Abstract
Patients diagnosed with coronavirus disease 2019 (COVID-19) associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection frequently experience symptom burden post-acute infection or post-hospitalisation. We aimed to identify optimal strategies for follow-up care that may positively impact the patient's quality of life (QoL). A European Respiratory Society (ERS) Task Force convened and prioritised eight clinical questions. A targeted search of the literature defined the timeline of “long COVID” as 1–6 months post-infection and identified clinical evidence in the follow-up of patients. Studies meeting the inclusion criteria report an association of characteristics of acute infection with persistent symptoms, thromboembolic events in the follow-up period, and evaluations of pulmonary physiology and imaging. Importantly, this statement reviews QoL consequences, symptom burden, disability and home care follow-up. Overall, the evidence for follow-up care for patients with long COVID is limited.
Short abstract
Follow-up care of patients infected with SARS-CoV-2 is crucial and may improve their quality of life. More evidence and research is emerging to understand the causes, mechanisms and risks of long COVID consequences. https://bit.ly/3J1WMWy
Scope of document
The European Respiratory Society (ERS) Task Force identified the need for a statement to identify approaches to optimise clinical follow-up care in patients with long COVID.
A multidisciplinary Task Force of ERS members, specialists in pneumonology, radiology and outcomes assessment, convened on 23 December 2020. Key clinical questions relating to the follow-up of patients with long COVID were identified and prioritised by consensus. The Task Force was approved as part of ERS TF-2020-14 (“The European Respiratory Society Guideline for Management of COVID-19”; Chairs J. Chalmers and N. Roche) and follows other ERS COVID-19 initiatives [1–3]. The Task Force reviewed features of acute disease that could predict long-term consequences, data on thromboembolic event risk, as well as infection control during the long COVID period. Further, the Task Force reviewed the evidence for cardiopulmonary and imaging techniques, and techniques for cognitive, psychological, disability and home care follow-up.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has infected over 300 million people and resulted in the deaths of over 6 million [4]. The natural history of COVID-19 and the long-term sequelae, with adverse health outcomes and impact on health-related quality of life (HRQoL), are not fully understood [5, 6].
Many patients who suffered from COVID-19 recover their baseline health status, but an uncertain proportion of COVID-19 survivors have persistent symptoms presenting a challenge for patients and physicians [7, 8]. These longer term consequences, thought to occur in ∼10% of people infected [9], appear to vary in severity, often impacting multiple organs. While the primary symptoms are often breathlessness, fatigue and sleeping difficulties, low-grade fever, depression, anxiety, and cardiac, pulmonary and renal anomalies have been reported [5], and there is no established nomenclature on how to define the lasting sequelae of COVID-19. Those proposed lack clear criteria of how to define this “condition” or how to stratify patients [10–13].
Guidelines
The National Institute for Health and Care Excellence, Scottish Intercollegiate Guidelines Network, and Royal College of General Practitioners in the UK published a rapid guideline on the management of the long-term effects of COVID-19 in December 2020 (updated November 2021) [14]. This guideline defines post-COVID-19 syndrome as “signs and symptoms that develop during or after an infection consistent with COVID-19, continue for more than 12 weeks and are not explained by an alternative diagnosis”. Referral to post-COVID-19 syndrome assessment clinics is recommended when symptoms persist for 6–12 weeks. Acute COVID-19 signs and symptoms are characterised to occur up to 4 weeks following diagnosis, while “long COVID” describes signs and symptoms that continue or develop after acute COVID-19, and “post-COVID-19 syndrome” encapsulates those with symptoms persisting >12 weeks [14].
The UK National Institute for Health Research (NIHR) [15] suggests that people experiencing long COVID may exhibit distinct clinical entities, such as post-intensive care syndrome (equivalent to the acute post-COVID-19 phase), post-viral fatigue syndrome (if fatigue is the predominant post-COVID-19 symptom), permanent organ damage (an underlying mechanism explaining long-term symptoms) and long-term COVID-19 syndrome (equivalent to long and persistent post-COVID-19 phases) based on the hypothesis that post-COVID-19 symptoms vary in intensity and duration and are not linear or sequential [16]. In this statement we use the term “long COVID” to incorporate elements most in need of clinical follow-up and to include subgroups with ongoing symptomatic COVID-19 and post-COVID-19 syndrome.
Guidelines for the follow-up of long COVID
The clinical management of long COVID is challenging due to a lack of evidence-based guidelines and standardisation in the pulmonary definition/terminology of the post-COVID-19 “condition”. The French Respiratory Medicine Society propose a complete lung evaluation in patients with symptoms persisting ≥12 weeks following infection (day 0 defined as the day of hospital admission or beginning of symptoms) [17]. The British Thoracic Society guidance supports algorithms for evaluating COVID-19 survivors in the first 3 months after hospital discharge based on the severity of acute COVID-19 and whether intensive care unit (ICU)-level care was delivered [18]. Algorithms for both severe and mild-to-moderate COVID-19 groups recommend clinical assessment and cardiopulmonary evaluation in all patients at 12 weeks, according to clinical judgement. Based on the findings of the 12-week assessment, patients either have further evaluation or are discharged. Prior to this, an earlier clinical assessment for respiratory, psychiatric and thromboembolic sequelae and rehabilitation needs is recommended at 4–6 weeks post-discharge for those with severe acute COVID-19, defined as those needing ICU- or high dependency unit-level care or those hospitalised with severe pneumonia, the elderly and all those with comorbidities [18]. Parameters for “elderly” were not defined. Interim guidance on rehabilitation in hospital and post-discharge published by an ERS/American Thoracic Society (ATS)-coordinated international Task Force recommends early bedside rehabilitation for patients affected by severe COVID-19, assessment of oxygen needs at discharge and more comprehensive assessment of rehabilitation needs including physical as well as mental status 6–8 weeks post-discharge [2].
Monitoring patients with COVID-19 after discharge is necessary to understand the extent and severity of long-term effects. In this statement, we will focus on long COVID follow-up 1–6 months post-acute COVID-19 infection. We define day 0 as the day of discharge or the day of the beginning of symptoms. We aim to address questions about potent predictors of long-term consequences, as well as optimal post-hospital assessment related to thromboembolic events, pulmonary physiology, imaging and infection control, based on current data. Further, we will appraise appropriate follow-up concerning cognitive and psychological functioning, quality of life (QoL), disability, and home care.
Methods
The Task Force consisted of 12 members (including an Early Career Member), experts in respiratory medicine, pulmonary physiology, radiology and outcomes assessment. Task Force members were selected by the Chairs based on their expertise and international representation.
The Chairs composed a list of 16 clinically important topics relevant to the follow-up of COVID-19 infection. Two meetings of Task Force members were convened and eight topics were selected according to clinical urgency and by consensus of the Task Force (table 1). Task Force members were divided into subgroups to address the topics. All questions were addressed following the ERS rules for statements. Statements are based on systematic literature searches (conducted by information specialists). A full systematic review with meta-analyses and grading of the evidence was not performed, and as a result this document does not contain recommendations for clinical practice. Individual literature searches for every question were designed by professional librarians, with the input of Task Force members. Systematic searches were conducted up to 26 March 2021 in MEDLINE and Cochrane CENTRAL. For the full search strategies, see appendix A in the supplementary material.
TABLE 1.
The eight clinical priorities and questions addressed by this Task Force
| 1 | Are there features of the acute disease characteristics which predict long-term consequences? |
| 2 | Which follow-up strategies relate to thromboembolic events? |
| 3 | Which follow-up strategies relate to pulmonary physiology? |
| 4 | Which follow-up strategies relate to imaging? |
| 5 | Which follow-up strategies relate to infection control? |
| 6 | Which follow-up strategies relate to cognitive, psychological and quality-of-life consequences? |
| 7 | Which follow-up strategies relate to disability? |
| 8 | Which follow-up strategies relate to home care follow-up (tele-medicine/tele-rehabilitation)? |
Studies meeting the inclusion criteria were published in English, reporting in adult populations, and on outcomes 1–6 months post-discharge in hospitalised and non-hospitalised patients. Case reports and case series were excluded, unless otherwise specified (supplementary table S1 in appendix B in the supplementary material). The study selection was finalised in April 2021. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) diagrams for each question are provided in appendix C in the supplementary material. Preliminary individual subsections were further discussed in a virtual meeting (May 2021) and revised until consensus among all co-authors was reached (June 2021). All co-authors critically revised and approved the final statement.
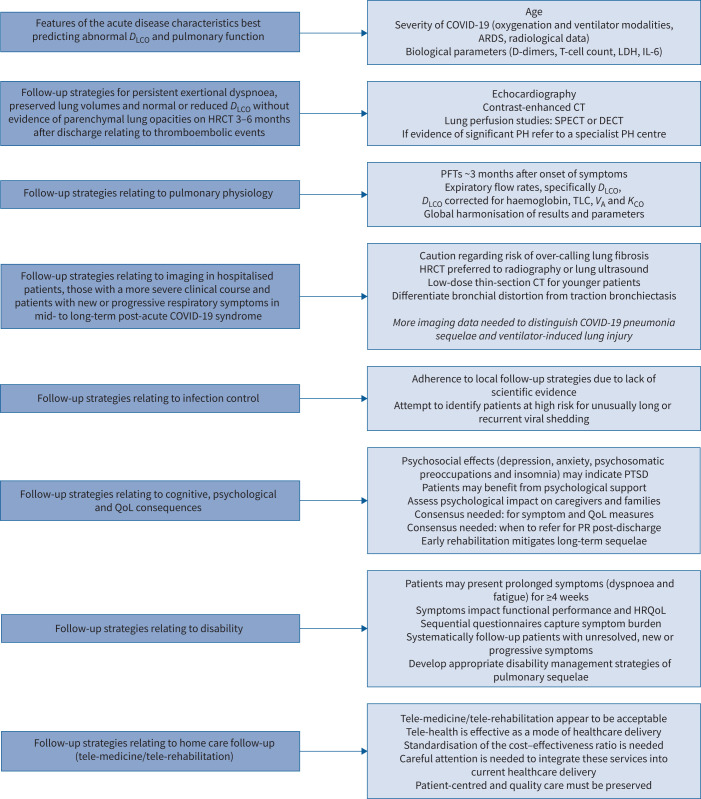
This ERS statement combines an evidence-based approach, clinical expertise of Task Force members, systematic search of the literature and critical discussions from virtual meetings. The statement summarises relevant literature and current practice by topic. It does not provide de novo recommendations for clinical practice but indicates where the Task Force members are in agreement with published guidance. Figure 1 illustrates the current practice of Task Force members. All members of the Task Force disclosed their conflicts of interest before initiation of the project and upon submission of the manuscript.
FIGURE 1.
Current practice of the Task Force members on their management of patients with long COVID-19, 1–6 months post-acute COVID-19 infection (based on the eight clinical priorities and questions listed in table 1). This figure describes the current practice of how the members of the Task Force treat patients with COVID-19 and is not intended as a recommendation for clinical practice. DLCO: diffusing capacity of the lung for carbon monoxide; ARDS: acute respiratory distress syndrome; LDH: lactate dehydrogenase; IL: interleukin; (HR)CT: (high-resolution) computed tomography; SPECT: single-photon emission computed tomography; DECT: dual-energy computed tomography; PH: pulmonary hypertension; PFT: pulmonary function test; VA: alveolar volume; KCO: transfer coefficient of the lung for carbon monoxide; PTSD: post-traumatic stress disorder; (HR)QoL: (health-related) quality of life; PR: pulmonary rehabilitation.
Clinical Question 1. Are there features of the acute disease characteristics which predict long-term consequences?
Evidence overview
Few data are available on predictors of long-term consequences of COVID-19. They mainly include pulmonary fibrosis, as described by reduced single breath diffusing capacity of the lung for carbon monoxide (DLCO), restrictive syndrome and persistent ground-glass opacities (GGO), and fatigue and/or anxiety 1–8 months post-COVID-19. Initially, 4524 records were identified. 12 eligible studies were included (one retrospective cohort study and 11 prospective studies) [8, 19–29], focusing directly on predicting factors of pulmonary fibrosis and/or persistent symptoms after a COVID-19 episode (appendix C in the supplementary material). Of the 12 studies, the majority reported on hospitalised patients and three reported on both hospitalised and non-hospitalised. No studies included non-hospitalised patients only.
The main persistent symptoms reported in the included studies were fatigue (50–65% of patients) and anxiety/depression (20–40%). Risk factors for COVID-19 persistent symptoms, especially fatigue, were not associated with initial severity [19], but with age, female gender and the number of symptoms during the first week of infection [19]. These results were not found in all studies [20]. Few studies focused on olfactory and gustatory late resolution. The initial grade of dysfunction (total or partial), gender and presence of nasal congestion appeared as potential predictive factors [21].
The vast majority of the studies included in this analysis focused on pulmonary fibrosis and reduced DLCO. The size of the cohorts and/or the design does not permit the calculation or estimation of the pulmonary fibrosis risk. Han et al. [22] reported (in 114 hospitalised patients) that one-third of patients had fibrotic-like lesions on a computed tomography (CT) scan done 6 months after discharge.
Among all possible factors, age was the most frequent predictor of long-term consequences [8, 22], possibly because the ageing lung is more susceptible to the development of a fibrotic response or elderly people may have a subclinical interstitial lung disease exacerbated by acute infection [30]. Presence of acute respiratory distress syndrome (ARDS) (OR 13, 95% CI 3.3–55) at the acute phase of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and severity of the initial disease [8, 22, 23] were two predictive factors of pulmonary fibrosis, but these data are also demonstrated in other causes of ARDS. According to these studies, COVID-19 severity, as a predictor of pulmonary fibrosis, is evaluated by need for mechanical ventilation, ventilation duration, opacity score at discharge and hospitalisation duration [8, 22]. Some biological parameters have been found to be associated with higher risk of pulmonary fibrosis: high lactate dehydrogenase (LDH) level on admission, low level of T-cells and prolonged elevation of interleukin-6 [24]. These parameters again reflect disease severity and indicate the dysregulated immune response [25]. LDH has already been considered as a marker of pulmonary injury [26].
In the studies focusing on DLCO decrease, the time to evaluate pulmonary function was variable between 1 and 8 months, so the long-term outcome of these abnormalities remains unknown. As for pulmonary fibrosis, initial disease severity [23, 27, 28], often evaluated by oxygenation modalities, sometimes by intensive care severity score and/or by other organ failure (e.g. renal failure), appeared as the main risk factor. High-flow oxygen therapy and mechanical ventilation (invasive and non-invasive ventilation) are associated to a higher risk of diffusion impairment (OR 4.6 in Huang et al. [8]). Biologically, a higher level of D-dimer at admission in a small cohort of 55 patients was an independent predictor of abnormal DLCO at 3 months [29].
Concluding remarks
Age, severity of COVID-19 (evaluated by a range of variables: oxygenation and ventilator modalities, ARDS, and radiological data) and some biological parameters (D-dimers, T-cell count, LDH and interleukin-6) appeared to be the best predictors of abnormal DLCO and pulmonary fibrosis occurrence. On the contrary, severity of initial disease was not associated with the persistence of symptoms, which appeared to be linked to gender, age or the number of symptoms during the first week. These data are based on a few small studies and will need to be confirmed in new, larger studies.
Clinical Question 2. Which follow-up strategies relate to thromboembolic events?
Evidence overview
Hypercoagulability is a frequent haematological alteration in hospitalised patients with COVID-19. Clinical manifestations include venous thromboembolism (VTE), disseminated intravascular coagulation, thrombosis of the lung microvascular circulation and arterial thrombosis. The systematic literature search identified 1181 studies on long-term consequences and patient follow-up. Six eligible studies (three prospective studies, two retrospective studies and one strategy proposition) [31–36] were included (appendix C in the supplementary material).
Four studies examined the rate of thrombosis after discharge. In an Italian follow-up study of 767 patients with COVID-19, 51% still reported symptoms at a median time of 81 days after discharge, with 38% of those having an elevated D-dimer level [31], including two with asymptomatic pulmonary thrombosis discovered by investigating striking D-dimer elevation. In a retrospective cohort study of 163 post-discharge patients with confirmed COVID-19 not receiving anticoagulation, the cumulative incidence of thrombosis (including arterial and venous events) at day 30 following discharge was 2.5% (95% CI 0.8–7.6%); the cumulative incidence of VTE alone was 0.6% (95% CI 0.1–4.6%). As the rate of haemorrhage appeared to be of the same magnitude, universal post-discharge thromboprophylaxis was not recommended [32]. Similar figures were demonstrated at 42 days follow-up in a cohort of 152 patients [33]. In a 6-week follow-up study of 33 patients discharged without anticoagulation, all patients with elevated D-dimer levels underwent ultrasound duplex scanning and a ventilation/perfusion scan to rule out VTE [34]. There were no thromboembolic complications and no echocardiographic impairments. Consequently, in the absence of other thrombotic risk factors, patients with COVID-19 are mostly discharged without prophylactic anticoagulation. If diagnosed with pulmonary embolism de novo during follow-up, patients should be treated in line with pulmonary embolism guidelines [37].
Only one of the identified studies reported on the long-term outcomes of patients with COVID-19 and VTE. This prospective observational study evaluated a composite of major bleeding and death at 90 days in 100 consecutive patients with VTE in the setting of COVID-19 (66 patients were hospitalised, 23 of them in the ICU; 64% pulmonary embolism) [35]. Mortality (24%) and major bleeding (11%) were high. The majority of complications occurred in the first 30 days. Most patients received direct oral anticoagulants (52%) or low-molecular-weight heparin (28%) at discharge. There were no VTE recurrences. The follow-up evaluation of patients with pulmonary embolism during acute COVID-19 draws from pulmonary embolism guidelines [37].
Concluding remarks
At this stage it is still unclear if pulmonary thromboembolism and inflammatory pulmonary microangiopathy [36] demonstrated in patients with severe COVID-19 will lead to sequelae such as chronic thromboembolic pulmonary hypertension (CTEPH) or pulmonary arterial hypertension. In patients with persistent exertional dyspnoea without evidence of parenchymal lung opacities on high-resolution CT 3–6 months after discharge and with pulmonary function tests (PFTs) documenting preserved lung volumes and normal or reduced DLCO, follow-up evaluation should include echocardiography and contrast-enhanced CT to identify significant pulmonary vascular involvement, as per proposed guidance [18]. As contrast-enhanced CT cannot exclude CTEPH [38], lung perfusion studies with single-photon emission CT or dual-energy CT are proposed to exclude vascular involvement in symptomatic post-COVID-19 patients, even in the absence of pulmonary embolism history during the acute illness [39]. As suggested by previous ERS/ATS guidelines, if there is evidence of significant pulmonary hypertension, patients should be considered for referral to a specialist pulmonary hypertension centre [40, 41].
Clinical Question 3. Which follow-up strategies relate to pulmonary physiology?
Evidence overview
Although SARS-CoV-2 can theoretically infect various organs after binding to the ubiquitous angiotensin-converting enzyme 2 cell membrane receptor, the respiratory system is the most frequently impacted due to the airborne nature of the infective agent. Regarding PFTs after the acute phase, 1578 records were identified (appendix C in the supplementary material). 39 eligible studies (one randomised controlled trial (RCT), three systematic reviews, 11 prospective cohort studies, seven retrospective studies, 15 cross-sectional studies and two case series) [8, 23, 27–29, 34, 42–74] were included. Two studies were longitudinal [56, 57] and 11 were prospective [23, 44–48, 56, 57, 59, 60, 74], including four multicentre [23, 45, 46, 56].
When measuring PFTs at rest, all investigators sought three main features, i.e. the existence of 1) the obstructive pattern, 2) the restrictive pattern and 3) lung gas exchange impairment [8, 23, 27–29, 34, 42–60, 66–68, 73, 74]. Some investigators also looked at more integrative responses to physical exercise, either using the 6-min walk test (6MWT) [8, 23, 34, 42–51, 61, 62, 73, 74] or cardiopulmonary exercise testing (CPET) [51, 63–65]. The main parameter used to define the bronchial obstructive pattern is the forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) ratio. The restrictive pattern was deemed to be present based on either reduction of total lung capacity (TLC) or the combination of low FVC and high FEV1/FVC ratio when TLC could not be measured. In some studies, reduced residual volume was also considered as part of the restrictive pattern. Lung gas exchange was mostly assessed using DLCO [8, 23, 27–29, 34, 42–50, 52–60, 73, 74]. Only one study examined diffusing capacity of the lung for nitric oxide (DLNO) combined with DLCO [69]. The earliest time-point after the acute phase of the disease was 1 month [27, 42, 73], with a majority of the studies reporting PFTs from 6 weeks to 4 months [23, 28, 29, 42–51, 53–60, 66, 67], with very few at 6 months [8, 52, 64] post-discharge or onset of disease. Patient inclusion criteria were inconsistent between studies. The majority of the studies included hospitalised patients, while two studies involved both hospitalised and non-hospitalised, and only one was performed on non-hospitalised patients. Patients with pre-existing chronic lung disease were not differentiated, thus making it difficult to relate abnormal PFTs results to either COVID-19 lung injury or possible pre-existing disease. There were disparities in reporting data either as absolute values or percentage of predicted values, with only a few reporting both absolute and percentage. Few studies adopted the Global Lung Function Initiative approach using the lower limits of normal threshold to distinguish abnormal values, while the majority retained 80% of predicted values for TLC, FVC and DLCO. Correcting DLCO for haemoglobin was not consistent. All studies reported a relatively high prevalence of reduced DLCO in 40–65% of patients compared with a medium to high prevalence of the restrictive pattern and an exceptionally low prevalence, if not absence, of the obstructive pattern. If the high prevalence of altered DLCO found at 1 month after discharge or the onset of the disease partly results from ongoing residual inflammation related to the initial lung injury, the persistent low values of DLCO at 3 and 6 months, even in patients with normalised chest CT, raise the need for further discussion [75]. DLCO is the product of the accessible alveolar volume (VA) and the transfer coefficient of the lung for carbon monoxide (KCO); altered DLCO can theoretically occur when either VA or KCO, or both, are reduced [76]. Deciphering between VA and KCO as the causal factor for reduced DLCO is therefore critical to infer the underlying lung structural changes with either interstitial abnormalities or pulmonary vascular abnormalities. If DLCO had been measured in all studies assessing PFTs, VA and KCO were seldom reported, thus compromising key messages regarding the pathological nature of impaired lung gas transfer. Another way to dissect the underlying mechanisms of reduced DLCO could be the simultaneous measurement of DLNO and DLCO. This test is only available in a small number of centres, which may explain the scarcity of DLNO papers in patients with COVID-19.
Concluding remarks
From the currently available literature [70–72], PFTs were performed on average 3 months after onset of COVID-19. PFT measurements including at least static lung volumes with ideally TLC measurement, expiratory flow rates and DLCO assessment are regarded as useful tools to assess long-term lung function sequelae in patients with COVID-19 by most investigators. An effort of global harmonisation to 1) express results, 2) choose criteria defining any anomaly, 3) refine patients’ inclusion criteria, 4) prospectively investigate lung function in 5) multiple centres is still insufficient. There is agreement in all studies on the high prevalence of altered lung gas exchange in patients with COVID-19 as the main feature of PFT anomalies. Most Task Force members provide DLCO results after correction for haemoglobin, and together with VA and KCO, this helps the reader to decipher the underlying causes of altered lung gas exchange. A large, international multicentre trial using DLNO and DLCO simultaneous measurement could provide more useful information.
Clinical Question 4. Which follow-up strategies relate to imaging?
Evidence overview
14 eligible studies (nine prospective cohort studies, four retrospective studies and one cross-sectional study) [19, 22, 43, 45, 47, 52, 77–84] were included on imaging follow-up of patients with COVID-19, following a search of 1300 initial records (appendix C in the supplementary material). A small number of studies reported high rates of persisting abnormalities on radiology at discharge, despite absence of symptoms [19, 52, 77–80]. Indeed, we know that radiological healing of pneumonitis is slower than clinical conversion. Further studies aimed to report the frequency of pulmonary abnormalities at 3- and 6-month CT [22, 43, 45, 52, 81, 82]. Studies of radiological abnormalities on CT at 3–6 months may overestimate the true frequency of persistent abnormalities as studies without systematic follow-up will be biased towards patients with severe disease and persistent symptoms. Only four of the studies also included non-hospitalised patients. In fact, residual lung lesions are more frequently observed in CT scans of patients who had extensive imaging abnormalities as well as severely altered clinical-laboratory markers of disease severity (including ICU admission and longer hospitalisation) during the acute phase [22, 52, 83].
The longitudinal behaviour of CT abnormalities mostly reflects the temporal evolution of diffuse alveolar damage and organising pneumonia, i.e. the main pathological patterns underlying COVID-19 pneumonia [84]. GGO is the most frequent finding at 3-month CT, followed by parenchymal bands, perilobular opacities and scant interlobular septal thickening. It was observed that these CT abnormalities diminish with time [22], while complete waning is still under investigation as more longitudinal data accumulate. Intriguingly, GGO may gradually increase in extent and reduce in density [22]. Terms such as “fibrosis” or “fibrotic-like” changes entered the literature, yet are still not justified for practical use and interpretation of patient management. CT features of lung fibrosis were interpreted in up to 47.1% at the 3-month follow-up [47]. Han et al. [22] reported a prospective cohort of 114 patients undergoing a 6-month follow-up CT scan [22]. This included 35% of the total cohort with “fibrotic-like” features (e.g. parenchymal bands, traction bronchiectasis, etc.). Indeed, it is unlikely that lung fibrosis occurs in such a large proportion of subjects who have had COVID-19 pneumonia and most of those “fibrotic-like” changes might be reversible at later follow-up.
Concluding remarks
Despite the current data, it is unclear if “fibrotic-like” CT features represent irreversible disease (e.g. post-ARDS) or slowly regressive infiltrate secondary to organising pneumonia, also seen with mild distortion mimicking actual fibrosis. The risk of over-calling lung fibrosis on follow-up CT scan seems more frequent in the presence of bronchial distortion within the areas of organising pneumonia features. Task Force members do not call such bronchial distortion traction bronchiectasis, which, by definition, represents an established CT feature of irreversible lung fibrosis. Therefore, most Task Force members are cautious when calling out fibrosis, especially in the early follow-up CT where parenchymal changes are encountered frequently and are more prone to resolve. Given the high proportion of subjects who either develop ARDS or undergo mechanical ventilation, the development of lung fibrosis remains a concern. More imaging data are needed to clearly distinguish between COVID-19 pneumonia sequelae and ventilator-induced lung injury. Guidelines are awaited to inform when and how imaging should be referred. Task Force members consider imaging follow-up in patients that were hospitalised and/or showed a more severe clinical disease course, or in patients presenting with new or progressive respiratory symptoms in the mid- to long-term after acute COVID-19 syndrome. There are recommendations to repeat CT scans at 12 weeks post-discharge in patients with persistent symptoms, to complement clinical assessment [18].
CT is the most utilised imaging technique to follow-up subjects who had COVID-19 pneumonia. In fact, it is undisputed that fine residual abnormalities such as GGO are best depicted with CT, rather than chest radiography or lung ultrasound. As this disease involves a sizeable proportion of younger subjects who might need repeated follow-up, it is worth underscoring that a low-dose thin-section CT protocol is used by the Task Force members.
Clinical Question 5. Which follow-up strategies relate to infection control?
Evidence overview
A large number of reports on COVID-19 have been published since the start of the pandemic, yet evidence regarding the dynamics of SARS-CoV-2 shedding in patients with COVID-19 in general and in specific patient subgroups in particular is scarce. Five eligible studies were included (two retrospective, two case studies and one guideline) [85–89] following a search of 815 initial studies (appendix C in the supplementary material). One further systematic review and meta-analysis [90] identified by the authors after completion of the literature searches is also included in this review.
In susceptible individuals, viral replication starts to increase rapidly only a few days after SARS-CoV-2 exposure. Viral load usually peaks ∼1 week after infection, in most patients ∼24 h before COVID-19 symptoms commence. In immune-competent patients, replication-competent SARS-CoV-2 can be isolated from the respiratory tract for 1 to several weeks after the onset of symptoms. More specifically, a recent meta-analysis of 79 reports on SARS-CoV-2 found that the mean duration of SARS-CoV-2 RNA shedding was 17.0 (95% CI 15.5–18.6) days in upper respiratory tract, 14.6 (95% CI 9.3–20.0) days in lower respiratory tract, 17.2 (95% CI 14.4–20.1) days in stool and 16.6 (95% CI 3.6–29.7) days in serum samples [90]. SARS-CoV-2 shedding duration was positively associated with age and COVID-19 severity. While these numbers suggest that after 3 weeks viral shedding usually concludes, it is noteworthy that the same meta-analysis reported a maximum shedding duration of 83 days in the upper respiratory tract, 59 days in the lower respiratory tract, 126 days in stools and 60 days in serum [90]. Importantly, viral shedding does not necessarily provide active infectious virus. Many studies did not detect live virus beyond day 9 of illness despite persistently high viral mRNA loads. One study observed shedding of infectious SARS-CoV-2 up to 70 days after initial diagnosis from an asymptomatic immunocompromised patient with cancer [85]. Other conditions of constitutive or acquired immunosuppression may predispose to prolonged shedding of live SARS-CoV-2 or reactivation of infection, such as cancer-directed therapies [86]. Further, time to SARS-CoV-2 clearance among cancer patients varies substantially depending on the criteria used [87]. Most evidence has been derived from other virus variants than the currently widespread delta variant; however, there is no evidence available for significant differences in the time course of viral load between SARS-CoV-2 variants.
Of note, positive SARS-CoV-2 PCR tests following temporary PCR negativity have been described in recovered patients with COVID-19. Several causes of recurrent positive tests for SARS-CoV-2 are suggested, including false-negative and false-positive PCR tests, detection of viral particles rather than replication-competent virus, and reactivation and re-infection with SARS-CoV-2, the latter two being more likely in immunocompromised patients than in healthy individuals. Depending on the specific healthcare setting, recurrent SARS-CoV-2 test positivity may preclude patients from treatment of underlying diseases over a longer time period. Considering the risk of increased morbidity and mortality of the underlying disease resulting from treatment delay, the Task Force members advocate for interpretation of the cycle threshold (Ct) value of real-time PCR in the clinical context. This will enable treatment of high-risk underlying disease, despite ongoing positive SARS-CoV-2 testing [88, 89].
Concluding remarks
The majority of Task Force members perform recurrent PCR testing after acute COVID-19 in specific subgroups of patients, on a case-by-case decision, given the lack of scientific evidence and universally agreed follow-up strategies for infection control in COVID-19. Particularly for immunodeficient patients including, but not restricted to, haematological, oncological, T- or B-cell incompetent patients, it is useful to undergo repetitive testing with individual frequency (e.g. once weekly) due to protracted high-risk status or recurrent viral shedding.
Clinical Question 6. Which follow-up strategies relate to cognitive, psychological and quality-of-life consequences?
Evidence overview
Published studies attempt to measure a range of symptoms occurring as a consequence of COVID-19, the impact of long COVID and evaluate the outcome measures used. 1402 studies were screened (appendix C in the supplementary material) and 19 studies were included (two reviews (one systematic), nine prospective cohort studies, three survey designs, three retrospective studies, one ambi-directional cohort study and one cross-sectional study) [8, 34, 49, 61, 70, 74, 91–103] reporting on symptom burden and QoL. All studies included hospitalised patients. The survey conducted by Machado et al. [97] extended to non-hospitalised patients and a small RCT (n=72) investigating the impact of rehabilitation on the elderly post-COVID-19 [74] did not differentiate hospitalisation status.
The spectrum of symptoms reported include fatigue [8, 61, 91–93], dyspnoea, cough [34, 91, 93], dysphagia [95], frailty [96], loss of memory [92], concentration [92], sleep disorders [8, 92, 97], anxiety and/or depression [8, 34, 74, 91, 98, 99], and pandemic-related stress factors [91, 94, 98], with several studies reporting on HRQoL [34, 49, 74, 93, 94, 99–101], specifically functional status [97, 102] or level of independence with activities of daily living (ADLs) [95]. A prospective cohort study of 183 patients (median age 57 years; 61.5% male, 54.1% White) reported older participants (aged 65–75 years: OR 8.666 (95% CI 2.216–33.884); p=0.0019) and women (male versus female: OR 0.462 (95% CI 0.225–0.949); p=0.0356) had statistically significant higher odds of experiencing persistent symptoms at 5 weeks post-discharge [93].
At 6 months post-acute COVID-19, survivors continued to experience fatigue, muscle weakness, sleep difficulties and anxiety or depression. A cross-sectional study of 1696 consecutive patients (mean±sd age 71.8±13.0 years; 56.1% female, 82.3% with comorbidities) reported that independence for ADLs was lower in those admitted to the ICU than the ward group (61.1% (95% CI 55.8–66.2%) versus 72.7% (95% CI 70.3–75.1%); p<0.001). Conversely dependence for ADLs was also more frequent in the ICU group (84.6% (95% CI 80.4–88.2%) versus 74.5% (95% CI 72.0–76.8%); p<0.001) [95]. Patients who were more severely ill during their hospital stay had more impaired DLCO and were the main target population for interventions of long-term recovery [8]. Those admitted to the ICU required more oxygen therapy (25.5% versus 12.6%; p<0.001), and experienced more dyspnoea during routine (45.2% versus 34.5%; p<0.001) and non-routine activities (66.3% versus 48.2%; p<0.001) [95].
Persistent symptoms of fatigue and sleep disturbance following severe COVID-19 pneumonia impacted HRQoL, productivity, physical activity and mental ill-health, and were associated with high rates of positive screening tests for anxiety, depression and post-traumatic stress disorder (PTSD) [91, 94, 98]. A systematic review and narrative synthesis recommended a prompt “general clinical” evaluation and risk assessment of patients presenting with neurological symptoms to minimise cognitive impairment and mental health, thereby improving prognosis and outcomes [103]. Causative mechanisms for adverse mental health outcomes following COVID-19 infection have not yet been established. Biological pathophysiological mechanisms, relating to cerebral vascular inflammation and thrombosis, survivor guilt, and isolation in COVID-19 survivors are cited as contributory factors of adverse mental health outcomes [91].
There is a lack of consistency in the selection of instruments to measure symptom burden, cognitive impairment, psychological wellbeing and QoL. A systematic review identified 33 outcome measures from 36 studies [70]. Most commonly used were the Hospital Anxiety and Depression Scale, Short Form-36 and St George's Respiratory Questionnaire (SGRQ). A summary of a broad range of instruments reported on in this review is provided in supplementary table S2. According to standardised questionnaires, patients experienced reduced QoL mainly due to decreased mobility (median (interquartile range (IQR)) SGRQ activity score 54 (19–78)) [34].
The battery of outcome measures implemented in some studies is recognised as being impractical for routine clinical use. Focused patient interviews were suggested as an alternative substitute for questionnaires [91]. Further, recommendations included a call to rationalise the approach to the selection/combination of outcome measures to capture all elements of COVID-19 to better understand the impact on survivors, and to plan timely and appropriate interventions to maximise functional return [70].
Few differences were observed between HRQoL for patients cared for on the ward and ICU [92]. Hospitalised individuals presented high levels of disability, dyspnoea, dysphagia and dependence [95]. Social disconnect appeared to predict the presence of post-traumatic stress symptoms (PTSS) (β 0.59 (95% CI 0.37–0.81); p<0.001) 1 month after hospitalisation but the severity of COVID-19 symptoms was not predicative for PTSS [98]. Depressive and anxiety symptoms decreased 1 month following hospitalisation. However, higher levels of anxiety (standardised β 1.15 (95% CI 0.81–1.49); p<0.001) and depression (β 0.97 (95% CI 0.63–1.31); p<0.001) during the first week of hospitalisation, feeling socially disconnected and longer hospitalisation period (β 0.25 (95% CI 0.03–0.47); p=0.026) predicted higher PTSS scores 1 month post-hospitalisation [98]. The need for social support during hospitalisation with a more robust approach to managing uncertainty regarding health status and family concerns is identified. Few studies explored the cognitive, psychological and QoL consequences of long COVID in non-hospitalised patients.
Concluding remarks
Few differences were observed between HRQoL for patients cared for on the ward and ICU; more in-depth exploration in larger cohorts of patients including more severe ICU patients is needed. A need was identified to target the reduction and avoidance of PTSD. The long-term psychosocial effects (e.g. depression, anxiety, psychosomatic preoccupations and insomnia) and an awareness of symptoms indicative of PTSD require prompt clinical follow-up as suggested by earlier guidelines [94]. Early rehabilitation helps to reduce PTSD and mitigates the long-term sequelae [102]. To date, pulmonary rehabilitation has been informed by experiences in other chronic respiratory conditions. Further studies and consensus on the approach are needed. Consensus is also needed on the selection of outcome measures to minimise clinical burden and standardise research.
Psychosocial implications should not be ignored, and particular attention should be paid to caregiver burden, family support, and the impact of recurrent and cross-infection.
HRQoL captures symptom experience and disease impacts that may result in disability. Further discussions on symptoms associated with disability are reviewed in Clinical Question 7.
Clinical Question 7. Which follow-up strategies relate to disability?
Evidence overview
1121 studies were screened (appendix C in the supplementary material) and 15 eligible studies were included (one RCT, one systematic review, 12 prospective studies and one retrospective study) [19, 23, 28, 43, 45, 47, 56, 74, 91, 104–110]. Nine studies included hospitalised patients, two included non-hospitalised patients, and four included both hospitalised and non-hospitalised. All studies report on disability (a physical or mental condition that limits a person's movements, senses or activities) due to persistent symptoms after recovering from acute COVID-19 infection, including fatigue and dyspnoea. Evidence is emerging of patients experiencing more than one symptom, resulting in disability with psychological and cognitive symptoms reported to affect functional abilities in the long term [19, 23, 28, 43, 45, 47, 56, 74, 91, 104–110].
In COVID-19 survivors, dyspnoea and associated disability is the most frequent persistent respiratory symptom regardless of the need for hospitalisation, ranging from 5.5% to 54% at 1–4 months [28, 45, 47, 56, 91, 105–107]. Tenforde et al. [108] reported shortness of breath at 3 weeks in 29% of patients never hospitalised. The most used measure to assess dyspnoea was the modified Medical Research Council scale [19, 23, 43, 45, 47, 56] followed by the modified Borg dyspnoea scale [19] and the Borg category dyspnoea scale [91, 106].
Follow-up and management of COVID-19 survivors presenting symptoms and subsequent disability is an urgent priority. There is emerging evidence of debilitating disability months after COVID-19 infection. Disability with limited physical performance due to dyspnoea or fatigue, or both, measured with at least one of the Short Physical Performance Battery (SPPB) score, 2-min walk test or 1-min sit-to-stand test (1MSTST), was found in 35% of COVID-19 survivors at 6 weeks [91], 14% at 3 months [105], 53.8% at 4 months [28] and 32% at 6 months [109]. The 6-min walk distance (6MWD) was within normal values at 75 days with only 3% of patients experiencing documented oxygen saturation <90% (median (IQR) modified Borg dyspnoea scale 3 (2–5)) [19, 104]. At 3 months follow-up, 6MWD was within normal values [45] and only one out of 62 patients had oxygen saturation <90% [47]. The 6MWT showed a significant reduction in distance walked and oxygen saturation levels in severely impaired COVID-19 patients compared to those with mild/moderate disease [23]. In a prospective observational study at 3 months after discharge [43], 22% of patients had 6MWD <80% predicted and 16% desaturated on exertion.
Disability persisted after a multidisciplinary rehabilitation programme: 1MSTST below normal value in 33.3% and SPPB in 53.3%. The Barthel Index showed poor performance in ADLs in 47.5% of COVID-19 survivors [110]. In an RCT [74], exercise capacity (6MWD) and ADLs (assessed with the Functional Independence Measure scale) were evaluated 6 months after COVID-19 infection: patients undergoing a 6-week rehabilitation programme (including respiratory muscle training, cough exercise, diaphragmatic training, stretching exercise and home exercise, but without specific training and whole body exercises) showed improvement in 6MWD when compared to the control group. Results on ADLs have also been reported in a prospective follow-up study [106] on 116 post-COVID-19 ICU patients which documented no limitations after 2 months from infection.
Concluding remarks
Evidence on follow-up of COVID-19 survivors suggests that patients recovering after the acute phase may present with prolonged symptoms for ≥4 weeks, causing disability with reduced functional performance and ADLs. This impacts some if not all aspects of HRQoL. Most Task Force members evaluate and systematically follow-up COVID-19 survivors with unresolved or new or progressive symptoms with related disability. Decline in exercise tolerance, weakness or reduced mobility define the assistance needed for ADLs (e.g. feeding, dressing, bathing, toileting, driving, housekeeping and grocery shopping) and help clinicians to develop appropriate disability management strategies of pulmonary sequelae. Rehabilitation programmes including exercise training could mitigate longer term disability. Long COVID clinics offer a one-stop shop for assessment and monitoring of disability. Longitudinal cohort studies are needed to determine the most effective interventions.
Clinical Question 8. Which follow-up strategies relate to home care follow-up (tele-medicine/tele-rehabilitation)?
Evidence overview
Patients with post-acute COVID-19 are at risk of long-term functional impairment and the rehabilitation community is calling for action preparing for a large increase of the rehabilitation needs in this patient population [94]. We screened 2057 records that were initially identified (appendix C in the supplementary material). According to the 29 eligible studies included (four systematic reviews, 15 prospective cohort studies, eight retrospective studies and two suggestion documents) [94, 95, 111–137], tele-health, often used as a broad term to include tele-visit, tele-medicine, tele-coaching, tele-nursing and tele-rehabilitation, offers an opportunity to follow-up patients while reducing the burden of travel for those patients affected by COVID-19, those with positive COVID-19 tests and those with COVID-19 sequelae. Virtual services may include asynchronous clinical communications, real-time virtual care, messaging, telephone or video conferencing, virtual health assessments and medication review. The COVID-19 pandemic forced a rapid adoption of tele/digital approaches over traditional in-person visits, although uptake is reported to be lower in communities with higher rates of poverty [111]. Tele-medicine/tele-rehabilitation have been proposed in COVID-19 patients who suffer from exercise dyspnoea, adequate stable condition, residual disability, displaced or isolated [112] provided they can ambulate independently, use technology, require minimal supplemental oxygen and are cognitively intact [113]. Tele-health may improve access and reduce barriers to healthcare access, overcome financial costs, increase medical care and follow-ups, and, most importantly, reduce the risk of COVID-19 transmission [114]. Approaches can monitor vital parameters (peripheral oxygen saturation, heart rate, blood pressure and respiratory rate). Specifically, pocket oximeters and smartphone-based systems need particular accuracy to avoid errors in pulse oximetry [115]. In selected cases, breath sounds can be analysed using advanced signal processing and analysis in tandem with new deep/machine learning [116]. Tele-health implemented in response to the COVID-19 pandemic in general resulted in high patient and provider satisfaction [114, 117–124]. Tele-medicine/tele-rehabilitation acceptance is related to increased accessibility, enhanced care, usefulness, ease of use and privacy/discomfort, whereas anxiety about COVID-19 is not [125]. Being male, having a history of both depression and anxiety, lower patient activation [120], and technical and administrative challenges [121] were significantly associated with a poor tele-health experience.
Mobile apps have been proposed for citizens, health professionals and decision makers to reduce the burden on hospitals, provide access to credible information, and track the symptoms and mental health of individuals [126–128], and help patients to improve their emotional resilience and subsequently their ability to cope with the trauma of their COVID-19 experiences [129]. During the pandemic the percentage of visits via tele-medicine increased 23-fold compared with the pre-pandemic period [111, 130]. As a majority of chronic respiratory patients are elderly and have multiple comorbidities, they are notably susceptible to severe complications of COVID-19 and, as such, have been advised to minimise social contact. This increased patients’ vulnerability to physical deconditioning, depression and social isolation. To address this major gap in care, some clinic-centred pulmonary rehabilitation programmes converted some or all of their educational packages to home-based tele-rehabilitation [131]. Tele-rehabilitation offers positive clinical results, even comparable to conventional face-to-face rehabilitation approaches [132], with general guides prepared in some countries [133]. In post-ICU patients, tele-rehabilitation consisted mostly of second opinions of psychologists (11.8%), physical therapists (8.0%), dietitians (6.8%) and speech language pathologists (4.6%) [95]. Tele-health has been used for patients experiencing depression and isolation to maintain sufficient relationships [134]. Barriers to medicine at distance have been described as: advanced age, poor confidence with technology, lack of communication, reduced confidence in doctors, additional burden for complex care, ethical issues and scepticism [114, 120, 135–137]. Several obstacles must be overcome for a wide use of tele-health: 1) the technology must be usable by the largest possible number of patients, 2) clarity on medico-legal liability and data privacy, 3) lack of economic reimbursement, 4) proper training of health professionals involved, 5) adequate caregiver support, and 6) infrastructure, operational challenges, regulatory, communication and legislative barriers [112, 118, 131].
Concluding remarks
Tele-health appointment experiences were often comparable to traditional in-person medical appointment experiences. Although tele-medicine/tele-rehabilitation appear to be acceptable, the level of agreement on standardisation remains unclear, in particular the cost–effectiveness ratio. Tele-health was effective as a mode of healthcare delivery during the pandemic and may be sustainable [112, 120]. Careful attention will be needed to integrate these services into current healthcare delivery systems while preserving patient-centred and quality care [122]. Further high-quality studies to enable the successful implementation of these modalities are needed [131].
Discussion
It is evident that long COVID is a substantial global public health problem with severe consequences for affected individuals. Emotional wellbeing and QoL are particularly impacted [103]. A standardised minimum set of outcomes for clinical care of patients with COVID-19 has recently been published by the International Consortium for Health Outcomes Measurement and is categorised into five domains: 1) functional status and QoL, 2) mental functioning, 3) social functioning, 4) clinical outcomes, and 5) symptoms [138]. While these outcomes may need some adjustment for specific comorbidities, treatment approaches and demographics, consensus recommendations could help guide clinical services and enable data comparison of patient-centric clinical outcomes.
Active management and strategies for the prevention of persistent symptoms and potential long-term complications continue to be explored as a more comprehensive understanding of COVID-19 sequelae emerges. The Post-hospitalisation COVID-19 study (PHOSP-COVID), a multicentre, long-term follow-up study of adults discharged in the UK with a clinical diagnosis of COVID-19, was published in October 2021 [139]. PHOSP-COVID determined that severe mental and physical impairments are independent of the degree of acute lung injury and could be related to persisting systemic inflammation. The investigators suggest a proactive approach and holistic clinical care that is stratified and personalised with access to interventions to improve mental, physical and cognitive health. At 6 months post-discharge, morbidity was more prevalent in females, the middle aged, and those with two or more comorbidities and more acute severe illness. Given the multisystemic nature of the post-COVID-19 condition, a multidisciplinary response is likely to be the optimal approach. Many post-COVID-19 multidisciplinary clinics are emerging globally [103], with post-COVID-19 clinical programmes designed to meet the needs of individuals characterised according to previous hospitalisation with COVID-19, non-hospitalisation with persistent respiratory symptoms post-COVID-19 and pre-existing lung disease complicated by COVID-19 [140].
Long COVID appears to overlap with complications of acute COVID-19, making it hard to define. Further evidence and research from multidisciplinary teams is crucial to understanding the causes, mechanisms, risks and consequences of long COVID [141]. The ultimate goal is to develop preventive measures, rehabilitation techniques and clinical management strategies. Individualised interventions in long COVID clinics with multiple specialties, including graded exercise, physical therapy, continuous check-ups and cognitive behavioural therapy, should be designed to address long COVID care [142]. Sensitivity to gender and age, and screening all patients regardless of COVID-19 severity across a range of physical and mental health symptoms, particularly anxiety and cognitive impairment, will be required to target interventions at an individual level and to groups of patients with similar post-COVID-19 profiles.
Preliminary reports confirm the feasibility and safety of a dedicated tele-rehabilitation programme for survivors of COVID-19 pneumonia with a clear improvement in exercise tolerance and dyspnoea [143]. Adapting tele-rehabilitation to the usual practice of physical therapy can be achieved through a paradigm change to ensure effective patient-based tele-rehabilitation [144].
From the clinical perspective, physicians should be aware of the symptoms, signs and biomarkers present in patients previously affected by COVID-19 to promptly assess, identify and halt long COVID progression, and to minimise the risk of chronic effects. Identification of possible biomarkers or laboratory tests would be useful, similar to those available for acute infection or post-acute hyperinflammatory illness [145]. Data showing that almost one-third of COVID-19 survivors will present with lung abnormalities 6 months after initial infection [22] raise questions. Does this high proportion of patients have clinically significant interstitial lung disease or should we interpret these findings with more scepticism [146]? Ongoing monitoring and regular follow-up will enable physicians to assess clinically significant pulmonary sequelae, identifying long COVID manifestations early and improving management.
This ERS statement intended to describe the evidence regarding eight clinical questions about long COVID consequences. The systematic search was completed in March 2021. Papers meeting the inclusion criteria but published after the cut-off date were not included. The aim of this document was to provide a rapid first overview of the clinical questions identified to help clinicians consider important aspects of follow-up care for patients with COVID-19. This statement is primarily focused on the respiratory system. Given the volume of publications related to COVID-19, we plan to update this statement and to further inform a clinical practice guideline with recommendations.
Recommendations for research
Post-acute sequelae COVID-19 research is a global priority, with the US National Institutes of Health investing USD 1.15 billion over 4 years and the UK NIHR investing GBP 38.5 million. Predisposing factors and symptom patterns associated with long-term sequelae remain difficult to determine. Importantly, the COVID-19 ERS Clinical Research Collaboration, END-COVID, aims to merge national long COVID initiatives in Europe. END-COVID intends to study the long-term effects of COVID-19 in post-hospitalisation survivors, both in those with pre-morbid lung conditions and in those with no previous lung disease and comorbidities.
It is a priority to identify determinants of those more likely to experience prolonged sequelae following SARS-CoV-2 infection. Some studies are under way to identify those at greatest risk [147], but the existence of intrinsic, extrinsic (biological, psychological and social) and factors associated with hospitalisation and interventions requires deeper investigation. Research on the respective contributions of physical/biological and functional somatic mechanisms to the expression of long COVID depending on the characteristics of this expression and of the acute disease is urgently needed. Histopathological phenotyping and genotyping are crucial, enabling deeper insights into the differences in pathogenesis and underlying immunological and tissue regenerative response patterns. The design of genetic association studies may unravel potent genetic correlations [148].
Large, multicentre studies will evaluate the long-term consequences of pulmonary physiology and imaging alongside patient factors, particularly disability and QoL. Further data are needed to determine the impact of COVID-19 vaccines for those diagnosed with long COVID.
Summary statements
Age and initial disease severity appear to correlate with long-term consequences, but not necessarily with the persistence of symptoms (larger studies required).
In patients with persistent exertional dyspnoea not explained by CT or PFT abnormalities 3–6 months after discharge, most Task Force members include echocardiography and contrast-enhanced CT in the follow-up evaluation to identify pulmonary vascular involvement.
PFT measurements, including static lung volumes, expiratory flow rates and DLCO assessment, are regarded as useful tools to assess long-term lung function sequelae in patients with COVID-19.
Task Force members agree with the proposed recommendations to repeat CT scans at 12 weeks after discharge in patients with persistent symptoms, as a complement to clinical-functional assessment. However, Task Force members are cautious when interpreting CT abnormalities taking into consideration the risk of over-calling lung fibrosis.
The majority of Task Force members perform recurrent PCR testing after acute COVID-19 in specific subgroups of patients, on a case-by-case decision, given the lack of scientific evidence.
Task Force members do not ignore the long-term psychological and psychosocial implications of infectious diseases, and most of them use symptom and QoL measures. There is a need for consensus on pulmonary rehabilitation that helps to reduce PTSD and mitigates long-term sequalae.
Based on current evidence, patients recovering after the acute phase may present prolonged symptoms affecting functional performance and daily life activities. Most Task Force members evaluate and systematically follow-up COVID-19 survivors with unresolved or new or progressive symptoms.
Tele-medicine and tele-rehabilitation appear to be acceptable, and tele-health was effective during the pandemic. Integration of these services into current healthcare delivery systems must preserve patient-centred and quality care.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-02174-2021.Supplement (523.8KB, pdf)
Shareable PDF
Acknowledgements
We thank Damien Basille (Amiens, France), Claudia Ravaglia (Forli, Italy) and Semeli Mastrodemou (Heraklion, Greece) for their help with the literature search and for their input to the draft. Our thanks go to Valerie Vaccaro from the ERS support team (ERS, Lausanne, Switzerland) for her assistance throughout this project.
Footnotes
This Statement was endorsed by the ERS Executive Committee on 19 January 2022.
Author contributions: K.M. Antoniou and A. Spanevello coordinated the project and collated the contributions from all authors. E. Vasarmidi and A-M. Russell had an equally major contribution in writing the manuscript. All the other authors contributed equally to the production of this Task Force report.
Conflict of interest: K.M. Antoniou declares consulting fees, payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events and support for attending meetings and/or travel from Boehringer Ingelheim and Roche, in the 36 months prior to manuscript submission; and an unpaid role as Secretary of the European Respiratory Society Interstitial Lung Diseases Assembly. E. Vasarmidi declares no competing interests. A-M. Russell declares payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events, and support for attending meetings and/or travel from Boehringer Ingelheim, Roche and the Irish Lung Fibrosis Association, in the 36 months prior to manuscript submission. C. Andrejak declares an honorarium for a lecture on COVID-19 for “Avancées en Pneumologie” (Actuality on Respiratory Disease) funded by AstraZeneca; support for attending meetings and/or travel from Aeris Medical, SOS Oxygène and Vitalaire; and participation on an advisory board on the French COVID Cohort, an institutional epidemiological study, in the 36 months prior to manuscript submission; in addition, they are a member of the French Public Health Council (COVID treatment group) and participate on an advisory board for an prospective cohort study on COVID sequelae (SEQ COV). B. Crestani reports grants to their institution from Boehringer Ingelheim and Roche; consulting fees from Apellis, Boehringer Ingelheim, Roche and Sanofi; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events, and support for attending meetings and/or travel from Boehringer Ingelheim, Roche, Novartis and Sanofi; and paid participation on a data safety monitoring board or advisory board for Apellis, Boehringer Ingelheim, Roche and Sanofi, in the 36 months prior to manuscript submission. M. Delcroix declares no competing interests. A.T. Dinh-Xuan declares payment or honoraria for lectures, presentations or educational events from Chiesi, Circassia, GlaxoSmithKline, Novartis and Sanofi, in the 36 months prior to manuscript submission. V. Poletti declares no competing interests. N. Sverzellati declares payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Boehringer Ingelheim, Roche and Chiesi; and support for attending meetings and/or travel from Boehringer Ingelheim, in the 36 months prior to manuscript submission. M. Vitacca declares no competing interests. M. Witzenrath declares grant funding from Deutsche Forschungsgemeinschaft, Bundesministerium für Bildung und Forschung, Deutsche Gesellschaft für Pneumologie, European Respiratory Society, Marie Curie Foundation, Else Kröner Fresenius Stiftung, Capnetz Stiftung, International Max Planck Research School, Quark Pharma, Takeda Pharma, Noxxon, Pantherna, Silence Therapeutics, Vaxxilon, Actelion, Bayer Health Care, Biotest and Boehringer Ingelheim; consulting fees from Noxxon, Pantherna, Silence Therapeutics, Vaxxilon, Aptarion, GlaxoSmithKline, Sinoxa and Biotest; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AstraZeneca, Berlin-Chemie, Chiesi, Novartis, Teva, Actelion, Boehringer Ingelheim, GlaxoSmithKline, Biotest and Bayer Health Care, in the 36 months prior to manuscript submission; and three patents relating to modulation of immune response in acute lung injury, inhibition of Ang-2 expression and treatment of SARS-CoV-2-infected lung cells, respectively. T. Tonia acts as a European Respiratory Society Methodologist. A. Spanevello declares payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events, and support for attending meetings and/or travel from Merck Sharp & Dohme, GlaxoSmithKline, AstraZeneca, Menarini, Guidotti and Chiesi, in the 36 months prior to manuscript submission.
Support statement: Funding support was provided by the European Respiratory Society (TF-2020-14). A-M. Russell is a NIHR 70@70 Senior Research Fellow. The views expressed in this publication are those of the author(s) and not necessarily those of the National Institute for Health Research or the Dept of Health and Social Care. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Chalmers JD, Crichton ML, Goeminne PC, et al. Management of hospitalised adults with coronavirus disease 2019 (COVID-19): a European Respiratory Society living guideline. Eur Respir J 2021; 57: 2100048. doi: 10.1183/13993003.00048-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spruit MA, Holland AE, Singh SJ, et al. COVID-19: interim guidance on rehabilitation in the hospital and post-hospital phase from a European Respiratory Society- and American Thoracic Society-coordinated international task force. Eur Respir J 2020; 56: 2002197. doi: 10.1183/13993003.02197-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janssen DJA, Ekström M, Currow DC, et al. COVID-19: guidance on palliative care from a European Respiratory Society international task force. Eur Respir J 2020; 56: 2002583. doi: 10.1183/13993003.02583-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization . WHO coronavirus (COVID-19) dashboard. 2022. https://covid19.who.int Date last accessed: 4 May 2022.
- 5.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med 2021; 27: 601–615. doi: 10.1038/s41591-021-01283-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carfì A, Bernabei R, Landi F, et al. Persistent symptoms in patients after acute COVID-19. JAMA 2020; 324: 603. doi: 10.1001/jama.2020.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soriano JB, Waterer G, Peñalvo JL, et al. Nefer, Sinuhe and clinical research assessing post COVID-19 condition. Eur Respir J 2021; 57: 2004423. doi: 10.1183/13993003.04423-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 2021; 397: 220–232. doi: 10.1016/S0140-6736(20)32656-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenhalgh T, Knight M, A'Court C, et al. Management of post-acute covid-19 in primary care. BMJ 2020; 370: m3026. doi: 10.1136/bmj.m3026 [DOI] [PubMed] [Google Scholar]
- 10.Fernández-de-las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V, et al. Defining post-COVID symptoms (post-acute COVID, long COVID, persistent post-COVID): an integrative classification. Int J Environ Res Public Health 2021; 18: 2621. doi: 10.3390/ijerph18052621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nabavi N. Long covid: how to define it and how to manage it. BMJ 2020; 370: m3489. doi: 10.1136/bmj.m3489 [DOI] [PubMed] [Google Scholar]
- 12.Ladds E, Rushforth A, Wieringa S, et al. Persistent symptoms after Covid-19: qualitative study of 114 “long Covid” patients and draft quality principles for services. BMC Health Serv Res 2020; 20: 1144. doi: 10.1186/s12913-020-06001-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amenta EM, Spallone A, Rodriguez-Barradas MC, et al. Postacute COVID-19: an overview and approach to classification. Open Forum Infect Dis 2020; 7: ofaa509. doi: 10.1093/ofid/ofaa509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Institute for Health and Care Excellence, Scottish Intercollegiate Guidelines Network, and the Royal College of General Practitioners . COVID-19 rapid guideline: managing the long-term effects of COVID-19. NICE guideline (NG188). 2020. www.nice.org.uk/guidance/ng188 Last updated: 11 November 2021. Date last accessed: 3 March 2022.
- 15.National Institute for Health Research . Living with Covid19 – second review. 2020. https://evidence.nihr.ac.uk/themedreview/living-with-covid19-second-review Date last accessed: 17 May 2021.
- 16.Mahase E. Long covid could be four different syndromes, review suggests. BMJ 2020; 371: m3981. doi: 10.1136/bmj.m3981 [DOI] [PubMed] [Google Scholar]
- 17.Andrejak C, Cottin V, Crestani B, et al. Guide de prise en charge des séquelles respiratoires post infection à SARS-CoV-2. Propositions de prise en charge élaborées par la Société de Pneumologie de Langue Française. Version du 10 novembre 2020. [Guide for management of patients with possible respiratory sequelae after a SARS-CoV-2 pneumonia. Support proposals developed by the French-speaking Respiratory Medicine Society. Version of 10 November 2020.]. Rev Mal Respir 2021; 38: 114–121. doi: 10.1016/j.rmr.2020.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.George PM, Barratt SL, Condliffe R, et al. Respiratory follow-up of patients with COVID-19 pneumonia. Thorax 2020; 75: 1009–1016. doi: 10.1136/thoraxjnl-2020-215314 [DOI] [PubMed] [Google Scholar]
- 19.Townsend L, Dowds J, O'Brien K, et al. Persistent poor health post-COVID-19 is not associated with respiratory complications or initial disease severity. Ann Am Thorac Soc 2021; 18: 997–1003. doi: 10.1513/AnnalsATS.202009-1175OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreno-Pérez O, Merino E, Leon-Ramirez J-M, et al. Post-acute COVID-19 syndrome. Incidence and risk factors: a Mediterranean cohort study. J Infect 2021; 82: 378–383. doi: 10.1016/j.jinf.2021.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paderno A, Mattavelli D, Rampinelli V, et al. Olfactory and gustatory outcomes in COVID-19: a prospective evaluation in nonhospitalized subjects. Otolaryngol Neck Surg 2020; 163: 1144–1149. doi: 10.1177/0194599820939538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han X, Fan Y, Alwalid O, et al. Six-month follow-up chest CT findings after severe COVID-19 pneumonia. Radiology 2021; 299: E177–E186. doi: 10.1148/radiol.2021203153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guler SA, Ebner L, Aubry-Beigelman C, et al. Pulmonary function and radiological features 4 months after COVID-19: first results from the national prospective observational Swiss COVID-19 lung study. Eur Respir J 2021; 57: 2003690. doi: 10.1183/13993003.03690-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liao B, Liu Z, Tang L, et al. Longitudinal clinical and radiographic evaluation reveals interleukin-6 as an indicator of persistent pulmonary injury in COVID-19. Int J Med Sci 2021; 18: 29–41. doi: 10.7150/ijms.49728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu X, Zhou H, Zhou Y, et al. Risk factors associated with disease severity and length of hospital stay in COVID-19 patients. J Infect 2020; 81: e95–e97. doi: 10.1016/j.jinf.2020.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong AW, Shah AS, Johnston JC, et al. Patient-reported outcome measures after COVID-19: a prospective cohort study. Eur Respir J 2020; 56: 2003276. doi: 10.1183/13993003.03276-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mo X, Jian W, Su Z, et al. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J 2020; 55: 2001217. doi: 10.1183/13993003.01217-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bellan M, Soddu D, Balbo PE, et al. Respiratory and psychophysical sequelae among patients with COVID-19 four months after hospital discharge. JAMA Netw Open 2021; 4: e2036142. doi: 10.1001/jamanetworkopen.2020.36142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao Y, Shang Y, Song W, et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine 2020; 25: 100463. doi: 10.1016/j.eclinm.2020.100463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meiners S, Eickelberg O, Königshoff M. Hallmarks of the ageing lung. Eur Respir J 2015; 45: 807–827. doi: 10.1183/09031936.00186914 [DOI] [PubMed] [Google Scholar]
- 31.Venturelli S, Benatti SV, Casati M, et al. Surviving COVID-19 in Bergamo province: a post-acute outpatient re-evaluation. Epidemiol Infect 2021; 149: e32. doi: 10.1017/S0950268821000145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patell R, Bogue T, Koshy A, et al. Postdischarge thrombosis and hemorrhage in patients with COVID-19. Blood 2020; 136: 1342–1346. doi: 10.1182/blood.2020007938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salisbury R, Iotchkova V, Jaafar S, et al. Incidence of symptomatic, image-confirmed venous thromboembolism following hospitalization for COVID-19 with 90-day follow-up. Blood Adv 2020; 4: 6230–6239. doi: 10.1182/bloodadvances.2020003349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daher A, Balfanz P, Cornelissen C, et al. Follow up of patients with severe coronavirus disease 2019 (COVID-19): pulmonary and extrapulmonary disease sequelae. Respir Med 2020; 174: 106197. doi: 10.1016/j.rmed.2020.106197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Demelo-Rodríguez P, Ordieres-Ortega L, Ji Z, et al. Long-term follow-up of patients with venous thromboembolism and COVID-19: analysis of risk factors for death and major bleeding. Eur J Haematol 2021; 106: 716–723. doi: 10.1111/ejh.13603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med 2020; 383: 120–128. doi: 10.1056/NEJMoa2015432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J 2020; 41: 543–603. doi: 10.1093/eurheartj/ehz405 [DOI] [PubMed] [Google Scholar]
- 38.Delcroix M, Torbicki A, Gopalan D, et al. ERS statement on chronic thromboembolic pulmonary hypertension. Eur Respir J 2021; 57: 2002828.doi: 10.1183/13993003.02828-2020 [DOI] [PubMed] [Google Scholar]
- 39.Dhawan RT, Gopalan D, Howard L, et al. Beyond the clot: perfusion imaging of the pulmonary vasculature after COVID-19. Lancet Respir Med 2021; 9: 107–116. doi: 10.1016/S2213-2600(20)30407-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Eur Respir J 2015; 46: 903–975. doi: 10.1183/13993003.01032-2015 [DOI] [PubMed] [Google Scholar]
- 41.Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Eur Heart J 2016; 37: 67–119. doi: 10.1093/eurheartj/ehv317 [DOI] [PubMed] [Google Scholar]
- 42.Cortés-Telles A, López-Romero S, Figueroa-Hurtado E, et al. Pulmonary function and functional capacity in COVID-19 survivors with persistent dyspnoea. Respir Physiol Neurobiol 2021; 288: 103644. doi: 10.1016/j.resp.2021.103644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van den Borst B, Peters JB, Brink M, et al. Comprehensive health assessment three months after recovery from acute COVID-19. Clin Infect Dis 2021; 73: e1089–e1098. doi: 10.1093/cid/ciaa1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shah AS, Wong AW, Hague CJ, et al. A prospective study of 12-week respiratory outcomes in COVID-19-related hospitalisations. Thorax 2021; 76: 402–404. doi: 10.1136/thoraxjnl-2020-216308 [DOI] [PubMed] [Google Scholar]
- 45.Lerum TV, Aaløkken TM, Brønstad E, et al. Dyspnoea, lung function and CT findings 34 months after hospital admission for COVID-19. Eur Respir J 2021; 57: 2003448. doi: 10.1183/13993003.03448-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Puchner B, Sahanic S, Kirchmair R, et al. Beneficial effects of multi-disciplinary rehabilitation in postacute COVID-19: an observational cohort study. Eur J Phys Rehabil Med 2021; 57: 189–198. doi: 10.23736/S1973-9087.21.06549-7 [DOI] [PubMed] [Google Scholar]
- 47.González J, Benítez ID, Carmona P, et al. Pulmonary function and radiologic features in survivors of critical COVID-19. Chest 2021; 160: 187–198. doi: 10.1016/j.chest.2021.02.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blanco J-R, Cobos-Ceballos M-J, Navarro F, et al. Pulmonary long-term consequences of COVID-19 infections after hospital discharge. Clin Microbiol Infect 2021; 27: 892–896. doi: 10.1016/j.cmi.2021.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Truffaut L, Demey L, Bruyneel AV, et al. Post-discharge critical COVID-19 lung function related to severity of radiologic lung involvement at admission. Respir Res 2021; 22: 29. doi: 10.1186/s12931-021-01625-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anastasio F, Barbuto S, Scarnecchia E, et al. Medium-term impact of COVID-19 on pulmonary function, functional capacity and quality of life. Eur Respir J 2021; 58: 2004015. doi: 10.1183/13993003.04015-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raman B, Cassar MP, Tunnicliffe EM, et al. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine 2021; 31: 100683. doi: 10.1016/j.eclinm.2020.100683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu Q, Zhong L, Li H, et al. A follow-up study of lung function and chest computed tomography at 64 months after discharge in patients with coronavirus disease 2019. Can Respir J 2021; 2021: 6692409. doi: 10.1155/2021/6692409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smet J, Stylemans D, Hanon S, et al. Clinical status and lung function 10 weeks after severe SARS-CoV-2 infection. Respir Med 2021; 176: 106276. doi: 10.1016/j.rmed.2020.106276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qin W, Chen S, Zhang Y, et al. Diffusion capacity abnormalities for carbon monoxide in patients with COVID-19 at 3-month follow-up. Eur Respir J 2021; 58: 2003677. doi: 10.1183/13993003.03677-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu J, Zhou M, Luo P, et al. Plasma metabolomic profiling of patients recovered from coronavirus disease 2019 (COVID-19) with pulmonary sequelae 34 months after discharge. Clin Infect Dis 2021; 73: 2228–2239. doi: 10.1093/cid/ciab147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sonnweber T, Sahanic S, Pizzini A, et al. Cardiopulmonary recovery after COVID-19: an observational prospective multicentre trial. Eur Respir J 2021; 57: 2003481. doi: 10.1183/13993003.03481-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van der Sar-van der Brugge S, Talman S, Boonman-de Winter L, et al. Pulmonary function and health-related quality of life after COVID-19 pneumonia. Respir Med 2021; 176: 106272. doi: 10.1016/j.rmed.2020.106272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Graaf MA, Antoni ML, ter Kuile MM, et al. Short-term outpatient follow-up of COVID-19 patients: a multidisciplinary approach. EClinicalMedicine 2021; 32: 100731. doi: 10.1016/j.eclinm.2021.100731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liang L, Yang B, Jiang N, et al. Three-month follow-up study of survivors of coronavirus disease 2019 after discharge. J Korean Med Sci 2020; 35: e418. doi: 10.3346/jkms.2020.35.e418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trinkmann F, Müller M, Reif A, et al. Residual symptoms and lower lung function in patients recovering from SARS-CoV-2 infection. Eur Respir J 2021; 57: 2003002. doi: 10.1183/13993003.03002-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Curci C, Pisano F, Bonacci E, et al. Early rehabilitation in post-acute COVID-19 patients: data from an Italian COVID-19 rehabilitation unit and proposal of a treatment protocol. Eur J Phys Rehabil Med 2020; 56: 633–641. doi: 10.23736/S1973-9087.20.06339-X [DOI] [PubMed] [Google Scholar]
- 62.Hermann M, Pekacka-Egli A-M, Witassek F, et al. Feasibility and efficacy of cardiopulmonary rehabilitation after COVID-19. Am J Phys Med Rehabil 2020; 99: 865–869. doi: 10.1097/PHM.0000000000001549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gao Y, Chen R, Geng Q, et al. Cardiopulmonary exercise testing might be helpful for interpretation of impaired pulmonary function in recovered COVID-19 patients. Eur Respir J 2021; 57: 2004265. doi: 10.1183/13993003.04265-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Debeaumont D, Boujibar F, Ferrand-Devouge E, et al. Cardiopulmonary exercise testing to assess persistent symptoms at 64 months in people with COVID-19 who survived hospitalization – a pilot study. Phys Ther 2021; 101: pzab099. doi: 10.1093/ptj/pzab099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Crameri GAG, Bielecki M, Züst R, et al. Reduced maximal aerobic capacity after COVID-19 in young adult recruits, Switzerland, May 2020. Euro Surveill 2020; 25; 2001542. doi: 10.2807/1560-7917.ES.2020.25.36.2001542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fumagalli A, Misuraca C, Bianchi A, et al. Pulmonary function in patients surviving to COVID-19 pneumonia. Infection 2021; 49: 153–157. doi: 10.1007/s15010-020-01474-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.You J, Zhang L, Ni-jia-Ti M, et al. Anormal pulmonary function and residual CT abnormalities in rehabilitating COVID-19 patients after discharge. J Infect 2020; 81: e150–e152. doi: 10.1016/j.jinf.2020.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li H, Zhao X, Wang Y, et al. Damaged lung gas exchange function of discharged COVID-19 patients detected by hyperpolarized 129 Xe MRI. Sci Adv 2021; 7: eabc8180. doi: 10.1126/sciadv.abc8180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barisione G, Brusasco V. Lung diffusing capacity for nitric oxide and carbon monoxide following mild-to-severe COVID-19. Physiol Rep 2021; 9: e14748. doi: 10.14814/phy2.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Patel K, Straudi S, Yee Sien N, et al. Applying the WHO ICF framework to the outcome measures used in the evaluation of long-term clinical outcomes in coronavirus outbreaks. Int J Environ Res Public Health 2020; 17: 6476. doi: 10.3390/ijerph17186476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Torres-Castro R, Vasconcello-Castillo L, Alsina-Restoy X, et al. Respiratory function in patients post-infection by COVID-19: a systematic review and meta-analysis. Pulmonology 2021; 27: 328–337. doi: 10.1016/j.pulmoe.2020.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.So M, Kabata H, Fukunaga K, et al. Radiological and functional lung sequelae of COVID-19: a systematic review and meta-analysis. BMC Pulm Med 2021; 21: 97. doi: 10.1186/s12890-021-01463-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang Y, Tan C, Wu J, et al. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir Res 2020; 21: 163. doi: 10.1186/s12931-020-01429-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu K, Zhang W, Yang Y, et al. Respiratory rehabilitation in elderly patients with COVID-19: a randomized controlled study. Complement Ther Clin Pract 2020; 39: 101166. doi: 10.1016/j.ctcp.2020.101166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chapman DG, Badal T, King GG, et al. Caution in interpretation of abnormal carbon monoxide diffusion capacity in COVID-19 patients. Eur Respir J 2021; 57: 2003263. doi: 10.1183/13993003.03263-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hughes JMB, Pride NB. Examination of the carbon monoxide diffusing capacity (DlCO) in relation to its Kco and Va components. Am J Respir Crit Care Med 2012; 186: 132–139. doi: 10.1164/rccm.201112-2160CI [DOI] [PubMed] [Google Scholar]
- 77.Alharthy A, Abuhamdah M, Balhamar A, et al. Residual lung injury in patients recovering from COVID-19 critical illness: a prospective longitudinal point-of-care lung ultrasound study. J Ultrasound Med 2020; 40: 1823–1838. doi: 10.1002/jum.15563 [DOI] [PubMed] [Google Scholar]
- 78.Rogliani P, Calzetta L, Coppola A, et al. Are there pulmonary sequelae in patients recovering from COVID-19? Respir Res 2020; 21: 286. doi: 10.1186/s12931-020-01550-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Goel N, Goyal N, Kumar R. Clinico-radiological evaluation of post COVID-19 at a tertiary pulmonary care centre in Delhi, India. Monaldi Arch Chest Dis 2021; 91: 1682. doi: 10.4081/monaldi.2021.1682 [DOI] [PubMed] [Google Scholar]
- 80.Pan Y, Xia L, Wang Y, et al. Dynamic changes in computed tomography manifestations of 105 patients with novel coronavirus pneumonia in Wuhan, China. J Int Med Res 2020; 48: 030006052097291. doi: 10.1177/0300060520972913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dai M, Liu X, Zhu X, et al. Temporal changes of CT findings between non-severe and severe cases of COVID-19 pneumonia: a multi-center, retrospective, longitudinal study. Int J Med Sci 2020; 17: 2653–2662. doi: 10.7150/ijms.51159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tabatabaei SMH, Rajebi H, Moghaddas F, et al. Chest CT in COVID-19 pneumonia: what are the findings in mid-term follow-up? Emerg Radiol 2020; 27: 711–719. doi: 10.1007/s10140-020-01869-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu N, He G, Yang X, et al. Dynamic changes of chest CT follow-up in coronavirus disease-19 (COVID-19) pneumonia: relationship to clinical typing. BMC Med Imaging 2020; 20: 92. doi: 10.1186/s12880-020-00491-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang Y, Jin C, Wu CC, et al. Organizing pneumonia of COVID-19: time-dependent evolution and outcome in CT findings. PLoS One 2020; 15: e0240347. doi: 10.1371/journal.pone.0240347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Avanzato VA, Matson MJ, Seifert SN, et al. Case study: prolonged infectious SARS-CoV-2 shedding from an asymptomatic immunocompromised individual with cancer. Cell 2020; 183: 1901–1912. doi: 10.1016/j.cell.2020.10.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wong W, Brieva C, May M, et al. A double-edged sword: prolonged detection of SARS-COV-2 in patients receiving cancer directed therapy. Semin Oncol 2021; 48: 166–170. doi: 10.1053/j.seminoncol.2020.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xu W, Piper-Vallillo AJ, Bindal P, et al. Time to SARS-CoV-2 clearance among patients with cancer and COVID-19. Cancer Med 2021; 10: 1545–1549. doi: 10.1002/cam4.3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Raveh Y, Simkins J, Vianna R, et al. A less restrictive policy for liver transplantation in coronavirus disease 2019 positive patients, based upon cycle threshold values. Transplant Proc 2021; 53: 1126–1131. doi: 10.1016/j.transproceed.2021.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Waghmare A, Abidi MZ, Boeckh M, et al. Guidelines for COVID-19 management in hematopoietic cell transplantation and cellular therapy recipients. Biol Blood Marrow Transplant 2020; 26: 1983–1994. doi: 10.1016/j.bbmt.2020.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cevik M, Tate M, Lloyd O, et al. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe 2021; 2: e13–e22. doi: 10.1016/S2666-5247(20)30172-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.D'Cruz RF, Waller MD, Perrin F, et al. Chest radiography is a poor predictor of respiratory symptoms and functional impairment in survivors of severe COVID-19 pneumonia. ERJ Open Res 2021; 7: 00655-2020. doi: 10.1183/23120541.00655-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Garrigues E, Janvier P, Kherabi Y, et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect 2020; 81: e4–e6. doi: 10.1016/j.jinf.2020.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jacobs LG, Gourna Paleoudis E, Lesky-Di Bari D, et al. Persistence of symptoms and quality of life at 35 days after hospitalization for COVID-19 infection. PLoS One 2020; 15: e0243882. doi: 10.1371/journal.pone.0243882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vitacca M, Lazzeri M, Guffanti E, et al. Italian suggestions for pulmonary rehabilitation in COVID-19 patients recovering from acute respiratory failure: results of a Delphi process. Monaldi Arch Chest Dis 2020; 90: 1444. doi: 10.4081/monaldi.2020.1444 [DOI] [PubMed] [Google Scholar]
- 95.Leite VF, Rampim DB, Jorge VC, et al. Persistent symptoms and disability after COVID-19 hospitalization: data from a comprehensive telerehabilitation program. Arch Phys Med Rehabil 2021; 102: 1308–1316. doi: 10.1016/j.apmr.2021.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Apea VJ, Wan YI, Dhairyawan R, et al. Ethnicity and outcomes in patients hospitalised with COVID-19 infection in East London: an observational cohort study. BMJ Open 2021; 11: e042140. doi: 10.1136/bmjopen-2020-042140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Machado FVC, Meys R, Delbressine JM, et al. Construct validity of the post-COVID-19 Functional Status Scale in adult subjects with COVID-19. Health Qual Life Outcomes 2021; 19: 40. doi: 10.1186/s12955-021-01691-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Matalon N, Dorman-Ilan S, Hasson-Ohayon I, et al. Trajectories of post-traumatic stress symptoms, anxiety, and depression in hospitalized COVID-19 patients: a one-month follow-up. J Psychosom Res 2021; 143: 110399. doi: 10.1016/j.jpsychores.2021.110399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Demeco A, Marotta N, Barletta M, et al. Rehabilitation of patients post-COVID-19 infection: a literature review. J Int Med Res 2020; 48: 030006052094838. doi: 10.1177/0300060520948382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Valent A, Dudoignon E, Ressaire Q, et al. Three-month quality of life in survivors of ARDS due to COVID-19: a preliminary report from a French academic centre. Anaesth Crit Care Pain Med 2020; 39: 740–741. doi: 10.1016/j.accpm.2020.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Arab-Zozani M, Hashemi F, Safari H, et al. Health-related quality of life and its associated factors in COVID-19 patients. Osong Public Health Res Perspect 2020; 11: 296–302. doi: 10.24171/j.phrp.2020.11.5.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Taboada M, Moreno E, Cariñena A, et al. Quality of life, functional status, and persistent symptoms after intensive care of COVID-19 patients. Br J Anaesth 2021; 126: e110–e113. doi: 10.1016/j.bja.2020.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Agostini F, Mangone M, Ruiu P, et al. Rehabilitation setting during and after Covid-19: an overview on recommendations. J Rehabil Med 2021; 53: jrm00141. doi: 10.2340/16501977-2776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Townsend L, Dowds J, O'Brien K, et al. Persistent poor health post-COVID-19 is not associated with respiratory complications or initial disease severity. Ann Am Thorac Soc 2021; 18: 997–1003. doi: 10.1513/AnnalsATS.202009-1175OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Arnold DT, Hamilton FW, Milne A, et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax 2021; 76: 399–401. doi: 10.1136/thoraxjnl-2020-216086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Monti G, Leggieri C, Fominskiy E, et al. Two-months quality of life of COVID-19 invasively ventilated survivors; an Italian single-center study. Acta Anaesthesiol Scand 2021; 65: 912–920. doi: 10.1111/aas.13812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Willi S, Lüthold R, Hunt A, et al. COVID-19 sequelae in adults aged less than 50 years: a systematic review. Travel Med Infect Dis 2021; 40: 101995. doi: 10.1016/j.tmaid.2021.101995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tenforde MW, Kim SS, Lindsell CJ, et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network – United States, March–June 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 993–998. doi: 10.15585/mmwr.mm6930e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Baricich A, Borg MB, Cuneo D, et al. Midterm functional sequelae and implications in rehabilitation after COVID-19: a cross-sectional study. Eur J Phys Rehabil Med 2021; 57: 199–207. doi: 10.23736/S1973-9087.21.06699-5 [DOI] [PubMed] [Google Scholar]
- 110.Belli S, Balbi B, Prince I, et al. Low physical functioning and impaired performance of activities of daily life in COVID-19 patients who survived hospitalisation. Eur Respir J 2020; 56: 2002096. doi: 10.1183/13993003.02096-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Patel SY, Mehrotra A, Huskamp HA, et al. Variation in telemedicine use and outpatient care during the COVID-19 pandemic in the United States: study examines variation in total US outpatient visits and telemedicine use across patient demographics, specialties, and conditions during the COVID-19 pandemic. Health Aff 2021; 40: 349–358. doi: 10.1377/hlthaff.2020.01786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Attipoe-Dorcoo S, Delgado R, Gupta A, et al. Mobile health clinic model in the COVID-19 pandemic: lessons learned and opportunities for policy changes and innovation. Int J Equity Health 2020; 19: 73. doi: 10.1186/s12939-020-01175-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rosen K, Patel M, Lawrence C, et al. Delivering telerehabilitation to COVID-19 inpatients: a retrospective chart review suggests it is a viable option. HSS J 2020; 16: 64–70. doi: 10.1007/s11420-020-09774-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Elhadi M, Msherghi A, Elhadi A, et al. Utilization of telehealth services in Libya in response to the COVID-19 pandemic: cross-sectional analysis. JMIR Med Inform 2021; 9: e23335. doi: 10.2196/23335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Luks AM, Swenson ER. Pulse oximetry for monitoring patients with COVID-19 at home. Potential pitfalls and practical guidance. Ann Am Thorac Soc 2020; 17: 1040–1046. doi: 10.1513/AnnalsATS.202005-418FR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Faezipour M, Abuzneid A. Smartphone-based self-testing of COVID-19 using breathing sounds. Telemed E-Health 2020; 26: 1202–1205. doi: 10.1089/tmj.2020.0114 [DOI] [PubMed] [Google Scholar]
- 117.Ebbert JO, Ramar P, Tulledge-Scheitel SM, et al. Patient preferences for telehealth services in a large multispecialty practice. J Telemed Telecare 2021; in press [ 10.1177/1357633X20980302]. doi: 10.1177/1357633X20980302 [DOI] [PubMed] [Google Scholar]
- 118.Hoel V, von Zweck C, Ledgerd R, et al. Was a global pandemic needed to adopt the use of telehealth in occupational therapy? Work 2021; 68: 13–20. doi: 10.3233/WOR-205268 [DOI] [PubMed] [Google Scholar]
- 119.Garcia-Huidobro D, Rivera S, Valderrama Chang S, et al. System-wide accelerated implementation of telemedicine in response to COVID-19: mixed methods evaluation. J Med Internet Res 2020; 22: e22146. doi: 10.2196/22146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Isautier JM, Copp T, Ayre J, et al. People's experiences and satisfaction with telehealth during the COVID-19 pandemic in Australia: cross-sectional survey study. J Med Internet Res 2020; 22: e24531. doi: 10.2196/24531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Barkai G, Gadot M, Amir H, et al. Patient and clinician experience with a rapidly implemented large-scale video consultation program during COVID-19. Int J Qual Health Care 2021; 33: mzaa165. doi: 10.1093/intqhc/mzaa165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Eddine IS, Zedan HS. Telehealth role during the COVID-19 pandemic: lessons learned from health care providers in Saudi Arabia. Telemed E-Health 2021; 27: 1249–1259. doi: 10.1089/tmj.2020.0489 [DOI] [PubMed] [Google Scholar]
- 123.Lian W, Wen L, Zhou Q, et al. Digital health technologies respond to the COVID-19 pandemic in a tertiary hospital in China: development and usability study. J Med Internet Res 2020; 22: e24505. doi: 10.2196/24505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sousa CS, Trigueiro-Barbosa M, Aguiar R, et al. What do asthmatic patients think about telemedicine visits? Eur Ann Allergy Clin Immunol 2021; 53: 138–142. doi: 10.23822/EurAnnACI.1764-1489.182 [DOI] [PubMed] [Google Scholar]
- 125.An MH, You SC, Park RW, et al. Using an extended technology acceptance model to understand the factors influencing telehealth utilization after flattening the COVID-19 curve in South Korea: cross-sectional survey study. JMIR Med Inform 2021; 9: e25435. doi: 10.2196/25435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kondylakis H, Katehakis DG, Kouroubali A, et al. COVID-19 mobile apps: a systematic review of the literature. J Med Internet Res 2020; 22: e23170. doi: 10.2196/23170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wynn R. E-health in Norway before and during the initial phase of the Covid-19 pandemic. Stud Health Technol Inform 2020; 272: 9–12. doi: 10.3233/SHTI200480 [DOI] [PubMed] [Google Scholar]
- 128.Manyati TK, Mutsau M. Exploring the effectiveness of telehealth interventions for diagnosis, contact tracing and care of Corona Virus Disease of 2019 (COVID19) patients in sub Saharan Africa: a rapid review. Health Technol 2021; 11: 341–348. doi: 10.1007/s12553-020-00485-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Philip P, Dupuy L, Morin CM, et al. Smartphone-based virtual agents to help individuals with sleep concerns during COVID-19 confinement: feasibility study. J Med Internet Res 2020; 22: e24268. doi: 10.2196/24268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jetty A, Jabbarpour Y, Westfall M, et al. Capacity of primary care to deliver telehealth in the United States. J Am Board Fam Med 2021; 34: Suppl., S48–S54. doi: 10.3122/jabfm.2021.S1.200202 [DOI] [PubMed] [Google Scholar]
- 131.Tsutsui M, Gerayeli F, Sin DD. Pulmonary rehabilitation in a post-COVID-19 world: telerehabilitation as a new standard in patients with COPD. Int J Chron Obstruct Pulmon Dis 2021; 16: 379–391. doi: 10.2147/COPD.S263031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jennings SC, Manning KM, Bettger JP, et al. Rapid transition to telehealth group exercise and functional assessments in response to COVID-19. Gerontol Geriatr Med 2020; 6: 233372142098031. doi: 10.1177/2333721420980313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Qureshi AZ, Ullah S, Aldajani AA, et al. Telerehabilitation guidelines in Saudi Arabia. Telemed E-Health 2021; 27: 1087–1098. doi: 10.1089/tmj.2020.0355 [DOI] [PubMed] [Google Scholar]
- 134.Franzosa E, Gorbenko K, Brody AA, et al. “At home, with care”: lessons from New York City home-based primary care practices managing COVID-19. J Am Geriatr Soc 2021; 69: 300–306. doi: 10.1111/jgs.16952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jørgensen BB, Gregersen M, Pallesen SH, et al. A group-based real-time videoconferencing telerehabilitation programme in recently discharged geriatric patients: a feasibility study. Eur Geriatr Med 2021; 12: 801–808. doi: 10.1007/s41999-020-00444-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fisk M, Livingstone A, Pit SW. Telehealth in the context of COVID-19: changing perspectives in Australia, the United Kingdom, and the United States. J Med Internet Res 2020; 22: e19264. doi: 10.2196/19264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Leochico CFD, Espiritu AI, Ignacio SD, et al. Challenges to the emergence of telerehabilitation in a developing country: a systematic review. Front Neurol 2020; 11: 1007. doi: 10.3389/fneur.2020.01007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Seligman WH, Fialho L, Sillett N, et al. Which outcomes are most important to measure in patients with COVID-19 and how and when should these be measured? Development of an international standard set of outcomes measures for clinical use in patients with COVID-19: a report of the International Consortium for Health Outcomes Measurement (ICHOM) COVID-19 Working Group. BMJ Open 2021; 11: e051065. doi: 10.1136/bmjopen-2021-051065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Evans RA, McAuley H, Harrison EM, et al. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): a UK multicentre, prospective cohort study. Lancet Respir Med 2021; 9: 1275–1287. doi: 10.1016/S2213-2600(21)00383-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Antoniou KM, Raghu G, Tzilas V, et al. Management of patients with interstitial lung disease in the midst of the COVID-19 pandemic. Respiration 2020; 99: 625–627. doi: 10.1159/000509523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Montani D, Savale L, Beurnier A, et al. Multidisciplinary approach for post-acute COVID-19 syndrome: time to break down the walls. Eur Respir J 2021; 58: 2101090. doi: 10.1183/13993003.01090-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Laine C, Cotton D. COVID-19: evaluation and care of patients with persistent symptoms following acute SARS-CoV-2 infection. Ann Intern Med 2021; 174: 1159–1160. doi: 10.7326/M21-2342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Paneroni M, Vitacca M, Bernocchi P, et al. Feasibility of tele-rehabilitation in survivors of COVID-19 pneumonia. Pulmonology 2022; 28: 152–154. doi: 10.1016/j.pulmoe.2021.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Suso-Martí L, La Touche R, Herranz-Gómez A, et al. Effectiveness of telerehabilitation in physical therapist practice: an umbrella and mapping review with meta–meta-analysis. Phys Ther 2021; 101: pzab075. doi: 10.1093/ptj/pzab075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mudatsir M, Fajar JK, Wulandari L, et al. Predictors of COVID-19 severity: a systematic review and meta-analysis. F1000Research 2021; 9: 1107. doi: 10.12688/f1000research.26186.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wells AU, Devaraj A, Desai SR. Interstitial lung disease after COVID-19 infection: a catalog of uncertainties. Radiology 2021; 299: E216–E218. doi: 10.1148/radiol.2021204482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med 2021; 27: 626–631. doi: 10.1038/s41591-021-01292-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.von der Thüsen J, Eerden M. Histopathology and genetic susceptibility in COVID-19 pneumonia. Eur J Clin Invest 2020; 50: e13259. doi: 10.1111/eci.13259 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-02174-2021.Supplement (523.8KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-02174-2021.Shareable (482.1KB, pdf)