Abstract
Background
Optogenetic modalities as well as optochemical and photopharmacological strategies, collectively termed optical methods, have revolutionized the control of cellular functions via light with great spatiotemporal precision. In comparison to the major advances in the photomodulation of signaling activities noted in neuroscience, similar applications to endocrine cells of the pancreas, particularly insulin-producing β-cells, have been limited. The availability of tools allowing light-mediated changes in the trafficking of ions such as K+ and Ca2+ and signaling intermediates such as cyclic adenosine monophosphate (cAMP), renders β-cells and their glucose-stimulated insulin secretion (GSIS) amenable to optoengineering for drug-free control of blood sugar.
Scope of review
The molecular circuit of the GSIS in β-cells is described with emphasis on intermediates which are targetable for optical intervention. Various pharmacological agents modifying the release of insulin are reviewed along with their documented side effects. These are contrasted with optical approaches, which have already been employed for engineering β-cell function or are considered for future such applications. Principal obstacles are also discussed as the implementation of optogenetics is pondered for tissue engineering and biology applications of the pancreas.
Major Conclusions
Notable advances in optogenetic, optochemical and photopharmacological tools are rendering feasible the smart engineering of pancreatic cells and tissues with light-regulated function paving the way for novel solutions for addressing pancreatic pathologies including diabetes.
Keywords: Optogenetics, Pancreas, Beta-cells, Insulin secretion, Diabetes
Abbreviations
- AC
adenylyl cyclase
- Ach
acetylcholine
- ADP
adenosine diphosphate
- ATP
adenosine triphosphate
- ATP/ADP
ATP/ADP ratio
- BldD
c-di-GMP–binding domain derived
- BLINK1
blue light-gated K+ channel 1
- BLINK2
blue light-gated K+ channel 2
- BLUF
blue light using flavin adenine dinucleotid
- bPAC
Beggiatoa photoactivatable adenylyl cyclase
- bReaChES
red-shifted channelrhodopsin
- cAMP
cyclic adenosine monophosphate
- cGMP
cyclic guanosine monophosphate
- ChR2
channelrhodopsin 2
- CRY2
cryptochrome 2
- DAG
diacylglycerol
- EPAC
exchange protein directly activated by cAMP
- EuPAC
Euglena gracilis photoactivatable adenylyl cyclase
- FAD
flavin adenine dinucleotide
- FADH
reduced flavin adenine dinucleotide
- GDL
D-glucono-δ-lactone
- GI
gastrointestinal
- GIP
gastric inhibitory polypeptide
- Gαi
receptors coupled Gi α subunit
- GLC
phospholipase C
- GLP-1
Glucagon-like peptide 1
- GLUT1
glucose transporter 1
- GLUT2
glucose transporter 2
- GPCR
G-protein-coupled receptor
- GPP4
dipeptidyl peptidase-4
- Gαq
receptors coupled Gq α subunit
- Gαs
receptors coupled Gs α subunit
- GSIS
glucose-stimulated insulin secretion
- HVCC
high voltage-gated Ca2+ channel
- IBMX
3-isobutyl-1-methylxanthine
- IP3
inositol trisphosphate
- JellyOp
rhodopsins from the jellyfish Carybea rastonii
- KATP+
ATP-sensitive K+ channel
- Kv
voltage-dependent K+ channel
- LAPD
light-activated PDE
- LED
light-emitting diodes
- LOV
light, oxygen, and voltage
- MGCTVSAE
myristoylation/palmitoylation domain
- MIN6-ChR2
MIN6 insulinoma cells transfected with the ChR2 gene
- NADH
Nicotinamide adenine dinucleotide
- NFAT
the nuclear factor of activated T cells
- NIR
near-infrared
- NIRW
near-infrared window
- OaPAC
Oscillatoria acuminata photoactivatable adenylyl cyclase
- p65
NF-κB–transactivating domain
- PAC
Photoactivatable adenylyl cyclase
- PAC-K
SthK and PAC system
- PDE
phosphodiesterase
- PEG
polyethylene glycol
- PHYB
phytochrome B
- PIP2
phospholipid phosphatidylinositol bisphosphate
- PKA
protein kinase A
- PKC
protein kinase C
- PLGA
polylactic-co-glycolic acid
- PMA
phorbol 12-myristate 13-acetate
- RP
reserve pool
- RRP
readily releasable pool
- SGLT
sodium-glucose cotransporter
- shGLP1
short variant of the human GLP-1
- SNARE
Soluble N-ethylmaleimide-sensitive factor attachment protein receptor
- SthK
small cAMP-gated K+ channel
- STZ
streptozotocin
- T1D
type 1 diabetes
- T2D
type 2 diabetes
- TCA
tricarboxylic acid
- TXNIP
thioredoxin-interacting protein
- VDCC
voltage-dependent calcium channels
- VP64
a tetramer of the VP16 activation domain;
1. Introduction
The regulation of major cellular functions using light has become possible through molecular customization of cells and advances in optogenetic and optochemical methods. These modalities afford superior spatial and temporal resolution allowing for precise stimulation of cells and tissues in contrast to pharmacological interventions. Optogenetic strategies were adopted early on in neuroscience with significant progress noted in their implementation [1,2], but recent successes have been reported for hearing restoration, bladder control, and cardiac muscle pacing [[3], [4], [5], [6]]. Moreover, light-mediated modulation of cellular signaling pathways has become part of the tool chest for understanding fundamental subcellular processes driving physiological responses by cells and cell ensembles.
Yet, the adoption of optical modulation systems in pancreas biology and tissue engineering has been relatively limited. While the exocrine pancreas produces digestive enzymes, the endocrine compartment supplies various hormones which are essential for homeostasis, including the control of blood sugar [7]. The pancreatic endocrine tissue comprises multiple cell types organized in clusters or islets traversed by blood vessels. Hormone secretion is orchestrated through interactions among neighboring cells and by extracellular stimuli. Greater understanding of this complex environment is necessary for developing effective therapies for pathologies of the pancreas. Among the endocrine cells, insulin-producing β-cells along with glucagon-secreting α-cells play a critical role in the maintenance of normoglycemia. This is evident in diabetes where extensive damage of the β-cells due to autoimmunity (type 1 diabetes; T1D) or insulin resistance (type 2 diabetes; T2D) mandates the administration of exogenous insulin in all T1D patients and over 30% of patients with advanced T2D. However, control of diabetes is suboptimal relying on manual calculation of the amount of insulin to be infused based on factors such as blood glucose, food caloric intake, etc. Patients with T2D are prescribed pharmacological regimens for boosting the amount of insulin released by their pancreas or facilitating the removal of excess glucose from the bloodstream. Nonetheless, compliance and side effects such as severe hypoglycemia, kidney dysfunction, and liver damage are vexing issues.
Basic research in pancreas biology and translation to clinically relevant technologies stand to benefit from advances in optogenetics. Islet hormone secretion is mediated by the trafficking of ions (e.g., Ca2+, K+) across compartments, membrane depolarization, and changes in metabolic and signaling intermediates (e.g., cyclic adenosine monophosphate (cAMP)). Conceivably, these molecular events can be paired with photoactivatable moieties such as opsins, which modify ionic fluxes across membranes, and proteins featuring domains of light, oxygen, and voltage (LOV), adenylyl cyclase activity (e.g., bPAC [8,9]), or interacting complementary regions (e.g., phytochrome B (PHYB), cryptochrome 2 (CRY2) [10], phytochrome interacting factor 6 (PIF6) [11]). For instance, elevated GSIS has been reported in β-cells expressing channelrhodopsin 2 (ChR2) where exposure to light activates ChR2 causing membrane depolarization and activation of Ca2+ channels [12]. Also, β-cells expressing bPAC exhibit higher insulin secretion rates when exposed to blue light [13] further supporting the use of optogenetic tools for advancing our knowledge of pancreas physiology and the development of medically relevant treatments.
It should be noted that this review focuses on β-cells, rather than other islet cell types, since amelioration of the reduction in/ablation of β-cell mass and insulin release is a major aim of relevant cell replacement strategies. To this end, the molecular pathway underpinning the glucose-stimulated insulin secretion (GSIS) of β-cells is presented with emphasis on intermediates amenable to optical intervention. Moreover, the drug-based modulation of these pathways is described along with documented off-target effects. Against this backdrop, we look mainly at reported optogenetic approaches, in addition to optochemical, and photopharmacological ones and how these have been or can be employed for engineering islet cell function. Major challenges to the realization of these objectives are discussed in the last section. As noted in the fields of brain biology and heart pathophysiology, optogenetics will help gain new insights regarding the complex functions of the pancreas, particularly of hormone-secreting islet cells. Conversely, light-regulated engineered cells and tissues can be part of therapeutic solutions for maladies of the pancreas.
2. Molecular circuit(s) governing GSIS of β-cells
2.1. Glucose metabolism in β-cells
Glucose sourced via ingestion of food in the small intestine enters the bloodstream and eventually reaches the liver and the pancreas. Plasma glucose levels of 4–5 mM are reported in the fasting state, and ∼7 mM is considered the threshold for metabolic events in pancreatic β-cells [14], which are the sole secretors of insulin in the body. A basic description is provided below of the molecular pathway(s) involved in β-cell GSIS with emphasis on major intermediates, which are potential targets for optogenetic intervention.
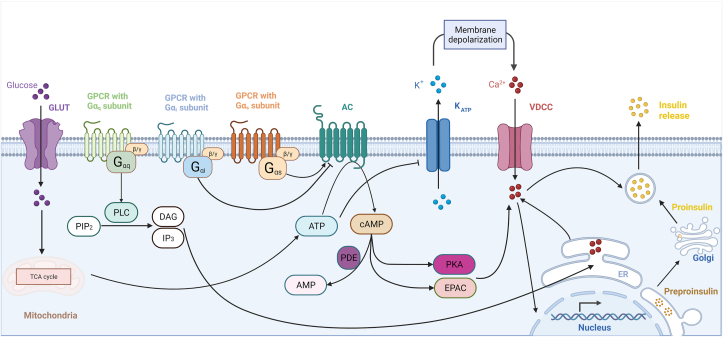
To enter β-cells (Figure 1), glucose undergoes facilitated diffusion through the transmembrane glucose transporter GLUT1 (in humans) or GLUT2 (in rodents). Intracellular glucose is phosphorylated by glucokinase (hexokinase IV in humans) in the first step of glycolysis, eventually leading to the generation of pyruvate. Pyruvate enters the Krebs’ (tricarboxylic acid; TCA) cycle in the mitochondria, producing nicotinamide adenine dinucleotide (NADH) and reduced Flavin adenine dinucleotide (FADH). These energy carriers are reduced by oxidative phosphorylation in a highly O2-intensive process to generate ATP [15]. Under suboptimal oxygen supply, animal cells can convert pyruvate to lactate, but β-cells express minimal levels of the catalyzing enzyme lactate dehydrogenase [16].
Figure 1.
Beta-cell GSIS molecular pathways. Glucose enters the β-cell from the bloodstream via the GLUT transporter and is catabolized yielding ATP. ATP induces the closure of KATP+ channels causing changes in membrane conductance leading to its depolarization. In response, VDCCs enhance Ca2+ influx, which triggers the migration and fusion of secretory granules to the cellular membrane, leading to insulin secretory granule exocytosis. Among various types of GPCRs, incretins that activate Gq can stimulate PLC to convert PIP2 to DAG and IP3, and release cytosolic [Ca2+]i that results in insulin release. On the other hand, Gi-coupled GPCR inhibits AC, while incretins, such as GLP-1, can bind to Gs, and stimulate AC activity. AC catalyzes the conversion of ATP to cAMP, which activates both PKA and EPAC enhancing VDCC activity, and eliciting insulin exocytosis. The PDEs degrade cAMP.
The primary effect of glucose catabolism is the increase in the ratio of ATP to ADP (ATP/ADP) with multiple downstream effects. ATP binds to an ATP-sensitive K+ (KATP+) channel, which consists of four pore-forming inward rectifier potassium channel subunits (Kir6.2; KCNJ11) and four sulfonylurea receptor subunits (SUR1; ABCC8) [14,17]. The Kir6.2 subunits bind metabolism-generated ATP causing the KATP+ channels to close. The SUR1 subunits feature nucleotide-binding sites inducing KATP+ closure upon interacting with insulin secretagogues such as sulfonylurea and glinide [14,17]. With KATP+ channels shut, membrane permeability is altered contributing to its depolarization. Hence, plasma glucose >6 mM reduces KATP+ channel activity by more than 75%. Conversely, β-cells are electrically silent in the absence of glucose due to active KATP+ channels and the maintenance of K+ efflux [14]. Serving as a halting mechanism of GSIS are the voltage-dependent K+ channels (Kv) and Ca2+-sensitive voltage-dependent K+ channels that repolarize the cell membrane, effectively suspending Ca2+ transport [14].
As the β-cell membrane depolarizes with elevated ATP/ADP, it reaches an activation threshold for voltage-dependent calcium channels (VDCC) to increase conductance and allow Ca2+ to enter the cell [14]. Membrane depolarization and intracellular Ca2+ concentration ([Ca2+]i) are both oscillatory rather than biphasic [17]. The rise in β-cell [Ca2+]i induces the fusion of insulin granules to the plasma membrane via a soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) and the eventual release of the hormone [17].
In addition to the KATP+-mediated insulin secretion, a parallel pathway (KATP+ channel-independent or metabolic amplifying pathway) in β-cells is responsible for regulating at least 50% of the total hormone release postprandially [18]. The pathway involves transmembrane G-protein-coupled receptors (GPCRs) which are important drug targets. Stimulated GPCRs proceed to activate heterotrimeric G-proteins composed of α, β, and γ subunits. The α subunits are divided into four functionally distinct classes: Gq, Gs, Gi, and G12/13. The type of activated Gα dictates the role of GPCR signaling on the β-cell signaling and insulin secretion [19,20].
GPCR-mediated triggering of Gq leads to phospholipase C (PLC)-catalyzed conversion of phospholipid phosphatidylinositol bisphosphate (PIP2) into diacylglycerol (DAG) and inositol trisphosphate (IP3) with subsequent release of Ca2+ from the endoplasmic reticulum (ER) triggering the release of insulin [19].
The Gs α-subunit can bind to the membrane-associated protein adenylyl cyclase (AC). There are nine transmembrane and one soluble isoforms of AC in different tissues with two main catalytic domains C1 and C2 [21]. Both insulinoma lines and primary β-cells in humans and rodents express different AC isoforms, including AC5 and AC6 [22] as well as the Ca2+- and calmodulin-activated isoforms AC1, AC3, and AC8 [23]. Activation of AC results in the conversion of ATP to cyclic adenosine monophosphate (cAMP). Conversely, phosphodiesterases (PDEs) continually degrade cAMP [24], bringing its concentration down to baseline once the incretin signal is attenuated. There are eleven PDE isoforms, of which human and rodent islets, and β-cell lines express: PDE1, PDE3, PDE4, PDE7, PDE8, PDE10, and PDE11 [25]. Among the PDE subtypes expressed in β-cell, PDE1, PDE3, and PDE4 are better studied and account largely for the regulation of cAMP levels in the context of GSIS [26]. Thus, Gs-targeting incretins promote the elevation of intracellular cAMP. In contrast, the activation of Gi results in inhibitory effect of AC, cAMP formation and insulin secretion.
The incretin-mediated increase in cAMP leads to activation of the protein kinase A (PKA), which phosphorylates and enhances the function of VDCCs, allowing for greater Ca2+ influx, and therefore stimulation of membrane fusion and exocytosis of insulin granules [18,27]. PKA also directly phosphorylates and closes KATP+ channels in an ADP-dependent manner resulting again in augmenting membrane depolarization and VDCC activity [18]. Cyclic AMP may enlarge the readily releasable pool (RRP) of granules in a concentration-dependent manner independent of Ca2+, priming β-cells at stimulatory ambient glucose concentration [17].
In addition to PKA, the exchange protein directly activated by cAMP (EPAC) is a cAMP sensor, which like PKA features a cAMP binding domain, and regulates functions such as cell adhesion and junctions, secretion, differentiation, gene expression, and apoptosis [28]. While the roles of EPAC and PKA in insulin secretion require further elucidation, EPAC is involved in the priming of vesicles, inhibiting KATP+ through interaction with SUR1, and regulating ryanodine-sensitive Ca2+ channels in Ca2+-induced Ca2+ release from internal stores [[29], [30], [31]].
The biphasic nature of insulin secretion begins with a largely Ca2+-dependent, high amplitude burst of insulin exocytosis over a short duration sourced from the RRP, whose granules are already docked and primed at the plasma membrane. The second phase consists of a lower amplitude period of insulin output over a longer duration and is derived from the supplementation of the RRP by the granule storage pool, or reserve pool (RP). Insulin secretion is also known to exhibit oscillatory behavior in intervals of approximately 5 min in human patients [32]. The secretion of insulin is concomitant to that of C-peptide – a byproduct of proinsulin processing representative of de novo insulin synthesis – in a 1:1 molar ratio.
3. Pharmacological/chemical agents modulating insulin secretion
There are several known secretagogues eliciting the release of insulin by β-cells, and some have been utilized in the treatment of T2D. Our discussion is limited to insulinotropic agents (Table 1) acknowledging that drugs in clinical use for managing blood glucose may target extrapancreatic tissues. For instance, biguanides (e.g., metformin) act on the liver, lessening glucose production, and sodium-glucose cotransporter (SGLT) inhibitors reduce the renal reabsorption of glucose [33,34].
Table 1.
Summary of agents that modulate various targets in GSIS pathway.
| Molecule | Target | Type | References |
|---|---|---|---|
| ChR2 | Ca2+↑ | Optogenetics | [12,[79], [80], [81], [82]] |
| red-shifted channelrhodopsin (bReaChES) | Ca2+↑ | Optogenetics | [78] |
| Dihydropyridine | Ca2+↑ | Pharmacological | [47] |
| Phenylalkylamine | Ca2+↑ | Pharmacological | [[47], [48], [49], [50]] |
| Benzothiazepine | Ca2+↑ | Pharmacological | [47,51] |
| Caged Ca2+ | Ca2+↑ | Optochemical | [102] |
| euPAC, OaPAC, bPAC, PACmn, NIRW-AC | cAMP↑ | Optogenetics | [8,9,13,[63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74]] |
| LA-PD | cAMP↓ | Optogenetics | [75] |
| PAC-K | cAMP↑, K+↓ | Optogenetics | [71] |
| Ach | GPCR with Gq ↑ | Pharmacological | [52,53] |
| GIP, GLP-1 | GPCR with Gs ↑ | Pharmacological | [14,15,18,27] |
| JellyOp | GPCR with Gs ↑ | Optogenetics | [55,56] |
| Short- and long-wavelength-sensitive opsins | GPCR with Gi ↑ | Optogenetics | [61,62] |
| Melanopsin | GPCR with Gq ↑ | Optogenetics | [58] |
| Meglitinides | KATP+↓ | Pharmacological | [44] |
| Sulfonylurea | KATP+↓ | Pharmacological | [[41], [42], [43]] |
| BLINK1, BLINK2 | KATP+↓ | Optogenetics | [76,77] |
| Azobenzene-sulfonylurea | KATP+↓ | Photopharmacological | [[94], [95], [96], [97]] |
| IBMX | PDE↓ | Pharmacological | [39,40] |
| PMA | PKC↑ | Pharmacological | [57] |
As discussed already, cAMP and its modulators, ACs and PDEs, are central to the circuit regulating β-cell insulin secretion. Transmembrane ACs are stimulated by the Gas subunit of GPCRs, which in turn are targeted by incretins such as the gastric inhibitory polypeptide (GIP) and the glucagon-like peptide 1 (GLP-1) released from the gut after food ingestion. The GLP-1, which is a Gs-binding incretin upregulates intracellular cAMP, in addition to stimulating GLUT2 and glucokinase. These actions result in inhibition of KATP+ and Kv channels (reducing membrane repolarization), promotion of insulin biosynthesis, suppression of apoptosis and stimulation of proliferation of β-cells, curbing glucagon secretion (thus, preventing the release of glucose into the blood from hepatic storage), and delaying gastric emptying [14,15,18,27]. Because GIP and GLP-1 are rapidly degraded by dipeptidyl peptidase-4 (DPP4) [35], longer-acting agonists of the incretins (such as exendin-4 [36]) have been used for T2D treatment while DPP4 inhibitors are in development. It is reported that the use of GLP-1 agonists is linked to a lower risk of hypoglycemia for T2D patients.
The natural diteprenoid forskolin also activates ACs in a rapid, dose-dependent and reversible manner by binding close to the enzyme's catalytic site promoting C1 and C2 assembly [37]. Because forskolin has a broad spectrum of actions including the inhibition of glucose transporters and ion channels [37], and the increase of acetylcholinesterase, its activity as an insulin secretagogue lacks high specificity [38].
Inhibitors of PDEs prolong elevated intracellular cAMP, thereby amplifying the secretion of insulin by β-cells. A competitive selective PDE inhibitor is 3-isobutyl-1-methylxanthine (IBMX), which acts on all isoforms except PDE 8 or 9 [39]. At 16.7 mM glucose, the addition of 5 μM forskolin or 25 μM IBMX to diabetic rat islets leads to a 1.5- or 1.8-fold increase in insulin release, respectively [40].
The rise in cAMP induces the closure of KATP+ channels evoking β-cell membrane depolarization, Ca2+ influx, and insulin release. Sulfonylureas, which are benzoic acid derivatives, bind to the SUR1 subunits of the KATP+ channel, inhibiting the K+ influx, triggering the same process train as elevated cAMP resulting in hormone secretion [41]. It should be noted, however, that this sulfonylurea-triggered production of insulin in β-cells is essentially independent of extracellular glucose levels, given that the binding of sulfonylureas diminishes the ability of KATP+ channels to sense intracellular ATP and ADP fluctuations in response to the rise and fall in extracellular glucose. This explains the risk for hypoglycemia and hyperinsulinism for T2D patients associated with this class of agents. Chronic administration of sulfonylureas also reduces SUR1 expression on the surface of β-cells exacerbating T2D, but this effect can be reversed by discontinuing the treatment. Moreover, several cell types beyond β-cells express KATP+ channels lowering the specificity of the anti-diabetic effect of sulfonylureas. Accordingly, sulfonylurea regimens have been linked to higher risks of adverse cardiovascular outcomes in T2D given that cardiomyocytes also express KATP+ channels [42]. Unlike the first generation of sulfonylureas (e.g., acetohexamide, chlorpropamide, tolazamide, and tolbutamide) which had a high dosage requirement (100–2,000 mg/daily), second-generation agents (e.g., glyburide, glipizide, gliclazide, and glimepiride) are significantly more effective and potent (1–20 mg per day) with fewer side effects [43].
Meglitinide was introduced in 1995 as another T2D drug binding to the SUR1 and stimulating insulin secretion. Meglitinide analogs such as repaglinide, nateglinide, and mitiglinide, bind to distinct sites of the SUR1 with lower affinity compared to sulfonylureas. The shorter half-life of meglitinides may point to a lower likelihood of cardiovascular implications for diabetic patients compared to sulfonylurea treatment [42]. Additionally, nateglinide is 1000-fold more selective for KATP+ channels in β-cells vs. cardiomyocytes. In albino mouse islets, 100 μM of tolbutamide, 1 μM of glipizide or 100 μM of meglitinide stimulates a rapid 8- to 9-fold increase of insulin secretion above basal values within 10 min in the presence of 10 mM glucose [44,45].
In pancreas, high voltage-gated Ca2+ channels (HVCCs) also play a significant role of in β-cell glucoregulatory insulin release, as the influx of calcium ions via HVCC initiate the Ca2+ -dependent exocytosis of insulin [46]. HVCCs are multi-protein complexes comprising several different subunits, including the primary pore-forming transmembrane α1 subunit, along with auxiliary extracellular (α2δ), intracellular (β), and transmembrane (γ) subunits [47]. Pharmacological Ca2+ channel blockers such as dihydropyridine (nifedipine), phenylalkylamine (Verapamil), and benzothiazepine (diltiazem), target L-type channels and are widely used to treat hypertension, inhibiting Ca2+ from entering vascular smooth muscle and myocardial cells [47]. An early study shows nifedipine has a stronger effect on β-cell electrical activity compared to Verapamil. However, upon glucose stimulation, murine β-cells show high sensitivity to Verapamil but not nifedipine [48]. In rodent β-cells and islets as well as in human islets, Verapamil decreases the expression of thioredoxin-interacting protein (TXNIP), prevents β cell apoptosis, and preserves functional β-cell mass [49]. In one pilot study involving recent-onset T1D patients, once-daily oral Verapamil (from 120 mg to 360 mg) reduces the need for exogenous insulin dosage, and frequencies of hypoglycemia compared to the placebo group [50]. The International Verapamil S.R./Trandolapril Study revealed an association between calcium channel blockers use and a lower risk for newly diagnosed T2DM However, intoxication by an overdose of Verapamil or diltiazem may result in hyperglycemia, as the agonists bind to the L-type HVCCs and diminish the release of insulin [51]. Among the GPCR-targeting agents to modulate [Ca2+]i, acetylcholine (Ach), for instance, is a ligand of M3 muscarinic ACh receptors (M3Rs) inciting Gq-type GPCRs, activating the PLC pathway thereby augmenting [Ca2+]i, and GSIS in rodent and human islets [52,53].
Other secretagogues operate beyond regulating the activity of cAMP, KATP+ or Ca2+ channels. Certain amino acids, including leucine, isoleucine, alanine, and arginine, enhance insulin secretion from primary islet cells and β-cell lines under specific conditions [54,55]. Cationically charged l-arginine directly triggers membrane depolarization at neutral pH only in the presence of glucose [56]. Other non-charged amino acids depolarize the cell membrane indirectly via a Na+ transport-dependent mechanism. The phorbol 12-myristate 13-acetate (PMA) diester triggers insulin secretion by activating the protein kinase C (PKC) isoforms α, δ and/or μ. Rat islets treated in 1 μM of PMA for 2 h exhibit enhanced insulin release both at baseline and after stimulation with glucose and tolbutamide. At the peak of first-phase insulin secretion, 20 mM glucose induces a 15-fold increase in PMA-treated islets vs. untreated islets. A less pronounced increase (3-fold) is also observed in the second phase [57].
While this is not an exhaustive review of pharmacological interventions for augmenting the secretion of insulin, it illustrates that these are associated with off-target effects and negative interactions, motivating research towards the generation of strategies for effective approaches characterized by greater specificity and fewer side effects.
4. Optogenetic approaches
4.1. Optogenetic regulation of GPCRs
Optogenetic tools have been reported for precise spatial and temporal control of GPCR signaling in β-cells. Mansouri et al. introduced melanopsin, a Gq-linked GPCR that can activate the PLC path which results in increases in cytosolic Ca2+ and insulin release, as demonstrated with the INSvesc cells, a subvariant of the human insulin-releasing β-cell line 1.1E7 [58]. The new cell line (iβ-cells) stably expressed a luciferase insulin reporter construct, and melanopsin that was excited at 475 nm or with a smartphone flashlight. Peak insulin release was observed within 15 min of illumination in vitro. When iβ-cells encapsulated in alginate-poly(l-lysine)-alginate beads were transplanted subcutaneously in STZ-treated diabetic C57BL/6JRj mice, blood insulin levels were elevated compared to STZ-mice alone and STZ-mice with transplanted cells but kept in the dark. Glucose tolerance testing showed that the hyperglycemia improved significantly with 15 min of flashlight illumination, even though INSvesc and iβ-cells cells per se are not capable to sense glucose. Of note, melanopsin is a promiscuous receptor activating Gq, Gi and Gs proteins [59] while the use of another opsin (Neuropsin/OPN5), which activates Gq signaling specifically [60], may increase the effectiveness of optogenetic insulin release by β-cells.
Gs protein-coupled rhodopsins such as JellyOp from the jellyfish Carybea rastonii [55] mediate Gs-signaling inducing transmembrane ACs to increase intracellular cAMP. Cardiomyocytes expressing JellyOp exhibited accelerated spontaneous beating with blue light illumination similar to β-adrenergic stimulation using pharmacological means [56]. Short- and long-wavelength-sensitive opsins have been used for control of Gi signaling cascades to inhibit AC, cAMP in neuronal systems [61] and cardiomyocytes to decrease the beating rate [62] yet such applications have not been reported in β-cells to date.
4.2. Optogenetic targeting of intra-β-cell cAMP
4.2.1. Photoactivatable ACs (PACs)
The central role of cAMP in the regulation of β-cell GSIS has motivated the search for tools that modulate this secondary messenger (Figure 2). Photoactivatable adenylyl cyclases (PACs) are expressed in bacteria and other microorganisms including Euglena gracilis (euPAC) [63], Oscillatoria acuminata (OaPAC) [64], and Beggiatoa (bPAC) [8,9] while artificial PACs resulting from protein engineering are also available (see below) [65]. The euPACs are large tetrameric proteins (>100 kDa) with two blue light using flavin adenine dinucleotide (BLUF) photoreceptor domains and two AC domains [66]. The complex exhibits baseline AC activity in the dark that is stimulated 80-fold in the light. Optostimulation of euPAC was demonstrated in Xenopus oocytes and HEK293 cells whereas its expression in the neuronal cells of Drosophila resulted in altered grooming behavior in response to blue light [67]. Yet, the large molecular mass, low solubility, significant dark activity, and moderate activation by light have prevented the wider use of euPACs in optogenetic applications.
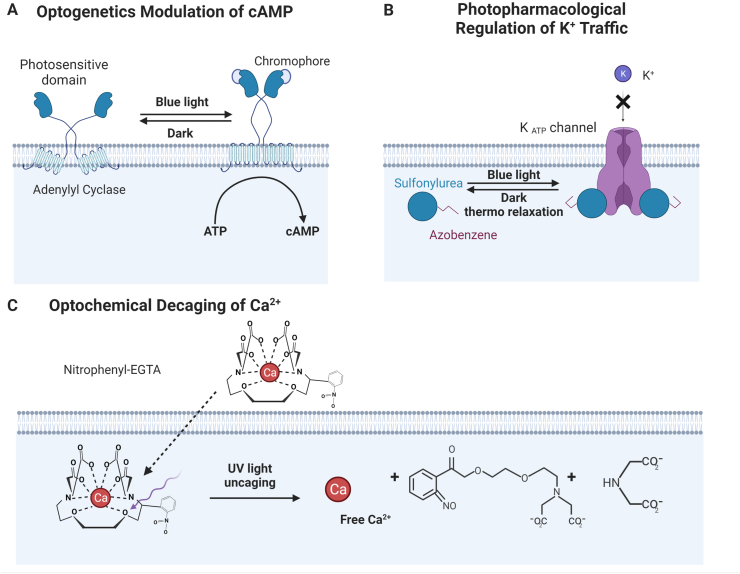
Figure 2.
Optical methods for modulating cell function using light. Three types of optical (optogenetics, photopharmacological and optochemical) approaches employed in the engineering of β-cells. (A) Light illumination stimulates PACs to upregulate conversion from ATP to intracellular cAMP thereby enhancing GSIS (See section 4.2.1). (B) Upon photoisomerization, azobenzene-based derivatives of sulfonylureas close the KATP+ channels leading to membrane depolarization, increases in [Ca2+]i and eventually the secretion of insulin [92]. (C) UV radiation cleaves photo-chelator nitrophenyl-EGTA with high affinity for Ca2+ and decages Ca2+ raising [Ca2+]i and leading to insulin release [99].
In comparison, bPAC is a smaller protein (350 aa) encoded by a ∼1 Kb gene, and it displays lower dark activity, high stimulation index (up to 300-fold increase in activity upon blue light stimulation), superior solubility, and short half-life of the active lit state. These attributes result in greater and longer augmentation of cAMP synthesis upon illumination [8,9,68,69]. The bPAC protein, originating in the sulfide-oxidizing Beggiatoa bacterium in sea floor environments, is a homodimer of an N-terminal BLUF photoreceptor domain and a C-terminal type III AC domain [8,68]. The flavin adenine dinucleotide (FAD) needed for BLUF activity is available natively in animal cells [9]. Absorption of photons and the resultant excitation cause secondary structural changes in the BLUF domain [[68], [69], [70]], which are required to activate the AC site. The absorption maximum of the original bPAC has been cited as 440 nm, indicating a high photosensitivity, possibly due to the bPAC's native deep-sea environment, where light exposure is likely minimal [8,68,70]. Various PACs have been used for modulation of cAMP in different cell types and in some instances, in combination with engineered ion channels (see PAC-K system [71] below).
In the context of β-cell GSIS, Zhang et al. expressed the bPAC gene in MIN6 insulinoma cells and murine islets. The expression of bPAC did not affect MIN6 cell growth, proliferation, and viability. Exposure of bPAC-expressing β-cells to blue light led to increased intracellular cAMP and insulin secretion. Photostimulation in the absence of glucose increased cAMP but did not elicit the production of insulin suggesting that glucose-independent hormone release is unlikely despite elevated cAMP. Interestingly, the oxygen consumption rate was not increased despite the blue light-mediated GSIS amplification. Upon transplantation of β-cells carrying bPAC to mice with streptozotocin (STZ)-induced diabetes, increased plasma insulin levels, heightened glucose tolerance and reduced blood glucose levels were measured [13,72].
Beyond natural PACs, the promise of proper optogenetic modulation of cAMP for controlling diverse functions of therapeutic interest has spurred the engineering of proteins featuring AC activity and photoactivatable moieties.
To this end, Yang et al. engineered PACmn to overcome the residual dark activity from natural soluble PACs [73]. PACmn is a modified bPAC with point mutations and membrane-anchoring peptides of the tyrosine kinase Lyn for membrane localization. Compared to the wild type bPAC, PACmn has a cyclase activity with three-fold lower light to dark ratio (>4,000) and shows a twice faster off-kinetics in oocytes. It is interesting that the construct that displays the highest light to dark activity ratio (>7,000) exhibits reduced light activity. In rat hippocampal neurons, PACmn shows no dark activity and accumulation of cAMP or PKA. The AC activity is observed by FRET in response to brief blue light illumination and diminishes within seconds for cAMP or ∼10 min for PKA.
Moreover, a near-infrared optical window (NIRW)-absorbing PAC was reported by Fomicheva et al. [74]. Compared to blue light, NIRW light penetrates tissues at a greater depth (several centimeters), rendering NIRW-stimulated ACs an attractive modality for biomedical applications. Additionally, red light is less energy-intensive than blue light easing the design demands of envisioned optogenetic devices. It should be noted that photoreceptors relevant to the NIRW light are absent in most animal cells – unlike blue light flavins, which are naturally occurring – lowering the potential for side effects (e.g., photooxidative damage) [65].
Engineered NIRW-ACs were constructed using bacteriophytochrome receptors with light sensitivity in the 670–760 nm spectral region and by fusing a type III homodimeric AC and a photosensory module from the bacteriophytochrome Rhodobacter sphaeroides [74]. Phytochromes bind covalently to bilin chromophores or (specific to bacteriophytochromes) biliverdin IXα, which is naturally occurring in animal cells [65]. In the dark state, NIRW-AC's enzymatic domain monomers are misaligned by photosensory modules preventing the formation of a homodimer. Upon absorption of NIRW photons, biliverdin IXα isomerization and alignment of the enzymatic domain monomers transpire, transitioning the synthetic AC into the active state [65,74].
The NIRW-AC engineered by Fomicheva et al., designated as IlaM5, mediated precise control of neuronal spindle waves in mice [74]. From the perspective of using IlaM5 in solutions for treating T2D, red spectral region-photoactivatable ACs would allow for greater tissue penetration, reduced energy of illumination, and potentially lower phototoxicity of β-cells [69,70].
4.2.2. Light-activated PDE
The Möglich group reported the generation of a synthetic light-activated PDE (LAPD) [75]. The LAPD was created by combining the photosensor module of Deinococcus radiodurans with the catalytic domain of the human PDE2A. Irradiation of LAPD with red light (650–700 nm) activates its hydrolytic activity of cAMP, whereas illumination with far-red light (theoretically 700–750 nm; however, an 850-nm LED was used in the study) reverts LAPD to its inactive state. It should be noted that for its function LAPD requires the chromophore biliverdin that is present in various animal tissues, but its supply can be augmented via co-expression of heme oxygenase, which converts heme to biliverdin. LAPD followed Michalis-Menten kinetics with a maximum reaction rate at substrate saturation of 1.8 and 6.5 μM/(min⋅nM LAPD) in the dark- and red-light-adapted states, respectively. The corresponding values for substate affinity (KM) were 470 and 180 μM. Upon expression of LAPD in zebrafish embryos, cAMP levels were reduced ∼40% with red light exposure vs. control embryos without LAPD. Conversely, no significant difference was noted in intracellular cAMP when embryos were illuminated with infrared light. Exogenous provision of biliverdin was not required.
This work shows that LAPD can be functionally expressed in vivo. However, the utilization of LAPD for the optogenetic regulation of cellular functions – including GSIS – is lacking. This may be due to various reasons including that LAPD has dual specificity catalyzing the hydrolysis of cAMP and cyclic guanosine monophosphate (cGMP) similar to the human PDE2A. The simultaneous activation by LAPD of cAMP- and cGMP-participating pathways may confound strategies intended for optogenetic control. Moreover, the red-light induction index of LAPD is more than one order of magnitude less than that of bPACs. Yet, the prospect is appealing of using LAPD to accelerate the reduction of cAMP levels following its excursion (e.g., in GSIS) thereby improving the overall kinetics of an optogenetically driven response.
4.3. Optogenetic regulation of K+ for insulin secretion
The secretion of insulin by β-cells involves the action of membrane potential-modulating K+ channels. In recent years, various groups have worked on engineering optogenetic channels with K+ trafficking activity. The Moroni group reported an engineered blue light-gated K+ channel (BLINK1), in which a LOV2 photoreceptor domain controls a miniature channel pore, Kcv, in a reversible manner [76]. BLINK1, which was tethered to the plasma membrane through a myristoylation/palmitoylation domain (MGCTVSAE), was expressed in oocytes and cultured HEK293T cells permitting repeated inhibition by blue light (455 nm) without inactivation. Moreover, BLINK1 RNA was injected in zebrafish embryos, which respond to touch with a burst of swimming (escape response). BLINK1-expressing embryos exhibited a reduced escape response when exposed to blue light, but not when kept in the dark.
A second version of the BLINK1 channel, coined BLINK2, was reported with a C-terminal signal and the ER export motif of the Kir2.1 and 14-3-3 (TASK1-3, KAT1) binding sites. BLINK2 exhibits improved surface export compared to BLINK1 and its post-illumination activity lasts tens of minutes [77] making this optogenetic K+ channel suitable for applications requiring durable light–off activity. To this end, rats with paclitaxel-induced allodynia, a neuropathic pain model, were intrathecally injected with a BLINK2 plasmid. Illumination with blue light reduced nociception for at least 30 min and up to 3 h. Given the disparate kinetics, a K+ channel like BLINK1, rather than BLINK2, with faster on/off kinetics, conceivably can be a suitable candidate for the photoregulation of insulin secretion in β-cells.
Light-controlled K+-based hyperpolarization has also been reported in conjunction with PAC activity [71]. The small cAMP-gated K+ channel SthK of the Spirochaeta thermophila confers strong repolarization/hyperpolarization. Co-expression of SthK and PAC (PAC-K system) in ventricular cardiomyocytes resulted in outward currents with a 10 ms exposure to 460 nm light suppressing electrically-evoked action potentials for extended periods, and silencing cardiomyocyte contractions. Similarly, PAC-K expression in cultured hippocampal neurons induced hyperpolarization with repetitive low-frequency or continuous exposure to low intensity blue light. In the same report, the PAC-K system was combined with a red-shifted channelrhodopsin (bReaChES) [78], which triggers action potentials by 550 nm light pulses, demonstrating dual-color optogenetic excitation and inhibition of membrane polarization-based cellular activity. Because the PAC-K system modulates both the intracellular cAMP and membrane potential, its consideration for use with different cell types including β-cells, warrants extensive investigation and significant tuning of its activity to avoid adverse non-specific effects.
4.4. Optogenetic regulation of Ca2+ for insulin release
Opsins are expressed across various organisms, from algae and fungi to animals. Channelrhodopsin-2 (ChR2; channelopsin-2 + retinal), which has been utilized extensively in optogenetic systems, acts as a cation channel that is activated by light. When exposed to blue light (∼460–480 nm) the C. reinhardtii-derived ChR2 augments its conductance for monovalent (e.g., Na+), and divalent cations (e.g., Ca2+, Ba2+). Generally, the permeability of ChR2 appears to decrease with increasing atomic radius of the cations [79]. Yet, ChR variants have been engineered to respond to light wavelengths beyond the blue light, significantly expanding the repertoire of optogenetic applications for ChRs [80,81].
The flux modulation of ions, especially of Ca2+, has motivated the use of ChRs for optogenetic control of β-cell membrane depolarization and insulin secretion. The insulin secreted by murine MIN6 insulinoma cells transfected with the ChR2 gene (MIN6-ChR2) increased by ∼60% upon irradiation with a 470 nm laser for 20 s vs. non-irradiated cells [12], notably though in the absence of glucose. Insulin secretion was mediated by the changes in [Ca2+]i due to ChR2 activation, as evidenced by patch-clamp analysis and the abrogation of insulin release in the presence of mibefradil dihydrochloride (a Ca2+ channel blocker). Upon subcutaneous delivery of MIN6-ChR2 cells to STZ-treated diabetic mice, blood glucose was lowered with irradiation (peak conc. of 18.6 mM) compared to that in animals receiving the cells but no light stimulation (peak conc. of 26.8 mM). The irradiation did not induce apoptosis of cells retrieved from the mice suggesting the lack of cytotoxicity due to ChR expression and activity. Reinbothe et al. examined islets isolated from transgenic mice with insulin promoter-driven expression of ChR2 [82]. As in MIN6 cells, exposure of islets from transgenic ChR2 mice in culture to blue light amplified [Ca2+]i and insulin secretion at low and intermediate but not at high concentrations of glucose. There was no change in glucagon release. In diabetic mice on high-fat diet, irradiation led to greater insulin secretion at high glucose indicating a compensatory potentiation of the Ca2+ response in these animals.
4.5. Non-β-cell optogenetic approaches
Extrapancreatic cells have also been targeted for optogenetic engineering and regulation of blood glucose with potential applications to diabetes. Elegant work by the Fussenegger group demonstrated the co-expression by HEK293 cells of melanopsin as well as a short variant of the human GLP-1 (shGLP1) gene driven by a promoter targeted by the nuclear factor of activated T cells (NFAT) [83]. Blue light activates a G-protein (melanopsin) cascade leading to the increase of [Ca2+]i. This in turn, induces calcineurin mobilizing the NFAT transcription factor to initiate the transcription of target genes. When supernatant from blue light-exposed HEK293 cells expressing melanopsin and the NFAT-targeted promoter/shGLP1 was transferred to βTC-6 β-cells, insulin secretion was evoked. Upon implantation of the engineered HEK293 cells to diabetic db/db mice, both circulating shGLP1 and insulin levels went up with 48 h of exposure to blue light. In a glucose tolerance assay, the reduction of blood sugar concentration after glucose injection was also higher in diabetic mice receiving the cells and illuminated with light vs. animals without photostimulation.
Along the same vein, Shao et al. reported a far-red light (FRL)-inducible system with activation of the engineered bacterial photoreceptor BphS, which converts GTP into c-di-GMP [84]. Increased c-di-GMP production triggers the binding of transactivating elements (BldD: a c-di-GMP–binding domain derived from sporulating actinomycetes; p65: the NF-κB–transactivating domain; VP64: a tetramer of the VP16 activation domain) to a promoter with BldD-specific operator DNA sites, turning on the expression of transgenes including shGLP-1 and mouse insulin. Alginate-encapsulated HEK293 cells carrying the requisite set of genes for reconstituting the aforementioned optogenetic circuit, were delivered subcutaneously to diabetic mice. The mice exhibited improved blood glucose profile in glucose tolerance tests 48 h post-implantation. Moreover, db/db mice with implanted cells had blood sugar levels close to those of wild-type animals for about 13 days with FRL illumination. The irradiation regimen was realized by combining smartphone technology, glucose sensing, and LED/hydrogel interfacing. Furthermore, a smartphone-based system (semi-automatic theranostic system) was reported that features blood glucose monitoring coupled to a wirelessly controlled LED for illumination of insulin-producing HEK293 cells entrapped in a hydrogel [85].
Yu et al. illustrated a similar system with FRL-induced BphS stably expressed in human mesenchymal stem cells which were implanted while encapsulated in poly-(l-lysine)-alginate microcapsules [86]. The implanted cells with exposure to light caused a reduction in hyperglycemia in a mouse model (STZ) of T1D for 40 days. Under illumination, the expression of the cardiac oxidative stress marker malondialdehyde was reduced, while superoxide dismutase, glutathione, and total antioxidant capacity showed significant increase, vs. those in STZ-treated animals without receiving cells, and those that received cells but were kept in the dark.
5. Optochemical and photopharmacological approaches
Parallel avenues for photocontrol of cellular activity are afforded by optochemistry (Figure 2), employing chemical constructs (rather than genetic as in optogenetics) with structural configuration that can be altered in response to light. For instance, caging compounds have been employed, which are capable of reversibly binding to a substrate or signaling intermediate of interest. Exposure to light induces the release of the bound substrate modifying a particular cellular function [87,88]. In addition to their high spatiotemporal precision and reversibility, optochemical methods rely on synthetic and thus more readily available agents. This is in contrast to optogenetic strategies entailing photosensitive proteins (e.g., PACs) whose expression requires molecular cloning and delivery of the corresponding genes for transcription and translation [89,90]. Yet, subcellular localization and photorelease efficiency are significant challenges of optochemical systems, as well as the formation of toxic byproduct(s) [91]. Optochemistry has notably been employed to aid several forms of biomodulation, including control of proteins, peptides, small molecules, and nucleic acids (see below) [92]. Current investigations of optochemistry focus on shifting the photoactivation to the near-infrared (NIR) region and increasing the decaging efficiency of caged groups through chemical synthesis and structure design. For example, the potential for orthogonal decaging and activation is enhanced with increased π-conjugation, and varying the caging group solubility improves intracellular or membrane localization of the optochemical moiety [89].
Exploiting the high spatiotemporal resolution and specificity of optochemical methods, the dynamic and synergistic nature of β-cell signaling and patterns for insulin secretion along with islet β-cell heterogeneity were studied, revealing contributory interactions such as the role of lipids in hormone release [91]. Exhibiting the significance of lipid localization in β-cells’ secretory function, Nadler et al. demonstrated that uncaging of photocaged lipid-arachidonic acid at the β-cell plasma membrane enhanced Ca2+ oscillation frequency, while uncaging within the cytoplasm conversely hindered Ca2+ oscillations [93].
This and other studies support the use of optochemistry as well as photopharmacology (Figure 2), i.e., the use of a photosensitive moiety as a drug or ligand, for altering GSIS. Among proposed applications are azobenzene-based derivatives of sulfonylureas [[94], [95], [96], [97]], diltiazem [98], and light-activated incretins [94,[99], [100], [101]] as photopharmacological methods; photocaged Ca2+, ATP, and secondary messengers such as cAMP [89,91,102]. These optical tools are expected to facilitate investigations of β-cell GSIS potency and pertinent signaling pathways in cells under normal and pathological conditions.
6. Outlook
The clinical realization of optogenetic approaches to treat diabetes share the challenges already faced in the field of islet transplantation, such as cell sourcing and delivery methods, and necessitates the progression of new technologies aligned for the photomodulatory nature of engineered cells, including illumination methods and seamless cellular interfacing with electronics.
Notable advances in the differentiation of human pluripotent stem cells (hPSCs) have brought us closer to the manufacturing of functional islet cells, strengthening the prospect of applying optomethods for control of hormone secretion in these cells. Several groups have reported the conversion of hPSCs to β-cell-like cells co-expressing biomarkers such as PDX1, MAFA, NKX6.1, and secreting variable amounts of insulin in response to glucose and other secretagogues [[103], [104], [105], [106]]. Major efforts also focus on the generation of clusters comprising multiple cell types akin to the native pancreatic islets, for instance, organoids mimicking the ultrastructural and functional characteristics of the pancreatic endocrine compartment [[107], [108], [109]].
The engineering of hPSC-derived islet cells for functional regulation with light will entail distinctive issues given that these cells have several features of adult islet cells but are not identical. Differentiated hPSC progeny typically exhibits an immature phenotype although these cells may undergo maturation in vivo. Insulin produced by hPSC-specified β-cells is detected at least 2–4 weeks post-transplantation [103,110]. Reaggregating INS+ cells after 20 days of differentiation, which induces maturation in culture, shortens this time frame, and C-peptide is detected 3 days after delivery to diabetic animals [104]. Hence, optogenetic modules for hPSC-derived insulin-producing cells may need to be compatible with the evolving phenotype until cells reach a fully mature state. Moreover, whether these islet cells maintain stable biochemical and functional properties in the long run is unclear. Successful optoregulation will rely on faithful recapitulation of the molecular circuitry of β-cell GSIS by insulin-producing cells from hPSCs. To this end, stem cell-derived β-cell-like cells reportedly increase their Ca2+ influx with glucose challenge, shut their KATP+ channels upon incubation with sulfonylureas, and secrete C-peptide in response to secretagogues such as KCl and exendin-4 [103,104]. However, detailed understanding of the GSIS cascade of stem cell-derived β-cells is warranted before optoengineering is considered. In fact, differential stimulation and sensing (e.g., ion channel activity), and disparate insulin content are documented among stem cell-derived and native islet cell populations [103,111,112]. This heterogeneity calls for optogenetic modulation that should evoke a desired response in cells falling on a spectrum of hormone production. Lastly, interactions should be considered among a multitude of cell types within clusters of stem cell-derived islet cells or organoids. For instance, glucagon production by α-cells and β-cell insulin secretion act synergistically for proper maintenance of glucose levels in vivo [113]. Hence, the effects of perturbations by optical switches on the physiology of multicellular assemblies and blood sugar control at the organism level should be researched.
Akin to the transplantation of islets, direct delivery of engineered β-cells will require chronic immunosuppression motivating encapsulation technologies. Since the study by Lim and Sun [114] of islet encapsulation in alginate microcapsules for transplantation in STZ-treated diabetic rats, relevant encapsulation modalities have advanced significantly. The selection of scaffolding materials is a critical parameter in the encapsulation of islet cells. Alginate, which is a polysaccharide derived from seaweed, has been reported extensively as a cell entrapment agent because of its minimal cytotoxicity and benign conditions for hydrogel formation. However, alginate implants, including these containing islets [115,116], promote the growth of fibrotic tissue around them. Other materials have also been utilized including agarose [117], hyaluronic acid [118], polylactic-co-glycolic acid (PLGA) [119], and polyethylene glycol (PEG) [120]. To reduce adverse immune reactions by the host, the delivery has been combined of cells with immunomodulatory factors such as TNFα and FasL [121,122].
The efficient exchange of nutrients, waste products, oxygen and insulin between the cells and their milieu is another important consideration for islet cell delivery technologies. Cells experience significant diffusion limitations over distances longer than 100–200 μm from vasculature [123]. Native islet microcirculation is realized by a network of capillaries traversing the islet core [124]. Hypoxia of encapsulated islets, which is a particularly vexing issue given the low solubility of O2 in aqueous environments and the high metabolic activity of islet cells, may be alleviated using perfluorocarbon emulsions, organosilicon compounds (e.g., PDMS) and other soluble factors.
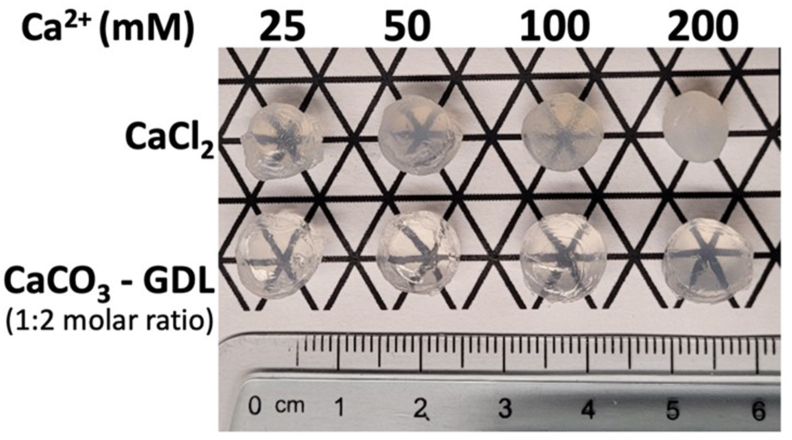
The photoregulation of cellular function requires materials with high light transmissibility, raising a unique challenge in the design of optogenetically engineered islet cell delivery systems. Constructs harboring islets with photosynthetic algae have been reported with the latter supplying O2 to islets with light delivery [125]. The murine islets and algae were co-encapsulated in sodium alginate beads based on the preparation method by Lim et al. [114], and placed in a chamber illuminated with an optical fiber, without details about the type of light. A 50% reduction of maximal stimulated insulin release was observed when the cells were kept in the dark for 30 min vs. cells with continuous exposure to light for the same interval. Alginate beads loaded with MIN6 cells expressing bPAC and implanted subcutaneously in STZ-treated mice, were successfully stimulated by an external blue LED array [72]. Animals with optogenetically engineered β-cells and exposed to blue light exhibited a better response to glucose tolerance test and higher plasma insulin levels vs. animals with the engineered β-cells but without photostimulation. Of note, alginate hydrogels formed with CaCO3 and D-glucono-δ-lactone (GDL) exhibit high transparency [126] with consistent compressive modulus and strength (Figure 3). To date, there are no reports of biomaterials besides alginate developed for use with optogenetically engineered islet cells.
Figure 3.
Alginate hydrogels for cell encapsulation. Alginate hydrogel formed with 1.5% (w/v) sodium alginate in PBS, and various concentrations of CaCl2 or CaCO3:GDL (1:2 molar ratio). Gels formed with CaCO3:GDL show higher transparency. Gelation with CaCO3 and GDL results in improved transparency compared to CaCl2-crosslinked alginate gels at higher Ca2+ concentration.
The intended site of implantation and light wavelength for optogenetic stimulation are also major factors in the design of constructs harboring optoengineered islet cells. Light source integration with the delivery scaffold increases its size. Subcutaneous implantation, while permitting stimulation of the delivered cells by an external light, is typically linked to poor vascularization and blood supply. Moreover, promising results have been reported upon islet cell implantation in the omentum [127], which affords high blood circulation, and accessibility for cell transplantation and retrieval. For optogenetically engineered cells, the proximity of the omentum to the skin bodes well for extracorporeal photostimulation. Implantation sites located deeper in the body will require the inclusion of a light source such as low energy light-emitting diodes (LEDs) in the delivery construct. This poses considerations about efficient powering of the light source. One potential solution is the wireless power transfer through inductive charging of the implanted light using an external charging device. Alternatively, cells may be engineered for stimulation by light in the infrared region (e.g., using a NIRW light-stimulated AC [74]) penetrating tissues deeper than light at shorter wavelengths.
Optogenetic device-based solutions envisioned for diabetes, feature control of the light source through coupling with continuous glucose monitoring of blood glucose, for instance, via readily available transdermal sensors (e.g., Dexcom Inc.), and wearable devices measuring glucose ocularly [128] or in biofluids such as sweat and saliva [129,130]. Complete linking of the cellular component, light source and glucose monitor can be achieved with software driving the operation of this closed loop for autonomous maintenance of normoglycemia, potentially eliminating error-prone user input and suboptimal sugar regulation. Advances in sensors, wireless communication and powering, and interfacing of biocomponents with electronics, demonstrate that interdisciplinary effort is pivotal for developing such closed-loop optogenetic systems. At the same time, the remarkable progress in photomodulation of cellular function and relevant technologies makes compelling the engineering of products ensuring reliable, robust and drug-free management of blood glucose in diabetic patients.
Funding
Grant numbers: National Science Foundation support to EST: CBET-1951104, CBET-2015849.
Contributor Information
Zijing Chen, Email: Zijing.Chen@tufts.edu.
Leah Truskinovsky, Email: Leah.Truskinovksy@tufts.edu.
Emmanuel S. Tzanakakis, Email: Emmanuel.Tzanakakis@tufts.edu.
Conflict of interest
None declared.
Data availability
No data was used for the research described in the article.
References
- 1.Boyden E.S., Zhang F., Bamberg E., Nagel G., Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nature Neuroscience. 2005;8(9):1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 2.Xu X., Mee T., Jia X. New era of optogenetics: from the central to peripheral nervous system. Critical Reviews in Biochemistry and Molecular Biology. 2020;55(1):1–16. doi: 10.1080/10409238.2020.1726279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wrobel C., Dieter A., Huet A., Keppeler D., Duque-Afonso C.J., Vogl C., et al. Optogenetic stimulation of cochlear neurons activates the auditory pathway and restores auditory-driven behavior in deaf adult gerbils. Science Translational Medicine. 2018;10(449) doi: 10.1126/scitranslmed.aao0540. [DOI] [PubMed] [Google Scholar]
- 4.Mickle A.D., Won S.M., Noh K.N., Yoon J., Meacham K.W., Xue Y., et al. A wireless closed-loop system for optogenetic peripheral neuromodulation. Nature. 2019;565(7739):361–365. doi: 10.1038/s41586-018-0823-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jang T.M., Lee J.H., Zhou H., Joo J., Lim B.H., Cheng H., et al. Expandable and implantable bioelectronic complex for analyzing and regulating real-time activity of the urinary bladder. Science Advances. 2020;6(46) doi: 10.1126/sciadv.abc9675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chua C.J., Han J.L., Li W., Liu W., Entcheva E. Integration of engineered "Spark-Cell" spheroids for optical pacing of cardiac tissue. Frontiers in Bioengineering and Biotechnology. 2021;9 doi: 10.3389/fbioe.2021.658594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henry B.M., Skinningsrud B., Saganiak K., Pekala P.A., Walocha J.A., Tomaszewski K.A. Development of the human pancreas and its vasculature - an integrated review covering anatomical, embryological, histological, and molecular aspects. Annals of Anatomy. 2019;221:115–124. doi: 10.1016/j.aanat.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Stierl M., Stumpf P., Udwari D., Gueta R., Hagedorn R., Losi A., et al. Light modulation of cellular cAMP by a small bacterial photoactivated adenylyl cyclase, bPAC, of the soil bacterium Beggiatoa. Journal of Biological Chemistry. 2011;286(2):1181–1188. doi: 10.1074/jbc.M110.185496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryu M.H., Moskvin O.V., Siltberg-Liberles J., Gomelsky M. Natural and engineered photoactivated nucleotidyl cyclases for optogenetic applications. Journal of Biological Chemistry. 2010;285(53):41501–41508. doi: 10.1074/jbc.M110.177600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes R.M., Vrana J.D., Song J., Tucker C.L. Light-dependent, dark-promoted interaction between Arabidopsis cryptochrome 1 and phytochrome B proteins. Journal of Biological Chemistry. 2012;287(26):22165–22172. doi: 10.1074/jbc.M112.360545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muller K., Engesser R., Metzger S., Schulz S., Kampf M.M., Busacker M., et al. A red/far-red light-responsive bi-stable toggle switch to control gene expression in mammalian cells. Nucleic Acids Research. 2013;41(7):e77. doi: 10.1093/nar/gkt002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kushibiki T., Okawa S., Hirasawa T., Ishihara M. Optogenetic control of insulin secretion by pancreatic beta-cells in vitro and in vivo. Gene Therapy. 2015;22(7):553–559. doi: 10.1038/gt.2015.23. [DOI] [PubMed] [Google Scholar]
- 13.Zhang F., Tzanakakis E.S. Optogenetic regulation of insulin secretion in pancreatic beta-cells. Scientific Reports. 2017;7(1):9357. doi: 10.1038/s41598-017-09937-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Velasco M., Diaz-Garcia C.M., Larque C., Hiriart M. Modulation of ionic channels and insulin secretion by drugs and hormones in pancreatic beta cells. Molecular Pharmacology. 2016;90(3):341–357. doi: 10.1124/mol.116.103861. [DOI] [PubMed] [Google Scholar]
- 15.Boland B.B., Rhodes C.J., Grimsby J.S. The dynamic plasticity of insulin production in beta-cells. Molecular Metabolism. 2017;6(9):958–973. doi: 10.1016/j.molmet.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Affourtit C., Alberts B., Barlow J., Carre J.E., Wynne A.G. Control of pancreatic beta-cell bioenergetics. Biochemical Society Transactions. 2018;46(3):555–564. doi: 10.1042/BST20170505. [DOI] [PubMed] [Google Scholar]
- 17.Komatsu M., Takei M., Ishii H., Sato Y. Glucose-stimulated insulin secretion: a newer perspective. J Diabetes Investig. 2013;4(6):511–516. doi: 10.1111/jdi.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim W., Egan J.M. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacological Reviews. 2008;60(4):470–512. doi: 10.1124/pr.108.000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahren B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nature Reviews Drug Discovery. 2009;8(5):369–385. doi: 10.1038/nrd2782. [DOI] [PubMed] [Google Scholar]
- 20.Winzell M.S., Ahrén B. G-protein-coupled receptors and islet function—implications for treatment of type 2 diabetes. Pharmacology & Therapeutics. 2007;116(3):437–448. doi: 10.1016/j.pharmthera.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Sunahara R.K., Dessauer C.W., Gilman A.G. Complexity and diversity of mammalian adenylyl cyclases. Annual Review of Pharmacology and Toxicology. 1996;36:461–480. doi: 10.1146/annurev.pa.36.040196.002333. [DOI] [PubMed] [Google Scholar]
- 22.Leech C.A., Castonguay M.A., Habener J.F. Expression of adenylyl cyclase subtypes in pancreatic beta-cells. Biochemical and Biophysical Research Communications. 1999;254(3):703–706. doi: 10.1006/bbrc.1998.9906. [DOI] [PubMed] [Google Scholar]
- 23.Lonsmann I., Bak L.K. Potential role of adenylyl cyclase 8 signaling complexes in regulating insulin secretion from pancreatic beta cells. Cellular Signalling. 2020;72 doi: 10.1016/j.cellsig.2020.109635. [DOI] [PubMed] [Google Scholar]
- 24.Sassone-Corsi P. The cyclic AMP pathway. Cold Spring Harbor Perspectives in Biology. 2012;4(12) doi: 10.1101/cshperspect.a011148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tengholm A. Cyclic AMP dynamics in the pancreatic β-cell. Upsala Journal of Medical Sciences. 2012;117(4):355–369. doi: 10.3109/03009734.2012.724732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pratt E.P., Harvey K.E., Salyer A.E., Hockerman G.H. Regulation of cAMP accumulation and activity by distinct phosphodiesterase subtypes in INS-1 cells and human pancreatic β-cells. PLoS One. 2019;14(8) doi: 10.1371/journal.pone.0215188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang G., Chepurny O.G., Holz G.G. cAMP-regulated guanine nucleotide exchange factor II (Epac2) mediates Ca2+-induced Ca2+ release in INS-1 pancreatic beta-cells. Journal of Physiology. 2001;536(Pt 2):375–385. doi: 10.1111/j.1469-7793.2001.0375c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng X., Ji Z., Tsalkova T., Mei F. Epac and PKA: a tale of two intracellular cAMP receptors. Acta Biochimica et Biophysica Sinica. 2008;40(7):651–662. doi: 10.1111/j.1745-7270.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ozaki N., Shibasaki T., Kashima Y., Miki T., Takahashi K., Ueno H., et al. cAMP-GEFII is a direct target of cAMP in regulated exocytosis. Nature Cell Biology. 2000;2(11):805–811. doi: 10.1038/35041046. [DOI] [PubMed] [Google Scholar]
- 30.Kang G., Chepurny O.G., Malester B., Rindler M.J., Rehmann H., Bos J.L., et al. cAMP sensor Epac as a determinant of ATP-sensitive potassium channel activity in human pancreatic beta cells and rat INS-1 cells. Journal of Physiology. 2006;573(Pt 3):595–609. doi: 10.1113/jphysiol.2006.107391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang G., Joseph J.W., Chepurny O.G., Monaco M., Wheeler M.B., Bos J.L., et al. Epac-selective cAMP analog 8-pCPT-2'-O-Me-cAMP as a stimulus for Ca2+-induced Ca2+ release and exocytosis in pancreatic beta-cells. Journal of Biological Chemistry. 2003;278(10):8279–8285. doi: 10.1074/jbc.M211682200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weir G.C., Bonner-Weir S. Islet beta cell mass in diabetes and how it relates to function, birth, and death. Annals of the New York Academy of Sciences. 2013;1281:92–105. doi: 10.1111/nyas.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scheen A.J. Pharmacodynamics, efficacy and safety of sodium-glucose co-transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus. Drugs. 2015;75(1):33–59. doi: 10.1007/s40265-014-0337-y. [DOI] [PubMed] [Google Scholar]
- 34.Blonde L., Dipp S., Cadena D. Combination glucose-lowering therapy plans in T2DM: case-based considerations. Advances in Therapy. 2018;35(7):939–965. doi: 10.1007/s12325-018-0694-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kieffer T.J., McIntosh C.H., Pederson R.A. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology. 1995;136(8):3585–3596. doi: 10.1210/endo.136.8.7628397. [DOI] [PubMed] [Google Scholar]
- 36.Egan J.M., Clocquet A.R., Elahi D. The insulinotropic effect of acute exendin-4 administered to humans: comparison of nondiabetic state to type 2 diabetes. Journal of Clinical Endocrinology and Metabolism. 2002;87(3):1282–1290. doi: 10.1210/jcem.87.3.8337. [DOI] [PubMed] [Google Scholar]
- 37.Pinto C., Papa D., Hubner M., Mou T.C., Lushington G.H., Seifert R. Activation and inhibition of adenylyl cyclase isoforms by forskolin analogs. Journal of Pharmacology and Experimental Therapeutics. 2008;325(1):27–36. doi: 10.1124/jpet.107.131904. [DOI] [PubMed] [Google Scholar]
- 38.Sapio L., Gallo M., Illiano M., Chiosi E., Naviglio D., Spina A., et al. The natural cAMP elevating compound forskolin in cancer therapy: is it time? Journal of Cellular Physiology. 2017;232(5):922–927. doi: 10.1002/jcp.25650. [DOI] [PubMed] [Google Scholar]
- 39.Lugnier C. Cyclic nucleotide phosphodiesterase (PDE) superfamily: a new target for the development of specific therapeutic agents. Pharmacology & Therapeutics. 2006;109(3):366–398. doi: 10.1016/j.pharmthera.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 40.Dachicourt N., Serradas P., Giroix M., Gangnerau M., Portha B. Decreased glucose-induced cAMP and insulin release in islets of diabetic rats: reversal by IBMX, glucagon, GIP. American Journal of Physiology - Endocrinology And Metabolism. 1996;271(4):E725–E732. doi: 10.1152/ajpendo.1996.271.4.E725. [DOI] [PubMed] [Google Scholar]
- 41.Zerangue N., Schwappach B., Jan Y.N., Jan L.Y. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane KATP channels. Neuron. 1999;22(3):537–548. doi: 10.1016/s0896-6273(00)80708-4. [DOI] [PubMed] [Google Scholar]
- 42.Evans J., Ogston S.A., Emslie-Smith A., Morris A.D. Risk of mortality and adverse cardiovascular outcomes in type 2 diabetes: a comparison of patients treated with sulfonylureas and metformin. Diabetologia. 2006;49(5):930. doi: 10.1007/s00125-006-0176-9. [DOI] [PubMed] [Google Scholar]
- 43.Sola D., Rossi L., Schianca G.P.C., Maffioli P., Bigliocca M., Mella R., et al. Sulfonylureas and their use in clinical practice. Archives of Medical Science: AMS. 2015;11(4):840. doi: 10.5114/aoms.2015.53304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Panten U., Burgfeld J., Goerke F., Rennicke M., Schwanstecher M., Wallasch A., et al. Control of insulin secretion by sulfonylureas, meglitinide and diazoxide in relation to their binding to the sulfonylurea receptor in pancreatic islets. Biochemical Pharmacology. 1989;38(8):1217–1229. doi: 10.1016/0006-2952(89)90327-4. [DOI] [PubMed] [Google Scholar]
- 45.Panten U., Zünkler B., Scheit S., Kirchhoff K., Lenzen S. Regulation of energy metabolism in pancreatic islets by glucose and tolbutamide. Diabetologia. 1986;29(9):648–654. doi: 10.1007/BF00869265. [DOI] [PubMed] [Google Scholar]
- 46.Rorsman P., Braun M., Zhang Q. Regulation of calcium in pancreatic α-and β-cells in health and disease. Cell Calcium. 2012;51(3–4):300–308. doi: 10.1016/j.ceca.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tuluc P., Theiner T., Jacobo-Piqueras N., Geisler S.M. Role of high voltage-gated Ca2+ channel subunits in pancreatic β-cell insulin release. From structure to function. Cells. 2021;10(8):2004. doi: 10.3390/cells10082004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vasseur M., Debuyser A., Joffre M. Sensitivity of pancreatic beta cell to calcium channel blockers. An electrophysiologic study of verapamil and nifedipine. Fundamental & Clinical Pharmacology. 1987;1(2):95–113. doi: 10.1111/j.1472-8206.1987.tb00549.x. [DOI] [PubMed] [Google Scholar]
- 49.Xu G., Chen J., Jing G., Shalev A. Preventing β-cell loss and diabetes with calcium channel blockers. Diabetes. 2012;61(4):848–856. doi: 10.2337/db11-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ovalle F., Grimes T., Xu G., Patel A.J., Grayson T.B., Thielen L.A., et al. Verapamil and beta cell function in adults with recent-onset type 1 diabetes. Nature Medicine. 2018;24(8):1108–1112. doi: 10.1038/s41591-018-0089-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Levine M., Boyer E.W., Pozner C.N., Geib A.-J., Thomsen T., Mick N., et al. Assessment of hyperglycemia after calcium channel blocker overdoses involving diltiazem or verapamil. Critical Care Medicine. 2007;35(9):2071–2075. doi: 10.1097/01.ccm.0000278916.04569.23. [DOI] [PubMed] [Google Scholar]
- 52.Gilon P., Henquin J.-C. Mechanisms and physiological significance of the cholinergic control of pancreatic β-cell function. Endocrine Reviews. 2001;22(5):565–604. doi: 10.1210/edrv.22.5.0440. [DOI] [PubMed] [Google Scholar]
- 53.Zhu L., Rossi M., Cohen A., Pham J., Zheng H., Dattaroy D., et al. Allosteric modulation of β-cell M3 muscarinic acetylcholine receptors greatly improves glucose homeostasis in lean and obese mice. Proceedings of the National Academy of Sciences. 2019;116(37):18684–18690. doi: 10.1073/pnas.1904943116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fajans S.S., Floyd J.C., Jr., Knopf R.F., Conn J.W. Elsevier; Berlin: 1969. Effect of amino acids and proteins on insulin secretion in man, Schering symposium on endocrinology; pp. 231–238. [Google Scholar]
- 55.Newsholme P., Bender K., Kiely A., Brennan L. Amino acid metabolism, insulin secretion and diabetes. Biochemical Society Transactions. 2007;35(5):1180–1186. doi: 10.1042/BST0351180. [DOI] [PubMed] [Google Scholar]
- 56.Sener A., Best L.C., Yates A.P., Kadiata M.M., Olivares E., Louchami K., et al. Stimulus-secretion coupling of arginine-induced insulin release. Endocrine. 2000;13(3):329–340. doi: 10.1385/ENDO:13:3:329. [DOI] [PubMed] [Google Scholar]
- 57.Zawalich W., Zawalich K., Ganesan S., Calle R., Rasmussen H. Effects of the phorbol ester phorbol 12-myristate 13-acetate (PMA) on islet-cell responsiveness. Biochemical Journal. 1991;278(1):49–56. doi: 10.1042/bj2780049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mansouri M., Xue S., Hussherr M.D., Strittmatter T., Camenisch G., Fussenegger M. Smartphone-Flashlight-Mediated Remote Control of Rapid Insulin Secretion Restores Glucose Homeostasis in Experimental Type-1 Diabetes. Small. 2021;17(35) doi: 10.1002/smll.202101939. [DOI] [PubMed] [Google Scholar]
- 59.McDowell R.J., Rodgers J., Milosavljevic N., Lucas R.J. Divergent G-protein selectivity across melanopsins from mice and humans. Journal of Cell Science. 2022;135(6) doi: 10.1242/jcs.258474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wagdi A., Malan D., Sathyanarayanan U., Beauchamp J.S., Vogt M., Zipf D., et al. Selective optogenetic control of Gq signaling using human Neuropsin. Nature Communications. 2022;13(1):1765. doi: 10.1038/s41467-022-29265-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Masseck O.A., Spoida K., Dalkara D., Maejima T., Rubelowski J.M., Wallhorn L., et al. Vertebrate cone opsins enable sustained and highly sensitive rapid control of Gi/o signaling in anxiety circuitry. Neuron. 2014;81(6):1263–1273. doi: 10.1016/j.neuron.2014.01.041. [DOI] [PubMed] [Google Scholar]
- 62.Cokić M., Bruegmann T., Sasse P., Malan D. Optogenetic stimulation of Gi signaling enables instantaneous modulation of cardiomyocyte pacemaking. Frontiers in Physiology. 2021;12 doi: 10.3389/fphys.2021.768495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Iseki M., Matsunaga S., Murakami A., Ohno K., Shiga K., Yoshida K., et al. A blue-light-activated adenylyl cyclase mediates photoavoidance in Euglena gracilis. Nature. 2002;415(6875):1047–1051. doi: 10.1038/4151047a. [DOI] [PubMed] [Google Scholar]
- 64.Ohki M., Sugiyama K., Kawai F., Tanaka H., Nihei Y., Unzai S., et al. Structural insight into photoactivation of an adenylate cyclase from a photosynthetic cyanobacterium. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(24):6659–6664. doi: 10.1073/pnas.1517520113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ryu M.H., Kang I.H., Nelson M.D., Jensen T.M., Lyuksyutova A.I., Siltberg-Liberles J., et al. Engineering adenylate cyclases regulated by near-infrared window light. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(28):10167–10172. doi: 10.1073/pnas.1324301111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gomelsky M., Klug G. BLUF: a novel FAD-binding domain involved in sensory transduction in microorganisms. Trends in biochemical sciences. 2002;27(10):497–500. doi: 10.1016/s0968-0004(02)02181-3. [DOI] [PubMed] [Google Scholar]
- 67.Schroder-Lang S., Schwarzel M., Seifert R., Strunker T., Kateriya S., Looser J., et al. Fast manipulation of cellular cAMP level by light in vivo. Nature Methods. 2007;4(1):39–42. doi: 10.1038/nmeth975. [DOI] [PubMed] [Google Scholar]
- 68.Lindner R., Hartmann E., Tarnawski M., Winkler A., Frey D., Reinstein J., et al. Photoactivation mechanism of a bacterial light-regulated adenylyl cyclase. Journal of Molecular Biology. 2017;429(9):1336–1351. doi: 10.1016/j.jmb.2017.03.020. [DOI] [PubMed] [Google Scholar]
- 69.Stierl M., Penzkofer A., Kennis J.T., Hegemann P., Mathes T. Key residues for the light regulation of the blue light-activated adenylyl cyclase from Beggiatoa sp. Biochemistry. 2014;53(31):5121–5130. doi: 10.1021/bi500479v. [DOI] [PubMed] [Google Scholar]
- 70.Hirano M., Takebe M., Ishido T., Ide T., Matsunaga S. The C-terminal region affects the activity of photoactivated adenylyl cyclase from Oscillatoria acuminata. Scientific Reports. 2019;9(1) doi: 10.1038/s41598-019-56721-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bernal Sierra Y.A., Rost B.R., Pofahl M., Fernandes A.M., Kopton R.A., Moser S., et al. Potassium channel-based optogenetic silencing. Nature Communications. 2018;9(1):4611. doi: 10.1038/s41467-018-07038-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang F., Tzanakakis E.S. Amelioration of diabetes in a murine model upon transplantation of pancreatic beta-cells with optogenetic control of cyclic adenosine monophosphate. ACS Synthetic Biology. 2019;8(10):2248–2255. doi: 10.1021/acssynbio.9b00262. [DOI] [PubMed] [Google Scholar]
- 73.Yang S., Constantin O.M., Sachidanandan D., Hofmann H., Kunz T.C., Kozjak-Pavlovic V., et al. PACmn for improved optogenetic control of intracellular cAMP. BMC Biology. 2021;19(1):1–17. doi: 10.1186/s12915-021-01151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fomicheva A., Zhou C., Sun Q.Q., Gomelsky M. Engineering adenylate cyclase activated by near-infrared window light for mammalian optogenetic applications. ACS Synthetic Biology. 2019;8(6):1314–1324. doi: 10.1021/acssynbio.8b00528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gasser C., Taiber S., Yeh C.M., Wittig C.H., Hegemann P., Ryu S., et al. Engineering of a red-light-activated human cAMP/cGMP-specific phosphodiesterase. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(24):8803–8808. doi: 10.1073/pnas.1321600111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cosentino C., Alberio L., Gazzarrini S., Aquila M., Romano E., Cermenati S., et al. Optogenetics. Engineering of a light-gated potassium channel. Science. 2015;348(6235):707–710. doi: 10.1126/science.aaa2787. [DOI] [PubMed] [Google Scholar]
- 77.Alberio L., Locarno A., Saponaro A., Romano E., Bercier V., Albadri S., et al. A light-gated potassium channel for sustained neuronal inhibition. Nature Methods. 2018;15(11):969–976. doi: 10.1038/s41592-018-0186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rajasethupathy P., Sankaran S., Marshel J.H., Kim C.K., Ferenczi E., Lee S.Y., et al. Projections from neocortex mediate top-down control of memory retrieval. Nature. 2015;526(7575):653–659. doi: 10.1038/nature15389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nagel G., Szellas T., Huhn W., Kateriya S., Adeishvili N., Berthold P., et al. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(24):13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Prigge M., Schneider F., Tsunoda S.P., Shilyansky C., Wietek J., Deisseroth K., et al. Color-tuned channelrhodopsins for multiwavelength optogenetics. Journal of Biological Chemistry. 2012;287(38):31804–31812. doi: 10.1074/jbc.M112.391185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schneider F., Grimm C., Hegemann P. Biophysics of channelrhodopsin. Annual Review of Biophysics. 2015;44:167–186. doi: 10.1146/annurev-biophys-060414-034014. [DOI] [PubMed] [Google Scholar]
- 82.Reinbothe T.M., Safi F., Axelsson A.S., Mollet I.G., Rosengren A.H. Optogenetic control of insulin secretion in intact pancreatic islets with beta-cell-specific expression of Channelrhodopsin-2. Islets. 2014;6(1) doi: 10.4161/isl.28095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ye H., Daoud-El Baba M., Peng R.W., Fussenegger M. A synthetic optogenetic transcription device enhances blood-glucose homeostasis in mice. Science. 2011;332(6037):1565–1568. doi: 10.1126/science.1203535. [DOI] [PubMed] [Google Scholar]
- 84.Shao J., Xue S., Yu G., Yu Y., Yang X., Bai Y., et al. Smartphone-controlled optogenetically engineered cells enable semiautomatic glucose homeostasis in diabetic mice. Science Translational Medicine. 2017;9(387) doi: 10.1126/scitranslmed.aal2298. [DOI] [PubMed] [Google Scholar]
- 85.Yu G., Yu Y., Ye H. Mammalian cell engineering. Springer; 2021. Constructing a smartphone-controlled semiautomatic theranostic system for glucose homeostasis in diabetic mice; pp. 141–158. [DOI] [PubMed] [Google Scholar]
- 86.Yu G., Zhang M., Gao L., Zhou Y., Qiao L., Yin J., et al. Far-red light-activated human islet-like designer cells enable sustained fine-tuned secretion of insulin for glucose control. Molecular Therapy. 2022;30(1):341–354. doi: 10.1016/j.ymthe.2021.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Deiters A. Special issue on optochemical and optogenetic control of cellular processes. ChemBioChem. 2018;19(12):1198–1200. doi: 10.1002/cbic.201800277. [DOI] [PubMed] [Google Scholar]
- 88.Caldwell R.M., Bermudez J.G., Thai D., Aonbangkhen C., Schuster B.S., Courtney T., et al. Optochemical control of protein localization and activity within cell-like compartments. Biochemistry. 2018;57(18):2590–2596. doi: 10.1021/acs.biochem.8b00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bardhan A., Deiters A. Development of photolabile protecting groups and their application to the optochemical control of cell signaling. Current Opinion in Structural Biology. 2019;57:164–175. doi: 10.1016/j.sbi.2019.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu P., Manna D. Optochemical control of protein degradation. ChemBioChem. 2020;21(16):2250–2252. doi: 10.1002/cbic.202000113. [DOI] [PubMed] [Google Scholar]
- 91.Frank J.A., Broichhagen J., Yushchenko D.A., Trauner D., Schultz C., Hodson D.J. Optical tools for understanding the complexity of beta-cell signalling and insulin release. Nature Reviews Endocrinology. 2018;14(12):721–737. doi: 10.1038/s41574-018-0105-2. [DOI] [PubMed] [Google Scholar]
- 92.Ankenbruck N., Courtney T., Naro Y., Deiters A. Optochemical control of biological processes in cells and animals. Angewandte Chemie International Edition in English. 2018;57(11):2768–2798. doi: 10.1002/anie.201700171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nadler A., Yushchenko D.A., Muller R., Stein F., Feng S., Mulle C., et al. Exclusive photorelease of signalling lipids at the plasma membrane. Nature Communications. 2015;6 doi: 10.1038/ncomms10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Broichhagen J., Schönberger M., Cork S.C., Frank J.A., Marchetti P., Bugliani M., et al. Optical control of insulin release using a photoswitchable sulfonylurea. Nature Communications. 2014;5(1):1–11. doi: 10.1038/ncomms6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Walczewska-Szewc K., Nowak W. Photo-switchable sulfonylureas binding to ATP-sensitive potassium channel reveal the mechanism of light-controlled insulin release. The Journal of Physical Chemistry B. 2021;125(48):13111–13121. doi: 10.1021/acs.jpcb.1c07292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Broichhagen J., Frank J., Johnston N., Mitchell R., Šmid K., Marchetti P., et al. A red-shifted photochromic sulfonylurea for the remote control of pancreatic beta cell function. Chemical Communications. 2015;51(27):6018–6021. doi: 10.1039/c5cc01224d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mehta Z.B., Johnston N.R., Nguyen-Tu M.-S., Broichhagen J., Schultz P., Larner D.P., et al. Remote control of glucose homeostasis in vivo using photopharmacology. Scientific Reports. 2017;7(1):1–11. doi: 10.1038/s41598-017-00397-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fehrentz T., Huber F.M.E., Hartrampf N., Bruegmann T., Frank J.A., Fine N.H.F., et al. Optical control of L-type Ca(2+) channels using a diltiazem photoswitch. Nature Chemical Biology. 2018;14(8):764–767. doi: 10.1038/s41589-018-0090-8. [DOI] [PubMed] [Google Scholar]
- 99.Broichhagen J., Johnston N.R., Von Ohlen Y., Meyer-Berg H., Jones B.J., Bloom S.R., et al. Allosteric optical control of a class BG-protein-coupled receptor. Angewandte Chemie International Edition. 2016;55(19):5865–5868. doi: 10.1002/anie.201600957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Broichhagen J., Podewin T., Meyer-Berg H., Von Ohlen Y., Johnston N.R., Jones B.J., et al. Optical control of insulin secretion using an incretin switch. Angewandte Chemie International Edition. 2015;54(51):15565–15569. doi: 10.1002/anie.201506384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jones B.J., Scopelliti R., Tomas A., Bloom S.R., Hodson D.J., Broichhagen J. Potent Prearranged Positive Allosteric Modulators of the Glucagon-like Peptide-1 Receptor. ChemistryOpen. 2017;6(4):501–505. doi: 10.1002/open.201700062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ellis-Davies G., Kaplan J.H. Nitrophenyl-EGTA, a photolabile chelator that selectively binds Ca2+ with high affinity and releases it rapidly upon photolysis. Proceedings of the National Academy of Sciences. 1994;91(1):187–191. doi: 10.1073/pnas.91.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pagliuca F.W., Millman J.R., Gurtler M., Segel M., Van Dervort A., Ryu J.H., et al. Generation of functional human pancreatic beta cells in vitro. Cell. 2014;159(2):428–439. doi: 10.1016/j.cell.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nair G.G., Liu J.S., Russ H.A., Tran S., Saxton M.S., Chen R., et al. Recapitulating endocrine cell clustering in culture promotes maturation of human stem-cell-derived beta cells. Nature Cell Biology. 2019;21(2):263–274. doi: 10.1038/s41556-018-0271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yabe S.G., Fukuda S., Takeda F., Nashiro K., Shimoda M., Okochi H. Efficient generation of functional pancreatic beta-cells from human induced pluripotent stem cells. Journal of Diabetes. 2017;9(2):168–179. doi: 10.1111/1753-0407.12400. [DOI] [PubMed] [Google Scholar]
- 106.Nair G.G., Tzanakakis E.S., Hebrok M. Emerging routes to the generation of functional beta-cells for diabetes mellitus cell therapy. Nature Reviews Endocrinology. 2020;16(9):506–518. doi: 10.1038/s41574-020-0375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lebreton F., Lavallard V., Bellofatto K., Bonnet R., Wassmer C.H., Perez L., et al. Insulin-producing organoids engineered from islet and amniotic epithelial cells to treat diabetes. Nature Communications. 2019;10(1):4491. doi: 10.1038/s41467-019-12472-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Soltanian A., Ghezelayagh Z., Mazidi Z., Halvaei M., Mardpour S., Ashtiani M.K., et al. Generation of functional human pancreatic organoids by transplants of embryonic stem cell derivatives in a 3D-printed tissue trapper. Journal of Cellular Physiology. 2019;234(6):9564–9576. doi: 10.1002/jcp.27644. [DOI] [PubMed] [Google Scholar]
- 109.Yoshihara E., O'Connor C., Gasser E., Wei Z., Oh T.G., Tseng T.W., et al. Immune-evasive human islet-like organoids ameliorate diabetes. Nature. 2020;586(7830):606–611. doi: 10.1038/s41586-020-2631-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rezania A., Bruin J.E., Arora P., Rubin A., Batushansky I., Asadi A., et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nature Biotechnology. 2014;32(11):1121–1133. doi: 10.1038/nbt.3033. [DOI] [PubMed] [Google Scholar]
- 111.Wojtusciszyn A., Armanet M., Morel P., Berney T., Bosco D. Insulin secretion from human beta cells is heterogeneous and dependent on cell-to-cell contacts. Diabetologia. 2008;51(10):1843–1852. doi: 10.1007/s00125-008-1103-z. [DOI] [PubMed] [Google Scholar]
- 112.Dorrell C., Schug J., Canaday P.S., Russ H.A., Tarlow B.D., Grompe M.T., et al. Human islets contain four distinct subtypes of beta cells. Nature Communications. 2016;7 doi: 10.1038/ncomms11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wendt A., Eliasson L. Pancreatic alpha-cells - the unsung heroes in islet function. Seminars in Cell & Developmental Biology. 2020;103:41–50. doi: 10.1016/j.semcdb.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 114.Lim F., Sun A.M. Microencapsulated islets as bioartificial endocrine pancreas. Science. 1980;210(4472):908–910. doi: 10.1126/science.6776628. [DOI] [PubMed] [Google Scholar]
- 115.Soon-Shiong P., Otterlie M., Skjak-Braek G., Smidsrod O., Heintz R., Lanza R.P., et al. An immunologic basis for the fibrotic reaction to implanted microcapsules. Transplantation Proceedings. 1991;23(1 Pt 1):758–759. [PubMed] [Google Scholar]
- 116.De Vos P., Van Straaten J.F., Nieuwenhuizen A.G., de Groot M., Ploeg R.J., De Haan B.J., et al. Why do microencapsulated islet grafts fail in the absence of fibrotic overgrowth? Diabetes. 1999;48(7):1381–1388. doi: 10.2337/diabetes.48.7.1381. [DOI] [PubMed] [Google Scholar]
- 117.Yang H., Iwata H., Shimizu H., Takagi T., Tsuji T., Ito F. Comparative studies of in vitro and in vivo function of three different shaped bioartificial pancreases made of agarose hydrogel. Biomaterials. 1994;15(2):113–120. doi: 10.1016/0142-9612(94)90259-3. [DOI] [PubMed] [Google Scholar]
- 118.Harrington S., Williams J., Rawal S., Ramachandran K., Stehno-Bittel L. Hyaluronic acid/collagen hydrogel as an alternative to alginate for long-term immunoprotected islet Transplantation<sup/>. Tissue Engineering Part A. 2017;23(19–20):1088–1099. doi: 10.1089/ten.tea.2016.0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dufour J.M., Rajotte R.V., Zimmerman M., Rezania A., Kin T., Dixon D.E., et al. Development of an ectopic site for islet transplantation, using biodegradable scaffolds. Tissue Engineering. 2005;11(9–10):1323–1331. doi: 10.1089/ten.2005.11.1323. [DOI] [PubMed] [Google Scholar]
- 120.Cruise G.M., Hegre O.D., Scharp D.S., Hubbell J.A. A sensitivity study of the key parameters in the interfacial photopolymerization of poly(ethylene glycol) diacrylate upon porcine islets. Biotechnology and Bioengineering. 1998;57(6):655–665. doi: 10.1002/(sici)1097-0290(19980320)57:6<655::aid-bit3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 121.Lin C.C., Metters A.T., Anseth K.S. Functional PEG-peptide hydrogels to modulate local inflammation induced by the pro-inflammatory cytokine TNFalpha. Biomaterials. 2009;30(28):4907–4914. doi: 10.1016/j.biomaterials.2009.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Headen D.M., Woodward K.B., Coronel M.M., Shrestha P., Weaver J.D., Zhao H., et al. Local immunomodulation Fas ligand-engineered biomaterials achieves allogeneic islet graft acceptance. Nature Materials. 2018;17(8):732–739. doi: 10.1038/s41563-018-0099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rouwkema J., Rivron N.C., van Blitterswijk C.A. Vascularization in tissue engineering. Trends in biotechnology. 2008;26(8):434–441. doi: 10.1016/j.tibtech.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 124.Liu Y.M., Guth P.H., Kaneko K., Livingston E.H., Brunicardi F.C. Dynamic in vivo observation of rat islet microcirculation. Pancreas. 1993;8(1):15–21. doi: 10.1097/00006676-199301000-00005. [DOI] [PubMed] [Google Scholar]
- 125.Bloch K., Papismedov E., Yavriyants K., Vorobeychik M., Beer S., Vardi P. Photosynthetic oxygen generator for bioartificial pancreas. Tissue Engineering. 2006;12(2):337–344. doi: 10.1089/ten.2006.12.337. [DOI] [PubMed] [Google Scholar]
- 126.Kuo C.K., Ma P.X. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: part 1. Structure, gelation rate and mechanical properties. Biomaterials. 2001;22(6):511–521. doi: 10.1016/s0142-9612(00)00201-5. [DOI] [PubMed] [Google Scholar]
- 127.Damyar K., Farahmand V., Whaley D., Alexander M., Lakey J.R.T. An overview of current advancements in pancreatic islet transplantation into the omentum. Islets. 2021;13(5–6):115–120. doi: 10.1080/19382014.2021.1954459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kim J., Kim M., Lee M.S., Kim K., Ji S., Kim Y.T., et al. Wearable smart sensor systems integrated on soft contact lenses for wireless ocular diagnostics. Nature Communications. 2017;8 doi: 10.1038/ncomms14997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bariya M., Nyein H.Y.Y., Javey A. Wearable sweat sensors. Nature Electronics. 2018;1(3):160–171. [Google Scholar]
- 130.Mani V., Beduk T., Khushaim W., Ceylan A.E., Timur S., Wolfbeis O.S., et al. Electrochemical sensors targeting salivary biomarkers: a comprehensive review. TrAC, Trends in Analytical Chemistry. 2021;135 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.