Abstract
Simple Summary
The microbiota can modulate immune responses and modify the physiology of the human organism, thereby increasing infective risks and a neoplastic predisposition. In this review, we focus on the composition of the cervical microbiota, to identify the risk of developing Cervical Intraepithelial Neoplasia and better understand the interaction between cervico-vaginal microbiota and human papillomavirus as a means of promoting the identification of new therapeutic strategies. In fact, no therapy for HPV is yet available. A better understanding of the cervical micro-environment could be a key element allowing complete viral clearance to be achieved in largely affected populations.
Abstract
The heterogeneity of the cervico-vaginal microbiota can be appreciated in various conditions, both pathological and non-pathological, and can vary according to biological and environmental factors. Attempts are still in course to define the interaction and role of the various factors that constitute this community of commensals in immune protection, inflammatory processes, and the onset of precancerous lesions of the cervical epithelium. Despite the many studies on the relationship between microbiota, immunity, and HPV-related cervical tumors, further aspects still need to be probed. In this review article, we will examine the principal characteristics of microorganisms commonly found in cervico-vaginal specimens (i) the factors that notoriously condition the diversity and composition of microbiota, (ii) the role that some families of organisms may play in the onset of HPV-dysplastic lesions and in neoplastic progression, and (iii) possible diagnostic-therapeutic approaches.
Keywords: microbiota, cervical microbiota, cervical intraepithelial neoplasia, CIN, cervical cancer
1. Epidemiology of HPV
Papillomaviruses are widely distributed in mammals and are species-specific [1]. Human papillomavirus (HPV) cannot be cultivated in tissue cultures or in common experimental animals [1]. They are members of the Papillomaviridae family, have no coating, measure from 50 to 55 nm in diameter, and have an icosahedral capsid of 72 capsomers [2]. Of the approximately 200 genotypes of HPV, subdivided into 14 species, about 40 can infect the epithelial cells (skin or mucous membranes) of the anogenital regions and other areas [3]. Differentiation into types is made based on the characteristics of the L1 protein [4]. HPV was the first virus to be recognized as responsible for cervical cancer (CC). According to the degree of association with invasive tumors, HPV genotypes have been subdivided into: high oncogenic risk (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68), related with an increased risk of developing CC [5]; low oncogenic risk (6, 11, 40, 42, 43, 44, 54, 61, 70, 72, 81, 89) associated with no disease most commonly or with benign epithelial lesions (such as anogenital and oropharyngeal warts) [6]; and, finally, HPV with an “undetermined risk” (3, 7, 10, 27, 28, 29, 30, 32, 34, 55, 57, 62, 67, 69, 71, 74, 77, 83, 84, 85, 86, 87, 90, 91) include those whose oncogenicity has not yet been fully defined [7]. It has been established that about 99.8% of CC have a high-risk HPV DeoxyriboNucleic Acid (DNA) sequence, particularly HPV 16 and 18, found in about 70% of invasive carcinomas [8]. The prevalence of infection is very high (70% of sexually active female patients over 25 years old), and most infections tend to regress spontaneously, with or without manifestations of dysplasia; only in some cases can HPV infection become persistent [9]. Data from the scientific literature show that in female patients over 30 years old, persistent high-risk HPV infections play a critical role in predicting the risk of developing CC [8,9,10]. The risk of developing in histopathologic high-grade cervical intraepithelial lesion HSIL (CIN2 and CIN3) or invasive CC is estimated to be much higher in women with persistent high-risk HPV infection, being 11 times higher in the 30–44 age group, 35-fold higher between 45 and 54 years, and 49-fold in those over 50 years of age [11]. The ability of HPV viruses, especially those at high risk, to integrate into infected cells, and to orchestrate a gene expression program that allows the transcription of oncogenic proteins (E6, E7), promotes carcinogenicity [12]. Cervical intraepithelial lesions (CIN) can regress spontaneously, or progress to invasive neoplasia in different percentages depending on their severity. More specifically, histopathologic low-grade cervical intraepithelial lesion LSIL (CIN1) tends to regress spontaneously, particularly in young patients. Ostor and coworkers [13] report that CIN1 subsides spontaneously in 60% of cases, persists in 30%, and can progress in 10% of cases. On the other hand, CIN2 regresses spontaneously in 40%, persists in another 40% of cases, and can progress in 20% of cases; finally, CIN3 can regress in 33% of cases and progress in more than 12% of cases.
2. Screening and Histopathology of Cervical HPV Lesions
The natural history of CC is typically characterized by the progression, over the years, of non-invasive HPV-related precancerous lesions to invasive carcinoma [7,8,9,10,11,12,13]. Cytology (Pap-Test) and human papillomavirus detection (HPV-DNA) are two screening tests whose purpose is to detect CC or precancerous changes at an early stage. Before the development of the HPV-DNA test, the Pap-Test alone was performed every 3 years in women after the onset of sexual activity or in any case from 25 years of age [14]. Today, according to guidelines from different countries, HPV-DNA tests are used for women over 30 or over 25, and a Pap-Test is only done if it gives a positive result [15,16,17,18,19,20]. In fact, globally the recommended method of primary CC screening is the HPV-DNA test, independently of resource settings [21], due to its sensitivity compared to the Pap-Test, even in the presence of a lower specificity especially for the identification of CIN2 and CIN3 lesions. HPV testing is recommended by the World Health Organization [14] and other guidelines [15,22] even with respect to the Pap-Test which is now considered a secondary test [14]. In fact, the Pap-Test even if it has allowed to tangibly reduce the incidence of invasive carcinoma, however, presenting a variability between different operators, it can lead to the diagnosis of false negatives as well as cases of invasive CC are also reported in the literature in women regularly investigated with the Pap-Test [23]. In view of the crucial role of persistent infection of hr-HPV, the focus has shifted to the use of HPV-DNA testing as a screening test [24] so that access to treatment is increasingly targeted and timely. According to the ASCO guidelines, if the HPV-DNA test results positive, genotyping for HPV 16/18 (with or without HPV 45) and/or Pap-Test are also indicated [21]. In the event of a positive or abnormal result, the HPV-DNA test procedure involves colposcopy and related biopsy [21]. Conversely, in discordant results between the HPV test and cytological examination, it is recommended to repeat the HPV-DNA test one year later, then repeat the test at 12–24 months in case of negativity or colposcopy in women who tested positive [21]. Finally, in cases of CIN2 histological diagnosis, patients should be offered a surgical solution followed by targeted follow-up over time [21]. According to data from the randomized study published by Ronco et al. [25], HPV-research-based screening is more effective than the Pap-Test in preventing CC in women aged 25–60, because it allows an earlier identification of high-grade persistent lesions. In fact, the execution of the HPV-DNA test is useful in stratifying the population according to the degree of risk: a negative test indicates a low risk of developing CC, and so in these controls can be made at longer intervals. Although with differences between the various settings, the HPV-DNA test should be started from the age of 30 in the general female population, regular screening being done with the HPV test validated every 5–10 years, versus 25 years of age in women living with HIV, who should be screened more frequently, every 3–5 years. CC is the fourth most common malignancy among women worldwide, accounting for approximately 7% of all female cancers [26,27]. As reported in the literature, most of the diagnoses of CC can be associated with the presence of HPV infection and, in some studies, these associations can reach levels comparable to almost all cases [28]. Among the high-risk HPV genotypes, variant 16 has the highest affinity for neoplastic progression with over 50% of cases, followed by variant 18 which occurs in 20% of cases; this association tends to vanish in the remaining high-risk genotype up to 5% and even less [29]. Conversely, there does not appear to be a significant difference between the HPV status and the histotype of the carcinomas except for squamous-cell carcinoma (SCC) which is unlikely to be HPV-negative [30], as well as in mixed adeno-squamous form where the HPV positivity may reach up to 86% [31] of cases and also in the vast majority of Adenocarcinoma in situ (AIS) [30]. Vice versa, the prevalence of HPV among adenocarcinoma (AD) types can vary and, according to the International Endocervical Adenocarcinoma Criteria and Classification, ADs are divided in two categories: HPV-associated (HPVA) and not HPV-associated (NHPVA), with well-defined characteristics due to histology HPVA shows more mitotic activity or apoptotic figures than NHPVA. If focal or equivocal HPVA features are visible at ×200, a tumor can be classified as a “limited HPVA” and provisionally diagnosed as NHPVA AD [32]. To this histotype belong different variants such as mucinous (that can show aspects of HPVA and NHPVA), gastric (prevalent NHPVA type), endometrioid and serous carcinomas (extraordinarily rare). NHPVA comprehends histological variants [32] such as gastric, clear cell, serous, endometrioid, or mesonephric carcinomas that notoriously tend to be HPV negative [33] compared to histotypes presenting glandular/villo-glandular/intestinal aspects that appear to have a higher percentage of HPV positivity.
Principal Biomarkers for Cervical Cancer
The study of viral and cellular biomarkers that could be useful for identification of specific stages of cervical intraepithelial lesions related to hrHPV infection is closely linked to the biology of HPV and the different stages from infection through intraepithelial lesion, and, if this is not properly treated, to invasive CC. After hrHPV infection, the infected cervical epithelium begins to proliferate, leading to the transformation into CIN1, 2, and 3 [34,35,36,37]. Progression from a transient to a transformation HPV infection is characterized by a sharp increase in HPV mRNA E6/E7 and protein expression [34,37]. With this in mind, several authors have assessed how the detection of mRNA transcripts belonging to E6/E7 proteins could be helpful for identifying cervical precancers [36,37,38,39,40,41,42]. From these works, we can deduce a good sensitivity and specificity of these tests; there are two platforms used for the study of this biomarker (PreTect. Proofer and APTIMA GenProbe). The p16INK4a protein is a cyclin-dependent kinase inhibitor that plays a key role in cell cycle regulation and is upregulated when E7 is overexpressed as in HPV infections, representing an ideal biomarker to define the nature of cervical lesions [43,44], especially if combined in a single test together with Ki-67 [45,46] which is generally used in immunohistochemistry to evaluate the cell proliferation index [47]. Double-stained cytology p16/Ki-67 (approved by FDA on 3 October 2020 [48]) is a qualitative immunocytochemical assay intended for the simultaneous detection of proteins P16INK4a (clone E6H4) and Ki-67 (clone 274-11AC3V1) in cervical specimens of women aged 25–65 years with positive HPV test (high oncogenic risk); it is also indicated for patients aged between 30 and 65 who have to postpone colposcopy or have other risk factors regardless of the result of the HPV test [48]. It is highly expressed in almost 100% of cases of CIN2, CIN3, squamous CC, but is rarely found in benign forms; it is highly expressed in 100% of AIS cases. Several studies have highlighted the ability of the p16 test to identify the neoplastic transformation of cervical cells infected with human papillomavirus, being able to predict with greater precision the underlying cervical intraepithelial neoplasia of grade 3 or worse [49,50]. Specifically, the ATHENA study, on a group of 7727 patients, showed that p16/Ki-67 dual-stained cytology was significantly (p < 0.0001) more sensitive than Pap-Test (74.9% vs. 51.9%) for the triage of HPV-positive women and that specificity was comparable between the two methods [50]. In studies directed by Bergeron and Petry [46,51], as well as by Wright and coworkers, the authors described a p16/Ki-67 dual-stained cytology, that either alone or combined with HPV16/18 genotyping, represents a promising approach as a sensitive and efficient triage for colposcopy of HPV-positive women when primary HPV screening is utilized [50]. These studies led the way in using p16/ki-67 as a reliable tool for risk stratification of HPV-positive women with cervical lesions, reporting elevated sensitivity values similar to those of the HPV test [51,52,53,54,55]. Furthermore, other authors have shown that p16/ki-67 had a higher specificity than the HPV test [45,53,54,55]. These data reinforce the diagnostic value of p16/Ki-67, which represents a useful tool in avoiding further diagnostic investigations such as unnecessary colposcopies given its ability to more precisely identify female patients at increased CIN2 risk. In the field of research into serum biomarkers for the early detection of CC, the activation of Macrophage-Colony Stimulating Factor (M-CSF) and Vascular Endothelial Growth Factor (VEGF) is likely involved in the pathogenesis and spread of CC. In particular, M-CSF is overexpressed in the CC lines compared to CIN and the blocking of the M-CSF receptor determines both a growth arrest and an increase in intratumoral apoptosis [56,57]. In various papers, Lawicki and coworkers [38,58], using the immunosorbent assay (ELAISA), evaluated the plasma levels of M-CSF as compared to commonly accepted tumor markers, such as CA 125 and SCC-Ag in CC patients before surgery and in healthy subjects. The M-CSF plasma level was significantly higher, as also CA 125 and SCC-Ag, in CC patients. The diagnostic sensitivity of M-CSF was higher than stem cell factor, CA 125, and SCC-Ag (25%, 30%, 40%, respectively). The diagnostic specificity was high and equal for all tested cytokines and CA 125 (92%). Positive and negative predictive values were higher for all tested parameters but highest for M-CSF (83% and 69.7%, respectively) [38]. More recent studies [59,60,61,62] confirmed that cytokine members of hematopoietic growth factors may have a diagnostic potential in CC. Zajkowska evaluated serological markers against CA 125 and SCC-Ag in 100 CC patients with chemiluminescent microparticle immunoassay and showed statistically significant M-CSF values from all parameters tested in the CC cohort compared to the control groups [59]. Similar results have also been described by Lubowicka et al. who investigated M-CSF, matrix metalloproteinase-2, and its inhibitor beyond CA 125 and SCC-Ag in 89 CC patients and in 50 healthy women (aged 22–61 years) reporting for M-CSF the highest specificity (86%) in the CC group [60]. Moreover, the association of these different markers increased in specificity, as observed in the combination between matrix metalloproteinase-2 and CA 125 in different CC stages [60]. While median levels of M-CSF and VEGF, as well as CA 125 and SCC-Ag are shown to be significantly different in women with CC, this relationship does not seem to be specific to SCC alone; in fact, the plasma levels of M-CSF and VEGF are also higher in AD than in the control group and, moreover, no significant differences were observed between SCC and AD [61]. Sidorkiewicz reported 81% sensitivity and 74% specificity for VEGF in the SCC group and 86% and 76% in the AD group [61]. Although the results of these studies are encouraging and suggest a diagnostic utility and a possible clinical applicability of serum biomarkers in patients with CC, to date they are not yet sufficiently studied, and more confirmations would be useful for their wider use than p16/Ki67 testing and mRNA detection.
3. Diversity and Influencing Factors of Vaginal Microbiota
The vaginal microbiota (VM) is characterized by a heterogeneous variety of microorganisms that are commonly found in cervicovaginal samples of female patients in both pathological and non-pathological conditions. Based on species composition and the relative abundance of bacterial population present in genital mucosae of 396 healthy women [40], using next-generation sequencing platforms, Ravel et al. analyzed and classified the microbiota in five clusters, also known as community state types (CSTs) [41]. In CST I, II, III, and V, Lactobacillus (L.) crispatus, L. gasseri, L. iners, and L. jensenii are more common, respectively, unlike CST IV, which features a high bacterial diversity including several anaerobic species such as Gardnerella, Megasphera, Atopobium, Prevotella, besides Pseudomonas, Shigella, Peptostreptococcus, Enterococcus, Streptococcus, Propionibacterium, Bifidobacterium, and Brevibacterium species [42]. The attempt to classify vaginal microorganisms in order to establish the role of VM in immune protection, inflammatory processes, and in cancer genesis is still ongoing today, owing to the different factors that notoriously influence the diversity and composition of VM [63,64]. The diversity of vaginal microbiota has a great geographic and ethnic variability [65,66] showing a greater variety in African women. Again, Lactobacillus species are significantly more frequent in Caucasian and Asian populations compared to Hispanic areas [41]. These differences, consisting of high VM diversity and variable abundances of Lactobacillus, called dysbiosis, may be due to hygiene practices, social, metabolic, and immune factors that influence vaginal homeostasis causing bacterial vaginosis (BV), with or without symptoms [67,68,69]. In this regard L. iners, that is unable to produce D-lactic acid and H2O2, seems to favor dysbiosis [70,71], although generally species of Lactobacillus produce lactic acid, hydrogen peroxide, and antimicrobial peptides, such as bacteriocins and biosurfactants, that inhibit the growth of bacteria and viruses [42,71,72], and regulate vaginal homeostasis. Vice versa, L. iners produces L-lactic acid and inerolysin (a pore-forming toxin cytolysin capable, like Gardnerella-released vaginosis, of prejudicing the integrity of the vaginal epithelium); both of these promote pathogenic proliferations and infections [70,73,74]. Although hormonal endogenous agents have a rather small impact on cervical and vaginal cancers’ development, female hormones and reproductive factors can still induce diverse female malignancies [75,76,77] and are one of the most important endogenous agents in the composition of the VM. In fact, estrogen receptors are widely expressed in the endometrium, where they regulate the growth and proliferation of epithelial cells and condition the stability of VM; estrogen-related factors such as menstruation, sexual activity [78], oral contraceptive use, pregnancy and lactation, or diseases such as diabetes mellitus and stress, cause hormonal fluctuations that influence the biological presence and the abundance of a bacterial community [71]. Age is clearly a crucial factor in relation to the action of the mechanisms underlying endocrine stimulation: low estrogen levels after birth cause a reduction in vaginal Lactobacillus and an increase in anaerobic populations until puberty [42], when there is an increase in estrogen and glycogen, further processed in lactic acid by Lactobacillus species [71,79]. During the reproductive age, the cyclic secretion of female hormones determines a great instability, especially during the menstrual phase when both the low levels of estrogen and progesterone, and the presence of menstrual blood, are linked to the decrease in some microorganisms and the enrichment of others [79,80]. Instead, high levels of estrogen and progesterone are a favorable factor for the stability of the VM during the female fertile age [79]. On the other hand, as described by David A MacIntyre et al. [81], a 100–1000-fold decrease in circulating estrogen concentrations postpartum triggers a significant increase in VM diversity. This condition also occurs in the menopausal state, when the sharp decrease in the production of female hormones causes atrophy of the vaginal epithelium and a relative absence of glycogen and Lactobacillus species [82,83,84]. Furthermore, exogenous factors can also influence the composition of the vaginal microbiota: the use of oral contraceptives is associated with an increased level of inflammatory cytokines in the cervix [85]. In fact, it has been shown that the use of oral contraceptives is associated with the risk of CC [86], even apart from the role played by the inflammatory microenvironment in influencing cell proliferation in different models of solid tumors [77,87,88,89,90]. In a meta-analysis conducted on the use of both combined hormonal contraceptives and progesterone-only hormonal contraceptives, Lenka A Vodstrcil et al. showed that, in general, they are equivalent, reducing recurrent BV by 31 and 32%, respectively [91]. These results are also reinforced by other studies showing the propensity of oral contraceptives to favor lactobacillus species [91,92,93], unlike contraceptives such as the intrauterine device and medroxyprogesterone acetate, which do not appear to affect the composition of the VM [94]. Furthermore, as previously mentioned, other exogenous factors seem to influence the biodiversity of the VM: in fact, in smoker women there is a greater diversity of species characterizing the VM, with a substantial reduction in L. crispatus [95] and an increase in species belonging to CST IV; Peptostreptococcus and Veillonella have been identified as most closely related to the use of tobacco [95]. Finally, even vaginal douches can affect not only the composition of the VM [96,97,98] but also the risk of related HPV infections or HPV-associated cervical diseases [98,99]. In fact, literature reports described a high production of proinflammatory cytokines in women with CST IV, which increased the recruitment of activated CD4+CCR5+ cells to the vaginal mucosa, increasing the risk of damage to the epithelial barrier and promoting HPV infection [42].
4. The Association of Cervical Microbiota with the Risk of CIN
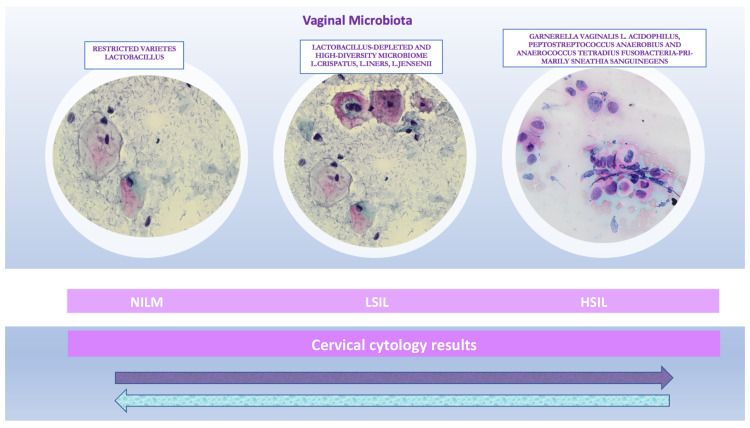
The human body is an extraordinary ecosystem constituted by trillions of microorganisms. The coevolution that occurred between man and microbes formed a complex communication network [100,101]. Most microorganisms are bacteria that live in the gut and have a strong impact on the health of their hosts [102]. Joshua Lederberg summed up this concept in 2001, when he coined the term “microbiota” referring to the community of commensal, symbiotic, and pathogenic microorganisms which share the same space and exert diversified interactions with the specific human tissue compartments where they are hosted [103]. In 1977, Carl R. Woese and George E. Fox had reported the first study utilizing the 16S rRNA gene to recognize these bacteria, and demonstrated that the gene could be used to detect microorganisms on the basis of molecular phylogeny [104]. This approach was innovative in the biology field because previously this gene had been largely used only for bacteria identification. In the literature, it has recently been shown that the microbiota can play a crucial role in cancer progression, by affecting the host immunity. The influence of the microbiota on HPV-induced gynecological neoplasms remains poorly understood. Therefore, several studies have focused on the VM since together with immune regulation, it could play an important role in HPV carcinogenesis [105]. The cervical microenvironment is complex, constituted by immune cells and the specific microbiota that regulate local immune responses [106]. Indeed, the immunologic status of the host and HPV-induced immune evasion could explain persistent HPV infection, but alone, these factors are not sufficient to determine cancer development [107]. In fact, not all HPV-infected patients develop CC since the immune system together with other additional factors influence the progression of cervical intraepithelial lesions to CC or else regression [108,109,110]. Therefore, several researchers explored the relationship between the VM and HPV infection, as the microbiota would seem to play a decisive role in neoplastic evolution [42,111,112]. Recently, studying virus–bacteria–host interaction models, two models were evaluated with the aim of better understanding the microbiota mechanisms in the development of virus-associated cancers [113]. The first model relied on the concept that the microbiota could affect viral infectivity through the release of bioproducts that could modulate virus–host interactions. The second model was based on the concept that bacteria–host interactions impact the host gene expression, increasing viral production and promoting tumorigenesis associated with the viral infection [114]. One of these studies characterized the microbiota in a population with HPV infection, using laboratory culture, and reported on the presence of ‘abnormal vaginal flora’ (that subsequent investigations would then identify using 16S rRNA analysis) [115,116]. Based on the microbiota composition, the microenvironment could also protect the host from viral infections. It is known that the main defense mechanisms of the cervicovaginal mucosa are antimicrobial peptides, microbiota dominated by lactobacilli and a pH of less than 4.5. An alteration of these protection mechanisms can result in physiochemical modifications that damage the cervical epithelium and vaginal mucosa [117]. Particularly, a reduction in lactic-acid-producing Lactobacilli, with a consequent increase in vaginal pH (4.5), can induce atypical bacterial growth and a decrease in protective flora [118], weakening the endogenous system of defense against viral infection. The most common type of cervicovaginal dysbiosis is BV, defined as a VM with scarce Lactobacillus, and an increase in anaerobes [119]. BV is associated with an increase in the levels of proinflammatory cytokines such as IL-1b and a decrease in the levels of the anti-inflammatory molecule, secretory leukocyte protease inhibitor, supporting the theory that BV causes changes in the immune system that can lead to a greater susceptibility to HPV and hence the development of high-grade cervical dysplasia [120]. Particularly, the authors also studied the oxidative DNA damage related to the natural history of HPV persistence and cervical carcinogenesis. BV-associated oxidative stress [121] could be involved in the generation of reactive oxygen species that could generate double-stranded DNA breaks in the host genome, as well as the HPV episome, accelerating HPV integration and neoplastic transformation, a mechanism also employed by the HPV E6 oncoprotein [122]. The integration produces a loss of E1 and E2 genes, which regulate E6 and E7 transcription. Subsequently, transcription of these oncoproteins goes unchecked after viral integration, leading to a greater cellular proliferation and a reduction in apoptosis [123]. Several studies in the literature support this evidence. Particularly, Mitra et al. assessed a group of 169 patients referred for colposcopy, who showed an increased bacterial diversity connected with diminished lactobacilli related to the gravity of the cytological lesion [124]. Oh et al. also highlighted the finding that the risk of L-SIL in HPV-positive patients with a high-risk microbial pattern was suggestively higher than in HPV-negative women with a low-risk microbial score (or: 34.1, 95% CI: 4.95–284.5). Therefore, the Lactobacillus genus (first described in 1892 by Döderlein) is usually abundant in the cervicovaginal microbiota [125]. Lactobacillus species such as L. crispatus, L. gasseri, and L. jensenii could generate lactic acid and hydrogen peroxide (H2O2), which limit the progression of viral and bacterial infections [71] (Figure 1).
Figure 1.
Cervical cytology results and corresponding cervical microbiota. The illustration is a summary chart of the uterine cervix microbiota community composition in physiological and precancerous stages, showing how it can influence both intraepithelial lesions progression and the stable condition of the cervical epithelium (cytological images at high magnification 40×). The arrows indicate the reversibility of the cervical changes: precancerous stages versus negative for intraepithelial lesion or malignancy (NILM).
Therefore, the microbiota is the first line of defense against infections. The second line is its composition, since it can produce lactic acid and H2O2, that have a defensive role against viral and bacterial infections [70]. On the contrary, L. iners was evaluated as a transitional species leading to the dysbiosis state [126]. Cross-sectional studies show a negative relationship between HPV infection and CIN with Lactobacillus dominance, except for L. iners, which shows the opposite tendency, being correlated with a higher frequency of HIV, HPV, and HSV-2 [126]. Therefore, in most HPV-infected women, the immune response can limit the infection, preventing high-grade lesions and tumors [127]. On the other hand, some dysbiotic bacterial communities seem to cause immune dysregulation, favoring a tumor-promoting microenvironment [128], and may play an important role in CC progression [129]. Indeed L. iners are frequently detected in patients diagnosed with CIN [130]. However, the role of this bacterium in cervicovaginal health is still uncertain, since it can be found in normal environments as well as in states of vaginal dysbiosis [131]. In fact, it has been demonstrated that L. iners has more complex nutritional needs and a more variable morphology than other Lactobacillus species. It has an unusually small genome, suggestive of a symbiotic or parasitic lifestyle [74]. Actually, L. iners may have clonal variants that in some cases promote health and in other cases are associated with dysbiosis and a predisposition to disease [74]. Moreover, literature reports suggest that the VM is different in female patients of diverse ethnicities and such variety may be associated to genetic differences among races including a few mitochondrial DNA haplotypes [131]. Consequently, distinguishing the VM in female patients of each race could justify the differing incidence of BV and sexually transmitted infections among diverse ethnicities and reveal the importance of genetic factors in influencing the VM of some individuals, making them prone to illnesses [130]. Female patients with dysbiosis can develop chronic inflammation, which may be an important factor for cancer development in different tissue types, including cervical tissue [42]. Moreover, in CST IV, there is also Gardnerella vaginalis, which produces sialidase that degrades the vaginal mucosal surface posing a physical barrier able to inhibit bacteria–host interactions [67,132]. Gao and coworkers tested 70 healthy women (32 HPV-negative and 38 HPV-positive) with normal cervical cytology and, using the Shannon–Weiner diversity index, they discovered that HPV-positive female patients had a greater biological diversity [91]. The major CSTs found in female patients with CIN were CSTs, characterized by Lactobacillus depletion, anaerobic bacteria predominance, and L. iners dominance [133]. On the other hand, L. crispatus was the predominant Lactobacillus species in Italian female patients, who were better able to clear HPV infection [133]. Indeed, Kwasniewski et al. [134] reported a study of the vaginal flora of 250 female patients, including 85 women with high-grade SIL and HPV positivity, 95 female patients with low-grade SIL and HPV positivity, and 70 healthy controls. In the control group, high levels of L. crispatus, L. iners, and L. taiwanensis and an absence of Gardnerella vaginalis and L. acidophilus were identified. In the low-grade-SIL group, L. crispatus was less numerous than in the control group and L. acidophilus and L. iners prevailed. In the high-grade-SIL group, Gardnerella vaginalis and L. acidophilus were prevalent, while the frequencies of L. iners, L. crispatus, and L. taiwanensis were lower than in the control group. Therefore, these results show a possible relationship between the VM, HPV infection, and CIN development. In particular, a microbiota dominated by Gardnerella vaginalis and with reduced L. iners, L. crispatus, and L. taiwanensis may play a role in HPV persistence, CIN development, and CC [134]. Additionally, Oh et al. [125] reported a cytology study on women with LSIL or HSIL vs. normal controls. The outcomes suggested that a paucity of L. crispatus and a predominance of A. vaginae and secondarily of Gardnerella vaginalis, and L. iners were linked to an almost 6-fold increase of the risk of cervical LSIL/HSIL disease (odds ratio (OR): 5.80, 95% CI: 1.73–19.4). The study also reported that the risk of SIL in HPV-positive women with a high-risk microbial pattern was significantly higher than in HPV-negative women with a low-risk microbial score (OR: 34.1, 95% CI: 4.95–284.5). These results were limited since risk factors were not evaluated, and the comparison groups combined different grades of disease severity [125]. Specific bacteria, such as Gardnerella, may be identified as biomarkers of cervical alterations to detect female patients with a high risk of developing persistent HPV infection/CIN and progression to cancer, while not all Lactobacillus species are uniformly protective [72]. On the other hand, in female patients with CC a prevalence of Fusobacterium species and a decreased abundance of Lactobacillus species were described. Both in women with CIN and in patients with CC, the VM was similar to that in female patients with BV [135]. Particularly, a Fusobacterium predominance was more commonly detected in CC patients and was shown to be correlated with a cytokine pattern of increased levels of IL-4 and TGF-b1 mRNA, suggesting a local immunosuppression state and supporting the concept of microbiota immunity [135]. BV is related to higher HPV infection rates, so an increase in the diversity of vaginal bacteria together with a decrease in Lactobacillus may be involved in the persistence of HPV infection [136]. Therefore, 16S-HTS and other methods, for example, molecular diagnostic tests such as direct probe assays and real-time PCR, can be used to analyze HPV+ women with a potential risk of developing cervical lesions or viral persistence [136]. These women may have an increased risk of persistence/progression of HPV-related lesions, so the results of such tests should impact diagnostic and therapeutic management. Another article by Piyathilake et al. correlated well-defined cytological groups of women with HSIL vs. LSIL in patients with high-risk HPV-positive infection [137]. In this study, the CST was not used to classify patients according to the VM structure but the Dirichlet multinomial mixture model was applied to partition the samples into four diverse metacommunities (partitions 1–4). Bacterial communities featuring principally L. iners and unclassified Lactobacillus species (partition 3) had a higher HSIL 1 level as compared to those with diverse-taxa-unclassified Lactobacillus, L. iners, Allobaculum, Clostridiales, and Bifidobacteriaceae (partition 1; OR 5 3.48, 95% CI: 1.27–9.55) [138].
5. Future Prospects
Despite the many studies conducted on the relationship between the microbiota and host immunity, the knowledge of the specific influence of HPV on gynecologic tumors remains limited [93]. Moreover, study of the microbiota and CC would need to be undertaken in large population samples in order to predict the development of precancerous lesions, since the progression of HPV infection to CC takes decades, and in many individuals never progresses to cancer at all [139]. Therefore, long-term longitudinal studies could allow the determination of early changes in the VM, that may help to evaluate the progression of precancerous lesions [93]. Furthermore, the novel advances in microbiota sequencing and sophisticated bioinformatics technologies have supported rapid progress in our understanding of the gut microbiota and the development of tumors [139,140]. Other research, using shotgun metagenomic sequencing, demonstrated that the VM community compositions and metagenomic profiles differed between patients with CC and individuals without cancer. Consequently, researchers understood that larger additional whole-genome shotgun studies are necessary to verify these relations [93]. Research into the VM can be further enhanced using metagenomic sequencing, rather than 16S (that sequences a specific 30S ribosome subunit of the human microbiota that is unique to prokaryotes and has regions that vary significantly between diverse species of bacteria and clusters the identified bacteria into operational taxonomic units) [121,122,141] or other targeted sequencing techniques, since these lack depth. Indeed, 16S amplification does not include microbes that lack a gene to match the primers (such as viruses, archaea, and eukaryotes). Since common medium- or large-scale VM analyses employed this technique, the role of non-bacterial constituents of the VM in HPV infection and disease has not yet been described. Therefore, proteomic analysis could be key to a more complete understanding of the VM and its influence on disease in the metabolic context [142,143]. Only after the pathogenic mechanisms of interaction between microbiota and HPV have been fully understood will it be possible to identify the most effective therapeutic strategy. Indeed, the execution of microbiota analysis in clinical practice in HPV-positive patients should make it possible to identify women at high risk of progression/persistence [120,121]. Consequently this “high risk” group could be candidates for cervical biopsy (for diagnostic confirmation), more restricted follow-up, and genotyping [144,145]. In addition, a more complete knowledge of the microbiota would permit the use of a targeted, personalized therapeutic approach using antibiotics and probiotics. Indeed, in women with BV, for example, there is a high diversity microbiota, and the usual cure is to prescribe antibiotics such as metronidazole and clindamycin. However, these antibiotics could prevent cervicovaginal recolonization by Lactobacillus species and could therefore, in turn, lead to relapse [144]. Indeed, high rates of BV relapse are already predictable after oral treatment with metronidazole [144]. Moreover, antibiotic therapy features both side effects [146,147,148] and a lack of efficacy due to resistant strains [149]. On the other hand, based on in vitro assays, L. crispatus LbV 88, L. gasseri LbV 150N, L. jensenii LbV [149], and L. rhamnosus LbV96 strains, were selected as relevant for vaginal health [150]. Consequently, a pilot clinical trial was performed in which a yoghurt preparation containing those beneficial microbes was administered to BV-diagnosed patients together with metronidazole. The study revealed that the group receiving probiotics had a considerably improved recovery rate from BV as compared to women treated only with antibiotics [151]. Likewise, in the literature, administration of probiotics is also helpful in vivo and in vitro to achieve a major CIN regression and total HPV clearance. In fact, the administration of probiotics for the manipulation of the microbiota may be a feasible possibility to induce HPV infection clearance and prevent progression to CC. Indeed, the authors revealed that the group receiving long-term probiotic treatment (6 months) showed not only a significantly higher chance of resolving cytological abnormalities but also presented increased rates of HPV clearance as compared to the group receiving short-term probiotic therapy (3 months) [152,153]. Although further clinical trials are required to elucidate this relationship in patients with high-risk microbiota, the concomitant intake of antibiotics and probiotics could lead to an effective impact on the microbiota, avoiding the persistence of HPV-related lesions and progression to CC [151]. Instead, the literature on the impact of vaccination on the microbiota is still limited. Particularly, a recent small Phase I study was performed to test whether the VM could influence vaccine responses and VM composition in women with biopsy-proven high-grade squamous intraepithelial lesions. There was no difference in bacterial diversity, but a significant increase in circulating T-helper type 1 cells, and a significant decrease in the HPV 16 viral load were reported. Further studies are needed to examine the role of the VM in response to HPV therapeutic vaccines [154]. Much evidence is available related to the microbiota, but there are still many shadows that require more sophisticated tools to gain a more prolonged insight. In particular, study of the microbiota with metagenomics may be the future roadmap, but it is also important to emphasize that these techniques are expensive and require specific funds for such research. This review of the literature was written to illustrate how a better understanding of the microbiota could be the key to personalized and specific management of cervical precancers, so even if the road is long, it may be worth investing in this direction.
Acknowledgments
Not applicable.
Author Contributions
Conceptualization, E.C. (Eliano Cascardi), G.C. (Gerardo Cazzato) and M.D.; literature review, E.C. (Eliano Cascardi), G.C. (Gerardo Cazzato), A.D., V.L., E.S. and M.D.; writing—original draft preparation, E.C. (Eliano Cascardi), G.C. (Gennaro Cormio) and M.D.; writing—review and editing, E.C. (Eliano Cascardi), G.C. (Gerardo Cazzato), G.C. (Gennaro Cormio), M.D., E.C. (Ettore Cicinelli), C.M., G.I., E.M., L.R., G.D.V., A.M., V.P. and S.S.; funding acquisition, A.D., E.S. and C.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was funded by the project financed by the Italian Ministero dello Sviluppo Economico (MISE, Ministry of Economic Development) “GENESI” code 092- Sviluppo di radiofarmaci e biomarker innovativi per la diagnosi dei tumori dell’apparato riproduttivo maschile e femminile (Development of innovative radiopharmaceuticals and biomarkers for the diagnosis of tumours of male and female reproductive system) (2021–2023).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brianti P., De Flammineis E., Mercuri S.R. Review of HPV-related diseases and cancers. New Microbiol. 2017;40:80–85. [PubMed] [Google Scholar]
- 2.Soheili M., Keyvani H., Soheili M., Nasseri S. Human papilloma virus: A review study of epidemiology, carcinogenesis, diagnostic methods, and treatment of all HPV-related cancers. Med. J. Islamic Repub. Iran. 2021;35:65. doi: 10.47176/mjiri.35.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schneider A., Koutsky L.A. Natural history and epidemiological features of genital HPV infection. IARC Sci. Publ. 1992;119:25–52. [PubMed] [Google Scholar]
- 4.McBride A.A. Oncogenic human papillomaviruses. Philos. Trans. R. Soc. B Biol. Sci. 2017;372:20160273. doi: 10.1098/rstb.2016.0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salambanga C., Zohoncon T.M., Traore I.M.A., Ouedraogo R.A., Djigma W.F., Ouedraog C., Simpore J. High prevalence of high-risk human papillomavirus (HPV) infection among sexually active women in Ouagadougou. Med. Sante Trop. 2019;29:302–305. doi: 10.1684/mst.2019.0920. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen A., Iftner T., Norgaard M., Munk C., Junge J., Kjaer S.K. The importance of low-risk HPV infection for the risk of abnormal cervical cytology/histology in more than 40,000 Danish women. Sex. Transm. Infect. 2012;88:627–632. doi: 10.1136/sextrans-2011-050307. [DOI] [PubMed] [Google Scholar]
- 7.Morandell D., Rostek U., Bouvard V., Campo-Fernandez B., Fiedler M., Jansen-Durr P., Zwerschke W. Human papillomavirus type 45 E7 is a transforming protein inducing retinoblastoma protein degradation and anchorage-independent cell cycle progression. Virology. 2008;379:20–29. doi: 10.1016/j.virol.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 8.de Sanjose S., Brotons M., Pavon M.A. The natural history of human papillomavirus infection. Best Pract. Res. Clin. Obstet. Gynaecol. 2018;47:2–13. doi: 10.1016/j.bpobgyn.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 9.Bosch F.X., de Sanjose S. Chapter 1: Human papillomavirus and cervical cancer--burden and assessment of causality. J. Natl. Cancer Inst. Monogr. 2003;2003:3–13. doi: 10.1093/oxfordjournals.jncimonographs.a003479. [DOI] [PubMed] [Google Scholar]
- 10.Munoz N., Bosch F.X., de Sanjose S., Herrero R., Castellsague X., Shah K.V., Snijders P.J., Meijer C.J. International Agency for Research on Cancer Multicenter Cervical Cancer Study, G. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Engl. J. Med. 2003;348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 11.Chen H.C., Schiffman M., Lin C.Y., Pan M.H., You S.L., Chuang L.C., Hsieh C.Y., Liaw K.L., Hsing A.W., Chen C.J., et al. Persistence of type-specific human papillomavirus infection and increased long-term risk of cervical cancer. J. Natl. Cancer Inst. 2011;103:1387–1396. doi: 10.1093/jnci/djr283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mbuya W., Held K., McHaro R.D., Haule A., Mhizde J., Mnkai J., Mahenge A., Mwakatima M., Sembo M., Mwalongo W., et al. Depletion of Human Papilloma Virus E6- and E7-Oncoprotein-Specific T-Cell Responses in Women Living With HIV. Front. Immunol. 2021;12:742861. doi: 10.3389/fimmu.2021.742861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ostor A.G. Natural history of cervical intraepithelial neoplasia: A critical review. Int. J. Gynecol. Pathol. 1993;12:186–192. doi: 10.1097/00004347-199304000-00018. [DOI] [PubMed] [Google Scholar]
- 14.WHO New Recommendations for Screening and Treatment to Prevent Cervical Cancer. 2021. [(accessed on 6 July 2021)]. Available online: https://www.who.int/news/item/06-07-2021-new-recommendations-for-screening-and-treatment-to-prevent-cervical-cancer.
- 15.Fontham E.T., Wolf A.M., Church T.R., Etzioni R., Flowers C.R., Herzig A., Guerra C.E., Oeffinger K.C., Shih Y.C.T., Walter L.C. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA A Cancer J. Clin. 2020;70:321–346. doi: 10.3322/caac.21628. [DOI] [PubMed] [Google Scholar]
- 16.Obermair H.M., Bennett K.F., Brotherton J.M., Smith M.A., McCaffery K.J., Dodd R.H. Australian National Cervical Screening Program renewal: Attitudes and experiences of general practitioners, and obstetricians and gynaecologists. Aust. N. Z. J. Obstet. Gynaecol. 2021;61:416–423. doi: 10.1111/ajo.13310. [DOI] [PubMed] [Google Scholar]
- 17.Brown R.F., Muller T.R., Olsen A. Australian women’s cervical cancer screening attendance as a function of screening barriers and facilitators. Soc. Sci. Med. 2019;220:396–402. doi: 10.1016/j.socscimed.2018.11.038. [DOI] [PubMed] [Google Scholar]
- 18.Gu C., Chan C.W., Chow K.M., Yang S., Luo Y., Cheng H., Wang H. Understanding the cervical screening behaviour of Chinese women: The role of health care system and health professions. Appl. Nurs. Res. 2018;39:58–64. doi: 10.1016/j.apnr.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Schiffman M., Wentzensen N. A Suggested Approach to Simplify and Improve Cervical Screening in the United States. J. Low. Genit. Tract Dis. 2016;20:1–7. doi: 10.1097/LGT.0000000000000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Karsa L., Arbyn M., De Vuyst H., Dillner J., Dillner L., Franceschi S., Patnick J., Ronco G., Segnan N., Suonio E. European guidelines for quality assurance in cervical cancer screening. Summary of the supplements on HPV screening and vaccination. Papillomavirus Res. 2015;1:22–31. doi: 10.1016/j.pvr.2015.06.006. [DOI] [Google Scholar]
- 21.ASCO Secondary Prevention of Cervical Cancer: American Society of Clinical Oncology Resource-Stratified Clinical Practice Guideline. 2016. [(accessed on 1 January 2016)]. Available online: https://www.asco.org/sites/new-www.asco.org/files/content-files/practice-and-guidelines/documents/2016-rs-secondary-prev-cervical-slides2.pdf.
- 22.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 23.Nayar R., Wilbur D.C. The Pap test and Bethesda 2014. Cancer Cytopathol. 2015;123:271–281. doi: 10.1002/cncy.21521. [DOI] [PubMed] [Google Scholar]
- 24.Tsikouras P., Zervoudis S., Manav B., Tomara E., Iatrakis G., Romanidis C., Bothou A., Galazios G. Cervical cancer: Screening, diagnosis and staging. J. BUON. 2016;21:320–325. [PubMed] [Google Scholar]
- 25.Ronco G., Giorgi-Rossi P., Carozzi F., Confortini M., Dalla Palma P., Del Mistro A., Ghiringhello B., Girlando S., Gillio-Tos A., De Marco L., et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: A randomised controlled trial. Lancet Oncol. 2010;11:249–257. doi: 10.1016/S1470-2045(09)70360-2. [DOI] [PubMed] [Google Scholar]
- 26.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J. Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 27.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 28.Monsonego J. Global challenges of cervical cancer prevention. Eur. J. Gynaec. Oncol.-IssN. 2000;392:2936. [PubMed] [Google Scholar]
- 29.De Martel C., Plummer M., Vignat J., Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer. 2017;141:664–670. doi: 10.1002/ijc.30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pirog E.C. Cervical adenocarcinoma: Diagnosis of human papillomavirus–positive and human papillomavirus–negative tumors. Arch. Pathol. Lab. Med. 2017;141:1653–1667. doi: 10.5858/arpa.2016-0356-RA. [DOI] [PubMed] [Google Scholar]
- 31.Holl K., Nowakowski A.M., Powell N., McCluggage W.G., Pirog E.C., Collas De Souza S., Tjalma W.A., Rosenlund M., Fiander A., Castro Sánchez M. Human papillomavirus prevalence and type-distribution in cervical glandular neoplasias: Results from a E uropean multinational epidemiological study. Int. J. Cancer. 2015;137:2858–2868. doi: 10.1002/ijc.29651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stolnicu S., Barsan I., Hoang L., Patel P., Terinte C., Pesci A., Aviel-Ronen S., Kiyokawa T., Alvarado-Cabrero I., Pike M.C. International Endocervical Adenocarcinoma Criteria and Classification (IECC): A new pathogenetic classification for invasive adenocarcinomas of the endocervix. Am. J. Surg. Pathol. 2018;42:214. doi: 10.1097/PAS.0000000000000986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tjalma W. HPV negative cervical cancers and primary HPV screening. Facts Views Vis. ObGyn. 2018;10:107. [PMC free article] [PubMed] [Google Scholar]
- 34.Szarewski A., Ambroisine L., Cadman L., Austin J., Ho L., Terry G., Liddle S., Dina R., McCarthy J., Buckley H. Comparison of predictors for high-grade cervical intraepithelial neoplasia in women with abnormal smears. Cancer Epidemiol. Prev. Biomark. 2008;17:3033–3042. doi: 10.1158/1055-9965.EPI-08-0508. [DOI] [PubMed] [Google Scholar]
- 35.Mayrand M.-H., Franco E.L. Integrating novel primary-and secondary-prevention strategies: The next challenge for cervical cancer control. Future Oncol. 2010;6:1725–1733. doi: 10.2217/fon.10.141. [DOI] [PubMed] [Google Scholar]
- 36.Wentzensen N., von Knebel Doeberitz M. Biomarkers in cervical cancer screening. Dis. Markers. 2007;23:315–330. doi: 10.1155/2007/678793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cuschieri K., Wentzensen N. Human papillomavirus mRNA and p16 detection as biomarkers for the improved diagnosis of cervical neoplasia. Cancer Epidemiol. Prev. Biomark. 2008;17:2536–2545. doi: 10.1158/1055-9965.EPI-08-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lawicki S., Bedkowska E., Gacuta-Szumarska E., Knapp P., Szmitkowski M. The plasma levels and diagnostic utility of stem cell factor (SCF) and macrophage-colony stimulating factor (M-CSF) in cervical cancer patients. Pol. Merkur. Lek. 2008;25:38–42. [PubMed] [Google Scholar]
- 39.Murphy N., Ring M., Heffron C.C., King B., Killalea A.G., Hughes C., Martin C.M., McGuinness E., Sheils O., O’Leary J.J. p16INK4A, CDC6, and MCM5: Predictive biomarkers in cervical preinvasive neoplasia and cervical cancer. J. Clin. Pathol. 2005;58:525–534. doi: 10.1136/jcp.2004.018895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gajer P., Brotman R.M., Bai G., Sakamoto J., Schutte U.M., Zhong X., Koenig S.S., Fu L., Ma Z.S., Zhou X., et al. Temporal dynamics of the human vaginal microbiota. Sci. Transl. Med. 2012;4:132ra152. doi: 10.1126/scitranslmed.3003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ravel J., Gajer P., Abdo Z., Schneider G.M., Koenig S.S., McCulle S.L., Karlebach S., Gorle R., Russell J., Tacket C.O., et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA. 2011;108((Suppl. S1)):4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitra A., MacIntyre D.A., Marchesi J.R., Lee Y.S., Bennett P.R., Kyrgiou M. The vaginal microbiota, human papillomavirus infection and cervical intraepithelial neoplasia: What do we know and where are we going next? Microbiome. 2016;4:58. doi: 10.1186/s40168-016-0203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalof A.N., Cooper K. p16INK4a immunoexpression: Surrogate marker of high-risk HPV and high-grade cervical intraepithelial neoplasia. Adv. Anat. Pathol. 2006;13:190–194. doi: 10.1097/00125480-200607000-00006. [DOI] [PubMed] [Google Scholar]
- 44.Trunk M.J., Dallenbach-Hellweg G., Ridder R., Petry K.U., Ikenberg H., Schneider V., von Knebel Doeberitz M. Morphologic characteristics of p16INK4a-positive cells in cervical cytology samples. Acta Cytol. 2004;48:771–782. doi: 10.1159/000326445. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt D., Bergeron C., Denton K.J., Ridder R., European C.C.S.G. p16/ki-67 dual-stain cytology in the triage of ASCUS and LSIL papanicolaou cytology: Results from the European equivocal or mildly abnormal Papanicolaou cytology study. Cancer Cytopathol. 2011;119:158–166. doi: 10.1002/cncy.20140. [DOI] [PubMed] [Google Scholar]
- 46.Bergeron C., Ikenberg H., Sideri M., Denton K., Bogers J., Schmidt D., Alameda F., Keller T., Rehm S., Ridder R., et al. Prospective evaluation of p16/Ki-67 dual-stained cytology for managing women with abnormal Papanicolaou cytology: PALMS study results. Cancer Cytopathol. 2015;123:373–381. doi: 10.1002/cncy.21542. [DOI] [PubMed] [Google Scholar]
- 47.Kruse A.J., Baak J.P., de Bruin P.C., Jiwa M., Snijders W.P., Boodt P.J., Fons G., Houben P.W., The H.S. Ki-67 immunoquantitation in cervical intraepithelial neoplasia (CIN): A sensitive marker for grading. J. Pathol. 2001;193:48–54. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH719>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 48.FDA Premarket Approval (PMA) [(accessed on 3 October 2020)];2020 Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P190024.
- 49.Peeters E., Wentzensen N., Bergeron C., Arbyn M. Meta-analysis of the accuracy of p16 or p16/Ki-67 immunocytochemistry versus HPV testing for the detection of CIN2+/CIN3+ in triage of women with minor abnormal cytology. Cancer Cytopathol. 2019;127:169–180. doi: 10.1002/cncy.22103. [DOI] [PubMed] [Google Scholar]
- 50.Wright T.C., Jr., Behrens C.M., Ranger-Moore J., Rehm S., Sharma A., Stoler M.H., Ridder R. Triaging HPV-positive women with p16/Ki-67 dual-stained cytology: Results from a sub-study nested into the ATHENA trial. Gynecol. Oncol. 2017;144:51–56. doi: 10.1016/j.ygyno.2016.10.031. [DOI] [PubMed] [Google Scholar]
- 51.Petry K.U., Schmidt D., Scherbring S., Luyten A., Reinecke-Luthge A., Bergeron C., Kommoss F., Loning T., Ordi J., Regauer S., et al. Triaging Pap cytology negative, HPV positive cervical cancer screening results with p16/Ki-67 Dual-stained cytology. Gynecol. Oncol. 2011;121:505–509. doi: 10.1016/j.ygyno.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 52.Wentzensen N., Schwartz L., Zuna R.E., Smith K., Mathews C., Gold M.A., Allen R.A., Zhang R., Dunn S.T., Walker J.L., et al. Performance of p16/Ki-67 immunostaining to detect cervical cancer precursors in a colposcopy referral population. Clin. Cancer Res. 2012;18:4154–4162. doi: 10.1158/1078-0432.CCR-12-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu L.L., Chen W., Lei X.Q., Qin Y., Wu Z.N., Pan Q.J., Zhang X., Chang B.F., Zhang S.K., Guo H.Q., et al. Evaluation of p16/Ki-67 dual staining in detection of cervical precancer and cancers: A multicenter study in China. Oncotarget. 2016;7:21181–21189. doi: 10.18632/oncotarget.8307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tay T.K.Y., Lim K.L., Hilmy M.H., Thike A.A., Goh S.T., Song L.H., Hwang J.S.G., Mantoo S. Comparison of the sensitivity and specificity of p16/Ki-67 dual staining and HPV DNA testing of abnormal cervical cytology in the detection of histology proven cervical intraepithelial neoplasia grade 2 and above (CIN 2+) Malays J. Pathol. 2017;39:257–265. [PubMed] [Google Scholar]
- 55.Possati-Resende J.C., Fregnani J.H., Kerr L.M., Mauad E.C., Longatto-Filho A., Scapulatempo-Neto C. The Accuracy of p16/Ki-67 and HPV Test in the Detection of CIN2/3 in Women Diagnosed with ASC-US or LSIL. PLoS ONE. 2015;10:e0134445. doi: 10.1371/journal.pone.0134445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hammes L.S., Tekmal R.R., Naud P., Edelweiss M.I., Kirma N., Valente P.T., Syrjanen K.J., Cunha-Filho J.S. Up-regulation of VEGF, c-fms and COX-2 expression correlates with severity of cervical cancer precursor (CIN) lesions and invasive disease. Gynecol. Oncol. 2008;110:445–451. doi: 10.1016/j.ygyno.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 57.Kirma N., Hammes L.S., Liu Y.G., Nair H.B., Valente P.T., Kumar S., Flowers L.C., Tekmal R.R. Elevated expression of the oncogene c-fms and its ligand, the macrophage colony-stimulating factor-1, in cervical cancer and the role of transforming growth factor-beta1 in inducing c-fms expression. Cancer Res. 2007;67:1918–1926. doi: 10.1158/0008-5472.CAN-06-1991. [DOI] [PubMed] [Google Scholar]
- 58.Lawicki S., Bedkowska G.E., Gacuta-Szumarska E., Knapp P., Szmitkowski M. Pretreatment plasma levels and diagnostic utility of hematopoietic cytokines in cervical cancer or cervical intraepithelial neoplasia patients. Folia Histochem. Cytobiol. 2012;50:213–219. doi: 10.5603/FHC.2012.0030. [DOI] [PubMed] [Google Scholar]
- 59.Zajkowska M., Zbucka-Kretowska M., Sidorkiewicz I., Lubowicka E., Gacuta E., Szmitkowski M., Chrostek L., Lawicki S. Plasma levels and diagnostic utility of macrophage-colony stimulating factor, matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 as tumor markers in cervical cancer patients. Tumour. Biol. 2018;40:1010428318790363. doi: 10.1177/1010428318790363. [DOI] [PubMed] [Google Scholar]
- 60.Lubowicka E., Zbucka-Kretowska M., Sidorkiewicz I., Zajkowska M., Gacuta E., Puchnarewicz A., Chrostek L., Szmitkowski M., Lawicki S. Diagnostic Power of Cytokine M-CSF, Metalloproteinase 2 (MMP-2) and Tissue Inhibitor-2 (TIMP-2) in Cervical Cancer Patients Based on ROC Analysis. Pathol. Oncol. Res. 2020;26:791–800. doi: 10.1007/s12253-019-00626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sidorkiewicz I., Zbucka-Kretowska M., Zareba K., Lubowicka E., Zajkowska M., Szmitkowski M., Gacuta E., Lawicki S. Plasma levels of M-CSF and VEGF in laboratory diagnostics and differentiation of selected histological types of cervical cancers. BMC Cancer. 2019;19:398. doi: 10.1186/s12885-019-5558-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Atoe K., Ehimigbai R., Ayinbuomwan E., Onovughakpo-Sakpa O. Assessing the utility of a cytokine in the diagnosis of cervical intraepithelial neoplasm among women in Benin city, Nigeria. Afr. J. Health Saf. Environ. 2020;1:101–112. doi: 10.52417/ajhse.v1i2.100. [DOI] [Google Scholar]
- 63.van de Wijgert J.H., Borgdorff H., Verhelst R., Crucitti T., Francis S., Verstraelen H., Jespers V. The vaginal microbiota: What have we learned after a decade of molecular characterization? PLoS ONE. 2014;9:e105998. doi: 10.1371/journal.pone.0105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brotman R.M. Vaginal microbiome and sexually transmitted infections: An epidemiologic perspective. J. Clin. Investig. 2011;121:4610–4617. doi: 10.1172/JCI57172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bradshaw C.S., Walker J., Fairley C.K., Chen M.Y., Tabrizi S.N., Donovan B., Kaldor J.M., McNamee K., Urban E., Walker S., et al. Prevalent and incident bacterial vaginosis are associated with sexual and contraceptive behaviours in young Australian women. PLoS ONE. 2013;8:e57688. doi: 10.1371/journal.pone.0057688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chico R.M., Mayaud P., Ariti C., Mabey D., Ronsmans C., Chandramohan D. Prevalence of malaria and sexually transmitted and reproductive tract infections in pregnancy in sub-Saharan Africa: A systematic review. JAMA. 2012;307:2079–2086. doi: 10.1001/jama.2012.3428. [DOI] [PubMed] [Google Scholar]
- 67.Fettweis J.M., Brooks J.P., Serrano M.G., Sheth N.U., Girerd P.H., Edwards D.J., Strauss J.F., The Vaginal Microbiome C., Jefferson K.K., Buck G.A. Differences in vaginal microbiome in African American women versus women of European ancestry. Microbiology (Reading) 2014;160:2272–2282. doi: 10.1099/mic.0.081034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.White B.A., Creedon D.J., Nelson K.E., Wilson B.A. The vaginal microbiome in health and disease. Trends Endocrinol. Metab. 2011;22:389–393. doi: 10.1016/j.tem.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ma B., Forney L.J., Ravel J. Vaginal microbiome: Rethinking health and disease. Annu. Rev. Microbiol. 2012;66:371–389. doi: 10.1146/annurev-micro-092611-150157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Petrova M.I., Reid G., Vaneechoutte M., Lebeer S. Lactobacillus iners: Friend or Foe? Trends Microbiol. 2017;25:182–191. doi: 10.1016/j.tim.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 71.Amabebe E., Anumba D.O.C. The Vaginal Microenvironment: The Physiologic Role of Lactobacilli. Front. Med. 2018;5:181. doi: 10.3389/fmed.2018.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tachedjian G., Aldunate M., Bradshaw C.S., Cone R.A. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res. Microbiol. 2017;168:782–792. doi: 10.1016/j.resmic.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 73.Rampersaud R., Planet P.J., Randis T.M., Kulkarni R., Aguilar J.L., Lehrer R.I., Ratner A.J. Inerolysin, a cholesterol-dependent cytolysin produced by Lactobacillus iners. J. Bacteriol. 2011;193:1034–1041. doi: 10.1128/JB.00694-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Macklaim J.M., Gloor G.B., Anukam K.C., Cribby S., Reid G. At the crossroads of vaginal health and disease, the genome sequence of Lactobacillus iners AB-1. Proc. Natl. Acad. Sci. USA. 2011;108((Suppl. S1)):4688–4695. doi: 10.1073/pnas.1000086107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Silvestris E., Dellino M., Cafforio P., Paradiso A.V., Cormio G., D’Oronzo S. Breast cancer: An update on treatment-related infertility. J. Cancer Res. Clin. Oncol. 2020;146:647–657. doi: 10.1007/s00432-020-03136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grassini D., Cascardi E., Sarotto I., Annaratone L., Sapino A., Berrino E., Marchiò C. Unusual patterns of HER2 expression in breast cancer: Insights and perspectives. Pathobiology. 2022;89:1–19. doi: 10.1159/000524227. [DOI] [PubMed] [Google Scholar]
- 77.Annaratone L., Cascardi E., Vissio E., Sarotto I., Chmielik E., Sapino A., Berrino E., Marchio C. The Multifaceted Nature of Tumor Microenvironment in Breast Carcinomas. Pathobiology. 2020;87:125–142. doi: 10.1159/000507055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mandar R., Punab M., Borovkova N., Lapp E., Kiiker R., Korrovits P., Metspalu A., Krjutskov K., Nolvak H., Preem J.K., et al. Complementary seminovaginal microbiome in couples. Res. Microbiol. 2015;166:440–447. doi: 10.1016/j.resmic.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 79.Hickey R.J., Zhou X., Settles M.L., Erb J., Malone K., Hansmann M.A., Shew M.L., Van Der Pol B., Fortenberry J.D., Forney L.J. Vaginal microbiota of adolescent girls prior to the onset of menarche resemble those of reproductive-age women. mBio. 2015;6:e00097-15. doi: 10.1128/mBio.00097-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Eschenbach D.A., Thwin S.S., Patton D.L., Hooton T.M., Stapleton A.E., Agnew K., Winter C., Meier A., Stamm W.E. Influence of the normal menstrual cycle on vaginal tissue, discharge, and microflora. Clin. Infect. Dis. 2000;30:901–907. doi: 10.1086/313818. [DOI] [PubMed] [Google Scholar]
- 81.MacIntyre D.A., Chandiramani M., Lee Y.S., Kindinger L., Smith A., Angelopoulos N., Lehne B., Arulkumaran S., Brown R., Teoh T.G., et al. The vaginal microbiome during pregnancy and the postpartum period in a European population. Sci. Rep. 2015;5:8988. doi: 10.1038/srep08988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brotman R.M., Shardell M.D., Gajer P., Fadrosh D., Chang K., Silver M.I., Viscidi R.P., Burke A.E., Ravel J., Gravitt P.E. Association between the vaginal microbiota, menopause status, and signs of vulvovaginal atrophy. Menopause. 2014;21:450–458. doi: 10.1097/GME.0b013e3182a4690b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Boskey E.R., Cone R.A., Whaley K.J., Moench T.R. Origins of vaginal acidity: High D/L lactate ratio is consistent with bacteria being the primary source. Hum. Reprod. 2001;16:1809–1813. doi: 10.1093/humrep/16.9.1809. [DOI] [PubMed] [Google Scholar]
- 84.Spear G.T., French A.L., Gilbert D., Zariffard M.R., Mirmonsef P., Sullivan T.H., Spear W.W., Landay A., Micci S., Lee B.H., et al. Human alpha-amylase present in lower-genital-tract mucosal fluid processes glycogen to support vaginal colonization by Lactobacillus. J. Infect. Dis. 2014;210:1019–1028. doi: 10.1093/infdis/jiu231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fichorova R.N., Chen P.L., Morrison C.S., Doncel G.F., Mendonca K., Kwok C., Chipato T., Salata R., Mauck C. The Contribution of Cervicovaginal Infections to the Immunomodulatory Effects of Hormonal Contraception. mBio. 2015;6:e00221-15. doi: 10.1128/mBio.00221-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moreno V., Bosch F.X., Munoz N., Meijer C.J., Shah K.V., Walboomers J.M., Herrero R., Franceschi S., International Agency for Research on Cancer Multicentric Cervical Cancer Study, G. Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: The IARC multicentric case-control study. Lancet. 2002;359:1085–1092. doi: 10.1016/S0140-6736(02)08150-3. [DOI] [PubMed] [Google Scholar]
- 87.Tamma R., Annese T., Ruggieri S., Brunetti O., Longo V., Cascardi E., Mastropasqua M.G., Maiorano E., Silvestris N., Ribatti D. Inflammatory cells infiltrate and angiogenesis in locally advanced and metastatic cholangiocarcinoma. Eur. J. Clin. Investig. 2019;49:e13087. doi: 10.1111/eci.13087. [DOI] [PubMed] [Google Scholar]
- 88.Tamma R., Limongelli L., Maiorano E., Pastore D., Cascardi E., Tempesta A., Carluccio P., Mastropasqua M.G., Capodiferro S., Covelli C., et al. Vascular density and inflammatory infiltrate in primary oral squamous cell carcinoma and after allogeneic hematopoietic stem cell transplantation. Ann. Hematol. 2019;98:979–986. doi: 10.1007/s00277-018-3575-3. [DOI] [PubMed] [Google Scholar]
- 89.Tamma R., Rutigliano M., Lucarelli G., Annese T., Ruggieri S., Cascardi E., Napoli A., Battaglia M., Ribatti D. Microvascular density, macrophages, and mast cells in human clear cell renal carcinoma with and without bevacizumab treatment. Urol. Oncol. 2019;37:355.e11–355.e19. doi: 10.1016/j.urolonc.2019.01.025. [DOI] [PubMed] [Google Scholar]
- 90.Verginelli F., Pisacane A., Gambardella G., D’Ambrosio A., Candiello E., Ferrio M., Panero M., Casorzo L., Benvenuti S., Cascardi E., et al. Cancer of unknown primary stem-like cells model multi-organ metastasis and unveil liability to MEK inhibition. Nat. Commun. 2021;12:2498. doi: 10.1038/s41467-021-22643-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vodstrcil L.A., Hocking J.S., Law M., Walker S., Tabrizi S.N., Fairley C.K., Bradshaw C.S. Hormonal contraception is associated with a reduced risk of bacterial vaginosis: A systematic review and meta-analysis. PLoS ONE. 2013;8:e73055. doi: 10.1371/journal.pone.0073055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.van de Wijgert J.H., Verwijs M.C., Turner A.N., Morrison C.S. Hormonal contraception decreases bacterial vaginosis but oral contraception may increase candidiasis: Implications for HIV transmission. AIDS. 2013;27:2141–2153. doi: 10.1097/QAD.0b013e32836290b6. [DOI] [PubMed] [Google Scholar]
- 93.Borgdorff H., Gautam R., Armstrong S.D., Xia D., Ndayisaba G.F., van Teijlingen N.H., Geijtenbeek T.B., Wastling J.M., van de Wijgert J.H. Cervicovaginal microbiome dysbiosis is associated with proteome changes related to alterations of the cervicovaginal mucosal barrier. Mucosal. Immunol. 2016;9:621–633. doi: 10.1038/mi.2015.86. [DOI] [PubMed] [Google Scholar]
- 94.Achilles S.L., Hillier S.L. The complexity of contraceptives: Understanding their impact on genital immune cells and vaginal microbiota. AIDS. 2013;27((Suppl. S1)):S5–S15. doi: 10.1097/QAD.0000000000000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brotman R.M., He X., Gajer P., Fadrosh D., Sharma E., Mongodin E.F., Ravel J., Glover E.D., Rath J.M. Association between cigarette smoking and the vaginal microbiota: A pilot study. BMC Infect. Dis. 2014;14:471. doi: 10.1186/1471-2334-14-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schwebke J.R., Desmond R.A., Oh M.K. Predictors of bacterial vaginosis in adolescent women who douche. Sex. Transm. Dis. 2004;31:433–436. doi: 10.1097/01.OLQ.0000129948.91055.9F. [DOI] [PubMed] [Google Scholar]
- 97.Brotman R.M., Ghanem K.G., Klebanoff M.A., Taha T.E., Scharfstein D.O., Zenilman J.M. The effect of vaginal douching cessation on bacterial vaginosis: A pilot study. Am. J. Obstet. Gynecol. 2008;198:628.e1–628.e7. doi: 10.1016/j.ajog.2007.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bui T.C., Thai T.N., Tran L.T., Shete S.S., Ramondetta L.M., Basen-Engquist K.M. Association Between Vaginal Douching and Genital Human Papillomavirus Infection Among Women in the United States. J. Infect. Dis. 2016;214:1370–1375. doi: 10.1093/infdis/jiw388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang J., Thomas A.G., Leybovich E. Vaginal douching and adverse health effects: A meta-analysis. Am. J. Public Health. 1997;87:1207–1211. doi: 10.2105/AJPH.87.7.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Foster K.R., Schluter J., Coyte K.Z., Rakoff-Nahoum S. The evolution of the host microbiome as an ecosystem on a leash. Nature. 2017;548:43–51. doi: 10.1038/nature23292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zeng Q., Wu S., Sukumaran J., Rodrigo A. Models of microbiome evolution incorporating host and microbial selection. Microbiome. 2017;5:127. doi: 10.1186/s40168-017-0343-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Backhed F., Ley R.E., Sonnenburg J.L., Peterson D.A., Gordon J.I. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 103.Lederberg J., McCray A.T. Ome SweetOmics—A genealogical treasury of words. Scientist. 2001;15:8. [Google Scholar]
- 104.Woese C.R., Fox G.E. Phylogenetic structure of the prokaryotic domain: The primary kingdoms. Proc. Natl. Acad. Sci. USA. 1977;74:5088–5090. doi: 10.1073/pnas.74.11.5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Curty G., de Carvalho P.S., Soares M.A. The role of the cervicovaginal microbiome on the genesis and as a biomarker of premalignant cervical intraepithelial neoplasia and invasive cervical cancer. Int. J. Mol. Sci. 2019;21:222. doi: 10.3390/ijms21010222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Audirac-Chalifour A., Torres-Poveda K., Bahena-Roman M., Tellez-Sosa J., Martinez-Barnetche J., Cortina-Ceballos B., Lopez-Estrada G., Delgado-Romero K., Burguete-Garcia A.I., Cantu D., et al. Cervical Microbiome and Cytokine Profile at Various Stages of Cervical Cancer: A Pilot Study. PLoS ONE. 2016;11:e0153274. doi: 10.1371/journal.pone.0153274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Conesa-Zamora P. Immune responses against virus and tumor in cervical carcinogenesis: Treatment strategies for avoiding the HPV-induced immune escape. Gynecol. Oncol. 2013;131:480–488. doi: 10.1016/j.ygyno.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 108.Shulzhenko N., Lyng H., Sanson G.F., Morgun A. Menage a trois: An evolutionary interplay between human papillomavirus, a tumor, and a woman. Trends Microbiol. 2014;22:345–353. doi: 10.1016/j.tim.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 109.Anahtar M.N., Byrne E.H., Doherty K.E., Bowman B.A., Yamamoto H.S., Soumillon M., Padavattan N., Ismail N., Moodley A., Sabatini M.E., et al. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity. 2015;42:965–976. doi: 10.1016/j.immuni.2015.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gosmann C., Anahtar M.N., Handley S.A., Farcasanu M., Abu-Ali G., Bowman B.A., Padavattan N., Desai C., Droit L., Moodley A., et al. Lactobacillus-Deficient Cervicovaginal Bacterial Communities Are Associated with Increased HIV Acquisition in Young South African Women. Immunity. 2017;46:29–37. doi: 10.1016/j.immuni.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Routy B., Le Chatelier E., Derosa L., Duong C.P.M., Alou M.T., Daillere R., Fluckiger A., Messaoudene M., Rauber C., Roberti M.P., et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 112.Kyrgiou M., Mitra A., Moscicki A.B. Does the vaginal microbiota play a role in the development of cervical cancer? Transl. Res. 2017;179:168–182. doi: 10.1016/j.trsl.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vyshenska D., Lam K.C., Shulzhenko N., Morgun A. Interplay between viruses and bacterial microbiota in cancer development. Semin. Immunol. 2017;32:14–24. doi: 10.1016/j.smim.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Curty G., Costa R.L., Siqueira J.D., Meyrelles A.I., Machado E.S., Soares E.A., Soares M.A. Analysis of the cervical microbiome and potential biomarkers from postpartum HIV-positive women displaying cervical intraepithelial lesions. Sci. Rep. 2017;7:17364. doi: 10.1038/s41598-017-17351-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gopalakrishnan V., Helmink B.A., Spencer C.N., Reuben A., Wargo J.A. The Influence of the Gut Microbiome on Cancer, Immunity, and Cancer Immunotherapy. Cancer Cell. 2018;33:570–580. doi: 10.1016/j.ccell.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gao W., Weng J., Gao Y., Chen X. Comparison of the vaginal microbiota diversity of women with and without human papillomavirus infection: A cross-sectional study. BMC Infect. Dis. 2013;13:271. doi: 10.1186/1471-2334-13-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schiffman M., Doorbar J., Wentzensen N., de Sanjose S., Fakhry C., Monk B.J., Stanley M.A., Franceschi S. Carcinogenic human papillomavirus infection. Nat. Rev. Dis. Primers. 2016;2:16086. doi: 10.1038/nrdp.2016.86. [DOI] [PubMed] [Google Scholar]
- 118.Laniewski P., Barnes D., Goulder A., Cui H., Roe D.J., Chase D.M., Herbst-Kralovetz M.M. Linking cervicovaginal immune signatures, HPV and microbiota composition in cervical carcinogenesis in non-Hispanic and Hispanic women. Sci. Rep. 2018;8:7593. doi: 10.1038/s41598-018-25879-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.van de Wijgert J., Jespers V. The global health impact of vaginal dysbiosis. Res. Microbiol. 2017;168:859–864. doi: 10.1016/j.resmic.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 120.Mitchell C., Marrazzo J. Bacterial vaginosis and the cervicovaginal immune response. Am. J. Reprod. Immunol. 2014;71:555–563. doi: 10.1111/aji.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Navas-Molina J.A., Peralta-Sanchez J.M., Gonzalez A., McMurdie P.J., Vazquez-Baeza Y., Xu Z., Ursell L.K., Lauber C., Zhou H., Song S.J., et al. Advancing our understanding of the human microbiome using QIIME. Methods Enzymol. 2013;531:371–444. doi: 10.1016/B978-0-12-407863-5.00019-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.The Human Microbiome Project Consortium Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.O’Hanlon D.E., Lanier B.R., Moench T.R., Cone R.A. Cervicovaginal fluid and semen block the microbicidal activity of hydrogen peroxide produced by vaginal lactobacilli. BMC Infect. Dis. 2010;10:120. doi: 10.1186/1471-2334-10-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mitra A., MacIntyre D.A., Lee Y.S., Smith A., Marchesi J.R., Lehne B., Bhatia R., Lyons D., Paraskevaidis E., Li J.V., et al. Cervical intraepithelial neoplasia disease progression is associated with increased vaginal microbiome diversity. Sci. Rep. 2015;5:16865. doi: 10.1038/srep16865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Oh H.Y., Kim B.S., Seo S.S., Kong J.S., Lee J.K., Park S.Y., Hong K.M., Kim H.K., Kim M.K. The association of uterine cervical microbiota with an increased risk for cervical intraepithelial neoplasia in Korea. Clin. Microbiol. Infect. 2015;21:674.e1–674.e9. doi: 10.1016/j.cmi.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 126.Borgdorff H., Tsivtsivadze E., Verhelst R., Marzorati M., Jurriaans S., Ndayisaba G.F., Schuren F.H., van de Wijgert J.H. Lactobacillus-dominated cervicovaginal microbiota associated with reduced HIV/STI prevalence and genital HIV viral load in African women. ISME J. 2014;8:1781–1793. doi: 10.1038/ismej.2014.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Insinga R.P., Perez G., Wheeler C.M., Koutsky L.A., Garland S.M., Leodolter S., Joura E.A., Ferris D.G., Steben M., Hernandez-Avila M., et al. Incident cervical HPV infections in young women: Transition probabilities for CIN and infection clearance. Cancer Epidemiol. Biomark. Prev. 2011;20:287–296. doi: 10.1158/1055-9965.EPI-10-0791. [DOI] [PubMed] [Google Scholar]
- 128.Garrett W.S. Cancer and the microbiota. Science. 2015;348:80–86. doi: 10.1126/science.aaa4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schwabe R.F., Jobin C. The microbiome and cancer. Nat. Rev. Cancer. 2013;13:800–812. doi: 10.1038/nrc3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Norenhag J., Du J., Olovsson M., Verstraelen H., Engstrand L., Brusselaers N. The vaginal microbiota, human papillomavirus and cervical dysplasia: A systematic review and network meta-analysis. BJOG. 2020;127:171–180. doi: 10.1111/1471-0528.15854. [DOI] [PubMed] [Google Scholar]
- 131.Skapinyecz J., Smid I., Horvath A., Jeney C., Kardos L., Kovacs P. Pelvic inflammatory disease is a risk factor for cervical cancer. Eur. J. Gynaecol. Oncol. 2003;24:401–404. [PubMed] [Google Scholar]
- 132.Klatt N.R., Cheu R., Birse K., Zevin A.S., Perner M., Noel-Romas L., Grobler A., Westmacott G., Xie I.Y., Butler J., et al. Vaginal bacteria modify HIV tenofovir microbicide efficacy in African women. Science. 2017;356:938–945. doi: 10.1126/science.aai9383. [DOI] [PubMed] [Google Scholar]
- 133.Brotman R.M., Shardell M.D., Gajer P., Tracy J.K., Zenilman J.M., Ravel J., Gravitt P.E. Interplay between the temporal dynamics of the vaginal microbiota and human papillomavirus detection. J. Infect. Dis. 2014;210:1723–1733. doi: 10.1093/infdis/jiu330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kwasniewski W., Wolun-Cholewa M., Kotarski J., Warchol W., Kuzma D., Kwasniewska A., Gozdzicka-Jozefiak A. Microbiota dysbiosis is associated with HPV-induced cervical carcinogenesis. Oncol. Lett. 2018;16:7035–7047. doi: 10.3892/ol.2018.9509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ranjan R., Rani A., Metwally A., McGee H.S., Perkins D.L. Analysis of the microbiome: Advantages of whole genome shotgun versus 16S amplicon sequencing. Biochem. Biophys. Res. Commun. 2016;469:967–977. doi: 10.1016/j.bbrc.2015.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang C., Liu Y., Gao W., Pan Y., Gao Y., Shen J., Xiong H. The direct and indirect association of cervical microbiota with the risk of cervical intraepithelial neoplasia. Cancer Med. 2018;7:2172–2179. doi: 10.1002/cam4.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Piyathilake C.J., Ollberding N.J., Kumar R., Macaluso M., Alvarez R.D., Morrow C.D. Cervical Microbiota Associated with Higher Grade Cervical Intraepithelial Neoplasia in Women Infected with High-Risk Human Papillomaviruses. Cancer Prev. Res. 2016;9:357–366. doi: 10.1158/1940-6207.CAPR-15-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tsay J.J., Wu B.G., Badri M.H., Clemente J.C., Shen N., Meyn P., Li Y., Yie T.A., Lhakhang T., Olsen E., et al. Airway Microbiota Is Associated with Upregulation of the PI3K Pathway in Lung Cancer. Am. J. Respir. Crit. Care Med. 2018;198:1188–1198. doi: 10.1164/rccm.201710-2118OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hyman R.W., Fukushima M., Jiang H., Fung E., Rand L., Johnson B., Vo K.C., Caughey A.B., Hilton J.F., Davis R.W., et al. Diversity of the vaginal microbiome correlates with preterm birth. Reprod. Sci. 2014;21:32–40. doi: 10.1177/1933719113488838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Buonomo B., Massarotti C., Dellino M., Anserini P., Ferrari A., Campanella M., Magnotti M., De Stefano C., Peccatori F.A., Lambertini M. Reproductive issues in carriers of germline pathogenic variants in the BRCA1/2 genes: An expert meeting. BMC Med. 2021;19:205. doi: 10.1186/s12916-021-02081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dellino M., Silvestris E., Loizzi V., Paradiso A., Loiacono R., Minoia C., Daniele A., Cormio G. Germinal ovarian tumors in reproductive age women: Fertility-sparing and outcome. Medicine. 2020;99:e22146. doi: 10.1097/MD.0000000000022146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Afiuni-Zadeh S., Boylan K.L.M., Jagtap P.D., Griffin T.J., Rudney J.D., Peterson M.L., Skubitz A.P.N. Evaluating the potential of residual Pap test fluid as a resource for the metaproteomic analysis of the cervical-vaginal microbiome. Sci. Rep. 2018;8:10868. doi: 10.1038/s41598-018-29092-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Silvestris E., Cormio G., Skrypets T., Dellino M., Paradiso A.V., Guarini A., Minoia C. Novel aspects on gonadotoxicity and fertility preservation in lymphoproliferative neoplasms. Crit. Rev. Oncol. Hematol. 2020;151:102981. doi: 10.1016/j.critrevonc.2020.102981. [DOI] [PubMed] [Google Scholar]
- 144.Bradshaw C.S., Morton A.N., Hocking J., Garland S.M., Morris M.B., Moss L.M., Horvath L.B., Kuzevska I., Fairley C.K. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J. Infect. Dis. 2006;193:1478–1486. doi: 10.1086/503780. [DOI] [PubMed] [Google Scholar]
- 145.Silvestris E., D’Oronzo S., Cafforio P., Kardhashi A., Dellino M., Cormio G. In Vitro Generation of Oocytes from Ovarian Stem Cells (OSCs): In Search of Major Evidence. Int. J. Mol. Sci. 2019;20:6225. doi: 10.3390/ijms20246225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Menard J.P. Antibacterial treatment of bacterial vaginosis: Current and emerging therapies. Int. J. Womens Health. 2011;3:295–305. doi: 10.2147/IJWH.S23814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dellino M., Minoia C., Paradiso A.V., De Palo R., Silvestris E. Fertility Preservation in Cancer Patients During the Coronavirus (COVID-19) Pandemic. Front. Oncol. 2020;10:1009. doi: 10.3389/fonc.2020.01009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bertuccini L., Russo R., Iosi F., Superti F. Effects of Lactobacillus rhamnosus and Lactobacillus acidophilus on bacterial vaginal pathogens. Int. J. Immunopathol. Pharmacol. 2017;30:163–167. doi: 10.1177/0394632017697987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Petrova M.I., Lievens E., Malik S., Imholz N., Lebeer S. Lactobacillus species as biomarkers and agents that can promote various aspects of vaginal health. Front. Physiol. 2015;6:81. doi: 10.3389/fphys.2015.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Domig K.J., Kiss H., Petricevic L., Viernstein H., Unger F., Kneifel W. Strategies for the evaluation and selection of potential vaginal probiotics from human sources: An exemplary study. Benef. Microbes. 2014;5:263–272. doi: 10.3920/BM2013.0069. [DOI] [PubMed] [Google Scholar]
- 151.Laue C., Papazova E., Liesegang A., Pannenbeckers A., Arendarski P., Linnerth B., Domig K.J., Kneifel W., Petricevic L., Schrezenmeir J. Effect of a yoghurt drink containing Lactobacillus strains on bacterial vaginosis in women—A double-blind, randomised, controlled clinical pilot trial. Benef. Microbes. 2018;9:35–50. doi: 10.3920/BM2017.0018. [DOI] [PubMed] [Google Scholar]
- 152.Palma E., Recine N., Domenici L., Giorgini M., Pierangeli A., Panici P.B. Long-term Lactobacillus rhamnosus BMX 54 application to restore a balanced vaginal ecosystem: A promising solution against HPV-infection. BMC Infect. Dis. 2018;18:13. doi: 10.1186/s12879-017-2938-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dellino M., Carriero C., Silvestris E., Capursi T., Paradiso A., Cormio G. Primary Vaginal Carcinoma Arising on Cystocele Mimicking Vulvar Cancer. J. Obstet. Gynaecol. Can. 2020;42:1543–1545. doi: 10.1016/j.jogc.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 154.Giraldo P.C., Sanches J.M., Sparvolli L.G., Amaral R., Migliorini I., Gil C.D., Taddei C.R., Witkin S.S., Discacciati M.G. Relationship between Papillomavirus vaccine, vaginal microbiome, and local cytokine response: An exploratory research. Braz. J. Microbiol. 2021;52:2363–2371. doi: 10.1007/s42770-021-00616-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.