Abstract

Common buckwheat (Fagopyrum esculentum Moench) seeds are important nutritious grains that are widely spread in several human food products and livestock feed. Their health benefits are mainly due to their bioactive phenolic compounds, especially rutin and quercetin, which have a positive impact on heart health, weight loss, and diabetes management. In this study, we evaluated different media and light treatments for the in vitro cultures of common buckwheat (CB) in order to find the most optimum one producing the highest yield with the highest purity of these compounds. The subcultured treated samples included in this study were shoots, leaves, stems, hairy roots, and calli. From the several treated samples and under different light stress conditions, the best production was achieved by growing the shoots of common buckwheat in hormone-free media containing activated charcoal and exposing to blue light, attaining 4.3 mg and 7.0 mg/g of extracts of rutin and quercetin, respectively, compared to 3.7 mg of rutin/g of extract and traces of quercetin in the seeds of CB. Continuous multiplication of CB shoots in the media containing charcoal and different concentrations of kinetin produced an extract with 161 mg/g of rutin and 26 mg/g of quercetin with an almost 20-fold increase in rutin content. The rutin content under these conditions reached up to 16% w/w of the extract. The hairy root cultures of the leaves exposed to red light showed a significantly high yield of quercetin attaining 10 mg/g of extract. Large-scale production of CB shootlets under the best conditions were carried out, which enabled the isolation of pure quercetin and rutin using a simple chromatographic procedure. The identity and purity of the isolated compounds were confirmed through NMR and HPLC analyses.
1. Introduction
Buckwheat seeds, belonging to the family Polygonaceae, have numerous nutritional and bioactive compounds with several health benefit effects, as for example lowering cholesterol, and decreasing constipation problems and obesity. Polyphenols, flavonoids (mainly rutin, quercetin, emodin, and fagopyrin),1,2 proteins, vitamins (thiamine, riboflavin, and pyridoxine),3 and minerals (sodium, potassium, copper, zinc, and magnesium)4 are examples of bioactive compounds in the different vegetative parts and seeds of buckwheat.1 It is safely consumed in their main diets by celiac-disease patients as it is free of gluten.5 The most two consumed species of buckwheat are common buckwheat (Fagopyrum esculentum Moench) and tartary buckwheat (Fagopyrum tataricum (L.) Gaertn).6 Generally, buckwheat is produced in China, France, and Eastern European countries such as Russia, Ukraine, and Poland.7 Regarding its phenolic content, buckwheat seeds grown at different locations contain more rutin (from 0.05 to 1.35%) than quercetin (from 0.01 to 0.17%) content.8
Rutin is a flavonol glycoside found in citrus, buckwheat, and other plants. It plays a vital role in human health such as antioxidant, treatment of capillary fragility, anti-Alzheimer, and anti-inflammatory effects.9 Quercetin is a flavonol possessing various pharmacological activities such as antihyperglycemic, anti-inflammatory, wound healing, anti-Alzheimer, antioxidant, treatment of capillary fragility, and cardiovascular diseases.10
However, as buckwheat cannot naturally grow in Egyptian soil, tissue culture presents a possible tool to introduce the plant to Egyptian market. In addition, tissue culture represents a renewable source for the production of plant secondary metabolites due to its increased commercial importance.11,12 Several biologically and commercially important secondary metabolites were produced through tissue culture techniques, such as ginsenosides, ginkolides, allicin, diosgenin, and caffeine.11 Production of phytochemicals under controlled conditions regardless of climatic changes is one of the advantages of using tissue culture in secondary metabolite production. In addition, production of compounds by tissue culture is more simple, reliable, and free from interfering compounds as in conventional methods from plants.11
Light is a significant abiotic elicitor in plants that influences plant growth and development, either directly or indirectly, particularly in large areas.13 Light has two primary effects on plants: light supplies the energy necessary by the plant for photosynthesis, and it controls growth, differentiation, and plant metabolism as it is a signal received by photoreceptors.14 Rutin and quercetin are bioactive flavonoids in buckwheat metabolites. When photosynthesis is higher, flavonoid deposition in the leaves increases as well. This is especially true in F. esculentum.(15) In typical buckwheat sprouts, blue light might be used to increase flavonoid content as well as antioxidant activity.16
This study aims to utilize plant biotechnology to usefully produce in vitro cultures from buckwheat and transform it into a platform producing rutin and quercetin in high yields and high purity. Initially, buckwheat in vitro cultures will be developed, and production of the target ingredients (rutin and quercetin) will be improved using different elicitors that were proved promising in preliminary work. In addition, extraction, purification, and identification of target compounds will be conducted.
2. Materials and Methods
2.1. General Experimental Procedures
The NMR experiments for all compounds were performed using a Bruker Ascend 400/R NMR spectrometer operating at a proton NMR frequency of 400 MHz and 13C-NMR (100 MHz). The NMR spectra were recorded in a suitable solvent (CD3OD) using TMS as internal standard and the chemical shifts are given in δ ppm values. An advion compact mass spectrometer (CMS) NY/USA instrument equipped with an electrospray ionization source (ESI) was employed to perform ESIMS analysis of quercetin and rutin. Precoated silica gel plates Kieselgel 60 F254 (0.25 mm, Merck, Darmstadt, Germany) were used for the thin-layer chromatographic (TLC) analysis. The compounds were detected by UV absorption at λmax 255 and 366 nm followed by spraying with a p-anisaldehyde:H2SO4 and AlCl3 spray reagent, and heating at 110 °C for 1–2 min.
2.2. Plant Material
Seeds of common buckwheat (F. esculentum Moench) were received from Dr. Oksana Sytar, Department of Plant Biology, Educational and Scientifc Center “Institute of Biology and Medicine”, Taras Shevchenko National University of Kyiv, Kyiv, Ukraine.
2.3. Surface Sterilization and Establishment of Shootlet Cultures
Common buckwheat seeds were used as plant material for starting in vitro cultures. The seeds were washed under running tap water for 10 min with a soap. Then, under air laminar flow, the seeds were immersed in 40% commercial Clorox (containing 5.25% sodium hypochlorite) for 20 min and finally washed three times with sterilized distilled water. The sterilized seeds were placed in 250 ml Erlenmeyer flasks contain 50 mL of solid-free MS basal17 medium (Duchefa M0222, including vitamins). The germinated seeds, after 10–14 days, were re-cultured in fresh hormone-free MS medium for three weeks.
2.4. Continuous Multiplication of Shoot Cultures
After three weeks in hormone-free MS medium, shoots of common buckwheat were transferred to MS medium (4.4 g/L) supplemented with two concentrations of kinetin (0.25 and 0.5 mg/L) + sucrose (25 g/L) + agar (9 g/L) with activated charcoal (0.5 g/L) and without activated charcoal to get stock plant material with a high multiplication rate without any tissue culture problems such as hyperhydricity (previously known as vitrification).
2.5. Multiplication Medium for Large-Scale Shoot Production
Thirty g/L sucrose + 3/4 MS medium including vitamins + 0.5 mg/L kinetin + 0.5 g/L AC +8 g/L agar pH = 5.7. Common buckwheat in blue florescent light was subcultured on 21.3.2021 and harvested on 8.4.2021.
2.6. Hairy Root Transformation
2.6.1. Preparation of Agrobacterium Rhizogenes
Agrobacterium rhizogenes A4 strain, originated from glycerol stock, was allowed to grow in YEBS liquid culture. The YEBS culture is composed of 1 g/L yeast extract, 5 g/L beef extract, 5 g/L sucrose, 5 g/L bacto-peptone, and 0.5 g/L magnesium sulfate. The pH 7 of this YEBS culture was maintained at 28 °C by consistent shaking at 150 rpm followed by addition of rifampicin as the appropriate antibiotic.18
2.6.2. Establishment of Hairy Root Cultures
Establishment of hairy root cultures, from leaf and stem explants previously raised from the shoot (Section 2.3), was carried out by following the previously described method.18
2.6.3. PCR Detection
Extraction of genomic DNA from the investigated cultures was done by using the DNA Kit (purification kit Wizard R genomic DNA, A1120, Promega) by following the previously described method.18
2.7. Callus Induction
For callus induction from the common buckwheat shoot (F. esculentum Moench), leaf and stem segments approximately 2 cm each, were excided from the in vitro growing shoots and used as explants. Two segments per jar of explants (i.e., shoot, leaf and stem) were plated on MS medium supplemented with different concentrations of plant growth regulators. We used different media compositions in order to determine the optimum one for callus induction as seen in Table 1. Cultures were maintained under white light fluorescence 16/8 h light/dark and under completely dark conditions for 3 subcultures, each subculture after 4 weeks to induce callus.
Table 1. Media Used for Production of Calli from Common Buckwheat.
| code | medium composition |
|---|---|
| M1 | 30 g/L sucrose + 4.3 g/L MS basal salts + 10 mg/L thiamine HCl + 100 mg/L myo inositol + 0.5 mg/L BA + 0.5 mg/L picloram +8 g/L Agar pH = 5.7 |
| M2 | 30 g/L sucrose + 4.3 g/L MS basal salts + 10 mg/L thiamine HCl + 100 mg/L myo inositol + 0.5 mg/L BA + 1 mg/L picloram +8 g/L Agar pH = 5.7 |
| M3 | 30 g/L sucrose + 4.3 g/L MS basal salts + 10 mg/L thiamine HCl + 100 mg/L myo inositol + 0.5 mg/L BA + 1.5 mg/L picloram +8 g/L Agar pH = 5.7 |
| M4 | 30 g/L sucrose + 4.3 g/L MS basal salts + 10 mg/L thiamine HCl + 100 mg/L myo inositol + 0.5 mg/L BA + 2 mg/L picloram +8 g/L Agar pH = 5.7 |
For callus induction from common buckwheat, stem segments approximately 2 cm each were excided from the in vitro growing shoots and used as explants. Two segments per jar of explants were plated on MS medium supplemented with different concentrations of picloram (Table 1).
Cultures were maintained under light 16/8 light/dark and under completely dark conditions for 3 subcultures, each subculture after 4 weeks to induce callus. Calli were exposed to different light conditions, viz., white, red, blue, and green colors, and the rutin and quercetin contents after different treatments were determined by HPLC.
2.8. Effect of Applied Light Quality on Shootlet Cultures, and Multiplied Shoot and Hairy Root Cultures
Fresh subculture shoot, multiplied shoot, and transformed hairy root cultures were incubated in a cabinet and exposed to continuing irradiation of different light spectra: white, blue, green, and red. The specific light conditions were maintained using TP 40 W lamps (TORNADO Fluorescent Lamp, El Araby Co., Egypt), with intensity 470 μmol/(m2 s). Blue fluorescent light = 470 nm, green fluorescent light = 550 nm, and red fluorescent light = 665 nm. Cultures were incubated under a photoperiod of 16/8 h light/dark. The cultures were evaluated after two subcultures (each one 4 weeks) of different light exposure. Cultures grown under white light were used as a control.
2.9. Extraction of Different Shootlets, Hairy Root, and Calli
All harvested tissue culture (shoot, stem, leaf, and calli) and hairy root treatments were freeze dried followed by extraction with methanol (3 × 100 mL) at room temperature. The pooled methanol extracts were evaporated under vacuum to yield the total methanol extract. Different extracts were separately dissolved in methanol and kept at 4 °C for further analysis using high-performance liquid chromatography (HPLC).
2.10. High-Performance Liquid Chromatography with a Diode-Array Detector (HPLC–DAD) Analysis of Different Treatments
For HPLC determination, analysis was performed on Agilent 1100 series HPLC system (Agilent Technologies, Palo Alto, CA) equipped with a quaternary pump G1311A, degasser G1322A, UV detector, and Agilent Chem Stations software. The separation was carried out on a LiChrospher RP-18 HPLC column (250 mm × 4.6 mm, 5 μm; Merck, Germany). The mobile phase was composed of solvent A (0.1% TFA in water) and solvent B (acetonitrile). The elution was carried out according to the following elution: 0 min 5% B till 12 min 100% B; the flow rate was 1.0 mL/min. The analysis was carried out in triplicates at room temperature, and the detection wavelength was set at 250, 280, and 320 nm. The concentration of each methanolic extract was 10 mg/mL. One milligram of each standard compound (rutin and quercetin) was accurately weighed, dissolved in methanol, and used to establish calibration curves at a concentration range of 7.0–500 μg/mL.
2.11. Isolation of Quercetin and Rutin from Shootlets
2.11.1. Extraction of Shootlets
The harvested tissue culture (buckwheat cultures under blue fluorescent light and the previously mentioned media) were freeze dried followed by extraction with methanol (3 × 100 mL) at room temperature. The pooled methanolic extracts were evaporated under vacuum to yield the total methanol extract (285 mg).
The produced methanolic extract was then suspended in water and partitioned with methylene chloride (3 × 200 mL), and the pooled methylene chloride extracts were evaporated under vaccum to obtain 50 mg of MCE. The remaining aqueous layer was extracted with ethyl acetate (3 × 200 mL). The pooled fractions were dried under vacuum to produce the ethyl acetate fraction (EtOAcF, 65 mg). The different fractions were investigated for their quercetin and/or rutin content using Co-TLC and HPLC against standard compounds.
2.11.2. Isolation of Pure Compounds
The isolation of quercetin and rutin was carried out from the methylene chloride and EtOAc fractions using semipreparative HPLC. Isolation was performed using the Agilent 1100 series HPLC system (Agilent Technologies, Palo Alto, CA) equipped with a quaternary pump G1311A, degasser G1322A, UV detector, and Agilent Chem Stations software. The separation was carried out on a Kromasil RP-18 HPLC column (250 mm × 10 mm, 5 μm; Sweden). The mobile phase was composed of solvent A (0.1% TFA in water) and solvent B (acetonitrile). The elution was carried out according to the following elution: 0 min 5% B till 15 min 100% B; the flow rate was 2.5 mL/min; DAD was adjusted to 250, 280, and 320 nm.
2.12. Statistical Analysis
All data are represented as means ± SD. One-way and two-way ANOVA were performed using Graph Pad prism version 9.0 followed by Tukey’s post hoc test for comparison of means.
3. Results
Our primary results revealed that seeds of the common buckwheat (F. esculentum) contain 3.7 mg of rutin/g of extract and traces of quercetin. However, these common buckwheat seeds cannot be cultivated naturally in Egypt. Therefore, our main target is to produce the plant in the laboratory using the tissue culture technique. In addition, subcultured samples (shoots, leaves, stems, hairy roots, and calli) were investigated for their rutin and quercetin contents compared to the seeds. Generally, the seeds were used as a plant material for starting the in vitro culture. Sterilized seeds were left to germinate in the basal MS medium17 under a photoperiod of 16/8 h light/dark and 25 ± 2 °C. The edible plant parts (stems and leaves) were used as sources for the hairy root transformation and callus induction. All of the treated samples, in addition to the seeds of the common buckwheat, were assessed for quantitative comparison of their contents of rutin and quercetin.
3.1. Multiplication of Shootlet Cultures
Only the healthy shoots with high multiplication rate were observed and selected on the MS medium supplemented with different concentrations of kinetin with or without activated charcoal (AC). It is worth declaring that a high multiplication rate implies increased shootlet height, number, and consequently, number of leaves per shootlet. We observed that shoots grown in the medium supplemented with activated charcoal were healthier than those grown in the medium without AC (Figure 1).
Figure 1.
(A) Seeds of common buckwheat, (B) shoot multiplication in media containing activated charcoal, and (C) highly proliferating hairy root transformation of buckwheat.
3.2. Establishment of Hairy Root Culture
Hairy roots were induced by injection or inoculation of the edible plant parts (stems and leaves) of the common buckwheat with Agrobacterium rhizogenes strain A4.18 Our observation reveals that hairy roots were induced within 10 days after inoculation emerging on the explants. Hairy roots were formed only on the wounded regions of the explants. Using young leaf explants resulted in greater enhancement of the transformation frequency than stem explants (Figure 1). The transformed hairy roots were established in free liquid or solid MS medium. They were subcultured in the same medium every 3 weeks and incubated at 25 °C in a rotary shaker at 100 rpm under dark condition to maintain hairy root lines. To confirm the insertion of rol genes, PCR-based analysis of rolb gene was conducted to assess the genetic transformation of the hairy root-transformed lines. Moreover, virD2 gene was used to confirm the absence of A. rhizogenes in the hairy root-transformed explants.
3.3. Callus Induction
Generally, callus cultures are produced from any differentiated plant structure such as leaf, stem, or root by placing the explants in media containing a relatively high level of auxin (to promote a physiological change in previously differentiated plant tissue, leading to cell differentiation and division) and low level of cytokinin. Also, the response of the explant in the culture media depends on the endogenous growth substances present at the time of the explant excision.
Regarding the effect of white light and dark conditions on callus induction, our results revealed that callus induction under white light condition is more effective than callus induction under complete dark condition. Also, callus induction from stem explant showed the fastest induction and the stem explant was more friable than other explants (shoot and leaf). In addition, the highest callus weight was produced from media containing 1.5 and 2.0 mg/L picloram (M3 and M4) in comparison with other media concentrations.
3.4. Determination of Rutin and Quercetin Contents in Different Treatments
3.4.1. Effect of Different Lights and Media on Rutin and Quercetin Contents in Shootlets
Shootlet cultures under green light did not show any significant accumulation of target compounds. However, under white, red, and blue lights, accumulation of rutin was 3 mg/g, 920 μg/g, and 2.1 mg/g extract, respectively (Figure 2). Therefore, only white, red, and blue lights were used in further multiplication of shootlets under different media compositions.
Figure 2.
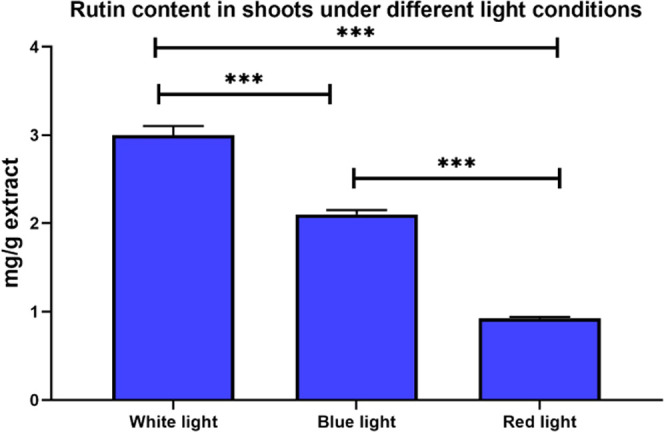
Concentrations of rutin (mg/g extract) in shoots under different light stress conditions. ***p < 0.001.
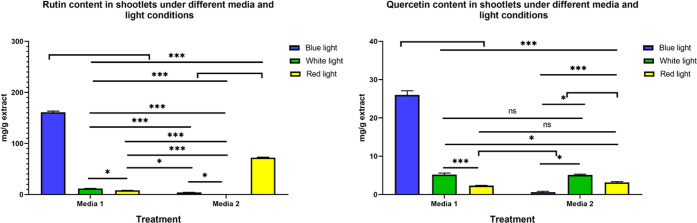
As mentioned before, shoots grown in the medium supplemented with activated charcoal (AC) were healthier than those grown in the medium without AC due to prevention of the vitrification (hyperhydricity) phenomena. After the continuous multiplication of common buckwheat shoots, under the previously mentioned light stress conditions, charcoal, and two kinetin concentrations, the highest yield of rutin (Figure 2) was 161 mg/g of extract (16% w/w; having almost 20-fold increase) observed in the multiplied shoots treated with blue light in media 1 (containing 0.25% kinetin), followed by 72 mg/g of extract in those treated with red light in media 2 (containing 0.5% kinetin), and 11.6 mg/g of extract in those grown under white light in media 1. In addition, quercetin was markedly accumulated in multiplied shoot cultures under different light treatments, with the highest content observed in the multiplied shoots treated with blue light (26 mg/g extract), followed by those grown under white light (5.1 and 5.2 mg/g of extract in media 1 and 2, respectively) (Figure 3).
Figure 3.
Concentrations of rutin and quercetin (mg/g extract) in the multiplied shoots grown in different media under different light stress conditions: media 1 = 0.25% kinetin; media 2 = 0.5% kinetin; ***p < 0.001, *p < 0.05; ns, nonsignificant.
3.4.2. Determination of Rutin and Quercetin Contents in Hairy Root Cultures
Regarding the hairy roots established from leaves and shoots, low yields of rutin were observed under white, green, and red lights. Surprisingly, the hairy root raised from leaves exposed to red light showed a markedly high yield of quercetin, attaining 10 mg/ g of extract with traces of rutin.
3.4.3. Determination of Rutin and Quercetin Content in Calli with Different Media and Light Conditions
According to Table 2, most of the treatments showed the presence of trace amounts of both compounds. However, common buckwheat treated with media containing 0.5 mg/L picloram and kept under white fluorescence showed the highest amount of rutin (5 mg/g extract) and 0.9 mg/g extract of quercetin.
Table 2. Rutin and Quercetin Contents in the Calli of Common Buckwheat under Different Light and Mediaa.
| light treatment | media | quercetin mg/g extract | rutin mg/g extract |
|---|---|---|---|
| white | M1 | 0.963 ± 0.02 | 5.954 ± 0.1 |
| M2 | 0.020 ± 0.01 | 0.168 ± 0.01 | |
| M3 | 0.098 ± 0.03 | 0.607 ± 0.03 | |
| M4 | ND | ND | |
| blue | M1 | ND | ND |
| M2 | ND | ND | |
| M3 | 0.012 ± 0.01 | 0.024 ± 0.02 | |
| M4 | 0.041 ± 0.04 | 0.059 ± 0.01 | |
| red | M1 | 0.025 ± 0.05 | 0.009 ± 0.01 |
| M2 | 0.034 ± 0.02 | 0.046 ± 0.01 | |
| M3 | 0.122 ± 0.06 | 0.191 ± 0.03 | |
| M4 | 0.014 ± 0.01 | 0.026 ± 0.01 | |
| Green | M1 | 0.057 ± 0.02 | 0.053 ± 0.02 |
| M2 | 0.033 ± 0.02 | 0.052 ± 0.02 | |
| M3 | ND | ND | |
| M4 | 0.079 ± 0.01 | 0.070 ± 0.01 |
ND; not detected.
3.5. Isolation of Rutin and Quercetin from Multiplied Shootlets
The methylene chloride and EtOAc fractions from the methanol extract of shootlets grown at blue fluorescence were identified with quercetin and rutin contents through CO-TLC and HPLC comparison with authentic compounds (Figure 4). Using semipreparative RP-HPLC, quercetin and rutin were isolated in high purity (>95%); rutin was eluted at 9.5 min, while quercetin was eluted at 12.4 min. The isolated compounds were separately evaporated under reduced pressure to obtain rutin (1.5 mg, yellowish powder) and quercetin (1 mg, yellow powder). The identity of the compounds was confirmed by comparing their UV spectra and Rt with those of the authentic compound (Supporting Figure S1). In addition, the identity of the compounds was further confirmed by NMR and ESI mass analysis of the isolated pure compounds (Supporting Figures S2–S4).
Figure 4.
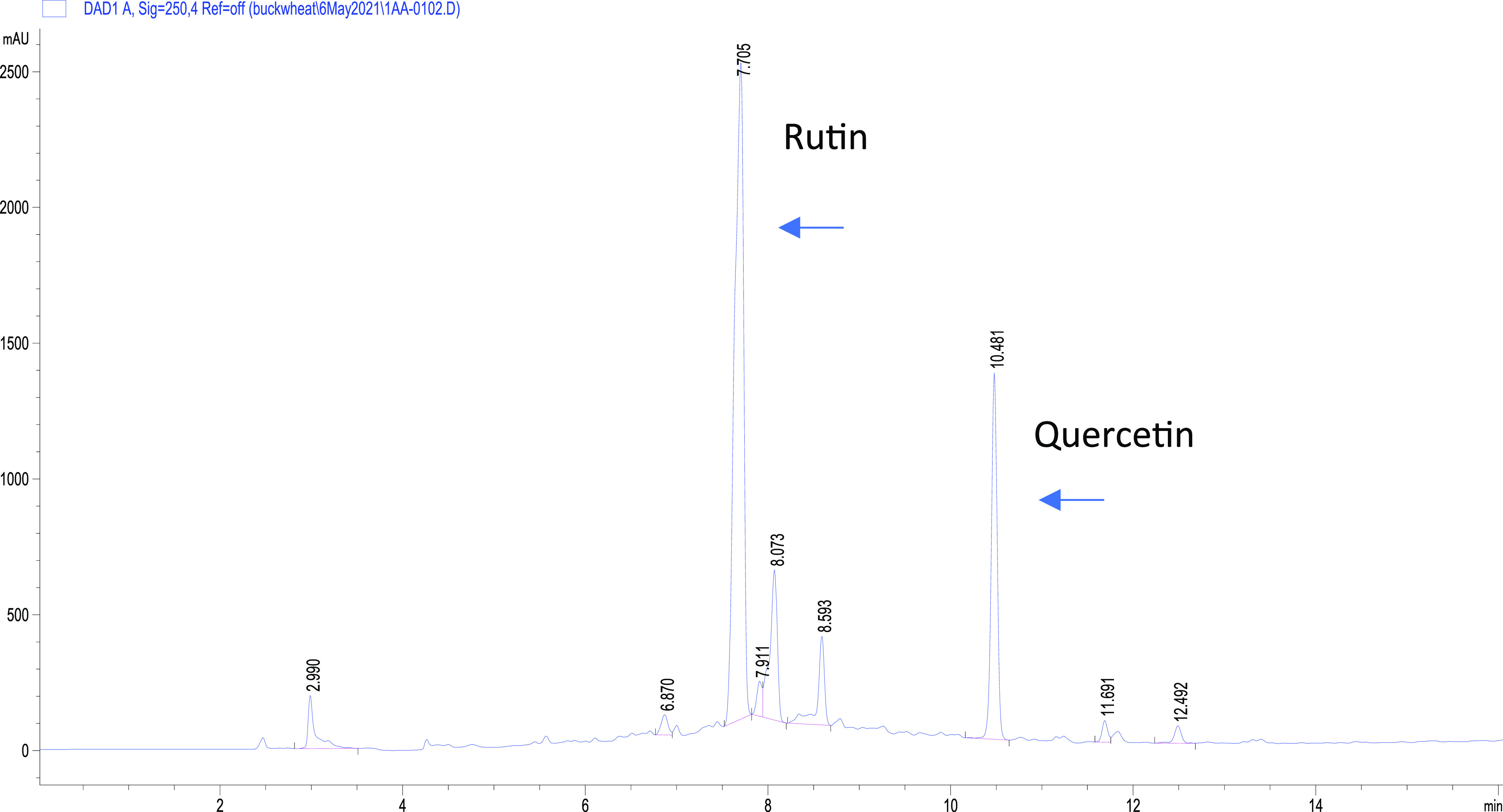
HPLC chromatogram of the ethyl acetate fraction of shootlet cultures.
3.6. Identification of Isolated Compounds
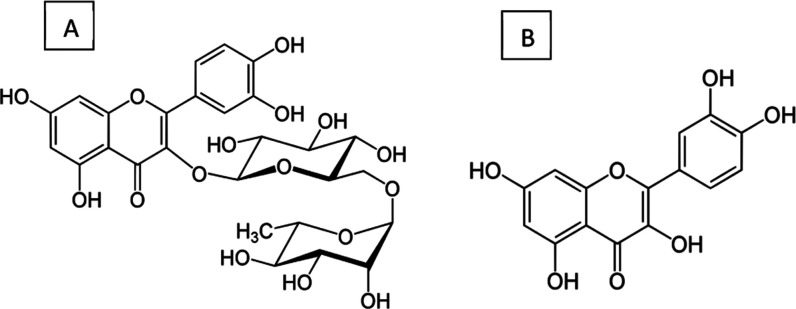
The isolated compounds (Figure 5) were identified using 1H-NMR and ESI mass spectrometry (Supporting Material, Figures S2–S4). The 1H-NMR spectrum of quercetin showed the characteristic signals for the aromatic protons at C-6 (6.2 ppm) and 8 (6.4 ppm) appearing as a doublet (m, J = 2.0 Hz), each integrated for 1 proton, in addition to the characteristic protons of the ABX system at ring B of the flavonoid nucleus appearing at 6.9 (1H, d, J = 8Hz, H-5), 7.65 (1H, dd, J = 8, 2 Hz, H6), and 7.75 (1H, d, J = 2 Hz, H-2) (Supporting Material, Figure S3). The ESI mass negative ion mode revealed the molecular ion peak at 301 reported for quercetin (Supporting Material, Figure S4).
Figure 5.
Chemical structures of flavonoids obtained from buckwheat seeds: (A) rutin and (B) quercetin.
The 1H-NMR spectra of rutin revealed signals similar to those of quercetin, representing the aglycone moiety. In addition, two anomeric protons at 5.15 (1H, d, J = 8 Hz) corresponding to the anomeric proton for glucose and at 4.53 (1H, d, J = 0.5 Hz) corresponding to the α-oriented anomeric proton of rhamnose were observed. The presence of rhamnose was further confirmed by the presence of the methyl at 1.13 (3H, d, J = 6 Hz). Moreover, the sugar protons appeared between 3.25 and 3.85.
4. Discussion
Plant growth regulators are essential for the growth and production of secondary metabolites from plant cultures. Benzyl amino purine and naphthalene acetic acid combination was found essential for callus production from the roots and leaves of Atropa acuminate.(19) Thidiazuron was found to be superior to both kinetin and benzyl adenine in the growth and production of photosynthetic pigments in organ cultures of Hyssopus officninalis.(20) The use of a combination of plant growth regulators such as kinetin and benzyl amino purine resulted in a marked effect on callus growth and the type of accumulated metabolites.21 In the current study, the use of kinetin in two concentrations showed a marked difference in the accumulation of both rutin and quercetin in cultured shoots.
The use of light stress is a common technique widely used for the production of secondary metabolites from plant cultures. Green light was found to be more effective than other light stress conditions in increasing the accumulation of saponins in ginseng callus.22 Similarly, chlorogenic acid was produced in high amounts from Gardenia jasmonides upon exposure to white LED light.23 Red/blue LED lights in the ratio of 1:1 increased the accumulation of 20-hydroxyecdysone in Brazilian ginseng cultures.24 Similar effects were found in Hypericum perforatum and Rehmannia elata.25,26 In the current study, changing light quality revealed a marked effect on the accumulation of secondary metabolites in buckwheat shoots. Initial culture trials showed that white and blue lights are superior to other lights in accumulation of rutin. Upon continuous multiplication of shootlets and using growth regulators such as kinetin, blue light showed a marked increase in both rutin and quercetin contents over red and white lights.
Several trials have been carried out for the production of secondary metabolites, especially from Tartary buckwheat; however, few studies have been done regarding the common buckwheat. In contrast to our results, blue light decreased quercetin and rutin contents in Tartary buckwheat, while both soluble and insoluble proanthocyanidins were increased,27 while red light increased the rutin content by 1.5-folds. Huang et al proved that UV-B irradiation increased rutin and quercetin accumulation in the hairy root cultures of Tartary buckwheat.28 In other reports, yeast polysaccharides and endophytic fungal elicitors were used to increase the accumulation of rutin and phenolics in buckwheat.29 Complementary to the previous trials, the current study introduces a promising method by using blue light in combination with media containing 0.25% of kinetin for the production of high amounts of both quercetin and rutin, which can be scaled up for preparative isolation and purification.
5. Conclusions
From the different biotechnological approach applied in this study, we concluded that activated charcoal is essential in the media in order to obtain the highest level of both flavonoids (rutin and quercetin). Also, blue light revealed its ability to produce about 4.3 mg and 7 mg/g of rutin and quercetin, respectively, in the shoots compared to 3.7 mg/g in the seeds of the common buckwheat. Indeed, the continuous multiplication of shoots helped to obtain 20-folds increase of rutin content (161 mg/g) and a higher level of quercetin (26 mg/g). However, other light conditions such as red light produced a high yield of quercetin (10 mg/g of extract) in the hairy root cultures of the leaves; meanwhile, other light conditions such as white, green, and blue did not show significant effects on the hairy roots raised from leaves of buckwheat.
Acknowledgments
This research is carried out and fully funded under the program of the National Strategy for Biotechnology and Genetic Engineering. The program is administered by the Academy of Scientific Research and Technology.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c02728.
HPLC chromatogram and UV spectra of alcoholic extract of shootlet cultures; NMR data of quercetin and rutin; mass spectra of quercetin and rutin (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Huda M. N.; Lu S.; Jahan T.; Ding M.; Jha R.; Zhang K.; Zhang W.; Georgiev M. I.; Park S. U.; Zhou M. Treasure from garden: Bioactive compounds of buckwheat. Food Chem. 2020, 335, 127653 10.1016/j.foodchem.2020.127653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthar Z.; Germ M.; Likar M.; Golob A.; Vogel-Mikuš K.; Pongrac P.; Kušar A.; Pravst I.; Kreft I. Breeding Buckwheat for Increased Levels of Rutin, Quercetin and Other Bioactive Compounds with Potential Antiviral Effects. Plants 2020, 9, 1638 10.3390/plants9121638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitane I.; Krumina-Zemture G. Evaluation of nutritional quality of raw and roasted buckwheat (Fagopyrum esculentum M.) fluor. J. Int. Sci. Publ.: Agric. Food 2017, 5, 51–56. [Google Scholar]
- Mota C.; Nascimento A. C.; Santos M.; Delgado I.; Coelho I.; Rego A.; Matos A. S.; Torres D.; Castanheira I. The effect of cooking methods on the mineral content of quinoa (Chenopodium quinoa), amaranth (Amaranthus sp.) and buckwheat (Fagopyrum esculentum). J. Food Compos. Anal. 2016, 49, 57–64. 10.1016/j.jfca.2016.02.006. [DOI] [Google Scholar]
- Ciacci C.; Ciclitira P.; Hadjivassiliou M.; Kaukinen K.; Ludvigsson J. F.; McGough N.; Sanders D. S.; Woodward J.; Leonard J. N.; Swift G. L. The gluten-free diet and its current application in coeliac disease and dermatitis herpetiformis. United Eur. Gastroenterol. J. 2015, 3, 121–135. 10.1177/2050640614559263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A.; Khalid N.; Ahmad A.; Abbasi N.; Latif M.; Randhawa M. Phytochemicals and biofunctional properties of buckwheat: a review. J. Agric. Sci. 2014, 152, 349–369. 10.1017/S0021859613000166. [DOI] [Google Scholar]
- Zhou M.; Kreft I.; Suvorova G.; Tang Y.; Woo S.-H.. Buckwheat Germplasm in the World; Academic Press, 2018. [Google Scholar]
- Bai C.; Feng M.; Hao X.; Zhong Q.; Tong L.; Wang Z. Rutin, quercetin, and free amino acid analysis in buckwheat (Fagopyrum) seeds from different locations. Genet. Mol. Res. 2015, 14, 19040–19048. 10.4238/2015.December.29.11. [DOI] [PubMed] [Google Scholar]
- Ganeshpurkar A.; Saluja A. K. The pharmacological potential of rutin. Saudi Pharm. J. 2017, 25, 149–164. 10.1016/j.jsps.2016.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi B.; Machin L.; Monzote L.; Sharifi-Rad J.; Ezzat S. M.; Salem M. A.; Merghany R. M.; El Mahdy N. M.; Kılıç C. S.; Sytar O.; et al. Therapeutic potential of quercetin: new insights and perspectives for human health. ACS Omega 2020, 5, 11849–11872. 10.1021/acsomega.0c01818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain M. S.; Fareed S.; Saba Ansari M.; Rahman A.; Ahmad I. Z.; Saeed M. Current approaches toward production of secondary plant metabolites. J. Pharm. BioAllied Sci. 2012, 4, 10 10.4103/0975-7406.92725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulabagal V.; Tsay H.-S. Plant cell cultures-an alternative and efficient source for the production of biologically important secondary metabolites. Inter. J. Appl. Sci. Eng. 2004, 2, 29–48. [Google Scholar]
- Stefano M.; Rosario M.. Effects of Light Quality on Micropropagation of Woody Species. In Micropropagation of Woody Trees and Fruits; Springer, 2003; pp 3–35. [Google Scholar]
- Gabr A. M.; Ebrahim H. S.; El-Ashry A. A.; El-Bahr M. K.. Importance of Artificial Environment Conditions on Plant Biotechnology, Plant Growth, and Secondary Metabolites. In Precision Agriculture Technologies for Food Security and Sustainability; IGI Global, 2021; pp 292–319. [Google Scholar]
- Tang Y.; Zhao G. Relationship between Phenylalanine Ammonialyase Activity and Flavone Content in Buckwheat. Plant Physiol. Commun. 1992, 28, 419–420. [Google Scholar]
- Nam T. G.; Kim D.-O.; Eom S. H. Effects of light sources on major flavonoids and antioxidant activity in common buckwheat sprouts. Food Sci. Biotechnol. 2018, 27, 169–176. 10.1007/s10068-017-0204-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T.; Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Gabr A. M. M.; Sytar O.; Ghareeb H.; Brestic M. Accumulation of amino acids and flavonoids in hairy root cultures of common buckwheat (Fagopyrum esculentum). Physiol. Mol. Biol. Plants 2019, 25, 787–797. 10.1007/s12298-019-00669-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar S. A.; Nawchoo I. A.; Tyub S.; Kamili A. N. Effect of plant growth regulators on in vitro induction and maintenance of callus from leaf and root explants of Atropa acuminata Royal ex Lindl. Biotechnol. Rep. 2021, 32, e00688 10.1016/j.btre.2021.e00688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoja H. M.; Shishavan H. K. Effects of different hormonal treatments on growth parameters and secondary metabolite production in organ culture of Hyssopus officinalis L. Biotechnologia 2021, 102, 33–41. 10.5114/bta.2021.103760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junairiah; Rahmawati R. K.; Manuhara Y. S. W.; Ni’matuzahroh; Pramudya M.; Sulistyorini L. Induction and identification of bioactive compounds from callus extract of Piper betle l. Var. nigra. Malays. J. Anal. Sci. 2020, 24, 1024–1034. [Google Scholar]
- Ren Y. Y.; Niu C.; Wang J. J.; Yang H.; Xu Y. H.; Liu Z. Effects of Different Light Qualities on Growth and Ginsenoside Contents in Callus of Panax ginseng. Spectros. Spectral Anal. 2022, 42, 1318–1322. 10.3964/j.issn.1000-0593(2022)04-1318-05. [DOI] [Google Scholar]
- El Ashry A. A.; Ebrahim H. S.; Rabie S. A. A.; El-Bahr M. K.; Gabr A. M. M. Influence of Light Quality on Growth and Secondary Metabolite in Tissue Cultures of Gardenia jasminoides, Variegata. Egypt. J. Chem. 2022, 65, 695–702. 10.21608/EJCHEM.2021.102068.4739. [DOI] [Google Scholar]
- Silva T. D.; Batista D. S.; Fortini E. A.; Castro K. M. D.; Felipe S. H. S.; Fernandes A. M.; Sousa R. M. D. J.; Chagas K.; Silva J. V. S. D.; Correia L. N. D. F.; et al. Blue and red light affects morphogenesis and 20-hydroxyecdisone content of in vitro Pfaffia glomerata accessions. J. Photochem. Photobiol., B 2020, 203, 111761 10.1016/j.jphotobiol.2019.111761. [DOI] [PubMed] [Google Scholar]
- Sobhani Najafabadi A.; Khanahmadi M.; Ebrahimi M.; Moradi K.; Behroozi P.; Noormohammadi N. Effect of different quality of light on growth and production of secondary metabolites in adventitious root cultivation of Hypericum perforatum. Plant Signal. Behav. 2019, 14, 1640561 10.1080/15592324.2019.1640561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piątczak E.; Kuźma; Kozłowska W.; Lisiecki P.; Szemraj M.; Płachno B. J.; Gonciarz W.; Chmiela M.; Matkowski A.; Zielińska S. Phenylethanoid and iridoid glycosides production in Rehmannia elata N.E.Brown ex Prein. in vitro shoot cultures and their biological activity. Ind. Crops Prod. 2020, 158, 113050 10.1016/j.indcrop.2020.113050. [DOI] [Google Scholar]
- Gumerova E. A.; Akulov A. N.; Rumyantseva N. I. Red and blue light have different effects on flavonols and proanthocyanidins accumulation in the cell culture of tartary buckwheat. AIP Conf. Proc. 2021, 2388, 030012 10.1063/5.0072174. [DOI] [Google Scholar]
- Huang X.; Yao J.; Zhao Y.; Xie D.; Jiang X.; Xu Z. Efficient rutin and quercetin biosynthesis through flavonoids-related gene expression in Fagopyrum tataricum gaertn. Hairy root cultures with UV-B irradiation. Front. Plant Sci. 2016, 7, 63 10.3389/fpls.2016.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J.; Xiang D.; Peng L.; Zou L.; Wang Y.; Zhao G. Enhancement of rutin production in fagopyrum tataricum hairy root cultures with its endophytic fungal elicitors. Prep. Biochem. Biotechnol. 2014, 44, 782–794. 10.1080/10826068.2013.867872. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.