rats of other strains.23 When we injected cells of one of these cell strains into syngeneic Wistar Furth rats and castrated half of them, a slight, nonsignificant delay in growth was found (Figure 7). Doubling time for 6 tumors in each group was 7.28 days (95% confidence interval: 6.20 to 8.81) in intact animals and 7.73 days (95% CI: 6.24 to 10.13). Tumor volumes at 43 days after cell injection were not different (P = .419 and .209 for 2- and 1-sided t test, respectively).
Table 8.
Comparison of Grossly Visible Tumors in Accessory Sex Glands of Wistar WU Rats Administered Chronic Low Dose Testosterone after Sequential Treatment with Cyproterone Acetate, Testosterone Propionate and MNU with or without Removal of the Testosterone at 28 Weeks after MNUa
| Number (%) of rats with: |
|||
|---|---|---|---|
| Testosterone treatment | Chronicb | Removed at 28 weeks/castrated | Removed at 28 weeks/intact |
| Number of Rats | 27 | 24 | 27 |
| Mean Survival, days (range) | 362 (228–497) | 416 (242–579) | 403 (231–579) |
| Median Survival, days (95% confidence interval) | 364 (339, 384) | 389 (363, 469) | 383 (361, 444) |
| All Accessory Sex Glands Combined (dorsolateral and anterior prostate plus seminal vesicle): | |||
| Grossly visible tumors | 14 (51.9)c | 5 (20.8)c. d | 5 (18.5)c, e |
| Dorsolateral Prostate Region (originating from dorsolateral, anterior prostate, and/or seminal vesicle): | |||
| Grossly visible tumors | 6 (22.2) | 1 (4.2) | 2 (7.4) |
| Anterior Prostate/Seminal Vesicle Region (originating from anterior prostate and/or seminal vesicle): | |||
| Grossly visible tumors | 4 (14.8) | 0 | 3 (11.1) |
| Dorsolateral Prostate and Anterior Prostate/Seminal Vesicle Region: | |||
| Grossly visible tumors | 4 (14.8) | 4 (16.7) | 0 |
| Seminal Vesicle Only (clearly confined to this gland): | |||
| Grossly visible tumors | 0 | 0 | 0 |
WU rats were obtained from Harlan Sprague Dawley-Netherlands, fed NIH-07 diet and sequentially treated with anti-androgen, one injection of testosterone propionate, and 40 mg/kg MNU followed by chronic treatment with testosterone by Silastic implants (2 implants filled over 2 cm length). In two groups the implants were removed 28 weeks after MNU treatment. Unfortunately, the microscopic slides of this experiment were lost during a long-distance move and we only have gross data showing a 60–64% reduction of tumors after removal of testosterone implants 28 weeks after MNU injection.
Data taken from a treatment group detailed in Table 2 that live for more than 29 weeks.
P = 0.013 (Chi Square test).
P = 0.022 for difference with chronic testosterone treatment (one sided Fisher exact test).
P = 0.011 for difference with chronic testosterone treatment (one sided Fisher exact test).
Figure 7.
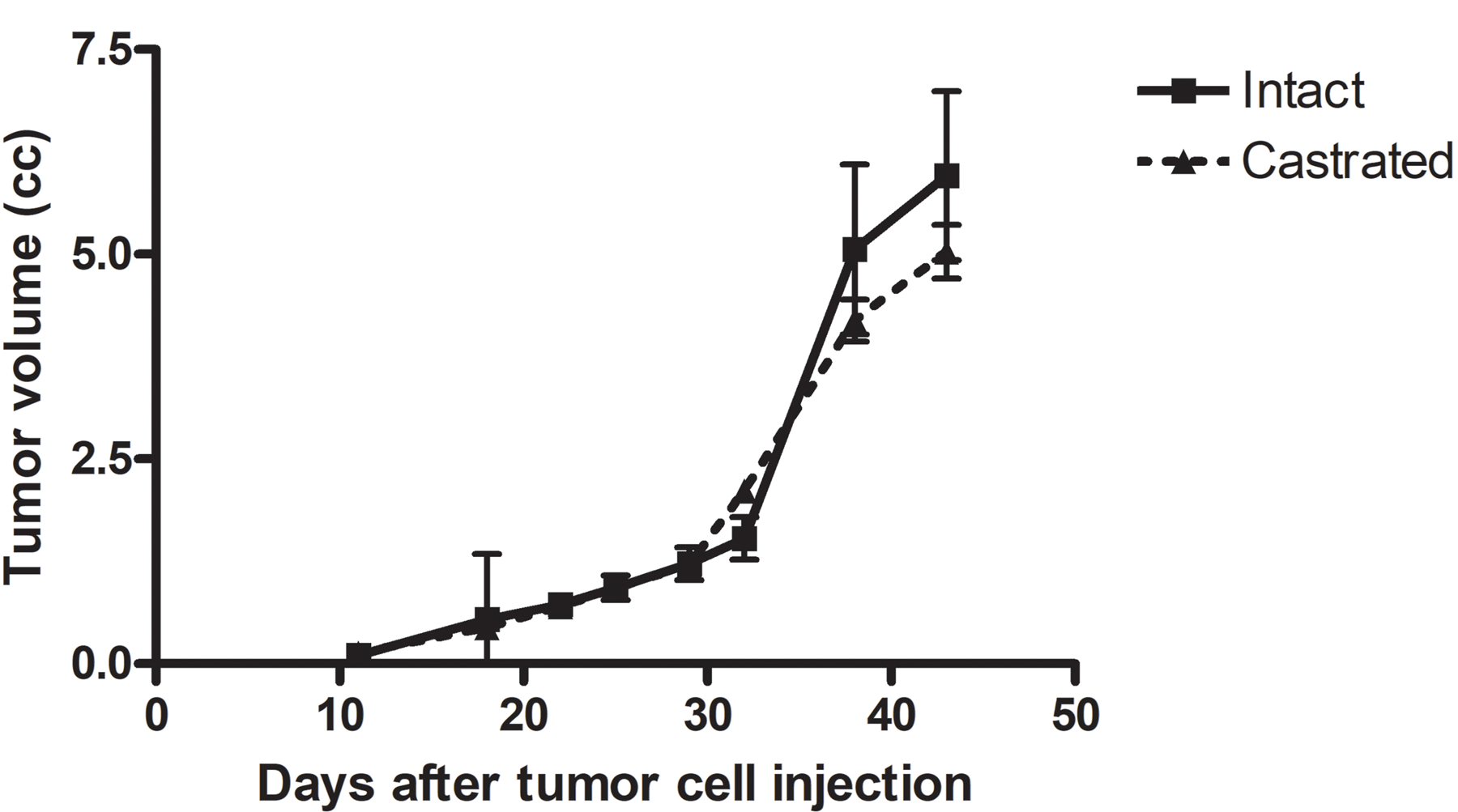
Effect of castration on in vivo growth of rat prostate cancer cells in syngeneic Wistar Furth rats (n = 6/group). Animals were castrated 11 days after tumor cell injection when tumors were first palpable. Tumor volume data are presented as mean ± standard error of the mean.
Results Summary
The MNU plus testosterone rat model produces a high incidence of metastatic prostatic adenocarcinomas in WU and F334 rats (and Wistar Furth and Lobund Wistar rats that are no longer available). The induction protocol requires androgen-induced stimulation of cell proliferation at the time of a single injection of MNU, even at low doses (20–30 mg/kg) of this carcinogen, and subsequent continuous treatment with testosterone by slow-release implants in a dose-related manner that plateaued at a dose resulting in circulating testosterone at the high end of physiological levels (~6 ng/mL).10 However, large adenocarcinomas are not very androgen dependent and androgen receptor-negative. Primary sites of metastasis are via the blood stream to the lungs and by seeding in abdominal structures, while the presence of osseous metastases is not clearly established.
Discussion
The incidence of cancers in the prostate and other accessory sex glands in WU rats was remarkably stable in studies performed over more than 25 years in four institutions using three different sources of WU rats and six different diets. Minor modifications to study protocols had relatively little impact on tumor incidences. After a 18- to 21-day course of Flutamide followed by one injection of testosterone propionate, an MNU dose of 30 mg/kg followed by two Silastic tubing implants tightly packed over 3 cm with crystalline testosterone is a reliable and reproducible protocol to induce a high incidence of adenocarcinomas originating in the anterior and dorsolateral prostate lobes. Outbred Wistar WU rats and inbred Fischer F344 rats develop the highest incidence and are commercially available strains; inbred Wistar Furth rats developed the highest incidence, but are currently only available as cryopreserved embryos. The current protocol used for induction of prostate cancer in these studies is provided in the Supplemental Material.
Spontaneous neoplasms in the accessory sex glands in WU rats are extremely rare. In 3,861 male Cpb:WU rats fed a natural ingredient stock diet22 in control groups of two-year and life-span toxicology and carcinogenicity studies performed in the 1970s and 1980s, only one dorsolateral prostate adenocarcinoma, one ventral prostate adenoma, and one carcinoma (not otherwise specified) were observed (unpublished data from the TNO-CIVO Toxicology and Nutrition Institute, Zeist, The Netherlands). In studies of background pathology in aging rats of most other strains, including the F344 strain, prostatic and seminal vesicle adenocarcinomas were very rare.29,32–35 Exceptions are Lobund Wistar rats and the Segaloff sub-strain of inbred ACI rats (ACI/segHapBR). Pollard36 has reported 27% incidence of spontaneous accessory sex gland carcinomas in untreated aging partially inbred Lobund Wistar rats and this was essentially reproduced by O’Sullivan et al.37 ACI/segHapBR rats have been reported to develop adenocarcinomas exclusively of the ventral prostate in a 30% to 40% incidence in animals older than 24 months.38
The malignant tumors induced in the dorsolateral and anterior prostate in the MNU plus testosterone rat model are adenocarcinomas that have the ability to metastasize, mostly via the bloodstream to the lungs and by seeding in the abdominal cavity, but not to the bone as often occurs in humans although skeletal metastases may have been missed in out studies. The rat dorsolateral and anterior prostate, unlike the ventral prostate lobe, are embryologically homologous with the human prostate.39,40 Some large tumors, particularly those originating in the seminal vesicle, were adenocarcinomas that merged into sarcomatoid neoplasms designated carcinosarcomas which are not common in humans; the seminal vesicle is an extremely rare tumor site in humans. The induction of prostate adenocarcinomas in this model clearly depends on the protracted, but modest, elevation of circulating testosterone.10 Consistent with this requirement, the incidence of grossly visible accessory sex gland tumors was markedly reduced by removal of testosterone implants, with or without castration, 28 weeks after carcinogen administration. These tumor characteristics strongly suggest androgen dependence which is a feature of virtually all human prostate cancers. However, androgen receptor expression was absent in large adenocarcinomas more than one year after MNU injection, and growth of xenografted cells derived from such tumors was only slightly reduced by castration of syngeneic hosts. Although not common, there are reports of undetectable androgen receptors in advanced-stage human prostate cancers,41,42 and approximately 6% to 15% of patients with previously untreated advanced stage prostate cancer do not respond to androgen deprivation therapy.43,44 Thus, the MNU plus testosterone rat model has many important similarities with human prostate cancer and originates in lobes that are embryologically homologous with the human prostate, but lacks the typical spread to bone and the high incidence of intraepithelial neoplasia that occur in humans.
Androgen is required for induction of prostate cancer in this model, but advanced large tumors do not seem androgen dependent. Although this is not comparable with human prostate cancer, the androgen requirements of untreated fatal advanced stage prostate tumors are not well established. Boileau, Clinton, and Erdman have used this model precisely following our protocol to study the effect of lycopene and tomato powder feeding and energy restriction on prostate carcinogenesis in WU rats.45 By 73 weeks into the experiment, 80% of control group rats had developed adenocarcinoma or in some rats’ carcinoma in situ or sarcoma in the prostate and seminal vesicle complex. The tumors had a progressively reduced expression of androgen receptor (% positive cells and % positive samples) with decreasing degree of differentiation,46 in line with our observations.
Pollard and co-workers have reported induction by MNU and testosterone of accessory sex gland adenocarcinomas in partially inbred Lobund Wistar rats that to the best of our knowledge are no longer available.47–49 Most accessory sex gland cancers in this model appear to arise in the seminal vesicle.36 This group also found that castration late in the process of tumor induction had no inhibitory effect50; this result is similar to our findings of androgen independence of advanced stage tumors in chemically-hormonally-induced prostate cancer. Only few other groups have used the Lobund-Wistar model37,51,52 and there are no reports on this model after 2015.53
Other similar models have been reported but have not been used by others than the authors of those reports, Pour and Stepan54 and Pour et al.55 and Shirai and coworkers.56–58 The carcinogen 3,2’-dimethyl-4-aminobiphenyl (DMAB) followed by testosterone propionate-filled Silastic implants induced adenocarcinomas in the dorsolateral prostate and seminal vesicle in Fischer F344 rats.56 Consistent with our findings, when DMAB plus testosterone propionate-treated animals were castrated late in the protocol, the incidence of dorsolateral and seminal vesicle carcinomas was not changed and the carcinomas did not express androgen receptor.57,58
Critical technical issues necessary to obtain prostate cancer in reproducible incidences include (1) the rat strain used; (2) carefully timed administration of testosterone to stimulate cell proliferation in the prostate at the time of MNU administration is essential for a robust cancer response; (3) assurance of carcinogen concentration and stability. MNU is both light and pH sensitive, and degrades rapidly if not handled under appropriate conditions. It is essential that MNU dosing solutions be prepared shortly prior to administration at acid pH and protected from light; (4) administration of testosterone in Silastic implants is most effectively performed using crystalline testosterone rather than testosterone propionate. Testosterone propionate is released from the Silastic capsule much more rapidly than is crystalline testosterone, which results in a short-term surge in plasma testosterone levels rather than a steady, long-term elevation10,59; (5) the diameter, wall thickness, and length of Silastic capsules determines the release testosterone release; the quantity of testosterone inside the tubing determines the duration of release59; (6) immersion and rinsing of the tubing before implantation avoids a large initial surge of testosterone release59; (7) neoplastic development requires at least 8 to 9 months (and more commonly, 12–13 months) after MNU injection; and (8) careful microscopic examination of the prostate-seminal vesicle complex using step sections to identify microscopic size lesions.11
Conclusions
The MNU plus testosterone rat model of prostate carcinogenesis is a robust, reproducible, reliable prostate cancer animal model that is representative of human prostate cancer in many respects and has been used extensively in chemoprevention studies up to the present day with good predictive value of findings in human chemoprevention clinical trials. The model may also be useful to generate prostate cancers for future therapeutic studies and as a source of rat prostate cancer cell lines.23 The induction protocol needs to be followed rather precisely and the rat strain used is of critical importance, with the commercially available outbred Wistar WU rats and the inbred Fischer F344 strain yielding the highest tumor incidences.
Supplementary Material
Figure 4.
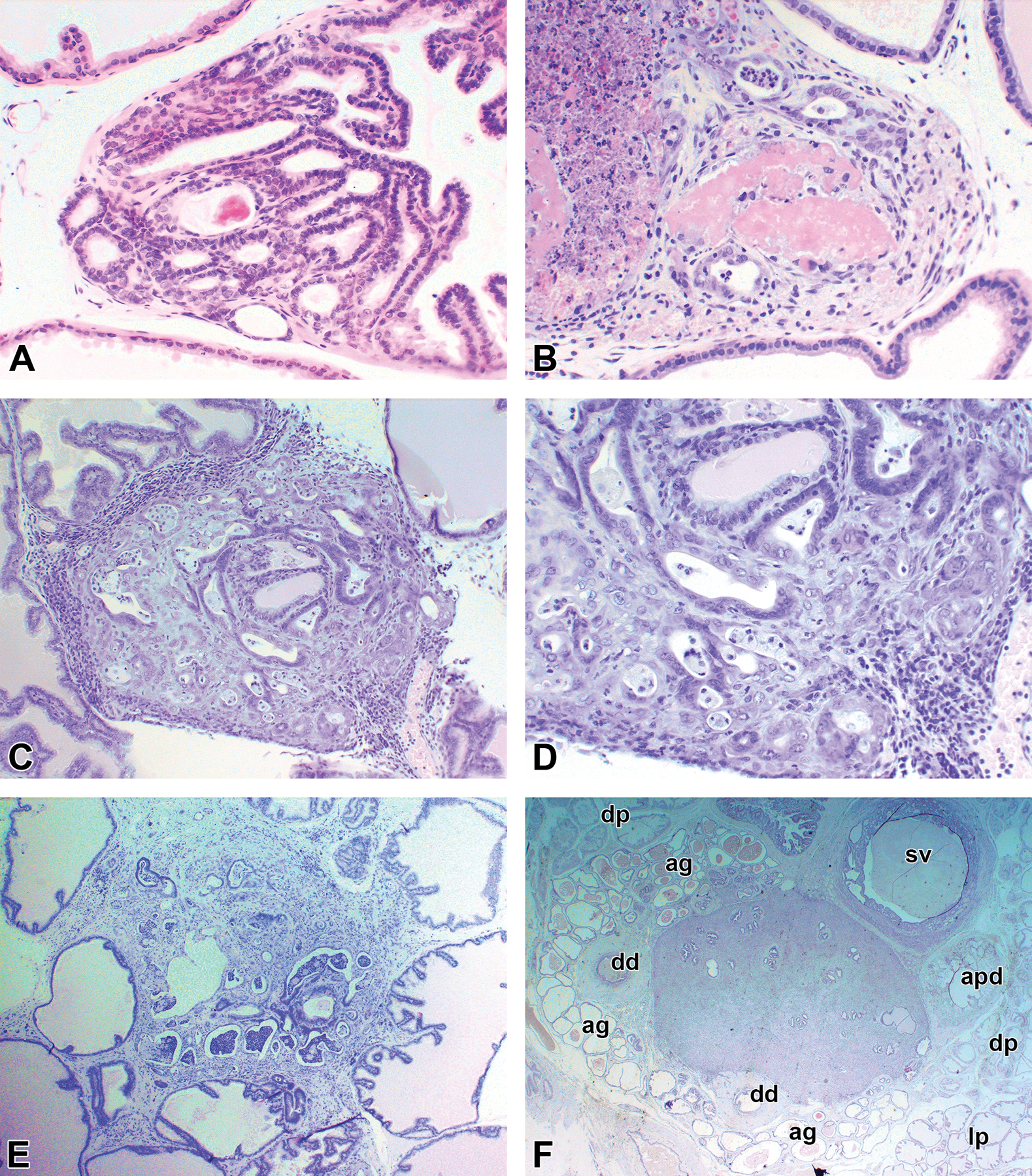
Examples of adenocarcinomas induced in the dorsolateral prostate. (A and B) The earliest lesions observed. (C and D) Lower and higher power of a small adenocarcinoma. (E) Larger small adenocarcinoma clearly located in the dorsolateral prostate. (F) Larger but still microscopic carcinoma involving several structures. ag indicates ampullary gland; ap, ducts of anterior prostate; dd, deferent duct; dp, dorsal prostate; lp, lateral prostate; sv, seminal vesicle.
Acknowledgments
The authors acknowledge the many critical contributions of the animal care staff at NYU, UIC, and IITRI and the histology facility staff at NYU and the Research Histology Core at UIC established with the support of the Vice Chancellor of Research. The authors dedicate this paper to honor the memory of Dr. Tomoyuki Shirai, a Japanese pathologist, who pioneered prostate carcinogenesis models in rats in Japan and made major contributions to prostate cancer research and toxicologic pathology.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The research described in this paper was supported in part by NIH/NCI grants R01CA43151, R01CA16087, R01 CA76426, R01CA104334, and R01 CA172169 (subcontract from Texas Tech University) to MCB, and P30CA16087 to New York University School of Medicine; and by the following contracts from NCI Division Cancer Prevention to DLM at IIT Research Institute: N01-CN-15110, N01-CN-25017, N01-CN-35566, N01-CN-43303, N01-CN-65120, N01-CN-85097, N01-CN-85177, N01-CN-95113, HHSN2612015000421, HHSN2612012000161, and 75N91019D00012.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplemental Material
Supplemental tables and figures can be found at http://tpx.sagepub.com/supplemental.
References
- 1.Pienta KJ, Abate-Shen C, Agus DB, et al. The current state of preclinical prostate cancer animal models. Prostate 2008;68(6):629–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arriaga JM, Abate-Shen C. Genetically engineered mouse models of prostate cancer in the postgenomic era. Cold Spring Harb Perspect Med 2019;9(2):a030528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grabowska MM, DeGraff DJ, Yu X, et al. Mouse models of prostate cancer: picking the best model for the question. Cancer Metastasis Rev 2014;33(2–3):377–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ittmann M, Huang J, Radaelli E, et al. Animal models of human prostate cancer: the consensus report of the New York meeting of the Mouse Models of Human Cancers Consortium Prostate Pathology Committee. Cancer Res 2013;73(9):2718–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein RD. The use of genetically engineered mouse models of prostate cancer for nutrition and cancer chemoprevention research. Mutat Res 2005;576(1–2):111–119. [DOI] [PubMed] [Google Scholar]
- 6.Lamb DJ, Zhang L. Challenges in prostate cancer research: animal models for nutritional studies of chemoprevention and disease progression. J Nutr 2005;135(suppl 12):3009S–3015S. [DOI] [PubMed] [Google Scholar]
- 7.Bosland MC, Prinsen MK, Kroes R. Adenocarcinomas of the prostate induced by N-nitroso-N-methylurea in rats pretreated with cyproterone acetate and testosterone. Cancer Lett 1983;18(1):69–78. [DOI] [PubMed] [Google Scholar]
- 8.Bosland MC, Prinsen MK. Induction of dorsolateral prostate adenocarcinomas and other accessory sex gland lesions in male Wistar rats by a single administration of N-methyl-N-nitrosourea, 7,12-dimethylbenz(a) anthracene, and 3,2’-dimethyl-4-aminobiphenyl after sequential treatment with cyproterone acetate and testosterone propionate. Cancer Res 1990;50(3):691–699. [PubMed] [Google Scholar]
- 9.Bosland MC, Prinsen MK, Rivenson A, et al. Induction of proliferative lesions of ventral prostate, seminal vesicle, and other accessory sex glands in rats by N-methyl-N-nitrosourea: effect of castration, pretreatment with cyproterone acetate and testosterone propionate and rat strain. Prostate 1992;20(4):339–353. [DOI] [PubMed] [Google Scholar]
- 10.Bosland MC. Testosterone treatment is a potent tumor promoter for the rat prostate. Endocrinology 2014;155(12):4629–4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCormick DL, Rao KV, Dooley L, et al. Influence of N-methyl-N-nitrosourea, testosterone, and N-(4-hydroxyphenyl)-all-trans-retinamide on prostate cancer induction in Wistar-Unilever rats. Cancer Res 1998;58(15):3282–3288. [PubMed] [Google Scholar]
- 12.McCormick DL, Rao KV, Steele VE, Lubet RA, Kelloff GJ, Bosland MC. Chemoprevention of rat prostate carcinogenesis by 9-cis-retinoic acid. Cancer Res 1999;59(3):521–524. [PubMed] [Google Scholar]
- 13.McCormick DL, Johnson WD, Lubet RA, Steele VE, Bosland MC. Differential chemopreventive activity of the antiandrogen, flutamide, and the antiestrogen, tamoxifen, in the rat prostate. Proc Am Assoc Cancer Res 2002;43:640. [Google Scholar]
- 14.McCormick DL, Johnson WD, Kozub NM, et al. Chemoprevention of rat prostate carcinogenesis by dietary 16alpha-fluoro-5-androsten-17-one (fluasterone), a minimally androgenic analog of dehydroepiandrosterone. Carcinogenesis 2007;28(2):398–403. [DOI] [PubMed] [Google Scholar]
- 15.McCormick DL, Johnson WD, Bosland MC, Lubet RA, Steele VE. Chemoprevention of rat prostate carcinogenesis by soy isoflavones and by Bowman-Birk inhibitor. Nutr Cancer 2007;57(2):184–193. [DOI] [PubMed] [Google Scholar]
- 16.McCormick DL, Johnson WD, Haryu TM, Bosland MC, Lubet RA, Steele VE. Null effect of dietary restriction on prostate carcinogenesis in the Wistar-Unilever rat. Nutr Cancer 2007;57(2):194–200. [DOI] [PubMed] [Google Scholar]
- 17.McCormick DL, Rao KV, Johnson WD, Bosland MC, Lubet RA, Steele VE. Null activity of selenium and vitamin E as cancer chemopreventive agents in the rat prostate. Cancer Prev Res (Phila) 2010;3(3):381–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murillo G, Peng X, Muzzio M, et al. Exceptional activity of ARN-509 (apalutamide) in prostate cancer prevention in rats. Cancer Res 2019;79(suppl 13):2730. [Google Scholar]
- 19.Rao KV, Johnson WD, Bosland MC, et al. Chemoprevention of rat prostate carcinogenesis by early and delayed administration of dehydroepian-drosterone. Cancer Res 1999;59(13):3084–3089. [PubMed] [Google Scholar]
- 20.Klein EA, Thompson IM Jr, Tangen CM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011;306(14):1549–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taneja SS, Morton R, Barnette G, Sieber P, Hancock ML, Steiner M. Prostate cancer diagnosis among men with isolated high-grade intraepithelial neoplasia enrolled onto a 3-year prospective phase III clinical trial of oral toremifene. J Clin Oncol 2013;31(5):523–529. [DOI] [PubMed] [Google Scholar]
- 22.de Groot AP, Feron VJ, Immel HR. Induction of hyperplasia in the bladder epithelium of rats by a dietary excess of acid or base: implications for toxicity/carcinogenicity testing. Food Chem Toxicol 1988;26(5):425–434. [DOI] [PubMed] [Google Scholar]
- 23.Condon MS, Kaplan LA, Crivello JF, Horton L, Bosland MC. Multiple pathways of prostate carcinogenesis analyzed by using cultured cells isolated from rats treated with N-methyl-N-nitrosourea and testosterone. Mol Carcinog 1999;25(3):179–186. [PubMed] [Google Scholar]
- 24.Tomayko MM, Reynolds CP. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol 1989;24(3):148–154. [DOI] [PubMed] [Google Scholar]
- 25.Bosland MC. Hyperplasia, prostate, rat. In: Jones TC, Mohr U, Hunt RD, eds. Genital System (Monographs on Pathology of Laboratory Animals) Berlin, Germany: Springer Verlag; 1987:267–272. [Google Scholar]
- 26.Bosland MC. Adenoma, prostate, rat. In: Jones TC, Mohr U, Hunt RD, eds. Genital System (Monographs on Pathology of Laboratory Animals) Berlin, Germany: Springer Verlag; 1987:261–266. [Google Scholar]
- 27.Bosland MC. Adenocarcinoma, prostate, rat. In: Jones TC, Mohr U, Hunt RD, eds. Genital System (Monographs on Pathology of Laboratory Animals) Berlin, Germany: Springer Verlag; 1987:272–275. [Google Scholar]
- 28.Bosland MC. Adenocarcinoma, seminal vesicle/coagulating gland, rat. In: Jones TC, Mohr U, Hunt RD, eds. Genital System (Monographs on Pathology of Laboratory Animals) Berlin, Germany: Springer Verlag; 1987:272–275. [Google Scholar]
- 29.Creasy D, Bube A, de Rijk E, et al. Proliferative and nonproliferative lesions of the rat and mouse male reproductive system. Toxicol Pathol 2012;40(suppl 6):40S–121S. [DOI] [PubMed] [Google Scholar]
- 30.Bosland MC, Tuomari DL, Elwell MR, Shirai T, Ward JM, McConnell RF. Proliferative lesions of the prostate and other accessory sex glands in male rats, URG-4. In: Guides for Toxicologic Pathology, STP/ARP/AFIP, Washington DC; 1998, pp 1–20. (https://www.toxpath.org/docs/SSNDC/MaleSexGlandsPro.pdf) [Google Scholar]
- 31.Bosland MC. The use of animal models in defining efficacy of chemo-prevention agents against prostate cancer. Europ Urol 1999;35(5–6):459–463. [DOI] [PubMed] [Google Scholar]
- 32.Mitsumori K, Elwell MR. Proliferative lesions in the male reproductive system of F344 rats and B6C3F1 mice: incidence and classification. Environ Health Perspect 1988;77:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haseman JK, Huff JE, Rao GN, Arnold JE, Boorman GA, McConnell EE. Neoplasms observed in untreated and corn oil gavage control groups of F344/N rats and (C57BL/6N X C3H/HeN)F1 (B6C3F1) mice. J Natl Cancer Inst 1985;75(5):975–984. [DOI] [PubMed] [Google Scholar]
- 34.Reznik G, Hamlin MH II, Ward JM, Stinson SF. Prostatic hyperplasia and neoplasia in aging F344 rats. Prostate 1981;2(3):261–268. [DOI] [PubMed] [Google Scholar]
- 35.Shoda T, Mitsumori K, Imazawa T, et al. A spontaneous seminal vesicle adenocarcinoma in an aged F344 rat. Toxicol Pathol 1998;26:448–451. [DOI] [PubMed] [Google Scholar]
- 36.Pollard M. Dihydrotestosterone prevents spontaneous adenocarcinomas in the prostate-seminal vesicle in aging L-W rats. Prostate 1998;36(3):168–171. [DOI] [PubMed] [Google Scholar]
- 37.O’Sullivan J, Sheridan J, Mulcahy H, Tenniswood M, Morrissey C. The effect of green tea on oxidative damage and tumour formation in Lobund-Wistar rats. Eur J Cancer Prev 2008;17(6):489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ward JM, Reznik G, Stinson SF, Lattuada CP, Longfellow DG, Cameron TP. Histogenesis and morphology of naturally occurring prostatic carcinoma in the ACI/segHapBR rat. Lab Invest 1980;43(6):517–522. [PubMed] [Google Scholar]
- 39.Cunha GR, Vezina CM, Isaacson D, et al. Development of the human prostate. Differentiation 2018;103:24–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Timms BG. Prostate development: a historical perspective. Differentiation 2008;76(6):565–577. [DOI] [PubMed] [Google Scholar]
- 41.Gorelic LS, Lamm DL, Ramzy I, Radwin HM, Shain SA. Androgen receptors in biopsy specimens of prostate adenocarcinoma. Heterogeneity of distribution and relation to prognostic significance of receptor measurements for survival of advanced cancer patients. Cancer 1987;60(2):211–219. [DOI] [PubMed] [Google Scholar]
- 42.van Aubel O, Bolt-de Vries J, Blankenstein MA, Schröder FH. Prediction of time to progression after orchiectomy by the nuclear androgen receptor content from multiple biopsy specimens in patients with advanced prostate cancer. Prostate 1988;12(3):191–198. [DOI] [PubMed] [Google Scholar]
- 43.Wasan HS, Waxman J. Hormonal management of prostate cancer. Clin Endocrinol 1992;37:477–480. [DOI] [PubMed] [Google Scholar]
- 44.Palmberg C, Koivisto P, Visakorpi T, Tammela TL. PSA decline is an independent prognostic marker in hormonally treated prostate cancer. Eur Urol 1999;36(3):191–196. [DOI] [PubMed] [Google Scholar]
- 45.Boileau TW, Liao Z, Kim S, Lemeshow S, Erdman JW Jr, Clinton SK. Prostate carcinogenesis in N-methyl-N-nitrosourea (NMU)-testosterone-treated rats fed tomato powder, lycopene, or energy-restricted diets. J Natl Cancer Inst 2003;95(21):1578–1586. [DOI] [PubMed] [Google Scholar]
- 46.Liao Z, Wang S, Boileau TW, Erdman JW Jr, Clinton SK. Increased phospho-AKT is associated with loss of the androgen receptor during the progression of N-methyl-N-nitrosourea-induced prostate carcinogenesis in rats. Prostate 2005;64(2):186–199. [DOI] [PubMed] [Google Scholar]
- 47.Pollard M, Luckert PH. Promotional effects of testosterone and high fat diet on the development of autochthonous prostate cancer in rats. Cancer Lett 1986;32(2):223–227. [DOI] [PubMed] [Google Scholar]
- 48.Pollard M, Suckow MA. Hormone-refractory prostate cancer in the Lobund-Wistar rat. Exp Biol Med (Maywood) 2005;230(8):520–526. [DOI] [PubMed] [Google Scholar]
- 49.Pollard M, Suckow MA. Dietary prevention of hormone refractory prostate cancer in Lobund-Wistar rats: a review of studies in a relevant animal model. Comp Med 2006;56(6):461–467. [PubMed] [Google Scholar]
- 50.Pollard M, Luckert PH, Snyder D. Prevention and treatment of experimental prostate cancer in Lobund-Wistar rats. I. Effects of estradiol, dihy-drotestosterone, and castration. Prostate 1989;15(2):95–103. [DOI] [PubMed] [Google Scholar]
- 51.Hoover DM, Best KL, McKenney BK, Tamura RN, Neubauer BL. Experimental induction of neoplasia in the accessory sex organs of male Lobund-Wistar rats. Cancer Res 1990;50(1):142–146. [PubMed] [Google Scholar]
- 52.Wang J, Eltoum IE, Carpenter M, Lamartiniere CA. Genistein mechanisms and timing of prostate cancer chemoprevention in Lobund-Wistar rats. Asian Pac J Cancer Prev 2009;10(1):143–150. [PubMed] [Google Scholar]
- 53.Wilson MJ, Lind J, Sinha AA. Induction of a heparin-stimulated serine proteinase in sex accessory gland tumors of the Lobund-Wistar rat. Exp Mol Pathol 2015;99(1):39–43. [DOI] [PubMed] [Google Scholar]
- 54.Pour PM, Stepan K. Induction of prostatic carcinomas and lower urinary tract neoplasms by combined treatment of intact and castrated rats with testosterone propionate and N-nitrosobis(2-oxopropyl)amine. Cancer Res 1987;47(21):5699–5706. [PubMed] [Google Scholar]
- 55.Pour PM, Groot K, Kazakoff K, Anderson K, Schally AV. Effects of high-fat diet on the patterns of prostatic cancer induced in rats by N-nitrosobis(2-oxopropyl)amine and testosterone. Cancer Res 1991;51(18):4757–4761. [PubMed] [Google Scholar]
- 56.Shirai T, Tamano S, Kato T, Iwasaki S, Takahashi S, Ito N. Induction of invasive carcinomas in the accessory sex organs other than the ventral prostate of rats given 3,2’-dimethyl-4-aminobiphenyl and testosterone propionate. Cancer Res 1991;51(4):1264–1269. [PubMed] [Google Scholar]
- 57.Iwasaki S, Kato K, Mori T, Takahashi S, Futakuchi M, Shirai T. Development of androgen-independent carcinomas from androgen-dependent preneoplastic lesions in the male accessory sex organs of rats treated with 3,2’-dimethyl-4-aminobiphenyl and testosterone propionate. Jpn J Cancer Res 1999;90(1):23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takahashi S, Inaguma S, Sakakibara M, et al. DNA methylation in the androgen receptor gene promoter region in rat prostate cancers. Prostate 2002;52(1):82–88. [DOI] [PubMed] [Google Scholar]
- 59.Dziuk PJ, Cook B. Passage of steroids through silicone rubber. Endocrinology 1966;78(1):208–211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


