Abstract
Designing and manufacturing efficient vaccines against coronavirus disease 2019 (COVID‐19) is a major objective. In this systematic review, we aimed to evaluate the most important vaccines under construction worldwide, their efficiencies and clinical results in healthy individuals and in those with specific underlying diseases. We conducted a comprehensive search in PubMed, Scopus, EMBASE, and Web of Sciences by 1 December 2021 to identify published research studies. The inclusion criteria were publications that evaluated the immune responses and safety of COVID‐19 vaccines in healthy individuals and in those with pre‐existing diseases. We also searched the VAERS database to estimate the incidence of adverse events of special interest (AESI) post COVID‐19 vaccination. Almost all investigated vaccines were well tolerated and developed good levels of both humoural and cellular responses. A protective and efficient humoural immune response develops after the second or third dose of vaccine and a longer interval (about 28 days) between the first and second injections of vaccine could induce higher antibody responses. The vaccines were less immunogenic in immunocompromised patients, particularly those with haematological malignancies. In addition, we found that venous and arterial thrombotic events, Bell's palsy, and myocarditis/pericarditis were the most common AESI. The results showed the potency of the SARS‐CoV‐2 vaccines to protect subjects against disease. The provision of further effective and safe vaccines is necessary in order to reach a high coverage of immunisation programs across the globe and to provide protection against infection itself.
Keywords: COVID‐19, efficacy, immune system, immunogenicity, SARS‐CoV‐2, vaccine
Abbreviations
- AEs
adverse events
- AESI
adverse event of special interest
- COVID‐19
coronavirus disease 2019
- CAMS
Chinese Academy of Medical Sciences
- IFNγ
interferon gamma
- IL
interleukin
- MMR
measles, mumps and rubella
- MERS‐CoV
Middle East respiratory syndrome coronavirus
- nAbs
neutralising antibodies
- rAd26
recombinant adenovirus type 26
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- TNF
tumour necrosis factor
- VLP
virus‐like particle
1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), is probably the most challenging disease of the current century, which has had irreversible effects on public health since 2 December019. 1 The present epidemic of COVID‐19 is the third well‐known spill over of an animal coronavirus onto humans in the last two decades, which has led to a major worrying pandemic worldwide. 2 The speed of transmission of SARS‐CoV‐2 and the daily increase in the number of confirmed cases is much faster than the other members of the SARS family. 3 At the time of writing this review article, this outbreak has spread to almost all countries and territories and has infected at least 262 million people so far and killed more than five million individuals globally. 4
Although transmission reduction strategies, including social‐distancing, which are performed in almost all countries, have prevented individuals from being affected, these strategies will paradoxically leave them unprotected from SARS‐CoV‐2 and thus vulnerable to infection. 5 In the meantime, health‐care workers, elderlies, and those with a defect or suppressed immune systems are at particularly high risk. 6 Therefore, the best way to control this pandemic is to create an effective and useful vaccine.
SARS‐CoV‐2 contains four major structural proteins, used in vaccine design (Figure 1). The general consensus is that the world will not return to its pre‐pandemic status until effective and safe vaccines become available and a global vaccination programme is successfully implemented.
FIGURE 1.
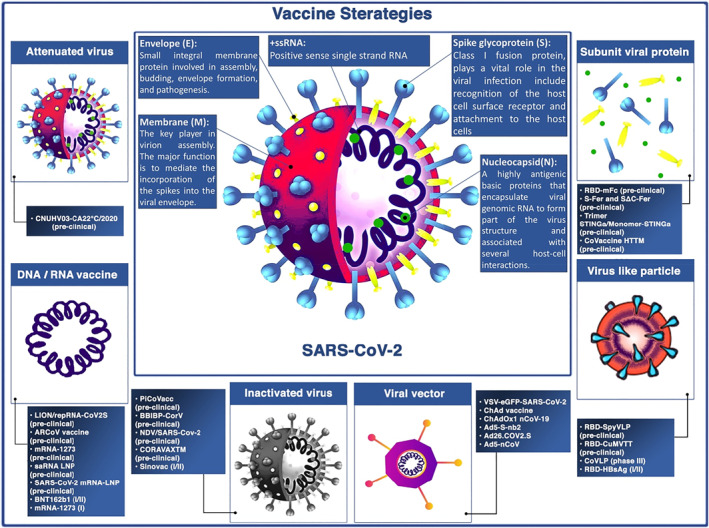
SARS‐CoV‐2 major structural proteins and summary of strategy types for COVID‐19 vaccine designing
Inactivated vaccines have received a lot of attention because they can elicit immune responses similar to those that occur against the virus particles. 7 These types of vaccines contain whole virus particles without replicating potential due to a lack of live genetic material. These vaccines are prepared by using virus inactivation approaches such as using chemical agents (e.g. formaldehyde, phenol, glutaraldehyde), radiation (e.g. ultra violet (UV), X‐ray, or gamma irradiation), or by physical methods (e.g. heat, pressure, or pH). 7
Nucleic acid‐based vaccines are new types of vaccines, that include RNA vaccines. 8 These types of vaccines include an mRNA strand coding for a specific antigen. They can be transcribed into a viral protein once they are delivered into human cells. To trigger an immune response, the viral protein could be presented on the cell surface where it is recognised by immune system components. 8 RNA‐based vaccines have been previously studied for viral infections such as influenza, rabies, and cytomegalovirus (CMV). 8
Two main types of viral vector‐based vaccines have been identified. 9 Non‐replicating vector vaccines just produce the vaccine antigen, and they are unable to produce new viral particles. Replicating vector vaccines could produce new viral particles and also enable to infect cells. Current developed viral vector vaccines for SARS‐CoV‐2 are non‐replicating viral vectors. 9
Prior attempts for development of vaccines against other Coronaviruses such as SARS‐CoV‐1 and Middle East respiratory syndrome coronavirus (MERS‐CoV), revealed that spike (S) proteins are appropriate targets for developing the SARS‐CoV‐2 subunit vaccines. 10 S protein contains two subunits, S1 and S2, which mediate receptor binding and membrane fusion, respectively. Subunit S1 consists of RBD that can stimulate tissue tropism of viruses as well as induction of neutralising antibodies (nAbs). 10
Virus‐like particles (VLPs) are artificially produced nanoparticles composed of a subset of viral components that roughly resemble the structure, size, and surface composition of natural viruses which accounts for their robust immunogenicity and induction of both antibody and cellular immune responses. 11 Different expression systems, including mammalian cell lines, bacteria, insect cell lines, yeast, and plant cells, have been used to construct VLPs. 12 Due to a lack of core genetic material, VLPs are non‐infectious which suggests them as a safer vaccine platform compared to several others, as well as makes them a safe and relevant model in performing molecular virus studies under biosafety level (BSL)‐2 conditions without biosafety protection. Besides being safe and efficacious, VLP vaccines also have the advantages of multivalency, inhaled vaccination potential, self‐adjuvant property, scalable manufacturing, and easily maintainable temperatures in the supply chain. 13 Human papillomavirus (HPV) and hepatitis B vaccines are of well‐known and commercially available FDA‐approved VLP vaccines 14 and many more types of VLP vaccine targeting other diseases, including COVID‐19, are currently being developed.
In this systematic review, we have evaluated the most important vaccines under construction worldwide, their efficacy and safety in healthy individuals and those with underlying diseases of interest.
2. METHODS
This systematic review was prepared according to the Preferred Reporting Items for systematic review and Meta‐Analysis (PRISMA) 2020 checklist (Table S1). 15
2.1. Search strategy
A comprehensive search strategy was performed in PubMed, Scopus, EMBASE, and Web of Sciences by 1 December 2021 to identify published publications regarding the immune response and safety of COVID‐19 vaccines in healthy individuals and those with underlying diseases. The search keywords are available in the supplementary data, Table S2.
2.2. Study eligibility criteria
Title and abstract screening were performed by two authors using the predefined inclusion criteria and excluded duplicate publications. Disagreement was resolved through discussion with two more authors.
To be eligible, studies had to meet the following criteria:
-
(1)
Clinical trials assessing the immunogenicity, safety, and efficacy of COVID‐19 vaccines that published their results.
-
(2)
Publications assessing the immunogenicity and safety of COVID‐19 vaccines in participants with underlying disorders.
Meanwhile, trials were excluded directly, if:
-
(1)
Received any types of therapies except SARS‐CoV‐2 vaccines.
-
(2)
Received vaccines against other types of coronaviruses except SARS‐CoV‐2.
-
(3)
Review articles, systematic reviews, hypothesis articles, abstracts, case reports, case series, and bioinformatics assessments.
2.3. Study quality assessment and data extraction
The quality of each publication was independently evaluated by two authors according to the PRISMA checklist. The full text of relevant studies was reviewed by three authors for evaluating the eligibility according to the inclusion and exclusion criteria. The following items were extracted from the included studies: first author's name, publication date, name of vaccine and the manufacture, number of participants, health status, schedule and dose of the vaccine received, immunogenicity, safety, and efficacy.
2.4. Analysing the incidence of adverse events of special interest post COVID‐19 vaccination in the USA
We utilised the data from the Vaccine Adverse Event Reporting System (VAERS) framework, which is a national post marketing spontaneous surveillance programme for vaccine safety. 16 Symptoms of adverse events (AEs) in VAERS are coded using the Medical Dictionary for Regulatory Activities (MedDRA), which is a highly validated and standardised terminology. 17 In order to estimate the incidence of some commonly reported adverse events of special interest post COVID‐19 immunisation with three vaccines (i.e. Pfizer‐BioNTech, Moderna, and Johnson & Johnson's Janssen), which have been authorised for emergency use in the USA, we searched the VAERS as of 22 November 2021. The number of reported venous and arterial thrombotic events, Bell's palsy, myocarditis/pericarditis, anaphylaxis, Guillian‐Barre syndrome, and transverse myelitis cases who received at least one dose of vaccine, were retrieved from the VAERSE database along with demographic information of the cases.
3. RESULTS
3.1. Immunogenicity, safety, and efficacy of SARS‐CoV‐2 vaccines
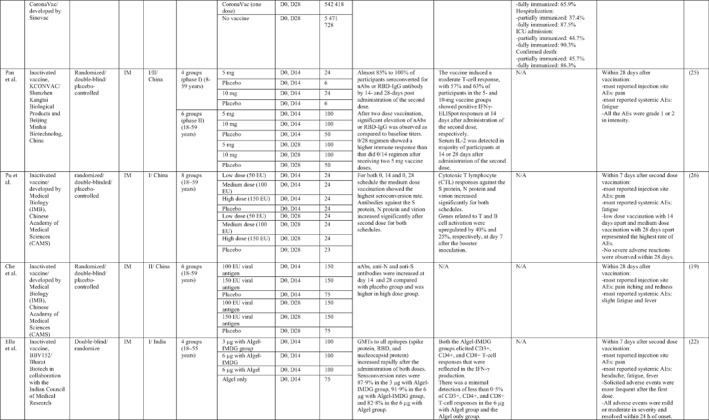
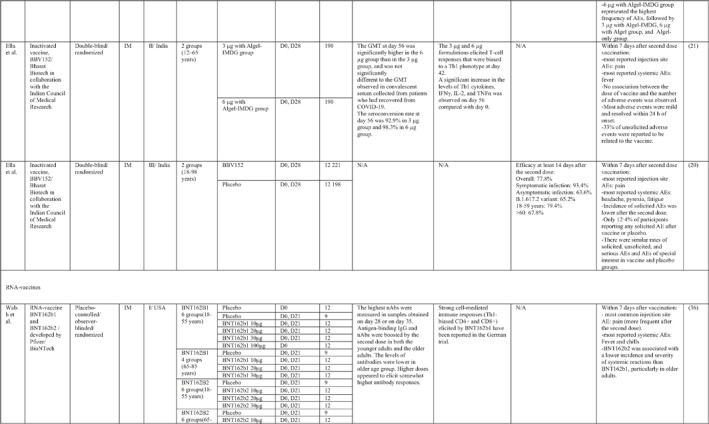
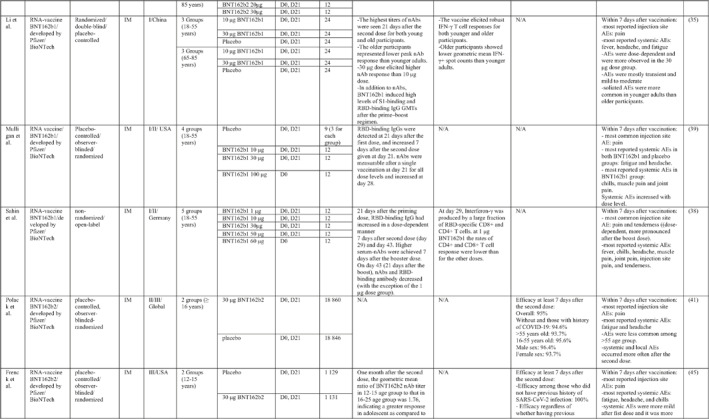
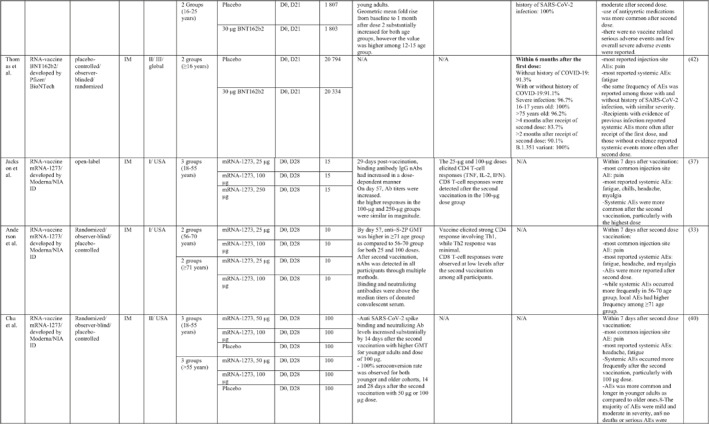
By 1 Dec 2021 from an initial screen of 132 identified studies, 88 studies provided clinical outcome data on the use of SARS‐CoV‐2 vaccines in healthy subjects. These results include inactivated vaccines (n = 15), RNA vaccines (n = 13), vector‐based vaccines (n = 16), and subunit vaccines (n = 10; Figure 2). Totally, 6,831,932 healthy subjects were enroled in these studies. Besides, 34 publications were also found which evaluated the immunogenicity and safety of COVID‐19 vaccines in individuals with underlying diseases. The characteristics of the included clinical studies, including the study type, clinical phase, country, route/schedule of the vaccination, days of injections, immunogenicity of the vaccines (humoural and cellular immune responses), efficacy, and related AEs are summarised in Table 1.
FIGURE 2.
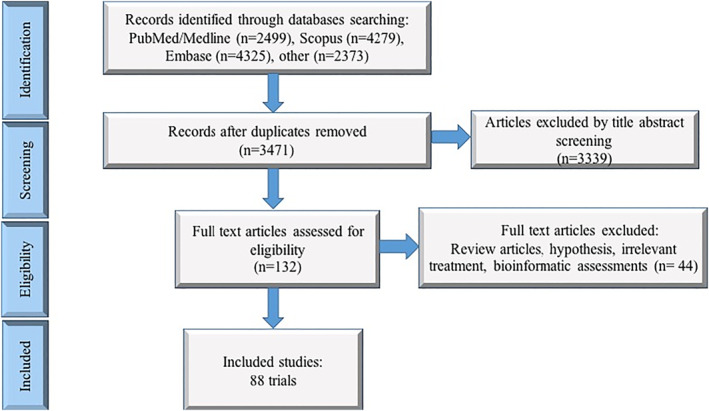
Flow diagram of study selection
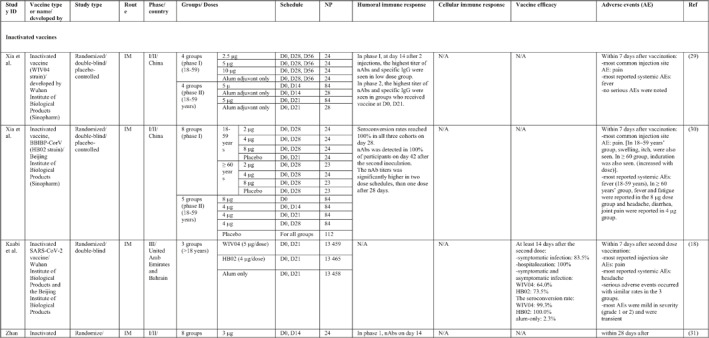
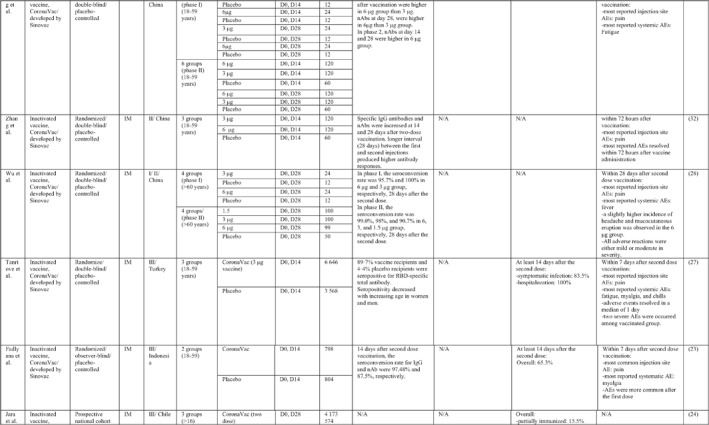
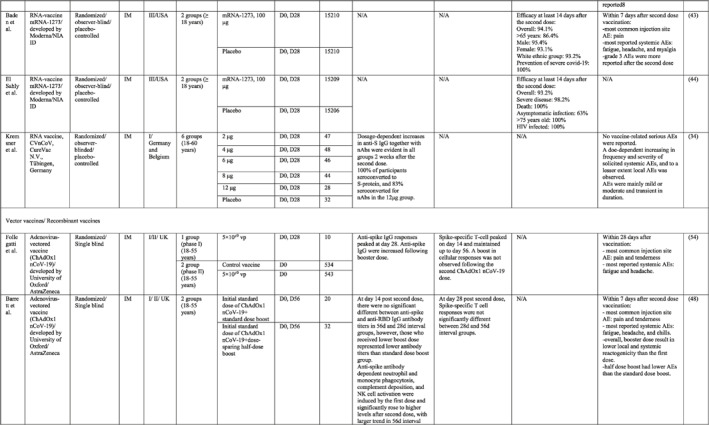
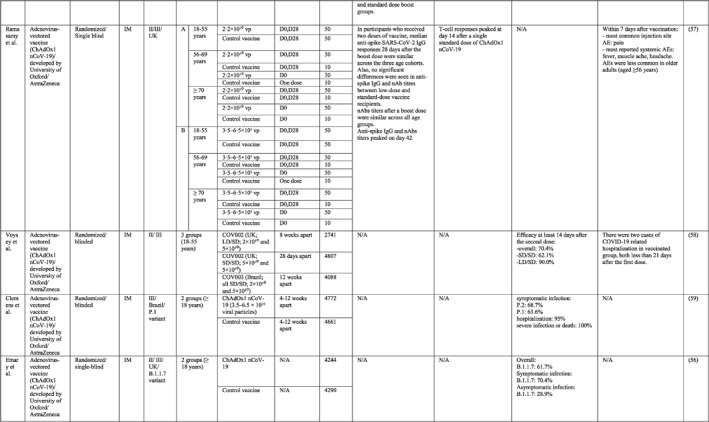
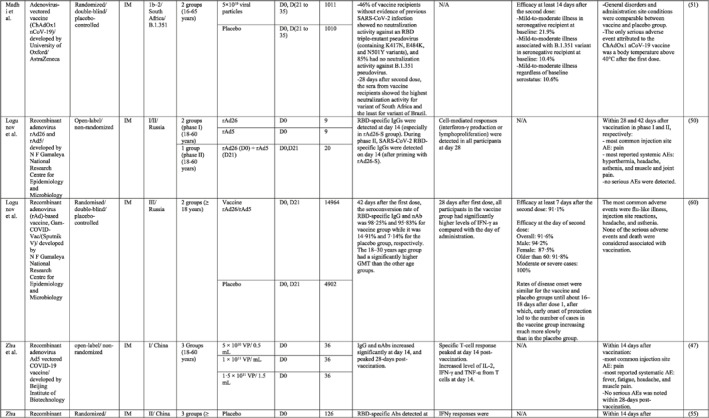
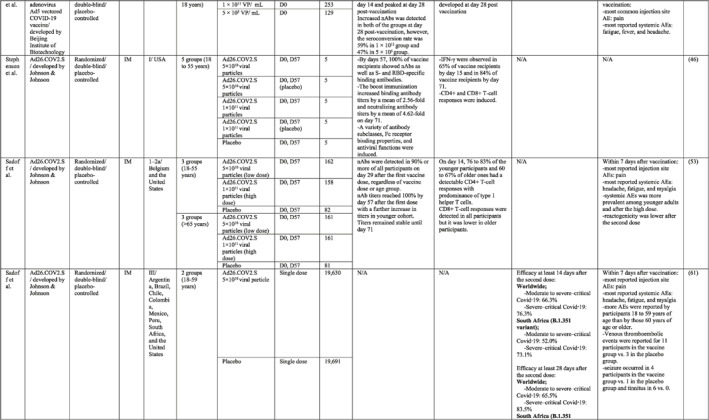
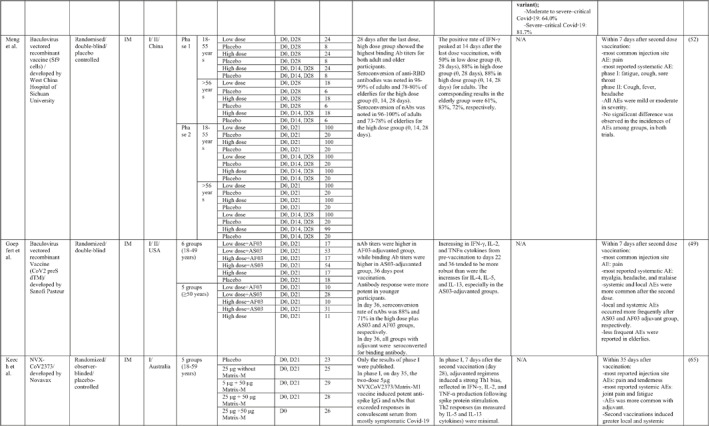
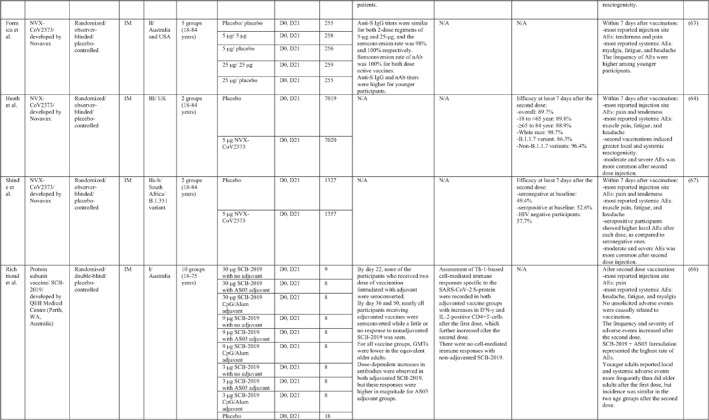
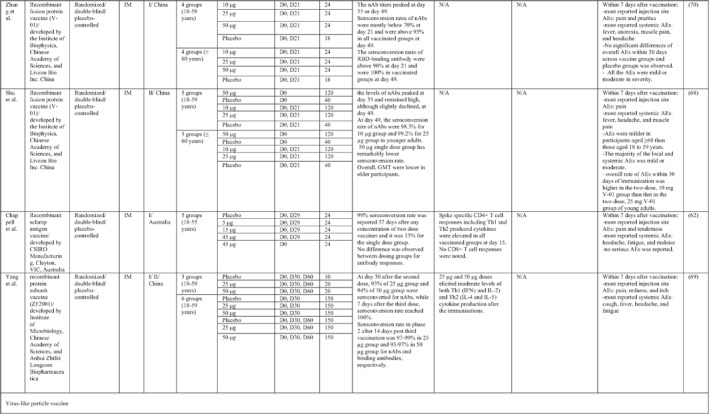
TABLE 1.
Immunogenicity, safety, and efficacy of COVID‐19 vaccines reported in recent clinical trials
|
|
|
|
|
|
|
|
|
|
|
|
|

|
Note: In this table, the study type, clinical phase, groups, immune responses, and adverse events related to current vaccines including inactivated vaccines, RNA‐vaccines, Vector‐based, recombinant, subunit, and virus‐like particle vaccines were separately addressed.
Abbreviations: Ab, antibody; AE, adverse event; IFNγ, Interferon gamma; IM, intra‐muscular; nAbs, neutralising antibodies; NP, number of participants; RBD, receptor binding domain; S, SARS‐CoV‐2 spike protein; VP, viral particle.
3.1.1. Inactivated whole virus vaccines
A total of 15 studies were identified that provided clinical outcomes as well as vaccine immunogenicity and efficacy on the use of SARS‐CoV‐2 inactivated vaccines in healthy subjects. 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 Two studies 22 , 26 were in the phase I trial, five studies 25 , 28 , 29 , 30 , 31 in the phase I/II trial, three studies in the phase II trial, 19 , 21 , 32 and five studies in the phase III trial. 18 , 20 , 23 , 24 , 27
The reports of phase I and II trials of an inactivated SARS‐CoV‐2 vaccine (CoronaVac) developed by Sinovac Biotech in China showed that the nAbs and specific IgG antibodies were increased at 14 and 28 days after two‐dose vaccination with either 6 μg or 3 μg formulations and that the higher increase in antibody titres was observed in 6 μg group. 31 , 32 For adults aged 60 years or older, the seroconversion rate for nAbs reached 99% in 6 μg group 28 days post second immunisation. 28 A phase III trial of the vaccine was conducted in three countries (Turkey, Indonesia, and Chile). The overall vaccine efficacy ranged from 65.3% to 83.5%, and the vaccine was efficient in preventing hospitalisation by 87.5%–100%. Pain at the injection site was the most common local AE, while fatigue and myalgia were frequently reported systemic AEs. 23 , 24 , 27
Similar results were found by the phase I/II trial of two inactivated SARS‐CoV‐2 vaccines (WIV04 and HB02 strains) developed by Sinopharm's vaccine‐making unit. The results of both phases showed that high levels of nAbs and specific IgG antibodies could be developed after two injections by 21 or 28 days intervals compared with a shorter interval schedule (14 days interval). 29 , 30 The phase 3 trial that was conducted in the United Arab Emirates and Bahrain reported the overall vaccine efficacy to be 64% for the vaccine based on the WIV04 strain and 73.5% for the HB02 strain, which could completely prevent hospitalisation. 18 The most reported injection site AE was pain, and the highest rate of systemic AEs was for fever and headache.
The results of the phases I and II trials of the inactivated vaccine developed by Institute of Medical Biology (IMB) and the Chinese Academy of Medical Sciences (CAMS) reported that two injections of vaccine at day D0/D14 or D0/D28 induced high levels of nAbs, anti‐N, and anti‐S antibodies that were higher in the medium dose group with a 14‐days interval for phase I trial. 26 However, the antibody titres were higher in the high dose group with a 28‐days interval schedule for the phase II trial. 19 The most common injection side AE was pain, which was mild and self‐limiting. Slight fatigue and fever were the most systemic AEs.
Three phases of another inactivated vaccine (BBV152) developed by Bharat Biotech in collaboration with the Indian Council of Medical Research were completely conducted in India. In phase 1 with a 14‐day interval schedule, the seroconversion rate was higher for the 6 μg group with Algel‐IMDG, while in phase 2 that the doses were 28 days apart, the 3 μg group with Algel‐IMDG were seroconverted at higher rates. Both 3 µg and 6 μg regimens elicited detectable CD4+ and CD8+ T‐cell responses. 21 , 22 The overall efficacy of vaccine in phase 3 trial was 77.8% and it was 65.2% for B.1.617.2 variant. 20 The most commonly reported local and systemic AEs were pain at the injection site and fever in addition to headache, in descending order of frequency.
The last inactivated vaccine (KCONVAC) was developed by Shenzhen Kangtai Biological Products and Beijing Minhai Biotechnology in China and its findings from phase I/II trials have been published. It revealed that a 5 μg regimen group with 28‐day intervals elicited higher antibody titres than that of 10 μg groups with 14‐day intervals. The vaccine induced a moderate T‐cell response with higher rates of detection of interleukin (IL)‐2 than interferon (IFN)‐γ, post second dose injection. 25 Pain at inoculation site and fatigue were highly reported AEs.
3.1.2. Nucleic acid‐based vaccines
A total of 13 studies were identified that provided clinical outcomes as well as vaccine immunogenicity and efficacy of SARS‐CoV‐2 RNA vaccines in healthy subjects. Five studies 33 , 34 , 35 , 36 , 37 were in the phase I trial, two studies in the phase I/II trial, 38 , 39 one study in the phase II trial, 40 two studies in the phase II/III trial, 41 , 42 and three studies in phase III trial. 43 , 44 , 45 The mRNA coding for the S‐protein of SARS‐CoV‐2 was the most common antigen in these trials.
Phases I/II trials conducted by Pfizer/BioNtech in the USA reported the safety, tolerability, and immunogenicity data of BNT162b1, an mRNA vaccine, among 45 healthy adults. An increased concentration of RBD‐binding IgG was reported on day 21 after the first dose, which substantially increased on day 7 after the second dose injection. SARS‐CoV‐2 neutralisation antibodies were increased at day 28 (i.e. 7 days after the second dose). 39
In order to evaluate the potency and safety of BNT162b1 and BNT162b2, a phase I trial was applied in two age groups including 18–55‐ and 65–85‐years’ old subjects in the USA by Pfizer/BioNTech. Although antigen‐binding IgG and virus‐neutralising responses were boosted by the second dose (highest titre on day 28 or 35) in both the younger adults and the older adults, the older ones had lower levels of antibodies than the younger ones. 36 In older adults, BNT162b2 was related to a lower incidence and severity of systemic adverse events than BNT162b1. Similar results were obtained from a phase 1 trial that was conducted in China to evaluate the potency and safety of the BNT162b1 vaccine. 35 In general, the most reported local AE was pain, and the most common systemic AEs were fever, fatigue, and headache, which followed a dose‐dependent trend with the highest incidence in the 30 μg group. In addition, older participants represented a higher incidence of AEs than younger adults.
Cellular immune responses against BNT162b1 were assessed in a phase I/II trial in Germany. 38 On day 29, IFNγ was produced by a large fraction of RBD‐specific CD8+ and CD4+ T cells. The rates of CD4+ and CD8+ T cell response were lower in low dose groups compared with high dose groups. Higher serum nAbs were achieved 7 days after the booster dose. Although RBD‐binding IgG had increased in a dose‐dependent manner 21 days after the first dose and showed the booster response 7 days after the second dose (i.e. day 29 and day 43), the titres decreased after day 43 (except for the low dose group). 38
After selection of BNT162b2 for further advancement to safety and efficacy evaluation, a phase II/III trial was conducted in a multicenter setting for participants aged 16 or older. 41 The overall efficacy was estimated to be 95%. The efficacy of the vaccine in adolescent recipients was assessed in another phase III trial, and it was found that the vaccine was 100% efficient regardless of whether the recipient had a previous history of SARS‐CoV‐2. 45 Moreover, a phase III investigation has revealed that the vaccine efficacy dropped to 91.1% within a period of 6 months after receiving the first dose. In terms of assessing the efficacy of vaccines against variants of concern, BNT162b2 showed 100% protection against the B.1.351 variant. 42
Phases I and II trials of mRNA‐1273 vaccine developed by Moderna in the USA showed both humoural and cellular immune responses induction post‐vaccination. Two doses of vaccine with 28‐day intervals schedule induced high levels of binding IgG antibody and neutralising antibody, CD4 T‐cell (TNF, IL‐2, IFNγ production), and CD8 T‐cell responses which were increased in a dose dependent manner, with 100 μg regimen showed the highest seroconversion rate. Furthermore, the immune response was more potent in younger recipients and higher reactogenicity was also reported in this group of participants. Pain at the site of injection together with fatigue, headache, and myalgia were the most often reported local and systemic AEs, in a descending order in frequency. 33 , 37 , 40
Two phase III trials of mRNA‐1273 vaccines were undertaken in the USA and reported an overall vaccine efficacy of 93.2% and 94.1%. Both trials have come to the conclusion that the vaccine was 100% efficient in preventing severe COVID‐19 and death. 43 , 44
Another published finding of an RNA vaccine was related to the phase I trial of the CVnCoV vaccine, which was carried out in Germany and Belgium. A dose‐dependent increase in antibody response and the incidence of AEs was noted. Meanwhile, 100% of participants seroconverted to anti‐S IgG, while 83% seroconverted to nAbs in the 12 μg group, 2 weeks after second inoculation. 34
3.1.3. Vector‐based SARS‐CoV‐2 vaccines
A total of 16 studies were identified that provided clinical outcomes as well as vaccine immunogenicity and efficacy on the use of vector‐based SARS‐CoV‐2 vaccines in healthy subjects. Two studies were in phase I trial, 46 , 47 seven studies in phase I/II trial, 48 , 49 , 50 , 51 , 52 , 53 , 54 one study in phase II trial, 55 three studies in phase II/III trial, 56 , 57 , 58 and three studies in phase III trial. 59 , 60 , 61
In a phase I/II trial by Folegatti et al. 54 in the UK, the immune response and reactogenicity of the ChAdOx1 nCoV‐19 (COV001) vaccine were evaluated. Spike‐specific T‐cell and anti‐spike IgG responses peaked on day 14 and day 28, respectively. Anti‐spike IgG responses were increased following the booster dose. Also, neutralising activity was seen in all participants after the second dose. Pain and tenderness, fatigue, and headache were the most commonly reported injection site and systemic reactions, respectively. Moreover, another phase I/II trial of the ChAdOx1 nCoV‐19 vaccine was tested in order to compare the immunogenicity and safety of standard dose or half dose booster vaccination. It was demonstrated that a half‐dose boost elicited lower immune responses with lower induction of AEs. 48 Results of phase II/III ChAdOx1 nCoV‐19 vaccine in the UK, aiming to investigate the effects of two different doses of vaccine in three age groups, showed no significant variations in anti‐spike IgG and nAb titres between low‐dose and standard‐dose vaccine recipients. Besides, within a week post second vaccination, older adults showed a lower incidence of AEs as compared to younger counterparts. 57
The overall vaccine efficacy was 70.4%, with a higher efficacy as 90% in the low dose prime vaccine regimen than 62.1% in standard dose prime vaccine regimen. Notably, three trials were conducted later in order to examine the efficacy of the vaccine against different types of SARS‐CoV‐2 variants. It has been estimated that ChAdOx1 nCoV‐19 is 63.6% efficient against the P.1 variant, 61.7% against the B.1.1.7 variant, and only 10.6% against the B.1.351 variant. 51 , 56 , 59
In a phase I/II trial, a heterologous COVID‐19 vaccine (Gam‐COVID‐Vac or Sputnik V) consisting of a recombinant adenovirus type 26 (rAd26) vector and a recombinant adenovirus type 5 (rAd5) vector, both carrying the spike glycoprotein gene of SARS‐CoV‐2, was tested in Russia. SARS‐CoV‐2 RBD‐specific IgGs were detected on day 14 after the last immunisation in all participants. Cell‐mediated responses (IFNγ production or lymphoproliferation) were detected in all participants on day 28. 50 The phase III of the vaccine showed that the seroconversion rate for RBD‐specific IgG and nAbs was 98.25% and 95.83%, respectively. Also, the trial reported 91.1% overall efficacy for the vaccine that could protect individuals against moderate or severe forms of COVID‐19 completely. 60 Common local AEs was pain at the injection site and headache and asthenia were frequently reported systemic AEs.
In other two clinical trials conducted in Belgium and the USA, the safety and potency of the Ad26 vectored COVID‐19 vaccine (Ad26.COV2.S) developed by Johnson & Johnson were tested in phase I/II trials. The vaccine induced acceptable titres of nAbs and T‐cell responses after the second dose of vaccine. 46 , 53 The worldwide efficacy analysis of the Ad26.COV2.S vaccine in a single dose manner estimated 66.3% protection against moderate to severe forms of the disease and 73.1% against severe to critical form. In addition, the vaccine was 52% efficient in preventing B.1.351 variant infection. 61 Pain at the site of injection together with headache, fatigue, and myalgia are considered the most commonly reported AEs.
In addition, in a first‐in‐human phase I trial conducted by Zhu et al. in China, the safety and immunogenicity of the Ad5 vectored COVID‐19 vaccine developed by Beijing Institute of Biotechnology (Beijing, China) and CanSino Biologics (Tianjin, China) were tested in healthy adults. 47 A single intramuscular (IM) injection of the vaccine was tested in a dose‐escalation manner, including three viral particle doses in phase I and two viral particle doses in phase II. 55 Antibody responses against RBD were detected from day 14, in all three groups and peaked at day 28. Produced IFNγ from CD4+ and CD8+ T cells was reported at day 14 and 28 post vaccination. In addition to the injection site pain as a frequent local AE, fever, fatigue, and headache were the most reported systemic AEs, with a higher incidence in the greater viral particle group.
Apart from adenoviral vectored vaccines, two other phase I/II trials utilised baculovirus as a vaccine vector. 49 , 52 The first trial, which was carried out in China, reported that the seroconversion rate of nAbs was 96%–100% in adults and 73%–78% in the elderly for the high dose group with triple injections. 52 The latter trial evaluated the immune response of the vaccine with two different adjuvants. It found that 36 days after vaccination, nAb titres were higher in the AF03‐adjuvanted group, while binding Ab titres were higher in the AS03‐adjuvanted group, with full seroconversion of all participants receiving the vaccine plus adjuvant. Furthermore, local and systemic AEs occurred more frequently after AS03 and AF03‐adjuvanted groups, respectively. 49
3.1.4. SARS‐CoV‐2 Subunit vaccines
A total of nine studies were identified that provided clinical outcomes as well as vaccine immunogenicity and efficacy on the use of vector‐based SARS‐CoV‐2 vaccines in healthy subjects. 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 Four studies were in phase I trials, 62 , 65 , 66 , 70 one in phase I/II trial, 69 three in phase II trials, 63 , 67 , 68 and one in phase III trial. 64
NVX‐CoV2373, a SARS‐CoV‐2 Recombinant Spike Protein Nanoparticle Vaccine, contains purified coronavirus spike (S) protein (named SARS‐CoV‐2 rS) adjuvanted with Novavax's patented saponin‐based Matrix‐M. The results of the phase I trial, which was conducted in Australia, showed that two‐doses of 5‐μg adjuvanted regimen elicited robust anti‐spike IgG and nAbs, which were numerically (4‐6 and 4‐fold, respectively) superior to those seen in human convalescent sera. Increased Th1 responses were reported at day 28 post vaccination. 65 The phase II trial assessed the immunogenicity and safety of the NVX‐CoV2373 vaccine in two different dose regimens as a single or double inoculation in healthy adults. No difference was observed in terms of anti‐S IgG titres for both 2‐dose regimens of 5‐μg and 25‐μg, and the seroconversion rate was 98% and 100%, respectively. Anti‐S IgG and nAb titres were higher for younger participants, with a higher frequency of AEs in this age group. 63 The result of the safety analysis revealed more severe AEs after the second dose injection. Findings of the NVX‐CoV2373 phase III trial noted an overall vaccine efficacy of 89.7% that dropped to 86.3% against the B.1.1.7 variant. 64 In terms of protecting against the B.1.351 variant, vaccine performed less effectively, such that the efficacy was 49.4% in the baseline seronegative group and 52.6% in the baseline seropositive group. 67
Phases I and II trials of a recombinant COVID‐19 vaccine (V‐01) containing fusion protein of the SARS‐CoV‐2 were conducted in China. Levels of nAb titres peaked 2 weeks after the second dose and remained high, although slightly declined, 4 weeks later. Almost all vaccinated participants developed RBD‐binding antibodies and nAb responses after the boost dose, with lower immunogenicity in single dose group. Vaccine related AEs were milder in older recipients and those who received higher dose regimens. In addition to pain as the most recorded local AE, fever, headache, and muscle pain were accounted as frequently reported systemic AEs. 68 , 70
Another protein subunit vaccine (SCB‐2019) was developed by QEII Medical Centre in Australia and tested in a phase I trial by Richmond et al. 66 They demonstrated that nearly all participants in vaccine group formulated with adjuvant were seroconverted while a little or no antibody response to non‐adjuvanted SCB‐2019 was seen. Likewise, IFN‐γ and IL‐2‐positive CD4+T‐cells were increased in adjuvanted groups with no cell‐mediated immune response for non‐adjuvanted group. Considering the safety profile of the vaccine, the frequency and severity of AEs increased after the second dose, with a predominance of pain at the injection site, headache, and fatigue. 66
Immunisation with a recombinant vaccine developed by Chappell et al., which comprises a trimeric glycosylated SARS‐CoV‐2 spike glycoprotein ectodomain, induced an effective antibody response in all immunised individuals with a booster dose, 4 weeks after the second vaccination, with no difference in antibody titres in dosing groups. Besides, the number of CD4+ T cells was found to be increased in all vaccine‐treated groups, and no CD8+ T cell responses were detected. Investigating the vaccine safety profile, none of the AEs were serious. 62
ZF2001, as a SARS‐CoV‐2 subunit vaccine, developed by the Institute of Microbiology in China. Triple dose regimens in the phase I/II trial showed a robust induction of both neutralising and binding antibodies and that higher titres were elicited in low dose group of 25 μg. In addition, the vaccine was able to evoke a moderated level of both Th1 (IFNγ and IL‐2) and Th2 (IL‐4 and IL‐5) cytokine production. Within a week after immunisation, pain, redness, and itching were more probable local AEs, while cough, fever, and headache were considered to be highly reported systemic AEs. 69
3.1.5. SARS‐CoV‐2 virus‐like particle (VLP) vaccines
One VLP‐based COVID‐19 vaccine candidate was produced by Medicago. 71 The immunogenicity results of the Phase I trial of Medicago's COVID‐19 vaccine candidate, which is a plant‐derived Coronavirus Virus‐Like Particle (CoVLP), demonstrated that CoVLP alone elicits weak humoural but modest cellular responses while adjuvanted formulations (GSK pandemic adjuvant (AS03), and Dynavax's CpG 1018™) have the potential to improve anti‐spike IgG and nAbs as well as IFNγ and IL4 responses. There were no serious solicited local and systemic AEs and pain was the most commonly reported injection site reaction. Their findings suggest that the CoVLP (3.75 μg)+AS03 regimen could be considered for further clinical assessment. 71
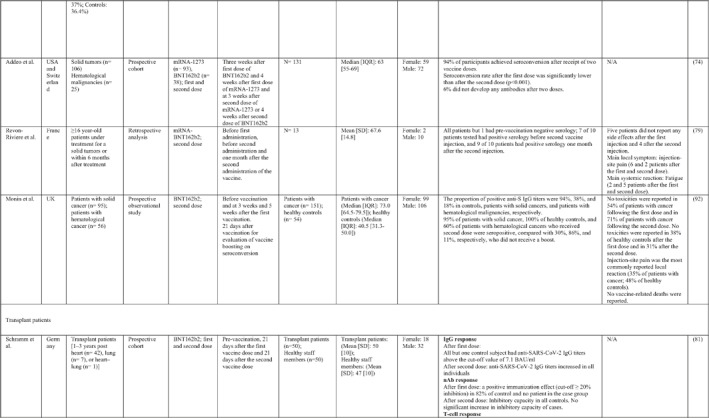
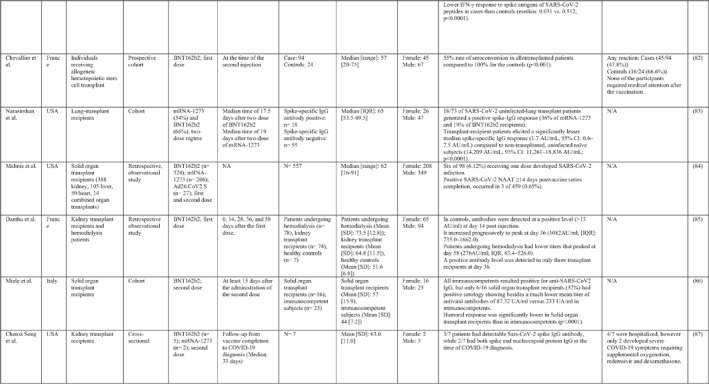
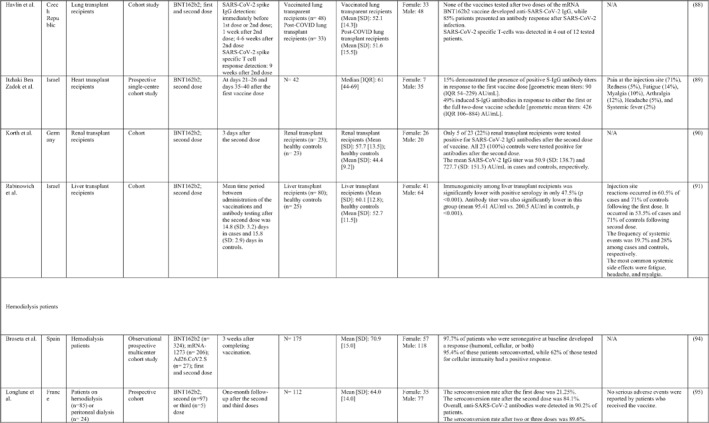
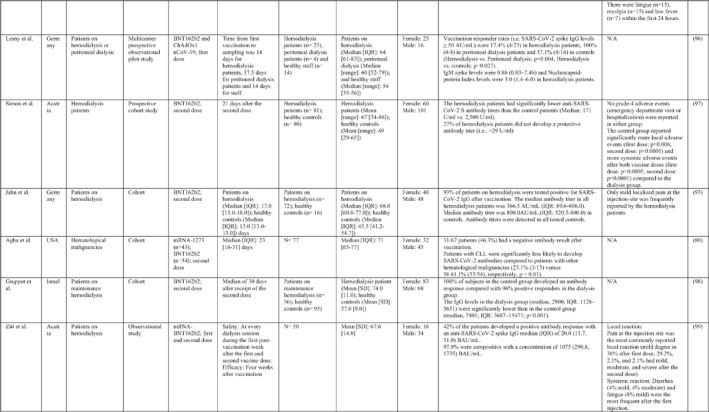
3.2. Immunogenicity and safety of COVID‐19 vaccines in patients with underlying diseases of interest
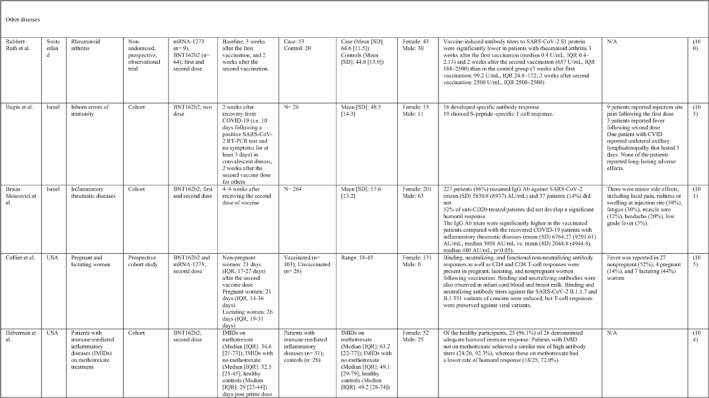
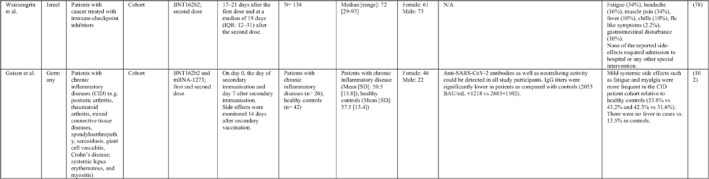
A total of 34 studies have assessed the immunogenicity and/or safety of COVID‐19 vaccines in patients with different underlying disorders or specific conditions such as malignancies (i.e. solid tumours and haematological malignancies), 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 transplant recipients, 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 individuals on dialysis, 85 , 94 , 95 , 96 , 97 , 98 , 99 inflammatory rheumatic diseases, 100 , 101 , 102 inborn errors of immunity, 103 immune‐mediated inflammatory diseases, 104 and pregnant or lactating women. 105 All studies evaluated the effects of mRNA COVID‐19 vaccines (i.e. BNT162b2 and mRNA‐1273 COVID‐19 vaccines) except for two studies that also assessed the COVID‐19 adenovirus platform (i.e. ChAdOx1/nCoV‐19) 96 , 106 (Table 2).
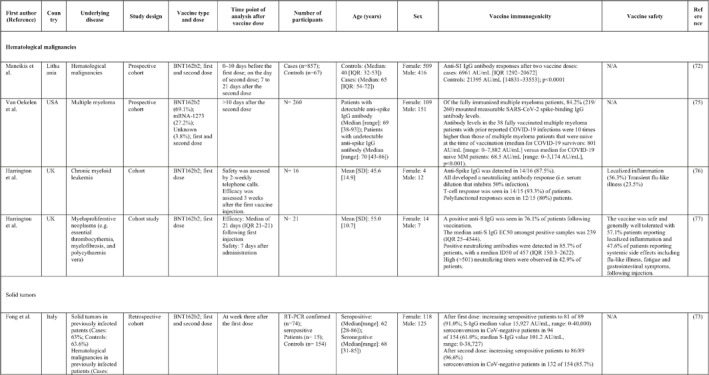
TABLE 2.
The immunogenicity and safety of COVID‐19 vaccines in individuals with specific underlying disorders
|
|
|
|
|
|
|
Note: Studies assessing the immunogenicity of vaccines in patients with underlying disease are reviewed separately in this table.
Abbreviations: CID, chronic inflammatory diseases; CLL, chronic lymphocytic leukaemia; CVID, common variable immunodeficiency; IFNγ, Interferon gamma; IMIDs, immune‐mediated inflammatory diseases; nAbs, neutralising antibodies; RT‐PCR, real time polymerase chain reaction.
In general, immunocompromised patients, such as organ transplantation patients and those suffering from cancers, particularly haematological malignancies, developed lower levels of immune response to COVID‐19 vaccines. A study that only included participants with multiple myeloma showed that previous SARS‐CoV‐2 infection led to a significantly higher SARS‐CoV‐2 spike‐binding IgG antibody levels than non‐infected patients (p < 0.001). 75 Also, treatment with anti‐CD38 agents was associated with antibody production among these patients (odds ratio (OR): 4.25; p = 0.005). 75 In another study on adolescents and young adults with cancer, 90% developed seroconversion 1 month after vaccination, while it was 80% 3 weeks following vaccination. 79 Among recipients of allogenic stem cell transplantation compared with controls, there was a significantly lower rate of seroconversion following the first dose of the BNT162b2 COVID‐19 vaccine (55% vs. 100%; p < 0.001) 82 (Table 2). Examining whether COVID‐19 vaccines are immunogenic enough in pregnant and lactating women, a recent study revealed that binding, neutralising, and functional non‐neutralising antibody responses as well as CD4+ and CD8+ T‐cell responses were present at an optimal level in this population. Binding and neutralising antibodies were also observed in infant cord blood and breast milk. 105
The most common local and systemic adverse events in patients with different underlying diseases of interest who received COVID‐19 vaccines were pain at the injection site and fatigue, respectively. 77 , 89 , 91 , 95 , 99 , 101 Other reactions like myalgia, arthralgia, headache, fever, and gastrointestinal disturbances were also reported, but none of them were serious or life‐threatening 78 , 89 , 101 (Table 2).
3.3. Incidence of AESI post COVID‐19 vaccination in the USA
As of 22 November 2021, 262.23 million doses of Pfizer/Biotech, 172.32 million doses of Moderna, and 16.44 doses of Janssen vaccines were administrated in the USA. 107 Overall, the AESI was more likely to occur after receiving the Janssen vaccine. Venous and arterial thrombotic events, Bell's palsy, and myocarditis/pericarditis were more common post immunisation. Although venous and arterial thrombotic events, Bell's palsy, Guillian‐Barre syndrome, and transverse myelitis were often reported in 65–79 age group, myocarditis/pericarditis and anaphylaxis were reported frequently in 18–29 age group and 30–39 age group, respectively. Unlike other AESI, the occurrence of myocarditis/pericarditis, and anaphylaxis followed a sex distribution pattern which mostly involved males. Table 3 summarises the comparative frequency of the AESI per million doses of the three vaccines, together with the frequency and rate of these events stratified by age and sex groups.
TABLE 3.
Adverse event of special interest post vaccination in the USA
| Venous and arterial thrombotic events | Bell's palsy | Myocarditis/pericarditis | Anaphylaxis | Guillian‐Barre syndrome | Transverse myelitis | |
|---|---|---|---|---|---|---|
| Vaccine manufacture | ||||||
| Pfizer/BioNtech | ||||||
| n | 2997 | 2457 | 1984 | 1076 | 307 | 84 |
| Per million dose | 11.43 | 9.37 | 7.57 | 4.1 | 1.17 | 0.32 |
| Moderna | ||||||
| n | 2338 | 1856 | 1056 | 684 | 224 | 67 |
| Per million dose | 13.57 | 10.77 | 6.13 | 3.97 | 1.3 | 0.39 |
| Johnson & Johnson's Janssen | ||||||
| n | 1695 | 417 | 134 | 112 | 203 | 26 |
| Per million dose | 103.1 | 25.35 | 8.15 | 6.81 | 12.35 | 1.58 |
| Sex | ||||||
| Male | ||||||
| n | 2845 | 1949 | 2186 | 1504 | 365 | 74 |
| % | 41.55 | 41.83 | 69.91 | 83.6 | 51.19 | 41.57 |
| Female | ||||||
| n | 4002 | 2710 | 941 | 295 | 348 | 104 |
| % | 58.45 | 58.17 | 30.1 | 16.4 | 48.81 | 58.43 |
| Age | ||||||
| <18 | ||||||
| n | 54 | 36 | 493 | 19 | 11 | 1 |
| % | 0.85 | 1.93 | 23.68 | 6.79 | 3.25 | 0.61 |
| 18–29 | ||||||
| n | 336 | 125 | 742 | 41 | 20 | 14 |
| % | 5.28 | 6.72 | 35.64 | 14.64 | 5.92 | 8.54 |
| 30–39 | ||||||
| n | 847 | 259 | 322 | 62 | 24 | 31 |
| % | 13.31 | 13.92 | 15.47 | 22.14 | 7.10 | 18.90 |
| 40–49 | ||||||
| n | 1146 | 352 | 172 | 43 | 55 | 27 |
| % | 18.01 | 18.91 | 8.26 | 15.36 | 16.27 | 16.46 |
| 50–59 | ||||||
| n | 1236 | 394 | 139 | 42 | 79 | 28 |
| % | 19.42 | 21.17 | 6.68 | 15 | 23.37 | 17.07 |
| 60–64 | ||||||
| n | 668 | 199 | 55 | 25 | 42 | 18 |
| % | 10.5 | 10.69 | 2.64 | 8.93 | 12.43 | 10.98 |
| 65–79 | ||||||
| n | 1574 | 401 | 140 | 40 | 87 | 37 |
| % | 24.74 | 21.55 | 6.72 | 14.29 | 25.74 | 22.56 |
| >80 | ||||||
| n | 502 | 95 | 19 | 8 | 20 | 8 |
| % | 7.9 | 5.10 | 0.91 | 2.86 | 5.92 | 4.88 |
4. COVID‐19 VACCINES AND OMICRON VARIANT
A new SARS‐CoV‐2 variant named Omicron (B.1.1.529), has been announced by WHO as a variant of concern on 26 November 2021. This newly emerged variant showed 32 amino acid alterations in the spike protein, which included three deletions and one insertion. As previously discussed, currently available COVID‐19 vaccines mostly target the S protein. The variant's ability to evade current vaccines may be considerably enhanced as a result of these changes. 108 , 109
However, research is currently underway into the effect of COVID‐19 vaccines on Omicron prevention. Viruses undergo continual mutations as they spread throughout time. This can lead to the emergence of novel varieties, such as the SARS‐CoV‐2 Omicron variant. The spike protein of Omicron has a lot of mutations, which makes it easier for it to attach to cells and infect them. As a result, Omicron is easier to disseminate and generates more infections than prior virus variants. COVID‐19 vaccines are being studied to see how well they protect against Omicron and other variants. To this end, studies are being conducted to investigate the humoural and cellular immunity induced by COVID‐19 vaccines against the Omicron variant. The current studies discovered evidence that existing vaccinations could induce cellular immunity against Omicron. In a study by Tarke A et al., T cell responses against COVID‐19 variants in vaccinated people were assessed. To this end, samples from 96 people who had gotten one of four vaccines (Pfizer‐BioNTech, Moderna, Johnson & Johnson/Janssen, or Novavax) were examined. Six months after immunisation, they found significantly fewer memory B cells and neutralising antibodies in people's blood. Unlike antibodies, T cell responses from the vaccinations detected all variations, including Delta and Omicron. When compared to early variants, 84% of CD4+ (helper) T cell responses and 85% of CD8+ (killer) T cell responses to Omicron remained the same 6 months after immunisation. 110
Another study by Liu J et al., examined samples from 47 people vaccinated with Johnson & Johnson or Pfizer‐BioNTech vaccines. These people showed robust T cell responses against Delta and Omicron variants following final vaccination. 111 Accordingly, Omicron was found to evade antibody neutralisation by the Pfizer–BioNTech vaccine in the live‐virus neutralisation experiments. 112 In addition, the effectiveness of the BNT162b2 vaccine has also been reported in preventing the hospitalisation of patients with the Omicron variant in South Africa. 113 Another study by Muik A et al, showed that three doses of the BNT162b2 could increase Omicron‐neutralising titres, suggesting probable protection against Omicron‐mediated COVID‐19. 114 These data could provide evidence that current vaccines have the potency to induce potent cellular immunity but low antibody responses to the SARS‐CoV‐2 Omicron variant.
5. DISCUSSION
The newly emerged coronavirus, SARS‐CoV‐2, and the associated disease, COVID‐19, have become a worldwide pandemic with a considerable rate of mortality and morbidity. 1 According to the World Health Organization (WHO), global dissemination of SARS‐CoV‐2 may continue until there is a high level of population immunity around the world. SARS‐CoV‐2 is an enveloped, positive‐sense, single‐stranded RNA virus that belongs to the family of coronaviruses. 2 For a superior understanding of the SARS‐CoV‐2, great research has been performed, and different strategies have been established with the aim of developing safe drugs and efficient vaccines. 115 According to our extensive search, a variety of COVID‐19 vaccines are being evaluated in different stages of clinical trials globally and will be authorised or approved only if they make it substantially less likely that we will get COVID‐19. According to the WHO reports, some of these vaccines have been approved for use in some countries (Table 4).
TABLE 4.
Eight approved SARS‐CoV‐2 vaccines by December 2021
| Vaccine name | Type | Developer | Number of countries approved the vaccine | Number of trials |
|---|---|---|---|---|
| BNT162b2 | RNA vaccine | Pfizer/BioNTech | 112 | 46 trials in 21 countries |
| mRNA1273 | RNA vaccine | Moderna | 78 | 33 trials in 8 countries |
| Ad26.COV2.S | Vector‐based | Johnson & Johnson | 85 | 16 trials in 18 countries |
| AZD1222 | Vector‐based | Oxford AstraZeneca | 127 | 50 trials in 23 countries |
| Covishield | Vector‐based | Oxford AstraZeneca formulation | 47 | 2 trials in 1 country |
| Covaxin | Inactivated vaccine | Bharat Biotech | 12 | 7 trials in 1 country |
| BBIBP‐CorV | Inactivated vaccine | Sinopharm‐Beijing | 72 | 19 trials in 10 countries |
| CoronaVac | Inactivated vaccine | Sinovac | 46 | 26 trials in 8 countries |
SARS‐CoV‐2 consists of sixteen non‐structural proteins, namely nsp1 to nsp16, and four structural proteins that form the main structure of the virus. 116 Among these structural and non‐structural proteins, the S protein is located on the surface of SARS‐CoV‐2 and binds to human angiotensin‐converting enzyme 2 (hACE2), which plays a critical role in the virulence of the virus 117 , 118 and elicits effective cellular and humoural immune responses. 119 , 120 S protein (especially RBD) is considered as an important target for SARS‐CoV‐2 vaccines. 121 On the other hand, the titres of neutralising antibodies and the levels of anti‐RBD IgG, and RBD‐specific IgG titres were significantly correlated with each other. 122 , 123 Therefore, RBD is considered a promising target for SARS‐CoV‐2 vaccines.
To provide an effective immune response to immunisation, it is necessary to stimulate both the innate and adaptive immune systems (Figure 3). 124 Humoural and cellular immune responses induced by SARS‐CoV‐2 vaccines have been reported mostly by the titters of RBD‐specific IgG, neutralising antibodies, and the levels of T cell cytokines, especially IFNγ after vaccination (Table 1).
FIGURE 3.
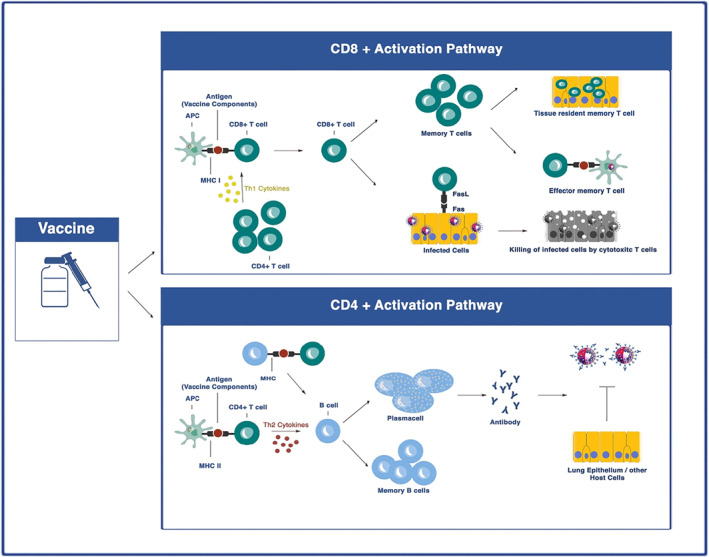
Vaccine‐induced immune responses. Antigen‐presenting cells (APCs) including dendritic cells (DC) can process and present vaccine antigens to both CD4+ and CD8+ T cells. CD4+ T cells that activated by SARS‐CoV‐2 vaccine antigens presented by APCs can production Th2 cytokines which help B cells to differentiate into plasma cells and memory B cells. The activated B cells can produce neutralising antibodies (nAbs). CD8+ T cells can be activated by Th1 cytokines and acquire the ability to attack and lysis the SARS‐CoV‐2 infected cells
Whole inactivated vaccines have been used effectively for immunisation against several viral diseases such as influenza, poliovirus, hepatitis A, SARS, and rabies. 125 , 126 , 127 Several advantages have made them popular, including rapid and easy production processes, high stability, strong immune responses, usability in immunodeficient subjects, and no risk for virus activation. 125 Humoural immunity and production of nAbs against virus antigens, especially S proteins of SARS‐CoV‐2, are the main immune responses against inactivated vaccines. Antibody titres against these types of vaccines diminish with time after the first vaccination. Therefore, a protective and efficient humoural immune response develops after the second or third dose of vaccine mixed with adjuvants, resulting in “boost” antibody titres. 29 , 128 In accordance with this, it has been shown that a longer interval (about 28 days) between the first and second injections of vaccine could induce higher antibody responses in humans and animal models. 32 , 129 , 130 Although a low dose of vaccine after two or three boosters can elicit an efficient immune response, participants who received high dose of inactivated vaccine had higher levels of nAbs and specific IgG antibodies in their sera. 19 In addition to considering the interval time, using appropriate adjuvants would also be an inevitable factor in eliciting robust immune responses. NDV/SARS‐CoV‐2, an inactivated SARS‐CoV‐2 vaccine induced the highest titre of S‐specific Ab in BALB/c mice when administered with R‐DOTAP as an adjuvant compared with using Addavax. 131 Of note, the frequent adjuvant which used in human trials is aluminium hydroxide (Al(OH)3). 19 , 29
In contrast to the inactivated vaccines, which needed several booster doses and an adjuvanted formulation, the protective immunity against live‐attenuated vaccines could be developed after a single, small dose of vaccine. The main concern about these vaccines is the possibility of a reversion of virus pathogenicity. In addition, these types of vaccines cannot be used in immunocompromised persons. 132 Live‐attenuated SARS‐CoV‐2 vaccines are currently being tested in pre‐clinical studies. 133 Some trials have been registered to evaluate the efficacy of live‐attenuated SARS‐CoV‐2 vaccines in healthy subjects (NCT04475081 and NCT04619628), but there are no published results yet.
Although subunit vaccines are safer than whole virus vaccines, these proteins are less immunogenic in the absence of other viral components. Therefore, subunit vaccines often require higher doses, booster schedule, and concomitant administration of adjuvants to enhance antigen‐specific immunity. 134 Phases I and II results of NVX‐CoV2373 developed by Novavax showed that two injection (D0,D21) of vaccine with adjuvant (Matrix‐M) could elevate humoural and cellular immune responses including high anti‐spike IgG and nAbs, and Th1 responses in adults, with an overall efficacy of 89.7%. 65 These studies showed that the efficacy and immunogenicity of the subunit vaccine greatly depend on how the subunit protein is presented to immune cells, co‐administration with adjuvants, adjuvant type, etc.
Nucleic acid‐based immunisation has emerged as a promising alternative to conventional vaccine approaches to protection against SARS‐CoV‐2. Virus particles are not used in the process of nucleic acid‐based vaccine production, so they are non‐infectious vaccines. Besides, the RNA strand could not integrate into the host genome, which is made of DNA, and it degrades when the protein is made. Importantly, production process of these vaccines is cost‐efficient and could be produced rapidly 8 and both cellular and humoural immune responses could be elicited by them. These advantages make them popular among conventional approaches, although it should be noted that these vaccines would need to be maintained frozen, which could affect vaccine distribution. Their immunogenicity and efficacy have been evaluated in several pre‐clinical and clinical studies. BNT162b2 developed by Pfizer and mRNA‐1273 developed by Moderna as human RNA vaccines could elicit a potent immune response against SARS‐CoV‐2. Although efficient immunity was shown to be induced after a single dose of some RNA vaccines such as mRNA1273 and mRNA‐LNP in pre‐clinical studies 135 , 136 but booster schedule effectively induced both humoural and cellular immunity in human adults. 36 , 37 , 38
Most clinical trials of SARS‐CoV‐2 RNA vaccines showed durable immunogenicity until about 28 days post‐vaccination. 36 , 38 , 39 mRNA‐1273 developed by Moderna/NIAD showed increased levels of antibody titres even on day 57 post‐vaccination. 37 BNT162b1 developed by Pfizer/BioNTech showed decreased levels of nAbs and RBD‐binding antibodies on day 43 (21 days after the boost) in most vaccinated groups (with the exception of the 1 μg dose group), 38 while it seems that RNA vaccines could be considered as potential immunisation approaches against SARS‐CoV‐2. In addition, these vaccine platforms showed a high efficacy against the most recent emerged infectious variants. 42
Viral vector‐based vaccines can increase immunogenicity without an adjuvant and elicit a strong cytotoxic T lymphocyte (CTL) response to eliminate virus‐infected cells. Several types of vectors have been used in order to deliver genetic code for antigens (e.g. the gene for spike protein) into human cells. Among them, adenoviruses are widely used as viral‐vectors due to their high transduction efficacy and high level of transgene expression. 9 Besides, a single dose of SARS‐CoV‐2 vaccine elicited both humoural and cellular immune responses in healthy adults. 47 , 50 , 54 , 55 As several studies show, the efficacy of vaccines could be affected by several factors. Selecting an appropriate antigen is an important factor for inducing a potent immune response.
As patients with pre‐existing diseases, particularly immunocompromised ones, were not enroled in the main trials, the efficacy and safety of COVID‐19 vaccines have yet to be clarified. These patients are at a high risk of mortality due to the effects of SARS‐CoV‐2 and the ineffectiveness of the vaccines, both due to immunosuppression status related to the therapeutic agents they receive and the underlying condition. Future trials should be guided towards adjusting the dose and time interval of administering vaccines for these patients to efficiently respond to immunisation.
Although approximately no serious AEs were reported in clinical trials of COVID‐19 vaccines, some rare events following public mass administrations have been reported in recent publications. Using the VAERS database, we demonstrated that venous and arterial thrombotic events, bell's palsy, and myocarditis/pericarditis were commonly reported AESI post immunisation. Therefore, it seems that follow‐up surveillance after a recipient of the COVID‐19 vaccine must be strengthened in order to prevent the occurrence or manage the condition at the earliest possible time. Furthermore, assigning several programs across the world to gather the characteristics, clinical presentation, progress, and outcomes of individuals affected by the AESI post administration of COVID‐19 vaccination would be necessary to provide clinicians with the most optimal way of treating patients and inform the expert advisory body regarding COVID‐19 vaccination.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
AUTHOR CONTRIBUTIONS
Nima Rezaei directed the project. Mona Sadeghalvad, Amir Hossein Mansourabadi and Nima Rezaei designed research. Data extraction performed by Mona Sadeghalvad, Amir Hossein Mansourabadi, Masoomeh Masoomikarimi, Masoumeh Alimohammadi, Maryam Noori, and Seyed Aria Nejadghaderi, The paper was draughted by Mona Sadeghalvad, Amir Hossein Mansourabadi, Masoomeh Masoomikarimi, Masoumeh Alimohammadi, Maryam Noori, and Seyed Aria Nejadghaderi. Nima Rezaei did critical revision of the paper. All the authors contributed to protocol development, read and finally approved the paper.
Supporting information
Supporting Information S1
ACKNOWLEDGEMENT
This study was supported by a research grant received from the of Tehran University of Medical Sciences, Tehran, Iran (No. 98‐01‐30‐41086).
Sadeghalvad M, Mansourabadi AH, Noori M, et al. Recent developments in SARS‐CoV‐2 vaccines: a systematic review of the current studies. Rev Med Virol. 2022;e2359. 10.1002/rmv.2359
Mona Sadeghalvad and Amir Hossein Mansourabadi contributed equally to the manuscript.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Mansourabadi AH, Sadeghalvad M, Mohammadi‐Motlagh H‐R, Rezaei N. The immune system as a target for therapy of SARS‐CoV‐2: a systematic review of the current immunotherapies for COVID‐19. Life Sci. 2020;2020:118185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gorbalenya AE, Baker SC, Baric RS, et al. The species Severe acute respiratory syndrome‐related coronavirus: classifying 2019‐nCoV and naming it SARS‐CoV‐2. Nat Microbiol. 2020;5(4):536‐544. 10.1038/s41564-020-0695-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yang L, Tian D, Liu W. Strategies for vaccine development of COVID‐19. Chin J Biotechnol. 2020;36(4):593. [DOI] [PubMed] [Google Scholar]
- 4. Organization WH. World health organization (WHO) coronavirus disease (COVID‐19). Dashboard 2021. https://covid19.who.int/
- 5. Jeyanathan M, Afkhami S, Smaill F, Miller MS, Lichty BD, Xing Z. Immunological considerations for COVID‐19 vaccine strategies. Nat Rev Immunol. 2020;20(10):615‐632. 10.1038/s41577-020-00434-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chou R, Dana T, Buckley DI, Selph S, Fu R, Totten AM. Epidemiology of and risk factors for coronavirus infection in health care workers: a living rapid review. Ann Intern Med. 2020;173(2):120‐136. 10.7326/m20-1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sanders B, Martin K, Schuitemaker H. Inactivated Viral Vaccines, p 45–80. Vaccine Analysis: Strategies, Principles, and Control. Springer; 2014. [Google Scholar]
- 8. Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261‐279. 10.1038/nrd.2017.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ura T, Okuda K, Shimada M. Developments in viral vector‐based vaccines. Vaccines (Basel). 2014;2(3):624‐641. 10.3390/vaccines2030624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khalaj‐Hedayati A. Protective immunity against SARS subunit vaccine candidates based on spike protein: lessons for coronavirus vaccine development. J Immunol Res. 2020;2020:7201752‐7201811. 10.1155/2020/7201752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mohsen MO, Zha L, Cabral‐Miranda G, Bachmann MF. Major findings and recent advances in virus‐like particle (VLP)‐based vaccines. Semin Immunol. 2017;34:123‐132. 10.1016/j.smim.2017.08.014 [DOI] [PubMed] [Google Scholar]
- 12. Xu R, Shi M, Li J, Song P, Li N. Construction of SARS‐CoV‐2 virus‐like particles by mammalian expression system. Front Bioeng Biotechnol. 2020;8:862. 10.3389/fbioe.2020.00862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gomes AC, Mohsen M, Bachmann MF. Harnessing nanoparticles for immunomodulation and vaccines. Vaccines (Basel). 2017;5(1):6. 10.3390/vaccines5010006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Donaldson B, Lateef Z, Walker GF, Young SL, Ward VK. Virus‐like particle vaccines: immunology and formulation for clinical translation. Expet Rev Vaccine. 2018;17(9):833‐849. 10.1080/14760584.2018.1516552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021:372. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Varricchio F, Iskander J, Destefano F, et al. Understanding vaccine safety information from the vaccine adverse event reporting system. Pediatr Infect Dis J. 2004;23(4):287‐294. 10.1097/00006454-200404000-00002 [DOI] [PubMed] [Google Scholar]
- 17. Shimabukuro TT, Nguyen M, Martin D, DeStefano F. Safety monitoring in the vaccine adverse event reporting system (VAERS). Vaccine. 2015;33(36):4398‐4405. 10.1016/j.vaccine.2015.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Al Kaabi N, Zhang Y, Xia S, et al. Effect of 2 inactivated SARS‐CoV‐2 vaccines on symptomatic COVID‐19 infection in adults: a randomized clinical trial. JAMA. 2021;326(1):35‐45. 10.1001/jama.2021.8565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Che Y, Liu X, Pu Y, et al. Randomized, double‐blinded and placebo‐controlled phase II trial of an inactivated SARS‐CoV‐2 vaccine in healthy adults. Clin Infect Dis. 2020;73(11):e3949‐e3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ella R, Reddy S, Blackwelder W, et al. Efficacy, safety, and lot‐to‐lot immunogenicity of an inactivated SARS‐CoV‐2 vaccine (BBV152): interim results of a randomised, double‐blind, controlled, phase 3 trial. Lancet. 2021;398(10317):2173‐2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ella R, Reddy S, Jogdand H, et al. Safety and immunogenicity of an inactivated SARS‐CoV‐2 vaccine, BBV152: interim results from a double‐blind, randomised, multicentre, phase 2 trial, and 3‐month follow‐up of a double‐blind, randomised phase 1 trial. Lancet Infect Dis. 2021;21(7):950‐961. 10.1016/s1473-3099(21)00070-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ella R, Vadrevu KM, Jogdand H, et al. Safety and immunogenicity of an inactivated SARS‐CoV‐2 vaccine, BBV152: a double‐blind, randomised, phase 1 trial. Lancet Infect Dis. 2021;21(5):637‐646. 10.1016/s1473-3099(20)30942-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fadlyana E, Rusmil K, Tarigan R, et al. A phase III, observer‐blind, randomized, placebo‐controlled study of the efficacy, safety, and immunogenicity of SARS‐CoV‐2 inactivated vaccine in healthy adults aged 18–59 years: an interim analysis in Indonesia. Vaccine. 2021;39(44):6520‐6528. 10.1016/j.vaccine.2021.09.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jara A, Undurraga EA, González C, et al. Effectiveness of an inactivated SARS‐CoV‐2 vaccine in Chile. N. Engl J Med. 2021;385(10):875‐884. 10.1056/nejmoa2107715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pan HX, Liu JK, Huang BY, et al. Immunogenicity and safety of a severe acute respiratory syndrome coronavirus 2 inactivated vaccine in healthy adults: randomized, double‐blind, and placebo‐controlled phase 1 and phase 2 clinical trials. Chin Med J (Engl). 2021;134(11):1289‐1298. 10.1097/cm9.0000000000001573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pu J, Yu Q, Yin Z, et al. The safety and immunogenicity of an inactivated SARS‐CoV‐2 vaccine in Chinese adults aged 18‐59 years: A phase I randomized, double‐blinded, controlled trial. Vaccine. 2021;39(20):2746‐2754. 10.1016/j.vaccine.2021.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tanriover MD, Doğanay HL, Akova M, et al. Efficacy and safety of an inactivated whole‐virion SARS‐CoV‐2 vaccine (CoronaVac): interim results of a double‐blind, randomised, placebo‐controlled, phase 3 trial in Turkey. Lancet. 2021;398(10296):213‐222. 10.1016/s0140-6736(21)01429-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu Z, Hu Y, Xu M, et al. Safety, tolerability, and immunogenicity of an inactivated SARS‐CoV‐2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double‐blind, placebo‐controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(6):803‐812. 10.1016/s1473-3099(20)30987-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xia S, Duan K, Zhang Y, et al. Effect of an inactivated vaccine against SARS‐CoV‐2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324(10):951‐960. 10.1001/jama.2020.15543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xia S, Zhang Y, Wang Y, et al. Safety and immunogenicity of an inactivated SARS‐CoV‐2 vaccine, BBIBP‐CorV: a randomised, double‐blind, placebo‐controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21(1):39‐51. 10.1016/s1473-3099(20)30831-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS‐CoV‐2 vaccine in healthy adults aged 18–59 years: a randomised, double‐blind, placebo‐controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(2):181‐192. 10.1016/s1473-3099(20)30843-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang, Y‐J , Zeng, G , Pan, H‐X , et al. Immunogenicity and safety of a SARS‐CoV‐2 inactivated vaccine in healthy adults aged 18‐59 years: report of the randomized, double‐blind, and placebo‐controlled phase 2 clinical trial. medRxiv. 2020:2020.07.31.20161216.
- 33. Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS‐CoV‐2 mRNA‐1273 vaccine in older adults. N Engl J Med. 2020;383(25):2427‐2438. 10.1056/nejmoa2028436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kremsner PG, Mann P, Kroidl A, et al. Safety and immunogenicity of an mRNA‐lipid nanoparticle vaccine candidate against SARS‐CoV‐2. Wien Klin Wochenschr. 2021;133(17‐18):931‐941. 10.1007/s00508-021-01922-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li J, Hui A, Zhang X, et al. Safety and immunogenicity of the SARS‐CoV‐2 BNT162b1 mRNA vaccine in younger and older Chinese adults: a randomized, placebo‐controlled, double‐blind phase 1 study. Nat Med. 2021;27(6):1062‐1070. 10.1038/s41591-021-01330-9 [DOI] [PubMed] [Google Scholar]
- 36. Walsh EE, Frenck RW, Falsey AR, et al. Safety and immunogenicity of Two RNA‐based Covid‐19 vaccine candidates. N. Engl J Med. 2020;383(25):2439‐2450. 10.1056/nejmoa2027906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS‐CoV‐2—preliminary report. N. Engl J Med. 2020;383(20):1920‐1931. 10.1056/nejmoa2022483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sahin U, Muik A, Derhovanessian E, et al. COVID‐19 vaccine BNT162b1 elicits human antibody and TH 1 T cell responses. Nature. 2020;586(7830):594‐599. 10.1038/s41586-020-2814-7 [DOI] [PubMed] [Google Scholar]
- 39. Mulligan MJ, Lyke KE, Kitchin N, et al. Phase I/II study of COVID‐19 RNA vaccine BNT162b1 in adults. Nature. 2020;586(7830):589‐593. 10.1038/s41586-020-2639-4 [DOI] [PubMed] [Google Scholar]
- 40. Chu L, McPhee R, Huang W, et al. A preliminary report of a randomized controlled phase 2 trial of the safety and immunogenicity of mRNA‐1273 SARS‐CoV‐2 vaccine. Vaccine. 2021;39(20):2791‐2799. 10.1016/j.vaccine.2021.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383(27):2603‐2615. 10.1056/nejmoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thomas SJ, Moreira ED, Jr , Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine through 6 months. N Engl J Med. 2021;385(19):1761‐1773. 10.1056/nejmoa2110345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med. 2020;384(5):403‐416. 10.1056/nejmoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. El Sahly HM, Baden LR, Essink B, et al. Efficacy of the mRNA‐1273 SARS‐CoV‐2 vaccine at completion of blinded phase. N Engl J Med. 2021;385(19):1774‐1785. 10.1056/nejmoa2113017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Frenck RW, Jr. , Klein NP, Kitchin N, et al. Safety, immunogenicity, and efficacy of the BNT162b2 Covid‐19 vaccine in adolescents. N Engl J Med. 2021;385(3):239‐250. 10.1056/nejmoa2107456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stephenson KE, Le Gars M, Sadoff J, et al. Immunogenicity of the Ad26.COV2.S vaccine for COVID‐19. JAMA. 2021;325(15):1535‐1544. 10.1001/jama.2021.3645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhu F‐C, Li Y‐H, Guan X‐H, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type‐5 vectored COVID‐19 vaccine: a dose‐escalation, open‐label, non‐randomised, first‐in‐human trial. Lancet. 2020;395(10240):1845‐1854. 10.1016/s0140-6736(20)31208-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Barrett JR, Belij‐Rammerstorfer S, Dold C, et al. Phase 1/2 trial of SARS‐CoV‐2 vaccine ChAdOx1 nCoV‐19 with a booster dose induces multifunctional antibody responses. Nat Med. 2021;27(2):279‐288. [DOI] [PubMed] [Google Scholar]
- 49. Goepfert PA, Fu B, Chabanon AL, et al. Safety and immunogenicity of SARS‐CoV‐2 recombinant protein vaccine formulations in healthy adults: interim results of a randomised, placebo‐controlled, phase 1‐2, dose‐ranging study. Lancet Infect Dis. 2021;21(9):1257‐1270. 10.1016/s1473-3099(21)00147-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Logunov DY, Dolzhikova IV, Zubkova OV, et al. Safety and immunogenicity of an rAd26 and rAd5 vector‐based heterologous prime‐boost COVID‐19 vaccine in two formulations: two open, non‐randomised phase 1/2 studies from Russia. Lancet. 2020;396(10255):887‐897. 10.1016/s0140-6736(20)31866-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Madhi SA, Baillie V, Cutland CL, et al. Efficacy of the ChAdOx1 nCoV‐19 Covid‐19 vaccine against the B.1.351 variant. N Engl J Med. 2021;384(20):1885‐1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Meng F‐Y, Gao F, Jia S‐Y, et al. Safety and immunogenicity of a recombinant COVID‐19 vaccine (Sf9 cells) in healthy population aged 18 years or older: two single‐center, randomised, double‐blind, placebo‐controlled, phase 1 and phase 2 trials. Signal Transduct Target Ther. 2021;6(1):271. 10.1038/s41392-021-00692-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sadoff J, Le Gars M, Shukarev G, et al. Interim results of a phase 1‐2a Trial of Ad26.COV2.S Covid‐19 vaccine. N. Engl J Med. 2021;384(19):1824‐1835. 10.1056/nejmoa2034201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV‐19 vaccine against SARS‐CoV‐2: a preliminary report of a phase 1/2, single‐blind, randomised controlled trial. Lancet (London, Engl). 2020;396(10249):467‐478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhu F‐C, Guan X‐H, Li Y‐H, et al. Immunogenicity and safety of a recombinant adenovirus type‐5‐vectored COVID‐19 vaccine in healthy adults aged 18 years or older: a randomised, double‐blind, placebo‐controlled, phase 2 trial. Lancet. 2020;396(10249):479‐488. 10.1016/s0140-6736(20)31605-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Emary KR, Golubchik T, Aley PK, et al. Efficacy of ChAdOx1 nCoV‐19 (AZD1222) vaccine against SARS‐CoV‐2 variant of concern 202012/01 (B. 1.1. 7): an exploratory analysis of a randomised controlled trial. Lancet. 2021;397(10282):1351‐1362. 10.1016/s0140-6736(21)00628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ramasamy MN, Minassian AM, Ewer KJ, et al. Safety and immunogenicity of ChAdOx1 nCoV‐19 vaccine administered in a prime‐boost regimen in young and old adults (COV002): a single‐blind, randomised, controlled, phase 2/3 trial. Lancet. 2020;396(10267):1979‐1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV‐19 vaccine (AZD1222) against SARS‐CoV‐2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Clemens SAC, Folegatti PM, Emary KRW, et al. Efficacy of ChAdOx1 nCoV‐19 (AZD1222) vaccine against SARS‐CoV‐2 lineages circulating in Brazil. Nat Commun. 2021;12(1):5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. Safety and efficacy of an rAd26 and rAd5 vector‐based heterologous prime‐boost COVID‐19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet (London, Engl). 2021;397(10275):671‐681. 10.1016/s0140-6736(21)00234-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single‐dose Ad26.COV2.S vaccine against Covid‐19. N Engl J Med. 2021;384(23):2187‐2201. 10.1056/nejmoa2101544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chappell KJ, Mordant FL, Li Z, et al. Safety and immunogenicity of an MF59‐adjuvanted spike glycoprotein‐clamp vaccine for SARS‐CoV‐2: a randomised, double‐blind, placebo‐controlled, phase 1 trial. Lancet Infect Dis. 2021;21(10):1383‐1394. 10.1016/s1473-3099(21)00200-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Formica N, Mallory R, Albert G, et al, for the 2019nCoV‐101 Study Group . Different dose regimens of a SARS‐CoV‐2 recombinant spike protein vaccine (NVX‐CoV2373) in younger and older adults: a phase 2 randomized placebo‐controlled trial. PLOS Med. 2021;18(10):e1003769. 10.1371/journal.pmed.1003769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Heath PT, Galiza EP, Baxter DN, et al. Safety and efficacy of NVX‐CoV2373 Covid‐19 vaccine. N. Engl J Med. 2021;385(13):1172‐1183. 10.1056/nejmoa2107659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Keech C, Albert G, Cho I, et al. Phase 1–2 Trial of a SARS‐CoV‐2 recombinant spike protein nanoparticle vaccine. N. Engl J Med. 2020;383(24):2320‐2332. 10.1056/nejmoa2026920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Richmond P, Hatchuel L, Dong M, et al. Safety and immunogenicity of S‐Trimer (SCB‐2019), a protein subunit vaccine candidate for COVID‐19 in healthy adults: a phase 1, randomised, double‐blind, placebo‐controlled trial. Lancet (London, Engl). 2021;397(10275):682‐694. 10.1016/s0140-6736(21)00241-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Shinde V, Bhikha S, Hoosain Z, et al. Efficacy of NVX‐CoV2373 Covid‐19 vaccine against the B.1.351 Variant. N. Engl J Med. 2021;384(20):1899‐1909. 10.1056/nejmoa2103055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shu YJ, He JF, Pei RJ, et al. Immunogenicity and safety of a recombinant fusion protein vaccine (V‐01) against coronavirus disease 2019 in healthy adults: a randomized, double‐blind, placebo‐controlled, phase II trial. Chin Med J (Engl). 2021;134(16):1967‐1976. 10.1097/cm9.0000000000001702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yang S, Li Y, Dai L, et al. Safety and immunogenicity of a recombinant tandem‐repeat dimeric RBD‐based protein subunit vaccine (ZF2001) against COVID‐19 in adults: two randomised, double‐blind, placebo‐controlled, phase 1 and 2 trials. Lancet Infect Dis. 2021;21(8):1107‐1119. 10.1016/s1473-3099(21)00127-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhang J, Hu Z, He J, et al. Safety and immunogenicity of a recombinant interferon‐armed RBD dimer vaccine (V‐01) for COVID‐19 in healthy adults: a randomized, double‐blind, placebo‐controlled, Phase I trial. Emerg Microbes Infect. 2021;10(1):1589‐1597. 10.1080/22221751.2021.1951126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ward, BJ , Gobeil, P , Séguin, A , et al. Phase 1 trial of a candidate recombinant virus‐like particle vaccine for Covid‐19 disease produced in plants. medRxiv. 2020:2020.11.04.20226282.
- 72. Maneikis K, Šablauskas K, Ringelevičiūtė U, et al. Immunogenicity of the BNT162b2 COVID‐19 mRNA vaccine and early clinical outcomes in patients with haematological malignancies in Lithuania: a national prospective cohort study. Lancet Haematol. 2021;8(8):e583‐e592. 10.1016/s2352-3026(21)00169-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Fong D, Mair MJ, Mitterer M. High levels of anti–SARS‐CoV‐2 IgG antibodies in previously infected patients with cancer after a single dose of BNT 162b2 vaccine. Eur J Cancer. 2021;154:4‐6. 10.1016/j.ejca.2021.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Addeo A, Shah PK, Bordry N, et al. Immunogenicity of SARS‐CoV‐2 messenger RNA vaccines in patients with cancer. Cancer Cell. 2021;39(8):1091‐1098.e2. 10.1016/j.ccell.2021.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Van Oekelen O, Gleason CR, Agte S, et al. Highly variable SARS‐CoV‐2 spike antibody responses to two doses of COVID‐19 RNA vaccination in patients with multiple myeloma. Cancer Cell. 2021;39(8):1028‐1030. 10.1016/j.ccell.2021.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Harrington P, Doores KJ, Radia D, et al. Single dose of BNT162b2 mRNA vaccine against severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) induces neutralising antibody and polyfunctional T‐cell responses in patients with chronic myeloid leukaemia. Br J Haematol. 2021;194(6):999‐1006. 10.1111/bjh.17568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Harrington P, de Lavallade H, Doores KJ, et al. Single dose of BNT162b2 mRNA vaccine against SARS‐CoV‐2 induces high frequency of neutralising antibody and polyfunctional T‐cell responses in patients with myeloproliferative neoplasms. Leukemia. 2021;35(12):3573‐3577. 10.1038/s41375-021-01300-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Waissengrin B, Agbarya A, Safadi E, Padova H, Wolf I. Short‐term safety of the BNT162b2 mRNA COVID‐19 vaccine in patients with cancer treated with immune checkpoint inhibitors. Lancet Oncol. 2021;22(5):581‐583. 10.1016/s1470-2045(21)00155-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Revon‐Riviere G, Ninove L, Min V, et al. The BNT162b2 mRNA COVID‐19 vaccine in adolescents and young adults with cancer: a monocentric experience. Eur J Cancer. 2021;154:30‐34. 10.1016/j.ejca.2021.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Agha, M , Blake, M , Chilleo, C , Wells, A , Haidar, G . Suboptimal response to COVID‐19 mRNA vaccines in hematologic malignancies patients. medRxiv: the preprint server for health sciences. 2021:2021.04.06.21254949
- 81. Schramm R, Costard‐Jäckle A, Rivinius R, et al. Poor humoral and T‐cell response to two‐dose SARS‐CoV‐2 messenger RNA vaccine BNT162b2 in cardiothoracic transplant recipients. Clin Res Cardiol. 2021;110(8):1142‐1149. 10.1007/s00392-021-01880-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Chevallier P, Coste‐Burel M, Le Bourgeois A, et al. Safety and immunogenicity of a first dose of SARS‐CoV‐2 mRNA vaccine in allogeneic hematopoietic stem‐cells recipients. eJHaem. 2021;2(3):520‐524. 10.1002/jha2.242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Narasimhan M, Mahimainathan L, Clark AE, et al. Serological response in lung transplant recipients after two doses of SARS‐CoV‐2 mRNA vaccines. Vaccines. 2021;9(7):708. 10.3390/vaccines9070708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Malinis M, Cohen E, Azar MM. Effectiveness of SARS‐CoV‐2 vaccination in fully vaccinated solid organ transplant recipients. Am J Transplant. 2021;21(8):2916‐2918. 10.1111/ajt.16713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Danthu C, Hantz S, Dahlem A, et al. Humoral response after SARS‐CoV‐2 mRNA vaccination in a cohort of hemodialysis patients and kidney transplant recipients. J Am Soc Nephrol. 2021;32(9):2153‐2158. 10.1681/asn.2021040490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Miele M, Busà R, Russelli G, et al. Impaired anti‐SARS‐CoV‐2 humoral and cellular immune response induced by Pfizer‐BioNTech BNT162b2 mRNA vaccine in solid organ transplanted patients. Am J Transplant. 2021;21(8):2919‐2921. 10.1111/ajt.16702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Chenxi Song C, Christensen J, Kumar D, Vissichelli N, Morales M, Gupta G. Early experience with SARs‐CoV‐2 mRNA vaccine breakthrough among kidney transplant recipients. Transpl Infect Dis. 2021;23(4):e13654. 10.1111/tid.13654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Havlin J, Svorcova M, Dvorackova E, et al. Immunogenicity of BNT162b2 mRNA COVID‐19 vaccine and SARS‐CoV‐2 infection in lung transplant recipients. J Heart Lung Transplant. 2021;40(8):754‐758. 10.1016/j.healun.2021.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Itzhaki Ben Zadok O, Shaul AA, Ben‐Avraham B, et al. Immunogenicity of the BNT162b2 mRNA vaccine in heart transplant recipients—a prospective cohort study. Eur J Heart Fail. 2021;23(9):1555‐1559. 10.1002/ejhf.2199 [DOI] [PubMed] [Google Scholar]
- 90. Korth J, Jahn M, Dorsch O, et al. Impaired humoral response in renal transplant recipients to SARS‐CoV‐2 vaccination with BNT162b2 (Pfizer‐BioNTech). Viruses. 2021;13(5):756. 10.3390/v13050756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Rabinowich L, Grupper A, Baruch R, et al. Low immunogenicity to SARS‐CoV‐2 vaccination among liver transplant recipients. J Hepatol. 2021;75(2):435‐438. 10.1016/j.jhep.2021.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Monin L, Laing AG, Muñoz‐Ruiz M, et al. Safety and immunogenicity of one versus two doses of the COVID‐19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22(6):765‐778. 10.1016/s1470-2045(21)00213-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Jahn M, Korth J, Dorsch O, et al. Humoral response to SARS‐CoV‐2‐vaccination with BNT162b2 (Pfizer‐BioNTech) in patients on hemodialysis. Vaccines. 2021;9(4):360. 10.3390/vaccines9040360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Broseta JJ, Rodríguez‐Espinosa D, Rodríguez N, et al. Humoral and cellular responses to mRNA‐1273 and BNT162b2 SARS‐CoV‐2 vaccines administered to hemodialysis patients. Am J Kidney Dis. 2021;78(4):571‐581. 10.1053/j.ajkd.2021.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Longlune N, Nogier MB, Miedougé M, et al. High immunogenicity of a messenger RNA‐based vaccine against SARS‐CoV‐2 in chronic dialysis patients. Nephrol Dial Transplant. 2021;36(9):1704‐1709. 10.1093/ndt/gfab193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lesny P, Anderson M, Cloherty G, et al. Immunogenicity of a first dose of mRNA‐ or vector‐based SARS‐CoV‐2 vaccination in dialysis patients: a multicenter prospective observational pilot study. J Nephrol. 2021;34(4):975‐983. 10.1007/s40620-021-01076-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Simon B, Rubey H, Treipl A, et al. Haemodialysis patients show a highly diminished antibody response after COVID‐19 mRNA vaccination compared with healthy controls. Nephrol Dial Transplant. 2021;36(9):1709‐1716. 10.1093/ndt/gfab179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Grupper A, Sharon N, Finn T, et al. Humoral response to the Pfizer BNT162b2 vaccine in patients undergoing maintenance hemodialysis. Clin J Am Soc Nephrol. 2021;16(7):1037‐1042. 10.2215/cjn.03500321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zitt E, Davidovic T, Schimpf J, et al. The safety and immunogenicity of the mRNA‐BNT162b2 SARS‐CoV‐2 vaccine in hemodialysis patients. Front Immunol. 2021;12(2390). 10.3389/fimmu.2021.704773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Rubbert‐Roth A, Vuilleumier N, Ludewig B, Schmiedeberg K, Haller C, von Kempis J. Anti‐SARS‐CoV‐2 mRNA vaccine in patients with rheumatoid arthritis. Lancet Rheumatol. 2021;3(7):e470‐e472. 10.1016/s2665-9913(21)00186-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Braun‐Moscovici Y, Kaplan M, Braun M, et al. Disease activity and humoral response in patients with inflammatory rheumatic diseases after two doses of the Pfizer mRNA vaccine against SARS‐CoV‐2. Ann Rheumatic Dis. 2021;80(10):1317‐1321. 10.1136/annrheumdis-2021-220503 [DOI] [PubMed] [Google Scholar]
- 102. Geisen UM, Berner DK, Tran F, et al. Immunogenicity and safety of anti‐SARS‐CoV‐2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheumatic Dis. 2021;80(10):1306‐1311. 10.1136/annrheumdis-2021-220272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hagin D, Freund T, Navon M, et al. Immunogenicity of Pfizer‐BioNTech COVID‐19 vaccine in patients with inborn errors of immunity. J Allergy Clin Immunol. 2021;148(3):739‐749. 10.1016/j.jaci.2021.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Haberman RH, Herati R, Simon D, et al. Methotrexate hampers immunogenicity to BNT162b2 mRNA COVID‐19 vaccine in immune‐mediated inflammatory disease. Ann Rheumatic Dis. 2021;80(10):1339‐1344. 10.1136/annrheumdis-2021-220597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Collier A‐rY, McMahan K, Yu J, et al. Immunogenicity of COVID‐19 mRNA vaccines in pregnant and lactating women. JAMA. 2021;325(23):2370‐2380. 10.1001/jama.2021.7563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Eyre DW, Lumley SF, Wei J, et al. Quantitative SARS‐CoV‐2 anti‐spike responses to Pfizer–BioNTech and Oxford–AstraZeneca vaccines by previous infection status. Clin Microbiol Infect. 2021;27(10):1516. e7‐e14. 10.1016/j.cmi.2021.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. OWi Data . Statistics and Research/Coronavirus (COVID‐19) Vaccinations 2021. https://ourworldindata.org/covid‐vaccinations [Google Scholar]
- 108. Dai L, Gao GF. Viral targets for vaccines against COVID‐19. Nat Rev Immunol. 2021;21(2):73‐82. 10.1038/s41577-020-00480-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Chen J, Wang R, Gilby NB, Wei G‐W. Omicron variant (B. 1.1. 529): infectivity, vaccine breakthrough, and antibody resistance. J Chem Inf Model. 2022;62(2):412‐422. 10.1021/acs.jcim.1c01451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Tarke A, Coelho CH, Zhang Z, et al. SARS‐CoV‐2 vaccination induces immunological T cell memory able to cross‐recognize variants from Alpha to Omicron. Cell. 2022;185(5):847‐859.e11. 10.1016/j.cell.2022.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Liu J, Chandrashekar A, Sellers D, et al. Vaccines elicit highly conserved cellular immunity to SARS‐CoV‐2 Omicron. Nature. 2022;603(7901):1‐7. 10.1038/s41586-022-04465-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Pulliam JR, van Schalkwyk C, Govender N, et al. Increased risk of SARS‐CoV‐2 reinfection associated with emergence of the Omicron variant in South Africa. MedRxiv; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Collie S, Champion J, Moultrie H, Bekker L‐G, Gray G. Effectiveness of BNT162b2 vaccine against omicron variant in South Africa. N. Engl J Med. 2022;386(5):494‐496. 10.1056/nejmc2119270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Muik A, Lui BG, Wallisch A‐K, et al. Neutralization of SARS‐CoV‐2 Omicron by BNT162b2 mRNA vaccine–elicited human sera. Science. 2022;eabn7591. 10.1126/science.abn7591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Nkengasong J. China’s response to a novel coronavirus stands in stark contrast to the 2002 SARS outbreak response. Nat Med. 2020;26(3):310‐311. 10.1038/s41591-020-0771-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wu A, Peng Y, Huang B, et al. Genome composition and divergence of the Novel Coronavirus (2019‐nCoV) Originating in China. Cell Host microbe. 2020;27(3):325‐328. 10.1016/j.chom.2020.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Liu Z, Xiao X, Wei X, et al. Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS‐CoV‐2. J Med Virol. 2020;92(6):595‐601. 10.1002/jmv.25726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Zhai X, Sun J, Yan Z, et al. Comparison of severe acute respiratory syndrome coronavirus 2 spike protein binding to ACE2 receptors from human, pets, farm animals, and putative intermediate hosts. J Virol. 2020;94(15):e00831‐20. 10.1128/jvi.00831-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Bisht H, Roberts A, Vogel L, et al. Severe acute respiratory syndrome coronavirus spike protein expressed by attenuated vaccinia virus protectively immunizes mice. Proc Natl Acad Sci USA. 2004;101(17):6641‐6646. 10.1073/pnas.0401939101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Yang ZY, Kong WP, Huang Y, et al. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature. 2004;428(6982):561‐564. 10.1038/nature02463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Amanat F, Krammer F. SARS‐CoV‐2 Vaccines: status report. Immunity. 2020;52(4):583‐589. 10.1016/j.immuni.2020.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Suthar MS, Zimmerman MG, Kauffman RC, et al. Rapid generation of neutralizing antibody responses in COVID‐19 patients. Cell Rep Med. 2020;1(3):100040. 10.1016/j.xcrm.2020.100040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Ni L, Ye F, Cheng ML, et al. Detection of SARS‐CoV‐2‐specific humoral and cellular immunity in COVID‐19 convalescent individuals. Immunity. 2020;52(6):971‐977. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Mohamed Khosroshahi L, Rezaei N. Dysregulation of the immune response in coronavirus disease 2019. Cell Biol Int. 2021;45(4):702‐707. 10.1002/cbin.11517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Sanders B, Koldijk M, Schuitemaker H. Inactivated viral vaccines. In: Nunnally BK, Turula VE, Sitrin RD, eds. Vaccine Analysis: Strategies, Principles, and Control. Springer; 2015:45‐80. [Google Scholar]
- 126. Keshavarz M, Mirzaei H, Salemi M, et al. Influenza vaccine: Where are we and where do we go? Rev Med Virol. 2019;29(1):e2014. 10.1002/rmv.2014 [DOI] [PubMed] [Google Scholar]
- 127. Jiang S, He Y, Liu S. SARS vaccine development. Emerg Infect Dis. 2005;11(7):1016‐1020. 10.3201/1107.050219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Xia S, Zhang Y, Wang Y, et al. Safety and immunogenicity of an inactivated SARS‐CoV‐2 vaccine, BBIBP‐CorV: a randomised, double‐blind, placebo‐controlled, phase 1/2 trial. Lancet Infect Dis; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Gao, Q , Bao, L , Mao, H , et al. Development of an inactivated vaccine candidate for SARS‐CoV‐2. Science. 2020;369:77‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Wang H, Zhang Y, Huang B, et al. Development of an inactivated vaccine candidate, BBIBP‐CorV, with potent protection against SARS‐CoV‐2. Cell. 2020;182(3):713‐721. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Sun W, McCroskery S, Liu W‐C, et al. A Newcastle disease virus (NDV) expressing membrane‐anchored spike as a cost‐effective inactivated SARS‐CoV‐2 vaccine. bioRxiv; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Jang YH, Seong BL. Principles underlying rational design of live attenuated influenza vaccines. Clin Exp Vaccine Res. 2012;1(1):35‐49. 10.7774/cevr.2012.1.1.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Seo SH, Jang Y. Cold‐adapted live attenuated SARS‐CoV‐2 vaccine completely protects human ACE2 transgenic mice from SARS‐CoV‐2 infection. Vaccines. 2020;8(4):584. 10.3390/vaccines8040584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Ye T, Zhong Z, García‐Sastre A, Schotsaert M, De Geest BG. Current Status of COVID‐19 (Pre). Clin Vaccine Dev. 2020;59(43):18885‐18897. 10.1002/anie.202008319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Corbett KS, Flynn B, Foulds KE, et al. Evaluation of the mRNA‐1273 vaccine against SARS‐CoV‐2 in nonhuman primates. N Engl J Med. 2020;383(16):1544‐1555. 10.1056/nejmoa2024671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Laczkó D, Hogan MJ, Toulmin SA, et al. A single immunization with nucleoside‐modified mRNA vaccines elicits strong cellular and humoral immune responses against SARS‐CoV‐2 in mice. Immunity. 2020;53(4):724‐732. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Ward BJ, Gobeil P, Séguin A, et al. Phase 1 randomized trial of a plant‐derived virus‐like particle vaccine for COVID‐19. Nat Med. 2021;27(6):1071‐1078. 10.1038/s41591-021-01370-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


