Abstract
Coronavirus disease 2019 (COVID‐19) often causes radiological and functional pulmonary sequelae. However, evidence on 1‐year follow‐up of pulmonary sequelae is limited. We aimed to investigate the characteristics and time‐course of pulmonary sequelae after recovery from COVID‐19 through 1‐year follow‐up. We searched PubMed and EMBASE databases on 25 February 2022, and included studies with computed tomography (CT) findings at the 1‐year follow‐up. The extracted data on CT findings were analysed using a one‐group meta‐analysis. We further analysed the data in relation to COVID‐19 severity, improvement rate and lung function. Fifteen eligible studies (N = 3134) were included. One year after COVID‐19, 32.6% (95% CI 24.0–42.6, I 2 = 92.9%) presented with residual CT abnormalities. Ground‐glass opacity and fibrotic‐like changes were frequently observed in 21.2% (95% CI 15.4–28.4, I 2 = 86.7%) and 20.6% (95% CI 11.0–35.2, I 2 = 91.9%), respectively. While the gradual recovery was seen on CT (52.9% [mid‐term] vs. 32.6% [1 year]), the frequency of CT abnormalities was higher in the severe/critical cases than in the mild/moderate cases (37.7% vs. 20.7%). In particular, fibrotic changes showed little improvement between 4–7 months and 1 year after COVID‐19. Pulmonary function tests at 1 year also showed the decline in diffusing capacity of the lung for carbon monoxide, especially in severe/critical cases. Our meta‐analysis indicated that residual CT abnormalities were common in hospitalized COVID‐19 patients 1 year after recovery, especially fibrotic changes in severe/critical cases. As these sequelae may last long, vigilant observations and longer follow‐up periods are warranted.
Keywords: computed tomography, coronavirus disease, COVID‐19, follow‐up, pulmonary function test, SARS‐CoV‐2, sequelae

INTRODUCTION
Coronavirus disease 2019 (COVID‐19) has caused a global pandemic with nearly 400 million confirmed cases and over 5.5 million deaths as of 5 February 2022. 1 Although COVID‐19 is known to cause multiple organ damage, pneumonia is the most common manifestation. 2 , 3 The severity of COVID‐19 pneumonia ranges from asymptomatic to critical respiratory failure requiring mechanical ventilatory support. 3
Not only can some cases present critical respiratory failure, but COVID‐19 pneumonia is also notorious for its tendency to persist after recovery. 4 , 5 A previous meta‐analysis investigated lung sequelae of COVID‐19 and demonstrated that more than half of the recovered patients still had chest computed tomography (CT) abnormalities 90 days after infection. 5 In particular, ground‐glass opacities (GGOs), parenchymal bands/fibrous stripes and reticulation were frequently observed. Furthermore, prospective studies have shown that patients with severe diseases tend to have long‐term lung abnormalities more frequently, and GGO and parenchymal bands take longer to resolve than consolidation or crazy‐paving patterns. 6 , 7
Although several prospective studies have sought to examine long‐term CT changes in COVID‐19, these findings were limited by small cohort sizes. 6 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 In addition, the interpretations of each study varied due to the wide range of severity. During the severe acute respiratory syndrome (SARS) outbreak in 2003, residual pulmonary fibrosis was found in 67% of patients 1 month after the infection, and some of those abnormalities were still observed 7 years later. 22 , 23 In contrast, the time‐course of pulmonary sequelae in the current COVID‐19 pandemic remains unclear.
Therefore, long‐term longitudinal follow‐up will help in understanding the overtime CT changes in COVID‐19. Since these radiological sequelae combined with pulmonary dysfunction can detrimentally affect patients' quality of life, elucidating the prevalence and time‐course of CT abnormalities in 1‐year follow‐up will be useful to determine the management plans for recovered patients. In this study, we conducted a systematic review and meta‐analysis of 1‐year follow‐up CT findings to investigate the changes in pulmonary sequelae in COVID‐19 patients in this time frame.
METHODS
Eligibility criteria
The included studies met the following criteria: (1) an observational study that was published in peer‐reviewed journals; (2) the study population included patients with SARS coronavirus 2 (SARS‐CoV‐2) infections confirmed by quantitative real‐time PCR; and (3) the study followed up patients for 1 year and evaluated chest CT findings. Articles that did not contain original patient data (e.g., guidelines, editorials and reviews) were excluded.
Information sources and search
Eligible studies were identified using a two‐level strategy. The PubMed and EMBASE databases were searched on 25 February 2022. Search items included ((SARS‐CoV‐2) OR (COVID‐19)) AND ((follow‐up) OR (long‐term)) AND ((1‐year) OR (one‐year) OR (1 year) OR (one year) OR (12‐month) OR (12 months)) AND ((CT) OR (computed tomography)). Relevant studies were further identified through a manual search of secondary sources, including references to initially identified articles, reviews and commentaries. Two independent authors (A. W. and M. S.) reviewed the search results separately and selected the studies based on the inclusion and exclusion criteria. Disagreements were resolved through consensus.
Risk of bias assessment
The risk of bias in individual studies was reviewed using the assessment of risk of bias in prevalence studies. 24 Publications bias was assessed using Egger's test.
Data items
Baseline characteristics including age, sex, comorbidities, severity of COVID‐19 and residual symptoms 1 year after COVID‐19 were collected. As for CT findings, follow‐up timing, frequency of overall CT abnormalities and each specific finding, including GGO, fibrotic‐like changes, consolidation, reticulation, interlobular septal thickening and bronchiectasis, were collected. The definitions of fibrotic‐like changes followed each study. We also collected pulmonary function test (PFT) results, including reduced (<80% of predicted value) diffusing capacity of the lung for carbon monoxide (DLCO), reduced forced expiratory volume in the first second (FEV1), reduced total lung capacity (TLC) and FEV1/forced vital capacity (FVC) ratio < 0.7. Guidelines from the National Institute of Health 25 and guidance from China, ‘Pneumonia diagnosis and treatment program for novel coronavirus infection (trail version 7)’, 26 were used to define the clinical severity of COVID‐19 as follows: (1) mild: no evidence of pneumonia on imaging; (2) moderate: evidence of lower respiratory disease during clinical assessment or imaging with oxygen saturation (SpO2) ≥ 93%; (3) severe: either respiratory distress, respiratory rate > 30/min, SpO2 < 93% at rest or arterial oxygen tension/fraction of inspired oxygen (PaO2/FiO2) < 300 mm Hg; (4) critical: either respiratory failure requiring ventilation, haemodynamic instability, other organ damage or intensive care unit admission.
Summary measures and synthesis of results
Comorbidities, residual COVID‐19 symptoms and the proportion of severe cases were calculated by dividing the total number of events by the number of patients in all studies wherein information was available. To calculate the frequency of residual abnormalities on follow‐up chest CT and PFT and to display the changes over time, we extracted data regarding the proportion of CT abnormalities both at mid‐term (4–7‐month) and long‐term (1‐year) follow‐ups, as well as each CT and PFT finding. When a patient who had normal imaging at mid‐term did not attend the 1‐year follow‐up, we considered that the CT at 1 year was normal. In addition, we stratified the percentage of residual CT abnormalities by severity. We pooled the logit of CT and PFT abnormalities, performed a one‐group meta‐analysis using the DerSimonian–Laird with a random‐effect model and back‐transformed them into the original scale. Meta‐analyses were conducted using OpenMetaAnalyst version 21.11.14. 27 Heterogeneity was assessed using I 2, with >50% being substantial. Meta‐regression was performed to detect the significant differences among the included studies with covariates of age, sex and follow‐up duration.
RESULTS
Study selection and study characteristics
We identified 183 articles that met our inclusion criteria using PubMed and EMBASE. We then reviewed them based on the title and abstract, and excluded 168 articles as they were irrelevant or did not contain original data. Finally, we reviewed the full text of 15 articles and included 15 observational studies (11 from China, three from Italy and one from the United Kingdom, N = 3134). Egger's test did not suggest a significant publication bias (p = 0.994) (Figure 1 and Figures S1 and S2 in the Supporting Information). 6 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21
FIGURE 1.
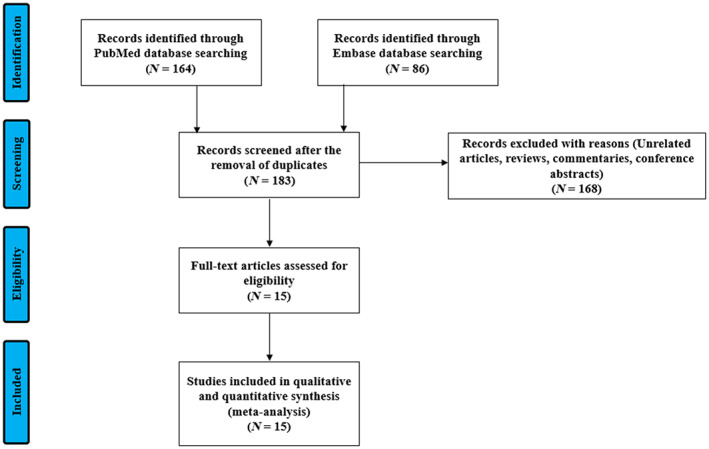
Flow diagram of study selection
Baseline characteristics and residual symptoms 1 year after COVID‐19
The baseline characteristics of the included study are shown in Table 1. The median age of the patients ranged from 39 to 64 years, and the percentage of men ranged from 19% to 89%. The most common comorbidity was hypertension (31.2% [859/2754]), followed by diabetes (14.2% [391/2760]), coronary heart disease (7.5% [161/2139]), chronic kidney disease (3.8% [68/1788]) and chronic obstructive pulmonary disease (3.4% [67/1975]). The severity of COVID‐19 in the pooled cohort (Table S1 in the Supporting Information) was as follows: severe/critical, 66.0% (2070/3134); mild/moderate, 30.6% (959/3134); and unknown severity, 3.4% (105/3134). Residual symptoms at 1‐year follow‐up included dyspnoea (either exertional or at rest), 32.8% (417/1273); fatigue, 22.0% (422/1915); insomnia, 21.1% (338/1604); musculoskeletal pain, 18.2% (359/1972); hair loss, 14.1% (219/1556); palpitations, 9.1% (140/1544); and smell or taste disorder, 5.9% (109/1850).
TABLE 1.
Baseline characteristics and CT findings at 1‐year follow‐up in COVID‐19 follow‐up studies
| Author | Country | Observational period | Cohort size | Age | Male, % | Severity, % (N) | Treatment for COVID‐19, % (N) | Follow‐up timing | Performed CT, N | Overall CT abnormalities, % (N) | GGO, % (N) | Fibrotic‐like changes, % (N) | Reticulation, % (N) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wu 8 | China | Feb–Mar 2020 | 83 | 60 [52–66] | 57 | Severe, 100 (83) |
Oseltamivir, 64 (53) Ribavirin, 100 (83) Ganciclovir, 51 (42) |
348 days | 83 | 24 (20) | 23 (19) | NA | 3.6 (3) |
| Han 9 | China | Dec 2019–Feb 2020 | 114 | 57 ± 10 | 55 |
Critical, 21 (24) Severe, 79 (90) |
NA | 363 [355–372] days | 62 | 73 (45) | 11 (7) | 56 (35) | 52 (32) |
| Chen 10 | China | Feb–Mar 2020 | 41 | 51 [38–59] | 59 |
Critical, 7.3 (3) Severe, 32 (13) Mild or moderate, 61 (25) |
Corticosteroids, 73 (30) | 1 year | 36 | 47 (17) | NA | NA | NA |
| Huang 11 | China | Jan–May 2020 | 1276 | 59 [49–67] | 53 |
Critical, 7.4 (94) Severe, 68 (864) Mild or moderate, 25 (318) |
Corticosteroids, 24 (307) Antivirals, 55 (705) Lopinavir‐ritonavir, 14 (173) IVIG, 20 (249) |
349 [337–361] days | 118 | 55 (65) | 46 (54) | NA | 3.4 (4) |
| Vijayakumar 20 | UK | Mar–Jun 2020 | 80 | 62 ± 11 | 66 |
Critical, 40 (32) Severe, 54 (43) Mild or moderate, 6.3 (5) |
NA | 364 [360–366] days | 32 | 84 (27) | NA | NA | NA |
| Pan 6 | China | Jan–Mar 2020 | 209 | 49 ± 13 | 45 |
Critical, 11 (22) Severe, 38 (80) Mild or moderate, 51 (107) |
NA | 1 year | 209 | 25 (53) | 24 (50) | NA | 13 (28) |
| Li 19 | China | Dec 2019–Apr 2020 | 141 | 59 [51–66] | 63 |
Critical, 26 (36) Unknown, 74 (105) |
Antivirals, 79 (111) Corticosteroids, 32 (45) |
351 [341–366] days | 25 | 52 (13) | 24 (6) | NA | 28 (7) |
| Shang 12 | China | Feb–Mar 2020 | 118 | 53 [44–61] | 41 |
Severe, 34 (40) Mild or moderate, 66 (78) |
NA | 349 [346–354] days | 99 | NA | 16 (16) | 18 (18) | NA |
| Zhao 13 | China | Jan–Feb 2020 | 94 | 48 | 57 |
Critical, 2.1 (2) Severe, 44 (41) Mild or moderate, 54 (51) |
Corticosteroids, 31 (29) IFN‐β, 82 (77) IVIG, 11 (10) |
366 [355–376] days | 94 | 71 (67) | 40 (38) | 8.5 (8) | 4.3 (4) |
| Zhan 14 | China | Jan–Mar 2020 | 121 | 49 [40–57] | 41 |
Critical, 0.8 (1) Severe, 15 (18) Mild or moderate, 84 (102) |
Antivirals, 89 (108) Corticosteroids, 14 (17) Hydroxychloroquine, 12 (14) |
348 [344–351] days | 121 | 8.3 (10) | NA | NA | NA |
| Liao 15 | China | Mar 2020 | 303 | 39 [33–48] | 19 |
Severe or critical, 63 (190) Mild or moderate, 37 (113) |
NA | 395 [382–408] days | 256 | 38 (96) | 25 (63) | 10 (26) | 0.8 (2) |
| Gamberini 16 | Italy | Feb–May 2020 | 178 | 64 [55–70] | 73 | Critical, 100 (178) | NA | 9–12 months | 37 | NA | 57 (21) | 70 (26) | NA |
| Bellan 17 | Italy | Mar–Jun 2020 | 200 | 62 [51–71] | 61 |
Critical, 35 (66) Severe, 41 (78) Mild or moderate, 29 (56) |
NA | 366 [363–369] days | 190 | 23 (44) | NA | NA | NA |
| Zhou 18 | China | Jan–Apr 2020 | 120 | 52 ± 11 | 41 |
Severe, 13 (16) Mild or moderate, 87 (104) |
NA | 315 [296–339] days | 97 | 57 (55) | 16 (16) | 18 (17) | NA |
| Zangrillo 21 | Italy | Feb–Apr 2020 | 56 | 56 ± 12 | 89 | Critical, 100 (56) |
Hydroxychloroquine, 96 (51) Tocilizumab, 15 (8) Antivirals, 92 (59) Corticosteroids, 30 (16) |
349 [343–356] days | 36 | NA | NA | 11 (4) | NA |
| Author | Interlobular septal thickening, % (N) | Bronchiectasis, % (N) | Consolidation, % (N) |
|---|---|---|---|
| Wu 8 | 4.8 (4) | 1.2 (1) | NA |
| Han 9 | 45 (28) | 44 (27) | 9.7 (6) |
| Chen 10 | NA | NA | 0 (0) |
| Huang 11 | 4.2 (5) | NA | 0.8 (1) |
| Vijayakumar 20 | NA | NA | NA |
| Pan 6 | 8.1 (17) | 6.7 (14) | 1.4 (3) |
| Li 19 | 36 (9) | 24 (6) | 0 (0) |
| Shang 12 | NA | 15 (15) | NA |
| Zhao 13 | 11 (10) | NA | 2.1 (2) |
| Zhan 14 | NA | NA | NA |
| Liao 15 | 8.6 (22) | 1.6 (4) | 3.2 (8) |
| Gamberini 16 | NA | 27 (10) | 8.1 (3) |
| Bellan 17 | NA | NA | NA |
| Zhou 18 | NA | 14 (14) | NA |
| Zangrillo 21 | NA | NA | NA |
Note: Values are shown as mean ± SD or median [Q1–Q3]. Observational periods represent the time when patients were hospitalized for acute phase of COVID‐19.
Abbreviations: COVID‐19, coronavirus disease 2019; CT, computed tomography; GGO, ground‐glass opacity; IFN, interferon; IVIG, intravenous immunoglobulin; NA, not applicable.
The frequency of abnormal CT findings 1 year after COVID‐19
Of the 3134 pooled subjects, 923 and 272 patients did not undergo CT because of random sampling and unspecified reasons, respectively. 11 Between the mid‐term and 1‐year follow‐up, 138 patients were lost to follow‐up; therefore, 1801 patients were evaluated for CT at 1 year (Figure S3 and Table S2 in the Supporting Information). Three studies did not report the proportion of overall residual CT abnormalities but reported other specific findings. 12 , 16 , 21 The proportion of patients with any residual CT abnormalities in 12 studies was estimated to be 32.6% (512/1629, 95% CI 24.0–42.6, I 2 = 92.9%; Figure 2A). The most prevalent residual CT finding was GGO (21.2% [290/1354], 95% CI 15.4–28.4, I 2 = 86.7%), followed by fibrotic‐like changes (definition: Table S3 in the Supporting Information) (20.6% [134/724], 95% CI 11.0–35.2, I 2 = 91.9%), bronchiectasis (9.6% [91/975], 95% CI 5.1–17.4, I 2 = 87.6%), interlobular septal thickening (8.4% [95/1121], 95% CI 4.6–14.9, I 2 = 87.6%), reticular opacity (5.5% [80/1121], 95% CI 2.1–13.3, I 2 = 92.3%) and consolidation (2.6% [23/1075], 95% CI 1.3–5.1, I 2 = 56.0%) (Figure 2B and Figure S4 in the Supporting Information). We performed a sensitivity analysis where CTs of the lost patients were added to the denominator to avoid overestimation, and the estimations were similar to the main analyses (Figure S5 in the Supporting Information). Given the substantial heterogeneity, we performed a meta‐regression to detect significant differences in baseline, but a significant relationship was not seen between the covariates and the proportion of overall residual CT abnormalities (Table S4 in the Supporting Information).
FIGURE 2.
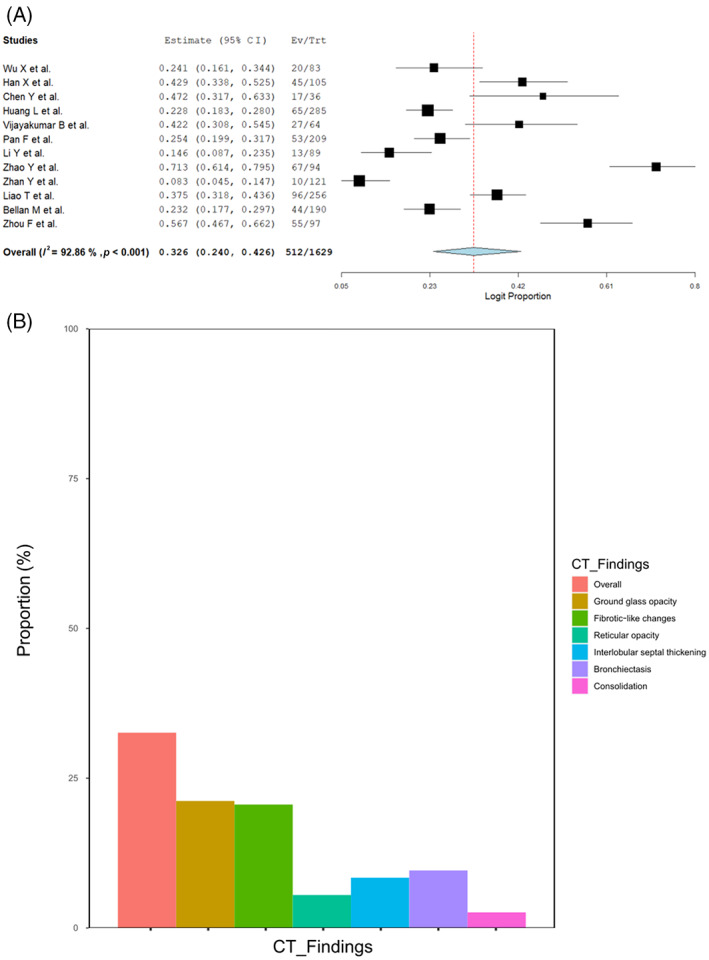
(A) Forest plots of overall residual computed tomography (CT) abnormalities at 1‐year follow‐up. (B) Bar graph showing the residual CT abnormalities at 1‐year follow‐up
Association between abnormal CT findings and the severity of COVID‐19
Next, 12 studies reported the proportion of abnormal CT findings at the 1‐year follow‐up according to the COVID‐19 severity. 6 , 8 , 9 , 10 , 11 , 13 , 14 , 15 , 16 , 18 , 20 , 21 Apart from the random sampling, 85.4% (950/1112) of severe/critical and 87.4% (560/641) of mild/moderate patients were included in our analyses. The proportion of residual CT abnormalities at 1‐year follow‐up was 37.7% (278/816, 95% CI 29.0–47.2, I 2 = 83.3%) in severe/critical patients and 20.7% (91/378, 95% CI 7.0–47.3, I 2 = 93.6%) in mild/moderate patients (Figure 3A,B). GGO was the major CT finding in patients with mild/moderate COVID‐19. In contrast, in patients with severe/critical COVID‐19, fibrotic‐like changes, bronchiectasis and interlobular septal thickening were observed in addition to GGO (Figure 3C and Figures S6 and S7 in the Supporting Information).
FIGURE 3.
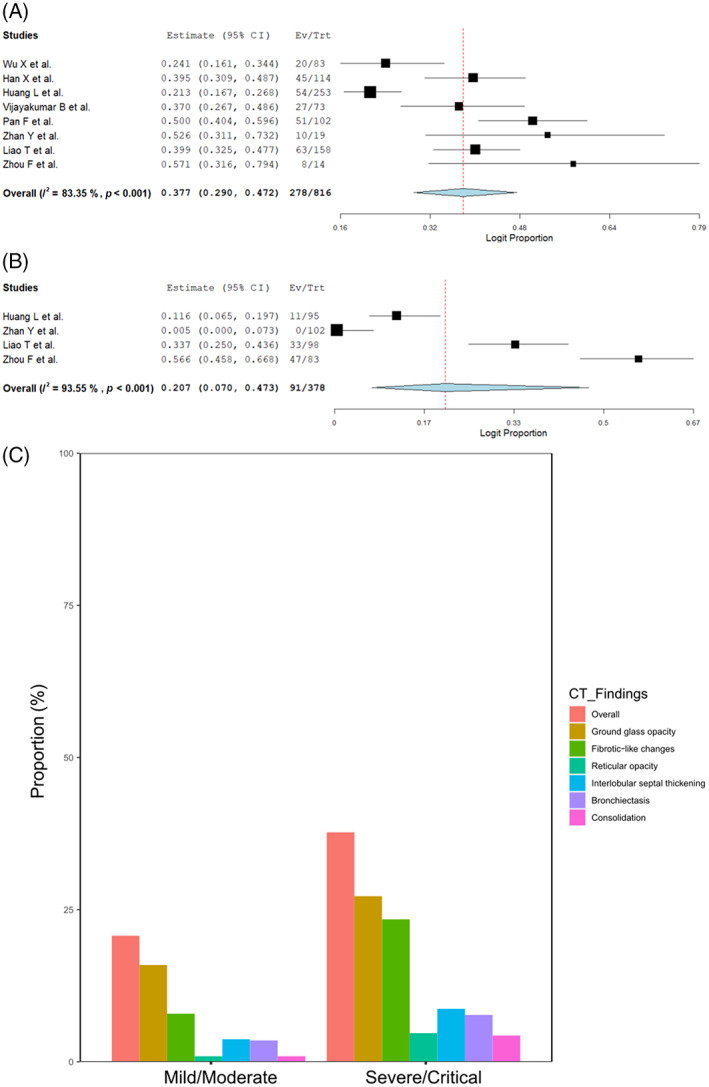
(A) Forest plots of overall residual computed tomography (CT) abnormalities at long‐term follow‐up in severe/critical patients. (B) Forest plots of overall residual CT abnormalities at long‐term follow‐up in mild/moderate patients. (C) Bar graph showing the differences in the proportion of each chest CT finding between severe/critical and mild/moderate patients at long‐term follow‐up
Time‐course of abnormal CT findings
Among the 15 studies, seven studies (N = 1014 patients) provided CT evaluation at 4–7‐month (ranging from 105 to 189 days) follow‐up 6 , 8 , 9 , 10 , 11 , 19 , 20 (Table S5 in the Supporting Information). One study did not report the proportion of overall CT abnormalities but reported specific findings. 10 The frequency of CT abnormalities 4–7 months after COVID‐19 was 52.9% (489/968, 95% CI 39.5–65.9, I 2 = 93.5%) (Figure 4A), which was higher than that at 1 year (32.6%). Notably, the frequency of GGO findings decreased from the 4–7‐month to 1‐year follow‐up (33.3% [337/968] vs. 21.2% [290/1354]), but there was little change in the frequency of fibrotic‐like changes (24.2% [51/187] vs. 20.6% [134/724]) and interlobular septal thickening (8.9% [70/757] vs. 8.4% [95/1121]) (Figure 4B and Figure S8 in the Supporting Information). Furthermore, the resolution rate of overall CT abnormalities was higher among mild/moderate patients at 0.60 (mid‐term: 51.6%, long‐term: 20.7%) than that of severe/critical patients at 0.37 (mid‐term: 59.7%, long‐term: 37.7%) (Figure S9 in the Supporting Information). While the proportion of patients with residual CT abnormalities decreased over time, severe/critical patients took longer to recover, especially patients with fibrotic‐like changes.
FIGURE 4.
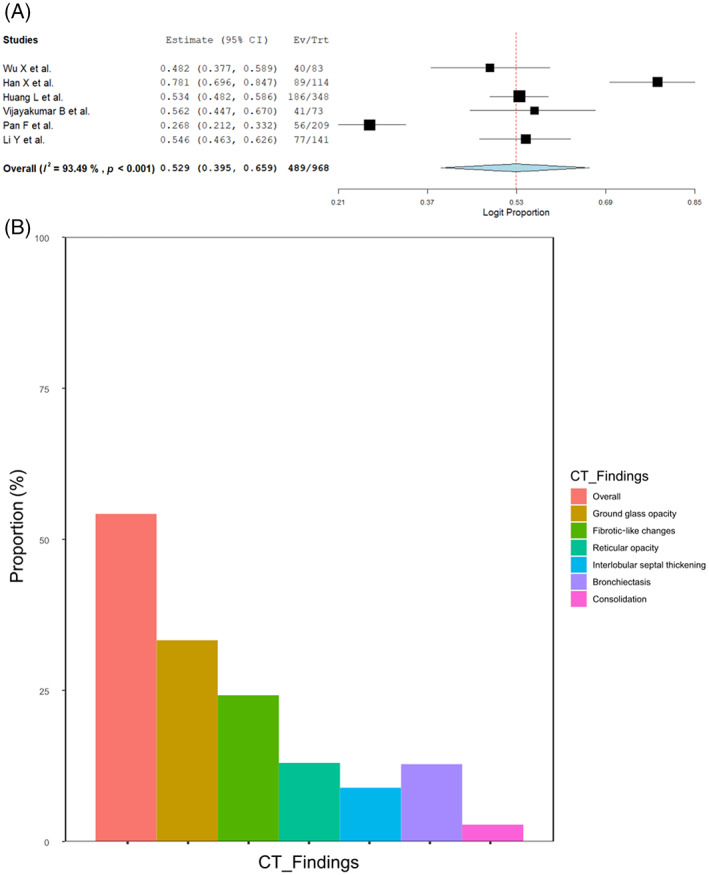
(A) Forest plots of overall residual computed tomography (CT) abnormalities at mid‐term follow‐up. (B) Bar graph showing the residual CT abnormalities at mid‐term follow‐up
Follow‐up PFT
Finally, we evaluated the PFT results at 1‐year follow‐up from 12 studies 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 20 (Table S6 in the Supporting Information). Reduced DLCO (<80% of predicted value), FEV1, FEV1/FVC < 0.7 and reduced TLC were observed in 30.5% (441/1052, 95% CI 24.5–37.2, I 2 = 81.1%), 9.1% (83/986, 95% CI 6.0–13.4, I 2 = 67.6%), 5.1% (50/986, 95% CI 3.1–8.2, I 2 = 53.1%) and 8.6% (85/994, 95% CI 6.1–12.0, I 2 = 54.2%), respectively (Figure 5A–D). Decreased DLCO was the major abnormal PFT finding 1 year after COVID‐19 and was more frequent in severe/critical cases than in mild/moderate cases (Figure 5E and Table S7 in the Supporting Information). Patients who recovered from severe COVID‐19 presented remaining CT abnormalities and prolonged functional abnormalities at 1‐year follow‐up.
FIGURE 5.
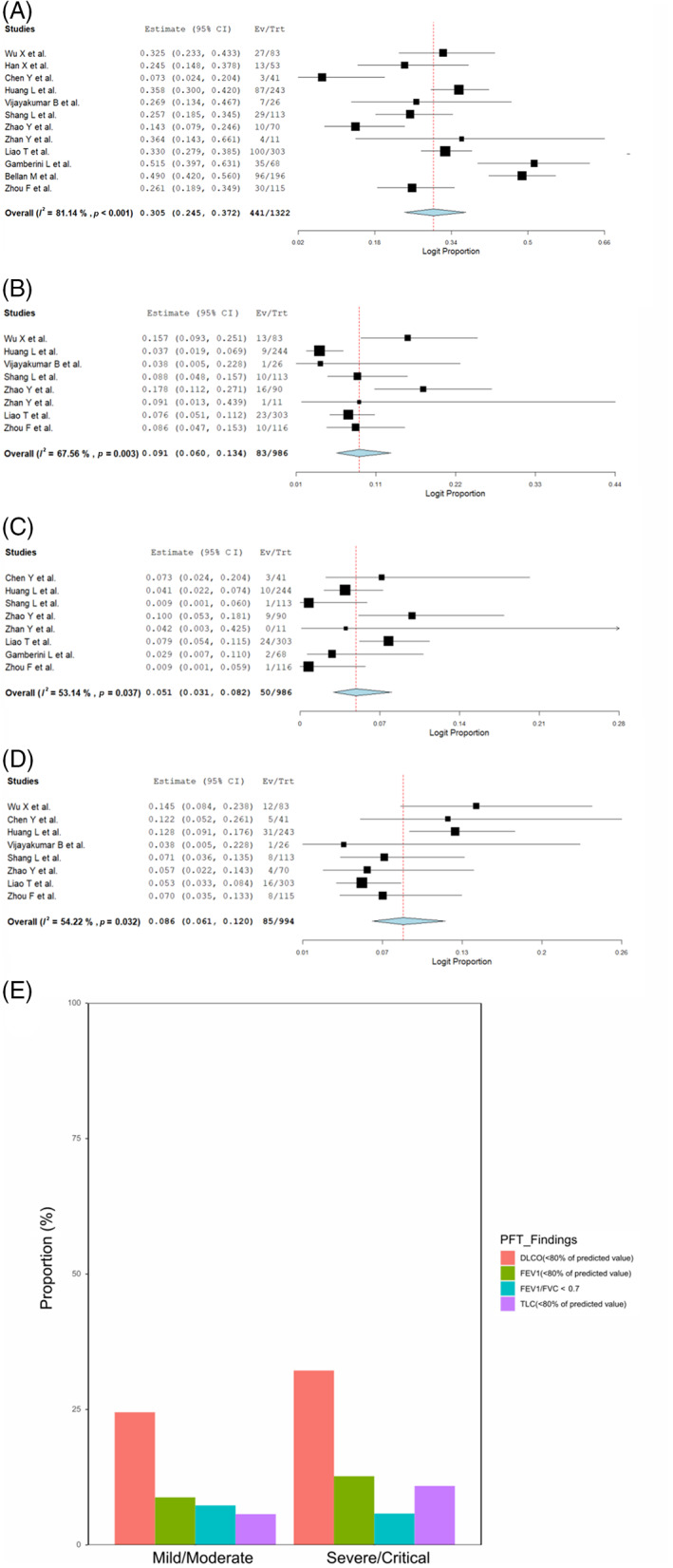
Forest plots of pulmonary function test (PFT) abnormalities at long‐term follow‐up (A: diffusing capacity of the lung for carbon monoxide [DLCO], B: forced expiratory volume in the first second [FEV1], C: FEV1/forced vital capacity [FVC], D: total lung capacity [TLC]). (E) Bar graph showing the differences in PFT abnormalities between severe/critical and mild/moderate patients at long‐term follow‐up
DISCUSSION
This meta‐analysis evaluated 1‐year follow‐up and overtime changes in CT abnormalities in recovered patients from COVID‐19. We elucidated that the frequency of CT abnormalities remained high 1 year after infection, especially among severe/critical patients for fibrotic changes. Furthermore, reflecting the fibrotic changes, the decline in DLCO persisted even after 1 year of COVID‐19.
Several prospective studies have investigated pulmonary sequelae 1 year after COVID‐19, 12 , 13 , 14 , 21 following a meta‐analysis on 90‐day follow‐up. 5 However, assessing the time‐course of CT abnormalities has been challenging due to a lack of multiple follow‐ups and a wide range of severity, with some studies including asymptomatic patients and others including only patients who required mechanical ventilation. The present study not only combined published data of multiple follow‐ups, but also enabled quantification in homogenous subgroups by sorting patients according to severity. In our study, while the gradual recovery was observed over time, residual CT abnormalities persisted in many patients at 1‐year follow‐up. The extent of CT findings also appeared to be related to severity. 10 , 16 Our results were similar to SARS‐CoV‐1 in 2003, where 30%–40% of survivors presented radiological abnormalities at 6 months to 1 year after recovery. At that time, patients with residual CT abnormalities at 1‐year follow‐up still had similar findings at the 15‐year follow‐up. 28 , 29 Provided this time course can be applied to COVID‐19, the tremendous number of the affected population is concerning.
In line with previous studies, fibrotic‐like changes, interstitial septal thickening and bronchiectasis were frequently observed in our study, possibly indicating evolving pulmonary fibrosis after COVID‐19. 6 , 9 , 10 , 30 , 31 , 32 , 33 A SARS‐CoV‐1 study showed that abnormal chest radiographs were present in 30% of survivors at the 6‐month follow‐up. 29 The high prevalence (62% [15/24]) of pulmonary fibrosis in another study was also noteworthy. 33 A Middle East respiratory syndrome (MERS) follow‐up study reported that pulmonary fibrosis (reticulation and linear opacities forming a mesh‐like pattern) was evident in 33% (12/36) of severe patients. 34 Not surprisingly, those findings that potentially indicate secondary pulmonary fibrosis were more common among severe patients in our study as well. In addition to severity, SARS and MERS studies identified older age as a potential risk factor for subsequent pulmonary fibrosis. 33 , 34 For COVID‐19, elderly, diabetes, obesity, lymphopenia, elevated d‐dimer, C‐reactive protein and lactate dehydrogenase have been suggested as possible risk factors for fibrotic changes as well as the severity. 6 , 12 , 13 , 15 , 19 , 35 These fibrotic findings may represent permanent lung damage, and the number of patients affected by COVID‐19 is substantial. 31 , 33 Hence, despite the argument that mechanical ventilation may cause barotrauma, resulting in iatrogenic fibrosis after acute respiratory distress syndrome, 20 , 36 the fibrotic signs on CT are not negligible. Understanding the impact and risk factors of pulmonary fibrosis after COVID‐19 pneumonia is vital because it will potentially enable us to take preventative measures (e.g., antifibrotic therapy) in appropriately selected patients and reduce the global burden of the consequences of COVID‐19. 37
We also extracted PFT data from the same cohorts and demonstrated consistently impaired diffusion capacity. Consistent with our findings, a longitudinal prospective study indicated that the proportion of reduced DLCO could remain unchanged from mid‐term to long‐term follow‐up, although the absolute value depended on the severity. 11 On the contrary, another study has shown the gradual functional recovery after COVID‐19. 8 According to their analysis, however, median DLCO was significantly lower among those with residual CT abnormalities at 1‐year follow‐up. Therefore, the disease severity and residual CT findings can be speculated to be interlinked with impaired diffusion capacity. Previous studies demonstrated a higher proportion of reduced DLCO after COVID‐19, regardless of restrictive or obstructive lung dysfunction, and indicated the possibility of underlying microthrombus formation as a cause of reduced DLCO. 5 , 38 , 39 , 40 Autopsy studies have also shown diffuse alveolar damages and focal pulmonary microthrombi in fatal COVID‐19. 40 , 41 Moreover, looking back at the previous outbreak, a SARS‐CoV‐1 follow‐up study indicated the relationship between the structural abnormality during the late recovery phase and lung diffusion impairment. 42 Considering the development of fibrotic‐like changes and hypercoagulable state in severe COVID‐19, 43 it is plausible that severe/critical patients present reduced DLCO frequently. Since extensive data on follow‐up longer than 1 year have not been obtained, the actual duration of lung dysfunction remains unclear; future investigations are awaited.
This study had several limitations. First, follow‐up regulations varied from study to study. Seven studies followed up patients twice or more, and four of them only invited the patients with mid‐term residual CT abnormalities to the 1‐year follow‐up. Nonetheless, some of the patients were lost to follow‐up. However, our sensitivity analyses, which counted the lost patients as complete recovery at 1 year, did not substantially differ from the main analyses, indicating that at least 30% of hospitalized patients due to COVID‐19 still had abnormal CT findings 1 year after infection. Second, the included studies were substantially heterogeneous. Although we have strived to modify the heterogeneity by classifying patient severity, sufficient changes in I 2 were not observed. Notwithstanding, our meta regression did not provide a statistically significant relationship between the covariates and the primary outcome and did not imply obvious confounding factors. Third, the CT evaluation method was not standardized. As speculations about fibrotic‐like changes rely primarily on CT findings, vague terminology and a lack of solid definition might have led to overstatements on ‘COVID‐related fibrosis’. 20 In addition, the extent of CT findings was not provided across the included studies. As this perspective is highly relevant to clinical practice, future studies focusing on this matter are warranted. Lastly, because the included patients were treated during the first wave, the treatment regimen and vaccination status are different from the current situation. Therefore, the percentage of the remaining CT and PFT abnormalities can change later in the pandemic. However, although treatment advancement can manage the acute phase more effectively, patients who progressed to severe conditions may still have a high proportion of pulmonary sequelae. Meticulous observations should be continued after a 1‐year follow‐up.
In conclusion, our meta‐analysis demonstrated a high frequency of residual CT abnormalities 1 year after COVID‐19. Severe and critical COVID‐19 cases may cause long‐term lung damage. Thus, physicians should remain vigilant regarding the sequelae of severe and critical COVID‐19 pneumonia. Further investigations with longer follow‐up periods will help understand the underlying mechanism of COVID‐19 sequelae and manage patients with these conditions.
AUTHOR CONTRIBUTION
Atsuyuki Watanabe: Conceptualization (equal); formal analysis (lead); investigation (lead); methodology (lead); software (lead); visualization (lead); writing – original draft (lead). Matsuo So: Conceptualization (equal); methodology (supporting); writing – review and editing (supporting). Masao Iwagami: Formal analysis (supporting); methodology (supporting); resources (lead); validation (equal); writing – review and editing (supporting). Koichi Fukunaga: Funding acquisition (supporting); writing – review and editing (supporting). Hisato Takagi: Formal analysis (equal); software (equal); supervision (supporting); writing – review and editing (supporting). Hiroki Kabata: Funding acquisition (lead); methodology (equal); project administration (equal); supervision (equal); visualization (supporting); writing – review and editing (lead). Toshiki Kuno: Conceptualization (lead); methodology (equal); project administration (lead); supervision (lead); writing – review and editing (lead).
CONFLICT OF INTEREST
None declared.
HUMAN ETHICS APPROVAL DECLARATION
Not applicable. This meta‐analysis was conducted under the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis guidelines. 44 PROSPERO systematic review registration: CRD42022313149 at https://www.crd.york.ac.uk/prospero/
Supporting information
Supporting information.
Visual Abstract One‐year follow‐up CT findings in COVID‐19 patients‐ A systematic review and meta‐analysis.
ACKNOWLEDGEMENTS
We would like to thank Editage (www.editage.com) for English language editing.
Research funding: The study was supported by Keio University Development Funds (Hiroki Kabata).
Watanabe A, So M, Iwagami M, Fukunaga K, Takagi H, Kabata H, et al. One‐year follow‐up CT findings in COVID‐19 patients: A systematic review and meta‐analysis. Respirology. 2022;27(8):605–616. 10.1111/resp.14311
Hiroki Kabata and Toshiki Kuno contributed equally to this research study.
Associate Editor: David Barnes; Senior Editor: Paul King
Funding information Keio University Academic Development Funds
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Johns Hopkins University . Home. Coronavirus Resource Center. 2022. Feb 5. Available from: https://coronavirus.jhu.edu/
- 2. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA. 2020;323:2052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sanchez‐Ramirez DC, Normand K, Zhaoyun Y, Torres‐Castro R. Long‐term impact of COVID‐19: a systematic review of the literature and meta‐analysis. Biomedicine. 2021;9:900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. So M, Kabata H, Fukunaga K, Takagi H, Kuno T. Radiological and functional lung sequelae of COVID‐19: a systematic review and meta‐analysis. BMC Pulm Med. 2021;21:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pan F, Yang L, Liang B, Ye T, Li L, Li L, et al. Chest CT patterns from diagnosis to 1 year of follow‐up in COVID‐19. Radiology. 2022;302:709–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. González J, Benítez ID, Carmona P, Santisteve S, Monge A, Moncusí‐Moix A, et al. Pulmonary function and radiologic features in survivors of critical COVID‐19: a 3‐month prospective cohort. Chest. 2021;160:187–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu X, Liu X, Zhou Y, Yu H, Li R, Zhan Q, et al. 3‐month, 6‐month, 9‐month, and 12‐month respiratory outcomes in patients following COVID‐19‐related hospitalisation: a prospective study. Lancet Respir Med. 2021;9:747–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Han X, Fan Y, Alwalid O, Zhang X, Jia X, Zheng Y, et al. Fibrotic interstitial lung abnormalities at 1‐year follow‐up CT after severe COVID‐19. Radiology. 2021;301:E438–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen Y, Ding C, Yu L, Guo W, Feng X, Yu L, et al. One‐year follow‐up of chest CT findings in patients after SARS‐CoV‐2 infection. BMC Med. 2021;19:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang L, Yao Q, Gu X, Wang Q, Ren L, Wang Y, et al. 1‐year outcomes in hospital survivors with COVID‐19: a longitudinal cohort study. Lancet. 2021;398:747–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shang L, Wang L, Zhou F, Li J, Liu Y, Yang S. Long‐term effects of obesity on COVID‐19 patients discharged from hospital. Immun Inflamm Dis. 2021;9:1678–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhao Y, Yang C, An X, Xiong Y, Shang Y, He J, et al. Follow‐up study on COVID‐19 survivors one year after discharge from hospital. Int J Infect Dis. 2021;112:173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhan Y, Zhu Y, Wang S, Jia S, Gao Y, Lu Y, et al. SARS‐CoV‐2 immunity and functional recovery of COVID‐19 patients 1‐year after infection. Signal Transduct Target Ther. 2021;6:368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liao T, Meng D, Xiong L, Wu S, Yang L, Wang S, et al. Long‐term effects of COVID‐19 on health care workers 1‐year post‐discharge in Wuhan. Infect Dis Ther. 2022;11:145–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gamberini L, Mazzoli CA, Prediletto I, Sintonen H, Scaramuzzo G, Allegri D, et al. Health‐related quality of life profiles, trajectories, persistent symptoms and pulmonary function one year after ICU discharge in invasively ventilated COVID‐19 patients, a prospective follow‐up study. Respir Med. 2021;189:106665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bellan M, Baricich A, Patrucco F, Zeppegno P, Gramaglia C, Balbo PE, et al. Long‐term sequelae are highly prevalent one year after hospitalization for severe COVID‐19. Sci Rep. 2021;11:22666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhou F, Tao M, Shang L, Liu Y, Pan G, Jin Y, et al. Assessment of sequelae of COVID‐19 nearly 1 year after diagnosis. Front Med. 2021;8:717194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li Y, Han X, Huang J, Alwalid O, Jia X, Yuan M, et al. Follow‐up study of pulmonary sequelae in discharged COVID‐19 patients with diabetes or secondary hyperglycemia. Eur J Radiol. 2021;144:109997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vijayakumar B, Tonkin J, Devaraj A, Philip KEJ, Orton CM, Desai SR, et al. CT lung abnormalities after COVID‐19 at 3 months and 1 year after hospital discharge. Radiology. 2021;5:211746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zangrillo A, Belletti A, Palumbo D, Calvi MR, Guzzo F, Fominskiy EV, et al. One‐year multidisciplinary follow‐up of patients with COVID‐19 requiring invasive mechanical ventilation. J Cardiothorac Vasc Anesth. 2021;27(36):1354–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ngai JC, Ko FW, Ng SS, To KW, Tong M, Hui DS. The long‐term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology. 2010;15:543–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu X, Dong D, Ma D. Thin‐section computed tomography manifestations during convalescence and long‐term follow‐up of patients with severe acute respiratory syndrome (SARS). Med Sci Monit. 2016;22:2793–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65:934–9. [DOI] [PubMed] [Google Scholar]
- 25. NIH . Clinical Spectrum. COVID‐19 Treatment Guidelines. 2022. Feb 26. Available from: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/
- 26. Diagnosis and treatment protocol for novel coronavirus pneumonia (trial version 7). Chin Med J. 2020;133:1087–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. OpenMeta[Analyst] . OpenMetaAnalyst. 2022 Jun 6. Available from: http://www.cebm.brown.edu/openmeta/
- 28. Zhang P, Li J, Liu H, Han N, Ju J, Kou Y, et al. Long‐term bone and lung consequences associated with hospital‐acquired severe acute respiratory syndrome: a 15‐year follow‐up from a prospective cohort study. Bone Res. 2020;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hui DS, Joynt GM, Wong KT, Gomersall CD, Li TS, Antonio G, et al. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax. 2005;60:401–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xie L, Liu Y, Fan B, Xiao Y, Tian Q, Chen L, et al. Dynamic changes of serum SARS‐coronavirus IgG, pulmonary function and radiography in patients recovering from SARS after hospital discharge. Respir Res. 2005;6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nöbauer‐Huhmann IM, Eibenberger K, Schaefer‐Prokop C, Steltzer H, Schlick W, Strasser K, et al. Changes in lung parenchyma after acute respiratory distress syndrome (ARDS): assessment with high‐resolution computed tomography. Eur Radiol. 2001;11:2436–43. [DOI] [PubMed] [Google Scholar]
- 32. Westcott JL, Cole SR. Traction bronchiectasis in end‐stage pulmonary fibrosis. Radiology. 1986;161:665–9. [DOI] [PubMed] [Google Scholar]
- 33. Antonio GE, Wong KT, Hui DSC, Wu A, Lee N, Yuen EH, et al. Thin‐section CT in patients with severe acute respiratory syndrome following hospital discharge: preliminary experience. Radiology. 2003;228:810–5. [DOI] [PubMed] [Google Scholar]
- 34. Das KM, Lee EY, Singh R, Enani MA, Al Dossari K, Van Gorkom K, et al. Follow‐up chest radiographic findings in patients with MERS‐CoV after recovery. Indian J Radiol Imaging. 2017;27:342–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Han X, Fan Y, Alwalid O, Li N, Jia X, Yuan M, et al. Six‐month follow‐up chest CT findings after severe COVID‐19 pneumonia. Radiology. 2021;299:E177–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Desai SR, Wells AU, Rubens MB, Evans TW, Hansell DM. Acute respiratory distress syndrome: CT abnormalities at long‐term follow‐up. Radiology. 1999;210:29–35. [DOI] [PubMed] [Google Scholar]
- 37. George PM, Wells AU, Jenkins RG. Pulmonary fibrosis and COVID‐19: the potential role for antifibrotic therapy. Lancet Respir Med. 2020;8:807–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhao YM, Shang YM, Song WB, Li QQ, Xie H, Xu QF, et al. Follow‐up study of the pulmonary function and related physiological characteristics of COVID‐19 survivors three months after recovery. EClinicalMedicine. 2020;25:100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid‐19. N Engl J Med. 2020;383:120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bradley BT, Maioli H, Johnston R, Chaudhry I, Fink SL, Xu H, et al. Histopathology and ultrastructural findings of fatal COVID‐19 infections in Washington State: a case series. Lancet. 2020;396:320–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Konopka KE, Nguyen T, Jentzen JM, Rayes O, Schmidt CJ, Wilson AM, et al. Diffuse alveolar damage (DAD) resulting from coronavirus disease 2019 infection is morphologically indistinguishable from other causes of DAD. Histopathology. 2020;77:570–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hsu HH, Tzao C, Wu CP, Chang WC, Tsai CL, Tung HJ, et al. Correlation of high‐resolution CT, symptoms, and pulmonary function in patients during recovery from severe acute respiratory syndrome. Chest. 2004;126:149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, et al. Hypercoagulability of COVID‐19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18:1738–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Visual Abstract One‐year follow‐up CT findings in COVID‐19 patients‐ A systematic review and meta‐analysis.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


