Abstract
Previous publications have reported a potent effect of COVID‐19 on platelet function and that the Spike protein enhances washed human platelet aggregation induced by various agonists. This study aims to evaluate whether mRNA vaccination for COVID‐19 affects human platelet‐rich plasma (hPRP) aggregation response, whether a recombinant Spike protein modulates PAF‐induced aggregation in hPRP and in washed rabbit platelets (WRP), and to investigate the effect of recombinant Spike protein on the PAF production in the U‐937 cell line. Our results showed that PRP from vaccinated individuals exhibited ex vivo lower EC50 values in response to PAF, ADP, and collagen. Platelet incubation with the Spike protein alone did not induce aggregation either in hPRP or in WRP, but resulted in augmentation of in vitro PAF‐induced aggregation in hPRP from non‐vaccinated individuals and in WRP. When PRP from vaccinated individuals was incubated with the Spike protein and PAF was subsequently added, elimination of the secondary wave of the biphasic aggregation curve was recorded compared with the aggregation induced by PAF alone. Collagen‐induced in vitro aggregation was dose‐dependently reduced when platelets were pre‐incubated with the Spike protein in all tested aggregation experiments. Stimulation of U‐937 by the Spike protein induced an increase in intracellular PAF production accompanied by elevation of the activities of all three PAF biosynthetic enzymes. In conclusion, since the Spike protein appears to modulate PAF production and activity, the use of compounds that act as PAF inhibitors, could be considered at least in mild cases of patients infected with SARS‐CoV‐2.
Keywords: ADP, collagen, coronavirus, human platelet‐rich plasma, monocytes, platelet‐activating factor, platelets, spike protein, thrombin, vaccines
Abbreviations
- AA
arachidonic acid
- ACE2
angiotensin‐converting enzyme 2
- ADP
adenosine diphosphate
- AT1
angiotensin II type 1 receptor
- BSA
bovine serum albumin
- COX
cyclooxygenases
- cPLA2
cytoplasmic phospholipase A2
- hPRP
human platelet‐ rich plasma
- IL‐1β
interleukin 1β
- IL‐8
interleukin 8
- LpPLA2
lipoprotein‐associated phospholipase A2
- Lyso‐PAFAT
acetyl‐CoA
lyso‐PAF acetyltransferase
- MCP‐1
monocyte chemoattractant protein‐1
- PAF
Platelet‐Activating Factor
- PAF‐AH
PAF acetylhydrolase
- PAF‐CPT
CDP‐choline: 1‐alkyl‐2‐acetyl‐sn‐glycerol cholinephosphotransferase
- PDGF
platelet‐derived growth factor
- PF4
platelet factor 4
- PGs
prostaglandins
- PLA2G7
phospholipase A2 group VII
- PPP
platelet‐poor plasma
- PRP
platelet‐ rich plasma
- RBD
receptor‐ binding domain
- SFM
serum‐ free medium
- TXs
thromboxanes
- TLR
Toll‐Like Receptors
- TNF‐α
tumor necrosis factor‐α
- TRAP
thrombin receptor activating peptide
- vWF
von Willebrand Factor
- WRP
washed rabbit platelets
1. INTRODUCTION
The ongoing coronavirus pandemic (COVID‐19) has already infected more than two hundred and seventy‐five million people and caused more than five million three hundred thousand deaths worldwide by December 2021, according to the John Hopkins Coronavirus Research Center.
Many publications report that COVID‐19 presents as a hypercoagulability state with a profound effect on platelet function characterized as a pre‐thrombotic state. 1 , 2 , 3 , 4 , 5 A recent review summarized the current state of knowledge of COVID‐19 and hemostasis. 6 Platelets have a key role in hemostasis and thrombosis and it has been reported that their aggregation is significantly increased in response to ADP, thrombin, and collagen in COVID‐19 patients. 7 Enhanced platelet aggregation was also observed when the Spike protein was added to washed human platelets before agonist‐induced stimulation. 8 However, there are conflicting results regarding the action of the Spike protein in the absence of any other agonist. 8 , 9 Even though, it is documented that the SARS‐CoV‐2 uses angiotensin‐converting enzyme 2 (ACE2) as its cellular receptor via the interaction of the Spike protein receptor‐binding domain (RBD), there is still controversy whether human platelets express ACE2. 7 , 8 On the other hand, human platelets express a spectrum of receptors that can recognize viruses such as toll‐like receptors (TLR) and it has been documented that the Spike protein binds TLR4 with high affinity leading to cell activation. 10 The Spike protein also binds to CD147, a co‐receptor involved in SARS‐CoV‐2 infection of epithelial cells 11 as well as the CD42b receptor, resulting in competitively antagonizing von Willebrand Factor (vWF). 9 Apart from platelet aggregation, SARS‐CoV‐2 and its Spike protein stimulate secretion of biologically active substances including platelet factor 4 (PF4), tumor necrosis factor‐α (TNF‐α), interleukin 8 (IL‐8), and interleukin 1β (IL‐1β) from platelets. 8
Platelet‐activating factor (PAF), chemically characterized as 1‐O‐alkyl‐2‐acetyl‐sn‐glycerol‐3‐phosphocholine, 12 is a potent phospholipid mediator produced by various cells including platelets under basal conditions or under appropriate stimuli and exerts pleiotropic effects. 13 PAF activates platelets resulting in their aggregation at concentrations as low as 10−12 M, in secretion of bioactive molecules from their granules, such as serotonin, PF4, thromboxane A2, and β‐thromboglobulin, as well as PAF itself resulting in further platelet activation. 14 , 15 , 16 PAF exerts its autocrine and paracrine actions through binding to its receptor (PAF‐R), a G‐protein‐coupled receptor that is expressed in plasma and nuclear membranes of many cell types. 13 Recent publications discussed PAF in the context of COVID‐19. 17 , 18 , 19 , 20 , 21
PAF levels in cells, tissues, and biological fluids are mainly controlled via its two biosynthetic pathways, namely, the remodeling and the de novo pathway, as well as the catabolic pathway. 13 In brief, the remodeling pathway begins with the action of cytoplasmic phospholipase A2 (cPLA2) on the existing membrane ether‐linked choline‐containing phospholipids resulting in the formation of lyso‐PAF which is then acetylated by acetyl‐CoA:lyso–platelet‐activating factor acetyltransferases (Lyso‐PAF AT) to generate PAF. Two isoforms of Lyso‐PAF AT are known; one of them is activated under inflammatory conditions, whereas the other is calcium‐independent and does not seem to be implicated in inflammatory processes. 22 It should be noted that by the action of the cPLA2, the most predominant polyunsaturated fatty acid, arachidonic acid (AA), is released and consequently enzymatically converted into a variety of eicosanoid mediators. Indeed, a recent untargeted metabolomic and lipidomic study showed the abundance of oleic acid and AA, and the upregulation of lyso‐phosphatidylcholines, both indicating that phospholipase A2 is activated in COVID‐19. 23 The de novo pathway is responsible for the constitutive production of PAF with the crucial enzyme, 1‐alkyl‐2‐acetyl‐sn‐glycerol cholinephosphotransferase (PAF‐CPT), catalyzing the synthesis of PAF from 1‐O‐alkyl‐2‐acetyl‐glycerol by adding phosphocholine. 24 PAF catabolism is performed by an intracellular PAF‐specific acetylhydrolase (PAF‐AH) and its plasma isoform lipoprotein‐associated phospholipase A2 (LpPLA2). 25 A recent study reported elevated levels of LpPLA2 in patients with COVID‐19 and also that the gene expression of phospholipase A2 group VII (PLA2G7) was correlated with the severity of COVID‐19. 26
Therefore, the aim of the present work is to examine the effect of the Spike protein on platelet aggregation, in human platelet‐rich plasma (hPRP) obtained from healthy vaccinated and non‐vaccinated volunteers, as well as in washed rabbit platelets (WRP). We also investigated the effect of the Spike protein on the production of PAF in the U‐937 promonocytic cell line.
2. MATERIALS AND METHODS
2.1. Materials
Bovine serum albumin Fraction V (FV‐BSA), PAF, ADP, and thrombin receptor activating peptide (TRAP) were obtained from Sigma‐Aldrich (St. Louis, MO) and collagen was obtained from Chrono‐Log (Havertown, PA). Recombinant HEK293‐derived SARS‐CoV‐2 Spike RBD protein (10500‐CV) was obtained from Bio‐Techne/R&D Systems (Minneapolis, MN) and was reconstituted in FV‐BSA. RPMI 1640 was purchased from Gibco BRL (Thermo Fischer Scientific, Waltham, MA) and newborn calf serum (NCS), glutamine, penicillin, Coomassie Brilliant Blue G‐250, other common reagents and all solvents were all obtained from Sigma (St. Louis, MO). Lyso‐PAF, free fatty acid low endotoxin bovine serum albumin (FFA‐BSA), acetyl‐coenzyme A (Acetyl‐CoA), cytidine 5′‐diphosphocholine (CDP‐choline), were obtained from Sigma (St. Louis, MO). 1‐O‐Hexadecyl‐2‐O‐acetyl‐sn‐glycerol (AAG) was purchased from Enzo Life Sciences Ltd (Farmingdale, NY).
2.2. Participants
Blood samples were obtained after overnight fasting from six healthy female volunteers before vaccination, three weeks and approximately five months after the second dose of the mRNA technology vaccines (n = 2 with Moderna and n = 4 with Pfizer/BioNTech). The experiments were performed after the second dose of the vaccine to achieve the full activation of the immune system. In addition, the short interval (3–4 weeks) between the two doses of mRNA vaccination accompanied by the possible use of medications such as painkillers with anti‐inflammatory action would affect the platelet activity. Volunteers aged 35–55 years old had not taken any medications known to affect platelet function at least 2.5 weeks before blood collection. They also gave their informed consent and the University Ethics Committee approved the experimental protocol.
2.3. Ex vivo and in vitro assessment of hPRP aggregation
Blood was collected in trisodium citrate as anticoagulant, and platelet‐rich plasma (PRP) was obtained by centrifugation at 140 × g without brake for 16 min. The pellet was recentrifuged at 1500 × g with brake for 15 min, to obtain platelet‐poor plasma (PPP). Platelet count of PRP was adjusted to 500.000 per μL with the addition of PPP. The aggregation, induced by various concentrations of PAF, ADP, collagen, and TRAP, was determined in human PRP by light transmission aggregometry in a Chrono‐Log (Havertown, PA) aggregometer (model 490‐4D) with four channels at 37°C with stirring at 1200 rpm. The height of the aggregation curve of minimum‐irreversible platelet aggregation of human PRP induced by an agonist was determined as the 100% aggregation. Consequently, the linear part of per cent aggregation (ranging from 20% to 80%) versus different concentrations of the agonist was constructed and the concentration of a specific agonist needed to induce 50% of platelet aggregation (EC50 or half maximal effective concentration) was calculated from the aggregation curve. The EC50 values were expressed in nM for PAF, μM for ADP and TRAP and in μg/ml for collagen.
To explore the effects of the Spike protein on agonists‐induced platelet aggregation, platelets were pre‐incubated for 1–10 min in the presence of the Spike protein (2–20 μg/ml) and various concentrations of platelet agonists were consequently added. In some experiments, the Spike protein (2–20 μg/ml) was added during agonist‐induced aggregation or before the secondary wave of agonist‐induced biphasic platelet aggregation. The experiments with the Spike protein were performed six times in hPRP obtained from three vaccinated as well as from three non‐vaccinated individuals.
2.4. In vitro assessment of washed rabbit platelets aggregation
Blood was collected from the main ear artery in polyethylene tubes containing anticoagulant (blood/anticoagulant 9:1 v/v) from white male California type rabbits, and platelets were isolated and washed by a previously described method. 27 The washed platelets were adjusted to 1.25 × 108 platelets/ml of Tyrode's buffer pH 7.2 and the aggregation, induced by various agonists was performed as described in the hPRP aggregation. The experiment was performed twice.
2.5. Culture and activation of the U‐937 cell line
U‐937 cells were cultured and maintained as previously described 28 and were rested in serum‐free medium (SFM) for 24 h before use. The final cell concentration for the experiments was 1.25 × 106 cells/ml and cells were incubated in the absence and presence of the Spike protein. The Spike protein was reconstituted in sterile phosphate‐buffered saline (PBS) and was added in cell cultures in final concentrations of 10 ng/ml and 100 ng/ml. After incubation with the Spike protein for 30 min, 3 h or 24 h, both cells and supernatant fluids were collected and centrifuged at room temperature at 500 × g for 10 min. The supernatant fluid was collected and treated with the Bligh‐Dyer method 29 to obtain the extracellular PAF (ePAF). The pellet was washed with 1 ml PBS and centrifuged as above; the supernatant was discarded and only the washed cells were kept. The pellet was reconstituted in 1 ml Tris–HCl 50 mM pH 7.4. Intracellular PAF (iPAF) was recovered by following the Bligh‐Dyer method in 360 μl of the cell suspension. Another 30 μl were stored in −20°C for protein determination by the Bradford method. 30 The rest of the cell suspension was homogenized by sonication. The homogenate was centrifuged at 4°C at 500 × g for 10 min. The supernatant fluids were kept at −80°C till the day of the enzyme activity determination. Both ePAF and iPAF were stored at 4°C until the HPLC analysis. The cytotoxicity of the Spike protein was tested in all concentrations using the 3‐(4,5‐dimethylthiazol‐2‐yl‐)‐5‐diphenyltetrazoliumbromide (MTT) assay according to the Mosmann method. 31 All assays were performed in triplicate.
The U‐937 human monocytes cell line was used in all cell experiments (kindly donated by Dr. Z. Varghese, Royal Free Hospital, Centre for Nephrology, University Collage Medical School, London, United Kingdom). Centrifugations were performed in a refrigerated Thermo Scientific SL16R centrifuge (Thermo Fischer Scientific, Waltham, MA). Homogenizations were carried out with an ultrasonic homogenizer, namely Sonoplus HD2070, carrying a MS73 sonication probe, all provided from Bandelin (Berlin, Germany).
2.6. Determination of PAF
The extracts obtained with the Bligh‐Dyer method, ePAF and iPAF, were subjected to HPLC separation according to the method of Demopoulos et al. 32 Briefly, the elution system was an isocratic solution of acetonitrile:methanol:water 61:35:4 (v/v/v) and a polar lipids standard was used to detect the time range where PAF is eluted. The purification of PAF by HPLC was performed at room temperature on an Agilent HPLC 1100 Series liquid chromatography model (Santa Clara, CA) equipped with a cation exchange column, namely, Partisil 10SCX 250 × 4.6 mm, from HiChrom Ltd (Reading, UK), a 100 μl Rheodyne (7725i) loop valve injector, a degasser G1322A, a quad gradient pump G1311A and a HP UV spectrophotometer G1314A as a detection system. The spectrophotometer was connected to a personal computer and Agilent ChemStation Rev.A.10.02 was used to control the HPLC system.
PAF levels were finally determined by measuring the aggregatory activity toward WRP. The quantification of PAF was based on a standard curve constructed with the use of known concentrations of synthetic PAF. The results are expressed as fmol PAF/μg of total protein.
2.7. Determination of PAF biosynthesis enzymes activity
PAF biosynthetic enzyme activity was based on the quantification of the produced PAF as described above except that the final step of PAF determination was performed with liquid chromatography‐mass spectrometry (LC–MS) as previously described. 33 Specifically, two isoforms of Lyso‐PAF AT were determined, 33 one of them is activated under inflammatory conditions (Lyso‐PAF ATC) and the assay was performed in the presence of 2.8 mM CaCl2, while the other one is calcium independent (Lyso‐PAF ATE), and the assay was performed in the presence of 1.4 mM EDTA. Also, the determination of PAF‐CPT activity was performed. 33 Protein determination was performed by the Bradford method. 30 All assays were performed in duplicate. The enzyme activities were expressed in pmol PAF /μg of total protein/min.
PAF‐18:0‐d4 (Cayman Chemical, Ann Arbor, MI) was used as an internal standard. LC–MS was performed using an Exactive™ Plus Orbitrap Mass Spectrometer with a Hypersil GOLD™ column (5 μm, 150 × 4.6 mm), all obtained from Thermo Fischer Scientific (Waltham, MA).
2.8. Statistical methods
Normality of values was tested with the Shapiro–Wilk criterion. Normally distributed continuous variables were presented as means ± SD. T‐test was applied for the comparisons while the paired t‐test was performed for testing the vaccination effect in EC50 values. All statistical analyses were performed using STATA version 15 statistical software (STATA Corp., Texas). The significance level was set to 0.05 for all tests.
3. RESULTS
3.1. Vaccination using mRNA technology enhances ex vivo hPRP aggregation
The effect of the vaccination on hPRP aggregation was agonist‐dependent (Table 1). The results show that 3 weeks after the vaccination the EC50 PAF values were dramatically lower by 83% (7.3 ± 1.3 nM versus 44.2 ± 14.8 nM before vaccination, p = 0.0001), the EC50 ADP values were significantly decreased by 29% (2.8 ± 0.3 μM versus 4.0 ± 0.5 μM, p = 0.0001) and the EC50 Collagen values were also decreased by 20% (0.47 ± 0.15 μg/ml versus 0.59 ± 0.15 μg/ml, p = 0.002). The EC50 values of all agonists returned to their initial values after 5 months (Table 1). The hPRP aggregation induced by TRAP was not affected by the vaccination.
TABLE 1.
EC50 values of hPRP aggregation before, three weeks and approximately five months after the second dose of the mRNA technology vaccines
| t0 before vaccination (N = 6) | Three weeks after the second dose (N = 6) | Five months after the second dose (N = 6) | pt0−3w | pt0−5m | |
|---|---|---|---|---|---|
| EC50 PAF (nΜ) | 44.2 ± 14.8 | 7.3 ± 1.3 | 54.7 ± 17.6 | 0.0001 | 0.11 |
| EC50 ADP (μΜ) | 4.0 ± 0.5 | 2.8 ± 0.3 | 3.8 ± 0.4 | 0.0001 | 0.08 |
| EC50 TRAP (μΜ) | 0.91 ± 0.15 | 0.95 ± 0.18 | 1.00 ± 0.17 | 0.24 | 0.15 |
| EC50 Collagen (μg/ml) | 0.59 ± 0.15 | 0.47 ± 0.15 | 0.62 ± 0.13 | 0.002 | 0.07 |
Note: Data are presented as means ± SD. Comparison to baseline values was performed by paired t‐test. Values in bold represent a statistically significant p‐value.
Abbreviations: EC50, Half maximal effective concentration; PAF, Platelet‐activating factor; TRAP, Thrombin receptor activating peptide.
3.2. SARS‐CoV‐2 spike protein decreases in vitro PAF‐ and collagen‐induced aggregation of hPRP from vaccinated individuals
Incubation with the Spike protein (final concentrations, 2 and 20 μg/ml) for 10 min did not induce hPRP aggregation.
When hPRP was pre‐incubated for 1 min with the Spike protein (2 μg/ml) and various concentrations of PAF that induce biphasic aggregation (final concentrations, 6–20 × 10−9 M) were added, a dose‐dependent decrease of aggregation was observed ranging from 50%–90%. When the Spike protein (2 μg/ml) was added just before the second phase of PAF‐induced biphasic aggregation, the secondary wave was completely eliminated (Figure 1A). When the Spike protein was added approximately 10 s after various concentrations of PAF inducing reversible and biphasic aggregation, a dose‐dependent decrease of aggregation was observed ranging from 39% to 65%. Interestingly, hPRP pre‐incubation for 10 min with the Spike protein (2 and 20 μg/ml) without stirring, did not affect the PAF‐induced biphasic aggregation.
FIGURE 1.
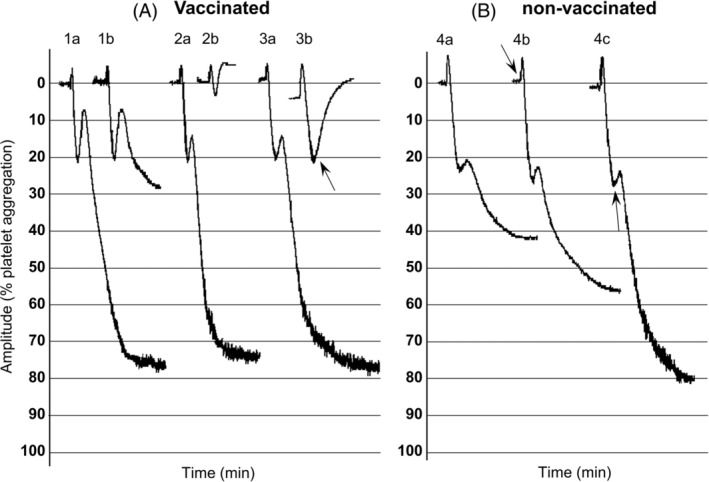
Effect of spike protein on PAF‐induced hPRP aggregation from vaccinated and non‐vaccinated individuals. Platelet aggregation induced by PAF was defined as the difference between the 0% (PRP) baseline and the 100% (PPP) baseline: (A) hPRP from vaccinated individuals. (1A): PAF‐ induced biphasic aggregation, 6 × 10−9 M. (1B) 1 min pre‐incubation with 2 μg/ml spike protein and addition of PAF 6 × 10−9 M. (2A) PAF‐induced biphasic aggregation, 20 × 10−9 M. (2B) 1 min pre‐incubation with 2 μg/ml spike protein and addition of PAF 20 × 10−9 M. (3A) PAF‐induced biphasic aggregation, 10 × 10−9 M. (3B) PAF 10 × 10−9 M, 2 μg/ml spike protein was added just before the second wave (see arrow). (B). hPRP from non‐vaccinated individuals. (4A) PAF‐induced biphasic aggregation, 80 × 10−9 M. (4B) PAF 80 × 10−9 M, 2 μg/ml spike protein added 10 s after PAF (see arrow). (4C) PAF 80 × 10−9 M, 2 μg/ml spike protein added just before the second wave (see arrow)
On the other hand, ADP‐induced aggregation (final concentrations, 3–6 × 10−6 M) was not affected by the Spike protein and only a slight increase by 15% was observed when hPRP was pre‐incubated for 1 min with the Spike protein (2 and 20 μg/ml).
A slight reduction by 13% was observed in TRAP‐induced aggregation (final concentrations, 1–1.2 × 10−6 M) when hPRP was pre‐incubated for 1 min with the Spike protein (2 μg/ml) and also when the Spike protein was added approximately 10 s after TRAP.
Lastly, hPRP pre‐incubation for 1 min with the Spike protein (2 μg/ml) resulted in 14% reduction of collagen‐induced aggregation (final concentrations, 0.4–0.8 μg/ml) while higher concentration of the Spike protein (20 μg/ml) was needed to obtain 45% reduction of collagen‐induced aggregation. The same effect was also observed when hPRP pre‐incubation was performed for 10 min without stirring with the Spike protein (2 and 20 μg/ml). A significant reduction by 65% was observed in collagen‐induced aggregation when the Spike protein (2 μg/ml) was added approximately 30 s after collagen (Figure 2A).
FIGURE 2.
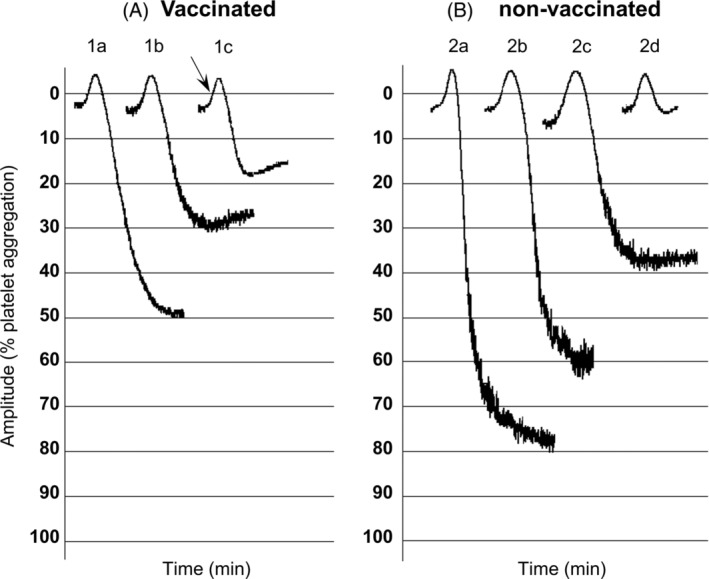
Effect of spike protein on collagen‐induced hPRP aggregation from vaccinated and non‐vaccinated individuals. Platelet aggregation with collagen was defined as the difference between the 0% (PRP) baseline and the 100% (PPP) baseline: (A). hPRP from vaccinated individuals. (1A) Collagen 0.8 μg/ml. (1B) 1 min pre‐incubation with 20 μg/ml spike protein and addition of collagen 0.8 μg/ml. (1C) Collagen 0.8 μg/ml, 2 μg/ml spike protein added 10 s after collagen (see arrow). (B). hPRP from non‐vaccinated individuals. (2A) Collagen 0.8 μg/ml. (2B) 1 min pre‐incubation with 2 μg/ml spike protein and addition of collagen 0.8 μg/ml. (2C) 1 min pre‐incubation with 20 μg/ml spike protein and addition of collagen 0.8 μg/ml. (2D) 10 min pre‐incubation with 20 μg/ml spike protein and addition of collagen 0.8 μg/ml
3.3. SARS‐CoV‐2 spike protein enhances in vitro PAF‐induced aggregation of hPRP from non‐vaccinated individuals
Incubation with the Spike protein (final concentrations, 2 and 20 μg/ml) for 10 min did not induce hPRP aggregation.
Biphasic aggregation induced by PAF (final concentrations, 2–8 × 10−8 M) was not affected by the hPRP pre‐incubation for 1 min with the Spike protein (2 and 20 μg/ml). On the contrary, when the Spike protein (2 μg/ml) was added, approximately 10 s after PAF or just before the second phase of PAF‐induced biphasic aggregation, an increase by 29% and 64%, respectively, was recorded (Figure 1B). Also, hPRP pre‐incubation for 10 min with the Spike protein (2 μg/ml) without stirring, resulted in 24% augmentation of PAF‐induced aggregation.
ADP‐induced aggregation (final concentrations, 6–10 × 10−6 M) was unaffected by the Spike protein (2 and 20 μg/ml).
TRAP‐induced aggregation (final concentrations, 1.2–2 × 10−6 M) was also unaffected by the Spike protein (2 μg/ml) and only a reduction of 20% was recorded with 20 μg/ml of the protein.
A dose‐dependent reduction of collagen‐induced aggregation (final concentrations, 0.8–1.6 μg/ml) ranging from 20%–90% was observed when hPRP pre‐incubation was performed for 1 min with the Spike protein (2 and 20 μg/ml) and also for 10 min pre‐incubation without stirring (Figure 2B).
3.4. SARS‐CoV‐2 spike protein enhances in vitro PAF‐induced washed rabbit platelets aggregation
Incubation with the Spike protein (2, 10, and 20 μg/ml) up to 7 min did not induce platelet aggregation in WRP.
Enhancement of PAF‐induced aggregation (0.6–1.2 × 10−11 M) in a range of 30%–48% was recorded when the Spike protein (2 μg/ml) was added simultaneously or approximately 3 and 10 s after PAF, as well as when rabbit platelets were pre‐incubated for 1 min with the Spike protein. Rabbit platelet pre‐incubation for 10 min with the Spike protein (2 and 20 μg/ml) without stirring, did not affect PAF‐induced aggregation (Figure 3A).
FIGURE 3.
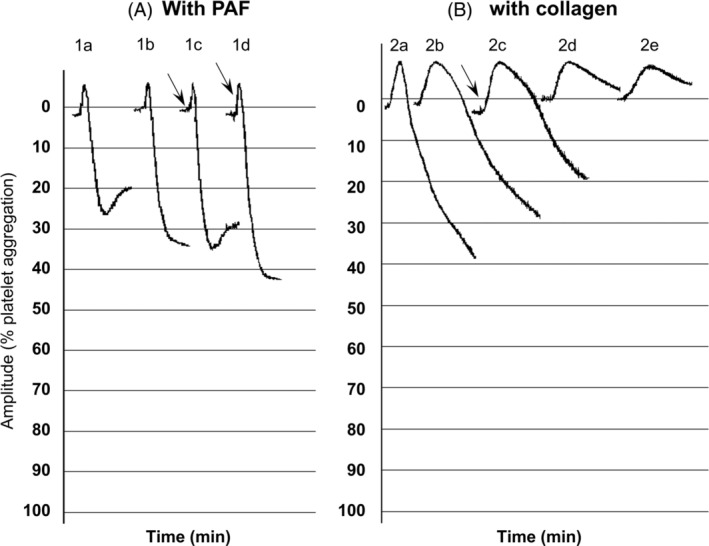
Effect of spike protein on PAF and collagen‐induced WRP aggregation. Platelet aggregation with PAF was defined as the difference between the 0% (PRP) baseline and the 100% (PPP) baseline: (A). PAF‐induced WRP aggregation. (1A) PAF, 1.2 × 10−11 M. (1B) 1 min pre‐incubation with 2 μg/ml spike protein and addition of PAF 1.2 × 10−11 M. (1C) PAF 1.2 × 10−11 M, 2 μg/ml spike protein added 3 s after PAF (see arrow). (1D) PAF 1.2 × 10−11 M, 2 μg/ml spike protein added 10 s after PAF (see arrow). (B). Collagen‐induced WRP aggregation. (2A) Collagen 0.08 μg/ml. (2B) 1 min pre‐incubation with 2 μg/ml spike protein and addition of collagen 0.08 μg/ml. (2C) Collagen 0.08 μg/ml, 2 μg/ml spike protein added 30 s after collagen (see arrow). (2D) 1 min pre‐incubation with 20 μg/ml spike protein and addition of collagen 0.08 μg/ml. (2E) 10 min pre‐incubation with 20 μg/ml spike protein and addition of collagen 0.08 μg/ml
TRAP‐induced aggregation (final concentrations, 0.2–0.4 × 10−6 M) was unaffected by the Spike protein (2 μg/ml).
Rabbit platelet pre‐incubation for 1–3 min with the Spike protein (2 μg/ml) resulted in 12% reduction of collagen‐induced aggregation (final concentration, 0.08 μg/ml) while higher concentration of the Spike protein (20 μg/ml) was needed to achieve 87% reduction. The same effect was also observed when pre‐incubation without stirring was performed for 10 min with the Spike protein (2 and 20 μg/ml). A reduction by 34% was also recorded in collagen‐induced aggregation when the Spike protein (2 μg/ml) was added approximately 30 s after collagen (Figure 3B).
3.5. SARS‐CoV‐2 spike protein stimulates PAF production in U‐937 cells
Two different concentrations of the Spike protein were tested, namely, 10 and 100 ng/ml that had no effect on the viability of the cells according to the MTT assay and did not induce cell proliferation according to the determination of cell protein (data not shown).
Treatment of U‐937 with the Spike protein was performed at 0.5, 3, or 24 h since we have previously demonstrated that intracellular PAF levels increased after IL‐1β stimulation at these time intervals. The Spike protein at both concentrations induced a two‐fold increase of intracellular PAF levels at 0.5 h compared to non‐stimulated cells (PAF baseline levels: 0.68 fmol/μg protein ±0.09, PAF levels after the 10 ng/ml Spike protein stimulation: 1.32 fmol/μg protein ±0.26, PAF levels after the 100 ng/ml Spike protein stimulation: 1.13 fmol/μg protein ±0.04) while a significant decrease of 77% and 50% was observed at 3 and 24 h, respectively with the highest Spike protein concentration (Figure 4). A substantial increase of extracellular PAF was recorded at 0.5 h (0.07 fmol/μg protein ±0.03 versus 0.096 fmol/μg protein ±0.05) and at 3 h with a four‐fold increase (0.007 fmol/μg protein ±0.003 versus 0.026 fmol/μg protein ±0.01) when the highest Spike protein concentration was used while at 24 h PAF was not detected in either non‐ or stimulated cells (Figure 4).
FIGURE 4.
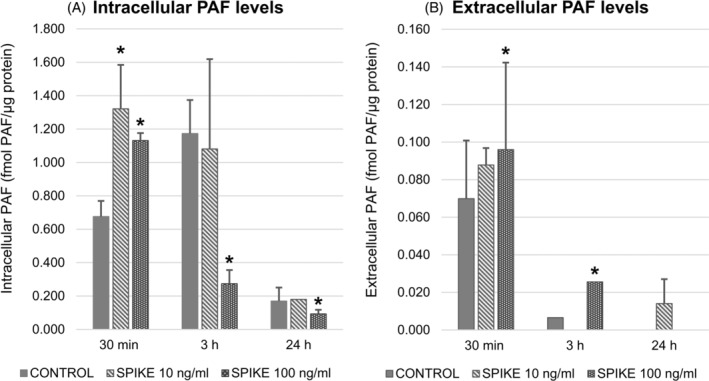
Intracellular and extracellular PAF levels in U‐937 stimulated with the spike protein (10 ng/ml and 100 ng/ml) at 0.5, 3 or 24 h. (A) The spike protein at 10 and 100 ng/ml induced an elevation of intracellular PAF levels at 0.5 h; a significant decrease of intracellular PAF levels at the order of 77% and 50% was observed at 3 and 24 h, respectively with 100 ng/ml spike protein. (B). Extracellular PAF levels were increased at 0.5 and 3 h with 100 ng/ml spike protein. The (*) symbol represents p ≤ 0.05 in comparison with non‐stimulated cells (baseline levels) within time group
Along with the two‐fold increase of intracellular PAF levels at 0.5 h, the specific activities of all three biosynthetic enzymes were also elevated. More specifically, stimulation with the 100 ng/ml of Spike protein resulted in higher PAF‐CPT by 76%, in Lyso‐PAF ATC by 30% and in Lyso‐PAF ATE by 47% compared with non‐stimulated cells (Figure 5).
FIGURE 5.
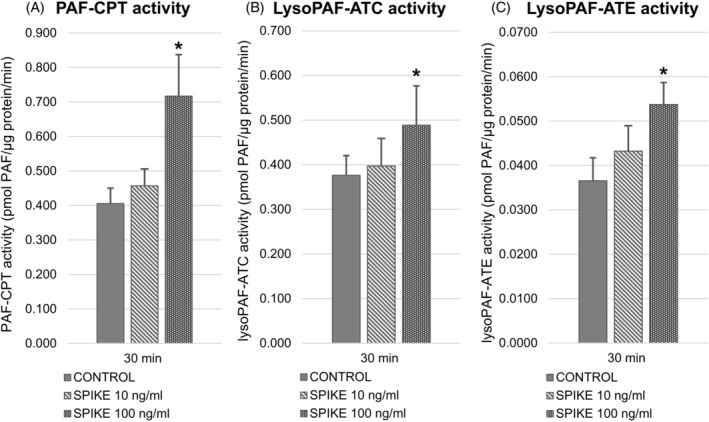
The specific activities of all three biosynthetic enzymes at 0.5 h after stimulation with the spike protein (10 and 100 ng/ml). Stimulation with the 100 ng/ml of spike protein resulted in higher PAF‐CPT by 76% (A) in Lyso‐PAF ATC by 29% (B) and in Lyso‐PAF ATE by 47% (C) compared with non‐stimulated cells. The (*) symbol represents p ≤ 0.05 in comparison with non‐stimulated cells (baseline levels). Lyso‐PAF ATC: Lyso‐PAF acetyltransferase in the presence of Ca2+; Lyso‐PAF ATE: Lyso‐PAF acetyltransferase in the presence of EDTA; PAF‐CPT: PAF‐cholinephosphotransferase
At 3 h, PAF‐CPT was decreased by 20% in the Spike protein stimulated cells. The activity of Lyso‐PAF ATC was not affected by the Spike protein stimulation at 3 h and 24 h in any tested concentration while the activity of its calcium‐independent isoform Lyso‐PAF ATE was increased by 29% at only 24 h with the highest concentration (data not shown).
4. DISCUSSION
Previous publications have reported that COVID‐19 was characterized by abnormal parameters such as decreased platelet counts, as well as by their hyperactivation since P‐selectin expression and integrin αIIbβ3 activation are both increased in most cases. Plasma levels of platelet‐derived growth factor (PDGF) are also elevated and washed platelet aggregation induced by ADP, thrombin and collagen was increased mainly in critically ill COVID‐19 patients. 4 , 5 , 7 , 34 Moreover, RBD Spike protein was reported to induce P‐selectin expression and integrin αIIbβ3 activation but there are conflicting results regarding its ability to induce platelet aggregation in human washed platelets in the absence of any other agonist. 8 , 9
Our results are in accordance with Zhang et al. 8 since the Spike protein for up to 10 min did not induce aggregation either in PRP obtained from healthy donors or in WRP. In addition, ex vivo platelet sensitivity, following the Spike protein production induced by mRNA vaccines, was enhanced in the presence of ADP, collagen, and PAF. The short‐term effect of vaccination was more profound in the case of PAF‐induced PRP aggregation where a significant augmentation of platelet sensitivity was observed. This phenomenon could be attributed to an enhancement of PAF production and/or to an upregulation of PAFR expression during B and T‐cell activation 35 , 36 since the mRNA vaccines function as both immunogen and adjuvant. 37
Augmentation of in vitro PAF‐induced aggregation was also recorded in the presence of the Spike protein either in hPRP from non‐vaccinated individuals or in WRP. In the case of hPRP from non‐vaccinated individuals, the increase in platelet aggregation was observed at the second wave of the biphasic aggregation curve. Unexpectedly, the Spike protein attenuated in vitro PAF‐induced aggregation in hPRP from vaccinated individuals and completely eliminated the secondary wave of the biphasic aggregation curve. The pre‐exposure to the Spike protein due to vaccination may have enhanced various reactions that could explain the different response to the second phase of the aggregation curves. 37 It should be noted that elimination of the second phase of PAF‐induced biphasic platelet aggregation has been previously reported in the presence of indomethacin and ADP scavengers, but without affecting PAF‐induced secretion of serotonin or PF4. 14 , 15 Based on the above, our results indicate that the Spike protein does not seem to bind to PAF receptor, but modulates PAF‐induced aggregation probably through platelet ADP release and other products derived from the cyclooxygenase pathway of arachidonic acid metabolism. It should be noted that in the cyclooxygenase pathway, a series of reactions catalyzed by cyclooxygenases (COX1/2) result in the synthesis of the different types of prostaglandins (PGs) and thromboxanes (TXs). The re‐exposure of vaccinated human PRP to the Spike protein might result to impaired balance between PGs and TXs, in favor of the vasodilator eicosanoids that also inhibit platelet aggregation through the increase of the intracellular cyclic adenosine 3′,5′‐monophosphate levels (c‐AMP).
On the other hand, ADP‐ and TRAP‐induced aggregation were not significantly affected by the Spike protein neither in PRP obtained from vaccinated individuals nor from non‐vaccinated ones. A slight potentiation by 15% of ADP‐induced aggregation along with a mild reduction by 13% in TRAP‐induced aggregation was recorded in vaccinated volunteers that could be attributed to the pre‐exposure to the Spike protein due to vaccination and secretion of ADP and/or impaired balance between PGs and TXs. TRAP‐induced aggregation was also unaffected in WRP aggregation.
Collagen‐induced aggregation was dose‐dependently diminished by the Spike protein in all tested aggregation experiments reaching approximately 90% decrease when 20 μg/ml of the Spike protein was used in hPRP from non‐vaccinated individuals and in WRP while a significant reduction at the order of 65% was observed in hPRP from vaccinated ones when 2 μg/ml of the Spike protein was added 30 s after collagen stimulation. While PAF, thrombin and ADP exert their primary effects directly, the stimulation with moderate amounts of collagen is depending to a considerable extent on arachidonic acid metabolism 38 , 39 that seems to be altered by the Spike protein. Zhang et al. reported that pre‐incubation for 5 min with the Spike protein and especially the Spike subunit 1 potentiated in vitro washed human platelet aggregation in response to one specific concentration of collagen, thrombin, and ADP with fibrinogen. The different results in our study could be attributed to different platelet preparations since we have used PRP, to different concentrations and type of agonists used in the case of ADP and thrombin, as well as to the different pre‐incubation time. Indeed, the formation of PGD2 that attenuates platelet aggregation has been reported in the presence of human serum albumin in plasma that favors the formation of PGD2 from the endoperoxides at the expense of other prostanoids. 39
Even though, it is still unclear whether human platelets express ACE2, the proposed ACE2 down‐regulation by the Spike protein, resulting in an imbalance between angiotensin II and angiotensin, 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 could also provide an explanation since angiotensin II has been reported to modulate PAF actions through PAF formation and binding, 40 and PAF increases ACE2 activity. 41 Angiotensin II type 1 receptor (AT1) antagonists inhibited monocyte chemoattractant protein‐1 (MCP‐1) production in human monocytes stimulated by PAF 42 and Mas receptor knock‐out mice were more susceptible to PAF. 43 Also, angiotensin 1 , 2 , 3 , 4 , 5 , 6 , 7 restored the lipopolysaccharide (LPS)‐induced decreases in platelet aggregation stimulated by collagen in endotoxaemic rats. 44
In addition, our results from U‐937 stimulated with the Spike protein revealed that it could induce a two‐fold increase in intracellular PAF production after 0.5 h incubation that was accompanied by the elevation of the specific activities of all three PAF biosynthetic enzymes. Specifically, the crucial enzyme of the de novo pathway, namely, PAF‐CPT was increased by 76%, while the two isoforms of acetyl‐CoA:lyso–PAF acetyltransferases, namely, the calcium‐ independent Lyso‐PAF ATE was increased by 47% and the calcium‐dependent Lyso‐PAF ATC by 30%. Even though, a four‐fold increase in extracellular PAF levels was recorded after 3 h incubation with the Spike protein, the percentage of the extracellular PAF levels compared with the total ones did not exceed 6%–13% at all experimental conditions. These results may have a clinical significance since we have previously reported that PAF induced mRNA expression and secretion of MCP‐1 from U‐937 cells, modulated their redox status and also increased mRNA expression of PAFR. 45 Other investigators have shown that monocytes and macrophages expressed high levels of proinflammatory cytokines in the presence of different viral proteins. 46 , 47 The ability of the Spike protein to induce the production of the potent mediator, PAF, along with other proinflammatory cytokines may in part mediate the effects of SARS‐CoV‐2 and potentially contribute to Long‐COVID syndrome. 48
5. LIMITATIONS
The number of volunteers studied was small and included only females. The hormonal status of the subjects (premenopausal or postmenopausal) was not taken into consideration. In the experiments with U‐937 stimulated with the Spike protein, the specific activity of the intracellular PAF‐specific acetylhydrolase (PAF‐AH) was not determined. Finally, we used mammalian recombinant RBD Spike protein, and not the full‐length Spike protein that could have additional effects via receptors other than ACE2.
6. CONCLUSION
Vaccination results in a short‐term augmentation of platelet sensitivity, especially when PAF is used as an agonist. SARS‐CoV‐2 Spike RBD protein exerts diverse effects in PAF‐induced aggregation depending on the status of vaccination, and an attenuation was recorded in PRP from vaccinated individuals. Lastly, the Spike protein stimulates the production of PAF in monocytes. Cells producing more PAF could increase the inflammatory response in the patient with COVID‐19, exacerbating the clinical condition. Since PAF exerts its action on a variety of cells such as platelets and mast cells, the use of rupatadine and other compounds of natural origin, that act as PAF, mast cell activation and platelet aggregation inhibitors (e.g., flavonoids, polar lipid extracts) 49 , 50 , 51 , 52 , 53 could be considered at least in mild cases of patients infected with SARS‐CoV‐2. Indeed, a recent paper reports that a novel integrative treatment approach including the dual histamine‐1 and PAF antagonist, rupatadine, as well as famotidine, misoprostol and vitamin D3, that suppress mast cell activation and the flavonoids luteolin and quercetin, with anti‐inflammatory and mast‐cell blocking properties, resulted in fully recovery of a severe COVID‐19 patient. 54
CONFLICT OF INTEREST
None of the authors declare any conflict of interest.
ACKNOWLEDGMENTS
We thank Margarita Christea for her technical assistance in blood sample collection. Recombinant HEK293‐derived SARS‐CoV‐2 Spike RBD protein (10500‐CV) was kindly provided by Bio‐Techne (Minneapolis MN).
Antonopoulou S, Petsini F, Detopoulou M, Theoharides TC, Demopoulos CA. Is there an interplay between the SARS‐CoV‐2 spike protein and Platelet‐Activating factor? BioFactors. 2022. 10.1002/biof.1877
DATA AVAILABILITY STATEMENT
Data available on request from the authors.
REFERENCES
- 1. Bowles L, Platton S, Yartey N, Dave M, Lee K, Hart DP, et al. Lupus anticoagulant and abnormal coagulation tests in patients with Covid‐19. N. Engl. J. Med. 2020;383:288–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ranucci M, Ballotta A, Di Dedda U, Baryshnikova E, Dei Poli M, et al. The procoagulant pattern of patients with COVID‐19 acute respiratory distress syndrome. J. Thromb. Haemost. 2020;18:1747–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid‐19. N. Engl. J. Med. 2020;382:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Litvinov RI, Evtugina NG, Peshkova AD, Safiullina SI, Andrianova IA, Khabirova AI, et al. Altered platelet and coagulation function in moderate‐to‐severe COVID‐19. Sci. Rep. 2021;11:16290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nicolai L, Leunig A, Brambs S, Kaiser R, Weinberger T, Weigand M, et al. Immunothrombotic dysregulation in COVID‐19 pneumonia is associated with respiratory failure and coagulopathy. Circulation. 2020;142:1176–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wool GD, Miller JL. The impact of COVID‐19 disease on platelets and coagulation. Pathobiology. 2021;88:15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Manne BK, Denorme F, Middleton EA, Portier I, Rowley JW, Stubben C, et al. Platelet gene expression and function in patients with COVID‐19. Blood. 2020;136:1317–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang S, Liu Y, Wang X, Yang L, Li H, Wang Y, et al. SARS‐CoV‐2 binds platelet ACE2 to enhance thrombosis in COVID‐19. J. Hematol. Oncol. 2020;13:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li T, Yang Y, Li Y, Wang Z, Ma F, Luo R, et al. Platelets mediate inflammatory monocyte activation by SARS‐CoV‐2 spike protein. J. Clin. Invest. 2022;132:e150101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao Y, Kuang M, Li J, Zhu L, Jia Z, Guo X, et al. SARS‐CoV‐2 spike protein interacts with and activates TLR4. Cell Res. 2021;31:818–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maugeri N, De Lorenzo R, Clementi N, Antonia Diotti R, Criscuolo E, et al. Unconventional CD147‐dependent platelet activation elicited by SARS‐CoV‐2 in COVID‐19. J. Thromb. Haemost. 2022;20:434–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Demopoulos CA, Pinckard RN, Hanahan DJ. Platelet‐activating factor. Evidence for 1‐O‐alkyl‐2‐acetyl‐sn‐glyceryl‐3‐phosphorylcholine as the active component (a new class of lipid chemical mediators). J. Biol. Chem. 1979;254:9355–8. [PubMed] [Google Scholar]
- 13. Antonopoulou S, Nomikos T, Karantonis HC, Fragopoulou E, Demopoulos CA. PAF, a potent lipid mediator. In: Tselepis A, editor. Bioactive phospholipids: role in inflammation and atherosclerosis. Kerala, India: Transworld Research Network; 2008. p. 85–134. [Google Scholar]
- 14. McManus LM, Hanahan DJ, Pinckard RN. Human platelet stimulation by acetyl glyceryl ether phosphorylcholine. J. Clin. Invest. 1981;67:903–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chesney CM, Pifer DD, Byers LW, Muirhead EE. Effect of platelet‐activating factor (PAF) on human platelets. Blood. 1982;59:582–5. [PubMed] [Google Scholar]
- 16. Shah N, Kumar K, Shah N. PAF physiology in target organ systems—a deep dive to understand the PAF mystery in pathogenesis of disease. Heart. 2021;2:551–60. [Google Scholar]
- 17. Theoharides TC, Antonopoulou S, Demopoulos CA. Coronavirus 2019, microthromboses, and platelet activating factor. Clin. Ther. 2020;42:1850–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Demopoulos C, Antonopoulou S, Theoharides TC. COVID‐19, microthromboses, inflammation, and platelet activating factor. Biofactors. 2020;46:927–33. [DOI] [PubMed] [Google Scholar]
- 19. Klein M, Dao V, Khan F. A review of platelet‐activating factor as a potential contributor to morbidity and mortality associated with severe COVID‐19. Clin. Appl. Thromb. Hemost. 2021;27:10760296211051764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zabetakis I, Lordan R, Norton C, Tsoupras A. COVID‐19: the inflammation link and the role of nutrition in potential mitigation. Nutrients. 2020;12:1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Detopoulou P, Demopoulos CA, Antonopoulou S. Micronutrients, phytochemicals and Mediterranean diet: a potential protective role against COVID‐19 through modulation of PAF actions and metabolism. Nutrients. 2021;13:462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harayama T, Shindou H, Ogasawara R, Suwabe A, Shimizu T. Identification of a novel noninflammatory biosynthetic pathway of platelet‐activating factor. J. Biol. Chem. 2008;283:11097–106. [DOI] [PubMed] [Google Scholar]
- 23. Barberis E, Timo S, Amede E, Vanella VV, Puricelli C, et al. Large‐scale plasma analysis revealed new mechanisms and molecules associated with the host response to SARS‐CoV‐2. Int. J. Mol. Sci. 2020;21:8623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Snyder F. CDP‐choline:alkylacetylglycerol cholinephosphotransferase catalyzes the final step in the de novo synthesis of platelet‐activating factor. Biochim. Biophys. Acta. 1997;1348:111–6. [DOI] [PubMed] [Google Scholar]
- 25. Stafforini DM. Biology of platelet‐activating factor acetylhydrolase (PAF‐AH, lipoprotein associated phospholipase A2). Cardiovasc. Drugs Ther. 2009;23:73–83. [DOI] [PubMed] [Google Scholar]
- 26. Li Y, Jiang Y, Zhang Y, Li N, Yin Q, Liu L, et al. Abnormal upregulation of cardiovascular disease biomarker PLA2G7 induced by proinflammatory macrophages in COVID‐19 patients. Sci. Rep. 2021;11:6811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Antonopoulou S, Nomikos T, Fragopoulou E, Karantonis HC. Isolation and characterization of cyanobacterial lipids with bioactivity towards platelet‐aggregation. In: Kleiner D, Mohanty P, editors. Protocols on algal and cyanobacterial research. New Delhi, India: Narosa Publishing House; 2010. p. 71–101. [Google Scholar]
- 28. Vlachogianni IC, Fragopoulou E, Stamatakis GM, Kostakis IK, Antonopoulou S. Platelet activating factor (PAF) biosynthesis is inhibited by phenolic compounds in U‐937 cells under inflammatory conditions. Prostaglandins Other Lipid Mediat. 2015;121:176–83. [DOI] [PubMed] [Google Scholar]
- 29. Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–7. [DOI] [PubMed] [Google Scholar]
- 30. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein‐dye binding. Anal. Biochem. 1976;72:248–54. [DOI] [PubMed] [Google Scholar]
- 31. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. [DOI] [PubMed] [Google Scholar]
- 32. Demopoulos CA, Andrikopoulos NK, Antonopoulou S. A simple and precise method for the routine determination of platelet‐activating factor in blood and urine. Lipids. 1994;29:305–9. [DOI] [PubMed] [Google Scholar]
- 33. Gavriil L, Detopoulou M, Petsini F, Antonopoulou S, Fragopoulou E. Consumption of plant extract supplement reduces platelet activating factor‐induced platelet aggregation and increases platelet activating factor catabolism: a randomised, double‐blind and placebo‐controlled trial. Br. J. Nutr. 2019;121:982–91. [DOI] [PubMed] [Google Scholar]
- 34. Hottz ED, Azevedo‐Quintanilha IG, Palhinha L, Teixeira L, Barreto EA, Pão CRR, et al. Platelet activation and platelet‐monocyte aggregate formation trigger tissue factor expression in patients with severe COVID‐19. Blood. 2020;136:1330–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nguer CM, Treton D, Rola‐Pleszczynski M, Mishal Z, Thomas Y, Galanaud P, et al. Regulation of platelet‐activating factor receptor expression in human B cells and B cell lines. Lipids. 1996;31:1051–8. [DOI] [PubMed] [Google Scholar]
- 36. Calabresse C, Nguer MC, Pellegrini O, Benveniste J, Richard Y, Thomas YN. Induction of high‐affinity paf receptor expression during T cell activation. Eur. J. Immunol. 1992;22:1349–55. [DOI] [PubMed] [Google Scholar]
- 37. Angeli F, Spanevello A, Reboldi G, Visca D, Verdecchia P. SARS‐CoV‐2 vaccines: lights and shadows. Eur. J. Intern. Med. 2021;88:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhou L, Schmaier AH. Platelet aggregation testing in platelet‐rich plasma: description of procedures with the aim to develop standards in the field. Am. J. Clin. Pathol. 2005;123:172–83. [DOI] [PubMed] [Google Scholar]
- 39. Orchard MA, Waddell KA, Lewis PJ, Blair IA. Thromboxane synthase inhibition causes re‐direction of prostaglandin endoperoxides to prostaglandin D2 during collagen stimulated aggregation of human platelet rich plasma. Thromb. Res. 1985;39:701–10. [DOI] [PubMed] [Google Scholar]
- 40. Sato A, Yokoyama I, Ebina K. Angiotensin peptides attenuate platelet‐activating factor‐induced inflammatory activity in rats. Peptides. 2015;73:60–6. [DOI] [PubMed] [Google Scholar]
- 41. Kawaguchi H, Sawa H, Yasuda H. Mechanism of increased angiotensin‐converting enzyme activity stimulated by platelet‐activating factor. Biochim. Biophys. Acta. 1990;1052:503–8. [DOI] [PubMed] [Google Scholar]
- 42. Proudfoot JM, Croft KD, Puddey IB, Beilin LJ. Angiotensin II type 1 receptor antagonists inhibit basal as well as low‐density lipoprotein and platelet‐activating factor‐stimulated human monocyte chemoattractant protein‐1. J. Pharmacol. Exp. Ther. 2003;305:846–53. [DOI] [PubMed] [Google Scholar]
- 43. Erfinanda L, Ravindran K, Kohse F, Gallo K, Preissner R, Walther T, et al. Oestrogen‐mediated upregulation of the mas receptor contributes to sex differences in acute lung injury and lung vascular barrier regulation. Eur. Respir. J. 2021;57:2000921. [DOI] [PubMed] [Google Scholar]
- 44. Tsai H‐J, Shih C‐C, Chang K‐Y, Liao M‐H, Liaw W‐J, Wu CC, et al. Angiotensin‐(1–7) treatment blocks lipopolysaccharide‐induced organ damage, platelet dysfunction, and IL‐6 and nitric oxide production in rats. Sci. Rep. 2021;11:610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Verouti SN, Fragopoulou E, Karantonis HC, Dimitriou AA, Tselepis AD, Antonopoulou S, et al. PAF effects on MCP‐1 and IL‐6 secretion in U‐937 monocytes in comparison with oxLDL and IL‐1β effects. Atherosclerosis. 2011;219:519–25. [DOI] [PubMed] [Google Scholar]
- 46. Karwaciak I, Sałkowska A, Karaś K, Dastych J, Ratajewski M. Nucleocapsid and spike proteins of the coronavirus SARS‐CoV‐2 induce IL6 in monocytes and macrophages‐potential implications for cytokine storm syndrome. Vaccines (Basel). 2021;9:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gowda P, Patrick S, Joshi SD, Kumawat RK, Sen E. Glycyrrhizin prevents SARS‐CoV‐2 S1 and Orf3a induced high mobility group box 1 (HMGB1) release and inhibits viral replication. Cytokine. 2021;142:155496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Theoharides TC. Could SARS‐CoV‐2 spike protein be responsible for long‐COVID syndrome? Mol. Neurobiol. 2022;59:1850–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Faggio C, Sureda A, Morabito S, Sanches‐Silva A, Mocan A, Nabavi SF, et al. Flavonoids and platelet aggregation: a brief review. Eur. J. Pharmacol. 2017;807:91–101. [DOI] [PubMed] [Google Scholar]
- 50. Antonopoulou S, Detopoulou M, Fragopoulou E, Nomikos T, Mikellidi Α, Yannakoulia M, et al. Consumption of yogurt enriched with polar lipids from olive oil by‐products reduces platelet sensitivity against platelet activating factor and inflammatory indices: a randomized, double‐blind clinical trial. Human Nutr Metab. 2022;28:200145. [Google Scholar]
- 51. Babic I, Bojic M, Males Z, Zadro R, Gojceta K, et al. Influence of flavonoids' lipophilicity on platelet aggregation. Acta Pharm. 2019;69:607–19. [DOI] [PubMed] [Google Scholar]
- 52. Balestrieri ML, Castaldo D, Balestrieri C, Quagliuolo L, Giovane A, Servillo L. Modulation by flavonoids of PAF and related phospholipids in endothelial cells during oxidative stress. J. Lipid Res. 2003;44:380–7. [DOI] [PubMed] [Google Scholar]
- 53. Alevizos M, Karagkouni A, Vasiadi M, Sismanopoulos N, Makris M, Kalogeromitros D, et al. Rupatadine inhibits inflammatory mediator release from human laboratory of allergic diseases 2 cultured mast cells stimulated by platelet‐activating factor. Ann. Allergy Asthma Immunol. 2013;111:542–7. [DOI] [PubMed] [Google Scholar]
- 54. Theoharides TC, Guerra L, Patel K. Successful treatment of a patient with severe COVID‐19 using an integrated approach addressing mast cells and their mediators. Int. J. Infect. Dis. 2022;118:164–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request from the authors.


