Abstract
Introduction
Detailed data on clinical characteristics in children with the omicron strain of SARS-COV-2 are limited.
Methods
We conducted a retrospective observational study of children with COVID-19 at the National Center for Child Health and Development to evaluate the clinical manifestations during and before the emergence of the omicron variant. Only symptomatic patients without underlying diseases were included. Participants were divided into two temporal groups: the “omicron era” (1/2022–2/2022) and the “pre-omicron era,” where the delta variant predominated (7/2021–11/2021). The patients were subclassified into an older vaccine-eligible group (aged 12–17 years), a younger vaccine-eligible group (aged 5–11 years), and a vaccine-ineligible group (aged 0–4 years).
Results
We compared 113 patients in the omicron era with 106 in the pre-omicron era. Most patients in both eras had non-severe disease, and no patients required mechanical ventilation or died. Among patients aged 0–4 years, sore throat and hoarseness were more common during the omicron era than the pre-omicron era (11.1% vs. 0.0% and 11.1% vs. 1.5%, respectively). Croup syndrome was diagnosed in all patients with hoarseness. Among patients aged 5–11 years, vomiting was more frequent during the omicron era (47.2%) than during the pre-omicron era (21.7%). Cough and rhinorrhea were less common during the omicron era in patients aged 0–4 and 5–11 years, respectively, than during the pre-omicron era.
Conclusions
In children with COVID-19, clinical manifestations differed between the omicron and pre-omicron eras. In the Omicron era, croup syndrome was more frequent in vaccine-ineligible children.
Keywords: Children, Coronavirus disease 2019, Croup, Delta variant of concern, Omicron variant of concern
Abbreviations
- COVID-19
coronavirus disease 2019
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- VOC
variant of concern
Authorship statement
HI wrote the first draft of the manuscript. MK and CO modified and reviewed the manuscript. CO supervised and revised the manuscript. All authors approved the final manuscript.
Funding sources
Financial support for this study was provided by the Grant of National Center for Child Health and Development (30E-1).
1. Introduction
The omicron strain (BA.1/B.1.1.529) of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a variant of concern (VOC); it is more transmissible than previous VOCs and has spread rapidly worldwide [[1], [2], [3]]. The omicron VOC contains up to 36 mutations in spike protein and has been speculated to differ from previous VOCs not only in infectivity but also in clinical manifestations [2,4].
A recent cohort study has suggested that the omicron VOC causes milder disease in children than did previous variants, with lower rates of admissions to intensive care units and of mechanical ventilation [5]. In contrast, surveillance across 14 U S. states revealed that hospitalization rates per population among patients aged 0–4 years with COVID-19 had been approximately five times higher after the emergence of the omicron VOC than before when the delta VOC predominated [6]. This may indicate that the omicron VOC has a substantial disease burden on children aged 0–4 years, who are not currently eligible for COVID-19 vaccination.
Data about the clinical characteristics in children infected with the omicron VOC are limited. Furthermore, it remains unclear whether the symptoms and severity caused by the omicron VOC differ according to age and whether the clinical features of the omicron VOC differ from those of the previous VOCs.
The purpose of this study was to investigate the clinical characteristics of pediatric patients with COVID-19 after the advent of the omicron VOC (the “omicron era”) and before (the “pre-omicron era”) in different age groups.
1.1. Patients and methods
We conducted a retrospective review of pediatric patients (<18 years old) with COVID-19 who visited the National Center for Child Health and Development in Tokyo, Japan, between July 1, 2021, and February 28, 2022. This study included both inpatients and outpatients. The diagnosis of COVID-19 was confirmed by polymerase chain reaction or an antigen test for SARS-CoV-2. We used patients’ electronic medical records to document medical history, age at presentation, sex, clinical manifestations, laboratory data, radiographic findings, and outcomes. Neither patients with underlying diseases nor those with onset of the illness in December 2021, the transition period, were included. Asymptomatic patients at presentation were also excluded. Patients eligible for the study were divided into two groups according to the timing of presentation: the pre-omicron era (July 1 to November 30, 2021) and the omicron era (January 1 to February 28, 2022). In Tokyo, where this study was conducted, of the SARS-CoV2 infections during the omicron era, 97.2% were with the omicron VOC and 2.8% were with the delta VOC; during the pre-omicron era, 0% of SARS-CoV2 infections were with the omicron VOC, 87% were with the delta VOC, and 13% were with the alpha VOC, according to the Division of Infectious Disease Control, Bureau of Social Welfare and Public Health, Tokyo Metropolitan Government [7]. The patients were further subdivided into three age groups: an older vaccine-eligible group (12–17 years of age), a younger vaccine-eligible group (5–11 years of age), and a vaccine-ineligible group (0–4 years of age). In Japan, vaccination against SARS-CoV-2 began in June 2021 for children aged 12 years and older and in March 2022 for children aged 5–11 years. Clinical manifestations were also classified according to the presence of respiratory symptoms (cough, rhinorrhea, sore throat, and hoarseness) and gastrointestinal symptom (diarrhea, vomiting, and abdominal pain). The severity of the manifestations was classified according to guidelines of the World Health Organization [8].
Values of continuous variables were calculated as medians with interquartile ranges (25th to 75th), and those of categorical variables were calculated as numbers and percentages. To compare continuous variables, we used the Mann–Whitney U test; to compare categorical data, we used Fisher's exact test. All P values were two-sided in tests, and P values of < .05 were considered statistically significant. All data were analyzed with SPSS software version 27 (IBM Corporation, Armonk, NY, USA).
Informed consent was obtained from the parents of all patients through an opt-out website. This study was approved by the Ethics Committees of the National Center for Child Health and Development in February 2022 (no. 2021–254).
2. Results
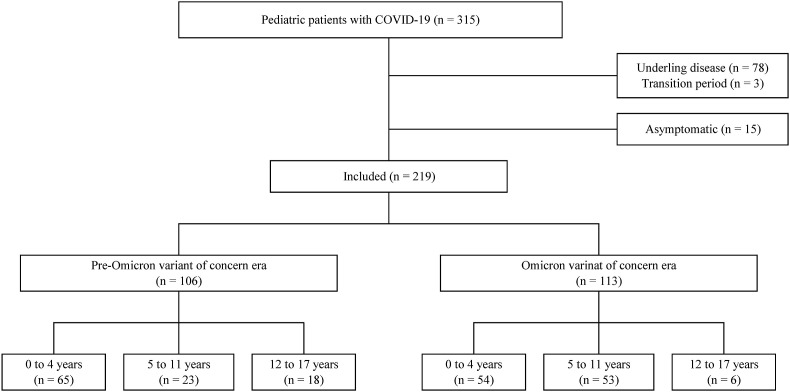
From July 1, 2021, to February 28, 2022, a total of 315 pediatric patients with COVID-19 presented to the National Center for Child Health and Development; we excluded 78 patients because of other underlying disease, 3 because they presented during the transition period (December 2021), and 15 because they had no symptoms. The remaining 219 patients were included: 113 who became infected during the omicron era and 106 infected during the pre-omicron era (Fig. 1 ). The omicron era had a larger proportion of patients aged 5–11 years and a smaller proportion of those aged 12–17 years (Supplementary Figs. 1 and 2). Demographic data, treatment, and outcomes are summarized in Table 1 . During both the omicron and pre-omicron eras, the majority of the patients were judged to have non-severe disease. Three patients were categorized as severe disease (two in the pre-omicron era and one in the omicron era), all of whom were treated with Remdesivir for pneumonia. Antibiotics were administered in four patients during the omicron era (two febrile patients under 3 months of age, one with laryngitis, and one with otitis media) and in three during the pre-omicron era (two with urinary tract infections and one with otitis media). Three patients required oxygen therapy in the omicron era (mean duration of 1.7 days) and six patients did in the pre-omicron era (mean duration of 2.8 days). No patients required mechanical ventilation or died. Only one 12-year-old patient in the pre-omicron era and no patients in the omicron era had received the COVID-19 vaccination. Clinical manifestations, laboratory data, and chest radiograph findings are depicted in Fig. 2, Fig. 3 and listed in Supplementary Tables 1–3.
Fig. 1.
Flowchart of the inclusion process.
Table 1.
Demographic and clinical data of patients with COVID-19.
| Pre–Omicron VOC era (n = 106) | Omicron VOC era (n = 113) | |
|---|---|---|
| Demographic data | ||
| Male, n (%) | 54 (50.9%) | 70 (61.9%) |
| Age at presentation, months, median (IQR) | 2.9 (1.3–7.6) | 5.3 (1.3–8.4) |
| 0–4 years, n | 65 | 54 |
| 5–11 years, n | 23 | 53 |
| 12–17 years, n | 18 | 6 |
| Vaccinated against SARS-CoV-2, n | 1 | 0 |
| Multiplex PCR, n, positive for non-SARS-CoV-2/obtained | 5/15 | 2/16 |
| Treatment and outcome during clinical course | ||
| Antibiotics, n | 3 | 4 |
| Remdesivir, n | 4 | 1 |
| Casirivimab/Imdevimab, n | 0 | 0 |
| Sotrovimab, n | 0 | 0 |
| Dexamethasone, n | 4 | 0 |
| ICU admission, n | 0 | 0 |
| High-flow nasal cannula, n | 0 | 0 |
| Mechanical ventilation, n | 0 | 0 |
| Severity | ||
| Non-severe, n (%) | 104 (98.1%) | 112 (99.2%) |
| Severe, n (%) | 2 (1.9%) | 1 (0.9%) |
| Critical, n (%) | 0 | 0 |
| Multisystem inflammatory syndrome in children, n | 0 | 0 |
| Deatha, n | 0 | 0 |
COVID-19, coronavirus disease 2019; VOC, variant of concern; IQR, interquartile range; PCR, polymerase chain reaction.
Complication up to 6 weeks after hospital visit.
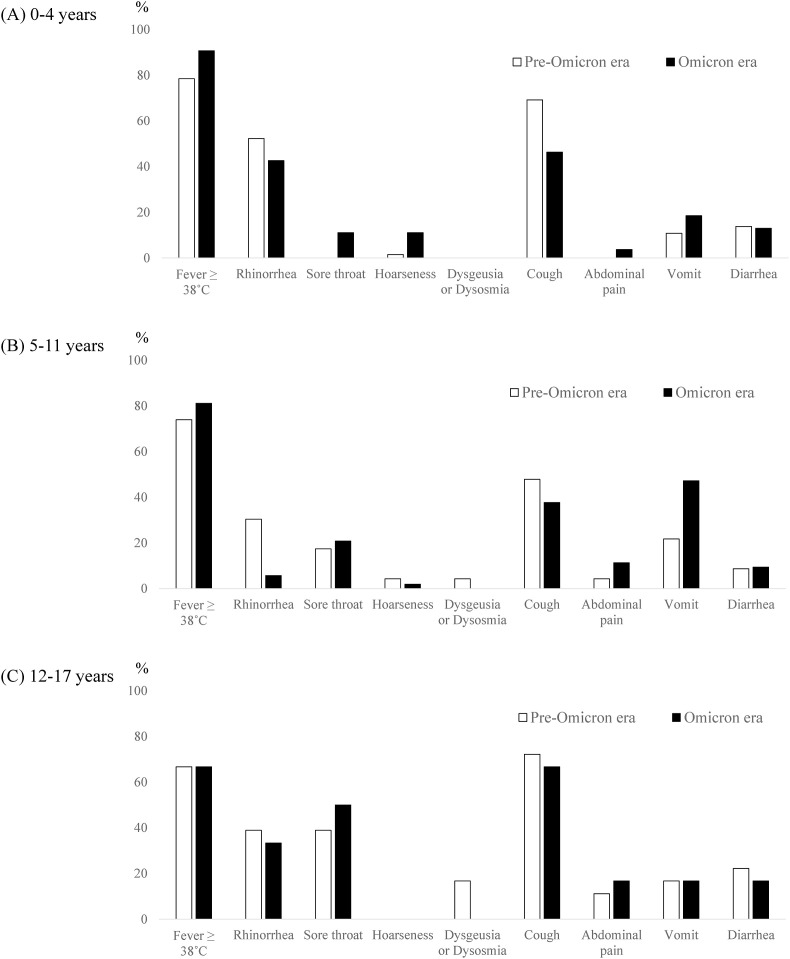
Fig. 2.
Symptoms of COVID-19 in each age group during the omicron and pre-omicron eras. A, 0–4 years of age (n = 65 in the pre-omicron era and n = 54 in the omicron era). B, 5–11 years of age (n = 23 in the pre-omicron era and n = 53 in the omicron era). C, 12–17 years of age (n = 18 in the pre-omicron era and n = 6 in the omicron era).
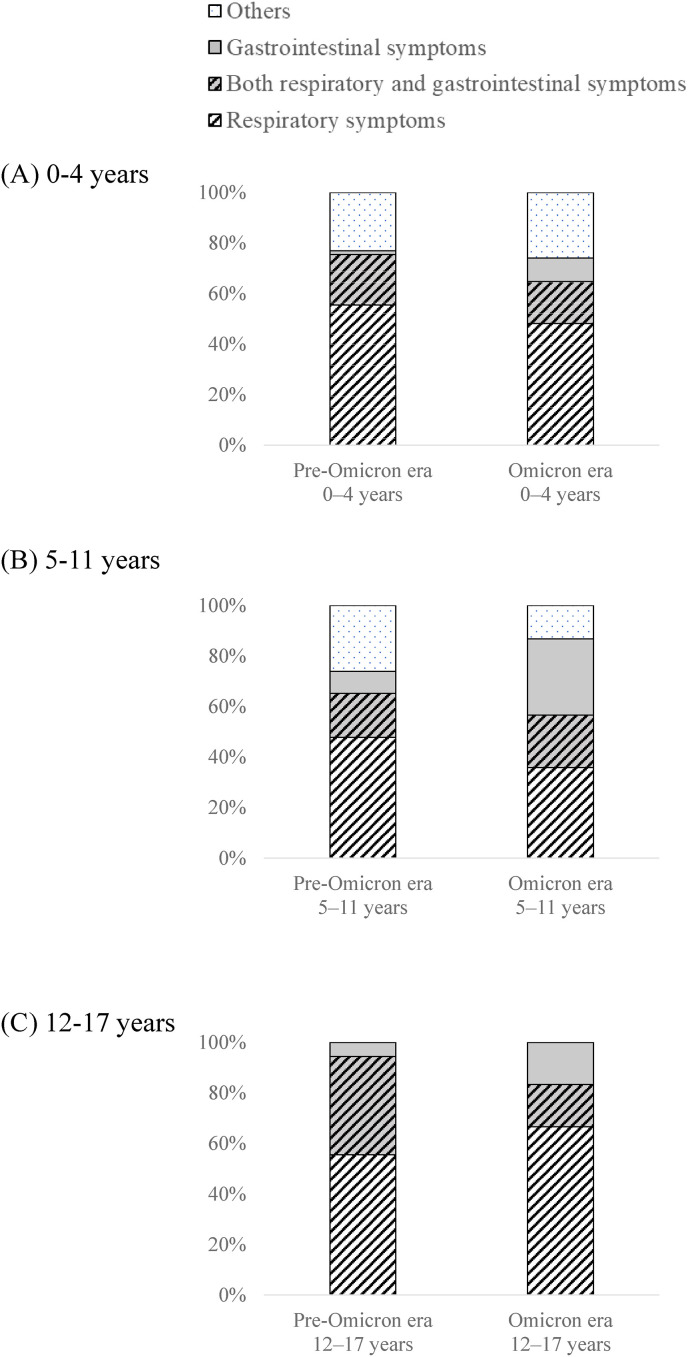
Fig. 3.
Distribution of clinical manifestations according to the presence of respiratory or gastrointestinal symptoms, or both. A, 0–4 years of age. B, 5–11 years of age. C, 12–17 years of age. Respiratory symptom included cough, rhinorrhea, sore throat, and hoarseness. Gastrointestinal symptom included diarrhea, vomit, and abdominal pain. Others included fever, fatigue, headache, rash, dysgeusia, and dysosmia.
Among patients aged 0–4 years, the incidence of sore throat was higher during the omicron era (11.1%) than during the pre-omicron era (0.0%), as was that of hoarseness (11.1% vs. 1.5%, respectively; Fig. 2A, and Supplementary Table 1). Croup syndrome was diagnosed in all patients with hoarseness. Among patients aged 5–11 years, the incidence of vomiting was higher during the omicron era (47.2%) than during the pre-omicron era (21.7%; Fig. 2B, and Supplementary Table 2) and gastrointestinal symptoms appeared quite common presentation regardless of the presence of respiratory symptoms during the omicron era (Fig. 3B). In contrast, in patients aged 0–4 and 5–11 years, cough and rhinorrhea, respectively, were less frequent during the omicron era (Fig. 2A and B, and Supplementary Tables 1 and 2). No difference in frequency of any symptoms was found in patients aged 12–17 years between the two eras (Fig. 2C, and Supplementary Table 3.
3. Discussion
In this study, we demonstrated that among pediatric patients with COVID-19, the incidences of croup syndrome and sore throat at ages 0–4 years and of vomiting at ages 5–11 years were higher during the omicron era than during the pre-omicron era. The majority of patients in both eras had non-severe disease.
Few patients with COVID-19 and croup syndrome were reported until the emergence of the omicron VOC. In Boston, the number of pediatric patients with croup syndrome increased with the emergence of the omicron variant [9]. Studies of adult patients have shown that the omicron VOC affects predominantly the upper airways and causes acute laryngitis, unlike previous VOCs, which often affected the lower respiratory tract [10]. These findings suggest that the omicron strain is more likely to cause laryngeal inflammation. Because the upper airways are narrower in children than in adults, inflammation of the larynx often leads to serious clinical manifestations such as croup syndrome, especially in younger children. Upper airway obstruction should therefore be considered in younger children when they are exposed to the omicron variant. Patients aged 0–4 years are not currently eligible for COVID-19 vaccination, and COVID-19–associated hospitalization rates in this age group increased dramatically when the omicron variant began to predominate in the United States [6]. Vaccination of eligible family members and caregivers is essential to prevent transmission of the virus to this age group.
The incidence of gastrointestinal symptoms, especially vomiting, was more frequent in the 5- to 11-year age group during the omicron era. Few studies have reported a higher frequency of vomiting in patients infected with the omicron variant in comparison with previous VOCs. In a review of adults before the emergence of omicron VOC, gastrointestinal symptoms were present in 1.1–49.5% of patients with COVID-19 (2–49.5% for diarrhea, 3.9–10.2% for nausea and vomiting, and 1.1–9.2% for abdominal pain) [11]. Angiotensin-converting enzyme 2 (ACE-2), the receptor for SARS-CoV, is expressed abundantly in the cells of the gastrointestinal tract, including the stomach, duodenum, and small and large intestines, presumably in association with nausea and vomiting [12]. Earlier studies have shown that the binding affinity of SARS-CoV-2 to ACE-2 is 10 times stronger than that of SARS-CoV-1 [13] and that SARS-CoV-2 productively infects human gut enterocytes [14]. Similarly, investigators using computational modeling and simulations has shown that the omicron VOC binds to human ACE-2 more strongly than does the wild type [15]. The higher incidence of vomiting in patients during the omicron era in this study may be attributable in part to the increased affinity of the omicron VOC to ACE-2 in intestinal cells. In this study, the reason for the difference in the incidence of gastrointestinal symptoms with age is unclear, but it may be partly because some symptoms are subjective.
Cough was less common in the 0 to 4-year age group during the omicron era than during the pre-omicron era. A study of primarily adult patients in the United Kingdom has shown that the omicron variant was associated with fewer lower respiratory tract symptoms [16]. An ex vivo study demonstrated that in comparison with previous variants, rates of replication competence of the omicron VOC in human lung cells were lower [17], and the other in vivo study using hamster bronchial cells showed that the omicron VOC was associated with attenuated lung disease [18]. These results have suggested that the lower incidence of cough during the omicron era in this study reflect that the omicron VOC is less likely to affect the lower respiratory tract.
This study had several limitations. It was a single-center, retrospective, observational study. The analysis in the adolescent age group was limited given the relatively small sample size, which was likely to prevent us to detect the small difference in specific symptoms (e.g., dysgeusia or dysosmia). We also could not evaluate the effects of the SARS-CoV-2 vaccine, although the smaller numbers of adolescents presenting with COVID-19 during the omicron era might have been attributable to the increased vaccination coverage of this age group. The information about SARS-CoV-2 strains was unavailable for individual patients. However, as described in the Methods section, the omicron and delta VOCs were primary strains in this catchment area during the omicron and pre-omicron eras, respectively. Therefore, we believe that our patients are largely representative of patients with those variants. Lastly, the populations of patients who presented during the omicron and pre-omicron eras might have differed in some ways. However, our hospital's policy to see patients did not change during the study period, and both patients with referrals and walk-ins were accepted. Thus, any potential difference between those populations is unlikely to substantially affect the results and conclusions of this study.
In conclusion, manifestations of COVID-19 during the omicron and pre-omicron eras differed according to age group. In younger children, the omicron VOC appeared to cause croup syndrome more frequently, important findings to protect this vulnerable population because COVID-19 vaccination is unavailable in this age group. Further studies are needed to distinguish the clinical characteristics of the omicron VOC from those of other VOCs, especially in adolescents.
Declaration of competing interest
All authors do not have any potential, perceived, or real conflicts of interest.
Acknowledgements
Financial support for this study was provided by the Grant of National Center for Child Health and Development (30E -1).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jiac.2022.07.016.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Viana R., Moyo S., Amoako D.G., Tegally H., Scheepers C., Althaus C.L., et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature. 2022;603:679–686. doi: 10.1038/s41586-022-04411-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dejnirattisai W., Huo J., Zhou D., Zahradník J., Supasa P., Liu C., et al. SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell. 2022;185:467–484. doi: 10.1016/j.cell.2021.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elliott P., Bodinier B., Eales O., Wang H., Haw D., Elliott J., et al. Rapid increase in omicron infections in England during december 2021: REACT-1 study. Science. 2022;375:1406–1411. doi: 10.1126/science.abn8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bittmann S., Luchter E., Moschuring-Alieva E., Bittmann L., Villalon G. What is new with omicron variant of SARS-CoV-2 in children? J Clin Med Res. 2022;14:108–109. doi: 10.14740/jocmr4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang L., Berger N.A., Kaelber D.C., Davis P.B., Volkow N.D., Xu R. Incidence rates and clinical outcomes of SARS-CoV-2 infection with the omicron and delta variants in children younger than 5 Years in the US. JAMA Pediatr. 2022 doi: 10.1001/jamapediatrics.2022.0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marks K.J., Whitaker M., Agathis N.T., Anglin O., Milucky J., Patel K., et al. Hospitalization of infants and children aged 0-4 Years with laboratory-confirmed COVID-19 - COVID-NET, 14 states, March 2020-february 2022. MMWR Morb Mortal Wkly Rep. 2022;71:429–436. doi: 10.15585/mmwr.mm7111e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tokyo metropolitan government disaster prevention information. https://www.bousai.metro.tokyo.lg.jp/_res/projects/default_project/_page_/001/021/131/81/20220303_10.pdf
- 8.World Health Organization Therapeutics and COVID-19: living guideline. https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2022.3 8 May 2022. [PubMed]
- 9.Brewster R.C.L., Parsons C., Laird-Gion J., Hilker S., Irwin M., Sommerschield A., et al. COVID-19-Associated croup in children. Pediatrics. 2022 doi: 10.1542/peds.2022-056492. [DOI] [PubMed] [Google Scholar]
- 10.Piersiala K., Kakabas L., Bruckova A., Starkhammar M., Cardell L.O. Acute odynophagia: a new symptom of COVID-19 during the SARS-CoV-2 Omicron variant wave in Sweden. J Intern Med. 2022 doi: 10.1111/joim.13470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sirinawasatien A., Chantarojanasiri T., Ekpanyapong S., Tivatunsakul N., Luvira V. Coronavirus disease 2019 gastrointestinal and liver manifestations in adults: a review. JGH Open. 2021;5:1257–1265. doi: 10.1002/jgh3.12671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang T., Liu D., Tian D., Xia L. The roles of nausea and vomiting in COVID-19: did we miss something? J Microbiol Immunol Infect. 2021;54:541–546. doi: 10.1016/j.jmii.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamers M.M., Beumer J., Vaart J., Knoops K., Puschhof J., Breugem T.I., et al. SARS-CoV-2 productively infects human gut enterocytes. Science. 2020;369:50–54. doi: 10.1126/science.abc1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lupala C.S., Ye Y., Chen H., Su X.D., Liu H. Mutations on RBD of SARS-CoV-2 Omicron variant result in stronger binding to human ACE2 receptor. Biochem Biophys Res Commun. 2022;29(590):34–41. doi: 10.1016/j.bbrc.2021.12.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vihta K.D., Pouwels K.B., Peto T.E.A., Pritchard E., House T., Studley R., et al. Omicron-associated changes in SARS-CoV-2 symptoms in the United Kingdom. medRxiv. 2022 doi: 10.1101/2022.01.18.22269082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hui K.P.Y., Ho J.C.W., Cheung M.C., Ng K.C., Ching R.H.H., Lai K.L., et al. SARS-CoV-2 Omicron variant replication in human bronchus and lung ex vivo. Nature. 2022;603:715–720. doi: 10.1038/s41586-022-04479-6. [DOI] [PubMed] [Google Scholar]
- 18.Diamond M., Halfmann P., Maemura T., Iwatsuki-Horimoto K., Iida S., Kiso M., et al. The SARS-CoV-2 B.1.1.529 Omicron virus causes attenuated infection and disease in mice and hamsters. Res Sq. 2021 doi: 10.21203/rs.3.rs-1211792/v1. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.