Abstract
Since its emergence in late 2019, the coronavirus disease 2019 (COVID‐19) pandemic has caused substantial morbidity and mortality. Despite the availability of efficacious vaccines, new variants with reduced sensitivity to vaccine‐induced protection are a troubling new reality. The Ad26.COV2.S vaccine is a recombinant, replication‐incompetent human adenovirus type 26 vector encoding a full‐length, membrane‐bound severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) spike protein in a prefusion‐stabilized conformation. This review discusses the immunogenicity and efficacy of Ad26.COV2.S as a single‐dose primary vaccination and as a homologous or heterologous booster vaccination. Ad26.COV2.S elicits broad humoral and cellular immune responses, which are associated with protective efficacy/effectiveness against SARS‐CoV‐2 infection, moderate to severe/critical COVID‐19, and COVID‐19–related hospitalization and death, including against emerging SARS‐CoV‐2 variants. The humoral immune responses elicited by Ad26.COV2.S vaccination are durable, continue to increase for at least 2‐3 months postvaccination, and involve a range of functional antibodies. Ad26.COV2.S given as a heterologous booster to mRNA vaccine–primed individuals markedly increases humoral and cellular immune responses. The use of Ad26.COV2.S as primary vaccination and as part of booster regimens is supporting the ongoing efforts to control and mitigate the COVID‐19 pandemic.
Keywords: COVID‐19, efficacy, immunogenicity, SARS‐CoV‐2, vaccination, vaccine
1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), has caused high morbidity and mortality globally. 1 Efficacious vaccines have been available since the end of 2020, profoundly changing the course of the pandemic in many parts of the world. However, large populations that are still unvaccinated and the rapid emergence of new variants with reduced sensitivity to vaccine‐elicited neutralizing antibodies demonstrate a clear need for continued global vaccination efforts. 2
The Ad26.COV2.S vaccine is a recombinant, replication‐incompetent human adenovirus type 26 (Ad26) vector encoding a full‐length, membrane‐bound SARS‐CoV‐2 spike protein in a prefusion‐stabilized conformation, based on the original Wuhan strain of SARS‐CoV‐2, 2 , 3 , 4 that has been authorized for emergency use worldwide. Ad26.COV2.S as both a single‐dose primary vaccination and a homologous booster dose was shown to be safe and immunogenic, eliciting humoral and cellular immune responses that are associated with protective efficacy against SARS‐CoV‐2 original strain and variant infection, moderate to severe COVID‐19, and COVID‐19–related hospitalization and death. 4 , 5 , 6 , 7 Here, we present an overview of the immunogenicity of Ad26.COV2.S as a single‐dose primary vaccination and as a homologous or heterologous booster. In addition, we discuss the association of Ad26.COV2.S‐elicited immune responses with efficacy or effectiveness against COVID‐19, including in the context of emerging SARS‐CoV‐2 variants.
2. IMMUNOGENICITY AND PROTECTIVE EFFICACY OF A SINGLE DOSE OF AD26.COV2.S
The quantity and quality of humoral and cellular immune responses elicited by Ad26.COV2.S were important parameters in the development of this vaccine. In multiple phase 1, 2, and 3 clinical trials, we have shown that Ad26.COV2.S elicits durable, broad, and functional humoral and cellular immune responses in adults ≥18 years of age. 4 , 8 , 9 , 10 , 11
2.1. Ad26.COV2.S‐elicited humoral immune responses against the SARS‐CoV‐2 reference strain
Humoral immune responses elicited by Ad26.COV2.S are characterized by the presence of SARS‐CoV‐2 spike–specific neutralizing antibodies. 4 , 8 , 10 , 11 Other nonneutralizing antibodies that bind the spike protein and may have fragment crystallizable (Fc)–mediated antiviral activity are also present. 4 , 8 , 10 , 11 Within 2 weeks after primary single‐dose vaccination with Ad26.COV2.S, response rates for neutralizing antibodies against the reference strain of SARS‐CoV‐2 ranged from 67% to 100%, with higher response rates in younger adults compared with older adults. 9 Overall geometric mean titers (GMTs) assessed by wild‐type virus neutralization assay (wtVNA) against the reference strain ranged from 224‐277 half‐maximal inhibitory concentrations (IC50). The kinetics with which these neutralizing antibodies developed after vaccination were somewhat slower in older adults but were in the same range as those of younger adults by Week 4. These data imply that, overall, younger and older adults responded equally well to vaccination. In both age groups, levels of neutralizing antibodies continued to increase and peaked by Day 57 (2 months) postvaccination (Figure 1).
FIGURE 1.
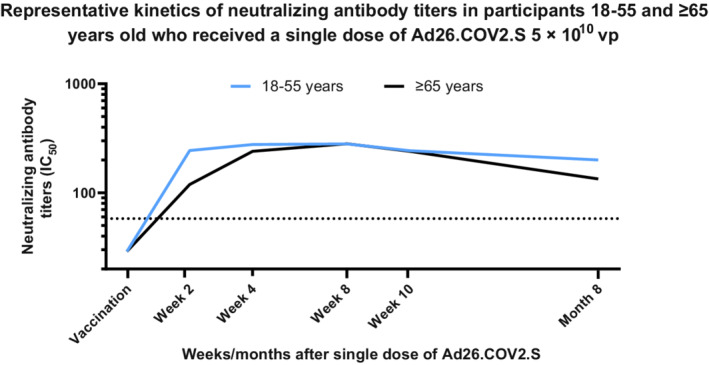
Representative kinetics of neutralizing antibody titers in participants aged 18‐55 and ≥65 years who received a single dose of Ad26.COV2.S 5 × 1010 vp. Participants in a phase 2 study aged 18‐55 and ≥65 years were administered a single dose of Ad26.COV2.S (5 × 1010 vp) at Day 1, as primary vaccination. Serum neutralizing antibody responses against SARS‐CoV‐2 were evaluated by wtVNA, up to 8 months post primary vaccination in a subset of participants aged 18‐55 (N = 25) and ≥65 (N = 22) years, respectively. Participants 18‐55 and ≥65 years of age are represented with blue and black lines, respectively. IC50, half‐maximal inhibitory concentration; SARS‐CoV‐2, severe acute respiratory coronavirus 2; vp, viral particles; wtVNA, wild‐type virus neutralization assay
Similar observations were made for the spike‐ and receptor‐binding domain (RBD)–specific antibodies, as measured by enzyme‐linked immunosorbent assay against the SARS‐CoV‐2 spike protein (S‐ELISA) or by MesoScale Discovery (MSD) assay. 4 , 9 , 10 For S‐ELISA, as an example, by 2 weeks after primary single‐dose vaccination, response rates for spike‐specific binding antibodies to the reference strain of SARS‐CoV‐2 ranged from 48% to 88%. Although response rates at 2 weeks postvaccination were higher in younger as compared with older adults, response rates were again comparable in both age groups by 4 weeks postvaccination. Similarly, the kinetics of the binding antibody response after vaccination were slower in older adults compared with younger adults but were similar across age groups by Week 4 (geometric mean concentrations in the trial for the total group ranged from 265‐423 EU/mL).
Spike‐ and RBD‐specific antibodies can be of various immunoglobulin isotypes and subclasses, such as IgA, IgG1, IgG2, IgG3, IgG4, and IgM, and can have neutralizing as well as nonneutralizing Fc‐mediated antiviral functions. 10 , 11 , 12 , 13 The Fc portion of the antibodies can bind to Fc receptors, which are present on innate immune cells and can trigger different immune effector functions leading to the clearance of virus and virus‐infected cells. 10 , 11 , 14 Important Fc effector functions mediated by these antigen‐specific antibodies include antibody‐dependent cellular phagocytosis (ADCP), antibody‐dependent neutrophil phagocytosis (ADNP), antibody‐dependent complement deposition (ADCD), and antibody‐dependent natural killer cell activation (ADNKA). Following immunization with Ad26.COV2.S, spike‐ and RBD‐specific immunoglobulins, including IgA1, IgA2, IgG1, IgG2, IgG3, IgG4, and IgM, could be detected. 10 These different subclasses of antibodies can bind to diverse Fc receptors and trigger ADCP, ADNP, ADCD, and ADNKA. 10 , 15 , 16
In conclusion, Ad26.COV2.S elicits high‐quality humoral immune responses with a range of functional antibodies that may contribute to protection against SARS‐CoV‐2 infection and COVID‐19. Moreover, a high correlation (Spearman correlation >0.7) was observed between the neutralizing, binding, and Fc‐mediated functional antibody levels at these early time points postvaccination, and the ratio of neutralizing to binding antibody remained stable over time, indicating that these are coordinated responses of highly functional antibodies.
2.2. Breadth of Ad26.COV2.S‐elicited humoral immunity: activity against SARS‐CoV‐2 variants
All first‐generation COVID‐19 vaccines currently available, including Ad26.COV2.S, are based on the spike protein sequence of the original Wuhan strain of SARS‐CoV‐2. 2 However, new variants of SARS‐CoV‐2 have emerged over time, some with significant sequence variation in the N‐terminal domain (NTD) and RBD of the spike protein. 17 These new strains have received considerable attention, as spike protein sequence variation may affect the sensitivity of SARS‐CoV‐2 variants to vaccine‐mediated immunity and impact vaccine efficacy against these variants. In a small cohort of adults 18‐55 years of age who received a single dose of Ad26.COV.S, sera obtained at Day 29 postvaccination showed lower neutralizing antibody titers (pseudovirion neutralization assay [psVNA]) against the Beta, Delta, Gamma, and Omicron variants of concern 18 as compared with the SARS‐CoV‐2 WA1/2020 strain (expressing a spike protein identical to the Wuhan‐Hu‐1 strain). The Omicron and Beta variants were the most resistant to neutralization, with an approximately 14‐fold decreased sensitivity as compared with the reference strain, while the Delta variant demonstrated a 7‐fold reduction.
The Omicron variant lineage of SARS‐CoV‐2 has, to date, demonstrated the largest number of substitutions in the spike protein. 19 Sera obtained 1 month after single‐dose primary vaccination with Ad26.COV2.S demonstrated very low to undetectable neutralizing activity against this variant; however, a higher proportion of sera obtained 8 months after primary vaccination demonstrated neutralizing activity, confirming an earlier observation of maturation and increasing breadth of the humoral immune response elicited by Ad26.COV2.S over time. 20 Indeed, in another cohort of the phase 1/2a study, Day 29 sera demonstrated lower neutralizing activity (wtVNA) against the Alpha, Beta, and Delta variants as compared with the reference strain (Figure 2, unpublished data). Notably, the neutralizing activity against variants increased with time since vaccination, thereby reducing the difference in neutralizing activity against the reference and variant strains. These findings suggest affinity maturation of neutralizing antibody–producing B cells over time, resulting in increased breadth and coverage of variants.
FIGURE 2.
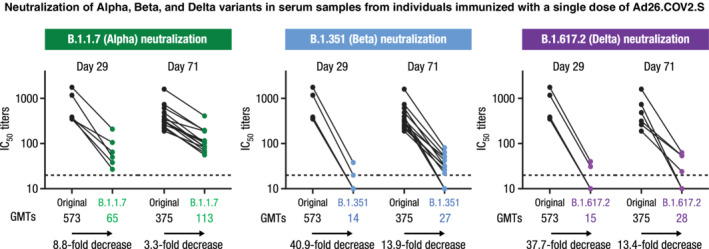
Neutralization of Alpha, Beta, and Delta variants in serum samples from individuals immunized with a single dose of Ad26.COV2.S. After vaccination with a single dose of Ad26.COV2.S, N = 6 samples at Day 29 and N = 14 samples at Day 71 were analyzed in wtVNA against the SARS‐CoV‐2 Victoria strain (D614, black dots), the B.1.1.7 (Alpha, green dots), the B.1.351 (Beta, blue dots), or the B.1.617.2 (Delta, purple dots) lineages. Dots represent IC50 titers per participant. GMTs are depicted below each figure, and the fold decrease in neutralizing activity between the Victoria strain and each lineage is shown below each arrow. GMT, geometric mean titer; IC50, half‐maximal inhibitory concentration; SARS‐CoV‐2, severe acute respiratory coronavirus 2; wtVNA, wild‐type virus neutralization assay
Interestingly, the time from vaccination had no discernible impact on overall spike‐specific antibody levels (S‐ELISA) across variants. Neutralizing antibodies that bind to the RBD, the region that contains most of the sequence variation in SARS‐CoV‐2 variants, likely represent only a small proportion of the overall antibody response elicited by the Ad26.COV2.S vaccine, which expresses the total spike protein. Nonneutralizing antibodies can also bind outside the highly variable regions of the spike and can have Fc‐mediated nonneutralizing antiviral activity. This concept is supported by the observation that Ad26.COV2.S‐elicited antibodies with Fc‐mediated functions, such as ADCP, ADNP, or ADNKA, are largely maintained against variants, with only a slight reduction in activity for the Alpha and Beta variants. 11
2.3. Cell‐mediated immune responses against SARS‐CoV‐2 reference strain
Cell‐mediated immune responses are crucial for the prevention and clearance of viral infections, such as those caused by SARS‐CoV‐2. CD4+ T helper cells may support B cells to produce SARS‐CoV‐2 spike–specific antibodies, either through direct cell‐to‐cell interaction or through secretion of B‐cell–stimulating cytokines. CD8+ cytotoxic T cells can directly interact with SARS‐CoV‐2–infected cells and destroy them through the secretion of cytokines and cytotoxic molecules, such as granzyme B and perforin. 21
By 2 weeks postvaccination with Ad26.COV2.S, cell‐mediated immune responses were detectable in a large proportion of individuals who were naïve for SARS‐CoV‐2 spike–specific immunity prior to vaccination (Figure 3). 4 CD4+ T‐cell responses, as measured by the percentage of interferon gamma (IFNγ)– and/or interleukin (IL)‐2–producing cells, were observed in 74% to 77% of vaccinated individuals by 14 days postvaccination, and rates were similar at 28 days. These response rates are considered to be high, as the responder definition is based on the very stringent Fisher’s exact test with Bonferroni adjustment, which compared nonstimulated conditions with spike peptide stimulation. 22 Similar response rates were observed between younger and older adults. 4
FIGURE 3.
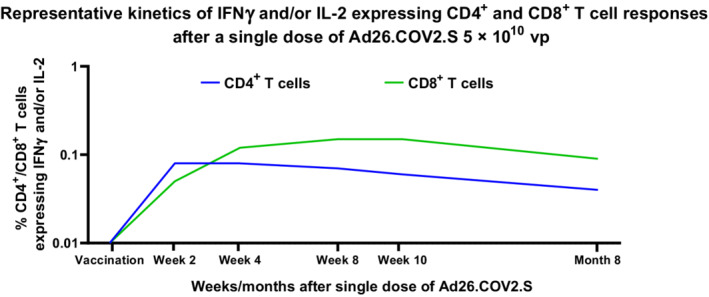
Representative kinetics of IFNγ and/or IL‐2 expressing CD4+ and CD8+ T‐cell responses after a single dose of Ad26.COV2.S 5 × 1010 vp. Participants aged 18‐55 years (N = ~40) and ≥65 years (N = ~40) in a phase 1/2a trial were given a single dose of Ad26.COV2.S (5 × 1010 vp) at Day 1, as primary vaccination. S‐specific T‐cell responses were measured at baseline and on Day 15 by intracellular cytokine staining in a subset of participants. In CD4+ and CD8+ T cells, a response was characterized by the expression of IFNγ or IL‐2 (or both). CD4+ and CD8+ T‐cell responses are indicated by blue and green lines, respectively. CD, cluster of differentiation; IFNγ, interferon gamma; IL, interleukin; vp, viral particles
Unlike CD4+ T‐cell responses, antibody and CD8+ T‐cell responses displayed similar kinetics early after vaccination. Response rates for CD8+ T cells ranged from 46% to 54% by 2 weeks postvaccination, as measured by the percentage of cells positive for IFNγ and/or IL‐2 (unpublished data). In contrast with the kinetics of CD4+ T‐cell responses, CD8+ T‐cell responses continued to increase, reaching a response rate of 74% to 82% by 2 months (Day 57) postvaccination, with increasing magnitude of IFNγ and/or IL‐2–positive CD8+ T cells, whereas CD4+ T‐cell responses peaked by 14 days postvaccination and thereafter slightly declined (unpublished data; Figure 3). 4 These data show different kinetics between CD4+ and CD8+ T‐cell responses, with a faster response for the CD4+ T cells, likely needed for interaction with B cells to support the generation of spike‐specific antibodies. The CD8+ T‐cell response in viral infection appears to be of greater magnitude and generally faster than the CD4+ T‐cell response, 23 , 24 whereas the CD4+ T‐cell response predominates in natural SARS‐CoV‐2 infection, 25 , 26 although the CD8+ T‐cell response can appear earlier. 27 Of note, Ad26.COV2.S‐elicited spike‐specific CD4+ and CD8+ T cells are polyfunctional, defined by their ability to produce multiple cytokines as determined by IFNγ, IL‐2, and/or tumor necrosis factor α (TNFα) production. 10 , 18 This is an important characteristic of immune responses elicited by Ad26‐based vaccines, as these different cytokines play key roles by inhibiting viral replication and viral‐infected cell cycles as well as promoting clearance. 28 Several studies point to the potential role of T cells in protection against severe COVID‐19, 21 , 26 , 29 , 30 , 31 and these cytokines could be part of the mechanism conferring protection.
A theoretical risk of vaccine‐associated enhanced respiratory disease (VAERD) 32 , 33 , 34 has been associated with poorly neutralizing humoral immunity and T helper‐2 (Th2)–dominant cellular immune responses against SARS‐CoV‐2. All Ad26.COV2.S‐elicited CD4+ T‐cell responses were Th1 dominant. 4 , 10 CD4+ T cells were shown to produce Th1 cytokines, such IFNγ, IL‐2, and/or TNFα, but not Th2 cytokines, such as IL‐4, IL‐5, or IL‐13, following Ad26.COV2.S vaccination. These results are in line with previous experience with the Ad26‐based vaccine platform. 34 , 35 , 36 The belief that the theoretical risk of VAERD is minimal is further supported by accompanying data showing that the ratio of neutralizing antibody titers to binding antibody titers remains very similar over time. Moreover, in efficacy studies of Ad26.COV2.S, no evidence of VAERD was detected, and Ad26.COV2.S vaccination reduced the severity of COVID‐19 based on a comparison of symptom scoring between vaccine and placebo recipients with breakthrough SARS‐CoV‐2 infection. 6 , 7
3. DURABILITY OF IMMUNE RESPONSES AFTER SINGLE‐DOSE PRIMARY VACCINATION WITH AD26.COV2.S
Overall, the efficacy and durability of protection of a vaccine are related to the quality and magnitude of the immune response. Durability of efficacy depends not only on the longevity of protective levels of antibodies and cell‐mediated immunity, but also on the vaccine’s ability to elicit high‐quality immune memory that will support an anamnestic or recall response in the event of future pathogen exposure. The ability of Ad26.COV2.S to elicit durable humoral and cellular immunity to SARS‐CoV‐2, as well as immune memory, were assessed in several phase 1 and 2 studies.
3.1. Durability of Ad26.COV2.S‐elicited humoral immune responses
As described above, the Ad26.COV2.S‐elicited humoral immune response continued to increase for at least 2‐3 months postvaccination. Antibody titers and response rates were stable until Day 183 (approximately 6 months) and were only slightly lower at Day 190 and Day 211 versus responses at Day 29, 9 , 37 thus confirming the durable humoral responses elicited by a single dose of Ad26.COV2.S. 8 In individuals aged 18‐55 years, a slight decline of approximately 1.5‐fold in neutralizing antibody levels (wtVNA and psVNA) was observed by 8 months after single‐dose vaccination as compared with the titers at 2‐3 months after vaccination. 9 Importantly, at 8 months postvaccination, neutralizing antibody titers were still detectable in 95% of participants 18‐55 years of age. In participants ≥65 years of age, the decline in antibody titers by 9 months postvaccination with Ad26.COV2.S was approximately 2‐fold compared with 1 month postvaccination, coinciding with a lower proportion of participants with detectable neutralizing antibody levels (68%), reflective of a steeper decline in neutralizing antibody levels in the older population. 9 Together, these observations confirm the overall durability of the antibody response, even if a slight decline is observed in the elderly population, after single‐dose primary vaccination with Ad26.COV2.S. Without more detailed knowledge regarding seroprotective levels, the clinical relevance of the slight decline by Months 8 and 9 remains to be established. The kinetics and durability of Ad26.COV2.S‐elicited humoral immune responses are in line with the immunogenicity of other Ad26 vector–based vaccines, such as Ad26.ZIKV.001. 38 Consistent durability of the antibody responses elicited by a single dose of Ad26.COV2.S was observed across different studies. 8 , 9
3.2. Durability of cell‐mediated immune responses
The durability of vaccine‐elicited cell‐mediated immune responses is an important factor in the immunogenicity of vaccines. As mentioned above, both CD4+ and CD8+ T‐cell responses fill critical functions in vaccine‐induced protection, through CD4+ T helper cell–mediated production of virus‐neutralizing antibodies and CD8+ T cell–mediated viral clearance by eliminating infected cells.
After primary vaccination with single‐dose Ad26.COV2.S, CD4+ and CD8+ T‐cell responses, as measured by intracellular cytokine staining (ICS) and IFNγ enzyme‐linked immune absorbent spot (ELISPOT) 4 , 8 , 18 were elicited. CD4+ T‐cell responses against vaccine‐matched SARS‐CoV‐2 spike protein as measured at 4 weeks after vaccination declined slightly by 8 months. A different kinetic was observed for CD8+ T‐cell responses, as spike‐specific CD8+ T cells expressing IFNγ and/or IL‐2 continued to increase between 1 and 8 months postvaccination.
In addition, IFNγ+ spike‐specific CD4+ and CD8+ central memory (CD45RA−CD27+) and effector memory (CD45RA−CD27−) T‐cell subpopulations elicited by Ad26.COV2.S were durable through at least 8 months after a single dose of Ad26.COV2.S in the vast majority of vaccinated participants.
3.3. Breadth of Ad26.COV2.S‐elicited cell‐mediated immune responses
Analysis of the amino acid sequences of SARS‐CoV‐2 variants of concern in comparison to the SARS‐CoV‐2 reference strain (Wuhan‐Hu‐1) has demonstrated that the majority of spike‐specific T‐cell epitopes are conserved in these newly emerging variants. 39 , 40 Likewise, Ad26.COV2.S‐elicited IFNγ+ spike‐specific CD4+ and CD8+ T‐cell responses, as assessed by ICS, were similar against the reference strain (WA1/2020), Delta, and Omicron. 18 These responses were durable through at least 8 months. Similar results were observed using IFNγ ELISPOT on the overall T‐cell responses. IL‐2– and TNFα–producing CD4+ and CD8+ T cells, with reactivity against Delta and Omicron variants, were similarly durable. IFNγ+ spike‐specific CD8+ and CD4+ T‐cell central memory (CD45RA−CD27+) and effector memory (CD45RA−CD27−) subpopulations elicited by Ad26.COV2.S exhibited cross‐reactivity to Delta and Omicron, with a similar level of memory T cells observed compared to 1 month postvaccination. 18
3.4. Anamnestic responses
Following vaccination or infection, immunity to SARS‐CoV‐2 infection or COVID‐19 may be explained by the presence of effective cellular immunity in combination with seroprotective levels of neutralizing and nonneutralizing Fc‐mediated functional antibodies in the upper and lower respiratory tracts, which are needed for viral clearance. In addition, primary vaccination or infection can imprint immune memory by inducing SARS‐CoV‐2–specific memory cells in both the B‐ and T‐cell compartments. 41 , 42 , 43 The presence of SARS‐CoV‐2–specific memory B cells after vaccination is indirectly shown by measuring the early kinetics of antibody responses to SARS‐CoV‐2 spike antigen exposure, irrespective of time since primary vaccination, which can be weeks, months, or even years. As compared with response kinetics after primary vaccination, an immediate immune response that displays faster kinetics upon re‐exposure to the same antigen provides evidence of immune memory established by primary vaccination. These so‐called anamnestic or recall responses play an important role in prolonged vaccine effectiveness, especially in protection against severe COVID‐19, as there is a period between the moment of infection and the development of symptoms.
To study the establishment of immune memory by Ad26.COV2.S, participants who had received a single‐dose primary vaccination with a full dose (5 × 1010 viral particles [vp]) of Ad26.COV2.S were given a low‐dose (1.25 × 1010 vp) Ad26.COV2.S vaccination as a mimic to SARS‐CoV‐2 spike antigen exposure in case of SARS‐CoV‐2 exposure or infection. By 7 days after administration of the low‐dose Ad26.COV2.S, a steep and robust increase in anti–SARS‐CoV‐2 antibodies was observed, as measured by wtVNA and ELISA. 9 Antibody levels further increased by 28 days postadministration of the low dose of Ad26.COV2.S, with 99% of participants having detectable titers, compared with 90% prior to administration of the low dose. The kinetics of the anamnestic response in older adults (aged ≥65 years) were somewhat slower compared with younger individuals. However, even in older adults with undetectable levels of antibody prior to re‐exposure to antigen (86% positivity rate), antibody titers by Day 29 after administration of low‐dose Ad26.COV2.S were similar to those in younger adults, with 97% of older adults having detectable titers. The slower kinetics of the anamnestic response in the elderly could be due to a reduced size and function of the germinal center response or due to an impaired innate immune response. 44 However, the similar titers by Day 29 may imply that the quality of immune memory established by Ad26.COV2.S primary vaccination is sufficient for protection, even in the elderly.
These data show that single‐dose primary vaccination with Ad26.COV2.S elicits durable antibody responses that mature over time, thereby increasing affinity and breadth and resulting in improved coverage of emerging variants of SARS‐CoV‐2. A single dose of Ad26.COV2.S also establishes long‐term B‐cell memory that supports rapid and robust production of spike‐specific antibodies upon re‐exposure to antigen. The durable humoral and cell‐mediated immune responses seen after primary vaccination with Ad26.COV2.S allow for flexible timing of subsequent booster vaccination; this flexibility opens opportunities to optimize immunogenicity/protection and strategic considerations of vaccine deployment.
4. EFFICACY OF AD26.COV2.S
In the phase 3 ENSEMBLE study conducted in the United States, South Africa, and 6 countries in Latin America, vaccine efficacy against COVID‐19 was evaluated following single‐dose primary vaccination with Ad26.COV2.S or placebo in 43 783 participants, of whom 38 295 were seronegative for SARS‐CoV‐2 at baseline, 34% were aged ≥60 years, and 41% had comorbidities. 6 Steps were taken to ensure a representative sample population, and study subjects were racially and ethnically diverse. Enrollment began September 21, 2020, and the data cutoff for final analysis of the double‐blind phase was July 9, 2021, with a median follow‐up of 121 days and 8940 participants followed for at least 6 months. Vaccine efficacy against the primary endpoint of moderate to severe/critical COVID‐19 from 28 days following vaccine administration was 52.9% (95% confidence interval [CI], 47.1‐58.1), an efficacy nearly identical to that observed against all symptomatic COVID‐19 (52.4%; 95% CI, 46.6‐57.6).
Based on Kaplan–Meier analysis, vaccine efficacy against moderate to severe/critical COVID‐19 persisted for approximately 6 to 7 months or longer; a modest decline in efficacy late in the trial was likely related to the emergence of neutralization‐resistant variants at that time. Consistent with this hypothesis, vaccine efficacy was stable through 195 days, with no evidence of waning against moderate to severe/critical COVID‐19 caused by the reference strain (Wuhan‐Hu‐1 sequence with the D614G mutation) and other strains with mutations not significant enough to warrant designation as a variant of interest or concern. This is consistent with the demonstrated persistence of Ad26.COV2.S‐elicited binding and neutralizing antibody titers and of cellular immunity for >7‐8 months. 4 , 8 , 9 , 18
Approximately 2 months after the completion of vaccination in ENSEMBLE, a new variant (Alpha) began circulating in the United States, and protection against moderate to severe/critical COVID‐19 was immediate after its emergence. In contrast, protection against the reference strain and the Beta variant that were circulating when vaccination in the study was ongoing began on Days 14 and 25, respectively. This finding suggests that existing levels of antibody and cellular immunity, potentially in combination with an anamnestic response, were adequate to control infection by the newly emerged Alpha variant. However, the later onset of protection against the Beta variant suggests that higher levels may be required for this variant. The involvement of different arms of the immune response may explain this difference in onset of protection.
Vaccine efficacy against symptomatic disease is impacted by the neutralization resistance of variant strains. 45 In the final analysis of the double‐blind phase of ENSEMBLE, vaccine efficacy against moderate to severe/critical COVID‐19 was 70.2% (95% CI, 35.3‐87.6) against the B.1.1.7 (Alpha) variant, which is relatively neutralization‐sensitive compared with the reference strain. 6 Efficacy was lower against the B.1.351 (Beta) variant (51.9%; 19.1‐72.2), the P.1 (Gamma) variant (36.5%; 14.1‐53.3), the C.37 (Lambda) variant (10.0%; −39.2‐42.1), the B.1.621 (Mu) variant (35.9%; 1.7‐58.7), and the B.1.617.2 (Delta) variant (−5.7%; −177.7‐59.2), although CIs were wide due to the limited number of observations for some strains. All of these variants exhibited a high relative resistance to serum neutralizing activity elicited by Wuhan‐Hu‐1 spike–based COVID‐19 vaccines.
Real‐world data for the relatively neutralization‐resistant Delta variant, which was predominant primarily outside the double‐blind efficacy portion of the ENSEMBLE trial, compared with Alpha and the reference strain, also indicates the impact of neutralization resistance on vaccine effectiveness against symptomatic infection. 46 , 47 , 48 , 49 Such data, due to the nature of the collection system upon which it is based, tend to measure effectiveness against more serious symptomatic manifestations across the spectrum of COVID‐19. Even with this caveat, estimates of the effectiveness of Ad26.COV2.S‐induced immune responses against symptomatic disease caused by the Delta variant ranged from 14% to 85%, indeed reflecting the impact of neutralization resistance on protection against symptomatic disease. 46 , 49 , 50 , 51
Overall vaccine efficacy against severe/critical COVID‐19 was 74.6% (95% CI, 64.7‐82.1), with Kaplan–Meier curves showing that this protection began approximately 7 days after vaccination, a time when antibodies can be detected, but only at very low levels. 6 This finding suggests that only minimal amounts of circulating antibody may be required to protect against lower respiratory tract and systemic infection and/or that cellular immunity and Fc‐mediated humoral immunity may play a larger role against more severe disease. Because the moment of infection is separated by an incubation period from the onset of clinical disease, the anamnestic response, which rapidly induces high levels of antibodies and cell‐mediated immunity upon repeated exposure to an antigen (e.g., after primary vaccination or prior infection), likely plays a central role in protection against severe COVID‐19. In addition, for this more severe clinical endpoint, there was no evidence of waning efficacy for at least 6‐7 months following vaccination, a result that reflects the durability of neutralizing antibody titers, Fc‐functional antibody titers, and cellular immunity as seen in the longer‐term immunologic measurements. There was evidence, however, that variant‐associated neutralization resistance also impacts vaccine efficacy against severe/critical COVID‐19, which was 93.1% (95% CI, 54.4‐99.8) for the reference strain; 71.8% (56.3‐82.3) for non–reference strain SARS‐CoV‐2 lineages, including those sequences with the E484K mutation; 78.4% (34.5‐94.7) for the Beta variant; 63.6% (18.8‐85.1) for the Gamma variant; 67.6% (−29.8‐94.4) for the Lambda variant; and 79.5% (38.5‐94.9) for the Mu variant. 6 These findings suggest that Ad26.COV2.S vaccination induces variant‐dependent seroprotective levels for more severe disease, and this protection is less impacted by neutralization resistance than is symptomatic COVID‐19. It seems most likely that incoming virus is contained in the upper respiratory tract by neutralizing antibodies; however, neutralization‐resistant variants may overcome this protection and reach the lower respiratory tract, where broad cellular immunity and Fc‐mediated antibody functions act as a second line of defense, still preventing more severe COVID‐19 in most, but not all, cases.
Vaccine efficacy against COVID‐19 resulting in medical intervention, which included hospitalization, was 75.6% (adjusted 95% CI, 54.3‐88.0) and remained stable for at least 6‐7 months. For COVID‐19–related death, vaccine efficacy was 82.8% (95% CI, 40.5‐96.8), with protection lasting through at least 6 months postvaccination. Both findings illustrate the effect of persistent immune responses following a single dose of Ad26.COV2.S. Persistence of protection against hospitalization after single‐dose Ad26.COV2.S is further illustrated by real‐world data, obtained outside of the double‐blind period (during a time when the Delta variant was dominant), where effectiveness against hospitalization due to the Delta variant was estimated to range from 60% to 95%, 46 , 50 , 52 , 53 , 54 and from 52% to 83% against death after SARS‐CoV‐2 infection. 51 , 53 Because this protection against the Delta variant was seen 5‐10 months after primary single‐dose immunization, these findings are consistent with the persistence of neutralizing antibody, Fc functional antibody, and cellular responses as seen in immunologic studies. In addition, the range of effectiveness estimates against Delta‐attributed hospitalization and death are in some cases below that seen against the reference strain in the double‐blind ENSEMBLE trial, indicating that neutralization resistance, as implied above, may impact protection against these more serious manifestations of COVID‐19 in addition to its more prominent effect on protection against all symptomatic infection.
Prior infection with SARS‐CoV‐2 is known to boost the magnitude and breadth of Ad26.COV2.S‐induced neutralizing antibodies, binding antibodies, and Fc effector function in a variant‐dependent manner. 55 Similarly, immunization of previously infected individuals can lead to very high levels of neutralizing antibody, 56 a phenomenon observed in the few individuals who were nucleocapsid (N)‐ELISA positive at baseline in immunologic studies with the Ad26.COVS.2 vaccine. 6 In the ENSEMBLE study, 2131 participants in the vaccine group who had no history of symptomatic infection but who were seropositive for SARS‐CoV‐2 N protein at baseline (indicating a prior asymptomatic infection) were compared with 18 924 baseline‐seronegative placebo group participants. This post hoc analysis indicated that having experienced asymptomatic infection could provide 90.4% (95% CI, 83.2‐95.1) protection against moderate to severe/critical COVID‐19 (comparing seropositive to seronegative placebo recipients). Protection further increased to 97.7% (93.3‐99.5) for seropositive individuals who were vaccinated. Furthermore, real‐world data indicate that effectiveness after single‐dose Ad26.COV2.S increases from 68% in those without prior exposure to SARS‐CoV‐2 to 95%‐100% in individuals with prior exposure. 57 These data suggest a strong relationship between immune responses and protection against symptomatic infection and underscore the added protection afforded by vaccination, even in an individual with previous asymptomatic infection.
5. HOMOLOGOUS AND HETEROLOGOUS BOOSTER DOSE: IMMUNOGENICITY AND VACCINE EFFICACY
In the development of Ad26.COV2.S, a conscious decision was made to test the efficacy of single‐dose primary vaccination, based on the target product profile published by the World Health Organization that listed a preference for single‐dose vaccines with at least 70% efficacy against severe COVID‐19. 58 Single‐dose vaccines relieve demands on production and logistics, reduce needs for vaccination capacity, and do not face the challenges of low compliance rates for additional doses of a multi‐dose primary vaccination regimen. However, to mitigate the potentially low efficacy of a single‐dose primary vaccination regimen, an Ad26.COV2.S booster given 2 months after the first dose was also studied for its impact on immunogenicity and vaccine efficacy. 7 , 37 Results from this study will be reviewed in this section.
During 2021, health authorities approved and recommended booster vaccinations to address declining antibody titers, decreasing vaccine efficacy, and emerging SARS‐CoV‐2 variants of concern that demonstrated reduced sensitivity to vaccine‐elicited humoral immunity. This recommendation for boosters was made for both single‐dose and two‐dose primary vaccination regimens according to different schedules. To support maximum flexibility in the use of vaccines as a booster, the impact of a single booster dose of Ad26.COV2.S is being investigated both as homologous (Ad26.COV2.S given to individuals who received single‐dose primary vaccination with the same vaccine) and as heterologous (Ad26.COV2.S given to individuals who received a 2‐dose primary vaccination with a non‐Ad26.COV2.S COVID‐19 vaccine) boosters, both for the impact on SARS‐CoV‐2 immune responses and for the impact on efficacy/effectiveness against COVID‐19. It is important to assess the durability of humoral immune responses elicited by a booster dose in the context of emerging SARS‐CoV‐2 variants, their resistance to vaccine‐elicited immunity, and the need for sustained protection against COVID‐19.
5.1. Impact of interval between primary vaccination and boosting on humoral immunity
To achieve optimal qualitative and quantitative increases in immune responses after homologous boosting with Ad26.COV2.S, we evaluated changes in two key parameters: the interval between single‐dose primary vaccination and booster administration, and the dose level of the booster. As noted above, the durability of humoral and cell‐mediated immune responses after primary vaccination with Ad26.COV2.S allows for flexibility in optimal timing of the subsequent booster.
In a phase 1/2a study, participants aged 18‐55 years who were boosted 2 months (Day 57) after primary vaccination with a single dose of Ad26.COV2.S4 showed an increase of approximately 3‐fold in neutralizing antibody responses (wtVNA) by 28 days postboost (Day 85) compared with the immediate preboost time point. 37 All participants had detectable neutralizing antibody titers postboost and were considered responders to the booster vaccination. Results were consistent with those observed in a phase 1 study in Japan (adults aged 20‐55 and ≥65 years) and a phase 2 study in Europe (adults aged 18‐55 and ≥65 years). 37 In both studies, a 2‐ to 3‐fold increase in neutralizing antibodies was observed by 14 or 28 days postboost compared with immediately preboost; overall neutralizing antibody titers were slightly lower in older versus younger age groups. In all three studies, spike‐binding antibody responses also increased after boosting at 2 months. Furthermore, neutralizing and binding antibody responses after the booster vaccination were higher than responses in convalescent serum from individuals with prior infection seen in the phase 1/2a study. 4
In the phase 3 ENSEMBLE2 study in adults aged ≥18 years with or without comorbidities, spike‐binding antibody concentrations following the Ad26.COV2.S booster given 2 months after primary vaccination increased 4.7‐fold by 14 days postboost (Day 71) compared with immediately preboost (Day 57). 37 Spike‐binding antibody levels were similar across different regions, such as Europe and the United States. Consistent with other studies, binding antibody concentrations at Day 29 after Dose 1 were generally lower for participants aged ≥60 years compared with those aged ≥18‐60 years. Antibody levels immediately preboost (Day 57) were generally comparable in participants ≥60 years of age and those 18‐60 years of age with comorbidities. Responder rates reached 100% by Day 71 (Day 14 postboost) in both age groups and comorbidity strata. 37 Although otherwise healthy individuals can experience severe COVID‐19, illness is more likely to be severe (requiring hospitalization, intensive care unit admission, or death) in patients with numerous common underlying risk factors, such as older age, male sex, lower socioeconomic background, Black and South Asian race, and underlying comorbidities, including chronic lung disease, cardiovascular disease, obesity, immunodeficiency or immunosuppression, chronic liver disease, chronic kidney disease, neurological conditions (dementia), pregnancy, and cancer. 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 Moreover, the risk of severe COVID‐19 illness increases with the number of underlying conditions affecting a patient in a dose‐response relationship. 62
Ad26.COV2.S given as a booster 3 months after primary vaccination in our phase 2 study in adults aged 18‐55 years and ≥65 years elicited an increase in neutralizing antibody levels of approximately 4‐fold compared with the immediate preboost time point. A booster dose administered 3 months after a single dose of Ad26.COV2.S elicited higher antibody titers and similar response/seropositivity rates compared with a booster dose of Ad26.COV2.S given at 2 months. Interestingly, participants ≥65 years of age seemed to have higher antibody titers compared with participants 18‐55 years of age, 37 suggesting that a longer interval between prime and boost is more beneficial in the older population. A follow‐up analysis 1 year postboost will permit assessment of the durability of humoral immune responses elicited by a booster dose administered at a 3‐month interval.
A booster dose of Ad26.COV2.S administered at a 6‐month interval after primary vaccination was also evaluated in a cohort aged 18‐55 years in our phase 1/2a study. By 7 days postboost (Day 190), mean binding antibody concentrations demonstrated a rapid and substantial 4.2‐fold increase from immediate preboost levels. 37 Similarly, neutralizing antibodies (psVNA) increased 3.5‐fold versus preboost levels. A further increase in binding antibody and neutralizing antibody concentrations was seen by 28 days postboost, representing 5.4‐fold and 5.0‐fold increases, respectively, from preboost levels.
Neutralizing antibody responses to the SARS‐CoV‐2 reference strain as well as SARS‐CoV‐2 variants (Beta, Delta, Gamma, and Lambda) were evaluated using an in‐house psVNA following the 6‐month booster dose in the cohort aged 18‐55 years (n = 17). 9 By 7 days postboost, a similar increase in neutralizing antibody levels was observed against all these variants compared with preboost, ranging from 1.6‐ to 2.2‐fold ( 37 and unpublished data). By 28 days postboost, neutralizing antibody titers against these variants had further increased, representing increases of 1.8‐, 3.0‐, 2.1‐, and 2.9‐fold from preboost for Beta, Delta, Gamma, and Lambda variants, respectively. Neutralizing antibody levels against all these variants were lower than for the reference strain by 28 days postboost (4.0‐, 2.0‐, 3.4‐, and 2.0‐fold lower for the Beta, Delta, Gamma, and Lambda variants, respectively ( 37 and unpublished data).
Spike‐binding antibody responses were also measured against the reference strain and the Beta and Delta variants pre‐ and postboosting at 6 months. 9 Unlike with neutralizing antibodies, no marked difference was observed between spike‐binding antibody levels to the reference strain and these 2 variants. This suggests that the differences in the spike protein between Beta and Delta variants and the reference strain did not impact the binding antibody levels, whereas RBD mutations associated with Beta and Delta reduce the neutralization of these variants by Ad26.COV2.S‐elicited antibodies ( 37 and unpublished data).
In summary, a booster dose of Ad26.COV2.S, administered 2 or 3 months after primary vaccination with Ad26.COV2.S, induced a rapid and substantial increase in humoral immune responses. 37 A 6‐month interval between primary vaccination and booster vaccination was associated with trends toward higher responses (Figure 4). Antibody responses to several variants of concern increased proportionally following a booster dose of Ad26.COV2.S. Compared with the reference strain, neutralization of these variants seems to be impacted by RBD mutations, while spike‐binding antibody levels are not affected. These results show the benefits of a homologous Ad26.COV2.S booster dose on humoral immune responses, with implications for neutralization of emerging variants.
FIGURE 4.
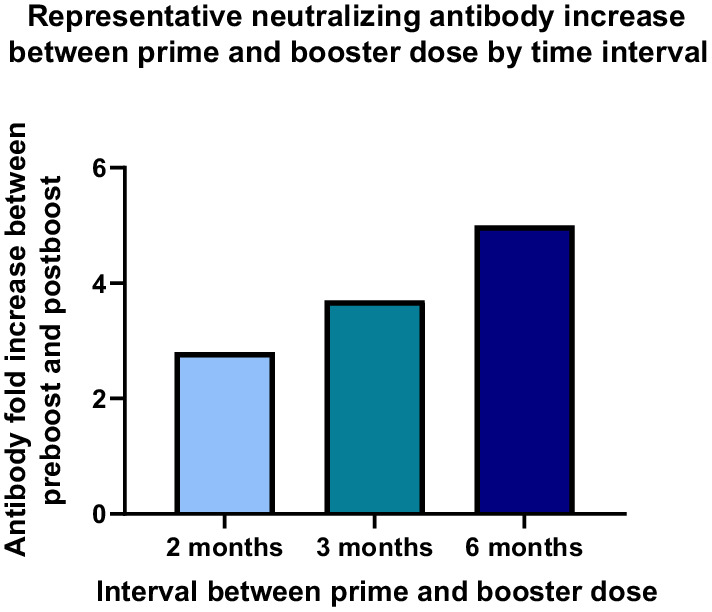
Representative neutralizing antibody increase between prime and booster dose by time interval. Participants in phase 1, phase 1/2a, and phase 2 studies aged 18‐55 years and ≥65 years were given a single dose of Ad26.COV2.S (5 × 1010 vp) at Day 1, followed by a booster dose of the same dose level at 2, 3, or 6 months after the primary dose. Neutralizing antibodies, as measured by wtVNA, were compared before boosting and at 28 days postboost. vp, viral particles; wtVNA, wild‐type virus neutralization assay
5.2. Impact of homologous booster with Ad26.COV2.S on cell‐mediated immune responses
While Ad26.COV2.S as a homologous booster resulted in a similar increase in humoral immune responses in both younger and older adults, albeit with different kinetics, the impact on cell‐mediated immune responses was more heterogeneous between the 2 age groups. In younger adults, a 2‐month boosting interval led to no apparent increase in response rate or magnitude in either IFNγ‐ and/or IL‐2–producing CD4+ T cells or CD8+ T cells by 14 days postboost (unpublished data) versus a single dose (approximately 70% response rate). 4 However, in adults ≥65 years of age boosted slightly later at a 3‐month interval, an increase was observed in both the proportion of participants with a CD4+ T‐cell response (from 42% preboost to 63% postboost) and in the magnitude of the IFNγ‐ and/or IL‐2–producing CD4+ T‐cell response. To a lesser extent, increased CD8+ T‐cell responses were also seen for the proportion of participants with IFNγ‐ and/or IL‐2–producing CD8+ T‐cell responses (55% preboost to 61% postboost) and for median CD8+ T‐cell response. By Day 29 postboost, the CD8+ T‐cell response rate increased to 71%, and median IFNγ‐ and/or IL‐2–producing CD8+ T cells increased to 0.23%; however, CD4+ T‐cell responses remained similar to 14 days postboost (unpublished data).
Due to a protocol deviation related to a clinical pause in the Ad26.COV2.S development program, the interval between primary single‐dose vaccination and homologous booster with Ad26.COV2.S differed between age cohorts. 37 The interval was 3 months in those aged ≥65 years but was 2 months (as per protocol) in those aged 18‐55 years; this difference may explain some of the variation observed in postboost cellular immune responses. Ongoing studies of different time intervals between primary and booster vaccination should clarify these differences. Moreover, additional cytokine and cytotoxic T‐cell markers, such as TNFα and granzyme B/perforin, are currently under evaluation. These are important markers for T‐cell function, and it is imperative to know if their expression is enhanced by a homologous booster dose of Ad26.COV2.S.
These results suggest that an interval of 3 months for a homologous booster dose of Ad26.COV2.S elicits an increase in CD4+ and CD8+ T‐cell responses compared with a 2‐month interval and/or that there are differences in cellular response to a homologous booster dose between younger and older individuals. A homologous booster dose of Ad26.COV2.S with larger intervals from primary vaccination may help clarify whether the interval plays a role in the postboost increase of CD4+ and CD8+ T‐cell responses.
5.3. Association between increased immune responses and vaccine efficacy/efficacy following a homologous booster dose
ENSEMBLE2 is an ongoing, randomized, double‐blind, placebo‐controlled, phase 3 efficacy trial conducted in 10 countries. 7 Enrollment began on November 16, 2020, and the cutoff date for analysis of the double‐blind phase was June 25, 2021. The study enrolled 31 300 participants, 14 492 of whom received 2 vaccinations or 2 doses of placebo, with an interval of 2 months between doses, and who were evaluable for efficacy (per‐protocol set, Ad26.COV2.S n = 7484; placebo n = 7008). Results of this study have been reported on a preprint server and have been submitted to a peer‐reviewed journal for publication. 7 These findings generally indicate that increased immunogenicity following the booster dose at 2 months translates into increased protection against symptomatic, severe/critical COVID‐19 and hospitalization due to SARS‐COV‐2, including infection caused by the Alpha variant and all other variant strains for which case numbers were sufficient to determine efficacy. This study had limited follow‐up due to an early need to immunize the control group after efficacy of the single‐dose regimen had been established.
In the large Sisonke 2 study among approximately 230 000 health care workers in South Africa, a single Ad26.COV2.S booster was given 4‐6 months after primary single‐dose vaccination. By 14‐27 days postboost, the vaccine was 74% effective against hospitalization during the Omicron wave in the 8113 Ad26.COV2.S recipients with PCR tests. 68 With longer follow‐up (1 to 2 months), estimates of efficacy against hospitalization declined minimally (72%). These real‐world data corroborate the concept that a booster immunization at 6 months following the primary immunization can induce immunologic responses that provide considerable protection against the highly neutralization‐resistant Omicron variant. The real‐world data from Sisonke comparing individuals who received a single dose of vaccine to those who received a booster dose is not currently available; however, given the moderate impact of neutralization resistance on protection (including against COVID‐19–related hospitalization and death) for other less neutralization‐resistant variants, it is reasonable to expect that the protection seen against Omicron‐related hospitalization following the booster will exceed protection following a single priming dose.
5.4. Heterologous prime‐boost regimens with Ad26.COV2.S
Given the availability of several approved COVID‐19 vaccines, multiple options exist for heterologous prime‐boost regimens. Ad26.COV2.S has been assessed in multiple studies as primary vaccination with a heterologous booster and a heterologous booster after a primary vaccination with an mRNA‐based COVID‐19 vaccine, for example, in an ongoing, Janssen‐sponsored study and the MixNMatch, COV‐BOOST, and RHH‐001 studies. 69 , 70 , 71
In an ongoing, Janssen‐sponsored, phase 2 clinical study in adults aged ≥18 years, a booster dose of Ad26.COV2.S was given at different dose levels (5 × 1010 vp, 2.5 × 1010 vp, or 1 × 1010 vp) at least 6 months after primary vaccination with 2 doses of the Pfizer/BioNTech mRNA‐based COVID‐19 vaccine BNT162b2. 18 , 72 , 73 Immediately prior to boosting, neutralizing antibodies (psVNA) to the reference strain (WA1/2020) and the Beta, Delta, and Omicron variants were detectable at low levels in the majority of participants. 73 By 2 weeks after boosting with Ad26.COV2.S, neutralizing antibody titers had increased significantly for the virus pseudotyped for the spike of the reference strain (~5‐fold), as well as for the viruses pseudotyped for the spike protein of Delta (~15‐fold), Beta (~15‐fold), and Omicron (~28‐fold) variants. As the study was still blinded at the time of the analysis, these data were aggregated for dose level. Four weeks after the Ad26.COV2.S booster, neutralizing antibody titers against the Omicron variant had further increased approximately 40‐fold compared with the immediate preboost time point. A different kinetic of neutralizing antibody response was observed when participants received a homologous booster with BNT162b2. At 2 weeks postboost, neutralizing antibody titers were higher after the BNT162b2 homologous booster than after a heterologous boost with Ad26.COV2.S. However, neutralizing antibody titers after the homologous BNT162b2 booster subsequently started to decline; by Week 4, these were similar to the neutralizing antibody titers after a booster with Ad26.COV2.S. Similar results were obtained for RBD‐specific binding antibodies, as measured by ELISA. In all measurements, antibody levels against the Omicron variant were lower than antibody levels against the Beta and Delta variants. As psVNA does not capture the impact of increased replicative capacity of a virus on its neutralization sensitivity, these findings may underestimate the true reduction in neutralization sensitivity for the Omicron variant.
In addition, T‐cell responses were evaluated by ICS. 73 Two weeks following the Ad26.COV2.S booster, a 4.3‐ and 5.5‐fold increase was seen in the percentage of IFNγ+ CD8+ T cells (pre‐ and postboost median of 0.018% and 0.078%, respectively, for the reference strain and pre‐ and postboost median of 0.017% and 0.093%, respectively, for Omicron. 73 A 3.2‐ and 3.1‐fold increase was also observed in the percentage of IFNγ+ CD4+ T cells specific to the reference strain and Omicron, respectively (pre‐ and postboost median of 0.030% and 0.096%, respectively, for the reference strain and pre‐ and postboost median of 0.030% and 0.092%, respectively, for Omicron). For both the reference strain and the Omicron variant, CD4+ and CD8+ memory T cells also increased following the Ad26.COV2.S booster dose. No increase in CD4+ T‐cell responses and a lower fold‐increase in CD8+ T‐cell responses (versus Ad26.COV2.S) were observed with a BNT162b2 booster dose compared with the immediate preboost time point. 18
An ongoing phase 1/2 heterologous booster study conducted by the National Institute of Health and the National Institute of Allergy and Infectious Diseases in the United States (DMID 21‐0012), also referred to as the MixNMatch study, is evaluating immune responses in adults who received a homologous or heterologous booster vaccination at least 12 weeks after primary vaccination with an approved mRNA COVID‐19 vaccine regimen (2 doses of Moderna‐mRNA‐1273 [100 μg] or 2 doses of BNT162b2 [30 μg]) or Ad26.COV2.S (1 dose of 5 × 1010 vp]). 70
On Day 15 after boosting with Ad26.COV2.S, and compared with immediate preboost levels, neutralizing antibody responses, as measured by psVNA (50% inhibitory dose [IU50] per milliliter) against the reference strain, had increased by 4‐, 6‐, and 13‐fold, with mean titers (IU50/mL) of 31 (95% CI, 22‐44), 382 (290‐503), and 216 (158‐297) in participants who had received Ad26.COV2.S, mRNA‐1273, and BNT162b2 as the primary vaccination regimen, respectively. Interestingly, binding antibody levels (GMTs) had further increased by Day 29 postboost to 369 (95% CI, 291‐467), 4560 (3544‐5867), and 2600 (2086‐3240), respectively. Thus, a heterologous booster with Ad26.COV2.S after an mRNA‐based primary vaccination results in higher neutralizing antibody levels than a homologous booster dose of Ad26.COV2.S.
In addition to the magnitude of the humoral immune responses, the kinetics of these responses are a key aspect to consider. By Day 15 postboost with mRNA‐1273 or BNT162b2, neutralizing and binding antibody responses were higher than after a booster with Ad26.COV2.S, irrespective of primary vaccination regimen. 70 However, by Day 29 postboost with mRNA‐1273 or BNT162b2 vaccines, neutralizing antibody titers had either slightly decreased or stabilized, whereas neutralizing antibody titers following a heterologous booster with Ad26.COV2.S had further increased between Days 15 and 29; thus, generally similar neutralizing antibody levels were reached by Day 29, irrespective of the heterologous regimen. As the kinetics of heterologous booster responses are different for Ad26.COV2.S and mRNA vaccines, it will be interesting to evaluate later postboost time points to see if these early differences in kinetics translate to differences in durability of the immune responses elicited by these booster doses. Durable immunity is highly desirable to reduce the need for frequent booster vaccinations.
Following heterologous boosting with Ad26.COV2.S, the increase of neutralizing antibody responses against the Beta, Delta, and Omicron variants demonstrated similar kinetics as the antibody responses against the reference strain. Relative to the reference strain, neutralizing activity was 2‐ to 4‐fold lower against Beta, Delta, and Omicron. 70 , 74
CD4+ and CD8+ T‐cell responses to the reference strain were also assessed by ICS in the MixNMatch study. 70 Prior to Ad26.COV2.S heterologous boosting, SARS‐CoV‐2 spike–specific Th1 (IFNγ and/or IL‐2) CD4+ T cells were found in 69% of individuals who received mRNA‐1273 or BNT162b2 primary vaccination. In those who were primed with mRNA‐1273 and boosted with Ad26.COV2.S, by 15 days postboost, the median of spike‐specific CD4+ T cells expressing IFNγ and/or IL‐2 increased from 0.34% to 0.39% from pre‐ to postboost, respectively. In those who were primed with BNT162b2 and boosted with Ad26.COV2, the median of spike‐specific CD4+ T cells expressing IFNγ and/or IL‐2 increased from 0.11% to 0.18% from pre‐ to postboost, respectively. No increase was observed in the total of participants who had positive Th1 responses compared to preboost in both arms, and no spike‐specific Th2 (IL‐4, IL‐5, or IL‐13) CD4+ T‐cell responses were observed.
Spike‐specific CD8+ T cells expressing IFNγ and/or IL‐2 were detected in 10% and 26% of the mRNA‐1273– and BNT162b2‐primed recipients, respectively, before Ad26.COV2.S boost. Booster immunization with Ad26.COV2.S increased the response rate to 60%‐63% in participants primarily vaccinated with mRNA‐1273 and BNT162b2. 70 The amount of spike‐specific CD8+ T cells also increased from 0.03% to 0.11% for participants primed with mRNA‐1273 and from 0.06% to 0.13% for participants primed with BNT162b2.
Compared with results from the ongoing, Janssen‐sponsored, phase 2 clinical study, these results show a slightly lower fold increase in the magnitude of T‐cell responses to the reference strain from pre to post heterologous boost with Ad26.COV2.S. 73 However, the intervals between primary vaccination and heterologous boost were shorter in the MixNMatch study compared with the ongoing, Janssen‐sponsored, phase 2 clinical study, potentially explaining this difference. Overall, these results show that Ad26.COV2.S administered as a heterologous booster sharply increases both antibody and T‐cell responses in mRNA vaccine–primed individuals.
The COV‐BOOST study 69 by the Vaccine Taskforce in the United Kingdom assessed the impact of a booster with Ad26.COV2.S at least 3 months after 2‐dose primary vaccination with either ChAdOx1 nCov‐19 or BNT162b2 on neutralizing antibody titers against the reference strain and the Delta variant. A steep increase in neutralizing and binding antibody levels against both viral strains was observed by Day 29 postboost, with fold increases relative to preboost levels ranging from 5‐ to 7‐fold and 8‐fold for participants receiving primary vaccination with ChAdOx1 and BNT162b2, respectively. Interestingly, the absolute levels of neutralizing and binding antibodies post Ad26.COV2.S booster were approximately 3‐fold higher in BNT162b2‐primed individuals compared with ChAdOx1‐primed individuals. Consistent with heterologous boosting data from the MixNMatch study, Ad26.COV2.S boosting elicits higher levels of antibodies in participants who previously received an mRNA vaccine as primary regimen compared with participants who received an adenoviral vector–based vaccine. Cell‐mediated immune responses to the reference strain, Beta variant, and Delta variant were measured by IFNγ ELISPOT and demonstrated approximately 2.5‐ and 3‐fold increases postboost with Ad26.COV2.S in participants who had received either ChAdOx1 nCov‐19 or BNT162b2 as primary vaccination, respectively.
The COV‐BOOST study showed that Ad26.COV2.S given as a heterologous booster at least 12 weeks after primary vaccination with an approved mRNA COVID‐19 vaccine induced an increase in humoral immune responses, supporting the idea that a heterologous booster with Ad26.COV2.S may improve protection, including against variants of concern, compared with mRNA primary vaccination.
The RHH‐001 study by the Ministry of Health in Brazil evaluated humoral immune responses 6 months after two doses of the CoronaVac inactivated SARS‐CoV‐2 vaccine followed by a homologous booster or heterologous booster with other vaccines, including Ad26.COV2.S. 71 Binding antibody concentrations were low immediately before boosting. After booster vaccination with single‐dose Ad26.COV2.S, spike binding IgG antibody concentrations rose considerably from baseline to Day 28 (geometric fold‐rise 77; 95% CI, 67‐88). Neutralizing antibody concentrations (psVNA) were also low immediately preboost; however, all heterologous boost regimens elicited high concentrations of neutralizing antibodies, with 100% seropositivity reached by Day 28. Antibody responses were greater for all heterologous regimens than the homologous CoronaVac regimen: the geometric mean ratio of Ad26.COV2.S relative to CoronaVac was 6.7 (95% CI, 5.8‐7.7) for spike binding IgG and 8.7 (5.9‐12.9) for neutralizing antibodies. Neutralizing antibody titers measured by a live virus assay in a subset analysis were detectable against the Omicron and Delta variants in 95% (19/20) and 90% (18/20) of samples, respectively, from participants who received the Ad26.COV2.S booster.
Overall, these data indicate that heterologous booster vaccination with Ad26.COV2.S elicits a significant increase in humoral and cellular immune responses against both the SARS‐CoV‐2 reference strain and the currently known variants of concern. The durability of these responses remains to be established. In addition, it is too early to tell whether heterologous boosting with Ad26.COV2.S improves the overall effectiveness of vaccination.
6. CONCLUSION
The Ad26.COV2.S vaccine as a single‐dose primary regimen and a homologous or heterologous booster dose elicits humoral and cellular immune responses that provide durable protection against SARS‐CoV‐2 infection, COVID‐19, and COVID‐19–related hospitalization and death, including against disease caused by emerging SARS‐CoV‐2 variants. As such, it is used worldwide as a prophylactic vaccine in the fight against the COVID‐19 pandemic.
While this review focuses on immunogenicity and protection against COVID‐19, it is important to note that postmarketing surveillance revealed a very rare but potentially fatal safety signal, now called vaccine‐induced thrombosis with thrombocytopenia (VITT) or thrombosis with thrombocytopenia syndrome (TTS) following vaccination with Ad26.COV2.S. 75 , 76 , 77 , 78 This safety signal was first described for ChAdOx1 nCov‐19, the COVID‐19 vaccine from AstraZeneca. 79 , 80 While the pathogenesis of VITT/TTS is being investigated, 81 , 82 , 83 which may reveal pathways to mitigate the risk, the continued use of Ad26.COV2.S is supported by a favorable benefit‐risk ratio. 84 , 85 This ratio can obviously vary per region, linked to the regional burden of COVID‐19, the background immunity in the population, and the availability of alternative vaccines.
All available COVID‐19 vaccines have contributed to the control of the pandemic. Whether additional vaccinations and/or updated vaccines for newly emerging variants will be needed in the future remains to be seen.
CONFLICT OF INTEREST
All authors are employees of Janssen Pharmaceutica NV and may hold Johnson & Johnson stock or stock options.
ACKNOWLEDGEMENTS
This work was supported by Janssen Vaccines & Prevention B.V. Clinical studies conducted by Janssen Vaccines & Prevention B.V. cited in this review may have been funded in whole or in part with federal funds from the Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority, under Other Transaction Agreement HHSO100201700018C and from the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health). Writing and editorial assistance were provided by Kurt Kunz, M.D., M.P.H., and Jill E. Kolesar, Ph.D., of Cello Health Communications/MedErgy and funded by Janssen Global Services, LLC.
Le Gars M, Hendriks J, Sadoff J, et al. Immunogenicity and efficacy of Ad26.COV2.S: An adenoviral vector–based COVID‐19 vaccine. Immunol Rev. 2022;00:1‐14. doi: 10.1111/imr.13088
*This article is part of a series of reviews covering SARS‐CoV‐2 Vaccines appearing in Volume 310 of Immunological Reviews.
Contributor Information
Mathieu Le Gars, Email: mlegars@its.jnj.com.
Hanneke Schuitemaker, Email: hschuite@its.jnj.com.
DATA AVAILABILITY STATEMENT
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical‐trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
REFERENCES
- 1. Karlinsky A, Kobak D. Tracking excess mortality across countries during the COVID‐19 pandemic with the World Mortality Dataset. Elife. 2021;10:e69336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tregoning JS, Flight KE, Higham SL, Wang Z, Pierce BF. Progress of the COVID‐19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat Rev Immunol. 2021;21:626‐636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bos R, Rutten L, van der Lubbe JEM, et al. Ad26 vector‐based COVID‐19 vaccine encoding a prefusion‐stabilized SARS‐CoV‐2 spike immunogen induces potent humoral and cellular immune responses. NPJ Vaccines. 2020;5:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sadoff J, Le Gars M, Shukarev G, et al. Interim results of a phase 1‐2a trial of Ad26.COV2.S COVID‐19 vaccine. N Engl J Med. 2021;384:1824‐1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single‐dose Ad26.COV2.S vaccine against Covid‐19. N Engl J Med. 2021;384:2187‐2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sadoff J, Gray G, Vandebosch A, et al. Final analysis of efficacy and safety of single‐dose Ad26.COV2.S. N Engl J Med. 2022;386:847‐860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hardt K, Vandebosch A, Sadoff J, et al. Efficacy and safety of a booster regimen of Ad26.COV2.S vaccine against Covid‐19. medRxiv. 2022. doi: 10.1101/2022.01.28.22270043 [DOI] [Google Scholar]
- 8. Barouch DH, Stephenson KE, Sadoff J, et al. Durable humoral and cellular immune responses 8 months after Ad26.COV2.S vaccination. N Engl J Med. 2021;385:951‐953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sadoff J, Le Gars M, Cardenas V, et al. Durability of antibody responses elicited by a single dose of Ad26.COV2.S and substantial increase following late boosting. medRxiv. 2021. doi: 10.1101/2021.08.25.21262569 [DOI] [Google Scholar]
- 10. Stephenson KE, Le Gars M, Sadoff J, et al. Immunogenicity of the Ad26.COV2.S vaccine for COVID‐19. JAMA. 2021;325:1535‐1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alter G, Yu J, Liu J, et al. Immunogenicity of Ad26.COV2.S vaccine against SARS‐CoV‐2 variants in humans. Nature. 2021;596:268‐272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Luo H, Jia T, Chen J, et al. The characterization of disease severity associated IgG subclasses response in COVID‐19 patients. Front Immunol. 2021;12:632814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bahnan W, Wrighton S, Sundwall M, et al. Spike‐dependent opsonization indicates both dose‐dependent inhibition of phagocytosis and that non‐neutralizing antibodies can confer protection to SARS‐CoV‐2. Front Immunol. 2021;12:808932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Keeler SP, Fox JM. Requirement of Fc‐Fc gamma receptor interaction for antibody‐based protection against emerging virus infections. Viruses. 2021;13:1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lu LL, Suscovich TJ, Fortune SM, Alter G. Beyond binding: antibody effector functions in infectious diseases. Nat Rev Immunol. 2018;18:46‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bournazos S, Gupta A, Ravetch JV. The role of IgG Fc receptors in antibody‐dependent enhancement. Nat Rev Immunol. 2020;20:633‐643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hirabara SM, Serdan TDA, Gorjao R, et al. SARS‐COV‐2 variants: differences and potential of immune evasion. Front Cell Infect Microbiol. 2021;11:781429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu J, Chandrashekar A, Sellers D, et al. Vaccines elicit highly conserved cellular immunity to SARS‐CoV‐2 Omicron. Nature. 2022;603:493‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sun Y, Lin W, Dong W, Xu J. Origin and evolutionary analysis of the SARS‐CoV‐2 Omicron variant. J Biosaf Biosecur. 2022;4:33‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schmidt F, Muecksch F, Weisblum Y, et al. Plasma neutralization of the SARS‐CoV‐2 Omicron variant. N Engl J Med. 2022;386:599‐601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sekine T, Perez‐Potti A, Rivera‐Ballesteros O, et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID‐19. Cell. 2020;183:158‐168.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Horton H, Thomas EP, Stucky JA, et al. Optimization and validation of an 8‐color intracellular cytokine staining (ICS) assay to quantify antigen‐specific T cells induced by vaccination. J Immunol Methods. 2007;323:39‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol. 2003;4:835‐842. [DOI] [PubMed] [Google Scholar]
- 24. Kaech SM, Wherry EJ, Ahmed R. Effector and memory T‐cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251‐262. [DOI] [PubMed] [Google Scholar]
- 25. Cohen KW, Linderman SL, Moodie Z, et al. Longitudinal analysis shows durable and broad immune memory after SARS‐CoV‐2 infection with persisting antibody responses and memory B and T cells. Cell Rep Med. 2021;2:100354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moss P. The T cell immune response against SARS‐CoV‐2. Nat Immunol. 2022;23:186‐193. [DOI] [PubMed] [Google Scholar]
- 27. Sette A, Crotty S. Adaptive immunity to SARS‐CoV‐2 and COVID‐19. Cell. 2021;184:861‐880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Seder RA, Darrah PA, Roederer M. T‐cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247‐258. [DOI] [PubMed] [Google Scholar]
- 29. McMahan K, Yu J, Mercado NB, et al. Correlates of protection against SARS‐CoV‐2 in rhesus macaques. Nature. 2021;590:630‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Swadling L, Diniz MO, Schmidt NM, et al. Pre‐existing polymerase‐specific T cells expand in abortive seronegative SARS‐CoV‐2. Nature. 2022;601:110‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bange EM, Han NA, Wileyto P, et al. CD8+ T cells contribute to survival in patients with COVID‐19 and hematologic cancer. Nat Med. 2021;27:1280‐1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Deming D, Sheahan T, Heise M, et al. Vaccine efficacy in senescent mice challenged with recombinant SARS‐CoV bearing epidemic and zoonotic spike variants. PLoS Med. 2006;3:e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Iwata‐Yoshikawa N, Uda A, Suzuki T, et al. Effects of toll‐like receptor stimulation on eosinophilic infiltration in lungs of BALB/c mice immunized with UV‐inactivated severe acute respiratory syndrome‐related coronavirus vaccine. J Virol. 2014;88:8597‐8614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yasui F, Kai C, Kitabatake M, et al. Prior immunization with severe acute respiratory syndrome (SARS)‐associated coronavirus (SARS‐CoV) nucleocapsid protein causes severe pneumonia in mice infected with SARS‐CoV. J Immunol. 2008;181:6337‐6348. [DOI] [PubMed] [Google Scholar]
- 35. Barouch DH, Tomaka FL, Wegmann F, et al. Evaluation of a mosaic HIV‐1 vaccine in a multicentre, randomised, double‐blind, placebo‐controlled, phase 1/2a clinical trial (APPROACH) and in rhesus monkeys (NHP 13‐19). Lancet. 2018;392:232‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Anywaine Z, Whitworth H, Kaleebu P, et al. Safety and immunogenicity of a 2‐dose heterologous vaccination regimen with Ad26.ZEBOV and MVA‐BN‐filo Ebola vaccines: 12‐month data from a phase 1 randomized clinical trial in Uganda and Tanzania. J Infect Dis. 2019;220:46‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Janssen . COVID‐19 vaccine Ad26.COV2.S VAC31518 (JNJ‐78436735). Janssen’s briefing materials: Vaccines and Related Biological Products Advisory Committee; 2021. https://www.fda.gov/media/152954/download
- 38. Salisch NC, Stephenson KE, Williams K, et al. A double‐blind, randomized, placebo‐controlled phase 1 study of Ad26.ZIKV.001, an Ad26‐vectored anti–Zika virus vaccine. Ann Intern Med. 2021;174:585‐594. [DOI] [PubMed] [Google Scholar]
- 39. Tarke A, Coelho CH, Zhang Z, et al. SARS‐CoV‐2 vaccination induces immunological T cell memory able to cross‐recognize variants from Alpha to Omicron. Cell. 2022;185:847‐859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Choi SJ, Kim DU, Noh JY, et al. T cell epitopes in SARS‐CoV‐2 proteins are substantially conserved in the Omicron variant. Cell Mol Immunol. 2022;19:447‐448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Turner JS, Kim W, Kalaidina E, et al. SARS‐CoV‐2 infection induces long‐lived bone marrow plasma cells in humans. Nature. 2021;595:421‐425. [DOI] [PubMed] [Google Scholar]
- 42. Goel RR, Apostolidis SA, Painter MM, et al. Distinct antibody and memory B cell responses in SARS‐CoV‐2 naive and recovered individuals following mRNA vaccination. Sci Immunol. 2021;6:eabi6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pape KA, Dileepan T, Kabage AJ, et al. High‐affinity memory B cells induced by SARS‐CoV‐2 infection produce more plasmablasts and atypical memory B cells than those primed by mRNA vaccines. Cell Rep. 2021;37:109823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lee JL, Linterman MA. Mechanisms underpinning poor antibody responses to vaccines in ageing. Immunol Lett. 2022;241:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fiolet T, Kherabi Y, MacDonald CJ, Ghosn J, Peiffer‐Smadja N. Comparing COVID‐19 vaccines for their characteristics, efficacy and effectiveness against SARS‐CoV‐2 and variants of concern: a narrative review. Clin Microbiol Infect. 2022;28:202‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Polinski JM, Weckstein AR, Batech M, et al. Durability of the single‐dose Ad26.COV2.S vaccine in the prevention of COVID‐19 infections and hospitalizations in the US before and during the Delta variant surge. JAMA Netw Open. 2022;5:e222959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lin DY, Gu Y, Wheeler B, et al. Effectiveness of Covid‐19 vaccines over a 9‐month period in North Carolina. N Engl J Med. 2022;386:933‐941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Risk M, Shen C, Hayek SS, et al. Comparative effectiveness of coronavirus disease (COVID‐19) vaccines against the Delta variant. Clin Infect Dis. 2022. Epub ahead of print. doi: 10.1093/cid/ciac106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Corchado‐Garcia J, Zemmour D, Hughes T, et al. Analysis of the effectiveness of the Ad26.COV2.S adenoviral vector vaccine for preventing COVID‐19. JAMA Netw Open. 2021;4:e2132540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rosenberg ES, Dorabawila V, Easton D, et al. Covid‐19 vaccine effectiveness in New York state. N Engl J Med. 2022;386:116‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cohn BA, Cirillo PM, Murphy CC, Krigbaum NY, Wallace AW. SARS‐CoV‐2 vaccine protection and deaths among US veterans during 2021. Science. 2022;375:331‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Grannis SJ, Rowley EA, Ong TC, et al. Interim estimates of COVID‐19 vaccine effectiveness against COVID‐19–associated emergency department or urgent care clinic encounters and hospitalizations among adults during SARS‐CoV‐2 B.1.617.2 (Delta) variant predominance—nine states, June–August 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1291‐1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bekker LG, Garrett N, Goga A, et al. Effectiveness of the Ad26.Cov2.S Vaccine in Health Care Workers in South Africa (November 17, 2021). Accessed January 5, 2022. doi: 10.2139/ssrn.3979291 [DOI]
- 54. de Gier B, Kooijman M, Kemmeren J, et al. COVID‐19 vaccine effectiveness against hospitalizations and ICU admissions in the Netherlands, April‐August 2021. medRxiv. 2021. doi: 10.1101/2021.09.15.21263613 [DOI] [Google Scholar]
- 55. Keeton R, Richardson SI, Moyo‐Gwete T, et al. Prior infection with SARS‐CoV‐2 boosts and broadens Ad26.COV2.S immunogenicity in a variant‐dependent manner. Cell Host Microbe. 2021;29:1611‐1619.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Crotty S. Hybrid immunity. Science. 2021;372:1392‐1393. [Google Scholar]
- 57. Wright BJ, Tideman S, Diaz GA, French T, Parsons GT, Robicsek A . Comparative vaccine effectiveness against severe COVID‐19 over time in US hospital administrative data: a case‐control study. Lancet Respir Med. 2022. Epub ahead of print. doi: 10.1016/S2213-2600(22)00042-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. World Health Organization (WHO) . WHO target product profiles for COVID‐19 vaccines 2020. Accessed December 15, 2021. https://www.who.int/publications/m/item/who‐target‐product‐profiles‐for‐covid‐19‐vaccines
- 59. U.S. Centers for Disease Control and Prevention (CDC) . Underlying medical conditions associated with higher risk for severe COVID‐19: information for healthcare professionals. Accessed March 13, 2022. https://www.cdc.gov/coronavirus/2019‐ncov/hcp/clinical‐care/underlyingconditions.html [PubMed]
- 60. Escobar GJ, Adams AS, Liu VX, et al. Racial disparities in COVID‐19 testing and outcomes: retrospective cohort study in an integrated health system. Ann Intern Med. 2021;174:786‐793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Harrison SL, Fazio‐Eynullayeva E, Lane DA, Underhill P, Lip GYH. Comorbidities associated with mortality in 31,461 adults with COVID‐19 in the United States: a federated electronic medical record analysis. PLoS Med. 2020;17:e1003321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kompaniyets L, Pennington AF, Goodman AB, et al. Underlying medical conditions and severe illness among 540,667 adults hospitalized with COVID‐19, March 2020–March 2021. Prev Chronic Dis. 2021;18:E66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta‐analysis. BMJ. 2020;370:m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kompaniyets L, Goodman AB, Belay B, et al. Body mass index and risk for COVID‐19–related hospitalization, intensive care unit admission, invasive mechanical ventilation, and death — United States, March–December 2020. MMWR Morb Mortal Wkly Rep. 2021;70:355‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID‐19‐related death using OpenSAFELY. Nature. 2020;584:430‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gold JAW, Rossen LM, Ahmad FB, et al. Race, ethnicity, and age trends in persons who died from COVID‐19 – United States, May–August 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1517‐1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. McMichael TM, Currie DW, Clark S, et al. Epidemiology of Covid‐19 in a long‐term care facility in King County, Washington. N Engl J Med. 2020;382:2005‐2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gray GE, Collie S, Garrett N, et al. Effectiveness of Ad26.COV2.S and BNT162b2 vaccines against Omicron variant in South Africa. N Engl J Med. 2022. Epub ahead of print. doi: 10.1056/NEJMc2202061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Munro APS, Janani L, Cornelius V, et al. Safety and immunogenicity of seven COVID‐19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov‐19 or BNT162b2 in the UK(COV‐BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021;398:2258‐2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Atmar RL, Lyke KE, Deming ME, et al. Homologous and heterologous covid‐19 booster vaccinations. N Engl J Med. 2022;386:1046‐1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Costa Clemens SA, Weckx L, Clemens R, et al. Heterologous versus homologous COVID‐19 booster vaccination in previous recipients of two doses of CoronaVac COVID‐19 vaccine in Brazil (RHH‐001): a phase 4, non‐inferiority, single blind, randomised study. Lancet. 2022;399:521‐529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Walsh EE, Frenck RW Jr, Falsey AR, et al. Safety and immunogenicity of two RNA‐based Covid‐19 vaccine candidates. N Engl J Med. 2020;383:2439‐2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tan CS, Collier AY, Liu J, et al. Homologous and heterologous vaccine boost strategies for humoral and cellular immunologic coverage of the SARS‐CoV‐2 Omicron variant. medRxiv. 2021. doi: 10.1101/2021.12.02.21267198 [DOI] [Google Scholar]
- 74. Lyke KE, Atmar RL, Islas CD, et al. SARS‐CoV‐2 Omicron neutralization after heterologous vaccine boosting. medRxiv. 2022. doi: 10.1101/2022.01.13.22268861 [DOI] [Google Scholar]
- 75. See I, Lale A, Marquez P, et al. Case series of thrombosis with thrombocytopenia syndrome after COVID‐19 vaccination—United States, December 2020 to August 2021. Ann Intern Med. 2022;175:513‐522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. See I, Su JR, Lale A, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325:2448‐2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Oliver SE, Wallace M, See I, et al. Use of the Janssen (Johnson & Johnson) COVID‐19 vaccine: updated interim recommendations from the Advisory Committee on Immunization Practices — United States, December 2021. MMWR Morb Mortal Wkly Rep. 2022;71:90‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. U.S. Centers for Disease Control and Prevention (CDC) . Selected adverse events reported after COVID‐19 vaccination. Accessed March 15, 2022. https://www.cdc.gov/coronavirus/2019‐ncov/vaccines/safety/adverse‐events.html
- 79. Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov‐19 vaccination. N Engl J Med. 2021;384:2092‐2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Schultz NH, Sorvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV‐19 vaccination. N Engl J Med. 2021;384:2124‐2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Huynh A, Kelton JG, Arnold DM, Daka M, Nazy I. Antibody epitopes in vaccine‐induced immune thrombotic thrombocytopaenia. Nature. 2021;596:565‐569. [DOI] [PubMed] [Google Scholar]
- 82. Greinacher A, Selleng K, Palankar R, et al. Insights in ChAdOx1 nCoV‐19 vaccine‐induced immune thrombotic thrombocytopenia. Blood. 2021;138:2256‐2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Baker AT, Boyd RJ, Sarkar D, et al. ChAdOx1 interacts with CAR and PF4 with implications for thrombosis with thrombocytopenia syndrome. Sci Adv. 2021;7:eabl8213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hippisley‐Cox J, Patone M, Mei XW, et al. Risk of thrombocytopenia and thromboembolism after Covid‐19 vaccination and SARS‐CoV‐2 positive testing: self‐controlled case series study. BMJ. 2021;374:n1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. MacNeil JR, Su JR, Broder KR, et al. Updated recommendations from the advisory committee on immunization practices for use of the Janssen (Johnson & Johnson) COVID‐19 vaccine after reports of thrombosis with thrombocytopenia syndrome among vaccine recipients — United States, April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:651‐656. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical‐trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.


