Abstract
In patients with gastrointestinal stromal tumors (GIST), it has become mandatory to determine the driver mutation in order to predict the response to standard treatment with tyrosine kinase inhibitors (TKI). A total of 10–15% of all GIST lack activating mutations in KIT proto-oncogene, receptor tyrosine kinase (KIT)/platelet-derived growth factor receptor alpha (PDGFRA) and have been classified as KIT/PDGFRA wild-type (WT) GIST. They are characterized by poor response to TKI. From a group of 119 metastatic GIST patients, 17 patients with KIT/PDGFRA/BRAF WT GIST as determined by reverse transcription-quantitative (RT-q) PCR and Sanger sequencing were profiled by a targeted next-generation sequencing (NGS) approach and their treatment outcome was assessed. In the present study, 41.2% of patients as KIT/PDGFRA/BRAF WT GIST examined with RT-qPCR and Sanger sequencing were confirmed to be carriers of pathogenic KIT/PDGFRA mutations by NGS and were responsive to TKI. The percentage of genuinely KIT/PDGFRA WT GIST in the present study thereby dropped from the initial 14.3% detected with the RT-qPCR and Sanger sequencing to 7.6% after NGS. Their outcome was universally poor. The reliability of RT-qPCR and direct Sanger sequencing results in this setting is therefore insufficient and it is recommended that NGS becomes a requirement for treatment decision at least in KIT/PDGFRA/BRAF WT GIST as determined by RT-qPCR and Sanger sequencing.
Keywords: gastrointestinal stromal tumors, wild-type, next-generation sequencing, tyrosine kinase inhibitors, imatinib
Introduction
Gastrointestinal stromal tumors (GIST) are the most common mesenchymal tumors affecting the gastrointestinal tract but still represent less than 1% of all gastrointestinal tract tumors. The estimated annual incidence of clinically relevant GIST is ~1/100,000 annually (1,2). A total of ~85% of GIST harbor mutually exclusive activating mutations of the genes encoding KIT proto-oncogene, receptor tyrosine kinase (KIT) or platelet-derived growth factor receptor alpha (PDGFRA) tyrosine kinase receptors leading to ligand-independent activation of these receptors and making tumor cells sensitive to tyrosine kinase inhibitors (TKI) (3,4). Imatinib mesylate inhibits the KIT/PDGFRA receptor by directly binding to the ATP-binding site within the amino-terminal domain of the kinase and thus competitively inhibits ATP binding (3). In prospective randomized clinical phase III trials, imatinib achieved disease control in 70–85% of patients with advanced KIT/PDGFRA-mutant GIST, and the median progression-free survival was 20–24 months. The median survival in patients with advanced disease who are treated with front-line imatinib is up to 5 years (5–7).
A total of ~10–15% of all GIST lack mutations in either of the aforementioned genes and have been classified as KIT/PDGFRA wild-type (WT) GIST (4,8–10). The molecular mechanism underlying tumorigenesis in this subgroup is not fully understood. ~A total of 20–40% of KIT/PDGFRA WT GIST represent succinate dehydrogenase complex (SDH)-deficient GIST. SDH-deficiency is caused by the loss of function mutations in one of the genes encoding the SDH subunits (SDHA, SDHB, SDHC and SDHD) or SDHC epimutation resulting in transcriptional silencing of the gene (11,12). Another subgroup of KIT/PDGFRA WT GIST with competent SDH complex activity is defined as RAS-pathway (RAS-P)-mutant GIST. This group includes GIST with inactivating mutation of the NF1 gene, an activating mutation of BRAF, or RAS (13,14). SDH-deficient and RAS mutant GIST represent half of the KIT/PDGFRA WT GIST. In the remaining KIT/PDGFRA WT GIST, the oncogenic driver remains unknown. Of late, certain extremely rare genetic alterations have been demonstrated, such as ETV6-NTRK3 fusion, and alterations in FGFR1, or FGF4, MAX and MEN1 genes (15–17). Metastatic KIT/PDGFRA WT GIST demonstrates a poor response to imatinib mesylate treatment, and there are no prospective data on response, disease progression and overall survival in this setting (18).
Standard methods currently used to determine activating mutations in the KIT and PDGFRA genes are reverse transcription-quantitative (RT-q) PCR and direct Sanger sequencing. The disadvantages of these methods are that they detect either predetermined mutations (mostly hot spot mutations) or in the case of Sanger sequencing, mutations in a specific gene segment, but only if allele frequency of mutation is at least 20% in the sample. They also do not detect all possible mutations in the KIT and PDGFRA genes. Alternatively, next-generation sequencing (NGS) enables the identification of numerous changes in a wider range of investigated genes that cannot be easily detected with standard methods.
This study was performed with the aim to identify somatic mutations using NGS in metastatic KIT/PDGFRA WT GIST patients and analyze their treatment outcomes in a real-world setting.
Materials and methods
Patients
Between January 2002 and December 2020, 119 patients with metastatic KIT-positive GIST started their treatment at the Institute of Oncology Ljubljana (Ljubljana, Slovenia). From this population of patients, only patients with KIT/PDGFRA/BRAF WT tumors as assessed by standard methods were included in further analysis. The present study was approved (approval no. 0120-204/2021/3) by the local Institutional Review Board of the Institute of Oncology Ljubljana and by the National Medical Ethics Committee of the Republic of Slovenia. All procedures in the present study were performed following the ethical standards of the responsible committees on human experimentation (institutional and national) and the Helsinki Declaration of 1975, as revised in 2013. Individual patient consent was waived for the present study as it was a retrospective study, the research involved no risk to the subjects, and the institutional informed consent forms for treatment included consent for the use of patient's data, materials, and/or test results for research purposes.
Immunohistochemistry (IHC)
Formalin-fixed, paraffin-embedded (FFPE) tissue samples were examined and evaluated by a dedicated pathologist from the Department of Pathology at the Institute of Oncology Ljubljana (Ljubljana, Slovenia). Diagnosis of GIST was confirmed based on microscopic findings and CD117 antigen expression as previously described by the authors (19). The determination of DOG1 antigen expression was performed after 2010 in selected cases.
In KIT/PDGFRA WT GIST patients, SDH deficiency was assessed using IHC. Specifically, CD117 and SDHB were detected on 2–4 µm FFPE tissue sections and dried at 56°C for 2 h. Until 2017, CD117 was detected according to a previously described protocol by the authors (19). From 2017, both CD117 and SDHB were detected using fully automated IHC system Ventana Benchmark XT (Ventana ROCHE Inc.). Both epitopes were retrieved on board employing HIER using high pH Cell Conditioning Solution 1 (cat. no. 950-124; Ventana ROCHE Inc.) for 88 min at 100°C. CD117 was detected using a commercially available rabbit monoclonal antibody CD117 (clone YR145; cat. no. 117R; Cell Marque; Merck KGaA). Primary antibody was diluted 1:200 using DAKO REAL™ antibody diluent (cat. no. S2022; DAKO Agilent Technologies, Inc.). SDHB was detected using commercially available rabbit monoclonal antibody SDHB (clone EP288; cat. no. 466R; Cell Marque; Merck KGaA). Primary antibody was diluted 1:100 using DAKO REAL™ antibody diluent (cat. no. S2022; DAKO Agilent Technologies, Inc.). Both antibodies were incubated on board for 60 min at 37°C. Bound primary antibodies were visualized using 3-step multimer detection system OptiView DAB IHC Detection kit (cat. no. 760-700; Ventana ROCHE Inc.) according to the manufacturer's protocol.
The determination of DOG1 was introduced in 2010 using a fully automated IHC system Ventana Benchmark XT (Ventana ROCHE Inc.). The epitope was retrieved on board employing HIER using high pH Cell Conditioning Solution 1 (cat. no. 950-124; Ventana ROCHE Inc.) for 88 min at 100°C. Until 2019, DOG1 was detected using a commercially available rabbit monoclonal antibody DOG-1 (clone SP31; cat. no. M331; Spring Bioscience; Abcam). Primary antibody was diluted 1:50 using DAKO REAL™ antibody diluent (cat. no. S2022; DAKO Agilent Technologies, Inc.). Since 2019, DOG1 was detected using a commercially available rabbit monoclonal antibody DOG-1 (clone SP31; cat. no. 244R; Cell Marque; Merck KGaA). Primary antibody was diluted 1:200 using DAKO REAL™ antibody diluent (cat. no. S2022; DAKO Agilent Technologies, Inc.). Both DOG1 antibodies were incubated on board for 60 min at 37°C. Bound primary antibody was visualized using 3-step multimer detection system OptiView DAB IHC Detection kit (cat. no. 760-700; Ventana ROCHE Inc.) according to the manufacturer's protocol.
Tumor genotyping
Genomic DNA and RNA for tumor molecular analysis were extracted from FFPE primary tumor samples. Macro-dissection of FFPE sample slides followed by tumor genomic DNA/RNA extraction was performed as previously described by the authors (19,20).
Detection of somatic mutations in KIT (exons 9, 11, 13 and 17) and PDGFRA (exons 12, 14 and 18) was performed in all GIST samples (n=119) from the present study by RT-qPCR and direct Sanger sequencing. Additionally, patients with KIT/PDGFRA WT GIST were tested for the presence of hot spot mutations in BRAF gene as previously described by the authors (19).
A total of 17 samples defined as KIT/PDGFRA/BRAF WT GIST (including the immunohistochemically SDHB deficient patients) were analyzed by NGS using TruSight Oncology 500 DNA kit (Illumina, Inc.): TSO500-DNA and 8 samples were additionally examined with TruSight Tumor 170 RNA kit (Illumina, Inc.): TST170-RNA. One sample was examined for germline alterations using TruSight Cancer Panel (Illumina, Inc.). Libraries were prepared according to the manufacturer's protocol and sequenced on NextSeq 550 Sequencing System. Read alignment to the hg19 reference genome and variant calling was performed using TruSight Tumor 170 v2 Local App software (Illumina, inc.). Bioinformatical analysis was performed as previously described by the authors (20). Targeted analysis of following genes was performed: KIT (LRG_307t1), PDGFRA (LRG_309t1), BRAF (LRG_299t1), SDHA (LRG_315t1), SDHB (LRG_316t1), SDHC (LRG_317t1), SDHD (LRG_9t1) and NF1 (LRG_214t1). Variants were described according to HGVS v2019.05 nomenclature (21).
Clinical evaluation, follow-up and outcome analysis
Patients were followed-up every 3–4 months with clinical evaluation, laboratory testing (complete blood count, biochemistry), abdominal ultrasound, abdominal contrast-enhanced computerized tomography. RECIST criteria until 2007 and Choi criteria were used thereafter for defining response and disease progression (22,23). In case of no disease progression, treatment was continued. In case of progression on imatinib, sunitinib has been used since 2007 and for those progressing on sunitinib, regorafenib since 2014. Patients with a documented local progression were managed surgically if that was feasible.
Statistical analysis
Overall survival was assessed from the beginning of treatment until the date of death from any cause by Kaplan-Meier test. To test the difference in survival between patients with KIT/PDGFRA/BRAF WT GIST vs. mutated tumors as assessed by RT-qPCR and Sanger sequencing, the log-rank test was used (24). P<0.05 was considered to indicate a statistically significant difference. Swimmer plot was applied to present the clinical outcome of patients with KIT/PDGFRA/BRAF WT tumors and their NGS molecular status (25). Statistical analyses were performed using SPSS Statistics for Windows (version 25; IBM Corp.).
Results
A total of 119 patients with metastatic GIST, all older than 18 years, were registered between 2002 and 2020. All 119 tumor samples stained positive for CD 117 staining (Fig. S1), the staining for DOG1 was performed after 2010 in 53 (44.5%) samples. The median follow-up at the time of analysis in October 2021 was 11.5 years. Clinicopathological characteristics of the whole group are presented in Table SI.
In 2 patients, tumor specimens were inadequate for molecular analysis. In the remaining 117 patients, mutational status and mutation location were assessed using standard methods RT-qPCR and Sanger sequencing. Single KIT or PDGFRA mutations were detected in 85% of all GIST patients: KIT exon 11 mutation in 81 patients (68.1%), KIT exon 9 mutation in 9 patients (7.6%), and KIT exon 13 in 1 patient (0.8%). A total of 2 patients had co-primary mutations: one in KIT exons 9 and 13 and one in KIT exons 11 and 13. In 3 patients, samples were taken and analyzed after the start of treatment with imatinib and revealed compound mutations: twice in KIT exons 11 and 17 and once in KIT exons 13 and 17. A total of 5 patients (4.1%) were PDGFRA mutated (one in PDGFRA exon 14, three in PDGFRA exon 18 and one in PDGFRA exon 12). None of the 17 KIT/PDGFRA WT patients was positive for BRAF. Thus, 17 patients (14.3%) were designated as KIT/PDGFRA/BRAF WT GIST (Table I). All 17 tumor samples stained positive for CD 117 antigen, while staining for DOG1 antigen was performed in 16 (94.1%) cases, one sample did not stain positive, probably due to an inadequate tissue sample.
Table I.
Mutational status and mutation location for all 119 patients with GIST, assessed by standard methods reverse transcription-quantitative PCR and Sanger sequencing.
| Mutation genotype | n (%) |
|---|---|
| KIT/PDGFRA/BRAF-mutant GIST | |
| KIT exon 11 | 81 (68.1) |
| KIT exon 9 | 9 (7.6) |
| KIT exon 13 | 1 (0.8) |
| KIT exons 9 and 13a | 1 (0.8) |
| KIT exons 11 and 17b | 2 (1.7) |
| KIT exons 13 and 17b | 1 (0.8) |
| PDGFRA exon 12 | 1 (0.8) |
| PDGFRA exon 14 | 1 (0.8) |
| PDGFRA exon 18 | 3 (2.5) |
| BRAF | 0 |
| KIT/PDGFRA/BRAF wild-type GIST | 17 (14.3) |
| Not determined/Unknown | 1 (0.8) |
two primary mutations;
sample taken and analyzed after beginning of treatment with imatinib; GIST, gastrointestinal stromal tumors.
The majority of 17 patients identified as KIT/PDGFRA/BRAF WT GIST were women (70.6%), their median age was 60 years, and 15 (88.2%) received therapy with imatinib. The majority of tumors were large, with a high mitotic rate and a high risk of progression. The most frequently affected sites were the small intestine in 47.0% and the stomach in 29.4%-similar to the whole study group. The clinicopathological characteristics of KIT/PDGFRA/BRAF WT GIST patients and their tumors are presented in Table II.
Table II.
Clinicopathological characteristics of 17 KIT/PDGFRA/BRAF wild-type GIST patients (as assessed by standard methods reverse transcription-quantitative PCR and Sanger sequencing) and their tumors.
| Clinicopathological characteristic | n (%) |
|---|---|
| Sex | |
| Male | 5 (29.4) |
| Female | 12 (70.6) |
| Age, years | |
| Median | 60 |
| Range | 32-76 |
| ≤60 | 9 (53) |
| >60 | 8 (47) |
| Imatinib initial therapy | |
| Yes | 15 (88.2) |
| No | 2 (11.8) |
| Primary tumor location | |
| Esophagus | 0 (0) |
| Stomach | 5 (29.4) |
| Small intestine | 8 (47.0) |
| Rectum | 2 (11.8) |
| Other | 2 (11.8) |
| Mitotic ratea | |
| ≤5 | 6 (35.3) |
| 5.1-10 | 2 (11.8) |
| >10 | 9 (52.9) |
| Primary tumor size, cm | |
| 0-5 | 1 (5.9) |
| 5.1-10 | 5 (29.4) |
| 10 | 11 (64.7) |
| Risk classificationb | |
| High | 11 (64.7) |
| Intermediate | 5 (29.4) |
| Low | 1 (5.9) |
| Very low | 0 (0) |
GIST, gastrointestinal stromal tumors;
Mitotic rate counted as the number of mitoses per 5 mm2;
Risk group for GIST adapted from Miettinen and Lasota (53).
The treatment decision in 15 KIT/PDGFRA/BRAF WT GIST patients as assessed by standard methods receiving initial therapy with imatinib differed regarding the period when they were treated. Before 2016, the decision for imatinib treatment in Slovenia was based on a pathohistological diagnosis of GIST solely (13 patients). After 2016, two patients were offered imatinib treatment based on reports of clinical responses to imatinib also in KIT/PDGFRA/BRAF WT patients (26,27). One of the two (2/17) KIT/PDGFRA/BRAF WT GIST patients not treated with imatinib, had a solitary lesion in the liver and was treated with radiofrequency ablation. The disease did not recur. The second patient was treated with upfront sunitinib. Altogether, five patients were treated with sunitinib occasionally in the course of their disease and none received regorafenib.
The median overall survival of patients with KIT/PDGFRA/BRAF WT GIST vs. mutated tumors as assessed by standard methods was 4.02 years (95% CI 0.17-7.86) and 5.98 years (95% CI 4.92-7.05), respectively (log-rank P=0.232; Fig. S2).
Results of IHC assessment of SDH competence/deficiency in 16 out of 17 KIT/PDGFRA/BRAF WT GIST patients as assessed by standard methods RT-qPCR and Sanger sequencing are provided in Table III. Specifically, loss of IHC staining for SDHB protein was detected in 4 patients.
Table III.
Mutational status, mutation location, allelic frequency, pathogenicity, and clinical benefit to imatinib in KIT/PDGFRA/BRAF WT GIST patients as assessed by standard methods reverse transcription-quantitative PCR and Sanger sequencing.
| n | SDH IHC | Clinically significant gene variants in GIST (NGS) | cDNA variant description | Predicted protein | Pathogenicity ACMG/AMPf | VAF (%) | TMB (mut/MB) ns/(ns+s) | Clinical benefit to imatinib | RECIST evaluation (22) |
|---|---|---|---|---|---|---|---|---|---|
| 1a | Loss | WT | 6/7 | NT | NA | ||||
| 2 | Comp. | WT | 1/2 | No | PD | ||||
| 3b | Comp. | WT | 1/1 | No | PD | ||||
| 4 | Comp. | WT | 0/1 | No | PD | ||||
| 5 | Comp. | WT | 2/3 | No | PD | ||||
| 6b,c | Comp. | NF1 (LRG_214t1) | c.4537C>T | p. (Arg1513*) R1513* | P | 96.27 | 2/2 | Yes | SD |
| 7 | Loss | SDHA (LRG_315t1) | c.776delinsGC | p. (Tyr259Cysfs*62) | LP | 69.66 | 4/4 | No | PD |
| Y259Cfs*62 | |||||||||
| 8 | Loss | SDHB (LRG_316t1) | c.268C>T | p. (Arg90*) R90* | LP | 30.4 | NA | No | PD |
| 9b,d | Loss | SDHB (LRG_316t1) | c.688C>T | p. (Arg230Cys) R230C | LP | 79.31 | 4/4 | NT | NA |
| 10b,g | Comp. | PDGFRA (LRG_309t1) | c.2525A>T | p. (Asp842Val) D584V | P | 41.17 | 0/0 | Yes | CR |
| 11b | Comp. | PDGFRA (LRG_309t1) | c.1682T>A | p. (Val561Asp) V561D | P | 84.61 | 2/3 | Yes | CR |
| 12b | Comp. | PDGFRA (LRG_309t1) | c.1936A>G | p. (Lys646Glu) K646E p. | LP LP | 40.56 | 2/3 | Yes | CR |
| c.1975A>C | (Asn659His) N659H | 41.27 | |||||||
| 13b | Comp. | PDGFRA (LRG_309t1) | c.1679T>A | p. (Val560Asp) V560D | P | 43.35 | 1/1 | Yes | CR |
| 14 | Comp. | KIT (LRG_307t1) | c.1669_1674del | p. (Trp557_Lys558del) | LP | 47.21 | 0/0 | Yes | PR |
| W557_K558del | |||||||||
| 15 | Comp. | KIT (LRG_307t1) | c.1671_1676del | p. (Trp557_Val559delinsCys) | LP | 41.2 | 1/3 | Yes | PR |
| W557_V559delinsC | |||||||||
| 16b | Comp. | KIT (LRG_307t1) | c.1648-3_1673del | p. (Lys550_Lys558del) | LP | 39.9 | 2/2 | Yes | PR |
| K550_K558del | |||||||||
| 17e | NA | NA | NA | NA | NA | NA | NA | No | PD |
WT, wild type; P, pathogenic; LP, likely pathogenic; VAF, variant allele frequency; TMB, tumor mutational burden; ns, nonsynonymous; s, synonymous; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; RECIST, response evaluation criteria in solid tumors; NA, not applicable; NT, not treated; comp., competent;
upfront treated with sunitinib;
besides DNA, also RNA was sequenced by TruSight Tumor 170-RNA;
confirmed germline mutation;
radiofrequency ablation of solitary liver metastasis;
not enough material for additional testing;
Pathogenicity determined according to ACMG/AMP guidelines (58);
resistant mutation PDGFRA D84.2V.
Molecular status of genes frequently altered in GIST was reevaluated using a targeted NGS approach in all 17 KIT/PDGFRA/BRAF WT GIST samples. In one case the quantity of remaining DNA was insufficient for NGS analysis. Mutational status, mutation location, variant allele frequency, and pathogenicity of detected alterations in 17 KIT/PDGFRA/BRAF WT GIST patients are presented in Table III. The deposition of the full sequences of KIT and PDGFRA in 17 GIST patients in the National Center for Biotechnology Information (NCBI) gene bank is in progress. A total of 7 out of 16 samples with sufficient DNA yield for NGS (43.8%) were found to carry a pathogenic alteration, either in KIT (4 patients) or PDGFRA (3 patients). They all responded to imatinib-most surprisingly, including the patient with PDGFRA D842V mutation. Besides pathogenic alterations in the aforementioned genes, NGS also revealed SDH alterations in 3 patients that had already been suspected based on the loss of IHC staining for SDHB protein (Fig. S3). One sample demonstrated a pathogenic variant in NF1, likely in the context of neurofibromatosis type 1. The detected NF1 pathogenic variant in this patient was confirmed to be of germline origin. No fusions in genes included in TruSightTumor 170-RNA kit were detected in examined samples. Only 5 out of 16 cases with sufficient DNA (31%) of KIT/PDGFRA/BRAF WT GIST were confirmed to be KIT/PDGFRA/BRAF/SDH/NF1 WT also by NGS, and none of them responded to TKIs. Their survival was typically poor except for one patient who remains alive with metastatic disease but has been treated surgically.
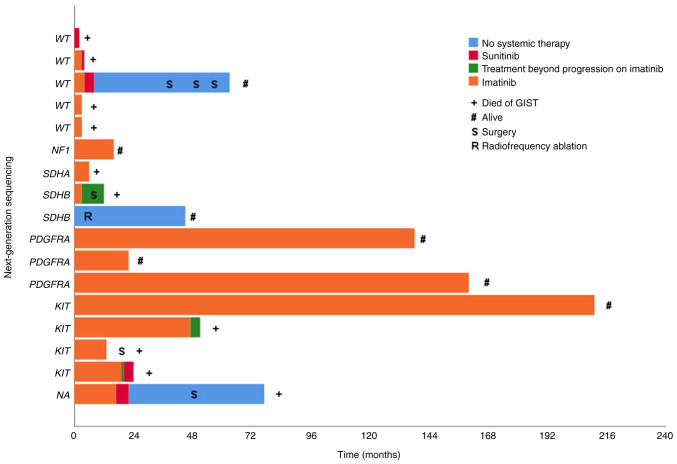
A total of 7 out of 8 patients that responded to imatinib had activating mutations in KIT or PDGFRA confirmed by NGS. One patient that had clinical benefit with stable disease, had the NF1 mutation (c.4537C>T; p. Arg1513*; Table III). Type of treatment, duration of response, survival, and NGS molecular status of 17 KIT/PDGFRA/BRAF WT GIST patients as assessed by standard methods is presented in Fig. 1.
Figure 1.
Clinical outcome in 17 KIT/PDGFRA/BRAF wild-type GIST patients (as assessed by standard methods reverse transcription-quantitative PCR and Sanger sequencing) and their next-generation sequencing molecular status. Patients are listed according to Table III. KIT, KIT proto-oncogene, receptor tyrosine kinase; PDGFRA, platelet-derived growth factor receptor alpha; GIST, gastrointestinal stromal tumors.
The percentage of KIT/PDGFRA/BRAF WT GIST patients as determined by standard methods (14.3%; 17 out of 119 patients) was reduced to 7.6% (9 out of 119 patients) after re-evaluation with NGS. However, only five out of 119 patients (4.2%) were truly WT GIST patients (excluding those without SDH and NF1 variants).
Discussion
In patients with GIST, it has become mandatory to determine the driver mutation in order to predict the response to TKI treatment. The European Society for Medical Oncology (ESMO) guidelines, however, do not specify the screening method for the detection of somatic mutations (28). RT-qPCR, direct Sanger sequencing or NGS may be used, but in the past, the first descriptions of gain of function mutations in KIT/PDGFRA were based on RT-qPCR and direct Sanger sequencing (4,29,30). These methods have remained the cornerstone for detecting oncogenic drivers in GIST until today. Nevertheless, it has been observed in routine clinical practice that there are patients with KIT/PDGFRA WT GIST by conventional RT-qPCR and direct Sanger sequencing that respond to imatinib. With these observations, the reliability of the results of RT-qPCR and direct Sanger sequencing in the identification of genuinely KIT/PDGFRA WT GIST are questioned.
In the present study, mutations detected by NGS as well as clinical outcomes of the KIT/PDGFRA/BRAF WT GIST were reported (n=17). Patients were selected from a larger series of GIST patients (n=119) analyzed for mutational status with conventional methods RT-qPCR and direct Sanger sequencing.
Among initially included 119 patients, the clinicopathological characteristics showed no difference when compared with published data. As previously reported, also in the present study, there was a slight male predominance (50.4%) (5,6,31). The age at diagnosis of our patients ranged from 32 to 89 years, with the median age being 63.5 years. The median age across most studies falls in the mid-60s (1,2,5–7). In our cohort, GIST most commonly arose in small intestine and stomach (37.0%) while in the previous study, the majority of GIST were found in the stomach and less commonly in the small intestine (1,2,8,32). This may be an area of interest for future research. There was also no difference in the proportion of KIT/PDGFRA/BRAF WT GIST as determined with conventional RT-qPCR and direct Sanger sequencing between our study (14.3%) and data published in the literature, where they observed 7.1–15% of WT GIST (2–4).
In the present study, KIT/PDGFRA/BRAF WT GIST by conventional RT-qPCR and direct Sanger sequencing were found to arise predominantly in females (70.6%), at a younger age than the whole study population (median age 60 years), while other clinical characteristics (primary location, mitotic count, risk group) did not differ from the whole study population. KIT/PDGFRA/BRAF WT GIST by conventional RT-qPCR and direct Sanger sequencing were reported to arise predominantly in the stomach if SDH mutant or epimutant and predominantly in the small intestine if SDH competent (33). In the present analysis, they were found more often in the small intestine than in the stomach, regardless of SDH status. Importantly, only adult GIST patients were included in the present analysis. The natural history of KIT/PDGFRA/BRAF WT GIST by conventional RT-qPCR and direct Sanger sequencing has been suggested to be more indolent than that of KIT/PDGFRA-mutated GIST, but the present study demonstrated a shorter (yet not significantly) overall survival compared with the whole study group (median overall survival 4.02 years vs. 5.87 years) (8,34,35). KIT/PDGFRA/BRAF WT GIST by conventional RT-qPCR and direct Sanger sequencing, that are also confirmed to be WT (for KIT/PDGFRA alterations) by NGS, can be associated with hereditary syndromes, such as type 1 neurofibromatosis (NF1), Carney triad (23), Carney Stratakis syndrome and hereditary paraganglioma/pheochromocytoma (HPGL/PCC) syndromes (8,33). In the present study, only one patient had a hereditary syndrome (NF1).
In the present study, 15 of total 17 KIT/PDGFRA/BRAF WT GIST patients by conventional RT-qPCR and direct Sanger sequencing were available for assessing response to imatinib. Patients (n=7) that responded to imatinib were, however, diagnosed to carry pathogenic alterations by NGS; either in KIT or PDGFRA. One patient with long-term, on-going complete response on imatinib was a carrier of PDGFRA D842V mutation. Patients with PDGFRA D842V mutation rarely respond to imatinib (34–37). Farag et al (37) reported partial response in 2 out of 16 PDGFRA D842V-mutated GIST patients and stable disease in 3 of totally 16 patients. The percentage of patients carrying pathogenic alterations either in KIT or PDGFRA in the present study (41.2%) was higher than the one reported by Heinrich et al (7) demonstrating pathogenic alterations either in KIT or PDGFRA in 3 of total 20 patients (15%) (5–7). No new mutations of KIT and PDGFRA were observed in the present study; all mutations discussed in Table III have already been previously reported in NCBI ClinVar and/or Cosmic and discussed (30,38–46).
One patient that had clinical benefit with stable disease was a carrier of NF1 germline pathogenic variant and the reason for benefit could only be hypothesized. Putatively, some of the lesions followed with CT scans could have been other entities (not verified) in the context of confirmed NF1 and not GIST metastases. A possible explanation for response to TKI could also be attributed to the increased KIT activation through phosphorylation. Namely, the efficacy of TKI in patients with increased KIT activation (detected in some of KIT/PDGFRA/BRAF WT tumors), suggested that in some of these patients KIT activation may play a role in the pathophysiology (26). Moreover, similar to our observation for the patient carrying pathogenic variant in NF1 exon 34 (c.4537C>T), Mussi et al (47) reported stable disease in one patient with NF1 exon 18 mutation after therapy with imatinib.
The remaining 7 out of 15 patients available for assessing response to imatinib did not respond. Among them, two samples demonstrated the SDH alteration which had already been suspected by IHC and was later verified with NGS. Patients with SDH alterations (mutation or epigenetic silencing) are known not to respond to imatinib (7,12). A total of 5 patients remained WT also by NGS, among whom 4 were initially treated with imatinib and did not respond, and one was treated with sunitinib and did not respond. This is consistent with the published literature stating that NGS WT GIST does not respond to TKI, regardless of whether SDH deficient or SDH competent by IHC (9). In the remaining not responding patient the quantity of DNA was not sufficient for NGS analysis.
In 16 GIST patients, the tumor mutational burden (TMB) was assessed. The calculated TMB was low in all patients (less than 10 mutations/MB). These results are in accordance with the previous study by Chalmers et al (48) but differ from the results by Feng et al (49). High TMB correlates with the response to immune checkpoint inhibitors in solid tumors and pembrolizumab was approved for adults and children with TMB-high solid tumors (50). In oncogene-driven disease, it would be expected that a lower TMB correlates with a longer response to TKI. At present, to the best of our knowledge, no aware study exists on the correlation between the TMB score and the largest clinical benefit of imatinib in GIST patients.
KIT/PDGFRA WT GIST has been reported to comprise 10–15% of all GIST with standard diagnostic methods RT-qPCR and direct Sanger sequencing. In large randomized clinical trials that establish the recommendations for the treatment of metastatic GIST, NGS has not been performed. In the phase III BFR14 trial, mutational status was determined by PCR and Sanger sequencing and identified 11.4% WT GIST (51). In the B2222 study, mutational status was determined by PCR and high-pressure liquid chromatography (HPLC) (3). Activating mutations were identified in 92.9%. In EORTC 62005 study mutations were screened by denaturing high-performance liquid chromatography (DHPLC) and characterized by bi-directional DNA sequencing (52). Activating mutations of KIT or PDGFRA were found in 84% of GIST. In SWOG S0033/CALGB 150105 study, a combination of PCR amplification, DHPLC screening, and sequencing was used and demonstrated 16.9% KIT/PDGFRA WT GIST (83.1% of samples carried KIT/PDGFRA mutations) (18). In the follow-up analysis of the SSG XVIII/AIO trial, conventional Sanger sequencing was used and identified 7% KIT/PDGFRA WT GIST (53). The results of the present study are absolutely in concordance with the results of the aforementioned studies. Namely, in the present study, 85% of samples were identified to be KIT/PDGFRA mutation-positive with RT-qPCR and Sanger sequencing, while 14.3% of samples were KIT/PDGFRA WT GIST.
However, in recent years, studies of NGS in GIST have been published, demonstrating that this technique has the potential to detect genetic alterations otherwise not detectable with conventional techniques, particularly in KIT/PDGFRA WT tumors (8,13,17,54,55). Indeed, ESMO guidelines recommend tumor molecular testing in GIST patients if TKI are considered as a part of the management, however, the preferential method is not specified (28). On the other hand, the latest National Comprehensive Cancer Network guidelines recommend that all GIST lacking a KIT or PDGFRA mutation should be examined for SDH deficiency and alternative driver mutations using NGS (56).
When comparing NGS with RT-qPCR and direct Sanger sequencing, NGS provides the highest positivity rate (43). This conclusion indicates a potential advantage of NGS for the detection of targetable mutations in real-world clinical routine setting. In the present study, using the NGS in 16 out of 17 KIT/PDGFRA/BRAF WT GIST as determined by conventional RT-qPCR and Sanger sequencing, mutated genotype was identified in 69% (11 patients). NGS identified 4 cases with KIT mutation, 3 cases with PDGFRA mutation, both missed in conventional analysis. It also identified 3 cases with SDH complex mutation (all suspicious on IHC staining) and 1 NF1 germline pathogenic variant. All patients with KIT/PDGFRA mutated genotype by NGS responded to imatinib. Similar results have also been reported by previous studies. In the report from Heinrich et al (18), the presumed causative mutation was identified with a combination of PCR and Sanger sequencing in 83% of GIST (KIT and PDGFRA analysis only). Using the NGS technologies, Heinrich et al (7) estimated that 97.5% of GIST can be assigned a mutated genotype. NGS of 20 cases originally classified as KIT/PDGFRA WT GIST disclosed 17 (85%) carrying a mutation, most commonly (in 60%) in SDH complex. Astolfi et al (43) indicated that ~20% of patients, assessed by Sanger and IHC, with KIT/PDGFRA/SDH/RAS-P WT GIST are carriers of pathogenic KIT mutations, thus expected to be eligible for and responsive to the various therapeutic lines of TKI in use for KIT/PDGFRA mutant GIST. Vanden Bempt et al (57) also demonstrated that the percentage of WT GIST decreases with the use of NGS. Optimizing detection assays of genetic alterations led to the identification of KIT or PDGFRA mutations in 92.0% of GIST samples and reduced the number of WT GIST to 1.2% only.
In conclusion, the results of the present study demonstrated that ~41.2% of patients diagnosed through standard methods (RT-qPCR and Sanger sequencing) with the KIT/PDGFRA/BRAF-pathway WT GIST are actually carriers of pathogenic KIT/PDGFRA mutations when re-evaluated by NGS, and are thus expected to be eligible for and responsive to the various therapeutic lines of TKI in use for KIT/PDGFRA-mutant GIST. The percentage of genuinely KIT/PDGFRA WT GIST in the present study decreased from the initial 14.3% when assessed with the RT-qPCR and Sanger sequencing to 7.6% after NGS. Therefore, it is strongly considered that NGS should become prerequisite for treatment decision at least in KIT/PDGFRA/BRAF-pathway WT GIST as determined by RT-qPCR and Sanger sequencing.
The limitation to the present study is a small number of NGS samples, as well as a long follow-up with different molecular methods used throughout the course of study, diverse methods of evaluation, and variable access to treatment.
Supplementary Material
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The data presented in this study are openly available in Sequence Read Archive (NCBI-SRA), accession number PRJNA824718.
Authors' contributions
MU, SN and BJN conceptualized the present study. Formal analysis, MU, AB, SN, VS, VSD, OB, GK and BJN performed formal analysis. MU, AB, SN, VS, VSD, OB, GK and BJN conducted investigation. MU, SN and BJN provided methodology. MU and BJN performed project administration. MU, AB, SN, VS, VSD, OB, GK and BJN provided resources, SN and BJN supervised the present study. MU and SN conducted visualization. MU wrote the original draft. MU, AB, SN, VS, VSD, OB, GK and BJN wrote, reviewed and edited the manuscript. All authors have read and approved the final version of the manuscript. SN and BJN confirm the authenticity of all the raw data.
Ethics approval and consent to participate
The present study was approved (approval no. 0120-204/2021/3) by the local Institutional Review Board of the Institute of Oncology Ljubljana and by the National Medical Ethics Committee of the Republic of Slovenia (Ljubljana, Slovenia). All procedures in the present study were done following the ethical standards of the responsible committees on human experimentation (institutional and national) and the Helsinki Declaration of 1975, as revised in 2013. Individual patient consent was waived for this study as it was a retrospective study, the research involved no risk to the subjects, and the institutional informed consent forms for treatment included consent for the use of patient's data, materials, and/or test results for research purposes.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Nilsson B, Bümming P, Meis-Kindblom JM, Odén A, Dortok A, Gustavsson B, Sablinska K, Kindblom LG. Gastrointestinal stromal tumors: The incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era-a population-based study in western Sweden. Cancer. 2005;103:821–829. doi: 10.1002/cncr.20862. [DOI] [PubMed] [Google Scholar]
- 2.Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol. 2004;22:3813–3825. doi: 10.1200/JCO.2004.05.140. [DOI] [PubMed] [Google Scholar]
- 3.Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: Origin and molecular oncology. Nat Rev Cancer. 2011;11:865–878. doi: 10.1038/nrc3143. [DOI] [PubMed] [Google Scholar]
- 4.Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, McGreevey LS, Chen CJ, Van den Abbeele AD, Druker BJ, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21:4342–4349. doi: 10.1200/JCO.2003.04.190. [DOI] [PubMed] [Google Scholar]
- 5.Casali PG, Zalcberg J, Le Cesne A, Reichardt P, Blay JY, Lindner LH, Judson IR, Schöffski P, Leyvraz S, Italiano A, et al. Ten-year progression-free and overall survival in patients with unresectable or metastatic GI stromal tumors: Long-term analysis of the European organisation for research and treatment of cancer, Italian sarcoma group, and australasian gastrointestinal trials group intergroup phase III randomized trial on imatinib at two dose levels. J Clin Oncol. 2017;35:1713–1720. doi: 10.1200/JCO.2016.71.0228. [DOI] [PubMed] [Google Scholar]
- 6.Blanke CD, Demetri GD, von Mehren M, Heinrich MC, Eisenberg B, Fletcher JA, Corless CL, Fletcher CD, Roberts PJ, Heinz D, et al. Long-term results from a randomized phase II trial of standard-versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol. 2008;26:620–625. doi: 10.1200/JCO.2007.13.4452. [DOI] [PubMed] [Google Scholar]
- 7.Heinrich MC, Rankin C, Blanke CD, Demetri GD, Borden EC, Ryan CW, von Mehren M, Blackstein ME, Priebat DA, Tap WD, et al. Correlation of long-term results of imatinib in advanced gastrointestinal stromal tumors with next-generation sequencing results: Analysis of phase 3 SWOG intergroup trial S0033. JAMA Oncol. 2017;3:944–952. doi: 10.1001/jamaoncol.2016.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nannini M, Biasco G, Astolfi A, Pantaleo MA. An overview on molecular biology of KIT/PDGFRA wild type (WT) gastrointestinal stromal tumours (GIST) J Med Genet. 2013;50:653–661. doi: 10.1136/jmedgenet-2013-101695. [DOI] [PubMed] [Google Scholar]
- 9.Szucs Z, Thway K, Fisher C, Bulusu R, Constantinidou A, Benson C, van der Graaf WT, Jones RL. Molecular subtypes of gastrointestinal stromal tumors and their prognostic and therapeutic implications. Future Oncol. 2017;13:93–107. doi: 10.2217/fon-2016-0192. [DOI] [PubMed] [Google Scholar]
- 10.Mei L, Smith SC, Faber AC, Trent J, Grossman SR, Stratakis CA, Boikos SA. Gastrointestinal stromal tumors: The GIST of precision medicine. Trends Cancer. 2018;4:74–91. doi: 10.1016/j.trecan.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Pantaleo MA, Astolfi A, Urbini M, Nannini M, Paterini P, Indio V, Saponara M, Formica S, Ceccarelli C, Casadio R, et al. Analysis of all subunits, SDHA, SDHB, SDHC, SDHD, of the succinate dehydrogenase complex in KIT/PDGFRA wild-type GIST. Eur J Hum Genet. 2014;22:32–39. doi: 10.1038/ejhg.2013.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janeway KA, Kim SY, Lodish M, Nosé V, Rustin P, Gaal J, Dahia PL, Liegl B, Ball ER, Raygada M, et al. Defects in succinate dehydrogenase in gastrointestinal stromal tumors lacking KIT and PDGFRA mutations. Proc Natl Acad Sci USA. 2011;108:314–318. doi: 10.1073/pnas.1009199108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pantaleo MA, Nannini M, Corless CL, Heinrich MC. Quadruple wild-type (WT) GIST: Defining the subset of GIST that lacks abnormalities of KIT, PDGFRA, SDH, or RAS signaling pathways. Cancer Med. 2015;4:101–103. doi: 10.1002/cam4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daniels M, Lurkin I, Pauli R, Erbstösser E, Hildebrandt U, Hellwig K, Zschille U, Lüders P, Krüger G, Knolle J, et al. Spectrum of KIT/PDGFRA/BRAF mutations and phosphatidylinositol-3-kinase pathway gene alterations in gastrointestinal stromal tumors (GIST) Cancer Lett. 2011;312:43–54. doi: 10.1016/j.canlet.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 15.Shin HT, Choi YL, Yun JW, Kim NKD, Kim SY, Jeon HJ, Nam JY, Lee C, Ryu D, Kim SC, et al. Prevalence and detection of low-allele-fraction variants in clinical cancer samples. Nat Commun. 2017;8:1377. doi: 10.1038/s41467-017-01470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brenca M, Rossi S, Polano M, Gasparotto D, Zanatta L, Racanelli D, Valori L, Lamon S, Dei Tos AP, Maestro R. Transcriptome sequencing identifies ETV6-NTRK3 as a gene fusion involved in GIST. J Pathol. 2016;238:543–549. doi: 10.1002/path.4677. [DOI] [PubMed] [Google Scholar]
- 17.Pantaleo MA, Urbini M, Indio V, Ravegnini G, Nannini M, De Luca M, Tarantino G, Angelini S, Gronchi A, Vincenzi B, et al. Genome-wide analysis identifies MEN1 and MAX mutations and a neuroendocrine-like molecular heterogeneity in quadruple WT GIST. Mol Cancer Res. 2017;15:553–562. doi: 10.1158/1541-7786.MCR-16-0376. [DOI] [PubMed] [Google Scholar]
- 18.Heinrich MC, Owzar K, Corless CL, Hollis D, Borden EC, Fletcher CD, Ryan CW, von Mehren M, Blanke CD, Rankin C, et al. Correlation of kinase genotype and clinical outcome in the North American intergroup phase III trial of imatinib mesylate for treatment of advanced gastrointestinal stromal tumor: CALGB 150105 study by cancer and leukemia group B and southwest oncology group. J Clin Oncol. 2008;26:5360–5367. doi: 10.1200/JCO.2008.17.4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bombac A, Zakotnik B, Bucic M, Setrajcic Dragos V, Gazic B, Stegel V, Klancar G, Novakovic S. Mutational spectrum and classification of novel mutations in patients with metastatic gastrointestinal stromal tumours. Int J Oncol. 2020;56:1468–1478. doi: 10.3892/ijo.2020.5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klančar G, Blatnik A, Šetrajčič Dragoš V, Vogrič V, Stegel V, Blatnik O, Drev P, Gazič B, Krajc M, Novaković S. A novel germline MLH1 in-frame deletion in a slovenian lynch syndrome family associated with uncommon isolated PMS2 loss in tumor tissue. Genes (Basel) 2020;11:325. doi: 10.3390/genes11030325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.den Dunnen JT, Dalgleish R, Maglott DR, Hart RK, Greenblatt MS, McGowan-Jordan J, Roux AF, Smith T, Antonarakis SE, Taschner PE. HGVS recommendations for the description of sequence variants: 2016 Update. Hum Mutat. 2016;37:564–569. doi: 10.1002/humu.22981. [DOI] [PubMed] [Google Scholar]
- 22.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 23.Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, Chen LL, Podoloff DA, Benjamin RS. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: Proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753–1759. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- 24.Bland JM, Altman DG. Survival probabilities (the Kaplan-Meier method) BMJ. 1998;317:1572. doi: 10.1136/bmj.317.7172.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chia PL, Gedye C, Boutros PC, Wheatley-Price P, John T. Current and evolving methods to visualize biological data in cancer research. J Natl Cancer Inst. 2016;108:djw031. doi: 10.1093/jnci/djw031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janeway KA, Liegl B, Harlow A, Le C, Perez-Atayde A, Kozakewich H, Corless CL, Heinrich MC, Fletcher JA. Pediatric KIT wild-type and platelet-derived growth factor receptor alpha-wild-type gastrointestinal stromal tumors share KIT activation but not mechanisms of genetic progression with adult gastrointestinal stromal tumors. Cancer Res. 2007;67:9084–9088. doi: 10.1158/0008-5472.CAN-07-1938. [DOI] [PubMed] [Google Scholar]
- 27.Murray M, Hatcher H, Jessop F, Williams D, Carroll N, Bulusu R, Judson I. Treatment of wild-type gastrointestinal stromal tumor (WT-GIST) with imatinib and sunitinib. Pediatr Blood Cancer. 2008;50:386–388. doi: 10.1002/pbc.21312. [DOI] [PubMed] [Google Scholar]
- 28.Casali PG, Blay JY, Abecassis N, Bajpai J, Bauer S, Biagini R, Bielack S, Bonvalot S, Boukovinas I, Bovee JVMG, et al. Gastrointestinal stromal tumours: ESMO-EURACAN-GENTURIS clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:20–33. doi: 10.1016/j.annonc.2021.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 30.Joensuu H, Wardelmann E, Sihto H, Eriksson M, Sundby Hall K, Reichardt A, Hartmann JT, Pink D, Cameron S, Hohenberger P, et al. Effect of KIT and PDGFRA mutations on survival in patients with gastrointestinal stromal tumors treated with adjuvant imatinib: An exploratory analysis of a randomized clinical trial. JAMA Oncol. 2017;3:602–609. doi: 10.1001/jamaoncol.2016.5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blanke CD, Rankin C, Demetri GD, Ryan CW, von Mehren M, Benjamin RS, Raymond AK, Bramwell VH, Baker LH, Maki RG, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26:626–632. doi: 10.1200/JCO.2007.13.4452. [DOI] [PubMed] [Google Scholar]
- 32.Boikos SA, Pappo AS, Killian JK, LaQuaglia MP, Weldon CB, George S, Trent JC, von Mehren M, Wright JA, Schiffman JD, et al. Molecular subtypes of KIT/PDGFRA wild-type gastrointestinal stromal tumors: A report from the national institutes of health gastrointestinal stromal tumor clinic. JAMA Oncol. 2016;2:922–928. doi: 10.1001/jamaoncol.2016.0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wada R, Arai H, Kure S, Peng WX, Naito Z. ‘Wild type’ GIST: Clinicopathological features and clinical practice. Pathol Int. 2016;66:431–437. doi: 10.1111/pin.12431. [DOI] [PubMed] [Google Scholar]
- 34.Yoo C, Ryu MH, Jo J, Park I, Ryoo BY, Kang YK. Efficacy of imatinib in patients with platelet-derived growth factor receptor alpha-mutated gastrointestinal stromal tumors. Cancer Res Treat. 2016;48:546–552. doi: 10.4143/crt.2015.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cassier PA, Fumagalli E, Rutkowski P, Schöffski P, Van Glabbeke M, Debiec-Rychter M, Emile JF, Duffaud F, Martin-Broto J, Landi B, et al. Outcome of patients with platelet-derived growth factor receptor alpha-mutated gastrointestinal stromal tumors in the tyrosine kinase inhibitor era. Clin Cancer Res. 2012;18:4458–4464. doi: 10.1158/1078-0432.CCR-11-3025. [DOI] [PubMed] [Google Scholar]
- 36.Corless CL, Schroeder A, Griffith D, Town A, McGreevey L, Harrell P, Shiraga S, Bainbridge T, Morich J, Heinrich MC. PDGFRA mutations in gastrointestinal stromal tumors: Frequency, spectrum and in vitro sensitivity to imatinib. J Clin Oncol. 2005;23:5357–5364. doi: 10.1200/JCO.2005.14.068. [DOI] [PubMed] [Google Scholar]
- 37.Farag S, Somaiah N, Choi H, Heeres B, Wang WL, van Boven H, Nederlof P, Benjamin R, van der Graaf W, Grunhagen D, et al. Clinical characteristics and treatment outcome in a large multicentre observational cohort of PDGFRA exon 18 mutated gastrointestinal stromal tumour patients. Eur J Cancer. 2017;76:76–83. doi: 10.1016/j.ejca.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 38.National Library of Medicine (NIH), corp-author ClinVar. NCBI; Bethesda, MD: 2022. [Google Scholar]
- 39.Cataloque of Somatic Mutations in Cancer (COSMIC), corp-author https://cancer.sanger.ac.uk/cosmic COSMIC v96. released 31-May-2022. [Google Scholar]
- 40.Joensuu H, Roberts PJ, Sarlomo-Rikala M, Andersson LC, Tervahartiala P, Tuveson D, Silberman S, Capdeville R, Dimitrijevic S, Druker B, Demetri GD. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344:1052–1056. doi: 10.1056/NEJM200104053441404. [DOI] [PubMed] [Google Scholar]
- 41.Mühlenberg T, Ketzer J, Heinrich MC, Grunewald S, Marino-Enriquez A, Trautmann M, Hartmann W, Wardelmann E, Treckmann J, Worm K, et al. KIT-dependent and KIT-independent genomic heterogeneity of resistance in gastrointestinal stromal tumors-TORC1/2 inhibition as salvage strategy. Mol Cancer Ther. 2019;18:1985–1996. doi: 10.1158/1535-7163.MCT-18-1224. [DOI] [PubMed] [Google Scholar]
- 42.Heinrich MC, Corless CL, Blanke CD, Demetri GD, Joensuu H, Roberts PJ, Eisenberg BL, von Mehren M, Fletcher CD, Sandau K, et al. Molecular correlates of imatinib resistance in gastrointestinal stromal tumors. J Clin Oncol. 2006;24:4764–4774. doi: 10.1200/JCO.2006.06.2265. [DOI] [PubMed] [Google Scholar]
- 43.Astolfi A, Indio V, Nannini M, Saponara M, Schipani A, De Leo A, Altimari A, Vincenzi B, Comandini D, Grignani G, et al. Targeted deep sequencing uncovers cryptic KIT mutations in KIT/PDGFRA/SDH/RAS-P wild-type GIST. Front Oncol. 2020;10:504. doi: 10.3389/fonc.2020.00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daum O, Zalud R, Grossmann P, Mukensnabl P, Michal M. A case of imatinib-naive ileal fibrous stromal tumor with unusual morphology and double PDGFRA mutation. Appl Immunohistochem Mol Morphol. 2010;18:484–485. doi: 10.1097/PAI.0b013e3181db500a. [DOI] [PubMed] [Google Scholar]
- 45.Ginori A, Scaramuzzino F, Marsili S, Tripodi S. Late hepatic metastasis from a duodenal gastrointestinal stromal tumor (29 years after surgery): Report of a case and review of the literature. Int J Surg Pathol. 2015;23:317–321. doi: 10.1177/1066896915573571. [DOI] [PubMed] [Google Scholar]
- 46.Nannini M, Astolfi A, Paterini P, Urbini M, Santini D, Catena F, Indio V, Casadio R, Pinna AD, Biasco G, Pantaleo MA. Expression of IGF-1 receptor in KIT/PDGF receptor-α wild-type gastrointestinal stromal tumors with succinate dehydrogenase complex dysfunction. Future Oncol. 2013;9:121–126. doi: 10.2217/fon.12.170. [DOI] [PubMed] [Google Scholar]
- 47.Mussi C, Schildhaus HU, Gronchi A, Wardelmann E, Hohenberger P. Therapeutic consequences from molecular biology for gastrointestinal stromal tumor patients affected by neurofibromatosis type 1. Clin Cancer Res. 2008;14:4550–4555. doi: 10.1158/1078-0432.CCR-08-0086. [DOI] [PubMed] [Google Scholar]
- 48.Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, Schrock A, Campbell B, Shlien A, Chmielecki J, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9:34. doi: 10.1186/s13073-017-0424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feng Y, Yao S, Pu Z, Cheng H, Fei B, Zou J, Huang Z. Identification of new tumor-related gene mutations in Chinese gastrointestinal stromal tumors. Front Cell Dev Biol. 2021;9:764275. doi: 10.3389/fcell.2021.764275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, Chung HC, Kindler HL, Lopez-Martin JA, Miller WH, Jr, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21:1353–1365. doi: 10.1016/S1470-2045(20)30445-9. [DOI] [PubMed] [Google Scholar]
- 51.Patrikidou A, Domont J, Chabaud S, Ray-Coquard I, Coindre JM, Bui-Nguyen B, Adenis A, Rios M, Bertucci F, Duffaud F, et al. Long-term outcome of molecular subgroups of GIST patients treated with standard-dose imatinib in the BFR14 trial of the French sarcoma group. Eur J Cancer. 2016;52:173–180. doi: 10.1016/j.ejca.2015.10.069. [DOI] [PubMed] [Google Scholar]
- 52.Debiec-Rychter M, Dumez H, Judson I, Wasag B, Verweij J, Brown M, Dimitrijevic S, Sciot R, Stul M, Vranck H, et al. Use of c-KIT/PDGFRA mutational analysis to predict the clinical response to imatinib in patients with advanced gastrointestinal stromal tumours entered on phase I and II studies of the EORTC soft tissue and bone sarcoma group. Eur J Cancer. 2004;40:689–695. doi: 10.1016/j.ejca.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 53.Miettinen M, Lasota J. Gastrointestinal stromal tumors: Pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23:70–83. doi: 10.1053/j.semdp.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 54.Nannini M, Urbini M, Astolfi A, Biasco G, Pantaleo MA. The progressive fragmentation of the KIT/PDGFRA wild-type (WT) gastrointestinal stromal tumors (GIST) J Transl Med. 2017;15:113. doi: 10.1186/s12967-017-1212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mavroeidis L, Metaxa-Mariatou V, Papoudou-Bai A, Lampraki AM, Kostadima L, Tsinokou I, Zarkavelis G, Papadaki A, Petrakis D, Gκoura S, et al. Comprehensive molecular screening by next generation sequencing reveals a distinctive mutational profile of KIT/PDGFRA genes and novel genomic alterations: Results from a 20-year cohort of patients with GIST from north-western Greece. ESMO Open. 2018;3:e000335. doi: 10.1136/esmoopen-2018-000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.National Comprehensive Cancer Network (NCCN), corp-author NCCN; Plymouth Meeting, PA: 2022. NCCN guidelines GIST v1.2022. [Google Scholar]
- 57.Vanden Bempt I, Vander Borght S, Sciot R, Spans L, Claerhout S, Brems H, Lehnert S, Dehaspe L, Fransis S, Neuville B, et al. Comprehensive targeted next-generation sequencing approach in the molecular diagnosis of gastrointestinal stromal tumor. Genes Chromosomes Cancer. 2021;60:239–249. doi: 10.1002/gcc.22923. [DOI] [PubMed] [Google Scholar]
- 58.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American college of medical genetics and genomics and the association for molecular pathology. Genet Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are openly available in Sequence Read Archive (NCBI-SRA), accession number PRJNA824718.