Abstract
There is emerging evidence that obstructive sleep apnea (OSA) is a risk factor for preclinical Alzheimer’s disease (AD). An American Thoracic Society workshop was convened that included clinicians, basic scientists, and epidemiologists with expertise in OSA, cognition, and dementia, with the overall objectives of summarizing the state of knowledge in the field, identifying important research gaps, and identifying potential directions for future research. Although currently available cognitive screening tests may allow for identification of cognitive impairment in patients with OSA, they should be interpreted with caution. Neuroimaging in OSA can provide surrogate measures of disease chronicity, but it has methodological limitations. Most data on the impact of OSA treatment on cognition are for continuous positive airway pressure (CPAP), with limited data for other treatments. The cognitive domains improving with CPAP show considerable heterogeneity across studies. OSA can negatively influence risk, manifestations, and possibly progression of AD and other forms of dementia. Sleep-dependent memory tasks need greater incorporation into OSA testing, with better delineation of sleep fragmentation versus intermittent hypoxia effects. Plasma biomarkers may prove to be sensitive, feasible, and scalable biomarkers for use in clinical trials. There is strong biological plausibility, but insufficient data, to prove bidirectional causality of the associations between OSA and aging pathology. Engaging, recruiting, and retaining diverse populations in health care and research may help to decrease racial and ethnic disparities in OSA and AD. Key recommendations from the workshop include research aimed at underlying mechanisms; longer-term longitudinal studies with objective assessment of OSA, sensitive cognitive markers, and sleep-dependent cognitive tasks; and pragmatic study designs for interventional studies that control for other factors that may impact cognitive outcomes and use novel biomarkers.
Keywords: obstructive sleep apnea, neurocognitive dysfunction, Alzheimer’s disease
Table of Contents
Overview
Introduction
Methods
Section 1: Epidemiology of Cognitive Impairment in OSA
Section 2: Screening Tests for Cognitive Impairment in OSA
Section 3: Brain Changes in OSA and CI
Section 4: Impact of OSA Treatment on Cognitive Function
-
Section 5: OSA and AD
Impact of OSA on Memory and Conversion to MCI
Biomarkers of CI and Screening for CI in OSA
Section 6: Racial and Ethnic Disparities in AD and OSA
-
Section 7: Future Directions
Approaches and challenges in planning a clinical trial
Alternative approaches to clinical trials
Conclusions
Overview
Obstructive sleep apnea (OSA) is a very common problem and is increasing in incidence worldwide. Mild cognitive impairment (MCI) is often considered a prodromal stage of Alzheimer’s disease (AD) and can progress to dementia at the rate of 10–15% per year. OSA is highly prevalent in patients with AD, and there is emerging evidence that OSA is a risk factor for preclinical AD. OSA has been associated with MCI, with some studies reporting a 25% prevalence of cognitive dysfunction in OSA. There has never before been an American Thoracic Society (ATS) workshop on this important topic of OSA and cognitive impairment. This workshop was convened with a strong multidisciplinary group of experts to summarize and review the state of knowledge in the field, identify important research gaps, and provide potential directions for future research.
The key conclusions from the workshop were:
-
1.
There is a growing body of evidence showing an association between OSA and cognitive impairment and decline.
-
2.
Currently available cognitive screening tests may allow for identification of MCI in patients with OSA, but caution is needed in using and interpreting these tests.
-
3.
Neuroimaging in OSA can provide surrogate measures of disease chronicity; however, several methodological challenges and limitations remain.
-
4.
Most data on the impact of OSA treatment on cognition are for continuous positive airway pressure (CPAP); very limited data exist for other treatments.
-
5.
There is considerable heterogeneity as to which cognitive domains improve with CPAP; this heterogeneity may relate to study design, cognitive reserve, the aspect of each cognitive domain that is studied, and other factors.
-
6.
OSA is recognized as a factor that negatively influences risk (through modulation of preclinical AD biomarkers), manifestations, and possibly progression of AD and other forms of dementia.
-
7.
Sleep-dependent memory tasks need greater incorporation into OSA testing, with better delineation of sleep fragmentation versus intermittent hypoxia effects.
-
8.
Plasma biomarkers may prove to be sensitive, feasible, and scalable biomarkers for use in clinical trials. Whether AD biomarkers can be used in young and middle-age populations with OSA where there is minimal AD pathology, or healthy elderly with normal cognition, remains a subject of debate.
-
9.
At the present time there is strong biological plausibility, but insufficient data, to prove the bidirectional causality of the associations between OSA and aging pathology, and a greater emphasis needs to be placed on understanding how AD biomarkers respond to treatment.
-
10.
Engaging, recruiting, and retaining diverse populations in healthcare and research may help to decrease the racial and ethnic disparities in OSA and AD.
-
11.
Key considerations for intervention studies include selection of the population to be recruited, use of pragmatic or alternative study designs, longer duration of intervention, new approaches to improve PAP adherence, and studying changes using more easily accessible biomarkers.
Introduction
There has never been a previous ATS Workshop on the important topic of OSA and cognitive impairment. Given the gaps in knowledge identified in this field through attendee surveys and by other means, and the advances in the field, assessment of this topic via an ATS workshop was warranted.
The overall objective and goal of this ATS Workshop was to assemble a strong multidisciplinary group of experts with varied expertise and perspectives to summarize and review the state of knowledge in the field, identify important research gaps, and provide potential directions for future research. The major areas of focus of the workshop group were: 1) epidemiological evidence linking OSA to cognitive decline; 2) the biomarkers of cognitive impairment (CI) in OSA, such as structural and functional brain imaging and genetic and proteomic biomarkers; 3) the effect of treatment of OSA and improved sleep on cognition; and 4) future directions for research in this field, particularly optimizing clinical trial design.
OSA is a very common disorder. Based on the American Academy of Sleep Medicine 2012 diagnostic criteria and apnea–hypopnea index (AHI) threshold values of ⩾5/h and ⩾15/h, a recent study found that 936 million adults aged 30–69 years have mild to severe OSA, and 425 million adults aged 30–69 years have moderate to severe OSA globally (1).
AD accounts for 60–70% of all dementias (2). AD is one of the major causes of disability/dependency among older people. OSA is highly prevalent in patients with MCI and AD (3). Studies have reported a 25% prevalence of cognitive dysfunction in OSA (4). The relationship between OSA and AD is a complex bidirectional one (5, 6).
Methods
The workshop proposal was peer reviewed and funded through the ATS Assembly project funding mechanism, with the primary project assembly being the Assembly on Sleep and Respiratory Neurobiology. An 8-hour workshop was held virtually on 2 separate days, for 4 hours each, in June 2021. The workshop included 15 participants who were identified based on their expertise and experience in OSA, cognition, and dementia and included both clinicians, and basic scientists. The panel was multidisciplinary, involving pulmonologists, neurologists, a psychiatrist, a clinical neuropsychologist, as well as clinical and basic science researchers. All workshop participants disclosed potential conflicts of interest, and these were managed in accordance with standard ATS policies.
The workshop involved presentations of the most updated information in the major areas of focus as outlined above, followed by an interactive discussion, with the goal of producing a cohesive document summarizing the findings of the panel, as well as delineating specific areas in which further research is needed. After the workshop, the presenters wrote their specific sections to create a workshop report to be submitted for publication. All workshop participants had the opportunity to contribute substantively to the report and to review and revise the manuscript in its final form.
This workshop report summarizes the findings of the workshop.
Section 1: Epidemiology of Cognitive Impairment in OSA
Growing evidence from population-based longitudinal studies have explored the association between OSA and cognitive decline. The first report was from the Study of Osteoporotic Fractures in 298 older women. Those with OSA (AHI ⩾ 15, n = 105) were at greater risk of developing MCI or dementia over 5 years (adjusted odds ratio [95% confidence interval], 1.85 [1.11–3.08]) than those without OSA (n = 193) (7). Some subsequent studies have also shown OSA to be associated with an increased risk of dementia (8, 9) or cognitive decline (10, 11); however, even within studies results can be inconsistent across cognitive domains, whereas others have shown no association (12). Inconsistent results are most likely due to OSA definitions used and varying study designs, population characteristics, and cognitive outcomes, as well as duration of follow-up in longitudinal studies. Meta-analyses, however, have consistently reported an association between OSA and future cognitive decline. In a pooled analysis of 212,943 individuals from six longitudinal studies, those with sleep-disordered breathing were 26% (risk ratio [95% confidence interval], 1.26 [1.05–1.50]) more likely to develop CI or dementia (4). Other meta-analyses have also shown consistent associations (13, 14), and studies in individuals with MCI specifically have shown higher rates of hypoxemia in such samples (15).
Section 2: Screening Tests for Cognitive Impairment in OSA
Screening tests for cognitive impairment take 3–20 minutes to administer and provide a broad overview of an individual’s gross cognitive functions. The presence of MCI is typically determined by comparing a patient’s total score to a validated threshold score. Although many screening tests have been developed, their validity to detect MCI may not translate to patient populations outside those used to develop them (16–20), and they may lack sensitivity to detect cognitive impairment in early stages of disease or in those with high levels of education or intellect. In these cases, neuropsychological assessment provides more sensitivity.
With respect to OSA, this limitation was highlighted by Gagnon and colleagues (16), who found the Mini-Mental State Examination (MMSE) had only fair to poor discriminatory ability to detect patients with OSA with MCI (determined via comprehensive neuropsychological examination). Moreover, the MMSE’s validated total score of <26 to indicate MCI (21) correctly identified only 60–62% of patients with OSA with MCI, with optimal threshold scores to detect MCI in mild and moderate-to-severe OSA found to be <30 and <29, respectively. However, these optimal threshold scores correctly identified only 61–64% of patients with OSA with MCI. By contrast, Gagnon and colleagues (16) found the Montreal Cognitive Assessment had good discriminatory ability to detect MCI in patients with OSA, although its validated total score of <26 to indicate MCI (22) correctly identified only 69–75% of patients with OSA with MCI. Threshold scores of <28 and <27 to indicate MCI were found to be optimal for patients with mild and moderate-to-severe OSA, although they still only correctly identified 72–81% of patients with OSA with MCI. Overall, the results of this study emphasize the difficulties of applying cognitive screening tests derived from one clinical population to another clinical setting, such as patients with OSA, and their potential lack of sensitivity in detecting more subtle cognitive deficits.
In summary, currently available cognitive screening tests may allow for early identification of MCI in patients with OSA. However, caution is needed in interpreting these tests, as they may lack sensitivity, and typical thresholds indicative of MCI may not translate directly to patients with OSA.
Section 3: Brain Changes in OSA and CI
Sleep loss or fragmentation is believed to lead to atrophy within the frontal and parietal regions (23), with hypoxemia being associated with an increase in sympathetic vasoconstriction and simultaneous decrease in vascular protective mechanisms (24–26). That said, at least one study demonstrated associations of OSA with increased gray matter volume, which may reflect inflammatory or edematous changes (27). These neuroanatomical changes could reflect the cognitive deficits associated with OSA (28).
Using magnetic resonance imaging (MRI), voxel-based morphometry studies report gray matter density reduction in frontal, parietal, temporal, and hippocampal regions in OSA (29–35), whereas white matter density reduction is reported in the bilateral hippocampus and frontotemporal regions (36). In older subjects, decreased thickness of the bilateral temporal pole has been observed, which was associated with hypoxemia and poorer verbal learning, as well as changes in functional connectivity of the default mode network (37, 38). Decreased thickness has also been reported in the pre- and postcentral gyri and the superior temporal gyrus (39). Fractional anisotropy values using diffusion imaging show loss of white matter integrity and structural connectivity (40, 41). Decreased concentrations of N-acetylaspartate, a marker of neuronal injury, and increased glutamate concentrations were observed in the hippocampal, thalamic, putamen, and midbrain regions in OSA (29). Together, this evidence suggests that the hippocampus is consistently impacted in OSA (36).
One month of CPAP treatment with lifestyle modifications and sleep hygiene education was shown to induce hypertrophy in the bilateral thalamus (42), whereas 3-month CPAP treatment was associated with increases in gray matter volume within the hippocampal and frontal structures (26). Conversely, O’Donoghue and colleagues reported no changes in gray matter density or volume after 6 months of CPAP treatment (35). Adherence to CPAP might underlie the discrepancy among these studies.
Although neuroimaging studies can reliably assess and quantify the abnormal brain changes and suggest that neuroanatomical changes are evident in OSA, it is worth noting the several methodological challenges and limitations that can constrain interpretation: association does not imply causation. The majority of the reported changes in patients with OSA draw parallels with those seen in healthy aging (43). Neuroimaging patients with OSA who do not show any symptoms or have fewer symptoms than similar-aged clinical populations is disputed. Furthermore, it should be noted that a majority of the cohorts in whom neuroimaging was undertaken were relatively younger than the elderly cohorts in whom reports of cognitive impairment are more common (38). Furthermore, because of a lack of long-term follow-up, progression of neurodegeneration in the brain regions observed to be impacted by OSA is not known. Studies that use T1-weighted structural MRI modality alone (particularly for gray matter density) should be supplemented by other modalities to separate the effects of myelin or iron content from neuronal loss (44).
In summary, neuroimaging studies in OSA feasibly provide surrogate markers of the chronicity of the disease. However, more intensive longitudinal studies that track neuroanatomical changes over longer periods of time are needed. Ideally, these studies would chart the ramifications of OSA and its treatment while delineating the expected changes with aging. In addition, research combining neuroimaging with high-density electroencephalogram or AD biomarker imaging with positron emission tomography (PET) (discussed in later sections) could better link these structural and functional brain changes in OSA with cognitive impairments and AD neurodegeneration.
Section 4: Impact of OSA Treatment on Cognitive Function
A meta-analysis of 13 randomized controlled trials (RCTs) (45) (n = 554 patients with OSA) only found a small significant effect on attention in favor of CPAP, with modest improvement in sleepiness. A systematic review and meta-analysis of RCTs in elderly patients with OSA (four RCTs, 680 participants) (46) compared CPAP to a control group. CPAP was found to be associated with a 2.62-point improvement in the Epworth Sleepiness Scale but only slight improvements in cognitive (digital span, digital symbol) tests.
In the PROOF (Prognostic Indicator of Cardiovascular and Cerebrovascular Events) cohort (126 subjects ⩾65 years of age) (47), cognitive testing was done in all subjects with OSA at baseline (2002–2003) and again 10 years later (between 2009 and 2012). CPAP treatment sustained maintenance of memory (delayed free recall), attention, and executive functioning.
A retrospective study used Medicare 5% fee-for-service claims data in 53,321 beneficiaries, aged ⩾65 years with an OSA diagnosis before 2011 (48). PAP adherence was associated with lower odds of a new diagnosis of AD between 2011 and 2013 (odds ratio, 0.65).
Three months of CPAP treatment showed a significant improvement in MMSE scores (49), episodic and short-term memory, as well as executive function (50) in patients with OSA. One month of CPAP treatment resulted in improved verbal episodic memory in patients with OSA (51) and in patients with MCI; improvements in verbal memory were seen after 12 weeks (52). However, with a shorter duration (2 wk) of treatment, cognitive benefits have not been found (53). Some cognitive domains appear to be more sensitive to the effect of OSA treatment than others (54). Difficulties with visuo-constructive abilities and executive function in OSA appear to be more chronic, despite treatment, and may reflect an underlying neuropathology (55). Data on the impact of non-PAP treatment, such as oral appliances (56), and upper airway surgery (57) on cognition in OSA are sparse, but one RCT showed improvements in processing speed with mandibular advancement splint (58).
The panel noted that cognitive reserve is difficult to measure and can change over a lifetime. Impact of circadian rhythm on cognitive task performance should be accounted for in clinical trials. It was noted that overnight motor learning has not been well studied in OSA.
In conclusion, most data on the impact of OSA treatment on cognition are for CPAP; very limited data exist for other treatments. Duration of CPAP treatment is important. Cognitive domains that have been shown to improve with CPAP vary across studies: this heterogeneity may relate to study design, cognitive reserve, and the particular cognitive domain that is studied, among other factors. Clinical trial designs should identify which cognitive domains are most impacted by OSA and most likely to show improvement. Endpoints such as treatment response, improvement in cognitive performance, progression to MCI, etc. need to be better defined. Minimum treatment duration required to produce optimal improvement in cognitive functioning needs to be identified.
Section 5: OSA and AD
Worldwide, at least 50 million people are believed to be living with AD or other dementias (59). The number of Americans with AD aged ⩾65 is 6.1 million, and the prevalence is expected to reach 13.8 million by 2060 (60). Almost two-thirds of Americans with AD are women (61), and for those ⩾65 years, 6.9% of whites, 9.4% of African Americans, and 11.5% of Hispanics have AD or other dementias (62).
The major AD risk factors are age, family history, and genetics. Of these factors, age is the greatest; family history represents 15–20% of late-onset AD, and the apolipoprotein E, epsilon 4 (APOE ε4) genotype is a genetic risk factor for AD (60).
The core clinical diagnostic criteria for probable AD are criteria for dementia (including functional decline), insidious onset, clear history of worsening cognition, cognitive deficits, and/or no evidence of neurologic conditions including other dementias (63). Brain changes can begin decades before the onset of overt cognitive symptoms. The National Institute on Aging and Alzheimer’s Association have proposed a framework that views AD through a biologic (rather than solely symptomatic) lens, allowing for characterization of the continuum of brain changes even in cognitively normal older individuals (64). This AT(N) model groups fluid and imaging biomarkers into those impacting brain amyloid (Aβ) deposition (A), pathologic tau (T), and neurodegeneration (N). Progression to AD is characterized by decreases in cerebrospinal fluid (CSF) and plasma concentrations of Aβ42 and Aβ40 (A), increases in phosphorylated tau (T), and increases in total tau and plasma neurofilament light (N). Brain deposits of Aβ and tau, as measured with PET imaging, increase with AD progression and neurodegeneration, which can be appreciated with standard structural brain MRI.
The continuum of Alzheimer’s disease, including the clinical symptoms, associated fluid and imaging biomarkers, and pathological features at each stage, is shown in Figure 1. The pathological course often follows this pattern (in years) before AD symptom onset: brain Aβ and tau deposition (65) (31.7), glucose metabolism decline (65) (14.1), and structural brain atrophy (4.5) (66). The clinical course is heterogeneous but typically follows the pattern of memory decline, as well as impairment in language, visuospatial, and executive functioning. Neuropsychiatric features may occur early in the disease course but become more pronounced with disease progression and include anxiety, depression, insomnia, agitation, and paranoia. A decline in activities of daily living occurs, and, in later stages, waking and swallowing difficulties and incontinence occur. Death, with the primary cause often an intercurrent illness (e.g., pneumonia), may occur 4 to ⩾10 years after diagnosis.
Figure 1.
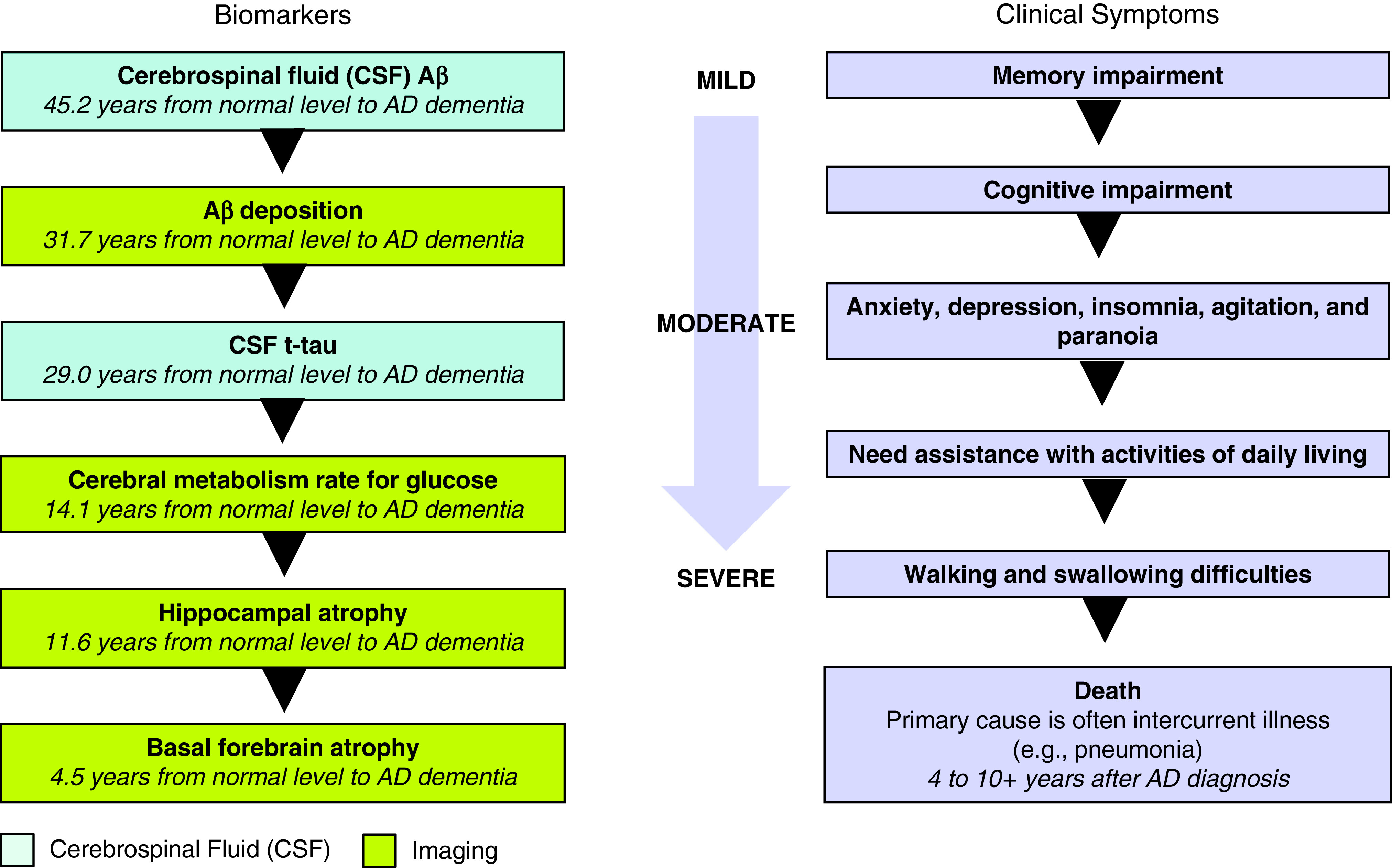
The continuum of Alzheimer’s disease (AD), including the clinical symptoms, associated fluid and imaging biomarkers, and pathological features at each stage (65, 66).
MCI is an at-risk state for AD, with progression rates of around 10–15% per year (67–69). It is characterized by objective CI beyond age and education expectations that occurs in the context of only minimal impairment in activities of daily living.
OSA is recognized as a factor that negatively influences risk (through modulation of preclinical AD biomarkers), manifestations, and possibly progression of AD (70).
Impact of OSA on Memory and Conversion to MCI
There is evidence that OSA impacts memory across declarative, episodic, spatial, emotional, and motor domains (71). In addition to memory domain, the extent to which OSA impacts memory depends on population demographics and memory testing paradigm. “Sleep-dependent” paradigms are those in which encoding and recall of memories are separated by a period of offline processing containing sleep. OSA has been shown to negatively impact a sleep-dependent form of spatial navigational memory that uses medial temporal lobe structures that include regions affected earliest by AD pathology, in particular tau tangle deposition (72). OSA negatively impacted the pattern of morning performance in a sleep-dependent spatial navigation task in a case/control study of cognitively normal older individuals (73). Using a sleep-dependent declarative verbal word-pairs task, OSA was linked to poorer overnight memory consolidation for cognitively intact control subjects but not for those with MCI (74), where hippocampal atrophy, daytime episodic memory, and sleep spindles were more pertinent predictors. OSA also negatively impacted overnight change in performance in a rapid eye movement–specific, within-subject, PAP withdrawal model in individuals with severe OSA (75). In a small clinical trial (RCT) of diet/exercise lifestyle modifications with or without PAP, those who received 3 months of PAP showed improvements in overnight consolidation of word pairs, with memory restored to levels observed in individuals without OSA (76). Improvements were also correlated with the increase in slow-wave sleep after starting PAP.
Subjects in the AD Neuroimaging Initiative were followed longitudinally for several years with clinical assessment of cognition and measurement of both neuroimaging and fluid biomarker assessments of AD risk. Among subjects converting to MCI, the average age of onset was significantly earlier (72.6 yr) in subjects with self-reported, untreated OSA compared with subjects without self-reported OSA (83.6 yr) (77). In those who used PAP, the average age of conversion to MCI was 82.1 years, similar to subjects without self-reported OSA.
In conclusion, the panel agreed that sleep-dependent memory tasks need greater incorporation into OSA testing, with better delineation of sleep fragmentation versus intermittent hypoxia effects. OSA treatment effects on memory remain understudied and can potentially be divided into effects on the prospective formation of new memories versus slowing the deterioration of established memories. OSA can have negative effects on additional aspects of cognition, such as attention or executive function, which can have indirect effects on memory.
Biomarkers of CI and Screening for CI in OSA
The use of AD biomarkers in patients with OSA is based on the assumption that OSA increases AD risk, but whether these biomarkers can be used in young and middle-age populations where there is minimal AD pathology, or healthy elderly with normal cognition, remains a subject of debate (78). In middle-aged and older adults, small sample size cross-sectional data suggest that there is an association between OSA and both established (amyloid PET [79–81], CSF Aβ [82, 83]) and novel (serum Aβ and tau [84, 85], CSF neuronal derived proteins [82], plasma cytokines [85]) biomarkers of AD pathology; the results seem more conclusive in those studies that included sleep clinic populations than those that were performed in community or memory clinic settings, and at least two negative studies have been published (86, 87).
In two studies performed in elder adults, OSA severity was associated with higher Aβ burden (measured as longitudinal decreases in CSF Aβ42 and increases in amyloid PET uptake) over a 2-year follow-up in cognitively normal elderly (88), whereas self-reported clinical diagnosis of OSA was associated with greater longitudinal increases in Aβ and tau burden by both CSF and PET imaging measured over a 2.5-year period in normal cognition and MCI groups (89). In middle-aged adults with newly diagnosed OSA beginning PAP treatment, there were significant associations between change in AHI4% (hypopneas as a decrease in airflow with ⩾4% desaturation) and change in CSF Aβ42, Aβ40, and T-tau, such that greater improvement in AHI4% was associated with a greater decline in each CSF AD biomarker from baseline (90). However, another study was not able to demonstrate associations between OSA diagnosed in middle age and adverse morphological brain changes 15 years later (91).
In patients with mild to moderate AD, recruited prospectively from a cognitive impairment unit, OSA was not associated with sleepiness or worse cognitive function, and APOE ε4 was not related to the presence or severity of OSA (3). However, there is strong biological plausibility for a bidirectional causal association between these conditions (92): arousals associated with OSA can regionally increase neural activity, which is believed to increase production of amyloid and tau (93). OSA may also reduce glymphatic flow, resulting in poorer clearance of amyloid and tau (94). Finally, OSA may cause direct neural injury through hypoxemia, free-radical generation from ischemia/reperfusion injury, or vascular/parenchymal inflammation (95). Conversely, the neurological deterioration that underlies aging and AD could affect breathing and the mechanisms causing OSA.
The panel discussed that at the present time there is strong biological plausibility, but insufficient data, to prove the bidirectional causality of the associations between OSA and aging pathology, and a greater emphasis needs to be placed on understanding how AD biomarkers respond to treatment. To do so would require a successful intervention for either condition (OSA or AD) as well as a medium- to long-term follow-up to observe clinical changes. However, blood-based AD biomarkers are becoming more reliably indicative of intracerebral amyloid and tau load, which may accelerate their utility as treatment outcomes in future OSA clinical trials; disease-modifying medications for AD are slowly unfolding, and the first was recently approved, albeit with some controversy, to treat AD.
Section 6: Racial and Ethnic Disparities in AD and OSA
Estimates show that the proportion of minoritized populations aged >65 years will increase from 22% to 45% by 2060 (96), although they currently remain underrepresented in biomedical (97–99) research. This projected exponential growth, alongside the extant research consistently demonstrating racial/ethnic disparities in OSA and AD, underscores the importance of determining and reducing disparities in these relationships.
Black/African American adults are 88% more likely to have OSA and 2.1 times more likely to have severe OSA, with the older adult populations maintaining the higher severity rates but demonstrating similar prevalence rates with their non-Hispanic White counterparts (100–102). A community-based study demonstrated that 18% of Hispanic/Latinx women and 33% of men had OSA (103). In addition, although they make up only 8–9% of older adults in the United States, Black/African American and Hispanic/Latinx individuals represent 12–18% of those diagnosed with AD (60, 61), with studies indicating a 1.5–2 times higher prevalence of AD (60, 61, 96, 104).
Emerging associations of OSA with increased Aβ (105), and AD neuropathology in the brainstem of individuals with OSA, provide the impetus for researching the bidirectional role of OSA in AD (106). These findings demonstrate the importance of OSA, a disorder disproportionately more prevalent in Black/African American and Hispanic/Latinx populations, as a possible mechanism in their demonstrated higher rates of AD.
In summary, evidence proposes increased risk related to the social/cultural construct of race/ethnicity may stem from disparities in health conditions, socioeconomics, and life experiences, secondary to systemic racism (60, 105). Therefore, the path toward reducing health disparities, especially in interrelated disorders disproportionally affecting minoritized populations like OSA and AD, should include increasing diversity and cultural competence; engaging, recruiting, and retaining diverse populations in health care and in research studies; and considering inclusion of measures of systemic racism as directly or indirectly impacting cognition, OSA, and AD.
Section 7: Future Directions
Approaches and Challenges in Planning a Clinical Trial
Research shows that up to 40% of dementia risk is due to modifiable risk factors (107), with OSA emerging as a key risk factor. Coupled with evidence that dementia pathology begins to accumulate in the brain up to 20 years before clinical symptoms emerge (108), robust trials targeting OSA for dementia prevention are warranted.
Study population and setting
Although ideal, prevention trials targeting OSA in cognitively normal individuals would need to be large-scale and therefore costly (109). A lengthy follow-up would be required to detect clinically significant benefits, which is often not feasible or well suited to the typical timeframes of funding bodies (i.e., 5–10 yr) (110, 111). Selective prevention trials may be more realistic.
Targeting individuals with OSA in sleep clinics would eliminate the need for large-scale community OSA screening and may yield participants with greater cognitive decline (112). Such approaches could be aligned with real-world translation goals, such as the implementation of cognitive screening in sleep clinics.
An alternative approach is to target individuals attending a memory clinic, such as those with MCI (113). In these settings, OSA is evident in up to 71% of patients with MCI (114). However, it is not yet clear if the critical window for intervention has passed and if targeting OSA in preclinical AD before the onset of CI may confer greater benefits.
Strategies that enrich for dementia risk are worth consideration, such as those with concomitant cardiovascular risk (115), positive for the APOE ε4 genotype (116), with more severe OSA or hypoxemia (37, 117), or with evidence of preclinical AD (118). For the latter, measurement of AD biomarkers using PET scanning or CSF could be used, but these are burdensome and costly (119–121). Blood-based biomarkers for AD could be a viable, scalable, and cost-effective screening alternative (122).
Selection of study outcomes
Because there are low base rates of Aβ in those in midlife (116), trials aiming to detect changes in AD biomarkers in midlife would need to incorporate very long treatment and follow-up periods or focus on those aged >60 years, where Aβ deposition is more evident (123). The choice of cognitive test is critical. Global cognitive scores (e.g., MMSE or Montreal Cognitive Assessment) and other composite scores lack sensitivity to detect short-term and domain-specific changes (124–126). Indeed, effects on memory may predominate (112, 127) in OSA, but executive functioning and processing speed outcomes also warrant consideration in midlife samples. The benefits of interventions on overnight memory consolidation should be considered (74), given emerging work showing the benefits of sleep for this critical process (74, 128), and cross-validation of digital cognitive tests or videoconference-based neuropsychological tests could be considered for large-scale trials and/or those in nonmetropolitan communities. Neuroimaging, although costly and less scalable, does represent another surrogate marker of brain health, especially if incorporating MRI markers of cerebrovascular disease (e.g., white matter lesions), hippocampal integrity, functional connectivity, or PET markers of neuroinflammation, glucose metabolism, or AD. As noted, CSF to measure AD pathology may be used but is invasive and not widely used worldwide. Plasma biomarkers such as neurofilament light (NFL), glial fibrillary acidic protein, and phosphorylated-tau assays (e.g., p-tau 181, 217, 231), may prove to be sensitive, feasible, and scalable biomarkers for use in clinical trials (129).
Future studies should also account for mediating factors, such as cerebrovascular disease, blood–brain barrier integrity, neuroinflammation, depression, substance use, and other factors that may impact cognition, brain health, or CPAP adherence and examine secondary impacts of treatments on overall functioning, as well as consider incorporating implementation science and health economics into trials to inform larger health system implementation.
In summary, treatment of OSA may slow the onset of dementia and AD, yet optimal design of randomized trials presents several challenges in terms of feasibility and cost. In addition, selection of study population and setting may require identifying individuals who are presymptomatic but at elevated risk of future dementia. Studies of sufficient size and duration to detect clinical outcomes are largely not feasible; therefore, careful consideration of surrogate outcomes, including more sensitive cognitive tests and biomarkers of AD and dementia, is essential.
Alternative Approaches to Clinical Trials
The impact of CPAP in improving cognitive function and preventing dementia is unclear. Observational studies suggest that CPAP may delay MCI by up to 10 years, but this finding was based on only 35 patients using CPAP (77). Large RCTs would be an ideal way to assess CPAP effectiveness.
However, conducting RCTs to address long-term health effects is challenging. Patients with substantial OSA symptoms and/or severe hypoxemia cannot ethically be randomized to control, given the efficacy of CPAP in improving symptoms and health outcomes (130–133). Furthermore, even patients with moderate symptoms may choose not to participate if they may be randomized to a control group for a prolonged time period. Excluding these patients from trials removes the subset of patients who might be most likely to experience robust CPAP benefit. Clinical trials tend to recruit minimally symptomatic patients, likely leading to lower adherence, as they do not generally feel better with CPAP (134). In the largest RCT of CPAP for cardiovascular disease prevention (N = 2,717) (135), CPAP adherence was low (3.3 h/night), likely accounting for the null results (133).
A complementary design to large RCTs would be long-term observational cohort studies that can recruit symptomatic and severe patients and enable rigorous propensity score (PS) matching (132).
PS matching (133, 136) estimates treatment effects by accounting for covariates that are associated with outcomes and that may differ between groups. A PS could be the probability of being CPAP adherent as a function of baseline covariates. A rich set of covariates known or suspected to be associated with both adherence and outcomes is required to develop a robust PS design. Selection into a PS subclass is the observational equivalent of being randomized (137–139). Several analysis methods using PS exist to reduce confounding in nonrandomized observational cohort studies (140).
PS models do not result in a balance of unmeasured confounders and are not a true substitute for RCTs. Nevertheless, they may be a useful alternative to RCTs, as they can be implemented in large database research cohorts and might be especially useful if randomization is not possible.
In summary, RCTs to study the impact of CPAP (and other OSA interventions) on long-term health effects is challenging because of issues with clinical equipoise (e.g., randomizing symptomatic patients for prolonged time periods) and adherence. Alternative designs such as rigorous observational studies may complement RCT and address some of these limitations.
Conclusions
The key conclusions from the workshop were:
-
1.
There is a growing body of evidence showing an association between OSA and cognitive impairment and decline
-
2.
Currently available cognitive screening tests may allow for early identification of MCI in patients with OSA, but caution is needed in interpreting these tests.
-
3.
Neuroimaging in OSA can provide surrogate measures of disease chronicity; however, several methodological challenges and limitations remain
-
4.
Most data on the impact of OSA treatment on cognition are for CPAP; very limited data exist for other treatments.
-
5.
There is considerable heterogeneity as to which cognitive domains have been shown to improve with CPAP; this heterogeneity may relate to study design, cognitive reserve, the aspect of each cognitive domain that is studied, and other factors.
-
6.
OSA is recognized as a factor that negatively influences risk (through modulation of preclinical AD biomarkers), manifestations, and possibly progression of AD.
-
7.
Sleep-dependent memory tasks need greater incorporation into OSA testing, with better delineation of sleep fragmentation versus intermittent hypoxia effects.
-
8.
Plasma biomarkers may prove to be sensitive, feasible, and scalable biomarkers for use in clinical trials. Whether AD biomarkers can be used in young and middle-age populations with OSA where there is minimal AD pathology, or healthy elderly with normal cognition, remains a subject of debate.
-
9.
At the present time there is strong biological plausibility, but insufficient data, to prove the bidirectional causality of the associations between OSA and aging pathology, and a greater emphasis needs to be placed on understanding how AD biomarkers respond to treatment.
-
10.
Engaging, recruiting, and retaining diverse populations in health care and research may help to decrease the racial and ethnic disparities in OSA and AD.
-
11.
Key considerations for intervention studies include selection of the population to be recruited, use of pragmatic or alternative study designs, longer duration of intervention, new approaches to improve PAP adherence, and studying changes in more easily accessible biomarkers.
Acknowledgments
This official workshop report was prepared by an ad hoc subcommittee of the ATS Assembly on Sleep and Respiratory Neurobiology Assembly.
Members of the subcommittee are as follows:
Chitra Lal, M.D. (Chair)1
Andrew W. Varga, M.D. (Co-Chair)2
Indu Ayappa, Ph.D.2*
Najib Ayas, M.D.3‡
Andrew E. Beaudin, Ph.D.4‡
Camilla Hoyos, Ph.D.5‡
Marta Kaminska, M.D.6*
Clete A. Kushida, M.D.7‡
Anna Mullins, Ph.D.2‡
Sharon L. Naismith, Ph.D.8‡
Ricardo S. Osorio, M.D.9‡
Ankit ParEkh, Ph.D.2*
Craig L. Phillips, Ph.D.10*
Katie L. Stone, Ph.D.11‡
Arlener D. Turner, Ph.D.12‡
*Participant.
‡Speaker.
1Medical University of South Carolina, Charleston, South Carolina; 2Icahn School of Medicine at Mount Sinai, New York, New York; 3University of British Columbia, Vancouver, British Columbia, Canada; 4University of Calgary, Calgary, Alberta, Canada; 5Woolcock Institute of Medical Research, University of Sydney, Sydney, New South Wales, Australia; 6McGill University, Montreal, Quebec, Canada; 7Stanford University, Redwood City, California; 8University of Sydney, Sydney, New South Wales, Australia; 9NYU Langone Medical Center, New York, New York; 10Macquarie University, Sydney, New South Wales, Australia; 11California Pacific Medical Center Research Institute, University of California, San Francisco, California; and 12University of Miami, Miami, Florida
Footnotes
This official workshop report of the American Thoracic Society was approved May 2022
Subcommittee Disclosures: C.L. served as a consultant and speaker for Chest/GlaxoSmithKline and Jazz Pharmaceuticals; served on a data safety and monitoring board for Jazz Pharmaceuticals; received research support from NIH Sleep smart, NIH LOFT HF study. I.A. served as a grant reviewer for AASM Foundation; received royalties from Fisher & Paykel for patent on CPAP algorithm; received research support from Fisher & Paykel. N.A. received research support from Signifier Medical. C.H. received research support from Advance Brain Monitoring, Fisher & Paykel, Sante Group. C.A.K. served as a consultant for Merck Sharp & Dohme and M^3 Public Benefit Corporation; served as a speaker for Itamar Medical and ResMed; holds stock options for M^3 Public Benefit Corp, Restful Robotics, Vivos Therapeutics. M.K. served on an advisory committee for Biron Soins du Sommeil; received research support from Fisher & Paykel, Philips, Signifier, VitalAire. S.L.N. served as a consultant for Brain Protection Company and University of Sunshine Coast; served as a speaker for AASBI, Nutricia, Roche; served on a data safety and monitoring board for Alzheimer’s Australia Research Foundation; serves in a leadership role for Australian Dementia Network; received research support from NHMRC, MRFF, US Alzheimer’s Drug Discovery Foundation, St Vincent’s Clinic Foundation, Cardiovascular Research Capacity Grants. A.P. served as a speaker for Health Advances; received research support from Itamar Medical. K.L.S. received research support from Merck. A.D.T. received research support from National Institute on Aging, National Institutes of Health. A.E.B., A.M., R.S.O., C.L.P., and A.W.V. reported no commercial or relevant non-commercial interests from ineligible companies.
References
- 1. Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med . 2019;7:687–698. doi: 10.1016/S2213-2600(19)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement . 2013;9:63–75.e2. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 3. Gaeta AM, Benítez ID, Jorge C, Torres G, Dakterzada F, Minguez O, et al. Prevalence of obstructive sleep apnea in Alzheimer’s disease patients. J Neurol . 2020;267:1012–1022. doi: 10.1007/s00415-019-09668-4. [DOI] [PubMed] [Google Scholar]
- 4. Leng Y, McEvoy CT, Allen IE, Yaffe K. Association of sleep-disordered breathing with cognitive function and risk of cognitive impairment: a systematic review and meta-analysis. JAMA Neurol . 2017;74:1237–1245. doi: 10.1001/jamaneurol.2017.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Polsek D, Gildeh N, Cash D, Winsky-Sommerer R, Williams SCR, Turkheimer F, et al. Obstructive sleep apnoea and Alzheimer’s disease: in search of shared pathomechanisms. Neurosci Biobehav Rev . 2018;86:142–149. doi: 10.1016/j.neubiorev.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ju YE, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology: a bidirectional relationship. Nat Rev Neurol . 2014;10:115–119. doi: 10.1038/nrneurol.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA . 2011;306:613–619. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chang WP, Liu ME, Chang WC, Yang AC, Ku YC, Pai JT, et al. Sleep apnea and the risk of dementia: a population-based 5-year follow-up study in Taiwan. PLoS One . 2013;8:e78655. doi: 10.1371/journal.pone.0078655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yaffe K, Nettiksimmons J, Yesavage J, Byers A. Sleep quality and risk of dementia among older male veterans. Am J Geriatr Psychiatry . 2015;23:651–654. doi: 10.1016/j.jagp.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 10. Martin MS, Sforza E, Roche F, Barthélémy JC, Thomas-Anterion C, PROOF study group Sleep breathing disorders and cognitive function in the elderly: an 8-year follow-up study. The proof-synapse cohort. Sleep (Basel) . 2015;38:179–187. doi: 10.5665/sleep.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blackwell T, Yaffe K, Laffan A, Redline S, Ancoli-Israel S, Ensrud KE, et al. Osteoporotic Fractures in Men Study Group Associations between sleep-disordered breathing, nocturnal hypoxemia, and subsequent cognitive decline in older community-dwelling men: the Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc . 2015;63:453–461. doi: 10.1111/jgs.13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lutsey PL, Bengtson LG, Punjabi NM, Shahar E, Mosley TH, Gottesman RF, et al. Obstructive sleep apnea and 15-year cognitive decline: the Atherosclerosis Risk in Communities (ARIC) study. Sleep (Basel) . 2016;39:309–316. doi: 10.5665/sleep.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shi L, Chen SJ, Ma MY, Bao YP, Han Y, Wang YM, et al. Sleep disturbances increase the risk of dementia: a systematic review and meta-analysis. Sleep Med Rev . 2018;40:4–16. doi: 10.1016/j.smrv.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 14. Bubu OM, Brannick M, Mortimer J, Umasabor-Bubu O, Sebastião YV, Wen Y, et al. Sleep, cognitive impairment, and Alzheimer’s disease: a systematic review and meta-analysis. Sleep (Basel) . 2017;40 doi: 10.1093/sleep/zsw032. [DOI] [PubMed] [Google Scholar]
- 15. D’Rozario AL, Chapman JL, Phillips CL, Palmer JR, Hoyos CM, Mowszowski L, et al. Objective measurement of sleep in mild cognitive impairment: a systematic review and meta-analysis. Sleep Med Rev . 2020;52:101308. doi: 10.1016/j.smrv.2020.101308. [DOI] [PubMed] [Google Scholar]
- 16. Gagnon K, Baril A-A, Montplaisir J, Carrier J, Chami S, Gauthier S, et al. Detection of mild cognitive impairment in middle-aged and older adults with obstructive sleep apnoea. Eur Respir J . 2018;52:1801137. doi: 10.1183/13993003.01137-2018. [DOI] [PubMed] [Google Scholar]
- 17. Beaudin AE, Raneri JK, Ayas NT, Skomro RP, Fox N, Hirsch Allen AJM, et al. Cognitive function in a sleep clinic cohort of patients with obstructive sleep apnea. Ann Am Thorac Soc . 2021;18:865–875. doi: 10.1513/AnnalsATS.202004-313OC. [DOI] [PubMed] [Google Scholar]
- 18. Ihle-Hansen H, Vigen T, Berge T, Einvik G, Aarsland D, Rønning OM, et al. Montreal Cognitive Assessment in a 63- to 65-year-old Norwegian Cohort from the general population: data from the Akershus Cardiac Examination 1950 study. Dement Geriatr Cogn Disord Extra . 2017;7:318–327. doi: 10.1159/000480496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Coen RF, Cahill R, Lawlor BA. Things to watch out for when using the Montreal Cognitive Assessment (MoCA) Int J Geriatr Psychiatry . 2011;26:107–108. doi: 10.1002/gps.2471. [DOI] [PubMed] [Google Scholar]
- 20. Rossetti HC, Lacritz LH, Cullum CM, Weiner MF. Normative data for the Montreal Cognitive Assessment (MoCA) in a population-based sample. Neurology . 2011;77:1272–1275. doi: 10.1212/WNL.0b013e318230208a. [DOI] [PubMed] [Google Scholar]
- 21. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res . 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 22. Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc . 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 23. Sexton CE, Storsve AB, Walhovd KB, Johansen-Berg H, Fjell AM. Poor sleep quality is associated with increased cortical atrophy in community-dwelling adults. Neurology . 2014;83:967–973. doi: 10.1212/WNL.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zimmerman ME, Aloia MS. A review of neuroimaging in obstructive sleep apnea. J Clin Sleep Med . 2006;2:461–471. [PubMed] [Google Scholar]
- 25. Lanfranchi P, Somers VK. Obstructive sleep apnea and vascular disease. Respir Res . 2001;2:315–319. doi: 10.1186/rr79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Canessa N, Castronovo V, Cappa SF, Aloia MS, Marelli S, Falini A, et al. Obstructive sleep apnea: brain structural changes and neurocognitive function before and after treatment. Am J Respir Crit Care Med . 2011;183:1419–1426. doi: 10.1164/rccm.201005-0693OC. [DOI] [PubMed] [Google Scholar]
- 27. Baril AA, Gagnon K, Brayet P, Montplaisir J, De Beaumont L, Carrier J, et al. Gray matter hypertrophy and thickening with obstructive sleep apnea in middle-aged and older adults. Am J Respir Crit Care Med . 2017;195:1509–1518. doi: 10.1164/rccm.201606-1271OC. [DOI] [PubMed] [Google Scholar]
- 28. Naismith S, Winter V, Gotsopoulos H, Hickie I, Cistulli P. Neurobehavioral functioning in obstructive sleep apnea: differential effects of sleep quality, hypoxemia and subjective sleepiness. J Clin Exp Neuropsychol . 2004;26:43–54. doi: 10.1076/jcen.26.1.43.23929. [DOI] [PubMed] [Google Scholar]
- 29. Macey PM, Sarma MK, Prasad JP, Ogren JA, Aysola R, Harper RM, et al. Obstructive sleep apnea is associated with altered midbrain chemical concentrations. Neuroscience . 2017;363:76–86. doi: 10.1016/j.neuroscience.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Macey PM, Kumar R, Woo MA, Valladares EM, Yan-Go FL, Harper RM. Brain structural changes in obstructive sleep apnea. Sleep . 2008;31:967–977. [PMC free article] [PubMed] [Google Scholar]
- 31. Macey PM, Henderson LA, Macey KE, Alger JR, Frysinger RC, Woo MA, et al. Brain morphology associated with obstructive sleep apnea. Am J Respir Crit Care Med . 2002;166:1382–1387. doi: 10.1164/rccm.200201-050OC. [DOI] [PubMed] [Google Scholar]
- 32. Castronovo V, Canessa N, Strambi LF, Aloia MS, Consonni M, Marelli S, et al. Brain activation changes before and after PAP treatment in obstructive sleep apnea. Sleep . 2009;32:1161–1172. doi: 10.1093/sleep/32.9.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gale SD, Hopkins RO. Effects of hypoxia on the brain: neuroimaging and neuropsychological findings following carbon monoxide poisoning and obstructive sleep apnea. J Int Neuropsychol Soc . 2004;10:60–71. doi: 10.1017/S1355617704101082. [DOI] [PubMed] [Google Scholar]
- 34. Yaouhi K, Bertran F, Clochon P, Mézenge F, Denise P, Foret J, et al. A combined neuropsychological and brain imaging study of obstructive sleep apnea. J Sleep Res . 2009;18:36–48. doi: 10.1111/j.1365-2869.2008.00705.x. [DOI] [PubMed] [Google Scholar]
- 35. O’Donoghue FJ, Briellmann RS, Rochford PD, Abbott DF, Pell GS, Chan CH, et al. Cerebral structural changes in severe obstructive sleep apnea. Am J Respir Crit Care Med . 2005;171:1185–1190. doi: 10.1164/rccm.200406-738OC. [DOI] [PubMed] [Google Scholar]
- 36. Weng HH, Tsai YH, Chen CF, Lin YC, Yang CT, Tsai YH, et al. Mapping gray matter reductions in obstructive sleep apnea: an activation likelihood estimation meta-analysis. Sleep (Basel) . 2014;37:167–175. doi: 10.5665/sleep.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Naismith SL, Duffy SL, Cross N, Grunstein R, Terpening Z, Hoyos C, et al. Nocturnal hypoxemia is associated with altered parahippocampal functional brain connectivity in older adults at risk for dementia. J Alzheimers Dis . 2020;73:571–584. doi: 10.3233/JAD-190747. [DOI] [PubMed] [Google Scholar]
- 38. Cross NE, Memarian N, Duffy SL, Paquola C, LaMonica H, D’Rozario A, et al. Structural brain correlates of obstructive sleep apnoea in older adults at risk for dementia. Eur Respir J . 2018;52:1800740. doi: 10.1183/13993003.00740-2018. [DOI] [PubMed] [Google Scholar]
- 39. Macey PM, Haris N, Kumar R, Thomas MA, Woo MA, Harper RM. Obstructive sleep apnea and cortical thickness in females and males. PLoS One . 2018;13:e0193854. doi: 10.1371/journal.pone.0193854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee MH, Yun CH, Min A, Hwang YH, Lee SK, Kim DY, et al. Altered structural brain network resulting from white matter injury in obstructive sleep apnea. Sleep (Basel) . 2019;42:zsz120. doi: 10.1093/sleep/zsz120. [DOI] [PubMed] [Google Scholar]
- 41. Lee MC, Shen YC, Wang JH, Li YY, Li TH, Chang ET, et al. Effects of continuous positive airway pressure on anxiety, depression, and major cardiac and cerebro-vascular events in obstructive sleep apnea patients with and without coronary artery disease. Ci Ji Yi Xue Za Zhi . 2017;29:218–222. doi: 10.4103/tcmj.tcmj_128_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rosenzweig I, Glasser M, Polsek D, Leschziner GD, Williams SC, Morrell MJ. Sleep apnoea and the brain: a complex relationship. Lancet Respir Med . 2015;3:404–414. doi: 10.1016/S2213-2600(15)00090-9. [DOI] [PubMed] [Google Scholar]
- 43. Veasey SC. Piecing together phenotypes of brain injury and dysfunction in obstructive sleep apnea. Front Neurol . 2012;3:139. doi: 10.3389/fneur.2012.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lambert C, Chowdhury R, Fitzgerald TH, Fleming SM, Lutti A, Hutton C, et al. Characterizing aging in the human brainstem using quantitative multimodal MRI analysis. Front Hum Neurosci . 2013;7:462. doi: 10.3389/fnhum.2013.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kylstra WA, Aaronson JA, Hofman WF, Schmand BA. Neuropsychological functioning after CPAP treatment in obstructive sleep apnea: a meta-analysis. Sleep Med Rev . 2013;17:341–347. doi: 10.1016/j.smrv.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 46. Labarca G, Saavedra D, Dreyse J, Jorquera J, Barbe F. Efficacy of CPAP for improvements in sleepiness, cognition, mood, and quality of life in elderly patients with OSA: systematic review and meta-analysis of randomized controlled trials. Chest . 2020;158:751–764. doi: 10.1016/j.chest.2020.03.049. [DOI] [PubMed] [Google Scholar]
- 47. Crawford-Achour E, Dauphinot V, Martin MS, Tardy M, Gonthier R, Barthelemy JC, et al. Protective effect of long-term CPAP therapy on cognitive performance in elderly patients with severe OSA: the PROOF study. J Clin Sleep Med . 2015;11:519–524. doi: 10.5664/jcsm.4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dunietz GL, Chervin RD, Burke JF, Conceicao AS, Braley TJ. Obstructive sleep apnea treatment and dementia risk in older adults. Sleep (Basel) . 2021;44:zsab076. doi: 10.1093/sleep/zsab076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kanbay A, Demir NC, Tutar N, Köstek O, Özer Şimşek Z, Buyukoglan H, et al. The effect of CPAP therapy on insulin-like growth factor and cognitive functions in obstructive sleep apnea patients. Clin Respir J . 2017;11:506–513. doi: 10.1111/crj.12365. [DOI] [PubMed] [Google Scholar]
- 50. Dalmases M, Solé-Padullés C, Torres M, Embid C, Nuñez MD, Martínez-Garcia MÁ, et al. Effect of CPAP on cognition, brain function, and structure among elderly patients with OSA: a randomized pilot study. Chest . 2015;148:1214–1223. doi: 10.1378/chest.15-0171. [DOI] [PubMed] [Google Scholar]
- 51. Rosenzweig I, Glasser M, Crum WR, Kempton MJ, Milosevic M, McMillan A, et al. Changes in neurocognitive architecture in patients with obstructive sleep apnea treated with continuous positive airway pressure. EBioMedicine . 2016;7:221–229. doi: 10.1016/j.ebiom.2016.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hoyos CM, Cross NE, Terpening Z, D’Rozario AL, Yee BJ, LaMonica H, et al. CPAP for cognition in sleep apnea and mild cognitive impairment: a pilot randomized crossover trial. Am J Respir Crit Care Med . 2022;205:1479–1482. doi: 10.1164/rccm.202111-2646LE. [DOI] [PubMed] [Google Scholar]
- 53. Lim W, Bardwell WA, Loredo JS, Kim EJ, Ancoli-Israel S, Morgan EE, et al. Neuropsychological effects of 2-week continuous positive airway pressure treatment and supplemental oxygen in patients with obstructive sleep apnea: a randomized placebo-controlled study. J Clin Sleep Med . 2007;3:380–386. [PMC free article] [PubMed] [Google Scholar]
- 54. Lau EY, Eskes GA, Morrison DL, Rajda M, Spurr KF. Executive function in patients with obstructive sleep apnea treated with continuous positive airway pressure. J Int Neuropsychol Soc . 2010;16:1077–1088. doi: 10.1017/S1355617710000901. [DOI] [PubMed] [Google Scholar]
- 55. Ferini-Strambi L, Baietto C, Di Gioia MR, Castaldi P, Castronovo C, Zucconi M, et al. Cognitive dysfunction in patients with obstructive sleep apnea (OSA): partial reversibility after continuous positive airway pressure (CPAP) Brain Res Bull . 2003;61:87–92. doi: 10.1016/s0361-9230(03)00068-6. [DOI] [PubMed] [Google Scholar]
- 56. Tegelberg A, Wilhelmsson B, Erixon-Lindroth N, Lindström LH. Improved cognitive functions after treatment with an oral appliance in obstructive sleep apnea. Nat Sci Sleep . 2012;4:89–96. doi: 10.2147/NSS.S33849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Alkan U, Nachalon Y, Weiss P, Ritter A, Feinmesser R, Gilat H, et al. Effects of surgery for obstructive sleep apnea on cognitive function and driving performance. Sleep Breath . 2021;25:1593–1600. doi: 10.1007/s11325-020-02285-w. [DOI] [PubMed] [Google Scholar]
- 58. Naismith SL, Winter VR, Hickie IB, Cistulli PA. Effect of oral appliance therapy on neurobehavioral functioning in obstructive sleep apnea: a randomized controlled trial. J Clin Sleep Med . 2005;1:374–380. [PubMed] [Google Scholar]
- 59.Patterson C. World Alzheimer report 2018. The state of the art of dementia research: new frontiers. An analysis of prevalence, incidence, cost and trends. London: Alzheimer’s Disease International; 2018. [Google Scholar]
- 60. Alzheimers Association. 2021 Alzheimer’s disease facts and figures. Alzheimers Dement . 2021;17:327–406. doi: 10.1002/alz.12328. [DOI] [PubMed] [Google Scholar]
- 61. Rajan KB, Weuve J, Barnes LL, McAninch EA, Wilson RS, Evans DA. Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020–2060) Alzheimers Dement . 2021;17:1966–1975. doi: 10.1002/alz.12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Alzheimers Association. 2017 Alzheimer’s disease facts and figures. Alzheimers Dement . 2017;13:325–373. [Google Scholar]
- 63. McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement . 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jack CR, Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. Contributors NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement . 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wang HF, Shen XN, Li JQ, Suckling J, Tan CC, Wang YJ, et al. Alzheimer’s Disease Neuroimaging Initiative Clinical and biomarker trajectories in sporadic Alzheimer’s disease: a longitudinal study. Alzheimers Dement (Amst) . 2020;12:e12095. doi: 10.1002/dad2.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hall AM, Moore RY, Lopez OL, Kuller L, Becker JT. Basal forebrain atrophy is a presymptomatic marker for Alzheimer’s disease. Alzheimers Dement . 2008;4:271–279. doi: 10.1016/j.jalz.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yaffe K, Petersen RC, Lindquist K, Kramer J, Miller B. Subtype of mild cognitive impairment and progression to dementia and death. Dement Geriatr Cogn Disord . 2006;22:312–319. doi: 10.1159/000095427. [DOI] [PubMed] [Google Scholar]
- 68. Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia--meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand . 2009;119:252–265. doi: 10.1111/j.1600-0447.2008.01326.x. [DOI] [PubMed] [Google Scholar]
- 69. Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, et al. Current concepts in mild cognitive impairment. Arch Neurol . 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 70. Andrade AG, Bubu OM, Varga AW, Osorio RS. The relationship between obstructive sleep apnea and Alzheimer’s disease. J Alzheimers Dis . 2018;64:S255–S270. doi: 10.3233/JAD-179936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ahuja S, Chen RK, Kam K, Pettibone WD, Osorio RS, Varga AW. Role of normal sleep and sleep apnea in human memory processing. Nat Sci Sleep . 2018;10:255–269. doi: 10.2147/NSS.S125299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol . 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mullins AE, Williams MK, Kam K, Parekh A, Bubu OM, Castillo B, et al. Effects of obstructive sleep apnea on human spatial navigational memory processing in cognitively normal older individuals. J Clin Sleep Med . 2021;17:939–948. doi: 10.5664/jcsm.9080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lam A, Haroutonian C, Grummitt L, Ireland C, Grunstein RR, Duffy S, et al. Sleep-dependent memory in older people with and without MCI: the relevance of sleep microarchitecture, OSA, hippocampal subfields, and episodic memory. Cereb Cortex . 2021;31:2993–3005. doi: 10.1093/cercor/bhaa406. [DOI] [PubMed] [Google Scholar]
- 75. Varga AW, Kishi A, Mantua J, Lim J, Koushyk V, Leibert DP, et al. Apnea-induced rapid eye movement sleep disruption impairs human spatial navigational memory. J Neurosci . 2014;34:14571–14577. doi: 10.1523/JNEUROSCI.3220-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Djonlagic IE, Guo M, Igue M, Kishore D, Stickgold R, Malhotra A. Continuous positive airway pressure restores declarative memory deficit in obstructive sleep apnea. Am J Respir Crit Care Med . 2021;203:1188–1190. doi: 10.1164/rccm.202011-4253LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Osorio RS, Gumb T, Pirraglia E, Varga AW, Lu SE, Lim J, et al. Alzheimer’s Disease Neuroimaging Initiative Sleep-disordered breathing advances cognitive decline in the elderly. Neurology . 2015;84:1964–1971. doi: 10.1212/WNL.0000000000001566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bubu OM, Andrade AG, Umasabor-Bubu OQ, Hogan MM, Turner AD, de Leon MJ, et al. Obstructive sleep apnea, cognition and Alzheimer’s disease: a systematic review integrating three decades of multidisciplinary research. Sleep Med Rev . 2020;50:101250. doi: 10.1016/j.smrv.2019.101250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yun CH, Lee HY, Lee SK, Kim H, Seo HS, Bang SA, et al. Amyloid burden in obstructive sleep apnea. J Alzheimers Dis . 2017;59:21–29. doi: 10.3233/JAD-161047. [DOI] [PubMed] [Google Scholar]
- 80. Jackson ML, Cavuoto M, Schembri R, Doré V, Villemagne VL, Barnes M, et al. Severe obstructive sleep apnea is associated with higher brain amyloid burden: a preliminary PET imaging study. J Alzheimers Dis . 2020;78:611–617. doi: 10.3233/JAD-200571. [DOI] [PubMed] [Google Scholar]
- 81. André C, Rehel S, Kuhn E, Landeau B, Moulinet I, Touron E, et al. Medit-Ageing Research Group Association of sleep-disordered breathing with Alzheimer disease biomarkers in community-dwelling older adults: a secondary analysis of a randomized clinical trial. JAMA Neurol . 2020;77:716–724. doi: 10.1001/jamaneurol.2020.0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ju YE, Finn MB, Sutphen CL, Herries EM, Jerome GM, Ladenson JH, et al. Obstructive sleep apnea decreases central nervous system-derived proteins in the cerebrospinal fluid. Ann Neurol . 2016;80:154–159. doi: 10.1002/ana.24672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Liguori C, Mercuri NB, Izzi F, Romigi A, Cordella A, Sancesario G, et al. Obstructive sleep apnea is associated with early but possibly modifiable Alzheimer’s disease biomarkers changes. Sleep (Basel) . 2017;40 doi: 10.1093/sleep/zsx011. [DOI] [PubMed] [Google Scholar]
- 84. Bu XL, Liu YH, Wang QH, Jiao SS, Zeng F, Yao XQ, et al. Serum amyloid-beta levels are increased in patients with obstructive sleep apnea syndrome. Sci Rep . 2015;5:13917. doi: 10.1038/srep13917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Motamedi V, Kanefsky R, Matsangas P, Mithani S, Jeromin A, Brock MS, et al. Elevated tau and interleukin-6 concentrations in adults with obstructive sleep apnea. Sleep Med . 2018;43:71–76. doi: 10.1016/j.sleep.2017.11.1121. [DOI] [PubMed] [Google Scholar]
- 86. Mendes A, Tezenas du Montcel S, Levy M, Bertrand A, Habert MO, Bertin H, et al. INSIGHT-PreAD study group Multimorbidity is associated with preclinical Alzheimer’s disease neuroimaging biomarkers. Dement Geriatr Cogn Disord . 2018;45:272–281. doi: 10.1159/000489007. [DOI] [PubMed] [Google Scholar]
- 87. Handa SS, Baba S, Yamashita K, Nishizaka M, Ando S. The severity of obstructive sleep apnea syndrome cannot predict the accumulation of brain amyloid by imaging with [11C]-Pittsburgh compound B PET computed tomography in patients with a normal cognitive function. Ann Nucl Med . 2019;33:541–544. doi: 10.1007/s12149-019-01349-6. [DOI] [PubMed] [Google Scholar]
- 88. Sharma RA, Varga AW, Bubu OM, Pirraglia E, Kam K, Parekh A, et al. Obstructive sleep apnea severity affects amyloid burden in cognitively normal elderly: a longitudinal study. Am J Respir Crit Care Med . 2018;197:933–943. doi: 10.1164/rccm.201704-0704OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bubu OM, Pirraglia E, Andrade AG, Sharma RA, Gimenez-Badia S, Umasabor-Bubu OQ, et al. Alzheimer’s Disease Neuroimaging Initiative Obstructive sleep apnea and longitudinal Alzheimer’s disease biomarker changes. Sleep (Basel) . 2019;42:zsz048. doi: 10.1093/sleep/zsz048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ju YS, Zangrilli MA, Finn MB, Fagan AM, Holtzman DM. Obstructive sleep apnea treatment, slow wave activity, and amyloid-β. Ann Neurol . 2019;85:291–295. doi: 10.1002/ana.25408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lutsey PL, Norby FL, Gottesman RF, Mosley T, MacLehose RF, Punjabi NM, et al. Sleep apnea, sleep duration and brain MRI markers of cerebral vascular disease and Alzheimer’s disease: the Atherosclerosis Risk in Communities study (ARIC) PLoS One . 2016;11:e0158758. doi: 10.1371/journal.pone.0158758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Özcan GG, Lim S, Leighton P, Allison WT, Rihel J. Sleep is bi-directionally modified by amyloid beta oligomers. eLife . 2020;9:e53995. doi: 10.7554/eLife.53995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Tampellini D. Synaptic activity and Alzheimer’s disease: a critical update. Front Neurosci . 2015;9:423. doi: 10.3389/fnins.2015.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Nedergaard M, Goldman SA. Glymphatic failure as a final common pathway to dementia. Science . 2020;370:50–56. doi: 10.1126/science.abb8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Mullins AE, Kam K, Parekh A, Bubu OM, Osorio RS, Varga AW. Obstructive sleep apnea and its treatment in aging: effects on Alzheimer’s disease biomarkers, cognition, brain structure and neurophysiology. Neurobiol Dis . 2020;145:105054. doi: 10.1016/j.nbd.2020.105054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Matthews KA, Xu W, Gaglioti AH, Holt JB, Croft JB, Mack D, et al. Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015-2060) in adults aged ≥65 years. Alzheimers Dement . 2019;15:17–24. doi: 10.1016/j.jalz.2018.06.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Konkel L. Racial and ethnic disparities in research studies: the challenge of creating more diverse cohorts. Environ Health Perspect . 2015;123:A297–A302. doi: 10.1289/ehp.123-A297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Burchard EG, Oh SS, Foreman MG, Celedón JC. Moving toward true inclusion of racial/ethnic minorities in federally funded studies: a key step for achieving respiratory health equality in the United States. Am J Respir Crit Care Med . 2015;191:514–521. doi: 10.1164/rccm.201410-1944PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Chen MS, Jr, Lara PN, Dang JH, Paterniti DA, Kelly K. Twenty years post-NIH Revitalization Act: enhancing minority participation in clinical trials (EMPaCT): laying the groundwork for improving minority clinical trial accrual: renewing the case for enhancing minority participation in cancer clinical trials. Cancer . 2014;120:1091–1096. doi: 10.1002/cncr.28575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Dudley KA, Patel SR. Disparities and genetic risk factors in obstructive sleep apnea. Sleep Med . 2016;18:96–102. doi: 10.1016/j.sleep.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Yaffe K, Nasrallah I, Hoang TD, Lauderdale DS, Knutson KL, Carnethon MR, et al. Sleep duration and white matter quality in middle-aged adults. Sleep (Basel) . 2016;39:1743–1747. doi: 10.5665/sleep.6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Pranathiageswaran S, Badr MS, Severson R, Rowley JA. The influence of race on the severity of sleep disordered breathing. J Clin Sleep Med . 2013;9:303–309. doi: 10.5664/jcsm.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Redline S, Sotres-Alvarez D, Loredo J, Hall M, Patel SR, Ramos A, et al. The Hispanic Community Health Study/Study of Latinos Sleep-disordered breathing in Hispanic/Latino individuals of diverse backgrounds. Am J Respir Crit Care Med . 2014;189:335–344. doi: 10.1164/rccm.201309-1735OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Alzheimers Association. 2020 Alzheimer’s disease facts and figures. Alzheimers Dement . 2020 10.1002/alz.12068 [Google Scholar]
- 105. Grandner MA, Hale L, Jackson N, Patel NP, Gooneratne NS, Troxel WM. Perceived racial discrimination as an independent predictor of sleep disturbance and daytime fatigue. Behav Sleep Med . 2012;10:235–249. doi: 10.1080/15402002.2012.654548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Owen JE, Benediktsdottir B, Cook E, Olafsson I, Gislason T, Robinson SR. Alzheimer’s disease neuropathology in the hippocampus and brainstem of people with obstructive sleep apnea. Sleep (Basel) . 2021;44:zsaa195. doi: 10.1093/sleep/zsaa195. [DOI] [PubMed] [Google Scholar]
- 107. Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet . 2020;396:413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O, et al. Australian Imaging Biomarkers and Lifestyle (AIBL) Research Group Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol . 2013;12:357–367. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- 109. Silverberg NB, Ryan LM, Carrillo MC, Sperling R, Petersen RC, Posner HB, et al. Assessment of cognition in early dementia. Alzheimers Dement . 2011;7:e60–e76. doi: 10.1016/j.jalz.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Coley N, Andrieu S, Jaros M, Weiner M, Cedarbaum J, Vellas B. Suitability of the Clinical Dementia Rating-Sum of Boxes as a single primary endpoint for Alzheimer’s disease trials. Alzheimers Dement . 2011;7:602–610.e2. doi: 10.1016/j.jalz.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 111. Huang L-K, Chao S-P, Hu C-J. Clinical trials of new drugs for Alzheimer disease. J Biomed Sci . 2020;27:18. doi: 10.1186/s12929-019-0609-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Cross N, Lampit A, Pye J, Grunstein RR, Marshall N, Naismith SL. Is obstructive sleep apnoea related to neuropsychological function in healthy older adults? A systematic review and meta-analysis. Neuropsychol Rev . 2017;27:389–402. doi: 10.1007/s11065-017-9344-6. [DOI] [PubMed] [Google Scholar]
- 113. Gauthier S, Reisberg B, Zaudig M, Petersen RC, Ritchie K, Broich K, et al. International Psychogeriatric Association Expert Conference on mild cognitive impairment Mild cognitive impairment. Lancet . 2006;367:1262–1270. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- 114. Mubashir T, Abrahamyan L, Niazi A, Piyasena D, Arif AA, Wong J, et al. The prevalence of obstructive sleep apnea in mild cognitive impairment: a systematic review. BMC Neurol . 2019;19:195. doi: 10.1186/s12883-019-1422-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology . 2005;64:277–281. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- 116. Jack CR, Jr, Wiste HJ, Weigand SD, Knopman DS, Vemuri P, Mielke MM, et al. Age, sex, and APOE ε4 effects on memory, brain structure, and β-amyloid across the adult life span. JAMA Neurol . 2015;72:511–519. doi: 10.1001/jamaneurol.2014.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Gosselin N, Baril A-A, Osorio RS, Kaminska M, Carrier J. Obstructive sleep apnea and the risk of cognitive decline in older adults. Am J Respir Crit Care Med . 2019;199:142–148. doi: 10.1164/rccm.201801-0204PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Jessen F, Wiese B, Bachmann C, Eifflaender-Gorfer S, Haller F, Kölsch H, et al. German Study on Aging, Cognition and Dementia in Primary Care Patients Study Group Prediction of dementia by subjective memory impairment: effects of severity and temporal association with cognitive impairment. Arch Gen Psychiatry . 2010;67:414–422. doi: 10.1001/archgenpsychiatry.2010.30. [DOI] [PubMed] [Google Scholar]
- 119. Knudsen GM, Ganz M, Appelhoff S, Boellaard R, Bormans G, Carson RE, et al. Guidelines for the content and format of PET brain data in publications and archives: a consensus paper. J Cereb Blood Flow Metab . 2020;40:1576–1585. doi: 10.1177/0271678X20905433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Berger M, Gould MK, Barnett PG. The cost of positron emission tomography in six United States Veterans Affairs hospitals and two academic medical centers. AJR Am J Roentgenol . 2003;181:359–365. doi: 10.2214/ajr.181.2.1810359. [DOI] [PubMed] [Google Scholar]
- 121. Blennow K, Dubois B, Fagan AM, Lewczuk P, de Leon MJ, Hampel H. Clinical utility of cerebrospinal fluid biomarkers in the diagnosis of early Alzheimer’s disease. Alzheimers Dement . 2015;11:58–69. doi: 10.1016/j.jalz.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Janelidze S, Stomrud E, Smith R, Palmqvist S, Mattsson N, Airey DC, et al. Cerebrospinal fluid p-tau217 performs better than p-tau181 as a biomarker of Alzheimer’s disease. Nat Commun . 2020;11:1683. doi: 10.1038/s41467-020-15436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Jack CR, Jr, Wiste HJ, Weigand SD, Rocca WA, Knopman DS, Mielke MM, et al. Age-specific population frequencies of cerebral β-amyloidosis and neurodegeneration among people with normal cognitive function aged 50-89 years: a cross-sectional study. Lancet Neurol . 2014;13:997–1005. doi: 10.1016/S1474-4422(14)70194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Devenney E, Hodges JR. The mini-mental state examination: pitfalls and limitations. Pract Neurol . 2017;17:79–80. doi: 10.1136/practneurol-2016-001520. [DOI] [PubMed] [Google Scholar]
- 125. Donohue MC, Sperling RA, Salmon DP, Rentz DM, Raman R, Thomas RG, et al. Australian Imaging, Biomarkers, and Lifestyle Flagship Study of Ageing Alzheimer’s Disease Neuroimaging Initiative; Alzheimer’s Disease Cooperative Study. The preclinical Alzheimer cognitive composite: measuring amyloid-related decline. JAMA Neurol . 2014;71:961–970. doi: 10.1001/jamaneurol.2014.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Schneider LS, Goldberg TE. Composite cognitive and functional measures for early stage Alzheimer’s disease trials. Alzheimers Dement (Amst) . 2020;12:e12017. doi: 10.1002/dad2.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Bucks RS, Olaithe M, Eastwood P. Neurocognitive function in obstructive sleep apnoea: a meta-review. Respirology . 2013;18:61–70. doi: 10.1111/j.1440-1843.2012.02255.x. [DOI] [PubMed] [Google Scholar]
- 128. Varga AW, Kam K. The importance of sleep-dependent memory testing in positive airway pressure treatment of obstructive sleep apnea. Am J Respir Crit Care Med . 2021;203:1064–1065. doi: 10.1164/rccm.202101-0078ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Chatterjee P, Pedrini S, Ashton NJ, Tegg M, Goozee K, Singh AK, et al. Diagnostic and prognostic plasma biomarkers for preclinical Alzheimer’s disease. Alzheimers Dement . 2021 doi: 10.1002/alz.12447. [DOI] [PubMed] [Google Scholar]
- 130. Alajmi M, Mulgrew AT, Fox J, Davidson W, Schulzer M, Mak E, et al. Impact of continuous positive airway pressure therapy on blood pressure in patients with obstructive sleep apnea hypopnea: a meta-analysis of randomized controlled trials. Lung . 2007;185:67–72. doi: 10.1007/s00408-006-0117-x. [DOI] [PubMed] [Google Scholar]
- 131. George CF. Reduction in motor vehicle collisions following treatment of sleep apnoea with nasal CPAP. Thorax . 2001;56:508–512. doi: 10.1136/thorax.56.7.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Singh B, Maislin G, Keenan BT, McArdle N, Mazzotti DR, Magalang U, et al. Sleep Apnea Global Interdisciplinary Consortium CPAP treatment and cardiovascular prevention: an alternate study design that includes excessively sleepy patients. Chest . 2020;157:1046–1047. doi: 10.1016/j.chest.2019.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Pack AI, Magalang UJ, Singh B, Kuna ST, Keenan BT, Maislin G. Randomized clinical trials of cardiovascular disease in obstructive sleep apnea: understanding and overcoming bias. Sleep (Basel) . 2021;44:zsaa229. doi: 10.1093/sleep/zsaa229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Mehrtash M, Bakker JP, Ayas N. Predictors of continuous positive airway pressure adherence in patients with obstructive sleep apnea. Lung . 2019;197:115–121. doi: 10.1007/s00408-018-00193-1. [DOI] [PubMed] [Google Scholar]
- 135. McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, et al. SAVE Investigators and Coordinators CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med . 2016;375:919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 136. Nakamura K, Nakamura H, Tohyama K, Takara C, Iseki C, Woodward M, et al. Survival benefit of continuous positive airway pressure in Japanese patients with obstructive sleep apnea: a propensity-score matching analysis. J Clin Sleep Med . 2021;17:211–218. doi: 10.5664/jcsm.8842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Keenan BT, Maislin G, Sunwoo BY, Arnardottir ES, Jackson N, Olafsson I, et al. Obstructive sleep apnoea treatment and fasting lipids: a comparative effectiveness study. Eur Respir J . 2014;44:405–414. doi: 10.1183/09031936.00043614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Pak VM, Keenan BT, Jackson N, Grandner MA, Maislin G, Teff K, et al. Adhesion molecule increases in sleep apnea: beneficial effect of positive airway pressure and moderation by obesity. Int J Obes . 2015;39:472–479. doi: 10.1038/ijo.2014.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Arnardottir ES, Lim DC, Keenan BT, Maislin G, Benediktsdottir B, Juliusson S, et al. Effects of obesity on the association between long-term sleep apnea treatment and changes in interleukin-6 levels: the Icelandic Sleep Apnea Cohort. J Sleep Res . 2015;24:148–159. doi: 10.1111/jsr.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Williamson EJ, Forbes A. Introduction to propensity scores. Respirology . 2014;19:625–635. doi: 10.1111/resp.12312. [DOI] [PubMed] [Google Scholar]


