Abstract
Objective:
The Veterans Health Administration (VHA) and the Centers for Medicare and Medicaid Services (CMS) each created initiatives to reduce off-label use of antipsychotics among patients with dementia in nursing homes. Although CMS has reported antipsychotic reductions, the impact on prescribing of antipsychotic and other central nervous system (CNS)-active medications in the VHA remains unclear. We evaluated national trends in antipsychotic and other CNS-active medication prescribing among nursing home patients with dementia in the VHA.
Methods:
All Veterans with dementia residing in VHA nursing homes for more than 30 days (n=35,742). Using an interrupted time-series design, the quarterly prevalence of antipsychotic, antidepressant, antiepileptic, anxiolytic, opioid, and memory medication prescribing was evaluated from FY2009-2018.
Results:
Antipsychotic prescribing in the VHA declined from FY2009-2018 (33.7% to 27.5%) with similar declines in anxiolytic prescribing (33.5% to 27.1%). During this period, prescribing of antiepileptics, antidepressants, and opioids increased significantly (antiepileptics: 26.8% to 43.3%; antidepressants: 56.8% to 63.4%; opioids: 32.6% to 41.2%). Gabapentin served as the main driver of antiepileptic increases (11.1% to 23.5%). Antidepressant prescribing increases included sertraline, mirtazapine, and trazodone prescribing. The overall prescribing of non-antipsychotic psychotropic medications grew from FY2009-2018 from 75.0% to 81.1%.
Conclusions:
Antipsychotic and anxiolytic prescribing to VHA nursing home residents with dementia declined, though overall prescribing of other psychotropic and opioid medications increased. Policies focused primarily on reducing antipsychotic use without considering use in context of other medications may contribute to growth in alternative medication classes with even less evidence of benefit and similar risks.
INTRODUCTION
The U.S. Food and Drug Administration (FDA) has not approved any drugs for behavioral and psychological symptoms of dementia (BPSD), though the widespread prescribing of psychotropic and opioid medications to persons with dementia could represent off-label attempts to address BPSD.1-3 Based upon mortality concerns, the U.S. FDA issued black box warnings for mortality risk associated with atypical (2005) and conventional (2008) antipsychotics when used to treat dementia-related psychosis.4 Given these safety concerns, providers may prescribe alternative central nervous system (CNS)-active medications in lieu of antipsychotics. However, commonly used alternatives, such as benzodiazepines and antiepileptics, have even poorer risk to benefit ratios.5 Ultimately, among psychotropic medications, atypical antipsychotics have the strongest evidence base for BPSD treatment, although their benefits may prove moderate at best.6 Numerous expert bodies and professional organizations, including the American Geriatrics Society, American Association of Geriatric Psychiatry, and American Psychiatric Association, have recommended non-pharmacological treatment strategies as first line to address BPSD.7,8
Prior to the first black box warning in 2005, 24-32% of nursing home residents received antipsychotics.9-11 The Centers for Medicare and Medicaid Services (CMS) launched the National Partnership to Improve Dementia Care in Nursing Homes (CMSNP) in 2012 to address concerns about persistently high rates of antipsychotic use and quality of care provided to nursing home residents with dementia.12 CMSNP included training materials and online resources with a goal of reducing antipsychotic prescribing in nursing homes. CMSNP appeared to lower antipsychotic prescribing in nursing homes from 24% in 2011 to 15% in 2018.13 Starting in 2015, the CMS Five Star Quality Rating System for nursing homes included a publicly reported measure of antipsychotic drug use by nursing homes through the Nursing Home Compare website, thus incentivizing facilities to reduce their antipsychotic prescribing.14
Whether the CMSNP recommendations translated into antipsychotic prescribing reductions within the Veterans Health Administration (VHA), which provides care to many Veterans with dementia in VHA nursing homes, named Community Living Centers (CLCs), remains unknown. The VHA maintains over 130 CLCs across the U.S. which provide both short- and long-term nursing and medical care for Veterans.15 In addition to potential impact of CMSNP within VHA, in December 2013 VHA launched the Psychotropic Drug Safety Initiative (PDSI), a nationwide psychopharmacology quality improvement initiative. From 2013 through 2017, PDSI included two targets relevant to Veterans with dementia: 1) the proportion prescribed antipsychotics, and 2) the proportion prescribed benzodiazepines. Further, similar to community nursing homes, CLCs complete the CMS Minimum Data Set 3.0 for clinical assessment of Veterans’ health and behaviors as well as to report CMS quality indicators. In 2018, the VHA developed a similar publicly-reported rating system adapted from the CMS Nursing Home Compare website, called CLC Compare to help evaluate the quality of care received by Veterans in CLCs.16
Therefore, both Medicare and VHA have used antipsychotic use as a proxy measure for quality of care among patients with dementia in nursing home settings.17 In community settings, antipsychotic reduction led to an increase in other types of CNS-active medication prescribing;18 it remains unknown whether this also occurred within VHA. We sought to evaluate national trends in antipsychotic and other CNS-active medication prescribing among Veterans with dementia residing in VHA nursing homes as well as understand how use of specific agents has changed over time.
METHODS
We used patient-level data from the VHA Corporate Data Warehouse (CDW) to evaluate changes in antipsychotic and other CNS-active medication prescribing from FY2009-2018 among Veterans with dementia residing in VHA nursing homes. The Veterans Affairs Ann Arbor Healthcare System institutional review board approved this study.
Study Cohort.
We used data from the VHA CDW for all Veterans from October 1, 2008 to September 31, 2018 (FY2009-2018). We first constructed quarterly cohorts of long-stay residents at VHA nursing facilities (i.e., CLCs). In community settings, “long-stay” is typically defined as a length of stay >100 nursing home days.19 However, only 28.9% of Veterans with dementia had a CLC length of stay >100 days. We therefore chose a length of stay >30 days to represent long-stay CLC stays, which represents 53.0% of Veterans with dementia in the CLC.
We included a Veteran in a given quarterly cohort if they met criteria for long-stay (>30 days) for at least one day during the quarter, were ≥65 years of age during the quarter, and had a dementia diagnosis (see eTable 1 for International Classification of Disease, Ninth and Tenth Revision codes).20 We determined comorbid clinical conditions (e.g., depression, anxiety, bipolar disorder, chronic pain) based on an inpatient or outpatient diagnosis code in the previous 12 months. (See eTable 1 for International Classification of Disease, Ninth and Tenth Revision codes for clinical conditions; we identified chronic pain using previously established codes.21,22) Further, we excluded those with a diagnosis of schizophrenia, Tourette’s syndrome, and Huntington’s disease to match the exclusion criteria that CMS uses for its antipsychotic quality measure (e.g., for Nursing Home Compare and the CMSNP).23 A long-stay CLC resident remained in the cohort (denominator) until death, the end of the long-stay period (e.g., discharge from CLC), or the end of the study period, whichever came first.
Outcomes.
We used Bar-Code Medication Administration data within CDW to identify medications of interest. Specifically, for each Veteran we determined quarterly CNS-active medication administration including antipsychotics, antidepressants, antiepileptics, anxiolytics (including benzodiazepines and sedative hypnotics), opioids, and memory medications (i.e., cholinesterase inhibitors and memantine; eTable 2).18 Additionally, we included a composite measure of any non-antipsychotic psychotropic medication use which included the following medication classes: antidepressants, antiepileptics, and anxiolytics, along with non-antipsychotic CNS-active medication polypharmacy defined as ≥3 medications from these classes and/or opioids during a 30-day rolling window.
Statistical Analyses.
We used interrupted time-series design to evaluate quarterly trends in prescribing rates from FY2009-2018. We began the analysis in FY2009, following the U.S. FDA black box warnings related to antipsychotic use among patients with dementia. To examine the association of CMSNP and VHA PDSI with CNS-active medication prescribing, we divided time into three periods: period 1 FY2009 to the CMSNP (2012 Q1), period 2 after the CMSNP (2012 Q2) to before the VHA PDSI (2013 Q3), and period 3 from the VHA PDSI (2013 Q4) to the end of the study period (2018 Q4).
We calculated prescribing rates as the percentage of long-stay CLC residents with dementia in each quarter with a prescription of interest. We then fit a three phase interrupted time series regression model to evaluate the association of the CMSNP and VHA PDSI, controlling for autocorrelation by assuming a first-order autoregressive process. We used quarterly percentage prescribed antipsychotics or other medications as our outcome of interest. Models included a linear time-trend variable, indicators for post-CMSNP and post-VHA PDSI, and terms for change in the linear time trend between the study periods (e.g., between the pre- and post-CMSNP periods). Lastly, we evaluated which specific medications increased or decreased over time by computing and plotting the percentage of Veterans that used each medication during each quarter. We determined statistical significance using 0.05 level two-tailed tests and used R version 4.0.2 for all analyses.
RESULTS
Our cohort included 35,742 Veterans with long-stay (>30 days) in a CLC, between FY2009-2018 (Table 1). The cohort was predominantly male (97.9%), non-Hispanic white (74.5%), and had a mean age of 80.5 years.
Table 1.
Characteristics of long-stay Community Living Center Veterans with dementia in the cohort, FY2009-2018
| Long-stay residents overalla N = 35,742 |
Period 1 (Q4 2008 to Q1 of 2012) N = 14,730 |
Period 2 (Q2 of 2012 to Q3 of 2013) N = 8,362 |
Period 3 (Q4 of 2013 to Q3 of 2018) N = 20,125 |
|||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | N | % | N | % | N | % | N | % |
| Age at nursing home entry, y (mean±S.D.) | 80.5 | 8.7 | 81.0 | 7.8 | 80.0 | 8.9 | 79.8 | 9.3 |
| Age at end of study period or death (mean±S.D.) | 82.6 | 8.4 | 84.0 | 7.4 | 83.3 | 8.4 | 81.8 | 8.9 |
| Sex | ||||||||
| Male | 34977 | 97.9 | 14378 | 97.6 | 8160 | 97.6 | 19719 | 98.0 |
| Female | 765 | 2.1 | 352 | 2.4 | 202 | 2.4 | 406 | 2.0 |
| Race/ethnicity | ||||||||
| White | 26641 | 74.5 | 11038 | 74.9 | 6242 | 74.6 | 14889 | 74.0 |
| Black | 5533 | 15.5 | 2032 | 13.8 | 1259 | 15.1 | 3396 | 16.9 |
| Hispanic | 1855 | 5.2 | 684 | 4.6 | 386 | 4.6 | 1105 | 5.5 |
| Other | 3568 | 10.0 | 1660 | 11.3 | 861 | 10.3 | 1840 | 9.1 |
| Clinical conditions b | ||||||||
| Anxiety | 8653 | 24.2 | 2814 | 19.1 | 1985 | 23.7 | 5754 | 28.6 |
| Depression | 4976 | 13.9 | 1045 | 7.1 | 703 | 8.4 | 3867 | 19.2 |
| Bipolar | 889 | 2.5 | 346 | 2.3 | 206 | 2.5 | 546 | 2.7 |
| Chronic pain | 24889 | 69.6 | 9361 | 63.6 | 5566 | 66.6 | 14777 | 73.4 |
| Charlson index (mean±S.D.) | 3.3 | 2.7 | 3.0 | 2.5 | 3.0 | 2.5 | 3.4 | 2.7 |
Presence determined based on the 30-day period required to establish patient as a long-stay CLC resident. The sum of Veterans across all three study periods yields a number larger than the overall study sample because Veterans could be included in more than one time period.
Diagnoses were based on all inpatient and outpatient visits that occurred during one year prior to the CLC admission date.
CNS-Active Medication Prescribing Among CLC Long-Stay Veterans with Dementia.
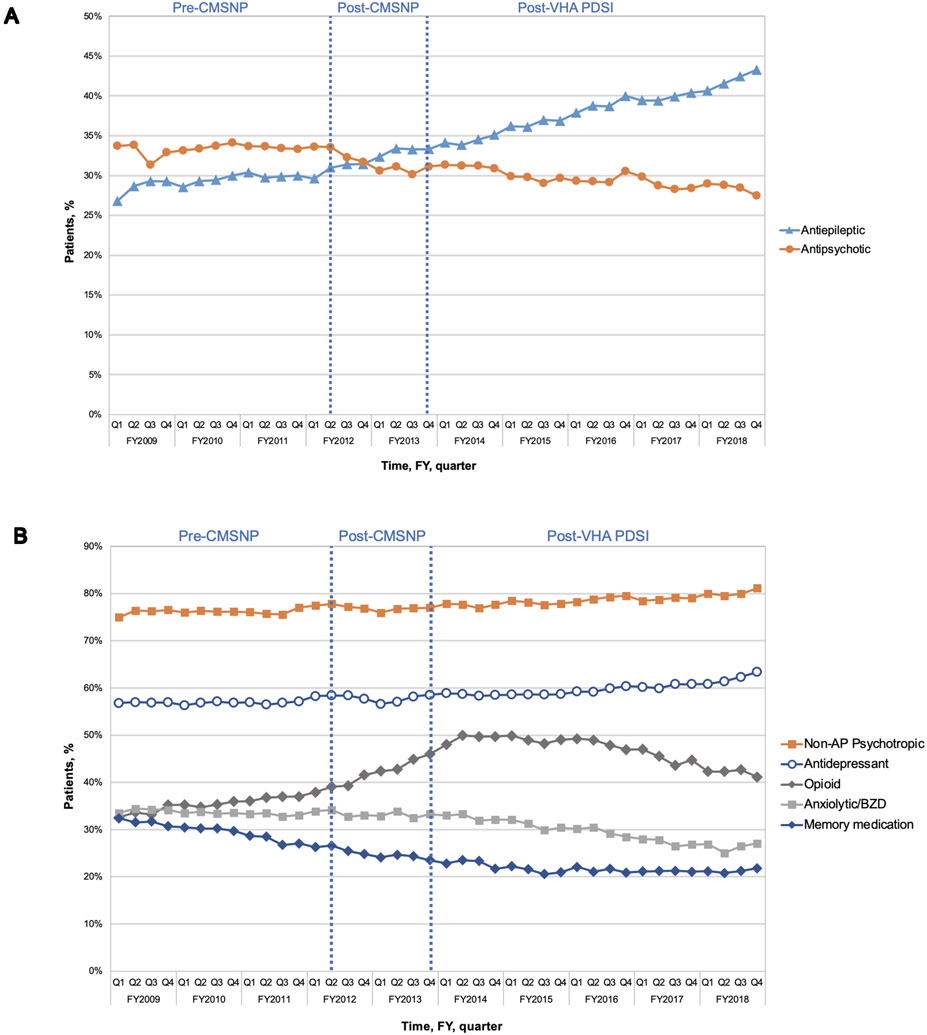
Figure 1 demonstrates the quarterly rates of CNS-active or memory medication prescribing among Veterans with dementia residing in CLCs throughout the three study periods (eTable 3 presents the quarterly rates represented in Figure 1). Table 2 depicts changes in the level and slope of medication prescribing before and after the start of the CMSNP and VHA PDSI.
Figure 1.
Antipsychotic, antiepileptic (A), and other CNS-active medication prescribing (B) among long-stay community living center Veterans with dementia, FY2009-2018
Any non-antipsychotic (non-AP) psychotropic includes a prescription for antianxiety, antidepressant, or antiepileptic medication
Table 2.
Rates and trends in quarterly antipsychotic and comparison medication prescribing among long-stay residents with dementia
| Period 1 (Q4 of 2008 to Q1 of 2012) | Period 2 (Q2 of 2012 to Q3 of 2013) | Period 3 (Q4 of 2013 to Q3 of 2018) | ||||||
|---|---|---|---|---|---|---|---|---|
| Medication class | Use, %a |
Slope (95% CI) | Use, % |
Slope (95% CI) | Slope change (95% CI) | Use, % |
Slope (95% CI) | Slope change (95% CI) |
| Antipsychotic | 33.7% | 0.06% (−0.03%, 0.15%) | 32.3% | −0.15% (−0.52%, 0.21%) | −0.21% (−0.60%, 0.18%) | 31.4% | −0.13% (−0.20%, −0.06%)*** | 0.03% (−0.31%, 0.37%) |
| Antiepileptic | 26.8% | 0.11% (0.02%, 0.20%) * | 31.4% | 0.38% (0.11%, 0.66%) ** | 0.28% (0.02%, 0.53%) * | 34.1% | 0.39% (0.25%, 0.54%) *** | 0.01% (−0.21%, 0.23%) |
| Antidepressant | 56.8% | 0.05% (−0.02%, 0.12%) | 58.4% | 0.22% (−0.00%, 0.44%) | 0.17% (−0.07%, 0.40%) | 58.8% | 0.07% (0.00%, 0.14%) * | −0.15% (−0.39%, 0.10%) |
| Anxiolytic | 33.5% | −0.06% (−0.16%, 0.04%) | 32.7% | 0.05% (−0.25%, 0.35%) | 0.11% (−0.20%, 0.42%) | 33.0% | −0.41% (−0.59%, −0.23%) *** | −0.46% (−0.78%, −0.14%) ** |
| Non-antipsychotic psychotropic | 75.0% | 0.06% (−0.03%, 0.14%) | 77.2% | 0.06% (−0.20%, 0.32%) | 0.00% (−0.28%, 0.29%) | 77.8% | 0.11% (0.04%, 0.17%) ** | 0.05% (−0.24%, 0.33%) |
| Opioids | 32.6% | 0.19% (0.05%, 0.33%) ** | 39.3% | 0.63% (0.20%, 1.06%) ** | 0.44% (0.03%, 0.85%) * | 48.0% | −0.26% (−0.36%, −0.17%) *** | −0.89% (−1.37%, −0.41%) *** |
| Memory Medications | 32.4% | −0.33 (−0.52%, −0.14%) ** | 25.5% | −0.17% (−0.48%, 0.13%) | 0.16% (−0.13%, 0.44%) | 22.8% | −0.05% (−0.11%, 0.00%) | 0.12% (−0.17%, 0.41%) |
| CNS Polypharmacy | 32.1% | 0.17 (0.04%, 0.30%) ** | 39.4% | 0.52% (0.18%, 0.87%) ** | 0.35% (0.01%, 0.69%) * | 44.6% | −0.12% (−0.18%, −0.06%) *** | −0.64% (−1.00%, −0.29%) *** |
Use reflects the first quarter of each period of interest. For example, in the first quarter of Period 1, 33.7% of long-stay veterans with dementia were prescribed an antipsychotic, and the estimated quarterly rate of change (i.e., slope) was 0.06 percentage points (95% CI, −0.03 to 0.15 percentage points); by the first quarter of Period 2, the prevalence of antipsychotic use was 32.3%, and during Period 2 the estimated quarterly rate of change was −0.15 percentage points (95% CI −0.52 to 0.21 percentage points). From Period 1 to Period 2, the quarterly rate of change decreased by −0.21 percentage points (95% CI −0.60 to 0.18 percentage points).
<0.05
<0.01
<0.001
Antipsychotics.
At the start of the study (period 1) in FY2009, 33.7% of Veterans had an antipsychotic medication prescription. By the end of the study (period 3), antipsychotic prescribing had decreased to 27.5%, representing an 6.2% absolute change from FY2009 to FY2018. (Figure 1a, eTable 3). The rate of antipsychotic prescribing decreased across all study periods, but only significantly declined following the start of VA PDSI (slope = −0.13, p<0.001).
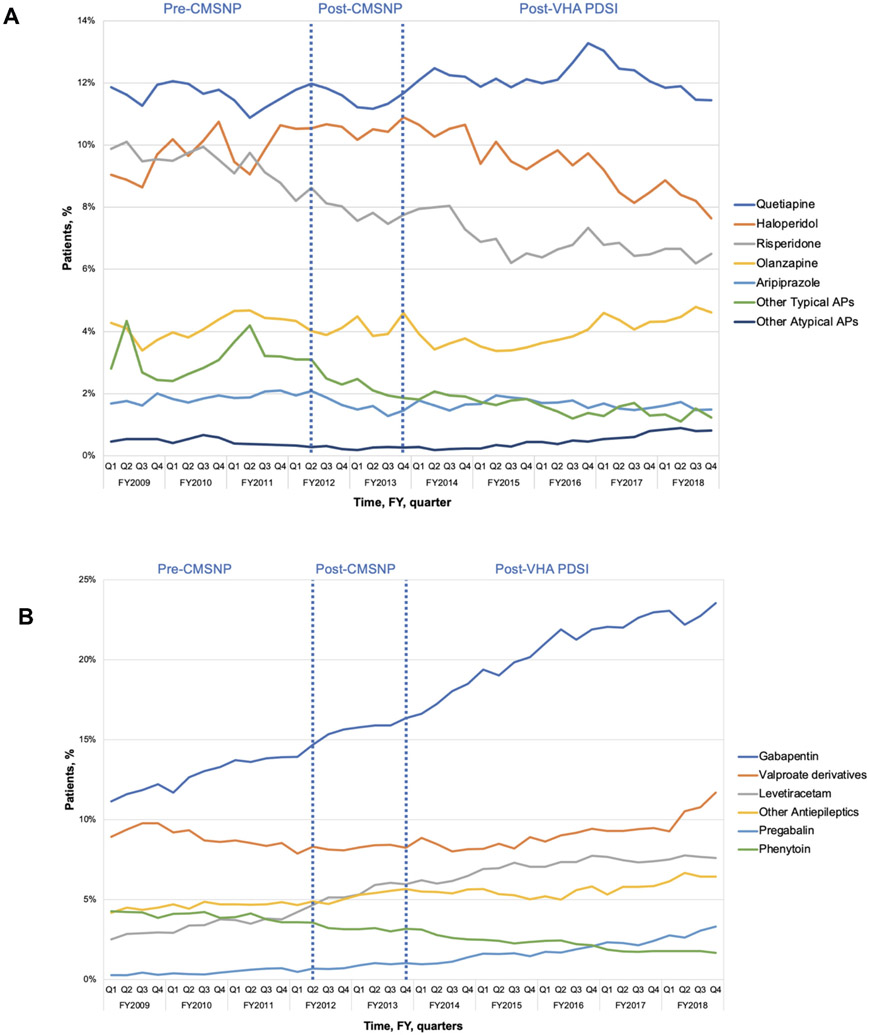
Quetiapine was the most commonly prescribed antipsychotic in all quarters, followed by haloperidol, risperidone, and olanzapine (Figure 2a). Prescribing of most antipsychotic agents remained stable, with the largest declines observed for risperidone (decline from 9.9% to 6.5%) and haloperidol (decline from 9.0% to 7.6%).
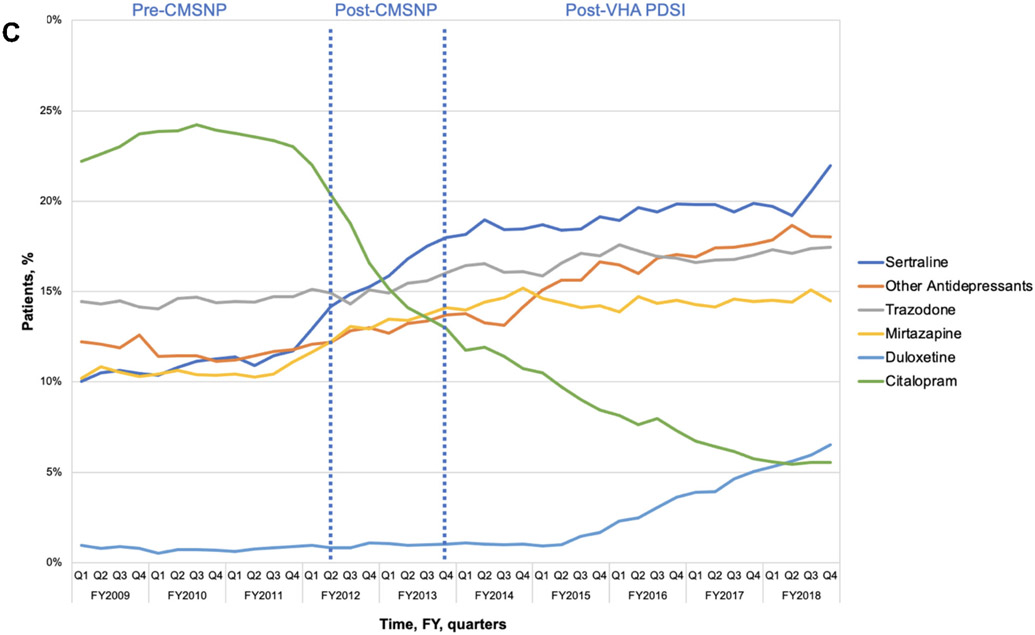
Figure 2A-C.
The most common antipsychotics (A), antiepileptics (B), and antidepressants (C) prescribed to long-stay community living center Veterans with dementia, FY2009-2018
Antiepileptics.
Among non-antipsychotic medications, antiepileptics showed the greatest growth in prescribing over the study period (Figure 1a). Among Veterans with dementia, antiepileptic use increased from 26.8% at the beginning of the study to 43.3% by the end of period 3, representing an 16.5% absolute change. Antiepileptic prescribing increased significantly during period 1 (i.e., before the start of the CMSNP; slope = 0.11, p=0.02) and continued to increase following the start of the CMSNP (period 2; slope change = 0.28, p=0.03). Following the start of VHA PDSI, antiepileptic prescribing continued to increase at a similar rate.
Prescribing of gabapentin, valproate derivatives, and levetiracetam had the largest increases during the study period (Figure 2b). Gabapentin was the most prescribed antiepileptic and prescribing grew from 11.1% to 23.5% during the study period.
Antidepressants.
Antidepressants were the most commonly prescribed CNS-active medication class at all study time points (Figure 1b). Overall use increased from 56.8% at the start of period 1 to 63.4% at the end of study period 3 (6.6% increase). The rate of antidepressant growth was highest in period 3 after the start of VHA PDSI (slope = 0.07, p=0.04; Table 2). Among antidepressant users, sertraline was most commonly prescribed by the post-VHA PDSI period (Figure 2c). Use of sedating antidepressants such as mirtazapine (increase from 10.2% to 14.5%) and trazodone (increase from 14.4% to 17.5%) increased, while citalopram declined markedly.
Anxiolytics.
Anxiolytic (including benzodiazepine) prescribing declined throughout the study period, decreasing from 33.5% at the start of period 1 to 27.1% at the end of the study. The greatest decline in anxiolytic prescribing was observed in period 3 after the start of VHA PDSI (slope change = −0.46, p=0.006).
Opioids.
Prescription of opioid use appeared common among long-stay Veterans with dementia and increased significantly during period 1 (increase from 32.6% to 39.1%; slope = 0.19, p=0.008) and period 2 (increase from 39.3% to 46.0%; slope change = 0.44, p=0.04). Following the start of period 3 (VHA PSDI), opioid prescribing began to decrease significantly from 48.0% to 41.2% at the end of the study (slope change = −0.89, p<0.001).
Memory Medications.
Prescribing of memory medications declined throughout the study from 32.4% at the start of period 1 to 21.8% at the end of period 3, representing 10.6% absolute decline. Memory medications were the least prescribed medication class to Veterans with dementia throughout the study time period.
Non-Antipsychotic Psychotropic Prescribing Overall and CNS-Active Polypharmacy.
The prescribing of non-antipsychotic psychotropic medication overall (i.e., antiepileptics, antidepressants, and anxiolytics) increased in period 1, steadied following the start of CMSNP in period 2, and increased significantly in period 3 following VHA PDSI (Figure 1b; Table 2). 81.1% of long-stay Veterans were prescribed a non-antipsychotic psychotropic at the end of the study period (increased from 75.0% at the start of period 1). Prescribing of non-antipsychotic CNS-active polypharmacy (i.e., ≥3 non-antipsychotic prescriptions including antidepressants, antiepileptics, anxiolytics, and opioids) demonstrated increased prescribing during the first and second period but declined in the third period following VHA PDSI (eFigure 2; Table 2). Prescribing of CNS-active polypharmacy overall increased from 32.1% at the start of period 1 to 41.3% at the end of period 3, representing an absolute increase of 9.2 percentage points.
DISCUSSION
This study provides national trends in CNS-active medication prescribing among Veterans with dementia in VHA CLCs. Our study found that antipsychotic use among Veterans with dementia declined over time, however, most significantly following VHA PDSI. Antidepressant prescribing increased following VHA PDSI while antiepileptic prescribing increased following both CMSNP and VHA PDSI. Overall prescribing of non-antipsychotic psychotropic medications in VHA CLCs remained high, with over 80% of Veterans with dementia prescribed a non-antipsychotic psychotropic medication at the end of the study period. Gabapentin, valproate derivatives, and sedative antidepressants had the largest contributions to growth in CNS-active medication prescribing. Anxiolytic prescribing declined following VHA PDSI whereas memory medication prescribing declined across all study periods and had a lower prescribing rate than any of the other medication classes observed. Lastly, opioid prescribing remained common and increasing prior to and during the CMSNP, only decreasing following the start of VHA PDSI.
Our findings in VHA are consistent with prior studies in non-VHA settings (one in the community, two in nursing homes),2,18,24 which found that initiatives focused on reducing antipsychotic use may have contributed to providers shifting to alternative medication classes with even less evidence of benefit and similar risks. A Canadian study showed that decreased antipsychotic use in nursing homes between 2004-2013 was offset by increased sedative antidepressant and antiepileptic use.24 A recent study in the U.S. examining Medicare beneficiaries found that antiepileptic use increased and accelerated after the initiation of CMSNP among nursing home residents.18 It appears that these trends also extend to the VHA CLC population as well, where even higher rates of antiepileptic prescribing occurred compared to care outside of the VHA.
When evaluating change in specific medications, quetiapine was the most commonly prescribed antipsychotic among patients with dementia, consistent with findings from community long-term care populations.25 While quetiapine has been found to have a lower mortality risk in dementia,26 studies also suggest it may have less evidence of benefit in reducing BPSD.27 Increases in antidepressant use were largely driven by prescribing of sertraline and sedating antidepressants (e.g., trazodone and mirtazapine), which may be used for treatment of depression or BPSD. Notable decreases in citalopram were observed following the 2011 U.S. FDA drug safety warning regarding concerns for QT prolongation with high dose citalopram use as has been reported in other studies.28,29
The growth of antiepileptic use was largely driven by gabapentin, for which prescribing doubled during the study period, likely off-label for pain, anxiety, or BPSD, rather than for seizure disorders.25 Significant increases in prescription opioid use were observed before and during the CMSNP. This is consistent with studies demonstrating high rates of opioid prescribing among patients with dementia in the community2 as well as population-based studies demonstrating significant growth in opioid use among patients with dementia.3 Such treatment may reflect improved recognition and treatment of pain in dementia or palliative approaches to dementia end-of-life care, however, this may also reflect another attempt to reduce BPSD.3,30 In addition to measures of the percent of long-stay residents with dementia prescribed an antipsychotic, VHA CLCs also have a publicly-reported quality measure of percent of long-stay CLC residents who self-report moderate or severe pain,16 further incentivizing treatment of pain.
A recent international panel of geriatric psychiatry and dementia care experts noted that antipsychotics are the most effective pharmacotherapy for BPSD including psychosis, agitation, and aggression.31 Studies reviewing the use of other medication classes such as antidepressants, benzodiazepines, antiepileptics, and opioids for treatment of BPSD show minimal benefit in reducing such symptoms.5,30,32 Additionally, these alternative medication classes substituted for antipsychotics can yield significant risks. Benzodiazepines can contribute to falls, worsened cognition, respiratory depression, and paradoxical disinhibition,33 whereas antiepileptics such as valproate derivatives can cause gait disturbances, tremor, cognitive changes and mortality.26,34 Gabapentin use can lead to ataxia, dizziness, drowsiness, fatigue, falls, and increased risk of respiratory suppression when combined with opioids.35 Reductions in prescribing of memory medications may reflect provider concerns about limited therapeutic benefit for patients with advanced dementia as well as concerns regarding side effects including nausea, loss of appetite, and diarrhea.36
The VHA may have achieved declines in several classes of CNS-active medications due to its multi-pronged efforts. In contrast with CMSNP, which focused on antipsychotic use only, VHA PDSI, in addition to an antipsychotic prescribing measure, another PDSI measure addresses benzodiazepine use, which may explain the observed anxiolytic reductions in the VHA. Further, VHA initiatives including the 2013 Opioid Safety Initiative (OSI) may explain reductions in opioid prescribing as well increased recognition about the dangers of opioid prescribing among patients with dementia.37 Additionally, in 2010, VHA launched an initiative to adapt an evidenced-based behavioral approach (Staff Training in Assisted Living Residences, or STAR38) for VHA to increase uptake of non-pharmacological strategies for BPSD. We do not know whether CLCs with STAR implementation decreased antipsychotic use, and if so, whether the decrease reflected increased use of non-pharmacologic strategies.39 Reports from long-term care staff outside of the VHA suggest considerable challenges in implementing such interventions given staff turnover as well as time and resources needed to implement non-pharmacologic strategies.40
Our analysis has several limitations. First, our results may not be directly comparable to prescribing in community nursing homes given the differences in the structure of VHA CLCs, including the presence of a national formulary and range of psychosocial services available to Veterans.15 Next, our sample includes a high proportion of men, and our study results may not generalize to other clinical populations. Third, prescriptions filled from pharmacy data can be an imprecise measure of actual drug exposure; medication fills may not reflect day-to-day use. However, this study focused on the impact of initiatives that influence prescribing rather than drug use. Next, we do not know the indication for medication prescribing (e.g., gabapentin could be prescribed for pain, seizures, or BPSD) or the appropriateness of such prescribing. However, the potential for medication related harms remains regardless of clinical indication. Further, prescribing trends may reflect changes in patient characteristics over time; however, our approach is consistent with CMSNP and VHA PDSI, neither of which case-mix adjust. Lastly, administrative data do not include reliable measures of non-pharmacological interventions, limiting our ability to examine if increased use of such interventions was associated with declines in antipsychotic prescribing.
CONCLUSIONS AND IMPLICATIONS
Although antipsychotic prescribing to Veterans with dementia in VHA nursing homes declined following both CMSNP and PDSI, concomitant increases in antiepileptic, antidepressant, and opioid prescribing occurred. Within the VHA, rates of anxiolytic and opioid prescribing to Veterans with dementia declined following PDSI, but the rate of any non-antipsychotic psychotropic use overall increased. Initiatives focused on improving quality of care for nursing home residents with BPSD both in the VHA and community should 1) monitor use of all CNS-active medication and other potentially sedating treatments used for sedation in dementia; and 2) consider how to incentivize and measure use of recommended evidence-based non-pharmacologic alternatives.6,7 The latter link to policy changes to stimulate a reorganization of dementia care, where providers are compensated for time spent in elucidating and addressing modifiable triggers to BPSD, and not perversely incentivized to utilize other medications with even less evidence for benefit in dementia.
Supplementary Material
eFigure 1. Study flow diagram
eFigure 2. CNS-active polypharmacy prescribing among long-stay community living center Veterans with dementia, FY2009-2018
eTable 1. International Classification of Disease, Ninth and Tenth Revision codes for dementia
eTable 2. List of medication classes
eTable 3. Percent of long-stay CLC Veterans with dementia prescribed antipsychotics or other medication classes (used for Figure 1)
Funding Source:
This work was supported by a VA Health Services Research and Development (VA IIR 15-330; Zivin). Dr. Gerlach was also support, in part, by grant K23AG066864 from the National Institute on Aging.
Role of the Funder/Sponsor:
The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
No related papers have been published or submitted from this study.
Conflict of Interest Disclosures: The authors have none to disclose.
REFERENCES
- 1.Maust DT, Langa KM, Blow FC, Kales HC. Psychotropic use and associated neuropsychiatric symptoms among patients with dementia in the USA. International Journal of Geriatric Psychiatry. 2017;32(2):164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maust DT, Strominger J, Bynum JPW, et al. Prevalence of Psychotropic and Opioid Prescription Fills Among Community-Dwelling Older Adults With Dementia in the US. JAMA. 2020;324(7):706–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jensen-Dahm C, Christensen AN, Gasse C, Waldemar G. The Use of Opioids and Antipsychotics in Elderly with Dementia - Have Opioids Replaced Antipsychotics in Treating Behavioral Symptoms in Dementia? J Alzheimers Dis. 2020;73(1):259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.U.S. Food and Drug Administration. Public Health Advisory: Deaths with antipsychotics in elderly patients with behavioral disturbances. Published April 11, 2005. Accessed April 1, 2021. Available at: http://psychrights.org/drugs/FDAantipsychotics4elderlywarning.htm.
- 5.Gerlach LB, Kales HC. Pharmacological Management of Neuropsychiatric Symptoms of Dementia. Current Treatment Options in Psychiatry. 2020;7(4):489–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. BMJ. 2015;350:h369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Geriatrics Society. American Association of Geriatric Psychiatry. Consensus statement on improving the quality of mental health care in U.S. nursing homes: management of depression and behavioral symptoms associated with dementia. Journal of the American Geriatrics Society. 2003;51(9):1287–98. [DOI] [PubMed] [Google Scholar]
- 8.Wisely Choosing. Five things physicians and patients should question. Arlington (VA): American Psychiatric Association; 2013. Available at: http://www.choosingwisely.org/societies/american-psychiatricassociation. Accessed June 15, 2020. [Google Scholar]
- 9.Chen Y, Briesacher BA, Field TS, Tjia J, Lau DT, Gurwitz JH. Unexplained variation across US nursing homes in antipsychotic prescribing rates. Archives of Internal Medicine. 2010;170(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng Z, Hirdes JP, Smith TF, et al. Use of physical restraints and antipsychotic medications in nursing homes: a cross-national study. International Journal of Geriatric Psychiatry. 2009;24(10):1110–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamble P, Chen H, Sherer J, Aparasu RR. Antipsychotic drug use among elderly nursing home residents in the United States. The American Journal of Geriatric Pharmacotherapy. 2008;6(4):187–197. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Medicare and Medicaid Services. CMS announces partnership to improve dementia care. Published May 30, 2012. Accessed on May 1, 2020. Available at: https://www.cms.gov/newsroom/press-releases/cms-announces-partnership-improve-dementia-care-nursing-homes.
- 13.Office of Government Accountability. Antipsychotic Drug Use: HHS Has Initiatives to Reduce Use among Older Adults in Nursing Homes, but Should Expand Efforts to Other Settings. Published January 2015. Accessed on September 15, 2020. Available at: https://www.gao.gov/assets/gao-15-211.pdf.
- 14.Centers for Medicare and Medicaid Services. Five Star Rating System. Published October 7, 2019. Accessed on March 11, 2019. Available at: https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/CertificationandComplianc/FSQRS.html.
- 15.Thomas KS, Cote D, Makineni R, et al. Change in VA Community Living Centers 2004-2011: Shifting Long-Term Care to the Community. J Aging Soc Policy. 2018;30(2):93–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.U.S. Department of Veterans Affairs. Quality of Care: Community Living Center Health Surveys. Published September 15, 2015. Accessed on February 2, 2021. Available at: https://www.va.gov/QUALITYOFCARE/apps/aspire/clcsurvey.aspx.
- 17.Rubin HR, Pronovost P, Diette GB. Methodology Matters. From a process of care to a measure: the development and testing of a quality indicator. International Journal for Quality in Health Care. 2001;13(6):489–496. [DOI] [PubMed] [Google Scholar]
- 18.Maust DT, Kim HM, Chiang C, Kales HC. Association of the Centers for Medicare & Medicaid Services' National Partnership to Improve Dementia Care With the Use of Antipsychotics and Other Psychotropics in Long-term Care in the United States From 2009 to 2014. JAMA Internal Medicine. 2018;178(5):640–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yun H, Kilgore ML, Curtis JR, et al. Identifying types of nursing facility stays using medicare claims data: an algorithm and validation. Health Services & Outcomes Research Methodology. 2010;10(1-2):100–110. [Google Scholar]
- 20.Taylor DH Jr., Fillenbaum GG, Ezell ME. The accuracy of medicare claims data in identifying Alzheimer's disease. Journal of Clinical Epidemiology. 2002;55(9):929–937. [DOI] [PubMed] [Google Scholar]
- 21.Maust DT, Kim HM, Wiechers IR, et al. Benzodiazepine Use among Medicare, Commercially Insured, and Veteran Older Adults, 2013-2017. J Am Geriatr Soc. 2021;69(1):98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ilgen MA, Kleinberg F, Ignacio RV, et al. Noncancer pain conditions and risk of suicide. JAMA Psychiatry. 2013;70(7):692–697. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Medicare and Medicaid Services. MDS 3.0 Quality Measures User’s Manual. Pulbished October 19, 2020. Accessed May 1, 2021. Available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/NursingHomeQualityInits/NHQIQualityMeasures.
- 24.Vasudeva SS, Kuan L, Burhan AM, Herrmann N, Leonard S, Mamdani M. Trends in psychotropic dispensing among older adults with dementia living in long-term care facilities: 2004-2013. American Journal of Geriatric Psychiatry. 2015;23(12):1259–1269. [DOI] [PubMed] [Google Scholar]
- 25.Gerlach LB, Kales HC, Kim HM, et al. Trends in Antipsychotic and Mood Stabilizer Prescribing in Long-Term Care in the U.S.: 2011-2014. Journal of the American Medical Directors Association. 2020;21(11):1629–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kales HC, Kim HM, Zivin K, et al. Risk of mortality among individual antipsychotics in patients with dementia. American Journal of Psychiatry. 2012;169(1):71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huybrechts KF, Gerhard T, Crystal S, et al. Differential risk of death in older residents in nursing homes prescribed specific antipsychotic drugs: population based cohort study. BMJ. 2012;344:e977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerlach LB, Kales HC, Maust DT, et al. Unintended Consequences of Adjusting Citalopram Prescriptions Following the 2011 FDA Warning. American Journal of Geriatric Psychiatry. 2017;25(4):407–414. [DOI] [PubMed] [Google Scholar]
- 29.Gerlach LB, Kim HM, Yosef M, et al. Assessing Responsiveness of Health Systems to Drug Safety Warnings. American Journal of Geriatric Psychiatry. 2018;26(4):476–483. [DOI] [PubMed] [Google Scholar]
- 30.Brown R, Howard R, Candy B, Sampson EL. Opioids for agitation in dementia. Cochrane Database of Systematic Reviews. 2015(5):Cd009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kales HC, Lyketsos CG, Miller EM, Ballard C. Management of behavioral and psychological symptoms in people with Alzheimer's disease: an international Delphi consensus. Int Psychogeriatr. 2018;31(1):83–90. [DOI] [PubMed] [Google Scholar]
- 32.Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA. Vol 2932005:596–608. [DOI] [PubMed] [Google Scholar]
- 33.Peisah C, Chan D, McKay R, Kurrle S, Reutens S. Practical guidelines for the acute emergency sedation of the severely agitated older patient. Internal Medicine Journal. 2011;41(9):651–657. [DOI] [PubMed] [Google Scholar]
- 34.Maust DT, Kim HM, Seyfried LS, et al. Antipsychotics, Other Psychotropics, and the Risk of Death in Patients With Dementia: Number Needed to Harm. JAMA Psychiatry. 2015;72(5):438–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.U.S. Food and Drug Administration. FDA warns about serious breathing problems with seizure and nerve pain medicines gabapentin (Neurontin, Gralise, Horizant) and pregabalin (Lyrica, Lyrica CR) when used with CNS depressants or in patients with lung problems. Published December 19, 2019. Available at: https://www.fda.gov/safety/medical-product-safety-information/neurontin-gralise-horizant-gabapentin-and-lyrica-lyrica-cr-pregabalin-drug-safety-communication. Accessed on January 20, 2020.
- 36.Renn BN, Asghar-Ali AA, Thielke S, et al. A Systematic Review of Practice Guidelines and Recommendations for Discontinuation of Cholinesterase Inhibitors in Dementia. American Journal of Geriatric Psychiatry. 2018;26(2):134–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sandbrink F, Oliva EM, McMullen TL, et al. Opioid Prescribing and Opioid Risk Mitigation Strategies in the Veterans Health Administration. Journal of General Internal Medicine. 2020;35(Suppl 3):927–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teri L, Huda P, Gibbons L, Young H, van Leynseele J. STAR: A Dementia-Specific Training Program for Staff in Assisted Living Residences Nancy Morrow-Howell, MSW, PhD, Editor. Gerontologist. 2005;45(5):686–693. [DOI] [PubMed] [Google Scholar]
- 39.Karel M, Teri L, McConnell E, Visnic S, Karlin B. Effectiveness of expanded implementation of STAR-VA for managing dementia-related behaviors among Veterans. Gerontologist. 2016;56(1):126–134. [DOI] [PubMed] [Google Scholar]
- 40.Simmons SF, Bonnett KR, Hollingsworth E, et al. Reducing Antipsychotic Medication Use in Nursing Homes: A Qualitative Study of Nursing Staff Perceptions. The Gerontologist. 2017;58(4):e239–e250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Study flow diagram
eFigure 2. CNS-active polypharmacy prescribing among long-stay community living center Veterans with dementia, FY2009-2018
eTable 1. International Classification of Disease, Ninth and Tenth Revision codes for dementia
eTable 2. List of medication classes
eTable 3. Percent of long-stay CLC Veterans with dementia prescribed antipsychotics or other medication classes (used for Figure 1)