Abstract
BACKGROUND:
Longitudinal studies examining the potential mediating roles of birth weight and breastfeeding duration on the pathways between maternal gestational weight gain (GWG) and offspring anthropometric outcomes are lacking.
METHODS:
We analyzed data from the mother–child pairs in the Infant Feeding Practices Study II (IFPS II) in late infancy (n = 1548) and at the Year 6 Follow-up (n = 1514) Study. Child anthropometrics included age- and sex-specific Z-scores for weight for age (WAZ), height /length for age, weight for height/length and body mass index (BMIZ). Structural equation models were used to estimate the total, direct and indirect effects of GWG on child anthropometrics through birth weight and breastfeeding duration.
RESULTS:
The total effect of GWG on offspring anthropometric outcomes was significant for WAZ (β = 0.107, 95% confidence interval (CI): 0.052, 0.161) at late infancy and for WAZ (β = 0.122, 95% CI: 0.066, 0.177) and BMIZ (β = 0.120, 95% CI: 0.063, 0.178) at 6 years old. The direct effects of GWG on offspring’s WAZ and BMIZ were observed only at 6 years old. The indirect effects of GWG through birth weight were significant on most of the offspring’s anthropometric measures. Compared to breastfeeding duration, birth weight was a stronger mediator on the pathways between GWG and all proposed anthropometric measures both in late infancy and in early childhood. Longer duration of breastfeeding was inversely associated with all offspring anthropometric outcomes at late infancy but not with those outcomes at 6 years old.
CONCLUSIONS:
Our findings suggest a stronger indirect rather than direct effect of GWG on children’s anthropometric outcomes mainly through birth weight, independent of maternal sociodemographic and reproductive factors. Longer duration of breastfeeding might suppress the positive relationship between GWG, birth weight and anthropometric outcomes in late infancy but not among 6 years old.
INTRODUCTION
Since 1990, obesity has become an epidemic among the pediatric population worldwide.1,2 Globally, the number of overweight or obese children under age 5 increased from 32 million in 1990 to 42 million in 2013, and is projected to reach 70 million (~11%) by 2025.3–5 In the United States, approximately one in every three children aged 2–19 years (~12.5 million) were overweight or obese in 2011–2012.6 Considering the rising prevalence of childhood obesity and its adverse health consequences, it is essential to identify the risk factors and seek effective ways to curtail this epidemic. Because the prevalence of childhood obesity has occurred among genetically stable populations in the past three decades, the prenatal and early life factors might have a more significant role than genetic factors in the current pediatric obesity epidemic.7–9
Gestational weight gain (GWG), a nutritional marker during pregnancy, is considered as an important modifiable risk factor to prevent childhood obesity and its associated health consequences in combination with pre-pregnancy body mass index (BMI).10–13 Studies examining the association of excessive GWG and the risk of childhood obesity have proliferated in the past decade. One recent meta-analysis of 12 cohort studies and systematic reviews14,15 reported that GWG is a strong predictor for childhood obesity. Even though a number of studies12,16–18 have examined the association of GWG and BMI Z-score (BMIZ), information on other anthropometric outcomes such as weight for age (WAZ), height for age (HAZ) and weight for height (WHZ) were limited.19,20 Because each anthropometric measure can be unique in its reflection of weight and height development at different ages, it is of great public health interest to examine the effects of GWG on various anthropometric measures during both infancy and childhood.
Epidemiological studies have also reported that gaining appropriate weight during pregnancy is an important factor to be considered for successful breastfeeding after birth.21,22 Moreover, many studies have found empirical evidence that breast-feeding is associated with reduced odds of pediatric overweight/obesity.23–25
The potential mediating roles of birth weight and breastfeeding duration on the pathways from maternal GWG to the development of overweight or obesity in offspring have been suggested by several studies.10,12,26,27 So far, only one study has examined the potential mediating roles of these two factors on offspring’s anthropometric outcomes.11 Zhu et al.11 reported that birth weight is a strong mediator between maternal GWG and anthropometric outcomes (that is, WAZ, HAZ, WHZ and BMIZ) measured at age 0–5.9 years, and a longer duration of breastfeeding is inversely associated with WAZ, WHZ and BMIZ. However, this study is a cross-sectional design and thus cannot detect potential different mediating effects of birth weight and breastfeeding over the ages.
Linkage between the Infant Feeding Practices Study II (IFPS II) and its Year 6 Follow-Up (Y6FU) allows us to examine the potential mediating roles of birth weight and breastfeeding duration on the association of maternal GWG with offspring anthropometric outcomes at both late infancy and age 6 years. The objectives for this study are to examine the following: (1) the effect of maternal GWG on offspring’s anthropometric outcomes in late infancy and 6 years after birth; and (2) the mediating roles of birth weight and breastfeeding duration in these associations.
SUBJECTS AND METHODS
Study population
The IFPS II and Y6FU were conducted by the Food and Drug Administration and the Centers for Disease Control and Prevention, in collaboration with other federal agencies in response to the nation’s continued need to understand and improve the health status of mothers and their offspring. The sample for IFPS II was drawn from a nationally distributed consumer opinion panel of 500 000 households. Detailed information about the study was described elsewhere.28,29 In brief, IFPS II was a longitudinal study that recruited a cohort of pregnant women between May 2005 and June 2007 during their third trimester and followed them throughout the first year of their baby’s life with a total of 1 prenatal and 10 postpartum mail questionnaires sent at ~ 1, 2, 3, 4, 5, 6, 7, 9, 10.5 and 12 months after birth. In 2012, mothers from IFPS II study were re-contacted to participate in Y6FU, a mail survey designed to follow-up IFPS children regarding their physical development, health condition and dietary patterns at 6 years old. The IFPS II had the following inclusion criteria: mothers aged 18 years or older at the time of the prenatal survey; having a singleton delivery of a full- or nearly full-term baby (⩾35 weeks’ gestation); newborn’s birth weight ⩾ 2.25 kg; both mother and baby being healthy at birth; and infants developing no illness or conditions, which are likely to affect feeding.
Maternal GWG
Maternal GWG was based on self-reported total weight gain during pregnancy obtained from the neonatal questionnaire administered about 3 weeks after delivery and treated as a continuous dependent variable in the analyses.
Offspring’s anthropometric outcomes
The weight and length measurements used for infancy were obtained from the month-12 survey of IFPS II, which asked mothers to report the values of these measurements from the most recent doctor’s visit and the date of the measurement. With the Y6FU questionnaire, mothers were sent a measuring tape and instructions on how to measure their child’s height and asked to report their child’s height in inches and to weigh their child on a scale without shoes and report the weight in pounds.30 BMI was calculated as weight (kg) divided by height in square meters. Because weight and length/height vary by sex and age, the sex- and age-specific Z-scores were calculated using preexisting SAS programs (version 9.4, SAS Institute Inc., Cary, NC, USA) to calculate WAZ, HAZ, WHZ and BMIZ scores among infants according to the 2006 World Health Organization Growth Charts,31 and WAZ, HAZ and BMIZ among 6 years old according to the 2000 Centers for Disease Control and Prevention Growth Charts.32
Mediators
Birth weight and duration of breastfeeding were proposed as potential mediating variables between maternal GWG and offspring anthropometrics. Birth weight was obtained within a week after birth through a short telephone interview. Breastfeeding practices were assessed throughout all postpartum IFPS II questionnaires and duration of breastfeeding to any extent was defined as the age of the infant in weeks when the mother completely stopped breastfeeding or pumping milk. For the infant cohort, breastfeeding duration was based on mothers’ responses to duration questions surveyed in IFPS II unless they were still breastfeeding at the last survey of IFPS II whom we used their answers to question ‘baby’s last age in weeks when baby was breastfed’. For the 6-year-old cohort, if mothers’ response to duration questions surveyed in Y6FU was ⩽ 52 weeks, we used their answer from IFPS II, which would have less reporting bias from relatively short time recall than Y6FU. If it was >52 weeks, we used their year 6 answer.
Covariates
The following covariates were considered as potential confounding variables and adjusted in the models. Maternal characteristics are all obtained from IFPS II, including age at delivery, pre-pregnancy BMI, race (white, black and other), maternal education (high school or less, some college, college graduate or higher), being married and cohabiting (yes or no), household income as percent of federal poverty level (<185%, 185–350% and >350%), parity (primiparous or multiparous), maternal smoking during pregnancy (yes or no), self-reported gestational diabetes (yes or no) and gestational age. Children’s characteristics consisted of infant sex and age at solid food introduction (<4 months, 4 to <6 months and ⩾ 6 months). Age at solid food introduction was calculated as the midpoint between the child’s age when the mother reported no solid food consumption and when she first reported her child had consumed solid foods in the previous 7 days on the basis of postnatal survey questionnaires completed by the mother monthly until infants reached 7 months and then completed at 9, 10.5 and 12 months. Solid foods included dairy foods other than milk (for example, yogurt, cheese and ice cream); soy foods other than soy milk (for example, tofu and frozen soy desserts); baby cereal; other cereals and starches (for example, breakfast cereals, teething biscuits, crackers, pasta and rice); fruits; vegetables; French fries; meat, chicken or combination dinners; fish or shellfish; peanut butter, other peanut foods, or nuts; eggs; and sweet foods (for example, candy, cookies and cake). Additional covariates included for modeling the year 6 anthropometric outcomes were days of the child doing physical activity in a typical week for a total of at least 60 min, daily hours of watching television, times of consuming sugar-sweetened beverages per week and days of fast food consumption per week.
Statistical analyses
A total of 1807 mother–child pairs completed the month-12 questionnaire of IFPS II and were eligible for our late infant cohort analysis. We limited our study sample to those pairs that had valid values of WAZ, HAZ, WHZ or BMIZ, which lead to 1548 pairs at late infancy. We further excluded those who had missing data or biologically implausible values (BIV) for each anthropometric measure: WAZ (5 BIV, 108 missing data); HAZ (45 BIV, 474 missing); WHZ (51 BIV, 433 missing data); and BMIZ (48 BIV, 475 missing data). For our 6-year-old cohort, among a total of 1542 women who completed Y6FU questionnaires, we applied similar exclusion criteria for anthropometric measures at 6 years old: WAZ (0 BIV, 177 missing data); HAZ (20 BIV, 208 missing data); and BMIZ (19 BIV, 236 missing data). The sample sizes for modeling each specific anthropometric Z-score were 1435, 1029, 1064 and 1025 for WAZ, HAZ, WHZ and BMIZ among infants, respectively; and were 1365, 1314 and 1287 for WAZ, HAZ and BMIZ among 6 years old, respectively. The BIV for each anthropometric measure was defined according to the World Health Organization’s recommendation for infancy (WAZ>5 or WAZ< −6, HAZ>6 or HAZ< −6, WHZ>5 or WHZ< −5, and BMIZ>5 or BMIZ< −5),33 and the Centers for Disease Control and Prevention’s recommendation34 for children at age 6 (WAZ>8 or WAZ< −5, HAZ>4 or HAZ< −5, and BMIZ>8 or BMIZ< −4). Continuous variables were summarized as mean and s.e., and categorical variables were described as percentages.
Structural equation modeling (SEM) is a statistical technique that can handle more complex relationships among variables, including mediation than traditional methods, such as generalized linear models. The primary advantage of the SEMs is its ability to decompose the total effect of GWG on anthropometric outcomes into direct and indirect effect via mediators simultaneously. The general mapping of these models is shown in the Figure 1, with Supplementary Materials offering more detailed setup of these models. Specifically, with the anthropometric outcome as the main model, we simultaneously examined three more models to estimate the direct effects of GWG on birth weight and breastfeeding duration after adjusting for other covariates listed in each model, as well as the direct effects of maternal demographic and pregnancy factors on GWG. Combining the effects from main model with the other three models, the pathways for the effect of GWG on the anthropometric outcome through birth weight and breastfeeding duration are then created as shown in the Figure 1. Our final models satisfied the common criteria in SEM, including the root mean square error of approximation estimate <0.08 and the standardized root mean square residual <0.08.13 We constructed the 95% confidence intervals (CIs) for the parameters and significance was set at the 0.05 level. All models were performed using Stata version 13 (StataCorp LP, College Station, TX, USA).
Figure 1.
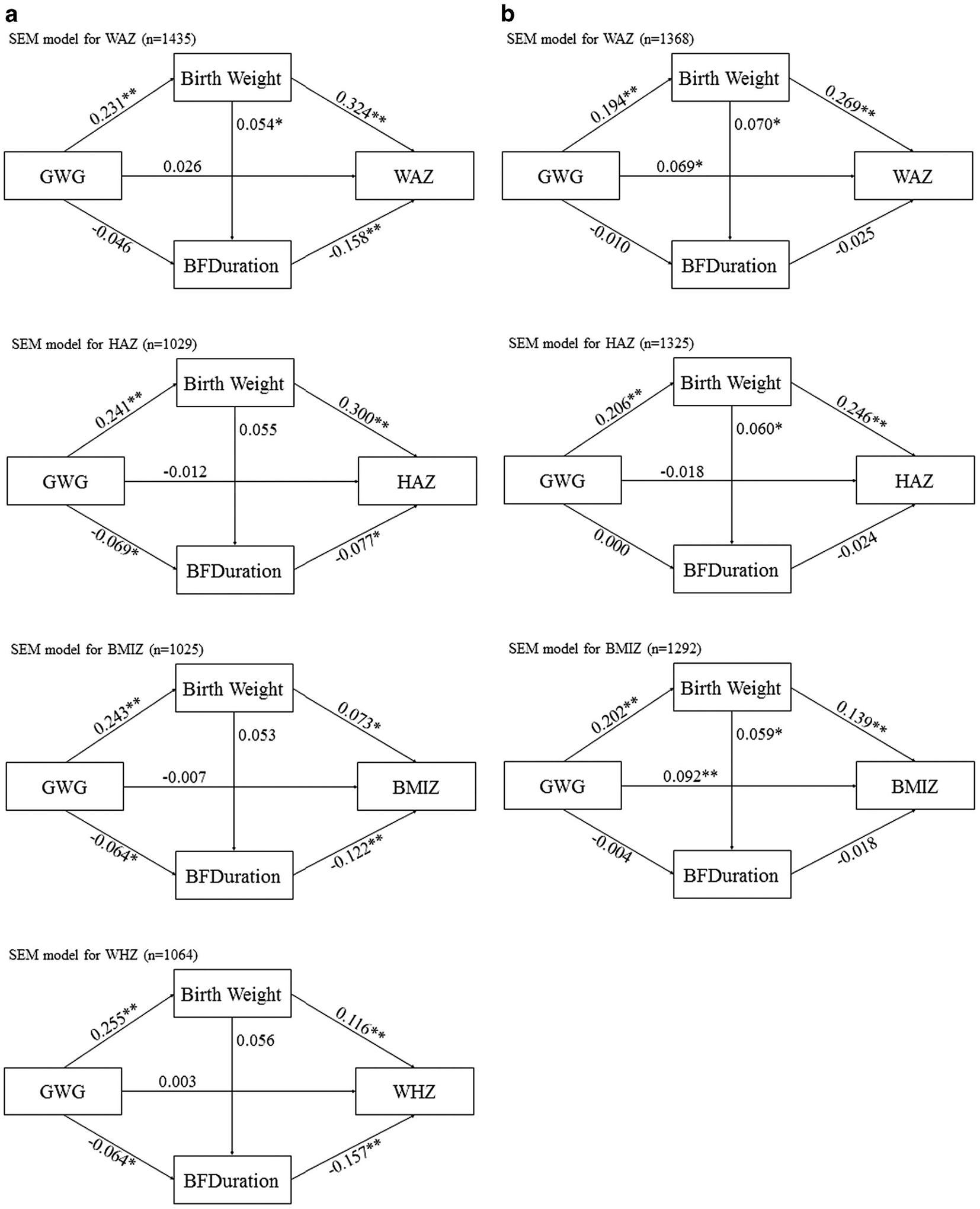
(a) Adjusted effects of maternal GWG, through mediators child’s birth weight and duration of breastfeeding on anthropometric outcomes (WAZ, HAZ, WHZ and BMIZ) for children at 1 year old. **P<0.01, *P<0.05. (b) Adjusted relationships among maternal GWG, child’s birth weight, duration of breastfeeding and WAZ, HAZ and BMIZ for children at 6 years old. **P<0.01, *P<0.05.
RESULTS
Table 1 summarized sample characteristics for each cohort of mothers at baseline and their offspring at late infancy and 6 years old, respectively. Approximately 90% of the women were White and half of the children were boys. The majority of women was married at pregnancy and had a college or higher education. The average weight mothers gained during pregnancy was around 14 kg in this study sample. The percentages of children who were overweight (BMIZ ⩾ 85th percentile) at late infancy and age 6 years were 30.47% and 24.01%, respectively (data not shown in the table). Almost 91% infants were introduced to solid foods before 6 months of age. Children at age 6 did ~ 5 days of physical activity in a regular week for at least 60 min a day, and the average screen time was around 1.8 h per day.
Table 1.
Sample characteristics by children’s age at the anthropometric measures
| Characteristics | Offspring | |
|---|---|---|
| One year old | Six years old | |
| n | 1548 | 1514 |
| Maternal characteristics at baseline | ||
| Maternal age in years, mean (s.d.) | 29.89 (5.28) | 30.06 (5.30) |
| Race, % | ||
| White | 89.12 | 88.68 |
| Black | 3.18 | 3.71 |
| Other | 7.69 | 7.62 |
| Married or cohabiting, % | 80.04 | 79.91 |
| Education, % | ||
| High school or less | 16.87 | 16.63 |
| Some college | 35.31 | 37.01 |
| College graduate or higher | 47.83 | 46.36 |
| Employed, % | 57.66 | 58.07 |
| Household income as percent of poverty level, % | ||
| < 185% | 35.01 | 35.23 |
| 185–350% | 37.02 | 37.61 |
| >350% | 27.97 | 27.16 |
| Parity, % | ||
| Primiparous | 29.83 | 28.40 |
| Multiparous | 70.17 | 71.60 |
| Maternal smoking during pregnancy, % | 7.38 | 7.29 |
| Pre-pregnancy BMI, mean (s.d.) | 27.37 (6.92) | 27.41 (7.00) |
| Total gestational weight gain (kg) at delivery, mean (s.d.) | 14.00 (6.29) | 13.89 (6.37) |
| Gestational diabetes, % | 6.75 | 7.83 |
| Characteristics of infants | ||
| Infant sex, % | ||
| Boy | 49.03 | 50.10 |
| Girl | 50.97 | 49.90 |
| Age at solid food introduction, % | ||
| < 4 months | 43.54 | 49.41 |
| 4 to < 6 months | 47.67 | 42.21 |
| ⩾ 6 months | 8.79 | 8.39 |
| Gestational age in weeks, mean (s.d.) | 39.29 (1.26) | 39.33 (1.26) |
| Infant’s birth weight in grams, mean (s.d.) | 3468.00 (471.38) | 3475.25 (486.75) |
| Duration of breastfeeding in weeks, mean (s.d.) | 28.24 (22.05) | 34.76 (36.05) |
| Characteristics of 6 years old | ||
| Days of doing physical activity for at least 60 min in a week | — | 5.44 (1.61) |
| Hours of watching television per day | — | 1.83 (1.16) |
| Times of sugar-sweetened beverages consumption per week | — | 3.65 (5.60) |
| Days of fast food consumption times per week | 0.86 (0.87) | |
| Anthropometric outcomes | ||
| Weight-for-age Z-score, mean (s.d.) | 0.27 (1.15) | 0.09 (1.13) |
| Height-for-age Z-score (s.d.) | 0.03 (1.65) | − 0.05 (1.21) |
| Weight-for-height Z-score (s.d.) | 0.45 (1.35) | — |
| BMI-for-age Z-score (s.d.) | 0.41 (1.42) | 0.13 (1.34) |
Abbreviation: BMI, body mass index.
Table 2 showed the results of total effect, direct effect, indirect effect and proportion of total effect mediated through both birth weight and duration of breastfeeding on the main associations of interest at late infancy and at 6 years of age. Overall, for the total effect, maternal GWG was positively associated with offspring’s WAZ with the β coefficients and 95% CIs of 0.107 (0.052, 0.161) at late infancy and 0.122 (0.066, 0.177) at age 6 years, respectively. Maternal GWG was positively associated with offspring’s BMIZ at 6 years of age only (β = 0.120, 95% CI: 0.063, 0.178). We observed significant direct effects between GWG with offspring’s WAZ (β = 0.069, 95% CI: 0.014, 0.128) and BMIZ (β = 0.092, 95% CI: 0.034, 0.151) at age 6 years only. In general, indirect effects of GWG on anthropometric outcomes in both late infancy and age 6 years through birth weight were significant while the effects through duration of breastfeeding were marginally significant only in late infancy (P-value <0.1, results not shown). These findings suggest that birth weight was a stronger mediator than breastfeeding duration. At late infancy, most of the effect of GWG on anthropometric outcomes came from its indirect effect through mediators, that is, birth weight and breastfeeding duration. More specifically, the indirect effect counted for 75.7%, 86.4%, 92.5% and 77.4% of the total effect, respectively, for WAZ, HAZ, WHZ and BMIZ. At age 6 years, the direct effect of GWG became stronger, while the indirect effect through birth weight still existed. Overall, the proportion of indirect effects of GWG (Table 2) through both birth weight and the duration of breastfeeding were greater in magnitude in late infancy compared with mediating effects at age 6.
Table 2.
Total, direct and indirect effects of total GWG on the offspring anthropometric outcomes at age 1 and 6 years old through birth weight and breastfeeding duration, IFPS II and its Y6FU study
| Outcomes | N | Mediators | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total effect of GWG | Direct effect of GWG | Indirect effect of GWG through birth weight | Indirect effect of GWG through breastfeeding duration | Proportion of total effect (%) | ||||||
| β | 95% CI | 95% CI | β | 95% CI | β | 95% CI | ||||
| Age at 1 | ||||||||||
| WAZ | 1435 | 0.107 | (0.052, 0.161) | 0.026 | (−0.027, 0.080) | 0.073 | (0.053, 0.093) | 0.007 | (−0.001, 0.016) | 75.70 |
| HAZ | 1029 | 0.065 | (−0.001, 0.130) | − 0.012 | (−0.077, 0.053) | 0.071 | (0.048, 0.095) | 0.005 | (−0.001, 0.012) | 86.36 |
| WHZ | 1064 | 0.040 | (−0.025, 0.105) | 0.003 | (−0.063, 0.069) | 0.027 | (0.009, 0.046) | 0.010 | (−0.000, 0.020) | 92.50 |
| BMIZ | 1025 | 0.017 | (−0.048, 0.083) | − 0.007 | (−0.074, 0.060) | 0.016 | (−0.001, 0.033) | 0.008 | (−0.001, 0.016) | 77.42 |
| Age at 6 | ||||||||||
| WAZ | 1368 | 0.122 | (0.066, 0.177) | 0.069 | (0.014, 0.124) | 0.052 | (0.035, 0.069) | 0.000 | (−0.001, 0.002) | 42.62 |
| HAZ | 1325 | 0.032 | (−0.026, 0.090) | − 0.018 | (−0.076, 0.040) | 0.051 | (0.033, 0.068) | 0.000 | (−0.001, 0.001) | 73.91 |
| BMIZ | 1292 | 0.120 | (0.063, 0.178) | 0.092 | (0.034, 0.151) | 0.028 | (0.014, 0.042) | 0.000 | (−0.001, 0.001) | 23.33 |
Abbreviations: BMIZ, body mass index Z-score; CI, confidence interval; GWG, gestational weight gain; HAZ, height-/length-for-age Z-score; IFPS II, Infant Feeding Practices Study II; WAZ, weight-for-age Z-score; WHZ, weight-for-height/-length Z-score; Y6FU, Year 6 Follow-Up study. All models adjusted for maternal age, pre-pregnancy BMI, race (white, black and other), maternal education (high school or less, some college, college graduate or higher), married and cohabiting (yes or no), income (<185%, 185–350% and >350%), parity (primiparous or multiparous), maternal smoking during pregnancy (yes or no), gestational diabetes (yes or no), age at solid food introduction (<4 months, 4 to <6 months and ⩾6 months), gestational age and infant sex. For children at age of 6 years, days of doing physical activity in a typical week for a total of at least 60 min, hours of watching television per day, times of sugar-sweetened beverages consumption per week and days of fast food consumption times per week were additionally adjusted.
Figure 1 indicates the estimates of regression coefficients and its significant levels for each direct effect of the exposure on its outcome. While the direct effect of GWG on each anthropometric measure shown in Table 2 was also presented in this Figure 1, the indirect effects shown in Table 2 were simply the multiplication of the direct effect of GWG on either birth weight or breastfeeding duration and the direct effect of the mediator on the anthropometric outcome presented in the figure. For example, the indirect effect of GWG through birth weight on WAZ at age 6 years is 0.052 (Table 2), which is 0.194 × 0.269 (Figure 1b).
Similar to Table 2, Figure 1a indicates that most of the indirect effects of GWG on anthropometric outcomes in late infancy were significant, either mediated through positive correlation with birth weight or negative correlation with breastfeeding duration (based on the significant direct effects shown with ‘*/**’ in the figure). The absolute magnitude of the mediating effect of birth weight is larger than breastfeeding duration, indicating that birth weight is a stronger mediator than breastfeeding duration. No direct association of GWG with offspring anthropometrics was observed among infants.
Figure 1b demonstrated the relationships of maternal GWG with offspring WAZ, HAZ and BMIZ at 6 years of age through birth weight and duration of breastfeeding. In contrast to the findings among infants, the negative association of breastfeeding duration and children’s anthropometrics was not statistically significant, and the GWG did not have a significant effect on the breastfeeding duration.
Sensitivity analysis was conducted that included the BIVs. For year 6, all the significance and direction of the estimated effects did not change. The effect of breastfeeding duration on HAZ at late infancy changed to be not significant, while the indirect effect of GWG through breastfeeding duration was still not significant. We looked further into this group of children; the mean breastfeeding duration for children with a BIV for HAZ was lower than the mean in this sample (21.93 versus 27.62).
DISCUSSION
This study shows that GWG might have an important role in children’s anthropometric development with significant overall associations of GWG with offspring WAZ during late infancy and with WAZ and BMIZ at 6 years old. While direct effects of maternal GWG were only observed on WAZ and BMIZ among 6 years old, its indirect effects via birth weight were observed for almost all the anthropometric outcomes measured in late infancy and at 6 years old indicating significant mediating role of birth weight on anthropometric development during childhood. In contrast, breastfeeding duration, as a potential mediator, was marginally significant only among late infancy suggesting birth weight is a stronger mediator than duration of breastfeeding. These findings advance our understanding of how birth weight and duration of breastfeeding may mediate the risk of GWG on childhood anthropometric outcomes in late infancy and at 6 years old.
Childhood obesity is a multi-system disease predisposing children to a myriad of short- and long-term adverse health outcomes such as insulin resistance syndrome, cardiovascular diseases, metabolic syndrome, diabetes and certain cancers later on in life.35,36 Similar to adult obesity, the etiology of childhood obesity is complex and may be influenced by both genetic and environmental/behavioral factors in prenatal and early life periods such as fetal growth, infant growth, diet, maternal feeding styles, physical activity and food environment.30,37–43 It is reported that the children born to mothers who gained excessive weight during pregnancy have higher birth weight,44,45 a strong predictor for obesity later on in life.46,47 Previous evidence supports adverse effects not only between excessive GWG and high birth weight but also between inadequate GWG and low birth weight.48 As the effects of GWG also depend upon pre-pregnancy BMI, Institute of Medicine recommends a range of optimal GWG for underweight, normal weight, overweight and obese women, respectively.49
Several studies have suggested that birth weight might act as a mediator on the pathways between maternal GWG and their offsprings’ adiposity due to the attenuated magnitude of the association after additional adjustment for birth weight.10,17,50,51 However, there is limited information regarding the decomposition of the total effect of maternal GWG on their offsprings’ anthropometric outcomes into direct and indirect effect through birth weight and duration of breastfeeding. The current study is one of a few11,12 that have examined the potential mediation effects of birth weight and duration of breastfeeding. Our findings were consistent in part with the findings from previous studies. Zhu and colleagues11 reported a significant overall association between GWG and offspring HAZ at ages 0–5.9 years based on a cross-sectional study. In addition, maternal GWG was positively associated with childhood anthropometrics, including WAZ, HAZ, WHZ and BMIZ via indirect pathways mainly through birth weight. Hinkle and colleagues12 examined how the overall association between GWG and offspring BMIZ at age 5 was decomposed into direct and indirect effects through birth weight and duration of breastfeeding using the data from the Early Childhood Longitudinal Study-Birth Cohort, a nationally representative cohort of children born in 2001. They observed a significant total association between GWG and BMIZ among both normal and overweight mothers, but the direct effect of GWG on BMIZ was significant only among mothers with normal weight, and the direct effect was stronger than the indirect effect through both birth weight and duration of breastfeeding. However, Hinkle et al. did not present separated mediating effects of birth weight from duration of breastfeeding in their paper.
Breastfeeding is considered an important modifiable factor in children’s early life that may help prevent childhood obesity.39 Previous studies also showed that breastfeeding duration has a mediating role on the pathway between GWG and child anthropometrics, which is of particular interest given that it might suppress/contradict the positive relationship between GWG and adiposity. In our study, the negative relationships observed in the pathway from GWG-breastfeeding duration-anthropometric outcomes during infancy implies that higher GWG is significantly associated with shorter breastfeeding duration, which in turn led to higher infant anthropometric values at 1 year. However, such associations were not observed among 6 years old. Similarly, Zhu et al.21 found that longer duration of breastfeeding suppressed the positive associations of GWG and birth weight with offspring WAZ, WHZ and BMIZ among children aged 0–5.9 years. However, Hinkle et al.22 did not observe a significant association between duration of breastfeeding and child BMIZ score at 5 years.
The current study has several strengths. First, the longitudinal design of IFPS II with detailed information collected about infants’ feeding practices reduced recall bias of measures in this study. Second, a large number of potential confounders were collected in this study with a relatively large sample size. Third, the linkage of the Y6FU data to the longitudinal IFPS II provides a unique opportunity to examine associations of maternal GWG and offspring anthropometric measures beyond infancy. Several potential limitations are worth noting. First, anthropometric measures in infancy and 6 years old, as well as GWG were reported by mothers, thus were susceptible to reporting errors even though all the anthropometric data during infancy were reported as the measures by clinicians during the latest clinic visit and Y6FU included detailed instruction with a standard tape for mothers to measure weight and height of their 6 years old. Given the longitudinal design of IFPS II, which asked mothers to report weight and height as measurements by the clinicians during doctor’s visit multiple times over the study period, the recall period for each anthropometric measurement was relatively short, which might limit the recall bias. Second, breastfeeding variable used in this study is duration of breastfeeding to any extent. Thus, it could not differentiate exclusive breastfeeding from partial breastfeeding to understand the impact of breastfeeding intensity on child growth. Third, we limited our study sample to the complete cases, as well as cases without BIV. However, sensitivity analysis conducted among all participants, including BIV cases had minimal impacts on the findings. Fourth, although well distributed throughout the United States, the data are not nationally representative of the US population. Hence, our results could not be generalized to the entire US population. Finally, we recognize that the same weight gain might have different health effects depending on the mother’s initial weight as suggested by the Institute of Medicine’s guidelines on GWG. As none of the interaction was tested significant in this study, we only controlled pre-pregnancy BMI as a covariate in the modeling.
In conclusion, our study shed lights on the association between maternal GWG and their offspring’s anthropometric development in both late infancy and early childhood by decomposing the total effect of GWG into direct and indirect effects through birth weight and breastfeeding duration. Our findings suggest a stronger indirect rather than direct effect of GWG on anthropometric outcomes at late infancy through birth weight, independent of maternal sociodemographic and reproductive factors. Although the direct effects of GWG were not significant for any anthropometric outcomes at late infancy, it became significant on WAZ and BMIZ at 6 years old. Furthermore, we found longer duration of breastfeeding might suppress the positive relationship between GWG and anthropometric outcomes in infancy but not at 6 years old. Therefore, promoting appropriate GWG, avoiding high birth weights and supporting longer duration of breastfeeding might help prevent childhood obesity.
Supplementary Material
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies this paper on International Journal of Obesity website (http://www.nature.com/ijo)
REFERENCES
- 1.de Onis M, Blossner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr 2010; 92: 1257–1264. [DOI] [PubMed] [Google Scholar]
- 2.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014; 384: 766–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Available at http://www.who.int/end-childhood-obesity/facts/en/ (accessed on 14 September 2015).
- 4.World Health Organization. Available at http://www.who.int/end-childhood-obesity/news/echo-second-meeting/en/ (accessed on 14 September 2015).
- 5.Obstetricians ACo and Gynecologists. ACOG Committee opinion no. 549: obesity in pregnancy. Obstet Gynecol 2013; 121: 213. [DOI] [PubMed] [Google Scholar]
- 6.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 2014; 311: 806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu F Genetic predictors of obesity. Obes Epidemiol 2008; 437–460. [Google Scholar]
- 8.Ebbeling CB, Pawlak DB, Ludwig DS. Childhood obesity: public-health crisis, common sense cure. Lancet 2002; 360: 473–482. [DOI] [PubMed] [Google Scholar]
- 9.Bammann K, Peplies J, De Henauw S, Hunsberger M, Molnar D, Moreno LA et al. Early life course risk factors for childhood obesity: the IDEFICS case-control study. PLoS ONE 2014; 9: e86914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wrotniak BH, Shults J, Butts S, Stettler N. Gestational weight gain and risk of overweight in the offspring at age 7 y in a multicenter, multiethnic cohort study. Am J Clin Nutr 2008; 87: 1818–1824. [DOI] [PubMed] [Google Scholar]
- 11.Zhu Y, Hernandez LM, Dong Y, Himes JH, Hirschfeld S, Forman MR. Longer breastfeeding duration reduces the positive relationships among gestational weight gain, birth weight and childhood anthropometrics. J Epidemiol Commun Health 2015; 69: 632–638. [DOI] [PubMed] [Google Scholar]
- 12.Hinkle SN, Sharma AJ, Swan DW, Schieve LA, Ramakrishnan U, Stein AD. Excess gestational weight gain is associated with child adiposity among mothers with normal and overweight prepregnancy weight status. J Nutr 2012; 142: 1851–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hooper D, Coughlan J, Mullen M. Structural equation modelling: guidelines for determining model fit. Articles 2008; 2. [Google Scholar]
- 14.Tie HT, Xia YY, Zeng YS, Zhang Y, Dai CL, Guo JJ et al. Risk of childhood over-weight or obesity associated with excessive weight gain during pregnancy: a meta-analysis. Archives of gynecology and obstetrics 2014; 289: 247–257. [DOI] [PubMed] [Google Scholar]
- 15.Lau EY, Liu J, Archer E, McDonald SM, Liu J. Maternal weight gain in pregnancy and risk of obesity among offspring: a systematic review. Journal of obesity 2014; 2014: 524939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beyerlein A, Nehring I, Rzehak P, Heinrich J, Muller MJ, Plachta-Danielzik S et al. Gestational weight gain and body mass index in children: results from three german cohort studies. PLoS ONE 2012; 7: e33205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Branum AM, Parker JD, Keim SA, Schempf AH. Prepregnancy body mass index and gestational weight gain in relation to child body mass index among siblings. Am J Epidemiol 2011; 174: 1159–1165. [DOI] [PubMed] [Google Scholar]
- 18.Ehrenthal DB, Maiden K, Rao A, West DW, Gidding SS, Bartoshesky L et al. Independent relation of maternal prenatal factors to early childhood obesity in the offspring. Obstet Gynecol 2013; 121: 115–121. [DOI] [PubMed] [Google Scholar]
- 19.Deierlein AL, Siega-Riz AM, Adair LS, Herring AH. Effects of pre-pregnancy body mass index and gestational weight gain on infant anthropometric outcomes. J Pediatr 2011; 158: 221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deierlein AL, Siega-Riz AM, Herring AH, Adair LS, Daniels JL. Gestational weight gain and predicted changes in offspring anthropometrics between early infancy and 3 years. Pediatr Obes 2012; 7: 134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hilson JA, Rasmussen KM, Kjolhede CL. Excessive weight gain during pregnancy is associated with earlier termination of breast-feeding among White women. J Nutr 2006; 136: 140–146. [DOI] [PubMed] [Google Scholar]
- 22.Winkvist A, Brantsaeter AL, Brandhagen M, Haugen M, Meltzer HM, Lissner L. Maternal prepregnant body mass index and gestational weight gain are associated with initiation and duration of breastfeeding among Norwegian mothers. J Nutr 2015; 145: 1263–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gillman MW, Rifas-Shiman SL, Camargo CA Jr, Berkey CS, Frazier AL, Rockett HR et al. Risk of overweight among adolescents who were breastfed as infants. JAMA 2001; 285: 2461–2467. [DOI] [PubMed] [Google Scholar]
- 24.Mayer-Davis EJ, Rifas-Shiman SL, Zhou L, Hu FB, Colditz GA, Gillman MW. Breast-feeding and risk for childhood obesity: does maternal diabetes or obesity status matter? Diabetes Care 2006; 29: 2231–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan J, Liu L, Zhu Y, Huang G, Wang PP. The association between breastfeeding and childhood obesity: a meta-analysis. BMC Public Health 2014; 14: 1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mgutshini NL Gestational weight gain and the risk of obesity among preschool children: is this mediated through birth weight? 2014. Available at http://scholarcommons.sc.edu/cgi/viewcontent.cgi?article=3687&context=etd.
- 27.Yu Z, Han S, Zhu G, Zhu C, Wang X, Cao X et al. Birth weight and subsequent risk of obesity: a systematic review and meta‐analysis. Obes Rev 2011; 12: 525–542. [DOI] [PubMed] [Google Scholar]
- 28.Fein SB, Labiner-Wolfe J, Shealy KR, Li R, Chen J, Grummer-Strawn LM. Infant Feeding Practices Study II: study methods. Pediatrics 2008; 122 (Suppl 2): S28–S35. [DOI] [PubMed] [Google Scholar]
- 29.Fein SB, Li R, Chen J, Scanlon KS, Grummer-Strawn LM. Methods for the year 6 follow-up study of children in the Infant Feeding Practices Study II. Pediatrics 2014; 134(Suppl 1): S4–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taveras EM, Rifas-Shiman SL, Sherry B, Oken E, Haines J, Kleinman K et al. Crossing growth percentiles in infancy and risk of obesity in childhood. Arch Pediatr Adolesc Med 2011; 165: 993–998. [DOI] [PubMed] [Google Scholar]
- 31.The Centers for Disease Prevention and Control. A SAS Program for the 2006 WHO Growth Charts. Available at http://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm (accessed on 11 January 2016).
- 32.The Centers for Disease Prevention and Control. A SAS Program for the 2000 CDC Growth Charts. Available at http://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm (accessed on 11 Novermber 2015).
- 33.Centers for Disease Control and Prevention. Available at http://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas-who.htm (accessed on 27 April 2016).
- 34.Centers for Disease Control and Prevention. Available at http://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm (accessed on 27 April 2016).
- 35.Ebbeling CB, Pawlak DB, Ludwig DS. Childhood obesity: public-health crisis, common sense cure. Lancet 2002; 360: 473–482. [DOI] [PubMed] [Google Scholar]
- 36.Han JC, Lawlor DA, Kimm SY. Childhood obesity. Lancet 2010; 375: 1737–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lytle LA. Examining the etiology of childhood obesity: The IDEA study. Am J Commun Psychol 2009; 44: 338–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bagchi D Global Perspectives on Childhood Obesity: Current Status, Consequences and Prevention. Academic Press: London, 2010. [Google Scholar]
- 39.Robinson SM, Crozier SR, Harvey NC, Barton BD, Law CM, Godfrey KM et al. Modifiable early-life risk factors for childhood adiposity and overweight: an analysis of their combined impact and potential for prevention. Am J Clin Nutr 2015; 101: 368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson L, Mander AP, Jones LR, Emmett PM, Jebb SA. Energy-dense, low-fiber, high-fat dietary pattern is associated with increased fatness in childhood. Am J Clin Nutr 2008; 87: 846–854. [DOI] [PubMed] [Google Scholar]
- 41.Rodgers RF, Paxton SJ, Massey R, Campbell KJ, Wertheim EH, Skouteris H et al. Maternal feeding practices predict weight gain and obesogenic eating behaviors in young children: a prospective study. Int J Behav Nutr Phys Act 2013; 10: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doornweerd S, RG IJ, van der Eijk L, Neter JE, van Dongen J, van der Ploeg HP et al. Physical activity and dietary intake in BMI discordant identical twins. Obesity 2016; 24: 1349–1355. [DOI] [PubMed] [Google Scholar]
- 43.Penney TL, Almiron-Roig E, Shearer C, McIsaac JL, Kirk SF. Modifying the food environment for childhood obesity prevention: challenges and opportunities. Proc Nutr Soc 2014; 73: 226–236. [DOI] [PubMed] [Google Scholar]
- 44.Shrestha I, Sunuwar L, Bhandary S, Sharma P. Correlation between gestational weight gain and birth weight of the infants. Nepal Med Coll J 2010; 12: 106–109. [PubMed] [Google Scholar]
- 45.Catov JM, Abatemarco D, Althouse A, Davis EM, Hubel C. Patterns of gestational weight gain related to fetal growth among women with overweight and obesity. Obesity 2015; 23: 1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rogers I, Group E-BS. The influence of birthweight and intrauterine environment on adiposity and fat distribution in later life. Int J Obes Relat Metab Disord 2003; 27: 755–777. [DOI] [PubMed] [Google Scholar]
- 47.Yu ZB, Han SP, Zhu GZ, Zhu C, Wang XJ, Cao XG et al. Birth weight and subsequent risk of obesity: a systematic review and meta-analysis. Obes Rev 2011; 12: 525–542. [DOI] [PubMed] [Google Scholar]
- 48.Siega-Riz AM, Viswanathan M, Moos MK, Deierlein A, Mumford S, Knaack J et al. A systematic review of outcomes of maternal weight gain according to the Institute of Medicine recommendations: birthweight, fetal growth, and postpartum weight retention. Am J Obstet Gynecol 2009; 201: e1–e14. [DOI] [PubMed] [Google Scholar]
- 49.Institute of Medicine. Weight Gain During Pregnancy: Reexamining the Guidelines. National Academies Press: Washington, DC, USA: 2009. [PubMed] [Google Scholar]
- 50.Crozier SR, Inskip HM, Godfrey KM, Cooper C, Harvey NC, Cole ZA et al. Weight gain in pregnancy and childhood body composition: findings from the Southampton Women’s Survey. Am J Clin Nutr 2010; 91: 1745–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schack-Nielsen L, Michaelsen KF, Gamborg M, Mortensen EL, Sorensen TI. Gestational weight gain in relation to offspring body mass index and obesity from infancy through adulthood. Int J Obes 2010; 34: 67–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


