ABSTRACT
Background
High gluten intake is associated with increased risk of celiac disease (CD) in children at genetic risk.
Objectives
We aimed to investigate if different dietary gluten sources up to age 2 y confer different risks of celiac disease autoimmunity (CDA) and CD in children at genetic risk.
Methods
Three-day food records were collected at ages 6, 9, 12, 18, and 24 mo from 2088 Swedish genetically at-risk children participating in a 15-y follow-up cohort study on type 1 diabetes and CD. Screening for CD was performed with tissue transglutaminase autoantibodies (tTGA). The primary outcome was CDA, defined as persistent tTGA positivity. The secondary outcome was CD, defined as having a biopsy specimen showing Marsh score ≥ 2 or an averaged tTGA level ≥ 100 Units. Cox regression adjusted for total gluten intake estimated HRs with 95% CIs for daily intake of gluten sources.
Results
During follow-up, 487 (23.3%) children developed CDA and 242 (11.6%) developed CD. Daily intake of ≤158 g porridge at age 9 mo was associated with increased risk of CDA (HR: 1.53; 95% CI: 1.05, 2.23; P = 0.026) compared with no intake. A high daily bread intake (>18.3 g) at age 12 mo was associated with increased risk of both CDA (HR: 1.47; 95% CI: 1.05, 2.05; P = 0.023) and CD (HR: 1.79; 95% CI: 1.10, 2.91; P = 0.019) compared with no intake. At age 18 mo, milk cereal drink was associated with an increased risk of CD (HR: 1.16; 95% CI: 1.00, 1.33; P = 0.047) per 200-g/d increased intake. No association was found for other gluten sources up to age 24 mo and risk of CDA or CD.
Conclusions
High daily intakes of bread at age 12 mo and of milk cereal drink during the second year of life are associated with increased risk of both CDA and CD in genetically at-risk children.
Keywords: celiac disease, children, gluten foods, HLA, TEDDY
Introduction
Celiac disease (CD) affects the intestinal epithelium and is caused by an immunologic response against gluten in individuals carrying the risk-haplotypes human leucocyte antigen (HLA)-DQA1*05:01-DQB*DQ02:01 (DQ2.5), DQA1*02:01-DQB*DQ02:01 (DQ2.2), and DQA1*03:01-DQB1*DQ03:02 (DQ8) (1). The global incidence of CD is increasing (2) and has been reported to be particularly high in countries with high wheat consumption (3), indicating that a high gluten intake confers increased risk of CD in individuals at genetic risk. Observations from prospective birth cohorts also demonstrate associations of increased risk of CD with higher amounts of gluten intake in early childhood in some (4–6), but not in all studies (7).
Gluten is a storage protein that belongs to the family of prolamins found in wheat, rye, and barley. The type and amount of gluten depend on the growing conditions and genetic polymorphism of the grain (8, 9). In the food industry, gluten is an important compound added and modified to enhance desired properties in specific foods, for example, to increase leavening in bread (10). Gluten is partly or fully resistant to enzymatic degradation in the human gut, where residual peptides may pass the small intestinal barrier and trigger the specific immunologic reaction observed in people with CD (11). The intestinal digestibility of gluten varies depending on the food matrix, content of water, and if other components, such as dietary fibers, are present (12–17).
The international observational TEDDY (The Environmental Determinants of Diabetes in the Young) study has demonstrated that Swedish children at genetic risk are more likely to develop CD than children in the United States (18). There could be several reasons for these phenomena, not necessarily attributable to variations in childhood gluten feeding. Yet, it is striking that Swedish children reported the highest gluten intake amounts in early childhood among the participating sites in TEDDY (4). Although no difference in CD risk was observed in a previous retrospective study comparing Swedish infants introduced to gluten in solid foods or in follow-on formula (19), it has not been studied if the risk of CD is associated with certain gluten-containing foods consumed in early childhood (20).
The aim of this study was to extend previous studies and examine whether intakes of different gluten-containing foods ≤2 y of age confer different risks of celiac disease autoimmunity (CDA) and CD in children at genetic risk.
Methods
Study population
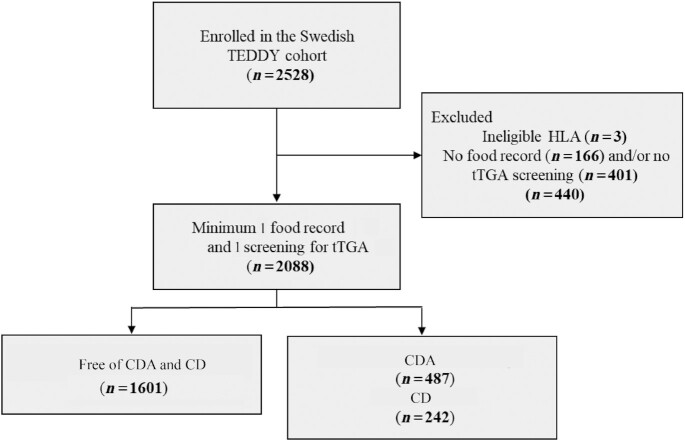
TEDDY study is a longitudinal birth cohort study with prospectively collected data from children born between 2004 and 2010 and with a genetic risk of type 1 diabetes and CD, as described elsewhere (21, 22). At the Swedish site, parents of 2528 (68%) eligible infants agreed to participate in the 15-y follow-up. Written informed consent was collected from primary caregivers for both the genetic screening and the follow-up study. TEDDY study was approved by local ethical review boards in each country (21). Included in the present cohort study were follow-up data collected at clinic visits as of 30 November, 2020, from 2088 (82.6%) Swedish children who had ≥1 completed 3-d food record ≤24 mo of age, and ≥1 sample analyzed for measurement of tissue transglutaminase autoantibodies (tTGA) (Figure 1).
FIGURE 1.
Flowchart of Swedish children in TEDDY study with ≥1 measure for tTGA and ≥1 three-day food record collected at age 6–24 mo. CD, celiac disease; CDA, celiac disease autoimmunity; HLA, human leucocyte antigen; TEDDY, The Environmental Determinants of Diabetes in the Young; tTGA, tissue transglutaminase autoantibodies.
Dietary assessment
Intake of gluten-containing foods and grains was assessed in grams per day by using 3-d food records collected at clinic visits at ages 6, 9, 12, 18, and 24 mo. Parents were instructed to document detailed information on foods and drinks consumed by the child, on 2 consecutive weekdays and 1 weekend day. Habitual food intake was encouraged. Parents were instructed to estimate portion sizes using household measures. To improve the estimations, a booklet with pictures of foods and shapes of food was provided, to be used both at home and in day-care. All food records were reviewed and entered into a food database by trained dietitians and nutritionists (23, 24).
Reported composite foods and dishes with gluten were broken down on both ingredient level (e.g., wheat, rye, barley) and food group level (e.g., white bread, pasta, porridge). Food groups were sorted into 7 main food groups and 26 subgroups. The gluten content in wheat, rye, and barley was estimated by multiplying the protein content in each grain by a factor of 0.8 (the gluten content in wheat) (4, 25).
For the purpose of this study, gluten-containing subgroups were combined into larger food groups. Subgroups with low proportions (<10%) of consumers (a consumer was defined as having an intake >0 g/d of a given food) and low intake levels (gluten-containing foods <10% of total intake) were combined with subgroups with a similar type of included gluten-containing grain, proportion of flour, and culinary use. For composite dishes, such as pizza, pie, and filled crêpes, a conversion factor based on Swedish standard recipes was applied to assess only the intake of the gluten-containing part of the dish. After excluding 3 subgroups not feasible for aggregation owing to few reporters and no similar food groups (dishes based on bread, pudding, and commercial baby fruit cereal), 8 gluten-containing food groups remained: porridge, milk cereal drink (a type of follow-on formula composed of skimmed milk powder and flour from different grains), bread, pasta, cookies and crackers, pancakes, sweet baked goods, and breakfast cereals (Supplemental Table 1). In total, 9432 three-day food records were reported within the frame of the study. All expected five 3-d food records were reported in 1485 (71.1%) of the children, and 41 children (2%) reported only 1 food record (Supplemental Table 2). The descriptive intake data of gluten-containing grains and foods presented excluded food records where the child had CDA or CD at the visit to reflect the dietary habits of healthy children in the cohort.
Assessment of CDA and CD
Annual screening for CD started at the age of 24 mo using tTGA as previously described (18). In children with a positive tTGA result (≥1.3 U), previously collected samples in TEDDY (26) were analyzed to find the closest time point of seroconversion to tTGA positivity. The primary outcome was CDA, defined as being persistently tTGA positive in 2 consecutive samples. All children with CDA were followed by a pediatric gastroenterologist at a tertiary hospital in the south of Sweden. The decision to perform an intestinal biopsy was outside the TEDDY study protocol but was recommended in children with tTGA levels ≥ 30, and in symptomatic children with CDA regardless of the tTGA level. Because not all children with CDA progress to CD (27–29), the secondary outcome was CD, defined either as having an intestinal biopsy specimen showing a Marsh score ≥ 2, or, if not performed, as a mean tTGA level ≥ 100 U in 2 consecutive samples (18).
Statistical analyses
Correlations between daily intake of a given gluten-containing food and total daily gluten intake were calculated using Spearman rank correlation coefficients to examine the contribution of gluten from each food group to the total daily gluten intake.
Cox regression was used to examine the associations of gluten-containing food groups and grains with the risks of the 2 study outcomes. Time to CDA was the age at the first of the 2 consecutive positive tTGA samples, and the right-censoring time was the age at the last negative tTGA sample. Time to CD was defined as the age at the diagnosis and the right-censoring time was the age at the last clinic visit.
Intake of gluten-containing foods and grains in absolute amounts at each visit was analyzed separately and modeled as follows: 1) if <10% were consumers, the food or grain was excluded from analysis; 2) if ≥10% and ≤50% were consumers, intakes were modeled as binary variables (0 g/d, >0 g/d); and 3) if >50% were consumers, intakes were modeled as categoric variables (0 g/d, median intake or less in consumers without CDA or CD at the visit, greater than median intake in consumers without CDA or CD at the visit), to represent no, low, and high intake. Foods and grains with ≥75% as consumers were in addition modeled as continuous variables. To account for differences in energy requirement and to reduce extraneous variation, Cox regressions were performed separately with food intakes standardized according to the nutrient density method to grams per 1000 kcal/d (food intake/energy intake × 1000 kcal) (30, 31). Modeling dietary intake data in absolute amounts or standardized to per 1000 kcal had small effects on the estimates, which were in the same direction, and resulted in similar precision. The results for food intakes were therefore presented in absolute amounts.
Analyses were adjusted for risk factors previously associated with CD in TEDDY (18): HLA risk genotypes [high- (homozygous for DQ2), moderate- (heterozygous for DQ2), and low-risk group (others)], female sex, and having a family history of CD (collected at the 9-mo visit) (18). Total energy intake (kcal/d) and total gluten intake (g/d) assessed from the corresponding food record were included to control for confounding by energy intake (30), and for gluten intake (4), respectively.
The Schoenfeld residuals from each Cox regression model were plotted and examined to evaluate the model fit. Two-sided nominal P values were presented and those < 0.05 were considered to be statistically significant. No adjustment in type 1 error was made for multiple comparisons because it was an exploratory study. Statistical analyses were performed in IBM SPSS Statistics for Windows, version 27.0 (IBM Corp., 2020).
Results
Characteristics of the study population
During follow-up to a mean ± SD age of 11.6 ± 3.3 y, 487 (23.3%) children developed CDA at median age 3.0 y (IQR: 1.9–5.0 y) and 242 (11.6%) were diagnosed with CD at median age 4.5 y (IQR: 2.9–6.7 y) (Table 1). Intestinal biopsy verified the diagnosis of CD in 239 children (98.8%). The median time from CDA onset to CD diagnosis was 13.9 mo (IQR: 10.5–18.9 mo).
TABLE 1.
Descriptive data of Swedish children at genetic risk of CD enrolled in The Environmental Determinants of Diabetes in the Young study1
| Cohort (n = 2088) | Free of CDA (n = 1601, 76.7%) | CDA (n = 487, 23.3%) | CD (n = 242, 11.6%) | |
|---|---|---|---|---|
| Females | 1021 (48.9) | 747 (46.7) | 274 (56.3) | 144 (59.5) |
| HLA risk group | ||||
| High (DR3-DQ2/DR3-DQ2)2 | 450 (21.6) | 240 (15.0) | 210 (43.1) | 116 (47.9) |
| Moderate (DR3-DQ2/DR4-DQ8)3 | 868 (41.6) | 709 (44.3) | 159 (32.6) | 73 (30.3) |
| Low (others)4 | 770 (36.9) | 652 (40.7) | 118 (24.2) | 53 (21.9) |
| Family history of CD5 | 39 (1.9) | 25 (1.6) | 14 (2.9) | 7 (2.9) |
| Breastfeeding duration,6 mo | 7.3 [4.0–10.0] | 7.1 [3.4–10.0] | 7.6 [5.0–10.0] | 7.3 [5.0–9.3] |
| Age at introduction of gluten, mo | 5.0 ± 1.0 | 5.0 ± 1.0 | 5.0 ± 1.0 | 4.9 ± 1.0 |
Values are n (%), median [IQR], or mean ± SD. Some percentages may not add up to 100 because of rounding. CD, celiac disease; CDA, celiac disease autoimmunity; HLA, human leucocyte antigen.
HLA risk group “high” includes genotype DR3*0501/0201*DR3*0501/0201.
HLA risk group “moderate” includes genotype DR4*030X/0302*DR3*0501/0201.
HLA risk group “low” includes genotypes DR4*030X/0302*DR4*030X/0302, DR4*030X/0302*DR4*030X/020X, DR4*030X/0302*DR8*0401/0402, DR4*030X/0302*DR1*0101/0501, DR4*030X/0302*DR13*0102/0604, DR4*030X/0302*DR4*030X/0304, DR4*030X/0302*DR9*030X/0303, and DR3*0501/0201*DR9*030X/0303.
Parent or sibling.
Of the cohort, 99.1% were breastfed.
Intake of food groups and grains with gluten
At the age of 6 mo, 71.6% of the children reported gluten consumption in the 3-d food records. At 9 mo of age and onwards, >99% reported daily gluten consumption. Wheat was the main source of gluten and the intake increased with age. From age 12 mo, most children consumed rye, although the intake was lower than that of wheat intake. The intake of barley was consistently low. Both the number of consumers and intake of the gluten-containing food groups differed with age. Porridge and milk cereal drink consumption dominated during the first year of life, whereas other gluten-containing food groups were more common in the older age groups (Table 2).
TABLE 2.
Descriptive dietary intake data of gluten-containing food groups and grains assessed by 3-d food records collected at ages 6–24 mo from 2088 Swedish children at genetic risk of CD1
| Age at clinic visit | ||||||
|---|---|---|---|---|---|---|
| 6 mo | 9 mo | 12 mo | 18 mo | 24 mo | Never reported intake2 | |
| Food records, n (missing %) | 2024 (3.0) | 1988 (3.6) | 1922 (6.2) | 1707 (10.8) | 1559 (12.2) | |
| intake, kcal/d | 676 ± 100 | 766 ± 130 | 854 ± 157 | 971 ± 189 | 1045 ± 203 | |
| Gluten, g/d | 0.5 [0.2–1.2] | 2.4 [1.4–3.4] | 3.9 [2.7–5.4] | 5.2 [3.9–6.7] | 5.4 [4.0–7.0] | |
| Intake of sources of gluten | ||||||
| Wheat, g/d | 5.7 [2.3–13.5] | 25.7 [15.7–36.6] | 38.8 [27.0–54.5] | 50.4 [36.3–67.3] | 54.0 [37.9–71.1] | |
| Reporters (%) | 71.5 | 98.7 | 99.7 | 99.7 | 99.9 | 0.1 |
| Rye, g/d | 1.0 [0.3–2.0] | 2.0 [1.0–4.3] | 6.3 [3.0–10.7] | 8.3 [4.7–12.3] | 8.3 [4.7–12.7] | |
| Reporters (%) | 6.2 | 46.3 | 84.6 | 95.7 | 95.5 | 0.8 |
| Barley, g/d | 0.5 [0.3–2.1] | 0.3 [0.3–1.0] | 0.7 [0.3–0.7] | 0.3 [0.3–0.7] | 0.7 [0.3–1.0] | |
| Reporters (%) | 0.3 | 2.1 | 2.1 | 6.0 | 8.8 | 84.5 |
| Porridge, g/d | 118 [53.3–167] | 158 [105–237] | 133 [86.0–197] | 83.3 [43.3–135] | 66.7 [33.3–112] | |
| Reporters (%) | 82.5 | 90.4 | 79.4 | 48.1 | 33.8 | 3.0 |
| Milk cereal drink, g/d | 220 [96–461] | 400 [233–497] | 410 [267–500] | 383 [240–460] | 360 [227–453] | |
| Reporters (%) | 40.1 | 74.8 | 78.9 | 75.6 | 66.6 | 13.0 |
| Bread, g/d | 4.0 [1.7–8.0] | 10.7 [5.7–20.1] | 18.3 [10.0–32.0] | 26.0 [14.8–40.7] | 30.0 [18.3–46.2] | |
| Reporters (%) | 12.1 | 60.1 | 84.6 | 94.8 | 96.7 | 1.2 |
| Cookies, crackers, g/d | 1.0 [0.7–2.0] | 2.0 [1.0–3.7] | 3.0 [1.5–5.7] | 5.0 [2.7–9.3] | 6.0 [3.3–11.3] | |
| Reporters (%) | 14.5 | 45.5 | 52.0 | 61.3 | 65.5 | 9.3 |
| Pasta, g/d | 7.7 [2.8–12.0] | 12.5 [6.2–22.1] | 15.3 [8.3–27.3] | 23.0 [13.3–35.6] | 25.0 [15.3–41.7] | |
| Reporters (%) | 3.4 | 21.1 | 41.4 | 66.5 | 69.5 | 11.2 |
| Sweet baked goods, g/d | 2.3 [1.7–4.3] | 6.7 [3.3–10.0] | 10.0 [5.0–18.3] | 11.7 [6.7–18.3] | 13.3 [7.6–23.3] | |
| Reporters (%) | 1.1 | 8.4 | 27.7 | 37.8 | 46.7 | 31.9 |
| Pancakes, g/d | 7.7 [5.0–10.8] | 11.7 [4.8–23.3] | 18.3 [10.0–33.0] | 23.3 [15.0–40.0] | 33.3 [17.3–46.7] | |
| Reporters (%) | 0.5 | 5.3 | 11.1 | 20.4 | 21.8 | 58.7 |
| Breakfast cereals, g/d | 0.3 [0.3–0.3] | 2.0 [1.0–4.7] | 1.7 [1.0–4.0] | 3.7 [2.0–6.7] | 4.0 [2.0–8.0] | |
| Reporters (%) | 0.1 | 1.8 | 10.5 | 37.8 | 49.5 | 39.8 |
Values are median [IQR], mean ± SD, or percentages, unless otherwise indicated. All intake data were reported only in consumers of a given food group (i.e., the child had an intake > 0 g/d). Food records collected at visits where the child had CD autoimmunity or CD were excluded. CD, celiac disease.
Did not report the specified food across all collected food records.
Spearman correlations between food groups and total gluten intake
Bread and pasta both strongly correlated with total gluten intake ≤24 mo of age (for bread, ρ: 0.44–0.34, P < 0.001; and for pasta, ρ: 0.72–0.63, P < 0.001). Milk cereal drink was less correlated with total gluten intake by age (ρ: 0.17–0.10, P < 0.05). Porridge correlated more strongly with total gluten intake before 12 mo of age (ρ: 0.51–0.27, P < 0.001) than after 12 mo of age (ρ: 0.05 to −0.04, P < 0.05) (Supplemental Table 3).
Cox regression estimates of sources of dietary gluten and association with CDA
At the age of 9 mo, a low daily intake of porridge for the age (i.e., ≤158 g/d) was associated with an increased risk of CDA (HR: 1.53; 95% CI: 1.05, 2.23; P = 0.026) compared with reporting no intake of porridge (Figure 2, Supplemental Table 4A–E). A high daily intake of bread for the age (i.e., >18.3 g/d) at 12 mo was associated with an increased risk of CDA (HR: 1.47; 95% CI: 1.05, 2.05; P = 0.023), compared with reporting no intake of bread. No association between daily intake of other gluten-containing food groups or grains and risk of CDA was found at other ages when total gluten intake was controlled for.
FIGURE 2.
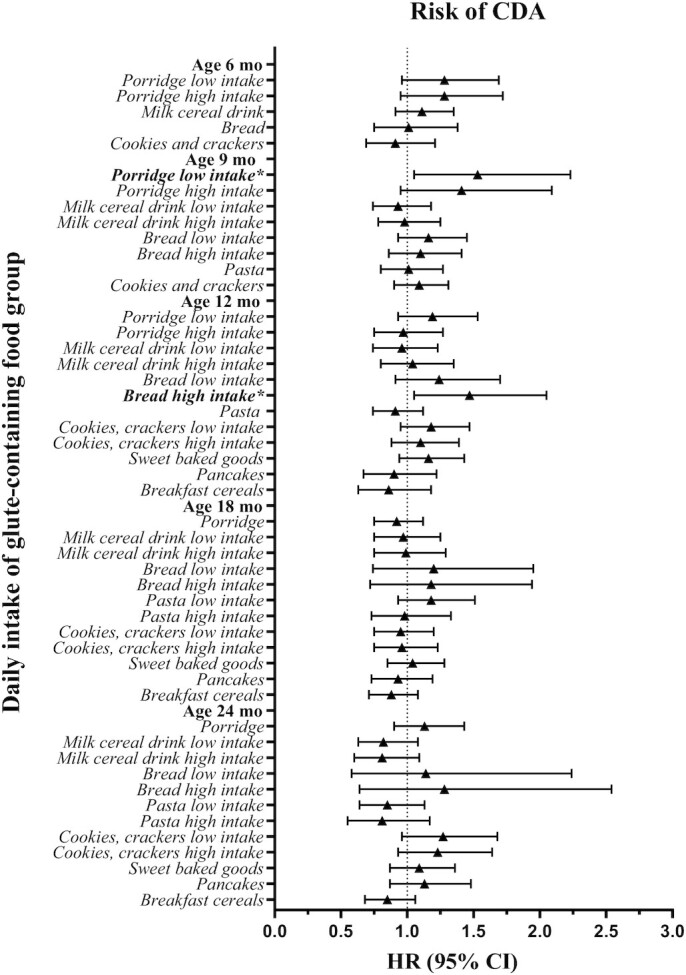
Summary plot of the estimated HRs and their related 95% CIs by Cox regressions of the association between daily intake of gluten-containing food groups assessed from 3-d food records at ages 6–24 mo and CDA (n = 487) in Swedish children (n = 2088) at genetic risk. Depending on the percentage of consumers (having an intake >0 g/d) at each age, intake variables were modeled as binary (if ≤50% were consumers; 0 g/d, >0 g/d) or categoric (if >50% were consumers; 0 g/d, median intake or less in participants without CDA or CD at the visit, greater than median intake in participants without CDA or CD at the visit) to represent no, low, and high intake. Included covariates in the analyses were human leucocyte antigen risk group, sex, having a parent or sibling with CD, and energy and gluten intake assessed by the respective food record. *Statistically significant result. Supplemental Table 4A–E summarizes detailed results. CD, celiac disease; CDA, celiac disease autoimmunity.
Cox regression estimates of sources of dietary gluten and risk of CD
A high daily intake of bread for the age (i.e., >18.3 g/d) at 12 mo was associated with an increased risk of CD (HR: 1.79; 95% CI: 1.10, 2.91; P = 0.019) compared with reporting no intake of bread (Figure 3, Supplemental Table 4A–E). At the age of 18 mo, milk cereal drink was associated with an increased risk of CD (HR: 1.16; 95% CI: 1.00, 1.33; P = 0.047) per every 200-g/d (corresponding to 1 bottle) increased intake. No association between daily intake of other gluten-containing food groups or grains and risk of CD was found for other ages when total gluten intake was controlled for.
FIGURE 3.
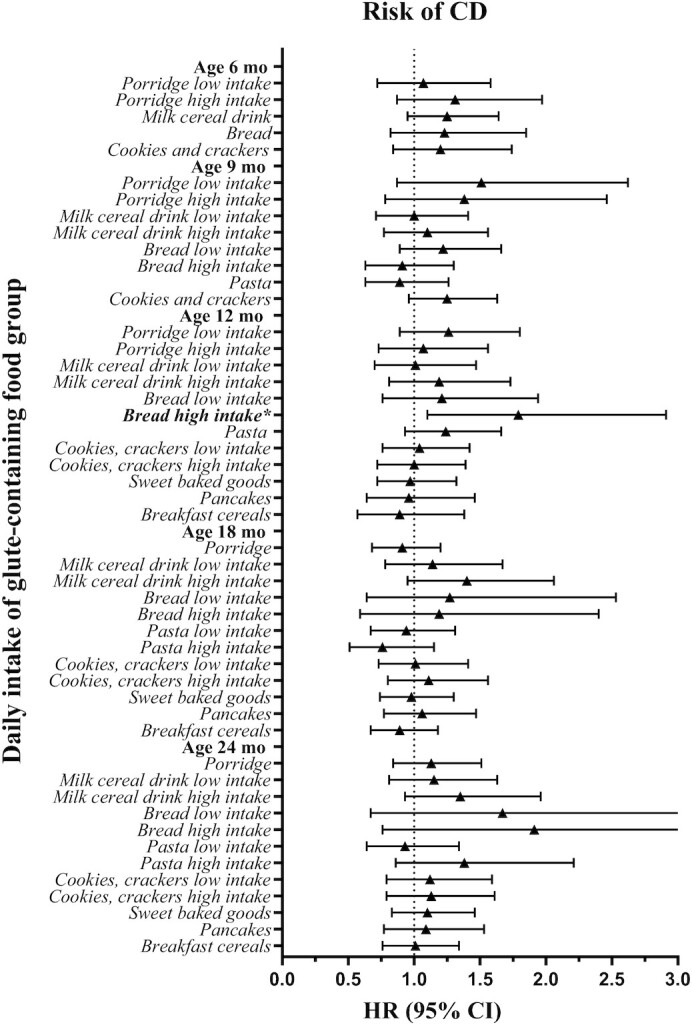
Summary plot of the estimated HRs and their related 95% CIs by Cox regressions of the association between daily intake of gluten-containing food groups assessed from 3-d food records at ages 6–24 mo and CD (n = 242) in Swedish children (n = 2088) at genetic risk. Depending on the percentage of consumers (having an intake >0 g/d) at each age, intake variables were modeled as binary (if ≤50% were consumers; 0 g/d, >0 g/d) or categoric (if >50% were consumers; 0 g/d, median intake or less in participants without CDA or CD at the visit, greater than median intake in participants without CDA or CD at the visit) to represent no, low, and high intake. Included covariates in the analyses were human leucocyte antigen risk group, sex, having a parent or sibling with CD, and energy and gluten intake assessed by the respective food record. *Statistically significant result. Supplemental Table 4A–E summarizes detailed results. CD, celiac disease; CDA, celiac disease autoimmunity.
Discussion
The present study on intake of gluten-containing foods and grains during the first 2 y of life found associations between intake of several gluten-containing foods and CDA and CD in Swedish children at genetic risk. This is in contrast to a previous retrospective Swedish study, which could not identify specific dietary sources of gluten that when introduced into infants’ diet conferred a subsequent risk of CD (19). However, the present study investigated gluten-containing foods up to age 2 y. Children reporting a high daily intake of bread for the age (corresponding to a gluten intake >1 g), compared with those with no bread consumption at age 12 mo, were at an almost 2-fold increased risk of developing CD. In addition, an association with increased risk of CD was also found for every additional bottle (i.e., 200 g) of daily intake of milk cereal drink (liquid infant cereals, with a gluten content ≤1.5 g/bottle) at age 18 mo. At the age of 9 mo, an intake up to the equivalent of 1.3 portions of porridge (i.e., ≤158 g, with a gluten content of ≤1.2 g), compared with no intake, was associated with a 50% increased risk of CDA.
High intake of porridge at age 9 mo was associated with CDA with a similar magnitude as for low intake, although not statistically significantly so. Together with the risk estimates for low and high intake and porridge at age 6 mo, the pattern suggests that the risk of CDA from the exposure of porridge in infancy may be irrespective of the amount consumed. For milk cereal drink, there was no association with CD when modeling the intake as a categoric variable at age 18 mo, although the risk estimates followed a dose-response pattern consistent with the result for the continuous intake. However, this may be the result of lost statistical power when categorizing data compared with modeling the continuous intake.
The associations found were not consistent over time, suggesting different effects of specific gluten-containing foods depending on age. However, another explanation may be that the diet interacts with other risk factors present at different ages.
Gluten-rich bread is a common staple food in the Swedish food culture, typically containing both wheat and rye. Whereas the gluten content in wheat is estimated to be 80% of the total protein, the gluten content is lower in rye (65% of the protein) and barley (50% of the protein) (32). We therefore further tested whether the grain source of gluten modified the risk of the study outcomes. Using the Swedish TEDDY cohort, we observed that the overall main grain consumed by 2 y of age was wheat. However, neither wheat nor rye were associated with a higher risk of CDA or CD beyond the content of gluten, which is in line with previous findings (33).
As expected, the bread food group with its high gluten content and common addition of extra gluten (10) was highly correlated with total gluten intake across all visits, which suggests that bread is a main source of gluten in the diet of Swedish children. Conversely, milk cereal drink was the food group that correlated least with total gluten intake but was still associated with an increased risk of CD at age 18 mo. In contrast, porridge was strongly correlated with gluten intake in the first years of life. These food groups mainly consisted of commercial infant cereals with various contents of different grains and gluten levels, thus with varying contributions to the total daily gluten intake. In the late 1990s, after the “Swedish celiac epidemic,” the content of wheat in commercial infant cereals in Sweden overall decreased, and thus also the amount of gluten (34).
Children eligible for and enrolled in the TEDDY study are genetically susceptible to type 1 diabetes and CD. So, there may be a risk that the dietary habits found in the present study may not reflect those of the general population. However, the reported intakes of the studied cohort were in line with those of healthy 4-y-old children according to the most recently performed Swedish national survey of dietary food habits (35). Previously performed studies have also reported that commercial infant cereals, including milk cereal drink and porridge, are common foods in the dietary habits of Swedish infants and toddlers (36–38). Moreover, among Swedish children born during and after the “Swedish celiac epidemic,” the intake of flour from milk cereal drinks in children younger than age 2 y was 38 g/d and 24 g/d, respectively (39). In the present study performed in children born 20 y later, we observed a similar daily intake of flour equivalent to 35 g/d by 2 y of age from milk cereal drink.
A major strength of this study is the repeated dietary assessments, which clearly reveal the transition in dietary intake and food habits from infancy to early childhood. The prospective study design is another strength, which allowed for investigating dietary factors before development of CDA when parents were unaware of their child's antibody status. However, in TEDDY, data from national food databases were harmonized on the ingredient level but not on the level of composite foods and dishes (40). This limited us to only including Swedish children and not the full TEDDY cohort, consisting of children from Finland, Germany, and the United States. Therefore, more studies on cohorts of other nationalities are warranted to validate our findings. Another limitation was that the amounts of gluten in individual foods were not estimated, albeit the total daily gluten intake was adjusted for in the analyses. Adjusting for the gluten amount in each food is a more precise measure and could potentially change the results. Because no adjustment for multiple comparisons was done in this exploratory study, findings should be interpreted as hypothesis-generating (41).
Dietary interventions have been suggested as primary prevention strategies in CD (42). The findings from this study propose that an intake of porridge, high intake of bread for the age, and intake of milk cereal drink during the second year of life, besides the effect of gluten in these foods, may modify the risk of CDA and CD. Together with previous findings in TEDDY on gluten amounts (4), it remains to be studied if replacing gluten-containing foods, such as breads and infant cereals, with nutritionally equivalent foods containing less gluten to reduce the total gluten intake consequently decreases this risk or only delays the age at onset of CD in children at genetic risk.
In conclusion, this prospective study in genetically at-risk Swedish children assessed for dietary sources of gluten during the first 2 y of life found that intake of porridge, high intakes of bread and milk cereal drink, but not pasta, breakfast cereals, pancakes, sweet baked goods, or cookies and crackers, may modify the risk of CDA and CD. Dietary intervention studies are therefore warranted to test the hypothesis that reduced intake of these foods in early childhood may be preventive of CD in children at genetic risk.
Supplementary Material
ACKNOWLEDGEMENTS
The TEDDY Study Group: Colorado Clinical Center: Marian Rewers, Aaron Barbour, Kimberly Bautista, Judith Baxter, Daniel Felipe-Morales, Brigitte I Frohnert, Patricia Gesualdo, Michelle Hoffman, Rachel Karban, Edwin Liu, Alondra Munoz, Jill Norris, Holly O'Donnell, Stesha Peacock, Hanan Shorrosh, Marisa Stahl, Andrea Steck, Megan Stern, Kathleen Waugh, University of Colorado, Anschutz Medical Campus, Barbara Davis Center for Childhood Diabetes. Finland Clinical Center: Jorma Toppari (PI),¥^ Olli G Simell, Sanna-Mari Aaltonen,^ Annika Adamsson,^ Suvi Ahonen,*±§ Mari Åkerlund,*±§ Leena Hakola,*± Anne Hekkala,µ¤ Henna Holappa,µ¤ Heikki Hyöty,*± Anni Ikonen,µ¤ Jorma Ilonen,¥¶ Sanna Jokipuu,^ Miia Kähönen,µ¤ Leena Karlsson,^ Jukka Kero,¥^ Mikael Knip,*± Minna-Liisa Koivikko,µ¤ Katja Kokkonen,*± Mirva Koreasalo,*±§ Jaakko J Koskenniemi,¥^ Merja Koskinen,*± Kalle Kurppa,*± Salla Kuusela,*± Jarita Kytölä,*± Jutta Laiho,* Tiina Latva-aho,µ¤ Laura Leppänen,^ Katri Lindfors,* Maria Lönnrot,*± Elina Mäntymäki,^ Markus Mattila,*± Maija Miettinen,§ Katja Multasuo,µ¤ Teija Mykkänen,µ¤ Tiina Niininen,±* Sari Niinistö,§ Mia Nyblom,*± Sami Oikarinen,*± Paula Ollikainen,µ¤ Zhian Othmani,¥ Sirpa Pohjola,µ¤ Jenna Rautanen,±§ Anne Riikonen,*±§ Minna Romo,^ Satu Simell,¥ Päivi Tossavainen,µ¤ Mari Vähä-Mäkilä,¥ Eeva Varjonen,^ Riitta Veijola,µ¤ Irene Viinikangas,µ¤ Suvi M Virtanen.*±§ ¥University of Turku, *Tampere University, µUniversity of Oulu, ^Turku University Hospital, Hospital District of Southwest Finland, ±Tampere University Hospital, ¤Oulu University Hospital, §Finnish Institute for Health and Welfare, Finland, ¶University of Kuopio. Georgia/Florida Clinical Center: Jin-Xiong She (PI), Desmond Schatz,* Diane Hopkins, Leigh Steed, Jennifer Bryant, Katherine Silvis, Michael Haller,* Melissa Gardiner, Richard McIndoe, Ashok Sharma, Stephen W Anderson,^ Laura Jacobsen,* John Marks,* PD Towe,* Center for Biotechnology and Genomic Medicine, Augusta University. *University of Florida, Pediatric Endocrinology. ^Pediatric Endocrine Associates, Atlanta. Germany Clinical Center: Anette G Ziegler (PI), Ezio Bonifacio,* Cigdem Gezginci, Anja Heublein, Eva Hohoff,¥ Sandra Hummel, Annette Knopff, Charlotte Koch, Sibylle Koletzko,¶ Claudia Ramminger, Roswith Roth, Jennifer Schmidt, Marlon Scholz, Joanna Stock, Katharina Warncke, Lorena Wendel, Christiane Winkler, Forschergruppe Diabetes eV and Institute of Diabetes Research, Helmholtz Zentrum München, Forschergruppe Diabetes, and Klinikum rechts der Isar, Technische Universität München. *Center for Regenerative Therapies, TU Dresden, ¶Dr. von Hauner Children's Hospital, Department of Gastroenterology, Ludwig Maximilians University Munich, ¥University of Bonn, Department of Nutritional Epidemiology. Sweden Clinical Center: Åke Lernmark (PI), Daniel Agardh, Carin Andrén Aronsson, Rasmus Bennet, Corrado Cilio, Susanne Dahlberg, Emelie Ericson-Hallström, Ulla Fält, Lina Fransson, Thomas Gard, Malin Goldman Tsubarah, Emina Halilovic, Gunilla Holmén, Susanne Hyberg, Berglind Jonsdottir, Naghmeh Karimi, Helena Elding Larsson, Marielle Lindström, Markus Lundgren, Marlena Maziarz, Maria Månsson Martinez, Jessica Melin, Zeliha Mestan, Caroline Nilsson, Yohanna Nordh, Kobra Rahmati, Anita Ramelius, Falastin Salami, Anette Sjöberg, Carina Törn, Ulrika Ulvenhag, Terese Wiktorsson, Åsa Wimar, Lund University. Washington Clinical Center: William A Hagopian (PI), Michael Killian, Claire Cowen Crouch, Jennifer Skidmore, Luka-Sophia Bowen, Mikeil Metcalf, Arlene Meyer, Jocelyn Meyer, Denise Mulenga, Nole Powell, Jared Radtke, Shreya Roy, Davey Schmitt, Preston Tucker, Pacific Northwest Research Institute. Pennsylvania Satellite Center: Dorothy Becker, Margaret Franciscus, MaryEllen Dalmagro-Elias Smith, Ashi Daftary, Mary Beth Klein, Chrystal Yates, Children's Hospital of Pittsburgh of University of Pittsburgh Medical Center. Data Coordinating Center: Jeffrey P Krischer (PI), Rajesh Adusumali, Sarah Austin-Gonzalez, Maryouri Avendano, Sandra Baethke, Brant Burkhardt, Martha Butterworth, Nicholas Cadigan, Joanna Clasen, Kevin Counts, Laura Gandolfo, Jennifer Garmeson, Veena Gowda, Christina Karges, Shu Liu, Xiang Liu, Kristian Lynch, Jamie Malloy, Cristina McCarthy, Jose Moreno, Lazarus Mramba, Hemang M Parikh, Cassandra Remedios, Chris Shaffer, Susan Smith, Noah Sulman, Roy Tamura, Dena Tewey, Michael Toth, Ulla Uusitalo, Kendra Vehik, Ponni Vijayakandipan, Melissa Wroble, Jimin Yang, Kenneth Young. Past staff: Michael Abbondondolo, Lori Ballard, Rasheedah Brown, David Cuthbertson, Stephen Dankyi, Christopher Eberhard, Steven Fiske, David Hadley, Kathleen Heyman, Belinda Hsiao, Francisco Perez Laras, Hye-Seung Lee, Qian Li, Colleen Maguire, Wendy McLeod, Aubrie Merrell, Steven Meulemans, Ryan Quigley, Laura Smith, University of South Florida. Project scientist: Beena Akolkar, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Autoantibody Reference Laboratories: Liping Yu,^ Dongmei Miao,^ Kathleen Gillespie,* Kyla Chandler,* Ilana Kelland,* Yassin Ben Khoud,* Matthew Randell.* ^Barbara Davis Center for Childhood Diabetes, University of Colorado Denver, *Bristol Medical School, University of Bristol. HLA Reference Laboratory: William Hagopian, Jared Radtke, Preston Tucker, Pacific Northwest Research Institute (previously Henry Erlich, Steven J Mack, Anna Lisa Fear, Center for Genetics, Children's Hospital Oakland Research Institute). Repository: Sandra Ke, Niveen Mulholland, NIDDK Biosample Repository at Fisher BioServices. Other contributors: Thomas Briese, Columbia University; Todd Brusko, University of Florida; Suzanne Bennett Johnson, Florida State University; Eoin McKinney, University of Cambridge; Tomi Pastinen, The Children's Mercy Hospital; Eric Triplett, University of Florida.
The authors’ responsibilities were as follows—EMHaS, UU, DA, and CAA: conducted the research; EMHaS: performed the statistical analyses and wrote the paper; XL: reviewed the statistical analysis plan and the interpretation of the results; EMHaS and CAA: had primary responsibility for the final content; and all authors: designed the research and read and approved the final manuscript. The authors report no conflicts of interest.
Notes
Supported in part by NIH/National Center for Advancing Translational Sciences (NCATS) Clinical and Translational Science Awards UL1 TR000064 (to the University of Florida) and UL1 TR002535 (to the University of Colorado).
The TEDDY Study is funded by grants U01 DK63829, U01 DK63861, U01 DK63821, U01 DK63865, U01 DK63863, U01 DK63836, U01 DK63790, UC4 DK63829, UC4 DK63861, UC4 DK63821, UC4 DK63865, UC4 DK63863, UC4 DK63836, UC4 DK95300, UC4 DK100238, UC4 DK106955, UC4 DK112243, UC4 DK117483, U01 DK124166, and U01 DK128847 and contract no. HHSN267200700014C from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Allergy and Infectious Diseases, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institute of Environmental Health Sciences, CDC, and JDRF. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Supplemental Tables 1–4 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: CD, celiac disease; CDA, celiac disease autoimmunity; HLA, human leucocyte antigen; TEDDY, The Environmental Determinants of Diabetes in the Young; tTGA, tissue transglutaminase autoantibodies.
Contributor Information
Elin M Hård af Segerstad, Department of Clinical Sciences, Lund University, Malmö, Sweden.
Xiang Liu, Health Informatics Institute, Department of Pediatrics, Morsani Collage of Medicine, University of South Florida, Tampa, FL, USA.
Ulla Uusitalo, Health Informatics Institute, Department of Pediatrics, Morsani Collage of Medicine, University of South Florida, Tampa, FL, USA.
Daniel Agardh, Department of Clinical Sciences, Lund University, Malmö, Sweden.
Carin Andrén Aronsson, Department of Clinical Sciences, Lund University, Malmö, Sweden.
for the TEDDY Study Group:
Marian Rewers, Aaron Barbour, Kimberly Bautista, Judith Baxter, Daniel Felipe-Morales, Brigitte I Frohnert Marisa Stahl, Patricia Gesualdo, Michelle Hoffman, Rachel Karban, Edwin Liu, Alondra Munoz, Jill Norris, Holly O'Donnell, Stesha Peacock, Hanan Shorrosh, Andrea Steck, Megan Stern, Kathleen Waugh, Jorma Toppari, Olli G Simell, Annika Adamsson, Sanna-Mari Aaltonen, Suvi Ahonen, Mari Åkerlund, Leena Hakola, Anne Hekkala, Henna Holappa, Heikki Hyöty, Anni Ikonen, Jorma Ilonen, Sanna Jokipuu, Leena Karlsson, Jukka Kero, Jaakko J Koskenniemi, Miia Kähönen, Mikael Knip, Minna-Liisa Koivikko, Katja Kokkonen, Merja Koskinen, Mirva Koreasalo, Kalle Kurppa, Salla Kuusela, Jarita Kytölä, Jutta Laiho, Tiina Latva-aho, Laura Leppänen, Katri Lindfors, Maria Lönnrot, Elina Mäntymäki, Markus Mattila, Maija Miettinen, Katja Multasuo, Teija Mykkänen, Tiina Niininen, Sari Niinistö, Mia Nyblom, Sami Oikarinen, Paula Ollikainen, Zhian Othmani, Sirpa Pohjola, Jenna Rautanen, Anne Riikonen, Minna Romo, Satu Simell, Päivi Tossavainen, Mari Vähä-Mäkilä, Eeva Varjonen, Riitta Veijola, Irene Viinikangas, Suvi M Virtanen, Jin-Xiong She, Desmond Schatz, Diane Hopkins, Leigh Steed, Jennifer Bryant, Katherine Silvis, Michael Haller, Melissa Gardiner, Richard McIndoe, Ashok Sharma, Stephen W Anderson, Laura Jacobsen, John Marks, P D Towe, Anette G Ziegler, Ezio Bonifacio, Cigdem Gezginci, Anja Heublein, Eva Hohoff, Sandra Hummel, Annette Knopff, Charlotte Koch, Sibylle Koletzko, Claudia Ramminger, Roswith Roth, Jennifer Schmidt, Marlon Scholz, Joanna Stock, Katharina Warncke, Lorena Wendel, Christiane Winkler, Åke Lernmark, Daniel Agardh, Carin Andrén Aronsson, Rasmus Bennet, Corrado Cilio, Susanne Dahlberg, Ulla Fält, Malin Goldman Tsubarah, Emelie Ericson-Hallström, Lina Fransson, Thomas Gard, Emina Halilovic, Gunilla Holmén, Susanne Hyberg, Berglind Jonsdottir, Naghmeh Karimi, Helena Elding Larsson, Marielle Lindström, Markus Lundgren, Marlena Maziarz, Maria Månsson Martinez, Jessica Melin, Zeliha Mestan, Caroline Nilsson, Yohanna Nordh, Kobra Rahmati, Anita Ramelius, Falastin Salami, Anette Sjöberg, Carina Törn, Ulrika Ulvenhag, Terese Wiktorsson, Åsa Wimar, William A Hagopian, Michael Killian, Claire Cowen Crouch, Jennifer Skidmore, Luka-Sophia Bowen, Mikeil Metcalf, Arlene Meyer, Jocelyn Meyer, Denise Mulenga, Nole Powell, Jared Radtke, Shreya Roy, Davey Schmitt, Preston Tucker, Dorothy Becker, Margaret Franciscus, MaryEllen Dalmagro-Elias Smith, Ashi Daftary, Mary Beth Klein, Chrystal Yates, Jeffrey P Krischer, Rajesh Adusumali, Sarah Austin-Gonzalez, Maryouri Avendano, Sandra Baethke, Brant Burkhardt, Martha Butterworth, Nicholas Cadigan, Joanna Clasen, Kevin Counts, Laura Gandolfo, Jennifer Garmeson, Veena Gowda, Christina Karges, Shu Liu, Xiang Liu, Kristian Lynch, Jamie Malloy, Lazarus Mramba, Cristina McCarthy, Jose Moreno, Hemang M Parikh, Cassandra Remedios, Chris Shaffer, Susan Smith, Noah Sulman, Roy Tamura, Dena Tewey, Michael Toth, Ulla Uusitalo, Kendra Vehik, Ponni Vijayakandipan, Melissa Wroble, Jimin Yang, Kenneth Young, Michael Abbondondolo, Lori Ballard, Rasheedah Brown, David Cuthbertson, Stephen Dankyi, Christopher Eberhard, Steven Fiske, David Hadley, Kathleen Heyman, Belinda Hsiao, Francisco Perez Laras, Hye-Seung Lee, Qian Li, Colleen Maguire, Wendy McLeod, Aubrie Merrell, Steven Meulemans, Ryan Quigley, Laura Smith, Beena Akolkar, Liping Yu, Dongmei Miao, Kathleen Gillespie, Kyla Chandler, Ilana Kelland, Yassin Ben Khoud, Matthew Randell, William Hagopian, Jared Radtke, Preston Tucker, Henry Erlich, Steven J Mack, Anna Lisa Fear, Sandra Ke, Niveen Mulholland, Thomas Briese, Todd Brusko, Suzanne Bennett Johnson, Eoin McKinney, Tomi Pastinen, and Eric Triplett
Data Availability
Data described in the article are deposited in the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Central Repository at https://repository.niddk.nih.gov/studies/teddy/.
References
- 1. Sollid LM. Coeliac disease: dissecting a complex inflammatory disorder. Nat Rev Immunol. 2002;2(9):647–55. [DOI] [PubMed] [Google Scholar]
- 2. King JA, Jeong J, Underwood FE, Quan J, Panaccione N, Windsor JWet al. . Incidence of celiac disease is increasing over time: a systematic review and meta-analysis. Am J Gastroenterol. 2020;115(4):507–25. [DOI] [PubMed] [Google Scholar]
- 3. Bradauskiene V, Vaiciulyte-Funk L, Martinaitiene D, Andruskiene J, Verma AK, Lima JPMet al. . Wheat consumption and prevalence of celiac disease: correlation from a multilevel analysis. Crit Rev Food Sci Nutr. 2021;1–15. [DOI] [PubMed] [Google Scholar]
- 4. Andrén Aronsson C, Lee HS, Hård Af Segerstad EM, Uusitalo U, Yang J, Koletzko Set al. . Association of gluten intake during the first 5 years of life with incidence of celiac disease autoimmunity and celiac disease among children at increased risk. JAMA. 2019;322(6):514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marild K, Dong F, Lund-Blix NA, Seifert J, Baron AE, Waugh KCet al. . Gluten intake and risk of celiac disease: long-term follow-up of an at-risk birth cohort. Am J Gastroenterol. 2019;114(8):1307–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lund-Blix NA, Marild K, Tapia G, Norris JM, Stene LC, Størdal K. Gluten intake in early childhood and risk of celiac disease in childhood: a nationwide cohort study. Am J Gastroenterol. 2019;114(8):1299–306. [DOI] [PubMed] [Google Scholar]
- 7. Crespo-Escobar P, Mearin ML, Hervas D, Auricchio R, Castillejo G, Gyimesi Jet al. . The role of gluten consumption at an early age in celiac disease development: a further analysis of the prospective PreventCD cohort study. Am J Clin Nutr. 2017;105(4):890–6. [DOI] [PubMed] [Google Scholar]
- 8. Shewry P. What is gluten—why is it special?. Front Nutr. 2019;6:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pronin D, Börner A, Weber H, Scherf KA. Wheat (Triticum aestivum L.) breeding from 1891 to 2010 contributed to increasing yield and glutenin contents but decreasing protein and gliadin contents. J Agric Food Chem. 2020;68(46):13247–56. [DOI] [PubMed] [Google Scholar]
- 10. Day L, Augustin MA, Batey IL, Wrigley CW. Wheat-gluten uses and industry needs. Trends Food Sci Technol. 2006;17(2):82–90. [Google Scholar]
- 11. Scherf KA, Catassi C, Chirdo F, Ciclitira PJ, Feighery C, Gianfrani Cet al. . Recent progress and recommendations on celiac disease from the Working Group on Prolamin Analysis and Toxicity. Front Nutr. 2020;7:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Smith F, Pan X, Bellido V, Toole GA, Gates FK, Wickham MSet al. . Digestibility of gluten proteins is reduced by baking and enhanced by starch digestion. Mol Nutr Food Res. 2015;59(10):2034–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ma S, Han W, Li L, Zheng X, Wang X. The thermal stability, structural changeability, and aggregability of glutenin and gliadin proteins induced by wheat bran dietary fiber. Food Funct. 2019;10(1):172–9. [DOI] [PubMed] [Google Scholar]
- 14. Prandi B, Faccini A, Tedeschi T, Cammerata A, Sgrulletta D, D'Egidio MGet al. . Qualitative and quantitative determination of peptides related to celiac disease in mixtures derived from different methods of simulated gastrointestinal digestion of wheat products. Anal Bioanal Chem. 2014;406(19):4765–75. [DOI] [PubMed] [Google Scholar]
- 15. Pasini G, Simonato B, Giannattasio M, Peruffo AD, Curioni A. Modifications of wheat flour proteins during in vitro digestion of bread dough, crumb, and crust: an electrophoretic and immunological study. J Agric Food Chem. 2001;49(5):2254–61. [DOI] [PubMed] [Google Scholar]
- 16. Wen W, Li S, Gu Y, Wang S, Wang J. Effects of starch on the digestibility of gluten under different thermal processing conditions. J Agric Food Chem. 2019;67(25):7120–7. [DOI] [PubMed] [Google Scholar]
- 17. Wang Y, Wang J, Wang S, Guo J, Wang S. Modification of glutenin and associated changes in digestibility due to methylglyoxal during heat processing. J Agric Food Chem. 2019;67(38):10734–43. [DOI] [PubMed] [Google Scholar]
- 18. Liu E, Lee H-S, Aronsson CA, Hagopian WA, Koletzko S, Rewers MJet al. . Risk of pediatric celiac disease according to HLA haplotype and country. N Engl J Med. 2014;371(1):42–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ivarsson A, Hernell O, Stenlund H, Persson LA. Breast-feeding protects against celiac disease. Am J Clin Nutr. 2002;75(5):914–21. [DOI] [PubMed] [Google Scholar]
- 20. Popp A, Mäki M. Changing pattern of childhood celiac disease epidemiology: contributing factors. Front Pediatr. 2019;7:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. TEDDY study group . The Environmental Determinants of Diabetes in the Young (TEDDY) study: study design. Pediatr Diabetes. 2007;8(5):286–98. [DOI] [PubMed] [Google Scholar]
- 22. Hagopian WA, Erlich H, Lernmark A, Rewers M, Ziegler AG, Simell Oet al. . The Environmental Determinants of Diabetes in the Young (TEDDY): genetic criteria and international diabetes risk screening of 421 000 infants. Pediatr Diabetes. 2011;12(8):733–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Andrén Aronsson C, Lee H-S, Koletzko S, Uusitalo U, Yang J, Virtanen SMet al. . Effects of gluten intake on risk of celiac disease: a case-control study on a Swedish birth cohort. Clin Gastroenterol Hepatol. 2016;14(3):403–9.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang J, Lynch KF, Uusitalo UM, Foterek K, Hummel S, Silvis Ket al. . Factors associated with longitudinal food record compliance in a paediatric cohort study. Public Health Nutr. 2016;19(5):804–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wieser H, Koehler P, Scherf KA. The two faces of wheat. Front Nutr. 2020;7:517313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vehik K, Fiske SW, Logan CA, Agardh D, Cilio CM, Hagopian Wet al. . Methods, quality control and specimen management in an international multicentre investigation of type 1 diabetes: TEDDY. Diabetes Metab Res Rev. 2013;29(7):557–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Simell S, Kupila A, Hoppu S, Hekkala A, Simell T, Stahlberg MRet al. . Natural history of transglutaminase autoantibodies and mucosal changes in children carrying HLA-conferred celiac disease susceptibility. Scand J Gastroenterol. 2005;40(10):1182–91. [DOI] [PubMed] [Google Scholar]
- 28. Liu E, Dong F, Barón AE, Taki I, Norris JM, Frohnert BIet al. . High incidence of celiac disease in a long-term study of adolescents with susceptibility genotypes. Gastroenterology. 2017;152(6):1329–36.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Björck S, Brundin C, Lörinc E, Lynch KF, Agardh D. Screening detects a high proportion of celiac disease in young HLA-genotyped children. J Pediatr Gastroenterol Nutr. 2010;50(1):49–53. [DOI] [PubMed] [Google Scholar]
- 30. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4):1220S–8S.; discussion 1229S–31S. [DOI] [PubMed] [Google Scholar]
- 31. Hörnell A, Berg C, Forsum E, Larsson C, Sonestedt E, Åkesson Aet al. . Perspective: an extension of the STROBE statement for observational studies in nutritional epidemiology (STROBE-nut): explanation and elaboration. Adv Nutr. 2017;8(5):652–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hoppe C, Trolle E, Gondolf UH, Husby S. Gluten intake in 6–36-month-old Danish infants and children based on a national survey. J Nutr Sci. 2013;2:e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hardy MY, Russell AK, Pizzey C, Jones CM, Watson KA, La Gruta NLet al. . Characterisation of clinical and immune reactivity to barley and rye ingestion in children with coeliac disease. Gut. 2020;69(5):830–40. [DOI] [PubMed] [Google Scholar]
- 34. Ivarsson A, Persson LÅ, Nyström L, Ascher H, Cavell B, Danielsson Let al. . Epidemic of coeliac disease in Swedish children. Acta Paediatr. 2000;89(2):165–71. [DOI] [PubMed] [Google Scholar]
- 35. Swedish Food Agency . National Food Survey of 2003: food and nutrient intake in Swedish children[Internet]. Uppsala, Sweden: Livsmedelsverket; 2003. Available from: https://www.livsmedelsverket.se/globalassets/matvanor-halsa-miljo/kostrad-matvanor/matvaneundersokningar/riksmaten-_-barn_2003_livsmedels_och_naringsintag_bland_barn_i_sverige1.pdf (cited June 7, 2021). [Google Scholar]
- 36. Almquist-Tangen G, Dahlgren J, Roswall J, Bergman S, Alm B. Milk cereal drink increases BMI risk at 12 and 18 months, but formula does not. Acta Paediatr. 2013;102(12):1174–9. [DOI] [PubMed] [Google Scholar]
- 37. Almquist-Tangen G, Bergman S, Dahlgren J, Lindholm A, Roswall J, Alm B. Consuming milk cereal drinks at one year of age was associated with a twofold risk of being overweight at the age of five. Acta Paediatr. 2019;108(6):1115–21. [DOI] [PubMed] [Google Scholar]
- 38. Ngwa CH, Panneh M, Eiben G, Rosén M, Hunsberger M. Milk cereal drink feeding practices: a descriptive study among Swedish children participating in the IDEFICS. Family study. J Food Sci Technol. 2021;6(1):293–8. [Google Scholar]
- 39. Ivarsson A, Myléus A, Norström F, van der Pals M, Rosén A, Högberg Let al. . Prevalence of childhood celiac disease and changes in infant feeding. Pediatrics. 2013;131(3):e687–94. [DOI] [PubMed] [Google Scholar]
- 40. Joslowski G, Yang J, Aronsson CA, Ahonen S, Butterworth M, Rautanen Jet al. . Development of a harmonized food grouping system for between-country comparisons in the TEDDY Study. J Food Compos Anal. 2017;63:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rothman K. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–6. [PubMed] [Google Scholar]
- 42. Auricchio R, Troncone R. Can celiac disease be prevented?. Front Immunol. 2021;12:672148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the article are deposited in the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Central Repository at https://repository.niddk.nih.gov/studies/teddy/.