Abstract
Nitrate has been shown to shunt the electron flow in Clostridium thermoaceticum from CO2 to nitrate, but it did not influence the levels of enzymes involved in the Wood-Ljungdahl pathway (J. M. Fröstl, C. Seifritz, and H. L. Drake, J. Bacteriol. 178:4597–4603, 1996). Here we show that under some growth conditions, nitrate does in fact repress proteins involved in the Wood-Ljungdahl pathway. The CO oxidation activity in crude extracts of nitrate (30 mM)–supplemented cultures was fivefold less than that of nitrate-free cultures, while the H2 oxidation activity was six- to sevenfold lower. The decrease in CO oxidation activity paralleled a decrease in CO dehydrogenase (CODH) protein level, as confirmed by Western blot analysis. Protein levels of CODH in nitrate-supplemented cultures were 50% lower than those in nitrate-free cultures. Western blots analyses showed that nitrate also decreased the levels of the corrinoid iron-sulfur protein (60%) and methyltransferase (70%). Surprisingly, the decrease in activity and protein levels upon nitrate supplementation was observed only when cultures were continuously sparged. Northern blot analysis indicates that the regulation of the proteins involved in the Wood-Ljungdahl pathway by nitrate is at the transcriptional level. At least a 10-fold decrease in levels of cytochrome b was observed with nitrate supplementation whether the cultures were sparged or stoppered. We also detected nitrate-inducible nitrate reductase activity (2 to 39 nmol min−1 mg−1) in crude extracts of C. thermoaceticum. Our results indicate that nitrate coordinately represses genes encoding enzymes and electron transport proteins in the Wood-Ljungdahl pathway and activates transcription of nitrate respiratory proteins. CO2 also appears to induce expression of the Wood-Ljungdahl pathway genes and repress nitrate reductase activity.
Acetogenic bacteria are strict anaerobes that can grow on CO2 as the electron acceptor. Two moles of CO2, together with 1 mol of coenzyme A (CoA), are converted to 1 mol of acetyl-CoA in an eight-electron reductive process called the Wood-Ljungdahl pathway (3, 39). The resultant acetyl-CoA can either be cleaved, yielding acetate, CoA, and 1 mol of ATP, or be used for biomass production. Key proteins in the Wood-Ljungdahl pathway are the bifunctional Ni-Fe/S enzyme CO dehydrogenase/acetyl-CoA synthase (CODH/ACS), a corrinoid iron-sulfur protein (CFeSP), and a methyltransferase (MeTr). The Clostridium thermoaceticum (renamed Moorella thermoacetica) genes encoding these three proteins are located on one large gene cluster (27).
In addition to CO2, acetogens can dissimilate a number of alternative substrates. Among these are fumarate (7, 21), methoxylated aromatic acids (21), malate (7), pyruvate (23), aromatic acrylates (22), inorganic sulfur compounds (17), and nitrate (32). Thus, acetogens appear to be a rather versatile and opportunistic group of bacteria. However, despite ample knowledge about the enzymology of the Wood-Ljungdahl pathway, the regulation (if any) of the proteins central to the Wood-Ljungdahl pathway has not been studied.
C. thermoaceticum is a strictly anaerobic, acetogenic thermophile that can either grow autotrophically or grow heterotrophically on substrates like glucose. In the latter case, the organism fixes CO2 to acetyl-CoA via the Wood-Ljungdahl pathway to dispose of its reducing equivalents. The organism can also use nitrate as its sole electron acceptor. When grown in the presence of both CO2 and nitrate, the latter was shown to be preferentially used as an electron acceptor over CO2 (32). In a later study, it was found that nitrate inhibited autotrophic growth of C. thermoaceticum and decreased membrane-bound cytochrome b levels, but it had no effect on the specific activity of enzymes engaged in the Wood-Ljungdahl pathway (10). Therefore, it was concluded that C. thermoaceticum cannot engage the carbon-fixing capacities of the Wood-Ljungdahl pathway in the presence of nitrate and that the nitrate block on the Wood-Ljungdahl pathway occurs via an alteration in electron transport.
In our laboratory, C. thermoaceticum is routinely grown under two different procedures. To maintain the organism, we grow it in stoppered 120-ml vials under a CO2 atmosphere. For enzyme purification purposes, we use a 14-liter fermentor that is continuously sparged with CO2. We found that extracts from nitrate-supplemented, sparged cultures invariably displayed significantly lower CO oxidation activity than sparged cultures that were not supplemented with nitrate. This prompted us to investigate the effect of nitrate on the expression of CODH/ACS and other proteins of the Wood-Ljungdahl pathway. The results of this study demonstrate that nitrate acts as a transcriptional repressor of the key enzymes of the Wood-Ljungdahl pathway.
MATERIALS AND METHODS
Growth conditions.
C. thermoaceticum (ATCC 39073) was cultivated under a 100% CO2 atmosphere at 55°C either in 120-ml serum vials that were closed with butyl rubber stoppers containing 100 ml of culture medium or in Erlenmeyer flasks that were continuously sparged with CO2. The medium was prepared essentially as described by Andreesen et al. (1) and contained (per liter) glucose (18 g), yeast extract (5 g), tryptone (5 g), K2HPO4 (3.5 g), KH2PO4 (2.8 g), NaHCO3 (8.4 g), thioglycolic acid (0.5 g), (NH4)2SO4 (1 g), NaCl (0.4 g), MgSO4 (0.25 g), CaCl (0.086 g), CoCl · 6H2O (0.03 g), nitrilotriacetic acid (0.075 g), zinc acetate (0.075 g), Fe(NH4)2(SO4)2 · 6H2O (0.039 g), NiCl2 (3 mg), Na2MoO4 · 2H2O (1.6 mg), Na2WO42 · H2O (2.2 mg), Na2SeO3 (13 mg), and the vitamins biotin, cyanocobalamin, flavin mononucleotide, folic acid, nicotinic acid, panthothenic acid, p-aminobenzoic acid, and thiamine pyrophosphate (200 μg of each). Nitrate was added as NaNO3. Gases were obtained from Lindweld, Lincoln, Neb.
Preparation of cell extracts.
Cells were harvested anaerobically at the end of exponential growth by centrifugation (Beckman model J2-HS centrifuge) at 10,000 × g for 20 min at room temperature. All ensuing steps were performed in an anaerobic glove box (oxygen tension, <5 ppm; Coy). The cell paste was suspended in three parts (weight/volume) lysis buffer containing Tris-HCl, pH 7.6 (50 mM), phenylmethylsulfonyl fluoride (10 mg/liter), lysozyme (1 g/liter), dithiothreitol (2 mM), sodium dithionite (2 mM), methyl viologen (0.1 mM), and DNase I (3 kU/liter). The suspension was sonicated for 5 min at 275 W (model XL2020; Heat Systems, Farmingdale, N.Y.) and the cell extract was centrifuged for 20 min at 14,000 × g at room temperature in a microcentrifuge (Eppendorf).
Enzyme activities.
CO oxidation was measured at 604 nm as described previously (5), using 10 mM methyl viologen. Nitrate reduction was measured at 578 nm following the oxidation of reduced benzyl viologen at 55°C. The nitrate reduction assay mixture contained 20 mM Tris-HCl (pH 6.8), 1 mM benzyl viologen, 0.1 mM sodium dithionite [from a freshly prepared 0.1 M stock solution in 0.2 M N-tris(hydroxymethyl)methyl-3-aminopropanesulfonic acid (pH 10)], and 5 μl of extract. The nonenzymatic oxidation of benzyl viologen was recorded for 1 min, and the reaction was started by adding 10 mM sodium nitrate. H2 oxidation was measured spectrophotometrically as described by Drake (8) at 55°C. One unit of activity is defined as 2 μmol of viologen oxidized or reduced per min.
Analytical procedures.
Unless otherwise stated, all chemicals were obtained from Sigma. Nitrite was determined by the diazo-coupling method (14). Nitrate was measured as nitrite after reduction with Cd filings in an anaerobic glove box (24). Cd filings were prepared as described by Green et al. (13), using Cd powder (100 mesh; Aldrich). Samples were diluted 1,000-fold in 5% (wt/vol) NH4Cl to yield a nitrate concentration of 30 μM or less. After adding Cd filings (10%, vol/vol), the suspension was vortexed for 1 min and briefly centrifuged, and the supernatant was assayed for nitrite. The conversion of nitrate to nitrate was >95%. Ammonium was measured enzymatically by monitoring NADH oxidation at 340 nm. The assay mixture (1 ml) contained glutamate dehydrogenase (type II, bovine liver) (2.8 U), NADH (0.1 mM), α-ketoglutarate (6 mM), and EDTA (0.5 mM) in 50 mM Tris-HCl, pH 7.6. Glucose was determined enzymatically by measuring the formation of NADPH at 340 nm. The assay mixture (1 ml, in 50 mM Tris-HCl [pH 7.6]) contained glucose-6-phosphate dehydrogenase (1.4 U), hexokinase (2.5 U), and NADP (0.2 mM). Acetate was measured by gas chromatography (Varian GC model 3700 gas chromatograph) on a capillary column (EC-1000; Alltech). The medium was clarified by centrifugation (10 min at 14,000 rpm) and diluted 50-fold in distilled water. The sample (1 ml) was acidified by adding 20 μl of concentrated HCl and centrifuged, and the supernatant (1 μl) was injected into the column. Chromatography conditions were as follows: injection port, 275°C; detection port, 250°C; column temperature, 125°C. After elution of acetate (∼30 s), the temperature was increased to 225°C at a speed of 25°C per min and then held at 225°C for 8 min. The column was allowed to cool to 125°C prior to a new injection. Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis was performed as described by Laemmli (20), using 12.5% glycine-buffered SDS-gels. Proteins separated on these gels were blotted onto a nitrocellulose membrane (37). The membranes were reacted with polyclonal antibodies raised against CODH/ACS, CFeSP, or MeTr, and bands were visualized by the enhanced chemiluminescence method as instructed by the manufacturer (Amersham), using horseradish peroxidase-conjugated anti-mouse and anti-rabbit antibodies (Bio-Rad) and chemiluminescence film (Boehringer Mannheim). Gels were scanned with a Molecular Dynamics Personal Densitometer. Protein was measured by the Rose Bengal assay (9).
Difference spectra of membranes.
Membranes were isolated as described in reference 10. Reduced-minus-oxidized spectra were obtained on a DW2000 double-beam spectrophotometer (Aminco) by adding a few grains of solid sodium dithionite to air-oxidized membranes (1 mg/ml).
Isolation of RNA and Northern blotting.
DNA probes (200 bp) were made by PCR amplification using a digoxigenin-dUTP PCR kit (Boehringer Mannheim) as specified by the manufacturer. Primers were purchased from Qiagen. PCR conditions were 35 cycles of 92°C for 20 s, 58°C for 1 min, and 72°C for 2 min. Probes (Table 1) against the α (82 kDa; ascB) and β (73 kDa; acsA) subunits of CODH/ACS were made by using plasmid pCt946A (27), and probes against MeTr (ascE) and the α (55 kDa; acsC) and β (33 kDa; acsD) subunits of CFeSP were made by using plasmid pCt946B (27). The specificity of the probes was tested by Southern hybridization (31). Cells of C. thermoaceticum were harvested in the logarithmic growth phase by rapid cooling on ice and centrifugation at 10,000 × g (Beckman model J2-HS centrifuge) for 8 min at 4°C. The cells were immediately frozen as pellets in liquid nitrogen and stored at −80°C until use. RNA was isolated by using an RNeasy midi kit (Qiagen). Frozen cells (0.1 g of wet cell mass) were ruptured under liquid nitrogen by grinding with a mortar and pestle for 15 min in the presence of sonication glass beads (Heat Systems), 2 ml of lysis buffer (RLT buffer; Qiagen), and 20 μl of 2-mercaptoethanol. All subsequent steps were performed according to the protocol of the manufacturer. RNA was blotted onto a positively charged nylon membrane by downward capillary transfer (2). Prior to hybridization, the membrane was blocked in 50% formamide–5× SSC (15 mM sodium citrate [pH 7.0], 0.15 M NaCl)–0.02% SDS–0.1% N-lauroylsarcosine–1% blocking reagent (Boehringer Mannheim). Hybridization using a probe concentration of 5 ng/ml was done in the same buffer. After hybridization, the membranes were washed (15 min for each washing step) twice in 2× SSC–0.1% SDS at room temperature and twice in 0.5× SSC–0.1% SDS at 68°C. The extent of hybridization was measured by using the chemiluminescent substrate CDP-Star and chemiluminescent film (both from Boehringer Mannheim). Gels were scanned with a Molecular Dynamics Personal Densitometer.
TABLE 1.
Primers used for construction of probes by PCR
| Genea | Sequence
|
|
|---|---|---|
| Upstream primer | Downstream primer | |
| acsA | 5′-TTCCGCGATCTCTCCCATAA-3′ | 5′-GAAACGGCAGCAAATACCTT-3′ |
| ascB | 5′-CCAGAAGGTAAAGAGCCGGT-3′ | 5′-AACAGCGAATAACCGGCAGG-3′ |
| acsC | 5′-CCTTTGACGGGACTGGAGAT-3′ | 5′-GGCGCCCAGGACTACCTTGG-3′ |
| ascD | 5′-GCCGTCCAGATTTTACGTGA-3′ | 5′-GGGCCAGTCGGGTACGATAT-3′ |
| acsE | 5′-CTCATTATCGGTGAACGGAT-3′ | 5′-GCTGACCTCCTGGGTGACTC-3′ |
acsA through acsE encode the β and α subunits of CODH, the α and β subunits of CFeSP, and MeTr, respectively.
RESULTS
Effect of nitrate on CODH levels.
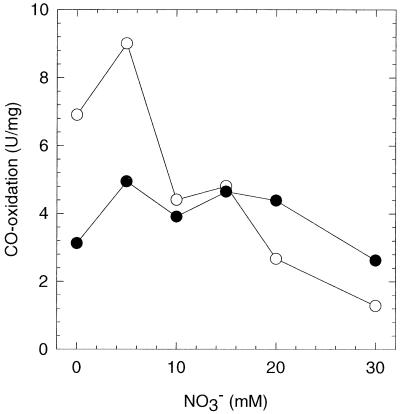
We cultivated C. thermoaceticum on 100 mM glucose in the presence or absence of nitrate in either stoppered or CO2-sparged vials. The cells were harvested after 3 days of growth when they were in the late exponential phase (optical density at 600 nm of approximately 4), and the crude extracts were tested for CODH activity (Fig. 1). When C. thermoaceticum was cultivated in stoppered vials under 100% CO2, as found earlier by Fröstl et al. (10), the CODH activities of extracts of nitrate-free and nitrate-supplemented cultures were approximately the same. However, when C. thermoaceticum was grown in CO2-sparged flasks, the CODH activity of extracts from cells grown in the presence of 30 mM nitrate was fivefold less than that of cells grown in the absence of nitrate. No further decrease in activity was observed at nitrate concentrations above 30 mM.
FIG. 1.
CODH activity in crude extracts of C. thermoaceticum grown in the presence of different concentrations of nitrate. Solid circles represent C. thermoaceticum cultures grown in stoppered vials under 100% CO2; open circles represent cultures grown in CO2-sparged vials.
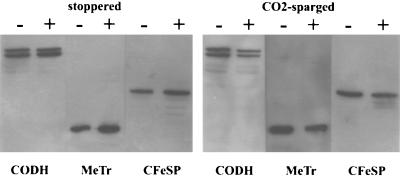
Western blot analysis showed that the decrease in CODH activity in nitrate-grown cultures is due to lower levels of protein (Fig. 2 and Table 2). In CO2-sparged cultures, the CODH levels for nitrate-supplemented cultures were 50% less than those for nitrate-free cultures. Nitrate had little effect on the CODH protein levels when cells were grown in stoppered vials. There was a slight increase in protein level upon addition of nitrate, although this is not reflected in CODH activity (Fig. 1).
FIG. 2.
Western blot analysis of total cell protein of C. thermoaceticum, grown in the absence (−) or presence (+) of nitrate (30 mM). Cells were grown under CO2 in stoppered vials or in CO2-sparged vials. Equal amounts (16 μg) of total cell protein were loaded on an SDS–12.5% gel and subsequently blotted onto a nitrocellulose filter. The blots were hybridized with polyclonal antibodies raised against CODH, MeTr, or CFeSP.
TABLE 2.
Western blot analysis of the effect of nitrate on Wood-Ljungdahl pathway protein levels
The genes encoding CODH/ACS (ascA and ascB) are located on a large gene cluster that also harbors the genes of at least two other key proteins of the Wood-Ljungdahl pathway, CFeSP (ascC and ascD) and MeTr (ascE) (28). That these genes are clustered may indicate that they are coregulated or are part of an operon. In support of this hypothesis, the levels of both CFeSP and MeTr were lower in nitrate-supplemented cultures than in nitrate-free cultures. In the case of stoppered vials, the protein levels increased rather than decreased (Table 2).
Effect of nitrate on hydrogenase and nitrate reductase.
Seifritz et al. found a decrease in H2 oxidation activity in extracts of autotrophically grown C. thermoaceticum cultures upon nitrate supplementation (5.4 versus 0.4 U/mg) (32). Table 3 shows that no appreciable change in H2 oxidation activity was observed in cells grown in stoppered cultures under 100% CO2, whereas nitrate decreased the hydrogenase specific activity in CO2-sparged cultures by sixfold.
TABLE 3.
Effects of nitrate on enzyme activities of CODH, hydrogenase, and nitrate reductase in cell extracts of C. thermoaceticum
| Culture condition | Concna
|
|||||
|---|---|---|---|---|---|---|
| CODH (U/mg)
|
Hydrogenase (U/mg)
|
Nitrate reductase (mU/mg)
|
||||
| − | + | − | + | − | + | |
| 100% CO2 (stoppered) | 3.1 ± 1.0 | 2.6 ± 0.7 | 0.32 ± 0.06 | 0.24 ± 0.14 | 2.1 ± 2.1 | 13 ± 5 |
| 100% N2 (stoppered)b | 1.6 ± 0.3 | 3.7 ± 0.5 | 0.02 ± 0.002 | 0.034 ± 0.031 | 23 ± 18 | 30 ± 6 |
| 100% CO2 (sparged) | 6.9 ± 2.2 | 1.3 ± 0.5 | 0.41 ± 0.18 | 0.07 ± 0.02 | 5.1 ± 4.5 | 39 ± 4 |
| 1% CO–99% CO2 (sparged) | 10 ± 2 | 2.6 ± 0.1 | 0.39 ± 0.12 | 0.032 ± 0.026 | 0.6 ± 0.2 | 26 ± 13 |
| 1% H2–99% CO2 (sparged) | 6.5 ± 0.5 | 5.1 ± 1.3 | 0.34 ± 0.13 | 0.14 ± 0.07 | 6.3 ± 0.5 | 22 ± 9 |
| 5% H2–95% CO2 (sparged) | 7.1 ± 0.1 | 2.1 ± 0.3 | 0.50 ± 0.28 | 0.11 ± 0.03 | 12 ± 6 | 38 ± 5 |
| 0.1% NO–99.9% CO2 (sparged) | 5.0c | 1.2 ± 0.1 | 0.12c | 0.59 ± 0.35 | 20c | 30 ± 3 |
Mean ± standard deviation from three independent experiments for cells grown without NO3− (−) or in the presence of 30 mM NO3− (+).
Cells grown without NaHCO3.
Single experiment.
We detected nitrate reductase in C. thermoaceticum cell extracts (Table 3). This activity has not been previously reported. Nitrate reductase appeared to be induced eightfold by addition of nitrate. In contrast to what we observed on the Wood-Ljungdahl pathway enzymes, we did not observe significant differences in specific activity or inducability of nitrate reductase between stoppered and CO2-sparged cultures.
Adding fumarate, trimethylamine-N-oxide, or dimethyl sulfoxide to the growth medium did not affect the activities or levels of the above-specified Wood-Ljungdahl pathway enzymes (data not shown).
The effect of nitrate on activities of CODH, hydrogenase, and nitrate reductase was slightly dependent on the growth phase. The magnitude of CODH repression and nitrate reductase induction was somewhat greater at the end of the growth phase, whereas the repression of hydrogenase was greater during the exponential phase (data not shown).
Roles of CO, H2, and NO in regulation of gene expression by nitrate.
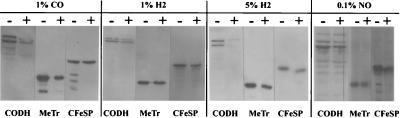
As described above, we observed striking differences in enzyme activities and protein levels as a function of nitrate availability between stoppered cultures under a CO2 atmosphere and CO2-sparged cultures. Since the only apparent difference between stoppered and sparged cultures is in their gas phase, we hypothesized that accumulation of a gas molecule in stoppered cultures may obviate the nitrate-dependent repression. Possible candidates for this putative gas molecule were H2, NO, and CO, since NO and CO are intermediates in nitrate and CO2 reduction, respectively. If one of these three gas molecules blocks the regulatory effect of nitrate, it should be possible to mimic a stoppered culture and alleviate the repression by nitrate in sparged cultures by bubbling the medium with this gas. However, low levels of the gases did not alter the nitrate-regulatory response seen in CO2-sparged cultures. The decreases in CODH and hydrogenase activity and protein levels in extracts of H2 (1% and 5%)- and CO (1%)-sparged cultures in response to nitrate supplementation were almost identical to the decrease observed for CO2-sparged cultures (Fig. 3 and Table 3), with the exception of hydrogenase activity in NO (0.1%)-sparged cultures, which increased rather than decreased in response to nitrate.
FIG. 3.
Western blot analysis of total cell protein of C. thermoaceticum, grown in the absence (−) or presence (+) of nitrate (30 mM). Cells were grown in vials which were sparged with either 1% CO, 1% H2, 5% H2, or 0.1% NO (gases balanced with CO2). Equal amounts (16 μg) of total cell protein were loaded on an SDS–12.5% gel and subsequently blotted onto a nitrocellulose filter. The blots were hybridized with polyclonal antibodies raised against CODH, MeTr, or CFeSP.
Effect of CO2 on regulation of gene expression by nitrate.
Figure 1 shows that the CODH activity from cells of CO2-sparged cultures is twofold higher than that from cells from stoppered cultures under CO2. Therefore, we tested the effect of CO2 on the activities of CODH, hydrogenase, and nitrate reductase. C. thermoaceticum was cultured in stoppered vials under a N2 atmosphere in medium from which sodium bicarbonate (otherwise always present at a initial concentration of 0.1 M) had been omitted (we were unable to maintain C. thermoaceticum in bicarbonate-free, N2-sparged cultures). Cells grown under N2 in stoppered, bicarbonate-free cultures grew slightly slower (doubling time of 8 h−1 versus 6 h−1) and to a lower final optical density (2 versus 4) (data not shown). The nitrate reductase activity of cells grown under N2 was comparable to that of cells grown under CO2 (Table 3). However, the hydrogenase activity of N2-grown cells in the presence or absence of nitrate was significantly (5- to 20-fold) reduced. The CODH activity of cells grown under N2 decreased twofold compared to stoppered cultures and four- to fivefold compared to sparged cultures.
Effect of nitrate on mRNA levels.
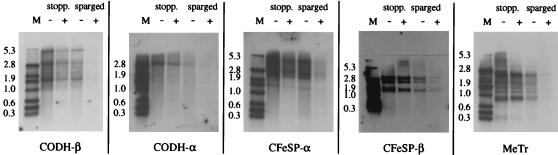
To examine whether the nitrate-dependent decrease in protein level upon nitrate supplementation is due to regulation at the gene level, we performed Northern blotting experiments. For stoppered cultures under CO2, the amount of mRNA of the five genes (acsA through acsE) was approximately the same for nitrate-free and nitrate-supplemented cultures, whereas for CO2-sparged cultures they decreased significantly upon nitrate supplementation (Fig. 4 and Table 4). These results indicate that the nitrate-dependent regulation of expression of Wood-Ljungdahl pathway genes is at the transcriptional level.
FIG. 4.
Northern blot analysis of isolated total mRNA (1 μg in each lane) of C. thermoaceticum, grown in the absence (−) or presence (+) of nitrate (30 mM). Cells were grown under CO2 in stoppered (stopp.) vials or in CO2-sparged vials. The blots were hybridized with a 200-bp digoxigenin-labeled DNA probe against the gene for the α or β subunit of CODH, α or β subunit of CFeSP, or MeTr. Equal loading was confirmed by comparing intensities of rRNA on a formaldehyde gel. The nitrate-dependent decrease in mRNA levels in CO2-sparged cultures was observed in three independent experiments.
TABLE 4.
Northern blot analysis of the effect of nitrate on Wood-Ljungdahl pathway mRNA levels
| Culture condition | Relative intensity (%)b
|
||||
|---|---|---|---|---|---|
| CODH β | CODH α | CFeSP α | CFeSP β | MeTr | |
| Stoppereda | |||||
| −NO3−b | 100 | 100 | 100 | 100 | 90 |
| +NO3−c | 40 | 90 | 90 | 70 | 100 |
| CO2 sparged | |||||
| −NO3− | 50 | 40 | 70 | 40 | 80 |
| +NO3− | 20 | 10 | 40 | 20 | 10 |
Under a CO2 atmosphere.
Obtained after scanning of Northern blots (Fig. 4). Data are normalized for each column.
Medium containing 30 mM NO3−.
Effect of nitrate on occurrence of cytochrome in membranes.
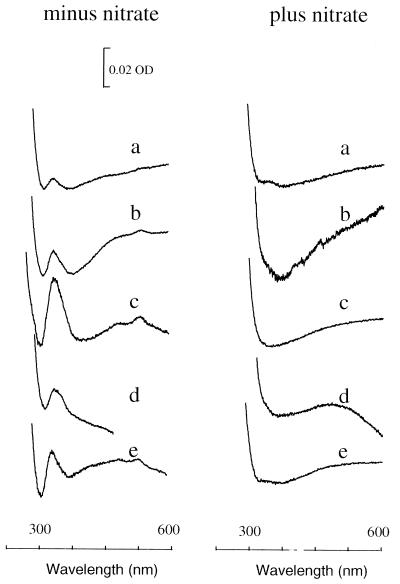
It was previously shown that nitrate supplementation results in the absence of a membrane-bound b-type cytochrome that is associated with acetogenesis (10). Membranes isolated from C. thermoaceticum cells grown in the absence of nitrate contained b-type cytochrome under all tested growth conditions (i.e., in stoppered vials or in vials sparged with 100% CO2, 1% CO, 1 or 5% H2, or 0.1% NO) (Fig. 5). The cytochrome level was threefold higher in CO-sparged cultures than in CO2-sparged cultures. When nitrate was present, cytochrome b was absent under all tested conditions. Infrequently, however, we have observed cytochrome in membrane preparations from cultures grown in the presence of nitrate.
FIG. 5.
Reduced-minus-oxidized spectra of C. thermoaceticum membranes (1 mg/ml). Cells were grown in the absence or presence of nitrate in vials that were stoppered under a CO2 atmosphere (a) or sparged with CO2 (b), CO (c), H2 (d), or NO (e). OD, optical density.
Products of nitrate respiration.
Nitrate was consumed by stoppered and CO2-sparged cultures of C. thermoaceticum (Table 5). In both cases, the detected end product of nitrate reduction was ammonium. We also detected some nitrite (4 mM in one case, but usually <0.1 mM). Note that in the nitrate-free cultures, there is net consumption of ammonium (8 to 9 mM; the initial ammonium concentration in the medium is 15 mM). Assuming that the consumption of ammonium is the same in nitrate-supplemented cultures as in nitrate-free cultures, we can estimate the formation of ammonium from nitrate by correcting for the consumption of ammonium. Thus, we find that the total levels of production of ammonium in stoppered and CO2-sparged cultures amount to approximately 14 and 25 mM, with N recoveries of ≈60 and 90%, respectively, indicating that ammonium is the predominant end product of nitrate reduction in heterotrophically grown cultures of C. thermoaceticum. Like the reduction of two molecules of CO2 to acetate, the reduction of nitrate to ammonium also involves eight electrons. The ratio of nitrate consumption to glucose consumption (0.6 and 1.3) and the high N recovery of nitrate to ammonium (60 and 90%) are therefore in accordance with the formation of 1 mol less acetate per mol of glucose consumed.
TABLE 5.
Effect of nitrate on formation of acetate, and fate of nitrate reduction in C. thermoaceticum
| Culture | NO3− medium (mM) | Mean ± SD (n = 3)
|
|||||
|---|---|---|---|---|---|---|---|
| Glucose consumed (mM) | Acetate formed (mM) | Acetate formed/ glucose consumed | NO3− consumed (mM) | NO3− consumed/ glucose consumed | ΔNH4+ (mM)b | ||
| Stoppered | 0 | 55 ± 4 | 160 ± 20 | 2.9 ± 0.3 | NAa | NA | −8.3 ± 0.5 |
| 30 | 40 ± 6 | 84 ± 7 | 2.1 ± 0.2 | 26 ± 5 | 0.6 ± 0.1 | +5.3 ± 3 | |
| CO2 sparged | 0 | 47 ± 7 | 130 ± 12 | 2.9 ± 0.4 | NA | NA | −9.3 ± 0.9 |
| 30 | 23 ± 1 | 51 ± 9 | 2.2 ± 0.3 | 31 ± 6 | 1.3 ± 0.2 | +16 ± 1 | |
NA, not applicable.
−, net consumption of ammonium; +, net formation of ammonium.
DISCUSSION
Despite their name, many acetogens are not strictly dependent on the reduction of CO2 for the disposal of electrons released by the oxidation of energy-rich compounds. Fumarate (7, 21), methoxylated aromatic acids (21), malate (7), pyruvate (23), aromatic acrylates (22), inorganic sulfur compounds (17), and nitrate (32) can all be dissimilated by certain acetogens. Of these alternative electron acceptors, nitrate is the best studied. Nitrate was shown to be the preferred electron acceptor for C. thermoaceticum and C. thermoautotrophicum (10). Moreover, membranes from cells grown in the presence of nitrate were devoid of a b-type cytochrome that is associated with the Wood-Ljungdahl pathway. It was suggested that the absence of this cytochrome could be one, although not necessarily the only, cause for the observed shift of electron flow from CO2 to nitrate. The preference to reduce nitrate over CO2 appears to be physiologically relevant since the former process is energetically more favorable (ΔΔG = −63 kJ/mol [32]). Interestingly, in the earlier studies (10), nitrate did not seem to affect the activities of the Wood-Ljungdahl pathway enzymes such as CODH in C. thermoaceticum. This is surprising because one would expect that when a metabolic pathway is not engaged, expression of the proteins involved in that pathway would be repressed. An example of this scenario is the regulation of respiratory enzymes in E. coli (reviewed by Gunsalus [15]). This facultative aerobe can utilize different respiratory substrates. These are, in decreasing order of potential energy, oxygen, nitrate, trimethyl-N-oxide, dimethyl sulfoxide, and fumarate. The regulation of the enzymes responsible for the reduction of these substrates is such that the cell will preferentially utilize the electron acceptor yielding most energy (according to the above series). In this way, no energy is wasted in assembling abundant and complex enzymes and cofactors that are not engaged.
Although C. thermoaceticum can reduce nitrate (10), its nitrate reductase activity has never been reported. The nitrate reductase activity in C. thermoaceticum cell extracts is similar to that reported for other respiratory nitrate reductases (4, 26, 33). Nitrate reductase appeared to be induced by nitrate supplementation, although basal enzyme activity was present in cells grown in the absence of nitrate. The latter observation appears to be inconsistent with the finding of Fröstl et al. that resting cells grown in the absence of nitrate are unable to reduce nitrate (10). A possible explanation is that the amount of nitrate consumed may have been below the detection limit because of low nitrate reductase activity (we measured 2 nmol/mg for stoppered vials). Nitrate induction of nitrate reductase activity has also been observed for other respiratory nitrate reductases (11, 19, 26, 29, 30, 34, 38).
The results described here demonstrate that under certain growth conditions, the proteins of the Wood-Ljungdahl pathway are coordinately regulated by nitrate. Based on activity data and Western blots, we found that nitrate decreases the levels of CODH and hydrogenase five- to sevenfold and the levels of CFeSP and MeTr approximately 50%. Northern blot analysis indicates that the observed repression is at the transcriptional level. Furthermore, nitrate addition results in an increase in nitrate reduction activity. Hence, nitrate appears to concomitantly repress some proteins (CODH, CFeSP, MeTr, cytochrome b, and hydrogenase) while inducing others (e.g., nitrate reductase). This situation is reminiscent of the aforementioned regulation of respiratory enzymes in E. coli, where nitrate acts simultaneously as a repressor (e.g., of fumarate reductase, dimethyl sulfoxide reductase, and trimethyl-N-oxide reductase) and as an inducer (e.g., of nitrate reductase and formate dehydrogenase) (6). In addition, nitrate regulation in E. coli is at the transcriptional level. Upon binding nitrate, two distinct nitrate sensors, NarQ and narX, can independently activate (phosphorylate) the response regulators NarP and NarL. The phosphorylated response regulators bind to DNA, thereby activating or repressing transcription of anaerobic respiratory pathway genes.
CO2 also appears to induce expression of the Wood-Ljungdahl pathway genes and repress nitrate reductase activity, except when NO or nitrate is also supplied. CODH activity in CO2-sparged cultures is twofold higher than in stoppered, CO2-supplemented cultures and fourfold higher than in CO2-free cultures (Table 3). CO2 also regulates the expression of genes involved in CO2 assimilation by the Calvin cycle (16, 35, 36). A transcriptional regulatory protein, CbbR, is a positive regulator of the Calvin cycle genes (12). Although the actual signal molecule that communicates with CbbR is not known, the redox state of the cell is clearly a component of the regulatory network (18, 25). This may be important in regulation of the acetyl-CoA pathway; however, fumarate, trimethylamine-N-oxide, and dimethyl sulfoxide do not affect the levels of the Wood-Ljungdahl pathway enzymes.
Are the effects of CO2 and nitrate interrelated? These electron acceptors have contrasting effects on gene expression. Moreover, the CODH activity in cells grown in the absence of CO2 is approximately equal to the activities observed with cells grown while sparging with CO2 in nitrate- or NO-supplemented medium. This finding suggests that there is a basal level of acetyl-CoA pathway enzymes and that nitrate might inhibit the induction of these genes by CO2. The regulation of hydrogenase expression follows the same pattern as CODH and thus could be explained in the same way. Similarly, the high level of nitrate reductase activity is approximately equal in cells grown in the absence of CO2 or in the presence of CO2 plus either nitrate or NO.
One surprising finding described here is that nitrate (and CO2)-dependent regulation of the acetyl-CoA pathway and nitrate reductase genes in C. thermoaceticum depends on culture conditions. In sparged vials, nitrate represses CODH, CFeSP, MeTr, and hydrogenase, but in stoppered vials, little or no decrease in either protein or mRNA levels is observed for any of these proteins. At this stage, we cannot explain why the regulation by nitrate is dependent on the method of cultivation. One possibility is that a gas produced during fermentation in respiration accumulates during growth in stoppered vials and interferes with the regulatory activity of nitrate. We did not observe any effect of H2, CO, and NO on the regulation by nitrate. Another possibility is that the intracellular redox potential is different in an open than in a closed culture system.
In summary, whether acetogenic cells reduce CO2 or nitrate is determined by adjusting the levels of the acetyl-CoA pathway enzymes (CODH/ACS, MeTr, and CFeSP), electron transfer proteins (cytochrome b), and nitrate respiratory enzymes (nitrate reductase). It is likely that the levels of all of these proteins are transcriptionally controlled; however, so far this has been demonstrated only for the acetyl-CoA pathway enzymes, since the genes encoding cytochrome b and nitrate reductase have not yet been cloned. The absence of cytochrome b in nitrate-supplemented cultures may play a more important role in shifting electron flow toward nitrate reduction than the decreased levels of the Wood-Ljungdahl pathway enzymes. This is because when C. thermoaceticum is grown on glucose, 3 mol of acetate are formed per mol of glucose consumed. Two moles acetate are formed by glycolysis, and the third acetate is formed by the Wood-Ljungdahl pathway of CO2 reduction. Previous studies have shown that when C. thermoaceticum is grown in the presence of nitrate, one-third less acetate is produced, indicating that nitrate is the preferred electron acceptor (32). In the present study, we found that both stoppered and CO2-sparged C. thermoaceticum cultures produced approximately one-third less moles of acetate when nitrate is present, indicating that there is no difference in the metabolic fate of nitrate between stoppered and sparged cultures (Table 5). Under both growth conditions, cytochrome b levels are severely reduced; however, in stoppered vials, the acetyl-CoA pathway enzymes are not repressed.
ACKNOWLEDGMENTS
We are grateful to Alan Penheiter for assistance with Southern and Northern blotting experiments. We thank Tom Elthon for use of the double-beam spectrometer and Saurabh Menon for raising of antibodies.
Financial support was provided by the Netherlands Organization for Scientific Research through a NATO fellowship (A.F.A.) and by NIH grant GM451 (S.W.R.).
We thank one of the reviewers for pointing out the possibility of CO2 induction.
REFERENCES
- 1.Andreesen J R, Schaupp A, Neurater C, Brown A, Ljungdahl L G. Fermentation of glucose, fructose, and xylose by Clostridium thermoaceticum: effect of metals on growth yield, enzymes, and the synthesis of acetate from CO2. J Bacteriol. 1973;114:743–751. doi: 10.1128/jb.114.2.743-751.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. I. New York, N.Y: John Wiley & Sons; 1989. [Google Scholar]
- 3.Barker H A, Kamen M D. Carbon dioxide utilization in the synthesis of acetic acid by Clostridium thermoaceticum. Proc Natl Acad Sci USA. 1945;31:219–225. doi: 10.1073/pnas.31.8.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrne M D, Nicholas D J D. A membrane-bound dissimilatory nitrate reductase from Rhodobacter sphaeroides f. sp. denitrificans. Biochim Biophys Acta. 1987;915:120–124. [Google Scholar]
- 5.Clark J E, Ragsdale S W, Ljungdahl L G, Wiegel J. Levels of enzymes involved in the synthesis of acetate from CO2 in Clostridium thermoaceticum. J Bacteriol. 1982;151:507–509. doi: 10.1128/jb.151.1.507-509.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darwin A J, Stewart V. The NAR modulon systems: nitrate and nitrite regulation of anaerobic gene expression. In: Lin E C C, Lynch A S, editors. Regulation of gene expression in Escherichia coli. R. G. New York, N.Y: Landes Company; 1996. pp. 343–359. [Google Scholar]
- 7.Dorn M, Andreesen J R, Gottschalk G. Fermentation of fumarate and l-malate by Clostridium formicoaceticum. J Bacteriol. 1978;133:26–32. doi: 10.1128/jb.133.1.26-32.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drake H L. Demonstration of hydrogenase in extracts of the homoacetate-fermenting bacterium Clostridium thermoaceticum. J Bacteriol. 1982;150:702–709. doi: 10.1128/jb.150.2.702-709.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elliott J I, Brewer J M. The inactivation of yeast enolase by 2,3-butanedione. Arch Biochem Biophys. 1978;190:351–357. doi: 10.1016/0003-9861(78)90285-0. [DOI] [PubMed] [Google Scholar]
- 10.Fröstl J M, Seifritz C, Drake H L. Effect of nitrate on the autotrophic metabolism of the acetogens Clostridium thermoautotrophicum and Clostridium thermoaceticum. J Bacteriol. 1996;178:4597–4603. doi: 10.1128/jb.178.15.4597-4603.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gauthier D K, Clark-Walker G D, Garrard J W T, Lascelles J. Nitrate reductase and soluble cytochrome c in Spirillum itersonii. J Bacteriol. 1970;102:797–803. doi: 10.1128/jb.102.3.797-803.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibson J L, Tabita F R. Nucleotide sequence and functional analysis of cbbR, a positive regulator of the Calvin cycle operons of Rhodobacter sphaeroides. J Bacteriol. 1993;175:5778–5784. doi: 10.1128/jb.175.18.5778-5784.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green L C, Wagner D A, Glogowski J, Skipper P L, Wishnok J S, Tannenbaum S T. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 14.Griess P. Bemerkungen zu der Abhandlung der HH. Weselksy und Benedikt “Ueber einige Azoverbindungen.”. Ber Dtsch Chem Ges. 1879;12:426–428. [Google Scholar]
- 15.Gunsalus R P. Control of electron flow in Escherichia coli: coordinated transcription of respiratory pathway genes. J Bacteriol. 1992;174:7069–7074. doi: 10.1128/jb.174.22.7069-7074.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hallenbeck P L, Lerchen R, Hessler P, Kaplan S. Roles of CfxA, CfxB, and external electron acceptors in regulation of ribulose 1,5-bisphosphate carboxylase/oxygenase expression in Rhodobacter sphaeroides. J Bacteriol. 1990;172:1736–1748. doi: 10.1128/jb.172.4.1736-1748.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heijthuijsen J H F G, Hansen T A. Selection of sulphur sources for the growth of Butyribacterium methylotrophicum and Acetobacterium woodii. Appl Microbiol Biotechnol. 1989;32:186–192. [Google Scholar]
- 18.Joshi H M, Tabita F R. A global two component signal transduction system that integrates the control of photosynthesis, carbon dioxide assimilation, and nitrogen fixation. Proc Natl Acad Sci USA. 1996;93:14515–14520. doi: 10.1073/pnas.93.25.14515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalman L V, Gunsalus R P. The frdR gene of Escherichia coli globally regulates several operons involved in anaerobic growth in response to nitrate. J Bacteriol. 1988;170:623–629. doi: 10.1128/jb.170.2.623-629.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laemmli U K. Cleavage of structural proteins during the assembly of the head bacteriophage T4. Nature (London) 1971;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Matthies C, Freiberger A, Drake H L. Fumarate dissimilation and differential reductant flow by Clostridium formicoaceticum and Clostridium aceticum. Arch Microbiol. 1993;160:273–278. [Google Scholar]
- 22.Misoph M, Daniel S L, Drake H L. Bidirectional usage of ferulate by the acetogen Peptostreptococcus productus U-1: CO2 and aromatic acrylate groups as competing electron accepters. Microbiology (United Kingdom) 1996;142:1983–1988. [Google Scholar]
- 23.Misoph M, Drake H L. Effect of CO2 on the fermentation capacities of the acetogen Peptostreptococcus productus U-1. J Bacteriol. 1996;178:3140–3145. doi: 10.1128/jb.178.11.3140-3145.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris A W, Riley J P. The determination of nitrate in sea water. Anal Chim Acta. 1963;29:272–279. [Google Scholar]
- 25.O’Gara J P, Eraso J M, Kaplan S. A redox-responsive pathway for aerobic regulation of photosynthesis gene expression in Rhodobacter sphaeroides 2.4.1. J Bacteriol. 1998;180:4044–4050. doi: 10.1128/jb.180.16.4044-4050.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reyes F, Roldán M D D, Klipp W, Castillo F, Moreno-Vivián C. Isolation of periplasmic nitrate reductase genes from Rhodobacter sphaeroides DSM 158: structural and functional differences among prokaryotic nitrate reductases. Mol Microbiol. 1996;19:1307–1318. doi: 10.1111/j.1365-2958.1996.tb02475.x. [DOI] [PubMed] [Google Scholar]
- 27.Roberts D L, James-Hagstrom J E, Smith D K, Gorst C M, Runquist J A, Baur J R, Haase F C, Ragsdale S W. Cloning and expression of the gene cluster encoding key proteins involved in acetyl-CoA synthesis in Clostridium thermoaceticum: CO dehydrogenase, the corrinoid/Fe-S protein, and methyltransferase. Proc Natl Acad Sci USA. 1989;86:32–36. doi: 10.1073/pnas.86.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts J R, Lu W-P, Ragsdale S W. Acetyl-coenzyme A synthesis from methyltetrahydrofolate, CO and coenzyme A by enzymes purified from Clostridium thermoaceticum: attainment of in vivo rates and identification of rate-limiting steps. J Bacteriol. 1992;174:4667–4676. doi: 10.1128/jb.174.14.4667-4676.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruiz-Herrera J, Salas-Vargas I. Regulation of nitrate reductase at the transcriptional and the translational levels in Escherichia coli. Biochim Biophys Acta. 1976;425:492–501. doi: 10.1016/0005-2787(76)90013-7. [DOI] [PubMed] [Google Scholar]
- 30.Sabaty M, Gagnon J, Verméglio A. Induction by nitrate of cytoplasmic and periplasmic proteins in the photodenitrifier Rhodobacter sphaeroides forma sp. denitrificans under anaerobic or aerobic conditions. Arch Microbiol. 1994;162:335–343. doi: 10.1007/BF00263781. [DOI] [PubMed] [Google Scholar]
- 31.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 32.Seifritz C, Daniel S L, Gößner A, Drake H L. Nitrate as a preferred electron sink for the acetogen Clostridium thermoaceticum. J Bacteriol. 1993;175:8008–8013. doi: 10.1128/jb.175.24.8008-8013.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seki-Chiba S, Ishimoto M. Studies on nitrate reductase of Clostridium perfringens. I. Purification, some properties and effects of tungstate on its formation. J Biochem. 1977;82:1663–1671. doi: 10.1093/oxfordjournals.jbchem.a131862. [DOI] [PubMed] [Google Scholar]
- 34.Steward V. Requirement of Fnr and NarL functions for nitrate reductase expression in Escherichia coli K-12. J Bacteriol. 1982;151:1320–1325. doi: 10.1128/jb.151.3.1320-1325.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stretton S, Goodman A E. Carbon dioxide as a regulator of gene expression in microorganisms. Antonie Leeuwenhoek. 1998;73:79–85. doi: 10.1023/a:1000610225458. [DOI] [PubMed] [Google Scholar]
- 36.Tabita F R. Molecular and cellular regulation of autotrophic carbon dioxide fixation in microorganisms. Microbiol Rev. 1988;52:155–189. doi: 10.1128/mr.52.2.155-189.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wimpenny W T, Cole J A. The regulation of metabolism in facultative bacteria. III. The effect of nitrate. Biochim Biophys Acta. 1967;148:233–242. doi: 10.1016/0304-4165(67)90298-x. [DOI] [PubMed] [Google Scholar]
- 39.Wood H G. Fermentation of 3,4-C14- and 1-C14-labeled glucose by Clostridium thermoaceticum. J Biol Chem. 1952;199:579–583. [PubMed] [Google Scholar]