Abstract
Although vaccination is widely accepted as an effective method of preventing and controlling the COVID‐19 pandemic, many people are concerned about possible cutaneous side‐effects, which can delay or prevent them from being vaccinated. The objectives of this systematic review were to assess the global prevalence and clinical manifestations of cutaneous adverse reactions following COVID‐19 vaccination. PubMed and Scopus databases were searched for articles published from 1 January 2019 to 31 December 2021, and reference lists for each selected article were screened. Case reports, case series, observational studies and randomized controlled trials that provided information on cutaneous adverse reactions following COVID‐19 vaccines were included. A total of 300 studies were included in a systematic review of which 32 studies with 946 366 participants were included in the meta‐analysis. The pooled prevalence of cutaneous manifestations following COVID‐19 vaccination was 3.8% (95% CI, 2.7%–5.3%). COVID‐19 vaccines based on the mRNA platform had a higher prevalence than other platforms at 6.9% (95% CI, 3.8%–12.3%). Various cutaneous manifestations have been reported from injection site reactions, which were the most common (72.16%) to uncommon adverse reactions such as delayed inflammatory reactions to tissue filler (0.07%) and flares of pre‐existing dermatoses (0.07%). Severe cutaneous reactions such as anaphylaxis have also been reported, but in rare cases (0.05%). In conclusion, cutaneous adverse reactions are common, especially in those receiving mRNA vaccines. Most reactions are mild and are not contraindications to subsequent vaccination except for anaphylaxis, which rarely occurs. COVID‐19 vaccination may also be associated with flares of pre‐existing dermatoses and delayed inflammatory reactions to tissue filler. Patients with a history of allergies, pre‐existing skin conditions or scheduled for filler injections should receive additional precounselling and monitoring. A better understanding of potential side‐effects may strengthen public confidence in those wary of new vaccine technologies.
Keywords: cutaneous adverse reactions, COVID‐19 vaccination, prevalence, clinical manifestation
Introduction
The emergence of the novel coronavirus known as severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) is currently a global pandemic and public health crisis. COVID‐19 causes significant morbidity and mortality with millions of deaths reported worldwide. 1
COVID‐19 vaccination represents a safe and effective way for disease prevention and mortality reduction. This public health emergency required urgent efforts globally to develop vaccines. More than 180 vaccine candidates using a wide range of technology platforms including nucleic acid (DNA and RNA), virus‐like particles, peptides, viral vectors (replicating and non‐replicating), recombinant protein, live attenuated virus and inactivated virus approaches are currently in development or have received emergency approval for use. 2
Establishing the safety of the COVID‐19 vaccines is crucial and plays an important role in gaining public trust for vaccinations since emergency approval is being granted without completing all phases of clinical trials. Speculations and reports about vaccine‐related side‐effects have arisen due to these very large‐scale vaccination programmes. Cutaneous adverse reactions to SARS‐CoV‐2 vaccinations are one of the most frequently reported adverse effects. 3 , 4
This study performed a systematic review and meta‐analysis of previously published studies to ascertain the prevalence of cutaneous adverse events associated with the COVID‐19 vaccines. Additionally, we summarized all clinical manifestations and therapeutic considerations, which may help guide clinicians with prevaccine counselling, prevention and management.
Methods
Data sources and search strategy
This study followed the Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) guidelines (Table S1). A systematic search was conducted on studies published from 1 January 2019 to 31 December 2021, in PubMed and Scopus databases. Search terms, such as “COVID‐19 vaccines”, “skin”, “cutaneous”, “derm*” and “rash” were used without any language restriction (Table S2). Records were managed by the Endnote X9.0 software to exclude duplicates. To identify missing studies, we scanned the reference lists for each selected article. Additional articles were obtained from manual searching.
Study selection
We included published studies that reported cases of COVID‐19 vaccine‐related cutaneous manifestations with no limit to the duration of follow‐up. The definition of adverse events following immunization (AEFI) by the World Health Organization (WHO) was employed. AEFI is regarded as any untoward medical occurrence, which follows immunization and which does not necessarily have a causal relationship with the usage of the vaccine. 5 Case reports, case series, case–control studies, retrospective/prospective cohort studies and randomized controlled trials were all eligible study designs. We excluded review articles and opinion articles that did not include original data and studies that reported on cases with insufficient information. Two authors (C.W. and J.T.) independently screened the title and abstract results from the initial search strategy. Comprehensive reviews of the full text of relevant articles were conducted using inclusion and exclusion criteria (Fig. 1). Disagreements were resolved by consensus or with the assistance of the third author (P.R.). The quantitative synthesis (meta‐analysis) included randomized controlled trials and observational studies that reported the prevalence of cutaneous manifestations following COVID‐19 vaccination. Case reports, case series, observational studies and randomized controlled trials that could not be analysed in terms of prevalence and used descriptive statistics to summarize the findings were excluded from the meta‐analysis.
Figure 1.
PRISMA study flow diagram.
Data extraction
Data extraction forms recorded details on the general information of studies (first author's surname, year of publication), study characteristics (country, study design, study phase), participant characteristics (age, sex, underlying disease), details of intervention (name of the vaccine, type of vaccine, manufacturer and dose of administration) and skin manifestations after vaccination (prevalence, clinical morphology, onset, duration and treatment).
Study quality assessment
The quality of the RCT was evaluated using the Jadad scale, 6 which ranges from 0 to 5, with a score of 3 or higher indicating a report of high quality. The risk of bias in observational studies was determined by the Newcastle–Ottawa quality assessment 7 where the maximum score is 9 and a score of 7 is the threshold denoting high quality (low risk of bias). The Joanna Briggs Institute (JBI) critical appraisal checklist 8 was used to assess the quality of case reports with a score of 0–8 and that of case series with a score of 0–10. Studies with a quality assessment score of 50% or higher (≥4 for case reports, ≥5 for case series) were included in the review. The level of evidence was assessed using the Oxford Centre for Evidence‐Based Medicine criteria. 9
Data synthesis and analysis
Quantitative synthesis
Odds ratios (ORs), pooled prevalence and 95% confidence intervals (95% CI) were used to summarize the weighted effect size for each study using the binary random‐effects model. Heterogeneity was assessed using the I 2 index and Q‐test P‐value. An I 2 index of ≥50% indicated medium to high heterogeneity. When heterogeneity was observed, it was investigated using subgroup analyses according to study design (randomized control trial versus observational study), type of skin manifestations (local injection site/near injection site reaction versus non‐injection site/generalized skin reaction), type of vaccination (inactivated SARS‐CoV‐2 versus mRNA‐based versus viral vector‐based), type of placebo control (aluminium hydroxide solution versus normal saline) and dose administration (first dose versus the second dose). Publication bias was formally assessed using the Egger test. All analyses were performed using Comprehensive Meta‐Analysis (version 2.0; Biostat, Englewood, NJ).
Qualitative analysis
Descriptive, categorical variables were reported as frequency and percentage, while continuous data were reported as mean (standard deviation [SD]) or median (range). All analyses were performed using STATA (version 15.1)
Results
Characteristics and quality of the studies
A total of 2181 publications were identified and screened for COVID‐19 vaccine‐related cutaneous manifestations using a database search and article reference lists (Fig. 1). Of these studies, 300 met the systematic review's inclusion criteria 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 , 156 , 157 , 158 , 159 , 160 , 161 , 162 , 163 , 164 , 165 , 166 , 167 , 168 , 169 , 170 , 171 , 172 , 173 , 174 , 175 , 176 , 177 , 178 , 179 , 180 , 181 , 182 , 183 , 184 , 185 , 186 , 187 , 188 , 189 , 190 , 191 , 192 , 193 , 194 , 195 , 196 , 197 , 198 , 199 , 200 , 201 , 202 , 203 , 204 , 205 , 206 , 207 , 208 , 209 , 210 , 211 , 212 , 213 , 214 , 215 , 216 , 217 , 218 , 219 , 220 , 221 , 222 , 223 , 224 , 225 , 226 , 227 , 228 , 229 , 230 , 231 , 232 , 233 , 234 , 235 , 236 , 237 , 238 , 239 , 240 , 241 , 242 , 243 , 244 , 245 , 246 , 247 , 248 , 249 , 250 , 251 , 252 , 253 , 254 , 255 , 256 , 257 , 258 , 259 , 260 , 261 , 262 , 263 , 264 , 265 , 266 , 267 , 268 , 269 , 270 , 271 , 272 , 273 , 274 , 275 , 276 , 277 , 278 , 279 , 280 , 281 , 282 , 283 , 284 , 285 , 286 , 287 , 288 , 289 , 290 , 291 , 292 , 293 , 294 , 295 , 296 , 297 , 298 , 299 , 300 , 301 , 302 , 303 , 304 , 305 , 306 , 307 , 308 , 309 (15 randomized control trials, 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 34 observational studies, 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 75 case series 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 and 176 case reports 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 , 156 , 157 , 158 , 159 , 160 , 161 , 162 , 163 , 164 , 165 , 166 , 167 , 168 , 169 , 170 , 171 , 172 , 173 , 174 , 175 , 176 , 177 , 178 , 179 , 180 , 181 , 182 , 183 , 184 , 185 , 186 , 187 , 188 , 189 , 190 , 191 , 192 , 193 , 194 , 195 , 196 , 197 , 198 , 199 , 200 , 201 , 202 , 203 , 204 , 205 , 206 , 207 , 208 , 209 , 210 , 211 , 212 , 213 , 214 , 215 , 216 , 217 , 218 , 219 , 220 , 221 , 222 , 223 , 224 , 225 , 226 , 227 , 228 , 229 , 230 , 231 , 232 , 233 , 234 , 235 , 236 , 237 , 238 , 239 , 240 , 241 , 242 , 243 , 244 , 245 , 246 , 247 , 248 , 249 , 250 , 251 , 252 , 253 , 254 , 255 , 256 , 257 , 258 , 259 , 260 , 261 , 262 , 263 , 264 , 265 , 266 , 267 , 268 , 269 , 270 , 271 , 272 , 273 , 274 , 275 , 276 , 277 , 278 , 279 , 280 , 281 , 282 , 283 , 284 , 285 , 286 , 287 , 288 , 289 , 290 , 291 , 292 , 293 , 294 , 295 , 296 , 297 , 298 , 299 , 300 , 301 , 302 , 303 , 304 , 305 , 306 , 307 , 308 , 309 ), while 32 studies 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 were included in the meta‐analysis (nine randomized control trials 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 and 23 observational studies 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 ). The characteristics of each study selected for the meta‐analysis are summarized in Table S3–S4. All studies were judged as meeting a high standard of quality (Tables S5–S8).
Prevalence of cutaneous adverse reactions following COVID‐19 vaccination
Thirty‐two studies 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 (23 observational studies 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 and intervention arms of 9 randomized control trials 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 ) resulting in 946 366 participants were included in the meta‐analysis for the pooled prevalence of overall cutaneous adverse reactions following COVID‐19 vaccination. There were seven studies (21.9%) on inactivated SARS‐CoV‐2 vaccines, 17 (53.1%) on mRNA‐based vaccines, 3 (9.4%) on viral vector‐based vaccines and 5 (15.6%) covering more than one platform. The pooled prevalence of overall cutaneous adverse reactions following COVID‐19 vaccination was 3.8% (95% CI, 2.7%–5.3%; I 2 = 99.77; Q‐test P < 0.001). (Table S9 illustrates the prevalence of overall cutaneous adverse reactions following COVID‐19 vaccination in each study.) The Egger test was not significant (P = 0.750), suggesting less publication bias.
Investigations of heterogeneity
Due to the substantial heterogeneity among studies, subgroup analyses by type of skin manifestations, dose administration, vaccine platforms and study design were performed. Skin reactions that were localized at the injection site or near the injection site were more common than non‐injection site or generalized skin reactions, with a pooled prevalence of 2.5% (95% CI, 1.4%–4.5%; I 2 = 99.85; Q‐test P < 0.001) vs. 1.6% (95% CI, 1.3%–2.0%; I 2 = 99.85; Q‐test P < 0.001). The rate of cutaneous adverse events was similar after each dose of the vaccine, with a pooled prevalence of 4.2% (95% CI, 2.8%–6.4%; I 2 = 99.85; Q‐test P < 0.001) for the first dose and 4.0% (95% CI, 2.1%–7.5%; I 2 = 99.85; Q‐test P < 0.001) for the second dose. COVID‐19 vaccines based on the mRNA platform had the highest prevalence of cutaneous adverse events at 6.9% (95% CI, 3.8%–12.3%; I 2 = 99.85; Q‐test P < 0.001) followed by viral vector‐based vaccines at 3.5% (95% CI, 0.2%–35.8%; I 2 = 99.78; Q‐test P < 0.001), and inactivated SARS‐CoV‐2 vaccine at 0.9% (95% CI, 0.1%–9.0%; I 2 = 99.32; Q‐test P < 0.001). The pooled prevalence from the 13 studies using multiple platforms was 2.4% (95% CI, 0.8%–6.7%; I 2 = 99.75; Q‐test P < 0.001). Observational studies reported a greater number of cutaneous adverse events with a pooled prevalence of 5.9% (95% CI, 3.8%–8.8%; I 2 = 99.83; Q‐test P < 0.001) compared to randomized control trials that reported a pooled prevalence of 1.1% (95% CI, 0.4%–3.4%; I 2 = 99.19; Q‐test P < 0.001) (Fig. 2).
Figure 2.
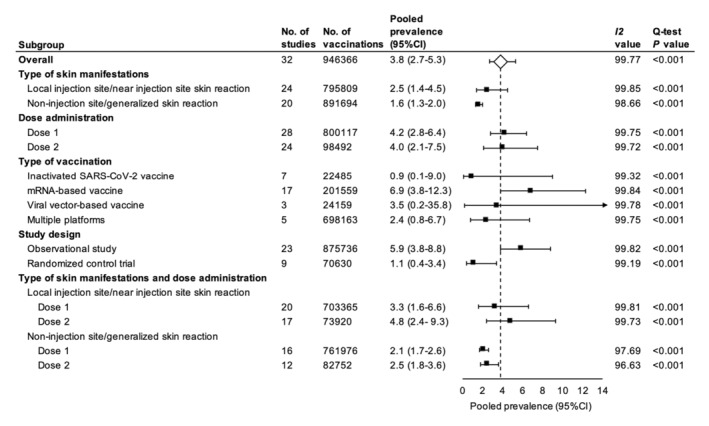
Forest plots of the pooled prevalence of cutaneous adverse events following COVID‐19 vaccination. *Square data markers represent prevalence rates. The diamond data marker represents the overall effect size based on included studies. Lines around the marker indicate 95% CIs. The arrow indicates that the upper confidence limit falls beyond the x‐axis.
Cutaneous adverse events following COVID‐19 vaccination compared to placebo
In the nine randomized control trials, 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 a total of 46 072 cases who received the COVID‐19 vaccine were compared to 41 401 controls who received a placebo. Aluminium hydroxide solution was used as a placebo in six studies, 10 , 11 , 12 while normal saline was used in three. 13 , 14 , 15 , 16 , 17 , 18 All vaccines and placebos were administered intramuscularly (IM). The pooled odds ratio of cutaneous adverse events following COVID‐19 vaccination between vaccine and placebo groups was 1.68 (95% CI, 0.47–5.95, P = 0.422; I 2 = 97.55; Q‐test P < 0.001). The Egger test was not significant (P = 0.240), suggesting less publication bias.
Investigations of heterogeneity
The pooled odds ratio was similar among studies using aluminium hydroxide solution as a placebo (pooled OR 1.78; 95% CI, 0.57–5.57; I 2 = 89.37; Q‐test P < 0.001) and those using normal saline (pooled OR 1.63; 95% CI, 0.19–14.29; I 2 = 94.64; Q‐test P < 0.001). Interestingly, when a subgroup analysis was performed on different platforms of the COVID‐19 vaccine, the mRNA‐based vaccine again had the highest rate of associated cutaneous adverse effects. Individuals who received the mRNA‐based vaccine were 7.2 times more likely to develop a cutaneous adverse event than those who received a placebo (95% CI, 6.35–8.19, P < 0.001; I 2 = 0; Q‐test P < 0.501). Local skin reactions to the mRNA vaccine at the injection site were more common than non‐injection site or generalized skin reactions (OR, 14.37; 95% CI, 11.97–17.25 vs. OR, 1.41; 95% CI 0.81–2.45). Second doses of mRNA caused injection site skin reactions 20.2 times more frequently than placebo (95% CI, 8.39–48.76, P < 0.001; I 2 = 17.08; Q‐test P < 0.299). In Fig. 3, the forest plot illustrates the cutaneous adverse events associated with each dose of the different types of vaccination.
Figure 3.
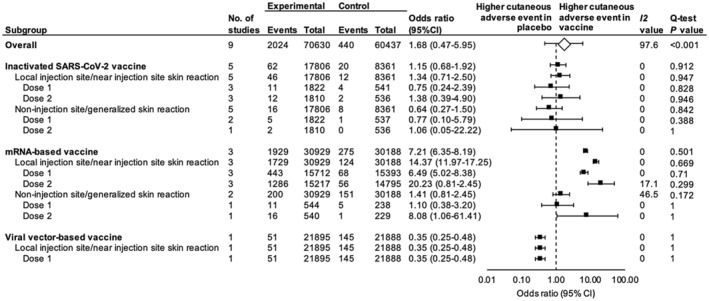
Forest plots of the pooled odds ratios for cutaneous adverse events in COVID‐19 vaccine recipients versus placebo. *Square data markers represent the odds ratios. The diamond data marker represents the overall effect size based on included studies. Lines around the marker indicate 95% CI.
Clinical manifestations and therapeutic considerations
From the systematic review, we identified 300 articles 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 , 156 , 157 , 158 , 159 , 160 , 161 , 162 , 163 , 164 , 165 , 166 , 167 , 168 , 169 , 170 , 171 , 172 , 173 , 174 , 175 , 176 , 177 , 178 , 179 , 180 , 181 , 182 , 183 , 184 , 185 , 186 , 187 , 188 , 189 , 190 , 191 , 192 , 193 , 194 , 195 , 196 , 197 , 198 , 199 , 200 , 201 , 202 , 203 , 204 , 205 , 206 , 207 , 208 , 209 , 210 , 211 , 212 , 213 , 214 , 215 , 216 , 217 , 218 , 219 , 220 , 221 , 222 , 223 , 224 , 225 , 226 , 227 , 228 , 229 , 230 , 231 , 232 , 233 , 234 , 235 , 236 , 237 , 238 , 239 , 240 , 241 , 242 , 243 , 244 , 245 , 246 , 247 , 248 , 249 , 250 , 251 , 252 , 253 , 254 , 255 , 256 , 257 , 258 , 259 , 260 , 261 , 262 , 263 , 264 , 265 , 266 , 267 , 268 , 269 , 270 , 271 , 272 , 273 , 274 , 275 , 276 , 277 , 278 , 279 , 280 , 281 , 282 , 283 , 284 , 285 , 286 , 287 , 288 , 289 , 290 , 291 , 292 , 293 , 294 , 295 , 296 , 297 , 298 , 299 , 300 , 301 , 302 , 303 , 304 , 305 , 306 , 307 , 308 , 309 (15 randomized control trials, 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 34 observational studies, 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 75 case series 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 and 176 case reports 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 , 156 , 157 , 158 , 159 , 160 , 161 , 162 , 163 , 164 , 165 , 166 , 167 , 168 , 169 , 170 , 171 , 172 , 173 , 174 , 175 , 176 , 177 , 178 , 179 , 180 , 181 , 182 , 183 , 184 , 185 , 186 , 187 , 188 , 189 , 190 , 191 , 192 , 193 , 194 , 195 , 196 , 197 , 198 , 199 , 200 , 201 , 202 , 203 , 204 , 205 , 206 , 207 , 208 , 209 , 210 , 211 , 212 , 213 , 214 , 215 , 216 , 217 , 218 , 219 , 220 , 221 , 222 , 223 , 224 , 225 , 226 , 227 , 228 , 229 , 230 , 231 , 232 , 233 , 234 , 235 , 236 , 237 , 238 , 239 , 240 , 241 , 242 , 243 , 244 , 245 , 246 , 247 , 248 , 249 , 250 , 251 , 252 , 253 , 254 , 255 , 256 , 257 , 258 , 259 , 260 , 261 , 262 , 263 , 264 , 265 , 266 , 267 , 268 , 269 , 270 , 271 , 272 , 273 , 274 , 275 , 276 , 277 , 278 , 279 , 280 , 281 , 282 , 283 , 284 , 285 , 286 , 287 , 288 , 289 , 290 , 291 , 292 , 293 , 294 , 295 , 296 , 297 , 298 , 299 , 300 , 301 , 302 , 303 , 304 , 305 , 306 , 307 , 308 , 309 ), resulting in a total of 44 582 cases that reported on COVID‐19 vaccine‐related cutaneous manifestations. The most frequent cutaneous manifestations were acute local injection site reactions (n = 32 173, 72.16%), followed by rash or unspecified skin eruption (n = 6158, 13.81%), urticaria or angio‐oedema (n = 2913, 6.53%), pruritus without skin lesion (n = 1009, 2.26%), delayed large local reactions (n = 847, 1.90%), maculopapular rash (n = 221, 0.50%), herpes zoster (n = 182, 0.41%), oral blister/ulcer/vesicle (n = 162, 0.36%), pityriasis rosea/pityriasis rosea‐like lesion (n = 108, 0.24%), vesiculobullous lesion (n = 86, 0.19%), petechiae/purpura/ecchymosis (n = 60, 0.14%), chilblains/chilblains‐like lesion (n = 58, 0.13%) and vasculitis/vasculitis‐like lesion (n = 46, 0.10%). Additional less common cutaneous manifestations are included in Table 1.
Table 1.
Cutaneous manifestations following COVID‐19 vaccination (n = number of cases)
| Cutaneous manifestations | Total (n = 44 582) | mRNA vaccine (n = 27 655) | Viral vector vaccine(n = 15 113) | Inactivated viral vaccine (n = 1112) | Protein subunit vaccine (n = 2) | Unidentified vaccine (n = 700) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | |
| Acute injection site reaction | 32 173 | 72.17 | 19 106 | 69.09 | 12 110 | 80.13 | 382 | 34.35 | 0 | 0.00 | 575 | 82.14 |
| Rash/unspecified skin eruption | 6158 | 13.81 | 3908 | 14.13 | 1803 | 11.93 | 404 | 36.33 | 0 | 0.00 | 43 | 6.14 |
| Urticaria and/or angio‐oedema | 2913 | 6.53 | 1812 | 6.55 | 920 | 6.09 | 165 | 14.84 | 0 | 0.00 | 16 | 2.29 |
| Pruritus without skin lesion | 1009 | 2.26 | 986 | 3.57 | 6 | 0.04 | 4 | 0.36 | 0 | 0.00 | 13 | 1.86 |
| Delayed large local reactions | 847 | 1.90 | 820 | 2.97 | 24 | 0.16 | 0 | 0.00 | 0 | 0.00 | 3 | 0.43 |
| Maculopapular rash | 221 | 0.50 | 162 | 0.59 | 36 | 0.24 | 17 | 1.53 | 0 | 0.00 | 6 | 0.86 |
| Herpes zoster | 182 | 0.41 | 134 | 0.49 | 28 | 0.19 | 12 | 1.08 | 0 | 0.00 | 8 | 1.14 |
| Oral blister/ulcer/vesicle | 162 | 0.36 | 121 | 0.44 | 38 | 0.25 | 0 | 0.00 | 0 | 0.00 | 3 | 0.43 |
| PR/PR‐like lesion | 108 | 0.24 | 65 | 0.24 | 12 | 0.08 | 26 | 2.34 | 0 | 0.00 | 5 | 0.71 |
| Vesiculobullous lesion | 86 | 0.19 | 76 | 0.28 | 7 | 0.05 | 3 | 0.27 | 0 | 0.00 | 0 | 0.00 |
| Petechiae/purpura/ecchymosis | 60 | 0.14 | 19 | 0.07 | 22 | 0.15 | 19 | 1.71 | 0 | 0.00 | 0 | 0.00 |
| Chilblains/chilblains‐like lesion | 58 | 0.13 | 43 | 0.16 | 7 | 0.05 | 3 | 0.27 | 0 | 0.00 | 5 | 0.71 |
| Vasculitis/vasculitic‐like lesion | 46 | 0.10 | 27 | 0.10 | 9 | 0.06 | 9 | 0.81 | 0 | 0.00 | 1 | 0.14 |
| CLE | 42 | 0.09 | 40 | 0.15 | 2 | 0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Eczema/eczematous lesion | 40 | 0.09 | 17 | 0.06 | 5 | 0.03 | 18 | 1.62 | 0 | 0.00 | 0 | 0.00 |
| Papulovesicular lesion | 35 | 0.08 | 19 | 0.07 | 9 | 0.06 | 7 | 0.63 | 0 | 0.00 | 0 | 0.00 |
| Erythromelalgia | 34 | 0.08 | 34 | 0.12 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Erythema multiforme | 31 | 0.07 | 18 | 0.07 | 4 | 0.03 | 4 | 0.36 | 0 | 0.00 | 5 | 0.71 |
| DIR to dermal filler | 31 | 0.07 | 30 | 0.11 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.14 |
| Psoriasis | 30 | 0.07 | 18 | 0.07 | 10 | 0.07 | 2 | 0.18 | 0 | 0.00 | 0 | 0.00 |
| Oral white/red plaque | 27 | 0.06 | 22 | 0.08 | 5 | 0.03 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Anaphylaxis | 23 | 0.05 | 3 | 0.01 | 5 | 0.03 | 15 | 1.35 | 0 | 0.00 | 0 | 0.00 |
| Contact dermatitis | 18 | 0.04 | 17 | 0.06 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.14 |
| Herpes simplex virus infection | 16 | 0.04 | 9 | 0.03 | 7 | 0.05 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Residual skin discoloration | 15 | 0.03 | 15 | 0.05 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Angular cheilitis | 14 | 0.03 | 13 | 0.05 | 1 | 0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Lichen planus | 14 | 0.03 | 11 | 0.04 | 1 | 0.01 | 0 | 0.00 | 1 | 50.00 | 1 | 0.14 |
| Bullous pemphigoid | 13 | 0.03 | 11 | 0.04 | 1 | 0.01 | 1 | 0.09 | 0 | 0.00 | 0 | 0.00 |
| SCARs | 12 | 0.03 | 4 | 0.01 | 5 | 0.03 | 1 | 0.09 | 1 | 50.00 | 1 | 0.14 |
| Burning gingiva | 10 | 0.02 | 10 | 0.04 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Alopecia | 11 | 0.03 | 5 | 0.02 | 4 | 0.03 | 0 | 0.00 | 0 | 0.00 | 2 | 0.29 |
| ITP | 8 | 0.02 | 6 | 0.02 | 2 | 0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Papulosquamous/pityriasiform lesion | 8 | 0.02 | 0 | 0.00 | 0 | 0.00 | 8 | 0.72 | 0 | 0.00 | 0 | 0.00 |
| Pemphigus Vulgaris | 7 | 0.02 | 5 | 0.02 | 2 | 0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Acne/acneiform lesion | 7 | 0.02 | 1 | <0.01 | 1 | 0.01 | 5 | 0.45 | 0 | 0.00 | 0 | 0.00 |
| Sweet's syndrome | 6 | 0.01 | 3 | 0.01 | 3 | 0.02 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Fixed drug eruption | 6 | 0.01 | 5 | 0.02 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.14 |
| PRP/PRP‐like lesion | 5 | 0.01 | 3 | 0.01 | 2 | 0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Hailey–Hailey | 5 | 0.01 | 5 | 0.02 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| SDRIFE | 4 | 0.01 | 2 | 0.01 | 1 | 0.01 | 1 | 0.09 | 0 | 0.00 | 0 | 0.00 |
| Vitiligo | 4 | 0.01 | 3 | 0.01 | 0 | 0.00 | 1 | 0.09 | 0 | 0.00 | 0 | 0.00 |
| Reaction to breast implant | 4 | 0.01 | 3 | 0.01 | 1 | 0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Rust‐like discoloration | 4 | 0.01 | 4 | 0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Alopecia areata | 4 | 0.01 | 1 | <0.01 | 3 | 0.02 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Erythema nodosum | 4 | 0.01 | 0 | 0.00 | 2 | 0.01 | 0 | 0.00 | 0 | 0.00 | 2 | 0.29 |
| Livedo reticularis | 4 | 0.01 | 3 | 0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.14 |
| Toxic erythema | 4 | 0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 4 | 0.57 |
| Granuloma annulare | 3 | 0.01 | 2 | 0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.14 |
| Skin necrosis | 3 | 0.01 | 2 | 0.01 | 1 | 0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Hay fever | 3 | 0.01 | 3 | 0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Still's disease | 2 | <0.01 | 1 | <0.01 | 1 | 0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Nicolau syndrome | 2 | <0.01 | 2 | 0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Multisystem inflammatory syndrome | 2 | <0.01 | 1 | <0.01 | 1 | 0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Radiation recall dermatitis | 2 | <0.01 | 0 | 0.00 | 1 | 0.01 | 1 | 0.09 | 0 | 0.00 | 0 | 0.00 |
| Exfoliation of the skin of the palms | 2 | <0.01 | 2 | 0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Local skin reaction on BCG scar | 2 | <0.01 | 2 | 0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Papulopustular lesion | 2 | <0.01 | 1 | <0.01 | 1 | 0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Lymphomatoid drug reaction | 2 | <0.01 | 1 | <0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.14 |
| Palmar erythema | 2 | <0.01 | 0 | 0.00 | 0 | 0.00 | 2 | 0.18 | 0 | 0.00 | 0 | 0.00 |
| Pityriasis lichenoides | 2 | <0.01 | 2 | 0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Eruptive cherry haemangiomatosis | 1 | <0.01 | 1 | <0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Erythema annulare centrifugum | 1 | <0.01 | 0 | 0.00 | 1 | 0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Livedo racemosa | 1 | <0.01 | 1 | <0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Pseudolymphoma | 1 | <0.01 | 1 | <0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Purpura annularis telangiectodes of Majocchi | 1 | <0.01 | 1 | <0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Rowell's syndrome | 1 | <0.01 | 1 | <0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Viral warts | 1 | <0.01 | 0 | 0.00 | 1 | 0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Psoriasiform eruption | 1 | <0.01 | 1 | <0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Darier's disease | 1 | <0.01 | 0 | 0.00 | 1 | 0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Erythema migrans | 1 | <0.01 | 1 | <0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Lichen striatus | 1 | <0.01 | 1 | <0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Primary cutaneous CD30‐positive lymphoproliferative disorder | 1 | <0.01 | 1 | <0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Eschar | 1 | <0.01 | 1 | <0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Lipschütz ulcer | 1 | <0.01 | 0 | 0.00 | 1 | 0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Acute localized exanthematous pustulosis | 1 | <0.01 | 0 | 0.00 | 1 | 0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Superficial venous thrombosis | 1 | <0.01 | 0 | 0.00 | 1 | 0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Eosinophilic cellulitis | 1 | <0.01 | 1 | <0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Pigmented purpuric dermatosis | 1 | <0.01 | 1 | <0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Serum sickness‐like reaction | 1 | <0.01 | 0 | 0.00 | 0 | 0.00 | 1 | 0.09 | 0 | 0.00 | 0 | 0.00 |
| Eosinophilic dermatosis | 1 | <0.01 | 0 | 0.00 | 1 | 0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Linear IgA bullous dermatosis | 1 | <0.01 | 0 | 0.00 | 1 | 0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Exuberant lichenoid eruption | 1 | <0.01 | 0 | 0.00 | 1 | 0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Multibacillary leprosy | 1 | <0.01 | 1 | <0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Subcutaneous nodule | 1 | <0.01 | 1 | <0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Insect bite | 1 | <0.01 | 0 | 0.00 | 1 | 0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Morphoea | 1 | <0.01 | 1 | <0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Raynaud | 1 | <0.01 | 1 | <0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Sarcoidosis | 1 | <0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.14 |
| Facial oedema | 1 | <0.01 | 1 | <0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Folliculitis | 1 | <0.01 | 0 | 0.00 | 0 | 0.00 | 1 | 0.09 | 0 | 0.00 | 0 | 0.00 |
CLE, cutaneous lupus erythematosus; DIR, delayed inflammatory reactions; ITP, idiopathic thrombocytopenic purpura; PR, pityriasis rosea; PRP, pityriasis rubra pilaris; SCARs, severe cutaneous adverse reactions; SDRIFE, systemic drug‐related intertriginous and flexural exanthema.
A total of 23 cases of anaphylaxis were reported from the systematic review with 12 cases coming from one study. 123 This study covered data from Thailand for a three‐month period during the initial rollout of the Sinovac inactivated vaccine that estimated a rate of one case of anaphylaxis per 2.2 million doses. 310
Demographic data, clinical presentations and therapeutic considerations of patients with COVID‐19 vaccine‐related cutaneous manifestations are summarized in Table 2.
Table 2.
Demographic data, clinical presentations and therapeutic considerations of patients with COVID‐19 vaccine‐related cutaneous manifestations (n = number of cases)
| Cutaneous manifestations, n | Age (years), Mean (±SD) | Male/Female/ND ratio | Onset (days), Mean (±SD) | Duration (days), Mean (±SD) | Dose, n (%) | Local symptoms, n (%) | Systemic symptoms, n (%) | Treatments, n (%) |
|---|---|---|---|---|---|---|---|---|
| Injection site reaction (n = 55) | 51.15 (15.10) | 4/23/28 | 2.50 (2.90) | 5.50 (2.12) | Dose 1: 36 (65.38), dose 2: 17 (30.77), both dose: 2 (3.85) | Pruritus 36 (65.45), pain 32 (58.18), burning 25 (45.45), no symptom 13 (23.6) | Fever 30 (54.55), headache 5 (9.09), myalgia 3 (5.45), no symptom 21 (38.18) | Topical corticosteroids 32 (58.18), antihistamine 30 (54.55), systemic corticosteroids 1 (1.82), spontaneous remission 30 (54.55) |
| Delayed injection site reaction (n = 82) | 51.05 (13.86) | 10/72 | 6.72 (3.88) | 5.13 (4.64) | Dose 1: 40 (48.78), dose 2: 12 (14.63), dose 3: 1 (1.21), both dose 1&2: 28 (34.14), not report: 1 (1.21) | Pruritus 23 (28.05), pain 25 (30.49), burning 4 (4.88), no symptom 15 (18.29) | Fever 17 (20.73), myalgia 16 (19.51), headache 11 (13.41), fatigue 12 (14.63), lymphadenopathy 6 (7.32), no symptom 36 (43.90) | Topical corticosteroids 27 (32.93), antihistamine 20 (24.39), systemic corticosteroids 5 (6.09), antibiotics 4 (4.87), antipyretic drugs 3 (3.66), analgesic drugs 2 (2.44), spontaneous remission 18 (21.95) |
| Urticaria and/or angio‐oedema (n = 46) | 40.67 (12.92) | 10/36 | 6.46 (16.97) | 24.28 (34.38) | Dose 1: 22 (47.82), dose 2: 8 (17.39), both dose: 10 (21.74), not report: 6 (13.04) | Pruritus 45 (97.82), no symptom 1 (2.17) | Fever 2 (4.35), myalgia 2 (4.35), fatigue 1 (2.17), diarrhoea 1 (2.17), no symptom 34 (73.91) | Oral antihistamine 12 (26.09), intravenous antihistamine 9 (19.57), systemic corticosteroids 8 (17.39), topical corticosteroids 2 (4.35), anti‐IgE monoclonal antibody 1 (2.17), spontaneous remission 20 (43.48) |
| Herpes zoster (n = 72) | 56.25 (18.17) | 35/37 | 7.76 (6.38) | 12.46 (6.81) | Dose 1: 37 (51.39), dose 2: 24 (33.33), both dose: 2 (2.78), not report: 9 (12.50) | Pain 36 (50.00), pruritus 15 (20.83), burning 11 (15.28), dysaesthesia 3 (4.17), no symptom 13 (18.06) | Myalgia 4 (5.56), fatigue 3 (4.17), fever 2 (2.78), headache 1 (1.39), no symptom 38 (52.78) | Antiviral agents 57 (79.17), anticonvulsants 12 (16.67), analgesic drugs 9 (12.50), systemic corticosteroids 5 (6.94), antibiotics 3 (4.17), topical corticosteroids 1 (1.38), spontaneous remission 3 (4.17) |
| PR/PR‐like lesion (n = 58) | 42.98 (13.03) | 26/32 | 9.64 (6.11) | 49.00 (24.09) | Dose 1: 32 (55.17), dose 2: 22 (37.93), both dose: 4 (6.90) | Pruritus 32 (55.17), no symptom 26 (44.83) | NA | Topical corticosteroids 7 (12.07), antihistamine 5 (8.62), systemic corticosteroids 1 (1.72), spontaneous remission 40 (68.97) |
| Psoriasis (n = 29) (new onset n = 5, flares of pre‐existing n = 24) | 62.24 (13.80) | 15/14 | 9.87 (8.03) | 14.50 (11.96) | Dose 1: 8 (27.59), dose 2: 19 (65.52), both dose: 2 (6.90) | Pruritus 18 (62.07), no symptom 11 (37.93) | Fever 4 (13.79), fatigue 3 (10.34), arthralgia 2 (6.89), 1 myalgia (3.44), no symptom 19 (65.51) | Vitamin D3 analogs 9 (31.03), topical corticosteroids 7 (24.13), biologics 5 (17.24), phototherapy 5 (17.24), antihistamine 4 (13.79), calcineurin inhibitors 2 (6.90), systemic corticosteroids 2 (6.90), vitamin A derivatives 1 (3.45) |
| Cutaneous vasculitis (n = 25) (new onset n = 24, flares of pre‐existing n = 1) | 53.24 (22.75) | 8/17 | 6.35 (6.24) | 15.21 (13.70) | Dose 1: 11 (44.00), dose 2: 8 (32.00), both dose 1&2: 3 (12.00), both dose 1&2&3: 1 (4.00), not report: 2 (8.00) | Pruritus 6 (24.00), pain 4 (16.00), burning 3 (12.00), no symptom 5 (20.00) | Arthralgia 5 (20.00), fever 4 (16.00), myalgia 4 (16.00), fatigue 2 (8.00), diarrhoea 1 (4.00), abdominal pain 1 (4.00), haematuria 1 (4.00), no symptom 3 (12.00) | Systemic corticosteroid 12 (48.00), topical corticosteroids 9 (36.00), antihistamine 6 (24.00), analgesic drugs 3 (12.00), antibiotics 2 (8.00), spontaneous remission 5 (20.00) |
| Chilblains/chilblains‐like lesion (n = 17) | 51.71 (14.11) | 9/7/1 | 4.94 (3.99) | 23.88 (13.93) | Dose 1: 7 (41.17), dose 2: 3 (17.65), both dose: 5 (29.41), not report: 2 (11.76) | Pruritus 4 (23.53), pain 4 (23.53), oedema 2 (11.76), burning 1 (5.88), no symptom 4 (23.53) | Fatigue 1 (5.88), headache 1 (5.88), no symptom 3 (17.65) | Topical corticosteroids 7 (58.33), antihistamine 1 (5.88), spontaneous remission 8 (47.06) |
| BP (n = 13) (new onset n = 9, flares of pre‐existing n = 4) | 77.77 (6.27) | 6/6/1 | 11.47 (10.89) | 55.50 (48.79) | Dose 1: 7 (53.85), dose 2: 2 (15.38), both dose 4 (30.77) | Pruritus 4 (30.17), no symptom 9 (69.23) | NA | Systemic corticosteroids 11 (84.6), topical corticosteroids 5 (38.46), antihistamine 2 (15.38), spontaneous remission 1 (7.69) |
| PV (n = 7) (new onset n = 4, flares of pre‐existing n = 3) | 57.71 (21.14) | 5/1/1 | 8.29 (9.74) | 117.00 (66.47) | Dose 1: 2 (28.57), dose 2: 2 (28.57), both dose: 1 (14.29), not report: 2 (28.57) | Pain 3 (42.86), no symptom 4 (57.14) | NA | Systemic corticosteroids 7 (100.00), rituximab 2 (28.57), mycophenolate mofetil 1 (14.29), azathioprine 1 (14.29) |
| Severe cutaneous adverse reactions (n = 11) | 49.55 (17.01) | 5/6 | 9.34 (15.38) | 20.83 (9.56) | Dose 1: 6 (54.54), dose 2: 2 (18.18), both dose: 1 (9.09), not report: 2 (18.18) | Pruritus 3 (27.27), pain 2 (18.18), burning 1 (9.09), no symptom 5 (45.45) | Fever 7 (63.63), myalgia 2 (18.18), fatigue 1 (9.09), lymphadenopathy 1 (9.09) | Topical corticosteroids 7 (63.63), systemic corticosteroid 5 (45.45), antihistamine 4 (36.36), analgesic drugs 2 (18.18), antipyretic drugs 2 (18.18), antibiotics 1 (9.09) |
| Erythema multiforme (n = 11) | 60.27 (19.31) | 3/7/1 | 5.00 (3.33) | 20.63 (19.31) | Dose 1: 7 (63.63), dose 2: 4 (36.36) | Pruritus 3 (27.27), pain 1 (9.09), no symptom 7 (63.63) | Fever 2 (18.18), myalgia 2 (18.18), fatigue 2 (18.18) | Topical corticosteroids 9 (81.81), systemic corticosteroid 5 (45.45), antihistamine 4 (36.36), spontaneous remission 4 (36.36) |
| Cutaneous lupus erythematosus (n = 8) | 47.88 (22.50) | 2/6 | 14.00 (12.96) | 15.75 (6.70) | Dose 1: 5 (62.5), dose 2: 2 (25.00), both dose: 1 (12.50) | Pruritus 3 (37.50), burning 2 (25.00), pain 1 (12.50), no symptom 2 (25.00) | Fatigue 5 (62.50), myalgia 2 (25.00), arthralgia 2 (25.00), lymphadenopathy 1 (12.50) | Systemic corticosteroids 6 (75.00), hydroxychloroquine 3 (37.50), topical corticosteroids 3 (37.50) |
| Delayed inflammatory reactions to dermal fillers (n = 7) | 42.00 (11.26) | 0/7 | 2.58 (2.69) | 11.14 (15.31) | Dose 1: 4 (57.14), dose 2: 2 (28.57), both dose: 1 (14.29) | Tender 4 (57.14), pain 2 (28.57), paraesthesia 1 (14.29) | Headache 2 (28.57), flu‐like symptoms 2 (28.57), slurred speech 2 (28.57), no symptom 3 (42.86) | Systemic corticosteroids 4 (57.14), hyaluronidase 2 (28.57), antihistamine 2 (28.57), ACE inhibitor 1 (14.28), antibiotics 1 (14.28), intralesional 5‐FU 1 (14.28) |
| Lichen planus (n = 6) (new onset n = 4, flares of pre‐existing n = 2) | 58.53 (7.20) | 5/0/1 | 4.17 (3.50) | NA | Dose 1: 2 (33.33), dose 2: 1 (16.67), both dose: 1 (16.67), not report: 2 (33.33) | Pruritus 3 (50.00), pain 1 (16.67), no symptom 2 (33.33) | NA | Topical corticosteroids 4 (66.67), systemic corticosteroids 1 (16.67), spontaneous remission 2 (33.33) |
| Sweet's syndrome (n = 6) | 57.50 (15.33) | 2/3/1 | 5.50 (3.62) | 29.88 (40.92) | Dose 1: 5 (83.33), not report: 1 (16.67) | Pain 2 (33.33), dysaesthesia 1 (16.67), no symptom 3 (50.00) | Fever 3 (50.00), headache 1 (16.67), dizziness 1 (16.67), arthralgia 1 (16.67), no symptom 2 (33.33) | Systemic corticosteroids 6 (100.00), topical corticosteroids 1 (16.67), antiviral agents 2 (33.33), antibiotics 2 (33.33) |
| Pityriasis rubra pilaris (PRP) and PRP‐like lesion (n = 5) | 66.00 (11.64) | 2/3 | 9.00 (7.07) | NA | Dose 1: 4 (80.00), both dose: 1 (20.00) | Pruritus 1 (20.00), no symptom 4 (80.00) | NA | Topical corticosteroids 3 (60.00), acitretin 2 (40.00), systemic corticosteroids 1 (20.00), methotrexate 1 (20.00) |
| Fixed drug eruption (n = 5) | 47.20 (23.18) | 0/5 | 11.67 (5.09) | 5.00 a | Dose 2: 2 (40.00), both dose: 3 (60.00) | Pruritus 2 (40.00), no symptom 3 (60.00) | NA | Topical corticosteroids 5 (100.00), antihistamine 2 (40.00) |
| Symmetrical drug‐related intertriginous and flexural exanthema (n = 4) | 52.25 (27.96) | 3/1 | 15.25 (18.68) | 27.00 (5.20) | Dose 2: 4 (100.00) | Pruritus 2 (50.00), pain 2 (50.00), burning 2 (50.00), no symptom 2 (50.00) | NA | Systemic corticosteroids 4 (100.00), topical corticosteroids 1 (25.00), antihistamine 1 (25.00) |
| Vitiligo (n = 4) (new onset n = 3, flares of pre‐existing n = 1) | 41.25 (17.21) | 2/2 | 8.00 (5.57) | NA | Dose 1: 1 (25.00), dose 2: 2 (50.00), both dose: 1 (25.00) | NA | NA | Phototherapy 2 (50.00), topical calcineurin inhibitor 1 (25.00), topical tacrolimus 1 (25.00) |
BP = bullous pemphigoid; PV = pemphigus vulgaris; ND, not identified; NA, not available.
This information was reported in only one case.
Discussion
Results from our meta‐analysis showed that cutaneous adverse reactions to COVID‐19 vaccines are common with the pooled prevalence of global cutaneous adverse events following COVID‐19 of 3.8% (95% CI, 2.7%–5.3%). This observed prevalence is close to previous estimates from vaccines. 311 A wide range of prevalence rates across studies, ranging from 0.04% 33 to 25.4%, 39 have been previously reported. We conducted a subgroup analysis and found that vaccine platform and study design may influence the prevalence of cutaneous adverse reactions, as cutaneous adverse events are much more prevalent in mRNA platforms than in other platforms, and observational studies reported a greater number of cutaneous adverse events than randomized control trials. A high degree of heterogeneity between studies was found to be present, which may be explained by different adverse event assessment methods, population and follow‐up time. Some studies may have underreported minor adverse events. As a result, caution is needed when generalizing our global findings to different subpopulations.
From the systematic review, this report has described various cutaneous manifestations associated with the COVID‐19 vaccine including injection site reactions, 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 which were the most common cutaneous adverse reaction in almost all vaccines, uncommon adverse reactions such as delayed inflammatory reactions to tissue filler, 73 , 127 , 228 , 279 flares of pre‐existing dermatoses (e.g. psoriasis, 103 , 133 , 137 , 141 , 215 , 239 , 248 , 252 , 282 , 312 bullous pemphigoid, 63 , 111 pemphigus vulgaris, 111 , 121 lichen planus, 85 , 164 , 245 cutaneous vasculitis, 163 vitiligo 293 and cutaneous lupus erythematosus 156 , 188 , 236 ) and viral reactivation (e.g. herpes zoster 74 , 81 , 85 , 92 , 99 , 100 , 102 , 107 , 108 , 113 , 116 , 131 , 191 , 268 ). In addition, more severe but rare reactions such as anaphylaxis 15 , 37 , 38 , 40 , 41 , 44 , 123 and severe cutaneous adverse reactions (SCARs) 136 , 185 , 231 , 271 have been reported as well. There is also some debate over whether some of the reported cutaneous adverse reactions following COVID‐19 vaccination were causal or temporally coincidental. We hope that our study may pave the way for further research to confirm any causal relationship. In terms of practical implications, almost all of the cutaneous adverse reactions are mild and self‐limiting or treatable with corticosteroids or antihistamines. Clinicians should emphasize to patients that the majority of cutaneous adverse reactions are not contraindications to subsequent vaccination. The only contraindication is severe allergic reactions to a previous dose of the COVID‐19 vaccine including anaphylaxis. While an immediate allergic reaction such as acute‐onset urticaria occurs within four hours of vaccine administration, clinicians should share decision‐making with patients to determine whether to administer vaccinations in full dose or graded doses or change platforms. It has previously been suggested that vaccine centres prepare for any possibility of immediate severe adverse reactions. 313
The results of our meta‐analysis showed that the prevalence of cutaneous adverse events following COVID‐19 immunization was substantially different between vaccine platforms, with the mRNA‐based vaccine exhibiting the highest prevalence of cutaneous adverse events. Previous research has indicated that cutaneous adverse reactions to the mRNA vaccines such as chilblains, erythromelalgia and pityriasis‐rosea‐like exanthems may mimic dermatologic manifestations of natural SARS‐CoV‐2 infection. As a result, dermatologic manifestations are more likely to occur as a result of an immune response rather than direct viral effects. 54 , 314 , 315 We therefore hypothesized that the observed differences in cutaneous adverse effects between different platforms of COVID‐19 vaccines may be explained by the differences in their immune‐mediated mechanisms. The mRNA‐based vaccines may elicit more robust immune responses, resulting in a higher prevalence of cutaneous adverse events.
Although no current research has been conducted to shed light on the distinct immuno‐dermatological mechanisms underlying each type of COVID‐19 vaccine, numerous studies on the cutaneous manifestations of the COVID‐19 vaccine support the pathophysiological hypothesis that the vaccine immunogenicity results in altered levels of chemokines and cytokines, which activate a variety of key players in the innate and adaptive immune systems. 316 At least four distinct types of cutaneous reactions have been proposed. The first type of reaction is a classical antiviral response characterized by a predominantly cellular immune response pattern involving CD8+ T cells and macrophages with a Th1‐polarized T‐helper cell profile. Interferon‐γ (IFN‐γ), tumour necrosis factor‐α (TNF‐α) and various interleukins, such as IL‐2 and IL‐6, are key mediators, which cause skin reactions such as cutaneous lupus erythematosus, lichen planus, maculopapular rash, pityriasis rosea and erythema multiforme. Second, numerous vaccine components, including adjuvants such as aluminium, may act as haptens, inducing a predominantly Th2‐polarized inflammatory response with high pro‐inflammatory cytokines IL‐4 and IL‐13. The allergic reaction might be immediately due to an IgE hypersensitivity reaction or delayed onset since mast cell degranulation occurs in certain individuals. Classic manifestations of this reaction are urticaria, atopic dermatitis, acute injection site reactions and autoimmune bullous dermatoses, whereas delayed injection‐site reactions (DIRs), also known as ‘COVID‐arms’, and distant reactions involving cosmetic dermal fillers are possible manifestations of delayed hypersensitivity. 317 , 318 Third, in susceptible individuals, skin‐resident memory T cells may be activated as a result of an active innate immune system, resulting in a Th17/Th22‐predominant environment, which causes skin reactions such as psoriasis, acute generalized exanthematous pustulosis and Sweet's syndrome. 319 Fourth, vaccine components may trigger inflammatory responses resulting in macrophages/histiocytes and granulomatous reactions. 316 , 319
Our data synthesis has several strengths. To our knowledge, this is the first meta‐analysis on the global prevalence of cutaneous adverse reactions following COVID‐19 vaccination, which included all high‐quality studies of randomized control trials and observational studies. The majority of these studies used a large sample size, ensuring that findings had adequate statistical power. Additionally, case reports and case series that could not be analysed in terms of prevalence were summarized using descriptive statistics in order to capture all characteristics of cutaneous manifestations, including uncommon reactions or flares of pre‐existing chronic inflammatory dermatoses. The study does have several limitations. First, some reports lacked additional details about individual patients, resulting in insufficient characteristic descriptions of some cutaneous manifestations. Second, although we performed subgroup analyses on the pooled prevalence of cutaneous adverse events, our meta‐analysis still had a high degree of heterogeneity. Caution is needed when generalizing our global findings to different subpopulations. Finally, in most reports the causal relationship between the skin manifestations and the vaccination was not confirmed by in vivo or in vitro testing. The diagnosis was made based on the occurrence of the skin reactions following the vaccination and that other possible causes were ruled out. Therefore, it was very challenging to confirm that the skin reactions were, in fact, induced by the vaccines, not a coincidence.
Conclusions
Cutaneous adverse reactions to COVID‐19 vaccines are common with a global prevalence rate of 3.8%. Various cutaneous manifestations have been reported with the mRNA‐based vaccine showing a higher prevalence than other platforms. The majority of cutaneous adverse reactions are mild and self‐limiting or treatable with corticosteroids or antihistamines. The only contraindication to subsequent vaccination is severe allergic reactions to a previous dose of the COVID‐19 vaccine, including anaphylaxis, which rarely occurs. In addition, COVID‐19 vaccination may be associated with flares of pre‐existing dermatoses and delayed inflammatory reactions to tissue filler. It is recommended patients with a history of allergies, pre‐existing inflammatory skin conditions, or scheduled for filler injection should receive additional precounselling and monitoring and that vaccine centre be prepared for even rare adverse events. A better understanding of potential side‐effects may strengthen public confidence among persons or communities reticent to receive new vaccine technologies.
Conflict of Interest
None reported.
Supporting information
Table S1 PRISMA 2020 Checklist
Table S2 Search strategy
Table S3 Characteristics and quality assessment of the randomized controlled trials included for meta‐analysis
Table S4 Characteristics and quality assessment of the observational studies included for meta‐analysis
Table S5 Quality assessment and level of evidence for case reports
Table S6 Quality assessment and level of evidence for case series
Table S7 Quality assessment and level of evidence for observational studies
Table S8 Quality assessment and level of evidence for randomized controlled trials
Table S9 Prevalence of overall cutaneous manifestations following COVID‐19 vaccination in each study
Acknowledgement
The authors thank the Skin and Allergy Research Unit for their support.
Conflict of interest
None.
Funding sources
None.
Data availability statement
The data that support the findings of this study are available in the supplementary material of this article.
References
- 1. Guan W, Ni Z, Hu Y et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pormohammad A, Zarei M, Ghorbani S et al. Efficacy and safety of COVID‐19 vaccines: a systematic review and meta‐analysis of randomized clinical trials. Vaccines (Basel) 2021; 9: 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gambichler T, Boms S, Susok L et al. Cutaneous findings following COVID‐19 vaccination: review of world literature and own experience. J Eur Acad Dermatol Venereol 2022; 36: 172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kaur RJ, Dutta S, Bhardwaj P et al. Adverse events reported from COVID‐19 vaccine trials: a systematic review. Indian J Clin Biochem 2021; 36: 427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization . (2018). Causality assessment of an adverse event following immunization (AEFI): user manual for the revised WHO classification, 2nd ed. World Health Organization. https://apps.who.int/iris/bitstream/handle/10665/259959/9789241513654‐eng.pdf License: CC BY‐NC‐SA 3.0 IGO.
- 6. Jadad AR, Moore RA, Carroll D et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17: 1–2. [DOI] [PubMed] [Google Scholar]
- 7. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P: The Newcastle‐Ottawa Scale for assessing the quality of nonrandomized studies in meta‐analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 8. Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. Chapter 7: Systematic reviews of etiology and risk. 2017. Joanna Briggs Institute Reviewer's Manual [Internet] Available from: https://reviewersmanualjoannabriggsorg.
- 9. Howick J, Chalmers I, Glasziou P, et al. Oxford Centre for Evidence‐Based Medicine Working Group. The Oxford 2011 Levels of Evidence 2011. http://www.cebm.net/index.aspx?o=5653.
- 10. Wu Z, Hu Y, Xu M et al. Safety, tolerability, and immunogenicity of an inactivated SARS‐CoV‐2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double‐blind, placebo‐controlled, phase 1/2 clinical trial. Lancet Infect Dis 2021; 21: 803–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pu J, Yu Q, Yin Z et al. The safety and immunogenicity of an inactivated SARS‐CoV‐2 vaccine in Chinese adults aged 18‐59 years: A phase I randomized, double‐blinded, controlled trial. Vaccine 2021; 39: 2746–2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang Y, Zeng G, Pan H et al. Safety, tolerability, and immunogenicity of an inactivated SARS‐CoV‐2 vaccine in healthy adults aged 18‐59 years: a randomised, double‐blind, placebo‐controlled, phase 1/2 clinical trial. Lancet Infect Dis 2021; 21: 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tanriover MD, Doğanay HL, Akova M et al. Efficacy and safety of an inactivated whole‐virion SARS‐CoV‐2 vaccine (CoronaVac): interim results of a double‐blind, randomised, placebo‐controlled, phase 3 trial in Turkey. Lancet 2021; 398: 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xia S, Zhang Y, Wang Y et al. Safety and immunogenicity of an inactivated COVID‐19 vaccine, BBIBP‐CorV, in people younger than 18 years: a randomised, double‐blind, controlled, phase 1/2 trial. Lancet Infect Dis 2022; 22: 196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baden LR, El Sahly HM, Essink B et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med 2021; 384: 403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chu L, McPhee R, Huang W et al. A preliminary report of a randomized controlled phase 2 trial of the safety and immunogenicity of mRNA‐1273 SARS‐CoV‐2 vaccine. Vaccine 2021; 39: 2791–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Walsh EE, Frenck RW Jr, Falsey AR et al. Safety and immunogenicity of two RNA‐based Covid‐19 vaccine candidates. N Engl J Med 2020; 383: 2439–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sadoff J, Gray G, Vandebosch A et al. Safety and efficacy of single‐dose Ad26.COV2.S vaccine against Covid‐19. N Engl J Med 2021; 384: 2187–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Polack FP, Thomas SJ, Kitchin N et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med 2020; 383: 2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Al Kaabi N, Zhang Y, Xia S et al. Effect of 2 inactivated SARS‐CoV‐2 vaccines on symptomatic COVID‐19 infection in adults: a randomized clinical trial. JAMA 2021; 326: 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ella R, Reddy S, Jogdand H et al. Safety and immunogenicity of an inactivated SARS‐CoV‐2 vaccine, BBV152: interim results from a double‐blind, randomised, multicentre, phase 2 trial, and 3‐month follow‐up of a double‐blind, randomised phase 1 trial. Lancet Infect Dis 2021; 21: 950–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Logunov DY, Dolzhikova IV, Shcheblyakov DV et al. Safety and efficacy of an rAd26 and rAd5 vector‐based heterologous prime‐boost COVID‐19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021; 397: 671–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jackson LA, Anderson EJ, Rouphael NG et al. An mRNA vaccine against SARS‐CoV‐2 ‐ preliminary report. N Engl J Med 2020; 383: 1920–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xia S, Zhang Y, Wang Y et al. Safety and immunogenicity of an inactivated SARS‐CoV‐2 vaccine, BBIBP‐CorV: a randomised, double‐blind, placebo‐controlled, phase 1/2 trial. Lancet Infect Dis 2021; 21: 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abu‐Hammad O, Alduraidi H, Abu‐Hammad S et al. Side effects reported by Jordanian healthcare workers who received COVID‐19 vaccines. Vaccines (Basel) 2021; 9: 557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Al Bahrani S, Albarrak A, Alghamdi OA et al. Safety and reactogenicity of the ChAdOx1 (AZD1222) COVID‐19 vaccine in Saudi Arabia. Int J Infect Dis 2021; 110: 359–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Robinson LB, Fu X, Hashimoto D et al. Incidence of cutaneous reactions after messenger RNA COVID‐19 vaccines. JAMA Dermatol 2021; 157: 1000–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Modenese A, Paduano S, Bargellini A et al. Neutralizing anti‐SARS‐CoV‐2 antibody titer and reported adverse effects, in a sample of Italian nursing home personnel after two doses of the BNT162b2 vaccine administered four weeks apart. Vaccines (Basel) 2021; 9: 652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nittner‐Marszalska M, Rosiek‐Biegus M, Kopeć A et al. Pfizer‐BioNTech COVID‐19 vaccine tolerance in allergic versus non‐allergic individuals. Vaccines (Basel) 2021; 9: 553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Waissengrin B, Agbarya A, Safadi E, Padova H, Wolf I. Short‐term safety of the BNT162b2 mRNA COVID‐19 vaccine in patients with cancer treated with immune checkpoint inhibitors. Lancet Oncol 2021; 22: 581–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Menni C, Klaser K, May A et al. Vaccine side‐effects and SARS‐CoV‐2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis 2021; 21: 939–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fernandez‐Nieto D, Hammerle J, Fernandez‐Escribano M et al. Skin manifestations of the BNT162b2 mRNA COVID‐19 vaccine in healthcare workers. 'COVID‐arm': a clinical and histological characterization. J Eur Acad Dermatol Venereol 2021; 35: e425–e427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang G, Zhu L, Zhu Y et al. Safety survey by clinical pharmacists on COVID‐19 vaccination from a single center in China. Hum Vaccin Immunother 2021; 17: 2863–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jęśkowiak I, Wiatrak B, Grosman‐Dziewiszek P, Szeląg A. The Incidence and Severity of Post‐Vaccination Reactions after Vaccination against COVID‐19. Vaccines (Basel) 2021; 9: 502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bookstein Peretz S, Regev N, Novick L et al. Short‐term outcome of pregnant women vaccinated with BNT162b2 mRNA COVID‐19 vaccine. Ultrasound Obstet Gynecol 2021; 58: 450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lim SM, Chan HC, Santosa A, Quek SC, Liu EHC, Somani J. Safety and side effect profile of Pfizer‐BioNTech COVID‐19 vaccination among healthcare workers: A tertiary hospital experience in Singapore. Ann Acad Med Singap 2021; 50: 703–711. [PubMed] [Google Scholar]
- 37. Rerknimitr P, Puaratanaarunkon T, Wongtada C et al. Cutaneous adverse reactions from 35,229 doses of Sinovac and AstraZeneca COVID‐19 vaccination: a prospective cohort study in healthcare workers. J Eur Acad Dermatol Venereol 2022; 36: e158–e161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Durmaz K, Aykut Temiz S, Metin Z, Dursun R, Abdelmaksoud A. Allergic and cutaneous reactions following inactivated SARS‐CoV‐2 vaccine (CoronaVac(®) ) in healthcare workers. Clin Exp Dermatol 2022; 47: 171–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Im JH, Kim E, Lee E et al. Adverse events with the Pfizer‐BioNTech COVID‐19 vaccine among Korean healthcare workers. Yonsei Med J 2021; 62: 1162–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vanegas E, Robles‐Velasco K, Osorio MF et al. Adverse reactions following COVID‐19 vaccination: An Ecuadorian experience. Ann Med Surg (Lond) 2021; 72: 103044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Blumenthal KG, Robinson LB, Camargo CA Jr et al. Acute allergic reactions to mRNA COVID‐19 vaccines. JAMA 2021; 325: 1562–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zavala‐Flores E, Salcedo‐Matienzo J, Quiroz‐Alva A, Berrocal‐Kasay A. Side effects and flares risk after SARS‐CoV‐2 vaccination in patients with systemic lupus erythematosus. Clin Rheumatol 2021; 41: 1349–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Solomon Y, Eshete T, Mekasha B, Assefa W. COVID‐19 vaccine: side effects after the first dose of the Oxford AstraZeneca vaccine among health professionals in low‐income country: ethiopia. J Multidiscip Healthc 2021; 14: 2577–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shavit R, Maoz‐Segal R, Iancovici‐Kidon M et al. Prevalence of allergic reactions after Pfizer‐BioNTech COVID‐19 vaccination among adults with high allergy risk. JAMA Netw Open 2021; 4: e2122255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cuschieri S, Borg M, Agius S, Souness J, Brincat A, Grech V. Adverse reactions to Pfizer‐BioNTech vaccination of healthcare workers at Malta's state hospital. Int J Clin Pract 2021; 75: e14605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pourani MR, Shahidi Dadras M, Salari M, Diab R, Namazi N, Abdollahimajd F. Cutaneous adverse events related to COVID‐19 vaccines: A cross‐sectional questionnaire‐based study of 867 patients. Dermatol Ther 2022; 35: e15223. [DOI] [PubMed] [Google Scholar]
- 47. Grieco T, Maddalena P, Sernicola A et al. Cutaneous adverse reactions after COVID‐19 vaccines in a cohort of 2740 Italian subjects: An observational study. Dermatol Ther 2021; 34: e15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Riad A, Pokorná A, Attia S, Klugarová J, Koščík M, Klugar M. Prevalence of COVID‐19 vaccine side effects among healthcare workers in the Czech Republic. J Clin Med 2021; 10: 1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Riad A, Pokorná A, Mekhemar M et al. Safety of ChAdOx1 nCoV‐19 vaccine: independent evidence from two EU states. Vaccines (Basel) 2021; 9: 673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kadali RAK, Janagama R, Peruru S, Malayala SV. Side effects of BNT162b2 mRNA COVID‐19 vaccine: A randomized, cross‐sectional study with detailed self‐reported symptoms from healthcare workers. Int J Infect Dis 2021; 106: 376–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Klugar M, Riad A, Mekhemar M et al. Side effects of mRNA‐based and viral vector‐based COVID‐19 vaccines among German healthcare workers. Biology (Basel) 2021; 10: 752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Riad A, Hocková B, Kantorová L et al. Side effects of mRNA‐based COVID‐19 vaccine: nationwide phase IV study among healthcare workers in Slovakia. Pharmaceuticals (Basel) 2021; 14: 873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang J, Hou Z, Liu J et al. Safety and immunogenicity of COVID‐19 vaccination in patients with non‐alcoholic fatty liver disease (CHESS2101): A multicenter study. J Hepatol 2021; 75: 439–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McMahon DE, Amerson E, Rosenbach M et al. Cutaneous reactions reported after Moderna and Pfizer COVID‐19 vaccination: A registry‐based study of 414 cases. J Am Acad Dermatol 2021; 85: 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Català A, Muñoz‐Santos C, Galván‐Casas C et al. Cutaneous reactions after SARS‐CoV‐2 vaccination: a cross‐sectional Spanish nationwide study of 405 cases. Br J Dermatol 2022; 186: 142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kadali RAK, Janagama R, Peruru S et al. Non‐life‐threatening adverse effects with COVID‐19 mRNA‐1273 vaccine: A randomized, cross‐sectional study on healthcare workers with detailed self‐reported symptoms. J Med Virol 2021; 93: 4420–4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Papadimitriou I, Bakirtzi K, Sotiriou E, Vakirlis E, Hatzibougias D, Ioannides D. Delayed localized hypersensitivity reactions to COVID‐19 mRNA vaccines: a 6‐month retrospective study. Clin Exp Dermatol 2022; 47: 157–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Freeman EE, Sun Q, McMahon DE et al. Skin reactions to COVID‐19 vaccines: An American Academy of Dermatology/International League of Dermatological Societies registry update on reaction location and COVID vaccine type. J Am Acad Dermatol 2021; 86: 165–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hoff NP, Freise NF, Schmidt AG et al. Delayed skin reaction after mRNA‐1273 vaccine against SARS‐CoV‐2: a rare clinical reaction. Eur J Med Res 2021; 26: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Coto‐Segura P, Fernández‐Prada M, Mir‐Bonafé M et al. Vesiculobullous skin reactions induced by COVID‐19 mRNA vaccine: report of four cases and review of the literature. Clin Exp Dermatol 2022; 47: 141–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rodríguez‐Jiménez P, Chicharro P, Cabrera LM et al. Varicella‐zoster virus reactivation after SARS‐CoV‐2 BNT162b2 mRNA vaccination: Report of 5 cases. JAAD Case Rep 2021; 12: 58–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Corbeddu M, Diociaiuti A, Vinci MR et al. Transient cutaneous manifestations after administration of Pfizer‐BioNTech COVID‐19 Vaccine: an Italian single‐centre case series. J Eur Acad Dermatol Venereol 2021; 35: e483–e485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Juay L, Chandran NS. Three cases of vesiculobullous non‐IgE‐mediated cutaneous reactions to tozinameran (Pfizer‐BioNTech COVID‐19 vaccine). J Eur Acad Dermatol Venereol 2021; 35: e855–e857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tomayko MM, Damsky W, Fathy R et al. Subepidermal blistering eruptions, including bullous pemphigoid, following COVID‐19 vaccination. J Allergy Clin Immunol 2021; 148: 750–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Patruno C, Napolitano M, Stingeni L, Fabbrocini G. Skin rashes after SARS‐CoV‐2 vaccine: which relationship, if any? Immun Inflamm Dis 2021; 9: 622–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lam M, Egail M, Bedlow AJ, Tso S. Ribonucleic acid COVID‐19 vaccine‐associated cutaneous adverse drug events: a case series of two patients. Clin Exp Dermatol 2021; 46: 1131–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Psichogiou M, Samarkos M, Mikos N, Hatzakis A. Reactivation of Varicella Zoster virus after vaccination for SARS‐CoV‐2. Vaccines (Basel) 2021; 9: 572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mazzatenta C, Piccolo V, Pace G, Romano I, Argenziano G, Bassi A. Purpuric lesions on the eyelids developed after BNT162b2 mRNA COVID‐19 vaccine: another piece of SARS‐CoV‐2 skin puzzle? J Eur Acad Dermatol Venereol 2021; 35: e543–e545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Busto‐Leis JM, Servera‐Negre G, Mayor‐Ibarguren A et al. Pityriasis rosea, COVID‐19 and vaccination: new keys to understand an old acquaintance. J Eur Acad Dermatol Venereol 2021; 35: e489–e491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cyrenne BM, Al‐Mohammedi F, DeKoven JG, Alhusayen R. Pityriasis rosea‐like eruptions following vaccination with BNT162b2 mRNA COVID‐19 Vaccine. J Eur Acad Dermatol Venereol 2021; 35: e546–e548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sidlow JS, Reichel M, Lowenstein EJ. Localized and generalized urticarial allergic dermatitis secondary to SARS‐CoV‐2 vaccination in a series of 6 patients. JAAD Case Rep 2021; 14: 13–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tammaro A, Adebanjo GAR, Parisella FR, De Marco G, Rello J. Local reactions to the second dose of the BNT162 COVID‐19 vaccine. Dermatol Ther 2021; 34: e15000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Michon A. Hyaluronic acid soft tissue filler delayed inflammatory reaction following COVID‐19 vaccination ‐ A case report. J Cosmet Dermatol 2021; 20: 2684–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. van Dam CS, Lede I, Schaar J, Al‐Dulaimy M, Rösken R, Smits M. Herpes zoster after COVID vaccination. Int J Infect Dis 2021; 111: 169–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Papamanoli A, Thorne M, Psevdos G. Delayed skin rash after receiving SARS‐CoV‐2 mRNA moderna vaccine. Infect Dis Clin Pract (Baltim Md) 2021; 29: e262–e263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Johnston MS, Galan A, Watsky KL, Little AJ. Delayed localized hypersensitivity reactions to the moderna COVID‐19 vaccine: a case series. JAMA Dermatol 2021; 157: 716–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Blumenthal KG, Freeman EE, Saff RR et al. Delayed large local reactions to mRNA‐1273 vaccine against SARS‐CoV‐2. N Engl J Med 2021; 384: 1273–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kim JE, Lee H, Paik SS, Moon JY, Yoon HJ, Kim SH. Delayed cutaneous reaction to ChAdOx1 nCoV‐19 vaccine: Is it an 'AstraZeneca arm'? J Eur Acad Dermatol Venereol 2021; 35: e711–e714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yu JN, Angeles CB, Lim HG, Chavez C, Roxas‐Rosete C. Cutaneous reactions to inactivated SARS‐CoV‐2 vaccine and ChAdOx1‐S (recombinant) vaccine against SARS‐CoV‐2: a case series from the Philippines. J Eur Acad Dermatol Venereol 2021; 35: e841–e845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Akdaş E, Öğüt B, Erdem Ö, Öztaş MO, İlter N. Cutaneous reactions following CoronaVac COVID‐19 vaccination: a case series of six healthcare workers from a single centre. J Eur Acad Dermatol Venereol 2021; 35: e861–e864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Farinazzo E, Ponis G, Zelin E et al. Cutaneous adverse reactions after m‐RNA COVID‐19 vaccine: early reports from Northeast Italy. J Eur Acad Dermatol Venereol 2021; 35: e548–e551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Soyfer V, Gutfeld O, Shamai S, Schlocker A, Merimsky O. COVID‐19 vaccine‐induced radiation recall phenomenon. Int J Radiat Oncol Biol Phys 2021; 110: 957–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zafar M, Ewnetu B, Ahmed S, Iqbal U, Whitehead M. COVID‐19 vaccination‐induced rash: does the choice of vaccine matter? Cureus 2021; 13: e15490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Shah SRA, Dolkar S, Mathew J, Vishnu P. COVID‐19 vaccination associated severe immune thrombocytopenia. Exp Hematol Oncol 2021; 10: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Burlando M, Russo R, Cozzani E, Parodi A. COVID‐19 "second wave" and vaccines: the dermatologists' perspective. Int J Dermatol 2021; 60: 889–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lindgren AL, Austin AH, Welsh KM. COVID arm: delayed hypersensitivity reactions to SARS‐CoV‐2 vaccines misdiagnosed as cellulitis. J Prim Care Community Health 2021; 12: 21501327211024431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Larson V, Seidenberg R, Caplan A, Brinster NK, Meehan SA, Kim RH. Clinical and histopathological spectrum of delayed adverse cutaneous reactions following COVID‐19 vaccination. J Cutan Pathol 2022; 49: 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lopatynsky‐Reyes EZ, Acosta‐Lazo H, Ulloa‐Gutierrez R, Ávila‐Aguero ML, Chacon‐Cruz E. BCG Scar local skin inflammation as a novel reaction following mRNA COVID‐19 vaccines in two international healthcare workers. Cureus 2021; 13: e14453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Temiz SA, Abdelmaksoud A, Dursun R, Vestita M. Acral chilblain‐like lesions following inactivated SARS‐CoV‐2 vaccination. Int J Dermatol 2021; 60: 1152–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Pasternack R, Pohjavaara S. A skin reaction with rust‐like discolouration to mRNA COVID‐19 vaccine. J Eur Acad Dermatol Venereol 2021; 35: e737–e738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Holmes GA, Desai M, Limone B et al. A case series of cutaneous COVID‐19 vaccine reactions at Loma Linda University Department of Dermatology. JAAD Case Rep 2021; 16: 53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lee C, Cotter D, Basa J, Greenberg HL. 20 Post‐COVID‐19 vaccine‐related shingles cases seen at the Las Vegas Dermatology clinic and sent to us via social media. J Cosmet Dermatol 2021; 20: 1960–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wei N, Fishman M, Wattenberg D, Gordon M, Lebwohl M. "COVID arm": A reaction to the Moderna vaccine. JAAD Case Rep 2021; 10: 92–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sechi A, Pierobon E, Pezzolo E et al. Abrupt onset of Sweet syndrome, pityriasis rubra pilaris, pityriasis lichenoides et varioliformis acuta and erythema multiforme: unravelling a possible common trigger, the COVID‐19 vaccine. Clin Exp Dermatol 2022; 47: 437–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Temiz SA, Abdelmaksoud A, Dursun R, Durmaz K, Sadoughifar R, Hasan A. Pityriasis rosea following SARS‐CoV‐2 vaccination: A case series. J Cosmet Dermatol 2021; 20: 3080–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Weitgasser L, Mahrhofer M, Schoeller T. Potential immune response to breast implants after immunization with COVID‐19 vaccines. Breast 2021; 59: 76–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Mehta H, Handa S, Malhotra P et al. Erythema nodosum, zoster duplex and pityriasis rosea as possible cutaneous adverse effects of Oxford‐AstraZeneca COVID‐19 vaccine: report of three cases from India. J Eur Acad Dermatol Venereol 2022; 36: e16–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Juárez Guerrero A, Domínguez Estirado A, Crespo Quirós J, Rojas‐Pérez‐Ezquerra P. Delayed cutaneous reactions after the administration of mRNA vaccines against COVID‐19. J Allergy Clin Immunol Pract 2021; 9: 3811–3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Bernardini N, Skroza N, Mambrin A et al. Herpes zoster ophthalmicus in two women after Pfizer‐BioNTech (BNT162b2) vaccine. J Med Virol 2021; 94: 817–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Koumaki D, Krueger‐Krasagakis SE, Papadakis M et al. Herpes zoster viral infection after AZD1222 and BNT162b2 coronavirus disease 2019 mRNA vaccines: a case series. J Eur Acad Dermatol Venereol 2022; 36: e85–e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Hai J, Shawa H, Kim‐Lim P et al. Systemic drug‐related intertriginous and flexural exanthema induced by the Pfizer‐BioNTech COVID‐19 vaccine: A report of 2 cases. JAAD Case Rep 2021; 18: 57–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Mohta A, Arora A, Srinivasa R, Mehta RD. Recurrent herpes zoster after COVID‐19 vaccination in patients with chronic urticaria being treated with cyclosporine‐A report of 3 cases. J Cosmet Dermatol 2021; 20: 3384–3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Nagrani P, Jindal R, Goyal D. Onset/flare of psoriasis following the ChAdOx1 nCoV‐19 Corona virus vaccine (Oxford‐AstraZeneca/Covishield): Report of two cases. Dermatol Ther 2021; 34: e15085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Sato T. Five Japanese cases of delayed large local reactions to coronavirus disease 2019 vaccines. J Dermatol 2021; 48: e558–e559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Brooks SG, Alhusayen R, Piguet V, Croitoru D. Pfizer/BioNTech‐associated perniosis in two young adults with re‐challenge evidence. J Eur Acad Dermatol Venereol 2022; 36: e84–e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Leasure AC, Cowper SE, McNiff J, Cohen JM. Generalized eczematous reactions to the Pfizer‐BioNTech COVID‐19 vaccine. J Eur Acad Dermatol Venereol 2021; 35: e716–e717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Vastarella M, Picone V, Martora F, Fabbrocini G. Herpes zoster after ChAdOx1 nCoV‐19 vaccine: a case series. J Eur Acad Dermatol Venereol 2021; 35: e845–e846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Alpalhão M, Filipe P. Herpes Zoster following SARS‐CoV‐2 vaccination – a series of four cases. J Eur Acad Dermatol Venereol 2021; 35: e750–e752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Gregoriou S, Kleidona IA, Tsimpidakis A, Nicolaidou E, Stratigos A, Rigopoulos D. 'COVID vaccine arm' may present after both mRNA vaccines vaccination. J Eur Acad Dermatol Venereol 2021; 35: e867–e868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Marcantonio‐Santa Cruz OY, Vidal‐Navarro A, Pesqué D, Giménez‐Arnau AM, Pujol RM, Martin‐Ezquerra G. Pityriasis rosea developing after COVID‐19 vaccination. J Eur Acad Dermatol Venereol 2021; 35: e721–e722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Damiani G, Pacifico A, Pelloni F, Iorizzo M. The first dose of COVID‐19 vaccine may trigger pemphigus and bullous pemphigoid flares: is the second dose therefore contraindicated? J Eur Acad Dermatol Venereol 2021; 35: e645–e647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Revilla‐Nebreda D, Roncero‐Riesco M, Santos‐Briz Á, Medina‐Migueláñez M, Segurado‐Tostón N, Román‐Curto C. New‐onset acral lesions on hands after administration of mRNA‐1273 vaccine against SARS‐CoV‐2: clinical images and histopathological study of three cases. J Eur Acad Dermatol Venereol 2021; 35: e747–e749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Palanivel JA. Herpes zoster after COVID‐19 vaccination‐Can the vaccine reactivate latent zoster virus? J Cosmet Dermatol 2021; 20: 3376–3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Bostan E, Elmas L, Yel B, Yalici‐Armagan B. Exacerbation of plaque psoriasis after inactivated and BNT162b2 mRNA COVID‐19 vaccines: A report of two cases. Dermatol Ther 2021; 34: e15110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Vaccaro M, Bertino L, Squeri R et al. Early atypical injection‐site reactions to COVID‐19 vaccine: a case series. J Eur Acad Dermatol Venereol 2022; 36: e24–e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Agarwal A, Panda M, Behera BK, Jena AK. Benign cutaneous reactions post‐COVID‐19 vaccination: A case series of 16 patients from a tertiary care center in India. J Cosmet Dermatol 2022; 21: 30–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Picone V, Martora F, Fabbrocini G, Marano L. "Covid arm": Abnormal side effect after Moderna COVID‐19 vaccine. Dermatol Ther 2022; 35: e15197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Martora F, Fabbrocini G, Marasca C. Pityriasis rosea after Moderna mRNA‐1273 vaccine: A case series. Dermatol Ther 2022; 35: e15225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Pesqué D, Lopez‐Trujillo E, Marcantonio O, Giménez‐Arnau AM, Pujol RM. New‐onset and exacerbations of psoriasis after mRNA COVID‐19 vaccines: two sides of the same coin? J Eur Acad Dermatol Venereol 2022; 36: e80–e81. [DOI] [PubMed] [Google Scholar]
- 120. Sy MY, Fromm E, Doan L, Rojek N, Brandt AU. Nicolau syndrome after glatiramer acetate injection in close proximity to administration of SARS‐CoV‐2 mRNA vaccine. Neurol Neuroimmunol Neuroinflamm 2022; 9: e1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Hatami P, Balighi K, Nicknam Asl H, Aryanian Z. COVID vaccination in patients under treatment with rituximab: A presentation of two cases from Iran and a review of the current knowledge with a specific focus on pemphigus. Dermatol Ther 2022; 35: e15216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Triwatcharikorn J, Puaratana‐Arunkon T, Punyaratabandhu P et al. Acute urticaria alone after CoronaVac COVID‐19 vaccination should not be a contraindication for revaccination. Clin Exp Dermatol 2021; 47: 735–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Laisuan W, Wongsa C, Chiewchalermsri C et al. CoronaVac COVID‐19 vaccine‐induced anaphylaxis: clinical characteristics and revaccination outcomes. J Asthma Allergy 2021; 14: 1209–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Niebel D, Wilhelmi J, De Vos L et al. Annular plaques mimicking Rowell's syndrome in the course of coronavirus disease 2019 mRNA vaccines: An overlooked phenomenon? J Dermatol 2022; 49: 151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Armoni‐Weiss G, Sheffer‐Levi S, Horev L et al. Exacerbation of Hailey‐Hailey disease following SARS‐CoV‐2 vaccination. Acta Derm Venereol 2021; 101: adv00554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Sandhu S, Bhatnagar A, Kumar H et al. Leukocytoclastic vasculitis as a cutaneous manifestation of ChAdOx1 nCoV‐19 corona virus vaccine (recombinant). Dermatol Ther 2021; 34: e15141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Munavalli GG, Guthridge R, Knutsen‐Larson S, Brodsky A, Matthew E, Landau M. COVID‐19/SARS‐CoV‐2 virus spike protein‐related delayed inflammatory reaction to hyaluronic acid dermal fillers: a challenging clinical conundrum in diagnosis and treatment. Arch Dermatol Res 2022; 314: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Bencharattanaphakhi R, Rerknimitr P. Sinovac COVID‐19 vaccine‐induced cutaneous leukocytoclastic vasculitis. JAAD Case Rep 2021; 18: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Annabi E, Dupin N, Sohier P et al. Rare cutaneous adverse effects of COVID‐19 vaccines: a case series and review of the literature. J Eur Acad Dermatol Venereol 2021; 35: e847–e850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Rossi A, Magri F, Michelini S et al. Recurrence of alopecia areata after covid‐19 vaccination: A report of three cases in Italy. J Cosmet Dermatol 2021; 20: 3753–3757. [DOI] [PubMed] [Google Scholar]
- 131. Özdemir AK, Kayhan S, Çakmak SK. Herpes zoster after inactivated SARS‐CoV‐2 vaccine in two healthy young adults. J Eur Acad Dermatol Venereol 2021; 35: e846–e847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ciccarese G, Drago F, Rebora A, Parodi A. Two cases of papulo‐pustular rosacea‐like eruptions following COVID‐19 vaccinations. J Eur Acad Dermatol Venereol 2021; 35: e868–e870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Sotiriou E, Tsentemeidou A, Bakirtzi K, Lallas A, Ioannides D, Vakirlis E. Psoriasis exacerbation after COVID‐19 vaccination: a report of 14 cases from a single centre. J Eur Acad Dermatol Venereol 2021; 35: e857–e859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. King ER, Towner E. A Case of Immune Thrombocytopenia After BNT162b2 mRNA COVID‐19 Vaccination. Am J Case Rep 2021; 22: e931478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Santovito LS, Pinna G. A case of reactivation of varicella‐zoster virus after BNT162b2 vaccine second dose? Inflamm Res 2021; 70: 935–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Lospinoso K, Nichols CS, Malachowski SJ, Mochel MC, Nutan F. A case of severe cutaneous adverse reaction following administration of the Janssen Ad26.COV2.S COVID‐19 vaccine. JAAD Case Rep 2021; 13: 134–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Onsun N, Kaya G, Işık BG, Güneş B. A generalized pustular psoriasis flare after CoronaVac COVID‐19 vaccination: Case report. Health Promot Perspect 2021; 11: 261–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Aksu SB, Öztürk GZ. A rare case of shingles after COVID‐19 vaccine: is it a possible adverse effect? Clin Exp Vaccine Res 2021; 10: 198–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Torrealba‐Acosta G, Martin JC, Huttenbach Y et al. Acute encephalitis, myoclonus and Sweet syndrome after mRNA‐1273 vaccine. BMJ Case Rep 2021; 14: e243173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Agaronov A, Makdesi C, Hall CS. Acute generalized exanthematous pustulosis induced by Moderna COVID‐19 messenger RNA vaccine. JAAD Case Rep 2021; 16: 96–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Perna D, Jones J, Schadt CR. Acute generalized pustular psoriasis exacerbated by the COVID‐19 vaccine. JAAD Case Rep 2021; 17: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Restivo V, Candore G, Barrale M et al. Allergy to polyethilenglicole of anti‐SARS CoV2 vaccine recipient: a case report of young adult recipient and the management of future exposure to SARS‐CoV2. Vaccines (Basel) 2021; 9: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Capassoni M, Ketabchi S, Cassisa A et al. AstraZeneca (AZD1222) COVID‐19 vaccine‐associated adverse drug event: A case report. J Med Virol 2021; 93: 5718–5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Abi Zeid Daou C, Natout MA, El Hadi N. Biphasic anaphylaxis after exposure to the first dose of Pfizer‐BioNTech COVID‐19 mRNA vaccine. J Med Virol 2021; 93: 6027–6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Kong J, Cuevas‐Castillo F, Nassar M et al. Bullous drug eruption after second dose of mRNA‐1273 (Moderna) COVID‐19 vaccine: Case report. J Infect Public Health 2021; 14: 1392–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Akinosoglou K, Tzivaki I, Marangos M. Covid‐19 vaccine and autoimmunity: Awakening the sleeping dragon. Clin Immunol 2021; 226: 108721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Wong CY, Rios EJ. Cutaneous hypersensitivity reaction with acute hepatitis following COVID‐19 vaccine. JAAD Case Rep 2021; 16: 44–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Vassallo C, Boveri E, Brazzelli V et al. Cutaneous lymphocytic vasculitis after administration of COVID‐19 mRNA vaccine. Dermatol Ther 2021; 34: e15076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. López‐Valle A, Falkenhain‐López D, Arranz CR. Cutaneous reaction to BNT162b2 mRNA COVID‐19 vaccine. Int J Dermatol 2021; 60: 891–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Berry CT, Eliliwi M, Gallagher S et al. Cutaneous small vessel vasculitis following single‐dose Janssen Ad26.COV2.S vaccination. JAAD Case Rep 2021; 15: 11–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Ramessur R, Saffar N, Czako B, Agarwal A, Batta K. Cutaneous thrombosis associated with skin necrosis following Oxford‐AstraZeneca COVID‐19 vaccination. Clin Exp Dermatol 2021; 46: 1610–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Elbæk MV, Vinding GR, Jemec GBE. Darier's disease flare following COVID‐19 vaccine. Case Rep Dermatol 2021; 13: 432–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Sprute R, Schumacher S, Pauls M, Pauls W, Cornely OA. Delayed Cutaneous hypersensitivity reaction to vaxzevria (ChAdOx1‐S) vaccine against SARS‐CoV‐2. Drugs R D 2021; 21: 371–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Grobusch MP, Schnyder J, Garcia‐Garrido HM et al. Delayed large local reaction to the adenovirus‐vectored (ChAdOx1) vaccine. Travel Med Infect Dis 2021; 43: 102093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Zengarini C, Artanidi C, Preci C, Gaspari V. Erythema migrans‐like rash after Moderna vaccine: An uncommon type of "COVID arm". Dermatol Ther 2021; 34: e15063. [DOI] [PubMed] [Google Scholar]
- 156. Niebel D, Ralser‐Isselstein V, Jaschke K, Braegelmann C, Bieber T, Wenzel J. Exacerbation of subacute cutaneous lupus erythematosus following vaccination with BNT162b2 mRNA vaccine. Dermatol Ther 2021; 34: e15017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Arora P, Sardana K, Mathachan SR, Malhotra P. Herpes zoster after inactivated COVID‐19 vaccine: A cutaneous adverse effect of the vaccine. J Cosmet Dermatol 2021; 20: 3389–3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Eid E, Abdullah L, Kurban M, Abbas O. Herpes zoster emergence following mRNA COVID‐19 vaccine. J Med Virol 2021; 93: 5231–5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Bostan E, Yalici‐Armagan B. Herpes zoster following inactivated COVID‐19 vaccine: A coexistence or coincidence? J Cosmet Dermatol 2021; 20: 1566–1567. [DOI] [PubMed] [Google Scholar]
- 160. Julian JA, Mathern DR, Fernando D. Idiopathic thrombocytopenic purpura and the moderna Covid‐19 vaccine. Ann Emerg Med 2021; 77: 654–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Hines A, Shen JG, Olazagasti C, Shams S. Immune thrombocytopenic purpura and acute liver injury after COVID‐19 vaccine. BMJ Case Rep 2021; 14: e242678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Weinstock‐Guttman B, Jakimovski D. Late‐onset cutaneous reaction to BNT162b2 mRNA COVID‐19 vaccine in an immunocompromised patient. Mult Scler 2021; 27: 2291–2292. [DOI] [PubMed] [Google Scholar]
- 163. Cohen SR, Prussick L, Kahn JS, Gao DX, Radfar A, Rosmarin D. Leukocytoclastic vasculitis flare following the COVID‐19 vaccine. Int J Dermatol 2021; 60: 1032–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Hiltun I, Sarriugarte J, Martínez‐de‐Espronceda I et al. Lichen planus arising after COVID‐19 vaccination. J Eur Acad Dermatol Venereol 2021; 35: e414–e415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Belina ME, Sarver MM, Al‐Rohil R, Fresco A. Lichen striatus post‐COVID‐19 vaccination. JAAD Case Rep 2021; 16: 16–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Tammaro A, Adebanjo GAR, Magri F, Parisella FR, Chello C, De Marco G. Local skin reaction to the AZD1222 vaccine in a patient who survived COVID‐19. J Cosmet Dermatol 2021; 20: 1965–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Edriss M, Farshchian M, Daveluy S. Localized cutaneous reaction to an mRNA COVID‐19 vaccine. J Cosmet Dermatol 2021; 20: 2380–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Jedlowski PM, Jedlowski MF. Morbilliform rash after administration of Pfizer‐BioNTech COVID‐19 mRNA vaccine. Dermatol Online J 2021; 27: 13030. [PubMed] [Google Scholar]
- 169. Nune A, Iyengar KP, Goddard C, Ahmed AE. Multisystem inflammatory syndrome in an adult following the SARS‐CoV‐2 vaccine (MIS‐V). BMJ Case Rep 2021; 14: e243888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Matsubara A, Sakurai M, Morita A. Neutrophilic urticarial dermatosis following BNT162b2 (Pfizer–BioNTech) COVID‐19 vaccination. J Cutaneous Immunol Allergy 2021; 4: 187–188. [Google Scholar]
- 171. Kha C, Itkin A. New‐onset chilblains in close temporal association to mRNA‐1273 vaccination. JAAD Case Rep 2021; 12: 12–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Al‐Ansari RY, Al‐Sharari M, Al‐Saadi T. Palms and soles itchiness as a side effect of COVID‐19 vaccination. J Infect Public Health 2021; 14: 1389–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Cameli N, Silvestri M, Mariano M, Nisticò SP, Cristaudo A. Pernio‐like skin lesions after the second dose of Pfizer‐BioNTech COVID‐19 vaccine. J Eur Acad Dermatol Venereol 2021; 35: e725–e727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Ackerman M, Henry D, Finon A, Binois R, Esteve E. Persistent maculopapular rash after the first dose of Pfizer‐BioNTech COVID‐19 vaccine. J Eur Acad Dermatol Venereol 2021; 35: e423–e425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Irvine NJ, Wiles BL. Petechiae and desquamation of fingers following immunization with BTN162b2 messenger RNA (mRNA) COVID‐19 vaccine. Cureus 2021; 13: e16858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Cebeci F, Kartal İ. Petechial skin rash associated with CoronaVac vaccination: first cutaneous side effect report before phase 3 results. Eur J Hosp Pharm 2021; 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Cohen OG, Clark AK, Milbar H, Tarlow M. Pityriasis rosea after administration of Pfizer‐BioNTech COVID‐19 vaccine. Hum Vaccin Immunother 2021; 17: 4097–4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Abdullah L, Hasbani D, Kurban M, Abbas O. Pityriasis rosea after mRNA COVID‐19 vaccination. Int J Dermatol 2021; 60: 1150–1151. [DOI] [PubMed] [Google Scholar]
- 179. Akdaş E, İlter N, Öğüt B, Erdem Ö. Pityriasis rosea following CoronaVac COVID‐19 vaccination: a case report. J Eur Acad Dermatol Venereol 2021; 35: e491–e493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Malayala SV, Mohan G, Vasireddy D, Atluri P. Purpuric rash and thrombocytopenia after the mRNA‐1273 (Moderna) COVID‐19 vaccine. Cureus 2021; 13: e14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Cazzato G, Romita P, Foti C et al. Purpuric skin rash in a patient undergoing Pfizer‐BioNTech COVID‐19 vaccination: histological evaluation and perspectives. Vaccines (Basel) 2021; 9: 760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Afacan E, Öğüt B, Üstün P, Şentürk E, Yazıcı O, Adışen E. Radiation recall dermatitis triggered by inactivated COVID‐19 vaccine. Clin Exp Dermatol 2021; 46: 1582–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Brumfiel CM, Patel MH, DiCaudo DJ, Rosenthal AC, Pittelkow MR, Mangold AR. Recurrence of primary cutaneous CD30‐positive lymphoproliferative disorder following COVID‐19 vaccination. Leuk Lymphoma 2021; 62: 2554–2555. [DOI] [PubMed] [Google Scholar]
- 184. Nanova K, Zlotogorski A, Ramot Y. Recurrent varicella following SARS‐CoV‐2 vaccination with BNT162b2. Int J Dermatol 2021; 60: 1148–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Mintoff D, Pisani D, Betts A, Scerri L. SARS‐CoV‐2 mRNA vaccine‐associated fixed drug eruption. J Eur Acad Dermatol Venereol 2021; 35: e560–e563. [DOI] [PubMed] [Google Scholar]
- 186. Helms JM, Ansteatt KT, Roberts JC et al. Severe, refractory immune thrombocytopenia occurring after SARS‐CoV‐2 vaccine. J Blood Med 2021; 12: 221–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Gruenstein D, Levitt J. Skin necrosis at both COVID‐19 vaccine injection sites. JAAD Case Rep 2021; 15: 67–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Joseph AK, Chong BF. Subacute cutaneous lupus erythematosus flare triggered by COVID‐19 vaccine. Dermatol Ther 2021; 34: e15114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. González‐Romero N, Morillo Montañes V, Vicente Sánchez I, García García M. Lipschütz ulcers after the AstraZeneca COVID‐19 vaccine. Actas Dermosifiliogr (Engl Ed) 2021; 113: 443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Song EJ, Wong AJS. Widespread annular eruption after Ad26.COV2.S COVID‐19 vaccine. JAAD Case Rep 2021; 13: 30–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Ardalan M, Moslemi H, Shafiei S, Tabrizi R, Moselmi M. Herpes‐like skin lesion after AstraZeneca vaccination for COVID‐19: A case report. Clin Case Rep 2021; 9: e04883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Bostan E, Gulseren D, Gokoz O. New‐onset leukocytoclastic vasculitis after COVID‐19 vaccine. Int J Dermatol 2021; 60: 1305–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Baffa ME, Maglie R, Giovannozzi N et al. Sweet syndrome following SARS‐CoV2 vaccination. Vaccines (Basel) 2021; 9: 1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Darrigade AS, Théophile H, Sanchez‐Pena P et al. Sweet syndrome induced by SARS‐CoV‐2 Pfizer‐BioNTech mRNA vaccine. Allergy 2021; 76: 3194–3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Falkenhain‐López D, Gutiérrez‐Collar C, Arroyo‐Andrés J, Gallego‐Gutiérrez I, Rodríguez‐Peralto JL, Sánchez‐Velázquez A. Widespread purpura annularis telangiectodes following mRNA SARS‐CoV‐2 vaccine. J Eur Acad Dermatol Venereol 2021; 35: e719–e721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Guzmán‐Pérez L, Puerta‐Peña M, Falkenhain‐López D et al. Small‐vessel vasculitis following Oxford‐AstraZeneca vaccination against SARS‐CoV‐2. J Eur Acad Dermatol Venereol 2021; 35: e741–e743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Kaminetsky J, Rudikoff D. New‐onset vitiligo following mRNA‐1273 (Moderna) COVID‐19 vaccination. Clin Case Rep 2021; 9: e04865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Tessas I, Kluger N. Ipsilateral herpes zoster after the first dose of BNT162b2 mRNA COVID‐19 vaccine. J Eur Acad Dermatol Venereol 2021; 35: e620–e622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Wu RW, Lin TK. Oxford‐AstraZeneca COVID‐19 vaccine‐induced acute localized exanthematous pustulosis. J Dermatol 2021; 48: e562–e563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Schmidt V, Blum R, Möhrenschlager M. Biphasic bullous pemphigoid starting after first dose and boosted by second dose of mRNA‐1273 vaccine in an 84‐year‐old female with polymorbidity and polypharmacy. J Eur Acad Dermatol Venereol 2022; 36: e88–e90. [DOI] [PubMed] [Google Scholar]
- 201. Sirufo MM, Raggiunti M, Magnanimi LM, Ginaldi L, De Martinis M. Henoch‐Schönlein purpura following the first dose of COVID‐19 viral vector vaccine: a case report. Vaccines (Basel) 2021; 9: 1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Stewart R, McDowell L. Radiation recall phenomenon following COVID‐19 vaccination. Int J Radiat Oncol Biol Phys 2021; 111: 835–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Thomas J, Thomas G, Chatim A, Shukla P, Mardiney M. Chronic spontaneous urticaria after COVID‐19 vaccine. Cureus 2021; 13: e18102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Waraich A, Williams G. Haematuria, a widespread petechial rash, and headaches following the Oxford AstraZeneca ChAdOx1 nCoV‐19 vaccination. BMJ Case Rep 2021; 14: e245440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Zengarini C, Misciali C, Lazzarotto T, Dika E. Eruptive angiomatosis after SARS‐CoV‐2 vaccine (Comirnaty, Pfizer). J Eur Acad Dermatol Venereol 2022; 36: e90–e91. [DOI] [PubMed] [Google Scholar]
- 206. Aoki N, Saruta Y, Tanaka S, Nakajima R, Sano H, Sano S. Skin ulcer at the injection site of BNT162b2 mRNA COVID‐19 vaccine. J Dermatol 2021; 48: e596–e597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Fata A, Jabbour G, Kourie H, Zoghbi M, Kassouf E, Zoghbi A. Rare cutaneous manifestation of COVID‐19 infection and Pfizer‐BioNTech COVID‐19 vaccine with a unique pattern similarity. Futur Virol 2021; 16: 741–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Mintoff D, Scerri L, Betts A. SARS‐CoV‐2 mRNA vaccine injection site pseudolymphoma. J Eur Acad Dermatol Venereol 2022; 36: e20–e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Borg L, Mercieca L, Mintoff D et al. Pfizer‐BioNTech SARS‐CoV‐2 mRNA vaccine‐associated erythema multiforme. J Eur Acad Dermatol Venereol 2022; 36: e22–e24. [DOI] [PubMed] [Google Scholar]
- 210. Agharbi FZ, Eljazouly M, Basri G et al. Bullous pemphigoid induced by the AstraZeneca COVID‐19 vaccine. Ann Dermatol Venereol 2022; 149: 56–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Chen YA, Huang HY, Wu SH, Huang TC. Thrombosis of the palmar digital vein after Oxford‐AstraZeneca COVID‐19 vaccination. Int J Dermatol 2021; 60: e469–e471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Badier L, Toledano A, Porel T et al. IgA vasculitis in adult patient following vaccination by ChadOx1 nCoV‐19. Autoimmun Rev 2021; 20: 102951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Buján Bonino C, Moreiras Arias N, López‐Pardo Rico M et al. Atypical erythema multiforme related to BNT162b2 (Pfizer‐BioNTech) COVID‐19 vaccine. Int J Dermatol 2021; 60: e466–e467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Cameli N, Silvestri M, Mariano M, Bennardo L, Nisticò SP, Cristaudo A. Erythema nodosum following the first dose of ChAdOx1‐S nCoV‐19 vaccine. J Eur Acad Dermatol Venereol 2022; 36: e161–e162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Fang WC, Chiu LW, Hu SC. Psoriasis exacerbation after first dose of AstraZeneca coronavirus disease 2019 vaccine. J Dermatol 2021; 48: e566–e567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Dash S, Behera B, Sethy M, Mishra J, Garg S. COVID‐19 vaccine‐induced urticarial vasculitis. Dermatol Ther 2021; 34: e15093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. de Montjoye L, Marot L, Baeck M. Eosinophilic cellulitis after BNT162b2 mRNA Covid‐19 vaccine. J Eur Acad Dermatol Venereol 2022; 36: e26–e28. [DOI] [PubMed] [Google Scholar]
- 218. Essam R, Ehab R, Al‐Razzaz R, Khater MW, Moustafa EA. Alopecia areata after ChAdOx1 nCoV‐19 vaccine (Oxford/AstraZeneca): a potential triggering factor? J Cosmet Dermatol 2021; 20: 3727–3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Guénin SH, Kresch M, Elbogen E, Lebwohl MG. Cutaneous reaction reported after third Moderna COVID‐19 vaccine. JAAD Case Rep 2021; 18: 49–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Hsu HT, Su HA, Chen YC. Erythema nodosum, after Medigen vaccination against COVID‐19? J Formos Med Assoc 2021; 121: 723–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Kar BR, Singh BS, Mohapatra L, Agrawal I. Cutaneous small‐vessel vasculitis following COVID‐19 vaccine. J Cosmet Dermatol 2021; 20: 3382–3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Kim JC, Lee SY, Kang SY, Kim HO, Park CW, Chung BY. Erythema annulare centrifugum induced by COVID‐19 vaccination. Clin Exp Dermatol 2021; 47: 591–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Kim MJ, Kim JW, Kim MS, Choi SY, Na JI. Generalized erythema multiforme‐like skin rash following the first dose of COVID‐19 vaccine (Pfizer‐BioNTech). J Eur Acad Dermatol Venereol 2022; 36: e98–e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Kouki C, Sellami K, Bahloul E, Amouri M, Masmoudi A, Turki H. Response to 'A skin reaction with rust‐like discolouration to mRNA COVID‐19 vaccine'. J Eur Acad Dermatol Venereol 2022; 36: e97–e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Lavery MJ, Nawimana S, Parslew R, Stewart L. A flare of pre‐existing erythema multiforme following BNT162b2 (Pfizer‐BioNTech) COVID‐19 vaccine. Clin Exp Dermatol 2021; 46: 1325–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Piccolo V, Mazzatenta C, Bassi A et al. COVID vaccine‐induced lichen planus on areas previously affected by vitiligo. J Eur Acad Dermatol Venereol 2022; 36: e28–e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Myrdal CN, Culpepper KS, Leyo DPS. Generalized dermal hypersensitivity reaction following Moderna COVID‐19 vaccination. Dermatol Ther 2021; 34: e15173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Osmond A, Kenny B. Reaction to dermal filler following COVID‐19 vaccination. J Cosmet Dermatol 2021; 20: 3751–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Patil S, Patil A. Systemic lupus erythematosus after COVID‐19 vaccination: A case report. J Cosmet Dermatol 2021; 20: 3103–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Płaszczyńska A, Sławińska M, Sobjanek M. Regression of common viral warts after ChAdOx1‐S COVID‐19 vaccine. J Eur Acad Dermatol Venereol 2022; 36: e162–e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Dash S, Sirka CS, Mishra S, Viswan P. COVID‐19 vaccine‐induced Stevens‐Johnson syndrome. Clin Exp Dermatol 2021; 46: 1615–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Bekkali N, Allard T, Lengellé C, Estève E. Eczematiform eruption after Pfizer‐BioNTech COVID‐19 vaccine. Therapie 2021; 76: 364–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Pileri A, Guglielmo A, Raone B, Patrizi A. Chilblain lesions after COVID‐19 mRNA vaccine. Br J Dermatol 2021; 185: e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Pérez‐López I, Moyano‐Bueno D, Ruiz‐Villaverde R. Bullous pemphigoid and COVID‐19 vaccine. Med Clin (Barc) 2021; 157: e333–e334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Hunjan MK, Roberts C, Karim S, Hague J. Pityriasis rubra pilaris‐like eruption following administration of the BNT163b2 (Pfizer‐BioNTech) mRNA COVID‐19 vaccine. Clin Exp Dermatol 2022; 47: 188–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Kreuter A, Licciardi‐Fernandez MJ, Burmann SN, Burkert B, Oellig F, Michalowitz AL. Induction and exacerbation of subacute cutaneous lupus erythematosus following mRNA‐based or adenoviral vector‐based SARS‐CoV‐2 vaccination. Clin Exp Dermatol 2022; 47: 161–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Lopez S, Vakharia P, Vandergriff T, Freeman EE, Vasquez R. Pernio after COVID‐19 vaccination. Br J Dermatol 2021; 185: 445–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Kreuter A, Burmann SN, Burkert B, Oellig F, Michalowitz AL. Transition of cutaneous into systemic lupus erythematosus following adenoviral vector‐based SARS‐CoV‐2 vaccination. J Eur Acad Dermatol Venereol 2021; 35: e733–e735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Krajewski PK, Matusiak Ł, Szepietowski JC. Psoriasis flare‐up associated with second dose of Pfizer‐BioNTech BNT16B2b2 COVID‐19 mRNA vaccine. J Eur Acad Dermatol Venereol 2021; 35: e632–e634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Young J, Mercieca L, Ceci M, Pisani D, Betts A, Boffa MJ. A case of bullous pemphigoid after the SARS‐CoV‐2 mRNA vaccine. J Eur Acad Dermatol Venereol 2022; 36: e13–e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. D'Cruz A, Parker H, Saha M. A Bullous Eruption following the Pfizer‐BioNTech COVID‐19 vaccination. J Eur Acad Dermatol Venereol 2021; 35: e864–e865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Piccolo V, Bassi A, Argenziano G et al. BNT162b2 mRNA COVID‐19 vaccine‐induced chilblain‐like lesions reinforces the hypothesis of their relationship with SARS‐CoV‐2. J Eur Acad Dermatol Venereol 2021; 35: e493–e494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Bostan E, Jarbou A. Atypical pityriasis rosea associated with mRNA COVID‐19 vaccine. J Med Virol 2021; 94: 814–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Gyldenløve M, Skov L, Hansen CB, Garred P. Recurrent injection‐site reactions after incorrect subcutaneous administration of a COVID‐19 vaccine. J Eur Acad Dermatol Venereol 2021; 35: e545–e546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Kulkarni R, Sollecito TP. COVID‐19 vaccination: possible short‐term exacerbations of oral mucosal diseases. Int J Dermatol 2021; 60: e335–e336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Nastro F, Fabbrocini G, di Vico F, Marasca C. Small vessel vasculitis related to varicella‐zoster virus after Pfizer‐BioNTech COVID‐19 vaccine. J Eur Acad Dermatol Venereol 2021; 35: e745–e747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Merhy R, Sarkis AS, Kaikati J, El Khoury L, Ghosn S, Stephan F. New‐onset cutaneous lichen planus triggered by COVID‐19 vaccination. J Eur Acad Dermatol Venereol 2021; 35: e729–e730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Mieczkowska K, Kaubisch A, McLellan BN. Exacerbation of psoriasis following COVID‐19 vaccination in a patient previously treated with PD‐1 inhibitor. Dermatol Ther 2021; 34: e15055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Wantavornprasert K, Noppakun N, Klaewsongkram J, Rerknimitr P. Generalized bullous fixed drug eruption after Oxford‐AstraZeneca (ChAdOx1 nCoV‐19) vaccination. Clin Exp Dermatol 2022; 47: 428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Lladó I, Butrón B, Sampedro‐Ruiz R, Fraga J, de Argila D. Pityriasis rubra pilaris after Vaxzevria® COVID‐19 vaccine. J Eur Acad Dermatol Venereol 2021; 35: e833–e835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Adya KA, Inamadar AC, Albadri W. Post Covid‐19 vaccination papulovesicular pityriasis rosea‐like eruption in a young male. Dermatol Ther 2021; 34: e15040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Quattrini L, Verardi L, Caldarola G, Peluso G, De Simone C, D'Agostino M. New onset of remitting seronegative symmetrical synovitis with pitting oedema and palmoplantar psoriasis flare‐up after Sars‐Cov‐2 vaccination. J Eur Acad Dermatol Venereol 2021; 35: e727–e729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Orenay OM, Balta I, Yigit D, Eksioglu M. Systemic drug‐related intertriginous and flexural exanthema like eruption after CoronaVac vaccine. J Eur Acad Dermatol Venereol 2021; 35: e634–e635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Lim PN, Wylie G. Symmetrical drug‐related intertriginous and flexural exanthema like eruption associated with COVID‐19 vaccination. Clin Exp Dermatol 2022; 47: 175–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Ohsawa R, Sano H, Ikeda M, Sano S. Clinical and histopathological views of morbilliform rash after COVID‐19 mRNA vaccination mimic those in SARS‐CoV‐2 virus infection‐associated cutaneous manifestations. J Dermatol Sci 2021; 103: 124–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Lesort C, Kanitakis J, Donzier L, Jullien D. Chilblain‐like lesions after BNT162b2 mRNA COVID‐19 vaccine: a case report suggesting that 'COVID toes' are due to the immune reaction to SARS‐CoV‐2. J Eur Acad Dermatol Venereol 2021; 35: e630–e632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Lopes NT, Pinilla CEO, Gerbase AC. Erythema multiforme after CoronaVac vaccination. J Eur Acad Dermatol Venereol 2021; 35: e717–e719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Lehmann M, Schorno P, Hunger RE, Heidemeyer K, Feldmeyer L, Yawalkar N. New onset of mainly guttate psoriasis after COVID‐19 vaccination: a case report. J Eur Acad Dermatol Venereol 2021; 35: e752–e755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Atak MF, Farabi B, Kalelioglu MB, Rao BK. Pigmented purpuric dermatosis after BNT162B2 mRNA COVID‐19 vaccine administration. J Cosmet Dermatol 2022; 21: 435–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Ayatollahi A, Robati RM, Firooz A. Plantar herpes zoster following heterologous recombinant adenovirus‐based COVID‐19 vaccine. J Cosmet Dermatol 2022; 21: 34–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Chaijaras S, Seree‐Aphinan C, Rutnin S, Ngamjanyaporn P, Rattanakaemakorn P. Serum sickness‐like reaction following an administration of the first dose of inactivated COVID19 vaccine. JAAD Case Rep 2022; 19: 21–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Ciccarese G, Drago F, Boldrin S, Pattaro M, Parodi A. Sudden onset of vitiligo after COVID‐19 vaccine. Dermatol Ther 2022; 35: e15196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Cinotti E, Perrot JL, Bruzziches F et al. Eosinophilic dermatosis after AstraZeneca COVID‐19 vaccination. J Eur Acad Dermatol Venereol 2022; 36: e171–e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Dell'Antonia M, Anedda S, Usai F, Atzori L, Ferreli C. Bullous pemphigoid triggered by COVID‐19 vaccine: Rapid resolution with corticosteroid therapy. Dermatol Ther 2022; 35: e15208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Kuzumi A, Yoshizaki A, Chiba K et al. Genital necrosis with cutaneous thrombosis after COVID‐19 mRNA vaccination. J Eur Acad Dermatol Venereol 2022; 36: e185–e186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Park JW, Yu SN, Chang SH, Ahn YH, Jeon MH. multisystem inflammatory syndrome in an adult after COVID‐19 vaccination: a case report and literature review. J Korean Med Sci 2021; 36: e312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Kherlopian A, Zhao C, Ge L, Forward E, Fischer G. A case of toxic epidermal necrolysis after ChAdOx1 nCov‐19 (AZD1222) vaccination. Australas J Dermatol 2022; 63: e93–e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Maranini B, Ciancio G, Cultrera R, Govoni M. Herpes zoster infection following mRNA COVID‐19 vaccine in a patient with ankylosing spondylitis. Reumatismo 2021; 73: 174–176. [DOI] [PubMed] [Google Scholar]
- 269. Hali F, Kerouach A, Alatawna H, Chiheb S, Lakhdar H. Linear IgA bullous dermatosis following Oxford AstraZeneca COVID‐19 vaccine. Clin Exp Dermatol 2022; 47: 611–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Grossman ME, Appel G, Little AJ, Ko CJ. Post‐COVID‐19 vaccination IgA vasculitis in an adult. J Cutan Pathol 2021; 49: 385–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Mansouri P, Chalangari R, Martits‐Chalangari K, Mozafari N. Stevens‐Johnson syndrome due to COVID‐19 vaccination. Clin Case Rep 2021; 9: e05099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Sernicola A, Dybala A, Gomes V et al. Lymphomatoid drug reaction developed after BNT162b2 (Comirnaty) COVID‐19 vaccine manifesting as pityriasis lichenoides et varioliformis acuta‐like eruption. J Eur Acad Dermatol Venereol 2022; 36: e172–e174. [DOI] [PubMed] [Google Scholar]
- 273. Koutlas IG, Camara R, Argyris PP, Davis MDP, Miller DD. Development of pemphigus vulgaris after the second dose of the mRNA‐1273 SARS‐Cov‐2 vaccine. Oral Dis 2021; 1–2. [DOI] [PubMed] [Google Scholar]
- 274. Doughty H, Barton D. Severe nonanaphylactic allergic reaction to the Pfizer‐BioNTech COVID‐19 vaccine. JAAD Case Rep 2022; 19: 84–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Souaid K, Oulès B, Sohier P, Deschamps L, Aractingi S, Dupin N. Type I interferon signature in chilblains following SARS‐CoV‐2 mRNA vaccine: a case report. Acta Derm Venereol 2021; 101: adv00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Shin SH, Hong JK, Hong SA, Li K, Yoo KH. Pityriasis Rosea shortly after mRNA‐1273 COVID‐19 vaccination. Int J Infect Dis 2022; 114: 88–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Abobaker A, Idris MA, Ogunjimi O. A localised vasculitic‐like skin rash following the second dose of COVID‐19 vaccine. Int J Infect Dis 2022; 114: 29–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Sharabi A, Shiber S, Molad Y. Adult‐onset Still's disease following mRNA COVID‐19 vaccination. Clin Immunol 2021; 233: 108878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Savva D, Battineni G, Amenta F, Nittari G. Hypersensitivity reaction to hyaluronic acid dermal filler after the Pfizer vaccination against SARS‐CoV‐2. Int J Infect Dis 2021; 113: 233–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Saifuddin A, Koesnoe S, Kurniati N, Sirait S, Arisanty R, Yunihastuti E. COVID arm after moderna booster in healthcare worker: a case report. Acta Med Indones 2021; 53: 326–330. [PubMed] [Google Scholar]
- 281. Zengarini C, Pileri A, Salamone FP, Piraccini BM, Vitale G, La Placa M. Subacute cutaneous lupus erythematosus induction after SARS‐CoV‐2 vaccine in a patient with primary biliary cholangitis. J Eur Acad Dermatol Venereol 2022; 36: e179–e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Yatsuzuka K, Murakami M, Kuroo Y et al. Flare‐up of generalized pustular psoriasis combined with systemic capillary leak syndrome after coronavirus disease 2019 mRNA vaccination. J Dermatol 2021; 49: 454–458. [DOI] [PubMed] [Google Scholar]
- 283. Jeon YH, Lim DH, Choi SW, Choi SJ. A flare of Still's disease following COVID‐19 vaccination in a 34‐year‐old patient. Rheumatol Int 2021; 42: 743–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Lopez ED, Javed N, Upadhyay S, Shekhar R, Sheikh AB. Acute exacerbation of psoriasis after COVID‐19 Pfizer vaccination. Baylor University Medical Center Proceedings 2021; 35: 199–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Sahni MK, Roy K, Asati DP, Khurana U. An old entity, a new trigger: Post COVID‐19 vaccine pityriasis rubra pilaris. Int J Risk Saf Med 2021; 32: 261–264. [DOI] [PubMed] [Google Scholar]
- 286. Wunderlich K, Dirschka T. Erythema multiforme following COVID‐19 vaccination (BNT162b2). Hautarzt 2022; 73: 68–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Colia R, Rotondo C, Corrado A, Cantatore FP. Cutaneous vasculitis after severe acute respiratory syndrome coronavirus 2 vaccine. Rheumatol Adv Pract 2021; 5: rkab050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Majid I, Mearaj S. Sweet syndrome after Oxford‐AstraZeneca COVID‐19 vaccine (AZD1222) in an elderly female. Dermatol Ther 2021; 34: e15146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Terashima T. Delayed large pruritic eruption to the BNT162b2 mRNA vaccine. IDCases 2021; 26: e01297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Pedrazini MC, da Silva MH. Pityriasis rosea‐like cutaneous eruption as a possible dermatological manifestation after Oxford‐AstraZeneca vaccine: Case report and brief literature review. Dermatol Ther 2021; 34: e15129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Nazzaro G, Maronese CA. Urticarial vasculitis following mRNA anti‐COVID‐19 vaccine. Dermatol Ther 2021; 35: e15282. [DOI] [PubMed] [Google Scholar]
- 292. Gökçek GE, Öksüm Solak E, Çölgeçen E. Pityriasis rosea like eruption: A dermatological manifestation of Coronavac‐COVID‐19 vaccine. Dermatol Ther 2022; 35: e15256. [DOI] [PubMed] [Google Scholar]
- 293. Okan G, Vural P. Worsening of the vitiligo following the second dose of the BNT162B2 mRNA COVID‐19 vaccine. Dermatol Ther 2021; 35: e15280. [DOI] [PubMed] [Google Scholar]
- 294. Correia C, Fernandes S, Soares‐de‐Almeida L, Filipe P. Exuberant lichenoid eruption after Oxford‐AstraZeneca COVID‐19 vaccine: a singular case. J Eur Acad Dermatol Venereol 2021; 36: e268–e270. [DOI] [PubMed] [Google Scholar]
- 295. Knechtl GV, Seyed Jafari SM, Berger T, Rammlmair A, Feldmeyer L, Borradori L. Development of pemphigus vulgaris following mRNA SARS‐CoV‐19 BNT162b2 vaccination in an 89‐year‐old patient. J Eur Acad Dermatol Venereol 2021; 36: e251–e253. [DOI] [PubMed] [Google Scholar]
- 296. Bostan E, Yel B, Akdogan N, Gokoz O. New‐onset bullous pemphigoid after inactivated Covid‐19 vaccine: Synergistic effect of the Covid‐19 vaccine and vildagliptin. Dermatol Ther 2022; 35: e15241. [DOI] [PubMed] [Google Scholar]
- 297. Oskay T, Isık M. Leukocytoclastic vasculitis after the third dose of CoronaVac vaccination. Clin Rheumatol 2021; 41: 1931–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. O'Connor T, O'Callaghan‐Maher M , Ryan P, Gibson G. Drug reaction with eosinophilia and systemic symptoms syndrome following vaccination with the AstraZeneca COVID‐19 vaccine. JAAD Case Rep 2022; 20: 14–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Koç Yıldırım S. A new‐onset vitiligo following the inactivated COVID‐19 vaccine. J Cosmet Dermatol 2022; 21: 429–430. [DOI] [PubMed] [Google Scholar]
- 300. Kharkar V, Vishwanath T, Mahajan S, Joshi R, Gole P. Asymmetrical cutaneous vasculitis following COVID‐19 vaccination with unusual eosinophil preponderance. Clin Exp Dermatol 2021; 46: 1596–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Nakamura K, Kosano M, Sakai Y et al. Case of bullous pemphigoid following coronavirus disease 2019 vaccination. J Dermatol 2021; 48: e606–e607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Masseran C, Calugareanu A, Caux F, Bohelay G. Extensive cutaneous lichen planus triggered by viral vector COVID‐19 vaccination (ChAdOx1 nCoV‐19). J Eur Acad Dermatol Venereol 2021; 36: e263–e265. [DOI] [PubMed] [Google Scholar]
- 303. Kothari RS, Subramani D, Asnani DR, Raman A, Deora MS, Gupta A. Erythema multiforme after Covishield/ChAdOx1 vaccination. Dermatol Ther 2021; 35: e15289. [DOI] [PubMed] [Google Scholar]
- 304. Aponso S, Hoou LC, Wei YY, Salahuddin SA, Yit PJ. Multibacillary leprosy unmasked by COVID‐19 vaccination. JAAD Case Rep 2022; 19: 87–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Fiorillo G, Pancetti S, Cortese A et al. Leukocytoclastic vasculitis (cutaneous small‐vessel vasculitis) after COVID‐19 vaccination. J Autoimmun 2022; 127: 102783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Ungari M, Pezzarossa E. Cutaneous lymphocytic vasculitis after administration of the second dose of AZD1222 (Oxford‐AstraZeneca) severe acute respiratory syndrome Coronavirus 2 vaccination: casuality or causality? Am J Dermatopathol 2022; 44: 80–82. [DOI] [PubMed] [Google Scholar]
- 307. Gambichler T, Scholl L, Dickel H, Ocker L, Stranzenbach R. Prompt onset of Rowell's syndrome following the first BNT162b2 SARS‐CoV‐2 vaccination. J Eur Acad Dermatol Venereol 2021; 35: e415–e416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Solimani F, Mansour Y, Didona D, Dilling A, Ghoreschi K, Meier K. Development of severe pemphigus vulgaris following SARS‐CoV‐2 vaccination with BNT162b2. J Eur Acad Dermatol Venereol 2021; 35: e649–e651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Krajewski PK, Szepietowski JC. Immune thrombocytopenic purpura associated with COVID‐19 Pfizer‐BioNTech BNT16B2b2 mRNA vaccine. J Eur Acad Dermatol Venereol 2021; 35: e626–e627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Data from the Ministry of Public Health . Thailand. Available from: https://ddc.moph.go.th/uploads/ckeditor2//files/Daily%20report%202021‐05‐30.pdf. Accessed July. 29, 2021.
- 311. Kelso JM, Greenhawt MJ, Li JT et al. Adverse reactions to vaccines practice parameter 2012 update. J Allergy Clin Immunol 2012; 130: 25–43. [DOI] [PubMed] [Google Scholar]
- 312. Wei N, Kresch M, Elbogen E, Lebwohl M. New onset and exacerbation of psoriasis after COVID‐19 vaccination. JAAD Case Rep 2022; 19: 74–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Greenhawt M, Abrams EM, Shaker M et al. The risk of allergic reaction to SARS‐CoV‐2 vaccines and recommended evaluation and management: a systematic review, meta‐analysis, GRADE assessment, and international consensus approach. J Allergy Clin Immunol Pract 2021; 9: 3546–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Welsh E, Cardenas‐de la Garza JA, Cuellar‐Barboza A, Franco‐Marquez R, Arvizu‐Rivera RI. SARS‐CoV‐2 spike protein positivity in pityriasis rosea‐like and urticaria‐like rashes of COVID‐19. Br J Dermatol 2021; 184: 1194–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Colmenero I, Santonja C, Alonso‐Riaño M et al. SARS‐CoV‐2 endothelial infection causes COVID‐19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol 2020; 183: 729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Niebel D, Novak N, Wilhelmi J et al. Cutaneous adverse reactions to COVID‐19 vaccines: insights from an immuno‐dermatological perspective. Vaccines (Basel) 2021; 9: 944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Kounis NG, Koniari I, de Gregorio C et al. Allergic reactions to current available COVID‐19 vaccinations: pathophysiology, causality, and therapeutic considerations. Vaccines (Basel) 2021; 9: 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Jeyanathan M, Afkhami S, Smaill F, Miller MS, Lichty BD, Xing Z. Immunological considerations for COVID‐19 vaccine strategies. Nat Rev Immunol 2020; 20: 615–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Eyerich K, Eyerich S. Immune response patterns in non‐communicable inflammatory skin diseases. J Eur Acad Dermatol Venereol 2018; 32: 692–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 PRISMA 2020 Checklist
Table S2 Search strategy
Table S3 Characteristics and quality assessment of the randomized controlled trials included for meta‐analysis
Table S4 Characteristics and quality assessment of the observational studies included for meta‐analysis
Table S5 Quality assessment and level of evidence for case reports
Table S6 Quality assessment and level of evidence for case series
Table S7 Quality assessment and level of evidence for observational studies
Table S8 Quality assessment and level of evidence for randomized controlled trials
Table S9 Prevalence of overall cutaneous manifestations following COVID‐19 vaccination in each study
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.


