Key Points
Question
Is damage from reactive oxygen species to crucial nucleic acids (DNA and RNA) increased in psychiatric disorders in adults?
Findings
In this systematic review and meta-analysis of 82 studies and 10 151 patient and 10 532 control observations, markers of DNA and RNA damage from oxidative stress were increased among individuals with psychiatric disorders. These increases were observed for peripheral biological matrices and central nervous system markers and across psychiatric disorder diagnostic groups.
Meaning
These findings suggest that there is an association with increased DNA and RNA damage from oxidative stress in adults with psychiatric disorders; this phenomenon may underlie the substantial burden of medical illness and accelerated aging associated with these disorders.
This meta-analysis uses peripheral biological matrices and central nervous system markers to examine the association of nucleic acid damage from oxidative stress with psychiatric disorders in adults.
Abstract
Importance
Nucleic acid damage from oxidative stress (NA-OXS) may be a molecular mechanism driving the severely increased morbidity and mortality from somatic causes in adults with psychiatric disorders.
Objective
To systematically retrieve and analyze data on NA-OXS across the psychiatric disorder diagnostic spectrum.
Data Sources
The PubMed, Embase, and PsycINFO databases were searched from inception to November 16, 2021. A hand search of reference lists of relevant articles was also performed.
Study Selection
Key study inclusion criteria in this meta-analysis were as follows: adult human study population, measurement of any marker of DNA or RNA damage from oxidative stress, and either a (1) cross-sectional design comparing patients with psychiatric disorders (any diagnosis) with a control group or (2) prospective intervention. Two authors screened the studies, and 2 senior authors read the relevant articles in full and assessed them for eligibility.
Data Extraction and Synthesis
The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines were followed. Two authors performed data extraction independently, and a senior coauthor was consulted in cases of disagreement. Data were synthesized with random-effects and multilevel meta-analyses.
Main Outcomes and Measures
The predefined hypothesis was that individuals with psychiatric disorders have increased NA-OXS levels. The main outcome was the standardized mean differences (SMDs) among patients and controls in nucleic acid oxidation markers compared across diagnostic groups. Analyses were divided into combinations of biological matrices and nucleic acids.
Results
Eighty-two studies fulfilled the inclusion criteria, comprising 205 patient vs control group comparisons and a total of 10 151 patient and 10 532 control observations. Overall, the data showed that patients with psychiatric disorders had higher NA-OXS levels vs controls across matrices and molecules. Pooled effect sizes ranged from moderate for urinary DNA markers (SMD = 0.44 [95% CI, 0.20-0.68]; P < .001) to very large for blood cell DNA markers (SMD = 1.12 [95% CI, 0.69-1.55; P < .001). Higher NA-OXS levels were observed among patients with dementias followed by psychotic and bipolar disorders. Sensitivity analyses excluding low-quality studies did not materially alter the results. Intervention studies were few and too heterogenous for meaningful meta-analysis.
Conclusions and Relevance
The results of this meta-analysis suggest that there is an association with increased NA-OXS levels in individuals across the psychiatric disorder diagnostic spectrum. NA-OXS may play a role in the somatic morbidity and mortality observed among individuals with psychiatric disorders.
Introduction
Epidemiologic studies consistently report increased mortality from somatic disease across the psychiatric disorder spectrum.1 Schizophrenia and bipolar disorders are associated with a life expectancy decrease of as much as 8 to 20 years, even in countries with relatively good access to health care.2,3,4 Severe unipolar depression is associated with a life expectancy decrease of 10 to 14 years.5 Personality disorders,6 substance use disorders,7,8 anxiety disorders,9 and posttraumatic stress disorder10 are also associated with increased mortality. The substantial impact of psychiatric morbidity on physical health is multicausal, with key contributors believed to be an unhealthy lifestyle, insufficient somatic care, metabolic side effects of psychotropic medications,11 and biological factors associated with the psychiatric disorder (eg, chronic neurohormonal stress).12 Finally, there are complex interactions between the primary disorder and comorbid conditions, such as substance use disorder.8
Regardless of the underlying causes, the somatic consequences of psychiatric disease can, in many aspects, be conceptualized as a state of accelerated aging.12,13,14 Across diagnostic groups of psychiatric illness, aging-like anatomical and biochemical alterations, such as progressive atrophic changes in the brain,15,16 systemic inflammation and immune dysregulation,17,18 insulin resistance,19,20 and osteoporosis,21,22 have been documented. Telomeres, the nucleotide repeat sequences that cap chromosomal ends and are shortened with cell division and increased age, undergo accelerated attrition in several psychiatric disorders, such as schizophrenia,23 bipolar disorder,24 and unipolar depression25 (although conflicting data exist26,27). Finally, medical conditions that occur at higher rates and at an earlier age among patients with psychiatric disorders (eg, circulatory, pulmonary, nervous, and endocrine system disorders) are also prevalent with increasing age in the general population.28,29 Therefore, it is relevant to ask: Are general cellular mechanisms of aging increased among individuals across the psychiatric disorder spectrum?
Nucleic acids (DNA and RNA) continuously undergo chemical modifications by reactive oxygen species. DNA damage from oxidation by reactive oxygen species has been established as a molecular mediator of aging per se.30 Downstream consequences of DNA damage from oxidation include mutations, telomere shortening, cellular senescence, and apoptosis.30 DNA damage from oxidation is considered a pathogenic event in a range of age-related disorders, such as type 2 diabetes, dementia, and cancer.30 Furthermore, there is a bidirectional relationship between oxidative stress on mitochondrial DNA (mtDNA) and mitochondrial dysfunction, which is another key event in aging.31 Oxidative modifications of messenger RNA may truncate proteins and reduce protein expression, whereas oxidation of noncoding RNAs may disrupt regulatory signaling functions and thereby potentially alter cell function.32 RNA damage from oxidative stress has gained increased attention as a potential pathogenic and early event in a range of aging-associated disorders, such as dementia33 and type 2 diabetes.34
Based on this evidence, we systematically retrieved and evaluated existing data on the association of psychiatric disorders and nucleic acid damage from oxidative stress (NA-OXS). Whereas previous reviews with or without meta-analyses focused on oxidative stress in specific psychiatric disorders,35,36,37 we applied a transdiagnostic approach because increased mortality and other signs of accelerated aging are present across the psychiatric disorder spectrum. We know of 2 comparable studies that both addressed only DNA damage in psychiatric disorders and were reviews without meta-analysis.38,39 We hypothesize that there is an association with increased NA-OXS levels overall and across diagnostic groups.
Methods
This meta-analysis was preregistered with the Open Science Framework (osf.io/tx4bz). This study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.
Eligibility Criteria
Studies were included if they (1) were in English, (2) included only human adults (age >18 years), (3) measured any marker of DNA or RNA damage from oxidative stress, (4) were either cross-sectional comparing psychiatric patients (any diagnosis) with a control group or a pre-post association with any specific intervention for the disorder in question (ie, naturalistic follow-up data were not included), and (5) disclosed information on age, gender (or biological sex), number of participants, biological matrix, and measurement methodology.
Search Strategy and Study Selection
We performed a search for relevant studies in the PubMed (from 1950), Embase (from 1974), and PsycINFO (from 1806) databases up to November 16, 2021. Furthermore, we conducted a hand search of reference lists of relevant articles. Two authors screened the studies, and 2 senior authors read the relevant articles in full and assessed them for eligibility. Two authors performed data extraction independently, and a senior coauthor was consulted in cases of disagreement. The eAppendix in the Supplement presents details on the search strategy, data extraction, and covariates. Abstracts and unpublished studies were not included.
We included all types of DNA (both nuclear DNA and mtDNA) or RNA oxidation markers. If multiple patient groups were included in a study and compared with the same control group, we extracted this combination as a separate comparison as described hereinafter. This strategy was also applied for studies comparing both a DNA marker and an RNA marker from the same matrix (eg, 8-oxo-7,8-dihydro-2-deoxyguanosine [8-oxodG] and 8-oxo-7,8-dihydroguanosine [8-oxoGuo] in urine) or the same marker in different matrices (eg, 8-oxodG in both urine and intra-DNA). However, if more than 1 NA-OXS marker was measured in the same matrix or anatomical region, only 1 marker was included in the analysis. In this case, we preferentially extracted a guanine oxidation marker because these are the most ubiquitous NA-OXS markers, are measured in a wide range of biological matrices (eg, in DNA and RNA from multiple cell types and as free molecules in urine, cerebrospinal fluid, and plasma),40 and have been validated extensively.41 Hence, the preferential use of guanine oxidation markers facilitates comparison across studies and matrices and reduces heterogeneity.
Statistical Analysis
We followed a preplanned analysis strategy. The primary study outcome was the standardized mean differences (SMDs) in nucleic acid oxidation markers for patients and controls compared across diagnostic groups. Diagnostic groups were coded as follows: dementia disorders (DEM), substance use disorders (SUB), psychotic disorders (PSY), bipolar disorders (BIP), major depressive disorders (MDD), and anxiety disorders (ANX). Analyses were divided into combinations of biological matrix and nucleic acid. Only matrix/molecule combinations with more than 2 studies were meta-analyzed. Secondary outcomes were SMDs vs measurement methodology, study quality, illness severity (eTable 1 in the Supplement), concurrent pharmacologic treatment, and cortical subregions.
SMDs were calculated as Hedges g effect sizes (95% CIs) and pooled using a random-effects model. For each matrix/molecule combination, pooled effects were calculated for each diagnostic subgroup and across all groups. We used the restricted maximum likelihood estimator to calculate heterogeneity variance τ2,42 and we used Knapp-Hartung adjustments to calculate the CIs.43 Heterogeneity is expressed with the I2 statistic, and the results are summarized in forest plots. Because some studies contributed more than 1 effect size in a given matrix/molecule combination, we performed a multilevel (or 3-level) meta-analysis with an integrated “study level,” thereby correcting the SMD estimates, CIs, and I2 values for the contribution of more than 1 effect size per study. A multilevel meta-regression analysis was conducted for the covariates age, gender, smoking status, and body mass index (BMI). Their contribution to heterogeneity was assessed by model comparisons using likelihood ratio tests. Risk of publication bias was assessed with a funnel plot and a multilevel Egger test. In a sensitivity analysis, all tests were performed excluding studies classified as low quality. We intended to analyze intervention effects with similar approaches, but the data did not allow for a meaningful meta-analysis. All analyses were performed in R, version 4.1.2 (R Foundation for Statistical Computing) with the “meta” and “metafor” packages.
Results
Literature Search
Details of the search results are presented in eFigure 1 in the Supplement. We identified 82 studies44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125 fulfilling the inclusion criteria, comprising 205 patient vs control group comparisons and a total of 10 151 patient and 10 532 control observations. Seven studies45,56,70,71,72,80,89 had interventions, comprising a total of 15 preintervention-postintervention comparisons. Included studies and their covariates are presented in eTable 2 (cross-sectional) and eTable 3 (interventions) in the Supplement.
Analyses of Nucleic Acid Oxidation Markers Across Diagnoses and Matrices
Results of the random-effects meta-analyses of matrix/molecule combinations with more than 2 studies are presented in Figure 1, Figure 2, Figure 3, and Figure 4 for peripheral markers (blood cells, plasma or serum, or urine) and in eFigure 2 in the Supplement for central nervous system markers. Results from multilevel meta-analyses are reported next to each forest plot, and subgroup results are provided in the Table and in eTable 4 in the Supplement.
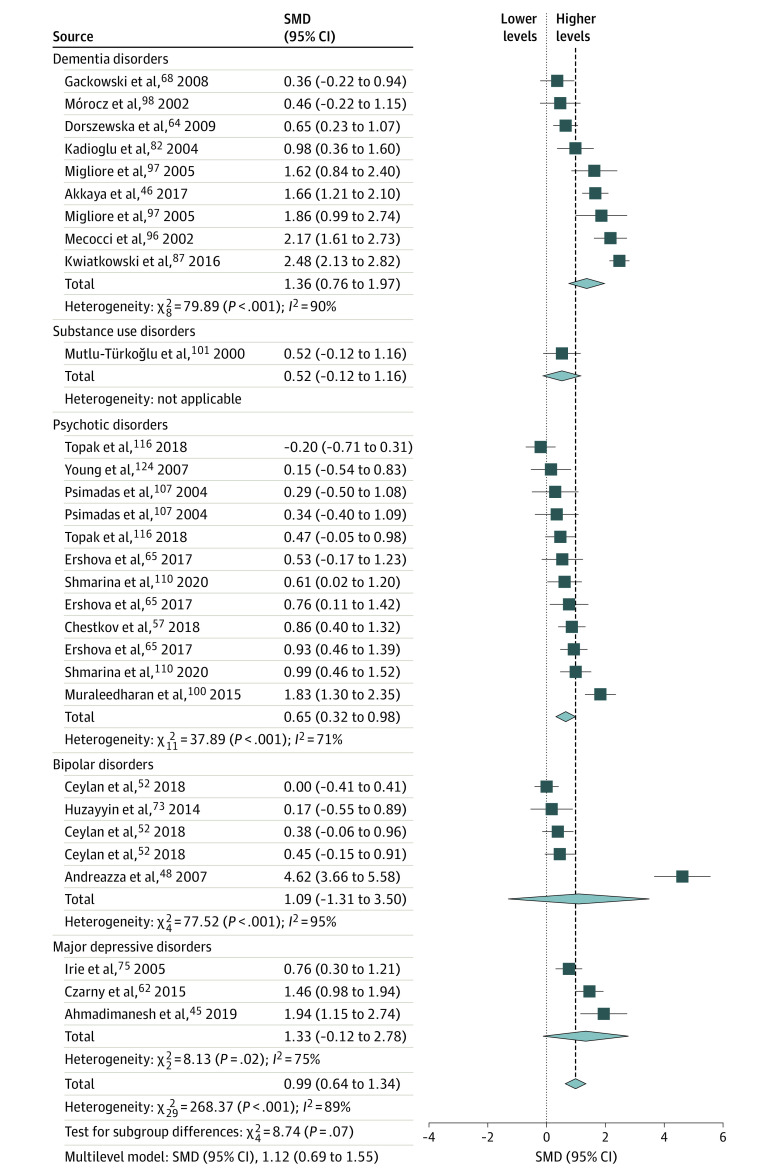
Figure 1. Forest Plot and Meta-analysis of Blood Cell Markers of DNA Damage From Oxidative Stress in Individuals With Psychiatric Disorders.
Standardized mean differences (SMDs) are given as Hedges g with 95% CIs. Heterogeneity is expressed by the I2 statistic. Results from the multilevel meta-analysis are given below each plot (study details in eTable 2 in the Supplement). The diamond size reflects the summary effect size.
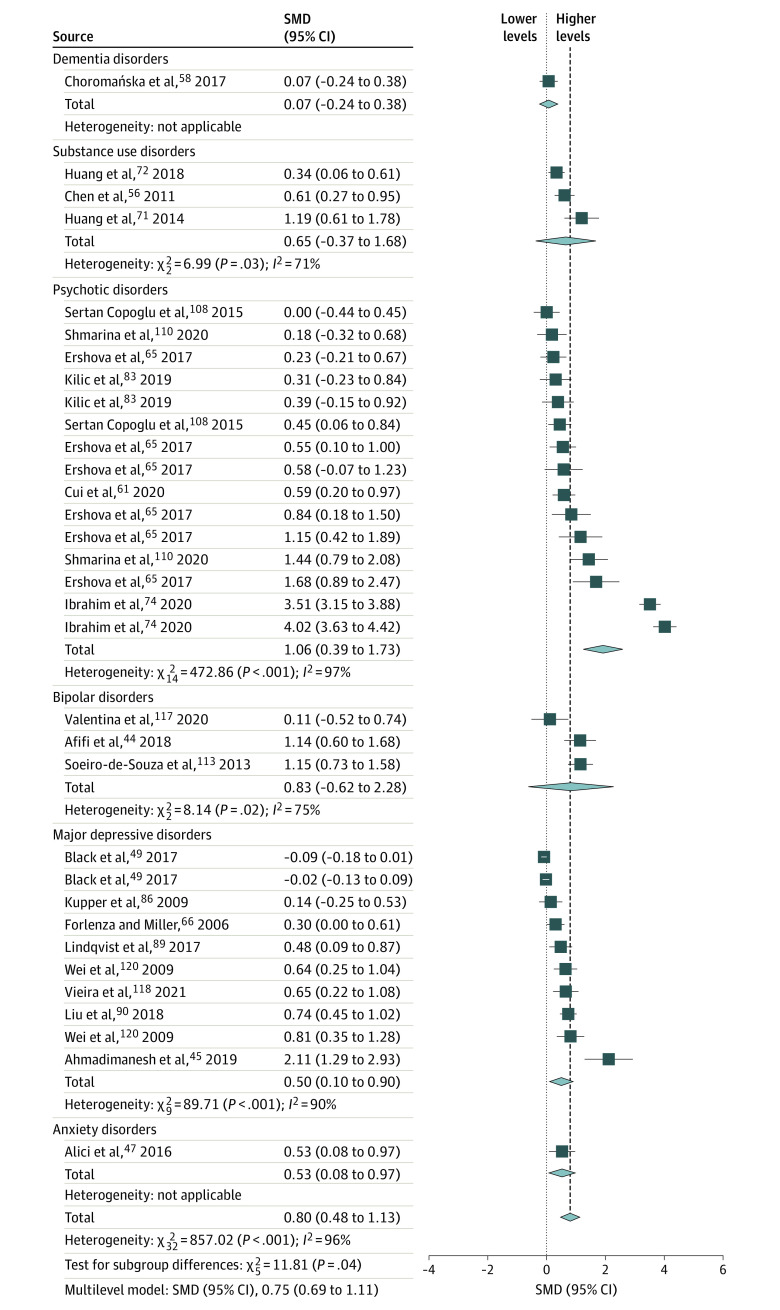
Figure 2. Forest Plot and Meta-analysis of Plasma or Serum Markers of DNA Damage From Oxidative Stress in Individuals With Psychiatric Disorders.
Standardized mean differences (SMDs) are given as Hedges g with 95% CIs. Heterogeneity is expressed by the I2 statistic. Results from the multilevel meta-analysis are presented below each plot (study details are provided in eTable 2 in the Supplement). The diamond size reflects the summary effect size.
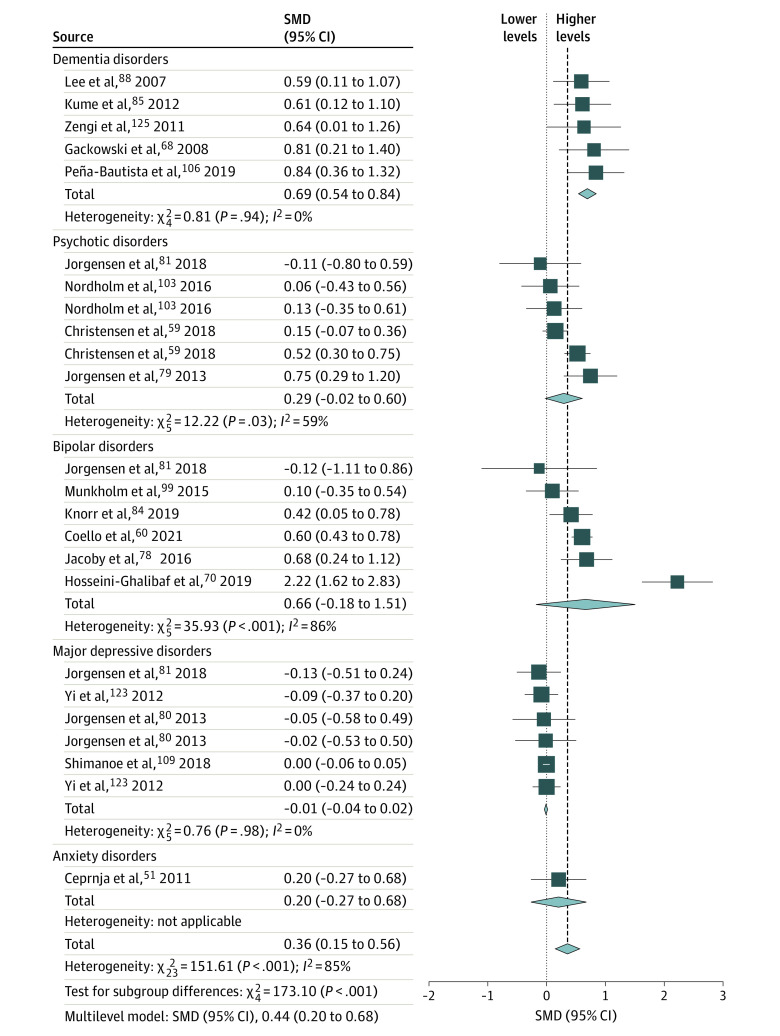
Figure 3. Forest Plot and Meta-analysis of Urinary Markers of DNA Damage From Oxidative Stress in Individuals With Psychiatric Disorders.
Standardized mean differences (SMDs) are given as Hedges g with 95% CIs. Heterogeneity is expressed by the I2 statistic. Results from the multilevel meta-analysis are presented below each plot (study details are provided in eTable 2 in the Supplement). The diamond size reflects the summary effect size.
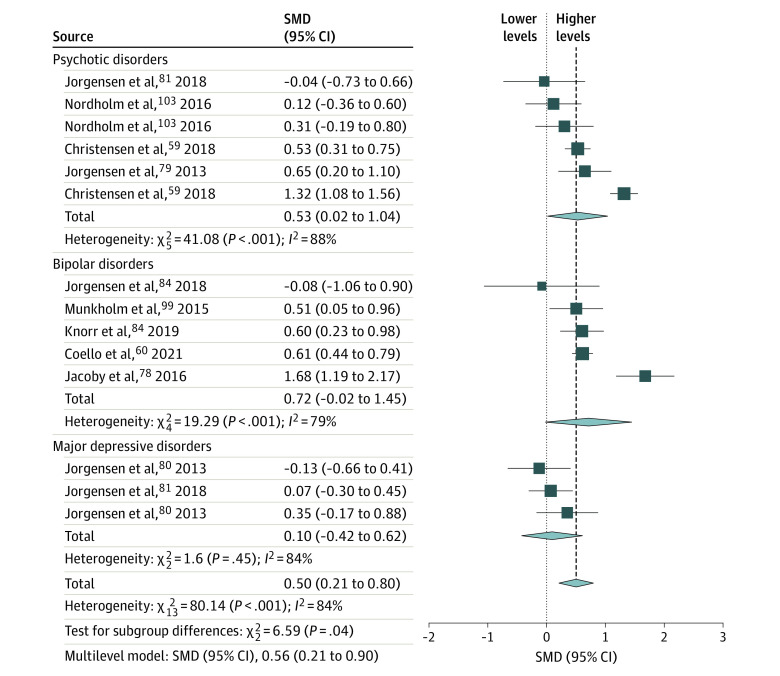
Figure 4. Forest Plot and Meta-analysis of Urinary Markers of RNA Damage From Oxidative Stress in Individuals With Psychiatric Disorders.
Standardized mean differences (SMDs) are given as Hedges g with 95% CIs. Heterogeneity is expressed by the I2 statistic. Results from the multilevel meta-analysis are presented below each plot (study details are provided in eTable 2 in the Supplement). The diamond size reflects the summary effect size.
Table. Multilevel Meta-analysis of Peripheral Markers of Nucleic Acid Damage From Oxidative Stress in Psychiatric Disorders, Including Subgroup Analyses and Sensitivity Analyses Excluding Low-Quality Studiesa.
| Characteristic | All studies | Medium- and high-quality studies | ||
|---|---|---|---|---|
| Hedges g (95% CI) | I2, % | Hedges g (95% CI) | I2, % | |
| Blood cell DNA markers | ||||
| No. of patient vs control group comparisons | 30b | NA | 14 | NA |
| Diagnostic group | ||||
| DEM | 1.32 (0.57 to 2.07)c | NA | NA | NA |
| SUB | 0.52 (−1.64 to 2.68) | NA | NA | NA |
| PSY | 0.70 (−0.10 to 1.50) | NA | 0.72 (−0.99 to 2.43) | NA |
| BIP | 1.54 (0.29 to 2.80)c | NA | 1.61 (−0.38 to 3.61) | NA |
| MDD | 1.37 (0.13 to 2.61)c | NA | 1.34 (−1.10 to 3.78) | NA |
| ANX | NA | NA | NA | NA |
| Total | 1.12 (0.69 to 1.55)c | 92 | 1.14 (0.14 to 2.15)c | 96 |
| Plasma or serum DNA markers | ||||
| No. of patient vs control group comparisons | 33b | NA | 27b | NA |
| Diagnostic group | ||||
| DEM | 0.07 (−1.77 to 1.91) | NA | 0.07 (−0.74 to 0.89) | NA |
| SUB | 0.70 (−0.38 to 1.78) | NA | 0.65 (0.16 to 1.15)c | NA |
| PSY | 1.08 (0.35 to 1.81)c | NA | 0.42 (−0.04 to 0.90) | NA |
| BIP | 0.81 (−0.28 to 1.91) | NA | 1.15 (0.51 to 1.79)c | NA |
| MDD | 0.61 (−0.01 to 1.24) | NA | 0.55 (0.26 to 0.83)c | NA |
| ANX | 0.53 (1.35 to 2.40) | NA | 0.53 (−0.36 to 1.41) | NA |
| Total | 0.75 (0.39 to 1.11)c | 96 | 0.57 (0.37 to 0.77)c | 84 |
| Urine DNA markers | ||||
| No. of patient vs control group comparisons | 24b | NA | 22b | NA |
| Diagnostic group | ||||
| DEM | 0.69 (0.29 to 1.10)c | NA | 0.70 (0.13 to 1.27)c | NA |
| SUB | NA | NA | NA | NA |
| PSY | 0.31 (0.09 to 0.70) | NA | 0.30 (−0.13 to 0.74) | NA |
| BIP | 0.66 (0.31 to 1.01)c | NA | 0.66 (0.27 to 1.05)c | NA |
| MDD | 0.02 (−0.33 to 0.38) | NA | 0.04 (−0.36 to 0.44) | NA |
| ANX | 0.20 (−0.66 to 1.07) | NA | 0.20 (−0.74 to 1.16) | NA |
| Total | 0.44 (0.20 to 0.68)c | 91 | 0.42 (0.15 to 0.68)c | 92 |
| Urine RNA markers | ||||
| No. of patient vs control group comparisons | 14b | NA | 14b | NA |
| Diagnostic group | ||||
| DEM | NA | NA | NA | NA |
| SUB | NA | NA | NA | NA |
| PSY | 0.52 (0.09 to 0.70)c | NA | 0.52 (0.09 to 0.70)c | NA |
| BIP | 0.73 (0.31 to 1.01)c | NA | 0.73 (0.31 to 1.01)c | NA |
| MDD | 0.21 (−0.33 to 0.38) | NA | 0.21 (−0.33 to 0.38) | NA |
| ANX | NA | NA | NA | NA |
| Total | 0.56 (0.21 to 0.90)c | 87 | 0.56 (0.21 to 0.90)c | 87 |
Abbreviations: ANX, anxiety disorders; BIP, bipolar disorders; DEM, dementia disorders; MDD, major depressive disorders; NA, not applicable; PSY, psychotic disorders; SUB, substance use disorders.
Data are standardized mean differences expressed as Hedges g (95% CIs), and heterogeneity is expressed by I2 values.
Tests of diagnostic group differences were significant (P < .05).
P < .05.
Overall, across matrices and molecules, patients with psychiatric disorders had higher NA-OXS levels than controls. All peripheral matrix/molecule combinations were statistically significant, with total pooled effect size estimates ranging from moderate for urinary DNA markers (SMD = 0.36 [95% CI, 0.15-0.56]) to very large for blood cell DNA markers (SMD = 0.99 [95% CI, 0.64-1.34]; Figures 1-4). Most tests for diagnostic group differences were statistically significant, with a general trend of higher NA-OXS levels in the DEM, PSY, and BIP diagnostic groups. The MDD diagnostic group showed statistically significant evidence of higher NA-OXS levels in blood cell and plasma or serum DNA markers (SMD = 0.50 [95% CI, 0.10-0.90]) but not in urinary DNA or RNA markers. The SUB and ANX diagnostic groups contained very few studies and were considered inconclusive. The multilevel meta-analysis yielded similar results, with total effect size estimates ranging from an SMD of 0.44 (95% CI, 0.20-0.68; P < .001) for urinary DNA markers to 1.12 (95% CI, 0.69-1.55; P < .001) for blood cell DNA markers (Table). The exclusion of low-quality studies in the sensitivity analysis did not change the overall effect sizes, although some subgroups changed significance status (Table). One extreme outlier112 was excluded from the plasma or serum DNA marker data set.
Central nervous system marker data were concentrated in fewer studies with lower numbers of participants (eFigure 2 in the Supplement). Neither cortical DNA nor RNA oxidation marker total pooled effects were statistically significant in the multilevel models. However, when low-quality studies were excluded, cortical RNA markers (only comprising DEM studies) reached statistical significance with a moderate to high effect size (SMD = 0.64 [95% CI, 0.05-1.23]). There was a statistically significant increase in cortical mtDNA markers in patients with psychiatric disorders (SMD = 1.03 [95% CI, 0.29-1.77]; all studies considered medium or high quality). The remaining matrix/molecule combinations contained few studies, and several diagnostic subgroups contained only 1 study (eFigure 2 in the Supplement). Only cerebellar RNA markers were statistically significant in the multilevel model (SMD = 0.82 [95% CI, 0.13-1.53]). However, hippocampal RNA marker studies were statistically significant after low-quality studies were excluded (SMD = 1.49 [95% CI, 1.19-1.80]; eTable 4 in the Supplement).
A meta-regression was performed on the full data set to study the covariates suspected to be associated with oxidative stress levels (age, gender, smoking status, and BMI; eTable 5 in the Supplement). Because only 37 (45%) and 20 (24%) studies disclosed information on smoking status and BMI, respectively, these factors were analyzed separately on the relevant subset of studies. The only covariate associated with effect sizes was age. However, the inclusion of age did not improve model performance as tested by a likelihood ratio test. The same was true for other covariates (eTable 5 in the Supplement).
For the 7 intervention studies, the intervention types included antidepressants for depression, alcohol detoxification for alcohol use disorder, and coenzyme Q10 supplementation for bipolar disorder (eTable 3 in the Supplement). These studies were therefore considered to be too heterogenous for meaningful meta-analysis.
Analyses of Secondary Outcomes and Risk of Publication Bias
Secondary outcomes were analyzed on the full data set (eTable 6 in the Supplement). We found statistically significant differences between measurement methods, study quality, illness severity, and pharmacologic treatment (P < .001 for all outcomes). With respect to measurement methodology, the comet assay was associated with the highest effect sizes (SMD = 1.31 [95% CI, 0.80-1.81]) and chromatography with the lowest effect sizes (SMD = 0.52 [95% CI, 0.23-0.80]). Low study quality was associated with higher effect sizes (SMD = 1.03 [95% CI, 0.70-1.36]) than medium (SMD = 0.90 [95% CI, 0.56-1.24]) and high (SMD = 0.60 [95% CI, 0.25-0.95]) study quality. Illness severity groups differed statistically significantly but in a nonlinear manner (moderate < mild < severe), and concurrent pharmacologic treatment was associated with higher effect sizes (SMD = 1.03 [95% CI, 0.46-1.30]) than no treatment (SMD = 0.79 [95% CI, 0.24-1.16]). An exploratory sensitivity analysis on studies controlled for BMI showed similar SMDs as in the full data set. We found no differences between cortical subregions with respect to DNA or RNA markers. A funnel plot (eFigure 3 in the Supplement) and significant multilevel Egger test results (P < .001) indicated publication bias. Finally, an exploratory analysis across all matrices showed statistically significant differences between diagnostic groups (P < .001), with DEM showing the highest pooled effect size (SMD = 1.01 [95% CI, 0.68-1.35]) followed by PSY (SMD = 0.93 [95% CI, 0.54-1.31]), BIP (SMD = 0.86 [95% CI, 0.42-1.30]), MDD (SMD = 0.64 [95% CI, 0.24-1.03]), SUB (SMD = 0.48 [95% CI, −0.36 to 1.32]), and ANX (SMD = 0.37 [95% CI, −1.00 to 1.73]; eTable 6 in the Supplement).
Discussion
The results of this transdiagnostic meta-analysis of 82 studies and more than 10 000 patient and control observations each suggest that there is an association with increased NA-OXS in patients with psychiatric disorders and that this phenomenon is not confined to a specific diagnostic group. An association with increased NA-OXS levels was evident in peripheral and central nervous system matrices. In many comparisons, SMDs in “nonorganic” psychiatric disorders (eg, psychotic and mood disorders) were of a magnitude similar to that of paradigmatic organic disorders of the dementias. Given the known roles of DNA and RNA damage from oxidation in molecular aging, ubiquitously increased NA-OXS could be an important biological mechanism driving the severely increased morbidity and mortality from age-related medical conditions in psychiatric disorders.1,29,126
This systematic review and meta-analysis was mainly based on cross-sectional studies and therefore cannot prove causality. Given that there was an association with NA-OXS across many different diagnoses and matrices, and given that NA-OXS was not specifically increased in the brain, we consider it more likely to be an epiphenomenon of the psychiatric conditions rather than a pathophysiologic factor underlying specific psychopathology. This finding is consistent with growing evidence showing general, transdiagnostic signs of accelerated aging and age-related illness in psychiatric disorders.13,126 Interestingly, a 2016 meta-analysis on DNA damage from oxidative stress in patients with cardiovascular disorders reported SMDs between patients and controls in the same order of magnitude as reported in our study.127
The molecular mechanisms underlying our observations of an association with increased NA-OXS cannot be inferred from this systematic review and meta-analysis. Although manifest medical illness that could cause oxidative stress (eg, type 2 diabetes128 or cardiovascular disease127) was usually an explicit exclusion criterion, a complex of risk factors commonly present in patients with psychiatric illness, such as obesity,129,130 chronic stress,31,131 smoking,132 and systemic inflammation,133 may converge to increase NA-OXS. Reporting of BMI and smoking status was sporadic, thus limiting the power of the meta-regression analysis of their potential influence. However, a sensitivity analysis of BMI-controlled studies suggested that an association of increased NA-OXS exists independent of differences in BMI. In addition, the state vs trait problem—that is, whether increased NA-OXS levels are a constant or fluctuating phenomenon—cannot be addressed by cross-sectional studies. Illness severity was not linearly associated with effect sizes (possibly owing to the low statistical power and heterogeneity in the underlying rating scales). We also observed that intervention studies, which could potentially shed light on these issues, were too heterogenous for meaningful meta-analysis. Finally, the evidence for publication bias merits caution in the interpretation of our results.
In line with existing evidence,41 we observed substantial differences in methodologies, with the comet and enzyme-linked immunosorbent assays being associated with the highest effect sizes and chromatographic methods associated with the lowest. Pharmacologic treatment was associated with higher effect sizes, but it cannot be determined whether this outcome is a causal phenomenon or a consequence of confounding by indication. Some studies indicate that antidepressant treatment may reduce oxidative stress levels,89,134 but it remains unknown whether and how psychiatric interventions influence NA-OXS and whether this treatment could mitigate the somatic consequences of psychiatric illness.
Our study integrates almost 3 decades of research on NA-OXS across multiple psychiatric diagnoses. The inclusion criteria were broad, and the included studies could thus be considered to span the majority of available evidence in the field. Using multilevel meta-analysis, we were able to include all group comparisons from each study, thereby considering all of the evidence available from the included studies.
Limitations
We acknowledge that by using overarching diagnostic, matrix, and brain region categories, relevant differences in relation to individual disorders, illness durations, and distribution of damage in brain subregions may be overlooked. This systematic review and meta-analysis did not include naturalistic, longitudinal data, which are rarely published (ie, available in only 7% of the included studies) and heterogenous in nature. Furthermore, we focused on products of NA-OXS and did not include markers of repair mechanisms (eg, DNA repair enzymes). Although levels of intra-DNA and intra-RNA damage represent a snapshot of the balance between damage and repair or degradation, levels of markers distributed in peripheral matrices are considered to represent—in steady state—the formation rate rather than the repair rate. We recently corroborated this notion in an in silico study,135 supporting the assertion that these peripheral markers can be interpreted as markers of oxidative stress. Additionally, we focused on NA-OXS because of its well-described roles in biological aging but did not address oxidative stress on other molecules (eg, lipids and proteins) or antioxidant markers.
Conclusions
The results of this systematic review and meta-analysis suggest that NA-OXS is increased across the psychiatric disorder diagnostic spectrum, with moderate to very high effect sizes. This observation may point to a common molecular mechanism underlying accelerated aging and increased mortality in individuals with psychiatric disorders. Future studies should elucidate the mechanistic links between psychiatric illness and NA-OXS.
eAppendix. Search Strategy, Data Extraction, and Covariates
eReferences
eTable 1. Illness Severity
eFigure 1. PRISMA Flow Diagram of the Literature Search and Study Selection
eTable 2. Characteristics of Included Cross-sectional Studies
eTable 3. Characteristics of Included Intervention Studies
eFigure 2. Forest Plots and Meta-analyses of Central Nervous System Markers
eTable 4. Multilevel Meta-analyses and Sensitivity Analysis for Central Nervous System Markers
eTable 5. Meta-regression Analyses of Covariates
eTable 6. Meta-analyses of Secondary Outcomes
eFigure 3. Funnel Plot of All Included Cross-sectional Studies
References
- 1.Plana-Ripoll O, Pedersen CB, Agerbo E, et al. A comprehensive analysis of mortality-related health metrics associated with mental disorders: a nationwide, register-based cohort study. Lancet. 2019;394(10211):1827-1835. doi: 10.1016/S0140-6736(19)32316-5 [DOI] [PubMed] [Google Scholar]
- 2.Wahlbeck K, Westman J, Nordentoft M, Gissler M, Laursen TM. Outcomes of Nordic mental health systems: life expectancy of patients with mental disorders. Br J Psychiatry. 2011;199(6):453-458. doi: 10.1192/bjp.bp.110.085100 [DOI] [PubMed] [Google Scholar]
- 3.Laursen TM, Munk-Olsen T, Nordentoft M, Mortensen PB. Increased mortality among patients admitted with major psychiatric disorders: a register-based study comparing mortality in unipolar depressive disorder, bipolar affective disorder, schizoaffective disorder, and schizophrenia. J Clin Psychiatry. 2007;68(6):899-907. doi: 10.4088/JCP.v68n0612 [DOI] [PubMed] [Google Scholar]
- 4.Kessing LV, Vradi E, Andersen PK. Life expectancy in bipolar disorder. Bipolar Disord. 2015;17(5):543-548. doi: 10.1111/bdi.12296 [DOI] [PubMed] [Google Scholar]
- 5.Laursen TM, Musliner KL, Benros ME, Vestergaard M, Munk-Olsen T. Mortality and life expectancy in persons with severe unipolar depression. J Affect Disord. 2016;193:203-207. doi: 10.1016/j.jad.2015.12.067 [DOI] [PubMed] [Google Scholar]
- 6.Høye A, Jacobsen BK, Bramness JG, Nesvåg R, Reichborn-Kjennerud T, Heiberg I. Total and cause-specific mortality in patients with personality disorders: the association between comorbid severe mental illness and substance use disorders. Soc Psychiatry Psychiatr Epidemiol. 2021;56(10):1809-1819. doi: 10.1007/s00127-021-02055-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holst C, Tolstrup JS, Sørensen HJ, Becker U. Alcohol dependence and risk of somatic diseases and mortality: a cohort study in 19 002 men and women attending alcohol treatment. Addiction. 2017;112(8):1358-1366. doi: 10.1111/add.13799 [DOI] [PubMed] [Google Scholar]
- 8.Iturralde E, Slama N, Kline-Simon AH, Young-Wolff KC, Mordecai D, Sterling SA. Premature mortality associated with severe mental illness or substance use disorder in an integrated health care system. Gen Hosp Psychiatry. 2021;68:1-6. doi: 10.1016/j.genhosppsych.2020.11.002 [DOI] [PubMed] [Google Scholar]
- 9.Meier SM, Mattheisen M, Mors O, Mortensen PB, Laursen TM, Penninx BW. Increased mortality among people with anxiety disorders: total population study. Br J Psychiatry. 2016;209(3):216-221. doi: 10.1192/bjp.bp.115.171975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lohr JB, Palmer BW, Eidt CA, et al. Is post-traumatic stress disorder associated with premature senescence? a review of the literature. Am J Geriatr Psychiatry. 2015;23(7):709-725. doi: 10.1016/j.jagp.2015.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Correll CU, Detraux J, De Lepeleire J, De Hert M. Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in people with schizophrenia, depression and bipolar disorder. World Psychiatry. 2015;14(2):119-136. doi: 10.1002/wps.20204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Epel ES. Psychological and metabolic stress: a recipe for accelerated cellular aging? Hormones (Athens). 2009;8(1):7-22. doi: 10.14310/horm.2002.1217 [DOI] [PubMed] [Google Scholar]
- 13.Wertz J, Caspi A, Ambler A, et al. Association of history of psychopathology with accelerated aging at midlife. JAMA Psychiatry. 2021;78(5):530-539. doi: 10.1001/jamapsychiatry.2020.4626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirkpatrick B, Messias E, Harvey PD, Fernandez-Egea E, Bowie CR. Is schizophrenia a syndrome of accelerated aging? Schizophr Bull. 2008;34(6):1024-1032. doi: 10.1093/schbul/sbm140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Velakoulis D, Wood SJ, Wong MT, et al. Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Arch Gen Psychiatry. 2006;63(2):139-149. doi: 10.1001/archpsyc.63.2.139 [DOI] [PubMed] [Google Scholar]
- 16.MacQueen GM, Campbell S, McEwen BS, et al. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci U S A. 2003;100(3):1387-1392. doi: 10.1073/pnas.0337481100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayley S, Hakim AM, Albert PR. Depression, dementia and immune dysregulation. Brain. 2021;144(3):746-760. doi: 10.1093/brain/awaa405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pillinger T, D’Ambrosio E, McCutcheon R, Howes OD. Is psychosis a multisystem disorder? a meta-review of central nervous system, immune, cardiometabolic, and endocrine alterations in first-episode psychosis and perspective on potential models. Mol Psychiatry. 2019;24(6):776-794. doi: 10.1038/s41380-018-0058-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hennekens CH, Hennekens AR, Hollar D, Casey DE. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. 2005;150(6):1115-1121. doi: 10.1016/j.ahj.2005.02.007 [DOI] [PubMed] [Google Scholar]
- 20.Moulton CD, Pickup JC, Ismail K. The link between depression and diabetes: the search for shared mechanisms. Lancet Diabetes Endocrinol. 2015;3(6):461-471. doi: 10.1016/S2213-8587(15)00134-5 [DOI] [PubMed] [Google Scholar]
- 21.Gomez L, Stubbs B, Shirazi A, Vancampfort D, Gaughran F, Lally J. Lower bone mineral density at the hip and lumbar spine in people with psychosis versus controls: a comprehensive review and skeletal site-specific meta-analysis. Curr Osteoporos Rep. 2016;14(6):249-259. doi: 10.1007/s11914-016-0325-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mezuk B. Affective disorders, bone metabolism, and osteoporosis. Clin Rev Bone Miner Metab. 2008;6(3-4):101-113. doi: 10.1007/s12018-009-9025-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ayora M, Fraguas D, Abregú-Crespo R, et al. Leukocyte telomere length in patients with schizophrenia and related disorders: a meta-analysis of case-control studies. Mol Psychiatry. 2022;27(7):2968-2975. doi: 10.1038/s41380-022-01541-7 [DOI] [PubMed] [Google Scholar]
- 24.Fries GR, Zamzow MJ, Andrews T, Pink O, Scaini G, Quevedo J. Accelerated aging in bipolar disorder: a comprehensive review of molecular findings and their clinical implications. Neurosci Biobehav Rev. 2020;112:107-116. doi: 10.1016/j.neubiorev.2020.01.035 [DOI] [PubMed] [Google Scholar]
- 25.Wolkowitz OM, Mellon SH, Epel ES, et al. Leukocyte telomere length in major depression: correlations with chronicity, inflammation and oxidative stress—preliminary findings. PLoS One. 2011;6(3):e17837. doi: 10.1371/journal.pone.0017837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindqvist D, Epel ES, Mellon SH, et al. Psychiatric disorders and leukocyte telomere length: underlying mechanisms linking mental illness with cellular aging. Neurosci Biobehav Rev. 2015;55:333-364. doi: 10.1016/j.neubiorev.2015.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Darrow SM, Verhoeven JE, Révész D, et al. The association between psychiatric disorders and telomere length: a meta-analysis involving 14,827 persons. Psychosom Med. 2016;78(7):776-787. doi: 10.1097/PSY.0000000000000356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kessing LV, Vradi E, McIntyre RS, Andersen PK. Causes of decreased life expectancy over the life span in bipolar disorder. J Affect Disord. 2015;180:142-147. doi: 10.1016/j.jad.2015.03.027 [DOI] [PubMed] [Google Scholar]
- 29.Momen NC, Plana-Ripoll O, Agerbo E, et al. Association between mental disorders and subsequent medical conditions. N Engl J Med. 2020;382(18):1721-1731. doi: 10.1056/NEJMoa1915784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schumacher B, Pothof J, Vijg J, Hoeijmakers JHJ. The central role of DNA damage in the ageing process. Nature. 2021;592(7856):695-703. doi: 10.1038/s41586-021-03307-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Picard M, Juster RP, McEwen BS. Mitochondrial allostatic load puts the ‘gluc’ back in glucocorticoids. Nat Rev Endocrinol. 2014;10(5):303-310. doi: 10.1038/nrendo.2014.22 [DOI] [PubMed] [Google Scholar]
- 32.Nunomura A, Lee HG, Zhu X, Perry G. Consequences of RNA oxidation on protein synthesis rate and fidelity: implications for the pathophysiology of neuropsychiatric disorders. Biochem Soc Trans. 2017;45(5):1053-1066. doi: 10.1042/BST20160433 [DOI] [PubMed] [Google Scholar]
- 33.Nunomura A, Moreira PI, Castellani RJ, et al. Oxidative damage to RNA in aging and neurodegenerative disorders. Neurotox Res. 2012;22(3):231-248. doi: 10.1007/s12640-012-9331-x [DOI] [PubMed] [Google Scholar]
- 34.Poulsen HE, Specht E, Broedbaek K, et al. RNA modifications by oxidation: a novel disease mechanism? Free Radic Biol Med. 2012;52(8):1353-1361. doi: 10.1016/j.freeradbiomed.2012.01.009 [DOI] [PubMed] [Google Scholar]
- 35.Murray AJ, Rogers JC, Katshu MZUH, Liddle PF, Upthegrove R. Oxidative stress and the pathophysiology and symptom profile of schizophrenia spectrum disorders. Front Psychiatry. 2021;12:703452. doi: 10.3389/fpsyt.2021.703452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiménez-Fernández S, Gurpegui M, Garrote-Rojas D, Gutiérrez-Rojas L, Carretero MD, Correll CU. Oxidative stress parameters and antioxidants in patients with bipolar disorder: results from a meta-analysis comparing patients, including stratification by polarity and euthymic status, with healthy controls. Bipolar Disord. 2021;23(2):117-129. doi: 10.1111/bdi.12980 [DOI] [PubMed] [Google Scholar]
- 37.Black CN, Bot M, Scheffer PG, Cuijpers P, Penninx BW. Is depression associated with increased oxidative stress? a systematic review and meta-analysis. Psychoneuroendocrinology. 2015;51:164-175. doi: 10.1016/j.psyneuen.2014.09.025 [DOI] [PubMed] [Google Scholar]
- 38.Czarny P, Bialek K, Ziolkowska S, Strycharz J, Sliwinski T. DNA damage and repair in neuropsychiatric disorders. What do we know and what are the future perspectives? Mutagenesis. 2020;35(1):79-106. doi: 10.1093/mutage/gez035 [DOI] [PubMed] [Google Scholar]
- 39.Raza MU, Tufan T, Wang Y, Hill C, Zhu MY. DNA damage in major psychiatric diseases. Neurotox Res. 2016;30(2):251-267. doi: 10.1007/s12640-016-9621-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chao MR, Evans MD, Hu CW, et al. Biomarkers of nucleic acid oxidation—a summary state-of-the-art. Redox Biol. 2021;42:101872. doi: 10.1016/j.redox.2021.101872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barregard L, Møller P, Henriksen T, et al. Human and methodological sources of variability in the measurement of urinary 8-oxo-7,8-dihydro-2′-deoxyguanosine. Antioxid Redox Signal. 2013;18(18):2377-2391. doi: 10.1089/ars.2012.4714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Viechtbauer W. Bias and efficiency of meta-analytic variance estimators in the random-effects model. J Educ Behav Stat. 2005;30(3):261-293. doi: 10.3102/10769986030003261 [DOI] [Google Scholar]
- 43.Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;22(17):2693-2710. doi: 10.1002/sim.1482 [DOI] [PubMed] [Google Scholar]
- 44.Afifi MA, Jiman-Fatani AA, Al-Rabia MW, Al-Hussainy NH, El Saadany S, Mayah W. More than an association: latent toxoplasmosis might provoke a local oxidative stress that triggers the development of bipolar disorder. J Microsc Ultrastruct. 2018;6(3):139-144. doi: 10.4103/JMAU.JMAU_22_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahmadimanesh M, Abbaszadegan MR, Morshedi Rad D, et al. Effects of selective serotonin reuptake inhibitors on DNA damage in patients with depression. J Psychopharmacol. 2019;33(11):1364-1376. doi: 10.1177/0269881119874461 [DOI] [PubMed] [Google Scholar]
- 46.Akkaya Ç, Yavuzer SS, Yavuzer H, Erkol G, Bozluolcay M, Dinçer Y. DNA damage, DNA susceptibility to oxidation and glutathione redox status in patients with Alzheimer’s disease treated with and without memantine. J Neurol Sci. 2017;378:158-162. doi: 10.1016/j.jns.2017.04.051 [DOI] [PubMed] [Google Scholar]
- 47.Alici D, Bulbul F, Virit O, et al. Evaluation of oxidative metabolism and oxidative DNA damage in patients with obsessive-compulsive disorder. Psychiatry Clin Neurosci. 2016;70(2):109-115. doi: 10.1111/pcn.12362 [DOI] [PubMed] [Google Scholar]
- 48.Andreazza AC, Frey BN, Erdtmann B, et al. DNA damage in bipolar disorder. Psychiatry Res. 2007;153(1):27-32. doi: 10.1016/j.psychres.2006.03.025 [DOI] [PubMed] [Google Scholar]
- 49.Black CN, Bot M, Scheffer PG, Penninx BW. Oxidative stress in major depressive and anxiety disorders, and the association with antidepressant use; results from a large adult cohort. Psychol Med. 2017;47(5):936-948. doi: 10.1017/S0033291716002828 [DOI] [PubMed] [Google Scholar]
- 50.Bradley-Whitman MA, Lovell MA. Increased oxidative damage in RNA in Alzheimer’s disease progression. J Anal Bioanal Tech. Published online October 30, 2013 doi: 10.4172/2155-9872.S2-004 [DOI] [Google Scholar]
- 51.Ceprnja M, Derek L, Unić A, et al. Oxidative stress markers in patients with post-traumatic stress disorder. Coll Antropol. 2011;35(4):1155-1160. [PubMed] [Google Scholar]
- 52.Ceylan D, Scola G, Tunca Z, et al. DNA redox modulations and global DNA methylation in bipolar disorder: effects of sex, smoking and illness state. Psychiatry Res. 2018;261:589-596. doi: 10.1016/j.psychres.2017.12.051 [DOI] [PubMed] [Google Scholar]
- 53.Chang CC, Jou SH, Lin TT, Lai TJ, Liu CS. Mitochondria DNA change and oxidative damage in clinically stable patients with major depressive disorder. PLoS One. 2015;10(5):e0125855. doi: 10.1371/journal.pone.0125855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chang CC, Jou SH, Lin TT, Liu CS. Mitochondrial DNA variation and increased oxidative damage in euthymic patients with bipolar disorder. Psychiatry Clin Neurosci. 2014;68(7):551-557. doi: 10.1111/pcn.12163 [DOI] [PubMed] [Google Scholar]
- 55.Che Y, Wang JF, Shao L, Young T. Oxidative damage to RNA but not DNA in the hippocampus of patients with major mental illness. J Psychiatry Neurosci. 2010;35(5):296-302. doi: 10.1503/jpn.090083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen CH, Pan CH, Chen CC, Huang MC. Increased oxidative DNA damage in patients with alcohol dependence and its correlation with alcohol withdrawal severity. Alcohol Clin Exp Res. 2011;35(2):338-344. doi: 10.1111/j.1530-0277.2010.01349.x [DOI] [PubMed] [Google Scholar]
- 57.Chestkov IV, Jestkova EM, Ershova ES, et al. ROS-induced DNA damage associates with abundance of mitochondrial DNA in white blood cells of the untreated schizophrenic patients. Oxid Med Cell Longev. 2018;2018:8587475. doi: 10.1155/2018/8587475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Choromańska M, Klimiuk A, Kostecka-Sochoń P, et al. Antioxidant defence, oxidative stress and oxidative damage in saliva, plasma and erythrocytes of dementia patients. Can salivary AGE be a marker of dementia? Int J Mol Sci. 2017;18(10):E2205. doi: 10.3390/ijms18102205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Christensen MR, Poulsen HE, Henriksen T, et al. Elevated levels of 8-oxoGuo and 8-oxodG in individuals with severe mental illness—an autopsy-based study. Free Radic Biol Med. 2018;126:372-378. doi: 10.1016/j.freeradbiomed.2018.08.029 [DOI] [PubMed] [Google Scholar]
- 60.Coello K, Bøgh HL, Stanislaus S, et al. Higher systemic oxidatively generated DNA and RNA damage in patients with newly diagnosed bipolar disorder and their unaffected first-degree relatives. Free Radic Biol Med. 2021;168:226-233. doi: 10.1016/j.freeradbiomed.2021.03.022 [DOI] [PubMed] [Google Scholar]
- 61.Cui G, Qing Y, Hu X, et al. Serum metabolomic profiling based on Fourier transform-ion cyclotron resonance-mass spectrometry: do the dysfunctions of metabolic pathways reveal a universal risk of oxidative stress in schizophrenia? Antioxid Redox Signal. 2020;33(10):679-688. doi: 10.1089/ars.2020.8141 [DOI] [PubMed] [Google Scholar]
- 62.Czarny P, Kwiatkowski D, Kacperska D, et al. Elevated level of DNA damage and impaired repair of oxidative DNA damage in patients with recurrent depressive disorder. Med Sci Monit. 2015;21:412-418. doi: 10.12659/MSM.892317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ding Q, Markesbery WR, Cecarini V, Keller JN. Decreased RNA, and increased RNA oxidation, in ribosomes from early Alzheimer’s disease. Neurochem Res. 2006;31(5):705-710. doi: 10.1007/s11064-006-9071-5 [DOI] [PubMed] [Google Scholar]
- 64.Dorszewska J, Kempisty B, Jaroszewska-Kolecka J, et al. Expression and polymorphisms of gene 8-oxoguanine glycosylase 1 and the level of oxidative DNA damage in peripheral blood lymphocytes of patients with Alzheimer’s disease. DNA Cell Biol. 2009;28(11):579-588. doi: 10.1089/dna.2009.0926 [DOI] [PubMed] [Google Scholar]
- 65.Ershova ES, Jestkova EM, Chestkov IV, et al. Quantification of cell-free DNA in blood plasma and DNA damage degree in lymphocytes to evaluate dysregulation of apoptosis in schizophrenia patients. J Psychiatr Res. 2017;87:15-22. doi: 10.1016/j.jpsychires.2016.12.006 [DOI] [PubMed] [Google Scholar]
- 66.Forlenza MJ, Miller GE. Increased serum levels of 8-hydroxy-2′-deoxyguanosine in clinical depression. Psychosom Med. 2006;68(1):1-7. doi: 10.1097/01.psy.0000195780.37277.2a [DOI] [PubMed] [Google Scholar]
- 67.Gabbita SP, Lovell MA, Markesbery WR. Increased nuclear DNA oxidation in the brain in Alzheimer’s disease. J Neurochem. 1998;71(5):2034-2040. doi: 10.1046/j.1471-4159.1998.71052034.x [DOI] [PubMed] [Google Scholar]
- 68.Gackowski D, Rozalski R, Siomek A, et al. Oxidative stress and oxidative DNA damage is characteristic for mixed Alzheimer disease/vascular dementia. J Neurol Sci. 2008;266(1-2):57-62. doi: 10.1016/j.jns.2007.08.041 [DOI] [PubMed] [Google Scholar]
- 69.Götz ME, Janetzky B, Pohli S, et al. Chronic alcohol consumption and cerebral indices of oxidative stress: is there a link? Alcohol Clin Exp Res. 2001;25(5):717-725. doi: 10.1111/j.1530-0277.2001.tb02272.x [DOI] [PubMed] [Google Scholar]
- 70.Hosseini-Ghalibaf A Sr, Yasrebifar F, Mirzaei E, Mirjalili M. Effect of coenzyme Q10 supplementation on urinary and salivary oxidative stress biomarkers in bipolar patients during the depressive episode. Nat Prod J. 2020;10(5):664-672. doi: 10.2174/2210315509666190624102012 [DOI] [Google Scholar]
- 71.Huang MC, Chen CC, Pan CH, Chen CH. Comparison of oxidative DNA damage between alcohol-dependent patients with and without delirium tremens. Alcohol Clin Exp Res. 2014;38(10):2523-2528. doi: 10.1111/acer.12539 [DOI] [PubMed] [Google Scholar]
- 72.Huang MC, Lai YC, Lin SK, Chen CH. Increased blood 8-hydroxy-2-deoxyguanosine levels in methamphetamine users during early abstinence. Am J Drug Alcohol Abuse. 2018;44(3):395-402. doi: 10.1080/00952990.2017.1344683 [DOI] [PubMed] [Google Scholar]
- 73.Huzayyin AA, Andreazza AC, Turecki G, et al. Decreased global methylation in patients with bipolar disorder who respond to lithium. Int J Neuropsychopharmacol. 2014;17(4):561-569. doi: 10.1017/S1461145713001569 [DOI] [PubMed] [Google Scholar]
- 74.Ibrahim RR, Amer RA, Abozeid AA, Elsharaby RM, Shafik NM. Micro RNA 146a gene variant/TNF-α/IL-6/IL-1 β; a cross-link axis in between oxidative stress, endothelial dysfunction and neuro-inflammation in acute ischemic stroke and chronic schizophrenic patients. Arch Biochem Biophys. 2020;679:108193. doi: 10.1016/j.abb.2019.108193 [DOI] [PubMed] [Google Scholar]
- 75.Irie M, Miyata M, Kasai H. Depression and possible cancer risk due to oxidative DNA damage. J Psychiatr Res. 2005;39(6):553-560. doi: 10.1016/j.jpsychires.2005.01.009 [DOI] [PubMed] [Google Scholar]
- 76.Isobe C, Abe T, Terayama Y. Homocysteine may contribute to pathogenesis of RNA damage in brains with Alzheimer’s disease. Neurodegener Dis. 2009;6(5-6):252-257. doi: 10.1159/000262443 [DOI] [PubMed] [Google Scholar]
- 77.Isobe C, Abe T, Terayama Y. Levels of reduced and oxidized coenzyme Q-10 and 8-hydroxy-2′-deoxyguanosine in the CSF of patients with Alzheimer’s disease demonstrate that mitochondrial oxidative damage and/or oxidative DNA damage contributes to the neurodegenerative process. J Neurol. 2010;257(3):399-404. doi: 10.1007/s00415-009-5333-x [DOI] [PubMed] [Google Scholar]
- 78.Jacoby AS, Vinberg M, Poulsen HE, Kessing LV, Munkholm K. Increased DNA and RNA damage by oxidation in patients with bipolar I disorder. Transl Psychiatry. 2016;6(8):e867. doi: 10.1038/tp.2016.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jorgensen A, Broedbaek K, Fink-Jensen A, et al. Increased systemic oxidatively generated DNA and RNA damage in schizophrenia. Psychiatry Res. 2013;209(3):417-423. doi: 10.1016/j.psychres.2013.01.033 [DOI] [PubMed] [Google Scholar]
- 80.Jorgensen A, Krogh J, Miskowiak K, et al. Systemic oxidatively generated DNA/RNA damage in clinical depression: associations to symptom severity and response to electroconvulsive therapy. J Affect Disord. 2013;149(1-3):355-362. doi: 10.1016/j.jad.2013.02.011 [DOI] [PubMed] [Google Scholar]
- 81.Jorgensen A, Siersma V, Davidsen AS, et al. Markers of DNA/RNA damage from oxidation as predictors of a registry-based diagnosis of psychiatric illness in type 2 diabetic patients. Psychiatry Res. 2018;259:370-376. doi: 10.1016/j.psychres.2017.11.017 [DOI] [PubMed] [Google Scholar]
- 82.Kadioglu E, Sardas S, Aslan S, Isik E, Esat Karakaya A. Detection of oxidative DNA damage in lymphocytes of patients with Alzheimer’s disease. Biomarkers. 2004;9(2):203-209. doi: 10.1080/13547500410001728390 [DOI] [PubMed] [Google Scholar]
- 83.Kilic OHT, Aksoy I, Elboga GC, Bulbul F. Oxidative parameters, oxidative DNA damage, and urotensin-II in schizoaffective disorder patients. Psychiatry Clin Psychopharmacol. 2019;29(2):151-157. doi: 10.1080/24750573.2018.1468637 [DOI] [Google Scholar]
- 84.Knorr U, Simonsen AH, Roos P, et al. Cerebrospinal fluid oxidative stress metabolites in patients with bipolar disorder and healthy controls: a longitudinal case-control study. Transl Psychiatry. 2019;9(1):325. doi: 10.1038/s41398-019-0664-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kume K, Kikukawa M, Hanyu H, et al. Telomere length shortening in patients with dementia with Lewy bodies. Eur J Neurol. 2012;19(6):905-910. doi: 10.1111/j.1468-1331.2011.03655.x [DOI] [PubMed] [Google Scholar]
- 86.Kupper N, Gidron Y, Winter J, Denollet J. Association between type D personality, depression, and oxidative stress in patients with chronic heart failure. Psychosom Med. 2009;71(9):973-980. doi: 10.1097/PSY.0b013e3181bee6dc [DOI] [PubMed] [Google Scholar]
- 87.Kwiatkowski D, Czarny P, Toma M, et al. Associations between DNA damage, DNA base excision repair gene variability and Alzheimer’s disease risk. Dement Geriatr Cogn Disord. 2016;41(3-4):152-171. doi: 10.1159/000443953 [DOI] [PubMed] [Google Scholar]
- 88.Lee SH, Kim I, Chung BC. Increased urinary level of oxidized nucleosides in patients with mild-to-moderate Alzheimer’s disease. Clin Biochem. 2007;40(13-14):936-938. doi: 10.1016/j.clinbiochem.2006.11.021 [DOI] [PubMed] [Google Scholar]
- 89.Lindqvist D, Dhabhar FS, James SJ, et al. Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinology. 2017;76:197-205. doi: 10.1016/j.psyneuen.2016.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu Z, Cai Y, He J. High serum levels of 8-OHdG are an independent predictor of post-stroke depression in Chinese stroke survivors. Neuropsychiatr Dis Treat. 2018;14:587-596. doi: 10.2147/NDT.S155144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lovell MA, Markesbery WR. Ratio of 8-hydroxyguanine in intact DNA to free 8-hydroxyguanine is increased in Alzheimer disease ventricular cerebrospinal fluid. Arch Neurol. 2001;58(3):392-396. doi: 10.1001/archneur.58.3.392 [DOI] [PubMed] [Google Scholar]
- 92.Lovell MA, Soman S, Bradley MA. Oxidatively modified nucleic acids in preclinical Alzheimer’s disease (PCAD) brain. Mech Ageing Dev. 2011;132(8-9):443-448. doi: 10.1016/j.mad.2011.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lyras L, Cairns NJ, Jenner A, Jenner P, Halliwell B. An assessment of oxidative damage to proteins, lipids, and DNA in brain from patients with Alzheimer’s disease. J Neurochem. 1997;68(5):2061-2069. doi: 10.1046/j.1471-4159.1997.68052061.x [DOI] [PubMed] [Google Scholar]
- 94.Lyras L, Perry RH, Perry EK, et al. Oxidative damage to proteins, lipids, and DNA in cortical brain regions from patients with dementia with Lewy bodies. J Neurochem. 1998;71(1):302-312. doi: 10.1046/j.1471-4159.1998.71010302.x [DOI] [PubMed] [Google Scholar]
- 95.Mecocci P, MacGarvey U, Beal MF. Oxidative damage to mitochondrial DNA is increased in Alzheimer’s disease. Ann Neurol. 1994;36(5):747-751. doi: 10.1002/ana.410360510 [DOI] [PubMed] [Google Scholar]
- 96.Mecocci P, Polidori MC, Cherubini A, et al. Lymphocyte oxidative DNA damage and plasma antioxidants in Alzheimer disease. Arch Neurol. 2002;59(5):794-798. doi: 10.1001/archneur.59.5.794 [DOI] [PubMed] [Google Scholar]
- 97.Migliore L, Fontana I, Trippi F, et al. Oxidative DNA damage in peripheral leukocytes of mild cognitive impairment and AD patients. Neurobiol Aging. 2005;26(5):567-573. doi: 10.1016/j.neurobiolaging.2004.07.016 [DOI] [PubMed] [Google Scholar]
- 98.Mórocz M, Kálmán J, Juhász A, et al. Elevated levels of oxidative DNA damage in lymphocytes from patients with Alzheimer’s disease. Neurobiol Aging. 2002;23(1):47-53. doi: 10.1016/s0197-4580(01)00257-3 [DOI] [PubMed] [Google Scholar]
- 99.Munkholm K, Poulsen HE, Kessing LV, Vinberg M. Elevated levels of urinary markers of oxidatively generated DNA and RNA damage in bipolar disorder. Bipolar Disord. 2015;17(3):257-268. doi: 10.1111/bdi.12245 [DOI] [PubMed] [Google Scholar]
- 100.Muraleedharan A, Menon V, Rajkumar RP, Chand P. Assessment of DNA damage and repair efficiency in drug naïve schizophrenia using comet assay. J Psychiatr Res. 2015;68:47-53. doi: 10.1016/j.jpsychires.2015.05.018 [DOI] [PubMed] [Google Scholar]
- 101.Mutlu-Türkoğlu U, Doğru-Abbasoğlu S, Aykaç-Toker G, Mirsal H, Beyazyürek M, Uysal M. Increased lipid and protein oxidation and DNA damage in patients with chronic alcoholism. J Lab Clin Med. 2000;136(4):287-291. doi: 10.1067/mlc.2000.109097 [DOI] [PubMed] [Google Scholar]
- 102.Nishioka N, Arnold SE. Evidence for oxidative DNA damage in the hippocampus of elderly patients with chronic schizophrenia. Am J Geriatr Psychiatry. 2004;12(2):167-175. doi: 10.1097/00019442-200403000-00008 [DOI] [PubMed] [Google Scholar]
- 103.Nordholm D, Poulsen HE, Hjorthøj C, et al. Systemic oxidative DNA and RNA damage are not increased during early phases of psychosis: a case control study. Psychiatry Res. 2016;241:201-206. doi: 10.1016/j.psychres.2016.04.062 [DOI] [PubMed] [Google Scholar]
- 104.Nunomura A, Chiba S, Lippa CF, et al. Neuronal RNA oxidation is a prominent feature of familial Alzheimer’s disease. Neurobiol Dis. 2004;17(1):108-113. doi: 10.1016/j.nbd.2004.06.003 [DOI] [PubMed] [Google Scholar]
- 105.Nunomura A, Tamaoki T, Motohashi N, et al. The earliest stage of cognitive impairment in transition from normal aging to Alzheimer disease is marked by prominent RNA oxidation in vulnerable neurons. J Neuropathol Exp Neurol. 2012;71(3):233-241. doi: 10.1097/NEN.0b013e318248e614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Peña-Bautista C, Tirle T, López-Nogueroles M, Vento M, Baquero M, Cháfer-Pericás C. Oxidative damage of DNA as early marker of Alzheimer’s disease. Int J Mol Sci. 2019;20(24):E6136. doi: 10.3390/ijms20246136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Psimadas D, Messini-Nikolaki N, Zafiropoulou M, Fortos A, Tsilimigaki S, Piperakis SM. DNA damage and repair efficiency in lymphocytes from schizophrenic patients. Cancer Lett. 2004;204(1):33-40. doi: 10.1016/j.canlet.2003.09.022 [DOI] [PubMed] [Google Scholar]
- 108.Sertan Copoglu U, Virit O, Hanifi Kokacya M, et al. Increased oxidative stress and oxidative DNA damage in non-remission schizophrenia patients. Psychiatry Res. 2015;229(1-2):200-205. doi: 10.1016/j.psychres.2015.07.036 [DOI] [PubMed] [Google Scholar]
- 109.Shimanoe C, Hara M, Nishida Y, et al. Perceived stress, depressive symptoms, and oxidative DNA damage. Psychosom Med. 2018;80(1):28-33. doi: 10.1097/PSY.0000000000000513 [DOI] [PubMed] [Google Scholar]
- 110.Shmarina GV, Orlova MD, Ershova ES, et al. NRF2 and HMOX1 gene expression against the background of systemic oxidative stress in patients with acute psychosis. Russ J Genet. 2020;56:96-102. doi: 10.1134/S102279542001010X [DOI] [Google Scholar]
- 111.Silva AR, Santos AC, Farfel JM, et al. Repair of oxidative DNA damage, cell-cycle regulation and neuronal death may influence the clinical manifestation of Alzheimer’s disease. PLoS One. 2014;9(6):e99897. doi: 10.1371/journal.pone.0099897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sliwinska A, Kwiatkowski D, Czarny P, et al. The levels of 7,8-dihydrodeoxyguanosine (8-oxoG) and 8-oxoguanine DNA glycosylase 1 (OGG1)—a potential diagnostic biomarkers of Alzheimer’s disease. J Neurol Sci. 2016;368:155-159. doi: 10.1016/j.jns.2016.07.008 [DOI] [PubMed] [Google Scholar]
- 113.Soeiro-de-Souza MG, Andreazza AC, Carvalho AF, Machado-Vieira R, Young LT, Moreno RA. Number of manic episodes is associated with elevated DNA oxidation in bipolar I disorder. Int J Neuropsychopharmacol. 2013;16(7):1505-1512. doi: 10.1017/S1461145713000047 [DOI] [PubMed] [Google Scholar]
- 114.Szebeni A, Szebeni K, DiPeri TP, et al. Elevated DNA oxidation and DNA repair enzyme expression in brain white matter in major depressive disorder. Int J Neuropsychopharmacol. 2017;20(5):363-373. doi: 10.1093/ijnp/pyw114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Te Koppele JM, Lucassen PJ, Sakkee AN, et al. 8OHdG levels in brain do not indicate oxidative DNA damage in Alzheimer’s disease. Neurobiol Aging. 1996;17(6):819-826. doi: 10.1016/S0197-4580(96)00165-0 [DOI] [PubMed] [Google Scholar]
- 116.Topak OZ, Ozdel O, Dodurga Y, Secme M. An evaluation of the differences in DNA damage in lymphocytes and repair efficiencies in patients with schizophrenia and schizoaffective disorder. Schizophr Res. 2018;202:99-105. doi: 10.1016/j.schres.2018.06.052 [DOI] [PubMed] [Google Scholar]
- 117.Valentina P, Ekaterina Y, Nikolay B, Evgenii P. The effect of organic lithium salts on plasma 8-hydroxy-2′-deoxyguanosine in bipolar patients in vitro. Psychopharmacol Bull. 2020;50(1):19-27. [PMC free article] [PubMed] [Google Scholar]
- 118.Vieira EL, Mendes-Silva AP, Ferreira JD, et al. Oxidative DNA damage is increased in older adults with a major depressive episode: a preliminary study. J Affect Disord. 2021;279:106-110. doi: 10.1016/j.jad.2020.09.084 [DOI] [PubMed] [Google Scholar]
- 119.Wang J, Markesbery WR, Lovell MA. Increased oxidative damage in nuclear and mitochondrial DNA in mild cognitive impairment. J Neurochem. 2006;96(3):825-832. doi: 10.1111/j.1471-4159.2005.03615.x [DOI] [PubMed] [Google Scholar]
- 120.Wei YC, Zhou FL, He DL, et al. Oxidative stress in depressive patients with gastric adenocarcinoma. Int J Neuropsychopharmacol. 2009;12(8):1089-1096. doi: 10.1017/S1461145709000091 [DOI] [PubMed] [Google Scholar]
- 121.Wei YC, Zhou FL, He DL, et al. The level of oxidative stress and the expression of genes involved in DNA-damage signaling pathways in depressive patients with colorectal carcinoma. J Psychosom Res. 2009;66(3):259-266. doi: 10.1016/j.jpsychores.2008.09.001 [DOI] [PubMed] [Google Scholar]
- 122.Weidner AM, Bradley MA, Beckett TL, et al. RNA oxidation adducts 8-OHG and 8-OHA change with Aβ42 levels in late-stage Alzheimer’s disease. PLoS One. 2011;6(9):e24930. doi: 10.1371/journal.pone.0024930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yi S, Nanri A, Matsushita Y, Kasai H, Kawai K, Mizoue T. Depressive symptoms and oxidative DNA damage in Japanese municipal employees. Psychiatry Res. 2012;200(2-3):318-322. doi: 10.1016/j.psychres.2012.05.035 [DOI] [PubMed] [Google Scholar]
- 124.Young J, McKinney SB, Ross BM, Wahle KW, Boyle SP. Biomarkers of oxidative stress in schizophrenic and control subjects. Prostaglandins Leukot Essent Fatty Acids. 2007;76(2):73-85. doi: 10.1016/j.plefa.2006.11.003 [DOI] [PubMed] [Google Scholar]
- 125.Zengi O, Karakas A, Ergun U, Senes M, Inan L, Yucel D. Urinary 8-hydroxy-2′-deoxyguanosine level and plasma paraoxonase 1 activity with Alzheimer’s disease. Clin Chem Lab Med. 2011;50(3):529-534. doi: 10.1515/CCLM.2011.792 [DOI] [PubMed] [Google Scholar]
- 126.Richmond-Rakerd LS, D’Souza S, Milne BJ, Caspi A, Moffitt TE. Longitudinal associations of mental disorders with dementia: 30-year analysis of 1.7 million New Zealand citizens. JAMA Psychiatry. 2022;79(4):333-340. doi: 10.1001/jamapsychiatry.2021.4377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Di Minno A, Turnu L, Porro B, et al. 8-Hydroxy-2-deoxyguanosine levels and cardiovascular disease: a systematic review and meta-analysis of the literature. Antioxid Redox Signal. 2016;24(10):548-555. doi: 10.1089/ars.2015.6508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Broedbaek K, Weimann A, Stovgaard ES, Poulsen HE. Urinary 8-oxo-7,8-dihydro-2′-deoxyguanosine as a biomarker in type 2 diabetes. Free Radic Biol Med. 2011;51(8):1473-1479. doi: 10.1016/j.freeradbiomed.2011.07.007 [DOI] [PubMed] [Google Scholar]
- 129.Jørs A, Lund MAV, Jespersen T, Hansen T, Poulsen HE, Holm JC. Urinary markers of nucleic acid oxidation increase with age, obesity and insulin resistance in Danish children and adolescents. Free Radic Biol Med. 2020;155:81-86. doi: 10.1016/j.freeradbiomed.2020.05.009 [DOI] [PubMed] [Google Scholar]
- 130.Carlsson ER, Fenger M, Henriksen T, et al. Reduction of oxidative stress on DNA and RNA in obese patients after Roux-en-Y gastric bypass surgery—an observational cohort study of changes in urinary markers. PLoS One. 2020;15(12):e0243918. doi: 10.1371/journal.pone.0243918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lin J, Epel E. Stress and telomere shortening: insights from cellular mechanisms. Ageing Res Rev. 2022;73:101507. doi: 10.1016/j.arr.2021.101507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ellegaard PK, Poulsen HE. Tobacco smoking and oxidative stress to DNA: a meta-analysis of studies using chromatographic and immunological methods. Scand J Clin Lab Invest. 2016;76(2):151-158. doi: 10.3109/00365513.2015.1127407 [DOI] [PubMed] [Google Scholar]
- 133.Sørensen AL, Hasselbalch HC, Nielsen CH, Poulsen HE, Ellervik C. Statin treatment, oxidative stress and inflammation in a Danish population. Redox Biol. 2019;21:101088. doi: 10.1016/j.redox.2018.101088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jiménez-Fernández S, Gurpegui M, Díaz-Atienza F, Pérez-Costillas L, Gerstenberg M, Correll CU. Oxidative stress and antioxidant parameters in patients with major depressive disorder compared to healthy controls before and after antidepressant treatment: results from a meta-analysis. J Clin Psychiatry. 2015;76(12):1658-1667. doi: 10.4088/JCP.14r09179 [DOI] [PubMed] [Google Scholar]
- 135.Jorgensen A, Thygesen MB, Kristiansen U, Poulsen HE. An in silico kinetic model of 8-oxo-7,8-dihydro-2-deoxyguanosine and 8-oxo-7,8-dihydroguanosine metabolism from intracellular formation to urinary excretion. Scand J Clin Lab Invest. 2021;81(7):540-545. doi: 10.1080/00365513.2021.1969682 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Search Strategy, Data Extraction, and Covariates
eReferences
eTable 1. Illness Severity
eFigure 1. PRISMA Flow Diagram of the Literature Search and Study Selection
eTable 2. Characteristics of Included Cross-sectional Studies
eTable 3. Characteristics of Included Intervention Studies
eFigure 2. Forest Plots and Meta-analyses of Central Nervous System Markers
eTable 4. Multilevel Meta-analyses and Sensitivity Analysis for Central Nervous System Markers
eTable 5. Meta-regression Analyses of Covariates
eTable 6. Meta-analyses of Secondary Outcomes
eFigure 3. Funnel Plot of All Included Cross-sectional Studies