Abstract
Ion signaling through Ca2+ and Na+ plays a key role in mechanotransduction and encourages a chondrogenic phenotype and tissue maturation. In this study, we propose that the pleiotropic effects of Ca2+ and Na+ modulation can be used to induce maturation and improvement of neocartilage derived from redifferentiated expanded chondrocytes from minipig rib cartilage. Three ion modulators were employed: (1) 4α-phorbol-12,13-didecanoate (4-αPDD), an agonist of the Ca2+-permeable transient receptor potential vanilloid 4 (TRPV4), (2) ouabain, an inhibitor of the Na+/K+ pump, and (3) ionomycin, a Ca2+ ionophore. These ion modulators were used individually or in combination. While no beneficial effects were observed when using combinations of the ion modulators, single treatment of constructs with the three ion modulators resulted in multiple effects in structure–function relationships. The most significant findings were related to ionomycin. Treatment of neocartilage with ionomycin produced 61% and 115% increases in glycosaminoglycan and pyridinoline crosslink content, respectively, compared with the control. Moreover, treatment with this Ca2+ ionophore resulted in a 45% increase of the aggregate modulus, and a 63% increase in the tensile Young's modulus, resulting in aggregate and Young's moduli of 567 kPa and 8.43 MPa, respectively. These results support the use of ion modulation to develop biomimetic neocartilage using expanded redifferentiated costal chondrocytes.
Impact Statement
New cost-effective, replicable, and highly controllable strategies are required to develop neocartilage with biomimetic properties akin to native tissue. Ion signaling plays a key role in mechanotransduction, promoting chondrogenic phenotype. Using rib cartilage, we proposed that Ca2+ and Na+ modulation could be used to induce maturation of neotissue derived from redifferentiated, expanded costal chondrocytes, improving its mechanical properties. Our results indicate that Ca2+ modulation with ionomycin, which stimulated extracellular matrix deposition and collagen crosslinking, improved morphological and mechanical features of neocartilage constructs, and holds potential as a powerful tool to engineer hyaline-like tissues.
Keywords: ion modulation, costal chondrocytes, self-assembling, neocartilage
Introduction
Stimulation of tissue maturation through mechanotransduction stands as a promising strategy to engineer neocartilage with appropriate biomechanical properties. Maturation of neotissues grown in vitro is a key step toward improving the mimicry of neocartilage to its native counterpart. For example, treatment of neocartilage constructs with Lysyl oxidase-like 2 (LoxL2), which catalyzes the pyridinoline (PYR) crosslink typically found in mature articular cartilage (AC), is highly efficient but has a narrow target effect.1 Conversely, the use of bioreactors to simulate mechanical cues found in the joint enables the activation of signaling cascades with pleiotropic effects in the neotissue.2 Stimuli, such as compression, tension, fluid shear, and hydrostatic pressure, have been employed to improve the biomechanical properties of engineered neocartilage.3–6
The activation of chondrogenic signaling pathways through mechanotransduction has been shown to favor a more cartilaginous extracellular matrix (ECM) in terms of composition (e.g., collagen types and content), macromolecule bonding (e.g., PYR crosslinks), and collagen alignment (anisotropy).7,8 Taking into consideration the multiple targets of mechanical stimulation, it is essential that a more critical breakdown of its various effects is carried out to inform cogent tissue engineering approaches.
Ion signaling plays a key role in mechanotransduction. The well-characterized mechanoelectrical transduction (MET) channels found in the stereocilia of hair cells are directly modulated by sound waves, which open the channels by bending of the stereocilia.9 Opening of the MET channels allows the influx of Ca2+, depolarizing the cell and converting the mechanical stimuli into an electrical signal. Piezo1, a depolarizing nonselective cationic channel permeable to Na+, K+, and Ca2+ that regulates vascular development, is activated by deformations in the plasma membrane induced by blood flow-derived shear stress.10
Similar to the MET channels, the mechanosensitive K+ TREK-1 channel responsible for pain perception in neurons is opened by cell swelling and membrane stretching, and has further shown sensitivity to changes in temperature and voltage.11,12 In chondrocytes, compressive loading on AC causes fluid loss and osmotic changes that induce an increase in intracellular Ca2+, which in turn drives gene expression toward ECM synthesis.13,14
Likewise, Na+ concentration is involved in the regulation of chondrocyte hydration and osmosis through interaction with positively charged molecules, such as glycosaminoglycan (GAG), thus attracting water.15 Modulation of intracellular ion concentrations, particularly Ca2+, is a common downstream effect of various responses to mechanical stimuli.
Ion modulation regulates the chondrogenic phenotype and encourages tissue development through multiple ion-specific and loading-specific mechanisms. For example, TRPV4 mediates the response to osmotic changes and mechanical stimuli through its interactions with integrins and other elements of the cytoskeleton, using Ca2+ as a secondary messenger.16 The current understanding points that the prochondrogenic gene expression is activated through the Ca2+/calmodulin pathway.17
Modulation of Ca2+ is also necessary for mechanotransduction of hydrostatic pressure, as evidenced by a lack of response in the presence of Ca2+ chelators.18 In this case, mechanosensitive Ca2+ channels induce an increase in the intracellular concentration of the cation. The increase in Ca2+ concentration activates secondary messengers such as calmodulin, calmodulin kinase type II, and calcineurin, which in turn initiate signaling cascades toward changes in gene expression, likely through Indian hedgehog (Ihh) and Parathyroid hormone-related peptide (PTHrP) pathways.19,20
Modulation of intracellular Na+ has also been implicated as an effector of mechanical cues in chondrocytes. Previous work has shown that hydrostatic pressure inhibits Na+/K+ pumps, producing an accumulation of intracellular Na+.21 Moreover, epithelial sodium channels (ENaC), which are expressed in articular chondrocytes, are also believed to enable mechanotransduction in chondrocytes.22 Colocalization of ENaC with integrins, voltage-activated Ca2+ channels, and Na+/K+ pumps has provided further support to ENaC playing a part in the molecular machinery of mechanotransduction. Other sodium channels that belong to the degenerin/epithelial sodium channel (DEG/ENaC) superfamily are acid-sensing ion channels (ASICs). A study published this year linked ASICs to transduction of mechanical cues.23
While the exact role of ASICs has yet to be determined, their functionality during mechanotransduction has been shown in gastrointestinal responses, bladder compliance, and vascular remodeling, among others.24–26 ASIC1 is expressed in chondrocytes, and a recent study has shown that deregulation of ASIC1 function is associated with the degradation of AC.27 In short, regardless of upstream activation or downstream effects, ion modulation has often associated with a prochondrogenic response.
Ion modulation, hence, could serve to improve tissue-engineered neocartilage. Using neocartilage constructs derived from primary bovine articular chondrocytes, the effects of Ca2+ modulation were determined using the TRPV4 agonist, 4α-phorbol-12,13-didecanoate (4-αPDD), to promote TRPV4 activation and Ca2+ influx.28,29 The increase in intracellular Ca2+ resulted in an increase in the collagen content and tensile properties. The involvement of the TRPV4 ion channel in remodeling and strengthening the matrix of neocartilage constructs was further shown using tensile bioreactors.4
Another tissue engineering study examining ion modulation consisted of Na+ and Ca2+ modulation using ouabain, an inhibitor of the Na+/K+ pump, and ionomycin, a Ca2+ ionophore.30–32 This work reported an increase in the tensile modulus compared with the untreated control by both ion modulators, and an increase in the ultimate tensile strength (UTS) by ouabain.
A more recent study aiming to induce maturation of cardiomyocytes with electrical stimulation found that Ca2+ influx mediated by ionomycin also triggers a comparable intracellular cation increase, possibly mediating a similar response.33 Ion modulation, as an effector of mechanotransduction, could bypass the need of mechanical stimulation bioreactors and be used as a downstream prompt to enhance engineered tissues.
In this work, we applied, for the first time, the Ca2+ modulators, 4-αPDD and ionomycin, and the Na+ modulator ouabain on neocartilage derived from minipig expanded and redifferentiated costal chondrocytes to improve the properties of the tissue-engineered cartilage. It was hypothesized that the modulation of Ca2+ and Na+ ions would modify the biochemical content of the neotissues, resulting in an improvement of the mechanical properties. This hypothesis was tested by determining the effects of 4-αPDD, ouabain, and ionomycin on their own (Phase 1) and in combination (Phase 2).
Methods
Isolation and expansion of costal chondrocytes
Juvenile porcine costal chondrocytes were isolated from the unmineralized portion of floating ribs of three juvenile minipigs obtained from Premier BioSource no later than 48 h after slaughter. Cartilage from ribs cleaned of all noncartilaginous tissue was cut into 1 mm3 pieces and washed three times with GlutaMAX Dulbecco's modified Eagle's medium containing 4.5 g/L glucose (DMEM; Gibco) and 1% (v/v) penicillin/streptomycin/fungizone (PSF; Lonza, Basel, Switzerland).
The cartilage was then digested with 0.4% pronase (Sigma, St. Louis, MO) in DMEM for 1 h at 37°C, and then in 0.2% collagenase type II (Worthington Biochemical, Lakewood, NJ) in DMEM supplemented with 3% (v/v) fetal bovine serum (FBS; Atlanta Biologicals, Lawrenceville, GA) for 18 h at 37°C. Cells were then strained through a 70 μm strainer, washed with red blood cell lysis buffer34 for 4 min, counted, and frozen in freezing medium containing 90% (v/v) FBS +10% (v/v) DMSO (Sigma).
Primary (P0) costal chondrocytes were thawed and seeded in T225 flasks at a density of ∼2.5 million cells per flask in chondrogenic medium (CHG) consisting of GlutaMAX DMEM, 1% (v/v) PSF, 1% (v/v) insulin–transferrin–selenium (BD Biosciences, San Jose, CA), 1% (v/v) nonessential amino acids (Thermo Fisher Scientific), 100 mg/mL sodium pyruvate (Thermo Fischer Scientific), 50 mg/mL ascorbate-2-phosphate (Sigma), 40 mg/mL L-proline (Sigma), and 100 nM dexamethasone (Sigma). Throughout expansion, medium was supplemented with 2% (v/v) FBS, 1 ng/mL TGF-β1, 5 ng/mL bFGF, and 10 ng/mL PDGF (PeproTech). Cells were cultured at 37°C in 10% CO2, and passaged to P3 at confluence using 0.05% trypsin-EDTA (Invitrogen) and 0.2% collagenase type II solutions.
Aggregate culture redifferentiation and neocartilage construct seeding
Passaged costal chondrocytes from each donor were seeded in equal parts on 1% (w/v) agarose-coated plates at a density of 750,000 cells/mL (30 mL total) per plate with CHG supplemented with 10 ng/mL TGF-β1, 100 ng/mL GDF-5, and 100 ng/mL BMP-2 (PeproTech). Plates were placed on an orbital shaker at 50 rpm for 24 h at 37°C in 10% CO2, and then kept static, changing medium every 4 days. At 14 days of aggregate redifferentiation, the aggregates were digested using 0.05% trypsin-EDTA and 0.2% collagenase type II solutions. Cells were strained through a 70 μm cell strainer, washed twice, and resuspended in CHG.
For the self-assembling of neocartilage constructs, 2 million cells were seeded in 5 mm diameter nonadherent 2% (w/v) agarose wells at a density of 20 million cells/mL. At 4 h after self-assembling, additional 400 μL of CHG (control) or CHG supplemented with 10 ng/mL TGF-β1 were added.
This medium was replaced daily until unconfinement (at day 3 after self-assembly), after which, constructs were kept in 2 mL of medium, replaced every 2 days. The CHG control group was maintained in CHG medium. For all other groups, TGF-β1, chondroitinase ABC (c-ABC), and LoxL2 treatment (termed TCL) was used as follows: TGF-β1 was applied at 10 ng/mL after seeding and until day 28, c-ABC (Sigma) was applied at 2 U per mL for 4 h in day 7, and LoxL2 (Signal Chem) was applied at 0.15 μg/mL between days 7 and 21 after self-assembly, together with 0.146 μg/mL hydroxylysine (Sigma) and 1.6 μg/mL copper sulfate (Sigma).
Chemical treatments
For all experiments, the constructs were randomly assigned across treatment groups. Modulation of ions in the constructs was performed between days 12 and 16 (i.e., for 5 days) after self-assembly. For every group in Phase 1, medium was replaced with 400 μL of CHG containing 10 μM 4-αPDD (4α; Enzo Life Sciences), 20 μM ouabain (Ou; Sigma), 0.3 μM ionomycin (Io; Sigma), or blank CHG (control), incubated for 1 h, and then washed twice using DMEM 1% (v/v) PSF before resuming culture in CHG media.
A full-factorial design was used in Phase 2 to determine any additive or synergistic effects across treatments. Ion modulation was performed at the same time using 400 μL of CHG containing the four possible combinations of 4-αPDD, ouabain, and ionomycin, and compared with an additional TCL-treated control group (control+), the current gold standard in tissue-engineered constructs, to examine further changes.
Mechanical testing
Tensile properties were determined using uniaxial tension in an Instron model 5565 (Instron, Canton, MA). Dog bone-shaped samples were obtained from every engineered cartilage construct with a gauge length of 1.55 mm and photographed to measure thickness and width in ImageJ. The ends of the dog bone were fixed to paper with cyanoacrylate to increase the gripping area for testing. A strain rate of 1% of the gauge length per second was used until failure. The Young's modulus was obtained from the linear region of the stress–strain curve and the ultimate tensile strength (UTS) was defined as the maximum stress obtained.
Compressive properties of the engineered cartilage constructs were determined by creep indentation testing. Briefly, 2.5 mm punches obtained from every construct were submerged in phosphate-buffered saline (PBS) until equilibrium and indented with a flat porous 0.5 mm diameter tip perpendicular to the surface of the sample to ∼10% strain. The aggregate modulus (HA) and shear modulus (μs) were obtained using a semianalytical, seminumerical, biphasic model and finite-element optimization.35,36
Biochemical testing
Cartilage constructs (∼2–3 mg) were weighed to obtain wet weight, lyophilized for 3 days, and weighed again to obtain dry weight. Lyophilized samples were digested in 125 μg/mL papain (Sigma) +5 mM N-acetyl-L-cysteine +5 mM EDTA in phosphate buffer pH 6.5 for 18 h at 60°C. GAG content was quantified using the Blyscan GAG Assay Kit (Biocolor, Newtownabbey, Northern Ireland). Total collagen content was quantified using a chloramine-T hydroxyproline assay (Accurate Chemical and Scientific Corp., Westbury, NY). DNA content was quantified with a PicoGreen assay (Thermo Fisher Scientific).
For the quantification of PYR crosslinks, separate tissue samples (∼0.2–1 mg) were weighed, lyophilized, and acid digested for 12 h in 6N HCl. After evaporation, the dried hydrolysate was resuspended in a 75%/25% (v/v) solution of 0.1% formic acid and acetonitrile. Samples were measured through mass spectrometry using a cogent diamond hydride column and a PYR standard.
Histology
Samples were fixed in 10% neutral-buffered formalin, embedded in paraffin, and sectioned at a thickness of 6 μm for histological evaluation. Sections were later processed and stained with Hematoxylin and Eosin (H&E), Safranin O, and Picrosirius Red using standard protocols.
Statistical analyses
All quantitative biochemical and biomechanical tests were performed using n = 6–8. All data are presented as mean ± standard deviations. A single-factor one-way ANOVA was employed in each phase of the study to assess differences among experimental groups. Multiple comparison was performed using a Dunnett's post hoc test. The Dunnett's test compares the mean from every experimental group against a control group mean to determine significant differences. All statistical analyses were performed using GraphPad Prism version 8.4.1 for Windows (GraphPad Software, San Diego, CA). Groups marked with * indicate that there are statistically significant differences compared with the control group (p < 0.05).
Experiment
Gross morphology and histology of stimulated neocartilage constructs
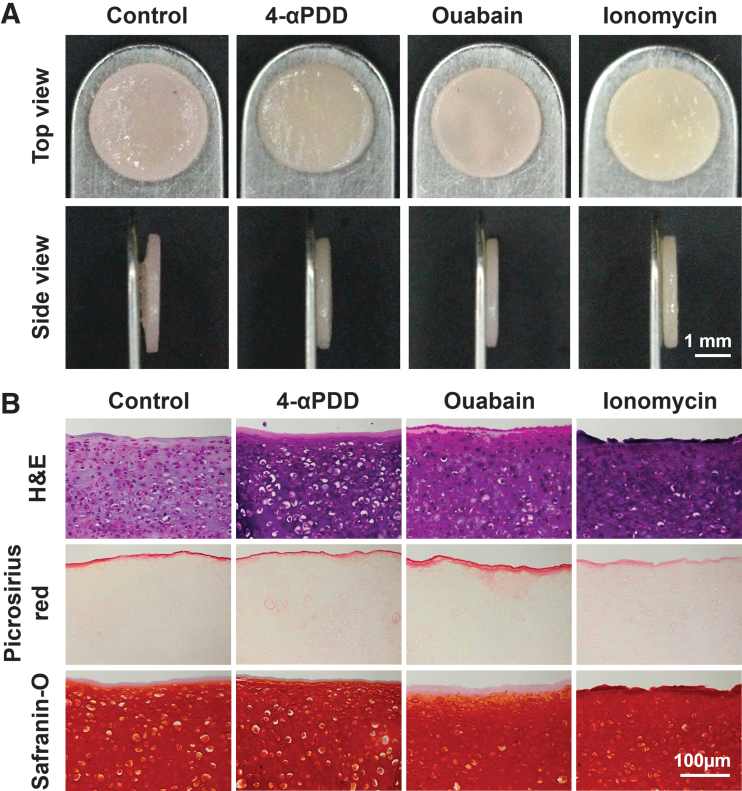
Neocartilage constructs engineered using expanded costal chondrocytes were exposed to exogenous stimuli corresponding to 4-αPDD, ouabain, or ionomycin to investigate their effects on improving functional properties. Gross morphology images of the self-assembled constructs of each group and overall values are shown in Figure 1A and Table 1. Treated groups showed on average a 3.33% reduction in the diameter compared with the untreated control group. However, treated groups were 18.5% thicker compared with the control group, with a significant increase in the 4-αPDD and ionomycin-treated constructs. A similar trend was observed in the mean wet weights among groups, with all constructs having on average a 70.5% water content.
FIG. 1.
(A) Gross morphology of neocartilage constructs: All constructs appear homogeneous, flat, and with no visible abnormalities. The ionomycin-treated constructs appear to be the thickest. (B) Histological analysis of neocartilage constructs: Overall cellularity and neotissue composition appears comparable among all groups as observed in Hematoxylin and Eosin staining. Picrosirius Red staining shows a localized distribution in untreated constructs and ouabain-treated constructs display. Safranin O intensity is higher in 4-αPDD and ionomycin-treated constructs. 4-αPDD, 4α-phorbol-12,13-didecanoate. Color images are available online.
Table 1.
Gross Morphology Values
| Diameter (mm) | Thickness (mm) | Wet weight (mg) | Hydration (%) | |
|---|---|---|---|---|
| Control | 5.51 ± 0.08 | 0.45 ± 0.02 | 13.52 ± 0.35 | 73.33 ± 6.85 |
| 4-Αpdd | 5.38 ± 0.05 | 0.51 ± 0.03* | 14.65 ± 0.57* | 72.33 ± 2.42 |
| Ouabain | 5.25 ± 0.06** | 0.50 ± 0.04 | 12.89 ± 0.48 | 67.66 ± 9.03 |
| Ionomycin | 5.35 ± 0.06 | 0.54 ± 0.03*** | 14.36 ± 0.67 | 68.67 ± 3.25 |
Data are presented as mean ± standard deviation. The asterisks denote significant differences compared with the control group based of Dunnett's post hoc test. *Denotes p < 0.05.
Denotes p < 0.01.
Denotes p < 0.005.
For histological evaluation, the neocartilage constructs were assessed using H&E, Safranin O, and Picrosirius Red staining (Fig. 1B). Picrosirius Red staining showed a localized increase in collagen deposition in the outermost region of untreated controls and, more markedly, in ouabain-treated constructs. Collagen deposition in 4-αPDD and in ionomycin-treated constructs appeared more homogeneous and evenly distributed throughout the construct. GAG deposition, as seen in Safranin O staining, appeared more intense in ionomycin-treated constructs, followed by 4-αPDD-treated constructs. In contrast to Picrosirius Red staining, the untreated constructs and ouabain-treated constructs showed a decrease in Safranin O intensity, especially in the outermost region.
Effect of ion modulation in the biochemical and mechanical properties of neocartilage constructs
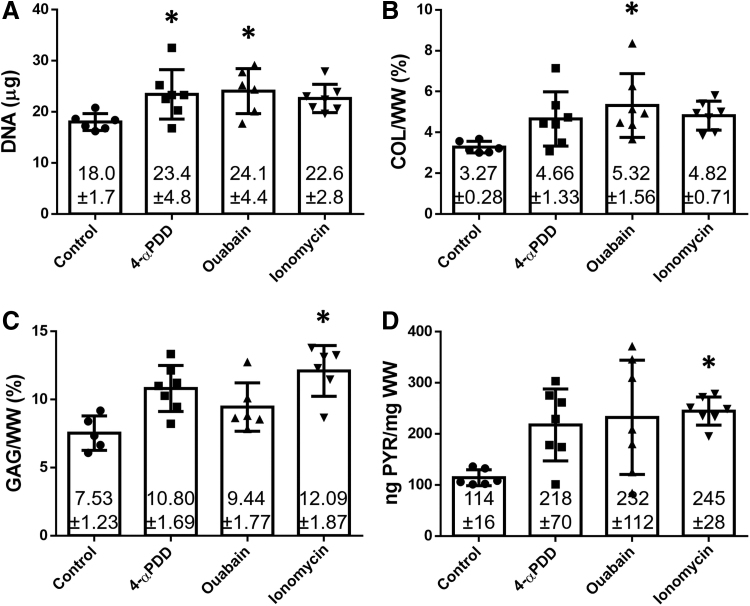
The amounts of DNA, total collagen, GAG, and PYR crosslink content of the different groups at 4 weeks were evaluated per wet weight (Fig. 2). All treated constructs showed an increase in the DNA content, with an averaged 34% increase in all groups compared with the control group. The amount of collagen was also increased with all treatments, but only significantly higher in constructs treated with ouabain, which showed a 63% increase over the untreated group.
FIG. 2.
Biochemical properties of neocartilage constructs treated with 4-αPDD, ouabain, or ionomycin. (A) DNA per construct, (B) collagen/WW, (C) GAG/WW, and (D) PYR/WW. Ouabain-treated constructs showed a 63% increase in the collagen content. Conversely, ionomycin-treated constructs showed a 61%, and a 115% increase in the amount of GAG and PYR crosslinks, respectively. The asterisks denotes significant differences compared to the control group based on Dunnett's post hoc test (* denotes p < 0.05). GAG, glycosaminoglycan; PYR, pyridinoline.
In concordance to the histological observations, all treatment groups showed an increase in the GAG content, with a 61% significant increase in ionomycin-treated constructs. Ionomycin also had an effect in the concentration of PYR crosslinks, which bond collagen triple helices through the oxidation of lysine residues by lysyl oxidases. The amount of PYR crosslinks was significantly higher in the presence of ionomycin, with a 115% increase compared with the control group.
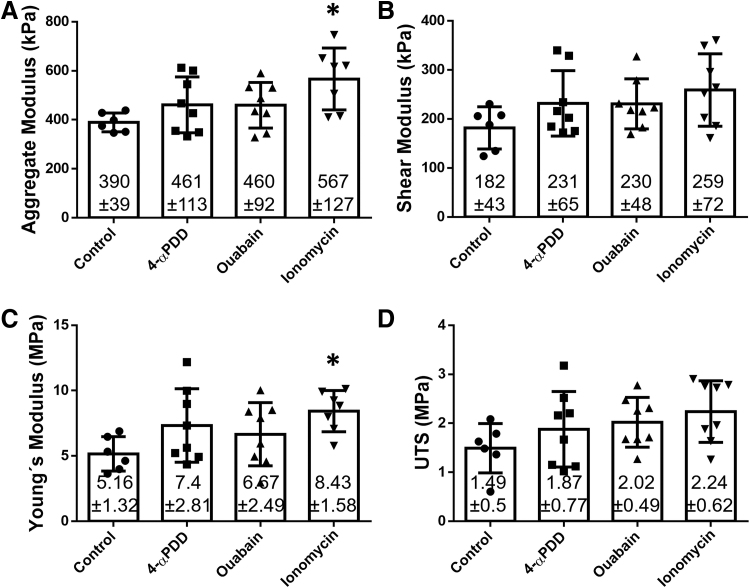
The HA increased an 18% with 4-αPDD or ouabain treatments, but only ionomycin induced a significant increase, with an HA of 567 kPa, resulting in a 45% increase over the control group (Fig. 3A). The same trend was observed in the μs, but with no significant differences (Fig. 3B). Likewise, 4-αPDD and ouabain resulted in higher Young's modulus values compared with the control group (7.37 and 6.67 MPa, respectively), although not significant. Ionomycin, however, produced a significant increase in the Young's modulus (8.43 MPa), corresponding to a 63% increase over the control group (5.16 MPa) (Fig. 3C). The UTS values of 4-αPDD, ouabain, and ionomycin corresponded to 1.87, 2.02, and 2.24 MPa, respectively, compared with the 1.79 MPa observed in the control group (Fig. 3D).
FIG. 3.
Biomechanical properties of neocartilage constructs treated with 4-αPDD, ouabain, or ionomycin. (A) Aggregate modulus, (B) shear modulus, (C) tensile Young's modulus, and (D) UTS. Ionomycin-treated constructs showed a 45% increase in the aggregate modulus and a 63% increase in the Young's modulus. The asterisks denotes significant differences compared to the control group based on Dunnett's post hoc test (* denotes p < 0.05).
Effect of treatment combinations in neocartilage constructs
To study possible synergistic effects among 4-αPDD, ouabain, and ionomycin, the four different combinations (“4α+Ou,” “4α+Io,” “Ou+Io,” and “4α+Ou+Io”) were tested. In consideration of the potential effects of the TCL treatment, data for TCL-treated constructs were additionally collected in Phase 2 as control (Control+). The values for the phase 2 untreated constructs (Control) for gross morphology (diameter: 5.96 ± 0.17 mm; thickness: 0.47 ± 0.04 mm; wet weight: 14.7 ± 2.03 mg; hydration: 80.85% ± 2.3%), biochemical content (DNA: 17.02 ± 2.19 μg; collagen: 2.4% ± 0.12%; GAG: 8.74% ± 1.03%; PYR: 113.6 ± 46.3 ng/mg), and mechanical properties (HA: 431.8 ± 37 kPa; Shear modulus: 208.7 ± 40.63 kPa; Young's modulus: 5.24 ± 1.25 MPa; UTS: 1.07 ± 0.49 MPa) were comparable to values obtained for the control group in Phase 1.
Uniformly, the biochemical and mechanical properties of the TCL-treated constructs (Control+) were higher than the values of the untreated control; the biochemical values for the TCL-treated constructs were DNA: 25.7 ± 4.3 μg; collagen: 5.01% ± 0.13%; GAG: 12.19% ± 0.97%; and PYR: 150.09 ± 76.51 ng/mg, and the mechanical values were HA: 499 ± 111 kPa; Shear modulus: 242 ± 61 kPa; Young's modulus: 7.8 ± 0.6 MPa; and UTS: 2.2 ± 0.3 MPa.
The properties of untreated and the TCL-treated constructs differ in accordance to the literature.37 For the test groups, diameter and thickness were unaffected by the treatment combinations (Table 2). Wet weight was significantly lower in the groups where “4α+Ou” and “4α+Ou+Io” were supplied, resulting in a 7% and 8% decrease, respectively (Table 2). Hydration values and cell/DNA content were unaffected by the different treatments, however. The amount of GAG was significantly lower in “Ou+Io”-treated constructs, showing a 34% decrease compared with the TCL control group (Table 2). The amount of total collagens remained stable, with no differences found with any treatments, however, the concentration of PYR crosslinks was significantly higher in the group treated with “4α+Ou,” with a 58% increase (Table 2).
Table 2.
Gross Morphology and Biochemical Data of Neocartilage Constructs Treated with Two or More Ion Modulators
| Diameter (mm) | Thickness (mm) | Wet weight (mg) | Hydration (%) | DNA/const. (μg) | Collagen (%WW) | GAG (%WW) | PYR (ng PYR/mg WW) | |
|---|---|---|---|---|---|---|---|---|
| Control+ | 5.28 ± 0.02 | 0.51 ± 0.02 | 14.5 ± 0.4 | 66.5 ± 7.1 | 25.7 ± 4.3 | 5.01 ± 0.13 | 12.19 ± 0.97 | 150.09 ± 76.51 |
| 4α+Ou | 5.32 ± 0.07 | 0.46 ± 0.05 | 13.3 ± 0.6* | 71.5 ± 2.5 | 21.1 ± 1.5 | 4.75 ± 0.57 | 10.27 ± 0.95 | 237.17 ± 24.54* |
| 4α+ Io | 5.36 ± 0.07 | 0.51 ± 0.03 | 14.4 ± 1.2 | 70.5 ± 4.0 | 19.2 ± 5.9 | 4.49 ± 0.68 | 11.76 ± 1.74 | 157.73 ± 60.74 |
| Ou+ Io | 5.29 ± 0.10 | 0.51 ± 0.03 | 13.7 ± 0.4 | 74.9 ± 8.2 | 22.2 ± 7.5 | 4.33 ± 1.39 | 8.00 ± 3.28* | 174.63 ± 64.84 |
| 4α+Ou+ Io | 5.35 ± 0.08 | 0.49 ± 0.04 | 13.5 ± 0.6* | 71.9 ± 8.4 | 27.3 ± 4.5 | 5.17 ± 1.31 | 9.92 ± 2.16 | 141.24 ± 33.71 |
Data are presented as mean ± standard deviation. The asterisks denote significant differences compared with the control+ group based of Dunnett's post hoc test. *Denotes p < 0.05.
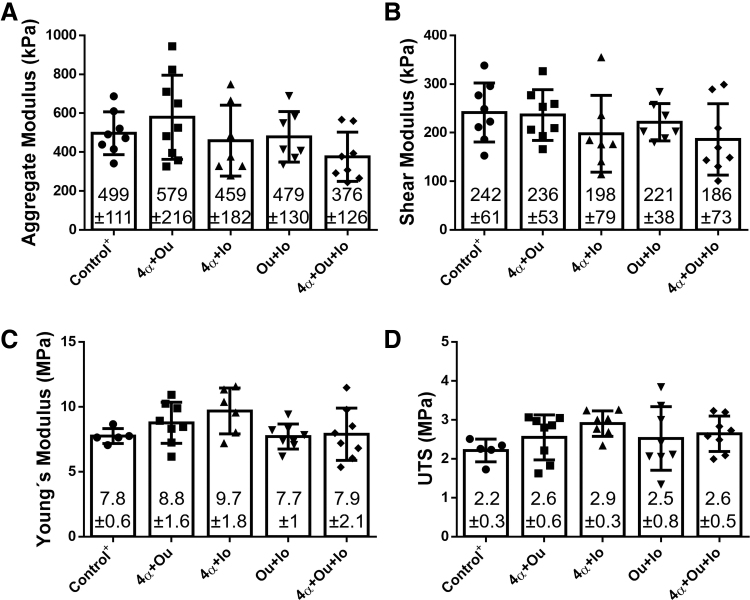
The compressive and tensile properties of constructs in the presence of two or more ion modulators were also tested (Fig. 4). The HA and μs showed no significant differences, whereas only one group, “4α+Ou,” displayed an increase in the HA, and all other combinations resulted in equal or lower compressive properties (Fig. 4A, B); the constructs treated with all three modulators showed a ∼25% decrease compared with the TCL control group in both HA and μs. On the contrary, and compared with the control group, all groups, except the “Ou+Io” group showed increases in the tensile Young's modulus and UTS, with 1–25% and 18–31% increases, respectively (Fig. 4C, D). Still, the combination of treatments did not show additive or synergistic effects toward improving the compressive or tensile properties.
FIG. 4.
Biomechanical properties of neocartilage constructs treated with combinations of 4-αPDD, ouabain, and ionomycin. (A) Aggregate modulus, (B) shear modulus, (C) tensile Young's modulus, and (D) tensile UTS. Abbreviations as follows: 4-αPDD (4α), ouabain (Ou), and ionomycin (Io). No statistical significance was found in any of the mechanical properties.
Discussion
The overall objective of this work was to employ ion modulation to develop biomimetic neocartilage using expanded redifferentiated costal chondrocytes. Expansion and redifferentiation of chondrocytes obtained from the rib tackles three longstanding issues in cartilage tissue engineering: (1) it eliminates donor-site morbidity in the joint, (2) it avoids cell-number limitations, and (3) it provides cells with a suitable chondrogenic phenotype toward building a hyaline cartilage.38 The ion modulators, 4-αPDD, ouabain, and ionomycin, change the intracellular ion concentration through different mechanisms (Fig. 5). This work is the first to quantify the effects of single and combined ion modulation in neocartilage constructs and to report the measurement of PYR crosslink, a key component of the mature cartilage ECM.39
FIG. 5.
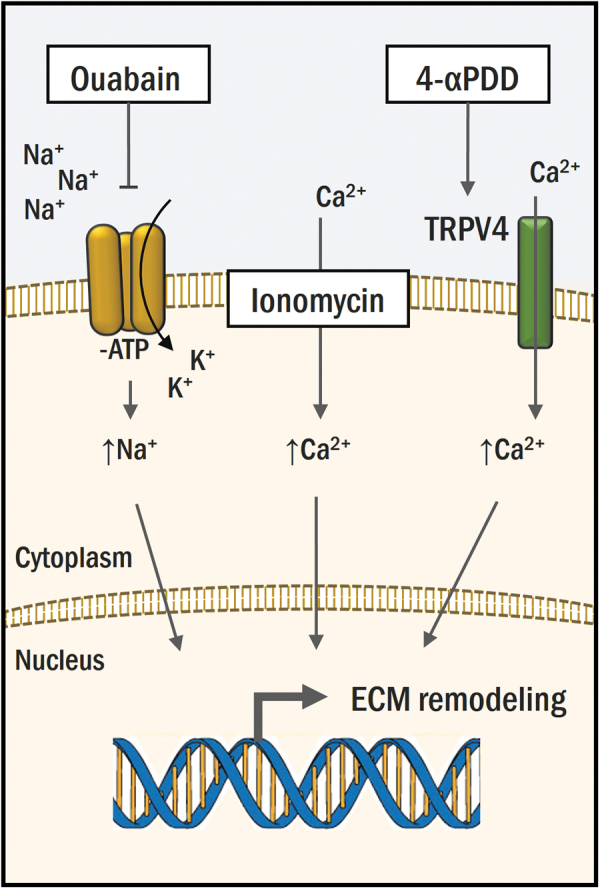
4-αPDD, ouabain, and ionomycin modulate intracellular ion concentrations. Four-αPDD activates the TRPV4 transmembrane receptor, promoting intake of Ca2+. Ouabain inhibits the Na+/K+ pump, enabling passive diffusion and accumulation of Na+ into the cell. Ionomycin allows influx of Ca2+ from the extracellular compartment and internal Ca2+ stores into the cytoplasm. Color images are available online.
The experimental results supported the hypothesis that the modulation of Ca2+ and Na+ ions result in biochemical content changes, which translate to an improvement of neotissue mechanical properties. Particularly, neocartilage constructs treated with ionomycin showed the highest improvement in mechanical properties.
Ionomycin-treated constructs showed the largest turnover of ECM components, with a 61% increase in GAG and a 115% increase in PYR crosslink content. These changes in the biochemical content were accompanied by an improvement in mechanical properties. Constructs treated with the Ca2+ ionophore showed a 45% improvement in the compressive properties, increasing the HA from 389.5 kPa (control) to 566.6 kPa. Furthermore, treatment with ionomycin also showed a 63% improvement in its tensile properties, increasing the Young's modulus from 5.16 MPa (control) to 8.43 MPa. The relationship between an increase in the GAG content and the HA has been reported in the past, as the negatively charged GAG attract water and provide compressive resistance to AC and high osmotic pressure.40,41
The connection between PYR crosslinks and compressive properties, however, has been less discussed; it is inferred here that a more mature collagen network with a higher presence of crosslinks provides a restraining structure that decreases GAG leaking and impedes interstitial fluid movement from the engineered constructs.42 Strategies that result in crosslink increases, such as hypoxia or in vivo maturation,39,43 support our observations that a higher crosslink content improves the compressive properties, and the opposite is observed in diseased intervertebral disk where crosslinks are lost.44 The increase in the Young's modulus, conversely, may be induced by a strengthening of the collagen network, also derived from the increase in PYR crosslink.45 These results demonstrate that Ca2+ modulation can be directed to enhance the mechanical properties in engineered cartilage.
Ionomycin treatment of engineered neocartilage also resulted in a 20% increase of construct thickness compared with the control group. In a similar fashion, 4-αPDD also produced a significant 13% increase in thickness. Therefore, it may be inferred that an intracellular increase in Ca2+ supports construct thickening. While diameter and shape can be successfully manipulated, control over the resulting thickness of self-assembled neotissues has proven more challenging.46,47
Measurements of human samples have reported the AC thickness to range between 0.35 and 6.25 mm in the tibial plateau and from 0.89 to 5.94 mm in the patella.48 Constructs that aim translation should span the thickness of human AC, and at this moment, any strategy that enables or supports the growth of neocartilage to treat larger and deeper chondral injuries (i.e., over the range from partial- to full-thickness defects) is highly desirable. Consequently, the improvement of gross morphology features also motivates the use of ion modulators toward clinical translation. Ca2+ signaling regulates many processes in chondrocytes, including division, migration, death, and differentiation, and more studies are required to determine the cause of this increase in thickness.49
Treatment with ouabain and 4-αPDD resulted in significant differences on the gross morphology and biochemical content of neocartilage constructs. Ouabain treatment was the only regime to affect construct size, reducing construct diameter by 5%, while 4-αPDD increased wet weight. Ouabain treatment was also the only regime to induce a significant variation in collagen content, with a 62% increase compared with control. Similar effects have been reported in cartilage constructs cultured in hypertonic medium, resulting in an increase of collagen content and Young's modulus.15 The self-assembling process used to generate the samples examined in this study has been demonstrated in ample prior work as yielding neocartilage with abundant collagen type II and no discernable collagen type I using immunohistochemistry and proteomic analysis.50,51
In our study, ouabain also increased neocartilage tensile and compressive properties, although not significantly. Due to the multiple signaling pathways in which both ouabain and the Na+/K+ ATP-dependent ion pump are involved, the mechanisms behind these effects require further study.52,53 Still, these results demonstrate that TRPV4 activation or Na+ modulation influence morphological and biochemical properties of neocartilage constructs.
The existence of different independent mechanisms of signaling through ion modulation that take place during mechanotransduction imply the potential of synergistic or additive effects between two or more modulators. In the Phase 2 study, only constructs treated with “4α+Ou” showed an increase in PYR crosslinks, but with no synergistic or additive effects in compressive or tensile properties. Although the PYR crosslink per wet weight was significantly different between 4α+Ou and the control group, the PYR crosslink per collagen only trended higher for the 4α+Ou group and not significantly different than the control, which may, in part, explain the observation that the compressive aggregate modulus for the 4α+Ou group only trended higher than the control as well but was not significant (Supplementary Fig. S1).
The observations reported in this second experiment may be due to cell saturation with ions and the desensitization of signaling pathways, inhibiting a further response regardless of the increase in cation influx. Furthermore, high magnitudes of mechanical stimulation can elicit damaging effects in cartilage constructs,54 and a similar effect may be replicated when two or more ion modulators are used simultaneously. Overstimulation with ion modulators did not elicit additive or synergistic effects on construct mechanical properties.
The effects of ion modulators on neocartilage derived from expanded and redifferentiated costal chondrocytes were partially comparable to those seen when employing primary articular chondrocytes. Treatment of constructs derived from primary bovine articular chondrocytes with 4-αPDD produced an 88% increase in collagen content and a 153% increase in tensile stiffness.29 It was also found that Na+ and Ca2+ modulation with ouabain and ionomycin, or their combination, increased the Young's modulus by 40–95% compared with untreated control.31 These observations differ from the results of the present study, and these variations may be due to differences in cell phenotype between bovine primary articular chondrocytes and minipig costal chondrocytes, which were expanded and redifferentiated. The present work suggests that the effect of ion modulators depends on the construct used, including cell type (articular vs. costal), passage (P0 vs. P3), chondrogenic state (primary vs. redifferentiated), and species (bovine vs. minipig).
The effects reported in this study are comparable to those observed when implementing mechanical stimuli on similar scaffoldless tissue-engineered constructs. For example, tension stimulation induced an increase in tensile properties of bovine neocartilage constructs. Constant tension resulted in a Young's modulus of 5.1 ± 2 MPa, which constitutes a 4.6-fold and a 1.6-fold increase in the modulus compared with untreated (1.1 ± 0.3 MPa) and TCL-treated controls (3.1 ± 1.1 MPa), respectively.4 Microarray analysis revealed that the TRPV4 ion channel was involved in the matrix remodeling initiated by the tensile stimulus, and inhibition of the channel with GSK205 abolished the tissue-level response to tension. In this study, 4-αPDD, known to activate TRPV4, was used.
Likewise, fluid-induced shear prompted a 2.7-fold increase in the Young's modulus (2.18 ± 0.74 MPa), a 1.9-fold increase in the aggregate modulus (64 ± 20 kPa), and a 1.4-fold increase in the collagen content (15 ± 3%/DW) compared with controls in human neocartilage.55 Analogous changes were found in this study; specifically, with ionomycin, we show a 1.6-fold increase in the tensile properties, a 1.5-fold increase in the aggregate modulus, and 1.6-fold increase in GAG content (12.09%/WW). In agreement with our hypothesis on using ionomycin in lieu of mechanotransduction, the RNA-seq data suggest that these changes may be induced by the perturbation of primary cilia and the opening of the PC1/2 complex, which allows the influx of Ca2+. The specific mechanisms that follow ion modulation remain ill defined, as multiple signaling pathways can interplay in accordance to the initial mechanical stimulus.56
While Wnt, TGF-β1, and YAP/TAZ pathways have been reported to become active following mechanical stresses, the activation of the Ihh pathway and the MAPK-ERK pathway may play a predominant role after ion modulation. With Ca2+ mediating the initial response, Ihh transduces the stimuli from the primary cilia.57,58 The MAPK-ERK pathway, on the other hand, is highly dependent on ion concentration, particularly Ca2+, to induce a transcriptional response.59 Future studies should aim at determining which specific pathways are involved in transducing the effects of both mechanically and chemically induced ion modulation and how these pathways compare.
Intracellular Ca2+ modulation presents an attractive and novel strategy to engineer robust neocartilage using expanded and redifferentiated chondrocytes from the rib, a non-AC cell source. The manipulation in ion concentration through the use of chemical modulators is an innovative method to enhance the mechanical properties of engineered cartilage compared with the use of targeted enzymatic treatments and bioreactors. Ionomycin may be used to replace mechanical stimulation bioreactors, often used ex vivo to induce neotissue maturation.60,61 In spite of exhibiting beneficial effects, bioreactors are prone to contamination and user error, whereas chemical ion modulation has multiple advantages: it is cost-effective, replicable, and highly controllable.
In summary, this study investigated whether ion modulation would improve the mechanical properties of engineered neocartilage constructs derived from expanded and redifferentiated costal chondrocytes. It was shown that ionomycin treatment resulted in an increase of neocartilage compressive and tensile properties. This increase may be a result of the higher GAG content coupled with an increase in the PYR crosslinking. The use of two or more ion modulators in combination did not produce additive nor synergistic effects. The results of this study demonstrate that ion modulation with ionomycin is a powerful tool in tissue engineering.
Supplementary Material
Disclosure Statement
No competing financial interests exist.
Funding Information
The authors acknowledge support from the National Institutes of Health (NIH) R01 AR067821 and Fulbright Chile scholarship.
Supplementary Material
References
- 1. Makris, E.A., MacBarb, R.F., Paschos, N.K., Hu, J.C., and Athanasiou, K.A.. Combined use of chondroitinase-ABC, TGF-beta1, and collagen crosslinking agent lysyl oxidase to engineer functional neotissues for fibrocartilage repair. Biomaterials 35, 6787, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Leipzig, N.D., and Athanasiou, K.A.. Static compression of single chondrocytes catabolically modifies single-cell gene expression. Biophys J 94, 2412, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huey, D.J., and Athanasiou, K.A.. Tension-compression loading with chemical stimulation results in additive increases to functional properties of anatomic meniscal constructs. PLoS One 6, e27857, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee, J.K., Huwe, L.W., Paschos, N., et al. . Tension stimulation drives tissue formation in scaffold-free systems. Nat Mater 16, 864, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ofek, G., Dowling, E.P., Raphael, R.M., McGarry, J.P., and Athanasiou, K.A.. Biomechanics of single chondrocytes under direct shear. Biomech Model Mechanobiol 9, 153, 2010. [DOI] [PubMed] [Google Scholar]
- 6. Elder, B.D., and Athanasiou, K.A.. Synergistic and additive effects of hydrostatic pressure and growth factors on tissue formation. PLoS One 3, e2341, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oinas, J., Ronkainen, A.P., Rieppo, L., et al. . Composition, structure and tensile biomechanical properties of equine articular cartilage during growth and maturation. Sci Rep 8, 11357, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huey, D.J., and Athanasiou, K.A.. Maturational growth of self-assembled, functional menisci as a result of TGF-beta1 and enzymatic chondroitinase-ABC stimulation. Biomaterials 32, 2052, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Qiu, X., and Muller, U.. Mechanically gated ion channels in mammalian hair cells. Front Cell Neurosci 12, 100, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Douguet, D., and Honore, E.. Mammalian mechanoelectrical transduction: structure and function of force-gated ion channels. Cell 179, 340, 2019. [DOI] [PubMed] [Google Scholar]
- 11. Djillani, A., Mazella, J., Heurteaux, C., and Borsotto, M.. Role of TREK-1 in health and disease, focus on the central nervous system. Front Pharmacol 10, 379, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alloui, A., Zimmermann, K., Mamet, J., et al. . TREK-1, a K+ channel involved in polymodal pain perception. EMBO J 25, 2368, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Madden, R.M., Han, S.K., and Herzog, W.. The effect of compressive loading magnitude on in situ chondrocyte calcium signaling. Biomech Model Mechanobiol 14, 135, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fitzgerald, J.B., Jin, M., Dean, D., et al. . Mechanical compression of cartilage explants induces multiple time-dependent gene expression patterns and involves intracellular calcium and cyclic AMP. J Biol Chem 279, 19502, 2004. [DOI] [PubMed] [Google Scholar]
- 15. Sampat, S.R., Dermksian, M.V., Oungoulian, S.R., et al. . Applied osmotic loading for promoting development of engineered cartilage. J Biomech 46, 2674, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O'Conor, C.J., Leddy, H.A., Benefield, H.C., Liedtke, W.B., and Guilak, F.. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proc Natl Acad Sci U S A 111, 1316, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Muramatsu, S., Wakabayashi, M., Ohno, T., et al. . Functional gene screening system identified TRPV4 as a regulator of chondrogenic differentiation. J Biol Chem 282, 32158, 2007. [DOI] [PubMed] [Google Scholar]
- 18. Steward, A.J., Kelly, D.J., and Wagner, D.R.. The role of calcium signalling in the chondrogenic response of mesenchymal stem cells to hydrostatic pressure. Eur Cell Mater 28, 358, 2014. [DOI] [PubMed] [Google Scholar]
- 19. Riddle, R.C., Taylor, A.F., Genetos, D.C., and Donahue, H.J.. MAP kinase and calcium signaling mediate fluid flow-induced human mesenchymal stem cell proliferation. Am J Physiol Cell Physiol 290, C776, 2006. [DOI] [PubMed] [Google Scholar]
- 20. Liu, Q., Yang, H.X., Wan, X.H., et al. . Calcium-/calmodulin-dependent protein kinase II in occlusion-induced degenerative cartilage of rat mandibular condyle. J Oral Rehabil 45, 442, 2018. [DOI] [PubMed] [Google Scholar]
- 21. Elder, B.D., and Athanasiou, K.A.. Hydrostatic pressure in articular cartilage tissue engineering: from chondrocytes to tissue regeneration. Tissue Eng Part B Rev 15, 43, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shakibaei, M., and Mobasheri, A.. Beta1-integrins co-localize with Na, K-ATPase, epithelial sodium channels (ENaC) and voltage activated calcium channels (VACC) in mechanoreceptor complexes of mouse limb-bud chondrocytes. Histol Histopathol 18, 343, 2003. [DOI] [PubMed] [Google Scholar]
- 23. Ruan, N., Tribble, J., Peterson, A.M., et al. . Acid-sensing ion channels and mechanosensation. Int J Mol Sci 22, 4810, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yoshiyama, M., Kobayashi, H., Takeda, M., and Araki, I.. Blockade of acid-sensing ion channels increases urinary bladder capacity with or without intravesical irritation in mice. Front Physiol 11, 592867, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jernigan, N.L., Herbert, L.M., Walker, B.R., and Resta, T.C.. Chronic hypoxia upregulates pulmonary arterial ASIC1: a novel mechanism of enhanced store-operated Ca2+ entry and receptor-dependent vasoconstriction. Am J Physiol Cell Physiol 302, C931, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alcaino, C., Farrugia, G., and Beyder, A.. Mechanosensitive piezo channels in the gastrointestinal tract. Curr Top Membr 79, 219, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu, Y., and Chen, F.. Acid-sensing ion channel-1a in articular chondrocytes and synovial fibroblasts: a novel therapeutic target for rheumatoid arthritis. Front Immunol 11, 580936, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Strotmann, R., Harteneck, C., Nunnenmacher, K., Schultz, G., and Plant, T.D.. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol 2, 695, 2000. [DOI] [PubMed] [Google Scholar]
- 29. Eleswarapu, S.V., and Athanasiou, K.A.. TRPV4 channel activation improves the tensile properties of self-assembled articular cartilage constructs. Acta Biomater 9, 5554, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sandtner, W., Egwolf, B., Khalili-Araghi, F., et al. . Ouabain binding site in a functioning Na+/K+ ATPase. J Biol Chem 286, 38177, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Natoli, R.M., Skaalure, S., Bijlani, S., et al. . Intracellular Na(+) and Ca(2+) modulation increases the tensile properties of developing engineered articular cartilage. Arthritis Rheum 62, 1097, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morgan, A.J., and Jacob, R.. Ionomycin enhances Ca2+ influx by stimulating store-regulated cation entry and not by a direct action at the plasma membrane. Biochem J 300(Pt 3), 665, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ma, R., Liang, J., Huang, W., et al. . Electrical stimulation enhances cardiac differentiation of human induced pluripotent stem cells for myocardial infarction therapy. Antioxid Redox Signal 28, 371, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brown, W.E., Hu, J.C., and Athanasiou, K.A.. Ammonium-chloride-potassium lysing buffer treatment of fully differentiated cells increases cell purity and resulting neotissue functional properties. Tissue Eng Part C Methods 22, 895, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Athanasiou, K.A., Niederauer, G.G., and Schenck, R.C.Jr. Biomechanical topography of human ankle cartilage. Ann Biomed Eng 23, 697, 1995. [DOI] [PubMed] [Google Scholar]
- 36. Mow, V.C., Kuei, S.C., Lai, W.M., and Armstrong, C.G.. Biphasic creep and stress relaxation of articular cartilage in compression? Theory and experiments. J Biomech Eng 102, 73, 1980. [DOI] [PubMed] [Google Scholar]
- 37. Kwon, H., O'Leary, S.A., Hu, J.C., and Athanasiou, K.A.. Translating the application of transforming growth factor-beta1, chondroitinase-ABC, and lysyl oxidase-like 2 for mechanically robust tissue-engineered human neocartilage. J Tissue Eng Regen Med 13, 283, 2019. [DOI] [PubMed] [Google Scholar]
- 38. Kwon, H., Brown, W.E., Lee, C.A., et al. . Surgical and tissue engineering strategies for articular cartilage and meniscus repair. Nat Rev Rheumatol 15, 550, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Makris, E.A., Hu, J.C., and Athanasiou, K.A.. Hypoxia-induced collagen crosslinking as a mechanism for enhancing mechanical properties of engineered articular cartilage. Osteoarthritis Cartilage 21, 634, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chahine, N.O., Chen, F.H., Hung, C.T., and Ateshian, G.A.. Direct measurement of osmotic pressure of glycosaminoglycan solutions by membrane osmometry at room temperature. Biophys J 89, 15430, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gu, W.Y., and Yao, H.. Effects of hydration and fixed charge density on fluid transport in charged hydrated soft tissues. Ann Biomed Eng 31, 1162, 2003. [DOI] [PubMed] [Google Scholar]
- 42. Han, E.H., Chen, S.S., Klisch, S.M., and Sah, R.L.. Contribution of proteoglycan osmotic swelling pressure to the compressive properties of articular cartilage. Biophys J 101, 916, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vapniarsky, N., Huwe, L.W., Arzi, B., et al. . Tissue engineering toward temporomandibular joint disc regeneration. Sci Transl Med 10, eaaq1802, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bezci, S.E., Nandy, A., and O'Connell, G.D.. Effect of hydration on healthy intervertebral disk mechanical stiffness. J Biomech Eng 137, 101007, 2015. [DOI] [PubMed] [Google Scholar]
- 45. Eleswarapu, S.V., Responte, D.J., and Athanasiou, K.A.. Tensile properties, collagen content, and crosslinks in connective tissues of the immature knee joint. PLoS One 6, e26178, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Huang, B.J., Brown, W.E., Keown, T., Hu, J.C., and Athanasiou, K.A.. Overcoming challenges in engineering large, scaffold-free neocartilage with functional properties. Tissue Eng Part A 24, 1652, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bryant, S.J., and Anseth, K.S.. The effects of scaffold thickness on tissue engineered cartilage in photocrosslinked poly(ethylene oxide) hydrogels. Biomaterials 22, 619, 2001. [DOI] [PubMed] [Google Scholar]
- 48. Ateshian, G.A., Soslowsky, L.J., and Mow, V.C.. Quantitation of articular surface topography and cartilage thickness in knee joints using stereophotogrammetry. J Biomech 24, 761, 1991. [DOI] [PubMed] [Google Scholar]
- 49. Lv, M., Zhou, Y., Chen, X., et al. . Calcium signaling of in situ chondrocytes in articular cartilage under compressive loading: roles of calcium sources and cell membrane ion channels. J Orthop Res 36, 730, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Natoli, R.M., Responte, D.J., Lu, B.Y., and Athanasiou, K.A.. Effects of multiple chondroitinase ABC applications on tissue engineered articular cartilage. J Orthop Res 27, 949, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Donahue, R.P., Nordberg, R.C., Bielajew, B.J., Hu, J.C., and Athanasiou, K.A. The effect of neonatal, juvenile, and adult donors on rejuvenated neocartilage functional properties. Tissue Eng Part A 2021 [Epub ahead of print]; DOI: 10.1089/ten.TEA.2021.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Contreras, R.G., Flores-Beni Tez, D., Flores-Maldonado, C., et al. . Na+,K+-ATPase and hormone ouabain: new roles for an old enzyme and an old inhibitor. Cell Mol Biol (Noisy-le-grand) 52, 31, 2006. [PubMed] [Google Scholar]
- 53. Ou, Y., Pan, C.X., Zuo, J., and van der Hoorn, F.A.. Ouabain affects cell migration via Na,K-ATPase-p130cas and via nucleus-centrosome association. PLoS One 12, e0183343, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Salinas, E.Y., Hu, J.C., and Athanasiou K.. A guide for using mechanical stimulation to enhance tissue-engineered articular cartilage properties. Tissue Eng Part B Rev 24, 345, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Salinas, E.Y., Aryaei, A., Paschos, N., et al. . Shear stress induced by fluid flow produces improvements in tissue-engineered cartilage. Biofabrication 12, 045010, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhao, Z., Li, Y., Wang, M., et al. . Mechanotransduction pathways in the regulation of cartilage chondrocyte homoeostasis. J Cell Mol Med 24, 5408, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Thompson, C.L., Chapple, J.P., and Knight, M.M.. Primary cilia disassembly down-regulates mechanosensitive hedgehog signalling: a feedback mechanism controlling ADAMTS-5 expression in chondrocytes. Osteoarthritis Cartilage 22, 490, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Shao, Y.Y., Wang, L., Welter, J.F., and Ballock, R.T.. Primary cilia modulate Ihh signal transduction in response to hydrostatic loading of growth plate chondrocytes. Bone 50, 79, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. He, Z., Leong, D.J., Zhuo, Z., et al. . Strain-induced mechanotransduction through primary cilia, extracellular ATP, purinergic calcium signaling, and ERK1/2 transactivates CITED2 and downregulates MMP-1 and MMP-13 gene expression in chondrocytes. Osteoarthritis Cartilage 24, 892, 2016. [DOI] [PubMed] [Google Scholar]
- 60. Darling, E.M., and Athanasiou, K.A.. Articular cartilage bioreactors and bioprocesses. Tissue Eng 9, 9, 2003. [DOI] [PubMed] [Google Scholar]
- 61. Mabvuure, N., Hindocha, S., and Khan, W.S.. The role of bioreactors in cartilage tissue engineering. Curr Stem Cell Res Ther 7, 287, 2012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.