Abstract
Objective:
Radiation therapy is commonplace for cancer treatment but often results in fibrosis and atrophy of surrounding soft tissue. Decellularized adipose matrices (DAMs) have been reported to improve these soft tissue defects through the promotion of adipogenesis. These matrices are decellularized by a combination of physical, chemical, and enzymatic methods to minimize their immunologic effects while promoting their regenerative effects. In this study, we aimed at exploring the regenerative ability of a DAM (renuva®; MTF biologics, Edison, NJ) in radiation-induced soft tissue injury.
Approach:
Fresh human lipoaspirate or DAM was injected into the irradiated scalp of CD-1 nude mice, and volume retention was monitored radiographically over 8 weeks. Explanted grafts were histologically assessed, and overlying skin was examined histologically and biomechanically. Irradiated human skin was also evaluated from patients after fat grafting or DAM injection. However, integrating data between murine and human skin in all cohorts is limited given the genetic variability between the two species.
Results:
Volume retention was found to be greater with fat grafts, though DAM retention was, nonetheless, appreciated at irradiated sites. Improvement in both mouse and human irradiated skin overlying fat and DAM grafts was observed in terms of biomechanical stiffness, dermal thickness, collagen density, collagen fiber networks, and skin vascularity.
Innovation:
This is the first demonstration of the use of DAMs for augmenting the regenerative potential of irradiated mouse and human skin.
Conclusions:
These findings support the use of DAMs to address soft tissue atrophy after radiation therapy. Morphological characteristics of the irradiated skin can also be improved with DAM grafting.
Keywords: radiation fibrosis, autologous fat grafting, allograft matrix, decellularized adipose matrix

Derrick C. Wan, MD

Rahim S. Nazerali, MD
INTRODUCTION
Substantial progress has been made in cancer therapy leading to better patient outcomes, but as cancer survival rates continue to rise, side effects associated with these treatments are more prevalent. Radiation therapy is a highly effective adjunct in oncologic treatment, and an estimated 50% of all cancer patients receive radiotherapy, of whom 90% experience some form of radiation-induced soft tissue injury.1,2 This often progresses into radiation-induced skin fibrosis (RIF) in the ensuing months to years post-treatment, and this can result in severe physiological and functional impairments, including skin retraction, contour deformities, induration, hypovascularity, restricted movement, and the development of chronic open wounds in irradiated fields.3–5
Autologous fat grafting is an established procedure that aids in the reconstruction and regeneration of irradiated tissue.6–8 Studies have demonstrated that adipose-derived stromal cells (ADSCs) within fat grafts may secrete pro-angiogenic and anti-fibrotic growth factors that contribute to increased vascularity, reorganization of collagen structure, improved skin pliability, and decreased hyperpigmentation and pain at the recipient sites.7–11 In addition, ADSC-enhanced fat grafting has been associated with improved aesthetic outcomes.12 This culminates in decreased fibrosis, enhanced dermal microvasculature, and an overall improved, regenerative local environment with direct clinical benefit seen in treating difficult, chronic wounds on previously irradiated skin.13 Thus, the outcomes that interest us in our preclinical studies would mirror these improvements. A drawback of fat graft-based soft tissue reconstruction is its widely variable retention rate, possibly necessitating repeat grafting and thus compounding the perioperative risks of multiple, repeated procedures.14,15
With the advent of structural and functional implantable scaffolds for tissue regeneration, significant progress has been made in developing and improving these three-dimensional frameworks as an alternative for filled radiated soft tissue defects. In fact, ex vivo tissue processing methods have allowed surgeon scientists to convert natural human tissues into implantable regeneration-promoting scaffolds.16,17 The administration of human decellularized adipose matrices (DAMs), as an injectable off-the-shelf filler without the need for autologous fat or ADSCs, has been developed as an alternative strategy for filling irradiated soft tissue defects.18–20 Use of DAMs may also eliminate concerns regarding potential morbidities associated with lipoaspiration.14 Previously described studies have revealed that adipose tissue contains abundant extracellular matrix components.21 Three-dimensional decellularized scaffolds prepared from discarded lipoaspirate retain most of their complex macromolecular network, and they can promote adhesion, proliferation, and adipogenic differentiation of progenitor cells by interacting with various mesenchymal stem cells and their microenvironments.21–23 Further, newly developed processing protocols for DAM preparation render allografts nonimmunogenic, while still preserving key growth factors including fibroblast growth factors (FGF)-1 and -2 and vascular endothelial growth factor.24,25 These retained growth factors have been described to diminish fibrosis and are integral in the de novo formation of adipose tissue.26–28 These findings highlight the potential use of allograft adipose matrices to address contour deficiencies and fibrosis at sites such as those seen after treatment for breast or head and neck cancer. Since DAMs alter the adipogenic milieu with their paracrine functions, a clinical benefit on irradiated tissue may be similarly observed to fat grafting.29 Therefore, in this study, we investigated the use of a DAM compared with traditional fat grafting, and we assessed its ability to deliver active growth factor components in irradiated fields, thus promoting its soft tissue regeneration capability.
CLINICAL PROBLEM ADDRESSED
Radiation therapy often results in collateral soft tissue fibrosis, which impacts the function and quality of life. Unfortunately, limited effective treatment options currently exist for this adverse sequela. In this study, we demonstrate that DAMs, with a shelf life of 3–4 months in clinical trials, have the capacity for both soft tissue volume restoration and regeneration of fibrotic, hypovascular tissue. These findings highlight the potential for this off-the-shelf therapeutic to improve radiation-damaged skin.
MATERIALS AND METHODS
Animals
Eight-week-old adult female CD-1 Nude immunocompromised mice (Crl:CD1-Foxn1nu; Charles River, Wilmington, MA) were used for experimentation (total n = 20 split into four conditions with n = 5 per group): (1) irradiated, (2) irradiated fat grafted, (3) irradiated DAM grafted, and (4) no irradiation or grafting. Mice were maintained at the Stanford University Research Animal Facility (four animals/cage) in sterile micro-insulators and were given water and rodent chow ad libitum, in accordance with Stanford University guidelines. All experiments were performed under an approved APLAC protocol (APLAC No. 31212) in accordance with the Stanford University Animal Care and Use Committee Guidelines.
Irradiation
CD-1 nude mice (n = 15) were treated with a total of 30 Gy external beam radiation, similar in these animals to standard whole breast radiation therapy in humans, to the scalp delivered as six fractionated doses of 5 Gy over 12 days (Kimtron Polaris SC-500; Kimtron, Inc., Oxford, CT), followed by 4 weeks of recovery to allow for the development of radiation-induced fibrosis, as previously described.6,15,30 Lead shielding was used to protect all other areas of the mouse besides the scalp. This site was selected, as there is minimal native subcutaneous fat compared with other areas, allowing for accurate radiographic graft volume tracking.
Processing of lipoaspirate and allograft preparation
Fresh human lipoaspirate was obtained from three healthy female patients with no medical comorbidities (mean age = 45.7 ± 8.6 years; body mass index 27.0 ± 2.0) with approval by the Stanford Institutional Review Board (IRB No. 2188). Lipoaspirate was incubated for 60 min at 4°C for separation and removal of the blood-aqueous layer and cellular debris. Dehydrated DAM (Renuva®) was obtained from MTF Biologics and rehydrated with sterile 0.9% saline in a 3:1 ratio as per manufacturer's instructions.
Grafting of irradiated mice
A 0.5 mm incision was made at the base of the skull with a 15 blade. A pocket was created in the subdermal space extending from the incision site to the superior aspect of the eyes. Grafting was performed with 200 μL of processed lipoaspirate or reconstituted DAM injected retrograde into this pocket (n = 5 of each respective condition) (Fig. 1).
Figure 1.
Schematic of experimental strategy for irradiation. Mice received 30 Gy radiation to the scalp and were allowed a 4-week recovery period. Mice were then split into three groups: (1) irradiation only control; (2) fat injected; and (3) DAM injected. A fourth mice group, nonirradiated and nongrafted (not depicted), was used as a control. DAM, decellularized adipose matrice.
Radiographic graft volume measurements
Micro-computed tomography (microCT) imaging was performed by using a Bruker Skyscan 1276 (Bruker, Kontich, Belgium). Mice were imaged immediately after fat grafting to determine baseline fat or DAM graft volume, and subsequent imaging was performed every 2 weeks until 8 weeks postgrafting. Hounsfield unit setting of −300 to +300 was utilized to monitor both fat graft volume and DAM graft volume, as the two cohorts are of similar density as previously described.31 Three-dimensional reconstructions were performed by using cubic-spline interpolation to determine fat graft volume as a percentage of the original transplanted volume.
Seventy-six-plex human cytokine luminex assay
Cytokine analysis was performed at the Human Immune Monitoring Center at Stanford University. Luminex-EMD Millipore H76 kits (EMD Millipore Corporation, Burlington, MA) were used according to the manufacturer's recommendations.
Murine tissue harvest and graft explantation
Twelve weeks after the completion of irradiation, mice were sacrificed, scalp skin was harvested, and grafts were explanted. Specimens for histology were fixed in 4% paraformaldehyde (Cat No. 15710; Electron Microscopy Sciences, Hatfield, PA) at 4°C for 18 h, washed with phosphate-buffered saline, dehydrated in gradients of alcohols, and embedded in paraffin for sectioning.
Human tissue collection
Irradiated skin overlying areas previously injected with DAM or autologous fat greater than 3 months earlier were obtained from three female patients (mean age 42.6 ± 10.1 years). DAM or autologous fat grafting was previously performed based on both surgeon and patient preference. DAM was grafted as per manufacturer's instructions. As seen earlier for mouse grafts, this involves reconstitution of small quantities (1.5–3 mL) of DAM in saline. This is achieved by thoroughly mixing, passing DAM and saline through two connected syringes back and forth, and then administering an injection similar to how autologous fat would be grafted. Patient selection criteria included: no other medical comorbidities, had previously undergone either lumpectomy or mastectomy with completion of standard local radiation treatment for cancer, and had received either autologous fat or DAM grafting for initial reconstruction (Fig. 2). Skin samples were obtained from patients who subsequently underwent further autologous breast reconstruction, at which time irradiated noninjected skin, irradiated skin overlying injected DAM, irradiated skin overlying injected autologous fat, and/or nonirradiated skin were resected and discarded. Specimens for histology were fixed in 4% paraformaldehyde at 4°C for 24 h, washed with PBS, dehydrated in alcohol gradients, and embedded in paraffin for sectioning. Skin specimens were all obtained under an approved Stanford University IRB protocol (IRB No. 25954).
Figure 2.
Representation of grafting sites in human subjects. Human samples were obtained from females who had previously undergone lumpectomy and/or mastectomy with radiation therapy for breast cancer. These patients subsequently underwent either autologous or decellularized adipose matrix grafting before delayed autologous breast reconstruction after completion of oncology treatment.
Mechanical analysis of skin
Harvested mouse and ex vivo human tissue samples were loaded into an MTS Bionix 200 (MTS Systems, Eden Prairie, MN) with an Interface SM-10 force transducer. Stress–strain curves were generated to calculate Young's moduli.
Dermal thickness and collagen density measurements
For assessment of dermal thickness, 6 μm sections of both human and murine skin specimens were stained with hematoxylin and eosin (H&E) (Cat. No. H-3502; Vector Laboratories, Burlingame, CA), and, for assessment of collagen density, specimens were stained with Masson's Trichrome (ab150686; Abcam, Cambridge, United Kingdom) and Picrosirius Red (ab150681; Abcam). The dermis was defined as the vertical distance from the basal layer of the epidermis to the underlying hypodermis and was measured on 10 randomly chosen sections per condition at the 10 × and 20 × objective by using a Leica DMI4000 B (Leica Microsystems, Wetzlar, Germany) to allow for significant power of statistical analyses. Masson's Trichrome stained skin was imaged at the 10 × and 20 × objectives, and integrated density measurements of stained collagen were measured on the same 10 chosen sections per condition by using the ImageJ color deconvolution plugin. Picrosirius-stained skin was imaged under a polarized light source by using a Leica DM5000 B light microscope (Leica Microsystems) at the 40 × objective (50 images per condition), and red pixel count was quantified through ImageJ by using a Color Detect macro.
Collagen fiber networks analysis
For assessment of fiber networks, 6 μm sections were stained with Picrosirius Red by using standard protocols. Picrosirius-stained skin was imaged at 40 × magnification (100 images per condition). Images of Picrosirius Red-stained slides were color deconvoluted, converted to gray scale, binarized, and skeletonized by using an algorithm run in MATLAB. From the skeletonized images, 13 parameters of collagen fibers, representing collagen maturation and organization, were extracted (length, width, branchpoints, brightness, number, persistence, angle, Euler number, extent, perimeter, solidity, eccentricity, equivalent diameter) and underwent dimensionality reduction to generate two-dimensional t-distributed stochastic neighbor embedding (TSNE) plots for visualization of how the collagen fiber network patterns were different between groups, as previously described.32
Skin vascularity assessment
Immunofluorescent staining for endothelial cells was performed on 6 μm sectioned paraffin slides that were blocked with 1 × Powerblock (HK083-50K; Biogenex, Fremont, CA) and incubated for 1 h at 37°C with unconjugated anti-CD31 (PECAM) (Ab28364; Abcam) at a 1:100 dilution in 0.1 × Powerblock. Specimens were washed in phosphate-buffered saline (10010023; Gibco®, Dublin, Ireland), incubated with an Alexa Fluor 647 conjugated secondary antibody (Ab10079; Abcam) for 1 h at 37°C, washed in PBS, and finally mounted onto glass slides in DAPI Fluromount-G (0100-20; SouthernBiotech, Birmingham, AL). Fluorescent images of the dermal layer were taken by using an LSM 880 inverted confocal microscope (Airyscan GaAsP detector, 880; Beckman Coulter, Pasadena, CA) at the 20 × objective (25 images per condition). The percentage of pixels positive for CD31 was calculated on each image by using ImageJ with a Color Detect macro to quantify vascularization.
Explanted graft histological analysis
The explanted fat grafts were sectioned into 6 μm slices and stained with H&E to assess for histological structure, and CD31 immunofluorescent staining was used to evaluate graft vascularization. H&E stained slides were imaged in bright field at 10 × and 20 × objectives on a Leica DMI4000 B microscope. Five blinded raters evaluated fat graft quality according to a previously published scoring system that grades: integrity, cyst/vacuoles, inflammation, and fibrosis (0–5 scale with “0” as lowest graft quality and “5” as highest graft quality).33 Raters included a combination of both clinical and basic science personnel at the postdoctoral level with experience handling and analyzing pathologic slides and specimens. Fifteen sections were selected at random from each subject in every conditional group for scoring and statistical comparison. In addition, these sections were stained with anti-CD31, mounted with DAPI Fluoromount-G, and imaged by using an LSM 880 inverted confocal microscope. The mean number of pixels positive for CD31 was calculated on each image by using ImageJ with a Color Detect macro to quantify graft vascularization.
Statistical analysis
Data are presented as means, with error bars representing standard deviation. Parametric analyses were performed by using two-tailed Student's t-tests for comparisons between two groups, and one-way analysis of variance followed by post hoc analysis using Tukey's multiple-comparisons test for multiple groups. Nonparametric analyses were performed with the Kruskal–Wallis test with post hoc Dunn's testing to compare means across groups. GraphPad Prism (GraphPad Software, San Diego, CA) was used to perform all statistical analyses. A value of *p < 0.05 was considered significant.
Results
Cytokine analysis
Cytokine and growth factor analysis was performed to determine potential DAM components contributing to reported cellular repopulation and vascularization after injection. DAM samples were evaluated by a multiplexed Luminex assay, which allows for analysis of 76 different cytokines per sample (Fig. 3A). Among the factors with the highest levels observed, FGF-2, Macrophage migration inhibitory factor (MIF), and platelet-derived growth factor (PDGF)-BB were all appreciated compared with control samples and each have been reported to play a role in regulating adipogenesis. Similarly, increased levels of epidermal growth factor were also observed compared with controls, which have been associated with increased adipogenic differentiation in vitro and greater adipocyte precursors in vivo.34,35 Further, FGF-2, MIF, and PDGF-AA/BB have all been described to have anti-fibrotic and angiogenic effects.27,36,37
Figure 3.
Longitudinal volumetric analysis, cytokine profile, and histological evaluation of grafts. (A) Seventy-six-plex human cytokine profile of DAM (three samples run in duplicates) using a multiplexed Luminex assay. Changes in secreted cytokine concentrations (pg/mL) are plotted in descending order from dark green (highest concentration) to light green (lowest concentration). (B) Micro-computed tomography representative images showing volume of fat grafts of mice receiving fat and DAM at baseline and 8 weeks postgrafting. (C) Graft retention over an 8-week period of each respective condition, volume retention of fat group was significantly greater than the DAM condition at week 4 (*p < 0.05), 6 (**p < 0.01), and 8 (**p < 0.01) postgrafting (n = 5/group). (D) Representative images of fat and DAM grafts stained with H&E. DAM image demonstrates de novo adipogenesis within the scaffold shown by the black arrow. (E) Graft scoring for integrity of intact adipocytes (best integrity = 5), cysts/vacuoles (least presence of cysts/vacuoles = 5), inflammation (least inflammation = 5), and fibrosis (least fibrosis = 5). Fat grafts had greater integrity, less inflammation, and less fibrosis (****p < 0.0001) compared with DAM grafts. (F) Immunofluorescence of representative explanted grafts stained with CD31 and DAPI. (G) CD31 pixel area quantification measuring graft vascularization. Fat grafts demonstrated greater vascularization than DAM scaffold (***p < 0.001). H&E, hematoxylin and eosin.
Longitudinal microCT volumetric analysis
To compare volume retention of fat grafts and DAM at irradiated recipient sites, in vivo graft retention was tracked radiographically for 8 weeks (Fig. 3B, C). Volume was noted to decrease in both groups, though by week 4 onward, volume retention was significantly greater in fat grafted mice compared with injected DAM. By week 8, fat graft volume retention was 59.4% ± 13.8% whereas volume retention of injected DAM was 28.2% ± 7.8% (*p < 0.05, **p < 0.01).
Graft histology
Fat grafts and injected DAM were explanted 8 weeks postgrafting and processed for histological analysis (Fig. 3D). Histologic scoring indicated that fat grafts had significantly greater integrity of adipocytes (****p < 0.0001), less inflammation (****p < 0.0001), and less fibrosis (****p < 0.0001) in comparison to the DAM grafts, whereas the presence of cyst/vacuoles was similar between groups (Fig. 3E). Immunofluorescent imaging of grafts indicated significantly greater amounts of staining for CD31 in fat grafts relative to DAM grafts (***p < 0.001), suggesting greater vascularity (Fig. 3F, G).
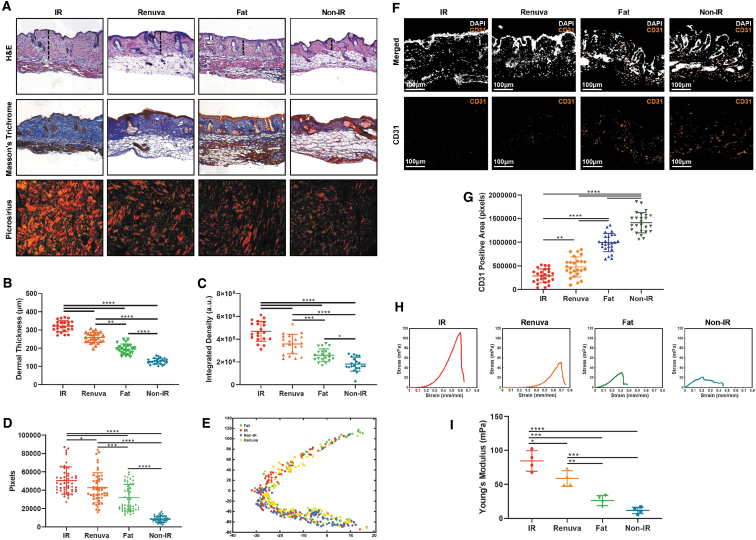
Mouse skin histology
Scalp skin overlying the fat grafts and injected DAM was harvested after 8 weeks and stained with H&E, Masson's Trichrome, and Picrosirius Red (Fig. 4A). Mean dermal thickness was greatest in irradiated nongrafted skin (mean = 320.3 ± 29.9 μm) and lowest in nonirradiated skin (mean = 128.6 ± 15.8 μm). Although a greater reduction in dermal thickness was appreciated in irradiated skin overlying fat grafts compared with DAM-injected mice, both fat (mean = 195.3 ± 27.4 μm) and DAM (mean = 258.6 ± 28.5 μm) grafted conditions demonstrated significant reductions in dermal thickness relative to the irradiated nongrafted cohort (both ****p < 0.0001) (Fig. 4B). Similarly, collagen density was lower in irradiated mouse skin overlying fat grafts compared with injected DAM (***p < 0.001), but both groups demonstrated significant improvement relative to irradiated nongrafted skin (both ****p < 0.001) (Fig. 4C). Quantification of Picrosirius Red stained skin confirmed that the collagen density in DAM or fat grafted irradiated mice skin was significantly lower than the nongrafted irradiated skin as well (*p < 0.05 and ****p < 0.0001, respectively) (Fig. 4D). Computational analysis of Picrosirius Red stained sections, as revealed by the TSNE plot, showed that collagen architecture in mouse skin of nonirradiated and irradiated skin was dissimilar and clustered relatively far apart 8 weeks after irradiation. Collagen ultrastructure of skin overlying fat grafts or DAM grafts was similar to each other and clustered closer to the nonirradiated healthy skin than to irradiated nongrafted skin (Fig. 4E). Finally, staining for CD31 was greatest in nonirradiated skin and lowest in the irradiated nongrafted skin. DAM and fat grafted irradiated mouse skin demonstrated significantly greater CD31 staining compared with irradiated non-grafted skin (**p < 0.01 and ****p < 0.0001, respectively) (Fig. 4F, G).
Figure 4.
Histological analysis and strength testing of mouse skin overlying grafts. (A) Representative histology images of scalp skin overlying fat grafts H&E (top row), Masson's Trichrome (middle row), and Picrosirius Red (bottom row). The dermal thickness of the skin (as measured with a dotted black line). H&E and Masson's Trichrome images were taken at 10 × objective and Picrosirius red at 40 × objective. (B) Dermal thickness measurements of H&E stained skin. DAM grafted conditions demonstrated a significant reduction in dermal thickness relative to the irradiated group (****p < 0.0001). (C) Collagen density measurements of Masson's Trichrome stained skin. DAM grafted condition demonstrated significantly lower integrated density of collagen compared with irradiated skin (****p < 0.001). (D) Collagen quantification of Picrosirius Red stained skin. Collagen quantity in DAM grafted irradiated mice skin was significantly lower than the nongrafted irradiated skin (*p < 0.05). (E) TSNE plot representing the grouping of collagen fiber network parameters in mice of all conditions. DAM and nonirradiated skin conditions exhibited greater clustering around each other, indicating similar collagen networks between groups. (F) Representative images of immunofluorescence of mice scalp skin stained with CD31 and DAPI. (G) Quantification of CD31 pixels positive area of all groups with the DAM grafted condition demonstrating higher vascularity than the irradiated condition (**p < 0.01). (H) Representative stress–strain curves depicting mechanical strength testing of mouse scalp skin for each respective condition. (I) Young's Modulus across all groups. The irradiated skin had significantly higher Young's Modulus when compared with skin from all of the other groups, including the DAM grafted condition (*p < 0.05). TSNE, t-distributed stochastic neighbor embedding.
Biomechanical analyses of murine skin
Tensile testing demonstrated increased stiffness in irradiated skin relative to nonirradiated skin, and fat grafting significantly improved irradiated skin stiffness (***p < 0.001) (Fig. 4H, I). Although reduction in stiffness of irradiated skin was not as dramatic with DAM-injected mice, biomechanical testing still demonstrated a significant improvement from irradiated non-grafted skin (*p < 0.05) (Fig. 4I).
Human skin histology
Ex vivo human skin specimens were stained with H&E, Masson's Trichrome, and Picrosirius Red (Fig. 5A). The dermal thickness of untreated irradiated skin (mean = 3.518 ± 0.717 mm) was significantly greater than the irradiated skin overlying injected DAM (2.919 ± 0.595 mm; **p < 0.01) and autologous fat grafts (mean = 2.847 ± 0.571 mm; ***p < 0.001) (Fig. 5B). Integrated collagen density values of Trichrome stained tissue also demonstrated irradiated skin overlying injected DAM or fat grafts were significantly lower than the nongrafted irradiated skin (*p < 0.05 and ***p < 0.001, respectively) (Fig. 5C). Analysis of the Picrosirius Red stained skin confirmed that the collagen density in irradiated skin overlying injected DAM or fat grafts was significantly lower than the nongrafted irradiated skin as well (***p < 0.001 and ****p < 0.0001, respectively) (Fig. 5D). Collagen network analysis, as depicted by the TNSE plot, demonstrated that untreated irradiated skin clustered differently from irradiated skin overlying injected DAM, irradiated skin overlying injected fat, and nonirradiated skin (Fig. 5E). This indicates that collagen matrix characteristics from nongrafted irradiated human skin were different compared with the other three conditions, which were relatively similar. Immunofluorescent imaging for CD31 revealed the greatest staining in nonirradiated skin and the least in the irradiated nongrafted skin. In addition, irradiated skin overlying injected DAM or fat grafts had significantly higher CD31 staining than non-grafted irradiated skin specimens (*p < 0.05 and ****p < 0.0001, respectively) (Fig. 5F, G).
Figure 5.
Histological analysis and mechanical strength testing of human tissue. (A) Representative histology images of human skin overlying fat grafts H&E (top row), Masson's Trichrome (middle), and Picrosirius Red (bottom). The dermal thickness of the skin (as measured with a dotted black line). H&E and Trichrome images were taken at 5 × objective and Picrosirius red at 40 × objective. (B) Dermal thickness measurements of H&E stained skin. DAM-injected human skin demonstrated a significant reduction in dermal thickness compared with the non-injected irradiated human skin (**p < 0.01). (C) Collagen density measurements of Trichrome-stained skin. DAM-injected irradiated skin demonstrated a significantly lower integrated density of collagen compared with irradiated skin (*p < 0.05). (D) Collagen quantification of Picrosirius Red stained skin. Collagen quantity in DAM-injected irradiated skin was significantly lower than irradiated skin (***p < 0.001). (E) TSNE plot representing the grouping of collagen fiber network parameters in human skin for all conditions. (F) Representative immunofluorescence images of human skin stained with CD31 and DAPI. (G) Percentage of CD31 pixels positive area in all groups. DAM grafted condition exhibiting higher vascularity than the irradiated condition (*p < 0.05). (H) Representative stress–strain curves of skin from human patients who received radiotherapy. (I) Young's Modulus across all groups. DAM-injected irradiated human skin exhibited a lower Young's Modulus compared with untreated irradiated skin (*p < 0.05).
Biomechanical analyses of ex vivo human skin
All conditional groups were tested for tensile strength (Fig. 5H). Irradiated skin overlying injected DAM or fat grafts exhibited a significantly lower Young's Modulus compared with irradiated nongrafted skin (*p < 0.05 and **p < 0.01, respectively) (Fig. 5I). Further, the stiffness of irradiated skin overlying injected DAM and fat grafts was more similar to nonirradiated skin than irradiated nongrafted skin.
DISCUSSION
Radiation therapy has greatly contributed to improved survival outcomes for oncologic patients, though collateral soft tissue injury after irradiation significantly impairs their quality of life. Over the past two decades, fat grafting has become a popular technique to address these soft tissue defects, and it has increasingly demonstrated a regenerative effect on overlying fibrotic skin.38–40 Multiple cytokines and growth factors secreted by ADSCs within fat have been postulated to drive observed improvements in vascularity and dermal thickness.39
The recent development of DAMs has provided an alternative, off-the-shelf approach to improve tissue deficits in patients without the need for additional donor site harvesting associated with autologous fat grafting or the risks and complications of general anesthesia. In addition, the majority of oncologic patients often undergo a significant amount of malnourishment and wasting of endogenous fat and muscle in the perioperative setting secondary to the stress of surgery, the chemotherapy and/or radiation regimens used to treat the malignancy, and increased metabolic demands placed on the body from the pathologic process of the malignancy itself. Recent developments have improved decellularization methods to extract cells, cellular components, and lipids, leaving a complex biologic scaffold enriched in collagen and various growth factors usually found in adipose tissue. Small-volume injections of DAMs have been used clinically to restore volume and cosmesis in soft tissue defects with a biocompatible framework that supports autologous cellular repopulation and vascularization while avoiding any additional risks listed earlier for this specific patient population.
In the present study, we evaluated the ability of a DAM to restore soft tissue volume and mitigate radiation-induced dermal fibrosis in a mouse model. Although less effective than autologous fat grafting, when the DAM was injected into an irradiated mouse scalp, improvements in the overlying radiation-injured skin were observed. Histological analysis revealed that the DAM reduced dermal thickness and excess collagen deposition, and it also altered the collagen architecture of the irradiated mouse skin. This was associated with decreased stiffness of the irradiated skin. In concordance with murine skin findings, human irradiated skin samples showed reduced dermal thickness, changes in collagen networks, and biomechanical improvement after DAM grafting. The antifibrotic effect of DAMs on the irradiated skin may be secondary to cytokines and growth factors retained in the bioscaffold. Cytokine and growth factor profiling demonstrated increased levels of FGF-2 and MIF in the DAMs. FGF-2 has been reported to have multiple antifibrotic activities and has been shown to enhance apoptosis of fibrotic myofibroblasts while decreasing pro-fibrotic TGF-β signaling pathways.27,41–43 Likewise, MIF has also been reported to possess antifibrotic effects via interactions with CD74.36 Finally, staining of CD31 in mouse and human irradiated skin confirmed that the injection of DAM scaffolds can promote angiogenesis. This observation may also be potentially attributed to the growth factors within the scaffold. Cytokine and growth factor profiling revealed a higher level of platelet-derived growth factor-AA/BB, which is known to improve vascularity in adipose tissue.37
Soft tissue volume retention was evaluated by microCT analysis, and by 8 weeks, ∼30% of initial volume was retained in the DAM group, which was half of the 60% retention seen in the fat graft group. Multiple emerging clinical studies have illustrated that DAMs can facilitate durable soft tissue restoration by promoting de novo adipogenesis. Kokai et al. conducted a randomized control trial to evaluate the safety of Renuva for soft tissue reconstruction after abdominoplasty in 10 patients.44 After 6 months, the decellularized scaffolds had remodeled and were composed of perilipin-positive adipocytes, representing native adipose tissue infiltration. Histological analysis confirmed adipogenesis and angiogenesis within the scaffold. In support of these findings, Gold et al. also demonstrated that DAM scaffolds can provide soft tissue replacement without complications in the temporal region of the face.45 However, the mechanism by which the DAMs interact with the surrounding adipose tissue and promote adipogenesis remains unclear. Kokai et al. found that non-commercial DAMs may promote adipogenesis due to the structural proteins in the extracellular matrix.46
Importantly, fat grafting was found to be superior to the DAM injection for restoring soft tissue volume and overcoming dermal fibrosis by almost every metric in our study. Though much of the extracellular collagen network and growth factors in adipose tissue are retained through the processing, DAMs such as Renuva are typically used where endogenous fat already exists. The minimal endogenous adipose tissue in the mouse scalp facilitates longitudinal radiographic analyses but may be insufficient in progenitor cell quantity to repopulate the matrix and facilitate de novo adipogenesis. Nonetheless, our study highlights that DAMs may provide an off-the-shelf alternative that promotes at least some level of volume retention and fibrotic tissue regeneration, even in irradiated recipient sites, throughout the DAMs 3–4 month shelf life.
The use of an athymic mouse model in this study limits the translatability of our findings in several ways. The speed of radiation injury progression differs greatly between humans and mice, and approximating acute, subacute, or chronic fibrosis timelines is difficult. The RIF may have continued to develop past our 4-week recovery period, and it may have been influenced by fat and DAM grafting. This could have confounded our results since nongrafted skin would appear worse comparatively because it did not receive any treatment that may have had an effect on RIF progression. The small sample size of our human tissue specimens also limits the human arm of our study. In particular, the number of breast cancer patients undergoing DAM injection post-oncologic resection is limited at our institution and the amount of time required to wait for an adequate sample size to greater power the human arm of our study is unpredictable and would not be feasible. Further, to prevent unnecessary risk to the human subjects, skin samples were only retrieved when excess skin matching a treatment condition was excised during the breast reconstruction phase of these patients' treatment course. Though the human subjects did not develop any clinical signs of wound complications at the time of their breast reconstruction after DAM grafting, monitoring for any significant late clinical effects of DAM grafting in a large, randomized control trial with varying time points of DAM grafting during radiation therapy would provide greater insight into its effectiveness in truly reversing RIF compared with autologous fat grafting. Nonetheless, our limited human samples still provided a proof of concept for our mouse findings and encourage additional interest in future investigations. In addition, we did not have the ability to investigate effects from an immunogenic standpoint in response to DAM injection, which will certainly be important to address for proper clinical use. Previous studies have demonstrated how DAMs shift macrophages toward the M2 phenotype or anti-inflammatory phenotype, but greater understanding into how allograft adipose matrices interact with infiltrating host cells may accelerate the optimization of DAM scaffolds, eventually mimicking the regenerative capabilities of fat grafts in fibrotic skin and likewise improving clinical outcomes.25 Finally, incorporation of additional soluble growth factors, beyond levels that exist in natural adipose tissue, may allow DAMs to promote adipogenesis and angiogenesis at harsher recipient sites such as those seen after radiation therapy.
INNOVATION
DAM scaffolds have the potential to improve radiation-induced fibrosis and restore cosmesis and functionality, especially when fat grafting may not be available or desirable. This study provides the first demonstration of the utility of DAMs in promoting regeneration of radiation-induced soft tissue injury in both mouse and human skin. Future work may aim at better delineating the mechanisms by which DAMs can promote adipogenesis and angiogenesis to provide an enhanced approach to the healing and regeneration of irradiated and fibrotic tissues.
KEY FINDINGS
DAMs retain cytokines and growth factors with adipogenic, antifibrotic, and angiogenic effects.
Both fat grafting and DAMs can be used to restore soft tissue volume at irradiated recipient sites. Though volume retention appears greater with fat grafting, it requires harvesting and introduces additional risk.
Both DAMs and fat grafts were capable of improving irradiated murine and human skin histologically, in addition to decreasing their biomechanical stiffness.
Abbreviations and Acronyms
- ADSC
adipose-derived stromal cells
- DAM
decellularized adipose matrix
- FGF-2
fibroblast growth factor-2
- H&E
hematoxylin and eosin
- microCT
micro-computed tomography
- MIF
macrophage inhibitor factor
- PDGF
platelet-derived growth factor
- RIF
radiation induced fibrosis
- TSNE
t-distributed stochastic neighbor embedding
AUTHORS' CONTRIBUTIONS/CONFIRMATION
S.A., M.R.B., R.S.N, and D.C.W. conceived, designed, and oversaw the experiments. S.A., N.M.D.D., M.R.B., C.V.L., S.M., D.B.A., A.H.S., R.A.P., and E.J.F. performed experiments and analyzed the data. S.A., M.G., and D.C.W. wrote the article. S.A., N.M.D.D., M.R.B., C.L., D.B.A., E.J.F., M.G., M.T.L., R.S.N., and D.C.W. edited and reviewed the article. All authors reviewed and approved of the final article.
ACKNOWLEDGMENTS AND FUNDING SOURCES
The authors would like to acknowledge Yael Romero and Jing Liang from the Stanford Human Immune Monitoring Center for assisting with the Luminex assays, the Dauskardt lab at Stanford University for use of the mechanical testing machines, and the Stanford Center for Innovation in in vivo Imaging (Sci3) small animal imaging center. This work was supported by the Oak Foundation and the Hagey Laboratory for Pediatric Regenerative Medicine. The NIH S10 Shared Instrumentation Grant (1S10OD02349701, PI Timothy C. Doyle) was used for purchase of the Bruker Skyscan 1276. M.R.B. was supported by the Plastic Surgery Research Foundation (PSRF). N.M.D.D. was supported by the California Institute for Regenerative Medicine (CIRM). A.H.S. was supported by funding from the Sarnoff Cardiovascular Foundation. M.T.L. was supported by the NIH Grant R01 GM116892, Gunn/Olivier Research Fund, Steinhart/Reed Fund, and the NIH Grant U01 HL099776 and U24 DE026914. R.S.N. and D.C.W. were supported by a PSF/MTF Biologics Allograft Tissue Research Grant. D.C.W. was also supported by NIH DE027346.
AUTHOR DISCLOSURE AND GHOST WRITING
R.S.N. and D.C.W. received funding from MTF Biologics in the form of an awarded PSF/MTF Biologics Tissue Allograft Research Grant to support research conducted in this article. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article. S.A., N.M.D.D., M.R.B., C.V.L, S.M., M.G., D.B.A., A.H.S., R.A.P, E.J.F., and M.T.L. have no commercial or financial relationships related to the research conducted that could be interpreted as a conflict of interest to report.
ABOUT THE AUTHORS
Sandeep Adem, MS, obtained his Bachelor of Science in Bioengineering from Santa Clara University and a Master of Science in Bioengineering from the University of California, San Diego. Darren B. Abbas, MD, received his Bachelor of Science with honors in Cellular and Molecular Biology from Tulane University and his Doctor of Medicine from Texas A&M University Health Science Center College of Medicine. He is currently a postdoctoral research fellow in the Department of Surgery at Stanford University. Rahim S. Nazerali, MD, received his medical degree from the Warren Alpert Medical School at Brown University and a Master's of Health Science in Public Health from the Johns Hopkins Medical Institute. He is a Clinical Assistant Professor of Surgery at Stanford University School of Medicine. Derrick C. Wan, MD, received his medical degree from Columbia University College of Physicians and Surgeons. He is a Hagey Family Faculty Scholar in Stem Cell Research and Regenerative Medicine and a Professor of Surgery at Stanford University School of Medicine.
REFERENCES
- 1. Hegedus F, Mathew LM, Schwartz RA. Radiation dermatitis: an overview. Int J Dermatol 2017;56:909–914. [DOI] [PubMed] [Google Scholar]
- 2. Baskar R, Lee KA, Yeo R, Yeoh K-W. Cancer and radiation therapy: current advances and future directions. Int J Med Sci 2012;9:193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brown KR, Rzucidlo E. Acute and chronic radiation injury. J Vasc Surg 2011;53:15S–21S. [DOI] [PubMed] [Google Scholar]
- 4. Wong RKS, Bensadoun RJ, Boers-Doets CB, et al. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Support Care Cancer 2013;21:2933–2948. [DOI] [PubMed] [Google Scholar]
- 5. Nazzal M, Osman MF, Albeshri H, Abbas DB, Angel CA. Wound healing. In: Brunicardi FC, et al., ed. Schwartz's Principles of Surgery. New York, NY: McGraw-Hill Education, 2019. [Google Scholar]
- 6. Garza RM, Paik KJ, Chung MT, et al. Studies in fat grafting: Part III. Fat grafting irradiated tissue—improved skin quality and decreased fat graft retention. Plast Reconstr Surg 2014;134:249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Phulpin B, Gangloff P, Tran N, et al. Rehabilitation of irradiated head and neck tissues by autologous fat transplantation. Plast Reconstr Surg 2009;123:1187–1197. [DOI] [PubMed] [Google Scholar]
- 8. Rigotti G, Galiè M, Baroni G, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg 2007;119:1409–1422. [DOI] [PubMed] [Google Scholar]
- 9. Borrelli MR, Patel RA, Blackshear C, et al. CD34+CD146+ adipose-derived stromal cells enhance engraftment of transplanted fat. Stem Cells Transl Med 2020;9:1389–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Borrelli MR, Patel RA, Adem S, et al. The antifibrotic adipose-derived stromal cell: grafted fat enriched with CD74+ adipose-derived stromal cells reduces chronic radiation-induced skin fibrosis. Stem Cells Transl Med 2020;9:1401–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Borrelli MR, Patel RA, Sokol J, et al. Fat chance: the rejuvenation of irradiated skin. Plast Reconstr Surg Glob Open 2019;7:e2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gentile P, Kothari A, Casella D, Calabrese C. Fat graft enhanced with adipose-derived stem cells in aesthetic breast augmentation: clinical, histological, and instrumental evaluation. Aesthet Surg J 2020;40:962–977. [DOI] [PubMed] [Google Scholar]
- 13. Jacobson LK, Johnson MB, Dedhia RD, Niknam-Bienia S, Wong AK. Impaired wound healing after radiation therapy: a systematic review of pathogenesis and treatment. JPRAS Open 2017;13:92–105. [Google Scholar]
- 14. Banyard DA, Borad V, Amezcua E, et al. Preparation, characterization, and clinical implications of human decellularized adipose tissue extracellular matrix (hDAM): a comprehensive review. Aesthet Surg J 2016;36:349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Flacco J, Chung N, Blackshear CP, et al. Deferoxamine preconditioning of irradiated tissue improves perfusion and fat graft retention. Plast Reconstr Surg 2018;141:655–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gentile P, Scioli MG, Bielli A, Orlandi A, Cervelli V. Concise review: the use of adipose-derived stromal vascular fraction cells and platelet rich plasma in regenerative plastic surgery. Stem Cells 2017;35:117–134. [DOI] [PubMed] [Google Scholar]
- 17. Gentile P, Sterodimas A, Pizzicannella J, et al. Systematic review: allogenic use of stromal vascular fraction (SVF) and decellularized extracellular matrices (ECM) as advanced therapy medicinal products (ATMP) in tissue regeneration. Int J Mol Sci 2020;21:4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Flynn LE. The use of decellularized adipose tissue to provide an inductive microenvironment for the adipogenic differentiation of human adipose-derived stem cells. Biomaterials 2010;31:4715–4724. [DOI] [PubMed] [Google Scholar]
- 19. Wang L, Johnson JA, Zhang Q, Beahm EK. Combining decellularized human adipose tissue extracellular matrix and adipose-derived stem cells for adipose tissue engineering. Acta Biomater 2013;9:8921–8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu I, Nahas Z, Kimmerling KA, Rosson GD, Elisseeff JH. An injectable adipose matrix for soft-tissue reconstruction. Plast Reconstr Surg 2012;129:1247–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jiang X, Lai XR, Lu JQ, et al. Decellularized adipose tissue: a key factor in promoting fat regeneration by recruiting and inducing mesenchymal stem cells. Biochem Biophys Res Commun 2021;541:63–69. [DOI] [PubMed] [Google Scholar]
- 22. Young DA,. Christman KL. Injectable biomaterials for adipose tissue engineering. Biomed. Mater 2012;7:024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Human decellularized dermal matrix seeded with adipose-derived stem cells enhances wound healing in a murine model: experimental study—ClinicalKey. https://www.clinicalkey.com/#!/content/playContent/1-s2.0-S2049080119301049?returnurl=https:%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS2049080119301049%3Fshowall%3Dtrue&referrer= (last accessed January 10, 2021). [DOI] [PMC free article] [PubMed]
- 24. Zhu J, Nathan C, Jin W, et al. Conversion of proepithelin to epithelins: roles of SLPI and elastase in host defense and wound repair. Cell 2002;111:867–878. [DOI] [PubMed] [Google Scholar]
- 25. Cicuéndez M, Casarrubios L, Feito MJ, et al. Effects of human and porcine adipose extracellular matrices decellularized by enzymatic or chemical methods on macrophage polarization and immunocompetence. Int J Mol Sci 2021;22:3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Voytik-Harbin SL, Brightman AO, Kraine MR, Waisner B, Badylak SF. Identification of extractable growth factors from small intestinal submucosa. J Cell Biochem 1997;67:478–491. [PubMed] [Google Scholar]
- 27. Dolivo DM, Larson SA, Dominko T. Fibroblast growth factor 2 as an antifibrotic: antagonism of myofibroblast differentiation and suppression of pro-fibrotic gene expression. Cytokine Growth Factor Rev 2017;38:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Murray LA, Habiel DM, Hohmann M, et al. Antifibrotic role of vascular endothelial growth factor in pulmonary fibrosis. JCI Insight 2017;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fahy EJ, Griffin M, Lavin C, et al. The adrenergic system in plastic and reconstructive surgery: physiology and clinical considerations. Ann Plast Surg 2021 [Epub ahead of print]; DOI: 10.1097/SAP.0000000000002706. [DOI] [PubMed] [Google Scholar]
- 30. Shen AH, Borrelli MR, Adem S, et al. Prophylactic treatment with transdermal deferoxamine mitigates radiation-induced skin fibrosis. Sci Rep 2020;10:12346. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31. Chung MT, Hyun JS, Lo DD, et al. Micro-computed tomography evaluation of human fat grafts in nude mice. Tissue Eng Part C Methods 2013;19:227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mascharak S, desJardin-Park HE, Januszyk M, et al. Divergent molecular signatures of regeneration and fibrosis during wound repair. bioRxiv 2020. [Epub ahead of print]; DOI: 10.1101/2020.12.17.423181. [DOI] [Google Scholar]
- 33. Panettiere P, Marchetti L, Accorsi D. The serial free fat transfer in irradiated prosthetic breast reconstructions. Aesthetic Plast Surg 2009;33:695–700. [DOI] [PubMed] [Google Scholar]
- 34. Adachi H, Kurachi H, Homma H, et al. Epidermal growth factor promotes adipogenesis of 3T3-L1 cell in vitro. Endocrinology 1994;135:1824–1830. [DOI] [PubMed] [Google Scholar]
- 35. Serrero G, Mills D. Physiological role of epidermal growth factor on adipose tissue development in vivo. Proc Natl Acad Sci U S A 1991;88:3912–3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Heinrichs D, Knauel M, Offermanns C, et al. Macrophage migration inhibitory factor (MIF) exerts antifibrotic effects in experimental liver fibrosis via CD74. Proc Natl Acad Sci U S A 2011;108:17444–17449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Watanabe E, Wada T, Okekawa A, et al. Stromal cell-derived factor 1 (SDF1) attenuates platelet-derived growth factor-B (PDGF-B)-induced vascular remodeling for adipose tissue expansion in obesity. Angiogenesis 2020;23:667–684. [DOI] [PubMed] [Google Scholar]
- 38. Griffin MF, Drago J, Almadori A, Kalavrezos N, Butler PE. Evaluation of the efficacy of lipotransfer to manage radiation-induced fibrosis and volume defects in head and neck oncology. Head Neck 2019;41:3647–3655. [DOI] [PubMed] [Google Scholar]
- 39. Almadori A, Griffin M, Ryan CM, et al. Stem cell enriched lipotransfer reverses the effects of fibrosis in systemic sclerosis. PLoS One 2019;14:e0218068. DOI: 10.1371/journal.pone.0218068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Trevor LV, Riches-Suman K, Mahajan AL, Thornton MJ. Adipose tissue: a source of stem cells with potential for regenerative therapies for wound healing. J Clin Med 2020;9:2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Akasaka Y, Ono I, Yamashita T, Jimbow K, Ishii T. Basic fibroblast growth factor promotes apoptosis and suppresses granulation tissue formation in acute incisional wounds. J Pathol 2004;203:710–720. [DOI] [PubMed] [Google Scholar]
- 42. Ishiguro S, Akasaka Y, Kiguchi H, et al. Basic fibroblast growth factor induces down-regulation of α-smooth muscle actin and reduction of myofibroblast areas in open skin wounds. Wound Repair Regen 2009;17:617–625. [DOI] [PubMed] [Google Scholar]
- 43. Svystonyuk DA, Ngu JM, Mewhort HE, et al. Fibroblast growth factor-2 regulates human cardiac myofibroblast-mediated extracellular matrix remodeling. J Transl Med 2015;13:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kokai LE, Sivak WN, Schilling BK, et al. Clinical evaluation of an off-the-shelf allogeneic adipose matrix for soft tissue reconstruction. Plast Reconstr Surg Glob Open 2020;8:e2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gold MH, Kinney BM, Kaminer MS, Rohrich RJ, D'Amico RA. A multi-center, open-label, pilot study of allograft adipose matrix for the correction of atrophic temples. J Cosmet Dermatol 2020;19:1044–1056. [DOI] [PubMed] [Google Scholar]
- 46. Kokai LE, Schilling BK, Chnari E, et al. Injectable allograft adipose matrix supports adipogenic tissue remodeling in the nude mouse and human. Plast Reconstr Surg 2019;143:299e. [DOI] [PMC free article] [PubMed] [Google Scholar]