Abstract
Ant colonies with permanent division of labour between castes and highly distinct roles of the sexes have been conceptualized to be superorganisms, but the cellular and molecular mechanisms that mediate caste/sex-specific behavioural specialization have remained obscure. Here we characterized the brain cell repertoire of queens, gynes (virgin queens), workers and males of Monomorium pharaonis by obtaining 206,367 single-nucleus transcriptomes. In contrast to Drosophila, the mushroom body Kenyon cells are abundant in ants and display a high diversity with most subtypes being enriched in worker brains, the evolutionarily derived caste. Male brains are as specialized as worker brains but with opposite trends in cell composition with higher abundances of all optic lobe neuronal subtypes, while the composition of gyne and queen brains remained generalized, reminiscent of solitary ancestors. Role differentiation from virgin gynes to inseminated queens induces abundance changes in roughly 35% of cell types, indicating active neurogenesis and/or programmed cell death during this transition. We also identified insemination-induced cell changes probably associated with the longevity and fecundity of the reproductive caste, including increases of ensheathing glia and a population of dopamine-regulated Dh31-expressing neurons. We conclude that permanent caste differentiation and extreme sex-differentiation induced major changes in the neural circuitry of ants.
Subject terms: Social behaviour, Social evolution, Gene expression profiling
Using single-cell transcriptomics, the authors generate a brain cell atlas for the pharaoh ant including individuals of different sexes and castes and show changes in cell composition underlying division of labour and reproductive specialization.
Main
Socially advanced ants appear to have brain cell numbers comparable to solitary fruit flies1,2 and their brains are smaller than in many weakly social or solitary wasps and bees1, indicating that social complexity is not obviously correlated with larger brains. Instead, remodelling of neural circuits and functional cellular innovations are probably more important predictors of social complexity3, particularly in social systems where brain development is caste-specific and developmentally hardwired. William Morton Wheeler was the first to identify that the highly divergent and complementary specialization of caste phenotypes resembles the ontogenetic differentiation of cell lineages in metazoans. This led him to coin the term superorganism for ant colonies to highlight the fundamental difference with animal societies where most individuals remain behaviourally and reproductively totipotent4,5. Permanent reproductive division of labour has indicated that the roles of the sexes have also become highly specialized and stereotyped6,7. It thus seems reasonable to propose that the superorganismal answer to social life of higher organizational complexity has been brain specialization rather than brain enlargement8.
Complex social behaviours are governed by neural circuits whose structure and function are determined by underlying gene regulatory networks, but the operational details remain poorly understood. Some recent studies have combined single-cell transcriptomics with neuroanatomy to better understand the organization of primate brains9–11, but such approaches have barely been developed for ants. Comparative transcriptomics have identified many differentially expressed genes (DEGs) across ant castes using whole-bodies12–14 or pooled brain tissues15–17, but have lacked the resolution to map the heterogeneity of brain cells and gene expression differences across cell populations. So far, only a single ant species, Harpegnathos saltator, has been interrogated at the single-cell level and only for the midbrains of workers and gamergates (inseminated and reproductively active workers)18. However, comprehensive profiling of whole brain single-cell transcriptomes across the full panel of distinct adult phenotypes of different sexes, castes and reproductive roles is necessary to understand how brain functions combine phenotypic specialization with integration in a superorganismal colony.
Inspired by Wheeler’s superorganism concept, we combined the power of massively parallel single-nucleus RNA-sequencing (snRNA-seq) with the unique biology of the pharaoh ant M. pharaonis to interrogate the neural correlates underlying obligate division of labour and reproductive specialization. Pharaoh ant queens are inseminated within the nest and establish new colonies through budding, rather than alone after mating flights19. Colonies are always highly polygynous: that is, many egg-laying queens coexist peacefully in a nest20. A typical pharaoh ant colony has three other phenotypes besides queens: gynes, workers and males. Gynes are virgin reproductives that will become queens after insemination, but will assume worker tasks and express reduced lifespans when they fail to become inseminated within a narrow time window after hatching from the pupal stage21,22. Workers are permanently sterile lacking both ovaries and sperm storage organs, and are responsible for all colony maintenance tasks20. Males are very short-lived and only meant to inseminate gynes23. The special social biology of M. pharaonis allowed mass rearing in the laboratory and collection of abundant brain tissues from all four adult phenotypes for comparative snRNA-seq analysis in a well-controlled sampling scheme. This allowed us to map important aspects of multi-brain complementarity and functional coordination in a superorganismal ant colony.
Results
Cell-type classification in M. pharaonis brains
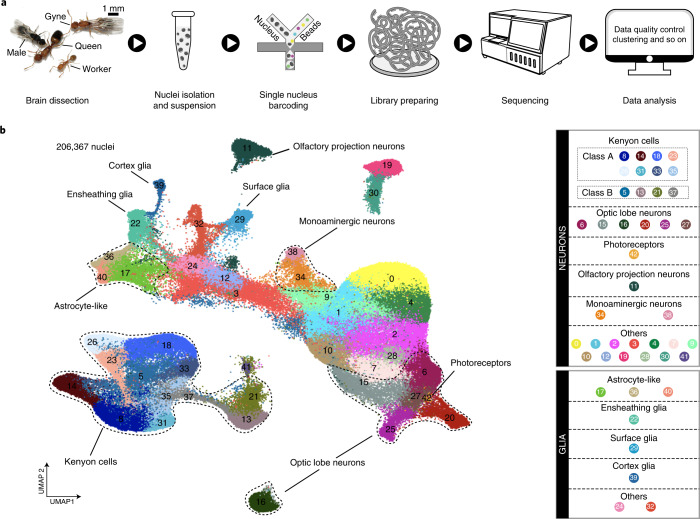
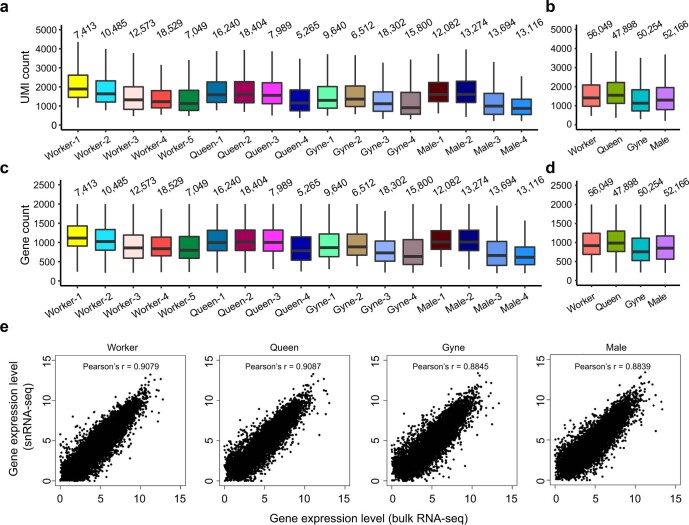
To create a comprehensive cell atlas, whole brain snRNA-seq was performed for four to five biological replicates of each adult phenotype: gynes (n = 4), queens (n = 4), males (n = 4) and workers (n = 5) (Fig. 1a and Supplementary Data 1). After stringent quality control and filtering, we obtained an average of roughly 50,000 high-quality nuclei from each of these four phenotypes, adding up to 206,367 nuclei (Extended Data Fig. 1a–d). This is 1.3 to 4 times the estimated cell number of 50,000–150,000 in a single individual ant brain1, and an order of magnitude higher than the recent study that obtained 18,583 cells for the midbrains (that is, optic lobes (OLs) removed) of the ant H. saltator18 (Supplementary Data 2). Correlations between gene expression quantifications via snRNA-seq and conventional bulk RNA-seq were high for each phenotype (Pearson’s r, 0.88–0.91), confirming that our snRNA-seq data were representative for the functionality of entire brains (Extended Data Fig. 1e).
Fig. 1. Transcriptomic classification of cell types in ant brains.
a, The four adult phenotypes of M. pharaonis and a schematic overview of the overall experimental design. Four to five biological replicates for each adult phenotype were prepared for snRNA-seq. For a single biological replicate of an adult phenotype, nuclei for snRNA-seq were isolated from a pool of 30 to 50 whole brains. b, UMAP plot of the 43 cell clusters generated by grouping the 206,367 nuclei obtained from brains of workers, queens, gynes and males. Each dot represents one nucleus. See legend for numerical and colour coding. See also Supplementary Data 1 for the number of nuclei per cluster in each replicate of the four adult phenotypes.
Extended Data Fig. 1. Quality control metrics of the M. pharaonis single-nucleus RNA-seq datasets.
(a-d) The number of transcripts (a, b) and genes (c, d) detected in the nuclei from each phenotype-specific biological replicate (a, c) and from each adult phenotype after combing biological replicates (b, d). The number of nuclei per category is shown above each box. For all box plots, the horizontal thick lines denote median values, the boxes show the range between the 25th and 75th percentile, and the whiskers represent 1.5× the interquartile range. (e) The correlation of gene expression between bulk RNA-seq data and snRNA-seq data.
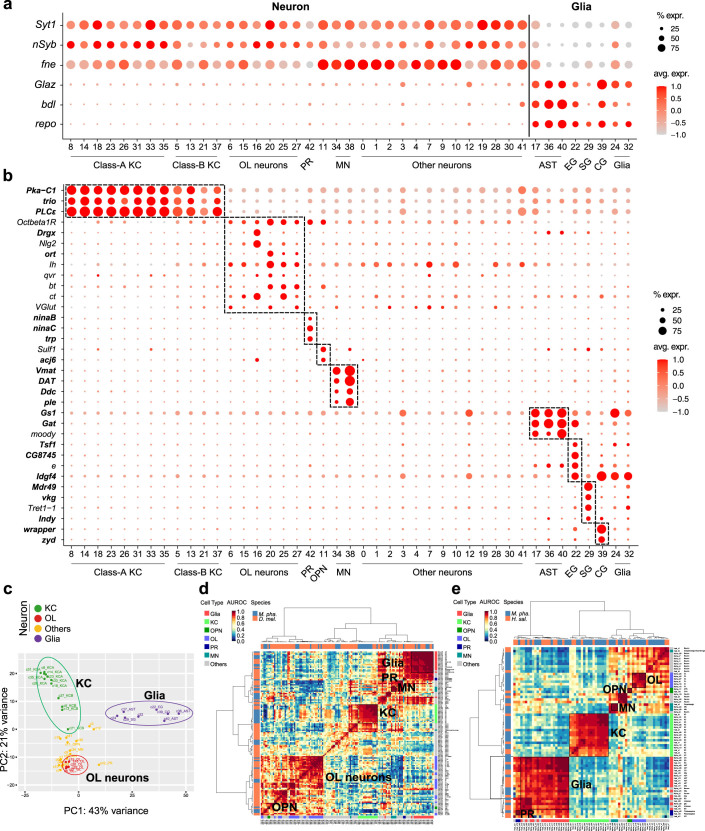
Overall, the 206,367 nuclei separated into 43 cell clusters with distinct gene expression patterns (Fig. 1b; see Supplementary Data 3 for a 3D view). All clusters showed high reproducibility across the biological replicates within phenotypes (Supplementary Data 1), suggesting that none of them are artefacts resulting from batch effects. By examining the expression of known marker genes from Drosophila and hymenopteran species, we could clearly distinguish the neurons from the glia (Extended Data Fig. 2a) and annotate many clusters to known cell types in insect brains, including Kenyon cells (KCs), olfactory projection neurons (OPNs), monoaminergic neurons, astrocytes, ensheathing glia, cortex glia, surface glia and photoreceptors (which may come from ocelli that were not completely removed) (Fig. 1b and Extended Data Fig. 2b,c).
Extended Data Fig. 2. Classification of the M. pharaonis clusters into major cell types.
(a) Dot plot showing the expression of neuronal (Syt1, nSyb, and fne) and glial markers (Glaz, bdl, and repo) across the M. pharaonis cell clusters. (b) Dot plot showing the expression of representative markers that define the major cell types. Gene symbols shown in bold font denotes known markers reported by previous studies in other insect species. Gene symbols shown in regular font denotes novel markers obtained from this study. (c) PCA based on average expression profile of each cluster. Clusters are coloured according to cell type category. (d-e) Pairwise transcriptional similarity (measured by AUROC scores) of cell clusters from Monomorium and Drosophila (d), and from Monomorium and Harpegnathos (e). The cell-cluster dendrogram trees were generated by hierarchical clustering using the Ward’s minimum variance method with the distance defined as 1-AUROC. KC: Kenyon cell; OL: optic lobe; PR: photoreceptor; OPN: olfactory projection neuron; MN: monoaminergic neuron; AST: astrocyte; EG: ensheathing glia; CG: cortex glia; SG: surface glia.
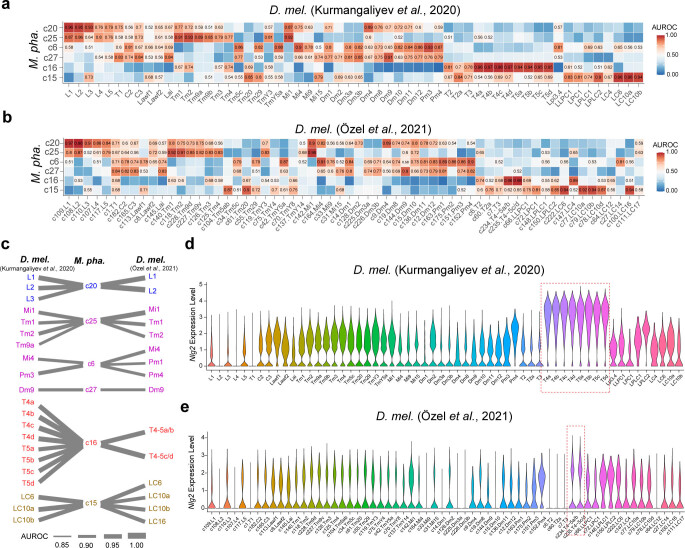
To annotate the OL cell types for which well-established marker genes are lacking, we mapped the Monomorium cell clusters to those identified in adult Drosophila melanogaster whole brains24 and H. saltator midbrains18 on the basis of transcriptional similarity of orthologous genes. The mean area under the receiver operator characteristic curve (AUROC) score acquired with MetaNeighbor25 was adopted to quantify the pairwise similarity of cell clusters between species. This revealed a total of 35 Monomorium clusters (81.4%) with high similarity (AUROC > 0.9) to at least one Drosophila cell cluster. Moreover, the cell-cluster dendrogram based on AUROC scores remained structured according to cell categories rather than species (Extended Data Fig. 2d), indicating that the main brain cell types are highly conserved across the two insects. A slightly lower proportion of the Monomorium cell clusters (31/43, 72.1%) could be mapped to Harpegnathos, probably due to all OL cell types being absent in the Harpegnathos midbrain dataset18. Nevertheless, these cross-species mapping analyses allowed us to identify six Monomorium clusters as putative OL neurons for ants, because they clearly grouped with the Drosophila OL clusters in the Monomorium-versus-Drosophila tree and formed a single clade in the Monomorium-versus-Harpegnathos tree (Extended Data Fig. 2d,e). Taken together, our combined efforts led to the annotation of 70% (30/43) of the cell clusters identified across the brains of the four adult Monomorium phenotypes.
Cell compositional differences between ant and fly brains
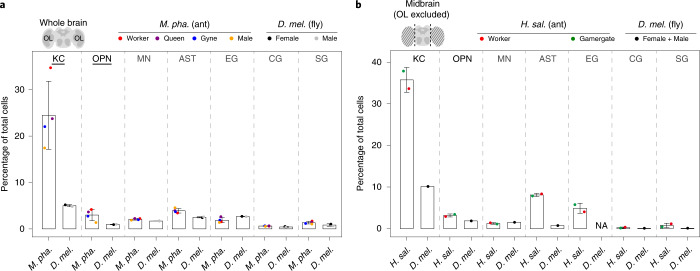
By comparing the relative abundances of cell types in adult brains of M. pharaonis, H. saltator and D. melanogaster, we found that the most striking difference between the ant and fly brains concerns the KCs: the intrinsic neurons of the mushroom bodies, the centre of associative learning and memory in insects26. The KCs alone represent roughly 24% of cells in the whole brains of Monomorium and roughly 36% of the Harpegnathos midbrain cells18, in sharp contrast to the mere 5 and 10% of cells in the Drosophila whole brains24 and midbrains27, respectively (Fig. 2a,b, Extended Data Fig. 3 and Supplementary Data 4). Another notable difference between the ant and fly brains was observed for the OPNs, a group of neurons that transfer olfactory information from the antennal lobes to the higher olfactory centres28,29. On average, the relative abundances of OPNs in entire Monomorium brains (roughly 3.0%) and Harpegnathos midbrains (roughly 3.2%) were three times and twice higher than in Drosophila, respectively (Fig. 2a,b). These higher abundances of KCs and OPNs observed in ant brains are consistent with the typical adaptations of ants to social life on the surface and underground where olfactory communication is key30, in contrast to the often airborne solitary flies.
Fig. 2. Cell compositional differences between brains of adult ants and flies.
a,b, Abundance of different cell types relative to total cells in entire brains of Monomorium and Drosophila24 (a) and in midbrains of Harpegnathos18 and Drosophila27 (b). M. pha., M. pharaonis; H. sal., H. saltator and D. mel., D. melanogaster. The schematic brains on the top left corners illustrate the anatomical differences between a whole brain and a midbrain. In the plots, each dot presents the relative abundance of a cell type in an ant adult phenotype or in a Drosophila sex. The relative abundance of a focal cell type in a specific phenotype or sex was measured as the percentage of cells belonging to the focal cell type out of the total number of cells in a specific phenotype or sex after combining cells from all biological replicates (or all libraries) of the ant phenotype or Drosophila sex. Accordingly, bars are the corresponding means ± s.d. across ant adult phenotypes (n = 4 for Monomorium and 2 for Harpegnathos) or Drosophila sexes (n = 2). Cell types with significant abundance difference assessed by scCODA and with a more than twofold change in relative abundance between species are underlined in a. Note that the original Drosophila midbrain dataset27 mixed cells from both sexes and that EG could not be detected in this dataset (Extended Data Fig. 3), so the scCODA assessment was not available for the midbrain datasets. MN, monoaminergic neuron; AST, astrocyte; EG, ensheathing glia; CG, cortex glia; SG, surface glia and NA, not available. See also Supplementary Data 4 for data related to this figure.
Extended Data Fig. 3. Re-analysis of the Drosophila midbrain single-cell dataset.
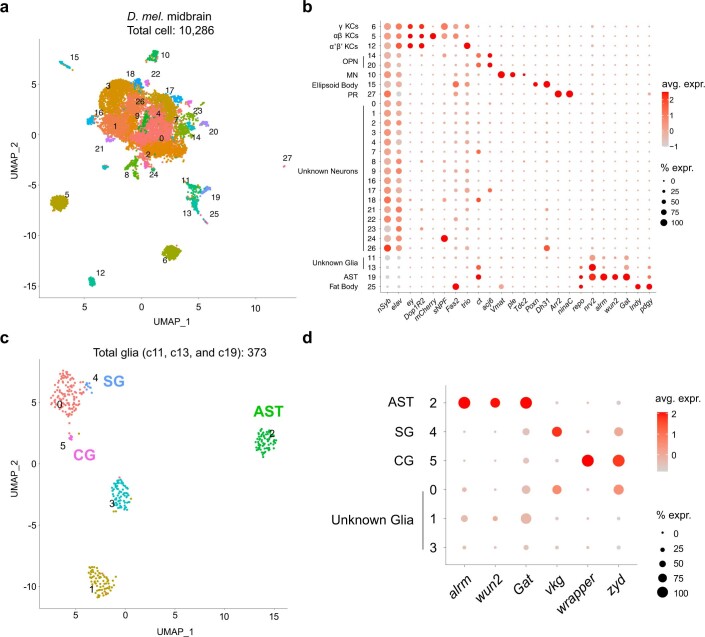
(a) UMAP plot showing the clustering result of 10,286 cells from Croset et al27, which are grouped into 28 clusters. Each dot represents one cell and dots are colored according to cluster identity. (b) Dot plot showing the expression of representative markers that define the known cell types in Drosophila brains. (c) Re-clustering of the glial clusters (c11, c13 and c19) identified three known glial subtypes. (d) Dot plot showing the expression of representative markers that define the known glial subtypes. KC: Kenyon cell; OPN: olfactory projection neuron; MN: monoaminergic neuron; PR: photoreceptor; AST: astrocyte; CG: cortex glia; SG: surface glia.
Diversification and evolution of mushroom body KCs
The Monomorium KCs are characterized by a high overall expression of Pka-C1, trio and PLCε (Extended Data Fig. 2b). RNA in situ hybridization (ISH) of Pka-C1 labelled the cell bodies of KCs around the calyces of the mushroom bodies (Extended Data Fig. 4a), consistent with previous observations in other hymenopteran insects18,31. The Monomorium KCs are highly diverse and could be divided into 12 transcriptionally differentiated cell clusters in the uniform manifold approximation and projection (UMAP) space (Fig. 1b). These 12 Monomorium KC clusters could be clearly separated into two distinct classes (class-A and class-B) according to gene expression-based clustering analysis (Fig. 3a). Class-A comprised eight of the 12 clusters and preferentially expressed CaMKII and Mblk-1, the marker genes of large-type (non-compact) KCs in adult honeybee brains32,33, whereas the class-B KCs were characterized by preferential expression of dati, Rbp6 and Cow (Fig. 3b). Moreover, the Monomorium class-A KCs could be divided further into three subclasses (KCA-1, KCA-2 and KCA-3; Fig. 3a). The class-A KCs were almost twice as abundant as the class-B KCs in all three female phenotypes, whereas they were slightly less abundant than class-B KCs in male brains (Fig. 3c), indicating a differential rate of neurogenesis of these two KC classes between the sexes during development.
Extended Data Fig. 4. Kenyon cells in three hymenopteran insects.
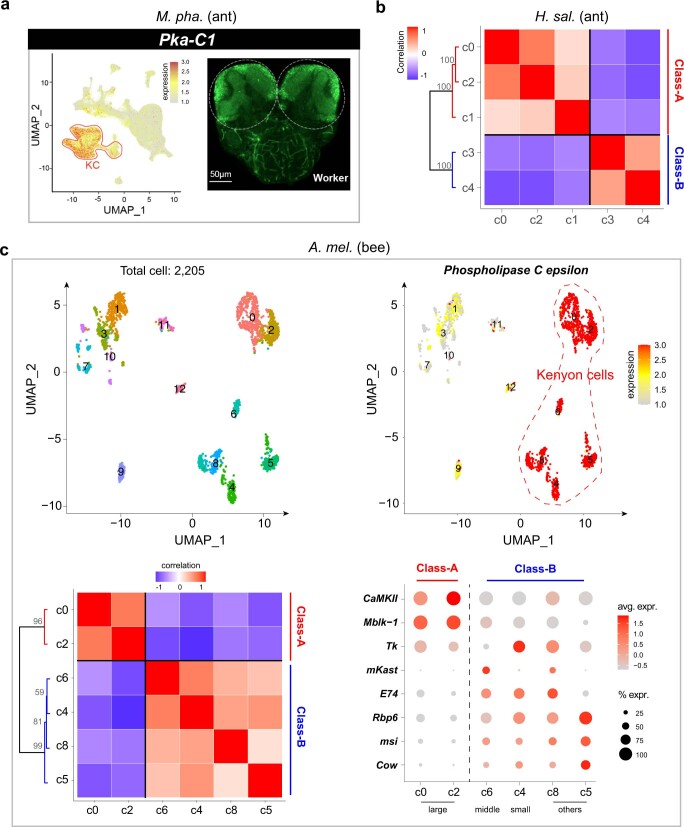
(a) Expression of the KC marker Pka-C1 across all cell clusters (left) and whole-mount RNA detection of Pka-C1 by in situ hybridization (right) in a M. pharaonis worker brain. UMAP plot is colored by gene expression (grey is low and red is high) with red solid line indicating the KC clusters. White dotted circles indicate paired mushroom bodies with strong hybridization signal. (b) Pairwise Pearson correlations and hierarchical clustering of the H. saltator KC clusters based on gene expression, showing a clear division into two major classes. The gray numbers at the branches are confidence values based on bootstrap method. (c) Re-analysis of the A. mellifera single-cell dataset. Top left: UMAP plot showing the clustering result of the 2,205 cells from Traniello et al35, which are grouped into 13 clusters. Top right: Expression of the honeybee KC marker Phospholipase C epsilon (PLCε) across the 2,205 cells. Dashed line indicates the six clusters that preferentially expressed PLCε. Bottom left: Pairwise Pearson correlations and hierarchical clustering of the six honeybee KC clusters in similar notation as panel b. Bottom right: Dot plot showing the expression of representative markers that define the known KC subtypes in honeybee brains.
Fig. 3. The diversity of KCs in ant brains.
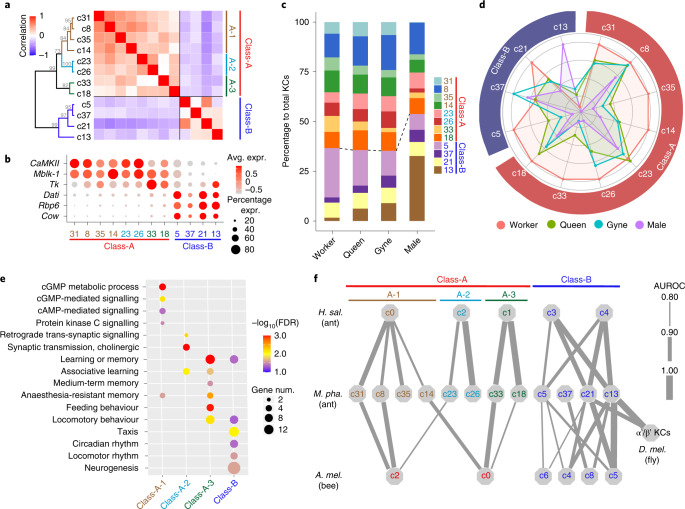
a, Pairwise Pearson correlations and hierarchical clustering of the 12 KC clusters in M. pharaonis brains based on gene expression, showing a clear division into two main classes (A and B). The grey numbers at the branches are bootstrap values. b, Dot plot showing the expression of representative DEGs between class-A and -B KCs. Dot colours represent average expression of a gene and dot sizes represent percentages of cells within each cluster expressing that gene. c, Bar plots showing the proportion of cells from each KC cluster against the total number of KCs in each of the four adult phenotypes, with the dashed line marking the boundary between class-A and -B KCs. d, Radar plot showing the variation in relative abundance of each KC subtype against total brain cells across Monomorium phenotypes. For each KC cluster, the mean across replicates for a phenotype was determined first and then divided by the maximum among the four phenotypes. See Supplementary Data 1 for the exact number of nuclei per KC cluster per phenotype. e, Representative GO terms enriched (FDR < 0.05) by the DEGs that were up-regulated in each KC (sub)class relative to the remaining KCs. Dot colours represent FDR values for each GO term, and dot sizes represent the number of DEGs associated with each GO term. f, Correspondence of KC clusters between Monomorium and Harpegnathos/Apis/Drosophila as predicted from the transcriptional similarities of orthologous genes by MetaNeighbor. A higher AUROC score means higher similarity. Each line links a Monomorium KC cluster to its top hit among the Harpegnathos/Apis/Drosophila KC clusters according to AUROC scores, with line thickness being proportional to the score. Only hits with AUROC > 0.80 are shown. A second hit is plotted as well when the difference between the top and second AUROC score was less than 0.05. AUROC scores for Drosophila α'/β' KCs are mean values across the three independent datasets (Extended Data Fig. 5d).
We next assessed the importance of each KC subtype for different social roles by comparing their relative abundances against total brain cells across the four adult Monomorium phenotypes. This showed that almost all class-A KC subtypes had the highest abundances in worker brains, whereas half of the class-B KC subtypes revealed higher abundancies in male or gyne brains (Fig. 3d). The class-A KCs might thus be particularly important for regulating worker behaviours, while some of the class-B KCs have probably been co-opted for mating-related behaviours in newly emerged reproductives. Consistent with this conjecture, functional enrichment analysis for the DEGs between KC (sub)classes showed that up-regulated DEGs in class-A KCs were enriched in cGMP- and cAMP-mediated signalling involved in memory formation34, associative learning and feeding behaviour, while those up-regulated in class-B KCs were enriched in taxis movement, circadian rhythm and neurogenesis (Fig. 3e).
To explore the evolutionary origin of the Monomorium KC subtypes, we next assessed the transcriptional similarity of KC clusters across M. pharaonis, H. saltator18, Apis mellifera (honeybee)35 and D. melanogaster24,27,36 (Extended Data Fig. 4b,c). Harpegnathos lives in small colonies and represents an early-branching ant lineage that has been separated from Monomorium for at least 130 million years37,38, while the honeybee independently evolved advanced superorganismal caste differentiation after the divergence of bees and ants roughly 160 million years ago39. In spite of these huge phylogenetic distances, we found that the Monomorium class-A and class-B KCs were very similar to two distinct groups of KC clusters in Harpegnathos and the honeybee (Fig. 3f and Extended Data Fig. 5a–c). This suggests that these two main KC classes evolved before the emergence of complex social life in the Hymenoptera. It is also notable that the three subclasses of class-A KCs were probably established early in ant evolution, because the Monomorium KCA-1, KCA-2 and KCA-3 were most similar to three distinct Harpegnathos class-A KC clusters (Fig. 3f). However, in contrast to class-A, the relationships of the class-B KC clusters across the three hymenopteran species were less clear (Fig. 3f), which indicates that the class-B KCs probably underwent independent diversification in these three distantly related hymenopteran lineages.
Extended Data Fig. 5. Comparison of Kenyon cells across species.
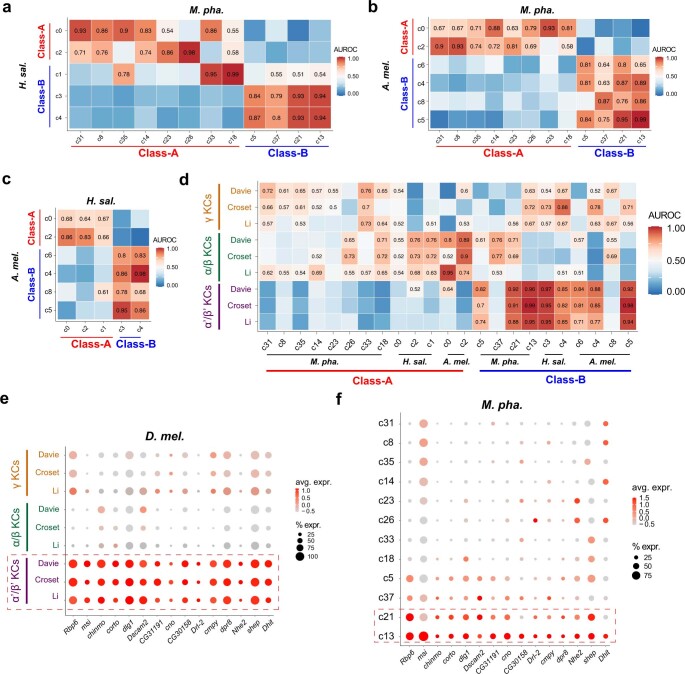
(a-c) Pairwise AUROC scores showing the cross-species transcriptional similarity of the KC subtypes from three hymenopteran insects (M. pharaonis, H. saltator and A. mellifera). Comparisons with AUROC scores > 0.5 are presented as exact values. (d) Pairwise AUROC scores showing the cross-species transcriptional similarity of hymenopteran and Drosophila KC subtypes. Drosophila KCs from three independent studies, namely Davie et al24, Croset et al27 and Li et al36, were used for the analysis (see also Supplementary Data 2). Comparisons with AUROC scores > 0.5 are presented as exact values. (e-f) Dot plots showing the expression of representative shared DEGs up-regulated in Drosophila α'/β' KCs (e) and Monomorium c13/c21 KCs (f) in relative to the remaining KC subtypes.
In adult Drosophila brains, KCs are classified into three subtypes (γ, α'/β' and α/β) on the basis of their axonal projection patterns40. Consistent with the substantial morphological differences of the mushroom bodies between ants and flies26, most Monomorium KC clusters showed low similarity to the three Drosophila KC subtypes. However, it was intriguing to see that the Monomorium c13 and c21 KCs showed high transcriptional similarity to the Drosophila α'/β' KCs (Fig. 3f), as validated by three independent Drosophila datasets24,27,36 with AUROC scores over 0.9 (Extended Data Fig. 5d). In fact, many marker genes were shared by Drosophila α'/β' KCs and Monomorium c13/c21 KCs, such as msi, Rbp6 and dlg1 (Extended Data Fig. 5e,f), indicating that the Monomorium c13/c21 KCs may account for similar functions to Drosophila α'/β' KCs that are important for adult life unrelated to sociality.
Insect behaviour regulation by conserved OL cells
The OL neurons formed another large cell population, varying from 3 to 28% of all cells in adult Monomorium brains. All Monomorium OL clusters showed high transcriptional similarity to at least one Drosophila OL cell type41,42, indicating functional conservation of these OL neurons in insects (Fig. 4a,b and Extended Data Fig. 6a–c).
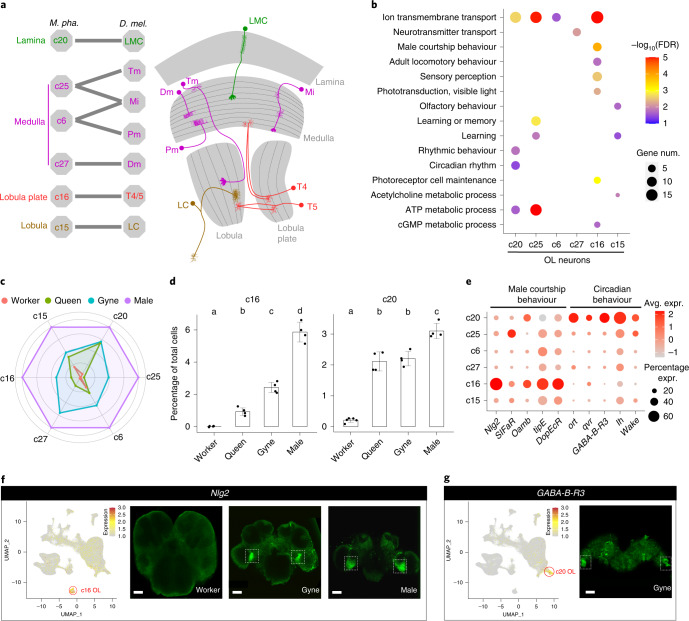
Fig. 4. The conserved OL neurons between Monomorium and Drosophila.
a, Annotation of the Monomorium OL clusters (left) based on transcriptional similarity comparison with two independent Drosophila OL single-cell datasets41,42 (Extended Data Fig. 6) and a schematic diagram of the Drosophila OL (right) highlighting the OL cell types conserved in Monomorium. Tm, transmedullary neuron; Mi, medulla intrinsic neuron; Pm, proximal medulla neuron and LC, lobula columnar cells. b, Representative GO terms enriched (FDR < 0.05) by the up-regulated DEGs in each OL cluster relative to the remaining OL neurons. Dot colours represent FDR values for each GO term, and dot sizes represent the number of DEGs associated with each GO term. c, Radar plot showing the variation in relative abundance of each OL cell type against total brain cells across phenotypes. For each OL cluster, the mean across replicates of a phenotype was determined first and then divided by the maximum among the four phenotypes. d, Percentage of cells from c16 (left) and c20 (right) against the total number of brain cells in each adult phenotype. Each dot presents the biological replicate value of an adult phenotype (n = 5 for workers, 4 for queens, 4 for gynes and 4 for males), bars are means ± s.d. across replicates and lowercase letters assign bars to different groups that were significantly different as assessed by scCODA. e, Expression of representative top DEGs from c16 and c20 across all OL clusters. Dot colours represent average expression level of a gene and dot sizes represent percentages of cells within each cluster expressing that gene. f,g, UMAP plot and whole-mount RNA in situ detection of Nlg2 (f) and GABA-B-R3 (g) in the brains of focal adult phenotypes. The UMAP plots are coloured by gene expression (grey is low and red is high), with red circles indicating the cell clusters that preferentially expressed the focal marker genes. White dotted boxes indicate the positions of hybridization signals in the brain images. Scale bars in f; 40, 50 and 50 μm, respectively and g, 50 μm.
Extended Data Fig. 6. Comparison of optic lobe neurons across species.
(a, b) Pairwise AUROC scores showing the cross-species transcriptional similarity of OL clusters between Monomorium and Drosophila. Drosophila OL cell clusters from two independent studies that focus exclusively on the Drosophila optic lobes, namely Kurmangaliyev et al41 and Özel et al42, were used for the analyses. Comparisons with AUROC scores > 0.5 are presented as exact values. (c) Correspondence of OL clusters between Monomorium and Drosophila as predicted by the AUROC scores in panel a and panel b. Each line links a Monomorium OL cluster to its top hit among the Drosophila OL clusters according to AUROC scores, with line thickness being proportional to the score. A second hit is plotted as well, when the difference between the top and second AUROC score was less than 0.05. (d, e) Violin plots showing the expression of Nlg2 across the Drosophila OL clusters. Dashed boxes indicate the T4/T5 neurons.
All Monomorium OL clusters displayed the highest abundances in male brains across the four adult phenotypes, and among them, c16 was most male-biased (Fig. 4c). This population of neurons, which occupies roughly 6% of the male brain cells, was completely absent in worker brains and was 2.5 and 5.8 times less represented in brains of gynes and queens, respectively (Fig. 4d). The DEGs up-regulated in c16 were enriched by genes involved in Drosophila male courtship behaviour (Fig. 4b), such as Nlg2, tipE and DopEcR (Fig. 4e). Nlg2-deficient male flies express less female-directed courtship and lower aggression to other males43. The Monomorium cl6 neurons showed the highest transcriptional similarity with Drosophila T4/T5 neurons, which mediate motion detection required for successful mating44,45 and also preferentially express Nlg2 (Fig. 4a and Extended Data Fig. 6d,e). In addition, the T4/T5 neurons are located near the lobula plate of Drosophila OLs46 (Fig. 4a), in almost the same location that we observed for the c16 neurons in Monomorium by RNA ISHs of Nlg2 (Fig. 4f). Our ISH assessments also confirmed the absence of c16 in workers and its higher abundance in males compared to gynes (Fig. 4f). These results indicate that this population of OL neurons probably play a conserved role in regulating male mating behaviour in insects regardless of sociality and that they are particularly important in the highly specialized males of ants.
Among the female phenotypes, gynes had the highest representation for all OL clusters. The only exception was c20, which was equally abundant in queens (Fig. 4d), suggesting that some vision-related functions are retained in mature egg-laying queens even though they mostly operate in the dark nest environment. The up-regulated DEGs in c20 were significantly enriched for genes involved in Drosophila circadian rhythm regulation (Fig. 4b), such as ort, qvr, GABA-B-R3 and wake (Fig. 4e). In addition, c20 displayed the highest transcriptional similarity to Drosophila lamina monopolar cells (LMCs), which reside near the lamina of Drosophila OLs46, as confirmed by RNA ISH of GABA-B-R3 and found to be also true in Monomorium (Fig. 4g). The LMCs in insects can dynamically optimize visual perception over a wide range of light levels47–50, indicating that these neurons are probably involved in circadian behaviour by responding to light. Consistent with the significant contraction of all OL clusters except for c20 in queens, we propose that most vision-related functions have degenerated in mature Monomorium queens, while the retained sensitivity to light intensity changes allows queens to assess the optimal time for nest-budding dispersal and to quickly retreat to the dark inner nest on unexpected nest disturbance51.
Specialization and complementation of social brains
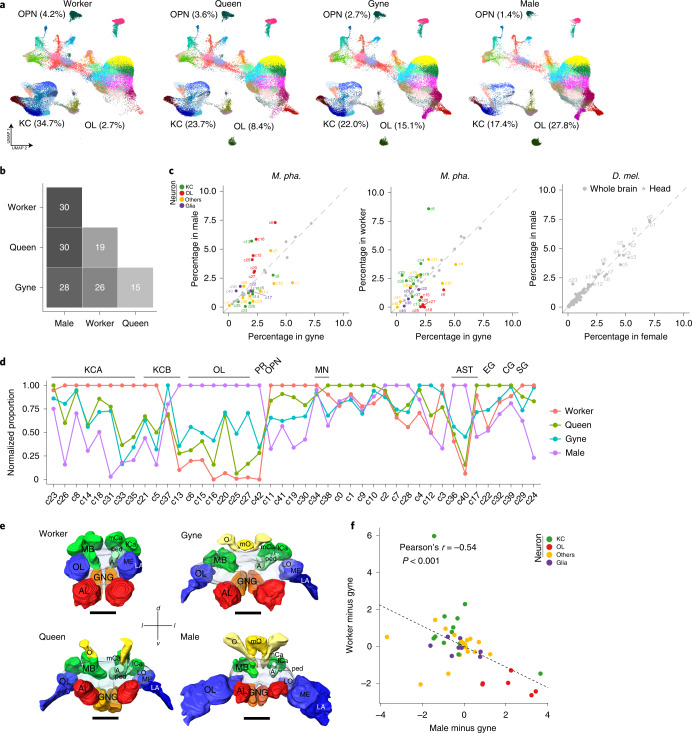
To investigate the extent to which ant brains are differentiated among colony members, we compared relative cell-type abundances across the four adult Monomorium phenotypes. We found that almost all 43 cell clusters are present in all phenotypes, except that c16 (T4/T5 neurons) and c42 (ocellus photoreceptors) were absent in worker brains (Fig. 5a and Supplementary Data 1). However, the abundances of up to 86% (37/43) of cell clusters showed significant differences across phenotypes as assessed by scCODA52 (Fig. 5b). The largest differences were observed between the sexes (males versus gynes/queens/workers), with 65–70% of cell clusters showing significant differences, followed by 49–56% differences between castes (workers versus gynes/queens). In contrast, we could not detect any cell clusters showing significant abundance differences between the sexes of Drosophila as assessed with two independent datasets24,36 (Fig. 5c and Supplementary Data 5). These results confirm that the sexual and caste phenotypes of Monomorium are developmentally specialized to a high extent, and these specializations might have been resulted from the differential investment of a common set of cell types during development.
Fig. 5. Specialization and complementarity of Monomorium brains.
a, UMAP plots of the 43 clusters in the brains of workers, queens, gynes and males. Each dot represents one nucleus and is coloured according to cell cluster as in Fig. 1b. The relative abundances of KCs, OL neurons and OPNs against total number of brain cells in each adult phenotype are also presented. b, The number of cell clusters showing significant abundance differences, assessed by scCODA and with >1.3-fold changes, between any two of the four adult phenotypes. c, Cell clusters that displayed significant abundance differences between the sexes (left) and castes (middle) in Monomorium (corresponding to b), with the differences between female and male Drosophila brains24 and heads36 as controls (right). Coloured dots represent cell clusters with significant abundance differences and grey dots/stars represent those with no significant differences. d, The variation in relative abundance of each cell type against total brain cells across phenotypes. For each cell cluster, the phenotype-specific mean across replicates was determined first and then divided by the maximum among the four phenotypes. e, 3D brain reconstructions of a worker, queen, gyne and male using confocal microscopy image stacks (an anterior view). MB, mushroom body; mCa, medial calyx of MB; lCa, lateral calyx of MB; ped, peduncle of MB; A, alpha lobe of MB; LO, lobula of OL; ME, medulla of OL; LA, lamina of OL; AL, antennal lobe; GNG, gnathal ganglia; O, ocelli; mO, medial ocelli; d, dorsal; l, lateral; v, ventral. Scale bars, 100 μm. f, Scatter plot showing that the abundance differences per cell cluster between males and gynes are negatively correlated with the same abundance differences between workers and gynes. Each dot represents one of the 43 cell clusters.
It appeared that the gyne and queen brains have a fairly generalized cell composition with moderate abundances for almost all cell clusters (Fig. 5d). By contrast, male brains had the highest abundances not only in all OL clusters, but also for two of the three astrocyte clusters, while they had the lowest abundances in OPNs and almost all KC-related clusters. These results clearly indicate that Monomorium males rely heavily on visually guided behaviours, even though nuptial flights appear to have lost in M. pharaonis53. Although mating in the laboratory happens easily within the same colony, these results made us speculate that M. pharaonis males have retained diurnal dispersal behaviour under natural conditions to prevent very close inbreeding. The worker brains were mostly characterized by cell-type preferences opposite to male brains, displaying the highest abundances in almost all KC clusters and the OPNs, but the lowest abundances in vision-related neurons, suggesting that learning, memorizing and processing of olfactory information are most important for worker behaviour. These data made us predict that worker brains should have the largest mushroom bodies and ALs while male brains should have the largest OLs in terms of relative volume, expectations that were confirmed by reconstructing the main neuropils of the four adult phenotypes with confocal microscopy image stacks (Fig. 5e, Extended Data Fig. 7 and Supplementary Data 6). We also observed an overall negative correlation between male versus gyne and worker versus gyne cell-type abundance changes, suggesting that male and worker brains are partially complementary to each other at cellular composition level in a Monomorium colony (Fig. 5f).
Extended Data Fig. 7. Relative volume of neuropil in different adult phenotypes.
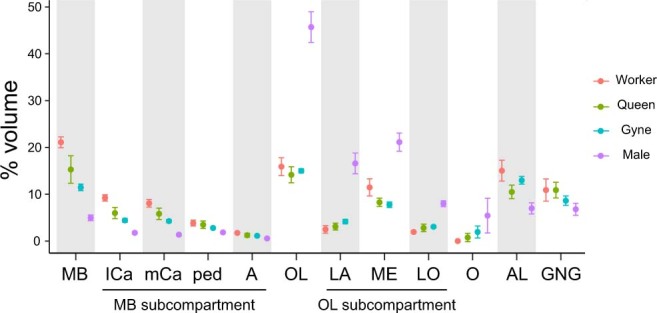
The relative volume of a neuropil in an individual brain was calculated by dividing the volume of the neuropil with the entire brain volume (n = 5 for worker, 7 for queen, 6 for gyne and 5 for male). Data are presented as mean ± s.d. across replicates. MB: mushroom body; mCa: medial calyx of MB; lCa: lateral calyx of MB; ped: peduncle of MB; A: alpha lobe of MB; OL: optic lobe; LA: lamina of OL; ME: medulla of OL; LO: lobula of OL; O: ocelli; AL: antennal lobe; GNG: gnathal ganglia.
Dopamine circuit remodelling induced by gyne insemination
Insemination is a crucial single step for gynes to become queens with full reproductive functions because ants never re-mate later in life7. A previous study has shown that gyne–queen role differentiation involves substantial brain anatomic changes and parallel remodelling of gene regulatory networks in M. pharaonis21. We detected significant abundance changes in 35% (15/43) of the cell clusters between gyne and queen brains (Fig. 6a), corroborating those previous findings21 and suggesting that active neurogenesis and/or programmed cell death might occur during this role differentiation process in adult ant reproductives. However, given that the queens (3–6 months old posteclosion) were much older than the gynes (5–10 days old posteclosion), some of these cellular changes could also reflect age rather than effects induced by insemination.
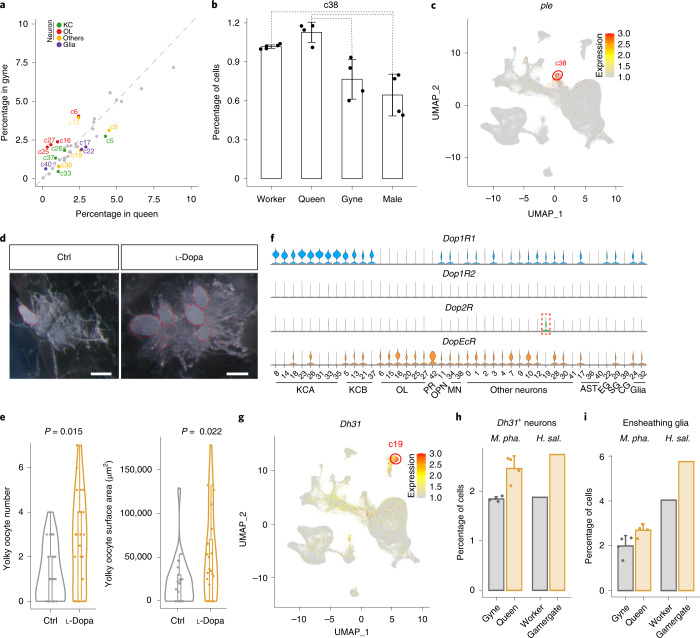
Fig. 6. Cell compositional differences between gyne and queen brains in Monomorium.
a, Cell clusters that display significant abundance differences, as assessed by scCODA and with >1.3-fold changes, between gyne and queen brains. Coloured dots represent cell clusters with significant abundance differences and grey dots represent those with no significant differences. b, The percentage of cells from c38 against total brain cells in each adult phenotype. Each dot represents the value of a phenotype-specific biological replicate (n = 5 for workers, 4 for queens, 4 for gynes and 4 for males), bars are means ± s.d. across replicates, and dotted lines indicate the comparison with significant differences in a. c, Expression level of ple across the 43 cell clusters. The UMAP plot is coloured by gene expression (grey is low and red is high) and the red circle indicates the cell cluster that preferentially expressed ple. d, Representative ovaries of control (Ctrl) and l-dopa treated gynes with yolky oocytes highlighted with red dotted ovals. Scale bar, 200 μm. e, Violin plots showing yolky oocyte number and total surface area in l-dopa treated gynes and control groups (n = 24 for both groups) with P values obtained from two-sided Student’s t-tests. For all box plots inside the kernel density plots, the horizontal thick lines denote median values, the boxes show the range between the 25th and 75th percentiles and the whiskers represent 1.5× the interquartile range. f, Expression of the four dopamine receptors across the 43 cell clusters, with the dashed box highlighting the only cell cluster with a preferential expression of Dop2R. g, Expression level of Dh31 across the 43 cell clusters, in similar notation to c. h,i, The convergent increases in relative abundance of Dh31+ neurons (h) and ensheathing glia (i) in M. pharaonis (accessed by scCODA and with >1.3-fold change) and H. saltator (accessed by Fisher’s exact test with FDR < 0.001 and >1.3-fold change) in reproductively active females compared with uninseminated females. Each dot represents the value of a phenotype-specific biological replicate (n = 4 for gynes and queens) and bars are means ± s.d. across replicates in M. pharaonis.
Among the cell clusters with increased abundance in queens, c38 stands out as it preferentially expressed ple (encoding the rate-limiting enzyme in dopamine synthesis54) and DAT (a dopamine transporter55), indicating that c38 primarily represents the dopaminergic neurons (Fig. 6b,c). The abundance increase of c38 in queens might therefore indicate an elevated activity of the dopamine circuit triggered by insemination. Previous studies of several ponerine ant species have found that dopamine titres are positively correlated with increased ovarian activity in reproductive workers (gamergates)56,57. We therefore tested whether dopamine administration would induce accelerated oocyte growth in M. pharaonis gynes by feeding 5-day-old gynes with 30 mg ml−1 l-dopa in 10% sucrose for 5 days. Compared to the control group, l-dopa treated gynes had more yolky oocytes and the total areas of these yolky oocytes were significantly enlarged (Fig. 6d,e), confirming that dopamine has a gonadotrophic function during or immediately following the gyne–queen transition induced by insemination.
The effects of dopamine depend on the downstream neurons that express the dopamine receptors58. The M. pharaonis genome encodes Dop1R1, Dop1R2, Dop2R and DopEcR similar to Drosophila (Extended Data Fig. 8a). We found that Dop2R was preferentially expressed in the c19 neurons (Fig. 6f), the abundance of which was significantly increased in queens compared to gynes (Fig. 6a). This cell cluster is also characterized by the preferential expression of Dh31, Prohormone-2, amon and 7B2, indicating that this dopamine-regulated cell cluster comprises a population of peptidergic neurons that mainly produce diuretic hormone (Fig. 6g). A recent study showed that neuronal knockdown of Dh31 led to a statistically significant decrease in egg laying in Drosophila59, indicating a potential role of this neuropeptide in ovulation. Overall, these results indicate that dopamine probably mediates insemination-induced gonadotrophic functions via the c19 Dh31-expressing neurons in M. pharaonis.
Extended Data Fig. 8. Phylogenetic analysis of dopamine receptors and expression analysis of Dh31.
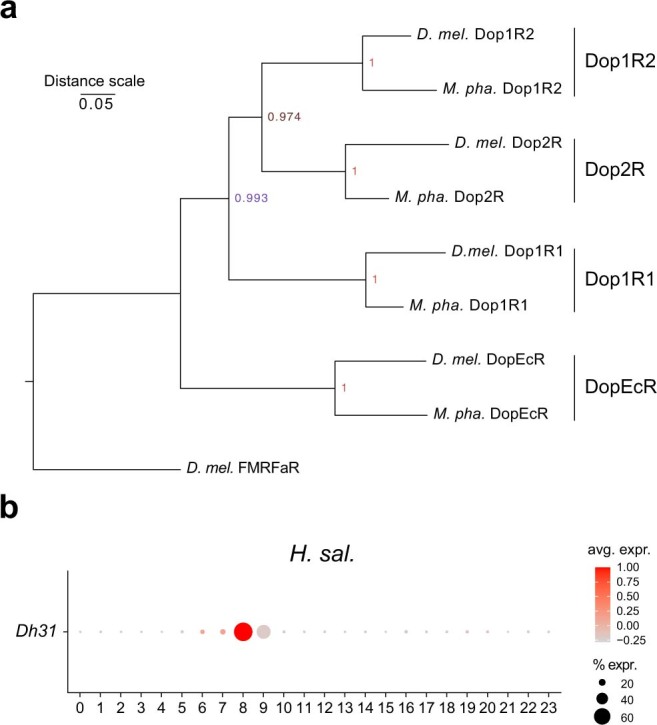
(a) Phylogenetic relationship of the dopamine receptors from M. pharaonis and D. melanogaster, indicating that the M. pharaonis genome encodes four distinct dopamine receptors as observed in D. melanogaster. The D. melanogaster FMRFaR protein is used as the outgroup. The phylogenetic tree was built with the GPR domain sequences after alignment by MUSCLE (v.3.8.31) and with the neighbor-joining method implemented in MEGAX. The reliability of the tree was estimated with 1,000 bootstrap replications. (b) Dot plot showing the expression of Dh31 across all the H. saltator cell clusters defined by Sheng et al18. Shade of dot represents mean expression within cluster, and size of dot represents percentage of cells within the cluster expressing that gene.
Convergent cellular changes in reproductive role transitions
While the gyne–queen transition in M. pharaonis represents a classic form of reproductive role differentiation21, several ant species such as H. saltator have secondarily evolved reproductive role differentiation within the worker caste60–62. It was therefore interesting to observe that the abundances of the Dh31-expressing neurons and the ensheathing glia were increased both in gamergates compared to workers of H. saltator and in queens relative to gynes of M. pharaonis (Fig. 6h,i, Extended Data Fig. 8b and Supplementary Data 7). This reminds that gamergates have co-opted the reproductive role differentiation gene regulatory network normally expressed in the gyne–queen transition21. In particular, the ensheathing glia, which play a neuroprotective role in adult Drosophila brains63, had the highest abundance in queens among the four adult phenotypes of M. pharaonis (Fig. 5d), suggesting a role in queen longevity. Ageing-associated decline of ensheathing glia has recently been reported in fruit flies and H. saltator workers, while the H. saltator gamergates are resistant to ageing with their ensheathing glia declining at a much slower rate18. Our finding of increased abundance of ensheathing glia in mature queens thus appears to corroborate the critical role of ensheathing glia for longevity in the reproductive castes of ants.
Discussion
Our study generated a superorganismal brain cell atlas by profiling all brain cells of the full panel of adult phenotypes that typically make up an ant colony. We found that the ant mushroom body KCs are abundant and transcriptionally diverse relative to the KCs of Drosophila. We also identified conserved OL neurons that probably play crucial roles in visual courtship behaviour and circadian rhythm regulation in ants. Our results are consistent with advanced brain-level division of labour in superorganismal colonies and shed new light on neural mechanisms associated with the lifespan differences between workers and queens.
Functional integration of superorganismal brains
As we outlined at the start, we expected that the major evolutionary transition to superorganismal colony organization in ancestral ants should have selected for specialization of neural circuitry rather than bigger brains per se. Our study provides direct evidence to support this hypothesis, with high degrees of specialization being detectable in the brain cellular composition of all four adult phenotypes of M. pharaonis ants. We found that 41 out of 43 cell types could be detected in all four brain phenotypes, albeit in different abundances. In particular, workers and males have evolved extreme forms of brain specialization and with almost opposite cell-type preferences. Worker brains had the most abundant KCs and OPNs and the least abundant OL neurons, all biases that were opposite in male brains. These cellular differences were consistent with anatomical brain structures reflecting the distinct social and sexual specialization in these two phenotypes. Males are extremely short-lived and do not take part in any colony maintenance tasks, as their only function is to find and inseminate a virgin queen. Ant males therefore function as ‘simple minded’ but extremely targeted sperm vectors7. In sharp contrast, workers engage in all the colony tasks except reproduction and need multipurpose brains, consistent with the KCs and OPNs in workers being biased for processing complex information associated with nursing, foraging, colony defence and social communication.
Relative to these extremes, gynes and queens had intermediate abundances for almost all brain cell types. This probably reflects that both gynes and queens have maintained functional brain repertoires for a large subset of the social tasks normally done in more advanced ways by workers. Many ants may have retained generalist queen brain functions because they have solitary lives during colony founding, so they need to nurse a first brood and in some species even to forage20. However, finding relatively generalist brains in M. pharaonis gynes and queens is remarkable because this species has lost that ancestral independent colony founding behaviour and never needs to operate without worker assistance. However, Monomorium colonies have very many queens, some of which may fail to become inseminated. Such failed queens are known to survive, albeit for less time than inseminated queens, and perform worker-like behaviours21, which may have selected for the maintenance of general cognitive abilities in the gyne/queen caste.
Overall, our results confirm the concept of complementary divergence in brain function between superorganismal colony members and strongly suggest that fine-tuned brain-level division of labour is an integrated part of sex, caste and reproductive role differentiation, in ways that are not expected to evolve in social systems where a variable number of colony members retain breeder potential even though they may first have helper roles. In many ways, the separate individual brains in colonies of ants such as M. pharaonis combine into a modularly coordinated super-neural organization maintained by advanced communication between colony members. Individual brains are continuously turned over when adult colony members hatch and die, but functional homeostasis and balanced interactions between modules continue, similar to how cells in a metazoan body are turned over without compromising overall body health, consistent with hypothetical comparisons by Wheeler more than a century ago5. The complementary functions of individual brains across the full panel of adult phenotypes are consistent with natural selection maximizing colony-level fitness, as expected for all superorganismal social insects, but not for animals that form societies without irreversible caste differentiation for life among all colony members4,5,64.
Neural effects on longevity/fecundity evoked by insemination
Gynes and queens represent two subsequent functional states of the same reproductive female caste, separated by a single insemination event that induces substantial brain transcriptome remodelling resulting in remarkable shifts in behaviour21. The gene regulatory network that mediates this reproductive role differentiation is insemination-specific rather than queen-specific, because it has been co-opted by distantly related ant species that secondarily shifted to reproduction via worker–gamergates rather than queens21. In the present study, we further explored this convergent evolutionary scenario across castes at the brain cell level. We found that there are parallel cellular shifts across these two caste-specific reproductive role transitions induced by insemination. In particular, a cluster of ensheathing glia with neuroprotective and anti-ageing functions was expanded in both M. pharaonis queens and H. saltator gamergates18. We therefore speculate that ensheathing glia modification might represent one of the proximate mechanisms that ancestrally prolonged queen longevity in ants and whose co-option secondarily extended worker lifespan when they became inseminated as gamergate reproductives. This quantitative reinforcement mechanism of particular neural modules in adulthood effectively decouples queen and worker ageing, so that extremely divergent caste-specific lifespans could evolve65.
Insemination also induced the expansion of dopamine neurons and a cluster of downstream Dop2R expressing neurons in M. pharaonis queens, and the counterpart cell cluster in H. saltator was found to be convergently expanded in gamergates as well. Our experimental confirmation of the gonadotrophic function of dopamine via feeding M. pharaonis gynes with l-dopa suggests that dormant ovary maturation in gynes may be switched into an accelerating trajectory by elevating functionality of dopamine neurons. The downstream Dop2R neurons also preferentially expressed Dh31, a diuretic hormone known to regulate ovulation in flies59. The simultaneously expanded dopamine neurons and downstream Dop2R neurons may thus constitute an integral and conserved neural module to realize enhanced reproductive potential in ant queens well beyond the normal fertility levels of solitary insects.
Methods
Biological samples
The original colony of M. pharaonis was collected in 2016 from a resident house in Mengla, Xishuangbanna, Yunnan Province, China, and split into hundreds of subcolonies in the laboratory in the subsequent years. All colonies were reared at 27 °C, 65% RH and a 12/12 h light/dark cycle. The rearing of gynes and males was induced in newly split colonies where inseminated and egg-laying queens were removed, and where easily recognizable male pupae were continuously removed to prevent the newly hatching gynes became inseminated. The eclosion date of males and gynes were recorded. The queens were collected from stable, mature colonies in which they were actively laying eggs. The demographic states of the colonies were frequently surveyed so the ages of queens could be estimated, albeit less accurately than the gynes and males. Workers were randomly collected from colonies, both inside and outside nests, so these samples covered both young (nursing) and old (foraging) workers. At the moment of dissection, gynes were 5–10 days posteclosion, queens were 3–6 months posteclosion and males were 3–14 days posteclosion, while the age of workers was not recorded. Four to five biological replicates were prepared for snRNA-seq for each of the four adult phenotypes (five for workers, four for queens, four for gynes and four for males). Nuclei for each single replicate were isolated from a pool of 30 to 50 whole brains for each specific category of adult phenotype.
Brain dissection, nuclei isolation and snRNA-seq
Ants were anaesthetized in a dissection dish on ice and washed with ethanol and PBS twice, after which brains were dissected in PBS on ice under a stereomicroscope (Nikon, SMZ645). We carefully removed the surrounding trachea (always present) and ocelli (absent in workers) after which ant brains were washed with 1 ml PBS to which 1 U µl−1 RNase inhibitor was added. All brain samples were collected during the daytime (9:00 to 16:00).
The single nuclei were prepared by mechanical extraction. Specifically, for a single replicate of a specific adult phenotype, 30 to 50 whole brains were pooled and infiltrated together with lysis buffer (10 mM Tris-HCl, pH 7.4, 10 mM NaCl, 3 mM MgCl2, 0.1% IGEPAL CA-630, 1% BSA, 1× protease inhibitor and 1 U µl−1 RNase inhibitor) for 5 min, followed by being pestled with a 2-ml Dounce homogenizer set. Then the nuclei were filtered through a 30-µm cell strainer (Sysmex CellTrics) and pelleted by centrifugation at 3,000g for 8 min. The nuclei were then resuspended (buffer 1% BSA, 2 U µl−1 RNase inhibitor and 6% ficoll in PBS) at a concentration of 1,000 nuclei per μl for single-nucleus library preparation.
The DNBelab C Series Single-Cell Library Prep Set (MGI Tech Co.) was used for the preparation of snRNA-seq libraries according to the manufacturer’s protocol (MGI Tech Co., Ltd). In brief, the single-nucleus suspensions were converted to barcoded snRNA-seq libraries through steps including droplet encapsulation, emulsion breakage, messenger RNA captured bead collection, reverse transcription, complementary DNA amplification and purification. Indexed sequencing libraries were constructed according to the manufacturer’s protocol. The libraries were quantified by Qubit single-strand DNA Assay Kit (Invitrogen) and paired-end sequenced on the DNBSEQ platform at China National GeneBank (Shenzhen, China). Read 1 was 30-bp in length and contained a 10-bp cell barcode 1, a 10-bp cell barcode 2 and a 10 bp unique molecular identifier (UMI). Read 2 was 100-bp in length and represented the transcript sequence.
snRNA-seq data processing and gene expression quantification
Before read alignment, Drop-seq_tools (v.1.13)66 was used to trim ploy(A) stretches, add cell and UMI barcodes to the reads, and remove the reads with barcodes that contained low-quality bases. The reads that passed quality control were then aligned to the M. pharaonis reference genome produced by Gao et al.67 using STAR (v.2.6.1a_08-27)68 with default parameters. To further ensure the accuracy of alignment, we used an in-house script to discard the reads that could be aligned to multiple positions of the reference genome, and to discard any spliced reads that spanned a gap >50 kb (because up to 99.5% of the M. pharaonis introns are shorter than 50 kb) or that detected splicing sites other than the canonical ones (that is, GT/AG, GC/AG and AT/AC). The TagReadWithGeneExon function of Drop-seq_tools was then used to add gene annotation tags to the aligned reads, and the DigitalExpression function to extract digital gene expression (DGE) data matrices (that is, the number of UMIs per gene per nucleus). Nuclei with fewer than 200 or more than 2,000 expressed genes, or with a high proportion (>1%) of UMI counts derived from mitochondrial genes were discarded.
Integration, clustering and cell-type annotation
After obtaining the filtered DGE matrices, we used Seurat (v.3.1.5)69 for normalization, integration, dimension reduction, clustering, visualization and marker gene analysis in the R (v.3.6) environment. Specifically, the Seurat NormalizeData and FindVariableGene functions were first executed for each of the 17 samples (that is, the five worker replicates, four queen replicates, four gyne replicates and four male replicates), after which the 17 samples were integrated into a single dataset using FindIntergrationAnchors and IntegrateData (parameters dims = 20, anchor.features = 4,000) to correct for batch effects. The integrated dataset was then scaled, followed by dimensionality reduction with the RunPCA function. The first 25 PCs were used to construct a shared nearest neighbour network, and clusters were identified using the Louvain algorithm that was implemented in the Seurat FindClusters function. The resulting clusters were visualized using the UMAP method70 by the RunUMAP function. Marker genes for each cluster were identified by the FindAllMarkers function with the Wilcoxon Rank Sum test (min.pct = 0.25, logfc.threshold = 0.25, test.use = ‘wilcox’, only.pos = TRUE).
To choose an appropriate resolution for clustering the 206,367 nuclei, we first generated different clustering versions using a series of resolutions (0.25–3.00 with a step of 0.25) and then manually checked the clustering results to find the versions that could best separate the main neuronal or glial cell types into different clusters. The examined cell types included KCs, OPNs, photoreceptors, monoaminergic neurons, astrocyte-like glia, ensheathing glia, cortex glia and surface glia, which have been well studied in insects with established marker genes as listed in Supplementary Data 1. We also required that all clusters in versions assessed as qualified contained nuclei from two or more of the 17 samples, to ensure that no cluster could result from batch effects. The combination of these considerations led us to finally choose the version generated by a resolution of 1.5 (see Fig. 1b for the cell-type annotations and Supplementary Data 1 for the number of nuclei per cluster per sample). The assignment of clusters to OL neurons, for which well-established marker genes are not available, was based on the transcriptional similarity of cell clusters between Monomorium and Drosophila and between Monomorium and Harpegnathos (see section Transcriptional similarity of cell clusters between species below for more details).
Correlations between snRNA-seq and bulk RNA-seq data
To evaluate the consistency of the gene expression quantification results obtained by snRNA-seq and conventional bulk RNA-seq (Extended Data Fig. 1e), we first generated pseudo-bulk data for each adult phenotype with the snRNA-seq data by accumulating the UMI counts by gene from all nuclei belonging to the same adult phenotype, after which the expression level of a given gene was calculated as CP10K (UMI counts per 10,000). Bulk whole brain RNA-seq reads of the four M. pharaonis adult phenotypes were retrieved from Wang et al.6. SOAPnuke (v.2.1.0)71 was first used to assess sequencing quality and filter reads of low quality with parameters (-G -l 20 -q 0.2 -E 60 -5 1 -Q 2). Clean reads were then aligned to the M. pharaonis reference genome produced by Gao et al.67 using Hisat2 (v.2.1.0)72,73 with default parameters. Read count matrices (that is, the number of uniquely mapped reads per gene per sample) were then obtained by an in-house script. After accumulating read counts by gene from all replicates belonging to the same adult phenotype, the expression level of a given gene was calculated as CP10K (read counts per 10,000). Finally, the correlations between gene expression as quantified by the snRNA-seq data (pseudo-bulk) and the bulk RNA-seq data were obtained as Pearson correlation coefficients after removing genes that had CP10K values <1 in both datasets and transforming the expression values to log2 [CP10K+1].
Principal component analysis (PCA) and hierarchical clustering analyses of cell clusters
For PCA presented in Extended Data Fig. 2c and hierarchical clustering analyses presented in Fig. 3a and Extended Data Fig. 4b,c, we generated a matrix representing the expression level of each gene in each cell cluster. These expression levels were calculated as , where ui is the percentage of UMI counts of a focal gene in each nucleus within the cell cluster, and n is the total nuclei number of a focal cluster. In Extended Data Fig. 2c, we first filtered the genes with narrow variance (standard deviation of expression level <1 across the 43 cell clusters) and then performed a variance stabilizing transformation with the vst function provided by DESeq2 (v.1.22.0)74. We finally generated the PCA plot with the vst-transformed matrix using the plotPCA function provided by DESeq2. In Fig. 3a and Extended Data Fig. 4b,c, only the top 8,000 highly expressed genes across all KC clusters were used for hierarchical clustering analysis, which was achieved using the R package ‘pvclust’ (v.2.2.0)75 with method.dist set to ‘cor’ and method.hclust set to ‘Ward.D’. Confidence levels of branches were estimated by the bootstrapping-based method implemented in pvclust.
Identification of DEGs between cell clusters
The FindMarkers function of Seurat (v.3.1.5) was used to identify DEGs between two (or two groups of) cell clusters with the MAST model76. P values were adjusted for false discovery rate (FDR) following the Benjamini–Hochberg procedure77. A gene was retained as a significant DEG when reporting an FDR < 0.05, showing an expression fold change >1.25 and being expressed in >20% of cells in the up-regulated cell cluster.
Orthologue identification
All analyses involving cross-species comparisons were restricted to one-to-one orthologues between species, built with the reciprocal best hit approach according to the bit scores obtained from all-versus-all BLASTP (blast-2.2.26) alignment with parameters (-F F -e 1 × 10−5).
Apart from M. pharaonis, other species used in the orthologue identification were H. saltator, D. melanogaster, A. mellifica, Caenorhabditis elegans and Homo sapiens. The gene sets of H. saltator (GCA_003227715.1_Hsal_v8.5) and A. mellifica (AJ489744) were downloaded from the National Center for Biotechnology Information (NCBI), and the gene sets of D. melanogaster, C. elegans and H. sapiens were obtained from Ensembl (release-100).
Gene ontology (GO) annotations and enrichment analyses
GO of the M. pharaonis protein-coding genes was assigned according to the GO annotation of their orthologues in D. melanogaster, C. elegans and H. sapiens obtained from the Ensembl database (release-100). One-to-one orthologues between M. pharaonis and D. melanogaster/C. elegans/H. sapiens were built as mentioned above. The GO annotation was assigned on the basis of the priority of D. melanogaster > C. elegans > H. sapiens when a M. pharaonis gene could find an orthologue in more than one species (that is according to the evolutionary distance against M. pharaonis from close to remote). The M. pharaonis genes that could not be annotated by the orthologous method were further aligned to the UniProt database (release-2020_04) using BLASTP with parameters (-F F -e 1 × 10−5). The best hit of each query gene was then retained, on the basis of its BLASTP bit score, and the GO annotations of that best hit was assigned to the query gene. The combination of these two methods allowed us to assign GO annotation to 76% of the M. pharaonis protein-coding genes, which is considerably higher than the 56% reported by Gao et al.67.
Fisher’s exact tests were used to examine whether the up-regulated DEGs in a focal cell cluster (or a group of clusters) were significantly enriched in a specific GO term in relation to the background genes. This procedure compared the number of up-regulated DEGs annotated to this GO term, the number of up-regulated DEGs not annotated to this GO term, the number of background genes annotated to this GO term and the number of background genes not annotated to this GO term. The background genes were defined as all genes except for the up-regulated DEGs with mean expression level (CP10K) > 1 across the cell clusters of interest (for example, the 12 KC clusters in Fig. 3e or the six OL clusters in Fig. 4b). P values were adjusted by FDR following the Benjamini–Hochberg procedure77, and GO terms with FDR < 0.05 and gene number ≥2 were considered to be significantly enriched.
Single-cell datasets of other insects
The single-cell datasets with cell-type annotation and gene expression information of each cell for the female and male whole brains of adult D. melanogaster24, the female and male heads of adult D. melanogaster36, the worker and gamergate midbrains of adult H. saltator18 and the OLs of D. melanogaster (the main dataset from Kurmangaliyev et al.41 and the adult dataset from Özel et al.42) were obtained from the original publications. Manual checks for possible mis-annotation of the cell clusters were performed for the D. melanogaster whole brain and H. saltator midbrain datasets before subsequent analyses (see Supplementary Data 2 for the cell numbers and final annotations for each cluster in each dataset). For the single-cell datasets of adult D. melanogaster midbrains27 as well as the whole brain and mushroom bodies of adult A. mellifera workers35, we obtained the digital-expression matrices and performed clustering analysis with Seurat following the parameters mentioned in the original publications (see Extended Data Figs. 3 and 4c for the clustering results; see Supplementary Data 2 for the cell numbers and annotations for each cluster). The initial clustering result of the D. melanogaster midbrain data could not separate glial subtypes into independent clusters except for astrocytes, so we reclustered all glial cells using a resolution of 1.1 and identified cell clusters of astrocytes, surface glia and cortex glia (Extended Data Fig. 3c,d). However, ensheathing glia were probably absent in this dataset27.
Transcriptional similarity of cell clusters between species
MetaNeighbor25 was used to assess the pairwise transcriptional similarity of cell clusters between two species (for example, between Monomorium and Drosophila or between Monomorium and Harpegnathos as presented in Extended Data Fig. 2d,e). The MetaNeighbor framework calculates the correlations between all pairs of cells within and between datasets on the basis of the expression of a set of genes, and produces a score of mean AUROC to quantify the similarity of cell-cluster pairs25. To improve the performance of MetaNeighbor, we used pseudo-cell rather than single-cell expression as suggested in previous studies78–80. This approach reduces the impact of data sparsity: a typical feature of high-throughput single-cell sequencing data. A pseudo-cell was generated by merging the data of ten cells that were randomly picked from the total cells within a cell cluster without replacement. The UMI counts from these ten cells were then summed by gene, after which gene expression levels were calculated as CP10K (UMI counts per 10,000) in a pseudo-cell. The pseudo-cell expression matrix of each species was then standardized by z-transformation as z = (x − m)/s.d., where x is the expression level of a focal gene in a pseudo-cell, and m and s.d. are the mean and the standard deviation of expression levels for the focal gene across all pseudo-cells. To maximize the differences between cell clusters, the orthologous genes that were identified as cell-cluster markers in at least one of the two compared species were used for the MetaNeighbor analysis. The correlations between pseudo-cell pairs were calculated as Pearson’s coefficients. Pairwise AUROC scores for all cell clusters within and between species were visualized as a heatmap generated by the heatmap.2 function of the R package ‘gplots’, and the dendrogram of the cell clusters was generated by hierarchical clustering using the Ward’s minimum variance method with the distance defined as 1-AUROC (Extended Data Fig. 2d,e).
By comparing all Monomorium cell clusters with all cell clusters defined in adult Drosophila whole brains (with OL cell types)24 and in adult Harpegnathos midbrains (without OL cell types)18 using the MetaNeighbor framework, we were also able to identify six Monomorium cell clusters that were derived from the OLs, because they clearly grouped with the Drosophila OL cell clusters in the Monomorium-versus-Drosophila tree and formed a single clade in the Monomorium-versus-Harpegnathos tree (Extended Data Fig. 2d,e).
Interspecies comparisons of KCs and OL neurons
To track the evolutionary origin of the Monomorium KC subtypes, we collected the KC clusters from two additional hymenopteran insects—the ant H. saltator that shares a common superorganismal ancestor with Monomorium18 and the honeybee A. mellifera that belongs to the corbiculate bee lineage that independently evolved superorganismal colonies35. We also collected the D. melanogaster γ, α'/β' and α/β KCs from three independent studies24,27,36 (Supplementary Data 2 and Extended Data Figs. 3 and 4). We then assessed the transcriptional similarity of KC clusters between any two of the four species (that is M. pharaonis, H. saltator, A. mellifera and D. melanogaster) with the MetaNeighbor framework as described above, except for limiting the pairwise AUROC calculation to KC clusters instead of all cell clusters and using the set of DEGs identified among the KC clusters to maximize the differences between KC subtypes. Pairwise similarities measured as AUROC scores for the focal cell clusters between species were visualized as heatmaps, which were generated with the R package ‘ggplot2’ (Extended Data Fig. 5a–d). We also used network plots to visualize the correspondence of KC clusters across species (Fig. 3f). These network plots were generated by Cytoscape (v.3.8.2)81, with the clusters of Monomorium as source nodes, clusters of other species as target nodes and AUROC scores as edges. Only edges with AUROC >0.80 were shown. Each edge linked a Monomorium KC cluster to its top hit among the KC clusters in another species according to AUROC scores. A second hit also was plotted when the difference between the top and second AUROC score was less than 0.05.
To improve the annotation of the Monomorium OL neurons, we compared the Monomorium OL clusters with the D. melanogaster OL cell clusters generated by two independent single-cell studies that focused exclusively on the D. melanogaster OLs41,42 (Supplementary Data 2), based on the MetaNeighbor framework as in the KC subtype analyses. The putative cell-type identity for the Monomorium OL clusters were summarized according to these two comparisons, which were generally consistent with each other (Extended Data Fig. 6a–c) and made us assign one Monomorium cluster (c20) as LMCs, three (c25, c6 and c27) as medulla neurons, one (c16) as lobula plate T4/T5 neurons and one (c15) as lobula columnar cells (Fig. 4a).
Differential abundance testing between phenotypes and sexes
scCODA (v.0.1.6)52, a Bayesian model based on hierarchical Dirichlet-multinomial distribution, was used to identify cell clusters with credible abundance differences between any two of the four Monomorium adult phenotypes when also taking the variation of the biological replicates into consideration. There are three important parameters to be considered when using scCODA. The first is the FDR level. In practice, an FDR level of 0.2 is deemed to be acceptable by the authors according to their applications of scCODA in five different real single-cell datasets52. The second is the Hamiltonian Monte Carlo (HMC) chain length, which is usually set according to the number of cell clusters. An HMC chain length of 800,000 with a burn-in of 10,000 was sufficient for our Monomorium dataset that contained 43 cell clusters. The third is the reference cell type, which is assumed to be unchanged in abundance across different samples. scCODA can automatically select an appropriate cell type as the reference or uses a prespecified reference cell type to identify compositional changes for the remaining cell types. As we did not have any previous knowledge about the best reference cell type for the four Monomorium adult phenotypes, the ‘automatic reference selection’ option was chosen. Finally, a cell cluster with the scCODA-inferred ‘Final parameter’ other than zero following the scCODA manual, and with >1.3-fold change in relative abundance, was considered to be showing a significant difference in abundance between two adult phenotypes (Fig. 5b,c and Supplementary Data 5).
The adult D. melanogaster whole brain dataset that contained five replicates for each sex24 and the adult D. melanogaster head dataset that contained six replicates for each sex36 (see Supplementary Data 2 for the number of cells per cluster per replicate) were used for the assessment of cell compositional differences between sexes in adult Drosophila flies. The same parameters as mentioned above for Monomorium were applied, which reported zero cell clusters as significant between female and male Drosophila flies in both datasets (Fig. 5b,c and Supplementary Data 5).
Differential abundance testing between species
The scCODA framework was also used to assess the cell compositional differences between the whole brains of adult M. pharaonis and adult D. melanogaster as presented in Fig. 2a. The examined cell types were KC, OPN, monoaminergic neuron, astrocyte, ensheathing glia, cortex glia and surface glia when the remaining cells were assigned as ‘others’. Considering that the M. pharaonis and D. melanogaster datasets were obtained by two different protocols, which might have sampling biases for different cell types, we raised the criteria for defining cell types with credible abundance differences. Specifically, the scCODA assessment was conducted as comparing the four M. pharaonis adult phenotypes with the two D. melanogaster sexes (see Supplementary Data 4 for data used for scCODA analysis) using an FDR level of 0.1, an HMC chain length of 800,000 with a burn-in of 10,000 and the ‘others’ category as the reference cell type. Then a cell type with the scCODA-inferred ‘Final parameter’ other than zero and more than twofold change in relative abundance was considered to be sufficient for showing a significant abundance difference between species.
Volumetric analysis of brain neuropils
Volumetric analyses of major brain neuropils were performed for the four representative adult phenotypes of M. pharaonis. Confocal image stacks were used to reconstruct the brain neuropils, which were obtained from whole head preparations to preserve the orientation of the brain in the head capsule. The preparation and imaging of the whole head samples were adapted from Smolla et al.82. The bleached head samples were imaged using a point-scanning confocal and multiphoton microscope (SP5-X MP, Leica Microsystems) with a ×20 objective. The microscope images were imported into the AMIRA (v.6.4) software, where the main brain compartments (mushroom body, ocellus, antennal lobe, gnathal ganglion) were located, labelled and reconstructed in 3D (Fig. 5e). The volume data of the brain compartments were exported to a Microsoft Excel table (Supplementary Data 6), after which the relative volumes of brain compartments were calculated by dividing compartment volumes by entire brain volumes (Extended Data Fig. 7).
ISH with tyramide signal amplification
Anti-sense probes were synthesized to detect the mRNA of Pka-C1 (XM_012678196) and GABA-B-R3 (XM_012679823). The lengths of the probes were 500–600 nt. The following primers were used to obtain PCR amplification products from M. pharaonis brain cDNA:
Pka-C1_F: CGTTTCTCGTGTCGTTGCG
Pka-C1_T7R: GGATCCTAATACGACTCACTATAGGTGTGGCCCTTGATGTCGTTT
GABA-B-R3_F: TGAATAATACAGGCGTTGCG
GABA-B-R3_T7R: GGATCCTAATACGACTCACTATAGGTATGCTTTTGTGCTTGCGA
The PCR amplification products were purified with the TIANgel midi purification kit (Tiangen DP209) and used as DNA template. Anti-sense probes were synthesized using the DIG RNA labelling kit (Roche catalogue no. 11175025910) with a purified DNA template.
ISH with tyramide signal amplification was performed step by step as follows: (1) dissect ant brains in ice-cold PBS (prepared in nuclease free water, unless otherwise indicated) and rinse the brains in PBSTw (0.1% tween 20 in PBS) twice for 5 min. (2) Fix them in 4% PFA (paraformaldehyde, 0.1% DEPC treated) supplemented with 10% DMSO for 20 min at room temperature, followed by a triple PBSTw rinse for 5 min. (3) Rinse with ice-cold methanol for 5 min, then with 3:1, 1:1, 1:3 methanol:PBSTw for 5 min each, then three times with PBSTw for 5 min. (4) Treat with 10 μg ml−1 proteinse K for 20 min, then stop the reaction with 20 mg ml−1 glycine rinse for 5 min, followed by three PBSTw rinses for 5 min. (5) Fix with 4% PFA (0.1% DEPC) for 20 min at room temperature, followed by four PBSTw rinses for 5 min. (6) Incubate with preheated in situ hybridization solution (ISHS) for 1 h at 55 °C. (7) Dissolve 4 μl (200 ng μl−1) of probe in 200 μl of ISHS, heat at 80 °C for 3 min, then cool on ice for 5 min, then remove 200 μl of prehybridization ISHS and add the probe solution. (8) Incubate the brains with probe solution for 16 h at 55 °C. (9) Rinse them with preheated ISHS, 3:1, 1:1, 1:3 ISHS:PBSTw and PBSTw for 15 min each at 55 °C. (10) Rinse twice with PBSTw for 15 min at room temperature. (11) Incubate with PAT (1% triton X-100, 1% BSA (g ml−1) in PBS) for 1 h at room temperature. (12) Incubate with the mouse anti-DIG primary antibody (1:400) for 2 h at room temperature. (13) Rinse three times with PBSTx (1% triton X-100 in PBS) for 15 min. (14) Incubate with poly-HRP conjugated anti-mouse IgG secondary antibody for 1 h at room temperature. (15) Rinse four times with PBS for 5 min. Thereafter, the tyramide signal amplification steps were conducted by strictly following the protocols provided in the Tyramide SuperBoost Kit (Alexa Fluor 488, poly-HRP conjugated goat anti-mouse IgG (Thermo Fisher B40912)). The stained brains were then imaged with a customized confocal microscope LSCM-1 (CASLIGHT, Suzhou Institute of Biomedical Engineering and Technology, Chinese Academy of Sciences).
Hybridization chain reaction (HCR)
The Nlg2 mRNA (XM_012685917) HCR probe set, amplifiers and buffers were commercially purchased from Molecular Instruments, Inc. We generally followed the HCR RNA-fluorescence ISH protocol provided by Molecular Instruments (www.molecularinstruments.com) for whole-mount fruit fly embryos in the HCR experiments, but with modifications for preparation steps of fixed whole-mount ant brains. Specifically, the ant brains were dissected in ice-cold PBS (prepared in nuclease free water), followed by two PBSTw (0.1% tween 20 in PBS) rinses for 5 min. The brains were then fixed in 4% PFA (paraformaldehyde, 0.1% DEPC treated) supplemented with 10% DMSO for 20 min at room temperature, followed by three PBSTw rinses of 5 min. Next, the brains were rinsed by ice-cold methanol for 5 min, then by 3:1, 1:1, 1:3 methanol:PBSTw for 5 min each, followed by two PBSTw rinses for 5 min. The brains were then treated with 5 μg ml−1 proteinse K for 5 min, followed by three PBSTw rinses for 5 min. After that, the brains were fixed in 4% PFA (0.1% DEPC treated) for 20 min at room temperature, followed by five PBSTw rinses for 5 min. Thereafter, the detection and amplification steps were performed following the referenced protocol. We used Alexa Fluor 488 for the detection of Nlg2. The stained brains were imaged with customized confocal microscope LSCM-1 (CASLIGHT, Suzhou Institute of Biomedical Engineering and Technology, Chinese Academy of Sciences).
Dopamine administration
We dissolved 30 mg ml−1 l-dopa (3,4-dihydroxy-l-phenylalanine, Sigma-Aldrich D9628-5G) and 10% (w/v) sucrose in distilled water with 0.9% HCl (w/v). One piece of Kimwipes paper was pressed to the bottom of a 15-ml conical tube (Falcon) and then soaked with l-dopa solution. Five-day old gynes were introduced into the tube that was subsequently plugged with a cotton ball, pushed into the tube to offer roughly 5 ml of volumetric space to the ants. The ants were transferred to new tubes every 2 days. The ovaries of 10-day old gynes were then dissected for estimation of the number of yolky oocytes and the total surface area of yolky oocytes. Control gynes were collected from the same colony on the same day and were treated the same except that they were fed in 10% sucrose with 0.9% HCl (w/v).
To measure the number of yolky oocytes and the total surface area of yolky oocytes, the dissected ovaries were spread out, exposing all ovarioles, and then imaged with an Oplenic digital camera mounted to a Nikon SMZ800N stereomicroscope. Yolky oocytes are growing oocytes in the process of absorbing nutrients from haemolymph. They appeared as opaque, oval-shaped areas in the images as indicated in Fig. 6d with red dotted ovals. The total surface area of yolky oocytes in an ovary was estimated as the summed area of these ovals. A total of 24 individuals were measured for each group and all the images were analysed using EZ-MET software (x64, v.6.0.7543).
Convergent cellular changes in Monomorium and Harpegnathos
Cell clusters with significant changes in abundance between Monomorium gyne and queen brains were identified by the scCODA framework as described above (Fig. 6a). The correspondence of cell clusters between Monomorium and Harpegnathos was assessed with MetaNeighbor analysis as mentioned above (AUROC score >0.9), followed by manual check of cell-cluster marker genes in both species, after which the corresponding cell clusters in Harpegnathos were subjected to examination of abundance change between the worker and gamergate brains (Supplementary Data 7). Fisher’s exact tests were used to assess the significance of abundance change for a given cell cluster between Harpegnathos gamergates and workers, by comparing the number of gamergate cells belonging to this cluster, the number gamergate cells not belonging to this cluster, the number of worker cells belonging to this cluster and the number of worker cells not belonging to this cluster. The raw P values were adjusted for FDR according to the Benjamini–Hochberg procedure, and the cell clusters with FDR < 0.001 and >1.3-fold changes in relative abundance between gamergate and worker brains were considered to reflect a significant change. Finally, the cell clusters with consistent direction of significant change between Monomorium gyne and queen brains and between Harpegnathos worker and gamergate brains were considered as evidence for convergent change during reproductive role differentiation in these two distantly related ant species.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Cell number and annotation for each Monomorium cell cluster.
Single-cell datasets of other insects used in this study.
A 3D view for the 43 Monomorium cell clusters.
Comparison of relative abundances of different cell types between ant and fly.
Cell clusters with significant differences in relatively abundance between Monomorium adult phenotypes and between Drosophila sexes tested by scCODA.
Volumetric measurements of brain neuropiles for the four adult phenotypes of Monomorium.
Abundance difference of each cell cluster between gamergate and worker brains of Harpegnathos.
Acknowledgements
We thank R. Bonasio from University of Pennsylvania for providing the single-cell gene expression data of H. saltator. We thank X. Zhan, J. Li, H. Yu and H. Pan from BGI-Shenzhen for technical help. This work was supported by the National Natural Science Foundation of China (grant nos. 31970573 to G.Z. and 31900399 to W.L.), and the Shenzhen Key Laboratory of Single-Cell Omics (ZDSYS20190902093613831 to L.L.). G.Z. was also funded by a Villum Investigator grant (no. 25900) from the Villum Foundation.
Extended data
Author contributions
G.Z., Q.L., W.L. and C.L. conceived the study. J.Z. maintained the ant colonies. W.L., T.W., W.D. and W.J. performed sample collection and brain dissection. M.W., C.L., Z.W. and X.W. conducted the snRNA-seq experiments and sequencing. Y.L., M.W., Q.L., L.H. and L.W. performed quality control, integration, clustering and annotation of the snRNA-seq data. Q.L., P.Z., Y.Z., Q.G. and N.X. conducted comparative analyses across species and adult phenotypes. W.L., X.D., X.Z. and W.Z. performed the ISH/HCR and dopamine administration experiments. M.N. and B.H.D. conducted volumetric analysis of brain neuropils. L.L., X.X., H.L., H.Y. and J.W. contributed reagents/materials/computing resources. Q.L. and W.L. wrote the manuscript with the inputs from all authors. G.Z. and J.J.B. revised the manuscript. All authors read and approved the final manuscript.
Peer review
Peer review information
Nature Ecology & Evolution thanks Hongjie Li and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
The raw snRNA-seq data of M. pharaonis generated in this study are deposited in NCBI Sequence Read Archive under BioProject accession no. PRJNA833256 and in the CNGB Nucleotide Sequence Archive under accession no. CNP0001472. The reference genome, gene models, functional annotations of protein-coding genes, gene expression matrix (the number of UMIs per gene per nucleus), full marker gene list of each cell cluster and all in-house scripts are deposited in the figshare repository83.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Qiye Li, Mingyue Wang, Pei Zhang, Yang Liu.
Contributor Information
Chuanyu Liu, Email: liuchuanyu@genomics.cn.
Guojie Zhang, Email: guojiezhang@zju.edu.cn.
Weiwei Liu, Email: liuweiwei@mail.kiz.ac.cn.
Extended data
are available for this paper at 10.1038/s41559-022-01784-1.
Supplementary information
The online version contains supplementary material available at 10.1038/s41559-022-01784-1.
References
- 1.Godfrey RK, Swartzlander M, Gronenberg W. Allometric analysis of brain cell number in Hymenoptera suggests ant brains diverge from general trends. Proc. Biol. Sci. / R. Soc. 2021;288:20210199. doi: 10.1098/rspb.2021.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raji JI, Potter CJ. The number of neurons in Drosophila and mosquito brains. PloS ONE. 2021;16:e0250381. doi: 10.1371/journal.pone.0250381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lihoreau M, Latty T, Chittka L. An exploration of the social brain hypothesis in insects. Front. Physiol. 2012;3:442. doi: 10.3389/fphys.2012.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wheeler, W. M. Ants: Their Structure, Development and Behavior (Columbia Univ. Press, 1910).
- 5.Wheeler WM. The ant‐colony as an organism. J. Morphol. 1911;22:307–325. doi: 10.1002/jmor.1050220206. [DOI] [Google Scholar]
- 6.Zayed A, Robinson GE. Understanding the relationship between brain gene expression and social behavior: lessons from the honey bee. Annu. Rev. Genet. 2012;46:591–615. doi: 10.1146/annurev-genet-110711-155517. [DOI] [PubMed] [Google Scholar]
- 7.Boomsma JJ, Baer B, Heinze J. The evolution of male traits in social insects. Annu. Rev. Entomol. 2005;50:395–420. doi: 10.1146/annurev.ento.50.071803.130416. [DOI] [PubMed] [Google Scholar]
- 8.Chittka L, Niven J. Are bigger brains better? Curr. Biol. 2009;19:R995–R1008. doi: 10.1016/j.cub.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 9.Khrameeva E, et al. Single-cell-resolution transcriptome map of human, chimpanzee, bonobo, and macaque brains. Genome Res. 2020;30:776–789. doi: 10.1101/gr.256958.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanton S, et al. Organoid single-cell genomic atlas uncovers human-specific features of brain development. Nature. 2019;574:418–422. doi: 10.1038/s41586-019-1654-9. [DOI] [PubMed] [Google Scholar]
- 11.Pollen AA, et al. Establishing cerebral organoids as models of human-specific brain evolution. Cell. 2019;176:743–756 e717. doi: 10.1016/j.cell.2019.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morandin C, et al. Comparative transcriptomics reveals the conserved building blocks involved in parallel evolution of diverse phenotypic traits in ants. Genome Biol. 2016;17:43. doi: 10.1186/s13059-016-0902-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feldmeyer B, Elsner D, Foitzik S. Gene expression patterns associated with caste and reproductive status in ants: worker-specific genes are more derived than queen-specific ones. Mol. Ecol. 2014;23:151–161. doi: 10.1111/mec.12490. [DOI] [PubMed] [Google Scholar]
- 14.Warner MR, Mikheyev AS, Linksvayer TA. Genomic signature of kin selection in an ant with obligately sterile workers. Mol. Biol. Evol. 2017;34:1780–1787. doi: 10.1093/molbev/msx123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qiu B, et al. Towards reconstructing the ancestral brain gene-network regulating caste differentiation in ants. Nat. Ecol. Evol. 2018;2:1782–1791. doi: 10.1038/s41559-018-0689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandra V, et al. Social regulation of insulin signaling and the evolution of eusociality in ants. Science. 2018;361:398–402. doi: 10.1126/science.aar5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang M, et al. Chromatin accessibility and transcriptome landscapes of Monomorium pharaonis brain. Sci. Data. 2020;7:217. doi: 10.1038/s41597-020-0556-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheng L, et al. Social reprogramming in ants induces longevity-associated glia remodeling. Sci. Adv. 2020;6:eaba9869. doi: 10.1126/sciadv.aba9869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pontieri, L. & Linksvayer, T. A. in Encyclopedia of Social Insects (ed. Starr, C.) 10.1007/978-3-319-90306-4_171-1 (Springer, 2019).
- 20.Hölldobler, B. & Wilson, E. O. The Ants (Harvard Univ. Press, 1990).
- 21.Nagel, M. et al. The gene expression network regulating queen brain remodeling after insemination and its parallel use in ants with reproductive workers. Sci. Adv.10.1126/sciadv.aaz5772 (2020). [DOI] [PMC free article] [PubMed]
- 22.Berndt KP, Nitschmann J. The physiology of reproduction in the pharaoh’s ant (Monomorium pharaonis L.) 2. The unmated queens. Insectes Sociaux. 1979;26:137–145. doi: 10.1007/BF02223507. [DOI] [Google Scholar]
- 23.Allard D, et al. Sperm transfer during mating in the pharaoh’s ant, Monomorium pharaonis. Physiological Entomol. 2006;31:294–298. doi: 10.1111/j.1365-3032.2006.00519.x. [DOI] [Google Scholar]
- 24.Davie K, et al. A single-cell transcriptome atlas of the aging Drosophila brain. Cell. 2018;174:982–998 e920. doi: 10.1016/j.cell.2018.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crow M, Paul A, Ballouz S, Huang ZJ, Gillis J. Characterizing the replicability of cell types defined by single cell RNA-sequencing data using MetaNeighbor. Nat. Commun. 2018;9:884. doi: 10.1038/s41467-018-03282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fahrbach SE. Structure of the mushroom bodies of the insect brain. Annu. Rev. Entomol. 2006;51:209–232. doi: 10.1146/annurev.ento.51.110104.150954. [DOI] [PubMed] [Google Scholar]
- 27.Croset, V., Treiber, C. D. & Waddell, S. Cellular diversity in the Drosophila midbrain revealed by single-cell transcriptomics. eLife10.7554/eLife.34550 (2018). [DOI] [PMC free article] [PubMed]
- 28.Abel R, Rybak J, Menzel R. Structure and response patterns of olfactory interneurons in the honeybee, Apis mellifera. J. Comp. Neurol. 2001;437:363–383. doi: 10.1002/cne.1289. [DOI] [PubMed] [Google Scholar]
- 29.Jefferis GS, et al. Comprehensive maps of Drosophila higher olfactory centers: spatially segregated fruit and pheromone representation. Cell. 2007;128:1187–1203. doi: 10.1016/j.cell.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson EO. Chemical communication in the social insects. Science. 1965;149:1064–1071. doi: 10.1126/science.149.3688.1064. [DOI] [PubMed] [Google Scholar]
- 31.Suenami S, Oya S, Kohno H, Kubo T. Kenyon cell subtypes/populations in the honeybee mushroom bodies: possible function based on their gene expression profiles, differentiation, possible evolution, and application of genome editing. Front Psychol. 2018;9:1717. doi: 10.3389/fpsyg.2018.01717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamikouchi A, Takeuchi H, Sawata M, Natori S, Kubo T. Concentrated expression of Ca2+/calmodulin-dependent protein kinase II and protein kinase C in the mushroom bodies of the brain of the honeybee Apis mellifera L. J. Comp. Neurol. 2000;417:501–510. doi: 10.1002/(SICI)1096-9861(20000221)417:4<501::AID-CNE8>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 33.Takeuchi H, et al. Identification of a novel gene, Mblk-1, that encodes a putative transcription factor expressed preferentially in the large-type Kenyon cells of the honeybee brain. Insect Mol. Biol. 2001;10:487–494. doi: 10.1046/j.0962-1075.2001.00288.x. [DOI] [PubMed] [Google Scholar]
- 34.Argyrousi EK, Heckman PRA, Prickaerts J. Role of cyclic nucleotides and their downstream signaling cascades in memory function: being at the right time at the right spot. Neurosci. Biobehav. Rev. 2020;113:12–38. doi: 10.1016/j.neubiorev.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 35.Traniello IM, et al. Meta-analysis of honey bee neurogenomic response links Deformed wing virus type A to precocious behavioral maturation. Sci. Rep. 2020;10:3101. doi: 10.1038/s41598-020-59808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li H, et al. Fly Cell Atlas: a single-nucleus transcriptomic atlas of the adult fruit fly. Science. 2022;375:eabk2432. doi: 10.1126/science.abk2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Branstetter MG, Longino JT, Ward PS, Faircloth BC. Enriching the ant tree of life: enhanced UCE bait set for genome‐scale phylogenetics of ants and other Hymenoptera. Meth. Ecol. Evol. 2017;8:768–776. doi: 10.1111/2041-210X.12742. [DOI] [Google Scholar]
- 38.Kumar S, Stecher G, Suleski M, Hedges SB. TimeTree: a resource for timelines, timetrees, and divergence times. Mol. Biol. Evol. 2017;34:1812–1819. doi: 10.1093/molbev/msx116. [DOI] [PubMed] [Google Scholar]
- 39.Peters RS, et al. Evolutionary history of the hymenoptera. Curr. Biol. 2017;27:1013–1018. doi: 10.1016/j.cub.2017.01.027. [DOI] [PubMed] [Google Scholar]
- 40.Lee T, Lee A, Luo L. Development of the Drosophila mushroom bodies: sequential generation of three distinct types of neurons from a neuroblast. Development. 1999;126:4065–4076. doi: 10.1242/dev.126.18.4065. [DOI] [PubMed] [Google Scholar]
- 41.Kurmangaliyev YZ, Yoo J, Valdes-Aleman J, Sanfilippo P, Zipursky SL. Transcriptional programs of circuit assembly in the Drosophila visual system. Neuron. 2020;108:1045–1057 e1046. doi: 10.1016/j.neuron.2020.10.006. [DOI] [PubMed] [Google Scholar]
- 42.Özel MN, et al. Neuronal diversity and convergence in a visual system developmental atlas. Nature. 2021;589:88–95. doi: 10.1038/s41586-020-2879-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hahn N, et al. Monogenic heritable autism gene neuroligin impacts Drosophila social behaviour. Behav. Brain Res. 2013;252:450–457. doi: 10.1016/j.bbr.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 44.Schnell B, Raghu SV, Nern A, Borst A. Columnar cells necessary for motion responses of wide-field visual interneurons in Drosophila. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2012;198:389–395. doi: 10.1007/s00359-012-0716-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ribeiro IMA, et al. Visual projection neurons mediating directed courtship in Drosophila. Cell. 2018;174:607–621 e618. doi: 10.1016/j.cell.2018.06.020. [DOI] [PubMed] [Google Scholar]
- 46.Davis, F. P. et al. A genetic, genomic, and computational resource for exploring neural circuit function. eLife10.7554/eLife.50901 (2020). [DOI] [PMC free article] [PubMed]
- 47.Stöckl AL, O’Carroll DC, Warrant EJ. Hawkmoth lamina monopolar cells act as dynamic spatial filters to optimize vision at different light levels. Sci. Adv. 2020;6:eaaz8645. doi: 10.1126/sciadv.aaz8645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greiner B, Ribi WA, Wcislo WT, Warrant EJ. Neural organisation in the first optic ganglion of the nocturnal bee Megalopta genalis. Cell Tissue Res. 2004;318:429–437. doi: 10.1007/s00441-004-0945-z. [DOI] [PubMed] [Google Scholar]
- 49.Greiner B, Ribi WA, Warrant EJ. A neural network to improve dim-light vision? Dendritic fields of first-order interneurons in the nocturnal bee Megalopta genalis. Cell Tissue Res. 2005;322:313–320. doi: 10.1007/s00441-005-0034-y. [DOI] [PubMed] [Google Scholar]
- 50.Stöckl AL, Ribi WA, Warrant EJ. Adaptations for nocturnal and diurnal vision in the hawkmoth lamina. J. Comp. Neurol. 2016;524:160–175. doi: 10.1002/cne.23832. [DOI] [PubMed] [Google Scholar]
- 51.Tay JW, Lee CY. Induced disturbances cause Monomorium pharaonis (Hymenoptera: Formicidae) nest relocation. J. Econ. Entomol. 2015;108:1237–1242. doi: 10.1093/jee/tov079. [DOI] [PubMed] [Google Scholar]
- 52.Büttner M, Ostner J, Müller CL, Theis FJ, Schubert B. scCODA is a Bayesian model for compositional single-cell data analysis. Nat. Commun. 2021;12:6876. doi: 10.1038/s41467-021-27150-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Passera, L. in Exotic Ants. Biology, Impact, and Control of Introduced Species (ed. Williams, D. F.) 23–43 (CRC Press, 1994).
- 54.Riemensperger T, et al. Behavioral consequences of dopamine deficiency in the Drosophila central nervous system. Proc. Natl Acad. Sci. USA. 2011;108:834–839. doi: 10.1073/pnas.1010930108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pörzgen P, Park SK, Hirsh J, Sonders MS, Amara SG. The antidepressant-sensitive dopamine transporter in Drosophila melanogaster: a primordial carrier for catecholamines. Mol. Pharm. 2001;59:83–95. doi: 10.1124/mol.59.1.83. [DOI] [PubMed] [Google Scholar]
- 56.Okada Y, et al. Social dominance and reproductive differentiation mediated by dopaminergic signaling in a queenless ant. J. Exp. Biol. 2015;218:1091–1098. doi: 10.1242/jeb.118414. [DOI] [PubMed] [Google Scholar]
- 57.Penick CA, Brent CS, Dolezal K, Liebig J. Neurohormonal changes associated with ritualized combat and the formation of a reproductive hierarchy in the ant Harpegnathos saltator. J. Exp. Biol. 2014;217:1496–1503. doi: 10.1242/jeb.098301. [DOI] [PubMed] [Google Scholar]
- 58.Jaber M, Robinson SW, Missale C, Caron MG. Dopamine receptors and brain function. Neuropharmacology. 1996;35:1503–1519. doi: 10.1016/S0028-3908(96)00100-1. [DOI] [PubMed] [Google Scholar]
- 59.Ma T, Matsuoka S, Drummond-Barbosa D. RNAi-based screens uncover a potential new role for the orphan neuropeptide receptor Moody in Drosophila female germline stem cell maintenance. PloS ONE. 2020;15:e0243756. doi: 10.1371/journal.pone.0243756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peeters C, Liebig J, Hölldobler B. Sexual reproduction by both queens and workers in the ponerine ant Harpegnathos saltator. Insectes Sociaux. 2000;47:325–332. doi: 10.1007/PL00001724. [DOI] [Google Scholar]
- 61.Tsuji K, Yamauchi K. Production of females by parthenogenesis in the ant, Cerapachys biroi. Insectes Sociaux. 1995;42:333–336. doi: 10.1007/BF01240430. [DOI] [Google Scholar]
- 62.Monnin T, Peeters C. Dominance hierarchy and reproductive conflicts among subordinates in a monogynous queenless ant. Behav. Ecol. 1999;10:323–332. doi: 10.1093/beheco/10.3.323. [DOI] [Google Scholar]
- 63.Doherty J, Logan MA, Taşdemir ÖE, Freeman MR. Ensheathing glia function as phagocytes in the adult Drosophila brain. J. Neurosci. 2009;29:4768–4781. doi: 10.1523/JNEUROSCI.5951-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boomsma JJ, Gawne R. Superorganismality and caste differentiation as points of no return: how the major evolutionary transitions were lost in translation. Biol. Rev. Camb. Philos. Soc. 2018;93:28–54. doi: 10.1111/brv.12330. [DOI] [PubMed] [Google Scholar]
- 65.Keller L, Genoud M. Extraordinary lifespans in ants: a test of evolutionary theories of ageing. Nature. 1997;389:958–960. doi: 10.1038/40130. [DOI] [Google Scholar]
- 66.Macosko EZ, et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell. 2015;161:1202–1214. doi: 10.1016/j.cell.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gao, Q. et al. High-quality chromosome-level genome assembly and full-length transcriptome analysis of the pharaoh ant Monomorium pharaonis. Gigascience10.1093/gigascience/giaa143 (2020). [DOI] [PMC free article] [PubMed]
- 68.Dobin A, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Butler A, Hoffman P, Smibert P, Papalexi E, Satija R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 2018;36:411–420. doi: 10.1038/nbt.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Becht, E. et al. Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol.10.1038/nbt.4314 (2018). [DOI] [PubMed]
- 71.Chen Y, et al. SOAPnuke: a MapReduce acceleration-supported software for integrated quality control and preprocessing of high-throughput sequencing data. Gigascience. 2018;7:gix120. doi: 10.1093/gigascience/gix120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods. 2015;12:357–360. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim D, Paggi JM, Park C, Bennett C, Salzberg SL. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019;37:907–915. doi: 10.1038/s41587-019-0201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Suzuki R, Shimodaira H. Pvclust: an R package for assessing the uncertainty in hierarchical clustering. Bioinformatics. 2006;22:1540–1542. doi: 10.1093/bioinformatics/btl117. [DOI] [PubMed] [Google Scholar]
- 76.Finak G, et al. MAST: a flexible statistical framework for assessing transcriptional changes and characterizing heterogeneity in single-cell RNA sequencing data. Genome Biol. 2015;16:278. doi: 10.1186/s13059-015-0844-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc.: Ser. B (Methodol.) 1995;57:289–300. [Google Scholar]
- 78.Wang J, et al. Tracing cell-type evolution by cross-species comparison of cell atlases. Cell Rep. 2021;34:108803. doi: 10.1016/j.celrep.2021.108803. [DOI] [PubMed] [Google Scholar]
- 79.Han X, et al. Construction of a human cell landscape at single-cell level. Nature. 2020;581:303–309. doi: 10.1038/s41586-020-2157-4. [DOI] [PubMed] [Google Scholar]
- 80.Tosches MA, et al. Evolution of pallium, hippocampus, and cortical cell types revealed by single-cell transcriptomics in reptiles. Science. 2018;360:881–888. doi: 10.1126/science.aar4237. [DOI] [PubMed] [Google Scholar]
- 81.Su G, Morris JH, Demchak B, Bader GD. Biological network exploration with Cytoscape 3. Curr. Protoc. Bioinforma. 2014;47:8 13 11–24. doi: 10.1002/0471250953.bi0813s47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Smolla M, Ruchty M, Nagel M, Kleineidam CJ. Clearing pigmented insect cuticle to investigate small insects’ organs in situ using confocal laser-scanning microscopy (CLSM) Arthropod Struct. Dev. 2014;43:175–181. doi: 10.1016/j.asd.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 83.Li, Q. et al. Supporting data for ‘A single-cell transcriptomic atlas tracking the neural basis of division of labor in an ant superorganism’. figshare10.6084/m9.figshare.16616353 (2022). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cell number and annotation for each Monomorium cell cluster.
Single-cell datasets of other insects used in this study.
A 3D view for the 43 Monomorium cell clusters.
Comparison of relative abundances of different cell types between ant and fly.
Cell clusters with significant differences in relatively abundance between Monomorium adult phenotypes and between Drosophila sexes tested by scCODA.
Volumetric measurements of brain neuropiles for the four adult phenotypes of Monomorium.
Abundance difference of each cell cluster between gamergate and worker brains of Harpegnathos.
Data Availability Statement
The raw snRNA-seq data of M. pharaonis generated in this study are deposited in NCBI Sequence Read Archive under BioProject accession no. PRJNA833256 and in the CNGB Nucleotide Sequence Archive under accession no. CNP0001472. The reference genome, gene models, functional annotations of protein-coding genes, gene expression matrix (the number of UMIs per gene per nucleus), full marker gene list of each cell cluster and all in-house scripts are deposited in the figshare repository83.