Abstract
Background
Vaccines are effective in preventing severe COVID‐19, a disease for which few treatments are available and which can lead to disability or death. Widespread vaccination against COVID‐19 may help protect those not yet able to get vaccinated. In addition, new and vaccine‐resistant mutations of SARS‐CoV‐2 may be less likely to develop if the spread of COVID‐19 is limited. Different vaccines are now widely available in many settings. However, vaccine hesitancy is a serious threat to the goal of nationwide vaccination in many countries and poses a substantial threat to population health. This scoping review maps interventions aimed at increasing COVID‐19 vaccine uptake and decreasing COVID‐19 vaccine hesitancy.
Objectives
To scope the existing research landscape on interventions to enhance the willingness of different populations to be vaccinated against COVID‐19, increase COVID‐19 vaccine uptake, or decrease COVID‐19 vaccine hesitancy, and to map the evidence according to addressed populations and intervention categories.
Search methods
We searched Cochrane COVID‐19 Study Register, Web of Science (Science Citation Index Expanded and Emerging Sources Citation Index), WHO COVID‐19 Global literature on coronavirus disease, PsycINFO, and CINAHL to 11 October 2021.
Selection criteria
We included studies that assess the impact of interventions implemented to enhance the willingness of different populations to be vaccinated against COVID‐19, increase vaccine uptake, or decrease COVID‐19 vaccine hesitancy. We included randomised controlled trials (RCTs), non‐randomised studies of intervention (NRSIs), observational studies and case studies with more than 100 participants. Furthermore, we included systematic reviews and meta‐analyses. We did not limit the scope of the review to a specific population or to specific outcomes assessed. We excluded interventions addressing hesitancy towards vaccines for diseases other than COVID‐19.
Data collection and analysis
Data were analysed according to a protocol uploaded to the Open Science Framework. We used an interactive scoping map to visualise the results of our scoping review. We mapped the identified interventions according to pre‐specified intervention categories, that were adapted to better fit the evidence. The intervention categories were: communication interventions, policy interventions, educational interventions, incentives (both financial and non‐financial), interventions to improve access, and multidimensional interventions. The study outcomes were also included in the mapping. Furthermore, we mapped the country in which the study was conducted, the addressed population, and whether the design was randomised‐controlled or not.
Main results
We included 96 studies in the scoping review, 35 of which are ongoing and 61 studies with published results. We did not identify any relevant systematic reviews. For an overview, please see the interactive scoping map (https://tinyurl.com/2p9jmx24).
Studies with published results
Of the 61 studies with published results, 46 studies were RCTs and 15 NRSIs. The interventions investigated in the studies were heterogeneous with most studies testing communication strategies to enhance COVID‐19 vaccine uptake. Most studies assessed the willingness to get vaccinated as an outcome. The majority of studies were conducted in English‐speaking high‐income countries. Moreover, most studies investigated digital interventions in an online setting. Populations that were addressed were diverse. For example, studies targeted healthcare workers, ethnic minorities in the USA, students, soldiers, at‐risk patients, or the general population.
Ongoing studies
Of the 35 ongoing studies, 29 studies are RCTs and six NRSIs. Educational and communication interventions were the most used types of interventions. The majority of ongoing studies plan to assess vaccine uptake as an outcome. Again, the majority of studies are being conducted in English‐speaking high‐income countries. In contrast to the studies with published results, most ongoing studies will not be conducted online. Addressed populations range from minority populations in the USA to healthcare workers or students. Eleven ongoing studies have estimated completion dates in 2022.
Authors' conclusions
We were able to identify and map a variety of heterogeneous interventions for increasing COVID‐19 vaccine uptake or decreasing vaccine hesitancy. Our results demonstrate that this is an active field of research with 61 published studies and 35 studies still ongoing. This review gives a comprehensive overview of interventions to increase COVID‐19 vaccine uptake and can be the foundation for subsequent systematic reviews on the effectiveness of interventions to increase COVID‐19 vaccine uptake.
A research gap was shown for studies conducted in low and middle‐income countries and studies investigating policy interventions and improved access, as well as for interventions addressing children and adolescents. As COVID‐19 vaccines become more widely available, these populations and interventions should not be neglected in research.
Plain language summary
Interventions to increase COVID‐19 vaccine uptake
Background
Vaccines are effective in preventing death or severe illness from COVID‐19, a disease for which few treatments are available. Widespread vaccination against COVID‐19 may help protect those not yet able to get vaccinated. However, many people do not want to get vaccinated against COVID‐19. This can put them at increased risk of severe disease and death.
What was our aim?
We wanted to find out which interventions to increase COVID‐19 vaccine uptake have been or are currently evaluated.
Methods
We searched medical databases and trial registries until the 11 of October 2021. We included all studies investigating interventions to increase COVID‐19 vaccine uptake. We excluded studies looking at other vaccines, for example, measles. We included all forms of studies as long as they had more than 100 participants.
Once we found the studies, we categorised the interventions into the following groups: communication interventions, policy interventions, interventions to improve access, educational interventions, incentives, and multidimensional interventions. We summarised the results in an interactive scoping map. Furthermore, we mapped the study outcomes, the country in which the study was conducted, the study population, and the study design.
Results
We included 96 studies in evidence mapping, 35 of which are ongoing and 61 studies with published results. The interventions tested in these studies are very diverse. Many studies used communication strategies to convince people to get vaccinated against COVID‐19. Interventions that included information on vaccination or a mixture of different strategies were also often used.
A majority of studies were conducted in English‐speaking countries of the global north, for example, the USA. Moreover, most studies investigated digital interventions in an online setting. The populations addressed varied across the studies. For example, studies addressed healthcare workers, ethnic minorities in the USA, students, soldiers, villagers, at‐risk patients, or the general population.
For an overview, please see the interactive scoping map (https://tinyurl.com/2p9jmx24).
Conclusion
We identified a large number of studies that investigate how COVID‐19 vaccine uptake might be increased. However, more studies are needed focusing on lower‐middle‐income countries and on children. Future research should compare the effectiveness of different interventions to improve COVID‐19 vaccine uptake.
Summary of findings
Summary of findings 1. Summary of findings.
| Intervention Group | Intervention | Outcomes | |||||||
| Vaccine hesitancy | Vaccine uptake | Willingness to get vaccinated | Agreement with COVID‐19 Policies | ||||||
| Proportion of participants with COVID‐19 vaccine hesitancy | Decrease in vaccine hesitancy | Reactance1 | Number of participants who got vaccinated | Number of participants indicating the intention to get vaccinated |
Vaccine trust | Further interest in vaccine information | Agreement with COVID‐19 vaccine passport | ||
| Communication strategies | Behavioural messaging | NCT04871776; NCT04895683 | |||||||
| Text messages | NCT04801524 | ||||||||
| Healthcare providers' communication about the COVID‐19 vaccine | NCT04706403 | ||||||||
| Framing | Borah 2021*; Chen 2021*; *Fox 2021*; Galasso 2021*; Gong 2021*; Huang 2021*; Palm 2021*; Strickland 2021* | ||||||||
| Information messaging | Argote 2021*; Bokemper 2021*; OCEAN*; Schwarzinger 2021*; Thorpe 2021*; NCT04813770 | ||||||||
| Framing videos | Yuan 2021* | ||||||||
| Expert claims | Robertson 2021b* | ||||||||
| Messaging about benefits | Ashworth 2021 | ||||||||
| Gain vs. Loss framing | Reichardt 2021* | Hong 2021* | Peng 2021*; Ye 2021* | ||||||
| HCW vaccine ambassadors | NCT04981392 | NCT04930965 (LA‐CEAL: HALT COVID) | |||||||
| Affect messaging | Capasso 2021* | ||||||||
| Public service messages | Jin 2021* | ||||||||
| Norm Framing | Ryoo 2021* | Sinclair 2021* | |||||||
| Framing and source of information | Pink 2021*; Thunström 2021* | ||||||||
| Risk framing | Sudharsanan 2021* | ||||||||
| Visual Illustrations with vaccine information | Ugwuoke 2021* | ||||||||
| Persuasive messages | Kachurka 2021* | ||||||||
| Personalised communication | Stein 2021*; Santos 2021*; NCT04805931 (VEText); NCT04834726; NCT04924803; NCT04939519 (SCALE‐UP Utah); ISRCTN15317247; NCT04952376; NCT04963790; NCT05027464 (CoVAcS) | Keppeler 2021* | |||||||
| Conversational agent | NCT04884750 | ||||||||
| Community influencer groups | PACTR202102846261362 | ||||||||
| Policy interventions | Mandatory vaccine policy | Sprengholz 2021* | |||||||
| Multidimensional interventions | High touch multi‐pronged behavioural intervention | NCT04732819 | |||||||
| Text messages for education outreach2 | NCT04800965 | ||||||||
| Nudging3 | NCT04867174 | NCT05037201 | Sotis 2021* | ||||||
| Culturally sensitive interventions | Marquez 2021*; NCT04542395; NCT04779138 | ||||||||
| Drawing attention to prosocial concerns | Jung 2021* | ||||||||
| Multidimensional information intervention | Kerr 2021* | ||||||||
| Vaccine education promotion management plan | NCT04761692 | ||||||||
| Phone‐based intervention for elders4 | NCT04870593 | ||||||||
| Incentives and nudging | Campos‐Mercade 2021* | ||||||||
| Multidimensional community intervention5 | NCT05022472 (2VIDA!) | ||||||||
| Multifaceted information intervention for HCW6 | Takamatsu 2021* | ||||||||
| Incentives and prosocial communication | Sprengholz 2021c* | ||||||||
| Multidimensional intervention for HCW7 | Howarth 2021* | ||||||||
| Education about herd immunity OR empathy condition | Pfattheicher 2021* | ||||||||
| Reminders and nudging | Senderey 2021* | ||||||||
| Incentives and easy access | Klüver 2021* | ||||||||
| Educational interventions | Entertainment‐education video | DRKS00023650 | |||||||
| Educational video | Witus 2021*; NCT04876885; NCT04960228; NCT04979416 | ||||||||
| Social marketing intervention | NCT04801030 | ||||||||
| Counselling | NCT04604743 | ||||||||
| Educational webinar | Kelkar 2021* | ||||||||
| Chatbot | Kobayashi 2021* | ||||||||
| Workshop | Talmy 2021* | ||||||||
| Rapid education | NCT04939506 | ||||||||
| Cultural‐appropriate Education | NCT04964154 (BRAVE) | ||||||||
| COVID‐19 vaccine information | Merkley 2021* | ||||||||
| Individualised information | Tran 2021* | ||||||||
| Incentives | Financial incentives8 | Kreps 2021*; Robertson 2021a*; Serra‐Garcia 2021*; Yu 2021*; Yu 2021b* | Duch 2021* | ||||||
| Lottery | Barber 2021*; Brehm 2021*; Sehgal 2021*; Thirumurthy 2021*; Walkey 2021*; NCT04951310 | ||||||||
*Studies have published results
1 Reactance was defined as "how frustrated, annoyed and disturbed participants felt about the vaccination situation" by the study authors (Sprengholz 2021).
2 Providing information as well as convincing people to get vaccinated via video, text messages and providing a link to schedule an appointment.
3 Nudges are behavioural interventions that influence people's choices from the perspective of policy‐makers or society, without restricting freedom of choice and changing the incentive system.
4 Calling elders to inform them of the vaccine and encouraging them to create buddy systems and gossip about the vaccine.
5Education and promotion (COVID‐19 awareness, education, health promotion), outreach and easy access (linkage to medical and supportive services, pop‐up vaccination sites)
6Education and information (lectures, educational sessions about the vaccine, informational leaflets), encouragement and risk reduction (vaccination‐encouraging announcements, allergy testing at risk of allergic reactions to the vaccine)
7Vaccine information (e.g. posters targeting vaccine misinformation, vaccine information packs) and vaccine role models (“Vaccine champions”, posters showing already vaccinated staff members
8Hypothetical financial fee or out‐of‐pocket cost for getting vaccinated
For an interactive version of this map, please see https://egmopenaccess.3ieimpact.org/evidence‐maps/interventions‐increase‐covid‐19‐vaccine‐uptake?
Background
Introduction
In March 2020, the World Health Organization (WHO) declared the current COVID‐19 outbreak a pandemic. The approval of the first COVID‐19 vaccine in December 2020 has been long‐awaited to help mitigate the effects of the COVID‐19 pandemic. Currently, 10 vaccines are recommended by the WHO (WHO 2021a), and many more are in development (WHO 2021b). Countries with limited vaccine access might not be in the position to create and implement vaccine campaigns raising awareness and willingness to be vaccinated.
Widespread COVID‐19 vaccination is crucial to protecting population health ‐ vaccination has been shown to be highly effective in preventing severe COVID‐19 illness and death from the disease (Public Health Ontario 2021). In nursing homes and hospitals, in particular, vaccines are supposed to protect high‐risk populations from severe illness, including healthcare workers. Furthermore, high vaccine uptake may indirectly protect people with a weak response to the vaccine, such as the elderly or immunosuppressed patients, from severe COVID‐19 disease. High levels of vaccination against COVID‐19 ought also to help ensure the smooth operation of health systems, as unvaccinated people are hospitalised for COVID‐19 more often than vaccinated people (Lopez 2021). In addition, new and vaccine‐resistant mutations of SARS‐CoV‐2 are more likely to develop if the spread of COVID‐19 is not limited. However, vaccine hesitancy is a serious threat to the goal of nationwide vaccination (Thunstrom 2020).
Vaccine hesitancy: reasons and prevalence
A recent systematic review of global COVID‐19 vaccine acceptance rates shows that populations widely differ in their acceptance of the COVID‐19 vaccines (Sallam 2021). For example, nearly the whole population of Ecuador, Malaysia and Indonesia are willing to get vaccinated against COVID‐19, while the projected acceptance rate among the French population was only 58.9%. In the USA, where COVID‐19 vaccines are widely available for the adult population, about 30% of the population remain unvaccinated (KFF 2022). Likewise, in countries where the vaccine is not yet widely available, a low willingness to be vaccinated against COVID‐19 is predicted by experts (Afolabi 2021).
In addition to a broad distrust in and doubts about vaccines in general, there seem to be specific reasons for hesitancy towards COVID‐19 vaccines. Studies show that people distrust the COVID‐19 vaccines as they believe the vaccines were manufactured too quickly (Nguyen 2021), or are sceptical of the new mRNA (messenger ribonucleic acid) technology used in some vaccines (Dror 2021). A number of conspiracy theories have been spread about the COVID‐19 vaccine that are likely influencing vaccination uptake (Romer 2020; Ullah 2021). For example, the mistaken belief that COVID‐19 is either a non‐existent or a harmless disease makes people unwilling to get vaccinated (Troiano 2021). Furthermore, people also express the fear of adverse vaccine reactions and long‐term harms of the COVID‐19 vaccine as a reason not to get vaccinated (Abu 2021).
Next to individual beliefs about COVID‐19, other characteristics are also associated with vaccine hesitancy. A survey conducted in the UK shows that factors such as negative experiences with the healthcare system and a general distrust of authorities are associated with COVID‐19 vaccine hesitancy (Freeman 2020). For example, people voting for anti‐establishment parties in Austria are also more likely to be vaccine‐hesitant (Schernhammer 2021). Likewise, political partisanship is predictive for COVID‐19 vaccine uptake in the USA (Hamel 2021), where Democrats are more likely to get vaccinated than Republicans. Distrust in authorities is also high among marginalised populations that have been affected disproportionally by the pandemic (Jaiswal 2020). Additionally, as reported by several studies, being female is linked with a higher hesitancy towards COVID‐19 vaccination (Bono 2021; Edwards 2020). Other demographic variables, such as age, education and ethnicity have also been linked to vaccine hesitancy (Nguyen 2021a; Reno 2021).
Why it is important to do this review
While attitudes towards the COVID‐19 vaccine have been thoroughly researched (Ahmed 2021; Akarsu 2021; Al‐Jayyousi 2021), it is still unclear whether there are effective interventions available to increase COVID‐19 vaccine uptake. The WHO identifies five factors that are central to the willingness to get vaccinated (Brewer 2017): social processes and people's emotions and thoughts impact their motivation to get vaccinated. Practical issues, such as costs and access, then influence if someone will get vaccinated or not. Each factor can be an important starting point for an intervention to increase vaccine uptake. A plethora of interventions have been proposed to increase the willingness to get vaccinated or to decrease vaccine hesitancy for other diseases. For example, financial incentives have been shown to increase vaccine uptake for human papillomavirus (HPV) vaccinations (Mantzari 2015), role models can increase vaccine uptake for hepatitis B (Vet 2010), and patient outreach has been utilised to increase pneumococcal vaccination (Winston 2007) and immunisation rates overall (Jacoboson Vann 2018). Moreover, Cochrane Reviews have demonstrated the effectiveness of different interventions, such as face‐to‐face communication, incentives or mandatory vaccinations to increase vaccine uptake for diverse populations (Abdullahi 2020; Jacoboson Vann 2018; Kaufman 2018; Oyo‐Ita 2016). Interventions can be categorised as communication‐based interventions, motivational interventions, or as structural interventions based on health policies and increased accessibility (Dubé 2015; Jarrett 2015; Odone 2015; Wigham 2014).
Rationale for conducting a scoping review
Research into measures to address COVID‐19 vaccine hesitancy is emerging rapidly. The methodology of scoping reviews can be used to identify and map available evidence (Anderson 2008; Munn 2018). Hence, a scoping review will allow us to obtain a rapid, comprehensive overview of possible interventions and populations targeted.
No systematic or scoping review has yet systematically identified and analysed these interventions in the context of COVID‐19. COVID‐19 vaccine hesitancy poses a substantial threat to population health and therefore, the evidence for interventions aimed at increasing COVID‐19 vaccine uptake needs to be investigated. This scoping review will help to define the scope of a subsequent systematic review.
Objectives
To scope the existing research landscape on interventions to enhance the willingness of different populations to be vaccinated against COVID‐19, increase COVID‐19 vaccine uptake, or decrease COVID‐19 vaccine hesitancy, and to map the evidence according to addressed populations and intervention categories.
Methods
Scoping review methodology
We followed the interim guidance on scoping reviews by Cochrane and the guidance of the Joanna Briggs Institute for conducting this review (Peters 2020). Furthermore, we consulted the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses extension for Scoping Reviews (PRISMA‐ScR) checklist (Tricco 2018) for the reporting of the results. Please see Appendix 1 for the completed checklist for this scoping review. The methods for this scoping review were published beforehand (Andreas 2021).
Inclusion criteria
Study design
We included studies that assess the impact of interventions implemented to enhance the willingness of different populations to be vaccinated against COVID‐19. As we wanted to get a broad overview of interventions being investigated and as no past reviews exist on this topic, we decided to include studies with the following designs: randomised controlled trials (RCTs), non‐randomised trials, observational studies (controlled pre‐post studies, interrupted time‐series studies, case‐control, cohort, and cross‐sectional studies), and single‐arm studies (uncontrolled pre‐post studies) with more than 100 participants. Furthermore, we included systematic reviews. For psychological experiments with hypothetical scenarios being tested, we decided to only include those studies that investigate scenarios that can be manipulated in a real‐world setting, so the findings can be translated into interventions. For example communication about vaccines can be manipulated, but vaccine efficacy cannot be manipulated.
Addressed population
In order to get a broad overview of all interventions and addressed populations, we did not limit the scope of our review by focusing on specific population groups. We also included studies testing interventions directed at healthcare providers, community leaders, or other role models to help these groups to increase COVID‐19 vaccine uptake more widely.
Interventions
We only included studies on interventions specifically addressing willingness to get vaccinated against COVID‐19, or intended to increase COVID‐19 vaccine uptake, or decrease COVID‐19 vaccine hesitancy. We excluded interventions addressing hesitancy towards vaccines for diseases other than COVID‐19.
Outcomes
We did not limit the scope of our review by focusing on specific outcomes in order to get a broad overview of the outcome measures assessed in studies.
An overview of inclusion and exclusion criteria is provided in Table 2 and Table 3, respectively.
1. Inclusion criteria .
| Criteria | Details |
| Study designs |
|
| Population | Any population, no restrictions |
| Setting | No restrictions |
| Interventions |
|
| Outcomes | No restrictions |
RCTs: randomised controlled trials
2. Exclusion criteria .
| Criteria | Details |
| Study designs |
|
| Population | No exclusion criteria |
| Setting | No exclusion criteria |
| Interventions |
|
| Outcomes | No exclusion criteria |
HPV: human papillomavirus
Identification of relevant studies
For the identification of evidence syntheses and completed and ongoing studies systematic searches were performed by our Information Specialist (IM). They were peer‐reviewed by a second Information Specialist as part of the editorial process for this manuscript.
We searched the following electronic databases for primary studies from 1 January 2020 to 11 October 2021:
-
Cochrane COVID‐19 Study Register (CCSR) (www.covid-19.cochrane.org)
Cochrane Central Register of Controlled Trials (CENTRAL);
PubMed;
Embase.com;
ClinicalTrials.gov (www.clinicaltrials.gov);
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (www.who.int/trialsearch);
medRxiv (www.medrxiv.org).
-
Web of Science Core Collection
Science Citation Index Expanded (1945 to present);
Emerging Sources Citation Index (2015 to present).
WHO COVID‐19 Global literature on coronavirus disease (search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/)
PsycINFO (Ovid 1806 to present);
CINAHL (EBSCO 1982 to present).
We searched the following electronic databases and websites for evidence syntheses from 1 January 2020 to 10 June 2021:
Evidence Aid Coronavirus (Covid‐19) (evidenceaid.org/evidence/coronavirus-covid-19/);
Usher Network for COVID‐19 Evidence Reviews (www.ed.ac.uk/usher/uncover/register-of-reviews);
Epistemonikos COVID‐19 L*OVE Platform (app.iloveevidence.com/loves);
MEDLINE (Ovid, 1 January 2020 to 10 June 2021) with a filter for systematic reviews (Wong 2006).
We searched for primary studies on 10 June 2021 and updated the search on 11 October 2021. We did not update the search for evidence syntheses, because we did not identify any relevant hits in the search of June. We began the search in January 2020 as this was when COVID‐19 was declared a Public Health Emergency of International Concern by the WHO.
Please see Appendix 2 for the search strategies for evidence syntheses and Appendix 3 for the search strategies for primary literature. The search was conducted in English, however, we did not exclude studies if they had not been published in English.
Study selection
Two review authors (MA, EB) independently screened titles and abstracts of identified records. We used the web‐based application Rayyan (https://rayyan.qcri.org/welcome) for title and abstract screening. Discrepancies between authors were discussed and in case the conflict persisted a third review author (CI) resolved conflicts. To ensure that all review authors screen consistently, we developed a guidance document to standardise the screening process. We then retrieved full‐text articles of all potentially included studies and assessed the eligibility of the remaining records against our pre‐defined eligibility criteria. This was also done independently and in duplicate. We documented reasons for the exclusion of full texts.
Extraction and presentation of data
Two authors (MA, EB) independently extracted the following information into a piloted data extraction sheet. Disagreements were resolved by discussion.
Study characteristics
-
Study design
Country of origin (where the study was conducted)
Characteristics of the population addressed by the intervention (age; gender; ethnicity)
Intervention details (timeframe; intervention category; intervention method)
Comparison details (if applicable)
Time of follow‐up (if applicable)
Outcomes (outcome measures; time points of outcome assessment)
Financial support and sponsoring
Disclosure of conflicts of interest (COIs)
If any of the above data were not available, we contacted the study authors for further information.
Summary and reporting of results
We presented the results in a tabular form using software by 3ie EGM (https://egmopenaccess.3ieimpact.org/evidence‐maps/interventions‐increase‐covid‐19‐vaccine‐uptake?) to create the evidence map. We mapped data according to the categories of interventions identified and the outcomes assessed. The classification of interventions was first based on a review by the SAGE working group for vaccine hesitancy (Larson 2014), and other systematic reviews addressing vaccine uptake (Dubé 2015; Jarrett 2015; Odone 2015; Wigham 2014; Winston 2007). We have added policy interventions (e.g. mandatory vaccine uptake) as we think these are especially relevant in the context of nationwide COVID‐19 vaccine rollouts. We adapted the categories originally published in our protocol to better fit the evidence we found. Thus, the final intervention categories were: education, policy interventions, communication interventions, incentives, interventions to improve access, and multidimensional interventions. Please see Table 4 for an overview and description of each category. Please see the Differences between protocol and review section for a justification of the changes made. Additionally, we mapped the region and country in which studies were conducted, the addressed population, as well as the study design.
3. Categorisation of interventions used in this review .
| Cetegory | Description |
|
Education |
Interventions aimed at educating or informing participants about COVID‐19, COVID‐19 vaccines, benefits of vaccine uptake and other aspects of the pandemic or vaccination. |
| Policy interventions | Interventions that can only be implemented across a whole jurisdiction by policymakers, such as mandatory vaccine policies. |
| Communication strategies | Interventions aiming to persuade people to get vaccinated. This can be in‐person communication but also communication used in different forms of media such as videos or written information. |
| Incentives |
|
| Interventions to improve access | Multidimensional interventions are interventions using more than one strategy. For example, educational interventions and communication strategies can be used together. |
Results
Results of the search
We identified 11,608 potentially relevant references. After the removal of duplicates (208), we screened 11,400 references based on their titles and abstracts, excluding 11,269 references because they did not meet the prespecified inclusion criteria. We screened the full texts of the remaining 131 references, or, if these were not available, abstract publications or trial registry entries. We excluded 30 studies after the full‐text screening. We identified 101 eligible studies, five of which were assessed as awaiting classification as it was unclear from trial registries if they really are intervention studies. In the end, we included 96 studies in the interactive scoping map, 61 studies with published results, and 35 of which are ongoing. The process and results of study selection are documented in the PRISMA flow diagram (Figure 1).
1.
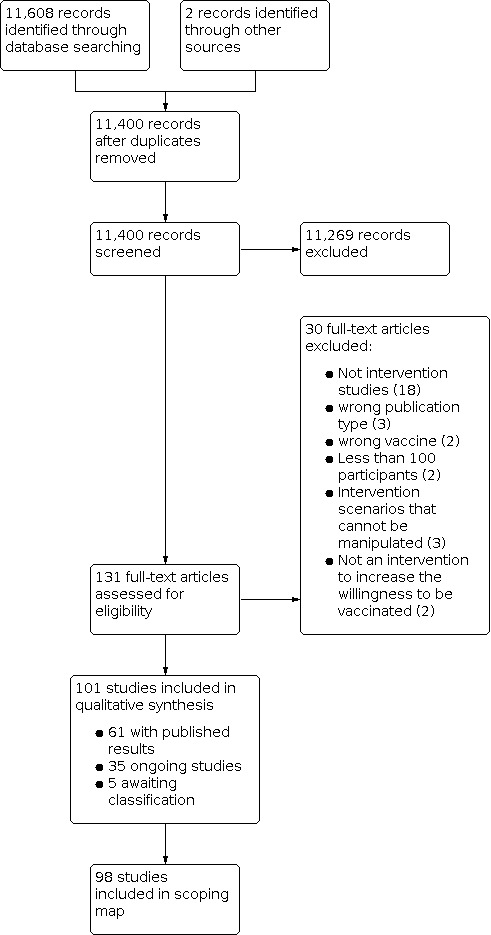
For an overview of all studies please see the Table 1 and the interactive scoping map.
Studies with published results
We included 61 studies with published results in the interactive scoping map. Of the studies with published results, 46 studies were randomised controlled trials (RCTs) (Argote 2021; Ashworth 2021; Bokemper 2021; Borah 2021; Campos‐Mercade 2021; Capasso 2021; Chen 2021; Duch 2021; Fox 2021; Galasso 2021; Gong 2021; Hong 2021; Huang 2021; OCEAN; Jin 2021; Jung 2021; Kachurka 2021; Kerr 2021; Keppeler 2021; Klüver 2021; Kreps 2021; Merkley 2021; Palm 2021; Peng 2021; Pfattheicher 2021; Pink 2021; Reichardt 2021; Robertson 2021a; Robertson 2021b; Santos 2021; Schwarzinger 2021; Sinclair 2021; Strickland 2021; Senderey 2021; Serra‐Garcia 2021; Sotis 2021; Sprengholz 2021; Sprengholz 2021c; Sudharsanan 2021; Thorpe 2021; Thunström 2021; Ye 2021; Yu 2021; Yu 2021b; Yuan 2021; Witus 2021). Fifteen studies were non‐randomised intervention studies. Of these, two studies were uncontrolled post‐intervention studies (Kobayashi 2021; Ryoo 2021), and nine were uncontrolled retrospective cohort studies (Barber 2021; Brehm 2021; Kelkar 2021; Marquez 2021; Sehgal 2021; Stein 2021; Talmy 2021; Thirumurthy 2021; Walkey 2021). One study was a controlled cohort study (Ugwuoke 2021). Three studies had a pre‐post interventional design (Howarth 2021; Takamatsu 2021; Tran 2021).
A majority (29 of 61) of studies were conducted in the USA. One study was conducted in the USA and Germany, and one in the USA and UK. Six studies were conducted in China. Six studies were conducted in the UK, two in France, two in Japan, one in Sweden, one in Poland, one in Italy, one in Canada, four in Germany, and two in Israel. Furthermore, one study was conducted in Pakistan and one in Nigeria. One multinational study was carried out in Australia, France, Germany, Italy, New Zealand, Poland, Sweden, the UK, and the USA. One study was carried out in Argentina, Brazil, Chile, Colombia, Mexico, and Peru. In summary, the majority of studies were conducted in high‐income countries. For an overview, please see our interactive scoping map, in which you can select regions and countries.
Forty‐one studies were conducted in an online setting, while 20 studies were conducted in person. Of the latter, one study was conducted in a university, five studies in a hospital setting, one study with a healthcare provider, and one study in a health system. Furthermore, one study was set in a military unit, one in Sweden, and one study in a Latinx community. Four studies were conducted in Ohio, USA and one study in 24 US states. One study was conducted via telephone in Germany, and one study via letters in a German municipality. One study was conducted in a camp for internally displaced persons. One study did not report a specific setting. In summary, a majority of published studies were conducted in an online setting, mostly testing hypothetical scenarios. We define online settings as interventions solely conducted online, for example, webinars or online surveys. For more detailed information, please see the Characteristics of included studies table.
Of the included studies, 11 were published on preprint servers (Argote 2021; Barber 2021; Duch 2021; Keppeler 2021; Kobayashi 2021; Marquez 2021; Senderey 2021; Serra‐Garcia 2021; Strickland 2021; Thirumurthy 2021; Witus 2021).
Ongoing studies
We identified 35 ongoing studies. Of the ongoing studies, 29 studies are RCTs (DRKS00023650; ISRCTN15317247; NCT04604743; NCT04706403; NCT04732819; NCT04761692; NCT04800965; NCT04801524; NCT04805931 (VEText); NCT04813770; NCT04834726; NCT04867174; NCT04870593; NCT04871776; NCT04884750; NCT04895683; NCT04924803; NCT04930965 (LA‐CEAL: HALT COVID); NCT04939519 (SCALE‐UP Utah); NCT04951310; NCT04952376; NCT04960228; NCT04963790; NCT04964154 (BRAVE); NCT04979416; NCT04981392; NCT05022472 (2VIDA!); NCT05027464 (CoVAcS); NCT05037201). Six studies have a non‐randomised intervention designs. Of those, one study will investigate the intervention in a prospective, two‐arm observational study (PACTR202102846261362). Three studies will utilise a non‐randomised trial design (NCT04876885; NCT04801030; NCT04939506). Two studies will use a pretest‐posttest design without a control group (NCT04542395; NCT04779138).
A majority (26 of 35) of ongoing studies are planned in the USA. One study will be conducted in the USA and China. Three studies will be conducted in the UK, three in Canada, one in Uganda, and one in India. In summary, a majority of ongoing studies are planned to be conducted in English‐speaking, high‐income countries.
Five of the ongoing studies will be conducted in an online setting, one study will be conducted via text messages and three via phone calls, while 11 studies are planned in person. Of the latter, two studies will be conducted in a hospital setting, two studies in a health centre, two studies within a healthcare provider, one in a primary care practice, one study in a skilled nursing facility, two in veteran health care, and one study in a rural clinic. Furthermore, two studies will be set in a church setting, one study in London, one in villages in Uganda, two in a university, one in a managed care setting, and two in public housing. One study will be conducted in southern California, one study will be conducted in Philadelphia, and one study will address vulnerable communities in Louisiana. Four studies did not report a specific setting. In summary, a majority of ongoing studies will address populations in person, most of them in a healthcare setting.
Eleven ongoing studies have estimated completion dates in 2022 (NCT04542395; NCT04964154 (BRAVE); NCT04800965; NCT04801524; NCT04867174; NCT04871776; NCT04895683; NCT04930965 (LA‐CEAL: HALT COVID); NCT04939519 (SCALE‐UP Utah); NCT04952376; NCT04963790). For the estimated completion dates of all ongoing studies, please see the characteristics of included studies table for each study.
Excluded studies
We excluded 30 studies for the following reasons.
Three studies were excluded because they were an ineligible publication type; one was an opinion piece (Hofer 2021), one was a letter to the editor (Sprengholz 2021b), and the other one was a correction (Loomba 2021). Furthermore, one study was excluded because it investigated interventions for influenza vaccine uptake (Yousuf 2021), and one because it investigated measles, mumps and rubella uptake (Kirkpatrick 2021). We excluded two uncontrolled studies with less than 100 participants (Ali 2021; Gakuba 2021). Batteux 2021, Davis 2021 and Wagner 2021 were excluded as the studies investigate scenarios that cannot be manipulated. Loomba 2021a and Thaker 2021 measure the effect of misinformation on vaccine intent and thus are not relevant to the research objective. The other 18 studies were excluded because they did not investigate interventions (American Society of Safety Professionals 2021; Bell 2021; ChiCTR2100043018; Community Practitioner 2021; Crawshaw 2021; Crawshaw 2021b; Gehrau 2021; Guelmami 2021; Kaplan 2021; Knight 2021; Kumar 2021; Lim 2020; NCT04694651; Rahmandad 2021; Salali 2021; Shmueli 2021; Vasquez 2021; Yuen 2021).
Studies awaiting assessment
We classified five studies as awaiting assessment because from the trial registrations and publications of these studies it is not clear whether these studies will test interventions to enhance COVID‐19 vaccine uptake (INFORMED; NCT04460703; NCT04731870; Larson 2020; Supraneni 2021).
Studies included in the scoping map
Please see our interactive scoping map (https://egmopenaccess.3ieimpact.org/evidence‐maps/interventions‐increase‐covid‐19‐vaccine‐uptake?) and the Table 1 for an overview of the interventions and outcomes used in studies as well as study location, population, and design. Please note that studies with published results as well as ongoing studies were included in the scoping map.
Studies with published results
Participants
The interventions investigated in the studies addressed a wide variety of different participants. Howarth 2021, Santos 2021, Takamatsu 2021, and Yu 2021b addressed healthcare workers. Kelkar 2021 and Stein 2021 included cancer patients. Patients in France were addressed in Tran 2021. Marquez 2021 included Latinx community members in San Francisco. Israeli soldiers participated in Talmy 2021, and American veterans and the general population in Thorpe 2021. Adult US citizens participated in Ashworth 2021, Barber 2021, Bokemper 2021, Borah 2021, Brehm 2021, Duch 2021, Fox 2021, Huang 2021, Jung 2021, Kreps 2021, Palm 2021, Pink 2021, Robertson 2021a, Robertson 2021b, Ryoo 2021, Sehgal 2021, Serra‐Garcia 2021, Sotis 2021, Strickland 2021, Thirumurthy 2021, Thunström 2021, Witus 2021, Walkey 2021, and Yuan 2021. Adult US and UK citizens participated in Sudharsanan 2021. Merkley 2021 included Canadian residents. Chen 2021, Gong 2021, and Peng 2021 included Chinese citizens and Yu 2021 Hong Kong citizens. Kerr 2021, Sinclair 2021, Pfattheicher 2021, and OCEAN included UK citizens. US citizens and German citizens participated in Sprengholz 2021 and German adults only in Keppeler 2021, Klüver 2021, Reichardt 2021, and Sprengholz 2021c. Japanese residents participated in Kobayashi 2021, Pakistani residents in Jin 2021, Polish residents in Kachurka 2021 and Swedish residents in Campos‐Mercade 2021. Israeli citizens were addressed by Senderey 2021. Internally displaced persons in Nigeria were addressed by Ugwuoke 2021. Argote 2021 included adults in Argentina, Brazil, Chile, Colombia, Mexico, and Peru and Galasso 2021 included adults in Australia, France, Germany, Italy, New Zealand, Poland, Sweden, the UK, and the USA. French citizens participated in Schwarzinger 2021 and Italian citizens in Capasso 2021. Young adults participated in Hong 2021 and Ye 2021.
All studies report a sample size of more than 100 participants. In summary, a majority of interventions were targeted towards US adults. For more detailed information on sample size, please see the characteristics of included studies tables. Population groups can also be selected in the interactive evidence map.
Interventions
Interventions were grouped as educational interventions, incentives, policies, communication strategies, increased access and multidimensional interventions. In summary, a majority of published studies tested communication strategies to increase COVID‐19 vaccine uptake. For more detailed information on the interventions, please see the evidence map as well as the characteristics of included studies table and Table 1.
Communication interventions
Thirty‐four studies tested communication strategies to increase the willingness to vaccinate against COVID‐19 (Argote 2021; Ashworth 2021; Bokemper 2021; Borah 2021; Capasso 2021; Chen 2021; Fox 2021; Galasso 2021; Gong 2021; Hong 2021; Huang 2021; Jin 2021; Kachurka 2021; Keppeler 2021; Merkley 2021; OCEAN; Palm 2021; Peng 2021; Pink 2021; Reichardt 2021; Robertson 2021b; Ryoo 2021; Santos 2021; Stein 2021; Sudharsanan 2021; Schwarzinger 2021; Strickland 2021; Sinclair 2021; Sotis 2021; Thunström 2021; Ugwuoke 2021; Witus 2021; Ye 2021; Yuan 2021), with most studies using framing as a method. Framing is the selection or highlighting of certain aspects of an issue to bring these to the forefront in communication and encourage particular interpretations (Entman 1993). For example, some studies compared gain and loss frames (Hong 2021; Peng 2021; Reichardt 2021; Ye 2021). Text messages, E‐mails, letters, webpages, posters, and face‐to‐face communication were used as media to transport messages about COVID‐19.
Incentives
Eleven studies investigated financial incentives to enhance COVID‐19 vaccine uptake (Barber 2021; Brehm 2021; Duch 2021; Kreps 2021; Robertson 2021a; Sehgal 2021; Serra‐Garcia 2021; Thirumurthy 2021; Walkey 2021; Yu 2021; Yu 2021b). Specifically, a majority of studies investigated vaccine lotteries, where it is possible to win a cash price for getting vaccinated. Other studies researched the effectiveness of financial incentives to motivate people to get vaccinated.
Multidimensional interventions
Ten studies investigated multidimensional interventions, that evaluated a mix of educational, communicational, and policy interventions as well as improved access (Howarth 2021; Jung 2021; Kerr 2021; Klüver 2021; Marquez 2021; Pfattheicher 2021; Senderey 2021; Serra‐Garcia 2021; Sprengholz 2021c; Takamatsu 2021). For example, one study combined incentives with easy access to vaccines (Klüver 2021).
Educational interventions
Five studies investigated educational interventions such as workshops or information texts. Specifically, Kelkar 2021 conducted a webinar, Talmy 2021 investigated the use of workshops to educate soldiers about COVID‐19 vaccines, Thorpe 2021 used online fact boxes to educate veterans, Kobayashi 2021 investigated a chatbot answering questions about COVID‐19 and vaccines, and Tran 2021 utilised an interactive web tool to offer individualised information for users.
Policy interventions
One study investigated a policy intervention (Sprengholz 2021), specifically the effects of a mandatory vaccine policy.
Interventions to improve access
We did not identify any studies with published results that investigate the effects of improved access.
Control conditions
Most studies used control arms in which other intervention strategies were tested or in which the intervention was slightly altered (Argote 2021; Ashworth 2021; Bokemper 2021; Borah 2021; Duch 2021; Campos‐Mercade 2021; Capasso 2021; Chen 2021; Fox 2021; Galasso 2021; Gong 2021; Hong 2021; Howarth 2021; Huang 2021; Jin 2021; Jung 2021; Kachurka 2021; Kelkar 2021; Keppeler 2021; Klüver 2021; Kreps 2021; Merkley 2021; OCEAN; Palm 2021; Peng 2021; Pfattheicher 2021; Pink 2021; Reichardt 2021; Robertson 2021a; Robertson 2021b; Ryoo 2021; Senderey 2021; Serra‐Garcia 2021; Sinclair 2021; Sotis 2021; Sprengholz 2021; Sprengholz 2021c; Strickland 2021; Sudharsanan 2021; Takamatsu 2021; Thorpe 2021; Thunström 2021; Tran 2021; Ye 2021; Yu 2021; Yu 2021b; Yuan 2021). Most studies had more than one control condition. Nine studies had control conditions with no intervention (Barber 2021; Brehm 2021; Kerr 2021; Schwarzinger 2021; Sehgal 2021; Thirumurthy 2021; Ugwuoke 2021; Walkey 2021; Witus 2021). One study had a delayed control condition (Santos 2021). Four studies were uncontrolled (Kobayashi 2021; Marquez 2021; Talmy 2021; Stein 2021).
Outcome measures
The willingness to get vaccinated for COVID‐19 was assessed as an outcome in 43 studies (Ashworth 2021; Argote 2021; Bokemper 2021; Borah 2021; Capasso 2021, Chen 2021; Fox 2021; Galasso 2021; Gong 2021; Howarth 2021; Huang 2021; Jin 2021; Jung 2021; Kachurka 2021; Klüver 2021; Kelkar 2021; Kerr 2021; Kobayashi 2021; Keppeler 2021; Kreps 2021; Merkley 2021; OCEAN; Palm 2021; Robertson 2021a; Robertson 2021b; Schwarzinger 2021; Serra‐Garcia 2021; Sudharsanan 2021; Sinclair 2021; Sprengholz 2021c; Strickland 2021; Tran 2021; Peng 2021; Pfattheicher 2021; Pink 2021; Thorpe 2021; Thunström 2021; Ugwuoke 2021; Witus 2021; Ye 2021; Yu 2021; Yu 2021b; Yuan 2021). Usually, this outcome was assessed with survey questions on the intention to get vaccinated.
A majority of studies (48 of 61) used an unvalidated questionnaire (e.g., “If there is a COVID‐19 vaccine available, are you willing to be vaccinated?” Gong 2021) to measure the willingness to get vaccinated or did not give any information on the source or their questionnaire. Some studies used only one question to measure the willingness to get vaccinated, while others used scales with more than one question. Validated questionnaires or questionnaires adapted from other studies were used in Borah 2021, Capasso 2021, Jin 2021, Kelkar 2021, Kerr 2021, OCEAN, Sinclair 2021, Peng 2021, Pfattheicher 2021, Sudharsanan 2021, Witus 2021, and Ye 2021. One study used the clicks on pages to register for a vaccine appointment as a proxy for the willingness to get vaccinated (Keppeler 2021).
Thirteen studies assessed vaccine uptake as an outcome (Barber 2021; Brehm 2021; Campos‐Mercade 2021; Hong 2021; Marquez 2021; Santos 2021; Sehgal 2021; Senderey 2021; Stein 2021; Takamatsu 2021; Talmy 2021; Thirumurthy 2021; Walkey 2021). This outcome was usually assessed using real‐world data on vaccination rates in the studied population. Reactance was measured in two studies (Reichardt 2021; Sprengholz 2021). Duch 2021 investigated further interest in vaccine information as an outcome. Vaccine hesitancy was measured in Ryoo 2021. Agreement with COVID‐19 passports was assessed in Sotis 2021.
Some studies also reported secondary outcomes. These were not mapped in the interactive scoping map and are further described in the Characteristics of included studies table.
Ongoing Studies
Please note that ongoing studies were also included in the mapping of the results.
Participants
Participant characteristics also differ between ongoing studies. African American and Latinx communities are addressed in four ongoing studies (NCT04761692; NCT04542395; NCT04779138; NCT05022472 (2VIDA!)). Furthermore, NCT04801030 plans to include African American adults and NCT04884750 churchgoers in Black churches in the USA. Ethnically diverse minority populations are being recruited in NCT04867174. Native American residents are recruited in NCT04964154 (BRAVE). NCT04871776 aims to address hospital patients, and NCT04834726 at‐risk patients. Nursing home residents and staff are participating in NCT04732819. NCT04876885 includes Ontario residents and healthcare professionals, and NCT05037201 employees in a US healthcare service. Patients are recruited in NCT04939519 (SCALE‐UP Utah), NCT04930965 (LA‐CEAL: HALT COVID), NCT04952376, NCT04963790, and NCT04981392. NCT04800965 and NCT04801524 aim to include university students. US veterans are participating in NCT04805931 (VEText). Adult US citizens participate in NCT04706403, NCT04951310, and NCT04979416, US and Chinese residents in DRKS00023650. ISRCTN15317247 includes UK citizens, NCT04895683 includes London citizens and NCT04813770 Scottish residents. US veterans are recruited in NCT05027464 (CoVAcS). Ugandan villagers participate in PACTR202102846261362. NCT04870593 aims to address elderly Indian residents. NCT04939506 addresses vaccine‐hesitant US adults. NCT04924803 aims to recruit drug users. All studies, except for NCT04604743 and NCT04960228 plan to include adults aged 18 years or older.
All ongoing studies report a sample size of more than 100 participants. Larger studies plan to recruit more than 20,000 participants (for example, DRKS00023650). For more detailed information on sample size, please see the Characteristics of ongoing studies tables.
Interventions
Interventions were grouped as educational interventions, incentives, policies, communication strategies, increased access and multidimensional interventions.
Communication interventions
Sixteen ongoing studies plan to test communication strategies to increase the willingness to vaccinate against COVID‐19. Nine of these studies plan the use of personalised communication strategies (ISRCTN15317247; NCT04939519 (SCALE‐UP Utah); NCT04924803; NCT04805931 (VEText); NCT04834726; NCT04895683; NCT04952376; NCT04963790; NCT05027464 (CoVAcS); ) such as personalised text messages as reminders. The other studies use different strategies to persuade people to get vaccinated (NCT04706403; NCT04801524; NCT04871776; NCT04981392; NCT04930965 (LA‐CEAL: HALT COVID); PACTR202102846261362; NCT04884750).
Educational interventions
Twelve ongoing studies aim to investigate educational interventions such as workshops or information texts (DRKS00023650; NCT04604743; NCT04779138; NCT04801030; NCT04813770; NCT04876885; NCT04939506; NCT04960228; NCT04979416; NCT04964154 (BRAVE); PACTR202102846261362; NCT04542395).
Multidimensional interventions
Six ongoing studies plan to investigate multidimensional interventions that mix different intervention categories (NCT04732819; NCT04867174; NCT04761692; NCT04800965; NCT04870593; NCT05022472 (2VIDA!)).
Incentives
One ongoing study plans to investigate a lottery as an incentive to get vaccinated (NCT04951310).
Interventions to improve access
We did not identify any ongoing studies that investigate the effects of improved access.
Control conditions
To control intervention effects, a majority (18 of 35) of ongoing studies plan to use an active control (DRKS00023650; ISRCTN15317247; NCT04939519 (SCALE‐UP Utah); NCT04981392; NCT04964154 (BRAVE); NCT04924803; NCT05022472 (2VIDA!); NCT04952376; NCT04963790; NCT05037201; NCT04761692; NCT04801524, NCT04813770; NCT04834726; NCT04867174; NCT04870593; NCT04884750; NCT04960228). Eleven studies compare the intervention to current practices (NCT04800965; NCT04801030; NCT04876885; NCT04732819; NCT04805931 (VEText); NCT04871776; NCT04895683; NCT04979416; NCT04951310; NCT05027464 (CoVAcS); NCT04930965 (LA‐CEAL: HALT COVID)). Three studies do not further specify the control condition (NCT04706403; PACTR202102846261362; NCT04604743) and three are uncontrolled (NCT04542395; NCT04779138; NCT04939506).
In summary, educational and multidimensional interventions are the most used types of interventions in ongoing studies. For a more detailed description of interventions, please see the Characteristics of ongoing studies table and the Table 1.
Outcome measures
Twenty‐four ongoing studies plan to assess vaccine uptake as an outcome (ISRCTN15317247; NCT04604743; NCT04779138; NCT04800965; NCT04801030; NCT04801524; NCT04805931 (VEText); NCT04834726; NCT04867174; NCT04884750; NCT04895683; NCT04870593; NCT04732819; NCT04761692; NCT04542395; NCT04939506; NCT04939519 (SCALE‐UP Utah); NCT04981392; NCT04964154 (BRAVE); NCT04924803; NCT04951310; NCT05027464 (CoVAcS); NCT04952376; NCT04963790). Usually, the studies plan to assess this outcome using real‐world data on vaccination rates in the studied population. Furthermore, the willingness to get vaccinated for COVID‐19 is assessed as an outcome in seven studies (NCT04706403; NCT04813770; NCT04876885; NCT05037201; NCT04960228; NCT04979416; NCT04930965 (LA‐CEAL: HALT COVID)). Usually, this outcome is assessed with survey questions on the intention to get vaccinated. One study aims to assess the decrease of vaccine hesitancy (DRKS00023650), and two studies the proportion of vaccine hesitancy among participants (NCT04801524; PACTR202102846261362). One study assesses vaccine confidence or trust (NCT05022472 (2VIDA!)). Some studies also reported secondary outcomes. These were not mapped in the interactive scoping mapand are further described in the Characteristics of ongoing studies table.
Discussion
Summary of main results
We identified 101 eligible studies and classified five studies of these as awaiting classification since it was unclear from trial registries if they really are intervention studies. Of the 96 included studies, 61 studies have published results and 35 are ongoing. Interventions to increase COVID‐19 vaccine uptake were very heterogeneous and included communication interventions (50), educational interventions (17), multidimensional interventions (16), and incentives (12), as well as policy interventions (1). The mapping of the results shows that interventions are mostly assessed with regard to their potential to increase the willingness to get vaccinated or COVID‐19 vaccine uptake. A smaller proportion of studies looked at interventions to decrease vaccine hesitancy. Only one study assessed the agreement with COVID‐19 policies as an outcome. A majority of studies was conducted in English‐speaking high‐income countries. Populations that were addressed were diverse with studies addressing healthcare workers, ethnic minorities in the US, students, soldiers, villagers, at‐risk patients, or the general population. A majority of the studies addressed adult participants. Most studies investigated the interventions in a randomised‐controlled setting.
Overall completeness and applicability of evidence
The evidence summarised here demonstrates a wide range of interventions addressing specific populations in English‐speaking high‐income countries. However, we could only identify a small number of studies set in low and middle‐income countries.
Moreover, we only identified four studies that focused specifically on young people. Since some vaccines against COVID‐19 are now also approved for young adults and children, it is of utmost importance to investigate interventions addressing COVID‐19 vaccine uptake in younger populations.
Until more evidence is available on the effects of interventions focused on increasing COVID‐19 vaccine uptake for lower‐middle‐income countries and younger populations, decision‐makers may want to draw on the findings of systematic reviews of related interventions to inform their decision‐making. For example, several Cochrane Systematic Reviews have investigated strategies to enhance vaccine uptake for other vaccines (Abdullahi 2020; Jacoboson Vann 2018; Kaufman 2018; Oyo‐Ita 2016; Thomas 2018).
None of the studies that we identified assessed the efficacy of policy interventions that have been implemented in 'real‐world' or practice settings. We only identified one study of policy interventions, and this investigated the effects of a hypothetical mandatory vaccine policy in a laboratory setting (Sprengholz 2021). With strategies such as mandatory vaccination or travel restrictions for unvaccinated people being discussed or implemented, studies to evaluate the impact of such strategies are becoming increasingly important. Moreover, we only identified one multidimensional study that investigated the effects of improved access (Klüver 2021) and no study only investigating improved access. Since interventions based on increased access to vaccines are effective in other contexts (Dubé 2015), this research gap should be addressed in future studies. Overall, the description of interventions was very heterogeneous and differed in detail. Especially for ongoing studies, interventions often were not well‐described and thus our categorization of them is preliminary.
Finally, while validated questionnaires to measure the willingness to get vaccinated or vaccine hesitancy are available, the majority of the included studies did not use these. Future studies should employ validated instruments to measure COVID‐19 vaccine hesitancy and willingness.
Potential biases in the review process
While we published a protocol for this review beforehand (Andreas 2021), it was not peer‐reviewed by external experts. Furthermore, although we registered the protocol in advance, the studies that we identified made a change to the intervention categorisation necessary. We adapted the intervention categories to better fit the evidence. Specifically, we added the category “multidimensional interventions” as many studies used a mixture of intervention categories. Additionally, instead of summarising education interventions under communication strategies, it became a separate category. We also added "interventions to improve access" as a separate category. These changes enabled us to more accurately map our findings.
We identified no other potential sources of bias in our review process.
Authors' conclusions
Implications for research and practice
We were able to identify and map a number of heterogeneous interventions for increasing COVID‐19 vaccine uptake or decreasing vaccine hesitancy. Our results demonstrate that this is an active field of research with 61 published studies and 35 studies still ongoing. The contexts and populations in which the interventions were tested were diverse and thus enable policymakers to identify evidence for specific populations.
While we could identify a heterogenous evidence base for interventions to increase COVID‐19 vaccine uptake in this scoping review, future research should address the following research gaps:
Developing and evaluating intervention strategies in low and middle‐income countries in order to inform health policies in these contexts
The majority of studies were conducted in experimental settings only. Studies are also needed on the effectiveness of interventions already in use in routine practice
Developing and evaluating interventions that address adolescents or children
Developing and evaluating interventions to improve access to vaccination
Developing and evaluating policy interventions
Few studies use validated questionnaires to measure outcomes such as the willingness to vaccinate. Future research should therefore develop and use validated instruments for these outcomes
Implications for a subsequent effectiveness review
This scoping review cannot answer the question of which interventions are most effective to increase COVID‐19 vaccine willingness. However, it provides a systematic overview of interventions, study design, populations, and settings of studies researching interventions to increase COVID‐19 vaccine uptake and willingness to vaccinate. We identified 61 published studies and an additional 35 ongoing studies, many of which are RCTs. Furthermore, many ongoing studies have estimated completion dates in 2022. Thus, a systematic review comparing the effectiveness of the various interventions to increase COVID‐19 vaccine uptake seems both feasible and warranted.
Nevertheless, this scoping review has also highlighted some challenges of conducting a subsequent systematic review. Firstly, the COVID‐19 pandemic is rapidly evolving, both within and between countries, making it hard to compare studies conducted in different contexts. Criteria such as the timing of the intervention should therefore be considered as subgroup analyses in a meta‐analysis. Secondly, this scoping review has demonstrated that a range of different measures to increase vaccine uptake exist. It follows that the comparison of different interventions in a systematic review with meta‐analysis might be difficult, especially regarding study heterogeneity. Thus, comparing effectiveness within an intervention category might be most appropriate for a subsequent systematic review. Furthermore, in the process of writing this review, we have adapted the categorisation system for interventions to better differentiate between the numerous intervention types we identified. The updated system can be used for a subsequent systematic review or similar scoping reviews; however, whether further adjustments will be required, remains to be seen.
Finally, the dynamic nature of the COVID‐19 pandemic highlights the importance of providing up‐to‐date evidence to inform policy decisions. New variants and new vaccines have emerged while we worked on this scoping review, that have likely impacted the ability of vaccines to block transmission and may have influenced willingness to get vaccinated. To best reflect the fast‐paced COVID‐19 pandemic, a living systematic review might be most appropriate.
In conclusion, as COVID‐19 vaccine hesitancy remains an urgent topic in most countries, a future systematic review can help to inform evidence‐based strategies to address the willingness to vaccinate against COVID‐19.
What's new
| Date | Event | Description |
|---|---|---|
| 5 August 2022 | Amended | Edits to faulty hyperlinks |
History
Review first published: Issue 8, 2022
Acknowledgements
This review was published in collaboration with the Cochrane Effective Practice and Organisation of Care (EPOC) group. We would like to thank the Co‐ordinating Editor Simon Lewin for his excellent editorial support and valuable comments on the review.
We would like to thank the International Initiative for Impact Evaluation (3ie) for making their software to create user evidence maps available to us and for supporting us throughout the process of creating the map.
We thank the team of the Referat für Medizinische Versorgung, Infektionsschutz, Hygiene from the Ministerium für Arbeit, Gesundheit und Soziales des Landes Nordrhein‐Westfalen for their participation in our stakeholder discussions and their valuable feedback on this scoping review.
The research was part of the Willie‐Vacc project supported by the German Research Foundation (Enhancing the willingness of healthcare workers to be vaccinated against COVID‐19 in Germany (SK 146/3‐1)).
We thank Jo Platt (Information Specialist, Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group) for peer‐reviewing the search strategy.
Editorial and peer‐reviewer contributions:
The following people conducted the editorial process for this article.
Sign‐off Editor (final editorial decision): Simon Lewin; Joint Co‐ordinating Editor of Cochrane Effective Practice and Organisation of Care (EPOC), Norweigan Institute for Public Health Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Helen Wakeford, Central Editorial Service, Cochrane Editorial Assistant (conducted editorial policy checks and supported editorial team): Leticia Rodrigues, Central Editorial Service, Cochrane Copy Editor (copy editing and production): Heather Maxwell, Cochrane Peer‐reviewers (provided comments and recommended an editorial decision): Jessica Kaufman, Murdoch Children's Research Institute, Holly Seale, University of New South Wales, and Majdi Sabahelzain, School of Health Sciences, Ahfad University for Women (clinical/content review), Solmaz Piri(consumer review), Chantelle Garrity (methods review), Robin Featherstone, Central Editorial Service (search review), Simon Lewin, Co‐ordinating Editor, Cochrane Effective Practice and Organisation of Care (EPOC).
Appendices
Appendix 1. PRISMA‐ ScR Checklist for this review
| Item | PRISMA‐ScR CHECKLIST ITEM | Done? | Section |
| 1 | Identify the report as a scoping review. | yes | Title |
| 2 | Provide a structured summary that includes (as applicable): background, objectives, eligibility criteria, sources of evidence, charting methods, results, and conclusions that relate to the review questions and objectives. | yes | Abstract |
| 3 | Describe the rationale for the review in the context of what is already known. Explain why the review questions/objectives lend themselves to a scoping review approach. | yes | Background |
| 4 | Provide an explicit statement of the questions and objectives being addressed with reference to their key elements (e.g., population or participants, concepts, and context) or other relevant key elements used to conceptualize the review questions and/or objectives. | yes | Objective |
| 5 | Indicate whether a review protocol exists; state if and where it can be accessed (e.g., a Web address); and if available, provide registration information, including the registration number. | yes | Methods |
| 6 | Specify characteristics of the sources of evidence used as eligibility criteria (e.g., years considered, language, and publication status), and provide a rationale. | yes | Methods |
| 7 | Describe all information sources in the search (e.g., databases with dates of coverage and contact with authors to identify additional sources), as well as the date the most recent search was executed. | yes | Methods |
| 8 | Present the full electronic search strategy for at least 1 database, including any limits used, such that it could be repeated. | yes | Appendix |
| 9 | State the process for selecting sources of evidence (i.e., screening and eligibility) included in the scoping review. | yes | Methods |
| 10 | Describe the methods of charting data from the included sources of evidence (e.g., calibrated forms or forms that have been tested by the team before their use, and whether data charting was done independently or in duplicate) and any processes for obtaining and confirming data from investigators. | yes | Mehods |
| 11 | List and define all variables for which data were sought and any assumptions and simplifications made. | yes | Methods |
| 12 | If done, provide a rationale for conducting a critical appraisal of included sources of evidence; describe the methods used and how this information was used in any data synthesis (if appropriate). | No; No critical appraisal conducted | |
| 13 | Describe the methods of handling and summarizing the data that were charted. | yes | Methods |
| 14 | Give numbers of sources of evidence screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally using a flow diagram. | yes | Figure 1: Flow diagram |
| 15 | For each source of evidence, present characteristics for which data were charted and provide the citations. | yes | Characteristics of included studies Table |
| 16 | If done, present data on critical appraisal of included sources of evidence (see item 12). | No; No critical appraisal conducted | |
| 17 | For each included source of evidence, present the relevant data that were charted that relate to the review questions and objectives. | yes | Summary of findings table, Interactive scoping map |
| 18 | Summarize and/or present the charting results as they relate to the review questions and objectives. | yes | Results |
| 19 | Summarize the main results (including an overview of concepts, themes, and types of evidence available), link to the review questions and objectives, and consider the relevance to key groups. | yes | Results |
| 20 | Discuss the limitations of the scoping review process. | yes | Discussion |
| 21 | Provide a general interpretation of the results with respect to the review questions and objectives, as well as potential implications and/or next steps. | yes | Discussion |
| 22 | Describe sources of funding for the included sources of evidence, as well as sources of funding for the scoping review. Describe the role of the funders of the scoping review. | yes | Sources of support, Acknowledgements |
[Enter text here]
Appendix 2. Search Strategies for Evidence Syntheses
Evidence Aid Coronavirus (Covid‐19) (evidenceaid.org/evidence/coronavirus-covid-19/)
searched by text word vaccin*
Usher Network for COVID‐19 Evidence Reviews (www.ed.ac.uk/usher/uncover/register-of-reviews)
searched by text word vaccin*
Epistemonikos L*OVE Covid‐19 (app.iloveevidence.com/loves)
search by text word vaccin* and limited to broad syntheses and systematic review
MEDLINE (Ovid 1946 to present)
# Searches
1 (COVID or "COVID‐19" or COVID19 or "SARS‐CoV‐2" or "SARS‐CoV2" or SARSCoV2 or "SARSCoV‐2").tw,kf.
2 (vaccin* adj5 (hesitanc* or hesitant* or hesitat* or uptake or "up‐take" or "take up" or trust* or distrust* or misinformation or barrier* or refusal or resist* or "anti‐vaccination" or "anti vaccine" or willing* or unwilling* or intent* or accept* or perception* or behaviour* or behavior* or belief* or view* or opinion or communication* or perspective* or attitude* or knowledge or concern or concerns or concerned or motivation or reject or confidence or "undecided" or "irresolute" or uncertain or nonintent or decide or deciding or decision* or consent* or perceiv* or aware*)).tw,kf.
3 cochrane database of systematic reviews.jn. or search*.tw. or meta analysis.pt. or medline.tw. or systematic review.tw. or systematic review.pt.
(Wong 2006 – systematic reviews filter – high specificity, 90,2% sens / 98,4% spec)
4 1 and 2 and 3
5 limit 4 to yr="2020 ‐Current"
Appendix 3. Search Strategies for Primary Literature
Cochrane COVID‐19 study register
vaccin*
AND
hesitanc* or hesitant* or hesitat* or uptake or "up‐take" or "take up" or trust* or distrust* or misinformation or barrier* or refusal or resist* or "anti‐vaccination" or "anti vaccine" or willing* or unwilling* or intent* or accept* or perception* or behaviour* or behavior* or belief* or believ* or view* or opinion* or communication* or perspective* or attitude* or knowledge or concern or concerns or concerned or motivation or reject or confidence or "undecided" or "irresolute" or uncertain or nonintent or decide or deciding or decision* or consent* or perceiv* or aware* or vaxxer* or "vaccination rates" or intend* or message* or encourage* or framing*
Web of Science
#1 TI=((vaccin* NEAR/5 (COVID OR COVID19 OR "SARS‐CoV‐2" OR "SARS‐CoV2" OR SARSCoV2 OR "SARSCoV‐2" OR "SARS coronavirus 2" OR "2019 nCoV" OR "2019nCoV" OR "2019‐novel CoV" OR "nCov 2019" OR "nCov 19" OR "severe acute respiratory syndrome coronavirus 2" OR "novel coronavirus disease" OR "novel corona virus disease" OR "corona virus disease 2019" OR "coronavirus disease 2019" OR "novel coronavirus pneumonia" OR "novel corona virus pneumonia" OR "severe acute respiratory syndrome coronavirus 2")))) OR AB=((vaccin* NEAR/5 (COVID OR COVID19 OR "SARS‐CoV‐2" OR "SARS‐CoV2" OR SARSCoV2 OR "SARSCoV‐2" OR "SARS coronavirus 2" OR "2019 nCoV" OR "2019nCoV" OR "2019‐novel CoV" OR "nCov 2019" OR "nCov 19" OR "severe acute respiratory syndrome coronavirus 2" OR "novel coronavirus disease" OR "novel corona virus disease" OR "corona virus disease 2019" OR "coronavirus disease 2019" OR "novel coronavirus pneumonia" OR "novel corona virus pneumonia" OR "severe acute respiratory syndrome coronavirus 2"))
#2 TI=((hesitanc* OR hesitant* OR hesitat* OR uptake OR "up‐take" OR "take up" OR trust* OR distrust* OR misinformation OR barrier* OR refusal OR resist* OR "anti‐vaccination" OR "anti vaccine" OR willing* OR unwilling* OR intent* OR accept* OR perception* OR behaviour* OR behavior* OR belief* OR believ* OR view* OR opinion* OR communication* OR perspective* OR attitude* OR knowledge OR concern OR concerns OR concerned OR motivation OR reject OR confidence OR "undecided" OR "irresolute" OR uncertain OR nonintent OR decide OR deciding OR decision* OR consent* OR perceiv* OR aware* OR vaxxer* OR "vaccination rates" OR intend* OR message* OR encourage* OR framing*))) OR AB=((hesitanc* OR hesitant* OR hesitat* OR uptake OR "up‐take" OR "take up" OR trust* OR distrust* OR misinformation OR barrier* OR refusal OR resist* OR "anti‐vaccination" OR "anti vaccine" OR willing* OR unwilling* OR intent* OR accept* OR perception* OR behaviour* OR behavior* OR belief* OR believ* OR view* OR opinion* OR communication* OR perspective* OR attitude* OR knowledge OR concern OR concerns OR concerned OR motivation OR reject OR confidence OR "undecided" OR "irresolute" OR uncertain OR nonintent OR decide OR deciding OR decision* OR consent* OR perceiv* OR aware* OR vaxxer* OR "vaccination rates" OR intend* OR message* OR encourage* OR framing*)
#3 #1 AND #2
WHO COVID‐19 global literature on coronavirus disease
Advanced search:
vaccin*
AND
hesitanc* or hesitant* or hesitat* or uptake or "up‐take" or "take up" or trust* or distrust* or misinformation or barrier* or refusal or resist* or "anti‐vaccination" or "anti vaccine" or willing* or unwilling* or intent* or accept* or perception* or behaviour* or behavior* or belief* or believ* or view* or opinion* or communication* or perspective* or attitude* or knowledge or concern or concerns or concerned or motivation or reject or confidence or "undecided" or "irresolute" or uncertain or nonintent or decide or deciding or decision* or consent* or perceiv* or aware* or vaxxer* or "vaccination rates" or intend* or message* or encourage* or framing*
PscycINFO Ovid
Search Strategy:
# Searches
1 immunization/
2 covid‐19/
3 (vaccin* adj8 (covid or covid‐19 or covid19 or "SARS‐CoV‐2" or "SARS‐CoV2" or SARSCoV2 or "SARSCoV‐2" or "SARS coronavirus 2")).mp.
4 (hesitanc* or hesitant* or hesitat* or uptake or "up‐take" or "take up" or trust* or distrust* or misinformation or barrier* or refusal or resist* or "anti‐vaccination" or "anti vaccine" or willing* or unwilling* or intent* or accept* or perception* or behaviour* or behavior* or belief* or believ* or view* or opinion* or communication* or perspective* or attitude* or knowledge or concern or concerns or concerned or motivation or reject or confidence or "undecided" or "irresolute" or uncertain or nonintent or decide or deciding or decision* or consent* or perceiv* or aware* or vaxxer* or "vaccination rates" or intend* or message* or encourage* or framing*).mp.
5 202*.up.
6 1 and 2
7 (3 or 6) and 4 and 5
CINAHL EBSCO
S1 MH "COVID‐19 Vaccines"
S2 MH "Anti‐Vaccination Movement"
S3 MM "Immunization"
S4 MH "COVID‐19"
S5 TX (vaccin* N8 (covid or covid‐19 or covid19 or "SARS‐CoV‐2" or "SARS‐CoV2" or SARSCoV2 or "SARSCoV‐2" or "SARS coronavirus 2"))
S6 S1 OR ( S4 AND (S2 OR S3) ) OR S5
S7 TX hesitanc* or hesitant* or hesitat* or uptake or "up‐take" or "take up" or trust* or distrust* or misinformation or barrier* or refusal or resist* or "anti‐vaccination" or "anti vaccine" or willing* or unwilling* or intent* or accept* or perception* or behaviour* or behavior* or belief* or believ* or view* or opinion* or communication* or perspective* or attitude* or knowledge or concern or concerns or concerned or motivation or reject or confidence or "undecided" or "irresolute" or uncertain or nonintent or decide or deciding or decision* or consent* or perceiv* or aware* or vaxxer* or "vaccination rates" or intend* or message* or encourage* or framing*
S8 S6 AND S7
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Argote 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Different information about COVID‐19 vaccine:
Motivation treatment:
|
|
| Population |
|
|
| Outcomes |
|
|
| Notes | COI: NR Funding: NR |
|
Ashworth 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Different messaging about benefits of the COVID‐19 vaccine:
|
|
| Population |
|
|
| Outcomes |
|
|
| Notes |
|
|
Barber 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Lottery | |
| Population |
|
|
| Outcomes | Vaccine uptake | |
| Notes |
|
|
Bokemper 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Experiment 1:
Experiment 2: The statement was randomly assigned to one of six values, (1) a positive or (2) negative statement by Dr. Anthony Fauci, (3) a positive or (4) negative statement by President Trump, (5) a joint positive statement by Trump and Speaker of the House Nancy Pelosi, or (6) a positive Trump statement and a negative Pelosi statement |
|
| Population |
|
|
| Outcomes | Primary outcome:
Secondary outcome:
|
|
| Notes | Sponsor/ funding: NR COI: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. |
|
Borah 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Framing
|
|
| Population |
|
|
| Outcomes |
|
|
| Notes |
|
|
Brehm 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | "Vax‐a‐Million" Lottery | |
| Population |
|
|
| Outcomes | Vaccine uptake | |
| Notes |
|
|
Campos‐Mercade 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions |
|
|
| Population |
|
|
| Outcomes |
|
|
| Notes | Funding: Danish National Research Foundation grant DNRF134 (PC), Swiss National Science Foundation Grant PZ00P1_201956(ANM), Swiss National Science Foundation grant 100018_185176 (FHS), Chazen Institute for Global Business at Columbia Business School (SM), Columbia Business School (SM), The Booth School of Business, University of Chicago(DP), and Riksbankens Jubileumsfond (EW) COI: none declared |
|
Capasso 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Persuasive messages focused on cognitive attitude plus anticipated affective reactions:
|
|
| Population |
|
|
| Outcomes |
|
|
| Notes |
|
|
Chen 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | News article about the development of the COVID‐19 vaccine with the following variables manipulated:
|
|
| Population |
|
|
| Outcomes |
|
|
| Notes | Funding: NR COI: none declared |
|
Duch 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Financial Incentives: Informational videos promoting vaccination with messages about:
|
|
| Population |
|
|
| Outcomes | Digital expression of further interest in vaccination information: choice between obtaining further information on being vaccinated in their state vs. ending the survey | |
| Notes |
|
|
Fox 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Framing
|
|
| Population |
|
|
| Outcomes |
|
|
| Notes |
|
|
Galasso 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Framing/Information provision
|
|
| Population |
|
|
| Outcomes |
|
|
| Notes |
|
|
Gong 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions |
|
|
| Population |
|
|
| Outcomes |
|
|
| Notes | Funding: none COI: none |
|
Hong 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Framing
|
|
| Population |
|
|
| Outcomes |
|
|
| Notes |
|
|
Howarth 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions |
|
|
| Population |
|
|
| Outcomes |
|
|
| Notes |
|
|
Huang 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Framing
|
|
| Population |
|
|
| Outcomes |
|
|
| Notes |
|
|
Jin 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Public service messages
|
|
| Population |
|
|
| Outcomes |
|
|
| Notes |
|
|
Jung 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Two scenarios:
|
|
| Population |
|
|
| Outcomes |
|
|
| Notes | Funding: National Institutes of Drug Abuse and the NIH COI: none declared |
|
Kachurka 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Persuasive messages:
Price information:
|
|
| Population |
|
|
| Outcomes | Willingness to get vaccinated | |
| Notes |
|
|
Kelkar 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions |
|
|
| Population |
|
|
| Outcomes |
|
|
| Notes | Funding: no external funding reported COI: none declared |
|
Keppeler 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions |
|
|
| Population |
|
|
| Outcomes | "Unique clicks," indicating the number of individuals who performed at least one click on each link (personalised link and corresponding QR code to the municipality’s information website, links to the digital scheduling software for vaccination appointments) | |
| Notes |
|
|
Kerr 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Study 1:
Study 2:
|
|
| Population |
|
|
| Outcomes | Study 1: Primary outcomes:
Secondary outcomes:
Study 2: Primary outcome:
Secondary outcomes:
|
|
| Notes | Funding: Winton Centre for Risk and Evidence Communication COI: none declared |
|
Klüver 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | 1. Freedoms – Control (0): there are no special regulations for vaccinated people even when the Corona incidence is high. For example, they cannot travel again, visit cinemas, restaurants or concerts and are still subject to contact restrictions. – Treatment (1): special regulations apply to vaccinated people. For example, even when the Corona incidence is high, they can travel again, visit cinemas, restaurants or concerts and are not subject to any contact restrictions. 2. Local Doctors – Control (0): eligible citizens can get vaccinated against Corona at the nearest vaccination centre but not at their local doctor. – Treatment (1): eligible citizens get vaccinated against Corona at the nearest vaccination centre or at their local doctor 3. Financial Incentives Control (0): citizens who are vaccinated will not receive any allowance after receiving the vaccination. – Treatment 1 (1): citizens who get vaccinated receive an expense allowance of 25 Euros after receiving the vaccination. – Treatment 2 (2): citizens who get vaccinated receive an expense allowance of 50 Euros after receiving the vaccination | |
| Population |
|
|
| Outcomes |
|
|
| Notes |
|
|
Kobayashi 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Corowa‐kun: a COVID‐19 vaccine information chatbot in a popular messenger app in Japan that answers commonly asked questions | |
| Population |
|
|
| Outcomes | Primary outcome: COVID‐19 vaccine hesitancy | |
| Notes | COI: none declared Funding:none | |
Kreps 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Manipulated variables:
|
|
| Population |
|
|
| Outcomes |
|
|
| Notes | Funding: Cornell Atkinson Center for Sustainability COI: the authors declare no competing interests. * This was the intervention we were interested in for this scoping review |
|
Marquez 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Community‐centered vaccination strategy that included mobilisation, vaccination, and activation components.
|
|
| Population |
|
|
| Outcomes | COVID‐19 vaccinations administered at the neighbourhood site during the evaluation period | |
| Notes | Funding: SFDPH, UCSF, private donors, and the Chan‐Zuckerberg Initiative COI: none |
|
Merkley 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions |
|
|
| Population |
|
|
| Outcomes |
|
|
| Notes |
|
|
OCEAN.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Control condition 1: Safety and effectiveness statement taken from the NHS website Condition 2: Adding the collective vaccination benefit of not personally getting ill Condition 3: Adding the collective vaccination benefit of not transmitting the virus to others Condition 4: Adding the collective vaccination benefits of not getting ill and not transmitting the virus (i.e.combining conditions 2 and 3) Condition 5: Adding the personal benefit of getting vaccinated Condition 6: Adding the seriousness of the pandemic (and that it is much more serious than seasonal influenza) Condition 7: Directly addressing concerns about vaccine safety related to the speed of development Condition 8: Indirectly addressing concerns about vaccine safety related to the speed of development Condition 9: Adding the collective and personal benefits together (i.e. combining conditions 4 and 5) Condition 10: Adding the information on the collective and personal benefits, the seriousness of the virus, and the information that indirectly addresses concerns about the speed of development (i.e. combining conditions 4, 5, 6, and 8) |
|
| Population |
|
|
| Outcomes | Primary outcome:
|
|
| Notes | Funding: NIHR Oxford Biomedical Research Centre and the NIHR Oxford Health Biomedical Research Centre. COI: AJP is Chair of UK Department Health and Social Care's Joint Committee on Vaccination & Immunisation, but does not participate in discussions on COVID‐19 vaccines, and is a member of the WHO's SAGE. Oxford University has entered into a partnership with AstraZeneca for the development of a coronavirus vaccine. All other authors declare no competing interests.. |
|
Palm 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Message in short article format on COVID‐19 vaccines were manipulated in the following conditions:
Control condition: no information |
|
| Population |
|
|
| Outcomes | Willingness to get vaccinated | |
| Notes | Funding: NR COI: none |
|
Peng 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Framing
|
|
| Population |
|
|
| Outcomes | Vaccination Intention measured on a Likert 5‐point scale | |
| Notes |
|
|
Pfattheicher 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Education about herd immunity or empathy condition
|
|
| Population |
|
|
| Outcomes |
|
|
| Notes |
|
|
Pink 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions |
|
|
| Population |
|
|
| Outcomes |
|
|
| Notes |
|
|
Reichardt 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Framing
|
|
| Population |
|
|
| Outcomes |
|
|
| Notes |
|
|
Robertson 2021a.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Financial incentives
|
|
| Population |
|
|
| Outcomes | Willingness to get vaccinated | |
| Notes |
|
|
Robertson 2021b.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Source of information
|
|
| Population |
|
|
| Outcomes | Vaccination intention | |
| Notes |
|
|
Ryoo 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Framing
|
|
| Population |
|
|
| Outcomes |
|
|
| Notes |
|
|
Santos 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions |
|
|
| Population |
|
|
| Outcomes | Primary outcome:
Secondary outcomes:
|
|
| Notes | Funding: NR COI: None |
|
Schwarzinger 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Survey section1 Variable 1: Information on herd immunity
Variable 2: General practitioner (GP) recommendation
Survey section 2: Vaccine with differing attributes:
|
|
| Population |
|
|
| Outcomes |
|
|
| Notes | COI: none declared Funding: French Public Health Agency (Santé Publique France) |
|
Sehgal 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | "Vax‐a‐Million” lottery | |
| Population |
|
|
| Outcomes | Vaccine uptake | |
| Notes |
|
|
Senderey 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Reminders and nudging
|
|
| Population |
|
|
| Outcomes | Vaccine uptake (CHS members who received (or reserved an appointment to receive) the first dose of the COVID‐19 vaccine | |
| Notes |
|
|
Serra‐Garcia 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Measures to increase support of COVID‐19 vaccination and testing
|
|
| Population |
|
|
| Outcomes |
|
|
| Notes | COI: NR Funding: NR | |
Sinclair 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Information saying that
|
|
| Population |
|
|
| Outcomes |
|
|
| Notes |
|
|
Sotis 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Nudging
|
|
| Population |
|
|
| Outcomes |
|
|
| Notes |
|
|
Sprengholz 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Study 1
Study 2
|
|
| Population |
|
|
| Outcomes | Study 1:
Study 2:
|
|
| Notes | COI: the authors declare that they have no conflicts of interest. Funding: Supported by the German Research Foundation (BE3970/12‐1), the Federal Centre for Health Education, the Robert Koch Institute, the Leibniz Institute for Psychology, the Klaus Tschira Stiftung, the University of Erfurt, and the University of Copenhagen |
|
Sprengholz 2021c.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Incentives and prosocial communication
|
|
| Population |
|
|
| Outcomes | Intention to get vaccinated against COVID‐19 | |
| Notes |
|
|
Stein 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Targeted vaccine outreach via informational telephone calls | |
| Population |
|
|
| Outcomes |
|
|
| Notes |
|
|
Strickland 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions |
|
|
| Population |
|
|
| Outcomes |
|
|
| Notes | Funding: Supported by the National Institute on Drug Abuse (NIDA) of the National Institutes of Health, General Research Fund award from the University of Kansas, and NIDA grant COI: None declared |
|
Sudharsanan 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | The risk of a future COVID‐19 vaccine is presented: (1) with a qualitative label (2) with a comparison risk (3) in absolute or relative terms (4) Status quo framing where the side effect risk mimics the media's communication in early April 2021 |
|
| Population |
|
|
| Outcomes | Primary:
Secondary
|
|
| Notes |
|
|
Takamatsu 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Multifaceted intervention
|
|
| Population |
|
|
| Outcomes | Vaccine uptake | |
| Notes |
|
|
Talmy 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions |
|
|
| Population |
|
|
| Outcomes | Vaccination rate | |
| Notes | COI: T.T. reports being an employee of Emedgene Technologies between 2017 and 2019, this affiliation has no connection or relevance to the currently submitted work. B.C, I.N and Y.B.M have no conflicts of interests to disclose. Funding: this research did not receive any specific grant or funding from any agency. |
|
Thirumurthy 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Lottery | |
| Population |
|
|
| Outcomes | Vaccine uptake (vaccine doses administered daily per 100,000 individuals) | |
| Notes |
|
|
Thorpe 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Information messaging:
|
|
| Population |
|
|
| Outcomes |
|
|
| Notes |
|
|
Thunström 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Framing and Information source
|
|
| Population |
|
|
| Outcomes |
|
|
| Notes |
|
|
Tran 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Interactive web tool
|
|
| Population |
|
|
| Outcomes |
|
|
| Notes |
|
|
Ugwuoke 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Visual illustration communications
|
|
| Population |
|
|
| Outcomes |
|
|
| Notes |
|
|
Walkey 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | "Vax‐a‐Million” lottery | |
| Population | Population
|
|
| Outcomes | Vaccine uptake | |
| Notes |
|
|
Witus 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Animated YouTube video explaining how COVID‐19 mRNA vaccines work:
|
|
| Population |
|
|
| Outcomes | Vaccination intention | |
| Notes | COI: none declared Funding: LSW is supported by a Cottrell Scholar Award from the Research Corporation for Science Advancement |
|
Ye 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Framing
|
|
| Population |
|
|
| Outcomes |
|
|
| Notes |
|
|
Yu 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Nine different scenarios of the COVID‐19 vaccine availability in Hong Kong:
|
|
| Population |
|
|
| Outcomes |
|
|
| Notes | COI: None declared Funding: The study was supported by internal research funding of the Centre for Health Behaviours Research. The funding source has no role in this study * For this scoping review, we are only interested in this intervention |
|
Yu 2021b.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Eight scenarios (S1–S8) combining vaccines’ effectiveness (80% versus 50%), safety (rare mild side effects versus common mild side effects), and cost (free versus 600 Yuan), and two scenarios of free or self‐paid COVID‐19 vaccination involving recommendations given by the government/hospitals (S9–S10)*. | |
| Population |
|
|
| Outcomes |
|
|
| Notes | COI: none declared
Funding: The study was supported by the internal research funding of the Centre for Health Behaviour Research, the Chinese University of Hong Kong *For this scoping review, we are only interested in the two scenarios of free or self‐paid COVID‐19 vaccination involving recommendations given by the government/hospitals. |
|
Yuan 2021.
| Study characteristics | ||
| Methods |
|
|
| Interventions | Distance framing via different videos:
|
|
| Population |
|
|
| Outcomes |
|
|
| Notes |
|
|
COI: conflict of interest;FDA: Food and Drug Administration; HCP: healthcare personnel;IQR: interquartile range; mRNA: messenger ribonucleic acid; NHS: National Health Service;NR: none reported;NI: no information; SD: standard deviation; vs.: versus.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ali 2021 | Single‐arm study with less than 100 participants (53) |
| American Society of Safety Professionals 2021 | Not an intervention study |
| Batteux 2021 | Investigates scenarios that cannot be manipulated |
| Bell 2021 | Not an intervention study |
| ChiCTR2100043018 | Not an intervention study |
| Community Practitioner 2021 | Not an intervention study |
| Crawshaw 2021 | Does not summarise interventions to increase uptake |
| Crawshaw 2021b | Does not summarise interventions to increase uptake |
| Davis 2021 | Hypothetical scenario that cannot be manipulated (vaccine efficacy) |
| Gakuba 2021 | Uncontrolled study with less than 100 participants (n = 61) |
| Gehrau 2021 | Not an intervention study |
| Guelmami 2021 | Not an intervention study |
| Hofer 2021 | Wrong publication type (opinion piece) |
| Kaplan 2021 | Investigates scenarios that cannot be manipulated |
| Kirkpatrick 2021 | Wrong vaccine (MMR) |
| Knight 2021 | Development but not testing of an intervention |
| Kumar 2021 | Not an intervention study |
| Lim 2020 | Not an intervention study |
| Loomba 2021 | Wrong publication type (correction) |
| Loomba 2021a | Measures the effect of misinformation on vaccine intent and thus not relevant to the research objective |
| NCT04694651 | Not an intervention study |
| Rahmandad 2021 | Not an intervention study |
| Salali 2021 | Not an intervention study |
| Shmueli 2021 | No intervention |
| Sprengholz 2021b | Wrong publication type |
| Thaker 2021 | Measures effect of vaccine misinformation |
| Vasquez 2021 | Not an intervention study |
| Wagner 2021 | Not an intervention study |
| Yousuf 2021 | Intervention targets influenza vaccine, not COVID‐19 vaccine |
| Yuen 2021 | Not an intervention study |
MMR: measles, mumps and rubella.
Characteristics of studies awaiting classification [ordered by study ID]
INFORMED.
| Methods |
|
| Objective | Develop and evaluate "INdividual and Family‐Oriented Responsive Messaging EDucation" (INFORMED) intervention in increasing knowledge about COVID‐19 testing and decreasing decisional conflicts of getting tested for COVID‐19. |
| Notes | Funding: University of California, San Francisco COI: NR |
Larson 2020.
| Methods |
|
| Objective | CONVINCE – COVID‐19 New Vaccine Information, Communication and Engagement – a rapidly expanding, voluntary global initiative to promote the use of effective public communications and engagement to build vaccine literacy and expedite immunisation programs to protect communities against the COVID‐19 Pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). |
| Notes | Funding: NR COI: NR |
NCT04460703.
| Methods |
|
| Objective | This study tests different messages about vaccinating against COVID‐19 once the vaccine becomes available. Participants are randomised to 1 of 12 arms, with one control arm and one baseline arm. We will compare the reported willingness to get a COVID‐19 vaccine at 3 and 6 months of it becoming available between the 10 intervention arms to the 2 control arms. |
| Notes | Funding: Yale University COI: NR |
NCT04731870.
| Methods |
|
| Objective | Explore perceptions, confidence, trust, and uptake of potential COVID‐19 vaccines among healthcare providers (nurses and doctors) and key at‐risk population subgroups (minority populations living in the rural south) and will develop and test vaccine messaging that boosts vaccine confidence and trust among these key at‐risk subgroups. |
| Notes | Funding: East Carolina University COI: NR |
Supraneni 2021.
| Methods |
|
| Objective | The study will help explore the burden of vaccine acceptance and hesitancy among individuals living in urban and rural settings of Chennai. Further, it will help to examine the variables that influence vaccine acceptance and hesitancy. Lastly, the findings will help to design and develop a user‐centred informatics platform that can deliver multimedia‐driven health education modules tailored to facilitate vaccine uptake in varied settings. |
| Notes | Funding: NI COI: none declared |
COI: conflict of interest;NR: not reported.
Characteristics of ongoing studies [ordered by study ID]
DRKS00023650.
| Study name | An entertainment‐education approach to improve vaccine confidence during the COVID‐19 pandemic: an online randomised controlled experiment with 24,000 participants |
| Starting date | April 2021 |
| Contact information | Stanford University, Ms. Dr. Maya Adam, 291 Campus Drive Li Ka Shing Building, 94305‐510 Stanford, USA |
| Methods |
|
| Intervention |
|
| Population |
|
| Outcomes | Primary outcome
Secondary outcomes
|
| Estimated completion date and number of participants | NR |
| Notes | COI: NR Sponsor: Heidelberg Institute of Global Health, University of Heidelberg (Institutional funding) |
ISRCTN15317247.
| Study name | Using text messages to boost COVID‐19 vaccine booking rate |
| Starting date | 3 June 2021 |
| Contact information | Hannah Behrendt, hannah.behrendt@bi.team |
| Methods |
|
| Intervention | Behaviourally‐informed SMS vaccination information
|
| Population |
|
| Outcomes | Primary outcome
Secondary outcomes
|
| Estimated completion date and number of participants | 4270,000 planned, completed 1 July 2021 |
| Notes |
|
NCT04542395.
| Study name | COVID‐2019 testing and vaccination among African American and Latinx public housing residents |
| Starting date | 1 June 2021 |
| Contact information | Mohsen Bazargan, (323) 563 5902, mohsenbazargan@cdrewu.edu |
| Methods |
|
| Intervention | Provide/enhance knowledge, modify attitudes, motivate and provide skills and resources to reduce COVID‐19 related risk and challenges and increase willingness and uptake in COVID‐19 testing and vaccination. |
| Population |
|
| Outcomes | Primary outcomes
|
| Estimated completion date and number of participants | 30 November 2022 with 310 participants |
| Notes | Funding: Charles Drew University of Medicine and Science COI: NR |
NCT04604743.
| Study name | Clinic‐based HPV and COVID‐19 vaccine promoting intervention for AfAm adolescents in Alabama |
| Starting date | 20 April 2021 |
| Contact information | Henna Budhwani, 205‐975‐7613, budhwani@uab.edu |
| Methods |
|
| Intervention | The intervention will target adolescents aged 15‐17 years who have not received at least one dose of the HPV vaccine. The intervention, when developed, will improve knowledge of HPV, COVID‐19, the HPV vaccine, and the COVID‐19 vaccine (Information), reduce stigma and distrust improving motivation (Motivation), leading to improved vaccine confidence and higher vaccination rates and lower vaccine hesitancy (Behavioural Skills). |
| Population |
|
| Outcomes |
|
| Estimated completion date and number of participants | 30 June 2023 with 120 participants |
| Notes | Funding: University of Alabama at Birmingham |
NCT04706403.
| Study name | If we build it, will they come? A pilot study to develop and test messages to maximize uptake of coronavirus vaccine when available |
| Starting date | 12 January 2021 |
| Contact information | NR |
| Methods |
|
| Intervention | Participants who express hesitation about getting vaccinated for COVID‐19 will be randomised to receive one of five different versions of messages from a healthcare provider (experimental groups) or a control message (control group). The messages that participants in each experimental group receive will vary slightly and systematically. Specific content and wording of these messages will be developed to address and mitigate concerns of those at risk for not being vaccinated. |
| Population | Inclusion criteria: adult (age 18 and over) who are members of an online panel (Prolific); able to complete an on‐line survey in English. |
| Outcomes | Primary outcome
Secondary outcome
|
| Estimated completion date and number of participants | 1 February 2021 with 1706 participants |
| Notes | Sponsor: University of Massachusetts, Worcester COI: NR |
NCT04732819.
| Study name | IMPACT‐C: Improving vaccine uptake in skilled nursing facilities |
| Starting date | 4 January 2021 |
| Contact information | NR |
| Methods |
|
| Intervention |
|
| Population |
|
| Outcomes | Primary outcome
Secondary outcomes
|
| Estimated completion date and number of participants | 16 April 2021 with 23,768 participants enrolled |
| Notes | Funding: Brown University COI: NR |
NCT04761692.
| Study name | Improving vaccine acceptance and uptake among underresourced African American and Latinx older adults: a multidisciplinary and culturally‐based training program for minority churches |
| Starting date | 1 October 2021 |
| Contact information | Mohsen Bazargan, 3233573655, mohsenbazargan@cdrewu.edu |
| Methods |
|
| Intervention |
|
| Population |
|
| Outcomes | Primary outcome
Secondary outcomes
|
| Estimated completion date and number of participants | 31 December 2026 with 570 participants |
| Notes | Sponsor: Charles Drew University of Medicine and Science COI: NR |
NCT04779138.
| Study name | Community partnered intervention to increase COVID‐19 vaccine uptake in low income underresourced African Americans and Latinx public housing residents |
| Starting date | 11 September 2021 |
| Contact information | Sharon Cobb, 3235683329, sharoncobb1@cdrewu.edu |
| Methods |
|
| Intervention | The proposed intervention will employ (1) culturally sensitive, (2) theoretically‐based intervention that will be jointly delivered by our ACTIVATE triad leaders and our researchers. We will use the Information, Motivation, and Behavioral Skills (IMB) model and the Transtheoretical Model to implement the intervention. |
| Population |
|
| Outcomes | Primary outcome
Secondary outcomes
|
| Estimated completion date and number of participants | 30 November2023 with 600 participants |
| Notes | Funding: Charles Drew University of Medicine and Science COI: NR |
NCT04800965.
| Study name | Text‐based interventions to promote COVID‐19 vaccinations |
| Starting date | 31 January 2021 |
| Contact information | NR |
| Methods |
|
| Intervention |
|
| Population |
|
| Outcomes | Primary outcome
Secondary outcomes
|
| Estimated completion date and number of participants | 1 January 2022 with 400,000 participants |
| Notes | Sponsors: University of California, Los Angeles and Carnegie Mellon University COI: NR |
NCT04801030.
| Study name | Using multi‐strategies to address COVID‐19 vaccine hesitancy among African Americans |
| Starting date | 1 June 12021 |
| Contact information | Jennifer C Erves, PhD6153275692, jerves@mmc.edu |
| Methods |
|
| Intervention |
|
| Population | Population: African American Inclusion criteria: unvaccinated for COVID‐19; Vaccine hesitant; 18 years and older; Speaks English. |
| Outcomes | Primary outcomes
Secondary outcome
|
| Estimated completion date and number of participants | 30 May 2023 with 300 participants |
| Notes | Funding: Meharry Medical College COI: NR |
NCT04801524.
| Study name | NCT04801524 |
| Starting date | 7 February 2021 |
| Contact information | UCLA Health Department of Medicine, Quality OfficeWestwood, California, United States, 90095 |
| Methods |
|
| Intervention |
|
| Population |
|
| Outcomes | Primaryooutcome
Secondary outcomes
|
| Estimated completion date and number of participants | 1 January 2022 with 250,000 participants |
| Notes | Sponsors: University of California, Los Angeles and Carnegie Mellon University COI: NR |
NCT04805931 (VEText).
| Study name | VEText message framing and COVID‐19 vaccine uptake among at‐risk veterans (VEText) |
| Starting date | 15 March 2021 |
| Contact information | Alaina Mori, BA(206) 247‐6782, alaina.mori@va.gov |
| Methods |
|
| Intervention |
|
| Population |
|
| Outcomes | Primary outcome
|
| Estimated completion date and number of participants | 1 November 2021 with 4311 participants |
| Notes | Funding: VA Puget Sound Health Care System COI: none |
NCT04813770.
| Study name | The impact of theory‐based messaging on COVID‐19 vaccination intentions |
| Starting date | 6 April 2021 |
| Contact information | NR |
| Methods |
|
| Intervention |
|
| Population |
|
| Outcomes | Primary outcome
Secondary outcomes
|
| Estimated completion date and number of participants | 26 April 2021 with 113 participants |
| Notes | Funding: University of Glasgow COI: NR |
NCT04834726.
| Study name | Pragmatic trial of COVID vaccine text outreach interventions |
| Starting date | 29 April 2021 |
| Contact information | NR |
| Methods |
|
| Intervention |
|
| Population |
|
| Outcomes | Primary outcome
Secondary outcomes
|
| Estimated completion date and number of participants | 30 July 2021 with 19,554 participants |
| Notes | Funding: University of Pennsylvania COI: NR |
NCT04867174.
| Study name | COVID‐19 vaccination take‐up in a county‐run Medicaid managed care population |
| Starting date | 24 May 2021 |
| Contact information | Mireille Jacobson, 213‐986‐6076, mireillj@usc.edu |
| Methods |
|
| Intervention |
|
| Population | Population: ethnically diverse minority populations; members of Contra Costa Health Plan (CCHP). Inclusion criteria: age 18 and over; no contraindications to vaccination, as determined by county health plan or other medical staff. |
| Outcomes | Primary outcome
Secondary outcomes
Other outcome
|
| Estimated completion date and number of participants | 24 May 2022 with 2825 participants |
| Notes | Funding: University of Southern California COI: NR |
NCT04870593.
| Study name | What works to get the elderly vaccinated against COVID‐19? Experimental evidence from India |
| Starting date | 17 April 2021 |
| Contact information | Esther Duflo, Professor, National Bureau of Economic Research, Inc. |
| Methods |
|
| Intervention |
|
| Population | Elderly population (≥55) of India (with a phone number) Inclusion criteria
Exclusion criteria
|
| Outcomes | Primary outcomes
|
| Estimated completion date and number of participants | 10 July 2021 with 3006 participants |
| Notes | Funding: National Bureau of Economic Research, Inc. COI: NR |
NCT04871776.
| Study name | Use of construal level theory to inform messaging to increase vaccination against COVID‐19 |
| Starting date | June 2021 |
| Contact information | Nancy Haff, 9782011244, nhaff@partners.org |
| Methods |
|
| Intervention |
|
| Population | Population: patients in the Mass General Brigham system Inclusion criteria: aged 18 and older; have not received a dose of any COVID vaccine; home address inside Massachusetts |
| Outcomes | Primary outcome
Secondary outcomes
|
| Estimated completion date and number of participants | June 2022 with 10,000 participants |
| Notes | Funding: Brigham and Women's Hospital COI: NR |
NCT04876885.
| Study name | The future of viral communications: video‐based health promotion strategies for COVID‐19 vaccinations |
| Starting date | 6 May 2021 |
| Contact information | Sarrah M Lal, 289.808.8597, lals2@mcmaster.ca |
| Methods |
|
| Intervention |
|
| Population | Population: general public and healthcare professionals Inclusion criteria
|
| Outcomes | Primary outcome
Secondary outcome
|
| Estimated completion date and number of participants | 6 June2021 with 100 participants |
| Notes | Funding: McMaster University COI: NR |
NCT04884750.
| Study name | Community‐based design and evaluation of a conversational agent to promote SARS‐COV2 vaccination in black churches |
| Starting date | July 1, 2022 |
| Contact information | Lin Shi, 408‐828‐7588, l.shi@northeastern.edu |
| Methods |
|
| Intervention | Intervention: a smartphone‐based embodied conversational agent that educates users and motivates them to obtain vaccinations for SARS‐CoV‐2 and Influenza, according to Boston Public Health Commission guidelines
|
| Population |
|
| Outcomes | Primary outcomes
Secondary outcomes
|
| Estimated completion date and number of participants | 31 January 2025 with 600 participants |
| Notes | Funding: Northeastern University COI: NR |
NCT04895683.
| Study name | Can Behavioural‐science Informed Text Messages Improve COVID‐19 Vaccination Uptake in North West London? A RCT |
| Starting date | May 11, 2021 |
| Contact information | Sarah Huf, 07496632732, s.huf@imperial.ac.uk |
| Methods |
|
| Intervention |
|
| Population |
|
| Outcomes | Primary outcome
Secondary outcomes
|
| Estimated completion date and number of participants | 11 May 2022 with 120,000 participants |
| Notes | Funding: Imperial College Healthcare NHS Trust COI: NR |
NCT04924803.
| Study name | Community developed technology‐based messaging to increase COVID‐19 vaccine uptake among people who inject drugs |
| Starting date | 14 June 2021 |
| Contact information | Ian D Aronson, ia14@nyu.edu |
| Methods |
|
| Intervention | weekly text messages and intervention videos
|
| Population |
|
| Outcomes |
|
| Estimated completion date and number of participants | December 2023, 500 planned |
| Notes |
|
NCT04930965 (LA‐CEAL: HALT COVID).
| Study name | Impact of LA‐CEAL HALT COVID‐19 Ambassador Program on likelihood to vaccinate |
| Starting date | June 18, 2021 |
| Contact information | Erin Peacock, epeacoc@tulane.edu Leslie Craig, lcraig1@tulane.edu |
| Methods |
|
| Intervention | HCW Vaccine Ambassadors
|
| Population |
|
| Outcomes |
|
| Estimated completion date and number of participants | 31cMarch 2022, 100 planned |
| Notes |
|
NCT04939506.
| Study name | COVID‐19 vaccine education at the point of testing to increase vaccine uptake in vulnerable communities in SE Louisiana |
| Starting date | 25 June, 2021 |
| Contact information | Sara Al‐Dahir, PharmD 5045205766 saaldah@xula.edu |
| Methods |
|
| Intervention | Rapid education
|
| Population |
|
| Outcomes | Primary outcome
Secondary outcome
|
| Estimated completion date and number of participants | June 2023, 375 planned |
| Notes |
|
NCT04939519 (SCALE‐UP Utah).
| Study name | SCALE‐UP Utah: Community‐Academic Partnership to address COVID‐19 vaccination rates among Utah Community Health Centers |
| Starting date | 25 June 2021 |
| Contact information | David Wetter |
| Methods |
|
| Intervention | Outreach text messages
|
| Population |
|
| Outcomes |
|
| Estimated completion date and number of participants | 22 September 2022, 110,270 recruited |
| Notes |
|
NCT04951310.
| Study name | COVID‐19 Vaccinations with a sweepstake |
| Starting date | 6 July 2021 |
| Contact information | University of Pennsylvania |
| Methods |
|
| Intervention | Lottery ("Philly Vax Sweepstakes")
|
| Population |
|
| Outcomes |
|
| Estimated completion date and number of participants | 14 August 2021, 3827,656 participants |
| Notes |
|
NCT04952376.
| Study name | Equitable access to COVID‐19 vaccines |
| Starting date | 7 July 2021 |
| Contact information | Farhia Omar, Omar.Farhia@mayo.edu Idali Cuellar, Cuellar.Idali@mayo.edu |
| Methods |
|
| Intervention | Personalised text messages
|
| Population |
|
| Outcomes | Vaccine uptake (Dose 1 COVID‐19 Vaccine (after 30 days), Dose 2 COVID‐19 Vaccine (after 60 days), Engagement (after 30 days). |
| Estimated completion date and number of participants | June 2022, 1500 planned |
| Notes |
|
NCT04960228.
| Study name | Exploring changes in COVID‐19 vaccination intentions by prompting altruistic motives using a video Intervention |
| Starting date | 13 July 2021 |
| Contact information | Zeev Rosberger, Sir Mortimer B. Davis ‐ Jewish General Hospital |
| Methods |
|
| Intervention | Video
|
| Population |
|
| Outcomes | Willingness to get vaccinated (Change in Vaccine Intentions ‐ Pre‐Post Intervention) |
| Estimated completion date and number of participants | 13 September 2021, 2630 planned |
| Notes |
|
NCT04963790.
| Study name | Enabling family physicians to reduce vaccine hesitancy andiIncrease Covid‐19 vaccine uptake |
| Starting date | 15 July 2021 |
| Contact information | Stephanie Chenail, stephaniechenail@montfort.on.ca |
| Methods |
|
| Intervention | Personalised text messages
|
| Population |
|
| Outcomes |
|
| Estimated completion date and number of participants | September 2022, 7200 planned |
| Notes |
|
NCT04964154 (BRAVE).
| Study name | Building Resiliency and Vital Equity (BRAVE) project: understanding Native Americans' perceptionsbBeliefs about COVID‐19 testing and vaccination study (BRAVE) |
| Starting date | 15 July 2021 |
| Contact information | Deepak Kumar, dkumar@ad.nccu.edu Tracie Locklear, tlockl12@nccu.edu |
| Methods |
|
| Intervention | Cultural appropriate educational information
|
| Population |
|
| Outcomes |
|
| Estimated completion date and number of participants | 30 June 2022, 4000 planned |
| Notes |
|
NCT04979416.
| Study name | Video messages and vaccination intention |
| Starting date | 28 July 2021 |
| Contact information | Mireille Jacobson, mireillj@usc.edu |
| Methods |
|
| Intervention | Video messaging
|
| Population |
|
| Outcomes | Vaccine intention (probability of getting vaccinated in the next 30 days) |
| Estimated completion date and number of participants | 22 July 021 1000 planned |
| Notes |
|
NCT04981392.
| Study name | Intervention to promote COVID‐19 vaccination |
| Starting date | 29 July 2021 |
| Contact information | Kimberly Fisher, kimberly.fisher@umassmemorial.org |
| Methods |
|
| Intervention | HCW Vaccine Ambassadors
|
| Population |
|
| Outcomes |
|
| Estimated completion date and number of participants | 29 February 2024, 38,292 participants |
| Notes |
|
NCT05022472 (2VIDA!).
| Study name | Project 2VIDA! COVID‐19 Vaccine Intervention Delivery for Adults in southern California (2VIDA!) |
| Starting date | 26 August 2021 |
| Contact information | Argentina E Servin, MD,MPH; 6195767211; arservin@ucsd.edu |
| Methods |
|
| Intervention | Multidimensional community intervention
|
| Population |
|
| Outcomes | Primary outcomes
Secondary outcomes
Other outcomes
|
| Estimated completion date and number of participants | 31 January 2026, 1000 participants |
| Notes |
|
NCT05027464 (CoVAcS).
| Study name | Developing and testing a COVID‐19 Vaccination Acceptance intervention (CoVAcS) |
| Starting date | 30 August 2021 |
| Contact information | Yasmin Jolly, Yasmin.Jolly@va.gov Nicole McCamish, Nicole.McCamish@va.gov |
| Methods |
|
| Intervention | Outreach calls
|
| Population |
|
| Outcomes | Primary outcome
Secondary outcomes
|
| Estimated completion date and number of participants | 30 September 2023, 2500 participants |
| Notes |
|
NCT05037201.
| Study name | Text message nudges for COVID‐19 vaccination |
| Starting date | 8 September, 2021 |
| Contact information | Mitesh Patel, MD, MBA 7343550817 Mitesh.Patel3@Ascension.org |
| Methods |
|
| Intervention | Nudging
|
| Population |
|
| Outcomes |
|
| Estimated completion date and number of participants | 8 November 2021, 2000 participants |
| Notes |
|
PACTR202102846261362.
| Study name | Using community influencer groups to address COVID‐19 misinformation and potential vaccine hesitancy in Uganda |
| Starting date | February 2021 |
| Contact information | Freddy Kitutu, kitutufred@gmail.com, +256705791777 |
| Methods |
|
| Intervention | Implementing community influencer groups (5 men, 5 women) that are educated to respond to COVID‐19 vaccine hesitancy and misinformation |
| Population | Population: Ugandans living in selected villages. Inclusion criteria: healthy men and women aged 18 years to 65 years older who normally reside in households in the selected villages, domestic servants who have slept for five nights a week or more in the households, and visitors who have slept in the household for at least the past four weeks. Exclusion criteria: men and women from households that are under COVID‐19 isolation or quarantine at the time of data collection will be excluded from the study if they do not have access to a phone for phone interviews to be conducted. |
| Outcomes | Primary outcomes
Secondary outcome
|
| Estimated completion date and number of participants | NR |
| Notes | Funding: Sabin Vaccine Institute, Makerere University |
Differences between protocol and review
We made several changes to the methods described in the protocol during the review process. The protocol was prospectively registered (Andreas 2021).
Methods: inclusion criteria; study design
For psychological experiments with hypothetical scenarios being tested, we later decided to only include those studies that investigate scenarios that can be manipulated in a real‐world setting. For example communication about vaccines can be manipulated, but vaccine efficacy cannot be manipulated. We made this change as we were unaware that this type of study exists and that it would be identified in our search.
In addition, we later decided not to include case studies, based on a suggestion with comprehensible reasoning from a reviewer.
Methods: Summary and reporting of results
The original intervention categories proposed in the protocol were based on past research on intervention strategies for vaccine uptake. However, after intensive discussion with Co‐authors and stakeholders, we realised that we would need more categories to adequately map our results. We, therefore, included the former subcategory "Education" of the category communication‐based interventions as a separate category. This enabled us to better portray the differences in the very diverse interventions that were communication‐based. Furthermore, we added the category of "multidimensional interventions", as many studies interventions use more than one strategy. We also added the category "interventions to improve access" because this was helpfully recommended by a peer‐reviewer. Please see Table 4 for an overview of the categories described in the protocol and those used in this review.
Contributions of authors
MA: methodological expertise and conception and writing of the review
CI: methodological expertise, writing and conception
VP: methodological expertise and conception, revision of review draft, and approval of final review draft
IM: developing of search strategy and conducting the search
EB: methodological expertise and conception and writing of the review
JJM: methodological expertise and conception, revision of review draft, and approval of final review draft
NS: methodological expertise and conception and writing of the review
Sources of support
Internal sources
-
University of Cologne, Germany
University of Cologne, Faculty of Medicine and University Hospital Cologne, Department I of Internal Medicine, Evidence‐based Oncology
External sources
-
Germany Research Foundation, Germany
This review is funded by the Germany Research Foundation (Deutsche Forschungsgemeinschaft; DFG) as part of the Willie‐Vacc project (Enhancing the willingness of healthcare workers to be vaccinated against COVID‐19; SK 146/3‐1).
Declarations of interest
MA: none known
CI: none known
VP: none known
EB: none known
JJM: member of the Standing Vaccination Committee (STIKO) in Germany
NS: none known
Edited (no change to conclusions)
References
References to studies included in this review
Argote 2021 {published data only}
- Argote P, Barham E, Daly S, Gerez J, Marshall J, Pocasangre O. Messaging interventions that increase COVID-19 vaccine willingness in Latin America: evidence from online experiments in six Latin American countries (preprint). SSRN 2021. [DOI: 10.2139/ssrn.3812023] [SSRN: 3812023] [DOI]
Ashworth 2021 {published data only}
- Ashworth M, Thunström L, Cherry TL, Newbold SC, Finnoff DC. Emphasize personal health benefits to boost COVID-19 vaccination rates. PNAS 2021;118(32):e2108225118. [DOI: 10.1073/pnas.2108225118] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barber 2021 {published data only}
- Barber A, West J. Conditional cash lotteries increase COVID-19 vaccination rates. Journal of Health Economics 2021;81:102578. [DOI: 10.1016/j.jhealeco.2021.102578.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bokemper 2021 {published data only}
- Bokemper SE, Huber GA, Gerber AS, James EK, Omer SB. Timing of COVID-19 vaccine approval and endorsement by public figures. Vaccine 2021;39(5):825-9. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Borah 2021 {published data only}
- Borah P, Hwang J, Hsu YC. COVID-19 vaccination attitudes and intention: message framing and the moderating role of perceived vaccine benefits. Journal of Health Communication 2021;26(8):523-33. [DOI: 10.1080/10810730.2021.1966687] [DOI] [PubMed] [Google Scholar]
Brehm 2021 {published data only}
- Brehm M, Brehm P, Saveedrah MH. The Ohio vaccine lottery and starting vaccination rates . SSRN 2021;(preprint):1-10. [DOI: 10.2139/ssrn.3875021] [SSRN: 3875021] [DOI] [Google Scholar]
Campos‐Mercade 2021 {published data only}
- Campos-Mercade P, Meier AN, Schneider FH, Meier S, Pope D, Wengström E. Monetary incentives increase COVID-19 vaccinations. Science 2021;published online:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Capasso 2021 {published data only}
- Capasso M, Caso D, Conner M. Anticipating pride or regret? Effects of anticipated affect focused persuasive messages on intention to get vaccinated against COVID-19. Social Science & Medicine 2021;289:114416. [DOI: 10.1016/j.socscimed.2021.114416] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2021 {published data only}
- Chen T, Dai M, Xia S, Zhou Y. Do messages matter? Investigating the combined effects of framing, outcome uncertainty, and number format on COVID-19 vaccination attitudes and intention. Health Communication 2021;January:1-8. [DOI: 10.1080/10410236.2021.1876814] [DOI] [PubMed] [Google Scholar]
Duch 2021 {published data only}
- Duch RM, Barnett A, Filipek M, Roope L, Violato M, Clarke P. Cash versus Lotteries: COVID-19 Vaccine Incentives Experiment . medRxiv 2021;(preprint):1-29. [DOI: 10.1101/2021.07.26.21250865] [DOI] [Google Scholar]
Fox 2021 {published data only}
- Fox A, Choi Y. Does framing coronavirus in terms of disparities reduce or increase vaccine hesitancy? Health Services Research 2021;56(S2):85-6. [DOI: 10.1111/1475-6773.13842] [DOI] [Google Scholar]
Galasso 2021 {published data only}
- Galasso V, Profeta P, Foucault M, Pons V. COVID-19 vaccine’s gender paradox (preprint). medRxiv 2021;March:1-27. [DOI: 10.1101/2021.03.26.21254380] [DOI] [Google Scholar]
Gong 2021 {published data only}
- Gong Z, Tang Z, Li J. What strategy is better for promoting COVID-19 vaccination? A comparison between gain-framed, loss-framed, and altruistic messages. Annals of Behavioral Medicine 16 August 2021;XX:1-7. [DOI: 10.1093/abm/kaab070] [DOI] [PubMed] [Google Scholar]
Hong 2021 {published data only}
- Hong Y, Hashimoto M. I will get myself vaccinated for others: the interplay of message frame, reference point, and perceived risk on intention for covid-19 vaccine. Health Communication 2021;Sep 22:1-11. [DOI: 10.1080/10410236.2021.1978668] [DOI] [PubMed] [Google Scholar]
Howarth 2021 {published data only}
- Howarth H, Stevens T, Gostick B, Stock L, Stone D. Improving attitudes towards the COVID-19 vaccine in forensic mental health workers: a quality improvement project. BJPsych Open 2021;7(1):194. [DOI: 10.1192/bjo.2021.523] [DOI] [Google Scholar]
Huang 2021 {published data only}
- Huang Y, Liu W. Promoting COVID-19 vaccination: the interplay of message framing, psychological uncertainty, and public agency as a message source. SAGE journals: Science Communication 2021;44(1):3-29. [DOI: 10.1177/10755470211048192] [DOI] [Google Scholar]
Jin 2021 {published data only}
- Jin Q, Raza SH, Yousaf M, Zaman U, Siang JM. Can communication strategies combat covid-19 vaccine hesitancy with trade-off between public service messages and public skepticism? Experimental evidence from Pakistan. Vaccines (Basel) 2021;9(7):757. [DOI: 10.3390/vaccines9070757] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jung 2021 {published data only}
- Jung H, Albarracín D . Concerns for others increases the likelihood of vaccination against influenza and COVID-19 more in sparsely rather than densely populated areas. Proceedings of the National Academy of Sciences 2021;118(1):1-8. [DOI: 10.1073/pnas.2007538118] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kachurka 2021 {published data only}
- Kachurka R, Krawczyk M, Rachubik J. Persuasive messages will not increase COVID-19 vaccine acceptance. Vaxccines (Basel) 2021;9(10):1113. [DOI: 10.3390/vaccines9101113] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelkar 2021 {published data only}
- Kelkar AH, Blake JA, Cherabuddi K, Cornett H, McKee BL, Cogle CR. Vaccine enthusiasm and hesitancy in cancer patients and the impact of a webinar. Healthcare 2021;9(3):351. [DOI: 10.3390/healthcare9030351] [DOI] [PMC free article] [PubMed] [Google Scholar]
Keppeler 2021 {published data only}
- Keppeler F, Sievert M, Jilke S. How local government vaccination campaigns can increase willingness to get vaccinated against Covid-19: a field experiment on psychological ownership. SSRN 2021;(preprint):1-35. [DOI: 10.2139/ssrn.3905470] [DOI]
Kerr 2021 {published data only}
- Kerr JR, Freeman AL, Marteau TM, Linden S. Effect of information about COVID-19 vaccine effectiveness and side effects on behavioural intentions: two online experiments. Vaccines 2021;9(4):379. [DOI: 10.3390/vaccines9040379] [DOI] [PMC free article] [PubMed] [Google Scholar]
Klüver 2021 {published data only}
- Klüver H, Hartmann F, Humphreys M, Geissler F, Giesecke J. Incentives can spur COVID-19 vaccination uptake. PNAS 2021;118(36):e2109543118. [DOI: 10.1073/pnas.2109543118] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kobayashi 2021 {published data only}
- Kobayashi T, Nishina, Y, Tomoi H, Harada K, Tanaka K, Matsumoto E, et al. Corowa-kun: Impact of a COVID-19 vaccine information chatbot on vaccine hesitancy (preprint). medRxiv 2021. [DOI: 10.1101/2021.05.26.21257854] [DOI] [PMC free article] [PubMed]
- Kobayashi T, Nishina Y, Tomoi H, Harada K, Matsumoto E, Inaba K, et al. Corowa-kun: impact of a COVID-19 vaccine information chatbot on vaccine hesitancy. Open Forum infectious Diseases 2021;8:321-2. [DOI: 10.1093/ofid/ofab466.638] [DOI] [Google Scholar]
Kreps 2021 {published data only}
- Kreps S, Dasgupta N, Brownstein JS, Hswen Y, Kriner DL. Public attitudes toward COVID-19 vaccination: the role of vaccine attributes, incentives, and misinformation. npj Vaccines 2021;6(1):1-7. [DOI: 10.1038/s41541-021-00335-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marquez 2021 {published data only}
- Marquez C, Kerkhoff AD, Naso J, Contreras MG, Castellanos E, Rojas S, et al. A multi-component, community-based strategy to facilitate COVID-19 vaccine uptake among Latinx populations: from theory to practice (preprint). medRxiv 2021. [DOI: 10.1101/2021.06.07.21258230] [DOI] [PMC free article] [PubMed]
Merkley 2021 {published data only}
- Merkley E, Loewen PJ. Assessment of communication strategies for mitigating COVID-19 vaccine-specific hesitancy in Canada. JAMA Network 2021;4(9):e2126635-e2126635. [DOI: 10.1001/jamanetworkopen.2021.26635] [DOI] [PMC free article] [PubMed] [Google Scholar]
OCEAN {published data only}
- Freeman D, Loe BS, Yu LM, Freeman J, Chadwick A, Vaccari C, Shanyinde M, et al. Effects of different types of written vaccination information on COVID-19 vaccine hesitancy in the UK (OCEANS-III): a single-blind, parallel-group, randomised controlled trial. Lancet Public Health 2021;6(6):e416-e427. [DOI: 10.1016/S2468-2667(21)00096-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
- In the general population, do specific forms of COVID-19 vaccine information, above simple information that they are safe and effective, increase willingness to be vaccinated? Cochrane COVID-19 Study Register 2021. [ISRCTN: 37254291]
Palm 2021 {published data only}
- Palm R, Bolsen T, Kingsland JT. The effect of frames on COVID-19 vaccine hesitancy (preprint). medRxiv 2021. [DOI: 10.1101/2021.01.04.21249241] [DOI]
- Palm R, Bolsen T, Kingsland JT. The effect of frames on COVID-19 vaccine resistance. Frontiers in Political Science 2021;13(3):41. [DOI: 10.3389/fpos.2021.661257] [DOI] [Google Scholar]
Peng 2021 {published data only}
- Peng L, Guo Y, Hu D. Information framing effect on public's intention to receive the COVID-19 vaccination in China. Vaccines 2021;9(9):995. [DOI: 10.3390/vaccines9090995] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pfattheicher 2021 {published data only}
- Pfattheicher S, Petersen MB, Böhm R. Information about herd immunity through vaccination and empathy promote COVID-19 vaccination intentions. Health Psychology 2021;epub ahead of print:1-10. [DOI: 10.1037/hea0001096] [DOI] [PubMed] [Google Scholar]
Pink 2021 {published data only}
- Pink SL, Chu J, Druckman JN, Rand DG, Willer R. Elite party cues increase vaccination intentions among republicans. PNAS 2021;118(32). [DOI: 10.1073/pnas.2106559118] [DOI] [PMC free article] [PubMed]
Reichardt 2021 {published data only}
- Reinhardt A, Rossmann C. Age-related framing effects: why vaccination against COVID-19 should be promoted differently in younger and older adults. Journal of Experimental Psychology 2021;27(4):10.1037/xap0000378. [DOI: 10.1037/xap0000378] [DOI] [PubMed] [Google Scholar]
Robertson 2021a {published data only}
- Robertson CT, Scheitrum D, Schaefer KA, Malone T, McFadden BR, Ferraro P, et al. Paying Americans to take the vaccine-would it help or backfire? Journal of Law and the Biosciences 2021;8:1-19. [DOI: 10.2139/ssrn.3798702] [SSRN: 3798702] [DOI] [PMC free article] [PubMed] [Google Scholar]
Robertson 2021b {published data only}
- Robertson CT, Bentele K, Meyerson B, Wood AS, Salwa J. Effects of political versus expert messaging on vaccination intentions of Trump voters. PLOS One 2019;16(9):e0257988. [DOI: 10.1371/journal.pone.0257988] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ryoo 2021 {published data only}
- Ryoo Y, Kim W. Using descriptive and injunctive norms to encourage covid-19 social distancing and vaccinations. Health Communication 2021;published online Sep 02:1-10. [DOI: 10.1080/10410236.2021.1973702] [DOI] [PubMed] [Google Scholar]
Santos 2021 {published data only}
- Emails promoting COVID-19 vaccination among healthcare workers. Clincaltrials.gov 2021. [NCT: 04728594]
- Santos HC, Goren A, Chabris CF, Meyer MN. Effect of targeted behavioral science messages on COVID-19 vaccination registration among employees of a large health system: a randomized trial. JAMA Network Open 2021;4(7):e2118702-e2118702. [DOI: 10.1001/jamanetworkopen.2021.18702] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schwarzinger 2021 {published data only}
- Schwarzinger M, Watson V, Arwidson P, Alla, Luchini S. COVID-19 vaccine hesitancy in a representative working-age population in France: a survey experiment based on vaccine characteristics. Lancet Public Health 2021;6(4):210-21. [DOI: 10.1016/S2468-2667(21)00012-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sehgal 2021 {published data only}
- Sehgal NK. Impact of Vax-a-Million lottery on COVID-19 vaccination rates in Ohio. American Journal of Medicine 2021;134(11):1424-6. [DOI: 10.1016/j.amjmed.2021.06.032] [DOI] [PMC free article] [PubMed] [Google Scholar]
Senderey 2021 {published data only}
- Senderey AB, Ohana R, Perchik S, Erev I, Balicer R. Encouraging uptake of the COVID-19 vaccine through behaviorally informed interventions: national real-world evidence from Israel (Preprint). SSRN 2021;March:1-8. [DOI: 10.2139/ssrn.3852345] [SSRN: 3852345] [DOI] [Google Scholar]
Serra‐Garcia 2021 {published data only}
- Serra-Garcia M, Szech N. Choice architecture and incentives increase COVID-19 vaccine intentions and test demand (preprint). SSRN 2021. [DOI: 10.2139/ssrn.3818182] [DOI]
Sinclair 2021 {published data only}
- Sinclair S, Agerström J. Do social norms influence young people's willingness to take the COVID-19 vaccine? Health Communication 2021;June:1-8. [DOI: 10.1080/10410236.2021.1937832] [DOI] [PubMed] [Google Scholar]
Sotis 2021 {published data only}
- Sotis C, Allena M, Reyes R, Romano A. COVID-19 vaccine passport and international traveling: the combined effect of two nudges on Americans' support for the pass. International Journal of Environmental Research and Public Health 2021;18(16):8800. [DOI: 10.3390/ijerph18168800] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sprengholz 2021 {published data only}
- Sprengholz P, Betsch C, Böhm R. Reactance revisited: consequences of mandatory and scarce vaccination in the case of COVID-19. Applied Psychology: Health and Well-Being 2021:1-10. [DOI: 10.1111/aphw.12285] [DOI] [PMC free article] [PubMed]
Sprengholz 2021c {published data only}
- Sprengholz P, Eitze S, Felgendreff L, Korn L, Betsch C. Money is not everything: experimental evidence that payments do not increase willingness to be vaccinated against COVID-19 response. Journal of Medical Ethics 2021;47(8):547-8. [DOI: 10.1136/medethics-2020-107122] [DOI] [PubMed] [Google Scholar]
Stein 2021 {published data only}
- Stein J, Fasold M, Daguerre KJ, Richardson J, Cheek S, Charlot M, et al. Use of an analytics and electronic health record-based approach for targeted COVID-19 vaccine outreach to marginalized populations. JAMA Oncology 2021;7(10):1570-2. [DOI: 10.1001/jamaoncol.2021.3833] [DOI] [PMC free article] [PubMed] [Google Scholar]
Strickland 2021 {published data only}
- Strickland JC, Reed DD, Hursh SR, Schwartz LP, Foster RN, Gelino BW, et al. Integrating operant and cognitive behavioral economics to inform infectious disease response: prevention, testing, and vaccination in the COVID-19 pandemic (preprint). medRxiv 2021. [DOI: 10.1101/2021.01.20.21250195] [DOI] [PMC free article] [PubMed]
Sudharsanan 2021 {published data only}
- Sudharsanan N, Favaretti C, Hachaturyan V, Bärnighausen T, Vandormael A. Effects of side-effect risk framing strategies on COVID-19 vaccine intentions: a randomized controlled trial (preprint). medRxiv 2021. [DOI: ] [DOI] [PMC free article] [PubMed]
- Sudharsanan N, Favaretti C, Hachaturyan V, Bärnighausen T, Vandormael A. The effect of framing and communicating COVID-19 vaccine side-effect risks on vaccine intentions for adults in the UK and the USA: a structured summary of a study protocol for a randomized controlled trial. Trials 2021;22(06 September 2021):592. [DOI: 10.1186/s13063-021-05484-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Takamatsu 2021 {published data only}
- Takamatsu A, Honda H, Kojima T, Murata K, Babcock HM. Promoting coronavirus disease 2019 (COVID-19) vaccination among healthcare personnel: a multifaceted intervention at a tertiary-care center in Japan. Infection Control & Hospital Epidemiology 2021;published online:1-6. [DOI: 10.1017/ice.2021.325] [DOI] [PMC free article] [PubMed] [Google Scholar]
Talmy 2021 {published data only}
- Talmy T, Cohen B, Nitzan I, Ben Michael Y. Primary care interventions to address COVID-19 vaccine hesitancy among Israel defense forces soldiers. Journal of Community Health 2021 May 14 [Epub ahead of print]:1-6. [DOI: 10.1007/s10900-021-01002-2] [DOI] [PMC free article] [PubMed]
Thirumurthy 2021 {published data only}
- Thirumurthy H, Milkman KL, Volpp K, Buttenheim A, Pope DG. Association between statewide financial incentive programs and COVID-19 vaccination rates . SSRN 2021;(preprint):1-10. [DOI: 10.2139/ssrn.3912786] [SSRN: 3912786] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorpe 2021 {published data only}
- Thorpe A, Fagerlin A, Butler J, Stevens V, Drews FA, Shoemaker H, et al. Communicating about COVID-19 vaccine development and safety . medRvix 2021;(Preprint):1-15. [DOI: 10.1101/2021.06.25.21259519] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thunström 2021 {published data only}
- Thunström L, Ashworth M, Finnoff D, Newbold SC. Hesitancy toward a COVID-19 vaccine. EcoHealth 2021;18(1):44-60. [DOI: 10.1007/s10393-021-01524-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tran 2021 {published data only}
- Tran VT, Sidorkiewicz S, Péan C, Ravaud P. Impact of an interactive web tool on patients' intention to receive COVID-19 vaccination: a before-and-after impact study among patients with chronic conditions in France. BMC Medical Informatics and Decision Making 2021;21(1):228. [DOI: 10.1186/s12911-021-01594-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ugwuoke 2021 {published data only}
- Ugwuoke JC, Talabi FO, Adelabu O, Sanusi BO, Gever VC, Onuora C. Expanding the boundaries of vaccine discourse: impact of visual illustrations communication intervention on intention towards COVID-19 vaccination among victims of insecurity in Nigeria. Human Vaccines & Immunotherapeutics 2021;17(10):3450-6. [DOI: 10.1080/21645515.2021.1886558] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walkey 2021 {published data only}
- Walkey AJ, Law A, Bosch NA. Lottery-based incentive in Ohio and COVID-19 vaccination rates. JAMA 2021;326(8):766-7. [DOI: 10.1001/jama.2021.11048] [DOI] [PMC free article] [PubMed] [Google Scholar]
Witus 2021 {published data only}
- Witus LS, Larson E. A randomized controlled trial of a video intervention shows evidence of increasing COVID-19 vaccination intention (preprint). medRxiv 2021. [DOI: 10.1101/2021.03.26.21254433] [DOI] [PMC free article] [PubMed]
Ye 2021 {published data only}
- Ye W, Li Q, Yu S. Persuasive effects of message framing and narrative format on promoting COVID-19 vaccination: a study on Chinese college students. International Journal of Environmental Research and Public Health 2021;18(18):9485. [DOI: 10.3390/ijerph18189485] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yu 2021 {published data only}
- Yu Y, Lau JT, Lau MM, Wong MC, Chan PK. Understanding the prevalence and associated factors of behavioral intention of COVID-19 vaccination under specific scenarios combining effectiveness, safety, and cost in the Hong Kong Chinese general population. International Journal of Health Policy and Management 2021 Jan 18 [Epub ahead of print]. [DOI: 10.34172/ijhpm.2021.02] [DOI] [PMC free article] [PubMed]
Yu 2021b {published data only}
- Yu Y, Lau JT, She R, Chen X, Li L, Li L, et al. Prevalence and associated factors of intention of COVID-19 vaccination among healthcare workers in China: application of the Health Belief Model. Human Vaccines & Immunotherapeutics 2021;17(9):2894. [DOI: 10.1080/21645515.2021.1909327] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yuan 2021 {published data only}
- Yuan S, Chu H. Vaccine for yourself, your community, or your country? Examining audiences' response to distance framing of COVID-19 vaccine messages. Patient Education and Counseling 2022;105(2):284-9. [DOI: 10.1016/j.pec.2021.08.019] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Ali 2021 {published data only}
- Ali N, Ashiru-Oredope D, Murdan S. Training university students as vaccination champions to promote vaccination in their multiple identities and help address vaccine hesitancy. Pharmacy Education 2021;21(1):407-19. [DOI: 10.46542/pe.2021.211.407419] [DOI] [Google Scholar]
American Society of Safety Professionals 2021 {published data only}
- CDC publishes COVID-19 vaccination communication tool kit. Available at: https://www.assp.org/news-and-articles/2021/01/26/cdc-publishes-covid-19-vaccination-communication-toolkit January 2021.
Batteux 2021 {published data only}
- Batteux E, Bilovich A, Johnson SG, Tuckett D. The negative consequences of failing to communicate uncertainties during a pandemic: the case of COVID-19 vaccines. Medrxiv.org 2021. [DOI: 10.1101/2021.02.28.21252616] [DOI] [PMC free article] [PubMed]
Bell 2021 {published data only}
- Bell S, Clarke RM, Ismail SA, Ojo-Aromokudu O, Naqvi H, Coghill Y, et al. COVID-19 vaccination beliefs, attitudes,and behaviours among health and social care workers in the UK: a mixed-methods study. medRxiv 2021. [DOI: 10.1101/2021.04.23.21255971] [DOI] [PMC free article] [PubMed]
ChiCTR2100043018 {published data only}
- Survey on the willingness and influencing factors of novel coronavirus pneumonia (COVID-19) inactivated vaccine among medical staff in Women's and Children's Hospital. Chinese Clinical Trial Registry 2021. [CHICTR: 2100043018]
Community Practitioner 2021 {published data only}
- Personal stories of vaccine side effects reduce the impact of hard science messages. Community Practicioner 2021;94(3):12.
Crawshaw 2021 {published data only}
- Crawshaw J, Castillo G, Grimshaw JM, Presseau J. Factors affecting healthcare worker COVID-19 vaccination acceptance and uptake: a living behavioural science evidence synthesis. Ottawa Hospital Research Institute, Ottawa [online]: Available from drive. google. com/file/d/10W5XDumdcwta5YMLWrXMb5UI3mHhKISa/view. 2021 2021.
Crawshaw 2021b {published data only}
- Crawshaw J, Castillo G, Grimshaw JM, Presseau J. Factors affecting healthcare worker COVID-19 vaccination acceptance and uptake:a living behavioural science evidence synthesis. Ottawa Hospital Research Institute, Ottawa [online]: Available from drive. google. com/file/d/10W5XDumdcwta5YMLWrXMb5UI3mHhKISa/view. 2021 2021.
Davis 2021 {published data only}
- Davis CJ, Golding M, McKay R. Efficacy information influences intention to take COVID‐19 vaccine. British Journal of Health Psychology 2021;1:1-20. [DOI: 10.1111/bjhp.12546] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gakuba 2021 {published data only}
- Gakuba C, Sar A, Gaborieau I, Hanouz JL, Verger P. Willingness to get a COVID-19 vaccine among critical care non-medical healthcare workers and impact of a vaccine information session. Anaesthesia, Critical Care & Pain Medicine 2021;40(3):100860. [DOI: 10.1016/j.accpm.2021.100860] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gehrau 2021 {published data only}
- Gehrau V, Fujarski S, Lorenz H, Schieb C, Blöbaum B. The impact of health information exposure and source credibility on COVID-19 vaccination intention in Germany. International Journal of Environmental Research and Public Health 2021;18(9):4678. [DOI: 10.3390/ijerph18094678] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guelmami 2021 {published data only}
- Guelmami N, Chalghaf N, Wu J, Kong JD, Mellado B, Jahrami H, et al. Social media COVID-19 information and vaccine decision: A latent class analysis (preprint). SSRN 2021:1-22. [DOI: 10.2139/ssrn.3841301] [DOI]
Hofer 2021 {published data only}
- Hofer, U. Make it personal to beat vaccine hesitancy. Nature Reviews Microbiology 2021;19:406. [DOI: 10.1038/s41579-021-00579-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaplan 2021 {published data only}
- Kaplan RM, Milstein A. Influence of a COVID-19 vaccine's effectiveness and safety profile on vaccination acceptance. PNAS 2021;118(10):e2021726118. [DOI: 10.1073/pnas.2021726118] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirkpatrick 2021 {published data only}
- Kirkpatrick AW, Park M, Domgaard S, Zhao W, Steinberg C, Hsu Y. Vaccine videos and information sharing: the effects of framing, evidence type, and speaker Expertise. Journal of Health Communication 2021;2(26):608-17. [DOI: 10.1080/10810730.2021.1983892] [DOI] [PubMed] [Google Scholar]
Knight 2021 {published data only}
- Knight H, Jia R, Ayling K, Bradbury K, Baker K, Chalder T, et al. Understanding and addressing vaccine hesitancy in the context of COVID-19: development of a digital intervention. medRxiv 2021. [DOI: 10.1101/2021.03.24.21254124] [DOI] [PMC free article] [PubMed]
Kumar 2021 {published data only}
- Kumar R, Panda S, Bhadauriya AS. Acceptance & hesitancy to COVID-19 vaccine among undergraduate students of ITM University in India (preprint). SSRN. [SSRN: https://ssrn.com/abstract=3836083]
Lim 2020 {published data only}
- Lim W, Zhang P. Herd immunity and a vaccination game: an experimental study. PLOS One 2020;15(5):e0232652. [DOI: 10.1371/journal.pone.0232652] [DOI] [PMC free article] [PubMed] [Google Scholar]
Loomba 2021 {published data only}
- Loomba S, Figueiredo A, Piatek SJ, Graaf K, Larson HJ. Measuring the impact of COVID-19 vaccine misinformation on vaccination intent in the UK and USA. Nature Human Behaviour 2021;5(3):407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Loomba 2021a {published data only}
- Loomba S, Figueiredo A, Piatek SJ, Graaf K, Larson HJ Measuring the impact of COVID-19 vaccine misinformation on vaccination intent in the UK and USA. Measuring the impact of COVID-19 vaccine misinformation on vaccination intent in the UK and USA. Nature Human Behavior 2021;5(7):337–48. [DOI: 10.1038/s41562-021-01056-1] [DOI] [PubMed] [Google Scholar]
NCT04694651 {published data only}
- Potential acceptance of COVID-19 vaccines in healthcare workers in Egypt. Clinicaltrials.gov 2021. [NCT: 04694651]
Rahmandad 2021 {published data only}
- Rahmandad H. Behavioral responses to risk promote vaccinating high-contact individuals first (preprint). medRxiv 2021. [DOI: 10.1101/2021.02.05.21251215] [DOI] [PMC free article] [PubMed]
Salali 2021 {published data only}
- Salali GD, Uysal MS. Effective incentives for increasing covid-19 vaccine uptake. Psychological Medicine 2021;published online:1-3. [DOI: 10.1017/S0033291721004013] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shmueli 2021 {published data only}
- Shmueli L The role of incentives in deciding to receive the available COVID-19 vaccine medRxiv 2021 Jan 1. The role of incentives in deciding to receive the available COVID-19 vaccine. medRxiv 2021;Jan 1:1. [DOI: 10.1101/2021.08.11.21261829] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sprengholz 2021b {published data only}
- Sprengholz P, Eitze S, Korn L, Siegers R, Betsch C. The power of choice: experimental evidence that freedom to choose a vaccine against COVID-19 improves willingness to be vaccinated. European Journal of Internal Medicine 2021;87:106-8. [DOI: 10.1016/j.ejim.2021.03.015] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thaker 2021 {published data only}
- Thaker J, Subramanian A. Vaccine hesitancy is as impactful as vaccine misinformation in inducing a decline in vaccination intentions in New Zealand: results from Pre-post between-groups randomized block experiment. Frontiers in Communication 2021;159:1-8. [DOI: 10.3389/fcomm.2021.721982] [DOI] [Google Scholar]
Vasquez 2021 {published data only}
- Vásquez WF, Trudeau JM. Will Americans get vaccinated? Predicting COVID-19 vaccine uptake rates under contingent scenarios. Value in Health 2021;24(11):1543-50. [DOI: 10.1016/j.jval.2021.05.007] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wagner 2021 {published data only}
- Wagner AL, Sheinfeld Gorin S, Boulton ML, Glover BA, Morenoff JD. Effect of vaccine effectiveness and safety on COVID-19 vaccine acceptance in Detroit, Michigan, July 2020. Human Vaccines and Immunotherapeutics 2021;17(9):2940-5. [DOI: 10.1080/21645515.2021.1917233] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yousuf 2021 {published data only}
- Yousuf H, Linden S, Bredius L, Essen GT, Sweep G, Preminger Z, et al. A media intervention applying debunking versus non-debunking content to combat vaccine misinformation in elderly in the Netherlands: a digital randomised trial. EClinicalMedicine 2021;35:100881. [DOI: 10.1016/j.eclinm.2021.100881] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yuen 2021 {published data only}
References to studies awaiting assessment
INFORMED {published data only}
- Getting Asian Americans INFORMED to facilitate COVID-19 testing and vaccination (INFORMED). Clinicaltrials.gov 2021. [NCT: 04893265]
Larson 2020 {published data only}
- Larson HJ, Lee N, Rabin KH, Rauh L, Scott C. Building Confidence to CONVINCE. Journal of Health Communication 2020;25(10):838-42. [DOI: 10.1080/10810730.2021.1884149] [DOI] [PubMed] [Google Scholar]
NCT04460703 {published data only}
- COVID-19 Vaccine Messaging, Part 1. Clinicaltrials.gov 2021. [NCT: 04460703]
NCT04731870 {published data only}
- Exploring vaccine confidence and uptake of potential COVID-19 vaccines. Clinicaltrials.gov 2021. [NCT: 04731870]
Supraneni 2021 {published data only}
- Surapaneni KM, Kaur M, Kaur R, Grover A, Joshi A. The impact of COVID-19 vaccine communication, acceptance, and practices (CO-VIN-CAP) on vaccine hesitancy in an Indian setting: protocol for a cross-sectional study. JMIR Research Protocols 2021;10(6):e29733. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
DRKS00023650 {published data only}
- Heidelberg Institute of Global Health. An entertainment-education approach to improve vaccine confidence during the COVID-19 pandemic: An online randomized controlled experiment with 24,000 participants. Deutsches Register Klinischer Studien 2021. [DRKS: 00023650]
ISRCTN15317247 {published data only}
- Using text messages to boost COVID-19 vaccine booking rate. Available from https://www.isrctn.com/ISRCTN15317247. [ISRCTN15317247]
NCT04542395 {published data only}
- COVID-2019 testing and vaccination among African American and Latinx public housing residents. Clinicaltrials.gov 2020. [NCT: 04542395]
NCT04604743 {published data only}
- Clinic-based HPV and COVID-19 vaccine promoting intervention for AfAm adolescents in Alabama. Clinicaltrials.gov 2021. [NCT: 04604743]
NCT04706403 {published data only}
- Views on COVID-19 and vaccination. Clinicaltrials.gov 2021. [NCT: 04706403]
NCT04732819 {published data only}
- Improving COVID-19 vaccine uptake in nursing homes. Clinicaltrials.gov 2021. [NCT: 04732819]
NCT04761692 {published data only}
- Increasing COVID-19, influenza, and pneumonia vaccine uptake. Clinicaltrials.gov 2021. [NCT: 04761692]
NCT04779138 {published data only}
- Increasing vaccine uptake in underresourced public housing areas. Clinicaltrials.gov 2021. [NCT: 04779138]
NCT04800965 {published data only}
- Dai H, Saccardo S, Han MA, Roh L, Raja N, Vangala S, et al. Behavioral nudges increase COVID-19 vaccinations: Two randomized controlled trials. medRxiv 2021. [DOI: 10.1101/2021.04.12.21254876] [DOI] [PMC free article] [PubMed]
- Text-based interventions to promote COVID-19 vaccinations. Cinicaltrials.gov 2021. [NCT: 04800965]
NCT04801030 {published data only}
- COVID-19 vaccine hesitancy among African Americans. Clinicaltrials.gov 2021. [NCT: 04801030]
NCT04801524 {published data only}
- Dai H, Saccardo S, Han M, Roh L, Raja N, Vangala S, et al. Behavioral nudges increase COVID-19 vaccinations: Two randomized controlled trials. medRxiv 2021. [DOI: 10.1101/2021.04.12.21254876] [DOI] [PMC free article] [PubMed]
- Text-based reminders to promote COVID-19 vaccinations. Clinicaltrials.gov 2021. [NCT: 04801524]
NCT04805931 (VEText) {published data only}
- VEText message framing and COVID-19 vaccine uptake among at risk veterans (VEText). Clinicaltrials.gov 2021. [NCT: 04805931]
NCT04813770 {published data only}
- The impact of theory-based messaging on COVID-19 vaccination intentions. Clinicaltrials.gov 2021. [NCT: 04813770]
- Young B, Kotzur M, Gatting L, Bonner C, Ayre J, McConnachie A, et al. The impact of theory-based messages on COVID-19 vaccination intentions: a structured summary of a study protocol for a randomised controlled trial. Trials 2021;22(1):311. [DOI: 10.1186/s13063-021-05277-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT04834726 {published data only}
- COVID-19 vaccine text outreach. Clinicaltrials.gov 2021. [NCT: 04834726]
NCT04867174 {published data only}
- COVID-19 vaccination take-up. Clinicaltrials.gov 2021. [NCT: 04867174]
NCT04870593 {published data only}
- What works to get the elderly vaccinated against COVID-19? Experimental evidence from India. Clinicaltrials.gov 2021. [NCT: 04870593]
NCT04871776 {published data only}
- Use of construal level theory to inform messaging to increase vaccination against COVID-19. Clinicaltrials.gov 2021. [NCT: 04871776]
NCT04876885 {published data only}
- The future of viral communications: video-based health promotion strategies for COVID-19 vaccinations. Clinicaltrials.gov 2021. [NCT: 04876885]
NCT04884750 {published data only}
- Conversational agent vaccine promotion RCT. Clinicaltrials.gov 2021. [NCT: 04884750]
NCT04895683 {published data only}
- Using text messages to improve COVID-19 vaccination uptake. Clinicaltrials.gov 2021. [NCT: 04895683]
NCT04924803 {published data only}
- Community developed technology-based messaging to increase COVID-19 vaccine uptake among people who inject drugs. Clinicaltrials.gov . [NCT: NCT04924803]
NCT04930965 (LA‐CEAL: HALT COVID) {published data only}
- Impact of LA-CEAL HALT COVID-19 Ambassador Program on likelihood to vaccinate. Clinicaltrials.gov . [NCT: NCT04930965]
NCT04939506 {published data only}
- COVID-19 vaccine education at the point of testing to increase vaccine uptake in vulnerable communities in SE Louisiana. Clinicaltrials.gov . [NCT: NCT04939506]
NCT04939519 (SCALE‐UP Utah) {published data only}
- SCALE-UP Utah: community-academic partnership to address COVID-19 vaccination rates among Utah Community Health Centers. Clinicaltrials.gov . [NCT: NCT04939519]
NCT04951310 {published data only}
- COVID-19 vaccinations with a sweepstakes. Clinicaltrials.gov . [NCT: NCT04951310]
NCT04952376 {published data only}
- Equitable access to COVID-19 vaccines. Clinicaltrials.gov. [NCT: NCT04952376]
NCT04960228 {published data only}
- Exploring changes in COVID-19 vaccination intentions by prompting altruistic motives using a video intervention. Clinicaltrials.gov. [NCT: NCT04960228]
NCT04963790 {published data only}
- Enabling family physicians to reduce vaccine hesitancy and increase Covid-19 vaccine uptake. Clinicaltrials.gov. [NCT: NCT04963790]
NCT04964154 (BRAVE) {published data only}
- Building Resiliency and Vital Equity (BRAVE) project: understanding Native Americans' perceptions/beliefs about COVID-19 testing and vaccination study (BRAVE). Clinicaltrials.gov. [NCT: NCT04964154]
NCT04979416 {published data only}
- Video Messages and Vaccination Intention. Clinicaltrials.gov. [NCT: NCT04979416]
NCT04981392 {published data only}
- Intervention to promote COVID-19 vaccination. Clinicaltrials.gov. [NCT: NCT04981392]
NCT05022472 (2VIDA!) {published data only}
- Project 2VIDA! COVID-19 vaccine intervention delivery for adults in Southern California (2VIDA!). Clinicaltrials.gov. [NCT: NCT05022472]
NCT05027464 (CoVAcS) {published data only}
- Developing and testing a COVID-19 Vaccination Acceptance intervention (CoVAcS). Clinicaltrials.gov. [NCT: NCT05027464]
NCT05037201 {published data only}
- Text message nudges for COVID-19 vaccination. Clinicaltrials.gov. [NCT: NCT05037201]
PACTR202102846261362 {published data only}
- Using community influencer groups to address COVID-19 misinformation and potential vaccine hesitancy in Uganda. Pan African Clinical Trials Registry 2021. [PACTR: 202102846261362]
Additional references
Abdullahi 2020
- Abdullahi LH, Kagina BM, Ndze VN, Hussey GD, Wiysonge CS. Improving vaccination uptake among adolescents. Cochrane Database of Systematic Reviews 2020, Issue 1. Art. No: CD011895. [DOI: 10.1002/14651858.CD011895] [DOI] [PMC free article] [PubMed] [Google Scholar]
Abu 2021
- Abu Farha Rana K, Alzoubi Karem H, Khabour Omar F, Alfaqih Mahmoud A. Exploring perception and hesitancy toward COVID-19 vaccine: a study from Jordan. Human Vaccines & Immunotherapeutics 2021;February :1-6. [DOI: 10.1080/21645515.2021.1888633] [DOI] [PMC free article] [PubMed] [Google Scholar]
Afolabi 2021
- Afolabi AA, Illesamni OS. Dealing with vaccine hesitancy in Africa: the prospective COVID-19 vaccine context. Pan African Medical Journal 2021;38:3. [DOI: 10.11604/pamj.2021.38.3.27401] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ahmed 2021
- Nehad JA, Faisal ZA, Abdulrahman AS, Ziyad A. Public knowledge and attitudes toward COVID-19 vaccination: a cross-sectional study. Medical Science 2021;25(108):279-84. [ISSN: 2321–7359] [Google Scholar]
Akarsu 2021
- Akarsu B, Canbay ÖD, Ayhan BD, Aksoy H, Fidancı I, Cankurtaran M. While studies on COVID-19 vaccine is ongoing, the public’s thoughts and attitudes to the future COVID-19 vaccine. International Journal of Clinical Practice 2021;75(4):e13891. [DOI: 10.1111/ijcp.13891] [DOI] [PMC free article] [PubMed] [Google Scholar]
Al‐Jayyousi 2021
- Al-Jayyousi GF, Sherbash MM, Ali LAM, El-Heneidy A, Alhussaini NW, Elhassan ME, et al. Factors influencing public attitudes towards COVID-19 vaccination: a scoping review informed by the socio-ecological model. Vaccines 2021;9(6):548. [DOI: 10.3390/vaccines9060548] [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderson 2008
- Anderson S, Allen P, Peckham S, Goodwin N. Asking the right questions: scoping studies in the commissioning of research on the organisation and delivery of health services. Health Research Policy and Systems 2008;6(1):7. [DOI: 10.1186/1478-4505-6-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Andreas 2021
- Andreas M, Iannizzi C, Monsef I, Bohndorf E, Meerpohl JJ, Piechotta et al. Interventions to increase COVID-19 vaccine uptake: protocol for a scoping review. Open Science Framework 2021. [DOI: 10.17605/OSF.IO/6825G] [DOI] [PMC free article] [PubMed]
Bono 2021
- Bono SA, Faria MV, Siau CS, Chen WS, Pengpid S, Hasan MT, et al. Factors affecting COVID-19 vaccine acceptance: an international survey among low- and middle-income countries. Vaccines 2021;9(5):515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brewer 2017
- Brewer NT, Chapman GB, Rothman AJ, Leask J, Kempe A (2017). Increasing vaccination: putting psychological science into action. Psychological Science for the Public Interest 2017;18(3):149-207. [DOI: 10.1177/1529100618760521] [DOI] [PubMed] [Google Scholar]
Dror 2021
- Dror AA, Daoud A, Morozov NG, Layous E, Eisenbach N, Mizrachi M, et al. Vaccine hesitancy due to vaccine country of origin, vaccine technology, and certification. European Journal of Epidemiology 2021;April:1-6. [DOI: 10.1007/s10654-021-00758-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dubé 2015
- Dubé E, Gagnon D, MacDonald NE. Strategies intended to address vaccine hesitancy: review of published reviews. Vaccine 2015;33(34):4191-203. [DOI: 10.1016/j.vaccine.2015.04.041] [DOI] [PubMed] [Google Scholar]
Edwards 2020
- Edwards B, Biddle N, Gray Ma, Sollis K. COVID-19 vaccine hesitancy and resistance: correlates in a nationally representative longitudinal survey of the Australian population. PLOS One 2020;16(3):e0248892. [DOI] [PMC free article] [PubMed] [Google Scholar]
Entman 1993
- Entman RM. Toward clarification of a fractured paradigm. Journal of Communication 1993;43(4):51-8. [Google Scholar]
Freeman 2020
- Freeman D, Loe BS, Chadwick A, Vaccari C, Waite F, Rosebrock L, et al. COVID-19 vaccine hesitancy in the UK: the Oxford coronavirus explanations, attitudes, and narratives survey (Oceans) II. Psychological Medicine 2020;December :1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hamel 2021
- Hamel L, Lopes L, Sparks G, Kirzinger A, Kearney A, Stokes M, et al. KFF COVID-19 vaccine monitor: profile of the unvaccinated. Available at: https://www.kff.org/coronavirus-covid-19/poll-finding/kff-covid-19-vaccine-monitor-september-2021/ (accessed 20.10.2021) 2021.
Jacoboson Vann 2018
- Jacobson Vann JC, Jacobson RM, Coyne‐Beasley T, Asafu‐Adjei JK, Szilagyi PG. Patient reminder and recall interventions to improve immunization rates. Cochrane Database of Systematic Reviews 2018, Issue 1. Art. No: CD003941. [DOI: 10.1002/14651858.CD003941.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jaiswal 2020
- Jaiswal J, LoSchiavo C, Perlman DC. Disinformation, misinformation and inequality-driven mistrust in the time of COVID-19: lessons unlearned from AIDS denialism. AIDS and Behavior 2020;24:2776-80. [DOI: 10.1007/s10461-020-02925-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jarrett 2015
- Jarrett C, Wilson R, O’Leary M, Eckersberger E, Larson HJ. Strategies for addressing vaccine hesitancy – a systematic review. Vaccine 2015;33(34):4180-90. [DOI] [PubMed] [Google Scholar]
Kaufman 2018
- Kaufman J, Ryan R, Walsh L, Horey D, Leask J, Robinson P, et al. Face‐to‐face interventions for informing or educating parents about early childhood vaccination. Cochrane Database of Systematic Reviews 2018, Issue 5. Art. No: CD010038. [DOI: 10.1002/14651858.CD010038] [DOI] [PMC free article] [PubMed] [Google Scholar]
KFF 2022
- KFF . KFF COVID-19 vaccine monitor dashboard . Available from: https://www.kff.org/coronavirus-covid-19/dashboard/kff-covid-19-vaccine-monitor-dashboard/#uptake (assessed 14.02.22).
Larson 2014
- Larson HJ, Jarrett C, Eckersberger E, Smith D, Paterson P. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: a systematic review of published literature, 2007–2012. Vaccine 2014;32(19):2150-9. [DOI] [PubMed] [Google Scholar]
Lopez 2021
- Lopez Bernal J, Andrews N, Gower C, Robertson C, Stowe J, Tessier E, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ 2021;373:n1088. [DOI: 10.1136/bmj.n1088] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mantzari 2015
- Mantzari E, Vogt F, Marteau TM. Financial incentives for increasing uptake of HPV vaccinations: a randomized controlled trial. Health Psychology 2015;34(2):160-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Munn 2018
- Munn Z, Peters M, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Medical Research Methodology 2018;18(1):143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nguyen 2021
- Nguyen KH, Srivastav A, Razzaghi H, Williams W, Lindley MC, Jorgensen C, et al. COVID-19 vaccination intent, perceptions, and reasons for not vaccinating among groups prioritized for early vaccination — United States, September and December 2020. American Journal of Transplantation 2021;21(4):1650-6. [DOI: 10.1111/ajt.16560] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nguyen 2021a
- Nguyen LH, Joshi AD, Drew DA, Merino J, Ma W, Lo CH, et al. Racial and ethnic differences in COVID-19 vaccine hesitancy and uptake. medRxiv 2021:2021.02.25.21252402. [DOI: 10.1101/2021.02.25.21252402] [DOI]
Odone 2015
- Odone A, Ferrari A, Spagnoli F, Visciarelli S, Shefer A, Pasquarella C, et al. Effectiveness of interventions that apply new media to improve vaccine uptake and vaccine coverage. Human Vaccines & Immunotherapeutics 2015;11(1):72-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oyo‐Ita 2016
- Oyo‐Ita A, Wiysonge CS, Oringanje C, Nwachukwu CE, Oduwole O, Meremikwu MM. Interventions for improving coverage of childhood immunisation in low‐ and middle‐income countries. Cochrane Database of Systematic Reviews 2016, Issue 7. Art. No: CD008145. [DOI: 10.1002/14651858.CD008145] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peters 2020
- Peters MD, Godfrey C, McInerney P, Munn Z, Tricco AC, Khalil H. Chapter 11: Scoping Reviews (2020 version). JBI Manual for Evidence Synthesis 2020.
Public Health Ontario 2021
- Ontario Agency for Health Protection and Promotion. COVID-19 real-world vaccine effectiveness – what we know so far. Toronto, ON: Queen’s Printer for Ontario 2021.
Reno 2021
- Reno C, Maietti E, Fantini MP, Savoia E, Manzoli L, Montalti M, et al. Enhancing COVID-19 vaccines acceptance: results from a survey on vaccine hesitancy in northern Italy. Vaccines 2021;9(4):378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Romer 2020
- Romer D, Jamieson KH. Conspiracy theories as barriers to controlling the spread of COVID-19 in the U.S. Social Science & Medicine 2020;263:113356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sallam 2021
- Sallam M. COVID-19 vaccine hesitancy worldwide: a concise systematic review of vaccine acceptance rates. Vaccines 2021;9(2):160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schernhammer 2021
- Schernhammer E, Weitzer J, Laubichler MD, Birmann BM, Bertau M, Zenk L, et al. Correlates of COVID-19 vaccine hesitancy in Austria: trust and the government. Jorunal of Public Health 2021 ;May 5 [Epub ahead of print]:1-11. [DOI: 10.1093/pubmed/fdab122] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomas 2018
- Thomas RE, Lorenzetti DL. Interventions to increase influenza vaccination rates of those 60 years and older in the community. Cochrane Database of Systematic Reviews 2018, Issue 5. Art. No: CD005188. [DOI: 10.1002/14651858.CD005188.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thunstrom 2020
- Thunstrom L, Ashworth M, Finnoff D, Newbold S. Hesitancy towards a COVID-19 vaccine and prospects for herd immunity. SSRN 2020. [DOI: 10.2139/ssrn.3593098] [DOI] [PMC free article] [PubMed]
Tricco 2018
- Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Annals of Internal Medicine 2018;169(7):467-73. [DOI] [PubMed] [Google Scholar]
Troiano 2021
- Troiano G, Nardi A. Vaccine hesitancy in the era of COVID-19. Public Health 2021;194:245-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ullah 2021
- Ullah I, Khan KS, Tahir MJ, Ahmed A, Harapan H. Myths and conspiracy theories on vaccines and COVID-19: potential effect on global vaccine refusals. Vacunas 2021;22(2):93-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vet 2010
- Vet R, Wit JBF, Das E. The efficacy of social role models to increase motivation to obtain vaccination against hepatitis B among men who have sex with men. Health Education Research 2010;26(2):192-200. [DOI] [PubMed] [Google Scholar]
WHO 2021a
- WHO. Prequalification of Medical products (IVDs, Medicines, vaccines and immunization devices, vector control). Available from: https://extranet.who.int/pqweb/vaccines/covid-19-vaccines (accessed 11.02.2022) 2021.
WHO 2021b
- WHO. COVID-19 vaccine tracker and landscape. Available from: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed 16.09.2021) 2021.
Wigham 2014
- Wigham S, Ternent L, Bryant A, Robalino S, Sniehotta FF, Adams J. Parental Financial Incentives for Increasing Preschool Vaccination Uptake: Systematic Review. Pediatrics 2014;134(4):e1117-e1128. [DOI] [PubMed] [Google Scholar]
Winston 2007
- Winston CA, Mims AD, Leatherwood KA. Increasing pneumococcal vaccination in managed care through telephone outreach. American Journal of Managed Care 2007;13(10):581. [PubMed] [Google Scholar]
Wong 2006
- Wong SS, Wilczynski NL, Haynes RB. Comparison of top-performing search strategies for detecting clinically sound treatment studies and systematic reviews in MEDLINE and EMBASE. Journal of the Medical Library Association 2006;94(4):451-5. [PMID: ] [PMC free article] [PubMed] [Google Scholar]


